User login
Next-gen genomic test plus bronchoscopy may improve lung nodule management
NEW ORLEANS – The use of a results of a recent clinical validation study suggest.
The Percepta Genomic Sequencing Classifier (GSC) was able to up- and down-classify probability of malignancy for a considerable proportion of nondiagnostic bronchoscopies in the study, Peter J. Mazzone MD, FCCP, reported at the annual meeting of the American College of Chest Physicians.
The test is seen as complementary to bronchoscopy, improving the sensitivity of bronchoscopy overall and showing a combined sensitivity of greater than 95% in low- and intermediate-risk groups, according to Dr. Mazzone.
While the clinical utility of this genomic test needs to be further tested, the eventual goal is to improve clinician decision making when bronchoscopy results don’t clearly classify nodules as malignant or benign, Dr. Mazzone said in an interview.
“In that situation, you’re often left wondering, ‘what should I do next? Can I just watch this, and see if it grows and changes, or do I have to be even more aggressive – do another biopsy, or have a surgery to take it out?’ ” he explained. “So the test hopes to help make a more informed decision by further stratifying those patients as being quite low risk and maybe safe to follow, or quite high risk and maybe you should be considering more aggressive management.”
The GSC improves on the performance of an earlier molecular test, the Percepta Bronchial Genomic Classifier, which uses a brushing of bronchial epithelium to enhance nodule management in smokers, according to the researcher.
The next-generation GSC uses 1,232 gene transcripts from whole-transcriptome RNA sequencing, along with clinical factors, to help with nodule diagnosis, he said.
To establish the diagnostic accuracy of the GSC, Dr. Mazzone and colleagues evaluated data on 412 patients from three independent cohorts, all of whom had bronchoscopies for lung nodule evaluation that were nondiagnostic. Of those patients, 5% had nodules that physicians had deemed as low probability of malignancy prior to bronchoscopy, 28% deemed intermediate risk, and 74% high risk.
They found that the Percepta GSC down-classified the low–pretest risk patients with 100% negative predictive value (NPV) and down-classified intermediate–pretest risk patients with a 91.0% NPV, Dr. Mazzone reported, while patients with intermediate pretest risk were up-classified with a 65.4% positive predictive value (PPV) and patients with high pretest risk were upclassified with a 91.5% PPV.
The proportion of patients reclassified was about 55% for the low-risk group, 42% for the intermediate-risk group, and 27% for the high-risk group, according to the report at the meeting.
These results suggest the Percepta GSC could help in the “sticky situation” where a bronchoscopy result is inconclusive, Dr. Mazzone told attendees.
“When a bronchoscopy is recommended, despite fantastic advances in navigation systems to get to those nodules, we often come back without a solid answer, and that leaves the clinician in a bit of a predicament,” he said in a late-breaking clinical trial presentation.
Dr. Mazzone provided disclosures related to Veracyte, Exact Sciences, SEER, Tencent, and PCORI (research support to institution).
SOURCE: Mazzone PJ et al. CHEST 2019, Abstract. doi: 10.1016/j.chest.2019.08.307.
NEW ORLEANS – The use of a results of a recent clinical validation study suggest.
The Percepta Genomic Sequencing Classifier (GSC) was able to up- and down-classify probability of malignancy for a considerable proportion of nondiagnostic bronchoscopies in the study, Peter J. Mazzone MD, FCCP, reported at the annual meeting of the American College of Chest Physicians.
The test is seen as complementary to bronchoscopy, improving the sensitivity of bronchoscopy overall and showing a combined sensitivity of greater than 95% in low- and intermediate-risk groups, according to Dr. Mazzone.
While the clinical utility of this genomic test needs to be further tested, the eventual goal is to improve clinician decision making when bronchoscopy results don’t clearly classify nodules as malignant or benign, Dr. Mazzone said in an interview.
“In that situation, you’re often left wondering, ‘what should I do next? Can I just watch this, and see if it grows and changes, or do I have to be even more aggressive – do another biopsy, or have a surgery to take it out?’ ” he explained. “So the test hopes to help make a more informed decision by further stratifying those patients as being quite low risk and maybe safe to follow, or quite high risk and maybe you should be considering more aggressive management.”
The GSC improves on the performance of an earlier molecular test, the Percepta Bronchial Genomic Classifier, which uses a brushing of bronchial epithelium to enhance nodule management in smokers, according to the researcher.
The next-generation GSC uses 1,232 gene transcripts from whole-transcriptome RNA sequencing, along with clinical factors, to help with nodule diagnosis, he said.
To establish the diagnostic accuracy of the GSC, Dr. Mazzone and colleagues evaluated data on 412 patients from three independent cohorts, all of whom had bronchoscopies for lung nodule evaluation that were nondiagnostic. Of those patients, 5% had nodules that physicians had deemed as low probability of malignancy prior to bronchoscopy, 28% deemed intermediate risk, and 74% high risk.
They found that the Percepta GSC down-classified the low–pretest risk patients with 100% negative predictive value (NPV) and down-classified intermediate–pretest risk patients with a 91.0% NPV, Dr. Mazzone reported, while patients with intermediate pretest risk were up-classified with a 65.4% positive predictive value (PPV) and patients with high pretest risk were upclassified with a 91.5% PPV.
The proportion of patients reclassified was about 55% for the low-risk group, 42% for the intermediate-risk group, and 27% for the high-risk group, according to the report at the meeting.
These results suggest the Percepta GSC could help in the “sticky situation” where a bronchoscopy result is inconclusive, Dr. Mazzone told attendees.
“When a bronchoscopy is recommended, despite fantastic advances in navigation systems to get to those nodules, we often come back without a solid answer, and that leaves the clinician in a bit of a predicament,” he said in a late-breaking clinical trial presentation.
Dr. Mazzone provided disclosures related to Veracyte, Exact Sciences, SEER, Tencent, and PCORI (research support to institution).
SOURCE: Mazzone PJ et al. CHEST 2019, Abstract. doi: 10.1016/j.chest.2019.08.307.
NEW ORLEANS – The use of a results of a recent clinical validation study suggest.
The Percepta Genomic Sequencing Classifier (GSC) was able to up- and down-classify probability of malignancy for a considerable proportion of nondiagnostic bronchoscopies in the study, Peter J. Mazzone MD, FCCP, reported at the annual meeting of the American College of Chest Physicians.
The test is seen as complementary to bronchoscopy, improving the sensitivity of bronchoscopy overall and showing a combined sensitivity of greater than 95% in low- and intermediate-risk groups, according to Dr. Mazzone.
While the clinical utility of this genomic test needs to be further tested, the eventual goal is to improve clinician decision making when bronchoscopy results don’t clearly classify nodules as malignant or benign, Dr. Mazzone said in an interview.
“In that situation, you’re often left wondering, ‘what should I do next? Can I just watch this, and see if it grows and changes, or do I have to be even more aggressive – do another biopsy, or have a surgery to take it out?’ ” he explained. “So the test hopes to help make a more informed decision by further stratifying those patients as being quite low risk and maybe safe to follow, or quite high risk and maybe you should be considering more aggressive management.”
The GSC improves on the performance of an earlier molecular test, the Percepta Bronchial Genomic Classifier, which uses a brushing of bronchial epithelium to enhance nodule management in smokers, according to the researcher.
The next-generation GSC uses 1,232 gene transcripts from whole-transcriptome RNA sequencing, along with clinical factors, to help with nodule diagnosis, he said.
To establish the diagnostic accuracy of the GSC, Dr. Mazzone and colleagues evaluated data on 412 patients from three independent cohorts, all of whom had bronchoscopies for lung nodule evaluation that were nondiagnostic. Of those patients, 5% had nodules that physicians had deemed as low probability of malignancy prior to bronchoscopy, 28% deemed intermediate risk, and 74% high risk.
They found that the Percepta GSC down-classified the low–pretest risk patients with 100% negative predictive value (NPV) and down-classified intermediate–pretest risk patients with a 91.0% NPV, Dr. Mazzone reported, while patients with intermediate pretest risk were up-classified with a 65.4% positive predictive value (PPV) and patients with high pretest risk were upclassified with a 91.5% PPV.
The proportion of patients reclassified was about 55% for the low-risk group, 42% for the intermediate-risk group, and 27% for the high-risk group, according to the report at the meeting.
These results suggest the Percepta GSC could help in the “sticky situation” where a bronchoscopy result is inconclusive, Dr. Mazzone told attendees.
“When a bronchoscopy is recommended, despite fantastic advances in navigation systems to get to those nodules, we often come back without a solid answer, and that leaves the clinician in a bit of a predicament,” he said in a late-breaking clinical trial presentation.
Dr. Mazzone provided disclosures related to Veracyte, Exact Sciences, SEER, Tencent, and PCORI (research support to institution).
SOURCE: Mazzone PJ et al. CHEST 2019, Abstract. doi: 10.1016/j.chest.2019.08.307.
REPORTING FROM CHEST 2019
The growing NP and PA workforce in hospital medicine
High rate of turnover among NPs, PAs
If you were a physician hospitalist in a group serving adults in 2017 you probably worked with nurse practitioners (NPs) and/or physician assistants (PAs). Seventy-seven percent of hospital medicine groups (HMGs) employed NPs and PAs that year.
In addition, the larger the group, the more likely the group was to have NPs and PAs as part of their practice model – 89% of hospital medicine groups with more than 30 physician had NPs and/or PAs as partners. In addition, the mean number of physicians for adult hospital medicine groups was 17.9. The same practices employed an average of 3.5 NPs, and 2.6 PAs.
Based on these numbers, there are just under three physicians per NP and PA in the typical HMG serving adults. This is all according to data from the 2018 State of Hospital Medicine (SoHM) report that was published in 2019 by the Society of Hospital Medicine.
These observations lead to a number of questions. One thing that is not clear from the SoHM is why NPs and PAs are becoming a larger part of the hospital medicine workforce, but there are some insights and conjecture that can be drawn from the data. The first is economics. Over 6 years, the median incomes of NPs and PAs have risen a relatively modest 10%; over the same period physician hospitalists have seen a whopping 23.6% median pay increase.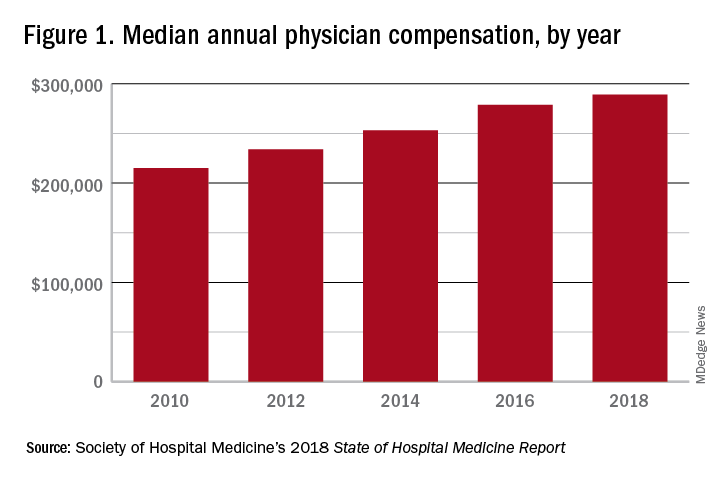
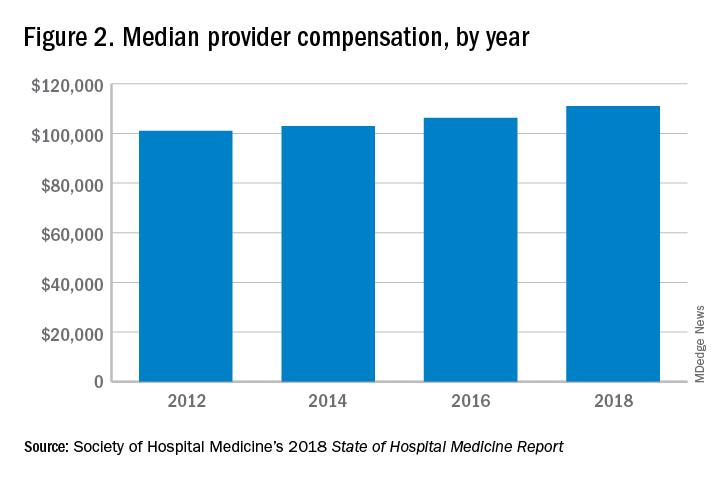
One argument against economics as a driving force behind greater use of NPs and PAs in the hospital medicine workforce is the billing patterns of HMGs that use NPs and PAs. Ten percent of HMGs do not have their NPs and PAs bill at all. The distribution of HMGs that predominantly bill NP and PA services as shared visits, versus having NPs and PAs bill independently, has also not changed much over the years, with 22% of HMGs having NPs and PAs bill independently as a predominant model. This would seem to suggest that some HMGs may not have learned how to deploy NPs and PAs effectively.
While inefficiency can be due to hospital bylaws, the culture of the hospital medicine group, or the skill set of the NPs and PAs working in HMGs – it would seem that if the driving force for the increase in the utilization of NPs and PAs in HMGs was financial, then that would also result in more of these providers billing independently, or alternatively, an increase in hospitalist physician productivity, which the data do not show. However, multistate HMGs may have this figured out better than some of the rest of us – 78% of these HMGs have NPs and PAs billing independently! All other categories of HMGs together are around 13%, with the next highest being hospital or health system integrated delivery systems, where NPs/PAs bill independently about 15% of the time.
In the last 2 years of the survey, there have been marked increases in the number of NPs and PAs at HMGs performing “nontraditional” services. For example, outpatient work has increased from 11% to 17%, and work in the postacute space has increased from 13% to 25%. Work in behavioral health and alcohol and drug rehab facilities has also increased, from 17% to 26%. As HMGs seek to rationalize their workforce while expanding, it is possible that decision makers have felt that it was either more economical to place NPs and PAs in positions where they are seeing these patients, or it was more aligned with the NP/PA skill set, or both. In any event, as the scope of hospital medicine broadens, the use of PAs and NPs has also increased – which is probably not coincidental.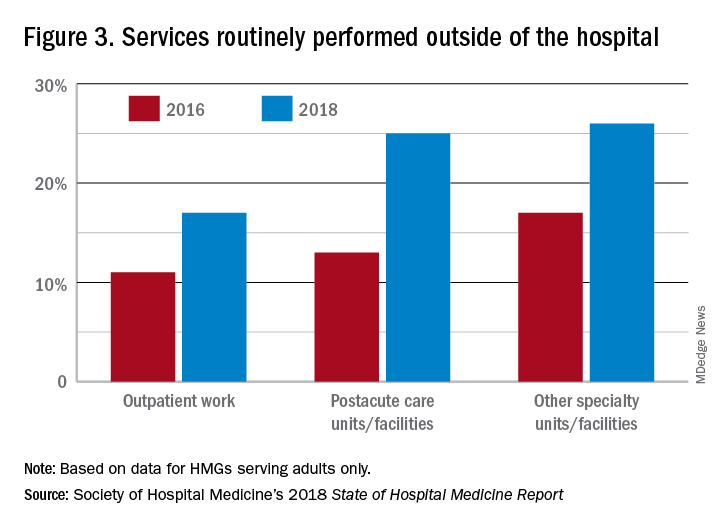
The average hospital medicine group continues to have staff openings. Workforce shortages may be leading to what in the past may have been considered physician openings being filled by NPs and PAs. Only 33% of HMGs reported having all their physician openings filled. Median physician shortage was 12% of total approved staffing. Given concerns in hospital medicine about provider burnout, the number of hospital medicine openings is no doubt a concern to HMG leaders and hospitalists. And necessity being the mother of invention, HMG leadership must be thinking differently than in the past about open positions and the skill mix needed to fill them. I believe this is leading to NPs and PAs being considered more often for a role that would have been open only to a physician in the past.
Just as open positions are a concern, so is turnover. One striking finding in the SoHM is the very high rate of turnover among NPs and PAs – a whopping 19.1% per year. For physicians, the same rate was 7.4% and has been declining every survey for many years. While NPs and PAs may be intended to stabilize the workforce, because of how this is being done in some groups, NPs and PAs may instead be a destabilizing factor. Rapid growth can lead to haphazard onboarding and less than clearly defined roles. NPs and PAs may often be placed into roles for which they are not yet prepared. In addition, the pay disparity between NPs and PAs and physicians has increased. As a new field, and with many HMGs still rapidly growing, increased thoughtfulness and maturity about how NPs and PAs are integrated into hospital medicine practices should lead to less turnover and better HMG stability in the future.
These observations could mark a future that includes higher pay for hospital medicine PAs and NPs (and potentially a slowdown in salary growth for physicians); HMGs taking steps to make the financial model more attractive by having NPs and PAs bill independently more often; and HMGs and their leaders engaging NPs and PAs by more clearly defining roles, shoring up onboarding and mentoring programs, and other measures that decrease turnover. This would help to make hospital medicine a career destination, rather than a stopping off point for NPs and PAs, much as it has become for internists over the past 20 years.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
High rate of turnover among NPs, PAs
High rate of turnover among NPs, PAs
If you were a physician hospitalist in a group serving adults in 2017 you probably worked with nurse practitioners (NPs) and/or physician assistants (PAs). Seventy-seven percent of hospital medicine groups (HMGs) employed NPs and PAs that year.
In addition, the larger the group, the more likely the group was to have NPs and PAs as part of their practice model – 89% of hospital medicine groups with more than 30 physician had NPs and/or PAs as partners. In addition, the mean number of physicians for adult hospital medicine groups was 17.9. The same practices employed an average of 3.5 NPs, and 2.6 PAs.
Based on these numbers, there are just under three physicians per NP and PA in the typical HMG serving adults. This is all according to data from the 2018 State of Hospital Medicine (SoHM) report that was published in 2019 by the Society of Hospital Medicine.
These observations lead to a number of questions. One thing that is not clear from the SoHM is why NPs and PAs are becoming a larger part of the hospital medicine workforce, but there are some insights and conjecture that can be drawn from the data. The first is economics. Over 6 years, the median incomes of NPs and PAs have risen a relatively modest 10%; over the same period physician hospitalists have seen a whopping 23.6% median pay increase.

One argument against economics as a driving force behind greater use of NPs and PAs in the hospital medicine workforce is the billing patterns of HMGs that use NPs and PAs. Ten percent of HMGs do not have their NPs and PAs bill at all. The distribution of HMGs that predominantly bill NP and PA services as shared visits, versus having NPs and PAs bill independently, has also not changed much over the years, with 22% of HMGs having NPs and PAs bill independently as a predominant model. This would seem to suggest that some HMGs may not have learned how to deploy NPs and PAs effectively.
While inefficiency can be due to hospital bylaws, the culture of the hospital medicine group, or the skill set of the NPs and PAs working in HMGs – it would seem that if the driving force for the increase in the utilization of NPs and PAs in HMGs was financial, then that would also result in more of these providers billing independently, or alternatively, an increase in hospitalist physician productivity, which the data do not show. However, multistate HMGs may have this figured out better than some of the rest of us – 78% of these HMGs have NPs and PAs billing independently! All other categories of HMGs together are around 13%, with the next highest being hospital or health system integrated delivery systems, where NPs/PAs bill independently about 15% of the time.
In the last 2 years of the survey, there have been marked increases in the number of NPs and PAs at HMGs performing “nontraditional” services. For example, outpatient work has increased from 11% to 17%, and work in the postacute space has increased from 13% to 25%. Work in behavioral health and alcohol and drug rehab facilities has also increased, from 17% to 26%. As HMGs seek to rationalize their workforce while expanding, it is possible that decision makers have felt that it was either more economical to place NPs and PAs in positions where they are seeing these patients, or it was more aligned with the NP/PA skill set, or both. In any event, as the scope of hospital medicine broadens, the use of PAs and NPs has also increased – which is probably not coincidental.
The average hospital medicine group continues to have staff openings. Workforce shortages may be leading to what in the past may have been considered physician openings being filled by NPs and PAs. Only 33% of HMGs reported having all their physician openings filled. Median physician shortage was 12% of total approved staffing. Given concerns in hospital medicine about provider burnout, the number of hospital medicine openings is no doubt a concern to HMG leaders and hospitalists. And necessity being the mother of invention, HMG leadership must be thinking differently than in the past about open positions and the skill mix needed to fill them. I believe this is leading to NPs and PAs being considered more often for a role that would have been open only to a physician in the past.
Just as open positions are a concern, so is turnover. One striking finding in the SoHM is the very high rate of turnover among NPs and PAs – a whopping 19.1% per year. For physicians, the same rate was 7.4% and has been declining every survey for many years. While NPs and PAs may be intended to stabilize the workforce, because of how this is being done in some groups, NPs and PAs may instead be a destabilizing factor. Rapid growth can lead to haphazard onboarding and less than clearly defined roles. NPs and PAs may often be placed into roles for which they are not yet prepared. In addition, the pay disparity between NPs and PAs and physicians has increased. As a new field, and with many HMGs still rapidly growing, increased thoughtfulness and maturity about how NPs and PAs are integrated into hospital medicine practices should lead to less turnover and better HMG stability in the future.
These observations could mark a future that includes higher pay for hospital medicine PAs and NPs (and potentially a slowdown in salary growth for physicians); HMGs taking steps to make the financial model more attractive by having NPs and PAs bill independently more often; and HMGs and their leaders engaging NPs and PAs by more clearly defining roles, shoring up onboarding and mentoring programs, and other measures that decrease turnover. This would help to make hospital medicine a career destination, rather than a stopping off point for NPs and PAs, much as it has become for internists over the past 20 years.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
If you were a physician hospitalist in a group serving adults in 2017 you probably worked with nurse practitioners (NPs) and/or physician assistants (PAs). Seventy-seven percent of hospital medicine groups (HMGs) employed NPs and PAs that year.
In addition, the larger the group, the more likely the group was to have NPs and PAs as part of their practice model – 89% of hospital medicine groups with more than 30 physician had NPs and/or PAs as partners. In addition, the mean number of physicians for adult hospital medicine groups was 17.9. The same practices employed an average of 3.5 NPs, and 2.6 PAs.
Based on these numbers, there are just under three physicians per NP and PA in the typical HMG serving adults. This is all according to data from the 2018 State of Hospital Medicine (SoHM) report that was published in 2019 by the Society of Hospital Medicine.
These observations lead to a number of questions. One thing that is not clear from the SoHM is why NPs and PAs are becoming a larger part of the hospital medicine workforce, but there are some insights and conjecture that can be drawn from the data. The first is economics. Over 6 years, the median incomes of NPs and PAs have risen a relatively modest 10%; over the same period physician hospitalists have seen a whopping 23.6% median pay increase.

One argument against economics as a driving force behind greater use of NPs and PAs in the hospital medicine workforce is the billing patterns of HMGs that use NPs and PAs. Ten percent of HMGs do not have their NPs and PAs bill at all. The distribution of HMGs that predominantly bill NP and PA services as shared visits, versus having NPs and PAs bill independently, has also not changed much over the years, with 22% of HMGs having NPs and PAs bill independently as a predominant model. This would seem to suggest that some HMGs may not have learned how to deploy NPs and PAs effectively.
While inefficiency can be due to hospital bylaws, the culture of the hospital medicine group, or the skill set of the NPs and PAs working in HMGs – it would seem that if the driving force for the increase in the utilization of NPs and PAs in HMGs was financial, then that would also result in more of these providers billing independently, or alternatively, an increase in hospitalist physician productivity, which the data do not show. However, multistate HMGs may have this figured out better than some of the rest of us – 78% of these HMGs have NPs and PAs billing independently! All other categories of HMGs together are around 13%, with the next highest being hospital or health system integrated delivery systems, where NPs/PAs bill independently about 15% of the time.
In the last 2 years of the survey, there have been marked increases in the number of NPs and PAs at HMGs performing “nontraditional” services. For example, outpatient work has increased from 11% to 17%, and work in the postacute space has increased from 13% to 25%. Work in behavioral health and alcohol and drug rehab facilities has also increased, from 17% to 26%. As HMGs seek to rationalize their workforce while expanding, it is possible that decision makers have felt that it was either more economical to place NPs and PAs in positions where they are seeing these patients, or it was more aligned with the NP/PA skill set, or both. In any event, as the scope of hospital medicine broadens, the use of PAs and NPs has also increased – which is probably not coincidental.
The average hospital medicine group continues to have staff openings. Workforce shortages may be leading to what in the past may have been considered physician openings being filled by NPs and PAs. Only 33% of HMGs reported having all their physician openings filled. Median physician shortage was 12% of total approved staffing. Given concerns in hospital medicine about provider burnout, the number of hospital medicine openings is no doubt a concern to HMG leaders and hospitalists. And necessity being the mother of invention, HMG leadership must be thinking differently than in the past about open positions and the skill mix needed to fill them. I believe this is leading to NPs and PAs being considered more often for a role that would have been open only to a physician in the past.
Just as open positions are a concern, so is turnover. One striking finding in the SoHM is the very high rate of turnover among NPs and PAs – a whopping 19.1% per year. For physicians, the same rate was 7.4% and has been declining every survey for many years. While NPs and PAs may be intended to stabilize the workforce, because of how this is being done in some groups, NPs and PAs may instead be a destabilizing factor. Rapid growth can lead to haphazard onboarding and less than clearly defined roles. NPs and PAs may often be placed into roles for which they are not yet prepared. In addition, the pay disparity between NPs and PAs and physicians has increased. As a new field, and with many HMGs still rapidly growing, increased thoughtfulness and maturity about how NPs and PAs are integrated into hospital medicine practices should lead to less turnover and better HMG stability in the future.
These observations could mark a future that includes higher pay for hospital medicine PAs and NPs (and potentially a slowdown in salary growth for physicians); HMGs taking steps to make the financial model more attractive by having NPs and PAs bill independently more often; and HMGs and their leaders engaging NPs and PAs by more clearly defining roles, shoring up onboarding and mentoring programs, and other measures that decrease turnover. This would help to make hospital medicine a career destination, rather than a stopping off point for NPs and PAs, much as it has become for internists over the past 20 years.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
Spotlight is on promising investigational antipsychotics
COPENHAGEN –
Two of the highlighted agents – pimavanserin and SEP-363856 – were designed to eschew the traditional antipsychotic target, the dopamine D2 receptor, in favor of other mechanisms of action aimed at the negative symptoms of schizophrenia, for which there is a long-recognized major unmet need for better therapies.
A third agent, known for now as ALKS 3831, is composed of a combination of olanzapine and samidorphan, an opioid receptor antagonist. This once-daily oral combination of olanzapine/samidorphan (OLA/SAM) is designed to retain the clinical efficacy of olanzapine while mitigating the drug’s limiting side effect of substantial weight gain.
OLA/SAM New Drug Application expected soon
Christine Graham, PhD, presented highlights of the pivotal phase 3 ENLIGHTEN-2 study, a double-blind clinical trial in which 661 U.S. outpatients with schizophrenia were randomized to OLA/SAM or olanzapine alone at 10 or 20 mg/day for 24 weeks, at which point everyone was switched to open-label OLA/SAM at 10 or 20 mg/10 mg for an additional 52-week extension safety study.
At 24 weeks, the OLA/SAM group had a mean 4.21% weight gain from baseline, significantly less than the 6.59% gain with olanzapine alone. A clinically meaningful and unwelcome weight gain of 7% or greater occurred in 27.5% of OLA/SAM patients, compared with 42.7% of controls, for an adjusted 50% reduction in risk in the group on the investigational medication. Similarly, a 10% or greater weight gain occurred in 17.8% of OLA/SAM patients and 29.8% of controls; once again, that represented a 50% relative risk reduction. The two therapies were equally effective, achieving roughly 10-point reductions in the Positive and Negative Syndrome Scale (PANSS) for schizophrenia total score.
Both treatments showed similar weight gain trajectories for the first 4 weeks. However, by week 6 the trajectories diverged, with body weight plateauing in the OLA/SAM group and remaining stable throughout the remainder of the 76-week, two-part study. Meanwhile, body weight continued to climb in the olanzapine-only group throughout the 24 weeks, reported Dr. Graham, senior clinical research scientist at Alkermes, in Waltham, Mass.
“The waist circumference results were surprising: We saw that waist circumference separated between the two groups as early as week 1, considerably earlier than the week 6 separation in weight. This suggests to us that even when weight gain is similar between the two treatments, OLA/SAM is showing an early effect at limiting central fat accumulation – and this has important health implications, as central fat has been shown to be potentially pathogenic for developing diabetes, cardiovascular disease, and even some forms of cancer,” she said.
The safety profile of OLA/SAM was essentially the same as for olanzapine-only, with the exception of the weight gain.
Alkermes is planning to submit its New Drug Application for OLA/SAM to the Food and Drug Administration before the year’s end. FDA officials have urged the company to broaden the application to include not only the treatment of schizophrenia, but bipolar I disorder as well, since olanzapine is an approved, well-established treatment for that disorder. Dr. Graham and coinvestigators have demonstrated that OLA/SAM has no clinically significant effect on the pharmacokinetics of lithium or valproate (Clin Drug Investig. 2019 Oct 4. doi: 10.1007/s40261-019-00860-y).
Phase 3 trial on pimavanserin underway
Pimavanserin is an oral selective serotonin inverse agonist, or SSIA, with a high affinity for 5-HT2A receptors, very low affinity for 5-HT2C receptors, and “absolutely no affinity” for dopaminergic, histaminergic, adrenergic, or muscarinic receptors, explained Dragana Bugarski-Kirola, MD, a psychiatrist and vice president of clinical development at Acadia Pharmaceuticals in San Diego.
“Those sites are thought to contribute to sedation, cognitive impairment, and orthostatic hypotension,” she noted.
Pimavanserin is at present FDA approved for a narrow indication: Treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. But the drug’s unique mechanism of action suggests broad efficacy across a range of psychiatric disorders.
Indeed, after a successful phase 2 clinical trial of pimavanserin for treatment of Alzheimer’s-related psychosis, a phase 3 randomized, double-blind, placebo-controlled clinical trial of the drug for relapse prevention in dementia-related psychosis is now enrolling a planned 360 outpatients at 95 centers in 13 countries. This 26-week study, known as HARMONY, is preceded by open-label psychotherapy to ensure that study participants truly need pharmacotherapy. Patients are eligible regardless of their type of dementia, because psychosis in patients with various forms of dementia is clinically pretty much the same, whether the underlying disorder is Alzheimer’s disease, vascular dementia, Parkinson’s disease, or Lewy body dementia, according to Dr. Bugarski-Kirola.
In addition, pimavanserin also is the subject of an ongoing phase 3 randomized trial in patients with major depressive disorder inadequately responsive to an selective serotonin reuptake inhibitor or a selective norepinephrine reuptake inhibitor. A 380-patient phase 2 study of the drug as adjunctive treatment for negative symptoms of schizophrenia also is underway based upon earlier promising results.
Across the board for these potential indications, the drug has been well tolerated, with a side effect profile similar to that of placebo. Importantly, pimavanserin has not been associated with cognitive impairment when used for dementia-related psychosis, unlike the antipsychotics now being used off label in clinical practice, the psychiatrist said.
SEP-363856 part of ‘novel class’
SEP-363856 is a nondopaminergic D2, trace amine-associated receptor agonist (TAAR1) under development for treatment of schizophrenia. Phase 3 trials in adults and adolescents with schizophrenia will begin before the end of the year on the strength of positive phase 2 results, according to Kenneth S. Koblan, PhD, head of global translational medicine and early development, as well as head of discovery sciences, at Sunovion Pharmaceuticals, Marlborough, Mass.
“We believe that SEP-363856 actually represents the first candidate in a novel class of antipsychotics. It’s a monoamine receptor activator, unlike the atypical antipsychotics, which work through blockade of the monoamine receptor via dopamine and serotonin. We believe that it’s the monoamine receptor activation that leads to the safety and efficacy of the class,” he explained.
In the four-country, double-blind, 4-week phase 2 trial conducted in 245 hospitalized acutely psychotic patients, oral SEP-363856 flexibly dosed at 50 or 75 mg/day had a side effect profile like that of placebo. Negative symptoms as assessed via the Brief Negative Symptom Scale improved by an average of 7.1 points at 4 weeks with SEP-363856, significantly more than the 2.7-point improvement with placebo. The PANSS total score improved by 17.2 points in the SEP-363856 group and 9.7 points in controls at 4 weeks, with a further 10-point drop in PANSS during a 6-month open-label extension phase of the study. Moreover, the SEP-363856 cohort showed significant functional improvement at 4 weeks in the UCSD Performance-Based Skills Assessment, with continued improvement during the open-label extension study.
Dr. Koblan said the pharmaceutical industry has overemphasized the development of dopaminergic D2-based drugs for schizophrenia. In the past 2 decades, roughly 30,000 patients have been enrolled in industry-sponsored, placebo-controlled, phase 2 or 3 randomized trials of drugs with that mechanism. Many of the those drugs have reached the marketplace. In contrast, there have been far fewer RCTs – and no product launches – of antipsychotics with non-D2 mechanisms of action.
“When you consider that the cost is about $50,000 per research subject and 50,000 subjects have been studied since 2000, the pharmaceutical industry has invested on the order of billions of dollars to try to come up with the next breakthrough medication,” he said.
COPENHAGEN –
Two of the highlighted agents – pimavanserin and SEP-363856 – were designed to eschew the traditional antipsychotic target, the dopamine D2 receptor, in favor of other mechanisms of action aimed at the negative symptoms of schizophrenia, for which there is a long-recognized major unmet need for better therapies.
A third agent, known for now as ALKS 3831, is composed of a combination of olanzapine and samidorphan, an opioid receptor antagonist. This once-daily oral combination of olanzapine/samidorphan (OLA/SAM) is designed to retain the clinical efficacy of olanzapine while mitigating the drug’s limiting side effect of substantial weight gain.
OLA/SAM New Drug Application expected soon
Christine Graham, PhD, presented highlights of the pivotal phase 3 ENLIGHTEN-2 study, a double-blind clinical trial in which 661 U.S. outpatients with schizophrenia were randomized to OLA/SAM or olanzapine alone at 10 or 20 mg/day for 24 weeks, at which point everyone was switched to open-label OLA/SAM at 10 or 20 mg/10 mg for an additional 52-week extension safety study.
At 24 weeks, the OLA/SAM group had a mean 4.21% weight gain from baseline, significantly less than the 6.59% gain with olanzapine alone. A clinically meaningful and unwelcome weight gain of 7% or greater occurred in 27.5% of OLA/SAM patients, compared with 42.7% of controls, for an adjusted 50% reduction in risk in the group on the investigational medication. Similarly, a 10% or greater weight gain occurred in 17.8% of OLA/SAM patients and 29.8% of controls; once again, that represented a 50% relative risk reduction. The two therapies were equally effective, achieving roughly 10-point reductions in the Positive and Negative Syndrome Scale (PANSS) for schizophrenia total score.
Both treatments showed similar weight gain trajectories for the first 4 weeks. However, by week 6 the trajectories diverged, with body weight plateauing in the OLA/SAM group and remaining stable throughout the remainder of the 76-week, two-part study. Meanwhile, body weight continued to climb in the olanzapine-only group throughout the 24 weeks, reported Dr. Graham, senior clinical research scientist at Alkermes, in Waltham, Mass.
“The waist circumference results were surprising: We saw that waist circumference separated between the two groups as early as week 1, considerably earlier than the week 6 separation in weight. This suggests to us that even when weight gain is similar between the two treatments, OLA/SAM is showing an early effect at limiting central fat accumulation – and this has important health implications, as central fat has been shown to be potentially pathogenic for developing diabetes, cardiovascular disease, and even some forms of cancer,” she said.
The safety profile of OLA/SAM was essentially the same as for olanzapine-only, with the exception of the weight gain.
Alkermes is planning to submit its New Drug Application for OLA/SAM to the Food and Drug Administration before the year’s end. FDA officials have urged the company to broaden the application to include not only the treatment of schizophrenia, but bipolar I disorder as well, since olanzapine is an approved, well-established treatment for that disorder. Dr. Graham and coinvestigators have demonstrated that OLA/SAM has no clinically significant effect on the pharmacokinetics of lithium or valproate (Clin Drug Investig. 2019 Oct 4. doi: 10.1007/s40261-019-00860-y).
Phase 3 trial on pimavanserin underway
Pimavanserin is an oral selective serotonin inverse agonist, or SSIA, with a high affinity for 5-HT2A receptors, very low affinity for 5-HT2C receptors, and “absolutely no affinity” for dopaminergic, histaminergic, adrenergic, or muscarinic receptors, explained Dragana Bugarski-Kirola, MD, a psychiatrist and vice president of clinical development at Acadia Pharmaceuticals in San Diego.
“Those sites are thought to contribute to sedation, cognitive impairment, and orthostatic hypotension,” she noted.
Pimavanserin is at present FDA approved for a narrow indication: Treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. But the drug’s unique mechanism of action suggests broad efficacy across a range of psychiatric disorders.
Indeed, after a successful phase 2 clinical trial of pimavanserin for treatment of Alzheimer’s-related psychosis, a phase 3 randomized, double-blind, placebo-controlled clinical trial of the drug for relapse prevention in dementia-related psychosis is now enrolling a planned 360 outpatients at 95 centers in 13 countries. This 26-week study, known as HARMONY, is preceded by open-label psychotherapy to ensure that study participants truly need pharmacotherapy. Patients are eligible regardless of their type of dementia, because psychosis in patients with various forms of dementia is clinically pretty much the same, whether the underlying disorder is Alzheimer’s disease, vascular dementia, Parkinson’s disease, or Lewy body dementia, according to Dr. Bugarski-Kirola.
In addition, pimavanserin also is the subject of an ongoing phase 3 randomized trial in patients with major depressive disorder inadequately responsive to an selective serotonin reuptake inhibitor or a selective norepinephrine reuptake inhibitor. A 380-patient phase 2 study of the drug as adjunctive treatment for negative symptoms of schizophrenia also is underway based upon earlier promising results.
Across the board for these potential indications, the drug has been well tolerated, with a side effect profile similar to that of placebo. Importantly, pimavanserin has not been associated with cognitive impairment when used for dementia-related psychosis, unlike the antipsychotics now being used off label in clinical practice, the psychiatrist said.
SEP-363856 part of ‘novel class’
SEP-363856 is a nondopaminergic D2, trace amine-associated receptor agonist (TAAR1) under development for treatment of schizophrenia. Phase 3 trials in adults and adolescents with schizophrenia will begin before the end of the year on the strength of positive phase 2 results, according to Kenneth S. Koblan, PhD, head of global translational medicine and early development, as well as head of discovery sciences, at Sunovion Pharmaceuticals, Marlborough, Mass.
“We believe that SEP-363856 actually represents the first candidate in a novel class of antipsychotics. It’s a monoamine receptor activator, unlike the atypical antipsychotics, which work through blockade of the monoamine receptor via dopamine and serotonin. We believe that it’s the monoamine receptor activation that leads to the safety and efficacy of the class,” he explained.
In the four-country, double-blind, 4-week phase 2 trial conducted in 245 hospitalized acutely psychotic patients, oral SEP-363856 flexibly dosed at 50 or 75 mg/day had a side effect profile like that of placebo. Negative symptoms as assessed via the Brief Negative Symptom Scale improved by an average of 7.1 points at 4 weeks with SEP-363856, significantly more than the 2.7-point improvement with placebo. The PANSS total score improved by 17.2 points in the SEP-363856 group and 9.7 points in controls at 4 weeks, with a further 10-point drop in PANSS during a 6-month open-label extension phase of the study. Moreover, the SEP-363856 cohort showed significant functional improvement at 4 weeks in the UCSD Performance-Based Skills Assessment, with continued improvement during the open-label extension study.
Dr. Koblan said the pharmaceutical industry has overemphasized the development of dopaminergic D2-based drugs for schizophrenia. In the past 2 decades, roughly 30,000 patients have been enrolled in industry-sponsored, placebo-controlled, phase 2 or 3 randomized trials of drugs with that mechanism. Many of the those drugs have reached the marketplace. In contrast, there have been far fewer RCTs – and no product launches – of antipsychotics with non-D2 mechanisms of action.
“When you consider that the cost is about $50,000 per research subject and 50,000 subjects have been studied since 2000, the pharmaceutical industry has invested on the order of billions of dollars to try to come up with the next breakthrough medication,” he said.
COPENHAGEN –
Two of the highlighted agents – pimavanserin and SEP-363856 – were designed to eschew the traditional antipsychotic target, the dopamine D2 receptor, in favor of other mechanisms of action aimed at the negative symptoms of schizophrenia, for which there is a long-recognized major unmet need for better therapies.
A third agent, known for now as ALKS 3831, is composed of a combination of olanzapine and samidorphan, an opioid receptor antagonist. This once-daily oral combination of olanzapine/samidorphan (OLA/SAM) is designed to retain the clinical efficacy of olanzapine while mitigating the drug’s limiting side effect of substantial weight gain.
OLA/SAM New Drug Application expected soon
Christine Graham, PhD, presented highlights of the pivotal phase 3 ENLIGHTEN-2 study, a double-blind clinical trial in which 661 U.S. outpatients with schizophrenia were randomized to OLA/SAM or olanzapine alone at 10 or 20 mg/day for 24 weeks, at which point everyone was switched to open-label OLA/SAM at 10 or 20 mg/10 mg for an additional 52-week extension safety study.
At 24 weeks, the OLA/SAM group had a mean 4.21% weight gain from baseline, significantly less than the 6.59% gain with olanzapine alone. A clinically meaningful and unwelcome weight gain of 7% or greater occurred in 27.5% of OLA/SAM patients, compared with 42.7% of controls, for an adjusted 50% reduction in risk in the group on the investigational medication. Similarly, a 10% or greater weight gain occurred in 17.8% of OLA/SAM patients and 29.8% of controls; once again, that represented a 50% relative risk reduction. The two therapies were equally effective, achieving roughly 10-point reductions in the Positive and Negative Syndrome Scale (PANSS) for schizophrenia total score.
Both treatments showed similar weight gain trajectories for the first 4 weeks. However, by week 6 the trajectories diverged, with body weight plateauing in the OLA/SAM group and remaining stable throughout the remainder of the 76-week, two-part study. Meanwhile, body weight continued to climb in the olanzapine-only group throughout the 24 weeks, reported Dr. Graham, senior clinical research scientist at Alkermes, in Waltham, Mass.
“The waist circumference results were surprising: We saw that waist circumference separated between the two groups as early as week 1, considerably earlier than the week 6 separation in weight. This suggests to us that even when weight gain is similar between the two treatments, OLA/SAM is showing an early effect at limiting central fat accumulation – and this has important health implications, as central fat has been shown to be potentially pathogenic for developing diabetes, cardiovascular disease, and even some forms of cancer,” she said.
The safety profile of OLA/SAM was essentially the same as for olanzapine-only, with the exception of the weight gain.
Alkermes is planning to submit its New Drug Application for OLA/SAM to the Food and Drug Administration before the year’s end. FDA officials have urged the company to broaden the application to include not only the treatment of schizophrenia, but bipolar I disorder as well, since olanzapine is an approved, well-established treatment for that disorder. Dr. Graham and coinvestigators have demonstrated that OLA/SAM has no clinically significant effect on the pharmacokinetics of lithium or valproate (Clin Drug Investig. 2019 Oct 4. doi: 10.1007/s40261-019-00860-y).
Phase 3 trial on pimavanserin underway
Pimavanserin is an oral selective serotonin inverse agonist, or SSIA, with a high affinity for 5-HT2A receptors, very low affinity for 5-HT2C receptors, and “absolutely no affinity” for dopaminergic, histaminergic, adrenergic, or muscarinic receptors, explained Dragana Bugarski-Kirola, MD, a psychiatrist and vice president of clinical development at Acadia Pharmaceuticals in San Diego.
“Those sites are thought to contribute to sedation, cognitive impairment, and orthostatic hypotension,” she noted.
Pimavanserin is at present FDA approved for a narrow indication: Treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. But the drug’s unique mechanism of action suggests broad efficacy across a range of psychiatric disorders.
Indeed, after a successful phase 2 clinical trial of pimavanserin for treatment of Alzheimer’s-related psychosis, a phase 3 randomized, double-blind, placebo-controlled clinical trial of the drug for relapse prevention in dementia-related psychosis is now enrolling a planned 360 outpatients at 95 centers in 13 countries. This 26-week study, known as HARMONY, is preceded by open-label psychotherapy to ensure that study participants truly need pharmacotherapy. Patients are eligible regardless of their type of dementia, because psychosis in patients with various forms of dementia is clinically pretty much the same, whether the underlying disorder is Alzheimer’s disease, vascular dementia, Parkinson’s disease, or Lewy body dementia, according to Dr. Bugarski-Kirola.
In addition, pimavanserin also is the subject of an ongoing phase 3 randomized trial in patients with major depressive disorder inadequately responsive to an selective serotonin reuptake inhibitor or a selective norepinephrine reuptake inhibitor. A 380-patient phase 2 study of the drug as adjunctive treatment for negative symptoms of schizophrenia also is underway based upon earlier promising results.
Across the board for these potential indications, the drug has been well tolerated, with a side effect profile similar to that of placebo. Importantly, pimavanserin has not been associated with cognitive impairment when used for dementia-related psychosis, unlike the antipsychotics now being used off label in clinical practice, the psychiatrist said.
SEP-363856 part of ‘novel class’
SEP-363856 is a nondopaminergic D2, trace amine-associated receptor agonist (TAAR1) under development for treatment of schizophrenia. Phase 3 trials in adults and adolescents with schizophrenia will begin before the end of the year on the strength of positive phase 2 results, according to Kenneth S. Koblan, PhD, head of global translational medicine and early development, as well as head of discovery sciences, at Sunovion Pharmaceuticals, Marlborough, Mass.
“We believe that SEP-363856 actually represents the first candidate in a novel class of antipsychotics. It’s a monoamine receptor activator, unlike the atypical antipsychotics, which work through blockade of the monoamine receptor via dopamine and serotonin. We believe that it’s the monoamine receptor activation that leads to the safety and efficacy of the class,” he explained.
In the four-country, double-blind, 4-week phase 2 trial conducted in 245 hospitalized acutely psychotic patients, oral SEP-363856 flexibly dosed at 50 or 75 mg/day had a side effect profile like that of placebo. Negative symptoms as assessed via the Brief Negative Symptom Scale improved by an average of 7.1 points at 4 weeks with SEP-363856, significantly more than the 2.7-point improvement with placebo. The PANSS total score improved by 17.2 points in the SEP-363856 group and 9.7 points in controls at 4 weeks, with a further 10-point drop in PANSS during a 6-month open-label extension phase of the study. Moreover, the SEP-363856 cohort showed significant functional improvement at 4 weeks in the UCSD Performance-Based Skills Assessment, with continued improvement during the open-label extension study.
Dr. Koblan said the pharmaceutical industry has overemphasized the development of dopaminergic D2-based drugs for schizophrenia. In the past 2 decades, roughly 30,000 patients have been enrolled in industry-sponsored, placebo-controlled, phase 2 or 3 randomized trials of drugs with that mechanism. Many of the those drugs have reached the marketplace. In contrast, there have been far fewer RCTs – and no product launches – of antipsychotics with non-D2 mechanisms of action.
“When you consider that the cost is about $50,000 per research subject and 50,000 subjects have been studied since 2000, the pharmaceutical industry has invested on the order of billions of dollars to try to come up with the next breakthrough medication,” he said.
REPORTING FROM ECNP 2019
ASCO to award $50,000 young investigator grant to study MCL
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
A cigarette in one hand and a Fitbit on the other
A cardiologist friend of mine told me a story about one of his patients. The man had recently been in to see him for an office visit. He had quite a scare needing two stents after an episode of prolonged chest pain and, during the office visit, apparently had said that he had “found religion” and was going to change his ways. He showed off the Fitbit that he had gotten and shared his excitement about using a new app to track his diet on his smart phone. His blood pressure was a little elevated, so my friend added a third antihypertensive in an effort to get his blood pressure under control. He referred the patient back to his primary care physician to address his elevated hemoglobin A1c.
My friend saw the patient again a couple of weeks later – this time at the mall. As he was driving through the parking lot, he noticed his patient sitting on a bench outside the entrance. He also noticed a cigarette in his patient’s right hand and saw the Fitbit still on his wrist. Now, it’s not that there is anything wrong with wearing a Fitbit, but …
My friend is an incredibly respectful person, and very nice. He decided not to say hello and risk embarrassing his patient, so he walked to a different door far from the bench and went inside. Nonetheless, the image bothered him. It bothered him enough to repeat the story to me 2 weeks later. It bothers me too.
The other day I was talking to a healthy young nurse with whom I work. She has been trying to get into shape, and her goal is to get to the gym 5 days a week after work. She read on a popular website that she should use a heart rate monitor to keep track of her training and that, if her heart rate is too slow, she should run faster and, if her heart rate is too fast, she should slow down. She was discouraged the other day, however, because her watch indicated that her pulse was going up to 170 while she was running hard, and she had heard that could be dangerous for her heart.
When she doesn’t push hard, though, she told me that her heart rate often plateaus at about 110, sometimes 115. She has been finding it difficult to achieve her calculated target heart rate of 120-160 beats per minute. She is frustrated and was going to skip her workout that evening. I explained to her that she should stop checking her pulse and just run – if she felt she was running too slow she could run faster.
With everything that we have learned about science and technology, the reality is that we are still people, with all our weaknesses and strengths. We often set goals with ambivalence, then rush forward hoping that a technological solution will move us in the direction we think we want to move. Unfortunately, owning a Fitbit will not make us more fit, and checking our pulse every five minutes while working out will not lead to a better exercise session. With the availability of so much technology for tracking our daily exercise, vital signs, and various other measures of health, we need to be more careful than ever to determine specifically what it is that we are trying to accomplish with the use of our technology.
When it comes to good health, it is the fundamentals that matter, and achieving the fundamentals requires being mindful and making repeated efforts to master them. For almost all adults, the most important habits to develop are still related to diet and exercise. Consuming the right diet and exercising adequately requires that the correct choices be made each and every day, all day long. Technology can help but will not do it for us. We need to be thoughtful about how we use technology and explicit about how we expect it to help. After a reasonable amount of time, we should evaluate to see if it is working for us. If it is, then we should continue to use it. If it is not, then we should stop using it or make a different change, like performing a new type of exercise.
Our goal should be to have intelligent empathic integration of technological and behavioral techniques to achieve an optimal health outcome. Putting running shoes by the bed at night is a great thing to do to encourage us to run in the morning. Choosing motivational music can help us get the energy and enthusiasm to go for that run (our favorites include the Rocky theme song and “I Didn’t Come this Far to Only Come this Far”). A visual reminder over the refrigerator can “nudge” us to make good choices as we open the door.
For those who want to learn more about how to integrate behavioral management into their advice for patients we highly recommend reading “Switch: How to Change Things When Change Is Hard” by Chip Heath and “Nudge: Improving Decisions About Health, Wealth, and Happiness” by Richard Thaler. We have always been, and remain, excited about the promise of technology to help us accomplish our goals. That said, we told the nurse to stop checking her pulse, to put on some music, and to appreciate the leaves on the trees this autumn while she was running. As for the gentleman outside the mall, well …
We are interested in your thoughts. Please email us at [email protected].
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A cardiologist friend of mine told me a story about one of his patients. The man had recently been in to see him for an office visit. He had quite a scare needing two stents after an episode of prolonged chest pain and, during the office visit, apparently had said that he had “found religion” and was going to change his ways. He showed off the Fitbit that he had gotten and shared his excitement about using a new app to track his diet on his smart phone. His blood pressure was a little elevated, so my friend added a third antihypertensive in an effort to get his blood pressure under control. He referred the patient back to his primary care physician to address his elevated hemoglobin A1c.
My friend saw the patient again a couple of weeks later – this time at the mall. As he was driving through the parking lot, he noticed his patient sitting on a bench outside the entrance. He also noticed a cigarette in his patient’s right hand and saw the Fitbit still on his wrist. Now, it’s not that there is anything wrong with wearing a Fitbit, but …
My friend is an incredibly respectful person, and very nice. He decided not to say hello and risk embarrassing his patient, so he walked to a different door far from the bench and went inside. Nonetheless, the image bothered him. It bothered him enough to repeat the story to me 2 weeks later. It bothers me too.
The other day I was talking to a healthy young nurse with whom I work. She has been trying to get into shape, and her goal is to get to the gym 5 days a week after work. She read on a popular website that she should use a heart rate monitor to keep track of her training and that, if her heart rate is too slow, she should run faster and, if her heart rate is too fast, she should slow down. She was discouraged the other day, however, because her watch indicated that her pulse was going up to 170 while she was running hard, and she had heard that could be dangerous for her heart.
When she doesn’t push hard, though, she told me that her heart rate often plateaus at about 110, sometimes 115. She has been finding it difficult to achieve her calculated target heart rate of 120-160 beats per minute. She is frustrated and was going to skip her workout that evening. I explained to her that she should stop checking her pulse and just run – if she felt she was running too slow she could run faster.
With everything that we have learned about science and technology, the reality is that we are still people, with all our weaknesses and strengths. We often set goals with ambivalence, then rush forward hoping that a technological solution will move us in the direction we think we want to move. Unfortunately, owning a Fitbit will not make us more fit, and checking our pulse every five minutes while working out will not lead to a better exercise session. With the availability of so much technology for tracking our daily exercise, vital signs, and various other measures of health, we need to be more careful than ever to determine specifically what it is that we are trying to accomplish with the use of our technology.
When it comes to good health, it is the fundamentals that matter, and achieving the fundamentals requires being mindful and making repeated efforts to master them. For almost all adults, the most important habits to develop are still related to diet and exercise. Consuming the right diet and exercising adequately requires that the correct choices be made each and every day, all day long. Technology can help but will not do it for us. We need to be thoughtful about how we use technology and explicit about how we expect it to help. After a reasonable amount of time, we should evaluate to see if it is working for us. If it is, then we should continue to use it. If it is not, then we should stop using it or make a different change, like performing a new type of exercise.
Our goal should be to have intelligent empathic integration of technological and behavioral techniques to achieve an optimal health outcome. Putting running shoes by the bed at night is a great thing to do to encourage us to run in the morning. Choosing motivational music can help us get the energy and enthusiasm to go for that run (our favorites include the Rocky theme song and “I Didn’t Come this Far to Only Come this Far”). A visual reminder over the refrigerator can “nudge” us to make good choices as we open the door.
For those who want to learn more about how to integrate behavioral management into their advice for patients we highly recommend reading “Switch: How to Change Things When Change Is Hard” by Chip Heath and “Nudge: Improving Decisions About Health, Wealth, and Happiness” by Richard Thaler. We have always been, and remain, excited about the promise of technology to help us accomplish our goals. That said, we told the nurse to stop checking her pulse, to put on some music, and to appreciate the leaves on the trees this autumn while she was running. As for the gentleman outside the mall, well …
We are interested in your thoughts. Please email us at [email protected].
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A cardiologist friend of mine told me a story about one of his patients. The man had recently been in to see him for an office visit. He had quite a scare needing two stents after an episode of prolonged chest pain and, during the office visit, apparently had said that he had “found religion” and was going to change his ways. He showed off the Fitbit that he had gotten and shared his excitement about using a new app to track his diet on his smart phone. His blood pressure was a little elevated, so my friend added a third antihypertensive in an effort to get his blood pressure under control. He referred the patient back to his primary care physician to address his elevated hemoglobin A1c.
My friend saw the patient again a couple of weeks later – this time at the mall. As he was driving through the parking lot, he noticed his patient sitting on a bench outside the entrance. He also noticed a cigarette in his patient’s right hand and saw the Fitbit still on his wrist. Now, it’s not that there is anything wrong with wearing a Fitbit, but …
My friend is an incredibly respectful person, and very nice. He decided not to say hello and risk embarrassing his patient, so he walked to a different door far from the bench and went inside. Nonetheless, the image bothered him. It bothered him enough to repeat the story to me 2 weeks later. It bothers me too.
The other day I was talking to a healthy young nurse with whom I work. She has been trying to get into shape, and her goal is to get to the gym 5 days a week after work. She read on a popular website that she should use a heart rate monitor to keep track of her training and that, if her heart rate is too slow, she should run faster and, if her heart rate is too fast, she should slow down. She was discouraged the other day, however, because her watch indicated that her pulse was going up to 170 while she was running hard, and she had heard that could be dangerous for her heart.
When she doesn’t push hard, though, she told me that her heart rate often plateaus at about 110, sometimes 115. She has been finding it difficult to achieve her calculated target heart rate of 120-160 beats per minute. She is frustrated and was going to skip her workout that evening. I explained to her that she should stop checking her pulse and just run – if she felt she was running too slow she could run faster.
With everything that we have learned about science and technology, the reality is that we are still people, with all our weaknesses and strengths. We often set goals with ambivalence, then rush forward hoping that a technological solution will move us in the direction we think we want to move. Unfortunately, owning a Fitbit will not make us more fit, and checking our pulse every five minutes while working out will not lead to a better exercise session. With the availability of so much technology for tracking our daily exercise, vital signs, and various other measures of health, we need to be more careful than ever to determine specifically what it is that we are trying to accomplish with the use of our technology.
When it comes to good health, it is the fundamentals that matter, and achieving the fundamentals requires being mindful and making repeated efforts to master them. For almost all adults, the most important habits to develop are still related to diet and exercise. Consuming the right diet and exercising adequately requires that the correct choices be made each and every day, all day long. Technology can help but will not do it for us. We need to be thoughtful about how we use technology and explicit about how we expect it to help. After a reasonable amount of time, we should evaluate to see if it is working for us. If it is, then we should continue to use it. If it is not, then we should stop using it or make a different change, like performing a new type of exercise.
Our goal should be to have intelligent empathic integration of technological and behavioral techniques to achieve an optimal health outcome. Putting running shoes by the bed at night is a great thing to do to encourage us to run in the morning. Choosing motivational music can help us get the energy and enthusiasm to go for that run (our favorites include the Rocky theme song and “I Didn’t Come this Far to Only Come this Far”). A visual reminder over the refrigerator can “nudge” us to make good choices as we open the door.
For those who want to learn more about how to integrate behavioral management into their advice for patients we highly recommend reading “Switch: How to Change Things When Change Is Hard” by Chip Heath and “Nudge: Improving Decisions About Health, Wealth, and Happiness” by Richard Thaler. We have always been, and remain, excited about the promise of technology to help us accomplish our goals. That said, we told the nurse to stop checking her pulse, to put on some music, and to appreciate the leaves on the trees this autumn while she was running. As for the gentleman outside the mall, well …
We are interested in your thoughts. Please email us at [email protected].
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Hormone therapy in transgender patients is safe for bone
ORLANDO – according to a presentation at the annual meeting of the American Society for Bone and Mineral Research.
“Hormonal treatment of transgender people is safe with respect to bone,” said Martin den Heijer, MD, PhD, of the VU University Medical Center in Amsterdam.
At baseline, transwomen have lower bone mass than do male reference populations, said Dr. den Heijer, citing a study that found 25 transwomen had less muscle mass (P less than or equal to .001), strength (P less than or equal to .05), and lower BMD at the hip, femoral neck, and spine (P less than .001), compared with 25 cisgender men in a control group and 941 men in a male reference population (Bone. 2013;54[1]:92-7). In a 2019 study from his own group, Dr. den Heijer said the z score in the lumbar spine for 711 transwomen was -0.9 and the incidence of osteoporosis was 14.2%, compared with a z score of 0.0 and 2.4% incidence of osteoporosis in 543 transmen (J Bone Min Res. 2019;34[3]:447-54).
In the prospective European Network for the Investigation of Gender Incongruence (ENIGI) study, researchers examined short-term effects of hormone therapy on BMD in 144 transwomen and 162 transmen who had a normal body mass index and were mostly white. The percentage of patients who reported they were current smokers was between 25% and 30%, and fewer than 10% said they consumed more than seven units of alcohol per week. Transwomen received estradiol (an oral estradiol valerate at a dose of 4 mg/day or an estradiol patch) together with 100 mg/day of cyproterone acetate, and transmen received testosterone in the form of a gel (50 mg/day), intramuscular esters (250 mg every 2-3 weeks), or intramuscular undecanoate at a dose of 1,000 mg every 12 weeks (J Sex Med. 2016;13[6]:994-9).
After 1 year of treatment, there were significant increases in BMD in transwomen in the lumbar spine (3.67%; 95% confidence interval, 3.20%-4.13%), femoral neck (1.86%; 95% CI, 1.41%-2.31%), and total hip (0.97%; 95% CI, 0.62%-1.31%). Transmen also had increased BMD in the lumbar spine (0.86%; 95% CI, 0.38%-1.35%) and total hip (1.04%; 95% CI, 0.64%-1.44%), with a slight decrease in femoral neck BMD (–0.46%; 95% CI, –1.07% to 0.16%).
Dr. den Heijer also discussed the long-term effects of hormone therapy on BMD in the Amsterdam Cohort of Dysphoria (ACOG) study, which consisted of 711 transwomen and 543 transmen and followed some patients out to 2 years, 5 years, and 10 years after beginning hormone therapy (J Sex Med. 2018;15[4]:582-90). Among transwomen, the median age was 33 years, 68.9% had begun hormone therapy, and 75.3% received a gonadectomy; among transmen, the median age was 25 years, 72.9% had begun hormone therapy, and 83.8% received a gonadectomy. Of these patients, dual-energy x-ray absorptiometry data were available for the lumbar spine BMD for 234 transwomen and 236 transmen at 2 years, 174 transwomen and 95 transmen at 5 years, and 102 transwomen and 70 transmen at 10 years.
Although there was no significant mean change in absolute BMD over the 10-year period, the concentration of estradiol in transwomen and transmen affected change in BMD the longer the transperson was receiving hormone therapy: Transwomen who received an estradiol concentration of 118 pmol/L had a decrease of –0.026% at 2 years, –0.044% at 5 years, and –0.009% at 10 years, compared with a dose of 443 pmol/L (+0.044% at 2 years, +0.025% at 5 years, +0.063% at 10 years), whereas transmen also had decreased BMD at the lowest estradiol concentrations of 95 pmol/L (–0.007% at 2 years, –0.024% at 5 years, +0.010% at 10 years), compared with transmen receiving the highest doses of 323 pmol/L (+0.028% at 2 years, +0.002% at 5 years, +0.053% at 10 years). There was no significant change in BMD in either group at any time point with regard to testosterone concentration.
When the investigators linked these patients to a national statistics database in the Netherlands to evaluate fracture incidence (J Bone Miner Res. 2019 Sep 5. doi: 10.1002/jbmr.3862), pairing five cisgender female controls and five cisgender male controls to every transgender patient, the researchers found transwomen had a higher incidence of osteoporotic fracture of the hip, spine, forearm, and humerus (41.8%), compared with cisgender men (26.6%; P = .014) and cisgender women (36.0%; P = .381). There was not enough information in the study to examine fracture information for transmen, Dr. den Heijer said. Transwomen and transmen who experienced a fracture were more likely to be a current smoker and have lower estradiol concentrations than were transwomen and transmen, respectively, who did not have a fracture.
“Attention for lifestyle factors remains important, especially smoking cessation, vitamin D intake, and regular exercise,” Dr. den Heijer said. “It remains important for everybody, but especially for transgender women.”
Dr. den Heijer reported no relevant conflicts of interest.
ORLANDO – according to a presentation at the annual meeting of the American Society for Bone and Mineral Research.
“Hormonal treatment of transgender people is safe with respect to bone,” said Martin den Heijer, MD, PhD, of the VU University Medical Center in Amsterdam.
At baseline, transwomen have lower bone mass than do male reference populations, said Dr. den Heijer, citing a study that found 25 transwomen had less muscle mass (P less than or equal to .001), strength (P less than or equal to .05), and lower BMD at the hip, femoral neck, and spine (P less than .001), compared with 25 cisgender men in a control group and 941 men in a male reference population (Bone. 2013;54[1]:92-7). In a 2019 study from his own group, Dr. den Heijer said the z score in the lumbar spine for 711 transwomen was -0.9 and the incidence of osteoporosis was 14.2%, compared with a z score of 0.0 and 2.4% incidence of osteoporosis in 543 transmen (J Bone Min Res. 2019;34[3]:447-54).
In the prospective European Network for the Investigation of Gender Incongruence (ENIGI) study, researchers examined short-term effects of hormone therapy on BMD in 144 transwomen and 162 transmen who had a normal body mass index and were mostly white. The percentage of patients who reported they were current smokers was between 25% and 30%, and fewer than 10% said they consumed more than seven units of alcohol per week. Transwomen received estradiol (an oral estradiol valerate at a dose of 4 mg/day or an estradiol patch) together with 100 mg/day of cyproterone acetate, and transmen received testosterone in the form of a gel (50 mg/day), intramuscular esters (250 mg every 2-3 weeks), or intramuscular undecanoate at a dose of 1,000 mg every 12 weeks (J Sex Med. 2016;13[6]:994-9).
After 1 year of treatment, there were significant increases in BMD in transwomen in the lumbar spine (3.67%; 95% confidence interval, 3.20%-4.13%), femoral neck (1.86%; 95% CI, 1.41%-2.31%), and total hip (0.97%; 95% CI, 0.62%-1.31%). Transmen also had increased BMD in the lumbar spine (0.86%; 95% CI, 0.38%-1.35%) and total hip (1.04%; 95% CI, 0.64%-1.44%), with a slight decrease in femoral neck BMD (–0.46%; 95% CI, –1.07% to 0.16%).
Dr. den Heijer also discussed the long-term effects of hormone therapy on BMD in the Amsterdam Cohort of Dysphoria (ACOG) study, which consisted of 711 transwomen and 543 transmen and followed some patients out to 2 years, 5 years, and 10 years after beginning hormone therapy (J Sex Med. 2018;15[4]:582-90). Among transwomen, the median age was 33 years, 68.9% had begun hormone therapy, and 75.3% received a gonadectomy; among transmen, the median age was 25 years, 72.9% had begun hormone therapy, and 83.8% received a gonadectomy. Of these patients, dual-energy x-ray absorptiometry data were available for the lumbar spine BMD for 234 transwomen and 236 transmen at 2 years, 174 transwomen and 95 transmen at 5 years, and 102 transwomen and 70 transmen at 10 years.
Although there was no significant mean change in absolute BMD over the 10-year period, the concentration of estradiol in transwomen and transmen affected change in BMD the longer the transperson was receiving hormone therapy: Transwomen who received an estradiol concentration of 118 pmol/L had a decrease of –0.026% at 2 years, –0.044% at 5 years, and –0.009% at 10 years, compared with a dose of 443 pmol/L (+0.044% at 2 years, +0.025% at 5 years, +0.063% at 10 years), whereas transmen also had decreased BMD at the lowest estradiol concentrations of 95 pmol/L (–0.007% at 2 years, –0.024% at 5 years, +0.010% at 10 years), compared with transmen receiving the highest doses of 323 pmol/L (+0.028% at 2 years, +0.002% at 5 years, +0.053% at 10 years). There was no significant change in BMD in either group at any time point with regard to testosterone concentration.
When the investigators linked these patients to a national statistics database in the Netherlands to evaluate fracture incidence (J Bone Miner Res. 2019 Sep 5. doi: 10.1002/jbmr.3862), pairing five cisgender female controls and five cisgender male controls to every transgender patient, the researchers found transwomen had a higher incidence of osteoporotic fracture of the hip, spine, forearm, and humerus (41.8%), compared with cisgender men (26.6%; P = .014) and cisgender women (36.0%; P = .381). There was not enough information in the study to examine fracture information for transmen, Dr. den Heijer said. Transwomen and transmen who experienced a fracture were more likely to be a current smoker and have lower estradiol concentrations than were transwomen and transmen, respectively, who did not have a fracture.
“Attention for lifestyle factors remains important, especially smoking cessation, vitamin D intake, and regular exercise,” Dr. den Heijer said. “It remains important for everybody, but especially for transgender women.”
Dr. den Heijer reported no relevant conflicts of interest.
ORLANDO – according to a presentation at the annual meeting of the American Society for Bone and Mineral Research.
“Hormonal treatment of transgender people is safe with respect to bone,” said Martin den Heijer, MD, PhD, of the VU University Medical Center in Amsterdam.
At baseline, transwomen have lower bone mass than do male reference populations, said Dr. den Heijer, citing a study that found 25 transwomen had less muscle mass (P less than or equal to .001), strength (P less than or equal to .05), and lower BMD at the hip, femoral neck, and spine (P less than .001), compared with 25 cisgender men in a control group and 941 men in a male reference population (Bone. 2013;54[1]:92-7). In a 2019 study from his own group, Dr. den Heijer said the z score in the lumbar spine for 711 transwomen was -0.9 and the incidence of osteoporosis was 14.2%, compared with a z score of 0.0 and 2.4% incidence of osteoporosis in 543 transmen (J Bone Min Res. 2019;34[3]:447-54).
In the prospective European Network for the Investigation of Gender Incongruence (ENIGI) study, researchers examined short-term effects of hormone therapy on BMD in 144 transwomen and 162 transmen who had a normal body mass index and were mostly white. The percentage of patients who reported they were current smokers was between 25% and 30%, and fewer than 10% said they consumed more than seven units of alcohol per week. Transwomen received estradiol (an oral estradiol valerate at a dose of 4 mg/day or an estradiol patch) together with 100 mg/day of cyproterone acetate, and transmen received testosterone in the form of a gel (50 mg/day), intramuscular esters (250 mg every 2-3 weeks), or intramuscular undecanoate at a dose of 1,000 mg every 12 weeks (J Sex Med. 2016;13[6]:994-9).
After 1 year of treatment, there were significant increases in BMD in transwomen in the lumbar spine (3.67%; 95% confidence interval, 3.20%-4.13%), femoral neck (1.86%; 95% CI, 1.41%-2.31%), and total hip (0.97%; 95% CI, 0.62%-1.31%). Transmen also had increased BMD in the lumbar spine (0.86%; 95% CI, 0.38%-1.35%) and total hip (1.04%; 95% CI, 0.64%-1.44%), with a slight decrease in femoral neck BMD (–0.46%; 95% CI, –1.07% to 0.16%).
Dr. den Heijer also discussed the long-term effects of hormone therapy on BMD in the Amsterdam Cohort of Dysphoria (ACOG) study, which consisted of 711 transwomen and 543 transmen and followed some patients out to 2 years, 5 years, and 10 years after beginning hormone therapy (J Sex Med. 2018;15[4]:582-90). Among transwomen, the median age was 33 years, 68.9% had begun hormone therapy, and 75.3% received a gonadectomy; among transmen, the median age was 25 years, 72.9% had begun hormone therapy, and 83.8% received a gonadectomy. Of these patients, dual-energy x-ray absorptiometry data were available for the lumbar spine BMD for 234 transwomen and 236 transmen at 2 years, 174 transwomen and 95 transmen at 5 years, and 102 transwomen and 70 transmen at 10 years.
Although there was no significant mean change in absolute BMD over the 10-year period, the concentration of estradiol in transwomen and transmen affected change in BMD the longer the transperson was receiving hormone therapy: Transwomen who received an estradiol concentration of 118 pmol/L had a decrease of –0.026% at 2 years, –0.044% at 5 years, and –0.009% at 10 years, compared with a dose of 443 pmol/L (+0.044% at 2 years, +0.025% at 5 years, +0.063% at 10 years), whereas transmen also had decreased BMD at the lowest estradiol concentrations of 95 pmol/L (–0.007% at 2 years, –0.024% at 5 years, +0.010% at 10 years), compared with transmen receiving the highest doses of 323 pmol/L (+0.028% at 2 years, +0.002% at 5 years, +0.053% at 10 years). There was no significant change in BMD in either group at any time point with regard to testosterone concentration.
When the investigators linked these patients to a national statistics database in the Netherlands to evaluate fracture incidence (J Bone Miner Res. 2019 Sep 5. doi: 10.1002/jbmr.3862), pairing five cisgender female controls and five cisgender male controls to every transgender patient, the researchers found transwomen had a higher incidence of osteoporotic fracture of the hip, spine, forearm, and humerus (41.8%), compared with cisgender men (26.6%; P = .014) and cisgender women (36.0%; P = .381). There was not enough information in the study to examine fracture information for transmen, Dr. den Heijer said. Transwomen and transmen who experienced a fracture were more likely to be a current smoker and have lower estradiol concentrations than were transwomen and transmen, respectively, who did not have a fracture.
“Attention for lifestyle factors remains important, especially smoking cessation, vitamin D intake, and regular exercise,” Dr. den Heijer said. “It remains important for everybody, but especially for transgender women.”
Dr. den Heijer reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASBMR 2019
GAO calls out HHS’ poor oversight of administrative costs of Medicaid work requirements
The Centers for Medicare & Medicaid Services needs to be doing a better job overseeing the administrative costs associated with the implementation of work requirements in Medicaid, the Government Accountability Office said in a new report.
The government watchdog found two key weaknesses in CMS’s oversight of the administrative costs of the Medicaid demonstration projects related to work requirements for Medicaid.
First, the GAO report notes that no consideration of the administrative costs of the work requirements is given during the administration of the approval process.
The GAO reports that, of five states’ approvals, the estimated administrative costs range from the low end of $6.1 million for New Hampshire (with 50,000 beneficiaries subject to the work requirement) to $271.6 million for Kentucky (with 620,000 beneficiaries subject to the work requirement). Indiana, with 420,000 beneficiaries subject to work requirements, has an estimated cost of $35.1 million.
A significant portion of Kentucky’s funding was for a the development of a new information technology system to help track work requirements.
“GAO found that CMS does not require states to provide projections of administrative costs when requesting demonstration approvals,” the report states. “Thus, the cost of administering demonstrations, including those with work requirements, is not transparent to the public or included in CMS’s assessment of whether a demonstration is budget neutral – that is, that federal spending will be no higher under the demonstration than it would have been without it.”
The GAO also reported that, by not requiring cost estimates, it also fails to meet the demonstration objective of transparency, something that goes hand in hand with budget neutrality.
The second weakness identified by GAO is that current procedures “may be insufficient to ensure that costs are allowable and matched at the correct rate.” Three of the five states examined in the report had received CMS approval for federal funds for administrative costs that were either not allowable for matching or were matched at higher rates than appropriate, based on CMS guidance.
The government watchdog noted that CMS did implement “procedures that may provide additional information on demonstrations’ administrative costs. ... However, it is unclear whether these efforts will result in data that improves CMS’s oversight.”
The GAO made three recommendations in the report. First, the CMS should require states to submit public projections of administrative costs when seeking approval for demonstration projects. Second, the administrative costs should be a part of the calculation for assessing the budget neutrality of demonstration project applications. Finally, CMS should do a better job assessing the risk that federal funds are being used to cover administrative costs that are not allowable and should improve oversight procedures as needed.
The GAO report included the Department of Health & Human Services’s response to the recommendations. To the first, the agency said that “its experience suggests that demonstration administrative costs will be a relatively small portion of total costs and therefore HHS believes making information about these costs available would provide stakeholders little to no value.”
Similarly, to the second recommendation, HHS countered that the information would provide little to no value given that administrative costs represent a relatively small portion of the total demonstration costs.
To the final recommendation on the need for better risk assessment, HHS said its existing approach “is appropriate for the low level of risk that administrative expenditures represent. ... CMS officials told us that they had not assessed wither current procedures sufficiently address risks posed by administrative costs for work requirements and had no plans to do so.”
The Centers for Medicare & Medicaid Services needs to be doing a better job overseeing the administrative costs associated with the implementation of work requirements in Medicaid, the Government Accountability Office said in a new report.
The government watchdog found two key weaknesses in CMS’s oversight of the administrative costs of the Medicaid demonstration projects related to work requirements for Medicaid.
First, the GAO report notes that no consideration of the administrative costs of the work requirements is given during the administration of the approval process.
The GAO reports that, of five states’ approvals, the estimated administrative costs range from the low end of $6.1 million for New Hampshire (with 50,000 beneficiaries subject to the work requirement) to $271.6 million for Kentucky (with 620,000 beneficiaries subject to the work requirement). Indiana, with 420,000 beneficiaries subject to work requirements, has an estimated cost of $35.1 million.
A significant portion of Kentucky’s funding was for a the development of a new information technology system to help track work requirements.
“GAO found that CMS does not require states to provide projections of administrative costs when requesting demonstration approvals,” the report states. “Thus, the cost of administering demonstrations, including those with work requirements, is not transparent to the public or included in CMS’s assessment of whether a demonstration is budget neutral – that is, that federal spending will be no higher under the demonstration than it would have been without it.”
The GAO also reported that, by not requiring cost estimates, it also fails to meet the demonstration objective of transparency, something that goes hand in hand with budget neutrality.
The second weakness identified by GAO is that current procedures “may be insufficient to ensure that costs are allowable and matched at the correct rate.” Three of the five states examined in the report had received CMS approval for federal funds for administrative costs that were either not allowable for matching or were matched at higher rates than appropriate, based on CMS guidance.
The government watchdog noted that CMS did implement “procedures that may provide additional information on demonstrations’ administrative costs. ... However, it is unclear whether these efforts will result in data that improves CMS’s oversight.”
The GAO made three recommendations in the report. First, the CMS should require states to submit public projections of administrative costs when seeking approval for demonstration projects. Second, the administrative costs should be a part of the calculation for assessing the budget neutrality of demonstration project applications. Finally, CMS should do a better job assessing the risk that federal funds are being used to cover administrative costs that are not allowable and should improve oversight procedures as needed.
The GAO report included the Department of Health & Human Services’s response to the recommendations. To the first, the agency said that “its experience suggests that demonstration administrative costs will be a relatively small portion of total costs and therefore HHS believes making information about these costs available would provide stakeholders little to no value.”
Similarly, to the second recommendation, HHS countered that the information would provide little to no value given that administrative costs represent a relatively small portion of the total demonstration costs.
To the final recommendation on the need for better risk assessment, HHS said its existing approach “is appropriate for the low level of risk that administrative expenditures represent. ... CMS officials told us that they had not assessed wither current procedures sufficiently address risks posed by administrative costs for work requirements and had no plans to do so.”
The Centers for Medicare & Medicaid Services needs to be doing a better job overseeing the administrative costs associated with the implementation of work requirements in Medicaid, the Government Accountability Office said in a new report.
The government watchdog found two key weaknesses in CMS’s oversight of the administrative costs of the Medicaid demonstration projects related to work requirements for Medicaid.
First, the GAO report notes that no consideration of the administrative costs of the work requirements is given during the administration of the approval process.
The GAO reports that, of five states’ approvals, the estimated administrative costs range from the low end of $6.1 million for New Hampshire (with 50,000 beneficiaries subject to the work requirement) to $271.6 million for Kentucky (with 620,000 beneficiaries subject to the work requirement). Indiana, with 420,000 beneficiaries subject to work requirements, has an estimated cost of $35.1 million.
A significant portion of Kentucky’s funding was for a the development of a new information technology system to help track work requirements.
“GAO found that CMS does not require states to provide projections of administrative costs when requesting demonstration approvals,” the report states. “Thus, the cost of administering demonstrations, including those with work requirements, is not transparent to the public or included in CMS’s assessment of whether a demonstration is budget neutral – that is, that federal spending will be no higher under the demonstration than it would have been without it.”
The GAO also reported that, by not requiring cost estimates, it also fails to meet the demonstration objective of transparency, something that goes hand in hand with budget neutrality.
The second weakness identified by GAO is that current procedures “may be insufficient to ensure that costs are allowable and matched at the correct rate.” Three of the five states examined in the report had received CMS approval for federal funds for administrative costs that were either not allowable for matching or were matched at higher rates than appropriate, based on CMS guidance.
The government watchdog noted that CMS did implement “procedures that may provide additional information on demonstrations’ administrative costs. ... However, it is unclear whether these efforts will result in data that improves CMS’s oversight.”
