User login
Lessons for patients with MS and COVID-19
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
FROM MSVirtual2020
Increasing hepatitis C treatment may curb hepatocellular carcinoma
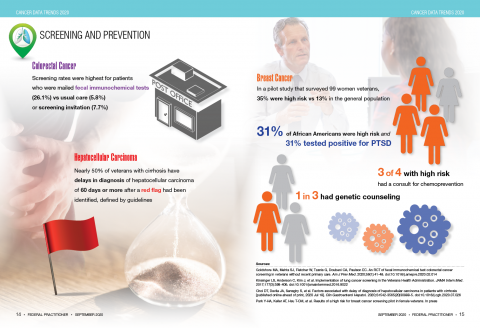
Widespread treatment of hepatitis C virus significantly reduced the risk of hepatocellular carcinoma, based on 18 years of data from patients in Veterans Health Administration hospitals.
Although eradication of hepatitis C virus (HCV) infections has been shown to reduce the risk of hepatocellular carcinoma (HCC), effective direct-acting antiviral therapies available since 2013 appear underused in the United States, with a 14% cure rate for HCV patients as of 2016, wrote Lauren A. Beste, MD, of Veterans Affairs Puget Sound Health Care System, Seattle, and colleagues.
However, “the Veterans Health Administration, the largest integrated health care system in the U.S., provides unrestricted access to HCV treatments and approximately 85% of its case load has achieved cure,” the researchers said.
In a letter published in JAMA, the researchers identified all patients in the VHA diagnosed with HCC based on electronic health records for each year between 2002 and 2018. HCV infection was based on any history of detectable viral load, and HCV cure was defined as a negative viral load at least 12 weeks following completion of antiviral treatment, the researchers said.
“We categorized patients into 3 groups as of the time of HCC diagnosis: HCC/HCV viremic (latest HCV RNA before HCC diagnosis was positive), HCC/HCV cured (HCV eradicated before HCC diagnosis), and HCC/non-HCV (no positive lifetime HCV RNA),” they explained.
The sum of HCC/HCV viremic plus HCC/HCV cured made up the HCC/HCV total. Overall, the incidence of HCC/HCV total increased from 2000 to 2015, peaked at 31.0 per 100,000 patients in 2015, and decreased to 21.8 per 100,000 in 2018 after the introduction of viral eradication efforts from 2014 to 2016.
HCV treatment shows value despite lack of causality
Although the study could not prove causality, “the timing of HCV eradication and declining HCC incidence, lack of decline in non–HCV-related HCC, and prior studies demonstrating that HCV eradication reduces HCC risk, provide indirect evidence that this decline may be related to widespread HCV treatment,” the researchers said.
The findings were limited by several factors including the observational study design, use of the International Classification of Diseases to define HCC, use of a VA population that might limit generalizability, and lack of data on the severity of disease prior to treatment, the researchers noted. However, “HCC incidence trends should continue to be monitored closely because patients cured of HCV may have yet to experience the full potential of risk reduction,” and the study results support large-scale campaigns to eliminate HCV as well as monitoring for HCC and HCV patients who achieve disease eradication, they concluded.
The study was supported in part by grants from the National Institutes of Health/National Cancer Institute and the Veterans Affairs Clinical Science Research and Development. The researchers had no financial conflicts to disclose.
SOURCE: Beste LA et al. JAMA. 2020 Sep 8;324(10):1003-5.
Widespread treatment of hepatitis C virus significantly reduced the risk of hepatocellular carcinoma, based on 18 years of data from patients in Veterans Health Administration hospitals.
Although eradication of hepatitis C virus (HCV) infections has been shown to reduce the risk of hepatocellular carcinoma (HCC), effective direct-acting antiviral therapies available since 2013 appear underused in the United States, with a 14% cure rate for HCV patients as of 2016, wrote Lauren A. Beste, MD, of Veterans Affairs Puget Sound Health Care System, Seattle, and colleagues.
However, “the Veterans Health Administration, the largest integrated health care system in the U.S., provides unrestricted access to HCV treatments and approximately 85% of its case load has achieved cure,” the researchers said.
In a letter published in JAMA, the researchers identified all patients in the VHA diagnosed with HCC based on electronic health records for each year between 2002 and 2018. HCV infection was based on any history of detectable viral load, and HCV cure was defined as a negative viral load at least 12 weeks following completion of antiviral treatment, the researchers said.
“We categorized patients into 3 groups as of the time of HCC diagnosis: HCC/HCV viremic (latest HCV RNA before HCC diagnosis was positive), HCC/HCV cured (HCV eradicated before HCC diagnosis), and HCC/non-HCV (no positive lifetime HCV RNA),” they explained.
The sum of HCC/HCV viremic plus HCC/HCV cured made up the HCC/HCV total. Overall, the incidence of HCC/HCV total increased from 2000 to 2015, peaked at 31.0 per 100,000 patients in 2015, and decreased to 21.8 per 100,000 in 2018 after the introduction of viral eradication efforts from 2014 to 2016.
HCV treatment shows value despite lack of causality
Although the study could not prove causality, “the timing of HCV eradication and declining HCC incidence, lack of decline in non–HCV-related HCC, and prior studies demonstrating that HCV eradication reduces HCC risk, provide indirect evidence that this decline may be related to widespread HCV treatment,” the researchers said.
The findings were limited by several factors including the observational study design, use of the International Classification of Diseases to define HCC, use of a VA population that might limit generalizability, and lack of data on the severity of disease prior to treatment, the researchers noted. However, “HCC incidence trends should continue to be monitored closely because patients cured of HCV may have yet to experience the full potential of risk reduction,” and the study results support large-scale campaigns to eliminate HCV as well as monitoring for HCC and HCV patients who achieve disease eradication, they concluded.
The study was supported in part by grants from the National Institutes of Health/National Cancer Institute and the Veterans Affairs Clinical Science Research and Development. The researchers had no financial conflicts to disclose.
SOURCE: Beste LA et al. JAMA. 2020 Sep 8;324(10):1003-5.
Widespread treatment of hepatitis C virus significantly reduced the risk of hepatocellular carcinoma, based on 18 years of data from patients in Veterans Health Administration hospitals.
Although eradication of hepatitis C virus (HCV) infections has been shown to reduce the risk of hepatocellular carcinoma (HCC), effective direct-acting antiviral therapies available since 2013 appear underused in the United States, with a 14% cure rate for HCV patients as of 2016, wrote Lauren A. Beste, MD, of Veterans Affairs Puget Sound Health Care System, Seattle, and colleagues.
However, “the Veterans Health Administration, the largest integrated health care system in the U.S., provides unrestricted access to HCV treatments and approximately 85% of its case load has achieved cure,” the researchers said.
In a letter published in JAMA, the researchers identified all patients in the VHA diagnosed with HCC based on electronic health records for each year between 2002 and 2018. HCV infection was based on any history of detectable viral load, and HCV cure was defined as a negative viral load at least 12 weeks following completion of antiviral treatment, the researchers said.
“We categorized patients into 3 groups as of the time of HCC diagnosis: HCC/HCV viremic (latest HCV RNA before HCC diagnosis was positive), HCC/HCV cured (HCV eradicated before HCC diagnosis), and HCC/non-HCV (no positive lifetime HCV RNA),” they explained.
The sum of HCC/HCV viremic plus HCC/HCV cured made up the HCC/HCV total. Overall, the incidence of HCC/HCV total increased from 2000 to 2015, peaked at 31.0 per 100,000 patients in 2015, and decreased to 21.8 per 100,000 in 2018 after the introduction of viral eradication efforts from 2014 to 2016.
HCV treatment shows value despite lack of causality
Although the study could not prove causality, “the timing of HCV eradication and declining HCC incidence, lack of decline in non–HCV-related HCC, and prior studies demonstrating that HCV eradication reduces HCC risk, provide indirect evidence that this decline may be related to widespread HCV treatment,” the researchers said.
The findings were limited by several factors including the observational study design, use of the International Classification of Diseases to define HCC, use of a VA population that might limit generalizability, and lack of data on the severity of disease prior to treatment, the researchers noted. However, “HCC incidence trends should continue to be monitored closely because patients cured of HCV may have yet to experience the full potential of risk reduction,” and the study results support large-scale campaigns to eliminate HCV as well as monitoring for HCC and HCV patients who achieve disease eradication, they concluded.
The study was supported in part by grants from the National Institutes of Health/National Cancer Institute and the Veterans Affairs Clinical Science Research and Development. The researchers had no financial conflicts to disclose.
SOURCE: Beste LA et al. JAMA. 2020 Sep 8;324(10):1003-5.
FROM JAMA
2020 Cancer Data Trends
Exposure to DMT may delay disability accumulation in primary progressive MS
Reducing the delay to treatment initiation, as well as treating younger patients, might improve long-term disability outcomes, according to a new study.
“To optimize treatment decision-making in primary progressive MS, further profiling of the best candidates for treatment is needed,” said the researchers. The study was presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Ocrelizumab remains the only treatment available for patients with primary progressive MS. In clinical trials, other drugs have failed to reduce disability progression in this population. Mattia Fonderico, a doctoral student and research assistant at the University of Florence (Italy), and colleagues reviewed data from the Italian MS Registry to examine whether DMT affects the attainment of given disability outcomes.
Patients with longer exposure were younger at baseline
Patients eligible for inclusion in the study had primary progressive MS, at least three evaluations using the Expanded Disability Status Scale (EDSS), and 3 years’ follow-up. The investigators defined the baseline for untreated patients as the first EDSS evaluation. For treated patients, the baseline was the date of DMT initiation.
Using multivariable Cox regression models, Ms. Fonderico and colleagues examined the effect of DMT on the risk of reaching EDSS scores of 6 (i.e., requirement for intermittent or unilateral constant walking assistance) and 7 (i.e., restriction to a wheelchair) as a dichotomous variable and as a time-dependent covariate. The researchers adjusted the data for age at baseline, sex, first EDSS score, symptoms at onset, annualized visit rate, and annualized relapse rate. They compared outcomes with an as-treated analysis and chose cohorts with similar baseline characteristics using propensity-score matching. In addition, Ms. Fonderico and colleagues also analyzed quartiles of DMT exposure.
