User login
FOLFOXIRI tops doublets as bevacizumab backbone for mCRC
investigators reported in the Journal of Clinical Oncology.
In analyzing data from five clinical trials, the investigators found a 4.4-month increase in median overall survival and an 11.6% increase in estimated 5-year overall survival with FOLFOXIRI versus doublets. The trade-off was a higher incidence of grade 3-4 adverse events with FOLFOXIRI.
FOLFOXIRI plus bevacizumab is already included among first-line options in most clinical guidelines and recommendations, but there was no “proper estimation of the magnitude of the overall survival benefit” because trials had other primary endpoints, according to study author Chiara Cremolini, MD, PhD, of University of Pisa (Italy), and colleagues.
“To fully appreciate the cost/benefit balance of this option,” the investigators wanted to see how the numbers played out when overall survival was the primary endpoint, so they pooled individual patient data from the CHARTA, OLIVIA, STEAM, TRIBE, and TRIBE2 trials.
Patient characteristics and treatment
The analysis included 1,697 patients. The median age was 61 years (range, 53-67 years).
About 99% of patients had an Eastern Cooperative Oncology Group performance score of 0 or 1. About 20% had left-sided RAS and BRAF wild-type tumors because of the increased use of anti–epidermal growth factor receptor antibodies as first-line therapy for that indication in recent years.
In all, 846 patients were randomized to FOLFOXIRI plus bevacizumab and 851 to bevacizumab with doublets: 69.9% to FOLFOX (leucovorin, fluorouracil, and oxaliplatin) and 30.1% to FOLFIRI (fluorouracil, leucovorin, and irinotecan).
The duration of induction in all five trials ranged from 4 to 6 months. It was followed by maintenance with a fluoropyrimidine (fluorouracil and leucovorin or capecitabine) plus bevacizumab.
Efficacy and safety
At a median follow-up of 39.9 months, the median overall survival was 28.9 months in the FOLFOXIRI group and 24.5 months in the doublet group (P < .001). The estimated 5-year overall survival rate was 22.3% and 10.7%, respectively.
The median progression-free survival was 12.2 months in the FOLFOXIRI group and 9.9 months in the doublet group (P < .001).
The objective response rate was higher with FOLFOXIRI (64.5% vs. 53.6%, P < .001), as was R0 resection rate (16.4% vs. 11.8%, P = .007).
The FOLFOXIRI group also had a higher incidence of grade 3-4 adverse events, including neutropenia (45.8% vs. 21.5%; P < .001), febrile neutropenia (6.3% vs. 3.7%; P = .019), nausea (5.5% vs. 3.0%; P = .016), mucositis (5.1% vs. 2.9%; P = .024), and diarrhea (17.8% vs. 8.4%; P < .001).
Even so, FOLFOXIRI plus bevacizumab was not associated with a significant increase in toxic deaths (2.3% vs. 1.4%; P = .277).
Patient selection is ‘critical’
Based on their findings, the investigators said the best candidates for first-line FOLFOXIRI plus bevacizumab may be younger patients with an ECOG performance status of 0 or 1 and right-sided and/or RAS-mutated tumors not exposed to a previous oxaliplatin-based adjuvant regimen.
FOLFOXIRI plus bevacizumab did not provide any additional benefit in patients with BRAF-mutant tumors, so the combination shouldn’t be the first choice in this group, the investigators wrote. “FOLFOX plus bevacizumab seems the preferable upfront option.”
For left-sided RAS and BRAF wild-type tumors, a chemotherapy doublet with an anti–epidermal growth factor receptor remains the preferred option, according to the investigators.
“The study does support the use of FOLFOXIRI and bevacizumab as a valuable first-line option, but patient selection is critical because there is a toxicity cost; this efficacy versus toxicity will constantly be a seesaw for us,” commented Aparna Parikh, MD, of Massachusetts General Hospital in Boston, when the study was presented at the American Society of Clinical Oncology annual meeting earlier this year.
There was no external funding for this analysis. Three of the original five trials were sponsored by Roche. The study authors had numerous industry ties. Among others, Dr. Cremolini is a consultant for and reported honoraria and travel expenses from Roche. One author was a Genentech employee. Dr. Parikh disclosed relationships with Lilly, Genentech, and other companies.
SOURCE: Cremolini C et al. J Clin Oncol. 2020 Aug 20. doi: 10.1200/JCO.20.01225.
investigators reported in the Journal of Clinical Oncology.
In analyzing data from five clinical trials, the investigators found a 4.4-month increase in median overall survival and an 11.6% increase in estimated 5-year overall survival with FOLFOXIRI versus doublets. The trade-off was a higher incidence of grade 3-4 adverse events with FOLFOXIRI.
FOLFOXIRI plus bevacizumab is already included among first-line options in most clinical guidelines and recommendations, but there was no “proper estimation of the magnitude of the overall survival benefit” because trials had other primary endpoints, according to study author Chiara Cremolini, MD, PhD, of University of Pisa (Italy), and colleagues.
“To fully appreciate the cost/benefit balance of this option,” the investigators wanted to see how the numbers played out when overall survival was the primary endpoint, so they pooled individual patient data from the CHARTA, OLIVIA, STEAM, TRIBE, and TRIBE2 trials.
Patient characteristics and treatment
The analysis included 1,697 patients. The median age was 61 years (range, 53-67 years).
About 99% of patients had an Eastern Cooperative Oncology Group performance score of 0 or 1. About 20% had left-sided RAS and BRAF wild-type tumors because of the increased use of anti–epidermal growth factor receptor antibodies as first-line therapy for that indication in recent years.
In all, 846 patients were randomized to FOLFOXIRI plus bevacizumab and 851 to bevacizumab with doublets: 69.9% to FOLFOX (leucovorin, fluorouracil, and oxaliplatin) and 30.1% to FOLFIRI (fluorouracil, leucovorin, and irinotecan).
The duration of induction in all five trials ranged from 4 to 6 months. It was followed by maintenance with a fluoropyrimidine (fluorouracil and leucovorin or capecitabine) plus bevacizumab.
Efficacy and safety
At a median follow-up of 39.9 months, the median overall survival was 28.9 months in the FOLFOXIRI group and 24.5 months in the doublet group (P < .001). The estimated 5-year overall survival rate was 22.3% and 10.7%, respectively.
The median progression-free survival was 12.2 months in the FOLFOXIRI group and 9.9 months in the doublet group (P < .001).
The objective response rate was higher with FOLFOXIRI (64.5% vs. 53.6%, P < .001), as was R0 resection rate (16.4% vs. 11.8%, P = .007).
The FOLFOXIRI group also had a higher incidence of grade 3-4 adverse events, including neutropenia (45.8% vs. 21.5%; P < .001), febrile neutropenia (6.3% vs. 3.7%; P = .019), nausea (5.5% vs. 3.0%; P = .016), mucositis (5.1% vs. 2.9%; P = .024), and diarrhea (17.8% vs. 8.4%; P < .001).
Even so, FOLFOXIRI plus bevacizumab was not associated with a significant increase in toxic deaths (2.3% vs. 1.4%; P = .277).
Patient selection is ‘critical’
Based on their findings, the investigators said the best candidates for first-line FOLFOXIRI plus bevacizumab may be younger patients with an ECOG performance status of 0 or 1 and right-sided and/or RAS-mutated tumors not exposed to a previous oxaliplatin-based adjuvant regimen.
FOLFOXIRI plus bevacizumab did not provide any additional benefit in patients with BRAF-mutant tumors, so the combination shouldn’t be the first choice in this group, the investigators wrote. “FOLFOX plus bevacizumab seems the preferable upfront option.”
For left-sided RAS and BRAF wild-type tumors, a chemotherapy doublet with an anti–epidermal growth factor receptor remains the preferred option, according to the investigators.
“The study does support the use of FOLFOXIRI and bevacizumab as a valuable first-line option, but patient selection is critical because there is a toxicity cost; this efficacy versus toxicity will constantly be a seesaw for us,” commented Aparna Parikh, MD, of Massachusetts General Hospital in Boston, when the study was presented at the American Society of Clinical Oncology annual meeting earlier this year.
There was no external funding for this analysis. Three of the original five trials were sponsored by Roche. The study authors had numerous industry ties. Among others, Dr. Cremolini is a consultant for and reported honoraria and travel expenses from Roche. One author was a Genentech employee. Dr. Parikh disclosed relationships with Lilly, Genentech, and other companies.
SOURCE: Cremolini C et al. J Clin Oncol. 2020 Aug 20. doi: 10.1200/JCO.20.01225.
investigators reported in the Journal of Clinical Oncology.
In analyzing data from five clinical trials, the investigators found a 4.4-month increase in median overall survival and an 11.6% increase in estimated 5-year overall survival with FOLFOXIRI versus doublets. The trade-off was a higher incidence of grade 3-4 adverse events with FOLFOXIRI.
FOLFOXIRI plus bevacizumab is already included among first-line options in most clinical guidelines and recommendations, but there was no “proper estimation of the magnitude of the overall survival benefit” because trials had other primary endpoints, according to study author Chiara Cremolini, MD, PhD, of University of Pisa (Italy), and colleagues.
“To fully appreciate the cost/benefit balance of this option,” the investigators wanted to see how the numbers played out when overall survival was the primary endpoint, so they pooled individual patient data from the CHARTA, OLIVIA, STEAM, TRIBE, and TRIBE2 trials.
Patient characteristics and treatment
The analysis included 1,697 patients. The median age was 61 years (range, 53-67 years).
About 99% of patients had an Eastern Cooperative Oncology Group performance score of 0 or 1. About 20% had left-sided RAS and BRAF wild-type tumors because of the increased use of anti–epidermal growth factor receptor antibodies as first-line therapy for that indication in recent years.
In all, 846 patients were randomized to FOLFOXIRI plus bevacizumab and 851 to bevacizumab with doublets: 69.9% to FOLFOX (leucovorin, fluorouracil, and oxaliplatin) and 30.1% to FOLFIRI (fluorouracil, leucovorin, and irinotecan).
The duration of induction in all five trials ranged from 4 to 6 months. It was followed by maintenance with a fluoropyrimidine (fluorouracil and leucovorin or capecitabine) plus bevacizumab.
Efficacy and safety
At a median follow-up of 39.9 months, the median overall survival was 28.9 months in the FOLFOXIRI group and 24.5 months in the doublet group (P < .001). The estimated 5-year overall survival rate was 22.3% and 10.7%, respectively.
The median progression-free survival was 12.2 months in the FOLFOXIRI group and 9.9 months in the doublet group (P < .001).
The objective response rate was higher with FOLFOXIRI (64.5% vs. 53.6%, P < .001), as was R0 resection rate (16.4% vs. 11.8%, P = .007).
The FOLFOXIRI group also had a higher incidence of grade 3-4 adverse events, including neutropenia (45.8% vs. 21.5%; P < .001), febrile neutropenia (6.3% vs. 3.7%; P = .019), nausea (5.5% vs. 3.0%; P = .016), mucositis (5.1% vs. 2.9%; P = .024), and diarrhea (17.8% vs. 8.4%; P < .001).
Even so, FOLFOXIRI plus bevacizumab was not associated with a significant increase in toxic deaths (2.3% vs. 1.4%; P = .277).
Patient selection is ‘critical’
Based on their findings, the investigators said the best candidates for first-line FOLFOXIRI plus bevacizumab may be younger patients with an ECOG performance status of 0 or 1 and right-sided and/or RAS-mutated tumors not exposed to a previous oxaliplatin-based adjuvant regimen.
FOLFOXIRI plus bevacizumab did not provide any additional benefit in patients with BRAF-mutant tumors, so the combination shouldn’t be the first choice in this group, the investigators wrote. “FOLFOX plus bevacizumab seems the preferable upfront option.”
For left-sided RAS and BRAF wild-type tumors, a chemotherapy doublet with an anti–epidermal growth factor receptor remains the preferred option, according to the investigators.
“The study does support the use of FOLFOXIRI and bevacizumab as a valuable first-line option, but patient selection is critical because there is a toxicity cost; this efficacy versus toxicity will constantly be a seesaw for us,” commented Aparna Parikh, MD, of Massachusetts General Hospital in Boston, when the study was presented at the American Society of Clinical Oncology annual meeting earlier this year.
There was no external funding for this analysis. Three of the original five trials were sponsored by Roche. The study authors had numerous industry ties. Among others, Dr. Cremolini is a consultant for and reported honoraria and travel expenses from Roche. One author was a Genentech employee. Dr. Parikh disclosed relationships with Lilly, Genentech, and other companies.
SOURCE: Cremolini C et al. J Clin Oncol. 2020 Aug 20. doi: 10.1200/JCO.20.01225.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Exorcising your ghosts
The COVID-19 pandemic has affected private medical practices on so many levels, not least of which is the loss of employees to illness, fear of illness, early retirement, and other reasons.
If you’re not hip to the slang, “ghosting” is the situation in which an employee disappears without any warning, notice, or explanation. It usually occurs after a candidate accepts a job offer, and you schedule their first day of work. That day dawns, but the new hire never arrives. Less commonly, an employee who has been with you for some time simply stops showing up and cannot be contacted.
Many employers think of ghosting as a relatively new phenomenon, and blame it on the irresponsibility of younger age groups – millennials, in particular. In fact, it has been an issue for many years across all age groups, and employers often share more of the responsibility than they think.
While total prevention is impossible, there are steps you can take as an employer to minimize ghosting in your practice.
- Your hiring process needs to be efficient. If you wait too long to schedule an interview with a promising candidate or to offer them the job, another job offer could lure them away. At the very least, a lengthy process or a lack of transparency may make the applicant apprehensive about accepting a job with you, particularly if other employers are pursuing them.
- Keep applicants in the loop. Follow up with every candidate; let them know where they are in your hiring process. Applicants who have no clue whether they have a shot at the job are going to start looking elsewhere. And make sure they know the job description and starting salary from the outset.
- Talk to new hires before their first day. Contact them personally to see if they have any questions or concerns, and let them know that you’re looking forward to their arrival.
- Once they start, make them feel welcome. An employee’s first few days on the job set the tone for the rest of the employment relationship. During this time, clearly communicate what the employee can expect from you and what you expect from them. Take time to discuss key issues, such as work schedules, timekeeping practices, how performance is measured, and dress codes. Introduce them to coworkers, and get them started shadowing more experienced staff members.
- Take a hard look at your supervision and your supervisors. Business people like to say that employees don’t quit their job, they quit their boss. If an employee quits – with or without notice – it may very well be because of a poor working relationship with you or the supervisor. To be effective, you and your supervisors need to be diligent in setting goals, managing performance, and applying workplace rules and policies. Numerous third-party companies provide training and guidance in these areas when needed.
- Recognize and reward. As I’ve written many times, positive feedback is a simple, low-cost way to improve employee retention. It demonstrates that you value an employee’s contributions and sets an excellent example for other employees. Effective recognition can come from anyone – including patients – and should be given openly. (Another old adage: “Praise publicly, criticize privately.”) It never hurts to catch an employee doing something right and acknowledge it.
- Don’t jump to conclusions. If a new hire or employee is absent without notice, don’t just assume you’ve been ghosted. There may be extenuating circumstances, such as an emergency or illness. In some states, an employee’s absence is protected under a law where the employee may not be required to provide advance notice, and taking adverse action could violate these laws. Establish procedures for attempting to contact absent employees, and make sure you’re complying with all applicable leave laws before taking any action.
If an employee does abandon their job, think before you act. Comply with all applicable laws. Act consistently with how you’ve handled similar situations in the past. Your attorney should be involved, especially if the decision involves termination. Notify the employee in writing. As with all employment decisions, keep adequate documentation in case the decision is ever challenged, or you need it to support future disciplinary decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. He has no disclosures related to this column. Write to him at [email protected].
The COVID-19 pandemic has affected private medical practices on so many levels, not least of which is the loss of employees to illness, fear of illness, early retirement, and other reasons.
If you’re not hip to the slang, “ghosting” is the situation in which an employee disappears without any warning, notice, or explanation. It usually occurs after a candidate accepts a job offer, and you schedule their first day of work. That day dawns, but the new hire never arrives. Less commonly, an employee who has been with you for some time simply stops showing up and cannot be contacted.
Many employers think of ghosting as a relatively new phenomenon, and blame it on the irresponsibility of younger age groups – millennials, in particular. In fact, it has been an issue for many years across all age groups, and employers often share more of the responsibility than they think.
While total prevention is impossible, there are steps you can take as an employer to minimize ghosting in your practice.
- Your hiring process needs to be efficient. If you wait too long to schedule an interview with a promising candidate or to offer them the job, another job offer could lure them away. At the very least, a lengthy process or a lack of transparency may make the applicant apprehensive about accepting a job with you, particularly if other employers are pursuing them.
- Keep applicants in the loop. Follow up with every candidate; let them know where they are in your hiring process. Applicants who have no clue whether they have a shot at the job are going to start looking elsewhere. And make sure they know the job description and starting salary from the outset.
- Talk to new hires before their first day. Contact them personally to see if they have any questions or concerns, and let them know that you’re looking forward to their arrival.
- Once they start, make them feel welcome. An employee’s first few days on the job set the tone for the rest of the employment relationship. During this time, clearly communicate what the employee can expect from you and what you expect from them. Take time to discuss key issues, such as work schedules, timekeeping practices, how performance is measured, and dress codes. Introduce them to coworkers, and get them started shadowing more experienced staff members.
- Take a hard look at your supervision and your supervisors. Business people like to say that employees don’t quit their job, they quit their boss. If an employee quits – with or without notice – it may very well be because of a poor working relationship with you or the supervisor. To be effective, you and your supervisors need to be diligent in setting goals, managing performance, and applying workplace rules and policies. Numerous third-party companies provide training and guidance in these areas when needed.
- Recognize and reward. As I’ve written many times, positive feedback is a simple, low-cost way to improve employee retention. It demonstrates that you value an employee’s contributions and sets an excellent example for other employees. Effective recognition can come from anyone – including patients – and should be given openly. (Another old adage: “Praise publicly, criticize privately.”) It never hurts to catch an employee doing something right and acknowledge it.
- Don’t jump to conclusions. If a new hire or employee is absent without notice, don’t just assume you’ve been ghosted. There may be extenuating circumstances, such as an emergency or illness. In some states, an employee’s absence is protected under a law where the employee may not be required to provide advance notice, and taking adverse action could violate these laws. Establish procedures for attempting to contact absent employees, and make sure you’re complying with all applicable leave laws before taking any action.
If an employee does abandon their job, think before you act. Comply with all applicable laws. Act consistently with how you’ve handled similar situations in the past. Your attorney should be involved, especially if the decision involves termination. Notify the employee in writing. As with all employment decisions, keep adequate documentation in case the decision is ever challenged, or you need it to support future disciplinary decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. He has no disclosures related to this column. Write to him at [email protected].
The COVID-19 pandemic has affected private medical practices on so many levels, not least of which is the loss of employees to illness, fear of illness, early retirement, and other reasons.
If you’re not hip to the slang, “ghosting” is the situation in which an employee disappears without any warning, notice, or explanation. It usually occurs after a candidate accepts a job offer, and you schedule their first day of work. That day dawns, but the new hire never arrives. Less commonly, an employee who has been with you for some time simply stops showing up and cannot be contacted.
Many employers think of ghosting as a relatively new phenomenon, and blame it on the irresponsibility of younger age groups – millennials, in particular. In fact, it has been an issue for many years across all age groups, and employers often share more of the responsibility than they think.
While total prevention is impossible, there are steps you can take as an employer to minimize ghosting in your practice.
- Your hiring process needs to be efficient. If you wait too long to schedule an interview with a promising candidate or to offer them the job, another job offer could lure them away. At the very least, a lengthy process or a lack of transparency may make the applicant apprehensive about accepting a job with you, particularly if other employers are pursuing them.
- Keep applicants in the loop. Follow up with every candidate; let them know where they are in your hiring process. Applicants who have no clue whether they have a shot at the job are going to start looking elsewhere. And make sure they know the job description and starting salary from the outset.
- Talk to new hires before their first day. Contact them personally to see if they have any questions or concerns, and let them know that you’re looking forward to their arrival.
