User login
The Importance of Emotional Intelligence When Leading in a Time of Crisis
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
© 2020 Society of Hospital Medicine
FDA Regulation of Predictive Clinical Decision-Support Tools: What Does It Mean for Hospitals?
Recent experiences in the transportation industry highlight the importance of getting right the regulation of decision-support systems in high-stakes environments. Two tragic plane crashes resulted in 346 deaths and were deemed, in part, to be related to a cockpit alert system that overwhelmed pilots with multiple notifications.1 Similarly, a driverless car struck and killed a pedestrian in the street, in part because the car was not programmed to look for humans outside of a crosswalk.2 These two bellwether events offer poignant lessons for the healthcare industry in which human lives also depend on decision-support systems.
Clinical decision-support (CDS) systems are computerized applications, often embedded in an electronic health record (EHR), that provide information to clinicians to inform care. Although CDS systems have been used for many years,3 they have never been subjected to any enforcement of formal testing requirements. However, a draft guidance document released in 2019 from the Food and Drug Administration (FDA) outlined new directions for the regulation of CDS systems.4 Although the FDA has thus far focused regulatory efforts on predictive systems developed by private manufacturers,5,6 this new document provides examples of software that would require regulation for CDS systems that hospitals are already using. Thus, this new guidance raises critical questions—will hospitals themselves be evaluated like private manufacturers, be exempted from federal regulation, or require their own specialized regulation? The FDA has not yet clarified its approach to hospitals or hospital-developed CDS systems, which leaves open numerous possibilities in a rapidly evolving regulatory environment.
Although the FDA has officially regulated CDS systems under section 201(h) of the Federal Food, Drug, and Cosmetic Act (1938), only recently has the FDA begun to sketch the shape of its regulatory efforts. This trend to actually regulate CDS systems began with the 21st Century Cures Act (2016) that amended the definition of software systems that qualify as medical devices and outlined criteria under which a system may be exempt from FDA oversight. For example, regulation would not apply to systems that support “population health” or a “healthy lifestyle” or to ones that qualify as “electronic patient records” as long as they do not “interpret or analyze” data within them.7 Following the rapid proliferation of many machine learning and other predictive technologies with medical applications, the FDA began the voluntary Digital Health Software Precertification (Pre-Cert) Program in 2017. Through this program, the FDA selected nine companies from more than 100 applicants and certified them across five domains of excellence. Notably, the Pre-Cert Program currently allows for certification of software manufacturers themselves and does not approve or test actual software devices directly. This regulatory pathway will eventually allow manufacturers to apply under a modified premarket review process for individual software as a medical device (SaMD) that use artificial intelligence (AI) and machine learning. In the meantime, however, many hospitals have developed and deployed their own predictive CDS systems that cross the boundaries into the FDA’s purview and, indeed, do “interpret or analyze” data for real-time EHR alerts, population health management, and other applications.
Regulatory oversight for hospitals could provide quality or safety standards where currently there are none. However, such regulations could also interfere with existing local care practices, hinder rapid development of new CDS systems, and may be perceived as interfering in hospital operations. With the current enthusiasm for AI-based technologies and the concurrent lack of evidence to suggest their effectiveness in practice, regulation could also prompt necessary scrutiny of potential harms of CDS systems, an area with even less evidence. At the same time, CDS developers—private or hospital based—may be able to avoid regulation for some devices with well-placed disclaimers about the intended use of the CDS, one of the FDA criteria for determining the degree of oversight. If the FDA were to regulate hospitals or hospital-developed CDS systems, there are several unanswered questions to consider so that such regulations have their intended impact.
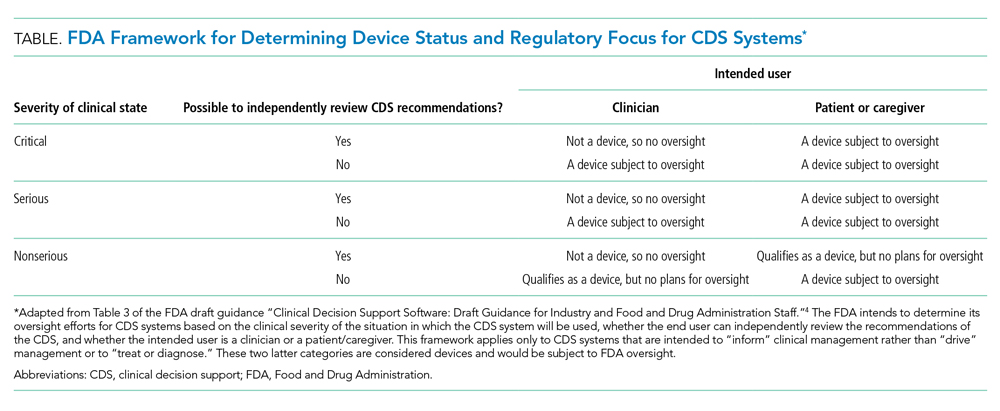
First, does the FDA intend to regulate hospitals and hospital-developed software at all? The framework for determining whether a CDS system will be regulated depends on the severity of the clinical scenario, the ability to independently evaluate the model output, and the intended user (Table). Notably, many types of CDS systems that would require regulation under this framework are already commonplace. For example, the FDA intends to regulate software that “identifies patients who may exhibit signs of opioid addiction,” a scenario similar to prediction models already developed at academic hospitals.8 The FDA also plans to regulate a software device even if it is not a CDS system if it is “intended to generate an alarm or an alert to notify a caregiver of a life-threatening condition, such as stroke, and the caregiver relies primarily on this alarm or alert to make a treatment decision.” Although there are no published reports of stroke-specific early warning systems in use, analogous nonspecific and sepsis-specific early warning systems to prompt urgent clinical care have been deployed by hospitals directly9 and developed for embedding in commercial EHRs.10 Hospitals need clarification on the FDA’s regulatory intentions for such CDS systems. FDA regulation of hospitals and hospital-developed CDS systems would fill a critical oversight need and potentially strengthen processes to improve safety and effectiveness. But burdensome regulations may also restrain hospitals from tackling complex problems in medicine for which they are uniquely suited.
Such a regulatory environment may be especially prohibitive for safety-net hospitals that could find themselves at a disadvantage in developing their own CDS systems relative to large academic medical centers that are typically endowed with greater resources. Additionally, CDS systems developed at academic medical centers may not generalize well to populations in the community setting, which could further deepen disparities in access to cutting-edge technologies. For example, racial bias in treatment and referral patterns could bias training labels for CDS systems focused on population health management.11 Similarly, the composition of patient skin color in one population may distort predictions of a model in another with a different distribution of skin color, even when the primary outcome of a prediction model is gender.12 Additional regulatory steps may apply for models that are adapted to new populations or recalibrated across locations and time.13 Until there is more data on the clinical impact of such CDS systems, it is unknown how potential differences in evaluation and approval would actually affect clinical outcomes.
Second, would hospitals be eligible for the Pre-Cert program, and if so, would they be held to the same standards as a private technology manufacturer? The domains of excellence required for precertification approval such as “patient safety,” “clinical responsibility,” and “proactive culture” are aligned with the efforts of hospitals that are already overseen and accredited by organizations like the Joint Commission on Accreditation of Healthcare Organizations and the American Nurses Credentialing Center. There is limited motivation for the FDA to be in the business of regulating these aspects of hospital functions. However, while domains like “product quality” and “cybersecurity” may be less familiar to some hospitals, these existing credentialing bodies may be better suited than the FDA to set and enforce standards for hospitals. In contrast, private manufacturers may have deep expertise in these latter domains. Therefore, as with public-private partnerships for the development of predictive radiology applications,14 synergies between hospitals and manufacturers may also prove useful for obtaining approvals in a competitive marketplace. Simultaneously, such collaborations would continue to raise questions about conflicts of interest and data privacy.
Finally, regardless of how the FDA will regulate hospitals, what will become of predictive CDS systems that fall outside of the FDA’s scope? Hospitals will continue to find themselves in the position of self-regulation without clear guidance. Although the FDA suggests that developers of unregulated CDS systems still follow best practices for software validation and cybersecurity, existing guidance documents in these domains do not cover the full range of concerns relevant to the development, deployment, and oversight of AI-based CDS systems in the clinical domain. Nor do most hospitals have the infrastructure or expertise to oversee their own CDS systems. Disparate recommendations for development, training, and oversight of AI-based medical systems have emerged but have yet to be endorsed by a federal regulatory body or become part of the hospital accreditation process.15 Optimal local oversight would require a collaboration between clinical experts, hospital operations leaders, statisticians, data scientists, and ethics experts to ensure effectiveness, safety, and fairness.
Hospitals will remain at the forefront of developing and implementing predictive CDS systems. The proposed FDA regulatory framework would mark an important step toward realizing benefit from such systems, but the FDA needs to clarify the requirements for hospitals and hospital-developed CDS systems to ensure reasonable standards that account for their differences from private software manufacturers. Should the FDA choose to focus regulation on private manufacturers only, hospitals leaders may both feel more empowered to develop their own local CDS tools and feel more comfortable buying CDS systems from vendors that have been precertified. This strategy would provide an optimal balance of assurance and flexibility while maintaining quality standards that ultimately improve patient care.
1. Sumwalt RL III, Landsbert B, Homendy J. Assumptions Used in the Safety Assessment Process and the Effects of Multiple Alerts and Indications on Pilot Performance. National Transportation Safety Board; 2019. https://www.ntsb.gov/investigations/AccidentReports/Reports/ASR1901.pdf
2. Becic E, Zych N, Ivarsson J. Vehicle Automation Report. National Transportation Safety Board; 2019. https://dms.ntsb.gov/public/62500-62999/62978/629713.pdf
3. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y
4. Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed October 15, 2019. https://www.fda.gov/media/109618/download
5. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. https://doi.org/10.1001/jama.2016.17216
6. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Medicine. 2018;1(1):39. https://doi.org/10.1038/s41746-018-0040-6
7. Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed March 18, 2020. https://www.fda.gov/media/109622/download
8. Lo-Ciganic W-H, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
9. Smith MEB, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454-1465. https://doi.org/10.1513/annalsats.201403-102oc
10. WAVE Clinical Platform 510(k) Premarket Notification. Food and Drug Administration. January 4, 2018. Accessed March 3, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K171056
11. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
12. Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. Proc Machine Learning Res. 2018;81:1-15.
13. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). Food and Drug Administration. April 2, 2019. Accessed April 6, 2020. https://www.regulations.gov/contentStreamer?documentId=FDA-2019-N-1185-0001&attachmentNumber=1&contentType=pdf
14. Allen B. The role of the FDA in ensuring the safety and efficacy of artificial intelligence software and devices. J Am Coll Radiol. 2019;16(2):208-210. https://doi.org/10.1016/j.jacr.2018.09.007
15. Reddy S, Allan S, Coghlan S, Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2019. https://doi.org/10.1093/jamia/ocz192
Recent experiences in the transportation industry highlight the importance of getting right the regulation of decision-support systems in high-stakes environments. Two tragic plane crashes resulted in 346 deaths and were deemed, in part, to be related to a cockpit alert system that overwhelmed pilots with multiple notifications.1 Similarly, a driverless car struck and killed a pedestrian in the street, in part because the car was not programmed to look for humans outside of a crosswalk.2 These two bellwether events offer poignant lessons for the healthcare industry in which human lives also depend on decision-support systems.
Clinical decision-support (CDS) systems are computerized applications, often embedded in an electronic health record (EHR), that provide information to clinicians to inform care. Although CDS systems have been used for many years,3 they have never been subjected to any enforcement of formal testing requirements. However, a draft guidance document released in 2019 from the Food and Drug Administration (FDA) outlined new directions for the regulation of CDS systems.4 Although the FDA has thus far focused regulatory efforts on predictive systems developed by private manufacturers,5,6 this new document provides examples of software that would require regulation for CDS systems that hospitals are already using. Thus, this new guidance raises critical questions—will hospitals themselves be evaluated like private manufacturers, be exempted from federal regulation, or require their own specialized regulation? The FDA has not yet clarified its approach to hospitals or hospital-developed CDS systems, which leaves open numerous possibilities in a rapidly evolving regulatory environment.
Although the FDA has officially regulated CDS systems under section 201(h) of the Federal Food, Drug, and Cosmetic Act (1938), only recently has the FDA begun to sketch the shape of its regulatory efforts. This trend to actually regulate CDS systems began with the 21st Century Cures Act (2016) that amended the definition of software systems that qualify as medical devices and outlined criteria under which a system may be exempt from FDA oversight. For example, regulation would not apply to systems that support “population health” or a “healthy lifestyle” or to ones that qualify as “electronic patient records” as long as they do not “interpret or analyze” data within them.7 Following the rapid proliferation of many machine learning and other predictive technologies with medical applications, the FDA began the voluntary Digital Health Software Precertification (Pre-Cert) Program in 2017. Through this program, the FDA selected nine companies from more than 100 applicants and certified them across five domains of excellence. Notably, the Pre-Cert Program currently allows for certification of software manufacturers themselves and does not approve or test actual software devices directly. This regulatory pathway will eventually allow manufacturers to apply under a modified premarket review process for individual software as a medical device (SaMD) that use artificial intelligence (AI) and machine learning. In the meantime, however, many hospitals have developed and deployed their own predictive CDS systems that cross the boundaries into the FDA’s purview and, indeed, do “interpret or analyze” data for real-time EHR alerts, population health management, and other applications.
Regulatory oversight for hospitals could provide quality or safety standards where currently there are none. However, such regulations could also interfere with existing local care practices, hinder rapid development of new CDS systems, and may be perceived as interfering in hospital operations. With the current enthusiasm for AI-based technologies and the concurrent lack of evidence to suggest their effectiveness in practice, regulation could also prompt necessary scrutiny of potential harms of CDS systems, an area with even less evidence. At the same time, CDS developers—private or hospital based—may be able to avoid regulation for some devices with well-placed disclaimers about the intended use of the CDS, one of the FDA criteria for determining the degree of oversight. If the FDA were to regulate hospitals or hospital-developed CDS systems, there are several unanswered questions to consider so that such regulations have their intended impact.
First, does the FDA intend to regulate hospitals and hospital-developed software at all? The framework for determining whether a CDS system will be regulated depends on the severity of the clinical scenario, the ability to independently evaluate the model output, and the intended user (Table). Notably, many types of CDS systems that would require regulation under this framework are already commonplace. For example, the FDA intends to regulate software that “identifies patients who may exhibit signs of opioid addiction,” a scenario similar to prediction models already developed at academic hospitals.8 The FDA also plans to regulate a software device even if it is not a CDS system if it is “intended to generate an alarm or an alert to notify a caregiver of a life-threatening condition, such as stroke, and the caregiver relies primarily on this alarm or alert to make a treatment decision.” Although there are no published reports of stroke-specific early warning systems in use, analogous nonspecific and sepsis-specific early warning systems to prompt urgent clinical care have been deployed by hospitals directly9 and developed for embedding in commercial EHRs.10 Hospitals need clarification on the FDA’s regulatory intentions for such CDS systems. FDA regulation of hospitals and hospital-developed CDS systems would fill a critical oversight need and potentially strengthen processes to improve safety and effectiveness. But burdensome regulations may also restrain hospitals from tackling complex problems in medicine for which they are uniquely suited.

Such a regulatory environment may be especially prohibitive for safety-net hospitals that could find themselves at a disadvantage in developing their own CDS systems relative to large academic medical centers that are typically endowed with greater resources. Additionally, CDS systems developed at academic medical centers may not generalize well to populations in the community setting, which could further deepen disparities in access to cutting-edge technologies. For example, racial bias in treatment and referral patterns could bias training labels for CDS systems focused on population health management.11 Similarly, the composition of patient skin color in one population may distort predictions of a model in another with a different distribution of skin color, even when the primary outcome of a prediction model is gender.12 Additional regulatory steps may apply for models that are adapted to new populations or recalibrated across locations and time.13 Until there is more data on the clinical impact of such CDS systems, it is unknown how potential differences in evaluation and approval would actually affect clinical outcomes.
Second, would hospitals be eligible for the Pre-Cert program, and if so, would they be held to the same standards as a private technology manufacturer? The domains of excellence required for precertification approval such as “patient safety,” “clinical responsibility,” and “proactive culture” are aligned with the efforts of hospitals that are already overseen and accredited by organizations like the Joint Commission on Accreditation of Healthcare Organizations and the American Nurses Credentialing Center. There is limited motivation for the FDA to be in the business of regulating these aspects of hospital functions. However, while domains like “product quality” and “cybersecurity” may be less familiar to some hospitals, these existing credentialing bodies may be better suited than the FDA to set and enforce standards for hospitals. In contrast, private manufacturers may have deep expertise in these latter domains. Therefore, as with public-private partnerships for the development of predictive radiology applications,14 synergies between hospitals and manufacturers may also prove useful for obtaining approvals in a competitive marketplace. Simultaneously, such collaborations would continue to raise questions about conflicts of interest and data privacy.
Finally, regardless of how the FDA will regulate hospitals, what will become of predictive CDS systems that fall outside of the FDA’s scope? Hospitals will continue to find themselves in the position of self-regulation without clear guidance. Although the FDA suggests that developers of unregulated CDS systems still follow best practices for software validation and cybersecurity, existing guidance documents in these domains do not cover the full range of concerns relevant to the development, deployment, and oversight of AI-based CDS systems in the clinical domain. Nor do most hospitals have the infrastructure or expertise to oversee their own CDS systems. Disparate recommendations for development, training, and oversight of AI-based medical systems have emerged but have yet to be endorsed by a federal regulatory body or become part of the hospital accreditation process.15 Optimal local oversight would require a collaboration between clinical experts, hospital operations leaders, statisticians, data scientists, and ethics experts to ensure effectiveness, safety, and fairness.
Hospitals will remain at the forefront of developing and implementing predictive CDS systems. The proposed FDA regulatory framework would mark an important step toward realizing benefit from such systems, but the FDA needs to clarify the requirements for hospitals and hospital-developed CDS systems to ensure reasonable standards that account for their differences from private software manufacturers. Should the FDA choose to focus regulation on private manufacturers only, hospitals leaders may both feel more empowered to develop their own local CDS tools and feel more comfortable buying CDS systems from vendors that have been precertified. This strategy would provide an optimal balance of assurance and flexibility while maintaining quality standards that ultimately improve patient care.
Recent experiences in the transportation industry highlight the importance of getting right the regulation of decision-support systems in high-stakes environments. Two tragic plane crashes resulted in 346 deaths and were deemed, in part, to be related to a cockpit alert system that overwhelmed pilots with multiple notifications.1 Similarly, a driverless car struck and killed a pedestrian in the street, in part because the car was not programmed to look for humans outside of a crosswalk.2 These two bellwether events offer poignant lessons for the healthcare industry in which human lives also depend on decision-support systems.
Clinical decision-support (CDS) systems are computerized applications, often embedded in an electronic health record (EHR), that provide information to clinicians to inform care. Although CDS systems have been used for many years,3 they have never been subjected to any enforcement of formal testing requirements. However, a draft guidance document released in 2019 from the Food and Drug Administration (FDA) outlined new directions for the regulation of CDS systems.4 Although the FDA has thus far focused regulatory efforts on predictive systems developed by private manufacturers,5,6 this new document provides examples of software that would require regulation for CDS systems that hospitals are already using. Thus, this new guidance raises critical questions—will hospitals themselves be evaluated like private manufacturers, be exempted from federal regulation, or require their own specialized regulation? The FDA has not yet clarified its approach to hospitals or hospital-developed CDS systems, which leaves open numerous possibilities in a rapidly evolving regulatory environment.
