User login
Novel chemo/PARP inhibitor strategy promising for advanced pancreatic cancer
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
REPORTING FROM AACR 2019
LGBT youth: Affirmation in your practice
Join us Wednesday, April 3, 2019, at 6:00 pm EST, for a Twitter discussion on caring for LGBT youth. Two pediatricians that are passionate about the LGBT community and children will be joining the conversation: Dr. Gayathri Chelvakumar and Dr. Gerald T. Montano.
Questions will include:
- How do people become aware they are gay, lesbian, bisexual, or transgender?
- How can we address specific health concerns of LGBT youth?
- How can we make our practices a safe space for LGBT youth?
- What is gender dysphoria, and how is it treated?
LGBT Youth Consult, by Dr. Gayathri Chelvakumar
- A primer on sexuality and gender identity
- Creating safe spaces for LGBTQ youth, families in health care settings
- How we can support our LGBTQ patients
- New research on health-related behaviors of sexual minority youth
- Promoting mental well-being in LGBTQ youth
New to Twitter Chats? Steps to join the conversation are below.

Join us Wednesday, April 3, 2019, at 6:00 pm EST, for a Twitter discussion on caring for LGBT youth. Two pediatricians that are passionate about the LGBT community and children will be joining the conversation: Dr. Gayathri Chelvakumar and Dr. Gerald T. Montano.
Questions will include:
- How do people become aware they are gay, lesbian, bisexual, or transgender?
- How can we address specific health concerns of LGBT youth?
- How can we make our practices a safe space for LGBT youth?
- What is gender dysphoria, and how is it treated?
LGBT Youth Consult, by Dr. Gayathri Chelvakumar
- A primer on sexuality and gender identity
- Creating safe spaces for LGBTQ youth, families in health care settings
- How we can support our LGBTQ patients
- New research on health-related behaviors of sexual minority youth
- Promoting mental well-being in LGBTQ youth
New to Twitter Chats? Steps to join the conversation are below.

Join us Wednesday, April 3, 2019, at 6:00 pm EST, for a Twitter discussion on caring for LGBT youth. Two pediatricians that are passionate about the LGBT community and children will be joining the conversation: Dr. Gayathri Chelvakumar and Dr. Gerald T. Montano.
Questions will include:
- How do people become aware they are gay, lesbian, bisexual, or transgender?
- How can we address specific health concerns of LGBT youth?
- How can we make our practices a safe space for LGBT youth?
- What is gender dysphoria, and how is it treated?
LGBT Youth Consult, by Dr. Gayathri Chelvakumar
- A primer on sexuality and gender identity
- Creating safe spaces for LGBTQ youth, families in health care settings
- How we can support our LGBTQ patients
- New research on health-related behaviors of sexual minority youth
- Promoting mental well-being in LGBTQ youth
New to Twitter Chats? Steps to join the conversation are below.

UNITY-NHL: Interim findings show activity, tolerability of umbralisib for R/R MZL
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
REPORTING FROM AACR 2019
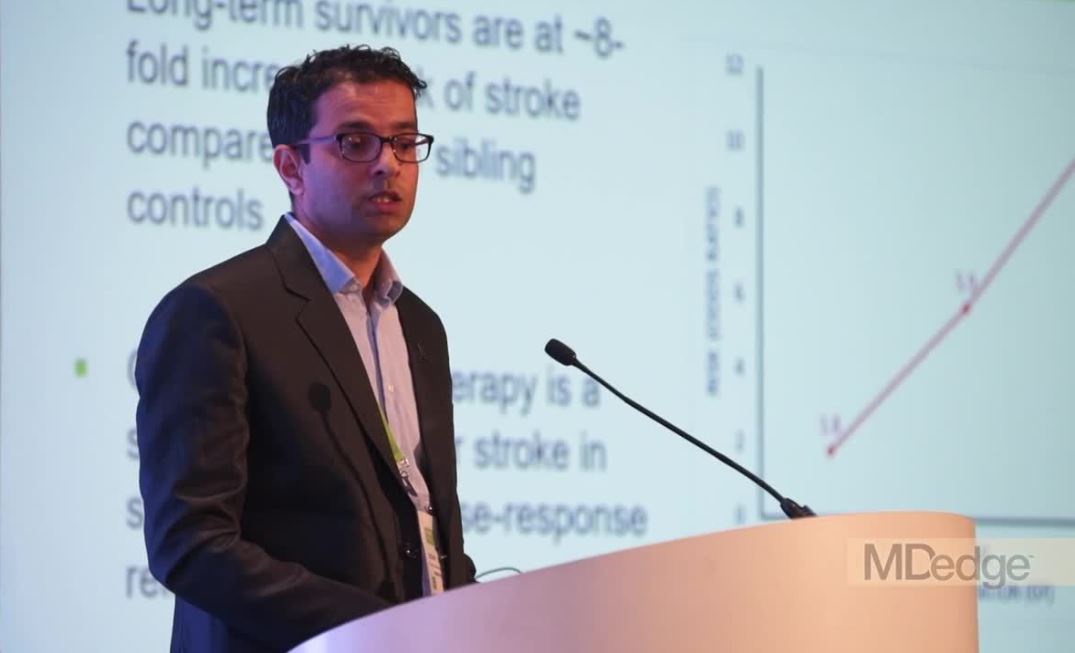
Genetic variant increases stroke risk in childhood cancer survivors
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
REPORTING FROM AACR 2019
2019 Update on prenatal exome sequencing
Prenatal diagnosis of genetic anomalies is important for diagnosing lethal genetic conditions before birth. It can provide information for parents regarding pregnancy options and allow for recurrence risk counseling and the potential use of preimplantation genetic testing in the next pregnancy. For decades, a karyotype was used to analyze amniocentesis and chorionic villus sampling specimens; in recent years, chromosomal microarray analysis provides more information about significant chromosomal abnormalities, including microdeletions and microduplications. However, microarrays also have limitations, as they do not identify base pair changes associated with single-gene disorders.
The advent of next-generation sequencing has substantially reduced the cost of DNA sequencing. Whole genome sequencing (WGS) can sequence the entire genome— both the coding (exonic) and noncoding (intronic) regions—while exome sequencing analyzes only the protein-coding exons, which make up 1% to 2% of the genome and about 85% of the protein-coding genes associated with known human disease. Exome sequencing increasingly is used in cases of suspected genetic disorders when other tests have been unrevealing.
In this Update, we review recent reports of prenatal exome sequencing, including studies exploring the yield in fetuses with structural anomalies; the importance of prenatal phenotyping; the perspectives of parents and health care professionals who were involved in prenatal exome sequencing studies; and a summary of a joint position statement from 3 societies regarding prenatal sequencing.
Prenatal whole exome sequencing has potential utility, with some limitations
Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
Exome sequencing has been shown to identify an underlying genetic cause in 25% to 30% of children with an undiagnosed suspected genetic disorder. Two studies recently published in the Lancet sought to determine the incremental diagnostic yield of prenatal whole exome sequencing (WES) in the setting of fetal structural anomalies when karyotype and microarray results were normal.
Continue to: Details of the studies...
Details of the studies
In a prospective cohort study by Petrovski and colleagues, DNA samples from 234 fetuses with a structural anomaly (identified on ultrasonography) and both parents (parent-fetus "trios") were used for analysis. WES identified diagnostic genetic variants in 24 trios (10%). An additional 46 (20%) had variants that indicated pathogenicity but without sufficient evidence to be considered diagnostic.
The anomalies with the highest frequency of a genetic diagnosis were lymphatic, 24%; skeletal, 24%; central nervous system, 22%; and renal, 16%; while cardiac anomalies had the lowest yield at 5%.
In another prospective cohort study, known as the Prenatal Assessment of Genomes and Exomes (PAGE), Lord and colleagues sequenced DNA samples from 610 parent-fetus trios, but they restricted sequencing to a predefined list of 1,628 genes. Diagnostic genetic variants were identified in 52 fetuses (8.5%), while 24 (3.9%) had a variant of uncertain significance that was thought to be of potential clinical usefulness.
Fetuses with multiple anomalies had the highest genetic yield (15.4%), followed by skeletal (15.4%) and cardiac anomalies (11.1%), with the lowest yield in fetuses with isolated increased nuchal translucency (3.2%).
Diagnostic yield is high, but prenatal utility is limited
Both studies showed a clinically significant diagnostic yield of 8% to 10% for prenatal exome sequencing in cases of fetal structural anomalies with normal karyotype and microarray testing. While this yield demonstrates the utility of prenatal exome sequencing, it is significantly lower than what has been reported in postnatal studies. One of the reasons for this is the inherent limitation of prenatal phenotyping (discussed below).
The cohort studies by both Petrovski and Lord and their colleagues show the feasibility and potential diagnostic utility of exome sequencing in cases of fetal structural anomalies where karyotype and microarray are not diagnostic. However, the lower yield found in these studies compared with those in postnatal studies highlights in part the limitations of prenatal phenotyping.
The importance of prenatal phenotyping
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In postnatal exome sequencing, the physical exam, imaging findings, and laboratory results are components of the phenotype that are used to interpret the sequencing data. Prenatal phenotyping, however, is limited to the use of fetal ultrasonography and, occasionally, the addition of magnetic resonance imaging. Prenatal phenotyping is without the benefit of an exam to detect more subtle anomalies or functional status, such as developmental delay, seizures, or failure to thrive.
When a structural anomaly is identified on prenatal ultrasonography, it is especially important that detailed imaging be undertaken to detect other anomalies, including more subtle facial features and dysmorphology.
Value of reanalyzing exome sequencing data
Aarabi and colleagues conducted a retrospective study of 20 fetuses with structural anomalies and normal karyotype and microarray. They performed trio exome sequencing first using information available only prenatally and then conducted a reanalysis using information available after delivery.
With prenatal phenotyping only, the investigators identified no pathogenic, or likely pathogenic, variants. On reanalysis of combined prenatal and postnatal findings, however, they identified pathogenic variants in 20% of cases.
Significance of the findings
This study highlights both the importance of a careful, detailed fetal ultrasonography study and the possible additional benefit of a postnatal examination (such as an autopsy) in order to yield improved results. In addition, the authors noted that the development of a prenatal phenotype-genotype database would significantly help exome sequencing interpretation in the prenatal setting.
Careful prenatal ultrasonography is crucial to help in the interpretation of prenatal exome sequencing. Patients who have undergone prenatal clinical exome sequencing may benefit from reanalysis of the genetic data based on detailed postnatal findings.
Social impact of WES: Parent and provider perspectives
Wou K, Weitz T, McCormack C, et al. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat Diagn. 2018;38:801-811.
Horn R, Parker M. Health professionals' and researchers' perspectives on prenatal whole genome and exome sequencing: 'We can't shut the door now, the genie's out, we need to refine it.' PLoS One. 2018;13:e0204158.
As health care providers enter a new era of prenatal genetic testing with exome sequencing, it is crucial to the path forward that we obtain perspectives from the parents and providers who participated in these studies. Notably, in both of the previously discussed Lancet reports, the authors interviewed the participants to discuss the challenges involved and identify strategies for improving future testing.
Continue to: What parents want...
What parents want
To ascertain the perceptions of couples who underwent prenatal WES, Wou and colleagues conducted semi-structured interviews with participants from the Fetal Sequencing Study regarding their experience. They interviewed 29 parents from 17 pregnancies, including a mix of those who had pathogenic prenatal results, terminated prior to receiving the results, and had normal results.
Expressed feelings and desires. Parents recalled feelings of anxiety and stress around the time of diagnosis and the need for help with coping while awaiting results. The majority of parents reported that they would like to be told about uncertain results, but that desire decreased as the certainty of results decreased.
Parents were overall satisfied with the prenatal genetic testing experience, but they added that they would have liked to receive written materials beforehand and a written report of the test results (including negative cases). They also would like to have connected with other families with similar experiences, to have received results sooner, and to have an in-person meeting after telephone disclosure of the results.
Health professionals articulate complexity of prenatal genomics
In a qualitative interview study to explore critical issues involved in the clinical practice use of prenatal genomics, Horn and Parker conducted interviews with 20 health care professionals who were involved in the previously described PAGE trial. Patient recruiters, midwives, genetic counselors, research assistants, and laboratory staff were included.
Interviewees cited numerous challenges involved in their day-to-day work with prenatal whole genome and exome sequencing, including:
- the complexity of achieving valid parental consent at a time of vulnerability
- management of parent expectations
- transmitting and comprehending complex information
- the usefulness of information
- the difficulty of a long turnaround time for study results.
All the interviewees agreed that prenatal exome sequencing studies contribute to knowledge generation and the advancement of technology.
The authors concluded that an appropriate next step would be the development of appropriate guidelines for good ethical practice that address the concerns encountered in genomics clinical practice.
The prenatal experience can be overwhelming for parents. Pretest and posttest counseling on genetic testing and results are of the utmost importance, as is finding ways to help support parents through this anxious time.
Societies offer guidance on using genome and exome sequencing
International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn. 2018;38:6-9.
In response to the rapid integration of exome sequencing for genetic diagnosis, several professional societies—the International Society for Prenatal Diagnosis, Society for Maternal Fetal Medicine, and Perinatal Quality Foundation—issued a joint statement addressing the clinical use of prenatal diagnostic genome wide sequencing, including exome sequencing.
Continue to: Guidance at a glance...
Guidance at a glance
The societies' recommendations are summarized as follows:
- Exome sequencing is best done as a trio analysis, with fetal and both parental samples sequenced and analyzed together.
- Extensive pretest education, counseling, and informed consent, as well as posttest counseling, are essential. This should include:
—the types of results to be conveyed (variants that are pathogenic, likely pathogenic, of uncertain significance, likely benign, and benign)
—the possibility that results will not be obtained or may not be available before the birth of the fetus
—realistic expectations regarding the likelihood that a significant result will be obtained
—the timeframe to results
—the option to include or exclude in the results incidental or secondary findings (such as an unexpected childhood disorder, cancer susceptibility genes, adult-onset disorders)
—the possibility of uncovering nonpaternity or consanguinity
—the potential reanalysis of results over time
—how data are stored, who has access, and for what purpose.
- Fetal sequencing may be beneficial in the following scenarios:
—multiple fetal anomalies or a single major anomaly suggestive of a genetic disorder, when the microarray is negative
—no microarray result is available, but the fetus exhibits a pattern of anomalies strongly suggestive of a single-gene disorder
—a prior undiagnosed fetus (or child) with anomalies suggestive of a genetic etiology, and with similar anomalies in the current pregnancy, with normal karyotype or microarray. Providers also can consider sequencing samples from both parents prior to preimplantation genetic testing to check for shared carrier status for autosomal recessive mutations, although obtaining exome sequencing from the prior affected fetus (or child) is ideal.
—history of recurrent stillbirths of unknown etiology, with a recurrent pattern of anomalies in the current pregnancy, with normal karyotype or microarray.
- Interpretation of results should be done using a multidisciplinary team-based approach, including clinical scientists, geneticists, genetic counselors, and experts in prenatal diagnosis.
- Where possible and after informed consent, reanalysis of results should be undertaken if a future pregnancy is planned or ongoing, and a significant amount of time has elapsed since the time the result was last reported.
- Parents should be given a written report of test results.
Three professional societies have convened to issue consensus opinion that includes current indications for prenatal exome sequencing and important factors to include in the consent process. We follow these guidelines in our own practice.
Summary
Exome sequencing is increasingly becoming mainstream in postnatal genetic testing, and it is emerging as the newest diagnostic frontier in prenatal genetic testing. However, there are limitations to prenatal exome sequencing, including issues with consent at a vulnerable time for parents, limited information available regarding the phenotype, and results that may not be available before the birth of a fetus. Providers should be familiar with the indications for testing, the possible results, the limitations of prenatal phenotyping, and the implications for future pregnancies.
Prenatal diagnosis of genetic anomalies is important for diagnosing lethal genetic conditions before birth. It can provide information for parents regarding pregnancy options and allow for recurrence risk counseling and the potential use of preimplantation genetic testing in the next pregnancy. For decades, a karyotype was used to analyze amniocentesis and chorionic villus sampling specimens; in recent years, chromosomal microarray analysis provides more information about significant chromosomal abnormalities, including microdeletions and microduplications. However, microarrays also have limitations, as they do not identify base pair changes associated with single-gene disorders.
The advent of next-generation sequencing has substantially reduced the cost of DNA sequencing. Whole genome sequencing (WGS) can sequence the entire genome— both the coding (exonic) and noncoding (intronic) regions—while exome sequencing analyzes only the protein-coding exons, which make up 1% to 2% of the genome and about 85% of the protein-coding genes associated with known human disease. Exome sequencing increasingly is used in cases of suspected genetic disorders when other tests have been unrevealing.
In this Update, we review recent reports of prenatal exome sequencing, including studies exploring the yield in fetuses with structural anomalies; the importance of prenatal phenotyping; the perspectives of parents and health care professionals who were involved in prenatal exome sequencing studies; and a summary of a joint position statement from 3 societies regarding prenatal sequencing.
Prenatal whole exome sequencing has potential utility, with some limitations
Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
Exome sequencing has been shown to identify an underlying genetic cause in 25% to 30% of children with an undiagnosed suspected genetic disorder. Two studies recently published in the Lancet sought to determine the incremental diagnostic yield of prenatal whole exome sequencing (WES) in the setting of fetal structural anomalies when karyotype and microarray results were normal.
Continue to: Details of the studies...
Details of the studies
In a prospective cohort study by Petrovski and colleagues, DNA samples from 234 fetuses with a structural anomaly (identified on ultrasonography) and both parents (parent-fetus "trios") were used for analysis. WES identified diagnostic genetic variants in 24 trios (10%). An additional 46 (20%) had variants that indicated pathogenicity but without sufficient evidence to be considered diagnostic.
The anomalies with the highest frequency of a genetic diagnosis were lymphatic, 24%; skeletal, 24%; central nervous system, 22%; and renal, 16%; while cardiac anomalies had the lowest yield at 5%.
In another prospective cohort study, known as the Prenatal Assessment of Genomes and Exomes (PAGE), Lord and colleagues sequenced DNA samples from 610 parent-fetus trios, but they restricted sequencing to a predefined list of 1,628 genes. Diagnostic genetic variants were identified in 52 fetuses (8.5%), while 24 (3.9%) had a variant of uncertain significance that was thought to be of potential clinical usefulness.
Fetuses with multiple anomalies had the highest genetic yield (15.4%), followed by skeletal (15.4%) and cardiac anomalies (11.1%), with the lowest yield in fetuses with isolated increased nuchal translucency (3.2%).
Diagnostic yield is high, but prenatal utility is limited
Both studies showed a clinically significant diagnostic yield of 8% to 10% for prenatal exome sequencing in cases of fetal structural anomalies with normal karyotype and microarray testing. While this yield demonstrates the utility of prenatal exome sequencing, it is significantly lower than what has been reported in postnatal studies. One of the reasons for this is the inherent limitation of prenatal phenotyping (discussed below).
The cohort studies by both Petrovski and Lord and their colleagues show the feasibility and potential diagnostic utility of exome sequencing in cases of fetal structural anomalies where karyotype and microarray are not diagnostic. However, the lower yield found in these studies compared with those in postnatal studies highlights in part the limitations of prenatal phenotyping.
The importance of prenatal phenotyping
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In postnatal exome sequencing, the physical exam, imaging findings, and laboratory results are components of the phenotype that are used to interpret the sequencing data. Prenatal phenotyping, however, is limited to the use of fetal ultrasonography and, occasionally, the addition of magnetic resonance imaging. Prenatal phenotyping is without the benefit of an exam to detect more subtle anomalies or functional status, such as developmental delay, seizures, or failure to thrive.
When a structural anomaly is identified on prenatal ultrasonography, it is especially important that detailed imaging be undertaken to detect other anomalies, including more subtle facial features and dysmorphology.
Value of reanalyzing exome sequencing data
Aarabi and colleagues conducted a retrospective study of 20 fetuses with structural anomalies and normal karyotype and microarray. They performed trio exome sequencing first using information available only prenatally and then conducted a reanalysis using information available after delivery.
With prenatal phenotyping only, the investigators identified no pathogenic, or likely pathogenic, variants. On reanalysis of combined prenatal and postnatal findings, however, they identified pathogenic variants in 20% of cases.
Significance of the findings
This study highlights both the importance of a careful, detailed fetal ultrasonography study and the possible additional benefit of a postnatal examination (such as an autopsy) in order to yield improved results. In addition, the authors noted that the development of a prenatal phenotype-genotype database would significantly help exome sequencing interpretation in the prenatal setting.
Careful prenatal ultrasonography is crucial to help in the interpretation of prenatal exome sequencing. Patients who have undergone prenatal clinical exome sequencing may benefit from reanalysis of the genetic data based on detailed postnatal findings.
Social impact of WES: Parent and provider perspectives
Wou K, Weitz T, McCormack C, et al. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat Diagn. 2018;38:801-811.
Horn R, Parker M. Health professionals' and researchers' perspectives on prenatal whole genome and exome sequencing: 'We can't shut the door now, the genie's out, we need to refine it.' PLoS One. 2018;13:e0204158.
As health care providers enter a new era of prenatal genetic testing with exome sequencing, it is crucial to the path forward that we obtain perspectives from the parents and providers who participated in these studies. Notably, in both of the previously discussed Lancet reports, the authors interviewed the participants to discuss the challenges involved and identify strategies for improving future testing.
Continue to: What parents want...
What parents want
To ascertain the perceptions of couples who underwent prenatal WES, Wou and colleagues conducted semi-structured interviews with participants from the Fetal Sequencing Study regarding their experience. They interviewed 29 parents from 17 pregnancies, including a mix of those who had pathogenic prenatal results, terminated prior to receiving the results, and had normal results.
Expressed feelings and desires. Parents recalled feelings of anxiety and stress around the time of diagnosis and the need for help with coping while awaiting results. The majority of parents reported that they would like to be told about uncertain results, but that desire decreased as the certainty of results decreased.
Parents were overall satisfied with the prenatal genetic testing experience, but they added that they would have liked to receive written materials beforehand and a written report of the test results (including negative cases). They also would like to have connected with other families with similar experiences, to have received results sooner, and to have an in-person meeting after telephone disclosure of the results.
Health professionals articulate complexity of prenatal genomics
In a qualitative interview study to explore critical issues involved in the clinical practice use of prenatal genomics, Horn and Parker conducted interviews with 20 health care professionals who were involved in the previously described PAGE trial. Patient recruiters, midwives, genetic counselors, research assistants, and laboratory staff were included.
Interviewees cited numerous challenges involved in their day-to-day work with prenatal whole genome and exome sequencing, including:
- the complexity of achieving valid parental consent at a time of vulnerability
- management of parent expectations
- transmitting and comprehending complex information
- the usefulness of information
- the difficulty of a long turnaround time for study results.
All the interviewees agreed that prenatal exome sequencing studies contribute to knowledge generation and the advancement of technology.
The authors concluded that an appropriate next step would be the development of appropriate guidelines for good ethical practice that address the concerns encountered in genomics clinical practice.
The prenatal experience can be overwhelming for parents. Pretest and posttest counseling on genetic testing and results are of the utmost importance, as is finding ways to help support parents through this anxious time.
Societies offer guidance on using genome and exome sequencing
International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn. 2018;38:6-9.
In response to the rapid integration of exome sequencing for genetic diagnosis, several professional societies—the International Society for Prenatal Diagnosis, Society for Maternal Fetal Medicine, and Perinatal Quality Foundation—issued a joint statement addressing the clinical use of prenatal diagnostic genome wide sequencing, including exome sequencing.
Continue to: Guidance at a glance...
Guidance at a glance
The societies' recommendations are summarized as follows:
- Exome sequencing is best done as a trio analysis, with fetal and both parental samples sequenced and analyzed together.
- Extensive pretest education, counseling, and informed consent, as well as posttest counseling, are essential. This should include:
—the types of results to be conveyed (variants that are pathogenic, likely pathogenic, of uncertain significance, likely benign, and benign)
—the possibility that results will not be obtained or may not be available before the birth of the fetus
—realistic expectations regarding the likelihood that a significant result will be obtained
—the timeframe to results
—the option to include or exclude in the results incidental or secondary findings (such as an unexpected childhood disorder, cancer susceptibility genes, adult-onset disorders)
—the possibility of uncovering nonpaternity or consanguinity
—the potential reanalysis of results over time
—how data are stored, who has access, and for what purpose.
- Fetal sequencing may be beneficial in the following scenarios:
—multiple fetal anomalies or a single major anomaly suggestive of a genetic disorder, when the microarray is negative
—no microarray result is available, but the fetus exhibits a pattern of anomalies strongly suggestive of a single-gene disorder
—a prior undiagnosed fetus (or child) with anomalies suggestive of a genetic etiology, and with similar anomalies in the current pregnancy, with normal karyotype or microarray. Providers also can consider sequencing samples from both parents prior to preimplantation genetic testing to check for shared carrier status for autosomal recessive mutations, although obtaining exome sequencing from the prior affected fetus (or child) is ideal.
—history of recurrent stillbirths of unknown etiology, with a recurrent pattern of anomalies in the current pregnancy, with normal karyotype or microarray.
- Interpretation of results should be done using a multidisciplinary team-based approach, including clinical scientists, geneticists, genetic counselors, and experts in prenatal diagnosis.
- Where possible and after informed consent, reanalysis of results should be undertaken if a future pregnancy is planned or ongoing, and a significant amount of time has elapsed since the time the result was last reported.
- Parents should be given a written report of test results.
Three professional societies have convened to issue consensus opinion that includes current indications for prenatal exome sequencing and important factors to include in the consent process. We follow these guidelines in our own practice.
Summary
Exome sequencing is increasingly becoming mainstream in postnatal genetic testing, and it is emerging as the newest diagnostic frontier in prenatal genetic testing. However, there are limitations to prenatal exome sequencing, including issues with consent at a vulnerable time for parents, limited information available regarding the phenotype, and results that may not be available before the birth of a fetus. Providers should be familiar with the indications for testing, the possible results, the limitations of prenatal phenotyping, and the implications for future pregnancies.
Prenatal diagnosis of genetic anomalies is important for diagnosing lethal genetic conditions before birth. It can provide information for parents regarding pregnancy options and allow for recurrence risk counseling and the potential use of preimplantation genetic testing in the next pregnancy. For decades, a karyotype was used to analyze amniocentesis and chorionic villus sampling specimens; in recent years, chromosomal microarray analysis provides more information about significant chromosomal abnormalities, including microdeletions and microduplications. However, microarrays also have limitations, as they do not identify base pair changes associated with single-gene disorders.
The advent of next-generation sequencing has substantially reduced the cost of DNA sequencing. Whole genome sequencing (WGS) can sequence the entire genome— both the coding (exonic) and noncoding (intronic) regions—while exome sequencing analyzes only the protein-coding exons, which make up 1% to 2% of the genome and about 85% of the protein-coding genes associated with known human disease. Exome sequencing increasingly is used in cases of suspected genetic disorders when other tests have been unrevealing.
In this Update, we review recent reports of prenatal exome sequencing, including studies exploring the yield in fetuses with structural anomalies; the importance of prenatal phenotyping; the perspectives of parents and health care professionals who were involved in prenatal exome sequencing studies; and a summary of a joint position statement from 3 societies regarding prenatal sequencing.
Prenatal whole exome sequencing has potential utility, with some limitations
Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
Exome sequencing has been shown to identify an underlying genetic cause in 25% to 30% of children with an undiagnosed suspected genetic disorder. Two studies recently published in the Lancet sought to determine the incremental diagnostic yield of prenatal whole exome sequencing (WES) in the setting of fetal structural anomalies when karyotype and microarray results were normal.
Continue to: Details of the studies...
Details of the studies
In a prospective cohort study by Petrovski and colleagues, DNA samples from 234 fetuses with a structural anomaly (identified on ultrasonography) and both parents (parent-fetus "trios") were used for analysis. WES identified diagnostic genetic variants in 24 trios (10%). An additional 46 (20%) had variants that indicated pathogenicity but without sufficient evidence to be considered diagnostic.
The anomalies with the highest frequency of a genetic diagnosis were lymphatic, 24%; skeletal, 24%; central nervous system, 22%; and renal, 16%; while cardiac anomalies had the lowest yield at 5%.
In another prospective cohort study, known as the Prenatal Assessment of Genomes and Exomes (PAGE), Lord and colleagues sequenced DNA samples from 610 parent-fetus trios, but they restricted sequencing to a predefined list of 1,628 genes. Diagnostic genetic variants were identified in 52 fetuses (8.5%), while 24 (3.9%) had a variant of uncertain significance that was thought to be of potential clinical usefulness.
Fetuses with multiple anomalies had the highest genetic yield (15.4%), followed by skeletal (15.4%) and cardiac anomalies (11.1%), with the lowest yield in fetuses with isolated increased nuchal translucency (3.2%).
Diagnostic yield is high, but prenatal utility is limited
Both studies showed a clinically significant diagnostic yield of 8% to 10% for prenatal exome sequencing in cases of fetal structural anomalies with normal karyotype and microarray testing. While this yield demonstrates the utility of prenatal exome sequencing, it is significantly lower than what has been reported in postnatal studies. One of the reasons for this is the inherent limitation of prenatal phenotyping (discussed below).
The cohort studies by both Petrovski and Lord and their colleagues show the feasibility and potential diagnostic utility of exome sequencing in cases of fetal structural anomalies where karyotype and microarray are not diagnostic. However, the lower yield found in these studies compared with those in postnatal studies highlights in part the limitations of prenatal phenotyping.
The importance of prenatal phenotyping
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In postnatal exome sequencing, the physical exam, imaging findings, and laboratory results are components of the phenotype that are used to interpret the sequencing data. Prenatal phenotyping, however, is limited to the use of fetal ultrasonography and, occasionally, the addition of magnetic resonance imaging. Prenatal phenotyping is without the benefit of an exam to detect more subtle anomalies or functional status, such as developmental delay, seizures, or failure to thrive.
When a structural anomaly is identified on prenatal ultrasonography, it is especially important that detailed imaging be undertaken to detect other anomalies, including more subtle facial features and dysmorphology.
Value of reanalyzing exome sequencing data
Aarabi and colleagues conducted a retrospective study of 20 fetuses with structural anomalies and normal karyotype and microarray. They performed trio exome sequencing first using information available only prenatally and then conducted a reanalysis using information available after delivery.
With prenatal phenotyping only, the investigators identified no pathogenic, or likely pathogenic, variants. On reanalysis of combined prenatal and postnatal findings, however, they identified pathogenic variants in 20% of cases.
Significance of the findings
This study highlights both the importance of a careful, detailed fetal ultrasonography study and the possible additional benefit of a postnatal examination (such as an autopsy) in order to yield improved results. In addition, the authors noted that the development of a prenatal phenotype-genotype database would significantly help exome sequencing interpretation in the prenatal setting.
Careful prenatal ultrasonography is crucial to help in the interpretation of prenatal exome sequencing. Patients who have undergone prenatal clinical exome sequencing may benefit from reanalysis of the genetic data based on detailed postnatal findings.
Social impact of WES: Parent and provider perspectives
Wou K, Weitz T, McCormack C, et al. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat Diagn. 2018;38:801-811.
Horn R, Parker M. Health professionals' and researchers' perspectives on prenatal whole genome and exome sequencing: 'We can't shut the door now, the genie's out, we need to refine it.' PLoS One. 2018;13:e0204158.
As health care providers enter a new era of prenatal genetic testing with exome sequencing, it is crucial to the path forward that we obtain perspectives from the parents and providers who participated in these studies. Notably, in both of the previously discussed Lancet reports, the authors interviewed the participants to discuss the challenges involved and identify strategies for improving future testing.
Continue to: What parents want...
What parents want
To ascertain the perceptions of couples who underwent prenatal WES, Wou and colleagues conducted semi-structured interviews with participants from the Fetal Sequencing Study regarding their experience. They interviewed 29 parents from 17 pregnancies, including a mix of those who had pathogenic prenatal results, terminated prior to receiving the results, and had normal results.
Expressed feelings and desires. Parents recalled feelings of anxiety and stress around the time of diagnosis and the need for help with coping while awaiting results. The majority of parents reported that they would like to be told about uncertain results, but that desire decreased as the certainty of results decreased.
Parents were overall satisfied with the prenatal genetic testing experience, but they added that they would have liked to receive written materials beforehand and a written report of the test results (including negative cases). They also would like to have connected with other families with similar experiences, to have received results sooner, and to have an in-person meeting after telephone disclosure of the results.
Health professionals articulate complexity of prenatal genomics
In a qualitative interview study to explore critical issues involved in the clinical practice use of prenatal genomics, Horn and Parker conducted interviews with 20 health care professionals who were involved in the previously described PAGE trial. Patient recruiters, midwives, genetic counselors, research assistants, and laboratory staff were included.
Interviewees cited numerous challenges involved in their day-to-day work with prenatal whole genome and exome sequencing, including:
- the complexity of achieving valid parental consent at a time of vulnerability
- management of parent expectations
- transmitting and comprehending complex information
- the usefulness of information
- the difficulty of a long turnaround time for study results.
All the interviewees agreed that prenatal exome sequencing studies contribute to knowledge generation and the advancement of technology.
The authors concluded that an appropriate next step would be the development of appropriate guidelines for good ethical practice that address the concerns encountered in genomics clinical practice.
The prenatal experience can be overwhelming for parents. Pretest and posttest counseling on genetic testing and results are of the utmost importance, as is finding ways to help support parents through this anxious time.
Societies offer guidance on using genome and exome sequencing
International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn. 2018;38:6-9.
In response to the rapid integration of exome sequencing for genetic diagnosis, several professional societies—the International Society for Prenatal Diagnosis, Society for Maternal Fetal Medicine, and Perinatal Quality Foundation—issued a joint statement addressing the clinical use of prenatal diagnostic genome wide sequencing, including exome sequencing.
Continue to: Guidance at a glance...
Guidance at a glance
The societies' recommendations are summarized as follows:
- Exome sequencing is best done as a trio analysis, with fetal and both parental samples sequenced and analyzed together.
- Extensive pretest education, counseling, and informed consent, as well as posttest counseling, are essential. This should include:
—the types of results to be conveyed (variants that are pathogenic, likely pathogenic, of uncertain significance, likely benign, and benign)
—the possibility that results will not be obtained or may not be available before the birth of the fetus
—realistic expectations regarding the likelihood that a significant result will be obtained
—the timeframe to results
—the option to include or exclude in the results incidental or secondary findings (such as an unexpected childhood disorder, cancer susceptibility genes, adult-onset disorders)
—the possibility of uncovering nonpaternity or consanguinity
—the potential reanalysis of results over time
—how data are stored, who has access, and for what purpose.
- Fetal sequencing may be beneficial in the following scenarios:
—multiple fetal anomalies or a single major anomaly suggestive of a genetic disorder, when the microarray is negative
—no microarray result is available, but the fetus exhibits a pattern of anomalies strongly suggestive of a single-gene disorder
—a prior undiagnosed fetus (or child) with anomalies suggestive of a genetic etiology, and with similar anomalies in the current pregnancy, with normal karyotype or microarray. Providers also can consider sequencing samples from both parents prior to preimplantation genetic testing to check for shared carrier status for autosomal recessive mutations, although obtaining exome sequencing from the prior affected fetus (or child) is ideal.
—history of recurrent stillbirths of unknown etiology, with a recurrent pattern of anomalies in the current pregnancy, with normal karyotype or microarray.
- Interpretation of results should be done using a multidisciplinary team-based approach, including clinical scientists, geneticists, genetic counselors, and experts in prenatal diagnosis.
- Where possible and after informed consent, reanalysis of results should be undertaken if a future pregnancy is planned or ongoing, and a significant amount of time has elapsed since the time the result was last reported.
- Parents should be given a written report of test results.
Three professional societies have convened to issue consensus opinion that includes current indications for prenatal exome sequencing and important factors to include in the consent process. We follow these guidelines in our own practice.
Summary
Exome sequencing is increasingly becoming mainstream in postnatal genetic testing, and it is emerging as the newest diagnostic frontier in prenatal genetic testing. However, there are limitations to prenatal exome sequencing, including issues with consent at a vulnerable time for parents, limited information available regarding the phenotype, and results that may not be available before the birth of a fetus. Providers should be familiar with the indications for testing, the possible results, the limitations of prenatal phenotyping, and the implications for future pregnancies.
What is the association of menopausal HT use and risk of Alzheimer disease?
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
Amyloid brain imaging changed clinical management in 60% of MCI and dementia patients
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
FROM THE JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION
Pre-exposure prophylaxis for the prevention of HIV infection: Ready for prime time
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
Prevention and Treatment of Traveler’s Diarrhea
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Combo could replace standard conditioning regimen for myeloma
Busulfan plus melphalan could replace melphalan alone as the standard conditioning regimen for multiple myeloma patients undergoing autologous hematopoietic cell transplant, according to researchers.
In a phase 3 trial, patients who received conditioning with busulfan plus melphalan had significantly longer median progression-free survival compared with patients who received melphalan alone – 64.7 months versus 43.5 months (P = .022).
However, there was no overall survival advantage with busulfan plus melphalan, and adverse events were more common with this regimen, reported Qaiser Bashir, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues. The report is in The Lancet Haematology.
To their knowledge, the researchers wrote, this was the first randomized, phase 3 trial showing a significant progression-free survival benefit for busulfan plus melphalan versus the standard of care of melphalan 200 mg/m2 pretransplantation conditioning. “These data suggest that busulfan plus melphalan conditioning can serve as a useful platform for further improvement of transplant outcomes in patients with myeloma.”
The current trial (NCT01413178) enrolled 205 multiple myeloma patients who were eligible for transplant. They were randomized to conditioning with melphalan alone or busulfan plus melphalan.
In all, 98 patients received melphalan alone, given at 200 mg/m2 on day –2. The 104 patients who received busulfan plus melphalan started with a test dose of busulfan at 32 mg/m2, which was followed by pharmacokinetically adjusted doses on days –7, –6, –5, and –4 to achieve a target daily area under the curve of 5,000 mmol/minute. These patients received melphalan at 70 mg/m2 per day on days –2 and –1.
The median age at transplant was 57.9 years (range, 31.7-70.9 years) in the busulfan group and 57.6 years (range, 34.3-70.6 years) in the melphalan-alone group.
The most common induction regimen used was bortezomib, lenalidomide, and dexamethasone, which was given to 60% of the busulfan group and 57% of the melphalan-alone group.
Most patients responded to induction – 96% of patients in the busulfan group and 94% of those in the melphalan-alone group.
There was no treatment-related mortality within 100 days of transplant.
At 90 days after transplant, the response rate was 98% in the busulfan group and 97% in the melphalan-alone group. The rate of complete remission/stringent complete remission was 27% and 34%, respectively.
Most patients received posttransplant maintenance. The most common maintenance regimen consisted of lenalidomide monotherapy, which was given to 57% of the busulfan group and 58% of the melphalan-alone group.
Patients continued maintenance until disease progression or unacceptable toxicity. The median duration of maintenance was 16.0 months in the busulfan group and 10.1 months in the melphalan-alone group.
The median follow-up was 22.6 months in the busulfan group and 20.2 months in the melphalan-alone group.
Progression-free survival was superior in the busulfan group. Median progression-free survival was 64.7 months in the busulfan group and 43.5 months in the melphalan-alone group (hazard ratio = 0.53; P = .022). The 3-year progression-free survival rate was 72% and 50%, respectively.
The median overall survival was not reached in either group. The 3-year overall survival rate was 91% in the busulfan group and 89% in the melphalan-alone group.
There were 10 deaths in the busulfan group, 7 due to progression and 3 due to infection. All 7 deaths in the melphalan-alone group were due to progression.
Grade 3-4 nonhematologic toxicity was more common in the busulfan group, occurring in 84% of that group and 33% of the melphalan-alone group (P less than .0001).
Grade 2-3 mucositis occurred in 74% of the busulfan group and 14% of the melphalan-alone group. There were no cases of grade 4 mucositis.
One patient in the busulfan group had grade 4 cardiac toxicity, an acute myocardial infarction, and ventricular fibrillation. However, the patient recovered and was in remission at last follow-up.
Two patients in the busulfan group developed second primary malignancies. One patient developed squamous cell skin cancer and rectal adenocarcinoma, and the other developed melanoma and basal cell skin carcinoma.
Three patients in the melphalan-alone group developed second primary malignancies. Two patients had squamous cell skin cancers and one had myelodysplastic syndrome.
Dr. Bashir and his colleagues noted that this study has limitations, including insufficient data to assess minimal residual disease and its impact on survival. It is a single-center study and induction and maintenance therapies were not prespecified.
“These results should be verified in a cooperative group or a multicenter, randomized study to assess the generalizability of our findings,” the researchers wrote.
Dr. Bashir and his colleagues reported having no competing financial interests. The trial was sponsored by MD Anderson Cancer Center in collaboration with Otsuka Pharmaceutical Development & Commercialization. The work was funded in part by the National Institutes of Health.
SOURCE: Bashir Q et al. Lancet Haematol. 2019 Mar 22. doi: 10.1016/S2352-3026(19)30023-7.
Busulfan plus melphalan could replace melphalan alone as the standard conditioning regimen for multiple myeloma patients undergoing autologous hematopoietic cell transplant, according to researchers.
In a phase 3 trial, patients who received conditioning with busulfan plus melphalan had significantly longer median progression-free survival compared with patients who received melphalan alone – 64.7 months versus 43.5 months (P = .022).
However, there was no overall survival advantage with busulfan plus melphalan, and adverse events were more common with this regimen, reported Qaiser Bashir, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues. The report is in The Lancet Haematology.
To their knowledge, the researchers wrote, this was the first randomized, phase 3 trial showing a significant progression-free survival benefit for busulfan plus melphalan versus the standard of care of melphalan 200 mg/m2 pretransplantation conditioning. “These data suggest that busulfan plus melphalan conditioning can serve as a useful platform for further improvement of transplant outcomes in patients with myeloma.”
The current trial (NCT01413178) enrolled 205 multiple myeloma patients who were eligible for transplant. They were randomized to conditioning with melphalan alone or busulfan plus melphalan.
In all, 98 patients received melphalan alone, given at 200 mg/m2 on day –2. The 104 patients who received busulfan plus melphalan started with a test dose of busulfan at 32 mg/m2, which was followed by pharmacokinetically adjusted doses on days –7, –6, –5, and –4 to achieve a target daily area under the curve of 5,000 mmol/minute. These patients received melphalan at 70 mg/m2 per day on days –2 and –1.
The median age at transplant was 57.9 years (range, 31.7-70.9 years) in the busulfan group and 57.6 years (range, 34.3-70.6 years) in the melphalan-alone group.
The most common induction regimen used was bortezomib, lenalidomide, and dexamethasone, which was given to 60% of the busulfan group and 57% of the melphalan-alone group.
Most patients responded to induction – 96% of patients in the busulfan group and 94% of those in the melphalan-alone group.
There was no treatment-related mortality within 100 days of transplant.
At 90 days after transplant, the response rate was 98% in the busulfan group and 97% in the melphalan-alone group. The rate of complete remission/stringent complete remission was 27% and 34%, respectively.
Most patients received posttransplant maintenance. The most common maintenance regimen consisted of lenalidomide monotherapy, which was given to 57% of the busulfan group and 58% of the melphalan-alone group.
Patients continued maintenance until disease progression or unacceptable toxicity. The median duration of maintenance was 16.0 months in the busulfan group and 10.1 months in the melphalan-alone group.
The median follow-up was 22.6 months in the busulfan group and 20.2 months in the melphalan-alone group.
Progression-free survival was superior in the busulfan group. Median progression-free survival was 64.7 months in the busulfan group and 43.5 months in the melphalan-alone group (hazard ratio = 0.53; P = .022). The 3-year progression-free survival rate was 72% and 50%, respectively.
The median overall survival was not reached in either group. The 3-year overall survival rate was 91% in the busulfan group and 89% in the melphalan-alone group.
There were 10 deaths in the busulfan group, 7 due to progression and 3 due to infection. All 7 deaths in the melphalan-alone group were due to progression.
Grade 3-4 nonhematologic toxicity was more common in the busulfan group, occurring in 84% of that group and 33% of the melphalan-alone group (P less than .0001).
Grade 2-3 mucositis occurred in 74% of the busulfan group and 14% of the melphalan-alone group. There were no cases of grade 4 mucositis.
One patient in the busulfan group had grade 4 cardiac toxicity, an acute myocardial infarction, and ventricular fibrillation. However, the patient recovered and was in remission at last follow-up.
Two patients in the busulfan group developed second primary malignancies. One patient developed squamous cell skin cancer and rectal adenocarcinoma, and the other developed melanoma and basal cell skin carcinoma.
Three patients in the melphalan-alone group developed second primary malignancies. Two patients had squamous cell skin cancers and one had myelodysplastic syndrome.
Dr. Bashir and his colleagues noted that this study has limitations, including insufficient data to assess minimal residual disease and its impact on survival. It is a single-center study and induction and maintenance therapies were not prespecified.
“These results should be verified in a cooperative group or a multicenter, randomized study to assess the generalizability of our findings,” the researchers wrote.
Dr. Bashir and his colleagues reported having no competing financial interests. The trial was sponsored by MD Anderson Cancer Center in collaboration with Otsuka Pharmaceutical Development & Commercialization. The work was funded in part by the National Institutes of Health.
SOURCE: Bashir Q et al. Lancet Haematol. 2019 Mar 22. doi: 10.1016/S2352-3026(19)30023-7.
Busulfan plus melphalan could replace melphalan alone as the standard conditioning regimen for multiple myeloma patients undergoing autologous hematopoietic cell transplant, according to researchers.
In a phase 3 trial, patients who received conditioning with busulfan plus melphalan had significantly longer median progression-free survival compared with patients who received melphalan alone – 64.7 months versus 43.5 months (P = .022).
However, there was no overall survival advantage with busulfan plus melphalan, and adverse events were more common with this regimen, reported Qaiser Bashir, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues. The report is in The Lancet Haematology.
To their knowledge, the researchers wrote, this was the first randomized, phase 3 trial showing a significant progression-free survival benefit for busulfan plus melphalan versus the standard of care of melphalan 200 mg/m2 pretransplantation conditioning. “These data suggest that busulfan plus melphalan conditioning can serve as a useful platform for further improvement of transplant outcomes in patients with myeloma.”
The current trial (NCT01413178) enrolled 205 multiple myeloma patients who were eligible for transplant. They were randomized to conditioning with melphalan alone or busulfan plus melphalan.
In all, 98 patients received melphalan alone, given at 200 mg/m2 on day –2. The 104 patients who received busulfan plus melphalan started with a test dose of busulfan at 32 mg/m2, which was followed by pharmacokinetically adjusted doses on days –7, –6, –5, and –4 to achieve a target daily area under the curve of 5,000 mmol/minute. These patients received melphalan at 70 mg/m2 per day on days –2 and –1.
The median age at transplant was 57.9 years (range, 31.7-70.9 years) in the busulfan group and 57.6 years (range, 34.3-70.6 years) in the melphalan-alone group.
The most common induction regimen used was bortezomib, lenalidomide, and dexamethasone, which was given to 60% of the busulfan group and 57% of the melphalan-alone group.
Most patients responded to induction – 96% of patients in the busulfan group and 94% of those in the melphalan-alone group.
There was no treatment-related mortality within 100 days of transplant.
At 90 days after transplant, the response rate was 98% in the busulfan group and 97% in the melphalan-alone group. The rate of complete remission/stringent complete remission was 27% and 34%, respectively.
Most patients received posttransplant maintenance. The most common maintenance regimen consisted of lenalidomide monotherapy, which was given to 57% of the busulfan group and 58% of the melphalan-alone group.
Patients continued maintenance until disease progression or unacceptable toxicity. The median duration of maintenance was 16.0 months in the busulfan group and 10.1 months in the melphalan-alone group.
The median follow-up was 22.6 months in the busulfan group and 20.2 months in the melphalan-alone group.
Progression-free survival was superior in the busulfan group. Median progression-free survival was 64.7 months in the busulfan group and 43.5 months in the melphalan-alone group (hazard ratio = 0.53; P = .022). The 3-year progression-free survival rate was 72% and 50%, respectively.
The median overall survival was not reached in either group. The 3-year overall survival rate was 91% in the busulfan group and 89% in the melphalan-alone group.
There were 10 deaths in the busulfan group, 7 due to progression and 3 due to infection. All 7 deaths in the melphalan-alone group were due to progression.
Grade 3-4 nonhematologic toxicity was more common in the busulfan group, occurring in 84% of that group and 33% of the melphalan-alone group (P less than .0001).
Grade 2-3 mucositis occurred in 74% of the busulfan group and 14% of the melphalan-alone group. There were no cases of grade 4 mucositis.
One patient in the busulfan group had grade 4 cardiac toxicity, an acute myocardial infarction, and ventricular fibrillation. However, the patient recovered and was in remission at last follow-up.
Two patients in the busulfan group developed second primary malignancies. One patient developed squamous cell skin cancer and rectal adenocarcinoma, and the other developed melanoma and basal cell skin carcinoma.
Three patients in the melphalan-alone group developed second primary malignancies. Two patients had squamous cell skin cancers and one had myelodysplastic syndrome.
Dr. Bashir and his colleagues noted that this study has limitations, including insufficient data to assess minimal residual disease and its impact on survival. It is a single-center study and induction and maintenance therapies were not prespecified.
“These results should be verified in a cooperative group or a multicenter, randomized study to assess the generalizability of our findings,” the researchers wrote.
Dr. Bashir and his colleagues reported having no competing financial interests. The trial was sponsored by MD Anderson Cancer Center in collaboration with Otsuka Pharmaceutical Development & Commercialization. The work was funded in part by the National Institutes of Health.
SOURCE: Bashir Q et al. Lancet Haematol. 2019 Mar 22. doi: 10.1016/S2352-3026(19)30023-7.
FROM LANCET HAEMATOLOGY