- Once they start, make them feel welcome. An employee’s first few days on the job set the tone for the rest of the employment relationship. During this time, clearly communicate what the employee can expect from you and what you expect from them. Take time to discuss key issues, such as work schedules, timekeeping practices, how performance is measured, and dress codes. Introduce them to coworkers, and get them started shadowing more experienced staff members.
- Take a hard look at your supervision and your supervisors. Business people like to say that employees don’t quit their job, they quit their boss. If an employee quits – with or without notice – it may very well be because of a poor working relationship with you or the supervisor. To be effective, you and your supervisors need to be diligent in setting goals, managing performance, and applying workplace rules and policies. Numerous third-party companies provide training and guidance in these areas when needed.
- Recognize and reward. As I’ve written many times, positive feedback is a simple, low-cost way to improve employee retention. It demonstrates that you value an employee’s contributions and sets an excellent example for other employees. Effective recognition can come from anyone – including patients – and should be given openly. (Another old adage: “Praise publicly, criticize privately.”) It never hurts to catch an employee doing something right and acknowledge it.
- Don’t jump to conclusions. If a new hire or employee is absent without notice, don’t just assume you’ve been ghosted. There may be extenuating circumstances, such as an emergency or illness. In some states, an employee’s absence is protected under a law where the employee may not be required to provide advance notice, and taking adverse action could violate these laws. Establish procedures for attempting to contact absent employees, and make sure you’re complying with all applicable leave laws before taking any action.
If an employee does abandon their job, think before you act. Comply with all applicable laws. Act consistently with how you’ve handled similar situations in the past. Your attorney should be involved, especially if the decision involves termination. Notify the employee in writing. As with all employment decisions, keep adequate documentation in case the decision is ever challenged, or you need it to support future disciplinary decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. He has no disclosures related to this column. Write to him at [email protected].
For lower-risk MDS, treat ‘what bugs patients most’
Clinicians who treat patients with lower-risk myelodysplastic syndrome should focus on “what bugs patients most,” with therapeutic goals reflecting and respecting the patients’ goals, a specialist in MDS recommended.
“There’s an uncomfortable truth in treating lower-risk MDS: No treatment that we have has ever been demonstrated in a prospective trial to prolong survival in lower-risk MDS, so in the end, what we’re doing is trying to improve transfusion needs and to improve quality of life,” said Michael A. Sekeres, MD, MS, from the Cleveland Clinic.
Dr. Sekeres described optimal therapy for patients with lower-risk MDS in an online presentation during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
He acknowledged that the definition of MDS as “a heterogeneous clonal hematopoietic disorder derived from an abnormal multipotent progenitor cell, characterized by a hyperproliferative bone marrow, dysplasia of the cellular elements, and ineffective hematopoiesis” can be confusing even for hematologists well versed in the disorder.
An easier-to-grasp explanation, he said, is that “MDS is considered a cancer, and like other cancers it has a clonal origin, involves the abnormal growth of cells that exceeds the growth of other cells around them and don’t know when to stop growing, and it takes over normal tissue, so that the normal tissues – in this case the hematopoietic precursors in the bone marrow – don’t function normally, resulting in cytopenias.”
‘Mild displeasure syndrome’
Approximately 95% of patients with MDS have a discrete genetic abnormality, but only one driver mutation, in the gene SF3B1, is considered to be a lower-risk abnormality, with a more favorable prognosis.
Treatment options for patients with lower-risk MDS, defined as an International Prognostic Scoring System score of 1 or less, or a Revised IPSS score of 3.5 or less, will depend on the patients’ transfusion needs and quality of life.
Patients with no transfusion requirements and a generally good quality of life may be followed by observation alone, with blood counts every 1 to 6 months depending on clinical presentation.
“We have some folks coming in who really don’t have very bad blood counts and have a good quality of life,” Dr. Sekeres said. “Those folks we would consider to have a very good risk type of MDS, which one of my patients referred to once as ‘mild displeasure syndrome.’ It was a displeasure to him to have to fight the traffic to come into Cleveland to see me every month, or 2 months, or 6 months, but beyond that we didn’t have to treat his MDS.”
Isolated cytopenias
Patients with isolated anemia, with hemoglobin less than 10 g/dL and/or transfusion dependence, and who are symptomatic should be started on an erythopoiesis-stimulating agent (ESA), either recombinant humanized erythropoietin or darbepoetin, or the erythroid-maturing agent luspatercept (Reblozyl).
The probability of a response to ESAs in this populations ranges from about 15% to 35%, with patients who have low baseline serum erythropoietin and no or few transfusions most likely to respond.
“On the other hand, patients who come into our clinic who are already dependent on red blood cell transfusions and have a sky-high [erythropoietin] level in the hundreds or even thousands have a very low likelihood of responding to exogenously administered ESAs,” he said.
Patients with no response to ESAs or luspatercept or a loss of response suggestive of disease progression should undergo repeat bone marrow biopsy. Patients who develop deletion 5q should be started on lenalidomide (Revlimid). In these patients, next-generation sequencing may also reveal targetable abnormalities.
For patients with isolated thrombocytopenia, thrombopoietin agonists such as romiplostim or eltrombopag may help to reduce platelet transfusion requirements and clinically significant bleeding events, but these agents come with a very important caveat: in addition to promoting platelet production, thrombopoietin receptor agonists can promote the growth of blasts, which could in turn promote the transformation of MDS to acute myeloid leukemia.
“This is an off-label use of romiplostim for the treatment of MDS with thrombocytopenia, and this drug should never, never, never be given to a patient who has excess blasts at baseline MDS; the same is true of its cousin eltrombopag.” Dr. Sekeres said.
Multlineage dysplasia
Patients with multilineage dysplasia can have good responses to hypomethylating agents, either azacitidine 75 mg/m2 IV or subcutaneously for 3 days every 4 weeks, or decitabine 20 mg/m2 IV for 3 days every 4 weeks.
“Another approach to treating patients with multilineage dysplasia is to consider the use of antithymocyte globulin; in other words, treat these patients as if they have aplastic anemia, because there are some types of MDS in which immune-mediated destruction of bone marrow plays a role,” Dr. Sekeres said.
“This is particularly appealing in patients who have a hyperplastic marrow, or those who have other autoimmune conditions that are going on that may indicate a broader autoimmune process that’s involved in the bone marrow,” he added.
Patients treated with antithymocyte globulin require hospitalization with discharge on steroids for 1 month to prevent serum sickness in response to the treatment, and maintenance on low-dose cyclosporine.
“In MDS, unfortunately, our understanding of the biology of the disease far exceeds what we can do about it, but we’re starting to catch up,” Dr. Sekeres said.
No funding source for the presentation was disclosed. Dr. Sekeres disclosed serving on advisory boards for Celegene/Bristol-Myers Squibb, Takeda/Millenium, and Pfizer.
Clinicians who treat patients with lower-risk myelodysplastic syndrome should focus on “what bugs patients most,” with therapeutic goals reflecting and respecting the patients’ goals, a specialist in MDS recommended.
“There’s an uncomfortable truth in treating lower-risk MDS: No treatment that we have has ever been demonstrated in a prospective trial to prolong survival in lower-risk MDS, so in the end, what we’re doing is trying to improve transfusion needs and to improve quality of life,” said Michael A. Sekeres, MD, MS, from the Cleveland Clinic.
Dr. Sekeres described optimal therapy for patients with lower-risk MDS in an online presentation during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
He acknowledged that the definition of MDS as “a heterogeneous clonal hematopoietic disorder derived from an abnormal multipotent progenitor cell, characterized by a hyperproliferative bone marrow, dysplasia of the cellular elements, and ineffective hematopoiesis” can be confusing even for hematologists well versed in the disorder.
An easier-to-grasp explanation, he said, is that “MDS is considered a cancer, and like other cancers it has a clonal origin, involves the abnormal growth of cells that exceeds the growth of other cells around them and don’t know when to stop growing, and it takes over normal tissue, so that the normal tissues – in this case the hematopoietic precursors in the bone marrow – don’t function normally, resulting in cytopenias.”
‘Mild displeasure syndrome’
Approximately 95% of patients with MDS have a discrete genetic abnormality, but only one driver mutation, in the gene SF3B1, is considered to be a lower-risk abnormality, with a more favorable prognosis.
Treatment options for patients with lower-risk MDS, defined as an International Prognostic Scoring System score of 1 or less, or a Revised IPSS score of 3.5 or less, will depend on the patients’ transfusion needs and quality of life.
Patients with no transfusion requirements and a generally good quality of life may be followed by observation alone, with blood counts every 1 to 6 months depending on clinical presentation.
“We have some folks coming in who really don’t have very bad blood counts and have a good quality of life,” Dr. Sekeres said. “Those folks we would consider to have a very good risk type of MDS, which one of my patients referred to once as ‘mild displeasure syndrome.’ It was a displeasure to him to have to fight the traffic to come into Cleveland to see me every month, or 2 months, or 6 months, but beyond that we didn’t have to treat his MDS.”
Isolated cytopenias
Patients with isolated anemia, with hemoglobin less than 10 g/dL and/or transfusion dependence, and who are symptomatic should be started on an erythopoiesis-stimulating agent (ESA), either recombinant humanized erythropoietin or darbepoetin, or the erythroid-maturing agent luspatercept (Reblozyl).
The probability of a response to ESAs in this populations ranges from about 15% to 35%, with patients who have low baseline serum erythropoietin and no or few transfusions most likely to respond.
“On the other hand, patients who come into our clinic who are already dependent on red blood cell transfusions and have a sky-high [erythropoietin] level in the hundreds or even thousands have a very low likelihood of responding to exogenously administered ESAs,” he said.
Patients with no response to ESAs or luspatercept or a loss of response suggestive of disease progression should undergo repeat bone marrow biopsy. Patients who develop deletion 5q should be started on lenalidomide (Revlimid). In these patients, next-generation sequencing may also reveal targetable abnormalities.
For patients with isolated thrombocytopenia, thrombopoietin agonists such as romiplostim or eltrombopag may help to reduce platelet transfusion requirements and clinically significant bleeding events, but these agents come with a very important caveat: in addition to promoting platelet production, thrombopoietin receptor agonists can promote the growth of blasts, which could in turn promote the transformation of MDS to acute myeloid leukemia.
“This is an off-label use of romiplostim for the treatment of MDS with thrombocytopenia, and this drug should never, never, never be given to a patient who has excess blasts at baseline MDS; the same is true of its cousin eltrombopag.” Dr. Sekeres said.
Multlineage dysplasia
Patients with multilineage dysplasia can have good responses to hypomethylating agents, either azacitidine 75 mg/m2 IV or subcutaneously for 3 days every 4 weeks, or decitabine 20 mg/m2 IV for 3 days every 4 weeks.
“Another approach to treating patients with multilineage dysplasia is to consider the use of antithymocyte globulin; in other words, treat these patients as if they have aplastic anemia, because there are some types of MDS in which immune-mediated destruction of bone marrow plays a role,” Dr. Sekeres said.
“This is particularly appealing in patients who have a hyperplastic marrow, or those who have other autoimmune conditions that are going on that may indicate a broader autoimmune process that’s involved in the bone marrow,” he added.
Patients treated with antithymocyte globulin require hospitalization with discharge on steroids for 1 month to prevent serum sickness in response to the treatment, and maintenance on low-dose cyclosporine.
“In MDS, unfortunately, our understanding of the biology of the disease far exceeds what we can do about it, but we’re starting to catch up,” Dr. Sekeres said.
No funding source for the presentation was disclosed. Dr. Sekeres disclosed serving on advisory boards for Celegene/Bristol-Myers Squibb, Takeda/Millenium, and Pfizer.
Clinicians who treat patients with lower-risk myelodysplastic syndrome should focus on “what bugs patients most,” with therapeutic goals reflecting and respecting the patients’ goals, a specialist in MDS recommended.
“There’s an uncomfortable truth in treating lower-risk MDS: No treatment that we have has ever been demonstrated in a prospective trial to prolong survival in lower-risk MDS, so in the end, what we’re doing is trying to improve transfusion needs and to improve quality of life,” said Michael A. Sekeres, MD, MS, from the Cleveland Clinic.
Dr. Sekeres described optimal therapy for patients with lower-risk MDS in an online presentation during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
He acknowledged that the definition of MDS as “a heterogeneous clonal hematopoietic disorder derived from an abnormal multipotent progenitor cell, characterized by a hyperproliferative bone marrow, dysplasia of the cellular elements, and ineffective hematopoiesis” can be confusing even for hematologists well versed in the disorder.
An easier-to-grasp explanation, he said, is that “MDS is considered a cancer, and like other cancers it has a clonal origin, involves the abnormal growth of cells that exceeds the growth of other cells around them and don’t know when to stop growing, and it takes over normal tissue, so that the normal tissues – in this case the hematopoietic precursors in the bone marrow – don’t function normally, resulting in cytopenias.”
‘Mild displeasure syndrome’
Approximately 95% of patients with MDS have a discrete genetic abnormality, but only one driver mutation, in the gene SF3B1, is considered to be a lower-risk abnormality, with a more favorable prognosis.
Treatment options for patients with lower-risk MDS, defined as an International Prognostic Scoring System score of 1 or less, or a Revised IPSS score of 3.5 or less, will depend on the patients’ transfusion needs and quality of life.
Patients with no transfusion requirements and a generally good quality of life may be followed by observation alone, with blood counts every 1 to 6 months depending on clinical presentation.
“We have some folks coming in who really don’t have very bad blood counts and have a good quality of life,” Dr. Sekeres said. “Those folks we would consider to have a very good risk type of MDS, which one of my patients referred to once as ‘mild displeasure syndrome.’ It was a displeasure to him to have to fight the traffic to come into Cleveland to see me every month, or 2 months, or 6 months, but beyond that we didn’t have to treat his MDS.”
Isolated cytopenias
Patients with isolated anemia, with hemoglobin less than 10 g/dL and/or transfusion dependence, and who are symptomatic should be started on an erythopoiesis-stimulating agent (ESA), either recombinant humanized erythropoietin or darbepoetin, or the erythroid-maturing agent luspatercept (Reblozyl).
The probability of a response to ESAs in this populations ranges from about 15% to 35%, with patients who have low baseline serum erythropoietin and no or few transfusions most likely to respond.
“On the other hand, patients who come into our clinic who are already dependent on red blood cell transfusions and have a sky-high [erythropoietin] level in the hundreds or even thousands have a very low likelihood of responding to exogenously administered ESAs,” he said.
Patients with no response to ESAs or luspatercept or a loss of response suggestive of disease progression should undergo repeat bone marrow biopsy. Patients who develop deletion 5q should be started on lenalidomide (Revlimid). In these patients, next-generation sequencing may also reveal targetable abnormalities.
For patients with isolated thrombocytopenia, thrombopoietin agonists such as romiplostim or eltrombopag may help to reduce platelet transfusion requirements and clinically significant bleeding events, but these agents come with a very important caveat: in addition to promoting platelet production, thrombopoietin receptor agonists can promote the growth of blasts, which could in turn promote the transformation of MDS to acute myeloid leukemia.
“This is an off-label use of romiplostim for the treatment of MDS with thrombocytopenia, and this drug should never, never, never be given to a patient who has excess blasts at baseline MDS; the same is true of its cousin eltrombopag.” Dr. Sekeres said.
Multlineage dysplasia
Patients with multilineage dysplasia can have good responses to hypomethylating agents, either azacitidine 75 mg/m2 IV or subcutaneously for 3 days every 4 weeks, or decitabine 20 mg/m2 IV for 3 days every 4 weeks.
“Another approach to treating patients with multilineage dysplasia is to consider the use of antithymocyte globulin; in other words, treat these patients as if they have aplastic anemia, because there are some types of MDS in which immune-mediated destruction of bone marrow plays a role,” Dr. Sekeres said.
“This is particularly appealing in patients who have a hyperplastic marrow, or those who have other autoimmune conditions that are going on that may indicate a broader autoimmune process that’s involved in the bone marrow,” he added.
Patients treated with antithymocyte globulin require hospitalization with discharge on steroids for 1 month to prevent serum sickness in response to the treatment, and maintenance on low-dose cyclosporine.
“In MDS, unfortunately, our understanding of the biology of the disease far exceeds what we can do about it, but we’re starting to catch up,” Dr. Sekeres said.
No funding source for the presentation was disclosed. Dr. Sekeres disclosed serving on advisory boards for Celegene/Bristol-Myers Squibb, Takeda/Millenium, and Pfizer.
FROM ASH HEMATOLOGIC MALIGNANCIES 2020
Studying in Dermatology Residency
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
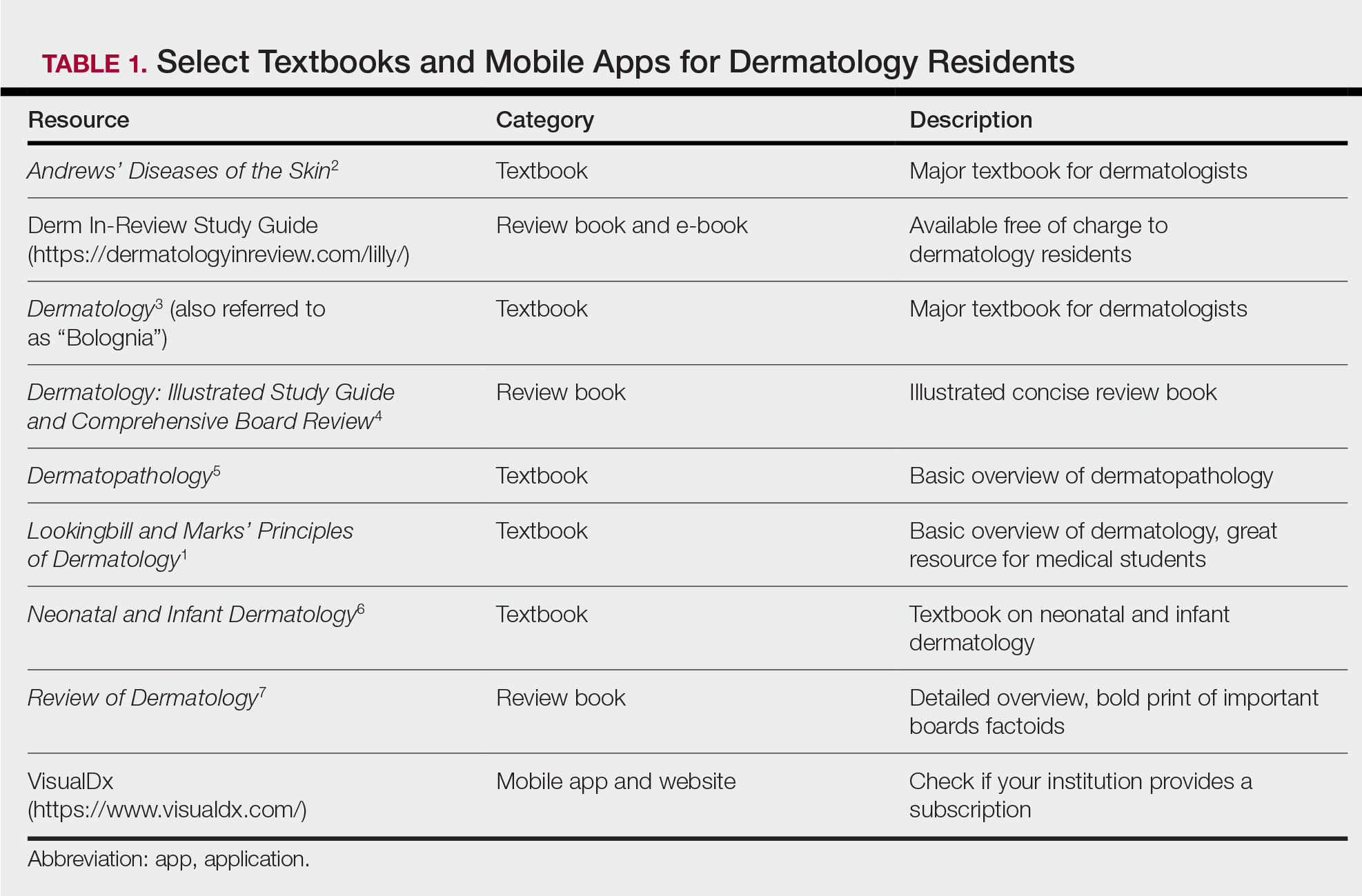
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.
First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
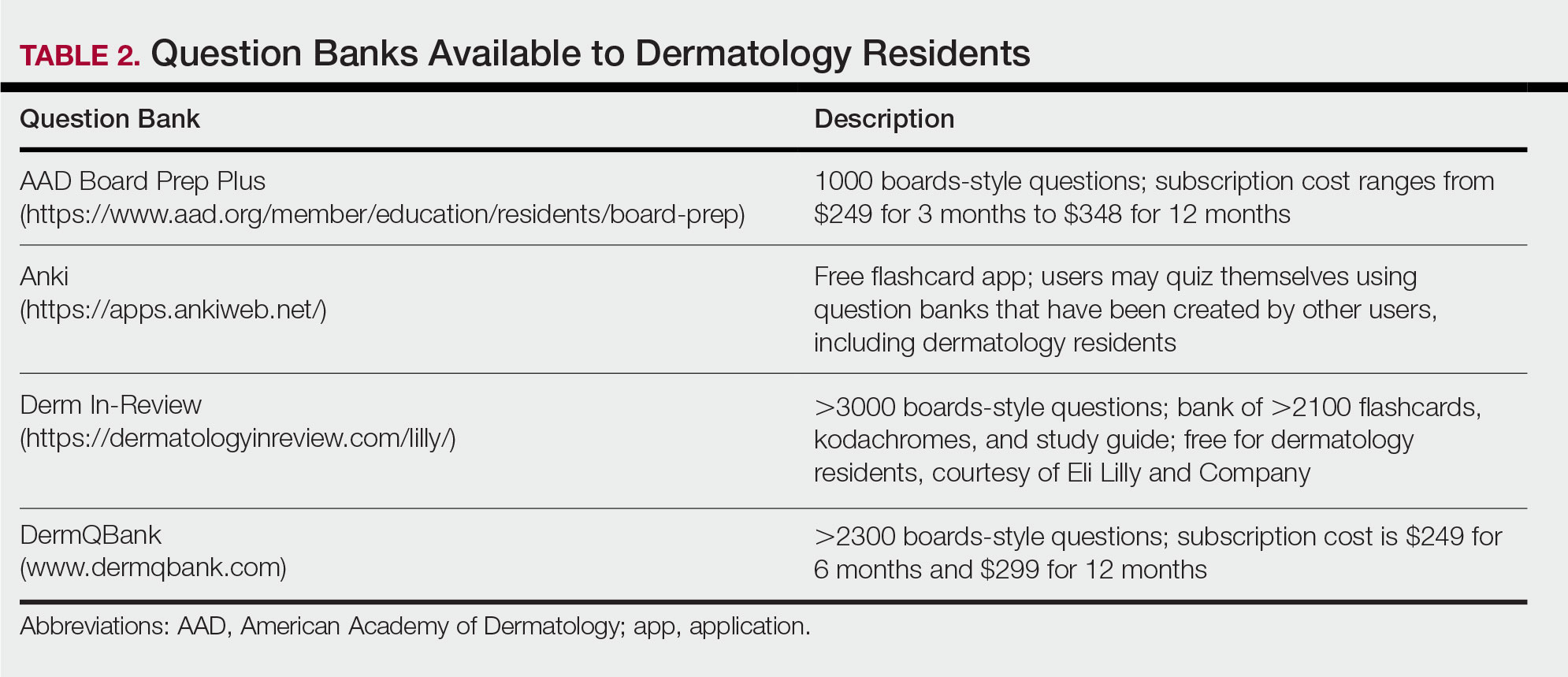
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.
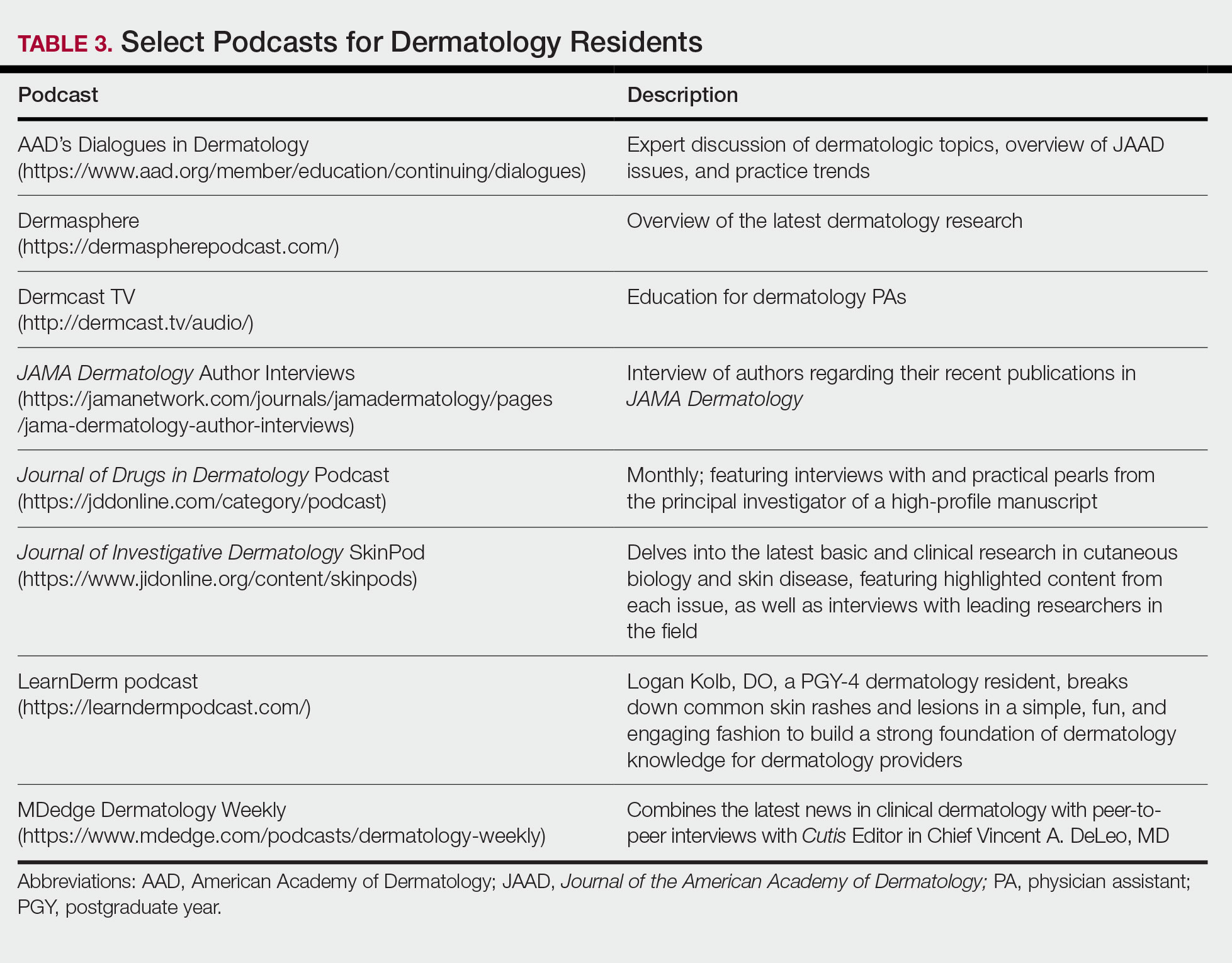
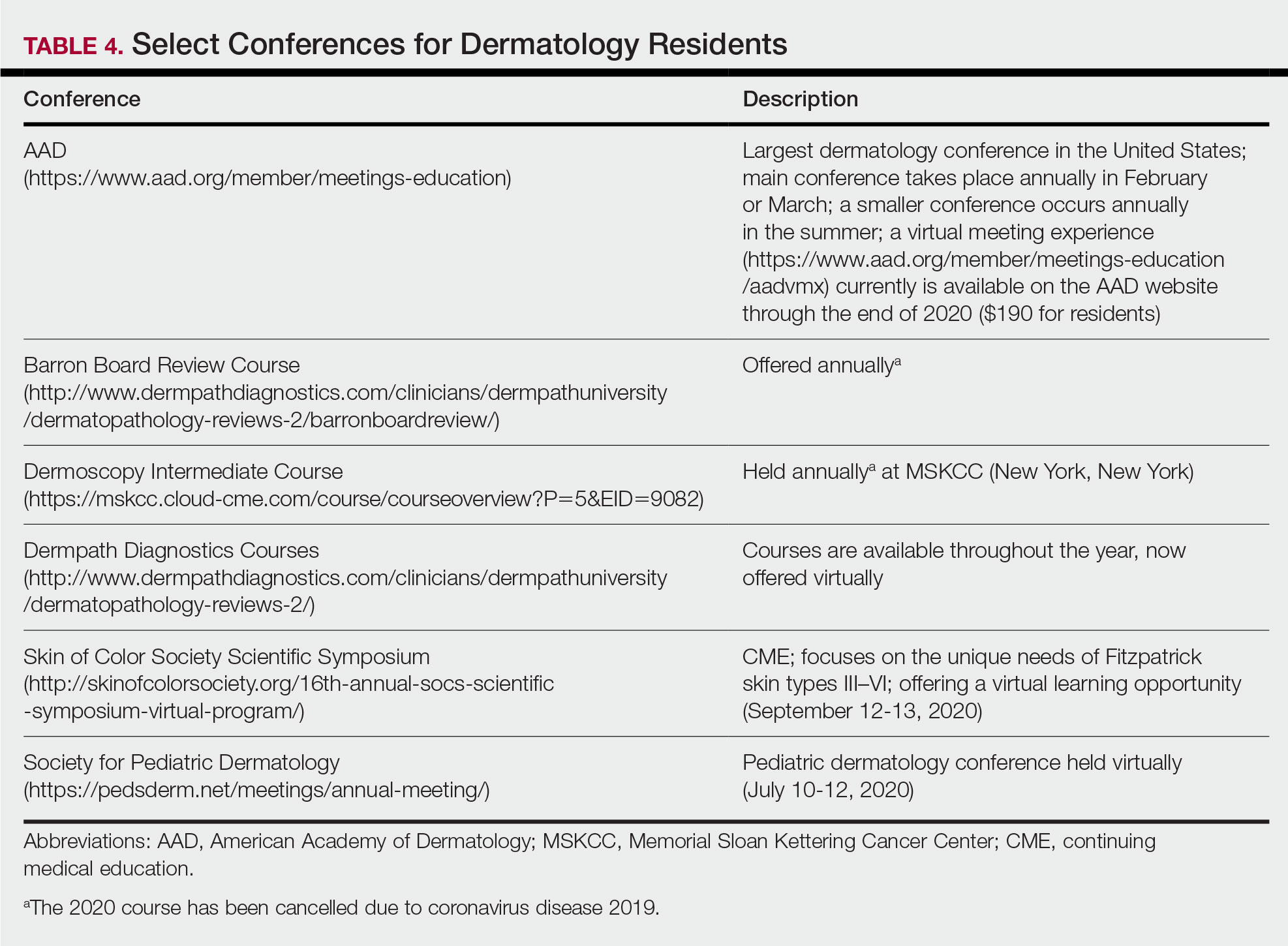
Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.