Although the FDA has officially regulated CDS systems under section 201(h) of the Federal Food, Drug, and Cosmetic Act (1938), only recently has the FDA begun to sketch the shape of its regulatory efforts. This trend to actually regulate CDS systems began with the 21st Century Cures Act (2016) that amended the definition of software systems that qualify as medical devices and outlined criteria under which a system may be exempt from FDA oversight. For example, regulation would not apply to systems that support “population health” or a “healthy lifestyle” or to ones that qualify as “electronic patient records” as long as they do not “interpret or analyze” data within them.7 Following the rapid proliferation of many machine learning and other predictive technologies with medical applications, the FDA began the voluntary Digital Health Software Precertification (Pre-Cert) Program in 2017. Through this program, the FDA selected nine companies from more than 100 applicants and certified them across five domains of excellence. Notably, the Pre-Cert Program currently allows for certification of software manufacturers themselves and does not approve or test actual software devices directly. This regulatory pathway will eventually allow manufacturers to apply under a modified premarket review process for individual software as a medical device (SaMD) that use artificial intelligence (AI) and machine learning. In the meantime, however, many hospitals have developed and deployed their own predictive CDS systems that cross the boundaries into the FDA’s purview and, indeed, do “interpret or analyze” data for real-time EHR alerts, population health management, and other applications.
Regulatory oversight for hospitals could provide quality or safety standards where currently there are none. However, such regulations could also interfere with existing local care practices, hinder rapid development of new CDS systems, and may be perceived as interfering in hospital operations. With the current enthusiasm for AI-based technologies and the concurrent lack of evidence to suggest their effectiveness in practice, regulation could also prompt necessary scrutiny of potential harms of CDS systems, an area with even less evidence. At the same time, CDS developers—private or hospital based—may be able to avoid regulation for some devices with well-placed disclaimers about the intended use of the CDS, one of the FDA criteria for determining the degree of oversight. If the FDA were to regulate hospitals or hospital-developed CDS systems, there are several unanswered questions to consider so that such regulations have their intended impact.
First, does the FDA intend to regulate hospitals and hospital-developed software at all? The framework for determining whether a CDS system will be regulated depends on the severity of the clinical scenario, the ability to independently evaluate the model output, and the intended user (Table). Notably, many types of CDS systems that would require regulation under this framework are already commonplace. For example, the FDA intends to regulate software that “identifies patients who may exhibit signs of opioid addiction,” a scenario similar to prediction models already developed at academic hospitals.8 The FDA also plans to regulate a software device even if it is not a CDS system if it is “intended to generate an alarm or an alert to notify a caregiver of a life-threatening condition, such as stroke, and the caregiver relies primarily on this alarm or alert to make a treatment decision.” Although there are no published reports of stroke-specific early warning systems in use, analogous nonspecific and sepsis-specific early warning systems to prompt urgent clinical care have been deployed by hospitals directly9 and developed for embedding in commercial EHRs.10 Hospitals need clarification on the FDA’s regulatory intentions for such CDS systems. FDA regulation of hospitals and hospital-developed CDS systems would fill a critical oversight need and potentially strengthen processes to improve safety and effectiveness. But burdensome regulations may also restrain hospitals from tackling complex problems in medicine for which they are uniquely suited.

Such a regulatory environment may be especially prohibitive for safety-net hospitals that could find themselves at a disadvantage in developing their own CDS systems relative to large academic medical centers that are typically endowed with greater resources. Additionally, CDS systems developed at academic medical centers may not generalize well to populations in the community setting, which could further deepen disparities in access to cutting-edge technologies. For example, racial bias in treatment and referral patterns could bias training labels for CDS systems focused on population health management.11 Similarly, the composition of patient skin color in one population may distort predictions of a model in another with a different distribution of skin color, even when the primary outcome of a prediction model is gender.12 Additional regulatory steps may apply for models that are adapted to new populations or recalibrated across locations and time.13 Until there is more data on the clinical impact of such CDS systems, it is unknown how potential differences in evaluation and approval would actually affect clinical outcomes.
Second, would hospitals be eligible for the Pre-Cert program, and if so, would they be held to the same standards as a private technology manufacturer? The domains of excellence required for precertification approval such as “patient safety,” “clinical responsibility,” and “proactive culture” are aligned with the efforts of hospitals that are already overseen and accredited by organizations like the Joint Commission on Accreditation of Healthcare Organizations and the American Nurses Credentialing Center. There is limited motivation for the FDA to be in the business of regulating these aspects of hospital functions. However, while domains like “product quality” and “cybersecurity” may be less familiar to some hospitals, these existing credentialing bodies may be better suited than the FDA to set and enforce standards for hospitals. In contrast, private manufacturers may have deep expertise in these latter domains. Therefore, as with public-private partnerships for the development of predictive radiology applications,14 synergies between hospitals and manufacturers may also prove useful for obtaining approvals in a competitive marketplace. Simultaneously, such collaborations would continue to raise questions about conflicts of interest and data privacy.
Finally, regardless of how the FDA will regulate hospitals, what will become of predictive CDS systems that fall outside of the FDA’s scope? Hospitals will continue to find themselves in the position of self-regulation without clear guidance. Although the FDA suggests that developers of unregulated CDS systems still follow best practices for software validation and cybersecurity, existing guidance documents in these domains do not cover the full range of concerns relevant to the development, deployment, and oversight of AI-based CDS systems in the clinical domain. Nor do most hospitals have the infrastructure or expertise to oversee their own CDS systems. Disparate recommendations for development, training, and oversight of AI-based medical systems have emerged but have yet to be endorsed by a federal regulatory body or become part of the hospital accreditation process.15 Optimal local oversight would require a collaboration between clinical experts, hospital operations leaders, statisticians, data scientists, and ethics experts to ensure effectiveness, safety, and fairness.
Hospitals will remain at the forefront of developing and implementing predictive CDS systems. The proposed FDA regulatory framework would mark an important step toward realizing benefit from such systems, but the FDA needs to clarify the requirements for hospitals and hospital-developed CDS systems to ensure reasonable standards that account for their differences from private software manufacturers. Should the FDA choose to focus regulation on private manufacturers only, hospitals leaders may both feel more empowered to develop their own local CDS tools and feel more comfortable buying CDS systems from vendors that have been precertified. This strategy would provide an optimal balance of assurance and flexibility while maintaining quality standards that ultimately improve patient care.
1. Sumwalt RL III, Landsbert B, Homendy J. Assumptions Used in the Safety Assessment Process and the Effects of Multiple Alerts and Indications on Pilot Performance. National Transportation Safety Board; 2019. https://www.ntsb.gov/investigations/AccidentReports/Reports/ASR1901.pdf
2. Becic E, Zych N, Ivarsson J. Vehicle Automation Report. National Transportation Safety Board; 2019. https://dms.ntsb.gov/public/62500-62999/62978/629713.pdf
3. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y
4. Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed October 15, 2019. https://www.fda.gov/media/109618/download
5. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. https://doi.org/10.1001/jama.2016.17216
6. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Medicine. 2018;1(1):39. https://doi.org/10.1038/s41746-018-0040-6
7. Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed March 18, 2020. https://www.fda.gov/media/109622/download
8. Lo-Ciganic W-H, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
9. Smith MEB, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454-1465. https://doi.org/10.1513/annalsats.201403-102oc
10. WAVE Clinical Platform 510(k) Premarket Notification. Food and Drug Administration. January 4, 2018. Accessed March 3, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K171056
11. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
12. Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. Proc Machine Learning Res. 2018;81:1-15.
13. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). Food and Drug Administration. April 2, 2019. Accessed April 6, 2020. https://www.regulations.gov/contentStreamer?documentId=FDA-2019-N-1185-0001&attachmentNumber=1&contentType=pdf
14. Allen B. The role of the FDA in ensuring the safety and efficacy of artificial intelligence software and devices. J Am Coll Radiol. 2019;16(2):208-210. https://doi.org/10.1016/j.jacr.2018.09.007
15. Reddy S, Allan S, Coghlan S, Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2019. https://doi.org/10.1093/jamia/ocz192
1. Sumwalt RL III, Landsbert B, Homendy J. Assumptions Used in the Safety Assessment Process and the Effects of Multiple Alerts and Indications on Pilot Performance. National Transportation Safety Board; 2019. https://www.ntsb.gov/investigations/AccidentReports/Reports/ASR1901.pdf
2. Becic E, Zych N, Ivarsson J. Vehicle Automation Report. National Transportation Safety Board; 2019. https://dms.ntsb.gov/public/62500-62999/62978/629713.pdf
3. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y
4. Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed October 15, 2019. https://www.fda.gov/media/109618/download
5. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. https://doi.org/10.1001/jama.2016.17216
6. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Medicine. 2018;1(1):39. https://doi.org/10.1038/s41746-018-0040-6
7. Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed March 18, 2020. https://www.fda.gov/media/109622/download
8. Lo-Ciganic W-H, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
9. Smith MEB, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454-1465. https://doi.org/10.1513/annalsats.201403-102oc
10. WAVE Clinical Platform 510(k) Premarket Notification. Food and Drug Administration. January 4, 2018. Accessed March 3, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K171056
11. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
12. Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. Proc Machine Learning Res. 2018;81:1-15.
13. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). Food and Drug Administration. April 2, 2019. Accessed April 6, 2020. https://www.regulations.gov/contentStreamer?documentId=FDA-2019-N-1185-0001&attachmentNumber=1&contentType=pdf
14. Allen B. The role of the FDA in ensuring the safety and efficacy of artificial intelligence software and devices. J Am Coll Radiol. 2019;16(2):208-210. https://doi.org/10.1016/j.jacr.2018.09.007
15. Reddy S, Allan S, Coghlan S, Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2019. https://doi.org/10.1093/jamia/ocz192
© 2020 Society of Hospital Medicine
Multiplying the Impact of Opioid Settlement Funds by Investing in Primary Prevention
There is growing momentum to hold drug manufacturers accountable for the more than 400,000 US opioid overdose deaths that have occurred since 1999.1 As state lawsuits against pharmaceutical manufacturers and distributors wind their way through the legal system, hospitals—which may benefit from settlement funds—have been paying close attention. Recently, former Governor John Kasich (R-Ohio), West Virginia University president E. Gordon Gee, and America’s Essential Hospitals argued that adequately compensating hospitals for the costs of being on the crisis’ “front lines” requires prioritizing them as settlement fund recipients.2
Hospitals should be laying the groundwork for how settlement funds might be used. They may consider enhancing some of the most promising, evidence-based services for individuals with opioid use disorders (OUDs), including improving treatment for commonly associated health conditions such as HIV and hepatitis C virus (HCV); expanding ambulatory long-term antibiotic treatment for endocarditis and other intravenous drug use–associated infections; more broadly adopting harm-reduction practices such as naloxone coprescribing; and applying best practices to caring for substance-exposed infants. They could also develop clinical services not already provided, including creating programs for OUD management during pregnancy and initiating medication for OUD in inpatient, emergency department, and ambulatory settings. In short, hospitals play a critical role in engaging people with OUD in treatment at every possible opportunity.3
When considering how to most effectively use opioid settlement funding, hospitals may consider adding or expanding these much-needed clinical services to address opioid-related harms; however, their efforts should not stop there. Investments made outside hospital walls could have a significant effect on the public’s health, especially if they target social determinants of health. By tackling factors in the pathway to developing OUD, such as lack of meaningful employment, affordable housing, and mental health care, hospitals can move beyond treating the downstream consequences of addiction and toward preventing community-level opioid-related harms. To accomplish this daunting goal, hospitals will need to strengthen existing relationships with community partners and build new ones. Yet in a 2015 study, only 54% of nonprofit hospitals proposed a strategy to address the overdose crisis that involved community partnering.4
In this Perspective, we describe the following three strategies hospitals can use to multiply the reach of their opioid settlement funding by addressing root causes of opioid use through primary prevention: (1) supporting economic opportunities in their communities, (2) expanding affordable housing options in surrounding neighborhoods, and (3) building capacity in ambulatory practices and pharmacies to prevent OUD (Table).

SUPPORTING ECONOMIC OPPORTUNITY IN THEIR COMMUNITIES
Lack of economic opportunity is one of many root causes of opioid use. For example, a recent study found that automotive assembly plant closures were associated with increases in opioid overdose mortality.5 To tackle this complex issue, hospitals can play a crucial role in expanding employment and career advancement options for members of their local communities. Specifically, hospitals can do the following:
- Create jobs within the healthcare system and preferentially recruit and hire from surrounding neighborhoods
- Establish structured career development programs to build skills among entry-level healthcare employees
- Award contracts of varying sizes to locally owned businesses
- Employ individuals with lived experience with substance use disorders, such as peer recovery coaches6
To illustrate how health systems are investing in enhancing career opportunities for members of their communities, hundreds of institutions have implemented “School at Work,” a 6-month career development program for entry-level healthcare employees.7 The hospitals’ Human Resources department trains participants in communication skills, reading and writing, patient safety and satisfaction, medical terminology, and strategies for success and career advancement. Evaluations of this program have demonstrated improved employee outcomes and a favorable return on investment for hospitals.8
As “anchor institutions” and large employers in many communities, hospitals can simultaneously enhance their own workforce and offer employment opportunities that can help break the cycle of addiction that commonly traps individuals and families in communities affected by the overdose crisis.
EXPANDING AFFORDABLE HOUSING OPTIONS
Hospitals are increasingly supporting interventions that fall outside their traditional purview as they seek to improve population health, such as developing safe green outdoor spaces and increasing access to healthy food options by supporting local farmers markets and grocers.9 Stable, decent, and affordable housing is critically important to health and well-being,10 and there is a well-documented association of opioid use disorder and opioid misuse with housing instability.11 Given evidence of improved outcomes with hospital-led housing interventions,12 a growing number of hospitals are partnering with housing authorities and community groups to help do the following13:
- Contribute to supportive housing options
- Provide environmental health assessments, repairs, and renovations
- Buy or develop affordable housing units
Boston Medical Center, where one in four inpatients are experiencing homelessnes and one in three pediatric emergency department patients are housing insecure, provides an example of how a hospital has invested in housing.14 In 2017, the hospital began a 5-year, $6.5 million investment in community partnerships in surrounding neighborhoods. Instead of building housing units or acting as a landlord, the hospital chose to invest funding in creative ways to increase the pool of affordable housing. It invested $1 million to rehabilitate permanent, supportive housing units for individuals with mental health conditions in a nearby Boston neighborhood and in a housing stabilization program for people with complex medical issues including substance use disorder. It provided resources to a homeless shelter near the hospital and to the Boston Health Care for the Homeless Program, which provides healthcare to individuals with housing instability. It also funded a community wellness advocate based at the hospital, who received training in substance use disorders and served as a liaison between the hospital and the Boston Housing Authority.
Housing instability is just one of the social determinants of health that hospitals have the capacity to address as they consider where to invest their opioid settlement funds.
BUILDING PREVENTION CAPACITY IN THE COMMUNITY
Finally, hospitals can partner with community ambulatory practices and pharmacies to prevent the progression to problematic opioid use and OUD. Specifically, hospitals can do as follows:
- Provide evidence-based training to community providers on safe prescribing practices for acute and chronic pain management, as well as postoperative, postprocedural, and postpartum pain management
- Support ambulatory providers in expanding office-based mental health treatment through direct care via telemedicine and in building mental health treatment capacity through consultation, continuing medical education, and telementorship (eg, Project ECHO15)
- Support ambulatory providers to implement risk reduction strategies to prevent initiation of problematic opioid use, particularly among adolescents and young adults
- Partner with local pharmacies to promote point-of-prescription counseling on the risks and benefits of opioids
Hospitals bring key strengths and resources to these prevention-oriented partnerships. First, they may have resources available for clinical research, implementation support, program evaluation, and quality improvement, bringing such expertise to partnerships with ambulatory practices and pharmacies. They likely have specific expertise among their staff, including areas such as pain management, obstetric care, pediatrics, and adolescent medicine, and can provide specialists for consultation services or telementoring initiatives. They also can organize continuing medical education and can offer in-service training at local practices and pharmacies.
Project ECHO is one example of telementoring to build capacity among community providers to manage chronic pain and address addiction and other related harms.16 The Project ECHO model includes virtual sessions with didactic content and case presentations during which specialists mentor community clinicians. Specific to primary prevention, telementoring has been shown to improve access to evidence-based treatment of chronic pain and mental health conditions,17,18 which could prevent the development of OUD. By equipping community clinicians with tools to prevent the development of problematic opioid use, hospitals can help reduce the downstream burden of OUD and its associated morbidity, mortality, and costs.
CONCLUSION
The opioid crisis has devastated families, reduced life expectancy in certain communities,19 and had a substantial financial impact on hospitals—resulting in an estimated $11 billion in costs to US hospitals each year.20 This ongoing crisis is only going to be compounded by the recent emergence of the SARS-CoV-2 virus. Hospital resources are being strained in unprecedented ways, which has required unprecedented responses in order to continue to serve their communities. Supporting economic opportunity, stable housing, and mental health treatment will be challenging in this new environment but has never been more urgently needed. If opioid settlement funds are targeted to US hospitals, they should be held accountable for where funds are spent because they have a unique opportunity to focus on primary prevention in their communities—confronting OUD before it begins.21 However, if hospitals use opioid settlement funding only to continue to provide services already offered, or fail to make bold investments in their communities, this public health crisis will continue to strain the resources of those providing clinical care on the front lines.
Acknowledgment
The authors wish to thank Hilary Peterson of the RAND Corporation for preparing the paper for submission. She was not compensated for her contribution.