The investigators included 1,214 patients (671 women) in their analysis. The population’s mean age at baseline was 48.7 years, and its mean EDSS score was 4.1. A total of 626 patients (52%) received DMT during follow-up. Approximately 57% of DMTs were platform therapies, and 43% were high-efficacy therapies.
Mean follow-up duration was 11.6 years. By the end of follow-up, 994 patients (82%) reached an EDSS score of 6, and 539 (44%) reached an EDSS score of 7. Multivariable Cox regression models indicated that DMT, analyzed as a dichotomous variable, did not affect the risk of reaching EDSS 6 (adjusted hazard ratio, 1.1) or EDSS 7 (aHR, 0.93). Longer DMT exposure, however, significantly reduced the risk of reaching EDSS 7 (aHR, 0.73).
Compared with patients with shorter exposure to DMT, patients in the highest quartile of DMT exposure were younger at baseline (mean age, 44.1 years) and initiated DMT closer to disease onset (mean time to DMT initiation was 6.8 years). The propensity score matching analysis confirmed these findings.
The investigators did not consider MRI variables, which Ms. Fonderico acknowledged was a weakness of the study. In addition, they did not analyze the effect of superimposed relapses.
A new perspective on primary progressive MS?
These results suggest that primary progressive MS behaves like relapsing-remitting MS, said Gavin Giovannoni, MD, PhD, chair of neurology at Queen Mary University of London. That is, they suggest that primary progressive MS “is modifiable by a DMT and that the earlier you treat, the better the outcome.” The results also indicate that neurologists commonly prescribe DMT off label in Italy, he added.
A weakness of the study is that it was not randomized. Furthermore, “EDSS [evaluations] tend not be done properly in routine clinical practice,” said Dr. Giovannoni. Still, the study raises an important question for future research. “Why have we missed the treatment effect in previous trials?” asked Dr. Giovannoni. Whether previous trials were too short or underpowered could be investigated, he added.
Study funding was not reported. Ms. Fonderico had no relevant disclosures. Dr. Giovannoni had no relevant disclosures.
Reducing the delay to treatment initiation, as well as treating younger patients, might improve long-term disability outcomes, according to a new study.
“To optimize treatment decision-making in primary progressive MS, further profiling of the best candidates for treatment is needed,” said the researchers. The study was presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Ocrelizumab remains the only treatment available for patients with primary progressive MS. In clinical trials, other drugs have failed to reduce disability progression in this population. Mattia Fonderico, a doctoral student and research assistant at the University of Florence (Italy), and colleagues reviewed data from the Italian MS Registry to examine whether DMT affects the attainment of given disability outcomes.
Patients with longer exposure were younger at baseline
Patients eligible for inclusion in the study had primary progressive MS, at least three evaluations using the Expanded Disability Status Scale (EDSS), and 3 years’ follow-up. The investigators defined the baseline for untreated patients as the first EDSS evaluation. For treated patients, the baseline was the date of DMT initiation.
Using multivariable Cox regression models, Ms. Fonderico and colleagues examined the effect of DMT on the risk of reaching EDSS scores of 6 (i.e., requirement for intermittent or unilateral constant walking assistance) and 7 (i.e., restriction to a wheelchair) as a dichotomous variable and as a time-dependent covariate. The researchers adjusted the data for age at baseline, sex, first EDSS score, symptoms at onset, annualized visit rate, and annualized relapse rate. They compared outcomes with an as-treated analysis and chose cohorts with similar baseline characteristics using propensity-score matching. In addition, Ms. Fonderico and colleagues also analyzed quartiles of DMT exposure.
The investigators included 1,214 patients (671 women) in their analysis. The population’s mean age at baseline was 48.7 years, and its mean EDSS score was 4.1. A total of 626 patients (52%) received DMT during follow-up. Approximately 57% of DMTs were platform therapies, and 43% were high-efficacy therapies.
Mean follow-up duration was 11.6 years. By the end of follow-up, 994 patients (82%) reached an EDSS score of 6, and 539 (44%) reached an EDSS score of 7. Multivariable Cox regression models indicated that DMT, analyzed as a dichotomous variable, did not affect the risk of reaching EDSS 6 (adjusted hazard ratio, 1.1) or EDSS 7 (aHR, 0.93). Longer DMT exposure, however, significantly reduced the risk of reaching EDSS 7 (aHR, 0.73).
Compared with patients with shorter exposure to DMT, patients in the highest quartile of DMT exposure were younger at baseline (mean age, 44.1 years) and initiated DMT closer to disease onset (mean time to DMT initiation was 6.8 years). The propensity score matching analysis confirmed these findings.
The investigators did not consider MRI variables, which Ms. Fonderico acknowledged was a weakness of the study. In addition, they did not analyze the effect of superimposed relapses.
A new perspective on primary progressive MS?
These results suggest that primary progressive MS behaves like relapsing-remitting MS, said Gavin Giovannoni, MD, PhD, chair of neurology at Queen Mary University of London. That is, they suggest that primary progressive MS “is modifiable by a DMT and that the earlier you treat, the better the outcome.” The results also indicate that neurologists commonly prescribe DMT off label in Italy, he added.
A weakness of the study is that it was not randomized. Furthermore, “EDSS [evaluations] tend not be done properly in routine clinical practice,” said Dr. Giovannoni. Still, the study raises an important question for future research. “Why have we missed the treatment effect in previous trials?” asked Dr. Giovannoni. Whether previous trials were too short or underpowered could be investigated, he added.
Study funding was not reported. Ms. Fonderico had no relevant disclosures. Dr. Giovannoni had no relevant disclosures.
Reducing the delay to treatment initiation, as well as treating younger patients, might improve long-term disability outcomes, according to a new study.
“To optimize treatment decision-making in primary progressive MS, further profiling of the best candidates for treatment is needed,” said the researchers. The study was presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Ocrelizumab remains the only treatment available for patients with primary progressive MS. In clinical trials, other drugs have failed to reduce disability progression in this population. Mattia Fonderico, a doctoral student and research assistant at the University of Florence (Italy), and colleagues reviewed data from the Italian MS Registry to examine whether DMT affects the attainment of given disability outcomes.
Patients with longer exposure were younger at baseline
Patients eligible for inclusion in the study had primary progressive MS, at least three evaluations using the Expanded Disability Status Scale (EDSS), and 3 years’ follow-up. The investigators defined the baseline for untreated patients as the first EDSS evaluation. For treated patients, the baseline was the date of DMT initiation.
Using multivariable Cox regression models, Ms. Fonderico and colleagues examined the effect of DMT on the risk of reaching EDSS scores of 6 (i.e., requirement for intermittent or unilateral constant walking assistance) and 7 (i.e., restriction to a wheelchair) as a dichotomous variable and as a time-dependent covariate. The researchers adjusted the data for age at baseline, sex, first EDSS score, symptoms at onset, annualized visit rate, and annualized relapse rate. They compared outcomes with an as-treated analysis and chose cohorts with similar baseline characteristics using propensity-score matching. In addition, Ms. Fonderico and colleagues also analyzed quartiles of DMT exposure.
The investigators included 1,214 patients (671 women) in their analysis. The population’s mean age at baseline was 48.7 years, and its mean EDSS score was 4.1. A total of 626 patients (52%) received DMT during follow-up. Approximately 57% of DMTs were platform therapies, and 43% were high-efficacy therapies.
Mean follow-up duration was 11.6 years. By the end of follow-up, 994 patients (82%) reached an EDSS score of 6, and 539 (44%) reached an EDSS score of 7. Multivariable Cox regression models indicated that DMT, analyzed as a dichotomous variable, did not affect the risk of reaching EDSS 6 (adjusted hazard ratio, 1.1) or EDSS 7 (aHR, 0.93). Longer DMT exposure, however, significantly reduced the risk of reaching EDSS 7 (aHR, 0.73).
Compared with patients with shorter exposure to DMT, patients in the highest quartile of DMT exposure were younger at baseline (mean age, 44.1 years) and initiated DMT closer to disease onset (mean time to DMT initiation was 6.8 years). The propensity score matching analysis confirmed these findings.
The investigators did not consider MRI variables, which Ms. Fonderico acknowledged was a weakness of the study. In addition, they did not analyze the effect of superimposed relapses.
A new perspective on primary progressive MS?
These results suggest that primary progressive MS behaves like relapsing-remitting MS, said Gavin Giovannoni, MD, PhD, chair of neurology at Queen Mary University of London. That is, they suggest that primary progressive MS “is modifiable by a DMT and that the earlier you treat, the better the outcome.” The results also indicate that neurologists commonly prescribe DMT off label in Italy, he added.
A weakness of the study is that it was not randomized. Furthermore, “EDSS [evaluations] tend not be done properly in routine clinical practice,” said Dr. Giovannoni. Still, the study raises an important question for future research. “Why have we missed the treatment effect in previous trials?” asked Dr. Giovannoni. Whether previous trials were too short or underpowered could be investigated, he added.
Study funding was not reported. Ms. Fonderico had no relevant disclosures. Dr. Giovannoni had no relevant disclosures.
FROM MSVirtual 2020
Disparities seen in COVID-19–related avoidance of care
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?
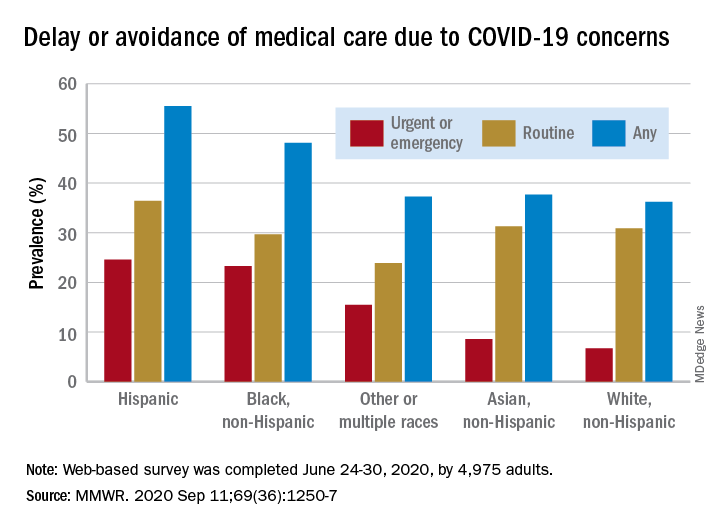
Turns out almost 41% of Americans delayed or avoided some form of medical care because of concerns about COVID-19, according to the results of a survey conducted June 24-30 by commercial survey company Qualtrics.
More specifically, the avoidance looks like this: 31.5% of the 4,975 adult respondents had avoided routine care and 12.0% had avoided urgent or emergency care, Mark E. Czeisler and associates said in the Morbidity and Mortality Weekly Report. The two categories were not mutually exclusive since respondents could select both routine care and urgent/emergency care.
There were, however, a number of significant disparities hidden among those numbers for the overall population. Blacks and Hispanics, with respective prevalences of 23.3% and 24.6%, were significantly more likely to delay or avoid urgent/emergency care than were Whites (6.7%), said Mr. Czeisler, a graduate student at Monash University, Melbourne, and associates.
Those differences “are especially concerning given increased COVID-19–associated mortality among Black adults and Hispanic adults,” they noted, adding that “age-adjusted COVID-19 hospitalization rates are approximately five times higher among Black persons and four times higher among Hispanic persons than” among Whites.
Other significant disparities in urgent/emergency care avoidance included the following:
- Unpaid caregivers for adults (29.8%) vs. noncaregivers (5.4%).
- Adults with two or more underlying conditions (22.7%) vs. those without such conditions (8.2%).
- Those with a disability (22.8%) vs. those without (8.9%).
- Those with health insurance (12.4%) vs. those without (7.8%).
The highest prevalence for all types of COVID-19–related delay and avoidance came from the adult caregivers (64.3%), followed by those with a disability (60.3%) and adults aged 18-24 years (57.2%). The lowest prevalence numbers were for adults with health insurance (24.8%) and those who were not caregivers for adults (32.2%), Mr. Czeisler and associates reported.
These reports of delayed and avoided care “might reflect adherence to community mitigation efforts such as stay-at-home orders, temporary closures of health facilities, or additional factors. However, if routine care avoidance were to be sustained, adults could miss opportunities for management of chronic conditions, receipt of routine vaccinations, or early detection of new conditions, which might worsen outcomes,” they wrote.
SOURCE: Czeisler ME et al. MMWR. 2020 Sep 11;69(36):1250-7.
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?

Turns out almost 41% of Americans delayed or avoided some form of medical care because of concerns about COVID-19, according to the results of a survey conducted June 24-30 by commercial survey company Qualtrics.
More specifically, the avoidance looks like this: 31.5% of the 4,975 adult respondents had avoided routine care and 12.0% had avoided urgent or emergency care, Mark E. Czeisler and associates said in the Morbidity and Mortality Weekly Report. The two categories were not mutually exclusive since respondents could select both routine care and urgent/emergency care.
There were, however, a number of significant disparities hidden among those numbers for the overall population. Blacks and Hispanics, with respective prevalences of 23.3% and 24.6%, were significantly more likely to delay or avoid urgent/emergency care than were Whites (6.7%), said Mr. Czeisler, a graduate student at Monash University, Melbourne, and associates.
Those differences “are especially concerning given increased COVID-19–associated mortality among Black adults and Hispanic adults,” they noted, adding that “age-adjusted COVID-19 hospitalization rates are approximately five times higher among Black persons and four times higher among Hispanic persons than” among Whites.
Other significant disparities in urgent/emergency care avoidance included the following:
- Unpaid caregivers for adults (29.8%) vs. noncaregivers (5.4%).
- Adults with two or more underlying conditions (22.7%) vs. those without such conditions (8.2%).
- Those with a disability (22.8%) vs. those without (8.9%).
- Those with health insurance (12.4%) vs. those without (7.8%).
The highest prevalence for all types of COVID-19–related delay and avoidance came from the adult caregivers (64.3%), followed by those with a disability (60.3%) and adults aged 18-24 years (57.2%). The lowest prevalence numbers were for adults with health insurance (24.8%) and those who were not caregivers for adults (32.2%), Mr. Czeisler and associates reported.
These reports of delayed and avoided care “might reflect adherence to community mitigation efforts such as stay-at-home orders, temporary closures of health facilities, or additional factors. However, if routine care avoidance were to be sustained, adults could miss opportunities for management of chronic conditions, receipt of routine vaccinations, or early detection of new conditions, which might worsen outcomes,” they wrote.
SOURCE: Czeisler ME et al. MMWR. 2020 Sep 11;69(36):1250-7.
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?

Turns out almost 41% of Americans delayed or avoided some form of medical care because of concerns about COVID-19, according to the results of a survey conducted June 24-30 by commercial survey company Qualtrics.
More specifically, the avoidance looks like this: 31.5% of the 4,975 adult respondents had avoided routine care and 12.0% had avoided urgent or emergency care, Mark E. Czeisler and associates said in the Morbidity and Mortality Weekly Report. The two categories were not mutually exclusive since respondents could select both routine care and urgent/emergency care.
There were, however, a number of significant disparities hidden among those numbers for the overall population. Blacks and Hispanics, with respective prevalences of 23.3% and 24.6%, were significantly more likely to delay or avoid urgent/emergency care than were Whites (6.7%), said Mr. Czeisler, a graduate student at Monash University, Melbourne, and associates.
Those differences “are especially concerning given increased COVID-19–associated mortality among Black adults and Hispanic adults,” they noted, adding that “age-adjusted COVID-19 hospitalization rates are approximately five times higher among Black persons and four times higher among Hispanic persons than” among Whites.
Other significant disparities in urgent/emergency care avoidance included the following:
- Unpaid caregivers for adults (29.8%) vs. noncaregivers (5.4%).
- Adults with two or more underlying conditions (22.7%) vs. those without such conditions (8.2%).
- Those with a disability (22.8%) vs. those without (8.9%).
- Those with health insurance (12.4%) vs. those without (7.8%).
The highest prevalence for all types of COVID-19–related delay and avoidance came from the adult caregivers (64.3%), followed by those with a disability (60.3%) and adults aged 18-24 years (57.2%). The lowest prevalence numbers were for adults with health insurance (24.8%) and those who were not caregivers for adults (32.2%), Mr. Czeisler and associates reported.
These reports of delayed and avoided care “might reflect adherence to community mitigation efforts such as stay-at-home orders, temporary closures of health facilities, or additional factors. However, if routine care avoidance were to be sustained, adults could miss opportunities for management of chronic conditions, receipt of routine vaccinations, or early detection of new conditions, which might worsen outcomes,” they wrote.
SOURCE: Czeisler ME et al. MMWR. 2020 Sep 11;69(36):1250-7.
New billing code for added COVID practice expense
The American Medical Association on Sept. 8 announced that a new code, 99072, is intended to cover additional supplies, materials, and clinical staff time over and above those usually included in an office visit when performed during a declared public health emergency, as defined by law, attributable to respiratory-transmitted infectious disease, the AMA said in a release.
Fifty national medical specialty societies and other organizations worked with the AMA’s Specialty Society RVS Update Committee over the summer to collect data on the costs of maintaining safe medical offices during the public health emergency. It has submitted recommendations to the Centers for Medicare & Medicaid Services seeking to persuade the federal agencies to recognize the new 99072 payment code.
The intention is to recognize the extra expenses involved in steps now routinely taken to reduce the risk for COVID transmission from office visits, Current Procedural Terminology Editorial Panel Chair Mark S. Synovec, MD, said in an interview. Some practices have adapted by having staff screen patients before they enter offices and making arrangements to keep patients at a safe distance from others during their visits, he said.
“Everyone’s life has significantly changed because of COVID and the health care system has dramatically changed,” Dr. Synovec said. “It was pretty clear that the status quo was not going to work.”
Physician practices will welcome this change, said Veronica Bradley, CPC, a senior industry adviser to the Medical Group Management Association. An office visit that in the past may have involved only basic infection control measures, such as donning a pair of gloves, now may involve clinicians taking the time to put on more extensive protective gear, she said.
“Now they are taking a heck of a lot more precautions, and there’s more time and more supplies being consumed,” Ms. Bradley said in an interview.
Code looks ahead to future use
The AMA explained how this new code differs from CPT code 99070, which is typically reported for supplies and materials that may be used or provided to patients during an office visit.
The new 99072 code applies only during declared public health emergencies and applies only to additional items required to support “a safe in-person provision” of evaluation, treatment, and procedures, the AMA said.
“These items contrast with those typically reported with code 99070, which focuses on additional supplies provided over and above those usually included with a specific service, such as drugs, intravenous catheters, or trays,” the AMA said.
The CPT panel sought to structure the new code for covering COVID practice expenses so that it could not be abused, and also looked ahead to the future, Dr. Synovec said.
“It’s a code that you would put on during a public health emergency as defined by law that would be related to a respiratory-transmitted infectious disease. Obviously we meant it for SARS-CoV-2,” he said. “Hopefully we can go another 100 years before we have another pandemic, but we also wanted to prepare something where if we have another airborne respiratory virus that requires additional practice expenses as seen this time, it would be available for use.”
The AMA also announced a second addition, CPT code 86413, that anticipates greater use of quantitative measurements of SARS-CoV-2 antibodies, as opposed to a qualitative assessment (positive/negative) provided by laboratory tests reported by other CPT codes.
More information is available on the AMA website.
A version of this article originally appeared on Medscape.com.
The American Medical Association on Sept. 8 announced that a new code, 99072, is intended to cover additional supplies, materials, and clinical staff time over and above those usually included in an office visit when performed during a declared public health emergency, as defined by law, attributable to respiratory-transmitted infectious disease, the AMA said in a release.
Fifty national medical specialty societies and other organizations worked with the AMA’s Specialty Society RVS Update Committee over the summer to collect data on the costs of maintaining safe medical offices during the public health emergency. It has submitted recommendations to the Centers for Medicare & Medicaid Services seeking to persuade the federal agencies to recognize the new 99072 payment code.
