User login
Negative colonoscopy linked with lower risk for more than 10 years
A negative colonoscopy result is associated with a reduced risk of colorectal cancer for more than 12 years after the examination, compared with an unscreened population, new research has found.
In a retrospective cohort study published in JAMA Internal Medicine, researchers analyzed data from 1,251,318 individuals at average risk of colorectal cancer who were eligible to participate in screening over more than 9 million person-years of follow-up.
They found that screened individuals with a negative result had an adjusted 46%-95% lower risk of colorectal cancer and 29%-96% lower risk of colorectal cancer mortality than unscreened individuals across more than 12 years of follow-up.
At 10 years post colonoscopy, participants who had a negative colonoscopy result still had a significant 46% lower risk of colorectal cancer (hazard ratio [HR], 0.54; 95% [confidence interval] CI: 0.31-0.94) and 88% lower risk of colorectal cancer mortality (HR, 0.12; 95% CI: 0.02-0.82). After more than 12 years, there was still a nonsignificant trend toward a lower risk of colorectal cancer incidence and mortality.
Jeffrey K. Lee, MD, of Kaiser Permanente San Francisco, and his coauthors suggested that their findings have implications for the timing of rescreening after a negative colonoscopy result.
“The current guideline-recommended 10-year rescreening interval is not based on a predetermined risk threshold, and while we observed a reduced risk of colorectal cancer and related deaths throughout the more than 12-year follow-up period, an examination of absolute risk [incidence] could provide another justification for the timing for rescreening,” they wrote. “Additional research is needed to evaluate the costs and benefits of earlier versus later rescreening, optimal rescreening tests following a negative colonoscopy result [e.g., another colonoscopy versus annual fecal immunochemical testing], and whether the benefits of rescreening vary between subgroups.”
The study showed that the rate of repeat endoscopic procedures increased at year 10, largely because of screening colonoscopies which are recommended at 10-year intervals. However, in a separate analysis, the authors excluded colonoscopies for a screening indication and still found a similar reduction in the risk of colorectal cancer, compared with the unscreened group.
The data also showed a 22%-87% lower risk of proximal colorectal cancer, a 50%-99% lower risk of distal cancer, a 31%-95% lower risk of early-stage colorectal cancer, and a 56%-96% reduced risk of advanced-stage colorectal cancer among those who had a negative result, compared with those who did not undergo screening.
The authors wrote that this pattern of greater risk reductions in the distal versus proximal cancer had been seen in previous studies and could be the result of incomplete examinations, inadequate bowel cleansing, challenges in identifying right colon polyps and sessile serrated adenomas, or differences in polyp biology in the proximal versus distal colon.
The incidence rates of colorectal cancer among those who had a negative result from colonoscopy ranged from 16.6 per 100,000 person-years (95% CI: 8.2-12.8) at 1 year after screening to 133.2 per 100,000 person-years (95% CI: 70.9-227.8) at the 10-year mark. In comparison, the incidence rates among the unscreened population increased from 62.9 per 100,000 person-years (95% CI: 55.7-70.0) at year 1 to 224.8 per 100,000 person-years (95% CI: 202.5-247.0) after year 12.
Mortality rates from colorectal cancer at year 1 were 6.8 per 100,000 person-years (95% CI: 0.8-12.7) in the negative results group and 10.5 (95% CI: 8.2-12.8) in the unscreened cohort. At year 12, that figure was 92.2 per 100,000 person-years (95% CI: 19.0-165.4) in the negative results cohort, while after year 12 in the unscreened cohort, colorectal cancer mortality rates increased to 192 per 100,000 person years (95% CI: 7-214.3).
While the study made use of a validated cancer registry to ensure they accurately captured cancers and mortality, the authors acknowledged that they weren’t able to adjust for residual confounding factors such as red-meat intake or smoking.
The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
The AGA GI Patient Center provides education materials that can help your patients better understand their colorectal cancer risk and prepare for a colonoscopy. Visit patient.gastro.org to review and download.
SOURCE: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
A negative colonoscopy result is associated with a reduced risk of colorectal cancer for more than 12 years after the examination, compared with an unscreened population, new research has found.
In a retrospective cohort study published in JAMA Internal Medicine, researchers analyzed data from 1,251,318 individuals at average risk of colorectal cancer who were eligible to participate in screening over more than 9 million person-years of follow-up.
They found that screened individuals with a negative result had an adjusted 46%-95% lower risk of colorectal cancer and 29%-96% lower risk of colorectal cancer mortality than unscreened individuals across more than 12 years of follow-up.
At 10 years post colonoscopy, participants who had a negative colonoscopy result still had a significant 46% lower risk of colorectal cancer (hazard ratio [HR], 0.54; 95% [confidence interval] CI: 0.31-0.94) and 88% lower risk of colorectal cancer mortality (HR, 0.12; 95% CI: 0.02-0.82). After more than 12 years, there was still a nonsignificant trend toward a lower risk of colorectal cancer incidence and mortality.
Jeffrey K. Lee, MD, of Kaiser Permanente San Francisco, and his coauthors suggested that their findings have implications for the timing of rescreening after a negative colonoscopy result.
“The current guideline-recommended 10-year rescreening interval is not based on a predetermined risk threshold, and while we observed a reduced risk of colorectal cancer and related deaths throughout the more than 12-year follow-up period, an examination of absolute risk [incidence] could provide another justification for the timing for rescreening,” they wrote. “Additional research is needed to evaluate the costs and benefits of earlier versus later rescreening, optimal rescreening tests following a negative colonoscopy result [e.g., another colonoscopy versus annual fecal immunochemical testing], and whether the benefits of rescreening vary between subgroups.”
The study showed that the rate of repeat endoscopic procedures increased at year 10, largely because of screening colonoscopies which are recommended at 10-year intervals. However, in a separate analysis, the authors excluded colonoscopies for a screening indication and still found a similar reduction in the risk of colorectal cancer, compared with the unscreened group.
The data also showed a 22%-87% lower risk of proximal colorectal cancer, a 50%-99% lower risk of distal cancer, a 31%-95% lower risk of early-stage colorectal cancer, and a 56%-96% reduced risk of advanced-stage colorectal cancer among those who had a negative result, compared with those who did not undergo screening.
The authors wrote that this pattern of greater risk reductions in the distal versus proximal cancer had been seen in previous studies and could be the result of incomplete examinations, inadequate bowel cleansing, challenges in identifying right colon polyps and sessile serrated adenomas, or differences in polyp biology in the proximal versus distal colon.
The incidence rates of colorectal cancer among those who had a negative result from colonoscopy ranged from 16.6 per 100,000 person-years (95% CI: 8.2-12.8) at 1 year after screening to 133.2 per 100,000 person-years (95% CI: 70.9-227.8) at the 10-year mark. In comparison, the incidence rates among the unscreened population increased from 62.9 per 100,000 person-years (95% CI: 55.7-70.0) at year 1 to 224.8 per 100,000 person-years (95% CI: 202.5-247.0) after year 12.
Mortality rates from colorectal cancer at year 1 were 6.8 per 100,000 person-years (95% CI: 0.8-12.7) in the negative results group and 10.5 (95% CI: 8.2-12.8) in the unscreened cohort. At year 12, that figure was 92.2 per 100,000 person-years (95% CI: 19.0-165.4) in the negative results cohort, while after year 12 in the unscreened cohort, colorectal cancer mortality rates increased to 192 per 100,000 person years (95% CI: 7-214.3).
While the study made use of a validated cancer registry to ensure they accurately captured cancers and mortality, the authors acknowledged that they weren’t able to adjust for residual confounding factors such as red-meat intake or smoking.
The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
The AGA GI Patient Center provides education materials that can help your patients better understand their colorectal cancer risk and prepare for a colonoscopy. Visit patient.gastro.org to review and download.
SOURCE: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
A negative colonoscopy result is associated with a reduced risk of colorectal cancer for more than 12 years after the examination, compared with an unscreened population, new research has found.
In a retrospective cohort study published in JAMA Internal Medicine, researchers analyzed data from 1,251,318 individuals at average risk of colorectal cancer who were eligible to participate in screening over more than 9 million person-years of follow-up.
They found that screened individuals with a negative result had an adjusted 46%-95% lower risk of colorectal cancer and 29%-96% lower risk of colorectal cancer mortality than unscreened individuals across more than 12 years of follow-up.
At 10 years post colonoscopy, participants who had a negative colonoscopy result still had a significant 46% lower risk of colorectal cancer (hazard ratio [HR], 0.54; 95% [confidence interval] CI: 0.31-0.94) and 88% lower risk of colorectal cancer mortality (HR, 0.12; 95% CI: 0.02-0.82). After more than 12 years, there was still a nonsignificant trend toward a lower risk of colorectal cancer incidence and mortality.
Jeffrey K. Lee, MD, of Kaiser Permanente San Francisco, and his coauthors suggested that their findings have implications for the timing of rescreening after a negative colonoscopy result.
“The current guideline-recommended 10-year rescreening interval is not based on a predetermined risk threshold, and while we observed a reduced risk of colorectal cancer and related deaths throughout the more than 12-year follow-up period, an examination of absolute risk [incidence] could provide another justification for the timing for rescreening,” they wrote. “Additional research is needed to evaluate the costs and benefits of earlier versus later rescreening, optimal rescreening tests following a negative colonoscopy result [e.g., another colonoscopy versus annual fecal immunochemical testing], and whether the benefits of rescreening vary between subgroups.”
The study showed that the rate of repeat endoscopic procedures increased at year 10, largely because of screening colonoscopies which are recommended at 10-year intervals. However, in a separate analysis, the authors excluded colonoscopies for a screening indication and still found a similar reduction in the risk of colorectal cancer, compared with the unscreened group.
The data also showed a 22%-87% lower risk of proximal colorectal cancer, a 50%-99% lower risk of distal cancer, a 31%-95% lower risk of early-stage colorectal cancer, and a 56%-96% reduced risk of advanced-stage colorectal cancer among those who had a negative result, compared with those who did not undergo screening.
The authors wrote that this pattern of greater risk reductions in the distal versus proximal cancer had been seen in previous studies and could be the result of incomplete examinations, inadequate bowel cleansing, challenges in identifying right colon polyps and sessile serrated adenomas, or differences in polyp biology in the proximal versus distal colon.
The incidence rates of colorectal cancer among those who had a negative result from colonoscopy ranged from 16.6 per 100,000 person-years (95% CI: 8.2-12.8) at 1 year after screening to 133.2 per 100,000 person-years (95% CI: 70.9-227.8) at the 10-year mark. In comparison, the incidence rates among the unscreened population increased from 62.9 per 100,000 person-years (95% CI: 55.7-70.0) at year 1 to 224.8 per 100,000 person-years (95% CI: 202.5-247.0) after year 12.
Mortality rates from colorectal cancer at year 1 were 6.8 per 100,000 person-years (95% CI: 0.8-12.7) in the negative results group and 10.5 (95% CI: 8.2-12.8) in the unscreened cohort. At year 12, that figure was 92.2 per 100,000 person-years (95% CI: 19.0-165.4) in the negative results cohort, while after year 12 in the unscreened cohort, colorectal cancer mortality rates increased to 192 per 100,000 person years (95% CI: 7-214.3).
While the study made use of a validated cancer registry to ensure they accurately captured cancers and mortality, the authors acknowledged that they weren’t able to adjust for residual confounding factors such as red-meat intake or smoking.
The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
The AGA GI Patient Center provides education materials that can help your patients better understand their colorectal cancer risk and prepare for a colonoscopy. Visit patient.gastro.org to review and download.
SOURCE: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Reduced rates of colorectal cancer after a negative colonoscopy persist beyond 10 years.
Major finding: After 10 years, a negative colonoscopy is still associated with an 88% lower risk of colorectal cancer mortality, compared with the unscreened.
Study details: A retrospective cohort study in 1,251,318 screening-eligible individuals.
Disclosures: The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
Source: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
Rheumatologist prescribing rates predict chronic opioid use in RA patients
CHICAGO – A physician’s baseline opioid prescribing rate strongly predicts future chronic opioid use in rheumatoid arthritis patients, an analysis of data from the Consortium of Rheumatology Researchers of North America (Corrona) Rheumatoid Arthritis Registry suggests.
The baseline 12-month opioid prescribing rate of 148 physicians in the initial cohort varied widely from 0% to 70% (median, 27%), and among 9,337 patients in the registry beyond the baseline 12 months, physician opioid prescribing rates during the baseline period were significantly associated with risk for chronic opioid use, Yvonne C. Lee, MD, reported at the annual meeting of the American College of Rheumatology. She and her colleagues defined chronic opioid use as any opioid use during at least two consecutive study visits.
“It is important to understand the relative contributions of patient vs. physician characteristics on chronic opioid use,” said Dr. Lee of Northwestern University, Chicago. She that the goals of the current study were to identify the extent to which rheumatologists in the United States varied in baseline opioid prescribing rates and to determine the implications of baseline prescribing rates with respect to future chronic opioid use.
Compared with the lowest quartile of baseline opioid prescribing (rate of 18% or less), the second, third, and fourth quartiles of prescribing were associated with increasing odds of chronic opioid use (odds ratios of 1.16, 1.89, and 2.01 for the quartiles, respectively) during the study period, she said.
The researchers saw similar relationships when they used a stricter definition of opioid use and when they extended the cutoff between the baseline and study periods to 18 months. The relationships persisted after adjusting for numerous patient characteristics, such as age, sex, race, insurance status, RA duration, and treatments used, she said.
Subgroup analyses were also conducted to examine heterogeneity across clinical characteristics, including Clinical Disease Activity Index score (10 or less vs. greater than 10), pain intensity (scores of 40 or less, greater than 40 to 60, and greater than 60 out of 100), and use vs. nonuse of antidepressant medication. The relationships between physician baseline prescribing and chronic opioid use were similar across subgroups, she noted.
The findings help to characterize the role of rheumatologists’ prescribing rates in the ongoing opioid crisis even though the conclusions that can be reached are limited by the fact that some patients may receive opioid prescriptions from physicians outside the registry, by a lack of data on specific opioid types and doses, and by a lack of detailed information about physician characteristics, Dr. Lee said.
Physicians were included in the analysis only if they had contributed at least 10 RA patients to the registry within their first year of participation, and patients were included if they were patients of those physicians, if they had at least 12 months of follow-up data available, and if they were not prevalent opioid users at study entry.
A long-term goal is to target interventions to appropriate subgroups, she said, noting that 21%-29% of patients who are prescribed opioids for chronic pain misuse them, and more than 33,000 Americans die of opioid overdoses each year.
“Implications [of the findings] are that, in addition to targeting patients, we may also really want to consider interventions that target high-intensity prescribers. This may be useful for helping to decrease chronic opioid use in patients,” she concluded.
Dr. Lee has an investigator-initiated grant from Pfizer and owns stock in Express Scripts.
SOURCE: Lee Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1917
CHICAGO – A physician’s baseline opioid prescribing rate strongly predicts future chronic opioid use in rheumatoid arthritis patients, an analysis of data from the Consortium of Rheumatology Researchers of North America (Corrona) Rheumatoid Arthritis Registry suggests.
The baseline 12-month opioid prescribing rate of 148 physicians in the initial cohort varied widely from 0% to 70% (median, 27%), and among 9,337 patients in the registry beyond the baseline 12 months, physician opioid prescribing rates during the baseline period were significantly associated with risk for chronic opioid use, Yvonne C. Lee, MD, reported at the annual meeting of the American College of Rheumatology. She and her colleagues defined chronic opioid use as any opioid use during at least two consecutive study visits.
“It is important to understand the relative contributions of patient vs. physician characteristics on chronic opioid use,” said Dr. Lee of Northwestern University, Chicago. She that the goals of the current study were to identify the extent to which rheumatologists in the United States varied in baseline opioid prescribing rates and to determine the implications of baseline prescribing rates with respect to future chronic opioid use.
Compared with the lowest quartile of baseline opioid prescribing (rate of 18% or less), the second, third, and fourth quartiles of prescribing were associated with increasing odds of chronic opioid use (odds ratios of 1.16, 1.89, and 2.01 for the quartiles, respectively) during the study period, she said.
The researchers saw similar relationships when they used a stricter definition of opioid use and when they extended the cutoff between the baseline and study periods to 18 months. The relationships persisted after adjusting for numerous patient characteristics, such as age, sex, race, insurance status, RA duration, and treatments used, she said.
Subgroup analyses were also conducted to examine heterogeneity across clinical characteristics, including Clinical Disease Activity Index score (10 or less vs. greater than 10), pain intensity (scores of 40 or less, greater than 40 to 60, and greater than 60 out of 100), and use vs. nonuse of antidepressant medication. The relationships between physician baseline prescribing and chronic opioid use were similar across subgroups, she noted.
The findings help to characterize the role of rheumatologists’ prescribing rates in the ongoing opioid crisis even though the conclusions that can be reached are limited by the fact that some patients may receive opioid prescriptions from physicians outside the registry, by a lack of data on specific opioid types and doses, and by a lack of detailed information about physician characteristics, Dr. Lee said.
Physicians were included in the analysis only if they had contributed at least 10 RA patients to the registry within their first year of participation, and patients were included if they were patients of those physicians, if they had at least 12 months of follow-up data available, and if they were not prevalent opioid users at study entry.
A long-term goal is to target interventions to appropriate subgroups, she said, noting that 21%-29% of patients who are prescribed opioids for chronic pain misuse them, and more than 33,000 Americans die of opioid overdoses each year.
“Implications [of the findings] are that, in addition to targeting patients, we may also really want to consider interventions that target high-intensity prescribers. This may be useful for helping to decrease chronic opioid use in patients,” she concluded.
Dr. Lee has an investigator-initiated grant from Pfizer and owns stock in Express Scripts.
SOURCE: Lee Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1917
CHICAGO – A physician’s baseline opioid prescribing rate strongly predicts future chronic opioid use in rheumatoid arthritis patients, an analysis of data from the Consortium of Rheumatology Researchers of North America (Corrona) Rheumatoid Arthritis Registry suggests.
The baseline 12-month opioid prescribing rate of 148 physicians in the initial cohort varied widely from 0% to 70% (median, 27%), and among 9,337 patients in the registry beyond the baseline 12 months, physician opioid prescribing rates during the baseline period were significantly associated with risk for chronic opioid use, Yvonne C. Lee, MD, reported at the annual meeting of the American College of Rheumatology. She and her colleagues defined chronic opioid use as any opioid use during at least two consecutive study visits.
“It is important to understand the relative contributions of patient vs. physician characteristics on chronic opioid use,” said Dr. Lee of Northwestern University, Chicago. She that the goals of the current study were to identify the extent to which rheumatologists in the United States varied in baseline opioid prescribing rates and to determine the implications of baseline prescribing rates with respect to future chronic opioid use.
Compared with the lowest quartile of baseline opioid prescribing (rate of 18% or less), the second, third, and fourth quartiles of prescribing were associated with increasing odds of chronic opioid use (odds ratios of 1.16, 1.89, and 2.01 for the quartiles, respectively) during the study period, she said.
The researchers saw similar relationships when they used a stricter definition of opioid use and when they extended the cutoff between the baseline and study periods to 18 months. The relationships persisted after adjusting for numerous patient characteristics, such as age, sex, race, insurance status, RA duration, and treatments used, she said.
Subgroup analyses were also conducted to examine heterogeneity across clinical characteristics, including Clinical Disease Activity Index score (10 or less vs. greater than 10), pain intensity (scores of 40 or less, greater than 40 to 60, and greater than 60 out of 100), and use vs. nonuse of antidepressant medication. The relationships between physician baseline prescribing and chronic opioid use were similar across subgroups, she noted.
The findings help to characterize the role of rheumatologists’ prescribing rates in the ongoing opioid crisis even though the conclusions that can be reached are limited by the fact that some patients may receive opioid prescriptions from physicians outside the registry, by a lack of data on specific opioid types and doses, and by a lack of detailed information about physician characteristics, Dr. Lee said.
Physicians were included in the analysis only if they had contributed at least 10 RA patients to the registry within their first year of participation, and patients were included if they were patients of those physicians, if they had at least 12 months of follow-up data available, and if they were not prevalent opioid users at study entry.
A long-term goal is to target interventions to appropriate subgroups, she said, noting that 21%-29% of patients who are prescribed opioids for chronic pain misuse them, and more than 33,000 Americans die of opioid overdoses each year.
“Implications [of the findings] are that, in addition to targeting patients, we may also really want to consider interventions that target high-intensity prescribers. This may be useful for helping to decrease chronic opioid use in patients,” she concluded.
Dr. Lee has an investigator-initiated grant from Pfizer and owns stock in Express Scripts.
SOURCE: Lee Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1917
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The odds of chronic opioid use increased with rising baseline prescribing rates (odds ratios, 1.16, 1.89, and 2.01 for 2nd, 3rd, and 4th quartiles vs. 1st quartile of prescribing, respectively).
Study details: An analysis of data from 148 physicians and 9,337 Corrona RA Registry patients.
Disclosures: Dr. Lee has an investigator-initiated grant from Pfizer and owns stock in Express Scripts.
Source: Lee Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1917
USPSTF issues draft research plan on opioid use disorder prevention
The U.S. Preventive Services Task Force posted a draft research plan on opioid use disorder prevention for public comment on Dec. 13, according to a USPSTF bulletin.
An estimated 2.1 million persons aged 12 years and older had an opioid use disorder in 2017, and opioids were involved in nearly two-thirds of more than 70,000 fatal drug overdoses, according to the task force. Prevention of unnecessary opioid use, opioid misuse, and opioid use disorder in primary care settings is necessary to effectively respond to the ongoing crisis.
In an extensive literature review, an independent research team will look for evidence on strategies for people not currently receiving opioids that can be implemented on the primary care level. The public is invited to submit comments on the research plan that will help focus and guide the literature review.
The USPSTF also will be updating the current 2008 recommendation statement on screening for illicit drugs and nonmedical prescription drugs in adolescents and young adults, including pregnant and postpartum women.
Comments can be submitted until Jan. 16, 2019, on the USPSTF website.
The U.S. Preventive Services Task Force posted a draft research plan on opioid use disorder prevention for public comment on Dec. 13, according to a USPSTF bulletin.
An estimated 2.1 million persons aged 12 years and older had an opioid use disorder in 2017, and opioids were involved in nearly two-thirds of more than 70,000 fatal drug overdoses, according to the task force. Prevention of unnecessary opioid use, opioid misuse, and opioid use disorder in primary care settings is necessary to effectively respond to the ongoing crisis.
In an extensive literature review, an independent research team will look for evidence on strategies for people not currently receiving opioids that can be implemented on the primary care level. The public is invited to submit comments on the research plan that will help focus and guide the literature review.
The USPSTF also will be updating the current 2008 recommendation statement on screening for illicit drugs and nonmedical prescription drugs in adolescents and young adults, including pregnant and postpartum women.
Comments can be submitted until Jan. 16, 2019, on the USPSTF website.
The U.S. Preventive Services Task Force posted a draft research plan on opioid use disorder prevention for public comment on Dec. 13, according to a USPSTF bulletin.
An estimated 2.1 million persons aged 12 years and older had an opioid use disorder in 2017, and opioids were involved in nearly two-thirds of more than 70,000 fatal drug overdoses, according to the task force. Prevention of unnecessary opioid use, opioid misuse, and opioid use disorder in primary care settings is necessary to effectively respond to the ongoing crisis.
In an extensive literature review, an independent research team will look for evidence on strategies for people not currently receiving opioids that can be implemented on the primary care level. The public is invited to submit comments on the research plan that will help focus and guide the literature review.
The USPSTF also will be updating the current 2008 recommendation statement on screening for illicit drugs and nonmedical prescription drugs in adolescents and young adults, including pregnant and postpartum women.
Comments can be submitted until Jan. 16, 2019, on the USPSTF website.
Altered Speech Examined in Persons with Migraine
Changes in speech occurred in almost half of individuals experiencing migraine attacks who were evaluated in a recent prospective, longitudinal, observational study. Participants provided speech samples 3 times per day using a speech elicitation tool included within a mobile app. Six complementary speech features that capture articulation and prosody were extracted from speech samples. Participants with migraine maintained a daily headache diary using the same app. A total of 56,767 speech samples were collected, including 43,102 from 15 individuals with migraine and 13,665 from matched healthy controls. They found:
- Significant group-level differences in speech features were identified between those with migraine and healthy controls and within the migraine group during the pre-attack vs attack vs interictal periods.
- Most consistently, speech changes occurred in the speaking rate, articulation rate and precision, and phonatory duration.
- Within-subject analysis revealed that 7 of 15 individuals with migraine showed significant change in at least 1 speech feature when comparing the migraine attack vs interictal phase and 4 showed similar changes when comparing the pre-attack vs interictal phases.
Schwedt TJ, Peplinski J, Garcia-Filion P, Berisha V. Altered speech with migraine attacks: A prospective, longitudinal study of episodic migraine without aura. [Published online ahead of print November 17, 2018]. Cephalalgia. doi:10.1177%2F0333102418815505.
Changes in speech occurred in almost half of individuals experiencing migraine attacks who were evaluated in a recent prospective, longitudinal, observational study. Participants provided speech samples 3 times per day using a speech elicitation tool included within a mobile app. Six complementary speech features that capture articulation and prosody were extracted from speech samples. Participants with migraine maintained a daily headache diary using the same app. A total of 56,767 speech samples were collected, including 43,102 from 15 individuals with migraine and 13,665 from matched healthy controls. They found:
- Significant group-level differences in speech features were identified between those with migraine and healthy controls and within the migraine group during the pre-attack vs attack vs interictal periods.
- Most consistently, speech changes occurred in the speaking rate, articulation rate and precision, and phonatory duration.
- Within-subject analysis revealed that 7 of 15 individuals with migraine showed significant change in at least 1 speech feature when comparing the migraine attack vs interictal phase and 4 showed similar changes when comparing the pre-attack vs interictal phases.
Schwedt TJ, Peplinski J, Garcia-Filion P, Berisha V. Altered speech with migraine attacks: A prospective, longitudinal study of episodic migraine without aura. [Published online ahead of print November 17, 2018]. Cephalalgia. doi:10.1177%2F0333102418815505.
Changes in speech occurred in almost half of individuals experiencing migraine attacks who were evaluated in a recent prospective, longitudinal, observational study. Participants provided speech samples 3 times per day using a speech elicitation tool included within a mobile app. Six complementary speech features that capture articulation and prosody were extracted from speech samples. Participants with migraine maintained a daily headache diary using the same app. A total of 56,767 speech samples were collected, including 43,102 from 15 individuals with migraine and 13,665 from matched healthy controls. They found:
- Significant group-level differences in speech features were identified between those with migraine and healthy controls and within the migraine group during the pre-attack vs attack vs interictal periods.
- Most consistently, speech changes occurred in the speaking rate, articulation rate and precision, and phonatory duration.
- Within-subject analysis revealed that 7 of 15 individuals with migraine showed significant change in at least 1 speech feature when comparing the migraine attack vs interictal phase and 4 showed similar changes when comparing the pre-attack vs interictal phases.
Schwedt TJ, Peplinski J, Garcia-Filion P, Berisha V. Altered speech with migraine attacks: A prospective, longitudinal study of episodic migraine without aura. [Published online ahead of print November 17, 2018]. Cephalalgia. doi:10.1177%2F0333102418815505.
Joint guidelines offer recommendations for treating peripheral artery disease
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New diabetes guidelines downgrade insulin as first-line injectable treatment
The American Diabetes Association is out with new standard-of-care guidelines that – among other things – reject injectable insulin as the main first-line treatment for type 2 diabetes mellitus (T2DM), debut a cardiac risk calculator, and offer new recommendations regarding medications for patients with kidney disease, clogged arteries, and heart failure.
The ADA’s newly released 2019 Standards of Medical Care in Diabetes “emphasize a patient-centered approach that considers the multiple health and life factors of each person living with diabetes,” said William T. Cefalu, MD, the ADA’s chief scientific, medical, and mission officer, in a statement.
The 193-page guidelines are now available online at the Diabetes Care website and will be available via an app and the print edition of the journal.
Here’s a closer look at a few of the many new and revised recommendations in the 2019 Standards of Care.
Diabetes treatment
In a new guideline, the standards of care says glucagonlike peptide–1 (GLP-1) receptor agonists should be “a first-line treatment” – ahead of insulin – “for most [type 2] patients who need the greater efficacy of an injectable medication.”
However, the recommendations note that the “high costs and tolerability issues are important barriers to the use of GLP-1 receptor agonists.”
A new recommendation suggests the use of sodium-glucose cotransporter 2 inhibitors or GLP-1 receptor agonists “with demonstrated cardiovascular disease benefit” in patients with type 2 diabetes who have confirmed atherosclerotic cardiovascular disease.
A related new recommendation says sodium-glucose cotransporter 2 inhibitors are the preferred treatment for these patients who have heart failure or are at high risk of developing it.
In a new recommendation, the ADA suggests that patients with type 2 diabetes and chronic kidney disease potentially take a sodium-glucose cotransporter 2 inhibitor or a GLP-1 receptor agonist, which has been shown to reduce the risk of chronic kidney disease progression, cardiac events, or both.
There’s a greater focus on insulin as the preferred treatment for hyperglycemia in gestational diabetes mellitus “as it does not cross the placenta to a measurable extent.” The ADA also warns against metformin and glyburide as first-line agents because they “both cross the placenta to the fetus.”
Diabetes monitoring and screening
The ADA now recommends use of the American College of Cardiology’s atherosclerotic cardiovascular disease risk calculator, the ASCVD Risk Estimator Plus. The calculator assesses the risk of this disease over 10 years and is “generally a useful tool.”
The ACA recommends screening for cardiac risk factors at least once a year in patients with diabetes.
Physicians are no longer advised to check the feet of patients with diabetes at every visit; now the recommendation is for those at high risk of ulceration only. However, an annual examination of feet is recommended for all patients with diabetes.
The ADA now recommends that patients with type 2 diabetes or prediabetes undergo screening for nonalcoholic steatohepatitis and liver fibrosis if they have elevated liver enzymes or an ultrasound examination shows signs of fatty liver.
Gabapentin is now listed along with pregabalin and duloxetine as first-line drug treatments for neuropathic pain in diabetes.
The American Diabetes Association is out with new standard-of-care guidelines that – among other things – reject injectable insulin as the main first-line treatment for type 2 diabetes mellitus (T2DM), debut a cardiac risk calculator, and offer new recommendations regarding medications for patients with kidney disease, clogged arteries, and heart failure.
The ADA’s newly released 2019 Standards of Medical Care in Diabetes “emphasize a patient-centered approach that considers the multiple health and life factors of each person living with diabetes,” said William T. Cefalu, MD, the ADA’s chief scientific, medical, and mission officer, in a statement.
The 193-page guidelines are now available online at the Diabetes Care website and will be available via an app and the print edition of the journal.
Here’s a closer look at a few of the many new and revised recommendations in the 2019 Standards of Care.
Diabetes treatment
In a new guideline, the standards of care says glucagonlike peptide–1 (GLP-1) receptor agonists should be “a first-line treatment” – ahead of insulin – “for most [type 2] patients who need the greater efficacy of an injectable medication.”
However, the recommendations note that the “high costs and tolerability issues are important barriers to the use of GLP-1 receptor agonists.”
A new recommendation suggests the use of sodium-glucose cotransporter 2 inhibitors or GLP-1 receptor agonists “with demonstrated cardiovascular disease benefit” in patients with type 2 diabetes who have confirmed atherosclerotic cardiovascular disease.
A related new recommendation says sodium-glucose cotransporter 2 inhibitors are the preferred treatment for these patients who have heart failure or are at high risk of developing it.
In a new recommendation, the ADA suggests that patients with type 2 diabetes and chronic kidney disease potentially take a sodium-glucose cotransporter 2 inhibitor or a GLP-1 receptor agonist, which has been shown to reduce the risk of chronic kidney disease progression, cardiac events, or both.
There’s a greater focus on insulin as the preferred treatment for hyperglycemia in gestational diabetes mellitus “as it does not cross the placenta to a measurable extent.” The ADA also warns against metformin and glyburide as first-line agents because they “both cross the placenta to the fetus.”
Diabetes monitoring and screening
The ADA now recommends use of the American College of Cardiology’s atherosclerotic cardiovascular disease risk calculator, the ASCVD Risk Estimator Plus. The calculator assesses the risk of this disease over 10 years and is “generally a useful tool.”
The ACA recommends screening for cardiac risk factors at least once a year in patients with diabetes.
Physicians are no longer advised to check the feet of patients with diabetes at every visit; now the recommendation is for those at high risk of ulceration only. However, an annual examination of feet is recommended for all patients with diabetes.
The ADA now recommends that patients with type 2 diabetes or prediabetes undergo screening for nonalcoholic steatohepatitis and liver fibrosis if they have elevated liver enzymes or an ultrasound examination shows signs of fatty liver.
Gabapentin is now listed along with pregabalin and duloxetine as first-line drug treatments for neuropathic pain in diabetes.
The American Diabetes Association is out with new standard-of-care guidelines that – among other things – reject injectable insulin as the main first-line treatment for type 2 diabetes mellitus (T2DM), debut a cardiac risk calculator, and offer new recommendations regarding medications for patients with kidney disease, clogged arteries, and heart failure.
The ADA’s newly released 2019 Standards of Medical Care in Diabetes “emphasize a patient-centered approach that considers the multiple health and life factors of each person living with diabetes,” said William T. Cefalu, MD, the ADA’s chief scientific, medical, and mission officer, in a statement.
The 193-page guidelines are now available online at the Diabetes Care website and will be available via an app and the print edition of the journal.
Here’s a closer look at a few of the many new and revised recommendations in the 2019 Standards of Care.
Diabetes treatment
In a new guideline, the standards of care says glucagonlike peptide–1 (GLP-1) receptor agonists should be “a first-line treatment” – ahead of insulin – “for most [type 2] patients who need the greater efficacy of an injectable medication.”
However, the recommendations note that the “high costs and tolerability issues are important barriers to the use of GLP-1 receptor agonists.”
A new recommendation suggests the use of sodium-glucose cotransporter 2 inhibitors or GLP-1 receptor agonists “with demonstrated cardiovascular disease benefit” in patients with type 2 diabetes who have confirmed atherosclerotic cardiovascular disease.
A related new recommendation says sodium-glucose cotransporter 2 inhibitors are the preferred treatment for these patients who have heart failure or are at high risk of developing it.
In a new recommendation, the ADA suggests that patients with type 2 diabetes and chronic kidney disease potentially take a sodium-glucose cotransporter 2 inhibitor or a GLP-1 receptor agonist, which has been shown to reduce the risk of chronic kidney disease progression, cardiac events, or both.
There’s a greater focus on insulin as the preferred treatment for hyperglycemia in gestational diabetes mellitus “as it does not cross the placenta to a measurable extent.” The ADA also warns against metformin and glyburide as first-line agents because they “both cross the placenta to the fetus.”
Diabetes monitoring and screening
The ADA now recommends use of the American College of Cardiology’s atherosclerotic cardiovascular disease risk calculator, the ASCVD Risk Estimator Plus. The calculator assesses the risk of this disease over 10 years and is “generally a useful tool.”
The ACA recommends screening for cardiac risk factors at least once a year in patients with diabetes.
Physicians are no longer advised to check the feet of patients with diabetes at every visit; now the recommendation is for those at high risk of ulceration only. However, an annual examination of feet is recommended for all patients with diabetes.
The ADA now recommends that patients with type 2 diabetes or prediabetes undergo screening for nonalcoholic steatohepatitis and liver fibrosis if they have elevated liver enzymes or an ultrasound examination shows signs of fatty liver.
Gabapentin is now listed along with pregabalin and duloxetine as first-line drug treatments for neuropathic pain in diabetes.
FROM DIABETES CARE
Migraine Treatment in Pregnant Women Evaluated
While the majority of pregnant women with acute migraine received medications considered relatively safe in pregnancy, there was variation in treatment choice and sequence, a recent study found. Researchers conducted a retrospective chart review of medication administration for pregnant women who presented to an acute care setting with a migraine attack and received neurology consultation between 2009 and 2014. They identified 72 pregnant women with migraine who were treated with pain medications and found:
- Fifty-one percent (37/72) were in the third trimester of pregnancy, 39% (28/72) in the second trimester, and 10% (7/72) in the first trimester.
- Thirty-two percent (23/72) had not tried any acute medications at home before coming to the hospital, and 47% (34/72) presented in status migrainosus.
- Patients received treatment in the hospital for a median of 23 hours.
- Acetaminophen was the most frequent medicine administered first (53%, 38/72).
- Thirty-eight percent (27/72) received an intravenous (IV) fluid bolus, 24% received IV magnesium (17/72), and 6% (4/72) had peripheral nerve blocks performed.
Hamilton KT, Robbins MS. Migraine treatment in pregnant women presenting to acute care: A retrospective observational study. [Published online ahead of print November 7, 2018]. Headache. doi:10.1111/head.13434.
While the majority of pregnant women with acute migraine received medications considered relatively safe in pregnancy, there was variation in treatment choice and sequence, a recent study found. Researchers conducted a retrospective chart review of medication administration for pregnant women who presented to an acute care setting with a migraine attack and received neurology consultation between 2009 and 2014. They identified 72 pregnant women with migraine who were treated with pain medications and found:
- Fifty-one percent (37/72) were in the third trimester of pregnancy, 39% (28/72) in the second trimester, and 10% (7/72) in the first trimester.
- Thirty-two percent (23/72) had not tried any acute medications at home before coming to the hospital, and 47% (34/72) presented in status migrainosus.
- Patients received treatment in the hospital for a median of 23 hours.
- Acetaminophen was the most frequent medicine administered first (53%, 38/72).
- Thirty-eight percent (27/72) received an intravenous (IV) fluid bolus, 24% received IV magnesium (17/72), and 6% (4/72) had peripheral nerve blocks performed.
Hamilton KT, Robbins MS. Migraine treatment in pregnant women presenting to acute care: A retrospective observational study. [Published online ahead of print November 7, 2018]. Headache. doi:10.1111/head.13434.
While the majority of pregnant women with acute migraine received medications considered relatively safe in pregnancy, there was variation in treatment choice and sequence, a recent study found. Researchers conducted a retrospective chart review of medication administration for pregnant women who presented to an acute care setting with a migraine attack and received neurology consultation between 2009 and 2014. They identified 72 pregnant women with migraine who were treated with pain medications and found:
- Fifty-one percent (37/72) were in the third trimester of pregnancy, 39% (28/72) in the second trimester, and 10% (7/72) in the first trimester.
- Thirty-two percent (23/72) had not tried any acute medications at home before coming to the hospital, and 47% (34/72) presented in status migrainosus.
- Patients received treatment in the hospital for a median of 23 hours.
- Acetaminophen was the most frequent medicine administered first (53%, 38/72).
- Thirty-eight percent (27/72) received an intravenous (IV) fluid bolus, 24% received IV magnesium (17/72), and 6% (4/72) had peripheral nerve blocks performed.
Hamilton KT, Robbins MS. Migraine treatment in pregnant women presenting to acute care: A retrospective observational study. [Published online ahead of print November 7, 2018]. Headache. doi:10.1111/head.13434.
Cardiac failure due to left atrial angiosarcoma
Abstract
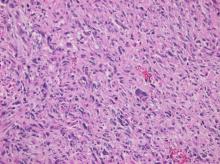
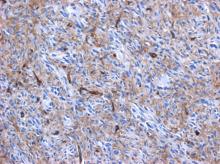
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
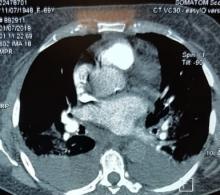
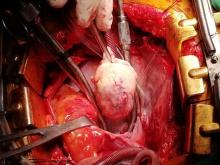
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
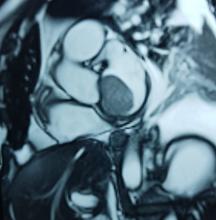
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
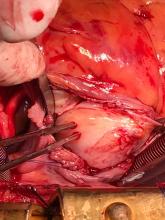
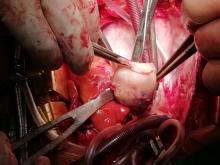
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: [email protected]
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: [email protected]
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: [email protected]
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
Texas judge strikes down ACA putting law in peril — again
The future of the Affordable Care Act is threatened – again – this time by a ruling Friday from a federal district court judge in Texas.
Judge Reed C. O’Connor struck down the law, siding with a group of 18 Republican state attorneys general and two GOP governors who brought the case. Judge O’Connor said the tax bill passed by Congress in December 2017 effectively rendered the entire health law unconstitutional.
That tax measure eliminated the penalty for not having insurance. An earlier Supreme Court decision upheld the ACA based on the view that the penalty was a tax and thus the law was valid because it relied on appropriate power allowed Congress under the Constitution. Judge O’Connor’s decision said that without that penalty, the law no longer met that constitutional test.
“In some ways, the question before the court involves the intent of both the 2010 and 2017 Congresses,” Judge O’Connor wrote in his 55-page decision. “The former enacted the ACA. The latter sawed off the last leg it stood on.”
The decision came just hours before the end of open enrollment for ACA plans in most states that use the federal HealthCare.gov insurance exchange. It is not expected that the ruling will impact the coverage for those people – the final decision will likely not come until the case reaches the Supreme Court again.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, which oversees those insurance exchanges, said in a tweet: “The recent federal court decision is still moving through the courts, and the exchanges are still open for business and we will continue with open enrollment. There is no impact to current coverage or coverage in a 2019 plan.”
The 16 Democratic state attorneys general who intervened in the case to defend the health law immediately vowed to appeal.
“The ACA has already survived more than 70 unsuccessful repeal attempts and withstood scrutiny in the Supreme Court,” said a statement from California Attorney General Xavier Becerra. “Today’s misguided ruling will not deter us: our coalition will continue to fight in court for the health and wellbeing of all Americans.”
It is all but certain the case will become the third time the Supreme Court decides a constitutional question related to the ACA. In addition to upholding the law in 2012, the court rejected another challenge to the law in 2015.
It is hard to overstate what would happen to the nation’s health care system if the decision is ultimately upheld. The Affordable Care Act touched almost every aspect of health care, from Medicare and Medicaid to generic biologic drugs, the Indian Health Service, and public health changes like calorie counts on menus.
The case, Texas v. United States, was filed in February. The plaintiffs argued that because the Supreme Court upheld the ACA in 2012 as a constitutional use of its taxing power, the elimination of the tax makes the rest of the law unconstitutional.
In June, the Justice Department announced it would not fully defend the law in court. While the Trump administration said it did not agree with the plaintiffs that the tax law meant the entire ACA was unconstitutional, it said that the provisions of the law guaranteeing that people with preexisting health conditions could purchase coverage at the same price as everyone else were so inextricably linked to the tax penalty that they should be struck.
The administration urged the court to declare those provisions invalid beginning Jan. 1, 2019. That is the day the tax penalty for not having insurance disappears.
The protections for people with preexisting conditions was one of the top health issues in the midterm elections in November. While the issue mostly played to the advantage of Democrats, one of the Republican plaintiffs, Missouri Attorney General Josh Hawley, defeated Democratic incumbent Sen. Claire McCaskill. Another plaintiff, West Virginia Attorney General Patrick Morrisey, lost to Democratic incumbent Sen. Joe Manchin.
President Donald Trump was quick to take a victory lap, and pressed Senate Majority Leader Mitch McConnell (R-Ky.) and presumed incoming House Speaker Nancy Pelosi (D-Calif.) to fix the problem. He tweeted Friday night that “As I predicted all along, Obamacare has been struck down as an UNCONSTITUTIONAL disaster! Now Congress must pass a STRONG law that provides GREAT healthcare and protects pre-existing conditions. Mitch and Nancy, get it done!”
But congressional leaders were quick to point out that the suit is far from over.
“The ruling seems to be based on faulty legal reasoning and hopefully it will be overturned,” said a statement from Senate Minority Leader Chuck Schumer (D-N.Y.).
Many legal experts agreed with that. “This is insanity in print, and it will not stand up on appeal,” tweeted University of Michigan Law School professor Nicholas Bagley, an expert in health law.
Even some conservatives were left scratching their heads. “Congress acted last year to repeal the mandate, but leave everything else in place and the courts should have deferred to that,” tweeted former congressional GOP aide Chris Jacobs.
AGA believes that Congress must include provisions to ensure patient access to specialty care and other essential patient protections in any new health care legislation. Read more at http://ow.ly/kzIz30n1cBo.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
The future of the Affordable Care Act is threatened – again – this time by a ruling Friday from a federal district court judge in Texas.
Judge Reed C. O’Connor struck down the law, siding with a group of 18 Republican state attorneys general and two GOP governors who brought the case. Judge O’Connor said the tax bill passed by Congress in December 2017 effectively rendered the entire health law unconstitutional.
That tax measure eliminated the penalty for not having insurance. An earlier Supreme Court decision upheld the ACA based on the view that the penalty was a tax and thus the law was valid because it relied on appropriate power allowed Congress under the Constitution. Judge O’Connor’s decision said that without that penalty, the law no longer met that constitutional test.
“In some ways, the question before the court involves the intent of both the 2010 and 2017 Congresses,” Judge O’Connor wrote in his 55-page decision. “The former enacted the ACA. The latter sawed off the last leg it stood on.”
The decision came just hours before the end of open enrollment for ACA plans in most states that use the federal HealthCare.gov insurance exchange. It is not expected that the ruling will impact the coverage for those people – the final decision will likely not come until the case reaches the Supreme Court again.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, which oversees those insurance exchanges, said in a tweet: “The recent federal court decision is still moving through the courts, and the exchanges are still open for business and we will continue with open enrollment. There is no impact to current coverage or coverage in a 2019 plan.”
The 16 Democratic state attorneys general who intervened in the case to defend the health law immediately vowed to appeal.
“The ACA has already survived more than 70 unsuccessful repeal attempts and withstood scrutiny in the Supreme Court,” said a statement from California Attorney General Xavier Becerra. “Today’s misguided ruling will not deter us: our coalition will continue to fight in court for the health and wellbeing of all Americans.”
It is all but certain the case will become the third time the Supreme Court decides a constitutional question related to the ACA. In addition to upholding the law in 2012, the court rejected another challenge to the law in 2015.
It is hard to overstate what would happen to the nation’s health care system if the decision is ultimately upheld. The Affordable Care Act touched almost every aspect of health care, from Medicare and Medicaid to generic biologic drugs, the Indian Health Service, and public health changes like calorie counts on menus.
The case, Texas v. United States, was filed in February. The plaintiffs argued that because the Supreme Court upheld the ACA in 2012 as a constitutional use of its taxing power, the elimination of the tax makes the rest of the law unconstitutional.
In June, the Justice Department announced it would not fully defend the law in court. While the Trump administration said it did not agree with the plaintiffs that the tax law meant the entire ACA was unconstitutional, it said that the provisions of the law guaranteeing that people with preexisting health conditions could purchase coverage at the same price as everyone else were so inextricably linked to the tax penalty that they should be struck.
The administration urged the court to declare those provisions invalid beginning Jan. 1, 2019. That is the day the tax penalty for not having insurance disappears.
The protections for people with preexisting conditions was one of the top health issues in the midterm elections in November. While the issue mostly played to the advantage of Democrats, one of the Republican plaintiffs, Missouri Attorney General Josh Hawley, defeated Democratic incumbent Sen. Claire McCaskill. Another plaintiff, West Virginia Attorney General Patrick Morrisey, lost to Democratic incumbent Sen. Joe Manchin.
President Donald Trump was quick to take a victory lap, and pressed Senate Majority Leader Mitch McConnell (R-Ky.) and presumed incoming House Speaker Nancy Pelosi (D-Calif.) to fix the problem. He tweeted Friday night that “As I predicted all along, Obamacare has been struck down as an UNCONSTITUTIONAL disaster! Now Congress must pass a STRONG law that provides GREAT healthcare and protects pre-existing conditions. Mitch and Nancy, get it done!”
But congressional leaders were quick to point out that the suit is far from over.
“The ruling seems to be based on faulty legal reasoning and hopefully it will be overturned,” said a statement from Senate Minority Leader Chuck Schumer (D-N.Y.).
Many legal experts agreed with that. “This is insanity in print, and it will not stand up on appeal,” tweeted University of Michigan Law School professor Nicholas Bagley, an expert in health law.
Even some conservatives were left scratching their heads. “Congress acted last year to repeal the mandate, but leave everything else in place and the courts should have deferred to that,” tweeted former congressional GOP aide Chris Jacobs.
AGA believes that Congress must include provisions to ensure patient access to specialty care and other essential patient protections in any new health care legislation. Read more at http://ow.ly/kzIz30n1cBo.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
The future of the Affordable Care Act is threatened – again – this time by a ruling Friday from a federal district court judge in Texas.
Judge Reed C. O’Connor struck down the law, siding with a group of 18 Republican state attorneys general and two GOP governors who brought the case. Judge O’Connor said the tax bill passed by Congress in December 2017 effectively rendered the entire health law unconstitutional.
That tax measure eliminated the penalty for not having insurance. An earlier Supreme Court decision upheld the ACA based on the view that the penalty was a tax and thus the law was valid because it relied on appropriate power allowed Congress under the Constitution. Judge O’Connor’s decision said that without that penalty, the law no longer met that constitutional test.
“In some ways, the question before the court involves the intent of both the 2010 and 2017 Congresses,” Judge O’Connor wrote in his 55-page decision. “The former enacted the ACA. The latter sawed off the last leg it stood on.”
