User login
Marcela Romero-Reyes, DDS, PhD, Comments on Peripheral and Central Headache Challenges
###
Dr. Rapoport: Do you commonly see patients who present with symptoms of both central and peripheral symptoms in practice?
Dr. Romero-Reyes: Yes, I see patients that present with temporomandibular disorders (TMD) and headache comorbidity, as well as patients with migraine, tension-type headache, and cervicogenic headache with myofascial pain.
Dr. Rapoport: Why do you think this condition is so challenging to treat?
Dr. Romero-Reyes: I think this is because of the lack of understanding and awareness that in addition to the multifactorial nature of headache disorders, other types of disorders that are not neurovascular in origin may influence trigeminovascular nociception, and these types of non-neurovascular disorders involve the skill and knowledge of other expertise.
Headaches receiving inputs from extracranial structures such as in TMD (temporomandibular joint [TMJ] and muscles of mastication) and/or cervical structures (cervical spine, cervical muscles) require multidisciplinary evaluation and management. In these cases, the management should involve a neurologist specialized in headache disorders, a dentist trained in TMD and orofacial pain disorders, and a physical therapist with special training in craniofacial and cervical Therapeutics. Multidisciplinary communication is key for successful management.
Another reason is that myofascial pain (MFP) is often overlooked in patients with headache disorders. In my experience, patients with episodic and chronic migraine, episodic and chronic tension-type headache, cervicogenic headache, and patients presenting TMD and headache comorbidity can present trigger points in the craniofacial and cervical muscles, an indication of MFP. It has been reported that these patients present a higher disability impact. The presence of MFP may be contributing to the activation of the trigeminovascular system and therefore facilitate, exacerbate, and perpetuate headache symptomatology and may accelerate the progression to a more chronic form of the disorder.
Dr. Rapoport: In your opinion, is this considered a controversial topic? Why or why not?
Dr. Romero-Reyes: Yes, I think it is necessary to clarify that tenderness in the back of the head or of neck muscles present in headache patients does not necessarily imply that it is due to a nerve compression. This could also be caused by local myalgia but more commonly, from latent or active myofascial trigger points present in the muscles of the area being palpated, or by referred pain beyond the area of the muscle being palpated. Suboccipital muscles (in the occiput area) are not the only muscle group that is associated with headache and neck pain symptomatology. For example, the trapezius muscle, which is an overlooked source of tension- type and cervicogenic headache, can present trigger points that can refer pain to the shoulder, neck, head, face and the eye. In addition, other craniofacial and cervical muscles such as the sternocleidomastoid (SCM) and temporalis muscles have been shown to be associated with headache symptomatology in the migraineur, as well as the chronic tension-type headache patient. Other muscles that also refer to the craniofacial area and can elicit headache and neck pain symptomatology include the masseter, occipitofrontalis, splenius capitis, splenius cervicis, semispinalis capitis, semispinalis cervicis and multifidi (cervical). The presence of trigger points in these muscles do not support or warrant the need to be removed or managed with non-conservative approaches.
Myofascial trigger points can result from muscle injury and overload, parafunctional activity, and poor head and neck posture. MFP is characterized by a regional pain and presence of localized tender areas (trigger points) in muscle, fascia or tendons that reproduce pain when palpated, and produce a pattern of regional pain spreading along the muscle palpated, or beyond the location boundary of the muscle palpated. It has been shown by microdyalisis that inflammatory mediators and neuropeptides are present in the area of an active trigger point. In addition, an increase of electromyography activity has been shown in trigger points in patients with chronic tension-type headache when compared with controls.
The importance of an evaluation by a skilled clinician in the craniofacial and cervical area to verify the source of pain is critical. The patient may be reporting pain in one area, but the source of the pain is in another area, and this is typical symptomatology present when there are active trigger points. In addition, an assessment of any contributing factors arising from the cervical spine (eg, poor posture) and craniofacial area (eg, TMD) that may exacerbate headache symptomatology is vital to proper diagnosis.
In my experience, patients with migraine, tension-type headache, cervicogenic headache, and TMD and headache comorbidity present MFP perpetuating headache symptomatology. MFP is not managed by surgical interventions. This perpetuating factor can be managed effectively with conservative measures. The plan is tailored for each patient’s needs. In general, the plan of management may include trigger point injections in the muscle with anesthetics, dry needling, and a physical therapy plan that may include education regarding habits and posture, exercises and physical therapy modalities, which are crucial to relieve pain and increase function. In cases of TMD and headache comorbidity, an occlusal appliance (stabilization appliance) can be included if necessary. We should also consider behavioral therapies (especially EMG biofeedback training) and some oral anti-inflammatories or muscle relaxants in the beginning of management, together with the plan of management mentioned above.
With these approaches to manage the MFP component in headache patients, I have been able to see that in migraineurs with MFP, the frequency and severity of the attacks decrease significantly. The patient may still experience migraine attacks, but feel happy to have the possibility to reduce medication intake and be in more control of their pain. In patients with tension-type headache, I have seen this even more dramatically.
This is telling us that headache pathophysiology involves a “conversation” between the peripheral and central nervous system, which influence each other. Peripheral nociceptive input coming from extracranial structures can induce trigeminovascular activation and therefore exacerbate a headache disorder and vice versa. Chronic myofascial pain may be the result of central sensitization due to the protracted peripheral nociceptive input (eg, poor posture, neck strain, parafunctional activity), therefore perpetuating the headache disorder even more.
Dr. Rapoport: Do you have any other comments about the article Treatment Challenges When Headache Has Central and Peripheral Involvement that you would like to share with our readers?
Dr. Romero-Reyes: It is simplistic to say migraine is either a peripheral or a central disorder, or that symptoms are either peripheral or central. Beyond thinking about migraine pain, migraine is fundamentally a brain (central) disorder. Its associated symptoms (nausea, phonophobia, photophobia) tell us this. Migraine headache is complex, and most likely the result of central mechanisms that can be influenced by peripheral inputs from the craniofacial and cervical region.
Embarking on surgical interventions for the management of headache disorders warrants a caution since it is still an experimental research question and the need of such therapies should be evaluated against conservative management. We are in a very exciting and hopeful time for migraine management. New evidence-based options from biological agents, such as anti-calcitonin gene-related peptide (CGRP) therapies, to non-pharmacological approaches, such as neuromodulation, can be offered to the patients. If the patient is experiencing pain in the neck area or other craniofacial area, it is recommended to have a thorough evaluation by a physical therapist with special training in cervical and craniofacial therapeutics and/or a dentist trained in TMD and orofacial pain disorders to work in consultation with a neurologist to elaborate a personalized management plan. Do not overlook the contribution of myofascial pain (trigger points) as well as TMD in the symptomatology of headache disorders. Few patients need to undergo surgical measures of peripheral nerves and muscles for improvement. An exhaustive evaluation must be undertaken first.
Resources for patients:
AHS
https://americanheadachesociety.org/
https://americanheadachesociety.org/wp-content/uploads/2018/06/Choosing-Wisely-Flyer.pdf
AAOP
https://aaop.clubexpress.com/content.aspx?sl=1152088466
PTBCTT
###
Dr. Rapoport: Do you commonly see patients who present with symptoms of both central and peripheral symptoms in practice?
Dr. Romero-Reyes: Yes, I see patients that present with temporomandibular disorders (TMD) and headache comorbidity, as well as patients with migraine, tension-type headache, and cervicogenic headache with myofascial pain.
Dr. Rapoport: Why do you think this condition is so challenging to treat?
Dr. Romero-Reyes: I think this is because of the lack of understanding and awareness that in addition to the multifactorial nature of headache disorders, other types of disorders that are not neurovascular in origin may influence trigeminovascular nociception, and these types of non-neurovascular disorders involve the skill and knowledge of other expertise.
Headaches receiving inputs from extracranial structures such as in TMD (temporomandibular joint [TMJ] and muscles of mastication) and/or cervical structures (cervical spine, cervical muscles) require multidisciplinary evaluation and management. In these cases, the management should involve a neurologist specialized in headache disorders, a dentist trained in TMD and orofacial pain disorders, and a physical therapist with special training in craniofacial and cervical Therapeutics. Multidisciplinary communication is key for successful management.
Another reason is that myofascial pain (MFP) is often overlooked in patients with headache disorders. In my experience, patients with episodic and chronic migraine, episodic and chronic tension-type headache, cervicogenic headache, and patients presenting TMD and headache comorbidity can present trigger points in the craniofacial and cervical muscles, an indication of MFP. It has been reported that these patients present a higher disability impact. The presence of MFP may be contributing to the activation of the trigeminovascular system and therefore facilitate, exacerbate, and perpetuate headache symptomatology and may accelerate the progression to a more chronic form of the disorder.
Dr. Rapoport: In your opinion, is this considered a controversial topic? Why or why not?
Dr. Romero-Reyes: Yes, I think it is necessary to clarify that tenderness in the back of the head or of neck muscles present in headache patients does not necessarily imply that it is due to a nerve compression. This could also be caused by local myalgia but more commonly, from latent or active myofascial trigger points present in the muscles of the area being palpated, or by referred pain beyond the area of the muscle being palpated. Suboccipital muscles (in the occiput area) are not the only muscle group that is associated with headache and neck pain symptomatology. For example, the trapezius muscle, which is an overlooked source of tension- type and cervicogenic headache, can present trigger points that can refer pain to the shoulder, neck, head, face and the eye. In addition, other craniofacial and cervical muscles such as the sternocleidomastoid (SCM) and temporalis muscles have been shown to be associated with headache symptomatology in the migraineur, as well as the chronic tension-type headache patient. Other muscles that also refer to the craniofacial area and can elicit headache and neck pain symptomatology include the masseter, occipitofrontalis, splenius capitis, splenius cervicis, semispinalis capitis, semispinalis cervicis and multifidi (cervical). The presence of trigger points in these muscles do not support or warrant the need to be removed or managed with non-conservative approaches.
Myofascial trigger points can result from muscle injury and overload, parafunctional activity, and poor head and neck posture. MFP is characterized by a regional pain and presence of localized tender areas (trigger points) in muscle, fascia or tendons that reproduce pain when palpated, and produce a pattern of regional pain spreading along the muscle palpated, or beyond the location boundary of the muscle palpated. It has been shown by microdyalisis that inflammatory mediators and neuropeptides are present in the area of an active trigger point. In addition, an increase of electromyography activity has been shown in trigger points in patients with chronic tension-type headache when compared with controls.
The importance of an evaluation by a skilled clinician in the craniofacial and cervical area to verify the source of pain is critical. The patient may be reporting pain in one area, but the source of the pain is in another area, and this is typical symptomatology present when there are active trigger points. In addition, an assessment of any contributing factors arising from the cervical spine (eg, poor posture) and craniofacial area (eg, TMD) that may exacerbate headache symptomatology is vital to proper diagnosis.
In my experience, patients with migraine, tension-type headache, cervicogenic headache, and TMD and headache comorbidity present MFP perpetuating headache symptomatology. MFP is not managed by surgical interventions. This perpetuating factor can be managed effectively with conservative measures. The plan is tailored for each patient’s needs. In general, the plan of management may include trigger point injections in the muscle with anesthetics, dry needling, and a physical therapy plan that may include education regarding habits and posture, exercises and physical therapy modalities, which are crucial to relieve pain and increase function. In cases of TMD and headache comorbidity, an occlusal appliance (stabilization appliance) can be included if necessary. We should also consider behavioral therapies (especially EMG biofeedback training) and some oral anti-inflammatories or muscle relaxants in the beginning of management, together with the plan of management mentioned above.
With these approaches to manage the MFP component in headache patients, I have been able to see that in migraineurs with MFP, the frequency and severity of the attacks decrease significantly. The patient may still experience migraine attacks, but feel happy to have the possibility to reduce medication intake and be in more control of their pain. In patients with tension-type headache, I have seen this even more dramatically.
This is telling us that headache pathophysiology involves a “conversation” between the peripheral and central nervous system, which influence each other. Peripheral nociceptive input coming from extracranial structures can induce trigeminovascular activation and therefore exacerbate a headache disorder and vice versa. Chronic myofascial pain may be the result of central sensitization due to the protracted peripheral nociceptive input (eg, poor posture, neck strain, parafunctional activity), therefore perpetuating the headache disorder even more.
Dr. Rapoport: Do you have any other comments about the article Treatment Challenges When Headache Has Central and Peripheral Involvement that you would like to share with our readers?
Dr. Romero-Reyes: It is simplistic to say migraine is either a peripheral or a central disorder, or that symptoms are either peripheral or central. Beyond thinking about migraine pain, migraine is fundamentally a brain (central) disorder. Its associated symptoms (nausea, phonophobia, photophobia) tell us this. Migraine headache is complex, and most likely the result of central mechanisms that can be influenced by peripheral inputs from the craniofacial and cervical region.
Embarking on surgical interventions for the management of headache disorders warrants a caution since it is still an experimental research question and the need of such therapies should be evaluated against conservative management. We are in a very exciting and hopeful time for migraine management. New evidence-based options from biological agents, such as anti-calcitonin gene-related peptide (CGRP) therapies, to non-pharmacological approaches, such as neuromodulation, can be offered to the patients. If the patient is experiencing pain in the neck area or other craniofacial area, it is recommended to have a thorough evaluation by a physical therapist with special training in cervical and craniofacial therapeutics and/or a dentist trained in TMD and orofacial pain disorders to work in consultation with a neurologist to elaborate a personalized management plan. Do not overlook the contribution of myofascial pain (trigger points) as well as TMD in the symptomatology of headache disorders. Few patients need to undergo surgical measures of peripheral nerves and muscles for improvement. An exhaustive evaluation must be undertaken first.
Resources for patients:
AHS
https://americanheadachesociety.org/
https://americanheadachesociety.org/wp-content/uploads/2018/06/Choosing-Wisely-Flyer.pdf
AAOP
https://aaop.clubexpress.com/content.aspx?sl=1152088466
PTBCTT
###
Dr. Rapoport: Do you commonly see patients who present with symptoms of both central and peripheral symptoms in practice?
Dr. Romero-Reyes: Yes, I see patients that present with temporomandibular disorders (TMD) and headache comorbidity, as well as patients with migraine, tension-type headache, and cervicogenic headache with myofascial pain.
Dr. Rapoport: Why do you think this condition is so challenging to treat?
Dr. Romero-Reyes: I think this is because of the lack of understanding and awareness that in addition to the multifactorial nature of headache disorders, other types of disorders that are not neurovascular in origin may influence trigeminovascular nociception, and these types of non-neurovascular disorders involve the skill and knowledge of other expertise.
Headaches receiving inputs from extracranial structures such as in TMD (temporomandibular joint [TMJ] and muscles of mastication) and/or cervical structures (cervical spine, cervical muscles) require multidisciplinary evaluation and management. In these cases, the management should involve a neurologist specialized in headache disorders, a dentist trained in TMD and orofacial pain disorders, and a physical therapist with special training in craniofacial and cervical Therapeutics. Multidisciplinary communication is key for successful management.
Another reason is that myofascial pain (MFP) is often overlooked in patients with headache disorders. In my experience, patients with episodic and chronic migraine, episodic and chronic tension-type headache, cervicogenic headache, and patients presenting TMD and headache comorbidity can present trigger points in the craniofacial and cervical muscles, an indication of MFP. It has been reported that these patients present a higher disability impact. The presence of MFP may be contributing to the activation of the trigeminovascular system and therefore facilitate, exacerbate, and perpetuate headache symptomatology and may accelerate the progression to a more chronic form of the disorder.
Dr. Rapoport: In your opinion, is this considered a controversial topic? Why or why not?
Dr. Romero-Reyes: Yes, I think it is necessary to clarify that tenderness in the back of the head or of neck muscles present in headache patients does not necessarily imply that it is due to a nerve compression. This could also be caused by local myalgia but more commonly, from latent or active myofascial trigger points present in the muscles of the area being palpated, or by referred pain beyond the area of the muscle being palpated. Suboccipital muscles (in the occiput area) are not the only muscle group that is associated with headache and neck pain symptomatology. For example, the trapezius muscle, which is an overlooked source of tension- type and cervicogenic headache, can present trigger points that can refer pain to the shoulder, neck, head, face and the eye. In addition, other craniofacial and cervical muscles such as the sternocleidomastoid (SCM) and temporalis muscles have been shown to be associated with headache symptomatology in the migraineur, as well as the chronic tension-type headache patient. Other muscles that also refer to the craniofacial area and can elicit headache and neck pain symptomatology include the masseter, occipitofrontalis, splenius capitis, splenius cervicis, semispinalis capitis, semispinalis cervicis and multifidi (cervical). The presence of trigger points in these muscles do not support or warrant the need to be removed or managed with non-conservative approaches.
Myofascial trigger points can result from muscle injury and overload, parafunctional activity, and poor head and neck posture. MFP is characterized by a regional pain and presence of localized tender areas (trigger points) in muscle, fascia or tendons that reproduce pain when palpated, and produce a pattern of regional pain spreading along the muscle palpated, or beyond the location boundary of the muscle palpated. It has been shown by microdyalisis that inflammatory mediators and neuropeptides are present in the area of an active trigger point. In addition, an increase of electromyography activity has been shown in trigger points in patients with chronic tension-type headache when compared with controls.
The importance of an evaluation by a skilled clinician in the craniofacial and cervical area to verify the source of pain is critical. The patient may be reporting pain in one area, but the source of the pain is in another area, and this is typical symptomatology present when there are active trigger points. In addition, an assessment of any contributing factors arising from the cervical spine (eg, poor posture) and craniofacial area (eg, TMD) that may exacerbate headache symptomatology is vital to proper diagnosis.
In my experience, patients with migraine, tension-type headache, cervicogenic headache, and TMD and headache comorbidity present MFP perpetuating headache symptomatology. MFP is not managed by surgical interventions. This perpetuating factor can be managed effectively with conservative measures. The plan is tailored for each patient’s needs. In general, the plan of management may include trigger point injections in the muscle with anesthetics, dry needling, and a physical therapy plan that may include education regarding habits and posture, exercises and physical therapy modalities, which are crucial to relieve pain and increase function. In cases of TMD and headache comorbidity, an occlusal appliance (stabilization appliance) can be included if necessary. We should also consider behavioral therapies (especially EMG biofeedback training) and some oral anti-inflammatories or muscle relaxants in the beginning of management, together with the plan of management mentioned above.
With these approaches to manage the MFP component in headache patients, I have been able to see that in migraineurs with MFP, the frequency and severity of the attacks decrease significantly. The patient may still experience migraine attacks, but feel happy to have the possibility to reduce medication intake and be in more control of their pain. In patients with tension-type headache, I have seen this even more dramatically.
This is telling us that headache pathophysiology involves a “conversation” between the peripheral and central nervous system, which influence each other. Peripheral nociceptive input coming from extracranial structures can induce trigeminovascular activation and therefore exacerbate a headache disorder and vice versa. Chronic myofascial pain may be the result of central sensitization due to the protracted peripheral nociceptive input (eg, poor posture, neck strain, parafunctional activity), therefore perpetuating the headache disorder even more.
Dr. Rapoport: Do you have any other comments about the article Treatment Challenges When Headache Has Central and Peripheral Involvement that you would like to share with our readers?
Dr. Romero-Reyes: It is simplistic to say migraine is either a peripheral or a central disorder, or that symptoms are either peripheral or central. Beyond thinking about migraine pain, migraine is fundamentally a brain (central) disorder. Its associated symptoms (nausea, phonophobia, photophobia) tell us this. Migraine headache is complex, and most likely the result of central mechanisms that can be influenced by peripheral inputs from the craniofacial and cervical region.
Embarking on surgical interventions for the management of headache disorders warrants a caution since it is still an experimental research question and the need of such therapies should be evaluated against conservative management. We are in a very exciting and hopeful time for migraine management. New evidence-based options from biological agents, such as anti-calcitonin gene-related peptide (CGRP) therapies, to non-pharmacological approaches, such as neuromodulation, can be offered to the patients. If the patient is experiencing pain in the neck area or other craniofacial area, it is recommended to have a thorough evaluation by a physical therapist with special training in cervical and craniofacial therapeutics and/or a dentist trained in TMD and orofacial pain disorders to work in consultation with a neurologist to elaborate a personalized management plan. Do not overlook the contribution of myofascial pain (trigger points) as well as TMD in the symptomatology of headache disorders. Few patients need to undergo surgical measures of peripheral nerves and muscles for improvement. An exhaustive evaluation must be undertaken first.
Resources for patients:
AHS
https://americanheadachesociety.org/
https://americanheadachesociety.org/wp-content/uploads/2018/06/Choosing-Wisely-Flyer.pdf
AAOP
https://aaop.clubexpress.com/content.aspx?sl=1152088466
PTBCTT
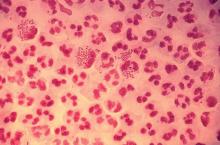
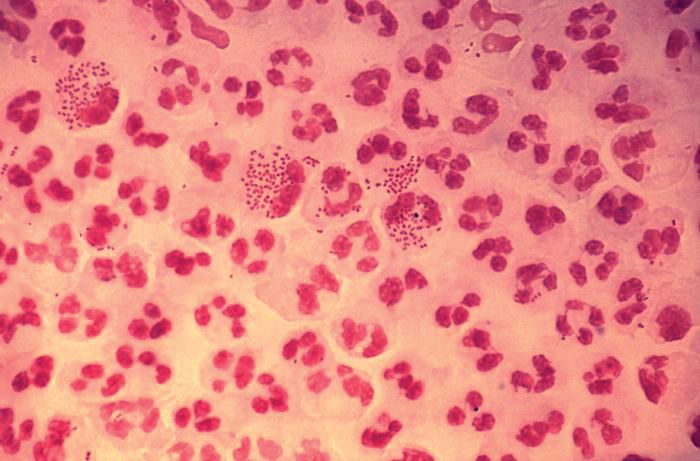
STIs pose complex challenge to HIV efforts
SEATTLE – Sexually-transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are on the rise among HIV-infected individuals, and emerging antimicrobial resistance in these organisms is presenting serious challenges to physicians. The issue may be traceable to the introduction of preexposure prophylaxis (PrEP) in 2011, which previous studies have shown to be associated with less condom use.
In the United States, a 2017 report by the Centers for Disease Control and Prevention showed rising incidences of chlamydia (+5% from 2015 to 2017), gonorrhea (+19%), and syphilis (+18%). “We have an incidence among men who have sex with men [MSM] that is above the pre-AIDS era estimates, and we have evidence of spread into heterosexual networks, and a very scary collision with the methamphetamine and heroine using networks,” said Jeanne Marrazzo, MD, professor of infectious diseases at the University of Alabama at Birmingham.
But the numbers alone don’t tell the whole story. “It’s not just the burden of these infections. What’s characterizing these trends is that we have continuing evolution of microbial resistance, which is really a crisis,” Dr. Marrazzo added during a plenary she delivered at the Conference on Retroviruses & Opportunistic Infections.
These infections also remain intricately linked with HIV. An analysis of syphilis cases found that 88% occurred in men. Of those, 80% were MSM. Of the cases in MSM, 46% were coinfected with HIV. “Those are incredible rates,” said Dr. Marrazzo. Among women, the trends are even more alarming. There has been a greater than 150% increase in primary/secondary and congenital syphilis between 2013 and 2017.
Resistance to ceftriaxone and azithromycin remains on the rise in gonorrhea, with 24% of countries reporting at least a 5% incidence of strains that are less susceptible or resistant to ceftriaxone, and 81% of countries reporting similar trends with azithromycin.
In the absence of new drugs to overcome that resistance, or vaccines that can prevent gonorrhea and other infections, what are clinicians to do?
One option may be postexposure doxycycline. One trial in MSM showed that a 200-mg dose taken 24-72 hours after sex was associated with about a 70% increase in both time to first chlamydia and time to first syphilis infection, though no effect was seen on gonorrhea infections. “We shouldn’t be surprised. We know that gonorrhea is classically resistant to tetracyclines, and the MSM population has the highest prevalence of antimicrobial resistance in gonorrhea,” said Dr. Marrazzo.
There are pros and cons to this strategy, of course. On the one hand, doxycycline works for chlamydia and syphilis, it’s safe, and it’s easy to administer. “We’re up a tree when it comes to syphilis, so why not?” opined Dr. Marrazzo. In fact, some MSM have read the literature and are already using it prophylactically. But there are downsides, including adverse effects such as esophagitis/ulceration and photosensitivity, and it is contraindicated in pregnant women. And then there’s the potential for evolving greater resistance. “The horse is out of the barn with respect to gonorrhea, but I think it’s worth thinking about resistance to other pathogens, where we still rely on doxycycline [to treat] in rare cases,” said Dr. Marrazzo.
Finally, Dr. Marrazzo discussed the role of STI treatment in the effort to eradicate HIV. Should the Getting to 0 strategies include aggressive prevention and treatment of STIs? Despite the potentiating role of some STIs in the spread HIV, some urban areas are approaching zero new infections even as other STIs remain a problem. It could be that undetectable = untransmittable, regardless of the presence an STI. Some view targeting STIs as a regressive practice in a setting where the U=U mantra has opened up an era of sexual freedom living with or at risk of HIV.
On the other hand, there are also good arguments to target STIs while trying to eliminate HIV. Results from high-resource locales such as San Francisco and New York City are unlikely to be replicated in places like Sub-Saharan Africa. The public health burden of STIs is extensive, and antibiotic resistance and antibiotic shortages can make treatment difficult. The situation is also different for women, who may experience impacts on fertility or pregnancies, and do not have the same freedom as men in many countries. “Stigma is highly operative and I would wager that sexual pleasure and freedom remain a very elusive goal for women across the globe,” said Dr. Marrazzo.
Dr. Marrazzo has a research grant/grant pending from Cepheid, and is on the advisory panels of BioFire and Gilead.
SEATTLE – Sexually-transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are on the rise among HIV-infected individuals, and emerging antimicrobial resistance in these organisms is presenting serious challenges to physicians. The issue may be traceable to the introduction of preexposure prophylaxis (PrEP) in 2011, which previous studies have shown to be associated with less condom use.
In the United States, a 2017 report by the Centers for Disease Control and Prevention showed rising incidences of chlamydia (+5% from 2015 to 2017), gonorrhea (+19%), and syphilis (+18%). “We have an incidence among men who have sex with men [MSM] that is above the pre-AIDS era estimates, and we have evidence of spread into heterosexual networks, and a very scary collision with the methamphetamine and heroine using networks,” said Jeanne Marrazzo, MD, professor of infectious diseases at the University of Alabama at Birmingham.
But the numbers alone don’t tell the whole story. “It’s not just the burden of these infections. What’s characterizing these trends is that we have continuing evolution of microbial resistance, which is really a crisis,” Dr. Marrazzo added during a plenary she delivered at the Conference on Retroviruses & Opportunistic Infections.
These infections also remain intricately linked with HIV. An analysis of syphilis cases found that 88% occurred in men. Of those, 80% were MSM. Of the cases in MSM, 46% were coinfected with HIV. “Those are incredible rates,” said Dr. Marrazzo. Among women, the trends are even more alarming. There has been a greater than 150% increase in primary/secondary and congenital syphilis between 2013 and 2017.
Resistance to ceftriaxone and azithromycin remains on the rise in gonorrhea, with 24% of countries reporting at least a 5% incidence of strains that are less susceptible or resistant to ceftriaxone, and 81% of countries reporting similar trends with azithromycin.
In the absence of new drugs to overcome that resistance, or vaccines that can prevent gonorrhea and other infections, what are clinicians to do?
One option may be postexposure doxycycline. One trial in MSM showed that a 200-mg dose taken 24-72 hours after sex was associated with about a 70% increase in both time to first chlamydia and time to first syphilis infection, though no effect was seen on gonorrhea infections. “We shouldn’t be surprised. We know that gonorrhea is classically resistant to tetracyclines, and the MSM population has the highest prevalence of antimicrobial resistance in gonorrhea,” said Dr. Marrazzo.
There are pros and cons to this strategy, of course. On the one hand, doxycycline works for chlamydia and syphilis, it’s safe, and it’s easy to administer. “We’re up a tree when it comes to syphilis, so why not?” opined Dr. Marrazzo. In fact, some MSM have read the literature and are already using it prophylactically. But there are downsides, including adverse effects such as esophagitis/ulceration and photosensitivity, and it is contraindicated in pregnant women. And then there’s the potential for evolving greater resistance. “The horse is out of the barn with respect to gonorrhea, but I think it’s worth thinking about resistance to other pathogens, where we still rely on doxycycline [to treat] in rare cases,” said Dr. Marrazzo.
Finally, Dr. Marrazzo discussed the role of STI treatment in the effort to eradicate HIV. Should the Getting to 0 strategies include aggressive prevention and treatment of STIs? Despite the potentiating role of some STIs in the spread HIV, some urban areas are approaching zero new infections even as other STIs remain a problem. It could be that undetectable = untransmittable, regardless of the presence an STI. Some view targeting STIs as a regressive practice in a setting where the U=U mantra has opened up an era of sexual freedom living with or at risk of HIV.
On the other hand, there are also good arguments to target STIs while trying to eliminate HIV. Results from high-resource locales such as San Francisco and New York City are unlikely to be replicated in places like Sub-Saharan Africa. The public health burden of STIs is extensive, and antibiotic resistance and antibiotic shortages can make treatment difficult. The situation is also different for women, who may experience impacts on fertility or pregnancies, and do not have the same freedom as men in many countries. “Stigma is highly operative and I would wager that sexual pleasure and freedom remain a very elusive goal for women across the globe,” said Dr. Marrazzo.
Dr. Marrazzo has a research grant/grant pending from Cepheid, and is on the advisory panels of BioFire and Gilead.
SEATTLE – Sexually-transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are on the rise among HIV-infected individuals, and emerging antimicrobial resistance in these organisms is presenting serious challenges to physicians. The issue may be traceable to the introduction of preexposure prophylaxis (PrEP) in 2011, which previous studies have shown to be associated with less condom use.
In the United States, a 2017 report by the Centers for Disease Control and Prevention showed rising incidences of chlamydia (+5% from 2015 to 2017), gonorrhea (+19%), and syphilis (+18%). “We have an incidence among men who have sex with men [MSM] that is above the pre-AIDS era estimates, and we have evidence of spread into heterosexual networks, and a very scary collision with the methamphetamine and heroine using networks,” said Jeanne Marrazzo, MD, professor of infectious diseases at the University of Alabama at Birmingham.
But the numbers alone don’t tell the whole story. “It’s not just the burden of these infections. What’s characterizing these trends is that we have continuing evolution of microbial resistance, which is really a crisis,” Dr. Marrazzo added during a plenary she delivered at the Conference on Retroviruses & Opportunistic Infections.
These infections also remain intricately linked with HIV. An analysis of syphilis cases found that 88% occurred in men. Of those, 80% were MSM. Of the cases in MSM, 46% were coinfected with HIV. “Those are incredible rates,” said Dr. Marrazzo. Among women, the trends are even more alarming. There has been a greater than 150% increase in primary/secondary and congenital syphilis between 2013 and 2017.
Resistance to ceftriaxone and azithromycin remains on the rise in gonorrhea, with 24% of countries reporting at least a 5% incidence of strains that are less susceptible or resistant to ceftriaxone, and 81% of countries reporting similar trends with azithromycin.
In the absence of new drugs to overcome that resistance, or vaccines that can prevent gonorrhea and other infections, what are clinicians to do?
One option may be postexposure doxycycline. One trial in MSM showed that a 200-mg dose taken 24-72 hours after sex was associated with about a 70% increase in both time to first chlamydia and time to first syphilis infection, though no effect was seen on gonorrhea infections. “We shouldn’t be surprised. We know that gonorrhea is classically resistant to tetracyclines, and the MSM population has the highest prevalence of antimicrobial resistance in gonorrhea,” said Dr. Marrazzo.
There are pros and cons to this strategy, of course. On the one hand, doxycycline works for chlamydia and syphilis, it’s safe, and it’s easy to administer. “We’re up a tree when it comes to syphilis, so why not?” opined Dr. Marrazzo. In fact, some MSM have read the literature and are already using it prophylactically. But there are downsides, including adverse effects such as esophagitis/ulceration and photosensitivity, and it is contraindicated in pregnant women. And then there’s the potential for evolving greater resistance. “The horse is out of the barn with respect to gonorrhea, but I think it’s worth thinking about resistance to other pathogens, where we still rely on doxycycline [to treat] in rare cases,” said Dr. Marrazzo.
Finally, Dr. Marrazzo discussed the role of STI treatment in the effort to eradicate HIV. Should the Getting to 0 strategies include aggressive prevention and treatment of STIs? Despite the potentiating role of some STIs in the spread HIV, some urban areas are approaching zero new infections even as other STIs remain a problem. It could be that undetectable = untransmittable, regardless of the presence an STI. Some view targeting STIs as a regressive practice in a setting where the U=U mantra has opened up an era of sexual freedom living with or at risk of HIV.
On the other hand, there are also good arguments to target STIs while trying to eliminate HIV. Results from high-resource locales such as San Francisco and New York City are unlikely to be replicated in places like Sub-Saharan Africa. The public health burden of STIs is extensive, and antibiotic resistance and antibiotic shortages can make treatment difficult. The situation is also different for women, who may experience impacts on fertility or pregnancies, and do not have the same freedom as men in many countries. “Stigma is highly operative and I would wager that sexual pleasure and freedom remain a very elusive goal for women across the globe,” said Dr. Marrazzo.
Dr. Marrazzo has a research grant/grant pending from Cepheid, and is on the advisory panels of BioFire and Gilead.
EXPERT ANALYSIS FROM CROI 2019
Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
CASE 1 Defining anatomic structures to assure surgical precision
A 44-year-old woman is scheduled for a vaginal hysterectomy and bilateral salpingectomy for abnormal uterine bleeding. In your academic practice, a resident routinely operates with you and is accompanied by a medical student. As this is your first case with each learner, you review the steps of the procedure along with pertinent anatomy. During this discussion, numerous anatomic terms are used to describe anterior cul-de-sac entry, including pubocervical fascia, vesicouterine fold, and vesicovaginal space. Which of these terms, if any, are correct? Is there a preferred term that should be used to teach future learners so we can all “speak” the same language?
What’s in a name?
ObGyns must thoroughly understand pelvic anatomy, since much of our patient care relates to structures in that region. We also must understand the terminology that most appropriately describes each pelvic structure so that we can communicate effectively with colleagues and other providers. The case described above lists several terms that are commonly found in gynecologic textbooks and surgical atlases to describe dissection for vaginal hysterectomy. Lack of a standardized vocabulary, however, often confuses teachers and learners alike, and it highlights the importance of having a universal language to ensure the safe, effective performance of surgical procedures.1
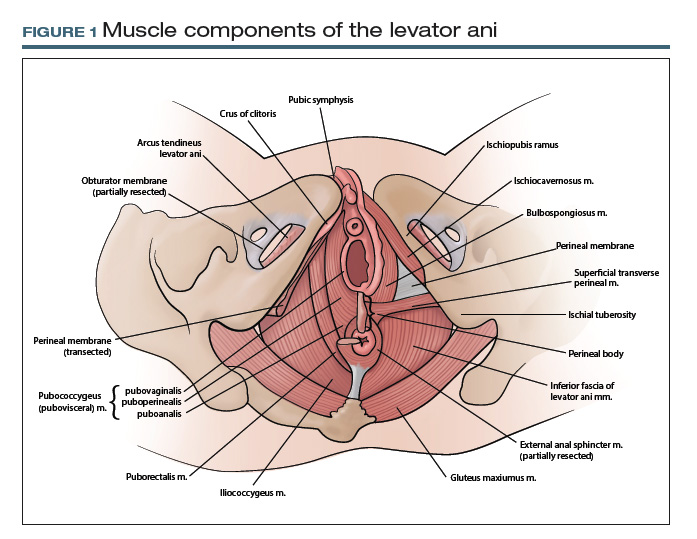
At first glance, it may seem that anatomic terms are inherently descriptive of the structure they represent; for example, the terms uterus and vagina seem rather obvious. However, many anatomic terms convey ambiguity. Which muscles, for example, constitute the levator ani: pubococcygeus, pubovisceral, pubovisceralis, puboperinealis, puboanalis, pubovaginalis, puborectalis, puborectal, iliococcygeus, ischiococcygeus? Do any of these terms redundantly describe the same structure, or does each term refer to an independent structure?
Standard terminology is essential
Anatomists long have recognized the need for standardized terminology to facilitate clear communication. To provide historical background, the term anatomy is derived from the Greek word for “dissection” or “to cut open.”2 Records on the scientific study of human anatomy date back thousands of years.
A brief review of current standardized terminology can be traced back to 1895, with the publication of Basle Terminologia Anatomica.3 That work was intended to provide a consolidated reference with clear direction regarding which anatomic terms should be used. It was updated several times during the ensuing century and was later published as Nomina Anatomica.
In 1990, an international committee was formed with representatives from many anatomical organizations, again with the intention of providing standardized anatomic terminology. Those efforts resulted in the publication of Terminologia Anatomica: International Anatomical Terminology, commonly referred to as TA, in 1998. TA continues to be the referent standard for human anatomic terminology; it was most recently updated in 2011.4
CASE 2 Conveying details of mesh erosion
A 52-year-old woman presents to the general gynecology clinic with a 10-year history of pelvic pain and dyspareunia after undergoing vaginal mesh surgery for prolapse and urinary incontinence. On examination, there is a visible ridge of mesh extending from the left side of the midurethra along the anterior and lateral vagina for a length of 1.5 cm. There also is a palpable tight band on the right vaginal wall near the ischial spine that reproduces her pain and causes spasm of the levator ani. You believe the patient needs a urogynecology referral for complications of vaginal mesh. How do you best describe your findings to your urogynecology colleague?
Continue to: Pelvic anatomy from the SGS perspective...
Pelvic anatomy from the SGS perspective
The Society of Gynecologic Surgeons (SGS) recognized the importance of standardizing terminology specific to the pelvis. The SGS Pelvic Anatomy Group thus was organized in 2016. The Pelvic Anatomy Group’s purpose is to help educate physicians about pelvic anatomy, with the overarching goal of compiling instructional materials, primarily from dissections (surgical or cadaveric), and radiologic imaging for all pelvic structures. Throughout the discussions on this initiative, it became clear that standardized terms needed to be established and used for pelvic structures.
While TA is an excellent reference work, it does not include all of the clinically relevant structures for gynecologic surgeons. As physicians, surgeons, and women’s health care providers, we read about and discuss pelvic anatomy structures in medical textbooks, medical literature, and clinical settings that are not necessarily included in TA. In addition, advances in information technology have facilitated the creation of clinically oriented computer-based anatomy programs and expanded the number and availability of electronic publications on surgical and clinical anatomy.5 As a result, there is a need not only to standardize nomenclature but also to continually revise and update terminology and integrate new terms, both from an anatomic and a clinical perspective.
The Pelvic Anatomy Group developed a novel approach to anatomic terminology. We decided to review the medical literature, identify the terms used, adjudicate the terms with current TA terms, and provide consensus for the terms and structures in the pelvis. Because of the volume of literature available and the existing number of terms, we divided the pelvis into 4 regions—anterior, apical, posterior, and vulvar—to improve the feasibility of reviewing the medical literature for the entire female pelvis.
Our process for tackling terminology
Our literature review started with the anterior compartment. (For complete details, see our prior publication.3) Modeled on a systematic review, we searched the MEDLINE database for terms related to the anterior pelvis, screened all associated abstracts, and then extracted terms from appropriate papers. We also identified several book chapters from various disciplines (anatomy, gynecology, urology, and radiology) to ensure wide representation of disciplines. We then extracted all terms pertinent to the anterior pelvis.
We organized the terms, with terms that referred to the same anatomic structure grouped together. Whenever possible, we used TA terms as the preferred terms. In this process, however, we identified several clinically relevant terms that were not included in TA: pelvic sidewall, pelvic bones, anterior compartment, pubourethral ligament, vaginal sulcus, and levator hiatus, among others. The new terms were then proposed and agreed on by members of the SGS Pelvic Anatomy Group and accepted by SGS members. We currently are completing a similar process for the apical pelvis, posterior pelvis, and vulvar regions.
TA code numbers pinpoint the nomenclature
As we move forward, we suggest that physicians use TA or other approved terms for patient and research communication. Such use will help standardize anatomic terms and also will improve communication between providers and education for learners.
Continue to: TA includes approved options...
TA includes approved options in English and Latin and lists a unique identification number for each term (shown in parentheses in the examples that follow). For instance, to answer the question posed earlier, the levator ani (A04.5.04.002) is comprised of the pubococcygeus (A04.5.04.003), puborectalis (A04.5.04.007), and iliococcygeus (A04.5.04.008) muscles (FIGURE 1).The terms pubovisceral and pubovisceralis are used synonymously in the literature with pubococcygeus (A04.5.04.003).3 The additional terms puboperinealis (A04.5.04.004), pubovaginalis (A04.5.04.005), and puboanalis (A04.5.04.006) are subcomponents of the pubococcygeus (A04.5.04.003), and this relationship is indicated in TA by indentation formatting.4 Finally, the ischiococcygeus (A04.5.04.011) muscle is not considered part of the levator ani (A04.5.04.002).
Revisiting the mesh erosion case: Reporting your findings
After reviewing the recommended terminology for the anterior pelvis,3,4 you might draft a report as follows: “A mesh erosion was visualized in anterior vaginal wall (A09.1.04.006) at the level of the mid-urethra extending into ‘anterior and lateral vaginal sulci’ (proposed term). In addition, there is a painful tight band in the ‘lateral vaginal wall’ (proposed term) near the ischial spine (A02.5.01.205). Palpation of this band reproduces the patient’s pain and causes secondary spasm of the levator ani (A04.5.04.002).” Certainly, TA identification numbers would not be expected to be included in medical communication; they are included here for reference.
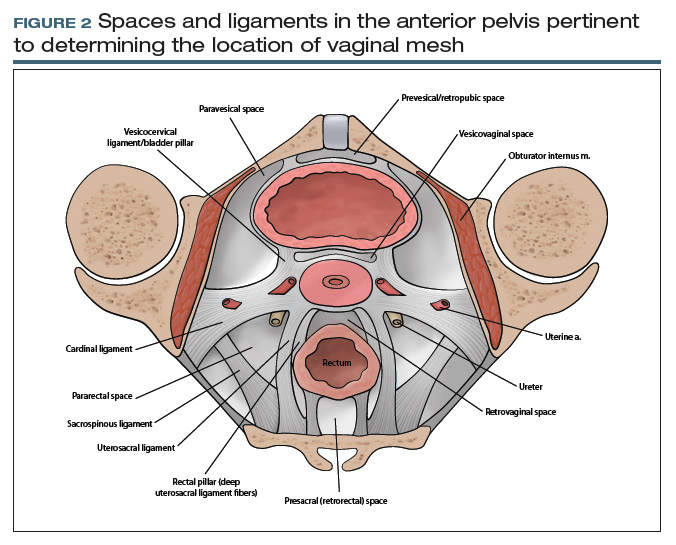
From your description, your urogynecology colleague has a better understanding of the location of your patient’s vaginal mesh and requests her operative report from an outside facility. In the operative report, the surgeon described “placement of mesh into the vagina, dissection through the rectal spaces, and anchoring of the mesh into the levator/pelvic muscles, the cervix, and lastly to the paraurethral ligaments,” and “passage of trocars through the cave of Retzius at the level of the midurethra” (FIGURE 2).
Based on this description, the urogynecologist ascertains that the mesh is located in the anterior vaginal wall (A09.1.04.006), with passage of anchoring arms through the bilateral sacrospinous ligaments (A03.6.03.007) and retropubic space (A10.1.01.003). Exposed mesh is visible, extending from the midurethra to the “anterior and lateral vaginal sulci” (proposed term).
This case clearly demonstrates the importance of communication between providers for patient care, since understanding the patient’s anatomy and the location of the vaginal mesh is important for planning surgical excision of the exposed mesh.
Additional initiatives
Outlining standardized terminology is just the first step toward improving the anatomic “language” used among providers. Ongoing efforts from the SGS Pelvic Anatomy Group include a special imaging group’s review of imaging modalities (ultrasonography, magnetic resonance imaging, computerized tomography) to improve standardization on reporting clinical anatomy. In addition, SGS has developed a group to create educational content related to the structures identified by the terminology group from cadaveric or surgical dissections. Educational materials will be compiled to help physicians and learners expand their anatomic understanding and improve their communication.
Further details of the Pelvic Anatomy Group’s efforts can be found on the SGS website at https://www.sgsonline.org.
- American Association of Clinical Anatomists, Educational Affairs Committee. The clinical anatomy of several invasive procedures. Clin Anat. 1999;12:43-54.
- Venes D, ed. Taber's Cyclopedic Medical Dictionary. 23rd ed. Philadelphia, PA: FA Davis Company; 2017.
- Jeppson PC, Balgobin S, Washington BB, et al; for the Society of Gynecologic Surgeons Pelvic Anatomy Group. Recommended standardized terminology of the anterior female pelvis based on a structured medical literature review. Am J Obstet Gynecol. 2018;219:26-39.
- Federative Committee on Anatomical Terminologies (FCAT). Terminologia Anatomica: International Anatomical Terminology. 2nd ed. Stuttgart, Germany: Thieme; 2011.
- Rosse C. Terminologia Anatomica: considered from the perspective of next-generation knowledge sources. Clin Anat. 2001;14:120-133.
CASE 1 Defining anatomic structures to assure surgical precision
A 44-year-old woman is scheduled for a vaginal hysterectomy and bilateral salpingectomy for abnormal uterine bleeding. In your academic practice, a resident routinely operates with you and is accompanied by a medical student. As this is your first case with each learner, you review the steps of the procedure along with pertinent anatomy. During this discussion, numerous anatomic terms are used to describe anterior cul-de-sac entry, including pubocervical fascia, vesicouterine fold, and vesicovaginal space. Which of these terms, if any, are correct? Is there a preferred term that should be used to teach future learners so we can all “speak” the same language?
What’s in a name?
ObGyns must thoroughly understand pelvic anatomy, since much of our patient care relates to structures in that region. We also must understand the terminology that most appropriately describes each pelvic structure so that we can communicate effectively with colleagues and other providers. The case described above lists several terms that are commonly found in gynecologic textbooks and surgical atlases to describe dissection for vaginal hysterectomy. Lack of a standardized vocabulary, however, often confuses teachers and learners alike, and it highlights the importance of having a universal language to ensure the safe, effective performance of surgical procedures.1
At first glance, it may seem that anatomic terms are inherently descriptive of the structure they represent; for example, the terms uterus and vagina seem rather obvious. However, many anatomic terms convey ambiguity. Which muscles, for example, constitute the levator ani: pubococcygeus, pubovisceral, pubovisceralis, puboperinealis, puboanalis, pubovaginalis, puborectalis, puborectal, iliococcygeus, ischiococcygeus? Do any of these terms redundantly describe the same structure, or does each term refer to an independent structure?
Standard terminology is essential
Anatomists long have recognized the need for standardized terminology to facilitate clear communication. To provide historical background, the term anatomy is derived from the Greek word for “dissection” or “to cut open.”2 Records on the scientific study of human anatomy date back thousands of years.
A brief review of current standardized terminology can be traced back to 1895, with the publication of Basle Terminologia Anatomica.3 That work was intended to provide a consolidated reference with clear direction regarding which anatomic terms should be used. It was updated several times during the ensuing century and was later published as Nomina Anatomica.
In 1990, an international committee was formed with representatives from many anatomical organizations, again with the intention of providing standardized anatomic terminology. Those efforts resulted in the publication of Terminologia Anatomica: International Anatomical Terminology, commonly referred to as TA, in 1998. TA continues to be the referent standard for human anatomic terminology; it was most recently updated in 2011.4
CASE 2 Conveying details of mesh erosion
A 52-year-old woman presents to the general gynecology clinic with a 10-year history of pelvic pain and dyspareunia after undergoing vaginal mesh surgery for prolapse and urinary incontinence. On examination, there is a visible ridge of mesh extending from the left side of the midurethra along the anterior and lateral vagina for a length of 1.5 cm. There also is a palpable tight band on the right vaginal wall near the ischial spine that reproduces her pain and causes spasm of the levator ani. You believe the patient needs a urogynecology referral for complications of vaginal mesh. How do you best describe your findings to your urogynecology colleague?
Continue to: Pelvic anatomy from the SGS perspective...
Pelvic anatomy from the SGS perspective
The Society of Gynecologic Surgeons (SGS) recognized the importance of standardizing terminology specific to the pelvis. The SGS Pelvic Anatomy Group thus was organized in 2016. The Pelvic Anatomy Group’s purpose is to help educate physicians about pelvic anatomy, with the overarching goal of compiling instructional materials, primarily from dissections (surgical or cadaveric), and radiologic imaging for all pelvic structures. Throughout the discussions on this initiative, it became clear that standardized terms needed to be established and used for pelvic structures.
While TA is an excellent reference work, it does not include all of the clinically relevant structures for gynecologic surgeons. As physicians, surgeons, and women’s health care providers, we read about and discuss pelvic anatomy structures in medical textbooks, medical literature, and clinical settings that are not necessarily included in TA. In addition, advances in information technology have facilitated the creation of clinically oriented computer-based anatomy programs and expanded the number and availability of electronic publications on surgical and clinical anatomy.5 As a result, there is a need not only to standardize nomenclature but also to continually revise and update terminology and integrate new terms, both from an anatomic and a clinical perspective.
The Pelvic Anatomy Group developed a novel approach to anatomic terminology. We decided to review the medical literature, identify the terms used, adjudicate the terms with current TA terms, and provide consensus for the terms and structures in the pelvis. Because of the volume of literature available and the existing number of terms, we divided the pelvis into 4 regions—anterior, apical, posterior, and vulvar—to improve the feasibility of reviewing the medical literature for the entire female pelvis.
Our process for tackling terminology
Our literature review started with the anterior compartment. (For complete details, see our prior publication.3) Modeled on a systematic review, we searched the MEDLINE database for terms related to the anterior pelvis, screened all associated abstracts, and then extracted terms from appropriate papers. We also identified several book chapters from various disciplines (anatomy, gynecology, urology, and radiology) to ensure wide representation of disciplines. We then extracted all terms pertinent to the anterior pelvis.
We organized the terms, with terms that referred to the same anatomic structure grouped together. Whenever possible, we used TA terms as the preferred terms. In this process, however, we identified several clinically relevant terms that were not included in TA: pelvic sidewall, pelvic bones, anterior compartment, pubourethral ligament, vaginal sulcus, and levator hiatus, among others. The new terms were then proposed and agreed on by members of the SGS Pelvic Anatomy Group and accepted by SGS members. We currently are completing a similar process for the apical pelvis, posterior pelvis, and vulvar regions.
TA code numbers pinpoint the nomenclature
As we move forward, we suggest that physicians use TA or other approved terms for patient and research communication. Such use will help standardize anatomic terms and also will improve communication between providers and education for learners.
Continue to: TA includes approved options...
TA includes approved options in English and Latin and lists a unique identification number for each term (shown in parentheses in the examples that follow). For instance, to answer the question posed earlier, the levator ani (A04.5.04.002) is comprised of the pubococcygeus (A04.5.04.003), puborectalis (A04.5.04.007), and iliococcygeus (A04.5.04.008) muscles (FIGURE 1).The terms pubovisceral and pubovisceralis are used synonymously in the literature with pubococcygeus (A04.5.04.003).3 The additional terms puboperinealis (A04.5.04.004), pubovaginalis (A04.5.04.005), and puboanalis (A04.5.04.006) are subcomponents of the pubococcygeus (A04.5.04.003), and this relationship is indicated in TA by indentation formatting.4 Finally, the ischiococcygeus (A04.5.04.011) muscle is not considered part of the levator ani (A04.5.04.002).

Revisiting the mesh erosion case: Reporting your findings
After reviewing the recommended terminology for the anterior pelvis,3,4 you might draft a report as follows: “A mesh erosion was visualized in anterior vaginal wall (A09.1.04.006) at the level of the mid-urethra extending into ‘anterior and lateral vaginal sulci’ (proposed term). In addition, there is a painful tight band in the ‘lateral vaginal wall’ (proposed term) near the ischial spine (A02.5.01.205). Palpation of this band reproduces the patient’s pain and causes secondary spasm of the levator ani (A04.5.04.002).” Certainly, TA identification numbers would not be expected to be included in medical communication; they are included here for reference.
From your description, your urogynecology colleague has a better understanding of the location of your patient’s vaginal mesh and requests her operative report from an outside facility. In the operative report, the surgeon described “placement of mesh into the vagina, dissection through the rectal spaces, and anchoring of the mesh into the levator/pelvic muscles, the cervix, and lastly to the paraurethral ligaments,” and “passage of trocars through the cave of Retzius at the level of the midurethra” (FIGURE 2).
Based on this description, the urogynecologist ascertains that the mesh is located in the anterior vaginal wall (A09.1.04.006), with passage of anchoring arms through the bilateral sacrospinous ligaments (A03.6.03.007) and retropubic space (A10.1.01.003). Exposed mesh is visible, extending from the midurethra to the “anterior and lateral vaginal sulci” (proposed term).
This case clearly demonstrates the importance of communication between providers for patient care, since understanding the patient’s anatomy and the location of the vaginal mesh is important for planning surgical excision of the exposed mesh.

Additional initiatives
Outlining standardized terminology is just the first step toward improving the anatomic “language” used among providers. Ongoing efforts from the SGS Pelvic Anatomy Group include a special imaging group’s review of imaging modalities (ultrasonography, magnetic resonance imaging, computerized tomography) to improve standardization on reporting clinical anatomy. In addition, SGS has developed a group to create educational content related to the structures identified by the terminology group from cadaveric or surgical dissections. Educational materials will be compiled to help physicians and learners expand their anatomic understanding and improve their communication.
Further details of the Pelvic Anatomy Group’s efforts can be found on the SGS website at https://www.sgsonline.org.
CASE 1 Defining anatomic structures to assure surgical precision
A 44-year-old woman is scheduled for a vaginal hysterectomy and bilateral salpingectomy for abnormal uterine bleeding. In your academic practice, a resident routinely operates with you and is accompanied by a medical student. As this is your first case with each learner, you review the steps of the procedure along with pertinent anatomy. During this discussion, numerous anatomic terms are used to describe anterior cul-de-sac entry, including pubocervical fascia, vesicouterine fold, and vesicovaginal space. Which of these terms, if any, are correct? Is there a preferred term that should be used to teach future learners so we can all “speak” the same language?
What’s in a name?
ObGyns must thoroughly understand pelvic anatomy, since much of our patient care relates to structures in that region. We also must understand the terminology that most appropriately describes each pelvic structure so that we can communicate effectively with colleagues and other providers. The case described above lists several terms that are commonly found in gynecologic textbooks and surgical atlases to describe dissection for vaginal hysterectomy. Lack of a standardized vocabulary, however, often confuses teachers and learners alike, and it highlights the importance of having a universal language to ensure the safe, effective performance of surgical procedures.1
At first glance, it may seem that anatomic terms are inherently descriptive of the structure they represent; for example, the terms uterus and vagina seem rather obvious. However, many anatomic terms convey ambiguity. Which muscles, for example, constitute the levator ani: pubococcygeus, pubovisceral, pubovisceralis, puboperinealis, puboanalis, pubovaginalis, puborectalis, puborectal, iliococcygeus, ischiococcygeus? Do any of these terms redundantly describe the same structure, or does each term refer to an independent structure?
Standard terminology is essential
Anatomists long have recognized the need for standardized terminology to facilitate clear communication. To provide historical background, the term anatomy is derived from the Greek word for “dissection” or “to cut open.”2 Records on the scientific study of human anatomy date back thousands of years.
A brief review of current standardized terminology can be traced back to 1895, with the publication of Basle Terminologia Anatomica.3 That work was intended to provide a consolidated reference with clear direction regarding which anatomic terms should be used. It was updated several times during the ensuing century and was later published as Nomina Anatomica.
In 1990, an international committee was formed with representatives from many anatomical organizations, again with the intention of providing standardized anatomic terminology. Those efforts resulted in the publication of Terminologia Anatomica: International Anatomical Terminology, commonly referred to as TA, in 1998. TA continues to be the referent standard for human anatomic terminology; it was most recently updated in 2011.4
CASE 2 Conveying details of mesh erosion
A 52-year-old woman presents to the general gynecology clinic with a 10-year history of pelvic pain and dyspareunia after undergoing vaginal mesh surgery for prolapse and urinary incontinence. On examination, there is a visible ridge of mesh extending from the left side of the midurethra along the anterior and lateral vagina for a length of 1.5 cm. There also is a palpable tight band on the right vaginal wall near the ischial spine that reproduces her pain and causes spasm of the levator ani. You believe the patient needs a urogynecology referral for complications of vaginal mesh. How do you best describe your findings to your urogynecology colleague?
Continue to: Pelvic anatomy from the SGS perspective...
Pelvic anatomy from the SGS perspective
The Society of Gynecologic Surgeons (SGS) recognized the importance of standardizing terminology specific to the pelvis. The SGS Pelvic Anatomy Group thus was organized in 2016. The Pelvic Anatomy Group’s purpose is to help educate physicians about pelvic anatomy, with the overarching goal of compiling instructional materials, primarily from dissections (surgical or cadaveric), and radiologic imaging for all pelvic structures. Throughout the discussions on this initiative, it became clear that standardized terms needed to be established and used for pelvic structures.
While TA is an excellent reference work, it does not include all of the clinically relevant structures for gynecologic surgeons. As physicians, surgeons, and women’s health care providers, we read about and discuss pelvic anatomy structures in medical textbooks, medical literature, and clinical settings that are not necessarily included in TA. In addition, advances in information technology have facilitated the creation of clinically oriented computer-based anatomy programs and expanded the number and availability of electronic publications on surgical and clinical anatomy.5 As a result, there is a need not only to standardize nomenclature but also to continually revise and update terminology and integrate new terms, both from an anatomic and a clinical perspective.
The Pelvic Anatomy Group developed a novel approach to anatomic terminology. We decided to review the medical literature, identify the terms used, adjudicate the terms with current TA terms, and provide consensus for the terms and structures in the pelvis. Because of the volume of literature available and the existing number of terms, we divided the pelvis into 4 regions—anterior, apical, posterior, and vulvar—to improve the feasibility of reviewing the medical literature for the entire female pelvis.
Our process for tackling terminology
Our literature review started with the anterior compartment. (For complete details, see our prior publication.3) Modeled on a systematic review, we searched the MEDLINE database for terms related to the anterior pelvis, screened all associated abstracts, and then extracted terms from appropriate papers. We also identified several book chapters from various disciplines (anatomy, gynecology, urology, and radiology) to ensure wide representation of disciplines. We then extracted all terms pertinent to the anterior pelvis.
We organized the terms, with terms that referred to the same anatomic structure grouped together. Whenever possible, we used TA terms as the preferred terms. In this process, however, we identified several clinically relevant terms that were not included in TA: pelvic sidewall, pelvic bones, anterior compartment, pubourethral ligament, vaginal sulcus, and levator hiatus, among others. The new terms were then proposed and agreed on by members of the SGS Pelvic Anatomy Group and accepted by SGS members. We currently are completing a similar process for the apical pelvis, posterior pelvis, and vulvar regions.
TA code numbers pinpoint the nomenclature
As we move forward, we suggest that physicians use TA or other approved terms for patient and research communication. Such use will help standardize anatomic terms and also will improve communication between providers and education for learners.
Continue to: TA includes approved options...
TA includes approved options in English and Latin and lists a unique identification number for each term (shown in parentheses in the examples that follow). For instance, to answer the question posed earlier, the levator ani (A04.5.04.002) is comprised of the pubococcygeus (A04.5.04.003), puborectalis (A04.5.04.007), and iliococcygeus (A04.5.04.008) muscles (FIGURE 1).The terms pubovisceral and pubovisceralis are used synonymously in the literature with pubococcygeus (A04.5.04.003).3 The additional terms puboperinealis (A04.5.04.004), pubovaginalis (A04.5.04.005), and puboanalis (A04.5.04.006) are subcomponents of the pubococcygeus (A04.5.04.003), and this relationship is indicated in TA by indentation formatting.4 Finally, the ischiococcygeus (A04.5.04.011) muscle is not considered part of the levator ani (A04.5.04.002).

Revisiting the mesh erosion case: Reporting your findings
After reviewing the recommended terminology for the anterior pelvis,3,4 you might draft a report as follows: “A mesh erosion was visualized in anterior vaginal wall (A09.1.04.006) at the level of the mid-urethra extending into ‘anterior and lateral vaginal sulci’ (proposed term). In addition, there is a painful tight band in the ‘lateral vaginal wall’ (proposed term) near the ischial spine (A02.5.01.205). Palpation of this band reproduces the patient’s pain and causes secondary spasm of the levator ani (A04.5.04.002).” Certainly, TA identification numbers would not be expected to be included in medical communication; they are included here for reference.
From your description, your urogynecology colleague has a better understanding of the location of your patient’s vaginal mesh and requests her operative report from an outside facility. In the operative report, the surgeon described “placement of mesh into the vagina, dissection through the rectal spaces, and anchoring of the mesh into the levator/pelvic muscles, the cervix, and lastly to the paraurethral ligaments,” and “passage of trocars through the cave of Retzius at the level of the midurethra” (FIGURE 2).
Based on this description, the urogynecologist ascertains that the mesh is located in the anterior vaginal wall (A09.1.04.006), with passage of anchoring arms through the bilateral sacrospinous ligaments (A03.6.03.007) and retropubic space (A10.1.01.003). Exposed mesh is visible, extending from the midurethra to the “anterior and lateral vaginal sulci” (proposed term).
This case clearly demonstrates the importance of communication between providers for patient care, since understanding the patient’s anatomy and the location of the vaginal mesh is important for planning surgical excision of the exposed mesh.

Additional initiatives
Outlining standardized terminology is just the first step toward improving the anatomic “language” used among providers. Ongoing efforts from the SGS Pelvic Anatomy Group include a special imaging group’s review of imaging modalities (ultrasonography, magnetic resonance imaging, computerized tomography) to improve standardization on reporting clinical anatomy. In addition, SGS has developed a group to create educational content related to the structures identified by the terminology group from cadaveric or surgical dissections. Educational materials will be compiled to help physicians and learners expand their anatomic understanding and improve their communication.
Further details of the Pelvic Anatomy Group’s efforts can be found on the SGS website at https://www.sgsonline.org.
- American Association of Clinical Anatomists, Educational Affairs Committee. The clinical anatomy of several invasive procedures. Clin Anat. 1999;12:43-54.
- Venes D, ed. Taber's Cyclopedic Medical Dictionary. 23rd ed. Philadelphia, PA: FA Davis Company; 2017.
- Jeppson PC, Balgobin S, Washington BB, et al; for the Society of Gynecologic Surgeons Pelvic Anatomy Group. Recommended standardized terminology of the anterior female pelvis based on a structured medical literature review. Am J Obstet Gynecol. 2018;219:26-39.
- Federative Committee on Anatomical Terminologies (FCAT). Terminologia Anatomica: International Anatomical Terminology. 2nd ed. Stuttgart, Germany: Thieme; 2011.
- Rosse C. Terminologia Anatomica: considered from the perspective of next-generation knowledge sources. Clin Anat. 2001;14:120-133.
- American Association of Clinical Anatomists, Educational Affairs Committee. The clinical anatomy of several invasive procedures. Clin Anat. 1999;12:43-54.
- Venes D, ed. Taber's Cyclopedic Medical Dictionary. 23rd ed. Philadelphia, PA: FA Davis Company; 2017.
- Jeppson PC, Balgobin S, Washington BB, et al; for the Society of Gynecologic Surgeons Pelvic Anatomy Group. Recommended standardized terminology of the anterior female pelvis based on a structured medical literature review. Am J Obstet Gynecol. 2018;219:26-39.
- Federative Committee on Anatomical Terminologies (FCAT). Terminologia Anatomica: International Anatomical Terminology. 2nd ed. Stuttgart, Germany: Thieme; 2011.
- Rosse C. Terminologia Anatomica: considered from the perspective of next-generation knowledge sources. Clin Anat. 2001;14:120-133.
Part 1: The Study in Question
Recently, my colleague Randy D. Danielsen, PhD, DFAAPA, PA-C Emeritus, shared a study from the American Journal of Emergency Medicine that focused on “the involvement of NPs and PAs who billed independently” in emergency departments (EDs).1 In casual conversation, several of us agreed the findings didn’t “pass the sniff test,” so I decided to do some investigating.
The context: Data from 2006-2009 indicate that in two-thirds of all EDs, NPs and PAs are involved in the care of 13.7% of all patients.2 Further analysis of Medicare Public Use Files from 2014 reveal that of 58,641 unique emergency medicine clinicians, 14,360 (24.5%) are advanced practice providers.3 All interesting statistics.
The American Journal of Emergency Medicine article, however, gave me (and several colleagues) pause. In it, the authors presented their analysis of Medicare provider utilization and payment data from 2012-2016.1 The researchers documented billing increases of 65% for NPs and 35% for PAs.
But what stopped me in my tracks was that the researchers emphasized an increase—from 18% to 24%—in NP/PA treatment of patients with the highest severity illness or injury (CPT code 99285).1 I discussed this finding with ED-based colleagues, and they too questioned its accuracy.
In fact, the more we parsed this study, the more questions we had … and the higher our eyebrows raised. What were the researchers examining and drawing conclusions on— independent billing by NPs and PAs, or independent practice? These are two very different measures. Were the authors in fact grousing about the increase in NP/PA providers in EDs?
There is a paucity of research on billing by NPs and PAs, and the discussion surrounding this particular study will undoubtedly prompt additional questions. Over the next 3 weeks, we invite you to join us on Thursdays as we continue our examination of this data—and encourage you to share your thoughts with us along the way!
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. Wilder JL, Rooks, SP, Ginde AA. Update on midlevel provider utilization in US emergency departments, 2006 to 2009. Academic Emerg Med. 2012;19(8):986-989.
3. Hall MK, Burns K, Carius M, et al. State of the national emergency department workforce: who provides care where? Ann Emerg Med. 2018;72(3):302-307.
Recently, my colleague Randy D. Danielsen, PhD, DFAAPA, PA-C Emeritus, shared a study from the American Journal of Emergency Medicine that focused on “the involvement of NPs and PAs who billed independently” in emergency departments (EDs).1 In casual conversation, several of us agreed the findings didn’t “pass the sniff test,” so I decided to do some investigating.
The context: Data from 2006-2009 indicate that in two-thirds of all EDs, NPs and PAs are involved in the care of 13.7% of all patients.2 Further analysis of Medicare Public Use Files from 2014 reveal that of 58,641 unique emergency medicine clinicians, 14,360 (24.5%) are advanced practice providers.3 All interesting statistics.
The American Journal of Emergency Medicine article, however, gave me (and several colleagues) pause. In it, the authors presented their analysis of Medicare provider utilization and payment data from 2012-2016.1 The researchers documented billing increases of 65% for NPs and 35% for PAs.
But what stopped me in my tracks was that the researchers emphasized an increase—from 18% to 24%—in NP/PA treatment of patients with the highest severity illness or injury (CPT code 99285).1 I discussed this finding with ED-based colleagues, and they too questioned its accuracy.
In fact, the more we parsed this study, the more questions we had … and the higher our eyebrows raised. What were the researchers examining and drawing conclusions on— independent billing by NPs and PAs, or independent practice? These are two very different measures. Were the authors in fact grousing about the increase in NP/PA providers in EDs?
There is a paucity of research on billing by NPs and PAs, and the discussion surrounding this particular study will undoubtedly prompt additional questions. Over the next 3 weeks, we invite you to join us on Thursdays as we continue our examination of this data—and encourage you to share your thoughts with us along the way!
Recently, my colleague Randy D. Danielsen, PhD, DFAAPA, PA-C Emeritus, shared a study from the American Journal of Emergency Medicine that focused on “the involvement of NPs and PAs who billed independently” in emergency departments (EDs).1 In casual conversation, several of us agreed the findings didn’t “pass the sniff test,” so I decided to do some investigating.
The context: Data from 2006-2009 indicate that in two-thirds of all EDs, NPs and PAs are involved in the care of 13.7% of all patients.2 Further analysis of Medicare Public Use Files from 2014 reveal that of 58,641 unique emergency medicine clinicians, 14,360 (24.5%) are advanced practice providers.3 All interesting statistics.
The American Journal of Emergency Medicine article, however, gave me (and several colleagues) pause. In it, the authors presented their analysis of Medicare provider utilization and payment data from 2012-2016.1 The researchers documented billing increases of 65% for NPs and 35% for PAs.
But what stopped me in my tracks was that the researchers emphasized an increase—from 18% to 24%—in NP/PA treatment of patients with the highest severity illness or injury (CPT code 99285).1 I discussed this finding with ED-based colleagues, and they too questioned its accuracy.
In fact, the more we parsed this study, the more questions we had … and the higher our eyebrows raised. What were the researchers examining and drawing conclusions on— independent billing by NPs and PAs, or independent practice? These are two very different measures. Were the authors in fact grousing about the increase in NP/PA providers in EDs?
There is a paucity of research on billing by NPs and PAs, and the discussion surrounding this particular study will undoubtedly prompt additional questions. Over the next 3 weeks, we invite you to join us on Thursdays as we continue our examination of this data—and encourage you to share your thoughts with us along the way!
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. Wilder JL, Rooks, SP, Ginde AA. Update on midlevel provider utilization in US emergency departments, 2006 to 2009. Academic Emerg Med. 2012;19(8):986-989.
3. Hall MK, Burns K, Carius M, et al. State of the national emergency department workforce: who provides care where? Ann Emerg Med. 2018;72(3):302-307.
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. Wilder JL, Rooks, SP, Ginde AA. Update on midlevel provider utilization in US emergency departments, 2006 to 2009. Academic Emerg Med. 2012;19(8):986-989.
3. Hall MK, Burns K, Carius M, et al. State of the national emergency department workforce: who provides care where? Ann Emerg Med. 2018;72(3):302-307.
Rising to the challenges in gynecologic surgical care
As the face of health care changes and physicians are presented with new challenges, we need to keep focused on our priorities: maintain outstanding patient care, continue to grow ourselves as physicians, and train the next generation of women’s health care providers. The theme of the SGS 2019 annual scientific meeting in Tucson, Arizona, “Looking Forward:
The excellent postgraduate workshops included courses on simulation of laparoscopic suturing, surgical strategies for fibroid management, and a quality improvement boot camp. In addition, Rebecca Rogers, MD, Cassandra Carberry, MD, and Danielle Antosh, MD, along with physical therapist Uchenna Ossai, PT, DPT, WCS, ran a course on pelvic surgery and its impact on sexual function, tackling an important, often difficult topic for gynecologic surgeons. In part 2 of this special section, these authors highlight current knowledge on sexual function related to surgery and offer an initial evaluation and treatment approach for women with sexual dysfunction after surgery.
Peter Jeppson, MD, Audra Jolyn Hill, MD, and Sunil Balgobin, MD, have been integral leaders of the SGS Pelvic Anatomy Group, which has a mission to educate physicians about pelvic anatomy. Early discussions made it clear that standardized terms needed to be established and used for pelvic structures. In this special section, these authors illustrate the importance of standard terminology to optimize patient care, and they review pertinent vaginal compartment structures for the gynecologist.
Along with outstanding plenary talks focusing on surgical education research by Gary Dunnington, MD, and health disparities in gynecologic surgery by Marcela del Carmen, MD, MPH, 2 special focus speakers were featured. Sean Dowdy, MD, highlighted advances in the perioperative care of gynecologic surgery patients. In this special section, he reviews best practices for enhanced recovery after surgery (ERAS) and describes his experience with implementing a successful ERAS program.
Cheryl Iglesia, MD, covered energy-based therapies in female genital cosmetic surgery. In part 2 of this special section, she highlights, with Sarah Ward, MD, the salient points from her presentation, including the mechanism of action of laser therapy on tissue remodeling as well as some therapeutic uses for and outcomes of laser therapy in gynecologic care.
I hope you enjoy the content of this special section (part 2 will follow in the May issue) and find that it helps you achieve excellence in gynecologic surgery for yourself, your learners, and your patients!
As the face of health care changes and physicians are presented with new challenges, we need to keep focused on our priorities: maintain outstanding patient care, continue to grow ourselves as physicians, and train the next generation of women’s health care providers. The theme of the SGS 2019 annual scientific meeting in Tucson, Arizona, “Looking Forward:
The excellent postgraduate workshops included courses on simulation of laparoscopic suturing, surgical strategies for fibroid management, and a quality improvement boot camp. In addition, Rebecca Rogers, MD, Cassandra Carberry, MD, and Danielle Antosh, MD, along with physical therapist Uchenna Ossai, PT, DPT, WCS, ran a course on pelvic surgery and its impact on sexual function, tackling an important, often difficult topic for gynecologic surgeons. In part 2 of this special section, these authors highlight current knowledge on sexual function related to surgery and offer an initial evaluation and treatment approach for women with sexual dysfunction after surgery.
Peter Jeppson, MD, Audra Jolyn Hill, MD, and Sunil Balgobin, MD, have been integral leaders of the SGS Pelvic Anatomy Group, which has a mission to educate physicians about pelvic anatomy. Early discussions made it clear that standardized terms needed to be established and used for pelvic structures. In this special section, these authors illustrate the importance of standard terminology to optimize patient care, and they review pertinent vaginal compartment structures for the gynecologist.
Along with outstanding plenary talks focusing on surgical education research by Gary Dunnington, MD, and health disparities in gynecologic surgery by Marcela del Carmen, MD, MPH, 2 special focus speakers were featured. Sean Dowdy, MD, highlighted advances in the perioperative care of gynecologic surgery patients. In this special section, he reviews best practices for enhanced recovery after surgery (ERAS) and describes his experience with implementing a successful ERAS program.
Cheryl Iglesia, MD, covered energy-based therapies in female genital cosmetic surgery. In part 2 of this special section, she highlights, with Sarah Ward, MD, the salient points from her presentation, including the mechanism of action of laser therapy on tissue remodeling as well as some therapeutic uses for and outcomes of laser therapy in gynecologic care.
I hope you enjoy the content of this special section (part 2 will follow in the May issue) and find that it helps you achieve excellence in gynecologic surgery for yourself, your learners, and your patients!
As the face of health care changes and physicians are presented with new challenges, we need to keep focused on our priorities: maintain outstanding patient care, continue to grow ourselves as physicians, and train the next generation of women’s health care providers. The theme of the SGS 2019 annual scientific meeting in Tucson, Arizona, “Looking Forward:
The excellent postgraduate workshops included courses on simulation of laparoscopic suturing, surgical strategies for fibroid management, and a quality improvement boot camp. In addition, Rebecca Rogers, MD, Cassandra Carberry, MD, and Danielle Antosh, MD, along with physical therapist Uchenna Ossai, PT, DPT, WCS, ran a course on pelvic surgery and its impact on sexual function, tackling an important, often difficult topic for gynecologic surgeons. In part 2 of this special section, these authors highlight current knowledge on sexual function related to surgery and offer an initial evaluation and treatment approach for women with sexual dysfunction after surgery.
Peter Jeppson, MD, Audra Jolyn Hill, MD, and Sunil Balgobin, MD, have been integral leaders of the SGS Pelvic Anatomy Group, which has a mission to educate physicians about pelvic anatomy. Early discussions made it clear that standardized terms needed to be established and used for pelvic structures. In this special section, these authors illustrate the importance of standard terminology to optimize patient care, and they review pertinent vaginal compartment structures for the gynecologist.
Along with outstanding plenary talks focusing on surgical education research by Gary Dunnington, MD, and health disparities in gynecologic surgery by Marcela del Carmen, MD, MPH, 2 special focus speakers were featured. Sean Dowdy, MD, highlighted advances in the perioperative care of gynecologic surgery patients. In this special section, he reviews best practices for enhanced recovery after surgery (ERAS) and describes his experience with implementing a successful ERAS program.
Cheryl Iglesia, MD, covered energy-based therapies in female genital cosmetic surgery. In part 2 of this special section, she highlights, with Sarah Ward, MD, the salient points from her presentation, including the mechanism of action of laser therapy on tissue remodeling as well as some therapeutic uses for and outcomes of laser therapy in gynecologic care.
I hope you enjoy the content of this special section (part 2 will follow in the May issue) and find that it helps you achieve excellence in gynecologic surgery for yourself, your learners, and your patients!
Has radial access PCI been overhyped?
NEW ORLEANS – , Michel R. Le May, MD, reported at the annual meeting of the American College of Cardiology.
Our findings suggest that adequately trained operators should be able to achieve similar results using either radial or femoral access for primary PCI,” declared Dr. Le May, professor of medicine at the University of Ottawa Heart Institute.
This is a controversial issue. European cardiologists have led a strong push for preferential use of radial access, citing reduced bleeding risk and an associated reduction in 30-day mortality. And this movement has spread to North America. But the evidence doesn’t convincingly support this position, the cardiologist said. He noted that of nine prior RCTs of radial versus the more traditional femoral access for primary PCI in STEMI, seven showed no difference in mortality. Nor did SAFARI-STEMI, which at 2,292 randomized STEMI patients was the second-largest trial to date.
SAFARI-STEMI was conducted at five high-volume Canadian PCI centers. Participating interventional cardiologists averaged 250 PCIs per year and were proficient in both access approaches. The study plan was to enroll 5,000 STEMI patients, but the trial was stopped after results were in for the first 2,292 because outcomes in the two study arms were so similar that the trial’s data safety monitoring board deemed it futile to continue.
The primary outcome was 30-day mortality. The rate was 1.5% in the radial access group and 1.3% in the femoral access group, with no differences among various subgroups.
Nor were there any between-group significant difference in the secondary endpoints of reinfarction (1.8% with radial, 1.6% with femoral), stroke (1.0% versus 0.4%), or the composite of death, reinfarction, or stroke, which occurred in 4.0% of the radial access group and 3.4% of the femoral group. Rates of non-CABG TIMI major or minor bleeding at 30 days were closely similar, as was need for transfusion. Definite or probable stent thrombosis occurred in 1.5% of the radial and 1.1% of the femoral groups.
Time from arrival at the PCI center to first balloon inflation was 47 minutes in the radial access group and significantly shorter at 44 minutes with femoral access, a noteworthy finding in the setting of STEMI, where time is myocardium. Fluoroscopy time was 1.2 minutes shorter in the femoral access group as well.
The reaction
Discussant Jacqueline E. Tamis-Holland, MD, said that, although she recently switched over to a radial access-first approach, her take away from SAFARI-STEMI is “It’s okay to do femoral.
“I think it’s comforting to the femoralists who are struggling to say, ‘I do a good femoral job and I don’t necessarily want to transition my STEMI patients to radial,’ ” said Dr. Tamis-Holland, associate director for the Mount Sinai St. Luke’s cardiac cath labs in New York.
Session cochair Martin B. Leon, MD, embraced the SAFARI-STEMI results with gusto.
“I’ve been tortured over the past 5 years by my junior interventional colleagues saying that, unless you’re doing transradial for STEMIs, that you’re not only out of step with the modern era of PCI, but you’re really moving against the evidence. And this study brings it back to a center position, where if you do a very-high-quality transfemoral approach, it is no different from transradial, not just from the standpoint of mortality but also bleeding complications. So I think we should aspire to be better transfemoral interventionalists, and if we do then there probably isn’t that much of a difference,” said Dr. Leon, professor of medicine at Columbia University in New York.
But discussant Sunil V. Rao, MD, who has championed radial access in the United States, was skeptical. “I think the results you achieved with femoral access in this trial are quite remarkable. We know from the registry data that those results are difficult to achieve in clinical practice.
“I would caution that the trial was stopped early, so I think it’s very challenging to try to apply this so as to influence our practice definitively,” said Dr. Rao of Duke University, Durham, N.C.
He asked Dr. Le May what advice he could give to femoralists in community practice to up their game and achieve results comparable to those in SAFARI-STEMI.
“We have to pay attention to their puncture,” Dr. Le May replied. “We use fluoroscopic guidance, and there are others who use ultrasound. We pay attention to the anticoagulation and antiplatelet therapy we use for these patients. We use GP IIb/IIIa inhibitors less today, and smaller sheaths. More than 90% of patients got ticagrelor before going to the cath lab. We’ve borrowed some of the techniques that the radial access people use.”
At a postpresentation ACC press conference, he indicated that it was difficult to recruit patients for the trial in the current strongly pro–radial access climate.
“I think there are people who think that, seriously, the horse is out of the barn, and it’s game over for the femoral. There is a mindset out there where people think that it’s just wrong to do a femoral approach,” said Dr. Le May. “We had comments that it’s not even ethical to randomize such patients.”
In fact, the issue is “very debatable,” he asserted, noting that radial artery occlusion is emerging as an important complication. And he suspects that cardiologists who strive to do 80%-90% of their percutaneous coronary interventions via the transradial route may become deskilled at using the femoral approach. That becomes a real concern when there is a problem in accessing the radial artery or need arises for a device that requires femoral access.
“I am of the school of thought that, given the results of our trial, we should teach people that you’re not a femoralist or a radialist. You should be an interventionalist that can do either and flip from one side to the other and be comfortable in doing that,” Dr. Le May concluded.
He reported having no conflicts regarding the study, funded by the Canadian Institutes of Health Research.
NEW ORLEANS – , Michel R. Le May, MD, reported at the annual meeting of the American College of Cardiology.
Our findings suggest that adequately trained operators should be able to achieve similar results using either radial or femoral access for primary PCI,” declared Dr. Le May, professor of medicine at the University of Ottawa Heart Institute.
This is a controversial issue. European cardiologists have led a strong push for preferential use of radial access, citing reduced bleeding risk and an associated reduction in 30-day mortality. And this movement has spread to North America. But the evidence doesn’t convincingly support this position, the cardiologist said. He noted that of nine prior RCTs of radial versus the more traditional femoral access for primary PCI in STEMI, seven showed no difference in mortality. Nor did SAFARI-STEMI, which at 2,292 randomized STEMI patients was the second-largest trial to date.
SAFARI-STEMI was conducted at five high-volume Canadian PCI centers. Participating interventional cardiologists averaged 250 PCIs per year and were proficient in both access approaches. The study plan was to enroll 5,000 STEMI patients, but the trial was stopped after results were in for the first 2,292 because outcomes in the two study arms were so similar that the trial’s data safety monitoring board deemed it futile to continue.
The primary outcome was 30-day mortality. The rate was 1.5% in the radial access group and 1.3% in the femoral access group, with no differences among various subgroups.
Nor were there any between-group significant difference in the secondary endpoints of reinfarction (1.8% with radial, 1.6% with femoral), stroke (1.0% versus 0.4%), or the composite of death, reinfarction, or stroke, which occurred in 4.0% of the radial access group and 3.4% of the femoral group. Rates of non-CABG TIMI major or minor bleeding at 30 days were closely similar, as was need for transfusion. Definite or probable stent thrombosis occurred in 1.5% of the radial and 1.1% of the femoral groups.
Time from arrival at the PCI center to first balloon inflation was 47 minutes in the radial access group and significantly shorter at 44 minutes with femoral access, a noteworthy finding in the setting of STEMI, where time is myocardium. Fluoroscopy time was 1.2 minutes shorter in the femoral access group as well.
The reaction
Discussant Jacqueline E. Tamis-Holland, MD, said that, although she recently switched over to a radial access-first approach, her take away from SAFARI-STEMI is “It’s okay to do femoral.
“I think it’s comforting to the femoralists who are struggling to say, ‘I do a good femoral job and I don’t necessarily want to transition my STEMI patients to radial,’ ” said Dr. Tamis-Holland, associate director for the Mount Sinai St. Luke’s cardiac cath labs in New York.
Session cochair Martin B. Leon, MD, embraced the SAFARI-STEMI results with gusto.
“I’ve been tortured over the past 5 years by my junior interventional colleagues saying that, unless you’re doing transradial for STEMIs, that you’re not only out of step with the modern era of PCI, but you’re really moving against the evidence. And this study brings it back to a center position, where if you do a very-high-quality transfemoral approach, it is no different from transradial, not just from the standpoint of mortality but also bleeding complications. So I think we should aspire to be better transfemoral interventionalists, and if we do then there probably isn’t that much of a difference,” said Dr. Leon, professor of medicine at Columbia University in New York.
But discussant Sunil V. Rao, MD, who has championed radial access in the United States, was skeptical. “I think the results you achieved with femoral access in this trial are quite remarkable. We know from the registry data that those results are difficult to achieve in clinical practice.
“I would caution that the trial was stopped early, so I think it’s very challenging to try to apply this so as to influence our practice definitively,” said Dr. Rao of Duke University, Durham, N.C.
He asked Dr. Le May what advice he could give to femoralists in community practice to up their game and achieve results comparable to those in SAFARI-STEMI.
“We have to pay attention to their puncture,” Dr. Le May replied. “We use fluoroscopic guidance, and there are others who use ultrasound. We pay attention to the anticoagulation and antiplatelet therapy we use for these patients. We use GP IIb/IIIa inhibitors less today, and smaller sheaths. More than 90% of patients got ticagrelor before going to the cath lab. We’ve borrowed some of the techniques that the radial access people use.”
At a postpresentation ACC press conference, he indicated that it was difficult to recruit patients for the trial in the current strongly pro–radial access climate.
“I think there are people who think that, seriously, the horse is out of the barn, and it’s game over for the femoral. There is a mindset out there where people think that it’s just wrong to do a femoral approach,” said Dr. Le May. “We had comments that it’s not even ethical to randomize such patients.”
In fact, the issue is “very debatable,” he asserted, noting that radial artery occlusion is emerging as an important complication. And he suspects that cardiologists who strive to do 80%-90% of their percutaneous coronary interventions via the transradial route may become deskilled at using the femoral approach. That becomes a real concern when there is a problem in accessing the radial artery or need arises for a device that requires femoral access.
“I am of the school of thought that, given the results of our trial, we should teach people that you’re not a femoralist or a radialist. You should be an interventionalist that can do either and flip from one side to the other and be comfortable in doing that,” Dr. Le May concluded.
He reported having no conflicts regarding the study, funded by the Canadian Institutes of Health Research.
NEW ORLEANS – , Michel R. Le May, MD, reported at the annual meeting of the American College of Cardiology.
Our findings suggest that adequately trained operators should be able to achieve similar results using either radial or femoral access for primary PCI,” declared Dr. Le May, professor of medicine at the University of Ottawa Heart Institute.
This is a controversial issue. European cardiologists have led a strong push for preferential use of radial access, citing reduced bleeding risk and an associated reduction in 30-day mortality. And this movement has spread to North America. But the evidence doesn’t convincingly support this position, the cardiologist said. He noted that of nine prior RCTs of radial versus the more traditional femoral access for primary PCI in STEMI, seven showed no difference in mortality. Nor did SAFARI-STEMI, which at 2,292 randomized STEMI patients was the second-largest trial to date.
SAFARI-STEMI was conducted at five high-volume Canadian PCI centers. Participating interventional cardiologists averaged 250 PCIs per year and were proficient in both access approaches. The study plan was to enroll 5,000 STEMI patients, but the trial was stopped after results were in for the first 2,292 because outcomes in the two study arms were so similar that the trial’s data safety monitoring board deemed it futile to continue.
The primary outcome was 30-day mortality. The rate was 1.5% in the radial access group and 1.3% in the femoral access group, with no differences among various subgroups.
Nor were there any between-group significant difference in the secondary endpoints of reinfarction (1.8% with radial, 1.6% with femoral), stroke (1.0% versus 0.4%), or the composite of death, reinfarction, or stroke, which occurred in 4.0% of the radial access group and 3.4% of the femoral group. Rates of non-CABG TIMI major or minor bleeding at 30 days were closely similar, as was need for transfusion. Definite or probable stent thrombosis occurred in 1.5% of the radial and 1.1% of the femoral groups.
Time from arrival at the PCI center to first balloon inflation was 47 minutes in the radial access group and significantly shorter at 44 minutes with femoral access, a noteworthy finding in the setting of STEMI, where time is myocardium. Fluoroscopy time was 1.2 minutes shorter in the femoral access group as well.
The reaction
Discussant Jacqueline E. Tamis-Holland, MD, said that, although she recently switched over to a radial access-first approach, her take away from SAFARI-STEMI is “It’s okay to do femoral.
“I think it’s comforting to the femoralists who are struggling to say, ‘I do a good femoral job and I don’t necessarily want to transition my STEMI patients to radial,’ ” said Dr. Tamis-Holland, associate director for the Mount Sinai St. Luke’s cardiac cath labs in New York.
Session cochair Martin B. Leon, MD, embraced the SAFARI-STEMI results with gusto.
“I’ve been tortured over the past 5 years by my junior interventional colleagues saying that, unless you’re doing transradial for STEMIs, that you’re not only out of step with the modern era of PCI, but you’re really moving against the evidence. And this study brings it back to a center position, where if you do a very-high-quality transfemoral approach, it is no different from transradial, not just from the standpoint of mortality but also bleeding complications. So I think we should aspire to be better transfemoral interventionalists, and if we do then there probably isn’t that much of a difference,” said Dr. Leon, professor of medicine at Columbia University in New York.
But discussant Sunil V. Rao, MD, who has championed radial access in the United States, was skeptical. “I think the results you achieved with femoral access in this trial are quite remarkable. We know from the registry data that those results are difficult to achieve in clinical practice.
“I would caution that the trial was stopped early, so I think it’s very challenging to try to apply this so as to influence our practice definitively,” said Dr. Rao of Duke University, Durham, N.C.
He asked Dr. Le May what advice he could give to femoralists in community practice to up their game and achieve results comparable to those in SAFARI-STEMI.
“We have to pay attention to their puncture,” Dr. Le May replied. “We use fluoroscopic guidance, and there are others who use ultrasound. We pay attention to the anticoagulation and antiplatelet therapy we use for these patients. We use GP IIb/IIIa inhibitors less today, and smaller sheaths. More than 90% of patients got ticagrelor before going to the cath lab. We’ve borrowed some of the techniques that the radial access people use.”
At a postpresentation ACC press conference, he indicated that it was difficult to recruit patients for the trial in the current strongly pro–radial access climate.
“I think there are people who think that, seriously, the horse is out of the barn, and it’s game over for the femoral. There is a mindset out there where people think that it’s just wrong to do a femoral approach,” said Dr. Le May. “We had comments that it’s not even ethical to randomize such patients.”
In fact, the issue is “very debatable,” he asserted, noting that radial artery occlusion is emerging as an important complication. And he suspects that cardiologists who strive to do 80%-90% of their percutaneous coronary interventions via the transradial route may become deskilled at using the femoral approach. That becomes a real concern when there is a problem in accessing the radial artery or need arises for a device that requires femoral access.
“I am of the school of thought that, given the results of our trial, we should teach people that you’re not a femoralist or a radialist. You should be an interventionalist that can do either and flip from one side to the other and be comfortable in doing that,” Dr. Le May concluded.
He reported having no conflicts regarding the study, funded by the Canadian Institutes of Health Research.
REPORTING FROM ACC 19
Beyond enhanced recovery after surgery
Our specialty is focusing now more intently on perioperative optimization, commonly referred to as enhanced recovery after surgery (ERAS), a concept championed first and most visibly by colorectal surgeons in the 1990s.1 Both academic and nonacademic practices are challenging long-held beliefs about perioperative management.
The 3 tenets of ERAS
In multiple surgical specialties, proper implementation of 3 tenets—early feeding, perioperative euvolemia, and multimodal pain control—reduces the length of hospital stay, improves patient satisfaction, reduces complications, lowers health care costs, and most importantly hastens patient recovery.
1 Early feeding
Just as athletes hydrate and carbohydrate load prior to a competition, patients benefit if fluids and calories are not withheld in anticipation of a physiologically stressful surgical procedure. Similarly, modest benefit is associated with carbohydrate loading as a liquid supplement 2 hours before surgery.2 The American Society of Anesthesiologists guidelines state that while solid foods should not be consumed after midnight before surgery, clear liquids safely may be withheld for only 2 hours prior to anesthesia induction, and systematic reviews have failed to show harm.3,4 All patients, including those undergoing colonic resections, are allowed to eat a general diet as tolerated the evening before surgery, supplemented with caloric-dense nutritional supplements.
2 Multimodal pain control
Postsurgical pain is a top patient concern. Pain control is critical for rapid recovery; it helps avoid upregulation of the sympathetic axis and permits ambulation and resumption of normal activities. Although opioids relieve pain, they should not be considered a primary pain control approach.
Responding to the opioid epidemic, in 2015 the Centers for Disease Control and Prevention identified opioid overdose prevention as one of the top 5 public health challenges; notably, approximately 6% of patients will experience new, persistent opioid use following surgery.5 Optimal pain management therefore should provide effective pain relief while minimizing opioid use.
Preemptive oral acetaminophen, gabapentin, and celecoxib should be used routinely prior to incision; nonsteroidal anti-inflammatory drugs should be scheduled postoperatively. Even after a complex cytoreductive laparotomy, pain may be controlled with oral rather than intravenous (IV) medications in most patients, with opioid requirements averaging just 2 to 4 tablets of oxycodone in the first 48 hours after surgery, in our experience. The most critical need for pain medications occurs in the first 48 hours after surgery, which highlights the importance of local or regional analgesia. In one investigation, implementation of multimodal pain management that included incisional injection of liposomal bupivacaine reduced patient-controlled analgesia use to less than 5% after laparotomy.6 The need for opioids more than a week postoperatively is uncommon even after a laparotomy.
3 Perioperative euvolemia
Maintaining euvolemia is a central and underrecognized tenet of enhanced recovery pathways, and it facilitates the other 2 tenets of early feeding and optimal pain control. Overhydrated patients have more pain and prolonged recovery of bowel function. Unfortunately, euvolemia is the most difficult ERAS component to implement, requiring seamless communication between all members of the surgical team.
Continue to: Fluid therapy...
Fluid therapy should be respected as a pharmacologic agent with both benefits and risks. Recognizing that a single liter of lactated Ringer’s solution contains the sodium load of more than 30 bags of potato chips (and normal saline contains far more), one can imagine the impact of 10 L of solution on peripheral and bowel edema and on overall recovery. Importantly, euvolemia must be initiated during surgery. A meta-analysis of nearly 1,000 randomly assigned patients showed that benefits were limited when euvolemia was initiated in the postoperative period.7
When it comes to maintaining euvolemia, particular care must be taken to avoid erring toward hyperadherence. No difference in hospital length of stay, complications, or ileus was observed when patients were randomly assigned to goal-directed fluid therapy or standard practice.8 However, differences in the volume of fluid administered were relatively small, and while there was evidence of underhydration (likely responsible for acute kidney injury), there was no evidence of overhydration. For example, 4 L of fluid is likely superior to 15 L, but it may not be clinically different from 4.5 L. A threshold of fluid restriction is likely to be reached; that is, additional benefit is not achieved and, instead, detrimental effects may occur.
Rather than a specific directive, a more clinically relevant goal may be to replace insensible fluid losses and to maintain perfusion and blood pressure with the lowest volume possible. Note that estimation of fluid requirements is vastly simplified by omitting mechanical bowel preparation. Postoperatively, permissive oliguria (20 mL/h) is allowed since reduced urine output is a normal response to surgery (as a result of inappropriate secretion of antidiuretic hormone) and does not necessitate administration of a fluid bolus. Above all, anesthesiologists should acknowledge that fluid administration’s effects on a patient extend past the postanesthesia care unit, and the entire surgical team should be invested in the patient’s long-term recovery.
Our experience with ERAS
In 2011, Mayo Clinic was the first institution to implement enhanced recovery on a large scale in gynecologic surgery. We have subsequently made multiple pathway modifications in the spirit of continuous improvement (FIGURE 1).
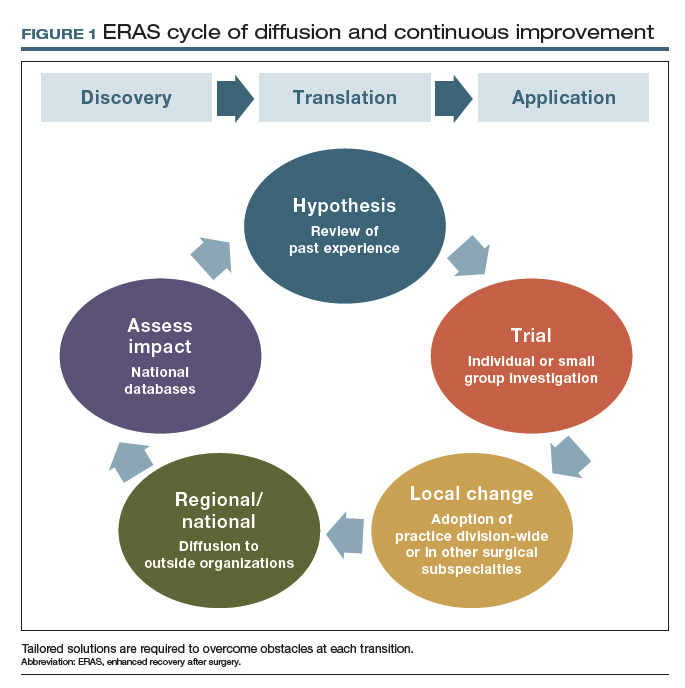
For patients with ovarian cancer requiring extended procedures for cytoreduction via laparotomy (such as colon resection, splenectomy, diaphragm resection), enhanced recovery reduced the median hospital stay by 3 days, patient-controlled IV analgesia use by 88%, and postoperative opioid requirements by 90%.9,10
At 48 hours after surgery, 40% of our patients require no opioids or tramadol, and epidurals are not utilized because of their effects on ambulation and the potential for hypotension. These reductions were met with stable to improved pain scores, a 60% decrease in nausea, and a 50% reduction in adynamic ileus.9,10
Our initial efforts reduced 30-day costs of care by more than $850,000 in just 6 months, with savings of more than $7,600 for each patient undergoing a complex cytoreduction. Furthermore, these improvements allowed consolidation of our inpatient unit with those of other surgical specialties, serving higher volumes of patients within a smaller inpatient footprint. This contraction of inpatient services has accounted for an additional $1.1 million in savings every year since implementation (FIGURE 2).9,10
Our group is not alone in realizing these benefits, and interest has intensified as demonstrated by the fact that the ERAS Society guidelines are among the all-time most downloaded articles in Gynecologic Oncology.11,12 Although our research to demonstrate safety has focused on women undergoing complex oncologic operations, ERAS nevertheless hastens recovery, improves patient satisfaction, and adds value for all patients undergoing gynecologic surgery.
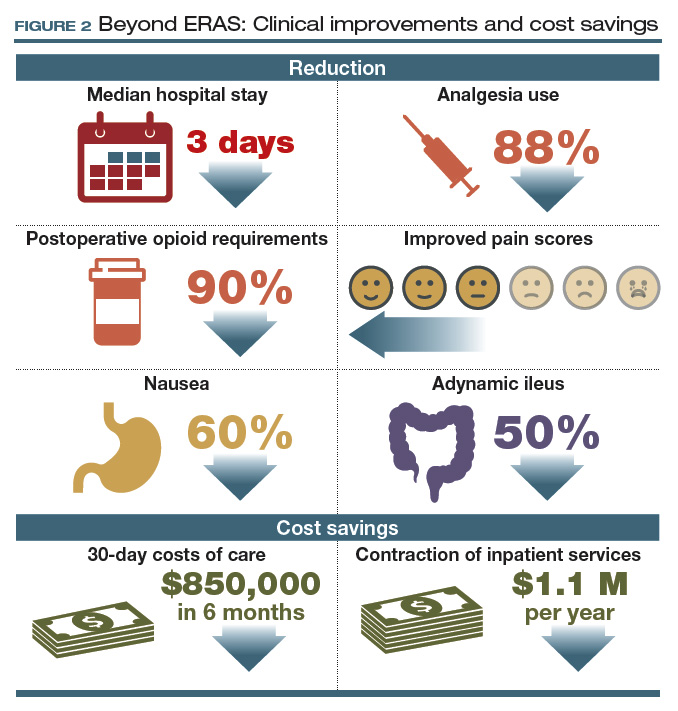
Continue to: Collateral improvements to practice...
Collateral improvements to practice
Clinical optimization using evidence-based practices such as enhanced recovery pathways can result in immediate patient benefit. Affecting such profound clinical improvements is energizing and creates a unique opportunity to transform the culture of the entire health care team. Irrespective of our provider roles (surgeon, anesthesiologist, nurse) or areas of interest (practice, research, education, leadership), we are united by a common purpose: to improve the human condition.13 Reaffirming this common purpose, through the collective effort involved in establishing a standardized enhanced recovery pathway, has allowed our practice and those of others to move beyond enhanced recovery and improve other areas of practice.
Other positive effects. The long-term collateral impact of this culture change at our institution is arguably more important than enhanced recovery itself. Examples of downstream impact include14,15:
- 80% reduction in surgical site infection
- 50% reduction in anastomotic leaks
- 60% reduction in blood utilization for patients undergoing surgery for ovarian cancer.
Team-based pragmatic strategies. Additionally, our willingness to make decisions as a division rather than as individuals facilitated universal implementation of sentinel lymph node biopsy for patients with endometrial cancer and standardized imaging, testing, and surgical decision making for patients with ovarian and endometrial cancer.
The interventions associated with these improvements were not tested in a randomized fashion; however, rather than await perfect data, we made informed decisions based on imperfect data together with a commitment to continuous data review. We find this to be an effective strategy if our goal is to ensure that tomorrow’s outcomes will be better than yesterday’s. In this way, pragmatic trials can be extremely effective in rural settings and tertiary centers.
Barriers to innovation
The widely reported benefits of enhanced recovery beg the question, Why has enhanced recovery not been adopted universally as standard of care? The answer is multifaceted and highlights long-standing shortcomings in our health care system.
Most importantly, our health care system lacks a robust interface to link discovery of new techniques, treatments, and workflows to clinical practice. Perhaps the best example of this is the adoption of minimally invasive surgery (MIS) for endometrial cancer. Ten years have passed since randomized trials showed MIS has equivalent oncologic outcomes and superior recovery compared to laparotomy, yet in the United States less than 50% of women with endometrial cancer benefit.16,17
However, even surgeons who are knowledgeable about recent innovations and genuinely wish to promote improvements may face near-insurmountable skepticism. Blind faith in our abilities and outcomes, overprotection of autonomy, close-mindedness, and satisfaction with the status quo are common responses to innovation and are the enemies of change. Resistance often comes from good intentions, but our desire to avoid complications may result in actions that could just as accurately be labeled superstitious as conservative. These observations suggest that developing methods to incorporate evidence-based practice into routine clinical use is the rate-limiting step in improving surgical quality.
The national Improving Surgical Care and Recovery program is available to specifically aid with ERAS implementation. A collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the American College of Surgeons, the program aims to diffuse enhanced recovery to 750 service lines in 4 surgical subspecialties, including gynecologic surgery, over the next 5 years. (Note: The author is the content expert for the gynecology portion of this program.) The program’s larger aim is to measurably improve patient outcomes, reduce health care utilization, and improve patient experience through the use of an adaptation to AHRQ’s Comprehensive Unit-based Safety Program (CUSP). The backbone for this program is the recent systematic review to establish best practices for gynecologic surgery.1 Free to all participants, the program includes resources such as webinars and coaching calls to assist with the inevitable barriers to ERAS implementation. For more information and to enroll, visit https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/enhanced-recovery /index.html. An important aspect of the program is a registry for tracking outcomes and identifying areas for improvement. For members who currently participate in the National Surgical Quality Improvement Program, clinical data are automatically uploaded into the database. Programs such as Improving Surgical Care and Recovery may be the most reliable way to facilitate diffusion of best practices and take collective responsibility for not only “my outcomes” but also for “our outcomes” as a national community of gynecologic surgeons.
Reference
1. Kalogera E, Nelson G, Liu J, et al. Surgical technical evidence review for gynecologic surgery conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery. Am J Obstet Gynecol. 2018;219:563.e1-563.e19.
Principles essential to change
Various methodologies have been described to manage change and facilitate implementation of new workflows and practices. Irrespective of the method used, including the more formal discipline of implementation science, at least 4 principles must be followed:
1. Teamwork. Mutual trust, mutual respect, and a sense of common purpose are minimum requirements for any successful initiative. Standardization is difficult or impossible without these elements. Thus, establishing a healthy team is the first step in implementing change.
2. Stakeholder analysis. Feedback from surgeons, nurses, residents, fellows, anesthesiologists, pharmacists, nurse anesthetists, and administrators is necessary to obtain diverse perspectives, facilitate engagement, and promote collaborative management. Negativity and resistance are common reactions to change, and it is particularly important to include those who are most skeptical in the stakeholder analysis to mitigate sabotage.
3. Concrete metrics. Success is possible only if defined a priori by specific and achievable goals. Counterbalances also are important to ensure that interventions do not have unintended consequences. Once a goal is met (for example, reduced hospital length of stay or costs), relevant metrics should be monitored after project completion for a minimum of 3 years to avoid regression to the pre-project state.
4. Leadership. The project champion responsible for the initiative must objectively facilitate all of the above and ensure excellent communication between stakeholders to nurture long-term engagement. Despite best efforts, if a minority of the group rejects compromise, this creates an opportunity to compare outcomes between those who do and do not accept the proposed change. Progress realized by early adopters may convince resistors to conform at a later time. Alternatively, the project champion also must have the insight to recognize when a proposed change is impossible at that point in time with that particular group. For example, our own initial attempts to implement enhanced recovery stalled in 2008, but they were successful 3 years later in a different environment.
Continue to: Although a discussion of leadership styles...
Although a discussion of leadership styles is beyond the scope of this article, in our experience, the most successful model is one of servant leadership that is team oriented rather than star dominated. Rather than being led by a single surgeon, each of the 4 quality improvement projects reviewed above (ERAS, and reductions in anastomotic leak, surgical site infection, and blood transfusion) that grew from enhanced recovery included trainees and was led by a different champion, encouraging teamwork and promoting career development. Such a model also supports the Accreditation Council for Graduate Medical Education’s emphasis on quality improvement education.
- Nygren J, Thacker J, Carli F, et al; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:285-305.
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114: 495-511.
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423.
- Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17:65-71.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504.
- Kalogera, E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331-341.
- Myles PS, Bellomo R, Corcoran T, et al; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263-2274.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part I. Gynecol Oncol. 2016;140:313-322.
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140:323-332.
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481.
- Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127:1135-1144.
- Kalogera E, Nitschmann CC, Dowdy SC, et al. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecol Oncol. 2017;144:343-347.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91-100.
Our specialty is focusing now more intently on perioperative optimization, commonly referred to as enhanced recovery after surgery (ERAS), a concept championed first and most visibly by colorectal surgeons in the 1990s.1 Both academic and nonacademic practices are challenging long-held beliefs about perioperative management.
The 3 tenets of ERAS
In multiple surgical specialties, proper implementation of 3 tenets—early feeding, perioperative euvolemia, and multimodal pain control—reduces the length of hospital stay, improves patient satisfaction, reduces complications, lowers health care costs, and most importantly hastens patient recovery.
1 Early feeding
Just as athletes hydrate and carbohydrate load prior to a competition, patients benefit if fluids and calories are not withheld in anticipation of a physiologically stressful surgical procedure. Similarly, modest benefit is associated with carbohydrate loading as a liquid supplement 2 hours before surgery.2 The American Society of Anesthesiologists guidelines state that while solid foods should not be consumed after midnight before surgery, clear liquids safely may be withheld for only 2 hours prior to anesthesia induction, and systematic reviews have failed to show harm.3,4 All patients, including those undergoing colonic resections, are allowed to eat a general diet as tolerated the evening before surgery, supplemented with caloric-dense nutritional supplements.
2 Multimodal pain control
Postsurgical pain is a top patient concern. Pain control is critical for rapid recovery; it helps avoid upregulation of the sympathetic axis and permits ambulation and resumption of normal activities. Although opioids relieve pain, they should not be considered a primary pain control approach.
Responding to the opioid epidemic, in 2015 the Centers for Disease Control and Prevention identified opioid overdose prevention as one of the top 5 public health challenges; notably, approximately 6% of patients will experience new, persistent opioid use following surgery.5 Optimal pain management therefore should provide effective pain relief while minimizing opioid use.
Preemptive oral acetaminophen, gabapentin, and celecoxib should be used routinely prior to incision; nonsteroidal anti-inflammatory drugs should be scheduled postoperatively. Even after a complex cytoreductive laparotomy, pain may be controlled with oral rather than intravenous (IV) medications in most patients, with opioid requirements averaging just 2 to 4 tablets of oxycodone in the first 48 hours after surgery, in our experience. The most critical need for pain medications occurs in the first 48 hours after surgery, which highlights the importance of local or regional analgesia. In one investigation, implementation of multimodal pain management that included incisional injection of liposomal bupivacaine reduced patient-controlled analgesia use to less than 5% after laparotomy.6 The need for opioids more than a week postoperatively is uncommon even after a laparotomy.
3 Perioperative euvolemia
Maintaining euvolemia is a central and underrecognized tenet of enhanced recovery pathways, and it facilitates the other 2 tenets of early feeding and optimal pain control. Overhydrated patients have more pain and prolonged recovery of bowel function. Unfortunately, euvolemia is the most difficult ERAS component to implement, requiring seamless communication between all members of the surgical team.
Continue to: Fluid therapy...
Fluid therapy should be respected as a pharmacologic agent with both benefits and risks. Recognizing that a single liter of lactated Ringer’s solution contains the sodium load of more than 30 bags of potato chips (and normal saline contains far more), one can imagine the impact of 10 L of solution on peripheral and bowel edema and on overall recovery. Importantly, euvolemia must be initiated during surgery. A meta-analysis of nearly 1,000 randomly assigned patients showed that benefits were limited when euvolemia was initiated in the postoperative period.7
When it comes to maintaining euvolemia, particular care must be taken to avoid erring toward hyperadherence. No difference in hospital length of stay, complications, or ileus was observed when patients were randomly assigned to goal-directed fluid therapy or standard practice.8 However, differences in the volume of fluid administered were relatively small, and while there was evidence of underhydration (likely responsible for acute kidney injury), there was no evidence of overhydration. For example, 4 L of fluid is likely superior to 15 L, but it may not be clinically different from 4.5 L. A threshold of fluid restriction is likely to be reached; that is, additional benefit is not achieved and, instead, detrimental effects may occur.
Rather than a specific directive, a more clinically relevant goal may be to replace insensible fluid losses and to maintain perfusion and blood pressure with the lowest volume possible. Note that estimation of fluid requirements is vastly simplified by omitting mechanical bowel preparation. Postoperatively, permissive oliguria (20 mL/h) is allowed since reduced urine output is a normal response to surgery (as a result of inappropriate secretion of antidiuretic hormone) and does not necessitate administration of a fluid bolus. Above all, anesthesiologists should acknowledge that fluid administration’s effects on a patient extend past the postanesthesia care unit, and the entire surgical team should be invested in the patient’s long-term recovery.
Our experience with ERAS
In 2011, Mayo Clinic was the first institution to implement enhanced recovery on a large scale in gynecologic surgery. We have subsequently made multiple pathway modifications in the spirit of continuous improvement (FIGURE 1).

For patients with ovarian cancer requiring extended procedures for cytoreduction via laparotomy (such as colon resection, splenectomy, diaphragm resection), enhanced recovery reduced the median hospital stay by 3 days, patient-controlled IV analgesia use by 88%, and postoperative opioid requirements by 90%.9,10
At 48 hours after surgery, 40% of our patients require no opioids or tramadol, and epidurals are not utilized because of their effects on ambulation and the potential for hypotension. These reductions were met with stable to improved pain scores, a 60% decrease in nausea, and a 50% reduction in adynamic ileus.9,10
Our initial efforts reduced 30-day costs of care by more than $850,000 in just 6 months, with savings of more than $7,600 for each patient undergoing a complex cytoreduction. Furthermore, these improvements allowed consolidation of our inpatient unit with those of other surgical specialties, serving higher volumes of patients within a smaller inpatient footprint. This contraction of inpatient services has accounted for an additional $1.1 million in savings every year since implementation (FIGURE 2).9,10
Our group is not alone in realizing these benefits, and interest has intensified as demonstrated by the fact that the ERAS Society guidelines are among the all-time most downloaded articles in Gynecologic Oncology.11,12 Although our research to demonstrate safety has focused on women undergoing complex oncologic operations, ERAS nevertheless hastens recovery, improves patient satisfaction, and adds value for all patients undergoing gynecologic surgery.

Continue to: Collateral improvements to practice...
Collateral improvements to practice
Clinical optimization using evidence-based practices such as enhanced recovery pathways can result in immediate patient benefit. Affecting such profound clinical improvements is energizing and creates a unique opportunity to transform the culture of the entire health care team. Irrespective of our provider roles (surgeon, anesthesiologist, nurse) or areas of interest (practice, research, education, leadership), we are united by a common purpose: to improve the human condition.13 Reaffirming this common purpose, through the collective effort involved in establishing a standardized enhanced recovery pathway, has allowed our practice and those of others to move beyond enhanced recovery and improve other areas of practice.
Other positive effects. The long-term collateral impact of this culture change at our institution is arguably more important than enhanced recovery itself. Examples of downstream impact include14,15:
- 80% reduction in surgical site infection
- 50% reduction in anastomotic leaks
- 60% reduction in blood utilization for patients undergoing surgery for ovarian cancer.
Team-based pragmatic strategies. Additionally, our willingness to make decisions as a division rather than as individuals facilitated universal implementation of sentinel lymph node biopsy for patients with endometrial cancer and standardized imaging, testing, and surgical decision making for patients with ovarian and endometrial cancer.
The interventions associated with these improvements were not tested in a randomized fashion; however, rather than await perfect data, we made informed decisions based on imperfect data together with a commitment to continuous data review. We find this to be an effective strategy if our goal is to ensure that tomorrow’s outcomes will be better than yesterday’s. In this way, pragmatic trials can be extremely effective in rural settings and tertiary centers.
Barriers to innovation
The widely reported benefits of enhanced recovery beg the question, Why has enhanced recovery not been adopted universally as standard of care? The answer is multifaceted and highlights long-standing shortcomings in our health care system.
Most importantly, our health care system lacks a robust interface to link discovery of new techniques, treatments, and workflows to clinical practice. Perhaps the best example of this is the adoption of minimally invasive surgery (MIS) for endometrial cancer. Ten years have passed since randomized trials showed MIS has equivalent oncologic outcomes and superior recovery compared to laparotomy, yet in the United States less than 50% of women with endometrial cancer benefit.16,17
However, even surgeons who are knowledgeable about recent innovations and genuinely wish to promote improvements may face near-insurmountable skepticism. Blind faith in our abilities and outcomes, overprotection of autonomy, close-mindedness, and satisfaction with the status quo are common responses to innovation and are the enemies of change. Resistance often comes from good intentions, but our desire to avoid complications may result in actions that could just as accurately be labeled superstitious as conservative. These observations suggest that developing methods to incorporate evidence-based practice into routine clinical use is the rate-limiting step in improving surgical quality.
The national Improving Surgical Care and Recovery program is available to specifically aid with ERAS implementation. A collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the American College of Surgeons, the program aims to diffuse enhanced recovery to 750 service lines in 4 surgical subspecialties, including gynecologic surgery, over the next 5 years. (Note: The author is the content expert for the gynecology portion of this program.) The program’s larger aim is to measurably improve patient outcomes, reduce health care utilization, and improve patient experience through the use of an adaptation to AHRQ’s Comprehensive Unit-based Safety Program (CUSP). The backbone for this program is the recent systematic review to establish best practices for gynecologic surgery.1 Free to all participants, the program includes resources such as webinars and coaching calls to assist with the inevitable barriers to ERAS implementation. For more information and to enroll, visit https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/enhanced-recovery /index.html. An important aspect of the program is a registry for tracking outcomes and identifying areas for improvement. For members who currently participate in the National Surgical Quality Improvement Program, clinical data are automatically uploaded into the database. Programs such as Improving Surgical Care and Recovery may be the most reliable way to facilitate diffusion of best practices and take collective responsibility for not only “my outcomes” but also for “our outcomes” as a national community of gynecologic surgeons.
Reference
1. Kalogera E, Nelson G, Liu J, et al. Surgical technical evidence review for gynecologic surgery conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery. Am J Obstet Gynecol. 2018;219:563.e1-563.e19.
Principles essential to change
Various methodologies have been described to manage change and facilitate implementation of new workflows and practices. Irrespective of the method used, including the more formal discipline of implementation science, at least 4 principles must be followed:
1. Teamwork. Mutual trust, mutual respect, and a sense of common purpose are minimum requirements for any successful initiative. Standardization is difficult or impossible without these elements. Thus, establishing a healthy team is the first step in implementing change.
2. Stakeholder analysis. Feedback from surgeons, nurses, residents, fellows, anesthesiologists, pharmacists, nurse anesthetists, and administrators is necessary to obtain diverse perspectives, facilitate engagement, and promote collaborative management. Negativity and resistance are common reactions to change, and it is particularly important to include those who are most skeptical in the stakeholder analysis to mitigate sabotage.
3. Concrete metrics. Success is possible only if defined a priori by specific and achievable goals. Counterbalances also are important to ensure that interventions do not have unintended consequences. Once a goal is met (for example, reduced hospital length of stay or costs), relevant metrics should be monitored after project completion for a minimum of 3 years to avoid regression to the pre-project state.
4. Leadership. The project champion responsible for the initiative must objectively facilitate all of the above and ensure excellent communication between stakeholders to nurture long-term engagement. Despite best efforts, if a minority of the group rejects compromise, this creates an opportunity to compare outcomes between those who do and do not accept the proposed change. Progress realized by early adopters may convince resistors to conform at a later time. Alternatively, the project champion also must have the insight to recognize when a proposed change is impossible at that point in time with that particular group. For example, our own initial attempts to implement enhanced recovery stalled in 2008, but they were successful 3 years later in a different environment.
Continue to: Although a discussion of leadership styles...
Although a discussion of leadership styles is beyond the scope of this article, in our experience, the most successful model is one of servant leadership that is team oriented rather than star dominated. Rather than being led by a single surgeon, each of the 4 quality improvement projects reviewed above (ERAS, and reductions in anastomotic leak, surgical site infection, and blood transfusion) that grew from enhanced recovery included trainees and was led by a different champion, encouraging teamwork and promoting career development. Such a model also supports the Accreditation Council for Graduate Medical Education’s emphasis on quality improvement education.
Our specialty is focusing now more intently on perioperative optimization, commonly referred to as enhanced recovery after surgery (ERAS), a concept championed first and most visibly by colorectal surgeons in the 1990s.1 Both academic and nonacademic practices are challenging long-held beliefs about perioperative management.
The 3 tenets of ERAS
In multiple surgical specialties, proper implementation of 3 tenets—early feeding, perioperative euvolemia, and multimodal pain control—reduces the length of hospital stay, improves patient satisfaction, reduces complications, lowers health care costs, and most importantly hastens patient recovery.
1 Early feeding
Just as athletes hydrate and carbohydrate load prior to a competition, patients benefit if fluids and calories are not withheld in anticipation of a physiologically stressful surgical procedure. Similarly, modest benefit is associated with carbohydrate loading as a liquid supplement 2 hours before surgery.2 The American Society of Anesthesiologists guidelines state that while solid foods should not be consumed after midnight before surgery, clear liquids safely may be withheld for only 2 hours prior to anesthesia induction, and systematic reviews have failed to show harm.3,4 All patients, including those undergoing colonic resections, are allowed to eat a general diet as tolerated the evening before surgery, supplemented with caloric-dense nutritional supplements.
2 Multimodal pain control
Postsurgical pain is a top patient concern. Pain control is critical for rapid recovery; it helps avoid upregulation of the sympathetic axis and permits ambulation and resumption of normal activities. Although opioids relieve pain, they should not be considered a primary pain control approach.
Responding to the opioid epidemic, in 2015 the Centers for Disease Control and Prevention identified opioid overdose prevention as one of the top 5 public health challenges; notably, approximately 6% of patients will experience new, persistent opioid use following surgery.5 Optimal pain management therefore should provide effective pain relief while minimizing opioid use.
Preemptive oral acetaminophen, gabapentin, and celecoxib should be used routinely prior to incision; nonsteroidal anti-inflammatory drugs should be scheduled postoperatively. Even after a complex cytoreductive laparotomy, pain may be controlled with oral rather than intravenous (IV) medications in most patients, with opioid requirements averaging just 2 to 4 tablets of oxycodone in the first 48 hours after surgery, in our experience. The most critical need for pain medications occurs in the first 48 hours after surgery, which highlights the importance of local or regional analgesia. In one investigation, implementation of multimodal pain management that included incisional injection of liposomal bupivacaine reduced patient-controlled analgesia use to less than 5% after laparotomy.6 The need for opioids more than a week postoperatively is uncommon even after a laparotomy.
3 Perioperative euvolemia
Maintaining euvolemia is a central and underrecognized tenet of enhanced recovery pathways, and it facilitates the other 2 tenets of early feeding and optimal pain control. Overhydrated patients have more pain and prolonged recovery of bowel function. Unfortunately, euvolemia is the most difficult ERAS component to implement, requiring seamless communication between all members of the surgical team.
Continue to: Fluid therapy...
Fluid therapy should be respected as a pharmacologic agent with both benefits and risks. Recognizing that a single liter of lactated Ringer’s solution contains the sodium load of more than 30 bags of potato chips (and normal saline contains far more), one can imagine the impact of 10 L of solution on peripheral and bowel edema and on overall recovery. Importantly, euvolemia must be initiated during surgery. A meta-analysis of nearly 1,000 randomly assigned patients showed that benefits were limited when euvolemia was initiated in the postoperative period.7
When it comes to maintaining euvolemia, particular care must be taken to avoid erring toward hyperadherence. No difference in hospital length of stay, complications, or ileus was observed when patients were randomly assigned to goal-directed fluid therapy or standard practice.8 However, differences in the volume of fluid administered were relatively small, and while there was evidence of underhydration (likely responsible for acute kidney injury), there was no evidence of overhydration. For example, 4 L of fluid is likely superior to 15 L, but it may not be clinically different from 4.5 L. A threshold of fluid restriction is likely to be reached; that is, additional benefit is not achieved and, instead, detrimental effects may occur.
Rather than a specific directive, a more clinically relevant goal may be to replace insensible fluid losses and to maintain perfusion and blood pressure with the lowest volume possible. Note that estimation of fluid requirements is vastly simplified by omitting mechanical bowel preparation. Postoperatively, permissive oliguria (20 mL/h) is allowed since reduced urine output is a normal response to surgery (as a result of inappropriate secretion of antidiuretic hormone) and does not necessitate administration of a fluid bolus. Above all, anesthesiologists should acknowledge that fluid administration’s effects on a patient extend past the postanesthesia care unit, and the entire surgical team should be invested in the patient’s long-term recovery.
Our experience with ERAS
In 2011, Mayo Clinic was the first institution to implement enhanced recovery on a large scale in gynecologic surgery. We have subsequently made multiple pathway modifications in the spirit of continuous improvement (FIGURE 1).

For patients with ovarian cancer requiring extended procedures for cytoreduction via laparotomy (such as colon resection, splenectomy, diaphragm resection), enhanced recovery reduced the median hospital stay by 3 days, patient-controlled IV analgesia use by 88%, and postoperative opioid requirements by 90%.9,10
At 48 hours after surgery, 40% of our patients require no opioids or tramadol, and epidurals are not utilized because of their effects on ambulation and the potential for hypotension. These reductions were met with stable to improved pain scores, a 60% decrease in nausea, and a 50% reduction in adynamic ileus.9,10
Our initial efforts reduced 30-day costs of care by more than $850,000 in just 6 months, with savings of more than $7,600 for each patient undergoing a complex cytoreduction. Furthermore, these improvements allowed consolidation of our inpatient unit with those of other surgical specialties, serving higher volumes of patients within a smaller inpatient footprint. This contraction of inpatient services has accounted for an additional $1.1 million in savings every year since implementation (FIGURE 2).9,10
Our group is not alone in realizing these benefits, and interest has intensified as demonstrated by the fact that the ERAS Society guidelines are among the all-time most downloaded articles in Gynecologic Oncology.11,12 Although our research to demonstrate safety has focused on women undergoing complex oncologic operations, ERAS nevertheless hastens recovery, improves patient satisfaction, and adds value for all patients undergoing gynecologic surgery.

Continue to: Collateral improvements to practice...
Collateral improvements to practice
Clinical optimization using evidence-based practices such as enhanced recovery pathways can result in immediate patient benefit. Affecting such profound clinical improvements is energizing and creates a unique opportunity to transform the culture of the entire health care team. Irrespective of our provider roles (surgeon, anesthesiologist, nurse) or areas of interest (practice, research, education, leadership), we are united by a common purpose: to improve the human condition.13 Reaffirming this common purpose, through the collective effort involved in establishing a standardized enhanced recovery pathway, has allowed our practice and those of others to move beyond enhanced recovery and improve other areas of practice.
Other positive effects. The long-term collateral impact of this culture change at our institution is arguably more important than enhanced recovery itself. Examples of downstream impact include14,15:
- 80% reduction in surgical site infection
- 50% reduction in anastomotic leaks
- 60% reduction in blood utilization for patients undergoing surgery for ovarian cancer.
Team-based pragmatic strategies. Additionally, our willingness to make decisions as a division rather than as individuals facilitated universal implementation of sentinel lymph node biopsy for patients with endometrial cancer and standardized imaging, testing, and surgical decision making for patients with ovarian and endometrial cancer.
The interventions associated with these improvements were not tested in a randomized fashion; however, rather than await perfect data, we made informed decisions based on imperfect data together with a commitment to continuous data review. We find this to be an effective strategy if our goal is to ensure that tomorrow’s outcomes will be better than yesterday’s. In this way, pragmatic trials can be extremely effective in rural settings and tertiary centers.
Barriers to innovation
The widely reported benefits of enhanced recovery beg the question, Why has enhanced recovery not been adopted universally as standard of care? The answer is multifaceted and highlights long-standing shortcomings in our health care system.
Most importantly, our health care system lacks a robust interface to link discovery of new techniques, treatments, and workflows to clinical practice. Perhaps the best example of this is the adoption of minimally invasive surgery (MIS) for endometrial cancer. Ten years have passed since randomized trials showed MIS has equivalent oncologic outcomes and superior recovery compared to laparotomy, yet in the United States less than 50% of women with endometrial cancer benefit.16,17
However, even surgeons who are knowledgeable about recent innovations and genuinely wish to promote improvements may face near-insurmountable skepticism. Blind faith in our abilities and outcomes, overprotection of autonomy, close-mindedness, and satisfaction with the status quo are common responses to innovation and are the enemies of change. Resistance often comes from good intentions, but our desire to avoid complications may result in actions that could just as accurately be labeled superstitious as conservative. These observations suggest that developing methods to incorporate evidence-based practice into routine clinical use is the rate-limiting step in improving surgical quality.
The national Improving Surgical Care and Recovery program is available to specifically aid with ERAS implementation. A collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the American College of Surgeons, the program aims to diffuse enhanced recovery to 750 service lines in 4 surgical subspecialties, including gynecologic surgery, over the next 5 years. (Note: The author is the content expert for the gynecology portion of this program.) The program’s larger aim is to measurably improve patient outcomes, reduce health care utilization, and improve patient experience through the use of an adaptation to AHRQ’s Comprehensive Unit-based Safety Program (CUSP). The backbone for this program is the recent systematic review to establish best practices for gynecologic surgery.1 Free to all participants, the program includes resources such as webinars and coaching calls to assist with the inevitable barriers to ERAS implementation. For more information and to enroll, visit https://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/enhanced-recovery /index.html. An important aspect of the program is a registry for tracking outcomes and identifying areas for improvement. For members who currently participate in the National Surgical Quality Improvement Program, clinical data are automatically uploaded into the database. Programs such as Improving Surgical Care and Recovery may be the most reliable way to facilitate diffusion of best practices and take collective responsibility for not only “my outcomes” but also for “our outcomes” as a national community of gynecologic surgeons.
Reference
1. Kalogera E, Nelson G, Liu J, et al. Surgical technical evidence review for gynecologic surgery conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery. Am J Obstet Gynecol. 2018;219:563.e1-563.e19.
Principles essential to change
Various methodologies have been described to manage change and facilitate implementation of new workflows and practices. Irrespective of the method used, including the more formal discipline of implementation science, at least 4 principles must be followed:
1. Teamwork. Mutual trust, mutual respect, and a sense of common purpose are minimum requirements for any successful initiative. Standardization is difficult or impossible without these elements. Thus, establishing a healthy team is the first step in implementing change.
2. Stakeholder analysis. Feedback from surgeons, nurses, residents, fellows, anesthesiologists, pharmacists, nurse anesthetists, and administrators is necessary to obtain diverse perspectives, facilitate engagement, and promote collaborative management. Negativity and resistance are common reactions to change, and it is particularly important to include those who are most skeptical in the stakeholder analysis to mitigate sabotage.
3. Concrete metrics. Success is possible only if defined a priori by specific and achievable goals. Counterbalances also are important to ensure that interventions do not have unintended consequences. Once a goal is met (for example, reduced hospital length of stay or costs), relevant metrics should be monitored after project completion for a minimum of 3 years to avoid regression to the pre-project state.
4. Leadership. The project champion responsible for the initiative must objectively facilitate all of the above and ensure excellent communication between stakeholders to nurture long-term engagement. Despite best efforts, if a minority of the group rejects compromise, this creates an opportunity to compare outcomes between those who do and do not accept the proposed change. Progress realized by early adopters may convince resistors to conform at a later time. Alternatively, the project champion also must have the insight to recognize when a proposed change is impossible at that point in time with that particular group. For example, our own initial attempts to implement enhanced recovery stalled in 2008, but they were successful 3 years later in a different environment.
Continue to: Although a discussion of leadership styles...
Although a discussion of leadership styles is beyond the scope of this article, in our experience, the most successful model is one of servant leadership that is team oriented rather than star dominated. Rather than being led by a single surgeon, each of the 4 quality improvement projects reviewed above (ERAS, and reductions in anastomotic leak, surgical site infection, and blood transfusion) that grew from enhanced recovery included trainees and was led by a different champion, encouraging teamwork and promoting career development. Such a model also supports the Accreditation Council for Graduate Medical Education’s emphasis on quality improvement education.
- Nygren J, Thacker J, Carli F, et al; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:285-305.
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114: 495-511.
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423.
- Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17:65-71.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504.
- Kalogera, E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331-341.
- Myles PS, Bellomo R, Corcoran T, et al; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263-2274.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part I. Gynecol Oncol. 2016;140:313-322.
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140:323-332.
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481.
- Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127:1135-1144.
- Kalogera E, Nitschmann CC, Dowdy SC, et al. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecol Oncol. 2017;144:343-347.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91-100.
- Nygren J, Thacker J, Carli F, et al; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37:285-305.
- American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114: 495-511.
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;(4):CD004423.
- Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17:65-71.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504.
- Kalogera, E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331-341.
- Myles PS, Bellomo R, Corcoran T, et al; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263-2274.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128:1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part I. Gynecol Oncol. 2016;140:313-322.
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140:323-332.
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481.
- Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127:1135-1144.
- Kalogera E, Nitschmann CC, Dowdy SC, et al. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecol Oncol. 2017;144:343-347.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91-100.
Malpractice: More lawsuits does not equal more relocations
David M. Studdert of Stanford (Calif.) University and his colleagues analyzed data from Medicare and the National Practitioner Data Bank (NPDB) to assess associations between the number of paid malpractice claims that doctors accrued and exits from medical practice, changes in clinical volume, geographic relocation, and change in practice-group size. The study population included 480,894 physicians who had 68,956 paid claims from 2003 to 2015. Of the study group, 89% had no claims, 9% had one claim, and the remaining 2% had two or more claims that accounted for 40% of all claims. Nearly three-quarters of the doctors studied were men, and the majority of specialties were internal medicine (17%), general practice/family medicine (15%), emergency medicine (7%), radiology (6%), and anesthesiology (6%).
Physicians with a higher number of claims against them did not relocate at a greater rate than physicians who had fewer or no claims, the investigators wrote in the New England Journal of Medicine.
More claims against a doctor were associated with a higher likelihood of leaving medicine and more shifts into smaller practice settings. For instance, physicians with one claim had 9% higher odds of leaving the practice than doctors with no claims, and physicians with five or more claims had a 45% higher chance of leaving medicine than doctors with no claims, the researchers found.
In addition, investigators found that doctors with two to four claims had 50%-60% higher odds of entering solo practice than physicians with no claims, and physicians with five or more claims had nearly 150% higher odds of moving to solo practice than doctors who had never been sued. Physicians with three or more claims were more likely to be male, work in surgical specialties, and be at least age 50 years.
The study addresses concerns that physicians with troubling legal records were moving across state lines for a fresh start, Mr. Studdert said in an interview. “We were surprised to find that physicians who accumulated multiple malpractice claims were no more likely to relocate their practices than physicians without claims. The National Practitioner Data Bank probably has something to do with that.”
Established by Congress in 1986, the NPDB was started, in part, to restrict the ability of incompetent physicians to move across states to hide their track records. By requiring hospitals to query doctors records before granting them clinical privileges and encouraging physician groups, health plans, and professional societies to do the same, the NPDB has “almost certainly increased the difficulty of relocation for physicians with legal problems,” the authors noted in the study.
A primary takeaway from the analysis is that, while a single malpractice claim is a relatively weak signal that a quality problem exists, multiple paid claims over a relatively short period of time are a strong signal that a physician may have a quality deficiency, Mr. Studdert said in the interview.
“Regulators and malpractice insurers should be paying closer attention to this signal,” he added. “To the extent that physicians are aware of a colleague’s checkered malpractice history, they may have a role to play too. Vigilance about signs of further problems, for one, but also careful thought about the wisdom of referring patients to such physicians.”
Michelle M. Mello, JD, PhD, and Mr. Studdert both reported receiving grants from SUMIT Insurance during the conduct of the study.
Source: Studdert DM et al. N Engl J Med. 2019 Mar 28. doi: 10.1056/NEJMsa1809981.
David M. Studdert of Stanford (Calif.) University and his colleagues analyzed data from Medicare and the National Practitioner Data Bank (NPDB) to assess associations between the number of paid malpractice claims that doctors accrued and exits from medical practice, changes in clinical volume, geographic relocation, and change in practice-group size. The study population included 480,894 physicians who had 68,956 paid claims from 2003 to 2015. Of the study group, 89% had no claims, 9% had one claim, and the remaining 2% had two or more claims that accounted for 40% of all claims. Nearly three-quarters of the doctors studied were men, and the majority of specialties were internal medicine (17%), general practice/family medicine (15%), emergency medicine (7%), radiology (6%), and anesthesiology (6%).
Physicians with a higher number of claims against them did not relocate at a greater rate than physicians who had fewer or no claims, the investigators wrote in the New England Journal of Medicine.
More claims against a doctor were associated with a higher likelihood of leaving medicine and more shifts into smaller practice settings. For instance, physicians with one claim had 9% higher odds of leaving the practice than doctors with no claims, and physicians with five or more claims had a 45% higher chance of leaving medicine than doctors with no claims, the researchers found.
In addition, investigators found that doctors with two to four claims had 50%-60% higher odds of entering solo practice than physicians with no claims, and physicians with five or more claims had nearly 150% higher odds of moving to solo practice than doctors who had never been sued. Physicians with three or more claims were more likely to be male, work in surgical specialties, and be at least age 50 years.
The study addresses concerns that physicians with troubling legal records were moving across state lines for a fresh start, Mr. Studdert said in an interview. “We were surprised to find that physicians who accumulated multiple malpractice claims were no more likely to relocate their practices than physicians without claims. The National Practitioner Data Bank probably has something to do with that.”
Established by Congress in 1986, the NPDB was started, in part, to restrict the ability of incompetent physicians to move across states to hide their track records. By requiring hospitals to query doctors records before granting them clinical privileges and encouraging physician groups, health plans, and professional societies to do the same, the NPDB has “almost certainly increased the difficulty of relocation for physicians with legal problems,” the authors noted in the study.
A primary takeaway from the analysis is that, while a single malpractice claim is a relatively weak signal that a quality problem exists, multiple paid claims over a relatively short period of time are a strong signal that a physician may have a quality deficiency, Mr. Studdert said in the interview.
“Regulators and malpractice insurers should be paying closer attention to this signal,” he added. “To the extent that physicians are aware of a colleague’s checkered malpractice history, they may have a role to play too. Vigilance about signs of further problems, for one, but also careful thought about the wisdom of referring patients to such physicians.”
Michelle M. Mello, JD, PhD, and Mr. Studdert both reported receiving grants from SUMIT Insurance during the conduct of the study.
Source: Studdert DM et al. N Engl J Med. 2019 Mar 28. doi: 10.1056/NEJMsa1809981.
David M. Studdert of Stanford (Calif.) University and his colleagues analyzed data from Medicare and the National Practitioner Data Bank (NPDB) to assess associations between the number of paid malpractice claims that doctors accrued and exits from medical practice, changes in clinical volume, geographic relocation, and change in practice-group size. The study population included 480,894 physicians who had 68,956 paid claims from 2003 to 2015. Of the study group, 89% had no claims, 9% had one claim, and the remaining 2% had two or more claims that accounted for 40% of all claims. Nearly three-quarters of the doctors studied were men, and the majority of specialties were internal medicine (17%), general practice/family medicine (15%), emergency medicine (7%), radiology (6%), and anesthesiology (6%).
Physicians with a higher number of claims against them did not relocate at a greater rate than physicians who had fewer or no claims, the investigators wrote in the New England Journal of Medicine.
More claims against a doctor were associated with a higher likelihood of leaving medicine and more shifts into smaller practice settings. For instance, physicians with one claim had 9% higher odds of leaving the practice than doctors with no claims, and physicians with five or more claims had a 45% higher chance of leaving medicine than doctors with no claims, the researchers found.
In addition, investigators found that doctors with two to four claims had 50%-60% higher odds of entering solo practice than physicians with no claims, and physicians with five or more claims had nearly 150% higher odds of moving to solo practice than doctors who had never been sued. Physicians with three or more claims were more likely to be male, work in surgical specialties, and be at least age 50 years.
The study addresses concerns that physicians with troubling legal records were moving across state lines for a fresh start, Mr. Studdert said in an interview. “We were surprised to find that physicians who accumulated multiple malpractice claims were no more likely to relocate their practices than physicians without claims. The National Practitioner Data Bank probably has something to do with that.”
Established by Congress in 1986, the NPDB was started, in part, to restrict the ability of incompetent physicians to move across states to hide their track records. By requiring hospitals to query doctors records before granting them clinical privileges and encouraging physician groups, health plans, and professional societies to do the same, the NPDB has “almost certainly increased the difficulty of relocation for physicians with legal problems,” the authors noted in the study.
A primary takeaway from the analysis is that, while a single malpractice claim is a relatively weak signal that a quality problem exists, multiple paid claims over a relatively short period of time are a strong signal that a physician may have a quality deficiency, Mr. Studdert said in the interview.
“Regulators and malpractice insurers should be paying closer attention to this signal,” he added. “To the extent that physicians are aware of a colleague’s checkered malpractice history, they may have a role to play too. Vigilance about signs of further problems, for one, but also careful thought about the wisdom of referring patients to such physicians.”
Michelle M. Mello, JD, PhD, and Mr. Studdert both reported receiving grants from SUMIT Insurance during the conduct of the study.
Source: Studdert DM et al. N Engl J Med. 2019 Mar 28. doi: 10.1056/NEJMsa1809981.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
MedPAC eyes scholarships, loan forgiveness to boost primary care ranks
WASHINGTON – according to a proposal presented at a meeting of the Medicare Payment Advisory Commission.
“A Medicare[-based] program would have a specific objective to encourage more physicians to enter primary care and provide primary care to beneficiaries,” MedPAC staffer Ariel Winter said. “By reducing educational debt, a Medicare-specific program would provide a financial incentive for physicians to choose primary care.”
Any program would face some challenges, Mr. Winter noted. Based on evidence, “it’s difficult to predict how physicians would respond if they were offered debt reduction in exchange for a commitment to practice primary care,” as financial considerations are not the only reason why physicians choose a specific career track.
Financing the program would also need to be considered. MedPAC staff recommended using a separate recommendation, one to end the Merit-based Incentive Payment System and use its $500 million put aside as MIPS bonuses to pay for any Medicare-based program.
Staff proposed a pilot program to “test the impact of different design choices on program operations, physician participation, and career choices,” he said. “Policymakers could use the results to improve the program and decide whether to expand it.”
MedPAC Vice Chairman Jon Christianson, PhD, suggested any program be tied to “physicians who practiced in areas where Medicare beneficiaries don’t have adequate access” to primary care doctors.
However, Mr. Winter noted that he is not aware of “any off-the-shelf system that identifies areas where there’s a problem, where there’s a shortage of clinicians for Medicare beneficiaries specifically. I am not sure how you would do that.”
MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, questioned whether nurse practitioners and physician assistants should be included in the program, as they “are beginning to subspecialize and get out of primary care.” Mr. Winter said it is open for consideration.
MedPAC member Pat Wang, president and CEO of Healthfirst in New York, questioned whether a new program was needed or whether fixing of existing programs, “making them work better” is the way to go given the evidence that the effect of student debt on decision making is mixed.
She suggested that rather than targeting loan forgiveness, maybe the program should be structured more as a bonus payment rather than debt forgiveness as a means of incentivizing people who may not be concerned with debt forgiveness.
Ms. Buto added that questions of autonomy might also need to be addressed. “Physicians often feel like they don’t have control in Medicare, that they’re required to do a lot of things, and that they are subject to the fee schedule. If there were some way to grant more autonomy, control, and convey status that way, whether it has to do with greater flexibility in whatever, payment models and so on, that’s where I think you can begin to shift the status within primary care.”
MedPAC Chairman Francis Crosson, MD, recalled his time at Kaiser Permanente and noted their programs showed success because of the combination of a significant amount of money and time commitment (10 years).
The time commitment became an important part because after that long, physicians became a part of their communities and tended to stay.
“Two or 3 years, from my perspective and my experience, doesn’t work very well,” Dr. Crosson said. “But a significant period of time does, and a significant amount of money does seem to work.”
WASHINGTON – according to a proposal presented at a meeting of the Medicare Payment Advisory Commission.
“A Medicare[-based] program would have a specific objective to encourage more physicians to enter primary care and provide primary care to beneficiaries,” MedPAC staffer Ariel Winter said. “By reducing educational debt, a Medicare-specific program would provide a financial incentive for physicians to choose primary care.”
Any program would face some challenges, Mr. Winter noted. Based on evidence, “it’s difficult to predict how physicians would respond if they were offered debt reduction in exchange for a commitment to practice primary care,” as financial considerations are not the only reason why physicians choose a specific career track.
Financing the program would also need to be considered. MedPAC staff recommended using a separate recommendation, one to end the Merit-based Incentive Payment System and use its $500 million put aside as MIPS bonuses to pay for any Medicare-based program.
Staff proposed a pilot program to “test the impact of different design choices on program operations, physician participation, and career choices,” he said. “Policymakers could use the results to improve the program and decide whether to expand it.”
MedPAC Vice Chairman Jon Christianson, PhD, suggested any program be tied to “physicians who practiced in areas where Medicare beneficiaries don’t have adequate access” to primary care doctors.
However, Mr. Winter noted that he is not aware of “any off-the-shelf system that identifies areas where there’s a problem, where there’s a shortage of clinicians for Medicare beneficiaries specifically. I am not sure how you would do that.”
MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, questioned whether nurse practitioners and physician assistants should be included in the program, as they “are beginning to subspecialize and get out of primary care.” Mr. Winter said it is open for consideration.
MedPAC member Pat Wang, president and CEO of Healthfirst in New York, questioned whether a new program was needed or whether fixing of existing programs, “making them work better” is the way to go given the evidence that the effect of student debt on decision making is mixed.
She suggested that rather than targeting loan forgiveness, maybe the program should be structured more as a bonus payment rather than debt forgiveness as a means of incentivizing people who may not be concerned with debt forgiveness.
Ms. Buto added that questions of autonomy might also need to be addressed. “Physicians often feel like they don’t have control in Medicare, that they’re required to do a lot of things, and that they are subject to the fee schedule. If there were some way to grant more autonomy, control, and convey status that way, whether it has to do with greater flexibility in whatever, payment models and so on, that’s where I think you can begin to shift the status within primary care.”
MedPAC Chairman Francis Crosson, MD, recalled his time at Kaiser Permanente and noted their programs showed success because of the combination of a significant amount of money and time commitment (10 years).
The time commitment became an important part because after that long, physicians became a part of their communities and tended to stay.
“Two or 3 years, from my perspective and my experience, doesn’t work very well,” Dr. Crosson said. “But a significant period of time does, and a significant amount of money does seem to work.”
WASHINGTON – according to a proposal presented at a meeting of the Medicare Payment Advisory Commission.
“A Medicare[-based] program would have a specific objective to encourage more physicians to enter primary care and provide primary care to beneficiaries,” MedPAC staffer Ariel Winter said. “By reducing educational debt, a Medicare-specific program would provide a financial incentive for physicians to choose primary care.”
Any program would face some challenges, Mr. Winter noted. Based on evidence, “it’s difficult to predict how physicians would respond if they were offered debt reduction in exchange for a commitment to practice primary care,” as financial considerations are not the only reason why physicians choose a specific career track.
Financing the program would also need to be considered. MedPAC staff recommended using a separate recommendation, one to end the Merit-based Incentive Payment System and use its $500 million put aside as MIPS bonuses to pay for any Medicare-based program.
Staff proposed a pilot program to “test the impact of different design choices on program operations, physician participation, and career choices,” he said. “Policymakers could use the results to improve the program and decide whether to expand it.”
MedPAC Vice Chairman Jon Christianson, PhD, suggested any program be tied to “physicians who practiced in areas where Medicare beneficiaries don’t have adequate access” to primary care doctors.
However, Mr. Winter noted that he is not aware of “any off-the-shelf system that identifies areas where there’s a problem, where there’s a shortage of clinicians for Medicare beneficiaries specifically. I am not sure how you would do that.”
MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, questioned whether nurse practitioners and physician assistants should be included in the program, as they “are beginning to subspecialize and get out of primary care.” Mr. Winter said it is open for consideration.
MedPAC member Pat Wang, president and CEO of Healthfirst in New York, questioned whether a new program was needed or whether fixing of existing programs, “making them work better” is the way to go given the evidence that the effect of student debt on decision making is mixed.
She suggested that rather than targeting loan forgiveness, maybe the program should be structured more as a bonus payment rather than debt forgiveness as a means of incentivizing people who may not be concerned with debt forgiveness.
Ms. Buto added that questions of autonomy might also need to be addressed. “Physicians often feel like they don’t have control in Medicare, that they’re required to do a lot of things, and that they are subject to the fee schedule. If there were some way to grant more autonomy, control, and convey status that way, whether it has to do with greater flexibility in whatever, payment models and so on, that’s where I think you can begin to shift the status within primary care.”
MedPAC Chairman Francis Crosson, MD, recalled his time at Kaiser Permanente and noted their programs showed success because of the combination of a significant amount of money and time commitment (10 years).
The time commitment became an important part because after that long, physicians became a part of their communities and tended to stay.
“Two or 3 years, from my perspective and my experience, doesn’t work very well,” Dr. Crosson said. “But a significant period of time does, and a significant amount of money does seem to work.”
REPORTING FROM A MEDPAC MEETING
Addressing insulin price spikes will require supply chain reform
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
REPORTING FROM A HOUSE ENERGY & COMMERCE SUBCOMMITTEE HEARING