The intention is to recognize the extra expenses involved in steps now routinely taken to reduce the risk for COVID transmission from office visits, Current Procedural Terminology Editorial Panel Chair Mark S. Synovec, MD, said in an interview. Some practices have adapted by having staff screen patients before they enter offices and making arrangements to keep patients at a safe distance from others during their visits, he said.
“Everyone’s life has significantly changed because of COVID and the health care system has dramatically changed,” Dr. Synovec said. “It was pretty clear that the status quo was not going to work.”
Physician practices will welcome this change, said Veronica Bradley, CPC, a senior industry adviser to the Medical Group Management Association. An office visit that in the past may have involved only basic infection control measures, such as donning a pair of gloves, now may involve clinicians taking the time to put on more extensive protective gear, she said.
“Now they are taking a heck of a lot more precautions, and there’s more time and more supplies being consumed,” Ms. Bradley said in an interview.
Code looks ahead to future use
The AMA explained how this new code differs from CPT code 99070, which is typically reported for supplies and materials that may be used or provided to patients during an office visit.
The new 99072 code applies only during declared public health emergencies and applies only to additional items required to support “a safe in-person provision” of evaluation, treatment, and procedures, the AMA said.
“These items contrast with those typically reported with code 99070, which focuses on additional supplies provided over and above those usually included with a specific service, such as drugs, intravenous catheters, or trays,” the AMA said.
The CPT panel sought to structure the new code for covering COVID practice expenses so that it could not be abused, and also looked ahead to the future, Dr. Synovec said.
“It’s a code that you would put on during a public health emergency as defined by law that would be related to a respiratory-transmitted infectious disease. Obviously we meant it for SARS-CoV-2,” he said. “Hopefully we can go another 100 years before we have another pandemic, but we also wanted to prepare something where if we have another airborne respiratory virus that requires additional practice expenses as seen this time, it would be available for use.”
The AMA also announced a second addition, CPT code 86413, that anticipates greater use of quantitative measurements of SARS-CoV-2 antibodies, as opposed to a qualitative assessment (positive/negative) provided by laboratory tests reported by other CPT codes.
More information is available on the AMA website.
A version of this article originally appeared on Medscape.com.
The American Medical Association on Sept. 8 announced that a new code, 99072, is intended to cover additional supplies, materials, and clinical staff time over and above those usually included in an office visit when performed during a declared public health emergency, as defined by law, attributable to respiratory-transmitted infectious disease, the AMA said in a release.
Fifty national medical specialty societies and other organizations worked with the AMA’s Specialty Society RVS Update Committee over the summer to collect data on the costs of maintaining safe medical offices during the public health emergency. It has submitted recommendations to the Centers for Medicare & Medicaid Services seeking to persuade the federal agencies to recognize the new 99072 payment code.
The intention is to recognize the extra expenses involved in steps now routinely taken to reduce the risk for COVID transmission from office visits, Current Procedural Terminology Editorial Panel Chair Mark S. Synovec, MD, said in an interview. Some practices have adapted by having staff screen patients before they enter offices and making arrangements to keep patients at a safe distance from others during their visits, he said.
“Everyone’s life has significantly changed because of COVID and the health care system has dramatically changed,” Dr. Synovec said. “It was pretty clear that the status quo was not going to work.”
Physician practices will welcome this change, said Veronica Bradley, CPC, a senior industry adviser to the Medical Group Management Association. An office visit that in the past may have involved only basic infection control measures, such as donning a pair of gloves, now may involve clinicians taking the time to put on more extensive protective gear, she said.
“Now they are taking a heck of a lot more precautions, and there’s more time and more supplies being consumed,” Ms. Bradley said in an interview.
Code looks ahead to future use
The AMA explained how this new code differs from CPT code 99070, which is typically reported for supplies and materials that may be used or provided to patients during an office visit.
The new 99072 code applies only during declared public health emergencies and applies only to additional items required to support “a safe in-person provision” of evaluation, treatment, and procedures, the AMA said.
“These items contrast with those typically reported with code 99070, which focuses on additional supplies provided over and above those usually included with a specific service, such as drugs, intravenous catheters, or trays,” the AMA said.
The CPT panel sought to structure the new code for covering COVID practice expenses so that it could not be abused, and also looked ahead to the future, Dr. Synovec said.
“It’s a code that you would put on during a public health emergency as defined by law that would be related to a respiratory-transmitted infectious disease. Obviously we meant it for SARS-CoV-2,” he said. “Hopefully we can go another 100 years before we have another pandemic, but we also wanted to prepare something where if we have another airborne respiratory virus that requires additional practice expenses as seen this time, it would be available for use.”
The AMA also announced a second addition, CPT code 86413, that anticipates greater use of quantitative measurements of SARS-CoV-2 antibodies, as opposed to a qualitative assessment (positive/negative) provided by laboratory tests reported by other CPT codes.
More information is available on the AMA website.
A version of this article originally appeared on Medscape.com.
In MS, serious adverse effects are more common in rituximab versus ocrelizumab
, a new postmarketing analysis finds, and AE-related deaths were not unusual. Serious AEs, and those linked to death, were more common in the rituximab group, although the reported infection rate was higher in the ocrelizumab group.
The analysis, published Aug. 21 in the Multiple Sclerosis Journal, highlights the importance of monitoring patients for infections and encouraging them to do the same, the authors said.
“This report points out the impact of treatments in terms of unrecognized or underappreciated complications,” said Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Patchogue, N.Y., who reviewed the study findings. “These medications have a significant downside.”
Lead author Natalia Gonzalez Caldito, MD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues analyzed AEs for the drugs in the Food and Drug Administration’s Adverse Event Reporting System. They only included cases in which the drugs were solely used to treat MS and were indicated as the cause of the AEs.
Rituximab (Rituxan) and ocrelizumab (Ocrevus) are both monoclonal antibodies. Rituximab is not FDA approved for MS but is used off label; ocrelizumab is approved for the relapsing forms of MS and primary progressive MS.
The researchers found 623 AE reports and 1,466 total AEs for rituximab and 7,948 and 23,613, respectively, for ocrelizumab. The average ages for the groups were 48.76 versus 43.89, respectively, (P < .001), and 71% in each group were women.
Among total AEs, serious AEs were more common in the rituximab group versus the ocrelizumab group (64.8% vs. 56.3%, respectively, P < .001). Adverse events that caused death were also more common in the rituximab group versus the ocrelizumab group (5.75% vs. 2.11%, P < .001).
Infections and infestations were more common in the ocrelizumab group than the rituximab group (21.93% vs. 11.05%, respectively, P < .001). However, certain AEs were more common in the rituximab group than the ocrelizumab group: Those in the blood and lymphatic system category (2.86% vs. 0.91%, respectively, P < .001), and those in the neoplasms category (4.02% vs. 1.28%, P < .001, respectively).
Researchers found a highly strong association between rituximab and a rare side effects – ear pruritus (itching, 0.8%). They also identified signals for infusion-related reaction (4.82%), throat irritation (4.01%) and throat tightness (1.44%), malignant melanoma (0.8%), breast cancer (1.77%) and neutropenia (2.57%).
Among the ocrelizumab AEs, researchers found the strongest association with oral herpes (2.21%), and they found other signals for herpes zoster (2.89%), urinary tract infection (10.52%), nasopharyngitis (9.79%), infusion-related reaction (4.76%), throat irritation (3.08%), and notably MS relapses (4.1%).
“Additional pharmacovigilance studies are needed to explore and further characterize these findings,” the researchers wrote. “Furthermore, these observations suggest that the AE profile of other second-generation anti-CD20 [monoclonal antibodies] may also differ from those of rituximab and ocrelizumab.”
Dr. Gudesblatt praised the analysis and said the findings make sense. “Use of B-cell–depleting agents lead to accumulative immune deficiency in routine care, which leads to higher rates of infection,” he said. He added that, “in the clinical trials for ocrelizumab, patients with IgG and IgM deficiency were excluded, but there is no advisement to exclude such patients in real care. The rates of infection in those patients with MS who have preexisting immune deficiencies and who are treated with these agents are unknown.”
The prospect of AEs is especially worrisome, he said, since “this information is only short term. Who knows what effect the prolonged use of unopposed B-cell depletion will have on infections in the long run?”
Neurologist Mitchell Wallin, MD, MPH, of George Washington University, Washington, and the University of Maryland, Baltimore County, said in an interview that the analysis is rigorous and especially useful because it includes a wider array of subjects – including those who are older and sicker – than took part in earlier clinical trials. “It’s really important to look at this real-world evidence,” he said, “and basically put this in the back of your head when you follow up with your patients.”
No study funding was reported. The corresponding author reported various disclosures. Dr. Gudesblatt and Dr. Wallin reported no disclosures.
SOURCE: Gonzalez Caldito N et al. Mult Scler J. 2020 Aug 21. doi: 10.1177/1352458520949986.
, a new postmarketing analysis finds, and AE-related deaths were not unusual. Serious AEs, and those linked to death, were more common in the rituximab group, although the reported infection rate was higher in the ocrelizumab group.
The analysis, published Aug. 21 in the Multiple Sclerosis Journal, highlights the importance of monitoring patients for infections and encouraging them to do the same, the authors said.
“This report points out the impact of treatments in terms of unrecognized or underappreciated complications,” said Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Patchogue, N.Y., who reviewed the study findings. “These medications have a significant downside.”
Lead author Natalia Gonzalez Caldito, MD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues analyzed AEs for the drugs in the Food and Drug Administration’s Adverse Event Reporting System. They only included cases in which the drugs were solely used to treat MS and were indicated as the cause of the AEs.
Rituximab (Rituxan) and ocrelizumab (Ocrevus) are both monoclonal antibodies. Rituximab is not FDA approved for MS but is used off label; ocrelizumab is approved for the relapsing forms of MS and primary progressive MS.
The researchers found 623 AE reports and 1,466 total AEs for rituximab and 7,948 and 23,613, respectively, for ocrelizumab. The average ages for the groups were 48.76 versus 43.89, respectively, (P < .001), and 71% in each group were women.
Among total AEs, serious AEs were more common in the rituximab group versus the ocrelizumab group (64.8% vs. 56.3%, respectively, P < .001). Adverse events that caused death were also more common in the rituximab group versus the ocrelizumab group (5.75% vs. 2.11%, P < .001).
Infections and infestations were more common in the ocrelizumab group than the rituximab group (21.93% vs. 11.05%, respectively, P < .001). However, certain AEs were more common in the rituximab group than the ocrelizumab group: Those in the blood and lymphatic system category (2.86% vs. 0.91%, respectively, P < .001), and those in the neoplasms category (4.02% vs. 1.28%, P < .001, respectively).
Researchers found a highly strong association between rituximab and a rare side effects – ear pruritus (itching, 0.8%). They also identified signals for infusion-related reaction (4.82%), throat irritation (4.01%) and throat tightness (1.44%), malignant melanoma (0.8%), breast cancer (1.77%) and neutropenia (2.57%).
Among the ocrelizumab AEs, researchers found the strongest association with oral herpes (2.21%), and they found other signals for herpes zoster (2.89%), urinary tract infection (10.52%), nasopharyngitis (9.79%), infusion-related reaction (4.76%), throat irritation (3.08%), and notably MS relapses (4.1%).
“Additional pharmacovigilance studies are needed to explore and further characterize these findings,” the researchers wrote. “Furthermore, these observations suggest that the AE profile of other second-generation anti-CD20 [monoclonal antibodies] may also differ from those of rituximab and ocrelizumab.”
Dr. Gudesblatt praised the analysis and said the findings make sense. “Use of B-cell–depleting agents lead to accumulative immune deficiency in routine care, which leads to higher rates of infection,” he said. He added that, “in the clinical trials for ocrelizumab, patients with IgG and IgM deficiency were excluded, but there is no advisement to exclude such patients in real care. The rates of infection in those patients with MS who have preexisting immune deficiencies and who are treated with these agents are unknown.”
The prospect of AEs is especially worrisome, he said, since “this information is only short term. Who knows what effect the prolonged use of unopposed B-cell depletion will have on infections in the long run?”
Neurologist Mitchell Wallin, MD, MPH, of George Washington University, Washington, and the University of Maryland, Baltimore County, said in an interview that the analysis is rigorous and especially useful because it includes a wider array of subjects – including those who are older and sicker – than took part in earlier clinical trials. “It’s really important to look at this real-world evidence,” he said, “and basically put this in the back of your head when you follow up with your patients.”
No study funding was reported. The corresponding author reported various disclosures. Dr. Gudesblatt and Dr. Wallin reported no disclosures.
SOURCE: Gonzalez Caldito N et al. Mult Scler J. 2020 Aug 21. doi: 10.1177/1352458520949986.
, a new postmarketing analysis finds, and AE-related deaths were not unusual. Serious AEs, and those linked to death, were more common in the rituximab group, although the reported infection rate was higher in the ocrelizumab group.
The analysis, published Aug. 21 in the Multiple Sclerosis Journal, highlights the importance of monitoring patients for infections and encouraging them to do the same, the authors said.
“This report points out the impact of treatments in terms of unrecognized or underappreciated complications,” said Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Patchogue, N.Y., who reviewed the study findings. “These medications have a significant downside.”
Lead author Natalia Gonzalez Caldito, MD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues analyzed AEs for the drugs in the Food and Drug Administration’s Adverse Event Reporting System. They only included cases in which the drugs were solely used to treat MS and were indicated as the cause of the AEs.
Rituximab (Rituxan) and ocrelizumab (Ocrevus) are both monoclonal antibodies. Rituximab is not FDA approved for MS but is used off label; ocrelizumab is approved for the relapsing forms of MS and primary progressive MS.
The researchers found 623 AE reports and 1,466 total AEs for rituximab and 7,948 and 23,613, respectively, for ocrelizumab. The average ages for the groups were 48.76 versus 43.89, respectively, (P < .001), and 71% in each group were women.
Among total AEs, serious AEs were more common in the rituximab group versus the ocrelizumab group (64.8% vs. 56.3%, respectively, P < .001). Adverse events that caused death were also more common in the rituximab group versus the ocrelizumab group (5.75% vs. 2.11%, P < .001).
Infections and infestations were more common in the ocrelizumab group than the rituximab group (21.93% vs. 11.05%, respectively, P < .001). However, certain AEs were more common in the rituximab group than the ocrelizumab group: Those in the blood and lymphatic system category (2.86% vs. 0.91%, respectively, P < .001), and those in the neoplasms category (4.02% vs. 1.28%, P < .001, respectively).
Researchers found a highly strong association between rituximab and a rare side effects – ear pruritus (itching, 0.8%). They also identified signals for infusion-related reaction (4.82%), throat irritation (4.01%) and throat tightness (1.44%), malignant melanoma (0.8%), breast cancer (1.77%) and neutropenia (2.57%).
Among the ocrelizumab AEs, researchers found the strongest association with oral herpes (2.21%), and they found other signals for herpes zoster (2.89%), urinary tract infection (10.52%), nasopharyngitis (9.79%), infusion-related reaction (4.76%), throat irritation (3.08%), and notably MS relapses (4.1%).
“Additional pharmacovigilance studies are needed to explore and further characterize these findings,” the researchers wrote. “Furthermore, these observations suggest that the AE profile of other second-generation anti-CD20 [monoclonal antibodies] may also differ from those of rituximab and ocrelizumab.”
Dr. Gudesblatt praised the analysis and said the findings make sense. “Use of B-cell–depleting agents lead to accumulative immune deficiency in routine care, which leads to higher rates of infection,” he said. He added that, “in the clinical trials for ocrelizumab, patients with IgG and IgM deficiency were excluded, but there is no advisement to exclude such patients in real care. The rates of infection in those patients with MS who have preexisting immune deficiencies and who are treated with these agents are unknown.”
The prospect of AEs is especially worrisome, he said, since “this information is only short term. Who knows what effect the prolonged use of unopposed B-cell depletion will have on infections in the long run?”
Neurologist Mitchell Wallin, MD, MPH, of George Washington University, Washington, and the University of Maryland, Baltimore County, said in an interview that the analysis is rigorous and especially useful because it includes a wider array of subjects – including those who are older and sicker – than took part in earlier clinical trials. “It’s really important to look at this real-world evidence,” he said, “and basically put this in the back of your head when you follow up with your patients.”
No study funding was reported. The corresponding author reported various disclosures. Dr. Gudesblatt and Dr. Wallin reported no disclosures.
SOURCE: Gonzalez Caldito N et al. Mult Scler J. 2020 Aug 21. doi: 10.1177/1352458520949986.
FROM MULTIPLE SCLEROSIS JOURNAL
Hospitalist movers and shakers – September 2020
The American Board of Internal Medicine has named David Pizzimenti, DO, to its board of trustees. The appointment comes with a 3-year term.
Dr. Pizzimenti has been a practicing internist in Mississippi since 2005. He currently serves as associate medical officer of acute care at North Mississippi Medical Center, Tupelo, where he also directs the hospitalist program and the internal medicine residency program. Prior to joining NMMC, he managed the same role at Magnolia Regional Health Center (Corinth, Miss.).
Dr. Pizzimenti is an inducted member of the American College of Osteopathic Internist College of Fellows, as well as a certified wound care specialist.
Tommy Ibrahim, MD, FHM, recently was named the new president and CEO for Bassett Healthcare Network, replacing William Streck, who had served in the role from 1984 to 2014, and then on an interim basis since 2018.
Dr. Ibrahim comes to Bassett from Integris Health, the largest nonprofit health care system in Oklahoma, where he was executive vice president and chief physician executive. He started his career as a hospitalist before moving into administration, and is a fellow in hospital medicine as well as a fellow of the American College of Healthcare Executives.
Bassett Healthcare Network is based at Bassett Medical Center in Cooperstown, N.Y., and includes four hospitals and more than two dozen primary care centers in eight New York counties.
Russell Kerbel, MD, MBA, has been named medical director for sepsis prevention at the University of California, Los Angeles. Since his arrival at UCLA in 2014, Dr. Kerbel – a hospitalist by training – has worked to increase awareness and standardize sepsis treatment through his advocacy, interdepartmental collaboration, and informatics knowledge.
Joshua Lenchus, DO, RPh, SFHM, was installed as vice president of the Florida Medical Association during the all-virtual 2020 FMA annual meeting in August. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Christopher Carpenter, MD, has been elevated to chief of staff at Natividad, a 172-bed, county-owned hospital in Salinas, Calif. Dr. Carpenter has served Natividad for the past 4 years, holding the positions of chief hospitalist, chief of service for pediatrics, vice chief of staff, and most recently director of pediatric services.
Dr. Carpenter’s term as chief of staff is limited to 2 years, during which he said his goals include promoting diversity within the facility’s leadership.
Prior to arriving at Natividad, Dr. Carpenter was instructor of pediatrics at Harvard Medical School, Boston, as well as associate director of the Boston Children’s Hospital Pediatric Global Health Fellowship.
David Fagan, MD, recently was promoted to medical director at Mid-State Health Center (Plymouth, N.H.), where he has served for the past 10 years. The 30-year medical veteran began working in his new role in May 2020.
Previously, Dr. Fagan has served the facility as an internist and hospitalist, and he has been among the leaders at Mid-State in ensuring safety for patients and staff during the COVID-19 response.
The Carroll County Memorial Hospital (Carrolton, Mo.) recently announced its new hospitalist program, which officially began on June 1, 2020. CCMH officials said the focus of the hospitalists will be to maintain communication with primary care physicians once patients leave the hospital facility.
CCMH added three physicians to its staff to work in the hospitalist program: Reuben I. Thaker, MD; Samuel C. Evans, MD; and Charles C. Glendenning, DO.
NorthShore University HealthSystem (Evanston, Ill.) has agreed to purchase Northwest Community Healthcare, a single-hospital health system located in Arlington Heights, Ill. NCH will become a hospital hub for NorthShore in the northwest Chicago suburbs.
When the agreement is finalized, NorthShore’s stable of hospitals will rise to six in and around Chicago. The system also provides outpatient care, labwork, and pharmacy services.
The American Board of Internal Medicine has named David Pizzimenti, DO, to its board of trustees. The appointment comes with a 3-year term.
Dr. Pizzimenti has been a practicing internist in Mississippi since 2005. He currently serves as associate medical officer of acute care at North Mississippi Medical Center, Tupelo, where he also directs the hospitalist program and the internal medicine residency program. Prior to joining NMMC, he managed the same role at Magnolia Regional Health Center (Corinth, Miss.).
Dr. Pizzimenti is an inducted member of the American College of Osteopathic Internist College of Fellows, as well as a certified wound care specialist.
Tommy Ibrahim, MD, FHM, recently was named the new president and CEO for Bassett Healthcare Network, replacing William Streck, who had served in the role from 1984 to 2014, and then on an interim basis since 2018.
Dr. Ibrahim comes to Bassett from Integris Health, the largest nonprofit health care system in Oklahoma, where he was executive vice president and chief physician executive. He started his career as a hospitalist before moving into administration, and is a fellow in hospital medicine as well as a fellow of the American College of Healthcare Executives.
Bassett Healthcare Network is based at Bassett Medical Center in Cooperstown, N.Y., and includes four hospitals and more than two dozen primary care centers in eight New York counties.
Russell Kerbel, MD, MBA, has been named medical director for sepsis prevention at the University of California, Los Angeles. Since his arrival at UCLA in 2014, Dr. Kerbel – a hospitalist by training – has worked to increase awareness and standardize sepsis treatment through his advocacy, interdepartmental collaboration, and informatics knowledge.
Joshua Lenchus, DO, RPh, SFHM, was installed as vice president of the Florida Medical Association during the all-virtual 2020 FMA annual meeting in August. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Christopher Carpenter, MD, has been elevated to chief of staff at Natividad, a 172-bed, county-owned hospital in Salinas, Calif. Dr. Carpenter has served Natividad for the past 4 years, holding the positions of chief hospitalist, chief of service for pediatrics, vice chief of staff, and most recently director of pediatric services.
Dr. Carpenter’s term as chief of staff is limited to 2 years, during which he said his goals include promoting diversity within the facility’s leadership.
Prior to arriving at Natividad, Dr. Carpenter was instructor of pediatrics at Harvard Medical School, Boston, as well as associate director of the Boston Children’s Hospital Pediatric Global Health Fellowship.
David Fagan, MD, recently was promoted to medical director at Mid-State Health Center (Plymouth, N.H.), where he has served for the past 10 years. The 30-year medical veteran began working in his new role in May 2020.
Previously, Dr. Fagan has served the facility as an internist and hospitalist, and he has been among the leaders at Mid-State in ensuring safety for patients and staff during the COVID-19 response.
The Carroll County Memorial Hospital (Carrolton, Mo.) recently announced its new hospitalist program, which officially began on June 1, 2020. CCMH officials said the focus of the hospitalists will be to maintain communication with primary care physicians once patients leave the hospital facility.
CCMH added three physicians to its staff to work in the hospitalist program: Reuben I. Thaker, MD; Samuel C. Evans, MD; and Charles C. Glendenning, DO.
NorthShore University HealthSystem (Evanston, Ill.) has agreed to purchase Northwest Community Healthcare, a single-hospital health system located in Arlington Heights, Ill. NCH will become a hospital hub for NorthShore in the northwest Chicago suburbs.
When the agreement is finalized, NorthShore’s stable of hospitals will rise to six in and around Chicago. The system also provides outpatient care, labwork, and pharmacy services.
The American Board of Internal Medicine has named David Pizzimenti, DO, to its board of trustees. The appointment comes with a 3-year term.
Dr. Pizzimenti has been a practicing internist in Mississippi since 2005. He currently serves as associate medical officer of acute care at North Mississippi Medical Center, Tupelo, where he also directs the hospitalist program and the internal medicine residency program. Prior to joining NMMC, he managed the same role at Magnolia Regional Health Center (Corinth, Miss.).
Dr. Pizzimenti is an inducted member of the American College of Osteopathic Internist College of Fellows, as well as a certified wound care specialist.
Tommy Ibrahim, MD, FHM, recently was named the new president and CEO for Bassett Healthcare Network, replacing William Streck, who had served in the role from 1984 to 2014, and then on an interim basis since 2018.
Dr. Ibrahim comes to Bassett from Integris Health, the largest nonprofit health care system in Oklahoma, where he was executive vice president and chief physician executive. He started his career as a hospitalist before moving into administration, and is a fellow in hospital medicine as well as a fellow of the American College of Healthcare Executives.
Bassett Healthcare Network is based at Bassett Medical Center in Cooperstown, N.Y., and includes four hospitals and more than two dozen primary care centers in eight New York counties.
Russell Kerbel, MD, MBA, has been named medical director for sepsis prevention at the University of California, Los Angeles. Since his arrival at UCLA in 2014, Dr. Kerbel – a hospitalist by training – has worked to increase awareness and standardize sepsis treatment through his advocacy, interdepartmental collaboration, and informatics knowledge.
Joshua Lenchus, DO, RPh, SFHM, was installed as vice president of the Florida Medical Association during the all-virtual 2020 FMA annual meeting in August. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Christopher Carpenter, MD, has been elevated to chief of staff at Natividad, a 172-bed, county-owned hospital in Salinas, Calif. Dr. Carpenter has served Natividad for the past 4 years, holding the positions of chief hospitalist, chief of service for pediatrics, vice chief of staff, and most recently director of pediatric services.
Dr. Carpenter’s term as chief of staff is limited to 2 years, during which he said his goals include promoting diversity within the facility’s leadership.
Prior to arriving at Natividad, Dr. Carpenter was instructor of pediatrics at Harvard Medical School, Boston, as well as associate director of the Boston Children’s Hospital Pediatric Global Health Fellowship.
David Fagan, MD, recently was promoted to medical director at Mid-State Health Center (Plymouth, N.H.), where he has served for the past 10 years. The 30-year medical veteran began working in his new role in May 2020.
Previously, Dr. Fagan has served the facility as an internist and hospitalist, and he has been among the leaders at Mid-State in ensuring safety for patients and staff during the COVID-19 response.
The Carroll County Memorial Hospital (Carrolton, Mo.) recently announced its new hospitalist program, which officially began on June 1, 2020. CCMH officials said the focus of the hospitalists will be to maintain communication with primary care physicians once patients leave the hospital facility.
CCMH added three physicians to its staff to work in the hospitalist program: Reuben I. Thaker, MD; Samuel C. Evans, MD; and Charles C. Glendenning, DO.
NorthShore University HealthSystem (Evanston, Ill.) has agreed to purchase Northwest Community Healthcare, a single-hospital health system located in Arlington Heights, Ill. NCH will become a hospital hub for NorthShore in the northwest Chicago suburbs.
When the agreement is finalized, NorthShore’s stable of hospitals will rise to six in and around Chicago. The system also provides outpatient care, labwork, and pharmacy services.
ASBMR 2020: Sequential osteoporosis meds, AI, bone cancer, and more
The virtual American Society for Bone and Mineral Research 2020 annual meeting “is full of highlights,” says Lorenz Hofbauer, MD, scientific chair, but “this year you won’t lose time in the hallways to switch between the talks,” he quipped.
Nevertheless, “although we won’t be coming together face to face this year, you will have the flexibility to virtually connect with peers and colleagues from around the world,” Teresita Bellido, PhD, ASBMR president emphasized in a message to members.
Like other medical organizations, with the advent of the COVID-19 pandemic, the ASBMR had to quickly pivot to provide a virtual meeting.
The meeting will take place September 11-15 and is free for ASBMR members.
Speaking to Medscape Medical News, Bellido and Hofbauer drew attention to some of the meeting’s major themes, key sessions, and top clinical oral abstracts.
Attendees at this year’s virtual meeting will hear the latest information on optimal sequential treatment for osteoporosis, the latest research using artificial intelligence (AI), and bone and cancer, among other topics.
Sequential osteoporosis treatment a recurring theme
According to Hofbauer, from Dresden Technical University, Germany, the September 13 Cutting Edge symposium entitled, “Optimizing Sequential Osteoporosis Treatment,” is not to be missed, and the topic “will be a leitmotiv [recurrent theme] for the entire meeting.”
During this session speakers will present findings from two perspectives – basic science and clinical applications – with the latter being another recurring theme at the meeting.
Bellido, from the University of Arkansas for Medical Sciences, in Little Rock, pointed out that romosozumab (Evenity, Amgen), recently approved by the US Food and Drug Administration, is an example of how basic laboratory research can lead to important new therapies.
Anabolic therapies for osteoporosis that “build up bone” include teriparatide, abaloparatide, and now romosozumab, whereas antiresorptive therapies that stop bone resorption include the bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) and the monoclonal antibody denosumab, Bellido explained.
As osteoporosis treatment options have expanded, the timing and sequencing of optimal therapies have become much more complex, and so this session on sequencing, as well as the September 13 Concurrent Orals session, “Issues of Long-term Treatment and Discontinuation,” is sure to spark interest.
The ASBMR/European Calcified Tissue Society debate, entitled, “A Treat to Target Approach is Helpful for Osteoporosis Management,” is also expected to be lively and generate wide interest, according to Bellido and Hofbauer.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, will argue against the motion and Celia Gregson, PhD, University of Bristol, UK, will argue for it. Attendees will be able to vote for/against the motion before and after the debate, and the result will indicate which speaker was more persuasive.
Bone cancer ultimately damages other tissues
The meeting will also offer attendees a close look at bone and cancer, which is an example of how “all the homeostatic processes that occur with bone not only affect bone but also impact other tissues and organs,” said Bellido.
In other words, “what happens in bone impacts other tissues – for example, skeletal muscle, the pancreas, and even frailty and fractures.”
Theresa A. Guise, MD, from the University of Texas MD Anderson Cancer Center, in Austin, will present the Louis V. Avioli Lecture on September 11, entitled, “Cancer, Bone and Beyond: An Integrated View of the Bone Microenvironment.”
“Local events in the bone microenvironment due to cancer and cancer treatment which result in pathologic bone destruction may have widespread systemic consequences that further increase morbidity and mortality,” Hofbauer noted.
Guise “will highlight cutting-edge concepts, potential mechanisms, and therapy for bone metastases,” he said.
These concepts will also be discussed in more detail during a 2-day virtual premeeting symposium, presented on September 9 and 10 by the ASBMR along with the Cancer and Bone Society, entitled, “The Seed and Soil: Therapeutic Targets for Cancer in Bone.”
The symposium will cover tumor dormancy, imaging, adiposity in the bone tumor microenvironment, a history of bone-targeted therapies in cancer, advances in breast cancer bone metastasis, and new approaches in myeloma bone disease.
“We have evidence from breast cancer, multiple myeloma, as well as from prostate cancer,” Bellido noted, that “all those cancer cells make their home in bone and transform the bone in such a way that not only the bone is damaged but also other tissues.”
“We have skeletal muscle weakness (that is directed by the effects that occur in bone), as well as changes in the pancreas – all directed by proteins and genes in bone cells.”
AI, bench to bedside research
“Every field is moving towards the use of AI,” Bellido noted, and the September 11 plenary symposium entitled, “Artificial Intelligence and Precision Medicine in Musculoskeletal Health,” will shed light on how AI is being used to study bone health.
The session “will give us a glimpse of the future,” said Hofbauer.
Session topics include principles of applications to research and clinical care in bone and mineral research; how AI can help detect rare diseases; and combining genomics with medical data using AI in precision medicine for drug discovery.
“The Bench to Bedside presentation on ‘Beta Blockers and Bone’ is a great example of translational research, while the Basic Symposium on ‘Bones, Guts and Brains’ provides inspiring and thought-provoking insights into novel physiology and tempting teleology,” Hofbauer explained.
“Another fascinating Cutting Edge symposium,” he added, “is on ‘Inspiring Mechanistic Bone Stories from Around the Animal Kingdom,’ a must-see for those employing preclinical animal models.”
For more insight into early research and a research pioneer, attendees can listen to Selma Masri, PhD, from the University of California, Irvine, who will deliver the Gerald D. Aurbach Lecture entitled, “The Scientific Legacy of Paolo Sassone-Corsi: A Tour Through the Fields of Transcriptional Regulation, Epigenetics, Metabolism and Circadian Rhythms.”
Masri’s lab is dissecting how genetic disruption of the circadian clock in mouse models affects cancer, and she will discuss the work and legacy of the late Sassone-Corsi, as well as the future of the field.
Rare disease, fragility fractures
The ASBMR meeting will also feature the latest research into rare diseases and fragility fractures.
Rare diseases are often about “more bone or less bone,” said Bellido. “Understanding the mechanisms of these rare diseases can give us very important clues of treating the more common diseases.”
A fragility fracture is a diagnosis of osteoporosis, but most are not treated, she continued. “This is equivalent to having, for example, a heart attack and leaving the hospital after the incident was resolved and not treating it.”
“We’re trying to address this gap,” she said, and a symposium on September 14 will present some of the latest knowledge.
During the “Long-term Management of Fragility Fracture” symposium, speakers will discuss reducing mortality with antiosteoporotic treatment, new scenarios to prevent postfracture frailty, as well as fracture and postfracture management – surgeon and nursing perspectives.
COVID-19, nutrition, microbiome, and top 5 clinical abstracts
In addition to plenary sessions and symposiums, there are many oral abstracts and posters on important studies in the field of bone health, including, for example, a topical study of vitamin D and COVID-19.
There are also many abstracts on nutrition, the microbiome, and treating bone loss, said Bellido.
“We have a huge increase in the number of abstracts submitted from South America and Australia compared to previous years,” she noted, “and a 10% increase (from 50% to 61%) in the number of abstracts submitted by young investigators, which is crucial.”
Close to 1000 abstracts (988) were submitted, two thirds of which were clinical.
The top 5 clinical abstracts reflect important current issues in the field, said Hofbauer.
“One major theme is on long-term and sequential therapy efficacy and safety,” he said. And “burosumab is a game-changing new drug, and nutritional aspects are evergreens [perennial favorites], especially in the elderly population.”
The top 5 clinical oral abstracts at the ASBMR 2020 meeting are:
- Dairy supplementation reduces fractures and falls in institutionalized older adults: A cluster-randomized placebo-controlled trial (abstract 1022).
- Treatment with zoledronate subsequent to denosumab in osteoporosis: A randomized trial (abstract 1065).
- Efficacy of burosumab in adults with X-linked hypophosphatemia (XLH): A subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study (abstract 1044).
- High-dose vitamin D supplementation affects bone density differently in females than males (abstract 1019).
- Bisphosphonate use and risk of atypical femoral fractures: A nationwide Danish analysis with blinded radiographic review (abstract 1061).
This article first appeared on Medscape.com.
The virtual American Society for Bone and Mineral Research 2020 annual meeting “is full of highlights,” says Lorenz Hofbauer, MD, scientific chair, but “this year you won’t lose time in the hallways to switch between the talks,” he quipped.
Nevertheless, “although we won’t be coming together face to face this year, you will have the flexibility to virtually connect with peers and colleagues from around the world,” Teresita Bellido, PhD, ASBMR president emphasized in a message to members.
Like other medical organizations, with the advent of the COVID-19 pandemic, the ASBMR had to quickly pivot to provide a virtual meeting.
The meeting will take place September 11-15 and is free for ASBMR members.
Speaking to Medscape Medical News, Bellido and Hofbauer drew attention to some of the meeting’s major themes, key sessions, and top clinical oral abstracts.
Attendees at this year’s virtual meeting will hear the latest information on optimal sequential treatment for osteoporosis, the latest research using artificial intelligence (AI), and bone and cancer, among other topics.
Sequential osteoporosis treatment a recurring theme
According to Hofbauer, from Dresden Technical University, Germany, the September 13 Cutting Edge symposium entitled, “Optimizing Sequential Osteoporosis Treatment,” is not to be missed, and the topic “will be a leitmotiv [recurrent theme] for the entire meeting.”
During this session speakers will present findings from two perspectives – basic science and clinical applications – with the latter being another recurring theme at the meeting.
Bellido, from the University of Arkansas for Medical Sciences, in Little Rock, pointed out that romosozumab (Evenity, Amgen), recently approved by the US Food and Drug Administration, is an example of how basic laboratory research can lead to important new therapies.
Anabolic therapies for osteoporosis that “build up bone” include teriparatide, abaloparatide, and now romosozumab, whereas antiresorptive therapies that stop bone resorption include the bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) and the monoclonal antibody denosumab, Bellido explained.
As osteoporosis treatment options have expanded, the timing and sequencing of optimal therapies have become much more complex, and so this session on sequencing, as well as the September 13 Concurrent Orals session, “Issues of Long-term Treatment and Discontinuation,” is sure to spark interest.
The ASBMR/European Calcified Tissue Society debate, entitled, “A Treat to Target Approach is Helpful for Osteoporosis Management,” is also expected to be lively and generate wide interest, according to Bellido and Hofbauer.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, will argue against the motion and Celia Gregson, PhD, University of Bristol, UK, will argue for it. Attendees will be able to vote for/against the motion before and after the debate, and the result will indicate which speaker was more persuasive.
Bone cancer ultimately damages other tissues
The meeting will also offer attendees a close look at bone and cancer, which is an example of how “all the homeostatic processes that occur with bone not only affect bone but also impact other tissues and organs,” said Bellido.
In other words, “what happens in bone impacts other tissues – for example, skeletal muscle, the pancreas, and even frailty and fractures.”
Theresa A. Guise, MD, from the University of Texas MD Anderson Cancer Center, in Austin, will present the Louis V. Avioli Lecture on September 11, entitled, “Cancer, Bone and Beyond: An Integrated View of the Bone Microenvironment.”
“Local events in the bone microenvironment due to cancer and cancer treatment which result in pathologic bone destruction may have widespread systemic consequences that further increase morbidity and mortality,” Hofbauer noted.
Guise “will highlight cutting-edge concepts, potential mechanisms, and therapy for bone metastases,” he said.
These concepts will also be discussed in more detail during a 2-day virtual premeeting symposium, presented on September 9 and 10 by the ASBMR along with the Cancer and Bone Society, entitled, “The Seed and Soil: Therapeutic Targets for Cancer in Bone.”
The symposium will cover tumor dormancy, imaging, adiposity in the bone tumor microenvironment, a history of bone-targeted therapies in cancer, advances in breast cancer bone metastasis, and new approaches in myeloma bone disease.
“We have evidence from breast cancer, multiple myeloma, as well as from prostate cancer,” Bellido noted, that “all those cancer cells make their home in bone and transform the bone in such a way that not only the bone is damaged but also other tissues.”
“We have skeletal muscle weakness (that is directed by the effects that occur in bone), as well as changes in the pancreas – all directed by proteins and genes in bone cells.”
AI, bench to bedside research
“Every field is moving towards the use of AI,” Bellido noted, and the September 11 plenary symposium entitled, “Artificial Intelligence and Precision Medicine in Musculoskeletal Health,” will shed light on how AI is being used to study bone health.
The session “will give us a glimpse of the future,” said Hofbauer.
Session topics include principles of applications to research and clinical care in bone and mineral research; how AI can help detect rare diseases; and combining genomics with medical data using AI in precision medicine for drug discovery.
“The Bench to Bedside presentation on ‘Beta Blockers and Bone’ is a great example of translational research, while the Basic Symposium on ‘Bones, Guts and Brains’ provides inspiring and thought-provoking insights into novel physiology and tempting teleology,” Hofbauer explained.
“Another fascinating Cutting Edge symposium,” he added, “is on ‘Inspiring Mechanistic Bone Stories from Around the Animal Kingdom,’ a must-see for those employing preclinical animal models.”
For more insight into early research and a research pioneer, attendees can listen to Selma Masri, PhD, from the University of California, Irvine, who will deliver the Gerald D. Aurbach Lecture entitled, “The Scientific Legacy of Paolo Sassone-Corsi: A Tour Through the Fields of Transcriptional Regulation, Epigenetics, Metabolism and Circadian Rhythms.”
Masri’s lab is dissecting how genetic disruption of the circadian clock in mouse models affects cancer, and she will discuss the work and legacy of the late Sassone-Corsi, as well as the future of the field.
Rare disease, fragility fractures
The ASBMR meeting will also feature the latest research into rare diseases and fragility fractures.
