User login
Organizing the P in a SOAP note
The Subjective, Objective, Assessment, Plan (SOAP) format of the progress note is widely recognized by clinicians in many specialties, including p
The Plan section should be organized in a way that is systematic and relevant across many psychiatric settings, including outpatient, inpatient, emergency room, jail, pediatric, geriatric, addiction, and consultation-liaison. To best accomplish this, I have designed a format for this section that consists of 6 categories:
1. Safety: Which safety issues need to be addressed?
Examples: If your patient is an inpatient, what precautions are required? If outpatient, Tarasoff? Involuntary hold? Police presence? Child or Adult Protective Services? Access to a firearm?
2. Collateral: Would it be helpful to obtain collateral information from any source?
Examples: Family? Friend? Caregiver? Teacher? Primary care clinician? Therapist? Past medical or psychiatric records?
3. Medical: Are there any medical tests or resources to consider?
Continue to: Examples...
Examples: Laboratory studies or imaging? Consult with a specialist from another field? Nursing orders?
4. Nonpharmacologic: What interventions or assessments would be helpful?
Examples: Psychotherapy? Cognitive testing? Social work? Case manager? Housing assistance? Job coach?
5. Pharmacologic: What interventions or assessments would be helpful? (I placed this category fifth to slow myself down and consider other strategies before quickly jumping to prescribe a medication.)
Examples: Medication? Long-acting injectable? Check pill count? Prescription drug monitoring program?
Continue to: 6. Disposition/follow-up...
6. Disposition/follow-up: What is the disposition/follow-up plan?
Examples: If outpatient, what is the time frame? If inpatient or an emergency room, when should the patient be discharged?
Using these 6 categories in the P section of my SOAP notes has helped me stay organized and think holistically about each patient I assess and treat. I hope other clinicians find this format helpful.
1. Pearce PF, Ferguson LA, George GS, et al. The essential SOAP note in an EHR age. Nurse Pract. 2016;41(2):29-36.
2. Foreman T, Dickstein LJ, Garakani A, et al (eds). A resident’s guide to surviving psychiatric training, 3rd ed. Washington, DC: American Psychiatric Association; 2015.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed August 20, 2018.
The Subjective, Objective, Assessment, Plan (SOAP) format of the progress note is widely recognized by clinicians in many specialties, including p
The Plan section should be organized in a way that is systematic and relevant across many psychiatric settings, including outpatient, inpatient, emergency room, jail, pediatric, geriatric, addiction, and consultation-liaison. To best accomplish this, I have designed a format for this section that consists of 6 categories:
1. Safety: Which safety issues need to be addressed?
Examples: If your patient is an inpatient, what precautions are required? If outpatient, Tarasoff? Involuntary hold? Police presence? Child or Adult Protective Services? Access to a firearm?
2. Collateral: Would it be helpful to obtain collateral information from any source?
Examples: Family? Friend? Caregiver? Teacher? Primary care clinician? Therapist? Past medical or psychiatric records?
3. Medical: Are there any medical tests or resources to consider?
Continue to: Examples...
Examples: Laboratory studies or imaging? Consult with a specialist from another field? Nursing orders?
4. Nonpharmacologic: What interventions or assessments would be helpful?
Examples: Psychotherapy? Cognitive testing? Social work? Case manager? Housing assistance? Job coach?
5. Pharmacologic: What interventions or assessments would be helpful? (I placed this category fifth to slow myself down and consider other strategies before quickly jumping to prescribe a medication.)
Examples: Medication? Long-acting injectable? Check pill count? Prescription drug monitoring program?
Continue to: 6. Disposition/follow-up...
6. Disposition/follow-up: What is the disposition/follow-up plan?
Examples: If outpatient, what is the time frame? If inpatient or an emergency room, when should the patient be discharged?
Using these 6 categories in the P section of my SOAP notes has helped me stay organized and think holistically about each patient I assess and treat. I hope other clinicians find this format helpful.
The Subjective, Objective, Assessment, Plan (SOAP) format of the progress note is widely recognized by clinicians in many specialties, including p
The Plan section should be organized in a way that is systematic and relevant across many psychiatric settings, including outpatient, inpatient, emergency room, jail, pediatric, geriatric, addiction, and consultation-liaison. To best accomplish this, I have designed a format for this section that consists of 6 categories:
1. Safety: Which safety issues need to be addressed?
Examples: If your patient is an inpatient, what precautions are required? If outpatient, Tarasoff? Involuntary hold? Police presence? Child or Adult Protective Services? Access to a firearm?
2. Collateral: Would it be helpful to obtain collateral information from any source?
Examples: Family? Friend? Caregiver? Teacher? Primary care clinician? Therapist? Past medical or psychiatric records?
3. Medical: Are there any medical tests or resources to consider?
Continue to: Examples...
Examples: Laboratory studies or imaging? Consult with a specialist from another field? Nursing orders?
4. Nonpharmacologic: What interventions or assessments would be helpful?
Examples: Psychotherapy? Cognitive testing? Social work? Case manager? Housing assistance? Job coach?
5. Pharmacologic: What interventions or assessments would be helpful? (I placed this category fifth to slow myself down and consider other strategies before quickly jumping to prescribe a medication.)
Examples: Medication? Long-acting injectable? Check pill count? Prescription drug monitoring program?
Continue to: 6. Disposition/follow-up...
6. Disposition/follow-up: What is the disposition/follow-up plan?
Examples: If outpatient, what is the time frame? If inpatient or an emergency room, when should the patient be discharged?
Using these 6 categories in the P section of my SOAP notes has helped me stay organized and think holistically about each patient I assess and treat. I hope other clinicians find this format helpful.
1. Pearce PF, Ferguson LA, George GS, et al. The essential SOAP note in an EHR age. Nurse Pract. 2016;41(2):29-36.
2. Foreman T, Dickstein LJ, Garakani A, et al (eds). A resident’s guide to surviving psychiatric training, 3rd ed. Washington, DC: American Psychiatric Association; 2015.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed August 20, 2018.
1. Pearce PF, Ferguson LA, George GS, et al. The essential SOAP note in an EHR age. Nurse Pract. 2016;41(2):29-36.
2. Foreman T, Dickstein LJ, Garakani A, et al (eds). A resident’s guide to surviving psychiatric training, 3rd ed. Washington, DC: American Psychiatric Association; 2015.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed August 20, 2018.
COMBS: Feeling positive about negative symptoms of schizophrenia
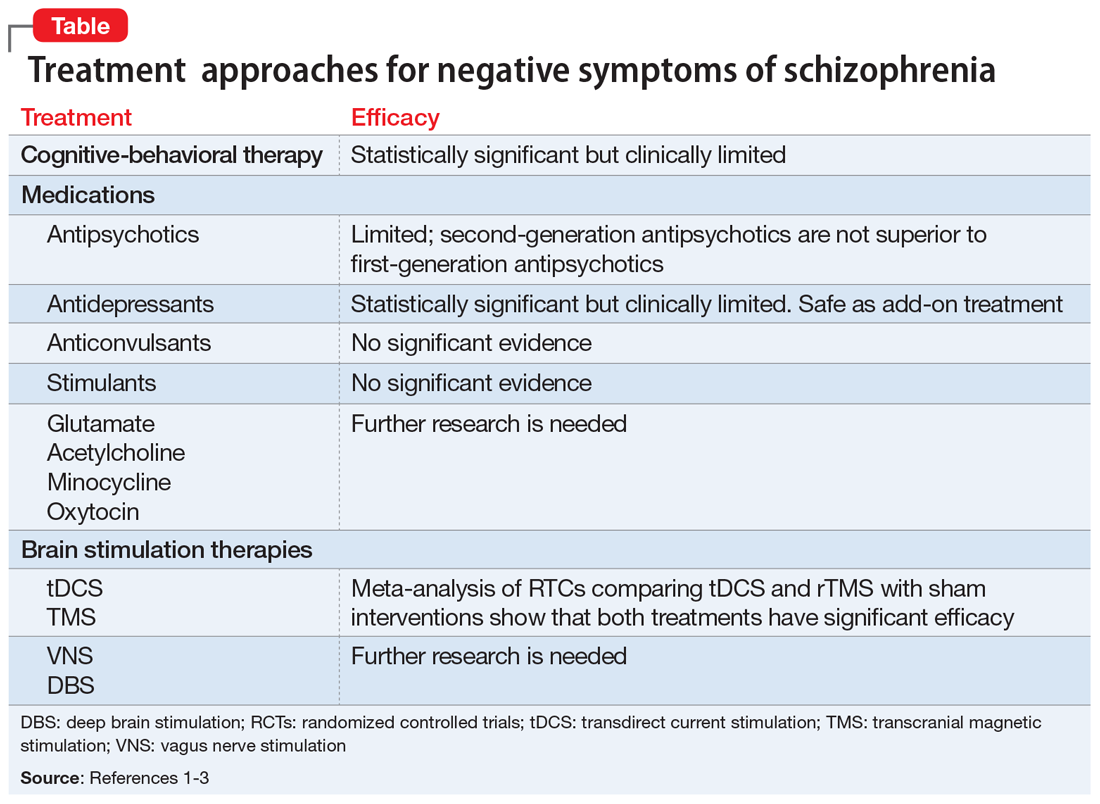
Negative symptoms of schizophrenia—such as social withdrawal, avolition, avoidance, lack of spontaneity, anhedonia, poverty of speech, and blunted affect—often persist after successful treatment of positive symptoms, such as hallucinations and delusions.1 Negative symptoms can be debilitating and are associated with poor social and occupational outcomes, as well as cognitive dysfunction. Currently, treatments for negative symptoms are not nearly as effective as treatments for positive symptoms. The mnemonic COMBS can be used to easily recall 3 treatment modalities often used to address negative symptoms.
COgnitive-behavioral therapy
Cognitive-behavioral therapy (CBT) and other psychosocial therapies derived from it, such as social skills training, recovery-oriented cognitive therapy, motivation and enhancement therapy, and cognitive-behavioral social skills training (CBSST), have shown to be effective for treating negative symptoms.2 In a study of 149 patients with schizophrenia, CBSST reduced symptoms of avolition and apathy and improved functioning outcomes.2
Medications
Antipsychotics. Although second-generation antipsychotics (SGAs) were initially promising, accumulating clinical experience and research have shown that these agents have limited efficacy for treating negative symptoms.1 Unlike first-generation antipsychotics, SGAs do not cause affective blunting, and are effective at treating depressive symptoms; however, depressive symptoms can sometimes be difficult to distinguish from negative symptoms. Improvement of depressive symptoms observed with SGA treatment could be mistakenly interpreted as alleviation of negative symptoms; however, clinical trials that focused specifically on treating negative symptoms have found no specific efficacy of SGAs.1
Antidepressants. Although clinical trials and meta-analyses have had mixed results,1 antidepressants appear to be safe add-on treatments with small efficacy for negative symptoms.
Anticonvulsants have long been used as augmentation to antipsychotics for patients with treatment-resistant schizophrenia; however, there is no evidence that these medications can improve negative symptoms.1
Stimulants. There is no strong evidence that stimulants could be an efficacious treatment for negative symptoms.1
Other pharmacologic agents,1 such as acetylcholine-related medications, oxytocin, and medications with a mechanism of action that is related to an inflammatory response and immunologic pathways (ie, minocycline), are being evaluated for treating negative symptoms. Research into the efficacy of glutamate-related agents also appears to be continuing.1
Continue to: Brain Stimulation therapies
Brain Stimulation therapies
Transcranial magnetic stimulation (TMS), transdirect current stimulation (tDCS), vagus nerve stimulation, and deep brain stimulation have been evaluated for treating negative symptoms. A recent meta-analysis of randomized controlled trials comparing the effects of brain stimulation with sham interventions in patients with schizophrenia found that TMS and tDCS that targeted the left dorsolateral prefrontal cortex effectively reduced the severity of negative symptoms.3
The Table1-3 summarizes available treatments for negative symptoms of schizophrenia and their efficacies. Although research investigating the improvement of negative symptoms is currently insufficient, CBT-related therapies and antidepressants appear to be helpful. For more information, see “Treating negative symptoms of schizophrenia” (C
1. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry. 2016;3:133-150.
2. Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophr Bull. 2018;44(3):653-661.
3. Kennedy NI, Lee WH. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77.
Negative symptoms of schizophrenia—such as social withdrawal, avolition, avoidance, lack of spontaneity, anhedonia, poverty of speech, and blunted affect—often persist after successful treatment of positive symptoms, such as hallucinations and delusions.1 Negative symptoms can be debilitating and are associated with poor social and occupational outcomes, as well as cognitive dysfunction. Currently, treatments for negative symptoms are not nearly as effective as treatments for positive symptoms. The mnemonic COMBS can be used to easily recall 3 treatment modalities often used to address negative symptoms.
COgnitive-behavioral therapy
Cognitive-behavioral therapy (CBT) and other psychosocial therapies derived from it, such as social skills training, recovery-oriented cognitive therapy, motivation and enhancement therapy, and cognitive-behavioral social skills training (CBSST), have shown to be effective for treating negative symptoms.2 In a study of 149 patients with schizophrenia, CBSST reduced symptoms of avolition and apathy and improved functioning outcomes.2
Medications
Antipsychotics. Although second-generation antipsychotics (SGAs) were initially promising, accumulating clinical experience and research have shown that these agents have limited efficacy for treating negative symptoms.1 Unlike first-generation antipsychotics, SGAs do not cause affective blunting, and are effective at treating depressive symptoms; however, depressive symptoms can sometimes be difficult to distinguish from negative symptoms. Improvement of depressive symptoms observed with SGA treatment could be mistakenly interpreted as alleviation of negative symptoms; however, clinical trials that focused specifically on treating negative symptoms have found no specific efficacy of SGAs.1
Antidepressants. Although clinical trials and meta-analyses have had mixed results,1 antidepressants appear to be safe add-on treatments with small efficacy for negative symptoms.
Anticonvulsants have long been used as augmentation to antipsychotics for patients with treatment-resistant schizophrenia; however, there is no evidence that these medications can improve negative symptoms.1
Stimulants. There is no strong evidence that stimulants could be an efficacious treatment for negative symptoms.1
Other pharmacologic agents,1 such as acetylcholine-related medications, oxytocin, and medications with a mechanism of action that is related to an inflammatory response and immunologic pathways (ie, minocycline), are being evaluated for treating negative symptoms. Research into the efficacy of glutamate-related agents also appears to be continuing.1
Continue to: Brain Stimulation therapies
Brain Stimulation therapies
Transcranial magnetic stimulation (TMS), transdirect current stimulation (tDCS), vagus nerve stimulation, and deep brain stimulation have been evaluated for treating negative symptoms. A recent meta-analysis of randomized controlled trials comparing the effects of brain stimulation with sham interventions in patients with schizophrenia found that TMS and tDCS that targeted the left dorsolateral prefrontal cortex effectively reduced the severity of negative symptoms.3
The Table1-3 summarizes available treatments for negative symptoms of schizophrenia and their efficacies. Although research investigating the improvement of negative symptoms is currently insufficient, CBT-related therapies and antidepressants appear to be helpful. For more information, see “Treating negative symptoms of schizophrenia” (C
Negative symptoms of schizophrenia—such as social withdrawal, avolition, avoidance, lack of spontaneity, anhedonia, poverty of speech, and blunted affect—often persist after successful treatment of positive symptoms, such as hallucinations and delusions.1 Negative symptoms can be debilitating and are associated with poor social and occupational outcomes, as well as cognitive dysfunction. Currently, treatments for negative symptoms are not nearly as effective as treatments for positive symptoms. The mnemonic COMBS can be used to easily recall 3 treatment modalities often used to address negative symptoms.
COgnitive-behavioral therapy
Cognitive-behavioral therapy (CBT) and other psychosocial therapies derived from it, such as social skills training, recovery-oriented cognitive therapy, motivation and enhancement therapy, and cognitive-behavioral social skills training (CBSST), have shown to be effective for treating negative symptoms.2 In a study of 149 patients with schizophrenia, CBSST reduced symptoms of avolition and apathy and improved functioning outcomes.2
Medications
Antipsychotics. Although second-generation antipsychotics (SGAs) were initially promising, accumulating clinical experience and research have shown that these agents have limited efficacy for treating negative symptoms.1 Unlike first-generation antipsychotics, SGAs do not cause affective blunting, and are effective at treating depressive symptoms; however, depressive symptoms can sometimes be difficult to distinguish from negative symptoms. Improvement of depressive symptoms observed with SGA treatment could be mistakenly interpreted as alleviation of negative symptoms; however, clinical trials that focused specifically on treating negative symptoms have found no specific efficacy of SGAs.1
Antidepressants. Although clinical trials and meta-analyses have had mixed results,1 antidepressants appear to be safe add-on treatments with small efficacy for negative symptoms.
Anticonvulsants have long been used as augmentation to antipsychotics for patients with treatment-resistant schizophrenia; however, there is no evidence that these medications can improve negative symptoms.1
Stimulants. There is no strong evidence that stimulants could be an efficacious treatment for negative symptoms.1
Other pharmacologic agents,1 such as acetylcholine-related medications, oxytocin, and medications with a mechanism of action that is related to an inflammatory response and immunologic pathways (ie, minocycline), are being evaluated for treating negative symptoms. Research into the efficacy of glutamate-related agents also appears to be continuing.1
Continue to: Brain Stimulation therapies
Brain Stimulation therapies
Transcranial magnetic stimulation (TMS), transdirect current stimulation (tDCS), vagus nerve stimulation, and deep brain stimulation have been evaluated for treating negative symptoms. A recent meta-analysis of randomized controlled trials comparing the effects of brain stimulation with sham interventions in patients with schizophrenia found that TMS and tDCS that targeted the left dorsolateral prefrontal cortex effectively reduced the severity of negative symptoms.3
The Table1-3 summarizes available treatments for negative symptoms of schizophrenia and their efficacies. Although research investigating the improvement of negative symptoms is currently insufficient, CBT-related therapies and antidepressants appear to be helpful. For more information, see “Treating negative symptoms of schizophrenia” (C
1. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry. 2016;3:133-150.
2. Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophr Bull. 2018;44(3):653-661.
3. Kennedy NI, Lee WH. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77.
1. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry. 2016;3:133-150.
2. Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophr Bull. 2018;44(3):653-661.
3. Kennedy NI, Lee WH. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77.
The angry disciple
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
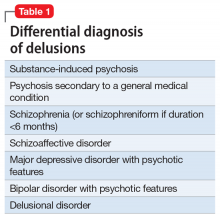
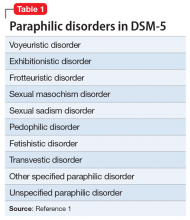
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
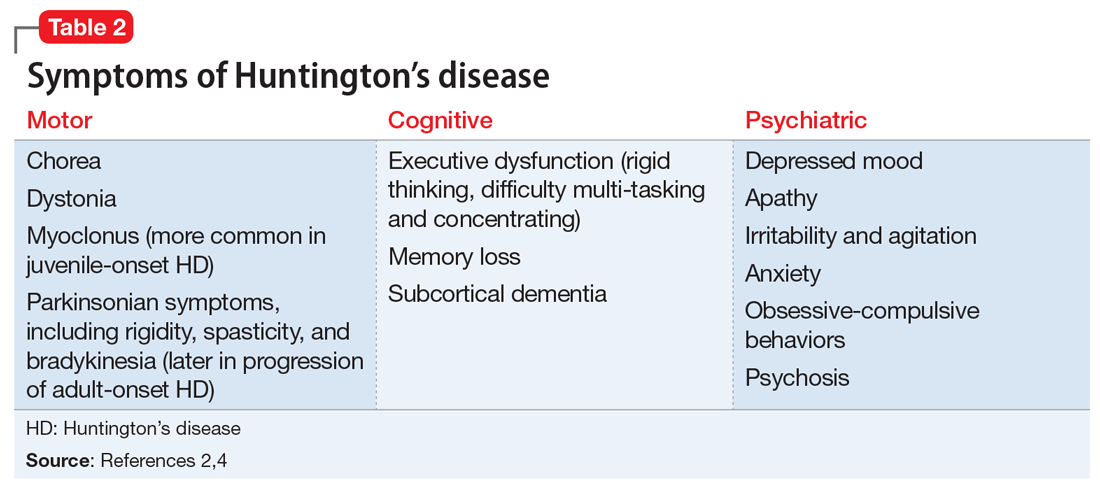
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
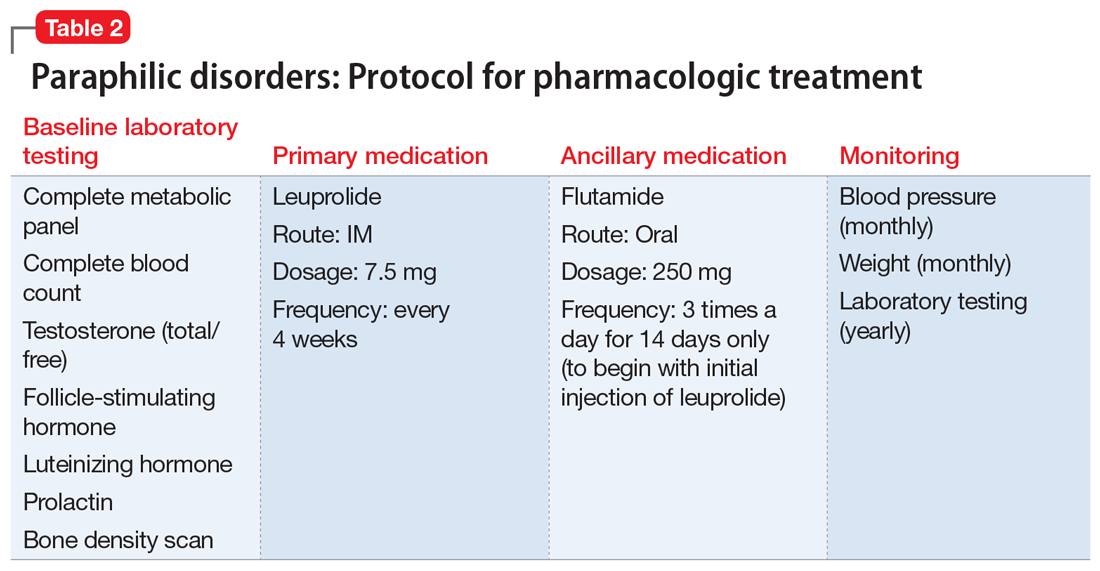
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
Pharmacogenomics testing: What the FDA says
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
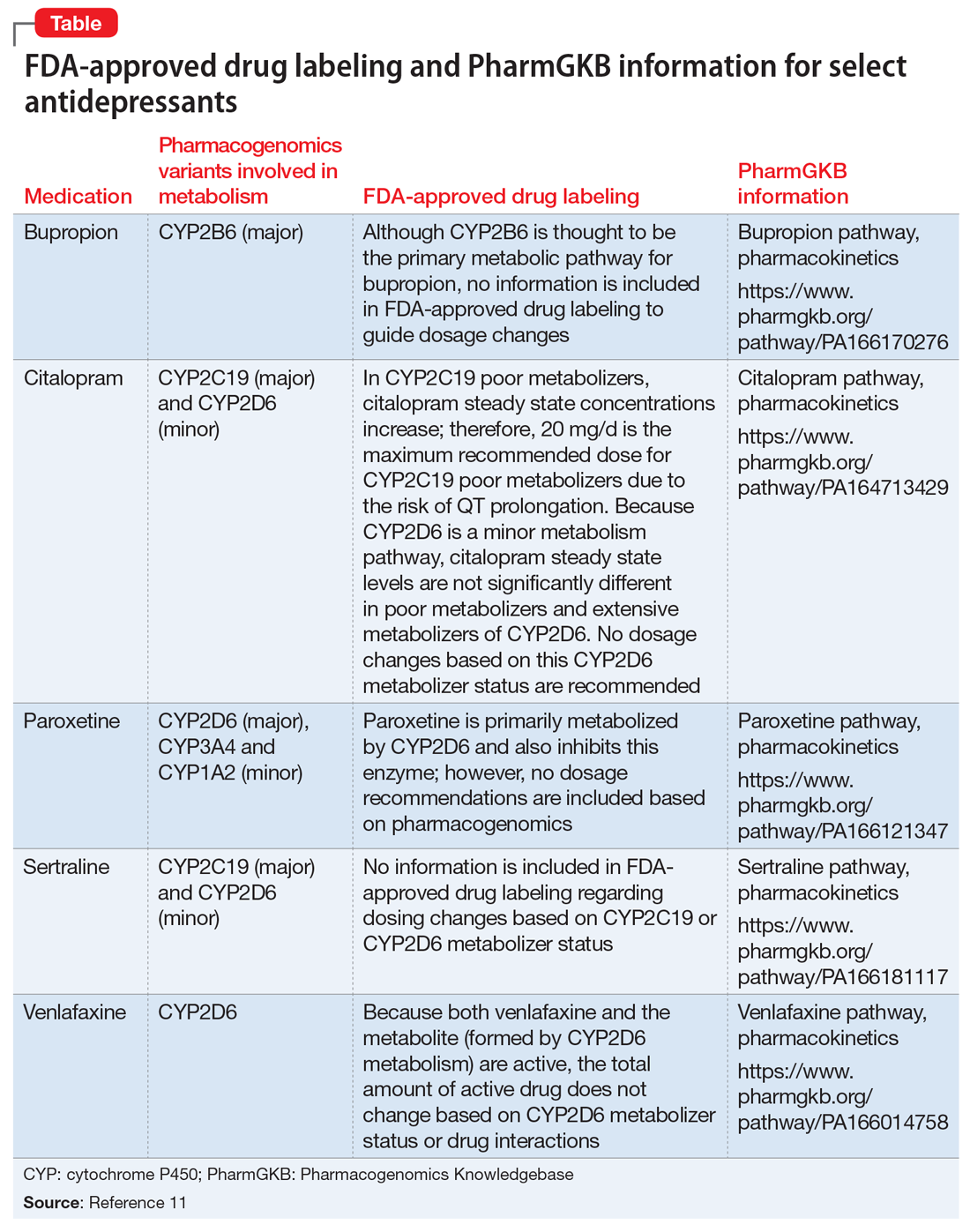
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
Pimavanserin: A potentially safer alternative to clozapine for refractory hallucinations and delusions
Up to 30% of patients with schizophrenia do not respond to dopamine antagonists, which include all first- and second-generation antipsychotics. They are labeled as “treatment-resistant” if they have a partial response, or “treatment-refractory” if their hallucinations and/or delusions do not improve at all despite multiple trials of antipsychotics.