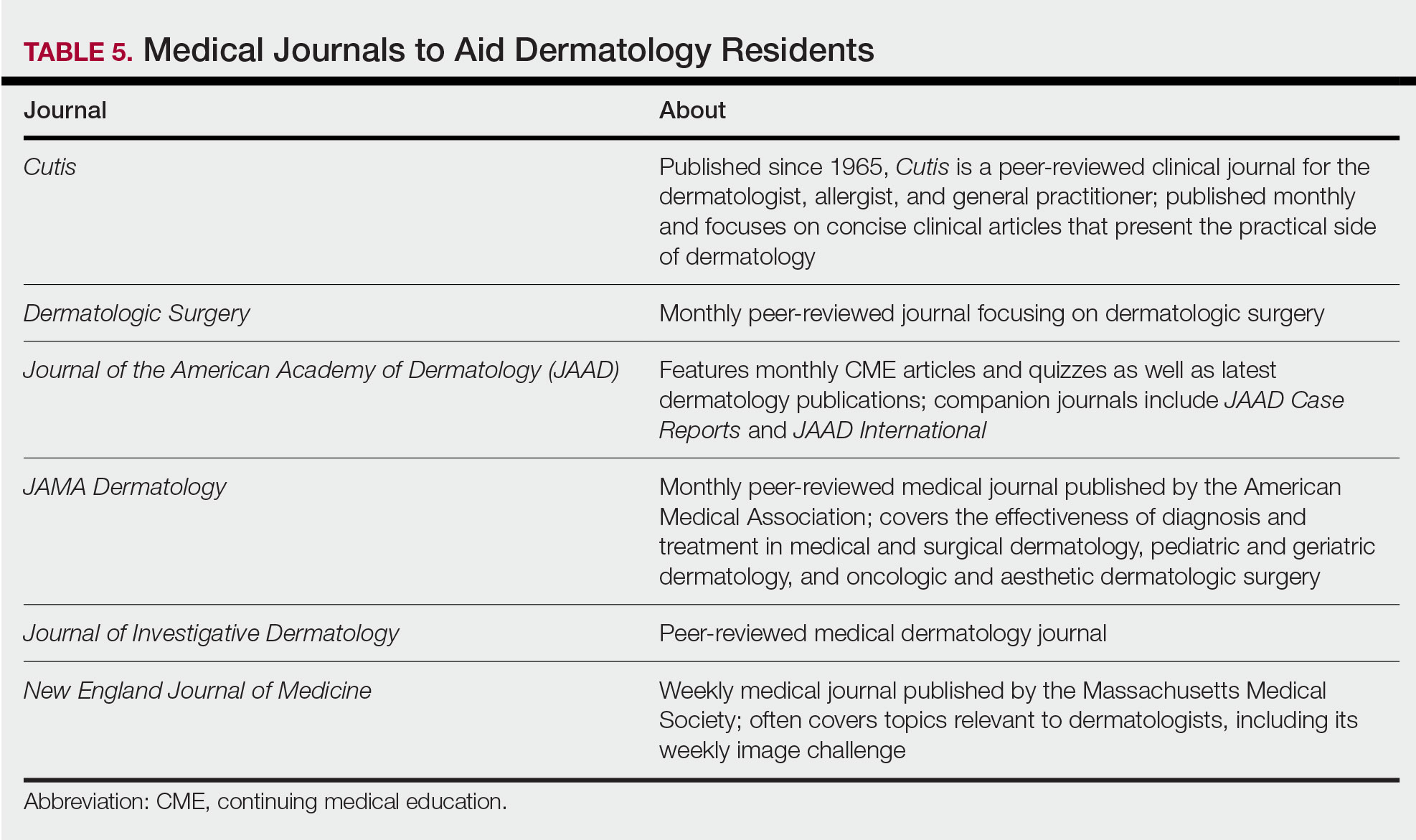
Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.

First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.



Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.

Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.

First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.



Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.

Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
Resident Pearls
- Independent study is a large component of dermatology residency.
- Consistent habits and a tailored approach will support optimal learning for each dermatology resident.
- The beginning of residency is a good time to explore a variety of resources to see what works best. Toward the end of residency, as studying becomes more targeted, residents may benefit from sticking to the resources with which they are most comfortable.
Dystrophic Calcinosis Cutis: Treatment With Intravenous Sodium Thiosulfate
To the Editor:
Severe dystrophic calcinosis cutis is a debilitating disease with no universally accepted therapeutic options. This case demonstrates the benefit of intravenous (IV) sodium thiosulfate in alleviating the calcified lesions as well as the associated pain and disability. This application of IV sodium thiosulfate with a favorable outcome is new and should be considered for the treatment of generalized dystrophic calcinosis cutis, especially when topical, procedural, or surgical options are not feasible.
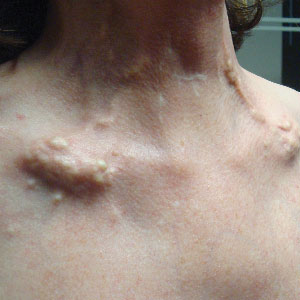
A 54-year-old woman with a history of well-controlled dermatomyositis and systemic lupus erythematosus presented with diffuse, hard, calcified lesions on the legs, arms, clavicular region, and neck that had slowly progressed over at least a 10-year period (Figure 1). The lesions were consistent with dystrophic calcinosis cutis. The patient was started on 12.5 g of IV sodium thiosulfate 3 times weekly infused over 30 minutes. Drastic diminution of the cutaneous calcification was observed at 3-month follow-up (Figure 2). She reported decreased pain and burning as well as increased overall functionality and improved sleep. The patient completed 8 months of therapy, but the treatment was stopped secondary to suspicion of a lupuslike flare, and the lesions recurred with more widespread involvement, including the trunk, tendons, bony prominences, and supraclavicular soft tissue. The patient reported burning pain and pruritus that resulted in impairment of daily activities such as getting dressed. Sodium thiosulfate was restarted once weekly, which again resulted in reduction of the dystrophic calcinosis cutis.
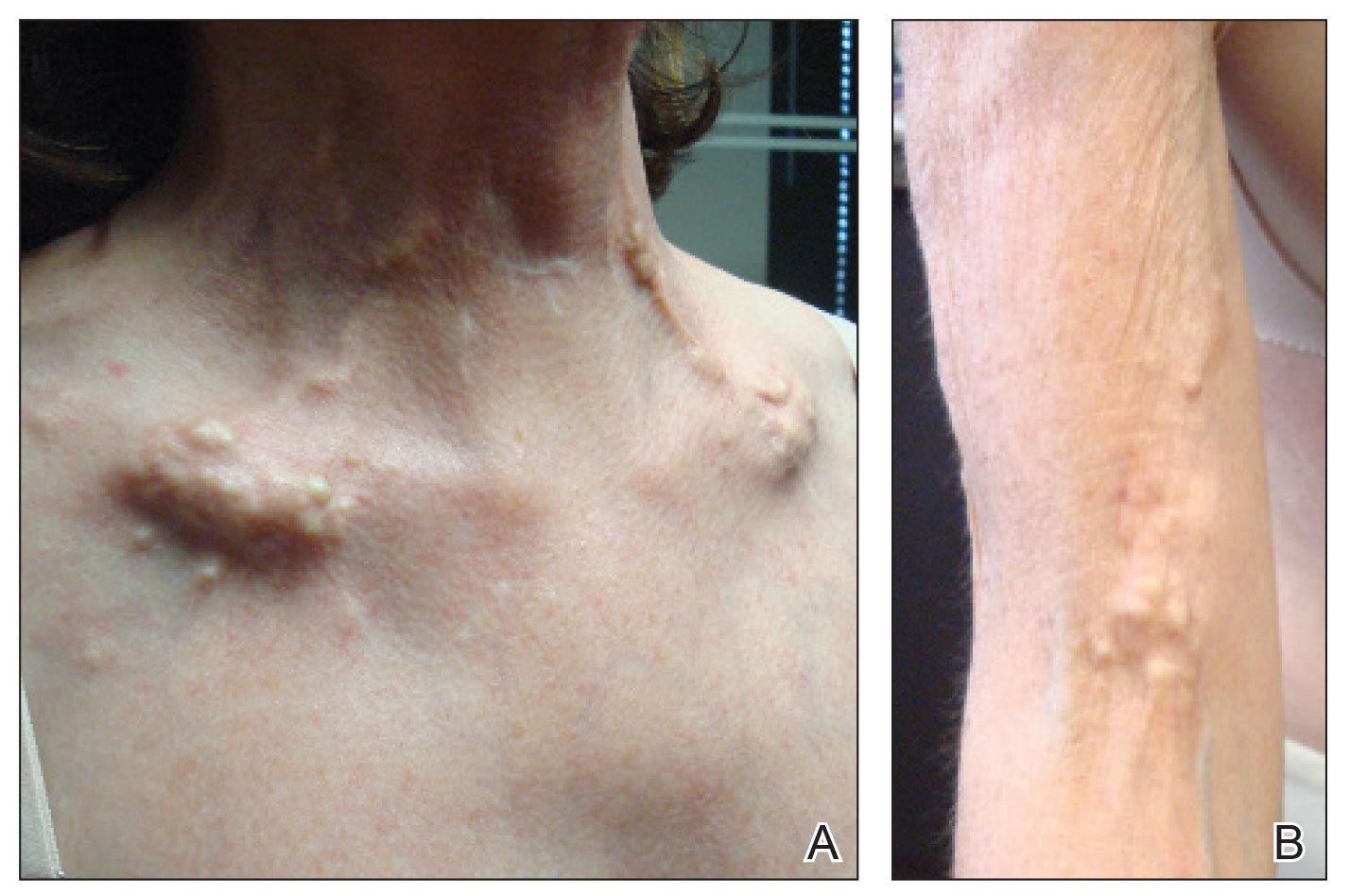
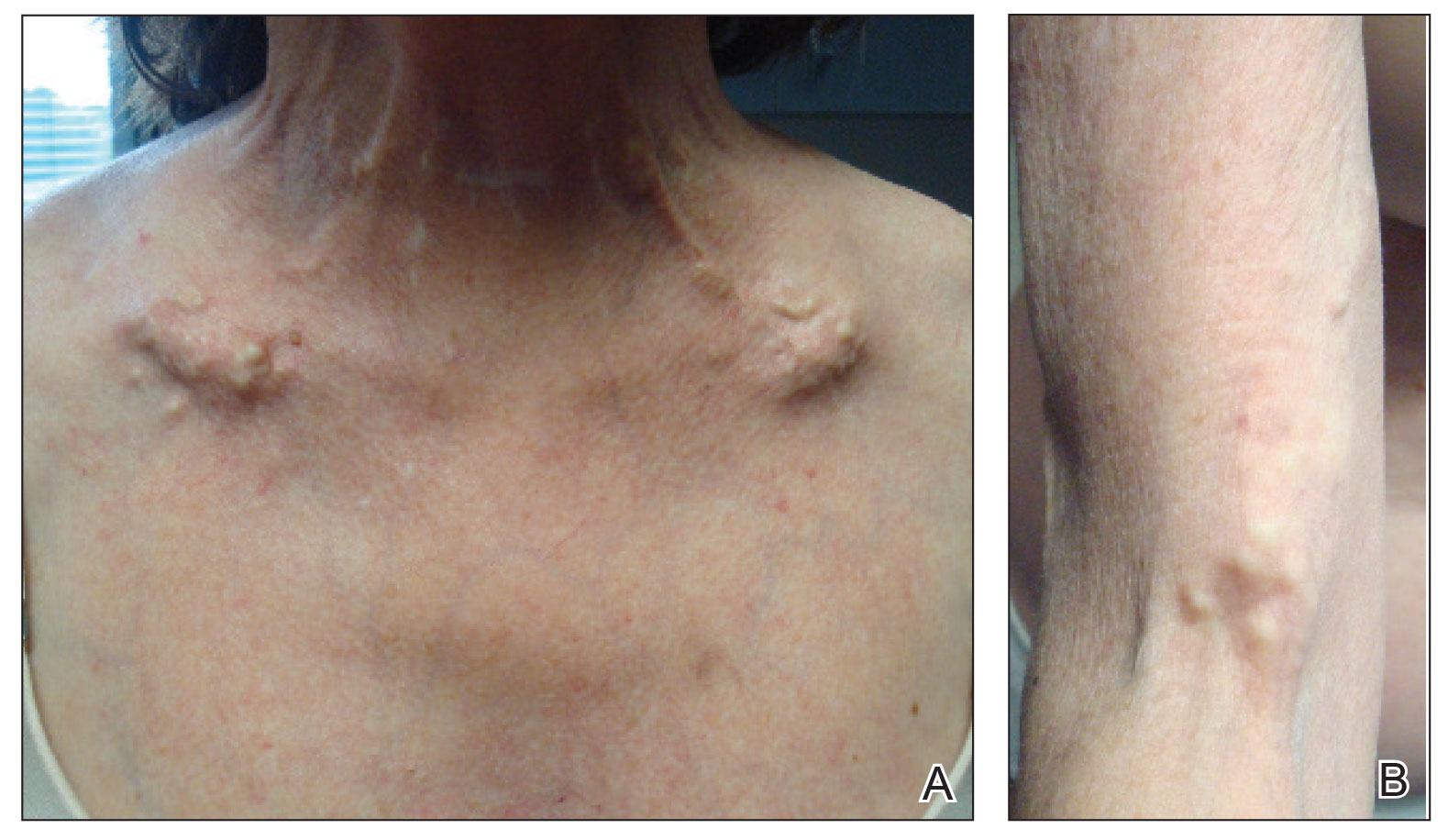
Dystrophic calcinosis cutis is a debilitating disease that results in considerable morbidity and pain with major implications on quality of life. The pathophysiology is unclear; calcium and phosphate serum levels generally are normal. A proposed mechanism is that chronic inflammation causes tissue damage and defective collagen synthesis, resulting in a distorted architecture that facilitates calcium deposition in the skin and subcutaneous tissues.1 Dystrophic calcinosis cutis most commonly is associated with systemic sclerosis and dermatomyositis but also can be seen in systemic lupus erythematosus, panniculitis, and other connective tissue diseases. It also can occur with skin neoplasms, collagen and elastin disorders, porphyria cutanea tarda, and pancreatic panniculitis.1 Progression of dystrophic calcinosis cutis usually is independent of the associated disease status.
Treatment is based on anecdotal evidence from case reports, as there is no universally accepted pharmacologic or procedural intervention available for dystrophic calcinosis cutis. Medications that have been reported to be helpful to varying degrees include diltiazem, colchicine, minocycline, IV immunoglobulin, ceftriaxone, aluminum hydroxide, probenecid, alendronic acid, etidronate disodium, warfarin, intralesional corticosteroids, and sodium thiosulfate. Procedural interventions also have been reported, such as surgical excision, extracorporeal shock wave lithotripsy, and CO2 and erbium: YAG lasers.1 Surgical excision of dystrophic calcinosis cutis is widely implemented but outcomes are poor. Moreover, in patients with widely diffuse calcinosis, targeted procedural therapy is impractical.
Intravenous sodium thiosulfate has been widely used for the treatment of calciphylaxis secondary to end-stage renal failure and tumoral calcinosis.2 It also has been reported to be effective in iatrogenic calcinosis cutis secondary to extravasation of calcium-containing solutions in a patient with T-cell acute lymphoblastic leukemia.3 However, reports of its use in treating dystrophic calcinosis cutis are limited. Intravenous sodium thiosulfate—10 g 3 times weekly for 2 weeks, followed by 15 g twice weekly for the next 3 months—was used with abatacept for treatment of dystrophic calcinosis cutis in a patient with juvenile dermatomyositis.4 Other formulations of sodium thiosulfate have been reported to result in clearance of calcified lesions, including a topical application compounded in zinc oxide5 and intradermal injection at the base of a nodule.6 We used 12.5 g over 30 minutes 3 times weekly; however, the dose can be increased to 25 g over 60 minutes if 3 to 4 treatments are tolerated, with nausea being the only notable side effect. Its mechanism of action in treating dystrophic calcinosis cutis is unclear, but it likely is due to its ability to chelate and dissolve calcium deposits. Topical and intradermal therapy is impractical for widespread, dystrophic calcinosis cutis as in our patient.
Our case highlights the successful use of IV sodium thiosulfate as a stand-alone treatment modality for generalized dystrophic calcinosis cutis in an adult patient. Both our patient and a child in a previously reported case who received the same treatment4 had dermatomyositis, but we suspect IV sodium thiosulfate also may be effective for dystrophic calcinosis cutis associated with other diseases. Sodium thiosulfate should be considered as a treatment for patients who experience tremendous pain and disability. It is safe, inexpensive, and easy to administer and is especially helpful in patients for whom topical, intradermal, or procedural therapy is not possible.
- Gutierrez A Jr, Wetter DA. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol Ther. 2012;25:195-206.
- Mageau A, Guigonis V, Ratzimbasafy V, et al. Intravenous sodium thiosulfate for treating tumoral calcinosis associated with systemic disorders: report of four cases. Joint Bone Spine. 2017;84:341-344.
Raffaella C, Annapaola C, Tullio I, et al. Successful treatment of severe iatrogenic calcinosis cutis with intravenous sodium thiosulfate in a child affected by T-acute lymphoblastic leukemia. Pediatr Dermatol. 2009;26:311-315. - Arabshahi B, Silverman RA, Jones OY, et al. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J Pediatrics. 2012;160:520-522.
- Bair B, Fivenson D. A novel treatment for ulcerative calcinosis cutis. J Drugs Dermatol. 2011;10:1042-1044.
- Smith GP. Intradermal sodium thiosulfate for exophytic calcinosis cutis of connective tissue disease. J Am Acad Dermatol. 2013;69:E146-E147.
To the Editor:
Severe dystrophic calcinosis cutis is a debilitating disease with no universally accepted therapeutic options. This case demonstrates the benefit of intravenous (IV) sodium thiosulfate in alleviating the calcified lesions as well as the associated pain and disability. This application of IV sodium thiosulfate with a favorable outcome is new and should be considered for the treatment of generalized dystrophic calcinosis cutis, especially when topical, procedural, or surgical options are not feasible.
A 54-year-old woman with a history of well-controlled dermatomyositis and systemic lupus erythematosus presented with diffuse, hard, calcified lesions on the legs, arms, clavicular region, and neck that had slowly progressed over at least a 10-year period (Figure 1). The lesions were consistent with dystrophic calcinosis cutis. The patient was started on 12.5 g of IV sodium thiosulfate 3 times weekly infused over 30 minutes. Drastic diminution of the cutaneous calcification was observed at 3-month follow-up (Figure 2). She reported decreased pain and burning as well as increased overall functionality and improved sleep. The patient completed 8 months of therapy, but the treatment was stopped secondary to suspicion of a lupuslike flare, and the lesions recurred with more widespread involvement, including the trunk, tendons, bony prominences, and supraclavicular soft tissue. The patient reported burning pain and pruritus that resulted in impairment of daily activities such as getting dressed. Sodium thiosulfate was restarted once weekly, which again resulted in reduction of the dystrophic calcinosis cutis.


Dystrophic calcinosis cutis is a debilitating disease that results in considerable morbidity and pain with major implications on quality of life. The pathophysiology is unclear; calcium and phosphate serum levels generally are normal. A proposed mechanism is that chronic inflammation causes tissue damage and defective collagen synthesis, resulting in a distorted architecture that facilitates calcium deposition in the skin and subcutaneous tissues.1 Dystrophic calcinosis cutis most commonly is associated with systemic sclerosis and dermatomyositis but also can be seen in systemic lupus erythematosus, panniculitis, and other connective tissue diseases. It also can occur with skin neoplasms, collagen and elastin disorders, porphyria cutanea tarda, and pancreatic panniculitis.1 Progression of dystrophic calcinosis cutis usually is independent of the associated disease status.
Treatment is based on anecdotal evidence from case reports, as there is no universally accepted pharmacologic or procedural intervention available for dystrophic calcinosis cutis. Medications that have been reported to be helpful to varying degrees include diltiazem, colchicine, minocycline, IV immunoglobulin, ceftriaxone, aluminum hydroxide, probenecid, alendronic acid, etidronate disodium, warfarin, intralesional corticosteroids, and sodium thiosulfate. Procedural interventions also have been reported, such as surgical excision, extracorporeal shock wave lithotripsy, and CO2 and erbium: YAG lasers.1 Surgical excision of dystrophic calcinosis cutis is widely implemented but outcomes are poor. Moreover, in patients with widely diffuse calcinosis, targeted procedural therapy is impractical.
Intravenous sodium thiosulfate has been widely used for the treatment of calciphylaxis secondary to end-stage renal failure and tumoral calcinosis.2 It also has been reported to be effective in iatrogenic calcinosis cutis secondary to extravasation of calcium-containing solutions in a patient with T-cell acute lymphoblastic leukemia.3 However, reports of its use in treating dystrophic calcinosis cutis are limited. Intravenous sodium thiosulfate—10 g 3 times weekly for 2 weeks, followed by 15 g twice weekly for the next 3 months—was used with abatacept for treatment of dystrophic calcinosis cutis in a patient with juvenile dermatomyositis.4 Other formulations of sodium thiosulfate have been reported to result in clearance of calcified lesions, including a topical application compounded in zinc oxide5 and intradermal injection at the base of a nodule.6 We used 12.5 g over 30 minutes 3 times weekly; however, the dose can be increased to 25 g over 60 minutes if 3 to 4 treatments are tolerated, with nausea being the only notable side effect. Its mechanism of action in treating dystrophic calcinosis cutis is unclear, but it likely is due to its ability to chelate and dissolve calcium deposits. Topical and intradermal therapy is impractical for widespread, dystrophic calcinosis cutis as in our patient.
Our case highlights the successful use of IV sodium thiosulfate as a stand-alone treatment modality for generalized dystrophic calcinosis cutis in an adult patient. Both our patient and a child in a previously reported case who received the same treatment4 had dermatomyositis, but we suspect IV sodium thiosulfate also may be effective for dystrophic calcinosis cutis associated with other diseases. Sodium thiosulfate should be considered as a treatment for patients who experience tremendous pain and disability. It is safe, inexpensive, and easy to administer and is especially helpful in patients for whom topical, intradermal, or procedural therapy is not possible.
To the Editor:
Severe dystrophic calcinosis cutis is a debilitating disease with no universally accepted therapeutic options. This case demonstrates the benefit of intravenous (IV) sodium thiosulfate in alleviating the calcified lesions as well as the associated pain and disability. This application of IV sodium thiosulfate with a favorable outcome is new and should be considered for the treatment of generalized dystrophic calcinosis cutis, especially when topical, procedural, or surgical options are not feasible.
A 54-year-old woman with a history of well-controlled dermatomyositis and systemic lupus erythematosus presented with diffuse, hard, calcified lesions on the legs, arms, clavicular region, and neck that had slowly progressed over at least a 10-year period (Figure 1). The lesions were consistent with dystrophic calcinosis cutis. The patient was started on 12.5 g of IV sodium thiosulfate 3 times weekly infused over 30 minutes. Drastic diminution of the cutaneous calcification was observed at 3-month follow-up (Figure 2). She reported decreased pain and burning as well as increased overall functionality and improved sleep. The patient completed 8 months of therapy, but the treatment was stopped secondary to suspicion of a lupuslike flare, and the lesions recurred with more widespread involvement, including the trunk, tendons, bony prominences, and supraclavicular soft tissue. The patient reported burning pain and pruritus that resulted in impairment of daily activities such as getting dressed. Sodium thiosulfate was restarted once weekly, which again resulted in reduction of the dystrophic calcinosis cutis.


Dystrophic calcinosis cutis is a debilitating disease that results in considerable morbidity and pain with major implications on quality of life. The pathophysiology is unclear; calcium and phosphate serum levels generally are normal. A proposed mechanism is that chronic inflammation causes tissue damage and defective collagen synthesis, resulting in a distorted architecture that facilitates calcium deposition in the skin and subcutaneous tissues.1 Dystrophic calcinosis cutis most commonly is associated with systemic sclerosis and dermatomyositis but also can be seen in systemic lupus erythematosus, panniculitis, and other connective tissue diseases. It also can occur with skin neoplasms, collagen and elastin disorders, porphyria cutanea tarda, and pancreatic panniculitis.1 Progression of dystrophic calcinosis cutis usually is independent of the associated disease status.
Treatment is based on anecdotal evidence from case reports, as there is no universally accepted pharmacologic or procedural intervention available for dystrophic calcinosis cutis. Medications that have been reported to be helpful to varying degrees include diltiazem, colchicine, minocycline, IV immunoglobulin, ceftriaxone, aluminum hydroxide, probenecid, alendronic acid, etidronate disodium, warfarin, intralesional corticosteroids, and sodium thiosulfate. Procedural interventions also have been reported, such as surgical excision, extracorporeal shock wave lithotripsy, and CO2 and erbium: YAG lasers.1 Surgical excision of dystrophic calcinosis cutis is widely implemented but outcomes are poor. Moreover, in patients with widely diffuse calcinosis, targeted procedural therapy is impractical.
Intravenous sodium thiosulfate has been widely used for the treatment of calciphylaxis secondary to end-stage renal failure and tumoral calcinosis.2 It also has been reported to be effective in iatrogenic calcinosis cutis secondary to extravasation of calcium-containing solutions in a patient with T-cell acute lymphoblastic leukemia.3 However, reports of its use in treating dystrophic calcinosis cutis are limited. Intravenous sodium thiosulfate—10 g 3 times weekly for 2 weeks, followed by 15 g twice weekly for the next 3 months—was used with abatacept for treatment of dystrophic calcinosis cutis in a patient with juvenile dermatomyositis.4 Other formulations of sodium thiosulfate have been reported to result in clearance of calcified lesions, including a topical application compounded in zinc oxide5 and intradermal injection at the base of a nodule.6 We used 12.5 g over 30 minutes 3 times weekly; however, the dose can be increased to 25 g over 60 minutes if 3 to 4 treatments are tolerated, with nausea being the only notable side effect. Its mechanism of action in treating dystrophic calcinosis cutis is unclear, but it likely is due to its ability to chelate and dissolve calcium deposits. Topical and intradermal therapy is impractical for widespread, dystrophic calcinosis cutis as in our patient.
Our case highlights the successful use of IV sodium thiosulfate as a stand-alone treatment modality for generalized dystrophic calcinosis cutis in an adult patient. Both our patient and a child in a previously reported case who received the same treatment4 had dermatomyositis, but we suspect IV sodium thiosulfate also may be effective for dystrophic calcinosis cutis associated with other diseases. Sodium thiosulfate should be considered as a treatment for patients who experience tremendous pain and disability. It is safe, inexpensive, and easy to administer and is especially helpful in patients for whom topical, intradermal, or procedural therapy is not possible.
- Gutierrez A Jr, Wetter DA. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol Ther. 2012;25:195-206.
- Mageau A, Guigonis V, Ratzimbasafy V, et al. Intravenous sodium thiosulfate for treating tumoral calcinosis associated with systemic disorders: report of four cases. Joint Bone Spine. 2017;84:341-344.
Raffaella C, Annapaola C, Tullio I, et al. Successful treatment of severe iatrogenic calcinosis cutis with intravenous sodium thiosulfate in a child affected by T-acute lymphoblastic leukemia. Pediatr Dermatol. 2009;26:311-315. - Arabshahi B, Silverman RA, Jones OY, et al. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J Pediatrics. 2012;160:520-522.
- Bair B, Fivenson D. A novel treatment for ulcerative calcinosis cutis. J Drugs Dermatol. 2011;10:1042-1044.
- Smith GP. Intradermal sodium thiosulfate for exophytic calcinosis cutis of connective tissue disease. J Am Acad Dermatol. 2013;69:E146-E147.
- Gutierrez A Jr, Wetter DA. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol Ther. 2012;25:195-206.
- Mageau A, Guigonis V, Ratzimbasafy V, et al. Intravenous sodium thiosulfate for treating tumoral calcinosis associated with systemic disorders: report of four cases. Joint Bone Spine. 2017;84:341-344.
Raffaella C, Annapaola C, Tullio I, et al. Successful treatment of severe iatrogenic calcinosis cutis with intravenous sodium thiosulfate in a child affected by T-acute lymphoblastic leukemia. Pediatr Dermatol. 2009;26:311-315. - Arabshahi B, Silverman RA, Jones OY, et al. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J Pediatrics. 2012;160:520-522.
- Bair B, Fivenson D. A novel treatment for ulcerative calcinosis cutis. J Drugs Dermatol. 2011;10:1042-1044.
- Smith GP. Intradermal sodium thiosulfate for exophytic calcinosis cutis of connective tissue disease. J Am Acad Dermatol. 2013;69:E146-E147.
Practice Points
- Dystrophic calcinosis cutis is a potentially debilitating condition with limited effective therapies.
- Consider intravenous sodium thiosulfate in patients with diffuse and severe dystrophic calcinosis cutis.
Novel calculator predicts cancer risk in patients with CVD
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
FROM ESC CONGRESS 2020
Physician income drops, burnout spikes globally in pandemic
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
The march of immunotherapy continues at ESMO 2020
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FROM ESMO 2020
Lusutrombopag found safe, effective for severe thrombocytopenia in patients with hepatocellular carcinoma
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Reworked OxyContin fails to cut overall opioid abuse, FDA panel says
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.