Rare diseases are often about “more bone or less bone,” said Bellido. “Understanding the mechanisms of these rare diseases can give us very important clues of treating the more common diseases.”
A fragility fracture is a diagnosis of osteoporosis, but most are not treated, she continued. “This is equivalent to having, for example, a heart attack and leaving the hospital after the incident was resolved and not treating it.”
“We’re trying to address this gap,” she said, and a symposium on September 14 will present some of the latest knowledge.
During the “Long-term Management of Fragility Fracture” symposium, speakers will discuss reducing mortality with antiosteoporotic treatment, new scenarios to prevent postfracture frailty, as well as fracture and postfracture management – surgeon and nursing perspectives.
COVID-19, nutrition, microbiome, and top 5 clinical abstracts
In addition to plenary sessions and symposiums, there are many oral abstracts and posters on important studies in the field of bone health, including, for example, a topical study of vitamin D and COVID-19.
There are also many abstracts on nutrition, the microbiome, and treating bone loss, said Bellido.
“We have a huge increase in the number of abstracts submitted from South America and Australia compared to previous years,” she noted, “and a 10% increase (from 50% to 61%) in the number of abstracts submitted by young investigators, which is crucial.”
Close to 1000 abstracts (988) were submitted, two thirds of which were clinical.
The top 5 clinical abstracts reflect important current issues in the field, said Hofbauer.
“One major theme is on long-term and sequential therapy efficacy and safety,” he said. And “burosumab is a game-changing new drug, and nutritional aspects are evergreens [perennial favorites], especially in the elderly population.”
The top 5 clinical oral abstracts at the ASBMR 2020 meeting are:
- Dairy supplementation reduces fractures and falls in institutionalized older adults: A cluster-randomized placebo-controlled trial (abstract 1022).
- Treatment with zoledronate subsequent to denosumab in osteoporosis: A randomized trial (abstract 1065).
- Efficacy of burosumab in adults with X-linked hypophosphatemia (XLH): A subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study (abstract 1044).
- High-dose vitamin D supplementation affects bone density differently in females than males (abstract 1019).
- Bisphosphonate use and risk of atypical femoral fractures: A nationwide Danish analysis with blinded radiographic review (abstract 1061).
This article first appeared on Medscape.com.
The virtual American Society for Bone and Mineral Research 2020 annual meeting “is full of highlights,” says Lorenz Hofbauer, MD, scientific chair, but “this year you won’t lose time in the hallways to switch between the talks,” he quipped.
Nevertheless, “although we won’t be coming together face to face this year, you will have the flexibility to virtually connect with peers and colleagues from around the world,” Teresita Bellido, PhD, ASBMR president emphasized in a message to members.
Like other medical organizations, with the advent of the COVID-19 pandemic, the ASBMR had to quickly pivot to provide a virtual meeting.
The meeting will take place September 11-15 and is free for ASBMR members.
Speaking to Medscape Medical News, Bellido and Hofbauer drew attention to some of the meeting’s major themes, key sessions, and top clinical oral abstracts.
Attendees at this year’s virtual meeting will hear the latest information on optimal sequential treatment for osteoporosis, the latest research using artificial intelligence (AI), and bone and cancer, among other topics.
Sequential osteoporosis treatment a recurring theme
According to Hofbauer, from Dresden Technical University, Germany, the September 13 Cutting Edge symposium entitled, “Optimizing Sequential Osteoporosis Treatment,” is not to be missed, and the topic “will be a leitmotiv [recurrent theme] for the entire meeting.”
During this session speakers will present findings from two perspectives – basic science and clinical applications – with the latter being another recurring theme at the meeting.
Bellido, from the University of Arkansas for Medical Sciences, in Little Rock, pointed out that romosozumab (Evenity, Amgen), recently approved by the US Food and Drug Administration, is an example of how basic laboratory research can lead to important new therapies.
Anabolic therapies for osteoporosis that “build up bone” include teriparatide, abaloparatide, and now romosozumab, whereas antiresorptive therapies that stop bone resorption include the bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) and the monoclonal antibody denosumab, Bellido explained.
As osteoporosis treatment options have expanded, the timing and sequencing of optimal therapies have become much more complex, and so this session on sequencing, as well as the September 13 Concurrent Orals session, “Issues of Long-term Treatment and Discontinuation,” is sure to spark interest.
The ASBMR/European Calcified Tissue Society debate, entitled, “A Treat to Target Approach is Helpful for Osteoporosis Management,” is also expected to be lively and generate wide interest, according to Bellido and Hofbauer.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, will argue against the motion and Celia Gregson, PhD, University of Bristol, UK, will argue for it. Attendees will be able to vote for/against the motion before and after the debate, and the result will indicate which speaker was more persuasive.
Bone cancer ultimately damages other tissues
The meeting will also offer attendees a close look at bone and cancer, which is an example of how “all the homeostatic processes that occur with bone not only affect bone but also impact other tissues and organs,” said Bellido.
In other words, “what happens in bone impacts other tissues – for example, skeletal muscle, the pancreas, and even frailty and fractures.”
Theresa A. Guise, MD, from the University of Texas MD Anderson Cancer Center, in Austin, will present the Louis V. Avioli Lecture on September 11, entitled, “Cancer, Bone and Beyond: An Integrated View of the Bone Microenvironment.”
“Local events in the bone microenvironment due to cancer and cancer treatment which result in pathologic bone destruction may have widespread systemic consequences that further increase morbidity and mortality,” Hofbauer noted.
Guise “will highlight cutting-edge concepts, potential mechanisms, and therapy for bone metastases,” he said.
These concepts will also be discussed in more detail during a 2-day virtual premeeting symposium, presented on September 9 and 10 by the ASBMR along with the Cancer and Bone Society, entitled, “The Seed and Soil: Therapeutic Targets for Cancer in Bone.”
The symposium will cover tumor dormancy, imaging, adiposity in the bone tumor microenvironment, a history of bone-targeted therapies in cancer, advances in breast cancer bone metastasis, and new approaches in myeloma bone disease.
“We have evidence from breast cancer, multiple myeloma, as well as from prostate cancer,” Bellido noted, that “all those cancer cells make their home in bone and transform the bone in such a way that not only the bone is damaged but also other tissues.”
“We have skeletal muscle weakness (that is directed by the effects that occur in bone), as well as changes in the pancreas – all directed by proteins and genes in bone cells.”
AI, bench to bedside research
“Every field is moving towards the use of AI,” Bellido noted, and the September 11 plenary symposium entitled, “Artificial Intelligence and Precision Medicine in Musculoskeletal Health,” will shed light on how AI is being used to study bone health.
The session “will give us a glimpse of the future,” said Hofbauer.
Session topics include principles of applications to research and clinical care in bone and mineral research; how AI can help detect rare diseases; and combining genomics with medical data using AI in precision medicine for drug discovery.
“The Bench to Bedside presentation on ‘Beta Blockers and Bone’ is a great example of translational research, while the Basic Symposium on ‘Bones, Guts and Brains’ provides inspiring and thought-provoking insights into novel physiology and tempting teleology,” Hofbauer explained.
“Another fascinating Cutting Edge symposium,” he added, “is on ‘Inspiring Mechanistic Bone Stories from Around the Animal Kingdom,’ a must-see for those employing preclinical animal models.”
For more insight into early research and a research pioneer, attendees can listen to Selma Masri, PhD, from the University of California, Irvine, who will deliver the Gerald D. Aurbach Lecture entitled, “The Scientific Legacy of Paolo Sassone-Corsi: A Tour Through the Fields of Transcriptional Regulation, Epigenetics, Metabolism and Circadian Rhythms.”
Masri’s lab is dissecting how genetic disruption of the circadian clock in mouse models affects cancer, and she will discuss the work and legacy of the late Sassone-Corsi, as well as the future of the field.
Rare disease, fragility fractures
The ASBMR meeting will also feature the latest research into rare diseases and fragility fractures.
Rare diseases are often about “more bone or less bone,” said Bellido. “Understanding the mechanisms of these rare diseases can give us very important clues of treating the more common diseases.”
A fragility fracture is a diagnosis of osteoporosis, but most are not treated, she continued. “This is equivalent to having, for example, a heart attack and leaving the hospital after the incident was resolved and not treating it.”
“We’re trying to address this gap,” she said, and a symposium on September 14 will present some of the latest knowledge.
During the “Long-term Management of Fragility Fracture” symposium, speakers will discuss reducing mortality with antiosteoporotic treatment, new scenarios to prevent postfracture frailty, as well as fracture and postfracture management – surgeon and nursing perspectives.
COVID-19, nutrition, microbiome, and top 5 clinical abstracts
In addition to plenary sessions and symposiums, there are many oral abstracts and posters on important studies in the field of bone health, including, for example, a topical study of vitamin D and COVID-19.
There are also many abstracts on nutrition, the microbiome, and treating bone loss, said Bellido.
“We have a huge increase in the number of abstracts submitted from South America and Australia compared to previous years,” she noted, “and a 10% increase (from 50% to 61%) in the number of abstracts submitted by young investigators, which is crucial.”
Close to 1000 abstracts (988) were submitted, two thirds of which were clinical.
The top 5 clinical abstracts reflect important current issues in the field, said Hofbauer.
“One major theme is on long-term and sequential therapy efficacy and safety,” he said. And “burosumab is a game-changing new drug, and nutritional aspects are evergreens [perennial favorites], especially in the elderly population.”
The top 5 clinical oral abstracts at the ASBMR 2020 meeting are:
- Dairy supplementation reduces fractures and falls in institutionalized older adults: A cluster-randomized placebo-controlled trial (abstract 1022).
- Treatment with zoledronate subsequent to denosumab in osteoporosis: A randomized trial (abstract 1065).
- Efficacy of burosumab in adults with X-linked hypophosphatemia (XLH): A subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study (abstract 1044).
- High-dose vitamin D supplementation affects bone density differently in females than males (abstract 1019).
- Bisphosphonate use and risk of atypical femoral fractures: A nationwide Danish analysis with blinded radiographic review (abstract 1061).
This article first appeared on Medscape.com.
Distinguishing COVID-19 from flu in kids remains challenging
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.