That’s why clozapine is considered a “lifesaver” for such patients, a last-resort medication that unshackles patients with refractory psychotic symptoms from the tyranny of auditory and/or visual hallucinations and the reality distortion of fixed false beliefs such as paranoid delusions.
Many long-suffering patients with refractory psychosis recover and return to their baseline, thanks to clozapine. In a past editorial, I discussed how one of my patients, Bethany, who had dropped out of college and became homeless for 4 years with refractory delusions and hallucinations, recovered completely when she received clozapine.1 She then returned to college, graduated with honors, and authored a book about her journey of recovery.2 She and I later established a nonprofit foundation we called CURESZ (Comprehensive Understanding via Research and Education in Schizophrenia), and assembled a panel of 80 clozapine experts across the country to provide access to clozapine for the hundreds of thousands of individuals with refractory psychosis who never received a trial of clozapine from their psychiatrists or psychiatric nurse practitioners. (Visit CURESZ.org for details.)
Bethany was very lucky to respond and recover completely, because only 40% of patients with refractory psychosis respond to clozapine. She does not mind having her blood drawn every week to measure her white blood cell count for early detection of potentially fatal agranulocytosis. Many refractory, often homeless patients with chronic schizophrenia refuse to have weekly phlebotomy and therefore are not treated with clozapine. Bethany was also fortunate to experience only 1 adverse effect of clozapine: extreme sedation that forced her to sleep up to 15 hours a day (this was reduced to 9 to 10 hours a day with adjunctive modafinil). Fortunately, she was spared the multiple other serious adverse effects of clozapine, which include excessive salivation, extreme weight gain, diabetes, hyperlipidemia, cardiomyopathy, pancreatitis, seizures, and ileus.3 Clozapine is also associated with sudden death more than any other antipsychotic agent.4
So, what can be done for patients with refractory hallucinations and delusions who are among the 60% who fail to respond to clozapine, or who experience intolerable adverse effects or safety problems, or who refuse to take clozapine and have their blood drawn every week? This is a desperately ill and seriously disabled group of patients who are deemed to be beyond the reach of medical intervention by psychiatry. They are often treated with various off-label medications as adjunctive therapy to clozapine, to which they failed to respond. This includes adding lamotrigine5 or benzoate,6 but none have been approved as an efficacious and safe monotherapy alternative to clozapine. So, what can be done for patients with refractory illness?
Enter pimavanserin. This new medication is an inverse agonist of serotonin 5-HT2A receptors and (to a lesser extent) serotonin 5-HT2C receptors. It was recently FDA-approved for treating the hallucinations and delusions of Parkinson’s disease psychosis,7 which is estimated to develop in up to 50% of individuals with Parkinson’s disease. It does not have any affinity to any dopamine receptors, which makes it an ideal antipsychotic for Parkinson’s disease, where any dopamine antagonism can worsen the motor symptoms (rigidity, hypokinesia, and tremors) associated with that movement disorder. Thus, pimavanserin became the first ever non-dopaminergic antipsychotic in the world and is indicated only for Parkinson’s disease psychosis.
Our clinical team made a serendipitous discovery about the efficacy of pimavanserin in patients with schizophrenia who failed to respond to clozapine therapy after several months at clinically adequate doses. Our findings were published online last month in the highly respected journal Schizophrenia Research.8 We reported the successful treatment with pimavanserin in 2 groups:
- patients who had not responded to clozapine received pimavanserin as an add-on to clozapine in doses of 34 mg/d, the same dose recommended for patients with Parkinson’s disease hallucinations and/or delusions.
- patients who had hallucinations and delusions that failed to respond to several non-clozapine antipsychotics received pimavanserin monotherapy instead of clozapine to avoid blood draws and serious adverse effects.
Continue to: Pimavanserin successfully treated...
Pimavanserin successfully treated the hallucinations and delusions of all 10 patients in both groups. Remission occurred within 1 month in most cases, and after 2 months in 1 patient. Those patients no longer required hospitalization as they did prior to taking pimavanserin, and they maintained their response for several months of follow-up. We were also pleased to note that most patients became more sociable and affable, with improved mood and affect, after their hallucinations and delusions disappeared with pimavanserin. We did have a few patients who did not respond to 34 mg/d of pimavanserin, and some who responded for several months but then showed signs of recurrence. We are considering increasing the dose to 68 mg/d in such patients because it is possible that a higher dose may be needed in some patients with refractory illness, who may vary in symptom severity or biology.
We are now planning to apply for a research grant to conduct a controlled trial to confirm our very encouraging clinical findings, and we hope other investigators will also conduct clinical trials in patients with refractory psychosis comparing pimavanserin with placebo or pimavanserin with clozapine in double-blind studies.
As a disclosure, our clinical findings were obtained without any knowledge of, or funding from, the company that makes pimavanserin (Acadia Pharmaceuticals Inc.). The company was informed of our findings only after our article was accepted for publication.
I hope this important finding of a potentially safer alternative to clozapine may address a major unmet need in psychiatry, involving the treatment of hundreds of thousands of patients with treatment-resistant or treatment-refractory psychosis, which includes patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21, 24-25.
2. Yeiser B. Mind estranged: my journey from schizophrenia and homelessness to recovery. Seattle, WA: Amazon; 2014.
3. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf. 2014;9(3):163-195.
4. Manu P, Kane JM, Corell CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936-941.
5. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2009;109(1-3):10-14.
6. Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2017;84(6):422-432.
7. Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in p atients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
8. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist [Epub ahead of print March 2, 2019]. Schizophrenia Res. 2019. doi: 10.1016/j.schres.2019.02.018.
Up to 30% of patients with schizophrenia do not respond to dopamine antagonists, which include all first- and second-generation antipsychotics. They are labeled as “treatment-resistant” if they have a partial response, or “treatment-refractory” if their hallucinations and/or delusions do not improve at all despite multiple trials of antipsychotics.
That’s why clozapine is considered a “lifesaver” for such patients, a last-resort medication that unshackles patients with refractory psychotic symptoms from the tyranny of auditory and/or visual hallucinations and the reality distortion of fixed false beliefs such as paranoid delusions.
Many long-suffering patients with refractory psychosis recover and return to their baseline, thanks to clozapine. In a past editorial, I discussed how one of my patients, Bethany, who had dropped out of college and became homeless for 4 years with refractory delusions and hallucinations, recovered completely when she received clozapine.1 She then returned to college, graduated with honors, and authored a book about her journey of recovery.2 She and I later established a nonprofit foundation we called CURESZ (Comprehensive Understanding via Research and Education in Schizophrenia), and assembled a panel of 80 clozapine experts across the country to provide access to clozapine for the hundreds of thousands of individuals with refractory psychosis who never received a trial of clozapine from their psychiatrists or psychiatric nurse practitioners. (Visit CURESZ.org for details.)
Bethany was very lucky to respond and recover completely, because only 40% of patients with refractory psychosis respond to clozapine. She does not mind having her blood drawn every week to measure her white blood cell count for early detection of potentially fatal agranulocytosis. Many refractory, often homeless patients with chronic schizophrenia refuse to have weekly phlebotomy and therefore are not treated with clozapine. Bethany was also fortunate to experience only 1 adverse effect of clozapine: extreme sedation that forced her to sleep up to 15 hours a day (this was reduced to 9 to 10 hours a day with adjunctive modafinil). Fortunately, she was spared the multiple other serious adverse effects of clozapine, which include excessive salivation, extreme weight gain, diabetes, hyperlipidemia, cardiomyopathy, pancreatitis, seizures, and ileus.3 Clozapine is also associated with sudden death more than any other antipsychotic agent.4
So, what can be done for patients with refractory hallucinations and delusions who are among the 60% who fail to respond to clozapine, or who experience intolerable adverse effects or safety problems, or who refuse to take clozapine and have their blood drawn every week? This is a desperately ill and seriously disabled group of patients who are deemed to be beyond the reach of medical intervention by psychiatry. They are often treated with various off-label medications as adjunctive therapy to clozapine, to which they failed to respond. This includes adding lamotrigine5 or benzoate,6 but none have been approved as an efficacious and safe monotherapy alternative to clozapine. So, what can be done for patients with refractory illness?
Enter pimavanserin. This new medication is an inverse agonist of serotonin 5-HT2A receptors and (to a lesser extent) serotonin 5-HT2C receptors. It was recently FDA-approved for treating the hallucinations and delusions of Parkinson’s disease psychosis,7 which is estimated to develop in up to 50% of individuals with Parkinson’s disease. It does not have any affinity to any dopamine receptors, which makes it an ideal antipsychotic for Parkinson’s disease, where any dopamine antagonism can worsen the motor symptoms (rigidity, hypokinesia, and tremors) associated with that movement disorder. Thus, pimavanserin became the first ever non-dopaminergic antipsychotic in the world and is indicated only for Parkinson’s disease psychosis.
Our clinical team made a serendipitous discovery about the efficacy of pimavanserin in patients with schizophrenia who failed to respond to clozapine therapy after several months at clinically adequate doses. Our findings were published online last month in the highly respected journal Schizophrenia Research.8 We reported the successful treatment with pimavanserin in 2 groups:
- patients who had not responded to clozapine received pimavanserin as an add-on to clozapine in doses of 34 mg/d, the same dose recommended for patients with Parkinson’s disease hallucinations and/or delusions.
- patients who had hallucinations and delusions that failed to respond to several non-clozapine antipsychotics received pimavanserin monotherapy instead of clozapine to avoid blood draws and serious adverse effects.
Continue to: Pimavanserin successfully treated...
Pimavanserin successfully treated the hallucinations and delusions of all 10 patients in both groups. Remission occurred within 1 month in most cases, and after 2 months in 1 patient. Those patients no longer required hospitalization as they did prior to taking pimavanserin, and they maintained their response for several months of follow-up. We were also pleased to note that most patients became more sociable and affable, with improved mood and affect, after their hallucinations and delusions disappeared with pimavanserin. We did have a few patients who did not respond to 34 mg/d of pimavanserin, and some who responded for several months but then showed signs of recurrence. We are considering increasing the dose to 68 mg/d in such patients because it is possible that a higher dose may be needed in some patients with refractory illness, who may vary in symptom severity or biology.
We are now planning to apply for a research grant to conduct a controlled trial to confirm our very encouraging clinical findings, and we hope other investigators will also conduct clinical trials in patients with refractory psychosis comparing pimavanserin with placebo or pimavanserin with clozapine in double-blind studies.
As a disclosure, our clinical findings were obtained without any knowledge of, or funding from, the company that makes pimavanserin (Acadia Pharmaceuticals Inc.). The company was informed of our findings only after our article was accepted for publication.
I hope this important finding of a potentially safer alternative to clozapine may address a major unmet need in psychiatry, involving the treatment of hundreds of thousands of patients with treatment-resistant or treatment-refractory psychosis, which includes patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder.
To comment on this editorial or other topics of interest: [email protected].
Up to 30% of patients with schizophrenia do not respond to dopamine antagonists, which include all first- and second-generation antipsychotics. They are labeled as “treatment-resistant” if they have a partial response, or “treatment-refractory” if their hallucinations and/or delusions do not improve at all despite multiple trials of antipsychotics.
That’s why clozapine is considered a “lifesaver” for such patients, a last-resort medication that unshackles patients with refractory psychotic symptoms from the tyranny of auditory and/or visual hallucinations and the reality distortion of fixed false beliefs such as paranoid delusions.
Many long-suffering patients with refractory psychosis recover and return to their baseline, thanks to clozapine. In a past editorial, I discussed how one of my patients, Bethany, who had dropped out of college and became homeless for 4 years with refractory delusions and hallucinations, recovered completely when she received clozapine.1 She then returned to college, graduated with honors, and authored a book about her journey of recovery.2 She and I later established a nonprofit foundation we called CURESZ (Comprehensive Understanding via Research and Education in Schizophrenia), and assembled a panel of 80 clozapine experts across the country to provide access to clozapine for the hundreds of thousands of individuals with refractory psychosis who never received a trial of clozapine from their psychiatrists or psychiatric nurse practitioners. (Visit CURESZ.org for details.)
Bethany was very lucky to respond and recover completely, because only 40% of patients with refractory psychosis respond to clozapine. She does not mind having her blood drawn every week to measure her white blood cell count for early detection of potentially fatal agranulocytosis. Many refractory, often homeless patients with chronic schizophrenia refuse to have weekly phlebotomy and therefore are not treated with clozapine. Bethany was also fortunate to experience only 1 adverse effect of clozapine: extreme sedation that forced her to sleep up to 15 hours a day (this was reduced to 9 to 10 hours a day with adjunctive modafinil). Fortunately, she was spared the multiple other serious adverse effects of clozapine, which include excessive salivation, extreme weight gain, diabetes, hyperlipidemia, cardiomyopathy, pancreatitis, seizures, and ileus.3 Clozapine is also associated with sudden death more than any other antipsychotic agent.4
So, what can be done for patients with refractory hallucinations and delusions who are among the 60% who fail to respond to clozapine, or who experience intolerable adverse effects or safety problems, or who refuse to take clozapine and have their blood drawn every week? This is a desperately ill and seriously disabled group of patients who are deemed to be beyond the reach of medical intervention by psychiatry. They are often treated with various off-label medications as adjunctive therapy to clozapine, to which they failed to respond. This includes adding lamotrigine5 or benzoate,6 but none have been approved as an efficacious and safe monotherapy alternative to clozapine. So, what can be done for patients with refractory illness?
Enter pimavanserin. This new medication is an inverse agonist of serotonin 5-HT2A receptors and (to a lesser extent) serotonin 5-HT2C receptors. It was recently FDA-approved for treating the hallucinations and delusions of Parkinson’s disease psychosis,7 which is estimated to develop in up to 50% of individuals with Parkinson’s disease. It does not have any affinity to any dopamine receptors, which makes it an ideal antipsychotic for Parkinson’s disease, where any dopamine antagonism can worsen the motor symptoms (rigidity, hypokinesia, and tremors) associated with that movement disorder. Thus, pimavanserin became the first ever non-dopaminergic antipsychotic in the world and is indicated only for Parkinson’s disease psychosis.
Our clinical team made a serendipitous discovery about the efficacy of pimavanserin in patients with schizophrenia who failed to respond to clozapine therapy after several months at clinically adequate doses. Our findings were published online last month in the highly respected journal Schizophrenia Research.8 We reported the successful treatment with pimavanserin in 2 groups:
- patients who had not responded to clozapine received pimavanserin as an add-on to clozapine in doses of 34 mg/d, the same dose recommended for patients with Parkinson’s disease hallucinations and/or delusions.
- patients who had hallucinations and delusions that failed to respond to several non-clozapine antipsychotics received pimavanserin monotherapy instead of clozapine to avoid blood draws and serious adverse effects.
Continue to: Pimavanserin successfully treated...
Pimavanserin successfully treated the hallucinations and delusions of all 10 patients in both groups. Remission occurred within 1 month in most cases, and after 2 months in 1 patient. Those patients no longer required hospitalization as they did prior to taking pimavanserin, and they maintained their response for several months of follow-up. We were also pleased to note that most patients became more sociable and affable, with improved mood and affect, after their hallucinations and delusions disappeared with pimavanserin. We did have a few patients who did not respond to 34 mg/d of pimavanserin, and some who responded for several months but then showed signs of recurrence. We are considering increasing the dose to 68 mg/d in such patients because it is possible that a higher dose may be needed in some patients with refractory illness, who may vary in symptom severity or biology.
We are now planning to apply for a research grant to conduct a controlled trial to confirm our very encouraging clinical findings, and we hope other investigators will also conduct clinical trials in patients with refractory psychosis comparing pimavanserin with placebo or pimavanserin with clozapine in double-blind studies.
As a disclosure, our clinical findings were obtained without any knowledge of, or funding from, the company that makes pimavanserin (Acadia Pharmaceuticals Inc.). The company was informed of our findings only after our article was accepted for publication.
I hope this important finding of a potentially safer alternative to clozapine may address a major unmet need in psychiatry, involving the treatment of hundreds of thousands of patients with treatment-resistant or treatment-refractory psychosis, which includes patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21, 24-25.
2. Yeiser B. Mind estranged: my journey from schizophrenia and homelessness to recovery. Seattle, WA: Amazon; 2014.
3. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf. 2014;9(3):163-195.
4. Manu P, Kane JM, Corell CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936-941.
5. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2009;109(1-3):10-14.
6. Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2017;84(6):422-432.
7. Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in p atients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
8. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist [Epub ahead of print March 2, 2019]. Schizophrenia Res. 2019. doi: 10.1016/j.schres.2019.02.018.
1. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21, 24-25.
2. Yeiser B. Mind estranged: my journey from schizophrenia and homelessness to recovery. Seattle, WA: Amazon; 2014.
3. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf. 2014;9(3):163-195.
4. Manu P, Kane JM, Corell CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936-941.
5. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2009;109(1-3):10-14.
6. Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2017;84(6):422-432.
7. Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in p atients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
8. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist [Epub ahead of print March 2, 2019]. Schizophrenia Res. 2019. doi: 10.1016/j.schres.2019.02.018.
Treating military members, veterans, and their families
I had the unique opportunity to attend a civilian medical school followed by residency and fellowship training along civilian providers, and I often was asked about my military experience. The more time I spent with civilian providers, the more I realized how unaware they are about the intricacies of military lifestyle and culture.
Of course, this makes sense. During the draft era, almost every family had a member who served, and more people were exposed to the uniqueness of military culture.1 However, with the shift to an all-volunteer military came a decrease in the number of both active duty members and veterans.2 Consequently, today’s society is generally less aware of the realities of the military lifestyle. This is especially true among people born after the Vietnam War, whose knowledge about military life is frequently limited to movies and video games. These movies and games are as accurate a reflection of military life as watching TV series such as ER or The Resident are for learning what it’s like to be a physician. To add to the problem, most medical schools and residency training curricula include little information about military culture.3 As a result, psychiatrists, like many other physicians, often feel unequipped to provide care for veterans, current military personnel, and their loved ones.4 At the very least, most psychiatrists are unaware of the differences between military and civilian cultures.
Veterans, current military members, and their families who seek mental health services outside the Veterans Affairs (VA) and military treatment facilities are more likely to encounter a clinician who does not feel comfortable with the nuances of the military lifestyle and its challenges.3 Facing a physician with limited familiarity with their experiences, and out of fear of being misunderstood, patients may not feel comfortable disclosing pertinent details.
The US military has its own culture, lingo, customs, rules, and regulations. Its structure is hierarchical and mission-oriented. The moment a person joins the military, he or she falls under a set of legal guidelines of the Uniform Code of Military Justice (UCMJ).5 For example, extra-marital sexual conduct, fistfighting (not in combat), disrespecting superior officers, and insubordination are all punishable under UCMJ.5,6 Active duty military members are also prohibited from suing the federal government for injuries.7 The Health Insurance Portability and Accountability Act (HIPAA) permits protected health information of Armed Forces personnel to be disclosed under special circumstances. These include fitness for duty determinations, fitness to perform a particular assignment, or other activities necessary for the military mission.8 A mental health provider’s understanding of the unique aspects of military culture can positively influence the patient-provider relationship whether the patient is still serving, has left the military, or is a family member of a current or former military member.
