User login
Native Americans appear to be at increased risk for AFib
Over 4 years, atrial fibrillation (AFib) developed significantly more often in a group of Native Americans men than it did among other racial and ethnic groups, a large longitudinal cohort study has found.
The overall incidence among Native Americans was 7.49 per 1,000 person-years – significantly higher than the incidence in a comparator cohort of black, white, Asian, and Hispanic men, Gregory M. Marcus, MD, of the University of California, San Francisco, and colleagues wrote in a research letter published in Circulation.
“We were surprised to find that American Indians experienced a higher risk of atrial fibrillation, compared to every other racial and ethnic group,” Dr. Marcus said in a press release that accompanied the study. “Understanding the mechanisms and factors by which American Indians experience this higher risk may help investigators better understand the fundamental causes of atrial fibrillation that prove useful to everyone at risk for AFib, regardless of their race or ethnicity.”
The team plumbed the Healthcare Cost and Utilization Project (HCUP) California state databases for information on more than 16 million cases of AFib that occurred during 2005-2011. Native Americans comprised just 0.6% of the cohort. Most of the patients (57.2%) were white; 8% were black, 25.6% Hispanic, and 8.6% Asian. After targeting only new-onset cases, there were 344,469 incident AFib episodes over a median follow-up of 4.1 years.
The overall incidence of AFib in Native Americans was 7.49 per 1,000 person-years, significantly higher than the 6.89 per 1000 person-years observed in the rest of the cohort ( P less than .0001). The difference remained significant even after the team controlled for age, sex, income, and heart and other diseases. Nor was it altered by a sensitivity analysis that controlled for place of presentation and patients who were aged at least 35 years with at least two encounters with medical facilities.
In an interaction analysis, the increased risk appeared to be driven by higher rates of diabetes and chronic kidney disease, the authors wrote.
“This may suggest that the presence of these two processes contributes some pathophysiology related to AF[ib] risk that may be similar to the heightened risk inherent among American Indians,” they wrote. “It is also important to note that there was no evidence of any other statistically significant interactions despite the inclusion of millions of patients.”
Supporting data for these associations were not included in the research letter.
The authors noted some limitations of their study. Race or ethnicity were self-reported and could not be independently confirmed, so there was no way to tease out the effects in multiracial patients. Also, the database didn’t record outpatient encounters, which might result in some selection bias.
“Last, because this was an observational study, these results should not be interpreted as evidence of causal effect,” they noted.
“In conclusion, we observed that American Indians had a higher risk of atrial fibrillation, compared with all other racial and ethnic group. The heightened risk … in American Indians persisted after multivariable adjustment for known conventional confounders and mediators, suggesting that an unidentified characteristic, including possible genetic or environmental factors, may be responsible,” the investigators wrote.
The HCUP database is supported by the Agency for Healthcare Research and Quality. Dr. Marcus reported receiving research support from Jawbone, Medtronic, Eight, and Baylis Medical, and is a consultant for and holds equity in InCarda Therapeutics. The other authors reported no conflicts of interest.
SOURCE: Marcus GM et al. Circulation. 2019 Oct 21. doi:10.1161/CIRCULATIONAHA.119.042882.
Over 4 years, atrial fibrillation (AFib) developed significantly more often in a group of Native Americans men than it did among other racial and ethnic groups, a large longitudinal cohort study has found.
The overall incidence among Native Americans was 7.49 per 1,000 person-years – significantly higher than the incidence in a comparator cohort of black, white, Asian, and Hispanic men, Gregory M. Marcus, MD, of the University of California, San Francisco, and colleagues wrote in a research letter published in Circulation.
“We were surprised to find that American Indians experienced a higher risk of atrial fibrillation, compared to every other racial and ethnic group,” Dr. Marcus said in a press release that accompanied the study. “Understanding the mechanisms and factors by which American Indians experience this higher risk may help investigators better understand the fundamental causes of atrial fibrillation that prove useful to everyone at risk for AFib, regardless of their race or ethnicity.”
The team plumbed the Healthcare Cost and Utilization Project (HCUP) California state databases for information on more than 16 million cases of AFib that occurred during 2005-2011. Native Americans comprised just 0.6% of the cohort. Most of the patients (57.2%) were white; 8% were black, 25.6% Hispanic, and 8.6% Asian. After targeting only new-onset cases, there were 344,469 incident AFib episodes over a median follow-up of 4.1 years.
The overall incidence of AFib in Native Americans was 7.49 per 1,000 person-years, significantly higher than the 6.89 per 1000 person-years observed in the rest of the cohort ( P less than .0001). The difference remained significant even after the team controlled for age, sex, income, and heart and other diseases. Nor was it altered by a sensitivity analysis that controlled for place of presentation and patients who were aged at least 35 years with at least two encounters with medical facilities.
In an interaction analysis, the increased risk appeared to be driven by higher rates of diabetes and chronic kidney disease, the authors wrote.
“This may suggest that the presence of these two processes contributes some pathophysiology related to AF[ib] risk that may be similar to the heightened risk inherent among American Indians,” they wrote. “It is also important to note that there was no evidence of any other statistically significant interactions despite the inclusion of millions of patients.”
Supporting data for these associations were not included in the research letter.
The authors noted some limitations of their study. Race or ethnicity were self-reported and could not be independently confirmed, so there was no way to tease out the effects in multiracial patients. Also, the database didn’t record outpatient encounters, which might result in some selection bias.
“Last, because this was an observational study, these results should not be interpreted as evidence of causal effect,” they noted.
“In conclusion, we observed that American Indians had a higher risk of atrial fibrillation, compared with all other racial and ethnic group. The heightened risk … in American Indians persisted after multivariable adjustment for known conventional confounders and mediators, suggesting that an unidentified characteristic, including possible genetic or environmental factors, may be responsible,” the investigators wrote.
The HCUP database is supported by the Agency for Healthcare Research and Quality. Dr. Marcus reported receiving research support from Jawbone, Medtronic, Eight, and Baylis Medical, and is a consultant for and holds equity in InCarda Therapeutics. The other authors reported no conflicts of interest.
SOURCE: Marcus GM et al. Circulation. 2019 Oct 21. doi:10.1161/CIRCULATIONAHA.119.042882.
Over 4 years, atrial fibrillation (AFib) developed significantly more often in a group of Native Americans men than it did among other racial and ethnic groups, a large longitudinal cohort study has found.
The overall incidence among Native Americans was 7.49 per 1,000 person-years – significantly higher than the incidence in a comparator cohort of black, white, Asian, and Hispanic men, Gregory M. Marcus, MD, of the University of California, San Francisco, and colleagues wrote in a research letter published in Circulation.
“We were surprised to find that American Indians experienced a higher risk of atrial fibrillation, compared to every other racial and ethnic group,” Dr. Marcus said in a press release that accompanied the study. “Understanding the mechanisms and factors by which American Indians experience this higher risk may help investigators better understand the fundamental causes of atrial fibrillation that prove useful to everyone at risk for AFib, regardless of their race or ethnicity.”
The team plumbed the Healthcare Cost and Utilization Project (HCUP) California state databases for information on more than 16 million cases of AFib that occurred during 2005-2011. Native Americans comprised just 0.6% of the cohort. Most of the patients (57.2%) were white; 8% were black, 25.6% Hispanic, and 8.6% Asian. After targeting only new-onset cases, there were 344,469 incident AFib episodes over a median follow-up of 4.1 years.
The overall incidence of AFib in Native Americans was 7.49 per 1,000 person-years, significantly higher than the 6.89 per 1000 person-years observed in the rest of the cohort ( P less than .0001). The difference remained significant even after the team controlled for age, sex, income, and heart and other diseases. Nor was it altered by a sensitivity analysis that controlled for place of presentation and patients who were aged at least 35 years with at least two encounters with medical facilities.
In an interaction analysis, the increased risk appeared to be driven by higher rates of diabetes and chronic kidney disease, the authors wrote.
“This may suggest that the presence of these two processes contributes some pathophysiology related to AF[ib] risk that may be similar to the heightened risk inherent among American Indians,” they wrote. “It is also important to note that there was no evidence of any other statistically significant interactions despite the inclusion of millions of patients.”
Supporting data for these associations were not included in the research letter.
The authors noted some limitations of their study. Race or ethnicity were self-reported and could not be independently confirmed, so there was no way to tease out the effects in multiracial patients. Also, the database didn’t record outpatient encounters, which might result in some selection bias.
“Last, because this was an observational study, these results should not be interpreted as evidence of causal effect,” they noted.
“In conclusion, we observed that American Indians had a higher risk of atrial fibrillation, compared with all other racial and ethnic group. The heightened risk … in American Indians persisted after multivariable adjustment for known conventional confounders and mediators, suggesting that an unidentified characteristic, including possible genetic or environmental factors, may be responsible,” the investigators wrote.
The HCUP database is supported by the Agency for Healthcare Research and Quality. Dr. Marcus reported receiving research support from Jawbone, Medtronic, Eight, and Baylis Medical, and is a consultant for and holds equity in InCarda Therapeutics. The other authors reported no conflicts of interest.
SOURCE: Marcus GM et al. Circulation. 2019 Oct 21. doi:10.1161/CIRCULATIONAHA.119.042882.
FROM CIRCULATION
Pulmonary Hemorrhage as the Initial Presentation of AIDS-Related Kaposi Sarcoma
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
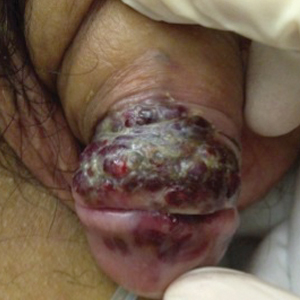
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.

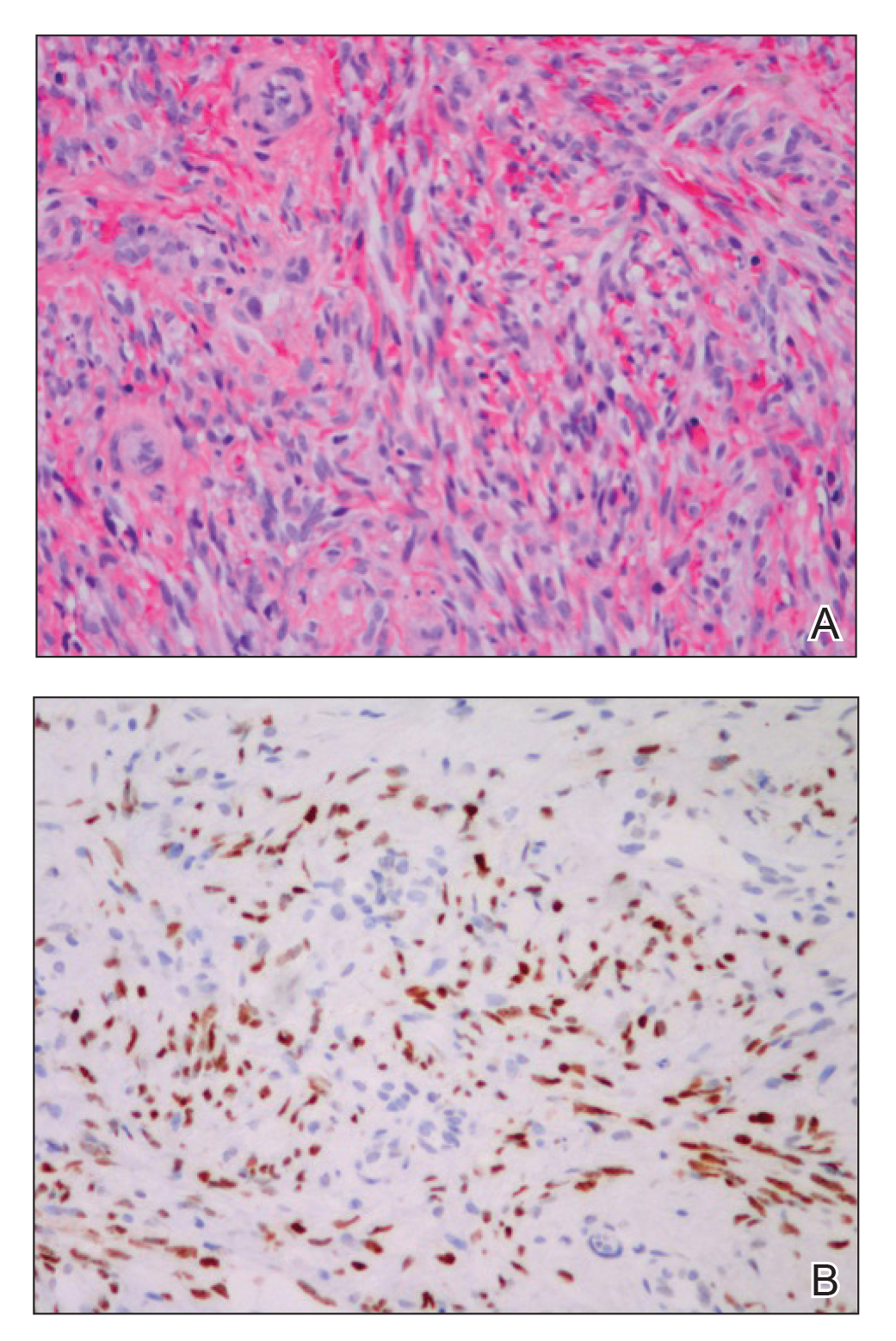
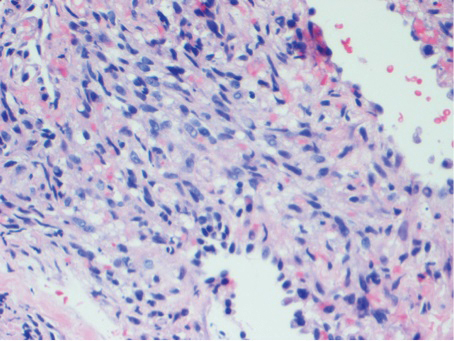
Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.
This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.

Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.


This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.

Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.


This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
Practice Points
- Visceral Kaposi sarcoma (KS) should be considered in patients with unexplained systemic symptoms in the setting of poorly controlled human immunodeficiency virus (HIV).
- If cutaneous KS is diagnosed in an HIV patient, a detailed history and physical examination should be undertaken to evaluate for signs of systemic disease.
Trastuzumab benefit lasts long-term in HER2+ breast cancer
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Will changing the names of psychiatric medications lead to better treatment?
Back in 1980, the American Psychiatric Association dropped the word “neurosis” from the DSM-III, so that if you had been neurotic, after 1980, you were neurotic no longer.
At the time, I discussed this on my daily radio show. For those folks who were nervous, worried, fearful, and full of anxieties about themselves, their families, welfare, health, and the environment around them, a new set of labels was introduced to more specifically describe one or more problems related to anxiety.
For codification, and at times, a clearer understanding of a specific problem, the change was made to be helpful. Certainly, for insurers and pharmacologic treatments, it worked. However, it’s interesting that the word and concept, neurosis, which still is used by some psychiatrists and psychologists – although not scientific – does offer a clear overall picture of a suffering, anxiety-ridden person who might have a combination of an anxiety disorder, panic attacks, somatic symptoms, and endless worry. This overlapping picture often is seen in clinical practice more than the multiple one-dimensional labels that are currently used. So be it.
This all leads me to what I’ve recently learned about the Neuroscience-based Nomenclature (NbN) Project. According to a recent article in the APA’s Psychiatric News, the group’s board of trustees has endorsed a proposal that would change or revise the names of psychiatric medications so that the names reflect their mechanism of action – a move seemingly focused on a pure biological model.
For example, according to the article, the medication perphenazine would be renamed a “D2 receptor antagonist” rather than an antipsychotic. For depression, we might have a serotonergic reuptake inhibitor, according to the report, and of course, the list of changes would go on – based on current knowledge of biological activity. It’s true that in general medicine, there are examples where mode of action is discussed. For example, in cardiology we have beta-blockers and alpha-blockers, which are descriptive of their actions. As doctors who have trained for years and know the mechanism of action of various medications, we will understand all this. But in patient care, both doctors and their patients often understand and feel comfortable using descriptive terms indicating the treatment modality, such as antibiotics, antivirals, antifungals, anti-inflammatory medications, as well as anti-itching, antiaging, and antispasmodic drugs.
So, I am concerned about these proposed changes. In an era focused on patient-centered care, where we seek to make it simpler for the patient/health care consumer, we might make it harder for the patient to grasp what’s going on.
It’s very important to keep in mind that we as physicians know the ins and outs of medications, and that even the most educated and bright patients who are not in medicine do not know what our education has taught us. For example, regardless of specialty, we all know the difference between left-sided and right-sided heart failure. Those outside of medicine, however, rarely know the difference. They understand heart disease as a rule. People in general might understand some general concepts, such as RBC, WBC, and platelets. A patient will speak of taking a blood thinner but rarely know or understand the differences between antiplatelets and anticoagulants. And why should they know this?
The point here is that I believe good patient care is keeping it simple and taking the time to explain what’s being treated, aiming to inform patients using down-to-earth, accessible language rather than the language of biochemistry.
It’s true that in psychiatry, wider use of certain medications than originally indicated has grown tremendously as well as off-label use. In light of that, the NbN idea is laudable. However, it would seem more practical to leave the traditional modes of action in place and expand our discussions with patients as to why we are using a specific medication. I have found a very simple and even rewarding way to explain to patients, for example, that yes, this is an antiseizure medication but it is now used in psychiatry as a mood stabilizer.
Another important point is the question of whether using nomenclature that describes the exact location of the problem is all that accurate. Currently, we know we still have a lot to learn about brain chemistry and neuronal transmission in mental disorders, just as in many medical disorders, there are gaps in our understanding of many illnesses and subsequent molecular changes.
Just as the DSM-III left behind the all-encompassing and descriptive word neurosis and the APA has changed labels in the DSM-IV and DSM-5, so the NbN project would change the nomenclature of current psychotropic medications. The intentions are good, but the idea that those changes will foster better patient understanding defies common sense. A better idea might be to continue use of both scientific names and names of commonly used actions of the medications, leaving both in place and letting clinicians decide what nomenclature best suits each patient.
It will be a sad day when psychiatrists become so medically and “scientifically” driven that we cannot explain to a patient, “I’m prescribing this antidepressant because it’s now used to treat anxiety,” or “Yes, this medicine is labeled ‘antipsychotic,’ but you’re not psychotic. It may help your mood swings and may even help you sleep better.” Now, is that hard? Is talking to a person and explaining the treatment no longer part of care? The take-home messages from the recent APA/Institute of Psychiatric Services meeting I attended seemed to suggest that human attention and care have great value. My father, a surgeon, always said that you learn a lot by simply talking to patients – and they learn from you.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019).
Back in 1980, the American Psychiatric Association dropped the word “neurosis” from the DSM-III, so that if you had been neurotic, after 1980, you were neurotic no longer.
At the time, I discussed this on my daily radio show. For those folks who were nervous, worried, fearful, and full of anxieties about themselves, their families, welfare, health, and the environment around them, a new set of labels was introduced to more specifically describe one or more problems related to anxiety.
For codification, and at times, a clearer understanding of a specific problem, the change was made to be helpful. Certainly, for insurers and pharmacologic treatments, it worked. However, it’s interesting that the word and concept, neurosis, which still is used by some psychiatrists and psychologists – although not scientific – does offer a clear overall picture of a suffering, anxiety-ridden person who might have a combination of an anxiety disorder, panic attacks, somatic symptoms, and endless worry. This overlapping picture often is seen in clinical practice more than the multiple one-dimensional labels that are currently used. So be it.
This all leads me to what I’ve recently learned about the Neuroscience-based Nomenclature (NbN) Project. According to a recent article in the APA’s Psychiatric News, the group’s board of trustees has endorsed a proposal that would change or revise the names of psychiatric medications so that the names reflect their mechanism of action – a move seemingly focused on a pure biological model.
For example, according to the article, the medication perphenazine would be renamed a “D2 receptor antagonist” rather than an antipsychotic. For depression, we might have a serotonergic reuptake inhibitor, according to the report, and of course, the list of changes would go on – based on current knowledge of biological activity. It’s true that in general medicine, there are examples where mode of action is discussed. For example, in cardiology we have beta-blockers and alpha-blockers, which are descriptive of their actions. As doctors who have trained for years and know the mechanism of action of various medications, we will understand all this. But in patient care, both doctors and their patients often understand and feel comfortable using descriptive terms indicating the treatment modality, such as antibiotics, antivirals, antifungals, anti-inflammatory medications, as well as anti-itching, antiaging, and antispasmodic drugs.
So, I am concerned about these proposed changes. In an era focused on patient-centered care, where we seek to make it simpler for the patient/health care consumer, we might make it harder for the patient to grasp what’s going on.
It’s very important to keep in mind that we as physicians know the ins and outs of medications, and that even the most educated and bright patients who are not in medicine do not know what our education has taught us. For example, regardless of specialty, we all know the difference between left-sided and right-sided heart failure. Those outside of medicine, however, rarely know the difference. They understand heart disease as a rule. People in general might understand some general concepts, such as RBC, WBC, and platelets. A patient will speak of taking a blood thinner but rarely know or understand the differences between antiplatelets and anticoagulants. And why should they know this?
The point here is that I believe good patient care is keeping it simple and taking the time to explain what’s being treated, aiming to inform patients using down-to-earth, accessible language rather than the language of biochemistry.
It’s true that in psychiatry, wider use of certain medications than originally indicated has grown tremendously as well as off-label use. In light of that, the NbN idea is laudable. However, it would seem more practical to leave the traditional modes of action in place and expand our discussions with patients as to why we are using a specific medication. I have found a very simple and even rewarding way to explain to patients, for example, that yes, this is an antiseizure medication but it is now used in psychiatry as a mood stabilizer.
Another important point is the question of whether using nomenclature that describes the exact location of the problem is all that accurate. Currently, we know we still have a lot to learn about brain chemistry and neuronal transmission in mental disorders, just as in many medical disorders, there are gaps in our understanding of many illnesses and subsequent molecular changes.
Just as the DSM-III left behind the all-encompassing and descriptive word neurosis and the APA has changed labels in the DSM-IV and DSM-5, so the NbN project would change the nomenclature of current psychotropic medications. The intentions are good, but the idea that those changes will foster better patient understanding defies common sense. A better idea might be to continue use of both scientific names and names of commonly used actions of the medications, leaving both in place and letting clinicians decide what nomenclature best suits each patient.
It will be a sad day when psychiatrists become so medically and “scientifically” driven that we cannot explain to a patient, “I’m prescribing this antidepressant because it’s now used to treat anxiety,” or “Yes, this medicine is labeled ‘antipsychotic,’ but you’re not psychotic. It may help your mood swings and may even help you sleep better.” Now, is that hard? Is talking to a person and explaining the treatment no longer part of care? The take-home messages from the recent APA/Institute of Psychiatric Services meeting I attended seemed to suggest that human attention and care have great value. My father, a surgeon, always said that you learn a lot by simply talking to patients – and they learn from you.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019).
Back in 1980, the American Psychiatric Association dropped the word “neurosis” from the DSM-III, so that if you had been neurotic, after 1980, you were neurotic no longer.
At the time, I discussed this on my daily radio show. For those folks who were nervous, worried, fearful, and full of anxieties about themselves, their families, welfare, health, and the environment around them, a new set of labels was introduced to more specifically describe one or more problems related to anxiety.
For codification, and at times, a clearer understanding of a specific problem, the change was made to be helpful. Certainly, for insurers and pharmacologic treatments, it worked. However, it’s interesting that the word and concept, neurosis, which still is used by some psychiatrists and psychologists – although not scientific – does offer a clear overall picture of a suffering, anxiety-ridden person who might have a combination of an anxiety disorder, panic attacks, somatic symptoms, and endless worry. This overlapping picture often is seen in clinical practice more than the multiple one-dimensional labels that are currently used. So be it.
This all leads me to what I’ve recently learned about the Neuroscience-based Nomenclature (NbN) Project. According to a recent article in the APA’s Psychiatric News, the group’s board of trustees has endorsed a proposal that would change or revise the names of psychiatric medications so that the names reflect their mechanism of action – a move seemingly focused on a pure biological model.
For example, according to the article, the medication perphenazine would be renamed a “D2 receptor antagonist” rather than an antipsychotic. For depression, we might have a serotonergic reuptake inhibitor, according to the report, and of course, the list of changes would go on – based on current knowledge of biological activity. It’s true that in general medicine, there are examples where mode of action is discussed. For example, in cardiology we have beta-blockers and alpha-blockers, which are descriptive of their actions. As doctors who have trained for years and know the mechanism of action of various medications, we will understand all this. But in patient care, both doctors and their patients often understand and feel comfortable using descriptive terms indicating the treatment modality, such as antibiotics, antivirals, antifungals, anti-inflammatory medications, as well as anti-itching, antiaging, and antispasmodic drugs.
So, I am concerned about these proposed changes. In an era focused on patient-centered care, where we seek to make it simpler for the patient/health care consumer, we might make it harder for the patient to grasp what’s going on.
It’s very important to keep in mind that we as physicians know the ins and outs of medications, and that even the most educated and bright patients who are not in medicine do not know what our education has taught us. For example, regardless of specialty, we all know the difference between left-sided and right-sided heart failure. Those outside of medicine, however, rarely know the difference. They understand heart disease as a rule. People in general might understand some general concepts, such as RBC, WBC, and platelets. A patient will speak of taking a blood thinner but rarely know or understand the differences between antiplatelets and anticoagulants. And why should they know this?
The point here is that I believe good patient care is keeping it simple and taking the time to explain what’s being treated, aiming to inform patients using down-to-earth, accessible language rather than the language of biochemistry.
It’s true that in psychiatry, wider use of certain medications than originally indicated has grown tremendously as well as off-label use. In light of that, the NbN idea is laudable. However, it would seem more practical to leave the traditional modes of action in place and expand our discussions with patients as to why we are using a specific medication. I have found a very simple and even rewarding way to explain to patients, for example, that yes, this is an antiseizure medication but it is now used in psychiatry as a mood stabilizer.
Another important point is the question of whether using nomenclature that describes the exact location of the problem is all that accurate. Currently, we know we still have a lot to learn about brain chemistry and neuronal transmission in mental disorders, just as in many medical disorders, there are gaps in our understanding of many illnesses and subsequent molecular changes.
Just as the DSM-III left behind the all-encompassing and descriptive word neurosis and the APA has changed labels in the DSM-IV and DSM-5, so the NbN project would change the nomenclature of current psychotropic medications. The intentions are good, but the idea that those changes will foster better patient understanding defies common sense. A better idea might be to continue use of both scientific names and names of commonly used actions of the medications, leaving both in place and letting clinicians decide what nomenclature best suits each patient.
It will be a sad day when psychiatrists become so medically and “scientifically” driven that we cannot explain to a patient, “I’m prescribing this antidepressant because it’s now used to treat anxiety,” or “Yes, this medicine is labeled ‘antipsychotic,’ but you’re not psychotic. It may help your mood swings and may even help you sleep better.” Now, is that hard? Is talking to a person and explaining the treatment no longer part of care? The take-home messages from the recent APA/Institute of Psychiatric Services meeting I attended seemed to suggest that human attention and care have great value. My father, a surgeon, always said that you learn a lot by simply talking to patients – and they learn from you.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019).
Is carpal tunnel syndrome the tip of the iceberg?
He takes the following medications: felodipine and atorvastatin. On exam, his blood pressure is 110/60 mm Hg, and his pulse is 90 beats per minute.
A cardiac examination found normal heart sounds with no murmurs.
A chest examination found dullness to percussion at both bases and rales.
A chest x-ray showed bilateral effusions and mild pulmonary edema.
The brain natriuretic peptide test found a level of 1,300 picograms/mL.
An ECG found increased ventricular wall thickness, an ejection fraction of 32%, and normal aortic and mitral valves.
What history would be the most helpful in making a diagnosis?
A. History of prostate cancer
B. History of carpal tunnel syndrome
C. History of playing professional football
D. History of hyperlipidemia
E. History of ulcerative colitis
The correct answer here would be B. history of carpal tunnel syndrome (CTS). This patient has clinical heart failure, without a history of clinical ischemic disease. The differential diagnosis for causes of heart failure is long, with the most common causes being chronic hypertension and ischemic heart disease. Other common causes include chronic untreated sleep apnea and valvular heart disease.
This patient really does not have clear reasons for having clinical heart failure. His cardiovascular risk factors have been well controlled, and no valvular disease was found on ECG.
Several recent reports have raised the importance of a history of CTS significantly increasing the likelihood of amyloidosis being the cause of underlying heart failure.
CTS is such a common clinical entity that it is easy to not appreciate its presence as a clue to possible amyloid cardiomyopathy. Fosbøl et al. reported that a diagnosis of CTS was associated with a higher incidence of heart failure (hazard ratio, 1.54; CI, 1.45-1.64).1 They found a highly increased risk of amyloid (HR, 12.2) in patients who had surgery for CTS.
Sperry et al. found that over 10% of patients who underwent carpal tunnel release stained for amyloid on biopsy specimens, and that concomitant cardiac evaluation identified patients with cardiac involvement.2
Pinney et al. found that 48% of patients with transthyretin amyloidosis had a history of CTS.3
In a retrospective study of patients with wild-type transthyretin amyloid (253), patients with hereditary transthyretin amyloid (136), and asymptomatic gene carriers (77), participants were screened for a history of spinal stenosis and CTS.4 Almost 60% of the patients with amyloid had a history of CTS, and 11% had a history of spinal stenosis. Patients with CTS and hereditary amyloid had thicker interventricular septums, higher left ventricular mass, and lower Karnovsky index than those without CTS.
The diagnosis of CTS, especially in those who need surgery for treatment or have bilateral disease, should make us consider the possibility of underlying amyloidosis.
Pearl: In patients who have heart failure and a history of CTS, amyloidosis should be considered as a cause.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Fosbøl EL et al. J Am Coll Cardiol. 2019;74:15-23.
2. Sperry BW et al. J Am Coll Cardiol. 2018 Oct 23;72(17):2040-50.
3. Pinney JH et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000098.
4. Aus dem Siepen F et al. Clin Res Cardiol. 2019 Apr 5. doi: 10.1007/s00392-019-01467-1.
He takes the following medications: felodipine and atorvastatin. On exam, his blood pressure is 110/60 mm Hg, and his pulse is 90 beats per minute.
A cardiac examination found normal heart sounds with no murmurs.
A chest examination found dullness to percussion at both bases and rales.
A chest x-ray showed bilateral effusions and mild pulmonary edema.
The brain natriuretic peptide test found a level of 1,300 picograms/mL.
An ECG found increased ventricular wall thickness, an ejection fraction of 32%, and normal aortic and mitral valves.
What history would be the most helpful in making a diagnosis?
A. History of prostate cancer
B. History of carpal tunnel syndrome
C. History of playing professional football
D. History of hyperlipidemia
E. History of ulcerative colitis
The correct answer here would be B. history of carpal tunnel syndrome (CTS). This patient has clinical heart failure, without a history of clinical ischemic disease. The differential diagnosis for causes of heart failure is long, with the most common causes being chronic hypertension and ischemic heart disease. Other common causes include chronic untreated sleep apnea and valvular heart disease.
This patient really does not have clear reasons for having clinical heart failure. His cardiovascular risk factors have been well controlled, and no valvular disease was found on ECG.
Several recent reports have raised the importance of a history of CTS significantly increasing the likelihood of amyloidosis being the cause of underlying heart failure.
CTS is such a common clinical entity that it is easy to not appreciate its presence as a clue to possible amyloid cardiomyopathy. Fosbøl et al. reported that a diagnosis of CTS was associated with a higher incidence of heart failure (hazard ratio, 1.54; CI, 1.45-1.64).1 They found a highly increased risk of amyloid (HR, 12.2) in patients who had surgery for CTS.
Sperry et al. found that over 10% of patients who underwent carpal tunnel release stained for amyloid on biopsy specimens, and that concomitant cardiac evaluation identified patients with cardiac involvement.2
Pinney et al. found that 48% of patients with transthyretin amyloidosis had a history of CTS.3
In a retrospective study of patients with wild-type transthyretin amyloid (253), patients with hereditary transthyretin amyloid (136), and asymptomatic gene carriers (77), participants were screened for a history of spinal stenosis and CTS.4 Almost 60% of the patients with amyloid had a history of CTS, and 11% had a history of spinal stenosis. Patients with CTS and hereditary amyloid had thicker interventricular septums, higher left ventricular mass, and lower Karnovsky index than those without CTS.
The diagnosis of CTS, especially in those who need surgery for treatment or have bilateral disease, should make us consider the possibility of underlying amyloidosis.
Pearl: In patients who have heart failure and a history of CTS, amyloidosis should be considered as a cause.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Fosbøl EL et al. J Am Coll Cardiol. 2019;74:15-23.
2. Sperry BW et al. J Am Coll Cardiol. 2018 Oct 23;72(17):2040-50.
3. Pinney JH et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000098.
4. Aus dem Siepen F et al. Clin Res Cardiol. 2019 Apr 5. doi: 10.1007/s00392-019-01467-1.
He takes the following medications: felodipine and atorvastatin. On exam, his blood pressure is 110/60 mm Hg, and his pulse is 90 beats per minute.
A cardiac examination found normal heart sounds with no murmurs.
A chest examination found dullness to percussion at both bases and rales.
A chest x-ray showed bilateral effusions and mild pulmonary edema.
The brain natriuretic peptide test found a level of 1,300 picograms/mL.
An ECG found increased ventricular wall thickness, an ejection fraction of 32%, and normal aortic and mitral valves.
What history would be the most helpful in making a diagnosis?
A. History of prostate cancer
B. History of carpal tunnel syndrome
C. History of playing professional football
D. History of hyperlipidemia
E. History of ulcerative colitis
The correct answer here would be B. history of carpal tunnel syndrome (CTS). This patient has clinical heart failure, without a history of clinical ischemic disease. The differential diagnosis for causes of heart failure is long, with the most common causes being chronic hypertension and ischemic heart disease. Other common causes include chronic untreated sleep apnea and valvular heart disease.
This patient really does not have clear reasons for having clinical heart failure. His cardiovascular risk factors have been well controlled, and no valvular disease was found on ECG.
Several recent reports have raised the importance of a history of CTS significantly increasing the likelihood of amyloidosis being the cause of underlying heart failure.
CTS is such a common clinical entity that it is easy to not appreciate its presence as a clue to possible amyloid cardiomyopathy. Fosbøl et al. reported that a diagnosis of CTS was associated with a higher incidence of heart failure (hazard ratio, 1.54; CI, 1.45-1.64).1 They found a highly increased risk of amyloid (HR, 12.2) in patients who had surgery for CTS.
Sperry et al. found that over 10% of patients who underwent carpal tunnel release stained for amyloid on biopsy specimens, and that concomitant cardiac evaluation identified patients with cardiac involvement.2
Pinney et al. found that 48% of patients with transthyretin amyloidosis had a history of CTS.3
In a retrospective study of patients with wild-type transthyretin amyloid (253), patients with hereditary transthyretin amyloid (136), and asymptomatic gene carriers (77), participants were screened for a history of spinal stenosis and CTS.4 Almost 60% of the patients with amyloid had a history of CTS, and 11% had a history of spinal stenosis. Patients with CTS and hereditary amyloid had thicker interventricular septums, higher left ventricular mass, and lower Karnovsky index than those without CTS.
The diagnosis of CTS, especially in those who need surgery for treatment or have bilateral disease, should make us consider the possibility of underlying amyloidosis.
Pearl: In patients who have heart failure and a history of CTS, amyloidosis should be considered as a cause.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Fosbøl EL et al. J Am Coll Cardiol. 2019;74:15-23.
2. Sperry BW et al. J Am Coll Cardiol. 2018 Oct 23;72(17):2040-50.
3. Pinney JH et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000098.
4. Aus dem Siepen F et al. Clin Res Cardiol. 2019 Apr 5. doi: 10.1007/s00392-019-01467-1.
Drug crisis continues to evolve beyond opioids
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.
“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Physician-researcher who promoted industry collaboration dies unexpectedly
Thomas Peter Stossel, MD, a physician-researcher known for his discovery of cellular proteins and advocacy for academic-industry relationships, died unexpectedly on Sept. 29 at the age of 78.
Dr. Stossel’s research was largely focused on cell motility. He is known for discovering the cellular proteins filamin and gelsolin, which regulate the assembly of actin. Dr. Stossel is also known for promoting relationships between physicians and industry. He believed these relationships accelerate medical innovation.
Dr. Stossel graduated from Princeton University and Harvard Medical School. He served as chief of the hematology-oncology unit at Massachusetts General Hospital, head of experimental medicine and codirector of the hematology and translational medicine units at Brigham and Women’s Hospital, and a professor at Harvard Medical School. When he died, Dr. Stossel was chief scientific officer of BioAegis Therapeutics, a company he cofounded.
Dr. Stossel also cofounded Options for Children in Zambia, a charity that works with Zambian partners to provide preventive dental and medical care. And he helped establish a sickle cell disease clinical and research center at University Teaching Hospital in Lusaka.
In happier news, Jeff Vacirca, MD, recently won the 2019 American Red Cross Greater New York Region’s Humanitarian Award. Dr. Vacirca received the award at the 2019 Heroes Among Us Gala on Oct. 16.
Dr. Vacirca is chief executive officer of New York Cancer & Blood Specialists. He is also a consulting physician for the Long Island Association for AIDS Care, medical director for AmerisourceBergen specialty group, a scientific advisory board member for Caris Life Sciences, director at OneOncology, director at Spectrum Pharmaceuticals, a medical board adviser for Flatiron Health, and vice-chairman of the board for Odonate Therapeutics, a company he cofounded in 2016. In 2014, Dr. Vacirca founded the New York Cancer Foundation, which provides financial assistance to patients undergoing cancer treatment.
Another award winner is Richard J. Bleicher, MD, who has won the 2020 Jamie Brooke Lieberman Remembrance Award from Susan G. Komen Philadelphia for his work related to breast cancer. Dr. Bleicher is scheduled to receive his award at the Susan G. Komen Philadelphia MORE THAN PINK Walk Kickoff event on March 18, 2020.
Dr. Bleicher is a professor, breast cancer surgeon, and clinical researcher at Fox Chase Cancer Center in Philadelphia. He is director of the breast fellowship and leader of the breast cancer program at Fox Chase. He sits on the board of the National Accreditation Program for Breast Centers and the Quality Improvement Committee, sits on the Commission for Cancer’s Operative Standards Committee, and is the vice-chair for the Society of Surgical Oncology’s breast fellowship programs for the United States and Canada.
Thomas Peter Stossel, MD, a physician-researcher known for his discovery of cellular proteins and advocacy for academic-industry relationships, died unexpectedly on Sept. 29 at the age of 78.
Dr. Stossel’s research was largely focused on cell motility. He is known for discovering the cellular proteins filamin and gelsolin, which regulate the assembly of actin. Dr. Stossel is also known for promoting relationships between physicians and industry. He believed these relationships accelerate medical innovation.
Dr. Stossel graduated from Princeton University and Harvard Medical School. He served as chief of the hematology-oncology unit at Massachusetts General Hospital, head of experimental medicine and codirector of the hematology and translational medicine units at Brigham and Women’s Hospital, and a professor at Harvard Medical School. When he died, Dr. Stossel was chief scientific officer of BioAegis Therapeutics, a company he cofounded.
Dr. Stossel also cofounded Options for Children in Zambia, a charity that works with Zambian partners to provide preventive dental and medical care. And he helped establish a sickle cell disease clinical and research center at University Teaching Hospital in Lusaka.
In happier news, Jeff Vacirca, MD, recently won the 2019 American Red Cross Greater New York Region’s Humanitarian Award. Dr. Vacirca received the award at the 2019 Heroes Among Us Gala on Oct. 16.
Dr. Vacirca is chief executive officer of New York Cancer & Blood Specialists. He is also a consulting physician for the Long Island Association for AIDS Care, medical director for AmerisourceBergen specialty group, a scientific advisory board member for Caris Life Sciences, director at OneOncology, director at Spectrum Pharmaceuticals, a medical board adviser for Flatiron Health, and vice-chairman of the board for Odonate Therapeutics, a company he cofounded in 2016. In 2014, Dr. Vacirca founded the New York Cancer Foundation, which provides financial assistance to patients undergoing cancer treatment.
Another award winner is Richard J. Bleicher, MD, who has won the 2020 Jamie Brooke Lieberman Remembrance Award from Susan G. Komen Philadelphia for his work related to breast cancer. Dr. Bleicher is scheduled to receive his award at the Susan G. Komen Philadelphia MORE THAN PINK Walk Kickoff event on March 18, 2020.
Dr. Bleicher is a professor, breast cancer surgeon, and clinical researcher at Fox Chase Cancer Center in Philadelphia. He is director of the breast fellowship and leader of the breast cancer program at Fox Chase. He sits on the board of the National Accreditation Program for Breast Centers and the Quality Improvement Committee, sits on the Commission for Cancer’s Operative Standards Committee, and is the vice-chair for the Society of Surgical Oncology’s breast fellowship programs for the United States and Canada.
Thomas Peter Stossel, MD, a physician-researcher known for his discovery of cellular proteins and advocacy for academic-industry relationships, died unexpectedly on Sept. 29 at the age of 78.
Dr. Stossel’s research was largely focused on cell motility. He is known for discovering the cellular proteins filamin and gelsolin, which regulate the assembly of actin. Dr. Stossel is also known for promoting relationships between physicians and industry. He believed these relationships accelerate medical innovation.
Dr. Stossel graduated from Princeton University and Harvard Medical School. He served as chief of the hematology-oncology unit at Massachusetts General Hospital, head of experimental medicine and codirector of the hematology and translational medicine units at Brigham and Women’s Hospital, and a professor at Harvard Medical School. When he died, Dr. Stossel was chief scientific officer of BioAegis Therapeutics, a company he cofounded.
Dr. Stossel also cofounded Options for Children in Zambia, a charity that works with Zambian partners to provide preventive dental and medical care. And he helped establish a sickle cell disease clinical and research center at University Teaching Hospital in Lusaka.
In happier news, Jeff Vacirca, MD, recently won the 2019 American Red Cross Greater New York Region’s Humanitarian Award. Dr. Vacirca received the award at the 2019 Heroes Among Us Gala on Oct. 16.
Dr. Vacirca is chief executive officer of New York Cancer & Blood Specialists. He is also a consulting physician for the Long Island Association for AIDS Care, medical director for AmerisourceBergen specialty group, a scientific advisory board member for Caris Life Sciences, director at OneOncology, director at Spectrum Pharmaceuticals, a medical board adviser for Flatiron Health, and vice-chairman of the board for Odonate Therapeutics, a company he cofounded in 2016. In 2014, Dr. Vacirca founded the New York Cancer Foundation, which provides financial assistance to patients undergoing cancer treatment.
Another award winner is Richard J. Bleicher, MD, who has won the 2020 Jamie Brooke Lieberman Remembrance Award from Susan G. Komen Philadelphia for his work related to breast cancer. Dr. Bleicher is scheduled to receive his award at the Susan G. Komen Philadelphia MORE THAN PINK Walk Kickoff event on March 18, 2020.
Dr. Bleicher is a professor, breast cancer surgeon, and clinical researcher at Fox Chase Cancer Center in Philadelphia. He is director of the breast fellowship and leader of the breast cancer program at Fox Chase. He sits on the board of the National Accreditation Program for Breast Centers and the Quality Improvement Committee, sits on the Commission for Cancer’s Operative Standards Committee, and is the vice-chair for the Society of Surgical Oncology’s breast fellowship programs for the United States and Canada.
How should anticoagulation be managed in a patient with cirrhosis?
DOACs may be a practical option for some CLD patients
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).
In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment
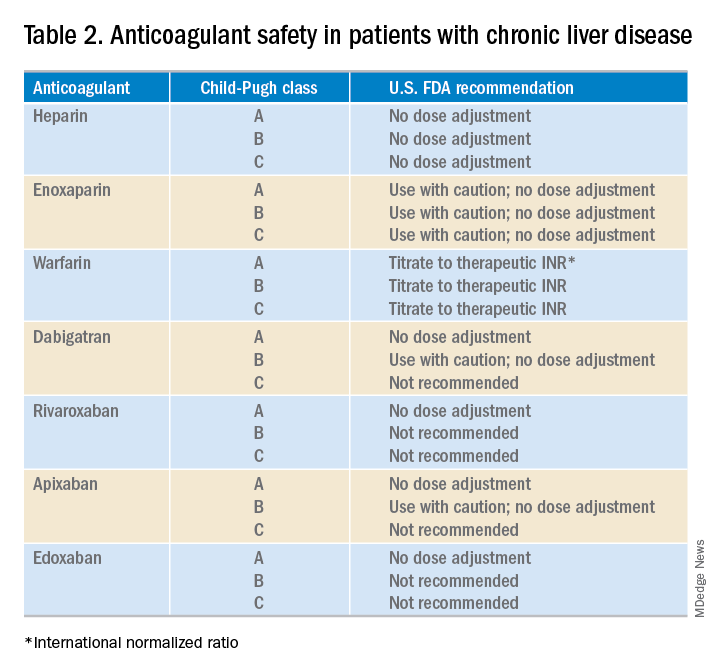
What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.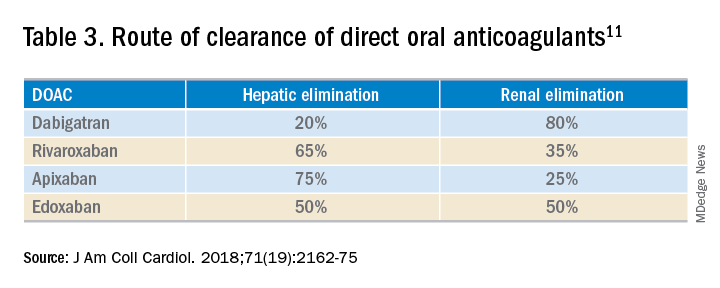
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
DOACs may be a practical option for some CLD patients
DOACs may be a practical option for some CLD patients
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).

In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment

What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).

In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment

What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
Crusted Demodicosis in an Immunocompetent Patient
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
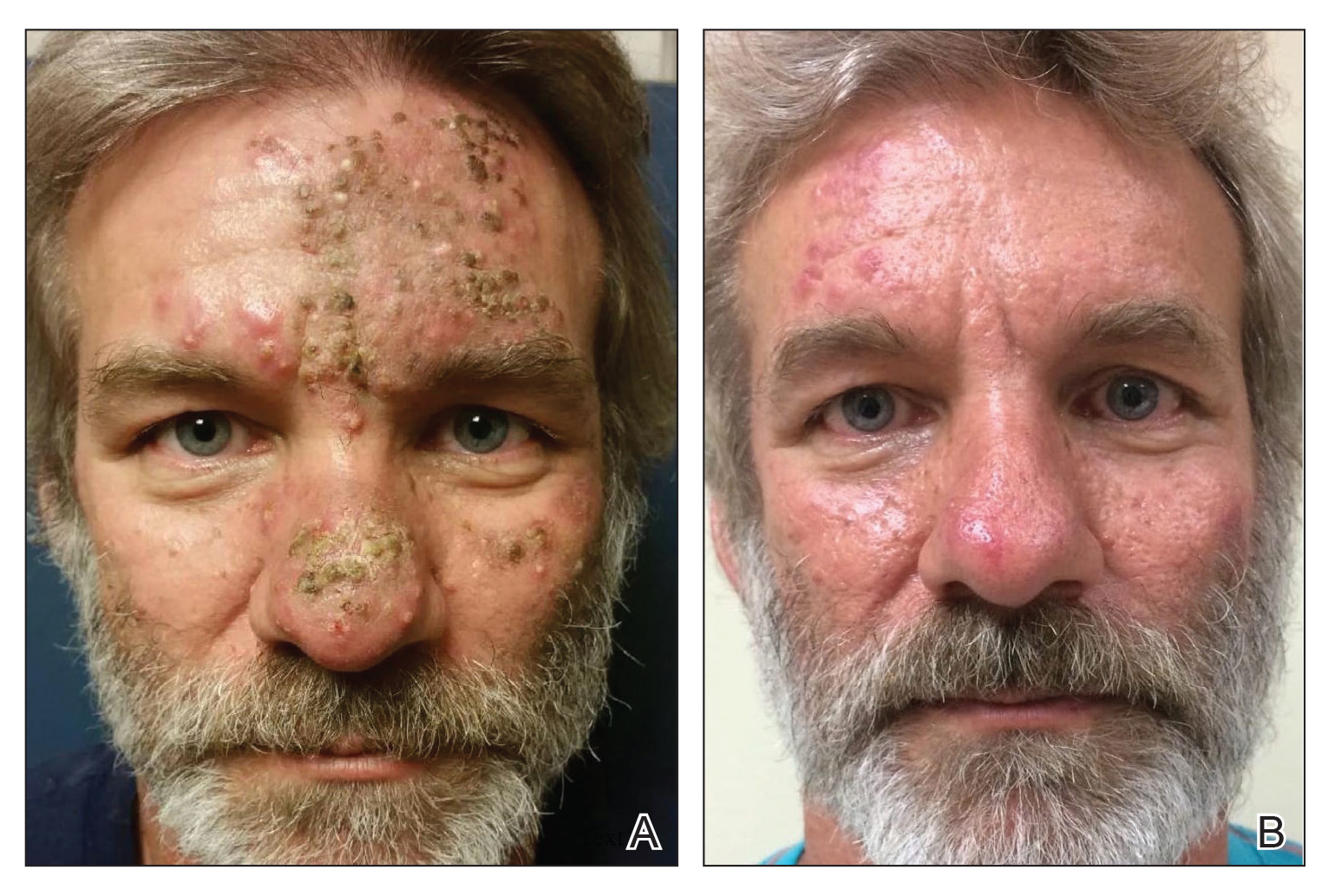
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
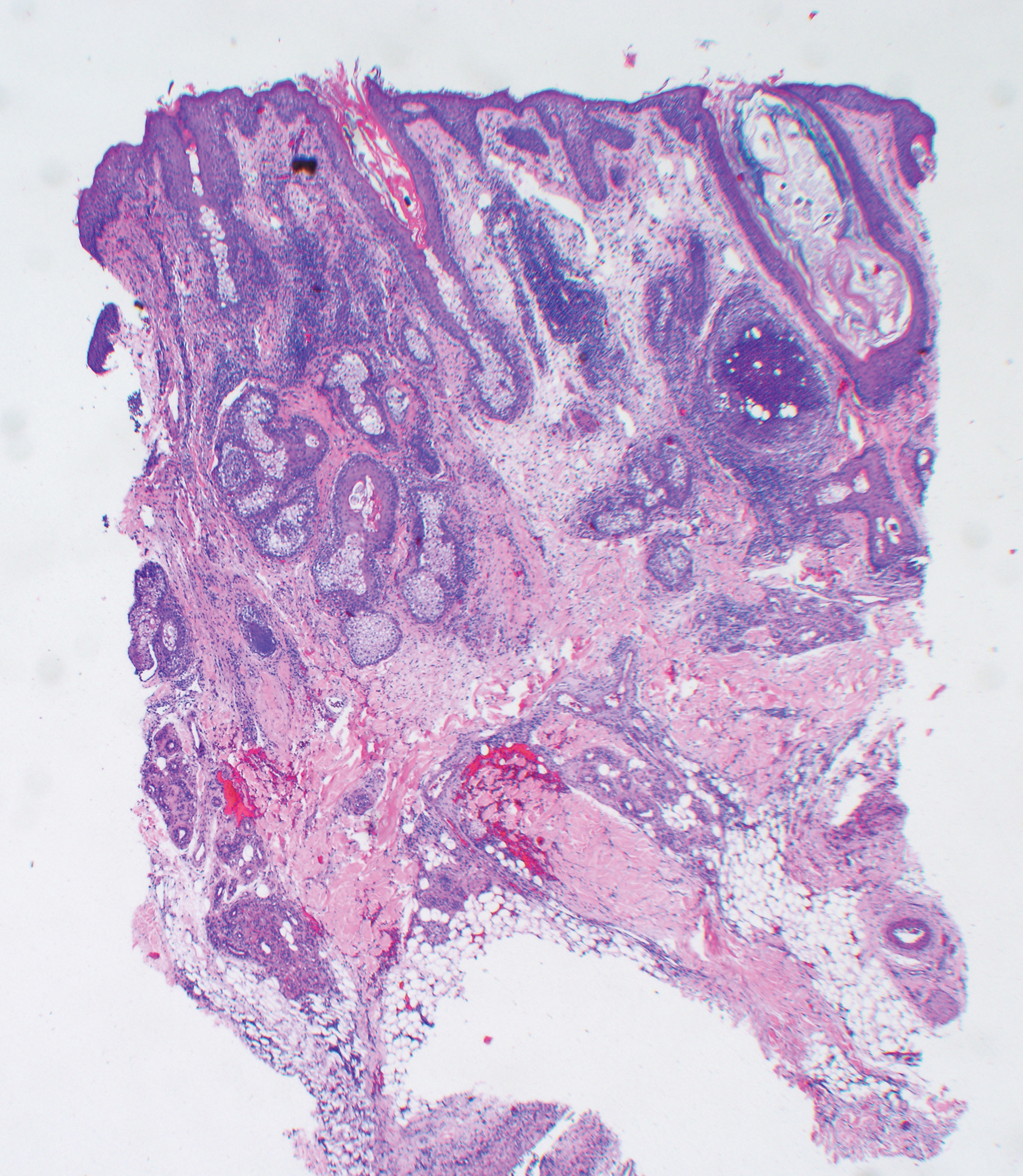
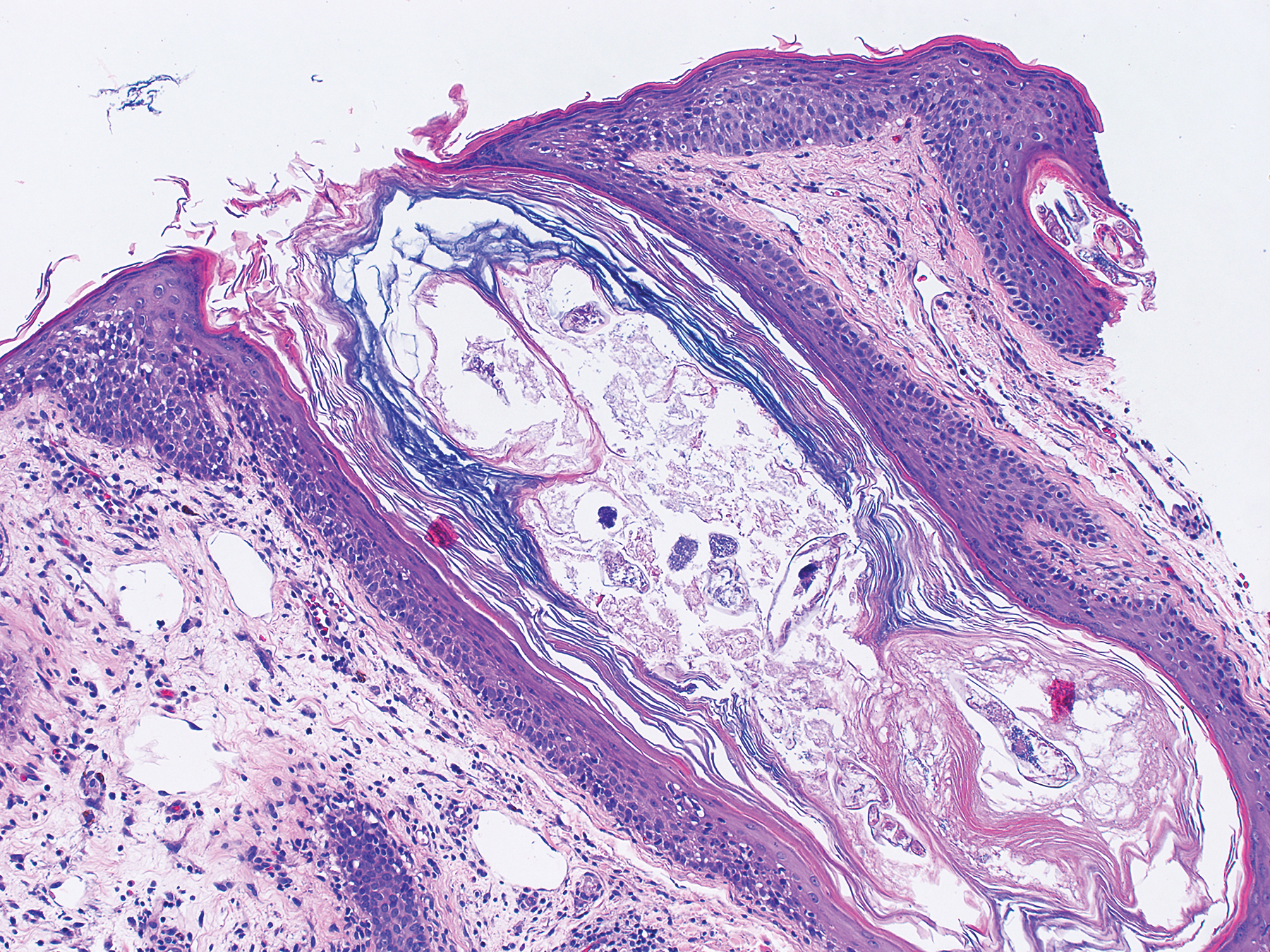
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).

The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.


Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).

The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.


Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
Practice Points
- The Demodex mite, believed to be a commensal species in humans, has the ability to shift to a pathologic form in immunocompromised patients.
- Demodicosis can manifest in a variety of forms including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis.