User login
A patient with severe adenomyosis requests uterine-sparing surgery
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.
Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
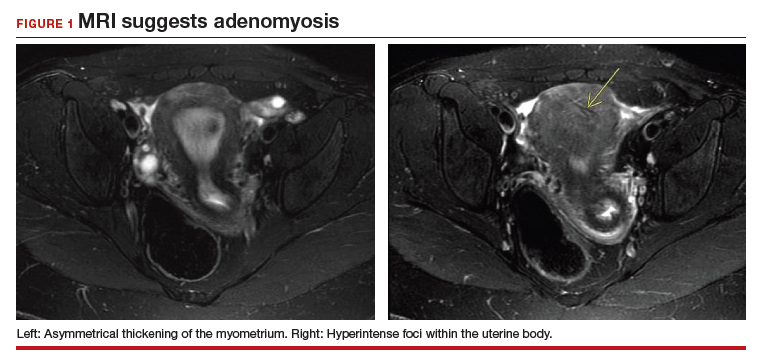
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25
Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
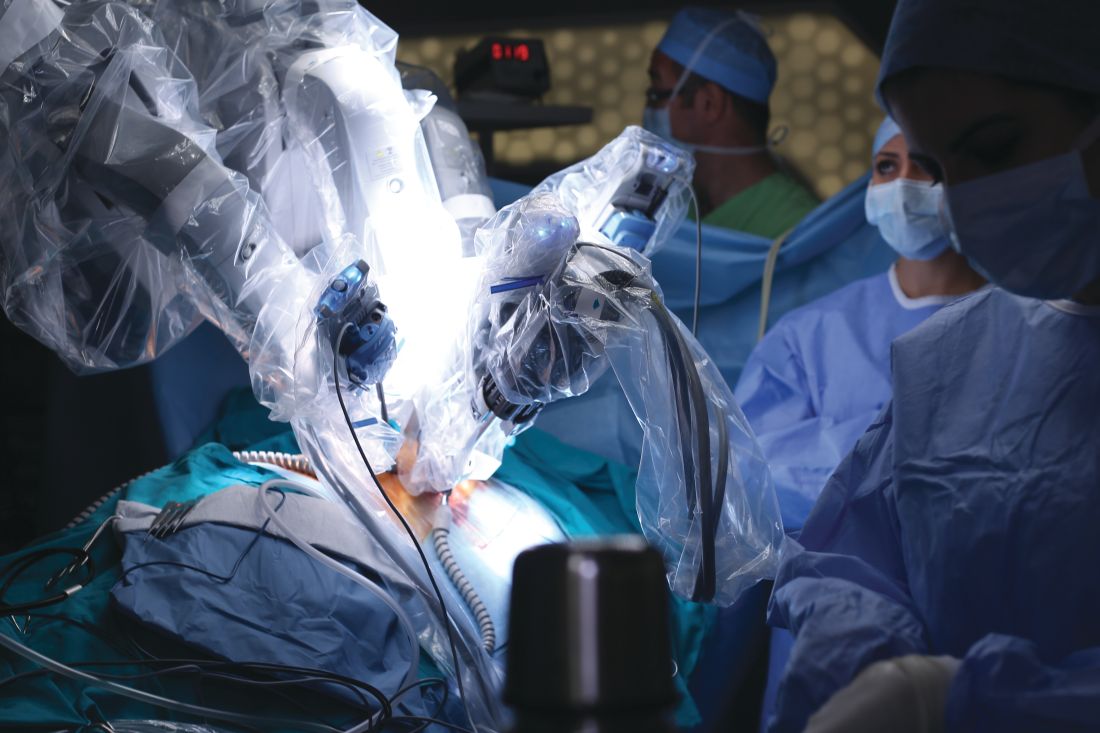
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
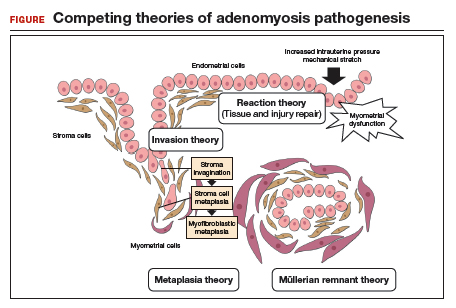
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
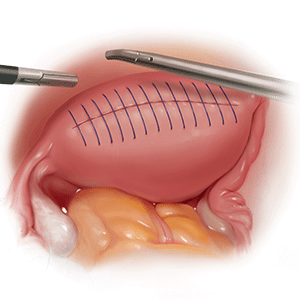
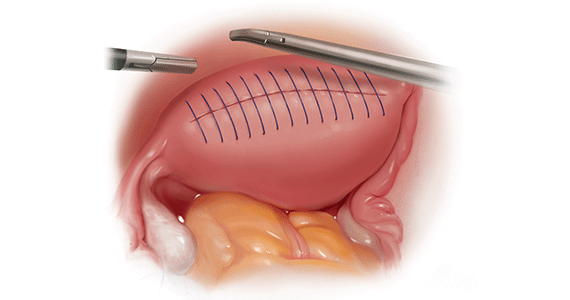
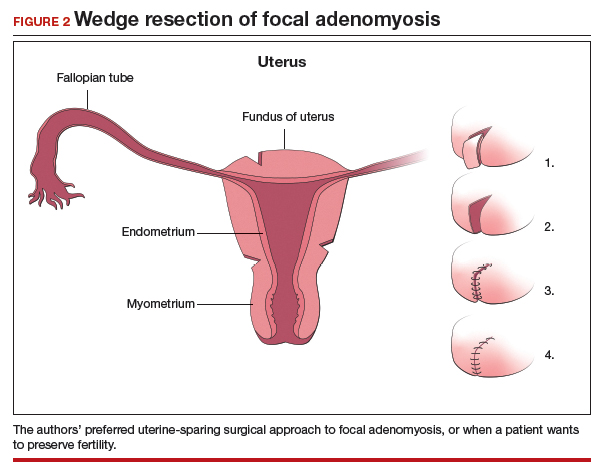
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.
CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
Intensive insulin added no benefit for hyperglycemia after ischemic stroke
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
REPORTING FROM ISC 2019
Key clinical point: Aggressive insulin management of hyperglycemia following an ischemic stroke gave no clinical benefit, compared with a standard approach.
Major finding: After 90 days, favorable outcomes occurred in 21% of patients on aggressive insulin treatment and 22% on standard treatment.
Study details: SHINE, a multicenter, randomized trial with 1,151 acute ischemic stroke patients.
Disclosures: SHINE received no commercial funding. Dr. Johnston had no disclosures.
Source: Johnston KC et al. ISC 2019, Abstract LB1.