The GAO made three recommendations in the report. First, the CMS should require states to submit public projections of administrative costs when seeking approval for demonstration projects. Second, the administrative costs should be a part of the calculation for assessing the budget neutrality of demonstration project applications. Finally, CMS should do a better job assessing the risk that federal funds are being used to cover administrative costs that are not allowable and should improve oversight procedures as needed.
The GAO report included the Department of Health & Human Services’s response to the recommendations. To the first, the agency said that “its experience suggests that demonstration administrative costs will be a relatively small portion of total costs and therefore HHS believes making information about these costs available would provide stakeholders little to no value.”
Similarly, to the second recommendation, HHS countered that the information would provide little to no value given that administrative costs represent a relatively small portion of the total demonstration costs.
To the final recommendation on the need for better risk assessment, HHS said its existing approach “is appropriate for the low level of risk that administrative expenditures represent. ... CMS officials told us that they had not assessed wither current procedures sufficiently address risks posed by administrative costs for work requirements and had no plans to do so.”
Crossword puzzle tools
Practice Puzzlers are an entertaining way to challenge your clinical expertise. To make the experience more enjoyable, here's how to use the tools located in the bar above the crossword.
- Away from your computer? Click “Print” to download and print a blank puzzle or the answer key to the puzzle.
- With the "group tool" invite colleagues to play with you and share final scores.
- To keep it fun, you can “Reveal” or “Check” a letter, word, or the entire grid; change the “Settings” to suit your preferences; or use the “Pencil” toggle to enter the answer in gray and later make it a normal entry when you’re sure about it.
Scoring
You get 10 points for each correct word completed. Revealing letters or words will cost you points. For each square you reveal, you lose 1 point, but you can still get the 10 points if you get the word right. You get 0 points if you reveal an entire word. The target time to complete this puzzle is 15 minutes. When you complete the puzzle, you will get a bonus of 15 points for every full minute under the target. There is no penalty for going over the time limit.
Practice Puzzlers are an entertaining way to challenge your clinical expertise. To make the experience more enjoyable, here's how to use the tools located in the bar above the crossword.
- Away from your computer? Click “Print” to download and print a blank puzzle or the answer key to the puzzle.
- With the "group tool" invite colleagues to play with you and share final scores.
- To keep it fun, you can “Reveal” or “Check” a letter, word, or the entire grid; change the “Settings” to suit your preferences; or use the “Pencil” toggle to enter the answer in gray and later make it a normal entry when you’re sure about it.
Scoring
You get 10 points for each correct word completed. Revealing letters or words will cost you points. For each square you reveal, you lose 1 point, but you can still get the 10 points if you get the word right. You get 0 points if you reveal an entire word. The target time to complete this puzzle is 15 minutes. When you complete the puzzle, you will get a bonus of 15 points for every full minute under the target. There is no penalty for going over the time limit.
Practice Puzzlers are an entertaining way to challenge your clinical expertise. To make the experience more enjoyable, here's how to use the tools located in the bar above the crossword.
- Away from your computer? Click “Print” to download and print a blank puzzle or the answer key to the puzzle.
- With the "group tool" invite colleagues to play with you and share final scores.
- To keep it fun, you can “Reveal” or “Check” a letter, word, or the entire grid; change the “Settings” to suit your preferences; or use the “Pencil” toggle to enter the answer in gray and later make it a normal entry when you’re sure about it.
Scoring
You get 10 points for each correct word completed. Revealing letters or words will cost you points. For each square you reveal, you lose 1 point, but you can still get the 10 points if you get the word right. You get 0 points if you reveal an entire word. The target time to complete this puzzle is 15 minutes. When you complete the puzzle, you will get a bonus of 15 points for every full minute under the target. There is no penalty for going over the time limit.
Atopic Dermatitis
Hematopoietic cell transplant offers realistic cure in secondary AML
yielding significantly better survival outcomes, according to findings from an observational study.
Although secondary AML has been identified as an independent predictor of poor prognosis, it is not included in current risk classifications that provide the basis of deciding when to perform HCT.
Christer Nilsson, MD, of Karolinska Institute, Stockholm, and colleagues, used two nationwide Swedish registries – the Swedish AML Registry and the Swedish Cancer Registry – to characterize how often HCT is performed in these patients and to evaluate its impact in a real-world setting. The registries include all patients with AML diagnosed between 1997 and 2013.
Their findings are in Biology of Blood and Marrow Transplantation.
The analysis included 3,337 adult patients with AML who were intensively treated and did not have acute promyelocytic leukemia. More than three-quarters of the patients had de novo AML and the remainder had secondary AML that was either therapy related or developed after an antecedent myeloid disease. In total, 100 patients with secondary AML underwent HCT while in first complete remission.
In terms of crude survival at 5 years after diagnosis, patients with secondary AML who did not undergo HCT did very poorly. The survival rate was 0% in those with AML preceded by myeloproliferative neoplasm (MPN-AML), 2% in patients with AML preceded by myelodysplastic syndrome (MDS-AML), and 4% in patients with therapy-related AML (t-AML). In contrast, the 5-year overall survival in patients who underwent HCT at any time point or disease stage was 32% for patients with MPN-AML, 18% for patients with MDS-AML, and 25% for patients t-AML.
These crude survival figures suggest that “HCT is the sole realistic curable treatment option for [secondary] AML,” the researchers wrote.
The researchers also performed a propensity score matching analysis of HCT versus chemotherapy consolidation in patients with secondary AML who had been in first complete remission for more than 90 days. The model matched 45 patients who underwent HCT with 66 patients treated with chemotherapy consolidation. The projected 5-year overall survival was 48% in the HCT group, compared with 20% in the consolidation group (P = .01). Similarly, 5-year relapse-free survival was also higher in the HCT group, compared with the consolidation group (43% vs. 21%, P = .02).
“Ideally, the role of transplantation in [secondary] AML should be evaluated in a prospective randomized trial, minimizing the risk of any bias,” the researchers wrote. “However, such a trial is lacking and most likely will never be performed.”
The researchers concluded that HCT should be considered for all patients with secondary AML who are eligible and fit for transplantation.
The study was supported by the Swedish Cancer Foundation, Swedish Research Council, Stockholm County Council, Gothenberg Medical Society, and Assar Gabrielsson Foundation. The researchers reported having no conflicts of interest.
SOURCE: Nilson C et al. Biol Blood Marrow Tranplant. 2019;25:1770-8.
yielding significantly better survival outcomes, according to findings from an observational study.
Although secondary AML has been identified as an independent predictor of poor prognosis, it is not included in current risk classifications that provide the basis of deciding when to perform HCT.
Christer Nilsson, MD, of Karolinska Institute, Stockholm, and colleagues, used two nationwide Swedish registries – the Swedish AML Registry and the Swedish Cancer Registry – to characterize how often HCT is performed in these patients and to evaluate its impact in a real-world setting. The registries include all patients with AML diagnosed between 1997 and 2013.
Their findings are in Biology of Blood and Marrow Transplantation.
The analysis included 3,337 adult patients with AML who were intensively treated and did not have acute promyelocytic leukemia. More than three-quarters of the patients had de novo AML and the remainder had secondary AML that was either therapy related or developed after an antecedent myeloid disease. In total, 100 patients with secondary AML underwent HCT while in first complete remission.
In terms of crude survival at 5 years after diagnosis, patients with secondary AML who did not undergo HCT did very poorly. The survival rate was 0% in those with AML preceded by myeloproliferative neoplasm (MPN-AML), 2% in patients with AML preceded by myelodysplastic syndrome (MDS-AML), and 4% in patients with therapy-related AML (t-AML). In contrast, the 5-year overall survival in patients who underwent HCT at any time point or disease stage was 32% for patients with MPN-AML, 18% for patients with MDS-AML, and 25% for patients t-AML.
These crude survival figures suggest that “HCT is the sole realistic curable treatment option for [secondary] AML,” the researchers wrote.
The researchers also performed a propensity score matching analysis of HCT versus chemotherapy consolidation in patients with secondary AML who had been in first complete remission for more than 90 days. The model matched 45 patients who underwent HCT with 66 patients treated with chemotherapy consolidation. The projected 5-year overall survival was 48% in the HCT group, compared with 20% in the consolidation group (P = .01). Similarly, 5-year relapse-free survival was also higher in the HCT group, compared with the consolidation group (43% vs. 21%, P = .02).
“Ideally, the role of transplantation in [secondary] AML should be evaluated in a prospective randomized trial, minimizing the risk of any bias,” the researchers wrote. “However, such a trial is lacking and most likely will never be performed.”
The researchers concluded that HCT should be considered for all patients with secondary AML who are eligible and fit for transplantation.
The study was supported by the Swedish Cancer Foundation, Swedish Research Council, Stockholm County Council, Gothenberg Medical Society, and Assar Gabrielsson Foundation. The researchers reported having no conflicts of interest.
SOURCE: Nilson C et al. Biol Blood Marrow Tranplant. 2019;25:1770-8.
yielding significantly better survival outcomes, according to findings from an observational study.
Although secondary AML has been identified as an independent predictor of poor prognosis, it is not included in current risk classifications that provide the basis of deciding when to perform HCT.
Christer Nilsson, MD, of Karolinska Institute, Stockholm, and colleagues, used two nationwide Swedish registries – the Swedish AML Registry and the Swedish Cancer Registry – to characterize how often HCT is performed in these patients and to evaluate its impact in a real-world setting. The registries include all patients with AML diagnosed between 1997 and 2013.
Their findings are in Biology of Blood and Marrow Transplantation.
The analysis included 3,337 adult patients with AML who were intensively treated and did not have acute promyelocytic leukemia. More than three-quarters of the patients had de novo AML and the remainder had secondary AML that was either therapy related or developed after an antecedent myeloid disease. In total, 100 patients with secondary AML underwent HCT while in first complete remission.
In terms of crude survival at 5 years after diagnosis, patients with secondary AML who did not undergo HCT did very poorly. The survival rate was 0% in those with AML preceded by myeloproliferative neoplasm (MPN-AML), 2% in patients with AML preceded by myelodysplastic syndrome (MDS-AML), and 4% in patients with therapy-related AML (t-AML). In contrast, the 5-year overall survival in patients who underwent HCT at any time point or disease stage was 32% for patients with MPN-AML, 18% for patients with MDS-AML, and 25% for patients t-AML.
These crude survival figures suggest that “HCT is the sole realistic curable treatment option for [secondary] AML,” the researchers wrote.
The researchers also performed a propensity score matching analysis of HCT versus chemotherapy consolidation in patients with secondary AML who had been in first complete remission for more than 90 days. The model matched 45 patients who underwent HCT with 66 patients treated with chemotherapy consolidation. The projected 5-year overall survival was 48% in the HCT group, compared with 20% in the consolidation group (P = .01). Similarly, 5-year relapse-free survival was also higher in the HCT group, compared with the consolidation group (43% vs. 21%, P = .02).
“Ideally, the role of transplantation in [secondary] AML should be evaluated in a prospective randomized trial, minimizing the risk of any bias,” the researchers wrote. “However, such a trial is lacking and most likely will never be performed.”
The researchers concluded that HCT should be considered for all patients with secondary AML who are eligible and fit for transplantation.
The study was supported by the Swedish Cancer Foundation, Swedish Research Council, Stockholm County Council, Gothenberg Medical Society, and Assar Gabrielsson Foundation. The researchers reported having no conflicts of interest.
SOURCE: Nilson C et al. Biol Blood Marrow Tranplant. 2019;25:1770-8.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION