User login
Liquid Biopsy for Colorectal Cancer Appears Promising But Still Lacks Robust Efficacy
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
Skin Lesions on the Face and Chest
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
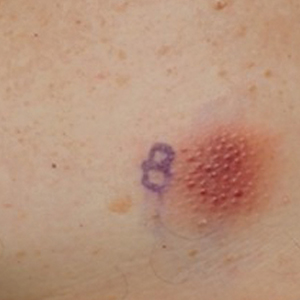
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.
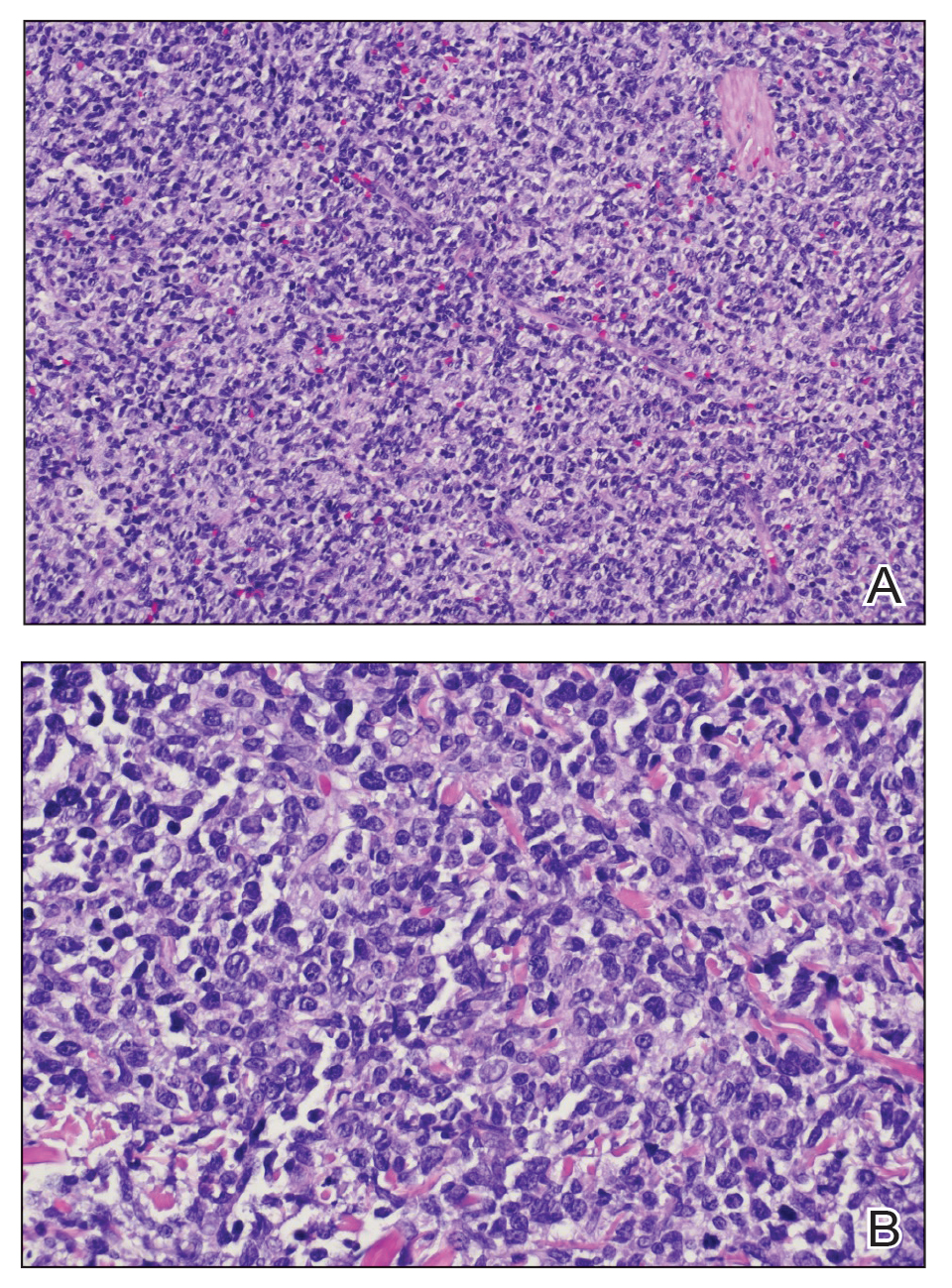
Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
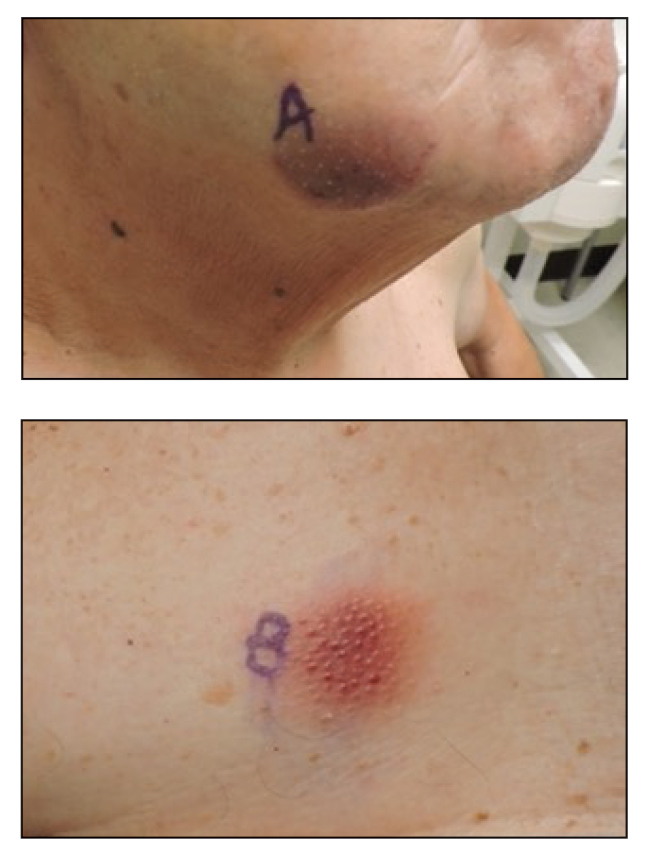
A 79-year-old man presented to the dermatology clinic with multiple skin lesions of 4 months’ duration. The patient had a history of monoclonal gammopathy and reported no changes in medication, travel, or trauma. He reported tenderness only when trying to comb hair over the left occipital nodule. He denied fevers, night sweats, weight loss, or poor appetite. Physical examination revealed 4 concerning skin lesions: a 3×3-cm violaceous nodule with underlying ecchymosis on the right medial jaw (top), a 3×2.5-cm violaceous nodule on the posterior occiput, a pink plaque with 1-mm vascular papules on the right mid-chest (bottom), and a 4×2.5-cm oval pink patch on the left side of the lower back. Punch biopsies were performed on the right medial jaw nodule and right mid-chest plaque.
Magnesium and Metabolic Syndrome: Any Connection?
TOPLINE:
Higher urinary magnesium loss, as indicated by an elevated magnesium depletion score (MDS), may be an independent risk factor for metabolic syndrome in US adults.
METHODOLOGY:
- Increasing evidence suggests that chronic hypomagnesemia may play a role in the pathogenesis of metabolic disorders, including overweight and obesity, insulin resistance, type 2 diabetes, hypertension, and dyslipidemia.
- Researchers examined the relationship between magnesium status and metabolic syndrome in 15,565 US adults (mean age, 47 years; half women) participating in the National Health and Nutrition Examination Survey (2003-2018), of whom 5438 had metabolic syndrome (mean age, 55 years).
- Magnesium deficiency was predicted by MDS, a four-factor score that aggregates diuretic use (one point), proton pump inhibitor (one point), kidney function (estimated glomerular filtration rate; one or two points), and heavy (one point).
- MDS was categorized into six levels (by scores 0-5), with a higher MDS indicating a more severe magnesium deficiency.
- Metabolic syndrome was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III report.
TAKEAWAY:
- The proportion of patients with MDS ≥ 2 was higher in the group with vs without metabolic syndrome (P < .05).
- Even after adjusting for potential confounding factors, each 1-unit increase in the MDS increased the odds of metabolic syndrome by about 30% (adjusted odds ratio, 1.31; 95% CI, 1.17-1.45).
- A dose-response relationship was observed between MDS and metabolic syndrome, with MDS level 1 being associated with 1.28-fold higher odds of metabolic syndrome (95% CI, 1.06-1.55) than MDS level 0; further escalation in the odds was noted for MDS levels 2, 3, and 4.
- The association between metabolic syndrome and MDS remained consistent across all population subgroups defined by age, gender, race (except Mexican American), body mass index, drinking status, or smoking status.
IN PRACTICE:
“It is possible to prevent and reduce MetS [metabolic syndrome] by supplementing with magnesium supplements or encouraging higher magnesium intake diet because the diet is a factor that can be changed,” the authors wrote.
SOURCE:
The study was led by Xiaohao Wang, Department of Geriatrics, the First Affiliated Hospital, School of Medicine, Southern University of Science and Technology (Shenzhen People’s Hospital), Shenzhen, China. It was published online in the Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study found no significant link between MDS level 5 and metabolic syndrome, likely due to the small sample size at this level. The study could not draw any causal relationship between metabolic syndrome and MDS owing to its cross-sectional nature. It also could not determine whether MDS was a better marker of magnesium deficiency than serum magnesium levels. MDS is a categorical, not continuous, variable.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China and the Natural Science Foundation of Shenzhen City, China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher urinary magnesium loss, as indicated by an elevated magnesium depletion score (MDS), may be an independent risk factor for metabolic syndrome in US adults.
METHODOLOGY:
- Increasing evidence suggests that chronic hypomagnesemia may play a role in the pathogenesis of metabolic disorders, including overweight and obesity, insulin resistance, type 2 diabetes, hypertension, and dyslipidemia.
- Researchers examined the relationship between magnesium status and metabolic syndrome in 15,565 US adults (mean age, 47 years; half women) participating in the National Health and Nutrition Examination Survey (2003-2018), of whom 5438 had metabolic syndrome (mean age, 55 years).
- Magnesium deficiency was predicted by MDS, a four-factor score that aggregates diuretic use (one point), proton pump inhibitor (one point), kidney function (estimated glomerular filtration rate; one or two points), and heavy (one point).
- MDS was categorized into six levels (by scores 0-5), with a higher MDS indicating a more severe magnesium deficiency.
- Metabolic syndrome was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III report.
TAKEAWAY:
- The proportion of patients with MDS ≥ 2 was higher in the group with vs without metabolic syndrome (P < .05).
- Even after adjusting for potential confounding factors, each 1-unit increase in the MDS increased the odds of metabolic syndrome by about 30% (adjusted odds ratio, 1.31; 95% CI, 1.17-1.45).
- A dose-response relationship was observed between MDS and metabolic syndrome, with MDS level 1 being associated with 1.28-fold higher odds of metabolic syndrome (95% CI, 1.06-1.55) than MDS level 0; further escalation in the odds was noted for MDS levels 2, 3, and 4.
- The association between metabolic syndrome and MDS remained consistent across all population subgroups defined by age, gender, race (except Mexican American), body mass index, drinking status, or smoking status.
IN PRACTICE:
“It is possible to prevent and reduce MetS [metabolic syndrome] by supplementing with magnesium supplements or encouraging higher magnesium intake diet because the diet is a factor that can be changed,” the authors wrote.
SOURCE:
The study was led by Xiaohao Wang, Department of Geriatrics, the First Affiliated Hospital, School of Medicine, Southern University of Science and Technology (Shenzhen People’s Hospital), Shenzhen, China. It was published online in the Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study found no significant link between MDS level 5 and metabolic syndrome, likely due to the small sample size at this level. The study could not draw any causal relationship between metabolic syndrome and MDS owing to its cross-sectional nature. It also could not determine whether MDS was a better marker of magnesium deficiency than serum magnesium levels. MDS is a categorical, not continuous, variable.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China and the Natural Science Foundation of Shenzhen City, China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher urinary magnesium loss, as indicated by an elevated magnesium depletion score (MDS), may be an independent risk factor for metabolic syndrome in US adults.
METHODOLOGY:
- Increasing evidence suggests that chronic hypomagnesemia may play a role in the pathogenesis of metabolic disorders, including overweight and obesity, insulin resistance, type 2 diabetes, hypertension, and dyslipidemia.
- Researchers examined the relationship between magnesium status and metabolic syndrome in 15,565 US adults (mean age, 47 years; half women) participating in the National Health and Nutrition Examination Survey (2003-2018), of whom 5438 had metabolic syndrome (mean age, 55 years).
- Magnesium deficiency was predicted by MDS, a four-factor score that aggregates diuretic use (one point), proton pump inhibitor (one point), kidney function (estimated glomerular filtration rate; one or two points), and heavy (one point).
- MDS was categorized into six levels (by scores 0-5), with a higher MDS indicating a more severe magnesium deficiency.
- Metabolic syndrome was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III report.
TAKEAWAY:
- The proportion of patients with MDS ≥ 2 was higher in the group with vs without metabolic syndrome (P < .05).
- Even after adjusting for potential confounding factors, each 1-unit increase in the MDS increased the odds of metabolic syndrome by about 30% (adjusted odds ratio, 1.31; 95% CI, 1.17-1.45).
- A dose-response relationship was observed between MDS and metabolic syndrome, with MDS level 1 being associated with 1.28-fold higher odds of metabolic syndrome (95% CI, 1.06-1.55) than MDS level 0; further escalation in the odds was noted for MDS levels 2, 3, and 4.
- The association between metabolic syndrome and MDS remained consistent across all population subgroups defined by age, gender, race (except Mexican American), body mass index, drinking status, or smoking status.
IN PRACTICE:
“It is possible to prevent and reduce MetS [metabolic syndrome] by supplementing with magnesium supplements or encouraging higher magnesium intake diet because the diet is a factor that can be changed,” the authors wrote.
SOURCE:
The study was led by Xiaohao Wang, Department of Geriatrics, the First Affiliated Hospital, School of Medicine, Southern University of Science and Technology (Shenzhen People’s Hospital), Shenzhen, China. It was published online in the Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study found no significant link between MDS level 5 and metabolic syndrome, likely due to the small sample size at this level. The study could not draw any causal relationship between metabolic syndrome and MDS owing to its cross-sectional nature. It also could not determine whether MDS was a better marker of magnesium deficiency than serum magnesium levels. MDS is a categorical, not continuous, variable.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China and the Natural Science Foundation of Shenzhen City, China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Restrictions Eased on Bariatric Surgery Because of GLP-1 Costs
Amid rising concern about the potential long-term costs of using glucagon-like peptide 1 (GLP-1) agonists to treat obesity, some insurers are making access to bariatric surgery easier.
While the relationship may not be entirely causal, data do suggest that at least for now, these new agents for treating obesity including semaglutide (Wegovy) and tirzepatide (Zepbound) are not cost-effective, whereas the surgery is.
According to GoodRx, Wegovy (semaglutide) has a list price of about $1350 for a 28-day supply. And the American Society for Metabolic and Bariatric Surgery (ASMBS) said that the average cost of bariatric surgery ranges between $17,000 and $26,000. But ASMBS added that third-party payers will recover those costs within 2-4 years due to reduction or elimination of obesity-related conditions and associated treatment costs, with an approximate 29% healthcare cost reduction at 5 years.
Recently, for example, Geisinger Health of Pennsylvania and Blue Cross/Blue Shield of Massachusetts expanded body mass index (BMI) eligibility for bariatric surgery procedures, while Blue Cross Blue Shield of Michigan dropped prior authorization requirements for several services, including bariatric surgery.
While most major health insurers cover Wegovy for obesity treatment, they typically require prior authorization and/or trials of other therapies first. Recently, some employers have denied coverage for the medications for treating obesity. Medicare does not cover these drugs. Coverage varies across state Medicaid plans.
“For years, insurers…have played games with the surgery, making people jump through hoops, hoping that they would just give up and go away. And now that health plans are getting concerned about [the cost of] drugs for obesity, and they’re getting so much attention, they’re thinking oh, maybe we shouldn’t be playing these games anymore,” obesity policy expert Ted Kyle, RPh, founder of ConscienHealth, told this news organization.
However, Christopher Doubet Still, DO, director of Nutrition and Weight Management at Geisinger Medical Center, Danville, Pennsylvania, told this news organization that Geisinger Health Plan’s change in May 2023 to lower the BMI surgery eligibility cutoff from 35 kg/m2 to 30 kg/m2 for people with comorbidities was not related to the cost of GLP-1 drugs.
“To date, bariatric surgery remains the most effective, enduring treatment of obesity, and most importantly, its comorbid medical problems. So that was really the reasoning. The weight loss is secondary to the profound medical benefits of bariatric surgery. I think that was the impetus of that change, not having really anything to do at the time with GLP-1s,” Dr. Still said.
The Geisinger Health Plan does not currently cover antiobesity medications, although Geisinger Health Plan Family, a state Medicaid plan, does because Pennsylvania is now one of a handful of states that cover the medications through Medicaid.
The Equation Keeps Changing
Health economist James Chambers, PhD, of Tufts University, Boston, Massachusetts, told this news organizations, “when you think about the value of a treatment, you don’t look at it in isolation. You’re looking at the difference in cost and benefits. So now that you have these expensive drugs, it’s not that surgeries become less expensive, but it does make you interpret the cost differently. When diet and exercise and counseling were the only real options, surgeries seemed like a much more expensive intervention. But with the advent of the GLP-1s, then, maybe plans consider the costs of surgery a little bit differently.”
And that equation is likely to change further, Dr. Still noted.
“I just think we’re dealing with a short-term financial problem because there’s basically only two main medications so the prices are high, but as more medications come on the market, the prices will come down,” he said.
Cristy Ms. Gallagher, MPAff, research project director of the STOP Obesity Alliance at the Milken Institute School of Public Health at George Washington University, Washington, DC, agreed.
“We have a lot of data on obesity treatment coverage from before 2023. But then this [GLP-1] explosion happened in 2023…The health payers are out there trying to figure out coverage, and they’re trying to figure out what this is going to look like for them,” Ms. Gallagher said.
However, she pointed out, “there is no treatment that fits everyone. The GLP-1s won’t work for every person because of the different stages of obesity, the side effects, and then because of the coverage. I think that you will not see a decrease in bariatric surgery in the near term, by any means.”
Ms. Gallagher also noted that although the data now suggest people will have to keep taking the drugs for life, there may be other future approaches.
“Once a person hits their goal weight, maybe then they could be transferred to a different pill form that might be cheaper, something that’s sort of more of a maintenance drug. I think that is a huge unknown right now,” she said.
And Mr. Kyle said that while bariatric surgery does provide the most durable benefit, “weight regain after surgery is a fact of life. People are still healthier 5 years later, but they do have some weight regain. And in those cases, you might want to follow-up with medicines…It’s not necessarily an either/or proposition any more than surgical treatment of cancer, surgical oncology is an either/or with medical oncology.”
A New View of Obesity
According to Mr. Kyle, all this attention on the new medications “is prompting people to rethink or think differently about obesity and consider it more carefully as a chronic medical condition and not a condition of appearance and behavior. And that’s all good, whether you’re thinking about it from the standpoint of bariatric surgery or from the standpoint of medical treatment of obesity because then people start considering options more rationally.”
This shifting view of obesity has meant that bariatric surgeons and medical obesity specialists are starting to work more collaboratively, he said.
“There is a trend that I detect toward more integrated approaches to obesity,” Mr. Kyle said.
He added, “We now have more tools. And we’re in the messy middle of figuring out how, as a practical matter, to use them.”
And as far as insurance coverage, “people are going to make mistakes. They are making mistakes. But I have been the eternal optimist, and I have faith that people are also figuring it out. It would be hard to do worse.”
For now, any initiatives to increase bariatric surgery rates in the GLP-1 era haven’t yet had an impact, American Society for Metabolic and Bariatric Surgery President Marina S. Kurian, MD, told this news organization in a statement.
“While we have heard of some insurers possibly changing their requirements for bariatric surgery, we have not seen a significant rise in procedures related to changes in insurance coverage for either antiobesity medications or metabolic and bariatric surgery,” Dr. Kurian said.
Mr. Kyle had accepted speaking or professional fees from Novo Nordisk, Behringer Ingelheim, Nutrisystem, Roman Health, and Emerald Lake Safety. Dr. Still was on the speaker’s bureau and did clinical trials for Eli Lilly and Novo Nordisk. Dr. Chambers and Gallagher had no disclosures.
A version of this article appeared on Medscape.com.
Amid rising concern about the potential long-term costs of using glucagon-like peptide 1 (GLP-1) agonists to treat obesity, some insurers are making access to bariatric surgery easier.
While the relationship may not be entirely causal, data do suggest that at least for now, these new agents for treating obesity including semaglutide (Wegovy) and tirzepatide (Zepbound) are not cost-effective, whereas the surgery is.
According to GoodRx, Wegovy (semaglutide) has a list price of about $1350 for a 28-day supply. And the American Society for Metabolic and Bariatric Surgery (ASMBS) said that the average cost of bariatric surgery ranges between $17,000 and $26,000. But ASMBS added that third-party payers will recover those costs within 2-4 years due to reduction or elimination of obesity-related conditions and associated treatment costs, with an approximate 29% healthcare cost reduction at 5 years.
Recently, for example, Geisinger Health of Pennsylvania and Blue Cross/Blue Shield of Massachusetts expanded body mass index (BMI) eligibility for bariatric surgery procedures, while Blue Cross Blue Shield of Michigan dropped prior authorization requirements for several services, including bariatric surgery.
While most major health insurers cover Wegovy for obesity treatment, they typically require prior authorization and/or trials of other therapies first. Recently, some employers have denied coverage for the medications for treating obesity. Medicare does not cover these drugs. Coverage varies across state Medicaid plans.
“For years, insurers…have played games with the surgery, making people jump through hoops, hoping that they would just give up and go away. And now that health plans are getting concerned about [the cost of] drugs for obesity, and they’re getting so much attention, they’re thinking oh, maybe we shouldn’t be playing these games anymore,” obesity policy expert Ted Kyle, RPh, founder of ConscienHealth, told this news organization.
However, Christopher Doubet Still, DO, director of Nutrition and Weight Management at Geisinger Medical Center, Danville, Pennsylvania, told this news organization that Geisinger Health Plan’s change in May 2023 to lower the BMI surgery eligibility cutoff from 35 kg/m2 to 30 kg/m2 for people with comorbidities was not related to the cost of GLP-1 drugs.
“To date, bariatric surgery remains the most effective, enduring treatment of obesity, and most importantly, its comorbid medical problems. So that was really the reasoning. The weight loss is secondary to the profound medical benefits of bariatric surgery. I think that was the impetus of that change, not having really anything to do at the time with GLP-1s,” Dr. Still said.
The Geisinger Health Plan does not currently cover antiobesity medications, although Geisinger Health Plan Family, a state Medicaid plan, does because Pennsylvania is now one of a handful of states that cover the medications through Medicaid.
The Equation Keeps Changing
Health economist James Chambers, PhD, of Tufts University, Boston, Massachusetts, told this news organizations, “when you think about the value of a treatment, you don’t look at it in isolation. You’re looking at the difference in cost and benefits. So now that you have these expensive drugs, it’s not that surgeries become less expensive, but it does make you interpret the cost differently. When diet and exercise and counseling were the only real options, surgeries seemed like a much more expensive intervention. But with the advent of the GLP-1s, then, maybe plans consider the costs of surgery a little bit differently.”
And that equation is likely to change further, Dr. Still noted.
“I just think we’re dealing with a short-term financial problem because there’s basically only two main medications so the prices are high, but as more medications come on the market, the prices will come down,” he said.
Cristy Ms. Gallagher, MPAff, research project director of the STOP Obesity Alliance at the Milken Institute School of Public Health at George Washington University, Washington, DC, agreed.
“We have a lot of data on obesity treatment coverage from before 2023. But then this [GLP-1] explosion happened in 2023…The health payers are out there trying to figure out coverage, and they’re trying to figure out what this is going to look like for them,” Ms. Gallagher said.
However, she pointed out, “there is no treatment that fits everyone. The GLP-1s won’t work for every person because of the different stages of obesity, the side effects, and then because of the coverage. I think that you will not see a decrease in bariatric surgery in the near term, by any means.”
Ms. Gallagher also noted that although the data now suggest people will have to keep taking the drugs for life, there may be other future approaches.
“Once a person hits their goal weight, maybe then they could be transferred to a different pill form that might be cheaper, something that’s sort of more of a maintenance drug. I think that is a huge unknown right now,” she said.
And Mr. Kyle said that while bariatric surgery does provide the most durable benefit, “weight regain after surgery is a fact of life. People are still healthier 5 years later, but they do have some weight regain. And in those cases, you might want to follow-up with medicines…It’s not necessarily an either/or proposition any more than surgical treatment of cancer, surgical oncology is an either/or with medical oncology.”
A New View of Obesity
According to Mr. Kyle, all this attention on the new medications “is prompting people to rethink or think differently about obesity and consider it more carefully as a chronic medical condition and not a condition of appearance and behavior. And that’s all good, whether you’re thinking about it from the standpoint of bariatric surgery or from the standpoint of medical treatment of obesity because then people start considering options more rationally.”
This shifting view of obesity has meant that bariatric surgeons and medical obesity specialists are starting to work more collaboratively, he said.
“There is a trend that I detect toward more integrated approaches to obesity,” Mr. Kyle said.
He added, “We now have more tools. And we’re in the messy middle of figuring out how, as a practical matter, to use them.”
And as far as insurance coverage, “people are going to make mistakes. They are making mistakes. But I have been the eternal optimist, and I have faith that people are also figuring it out. It would be hard to do worse.”
For now, any initiatives to increase bariatric surgery rates in the GLP-1 era haven’t yet had an impact, American Society for Metabolic and Bariatric Surgery President Marina S. Kurian, MD, told this news organization in a statement.
“While we have heard of some insurers possibly changing their requirements for bariatric surgery, we have not seen a significant rise in procedures related to changes in insurance coverage for either antiobesity medications or metabolic and bariatric surgery,” Dr. Kurian said.
Mr. Kyle had accepted speaking or professional fees from Novo Nordisk, Behringer Ingelheim, Nutrisystem, Roman Health, and Emerald Lake Safety. Dr. Still was on the speaker’s bureau and did clinical trials for Eli Lilly and Novo Nordisk. Dr. Chambers and Gallagher had no disclosures.
A version of this article appeared on Medscape.com.
Amid rising concern about the potential long-term costs of using glucagon-like peptide 1 (GLP-1) agonists to treat obesity, some insurers are making access to bariatric surgery easier.
While the relationship may not be entirely causal, data do suggest that at least for now, these new agents for treating obesity including semaglutide (Wegovy) and tirzepatide (Zepbound) are not cost-effective, whereas the surgery is.
According to GoodRx, Wegovy (semaglutide) has a list price of about $1350 for a 28-day supply. And the American Society for Metabolic and Bariatric Surgery (ASMBS) said that the average cost of bariatric surgery ranges between $17,000 and $26,000. But ASMBS added that third-party payers will recover those costs within 2-4 years due to reduction or elimination of obesity-related conditions and associated treatment costs, with an approximate 29% healthcare cost reduction at 5 years.
Recently, for example, Geisinger Health of Pennsylvania and Blue Cross/Blue Shield of Massachusetts expanded body mass index (BMI) eligibility for bariatric surgery procedures, while Blue Cross Blue Shield of Michigan dropped prior authorization requirements for several services, including bariatric surgery.
While most major health insurers cover Wegovy for obesity treatment, they typically require prior authorization and/or trials of other therapies first. Recently, some employers have denied coverage for the medications for treating obesity. Medicare does not cover these drugs. Coverage varies across state Medicaid plans.
“For years, insurers…have played games with the surgery, making people jump through hoops, hoping that they would just give up and go away. And now that health plans are getting concerned about [the cost of] drugs for obesity, and they’re getting so much attention, they’re thinking oh, maybe we shouldn’t be playing these games anymore,” obesity policy expert Ted Kyle, RPh, founder of ConscienHealth, told this news organization.
However, Christopher Doubet Still, DO, director of Nutrition and Weight Management at Geisinger Medical Center, Danville, Pennsylvania, told this news organization that Geisinger Health Plan’s change in May 2023 to lower the BMI surgery eligibility cutoff from 35 kg/m2 to 30 kg/m2 for people with comorbidities was not related to the cost of GLP-1 drugs.
“To date, bariatric surgery remains the most effective, enduring treatment of obesity, and most importantly, its comorbid medical problems. So that was really the reasoning. The weight loss is secondary to the profound medical benefits of bariatric surgery. I think that was the impetus of that change, not having really anything to do at the time with GLP-1s,” Dr. Still said.
The Geisinger Health Plan does not currently cover antiobesity medications, although Geisinger Health Plan Family, a state Medicaid plan, does because Pennsylvania is now one of a handful of states that cover the medications through Medicaid.
The Equation Keeps Changing
Health economist James Chambers, PhD, of Tufts University, Boston, Massachusetts, told this news organizations, “when you think about the value of a treatment, you don’t look at it in isolation. You’re looking at the difference in cost and benefits. So now that you have these expensive drugs, it’s not that surgeries become less expensive, but it does make you interpret the cost differently. When diet and exercise and counseling were the only real options, surgeries seemed like a much more expensive intervention. But with the advent of the GLP-1s, then, maybe plans consider the costs of surgery a little bit differently.”
And that equation is likely to change further, Dr. Still noted.
“I just think we’re dealing with a short-term financial problem because there’s basically only two main medications so the prices are high, but as more medications come on the market, the prices will come down,” he said.
Cristy Ms. Gallagher, MPAff, research project director of the STOP Obesity Alliance at the Milken Institute School of Public Health at George Washington University, Washington, DC, agreed.
“We have a lot of data on obesity treatment coverage from before 2023. But then this [GLP-1] explosion happened in 2023…The health payers are out there trying to figure out coverage, and they’re trying to figure out what this is going to look like for them,” Ms. Gallagher said.
However, she pointed out, “there is no treatment that fits everyone. The GLP-1s won’t work for every person because of the different stages of obesity, the side effects, and then because of the coverage. I think that you will not see a decrease in bariatric surgery in the near term, by any means.”
Ms. Gallagher also noted that although the data now suggest people will have to keep taking the drugs for life, there may be other future approaches.
“Once a person hits their goal weight, maybe then they could be transferred to a different pill form that might be cheaper, something that’s sort of more of a maintenance drug. I think that is a huge unknown right now,” she said.
And Mr. Kyle said that while bariatric surgery does provide the most durable benefit, “weight regain after surgery is a fact of life. People are still healthier 5 years later, but they do have some weight regain. And in those cases, you might want to follow-up with medicines…It’s not necessarily an either/or proposition any more than surgical treatment of cancer, surgical oncology is an either/or with medical oncology.”
A New View of Obesity
According to Mr. Kyle, all this attention on the new medications “is prompting people to rethink or think differently about obesity and consider it more carefully as a chronic medical condition and not a condition of appearance and behavior. And that’s all good, whether you’re thinking about it from the standpoint of bariatric surgery or from the standpoint of medical treatment of obesity because then people start considering options more rationally.”
This shifting view of obesity has meant that bariatric surgeons and medical obesity specialists are starting to work more collaboratively, he said.
“There is a trend that I detect toward more integrated approaches to obesity,” Mr. Kyle said.
He added, “We now have more tools. And we’re in the messy middle of figuring out how, as a practical matter, to use them.”
And as far as insurance coverage, “people are going to make mistakes. They are making mistakes. But I have been the eternal optimist, and I have faith that people are also figuring it out. It would be hard to do worse.”
For now, any initiatives to increase bariatric surgery rates in the GLP-1 era haven’t yet had an impact, American Society for Metabolic and Bariatric Surgery President Marina S. Kurian, MD, told this news organization in a statement.
“While we have heard of some insurers possibly changing their requirements for bariatric surgery, we have not seen a significant rise in procedures related to changes in insurance coverage for either antiobesity medications or metabolic and bariatric surgery,” Dr. Kurian said.
Mr. Kyle had accepted speaking or professional fees from Novo Nordisk, Behringer Ingelheim, Nutrisystem, Roman Health, and Emerald Lake Safety. Dr. Still was on the speaker’s bureau and did clinical trials for Eli Lilly and Novo Nordisk. Dr. Chambers and Gallagher had no disclosures.
A version of this article appeared on Medscape.com.
Ovarian Cancer Red Flags: What to Know to Quicken Diagnoses
One in seven women will die within 2 months of being diagnosed with ovarian cancer, a new report from the United Kingdom states. But if diagnosed at the earliest stage, 9 in 10 women will survive. Two thirds of women are now diagnosed late, when the cancer is harder to treat.
Diagnosis is difficult for many reasons, among them that women sometimes think symptoms are a natural part of menopause and don’t acknowledge or report them. Clinicians may mistake abdominal symptoms for those of a bowel condition or bladder problem. Almost half of GPs (46%) in the UK mistakenly believe that ovarian cancer symptoms present in only the later stages of the disease.
Cervical Screening Does Not Detect Ovarian Cancer
Additionally, there are misconceptions regarding cervical cancer screening — one study found that “40% of women in the general public mistakenly believe that cervical screening detects ovarian cancer.” But there is no current screening program for ovarian cancer in the UK or United States.
During a pelvic exam, the physician feels the ovaries and uterus for size, shape, and consistency and that can be useful in finding some cancers early, but most early ovarian tumors are difficult or impossible to feel, the American Cancer Society notes.
Recognizing the Red Flags
Victoria Barber, MBBS, a general practitioner in Northamptonshire and a Primary Care Advisory Board member with the Target Ovarian Cancer program in the UK published a paper in the British Journal of Nursing (2024 Mar 7. doi: 10.12968/bjon.2024.33.5.S16) on the program’s efforts to urge clinicians to recognize ovarian cancer red flags and to “never diagnose new-onset irritable bowel syndrome or overactive bladder in women over 50 without ruling out ovarian cancer.”
She says nurses should be involved to help with earlier diagnosis of ovarian cancer as they are often involved in evaluating urine samples. Nurse practitioners, she notes, are typically included in consultations for abdominal symptoms and potential urinary tract infections.
“If the woman is recurrently presenting with urinary symptoms, sterile midstream urine samples should raise alarm,” she says. “The woman may have diabetes, an overactive bladder, or interstitial cystitis; however, urgency and frequency are some of the symptoms of ovarian cancer, and they need investigation.”
Persistent Systems Over Age 50
The paper lists ovarian cancer symptoms from the UK’s National Institute for Health and Care Excellence and notes that among red flags are having any of the following persistently/frequently (particularly more than 12 times per month and especially if the woman is 50 years or older):
- Early satiety and/or loss of appetite
- Abdominal bloating
- Pelvic or abdominal pain
- Urinary urgency/frequency
Other symptoms could include:
- Changes in bowel habits (e.g., diarrhea or constipation)
- Extreme fatigue
- Unexplained weight loss
Diagnosis Challenges Similar in US
Ernst Lengyel, MD, PhD, UChicago Medicine’s Chairman of the Department of Obstetrics and Gynecology in Chicago, Illinois, who was not involved with the paper, said the situation in the United States is similar to that described in the UK.
“The diagnosis is delayed because the symptoms are unspecific. The problem is that ovarian cancer is so rare, and primary care physicians or nurse practitioners have to consider over 100 differential diagnoses,” he says.
In the US, he says, it is likely easier to get in and see a physician because of the private insurance options and because there are more gynecologic oncologists in large urban areas. Getting imaging approved — such as ultrasound and computed tomography scans — is also easier in the US.
Still, “there is no effective way to diagnose ovarian cancer early,” he says. “No single test or combination of symptoms can be used as a screening test.”
The CA-125 blood test measures proteins that can be linked with ovarian cancer, but is not a screening test, he notes.
“Large UK and US studies have not been able to show a survival benefit with ultrasound, serial CA-125, or a combination thereof,” Dr. Lengyel said.
Weight Gain May Also be a Sign
A broad range of clinicians should be aware of the symptoms the author mentions, he says, especially primary care physicians, nurse practitioners, and obstetrician/gynecologists.
“Too often, symptoms that women report are ignored and treated as unspecific or psychosomatic,” Dr. Lengyel says. “It is easy to disregard recurrent complaints and move on instead of being vigilant and working them up. Ironically, women with ovarian cancer can initially gain weight, which is counterintuitive as most doctors believe that patients with cancer lose weight. However, if they develop abdominal fluid, a patient often gains weight.”
Dr. Barber and Dr. Lengyel report no relevant financial relationships.
One in seven women will die within 2 months of being diagnosed with ovarian cancer, a new report from the United Kingdom states. But if diagnosed at the earliest stage, 9 in 10 women will survive. Two thirds of women are now diagnosed late, when the cancer is harder to treat.
Diagnosis is difficult for many reasons, among them that women sometimes think symptoms are a natural part of menopause and don’t acknowledge or report them. Clinicians may mistake abdominal symptoms for those of a bowel condition or bladder problem. Almost half of GPs (46%) in the UK mistakenly believe that ovarian cancer symptoms present in only the later stages of the disease.
Cervical Screening Does Not Detect Ovarian Cancer
Additionally, there are misconceptions regarding cervical cancer screening — one study found that “40% of women in the general public mistakenly believe that cervical screening detects ovarian cancer.” But there is no current screening program for ovarian cancer in the UK or United States.
During a pelvic exam, the physician feels the ovaries and uterus for size, shape, and consistency and that can be useful in finding some cancers early, but most early ovarian tumors are difficult or impossible to feel, the American Cancer Society notes.
Recognizing the Red Flags
Victoria Barber, MBBS, a general practitioner in Northamptonshire and a Primary Care Advisory Board member with the Target Ovarian Cancer program in the UK published a paper in the British Journal of Nursing (2024 Mar 7. doi: 10.12968/bjon.2024.33.5.S16) on the program’s efforts to urge clinicians to recognize ovarian cancer red flags and to “never diagnose new-onset irritable bowel syndrome or overactive bladder in women over 50 without ruling out ovarian cancer.”
She says nurses should be involved to help with earlier diagnosis of ovarian cancer as they are often involved in evaluating urine samples. Nurse practitioners, she notes, are typically included in consultations for abdominal symptoms and potential urinary tract infections.
“If the woman is recurrently presenting with urinary symptoms, sterile midstream urine samples should raise alarm,” she says. “The woman may have diabetes, an overactive bladder, or interstitial cystitis; however, urgency and frequency are some of the symptoms of ovarian cancer, and they need investigation.”
Persistent Systems Over Age 50
The paper lists ovarian cancer symptoms from the UK’s National Institute for Health and Care Excellence and notes that among red flags are having any of the following persistently/frequently (particularly more than 12 times per month and especially if the woman is 50 years or older):
- Early satiety and/or loss of appetite
- Abdominal bloating
- Pelvic or abdominal pain
- Urinary urgency/frequency
Other symptoms could include:
- Changes in bowel habits (e.g., diarrhea or constipation)
- Extreme fatigue
- Unexplained weight loss
Diagnosis Challenges Similar in US
Ernst Lengyel, MD, PhD, UChicago Medicine’s Chairman of the Department of Obstetrics and Gynecology in Chicago, Illinois, who was not involved with the paper, said the situation in the United States is similar to that described in the UK.
“The diagnosis is delayed because the symptoms are unspecific. The problem is that ovarian cancer is so rare, and primary care physicians or nurse practitioners have to consider over 100 differential diagnoses,” he says.
In the US, he says, it is likely easier to get in and see a physician because of the private insurance options and because there are more gynecologic oncologists in large urban areas. Getting imaging approved — such as ultrasound and computed tomography scans — is also easier in the US.
Still, “there is no effective way to diagnose ovarian cancer early,” he says. “No single test or combination of symptoms can be used as a screening test.”
The CA-125 blood test measures proteins that can be linked with ovarian cancer, but is not a screening test, he notes.
“Large UK and US studies have not been able to show a survival benefit with ultrasound, serial CA-125, or a combination thereof,” Dr. Lengyel said.
Weight Gain May Also be a Sign
A broad range of clinicians should be aware of the symptoms the author mentions, he says, especially primary care physicians, nurse practitioners, and obstetrician/gynecologists.
“Too often, symptoms that women report are ignored and treated as unspecific or psychosomatic,” Dr. Lengyel says. “It is easy to disregard recurrent complaints and move on instead of being vigilant and working them up. Ironically, women with ovarian cancer can initially gain weight, which is counterintuitive as most doctors believe that patients with cancer lose weight. However, if they develop abdominal fluid, a patient often gains weight.”
Dr. Barber and Dr. Lengyel report no relevant financial relationships.
One in seven women will die within 2 months of being diagnosed with ovarian cancer, a new report from the United Kingdom states. But if diagnosed at the earliest stage, 9 in 10 women will survive. Two thirds of women are now diagnosed late, when the cancer is harder to treat.
Diagnosis is difficult for many reasons, among them that women sometimes think symptoms are a natural part of menopause and don’t acknowledge or report them. Clinicians may mistake abdominal symptoms for those of a bowel condition or bladder problem. Almost half of GPs (46%) in the UK mistakenly believe that ovarian cancer symptoms present in only the later stages of the disease.
Cervical Screening Does Not Detect Ovarian Cancer
Additionally, there are misconceptions regarding cervical cancer screening — one study found that “40% of women in the general public mistakenly believe that cervical screening detects ovarian cancer.” But there is no current screening program for ovarian cancer in the UK or United States.
During a pelvic exam, the physician feels the ovaries and uterus for size, shape, and consistency and that can be useful in finding some cancers early, but most early ovarian tumors are difficult or impossible to feel, the American Cancer Society notes.
Recognizing the Red Flags
Victoria Barber, MBBS, a general practitioner in Northamptonshire and a Primary Care Advisory Board member with the Target Ovarian Cancer program in the UK published a paper in the British Journal of Nursing (2024 Mar 7. doi: 10.12968/bjon.2024.33.5.S16) on the program’s efforts to urge clinicians to recognize ovarian cancer red flags and to “never diagnose new-onset irritable bowel syndrome or overactive bladder in women over 50 without ruling out ovarian cancer.”
She says nurses should be involved to help with earlier diagnosis of ovarian cancer as they are often involved in evaluating urine samples. Nurse practitioners, she notes, are typically included in consultations for abdominal symptoms and potential urinary tract infections.
“If the woman is recurrently presenting with urinary symptoms, sterile midstream urine samples should raise alarm,” she says. “The woman may have diabetes, an overactive bladder, or interstitial cystitis; however, urgency and frequency are some of the symptoms of ovarian cancer, and they need investigation.”
Persistent Systems Over Age 50
The paper lists ovarian cancer symptoms from the UK’s National Institute for Health and Care Excellence and notes that among red flags are having any of the following persistently/frequently (particularly more than 12 times per month and especially if the woman is 50 years or older):
- Early satiety and/or loss of appetite
- Abdominal bloating
- Pelvic or abdominal pain
- Urinary urgency/frequency
Other symptoms could include:
- Changes in bowel habits (e.g., diarrhea or constipation)
- Extreme fatigue
- Unexplained weight loss
Diagnosis Challenges Similar in US
Ernst Lengyel, MD, PhD, UChicago Medicine’s Chairman of the Department of Obstetrics and Gynecology in Chicago, Illinois, who was not involved with the paper, said the situation in the United States is similar to that described in the UK.
“The diagnosis is delayed because the symptoms are unspecific. The problem is that ovarian cancer is so rare, and primary care physicians or nurse practitioners have to consider over 100 differential diagnoses,” he says.
In the US, he says, it is likely easier to get in and see a physician because of the private insurance options and because there are more gynecologic oncologists in large urban areas. Getting imaging approved — such as ultrasound and computed tomography scans — is also easier in the US.
Still, “there is no effective way to diagnose ovarian cancer early,” he says. “No single test or combination of symptoms can be used as a screening test.”
The CA-125 blood test measures proteins that can be linked with ovarian cancer, but is not a screening test, he notes.
“Large UK and US studies have not been able to show a survival benefit with ultrasound, serial CA-125, or a combination thereof,” Dr. Lengyel said.
Weight Gain May Also be a Sign
A broad range of clinicians should be aware of the symptoms the author mentions, he says, especially primary care physicians, nurse practitioners, and obstetrician/gynecologists.
“Too often, symptoms that women report are ignored and treated as unspecific or psychosomatic,” Dr. Lengyel says. “It is easy to disregard recurrent complaints and move on instead of being vigilant and working them up. Ironically, women with ovarian cancer can initially gain weight, which is counterintuitive as most doctors believe that patients with cancer lose weight. However, if they develop abdominal fluid, a patient often gains weight.”
Dr. Barber and Dr. Lengyel report no relevant financial relationships.
FROM THE BRITISH JOURNAL OF NURSING
Savolitinib Active Against MET Ex14 Mutated NSCLC
Savolitinib, a selective oral tyrosine kinase inhibitor, showed good activity against locally advanced or metastatic non–small cell lung cancer (NSCLC) bearing MET exon 14 mutations as both first-line therapy for treatment-naive patients and in second-line of therapy for previously treated patients
“The phase 3b study results further confirm savolitinib as a valuable targeted therapy option for naive and previously-treated non–small cell lung cancer with MET 14 exon mutations,” Yongchang Zhang, MD, said while presenting the final results of the trial at the European Lung Cancer Congress 2024.
For 87 previously untreated patients the objective response rate (ORR) as assessed by independent review, the primary endpoint, was 62.1%. For 79 patients receiving savolitinib in the second line, the ORR was 39.2%, reported Dr. Zhang, MD, of the Hunan Cancer Hospital in Changsha, China.
Preliminary results of this trial were reported at the World Conference on Lung Cancer in 2023.
Selective Inhibitor
Savolitinib (AZD6094, also called volitinib) is reported to be a highly selective oral inhibitor of the MET receptor tyrosine kinase (TKI). It is approved in China for the treatment of patients with NSCLC harboring MET exon 14 mutations that has progressed on prior systemic therapy, or patients who are unable to tolerate platinum-based chemotherapy.
In the phase 3b study, patients with MET ex14-positive tumors who were negative for EGFR, ALK or ROS1 alterations and were naive to a MET inhibitor were enrolled. Those who weighed 50 kg or greater received 600 mg savolitinib orally once daily for each 21-day cycle, while patients who weighed less than 50 kg received a 400-mg daily dose. Therapy continued until disease progression, death, or unacceptable toxicity.
Tumors were evaluated by investigators every 6 weeks for the first year, than every 12 weeks thereafter.
As noted before, ORR by independent review was 62.1% for treatment-naive patients and 39.2% for previously treated patients. The respective ORRs by investigator assessment were 59.8% and 43%. All responses in each arm were partial responses.
Median progression-free survival (PFS) after a median follow-up of 18 months for treatment-naive patients and 11 months for treatment-experienced patients was 13.7 months and 11 months, respectively.
Overall survival after a median follow-up of 20.8 months for treatment-naive patients and 12.5 months for previously treated patients was not reached in treatment-naive patients and not mature in treatment-experienced patients.
Grade 3 or greater treatment-emergent adverse events occurred in 74.1% of patients, including 3 events (1.8%) leading to death. Dose modifications were required for 74.7% of patients.
Grade 3 or greater adverse events included peripheral edema, liver enzyme elevations, abnormal liver function, decreased platelet and white blood cell counts, and vomiting.
Which TKI is Best?
Invited discussant Antonio Passaro, MD, PhD, from the European Institute of Oncology in Milan, noted that eligibility for treatment with savolitinib or other MET exon 14-targeting TKIs is limited to about 3% of patients with NSCLC of adenocarcinoma histology.
He said that savolitinib appears to be similar in performance to two other TKIs for NSCLC with MET exon-14 skipping mutations that are currently on the market in the United States, Europe, and Japan: capmatinib (Tabrecta) and tepotinib (Tepmetko).
“Globally, all the results show a numerically better performance when we use a selective TKI in first-line treatment over the second-line treatment, in particular for overall response rate,” he said.
Dr. Passaro noted that savolitinib differs from the other two MET TKIs in that PFS with savolitinib is similar for treatment-naive and previously treated patients.
He added, however, that “today it’s very difficult” to determine which is the “perfect” agent for a specific disease presentation, particularly since MET exon 14 skipping mutations can also be found in patients with squamous cell carcinomas and those with a history of smoking.
To get a better sense of which drug to use in a specific situation, it would be helpful to analyze trial results in the context of tumor histology, smoking history, programmed death protein 1-ligand 1 status, and co-mutations, he said.
The study was sponsored by Hutchmed. Dr. Zhang reported having no conflicts of interest. Dr. Passaro reported a consulting, advisory, or speakers bureau role for multiple companies, not including Hutchmed.
Savolitinib, a selective oral tyrosine kinase inhibitor, showed good activity against locally advanced or metastatic non–small cell lung cancer (NSCLC) bearing MET exon 14 mutations as both first-line therapy for treatment-naive patients and in second-line of therapy for previously treated patients
“The phase 3b study results further confirm savolitinib as a valuable targeted therapy option for naive and previously-treated non–small cell lung cancer with MET 14 exon mutations,” Yongchang Zhang, MD, said while presenting the final results of the trial at the European Lung Cancer Congress 2024.
For 87 previously untreated patients the objective response rate (ORR) as assessed by independent review, the primary endpoint, was 62.1%. For 79 patients receiving savolitinib in the second line, the ORR was 39.2%, reported Dr. Zhang, MD, of the Hunan Cancer Hospital in Changsha, China.
Preliminary results of this trial were reported at the World Conference on Lung Cancer in 2023.
Selective Inhibitor
Savolitinib (AZD6094, also called volitinib) is reported to be a highly selective oral inhibitor of the MET receptor tyrosine kinase (TKI). It is approved in China for the treatment of patients with NSCLC harboring MET exon 14 mutations that has progressed on prior systemic therapy, or patients who are unable to tolerate platinum-based chemotherapy.
In the phase 3b study, patients with MET ex14-positive tumors who were negative for EGFR, ALK or ROS1 alterations and were naive to a MET inhibitor were enrolled. Those who weighed 50 kg or greater received 600 mg savolitinib orally once daily for each 21-day cycle, while patients who weighed less than 50 kg received a 400-mg daily dose. Therapy continued until disease progression, death, or unacceptable toxicity.
Tumors were evaluated by investigators every 6 weeks for the first year, than every 12 weeks thereafter.
As noted before, ORR by independent review was 62.1% for treatment-naive patients and 39.2% for previously treated patients. The respective ORRs by investigator assessment were 59.8% and 43%. All responses in each arm were partial responses.
Median progression-free survival (PFS) after a median follow-up of 18 months for treatment-naive patients and 11 months for treatment-experienced patients was 13.7 months and 11 months, respectively.
Overall survival after a median follow-up of 20.8 months for treatment-naive patients and 12.5 months for previously treated patients was not reached in treatment-naive patients and not mature in treatment-experienced patients.
Grade 3 or greater treatment-emergent adverse events occurred in 74.1% of patients, including 3 events (1.8%) leading to death. Dose modifications were required for 74.7% of patients.
Grade 3 or greater adverse events included peripheral edema, liver enzyme elevations, abnormal liver function, decreased platelet and white blood cell counts, and vomiting.
Which TKI is Best?
Invited discussant Antonio Passaro, MD, PhD, from the European Institute of Oncology in Milan, noted that eligibility for treatment with savolitinib or other MET exon 14-targeting TKIs is limited to about 3% of patients with NSCLC of adenocarcinoma histology.
He said that savolitinib appears to be similar in performance to two other TKIs for NSCLC with MET exon-14 skipping mutations that are currently on the market in the United States, Europe, and Japan: capmatinib (Tabrecta) and tepotinib (Tepmetko).
“Globally, all the results show a numerically better performance when we use a selective TKI in first-line treatment over the second-line treatment, in particular for overall response rate,” he said.
Dr. Passaro noted that savolitinib differs from the other two MET TKIs in that PFS with savolitinib is similar for treatment-naive and previously treated patients.
He added, however, that “today it’s very difficult” to determine which is the “perfect” agent for a specific disease presentation, particularly since MET exon 14 skipping mutations can also be found in patients with squamous cell carcinomas and those with a history of smoking.
To get a better sense of which drug to use in a specific situation, it would be helpful to analyze trial results in the context of tumor histology, smoking history, programmed death protein 1-ligand 1 status, and co-mutations, he said.
The study was sponsored by Hutchmed. Dr. Zhang reported having no conflicts of interest. Dr. Passaro reported a consulting, advisory, or speakers bureau role for multiple companies, not including Hutchmed.
Savolitinib, a selective oral tyrosine kinase inhibitor, showed good activity against locally advanced or metastatic non–small cell lung cancer (NSCLC) bearing MET exon 14 mutations as both first-line therapy for treatment-naive patients and in second-line of therapy for previously treated patients
“The phase 3b study results further confirm savolitinib as a valuable targeted therapy option for naive and previously-treated non–small cell lung cancer with MET 14 exon mutations,” Yongchang Zhang, MD, said while presenting the final results of the trial at the European Lung Cancer Congress 2024.
For 87 previously untreated patients the objective response rate (ORR) as assessed by independent review, the primary endpoint, was 62.1%. For 79 patients receiving savolitinib in the second line, the ORR was 39.2%, reported Dr. Zhang, MD, of the Hunan Cancer Hospital in Changsha, China.
Preliminary results of this trial were reported at the World Conference on Lung Cancer in 2023.
Selective Inhibitor
Savolitinib (AZD6094, also called volitinib) is reported to be a highly selective oral inhibitor of the MET receptor tyrosine kinase (TKI). It is approved in China for the treatment of patients with NSCLC harboring MET exon 14 mutations that has progressed on prior systemic therapy, or patients who are unable to tolerate platinum-based chemotherapy.
In the phase 3b study, patients with MET ex14-positive tumors who were negative for EGFR, ALK or ROS1 alterations and were naive to a MET inhibitor were enrolled. Those who weighed 50 kg or greater received 600 mg savolitinib orally once daily for each 21-day cycle, while patients who weighed less than 50 kg received a 400-mg daily dose. Therapy continued until disease progression, death, or unacceptable toxicity.
Tumors were evaluated by investigators every 6 weeks for the first year, than every 12 weeks thereafter.
As noted before, ORR by independent review was 62.1% for treatment-naive patients and 39.2% for previously treated patients. The respective ORRs by investigator assessment were 59.8% and 43%. All responses in each arm were partial responses.
Median progression-free survival (PFS) after a median follow-up of 18 months for treatment-naive patients and 11 months for treatment-experienced patients was 13.7 months and 11 months, respectively.
Overall survival after a median follow-up of 20.8 months for treatment-naive patients and 12.5 months for previously treated patients was not reached in treatment-naive patients and not mature in treatment-experienced patients.
Grade 3 or greater treatment-emergent adverse events occurred in 74.1% of patients, including 3 events (1.8%) leading to death. Dose modifications were required for 74.7% of patients.
Grade 3 or greater adverse events included peripheral edema, liver enzyme elevations, abnormal liver function, decreased platelet and white blood cell counts, and vomiting.
Which TKI is Best?
Invited discussant Antonio Passaro, MD, PhD, from the European Institute of Oncology in Milan, noted that eligibility for treatment with savolitinib or other MET exon 14-targeting TKIs is limited to about 3% of patients with NSCLC of adenocarcinoma histology.
He said that savolitinib appears to be similar in performance to two other TKIs for NSCLC with MET exon-14 skipping mutations that are currently on the market in the United States, Europe, and Japan: capmatinib (Tabrecta) and tepotinib (Tepmetko).
“Globally, all the results show a numerically better performance when we use a selective TKI in first-line treatment over the second-line treatment, in particular for overall response rate,” he said.
Dr. Passaro noted that savolitinib differs from the other two MET TKIs in that PFS with savolitinib is similar for treatment-naive and previously treated patients.
He added, however, that “today it’s very difficult” to determine which is the “perfect” agent for a specific disease presentation, particularly since MET exon 14 skipping mutations can also be found in patients with squamous cell carcinomas and those with a history of smoking.
To get a better sense of which drug to use in a specific situation, it would be helpful to analyze trial results in the context of tumor histology, smoking history, programmed death protein 1-ligand 1 status, and co-mutations, he said.
The study was sponsored by Hutchmed. Dr. Zhang reported having no conflicts of interest. Dr. Passaro reported a consulting, advisory, or speakers bureau role for multiple companies, not including Hutchmed.
FROM ELCC 2024
Alzheimer’s Prevalence Predicted to Double by 2050
An estimated 6.9 million older adults are living with Alzheimer’s disease (AD) in the United States, and another 200,000 people under age 65 have younger-onset AD, new data showed.
The report also included sobering statistics on AD-related mortality — which increased 141% between 2001 and 2021 — and described “dementia neurology deserts” that will leave some states with less than 10 neurologists per 10,000 people with dementia as early as 2025. The shortages extend to other specialties, clinical professionals, and direct care workers, the report authors wrote.
“Dementia healthcare is a complex maze composed of primary care providers, specialists, social services, medication management, and caregiver support,” Sam Fazio, PhD, senior director, psychosocial research and quality care, Alzheimer’s Association, said in a press release.
“As the number of individuals living with Alzheimer’s continues to grow, ensuring patients, their caregivers, and families have a clear understanding of how to navigate dementia care resources is critical to improving health outcomes,” Dr. Fazio added.
The “2024 Alzheimer’s Disease Facts and Figures” study and accompanying report “Mapping a Better Future for Dementia Care Navigation” were published online on March 20 by the Alzheimer’s Association and will appear in the May issue of Alzheimer’s & Dementia.
Significant Increase in Mortality
The number of people over 65 with AD rose slightly in 2024 to 6.9 million from 6.7 million in 2023. The number of younger-onset AD cases remained roughly the same.
States and counties in the eastern and southeastern United States have the highest percentage of people over 65 with AD, with the District of Columbia reporting 16.8% and New York, Florida, and Mississippi between 12.5% and 12.7%. Alaska has the lowest with 8.8%.
Based on an analysis of death certificate data, the number of deaths from AD increased 141% between 2000 and 2021, while deaths from heart disease — the number-one cause of death — decreased 2.1%. Among people aged 70, 61% of those with AD are expected to die before age 80 compared with 30% of those without AD.
The cost of health and long-term care for people with AD has also risen, the data suggested, with a projected total for 2024 of $360 billion, a $15 billion increase since 2023. That figure does not include unpaid caregiving by family and friends, which the report valued at nearly $350 billion.
With the prevalence of AD expected to rise — the report projected 11.2 million by 2040 and 12.7 million by 2050 — mortality, morbidity, and healthcare costs will only continue to go up. Without new treatments and advancements in care, study authors estimated the cost will reach $1 trillion in 2050.
The report also waded into the issue of workforce deficits. Between 2012 and 2022, the number of direct care workers in the United States increased from 3.2 million to 4.8 million. Study authors estimated more than 1 million additional direct care workers will be needed before 2031.
There is a shortage of clinicians as well, especially for geriatricians, specially trained family physicians, or board-certified internists who can screen for, detect, and diagnose possible dementia. The National Center for Health Workforce Analysis (NCHWA) determined shortages in that specialty began a decade ago, and the projected need for geriatricians is expected to far exceed the supply in every region of the United States by 2050.
The NCHWA also projected a shortfall of neurologists by 2025. The report listed 20 US states as “dementia neurology deserts,” meaning they’re projected to have fewer than 10 neurologists per 10,000 people with dementia in 2025.
Several factors may contribute to the scarcity of specialists. In addition to an aging population, contributors include lower pay for geriatricians and neurologists compared with other specialists, an inadequate number of clinician educators with relevant specialties on faculties of health professional schools, and limited incentives to choose these specialties.
Underestimating a ‘Serious Problem’
The report “probably underestimates” the “serious problem with dementia specialty care in the United States,” David S. Knopman, MD, professor of neurology, Mayo Clinic, Rochester, Minnesota, told this news organization.
Given the complexity of managing treatments for AD, such as the monoclonal antibody lecanemab, or those for dementia with Lewy bodies, “my sense is that very few geriatricians are likely to take an active role in dementia care,” said Dr. Knopman.
Very few neurologists have specialty training in dementia diagnosis and care, he added, and neurologists who do specialize in dementia are generally located exclusively in tertiary medical centers.
“While neurologists are more likely to be able to diagnose dementia subtypes compared to geriatricians or general internists or family physicians, non-specialty neurologists are also unlikely to have the expertise to manage lecanemab therapy or to deal with diagnosis and management of dementia subtypes,” Dr. Knopman said.
“Filling the pipeline with new trainees is going to take a long, long time,” he added.
As it stands, most dementia diagnoses are not made by specialists. The report cited a study of Medicare beneficiaries that found 85% of people living with dementia were diagnosed by providers such as primary care physicians (PCPs).
Barriers to Care
Although screening is now a reimbursable service by Medicare, PCPs experience numerous barriers to detecting cognitive impairment and diagnosing dementia. Routinely used cognitive assessments are time-consuming and labor-intensive, making them challenging to use in a busy clinical setting.
“Even if dementia is diagnosed, providers sometimes wait to disclose this information to the patient due to diagnostic uncertainty, time constraints, stigma, and fear of causing emotional distress,” the authors wrote.
A previous survey by the Alzheimer’s Association uncovered a high degree of uncertainty and discomfort among PCPs in making a dementia diagnosis. While almost a third reported referring patients to specialists, 55% said there were not enough geriatricians and other specialists in their area to meet the demand.
In tackling the theme of dementia care navigation, the report included a survey of 1204 nonphysician healthcare workers, including nurses, physician assistants, and social workers.
About 60% believed the US healthcare system isn’t effectively helping patients and families navigate the system and that training in dementia care navigation is lacking and not standardized. Respondents also said nonmedical professionals are best suited to help people with dementia and their caregivers navigate care.
Respondents identified a range of barriers that make navigating dementia care difficult for patients and families. More than three in four (77%) identified a lack of community-based resources as a barrier. And 70% called out restrictions in current payment models as a barrier, with 41% saying this was the greatest barrier.
Alternative Model
In July, the Centers for Medicare & Medicaid Services will launch a pilot model in dementia care management, the Guiding an Improved Dementia Experience. The program will test a monthly per-patient payment model as a fee-for-service replacement.
Healthcare providers who participate in the program will deliver supportive services to people living with dementia and provide access to a care navigator to help patients and caregivers access services and support.
“There is growing momentum in this country to enhance dementia care navigation,” Dr. Fazio said in the release. “Dementia care navigation programs have shown they can be a huge benefit to people living with dementia and their caregivers.”
These programs are unfortunately not widespread across the country, but the Alzheimer’s Association hopes this report “will be a catalyst for change,” Dr. Fazio added.
A separate survey of dementia caregivers found they would overwhelmingly welcome navigator support. The vast majority (97%) said they would find navigation services helpful.
Such services may also go a long way to alleviating stresses involved in dementia caregiving, a top stressor being care coordination, the report noted. Seven in 10 caregiver survey respondents (70%) reported coordinating care is stressful. More than half (53%) said navigating healthcare is difficult, and two-thirds (66%) said they have difficulty finding resources and supports.
Around-the-clock support in addition to care coordination and help understanding their care recipient’s condition are among the top services dementia caregiver respondents cited as being most helpful.
Dr. Knopman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
An estimated 6.9 million older adults are living with Alzheimer’s disease (AD) in the United States, and another 200,000 people under age 65 have younger-onset AD, new data showed.
The report also included sobering statistics on AD-related mortality — which increased 141% between 2001 and 2021 — and described “dementia neurology deserts” that will leave some states with less than 10 neurologists per 10,000 people with dementia as early as 2025. The shortages extend to other specialties, clinical professionals, and direct care workers, the report authors wrote.
“Dementia healthcare is a complex maze composed of primary care providers, specialists, social services, medication management, and caregiver support,” Sam Fazio, PhD, senior director, psychosocial research and quality care, Alzheimer’s Association, said in a press release.
“As the number of individuals living with Alzheimer’s continues to grow, ensuring patients, their caregivers, and families have a clear understanding of how to navigate dementia care resources is critical to improving health outcomes,” Dr. Fazio added.
The “2024 Alzheimer’s Disease Facts and Figures” study and accompanying report “Mapping a Better Future for Dementia Care Navigation” were published online on March 20 by the Alzheimer’s Association and will appear in the May issue of Alzheimer’s & Dementia.
Significant Increase in Mortality
The number of people over 65 with AD rose slightly in 2024 to 6.9 million from 6.7 million in 2023. The number of younger-onset AD cases remained roughly the same.
States and counties in the eastern and southeastern United States have the highest percentage of people over 65 with AD, with the District of Columbia reporting 16.8% and New York, Florida, and Mississippi between 12.5% and 12.7%. Alaska has the lowest with 8.8%.
Based on an analysis of death certificate data, the number of deaths from AD increased 141% between 2000 and 2021, while deaths from heart disease — the number-one cause of death — decreased 2.1%. Among people aged 70, 61% of those with AD are expected to die before age 80 compared with 30% of those without AD.
The cost of health and long-term care for people with AD has also risen, the data suggested, with a projected total for 2024 of $360 billion, a $15 billion increase since 2023. That figure does not include unpaid caregiving by family and friends, which the report valued at nearly $350 billion.
With the prevalence of AD expected to rise — the report projected 11.2 million by 2040 and 12.7 million by 2050 — mortality, morbidity, and healthcare costs will only continue to go up. Without new treatments and advancements in care, study authors estimated the cost will reach $1 trillion in 2050.
The report also waded into the issue of workforce deficits. Between 2012 and 2022, the number of direct care workers in the United States increased from 3.2 million to 4.8 million. Study authors estimated more than 1 million additional direct care workers will be needed before 2031.
There is a shortage of clinicians as well, especially for geriatricians, specially trained family physicians, or board-certified internists who can screen for, detect, and diagnose possible dementia. The National Center for Health Workforce Analysis (NCHWA) determined shortages in that specialty began a decade ago, and the projected need for geriatricians is expected to far exceed the supply in every region of the United States by 2050.
The NCHWA also projected a shortfall of neurologists by 2025. The report listed 20 US states as “dementia neurology deserts,” meaning they’re projected to have fewer than 10 neurologists per 10,000 people with dementia in 2025.
Several factors may contribute to the scarcity of specialists. In addition to an aging population, contributors include lower pay for geriatricians and neurologists compared with other specialists, an inadequate number of clinician educators with relevant specialties on faculties of health professional schools, and limited incentives to choose these specialties.
Underestimating a ‘Serious Problem’
The report “probably underestimates” the “serious problem with dementia specialty care in the United States,” David S. Knopman, MD, professor of neurology, Mayo Clinic, Rochester, Minnesota, told this news organization.
Given the complexity of managing treatments for AD, such as the monoclonal antibody lecanemab, or those for dementia with Lewy bodies, “my sense is that very few geriatricians are likely to take an active role in dementia care,” said Dr. Knopman.
Very few neurologists have specialty training in dementia diagnosis and care, he added, and neurologists who do specialize in dementia are generally located exclusively in tertiary medical centers.
“While neurologists are more likely to be able to diagnose dementia subtypes compared to geriatricians or general internists or family physicians, non-specialty neurologists are also unlikely to have the expertise to manage lecanemab therapy or to deal with diagnosis and management of dementia subtypes,” Dr. Knopman said.
“Filling the pipeline with new trainees is going to take a long, long time,” he added.
As it stands, most dementia diagnoses are not made by specialists. The report cited a study of Medicare beneficiaries that found 85% of people living with dementia were diagnosed by providers such as primary care physicians (PCPs).
Barriers to Care
Although screening is now a reimbursable service by Medicare, PCPs experience numerous barriers to detecting cognitive impairment and diagnosing dementia. Routinely used cognitive assessments are time-consuming and labor-intensive, making them challenging to use in a busy clinical setting.
“Even if dementia is diagnosed, providers sometimes wait to disclose this information to the patient due to diagnostic uncertainty, time constraints, stigma, and fear of causing emotional distress,” the authors wrote.
A previous survey by the Alzheimer’s Association uncovered a high degree of uncertainty and discomfort among PCPs in making a dementia diagnosis. While almost a third reported referring patients to specialists, 55% said there were not enough geriatricians and other specialists in their area to meet the demand.
In tackling the theme of dementia care navigation, the report included a survey of 1204 nonphysician healthcare workers, including nurses, physician assistants, and social workers.
About 60% believed the US healthcare system isn’t effectively helping patients and families navigate the system and that training in dementia care navigation is lacking and not standardized. Respondents also said nonmedical professionals are best suited to help people with dementia and their caregivers navigate care.
Respondents identified a range of barriers that make navigating dementia care difficult for patients and families. More than three in four (77%) identified a lack of community-based resources as a barrier. And 70% called out restrictions in current payment models as a barrier, with 41% saying this was the greatest barrier.
Alternative Model
In July, the Centers for Medicare & Medicaid Services will launch a pilot model in dementia care management, the Guiding an Improved Dementia Experience. The program will test a monthly per-patient payment model as a fee-for-service replacement.
Healthcare providers who participate in the program will deliver supportive services to people living with dementia and provide access to a care navigator to help patients and caregivers access services and support.
“There is growing momentum in this country to enhance dementia care navigation,” Dr. Fazio said in the release. “Dementia care navigation programs have shown they can be a huge benefit to people living with dementia and their caregivers.”
These programs are unfortunately not widespread across the country, but the Alzheimer’s Association hopes this report “will be a catalyst for change,” Dr. Fazio added.
A separate survey of dementia caregivers found they would overwhelmingly welcome navigator support. The vast majority (97%) said they would find navigation services helpful.
Such services may also go a long way to alleviating stresses involved in dementia caregiving, a top stressor being care coordination, the report noted. Seven in 10 caregiver survey respondents (70%) reported coordinating care is stressful. More than half (53%) said navigating healthcare is difficult, and two-thirds (66%) said they have difficulty finding resources and supports.
Around-the-clock support in addition to care coordination and help understanding their care recipient’s condition are among the top services dementia caregiver respondents cited as being most helpful.
Dr. Knopman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
An estimated 6.9 million older adults are living with Alzheimer’s disease (AD) in the United States, and another 200,000 people under age 65 have younger-onset AD, new data showed.
The report also included sobering statistics on AD-related mortality — which increased 141% between 2001 and 2021 — and described “dementia neurology deserts” that will leave some states with less than 10 neurologists per 10,000 people with dementia as early as 2025. The shortages extend to other specialties, clinical professionals, and direct care workers, the report authors wrote.
“Dementia healthcare is a complex maze composed of primary care providers, specialists, social services, medication management, and caregiver support,” Sam Fazio, PhD, senior director, psychosocial research and quality care, Alzheimer’s Association, said in a press release.
“As the number of individuals living with Alzheimer’s continues to grow, ensuring patients, their caregivers, and families have a clear understanding of how to navigate dementia care resources is critical to improving health outcomes,” Dr. Fazio added.
The “2024 Alzheimer’s Disease Facts and Figures” study and accompanying report “Mapping a Better Future for Dementia Care Navigation” were published online on March 20 by the Alzheimer’s Association and will appear in the May issue of Alzheimer’s & Dementia.
Significant Increase in Mortality
The number of people over 65 with AD rose slightly in 2024 to 6.9 million from 6.7 million in 2023. The number of younger-onset AD cases remained roughly the same.
States and counties in the eastern and southeastern United States have the highest percentage of people over 65 with AD, with the District of Columbia reporting 16.8% and New York, Florida, and Mississippi between 12.5% and 12.7%. Alaska has the lowest with 8.8%.
Based on an analysis of death certificate data, the number of deaths from AD increased 141% between 2000 and 2021, while deaths from heart disease — the number-one cause of death — decreased 2.1%. Among people aged 70, 61% of those with AD are expected to die before age 80 compared with 30% of those without AD.
The cost of health and long-term care for people with AD has also risen, the data suggested, with a projected total for 2024 of $360 billion, a $15 billion increase since 2023. That figure does not include unpaid caregiving by family and friends, which the report valued at nearly $350 billion.
With the prevalence of AD expected to rise — the report projected 11.2 million by 2040 and 12.7 million by 2050 — mortality, morbidity, and healthcare costs will only continue to go up. Without new treatments and advancements in care, study authors estimated the cost will reach $1 trillion in 2050.
The report also waded into the issue of workforce deficits. Between 2012 and 2022, the number of direct care workers in the United States increased from 3.2 million to 4.8 million. Study authors estimated more than 1 million additional direct care workers will be needed before 2031.
There is a shortage of clinicians as well, especially for geriatricians, specially trained family physicians, or board-certified internists who can screen for, detect, and diagnose possible dementia. The National Center for Health Workforce Analysis (NCHWA) determined shortages in that specialty began a decade ago, and the projected need for geriatricians is expected to far exceed the supply in every region of the United States by 2050.
The NCHWA also projected a shortfall of neurologists by 2025. The report listed 20 US states as “dementia neurology deserts,” meaning they’re projected to have fewer than 10 neurologists per 10,000 people with dementia in 2025.
Several factors may contribute to the scarcity of specialists. In addition to an aging population, contributors include lower pay for geriatricians and neurologists compared with other specialists, an inadequate number of clinician educators with relevant specialties on faculties of health professional schools, and limited incentives to choose these specialties.
Underestimating a ‘Serious Problem’
The report “probably underestimates” the “serious problem with dementia specialty care in the United States,” David S. Knopman, MD, professor of neurology, Mayo Clinic, Rochester, Minnesota, told this news organization.
Given the complexity of managing treatments for AD, such as the monoclonal antibody lecanemab, or those for dementia with Lewy bodies, “my sense is that very few geriatricians are likely to take an active role in dementia care,” said Dr. Knopman.
Very few neurologists have specialty training in dementia diagnosis and care, he added, and neurologists who do specialize in dementia are generally located exclusively in tertiary medical centers.
“While neurologists are more likely to be able to diagnose dementia subtypes compared to geriatricians or general internists or family physicians, non-specialty neurologists are also unlikely to have the expertise to manage lecanemab therapy or to deal with diagnosis and management of dementia subtypes,” Dr. Knopman said.
“Filling the pipeline with new trainees is going to take a long, long time,” he added.
As it stands, most dementia diagnoses are not made by specialists. The report cited a study of Medicare beneficiaries that found 85% of people living with dementia were diagnosed by providers such as primary care physicians (PCPs).
Barriers to Care
Although screening is now a reimbursable service by Medicare, PCPs experience numerous barriers to detecting cognitive impairment and diagnosing dementia. Routinely used cognitive assessments are time-consuming and labor-intensive, making them challenging to use in a busy clinical setting.
“Even if dementia is diagnosed, providers sometimes wait to disclose this information to the patient due to diagnostic uncertainty, time constraints, stigma, and fear of causing emotional distress,” the authors wrote.
A previous survey by the Alzheimer’s Association uncovered a high degree of uncertainty and discomfort among PCPs in making a dementia diagnosis. While almost a third reported referring patients to specialists, 55% said there were not enough geriatricians and other specialists in their area to meet the demand.
In tackling the theme of dementia care navigation, the report included a survey of 1204 nonphysician healthcare workers, including nurses, physician assistants, and social workers.
About 60% believed the US healthcare system isn’t effectively helping patients and families navigate the system and that training in dementia care navigation is lacking and not standardized. Respondents also said nonmedical professionals are best suited to help people with dementia and their caregivers navigate care.
Respondents identified a range of barriers that make navigating dementia care difficult for patients and families. More than three in four (77%) identified a lack of community-based resources as a barrier. And 70% called out restrictions in current payment models as a barrier, with 41% saying this was the greatest barrier.
Alternative Model
In July, the Centers for Medicare & Medicaid Services will launch a pilot model in dementia care management, the Guiding an Improved Dementia Experience. The program will test a monthly per-patient payment model as a fee-for-service replacement.
Healthcare providers who participate in the program will deliver supportive services to people living with dementia and provide access to a care navigator to help patients and caregivers access services and support.
“There is growing momentum in this country to enhance dementia care navigation,” Dr. Fazio said in the release. “Dementia care navigation programs have shown they can be a huge benefit to people living with dementia and their caregivers.”
These programs are unfortunately not widespread across the country, but the Alzheimer’s Association hopes this report “will be a catalyst for change,” Dr. Fazio added.
A separate survey of dementia caregivers found they would overwhelmingly welcome navigator support. The vast majority (97%) said they would find navigation services helpful.
Such services may also go a long way to alleviating stresses involved in dementia caregiving, a top stressor being care coordination, the report noted. Seven in 10 caregiver survey respondents (70%) reported coordinating care is stressful. More than half (53%) said navigating healthcare is difficult, and two-thirds (66%) said they have difficulty finding resources and supports.
Around-the-clock support in addition to care coordination and help understanding their care recipient’s condition are among the top services dementia caregiver respondents cited as being most helpful.
Dr. Knopman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Hair-Straightening Products Entail Acute Kidney Failure Risk
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Skin Test Accurately Detects Parkinson’s, Other Neurodegenerative Disorders
A simple skin biopsy test is able to detect an abnormal form of alpha-synuclein with high accuracy in individuals with neurodegenerative disorders such as Parkinson’s disease (PD).
Synucleinopathies include PD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and pure autonomic failure (PAF).
“Each year, there are nearly 200,000 people in the U.S. who face a diagnosis of Parkinson’s disease, dementia with Lewy bodies, and related disorders,” study investigator Christopher H. Gibbons, MD, professor of neurology at Harvard Medical School in Boston, said in a press release.
He explained that patients often experience delays in diagnosis or are misdiagnosed due to the complexity of synucleinopathies.
“With a simple, minimally invasive skin biopsy test, this blinded, multicenter study demonstrated how we can more objectively identify the underlying pathology of synucleinopathies and offer better diagnostic answers and care for patients.”
The findings were published online on March 20 in JAMA.
An Urgent Priority
Affecting an estimated 2.5 million people in the United States, synucleinopathies are progressive neurodegenerative diseases with varying prognoses, so identifying a reliable diagnostic biomarker is an “urgent unmet priority,” the researchers noted.
The disorders share some symptoms such as tremors and cognitive changes, and all are characterized by P-SYN, an abnormal protein found in the cutaneous nerve fibers.
The study included 428 adults aged 40-99 years (mean age, 70 years) recruited from 30 academic and community-based neurology practices across the United States, with 277 diagnosed with PD, DLB, MSA, or PAF. It also included a control group of 120 participants with no symptoms suggestive of synucleinopathy.
Investigators used the commercially available Syn-One Test, developed in 2019 by CND Life Sciences, to analyze levels of P-SYN via 3-mm punch skin biopsies from each participant.
The test detected P-SYN in 95.5% of study participants overall, including 89 of 96 (92.7%) with PD, 54 of 55 (98.2%) with MSA, 48 of 50 (96%) with DLB, 22 of 22 (100%) with PAF, and 4 of 120 (3.3%) of the controls with no synucleinopathy.
The investigators said it is possible that some of the controls who tested positive had a subclinical form of synucleinopathy, which would explain the false positives.
Study limitations include clinical consensus diagnostic criteria without video or autopsy confirmation, a lack of genetic testing on participants (some genetic forms of PD do not have alpha-synuclein deposition), and the fact that controls were younger than those in disease groups.
“Further research is needed in unselected clinical populations to externally validate the findings and fully characterize the potential role of skin biopsy detection of P-SYN in clinical care,” the authors wrote.
Syn-One is not approved by the US Food and Drug Administration as a diagnostic test for PD but is available as a pathologic assay that determines whether a tissue sample contains phosphorylated alpha-synuclein and can be billed through Medicare.
The study was funded by the National Institutes of Health. Dr. Gibbons reported having stock options in CND Life Sciences outside the submitted work. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
A simple skin biopsy test is able to detect an abnormal form of alpha-synuclein with high accuracy in individuals with neurodegenerative disorders such as Parkinson’s disease (PD).
Synucleinopathies include PD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and pure autonomic failure (PAF).
“Each year, there are nearly 200,000 people in the U.S. who face a diagnosis of Parkinson’s disease, dementia with Lewy bodies, and related disorders,” study investigator Christopher H. Gibbons, MD, professor of neurology at Harvard Medical School in Boston, said in a press release.
He explained that patients often experience delays in diagnosis or are misdiagnosed due to the complexity of synucleinopathies.
“With a simple, minimally invasive skin biopsy test, this blinded, multicenter study demonstrated how we can more objectively identify the underlying pathology of synucleinopathies and offer better diagnostic answers and care for patients.”
The findings were published online on March 20 in JAMA.
An Urgent Priority
Affecting an estimated 2.5 million people in the United States, synucleinopathies are progressive neurodegenerative diseases with varying prognoses, so identifying a reliable diagnostic biomarker is an “urgent unmet priority,” the researchers noted.
The disorders share some symptoms such as tremors and cognitive changes, and all are characterized by P-SYN, an abnormal protein found in the cutaneous nerve fibers.
The study included 428 adults aged 40-99 years (mean age, 70 years) recruited from 30 academic and community-based neurology practices across the United States, with 277 diagnosed with PD, DLB, MSA, or PAF. It also included a control group of 120 participants with no symptoms suggestive of synucleinopathy.
Investigators used the commercially available Syn-One Test, developed in 2019 by CND Life Sciences, to analyze levels of P-SYN via 3-mm punch skin biopsies from each participant.
The test detected P-SYN in 95.5% of study participants overall, including 89 of 96 (92.7%) with PD, 54 of 55 (98.2%) with MSA, 48 of 50 (96%) with DLB, 22 of 22 (100%) with PAF, and 4 of 120 (3.3%) of the controls with no synucleinopathy.
The investigators said it is possible that some of the controls who tested positive had a subclinical form of synucleinopathy, which would explain the false positives.
Study limitations include clinical consensus diagnostic criteria without video or autopsy confirmation, a lack of genetic testing on participants (some genetic forms of PD do not have alpha-synuclein deposition), and the fact that controls were younger than those in disease groups.
“Further research is needed in unselected clinical populations to externally validate the findings and fully characterize the potential role of skin biopsy detection of P-SYN in clinical care,” the authors wrote.
Syn-One is not approved by the US Food and Drug Administration as a diagnostic test for PD but is available as a pathologic assay that determines whether a tissue sample contains phosphorylated alpha-synuclein and can be billed through Medicare.
The study was funded by the National Institutes of Health. Dr. Gibbons reported having stock options in CND Life Sciences outside the submitted work. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
A simple skin biopsy test is able to detect an abnormal form of alpha-synuclein with high accuracy in individuals with neurodegenerative disorders such as Parkinson’s disease (PD).
Synucleinopathies include PD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and pure autonomic failure (PAF).
“Each year, there are nearly 200,000 people in the U.S. who face a diagnosis of Parkinson’s disease, dementia with Lewy bodies, and related disorders,” study investigator Christopher H. Gibbons, MD, professor of neurology at Harvard Medical School in Boston, said in a press release.
He explained that patients often experience delays in diagnosis or are misdiagnosed due to the complexity of synucleinopathies.
“With a simple, minimally invasive skin biopsy test, this blinded, multicenter study demonstrated how we can more objectively identify the underlying pathology of synucleinopathies and offer better diagnostic answers and care for patients.”
The findings were published online on March 20 in JAMA.
An Urgent Priority
Affecting an estimated 2.5 million people in the United States, synucleinopathies are progressive neurodegenerative diseases with varying prognoses, so identifying a reliable diagnostic biomarker is an “urgent unmet priority,” the researchers noted.
The disorders share some symptoms such as tremors and cognitive changes, and all are characterized by P-SYN, an abnormal protein found in the cutaneous nerve fibers.
The study included 428 adults aged 40-99 years (mean age, 70 years) recruited from 30 academic and community-based neurology practices across the United States, with 277 diagnosed with PD, DLB, MSA, or PAF. It also included a control group of 120 participants with no symptoms suggestive of synucleinopathy.
Investigators used the commercially available Syn-One Test, developed in 2019 by CND Life Sciences, to analyze levels of P-SYN via 3-mm punch skin biopsies from each participant.
The test detected P-SYN in 95.5% of study participants overall, including 89 of 96 (92.7%) with PD, 54 of 55 (98.2%) with MSA, 48 of 50 (96%) with DLB, 22 of 22 (100%) with PAF, and 4 of 120 (3.3%) of the controls with no synucleinopathy.
The investigators said it is possible that some of the controls who tested positive had a subclinical form of synucleinopathy, which would explain the false positives.
Study limitations include clinical consensus diagnostic criteria without video or autopsy confirmation, a lack of genetic testing on participants (some genetic forms of PD do not have alpha-synuclein deposition), and the fact that controls were younger than those in disease groups.
“Further research is needed in unselected clinical populations to externally validate the findings and fully characterize the potential role of skin biopsy detection of P-SYN in clinical care,” the authors wrote.
Syn-One is not approved by the US Food and Drug Administration as a diagnostic test for PD but is available as a pathologic assay that determines whether a tissue sample contains phosphorylated alpha-synuclein and can be billed through Medicare.
The study was funded by the National Institutes of Health. Dr. Gibbons reported having stock options in CND Life Sciences outside the submitted work. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
Commentary: Choosing Treatments of AD, and Possible Connection to Learning Issues, April 2024
Not everyone with AD treated with dupilumab gets clear or almost clear in clinical trials. The study by Cork and colleagues looked to see whether those patients who did not get to clear or almost clear were still having clinically meaningful improvement. To test this, the investigators looked at patients who still had mild or worse disease and then at the proportion of those patients at week 16 who achieved a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms, and quality of life: ≥50% improvement in Eczema Area and Severity Index or ≥4-point reduction in worst scratch/itch numerical rating scale, or ≥6-point reduction in Children's Dermatology Life Quality Index/Infants' Dermatitis Quality of Life Index. Significantly more patients, both clinically and statistically significantly more, receiving dupilumab vs placebo achieved the composite endpoint (77.7% vs 24.6%; P < .0001).
The "success rate" reported in clinical trials underestimates how often patients can be successfully treated with dupilumab. I don't need a complicated composite outcome to know this. I just use the standardized 2-point Patient Global Assessment measure. I ask patients, "How are you doing?" If they say "Great," that's success. If they say, "Not so good," that's failure. I think about 80% of patients with AD treated with dupilumab have success based on this standard.
Hand dermatitis can be quite resistant to treatment. Even making a diagnosis can be challenging, as psoriasis and dermatitis of the hands looks so similar to me (and when I used to send biopsies and ask the pathologist whether it's dermatitis or psoriasis, invariably the dermatopathologist responded "yes"). The study by Kamphuis and colleagues examined the efficacy of abrocitinib in just over 100 patients with hand eczema who were enrolled in the BioDay registry. Such registries are very helpful for assessing real-world results. The drug seemed reasonably successful, with only about 30% discontinuing treatment. About two thirds of the discontinuations were due to inefficacy and about one third to an adverse event.
I think there's real value in prescribing the treatments patients want. Studies like the one by Ameen and colleagues, using a discrete-choice methodology, allows one to determine patients' average preferences. In this study, the discrete-choice approach found that patients prefer safety over other attributes. Some years ago, my colleagues and I queried patients to get a sense of their quantitative preferences for different treatments. Our study also found that patients preferred safety over other attributes. However, when we asked them to choose among different treatment options, they didn't choose the safest one. I think they believe that they prefer safety, but I'm not sure they really do. In any case, the average preference of the entire population of people with AD isn't really all that important when we've got just one patient sitting in front of us. It's that particular patient's preference that should drive the treatment plan.
Not everyone with AD treated with dupilumab gets clear or almost clear in clinical trials. The study by Cork and colleagues looked to see whether those patients who did not get to clear or almost clear were still having clinically meaningful improvement. To test this, the investigators looked at patients who still had mild or worse disease and then at the proportion of those patients at week 16 who achieved a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms, and quality of life: ≥50% improvement in Eczema Area and Severity Index or ≥4-point reduction in worst scratch/itch numerical rating scale, or ≥6-point reduction in Children's Dermatology Life Quality Index/Infants' Dermatitis Quality of Life Index. Significantly more patients, both clinically and statistically significantly more, receiving dupilumab vs placebo achieved the composite endpoint (77.7% vs 24.6%; P < .0001).
The "success rate" reported in clinical trials underestimates how often patients can be successfully treated with dupilumab. I don't need a complicated composite outcome to know this. I just use the standardized 2-point Patient Global Assessment measure. I ask patients, "How are you doing?" If they say "Great," that's success. If they say, "Not so good," that's failure. I think about 80% of patients with AD treated with dupilumab have success based on this standard.
Hand dermatitis can be quite resistant to treatment. Even making a diagnosis can be challenging, as psoriasis and dermatitis of the hands looks so similar to me (and when I used to send biopsies and ask the pathologist whether it's dermatitis or psoriasis, invariably the dermatopathologist responded "yes"). The study by Kamphuis and colleagues examined the efficacy of abrocitinib in just over 100 patients with hand eczema who were enrolled in the BioDay registry. Such registries are very helpful for assessing real-world results. The drug seemed reasonably successful, with only about 30% discontinuing treatment. About two thirds of the discontinuations were due to inefficacy and about one third to an adverse event.
I think there's real value in prescribing the treatments patients want. Studies like the one by Ameen and colleagues, using a discrete-choice methodology, allows one to determine patients' average preferences. In this study, the discrete-choice approach found that patients prefer safety over other attributes. Some years ago, my colleagues and I queried patients to get a sense of their quantitative preferences for different treatments. Our study also found that patients preferred safety over other attributes. However, when we asked them to choose among different treatment options, they didn't choose the safest one. I think they believe that they prefer safety, but I'm not sure they really do. In any case, the average preference of the entire population of people with AD isn't really all that important when we've got just one patient sitting in front of us. It's that particular patient's preference that should drive the treatment plan.
Not everyone with AD treated with dupilumab gets clear or almost clear in clinical trials. The study by Cork and colleagues looked to see whether those patients who did not get to clear or almost clear were still having clinically meaningful improvement. To test this, the investigators looked at patients who still had mild or worse disease and then at the proportion of those patients at week 16 who achieved a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms, and quality of life: ≥50% improvement in Eczema Area and Severity Index or ≥4-point reduction in worst scratch/itch numerical rating scale, or ≥6-point reduction in Children's Dermatology Life Quality Index/Infants' Dermatitis Quality of Life Index. Significantly more patients, both clinically and statistically significantly more, receiving dupilumab vs placebo achieved the composite endpoint (77.7% vs 24.6%; P < .0001).
The "success rate" reported in clinical trials underestimates how often patients can be successfully treated with dupilumab. I don't need a complicated composite outcome to know this. I just use the standardized 2-point Patient Global Assessment measure. I ask patients, "How are you doing?" If they say "Great," that's success. If they say, "Not so good," that's failure. I think about 80% of patients with AD treated with dupilumab have success based on this standard.
Hand dermatitis can be quite resistant to treatment. Even making a diagnosis can be challenging, as psoriasis and dermatitis of the hands looks so similar to me (and when I used to send biopsies and ask the pathologist whether it's dermatitis or psoriasis, invariably the dermatopathologist responded "yes"). The study by Kamphuis and colleagues examined the efficacy of abrocitinib in just over 100 patients with hand eczema who were enrolled in the BioDay registry. Such registries are very helpful for assessing real-world results. The drug seemed reasonably successful, with only about 30% discontinuing treatment. About two thirds of the discontinuations were due to inefficacy and about one third to an adverse event.
I think there's real value in prescribing the treatments patients want. Studies like the one by Ameen and colleagues, using a discrete-choice methodology, allows one to determine patients' average preferences. In this study, the discrete-choice approach found that patients prefer safety over other attributes. Some years ago, my colleagues and I queried patients to get a sense of their quantitative preferences for different treatments. Our study also found that patients preferred safety over other attributes. However, when we asked them to choose among different treatment options, they didn't choose the safest one. I think they believe that they prefer safety, but I'm not sure they really do. In any case, the average preference of the entire population of people with AD isn't really all that important when we've got just one patient sitting in front of us. It's that particular patient's preference that should drive the treatment plan.