Not all military veterans qualify for VA health care. For example, those who didn’t serve the required time on active duty, those whose injury existed prior to joining the military and was not worsened by their military service, and those discharged under other-than-honorable, bad conduct, or dishonorable conditions are unlikely to qualify.9 Other veterans simply prefer to be privately treated outside the VA. However, despite where a veteran receives treatment, the clinician’s knowledge of important military concepts can facilitate rapport-building and providing a safe space for disclosure of pertinent history. Obtaining a military history that includes (for example) years of service, number and location of deployments, combat experience, and number of transfers can help with understanding the biopsychosocial factors contributing to the diagnosis and important treatment needs.
While military dependents (spouses and children) don’t wear uniforms, they are also affected by the service and sacrifices of the military member. Spouses have to deal with adjusting to the military lifestyle, searching for new housing and jobs, finding schools for children, and separation and reconnection with a military member. Military children are not spared, either. They, too, have to leave their friends and find new ones, and adjust to new places, routines, and schools, knowing that in 2 to 3 years they likely will have to move again.
As a military member, mother, and spouse of a former military member, I know how life-changing military service can be for the entire family. I encourage all physicians to start routinely asking if their patient or his or her loved ones have ever been in the military, because a positive answer could help you to better understand the patient and provide the most appropriate, person-centered, culturally-informed treatment.
1. Pew Research Center. The military-civilian gap: War and sacrifice in the post-9/11 era. http://www.pewsocialtrends.org/2011/10/05/war-and-sacrifice-in-the-post-911-era. Published October 5, 2011. Accessed March 12, 2019.
2. Bialik K. The changing face of America’s veteran population. http://www.pewresearch.org/fact-tank/2017/11/10/the-changing-face-of-americas-veteran-population. Published November 10, 2017. Accessed March 12, 2019.
3. Meyer EG, Writer BW, Brim W. The importance of military cultural competence. Curr Psychiatry Rep. 2016;18(3):26.
4. Tanielian T, Farris C, Epley C, et al; RAND Corporation. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf. Accessed December 10, 2018.
5. The Uniform Code of Military Justice. http://www.ucmj.us. Accessed March 4, 2019.
6. Myers M. Here’s what you need to know about the biggest update to UCMJ in decades. Military Times. https://www.militarytimes.com/news/your-army/2019/01/15/heres-what-you-need-to-know-about-the-biggest-update-to-ucmj-in-decades/. Published Jan 15, 2019. Accessed March 12, 2019.
7. Information Institute. Feres Doctrine. https://www.law.cornell.edu/wex/feres_doctrine. Accessed March 12, 2019.
8. Defense Health Agency Privacy and Civil Liberties Office. The military command exception and disclosing PHI of armed forces personnel. https://health.mil/Reference-Center/Fact-Sheets/2015/05/13/Info-Paper-Military-Command-Exception-and-Disclosing-PHI-of-Armed-Forces-Personnel. Published May 13, 2015. Accessed March 12, 2019.
9. Veterans Benefits Administration. Applying for benefits and your character of discharge. https://www.benefits.va.gov/benefits/character_of_discharge.asp. Updated May 19, 2015. Accessed March 12, 2019.
I had the unique opportunity to attend a civilian medical school followed by residency and fellowship training along civilian providers, and I often was asked about my military experience. The more time I spent with civilian providers, the more I realized how unaware they are about the intricacies of military lifestyle and culture.
Of course, this makes sense. During the draft era, almost every family had a member who served, and more people were exposed to the uniqueness of military culture.1 However, with the shift to an all-volunteer military came a decrease in the number of both active duty members and veterans.2 Consequently, today’s society is generally less aware of the realities of the military lifestyle. This is especially true among people born after the Vietnam War, whose knowledge about military life is frequently limited to movies and video games. These movies and games are as accurate a reflection of military life as watching TV series such as ER or The Resident are for learning what it’s like to be a physician. To add to the problem, most medical schools and residency training curricula include little information about military culture.3 As a result, psychiatrists, like many other physicians, often feel unequipped to provide care for veterans, current military personnel, and their loved ones.4 At the very least, most psychiatrists are unaware of the differences between military and civilian cultures.
Veterans, current military members, and their families who seek mental health services outside the Veterans Affairs (VA) and military treatment facilities are more likely to encounter a clinician who does not feel comfortable with the nuances of the military lifestyle and its challenges.3 Facing a physician with limited familiarity with their experiences, and out of fear of being misunderstood, patients may not feel comfortable disclosing pertinent details.
The US military has its own culture, lingo, customs, rules, and regulations. Its structure is hierarchical and mission-oriented. The moment a person joins the military, he or she falls under a set of legal guidelines of the Uniform Code of Military Justice (UCMJ).5 For example, extra-marital sexual conduct, fistfighting (not in combat), disrespecting superior officers, and insubordination are all punishable under UCMJ.5,6 Active duty military members are also prohibited from suing the federal government for injuries.7 The Health Insurance Portability and Accountability Act (HIPAA) permits protected health information of Armed Forces personnel to be disclosed under special circumstances. These include fitness for duty determinations, fitness to perform a particular assignment, or other activities necessary for the military mission.8 A mental health provider’s understanding of the unique aspects of military culture can positively influence the patient-provider relationship whether the patient is still serving, has left the military, or is a family member of a current or former military member.
Not all military veterans qualify for VA health care. For example, those who didn’t serve the required time on active duty, those whose injury existed prior to joining the military and was not worsened by their military service, and those discharged under other-than-honorable, bad conduct, or dishonorable conditions are unlikely to qualify.9 Other veterans simply prefer to be privately treated outside the VA. However, despite where a veteran receives treatment, the clinician’s knowledge of important military concepts can facilitate rapport-building and providing a safe space for disclosure of pertinent history. Obtaining a military history that includes (for example) years of service, number and location of deployments, combat experience, and number of transfers can help with understanding the biopsychosocial factors contributing to the diagnosis and important treatment needs.
While military dependents (spouses and children) don’t wear uniforms, they are also affected by the service and sacrifices of the military member. Spouses have to deal with adjusting to the military lifestyle, searching for new housing and jobs, finding schools for children, and separation and reconnection with a military member. Military children are not spared, either. They, too, have to leave their friends and find new ones, and adjust to new places, routines, and schools, knowing that in 2 to 3 years they likely will have to move again.
As a military member, mother, and spouse of a former military member, I know how life-changing military service can be for the entire family. I encourage all physicians to start routinely asking if their patient or his or her loved ones have ever been in the military, because a positive answer could help you to better understand the patient and provide the most appropriate, person-centered, culturally-informed treatment.
I had the unique opportunity to attend a civilian medical school followed by residency and fellowship training along civilian providers, and I often was asked about my military experience. The more time I spent with civilian providers, the more I realized how unaware they are about the intricacies of military lifestyle and culture.
Of course, this makes sense. During the draft era, almost every family had a member who served, and more people were exposed to the uniqueness of military culture.1 However, with the shift to an all-volunteer military came a decrease in the number of both active duty members and veterans.2 Consequently, today’s society is generally less aware of the realities of the military lifestyle. This is especially true among people born after the Vietnam War, whose knowledge about military life is frequently limited to movies and video games. These movies and games are as accurate a reflection of military life as watching TV series such as ER or The Resident are for learning what it’s like to be a physician. To add to the problem, most medical schools and residency training curricula include little information about military culture.3 As a result, psychiatrists, like many other physicians, often feel unequipped to provide care for veterans, current military personnel, and their loved ones.4 At the very least, most psychiatrists are unaware of the differences between military and civilian cultures.
Veterans, current military members, and their families who seek mental health services outside the Veterans Affairs (VA) and military treatment facilities are more likely to encounter a clinician who does not feel comfortable with the nuances of the military lifestyle and its challenges.3 Facing a physician with limited familiarity with their experiences, and out of fear of being misunderstood, patients may not feel comfortable disclosing pertinent details.
The US military has its own culture, lingo, customs, rules, and regulations. Its structure is hierarchical and mission-oriented. The moment a person joins the military, he or she falls under a set of legal guidelines of the Uniform Code of Military Justice (UCMJ).5 For example, extra-marital sexual conduct, fistfighting (not in combat), disrespecting superior officers, and insubordination are all punishable under UCMJ.5,6 Active duty military members are also prohibited from suing the federal government for injuries.7 The Health Insurance Portability and Accountability Act (HIPAA) permits protected health information of Armed Forces personnel to be disclosed under special circumstances. These include fitness for duty determinations, fitness to perform a particular assignment, or other activities necessary for the military mission.8 A mental health provider’s understanding of the unique aspects of military culture can positively influence the patient-provider relationship whether the patient is still serving, has left the military, or is a family member of a current or former military member.
Not all military veterans qualify for VA health care. For example, those who didn’t serve the required time on active duty, those whose injury existed prior to joining the military and was not worsened by their military service, and those discharged under other-than-honorable, bad conduct, or dishonorable conditions are unlikely to qualify.9 Other veterans simply prefer to be privately treated outside the VA. However, despite where a veteran receives treatment, the clinician’s knowledge of important military concepts can facilitate rapport-building and providing a safe space for disclosure of pertinent history. Obtaining a military history that includes (for example) years of service, number and location of deployments, combat experience, and number of transfers can help with understanding the biopsychosocial factors contributing to the diagnosis and important treatment needs.
While military dependents (spouses and children) don’t wear uniforms, they are also affected by the service and sacrifices of the military member. Spouses have to deal with adjusting to the military lifestyle, searching for new housing and jobs, finding schools for children, and separation and reconnection with a military member. Military children are not spared, either. They, too, have to leave their friends and find new ones, and adjust to new places, routines, and schools, knowing that in 2 to 3 years they likely will have to move again.
As a military member, mother, and spouse of a former military member, I know how life-changing military service can be for the entire family. I encourage all physicians to start routinely asking if their patient or his or her loved ones have ever been in the military, because a positive answer could help you to better understand the patient and provide the most appropriate, person-centered, culturally-informed treatment.
1. Pew Research Center. The military-civilian gap: War and sacrifice in the post-9/11 era. http://www.pewsocialtrends.org/2011/10/05/war-and-sacrifice-in-the-post-911-era. Published October 5, 2011. Accessed March 12, 2019.
2. Bialik K. The changing face of America’s veteran population. http://www.pewresearch.org/fact-tank/2017/11/10/the-changing-face-of-americas-veteran-population. Published November 10, 2017. Accessed March 12, 2019.
3. Meyer EG, Writer BW, Brim W. The importance of military cultural competence. Curr Psychiatry Rep. 2016;18(3):26.
4. Tanielian T, Farris C, Epley C, et al; RAND Corporation. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf. Accessed December 10, 2018.
5. The Uniform Code of Military Justice. http://www.ucmj.us. Accessed March 4, 2019.
6. Myers M. Here’s what you need to know about the biggest update to UCMJ in decades. Military Times. https://www.militarytimes.com/news/your-army/2019/01/15/heres-what-you-need-to-know-about-the-biggest-update-to-ucmj-in-decades/. Published Jan 15, 2019. Accessed March 12, 2019.
7. Information Institute. Feres Doctrine. https://www.law.cornell.edu/wex/feres_doctrine. Accessed March 12, 2019.
8. Defense Health Agency Privacy and Civil Liberties Office. The military command exception and disclosing PHI of armed forces personnel. https://health.mil/Reference-Center/Fact-Sheets/2015/05/13/Info-Paper-Military-Command-Exception-and-Disclosing-PHI-of-Armed-Forces-Personnel. Published May 13, 2015. Accessed March 12, 2019.
9. Veterans Benefits Administration. Applying for benefits and your character of discharge. https://www.benefits.va.gov/benefits/character_of_discharge.asp. Updated May 19, 2015. Accessed March 12, 2019.
1. Pew Research Center. The military-civilian gap: War and sacrifice in the post-9/11 era. http://www.pewsocialtrends.org/2011/10/05/war-and-sacrifice-in-the-post-911-era. Published October 5, 2011. Accessed March 12, 2019.
2. Bialik K. The changing face of America’s veteran population. http://www.pewresearch.org/fact-tank/2017/11/10/the-changing-face-of-americas-veteran-population. Published November 10, 2017. Accessed March 12, 2019.
3. Meyer EG, Writer BW, Brim W. The importance of military cultural competence. Curr Psychiatry Rep. 2016;18(3):26.
4. Tanielian T, Farris C, Epley C, et al; RAND Corporation. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf. Accessed December 10, 2018.
5. The Uniform Code of Military Justice. http://www.ucmj.us. Accessed March 4, 2019.
6. Myers M. Here’s what you need to know about the biggest update to UCMJ in decades. Military Times. https://www.militarytimes.com/news/your-army/2019/01/15/heres-what-you-need-to-know-about-the-biggest-update-to-ucmj-in-decades/. Published Jan 15, 2019. Accessed March 12, 2019.
7. Information Institute. Feres Doctrine. https://www.law.cornell.edu/wex/feres_doctrine. Accessed March 12, 2019.
8. Defense Health Agency Privacy and Civil Liberties Office. The military command exception and disclosing PHI of armed forces personnel. https://health.mil/Reference-Center/Fact-Sheets/2015/05/13/Info-Paper-Military-Command-Exception-and-Disclosing-PHI-of-Armed-Forces-Personnel. Published May 13, 2015. Accessed March 12, 2019.
9. Veterans Benefits Administration. Applying for benefits and your character of discharge. https://www.benefits.va.gov/benefits/character_of_discharge.asp. Updated May 19, 2015. Accessed March 12, 2019.
Paraphilic disorders: A better understanding
In my role as the Director of The Johns Hopkins Sex and Gender Clinic, I have had the opportunity to provide care to 3 broad categories of patients: patients with sexual dysfunctions, patients experiencing gender dysphoria, and patients manifesting a paraphilic disorder. This article will not address sexual dysfunctions or gender dysphoria, but these terms are defined in the Box1-3 to clearly distinguish them from paraphilic disorders.
Box
Individuals with a sexual dysfunction (eg, erectile dysfunction or anorgasmia) generally experience conventional sexual feelings, but they may have difficulty performing sexually.1 Although ordinarily capable of adequate sexual performance, persons with a paraphilic disorder experience atypical erotic cravings.2 Such cravings can either be for an atypical or unacceptable category of potential partner (eg, animals or children), or for an atypical or unacceptable type of behavior (eg, crossdressing or public exhibitionism). Individuals with gender dysphoria frequently experience distress because their internal sense of feeling either male or female is not congruent with their external physical anatomy.3 The primary concerns of individuals experiencing gender dysphoria relate to feelings of gender identity, as opposed to problems involving erotic arousal.
Persons with paraphilic disorders (predominantly males) experience recurrent atypical sexual fantasies and urges that cause clinically significant impairment or distress.1 Those atypical fantasies and urges may be directed towards unacceptable partners such as animals or children, or towards unacceptable behaviors such as public exhibitionism. Table 11 lists the paraphilic disorders identified in DSM-5. This article focuses primarily, though not exclusively, upon pedophilic disorder, and its pharmacologic treatment. However, the rationale underlying such treatment is applicable across the paraphilic spectrum. Before providing such treatment, it is important for clinicians to have a clear conceptual understanding of paraphilic disorders.
When is a difference a disorder?
Cancer and respiration are 2 different biologic phenomenon. Cancer causes suffering and impairment, and as a consequence, we label it a disorder. We do so in the hope of learning more about it, and being able to successfully treat it. We do not classify respiration as a disorder because we do not consider it to be harmful.
The spectrum of human sexuality is quite broad, and psychiatry is generally not concerned with private sexual thoughts and behaviors involving consenting adults that do not cause suffering or impairment. When adults choose to engage in “kinky sex” that causes neither harm nor distress, so be it.
Some individuals may be privately aware of experiencing either an exclusive or nonexclusive sexual attraction to children. Some of these individuals may not be distressed by experiencing such attractions, and may be fully capable of resisting the temptation to enact them. In such an instance, even though an individual may be experiencing sexual attractions that are different from the norm, there may not be a sufficient basis for diagnosing pedophilic disorder. However, that difference in sexual phenomenology (ie, mental experience) could rise to the level of a diagnosable disorder if the individual in question expresses distress about experiencing such attractions, and/or if his capacity to resist acting upon them is impaired.4 Under such circumstances, treatment would be warranted.
Patients with paraphilic disorders deserve treatment
Prior to establishment of the Betty Ford Clinic in 1982, individuals who were drug- or alcohol-dependent were often portrayed in a negative light and referred to by derogatory pejoratives such as “bum” or “pothead.”5 Over time, society came to appreciate that good people, deserving of treatment, can become dependent upon substances, and in recent years there has been considerable support for related research initiatives and humane care. However, there has not been analogous support for individuals who manifest paraphilic disorders, especially those with pedophilic disorder. Instead, such individuals are often perceived as undeserving of mental health care and resources. This has been the case, even though successful treatment of a pedophilic disorder could help prevent the serious consequences of child molestation from occurring.
In contemporary society, the term pedophilia, which is a psychiatric specifier intended to guide research and treatment, has been hijacked by the nonmedical community and turned into a demeaning pejorative. In the collective consciousness of the public, the term pedophilia is routinely and mistakenly equated with the behavior of child molestation. Just as all alcoholics are not drunk drivers, all individuals with pedophilic disorder are not “child molesters.” Conversely, not all “child molesters” have pedophilic disorder.
Continue to: Individuals with other types...
Individuals with other types of paraphilic disorders are frequently similarly maligned and referred to as “perverts” or “deviants.” Public service announcements are frequently aired to reach out to individuals who are depressed, or drug- or alcohol-dependent, or suffering with other forms of mental disorders. When does one hear a public service announcement that encourages young people who may be experiencing disturbing or unacceptable sexual feelings to seek psychiatric treatment? There is a support group on the internet called Virtuous Pedophiles.6 That organization is unequivocally opposed to child molestation, while supporting efforts to improve the mental well-being of individuals who, through no fault of their own, experience unwanted pedophilic feelings.
Causes and noncauses
In attempting to elucidate etiology, researchers typically investigate nature (biology) and/or nurture (life experiences). In terms of the development of pedophilic disorder, there is evidence that both nature and nurture can play a role. Researchers have found that boys who are sexually abused are at increased risk for developing pedophilic disorder, and evidence of temporal lobe disturbances has also been documented in some instances.7,8
From clinical, societal, and forensic perspectives, it may be equally important to identify noncausal factors. Paraphilic disorders, including pedophilic disorder, do not develop as a consequence of volitional choice.9 For example, none of us decide which category, or categories, of potential partners are going to attract us sexually. Rather, in maturing we discover the nature of our own sexuality. Children do not ponder their options, somehow deciding while growing up to be attracted to the opposite sex (heterosexuality), the same sex (homosexuality), or both sexes (bisexuality). Similarly, in maturing into adulthood, individuals do not decide to become sexually attracted to prepubescent children. Who would decide to do that? Instead, unlike most of us, some individuals discover this about themselves; this often is a deeply disturbing insight.
It is not an individual’s fault that he or she has a paraphilic disorder. It is, however, his or her responsibility to do something about it. This may require accessing appropriate psychiatric care.
Why treatment may be needed
Sex is a powerful, biologically based appetite that recurrently craves satiation. God or nature has put that drive into all of us to ensure the survival of humanity. Even when that powerful biologic drive becomes misdirected (for example, towards children, or towards a desire to engage in public exhibitionism), it still recurrently craves satisfaction. It does not require mental health expertise to appreciate what a problematic situation this could become.
Continue to: Some individuals need help...
Some individuals need help in overcoming cravings related to nonsexual appetites. For example, Americans spend millions of dollars each year trying to diet; they often require some form of assistance in order to succeed. Individuals who crave drugs or alcohol often require mental health interventions to abstain because they are unable to consistently resist through willpower alone the powerful biologic urges that drive their actions.
The fundamental mental characteristic of any paraphilic disorder is the presence of intense, recurrent, sexual urges of an atypical nature. In the case of a pedophilic disorder, those urges involve sexual feelings about children.2 In the case of an exhibitionistic disorder, the afflicted individual experiences intense, recurrent sexual fantasies/urges related to exposing his genitals in public.1 Clearly, most men do not have to recurrently fight off the urge to act in such a fashion. Given the driven nature of intense erotic cravings, individuals who experience such cravings will frequently require access to competent mental health care.
Pharmacologic treatment of paraphilic disorders
In the future, we may develop a scientifically based understanding of the biologic factors that underlie qualitative differences in sexuality. At that point, it may become possible to intervene pharmacologically, changing the qualitative nature of a sexual urge with pharmacologic interventions. This cannot yet be done. H
In 1939, a Nobel Prize in Chemistry was awarded for the identification and isolation of the hormone testosterone, which energizes sexual drive.10 If an individual is hungering sexually to expose himself, to view child pornography, or to engage in sexual acts with children, the intensity of such hungers can be significantly reduced by lowering testosterone, thereby enhancing the capacity for successful sexual self-control.
A large body of scientific data has documented a marked decrease in sexually motivated behaviors when testosterone levels are significantly diminished.11 There is also evidence that recidivism rates of sexually motivated crimes can be significantly reduced when testosterone-lowering interventions are used.12
Continue to: Historically, removal of the testes...
Historically, removal of the testes (surgical castration) had been the only effective way to reliably lower testosterone. Today, this can be achieved pharmacologically. Use of a sex drive–lowering medication should be considered when either the clinician or the patient is concerned that a nonpharmacologic approach may be inadequate. In all instances, a patient with a paraphilic disorder should be informed that pharmacologic treatment is an option. A protocol for the pharmacologic treatment of paraphilic disorders that is based on my clinical experience is summarized in Table 2.
Leuprolide. A depot form of leuprolide is the most commonly employed agent to pharmacologically lower testosterone to treat a paraphilic disorder.13 When injected into muscle, leuprolide binds to it before gradually being released into the bloodstream. Previously, a depot medroxyprogesterone (a form of progesterone) had been used to treat paraphilic disorders.14 However, that had required weekly rather than monthly injections, and carried an increased risk of thrombotic emboli.
Prescribing leuprolide to treat a paraphilic disorder falls under FDA guidelines regarding the use of an approved drug for an “off-label” indication, and therefore is not considered investigational. For treating a paraphilic disorder, an effective dosage of leuprolide is 7.5 mg IM every 4 weeks. Long-term treatment is generally required, analogous to the management of diabetes. Because the initial injection of the series can cause a transient increase in testosterone (prior to its sustained decline), flutamide, a testosterone receptor blocking agent, is ordinarily prescribed for the first 14 days only, following initiation of treatment with depot leuprolide.15 Using flutamide in this fashion prevents the transient increase in testosterone from transiently increasing sexual drive. Flutamide should be discontinued after 14 days because long-term use can result in liver toxicity.
Some clinicians have been hesitant to prescribe leuprolide because of negative connotations associated with the term “chemical castration.” Unlike surgical castration, use of leuprolide is not a physically irreversible intervention, and does not result in sterility (although there may be an increase of atypical sperm and a decrease in total sperm production). The dosage can sometimes be titrated without a loss of efficacy.
In general, leuprolide’s safety protocol is well within the range associated with psychotropic medications.13 Low-risk adverse effects, such as hot flashes or cold sweats, may occur, especially during the period when hormone levels are in transition. There are no absolute contraindications to the use of leuprolide.
Continue to: Other medications
Other medications. Some researchers have suggested treating paraphilic disorders with psychotropic medications known to lower libido, such as selective serotonin reuptake inhibitors (SSRIs).16 However, leuprolide is far more reliable in consistently lowering testosterone and lowering the frequency and intensity of sexual urges. Although psychiatrists unfamiliar with treating paraphilic disorders may feel more comfortable initiating treatment with an SSRI, in my clinical experience, SSRIs have often proven inadequate for this purpose. When it comes to those paraphilic disorders in which treatment failure can result in significant harm (eg, pedophilic disorder), in my judgment, leuprolide should be the pharmacologic treatment of choice.
The opioid antagonist naltrexone has been used clinically to reduce cravings, primarily cravings for alcohol or drugs.17 However, I have not seen convincing evidence that it can be reliably beneficial in treating paraphilic disorders.
Tests to order before starting leuprolide
Long-term use of leuprolide can increase the risk of osteoporosis. Therefore, a baseline bone density scan should be performed before starting a patient on leuprolide. Baseline levels of testosterone, follicle-stimulating hormone, and luteinizing hormone also should be obtained. Patients should have yearly physical examinations, with accompanying laboratory testing. Hematocrit levels are often marginally low after beginning treatment, but not in a clinically significant way. Patients should also undergo routine monitoring for possible weight gain and the potential for associated hypertension. Treatment is predicated upon the known testosterone-suppressing effects of leuprolide, not upon routine monitoring of blood androgen levels.
Pharmacologic treatment of a paraphilic disorder should ordinarily occur in conjunction with nonpharmacologic modalities. One such modality would be group therapy, similar to the type frequently used to treat other craving disorders, such as drug or alcohol dependency.
In recent years, I have seen increasing numbers of patients presenting with a history of accessing and viewing child pornography. Once they have become more aware of the serious consequences of this behavior, most patients have been able to discontinue doing so without pharmacologic treatment. However, for patients in whom that behavior has seemed more driven (suggestive of a variant of voyeuristic disorder), prescription of leuprolide has been beneficial. Under such circumstances, I have diagnosed the patient’s condition as “other specified paraphilic disorder” with elements of pedophilia and voyeurism—the associated behaviors restricted to the voyeuristic viewing of child pornography.18
Continue to: Can treated patients still be sexual?
Can treated patients still be sexual?
If pharmacologic treatment of a paraphilic disorder results in erectile dysfunction, prescription of a medication such as sildenafil can be considered for patients who are in a consenting adult relationship, generally with the knowledge of their partner. Sildenafil can facilitate erectile capacity without increasing sexual drive. It can be helpful to explain to a patient that the purpose of pharmacologic treatment is not to prevent the enjoyment of sexual feelings within the context of a healthy, consenting, adult relationship, but instead to lower the intensity of problematic sexual urges, thereby facilitating sexual self-control. Just as lowering the appetite for food can make it easier to diet but not impossible to eat, lowering sexual appetite can facilitate successful self-control without necessarily interfering with erotic feelings experienced during sexual intimacy.
Bottom Line
Paraphilic disorders are not manifestations of a character flaw, but manifestations of unchosen qualitative differences in the nature of one’s sexual cravings. Not enough is yet known about the biology of sex to be able to pharmacologically alter its qualitative nature. However, pharmacologically lowering the intensity of a patient’s sexual drive can facilitate successful sexual self-regulation.
Related Resources
- LeVay S. Gay, straight and the reason why: the science of sexual orientation. London, UK: Oxford University Press; 2011.
- Rosler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338(7):416-422.
- Brown GR. Overview of paraphilic disorders (paraphilias). https://www.merckmanuals.com/professional/psychiatricdisorders/sexuality,-gender-dysphoria,-and-paraphilias/overview-of-paraphilic-disorders.
Drug Brand Names
Flutamide • Eulexin
Leuprolide injection •
Eligard, Lupron Depot
Medroxyprogesterone •
Provera
Naltrexone • Revia, Vivitrol
Progesterone • Prometrium
Sildenafil • Viagra
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:423-450.
2. Berlin FS. Pedophilia: criminal mind-set or mental disorder? A conceptual review. American Journal of Forensic Psychiatry. 2001;32(2):3-25.
3. Berlin FS. A conceptual overview and commentary on gender dysphoria. J Am Acad Psychiatry Law. 2016;44(2):246-252
4. Berlin FS. Pedophilia: when is a difference a disorder: Peer commentaries on Green (2002) and Schmidt (2002). Arch Sex Behav. 2002;31:1-2.
5. Ford B, Chase C. Betty: a glad awakening. New York, NY: Doubleday; 1987.
6. Virtuous Pedophiles. https://www.virped.org/. Accessed September 28, 2018.
7. Freund K, Kuban M. The basis of the abused abuser theory of pedophilia: A further elaboration of an earlier study. Arch Sex Behav. 1994;23(5):553-563.
8. Mendes MF, Chow T, Ringman T, et al. Pedophilia and temporal lobe disturbances. J Neuropsychiatry Clin Neurosci. 2000;12(1):71-76.
9. Money J. Love and love sickness: The science of sex, gender differences, and pair bonding. Baltimore, MD: Johns Hopkins University Press; 1980.
10. The Nobel Prize in Chemistry 1939. https://www.nobelprize.org/prizes/chemistry/1939/summary/. Accessed September 29, 2018.
11. Berlin FS. Commentary: The impact of surgical castration on sexual recidivism risk among civilly committed sex offenders. J Am Acad Psychiatry Law. 2005;33(1):37-41.
12. Hansen H. Treatment of dangerous sexual offenders. In: Seminar on Prison Health Services in Tampere, Finland. Helsinki, Finland: Ministry of Justice, Government Printing Centre; 1991:33-38.
13. Berlin FS. Risk/benefit ratio of androgen deprivation treatment for sex offenders. J Am Acad Psychiatry Law. 2009;37(1):59-62.
14. Berlin FS, Meinecke CF. Treatment of sex offenders with antiandrogenic medication: conceptualization, review of treatment modalities, and preliminary findings. Am J Psychiatry. 1981;138(5):601-607.
15. Neri R. Pharmacology and pharmacokinetics of flutamide. Urology. 1989;34(suppl 4):19-21; discussion 46-56.
16. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost consequences of selective serotonin receptor reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
17. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
18. Berlin FS. Commentary on pedophilia diagnostic criteria in DSM-5. J Am Acad Psychiatry Law. 2011;39(2):242-244.
In my role as the Director of The Johns Hopkins Sex and Gender Clinic, I have had the opportunity to provide care to 3 broad categories of patients: patients with sexual dysfunctions, patients experiencing gender dysphoria, and patients manifesting a paraphilic disorder. This article will not address sexual dysfunctions or gender dysphoria, but these terms are defined in the Box1-3 to clearly distinguish them from paraphilic disorders.
Box
Individuals with a sexual dysfunction (eg, erectile dysfunction or anorgasmia) generally experience conventional sexual feelings, but they may have difficulty performing sexually.1 Although ordinarily capable of adequate sexual performance, persons with a paraphilic disorder experience atypical erotic cravings.2 Such cravings can either be for an atypical or unacceptable category of potential partner (eg, animals or children), or for an atypical or unacceptable type of behavior (eg, crossdressing or public exhibitionism). Individuals with gender dysphoria frequently experience distress because their internal sense of feeling either male or female is not congruent with their external physical anatomy.3 The primary concerns of individuals experiencing gender dysphoria relate to feelings of gender identity, as opposed to problems involving erotic arousal.
Persons with paraphilic disorders (predominantly males) experience recurrent atypical sexual fantasies and urges that cause clinically significant impairment or distress.1 Those atypical fantasies and urges may be directed towards unacceptable partners such as animals or children, or towards unacceptable behaviors such as public exhibitionism. Table 11 lists the paraphilic disorders identified in DSM-5. This article focuses primarily, though not exclusively, upon pedophilic disorder, and its pharmacologic treatment. However, the rationale underlying such treatment is applicable across the paraphilic spectrum. Before providing such treatment, it is important for clinicians to have a clear conceptual understanding of paraphilic disorders.
When is a difference a disorder?
Cancer and respiration are 2 different biologic phenomenon. Cancer causes suffering and impairment, and as a consequence, we label it a disorder. We do so in the hope of learning more about it, and being able to successfully treat it. We do not classify respiration as a disorder because we do not consider it to be harmful.
The spectrum of human sexuality is quite broad, and psychiatry is generally not concerned with private sexual thoughts and behaviors involving consenting adults that do not cause suffering or impairment. When adults choose to engage in “kinky sex” that causes neither harm nor distress, so be it.
Some individuals may be privately aware of experiencing either an exclusive or nonexclusive sexual attraction to children. Some of these individuals may not be distressed by experiencing such attractions, and may be fully capable of resisting the temptation to enact them. In such an instance, even though an individual may be experiencing sexual attractions that are different from the norm, there may not be a sufficient basis for diagnosing pedophilic disorder. However, that difference in sexual phenomenology (ie, mental experience) could rise to the level of a diagnosable disorder if the individual in question expresses distress about experiencing such attractions, and/or if his capacity to resist acting upon them is impaired.4 Under such circumstances, treatment would be warranted.
Patients with paraphilic disorders deserve treatment
Prior to establishment of the Betty Ford Clinic in 1982, individuals who were drug- or alcohol-dependent were often portrayed in a negative light and referred to by derogatory pejoratives such as “bum” or “pothead.”5 Over time, society came to appreciate that good people, deserving of treatment, can become dependent upon substances, and in recent years there has been considerable support for related research initiatives and humane care. However, there has not been analogous support for individuals who manifest paraphilic disorders, especially those with pedophilic disorder. Instead, such individuals are often perceived as undeserving of mental health care and resources. This has been the case, even though successful treatment of a pedophilic disorder could help prevent the serious consequences of child molestation from occurring.
In contemporary society, the term pedophilia, which is a psychiatric specifier intended to guide research and treatment, has been hijacked by the nonmedical community and turned into a demeaning pejorative. In the collective consciousness of the public, the term pedophilia is routinely and mistakenly equated with the behavior of child molestation. Just as all alcoholics are not drunk drivers, all individuals with pedophilic disorder are not “child molesters.” Conversely, not all “child molesters” have pedophilic disorder.
Continue to: Individuals with other types...
Individuals with other types of paraphilic disorders are frequently similarly maligned and referred to as “perverts” or “deviants.” Public service announcements are frequently aired to reach out to individuals who are depressed, or drug- or alcohol-dependent, or suffering with other forms of mental disorders. When does one hear a public service announcement that encourages young people who may be experiencing disturbing or unacceptable sexual feelings to seek psychiatric treatment? There is a support group on the internet called Virtuous Pedophiles.6 That organization is unequivocally opposed to child molestation, while supporting efforts to improve the mental well-being of individuals who, through no fault of their own, experience unwanted pedophilic feelings.
Causes and noncauses
In attempting to elucidate etiology, researchers typically investigate nature (biology) and/or nurture (life experiences). In terms of the development of pedophilic disorder, there is evidence that both nature and nurture can play a role. Researchers have found that boys who are sexually abused are at increased risk for developing pedophilic disorder, and evidence of temporal lobe disturbances has also been documented in some instances.7,8
From clinical, societal, and forensic perspectives, it may be equally important to identify noncausal factors. Paraphilic disorders, including pedophilic disorder, do not develop as a consequence of volitional choice.9 For example, none of us decide which category, or categories, of potential partners are going to attract us sexually. Rather, in maturing we discover the nature of our own sexuality. Children do not ponder their options, somehow deciding while growing up to be attracted to the opposite sex (heterosexuality), the same sex (homosexuality), or both sexes (bisexuality). Similarly, in maturing into adulthood, individuals do not decide to become sexually attracted to prepubescent children. Who would decide to do that? Instead, unlike most of us, some individuals discover this about themselves; this often is a deeply disturbing insight.
It is not an individual’s fault that he or she has a paraphilic disorder. It is, however, his or her responsibility to do something about it. This may require accessing appropriate psychiatric care.
Why treatment may be needed
Sex is a powerful, biologically based appetite that recurrently craves satiation. God or nature has put that drive into all of us to ensure the survival of humanity. Even when that powerful biologic drive becomes misdirected (for example, towards children, or towards a desire to engage in public exhibitionism), it still recurrently craves satisfaction. It does not require mental health expertise to appreciate what a problematic situation this could become.
Continue to: Some individuals need help...
Some individuals need help in overcoming cravings related to nonsexual appetites. For example, Americans spend millions of dollars each year trying to diet; they often require some form of assistance in order to succeed. Individuals who crave drugs or alcohol often require mental health interventions to abstain because they are unable to consistently resist through willpower alone the powerful biologic urges that drive their actions.
The fundamental mental characteristic of any paraphilic disorder is the presence of intense, recurrent, sexual urges of an atypical nature. In the case of a pedophilic disorder, those urges involve sexual feelings about children.2 In the case of an exhibitionistic disorder, the afflicted individual experiences intense, recurrent sexual fantasies/urges related to exposing his genitals in public.1 Clearly, most men do not have to recurrently fight off the urge to act in such a fashion. Given the driven nature of intense erotic cravings, individuals who experience such cravings will frequently require access to competent mental health care.
Pharmacologic treatment of paraphilic disorders
In the future, we may develop a scientifically based understanding of the biologic factors that underlie qualitative differences in sexuality. At that point, it may become possible to intervene pharmacologically, changing the qualitative nature of a sexual urge with pharmacologic interventions. This cannot yet be done. H
In 1939, a Nobel Prize in Chemistry was awarded for the identification and isolation of the hormone testosterone, which energizes sexual drive.10 If an individual is hungering sexually to expose himself, to view child pornography, or to engage in sexual acts with children, the intensity of such hungers can be significantly reduced by lowering testosterone, thereby enhancing the capacity for successful sexual self-control.
A large body of scientific data has documented a marked decrease in sexually motivated behaviors when testosterone levels are significantly diminished.11 There is also evidence that recidivism rates of sexually motivated crimes can be significantly reduced when testosterone-lowering interventions are used.12
Continue to: Historically, removal of the testes...
Historically, removal of the testes (surgical castration) had been the only effective way to reliably lower testosterone. Today, this can be achieved pharmacologically. Use of a sex drive–lowering medication should be considered when either the clinician or the patient is concerned that a nonpharmacologic approach may be inadequate. In all instances, a patient with a paraphilic disorder should be informed that pharmacologic treatment is an option. A protocol for the pharmacologic treatment of paraphilic disorders that is based on my clinical experience is summarized in Table 2.
Leuprolide. A depot form of leuprolide is the most commonly employed agent to pharmacologically lower testosterone to treat a paraphilic disorder.13 When injected into muscle, leuprolide binds to it before gradually being released into the bloodstream. Previously, a depot medroxyprogesterone (a form of progesterone) had been used to treat paraphilic disorders.14 However, that had required weekly rather than monthly injections, and carried an increased risk of thrombotic emboli.
Prescribing leuprolide to treat a paraphilic disorder falls under FDA guidelines regarding the use of an approved drug for an “off-label” indication, and therefore is not considered investigational. For treating a paraphilic disorder, an effective dosage of leuprolide is 7.5 mg IM every 4 weeks. Long-term treatment is generally required, analogous to the management of diabetes. Because the initial injection of the series can cause a transient increase in testosterone (prior to its sustained decline), flutamide, a testosterone receptor blocking agent, is ordinarily prescribed for the first 14 days only, following initiation of treatment with depot leuprolide.15 Using flutamide in this fashion prevents the transient increase in testosterone from transiently increasing sexual drive. Flutamide should be discontinued after 14 days because long-term use can result in liver toxicity.
Some clinicians have been hesitant to prescribe leuprolide because of negative connotations associated with the term “chemical castration.” Unlike surgical castration, use of leuprolide is not a physically irreversible intervention, and does not result in sterility (although there may be an increase of atypical sperm and a decrease in total sperm production). The dosage can sometimes be titrated without a loss of efficacy.
In general, leuprolide’s safety protocol is well within the range associated with psychotropic medications.13 Low-risk adverse effects, such as hot flashes or cold sweats, may occur, especially during the period when hormone levels are in transition. There are no absolute contraindications to the use of leuprolide.
Continue to: Other medications
Other medications. Some researchers have suggested treating paraphilic disorders with psychotropic medications known to lower libido, such as selective serotonin reuptake inhibitors (SSRIs).16 However, leuprolide is far more reliable in consistently lowering testosterone and lowering the frequency and intensity of sexual urges. Although psychiatrists unfamiliar with treating paraphilic disorders may feel more comfortable initiating treatment with an SSRI, in my clinical experience, SSRIs have often proven inadequate for this purpose. When it comes to those paraphilic disorders in which treatment failure can result in significant harm (eg, pedophilic disorder), in my judgment, leuprolide should be the pharmacologic treatment of choice.
The opioid antagonist naltrexone has been used clinically to reduce cravings, primarily cravings for alcohol or drugs.17 However, I have not seen convincing evidence that it can be reliably beneficial in treating paraphilic disorders.
Tests to order before starting leuprolide
Long-term use of leuprolide can increase the risk of osteoporosis. Therefore, a baseline bone density scan should be performed before starting a patient on leuprolide. Baseline levels of testosterone, follicle-stimulating hormone, and luteinizing hormone also should be obtained. Patients should have yearly physical examinations, with accompanying laboratory testing. Hematocrit levels are often marginally low after beginning treatment, but not in a clinically significant way. Patients should also undergo routine monitoring for possible weight gain and the potential for associated hypertension. Treatment is predicated upon the known testosterone-suppressing effects of leuprolide, not upon routine monitoring of blood androgen levels.
Pharmacologic treatment of a paraphilic disorder should ordinarily occur in conjunction with nonpharmacologic modalities. One such modality would be group therapy, similar to the type frequently used to treat other craving disorders, such as drug or alcohol dependency.
In recent years, I have seen increasing numbers of patients presenting with a history of accessing and viewing child pornography. Once they have become more aware of the serious consequences of this behavior, most patients have been able to discontinue doing so without pharmacologic treatment. However, for patients in whom that behavior has seemed more driven (suggestive of a variant of voyeuristic disorder), prescription of leuprolide has been beneficial. Under such circumstances, I have diagnosed the patient’s condition as “other specified paraphilic disorder” with elements of pedophilia and voyeurism—the associated behaviors restricted to the voyeuristic viewing of child pornography.18
Continue to: Can treated patients still be sexual?
Can treated patients still be sexual?
If pharmacologic treatment of a paraphilic disorder results in erectile dysfunction, prescription of a medication such as sildenafil can be considered for patients who are in a consenting adult relationship, generally with the knowledge of their partner. Sildenafil can facilitate erectile capacity without increasing sexual drive. It can be helpful to explain to a patient that the purpose of pharmacologic treatment is not to prevent the enjoyment of sexual feelings within the context of a healthy, consenting, adult relationship, but instead to lower the intensity of problematic sexual urges, thereby facilitating sexual self-control. Just as lowering the appetite for food can make it easier to diet but not impossible to eat, lowering sexual appetite can facilitate successful self-control without necessarily interfering with erotic feelings experienced during sexual intimacy.
Bottom Line
Paraphilic disorders are not manifestations of a character flaw, but manifestations of unchosen qualitative differences in the nature of one’s sexual cravings. Not enough is yet known about the biology of sex to be able to pharmacologically alter its qualitative nature. However, pharmacologically lowering the intensity of a patient’s sexual drive can facilitate successful sexual self-regulation.
Related Resources
- LeVay S. Gay, straight and the reason why: the science of sexual orientation. London, UK: Oxford University Press; 2011.
- Rosler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338(7):416-422.
- Brown GR. Overview of paraphilic disorders (paraphilias). https://www.merckmanuals.com/professional/psychiatricdisorders/sexuality,-gender-dysphoria,-and-paraphilias/overview-of-paraphilic-disorders.
Drug Brand Names
Flutamide • Eulexin
Leuprolide injection •
Eligard, Lupron Depot
Medroxyprogesterone •
Provera
Naltrexone • Revia, Vivitrol
Progesterone • Prometrium
Sildenafil • Viagra
In my role as the Director of The Johns Hopkins Sex and Gender Clinic, I have had the opportunity to provide care to 3 broad categories of patients: patients with sexual dysfunctions, patients experiencing gender dysphoria, and patients manifesting a paraphilic disorder. This article will not address sexual dysfunctions or gender dysphoria, but these terms are defined in the Box1-3 to clearly distinguish them from paraphilic disorders.
Box
Individuals with a sexual dysfunction (eg, erectile dysfunction or anorgasmia) generally experience conventional sexual feelings, but they may have difficulty performing sexually.1 Although ordinarily capable of adequate sexual performance, persons with a paraphilic disorder experience atypical erotic cravings.2 Such cravings can either be for an atypical or unacceptable category of potential partner (eg, animals or children), or for an atypical or unacceptable type of behavior (eg, crossdressing or public exhibitionism). Individuals with gender dysphoria frequently experience distress because their internal sense of feeling either male or female is not congruent with their external physical anatomy.3 The primary concerns of individuals experiencing gender dysphoria relate to feelings of gender identity, as opposed to problems involving erotic arousal.
Persons with paraphilic disorders (predominantly males) experience recurrent atypical sexual fantasies and urges that cause clinically significant impairment or distress.1 Those atypical fantasies and urges may be directed towards unacceptable partners such as animals or children, or towards unacceptable behaviors such as public exhibitionism. Table 11 lists the paraphilic disorders identified in DSM-5. This article focuses primarily, though not exclusively, upon pedophilic disorder, and its pharmacologic treatment. However, the rationale underlying such treatment is applicable across the paraphilic spectrum. Before providing such treatment, it is important for clinicians to have a clear conceptual understanding of paraphilic disorders.
When is a difference a disorder?
Cancer and respiration are 2 different biologic phenomenon. Cancer causes suffering and impairment, and as a consequence, we label it a disorder. We do so in the hope of learning more about it, and being able to successfully treat it. We do not classify respiration as a disorder because we do not consider it to be harmful.
The spectrum of human sexuality is quite broad, and psychiatry is generally not concerned with private sexual thoughts and behaviors involving consenting adults that do not cause suffering or impairment. When adults choose to engage in “kinky sex” that causes neither harm nor distress, so be it.
Some individuals may be privately aware of experiencing either an exclusive or nonexclusive sexual attraction to children. Some of these individuals may not be distressed by experiencing such attractions, and may be fully capable of resisting the temptation to enact them. In such an instance, even though an individual may be experiencing sexual attractions that are different from the norm, there may not be a sufficient basis for diagnosing pedophilic disorder. However, that difference in sexual phenomenology (ie, mental experience) could rise to the level of a diagnosable disorder if the individual in question expresses distress about experiencing such attractions, and/or if his capacity to resist acting upon them is impaired.4 Under such circumstances, treatment would be warranted.
Patients with paraphilic disorders deserve treatment
Prior to establishment of the Betty Ford Clinic in 1982, individuals who were drug- or alcohol-dependent were often portrayed in a negative light and referred to by derogatory pejoratives such as “bum” or “pothead.”5 Over time, society came to appreciate that good people, deserving of treatment, can become dependent upon substances, and in recent years there has been considerable support for related research initiatives and humane care. However, there has not been analogous support for individuals who manifest paraphilic disorders, especially those with pedophilic disorder. Instead, such individuals are often perceived as undeserving of mental health care and resources. This has been the case, even though successful treatment of a pedophilic disorder could help prevent the serious consequences of child molestation from occurring.
In contemporary society, the term pedophilia, which is a psychiatric specifier intended to guide research and treatment, has been hijacked by the nonmedical community and turned into a demeaning pejorative. In the collective consciousness of the public, the term pedophilia is routinely and mistakenly equated with the behavior of child molestation. Just as all alcoholics are not drunk drivers, all individuals with pedophilic disorder are not “child molesters.” Conversely, not all “child molesters” have pedophilic disorder.
Continue to: Individuals with other types...
Individuals with other types of paraphilic disorders are frequently similarly maligned and referred to as “perverts” or “deviants.” Public service announcements are frequently aired to reach out to individuals who are depressed, or drug- or alcohol-dependent, or suffering with other forms of mental disorders. When does one hear a public service announcement that encourages young people who may be experiencing disturbing or unacceptable sexual feelings to seek psychiatric treatment? There is a support group on the internet called Virtuous Pedophiles.6 That organization is unequivocally opposed to child molestation, while supporting efforts to improve the mental well-being of individuals who, through no fault of their own, experience unwanted pedophilic feelings.
Causes and noncauses
In attempting to elucidate etiology, researchers typically investigate nature (biology) and/or nurture (life experiences). In terms of the development of pedophilic disorder, there is evidence that both nature and nurture can play a role. Researchers have found that boys who are sexually abused are at increased risk for developing pedophilic disorder, and evidence of temporal lobe disturbances has also been documented in some instances.7,8
From clinical, societal, and forensic perspectives, it may be equally important to identify noncausal factors. Paraphilic disorders, including pedophilic disorder, do not develop as a consequence of volitional choice.9 For example, none of us decide which category, or categories, of potential partners are going to attract us sexually. Rather, in maturing we discover the nature of our own sexuality. Children do not ponder their options, somehow deciding while growing up to be attracted to the opposite sex (heterosexuality), the same sex (homosexuality), or both sexes (bisexuality). Similarly, in maturing into adulthood, individuals do not decide to become sexually attracted to prepubescent children. Who would decide to do that? Instead, unlike most of us, some individuals discover this about themselves; this often is a deeply disturbing insight.
It is not an individual’s fault that he or she has a paraphilic disorder. It is, however, his or her responsibility to do something about it. This may require accessing appropriate psychiatric care.
Why treatment may be needed
Sex is a powerful, biologically based appetite that recurrently craves satiation. God or nature has put that drive into all of us to ensure the survival of humanity. Even when that powerful biologic drive becomes misdirected (for example, towards children, or towards a desire to engage in public exhibitionism), it still recurrently craves satisfaction. It does not require mental health expertise to appreciate what a problematic situation this could become.
Continue to: Some individuals need help...
Some individuals need help in overcoming cravings related to nonsexual appetites. For example, Americans spend millions of dollars each year trying to diet; they often require some form of assistance in order to succeed. Individuals who crave drugs or alcohol often require mental health interventions to abstain because they are unable to consistently resist through willpower alone the powerful biologic urges that drive their actions.
The fundamental mental characteristic of any paraphilic disorder is the presence of intense, recurrent, sexual urges of an atypical nature. In the case of a pedophilic disorder, those urges involve sexual feelings about children.2 In the case of an exhibitionistic disorder, the afflicted individual experiences intense, recurrent sexual fantasies/urges related to exposing his genitals in public.1 Clearly, most men do not have to recurrently fight off the urge to act in such a fashion. Given the driven nature of intense erotic cravings, individuals who experience such cravings will frequently require access to competent mental health care.
Pharmacologic treatment of paraphilic disorders
In the future, we may develop a scientifically based understanding of the biologic factors that underlie qualitative differences in sexuality. At that point, it may become possible to intervene pharmacologically, changing the qualitative nature of a sexual urge with pharmacologic interventions. This cannot yet be done. H
In 1939, a Nobel Prize in Chemistry was awarded for the identification and isolation of the hormone testosterone, which energizes sexual drive.10 If an individual is hungering sexually to expose himself, to view child pornography, or to engage in sexual acts with children, the intensity of such hungers can be significantly reduced by lowering testosterone, thereby enhancing the capacity for successful sexual self-control.
A large body of scientific data has documented a marked decrease in sexually motivated behaviors when testosterone levels are significantly diminished.11 There is also evidence that recidivism rates of sexually motivated crimes can be significantly reduced when testosterone-lowering interventions are used.12
Continue to: Historically, removal of the testes...
Historically, removal of the testes (surgical castration) had been the only effective way to reliably lower testosterone. Today, this can be achieved pharmacologically. Use of a sex drive–lowering medication should be considered when either the clinician or the patient is concerned that a nonpharmacologic approach may be inadequate. In all instances, a patient with a paraphilic disorder should be informed that pharmacologic treatment is an option. A protocol for the pharmacologic treatment of paraphilic disorders that is based on my clinical experience is summarized in Table 2.
Leuprolide. A depot form of leuprolide is the most commonly employed agent to pharmacologically lower testosterone to treat a paraphilic disorder.13 When injected into muscle, leuprolide binds to it before gradually being released into the bloodstream. Previously, a depot medroxyprogesterone (a form of progesterone) had been used to treat paraphilic disorders.14 However, that had required weekly rather than monthly injections, and carried an increased risk of thrombotic emboli.
Prescribing leuprolide to treat a paraphilic disorder falls under FDA guidelines regarding the use of an approved drug for an “off-label” indication, and therefore is not considered investigational. For treating a paraphilic disorder, an effective dosage of leuprolide is 7.5 mg IM every 4 weeks. Long-term treatment is generally required, analogous to the management of diabetes. Because the initial injection of the series can cause a transient increase in testosterone (prior to its sustained decline), flutamide, a testosterone receptor blocking agent, is ordinarily prescribed for the first 14 days only, following initiation of treatment with depot leuprolide.15 Using flutamide in this fashion prevents the transient increase in testosterone from transiently increasing sexual drive. Flutamide should be discontinued after 14 days because long-term use can result in liver toxicity.
Some clinicians have been hesitant to prescribe leuprolide because of negative connotations associated with the term “chemical castration.” Unlike surgical castration, use of leuprolide is not a physically irreversible intervention, and does not result in sterility (although there may be an increase of atypical sperm and a decrease in total sperm production). The dosage can sometimes be titrated without a loss of efficacy.
In general, leuprolide’s safety protocol is well within the range associated with psychotropic medications.13 Low-risk adverse effects, such as hot flashes or cold sweats, may occur, especially during the period when hormone levels are in transition. There are no absolute contraindications to the use of leuprolide.
Continue to: Other medications
Other medications. Some researchers have suggested treating paraphilic disorders with psychotropic medications known to lower libido, such as selective serotonin reuptake inhibitors (SSRIs).16 However, leuprolide is far more reliable in consistently lowering testosterone and lowering the frequency and intensity of sexual urges. Although psychiatrists unfamiliar with treating paraphilic disorders may feel more comfortable initiating treatment with an SSRI, in my clinical experience, SSRIs have often proven inadequate for this purpose. When it comes to those paraphilic disorders in which treatment failure can result in significant harm (eg, pedophilic disorder), in my judgment, leuprolide should be the pharmacologic treatment of choice.
The opioid antagonist naltrexone has been used clinically to reduce cravings, primarily cravings for alcohol or drugs.17 However, I have not seen convincing evidence that it can be reliably beneficial in treating paraphilic disorders.
Tests to order before starting leuprolide
Long-term use of leuprolide can increase the risk of osteoporosis. Therefore, a baseline bone density scan should be performed before starting a patient on leuprolide. Baseline levels of testosterone, follicle-stimulating hormone, and luteinizing hormone also should be obtained. Patients should have yearly physical examinations, with accompanying laboratory testing. Hematocrit levels are often marginally low after beginning treatment, but not in a clinically significant way. Patients should also undergo routine monitoring for possible weight gain and the potential for associated hypertension. Treatment is predicated upon the known testosterone-suppressing effects of leuprolide, not upon routine monitoring of blood androgen levels.
Pharmacologic treatment of a paraphilic disorder should ordinarily occur in conjunction with nonpharmacologic modalities. One such modality would be group therapy, similar to the type frequently used to treat other craving disorders, such as drug or alcohol dependency.
In recent years, I have seen increasing numbers of patients presenting with a history of accessing and viewing child pornography. Once they have become more aware of the serious consequences of this behavior, most patients have been able to discontinue doing so without pharmacologic treatment. However, for patients in whom that behavior has seemed more driven (suggestive of a variant of voyeuristic disorder), prescription of leuprolide has been beneficial. Under such circumstances, I have diagnosed the patient’s condition as “other specified paraphilic disorder” with elements of pedophilia and voyeurism—the associated behaviors restricted to the voyeuristic viewing of child pornography.18
Continue to: Can treated patients still be sexual?
Can treated patients still be sexual?
If pharmacologic treatment of a paraphilic disorder results in erectile dysfunction, prescription of a medication such as sildenafil can be considered for patients who are in a consenting adult relationship, generally with the knowledge of their partner. Sildenafil can facilitate erectile capacity without increasing sexual drive. It can be helpful to explain to a patient that the purpose of pharmacologic treatment is not to prevent the enjoyment of sexual feelings within the context of a healthy, consenting, adult relationship, but instead to lower the intensity of problematic sexual urges, thereby facilitating sexual self-control. Just as lowering the appetite for food can make it easier to diet but not impossible to eat, lowering sexual appetite can facilitate successful self-control without necessarily interfering with erotic feelings experienced during sexual intimacy.
Bottom Line
Paraphilic disorders are not manifestations of a character flaw, but manifestations of unchosen qualitative differences in the nature of one’s sexual cravings. Not enough is yet known about the biology of sex to be able to pharmacologically alter its qualitative nature. However, pharmacologically lowering the intensity of a patient’s sexual drive can facilitate successful sexual self-regulation.
Related Resources
- LeVay S. Gay, straight and the reason why: the science of sexual orientation. London, UK: Oxford University Press; 2011.
- Rosler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338(7):416-422.
- Brown GR. Overview of paraphilic disorders (paraphilias). https://www.merckmanuals.com/professional/psychiatricdisorders/sexuality,-gender-dysphoria,-and-paraphilias/overview-of-paraphilic-disorders.
Drug Brand Names
Flutamide • Eulexin
Leuprolide injection •
Eligard, Lupron Depot
Medroxyprogesterone •
Provera
Naltrexone • Revia, Vivitrol
Progesterone • Prometrium
Sildenafil • Viagra
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:423-450.
2. Berlin FS. Pedophilia: criminal mind-set or mental disorder? A conceptual review. American Journal of Forensic Psychiatry. 2001;32(2):3-25.
3. Berlin FS. A conceptual overview and commentary on gender dysphoria. J Am Acad Psychiatry Law. 2016;44(2):246-252
4. Berlin FS. Pedophilia: when is a difference a disorder: Peer commentaries on Green (2002) and Schmidt (2002). Arch Sex Behav. 2002;31:1-2.
5. Ford B, Chase C. Betty: a glad awakening. New York, NY: Doubleday; 1987.
6. Virtuous Pedophiles. https://www.virped.org/. Accessed September 28, 2018.
7. Freund K, Kuban M. The basis of the abused abuser theory of pedophilia: A further elaboration of an earlier study. Arch Sex Behav. 1994;23(5):553-563.
8. Mendes MF, Chow T, Ringman T, et al. Pedophilia and temporal lobe disturbances. J Neuropsychiatry Clin Neurosci. 2000;12(1):71-76.
9. Money J. Love and love sickness: The science of sex, gender differences, and pair bonding. Baltimore, MD: Johns Hopkins University Press; 1980.
10. The Nobel Prize in Chemistry 1939. https://www.nobelprize.org/prizes/chemistry/1939/summary/. Accessed September 29, 2018.
11. Berlin FS. Commentary: The impact of surgical castration on sexual recidivism risk among civilly committed sex offenders. J Am Acad Psychiatry Law. 2005;33(1):37-41.
12. Hansen H. Treatment of dangerous sexual offenders. In: Seminar on Prison Health Services in Tampere, Finland. Helsinki, Finland: Ministry of Justice, Government Printing Centre; 1991:33-38.
13. Berlin FS. Risk/benefit ratio of androgen deprivation treatment for sex offenders. J Am Acad Psychiatry Law. 2009;37(1):59-62.
14. Berlin FS, Meinecke CF. Treatment of sex offenders with antiandrogenic medication: conceptualization, review of treatment modalities, and preliminary findings. Am J Psychiatry. 1981;138(5):601-607.
15. Neri R. Pharmacology and pharmacokinetics of flutamide. Urology. 1989;34(suppl 4):19-21; discussion 46-56.
16. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost consequences of selective serotonin receptor reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
17. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
18. Berlin FS. Commentary on pedophilia diagnostic criteria in DSM-5. J Am Acad Psychiatry Law. 2011;39(2):242-244.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:423-450.
2. Berlin FS. Pedophilia: criminal mind-set or mental disorder? A conceptual review. American Journal of Forensic Psychiatry. 2001;32(2):3-25.
3. Berlin FS. A conceptual overview and commentary on gender dysphoria. J Am Acad Psychiatry Law. 2016;44(2):246-252
4. Berlin FS. Pedophilia: when is a difference a disorder: Peer commentaries on Green (2002) and Schmidt (2002). Arch Sex Behav. 2002;31:1-2.
5. Ford B, Chase C. Betty: a glad awakening. New York, NY: Doubleday; 1987.
6. Virtuous Pedophiles. https://www.virped.org/. Accessed September 28, 2018.
7. Freund K, Kuban M. The basis of the abused abuser theory of pedophilia: A further elaboration of an earlier study. Arch Sex Behav. 1994;23(5):553-563.
8. Mendes MF, Chow T, Ringman T, et al. Pedophilia and temporal lobe disturbances. J Neuropsychiatry Clin Neurosci. 2000;12(1):71-76.
9. Money J. Love and love sickness: The science of sex, gender differences, and pair bonding. Baltimore, MD: Johns Hopkins University Press; 1980.
10. The Nobel Prize in Chemistry 1939. https://www.nobelprize.org/prizes/chemistry/1939/summary/. Accessed September 29, 2018.
11. Berlin FS. Commentary: The impact of surgical castration on sexual recidivism risk among civilly committed sex offenders. J Am Acad Psychiatry Law. 2005;33(1):37-41.
12. Hansen H. Treatment of dangerous sexual offenders. In: Seminar on Prison Health Services in Tampere, Finland. Helsinki, Finland: Ministry of Justice, Government Printing Centre; 1991:33-38.
13. Berlin FS. Risk/benefit ratio of androgen deprivation treatment for sex offenders. J Am Acad Psychiatry Law. 2009;37(1):59-62.
14. Berlin FS, Meinecke CF. Treatment of sex offenders with antiandrogenic medication: conceptualization, review of treatment modalities, and preliminary findings. Am J Psychiatry. 1981;138(5):601-607.
15. Neri R. Pharmacology and pharmacokinetics of flutamide. Urology. 1989;34(suppl 4):19-21; discussion 46-56.
16. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost consequences of selective serotonin receptor reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
17. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
18. Berlin FS. Commentary on pedophilia diagnostic criteria in DSM-5. J Am Acad Psychiatry Law. 2011;39(2):242-244.
Cost-Effective Treatment Option for von Willebrand Disease
Click here to read the supplement.
What can be done to provide a cost-effective treatment option for von Willebrand Disease?
Topics include:
- Review of Key Clinical Studies and Surgical Procedures
- Pharmacokinetics Comparison
- Pharmacoeconomic Comparisons
Click here to read the supplement.
Click here to read the supplement.
What can be done to provide a cost-effective treatment option for von Willebrand Disease?
Topics include:
- Review of Key Clinical Studies and Surgical Procedures
- Pharmacokinetics Comparison
- Pharmacoeconomic Comparisons
Click here to read the supplement.
Click here to read the supplement.
What can be done to provide a cost-effective treatment option for von Willebrand Disease?
Topics include:
- Review of Key Clinical Studies and Surgical Procedures
- Pharmacokinetics Comparison
- Pharmacoeconomic Comparisons
Click here to read the supplement.
Enhancing Opportunities for Physical Activity Among Long-Term Care Residents: A Narrative Review
From the Geriatric Education and Research in Aging Sciences (GERAS) Centre for Aging Research, McMaster University, Hamilton, ON.
Abstract
- Objective. To summarize the literature on improving opportunities for physical activity for residents in long-term care (LTC).
- Method. Narrative review of the literature.
- Results. Residents in LTC spend much of their time in sedentary activities such as sitting or lying in bed. Physical activity is important to help decrease the negative effects of sedentary time, such as poor mood and increased risk of death, and to improve physical function. This review identifies several strategies for promoting physical activity for LTC residents: incorporating simple strategies into daily activities, participating in group activities (eg, exercise, dance, or music therapy), using motivational strategies to encourage staff to promote activity, leveraging the physical environment, reducing physical and chemical restraints, and using innovative solutions such as robots or interactive technology.
- Conclusion. While the quality of evidence to date is limited, preliminary work suggests that the strategies identified in this article could be included as part of a multifactorial approach to increasing physical activity in LTC.
Keywords: long-term care; nursing homes; physical activity; sedentary; mobility.
The United Nations estimates that between 2013 and 2050 the population aged 60 years or older will double.1 Furthermore, the fastest growth rate will be seen in older adults over the age of 80 years.1 With this demographic shift, a growing number of older adults will require supportive housing, such as long-term care (LTC). Indeed, it is projected that the number of older adults requiring LTC will double by 2036.2
Residents in LTC are often medically complex and experience multimorbidity, cognitive impairment, and functional decline,3 making it difficult for them to engage in physical activity. LTC residents spend approximately 75% of their waking time in sedentary activities (eg, sitting, lying down, watching TV), which amounts to more than 12 hours per day.4-6 Residents with cognitive impairment are even more sedentary, spending as little as 1 minute per day in moderate physical activity and approximately 87% of their time in sedentary activities.7 Additionally, a high prevalence of use of psychotropic drugs and physical restraints contributes to high levels of physical inactivity for residents in LTC.8 Increased time spent in sedentary activities has been associated with adverse health outcomes, such as incidence of cardiovascular disease and type 2 diabetes, and mortality.9-11 Moreover, bed and chair rest are associated with muscle disuse, which can lead to functional impairment.12,13
Given the large amount of time LTC residents spend in sedentary activities and the negative consequences this has on their health, it is essential to find opportunities to engage residents in physical activity throughout the day. This article summarizes evidence about increasing opportunities for physical activity for LTC residents. Physical activity is defined as “any bodily movement produced by skeletal muscles that results in energy expenditure,” while exercise, which is a subset of physical activity, is purposefully planned, structured, and repetitive and has a goal of maintaining or improving physical fitness.14 Previous work has described exercise among LTC residents in detail,8,15,16 and thus exercise is not addressed here. Also, as a narrative review, this article provides an overview of available interventions to improve physical activity for LTC residents and does not provide comments on efficacy or an exhaustive list of potential interventions. Rather, it provides a starting point for LTC homes to consider when providing opportunities to improve physical activity for their residents.
Guidelines for Increasing Physical Activity
There are currently no published evidence-based guidelines for increasing physical activity and reducing sedentary time for residents of LTC homes. However, an international task force of experts in geriatrics, exercise, and LTC research convened in 2015 and made recommendations on this matter.8 They emphasize the importance of considering the needs of residents, family members, health care professionals, LTC staff, and policy-makers when designing strategies to promote movement in LTC.8 This will ensure that the strategies to promote movement will be realistic and sustainable. Additionally, the task force identified motivation and pleasure as key to engaging residents in physical activities, and recommended that interests and preferences should be used to guide the selection of activities.8 The following sections describe example strategies to improve physical activity for residents in LTC that LTC homes can use to help facilitate movement for their residents.
Strategies for Promoting Physical Activity
Leveraging Daily Activities
One approach to promoting physical activity in LTC homes is to systematically use simple strategies embedded within routine care to engage residents in movement.8 Function-focused, or restorative care,17 is a philosophy of care that promotes increasing physical activity and maintaining functional abilities based on the resident’s abilities. Examples include walking with residents to the dining room rather than pushing them in a wheelchair where appropriate, inviting residents to events that require them to leave their room, improving independent wheelchair propulsion for residents who cannot walk, and increasing opportunities for sit-to-stand activities where possible. These activities are scaled to the resident’s underlying physical and cognitive capabilities. A systematic review of function-focused care revealed that it can help maintain functional skills for residents in LTC, and there is no significant risk associated with implementation.18 In a study by Slaughter et al19 that examined the effectiveness of techniques to encourage mobility by residents’ usual caregivers, health care aides prompted residents to perform the sit-to-stand activity 4 times per day, with the number of repetitions individualized based on resident ability, fatigue, and motivation. Residents who completed the sit-to-stand activity had smaller declines in mobility and functional outcomes (ie, less decline on the Functional Independence Measure).19 This study included residents with Alzheimer’s disease and dementia who could transfer independently or with the assistance of one person,20 indicating that this type of intervention is feasible and appropriate for residents with cognitive impairment.
Group Activities
Group activities in LTC homes are another way of engaging residents in physical activity in a motivating and pleasant setting that also encourages social engagement among residents and LTC staff. Group exercise classes can be effective for improving mood and functional outcomes. For example, a systematic review of dance classes in LTC homes revealed an improvement in problematic behaviors, mood, cognition, communication, and socialization.21 Most studies included participants with dementia, and no adverse events were reported, supporting the feasibility and safety of implementing group dance activities for residents with cognitive impairment. Group exercise is the most common delivery method for exercise within LTC homes22 and has been demonstrated to have small positive effects on activities of daily living (ADL; ie, improvement in ADL independence equivalent to 1.3 points on the Barthel Index).23 Other group activities, such as music therapy, have demonstrated improvements in depressive symptoms, emotional well-being, and anxiety for LTC residents with dementia.24 Group activities also provide the opportunity for movement as residents leave their rooms, walk to a new location (if able), and return to their rooms when the activity is complete.
Barriers to Physical Activity and Strategies to Overcome Them
Caregiver-related Factors
LTC staff have limited time to spend promoting physical activity since residents often have complex health care needs and staffing levels are often constrained.25 Indeed, having lower staffing levels has been associated with lower levels of physical activity for residents.26,27 LTC staff have identified a lack of time to walk with residents28,29 and having other tasks to do (eg, clean) as barriers to promoting movement.28,29 However, asking residents to help staff with small household chores, such as folding laundry or clearing dishes, was a facilitator to promoting movement.30 Activating residents by helping them transfer to a wheelchair for independent mobilization around the home or by assisting them to walk where appropriate were also facilitators.30,31 Leveraging facilitators will help staff who have limited time to help residents engage in more physical activity.
Motivation of LTC staff can also be a barrier to encouraging physical activity for residents in LTC. Fear that increasing physical activity will cause adverse events like falls, illness, or exacerbation of symptoms often decreases motivation for staff to facilitate physical activity.32,33 Another potential barrier is the conceptualization of the role of nursing in LTC as protecting residents from harm by encouraging them to engage in “risk-free” activities like staying in bed.34-39 Strategies to increase staff motivation to engage LTC residents in physical activity that have been shown to be effective are verbal prompts, modelling behaviors, goal setting, and home champions to promote function-focused care.17,33,40-43
The Physical Environment
Aspects of the physical environment of LTC homes may facilitate or limit residents’ ability to be physically active. A 2017 systematic review examined elements of the physical environment that acted as barriers and facilitators to physical activity for older adults living in LTC.30 The authors found that the person-environment fit, security, accessibility, and comfort were key components of the physical environment that were associated with residents’ physical activity levels.30 First, an appropriate fit between the residents’ abilities and the demands of the environment was related to improved activity as measured by actigraphy.44 For example, having long hallways between residents’ rooms and common spaces discourages residents who can only walk short distances from walking to these locations. However, residents were more active in larger-scaled LTC homes with shorter distances between different areas (eg, resident rooms and dining rooms).45 Clearly, there must be enough space to encourage walking between areas, but not so much space that walking is not feasible. Residents participating in a focus group identified accessibility and comfort features as being facilitators for walking in the corridor, such as wide corridors, sturdy handrails, carpet, chairs placed at short intervals for seated breaks, windows to look out, plants, and accessible activity rooms and restrooms.45,46 On the other hand, limited things to see and do indoors and outdoors, along with restricted walking areas, were identified as barriers to corridor walking by residents.46
One method for optimizing LTC home architecture to promote movement is to provide therapeutic outdoor spaces, such as gardens. Indeed, therapeutic gardens have been studied as a nonpharmacological method of engaging LTC residents with dementia and have been shown to benefit mood, pain, and fall prevention.47 Secure therapeutic gardens or outdoor spaces provide opportunities for various activities to increase movement, including gardening, animal care, and walking.48 However, there is a higher propensity for residents who use walkers or wheelchairs to slide off paths or become stuck in mud or mulch.49 Residents with physical limitations may require additional supervision in garden spaces, and as such spaces should be designed with improved safety in mind (eg, barriers between paths and places where mud could accumulate). The number of available indoor (eg, a physical therapy gym) and outdoor (eg, gardens) spaces was also found to be positively related to residents’ physical activity levels.50 However, these relationships were mediated by the number of activity programs available in the LTC homes.50 Therefore, having staff available to facilitate activities is also important for promoting physical activity.
Chemical and Physical Restraints
Physical and chemical restraints (eg, antipsychotics and sedatives) are sometimes used to manage the behavioral and psychological symptoms of dementia,51,52 which many residents in LTC experience.3 Though there has been an emphasis in North America to decrease their use, physical and chemical restraints are still used in LTC.53 Physical restraint use is associated with a higher risk of functional and cognitive decline.53,54 Residents who are both physically and chemically restrained through antipsychotic use are at even higher risk for these declines.54 Thus, to improve opportunities for movement in LTC, physical restraint use should be minimized. The risks and benefits of using psychotropic medications that often decrease residents’ physical activity levels must be evaluated individually, and other nonpharmacological strategies should be used to manage the behavioral and psychological symptoms of dementia. These could include functional analysis-based interventions (ie, individualized interventions aimed at identifying unmet needs, causes, antecedents, and consequences of the behavior),55 music therapy,55 or other interventions described above.
Emerging Innovative Interventions
Robots are an emerging nonpharmacological intervention for improving the behavioral and psychological symptoms of dementia and facilitating physical activity in LTC. Robotic animal interventions, where LTC residents interact with robotic animals in an individual or group setting, have been shown to reduce negative behaviors and increase positive mood.56 Additionally, robots are being used in rehabilitation to provide exercise post-stroke57 and could easily be transitioned to do similar tasks in LTC. Robotic interventions are attractive for the LTC sector as they could help relieve the workload demands on an often overloaded sector, and, in the case of pet therapy, surmount regulations for bringing live animals into a LTC home. Though studies examining the use of robots in LTC have mainly focused on the effect of pet therapy on reducing behavioral symptoms, the use of robots to promote physical activity and exercise in LTC is a natural progression for the work that has been done in inpatient rehabilitation.57 On a similar note, an interactive technology (similar to a Kinect system) used to promote 30-minute, twice-weekly physical activity sessions has demonstrated improvements in physical function (Short Physical Performance Battery [SPPB]) for pre-disabled (SPPB of 6 to 9) residents in LTC without dementia.58 The role of technology to promote physical activity in LTC is an emerging area of interest, and future innovations in this area will continue to help facilitate movement.
Quality of Evidence
Most studies aimed at improving physical activity for LTC residents to date are small, have nonrandomized designs, and have limited generalizability and evidence to support the efficacy of the interventions. For example, most studies included in systematic reviews for function-focused care, dance, group exercise, and music therapy are small, observational, or quasi-experimental studies with methodological issues resulting in bias.18,21,23,24 Likewise, the evidence surrounding nonpharmacological interventions for reducing behavioral and psychological symptoms of dementia is of very low to moderate quality.55 Innovative interventions, such as robotics and interactive technology, to promote physical activity in LTC are in their infancy. There are no data syntheses available to date to summarize the available literature on this topic, and conclusions rely on small, nonrandomized designs or extrapolations of results from similar sectors (eg, inpatient rehabilitation). Thus, the studies described in this review can be used as preliminary evidence to support the implementation of interventions to improve physical activity, but discretion should be used when interpreting the efficacy of these interventions.
Discussion
This review identifies several strategies for promoting physical activity for LTC residents, including incorporating simple strategies into daily activities, participating in group activities (eg, exercise, dance, or music therapy), using motivational strategies to encourage staff to promote activity, leveraging the physical environment, reducing physical and chemical restraints, and using innovation solutions like robots or interactive technology. While the quality of evidence to date is limited, preliminary work suggests that strategies identified in this paper could be included as part of a multifactorial approach to increasing physical activity in LTC.
The current evidence does not suggest that any one strategy is more effective at improving physical activity, and it is likely that LTC homes will need to employ a combination of strategies to help residents move more. Additionally, residents’ preferences, goals, and capabilities should always be considered when designing an individualized physical activity plan. For example, if a resident does not like to be outdoors or gardening but enjoys dancing and music, then their physical activity plan should include group dance class and music therapy rather than gardening. LTC homes will need to have a menu of opportunities for movement that residents can choose from so that activities are pleasant and motivating, and therefore more likely to be completed.
Many of the interventions described in this review are safe and feasible to implement with residents who have physical or cognitive impairments. Function-focused care is scaled to the residents’ capabilities and did not increase the risk of falling, though LTC staff require the skills to scale physical activities appropriately.18 Likewise, group dance activities and music therapy were tested with residents with dementia, with no adverse events reported.21,24 However, more work is needed to determine the feasibility of implementing emerging methods, such as robotics and interactive technology, for increasing physical activity for residents with physical and cognitive impairments. Most studies to date have included mobile residents or those with minimal cognitive impairment. Similarly, outdoor garden spaces may be less safe for residents who use walkers or wheelchairs if there is an opportunity for them to slip off paths or get stuck in mud or mulch. LTC homes implementing any of these interventions should evaluate the benefits and risks of each intervention, the resources available within the home to support them (eg, trained staff), and the target residents’ physical and cognitive capabilities.
While increasing physical activity is important, structured exercise is needed to see gains in components of physical fitness such as strength, aerobic capacity, and balance. Indeed, one major consideration highlighted by the aforementioned task force is that every resident who does not have contraindications must also have a personalized multicomponent exercise program as part of their care plan.8 The task force recommends moderate- to high-intensity strength, aerobic, and balance exercises 2 times per week for 35 to 45 minutes per session.8 There is an interrelationship between physical activity and structured exercise: structured exercise programs can certainly be part of a physical activity plan, but physical activity can include more than structured exercise. Physical activity also includes any activity that involves movement, such as walking in gardens or between home areas, or physically participating more in personal care activities (eg, assisting with bathing or dressing).14 Both structured exercise and physical activity are important for LTC residents. Structured exercise provides an opportunity to improve strength and cardiovascular fitness, which aim to decrease the negative effects of sarcopenia and cardiovascular disease, such as disability and death.59,60 However, structured exercise should not be done daily for the same muscle groups.8 Rather, it is recommended for LTC residents to engage in structured exercise 2 times per week.8 Increasing physical activity is a daily goal, as daily physical activity decreases sedentary time, which has negative consequences such as decreased mood61 and increased mortality.62 LTC homes should incorporate strategies to both increase daily physical activity and promote individualized, structured exercise programs.
Conclusion
Residents in LTC spend much of their time in sedentary activities such as sitting or lying in bed. Physical activity is important to help decrease the negative effects of sedentary time, like poor mood and increased risk of death, and to improve physical function. This review describes several strategies to promote physical activity within LTC homes, such as leveraging daily activities and the physical environment, providing group activities, reducing physical and chemical restraint use, and using innovative technology such as robots. LTC homes can use the information in this review to plan strategies to promote physical activity.
Corresponding author: Caitlin McArthur, MScPT, PhD, Hamilton Health Sciences, St. Peter’s Hospital, 88 Maplewood Avenue, Hamilton, ON L8M 1W9; [email protected].
Financial disclosures: None.
1. United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2013. New York, NY: United Nations; 2013.
2. Pickard L, Comas-Herrera A, Costa-Font J, et al. Modelling an entitlement to long-term care services for older people in Europe: projections for long-term care expenditure to 2050. J Eur Soc Policy. 2007;17:33-48.
3. Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Can J Aging. 2011;30:371-390.
4. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr. 2006;6:9.
5. Ikezoe T, Asakawa Y, Shima H, Kishibuchi K, Ichihashi N. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr. 2013;57:221-225.
6. Keogh JW, Senior H, Beller EM, Henwood T. Prevalence and risk factors for low habitual walking speed in nursing home residents: an observational study. Arch Phys Med Rehabil. 2015;96:1993-1999.
7. Marmeleira J, Ferreira S, Raimundo A. Physical activity and physical fitness of nursing home residents with cognitive impairment: A pilot study. Exp Gerontol. 2017;100:63-69.
8. de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc. 2016;17:381-392.
9. van der Ploeg HP, Chey T, Korda RJ, et al. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494-500.
10. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8:e80000.
11. Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448-2455.
12. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82:418-423.
13. Wall BT, Dirks ML, van Loon LJC. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12:898-906.
14. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131.
15. McArthur C, Giangregorio LM. Improving strength and balance for long-term care residents at risk for falling: Suggestions for practice. J Clin Outcomes Manag. 2018;25:28-38.
16. Crocker T, Forster A, Young J, et al. Physical rehabilitation for older people in long-term care. Cochrane Database Syst Rev. 2013;2:CD004294.
17. Resnick B, Galik E, Boltz M, Pretzer-Aboff IE. Implementing Restorative Care Nursing in All Settings. 2nd ed. New York, NY: Spring Publishing Company; 2011.
18. Resnick B, Galik E, Boltz M. Function focused care approaches: literature review of progress and future possibilities. J Am Med Dir Assoc. 2013;14:313-318.
19. Slaughter SE, Estabrooks CA, Jones CA, Wagg AS. Mobility of Vulnerable Elders (MOVE): study protocol to evaluate the implementation and outcomes of a mobility intervention in long-term care facilities. BMC Geriatr. 2011;11:84.
20. Slaughter SE, Wagg AS, Jones CA, et al. Mobility of Vulnerable Elders study: effect of the sit-to-stand activity on mobility, function, and quality of life. J Am Med Dir Assoc. 2015;16(2):138-143.
21. Guzmán-García A, Hughes JC, James IA, Rochester L. Dancing as a psychosocial intervention in care homes: a systematic review of the literature. Int J Geriatr Psychiatry. 2013;28:914-924.
22. McArthur C, Gibbs JC, Patel R, et al. A scoping review of physical rehabilitation in long-term care: interventions, outcomes, tools. Can J Aging/La Rev Can du Vieil. 2017;36:435-452.
23. Crocker T, Young J, Forster A, et al. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: Systematic review with meta-analysis. Age Ageing. 2013;42:682-688.
24. van der Steen JT, Smaling HJ, van der Wouden JC, et al. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. 2018;7:CD003477.
25. Froggatt K, Davies S, Meyer J. Understanding Care Homes, A Research and Development Perspective. London: Jessica Kingsley Publishers; 2009.
26. Shore BA, Lerman DC, Smith RG, et al. Direct assessment of quality of care in a geriatric nursing home. J Appl Behav Anal. 1995;28:435-448.
27. Bates-Jensen BM, Schnelle JF, Alessi CA, et al. The effects of staffing on in-bed times of nursing home residents. J Am Geriatr Soc. 2004;52:931-938.
28. Ericson-Lidman E, Renström A-S, Åhlin J, Strandberg G. Relatives’ perceptions of residents’ life in a municipal care facility for older people with a focus on quality of life and care environment. Int J Older People Nurs. 2015;10:160-169.
29. Häggström E, Kihlgren A, Kihlgren M, Sörlie V. Relatives’ struggle for an improved and more just care for older people in community care. J Clin Nurs. 2007;16:1749-1757.
30. Douma JG, Volkers KM, Engels G, et al. Setting-related influences on physical inactivity of older adults in residential care settings: a review. BMC Geriatr. 2017;17:97.
31. Zegelin A. ’Tied down’- the process of becoming bedridden through gradual local confinement. J Clin Nurs. 2008;17:2294-2301.
32. Resnick B, Galik E, Gruber-Baldini AL, Zimmerman S. Falls and fall-related injuries associated with function-focused care. Clin Nurs Res. 2012;21:43-63.
33. Pretzer-Aboff I, Galik E. Feasibility and impact of a function focused care intervention for Parkinson’s disease in the community. Nursing Res. 2011;60:276-283.
34. Leditschke IA, Green M, Irvine J, et al. What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J. 2012;23:26-29.
35. Mittmann N, Seung SJ, Pisterzi LF, et al. Nursing workload associated with hospital patient care. Dis Manag Heal Outcomes. 2008;16:53-61.
36. Dykes PC, Carroll DL, Hurley AC, et al. Why do patients in acute care hospitals fall? Can falls be prevented? J Nurs Adm. 2009;39:299-304.
37. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging. 2013;8:1-10.
38. Wakefield BJ, Holman JE. Functional trajectories associated with hospitalization in older adults. West J Nurs Res. 2007;29:161-177.
39. Boltz M, Resnick B, Capezuti E, Shuluk J. Activity restriction vs. self-direction: hospitalised older adults’ response to fear of falling. Int J Older People Nurs. 2014;9:44-53.
40. Resnick B, Galik E, Gruber-Baldini A, Zimmerman S. Testing the effect of function-focused care in assisted living. J Am Geriatr Soc. 2011;59:2233-2240.
41. Galik EM, Resnick B, Gruber-Baldini A, et al. Pilot testing of the restorative care intervention for the cognitively impaired. J Am Med Dir Assoc. 2008;9:516-522.
42. Resnick B, Galik E, Gruber-Baldini AL, Zimmerman S. Implementing a restorative care philosophy of care in assisted living: pilot testing of Res-Care-AL. J Am Acad Nurse Pract. 2009;21:123-133.
43. Resnick B, Gruber-Baldini AL, Zimmerman S, et al. Nursing home resident outcomes from the Res-Care intervention. J Am Geriatr Soc. 2009;57:1156-1165.
44. Pomeroy SH, Scherer Y, Runkawatt V, et al. Person-environment fit and functioning among older adults in a long-term care setting. Geriatr Nurs. 2011;32:368-378.
45. Moos RH, David TG, Lemke S, Postle E. Coping with an intra-institutional relocation: changes in resident and staff behavior patterns. Gerontologist. 1984;24:495-502.
46. Lu Z, Rodiek SD, Shepley MM, Duffy M. Influences of physical environment on corridor walking among assisted living residents. J Appl Gerontol. 2011;30:463-484.
47. Detweiler MB, Sharma T, Detweiler JG, et al. What is the evidence to support the use of therapeutic gardens for the elderly? Psychiatry Investig. 2012;9:100.
48. Blake M, Mitchell G. Horticultural therapy in dementia care: a literature review. Nurs Stand. 2016;30:41-47.
49. Detweiler MB, Murphy PF, Myers LC, Kim KY. Does a wander garden influence inappropriate behaviors in dementia residents? Am J Alzheimers Dis Other Demen. 2008;23:31-45.
50. Joseph A, Zimring C, Harris-Kojetin L, Kiefer K. Presence and visibility of outdoor and indoor physical activity features and participation in physical activity among older adults in retirement communities. J Hous Elderly. 2006;19:141-165.
51. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24:1110-1118.
52. Herrmann N. Recommendations for the management of behavioral and psychological symptoms of dementia. Can J Neurol Sci. 2001;28 Suppl 1:S96-107.
53. Freeman S, Spirgiene L, Martin-Khan M, Hirdes JP. Relationship between restraint use, engagement in social activity, and decline in cognitive status among residents newly admitted to long-term care facilities. Geriatr Gerontol Int. 2017;17:246-255.
54. Foebel AD, Onder G, Finne-Soveri H, et al. Physical restraint and antipsychotic medication use among nursing home residents with dementia. J Am Med Dir Assoc. 2016;17:184.e9-184.e14.
55. Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30:295-309.
56. Robinson H, MacDonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667.
57. Lo K, Stephenson M, Lockwood C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients. JBI Database System Rev Implement Rep. 2017;15:3049-3091.
58. Valiani V, Lauzé M, Martel D, et al. A new adaptive home-based exercise technology among older adults living in nursing home: a pilot study on feasibility, acceptability and physical performance. J Nutr Health Aging. 2017;21:819-824.
59. Locquet M, Beaudart C, Hajaoui M, et al. Three-year adverse health consequences of sarcopenia in community-dwelling older adults according to 5 diagnosis definitions. J Am Med Dir Assoc. 2019;20:43-46.e2.
60. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510-1530.
61. Park S-Y, Lee K, Um YJ, Paek S, Ryou IS. Association between physical activity and depressive mood among Korean adults with chronic diseases. Korean J Fam Med. 2018;39:185-190.
62. Loprinzi PD. Light-intensity physical activity and all-cause mortality. Am J Health Promot. 2017;31:340-342.
From the Geriatric Education and Research in Aging Sciences (GERAS) Centre for Aging Research, McMaster University, Hamilton, ON.
Abstract
- Objective. To summarize the literature on improving opportunities for physical activity for residents in long-term care (LTC).
- Method. Narrative review of the literature.
- Results. Residents in LTC spend much of their time in sedentary activities such as sitting or lying in bed. Physical activity is important to help decrease the negative effects of sedentary time, such as poor mood and increased risk of death, and to improve physical function. This review identifies several strategies for promoting physical activity for LTC residents: incorporating simple strategies into daily activities, participating in group activities (eg, exercise, dance, or music therapy), using motivational strategies to encourage staff to promote activity, leveraging the physical environment, reducing physical and chemical restraints, and using innovative solutions such as robots or interactive technology.
- Conclusion. While the quality of evidence to date is limited, preliminary work suggests that the strategies identified in this article could be included as part of a multifactorial approach to increasing physical activity in LTC.
Keywords: long-term care; nursing homes; physical activity; sedentary; mobility.
The United Nations estimates that between 2013 and 2050 the population aged 60 years or older will double.1 Furthermore, the fastest growth rate will be seen in older adults over the age of 80 years.1 With this demographic shift, a growing number of older adults will require supportive housing, such as long-term care (LTC). Indeed, it is projected that the number of older adults requiring LTC will double by 2036.2
Residents in LTC are often medically complex and experience multimorbidity, cognitive impairment, and functional decline,3 making it difficult for them to engage in physical activity. LTC residents spend approximately 75% of their waking time in sedentary activities (eg, sitting, lying down, watching TV), which amounts to more than 12 hours per day.4-6 Residents with cognitive impairment are even more sedentary, spending as little as 1 minute per day in moderate physical activity and approximately 87% of their time in sedentary activities.7 Additionally, a high prevalence of use of psychotropic drugs and physical restraints contributes to high levels of physical inactivity for residents in LTC.8 Increased time spent in sedentary activities has been associated with adverse health outcomes, such as incidence of cardiovascular disease and type 2 diabetes, and mortality.9-11 Moreover, bed and chair rest are associated with muscle disuse, which can lead to functional impairment.12,13
Given the large amount of time LTC residents spend in sedentary activities and the negative consequences this has on their health, it is essential to find opportunities to engage residents in physical activity throughout the day. This article summarizes evidence about increasing opportunities for physical activity for LTC residents. Physical activity is defined as “any bodily movement produced by skeletal muscles that results in energy expenditure,” while exercise, which is a subset of physical activity, is purposefully planned, structured, and repetitive and has a goal of maintaining or improving physical fitness.14 Previous work has described exercise among LTC residents in detail,8,15,16 and thus exercise is not addressed here. Also, as a narrative review, this article provides an overview of available interventions to improve physical activity for LTC residents and does not provide comments on efficacy or an exhaustive list of potential interventions. Rather, it provides a starting point for LTC homes to consider when providing opportunities to improve physical activity for their residents.
Guidelines for Increasing Physical Activity
There are currently no published evidence-based guidelines for increasing physical activity and reducing sedentary time for residents of LTC homes. However, an international task force of experts in geriatrics, exercise, and LTC research convened in 2015 and made recommendations on this matter.8 They emphasize the importance of considering the needs of residents, family members, health care professionals, LTC staff, and policy-makers when designing strategies to promote movement in LTC.8 This will ensure that the strategies to promote movement will be realistic and sustainable. Additionally, the task force identified motivation and pleasure as key to engaging residents in physical activities, and recommended that interests and preferences should be used to guide the selection of activities.8 The following sections describe example strategies to improve physical activity for residents in LTC that LTC homes can use to help facilitate movement for their residents.
Strategies for Promoting Physical Activity
Leveraging Daily Activities
One approach to promoting physical activity in LTC homes is to systematically use simple strategies embedded within routine care to engage residents in movement.8 Function-focused, or restorative care,17 is a philosophy of care that promotes increasing physical activity and maintaining functional abilities based on the resident’s abilities. Examples include walking with residents to the dining room rather than pushing them in a wheelchair where appropriate, inviting residents to events that require them to leave their room, improving independent wheelchair propulsion for residents who cannot walk, and increasing opportunities for sit-to-stand activities where possible. These activities are scaled to the resident’s underlying physical and cognitive capabilities. A systematic review of function-focused care revealed that it can help maintain functional skills for residents in LTC, and there is no significant risk associated with implementation.18 In a study by Slaughter et al19 that examined the effectiveness of techniques to encourage mobility by residents’ usual caregivers, health care aides prompted residents to perform the sit-to-stand activity 4 times per day, with the number of repetitions individualized based on resident ability, fatigue, and motivation. Residents who completed the sit-to-stand activity had smaller declines in mobility and functional outcomes (ie, less decline on the Functional Independence Measure).19 This study included residents with Alzheimer’s disease and dementia who could transfer independently or with the assistance of one person,20 indicating that this type of intervention is feasible and appropriate for residents with cognitive impairment.
Group Activities
Group activities in LTC homes are another way of engaging residents in physical activity in a motivating and pleasant setting that also encourages social engagement among residents and LTC staff. Group exercise classes can be effective for improving mood and functional outcomes. For example, a systematic review of dance classes in LTC homes revealed an improvement in problematic behaviors, mood, cognition, communication, and socialization.21 Most studies included participants with dementia, and no adverse events were reported, supporting the feasibility and safety of implementing group dance activities for residents with cognitive impairment. Group exercise is the most common delivery method for exercise within LTC homes22 and has been demonstrated to have small positive effects on activities of daily living (ADL; ie, improvement in ADL independence equivalent to 1.3 points on the Barthel Index).23 Other group activities, such as music therapy, have demonstrated improvements in depressive symptoms, emotional well-being, and anxiety for LTC residents with dementia.24 Group activities also provide the opportunity for movement as residents leave their rooms, walk to a new location (if able), and return to their rooms when the activity is complete.
Barriers to Physical Activity and Strategies to Overcome Them
Caregiver-related Factors
LTC staff have limited time to spend promoting physical activity since residents often have complex health care needs and staffing levels are often constrained.25 Indeed, having lower staffing levels has been associated with lower levels of physical activity for residents.26,27 LTC staff have identified a lack of time to walk with residents28,29 and having other tasks to do (eg, clean) as barriers to promoting movement.28,29 However, asking residents to help staff with small household chores, such as folding laundry or clearing dishes, was a facilitator to promoting movement.30 Activating residents by helping them transfer to a wheelchair for independent mobilization around the home or by assisting them to walk where appropriate were also facilitators.30,31 Leveraging facilitators will help staff who have limited time to help residents engage in more physical activity.
Motivation of LTC staff can also be a barrier to encouraging physical activity for residents in LTC. Fear that increasing physical activity will cause adverse events like falls, illness, or exacerbation of symptoms often decreases motivation for staff to facilitate physical activity.32,33 Another potential barrier is the conceptualization of the role of nursing in LTC as protecting residents from harm by encouraging them to engage in “risk-free” activities like staying in bed.34-39 Strategies to increase staff motivation to engage LTC residents in physical activity that have been shown to be effective are verbal prompts, modelling behaviors, goal setting, and home champions to promote function-focused care.17,33,40-43
The Physical Environment
Aspects of the physical environment of LTC homes may facilitate or limit residents’ ability to be physically active. A 2017 systematic review examined elements of the physical environment that acted as barriers and facilitators to physical activity for older adults living in LTC.30 The authors found that the person-environment fit, security, accessibility, and comfort were key components of the physical environment that were associated with residents’ physical activity levels.30 First, an appropriate fit between the residents’ abilities and the demands of the environment was related to improved activity as measured by actigraphy.44 For example, having long hallways between residents’ rooms and common spaces discourages residents who can only walk short distances from walking to these locations. However, residents were more active in larger-scaled LTC homes with shorter distances between different areas (eg, resident rooms and dining rooms).45 Clearly, there must be enough space to encourage walking between areas, but not so much space that walking is not feasible. Residents participating in a focus group identified accessibility and comfort features as being facilitators for walking in the corridor, such as wide corridors, sturdy handrails, carpet, chairs placed at short intervals for seated breaks, windows to look out, plants, and accessible activity rooms and restrooms.45,46 On the other hand, limited things to see and do indoors and outdoors, along with restricted walking areas, were identified as barriers to corridor walking by residents.46
One method for optimizing LTC home architecture to promote movement is to provide therapeutic outdoor spaces, such as gardens. Indeed, therapeutic gardens have been studied as a nonpharmacological method of engaging LTC residents with dementia and have been shown to benefit mood, pain, and fall prevention.47 Secure therapeutic gardens or outdoor spaces provide opportunities for various activities to increase movement, including gardening, animal care, and walking.48 However, there is a higher propensity for residents who use walkers or wheelchairs to slide off paths or become stuck in mud or mulch.49 Residents with physical limitations may require additional supervision in garden spaces, and as such spaces should be designed with improved safety in mind (eg, barriers between paths and places where mud could accumulate). The number of available indoor (eg, a physical therapy gym) and outdoor (eg, gardens) spaces was also found to be positively related to residents’ physical activity levels.50 However, these relationships were mediated by the number of activity programs available in the LTC homes.50 Therefore, having staff available to facilitate activities is also important for promoting physical activity.
Chemical and Physical Restraints
Physical and chemical restraints (eg, antipsychotics and sedatives) are sometimes used to manage the behavioral and psychological symptoms of dementia,51,52 which many residents in LTC experience.3 Though there has been an emphasis in North America to decrease their use, physical and chemical restraints are still used in LTC.53 Physical restraint use is associated with a higher risk of functional and cognitive decline.53,54 Residents who are both physically and chemically restrained through antipsychotic use are at even higher risk for these declines.54 Thus, to improve opportunities for movement in LTC, physical restraint use should be minimized. The risks and benefits of using psychotropic medications that often decrease residents’ physical activity levels must be evaluated individually, and other nonpharmacological strategies should be used to manage the behavioral and psychological symptoms of dementia. These could include functional analysis-based interventions (ie, individualized interventions aimed at identifying unmet needs, causes, antecedents, and consequences of the behavior),55 music therapy,55 or other interventions described above.
Emerging Innovative Interventions
Robots are an emerging nonpharmacological intervention for improving the behavioral and psychological symptoms of dementia and facilitating physical activity in LTC. Robotic animal interventions, where LTC residents interact with robotic animals in an individual or group setting, have been shown to reduce negative behaviors and increase positive mood.56 Additionally, robots are being used in rehabilitation to provide exercise post-stroke57 and could easily be transitioned to do similar tasks in LTC. Robotic interventions are attractive for the LTC sector as they could help relieve the workload demands on an often overloaded sector, and, in the case of pet therapy, surmount regulations for bringing live animals into a LTC home. Though studies examining the use of robots in LTC have mainly focused on the effect of pet therapy on reducing behavioral symptoms, the use of robots to promote physical activity and exercise in LTC is a natural progression for the work that has been done in inpatient rehabilitation.57 On a similar note, an interactive technology (similar to a Kinect system) used to promote 30-minute, twice-weekly physical activity sessions has demonstrated improvements in physical function (Short Physical Performance Battery [SPPB]) for pre-disabled (SPPB of 6 to 9) residents in LTC without dementia.58 The role of technology to promote physical activity in LTC is an emerging area of interest, and future innovations in this area will continue to help facilitate movement.
Quality of Evidence
Most studies aimed at improving physical activity for LTC residents to date are small, have nonrandomized designs, and have limited generalizability and evidence to support the efficacy of the interventions. For example, most studies included in systematic reviews for function-focused care, dance, group exercise, and music therapy are small, observational, or quasi-experimental studies with methodological issues resulting in bias.18,21,23,24 Likewise, the evidence surrounding nonpharmacological interventions for reducing behavioral and psychological symptoms of dementia is of very low to moderate quality.55 Innovative interventions, such as robotics and interactive technology, to promote physical activity in LTC are in their infancy. There are no data syntheses available to date to summarize the available literature on this topic, and conclusions rely on small, nonrandomized designs or extrapolations of results from similar sectors (eg, inpatient rehabilitation). Thus, the studies described in this review can be used as preliminary evidence to support the implementation of interventions to improve physical activity, but discretion should be used when interpreting the efficacy of these interventions.
Discussion
This review identifies several strategies for promoting physical activity for LTC residents, including incorporating simple strategies into daily activities, participating in group activities (eg, exercise, dance, or music therapy), using motivational strategies to encourage staff to promote activity, leveraging the physical environment, reducing physical and chemical restraints, and using innovation solutions like robots or interactive technology. While the quality of evidence to date is limited, preliminary work suggests that strategies identified in this paper could be included as part of a multifactorial approach to increasing physical activity in LTC.
The current evidence does not suggest that any one strategy is more effective at improving physical activity, and it is likely that LTC homes will need to employ a combination of strategies to help residents move more. Additionally, residents’ preferences, goals, and capabilities should always be considered when designing an individualized physical activity plan. For example, if a resident does not like to be outdoors or gardening but enjoys dancing and music, then their physical activity plan should include group dance class and music therapy rather than gardening. LTC homes will need to have a menu of opportunities for movement that residents can choose from so that activities are pleasant and motivating, and therefore more likely to be completed.
Many of the interventions described in this review are safe and feasible to implement with residents who have physical or cognitive impairments. Function-focused care is scaled to the residents’ capabilities and did not increase the risk of falling, though LTC staff require the skills to scale physical activities appropriately.18 Likewise, group dance activities and music therapy were tested with residents with dementia, with no adverse events reported.21,24 However, more work is needed to determine the feasibility of implementing emerging methods, such as robotics and interactive technology, for increasing physical activity for residents with physical and cognitive impairments. Most studies to date have included mobile residents or those with minimal cognitive impairment. Similarly, outdoor garden spaces may be less safe for residents who use walkers or wheelchairs if there is an opportunity for them to slip off paths or get stuck in mud or mulch. LTC homes implementing any of these interventions should evaluate the benefits and risks of each intervention, the resources available within the home to support them (eg, trained staff), and the target residents’ physical and cognitive capabilities.
While increasing physical activity is important, structured exercise is needed to see gains in components of physical fitness such as strength, aerobic capacity, and balance. Indeed, one major consideration highlighted by the aforementioned task force is that every resident who does not have contraindications must also have a personalized multicomponent exercise program as part of their care plan.8 The task force recommends moderate- to high-intensity strength, aerobic, and balance exercises 2 times per week for 35 to 45 minutes per session.8 There is an interrelationship between physical activity and structured exercise: structured exercise programs can certainly be part of a physical activity plan, but physical activity can include more than structured exercise. Physical activity also includes any activity that involves movement, such as walking in gardens or between home areas, or physically participating more in personal care activities (eg, assisting with bathing or dressing).14 Both structured exercise and physical activity are important for LTC residents. Structured exercise provides an opportunity to improve strength and cardiovascular fitness, which aim to decrease the negative effects of sarcopenia and cardiovascular disease, such as disability and death.59,60 However, structured exercise should not be done daily for the same muscle groups.8 Rather, it is recommended for LTC residents to engage in structured exercise 2 times per week.8 Increasing physical activity is a daily goal, as daily physical activity decreases sedentary time, which has negative consequences such as decreased mood61 and increased mortality.62 LTC homes should incorporate strategies to both increase daily physical activity and promote individualized, structured exercise programs.
Conclusion
Residents in LTC spend much of their time in sedentary activities such as sitting or lying in bed. Physical activity is important to help decrease the negative effects of sedentary time, like poor mood and increased risk of death, and to improve physical function. This review describes several strategies to promote physical activity within LTC homes, such as leveraging daily activities and the physical environment, providing group activities, reducing physical and chemical restraint use, and using innovative technology such as robots. LTC homes can use the information in this review to plan strategies to promote physical activity.
Corresponding author: Caitlin McArthur, MScPT, PhD, Hamilton Health Sciences, St. Peter’s Hospital, 88 Maplewood Avenue, Hamilton, ON L8M 1W9; [email protected].
Financial disclosures: None.
From the Geriatric Education and Research in Aging Sciences (GERAS) Centre for Aging Research, McMaster University, Hamilton, ON.
Abstract
- Objective. To summarize the literature on improving opportunities for physical activity for residents in long-term care (LTC).
- Method. Narrative review of the literature.
- Results. Residents in LTC spend much of their time in sedentary activities such as sitting or lying in bed. Physical activity is important to help decrease the negative effects of sedentary time, such as poor mood and increased risk of death, and to improve physical function. This review identifies several strategies for promoting physical activity for LTC residents: incorporating simple strategies into daily activities, participating in group activities (eg, exercise, dance, or music therapy), using motivational strategies to encourage staff to promote activity, leveraging the physical environment, reducing physical and chemical restraints, and using innovative solutions such as robots or interactive technology.
- Conclusion. While the quality of evidence to date is limited, preliminary work suggests that the strategies identified in this article could be included as part of a multifactorial approach to increasing physical activity in LTC.
Keywords: long-term care; nursing homes; physical activity; sedentary; mobility.
The United Nations estimates that between 2013 and 2050 the population aged 60 years or older will double.1 Furthermore, the fastest growth rate will be seen in older adults over the age of 80 years.1 With this demographic shift, a growing number of older adults will require supportive housing, such as long-term care (LTC). Indeed, it is projected that the number of older adults requiring LTC will double by 2036.2
Residents in LTC are often medically complex and experience multimorbidity, cognitive impairment, and functional decline,3 making it difficult for them to engage in physical activity. LTC residents spend approximately 75% of their waking time in sedentary activities (eg, sitting, lying down, watching TV), which amounts to more than 12 hours per day.4-6 Residents with cognitive impairment are even more sedentary, spending as little as 1 minute per day in moderate physical activity and approximately 87% of their time in sedentary activities.7 Additionally, a high prevalence of use of psychotropic drugs and physical restraints contributes to high levels of physical inactivity for residents in LTC.8 Increased time spent in sedentary activities has been associated with adverse health outcomes, such as incidence of cardiovascular disease and type 2 diabetes, and mortality.9-11 Moreover, bed and chair rest are associated with muscle disuse, which can lead to functional impairment.12,13
Given the large amount of time LTC residents spend in sedentary activities and the negative consequences this has on their health, it is essential to find opportunities to engage residents in physical activity throughout the day. This article summarizes evidence about increasing opportunities for physical activity for LTC residents. Physical activity is defined as “any bodily movement produced by skeletal muscles that results in energy expenditure,” while exercise, which is a subset of physical activity, is purposefully planned, structured, and repetitive and has a goal of maintaining or improving physical fitness.14 Previous work has described exercise among LTC residents in detail,8,15,16 and thus exercise is not addressed here. Also, as a narrative review, this article provides an overview of available interventions to improve physical activity for LTC residents and does not provide comments on efficacy or an exhaustive list of potential interventions. Rather, it provides a starting point for LTC homes to consider when providing opportunities to improve physical activity for their residents.
Guidelines for Increasing Physical Activity
There are currently no published evidence-based guidelines for increasing physical activity and reducing sedentary time for residents of LTC homes. However, an international task force of experts in geriatrics, exercise, and LTC research convened in 2015 and made recommendations on this matter.8 They emphasize the importance of considering the needs of residents, family members, health care professionals, LTC staff, and policy-makers when designing strategies to promote movement in LTC.8 This will ensure that the strategies to promote movement will be realistic and sustainable. Additionally, the task force identified motivation and pleasure as key to engaging residents in physical activities, and recommended that interests and preferences should be used to guide the selection of activities.8 The following sections describe example strategies to improve physical activity for residents in LTC that LTC homes can use to help facilitate movement for their residents.
Strategies for Promoting Physical Activity
Leveraging Daily Activities
One approach to promoting physical activity in LTC homes is to systematically use simple strategies embedded within routine care to engage residents in movement.8 Function-focused, or restorative care,17 is a philosophy of care that promotes increasing physical activity and maintaining functional abilities based on the resident’s abilities. Examples include walking with residents to the dining room rather than pushing them in a wheelchair where appropriate, inviting residents to events that require them to leave their room, improving independent wheelchair propulsion for residents who cannot walk, and increasing opportunities for sit-to-stand activities where possible. These activities are scaled to the resident’s underlying physical and cognitive capabilities. A systematic review of function-focused care revealed that it can help maintain functional skills for residents in LTC, and there is no significant risk associated with implementation.18 In a study by Slaughter et al19 that examined the effectiveness of techniques to encourage mobility by residents’ usual caregivers, health care aides prompted residents to perform the sit-to-stand activity 4 times per day, with the number of repetitions individualized based on resident ability, fatigue, and motivation. Residents who completed the sit-to-stand activity had smaller declines in mobility and functional outcomes (ie, less decline on the Functional Independence Measure).19 This study included residents with Alzheimer’s disease and dementia who could transfer independently or with the assistance of one person,20 indicating that this type of intervention is feasible and appropriate for residents with cognitive impairment.
Group Activities
Group activities in LTC homes are another way of engaging residents in physical activity in a motivating and pleasant setting that also encourages social engagement among residents and LTC staff. Group exercise classes can be effective for improving mood and functional outcomes. For example, a systematic review of dance classes in LTC homes revealed an improvement in problematic behaviors, mood, cognition, communication, and socialization.21 Most studies included participants with dementia, and no adverse events were reported, supporting the feasibility and safety of implementing group dance activities for residents with cognitive impairment. Group exercise is the most common delivery method for exercise within LTC homes22 and has been demonstrated to have small positive effects on activities of daily living (ADL; ie, improvement in ADL independence equivalent to 1.3 points on the Barthel Index).23 Other group activities, such as music therapy, have demonstrated improvements in depressive symptoms, emotional well-being, and anxiety for LTC residents with dementia.24 Group activities also provide the opportunity for movement as residents leave their rooms, walk to a new location (if able), and return to their rooms when the activity is complete.
Barriers to Physical Activity and Strategies to Overcome Them
Caregiver-related Factors
LTC staff have limited time to spend promoting physical activity since residents often have complex health care needs and staffing levels are often constrained.25 Indeed, having lower staffing levels has been associated with lower levels of physical activity for residents.26,27 LTC staff have identified a lack of time to walk with residents28,29 and having other tasks to do (eg, clean) as barriers to promoting movement.28,29 However, asking residents to help staff with small household chores, such as folding laundry or clearing dishes, was a facilitator to promoting movement.30 Activating residents by helping them transfer to a wheelchair for independent mobilization around the home or by assisting them to walk where appropriate were also facilitators.30,31 Leveraging facilitators will help staff who have limited time to help residents engage in more physical activity.
Motivation of LTC staff can also be a barrier to encouraging physical activity for residents in LTC. Fear that increasing physical activity will cause adverse events like falls, illness, or exacerbation of symptoms often decreases motivation for staff to facilitate physical activity.32,33 Another potential barrier is the conceptualization of the role of nursing in LTC as protecting residents from harm by encouraging them to engage in “risk-free” activities like staying in bed.34-39 Strategies to increase staff motivation to engage LTC residents in physical activity that have been shown to be effective are verbal prompts, modelling behaviors, goal setting, and home champions to promote function-focused care.17,33,40-43
The Physical Environment
Aspects of the physical environment of LTC homes may facilitate or limit residents’ ability to be physically active. A 2017 systematic review examined elements of the physical environment that acted as barriers and facilitators to physical activity for older adults living in LTC.30 The authors found that the person-environment fit, security, accessibility, and comfort were key components of the physical environment that were associated with residents’ physical activity levels.30 First, an appropriate fit between the residents’ abilities and the demands of the environment was related to improved activity as measured by actigraphy.44 For example, having long hallways between residents’ rooms and common spaces discourages residents who can only walk short distances from walking to these locations. However, residents were more active in larger-scaled LTC homes with shorter distances between different areas (eg, resident rooms and dining rooms).45 Clearly, there must be enough space to encourage walking between areas, but not so much space that walking is not feasible. Residents participating in a focus group identified accessibility and comfort features as being facilitators for walking in the corridor, such as wide corridors, sturdy handrails, carpet, chairs placed at short intervals for seated breaks, windows to look out, plants, and accessible activity rooms and restrooms.45,46 On the other hand, limited things to see and do indoors and outdoors, along with restricted walking areas, were identified as barriers to corridor walking by residents.46
One method for optimizing LTC home architecture to promote movement is to provide therapeutic outdoor spaces, such as gardens. Indeed, therapeutic gardens have been studied as a nonpharmacological method of engaging LTC residents with dementia and have been shown to benefit mood, pain, and fall prevention.47 Secure therapeutic gardens or outdoor spaces provide opportunities for various activities to increase movement, including gardening, animal care, and walking.48 However, there is a higher propensity for residents who use walkers or wheelchairs to slide off paths or become stuck in mud or mulch.49 Residents with physical limitations may require additional supervision in garden spaces, and as such spaces should be designed with improved safety in mind (eg, barriers between paths and places where mud could accumulate). The number of available indoor (eg, a physical therapy gym) and outdoor (eg, gardens) spaces was also found to be positively related to residents’ physical activity levels.50 However, these relationships were mediated by the number of activity programs available in the LTC homes.50 Therefore, having staff available to facilitate activities is also important for promoting physical activity.
Chemical and Physical Restraints
Physical and chemical restraints (eg, antipsychotics and sedatives) are sometimes used to manage the behavioral and psychological symptoms of dementia,51,52 which many residents in LTC experience.3 Though there has been an emphasis in North America to decrease their use, physical and chemical restraints are still used in LTC.53 Physical restraint use is associated with a higher risk of functional and cognitive decline.53,54 Residents who are both physically and chemically restrained through antipsychotic use are at even higher risk for these declines.54 Thus, to improve opportunities for movement in LTC, physical restraint use should be minimized. The risks and benefits of using psychotropic medications that often decrease residents’ physical activity levels must be evaluated individually, and other nonpharmacological strategies should be used to manage the behavioral and psychological symptoms of dementia. These could include functional analysis-based interventions (ie, individualized interventions aimed at identifying unmet needs, causes, antecedents, and consequences of the behavior),55 music therapy,55 or other interventions described above.
Emerging Innovative Interventions
Robots are an emerging nonpharmacological intervention for improving the behavioral and psychological symptoms of dementia and facilitating physical activity in LTC. Robotic animal interventions, where LTC residents interact with robotic animals in an individual or group setting, have been shown to reduce negative behaviors and increase positive mood.56 Additionally, robots are being used in rehabilitation to provide exercise post-stroke57 and could easily be transitioned to do similar tasks in LTC. Robotic interventions are attractive for the LTC sector as they could help relieve the workload demands on an often overloaded sector, and, in the case of pet therapy, surmount regulations for bringing live animals into a LTC home. Though studies examining the use of robots in LTC have mainly focused on the effect of pet therapy on reducing behavioral symptoms, the use of robots to promote physical activity and exercise in LTC is a natural progression for the work that has been done in inpatient rehabilitation.57 On a similar note, an interactive technology (similar to a Kinect system) used to promote 30-minute, twice-weekly physical activity sessions has demonstrated improvements in physical function (Short Physical Performance Battery [SPPB]) for pre-disabled (SPPB of 6 to 9) residents in LTC without dementia.58 The role of technology to promote physical activity in LTC is an emerging area of interest, and future innovations in this area will continue to help facilitate movement.
Quality of Evidence
Most studies aimed at improving physical activity for LTC residents to date are small, have nonrandomized designs, and have limited generalizability and evidence to support the efficacy of the interventions. For example, most studies included in systematic reviews for function-focused care, dance, group exercise, and music therapy are small, observational, or quasi-experimental studies with methodological issues resulting in bias.18,21,23,24 Likewise, the evidence surrounding nonpharmacological interventions for reducing behavioral and psychological symptoms of dementia is of very low to moderate quality.55 Innovative interventions, such as robotics and interactive technology, to promote physical activity in LTC are in their infancy. There are no data syntheses available to date to summarize the available literature on this topic, and conclusions rely on small, nonrandomized designs or extrapolations of results from similar sectors (eg, inpatient rehabilitation). Thus, the studies described in this review can be used as preliminary evidence to support the implementation of interventions to improve physical activity, but discretion should be used when interpreting the efficacy of these interventions.
Discussion
This review identifies several strategies for promoting physical activity for LTC residents, including incorporating simple strategies into daily activities, participating in group activities (eg, exercise, dance, or music therapy), using motivational strategies to encourage staff to promote activity, leveraging the physical environment, reducing physical and chemical restraints, and using innovation solutions like robots or interactive technology. While the quality of evidence to date is limited, preliminary work suggests that strategies identified in this paper could be included as part of a multifactorial approach to increasing physical activity in LTC.
The current evidence does not suggest that any one strategy is more effective at improving physical activity, and it is likely that LTC homes will need to employ a combination of strategies to help residents move more. Additionally, residents’ preferences, goals, and capabilities should always be considered when designing an individualized physical activity plan. For example, if a resident does not like to be outdoors or gardening but enjoys dancing and music, then their physical activity plan should include group dance class and music therapy rather than gardening. LTC homes will need to have a menu of opportunities for movement that residents can choose from so that activities are pleasant and motivating, and therefore more likely to be completed.
Many of the interventions described in this review are safe and feasible to implement with residents who have physical or cognitive impairments. Function-focused care is scaled to the residents’ capabilities and did not increase the risk of falling, though LTC staff require the skills to scale physical activities appropriately.18 Likewise, group dance activities and music therapy were tested with residents with dementia, with no adverse events reported.21,24 However, more work is needed to determine the feasibility of implementing emerging methods, such as robotics and interactive technology, for increasing physical activity for residents with physical and cognitive impairments. Most studies to date have included mobile residents or those with minimal cognitive impairment. Similarly, outdoor garden spaces may be less safe for residents who use walkers or wheelchairs if there is an opportunity for them to slip off paths or get stuck in mud or mulch. LTC homes implementing any of these interventions should evaluate the benefits and risks of each intervention, the resources available within the home to support them (eg, trained staff), and the target residents’ physical and cognitive capabilities.
While increasing physical activity is important, structured exercise is needed to see gains in components of physical fitness such as strength, aerobic capacity, and balance. Indeed, one major consideration highlighted by the aforementioned task force is that every resident who does not have contraindications must also have a personalized multicomponent exercise program as part of their care plan.8 The task force recommends moderate- to high-intensity strength, aerobic, and balance exercises 2 times per week for 35 to 45 minutes per session.8 There is an interrelationship between physical activity and structured exercise: structured exercise programs can certainly be part of a physical activity plan, but physical activity can include more than structured exercise. Physical activity also includes any activity that involves movement, such as walking in gardens or between home areas, or physically participating more in personal care activities (eg, assisting with bathing or dressing).14 Both structured exercise and physical activity are important for LTC residents. Structured exercise provides an opportunity to improve strength and cardiovascular fitness, which aim to decrease the negative effects of sarcopenia and cardiovascular disease, such as disability and death.59,60 However, structured exercise should not be done daily for the same muscle groups.8 Rather, it is recommended for LTC residents to engage in structured exercise 2 times per week.8 Increasing physical activity is a daily goal, as daily physical activity decreases sedentary time, which has negative consequences such as decreased mood61 and increased mortality.62 LTC homes should incorporate strategies to both increase daily physical activity and promote individualized, structured exercise programs.
Conclusion
Residents in LTC spend much of their time in sedentary activities such as sitting or lying in bed. Physical activity is important to help decrease the negative effects of sedentary time, like poor mood and increased risk of death, and to improve physical function. This review describes several strategies to promote physical activity within LTC homes, such as leveraging daily activities and the physical environment, providing group activities, reducing physical and chemical restraint use, and using innovative technology such as robots. LTC homes can use the information in this review to plan strategies to promote physical activity.
Corresponding author: Caitlin McArthur, MScPT, PhD, Hamilton Health Sciences, St. Peter’s Hospital, 88 Maplewood Avenue, Hamilton, ON L8M 1W9; [email protected].
Financial disclosures: None.
1. United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2013. New York, NY: United Nations; 2013.
2. Pickard L, Comas-Herrera A, Costa-Font J, et al. Modelling an entitlement to long-term care services for older people in Europe: projections for long-term care expenditure to 2050. J Eur Soc Policy. 2007;17:33-48.
3. Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Can J Aging. 2011;30:371-390.
4. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr. 2006;6:9.
5. Ikezoe T, Asakawa Y, Shima H, Kishibuchi K, Ichihashi N. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr. 2013;57:221-225.
6. Keogh JW, Senior H, Beller EM, Henwood T. Prevalence and risk factors for low habitual walking speed in nursing home residents: an observational study. Arch Phys Med Rehabil. 2015;96:1993-1999.
7. Marmeleira J, Ferreira S, Raimundo A. Physical activity and physical fitness of nursing home residents with cognitive impairment: A pilot study. Exp Gerontol. 2017;100:63-69.
8. de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc. 2016;17:381-392.
9. van der Ploeg HP, Chey T, Korda RJ, et al. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494-500.
10. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8:e80000.
11. Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448-2455.
12. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82:418-423.
13. Wall BT, Dirks ML, van Loon LJC. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12:898-906.
14. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131.
15. McArthur C, Giangregorio LM. Improving strength and balance for long-term care residents at risk for falling: Suggestions for practice. J Clin Outcomes Manag. 2018;25:28-38.
16. Crocker T, Forster A, Young J, et al. Physical rehabilitation for older people in long-term care. Cochrane Database Syst Rev. 2013;2:CD004294.
17. Resnick B, Galik E, Boltz M, Pretzer-Aboff IE. Implementing Restorative Care Nursing in All Settings. 2nd ed. New York, NY: Spring Publishing Company; 2011.
18. Resnick B, Galik E, Boltz M. Function focused care approaches: literature review of progress and future possibilities. J Am Med Dir Assoc. 2013;14:313-318.
19. Slaughter SE, Estabrooks CA, Jones CA, Wagg AS. Mobility of Vulnerable Elders (MOVE): study protocol to evaluate the implementation and outcomes of a mobility intervention in long-term care facilities. BMC Geriatr. 2011;11:84.
20. Slaughter SE, Wagg AS, Jones CA, et al. Mobility of Vulnerable Elders study: effect of the sit-to-stand activity on mobility, function, and quality of life. J Am Med Dir Assoc. 2015;16(2):138-143.
21. Guzmán-García A, Hughes JC, James IA, Rochester L. Dancing as a psychosocial intervention in care homes: a systematic review of the literature. Int J Geriatr Psychiatry. 2013;28:914-924.
22. McArthur C, Gibbs JC, Patel R, et al. A scoping review of physical rehabilitation in long-term care: interventions, outcomes, tools. Can J Aging/La Rev Can du Vieil. 2017;36:435-452.
23. Crocker T, Young J, Forster A, et al. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: Systematic review with meta-analysis. Age Ageing. 2013;42:682-688.
24. van der Steen JT, Smaling HJ, van der Wouden JC, et al. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. 2018;7:CD003477.
25. Froggatt K, Davies S, Meyer J. Understanding Care Homes, A Research and Development Perspective. London: Jessica Kingsley Publishers; 2009.
26. Shore BA, Lerman DC, Smith RG, et al. Direct assessment of quality of care in a geriatric nursing home. J Appl Behav Anal. 1995;28:435-448.
27. Bates-Jensen BM, Schnelle JF, Alessi CA, et al. The effects of staffing on in-bed times of nursing home residents. J Am Geriatr Soc. 2004;52:931-938.
28. Ericson-Lidman E, Renström A-S, Åhlin J, Strandberg G. Relatives’ perceptions of residents’ life in a municipal care facility for older people with a focus on quality of life and care environment. Int J Older People Nurs. 2015;10:160-169.
29. Häggström E, Kihlgren A, Kihlgren M, Sörlie V. Relatives’ struggle for an improved and more just care for older people in community care. J Clin Nurs. 2007;16:1749-1757.
30. Douma JG, Volkers KM, Engels G, et al. Setting-related influences on physical inactivity of older adults in residential care settings: a review. BMC Geriatr. 2017;17:97.
31. Zegelin A. ’Tied down’- the process of becoming bedridden through gradual local confinement. J Clin Nurs. 2008;17:2294-2301.
32. Resnick B, Galik E, Gruber-Baldini AL, Zimmerman S. Falls and fall-related injuries associated with function-focused care. Clin Nurs Res. 2012;21:43-63.
33. Pretzer-Aboff I, Galik E. Feasibility and impact of a function focused care intervention for Parkinson’s disease in the community. Nursing Res. 2011;60:276-283.
34. Leditschke IA, Green M, Irvine J, et al. What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J. 2012;23:26-29.
35. Mittmann N, Seung SJ, Pisterzi LF, et al. Nursing workload associated with hospital patient care. Dis Manag Heal Outcomes. 2008;16:53-61.
36. Dykes PC, Carroll DL, Hurley AC, et al. Why do patients in acute care hospitals fall? Can falls be prevented? J Nurs Adm. 2009;39:299-304.
37. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging. 2013;8:1-10.
38. Wakefield BJ, Holman JE. Functional trajectories associated with hospitalization in older adults. West J Nurs Res. 2007;29:161-177.
39. Boltz M, Resnick B, Capezuti E, Shuluk J. Activity restriction vs. self-direction: hospitalised older adults’ response to fear of falling. Int J Older People Nurs. 2014;9:44-53.
40. Resnick B, Galik E, Gruber-Baldini A, Zimmerman S. Testing the effect of function-focused care in assisted living. J Am Geriatr Soc. 2011;59:2233-2240.
41. Galik EM, Resnick B, Gruber-Baldini A, et al. Pilot testing of the restorative care intervention for the cognitively impaired. J Am Med Dir Assoc. 2008;9:516-522.
42. Resnick B, Galik E, Gruber-Baldini AL, Zimmerman S. Implementing a restorative care philosophy of care in assisted living: pilot testing of Res-Care-AL. J Am Acad Nurse Pract. 2009;21:123-133.
43. Resnick B, Gruber-Baldini AL, Zimmerman S, et al. Nursing home resident outcomes from the Res-Care intervention. J Am Geriatr Soc. 2009;57:1156-1165.
44. Pomeroy SH, Scherer Y, Runkawatt V, et al. Person-environment fit and functioning among older adults in a long-term care setting. Geriatr Nurs. 2011;32:368-378.
45. Moos RH, David TG, Lemke S, Postle E. Coping with an intra-institutional relocation: changes in resident and staff behavior patterns. Gerontologist. 1984;24:495-502.
46. Lu Z, Rodiek SD, Shepley MM, Duffy M. Influences of physical environment on corridor walking among assisted living residents. J Appl Gerontol. 2011;30:463-484.
47. Detweiler MB, Sharma T, Detweiler JG, et al. What is the evidence to support the use of therapeutic gardens for the elderly? Psychiatry Investig. 2012;9:100.
48. Blake M, Mitchell G. Horticultural therapy in dementia care: a literature review. Nurs Stand. 2016;30:41-47.
49. Detweiler MB, Murphy PF, Myers LC, Kim KY. Does a wander garden influence inappropriate behaviors in dementia residents? Am J Alzheimers Dis Other Demen. 2008;23:31-45.
50. Joseph A, Zimring C, Harris-Kojetin L, Kiefer K. Presence and visibility of outdoor and indoor physical activity features and participation in physical activity among older adults in retirement communities. J Hous Elderly. 2006;19:141-165.
51. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24:1110-1118.
52. Herrmann N. Recommendations for the management of behavioral and psychological symptoms of dementia. Can J Neurol Sci. 2001;28 Suppl 1:S96-107.
53. Freeman S, Spirgiene L, Martin-Khan M, Hirdes JP. Relationship between restraint use, engagement in social activity, and decline in cognitive status among residents newly admitted to long-term care facilities. Geriatr Gerontol Int. 2017;17:246-255.
54. Foebel AD, Onder G, Finne-Soveri H, et al. Physical restraint and antipsychotic medication use among nursing home residents with dementia. J Am Med Dir Assoc. 2016;17:184.e9-184.e14.
55. Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30:295-309.
56. Robinson H, MacDonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667.
57. Lo K, Stephenson M, Lockwood C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients. JBI Database System Rev Implement Rep. 2017;15:3049-3091.
58. Valiani V, Lauzé M, Martel D, et al. A new adaptive home-based exercise technology among older adults living in nursing home: a pilot study on feasibility, acceptability and physical performance. J Nutr Health Aging. 2017;21:819-824.
59. Locquet M, Beaudart C, Hajaoui M, et al. Three-year adverse health consequences of sarcopenia in community-dwelling older adults according to 5 diagnosis definitions. J Am Med Dir Assoc. 2019;20:43-46.e2.
60. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510-1530.
61. Park S-Y, Lee K, Um YJ, Paek S, Ryou IS. Association between physical activity and depressive mood among Korean adults with chronic diseases. Korean J Fam Med. 2018;39:185-190.
62. Loprinzi PD. Light-intensity physical activity and all-cause mortality. Am J Health Promot. 2017;31:340-342.
1. United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2013. New York, NY: United Nations; 2013.
2. Pickard L, Comas-Herrera A, Costa-Font J, et al. Modelling an entitlement to long-term care services for older people in Europe: projections for long-term care expenditure to 2050. J Eur Soc Policy. 2007;17:33-48.
3. Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Can J Aging. 2011;30:371-390.
4. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr. 2006;6:9.
5. Ikezoe T, Asakawa Y, Shima H, Kishibuchi K, Ichihashi N. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr. 2013;57:221-225.
6. Keogh JW, Senior H, Beller EM, Henwood T. Prevalence and risk factors for low habitual walking speed in nursing home residents: an observational study. Arch Phys Med Rehabil. 2015;96:1993-1999.
7. Marmeleira J, Ferreira S, Raimundo A. Physical activity and physical fitness of nursing home residents with cognitive impairment: A pilot study. Exp Gerontol. 2017;100:63-69.
8. de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc. 2016;17:381-392.
9. van der Ploeg HP, Chey T, Korda RJ, et al. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494-500.
10. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8:e80000.
11. Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448-2455.
12. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82:418-423.
13. Wall BT, Dirks ML, van Loon LJC. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12:898-906.
14. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131.
15. McArthur C, Giangregorio LM. Improving strength and balance for long-term care residents at risk for falling: Suggestions for practice. J Clin Outcomes Manag. 2018;25:28-38.
16. Crocker T, Forster A, Young J, et al. Physical rehabilitation for older people in long-term care. Cochrane Database Syst Rev. 2013;2:CD004294.
17. Resnick B, Galik E, Boltz M, Pretzer-Aboff IE. Implementing Restorative Care Nursing in All Settings. 2nd ed. New York, NY: Spring Publishing Company; 2011.
18. Resnick B, Galik E, Boltz M. Function focused care approaches: literature review of progress and future possibilities. J Am Med Dir Assoc. 2013;14:313-318.
19. Slaughter SE, Estabrooks CA, Jones CA, Wagg AS. Mobility of Vulnerable Elders (MOVE): study protocol to evaluate the implementation and outcomes of a mobility intervention in long-term care facilities. BMC Geriatr. 2011;11:84.
20. Slaughter SE, Wagg AS, Jones CA, et al. Mobility of Vulnerable Elders study: effect of the sit-to-stand activity on mobility, function, and quality of life. J Am Med Dir Assoc. 2015;16(2):138-143.
21. Guzmán-García A, Hughes JC, James IA, Rochester L. Dancing as a psychosocial intervention in care homes: a systematic review of the literature. Int J Geriatr Psychiatry. 2013;28:914-924.
22. McArthur C, Gibbs JC, Patel R, et al. A scoping review of physical rehabilitation in long-term care: interventions, outcomes, tools. Can J Aging/La Rev Can du Vieil. 2017;36:435-452.
23. Crocker T, Young J, Forster A, et al. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: Systematic review with meta-analysis. Age Ageing. 2013;42:682-688.
24. van der Steen JT, Smaling HJ, van der Wouden JC, et al. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. 2018;7:CD003477.
25. Froggatt K, Davies S, Meyer J. Understanding Care Homes, A Research and Development Perspective. London: Jessica Kingsley Publishers; 2009.
26. Shore BA, Lerman DC, Smith RG, et al. Direct assessment of quality of care in a geriatric nursing home. J Appl Behav Anal. 1995;28:435-448.
27. Bates-Jensen BM, Schnelle JF, Alessi CA, et al. The effects of staffing on in-bed times of nursing home residents. J Am Geriatr Soc. 2004;52:931-938.
28. Ericson-Lidman E, Renström A-S, Åhlin J, Strandberg G. Relatives’ perceptions of residents’ life in a municipal care facility for older people with a focus on quality of life and care environment. Int J Older People Nurs. 2015;10:160-169.
29. Häggström E, Kihlgren A, Kihlgren M, Sörlie V. Relatives’ struggle for an improved and more just care for older people in community care. J Clin Nurs. 2007;16:1749-1757.
30. Douma JG, Volkers KM, Engels G, et al. Setting-related influences on physical inactivity of older adults in residential care settings: a review. BMC Geriatr. 2017;17:97.
31. Zegelin A. ’Tied down’- the process of becoming bedridden through gradual local confinement. J Clin Nurs. 2008;17:2294-2301.
32. Resnick B, Galik E, Gruber-Baldini AL, Zimmerman S. Falls and fall-related injuries associated with function-focused care. Clin Nurs Res. 2012;21:43-63.
33. Pretzer-Aboff I, Galik E. Feasibility and impact of a function focused care intervention for Parkinson’s disease in the community. Nursing Res. 2011;60:276-283.
34. Leditschke IA, Green M, Irvine J, et al. What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J. 2012;23:26-29.
35. Mittmann N, Seung SJ, Pisterzi LF, et al. Nursing workload associated with hospital patient care. Dis Manag Heal Outcomes. 2008;16:53-61.
36. Dykes PC, Carroll DL, Hurley AC, et al. Why do patients in acute care hospitals fall? Can falls be prevented? J Nurs Adm. 2009;39:299-304.
37. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging. 2013;8:1-10.
38. Wakefield BJ, Holman JE. Functional trajectories associated with hospitalization in older adults. West J Nurs Res. 2007;29:161-177.
39. Boltz M, Resnick B, Capezuti E, Shuluk J. Activity restriction vs. self-direction: hospitalised older adults’ response to fear of falling. Int J Older People Nurs. 2014;9:44-53.
40. Resnick B, Galik E, Gruber-Baldini A, Zimmerman S. Testing the effect of function-focused care in assisted living. J Am Geriatr Soc. 2011;59:2233-2240.
41. Galik EM, Resnick B, Gruber-Baldini A, et al. Pilot testing of the restorative care intervention for the cognitively impaired. J Am Med Dir Assoc. 2008;9:516-522.
42. Resnick B, Galik E, Gruber-Baldini AL, Zimmerman S. Implementing a restorative care philosophy of care in assisted living: pilot testing of Res-Care-AL. J Am Acad Nurse Pract. 2009;21:123-133.
43. Resnick B, Gruber-Baldini AL, Zimmerman S, et al. Nursing home resident outcomes from the Res-Care intervention. J Am Geriatr Soc. 2009;57:1156-1165.
44. Pomeroy SH, Scherer Y, Runkawatt V, et al. Person-environment fit and functioning among older adults in a long-term care setting. Geriatr Nurs. 2011;32:368-378.
45. Moos RH, David TG, Lemke S, Postle E. Coping with an intra-institutional relocation: changes in resident and staff behavior patterns. Gerontologist. 1984;24:495-502.
46. Lu Z, Rodiek SD, Shepley MM, Duffy M. Influences of physical environment on corridor walking among assisted living residents. J Appl Gerontol. 2011;30:463-484.
47. Detweiler MB, Sharma T, Detweiler JG, et al. What is the evidence to support the use of therapeutic gardens for the elderly? Psychiatry Investig. 2012;9:100.
48. Blake M, Mitchell G. Horticultural therapy in dementia care: a literature review. Nurs Stand. 2016;30:41-47.
49. Detweiler MB, Murphy PF, Myers LC, Kim KY. Does a wander garden influence inappropriate behaviors in dementia residents? Am J Alzheimers Dis Other Demen. 2008;23:31-45.
50. Joseph A, Zimring C, Harris-Kojetin L, Kiefer K. Presence and visibility of outdoor and indoor physical activity features and participation in physical activity among older adults in retirement communities. J Hous Elderly. 2006;19:141-165.
51. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24:1110-1118.
52. Herrmann N. Recommendations for the management of behavioral and psychological symptoms of dementia. Can J Neurol Sci. 2001;28 Suppl 1:S96-107.
53. Freeman S, Spirgiene L, Martin-Khan M, Hirdes JP. Relationship between restraint use, engagement in social activity, and decline in cognitive status among residents newly admitted to long-term care facilities. Geriatr Gerontol Int. 2017;17:246-255.
54. Foebel AD, Onder G, Finne-Soveri H, et al. Physical restraint and antipsychotic medication use among nursing home residents with dementia. J Am Med Dir Assoc. 2016;17:184.e9-184.e14.
55. Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30:295-309.
56. Robinson H, MacDonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667.
57. Lo K, Stephenson M, Lockwood C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients. JBI Database System Rev Implement Rep. 2017;15:3049-3091.
58. Valiani V, Lauzé M, Martel D, et al. A new adaptive home-based exercise technology among older adults living in nursing home: a pilot study on feasibility, acceptability and physical performance. J Nutr Health Aging. 2017;21:819-824.
59. Locquet M, Beaudart C, Hajaoui M, et al. Three-year adverse health consequences of sarcopenia in community-dwelling older adults according to 5 diagnosis definitions. J Am Med Dir Assoc. 2019;20:43-46.e2.
60. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510-1530.
61. Park S-Y, Lee K, Um YJ, Paek S, Ryou IS. Association between physical activity and depressive mood among Korean adults with chronic diseases. Korean J Fam Med. 2018;39:185-190.
62. Loprinzi PD. Light-intensity physical activity and all-cause mortality. Am J Health Promot. 2017;31:340-342.
Management of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; [email protected].
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.