User login
Initial response to novel agents preps MM patients for favorable transplant outcomes
For patients with multiple myeloma, triplet induction with novel agents beat doublet with regards to early results, according to Lalit Kumar, MD, and colleagues at the All India Institute of Medical sciences Myeloma Group, New Delhi.
The study analyzed 326 multiple myeloma patients who received high-dose, novel agent–based induction therapy prior to autologous stem cell transplant (ASCT) at a single institution, according to a report published in Clinical Lymphoma, Myeloma and Leukemia.
Between January 2005 and December 2018, 326 consecutive patients underwent high-dose chemotherapy and autologous stem cell transplant. The median age of the patients was 52 years; 66% were men, nearly 33% had Revised ISS III disease; almost 16% had high-risk cytogenetics and 23% underwent transplant in second remission after salvage therapy for relapse. A total of 194 patients (59.5%) received induction with two novel agents (thalidomide/dexamethasone, n = 95; lenalidomide/dexamethasone, n = 63; bortezomib/dexamethasone, n = 36) and 132 (40.5%) received three drugs (bortezomib/lenalidomide/dexamethasone, n = 53; bortezomib/liposomal doxorubicin/dexamethasone, n = 42; bortezomib/thalidomide/dexamethasone, n = 31; other n = 3).
Outcomes favorable
After transplant 227 (69.8%) patients achieved a complete response; 48 (14.7%) had a very good partial response, 32 (9.8%) had a partial response, and 9 (2.8%) patients had stable disease. Ten (3.1%) patients died of transplant-related complications (before day 100). Triplet induction beat doublet with regards to early response (95.4% vs. 84.02% [doublets], P < .003), stem cell mobilization (88.6% vs. 76.8%, P < .005) and lower day-100 transplant-related mortality (P < .001), However, at a median follow-up of 62.5 months, the median overall response rate (97.5 months triplet vs. 100.0 months doublet) and the median progression free survival (54.5 months vs. 57 months) were not statistically different between the two induction-treatment groups.
Patients who had undergone transplant in a recent period (2016-18) had a better outcome, compared with initial years, which possibly reflects a combined effect of learning curve, use of triplets, and gradual reduction in day-100 mortality, the authors stated.
“Whether newer regimens incorporating monoclonal antibodies (associated with higher [complete response] rate and [minimal residual disease] negativity) would result in further improvement in survival, needs to be determined in future studies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Kumar L et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 18. doi: 10.1016/j.clml.2020.08.021.
For patients with multiple myeloma, triplet induction with novel agents beat doublet with regards to early results, according to Lalit Kumar, MD, and colleagues at the All India Institute of Medical sciences Myeloma Group, New Delhi.
The study analyzed 326 multiple myeloma patients who received high-dose, novel agent–based induction therapy prior to autologous stem cell transplant (ASCT) at a single institution, according to a report published in Clinical Lymphoma, Myeloma and Leukemia.
Between January 2005 and December 2018, 326 consecutive patients underwent high-dose chemotherapy and autologous stem cell transplant. The median age of the patients was 52 years; 66% were men, nearly 33% had Revised ISS III disease; almost 16% had high-risk cytogenetics and 23% underwent transplant in second remission after salvage therapy for relapse. A total of 194 patients (59.5%) received induction with two novel agents (thalidomide/dexamethasone, n = 95; lenalidomide/dexamethasone, n = 63; bortezomib/dexamethasone, n = 36) and 132 (40.5%) received three drugs (bortezomib/lenalidomide/dexamethasone, n = 53; bortezomib/liposomal doxorubicin/dexamethasone, n = 42; bortezomib/thalidomide/dexamethasone, n = 31; other n = 3).
Outcomes favorable
After transplant 227 (69.8%) patients achieved a complete response; 48 (14.7%) had a very good partial response, 32 (9.8%) had a partial response, and 9 (2.8%) patients had stable disease. Ten (3.1%) patients died of transplant-related complications (before day 100). Triplet induction beat doublet with regards to early response (95.4% vs. 84.02% [doublets], P < .003), stem cell mobilization (88.6% vs. 76.8%, P < .005) and lower day-100 transplant-related mortality (P < .001), However, at a median follow-up of 62.5 months, the median overall response rate (97.5 months triplet vs. 100.0 months doublet) and the median progression free survival (54.5 months vs. 57 months) were not statistically different between the two induction-treatment groups.
Patients who had undergone transplant in a recent period (2016-18) had a better outcome, compared with initial years, which possibly reflects a combined effect of learning curve, use of triplets, and gradual reduction in day-100 mortality, the authors stated.
“Whether newer regimens incorporating monoclonal antibodies (associated with higher [complete response] rate and [minimal residual disease] negativity) would result in further improvement in survival, needs to be determined in future studies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Kumar L et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 18. doi: 10.1016/j.clml.2020.08.021.
For patients with multiple myeloma, triplet induction with novel agents beat doublet with regards to early results, according to Lalit Kumar, MD, and colleagues at the All India Institute of Medical sciences Myeloma Group, New Delhi.
The study analyzed 326 multiple myeloma patients who received high-dose, novel agent–based induction therapy prior to autologous stem cell transplant (ASCT) at a single institution, according to a report published in Clinical Lymphoma, Myeloma and Leukemia.
Between January 2005 and December 2018, 326 consecutive patients underwent high-dose chemotherapy and autologous stem cell transplant. The median age of the patients was 52 years; 66% were men, nearly 33% had Revised ISS III disease; almost 16% had high-risk cytogenetics and 23% underwent transplant in second remission after salvage therapy for relapse. A total of 194 patients (59.5%) received induction with two novel agents (thalidomide/dexamethasone, n = 95; lenalidomide/dexamethasone, n = 63; bortezomib/dexamethasone, n = 36) and 132 (40.5%) received three drugs (bortezomib/lenalidomide/dexamethasone, n = 53; bortezomib/liposomal doxorubicin/dexamethasone, n = 42; bortezomib/thalidomide/dexamethasone, n = 31; other n = 3).
Outcomes favorable
After transplant 227 (69.8%) patients achieved a complete response; 48 (14.7%) had a very good partial response, 32 (9.8%) had a partial response, and 9 (2.8%) patients had stable disease. Ten (3.1%) patients died of transplant-related complications (before day 100). Triplet induction beat doublet with regards to early response (95.4% vs. 84.02% [doublets], P < .003), stem cell mobilization (88.6% vs. 76.8%, P < .005) and lower day-100 transplant-related mortality (P < .001), However, at a median follow-up of 62.5 months, the median overall response rate (97.5 months triplet vs. 100.0 months doublet) and the median progression free survival (54.5 months vs. 57 months) were not statistically different between the two induction-treatment groups.
Patients who had undergone transplant in a recent period (2016-18) had a better outcome, compared with initial years, which possibly reflects a combined effect of learning curve, use of triplets, and gradual reduction in day-100 mortality, the authors stated.
“Whether newer regimens incorporating monoclonal antibodies (associated with higher [complete response] rate and [minimal residual disease] negativity) would result in further improvement in survival, needs to be determined in future studies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Kumar L et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 18. doi: 10.1016/j.clml.2020.08.021.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
New first-line standard of care for esophageal cancer?
Pembrolizumab (Keytruda) is already approved for use in the treatment of esophageal cancer, but in the second-line setting.
New results show that it also improves outcomes when used in the first-line setting, and the investigators suggest that pembrolizumab in combination with chemotherapy should be the new standard of care.
The results come from an interim analysis of the phase 3 KEYNOTE-590 trial, conducted with 740 patients who had advanced cancers of the esophagus or esophagogastric junction (EGJ). The patients were followed for a median of 10.8 months.
The findings show that the combination offered patients a modest but distinct survival benefit over chemotherapy alone.
Median overall survival (OS), one of two primary endpoints for the trial, was 12.4 months for pembrolizumab plus chemotherapy, compared with 9.8 months for patients who received chemotherapy plus placebo.
This difference translated into a hazard ratio (HR) for death with pembrolizumab of 0.73 (P < .0001), reported Peter Enzinger, MD, from the Dana-Farber Cancer Institute in Boston. Progression-free survival (PFS), the trial’s other primary endpoint, was superior with the combination, at a median of 6.3 months compared with 5.8 months for chemotherapy alone, translating into an HR for progression with pembrolizumab of 0.65 (P < .0001).
“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy in patients with locally advanced unresectable or metastastic esophageal cancer, including the EGJ, regardless of the histology and biomarker status,” Enzinger concluded.
He was speaking at a press briefing prior to a presentation of the data in a presidential symposium at the European Society of Medical Oncology (ESMO) Virtual Congress 2020. (The data were presented in oral session by Ken Kato, MD, from the National Cancer Center Hospital in Tokyo, Japan.)
Little change in treatment
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long period of time,” Enzinger said.
Standard first-line therapy for patients with advanced esophageal, EGJ, or gastric adenocarcinoma is primarily a chemotherapy doublet consisting of a fluoropyrimidine plus a platinum agent.
Pembrolizumab was previously shown to have some activity as monotherapy against advanced or metastatic esophageal cancer in the third line in the KEYNOTE-180 trial. It yielded an overall response rate (ORR) of 14%. A median duration of response was not reached among patients with esophageal squamous cell carcinoma (ESCC) and tumors with programmed cell death–ligand-1 (PD-L1) that had combined positive scores (CPSs) of 10 or higher.
Another previous trial, KEYNOTE-181, showed that pembrolizumab monotherapy in the second line was associated with an ORR of 22% vs 7% for chemotherapy and respective median OS of 10.3 vs 6.7 months. This trial led to US Food and Drug Administration approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic ESCC with PD-L1 CPS ≥10 who have experienced disease progression after at least one prior line of therapy.
The trial now being reported, KEYNOTE-590, assessed use of the drug in the first-line setting. It was designed to see whether combining standard-of-care chemotherapy with pembrolizumab would improve outcomes.
Study details
Patients were randomly assigned to receive chemotherapy with 5-fluorauracil (5-FU) 800 mg/m2 intravenously on days 1–5 every 3 weeks for up to 35 cycles and cisplatin 80 mg/m2 IV every 3 weeks for up to 6 cycles, plus either pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles or saline placebo IV.
Rates of treatment-related adverse events of grade 3 or higher were similar between study arms, occurring in 71.9% with the combination and in 67.6% with chemotherapy alone. Adverse events leading to drug discontinuation occurred in 19.5% and 11.6%, respectively. Fatal adverse events occurred in 2.4% of patients who received the combination and in 1.4% of patients who received chemotherapy and placebo.
Immune-mediated adverse events of grade 3 or higher and infusion reactions occurred in 7% and 2.2% of patients, respectively.
Patient-reported quality of life was similar between the groups.
Mixed histologies muddy results
The improvement in OS was observed across all patient populations, including patients with ESCC, esophageal adenocarcinoma (EAC), and EGJ tumors, the researchers noted.
However, invited discussant Andres Cervantes, MD, PhD, from the University of Valencia, Spain, commented that ESCC and EAC have very different histologies and that including patients with both in a single trial can make the results very confusing.
He pointed out that, in the KEYNOTE-590 population, 73% of patients had ESCCs and 27% had EACs.
The OS improvement was seen regardless of PD-L1 expression, although the best results occurred in patients with high expression.
However, Cervantes noted that PD-L1 expression was not used as a stratification factor prior to randomization, even though it was included in the complex, step-wise statistical plan for the study.
Another expert, Salah-Eddin Al-Batran, MD, from the Krankenhaus Nordwest in Frankfurt, Germany, questioned the researchers’ recommendation that all patients with esophageal cancer receive this regimen, regardless of PD-L1 expression.
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high [PD-L1] expressers,” he said at the press briefing.
He said the trial report also leaves open the question of efficacy in tumors with microsatellite instability and/or high tumor mutational burden.
“I think these questions have to be addressed to give us a clear picture of how to treat a patient sitting in front of us as doctors,” he said.
Also adding to the concerns about this trial is the fact that the “selected chemotherapy schedule is not much currently used as the standard of care,” said Cervantes, although he added that it “is acceptable for this protocol.”
Despite these caveats, the trial was clearly positive, Cervantes said. The greatest benefit appeared to accrue for patients with ESCC.
“The addition of pembrolizumab to platinum-based chemotherapy significantly increases response rate, PFS, and OS in patients with advanced esophageal carcinomas over chemotherapy alone,” he said, and therefore he agreed with the investigators that “this is a new standard of care.”
The study was funded by Merck Sharp & Dohme. Enzinger disclosed honoraria and advisory or consulting roles for Merck and others. Cervantes disclosed consulting or advising and research funding from Merck Serono and others. He is president-elect of ESMO. Al-Batran has disclosed no relevant financial relationships.
This story first appeared on Medscape.com.
Pembrolizumab (Keytruda) is already approved for use in the treatment of esophageal cancer, but in the second-line setting.
New results show that it also improves outcomes when used in the first-line setting, and the investigators suggest that pembrolizumab in combination with chemotherapy should be the new standard of care.
The results come from an interim analysis of the phase 3 KEYNOTE-590 trial, conducted with 740 patients who had advanced cancers of the esophagus or esophagogastric junction (EGJ). The patients were followed for a median of 10.8 months.
The findings show that the combination offered patients a modest but distinct survival benefit over chemotherapy alone.
Median overall survival (OS), one of two primary endpoints for the trial, was 12.4 months for pembrolizumab plus chemotherapy, compared with 9.8 months for patients who received chemotherapy plus placebo.
This difference translated into a hazard ratio (HR) for death with pembrolizumab of 0.73 (P < .0001), reported Peter Enzinger, MD, from the Dana-Farber Cancer Institute in Boston. Progression-free survival (PFS), the trial’s other primary endpoint, was superior with the combination, at a median of 6.3 months compared with 5.8 months for chemotherapy alone, translating into an HR for progression with pembrolizumab of 0.65 (P < .0001).
“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy in patients with locally advanced unresectable or metastastic esophageal cancer, including the EGJ, regardless of the histology and biomarker status,” Enzinger concluded.
He was speaking at a press briefing prior to a presentation of the data in a presidential symposium at the European Society of Medical Oncology (ESMO) Virtual Congress 2020. (The data were presented in oral session by Ken Kato, MD, from the National Cancer Center Hospital in Tokyo, Japan.)
Little change in treatment
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long period of time,” Enzinger said.
Standard first-line therapy for patients with advanced esophageal, EGJ, or gastric adenocarcinoma is primarily a chemotherapy doublet consisting of a fluoropyrimidine plus a platinum agent.
Pembrolizumab was previously shown to have some activity as monotherapy against advanced or metastatic esophageal cancer in the third line in the KEYNOTE-180 trial. It yielded an overall response rate (ORR) of 14%. A median duration of response was not reached among patients with esophageal squamous cell carcinoma (ESCC) and tumors with programmed cell death–ligand-1 (PD-L1) that had combined positive scores (CPSs) of 10 or higher.
Another previous trial, KEYNOTE-181, showed that pembrolizumab monotherapy in the second line was associated with an ORR of 22% vs 7% for chemotherapy and respective median OS of 10.3 vs 6.7 months. This trial led to US Food and Drug Administration approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic ESCC with PD-L1 CPS ≥10 who have experienced disease progression after at least one prior line of therapy.
The trial now being reported, KEYNOTE-590, assessed use of the drug in the first-line setting. It was designed to see whether combining standard-of-care chemotherapy with pembrolizumab would improve outcomes.
Study details
Patients were randomly assigned to receive chemotherapy with 5-fluorauracil (5-FU) 800 mg/m2 intravenously on days 1–5 every 3 weeks for up to 35 cycles and cisplatin 80 mg/m2 IV every 3 weeks for up to 6 cycles, plus either pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles or saline placebo IV.
Rates of treatment-related adverse events of grade 3 or higher were similar between study arms, occurring in 71.9% with the combination and in 67.6% with chemotherapy alone. Adverse events leading to drug discontinuation occurred in 19.5% and 11.6%, respectively. Fatal adverse events occurred in 2.4% of patients who received the combination and in 1.4% of patients who received chemotherapy and placebo.
Immune-mediated adverse events of grade 3 or higher and infusion reactions occurred in 7% and 2.2% of patients, respectively.
Patient-reported quality of life was similar between the groups.
Mixed histologies muddy results
The improvement in OS was observed across all patient populations, including patients with ESCC, esophageal adenocarcinoma (EAC), and EGJ tumors, the researchers noted.
However, invited discussant Andres Cervantes, MD, PhD, from the University of Valencia, Spain, commented that ESCC and EAC have very different histologies and that including patients with both in a single trial can make the results very confusing.
He pointed out that, in the KEYNOTE-590 population, 73% of patients had ESCCs and 27% had EACs.
The OS improvement was seen regardless of PD-L1 expression, although the best results occurred in patients with high expression.
However, Cervantes noted that PD-L1 expression was not used as a stratification factor prior to randomization, even though it was included in the complex, step-wise statistical plan for the study.
Another expert, Salah-Eddin Al-Batran, MD, from the Krankenhaus Nordwest in Frankfurt, Germany, questioned the researchers’ recommendation that all patients with esophageal cancer receive this regimen, regardless of PD-L1 expression.
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high [PD-L1] expressers,” he said at the press briefing.
He said the trial report also leaves open the question of efficacy in tumors with microsatellite instability and/or high tumor mutational burden.
“I think these questions have to be addressed to give us a clear picture of how to treat a patient sitting in front of us as doctors,” he said.
Also adding to the concerns about this trial is the fact that the “selected chemotherapy schedule is not much currently used as the standard of care,” said Cervantes, although he added that it “is acceptable for this protocol.”
Despite these caveats, the trial was clearly positive, Cervantes said. The greatest benefit appeared to accrue for patients with ESCC.
“The addition of pembrolizumab to platinum-based chemotherapy significantly increases response rate, PFS, and OS in patients with advanced esophageal carcinomas over chemotherapy alone,” he said, and therefore he agreed with the investigators that “this is a new standard of care.”
The study was funded by Merck Sharp & Dohme. Enzinger disclosed honoraria and advisory or consulting roles for Merck and others. Cervantes disclosed consulting or advising and research funding from Merck Serono and others. He is president-elect of ESMO. Al-Batran has disclosed no relevant financial relationships.
This story first appeared on Medscape.com.
Pembrolizumab (Keytruda) is already approved for use in the treatment of esophageal cancer, but in the second-line setting.
New results show that it also improves outcomes when used in the first-line setting, and the investigators suggest that pembrolizumab in combination with chemotherapy should be the new standard of care.
The results come from an interim analysis of the phase 3 KEYNOTE-590 trial, conducted with 740 patients who had advanced cancers of the esophagus or esophagogastric junction (EGJ). The patients were followed for a median of 10.8 months.
The findings show that the combination offered patients a modest but distinct survival benefit over chemotherapy alone.
Median overall survival (OS), one of two primary endpoints for the trial, was 12.4 months for pembrolizumab plus chemotherapy, compared with 9.8 months for patients who received chemotherapy plus placebo.
This difference translated into a hazard ratio (HR) for death with pembrolizumab of 0.73 (P < .0001), reported Peter Enzinger, MD, from the Dana-Farber Cancer Institute in Boston. Progression-free survival (PFS), the trial’s other primary endpoint, was superior with the combination, at a median of 6.3 months compared with 5.8 months for chemotherapy alone, translating into an HR for progression with pembrolizumab of 0.65 (P < .0001).
“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy in patients with locally advanced unresectable or metastastic esophageal cancer, including the EGJ, regardless of the histology and biomarker status,” Enzinger concluded.
He was speaking at a press briefing prior to a presentation of the data in a presidential symposium at the European Society of Medical Oncology (ESMO) Virtual Congress 2020. (The data were presented in oral session by Ken Kato, MD, from the National Cancer Center Hospital in Tokyo, Japan.)
Little change in treatment
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long period of time,” Enzinger said.
Standard first-line therapy for patients with advanced esophageal, EGJ, or gastric adenocarcinoma is primarily a chemotherapy doublet consisting of a fluoropyrimidine plus a platinum agent.
Pembrolizumab was previously shown to have some activity as monotherapy against advanced or metastatic esophageal cancer in the third line in the KEYNOTE-180 trial. It yielded an overall response rate (ORR) of 14%. A median duration of response was not reached among patients with esophageal squamous cell carcinoma (ESCC) and tumors with programmed cell death–ligand-1 (PD-L1) that had combined positive scores (CPSs) of 10 or higher.
Another previous trial, KEYNOTE-181, showed that pembrolizumab monotherapy in the second line was associated with an ORR of 22% vs 7% for chemotherapy and respective median OS of 10.3 vs 6.7 months. This trial led to US Food and Drug Administration approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic ESCC with PD-L1 CPS ≥10 who have experienced disease progression after at least one prior line of therapy.
The trial now being reported, KEYNOTE-590, assessed use of the drug in the first-line setting. It was designed to see whether combining standard-of-care chemotherapy with pembrolizumab would improve outcomes.
Study details
Patients were randomly assigned to receive chemotherapy with 5-fluorauracil (5-FU) 800 mg/m2 intravenously on days 1–5 every 3 weeks for up to 35 cycles and cisplatin 80 mg/m2 IV every 3 weeks for up to 6 cycles, plus either pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles or saline placebo IV.
Rates of treatment-related adverse events of grade 3 or higher were similar between study arms, occurring in 71.9% with the combination and in 67.6% with chemotherapy alone. Adverse events leading to drug discontinuation occurred in 19.5% and 11.6%, respectively. Fatal adverse events occurred in 2.4% of patients who received the combination and in 1.4% of patients who received chemotherapy and placebo.
Immune-mediated adverse events of grade 3 or higher and infusion reactions occurred in 7% and 2.2% of patients, respectively.
Patient-reported quality of life was similar between the groups.
Mixed histologies muddy results
The improvement in OS was observed across all patient populations, including patients with ESCC, esophageal adenocarcinoma (EAC), and EGJ tumors, the researchers noted.
However, invited discussant Andres Cervantes, MD, PhD, from the University of Valencia, Spain, commented that ESCC and EAC have very different histologies and that including patients with both in a single trial can make the results very confusing.
He pointed out that, in the KEYNOTE-590 population, 73% of patients had ESCCs and 27% had EACs.
The OS improvement was seen regardless of PD-L1 expression, although the best results occurred in patients with high expression.
However, Cervantes noted that PD-L1 expression was not used as a stratification factor prior to randomization, even though it was included in the complex, step-wise statistical plan for the study.
Another expert, Salah-Eddin Al-Batran, MD, from the Krankenhaus Nordwest in Frankfurt, Germany, questioned the researchers’ recommendation that all patients with esophageal cancer receive this regimen, regardless of PD-L1 expression.
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high [PD-L1] expressers,” he said at the press briefing.
He said the trial report also leaves open the question of efficacy in tumors with microsatellite instability and/or high tumor mutational burden.
“I think these questions have to be addressed to give us a clear picture of how to treat a patient sitting in front of us as doctors,” he said.
Also adding to the concerns about this trial is the fact that the “selected chemotherapy schedule is not much currently used as the standard of care,” said Cervantes, although he added that it “is acceptable for this protocol.”
Despite these caveats, the trial was clearly positive, Cervantes said. The greatest benefit appeared to accrue for patients with ESCC.
“The addition of pembrolizumab to platinum-based chemotherapy significantly increases response rate, PFS, and OS in patients with advanced esophageal carcinomas over chemotherapy alone,” he said, and therefore he agreed with the investigators that “this is a new standard of care.”
The study was funded by Merck Sharp & Dohme. Enzinger disclosed honoraria and advisory or consulting roles for Merck and others. Cervantes disclosed consulting or advising and research funding from Merck Serono and others. He is president-elect of ESMO. Al-Batran has disclosed no relevant financial relationships.
This story first appeared on Medscape.com.
FROM ESMO 2020
Understanding the enduring power of caste
Isabel Wilkerson’s naming of the malady facilitates space for a shift in thinking.
America has been struggling to understand its racial dynamics since the arrival of enslaved Africans more than 400 years ago. Today, with much of the world more polarized than ever, and certainly in our United States, there is a need for something to shift us from our fear and survival paranoid schizoid (us-vs.-them) position to an integrated form if we are to come out of this unusual democratic and societal unrest whole.
Yet, we’ve never had the lexicon to adequately describe the sociopolitical dynamics rooted in race and racism and their power to shape the thinking of all who originate in this country and all who enter its self-made borders whether forcefully or voluntarily. Enter Isabel Wilkerson, a Pulitzer Prize–winning, former New York Times Chicago bureau chief, and author of “The Warmth of Other Suns: The Epic Story of America’s Great Migration” (New York: Random House, 2010) with her second book, “Caste: The Origins of Our Discontents” (New York: Random House, 2020).
Ms. Wilkerson quickly gets to work in an engaging storytelling style of weaving past to present with ideas she supports with letters from the past, historians’ impressions, research studies, and data. Her observations and research are bookended by the lead up to the 2016 presidential election and its aftermath on the one end, and the impending 2020 presidential election on the other. In her view, the reemergence of violence that has accelerated in the 21st century and the renewed commitment to promote white supremacy can be understood if we expand our view of race and racism to consider the enduring power of caste. For, in Ms. Wilkerson’s view, the fear of the 2042 U.S. census (which is predicted to reflect for the first time a non-White majority) is a driving force behind the dominant caste’s determination to maintain the status quo power dynamics in the United States.
In an effort to explain American’s racial hierarchy, Ms. Wilkerson explains the need for a new lexicon “that may sound like a foreign language,” but this is intentional on her part. She writes:
“To recalibrate how we see ourselves, I use language that may be more commonly associated with people in other cultures, to suggest a new way of understanding our hierarchy: Dominant caste, ruling majority, favored caste, or upper caste, instead of, or in addition to, white. Middle castes instead of, or in addition to, Asian or Latino. Subordinate caste, lowest caste, bottom caste, disfavored caste, historically stigmatized instead of African-American. Original, conquered, or indigenous peoples instead of, or in addition to, Native American. Marginalized people in addition to, or instead of, women of any race, or minorities of any kind.”
Early in the book Ms. Wilkerson anchors her argument in Rev. Dr. Martin Luther King Jr.’s sojourn to India. Rather than focus on the known history of Dr. King’s admiration of Mohandas Gandhi, Ms. Wilkerson directs our attention to Dr. King’s discovery of his connection to Dalits, those who had been considered “untouchables” until Bhimrao Ramji Ambedkar, the Indian economist, jurist, social reformer, and Dalit leader, fiercely and successfully advocated for a rebranding of his caste of origin; instead of “untouchables” they would be considered Dalits or “broken people.” Dr. King did not meet Mr. Ambedkar, who died 3 years before this journey, but Ms. Wilkerson writes that Dr. King acknowledged the kinship, “And he said unto himself, Yes, I am an untouchable, and every Negro in the United States is an untouchable.” The Dalits and Dr. King recognized in each other their shared positions as subordinates in a global caste system.
In answering the question about the difference between racism and casteism, Ms. Wilkerson writes:
“Because caste and race are interwoven in America, it can be hard to separate the two. ... Casteism is the investment in keeping the hierarchy as it is in order to maintain your own ranking, advantage, privilege, or to elevate yourself above others or to keep others beneath you.”
Reading “Caste: The Origins of Our Discontents” is akin to the experience of gaining relief after struggling for years with a chronic malady that has a fluctuating course: Under the surface is low-grade pain that is compartmentalized and often met with denial or gaslighting when symptoms and systems are reported to members of the dominant caste. Yet, when there are acute flare-ups and increasingly frequent deadly encounters, the defenses of denial are painfully revealed; structures are broken and sometimes burned down. This has been the clinical course of racism, particularly in the United States. In that vein, an early reaction while reading “Caste” might be comparable to hearing an interpretation that educates, clarifies, resonates, and lands perfectly on the right diagnosis at the right moment.
Approach proves clarifying
In conceptualizing the malady as one of caste, Ms. Wilkerson achieves several things simultaneously – she names the malady, thus providing a lexicon, describes its symptoms, and most importantly, in our opinion, shares some of the compelling data from her field studies. By focusing on India, Nazi Germany, and the United States, she describes how easily one system influences another in the global effort to maintain power among the privileged.
This is not a new way of conceptualizing racial hierarchy; however, what is truly persuasive is Ms. Wilkerson’s ability to weave her rigorous research, sociopolitical analysis, and cogent psychological insights and interpretations to explain the 400-year trajectory of racialized caste in the United States. She achieves this exigent task with beautiful prose that motivates the reader to return time and time again to learn gut-wrenching painful historical details. She summarizes truths that have been unearthed (again) about Germany, India, and, in particular, the United States during her research and travels around the world. In doing so, she provides vivid examples of racism layered on caste. Consider the following:
“The Nazis were impressed by the American custom of lynching its subordinate caste of African-Americans, having become aware of the ritual torture and mutilations that typically accompanied them. Hitler especially marveled at the American ‘knack for maintaining an air of robust innocence in the wake of mass death.’ ” Ms. Wilkerson informs us that Hitler sent emissaries to study America’s Jim Crow system and then imported some features to orchestrate the Holocaust in Nazi Germany.
and a corresponding sense of inadequacy in the presence of someone who is considered to be from a higher caste.
A painful account of interpersonal racism is captured as Ms. Wilkerson recounts her experience after a routine business flight from Chicago to Detroit. She details her difficulty leaving a rental car parking lot because she had become so disoriented after being profiled and accosted by Drug Enforcement Administration agents who had intercepted her in the airport terminal and followed her onto the airport shuttle bus as she attempted to reach her destination. She provides a description of “getting turned around in a parking lot that I had been to dozens of times, going in circles, not able to get out, not registering the signs to the exit, not seeing how to get to Interstate 94, when I knew full well how to get to I-94 after all the times I’d driven it. ... This was the thievery of caste, stealing the time and psychic resources of the marginalized, draining energy in an already uphill competition. They were not, like me, frozen and disoriented, trying to make sense of a public violation that seemed all the more menacing now that I could see it in full. The quiet mundanity of that terror has never left me, the scars outliving the cut.”
This account is consistent with the dissociative, disorienting dynamics of race-based trauma. Her experience is not uncommon and helps to explain the activism of those in the subordinate caste who have attained some measure of wealth, power, and influence, and are motivated to expend their resources (energy, time, fame, and/or wealth) to raise awareness about social and political injustices by calling out structural racism in medicine, protesting police use of force by taking a knee, boycotting sporting events, and even demanding that football stadiums be used as polling sites. At the end of the day, all of us who have “made it” know that when we leave our homes, our relegation to the subordinate caste determines how we are perceived and what landmines we must navigate to make it through the day and that determine whether we will make it home.
This tour de force work of art has the potential to be a game changer in the way that we think about racial polarization in the United States. It is hoped that this new language opens up a space that allows each of us to explore this hegemony while identifying our placement and actions we take to maintain it, for each of us undeniably has a position in this caste system.
Having this new lexicon summons to mind the reactions of patients who gain immediate relief from having their illnesses named. In the case of the U.S. malady that has gripped us all, Ms. Wilkerson reiterates the importance of naming the condition. She writes:
“Because, to truly understand America, we must open our eyes to the hidden work of a caste system that has gone unnamed but prevails among us to our collective detriment, to see that we have more in common with each other and with cultures that we might otherwise dismiss, and to summon the courage to consider that therein may lie the answers.”
The naming allows both doctor and patient to have greater insight, understanding its origins and course, as well as having hope that there is a remedy. Naming facilitates the space for a shift in thinking and implementation of treatment protocols, such as Nazi Germany’s “zero tolerance policy” of swastikas in comparison to the ongoing U.S. controversy about the display of Confederate symbols. At this point in history, we welcome a diagnosis that has the potential to shift us from these poles of dominant and subordinate, black and white, good and bad, toward integration and wholeness of the individual psyche and collective global community. This is similar to what Melanie Klein calls the depressive position. Ms. Wilkerson suggests, in relinquishing these polar splits, we increase our capacity to shift to a space where our psychic integration occurs and our inextricable interdependence and responsibility for one another are honored.
Dr. Dunlap is a psychiatrist and psychoanalyst, and clinical professor of psychiatry and behavioral sciences at George Washington University. She is interested in the management of “difference” – race, gender, ethnicity, and intersectionality – in dyadic relationships and group dynamics; and the impact of racism on interpersonal relationships in institutional structures. Dr. Dunlap practices in Washington and has no disclosures. Dr. Dennis is a clinical psychologist and psychoanalyst. Her interests are in gender and ethnic diversity, health equity, and supervision and training. Dr. Dennis practices in Washington and has no disclosures.
Isabel Wilkerson’s naming of the malady facilitates space for a shift in thinking.
Isabel Wilkerson’s naming of the malady facilitates space for a shift in thinking.
America has been struggling to understand its racial dynamics since the arrival of enslaved Africans more than 400 years ago. Today, with much of the world more polarized than ever, and certainly in our United States, there is a need for something to shift us from our fear and survival paranoid schizoid (us-vs.-them) position to an integrated form if we are to come out of this unusual democratic and societal unrest whole.
Yet, we’ve never had the lexicon to adequately describe the sociopolitical dynamics rooted in race and racism and their power to shape the thinking of all who originate in this country and all who enter its self-made borders whether forcefully or voluntarily. Enter Isabel Wilkerson, a Pulitzer Prize–winning, former New York Times Chicago bureau chief, and author of “The Warmth of Other Suns: The Epic Story of America’s Great Migration” (New York: Random House, 2010) with her second book, “Caste: The Origins of Our Discontents” (New York: Random House, 2020).
Ms. Wilkerson quickly gets to work in an engaging storytelling style of weaving past to present with ideas she supports with letters from the past, historians’ impressions, research studies, and data. Her observations and research are bookended by the lead up to the 2016 presidential election and its aftermath on the one end, and the impending 2020 presidential election on the other. In her view, the reemergence of violence that has accelerated in the 21st century and the renewed commitment to promote white supremacy can be understood if we expand our view of race and racism to consider the enduring power of caste. For, in Ms. Wilkerson’s view, the fear of the 2042 U.S. census (which is predicted to reflect for the first time a non-White majority) is a driving force behind the dominant caste’s determination to maintain the status quo power dynamics in the United States.
In an effort to explain American’s racial hierarchy, Ms. Wilkerson explains the need for a new lexicon “that may sound like a foreign language,” but this is intentional on her part. She writes:
“To recalibrate how we see ourselves, I use language that may be more commonly associated with people in other cultures, to suggest a new way of understanding our hierarchy: Dominant caste, ruling majority, favored caste, or upper caste, instead of, or in addition to, white. Middle castes instead of, or in addition to, Asian or Latino. Subordinate caste, lowest caste, bottom caste, disfavored caste, historically stigmatized instead of African-American. Original, conquered, or indigenous peoples instead of, or in addition to, Native American. Marginalized people in addition to, or instead of, women of any race, or minorities of any kind.”
Early in the book Ms. Wilkerson anchors her argument in Rev. Dr. Martin Luther King Jr.’s sojourn to India. Rather than focus on the known history of Dr. King’s admiration of Mohandas Gandhi, Ms. Wilkerson directs our attention to Dr. King’s discovery of his connection to Dalits, those who had been considered “untouchables” until Bhimrao Ramji Ambedkar, the Indian economist, jurist, social reformer, and Dalit leader, fiercely and successfully advocated for a rebranding of his caste of origin; instead of “untouchables” they would be considered Dalits or “broken people.” Dr. King did not meet Mr. Ambedkar, who died 3 years before this journey, but Ms. Wilkerson writes that Dr. King acknowledged the kinship, “And he said unto himself, Yes, I am an untouchable, and every Negro in the United States is an untouchable.” The Dalits and Dr. King recognized in each other their shared positions as subordinates in a global caste system.
In answering the question about the difference between racism and casteism, Ms. Wilkerson writes:
“Because caste and race are interwoven in America, it can be hard to separate the two. ... Casteism is the investment in keeping the hierarchy as it is in order to maintain your own ranking, advantage, privilege, or to elevate yourself above others or to keep others beneath you.”
Reading “Caste: The Origins of Our Discontents” is akin to the experience of gaining relief after struggling for years with a chronic malady that has a fluctuating course: Under the surface is low-grade pain that is compartmentalized and often met with denial or gaslighting when symptoms and systems are reported to members of the dominant caste. Yet, when there are acute flare-ups and increasingly frequent deadly encounters, the defenses of denial are painfully revealed; structures are broken and sometimes burned down. This has been the clinical course of racism, particularly in the United States. In that vein, an early reaction while reading “Caste” might be comparable to hearing an interpretation that educates, clarifies, resonates, and lands perfectly on the right diagnosis at the right moment.
Approach proves clarifying
In conceptualizing the malady as one of caste, Ms. Wilkerson achieves several things simultaneously – she names the malady, thus providing a lexicon, describes its symptoms, and most importantly, in our opinion, shares some of the compelling data from her field studies. By focusing on India, Nazi Germany, and the United States, she describes how easily one system influences another in the global effort to maintain power among the privileged.
This is not a new way of conceptualizing racial hierarchy; however, what is truly persuasive is Ms. Wilkerson’s ability to weave her rigorous research, sociopolitical analysis, and cogent psychological insights and interpretations to explain the 400-year trajectory of racialized caste in the United States. She achieves this exigent task with beautiful prose that motivates the reader to return time and time again to learn gut-wrenching painful historical details. She summarizes truths that have been unearthed (again) about Germany, India, and, in particular, the United States during her research and travels around the world. In doing so, she provides vivid examples of racism layered on caste. Consider the following:
“The Nazis were impressed by the American custom of lynching its subordinate caste of African-Americans, having become aware of the ritual torture and mutilations that typically accompanied them. Hitler especially marveled at the American ‘knack for maintaining an air of robust innocence in the wake of mass death.’ ” Ms. Wilkerson informs us that Hitler sent emissaries to study America’s Jim Crow system and then imported some features to orchestrate the Holocaust in Nazi Germany.
and a corresponding sense of inadequacy in the presence of someone who is considered to be from a higher caste.
A painful account of interpersonal racism is captured as Ms. Wilkerson recounts her experience after a routine business flight from Chicago to Detroit. She details her difficulty leaving a rental car parking lot because she had become so disoriented after being profiled and accosted by Drug Enforcement Administration agents who had intercepted her in the airport terminal and followed her onto the airport shuttle bus as she attempted to reach her destination. She provides a description of “getting turned around in a parking lot that I had been to dozens of times, going in circles, not able to get out, not registering the signs to the exit, not seeing how to get to Interstate 94, when I knew full well how to get to I-94 after all the times I’d driven it. ... This was the thievery of caste, stealing the time and psychic resources of the marginalized, draining energy in an already uphill competition. They were not, like me, frozen and disoriented, trying to make sense of a public violation that seemed all the more menacing now that I could see it in full. The quiet mundanity of that terror has never left me, the scars outliving the cut.”
This account is consistent with the dissociative, disorienting dynamics of race-based trauma. Her experience is not uncommon and helps to explain the activism of those in the subordinate caste who have attained some measure of wealth, power, and influence, and are motivated to expend their resources (energy, time, fame, and/or wealth) to raise awareness about social and political injustices by calling out structural racism in medicine, protesting police use of force by taking a knee, boycotting sporting events, and even demanding that football stadiums be used as polling sites. At the end of the day, all of us who have “made it” know that when we leave our homes, our relegation to the subordinate caste determines how we are perceived and what landmines we must navigate to make it through the day and that determine whether we will make it home.
This tour de force work of art has the potential to be a game changer in the way that we think about racial polarization in the United States. It is hoped that this new language opens up a space that allows each of us to explore this hegemony while identifying our placement and actions we take to maintain it, for each of us undeniably has a position in this caste system.
Having this new lexicon summons to mind the reactions of patients who gain immediate relief from having their illnesses named. In the case of the U.S. malady that has gripped us all, Ms. Wilkerson reiterates the importance of naming the condition. She writes:
“Because, to truly understand America, we must open our eyes to the hidden work of a caste system that has gone unnamed but prevails among us to our collective detriment, to see that we have more in common with each other and with cultures that we might otherwise dismiss, and to summon the courage to consider that therein may lie the answers.”
The naming allows both doctor and patient to have greater insight, understanding its origins and course, as well as having hope that there is a remedy. Naming facilitates the space for a shift in thinking and implementation of treatment protocols, such as Nazi Germany’s “zero tolerance policy” of swastikas in comparison to the ongoing U.S. controversy about the display of Confederate symbols. At this point in history, we welcome a diagnosis that has the potential to shift us from these poles of dominant and subordinate, black and white, good and bad, toward integration and wholeness of the individual psyche and collective global community. This is similar to what Melanie Klein calls the depressive position. Ms. Wilkerson suggests, in relinquishing these polar splits, we increase our capacity to shift to a space where our psychic integration occurs and our inextricable interdependence and responsibility for one another are honored.
Dr. Dunlap is a psychiatrist and psychoanalyst, and clinical professor of psychiatry and behavioral sciences at George Washington University. She is interested in the management of “difference” – race, gender, ethnicity, and intersectionality – in dyadic relationships and group dynamics; and the impact of racism on interpersonal relationships in institutional structures. Dr. Dunlap practices in Washington and has no disclosures. Dr. Dennis is a clinical psychologist and psychoanalyst. Her interests are in gender and ethnic diversity, health equity, and supervision and training. Dr. Dennis practices in Washington and has no disclosures.
America has been struggling to understand its racial dynamics since the arrival of enslaved Africans more than 400 years ago. Today, with much of the world more polarized than ever, and certainly in our United States, there is a need for something to shift us from our fear and survival paranoid schizoid (us-vs.-them) position to an integrated form if we are to come out of this unusual democratic and societal unrest whole.
Yet, we’ve never had the lexicon to adequately describe the sociopolitical dynamics rooted in race and racism and their power to shape the thinking of all who originate in this country and all who enter its self-made borders whether forcefully or voluntarily. Enter Isabel Wilkerson, a Pulitzer Prize–winning, former New York Times Chicago bureau chief, and author of “The Warmth of Other Suns: The Epic Story of America’s Great Migration” (New York: Random House, 2010) with her second book, “Caste: The Origins of Our Discontents” (New York: Random House, 2020).
Ms. Wilkerson quickly gets to work in an engaging storytelling style of weaving past to present with ideas she supports with letters from the past, historians’ impressions, research studies, and data. Her observations and research are bookended by the lead up to the 2016 presidential election and its aftermath on the one end, and the impending 2020 presidential election on the other. In her view, the reemergence of violence that has accelerated in the 21st century and the renewed commitment to promote white supremacy can be understood if we expand our view of race and racism to consider the enduring power of caste. For, in Ms. Wilkerson’s view, the fear of the 2042 U.S. census (which is predicted to reflect for the first time a non-White majority) is a driving force behind the dominant caste’s determination to maintain the status quo power dynamics in the United States.
In an effort to explain American’s racial hierarchy, Ms. Wilkerson explains the need for a new lexicon “that may sound like a foreign language,” but this is intentional on her part. She writes:
“To recalibrate how we see ourselves, I use language that may be more commonly associated with people in other cultures, to suggest a new way of understanding our hierarchy: Dominant caste, ruling majority, favored caste, or upper caste, instead of, or in addition to, white. Middle castes instead of, or in addition to, Asian or Latino. Subordinate caste, lowest caste, bottom caste, disfavored caste, historically stigmatized instead of African-American. Original, conquered, or indigenous peoples instead of, or in addition to, Native American. Marginalized people in addition to, or instead of, women of any race, or minorities of any kind.”
Early in the book Ms. Wilkerson anchors her argument in Rev. Dr. Martin Luther King Jr.’s sojourn to India. Rather than focus on the known history of Dr. King’s admiration of Mohandas Gandhi, Ms. Wilkerson directs our attention to Dr. King’s discovery of his connection to Dalits, those who had been considered “untouchables” until Bhimrao Ramji Ambedkar, the Indian economist, jurist, social reformer, and Dalit leader, fiercely and successfully advocated for a rebranding of his caste of origin; instead of “untouchables” they would be considered Dalits or “broken people.” Dr. King did not meet Mr. Ambedkar, who died 3 years before this journey, but Ms. Wilkerson writes that Dr. King acknowledged the kinship, “And he said unto himself, Yes, I am an untouchable, and every Negro in the United States is an untouchable.” The Dalits and Dr. King recognized in each other their shared positions as subordinates in a global caste system.
In answering the question about the difference between racism and casteism, Ms. Wilkerson writes:
“Because caste and race are interwoven in America, it can be hard to separate the two. ... Casteism is the investment in keeping the hierarchy as it is in order to maintain your own ranking, advantage, privilege, or to elevate yourself above others or to keep others beneath you.”
Reading “Caste: The Origins of Our Discontents” is akin to the experience of gaining relief after struggling for years with a chronic malady that has a fluctuating course: Under the surface is low-grade pain that is compartmentalized and often met with denial or gaslighting when symptoms and systems are reported to members of the dominant caste. Yet, when there are acute flare-ups and increasingly frequent deadly encounters, the defenses of denial are painfully revealed; structures are broken and sometimes burned down. This has been the clinical course of racism, particularly in the United States. In that vein, an early reaction while reading “Caste” might be comparable to hearing an interpretation that educates, clarifies, resonates, and lands perfectly on the right diagnosis at the right moment.
Approach proves clarifying
In conceptualizing the malady as one of caste, Ms. Wilkerson achieves several things simultaneously – she names the malady, thus providing a lexicon, describes its symptoms, and most importantly, in our opinion, shares some of the compelling data from her field studies. By focusing on India, Nazi Germany, and the United States, she describes how easily one system influences another in the global effort to maintain power among the privileged.
This is not a new way of conceptualizing racial hierarchy; however, what is truly persuasive is Ms. Wilkerson’s ability to weave her rigorous research, sociopolitical analysis, and cogent psychological insights and interpretations to explain the 400-year trajectory of racialized caste in the United States. She achieves this exigent task with beautiful prose that motivates the reader to return time and time again to learn gut-wrenching painful historical details. She summarizes truths that have been unearthed (again) about Germany, India, and, in particular, the United States during her research and travels around the world. In doing so, she provides vivid examples of racism layered on caste. Consider the following:
“The Nazis were impressed by the American custom of lynching its subordinate caste of African-Americans, having become aware of the ritual torture and mutilations that typically accompanied them. Hitler especially marveled at the American ‘knack for maintaining an air of robust innocence in the wake of mass death.’ ” Ms. Wilkerson informs us that Hitler sent emissaries to study America’s Jim Crow system and then imported some features to orchestrate the Holocaust in Nazi Germany.
and a corresponding sense of inadequacy in the presence of someone who is considered to be from a higher caste.
A painful account of interpersonal racism is captured as Ms. Wilkerson recounts her experience after a routine business flight from Chicago to Detroit. She details her difficulty leaving a rental car parking lot because she had become so disoriented after being profiled and accosted by Drug Enforcement Administration agents who had intercepted her in the airport terminal and followed her onto the airport shuttle bus as she attempted to reach her destination. She provides a description of “getting turned around in a parking lot that I had been to dozens of times, going in circles, not able to get out, not registering the signs to the exit, not seeing how to get to Interstate 94, when I knew full well how to get to I-94 after all the times I’d driven it. ... This was the thievery of caste, stealing the time and psychic resources of the marginalized, draining energy in an already uphill competition. They were not, like me, frozen and disoriented, trying to make sense of a public violation that seemed all the more menacing now that I could see it in full. The quiet mundanity of that terror has never left me, the scars outliving the cut.”
This account is consistent with the dissociative, disorienting dynamics of race-based trauma. Her experience is not uncommon and helps to explain the activism of those in the subordinate caste who have attained some measure of wealth, power, and influence, and are motivated to expend their resources (energy, time, fame, and/or wealth) to raise awareness about social and political injustices by calling out structural racism in medicine, protesting police use of force by taking a knee, boycotting sporting events, and even demanding that football stadiums be used as polling sites. At the end of the day, all of us who have “made it” know that when we leave our homes, our relegation to the subordinate caste determines how we are perceived and what landmines we must navigate to make it through the day and that determine whether we will make it home.
This tour de force work of art has the potential to be a game changer in the way that we think about racial polarization in the United States. It is hoped that this new language opens up a space that allows each of us to explore this hegemony while identifying our placement and actions we take to maintain it, for each of us undeniably has a position in this caste system.
Having this new lexicon summons to mind the reactions of patients who gain immediate relief from having their illnesses named. In the case of the U.S. malady that has gripped us all, Ms. Wilkerson reiterates the importance of naming the condition. She writes:
“Because, to truly understand America, we must open our eyes to the hidden work of a caste system that has gone unnamed but prevails among us to our collective detriment, to see that we have more in common with each other and with cultures that we might otherwise dismiss, and to summon the courage to consider that therein may lie the answers.”
The naming allows both doctor and patient to have greater insight, understanding its origins and course, as well as having hope that there is a remedy. Naming facilitates the space for a shift in thinking and implementation of treatment protocols, such as Nazi Germany’s “zero tolerance policy” of swastikas in comparison to the ongoing U.S. controversy about the display of Confederate symbols. At this point in history, we welcome a diagnosis that has the potential to shift us from these poles of dominant and subordinate, black and white, good and bad, toward integration and wholeness of the individual psyche and collective global community. This is similar to what Melanie Klein calls the depressive position. Ms. Wilkerson suggests, in relinquishing these polar splits, we increase our capacity to shift to a space where our psychic integration occurs and our inextricable interdependence and responsibility for one another are honored.
Dr. Dunlap is a psychiatrist and psychoanalyst, and clinical professor of psychiatry and behavioral sciences at George Washington University. She is interested in the management of “difference” – race, gender, ethnicity, and intersectionality – in dyadic relationships and group dynamics; and the impact of racism on interpersonal relationships in institutional structures. Dr. Dunlap practices in Washington and has no disclosures. Dr. Dennis is a clinical psychologist and psychoanalyst. Her interests are in gender and ethnic diversity, health equity, and supervision and training. Dr. Dennis practices in Washington and has no disclosures.
Suicide rates up significantly among adolescents, young adults
Suicide rates in young people aged 10-24 years increased significantly in 42 states from 2007-2009 to 2016-2018, according to a recent analysis from the National Center for Health Statistics.
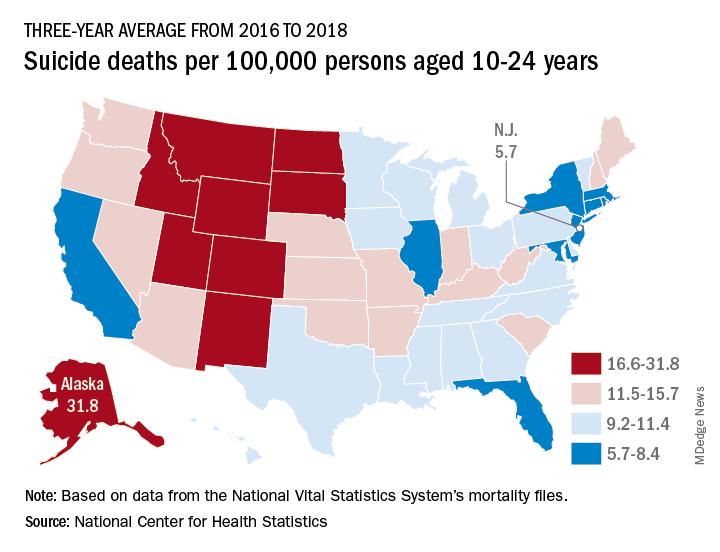
Nationally, the suicide rate jumped 47%, based on the averages for the two 3-year periods, rising from 7.0 per 100,000 persons aged 10-24 years to 10.3 per 100,000. For all ages, the corresponding increase was 47%, Sally C. Curtin, MA, of the NCHS, said in a National Vital Statistics Report.
There was no state with a decrease in suicide rates for adolescents and young adults, as the other eight all had nonsignificant increases, the smallest being 14% in South Dakota. Three-year averages were used to increase statistical power for states with relatively small numbers of deaths but were still not enough to show significance for some large increases, such as the 48% rise in Delaware, Ms. Curtin noted.
In 2016-2018, Alaska’s suicide rate of 31.8 per 100,000 persons aged 10-24 years was the highest in the country, followed by South Dakota (23.6), Montana (23.2), and Wyoming (20.5). New Jersey had the lowest rate at 5.7 per 100,000, with New York and Rhode Island both slightly higher at 5.9 and Connecticut at 6.3, based on data from the National Vital Statistics System.
Even the low numbers, however, hide some large changes, as New Jersey (up by 39%) and New York (up by 44%) were among the 42 states with statistically significant increases, which ranged from 21.7% in Maryland to 110% in New Hampshire, Ms. Curtin said in the report. The increases seen in this analysis contrast with data from the preceding time period, as “the suicide rate among persons aged 10-24 was statistically stable from 2000 to 2007.”
SOURCE: Curtin SC. National Vital Statistics Reports. 2020;69(11)1-9.
Suicide rates in young people aged 10-24 years increased significantly in 42 states from 2007-2009 to 2016-2018, according to a recent analysis from the National Center for Health Statistics.

Nationally, the suicide rate jumped 47%, based on the averages for the two 3-year periods, rising from 7.0 per 100,000 persons aged 10-24 years to 10.3 per 100,000. For all ages, the corresponding increase was 47%, Sally C. Curtin, MA, of the NCHS, said in a National Vital Statistics Report.
There was no state with a decrease in suicide rates for adolescents and young adults, as the other eight all had nonsignificant increases, the smallest being 14% in South Dakota. Three-year averages were used to increase statistical power for states with relatively small numbers of deaths but were still not enough to show significance for some large increases, such as the 48% rise in Delaware, Ms. Curtin noted.
In 2016-2018, Alaska’s suicide rate of 31.8 per 100,000 persons aged 10-24 years was the highest in the country, followed by South Dakota (23.6), Montana (23.2), and Wyoming (20.5). New Jersey had the lowest rate at 5.7 per 100,000, with New York and Rhode Island both slightly higher at 5.9 and Connecticut at 6.3, based on data from the National Vital Statistics System.
Even the low numbers, however, hide some large changes, as New Jersey (up by 39%) and New York (up by 44%) were among the 42 states with statistically significant increases, which ranged from 21.7% in Maryland to 110% in New Hampshire, Ms. Curtin said in the report. The increases seen in this analysis contrast with data from the preceding time period, as “the suicide rate among persons aged 10-24 was statistically stable from 2000 to 2007.”
SOURCE: Curtin SC. National Vital Statistics Reports. 2020;69(11)1-9.
Suicide rates in young people aged 10-24 years increased significantly in 42 states from 2007-2009 to 2016-2018, according to a recent analysis from the National Center for Health Statistics.

Nationally, the suicide rate jumped 47%, based on the averages for the two 3-year periods, rising from 7.0 per 100,000 persons aged 10-24 years to 10.3 per 100,000. For all ages, the corresponding increase was 47%, Sally C. Curtin, MA, of the NCHS, said in a National Vital Statistics Report.
There was no state with a decrease in suicide rates for adolescents and young adults, as the other eight all had nonsignificant increases, the smallest being 14% in South Dakota. Three-year averages were used to increase statistical power for states with relatively small numbers of deaths but were still not enough to show significance for some large increases, such as the 48% rise in Delaware, Ms. Curtin noted.
In 2016-2018, Alaska’s suicide rate of 31.8 per 100,000 persons aged 10-24 years was the highest in the country, followed by South Dakota (23.6), Montana (23.2), and Wyoming (20.5). New Jersey had the lowest rate at 5.7 per 100,000, with New York and Rhode Island both slightly higher at 5.9 and Connecticut at 6.3, based on data from the National Vital Statistics System.
Even the low numbers, however, hide some large changes, as New Jersey (up by 39%) and New York (up by 44%) were among the 42 states with statistically significant increases, which ranged from 21.7% in Maryland to 110% in New Hampshire, Ms. Curtin said in the report. The increases seen in this analysis contrast with data from the preceding time period, as “the suicide rate among persons aged 10-24 was statistically stable from 2000 to 2007.”
SOURCE: Curtin SC. National Vital Statistics Reports. 2020;69(11)1-9.
Is psychiatry coddling the American mind?
A trainee recently observed that psychiatrists frequently seem motivated to protect patients from emotional and internal disruption. He suggested that we often did so by validating their maladaptive perspectives regarding their impaired relationships to society and close attachments. These maneuvers were justified by referring to the need to establish a therapeutic alliance and reduce patients’ suffering.
As an example, he mentioned a patient with alcohol use disorder. The patient came in with complaints that he could not stay sober with his current level of depression. The patient also complained of a family member who was setting limits. To the trainee’s surprise, the patient was not challenged on his perceived victimhood and his fantasy that a sober life should mean a life without negative effect. Instead, the patient was validated in his anger toward the family member. In addition, his medications were adjusted, seemingly confirming to the patient that one could only ask for sobriety once life is empty of pain.
The observation of the trainee reminded us of the three great “untruths” mentioned by Greg Lukianoff and Jonathan Haidt, PhD, in their famous book, “The Coddling of the American Mind.”1 In the book they warn against the idea of fragility – what doesn’t kill you makes you weaker; emotional reasoning – always trust your feelings; and us-versus-them thinking – life is a battle between good people and evil people. The authors compare these three great untruths with the cognitive distortions of cognitive-behavioral therapy.
We ponder the trainee’s observation that psychiatrists appear to encourage the untruths rather than challenge them. Part of psychiatric and all medical training involves learning nonjudgmental approaches to human suffering and an identification with individual needs over societal demands. Our suspicion is that a nonjudgmental approach to the understanding of the human condition generates a desire to protect patients from a moralistic shaming position. However, we wonder if, at times, psychiatry takes this approach too far.
Reconceptualizing shame
Shame can be a toxic presence in the overwhelmed superego of a patient, but it can serve an important role in psychic development and should not be avoided out of hand. We suggest that it can be appropriate for a patient with an alcohol use disorder to feel some shame for the harm caused by their drinking, and we question the limit of psychiatry’s current pursuit of incessant validation. As an extreme example, would modern psychiatry discourage a patient who killed someone while driving in an intoxicated state from feeling remorse and shame?
Modern psychiatry appears to have other examples of the three great untruths on display. In our work, we are often faced with patients who are prematurely placed on disability, an example of fragility. Instead of encouraging patients to return to the workforce, they are “protected” from the emotional difficulty of work. In many patients this results in a decline in functioning and worsening of psychiatric symptoms. We are also confronted with patients who define themselves by how they feel, an example of emotional reasoning. Instead of using our clinical judgments to define and assess symptoms, patients are left to decide for themselves through self-rated scales with questionable validity.2 This can result in patients having their emotional experiences not only validated when inappropriate but can also give emotional reasoning a false sense of medical legitimacy.
Finally, we commonly see patients who endlessly blame family members and others for any life difficulties, a form of us-versus-them thinking. Instead of acknowledging and then integrating life challenges to achieve recovery, patients are affirmed despite clinicians having little evidence on the veracity of the patients’ perspective. As a consequence, patients can be further isolated from their greatest source of support.
In some ways, a mindlessly validating approach in psychiatry is unexpected since the practice of psychiatry would seem to promote the development of strong attachments and resilience. After all, connections to family, employment, social institutions, and even religious worship are associated with vastly better outcomes. Those who have become alienated to these pillars of social cohesion fare much worse. One may deplore the static and at times oppressive nature of these institutions but the empirical experience of practicing psychiatry leads one to a healthy respect for the stabilizing influence they accord for individuals struggling with life’s vicissitudes, unpredictability, and loneliness. Overcoming the fear of responsibility, living up to the demands and expectations of society, and having the strength to overcome difficult emotions should be the standard goals of psychiatric treatment.
From the knowledge gained from working with patients struggling from psychic pain, we wonder how to encourage patients to pursue those adaptive approaches to life. We argue that a stoic emphasis on learning to manage one’s affective and mental response to the inevitable changes of life is key to achieving wisdom and stability in our humble lives. This perspective is a common denominator of multiple different psychotherapies. The goal is to provide patients with the ability to be in a place where they are engaged with the world in a meaningful way that is not overwhelmed by distorted, self-absorbed psychic anguish. This perspective discourages externalization as a relatively low-yield way to understand and overcome one’s problems. One identifies childhood experiences with one’s mother as a source of adult distress not for the purpose of blaming her, but for the purpose of recognizing one’s own childish motivations for making maladaptive decisions as an adult.
For many patients, the goal should be to emphasize an internal locus of control and responsibility. We should also avoid constantly relying on society and government’s role in helping the individual achieve a satisfactory life. We wonder if this endless pursuit of nonjudgment and validation corrupts the doctor-patient interaction. In other words, and individual responsibility for their own psychic development. Psychotherapy that ends with the patient being able to identify all the traumas that led to their sorrow has simply left the patient in the role of helpless and sorrowful victim. Instead, we should allow patients to proceed to the next step, which is empowerment and transformation. From this angle, the field of psychiatry should be cautious of encouraging movements that promote victimhood and grievance as a meaningful psychic position to take in society.
Mr. Lukianoff and Dr. Haidt use cognitive therapy as an analogy throughout their books for how to confront the great untruths. They perceive those modern forms of thinking as cognitive distortions, which can be remedied using the techniques of cognitive restructuring found in cognitive-behavioral therapy. They encourage us to recognize those maladaptive thoughts, and create more accurate and adaptive ways of viewing the world – a view that would be able to grow from challenges not just survive them; a view referred to as antifragile.3 We believe that those techniques and others would certainly be of assistance in our current times. However, the first step is to recognize our problem – a problem that is not rooted in the DSM, research, or biology but in an exaggerated intention to be patient centered. We should, however, remember that, when a patient has negative schemas, being too patient centered can be encouraging to maladaptive behaviors.
In conclusion, we wonder what modern psychiatry could look like if it made a concerted effort at also treating mental illness by reinforcing the importance of individual agency and responsibility. Modern psychiatry has been so focused on describing biological symptoms needing biological treatments that we sometimes forget that having no symptom (being asymptomatic) is not the only goal. Having a fulfilling and meaningful life, which is resilient to future symptoms is just as important to patients. We seem to have entrenched ourselves so deeply in an overly basic approach of problem-solution and diagnosis-treatment paradigm. However, we don’t need to renege on modern advances to promote the patient’s strength, adaptability, and antifragility. An emphasis on patient growth can complement the medical model. We wonder what effect such an approach would have if the trainee’s patient with alcohol use disorder was instead told: “Given the suffering you have and have caused because of your alcohol use disorder, how do you plan to make changes in your life to help the treatment plan we create together?”
Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He also is the course director for the UCSD third-year medical student psychiatry clerkship. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at UCSD and the University of San Diego. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas are Setting Up a Generation for Failure. New York: Penguin Books, 2019.
2. Levis B et al. J Clin Epidemiol. 2020 Jun;122:115-128.e1.,
3. Taleb NN. Antifragile: Things That Gain from Disorder. Vol. 3. New York: Random House, 2012.
A trainee recently observed that psychiatrists frequently seem motivated to protect patients from emotional and internal disruption. He suggested that we often did so by validating their maladaptive perspectives regarding their impaired relationships to society and close attachments. These maneuvers were justified by referring to the need to establish a therapeutic alliance and reduce patients’ suffering.
As an example, he mentioned a patient with alcohol use disorder. The patient came in with complaints that he could not stay sober with his current level of depression. The patient also complained of a family member who was setting limits. To the trainee’s surprise, the patient was not challenged on his perceived victimhood and his fantasy that a sober life should mean a life without negative effect. Instead, the patient was validated in his anger toward the family member. In addition, his medications were adjusted, seemingly confirming to the patient that one could only ask for sobriety once life is empty of pain.
The observation of the trainee reminded us of the three great “untruths” mentioned by Greg Lukianoff and Jonathan Haidt, PhD, in their famous book, “The Coddling of the American Mind.”1 In the book they warn against the idea of fragility – what doesn’t kill you makes you weaker; emotional reasoning – always trust your feelings; and us-versus-them thinking – life is a battle between good people and evil people. The authors compare these three great untruths with the cognitive distortions of cognitive-behavioral therapy.
We ponder the trainee’s observation that psychiatrists appear to encourage the untruths rather than challenge them. Part of psychiatric and all medical training involves learning nonjudgmental approaches to human suffering and an identification with individual needs over societal demands. Our suspicion is that a nonjudgmental approach to the understanding of the human condition generates a desire to protect patients from a moralistic shaming position. However, we wonder if, at times, psychiatry takes this approach too far.
Reconceptualizing shame
Shame can be a toxic presence in the overwhelmed superego of a patient, but it can serve an important role in psychic development and should not be avoided out of hand. We suggest that it can be appropriate for a patient with an alcohol use disorder to feel some shame for the harm caused by their drinking, and we question the limit of psychiatry’s current pursuit of incessant validation. As an extreme example, would modern psychiatry discourage a patient who killed someone while driving in an intoxicated state from feeling remorse and shame?
Modern psychiatry appears to have other examples of the three great untruths on display. In our work, we are often faced with patients who are prematurely placed on disability, an example of fragility. Instead of encouraging patients to return to the workforce, they are “protected” from the emotional difficulty of work. In many patients this results in a decline in functioning and worsening of psychiatric symptoms. We are also confronted with patients who define themselves by how they feel, an example of emotional reasoning. Instead of using our clinical judgments to define and assess symptoms, patients are left to decide for themselves through self-rated scales with questionable validity.2 This can result in patients having their emotional experiences not only validated when inappropriate but can also give emotional reasoning a false sense of medical legitimacy.
Finally, we commonly see patients who endlessly blame family members and others for any life difficulties, a form of us-versus-them thinking. Instead of acknowledging and then integrating life challenges to achieve recovery, patients are affirmed despite clinicians having little evidence on the veracity of the patients’ perspective. As a consequence, patients can be further isolated from their greatest source of support.
In some ways, a mindlessly validating approach in psychiatry is unexpected since the practice of psychiatry would seem to promote the development of strong attachments and resilience. After all, connections to family, employment, social institutions, and even religious worship are associated with vastly better outcomes. Those who have become alienated to these pillars of social cohesion fare much worse. One may deplore the static and at times oppressive nature of these institutions but the empirical experience of practicing psychiatry leads one to a healthy respect for the stabilizing influence they accord for individuals struggling with life’s vicissitudes, unpredictability, and loneliness. Overcoming the fear of responsibility, living up to the demands and expectations of society, and having the strength to overcome difficult emotions should be the standard goals of psychiatric treatment.
From the knowledge gained from working with patients struggling from psychic pain, we wonder how to encourage patients to pursue those adaptive approaches to life. We argue that a stoic emphasis on learning to manage one’s affective and mental response to the inevitable changes of life is key to achieving wisdom and stability in our humble lives. This perspective is a common denominator of multiple different psychotherapies. The goal is to provide patients with the ability to be in a place where they are engaged with the world in a meaningful way that is not overwhelmed by distorted, self-absorbed psychic anguish. This perspective discourages externalization as a relatively low-yield way to understand and overcome one’s problems. One identifies childhood experiences with one’s mother as a source of adult distress not for the purpose of blaming her, but for the purpose of recognizing one’s own childish motivations for making maladaptive decisions as an adult.
For many patients, the goal should be to emphasize an internal locus of control and responsibility. We should also avoid constantly relying on society and government’s role in helping the individual achieve a satisfactory life. We wonder if this endless pursuit of nonjudgment and validation corrupts the doctor-patient interaction. In other words, and individual responsibility for their own psychic development. Psychotherapy that ends with the patient being able to identify all the traumas that led to their sorrow has simply left the patient in the role of helpless and sorrowful victim. Instead, we should allow patients to proceed to the next step, which is empowerment and transformation. From this angle, the field of psychiatry should be cautious of encouraging movements that promote victimhood and grievance as a meaningful psychic position to take in society.
Mr. Lukianoff and Dr. Haidt use cognitive therapy as an analogy throughout their books for how to confront the great untruths. They perceive those modern forms of thinking as cognitive distortions, which can be remedied using the techniques of cognitive restructuring found in cognitive-behavioral therapy. They encourage us to recognize those maladaptive thoughts, and create more accurate and adaptive ways of viewing the world – a view that would be able to grow from challenges not just survive them; a view referred to as antifragile.3 We believe that those techniques and others would certainly be of assistance in our current times. However, the first step is to recognize our problem – a problem that is not rooted in the DSM, research, or biology but in an exaggerated intention to be patient centered. We should, however, remember that, when a patient has negative schemas, being too patient centered can be encouraging to maladaptive behaviors.
In conclusion, we wonder what modern psychiatry could look like if it made a concerted effort at also treating mental illness by reinforcing the importance of individual agency and responsibility. Modern psychiatry has been so focused on describing biological symptoms needing biological treatments that we sometimes forget that having no symptom (being asymptomatic) is not the only goal. Having a fulfilling and meaningful life, which is resilient to future symptoms is just as important to patients. We seem to have entrenched ourselves so deeply in an overly basic approach of problem-solution and diagnosis-treatment paradigm. However, we don’t need to renege on modern advances to promote the patient’s strength, adaptability, and antifragility. An emphasis on patient growth can complement the medical model. We wonder what effect such an approach would have if the trainee’s patient with alcohol use disorder was instead told: “Given the suffering you have and have caused because of your alcohol use disorder, how do you plan to make changes in your life to help the treatment plan we create together?”
Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He also is the course director for the UCSD third-year medical student psychiatry clerkship. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at UCSD and the University of San Diego. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas are Setting Up a Generation for Failure. New York: Penguin Books, 2019.
2. Levis B et al. J Clin Epidemiol. 2020 Jun;122:115-128.e1.,
3. Taleb NN. Antifragile: Things That Gain from Disorder. Vol. 3. New York: Random House, 2012.
A trainee recently observed that psychiatrists frequently seem motivated to protect patients from emotional and internal disruption. He suggested that we often did so by validating their maladaptive perspectives regarding their impaired relationships to society and close attachments. These maneuvers were justified by referring to the need to establish a therapeutic alliance and reduce patients’ suffering.
As an example, he mentioned a patient with alcohol use disorder. The patient came in with complaints that he could not stay sober with his current level of depression. The patient also complained of a family member who was setting limits. To the trainee’s surprise, the patient was not challenged on his perceived victimhood and his fantasy that a sober life should mean a life without negative effect. Instead, the patient was validated in his anger toward the family member. In addition, his medications were adjusted, seemingly confirming to the patient that one could only ask for sobriety once life is empty of pain.
The observation of the trainee reminded us of the three great “untruths” mentioned by Greg Lukianoff and Jonathan Haidt, PhD, in their famous book, “The Coddling of the American Mind.”1 In the book they warn against the idea of fragility – what doesn’t kill you makes you weaker; emotional reasoning – always trust your feelings; and us-versus-them thinking – life is a battle between good people and evil people. The authors compare these three great untruths with the cognitive distortions of cognitive-behavioral therapy.
We ponder the trainee’s observation that psychiatrists appear to encourage the untruths rather than challenge them. Part of psychiatric and all medical training involves learning nonjudgmental approaches to human suffering and an identification with individual needs over societal demands. Our suspicion is that a nonjudgmental approach to the understanding of the human condition generates a desire to protect patients from a moralistic shaming position. However, we wonder if, at times, psychiatry takes this approach too far.
Reconceptualizing shame
Shame can be a toxic presence in the overwhelmed superego of a patient, but it can serve an important role in psychic development and should not be avoided out of hand. We suggest that it can be appropriate for a patient with an alcohol use disorder to feel some shame for the harm caused by their drinking, and we question the limit of psychiatry’s current pursuit of incessant validation. As an extreme example, would modern psychiatry discourage a patient who killed someone while driving in an intoxicated state from feeling remorse and shame?
Modern psychiatry appears to have other examples of the three great untruths on display. In our work, we are often faced with patients who are prematurely placed on disability, an example of fragility. Instead of encouraging patients to return to the workforce, they are “protected” from the emotional difficulty of work. In many patients this results in a decline in functioning and worsening of psychiatric symptoms. We are also confronted with patients who define themselves by how they feel, an example of emotional reasoning. Instead of using our clinical judgments to define and assess symptoms, patients are left to decide for themselves through self-rated scales with questionable validity.2 This can result in patients having their emotional experiences not only validated when inappropriate but can also give emotional reasoning a false sense of medical legitimacy.
Finally, we commonly see patients who endlessly blame family members and others for any life difficulties, a form of us-versus-them thinking. Instead of acknowledging and then integrating life challenges to achieve recovery, patients are affirmed despite clinicians having little evidence on the veracity of the patients’ perspective. As a consequence, patients can be further isolated from their greatest source of support.
In some ways, a mindlessly validating approach in psychiatry is unexpected since the practice of psychiatry would seem to promote the development of strong attachments and resilience. After all, connections to family, employment, social institutions, and even religious worship are associated with vastly better outcomes. Those who have become alienated to these pillars of social cohesion fare much worse. One may deplore the static and at times oppressive nature of these institutions but the empirical experience of practicing psychiatry leads one to a healthy respect for the stabilizing influence they accord for individuals struggling with life’s vicissitudes, unpredictability, and loneliness. Overcoming the fear of responsibility, living up to the demands and expectations of society, and having the strength to overcome difficult emotions should be the standard goals of psychiatric treatment.
From the knowledge gained from working with patients struggling from psychic pain, we wonder how to encourage patients to pursue those adaptive approaches to life. We argue that a stoic emphasis on learning to manage one’s affective and mental response to the inevitable changes of life is key to achieving wisdom and stability in our humble lives. This perspective is a common denominator of multiple different psychotherapies. The goal is to provide patients with the ability to be in a place where they are engaged with the world in a meaningful way that is not overwhelmed by distorted, self-absorbed psychic anguish. This perspective discourages externalization as a relatively low-yield way to understand and overcome one’s problems. One identifies childhood experiences with one’s mother as a source of adult distress not for the purpose of blaming her, but for the purpose of recognizing one’s own childish motivations for making maladaptive decisions as an adult.
For many patients, the goal should be to emphasize an internal locus of control and responsibility. We should also avoid constantly relying on society and government’s role in helping the individual achieve a satisfactory life. We wonder if this endless pursuit of nonjudgment and validation corrupts the doctor-patient interaction. In other words, and individual responsibility for their own psychic development. Psychotherapy that ends with the patient being able to identify all the traumas that led to their sorrow has simply left the patient in the role of helpless and sorrowful victim. Instead, we should allow patients to proceed to the next step, which is empowerment and transformation. From this angle, the field of psychiatry should be cautious of encouraging movements that promote victimhood and grievance as a meaningful psychic position to take in society.
Mr. Lukianoff and Dr. Haidt use cognitive therapy as an analogy throughout their books for how to confront the great untruths. They perceive those modern forms of thinking as cognitive distortions, which can be remedied using the techniques of cognitive restructuring found in cognitive-behavioral therapy. They encourage us to recognize those maladaptive thoughts, and create more accurate and adaptive ways of viewing the world – a view that would be able to grow from challenges not just survive them; a view referred to as antifragile.3 We believe that those techniques and others would certainly be of assistance in our current times. However, the first step is to recognize our problem – a problem that is not rooted in the DSM, research, or biology but in an exaggerated intention to be patient centered. We should, however, remember that, when a patient has negative schemas, being too patient centered can be encouraging to maladaptive behaviors.
In conclusion, we wonder what modern psychiatry could look like if it made a concerted effort at also treating mental illness by reinforcing the importance of individual agency and responsibility. Modern psychiatry has been so focused on describing biological symptoms needing biological treatments that we sometimes forget that having no symptom (being asymptomatic) is not the only goal. Having a fulfilling and meaningful life, which is resilient to future symptoms is just as important to patients. We seem to have entrenched ourselves so deeply in an overly basic approach of problem-solution and diagnosis-treatment paradigm. However, we don’t need to renege on modern advances to promote the patient’s strength, adaptability, and antifragility. An emphasis on patient growth can complement the medical model. We wonder what effect such an approach would have if the trainee’s patient with alcohol use disorder was instead told: “Given the suffering you have and have caused because of your alcohol use disorder, how do you plan to make changes in your life to help the treatment plan we create together?”
Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He also is the course director for the UCSD third-year medical student psychiatry clerkship. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at UCSD and the University of San Diego. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas are Setting Up a Generation for Failure. New York: Penguin Books, 2019.
2. Levis B et al. J Clin Epidemiol. 2020 Jun;122:115-128.e1.,
3. Taleb NN. Antifragile: Things That Gain from Disorder. Vol. 3. New York: Random House, 2012.
No prior use of insulin predicts postsurgical diabetes remission
Type 2 diabetes patients who had never used insulin showed sustained remission 10 years after bariatric surgery in a prospective study of 85 patients.
Having diabetes for less than 5 years was also predictive of achieving long-term diabetes remission, Diego Moriconi, MD, of the University of Pisa (Italy) and presenting study investigator, reported at the virtual annual meeting of the European Association for the Study of Diabetes.
“Weight loss was associated with type 2 diabetes remission 1 year after surgery, but it had no impact on the long-term relapse of diabetes,” Dr. Moriconi said.
The findings are important, commented Tina Vilsbøll, MD, DMSc, chief consultant at the Steno Diabetes Centre Copenhagen, who chaired the session. They’re important because they would help “to set the expectations for patients before they have surgery, what to expect in respect to resolution or remission of diabetes.”
If patients were taking insulin, for instance, the take home would seem to be not to expect too much in terms of remission of their diabetes, Dr. Vilsbøll said. She added: “Usually I am not a big fan of [relying on] diabetes duration, because often we know that patients with type 2 diabetes have had diabetes for a long time before they’re actually diagnosed.” However, “it seems to be very important here.”
Dr. Moriconi reported the findings of an observational study that had started in 2006 and recruited individuals about to undergo bariatric surgery for type 2 diabetes. Participants were evaluated before surgery and every 6-12 months after, undergoing various clinical and laboratory investigations, for a period of 10 years.
The majority of the recruited patients (76%) were women. Most (also 76%) had undergone gastric bypass (Roux-en-Y) surgery, and the remainder had undergone sleeve gastrectomy. Both types of surgery were equally as good at getting people into remission, as defined by the American Diabetes Association Standards of Medical Care in Diabetes, Dr. Moriconi said. As such, remission was achieved if the fasting blood glucose fell below 100 mg/dL and the hemoglobin A1c below 5.7%.
In the first year following surgery, 75% of patients had met diabetes remission criteria. This fell to 61% of patients after 5 years, and to 55% at 10 years. At each of these time points, 25% of patients had type 2 diabetes, with 14% relapsing back at 5 years and 20% at 10 years.
Dr. Moriconi pointed out some of the different characteristics of the 47 patients who had achieved diabetes remission at 10 years, compared with the 17 who had “relapsed” back to having type 2 diabetes and the 21 who had remained with type 2 diabetes.
The decrease in body mass index achieved at 10 years was no different between the three groups. However, 1 year after surgery, there had been a significantly greater drop in body in those who achieved remission, compared with those who did not (P = .04).
“Glycemic control improved with time in all the three groups after bariatric surgery, although more markedly so in the remission group,” Dr. Moriconi said.
He highlighted how none of the patients who had achieved remission had used insulin, whereas 12% of those who had relapsed and half (52%) of those who remained with type 2 diabetes had used insulin (P < .0001).
Patients who achieved remission at 1, 5, and 10 years were more likely to have had diabetes for less than 5 years than those who remained with type 2 diabetes. The average duration of diabetes was 2 years in those achieving remission versus 8 years in those who had relapsed and 13 years in those who had remained diabetic (P < .0001).
Logistic regression analysis, which adjusted for all major confounding factors such as age, sex, and type of surgery, showed that only the duration of diabetes and insulin therapy before surgery were the only predictors of long-term diabetes remission.
The study had no commercial funding. Dr. Moriconi and Dr. Vilsbøll had no conflicts of interest to disclose.
SOURCE: Moriconi D. EASD 2020, Oral presentation 120.
Type 2 diabetes patients who had never used insulin showed sustained remission 10 years after bariatric surgery in a prospective study of 85 patients.
Having diabetes for less than 5 years was also predictive of achieving long-term diabetes remission, Diego Moriconi, MD, of the University of Pisa (Italy) and presenting study investigator, reported at the virtual annual meeting of the European Association for the Study of Diabetes.
“Weight loss was associated with type 2 diabetes remission 1 year after surgery, but it had no impact on the long-term relapse of diabetes,” Dr. Moriconi said.
The findings are important, commented Tina Vilsbøll, MD, DMSc, chief consultant at the Steno Diabetes Centre Copenhagen, who chaired the session. They’re important because they would help “to set the expectations for patients before they have surgery, what to expect in respect to resolution or remission of diabetes.”
If patients were taking insulin, for instance, the take home would seem to be not to expect too much in terms of remission of their diabetes, Dr. Vilsbøll said. She added: “Usually I am not a big fan of [relying on] diabetes duration, because often we know that patients with type 2 diabetes have had diabetes for a long time before they’re actually diagnosed.” However, “it seems to be very important here.”
Dr. Moriconi reported the findings of an observational study that had started in 2006 and recruited individuals about to undergo bariatric surgery for type 2 diabetes. Participants were evaluated before surgery and every 6-12 months after, undergoing various clinical and laboratory investigations, for a period of 10 years.
The majority of the recruited patients (76%) were women. Most (also 76%) had undergone gastric bypass (Roux-en-Y) surgery, and the remainder had undergone sleeve gastrectomy. Both types of surgery were equally as good at getting people into remission, as defined by the American Diabetes Association Standards of Medical Care in Diabetes, Dr. Moriconi said. As such, remission was achieved if the fasting blood glucose fell below 100 mg/dL and the hemoglobin A1c below 5.7%.
In the first year following surgery, 75% of patients had met diabetes remission criteria. This fell to 61% of patients after 5 years, and to 55% at 10 years. At each of these time points, 25% of patients had type 2 diabetes, with 14% relapsing back at 5 years and 20% at 10 years.
Dr. Moriconi pointed out some of the different characteristics of the 47 patients who had achieved diabetes remission at 10 years, compared with the 17 who had “relapsed” back to having type 2 diabetes and the 21 who had remained with type 2 diabetes.
The decrease in body mass index achieved at 10 years was no different between the three groups. However, 1 year after surgery, there had been a significantly greater drop in body in those who achieved remission, compared with those who did not (P = .04).
“Glycemic control improved with time in all the three groups after bariatric surgery, although more markedly so in the remission group,” Dr. Moriconi said.
He highlighted how none of the patients who had achieved remission had used insulin, whereas 12% of those who had relapsed and half (52%) of those who remained with type 2 diabetes had used insulin (P < .0001).
Patients who achieved remission at 1, 5, and 10 years were more likely to have had diabetes for less than 5 years than those who remained with type 2 diabetes. The average duration of diabetes was 2 years in those achieving remission versus 8 years in those who had relapsed and 13 years in those who had remained diabetic (P < .0001).
Logistic regression analysis, which adjusted for all major confounding factors such as age, sex, and type of surgery, showed that only the duration of diabetes and insulin therapy before surgery were the only predictors of long-term diabetes remission.
The study had no commercial funding. Dr. Moriconi and Dr. Vilsbøll had no conflicts of interest to disclose.
SOURCE: Moriconi D. EASD 2020, Oral presentation 120.
Type 2 diabetes patients who had never used insulin showed sustained remission 10 years after bariatric surgery in a prospective study of 85 patients.
Having diabetes for less than 5 years was also predictive of achieving long-term diabetes remission, Diego Moriconi, MD, of the University of Pisa (Italy) and presenting study investigator, reported at the virtual annual meeting of the European Association for the Study of Diabetes.
“Weight loss was associated with type 2 diabetes remission 1 year after surgery, but it had no impact on the long-term relapse of diabetes,” Dr. Moriconi said.
The findings are important, commented Tina Vilsbøll, MD, DMSc, chief consultant at the Steno Diabetes Centre Copenhagen, who chaired the session. They’re important because they would help “to set the expectations for patients before they have surgery, what to expect in respect to resolution or remission of diabetes.”
If patients were taking insulin, for instance, the take home would seem to be not to expect too much in terms of remission of their diabetes, Dr. Vilsbøll said. She added: “Usually I am not a big fan of [relying on] diabetes duration, because often we know that patients with type 2 diabetes have had diabetes for a long time before they’re actually diagnosed.” However, “it seems to be very important here.”
Dr. Moriconi reported the findings of an observational study that had started in 2006 and recruited individuals about to undergo bariatric surgery for type 2 diabetes. Participants were evaluated before surgery and every 6-12 months after, undergoing various clinical and laboratory investigations, for a period of 10 years.
The majority of the recruited patients (76%) were women. Most (also 76%) had undergone gastric bypass (Roux-en-Y) surgery, and the remainder had undergone sleeve gastrectomy. Both types of surgery were equally as good at getting people into remission, as defined by the American Diabetes Association Standards of Medical Care in Diabetes, Dr. Moriconi said. As such, remission was achieved if the fasting blood glucose fell below 100 mg/dL and the hemoglobin A1c below 5.7%.
In the first year following surgery, 75% of patients had met diabetes remission criteria. This fell to 61% of patients after 5 years, and to 55% at 10 years. At each of these time points, 25% of patients had type 2 diabetes, with 14% relapsing back at 5 years and 20% at 10 years.
Dr. Moriconi pointed out some of the different characteristics of the 47 patients who had achieved diabetes remission at 10 years, compared with the 17 who had “relapsed” back to having type 2 diabetes and the 21 who had remained with type 2 diabetes.
The decrease in body mass index achieved at 10 years was no different between the three groups. However, 1 year after surgery, there had been a significantly greater drop in body in those who achieved remission, compared with those who did not (P = .04).
“Glycemic control improved with time in all the three groups after bariatric surgery, although more markedly so in the remission group,” Dr. Moriconi said.
He highlighted how none of the patients who had achieved remission had used insulin, whereas 12% of those who had relapsed and half (52%) of those who remained with type 2 diabetes had used insulin (P < .0001).
Patients who achieved remission at 1, 5, and 10 years were more likely to have had diabetes for less than 5 years than those who remained with type 2 diabetes. The average duration of diabetes was 2 years in those achieving remission versus 8 years in those who had relapsed and 13 years in those who had remained diabetic (P < .0001).
Logistic regression analysis, which adjusted for all major confounding factors such as age, sex, and type of surgery, showed that only the duration of diabetes and insulin therapy before surgery were the only predictors of long-term diabetes remission.
The study had no commercial funding. Dr. Moriconi and Dr. Vilsbøll had no conflicts of interest to disclose.
SOURCE: Moriconi D. EASD 2020, Oral presentation 120.
FROM EASD 2020
Address root causes to manage NASH
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM DIGESTIVE DISEASES: NEW ADVANCES
CDC playbook prepares states for rollout of COVID-19 vaccine if one is approved
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
Hair loss and scalp papules
The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.

The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
Top AGA Community patient cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. The upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field.
In case you missed it, here are some clinical discussions and Roundtables in the newsfeed this month:
- Patient case: Crohn’s patient with three different strictures (https://community.gastro.org/posts/22491)
- Patient case: Alcoholic hepatitis and positive anti-smooth muscle antibody (https://community.gastro.org/posts/22407)
- COVID-19: The importance of preparedness in independent GI practices (https://community.gastro.org/posts/22340)
- Patient case: Crohn’s patient with no tissue (https://community.gastro.org/posts/22472)
Roundtables (https://community.gastro.org/discussions/)
- Roadmap for the future of colorectal cancer screening in the U.S.
- Windows on Clinical GI lecture series: NAFLD, Crohn’s disease and gastroparesis
View all upcoming Roundtables in the community at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. The upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field.
In case you missed it, here are some clinical discussions and Roundtables in the newsfeed this month:
- Patient case: Crohn’s patient with three different strictures (https://community.gastro.org/posts/22491)
- Patient case: Alcoholic hepatitis and positive anti-smooth muscle antibody (https://community.gastro.org/posts/22407)
- COVID-19: The importance of preparedness in independent GI practices (https://community.gastro.org/posts/22340)
- Patient case: Crohn’s patient with no tissue (https://community.gastro.org/posts/22472)
Roundtables (https://community.gastro.org/discussions/)
- Roadmap for the future of colorectal cancer screening in the U.S.
- Windows on Clinical GI lecture series: NAFLD, Crohn’s disease and gastroparesis
View all upcoming Roundtables in the community at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. The upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field.
In case you missed it, here are some clinical discussions and Roundtables in the newsfeed this month:
- Patient case: Crohn’s patient with three different strictures (https://community.gastro.org/posts/22491)
- Patient case: Alcoholic hepatitis and positive anti-smooth muscle antibody (https://community.gastro.org/posts/22407)
- COVID-19: The importance of preparedness in independent GI practices (https://community.gastro.org/posts/22340)
- Patient case: Crohn’s patient with no tissue (https://community.gastro.org/posts/22472)
Roundtables (https://community.gastro.org/discussions/)
- Roadmap for the future of colorectal cancer screening in the U.S.
- Windows on Clinical GI lecture series: NAFLD, Crohn’s disease and gastroparesis
View all upcoming Roundtables in the community at https://community.gastro.org/discussions.