New cryoablating catheter shows promising AF efficacy, safety
BOSTON – An ultra-low-temperature ablation catheter was safe and effective in the first 48 atrial fibrillation (AF) patients to undergo treatment with the device.
The ultra-low-temperature catheter, cooled by liquid nitrogen to –196° C, showed excellent safety and a high success rate, in the modest number of patients who have been followed for 6 or 12 months, Tom De Potter, MD, said at the annual International AF Symposium. The catheter is capable of delivering both focal and linear lesions, said Dr. De Potter, a cardiac electrophysiologist at the Cardiovascular Center of OLV Hospital in Aalst, Belgium.
He reported results on the first 48 AF patients treated with the catheter at either his center or at St. Antonius Hospital in Nieuwegein, the Netherlands, in the CryoCure2 (Investigation of the Adagio Cryoablation System in Patients With Atrial Fibrillation) study. The investigators included safety findings only from the first 13 patients treated, but they assessed both safety and efficacy for the following 35 patients.
The safety review showed one patient with a groin-puncture injury, and two patients with phrenic nerve palsy, but no cases of phrenic nerve palsy in the most recent 41 patients who were treated with an updated version of the catheter that included a cryomapping feature. None of the 48 patients had a stroke, transient ischemic attack, esophageal injury, tamponade, or MI.
The 35 patients reviewed for efficacy included 10 patients with paroxysmal AF and 25 with persistent AF. All five of the paroxysmal AF patients followed for at least 6 months, and all four of those patients followed for 12 months, showed no AF recurrences. One of the persistent AF patients never achieved cardioversion at the time of the ablation procedure. All 17 other persistent AF patients followed for at least 6 months remained in sinus rhythm at 6 months. Among the eight of these patients followed for at least 12 months after treatment, one patient had AF recurrence, so the current 12-month rate of freedom from recurrence is seven of nine patients (including the one who never achieved cardioversion), or 78%.
The average procedure time to perform pulmonary vein isolation (PVI) only was 106 minutes, and procedure time was 116 minutes in the patients who underwent PVI plus additional ablation procedures. Average ablation time was 12 minutes among those just having PVI, and 14 minutes in those who underwent PVI plus other ablations. The cryoablation catheter comes with 20 electrodes that allow it to also record electrograms.
The attraction of the ultra-low-temperature cryoablation catheter used in the study is it “can achieve complete PVI considerably faster” than other ablation methods while also achieving “unsurpassed efficacy,” Dr. De Potter said in a written statement. In addition, “the new, adjustable diagnostic capacity simplifies the procedure and makes it much less operator dependent,” he added.
BOSTON – An ultra-low-temperature ablation catheter was safe and effective in the first 48 atrial fibrillation (AF) patients to undergo treatment with the device.
The ultra-low-temperature catheter, cooled by liquid nitrogen to –196° C, showed excellent safety and a high success rate, in the modest number of patients who have been followed for 6 or 12 months, Tom De Potter, MD, said at the annual International AF Symposium. The catheter is capable of delivering both focal and linear lesions, said Dr. De Potter, a cardiac electrophysiologist at the Cardiovascular Center of OLV Hospital in Aalst, Belgium.
He reported results on the first 48 AF patients treated with the catheter at either his center or at St. Antonius Hospital in Nieuwegein, the Netherlands, in the CryoCure2 (Investigation of the Adagio Cryoablation System in Patients With Atrial Fibrillation) study. The investigators included safety findings only from the first 13 patients treated, but they assessed both safety and efficacy for the following 35 patients.
The safety review showed one patient with a groin-puncture injury, and two patients with phrenic nerve palsy, but no cases of phrenic nerve palsy in the most recent 41 patients who were treated with an updated version of the catheter that included a cryomapping feature. None of the 48 patients had a stroke, transient ischemic attack, esophageal injury, tamponade, or MI.
The 35 patients reviewed for efficacy included 10 patients with paroxysmal AF and 25 with persistent AF. All five of the paroxysmal AF patients followed for at least 6 months, and all four of those patients followed for 12 months, showed no AF recurrences. One of the persistent AF patients never achieved cardioversion at the time of the ablation procedure. All 17 other persistent AF patients followed for at least 6 months remained in sinus rhythm at 6 months. Among the eight of these patients followed for at least 12 months after treatment, one patient had AF recurrence, so the current 12-month rate of freedom from recurrence is seven of nine patients (including the one who never achieved cardioversion), or 78%.
The average procedure time to perform pulmonary vein isolation (PVI) only was 106 minutes, and procedure time was 116 minutes in the patients who underwent PVI plus additional ablation procedures. Average ablation time was 12 minutes among those just having PVI, and 14 minutes in those who underwent PVI plus other ablations. The cryoablation catheter comes with 20 electrodes that allow it to also record electrograms.
The attraction of the ultra-low-temperature cryoablation catheter used in the study is it “can achieve complete PVI considerably faster” than other ablation methods while also achieving “unsurpassed efficacy,” Dr. De Potter said in a written statement. In addition, “the new, adjustable diagnostic capacity simplifies the procedure and makes it much less operator dependent,” he added.
BOSTON – An ultra-low-temperature ablation catheter was safe and effective in the first 48 atrial fibrillation (AF) patients to undergo treatment with the device.
The ultra-low-temperature catheter, cooled by liquid nitrogen to –196° C, showed excellent safety and a high success rate, in the modest number of patients who have been followed for 6 or 12 months, Tom De Potter, MD, said at the annual International AF Symposium. The catheter is capable of delivering both focal and linear lesions, said Dr. De Potter, a cardiac electrophysiologist at the Cardiovascular Center of OLV Hospital in Aalst, Belgium.
He reported results on the first 48 AF patients treated with the catheter at either his center or at St. Antonius Hospital in Nieuwegein, the Netherlands, in the CryoCure2 (Investigation of the Adagio Cryoablation System in Patients With Atrial Fibrillation) study. The investigators included safety findings only from the first 13 patients treated, but they assessed both safety and efficacy for the following 35 patients.
The safety review showed one patient with a groin-puncture injury, and two patients with phrenic nerve palsy, but no cases of phrenic nerve palsy in the most recent 41 patients who were treated with an updated version of the catheter that included a cryomapping feature. None of the 48 patients had a stroke, transient ischemic attack, esophageal injury, tamponade, or MI.
The 35 patients reviewed for efficacy included 10 patients with paroxysmal AF and 25 with persistent AF. All five of the paroxysmal AF patients followed for at least 6 months, and all four of those patients followed for 12 months, showed no AF recurrences. One of the persistent AF patients never achieved cardioversion at the time of the ablation procedure. All 17 other persistent AF patients followed for at least 6 months remained in sinus rhythm at 6 months. Among the eight of these patients followed for at least 12 months after treatment, one patient had AF recurrence, so the current 12-month rate of freedom from recurrence is seven of nine patients (including the one who never achieved cardioversion), or 78%.
The average procedure time to perform pulmonary vein isolation (PVI) only was 106 minutes, and procedure time was 116 minutes in the patients who underwent PVI plus additional ablation procedures. Average ablation time was 12 minutes among those just having PVI, and 14 minutes in those who underwent PVI plus other ablations. The cryoablation catheter comes with 20 electrodes that allow it to also record electrograms.
The attraction of the ultra-low-temperature cryoablation catheter used in the study is it “can achieve complete PVI considerably faster” than other ablation methods while also achieving “unsurpassed efficacy,” Dr. De Potter said in a written statement. In addition, “the new, adjustable diagnostic capacity simplifies the procedure and makes it much less operator dependent,” he added.
REPORTING FROM THE AF SYMPOSIUM 2019
Key clinical point:
Major finding: A year after cryoablation, seven of nine treated patients with persistent atrial fibrillation remained recurrence free.
Study details: CryoCure2, a single arm, multicenter, European study with 48 patients.
Disclosures: The CryoCure2 study is sponsored by Adagio Medical, the company developing the cryoablation catheter. Dr. De Potter has received travel support from Adagio, and he has received research funding from Boston Scientific and Johnson & Johnson.
Benefiting from an egalitarian hospital culture
Cultural change linked to improved outcomes
Health care experts have long known of a link between patient outcomes and a hospital’s organizational culture, according to an article in the New York Times by Pauline W. Chen, MD.
“Heart attack patients who are treated at hospitals where nurses feel powerless and senior management is only sporadically involved in patient care tend to fare more poorly than patients hospitalized at institutions where nurses are asked regularly for their input and chief executives hold regular meetings with clinicians to review patient results,” she wrote.
But there is hope for change, Dr. Chen noted, and it’s demonstrable, citing a group of researchers that has written about strategies targeting hospital organizational culture called “Leadership Saves Lives.” The researchers showed hospitals could create significant cultural changes, which could impact patient outcomes, in just 2 years.
“Leadership Saves Lives requires that each hospital create a ‘Guiding Coalition,’ a group of staff members from across the entire institution. The coalition members participate in regular workshops, discussions, and national forums on ways hospitals might improve, then help their respective hospital translate newfound ideas and information into clinical practice,” she wrote.
The researchers monitored heart attack patients to assess the effect of Leadership Saves Lives in 10 hospitals that had below average patient outcomes. Over 2 years, all 10 hospitals changed significantly, but 6 hospitals experienced particularly profound cultural transformations.
“The staff of these hospitals spoke of an institutional shift from ‘because I said so’ to ‘focusing on the why’s,’ ” Dr. Chen wrote. “Instead of accepting that every heart attack patient had to undergo certain testing or take specific drugs because the chief of the department or administrator had previously established such clinical protocols, for example, it became more important to provide the data that proved such rituals were actually helpful. Staff members in these hospitals also said they received, and appreciated, increased support from senior management and a newfound freedom to voice opinions in ‘more of an equal role, no matter what position you are.’ ”
The degree of an institution’s cultural change was directly linked to patient outcomes, the researchers found. Indeed, hospitals that made more substantial changes in their work culture realized larger and more sustained drops in heart attack mortality rates.
References
1. Chen PW. A More Egalitarian Hospital Culture Is Better for Everyone. New York Times. https://www.nytimes.com/2018/05/31/well/live/doctors-patients-hospital-culture-better-health.html. Published May 31, 2018. Accessed June 1, 2018.
2. Curry LA et al. Organizational culture change in U.S. hospitals: A mixed methods longitudinal intervention study. Implementation Science. 2015 Mar 7. doi: 10.1186/s13012-015-0218-0. Accessed June 18, 2018.
Cultural change linked to improved outcomes
Cultural change linked to improved outcomes
Health care experts have long known of a link between patient outcomes and a hospital’s organizational culture, according to an article in the New York Times by Pauline W. Chen, MD.
“Heart attack patients who are treated at hospitals where nurses feel powerless and senior management is only sporadically involved in patient care tend to fare more poorly than patients hospitalized at institutions where nurses are asked regularly for their input and chief executives hold regular meetings with clinicians to review patient results,” she wrote.
But there is hope for change, Dr. Chen noted, and it’s demonstrable, citing a group of researchers that has written about strategies targeting hospital organizational culture called “Leadership Saves Lives.” The researchers showed hospitals could create significant cultural changes, which could impact patient outcomes, in just 2 years.
“Leadership Saves Lives requires that each hospital create a ‘Guiding Coalition,’ a group of staff members from across the entire institution. The coalition members participate in regular workshops, discussions, and national forums on ways hospitals might improve, then help their respective hospital translate newfound ideas and information into clinical practice,” she wrote.
The researchers monitored heart attack patients to assess the effect of Leadership Saves Lives in 10 hospitals that had below average patient outcomes. Over 2 years, all 10 hospitals changed significantly, but 6 hospitals experienced particularly profound cultural transformations.
“The staff of these hospitals spoke of an institutional shift from ‘because I said so’ to ‘focusing on the why’s,’ ” Dr. Chen wrote. “Instead of accepting that every heart attack patient had to undergo certain testing or take specific drugs because the chief of the department or administrator had previously established such clinical protocols, for example, it became more important to provide the data that proved such rituals were actually helpful. Staff members in these hospitals also said they received, and appreciated, increased support from senior management and a newfound freedom to voice opinions in ‘more of an equal role, no matter what position you are.’ ”
The degree of an institution’s cultural change was directly linked to patient outcomes, the researchers found. Indeed, hospitals that made more substantial changes in their work culture realized larger and more sustained drops in heart attack mortality rates.
References
1. Chen PW. A More Egalitarian Hospital Culture Is Better for Everyone. New York Times. https://www.nytimes.com/2018/05/31/well/live/doctors-patients-hospital-culture-better-health.html. Published May 31, 2018. Accessed June 1, 2018.
2. Curry LA et al. Organizational culture change in U.S. hospitals: A mixed methods longitudinal intervention study. Implementation Science. 2015 Mar 7. doi: 10.1186/s13012-015-0218-0. Accessed June 18, 2018.
Health care experts have long known of a link between patient outcomes and a hospital’s organizational culture, according to an article in the New York Times by Pauline W. Chen, MD.
“Heart attack patients who are treated at hospitals where nurses feel powerless and senior management is only sporadically involved in patient care tend to fare more poorly than patients hospitalized at institutions where nurses are asked regularly for their input and chief executives hold regular meetings with clinicians to review patient results,” she wrote.
But there is hope for change, Dr. Chen noted, and it’s demonstrable, citing a group of researchers that has written about strategies targeting hospital organizational culture called “Leadership Saves Lives.” The researchers showed hospitals could create significant cultural changes, which could impact patient outcomes, in just 2 years.
“Leadership Saves Lives requires that each hospital create a ‘Guiding Coalition,’ a group of staff members from across the entire institution. The coalition members participate in regular workshops, discussions, and national forums on ways hospitals might improve, then help their respective hospital translate newfound ideas and information into clinical practice,” she wrote.
The researchers monitored heart attack patients to assess the effect of Leadership Saves Lives in 10 hospitals that had below average patient outcomes. Over 2 years, all 10 hospitals changed significantly, but 6 hospitals experienced particularly profound cultural transformations.
“The staff of these hospitals spoke of an institutional shift from ‘because I said so’ to ‘focusing on the why’s,’ ” Dr. Chen wrote. “Instead of accepting that every heart attack patient had to undergo certain testing or take specific drugs because the chief of the department or administrator had previously established such clinical protocols, for example, it became more important to provide the data that proved such rituals were actually helpful. Staff members in these hospitals also said they received, and appreciated, increased support from senior management and a newfound freedom to voice opinions in ‘more of an equal role, no matter what position you are.’ ”
The degree of an institution’s cultural change was directly linked to patient outcomes, the researchers found. Indeed, hospitals that made more substantial changes in their work culture realized larger and more sustained drops in heart attack mortality rates.
References
1. Chen PW. A More Egalitarian Hospital Culture Is Better for Everyone. New York Times. https://www.nytimes.com/2018/05/31/well/live/doctors-patients-hospital-culture-better-health.html. Published May 31, 2018. Accessed June 1, 2018.
2. Curry LA et al. Organizational culture change in U.S. hospitals: A mixed methods longitudinal intervention study. Implementation Science. 2015 Mar 7. doi: 10.1186/s13012-015-0218-0. Accessed June 18, 2018.
Cobomarsen shows early promise for treating ATLL
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen.
Study details: Phase 1 trial including eight ATLL patients.
Disclosures: The trial is sponsored by miRagen Therapeutics.
Nail Psoriasis Tips
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
Enoblituzumab plus pembrolizumab shows promise for select solid tumors
WASHINGTON – Combination enoblituzumab and pembrolizumab showed acceptable safety and encouraging antitumor activity in select patients with B7-H3-expressing non–small-cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and other solid tumors in a phase 1 dose-escalation and expansion study.
Of note, the combination showed activity in patients anticipated to be poorly responsive to checkpoint inhibitor therapy alone, Charu Aggarwal, MD, of the University of Pennsylvania, Philadelphia, reported at the annual meeting of the Society for Immunotherapy of Cancer.
In all, 133 patients were treated weekly with 3, 10 or 15 mg/kg intravenous doses of the investigational anti-B7-H3 monoclonal antibody enoblituzumab plus 2 mg/kg intravenous doses of the programmed death-1 (PD-1) inhibitor pembrolizumab every 3 weeks (the standard dose at the time) for up to 1 year in the open-label dose-escalation study. Of those, 85% experienced treatment-related adverse events (AEs), and 27.1% experienced grade 3 or higher AEs, Dr. Aggarwal said.
These AEs were mostly infusion-related reactions typically seen with the first dose, and they were not usually cumulative, she said, noting that the rates of immune-related adverse events were less than 5%, about 7% of patients discontinued treatment because of a drug-related AE, and 1 treatment-related death due to pneumonitis occurred.
“No maximum tolerated dose was reached,” she added. “What I want to emphasize is that this is a combination immunotherapy approach, and what we found was that despite a combination approach, the nature, rate, and incidence of immune-related adverse events was not different than what is expected compared to single-agent [therapy] alone.”
Patients were then divided into disease-specific dose-expansion cohorts and the SCCHN and NSCLC cohorts were further stratified based on whether or not they had prior exposure to PD-1 inhibitor therapy.
Antitumor activity was noted in anti-PD-1-naive SCCHN patients, and objective responses were also seen in NSCLC patient with tumor programmed death-ligand 1 (PD-L1) expression of less than 1% and patients with checkpoint inhibitor–refractory urothelial carcinoma.
“We saw a 33.3% response rate in IO [immunotherapy]-naive head and neck cancer patients, and a 35.7% response rate in patients with PD-L1-negative immunotherapy-naive non–small-cell lung cancer patients,” she said.
No objective radiographic responses were seen in the IO-exposed SCCHN patients, but a few were seen in the other tumor cohorts, she noted.
“What was more interesting is this prolonged and high level of stable disease that we found in patients who had been previously treated with IO and had actually experienced significant clinical and radiographic progression then experienced stability with this combination,” she said, noting that “a fair amount of stable disease” was also seen in the IO-naive SCCHN and NSCLC patients.
In the immunotherapy-naive SCCHN patients, responses were seen regardless of human papillomavirus status, and four are still on treatment. One had a confirmed complete response.
“The majority of our patients on our trial and in this cohort were B7-H3-positive, she said, adding that the responses that were seen, including in those with stable disease, were “sustained and durable.”
Responses were similar in the immunotherapy-naive NSCLC patients who were PD-L1 negative, she said.
“Responses were seen irrespective of histology ... and many of these patients are still on treatment,” she added, noting that most were B7-H3-positive, which “seems to select our patients who have an even higher response rate of about 45%.”
The responses in that cohort also occurred early, were durable, and are sustained, and some patients remain on treatment and “are enjoying the clinical benefit afforded by this combination,” she said.
The findings are notable, because B7-H3 is highly expressed in many solid tumors, and monotherapy with enoblituzumab, which targets B7-H3 and is engineered to enhance antibody-dependent cell-mediated cytotoxicity, has demonstrated antitumor activity with an acceptable safety profile in patients with selected solid tumors.
In this study it was combined with pembrolizumab to test the hypothesis that coordinated engagement of both innate and adaptive immunity via the targeting of two distinct members of the B7 family could achieve greater antitumor activity than either agent alone, she explained.
The results “benchmark favorably” versus prior experience with PD-1 agents; pembrolizumab and nivolumab each lead to activity of about 13%-16%, she said.
“We acknowledge that our study has very small numbers. Nevertheless, these are encouraging data ... in this very tough-to-treat population,” Dr. Aggarwal said, adding that “further investigation of enoblituzumab with an anti-PD-1 molecule is warranted in both head and neck and lung cancer patients, perhaps including in combination with chemotherapy.”
Further, given the expression patterns of B7-H3 on a wide variety of solid tumors, further investigation of this combination ... is warranted in other tumor types, including in both checkpoint-naive and -treated populations, she concluded.
This study was sponsored by MacroGenics. Dr. Aggarwal reported receiving consulting fees from BMS.
SOURCE: Aggarwal C et al., SITC 2018 Abstract O24.
WASHINGTON – Combination enoblituzumab and pembrolizumab showed acceptable safety and encouraging antitumor activity in select patients with B7-H3-expressing non–small-cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and other solid tumors in a phase 1 dose-escalation and expansion study.
Of note, the combination showed activity in patients anticipated to be poorly responsive to checkpoint inhibitor therapy alone, Charu Aggarwal, MD, of the University of Pennsylvania, Philadelphia, reported at the annual meeting of the Society for Immunotherapy of Cancer.
In all, 133 patients were treated weekly with 3, 10 or 15 mg/kg intravenous doses of the investigational anti-B7-H3 monoclonal antibody enoblituzumab plus 2 mg/kg intravenous doses of the programmed death-1 (PD-1) inhibitor pembrolizumab every 3 weeks (the standard dose at the time) for up to 1 year in the open-label dose-escalation study. Of those, 85% experienced treatment-related adverse events (AEs), and 27.1% experienced grade 3 or higher AEs, Dr. Aggarwal said.
These AEs were mostly infusion-related reactions typically seen with the first dose, and they were not usually cumulative, she said, noting that the rates of immune-related adverse events were less than 5%, about 7% of patients discontinued treatment because of a drug-related AE, and 1 treatment-related death due to pneumonitis occurred.
“No maximum tolerated dose was reached,” she added. “What I want to emphasize is that this is a combination immunotherapy approach, and what we found was that despite a combination approach, the nature, rate, and incidence of immune-related adverse events was not different than what is expected compared to single-agent [therapy] alone.”
Patients were then divided into disease-specific dose-expansion cohorts and the SCCHN and NSCLC cohorts were further stratified based on whether or not they had prior exposure to PD-1 inhibitor therapy.
Antitumor activity was noted in anti-PD-1-naive SCCHN patients, and objective responses were also seen in NSCLC patient with tumor programmed death-ligand 1 (PD-L1) expression of less than 1% and patients with checkpoint inhibitor–refractory urothelial carcinoma.
“We saw a 33.3% response rate in IO [immunotherapy]-naive head and neck cancer patients, and a 35.7% response rate in patients with PD-L1-negative immunotherapy-naive non–small-cell lung cancer patients,” she said.
No objective radiographic responses were seen in the IO-exposed SCCHN patients, but a few were seen in the other tumor cohorts, she noted.
“What was more interesting is this prolonged and high level of stable disease that we found in patients who had been previously treated with IO and had actually experienced significant clinical and radiographic progression then experienced stability with this combination,” she said, noting that “a fair amount of stable disease” was also seen in the IO-naive SCCHN and NSCLC patients.
In the immunotherapy-naive SCCHN patients, responses were seen regardless of human papillomavirus status, and four are still on treatment. One had a confirmed complete response.
“The majority of our patients on our trial and in this cohort were B7-H3-positive, she said, adding that the responses that were seen, including in those with stable disease, were “sustained and durable.”
Responses were similar in the immunotherapy-naive NSCLC patients who were PD-L1 negative, she said.
“Responses were seen irrespective of histology ... and many of these patients are still on treatment,” she added, noting that most were B7-H3-positive, which “seems to select our patients who have an even higher response rate of about 45%.”
The responses in that cohort also occurred early, were durable, and are sustained, and some patients remain on treatment and “are enjoying the clinical benefit afforded by this combination,” she said.
The findings are notable, because B7-H3 is highly expressed in many solid tumors, and monotherapy with enoblituzumab, which targets B7-H3 and is engineered to enhance antibody-dependent cell-mediated cytotoxicity, has demonstrated antitumor activity with an acceptable safety profile in patients with selected solid tumors.
In this study it was combined with pembrolizumab to test the hypothesis that coordinated engagement of both innate and adaptive immunity via the targeting of two distinct members of the B7 family could achieve greater antitumor activity than either agent alone, she explained.
The results “benchmark favorably” versus prior experience with PD-1 agents; pembrolizumab and nivolumab each lead to activity of about 13%-16%, she said.
“We acknowledge that our study has very small numbers. Nevertheless, these are encouraging data ... in this very tough-to-treat population,” Dr. Aggarwal said, adding that “further investigation of enoblituzumab with an anti-PD-1 molecule is warranted in both head and neck and lung cancer patients, perhaps including in combination with chemotherapy.”
Further, given the expression patterns of B7-H3 on a wide variety of solid tumors, further investigation of this combination ... is warranted in other tumor types, including in both checkpoint-naive and -treated populations, she concluded.
This study was sponsored by MacroGenics. Dr. Aggarwal reported receiving consulting fees from BMS.
SOURCE: Aggarwal C et al., SITC 2018 Abstract O24.
WASHINGTON – Combination enoblituzumab and pembrolizumab showed acceptable safety and encouraging antitumor activity in select patients with B7-H3-expressing non–small-cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and other solid tumors in a phase 1 dose-escalation and expansion study.
Of note, the combination showed activity in patients anticipated to be poorly responsive to checkpoint inhibitor therapy alone, Charu Aggarwal, MD, of the University of Pennsylvania, Philadelphia, reported at the annual meeting of the Society for Immunotherapy of Cancer.
In all, 133 patients were treated weekly with 3, 10 or 15 mg/kg intravenous doses of the investigational anti-B7-H3 monoclonal antibody enoblituzumab plus 2 mg/kg intravenous doses of the programmed death-1 (PD-1) inhibitor pembrolizumab every 3 weeks (the standard dose at the time) for up to 1 year in the open-label dose-escalation study. Of those, 85% experienced treatment-related adverse events (AEs), and 27.1% experienced grade 3 or higher AEs, Dr. Aggarwal said.
These AEs were mostly infusion-related reactions typically seen with the first dose, and they were not usually cumulative, she said, noting that the rates of immune-related adverse events were less than 5%, about 7% of patients discontinued treatment because of a drug-related AE, and 1 treatment-related death due to pneumonitis occurred.
“No maximum tolerated dose was reached,” she added. “What I want to emphasize is that this is a combination immunotherapy approach, and what we found was that despite a combination approach, the nature, rate, and incidence of immune-related adverse events was not different than what is expected compared to single-agent [therapy] alone.”
Patients were then divided into disease-specific dose-expansion cohorts and the SCCHN and NSCLC cohorts were further stratified based on whether or not they had prior exposure to PD-1 inhibitor therapy.
Antitumor activity was noted in anti-PD-1-naive SCCHN patients, and objective responses were also seen in NSCLC patient with tumor programmed death-ligand 1 (PD-L1) expression of less than 1% and patients with checkpoint inhibitor–refractory urothelial carcinoma.
“We saw a 33.3% response rate in IO [immunotherapy]-naive head and neck cancer patients, and a 35.7% response rate in patients with PD-L1-negative immunotherapy-naive non–small-cell lung cancer patients,” she said.
No objective radiographic responses were seen in the IO-exposed SCCHN patients, but a few were seen in the other tumor cohorts, she noted.
“What was more interesting is this prolonged and high level of stable disease that we found in patients who had been previously treated with IO and had actually experienced significant clinical and radiographic progression then experienced stability with this combination,” she said, noting that “a fair amount of stable disease” was also seen in the IO-naive SCCHN and NSCLC patients.
In the immunotherapy-naive SCCHN patients, responses were seen regardless of human papillomavirus status, and four are still on treatment. One had a confirmed complete response.
“The majority of our patients on our trial and in this cohort were B7-H3-positive, she said, adding that the responses that were seen, including in those with stable disease, were “sustained and durable.”
Responses were similar in the immunotherapy-naive NSCLC patients who were PD-L1 negative, she said.
“Responses were seen irrespective of histology ... and many of these patients are still on treatment,” she added, noting that most were B7-H3-positive, which “seems to select our patients who have an even higher response rate of about 45%.”
The responses in that cohort also occurred early, were durable, and are sustained, and some patients remain on treatment and “are enjoying the clinical benefit afforded by this combination,” she said.
The findings are notable, because B7-H3 is highly expressed in many solid tumors, and monotherapy with enoblituzumab, which targets B7-H3 and is engineered to enhance antibody-dependent cell-mediated cytotoxicity, has demonstrated antitumor activity with an acceptable safety profile in patients with selected solid tumors.
In this study it was combined with pembrolizumab to test the hypothesis that coordinated engagement of both innate and adaptive immunity via the targeting of two distinct members of the B7 family could achieve greater antitumor activity than either agent alone, she explained.
The results “benchmark favorably” versus prior experience with PD-1 agents; pembrolizumab and nivolumab each lead to activity of about 13%-16%, she said.
“We acknowledge that our study has very small numbers. Nevertheless, these are encouraging data ... in this very tough-to-treat population,” Dr. Aggarwal said, adding that “further investigation of enoblituzumab with an anti-PD-1 molecule is warranted in both head and neck and lung cancer patients, perhaps including in combination with chemotherapy.”
Further, given the expression patterns of B7-H3 on a wide variety of solid tumors, further investigation of this combination ... is warranted in other tumor types, including in both checkpoint-naive and -treated populations, she concluded.
This study was sponsored by MacroGenics. Dr. Aggarwal reported receiving consulting fees from BMS.
SOURCE: Aggarwal C et al., SITC 2018 Abstract O24.
REPORTING FROM SITC 2018
Key clinical point: Enoblituzumab plus pembrolizumab shows promise in select patients with B7-H3-expressing solid tumors.
Major finding: The ORRs were 33.3% in IO-naive SCCHN patients and 35.7% in PD-L1-negative IO-naive NSCLC patients.
Study details: A phase 1 dose-escalation and expansion study of 133 patients.
Disclosures: This study was sponsored by MacroGenics. Dr. Aggarwal reported receiving consulting fees from BMS.
Source: Aggarwal C et al. SITC 2018 Abstract O24.
Granuloma Annulare: A Retrospective Series of 133 Patients
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).
There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).
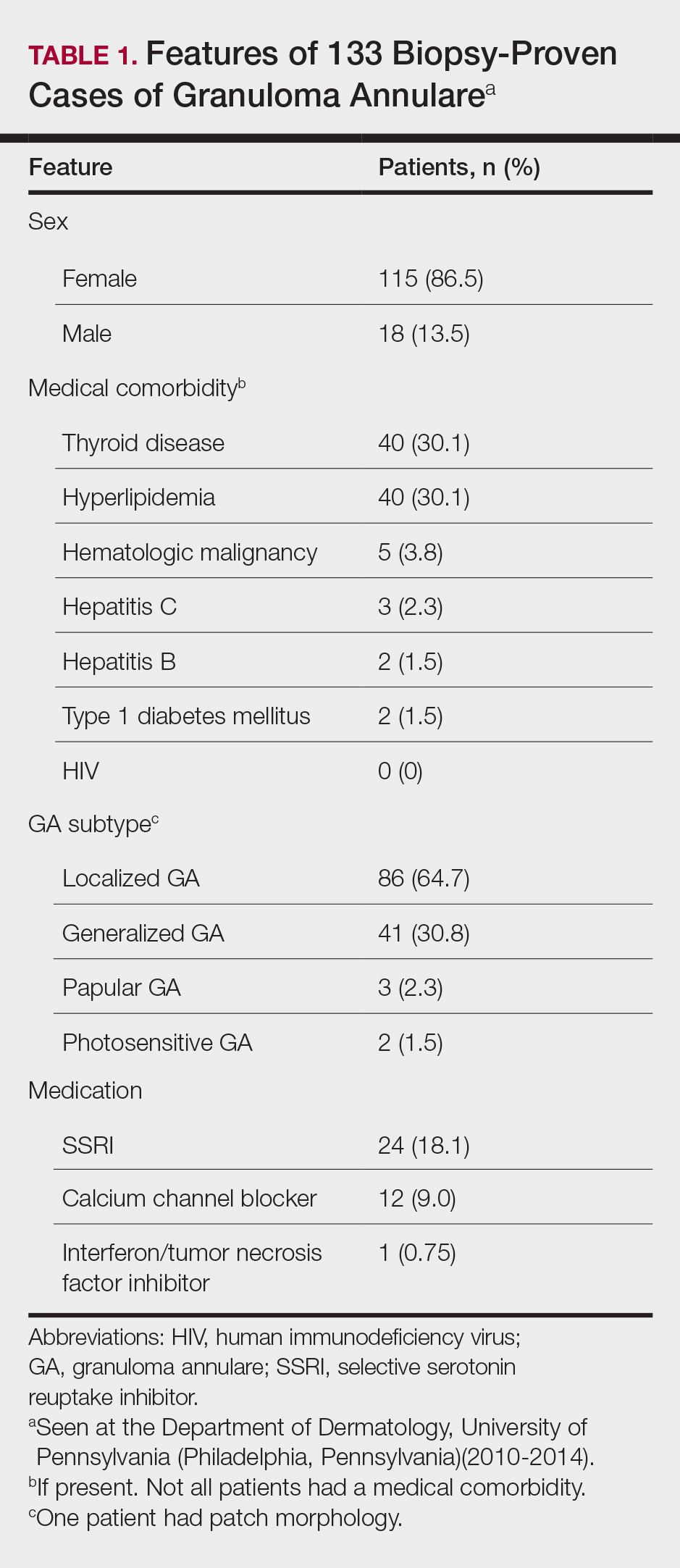
The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).
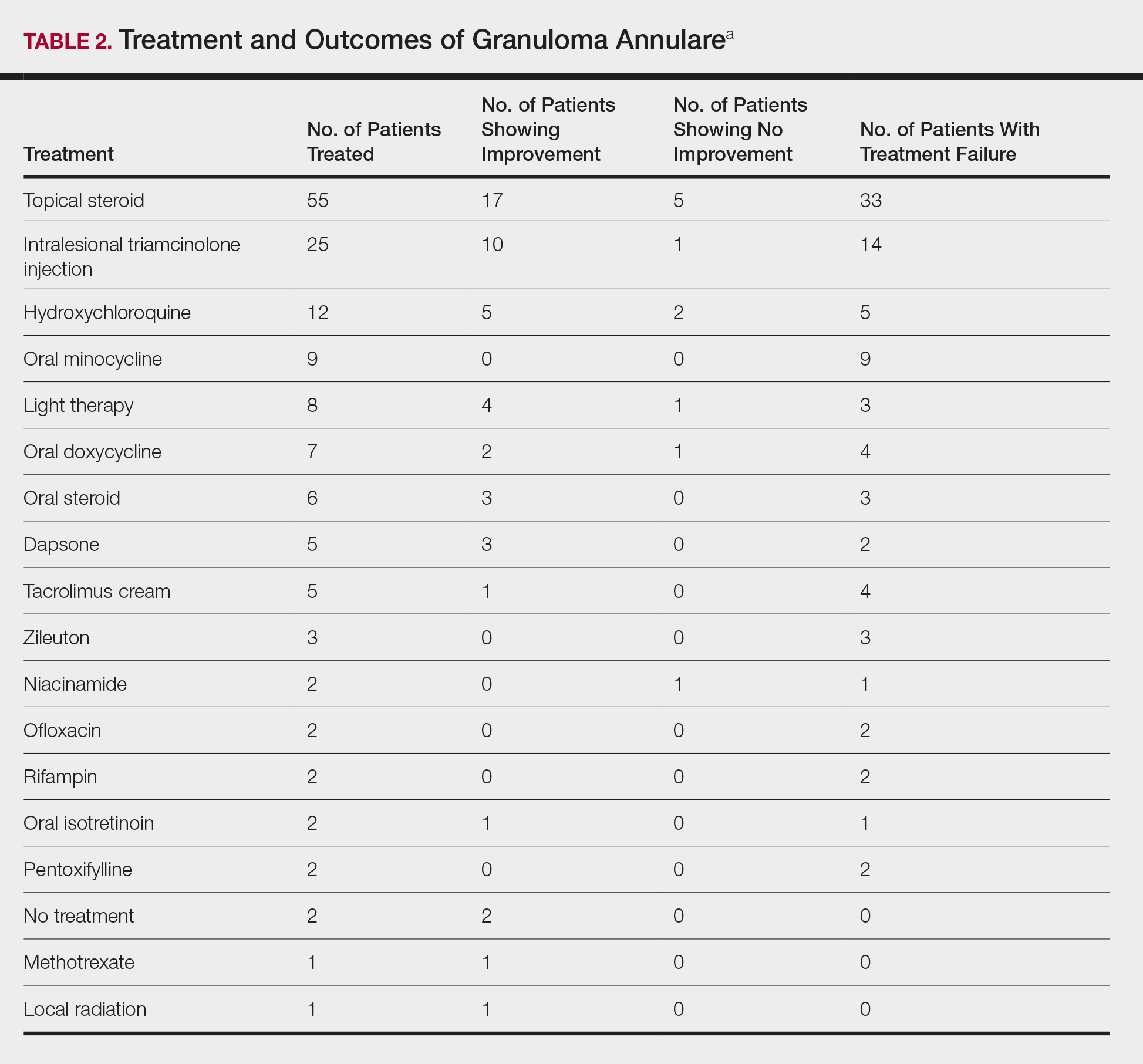
Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).

There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).

The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).

Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).

There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).

The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).

Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
Practice Points
- Although the pathogenesis of granuloma annulare (GA) is unknown, associations between the disorder and underlying systemic processes (eg, diabetes mellitus, hyperlipidemia, thyroid disease, human immunodeficiency virus) have been proposed.
- This study elicited a period prevalence of GA of 0.22% to 0.27%.
- The most commonly used treatments of GA were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine.
Therapy ups breast cancer survivors’ cardiac risks
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Oncologists should work with cardiologists to mitigate heart disease risk.
Major finding: Anthracyclines followed by trastuzumab significantly increase risk of HF.
Study details: Review of risk for heart disease in breast cancer survivors.
Disclosures: Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
Practice makes better: Robotic lobectomy can shorten OR times
A new single-surgeon study suggests that and shave about 90 minutes off adjusted operating time.
The findings provide “further support to the adaptation of formalized robotic training and credentialing procedures,” wrote the authors of the retrospective, single-center study, which was presented at the 2018 Academic Surgical Congress and published in Surgery.
According to the study authors, advantages of robotic surgery, compared with thoracoscopic surgery, include “3-dimensional visualization, enhanced maneuverability in small spaces, and the ease of the hilar and mediastinal dissection. Disadvantages include the lack of haptic feedback, increased cost, and increased operative time.”
In the new study, the authors, led by thoracic surgeon Brian N. Arnold, MD, of Yale University, New Haven, Conn., attempted to quantify the learning curve in RATS pulmonary lobectomies by using a more “statistically rigorous” technique than previous studies.
The study tracked 101 of 116 consecutive patients who underwent RATS pulmonary lobectomy at a single unnamed center from 2010 to 2016. Some patients, such as those who underwent a right middle lobectomy that is considered an easier procedure, were excluded. All patients were treated by the same unidentified surgeon.
Researchers identified three phases of the RATS learning curve: cases 1-22, cases 23-63, and cases 64-101.
On average, the patients were aged 69 years; 52% were female. Overall, a third of the patients developed complications.
After controlling for various factors, the researchers found that adjusted operating time and estimated blood loss were statistically different between the first and second phases (P less than .05 and P = .016, respectively). They were also different between the first and third phases (P less than .05 and P = .006, respectively).
Specifically, operating time in the first phase was a mean of 256 minutes versus 195 minutes in the second phase (P = .0002) and 168 minutes in the third phase (P less than .0001). Blood loss was 200 mL (interquartile range, 150-300 mL) in the first phase versus 150 mL (IQR, 75-200 mL; P = .0219) in the second phase and 150 mL (IQR, 100-150 mL; P = .0096) in the third phase.
The researchers found no statistically significant evidence that the surgeon’s growing experience affected length of stay, postoperative complications, chest tube duration, or conversion rate. No patients died within 30 or 90 days.
The researchers also compared operating time, length of stay, and complication rate in the RATS procedures with those in video-assisted thoracoscopic (VATS) lobectomies performed at the same institution from 2008 to 2014. There was only a statistically significant difference in mean operating time (RATS, 319 minutes; VATS, 253 minutes; P less than .001)
The study authors noted that the surgeon had extensive previous experience with VATS procedures. “Therefore, for better or for worse, the results may not apply to surgeons without this experience who move from open surgery to robotic surgery.”
Study funding and disclosures were not reported.
SOURCE: Arnold BN et al. Surgery. 2019 Feb;165(2):450-4.
A new single-surgeon study suggests that and shave about 90 minutes off adjusted operating time.
The findings provide “further support to the adaptation of formalized robotic training and credentialing procedures,” wrote the authors of the retrospective, single-center study, which was presented at the 2018 Academic Surgical Congress and published in Surgery.
According to the study authors, advantages of robotic surgery, compared with thoracoscopic surgery, include “3-dimensional visualization, enhanced maneuverability in small spaces, and the ease of the hilar and mediastinal dissection. Disadvantages include the lack of haptic feedback, increased cost, and increased operative time.”
In the new study, the authors, led by thoracic surgeon Brian N. Arnold, MD, of Yale University, New Haven, Conn., attempted to quantify the learning curve in RATS pulmonary lobectomies by using a more “statistically rigorous” technique than previous studies.
The study tracked 101 of 116 consecutive patients who underwent RATS pulmonary lobectomy at a single unnamed center from 2010 to 2016. Some patients, such as those who underwent a right middle lobectomy that is considered an easier procedure, were excluded. All patients were treated by the same unidentified surgeon.
Researchers identified three phases of the RATS learning curve: cases 1-22, cases 23-63, and cases 64-101.
On average, the patients were aged 69 years; 52% were female. Overall, a third of the patients developed complications.
After controlling for various factors, the researchers found that adjusted operating time and estimated blood loss were statistically different between the first and second phases (P less than .05 and P = .016, respectively). They were also different between the first and third phases (P less than .05 and P = .006, respectively).
Specifically, operating time in the first phase was a mean of 256 minutes versus 195 minutes in the second phase (P = .0002) and 168 minutes in the third phase (P less than .0001). Blood loss was 200 mL (interquartile range, 150-300 mL) in the first phase versus 150 mL (IQR, 75-200 mL; P = .0219) in the second phase and 150 mL (IQR, 100-150 mL; P = .0096) in the third phase.
The researchers found no statistically significant evidence that the surgeon’s growing experience affected length of stay, postoperative complications, chest tube duration, or conversion rate. No patients died within 30 or 90 days.
The researchers also compared operating time, length of stay, and complication rate in the RATS procedures with those in video-assisted thoracoscopic (VATS) lobectomies performed at the same institution from 2008 to 2014. There was only a statistically significant difference in mean operating time (RATS, 319 minutes; VATS, 253 minutes; P less than .001)
The study authors noted that the surgeon had extensive previous experience with VATS procedures. “Therefore, for better or for worse, the results may not apply to surgeons without this experience who move from open surgery to robotic surgery.”
Study funding and disclosures were not reported.
SOURCE: Arnold BN et al. Surgery. 2019 Feb;165(2):450-4.
A new single-surgeon study suggests that and shave about 90 minutes off adjusted operating time.
The findings provide “further support to the adaptation of formalized robotic training and credentialing procedures,” wrote the authors of the retrospective, single-center study, which was presented at the 2018 Academic Surgical Congress and published in Surgery.
According to the study authors, advantages of robotic surgery, compared with thoracoscopic surgery, include “3-dimensional visualization, enhanced maneuverability in small spaces, and the ease of the hilar and mediastinal dissection. Disadvantages include the lack of haptic feedback, increased cost, and increased operative time.”
In the new study, the authors, led by thoracic surgeon Brian N. Arnold, MD, of Yale University, New Haven, Conn., attempted to quantify the learning curve in RATS pulmonary lobectomies by using a more “statistically rigorous” technique than previous studies.
The study tracked 101 of 116 consecutive patients who underwent RATS pulmonary lobectomy at a single unnamed center from 2010 to 2016. Some patients, such as those who underwent a right middle lobectomy that is considered an easier procedure, were excluded. All patients were treated by the same unidentified surgeon.
Researchers identified three phases of the RATS learning curve: cases 1-22, cases 23-63, and cases 64-101.
On average, the patients were aged 69 years; 52% were female. Overall, a third of the patients developed complications.
After controlling for various factors, the researchers found that adjusted operating time and estimated blood loss were statistically different between the first and second phases (P less than .05 and P = .016, respectively). They were also different between the first and third phases (P less than .05 and P = .006, respectively).
Specifically, operating time in the first phase was a mean of 256 minutes versus 195 minutes in the second phase (P = .0002) and 168 minutes in the third phase (P less than .0001). Blood loss was 200 mL (interquartile range, 150-300 mL) in the first phase versus 150 mL (IQR, 75-200 mL; P = .0219) in the second phase and 150 mL (IQR, 100-150 mL; P = .0096) in the third phase.
The researchers found no statistically significant evidence that the surgeon’s growing experience affected length of stay, postoperative complications, chest tube duration, or conversion rate. No patients died within 30 or 90 days.
The researchers also compared operating time, length of stay, and complication rate in the RATS procedures with those in video-assisted thoracoscopic (VATS) lobectomies performed at the same institution from 2008 to 2014. There was only a statistically significant difference in mean operating time (RATS, 319 minutes; VATS, 253 minutes; P less than .001)
The study authors noted that the surgeon had extensive previous experience with VATS procedures. “Therefore, for better or for worse, the results may not apply to surgeons without this experience who move from open surgery to robotic surgery.”
Study funding and disclosures were not reported.
SOURCE: Arnold BN et al. Surgery. 2019 Feb;165(2):450-4.
FROM SURGERY
Key clinical point: Extensive experience in robot-assisted thoracoscopic (RATS) pulmonary lobectomies could lead to dramatically shorter adjusted operating time.
Major finding: From a surgeon’s first 22 surgeries to cases 64-101, mean operating time fell from 256 minutes to 168 minutes, (P less than .05).
Study details: A retrospective, single-center, single-surgeon study of 101 patients who underwent robot-assisted thoracoscopic pulmonary lobectomies from 2010 to 2016.
Disclosures: Study funding and disclosures were not reported.
Source: Arnold BN et al. Surgery. 2019 Feb;165(2):450-4.