Disclosures
The authors report being supported by grants from the National Institute on Drug Abuse of the National Institutes of Health under awards R21DA045212 (Dr Faherty), K23DA045085 (Dr Hadland), L40DA042434 (Dr Hadland), K23DA038720 (Dr Patrick), R01DA045729 (Dr Patrick), and P50DA046351 (Dr Stein). Dr Hadland also reports grant support from the Thrasher Research Fund and the Academic Pediatric Association. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. http://doi.org/10.15585/mmwr.mm675152e1
2. Kasich J, Gee EG. Don’t forget our frontline caregivers in the opioid epidemic. New York Times. Published September 18, 2019. Accessed December 16, 2019. https://www.nytimes.com/2019/09/17/opinion/opioid-settlement-hospitals.html
3. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
4. Franz B, Cronin CE, Wainwright A, Pagan JA. Measuring efforts of nonprofit hospitals to address opioid abuse after the Affordable Care Act. J Prim Care Communit. 2019;10:2150132719863611. https://doi.org/10.1177/2150132719863611
5. Venkataramani AS, Bair EF, O’Brien RL, Tsai ALC. Association between automotive assembly plant closures and opioid overdose mortality in the United States a difference-in-differences analysis. JAMA Intern Med. 2020;180(2):254-262. https://doi.org/10.1001/jamainternmed.2019.5686
6. Englander H, Gregg J, Gullickson J, et al. Recommendations for integrating peer mentors in hospital-based addiction care. Subst Abus. 2019:1-6. https://doi.org/10.1080/08897077.2019.1635968
7. Geisinger investing in employees’ careers with School at Work program. News Release. Geisinger; November 5, 2018. Updated November 5, 2018. Accessed February 17, 2020. https://www.geisinger.org/about-geisinger/news-and-media/news-releases/2018/11/19/17/31/geisinger-investing-in-employees-careers-with-school-at-work-program
8. Jackson A, Brasfield-Gorrigan H. Investing in the Future of the Healthcare Workforce: An Analysis of the Business Impact of Select Employee Development Programs at TriHealth in 2013. TriHealth. March 30, 2015. Accessed 20 April 2020. http://www.catalystlearning.com/Portals/0/Documents/TriHealth%20RoI%20Study%20Updated%20Version.pdf
9. Roy B, Stanojevich J, Stange P, Jiwani N, King R, Koo D. Development of the Community Health Improvement Navigator Database of Interventions. MMWR Suppl. 2016;65:1-9. http://doi.org/10.15585/mmwr.su6502a1
10. Sandel M, Desmond M. Investing in housing for health improves both mission and margin. JAMA. 2017;318(23):2291-2292. https://doi.org/10.1001/jama.2017.15771
11. Vijayaraghavan M, Penko J, Bangsberg DR, Miaskowski C, Kushel MB. Opioid analgesic misuse in a community-based cohort of HIV-infected indigent adults. JAMA Intern Med. 2013;173(3):235-237. https://doi.org/10.1001/jamainternmed.2013.1576
12. Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults a randomized trial. JAMA. 2009;301(17):1771-1778. https://doi.org/10.1001/jama.2009.561
13. Health Research & Educational Trust. Social Determinants of Health Series: Housing and the Role of Hospitals. American Hospital Association. August 2017. Accessed December 16, 2019. https://www.aha.org/ahahret-guides/2017-08-22-social-determinants-health-series-housing-and-role-hospitals
14. Boston Medical Center to Invest $6.5 Million in Affordable Housing to Improve Community Health and Patient Outcomes, Reduce Medical Costs. Press release. Boston Medical Center; December 7, 2017. Accessed March 4, 2020. https://www.bmc.org/news/press-releases/2017/12/07/boston-medical-center-invest-65-million-affordable-housing-improve
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207. https://doi.org/10.1056/nejmoa1009370
16. Chronic Pain and Opioid Management. Project ECHO. Accessed February 16, 2020. https://echo.unm.edu/teleecho-programs/chronic-pain
17. Anderson D, Zlateva I, Davis B, et al. Improving pain care with Project ECHO in community health centers. Pain Med. 2017;18(10):1882-1889. https://doi.org/10.1093/pm/pnx187
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100. https://doi.org/10.1111/pme.12715
19. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. https://doi.org/10.1001/jama.2019.16932
20. Opioid Overdoses Costing US Hospitals an Estimated $11 Billion Annually. Press Release. Premier; January 3, 2019. Accessed March 4, 2020. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
21. Butler JC. 2017 ASTHO president’s challenge: public health approaches to preventing substance misuse and addiction. J Public Health Manag Pract. 2017;23(5):531-536. https://doi.org/10.1097/phh.0000000000000631
There is growing momentum to hold drug manufacturers accountable for the more than 400,000 US opioid overdose deaths that have occurred since 1999.1 As state lawsuits against pharmaceutical manufacturers and distributors wind their way through the legal system, hospitals—which may benefit from settlement funds—have been paying close attention. Recently, former Governor John Kasich (R-Ohio), West Virginia University president E. Gordon Gee, and America’s Essential Hospitals argued that adequately compensating hospitals for the costs of being on the crisis’ “front lines” requires prioritizing them as settlement fund recipients.2
Hospitals should be laying the groundwork for how settlement funds might be used. They may consider enhancing some of the most promising, evidence-based services for individuals with opioid use disorders (OUDs), including improving treatment for commonly associated health conditions such as HIV and hepatitis C virus (HCV); expanding ambulatory long-term antibiotic treatment for endocarditis and other intravenous drug use–associated infections; more broadly adopting harm-reduction practices such as naloxone coprescribing; and applying best practices to caring for substance-exposed infants. They could also develop clinical services not already provided, including creating programs for OUD management during pregnancy and initiating medication for OUD in inpatient, emergency department, and ambulatory settings. In short, hospitals play a critical role in engaging people with OUD in treatment at every possible opportunity.3
When considering how to most effectively use opioid settlement funding, hospitals may consider adding or expanding these much-needed clinical services to address opioid-related harms; however, their efforts should not stop there. Investments made outside hospital walls could have a significant effect on the public’s health, especially if they target social determinants of health. By tackling factors in the pathway to developing OUD, such as lack of meaningful employment, affordable housing, and mental health care, hospitals can move beyond treating the downstream consequences of addiction and toward preventing community-level opioid-related harms. To accomplish this daunting goal, hospitals will need to strengthen existing relationships with community partners and build new ones. Yet in a 2015 study, only 54% of nonprofit hospitals proposed a strategy to address the overdose crisis that involved community partnering.4
In this Perspective, we describe the following three strategies hospitals can use to multiply the reach of their opioid settlement funding by addressing root causes of opioid use through primary prevention: (1) supporting economic opportunities in their communities, (2) expanding affordable housing options in surrounding neighborhoods, and (3) building capacity in ambulatory practices and pharmacies to prevent OUD (Table).

SUPPORTING ECONOMIC OPPORTUNITY IN THEIR COMMUNITIES
Lack of economic opportunity is one of many root causes of opioid use. For example, a recent study found that automotive assembly plant closures were associated with increases in opioid overdose mortality.5 To tackle this complex issue, hospitals can play a crucial role in expanding employment and career advancement options for members of their local communities. Specifically, hospitals can do the following:
- Create jobs within the healthcare system and preferentially recruit and hire from surrounding neighborhoods
- Establish structured career development programs to build skills among entry-level healthcare employees
- Award contracts of varying sizes to locally owned businesses
- Employ individuals with lived experience with substance use disorders, such as peer recovery coaches6
To illustrate how health systems are investing in enhancing career opportunities for members of their communities, hundreds of institutions have implemented “School at Work,” a 6-month career development program for entry-level healthcare employees.7 The hospitals’ Human Resources department trains participants in communication skills, reading and writing, patient safety and satisfaction, medical terminology, and strategies for success and career advancement. Evaluations of this program have demonstrated improved employee outcomes and a favorable return on investment for hospitals.8
As “anchor institutions” and large employers in many communities, hospitals can simultaneously enhance their own workforce and offer employment opportunities that can help break the cycle of addiction that commonly traps individuals and families in communities affected by the overdose crisis.
EXPANDING AFFORDABLE HOUSING OPTIONS
Hospitals are increasingly supporting interventions that fall outside their traditional purview as they seek to improve population health, such as developing safe green outdoor spaces and increasing access to healthy food options by supporting local farmers markets and grocers.9 Stable, decent, and affordable housing is critically important to health and well-being,10 and there is a well-documented association of opioid use disorder and opioid misuse with housing instability.11 Given evidence of improved outcomes with hospital-led housing interventions,12 a growing number of hospitals are partnering with housing authorities and community groups to help do the following13:
- Contribute to supportive housing options
- Provide environmental health assessments, repairs, and renovations
- Buy or develop affordable housing units
Boston Medical Center, where one in four inpatients are experiencing homelessnes and one in three pediatric emergency department patients are housing insecure, provides an example of how a hospital has invested in housing.14 In 2017, the hospital began a 5-year, $6.5 million investment in community partnerships in surrounding neighborhoods. Instead of building housing units or acting as a landlord, the hospital chose to invest funding in creative ways to increase the pool of affordable housing. It invested $1 million to rehabilitate permanent, supportive housing units for individuals with mental health conditions in a nearby Boston neighborhood and in a housing stabilization program for people with complex medical issues including substance use disorder. It provided resources to a homeless shelter near the hospital and to the Boston Health Care for the Homeless Program, which provides healthcare to individuals with housing instability. It also funded a community wellness advocate based at the hospital, who received training in substance use disorders and served as a liaison between the hospital and the Boston Housing Authority.
Housing instability is just one of the social determinants of health that hospitals have the capacity to address as they consider where to invest their opioid settlement funds.
BUILDING PREVENTION CAPACITY IN THE COMMUNITY
Finally, hospitals can partner with community ambulatory practices and pharmacies to prevent the progression to problematic opioid use and OUD. Specifically, hospitals can do as follows:
- Provide evidence-based training to community providers on safe prescribing practices for acute and chronic pain management, as well as postoperative, postprocedural, and postpartum pain management
- Support ambulatory providers in expanding office-based mental health treatment through direct care via telemedicine and in building mental health treatment capacity through consultation, continuing medical education, and telementorship (eg, Project ECHO15)
- Support ambulatory providers to implement risk reduction strategies to prevent initiation of problematic opioid use, particularly among adolescents and young adults
- Partner with local pharmacies to promote point-of-prescription counseling on the risks and benefits of opioids
Hospitals bring key strengths and resources to these prevention-oriented partnerships. First, they may have resources available for clinical research, implementation support, program evaluation, and quality improvement, bringing such expertise to partnerships with ambulatory practices and pharmacies. They likely have specific expertise among their staff, including areas such as pain management, obstetric care, pediatrics, and adolescent medicine, and can provide specialists for consultation services or telementoring initiatives. They also can organize continuing medical education and can offer in-service training at local practices and pharmacies.
Project ECHO is one example of telementoring to build capacity among community providers to manage chronic pain and address addiction and other related harms.16 The Project ECHO model includes virtual sessions with didactic content and case presentations during which specialists mentor community clinicians. Specific to primary prevention, telementoring has been shown to improve access to evidence-based treatment of chronic pain and mental health conditions,17,18 which could prevent the development of OUD. By equipping community clinicians with tools to prevent the development of problematic opioid use, hospitals can help reduce the downstream burden of OUD and its associated morbidity, mortality, and costs.
CONCLUSION
The opioid crisis has devastated families, reduced life expectancy in certain communities,19 and had a substantial financial impact on hospitals—resulting in an estimated $11 billion in costs to US hospitals each year.20 This ongoing crisis is only going to be compounded by the recent emergence of the SARS-CoV-2 virus. Hospital resources are being strained in unprecedented ways, which has required unprecedented responses in order to continue to serve their communities. Supporting economic opportunity, stable housing, and mental health treatment will be challenging in this new environment but has never been more urgently needed. If opioid settlement funds are targeted to US hospitals, they should be held accountable for where funds are spent because they have a unique opportunity to focus on primary prevention in their communities—confronting OUD before it begins.21 However, if hospitals use opioid settlement funding only to continue to provide services already offered, or fail to make bold investments in their communities, this public health crisis will continue to strain the resources of those providing clinical care on the front lines.
Acknowledgment
The authors wish to thank Hilary Peterson of the RAND Corporation for preparing the paper for submission. She was not compensated for her contribution.
Disclosures
The authors report being supported by grants from the National Institute on Drug Abuse of the National Institutes of Health under awards R21DA045212 (Dr Faherty), K23DA045085 (Dr Hadland), L40DA042434 (Dr Hadland), K23DA038720 (Dr Patrick), R01DA045729 (Dr Patrick), and P50DA046351 (Dr Stein). Dr Hadland also reports grant support from the Thrasher Research Fund and the Academic Pediatric Association. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
There is growing momentum to hold drug manufacturers accountable for the more than 400,000 US opioid overdose deaths that have occurred since 1999.1 As state lawsuits against pharmaceutical manufacturers and distributors wind their way through the legal system, hospitals—which may benefit from settlement funds—have been paying close attention. Recently, former Governor John Kasich (R-Ohio), West Virginia University president E. Gordon Gee, and America’s Essential Hospitals argued that adequately compensating hospitals for the costs of being on the crisis’ “front lines” requires prioritizing them as settlement fund recipients.2
Hospitals should be laying the groundwork for how settlement funds might be used. They may consider enhancing some of the most promising, evidence-based services for individuals with opioid use disorders (OUDs), including improving treatment for commonly associated health conditions such as HIV and hepatitis C virus (HCV); expanding ambulatory long-term antibiotic treatment for endocarditis and other intravenous drug use–associated infections; more broadly adopting harm-reduction practices such as naloxone coprescribing; and applying best practices to caring for substance-exposed infants. They could also develop clinical services not already provided, including creating programs for OUD management during pregnancy and initiating medication for OUD in inpatient, emergency department, and ambulatory settings. In short, hospitals play a critical role in engaging people with OUD in treatment at every possible opportunity.3
When considering how to most effectively use opioid settlement funding, hospitals may consider adding or expanding these much-needed clinical services to address opioid-related harms; however, their efforts should not stop there. Investments made outside hospital walls could have a significant effect on the public’s health, especially if they target social determinants of health. By tackling factors in the pathway to developing OUD, such as lack of meaningful employment, affordable housing, and mental health care, hospitals can move beyond treating the downstream consequences of addiction and toward preventing community-level opioid-related harms. To accomplish this daunting goal, hospitals will need to strengthen existing relationships with community partners and build new ones. Yet in a 2015 study, only 54% of nonprofit hospitals proposed a strategy to address the overdose crisis that involved community partnering.4
In this Perspective, we describe the following three strategies hospitals can use to multiply the reach of their opioid settlement funding by addressing root causes of opioid use through primary prevention: (1) supporting economic opportunities in their communities, (2) expanding affordable housing options in surrounding neighborhoods, and (3) building capacity in ambulatory practices and pharmacies to prevent OUD (Table).

SUPPORTING ECONOMIC OPPORTUNITY IN THEIR COMMUNITIES
Lack of economic opportunity is one of many root causes of opioid use. For example, a recent study found that automotive assembly plant closures were associated with increases in opioid overdose mortality.5 To tackle this complex issue, hospitals can play a crucial role in expanding employment and career advancement options for members of their local communities. Specifically, hospitals can do the following:
- Create jobs within the healthcare system and preferentially recruit and hire from surrounding neighborhoods
- Establish structured career development programs to build skills among entry-level healthcare employees
- Award contracts of varying sizes to locally owned businesses
- Employ individuals with lived experience with substance use disorders, such as peer recovery coaches6
To illustrate how health systems are investing in enhancing career opportunities for members of their communities, hundreds of institutions have implemented “School at Work,” a 6-month career development program for entry-level healthcare employees.7 The hospitals’ Human Resources department trains participants in communication skills, reading and writing, patient safety and satisfaction, medical terminology, and strategies for success and career advancement. Evaluations of this program have demonstrated improved employee outcomes and a favorable return on investment for hospitals.8
As “anchor institutions” and large employers in many communities, hospitals can simultaneously enhance their own workforce and offer employment opportunities that can help break the cycle of addiction that commonly traps individuals and families in communities affected by the overdose crisis.
EXPANDING AFFORDABLE HOUSING OPTIONS
Hospitals are increasingly supporting interventions that fall outside their traditional purview as they seek to improve population health, such as developing safe green outdoor spaces and increasing access to healthy food options by supporting local farmers markets and grocers.9 Stable, decent, and affordable housing is critically important to health and well-being,10 and there is a well-documented association of opioid use disorder and opioid misuse with housing instability.11 Given evidence of improved outcomes with hospital-led housing interventions,12 a growing number of hospitals are partnering with housing authorities and community groups to help do the following13:
- Contribute to supportive housing options
- Provide environmental health assessments, repairs, and renovations
- Buy or develop affordable housing units
Boston Medical Center, where one in four inpatients are experiencing homelessnes and one in three pediatric emergency department patients are housing insecure, provides an example of how a hospital has invested in housing.14 In 2017, the hospital began a 5-year, $6.5 million investment in community partnerships in surrounding neighborhoods. Instead of building housing units or acting as a landlord, the hospital chose to invest funding in creative ways to increase the pool of affordable housing. It invested $1 million to rehabilitate permanent, supportive housing units for individuals with mental health conditions in a nearby Boston neighborhood and in a housing stabilization program for people with complex medical issues including substance use disorder. It provided resources to a homeless shelter near the hospital and to the Boston Health Care for the Homeless Program, which provides healthcare to individuals with housing instability. It also funded a community wellness advocate based at the hospital, who received training in substance use disorders and served as a liaison between the hospital and the Boston Housing Authority.
Housing instability is just one of the social determinants of health that hospitals have the capacity to address as they consider where to invest their opioid settlement funds.
BUILDING PREVENTION CAPACITY IN THE COMMUNITY
Finally, hospitals can partner with community ambulatory practices and pharmacies to prevent the progression to problematic opioid use and OUD. Specifically, hospitals can do as follows:
- Provide evidence-based training to community providers on safe prescribing practices for acute and chronic pain management, as well as postoperative, postprocedural, and postpartum pain management
- Support ambulatory providers in expanding office-based mental health treatment through direct care via telemedicine and in building mental health treatment capacity through consultation, continuing medical education, and telementorship (eg, Project ECHO15)
- Support ambulatory providers to implement risk reduction strategies to prevent initiation of problematic opioid use, particularly among adolescents and young adults
- Partner with local pharmacies to promote point-of-prescription counseling on the risks and benefits of opioids
Hospitals bring key strengths and resources to these prevention-oriented partnerships. First, they may have resources available for clinical research, implementation support, program evaluation, and quality improvement, bringing such expertise to partnerships with ambulatory practices and pharmacies. They likely have specific expertise among their staff, including areas such as pain management, obstetric care, pediatrics, and adolescent medicine, and can provide specialists for consultation services or telementoring initiatives. They also can organize continuing medical education and can offer in-service training at local practices and pharmacies.
Project ECHO is one example of telementoring to build capacity among community providers to manage chronic pain and address addiction and other related harms.16 The Project ECHO model includes virtual sessions with didactic content and case presentations during which specialists mentor community clinicians. Specific to primary prevention, telementoring has been shown to improve access to evidence-based treatment of chronic pain and mental health conditions,17,18 which could prevent the development of OUD. By equipping community clinicians with tools to prevent the development of problematic opioid use, hospitals can help reduce the downstream burden of OUD and its associated morbidity, mortality, and costs.
CONCLUSION
The opioid crisis has devastated families, reduced life expectancy in certain communities,19 and had a substantial financial impact on hospitals—resulting in an estimated $11 billion in costs to US hospitals each year.20 This ongoing crisis is only going to be compounded by the recent emergence of the SARS-CoV-2 virus. Hospital resources are being strained in unprecedented ways, which has required unprecedented responses in order to continue to serve their communities. Supporting economic opportunity, stable housing, and mental health treatment will be challenging in this new environment but has never been more urgently needed. If opioid settlement funds are targeted to US hospitals, they should be held accountable for where funds are spent because they have a unique opportunity to focus on primary prevention in their communities—confronting OUD before it begins.21 However, if hospitals use opioid settlement funding only to continue to provide services already offered, or fail to make bold investments in their communities, this public health crisis will continue to strain the resources of those providing clinical care on the front lines.
Acknowledgment
The authors wish to thank Hilary Peterson of the RAND Corporation for preparing the paper for submission. She was not compensated for her contribution.
Disclosures
The authors report being supported by grants from the National Institute on Drug Abuse of the National Institutes of Health under awards R21DA045212 (Dr Faherty), K23DA045085 (Dr Hadland), L40DA042434 (Dr Hadland), K23DA038720 (Dr Patrick), R01DA045729 (Dr Patrick), and P50DA046351 (Dr Stein). Dr Hadland also reports grant support from the Thrasher Research Fund and the Academic Pediatric Association. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. http://doi.org/10.15585/mmwr.mm675152e1
2. Kasich J, Gee EG. Don’t forget our frontline caregivers in the opioid epidemic. New York Times. Published September 18, 2019. Accessed December 16, 2019. https://www.nytimes.com/2019/09/17/opinion/opioid-settlement-hospitals.html
3. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
4. Franz B, Cronin CE, Wainwright A, Pagan JA. Measuring efforts of nonprofit hospitals to address opioid abuse after the Affordable Care Act. J Prim Care Communit. 2019;10:2150132719863611. https://doi.org/10.1177/2150132719863611
5. Venkataramani AS, Bair EF, O’Brien RL, Tsai ALC. Association between automotive assembly plant closures and opioid overdose mortality in the United States a difference-in-differences analysis. JAMA Intern Med. 2020;180(2):254-262. https://doi.org/10.1001/jamainternmed.2019.5686
6. Englander H, Gregg J, Gullickson J, et al. Recommendations for integrating peer mentors in hospital-based addiction care. Subst Abus. 2019:1-6. https://doi.org/10.1080/08897077.2019.1635968
7. Geisinger investing in employees’ careers with School at Work program. News Release. Geisinger; November 5, 2018. Updated November 5, 2018. Accessed February 17, 2020. https://www.geisinger.org/about-geisinger/news-and-media/news-releases/2018/11/19/17/31/geisinger-investing-in-employees-careers-with-school-at-work-program
8. Jackson A, Brasfield-Gorrigan H. Investing in the Future of the Healthcare Workforce: An Analysis of the Business Impact of Select Employee Development Programs at TriHealth in 2013. TriHealth. March 30, 2015. Accessed 20 April 2020. http://www.catalystlearning.com/Portals/0/Documents/TriHealth%20RoI%20Study%20Updated%20Version.pdf
9. Roy B, Stanojevich J, Stange P, Jiwani N, King R, Koo D. Development of the Community Health Improvement Navigator Database of Interventions. MMWR Suppl. 2016;65:1-9. http://doi.org/10.15585/mmwr.su6502a1
10. Sandel M, Desmond M. Investing in housing for health improves both mission and margin. JAMA. 2017;318(23):2291-2292. https://doi.org/10.1001/jama.2017.15771
11. Vijayaraghavan M, Penko J, Bangsberg DR, Miaskowski C, Kushel MB. Opioid analgesic misuse in a community-based cohort of HIV-infected indigent adults. JAMA Intern Med. 2013;173(3):235-237. https://doi.org/10.1001/jamainternmed.2013.1576
12. Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults a randomized trial. JAMA. 2009;301(17):1771-1778. https://doi.org/10.1001/jama.2009.561
13. Health Research & Educational Trust. Social Determinants of Health Series: Housing and the Role of Hospitals. American Hospital Association. August 2017. Accessed December 16, 2019. https://www.aha.org/ahahret-guides/2017-08-22-social-determinants-health-series-housing-and-role-hospitals
14. Boston Medical Center to Invest $6.5 Million in Affordable Housing to Improve Community Health and Patient Outcomes, Reduce Medical Costs. Press release. Boston Medical Center; December 7, 2017. Accessed March 4, 2020. https://www.bmc.org/news/press-releases/2017/12/07/boston-medical-center-invest-65-million-affordable-housing-improve
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207. https://doi.org/10.1056/nejmoa1009370
16. Chronic Pain and Opioid Management. Project ECHO. Accessed February 16, 2020. https://echo.unm.edu/teleecho-programs/chronic-pain
17. Anderson D, Zlateva I, Davis B, et al. Improving pain care with Project ECHO in community health centers. Pain Med. 2017;18(10):1882-1889. https://doi.org/10.1093/pm/pnx187
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100. https://doi.org/10.1111/pme.12715
19. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. https://doi.org/10.1001/jama.2019.16932
20. Opioid Overdoses Costing US Hospitals an Estimated $11 Billion Annually. Press Release. Premier; January 3, 2019. Accessed March 4, 2020. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
21. Butler JC. 2017 ASTHO president’s challenge: public health approaches to preventing substance misuse and addiction. J Public Health Manag Pract. 2017;23(5):531-536. https://doi.org/10.1097/phh.0000000000000631
1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. http://doi.org/10.15585/mmwr.mm675152e1
2. Kasich J, Gee EG. Don’t forget our frontline caregivers in the opioid epidemic. New York Times. Published September 18, 2019. Accessed December 16, 2019. https://www.nytimes.com/2019/09/17/opinion/opioid-settlement-hospitals.html
3. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
4. Franz B, Cronin CE, Wainwright A, Pagan JA. Measuring efforts of nonprofit hospitals to address opioid abuse after the Affordable Care Act. J Prim Care Communit. 2019;10:2150132719863611. https://doi.org/10.1177/2150132719863611
5. Venkataramani AS, Bair EF, O’Brien RL, Tsai ALC. Association between automotive assembly plant closures and opioid overdose mortality in the United States a difference-in-differences analysis. JAMA Intern Med. 2020;180(2):254-262. https://doi.org/10.1001/jamainternmed.2019.5686
6. Englander H, Gregg J, Gullickson J, et al. Recommendations for integrating peer mentors in hospital-based addiction care. Subst Abus. 2019:1-6. https://doi.org/10.1080/08897077.2019.1635968
7. Geisinger investing in employees’ careers with School at Work program. News Release. Geisinger; November 5, 2018. Updated November 5, 2018. Accessed February 17, 2020. https://www.geisinger.org/about-geisinger/news-and-media/news-releases/2018/11/19/17/31/geisinger-investing-in-employees-careers-with-school-at-work-program
8. Jackson A, Brasfield-Gorrigan H. Investing in the Future of the Healthcare Workforce: An Analysis of the Business Impact of Select Employee Development Programs at TriHealth in 2013. TriHealth. March 30, 2015. Accessed 20 April 2020. http://www.catalystlearning.com/Portals/0/Documents/TriHealth%20RoI%20Study%20Updated%20Version.pdf
9. Roy B, Stanojevich J, Stange P, Jiwani N, King R, Koo D. Development of the Community Health Improvement Navigator Database of Interventions. MMWR Suppl. 2016;65:1-9. http://doi.org/10.15585/mmwr.su6502a1
10. Sandel M, Desmond M. Investing in housing for health improves both mission and margin. JAMA. 2017;318(23):2291-2292. https://doi.org/10.1001/jama.2017.15771
11. Vijayaraghavan M, Penko J, Bangsberg DR, Miaskowski C, Kushel MB. Opioid analgesic misuse in a community-based cohort of HIV-infected indigent adults. JAMA Intern Med. 2013;173(3):235-237. https://doi.org/10.1001/jamainternmed.2013.1576
12. Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults a randomized trial. JAMA. 2009;301(17):1771-1778. https://doi.org/10.1001/jama.2009.561
13. Health Research & Educational Trust. Social Determinants of Health Series: Housing and the Role of Hospitals. American Hospital Association. August 2017. Accessed December 16, 2019. https://www.aha.org/ahahret-guides/2017-08-22-social-determinants-health-series-housing-and-role-hospitals
14. Boston Medical Center to Invest $6.5 Million in Affordable Housing to Improve Community Health and Patient Outcomes, Reduce Medical Costs. Press release. Boston Medical Center; December 7, 2017. Accessed March 4, 2020. https://www.bmc.org/news/press-releases/2017/12/07/boston-medical-center-invest-65-million-affordable-housing-improve
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207. https://doi.org/10.1056/nejmoa1009370
16. Chronic Pain and Opioid Management. Project ECHO. Accessed February 16, 2020. https://echo.unm.edu/teleecho-programs/chronic-pain
17. Anderson D, Zlateva I, Davis B, et al. Improving pain care with Project ECHO in community health centers. Pain Med. 2017;18(10):1882-1889. https://doi.org/10.1093/pm/pnx187
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100. https://doi.org/10.1111/pme.12715
19. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. https://doi.org/10.1001/jama.2019.16932
20. Opioid Overdoses Costing US Hospitals an Estimated $11 Billion Annually. Press Release. Premier; January 3, 2019. Accessed March 4, 2020. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
21. Butler JC. 2017 ASTHO president’s challenge: public health approaches to preventing substance misuse and addiction. J Public Health Manag Pract. 2017;23(5):531-536. https://doi.org/10.1097/phh.0000000000000631
© 2020 Society of Hospital Medicine
COVID-19: A Dermatologist’s Experience From the US Epicenter
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
Practice Points
- Coronavirus disease 2019 (COVID-19) can spread quickly, creating chaos in the health care system and leading to critical supply shortages within a short amount of time.
- Social distancing, quarantine, and isolation appear to be powerful tools in reducing the spread of COVID-19.
ASCO updates guideline for metastatic pancreatic cancer
Early testing for actionable genomic alterations is now recommended for metastatic pancreatic cancer patients who progress on therapy or experience intolerable toxicity and who are potential candidates for additional treatment after first-line therapy, according to an American Society of Clinical Oncology guideline update.
Both germline and somatic testing, including for microsatellite instability/mismatch repair deficiency, BRCA mutations with known significance, and NTRK gene fusions, are recommended in this population, reported Davendra P.S. Sohal, MD, MPH, of the University of Cincinnati, and colleagues on ASCO’s expert panel. The update was published online Aug. 5 in the Journal of Clinical Oncology.
The ASCO guideline on clinical decision making for patients with metastatic pancreatic cancer was first published in 2016 to address initial assessment and first- and second-line treatment options, supportive care, and follow-up and was updated in 2018.
The phase 3 POLO trial, for example, showed significantly improved progression-free survival with the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib for maintenance therapy after first-line treatment in patients with a germline BRCA1 or BRCA2 mutation and metastatic pancreatic cancer that had not progressed during first-line platinum-based chemotherapy. An integrated analysis of three studies showed that entrectinib, a potent inhibitor of tropomyosin receptor kinase (TRK) A, B, and C, safely induced durable and clinically meaningful responses in patients with NTRK fusion-positive solid tumors, and a phase 1-2 study showed that the highly selective TRK inhibitor larotrectinib had marked and durable antitumor activity in both children and adults with TRK fusion-positive solid tumors.
With respect to the new recommendation endorsing early testing for actionable genomic alterations (Recommendation 1.5), the authors noted that the results of testing can lead to treatment with PARP inhibitors, programmed death-1 (PD-1) checkpoint inhibitor therapy, TRK fusion inhibitors, and clinical trials of targeted therapies.
“Genomic testing is recommended as part of an initial assessment to ensure that the results of testing are available at the time of treatment decision where applicable after first-line therapy,” the new recommendation states.
A “qualifying statement” further notes that the decision to test should “involve a discussion between the patient and physician regarding the frequency of actionable findings, treatment implications of testing results, and genetic counseling related to germline testing.”
Recommendation 1.5 is rated by the panel as “strong” and is based on informal consensus.
The panel also added two recommendations on treatment options after first-line therapy:
- Recommendation 3.1 calls for treatment with larotrectinib or entrectinib in patient with tumors harboring NTRK fusions.
- Recommendation 3.3 states that patients with a germline BRCA1 or BCA2 mutation who have received first-line platinum-based chemotherapy without disease progression for at least 16 weeks can receive chemotherapy or PARP inhibition with olaparib.
The relevant evidence for these two recommendations is of low quality, but shows that the benefits outweigh the harms; the strength of both recommendations is “moderate.”
A qualifying statement for the latter notes that “the decision to continue treatment with chemotherapy or proceed to maintenance therapy with olaparib should be based on a discussion between the patient and the oncologist, including consideration of whether a maximum response and plateau in response to chemotherapy have been achieved, the level of cumulative toxicities associated with chemotherapy treatment, patient preference, convenience, toxicity, goals of care, cost, and clinical evidence, including a lack of overall survival benefit demonstrated in the POLO randomized controlled trial.”
This focused update includes minor modifications to three existing recommendations:
- In addition to capecitabine or erlotinib, nab-paclitaxel is now included in Recommendation 2.3 as another possible add-on to gemcitabine alone for patients with either an Eastern Cooperative Oncology Group (ECOG) performance score of 2 or a comorbidity profile that precludes more aggressive regimens. The recommendation was also updated to encourage proactive dose and schedule adjustments to minimize toxicities.
- Recommendation 3.5 now includes patients treated previously with a gemcitabine-based regimen in the criteria for the preferred second-line treatment combination of fluorouracil plus nanoliposomal irinotecan or fluorouracil plus irinotecan “where the former is unavailable.”
- Recommendation 3.7 now includes nab-paclitaxel as an add-on option to gemcitabine, and nanoliposomal irinotecan as an add-on option to fluorouracil for second-line therapy – with proactive dose and schedule adjustments to minimize toxicities – in patients with ECOG performance score of 2 or a comorbidity profile that precludes more aggressive regimens.
These three minor modifications reflect new evidence in the first-line treatment setting, including from the FRAGRANCE trial, and are based on expert panel consensus. All other recommendations in the 2018 update are endorsed for the current update, which is available at the ASCO website.
Dr. Sohal reported honoraria from Foundation Medicine, and consulting or advisory roles with Perthera, Ability Pharma, and PierianDx. He reported research funding to his institution from Novartis, Celgene, OncoMed, Bayer, Genentech, Bristol Myers Squibb, Agios, Incyte, Loxo, and Rafael Pharmaceuticals.
SOURCE: Sohal D et al. J Clin Oncol. 2020 Aug 5. doi: 10.1200/JCO.20.01364.
Early testing for actionable genomic alterations is now recommended for metastatic pancreatic cancer patients who progress on therapy or experience intolerable toxicity and who are potential candidates for additional treatment after first-line therapy, according to an American Society of Clinical Oncology guideline update.
Both germline and somatic testing, including for microsatellite instability/mismatch repair deficiency, BRCA mutations with known significance, and NTRK gene fusions, are recommended in this population, reported Davendra P.S. Sohal, MD, MPH, of the University of Cincinnati, and colleagues on ASCO’s expert panel. The update was published online Aug. 5 in the Journal of Clinical Oncology.
The ASCO guideline on clinical decision making for patients with metastatic pancreatic cancer was first published in 2016 to address initial assessment and first- and second-line treatment options, supportive care, and follow-up and was updated in 2018.
The phase 3 POLO trial, for example, showed significantly improved progression-free survival with the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib for maintenance therapy after first-line treatment in patients with a germline BRCA1 or BRCA2 mutation and metastatic pancreatic cancer that had not progressed during first-line platinum-based chemotherapy. An integrated analysis of three studies showed that entrectinib, a potent inhibitor of tropomyosin receptor kinase (TRK) A, B, and C, safely induced durable and clinically meaningful responses in patients with NTRK fusion-positive solid tumors, and a phase 1-2 study showed that the highly selective TRK inhibitor larotrectinib had marked and durable antitumor activity in both children and adults with TRK fusion-positive solid tumors.
With respect to the new recommendation endorsing early testing for actionable genomic alterations (Recommendation 1.5), the authors noted that the results of testing can lead to treatment with PARP inhibitors, programmed death-1 (PD-1) checkpoint inhibitor therapy, TRK fusion inhibitors, and clinical trials of targeted therapies.
“Genomic testing is recommended as part of an initial assessment to ensure that the results of testing are available at the time of treatment decision where applicable after first-line therapy,” the new recommendation states.
A “qualifying statement” further notes that the decision to test should “involve a discussion between the patient and physician regarding the frequency of actionable findings, treatment implications of testing results, and genetic counseling related to germline testing.”
Recommendation 1.5 is rated by the panel as “strong” and is based on informal consensus.
The panel also added two recommendations on treatment options after first-line therapy:
- Recommendation 3.1 calls for treatment with larotrectinib or entrectinib in patient with tumors harboring NTRK fusions.
- Recommendation 3.3 states that patients with a germline BRCA1 or BCA2 mutation who have received first-line platinum-based chemotherapy without disease progression for at least 16 weeks can receive chemotherapy or PARP inhibition with olaparib.
The relevant evidence for these two recommendations is of low quality, but shows that the benefits outweigh the harms; the strength of both recommendations is “moderate.”
A qualifying statement for the latter notes that “the decision to continue treatment with chemotherapy or proceed to maintenance therapy with olaparib should be based on a discussion between the patient and the oncologist, including consideration of whether a maximum response and plateau in response to chemotherapy have been achieved, the level of cumulative toxicities associated with chemotherapy treatment, patient preference, convenience, toxicity, goals of care, cost, and clinical evidence, including a lack of overall survival benefit demonstrated in the POLO randomized controlled trial.”
This focused update includes minor modifications to three existing recommendations:
- In addition to capecitabine or erlotinib, nab-paclitaxel is now included in Recommendation 2.3 as another possible add-on to gemcitabine alone for patients with either an Eastern Cooperative Oncology Group (ECOG) performance score of 2 or a comorbidity profile that precludes more aggressive regimens. The recommendation was also updated to encourage proactive dose and schedule adjustments to minimize toxicities.
- Recommendation 3.5 now includes patients treated previously with a gemcitabine-based regimen in the criteria for the preferred second-line treatment combination of fluorouracil plus nanoliposomal irinotecan or fluorouracil plus irinotecan “where the former is unavailable.”
- Recommendation 3.7 now includes nab-paclitaxel as an add-on option to gemcitabine, and nanoliposomal irinotecan as an add-on option to fluorouracil for second-line therapy – with proactive dose and schedule adjustments to minimize toxicities – in patients with ECOG performance score of 2 or a comorbidity profile that precludes more aggressive regimens.
These three minor modifications reflect new evidence in the first-line treatment setting, including from the FRAGRANCE trial, and are based on expert panel consensus. All other recommendations in the 2018 update are endorsed for the current update, which is available at the ASCO website.
Dr. Sohal reported honoraria from Foundation Medicine, and consulting or advisory roles with Perthera, Ability Pharma, and PierianDx. He reported research funding to his institution from Novartis, Celgene, OncoMed, Bayer, Genentech, Bristol Myers Squibb, Agios, Incyte, Loxo, and Rafael Pharmaceuticals.
SOURCE: Sohal D et al. J Clin Oncol. 2020 Aug 5. doi: 10.1200/JCO.20.01364.
Early testing for actionable genomic alterations is now recommended for metastatic pancreatic cancer patients who progress on therapy or experience intolerable toxicity and who are potential candidates for additional treatment after first-line therapy, according to an American Society of Clinical Oncology guideline update.
Both germline and somatic testing, including for microsatellite instability/mismatch repair deficiency, BRCA mutations with known significance, and NTRK gene fusions, are recommended in this population, reported Davendra P.S. Sohal, MD, MPH, of the University of Cincinnati, and colleagues on ASCO’s expert panel. The update was published online Aug. 5 in the Journal of Clinical Oncology.
The ASCO guideline on clinical decision making for patients with metastatic pancreatic cancer was first published in 2016 to address initial assessment and first- and second-line treatment options, supportive care, and follow-up and was updated in 2018.
The phase 3 POLO trial, for example, showed significantly improved progression-free survival with the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib for maintenance therapy after first-line treatment in patients with a germline BRCA1 or BRCA2 mutation and metastatic pancreatic cancer that had not progressed during first-line platinum-based chemotherapy. An integrated analysis of three studies showed that entrectinib, a potent inhibitor of tropomyosin receptor kinase (TRK) A, B, and C, safely induced durable and clinically meaningful responses in patients with NTRK fusion-positive solid tumors, and a phase 1-2 study showed that the highly selective TRK inhibitor larotrectinib had marked and durable antitumor activity in both children and adults with TRK fusion-positive solid tumors.
With respect to the new recommendation endorsing early testing for actionable genomic alterations (Recommendation 1.5), the authors noted that the results of testing can lead to treatment with PARP inhibitors, programmed death-1 (PD-1) checkpoint inhibitor therapy, TRK fusion inhibitors, and clinical trials of targeted therapies.
“Genomic testing is recommended as part of an initial assessment to ensure that the results of testing are available at the time of treatment decision where applicable after first-line therapy,” the new recommendation states.
A “qualifying statement” further notes that the decision to test should “involve a discussion between the patient and physician regarding the frequency of actionable findings, treatment implications of testing results, and genetic counseling related to germline testing.”
Recommendation 1.5 is rated by the panel as “strong” and is based on informal consensus.
The panel also added two recommendations on treatment options after first-line therapy:
- Recommendation 3.1 calls for treatment with larotrectinib or entrectinib in patient with tumors harboring NTRK fusions.
- Recommendation 3.3 states that patients with a germline BRCA1 or BCA2 mutation who have received first-line platinum-based chemotherapy without disease progression for at least 16 weeks can receive chemotherapy or PARP inhibition with olaparib.
The relevant evidence for these two recommendations is of low quality, but shows that the benefits outweigh the harms; the strength of both recommendations is “moderate.”
A qualifying statement for the latter notes that “the decision to continue treatment with chemotherapy or proceed to maintenance therapy with olaparib should be based on a discussion between the patient and the oncologist, including consideration of whether a maximum response and plateau in response to chemotherapy have been achieved, the level of cumulative toxicities associated with chemotherapy treatment, patient preference, convenience, toxicity, goals of care, cost, and clinical evidence, including a lack of overall survival benefit demonstrated in the POLO randomized controlled trial.”
This focused update includes minor modifications to three existing recommendations:
- In addition to capecitabine or erlotinib, nab-paclitaxel is now included in Recommendation 2.3 as another possible add-on to gemcitabine alone for patients with either an Eastern Cooperative Oncology Group (ECOG) performance score of 2 or a comorbidity profile that precludes more aggressive regimens. The recommendation was also updated to encourage proactive dose and schedule adjustments to minimize toxicities.
- Recommendation 3.5 now includes patients treated previously with a gemcitabine-based regimen in the criteria for the preferred second-line treatment combination of fluorouracil plus nanoliposomal irinotecan or fluorouracil plus irinotecan “where the former is unavailable.”
- Recommendation 3.7 now includes nab-paclitaxel as an add-on option to gemcitabine, and nanoliposomal irinotecan as an add-on option to fluorouracil for second-line therapy – with proactive dose and schedule adjustments to minimize toxicities – in patients with ECOG performance score of 2 or a comorbidity profile that precludes more aggressive regimens.
These three minor modifications reflect new evidence in the first-line treatment setting, including from the FRAGRANCE trial, and are based on expert panel consensus. All other recommendations in the 2018 update are endorsed for the current update, which is available at the ASCO website.
Dr. Sohal reported honoraria from Foundation Medicine, and consulting or advisory roles with Perthera, Ability Pharma, and PierianDx. He reported research funding to his institution from Novartis, Celgene, OncoMed, Bayer, Genentech, Bristol Myers Squibb, Agios, Incyte, Loxo, and Rafael Pharmaceuticals.
SOURCE: Sohal D et al. J Clin Oncol. 2020 Aug 5. doi: 10.1200/JCO.20.01364.
FROM JOURNAL OF CLINICAL ONCOLOGY
Incidence, prognosis of second lung cancers support long-term surveillance
Second lung cancers occurring up to a decade after the first are on the rise, but their prognosis is similar – especially when detected early – which supports long-term surveillance in survivors, finds a large population-based study.
Although guidelines recommend continued annual low-dose CT scan surveillance extending beyond 4 years for this population based on expert consensus, long-term evidence of benefit is lacking.
Investigators led by John M. Varlotto, MD, a radiation oncologist at the University of Massachusetts Medical Center, Worcester, analyzed Surveillance, Epidemiology & End Results (SEER) data for more than 58,000 patients with first and sometimes second non–small cell lung cancers initially treated by surgical resection.
Study results reported in Lung Cancer showed that the age-adjusted incidence of second lung cancers occurring 4-10 years after the first lung cancer rose sharply during the 1985-2014 study period, driven by a large uptick in women patients.
Among all patients, second lung cancers had similar overall survival as first lung cancers, but poorer lung cancer–specific survival. However, among the subset of patients having early-stage resectable disease (tumors measuring less than 4 cm with negative nodes), both outcomes were statistically indistinguishable.
“Because our investigation noted that the overall survival of patients undergoing a second lung cancer operation was similar to those patients undergoing a first operation, and because there is a rising rate of second lung cancer in lung cancer survivors, we feel that continued surveillance beyond the 4-year interval as recommended by the American Association for Thoracic Surgery as well as the [National Comprehensive Cancer Network] guidelines would be beneficial to long-term survivors of early-stage lung cancer,” Dr. Varlotto and coinvestigators wrote.
“The recent results from recent lung cancer screening studies demonstrate that females may benefit preferentially from screening … and our study suggests that these preferential benefits of increased CT scan surveillance may extend to females who are long-term survivors of lung cancer as well,” they added.
Findings in context
“As this is an observational study, it is challenging to understand what is driving the rise in prevalence of second lung cancers,” Mara Antonoff, MD, of The University of Texas MD Anderson Cancer Center in Houston commented in an interview.
“Overall, the findings are very important, as they suggest that we should continue to perform surveillance imaging for patients beyond recommended guidelines, which may allow us to achieve better survival outcomes for those individuals who develop a second lung cancer years after the first lung cancer,” she agreed.
“Just as lung cancer screening is important to identifying lung cancers at an earlier stage when they are more easily treatable and more likely to be cured, surveillance after an initial treatment for lung cancer would allow a diagnosis of second lung cancers at an earlier stage, so the patients can again achieve durable cure,” Dr. Antonoff concluded.
Study details
For the study, Dr. Varlotto and coinvestigators used data from SEER-13 and SEER-18 to identify patients with a lung cancer diagnosis during 1998-2013, and data from SEER-9, covering the years 1985-2014, to calculate rates of second cancers occurring 4-10 years after a first lung cancer.
Analyses were based on 58,758 patients with a surgically resected first primary lung cancer (55.9% with early-stage disease) and 384 patients with a surgically resected second primary lung cancer (77.6% with early-stage disease). Median follow-up was 76 months for the former and 46 months for the latter.
Results showed that in the 4-10 years after a first lung cancer diagnosis, the age-adjusted incidence of second lung cancers rose by study year but remained less than that of all other second cancers combined until the mid-2000s. Among women, incidence started rising sharply in 2001 and significantly exceeded that of all other second cancers starting in 2005.
In the entire population of study patients, propensity-adjusted analyses showed that second lung cancers were similar to first lung cancers on overall survival (P = .1726) but had worse lung cancer–specific survival (P = .0143). However, in the subset of patients with early-stage resectable disease, second and first lung cancers were similar on both overall survival (P = .3872) and lung cancer–specific survival (P = .1276).
Dr. Varlotto disclosed that he had no conflicts of interest. The study was funded by the Department of Radiation Oncology, University of Massachusetts. Dr. Antonoff disclosed that she had no relevant conflicts of interest.
SOURCE: Varlotto JM et al. Lung Cancer. 2020;147:115-122.
Second lung cancers occurring up to a decade after the first are on the rise, but their prognosis is similar – especially when detected early – which supports long-term surveillance in survivors, finds a large population-based study.
Although guidelines recommend continued annual low-dose CT scan surveillance extending beyond 4 years for this population based on expert consensus, long-term evidence of benefit is lacking.
Investigators led by John M. Varlotto, MD, a radiation oncologist at the University of Massachusetts Medical Center, Worcester, analyzed Surveillance, Epidemiology & End Results (SEER) data for more than 58,000 patients with first and sometimes second non–small cell lung cancers initially treated by surgical resection.
Study results reported in Lung Cancer showed that the age-adjusted incidence of second lung cancers occurring 4-10 years after the first lung cancer rose sharply during the 1985-2014 study period, driven by a large uptick in women patients.
Among all patients, second lung cancers had similar overall survival as first lung cancers, but poorer lung cancer–specific survival. However, among the subset of patients having early-stage resectable disease (tumors measuring less than 4 cm with negative nodes), both outcomes were statistically indistinguishable.
“Because our investigation noted that the overall survival of patients undergoing a second lung cancer operation was similar to those patients undergoing a first operation, and because there is a rising rate of second lung cancer in lung cancer survivors, we feel that continued surveillance beyond the 4-year interval as recommended by the American Association for Thoracic Surgery as well as the [National Comprehensive Cancer Network] guidelines would be beneficial to long-term survivors of early-stage lung cancer,” Dr. Varlotto and coinvestigators wrote.
“The recent results from recent lung cancer screening studies demonstrate that females may benefit preferentially from screening … and our study suggests that these preferential benefits of increased CT scan surveillance may extend to females who are long-term survivors of lung cancer as well,” they added.
Findings in context
“As this is an observational study, it is challenging to understand what is driving the rise in prevalence of second lung cancers,” Mara Antonoff, MD, of The University of Texas MD Anderson Cancer Center in Houston commented in an interview.
“Overall, the findings are very important, as they suggest that we should continue to perform surveillance imaging for patients beyond recommended guidelines, which may allow us to achieve better survival outcomes for those individuals who develop a second lung cancer years after the first lung cancer,” she agreed.
“Just as lung cancer screening is important to identifying lung cancers at an earlier stage when they are more easily treatable and more likely to be cured, surveillance after an initial treatment for lung cancer would allow a diagnosis of second lung cancers at an earlier stage, so the patients can again achieve durable cure,” Dr. Antonoff concluded.
Study details
For the study, Dr. Varlotto and coinvestigators used data from SEER-13 and SEER-18 to identify patients with a lung cancer diagnosis during 1998-2013, and data from SEER-9, covering the years 1985-2014, to calculate rates of second cancers occurring 4-10 years after a first lung cancer.
Analyses were based on 58,758 patients with a surgically resected first primary lung cancer (55.9% with early-stage disease) and 384 patients with a surgically resected second primary lung cancer (77.6% with early-stage disease). Median follow-up was 76 months for the former and 46 months for the latter.
Results showed that in the 4-10 years after a first lung cancer diagnosis, the age-adjusted incidence of second lung cancers rose by study year but remained less than that of all other second cancers combined until the mid-2000s. Among women, incidence started rising sharply in 2001 and significantly exceeded that of all other second cancers starting in 2005.
In the entire population of study patients, propensity-adjusted analyses showed that second lung cancers were similar to first lung cancers on overall survival (P = .1726) but had worse lung cancer–specific survival (P = .0143). However, in the subset of patients with early-stage resectable disease, second and first lung cancers were similar on both overall survival (P = .3872) and lung cancer–specific survival (P = .1276).
Dr. Varlotto disclosed that he had no conflicts of interest. The study was funded by the Department of Radiation Oncology, University of Massachusetts. Dr. Antonoff disclosed that she had no relevant conflicts of interest.
SOURCE: Varlotto JM et al. Lung Cancer. 2020;147:115-122.
Second lung cancers occurring up to a decade after the first are on the rise, but their prognosis is similar – especially when detected early – which supports long-term surveillance in survivors, finds a large population-based study.
Although guidelines recommend continued annual low-dose CT scan surveillance extending beyond 4 years for this population based on expert consensus, long-term evidence of benefit is lacking.
Investigators led by John M. Varlotto, MD, a radiation oncologist at the University of Massachusetts Medical Center, Worcester, analyzed Surveillance, Epidemiology & End Results (SEER) data for more than 58,000 patients with first and sometimes second non–small cell lung cancers initially treated by surgical resection.
Study results reported in Lung Cancer showed that the age-adjusted incidence of second lung cancers occurring 4-10 years after the first lung cancer rose sharply during the 1985-2014 study period, driven by a large uptick in women patients.
Among all patients, second lung cancers had similar overall survival as first lung cancers, but poorer lung cancer–specific survival. However, among the subset of patients having early-stage resectable disease (tumors measuring less than 4 cm with negative nodes), both outcomes were statistically indistinguishable.
“Because our investigation noted that the overall survival of patients undergoing a second lung cancer operation was similar to those patients undergoing a first operation, and because there is a rising rate of second lung cancer in lung cancer survivors, we feel that continued surveillance beyond the 4-year interval as recommended by the American Association for Thoracic Surgery as well as the [National Comprehensive Cancer Network] guidelines would be beneficial to long-term survivors of early-stage lung cancer,” Dr. Varlotto and coinvestigators wrote.
“The recent results from recent lung cancer screening studies demonstrate that females may benefit preferentially from screening … and our study suggests that these preferential benefits of increased CT scan surveillance may extend to females who are long-term survivors of lung cancer as well,” they added.
Findings in context
“As this is an observational study, it is challenging to understand what is driving the rise in prevalence of second lung cancers,” Mara Antonoff, MD, of The University of Texas MD Anderson Cancer Center in Houston commented in an interview.
“Overall, the findings are very important, as they suggest that we should continue to perform surveillance imaging for patients beyond recommended guidelines, which may allow us to achieve better survival outcomes for those individuals who develop a second lung cancer years after the first lung cancer,” she agreed.
“Just as lung cancer screening is important to identifying lung cancers at an earlier stage when they are more easily treatable and more likely to be cured, surveillance after an initial treatment for lung cancer would allow a diagnosis of second lung cancers at an earlier stage, so the patients can again achieve durable cure,” Dr. Antonoff concluded.
Study details
For the study, Dr. Varlotto and coinvestigators used data from SEER-13 and SEER-18 to identify patients with a lung cancer diagnosis during 1998-2013, and data from SEER-9, covering the years 1985-2014, to calculate rates of second cancers occurring 4-10 years after a first lung cancer.
Analyses were based on 58,758 patients with a surgically resected first primary lung cancer (55.9% with early-stage disease) and 384 patients with a surgically resected second primary lung cancer (77.6% with early-stage disease). Median follow-up was 76 months for the former and 46 months for the latter.
Results showed that in the 4-10 years after a first lung cancer diagnosis, the age-adjusted incidence of second lung cancers rose by study year but remained less than that of all other second cancers combined until the mid-2000s. Among women, incidence started rising sharply in 2001 and significantly exceeded that of all other second cancers starting in 2005.
In the entire population of study patients, propensity-adjusted analyses showed that second lung cancers were similar to first lung cancers on overall survival (P = .1726) but had worse lung cancer–specific survival (P = .0143). However, in the subset of patients with early-stage resectable disease, second and first lung cancers were similar on both overall survival (P = .3872) and lung cancer–specific survival (P = .1276).
Dr. Varlotto disclosed that he had no conflicts of interest. The study was funded by the Department of Radiation Oncology, University of Massachusetts. Dr. Antonoff disclosed that she had no relevant conflicts of interest.
SOURCE: Varlotto JM et al. Lung Cancer. 2020;147:115-122.
FROM LUNG CANCER
How dogs can teach parenting
Have you ever wished you could prescribe dog training classes to any of the parents of your pediatric patients? As one of the myriad people adopting a dog during COVID-19 quarantine, I have had the amusing and poignant chance to relive the principles basic to effective parenting of young children that I have been coaching about for decades.
Managing a dog instead of a child strips away layers that obfuscate parenting (e.g. child from unwanted pregnancy, fears about health issues, hopes for Harvard, wishes for the other gender, projection of expectations based on relatives, etc.) thereby making the lessons crystal clear. Unlike our perceptions for children, dog behavior does not mean anything (dog aficionados who differ, please allow poetic license). When a dog is hyper it indicates time to play or eat, not intentional defiance. Understanding this, we tend to respond more rationally.
With a dog of any age post weaning, one starts with the same basic learning abilities that will ever be present. An infant soaks up one’s caregiving for months before much training can begin, lulling parents into a mindset of having perfect skills that later requires a wrenching transition and new techniques when toddlerhood strikes.
Without expressive language feedback from a dog, we are forced to observe closely, and consciously use behavior modification techniques to get the desired behavior, but we have the advantage of seeing the effects of our management in days, not years later as for children!
Get her attention
It becomes obvious that to teach something, we need to get a dog’s attention first. A smell, appearance of a rabbit during a walk, a raindrop on the dog’s head all need to pass before a verbal command has a chance. Somehow the fact that children from toddler age on understand language (most of the time) makes parents forget that something else may be more interesting at the moment. We understand we need to teach a dog in a nondistracting environment without judging them for this requirement. In fact, trying to see what is engaging a dog or a toddler can enhance our appreciation of the world. But we stay curious about a dog’s distraction – not expecting to sense all a dog can – yet we may label a child’s repeated distraction as a flaw. Not being dogs ourselves allows us to give them the gift of being nonjudgmental.
Humans are inclined to talk to their young from birth, and, in general, the more talk the better for the child’s long-term development. Dogs can readily learn some human language but dog trainers all instruct us, when trying to teach a command, to give a single word instruction once, the same way each time, maintaining the animal’s attention, then waiting for at least a partially correct response (shaping) before rewarding. Inherent in this method is consistency and avoiding messages that are confusing because of extraneous words or emotions. While providing complex language that includes emotions is important for children overall, parents often do not differentiate times when they are actually giving an instruction from general banter, yet are upset when the child fails to follow through.
Be positive
Rather than relying on words to teach, using routines is the secret to desirable behavior in dogs. Dogs quickly develop habits (such as pooping on a certain rug) that can take many repetitions of humans supplying an alternative acceptable routine (pooping only in part of the yard) to change. Supplying an approximate alternative (rag toy instead of shoelaces), particularly if it is more exciting by being relatively novel and unavailable at other times, is far more effective than saying “No.” In fact, yelling at or hitting a dog is rarely effective because of short memory and lack of causal thinking and, in addition, can result in anxiety, shying away from interacting, or aggression; all consequences of harsh punishment in children as well.
Reinforcement works
Whatever your beliefs about dogs loving their humans, dogs understand only a small human vocabulary and are instead reinforced mainly by our attention to them that has become strongly associated with getting food or treats through instrumental conditioning. Because dogs have short memories, the most effective tools in changing their behavior are immediate attention, praise, and treats; this is also is true for children. The opposite of attention – ignoring – is very powerful in extinguishing an undesired behavior. We are told to wait at least 2 minutes after an undesired dog behavior before re-engaging. Why does this not seem to work in child rearing? Actually, it works well but is very hard for parents to do as our hearts go out to the begging child, who is part of our soul and closest kin. Soft-hearted dog owners have the same problem and often create obnoxious barking, begging, and nipping dogs as a result. These are all behaviors that could otherwise be extinguished.
Consistency is key
Behavior management works best and fastest if all the humans agree on the rules and follow them. This kind of consistency can be difficult for people training dogs as well as raising children, for many reasons. Most often there is a failure to take the time to explicitly decide on the rules; in other cases, it is lower thresholds for being annoyed and an inability to ignore a behavior. There may have been past experiences with being harshly punished, ignored, or coddled that people are are trying to overcome or reproduce; covert disagreements or desires to undermine a plan whether for the dog, the child, or the relationship; or even a desire for the dog or child to favor them by giving more treats. Sound familiar in pediatrics? With animals, objectivity and agreement may be easier to achieve because unwanted animal behavior is immediately more obviously related to training consistency than for children and may include big disincentives for humans such as barking, biting, or defecating. When these overt or covert disagreements occur in parenting children, our pediatric counseling or even family therapy may be needed. A similar acceleration plan may be available for people and their dogs (but not covered by insurance)!
While a dog may run down the stairs after a ball or a treat day after day, having forgotten that he will inevitably end up being locked in the basement for the night, we are taking advantage of the fact that dogs generally do not anticipate consequences. Yet, parents often scold even young children for a similar level of comprehension: “Didn’t you know that would break?” Fortunately, talking about consequences is educational over time for children but it needs to be done kindly with the understanding that, as with dogs, doing the same undesirable thing repeatedly is not necessarily defiance in young children but failure of our teaching. If behavior is not what you hoped for, look at what you are doing to promote it.
Much of what we call temperament is genetic in children as well as dogs. People know what to expect adopting a Jack Russell Terrier vs. a Labrador Retriever. With children we just don’t get to pick. Acceptance of what we got will make the journey easier.
We have much to cherish about dogs and children. If we lose it over the location of their poop, their forgiveness is quick. There is no such thing as too much affection. And joy is always available from both.
So why do I wish I could recommend dog training? Besides all the principles above, raising a dog together allows adults to discover mismatches in behavior management philosophies and to have a chance to see if they can negotiate a plan acceptable to both. Maybe it should be a premarital recommendation.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Have you ever wished you could prescribe dog training classes to any of the parents of your pediatric patients? As one of the myriad people adopting a dog during COVID-19 quarantine, I have had the amusing and poignant chance to relive the principles basic to effective parenting of young children that I have been coaching about for decades.
Managing a dog instead of a child strips away layers that obfuscate parenting (e.g. child from unwanted pregnancy, fears about health issues, hopes for Harvard, wishes for the other gender, projection of expectations based on relatives, etc.) thereby making the lessons crystal clear. Unlike our perceptions for children, dog behavior does not mean anything (dog aficionados who differ, please allow poetic license). When a dog is hyper it indicates time to play or eat, not intentional defiance. Understanding this, we tend to respond more rationally.
With a dog of any age post weaning, one starts with the same basic learning abilities that will ever be present. An infant soaks up one’s caregiving for months before much training can begin, lulling parents into a mindset of having perfect skills that later requires a wrenching transition and new techniques when toddlerhood strikes.
Without expressive language feedback from a dog, we are forced to observe closely, and consciously use behavior modification techniques to get the desired behavior, but we have the advantage of seeing the effects of our management in days, not years later as for children!
Get her attention
It becomes obvious that to teach something, we need to get a dog’s attention first. A smell, appearance of a rabbit during a walk, a raindrop on the dog’s head all need to pass before a verbal command has a chance. Somehow the fact that children from toddler age on understand language (most of the time) makes parents forget that something else may be more interesting at the moment. We understand we need to teach a dog in a nondistracting environment without judging them for this requirement. In fact, trying to see what is engaging a dog or a toddler can enhance our appreciation of the world. But we stay curious about a dog’s distraction – not expecting to sense all a dog can – yet we may label a child’s repeated distraction as a flaw. Not being dogs ourselves allows us to give them the gift of being nonjudgmental.
Humans are inclined to talk to their young from birth, and, in general, the more talk the better for the child’s long-term development. Dogs can readily learn some human language but dog trainers all instruct us, when trying to teach a command, to give a single word instruction once, the same way each time, maintaining the animal’s attention, then waiting for at least a partially correct response (shaping) before rewarding. Inherent in this method is consistency and avoiding messages that are confusing because of extraneous words or emotions. While providing complex language that includes emotions is important for children overall, parents often do not differentiate times when they are actually giving an instruction from general banter, yet are upset when the child fails to follow through.
Be positive
Rather than relying on words to teach, using routines is the secret to desirable behavior in dogs. Dogs quickly develop habits (such as pooping on a certain rug) that can take many repetitions of humans supplying an alternative acceptable routine (pooping only in part of the yard) to change. Supplying an approximate alternative (rag toy instead of shoelaces), particularly if it is more exciting by being relatively novel and unavailable at other times, is far more effective than saying “No.” In fact, yelling at or hitting a dog is rarely effective because of short memory and lack of causal thinking and, in addition, can result in anxiety, shying away from interacting, or aggression; all consequences of harsh punishment in children as well.
Reinforcement works
Whatever your beliefs about dogs loving their humans, dogs understand only a small human vocabulary and are instead reinforced mainly by our attention to them that has become strongly associated with getting food or treats through instrumental conditioning. Because dogs have short memories, the most effective tools in changing their behavior are immediate attention, praise, and treats; this is also is true for children. The opposite of attention – ignoring – is very powerful in extinguishing an undesired behavior. We are told to wait at least 2 minutes after an undesired dog behavior before re-engaging. Why does this not seem to work in child rearing? Actually, it works well but is very hard for parents to do as our hearts go out to the begging child, who is part of our soul and closest kin. Soft-hearted dog owners have the same problem and often create obnoxious barking, begging, and nipping dogs as a result. These are all behaviors that could otherwise be extinguished.
Consistency is key
Behavior management works best and fastest if all the humans agree on the rules and follow them. This kind of consistency can be difficult for people training dogs as well as raising children, for many reasons. Most often there is a failure to take the time to explicitly decide on the rules; in other cases, it is lower thresholds for being annoyed and an inability to ignore a behavior. There may have been past experiences with being harshly punished, ignored, or coddled that people are are trying to overcome or reproduce; covert disagreements or desires to undermine a plan whether for the dog, the child, or the relationship; or even a desire for the dog or child to favor them by giving more treats. Sound familiar in pediatrics? With animals, objectivity and agreement may be easier to achieve because unwanted animal behavior is immediately more obviously related to training consistency than for children and may include big disincentives for humans such as barking, biting, or defecating. When these overt or covert disagreements occur in parenting children, our pediatric counseling or even family therapy may be needed. A similar acceleration plan may be available for people and their dogs (but not covered by insurance)!
While a dog may run down the stairs after a ball or a treat day after day, having forgotten that he will inevitably end up being locked in the basement for the night, we are taking advantage of the fact that dogs generally do not anticipate consequences. Yet, parents often scold even young children for a similar level of comprehension: “Didn’t you know that would break?” Fortunately, talking about consequences is educational over time for children but it needs to be done kindly with the understanding that, as with dogs, doing the same undesirable thing repeatedly is not necessarily defiance in young children but failure of our teaching. If behavior is not what you hoped for, look at what you are doing to promote it.
Much of what we call temperament is genetic in children as well as dogs. People know what to expect adopting a Jack Russell Terrier vs. a Labrador Retriever. With children we just don’t get to pick. Acceptance of what we got will make the journey easier.
We have much to cherish about dogs and children. If we lose it over the location of their poop, their forgiveness is quick. There is no such thing as too much affection. And joy is always available from both.
So why do I wish I could recommend dog training? Besides all the principles above, raising a dog together allows adults to discover mismatches in behavior management philosophies and to have a chance to see if they can negotiate a plan acceptable to both. Maybe it should be a premarital recommendation.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Have you ever wished you could prescribe dog training classes to any of the parents of your pediatric patients? As one of the myriad people adopting a dog during COVID-19 quarantine, I have had the amusing and poignant chance to relive the principles basic to effective parenting of young children that I have been coaching about for decades.
Managing a dog instead of a child strips away layers that obfuscate parenting (e.g. child from unwanted pregnancy, fears about health issues, hopes for Harvard, wishes for the other gender, projection of expectations based on relatives, etc.) thereby making the lessons crystal clear. Unlike our perceptions for children, dog behavior does not mean anything (dog aficionados who differ, please allow poetic license). When a dog is hyper it indicates time to play or eat, not intentional defiance. Understanding this, we tend to respond more rationally.
With a dog of any age post weaning, one starts with the same basic learning abilities that will ever be present. An infant soaks up one’s caregiving for months before much training can begin, lulling parents into a mindset of having perfect skills that later requires a wrenching transition and new techniques when toddlerhood strikes.
Without expressive language feedback from a dog, we are forced to observe closely, and consciously use behavior modification techniques to get the desired behavior, but we have the advantage of seeing the effects of our management in days, not years later as for children!
Get her attention
It becomes obvious that to teach something, we need to get a dog’s attention first. A smell, appearance of a rabbit during a walk, a raindrop on the dog’s head all need to pass before a verbal command has a chance. Somehow the fact that children from toddler age on understand language (most of the time) makes parents forget that something else may be more interesting at the moment. We understand we need to teach a dog in a nondistracting environment without judging them for this requirement. In fact, trying to see what is engaging a dog or a toddler can enhance our appreciation of the world. But we stay curious about a dog’s distraction – not expecting to sense all a dog can – yet we may label a child’s repeated distraction as a flaw. Not being dogs ourselves allows us to give them the gift of being nonjudgmental.
Humans are inclined to talk to their young from birth, and, in general, the more talk the better for the child’s long-term development. Dogs can readily learn some human language but dog trainers all instruct us, when trying to teach a command, to give a single word instruction once, the same way each time, maintaining the animal’s attention, then waiting for at least a partially correct response (shaping) before rewarding. Inherent in this method is consistency and avoiding messages that are confusing because of extraneous words or emotions. While providing complex language that includes emotions is important for children overall, parents often do not differentiate times when they are actually giving an instruction from general banter, yet are upset when the child fails to follow through.
Be positive
Rather than relying on words to teach, using routines is the secret to desirable behavior in dogs. Dogs quickly develop habits (such as pooping on a certain rug) that can take many repetitions of humans supplying an alternative acceptable routine (pooping only in part of the yard) to change. Supplying an approximate alternative (rag toy instead of shoelaces), particularly if it is more exciting by being relatively novel and unavailable at other times, is far more effective than saying “No.” In fact, yelling at or hitting a dog is rarely effective because of short memory and lack of causal thinking and, in addition, can result in anxiety, shying away from interacting, or aggression; all consequences of harsh punishment in children as well.
Reinforcement works
Whatever your beliefs about dogs loving their humans, dogs understand only a small human vocabulary and are instead reinforced mainly by our attention to them that has become strongly associated with getting food or treats through instrumental conditioning. Because dogs have short memories, the most effective tools in changing their behavior are immediate attention, praise, and treats; this is also is true for children. The opposite of attention – ignoring – is very powerful in extinguishing an undesired behavior. We are told to wait at least 2 minutes after an undesired dog behavior before re-engaging. Why does this not seem to work in child rearing? Actually, it works well but is very hard for parents to do as our hearts go out to the begging child, who is part of our soul and closest kin. Soft-hearted dog owners have the same problem and often create obnoxious barking, begging, and nipping dogs as a result. These are all behaviors that could otherwise be extinguished.
Consistency is key
Behavior management works best and fastest if all the humans agree on the rules and follow them. This kind of consistency can be difficult for people training dogs as well as raising children, for many reasons. Most often there is a failure to take the time to explicitly decide on the rules; in other cases, it is lower thresholds for being annoyed and an inability to ignore a behavior. There may have been past experiences with being harshly punished, ignored, or coddled that people are are trying to overcome or reproduce; covert disagreements or desires to undermine a plan whether for the dog, the child, or the relationship; or even a desire for the dog or child to favor them by giving more treats. Sound familiar in pediatrics? With animals, objectivity and agreement may be easier to achieve because unwanted animal behavior is immediately more obviously related to training consistency than for children and may include big disincentives for humans such as barking, biting, or defecating. When these overt or covert disagreements occur in parenting children, our pediatric counseling or even family therapy may be needed. A similar acceleration plan may be available for people and their dogs (but not covered by insurance)!
While a dog may run down the stairs after a ball or a treat day after day, having forgotten that he will inevitably end up being locked in the basement for the night, we are taking advantage of the fact that dogs generally do not anticipate consequences. Yet, parents often scold even young children for a similar level of comprehension: “Didn’t you know that would break?” Fortunately, talking about consequences is educational over time for children but it needs to be done kindly with the understanding that, as with dogs, doing the same undesirable thing repeatedly is not necessarily defiance in young children but failure of our teaching. If behavior is not what you hoped for, look at what you are doing to promote it.
Much of what we call temperament is genetic in children as well as dogs. People know what to expect adopting a Jack Russell Terrier vs. a Labrador Retriever. With children we just don’t get to pick. Acceptance of what we got will make the journey easier.
We have much to cherish about dogs and children. If we lose it over the location of their poop, their forgiveness is quick. There is no such thing as too much affection. And joy is always available from both.
So why do I wish I could recommend dog training? Besides all the principles above, raising a dog together allows adults to discover mismatches in behavior management philosophies and to have a chance to see if they can negotiate a plan acceptable to both. Maybe it should be a premarital recommendation.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Beyond baseline, DBT no better than mammography for dense breasts
In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the baseline exam, according to a review of nearly 1.6 million screenings.
At baseline, DBT improved recall and cancer detection rates for all women. On subsequent exams, differences in screening performance between DBT and DM varied by age and density subgroups. However, there were no significant differences in recall or cancer detection rates among women with extremely dense breasts in any age group.
Kathryn Lowry, MD, of the University of Washington in Seattle, and colleagues reported these findings in JAMA Network Open.
“Our findings suggest that density likely should not be used as a criterion to triage use of DBT for routine screening in settings where DBT is not universally available, as has been reported in physician surveys,” the authors wrote. “The largest absolute improvements of DBT screening were achieved on the baseline screening examination, suggesting that women presenting for their first screening examination are particularly important to prioritize for DBT,” regardless of breast density or age.
Study details
Dr. Lowry and colleagues reviewed 1,584,079 screenings in women aged 40-79 years. The exams were done from January 2010 to April 2018 at Breast Cancer Surveillance Consortium facilities across the United States.
Sixty-five percent of the exams were in White, non-Hispanic women, 25.2% were in women younger than 50 years, and 42.4% were in women with heterogeneously dense or extremely dense breasts. Subjects had no history of breast cancer, mastectomy, or breast augmentation.
The investigators compared the performance of 1,273,492 DMs with 310,587 DBTs across the four Breast Imaging Reporting and Database System density types: almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Findings were adjusted for race, family breast cancer history, and other potential confounders.
Recall and cancer detection rates
At baseline, recall and cancer detection rates were better with DBT than with DM, regardless of breast density subtype or patient age.
For instance, in women aged 50-59 years, screening recalls per 1,000 exams dropped from 241 with DM to 204 with DBT (relative risk, 0.84; 95% confidence interval, 0.73-0.98). Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT (RR, 1.50; 95% CI, 1.10-2.08).
On follow-up exams, recall rates were lower with DBT for women with scattered fibroglandular density and heterogeneously dense breasts in all age groups, as well as in women with almost entirely fatty breasts aged 50-79 years.
“By contrast, there were no significant differences in recall rates in women with extremely dense breasts in any age group,” the authors wrote.
Cancer detection rates on follow-up exams varied by age and breast density.
Cancer detection rates were higher with DBT than with DM in women with heterogeneously dense breasts in all age groups and in women with scattered fibroglandular density at 50-59 years of age and 60-79 years of age. However, cancer detection rates were not significantly different with DBT or DM for women with almost entirely fatty breasts or extremely dense breasts of any age.
Implications and next steps
Dr. Lowry and colleagues noted that use of DBT has increased steadily since it was approved by the Food and Drug Administration in 2011, driven by studies demonstrating, among other things, earlier detection of invasive cancers.
The problem has been that previous investigations “largely dichotomized dense (heterogeneously dense and extremely dense) and nondense (almost entirely fat and scattered fibroglandular densities) categories,” the authors wrote. Therefore, the nuance of benefit across density subtypes hasn’t been clear.
The finding that “screening benefits of DBT differ for women with heterogeneously dense breasts [versus] extremely dense breasts is especially important in the current landscape of density legislation and demand for supplemental screening tests beyond mammography. To date, most state mandates and ... proposed federal legislation have uniformly grouped women with heterogeneously dense breasts and those with extremely dense breasts as a single population,” the authors wrote.
As the new findings suggest, “there are important differences in performance that may not be appreciated by combining density categories,” the authors added.
The results “suggest that women with extremely dense breast tissue may benefit more from additional screening than women with heterogeneously dense breasts who undergo tomosynthesis mammography,” Catherine Tuite, MD, of Fox Chase Cancer Center in Philadelphia, and colleagues wrote in a related editorial.
“Research to determine density and risk-specific outcomes for supplemental screening methods, such as magnetic resonance imaging ... molecular breast imaging, or ultrasonography is necessary to understand which screening method beyond DBT is best for average-risk women with heterogeneous or extremely dense breasts,” the editorialists wrote.
This research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. Dr. Lowry reported grants from GE Healthcare outside the submitted work. The editorialists didn’t have any disclosures.
SOURCE: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the baseline exam, according to a review of nearly 1.6 million screenings.
At baseline, DBT improved recall and cancer detection rates for all women. On subsequent exams, differences in screening performance between DBT and DM varied by age and density subgroups. However, there were no significant differences in recall or cancer detection rates among women with extremely dense breasts in any age group.
Kathryn Lowry, MD, of the University of Washington in Seattle, and colleagues reported these findings in JAMA Network Open.
“Our findings suggest that density likely should not be used as a criterion to triage use of DBT for routine screening in settings where DBT is not universally available, as has been reported in physician surveys,” the authors wrote. “The largest absolute improvements of DBT screening were achieved on the baseline screening examination, suggesting that women presenting for their first screening examination are particularly important to prioritize for DBT,” regardless of breast density or age.
Study details
Dr. Lowry and colleagues reviewed 1,584,079 screenings in women aged 40-79 years. The exams were done from January 2010 to April 2018 at Breast Cancer Surveillance Consortium facilities across the United States.
Sixty-five percent of the exams were in White, non-Hispanic women, 25.2% were in women younger than 50 years, and 42.4% were in women with heterogeneously dense or extremely dense breasts. Subjects had no history of breast cancer, mastectomy, or breast augmentation.
The investigators compared the performance of 1,273,492 DMs with 310,587 DBTs across the four Breast Imaging Reporting and Database System density types: almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Findings were adjusted for race, family breast cancer history, and other potential confounders.
Recall and cancer detection rates
At baseline, recall and cancer detection rates were better with DBT than with DM, regardless of breast density subtype or patient age.
For instance, in women aged 50-59 years, screening recalls per 1,000 exams dropped from 241 with DM to 204 with DBT (relative risk, 0.84; 95% confidence interval, 0.73-0.98). Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT (RR, 1.50; 95% CI, 1.10-2.08).
On follow-up exams, recall rates were lower with DBT for women with scattered fibroglandular density and heterogeneously dense breasts in all age groups, as well as in women with almost entirely fatty breasts aged 50-79 years.
“By contrast, there were no significant differences in recall rates in women with extremely dense breasts in any age group,” the authors wrote.
Cancer detection rates on follow-up exams varied by age and breast density.
Cancer detection rates were higher with DBT than with DM in women with heterogeneously dense breasts in all age groups and in women with scattered fibroglandular density at 50-59 years of age and 60-79 years of age. However, cancer detection rates were not significantly different with DBT or DM for women with almost entirely fatty breasts or extremely dense breasts of any age.
Implications and next steps
Dr. Lowry and colleagues noted that use of DBT has increased steadily since it was approved by the Food and Drug Administration in 2011, driven by studies demonstrating, among other things, earlier detection of invasive cancers.
The problem has been that previous investigations “largely dichotomized dense (heterogeneously dense and extremely dense) and nondense (almost entirely fat and scattered fibroglandular densities) categories,” the authors wrote. Therefore, the nuance of benefit across density subtypes hasn’t been clear.
The finding that “screening benefits of DBT differ for women with heterogeneously dense breasts [versus] extremely dense breasts is especially important in the current landscape of density legislation and demand for supplemental screening tests beyond mammography. To date, most state mandates and ... proposed federal legislation have uniformly grouped women with heterogeneously dense breasts and those with extremely dense breasts as a single population,” the authors wrote.
As the new findings suggest, “there are important differences in performance that may not be appreciated by combining density categories,” the authors added.
The results “suggest that women with extremely dense breast tissue may benefit more from additional screening than women with heterogeneously dense breasts who undergo tomosynthesis mammography,” Catherine Tuite, MD, of Fox Chase Cancer Center in Philadelphia, and colleagues wrote in a related editorial.
“Research to determine density and risk-specific outcomes for supplemental screening methods, such as magnetic resonance imaging ... molecular breast imaging, or ultrasonography is necessary to understand which screening method beyond DBT is best for average-risk women with heterogeneous or extremely dense breasts,” the editorialists wrote.
This research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. Dr. Lowry reported grants from GE Healthcare outside the submitted work. The editorialists didn’t have any disclosures.
SOURCE: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the baseline exam, according to a review of nearly 1.6 million screenings.
At baseline, DBT improved recall and cancer detection rates for all women. On subsequent exams, differences in screening performance between DBT and DM varied by age and density subgroups. However, there were no significant differences in recall or cancer detection rates among women with extremely dense breasts in any age group.
Kathryn Lowry, MD, of the University of Washington in Seattle, and colleagues reported these findings in JAMA Network Open.
“Our findings suggest that density likely should not be used as a criterion to triage use of DBT for routine screening in settings where DBT is not universally available, as has been reported in physician surveys,” the authors wrote. “The largest absolute improvements of DBT screening were achieved on the baseline screening examination, suggesting that women presenting for their first screening examination are particularly important to prioritize for DBT,” regardless of breast density or age.
Study details
Dr. Lowry and colleagues reviewed 1,584,079 screenings in women aged 40-79 years. The exams were done from January 2010 to April 2018 at Breast Cancer Surveillance Consortium facilities across the United States.
Sixty-five percent of the exams were in White, non-Hispanic women, 25.2% were in women younger than 50 years, and 42.4% were in women with heterogeneously dense or extremely dense breasts. Subjects had no history of breast cancer, mastectomy, or breast augmentation.
The investigators compared the performance of 1,273,492 DMs with 310,587 DBTs across the four Breast Imaging Reporting and Database System density types: almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Findings were adjusted for race, family breast cancer history, and other potential confounders.
Recall and cancer detection rates
At baseline, recall and cancer detection rates were better with DBT than with DM, regardless of breast density subtype or patient age.
For instance, in women aged 50-59 years, screening recalls per 1,000 exams dropped from 241 with DM to 204 with DBT (relative risk, 0.84; 95% confidence interval, 0.73-0.98). Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT (RR, 1.50; 95% CI, 1.10-2.08).
On follow-up exams, recall rates were lower with DBT for women with scattered fibroglandular density and heterogeneously dense breasts in all age groups, as well as in women with almost entirely fatty breasts aged 50-79 years.
“By contrast, there were no significant differences in recall rates in women with extremely dense breasts in any age group,” the authors wrote.
Cancer detection rates on follow-up exams varied by age and breast density.
Cancer detection rates were higher with DBT than with DM in women with heterogeneously dense breasts in all age groups and in women with scattered fibroglandular density at 50-59 years of age and 60-79 years of age. However, cancer detection rates were not significantly different with DBT or DM for women with almost entirely fatty breasts or extremely dense breasts of any age.
Implications and next steps
Dr. Lowry and colleagues noted that use of DBT has increased steadily since it was approved by the Food and Drug Administration in 2011, driven by studies demonstrating, among other things, earlier detection of invasive cancers.
The problem has been that previous investigations “largely dichotomized dense (heterogeneously dense and extremely dense) and nondense (almost entirely fat and scattered fibroglandular densities) categories,” the authors wrote. Therefore, the nuance of benefit across density subtypes hasn’t been clear.
The finding that “screening benefits of DBT differ for women with heterogeneously dense breasts [versus] extremely dense breasts is especially important in the current landscape of density legislation and demand for supplemental screening tests beyond mammography. To date, most state mandates and ... proposed federal legislation have uniformly grouped women with heterogeneously dense breasts and those with extremely dense breasts as a single population,” the authors wrote.
As the new findings suggest, “there are important differences in performance that may not be appreciated by combining density categories,” the authors added.
The results “suggest that women with extremely dense breast tissue may benefit more from additional screening than women with heterogeneously dense breasts who undergo tomosynthesis mammography,” Catherine Tuite, MD, of Fox Chase Cancer Center in Philadelphia, and colleagues wrote in a related editorial.
“Research to determine density and risk-specific outcomes for supplemental screening methods, such as magnetic resonance imaging ... molecular breast imaging, or ultrasonography is necessary to understand which screening method beyond DBT is best for average-risk women with heterogeneous or extremely dense breasts,” the editorialists wrote.
This research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. Dr. Lowry reported grants from GE Healthcare outside the submitted work. The editorialists didn’t have any disclosures.
SOURCE: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
FROM THE JAMA OPEN NETWORK
Quality improvement program expands early childhood screening
Primary care screening in several key areas including maternal depression and developmental delay increased significantly after practices implemented a quality improvement (QI) program, according to data from 19 pediatric primary care practices in 12 states.
Screening for developmental delay, maternal depression, and autism spectrum disorder are recommended by the American Academy of Pediatrics; screening for social-emotional problems and social determinants of health also are recommended. However, “Practices face challenges in implementing recommended screenings simultaneously,” wrote Kori B. Flower, MD, MPH, of the University of North Carolina at Chapel Hill, and colleagues in Pediatrics.
To support practices in screening, the researchers developed a national QI collaborative. “Aims were to improve screening processes, including screening, discussion, referral, and follow-up,” the researchers wrote.
In the study published in Pediatrics, the researchers reviewed data from 19 pediatric practices in 12 states, including independent, academic, hospital-affiliated, and multispecialty group practices and community health centers for diversity in type, size, location, and patient population.
The improvement program included two full-day sessions of in-person learning, separated by a 9-month action period that included virtual learning through webinars and online resources, monthly data collection to assess progress, and coaching. “Coaches used reports to guide virtual learning content and provide individual feedback to practices,” the researchers said.
Overall, Screening also increased significantly for developmental delays (from 60% to 93%), and autism spectrum disorder (from 74% to 95%).
Statistically significant increases in discussion of screening results occurred for all screening areas: developmental delays (from 63% to 97%), autism spectrum disorder (from 51% to 93%), maternal depression (from 46% to 90%), and social determinants of health (from 19% to 73%).
In addition, significant increases in referrals were seen for development (from 53% to 86%) and maternal depression (from 23% to 100%).
EHR packages deficiencies seen as barrier
“Standard EHR packages often lack features for documenting and tracking screenings, and this was a persistent barrier to screening improvement,” Dr. Flower and associates noted. However, the percentage of practices citing EHR challenges as a barrier to screening decreased from 41% at baseline to 24% after the intervention.
Parents also reported increased discussion of screening and referrals, but “[o]n overall rating of care, the percentage of parents rating care as above average or best did not change,” but parents were not asked reasons for their care rating, the researchers wrote.
The study findings were limited by several factors including limited data quality control and insufficient data to assess the effects of screening interventions on other preventive services or other office-based factors such as revenue, the researchers noted. However, the results suggest that shared learning can help primary care practices increase screening.
“Careful attention to integrating screenings in visit flow and emphasizing their potential impact on child health can make implementation possible in multiple screening areas,” Dr. Flower and colleagues concluded.
Making measurable, meaningful practice change
Barbara J. Howard, MD, commented: “It is clear that using validated tools to screen have benefits in accuracy, equity, efficiency, and income. Increasingly, practices are being judged and paid based on ‘value,’ which is especially difficult to measure in pediatrics with its low rates of serious chronic conditions to assess. We pediatricians will be judged on use of proven methods instead, and screening is a major criterion and also, fortunately, one that is within our power to change.
“However, as this study shows, a great deal of effort and teamwork is needed to shift office workflows to incorporate screening, discussions, referral, and follow-up – all necessary processes for screening to be of value. It is broadly recognized in all industries, not just health care, that use of QI processes is a major force in facilitating change in standard practices. The American Board of Pediatrics, as well as the American Academy of Pediatrics, recognizes this need and has been assisting as well as requiring use of QI methods.
“This study specifically selected a range of practices characteristic of U.S. providers to demonstrate that both screening for multiple child health risk factors simultaneously and use of methods of QI can be feasible and effective for measurable and meaningful practice change. This should give all pediatricians encouragement to move forward in implementing changes in screening,” Dr. Howard, of Johns Hopkins University, Baltimore, said in an interview.
This study not only showed the effectiveness of change management, but also detailed the effort it required, including:
- Use of monthly team meetings.
- Collecting data from patients and team members.
- Soliciting parent feedback.
- Implementing new templates for care.
- Use of tool translations or translator support.
- Involving colocated professionals, residents, and students.
- Assembling resources.
- Attempting to invoke change in EHR vendor.
“There were expert coaches involved of national prominence and extensive QI experience. Even with all this support and effort, it should be noted that 74% of practices had participated in QI efforts previously, which should have made this project easier, and even then it took 6-7 months before measurable change in practice could be documented. In spite of the fact that actually getting help for problems identified is the goal, referrals were only marginally improved, and the tracking of referrals was not significantly improved even with all this effort,” Dr. Howard noted.
“Of note, the practices reported at the end of the project that fewer practices reported lack of time or resources for screening and referral. As a result of this publication, a slimmed down set of practice report measures might be chosen to make future QI efforts work and be measurable in meaningful ways. Instead of paper chart reviews, data from electronic screening could be automatically collected in the course of care. Referral processes could likewise be made electronic and automated, including tracking their success, not just those through a local EHR. Integration of Software as a Service with EHRs could make this data collection – that is essential to both QI and actual good care – seamless. Templates and checklists, as well as more incidental knowledge gained from this and other QI projects in pediatric practices, should be shared. While each practice operates somewhat differently, the differences are not that great and, in some cases, traditional ways of doing things would be fruitfully discarded,” suggested Dr. Howard, who was not involved in the study.
“While the pediatricians participated in the QI sessions, it is clear that the QI processes depend on the entire practice team, and generally, the team members more critical to success are not the doctor but the front desk receptionist, medical assistants, and the practice managers – as these individuals conduct or oversee workflow activities. Future QI interventions might include reinforcement and acknowledgment of these team members through inclusion in parallel continuing education activities from the American Association of Medical Assistants and the Medical Group Management Association continuing education credits,” she said.
Dr. Howard continued, “Of note, these studies were completed prior to the pandemic-related workflow changes including telehealth visits and requirements to minimize waiting room time and activities for the safety of patients and staff. These disruptive forces and the likelihood that telehealth alternatives will persist in primary care suggest that the traditional paper waiting room questionnaires are likely to have to give way to electronic alternatives. Using all electronic [approaches] will be the best unified workflow.”
The study was supported by the JPB Foundation through support to the American Academy of Pediatrics. The researchers had no financial conflicts to disclose. Dr. Howard is a pediatric founder of CHADIS, an online screening, decision support, patient education, and referral/tracking system in use nationally and implemented using QI processes. CHADIS is distributed by Total Child Health, of which Dr. Howard is president. Use of CHADIS for Part 4 Maintenance of Certification QI programs is under the ABMS portfolio sponsorship of the nonprofit Center for Promotion of Child Development through Primary Care, directed by her husband, Raymond Sturner, MD.
SOURCE: Flower KB et al. Pediatrics. 2020 Aug 7. doi: 10.1542/peds.2019-2328.
Primary care screening in several key areas including maternal depression and developmental delay increased significantly after practices implemented a quality improvement (QI) program, according to data from 19 pediatric primary care practices in 12 states.
Screening for developmental delay, maternal depression, and autism spectrum disorder are recommended by the American Academy of Pediatrics; screening for social-emotional problems and social determinants of health also are recommended. However, “Practices face challenges in implementing recommended screenings simultaneously,” wrote Kori B. Flower, MD, MPH, of the University of North Carolina at Chapel Hill, and colleagues in Pediatrics.
To support practices in screening, the researchers developed a national QI collaborative. “Aims were to improve screening processes, including screening, discussion, referral, and follow-up,” the researchers wrote.
In the study published in Pediatrics, the researchers reviewed data from 19 pediatric practices in 12 states, including independent, academic, hospital-affiliated, and multispecialty group practices and community health centers for diversity in type, size, location, and patient population.
The improvement program included two full-day sessions of in-person learning, separated by a 9-month action period that included virtual learning through webinars and online resources, monthly data collection to assess progress, and coaching. “Coaches used reports to guide virtual learning content and provide individual feedback to practices,” the researchers said.
Overall, Screening also increased significantly for developmental delays (from 60% to 93%), and autism spectrum disorder (from 74% to 95%).
Statistically significant increases in discussion of screening results occurred for all screening areas: developmental delays (from 63% to 97%), autism spectrum disorder (from 51% to 93%), maternal depression (from 46% to 90%), and social determinants of health (from 19% to 73%).
In addition, significant increases in referrals were seen for development (from 53% to 86%) and maternal depression (from 23% to 100%).
EHR packages deficiencies seen as barrier
“Standard EHR packages often lack features for documenting and tracking screenings, and this was a persistent barrier to screening improvement,” Dr. Flower and associates noted. However, the percentage of practices citing EHR challenges as a barrier to screening decreased from 41% at baseline to 24% after the intervention.
Parents also reported increased discussion of screening and referrals, but “[o]n overall rating of care, the percentage of parents rating care as above average or best did not change,” but parents were not asked reasons for their care rating, the researchers wrote.
The study findings were limited by several factors including limited data quality control and insufficient data to assess the effects of screening interventions on other preventive services or other office-based factors such as revenue, the researchers noted. However, the results suggest that shared learning can help primary care practices increase screening.
“Careful attention to integrating screenings in visit flow and emphasizing their potential impact on child health can make implementation possible in multiple screening areas,” Dr. Flower and colleagues concluded.
Making measurable, meaningful practice change
Barbara J. Howard, MD, commented: “It is clear that using validated tools to screen have benefits in accuracy, equity, efficiency, and income. Increasingly, practices are being judged and paid based on ‘value,’ which is especially difficult to measure in pediatrics with its low rates of serious chronic conditions to assess. We pediatricians will be judged on use of proven methods instead, and screening is a major criterion and also, fortunately, one that is within our power to change.
“However, as this study shows, a great deal of effort and teamwork is needed to shift office workflows to incorporate screening, discussions, referral, and follow-up – all necessary processes for screening to be of value. It is broadly recognized in all industries, not just health care, that use of QI processes is a major force in facilitating change in standard practices. The American Board of Pediatrics, as well as the American Academy of Pediatrics, recognizes this need and has been assisting as well as requiring use of QI methods.
“This study specifically selected a range of practices characteristic of U.S. providers to demonstrate that both screening for multiple child health risk factors simultaneously and use of methods of QI can be feasible and effective for measurable and meaningful practice change. This should give all pediatricians encouragement to move forward in implementing changes in screening,” Dr. Howard, of Johns Hopkins University, Baltimore, said in an interview.
This study not only showed the effectiveness of change management, but also detailed the effort it required, including:
- Use of monthly team meetings.
- Collecting data from patients and team members.
- Soliciting parent feedback.
- Implementing new templates for care.
- Use of tool translations or translator support.
- Involving colocated professionals, residents, and students.
- Assembling resources.
- Attempting to invoke change in EHR vendor.
“There were expert coaches involved of national prominence and extensive QI experience. Even with all this support and effort, it should be noted that 74% of practices had participated in QI efforts previously, which should have made this project easier, and even then it took 6-7 months before measurable change in practice could be documented. In spite of the fact that actually getting help for problems identified is the goal, referrals were only marginally improved, and the tracking of referrals was not significantly improved even with all this effort,” Dr. Howard noted.
“Of note, the practices reported at the end of the project that fewer practices reported lack of time or resources for screening and referral. As a result of this publication, a slimmed down set of practice report measures might be chosen to make future QI efforts work and be measurable in meaningful ways. Instead of paper chart reviews, data from electronic screening could be automatically collected in the course of care. Referral processes could likewise be made electronic and automated, including tracking their success, not just those through a local EHR. Integration of Software as a Service with EHRs could make this data collection – that is essential to both QI and actual good care – seamless. Templates and checklists, as well as more incidental knowledge gained from this and other QI projects in pediatric practices, should be shared. While each practice operates somewhat differently, the differences are not that great and, in some cases, traditional ways of doing things would be fruitfully discarded,” suggested Dr. Howard, who was not involved in the study.
“While the pediatricians participated in the QI sessions, it is clear that the QI processes depend on the entire practice team, and generally, the team members more critical to success are not the doctor but the front desk receptionist, medical assistants, and the practice managers – as these individuals conduct or oversee workflow activities. Future QI interventions might include reinforcement and acknowledgment of these team members through inclusion in parallel continuing education activities from the American Association of Medical Assistants and the Medical Group Management Association continuing education credits,” she said.
Dr. Howard continued, “Of note, these studies were completed prior to the pandemic-related workflow changes including telehealth visits and requirements to minimize waiting room time and activities for the safety of patients and staff. These disruptive forces and the likelihood that telehealth alternatives will persist in primary care suggest that the traditional paper waiting room questionnaires are likely to have to give way to electronic alternatives. Using all electronic [approaches] will be the best unified workflow.”
The study was supported by the JPB Foundation through support to the American Academy of Pediatrics. The researchers had no financial conflicts to disclose. Dr. Howard is a pediatric founder of CHADIS, an online screening, decision support, patient education, and referral/tracking system in use nationally and implemented using QI processes. CHADIS is distributed by Total Child Health, of which Dr. Howard is president. Use of CHADIS for Part 4 Maintenance of Certification QI programs is under the ABMS portfolio sponsorship of the nonprofit Center for Promotion of Child Development through Primary Care, directed by her husband, Raymond Sturner, MD.
SOURCE: Flower KB et al. Pediatrics. 2020 Aug 7. doi: 10.1542/peds.2019-2328.
Primary care screening in several key areas including maternal depression and developmental delay increased significantly after practices implemented a quality improvement (QI) program, according to data from 19 pediatric primary care practices in 12 states.
Screening for developmental delay, maternal depression, and autism spectrum disorder are recommended by the American Academy of Pediatrics; screening for social-emotional problems and social determinants of health also are recommended. However, “Practices face challenges in implementing recommended screenings simultaneously,” wrote Kori B. Flower, MD, MPH, of the University of North Carolina at Chapel Hill, and colleagues in Pediatrics.
To support practices in screening, the researchers developed a national QI collaborative. “Aims were to improve screening processes, including screening, discussion, referral, and follow-up,” the researchers wrote.
In the study published in Pediatrics, the researchers reviewed data from 19 pediatric practices in 12 states, including independent, academic, hospital-affiliated, and multispecialty group practices and community health centers for diversity in type, size, location, and patient population.
The improvement program included two full-day sessions of in-person learning, separated by a 9-month action period that included virtual learning through webinars and online resources, monthly data collection to assess progress, and coaching. “Coaches used reports to guide virtual learning content and provide individual feedback to practices,” the researchers said.
Overall, Screening also increased significantly for developmental delays (from 60% to 93%), and autism spectrum disorder (from 74% to 95%).
Statistically significant increases in discussion of screening results occurred for all screening areas: developmental delays (from 63% to 97%), autism spectrum disorder (from 51% to 93%), maternal depression (from 46% to 90%), and social determinants of health (from 19% to 73%).
In addition, significant increases in referrals were seen for development (from 53% to 86%) and maternal depression (from 23% to 100%).
EHR packages deficiencies seen as barrier
“Standard EHR packages often lack features for documenting and tracking screenings, and this was a persistent barrier to screening improvement,” Dr. Flower and associates noted. However, the percentage of practices citing EHR challenges as a barrier to screening decreased from 41% at baseline to 24% after the intervention.
Parents also reported increased discussion of screening and referrals, but “[o]n overall rating of care, the percentage of parents rating care as above average or best did not change,” but parents were not asked reasons for their care rating, the researchers wrote.
The study findings were limited by several factors including limited data quality control and insufficient data to assess the effects of screening interventions on other preventive services or other office-based factors such as revenue, the researchers noted. However, the results suggest that shared learning can help primary care practices increase screening.
“Careful attention to integrating screenings in visit flow and emphasizing their potential impact on child health can make implementation possible in multiple screening areas,” Dr. Flower and colleagues concluded.
Making measurable, meaningful practice change
Barbara J. Howard, MD, commented: “It is clear that using validated tools to screen have benefits in accuracy, equity, efficiency, and income. Increasingly, practices are being judged and paid based on ‘value,’ which is especially difficult to measure in pediatrics with its low rates of serious chronic conditions to assess. We pediatricians will be judged on use of proven methods instead, and screening is a major criterion and also, fortunately, one that is within our power to change.
“However, as this study shows, a great deal of effort and teamwork is needed to shift office workflows to incorporate screening, discussions, referral, and follow-up – all necessary processes for screening to be of value. It is broadly recognized in all industries, not just health care, that use of QI processes is a major force in facilitating change in standard practices. The American Board of Pediatrics, as well as the American Academy of Pediatrics, recognizes this need and has been assisting as well as requiring use of QI methods.
“This study specifically selected a range of practices characteristic of U.S. providers to demonstrate that both screening for multiple child health risk factors simultaneously and use of methods of QI can be feasible and effective for measurable and meaningful practice change. This should give all pediatricians encouragement to move forward in implementing changes in screening,” Dr. Howard, of Johns Hopkins University, Baltimore, said in an interview.
This study not only showed the effectiveness of change management, but also detailed the effort it required, including:
- Use of monthly team meetings.
- Collecting data from patients and team members.
- Soliciting parent feedback.
- Implementing new templates for care.
- Use of tool translations or translator support.
- Involving colocated professionals, residents, and students.
- Assembling resources.
- Attempting to invoke change in EHR vendor.
“There were expert coaches involved of national prominence and extensive QI experience. Even with all this support and effort, it should be noted that 74% of practices had participated in QI efforts previously, which should have made this project easier, and even then it took 6-7 months before measurable change in practice could be documented. In spite of the fact that actually getting help for problems identified is the goal, referrals were only marginally improved, and the tracking of referrals was not significantly improved even with all this effort,” Dr. Howard noted.
“Of note, the practices reported at the end of the project that fewer practices reported lack of time or resources for screening and referral. As a result of this publication, a slimmed down set of practice report measures might be chosen to make future QI efforts work and be measurable in meaningful ways. Instead of paper chart reviews, data from electronic screening could be automatically collected in the course of care. Referral processes could likewise be made electronic and automated, including tracking their success, not just those through a local EHR. Integration of Software as a Service with EHRs could make this data collection – that is essential to both QI and actual good care – seamless. Templates and checklists, as well as more incidental knowledge gained from this and other QI projects in pediatric practices, should be shared. While each practice operates somewhat differently, the differences are not that great and, in some cases, traditional ways of doing things would be fruitfully discarded,” suggested Dr. Howard, who was not involved in the study.
“While the pediatricians participated in the QI sessions, it is clear that the QI processes depend on the entire practice team, and generally, the team members more critical to success are not the doctor but the front desk receptionist, medical assistants, and the practice managers – as these individuals conduct or oversee workflow activities. Future QI interventions might include reinforcement and acknowledgment of these team members through inclusion in parallel continuing education activities from the American Association of Medical Assistants and the Medical Group Management Association continuing education credits,” she said.
Dr. Howard continued, “Of note, these studies were completed prior to the pandemic-related workflow changes including telehealth visits and requirements to minimize waiting room time and activities for the safety of patients and staff. These disruptive forces and the likelihood that telehealth alternatives will persist in primary care suggest that the traditional paper waiting room questionnaires are likely to have to give way to electronic alternatives. Using all electronic [approaches] will be the best unified workflow.”
The study was supported by the JPB Foundation through support to the American Academy of Pediatrics. The researchers had no financial conflicts to disclose. Dr. Howard is a pediatric founder of CHADIS, an online screening, decision support, patient education, and referral/tracking system in use nationally and implemented using QI processes. CHADIS is distributed by Total Child Health, of which Dr. Howard is president. Use of CHADIS for Part 4 Maintenance of Certification QI programs is under the ABMS portfolio sponsorship of the nonprofit Center for Promotion of Child Development through Primary Care, directed by her husband, Raymond Sturner, MD.
SOURCE: Flower KB et al. Pediatrics. 2020 Aug 7. doi: 10.1542/peds.2019-2328.
FROM PEDIATRICS
Coping with COVID-19, racism, and other stressors
The start of a new school year is usually a time of excitement and return to routine, structure, and consistency for children, teenagers, and families. With the current COVID-19 pandemic, this year is anything but typical. Face masks, hand washing, physical distancing, remote learning, and restrictions on extracurricular activities are just a few of the changes experienced by children in schools. At home, the disruptions and uncertainty for families are equally dramatic with loss of employment, limited child care, risk of eviction and foreclosure, food insecurity, and growing numbers of families directly impacted by loss of health and life due to the coronavirus.
While every family is impacted by the current global pandemic, the realities of the pandemic have thrown increasing light on the racial, social, and structural injustices in our system. People of color are much more likely to be infected, have more severe disease, and die from COVID-19; they are more likely to experience the socioeconomic impacts.1 Centuries of racial injustice and inequity have been highlighted not just by this pandemic but by ongoing differential treatment of people of color in our education, health, justice, economic, and housing systems. The murders of George Floyd, Breonna Taylor, Ahmaud Arbery, and too many others are just one source of the constant stress facing children and families of color.
While each family and individual currently faces a distinct combination of stressors and adversity, no one has been spared from these disruptions. International, national, and local communities all need to continue efforts to overcome the current pandemic and systemic racism. As providers, we have a profound opportunity and responsibility to engage both in advocacy for our communities and the individual care of children and families. We are aware of the negative impacts of acute and chronic stress on long-term health outcomes but are equally familiar with the power of resilience.
Resilience has broadly been defined as the “process of adapting well in the face of adversity, trauma, tragedy, threats or significant sources of stress.”2 Some have argued that resilience should be further defined to include an individual making a “conscious effort to move forward” after or during adversity.3 Another definition with particular utility in considering how to develop and promote resilience describes it as “a process to harness resources to sustain well-being.”3 This definition not only discusses the end result, but the need to reach beyond the current capacity of an individual by harnessing both internal and external resources. These resources may be as tangible as money, food, infrastructure, or treatment, but also can include relationships, social capital, and the lived experience of others. Social supports, mature mentors, and solid bonds with parents/caregivers are critical resources for the development of child and adolescent resilience.4,5
by both being a resource and helping them harness other resources that can lead to physical, emotional, and relationship well-being. To do this, consider incorporating the following into your practice:
Help children and adolescents identify and reach out to positive supports
Research has shown the importance of a stable adult figure in the development of resilience in children.4,5 Ideally, parents will be a major positive support to their children in times of crisis. When parents are not appropriate supports, teachers, coaches, mentors, grandparents, or other extended family members can provide the needed support for children to be resilient across educational, emotional, and relationship domains.4 To find out who your patients have as a stable adult figure, ask the following or a related question: “Who do you have in your life who you can talk to or get support from on a regular basis?”
Screen for substance use and mental health challenges
Do this for children, adolescents, AND adults. Then treat and refer to appropriate treatment as indicated. Rates of depression, anxiety, suicide, substance use, and overdose all have increased with recent events.6 Treating parents with mental health and substance use disorders will not only facilitate their ability to be a positive support and role model for their children and promote resilience, but it has been shown to decrease child psychopathology.7 Providing parents with referrals for substance use and mental health services as well as educating them on the importance of self-care is vital for helping the development of children.
Provide parents with resources on how to cope with ongoing stressors
These stressors may be related to the COVID-19 pandemic, racism, or both. By providing resources to parents, they can better help their children overcome stressors. Multiple organizations have free online collections to support parents and families including the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and many others (See below for a list of resources).
Encourage families to find and develop purpose and meaning during this time. Children and families have devoted their time to many activities, some more adaptive and health promoting than others. If we think of resilience as the process of “moving forward” then developing goals and plans to be productive can be helpful and “meaning-making.”3 Spending time together as families, developing skills, accomplishing goals, becoming involved in important social movements, or volunteering all can be ways that individuals and families can develop feelings of self-worth, purpose, and accomplishment.2
Dr. Heward is a child and adolescent psychiatrist at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
Resources: Coping with COVID-19
1. American Academy of Pediatrics HealthyChildren.org page on COVID-19.
2. American Academy of Child and Adolescent Psychiatry COVID-19 Resources for Families.
3. American Psychiatric Association COVID-19 Resources for Families.
4. American Psychological Association COVID-19 Information and Resources.
Resources: Racism and discrimination
1. American Academy of Pediatrics Talking to Children About Racial Bias.
2. American Academy of Child and Adolescent Psychiatry Racism Resource Library.
3. American Psychological Association Bias, Discrimination, and Equity Resources.
References
1. “Double jeopardy: COVID-19 and behavioral health disparities for Black and Latino communities in the U.S.” Substance Abuse and Mental Health Services Administration. (Submitted by Office of Behavioral Health Equity).
2. “Building your resilience.” American Psychological Association.
3. Eur J Psychotraumatol. 2014 Oct 1. doi: 10.3402/ejpt.v5.25338.
4. Psychological and biological factors associated with resilience to stress and trauma, in “The Unbroken Soul: Tragedy, Trauma, and Human Resilience” (Lanham, Md.: Jason Aronson, 2008, pp.129-51).
5. Biol Psychiatry. 2019 Sep 15. doi: 10.1016/j.biopsych.2019.07.012.
6. MMWR Morb Mortal Wkly Rep. 2020;69:1049-57.
7. J Am Acad Child Adolesc Psychiatry. 2008 Apr;47(4):379-89.
The start of a new school year is usually a time of excitement and return to routine, structure, and consistency for children, teenagers, and families. With the current COVID-19 pandemic, this year is anything but typical. Face masks, hand washing, physical distancing, remote learning, and restrictions on extracurricular activities are just a few of the changes experienced by children in schools. At home, the disruptions and uncertainty for families are equally dramatic with loss of employment, limited child care, risk of eviction and foreclosure, food insecurity, and growing numbers of families directly impacted by loss of health and life due to the coronavirus.
While every family is impacted by the current global pandemic, the realities of the pandemic have thrown increasing light on the racial, social, and structural injustices in our system. People of color are much more likely to be infected, have more severe disease, and die from COVID-19; they are more likely to experience the socioeconomic impacts.1 Centuries of racial injustice and inequity have been highlighted not just by this pandemic but by ongoing differential treatment of people of color in our education, health, justice, economic, and housing systems. The murders of George Floyd, Breonna Taylor, Ahmaud Arbery, and too many others are just one source of the constant stress facing children and families of color.
While each family and individual currently faces a distinct combination of stressors and adversity, no one has been spared from these disruptions. International, national, and local communities all need to continue efforts to overcome the current pandemic and systemic racism. As providers, we have a profound opportunity and responsibility to engage both in advocacy for our communities and the individual care of children and families. We are aware of the negative impacts of acute and chronic stress on long-term health outcomes but are equally familiar with the power of resilience.
Resilience has broadly been defined as the “process of adapting well in the face of adversity, trauma, tragedy, threats or significant sources of stress.”2 Some have argued that resilience should be further defined to include an individual making a “conscious effort to move forward” after or during adversity.3 Another definition with particular utility in considering how to develop and promote resilience describes it as “a process to harness resources to sustain well-being.”3 This definition not only discusses the end result, but the need to reach beyond the current capacity of an individual by harnessing both internal and external resources. These resources may be as tangible as money, food, infrastructure, or treatment, but also can include relationships, social capital, and the lived experience of others. Social supports, mature mentors, and solid bonds with parents/caregivers are critical resources for the development of child and adolescent resilience.4,5
by both being a resource and helping them harness other resources that can lead to physical, emotional, and relationship well-being. To do this, consider incorporating the following into your practice:
Help children and adolescents identify and reach out to positive supports
Research has shown the importance of a stable adult figure in the development of resilience in children.4,5 Ideally, parents will be a major positive support to their children in times of crisis. When parents are not appropriate supports, teachers, coaches, mentors, grandparents, or other extended family members can provide the needed support for children to be resilient across educational, emotional, and relationship domains.4 To find out who your patients have as a stable adult figure, ask the following or a related question: “Who do you have in your life who you can talk to or get support from on a regular basis?”
Screen for substance use and mental health challenges
Do this for children, adolescents, AND adults. Then treat and refer to appropriate treatment as indicated. Rates of depression, anxiety, suicide, substance use, and overdose all have increased with recent events.6 Treating parents with mental health and substance use disorders will not only facilitate their ability to be a positive support and role model for their children and promote resilience, but it has been shown to decrease child psychopathology.7 Providing parents with referrals for substance use and mental health services as well as educating them on the importance of self-care is vital for helping the development of children.
Provide parents with resources on how to cope with ongoing stressors
These stressors may be related to the COVID-19 pandemic, racism, or both. By providing resources to parents, they can better help their children overcome stressors. Multiple organizations have free online collections to support parents and families including the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and many others (See below for a list of resources).
Encourage families to find and develop purpose and meaning during this time. Children and families have devoted their time to many activities, some more adaptive and health promoting than others. If we think of resilience as the process of “moving forward” then developing goals and plans to be productive can be helpful and “meaning-making.”3 Spending time together as families, developing skills, accomplishing goals, becoming involved in important social movements, or volunteering all can be ways that individuals and families can develop feelings of self-worth, purpose, and accomplishment.2
Dr. Heward is a child and adolescent psychiatrist at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
Resources: Coping with COVID-19
1. American Academy of Pediatrics HealthyChildren.org page on COVID-19.
2. American Academy of Child and Adolescent Psychiatry COVID-19 Resources for Families.
3. American Psychiatric Association COVID-19 Resources for Families.
4. American Psychological Association COVID-19 Information and Resources.
Resources: Racism and discrimination
1. American Academy of Pediatrics Talking to Children About Racial Bias.
2. American Academy of Child and Adolescent Psychiatry Racism Resource Library.
3. American Psychological Association Bias, Discrimination, and Equity Resources.
References
1. “Double jeopardy: COVID-19 and behavioral health disparities for Black and Latino communities in the U.S.” Substance Abuse and Mental Health Services Administration. (Submitted by Office of Behavioral Health Equity).
2. “Building your resilience.” American Psychological Association.
3. Eur J Psychotraumatol. 2014 Oct 1. doi: 10.3402/ejpt.v5.25338.
4. Psychological and biological factors associated with resilience to stress and trauma, in “The Unbroken Soul: Tragedy, Trauma, and Human Resilience” (Lanham, Md.: Jason Aronson, 2008, pp.129-51).
5. Biol Psychiatry. 2019 Sep 15. doi: 10.1016/j.biopsych.2019.07.012.
6. MMWR Morb Mortal Wkly Rep. 2020;69:1049-57.
7. J Am Acad Child Adolesc Psychiatry. 2008 Apr;47(4):379-89.
The start of a new school year is usually a time of excitement and return to routine, structure, and consistency for children, teenagers, and families. With the current COVID-19 pandemic, this year is anything but typical. Face masks, hand washing, physical distancing, remote learning, and restrictions on extracurricular activities are just a few of the changes experienced by children in schools. At home, the disruptions and uncertainty for families are equally dramatic with loss of employment, limited child care, risk of eviction and foreclosure, food insecurity, and growing numbers of families directly impacted by loss of health and life due to the coronavirus.
While every family is impacted by the current global pandemic, the realities of the pandemic have thrown increasing light on the racial, social, and structural injustices in our system. People of color are much more likely to be infected, have more severe disease, and die from COVID-19; they are more likely to experience the socioeconomic impacts.1 Centuries of racial injustice and inequity have been highlighted not just by this pandemic but by ongoing differential treatment of people of color in our education, health, justice, economic, and housing systems. The murders of George Floyd, Breonna Taylor, Ahmaud Arbery, and too many others are just one source of the constant stress facing children and families of color.
While each family and individual currently faces a distinct combination of stressors and adversity, no one has been spared from these disruptions. International, national, and local communities all need to continue efforts to overcome the current pandemic and systemic racism. As providers, we have a profound opportunity and responsibility to engage both in advocacy for our communities and the individual care of children and families. We are aware of the negative impacts of acute and chronic stress on long-term health outcomes but are equally familiar with the power of resilience.
Resilience has broadly been defined as the “process of adapting well in the face of adversity, trauma, tragedy, threats or significant sources of stress.”2 Some have argued that resilience should be further defined to include an individual making a “conscious effort to move forward” after or during adversity.3 Another definition with particular utility in considering how to develop and promote resilience describes it as “a process to harness resources to sustain well-being.”3 This definition not only discusses the end result, but the need to reach beyond the current capacity of an individual by harnessing both internal and external resources. These resources may be as tangible as money, food, infrastructure, or treatment, but also can include relationships, social capital, and the lived experience of others. Social supports, mature mentors, and solid bonds with parents/caregivers are critical resources for the development of child and adolescent resilience.4,5
by both being a resource and helping them harness other resources that can lead to physical, emotional, and relationship well-being. To do this, consider incorporating the following into your practice:
Help children and adolescents identify and reach out to positive supports
Research has shown the importance of a stable adult figure in the development of resilience in children.4,5 Ideally, parents will be a major positive support to their children in times of crisis. When parents are not appropriate supports, teachers, coaches, mentors, grandparents, or other extended family members can provide the needed support for children to be resilient across educational, emotional, and relationship domains.4 To find out who your patients have as a stable adult figure, ask the following or a related question: “Who do you have in your life who you can talk to or get support from on a regular basis?”
Screen for substance use and mental health challenges
Do this for children, adolescents, AND adults. Then treat and refer to appropriate treatment as indicated. Rates of depression, anxiety, suicide, substance use, and overdose all have increased with recent events.6 Treating parents with mental health and substance use disorders will not only facilitate their ability to be a positive support and role model for their children and promote resilience, but it has been shown to decrease child psychopathology.7 Providing parents with referrals for substance use and mental health services as well as educating them on the importance of self-care is vital for helping the development of children.
Provide parents with resources on how to cope with ongoing stressors
These stressors may be related to the COVID-19 pandemic, racism, or both. By providing resources to parents, they can better help their children overcome stressors. Multiple organizations have free online collections to support parents and families including the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and many others (See below for a list of resources).
Encourage families to find and develop purpose and meaning during this time. Children and families have devoted their time to many activities, some more adaptive and health promoting than others. If we think of resilience as the process of “moving forward” then developing goals and plans to be productive can be helpful and “meaning-making.”3 Spending time together as families, developing skills, accomplishing goals, becoming involved in important social movements, or volunteering all can be ways that individuals and families can develop feelings of self-worth, purpose, and accomplishment.2
Dr. Heward is a child and adolescent psychiatrist at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
Resources: Coping with COVID-19
1. American Academy of Pediatrics HealthyChildren.org page on COVID-19.
2. American Academy of Child and Adolescent Psychiatry COVID-19 Resources for Families.
3. American Psychiatric Association COVID-19 Resources for Families.
4. American Psychological Association COVID-19 Information and Resources.
Resources: Racism and discrimination
1. American Academy of Pediatrics Talking to Children About Racial Bias.
2. American Academy of Child and Adolescent Psychiatry Racism Resource Library.
3. American Psychological Association Bias, Discrimination, and Equity Resources.
References
1. “Double jeopardy: COVID-19 and behavioral health disparities for Black and Latino communities in the U.S.” Substance Abuse and Mental Health Services Administration. (Submitted by Office of Behavioral Health Equity).
2. “Building your resilience.” American Psychological Association.
3. Eur J Psychotraumatol. 2014 Oct 1. doi: 10.3402/ejpt.v5.25338.
4. Psychological and biological factors associated with resilience to stress and trauma, in “The Unbroken Soul: Tragedy, Trauma, and Human Resilience” (Lanham, Md.: Jason Aronson, 2008, pp.129-51).
5. Biol Psychiatry. 2019 Sep 15. doi: 10.1016/j.biopsych.2019.07.012.
6. MMWR Morb Mortal Wkly Rep. 2020;69:1049-57.
7. J Am Acad Child Adolesc Psychiatry. 2008 Apr;47(4):379-89.










