User login
FDA authorizes new saliva COVID-19 test
The FDA authorized a new type of saliva-based coronavirus test on August 15 that could cut down on the cost of testing and the time it takes to process results.
The emergency use authorization is for SalivaDirect, a diagnostic test created by the Yale School of Public Health. The test doesn’t require a special type of swab or collection tube — saliva can be collected in any sterile container, according to the FDA announcement.
The new test is “yet another testing innovation game changer that will reduce the demand for scarce testing resources,” Admiral Brett Giroir, MD, the assistant secretary for health and the COVID-19 testing coordinator, said in the statement.
The test also doesn’t require a special type of extractor, which is helpful because the extraction kits used to process other tests have faced shortages during the pandemic. The test can be used with different types of reagents and instruments already found in labs.
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” Stephen Hahn, MD, the FDA commissioner, also said in the statement.
Yale will provide the instructions to labs as an “open source” protocol. The test doesn’t require any proprietary equipment or testing components, so labs across the country can assemble and use it based on the FDA guidelines. The testing method is available immediately and could be scaled up quickly in the next few weeks, according to a statement from Yale.
“This is a huge step forward to make testing more accessible,” Chantal Vogels, a postdoctoral fellow at Yale who led the lab development and test validation efforts, said in the statement.
The Yale team is further testing whether the saliva method can be used to find coronavirus cases among people who don’t have any symptoms and has been working with players and staff from the NBA. So far, the results have been accurate and similar to the nasal swabs for COVID-19, according to a preprint study published on medRxiv.
The research team wanted to get rid of the expensive collection tubes that other companies use to preserve the virus during processing, according to the Yale statement. They found that the virus is stable in saliva for long periods of time at warm temperatures and that special tubes aren’t necessary.
The FDA has authorized other saliva-based tests, according to ABC News, but SalivaDirect is the first that doesn’t require the extraction process used to test viral genetic material. Instead, the Yale process breaks down the saliva with an enzyme and applied heat. This type of testing could cost about $10, the Yale researchers said, and people can collect the saliva themselves under supervision.
“This, I hope, is a turning point,” Anne Wyllie, PhD, one of the lead researchers at Yale, told the news station.* “Expand testing capacity, inspire creativity and we can take competition to those labs charging a lot and bring prices down.”
This article first appeared on WebMD.com.
Correction, 8/25/20: An earlier version of this article misstated Dr. Wylie's academic degree.
The FDA authorized a new type of saliva-based coronavirus test on August 15 that could cut down on the cost of testing and the time it takes to process results.
The emergency use authorization is for SalivaDirect, a diagnostic test created by the Yale School of Public Health. The test doesn’t require a special type of swab or collection tube — saliva can be collected in any sterile container, according to the FDA announcement.
The new test is “yet another testing innovation game changer that will reduce the demand for scarce testing resources,” Admiral Brett Giroir, MD, the assistant secretary for health and the COVID-19 testing coordinator, said in the statement.
The test also doesn’t require a special type of extractor, which is helpful because the extraction kits used to process other tests have faced shortages during the pandemic. The test can be used with different types of reagents and instruments already found in labs.
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” Stephen Hahn, MD, the FDA commissioner, also said in the statement.
Yale will provide the instructions to labs as an “open source” protocol. The test doesn’t require any proprietary equipment or testing components, so labs across the country can assemble and use it based on the FDA guidelines. The testing method is available immediately and could be scaled up quickly in the next few weeks, according to a statement from Yale.
“This is a huge step forward to make testing more accessible,” Chantal Vogels, a postdoctoral fellow at Yale who led the lab development and test validation efforts, said in the statement.
The Yale team is further testing whether the saliva method can be used to find coronavirus cases among people who don’t have any symptoms and has been working with players and staff from the NBA. So far, the results have been accurate and similar to the nasal swabs for COVID-19, according to a preprint study published on medRxiv.
The research team wanted to get rid of the expensive collection tubes that other companies use to preserve the virus during processing, according to the Yale statement. They found that the virus is stable in saliva for long periods of time at warm temperatures and that special tubes aren’t necessary.
The FDA has authorized other saliva-based tests, according to ABC News, but SalivaDirect is the first that doesn’t require the extraction process used to test viral genetic material. Instead, the Yale process breaks down the saliva with an enzyme and applied heat. This type of testing could cost about $10, the Yale researchers said, and people can collect the saliva themselves under supervision.
“This, I hope, is a turning point,” Anne Wyllie, PhD, one of the lead researchers at Yale, told the news station.* “Expand testing capacity, inspire creativity and we can take competition to those labs charging a lot and bring prices down.”
This article first appeared on WebMD.com.
Correction, 8/25/20: An earlier version of this article misstated Dr. Wylie's academic degree.
The FDA authorized a new type of saliva-based coronavirus test on August 15 that could cut down on the cost of testing and the time it takes to process results.
The emergency use authorization is for SalivaDirect, a diagnostic test created by the Yale School of Public Health. The test doesn’t require a special type of swab or collection tube — saliva can be collected in any sterile container, according to the FDA announcement.
The new test is “yet another testing innovation game changer that will reduce the demand for scarce testing resources,” Admiral Brett Giroir, MD, the assistant secretary for health and the COVID-19 testing coordinator, said in the statement.
The test also doesn’t require a special type of extractor, which is helpful because the extraction kits used to process other tests have faced shortages during the pandemic. The test can be used with different types of reagents and instruments already found in labs.
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” Stephen Hahn, MD, the FDA commissioner, also said in the statement.
Yale will provide the instructions to labs as an “open source” protocol. The test doesn’t require any proprietary equipment or testing components, so labs across the country can assemble and use it based on the FDA guidelines. The testing method is available immediately and could be scaled up quickly in the next few weeks, according to a statement from Yale.
“This is a huge step forward to make testing more accessible,” Chantal Vogels, a postdoctoral fellow at Yale who led the lab development and test validation efforts, said in the statement.
The Yale team is further testing whether the saliva method can be used to find coronavirus cases among people who don’t have any symptoms and has been working with players and staff from the NBA. So far, the results have been accurate and similar to the nasal swabs for COVID-19, according to a preprint study published on medRxiv.
The research team wanted to get rid of the expensive collection tubes that other companies use to preserve the virus during processing, according to the Yale statement. They found that the virus is stable in saliva for long periods of time at warm temperatures and that special tubes aren’t necessary.
The FDA has authorized other saliva-based tests, according to ABC News, but SalivaDirect is the first that doesn’t require the extraction process used to test viral genetic material. Instead, the Yale process breaks down the saliva with an enzyme and applied heat. This type of testing could cost about $10, the Yale researchers said, and people can collect the saliva themselves under supervision.
“This, I hope, is a turning point,” Anne Wyllie, PhD, one of the lead researchers at Yale, told the news station.* “Expand testing capacity, inspire creativity and we can take competition to those labs charging a lot and bring prices down.”
This article first appeared on WebMD.com.
Correction, 8/25/20: An earlier version of this article misstated Dr. Wylie's academic degree.
A pandemic playbook for residency programs in the COVID-19 era: Lessons learned from ObGyn programs at the epicenter
The 2020 pandemic of coronavirus disease 2019 (COVID-19) has presented significant challenges to the health care workforce.1,2 As New York City and its environs became the epicenter of the pandemic in the United States, we continued to care for our patients while simultaneously maintaining the education and well-being of our residents.3 Keeping this balance significantly strained resources and presented new challenges for education and service in residency education. What first emerged as an acute emergency has become a chronic disruption in the clinical learning environment. Programs are working to respond to the critical patient needs while ensuring continued progress toward training goals.
Since pregnancy is one condition for which healthy patients continued to require both outpatient visits and inpatient hospitalization, volume was not anticipated to be significantly decreased on our units. Thus, our ObGyn residency programs sought to expeditiously restructure our workforce and educational methods to address the demands of the pandemic. We were aided in our efforts by the Accreditation Council for Graduate Medical Education (ACGME) Extraordinary Circumstances policy. Our institutions were deemed to be functioning at Stage 3 Pandemic Emergency Status, a state in which “the increase in volume and/or severity of illness creates an extraordinary circumstance where routine care, education, and delivery must be reconfigured to focus only on patient care.”4
As of May 18, 2020, 26% of residency and fellowship programs in the United States were under Stage 3 COVID-19 Pandemic Emergency Status.5 Accordingly, our patient care delivery and educational processes were reconfigured within the context of Stage 3 Status, governed by the overriding principles of ensuring appropriate resources and training, adhering to work hour limits, providing adequate supervision, and credentialing fellows to function in our core specialty.
As ObGyn education leaders from 5 academic medical centers within the COVID-19 epicenter, we present a summary of best practices, based on our experiences, for each of the 4 categories of Stage 3 Status outlined by the ACGME. In an era of globalization, we must learn from pandemics, a call made after the Ebola outbreak in 2015.6 We recognize that this type of disruption could happen again with a possible second wave of COVID-19 or another emerging disease.7 Thus, we emphasize “lessons learned” that are applicable to a wide range of residency training programs facing various clinical crises.
Ensuring adequate resources and training
Within the context of Stage 3 Status, residency programs have the flexibility to increase residents’ availability in the clinical care setting. However, programs must ensure the safety of both patients and residents.
Continue to: Measures to decrease risk of infection...
Measures to decrease risk of infection
One critical resource needed to protect patients and residents is personal protective equipment (PPE). Online instruction and in-person training were used to educate residents and staff on appropriate techniques for donning, doffing, and conserving PPE. Surgical teams were limited to 1 surgeon and 1 resident in each case. In an effort to limit direct contact with COVID-19 infected patients, the number of health care providers rounding on inpatients was restricted, and phone or video conversations were used for communication.
The workforce was modified to decrease exposure to infection and maintain a reserve of healthy residents who were working from home—anticipating that some residents would become ill and this reserve would be called for duty. Similar to other specialties, our programs organized the workforce by arranging residents into teams in which residents worked a number of shifts in a row.8-12 Regular block schedules were disrupted and non-core rotations were deferred.
As surgeries were canceled and outpatient visits curtailed, many rotations required less resident coverage. Residents were reassigned from rotations where clinical work was suspended to accommodate increased staffing needs in other areas, while accounting for residents who were ill or on leave for postexposure quarantine. Typically, residents worked 12-hour shifts for 3 to 6 days followed by several days off or days working remotely. This team-based strategy decreased the number of residents exposed to COVID-19 at one time, provided time for recuperation, encouraged camaraderie, and enabled residents working remotely to coordinate care and participate in telehealth without direct patient contact.
To minimize high-risk exposure of pregnant residents or residents with underlying health conditions, these residents also worked remotely. Similar to other specialties, it was important to determine essential resident duties and enlist assistance from other clinicians, such as fellows, nurse practitioners, physician assistants, and midwives.
To protect residents and patients, maximizing testing of patients for COVID-19 was an important strategy. Based on early experience at 1 center with patients who were initially asymptomatic but later developed symptoms and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), universal testing was implemented and endorsed by the New York State COVID-19 Maternity Task Force.13 Notably, 87.9% of patients who were positive for SARS-CoV-2 at the time of admission had no symptoms of COVID-19 at presentation. Because the asymptomatic carrier rate appears to be high in obstetric patients, testing of patients is paramount.3,14 Finally, suspending visitation (except for 1 support person) also was instrumental in decreasing the risk of infection to residents.13
Resources for residents with COVID-19
This pandemic placed residency program directors in an unusual situation as frontline caregivers for their own residents. It was imperative to track residents with physical symptoms, conduct testing when possible, and follow the course of residents with confirmed or suspected COVID-19. As serious illness and death have been reported among otherwise healthy young people, we ensured that our homebound residents were frequently monitored.15 At several of our centers, residents with COVID-19 from any program who chose to separate from their families were provided with alternative housing accommodations. In addition, some of our graduate medical education offices identified specific physicians to care for residents with COVID-19 who did not require hospitalization.
Continue to: Deployment to other specialties...
Deployment to other specialties
Several hospitals in the United States redeployed residents because of staffing shortages in high-impact settings.12 It was important for ObGyns to emphasize that the labor and delivery unit functions as the emergency ward for pregnant women, and that ObGyn residents possess skills specific to the care of these patients.
For our departments, we highlighted that external redeployment could adversely affect our workforce restructuring and, ultimately, patient care. We focused efforts on internal deployment or reassignment as much as possible. Some faculty and fellows in nonobstetric subspecialty areas were redirected to provide care on our inpatient obstetric services.
Educating residents
To maintain educational efforts with social distancing, we used videoconferencing to preserve the protected didactic education time that existed for our residents before the pandemic. This regularly scheduled, nonclinical time also was utilized to instruct residents on the rapidly changing clinical guidelines and to disseminate information about new institutional policies and procedures, ensuring that residents were adequately prepared for their new clinical work.
Work hour requirements
The ACGME requires that work hour limitations remain unchanged during Stage 3 Pandemic Emergency Status. As the pandemic presented new challenges and stressors for residents inside and outside the workplace, ensuring adequate time off to rest and recover was critical for maintaining the resident workforce’s health and wellness.
Thus, our workforce restructuring plans accounted for work hour limitations. As detailed above, the restructuring was accomplished by cohorting residents into small teams that remained unchanged for several weeks. Most shifts were limited to 12 hours, residents continued to be assigned at least 1 day off each week, and daily schedules were structured to ensure at least 10 hours off between shifts. Time spent working remotely was included in work hour calculations.
In addition, residents on “jeopardy” who were available for those who needed to be removed from direct patient care were given at least 1 day off per week in which they could not be pulled for clinical duty. Finally, prolonged inpatient assignments were limited; after these assignments, residents were given increased time for rest and recuperation.
Ensuring adequate supervision
The expectation during Stage 3 Pandemic Emergency Status is that residents, with adequate supervision, provide care that is appropriate for their level of training. To adequately and safely supervise residents, faculty needed training to remain well informed about the clinical care of COVID-19 patients. This was accomplished through frequent communication and consultation with colleagues in infectious disease, occupational health, and guidance from national organizations, such as the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, and information from our state health departments.
Faculty members were trained in safe donning and doffing of PPE and infection control strategies to ensure they could safely oversee and train residents in these practices. Faculty schedules were significantly altered to ensure an adequate workforce and adequate resident supervision. Faculty efforts were focused on areas of critical need—in our case inpatient obstetrics—with a smaller workforce assigned to outpatient services and inpatient gynecology and gynecologic oncology. Many ObGyn subspecialist faculty were redeployed to general ObGyn inpatient units, thus permitting appropriate resident supervision at all times. In the outpatient setting, faculty adjusted to the changing demands and learned to conduct and supervise telehealth visits.
Finally, for those whose residents were deployed to other services (for example, internal medicine, emergency medicine, or critical care), supervision became paramount. We checked in with our deployed residents daily to be sure that their supervision on those services was adequate. Considering the extreme complexity, rapidly changing understanding of the disease, and often tragic patient outcomes, it was essential to ensure appropriate support and supervision on “off service” deployment.
Continue to: Fellows functioning in core specialty...
Fellows functioning in core specialty
Anticipating the increased need for clinicians on the obstetric services, fellows in subspecialty areas were granted emergency privileges to act as attending faculty in the core specialty, supervising residents and providing patient care. On the other hand, some of those fellows, primarily in gynecologic oncology, were externally redeployed out of core specialty to internal medicine and critical care units. Careful consideration of the fellows’ needs for supervision and support in these roles was essential, and similar support measures that were put in place for our residents were offered to fellows.
In conclusion
The COVID-19 pandemic has presented diverse and complex challenges to the entire health care workforce. Because this crisis is widespread and likely will be lengthy, a sustained and organized response is required.16 We have highlighted unique challenges specific to residency programs and presented collective best practices from our experiences in ObGyn navigating these obstacles, which are applicable to many other programs.
The flexibility and relief afforded by the ACGME Stage 3 Pandemic Emergency Status designation allowed us to meet the needs of the surge of patients that required care while we maintained our educational framework and tenets of providing adequate resources and training, working within the confines of safe work hours, ensuring proper supervision, and granting attending privileges to fellows in their core specialty. ●
- Panahi L, Amiri M, Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Arch Acad Emerg Med. 2020;8e34.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415-426.
- Sutton D, Fuchs K, D'Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163-2164.
- Accreditation Council for Graduate Medical Education. Three stages of GME during the COVID-19 pandemic. https://www.acgme.org/COVID-19/Three-Stages-of-GME-During-the-COVID-19-Pandemic. Accessed May 28, 2020.
- Accreditation Council for Graduate Medical Education. Emergency category maps/5-18-20: percentage of residents in each state/territory under pandemic emergency status. Percentage of residency and fellowship programs under ACGME COVID-19 pandemic emergency status (stage 3). https://dl.acgme.org/learn/course/sponsoring-institution-idea-exchange/emergency-category-maps/5-18-20-percentage-of-residents-in-each-state-territory-under-pandemic-emergency-status. Accessed May 28, 2020.
- Gates B. The next epidemic--lessons from Ebola. N Engl J Med. 2015;372:1381-1384.
- Pepe D, Martinello RA, Juthani-Mehta M. Involving physicians-in-training in the care of patients during epidemics. J Grad Med Educ. 2019;11:632-634.
- Crosby DL, Sharma A. Insights on otolaryngology residency training during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020;163:38-41.
- Kim CS, Lynch JB, Seth C, et al. One academic health system's early (and ongoing) experience responding to COVID-19: recommendations from the initial epicenter of the pandemic in the United States. Acad Med. 2020;95:1146-1148.
- Kogan M, Klein SE, Hannon CP, et al. Orthopaedic education during the COVID-19 pandemic. J Am Acad Orthop Surg. 2020; 28:e456-e464.
- Vargo E, Ali M, Henry F, et al. Cleveland Clinic Akron general urology residency program's COVID-19 experience. Urology. 2020;140:1-3.
- Zarzaur BL, Stahl CC, Greenberg JA, et al. Blueprint for restructuring a department of surgery in concert with the health care system during a pandemic: the University of Wisconsin experience. JAMA Surg. 2020. doi: 10.1001/jamasurg.2020.1386.
- New York State COVID-19 Maternity Task Force. Recommendations to the governor to promote increased choice and access to safe maternity care during the COVID-19 pandemic. https://www.governor.ny.gov/sites/governor.ny.gov/files/atoms/files/042920_CMTF_Recommendations.pdf. Accessed May 28, 2020.
- Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in southern Connecticut. JAMA. 2020;323:2520-2522.
- CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)--United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-346.
- Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860-868.
The 2020 pandemic of coronavirus disease 2019 (COVID-19) has presented significant challenges to the health care workforce.1,2 As New York City and its environs became the epicenter of the pandemic in the United States, we continued to care for our patients while simultaneously maintaining the education and well-being of our residents.3 Keeping this balance significantly strained resources and presented new challenges for education and service in residency education. What first emerged as an acute emergency has become a chronic disruption in the clinical learning environment. Programs are working to respond to the critical patient needs while ensuring continued progress toward training goals.
Since pregnancy is one condition for which healthy patients continued to require both outpatient visits and inpatient hospitalization, volume was not anticipated to be significantly decreased on our units. Thus, our ObGyn residency programs sought to expeditiously restructure our workforce and educational methods to address the demands of the pandemic. We were aided in our efforts by the Accreditation Council for Graduate Medical Education (ACGME) Extraordinary Circumstances policy. Our institutions were deemed to be functioning at Stage 3 Pandemic Emergency Status, a state in which “the increase in volume and/or severity of illness creates an extraordinary circumstance where routine care, education, and delivery must be reconfigured to focus only on patient care.”4
As of May 18, 2020, 26% of residency and fellowship programs in the United States were under Stage 3 COVID-19 Pandemic Emergency Status.5 Accordingly, our patient care delivery and educational processes were reconfigured within the context of Stage 3 Status, governed by the overriding principles of ensuring appropriate resources and training, adhering to work hour limits, providing adequate supervision, and credentialing fellows to function in our core specialty.
As ObGyn education leaders from 5 academic medical centers within the COVID-19 epicenter, we present a summary of best practices, based on our experiences, for each of the 4 categories of Stage 3 Status outlined by the ACGME. In an era of globalization, we must learn from pandemics, a call made after the Ebola outbreak in 2015.6 We recognize that this type of disruption could happen again with a possible second wave of COVID-19 or another emerging disease.7 Thus, we emphasize “lessons learned” that are applicable to a wide range of residency training programs facing various clinical crises.
Ensuring adequate resources and training
Within the context of Stage 3 Status, residency programs have the flexibility to increase residents’ availability in the clinical care setting. However, programs must ensure the safety of both patients and residents.
Continue to: Measures to decrease risk of infection...
Measures to decrease risk of infection
One critical resource needed to protect patients and residents is personal protective equipment (PPE). Online instruction and in-person training were used to educate residents and staff on appropriate techniques for donning, doffing, and conserving PPE. Surgical teams were limited to 1 surgeon and 1 resident in each case. In an effort to limit direct contact with COVID-19 infected patients, the number of health care providers rounding on inpatients was restricted, and phone or video conversations were used for communication.
The workforce was modified to decrease exposure to infection and maintain a reserve of healthy residents who were working from home—anticipating that some residents would become ill and this reserve would be called for duty. Similar to other specialties, our programs organized the workforce by arranging residents into teams in which residents worked a number of shifts in a row.8-12 Regular block schedules were disrupted and non-core rotations were deferred.
As surgeries were canceled and outpatient visits curtailed, many rotations required less resident coverage. Residents were reassigned from rotations where clinical work was suspended to accommodate increased staffing needs in other areas, while accounting for residents who were ill or on leave for postexposure quarantine. Typically, residents worked 12-hour shifts for 3 to 6 days followed by several days off or days working remotely. This team-based strategy decreased the number of residents exposed to COVID-19 at one time, provided time for recuperation, encouraged camaraderie, and enabled residents working remotely to coordinate care and participate in telehealth without direct patient contact.
To minimize high-risk exposure of pregnant residents or residents with underlying health conditions, these residents also worked remotely. Similar to other specialties, it was important to determine essential resident duties and enlist assistance from other clinicians, such as fellows, nurse practitioners, physician assistants, and midwives.
To protect residents and patients, maximizing testing of patients for COVID-19 was an important strategy. Based on early experience at 1 center with patients who were initially asymptomatic but later developed symptoms and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), universal testing was implemented and endorsed by the New York State COVID-19 Maternity Task Force.13 Notably, 87.9% of patients who were positive for SARS-CoV-2 at the time of admission had no symptoms of COVID-19 at presentation. Because the asymptomatic carrier rate appears to be high in obstetric patients, testing of patients is paramount.3,14 Finally, suspending visitation (except for 1 support person) also was instrumental in decreasing the risk of infection to residents.13
Resources for residents with COVID-19
This pandemic placed residency program directors in an unusual situation as frontline caregivers for their own residents. It was imperative to track residents with physical symptoms, conduct testing when possible, and follow the course of residents with confirmed or suspected COVID-19. As serious illness and death have been reported among otherwise healthy young people, we ensured that our homebound residents were frequently monitored.15 At several of our centers, residents with COVID-19 from any program who chose to separate from their families were provided with alternative housing accommodations. In addition, some of our graduate medical education offices identified specific physicians to care for residents with COVID-19 who did not require hospitalization.
Continue to: Deployment to other specialties...
Deployment to other specialties
Several hospitals in the United States redeployed residents because of staffing shortages in high-impact settings.12 It was important for ObGyns to emphasize that the labor and delivery unit functions as the emergency ward for pregnant women, and that ObGyn residents possess skills specific to the care of these patients.
For our departments, we highlighted that external redeployment could adversely affect our workforce restructuring and, ultimately, patient care. We focused efforts on internal deployment or reassignment as much as possible. Some faculty and fellows in nonobstetric subspecialty areas were redirected to provide care on our inpatient obstetric services.
Educating residents
To maintain educational efforts with social distancing, we used videoconferencing to preserve the protected didactic education time that existed for our residents before the pandemic. This regularly scheduled, nonclinical time also was utilized to instruct residents on the rapidly changing clinical guidelines and to disseminate information about new institutional policies and procedures, ensuring that residents were adequately prepared for their new clinical work.
Work hour requirements
The ACGME requires that work hour limitations remain unchanged during Stage 3 Pandemic Emergency Status. As the pandemic presented new challenges and stressors for residents inside and outside the workplace, ensuring adequate time off to rest and recover was critical for maintaining the resident workforce’s health and wellness.
Thus, our workforce restructuring plans accounted for work hour limitations. As detailed above, the restructuring was accomplished by cohorting residents into small teams that remained unchanged for several weeks. Most shifts were limited to 12 hours, residents continued to be assigned at least 1 day off each week, and daily schedules were structured to ensure at least 10 hours off between shifts. Time spent working remotely was included in work hour calculations.
In addition, residents on “jeopardy” who were available for those who needed to be removed from direct patient care were given at least 1 day off per week in which they could not be pulled for clinical duty. Finally, prolonged inpatient assignments were limited; after these assignments, residents were given increased time for rest and recuperation.
Ensuring adequate supervision
The expectation during Stage 3 Pandemic Emergency Status is that residents, with adequate supervision, provide care that is appropriate for their level of training. To adequately and safely supervise residents, faculty needed training to remain well informed about the clinical care of COVID-19 patients. This was accomplished through frequent communication and consultation with colleagues in infectious disease, occupational health, and guidance from national organizations, such as the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, and information from our state health departments.
Faculty members were trained in safe donning and doffing of PPE and infection control strategies to ensure they could safely oversee and train residents in these practices. Faculty schedules were significantly altered to ensure an adequate workforce and adequate resident supervision. Faculty efforts were focused on areas of critical need—in our case inpatient obstetrics—with a smaller workforce assigned to outpatient services and inpatient gynecology and gynecologic oncology. Many ObGyn subspecialist faculty were redeployed to general ObGyn inpatient units, thus permitting appropriate resident supervision at all times. In the outpatient setting, faculty adjusted to the changing demands and learned to conduct and supervise telehealth visits.
Finally, for those whose residents were deployed to other services (for example, internal medicine, emergency medicine, or critical care), supervision became paramount. We checked in with our deployed residents daily to be sure that their supervision on those services was adequate. Considering the extreme complexity, rapidly changing understanding of the disease, and often tragic patient outcomes, it was essential to ensure appropriate support and supervision on “off service” deployment.
Continue to: Fellows functioning in core specialty...
Fellows functioning in core specialty
Anticipating the increased need for clinicians on the obstetric services, fellows in subspecialty areas were granted emergency privileges to act as attending faculty in the core specialty, supervising residents and providing patient care. On the other hand, some of those fellows, primarily in gynecologic oncology, were externally redeployed out of core specialty to internal medicine and critical care units. Careful consideration of the fellows’ needs for supervision and support in these roles was essential, and similar support measures that were put in place for our residents were offered to fellows.
In conclusion
The COVID-19 pandemic has presented diverse and complex challenges to the entire health care workforce. Because this crisis is widespread and likely will be lengthy, a sustained and organized response is required.16 We have highlighted unique challenges specific to residency programs and presented collective best practices from our experiences in ObGyn navigating these obstacles, which are applicable to many other programs.
The flexibility and relief afforded by the ACGME Stage 3 Pandemic Emergency Status designation allowed us to meet the needs of the surge of patients that required care while we maintained our educational framework and tenets of providing adequate resources and training, working within the confines of safe work hours, ensuring proper supervision, and granting attending privileges to fellows in their core specialty. ●
The 2020 pandemic of coronavirus disease 2019 (COVID-19) has presented significant challenges to the health care workforce.1,2 As New York City and its environs became the epicenter of the pandemic in the United States, we continued to care for our patients while simultaneously maintaining the education and well-being of our residents.3 Keeping this balance significantly strained resources and presented new challenges for education and service in residency education. What first emerged as an acute emergency has become a chronic disruption in the clinical learning environment. Programs are working to respond to the critical patient needs while ensuring continued progress toward training goals.
Since pregnancy is one condition for which healthy patients continued to require both outpatient visits and inpatient hospitalization, volume was not anticipated to be significantly decreased on our units. Thus, our ObGyn residency programs sought to expeditiously restructure our workforce and educational methods to address the demands of the pandemic. We were aided in our efforts by the Accreditation Council for Graduate Medical Education (ACGME) Extraordinary Circumstances policy. Our institutions were deemed to be functioning at Stage 3 Pandemic Emergency Status, a state in which “the increase in volume and/or severity of illness creates an extraordinary circumstance where routine care, education, and delivery must be reconfigured to focus only on patient care.”4
As of May 18, 2020, 26% of residency and fellowship programs in the United States were under Stage 3 COVID-19 Pandemic Emergency Status.5 Accordingly, our patient care delivery and educational processes were reconfigured within the context of Stage 3 Status, governed by the overriding principles of ensuring appropriate resources and training, adhering to work hour limits, providing adequate supervision, and credentialing fellows to function in our core specialty.
As ObGyn education leaders from 5 academic medical centers within the COVID-19 epicenter, we present a summary of best practices, based on our experiences, for each of the 4 categories of Stage 3 Status outlined by the ACGME. In an era of globalization, we must learn from pandemics, a call made after the Ebola outbreak in 2015.6 We recognize that this type of disruption could happen again with a possible second wave of COVID-19 or another emerging disease.7 Thus, we emphasize “lessons learned” that are applicable to a wide range of residency training programs facing various clinical crises.
Ensuring adequate resources and training
Within the context of Stage 3 Status, residency programs have the flexibility to increase residents’ availability in the clinical care setting. However, programs must ensure the safety of both patients and residents.
Continue to: Measures to decrease risk of infection...
Measures to decrease risk of infection
One critical resource needed to protect patients and residents is personal protective equipment (PPE). Online instruction and in-person training were used to educate residents and staff on appropriate techniques for donning, doffing, and conserving PPE. Surgical teams were limited to 1 surgeon and 1 resident in each case. In an effort to limit direct contact with COVID-19 infected patients, the number of health care providers rounding on inpatients was restricted, and phone or video conversations were used for communication.
The workforce was modified to decrease exposure to infection and maintain a reserve of healthy residents who were working from home—anticipating that some residents would become ill and this reserve would be called for duty. Similar to other specialties, our programs organized the workforce by arranging residents into teams in which residents worked a number of shifts in a row.8-12 Regular block schedules were disrupted and non-core rotations were deferred.
As surgeries were canceled and outpatient visits curtailed, many rotations required less resident coverage. Residents were reassigned from rotations where clinical work was suspended to accommodate increased staffing needs in other areas, while accounting for residents who were ill or on leave for postexposure quarantine. Typically, residents worked 12-hour shifts for 3 to 6 days followed by several days off or days working remotely. This team-based strategy decreased the number of residents exposed to COVID-19 at one time, provided time for recuperation, encouraged camaraderie, and enabled residents working remotely to coordinate care and participate in telehealth without direct patient contact.
To minimize high-risk exposure of pregnant residents or residents with underlying health conditions, these residents also worked remotely. Similar to other specialties, it was important to determine essential resident duties and enlist assistance from other clinicians, such as fellows, nurse practitioners, physician assistants, and midwives.
To protect residents and patients, maximizing testing of patients for COVID-19 was an important strategy. Based on early experience at 1 center with patients who were initially asymptomatic but later developed symptoms and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), universal testing was implemented and endorsed by the New York State COVID-19 Maternity Task Force.13 Notably, 87.9% of patients who were positive for SARS-CoV-2 at the time of admission had no symptoms of COVID-19 at presentation. Because the asymptomatic carrier rate appears to be high in obstetric patients, testing of patients is paramount.3,14 Finally, suspending visitation (except for 1 support person) also was instrumental in decreasing the risk of infection to residents.13
Resources for residents with COVID-19
This pandemic placed residency program directors in an unusual situation as frontline caregivers for their own residents. It was imperative to track residents with physical symptoms, conduct testing when possible, and follow the course of residents with confirmed or suspected COVID-19. As serious illness and death have been reported among otherwise healthy young people, we ensured that our homebound residents were frequently monitored.15 At several of our centers, residents with COVID-19 from any program who chose to separate from their families were provided with alternative housing accommodations. In addition, some of our graduate medical education offices identified specific physicians to care for residents with COVID-19 who did not require hospitalization.
Continue to: Deployment to other specialties...
Deployment to other specialties
Several hospitals in the United States redeployed residents because of staffing shortages in high-impact settings.12 It was important for ObGyns to emphasize that the labor and delivery unit functions as the emergency ward for pregnant women, and that ObGyn residents possess skills specific to the care of these patients.
For our departments, we highlighted that external redeployment could adversely affect our workforce restructuring and, ultimately, patient care. We focused efforts on internal deployment or reassignment as much as possible. Some faculty and fellows in nonobstetric subspecialty areas were redirected to provide care on our inpatient obstetric services.
Educating residents
To maintain educational efforts with social distancing, we used videoconferencing to preserve the protected didactic education time that existed for our residents before the pandemic. This regularly scheduled, nonclinical time also was utilized to instruct residents on the rapidly changing clinical guidelines and to disseminate information about new institutional policies and procedures, ensuring that residents were adequately prepared for their new clinical work.
Work hour requirements
The ACGME requires that work hour limitations remain unchanged during Stage 3 Pandemic Emergency Status. As the pandemic presented new challenges and stressors for residents inside and outside the workplace, ensuring adequate time off to rest and recover was critical for maintaining the resident workforce’s health and wellness.
Thus, our workforce restructuring plans accounted for work hour limitations. As detailed above, the restructuring was accomplished by cohorting residents into small teams that remained unchanged for several weeks. Most shifts were limited to 12 hours, residents continued to be assigned at least 1 day off each week, and daily schedules were structured to ensure at least 10 hours off between shifts. Time spent working remotely was included in work hour calculations.
In addition, residents on “jeopardy” who were available for those who needed to be removed from direct patient care were given at least 1 day off per week in which they could not be pulled for clinical duty. Finally, prolonged inpatient assignments were limited; after these assignments, residents were given increased time for rest and recuperation.
Ensuring adequate supervision
The expectation during Stage 3 Pandemic Emergency Status is that residents, with adequate supervision, provide care that is appropriate for their level of training. To adequately and safely supervise residents, faculty needed training to remain well informed about the clinical care of COVID-19 patients. This was accomplished through frequent communication and consultation with colleagues in infectious disease, occupational health, and guidance from national organizations, such as the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, and information from our state health departments.
Faculty members were trained in safe donning and doffing of PPE and infection control strategies to ensure they could safely oversee and train residents in these practices. Faculty schedules were significantly altered to ensure an adequate workforce and adequate resident supervision. Faculty efforts were focused on areas of critical need—in our case inpatient obstetrics—with a smaller workforce assigned to outpatient services and inpatient gynecology and gynecologic oncology. Many ObGyn subspecialist faculty were redeployed to general ObGyn inpatient units, thus permitting appropriate resident supervision at all times. In the outpatient setting, faculty adjusted to the changing demands and learned to conduct and supervise telehealth visits.
Finally, for those whose residents were deployed to other services (for example, internal medicine, emergency medicine, or critical care), supervision became paramount. We checked in with our deployed residents daily to be sure that their supervision on those services was adequate. Considering the extreme complexity, rapidly changing understanding of the disease, and often tragic patient outcomes, it was essential to ensure appropriate support and supervision on “off service” deployment.
Continue to: Fellows functioning in core specialty...
Fellows functioning in core specialty
Anticipating the increased need for clinicians on the obstetric services, fellows in subspecialty areas were granted emergency privileges to act as attending faculty in the core specialty, supervising residents and providing patient care. On the other hand, some of those fellows, primarily in gynecologic oncology, were externally redeployed out of core specialty to internal medicine and critical care units. Careful consideration of the fellows’ needs for supervision and support in these roles was essential, and similar support measures that were put in place for our residents were offered to fellows.
In conclusion
The COVID-19 pandemic has presented diverse and complex challenges to the entire health care workforce. Because this crisis is widespread and likely will be lengthy, a sustained and organized response is required.16 We have highlighted unique challenges specific to residency programs and presented collective best practices from our experiences in ObGyn navigating these obstacles, which are applicable to many other programs.
The flexibility and relief afforded by the ACGME Stage 3 Pandemic Emergency Status designation allowed us to meet the needs of the surge of patients that required care while we maintained our educational framework and tenets of providing adequate resources and training, working within the confines of safe work hours, ensuring proper supervision, and granting attending privileges to fellows in their core specialty. ●
- Panahi L, Amiri M, Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Arch Acad Emerg Med. 2020;8e34.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415-426.
- Sutton D, Fuchs K, D'Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163-2164.
- Accreditation Council for Graduate Medical Education. Three stages of GME during the COVID-19 pandemic. https://www.acgme.org/COVID-19/Three-Stages-of-GME-During-the-COVID-19-Pandemic. Accessed May 28, 2020.
- Accreditation Council for Graduate Medical Education. Emergency category maps/5-18-20: percentage of residents in each state/territory under pandemic emergency status. Percentage of residency and fellowship programs under ACGME COVID-19 pandemic emergency status (stage 3). https://dl.acgme.org/learn/course/sponsoring-institution-idea-exchange/emergency-category-maps/5-18-20-percentage-of-residents-in-each-state-territory-under-pandemic-emergency-status. Accessed May 28, 2020.
- Gates B. The next epidemic--lessons from Ebola. N Engl J Med. 2015;372:1381-1384.
- Pepe D, Martinello RA, Juthani-Mehta M. Involving physicians-in-training in the care of patients during epidemics. J Grad Med Educ. 2019;11:632-634.
- Crosby DL, Sharma A. Insights on otolaryngology residency training during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020;163:38-41.
- Kim CS, Lynch JB, Seth C, et al. One academic health system's early (and ongoing) experience responding to COVID-19: recommendations from the initial epicenter of the pandemic in the United States. Acad Med. 2020;95:1146-1148.
- Kogan M, Klein SE, Hannon CP, et al. Orthopaedic education during the COVID-19 pandemic. J Am Acad Orthop Surg. 2020; 28:e456-e464.
- Vargo E, Ali M, Henry F, et al. Cleveland Clinic Akron general urology residency program's COVID-19 experience. Urology. 2020;140:1-3.
- Zarzaur BL, Stahl CC, Greenberg JA, et al. Blueprint for restructuring a department of surgery in concert with the health care system during a pandemic: the University of Wisconsin experience. JAMA Surg. 2020. doi: 10.1001/jamasurg.2020.1386.
- New York State COVID-19 Maternity Task Force. Recommendations to the governor to promote increased choice and access to safe maternity care during the COVID-19 pandemic. https://www.governor.ny.gov/sites/governor.ny.gov/files/atoms/files/042920_CMTF_Recommendations.pdf. Accessed May 28, 2020.
- Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in southern Connecticut. JAMA. 2020;323:2520-2522.
- CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)--United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-346.
- Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860-868.
- Panahi L, Amiri M, Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Arch Acad Emerg Med. 2020;8e34.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415-426.
- Sutton D, Fuchs K, D'Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163-2164.
- Accreditation Council for Graduate Medical Education. Three stages of GME during the COVID-19 pandemic. https://www.acgme.org/COVID-19/Three-Stages-of-GME-During-the-COVID-19-Pandemic. Accessed May 28, 2020.
- Accreditation Council for Graduate Medical Education. Emergency category maps/5-18-20: percentage of residents in each state/territory under pandemic emergency status. Percentage of residency and fellowship programs under ACGME COVID-19 pandemic emergency status (stage 3). https://dl.acgme.org/learn/course/sponsoring-institution-idea-exchange/emergency-category-maps/5-18-20-percentage-of-residents-in-each-state-territory-under-pandemic-emergency-status. Accessed May 28, 2020.
- Gates B. The next epidemic--lessons from Ebola. N Engl J Med. 2015;372:1381-1384.
- Pepe D, Martinello RA, Juthani-Mehta M. Involving physicians-in-training in the care of patients during epidemics. J Grad Med Educ. 2019;11:632-634.
- Crosby DL, Sharma A. Insights on otolaryngology residency training during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020;163:38-41.
- Kim CS, Lynch JB, Seth C, et al. One academic health system's early (and ongoing) experience responding to COVID-19: recommendations from the initial epicenter of the pandemic in the United States. Acad Med. 2020;95:1146-1148.
- Kogan M, Klein SE, Hannon CP, et al. Orthopaedic education during the COVID-19 pandemic. J Am Acad Orthop Surg. 2020; 28:e456-e464.
- Vargo E, Ali M, Henry F, et al. Cleveland Clinic Akron general urology residency program's COVID-19 experience. Urology. 2020;140:1-3.
- Zarzaur BL, Stahl CC, Greenberg JA, et al. Blueprint for restructuring a department of surgery in concert with the health care system during a pandemic: the University of Wisconsin experience. JAMA Surg. 2020. doi: 10.1001/jamasurg.2020.1386.
- New York State COVID-19 Maternity Task Force. Recommendations to the governor to promote increased choice and access to safe maternity care during the COVID-19 pandemic. https://www.governor.ny.gov/sites/governor.ny.gov/files/atoms/files/042920_CMTF_Recommendations.pdf. Accessed May 28, 2020.
- Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in southern Connecticut. JAMA. 2020;323:2520-2522.
- CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)--United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-346.
- Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860-868.
Comment & Controversy
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Is it reasonable to choose the age of 40 for proposing an anticipation of labor induction?
In physiologic ongoing pregnancies (whether they are spontaneous or autologous in vitro fertilization [IVF] or heterologous IVF), the evidence for anticipating labor induction based upon the only factor of age (after 40 years) is missing. Nonetheless, the number of women becoming pregnant at an older age is expected to increase, and from my perspective, to induce all physiologic pregnancies at term by 41 weeks and 5 days’ gestation does not appear to be best practice. I favor the idea of all women aged 40 and older to start labor induction earlier (for instance, to offer labor induction, with proper informed consent, by 41+ 0 and not 41+ 5 through 42+ 0 weeks of pregnancy).
Luca Bernardini, MD
La Spezia, Italy
Dr. Barbieri responds
At Brigham and Women’s Hospital in Boston, Massachusetts, our approach is to offer women ≥40 years of age induction of labor (IOL) at 39 weeks’ gestation, unless there is an obstetric contraindication to IOL. We believe that IOL at 39 weeks’ gestation is associated with a reduced risk of both cesarean delivery and a new diagnosis of hypertension.1
Reference
- Grobman WA, Rice MM, Reddy, UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
What is the optimal hormonal treatment for women with polycystic ovary syndrome?
ROBERT L. BARBIERI, MD
(EDITORIAL; JANUARY 2020)
OCs and spironolactone study
I often recommend oral contraceptives (OCs) containing drospirenone for patients with polycyctic ovary syndrome (PCOS)-associated mild acne and hirsutism—since OCs are already approved by the US Food and Drug Administration for acne, with similar effects as spironolactone. My patients seem to do well on an OC, and require only one medication. Of course, I would add spironolactone to the treatment regimen and switch OCs if she was not responding well.
Michael T. Cane, MD
Arlington, Texas
Dr. Barbieri responds
The Endocrine Society agrees with Dr. Cane’s approach, recommending the initiation of monotherapy with an estrogen-progestin followed by the addition of spironolactone if 6 months of monotherapy produces insufficient improvement in dermatologic symptoms of PCOS, including hirsutism and acne. Most contraceptives contain 3 mg or 4 mg of drospirenone, which is thought to have antiandrogenic effects similar to spironolactone 25 mg. I believe that spironolactone 100 mg provides more complete and rapid resolution of the dermatologic symptoms caused by PCOS. Hence, I initiate both an estrogen-progestin contraceptive with spironolactone.
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Is it reasonable to choose the age of 40 for proposing an anticipation of labor induction?
In physiologic ongoing pregnancies (whether they are spontaneous or autologous in vitro fertilization [IVF] or heterologous IVF), the evidence for anticipating labor induction based upon the only factor of age (after 40 years) is missing. Nonetheless, the number of women becoming pregnant at an older age is expected to increase, and from my perspective, to induce all physiologic pregnancies at term by 41 weeks and 5 days’ gestation does not appear to be best practice. I favor the idea of all women aged 40 and older to start labor induction earlier (for instance, to offer labor induction, with proper informed consent, by 41+ 0 and not 41+ 5 through 42+ 0 weeks of pregnancy).
Luca Bernardini, MD
La Spezia, Italy
Dr. Barbieri responds
At Brigham and Women’s Hospital in Boston, Massachusetts, our approach is to offer women ≥40 years of age induction of labor (IOL) at 39 weeks’ gestation, unless there is an obstetric contraindication to IOL. We believe that IOL at 39 weeks’ gestation is associated with a reduced risk of both cesarean delivery and a new diagnosis of hypertension.1
Reference
- Grobman WA, Rice MM, Reddy, UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
What is the optimal hormonal treatment for women with polycystic ovary syndrome?
ROBERT L. BARBIERI, MD
(EDITORIAL; JANUARY 2020)
OCs and spironolactone study
I often recommend oral contraceptives (OCs) containing drospirenone for patients with polycyctic ovary syndrome (PCOS)-associated mild acne and hirsutism—since OCs are already approved by the US Food and Drug Administration for acne, with similar effects as spironolactone. My patients seem to do well on an OC, and require only one medication. Of course, I would add spironolactone to the treatment regimen and switch OCs if she was not responding well.
Michael T. Cane, MD
Arlington, Texas
Dr. Barbieri responds
The Endocrine Society agrees with Dr. Cane’s approach, recommending the initiation of monotherapy with an estrogen-progestin followed by the addition of spironolactone if 6 months of monotherapy produces insufficient improvement in dermatologic symptoms of PCOS, including hirsutism and acne. Most contraceptives contain 3 mg or 4 mg of drospirenone, which is thought to have antiandrogenic effects similar to spironolactone 25 mg. I believe that spironolactone 100 mg provides more complete and rapid resolution of the dermatologic symptoms caused by PCOS. Hence, I initiate both an estrogen-progestin contraceptive with spironolactone.
How do you feel about expectantly managing a well-dated pregnancy past 41 weeks’ gestation?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Is it reasonable to choose the age of 40 for proposing an anticipation of labor induction?
In physiologic ongoing pregnancies (whether they are spontaneous or autologous in vitro fertilization [IVF] or heterologous IVF), the evidence for anticipating labor induction based upon the only factor of age (after 40 years) is missing. Nonetheless, the number of women becoming pregnant at an older age is expected to increase, and from my perspective, to induce all physiologic pregnancies at term by 41 weeks and 5 days’ gestation does not appear to be best practice. I favor the idea of all women aged 40 and older to start labor induction earlier (for instance, to offer labor induction, with proper informed consent, by 41+ 0 and not 41+ 5 through 42+ 0 weeks of pregnancy).
Luca Bernardini, MD
La Spezia, Italy
Dr. Barbieri responds
At Brigham and Women’s Hospital in Boston, Massachusetts, our approach is to offer women ≥40 years of age induction of labor (IOL) at 39 weeks’ gestation, unless there is an obstetric contraindication to IOL. We believe that IOL at 39 weeks’ gestation is associated with a reduced risk of both cesarean delivery and a new diagnosis of hypertension.1
Reference
- Grobman WA, Rice MM, Reddy, UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
What is the optimal hormonal treatment for women with polycystic ovary syndrome?
ROBERT L. BARBIERI, MD
(EDITORIAL; JANUARY 2020)
OCs and spironolactone study
I often recommend oral contraceptives (OCs) containing drospirenone for patients with polycyctic ovary syndrome (PCOS)-associated mild acne and hirsutism—since OCs are already approved by the US Food and Drug Administration for acne, with similar effects as spironolactone. My patients seem to do well on an OC, and require only one medication. Of course, I would add spironolactone to the treatment regimen and switch OCs if she was not responding well.
Michael T. Cane, MD
Arlington, Texas
Dr. Barbieri responds
The Endocrine Society agrees with Dr. Cane’s approach, recommending the initiation of monotherapy with an estrogen-progestin followed by the addition of spironolactone if 6 months of monotherapy produces insufficient improvement in dermatologic symptoms of PCOS, including hirsutism and acne. Most contraceptives contain 3 mg or 4 mg of drospirenone, which is thought to have antiandrogenic effects similar to spironolactone 25 mg. I believe that spironolactone 100 mg provides more complete and rapid resolution of the dermatologic symptoms caused by PCOS. Hence, I initiate both an estrogen-progestin contraceptive with spironolactone.
Pregnancy of unknown location: Evidence-based evaluation and management
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.
Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.
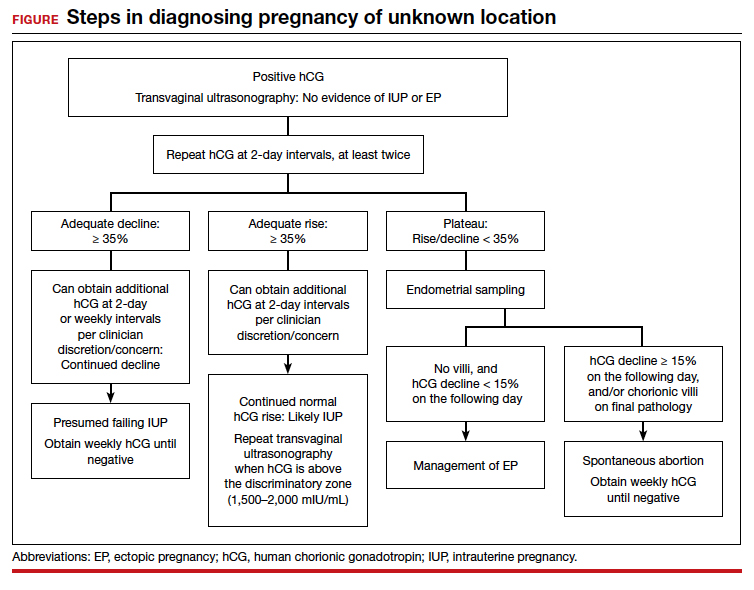
Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.

Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.

Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
CASE Woman with bleeding in early pregnancy
A 31-year-old woman (G1P0) presents to the local emergency department (ED) due to bleeding in pregnancy. She reports a prior open appendectomy for ruptured appendix; she denies a history of sexually transmitted infections, smoking, and contraception use. She reports having regular menstrual cycles and trying to conceive with her husband for 18 months without success until now.
The patient reports that the previous week she took a home pregnancy test that was positive; she endorses having dark brown spotting for the past 2 days but denies pain. Based on the date of her last menstrual period, gestational age is estimated to be 5 weeks and 1 day. Her human chorionic gonadotropin (hCG) level is 1,670 mIU/mL. Transvaginal ultrasonography demonstrates a normal uterus with an endometrial thickness of 10 mm, no evidence of an intrauterine pregnancy (IUP), normal adnexa bilaterally, and scant free fluid in the pelvis.
Identifying and evaluating pregnancy of unknown location
A pregnancy of unknown location (PUL) is defined by a positive serum hCG level in the absence of a visualized IUP or ectopic pregnancy (EP) by pelvic ultrasonography.
Because of variations in screening tools and clinical practices between institutions and care settings (for example, EDs versus specialized outpatient offices), the incidence of PUL is difficult to capture. In specialized early pregnancy clinics, the rate is 8% to 10%, whereas in the ED setting, the PUL rate has been reported to be as high as 42%.1-6 While approximately 98% to 99% of all pregnancies are intrauterine, only 30% of PULs will continue to develop as viable ongoing intrauterine gestations.7-9 The remainder are revealed as failing IUPs or EPs. To counsel patients, set expectations, and triage to appropriate management, it is critical to diagnose pregnancy location as efficiently as possible.
Ectopic pregnancy
Ectopic pregnancies represent only 1% to 2% of conceptions (both spontaneous and through assisted reproduction) and occur most commonly in the fallopian tube, although EPs also can implant in the cornua of the uterus, the cervix, cesarean scar, and more rarely on the ovary or abdominal viscera.10,11 Least common, heterotopic pregnancies—in which an IUP coexists with an EP—occur in 1 in 4,000 to 30,000 pregnancies, more commonly in women who used assisted reproduction.11
Major risk factors for EP include a history of tubal surgery, sexually transmitted infections (particularly Chlamydia trachomatis), pelvic inflammatory disease, conception with an intrauterine device in situ, and a history of prior EP or tubal surgery, particularly prior tubal ligation; minor risk factors include a history of infertility (excluding known tubal factor infertility) or smoking (in a dose-dependent manner).11,12 The concern for an EP is heightened in patients with these risk factors.
Because of the possibility of rupture and life-threatening hemorrhage, EP carries a risk of significant morbidity and mortality.13 Ruptured EPs account for approximately 2.7% of all maternal deaths each year.14 When diagnosed sufficiently early in a stable patient, most EPs can be managed medically with methotrexate, a folic acid antagonist.15 Ectopic pregnancies also may be managed surgically, and emergency surgery is indicated in women with evidence of EP rupture and intraperitoneal bleeding.

Continue to: Intrauterine pregnancy...
Intrauterine pregnancy
While excluding EP is critical, it is equally important to diagnose an IUP as expeditiously as possible to avoid inadvertent, destructive intervention. Diagnosis and management of a PUL can involve endometrial aspiration, which would interrupt an IUP and should be avoided until the possibility of a viable IUP has been eliminated in desired pregnancies. The inadvertent administration of methotrexate, a known teratogen, to a patient with an undiagnosed viable IUP can result in miscarriage, elective termination, or a live-born infant with significant malformations, all of which expose the administering physician to malpractice litigation.16,17
In desired pregnancies, it is essential to differentiate between a viable IUP, a nonviable IUP, and an EP to guide appropriate management and ensure patient safety, whereas exclusion of EP is the priority in undesired pregnancies.
Tools for diagnosing pregnancy location
For diagnosing pregnancy location, serial hCG measurement, transvaginal pelvic ultrasonography, and outpatient endometrial aspiration are all relevant clinical tools. Pregnancy location can be diagnosed with either direct visualization of an IUP or EP by ultrasonography or with confirmed pathology (chorionic villi or trophoblast cells) from endometrial aspiration (FIGURE). A decline in hCG to an undetectable level following endometrial aspiration also is considered sufficient to diagnose a failed IUP, even in the absence of a confirmatory ultrasonography.

Trending hCG values
In stable patients with PUL, serum hCG levels are commonly measured at 2-day intervals, ideally for a minimum of 3 values. Conventional wisdom dictates that in viable IUPs, the hCG level should roughly double every 2 days. However, more recent data suggest that the threshold for minimum expected hCG rise for an ongoing IUP should be far lower when the pregnancy is desired.18 A less conservative cutoff can be considered when a pregnancy is not desired.
In a multisite cohort study of 1,005 women with PUL, a minimum hCG rise of 35% in 2 days captured the majority of IUPs, with a negative predictive value of 97.2% for IUP.19 Of note, although the cutoff of 35% was selected to reduce the risk of misdiagnosing an IUP as an EP, 7.7% of IUPs (and 16.8% of EPs) were still misclassified, showing that hCG trends must be interpreted in the context of other clinical data, including ultrasonography findings and patient symptoms and history.
A follow-up study demonstrated that hCG rises are lower (but still within this normal range) when the initial hCG value is higher, particularly greater than 3,000 mIU/mL.20
Studies show that the rate of spontaneous hCG decline in failing IUPs ranges from 12% to 47% in 2 days, falling more rapidly from higher starting hCG values.19,21 In a retrospective review of 443 women with spontaneously resolving PUL (presumed to be failing IUPs), the minimum 2-day decline in hCG was 35%.22 Any spontaneous hCG decline less than 35% in 2 days in a PUL should raise physician concern for EP.
Conversely, EPs do not demonstrate predictable hCG trends and can mimic the hCG trends of viable or failing IUPs. Although typically half of EPs present with an increasing hCG value and half present with a decreasing hCG value, the majority (71%) demonstrate a slower rate of change than either a viable IUP or a miscarriage.11 This slower change (plateau) should heighten the clinician’s suspicion for an EP.
Continue to: Progesterone levels...
Progesterone levels
A progesterone level often is used to attempt to determine pregnancy viability in women who are not receiving progesterone supplementation, although it ultimately has limited utility. While far less sensitive than an hCG value trend, a serum progesterone level of less than 5 to 10 ng/mL is a rough marker of nonviable pregnancy.23
In a large meta-analysis of women with pain and bleeding, 96.8% of pregnancies with a single progesterone level of less than 10 ng/mL were nonviable.23 When an inconclusive ultrasonography was documented in addition to symptoms of pain and bleeding, 99.2% of pregnancies with a progesterone level of less than 3.2 to 6 ng/mL were nonviable.
Progesterone’s usefulness in assessing for a PUL is limited: While progesterone levels may indicate nonviability, they provide no indication of pregnancy location (intrauterine or ectopic).
Alternative serologic markers
Various other reproductive and pregnancy-related hormones have been investigated for use in the diagnosis of pregnancy location in PULs, including activin A, inhibin A, pregnancy-associated plasma protein A (PAPP-A), placental-like growth factor, vascular endothelial growth factor, follistatin, and various microRNAs.24,25 While research into these biomarkers is ongoing, none have been studied in prospective trials, and they are not for use in current clinical care.
Pelvic ultrasonography
Pelvic ultrasonography is a crucial part of PUL assessment. Transvaginal ultrasonography should be interpreted in the context of the estimated gestational age of the pregnancy and serial hCG values, if available; the patient’s symptoms; and the sensitivity of the ultrasonography equipment, which also may be affected by variables that can reduce visualization, such as uterine fibroids and obesity.
The “discriminatory zone” refers to the hCG value above which an IUP should be visualized by ultrasonography. Generally, with an hCG value of 1,500 to 2,000 mIU/mL or greater, an IUP is expected to be seen with transvaginal sonography.3,26 Many exceptions to the discriminatory zone have been reported, however, including multiple pregnancies, which will have a higher hCG value at an earlier gestational age. Even in singleton pregnancies, viable IUPs have been documented as developing from PULs with an elevated initial hCG value as high as 4,300 mIU/mL.27 The discriminatory zone may vary among clinical hCG assays, and it also is affected by the quality and modernity of the ultrasonography equipment as well as by the ultrasonography operator’s experience and skill.28,29
The estimated gestational age, based on either the last menstrual period or assisted reproduction procedure, provides a helpful data point to guide expectations for ultrasonography findings.30 Using transvaginal ultrasonography in a normally progressing IUP, a gestational sac—typically measuring 2 to 3 mm—should be visualized at 5 weeks.15,30 At approximately 5.5 weeks, a yolk sac measuring 3 to 5 mm should appear. At 6 weeks, an embryo with cardiac activity should be visualized.
In a pregnancy reliably dated beyond 5 weeks, the lack of an intrauterine gestational sac is suspicious for, but not diagnostic of, an EP. Conversely, the visualization of a gestational sac alone (without a yolk sac) is insufficient to definitively exclude an EP, since a small fluid collection in the endometrium (a “pseudosac”) can convincingly mimic the appearance of a gestational sac, and a follow-up ultrasonography should be performed in such cases.
Among patients without ultrasonographic evidence of an IUP, endometrial thickness has been posited as a way to differentiate between IUP and EP.31,32 Evidence suggests that an endometrial stripe of at least 8 to 10 mm may be somewhat predictive of an IUP, while endometrial thickness below 8 mm is more concerning for EP. This clinical variable, however, has been shown repeatedly to lack sufficient sensitivity and specificity for IUP and should be considered only within the entire clinical context.
Continue to: Endometrial aspiration...
Endometrial aspiration
A persistently abnormal hCG trend and an ultrasonography without evidence of an IUP—particularly with an hCG value above the discriminatory zone and/or with reliable pregnancy dating beyond 5 to 6 weeks—is highly concerning for either a failing IUP or an EP. Once a viable desired IUP is excluded beyond reasonable doubt through these measures, endometrial aspiration to determine pregnancy location is a reasonable next step in PUL management.
Endometrial aspiration can identify a failing IUP by detection of trophoblasts or chorionic villi on pathology and/or by a decline of at least 15% in hCG, measured on the day of endometrial aspiration and again the following day. Endometrial aspiration is effective even in clinical care settings that do not have rapid pathologic analysis available, as hCG measurement before and within 24 hours after the procedure still can be performed.
Vacuum aspiration (electric or manual) in an operating room or office setting is an effective tool for diagnosing pregnancy location.33,34 The use of an endometrial Pipelle for endometrial sampling (typically used for an office endometrial biopsy to diagnose hyperplasia or malignancy) is insufficient for determining pregnancy location.35 For all patients managed with this protocol, the hCG value ideally should be followed until it is undetectable, regardless of whether an EP or failing IUP was diagnosed. In rare cases, an EP may be diagnosed by a late plateau in hCG values, following an initial decline consistent with a failing IUP.
Utility for diagnosis. Retrospective studies in patients with PUL following in vitro fertilization have established the utility of outpatient endometrial aspiration with a Karman cannula, followed by a repeat hCG measurement on the day after the procedure.34,36 These data demonstrate that between 42% and 69% of women were ultimately diagnosed with a failed IUP following endometrial aspiration, thereby sparing them unnecessary exposure to methotrexate.
A decline in hCG levels of at least 15% within 24 hours after the procedure indicates that a failed IUP is the most likely diagnosis and further intervention is not indicated (although falling hCG values should be monitored for confirmation); confirmatory pathology with chorionic villi or trophoblasts was present in less than half of these women and is not necessary to diagnose a failed IUP.36 Women diagnosed with a failed IUP after endometrial aspiration also benefitted from a shorter time to resolution of the nonviable pregnancy by approximately 2 weeks.36
Despite the efficacy of endometrial aspiration for the diagnosis of pregnancy location, recent data show that physicians have highly variable approaches to PUL with an hCG plateaued above the discriminatory zone: One-third would first perform endometrial aspiration, while one-third would give methotrexate without further diagnostics.37 Academic physicians were 4 times more likely to recommend endometrial aspiration.37
Presumed EP. Following endometrial aspiration, if pathology does not confirm an intrauterine gestation and the hCG fails to decline by at least 15%, the diagnosis of a presumed EP is made.
For stable patients with neither evidence of intra-abdominal bleeding nor contraindications to methotrexate (such as blood dyscrasias, hepatic or renal insufficiency, active pulmonary or peptic ulcer disease, breastfeeding, or a known intolerance to the medication), methotrexate is recommended for medical management.26 Following screening blood work that includes a complete blood count and liver function and renal function tests, the typical methotrexate dose is 50 mg/m2 of body surface area. The single-dose regimen entails checking hCG on the day of methotrexate administration and again on days 4 and 7 thereafter. A minimum decline in hCG of 15% between days 4 and 7 indicates successful treatment; if the hCG decline is below 15%, the patient should receive an additional dose of methotrexate.
There are several published alternative regimens for methotrexate administration, including 2-dose and multidose regimens; the 2-dose protocol (2 doses within 7 days) may be more effective in women with higher hCG (> 3,000 mIU/mL) or known adnexal mass.26,38
Continue to: Contraindications to methotrexate...
Contraindications to methotrexate. In addition to strict medical contraindications to methotrexate, relative contraindications that indicate a higher risk of methotrexate failure include the presence of fetal cardiac activity, EP mass greater than 4 cm, and serum hCG above 5,000 mIU/mL.26 Because of the potential risk of tubal rupture during medical management, relative contraindications also include patient inability to follow up as an outpatient and patient refusal of blood transfusion.26 Patients with contraindications to methotrexate, hemodynamic instability, ultrasonographic or clinical evidence of EP rupture, or those electing for surgical management may be managed with laparoscopy.11 Discussion of surgical management of EP is beyond the scope of this article.
Follow the hCG level. In patients with a failing IUP or an EP treated with methotrexate or salpingostomy, the hCG level should always be followed until it is negative, usually by weekly measurements once the diagnosis is made. In some cases, the hCG level may plateau after an initial decline, alerting the clinician to failed treatment for a known EP or the need for recategorization of a failed IUP as an EP.
CASE Concluded
The patient’s second and third hCG measurements at 2-day intervals were 1,903 mIU/mL (14% rise) and 2,264 mIU/mL (16% rise). At that point, a repeat transvaginal ultrasonography showed no IUP, adnexal mass, or free fluid. The patient was counseled for outpatient endometrial aspiration, which was performed using manual vacuum aspiration. The serum hCG level on the morning of the procedure was 2,420 mIU/mL. On postprocedure day 1, the serum hCG level fell to 1,615 mIU/mL, a 33% decline. The patient was counseled that this decline in hCG indicated a failing IUP. The final pathologic analysis was returned 3 days later, showing no evidence of trophoblasts and chorionic villi. Regardless, the diagnosis of failing IUP remained given the rapid hCG decline; the tissue from the disrupted failing IUP was likely very scant or simply not drawn into the cannula. Serum hCG levels repeated at weekly intervals revealed ongoing decline, and after 4 weeks, the serum hCG was negative.
In summary
For women diagnosed with PUL, the primary goal is to distinguish an IUP from an EP to reduce the risk of EP rupture through expeditious diagnosis and treatment. In women for whom the pregnancy is desired, distinguishing a viable IUP from a nonviable IUP or an EP is the more specific goal to avoid intervention on a viable IUP (with methotrexate or endometrial aspiration). In women with abnormal hCG trends and indeterminate ultrasonography results (particularly with a serum hCG above the discriminatory zone), outpatient endometrial aspiration is a highly effective way to determine pregnancy location, which dictates further treatment. ●
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20:250-261.
- Kirk E, Condous G, Bourne T. Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 2009;23:493-499.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Banerjee S, Aslam N, Zosmer N, et al. The expectant management of women with early pregnancy of unknown location. Ultrasound Obstet Gynecol. 1999;14:231-236.
- Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
- Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril. 1998;70:972-981.
- Kirk E, Condous G, Van Calster B, et al. Rationalizing the follow-up of pregnancies of unknown location. Hum Reprod. 2007;22:1744-1750.
- Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril. 2014;102:1671-1676.
- Zeng MF, Li LM. Frozen blastocyst transfer reduces incidence of ectopic pregnancy compared with fresh blastocyst transfer: a meta-analysis. Gynecol Endocrinol. 2019;35:93-99.
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583-591.
- Barnhart KT. Ectopic pregnancy. N Engl J Med. 2009;361:379-387.
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373.
- Brady PC. Handbook of Consult and Inpatient Gynecology. Switzerland: Springer International Publishing; 2016.
- Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675-684.
- Nurmohamed L, Moretti ME, Schechter T, et al. Outcome following high-dose methotrexate in pregnancies misdiagnosed as ectopic. Am J Obstet Gynecol. 2011;205:533.e1-533.e3.
- Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
- Morse CB, Sammel MD, Shaunik A, et al. Performance of human chorionic gonadotropin curves in women at risk for ectopic pregnancy: exceptions to the rules. Fertil Steril. 2012;97:101-6.e2.
- Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128:504-511.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5, pt 1):975-981.
- Butts SF, Guo W, Cary MS, et al. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013;122(2 pt 1):337-343.
- Verhaegen J, Gallos ID, van Mello NM, et al. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ. 2012;345:e6077.
- Senapati S, Sammel MD, Butts SF, et al. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106:1725-1732.e3.
- Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta. 2020;500:69-74.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638-644.
- Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med. 2011;30:1637-1642.
- Desai D, Lu J, Wyness SP, et al. Human chorionic gonadotropin discriminatory zone in ectopic pregnancy: does assay harmonization matter? Fertil Steril. 2014;101:1671-1674.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33:465-471.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Moschos E, Twickler DM. Endometrial thickness predicts intrauterine pregnancy in patients with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2008;32:929-934.
- Ellaithy M, Abdelaziz A, Hassan MF. Outcome prediction in pregnancies of unknown location using endometrial thickness measurement: is this of real clinical value? Eur J Obstet Gynecol Reprod Biol. 2013;168:68-74.
- Shaunik A, Kulp J, Appleby DH, et al. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204:130.e1-130.e6.
- Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101:420-426.
- Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
- Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol. 2017;217:185.e1-185.e9.
- Parks MA, Barnhart KT, Howard DL. Trends in the management of nonviable pregnancies of unknown location in the United States. Gynecol Obstet Invest. 2018;83:552-557.
- Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol. 2019;221:95-108.e2.
Telemedicine checklist may smooth visits with older patients
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
PPE shortage could last years without strategic plan, experts warn
Officials said logistical challenges continue 7 months after the coronavirus reached the United States, as the flu season approaches and as some state emergency management agencies prepare for a fall surge in COVID-19 cases.
Although the disarray is not as widespread as it was this spring, hospitals said rolling shortages of supplies range from specialized beds to disposable isolation gowns to thermometers.
“A few weeks ago, we were having a very difficult time getting the sanitary wipes. You just couldn’t get them,” said Bernard Klein, MD, chief executive of Providence Holy Cross Medical Center in Mission Hills, Calif., near Los Angeles. “We actually had to manufacture our own.”
This same dynamic has played out across a number of critical supplies in his hospital. First masks, then isolation gowns and now a specialized bed that allows nurses to turn COVID-19 patients onto their bellies – equipment that helps workers with what can otherwise be a six-person job.
“We’ve seen whole families come to our hospital with COVID, and several members hospitalized at the same time,” said Dr. Klein. “It’s very, very sad.”
Testing supplies ran short as the predominantly Latino community served by Providence Holy Cross was hit hard by COVID, and even as nearby hospitals could process 15-minute tests.
“If we had a more coordinated response with a partnership between the medical field, the government and the private industry, it would help improve the supply chain to the areas that need it most,” Dr. Klein said.
Dr. Klein said he expected to deal with equipment and supply shortages throughout 2021, especially as flu season approaches.
“Most people focus on those N95 respirators,” said Carmela Coyle, CEO of the California Hospital Association, an industry group that represents more than 400 hospitals across one of America’s hardest-hit states.
She said she believed COVID-19-related supply challenges will persist through 2022.
“We have been challenged with shortages of isolation gowns, face shields, which you’re now starting to see in public places. Any one piece that’s in shortage or not available creates risk for patients and for health care workers,” said Ms. Coyle.
At the same time, trade associations representing manufacturers said persuading customers to shift to American suppliers had been difficult.
“I also have industry that’s working only at 10-20% capacity, who can make PPE in our own backyard, but have no orders,” said Kim Glas, CEO of the National Council of Textile Organizations, whose members make reusable cloth gowns.
Manufacturers in her organization have made “hundreds of millions of products,” but, without long-term government contracts, many are apprehensive to invest in the equipment needed to scale up the business and eventually lower prices.
“If there continues to be an upward trajectory of COVID-19 cases, not just in the U.S. but globally, you can see those supply chains breaking down again,” Ms. Glas said. “It is a health care security issue.”
For the past 2 decades, personal protective equipment was supplied to health care institutions in lean supply chains in the same way toilet paper was to grocery stores. Chains between major manufacturers and end users were so efficient, there was no need to stockpile goods.
But in March, the supply chain broke when major Asian PPE exporters embargoed materials or shut down just as demand increased exponentially. Thus, health care institutions were in much the same position as regular grocery shoppers, who were trying to buy great quantities of a product they never needed to stockpile before.
“I am very concerned about long-term PPE shortages for the foreseeable future,” said Susan Bailey, MD, president of the American Medical Association.
“There’s no question the situation is better than it was a couple of months ago,” said Bailey. However, many health care organizations, including her own, have struggled to obtain PPE. Bailey practices at a 10-doctor allergy clinic and was met with a 10,000-mask minimum when they tried to order N95 respirators.
“We have not seen evidence of a long-term strategic plan for the manufacture, acquisition and distribution of PPE” from the government, said Dr. Bailey. “The supply chain needs to be strengthened dramatically, and we need less dependence on foreign goods to manufacture our own PPE in the U.S.”
Some products have now come back to be made in the United States – although factories are not expected to be able to reach demand until mid-2021.
“A lot has been done in the last 6 months. We are largely out of the hole, and we have planted the seeds to render the United States self-sufficient,” said Dave Rousse, president of the Association of the Nonwoven Fabrics Industry.
In 2019, 850 tons of the material used in disposable masks was made in the United States. Around 10,000 tons is expected to be made in 2021, satisfying perhaps 80% of demand. But PPE is a suite of items – including gloves, gowns and face shields – not all of which have seen the same success.
“Thermometers are becoming a real issue,” said Cindy Juhas, chief strategy officer of CME, an American health care product distributor. “They’re expecting even a problem with needles and syringes for the amount of vaccines they have to make.”
Federal government efforts to address the supply chain have foundered. The Federal Emergency Management Agency, in charge of the COVID-19 response, told congressional interviewers in June it had “no involvement” in distributing PPE to hot spots.
Project Airbridge, an initiative headed by Jared Kushner, President Donald Trump’s son-in-law, flew PPE from international suppliers to the U.S. at taxpayer expense but was phased out. And the government has not responded to the AMA’s calls for more distribution data.
Arguably, Dr. Klein is among the best placed to weather such disruptions. He is part of a 51-hospital chain with purchasing power, and among the institutions that distributors prioritize when selling supplies. But tribulations continue even in hospitals, as shortages have pushed buyers to look directly for manufacturers, often through a swamp of companies that have sprung up overnight.
Now distributors are being called upon not just by their traditional customers – hospitals and long-term care homes – but by nearly every segment of society. First responders, schools, clinics and even food businesses are all buying medical equipment now.
“There’s going to be lots of other shortages we haven’t even thought about,” said Ms. Juhas.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Officials said logistical challenges continue 7 months after the coronavirus reached the United States, as the flu season approaches and as some state emergency management agencies prepare for a fall surge in COVID-19 cases.
Although the disarray is not as widespread as it was this spring, hospitals said rolling shortages of supplies range from specialized beds to disposable isolation gowns to thermometers.
“A few weeks ago, we were having a very difficult time getting the sanitary wipes. You just couldn’t get them,” said Bernard Klein, MD, chief executive of Providence Holy Cross Medical Center in Mission Hills, Calif., near Los Angeles. “We actually had to manufacture our own.”
This same dynamic has played out across a number of critical supplies in his hospital. First masks, then isolation gowns and now a specialized bed that allows nurses to turn COVID-19 patients onto their bellies – equipment that helps workers with what can otherwise be a six-person job.
“We’ve seen whole families come to our hospital with COVID, and several members hospitalized at the same time,” said Dr. Klein. “It’s very, very sad.”
Testing supplies ran short as the predominantly Latino community served by Providence Holy Cross was hit hard by COVID, and even as nearby hospitals could process 15-minute tests.
“If we had a more coordinated response with a partnership between the medical field, the government and the private industry, it would help improve the supply chain to the areas that need it most,” Dr. Klein said.
Dr. Klein said he expected to deal with equipment and supply shortages throughout 2021, especially as flu season approaches.
“Most people focus on those N95 respirators,” said Carmela Coyle, CEO of the California Hospital Association, an industry group that represents more than 400 hospitals across one of America’s hardest-hit states.
She said she believed COVID-19-related supply challenges will persist through 2022.
“We have been challenged with shortages of isolation gowns, face shields, which you’re now starting to see in public places. Any one piece that’s in shortage or not available creates risk for patients and for health care workers,” said Ms. Coyle.
At the same time, trade associations representing manufacturers said persuading customers to shift to American suppliers had been difficult.
“I also have industry that’s working only at 10-20% capacity, who can make PPE in our own backyard, but have no orders,” said Kim Glas, CEO of the National Council of Textile Organizations, whose members make reusable cloth gowns.
Manufacturers in her organization have made “hundreds of millions of products,” but, without long-term government contracts, many are apprehensive to invest in the equipment needed to scale up the business and eventually lower prices.
“If there continues to be an upward trajectory of COVID-19 cases, not just in the U.S. but globally, you can see those supply chains breaking down again,” Ms. Glas said. “It is a health care security issue.”
For the past 2 decades, personal protective equipment was supplied to health care institutions in lean supply chains in the same way toilet paper was to grocery stores. Chains between major manufacturers and end users were so efficient, there was no need to stockpile goods.
But in March, the supply chain broke when major Asian PPE exporters embargoed materials or shut down just as demand increased exponentially. Thus, health care institutions were in much the same position as regular grocery shoppers, who were trying to buy great quantities of a product they never needed to stockpile before.
“I am very concerned about long-term PPE shortages for the foreseeable future,” said Susan Bailey, MD, president of the American Medical Association.
“There’s no question the situation is better than it was a couple of months ago,” said Bailey. However, many health care organizations, including her own, have struggled to obtain PPE. Bailey practices at a 10-doctor allergy clinic and was met with a 10,000-mask minimum when they tried to order N95 respirators.
“We have not seen evidence of a long-term strategic plan for the manufacture, acquisition and distribution of PPE” from the government, said Dr. Bailey. “The supply chain needs to be strengthened dramatically, and we need less dependence on foreign goods to manufacture our own PPE in the U.S.”
Some products have now come back to be made in the United States – although factories are not expected to be able to reach demand until mid-2021.
“A lot has been done in the last 6 months. We are largely out of the hole, and we have planted the seeds to render the United States self-sufficient,” said Dave Rousse, president of the Association of the Nonwoven Fabrics Industry.
In 2019, 850 tons of the material used in disposable masks was made in the United States. Around 10,000 tons is expected to be made in 2021, satisfying perhaps 80% of demand. But PPE is a suite of items – including gloves, gowns and face shields – not all of which have seen the same success.
“Thermometers are becoming a real issue,” said Cindy Juhas, chief strategy officer of CME, an American health care product distributor. “They’re expecting even a problem with needles and syringes for the amount of vaccines they have to make.”
Federal government efforts to address the supply chain have foundered. The Federal Emergency Management Agency, in charge of the COVID-19 response, told congressional interviewers in June it had “no involvement” in distributing PPE to hot spots.
Project Airbridge, an initiative headed by Jared Kushner, President Donald Trump’s son-in-law, flew PPE from international suppliers to the U.S. at taxpayer expense but was phased out. And the government has not responded to the AMA’s calls for more distribution data.
Arguably, Dr. Klein is among the best placed to weather such disruptions. He is part of a 51-hospital chain with purchasing power, and among the institutions that distributors prioritize when selling supplies. But tribulations continue even in hospitals, as shortages have pushed buyers to look directly for manufacturers, often through a swamp of companies that have sprung up overnight.
Now distributors are being called upon not just by their traditional customers – hospitals and long-term care homes – but by nearly every segment of society. First responders, schools, clinics and even food businesses are all buying medical equipment now.
“There’s going to be lots of other shortages we haven’t even thought about,” said Ms. Juhas.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Officials said logistical challenges continue 7 months after the coronavirus reached the United States, as the flu season approaches and as some state emergency management agencies prepare for a fall surge in COVID-19 cases.
Although the disarray is not as widespread as it was this spring, hospitals said rolling shortages of supplies range from specialized beds to disposable isolation gowns to thermometers.
“A few weeks ago, we were having a very difficult time getting the sanitary wipes. You just couldn’t get them,” said Bernard Klein, MD, chief executive of Providence Holy Cross Medical Center in Mission Hills, Calif., near Los Angeles. “We actually had to manufacture our own.”
This same dynamic has played out across a number of critical supplies in his hospital. First masks, then isolation gowns and now a specialized bed that allows nurses to turn COVID-19 patients onto their bellies – equipment that helps workers with what can otherwise be a six-person job.
“We’ve seen whole families come to our hospital with COVID, and several members hospitalized at the same time,” said Dr. Klein. “It’s very, very sad.”
Testing supplies ran short as the predominantly Latino community served by Providence Holy Cross was hit hard by COVID, and even as nearby hospitals could process 15-minute tests.
“If we had a more coordinated response with a partnership between the medical field, the government and the private industry, it would help improve the supply chain to the areas that need it most,” Dr. Klein said.
Dr. Klein said he expected to deal with equipment and supply shortages throughout 2021, especially as flu season approaches.
“Most people focus on those N95 respirators,” said Carmela Coyle, CEO of the California Hospital Association, an industry group that represents more than 400 hospitals across one of America’s hardest-hit states.
She said she believed COVID-19-related supply challenges will persist through 2022.
“We have been challenged with shortages of isolation gowns, face shields, which you’re now starting to see in public places. Any one piece that’s in shortage or not available creates risk for patients and for health care workers,” said Ms. Coyle.
At the same time, trade associations representing manufacturers said persuading customers to shift to American suppliers had been difficult.
“I also have industry that’s working only at 10-20% capacity, who can make PPE in our own backyard, but have no orders,” said Kim Glas, CEO of the National Council of Textile Organizations, whose members make reusable cloth gowns.
Manufacturers in her organization have made “hundreds of millions of products,” but, without long-term government contracts, many are apprehensive to invest in the equipment needed to scale up the business and eventually lower prices.
“If there continues to be an upward trajectory of COVID-19 cases, not just in the U.S. but globally, you can see those supply chains breaking down again,” Ms. Glas said. “It is a health care security issue.”
For the past 2 decades, personal protective equipment was supplied to health care institutions in lean supply chains in the same way toilet paper was to grocery stores. Chains between major manufacturers and end users were so efficient, there was no need to stockpile goods.
But in March, the supply chain broke when major Asian PPE exporters embargoed materials or shut down just as demand increased exponentially. Thus, health care institutions were in much the same position as regular grocery shoppers, who were trying to buy great quantities of a product they never needed to stockpile before.
“I am very concerned about long-term PPE shortages for the foreseeable future,” said Susan Bailey, MD, president of the American Medical Association.
“There’s no question the situation is better than it was a couple of months ago,” said Bailey. However, many health care organizations, including her own, have struggled to obtain PPE. Bailey practices at a 10-doctor allergy clinic and was met with a 10,000-mask minimum when they tried to order N95 respirators.
“We have not seen evidence of a long-term strategic plan for the manufacture, acquisition and distribution of PPE” from the government, said Dr. Bailey. “The supply chain needs to be strengthened dramatically, and we need less dependence on foreign goods to manufacture our own PPE in the U.S.”
Some products have now come back to be made in the United States – although factories are not expected to be able to reach demand until mid-2021.
“A lot has been done in the last 6 months. We are largely out of the hole, and we have planted the seeds to render the United States self-sufficient,” said Dave Rousse, president of the Association of the Nonwoven Fabrics Industry.
In 2019, 850 tons of the material used in disposable masks was made in the United States. Around 10,000 tons is expected to be made in 2021, satisfying perhaps 80% of demand. But PPE is a suite of items – including gloves, gowns and face shields – not all of which have seen the same success.
“Thermometers are becoming a real issue,” said Cindy Juhas, chief strategy officer of CME, an American health care product distributor. “They’re expecting even a problem with needles and syringes for the amount of vaccines they have to make.”
Federal government efforts to address the supply chain have foundered. The Federal Emergency Management Agency, in charge of the COVID-19 response, told congressional interviewers in June it had “no involvement” in distributing PPE to hot spots.
Project Airbridge, an initiative headed by Jared Kushner, President Donald Trump’s son-in-law, flew PPE from international suppliers to the U.S. at taxpayer expense but was phased out. And the government has not responded to the AMA’s calls for more distribution data.
Arguably, Dr. Klein is among the best placed to weather such disruptions. He is part of a 51-hospital chain with purchasing power, and among the institutions that distributors prioritize when selling supplies. But tribulations continue even in hospitals, as shortages have pushed buyers to look directly for manufacturers, often through a swamp of companies that have sprung up overnight.
Now distributors are being called upon not just by their traditional customers – hospitals and long-term care homes – but by nearly every segment of society. First responders, schools, clinics and even food businesses are all buying medical equipment now.
“There’s going to be lots of other shortages we haven’t even thought about,” said Ms. Juhas.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Erratum
Grandhi R, Shamloul N, Powell M. Purpuric bullae on the lower extremities. Cutis. 2020;105:282, 286-287.
In the article above from the June 2020 issue, the images were incorrect. The correct images appear below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Quiz Images
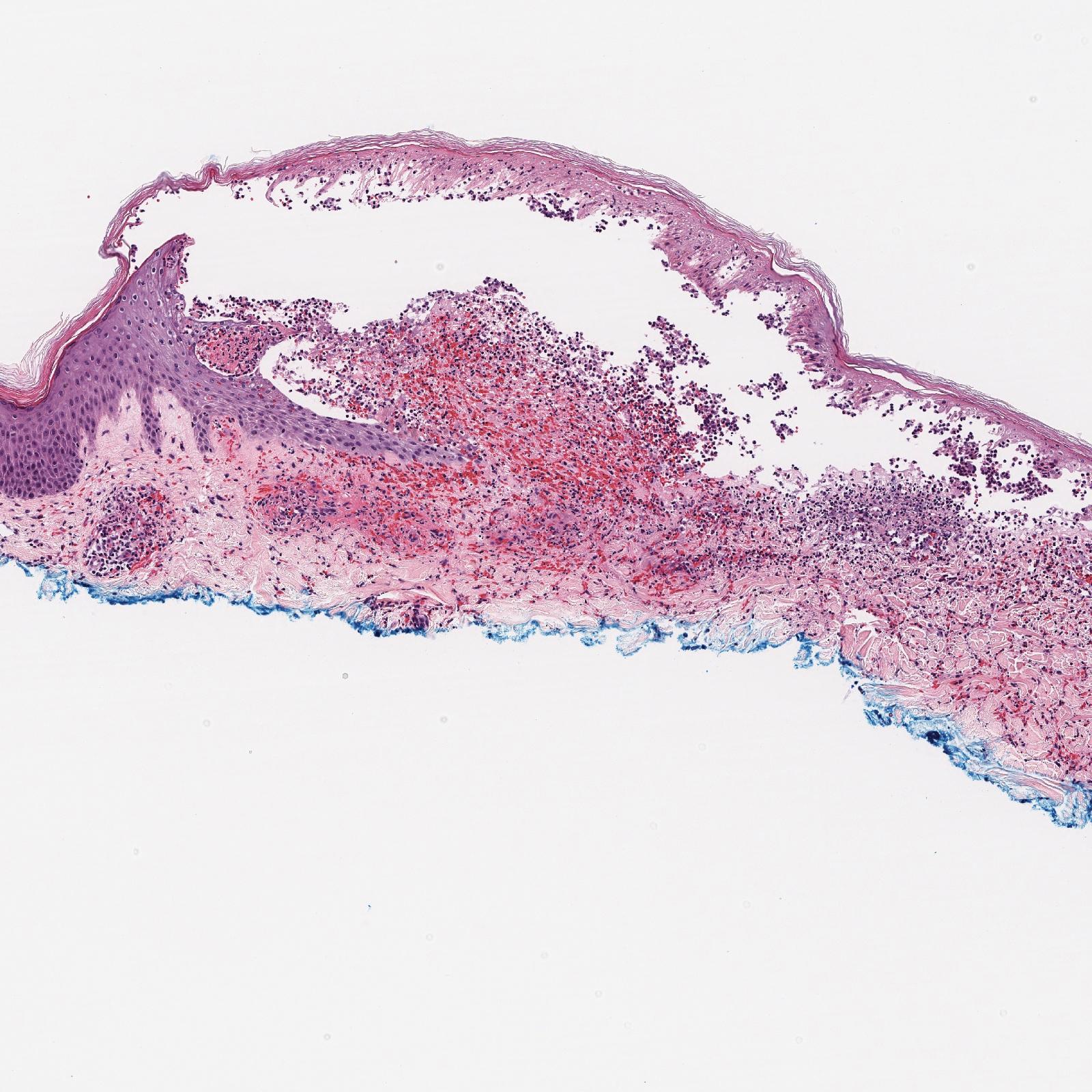
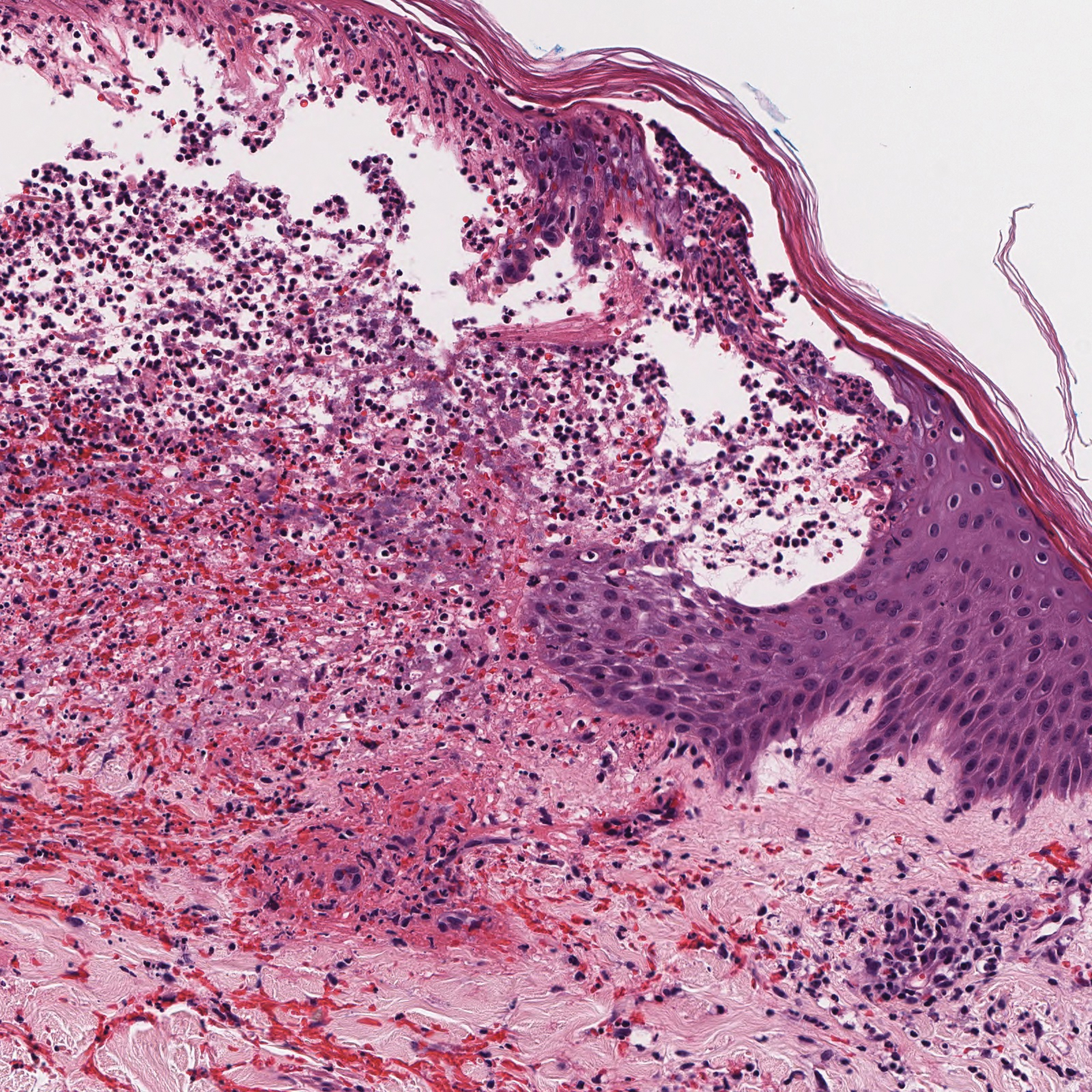
Discussion Images




Grandhi R, Shamloul N, Powell M. Purpuric bullae on the lower extremities. Cutis. 2020;105:282, 286-287.
In the article above from the June 2020 issue, the images were incorrect. The correct images appear below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Quiz Images


Discussion Images




Grandhi R, Shamloul N, Powell M. Purpuric bullae on the lower extremities. Cutis. 2020;105:282, 286-287.
In the article above from the June 2020 issue, the images were incorrect. The correct images appear below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Quiz Images


Discussion Images




The evidence is not clear: Rheumatic diseases, drugs, and COVID-19
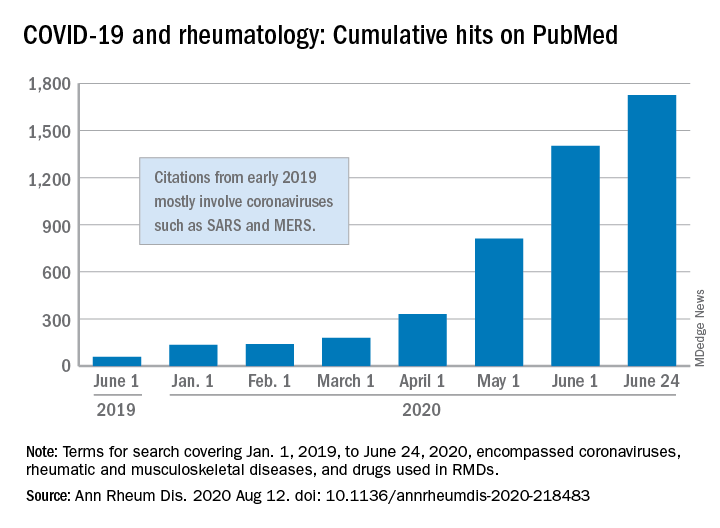
“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.

“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.

“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.
FROM ANNALS OF THE RHEUMATIC DISEASES
A Roundabout Journey to Diagnosis
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
After journeying for several months from Honduras, this 30-year-old woman visits the clinic for evaluation of a lesion that has been growing on her cheek since before she started traveling. She saw several providers—mostly in NGO clinics—along her journey. The diagnosis they gave was consistently “ringworm.” She was offered various topical creams, none of which produced any results.
Though the lesion is not painful, it causes some itching. The patient is much more concerned about its appearance. Through interpreters, she claims to be in otherwise good health. She has no other lesions, joint pain, fever, or malaise. She reports neither her family nor fellow travelers have such lesions.
Examination reveals an impressive 3-cm, round, papulosquamous plaque on the right side of her face (below the malar area). The lesion is neither tender nor notably warm. There are no palpable lymph nodes in the area. The scaling is mostly on the periphery. A KOH prep of the scaling shows no fungal elements.
Mammography starting at 40 cuts risk of breast cancer death
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.