User login
Chronic, nonhealing leg ulcer
An 80-year-old woman with a history of hypertension, hyperlipidemia, psoriasis vulgaris with associated pruritus, and well-controlled type 2 diabetes mellitus presented with a slowly enlarging ulceration on her left leg of 1 year’s duration. She noted that this lesion healed less rapidly than previous stasis leg ulcerations, despite using the same treatment approach that included dressings, elevation, and diuretics to decrease pedal edema.
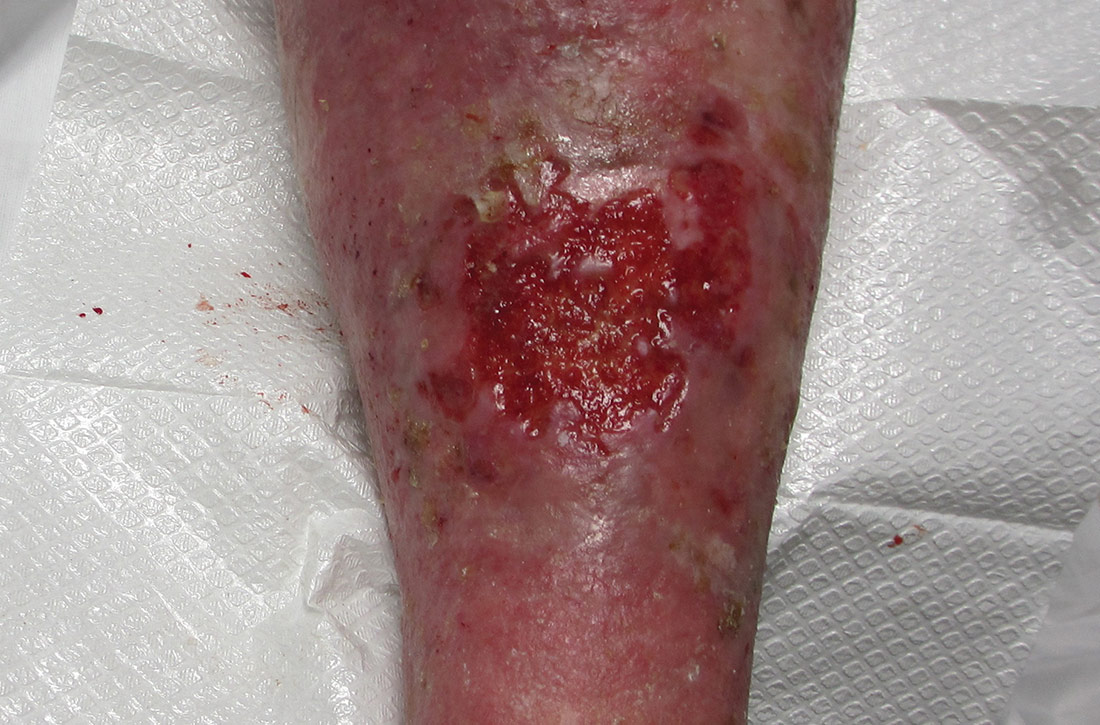
Physical examination revealed plaques with white micaceous scaling over her extensor surfaces and scalp, as well as guttate lesions on the trunk, typical of psoriasis vulgaris. A 5.8 × 7.2-cm malodorous ulceration was superimposed on a large psoriatic plaque on her left anterior lower leg (FIGURE 1). A 4-mm punch biopsy was obtained from the peripheral margin.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Basal cell carcinoma
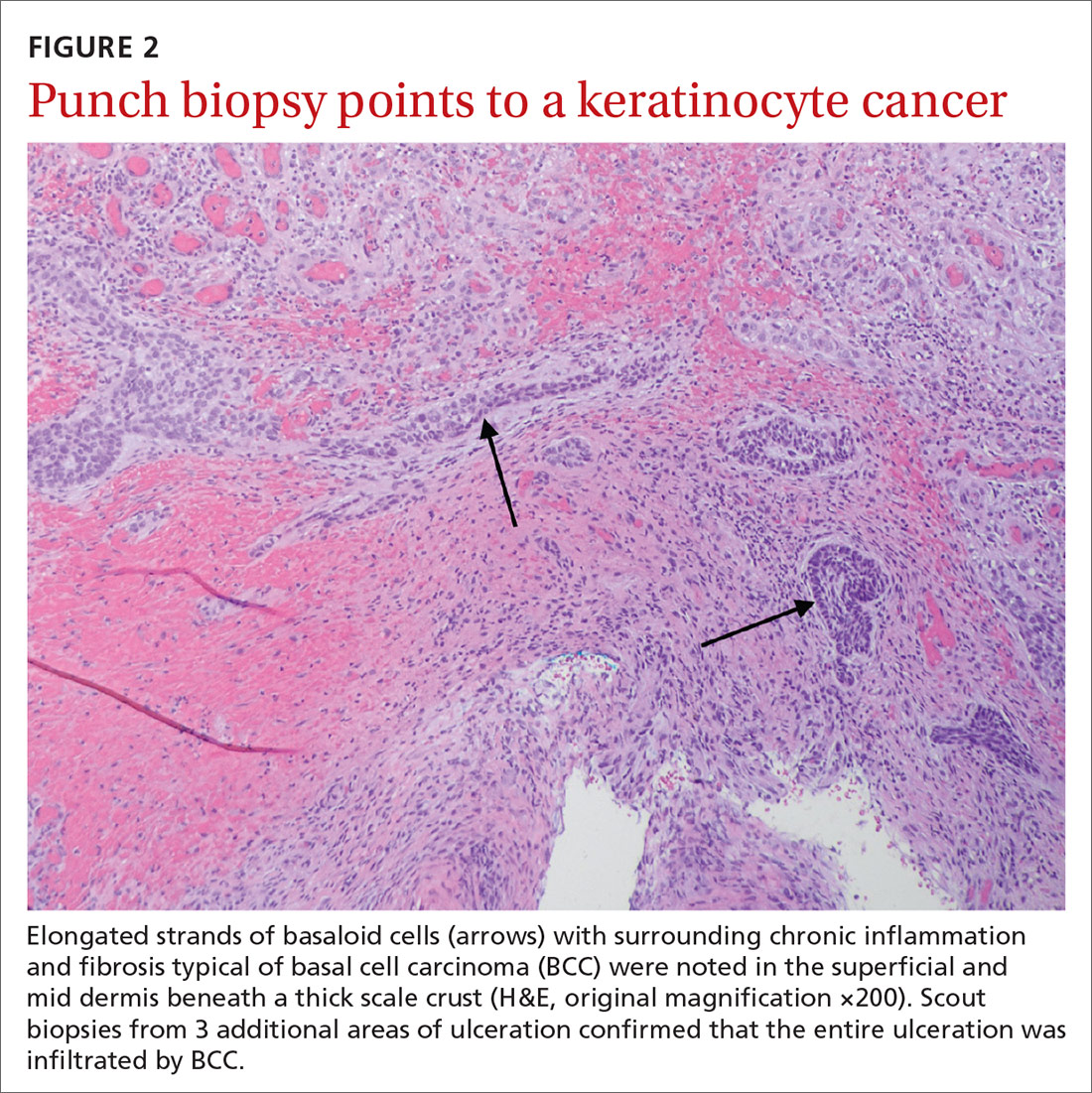
Histopathological examination revealed elongated strands of closely packed basaloid cells embedded in a dense fibrous stroma with overlying ulceration and crusting (FIGURE 2). Immunohistochemical staining with cytokeratin (CK) 5/6 decorated the cytoplasm of the tumor cells, which confirmed that the tumor was a keratinocyte cancer. CK 20 was negative, excluding the possibility of a Merkel cell carcinoma. Scout biopsies from 3 additional areas of ulceration confirmed that the entire ulceration was infiltrated by basal cell carcinoma (BCC).
A surprise hidden in a chronic ulcer
More than 6 million Americans have chronic ulcers and most occur on the legs.1 The majority of these chronic ulcerations are etiologically related to venous stasis, arterial insufficiency, or neuropathy.2
Bacterial pyoderma, chronic infection caused by atypical acid-fast bacilli or deep fungal infection, pyoderma gangrenosum, cutaneous vasculitis, calciphylaxis, and venous ulceration were all considered to explain this patient’s nonhealing wound. A biopsy was required to fully assess these possibilities.
Don’t overlook the possibility of malignancy. In a cross-sectional, multicenter study by Senet et al,3 144 patients with 154 total chronic leg ulcers were evaluated in tertiary care centers for malignancy, which was found to occur at a rate of 10.4%. Similarly, Ghasemi et al4 demonstrated a malignancy rate of 16.1% in 124 patients who underwent biopsy; the anterior shin was determined to be the most frequent location for malignancy. The most common skin cancer identified within the setting of chronic ulcers is squamous cell carcinoma.3 Although rare, there are reports of BCC identified in chronic wounds.3-7
Morphological signs suggestive of malignancy in chronic ulcerations include hyperkeratosis, granulation tissue surrounded by a raised border, unusual pain or bleeding, and increased tissue friability. Our patient had none of these signs and symptoms. However, it is possible that she had a tumor that ulcerated and would not heal.
Continue to: Which came first?
Which came first? It’s difficult to know in this case whether a persistent BCC ulcerated, forming this lesion, or if scarring associated with a chronic ulceration led to the development of the BCC.6 Based on biopsies taken at an earlier date, Schnirring-Judge and Belpedio7 concluded that a chronic leg ulcer could, indeed, transform into a BCC; however, pre-existing BCC more commonly ulcerates and then does not heal.
Treatment options
While smaller, superficial BCCs can be treated with topical imiquimod, photodynamic therapy, or electrodesiccation and curettage, larger lesions should be treated with Mohs micrographic surgery and excisional surgery with grafting. Inoperable tumors may be treated with radiation therapy and vismodegib.
Our patient. Once the diagnosis of BCC was established, treatment options were discussed, including excision, local radiation therapy, and oral hedgehog inhibitor drug therapy.8 Our patient opted to undergo a wide local excision of the lesion followed by negative-pressure wound therapy, which led to complete healing.
CORRESPONDENCE
David Crasto, DO, William Carey University College of Osteopathic Medicine, 498 Tuscan Avenue, Hattiesburg, MS 39401; [email protected]
1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
2. Fox JD, Baquerizo Nole KL, Berriman SJ, et al. Chronic wounds: the need for greater emphasis in medical schools, post-graduate training and public health discussions. Ann Surg. 2016;264:241-243.
3. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers. Arch Dermatol. 2012;148:704-708.
4. Ghasemi F, Anooshirvani N, Sibbald RG, et al. The point prevalence of malignancy in a wound clinic. Int J Low Extrem Wounds. 2016;15:58-62.
5. Labropoulos N, Manalo D, Patel N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
6. Tchanque-Fossuo CN, Millsop J, Johnson MA, et al. Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Adv Skin Wound Care. 2018;31:130-134.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case and review of the pathophysiology. J Foot Ankle Surg. 2010;49:75-79.
8. Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497-503.
An 80-year-old woman with a history of hypertension, hyperlipidemia, psoriasis vulgaris with associated pruritus, and well-controlled type 2 diabetes mellitus presented with a slowly enlarging ulceration on her left leg of 1 year’s duration. She noted that this lesion healed less rapidly than previous stasis leg ulcerations, despite using the same treatment approach that included dressings, elevation, and diuretics to decrease pedal edema.
Physical examination revealed plaques with white micaceous scaling over her extensor surfaces and scalp, as well as guttate lesions on the trunk, typical of psoriasis vulgaris. A 5.8 × 7.2-cm malodorous ulceration was superimposed on a large psoriatic plaque on her left anterior lower leg (FIGURE 1). A 4-mm punch biopsy was obtained from the peripheral margin.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Basal cell carcinoma
Histopathological examination revealed elongated strands of closely packed basaloid cells embedded in a dense fibrous stroma with overlying ulceration and crusting (FIGURE 2). Immunohistochemical staining with cytokeratin (CK) 5/6 decorated the cytoplasm of the tumor cells, which confirmed that the tumor was a keratinocyte cancer. CK 20 was negative, excluding the possibility of a Merkel cell carcinoma. Scout biopsies from 3 additional areas of ulceration confirmed that the entire ulceration was infiltrated by basal cell carcinoma (BCC).

A surprise hidden in a chronic ulcer
More than 6 million Americans have chronic ulcers and most occur on the legs.1 The majority of these chronic ulcerations are etiologically related to venous stasis, arterial insufficiency, or neuropathy.2
Bacterial pyoderma, chronic infection caused by atypical acid-fast bacilli or deep fungal infection, pyoderma gangrenosum, cutaneous vasculitis, calciphylaxis, and venous ulceration were all considered to explain this patient’s nonhealing wound. A biopsy was required to fully assess these possibilities.
Don’t overlook the possibility of malignancy. In a cross-sectional, multicenter study by Senet et al,3 144 patients with 154 total chronic leg ulcers were evaluated in tertiary care centers for malignancy, which was found to occur at a rate of 10.4%. Similarly, Ghasemi et al4 demonstrated a malignancy rate of 16.1% in 124 patients who underwent biopsy; the anterior shin was determined to be the most frequent location for malignancy. The most common skin cancer identified within the setting of chronic ulcers is squamous cell carcinoma.3 Although rare, there are reports of BCC identified in chronic wounds.3-7
Morphological signs suggestive of malignancy in chronic ulcerations include hyperkeratosis, granulation tissue surrounded by a raised border, unusual pain or bleeding, and increased tissue friability. Our patient had none of these signs and symptoms. However, it is possible that she had a tumor that ulcerated and would not heal.
Continue to: Which came first?
Which came first? It’s difficult to know in this case whether a persistent BCC ulcerated, forming this lesion, or if scarring associated with a chronic ulceration led to the development of the BCC.6 Based on biopsies taken at an earlier date, Schnirring-Judge and Belpedio7 concluded that a chronic leg ulcer could, indeed, transform into a BCC; however, pre-existing BCC more commonly ulcerates and then does not heal.
Treatment options
While smaller, superficial BCCs can be treated with topical imiquimod, photodynamic therapy, or electrodesiccation and curettage, larger lesions should be treated with Mohs micrographic surgery and excisional surgery with grafting. Inoperable tumors may be treated with radiation therapy and vismodegib.
Our patient. Once the diagnosis of BCC was established, treatment options were discussed, including excision, local radiation therapy, and oral hedgehog inhibitor drug therapy.8 Our patient opted to undergo a wide local excision of the lesion followed by negative-pressure wound therapy, which led to complete healing.
CORRESPONDENCE
David Crasto, DO, William Carey University College of Osteopathic Medicine, 498 Tuscan Avenue, Hattiesburg, MS 39401; [email protected]
An 80-year-old woman with a history of hypertension, hyperlipidemia, psoriasis vulgaris with associated pruritus, and well-controlled type 2 diabetes mellitus presented with a slowly enlarging ulceration on her left leg of 1 year’s duration. She noted that this lesion healed less rapidly than previous stasis leg ulcerations, despite using the same treatment approach that included dressings, elevation, and diuretics to decrease pedal edema.
Physical examination revealed plaques with white micaceous scaling over her extensor surfaces and scalp, as well as guttate lesions on the trunk, typical of psoriasis vulgaris. A 5.8 × 7.2-cm malodorous ulceration was superimposed on a large psoriatic plaque on her left anterior lower leg (FIGURE 1). A 4-mm punch biopsy was obtained from the peripheral margin.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Basal cell carcinoma
Histopathological examination revealed elongated strands of closely packed basaloid cells embedded in a dense fibrous stroma with overlying ulceration and crusting (FIGURE 2). Immunohistochemical staining with cytokeratin (CK) 5/6 decorated the cytoplasm of the tumor cells, which confirmed that the tumor was a keratinocyte cancer. CK 20 was negative, excluding the possibility of a Merkel cell carcinoma. Scout biopsies from 3 additional areas of ulceration confirmed that the entire ulceration was infiltrated by basal cell carcinoma (BCC).

A surprise hidden in a chronic ulcer
More than 6 million Americans have chronic ulcers and most occur on the legs.1 The majority of these chronic ulcerations are etiologically related to venous stasis, arterial insufficiency, or neuropathy.2
Bacterial pyoderma, chronic infection caused by atypical acid-fast bacilli or deep fungal infection, pyoderma gangrenosum, cutaneous vasculitis, calciphylaxis, and venous ulceration were all considered to explain this patient’s nonhealing wound. A biopsy was required to fully assess these possibilities.
Don’t overlook the possibility of malignancy. In a cross-sectional, multicenter study by Senet et al,3 144 patients with 154 total chronic leg ulcers were evaluated in tertiary care centers for malignancy, which was found to occur at a rate of 10.4%. Similarly, Ghasemi et al4 demonstrated a malignancy rate of 16.1% in 124 patients who underwent biopsy; the anterior shin was determined to be the most frequent location for malignancy. The most common skin cancer identified within the setting of chronic ulcers is squamous cell carcinoma.3 Although rare, there are reports of BCC identified in chronic wounds.3-7
Morphological signs suggestive of malignancy in chronic ulcerations include hyperkeratosis, granulation tissue surrounded by a raised border, unusual pain or bleeding, and increased tissue friability. Our patient had none of these signs and symptoms. However, it is possible that she had a tumor that ulcerated and would not heal.
Continue to: Which came first?
Which came first? It’s difficult to know in this case whether a persistent BCC ulcerated, forming this lesion, or if scarring associated with a chronic ulceration led to the development of the BCC.6 Based on biopsies taken at an earlier date, Schnirring-Judge and Belpedio7 concluded that a chronic leg ulcer could, indeed, transform into a BCC; however, pre-existing BCC more commonly ulcerates and then does not heal.
Treatment options
While smaller, superficial BCCs can be treated with topical imiquimod, photodynamic therapy, or electrodesiccation and curettage, larger lesions should be treated with Mohs micrographic surgery and excisional surgery with grafting. Inoperable tumors may be treated with radiation therapy and vismodegib.
Our patient. Once the diagnosis of BCC was established, treatment options were discussed, including excision, local radiation therapy, and oral hedgehog inhibitor drug therapy.8 Our patient opted to undergo a wide local excision of the lesion followed by negative-pressure wound therapy, which led to complete healing.
CORRESPONDENCE
David Crasto, DO, William Carey University College of Osteopathic Medicine, 498 Tuscan Avenue, Hattiesburg, MS 39401; [email protected]
1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
2. Fox JD, Baquerizo Nole KL, Berriman SJ, et al. Chronic wounds: the need for greater emphasis in medical schools, post-graduate training and public health discussions. Ann Surg. 2016;264:241-243.
3. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers. Arch Dermatol. 2012;148:704-708.
4. Ghasemi F, Anooshirvani N, Sibbald RG, et al. The point prevalence of malignancy in a wound clinic. Int J Low Extrem Wounds. 2016;15:58-62.
5. Labropoulos N, Manalo D, Patel N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
6. Tchanque-Fossuo CN, Millsop J, Johnson MA, et al. Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Adv Skin Wound Care. 2018;31:130-134.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case and review of the pathophysiology. J Foot Ankle Surg. 2010;49:75-79.
8. Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497-503.
1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
2. Fox JD, Baquerizo Nole KL, Berriman SJ, et al. Chronic wounds: the need for greater emphasis in medical schools, post-graduate training and public health discussions. Ann Surg. 2016;264:241-243.
3. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers. Arch Dermatol. 2012;148:704-708.
4. Ghasemi F, Anooshirvani N, Sibbald RG, et al. The point prevalence of malignancy in a wound clinic. Int J Low Extrem Wounds. 2016;15:58-62.
5. Labropoulos N, Manalo D, Patel N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
6. Tchanque-Fossuo CN, Millsop J, Johnson MA, et al. Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Adv Skin Wound Care. 2018;31:130-134.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case and review of the pathophysiology. J Foot Ankle Surg. 2010;49:75-79.
8. Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497-503.
HPV vaccination remains below Healthy People goals despite increases
and vary widely across states based on data from a nested cohort study including more than 7 million children.
“Understanding regional and temporal variations in HPV vaccination coverage may help improve HPV vaccination uptake by informing public health policy,” Szu-Ta Chen, MD, of Harvard University, Boston, and colleagues wrote in Pediatrics.
To identify trends in one-dose and two-dose human papillomavirus (HPV) vaccination coverage, the researchers reviewed data from the MarketScan health care database between January 2003 and December 2017 that included 7,837,480 children and 19,843,737 person-years. The children were followed starting at age 9, when HPV vaccination could begin, and ending at one of the following: the first or second vaccination, insurance disenrollment, December 2017, or the end of the year in which they turned 17.
Overall, the proportion of 15-year-old girls and boys with at least a one-dose HPV vaccination increased from 38% and 5%, respectively, in 2011 to 57% and 51%, respectively, in 2017. The comparable proportions of girls and boys with at least a two-dose vaccination increased from 30% and 2%, respectively, in 2011 to 46% and 39%, respectively, in 2017.
Coverage lacks consistency across states
However, the vaccination coverage varied widely across states; two-dose HPV vaccination coverage ranged from 80% of girls in the District of Columbia to 15% of boys in Mississippi. In general, states with more HPV vaccine interventions had higher levels of vaccination, the researchers noted.
Legislation to improve vaccination education showed the strongest association with coverage; an 8.8% increase in coverage for girls and an 8.7% increase for boys. Pediatrician availability also was a factor associated with a 1.1% increase in coverage estimated for every pediatrician per 10,000 children.
Cumulative HPV vaccinations seen among children continuously enrolled in the study were similar to the primary analysis, the Dr. Chen and associates said. “After the initial HPV vaccination, 87% of girls and 82% of boys received a second dose by age 17 in the most recent cohorts.”
However, the HPV vaccination coverage remains below the Healthy People 2020 goal of 80% of children vaccinated by age 15 years, the researchers said. Barriers to vaccination may include a lack of routine clinical encounters in adolescents aged 11-17 years. HPV vaccination coverage was higher in urban populations, compared with rural, which may be related to a lack of providers in rural areas.
“Thus, measures beyond recommending routine vaccination at annual check-ups might be necessary to attain sufficient HPV vaccine coverage, and the optimal strategy may differ by state characteristics,” they wrote.
The study findings were limited by several factors including the use of data from only commercially-insured children and lack of data on vaccines received outside of insurance, the researchers noted.
However, the results were strengthened by the large, population-based sample, and support the need for increased efforts in HPV vaccination. “Most states will not achieve the Healthy People 2020 goal of 80% coverage with at least two HPV vaccine doses by 2020,” Dr. Chen and associates concluded.
Vaccination goals are possible with effort in the right places
The fact of below-target vaccination for HPV in the United States may be old news, but the current study offers new insights on HPV uptake, Amanda F. Dempsey, MD, PhD, of the University of Colorado at Denver, in Aurora, wrote in an accompanying editorial.
“A unique feature of this study is the ability of its researchers to study individuals over time, particularly at a national scope,” which yielded two key messages, she said.
The longitudinal examination of vaccination levels among birth cohorts showed that similar vaccination levels were achieved more quickly each year.
“For example, among the birth cohort from the year 2000, representing 17-year-olds at the time data were abstracted for the study, 40% vaccination coverage was achieved when this group was 14 years old. In contrast, among the birth cohort from the year 2005, representing 12-year-olds at the time of data abstraction, 40% vaccination coverage was reached at the age of 12,” Dr. Dempsey explained.
In addition, the study design allowed the researchers to model future vaccine coverage based on current trends, said Dr. Dempsey. “The authors estimate that, by the year 2022, the 2012 birth cohort will have reached 80% coverage for the first dose in the HPV vaccine series.”
Dr. Dempsey said she was surprised that the models did not support the hypothesis that school mandates for vaccination would increase coverage; however, there were few states in this category.
Although the findings were limited by the lack of data on uninsured children and those insured by Medicaid, the state-by-state results show that the achievement of national vaccination goals is possible, Dr. Dempsey said. In addition, the findings “warrant close consideration by policy makers and the medical community at large regarding vaccination policies and workforce,” she emphasized.The study received no outside funding. Dr. Chen had no financial conflicts to disclose. Several coauthors reported research grants to their institutions from pharmaceutical companies or being consultants to such companies. Dr. Dempsey disclosed serving on the advisory boards for Merck, Pfizer, and Sanofi Pasteur.
SOURCE: Chen S-T et al. Pediatrics. 2020 Sep 14. doi: 10.1542/peds.2019-3557.
and vary widely across states based on data from a nested cohort study including more than 7 million children.
“Understanding regional and temporal variations in HPV vaccination coverage may help improve HPV vaccination uptake by informing public health policy,” Szu-Ta Chen, MD, of Harvard University, Boston, and colleagues wrote in Pediatrics.
To identify trends in one-dose and two-dose human papillomavirus (HPV) vaccination coverage, the researchers reviewed data from the MarketScan health care database between January 2003 and December 2017 that included 7,837,480 children and 19,843,737 person-years. The children were followed starting at age 9, when HPV vaccination could begin, and ending at one of the following: the first or second vaccination, insurance disenrollment, December 2017, or the end of the year in which they turned 17.
Overall, the proportion of 15-year-old girls and boys with at least a one-dose HPV vaccination increased from 38% and 5%, respectively, in 2011 to 57% and 51%, respectively, in 2017. The comparable proportions of girls and boys with at least a two-dose vaccination increased from 30% and 2%, respectively, in 2011 to 46% and 39%, respectively, in 2017.
Coverage lacks consistency across states
However, the vaccination coverage varied widely across states; two-dose HPV vaccination coverage ranged from 80% of girls in the District of Columbia to 15% of boys in Mississippi. In general, states with more HPV vaccine interventions had higher levels of vaccination, the researchers noted.
Legislation to improve vaccination education showed the strongest association with coverage; an 8.8% increase in coverage for girls and an 8.7% increase for boys. Pediatrician availability also was a factor associated with a 1.1% increase in coverage estimated for every pediatrician per 10,000 children.
Cumulative HPV vaccinations seen among children continuously enrolled in the study were similar to the primary analysis, the Dr. Chen and associates said. “After the initial HPV vaccination, 87% of girls and 82% of boys received a second dose by age 17 in the most recent cohorts.”
However, the HPV vaccination coverage remains below the Healthy People 2020 goal of 80% of children vaccinated by age 15 years, the researchers said. Barriers to vaccination may include a lack of routine clinical encounters in adolescents aged 11-17 years. HPV vaccination coverage was higher in urban populations, compared with rural, which may be related to a lack of providers in rural areas.
“Thus, measures beyond recommending routine vaccination at annual check-ups might be necessary to attain sufficient HPV vaccine coverage, and the optimal strategy may differ by state characteristics,” they wrote.
The study findings were limited by several factors including the use of data from only commercially-insured children and lack of data on vaccines received outside of insurance, the researchers noted.
However, the results were strengthened by the large, population-based sample, and support the need for increased efforts in HPV vaccination. “Most states will not achieve the Healthy People 2020 goal of 80% coverage with at least two HPV vaccine doses by 2020,” Dr. Chen and associates concluded.
Vaccination goals are possible with effort in the right places
The fact of below-target vaccination for HPV in the United States may be old news, but the current study offers new insights on HPV uptake, Amanda F. Dempsey, MD, PhD, of the University of Colorado at Denver, in Aurora, wrote in an accompanying editorial.
“A unique feature of this study is the ability of its researchers to study individuals over time, particularly at a national scope,” which yielded two key messages, she said.
The longitudinal examination of vaccination levels among birth cohorts showed that similar vaccination levels were achieved more quickly each year.
“For example, among the birth cohort from the year 2000, representing 17-year-olds at the time data were abstracted for the study, 40% vaccination coverage was achieved when this group was 14 years old. In contrast, among the birth cohort from the year 2005, representing 12-year-olds at the time of data abstraction, 40% vaccination coverage was reached at the age of 12,” Dr. Dempsey explained.
In addition, the study design allowed the researchers to model future vaccine coverage based on current trends, said Dr. Dempsey. “The authors estimate that, by the year 2022, the 2012 birth cohort will have reached 80% coverage for the first dose in the HPV vaccine series.”
Dr. Dempsey said she was surprised that the models did not support the hypothesis that school mandates for vaccination would increase coverage; however, there were few states in this category.
Although the findings were limited by the lack of data on uninsured children and those insured by Medicaid, the state-by-state results show that the achievement of national vaccination goals is possible, Dr. Dempsey said. In addition, the findings “warrant close consideration by policy makers and the medical community at large regarding vaccination policies and workforce,” she emphasized.The study received no outside funding. Dr. Chen had no financial conflicts to disclose. Several coauthors reported research grants to their institutions from pharmaceutical companies or being consultants to such companies. Dr. Dempsey disclosed serving on the advisory boards for Merck, Pfizer, and Sanofi Pasteur.
SOURCE: Chen S-T et al. Pediatrics. 2020 Sep 14. doi: 10.1542/peds.2019-3557.
and vary widely across states based on data from a nested cohort study including more than 7 million children.
“Understanding regional and temporal variations in HPV vaccination coverage may help improve HPV vaccination uptake by informing public health policy,” Szu-Ta Chen, MD, of Harvard University, Boston, and colleagues wrote in Pediatrics.
To identify trends in one-dose and two-dose human papillomavirus (HPV) vaccination coverage, the researchers reviewed data from the MarketScan health care database between January 2003 and December 2017 that included 7,837,480 children and 19,843,737 person-years. The children were followed starting at age 9, when HPV vaccination could begin, and ending at one of the following: the first or second vaccination, insurance disenrollment, December 2017, or the end of the year in which they turned 17.
Overall, the proportion of 15-year-old girls and boys with at least a one-dose HPV vaccination increased from 38% and 5%, respectively, in 2011 to 57% and 51%, respectively, in 2017. The comparable proportions of girls and boys with at least a two-dose vaccination increased from 30% and 2%, respectively, in 2011 to 46% and 39%, respectively, in 2017.
Coverage lacks consistency across states
However, the vaccination coverage varied widely across states; two-dose HPV vaccination coverage ranged from 80% of girls in the District of Columbia to 15% of boys in Mississippi. In general, states with more HPV vaccine interventions had higher levels of vaccination, the researchers noted.
Legislation to improve vaccination education showed the strongest association with coverage; an 8.8% increase in coverage for girls and an 8.7% increase for boys. Pediatrician availability also was a factor associated with a 1.1% increase in coverage estimated for every pediatrician per 10,000 children.
Cumulative HPV vaccinations seen among children continuously enrolled in the study were similar to the primary analysis, the Dr. Chen and associates said. “After the initial HPV vaccination, 87% of girls and 82% of boys received a second dose by age 17 in the most recent cohorts.”
However, the HPV vaccination coverage remains below the Healthy People 2020 goal of 80% of children vaccinated by age 15 years, the researchers said. Barriers to vaccination may include a lack of routine clinical encounters in adolescents aged 11-17 years. HPV vaccination coverage was higher in urban populations, compared with rural, which may be related to a lack of providers in rural areas.
“Thus, measures beyond recommending routine vaccination at annual check-ups might be necessary to attain sufficient HPV vaccine coverage, and the optimal strategy may differ by state characteristics,” they wrote.
The study findings were limited by several factors including the use of data from only commercially-insured children and lack of data on vaccines received outside of insurance, the researchers noted.
However, the results were strengthened by the large, population-based sample, and support the need for increased efforts in HPV vaccination. “Most states will not achieve the Healthy People 2020 goal of 80% coverage with at least two HPV vaccine doses by 2020,” Dr. Chen and associates concluded.
Vaccination goals are possible with effort in the right places
The fact of below-target vaccination for HPV in the United States may be old news, but the current study offers new insights on HPV uptake, Amanda F. Dempsey, MD, PhD, of the University of Colorado at Denver, in Aurora, wrote in an accompanying editorial.
“A unique feature of this study is the ability of its researchers to study individuals over time, particularly at a national scope,” which yielded two key messages, she said.
The longitudinal examination of vaccination levels among birth cohorts showed that similar vaccination levels were achieved more quickly each year.
“For example, among the birth cohort from the year 2000, representing 17-year-olds at the time data were abstracted for the study, 40% vaccination coverage was achieved when this group was 14 years old. In contrast, among the birth cohort from the year 2005, representing 12-year-olds at the time of data abstraction, 40% vaccination coverage was reached at the age of 12,” Dr. Dempsey explained.
In addition, the study design allowed the researchers to model future vaccine coverage based on current trends, said Dr. Dempsey. “The authors estimate that, by the year 2022, the 2012 birth cohort will have reached 80% coverage for the first dose in the HPV vaccine series.”
Dr. Dempsey said she was surprised that the models did not support the hypothesis that school mandates for vaccination would increase coverage; however, there were few states in this category.
Although the findings were limited by the lack of data on uninsured children and those insured by Medicaid, the state-by-state results show that the achievement of national vaccination goals is possible, Dr. Dempsey said. In addition, the findings “warrant close consideration by policy makers and the medical community at large regarding vaccination policies and workforce,” she emphasized.The study received no outside funding. Dr. Chen had no financial conflicts to disclose. Several coauthors reported research grants to their institutions from pharmaceutical companies or being consultants to such companies. Dr. Dempsey disclosed serving on the advisory boards for Merck, Pfizer, and Sanofi Pasteur.
SOURCE: Chen S-T et al. Pediatrics. 2020 Sep 14. doi: 10.1542/peds.2019-3557.
FROM PEDIATRICS
28-year-old woman • weakness • anxiety • altered mental status • Dx?
THE CASE
A 28-year-old woman with an extensive psychiatric history—including generalized anxiety disorder, panic disorder, and recent postpartum depression—presented with a chief complaint of right leg weakness. She stated this weakness had begun 4 days earlier. It occurred episodically and was preceded by tingling and cramping sensations. Each episode lasted a couple of minutes and spontaneously resolved. Associated with it, she experienced slurred speech and altered mentation. There was no loss of consciousness and no pain. A panic attack usually followed, consisting of feelings of impending doom, rapid breathing, palpitations, and nausea.
She had 3 prior diagnostic evaluations for this same chief complaint, twice in an emergency department (ED) and once with her primary care physician. These evaluations included lab work and extensive head imaging, which demonstrated no acute intracranial pathology. At each previous presentation, the diagnosis was an exacerbation of her anxiety disorder, and she was treated with lorazepam.
At the current presentation, her vital signs were stable. Examination revealed a notably anxious patient. She repeatedly expressed concern that she might have a brain tumor or some other deadly disease, as she had a family history of brain cancer. Her physical exam was entirely normal, including normal strength, sensation, and reflexes in all extremities.
Further head imaging (computed tomography, CT angiography, and magnetic resonance imaging of the brain) failed to reveal an etiology of her symptoms. With no clear organic cause, her medical providers again suspected an anxiety or panic episode. She was given reassurance, and an outpatient neurology consult was arranged.
THE DIAGNOSIS
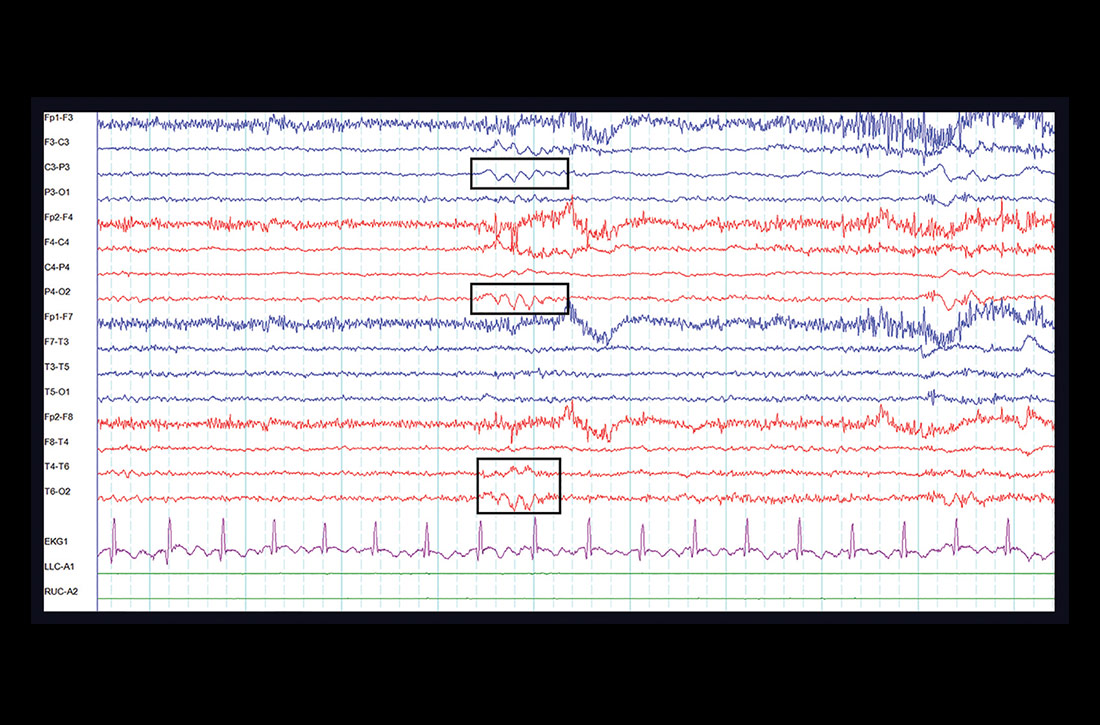
One week later, at her outpatient neurology appointment, an electroencephalogram (EEG) was performed. Following photic stimulation, the EEG showed multiple right- and left-hemisphere foci of cortical hyperexcitability including a subtle sharp component (see FIGURE). Immediately following the longest of these episodes, the patient expressed a sense of anxiety and an altered sensorium similar to her prior presentations.

The EEG findings, in addition to the postictal anxiety symptoms and clinical history, were all important components that led the treating neurologist to the diagnosis of localization-related (focal) epilepsy.1 The patient was started on oxcarbazepine, a first-line anti-epileptic medication used in the treatment of focal epilepsy.2 She is being followed by a neurologist regularly and after optimizing her anti-epileptic medication, is no longer having seizures.
DISCUSSION
The difficulty of this case stems from the atypical presentation of the patient’s seizures. The key step to the correct diagnosis was a neurological consultation and an ensuing EEG. However, the patient received a vast spectrum of care, including multiple work-ups, prior to a conclusive diagnosis—which highlights an important issue health care providers must address.
Continue to: The role of bias
The role of bias. From the patient’s initial visits to the ED to her hospital admission, there was a prominent affixation, known as the anchoring bias,3 by the clinicians providing her care: All were focused heavily on her psychiatric features. Conversely, the evaluation for patients with suspected psychiatric diagnoses should focus on successfully ruling out major organic etiology with a broad differential diagnosis. It is crucial for providers to take a step back and make a conscious attempt to avoid fixation on a particular diagnosis, especially when it is psychiatric in nature. This allows the provider to actively consider alternative explanations for a patient presentation and work through a more encompassing differential.
The distinguishing symptoms. There is a common association between comorbid mood disorders (eg, depression, anxiety) and epilepsy.4 Another clue is ictal anxiety or nervousness, which is commonly observed in patients with partial seizures (and occurred with our patient).
These ictal episodes can be difficult to identify within the context of an isolated psychiatric diagnosis.5 The distinction can be clarified by the presence of associated somatic symptoms, which in this case included unilateral cramping, paresthesia, and weakness. These symptoms should clue in a practitioner to the possibility of underlying neurologic pathology, which should prompt the ordering of either an EEG or, at minimum, a neurological consultation.
THE TAKEAWAY
This case report shows how anchoring bias can lead to a delay in diagnosis and treatment. Avoidance of this type of bias requires heightened cognitive awareness by medical providers. A more system-based approach is to have structured diagnostic assessments,6 such as conducting a thorough neurological exam for patients with somatic symptoms and exacerbating comorbid psychiatric conditions.
It may also help to review cases like this with colleagues from diverse disciplinary backgrounds, highlighting thought processes and sharing uncertainty.3 These processes may shed light on confounding diagnoses that might be playing a role in a patient’s presentation and ultimately aid in the decision-making process.
CORRESPONDENCE
Paimon Ameli, DO, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134; [email protected]
1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530.
2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000-1015.
3. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
4. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
5. López-Gómez M, Espinola M, Ramirez-Bermudez J, et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008;4:1235-1239.
6. Etchells E. Anchoring bias with critical implications. Published June 2015. Patient Safety Network. https://psnet.ahrq.gov/web-mm/anchoring-bias-critical-implications. Accessed September 29, 2020.
THE CASE
A 28-year-old woman with an extensive psychiatric history—including generalized anxiety disorder, panic disorder, and recent postpartum depression—presented with a chief complaint of right leg weakness. She stated this weakness had begun 4 days earlier. It occurred episodically and was preceded by tingling and cramping sensations. Each episode lasted a couple of minutes and spontaneously resolved. Associated with it, she experienced slurred speech and altered mentation. There was no loss of consciousness and no pain. A panic attack usually followed, consisting of feelings of impending doom, rapid breathing, palpitations, and nausea.
She had 3 prior diagnostic evaluations for this same chief complaint, twice in an emergency department (ED) and once with her primary care physician. These evaluations included lab work and extensive head imaging, which demonstrated no acute intracranial pathology. At each previous presentation, the diagnosis was an exacerbation of her anxiety disorder, and she was treated with lorazepam.
At the current presentation, her vital signs were stable. Examination revealed a notably anxious patient. She repeatedly expressed concern that she might have a brain tumor or some other deadly disease, as she had a family history of brain cancer. Her physical exam was entirely normal, including normal strength, sensation, and reflexes in all extremities.
Further head imaging (computed tomography, CT angiography, and magnetic resonance imaging of the brain) failed to reveal an etiology of her symptoms. With no clear organic cause, her medical providers again suspected an anxiety or panic episode. She was given reassurance, and an outpatient neurology consult was arranged.
THE DIAGNOSIS
One week later, at her outpatient neurology appointment, an electroencephalogram (EEG) was performed. Following photic stimulation, the EEG showed multiple right- and left-hemisphere foci of cortical hyperexcitability including a subtle sharp component (see FIGURE). Immediately following the longest of these episodes, the patient expressed a sense of anxiety and an altered sensorium similar to her prior presentations.

The EEG findings, in addition to the postictal anxiety symptoms and clinical history, were all important components that led the treating neurologist to the diagnosis of localization-related (focal) epilepsy.1 The patient was started on oxcarbazepine, a first-line anti-epileptic medication used in the treatment of focal epilepsy.2 She is being followed by a neurologist regularly and after optimizing her anti-epileptic medication, is no longer having seizures.
DISCUSSION
The difficulty of this case stems from the atypical presentation of the patient’s seizures. The key step to the correct diagnosis was a neurological consultation and an ensuing EEG. However, the patient received a vast spectrum of care, including multiple work-ups, prior to a conclusive diagnosis—which highlights an important issue health care providers must address.
Continue to: The role of bias
The role of bias. From the patient’s initial visits to the ED to her hospital admission, there was a prominent affixation, known as the anchoring bias,3 by the clinicians providing her care: All were focused heavily on her psychiatric features. Conversely, the evaluation for patients with suspected psychiatric diagnoses should focus on successfully ruling out major organic etiology with a broad differential diagnosis. It is crucial for providers to take a step back and make a conscious attempt to avoid fixation on a particular diagnosis, especially when it is psychiatric in nature. This allows the provider to actively consider alternative explanations for a patient presentation and work through a more encompassing differential.
The distinguishing symptoms. There is a common association between comorbid mood disorders (eg, depression, anxiety) and epilepsy.4 Another clue is ictal anxiety or nervousness, which is commonly observed in patients with partial seizures (and occurred with our patient).
These ictal episodes can be difficult to identify within the context of an isolated psychiatric diagnosis.5 The distinction can be clarified by the presence of associated somatic symptoms, which in this case included unilateral cramping, paresthesia, and weakness. These symptoms should clue in a practitioner to the possibility of underlying neurologic pathology, which should prompt the ordering of either an EEG or, at minimum, a neurological consultation.
THE TAKEAWAY
This case report shows how anchoring bias can lead to a delay in diagnosis and treatment. Avoidance of this type of bias requires heightened cognitive awareness by medical providers. A more system-based approach is to have structured diagnostic assessments,6 such as conducting a thorough neurological exam for patients with somatic symptoms and exacerbating comorbid psychiatric conditions.
It may also help to review cases like this with colleagues from diverse disciplinary backgrounds, highlighting thought processes and sharing uncertainty.3 These processes may shed light on confounding diagnoses that might be playing a role in a patient’s presentation and ultimately aid in the decision-making process.
CORRESPONDENCE
Paimon Ameli, DO, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134; [email protected]
THE CASE
A 28-year-old woman with an extensive psychiatric history—including generalized anxiety disorder, panic disorder, and recent postpartum depression—presented with a chief complaint of right leg weakness. She stated this weakness had begun 4 days earlier. It occurred episodically and was preceded by tingling and cramping sensations. Each episode lasted a couple of minutes and spontaneously resolved. Associated with it, she experienced slurred speech and altered mentation. There was no loss of consciousness and no pain. A panic attack usually followed, consisting of feelings of impending doom, rapid breathing, palpitations, and nausea.
She had 3 prior diagnostic evaluations for this same chief complaint, twice in an emergency department (ED) and once with her primary care physician. These evaluations included lab work and extensive head imaging, which demonstrated no acute intracranial pathology. At each previous presentation, the diagnosis was an exacerbation of her anxiety disorder, and she was treated with lorazepam.
At the current presentation, her vital signs were stable. Examination revealed a notably anxious patient. She repeatedly expressed concern that she might have a brain tumor or some other deadly disease, as she had a family history of brain cancer. Her physical exam was entirely normal, including normal strength, sensation, and reflexes in all extremities.
Further head imaging (computed tomography, CT angiography, and magnetic resonance imaging of the brain) failed to reveal an etiology of her symptoms. With no clear organic cause, her medical providers again suspected an anxiety or panic episode. She was given reassurance, and an outpatient neurology consult was arranged.
THE DIAGNOSIS
One week later, at her outpatient neurology appointment, an electroencephalogram (EEG) was performed. Following photic stimulation, the EEG showed multiple right- and left-hemisphere foci of cortical hyperexcitability including a subtle sharp component (see FIGURE). Immediately following the longest of these episodes, the patient expressed a sense of anxiety and an altered sensorium similar to her prior presentations.

The EEG findings, in addition to the postictal anxiety symptoms and clinical history, were all important components that led the treating neurologist to the diagnosis of localization-related (focal) epilepsy.1 The patient was started on oxcarbazepine, a first-line anti-epileptic medication used in the treatment of focal epilepsy.2 She is being followed by a neurologist regularly and after optimizing her anti-epileptic medication, is no longer having seizures.
DISCUSSION
The difficulty of this case stems from the atypical presentation of the patient’s seizures. The key step to the correct diagnosis was a neurological consultation and an ensuing EEG. However, the patient received a vast spectrum of care, including multiple work-ups, prior to a conclusive diagnosis—which highlights an important issue health care providers must address.
Continue to: The role of bias
The role of bias. From the patient’s initial visits to the ED to her hospital admission, there was a prominent affixation, known as the anchoring bias,3 by the clinicians providing her care: All were focused heavily on her psychiatric features. Conversely, the evaluation for patients with suspected psychiatric diagnoses should focus on successfully ruling out major organic etiology with a broad differential diagnosis. It is crucial for providers to take a step back and make a conscious attempt to avoid fixation on a particular diagnosis, especially when it is psychiatric in nature. This allows the provider to actively consider alternative explanations for a patient presentation and work through a more encompassing differential.
The distinguishing symptoms. There is a common association between comorbid mood disorders (eg, depression, anxiety) and epilepsy.4 Another clue is ictal anxiety or nervousness, which is commonly observed in patients with partial seizures (and occurred with our patient).
These ictal episodes can be difficult to identify within the context of an isolated psychiatric diagnosis.5 The distinction can be clarified by the presence of associated somatic symptoms, which in this case included unilateral cramping, paresthesia, and weakness. These symptoms should clue in a practitioner to the possibility of underlying neurologic pathology, which should prompt the ordering of either an EEG or, at minimum, a neurological consultation.
THE TAKEAWAY
This case report shows how anchoring bias can lead to a delay in diagnosis and treatment. Avoidance of this type of bias requires heightened cognitive awareness by medical providers. A more system-based approach is to have structured diagnostic assessments,6 such as conducting a thorough neurological exam for patients with somatic symptoms and exacerbating comorbid psychiatric conditions.
It may also help to review cases like this with colleagues from diverse disciplinary backgrounds, highlighting thought processes and sharing uncertainty.3 These processes may shed light on confounding diagnoses that might be playing a role in a patient’s presentation and ultimately aid in the decision-making process.
CORRESPONDENCE
Paimon Ameli, DO, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134; [email protected]
1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530.
2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000-1015.
3. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
4. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
5. López-Gómez M, Espinola M, Ramirez-Bermudez J, et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008;4:1235-1239.
6. Etchells E. Anchoring bias with critical implications. Published June 2015. Patient Safety Network. https://psnet.ahrq.gov/web-mm/anchoring-bias-critical-implications. Accessed September 29, 2020.
1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522-530.
2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000-1015.
3. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
4. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
5. López-Gómez M, Espinola M, Ramirez-Bermudez J, et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008;4:1235-1239.
6. Etchells E. Anchoring bias with critical implications. Published June 2015. Patient Safety Network. https://psnet.ahrq.gov/web-mm/anchoring-bias-critical-implications. Accessed September 29, 2020.
Don’t discount ultrapotent topical corticosteroids for vulvar lichen sclerosus
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
EXPERT ANALYSIS FROM THE ISSVD BIENNIAL CONFERENCE
Prospects and challenges for the upcoming influenza season
The 2020-2021 influenza season is shaping up to be challenging. Its likely concurrence with the ongoing severe acute respiratory syndrome-coronavirus 2 (SARS-coV-2) pandemic (COVID-19) will pose diagnostic and therapeutic dilemmas and could overload the hospital system. But there could also be potential synergies in preventing morbidity and mortality from each disease.
A consistent pattern overthe past few influenza seasons
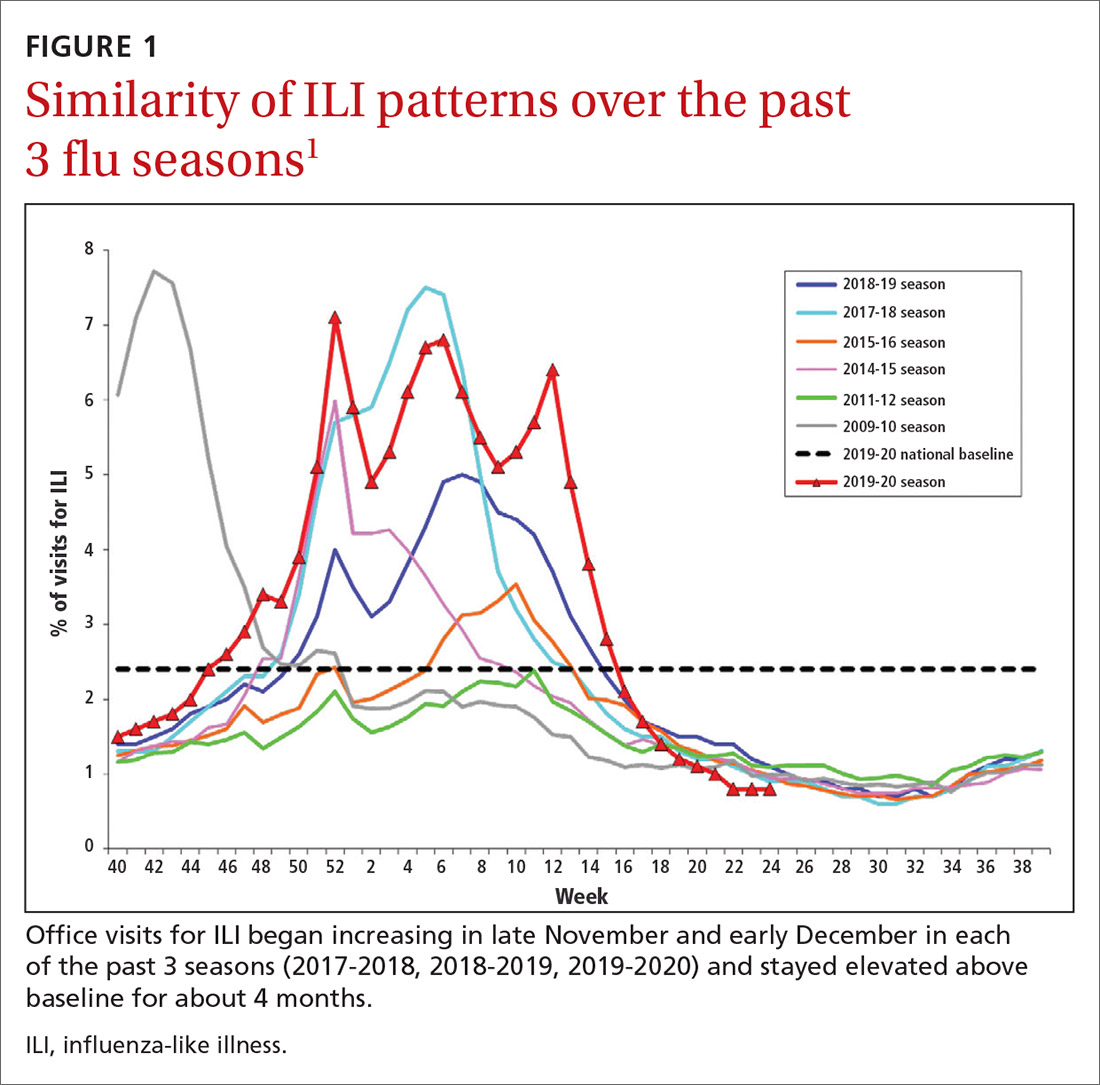
During the 2019-2020 flu season, there were an estimated 410,000 to 740,000 hospitalizations and 24,000 to 62,000 deaths attributed to influenza.1 As seen in FIGURE 1, office visits for influenza-like illness (ILI) began to increase in late November and early December in each of the last 3 years (2017-2018, 2018-2019, 2019-2020) and stayed elevated above baseline for about 4 months each season.1
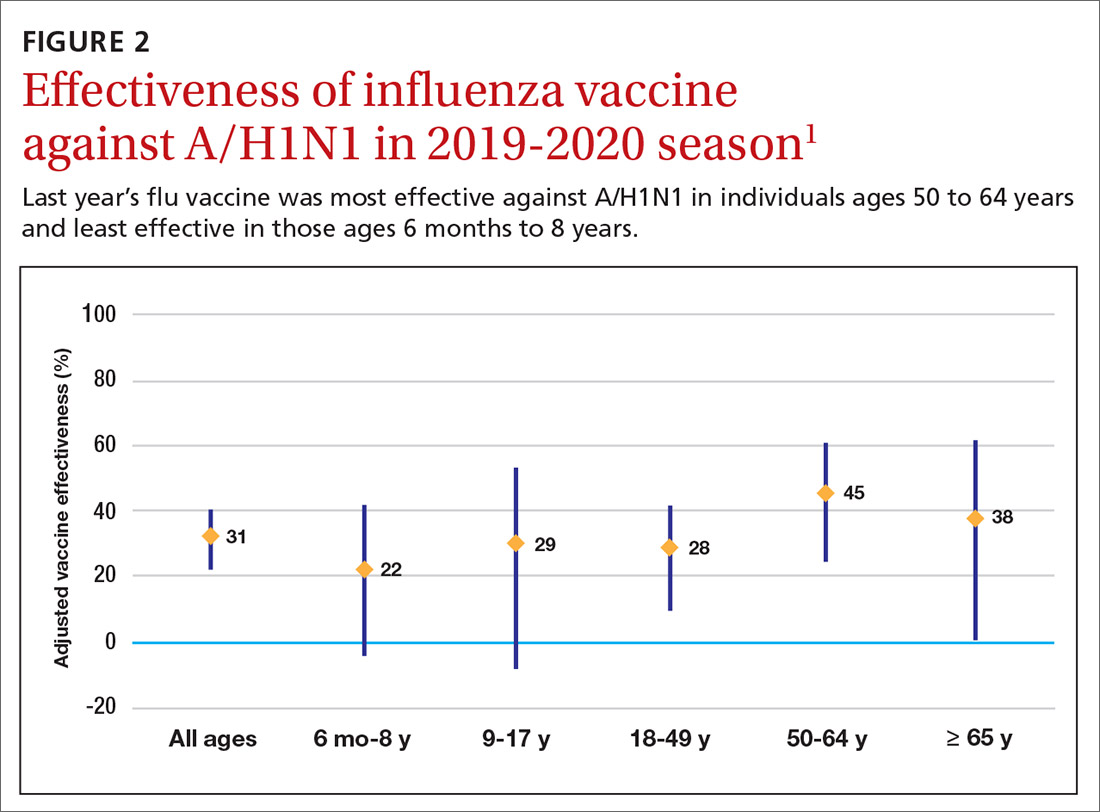
The effectiveness of influenza vaccine during the 2019-2020 season is being estimated using the US Flu Vaccine Effectiveness Network, which has close to 9000 enrollees. Overall, it appears the vaccine was 39% effective against medically attended influenza, with a higher effectiveness against influenza B (44%) than against A/H1N1 (31%). Effectiveness against influenza B was similar in all age groups, but effectiveness against A/H1N1 was highest for those ages 50 to 64 years (45%) and lowest for those ages 6 months through 8 years (22%), although 95% confidence intervals overlapped for all age groups (FIGURE 2). These preliminary effectiveness rates were presented at the summer meeting of the Advisory Committee on Immunization Practices (ACIP).1
Influenza vaccine safety data for 2019-2020 were based on the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system, and on the Vaccine Safety Datalink (VSD) system, an active surveillance system involving close to 6 million doses administered at VSD sites. No safety concerns were identified for any of the different vaccine types. Both the VAERS and VSD surveillance systems have been described in more detail in a previous Practice Alert.2
Recommendations for 2020-2021
The composition of the influenza vaccines for this year’s flu season will be different for 3 of the 4 antigens: A/H1N1, A/H2N2 and B/Victoria.3 The antigens included in the influenza vaccines each year are decided on in the spring, based on surveillance of circulating strains around the world. The effectiveness of the vaccine each year largely depends on how well the strains included in the vaccine match those circulating in the United States during the influenza season.
The main immunization recommendation for preventing morbidity and mortality from influenza has not changed: All individuals ages 6 months and older without a contraindication should receive an influenza vaccine.4 The Centers for Disease Control and Prevention (CDC) recommends that patients receive the vaccine by the end of October.4 This includes the second dose for those children younger than 9 years who need 2 doses—ie, those who have received fewer than 2 doses of influenza vaccine prior to July 2020. Vaccination should continue through the end of the season for anyone who has not received a 2020-2021 influenza vaccine.
Two new influenza vaccine products are available for use in those ages 65 years and older: Fluzone high-dose quadrivalent and Fluad Quadrivalent (adjuvanted).4 Both of these products were available last year as trivalent options. Currently no specific vaccine product is listed as preferred by ACIP for those ages 65 and older.
Continue to: New vaccine contraindications
New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
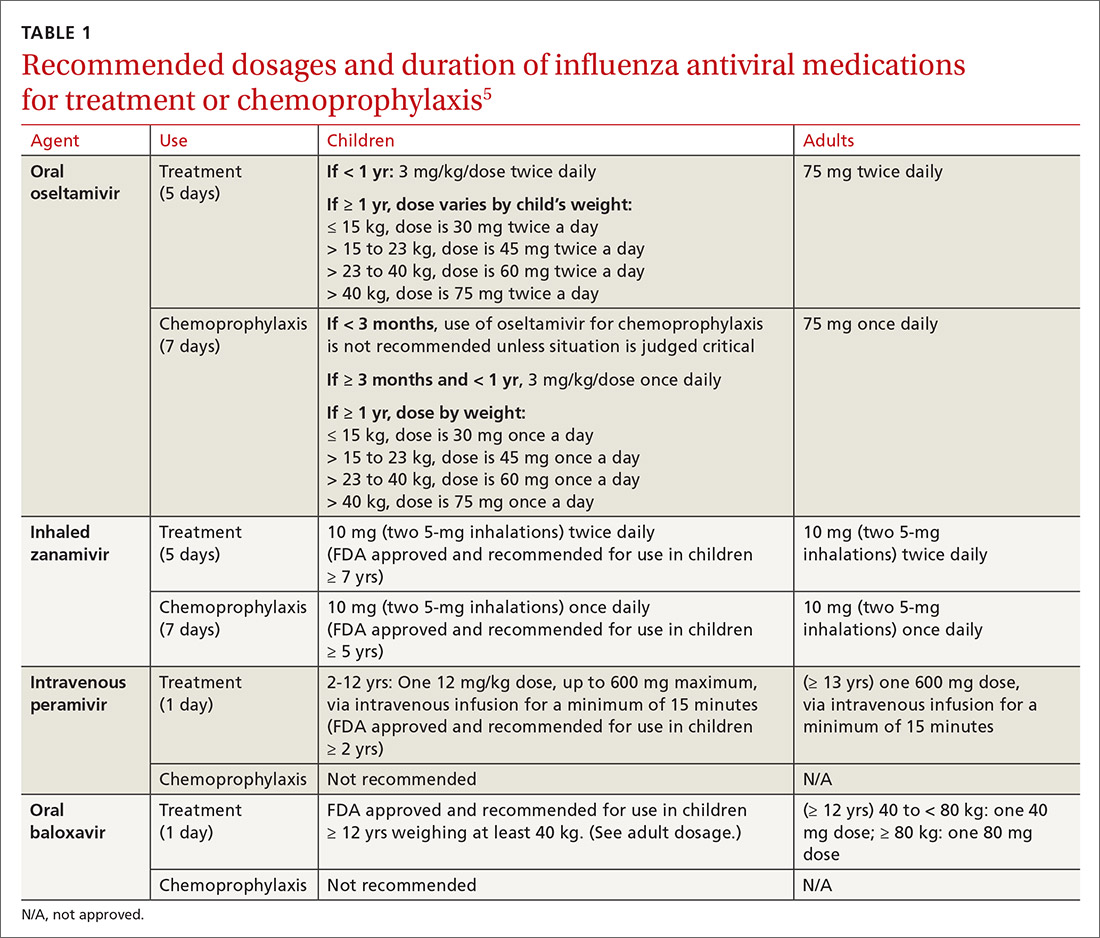
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.
The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.

Continue to: A potential perfect storm
A potential perfect storm: Concurrence of influenza and SARS-coV-19
While we have vaccines and antivirals to prevent influenza, and have effective antivirals for treatment, no prevention or treatment options exist for COVID-19, except, possibly, dexamethasone to reduce mortality among those seriously ill.6 The concurrence of influenza and COVID-19 will present unique challenges for the health care system.
Action steps. Keep abreast of the incidences of circulating SARS-coV-19 and influenza viruses in your community. The similar signs and symptoms of these 2 infectious agents will complicate diagnosis. Rapid, or point-of-care, tests for influenza are widely available, but their accuracy varies and not all tests detect both influenza A and B. The CDC lists approved point-of-care tests at www.cdc.gov/flu/professionals/diagnosis/table-ridt.html and advises on how to interpret these test results when influenza is and is not circulating in the community, at www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm.
Clinical practice advice for both conditions should be implemented when any patient presents with ILI:7
- Most patients who are not seriously ill and have no conditions that place them at high risk for adverse outcomes can be treated symptomatically at home.
- Those with ILI should be tested for both influenza virus and SARS-CoV-2 if testing is available. It is possible to be co-infected.
- Sick patients should self-isolate at home for the duration of their symptoms.
- If others live in the house, the sick person should stay in a separate room and wear a mask. Everyone in the house should cover coughs and sneezes (if not wearing a mask), dispose of used tissues in a trash can (rather than leaving them on night stands and countertops), and wash hands frequently.
- All household members should be vaccinated against influenza. Those who are unvaccinated, and those at high risk who have been recently vaccinated, can consider influenza antiviral prophylaxis. If the sick family member is confirmed to have COVID-19, with no co-existing influenza, anti-influenza antiviral prophylaxis may be discontinued.
- Clinical infection control practices should be the same for anyone presenting with ILI.7 Enhanced clinic-based infection control practices to prevent spread of SARS-CoV-2 are listed in TABLE 3.8
Since there currently are no preventive medications proven to work for COVID-19, the main clinical decision physicians will have to make when a patient presents with ILI is whether to use antivirals to treat those who are at risk for complications based on the result of rapid, on-site influenza testing, or clinical presentation, or both. In this situation, knowledge of which viruses are circulating at high rates in the community could be valuable.
Milder season or perfect storm? The society-wide interventions that have been encouraged (although not mandated everywhere) to prevent community spread of SARS-CoV-2 should help prevent the community spread of influenza as well, and, if adhered to, may lead to a milder influenza season than would otherwise have occurred. However, given the uncertainties, the combination of influenza and coronavirus could present a perfect storm for the health care system and result in higher-than-normal morbidity and mortality from ILI and pneumonia overall.
Continue to: The possibility that one or more vaccines...
The possibility that one or more vaccines to prevent COVID-19 may be available in late 2020 or early 2021 offers hope. However, in current testing, the vaccine is not being given simultaneously with the influenza vaccine. If the potential for adverse interaction exists between the vaccines, it is important that influenza vaccine be given by mid- to late-October to avoid such an interaction if and when the new SARS-CoV-2 vaccine becomes available. Individuals who have symptoms of COVID-19 should not be vaccinated with influenza vaccine until they are considered noninfectious.
Encourage influenza vaccination. The COVID-19 pandemic may make it difficult to achieve desired community influenza vaccine levels because of decreased visits to medical facilities for preventive care, possible lower insurance coverage due to loss of employment, and a decrease in worksite mass vaccination programs. This makes it important for family physicians to encourage and offer influenza vaccines at their clinical sites.
Several evidence-based practices have been shown to improve vaccine uptake. Examples of such practices include patient reminder and recall systems that provide feedback to clinicians about rates of vaccination among patients, and establishing standing orders for vaccine administration that allow other health care providers to assess a patient’s immunization status and administer vaccinations according to a protocol.9 Finally, the CDC provides a video on how to recommend influenza vaccine to those who may be resistant (www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html).
SIDEBAR
CDC influenza resources
Point-of-care tests that detect both influenza A and B viruses approved by the CDC
www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
Advice on how to interpret the test results
www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
How to recommend influenza vaccine to reluctant patients
www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html
CDC, Centers for Disease Control and Prevention.
1. Grohskopf L. Influenza work groups: updates, considerations, and proposed recommendations for the 2020-2021 season. Presented at the ACIP meeting June 24, 2020. www.youtube.com/watch?v=W1SV2DSJsaQ&list=PLvrp9iOILTQb6D9e1YZWpbUvzfptNMKx2&index=8&t=0s. [Time stamp: 1:26:48] Accessed Septemeber 29, 2020.
2. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
3. Grohskopf L, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
4. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21 Summary of Recommendations. www.cdc.gov/flu/pdf/professionals/acip/acip-2020-21-summary-of-recommendations.pdf. Accessed September 29, 2020.
5. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2020.
6. NIH. COVID-19 treatment guidelines. Corticosteroids. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 29, 2020.
7. CDC. Infection control. www.cdc.gov/infectioncontrol/. Accessed September 29, 2020.
8. CDC. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Accessed September 29, 2020.
9. HHS. CPSTF findings for increasing vaccination. www.thecommunityguide.org/content/task-force-findings-increasing-vaccination. Accessed September 29, 2020.
The 2020-2021 influenza season is shaping up to be challenging. Its likely concurrence with the ongoing severe acute respiratory syndrome-coronavirus 2 (SARS-coV-2) pandemic (COVID-19) will pose diagnostic and therapeutic dilemmas and could overload the hospital system. But there could also be potential synergies in preventing morbidity and mortality from each disease.
A consistent pattern overthe past few influenza seasons
During the 2019-2020 flu season, there were an estimated 410,000 to 740,000 hospitalizations and 24,000 to 62,000 deaths attributed to influenza.1 As seen in FIGURE 1, office visits for influenza-like illness (ILI) began to increase in late November and early December in each of the last 3 years (2017-2018, 2018-2019, 2019-2020) and stayed elevated above baseline for about 4 months each season.1

The effectiveness of influenza vaccine during the 2019-2020 season is being estimated using the US Flu Vaccine Effectiveness Network, which has close to 9000 enrollees. Overall, it appears the vaccine was 39% effective against medically attended influenza, with a higher effectiveness against influenza B (44%) than against A/H1N1 (31%). Effectiveness against influenza B was similar in all age groups, but effectiveness against A/H1N1 was highest for those ages 50 to 64 years (45%) and lowest for those ages 6 months through 8 years (22%), although 95% confidence intervals overlapped for all age groups (FIGURE 2). These preliminary effectiveness rates were presented at the summer meeting of the Advisory Committee on Immunization Practices (ACIP).1

Influenza vaccine safety data for 2019-2020 were based on the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system, and on the Vaccine Safety Datalink (VSD) system, an active surveillance system involving close to 6 million doses administered at VSD sites. No safety concerns were identified for any of the different vaccine types. Both the VAERS and VSD surveillance systems have been described in more detail in a previous Practice Alert.2
Recommendations for 2020-2021
The composition of the influenza vaccines for this year’s flu season will be different for 3 of the 4 antigens: A/H1N1, A/H2N2 and B/Victoria.3 The antigens included in the influenza vaccines each year are decided on in the spring, based on surveillance of circulating strains around the world. The effectiveness of the vaccine each year largely depends on how well the strains included in the vaccine match those circulating in the United States during the influenza season.
The main immunization recommendation for preventing morbidity and mortality from influenza has not changed: All individuals ages 6 months and older without a contraindication should receive an influenza vaccine.4 The Centers for Disease Control and Prevention (CDC) recommends that patients receive the vaccine by the end of October.4 This includes the second dose for those children younger than 9 years who need 2 doses—ie, those who have received fewer than 2 doses of influenza vaccine prior to July 2020. Vaccination should continue through the end of the season for anyone who has not received a 2020-2021 influenza vaccine.
Two new influenza vaccine products are available for use in those ages 65 years and older: Fluzone high-dose quadrivalent and Fluad Quadrivalent (adjuvanted).4 Both of these products were available last year as trivalent options. Currently no specific vaccine product is listed as preferred by ACIP for those ages 65 and older.
Continue to: New vaccine contraindications
New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.

The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.

Continue to: A potential perfect storm
A potential perfect storm: Concurrence of influenza and SARS-coV-19
While we have vaccines and antivirals to prevent influenza, and have effective antivirals for treatment, no prevention or treatment options exist for COVID-19, except, possibly, dexamethasone to reduce mortality among those seriously ill.6 The concurrence of influenza and COVID-19 will present unique challenges for the health care system.
Action steps. Keep abreast of the incidences of circulating SARS-coV-19 and influenza viruses in your community. The similar signs and symptoms of these 2 infectious agents will complicate diagnosis. Rapid, or point-of-care, tests for influenza are widely available, but their accuracy varies and not all tests detect both influenza A and B. The CDC lists approved point-of-care tests at www.cdc.gov/flu/professionals/diagnosis/table-ridt.html and advises on how to interpret these test results when influenza is and is not circulating in the community, at www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm.
Clinical practice advice for both conditions should be implemented when any patient presents with ILI:7
- Most patients who are not seriously ill and have no conditions that place them at high risk for adverse outcomes can be treated symptomatically at home.
- Those with ILI should be tested for both influenza virus and SARS-CoV-2 if testing is available. It is possible to be co-infected.
- Sick patients should self-isolate at home for the duration of their symptoms.
- If others live in the house, the sick person should stay in a separate room and wear a mask. Everyone in the house should cover coughs and sneezes (if not wearing a mask), dispose of used tissues in a trash can (rather than leaving them on night stands and countertops), and wash hands frequently.
- All household members should be vaccinated against influenza. Those who are unvaccinated, and those at high risk who have been recently vaccinated, can consider influenza antiviral prophylaxis. If the sick family member is confirmed to have COVID-19, with no co-existing influenza, anti-influenza antiviral prophylaxis may be discontinued.
- Clinical infection control practices should be the same for anyone presenting with ILI.7 Enhanced clinic-based infection control practices to prevent spread of SARS-CoV-2 are listed in TABLE 3.8

Since there currently are no preventive medications proven to work for COVID-19, the main clinical decision physicians will have to make when a patient presents with ILI is whether to use antivirals to treat those who are at risk for complications based on the result of rapid, on-site influenza testing, or clinical presentation, or both. In this situation, knowledge of which viruses are circulating at high rates in the community could be valuable.
Milder season or perfect storm? The society-wide interventions that have been encouraged (although not mandated everywhere) to prevent community spread of SARS-CoV-2 should help prevent the community spread of influenza as well, and, if adhered to, may lead to a milder influenza season than would otherwise have occurred. However, given the uncertainties, the combination of influenza and coronavirus could present a perfect storm for the health care system and result in higher-than-normal morbidity and mortality from ILI and pneumonia overall.
Continue to: The possibility that one or more vaccines...
The possibility that one or more vaccines to prevent COVID-19 may be available in late 2020 or early 2021 offers hope. However, in current testing, the vaccine is not being given simultaneously with the influenza vaccine. If the potential for adverse interaction exists between the vaccines, it is important that influenza vaccine be given by mid- to late-October to avoid such an interaction if and when the new SARS-CoV-2 vaccine becomes available. Individuals who have symptoms of COVID-19 should not be vaccinated with influenza vaccine until they are considered noninfectious.
Encourage influenza vaccination. The COVID-19 pandemic may make it difficult to achieve desired community influenza vaccine levels because of decreased visits to medical facilities for preventive care, possible lower insurance coverage due to loss of employment, and a decrease in worksite mass vaccination programs. This makes it important for family physicians to encourage and offer influenza vaccines at their clinical sites.
Several evidence-based practices have been shown to improve vaccine uptake. Examples of such practices include patient reminder and recall systems that provide feedback to clinicians about rates of vaccination among patients, and establishing standing orders for vaccine administration that allow other health care providers to assess a patient’s immunization status and administer vaccinations according to a protocol.9 Finally, the CDC provides a video on how to recommend influenza vaccine to those who may be resistant (www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html).
SIDEBAR
CDC influenza resources
Point-of-care tests that detect both influenza A and B viruses approved by the CDC
www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
Advice on how to interpret the test results
www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
How to recommend influenza vaccine to reluctant patients
www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html
CDC, Centers for Disease Control and Prevention.
The 2020-2021 influenza season is shaping up to be challenging. Its likely concurrence with the ongoing severe acute respiratory syndrome-coronavirus 2 (SARS-coV-2) pandemic (COVID-19) will pose diagnostic and therapeutic dilemmas and could overload the hospital system. But there could also be potential synergies in preventing morbidity and mortality from each disease.
A consistent pattern overthe past few influenza seasons
During the 2019-2020 flu season, there were an estimated 410,000 to 740,000 hospitalizations and 24,000 to 62,000 deaths attributed to influenza.1 As seen in FIGURE 1, office visits for influenza-like illness (ILI) began to increase in late November and early December in each of the last 3 years (2017-2018, 2018-2019, 2019-2020) and stayed elevated above baseline for about 4 months each season.1

The effectiveness of influenza vaccine during the 2019-2020 season is being estimated using the US Flu Vaccine Effectiveness Network, which has close to 9000 enrollees. Overall, it appears the vaccine was 39% effective against medically attended influenza, with a higher effectiveness against influenza B (44%) than against A/H1N1 (31%). Effectiveness against influenza B was similar in all age groups, but effectiveness against A/H1N1 was highest for those ages 50 to 64 years (45%) and lowest for those ages 6 months through 8 years (22%), although 95% confidence intervals overlapped for all age groups (FIGURE 2). These preliminary effectiveness rates were presented at the summer meeting of the Advisory Committee on Immunization Practices (ACIP).1

Influenza vaccine safety data for 2019-2020 were based on the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system, and on the Vaccine Safety Datalink (VSD) system, an active surveillance system involving close to 6 million doses administered at VSD sites. No safety concerns were identified for any of the different vaccine types. Both the VAERS and VSD surveillance systems have been described in more detail in a previous Practice Alert.2
Recommendations for 2020-2021
The composition of the influenza vaccines for this year’s flu season will be different for 3 of the 4 antigens: A/H1N1, A/H2N2 and B/Victoria.3 The antigens included in the influenza vaccines each year are decided on in the spring, based on surveillance of circulating strains around the world. The effectiveness of the vaccine each year largely depends on how well the strains included in the vaccine match those circulating in the United States during the influenza season.
The main immunization recommendation for preventing morbidity and mortality from influenza has not changed: All individuals ages 6 months and older without a contraindication should receive an influenza vaccine.4 The Centers for Disease Control and Prevention (CDC) recommends that patients receive the vaccine by the end of October.4 This includes the second dose for those children younger than 9 years who need 2 doses—ie, those who have received fewer than 2 doses of influenza vaccine prior to July 2020. Vaccination should continue through the end of the season for anyone who has not received a 2020-2021 influenza vaccine.
Two new influenza vaccine products are available for use in those ages 65 years and older: Fluzone high-dose quadrivalent and Fluad Quadrivalent (adjuvanted).4 Both of these products were available last year as trivalent options. Currently no specific vaccine product is listed as preferred by ACIP for those ages 65 and older.
Continue to: New vaccine contraindications
New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.

The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.

Continue to: A potential perfect storm
A potential perfect storm: Concurrence of influenza and SARS-coV-19
While we have vaccines and antivirals to prevent influenza, and have effective antivirals for treatment, no prevention or treatment options exist for COVID-19, except, possibly, dexamethasone to reduce mortality among those seriously ill.6 The concurrence of influenza and COVID-19 will present unique challenges for the health care system.
Action steps. Keep abreast of the incidences of circulating SARS-coV-19 and influenza viruses in your community. The similar signs and symptoms of these 2 infectious agents will complicate diagnosis. Rapid, or point-of-care, tests for influenza are widely available, but their accuracy varies and not all tests detect both influenza A and B. The CDC lists approved point-of-care tests at www.cdc.gov/flu/professionals/diagnosis/table-ridt.html and advises on how to interpret these test results when influenza is and is not circulating in the community, at www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm.
Clinical practice advice for both conditions should be implemented when any patient presents with ILI:7
- Most patients who are not seriously ill and have no conditions that place them at high risk for adverse outcomes can be treated symptomatically at home.
- Those with ILI should be tested for both influenza virus and SARS-CoV-2 if testing is available. It is possible to be co-infected.
- Sick patients should self-isolate at home for the duration of their symptoms.
- If others live in the house, the sick person should stay in a separate room and wear a mask. Everyone in the house should cover coughs and sneezes (if not wearing a mask), dispose of used tissues in a trash can (rather than leaving them on night stands and countertops), and wash hands frequently.
- All household members should be vaccinated against influenza. Those who are unvaccinated, and those at high risk who have been recently vaccinated, can consider influenza antiviral prophylaxis. If the sick family member is confirmed to have COVID-19, with no co-existing influenza, anti-influenza antiviral prophylaxis may be discontinued.
- Clinical infection control practices should be the same for anyone presenting with ILI.7 Enhanced clinic-based infection control practices to prevent spread of SARS-CoV-2 are listed in TABLE 3.8

Since there currently are no preventive medications proven to work for COVID-19, the main clinical decision physicians will have to make when a patient presents with ILI is whether to use antivirals to treat those who are at risk for complications based on the result of rapid, on-site influenza testing, or clinical presentation, or both. In this situation, knowledge of which viruses are circulating at high rates in the community could be valuable.
Milder season or perfect storm? The society-wide interventions that have been encouraged (although not mandated everywhere) to prevent community spread of SARS-CoV-2 should help prevent the community spread of influenza as well, and, if adhered to, may lead to a milder influenza season than would otherwise have occurred. However, given the uncertainties, the combination of influenza and coronavirus could present a perfect storm for the health care system and result in higher-than-normal morbidity and mortality from ILI and pneumonia overall.
Continue to: The possibility that one or more vaccines...
The possibility that one or more vaccines to prevent COVID-19 may be available in late 2020 or early 2021 offers hope. However, in current testing, the vaccine is not being given simultaneously with the influenza vaccine. If the potential for adverse interaction exists between the vaccines, it is important that influenza vaccine be given by mid- to late-October to avoid such an interaction if and when the new SARS-CoV-2 vaccine becomes available. Individuals who have symptoms of COVID-19 should not be vaccinated with influenza vaccine until they are considered noninfectious.
Encourage influenza vaccination. The COVID-19 pandemic may make it difficult to achieve desired community influenza vaccine levels because of decreased visits to medical facilities for preventive care, possible lower insurance coverage due to loss of employment, and a decrease in worksite mass vaccination programs. This makes it important for family physicians to encourage and offer influenza vaccines at their clinical sites.
Several evidence-based practices have been shown to improve vaccine uptake. Examples of such practices include patient reminder and recall systems that provide feedback to clinicians about rates of vaccination among patients, and establishing standing orders for vaccine administration that allow other health care providers to assess a patient’s immunization status and administer vaccinations according to a protocol.9 Finally, the CDC provides a video on how to recommend influenza vaccine to those who may be resistant (www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html).
SIDEBAR
CDC influenza resources
Point-of-care tests that detect both influenza A and B viruses approved by the CDC
www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
Advice on how to interpret the test results
www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
How to recommend influenza vaccine to reluctant patients
www.cdc.gov/vaccines/howirecommend/adult-vacc-videos.html
CDC, Centers for Disease Control and Prevention.
1. Grohskopf L. Influenza work groups: updates, considerations, and proposed recommendations for the 2020-2021 season. Presented at the ACIP meeting June 24, 2020. www.youtube.com/watch?v=W1SV2DSJsaQ&list=PLvrp9iOILTQb6D9e1YZWpbUvzfptNMKx2&index=8&t=0s. [Time stamp: 1:26:48] Accessed Septemeber 29, 2020.
2. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
3. Grohskopf L, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
4. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21 Summary of Recommendations. www.cdc.gov/flu/pdf/professionals/acip/acip-2020-21-summary-of-recommendations.pdf. Accessed September 29, 2020.
5. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2020.
6. NIH. COVID-19 treatment guidelines. Corticosteroids. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 29, 2020.
7. CDC. Infection control. www.cdc.gov/infectioncontrol/. Accessed September 29, 2020.
8. CDC. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Accessed September 29, 2020.
9. HHS. CPSTF findings for increasing vaccination. www.thecommunityguide.org/content/task-force-findings-increasing-vaccination. Accessed September 29, 2020.
1. Grohskopf L. Influenza work groups: updates, considerations, and proposed recommendations for the 2020-2021 season. Presented at the ACIP meeting June 24, 2020. www.youtube.com/watch?v=W1SV2DSJsaQ&list=PLvrp9iOILTQb6D9e1YZWpbUvzfptNMKx2&index=8&t=0s. [Time stamp: 1:26:48] Accessed Septemeber 29, 2020.
2. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
3. Grohskopf L, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
4. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21 Summary of Recommendations. www.cdc.gov/flu/pdf/professionals/acip/acip-2020-21-summary-of-recommendations.pdf. Accessed September 29, 2020.
5. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2020.
6. NIH. COVID-19 treatment guidelines. Corticosteroids. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 29, 2020.
7. CDC. Infection control. www.cdc.gov/infectioncontrol/. Accessed September 29, 2020.
8. CDC. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Accessed September 29, 2020.
9. HHS. CPSTF findings for increasing vaccination. www.thecommunityguide.org/content/task-force-findings-increasing-vaccination. Accessed September 29, 2020.
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
Returning to competition
As we continue to stumble around trying to find our way out of the COVID-19 pandemic, it has become clear that the journey has been a never-ending continuum of exercises in risk/benefit assessment. The population always has sorted itself into a bell-shaped curve from those who are risk averse to those who revel in risk taking. And, of course, with a paucity of facts on which we can base our assessment of risk, the discussion often shifts to our gut feelings about the benefits.
When faced with the question of when it is time for children to return to in-person schooling, there seems to be reasonably good agreement about the benefits of face-to-face learning. The level of risk is still to be determined.
When it comes to the issue of when to return to competitive school sports, the risks are equally indeterminate but there is less agreement on the benefits. This lack of uniformity reflects a long-standing dichotomy between those parents and students with a passion for competitive sports and those who see them as nonessential. This existential tug-of-war has gone on in almost every school system I am aware of when the school budget comes up for a vote.
The debate about a return to competitive sports on a collegiate and professional level unfortunately is colored by enormous revenues from media contracts, which means that high school and middle schools can’t look to what are essentially businesses for guidance. The delay created confusion, fluctuating angst and disappointment, but the end product made some sense. Volleyball (indoor) and football were indefinitely delayed. Heavy breathing between competitors separated by a couple of feet and protected only by a flimsy net or helmet cage seems like a risk not worth taking – at least until we have more information.
Other sports were allowed to start with restrictions based on existing social distancing mandates which include no locker rooms and no fans. Some rules such as no throw-ins for soccer didn’t make sense given what we are learning about the virus. But, for the most part, the compromises should result in a chance to reap the benefits of competition for the students whose families are willing to expose them to the yet to be fully determined risks.
There has been some grumbling from parents who see the no-fans mandate as a step too far. Until we know more about the risk of group gatherings outdoors, having no fans, including parents and grandparents, makes sense. In fact, to me it is a step long overdue and a rare sliver of silver lining to the pandemic. Competitive youth sports are for the kids. They are not meant to be entertainment events. Too often children are exposed to parental pressure (voiced and unvoiced) about their “performance” on the field. Neither my younger sister nor I can remember our parents going to any of my away football games in high school or any of my lacrosse games in college. I never felt the loss.
Will I miss watching my grandchildren compete? Of course I will miss it badly. However, giving kids some space to learn and enjoy the competition for itself in an atmosphere free of parental over-involvement will be a breath of fresh air. Something we need badly during this pandemic.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
As we continue to stumble around trying to find our way out of the COVID-19 pandemic, it has become clear that the journey has been a never-ending continuum of exercises in risk/benefit assessment. The population always has sorted itself into a bell-shaped curve from those who are risk averse to those who revel in risk taking. And, of course, with a paucity of facts on which we can base our assessment of risk, the discussion often shifts to our gut feelings about the benefits.
When faced with the question of when it is time for children to return to in-person schooling, there seems to be reasonably good agreement about the benefits of face-to-face learning. The level of risk is still to be determined.
When it comes to the issue of when to return to competitive school sports, the risks are equally indeterminate but there is less agreement on the benefits. This lack of uniformity reflects a long-standing dichotomy between those parents and students with a passion for competitive sports and those who see them as nonessential. This existential tug-of-war has gone on in almost every school system I am aware of when the school budget comes up for a vote.
The debate about a return to competitive sports on a collegiate and professional level unfortunately is colored by enormous revenues from media contracts, which means that high school and middle schools can’t look to what are essentially businesses for guidance. The delay created confusion, fluctuating angst and disappointment, but the end product made some sense. Volleyball (indoor) and football were indefinitely delayed. Heavy breathing between competitors separated by a couple of feet and protected only by a flimsy net or helmet cage seems like a risk not worth taking – at least until we have more information.
Other sports were allowed to start with restrictions based on existing social distancing mandates which include no locker rooms and no fans. Some rules such as no throw-ins for soccer didn’t make sense given what we are learning about the virus. But, for the most part, the compromises should result in a chance to reap the benefits of competition for the students whose families are willing to expose them to the yet to be fully determined risks.
There has been some grumbling from parents who see the no-fans mandate as a step too far. Until we know more about the risk of group gatherings outdoors, having no fans, including parents and grandparents, makes sense. In fact, to me it is a step long overdue and a rare sliver of silver lining to the pandemic. Competitive youth sports are for the kids. They are not meant to be entertainment events. Too often children are exposed to parental pressure (voiced and unvoiced) about their “performance” on the field. Neither my younger sister nor I can remember our parents going to any of my away football games in high school or any of my lacrosse games in college. I never felt the loss.
Will I miss watching my grandchildren compete? Of course I will miss it badly. However, giving kids some space to learn and enjoy the competition for itself in an atmosphere free of parental over-involvement will be a breath of fresh air. Something we need badly during this pandemic.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
As we continue to stumble around trying to find our way out of the COVID-19 pandemic, it has become clear that the journey has been a never-ending continuum of exercises in risk/benefit assessment. The population always has sorted itself into a bell-shaped curve from those who are risk averse to those who revel in risk taking. And, of course, with a paucity of facts on which we can base our assessment of risk, the discussion often shifts to our gut feelings about the benefits.
When faced with the question of when it is time for children to return to in-person schooling, there seems to be reasonably good agreement about the benefits of face-to-face learning. The level of risk is still to be determined.
When it comes to the issue of when to return to competitive school sports, the risks are equally indeterminate but there is less agreement on the benefits. This lack of uniformity reflects a long-standing dichotomy between those parents and students with a passion for competitive sports and those who see them as nonessential. This existential tug-of-war has gone on in almost every school system I am aware of when the school budget comes up for a vote.
The debate about a return to competitive sports on a collegiate and professional level unfortunately is colored by enormous revenues from media contracts, which means that high school and middle schools can’t look to what are essentially businesses for guidance. The delay created confusion, fluctuating angst and disappointment, but the end product made some sense. Volleyball (indoor) and football were indefinitely delayed. Heavy breathing between competitors separated by a couple of feet and protected only by a flimsy net or helmet cage seems like a risk not worth taking – at least until we have more information.
Other sports were allowed to start with restrictions based on existing social distancing mandates which include no locker rooms and no fans. Some rules such as no throw-ins for soccer didn’t make sense given what we are learning about the virus. But, for the most part, the compromises should result in a chance to reap the benefits of competition for the students whose families are willing to expose them to the yet to be fully determined risks.
There has been some grumbling from parents who see the no-fans mandate as a step too far. Until we know more about the risk of group gatherings outdoors, having no fans, including parents and grandparents, makes sense. In fact, to me it is a step long overdue and a rare sliver of silver lining to the pandemic. Competitive youth sports are for the kids. They are not meant to be entertainment events. Too often children are exposed to parental pressure (voiced and unvoiced) about their “performance” on the field. Neither my younger sister nor I can remember our parents going to any of my away football games in high school or any of my lacrosse games in college. I never felt the loss.
Will I miss watching my grandchildren compete? Of course I will miss it badly. However, giving kids some space to learn and enjoy the competition for itself in an atmosphere free of parental over-involvement will be a breath of fresh air. Something we need badly during this pandemic.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Patients with non-advanced LC. Boxed warning for montelukast. The happy hypoxic. COVID-19 and pulmonary vasculature.
Interventional chest and diagnostic procedures
Impact of COVID-19 pandemic in patients with non-advanced LC
The COVID-19 pandemic has challenged the way we screen for, diagnose, and treat lung cancer.1, 2 Knowing that these patients are at higher risk of respiratory failure, and that COVID-19 causes poor outcomes in cancer patients,1,3,4 valid concerns regarding viral transmission to patients and health-care workers have hampered the expedited care this population needs.
In recent months, efforts to manage the pandemic have been herculean. With the goal of limiting transmission, expert panels have offered guidance including limiting access to medical facilities, decreasing aerosolizing procedures, and prioritizing curative treatments.2,5 In general, lung cancer screening should be delayed, and patients with highly suspicious localized pulmonary lesions could receive empiric regimens, surgery, or stereotactic radiotherapy.1,3-5
The conundrum occurs when diagnostic bronchoscopy is required for staging, acquiring tissue for targeted therapy, or a moderate-risk pulmonary nodule with indeterminate PET-CT and/or high-risk for CT-guided biopsy. Thoughtful balancing of risks and benefits depends on patient comorbidities, hospital resources – such preprocedural COVID screening, adequate protective personal equipment- and rate of local viral prevalence.6,7 Delaying diagnosis and staging could lead to progression of cancer and preclude curative or adjuvant therapy for appropriate candidates. Furthermore, we should not dismiss the appalling psychological impact of delayed care on our patients.
While the pandemic continues and challenges arise in the care of patients with lung cancer, the value of a multidisciplinary input and individualized care cannot be overstated, with focus on providing the best care possible while both minimizing transmission and increasing the chances of acceptable outcomes.
Jose De Cardenas MD, FCCP – Steering Committee Member
Abdul Hamid Alraiyes MD, FCCP – Steering Committee Member
References
1. Mazzone PJ, et al. Chest. 2020;158(1):406-415. doi: 10.1016/j.chest.2020.04.020.
2. Banna G, et al. ESMO Open. 2020;5(2):e000765. doi: 10.1136/esmoopen-2020-000765.
3. Liang W, et al. Lancet Oncol. 2020;21(3):335-337. doi: 10.1016/S1470-2045(20)30096-6.
4. Singh AP, et al. JCO Oncol Pract. 2020 May 26;OP2000286. doi: 10.1200/OP.20.00286.
5. Dingemans AC, et al. J Thorac Oncol. 2020;15(7):1119-1136. doi: 10.1016/j.jtho.2020.05.001.
6. Wahidi MM, et al. J Bronchology Interv Pulmonol. 2020 Mar 18. doi: 10.1097/LBR.0000000000000681.
7. Pritchett MA, et al. J Thorac Dis. 2020;12(5):1781-1798. doi: 10.21037/jtd.2020.04.32.
Pediatric chest medicine
FDA strengthens the boxed warning for montelukast
Early this year the Food and Drug Administration (FDA) updated the boxed warning for montelukast (Singulair), related to the potential for serious mental health side effects, such as agitation, aggressive behavior, depression, hallucinations, and suicidal thoughts and actions. Since its approval in 1998, montelukast is part of the therapeutic approach for persistent asthma in children age 1 year and older, allergic rhinitis from 6 months and older, and exercises induced bronchospasm in children age 6 years and older. In 2018, around 2.3 million children younger than 17 years received a prescription for montelukast.
The FDA reviewed data from their Sentinel System comparing children receiving montelukast vs inhaled corticosteroids, and this study failed to demonstrate significant increased risk of hospitalized depressive disorders, outpatient depressive disorders, self-harm, or suicide. However, a focused evaluation by the FDA of suicides identified 82 cases of completed suicides associated with montelukast, and 19 of these cases were in children younger than 17 years of age.
Post-marketing case reports submitted to the FDA, published observational and animal studies were evaluated along with the Sentinel System study that led to the new recommendations.
Finally, on March 4, 2020, the FDA updated the Singulair®/montelukast black box warning, focusing on the importance of advising patients and caregivers about the potential for serious neuropsychiatric side effects and advice to immediately discontinue use if symptoms occurred. The warning contains a strong recommendation to reserve use of Singulair®/montelukast to patients with allergic rhinitis who have an inadequate response or intolerance to alternate therapies.
Endy Dominguez Silveyra, MD - Fellow-in-Training Member
References
1. FDA requires boxed warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. FDA Drug Safety Communication, March 4, 2020.
2. Neuropsychiatric events following montelukast use: A propensity score matched analysis. Sentinel, Sept. 27, 2019.
Pulmonary physiology, function, and rehabilitation
The happy hypoxic
In early December 2019, the novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified. Over the ensuing months, SARS-CoV-2 would cause a wide range of pulmonary symptoms from cough and mild shortness of breath to acute respiratory distress syndrome (ARDS) with severe hypoxia that puzzled intensivists worldwide.
One such mystifying presentation was finding patients with critically low oxygen levels who did not appear to be short of breath. This concept was dubbed “happy or silent hypoxemia.” Novel mechanisms of the SARS-Co-V-2 virus on the respiratory system have been proposed to explain this paradox, but recent literature suggests that foundational pulmonary physiology concepts can explain most of these findings.1
Breathing is centrally controlled by the respiratory center in the brain stem and is influenced mainly by dissolved carbon dioxide and pH.2 Hypercapnia is, therefore, a powerful stimulus to breathe and increase minute ventilation. It can cause dyspnea if this demand is not met.3
Hypoxia, on the other hand, is less powerful and does not evoke dyspnea until the PaO2 drops below 60 mm Hg.4 Hypercapnia potentiates this response: the higher the PaCO2, the higher the hypoxic response. Patients with a PaCO2 of 39 mm Hg or less may not experience dyspnea even when hypoxia is severe.1
Other possible explanations for silent hypoxemia include the poor accuracy of the pulse oximeter for estimating oxygen saturation of less than 80%,1 especially in the critically ill5 and the leftward shift of the oxygen dissociation curve due to fever, making the oxygen saturation lower for any given PaO2.1
In conclusion, the clinical management of COVID-19 pneumonia with a broad range of clinical features presents many unknowns, but it is reassuring to find an anchor in good old pulmonary physiology concepts.5
It is back to the basics for us all and that might be a good thing.
Oriade Adeoye, MD – Fellow-in-Training Member
References
1. Tobin MJ, et al. Am J Respir Crit Care Med. 2020;202(3):356-360. doi: 10.1164/rccm.202006-2157CP.
2. Vaporidi K, et al. Am J Respir Crit Care Med. 2020;201(1):20-32. doi: 10.1164/rccm.201903-0596SO.
3. Dhont S, et al. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5.
4. Weil JV, et al. J Clin Invest. 1970;49(6):1061-1072. doi:10.1172/JCI106322.
5. Tobin MJ. Am J Respir Crit Care Med. 2020;201(11):1319-1320. doi:10.1164/rccm.202004-1076ED.
Pulmonary vascular disease
COVID-19 and pulmonary vasculature: an intriguing relationship
Hypoxemia is the cardinal symptom in patients with severe coronavirus disease-2019 (COVID-19). However, hypoxemia disproportionate to radiographic opacities has led to growing suspicion that involvement of pulmonary vasculature (PV), leading to shunt physiology, may be a driver of this marked hypoxemia.
The virus’s affinity for PV is explained by presence of angiotensin-converting enzyme 2 receptor, which serves as the functional receptor for SARS-CoV-2, on pulmonary endothelium (Provencher, et al. Pulm Circ. 2020 Jun 10;10[3]:2045894020933088. doi: 10.1177/2045894020933088).
This increased affinity predisposes PV to pathologic effects of SARS-CoV-2, noted in COVID-19 patients’ autopsies, which revealed pulmonary endothelial injury and abnormal vessel growth (intussusceptive angiogenesis). These changes, along with profound inflammatory response, further predispose the PV to thrombosis and microangiopathy in COVID-19 (Ackermann, et al. N Engl J Med. 2020 Jul 9;383[2]:120-128).
These autopsy results also explain the radiologic findings of PV in COVID-19. Dual energy CT scanning, used to evaluate lung perfusion in these patients, has demonstrated PV thickening, mosaicism, and pulmonary vessel dilation; the latter likely occurring due to aberrations in physiologic hypoxic pulmonary vasoconstriction (Lang, et al. Lancet. 2020 Apr 30;S1473-3099[20]30367).
Despite PV’s involvement, only few cases of COVID-19 have been reported in patients with pulmonary arterial hypertension (PAH) , leading to the hypothesis that pre-existing vascular changes may have a protective effect in PAH patients (Horn, et al. Pulm Circ. 2020;10(2):1-2).
The above discussion details the complex and multifaceted relationship between COVID-19 and PV which underscores the value of understanding this interaction further and may prove to be insightful for discovering potential therapeutic targets in COVID-19.
Humna Abid Memon, MD – Fellow-in-Training Member
Interventional chest and diagnostic procedures
Impact of COVID-19 pandemic in patients with non-advanced LC
The COVID-19 pandemic has challenged the way we screen for, diagnose, and treat lung cancer.1, 2 Knowing that these patients are at higher risk of respiratory failure, and that COVID-19 causes poor outcomes in cancer patients,1,3,4 valid concerns regarding viral transmission to patients and health-care workers have hampered the expedited care this population needs.
In recent months, efforts to manage the pandemic have been herculean. With the goal of limiting transmission, expert panels have offered guidance including limiting access to medical facilities, decreasing aerosolizing procedures, and prioritizing curative treatments.2,5 In general, lung cancer screening should be delayed, and patients with highly suspicious localized pulmonary lesions could receive empiric regimens, surgery, or stereotactic radiotherapy.1,3-5
The conundrum occurs when diagnostic bronchoscopy is required for staging, acquiring tissue for targeted therapy, or a moderate-risk pulmonary nodule with indeterminate PET-CT and/or high-risk for CT-guided biopsy. Thoughtful balancing of risks and benefits depends on patient comorbidities, hospital resources – such preprocedural COVID screening, adequate protective personal equipment- and rate of local viral prevalence.6,7 Delaying diagnosis and staging could lead to progression of cancer and preclude curative or adjuvant therapy for appropriate candidates. Furthermore, we should not dismiss the appalling psychological impact of delayed care on our patients.
While the pandemic continues and challenges arise in the care of patients with lung cancer, the value of a multidisciplinary input and individualized care cannot be overstated, with focus on providing the best care possible while both minimizing transmission and increasing the chances of acceptable outcomes.
Jose De Cardenas MD, FCCP – Steering Committee Member
Abdul Hamid Alraiyes MD, FCCP – Steering Committee Member
References
1. Mazzone PJ, et al. Chest. 2020;158(1):406-415. doi: 10.1016/j.chest.2020.04.020.
2. Banna G, et al. ESMO Open. 2020;5(2):e000765. doi: 10.1136/esmoopen-2020-000765.
3. Liang W, et al. Lancet Oncol. 2020;21(3):335-337. doi: 10.1016/S1470-2045(20)30096-6.
4. Singh AP, et al. JCO Oncol Pract. 2020 May 26;OP2000286. doi: 10.1200/OP.20.00286.
5. Dingemans AC, et al. J Thorac Oncol. 2020;15(7):1119-1136. doi: 10.1016/j.jtho.2020.05.001.
6. Wahidi MM, et al. J Bronchology Interv Pulmonol. 2020 Mar 18. doi: 10.1097/LBR.0000000000000681.
7. Pritchett MA, et al. J Thorac Dis. 2020;12(5):1781-1798. doi: 10.21037/jtd.2020.04.32.
Pediatric chest medicine
FDA strengthens the boxed warning for montelukast
Early this year the Food and Drug Administration (FDA) updated the boxed warning for montelukast (Singulair), related to the potential for serious mental health side effects, such as agitation, aggressive behavior, depression, hallucinations, and suicidal thoughts and actions. Since its approval in 1998, montelukast is part of the therapeutic approach for persistent asthma in children age 1 year and older, allergic rhinitis from 6 months and older, and exercises induced bronchospasm in children age 6 years and older. In 2018, around 2.3 million children younger than 17 years received a prescription for montelukast.
The FDA reviewed data from their Sentinel System comparing children receiving montelukast vs inhaled corticosteroids, and this study failed to demonstrate significant increased risk of hospitalized depressive disorders, outpatient depressive disorders, self-harm, or suicide. However, a focused evaluation by the FDA of suicides identified 82 cases of completed suicides associated with montelukast, and 19 of these cases were in children younger than 17 years of age.
Post-marketing case reports submitted to the FDA, published observational and animal studies were evaluated along with the Sentinel System study that led to the new recommendations.
Finally, on March 4, 2020, the FDA updated the Singulair®/montelukast black box warning, focusing on the importance of advising patients and caregivers about the potential for serious neuropsychiatric side effects and advice to immediately discontinue use if symptoms occurred. The warning contains a strong recommendation to reserve use of Singulair®/montelukast to patients with allergic rhinitis who have an inadequate response or intolerance to alternate therapies.
Endy Dominguez Silveyra, MD - Fellow-in-Training Member
References
1. FDA requires boxed warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. FDA Drug Safety Communication, March 4, 2020.
2. Neuropsychiatric events following montelukast use: A propensity score matched analysis. Sentinel, Sept. 27, 2019.
Pulmonary physiology, function, and rehabilitation
The happy hypoxic
In early December 2019, the novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified. Over the ensuing months, SARS-CoV-2 would cause a wide range of pulmonary symptoms from cough and mild shortness of breath to acute respiratory distress syndrome (ARDS) with severe hypoxia that puzzled intensivists worldwide.
One such mystifying presentation was finding patients with critically low oxygen levels who did not appear to be short of breath. This concept was dubbed “happy or silent hypoxemia.” Novel mechanisms of the SARS-Co-V-2 virus on the respiratory system have been proposed to explain this paradox, but recent literature suggests that foundational pulmonary physiology concepts can explain most of these findings.1
Breathing is centrally controlled by the respiratory center in the brain stem and is influenced mainly by dissolved carbon dioxide and pH.2 Hypercapnia is, therefore, a powerful stimulus to breathe and increase minute ventilation. It can cause dyspnea if this demand is not met.3
Hypoxia, on the other hand, is less powerful and does not evoke dyspnea until the PaO2 drops below 60 mm Hg.4 Hypercapnia potentiates this response: the higher the PaCO2, the higher the hypoxic response. Patients with a PaCO2 of 39 mm Hg or less may not experience dyspnea even when hypoxia is severe.1
Other possible explanations for silent hypoxemia include the poor accuracy of the pulse oximeter for estimating oxygen saturation of less than 80%,1 especially in the critically ill5 and the leftward shift of the oxygen dissociation curve due to fever, making the oxygen saturation lower for any given PaO2.1
In conclusion, the clinical management of COVID-19 pneumonia with a broad range of clinical features presents many unknowns, but it is reassuring to find an anchor in good old pulmonary physiology concepts.5
It is back to the basics for us all and that might be a good thing.
Oriade Adeoye, MD – Fellow-in-Training Member
References
1. Tobin MJ, et al. Am J Respir Crit Care Med. 2020;202(3):356-360. doi: 10.1164/rccm.202006-2157CP.
2. Vaporidi K, et al. Am J Respir Crit Care Med. 2020;201(1):20-32. doi: 10.1164/rccm.201903-0596SO.
3. Dhont S, et al. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5.
4. Weil JV, et al. J Clin Invest. 1970;49(6):1061-1072. doi:10.1172/JCI106322.
5. Tobin MJ. Am J Respir Crit Care Med. 2020;201(11):1319-1320. doi:10.1164/rccm.202004-1076ED.
Pulmonary vascular disease
COVID-19 and pulmonary vasculature: an intriguing relationship
Hypoxemia is the cardinal symptom in patients with severe coronavirus disease-2019 (COVID-19). However, hypoxemia disproportionate to radiographic opacities has led to growing suspicion that involvement of pulmonary vasculature (PV), leading to shunt physiology, may be a driver of this marked hypoxemia.
The virus’s affinity for PV is explained by presence of angiotensin-converting enzyme 2 receptor, which serves as the functional receptor for SARS-CoV-2, on pulmonary endothelium (Provencher, et al. Pulm Circ. 2020 Jun 10;10[3]:2045894020933088. doi: 10.1177/2045894020933088).
This increased affinity predisposes PV to pathologic effects of SARS-CoV-2, noted in COVID-19 patients’ autopsies, which revealed pulmonary endothelial injury and abnormal vessel growth (intussusceptive angiogenesis). These changes, along with profound inflammatory response, further predispose the PV to thrombosis and microangiopathy in COVID-19 (Ackermann, et al. N Engl J Med. 2020 Jul 9;383[2]:120-128).
These autopsy results also explain the radiologic findings of PV in COVID-19. Dual energy CT scanning, used to evaluate lung perfusion in these patients, has demonstrated PV thickening, mosaicism, and pulmonary vessel dilation; the latter likely occurring due to aberrations in physiologic hypoxic pulmonary vasoconstriction (Lang, et al. Lancet. 2020 Apr 30;S1473-3099[20]30367).
Despite PV’s involvement, only few cases of COVID-19 have been reported in patients with pulmonary arterial hypertension (PAH) , leading to the hypothesis that pre-existing vascular changes may have a protective effect in PAH patients (Horn, et al. Pulm Circ. 2020;10(2):1-2).
The above discussion details the complex and multifaceted relationship between COVID-19 and PV which underscores the value of understanding this interaction further and may prove to be insightful for discovering potential therapeutic targets in COVID-19.
Humna Abid Memon, MD – Fellow-in-Training Member
Interventional chest and diagnostic procedures
Impact of COVID-19 pandemic in patients with non-advanced LC
The COVID-19 pandemic has challenged the way we screen for, diagnose, and treat lung cancer.1, 2 Knowing that these patients are at higher risk of respiratory failure, and that COVID-19 causes poor outcomes in cancer patients,1,3,4 valid concerns regarding viral transmission to patients and health-care workers have hampered the expedited care this population needs.
In recent months, efforts to manage the pandemic have been herculean. With the goal of limiting transmission, expert panels have offered guidance including limiting access to medical facilities, decreasing aerosolizing procedures, and prioritizing curative treatments.2,5 In general, lung cancer screening should be delayed, and patients with highly suspicious localized pulmonary lesions could receive empiric regimens, surgery, or stereotactic radiotherapy.1,3-5
The conundrum occurs when diagnostic bronchoscopy is required for staging, acquiring tissue for targeted therapy, or a moderate-risk pulmonary nodule with indeterminate PET-CT and/or high-risk for CT-guided biopsy. Thoughtful balancing of risks and benefits depends on patient comorbidities, hospital resources – such preprocedural COVID screening, adequate protective personal equipment- and rate of local viral prevalence.6,7 Delaying diagnosis and staging could lead to progression of cancer and preclude curative or adjuvant therapy for appropriate candidates. Furthermore, we should not dismiss the appalling psychological impact of delayed care on our patients.
While the pandemic continues and challenges arise in the care of patients with lung cancer, the value of a multidisciplinary input and individualized care cannot be overstated, with focus on providing the best care possible while both minimizing transmission and increasing the chances of acceptable outcomes.
Jose De Cardenas MD, FCCP – Steering Committee Member
Abdul Hamid Alraiyes MD, FCCP – Steering Committee Member
References
1. Mazzone PJ, et al. Chest. 2020;158(1):406-415. doi: 10.1016/j.chest.2020.04.020.
2. Banna G, et al. ESMO Open. 2020;5(2):e000765. doi: 10.1136/esmoopen-2020-000765.
3. Liang W, et al. Lancet Oncol. 2020;21(3):335-337. doi: 10.1016/S1470-2045(20)30096-6.
4. Singh AP, et al. JCO Oncol Pract. 2020 May 26;OP2000286. doi: 10.1200/OP.20.00286.
5. Dingemans AC, et al. J Thorac Oncol. 2020;15(7):1119-1136. doi: 10.1016/j.jtho.2020.05.001.
6. Wahidi MM, et al. J Bronchology Interv Pulmonol. 2020 Mar 18. doi: 10.1097/LBR.0000000000000681.
7. Pritchett MA, et al. J Thorac Dis. 2020;12(5):1781-1798. doi: 10.21037/jtd.2020.04.32.
Pediatric chest medicine
FDA strengthens the boxed warning for montelukast
Early this year the Food and Drug Administration (FDA) updated the boxed warning for montelukast (Singulair), related to the potential for serious mental health side effects, such as agitation, aggressive behavior, depression, hallucinations, and suicidal thoughts and actions. Since its approval in 1998, montelukast is part of the therapeutic approach for persistent asthma in children age 1 year and older, allergic rhinitis from 6 months and older, and exercises induced bronchospasm in children age 6 years and older. In 2018, around 2.3 million children younger than 17 years received a prescription for montelukast.
The FDA reviewed data from their Sentinel System comparing children receiving montelukast vs inhaled corticosteroids, and this study failed to demonstrate significant increased risk of hospitalized depressive disorders, outpatient depressive disorders, self-harm, or suicide. However, a focused evaluation by the FDA of suicides identified 82 cases of completed suicides associated with montelukast, and 19 of these cases were in children younger than 17 years of age.
Post-marketing case reports submitted to the FDA, published observational and animal studies were evaluated along with the Sentinel System study that led to the new recommendations.
Finally, on March 4, 2020, the FDA updated the Singulair®/montelukast black box warning, focusing on the importance of advising patients and caregivers about the potential for serious neuropsychiatric side effects and advice to immediately discontinue use if symptoms occurred. The warning contains a strong recommendation to reserve use of Singulair®/montelukast to patients with allergic rhinitis who have an inadequate response or intolerance to alternate therapies.
Endy Dominguez Silveyra, MD - Fellow-in-Training Member
References
1. FDA requires boxed warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. FDA Drug Safety Communication, March 4, 2020.
2. Neuropsychiatric events following montelukast use: A propensity score matched analysis. Sentinel, Sept. 27, 2019.
Pulmonary physiology, function, and rehabilitation
The happy hypoxic
In early December 2019, the novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified. Over the ensuing months, SARS-CoV-2 would cause a wide range of pulmonary symptoms from cough and mild shortness of breath to acute respiratory distress syndrome (ARDS) with severe hypoxia that puzzled intensivists worldwide.
One such mystifying presentation was finding patients with critically low oxygen levels who did not appear to be short of breath. This concept was dubbed “happy or silent hypoxemia.” Novel mechanisms of the SARS-Co-V-2 virus on the respiratory system have been proposed to explain this paradox, but recent literature suggests that foundational pulmonary physiology concepts can explain most of these findings.1
Breathing is centrally controlled by the respiratory center in the brain stem and is influenced mainly by dissolved carbon dioxide and pH.2 Hypercapnia is, therefore, a powerful stimulus to breathe and increase minute ventilation. It can cause dyspnea if this demand is not met.3
Hypoxia, on the other hand, is less powerful and does not evoke dyspnea until the PaO2 drops below 60 mm Hg.4 Hypercapnia potentiates this response: the higher the PaCO2, the higher the hypoxic response. Patients with a PaCO2 of 39 mm Hg or less may not experience dyspnea even when hypoxia is severe.1
Other possible explanations for silent hypoxemia include the poor accuracy of the pulse oximeter for estimating oxygen saturation of less than 80%,1 especially in the critically ill5 and the leftward shift of the oxygen dissociation curve due to fever, making the oxygen saturation lower for any given PaO2.1
In conclusion, the clinical management of COVID-19 pneumonia with a broad range of clinical features presents many unknowns, but it is reassuring to find an anchor in good old pulmonary physiology concepts.5
It is back to the basics for us all and that might be a good thing.
Oriade Adeoye, MD – Fellow-in-Training Member
References
1. Tobin MJ, et al. Am J Respir Crit Care Med. 2020;202(3):356-360. doi: 10.1164/rccm.202006-2157CP.
2. Vaporidi K, et al. Am J Respir Crit Care Med. 2020;201(1):20-32. doi: 10.1164/rccm.201903-0596SO.
3. Dhont S, et al. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5.
4. Weil JV, et al. J Clin Invest. 1970;49(6):1061-1072. doi:10.1172/JCI106322.
5. Tobin MJ. Am J Respir Crit Care Med. 2020;201(11):1319-1320. doi:10.1164/rccm.202004-1076ED.
Pulmonary vascular disease
COVID-19 and pulmonary vasculature: an intriguing relationship
Hypoxemia is the cardinal symptom in patients with severe coronavirus disease-2019 (COVID-19). However, hypoxemia disproportionate to radiographic opacities has led to growing suspicion that involvement of pulmonary vasculature (PV), leading to shunt physiology, may be a driver of this marked hypoxemia.
The virus’s affinity for PV is explained by presence of angiotensin-converting enzyme 2 receptor, which serves as the functional receptor for SARS-CoV-2, on pulmonary endothelium (Provencher, et al. Pulm Circ. 2020 Jun 10;10[3]:2045894020933088. doi: 10.1177/2045894020933088).
This increased affinity predisposes PV to pathologic effects of SARS-CoV-2, noted in COVID-19 patients’ autopsies, which revealed pulmonary endothelial injury and abnormal vessel growth (intussusceptive angiogenesis). These changes, along with profound inflammatory response, further predispose the PV to thrombosis and microangiopathy in COVID-19 (Ackermann, et al. N Engl J Med. 2020 Jul 9;383[2]:120-128).
These autopsy results also explain the radiologic findings of PV in COVID-19. Dual energy CT scanning, used to evaluate lung perfusion in these patients, has demonstrated PV thickening, mosaicism, and pulmonary vessel dilation; the latter likely occurring due to aberrations in physiologic hypoxic pulmonary vasoconstriction (Lang, et al. Lancet. 2020 Apr 30;S1473-3099[20]30367).
Despite PV’s involvement, only few cases of COVID-19 have been reported in patients with pulmonary arterial hypertension (PAH) , leading to the hypothesis that pre-existing vascular changes may have a protective effect in PAH patients (Horn, et al. Pulm Circ. 2020;10(2):1-2).
The above discussion details the complex and multifaceted relationship between COVID-19 and PV which underscores the value of understanding this interaction further and may prove to be insightful for discovering potential therapeutic targets in COVID-19.
Humna Abid Memon, MD – Fellow-in-Training Member
Bronchoscopy and tracheostomy in the COVID-19 era
The coronavirus disease 2019 (COVID-19) pandemic has changed the way we deliver healthcare for the foreseeable future. Not only have we had to rapidly learn how to evaluate, diagnose, and treat this new disease, we have also had to shift how we screen, triage, and care for other patients for both their safety and ours. As the virus is primarily spread via respiratory droplets, aerosol-generating procedures (AGP), such as bronchoscopy and tracheostomy, are high-risk for viral transmission. We have therefore had to reassess the risk/benefit ratio of performing these procedures – what is the risk to the patient by procedure postponement vs the risk to the health-care personnel (HCP) involved by moving ahead with the procedure? And, if proceeding, how should we protect ourselves? How do we screen patients to help us stratify risk? In order to answer these questions, we generally divide patients into three categories: the asymptomatic outpatient, the symptomatic patient, and the critically ill patient.
The asymptomatic outpatient
Early in the pandemic as cases began to spike in the US, many hospitals decided to postpone all elective procedures and surgeries. Guidelines quickly emerged stratifying bronchoscopic procedures into emergent, urgent, acute, subacute, and truly elective with recommendations on the subsequent timing of those procedures (Pritchett MA, et al. J Thorac Dis. 2020 May;12[5]:1781-1798). As we have obtained further data and our infrastructure has been bolstered, many physicians have begun performing more routine procedures. Preprocedural screening, both with symptom questionnaires and nasopharyngeal swabs, has been enacted as a measure to prevent inadvertent exposure to infected patients. While there are limited data regarding the reliability of this measure, emerging data have shown good concordance between nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) swabs and bronchoalveolar lavage (BAL) samples in low-risk patients (Oberg, et al. Personal communication, Sept 2020). Emergency procedures, such as foreign body aspiration, critical airway obstruction, and massive hemoptysis, were generally performed without delay throughout the pandemic. More recently, emphasis has been placed on prioritizing procedures for acute clinical diagnoses, such as biopsies for concerning lung nodules or masses in potentially early-stage patients, in those where staging is needed and in those where disease progression is suspected. Subacute procedures, such as inspection bronchoscopy for cough, minor hemoptysis, or airway stent surveillance, have generally been reintroduced while elective procedures, such as bronchial thermoplasty and bronchoscopic lung volume reduction, are considered elective, and their frequency and timing is determined mostly by the number of new cases of COVID-19 in the local community.
For all procedures, general modifications have been made. High-efficiency particulate air (HEPA) filters should be placed on all ventilatory circuits. When equivalent, flexible bronchoscopy is preferred over rigid bronchoscopy due to the closed circuit. Enhanced personal protective equipment (PPE) for all procedures is recommended – this typically includes a gown, gloves, hair bonnet, N-95 mask, and a face shield. Strict adherence to the Centers for Disease Control and Prevention (CDC) guidelines for postprocedure cleaning and sterilization is strongly recommended. In some cases, single-use bronchoscopes are being preferentially used, though no strong recommendations exist for this.
The symptomatic COVID-19 patient
In patients who have been diagnosed with SARS-CoV-2, we generally recommend postponing all procedures other than for life-threatening indications. For outpatients, we generally wait for two negative nasopharyngeal swabs prior to performing any nonemergent procedure. In inpatients, similar recommendations exist. Potential inpatient indications for bronchoscopy include diagnostic evaluation for alternate or coinfections, and therapeutic aspiration of clinically significant secretions. These should be carefully considered and performed only if deemed absolutely necessary. If bronchoscopy is needed in a patient with suspected or confirmed COVID-19, at a minimum, gown, gloves, head cover, face shield, and an N-95 mask should be worn. A powered air purifying respirator (PAPR) can be used and may provide increased protection. Proper donning and doffing techniques should be reviewed prior to any procedure. Personnel involved in the case should be limited to the minimum required. The procedure should be performed by experienced operators and limited in length. Removal and reinsertion of the bronchoscope should be minimized.
The critically ill COVID-19 patient
While the majority of patients infected with SARS-CoV-2 will have only mild symptoms, we know that a subset of patients will develop respiratory failure. Of those, a small but significant number will require prolonged mechanical ventilation during their clinical course. Thus, the consideration for tracheostomy comes into play.
Multiple issues arise when discussing tracheostomy placement in the COVID-19 world. Should it be done at all? If yes, what is the best technique and who should do it? When and where should it be done? Importantly – how do we care for patients once it is in place to facilitate recovery and, hopefully, decannulation?
Tracheostomy tubes are used in the ICU for patients who require prolonged mechanical ventilation for many reasons – patient comfort, decreased need for sedation, and to facilitate transfer out of the ICU to less acute care areas. These reasons are just as important in patients afflicted with respiratory failure from COVID-19, if not more so. As the patient volumes surge, health-care systems can quickly become overwhelmed. The ability to safely move patients out of the ICU frees up those resources for others who are more acutely ill.
The optimal technique for tracheostomy placement largely depends on the technological and human capital of each institution. Emphasis should be placed on procedural experience, efficiency, safety, and minimizing risk to HCP. While mortality rates do not differ between the surgical and percutaneous techniques, the percutaneous approach has been shown to require less procedural time (Iftikhar IH, et al. Lung. 2019[Jun];197[3]:267-275), an important infection control advantage in COVID-19 patients. Additionally, percutaneous tracheostomies are typically performed at the bedside, which offers the immediate benefit of minimizing patient transfer. This decreases exposure to multiple HCP, as well as contamination of other health-care areas. If performing a bronchoscopic-guided percutaneous tracheostomy, apnea should be maintained from insertion of the guiding catheter to tracheostomy insertion in order to minimize aerosolization. A novel technique involving placing the bronchoscope beside the endotracheal tube instead of through it has also been described (Angel L, et al. Ann Thorac Surg. 2020[Sep];110[3]:1006–1011).
Timing of tracheostomy placement in COVID-19 patients has varied widely. Initially, concern for the safety of HCP performing these procedures led to recommendations of waiting at least 21 days of intubation or until COVID-19 testing became negative. However, more recently, multiple recommendations have been made for tracheostomy placement after day 10 of intubation (McGrath, et al. Lancet Respir Med. 2020[Jul];8[7]:717-725).
Finally, once a tracheostomy tube has been placed, the care does not stop there. As patients are transitioned to rehabilitation centers or skilled nursing facilities and are assessed for weaning, downsizing, and decannulation, care should be taken to avoid virus aerosolization during key high-risk steps. Modifications such as performing spontaneous breathing trials using pressure support (a closed circuit) rather than tracheostomy mask, bypassing speaking valve trials in favor of direct tracheostomy capping, and avoiding routine tracheostomy downsizing are examples of simple steps that can be taken to facilitate patient progress while minimizing HCP risk (Divo, et al. Respir Care. 2020[Aug]5;respcare.08157).
What’s ahead?
As we move forward, we will continue to balance caring for patients effectively and efficiently while minimizing risk to ourselves and others. Ultimately until a vaccine exists, we will have to focus on prevention of infection and spread; therefore, the core principles of hand hygiene, mask wearing, and social distancing have never been more important. We encourage continued study, scrutiny, and collaboration in order to optimize procedural techniques as more information becomes available.
Dr. Oberg is with the Section of Interventional Pulmonology, David Geffen School of Medicine at UCLA; Dr. Beattie is with the Section of Interventional Pulmonology, Memorial Sloan Kettering Cancer Center, New York; and Dr. Folch is with the Section of Interventional Pulmonology, Massachusetts General Hospital, Harvard Medical School.
The coronavirus disease 2019 (COVID-19) pandemic has changed the way we deliver healthcare for the foreseeable future. Not only have we had to rapidly learn how to evaluate, diagnose, and treat this new disease, we have also had to shift how we screen, triage, and care for other patients for both their safety and ours. As the virus is primarily spread via respiratory droplets, aerosol-generating procedures (AGP), such as bronchoscopy and tracheostomy, are high-risk for viral transmission. We have therefore had to reassess the risk/benefit ratio of performing these procedures – what is the risk to the patient by procedure postponement vs the risk to the health-care personnel (HCP) involved by moving ahead with the procedure? And, if proceeding, how should we protect ourselves? How do we screen patients to help us stratify risk? In order to answer these questions, we generally divide patients into three categories: the asymptomatic outpatient, the symptomatic patient, and the critically ill patient.
The asymptomatic outpatient
Early in the pandemic as cases began to spike in the US, many hospitals decided to postpone all elective procedures and surgeries. Guidelines quickly emerged stratifying bronchoscopic procedures into emergent, urgent, acute, subacute, and truly elective with recommendations on the subsequent timing of those procedures (Pritchett MA, et al. J Thorac Dis. 2020 May;12[5]:1781-1798). As we have obtained further data and our infrastructure has been bolstered, many physicians have begun performing more routine procedures. Preprocedural screening, both with symptom questionnaires and nasopharyngeal swabs, has been enacted as a measure to prevent inadvertent exposure to infected patients. While there are limited data regarding the reliability of this measure, emerging data have shown good concordance between nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) swabs and bronchoalveolar lavage (BAL) samples in low-risk patients (Oberg, et al. Personal communication, Sept 2020). Emergency procedures, such as foreign body aspiration, critical airway obstruction, and massive hemoptysis, were generally performed without delay throughout the pandemic. More recently, emphasis has been placed on prioritizing procedures for acute clinical diagnoses, such as biopsies for concerning lung nodules or masses in potentially early-stage patients, in those where staging is needed and in those where disease progression is suspected. Subacute procedures, such as inspection bronchoscopy for cough, minor hemoptysis, or airway stent surveillance, have generally been reintroduced while elective procedures, such as bronchial thermoplasty and bronchoscopic lung volume reduction, are considered elective, and their frequency and timing is determined mostly by the number of new cases of COVID-19 in the local community.
For all procedures, general modifications have been made. High-efficiency particulate air (HEPA) filters should be placed on all ventilatory circuits. When equivalent, flexible bronchoscopy is preferred over rigid bronchoscopy due to the closed circuit. Enhanced personal protective equipment (PPE) for all procedures is recommended – this typically includes a gown, gloves, hair bonnet, N-95 mask, and a face shield. Strict adherence to the Centers for Disease Control and Prevention (CDC) guidelines for postprocedure cleaning and sterilization is strongly recommended. In some cases, single-use bronchoscopes are being preferentially used, though no strong recommendations exist for this.
The symptomatic COVID-19 patient
In patients who have been diagnosed with SARS-CoV-2, we generally recommend postponing all procedures other than for life-threatening indications. For outpatients, we generally wait for two negative nasopharyngeal swabs prior to performing any nonemergent procedure. In inpatients, similar recommendations exist. Potential inpatient indications for bronchoscopy include diagnostic evaluation for alternate or coinfections, and therapeutic aspiration of clinically significant secretions. These should be carefully considered and performed only if deemed absolutely necessary. If bronchoscopy is needed in a patient with suspected or confirmed COVID-19, at a minimum, gown, gloves, head cover, face shield, and an N-95 mask should be worn. A powered air purifying respirator (PAPR) can be used and may provide increased protection. Proper donning and doffing techniques should be reviewed prior to any procedure. Personnel involved in the case should be limited to the minimum required. The procedure should be performed by experienced operators and limited in length. Removal and reinsertion of the bronchoscope should be minimized.
The critically ill COVID-19 patient
While the majority of patients infected with SARS-CoV-2 will have only mild symptoms, we know that a subset of patients will develop respiratory failure. Of those, a small but significant number will require prolonged mechanical ventilation during their clinical course. Thus, the consideration for tracheostomy comes into play.
Multiple issues arise when discussing tracheostomy placement in the COVID-19 world. Should it be done at all? If yes, what is the best technique and who should do it? When and where should it be done? Importantly – how do we care for patients once it is in place to facilitate recovery and, hopefully, decannulation?
Tracheostomy tubes are used in the ICU for patients who require prolonged mechanical ventilation for many reasons – patient comfort, decreased need for sedation, and to facilitate transfer out of the ICU to less acute care areas. These reasons are just as important in patients afflicted with respiratory failure from COVID-19, if not more so. As the patient volumes surge, health-care systems can quickly become overwhelmed. The ability to safely move patients out of the ICU frees up those resources for others who are more acutely ill.
The optimal technique for tracheostomy placement largely depends on the technological and human capital of each institution. Emphasis should be placed on procedural experience, efficiency, safety, and minimizing risk to HCP. While mortality rates do not differ between the surgical and percutaneous techniques, the percutaneous approach has been shown to require less procedural time (Iftikhar IH, et al. Lung. 2019[Jun];197[3]:267-275), an important infection control advantage in COVID-19 patients. Additionally, percutaneous tracheostomies are typically performed at the bedside, which offers the immediate benefit of minimizing patient transfer. This decreases exposure to multiple HCP, as well as contamination of other health-care areas. If performing a bronchoscopic-guided percutaneous tracheostomy, apnea should be maintained from insertion of the guiding catheter to tracheostomy insertion in order to minimize aerosolization. A novel technique involving placing the bronchoscope beside the endotracheal tube instead of through it has also been described (Angel L, et al. Ann Thorac Surg. 2020[Sep];110[3]:1006–1011).
Timing of tracheostomy placement in COVID-19 patients has varied widely. Initially, concern for the safety of HCP performing these procedures led to recommendations of waiting at least 21 days of intubation or until COVID-19 testing became negative. However, more recently, multiple recommendations have been made for tracheostomy placement after day 10 of intubation (McGrath, et al. Lancet Respir Med. 2020[Jul];8[7]:717-725).
Finally, once a tracheostomy tube has been placed, the care does not stop there. As patients are transitioned to rehabilitation centers or skilled nursing facilities and are assessed for weaning, downsizing, and decannulation, care should be taken to avoid virus aerosolization during key high-risk steps. Modifications such as performing spontaneous breathing trials using pressure support (a closed circuit) rather than tracheostomy mask, bypassing speaking valve trials in favor of direct tracheostomy capping, and avoiding routine tracheostomy downsizing are examples of simple steps that can be taken to facilitate patient progress while minimizing HCP risk (Divo, et al. Respir Care. 2020[Aug]5;respcare.08157).
What’s ahead?
As we move forward, we will continue to balance caring for patients effectively and efficiently while minimizing risk to ourselves and others. Ultimately until a vaccine exists, we will have to focus on prevention of infection and spread; therefore, the core principles of hand hygiene, mask wearing, and social distancing have never been more important. We encourage continued study, scrutiny, and collaboration in order to optimize procedural techniques as more information becomes available.
Dr. Oberg is with the Section of Interventional Pulmonology, David Geffen School of Medicine at UCLA; Dr. Beattie is with the Section of Interventional Pulmonology, Memorial Sloan Kettering Cancer Center, New York; and Dr. Folch is with the Section of Interventional Pulmonology, Massachusetts General Hospital, Harvard Medical School.
The coronavirus disease 2019 (COVID-19) pandemic has changed the way we deliver healthcare for the foreseeable future. Not only have we had to rapidly learn how to evaluate, diagnose, and treat this new disease, we have also had to shift how we screen, triage, and care for other patients for both their safety and ours. As the virus is primarily spread via respiratory droplets, aerosol-generating procedures (AGP), such as bronchoscopy and tracheostomy, are high-risk for viral transmission. We have therefore had to reassess the risk/benefit ratio of performing these procedures – what is the risk to the patient by procedure postponement vs the risk to the health-care personnel (HCP) involved by moving ahead with the procedure? And, if proceeding, how should we protect ourselves? How do we screen patients to help us stratify risk? In order to answer these questions, we generally divide patients into three categories: the asymptomatic outpatient, the symptomatic patient, and the critically ill patient.
The asymptomatic outpatient
Early in the pandemic as cases began to spike in the US, many hospitals decided to postpone all elective procedures and surgeries. Guidelines quickly emerged stratifying bronchoscopic procedures into emergent, urgent, acute, subacute, and truly elective with recommendations on the subsequent timing of those procedures (Pritchett MA, et al. J Thorac Dis. 2020 May;12[5]:1781-1798). As we have obtained further data and our infrastructure has been bolstered, many physicians have begun performing more routine procedures. Preprocedural screening, both with symptom questionnaires and nasopharyngeal swabs, has been enacted as a measure to prevent inadvertent exposure to infected patients. While there are limited data regarding the reliability of this measure, emerging data have shown good concordance between nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) swabs and bronchoalveolar lavage (BAL) samples in low-risk patients (Oberg, et al. Personal communication, Sept 2020). Emergency procedures, such as foreign body aspiration, critical airway obstruction, and massive hemoptysis, were generally performed without delay throughout the pandemic. More recently, emphasis has been placed on prioritizing procedures for acute clinical diagnoses, such as biopsies for concerning lung nodules or masses in potentially early-stage patients, in those where staging is needed and in those where disease progression is suspected. Subacute procedures, such as inspection bronchoscopy for cough, minor hemoptysis, or airway stent surveillance, have generally been reintroduced while elective procedures, such as bronchial thermoplasty and bronchoscopic lung volume reduction, are considered elective, and their frequency and timing is determined mostly by the number of new cases of COVID-19 in the local community.
For all procedures, general modifications have been made. High-efficiency particulate air (HEPA) filters should be placed on all ventilatory circuits. When equivalent, flexible bronchoscopy is preferred over rigid bronchoscopy due to the closed circuit. Enhanced personal protective equipment (PPE) for all procedures is recommended – this typically includes a gown, gloves, hair bonnet, N-95 mask, and a face shield. Strict adherence to the Centers for Disease Control and Prevention (CDC) guidelines for postprocedure cleaning and sterilization is strongly recommended. In some cases, single-use bronchoscopes are being preferentially used, though no strong recommendations exist for this.
The symptomatic COVID-19 patient
In patients who have been diagnosed with SARS-CoV-2, we generally recommend postponing all procedures other than for life-threatening indications. For outpatients, we generally wait for two negative nasopharyngeal swabs prior to performing any nonemergent procedure. In inpatients, similar recommendations exist. Potential inpatient indications for bronchoscopy include diagnostic evaluation for alternate or coinfections, and therapeutic aspiration of clinically significant secretions. These should be carefully considered and performed only if deemed absolutely necessary. If bronchoscopy is needed in a patient with suspected or confirmed COVID-19, at a minimum, gown, gloves, head cover, face shield, and an N-95 mask should be worn. A powered air purifying respirator (PAPR) can be used and may provide increased protection. Proper donning and doffing techniques should be reviewed prior to any procedure. Personnel involved in the case should be limited to the minimum required. The procedure should be performed by experienced operators and limited in length. Removal and reinsertion of the bronchoscope should be minimized.
The critically ill COVID-19 patient
While the majority of patients infected with SARS-CoV-2 will have only mild symptoms, we know that a subset of patients will develop respiratory failure. Of those, a small but significant number will require prolonged mechanical ventilation during their clinical course. Thus, the consideration for tracheostomy comes into play.
Multiple issues arise when discussing tracheostomy placement in the COVID-19 world. Should it be done at all? If yes, what is the best technique and who should do it? When and where should it be done? Importantly – how do we care for patients once it is in place to facilitate recovery and, hopefully, decannulation?
Tracheostomy tubes are used in the ICU for patients who require prolonged mechanical ventilation for many reasons – patient comfort, decreased need for sedation, and to facilitate transfer out of the ICU to less acute care areas. These reasons are just as important in patients afflicted with respiratory failure from COVID-19, if not more so. As the patient volumes surge, health-care systems can quickly become overwhelmed. The ability to safely move patients out of the ICU frees up those resources for others who are more acutely ill.
The optimal technique for tracheostomy placement largely depends on the technological and human capital of each institution. Emphasis should be placed on procedural experience, efficiency, safety, and minimizing risk to HCP. While mortality rates do not differ between the surgical and percutaneous techniques, the percutaneous approach has been shown to require less procedural time (Iftikhar IH, et al. Lung. 2019[Jun];197[3]:267-275), an important infection control advantage in COVID-19 patients. Additionally, percutaneous tracheostomies are typically performed at the bedside, which offers the immediate benefit of minimizing patient transfer. This decreases exposure to multiple HCP, as well as contamination of other health-care areas. If performing a bronchoscopic-guided percutaneous tracheostomy, apnea should be maintained from insertion of the guiding catheter to tracheostomy insertion in order to minimize aerosolization. A novel technique involving placing the bronchoscope beside the endotracheal tube instead of through it has also been described (Angel L, et al. Ann Thorac Surg. 2020[Sep];110[3]:1006–1011).
Timing of tracheostomy placement in COVID-19 patients has varied widely. Initially, concern for the safety of HCP performing these procedures led to recommendations of waiting at least 21 days of intubation or until COVID-19 testing became negative. However, more recently, multiple recommendations have been made for tracheostomy placement after day 10 of intubation (McGrath, et al. Lancet Respir Med. 2020[Jul];8[7]:717-725).
Finally, once a tracheostomy tube has been placed, the care does not stop there. As patients are transitioned to rehabilitation centers or skilled nursing facilities and are assessed for weaning, downsizing, and decannulation, care should be taken to avoid virus aerosolization during key high-risk steps. Modifications such as performing spontaneous breathing trials using pressure support (a closed circuit) rather than tracheostomy mask, bypassing speaking valve trials in favor of direct tracheostomy capping, and avoiding routine tracheostomy downsizing are examples of simple steps that can be taken to facilitate patient progress while minimizing HCP risk (Divo, et al. Respir Care. 2020[Aug]5;respcare.08157).
What’s ahead?
As we move forward, we will continue to balance caring for patients effectively and efficiently while minimizing risk to ourselves and others. Ultimately until a vaccine exists, we will have to focus on prevention of infection and spread; therefore, the core principles of hand hygiene, mask wearing, and social distancing have never been more important. We encourage continued study, scrutiny, and collaboration in order to optimize procedural techniques as more information becomes available.
Dr. Oberg is with the Section of Interventional Pulmonology, David Geffen School of Medicine at UCLA; Dr. Beattie is with the Section of Interventional Pulmonology, Memorial Sloan Kettering Cancer Center, New York; and Dr. Folch is with the Section of Interventional Pulmonology, Massachusetts General Hospital, Harvard Medical School.
CHEST 2020 is coming to YOU
Expert-driven education—reimagined
CHEST’s premier event in pulmonary, critical care, and sleep medicine is just around the corner! Join us for CHEST Annual Meeting 2020, taking place October 18-21. We know it’s hard to plan out your schedule during an ever-changing pandemic, which is why this year’s meeting is being brought to you on a virtual platform. You’ll be able to access the meeting content from any device, in any location, at any time. It’s that convenient! Plus, you can join in immersive, interactive live sessions taught by expert faculty and followed by Q&As, or listen to prerecorded content at your own pace. Don’t worry if you’re unable to attend a session — all meeting content will be available to registrants until January 2021.
This year, you can expect:
• A keynote address by Anthony Fauci, MD, covering COVID-19.
• Over 88 live sessions, including panel and case-based discussions.
• Critically relevant sessions focusing on COVID-19 and cultural diversity.
• Original investigation presentations with new, unpublished science.
• Unique networking opportunities.
• Fun and interactive CHEST Games.
Register Today
Chestmeeting.chestnet.org
Expert-driven education—reimagined
Expert-driven education—reimagined
CHEST’s premier event in pulmonary, critical care, and sleep medicine is just around the corner! Join us for CHEST Annual Meeting 2020, taking place October 18-21. We know it’s hard to plan out your schedule during an ever-changing pandemic, which is why this year’s meeting is being brought to you on a virtual platform. You’ll be able to access the meeting content from any device, in any location, at any time. It’s that convenient! Plus, you can join in immersive, interactive live sessions taught by expert faculty and followed by Q&As, or listen to prerecorded content at your own pace. Don’t worry if you’re unable to attend a session — all meeting content will be available to registrants until January 2021.
This year, you can expect:
• A keynote address by Anthony Fauci, MD, covering COVID-19.
• Over 88 live sessions, including panel and case-based discussions.
• Critically relevant sessions focusing on COVID-19 and cultural diversity.
• Original investigation presentations with new, unpublished science.
• Unique networking opportunities.
• Fun and interactive CHEST Games.
Register Today
Chestmeeting.chestnet.org
CHEST’s premier event in pulmonary, critical care, and sleep medicine is just around the corner! Join us for CHEST Annual Meeting 2020, taking place October 18-21. We know it’s hard to plan out your schedule during an ever-changing pandemic, which is why this year’s meeting is being brought to you on a virtual platform. You’ll be able to access the meeting content from any device, in any location, at any time. It’s that convenient! Plus, you can join in immersive, interactive live sessions taught by expert faculty and followed by Q&As, or listen to prerecorded content at your own pace. Don’t worry if you’re unable to attend a session — all meeting content will be available to registrants until January 2021.
This year, you can expect:
• A keynote address by Anthony Fauci, MD, covering COVID-19.
• Over 88 live sessions, including panel and case-based discussions.
• Critically relevant sessions focusing on COVID-19 and cultural diversity.
• Original investigation presentations with new, unpublished science.
• Unique networking opportunities.
• Fun and interactive CHEST Games.
Register Today
Chestmeeting.chestnet.org