The decision came just hours before the end of open enrollment for ACA plans in most states that use the federal HealthCare.gov insurance exchange. It is not expected that the ruling will impact the coverage for those people – the final decision will likely not come until the case reaches the Supreme Court again.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, which oversees those insurance exchanges, said in a tweet: “The recent federal court decision is still moving through the courts, and the exchanges are still open for business and we will continue with open enrollment. There is no impact to current coverage or coverage in a 2019 plan.”
The 16 Democratic state attorneys general who intervened in the case to defend the health law immediately vowed to appeal.
“The ACA has already survived more than 70 unsuccessful repeal attempts and withstood scrutiny in the Supreme Court,” said a statement from California Attorney General Xavier Becerra. “Today’s misguided ruling will not deter us: our coalition will continue to fight in court for the health and wellbeing of all Americans.”
It is all but certain the case will become the third time the Supreme Court decides a constitutional question related to the ACA. In addition to upholding the law in 2012, the court rejected another challenge to the law in 2015.
It is hard to overstate what would happen to the nation’s health care system if the decision is ultimately upheld. The Affordable Care Act touched almost every aspect of health care, from Medicare and Medicaid to generic biologic drugs, the Indian Health Service, and public health changes like calorie counts on menus.
The case, Texas v. United States, was filed in February. The plaintiffs argued that because the Supreme Court upheld the ACA in 2012 as a constitutional use of its taxing power, the elimination of the tax makes the rest of the law unconstitutional.
In June, the Justice Department announced it would not fully defend the law in court. While the Trump administration said it did not agree with the plaintiffs that the tax law meant the entire ACA was unconstitutional, it said that the provisions of the law guaranteeing that people with preexisting health conditions could purchase coverage at the same price as everyone else were so inextricably linked to the tax penalty that they should be struck.
The administration urged the court to declare those provisions invalid beginning Jan. 1, 2019. That is the day the tax penalty for not having insurance disappears.
The protections for people with preexisting conditions was one of the top health issues in the midterm elections in November. While the issue mostly played to the advantage of Democrats, one of the Republican plaintiffs, Missouri Attorney General Josh Hawley, defeated Democratic incumbent Sen. Claire McCaskill. Another plaintiff, West Virginia Attorney General Patrick Morrisey, lost to Democratic incumbent Sen. Joe Manchin.
President Donald Trump was quick to take a victory lap, and pressed Senate Majority Leader Mitch McConnell (R-Ky.) and presumed incoming House Speaker Nancy Pelosi (D-Calif.) to fix the problem. He tweeted Friday night that “As I predicted all along, Obamacare has been struck down as an UNCONSTITUTIONAL disaster! Now Congress must pass a STRONG law that provides GREAT healthcare and protects pre-existing conditions. Mitch and Nancy, get it done!”
But congressional leaders were quick to point out that the suit is far from over.
“The ruling seems to be based on faulty legal reasoning and hopefully it will be overturned,” said a statement from Senate Minority Leader Chuck Schumer (D-N.Y.).
Many legal experts agreed with that. “This is insanity in print, and it will not stand up on appeal,” tweeted University of Michigan Law School professor Nicholas Bagley, an expert in health law.
Even some conservatives were left scratching their heads. “Congress acted last year to repeal the mandate, but leave everything else in place and the courts should have deferred to that,” tweeted former congressional GOP aide Chris Jacobs.
AGA believes that Congress must include provisions to ensure patient access to specialty care and other essential patient protections in any new health care legislation. Read more at http://ow.ly/kzIz30n1cBo.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
Active migraine in women linked to lower risk of developing T2DM
Women with active migraines are less likely to have type 2 diabetes mellitus (T2DM) and show a decrease in migraine symptoms prior to diagnosis of T2DM, indicating an inverse relationship between hyperglycemia, hyperinsulinism, and migraines, according to recent research published in JAMA Neurology.
“Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease,” Guy Fagherazzi, PhD, at the Center for Research in Epidemiology and Population Health at the Gustave Roussy Institute in Villejuif, France, and his colleagues wrote in their study. “Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.”
The researchers used data from the prospective Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study, initiated in 1990 and identified 74,247 women (mean age, 61 years old) with self-reported migraine in a 2002 follow-up questionnaire who had 10-year follow-up data during 2004-2014. The women in the cohort were born during 1925-1950 and completed biennial questionnaires about their health, including migraine status and medications, since 1992. The participants were divided into groups based on no migraine (49,199 participants), active migraine (7,839 participants), or prior migraine history (17,209 participants), and patients with T2DM at baseline were excluded.
Dr. Fagherazzi and his colleagues found 2,372 cases of type 2 diabetes over the follow-up period. Women who had active migraine status were less likely to have T2DM (hazard ratio, 0.80; 95% confidence interval, 0.67-0.96) than were the participants who did not have migraines, and this inverse association persisted after the researchers adjusted for factors such as myocardial infarction, education level, family history of diabetes, body mass index, smoking status, hypertension, physical activity, oral contraceptive use, menopausal status, menopausal hormone therapy, handedness, antimigraine preparations, and other prescribed migraine drugs (HR, 0.70; 95% CI, 0.58-0.85).
In the participants who developed T2DM, the researchers also found that there was a decrease in the prevalence of active migraine in the 24 years prior to T2DM diagnosis from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) after adjusting for T2DM risk factors, which was then followed by an up to 22-year plateau in migraine prevalence of 11% for these participants.
“The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations,” Dr. Fagherazzi and his colleagues wrote. “The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.”
The researchers noted limitations in the study, such as self-reported migraine by participants in the cohort, exclusion of non–pharmacologically treated T2DM cases, observational nature of the study, and homogenized population in the E3N cohort consisting of mainly women in menopause who were teachers and belonged to the same health insurance plan.
This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the “Mutuelle Générale de l’Education Nationale,” European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided BMJ with editorial services.
SOURCE: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
Although it has been noted for some time in the clinical setting, researchers are still unsure why there is an inverse association between active migraine and type 2 diabetes mellitus, as noted by Fagherazzi et al. in a recent study.
One explanation is the presence of calcitonin gene–related peptide in both animal models of energy metabolism and the pathophysiology of migraine. It is possible that insulin resistance and hyperglycemia damage the sensory neurons that produce the peptide. If these damaged nerves are soothed, migraine may resolve.
Other silver linings associated with active migraine include an increased likelihood of having a healthy cardiovascular system and decreased alcohol consumption.
The epidemiology of migraine and findings like those in this study prompt the question: What is migraine good for?
Amy A. Gelfand, MD , of the University of California, San Francisco, and Elizabeth Loder, MD , MPH, of Harvard Medical School in Boston made these comments in an editorial accompanying Dr. Fagherazzi’s study. They disclosed a number of financial relationships with companies marketing treatments for migraine.
Although it has been noted for some time in the clinical setting, researchers are still unsure why there is an inverse association between active migraine and type 2 diabetes mellitus, as noted by Fagherazzi et al. in a recent study.
One explanation is the presence of calcitonin gene–related peptide in both animal models of energy metabolism and the pathophysiology of migraine. It is possible that insulin resistance and hyperglycemia damage the sensory neurons that produce the peptide. If these damaged nerves are soothed, migraine may resolve.
Other silver linings associated with active migraine include an increased likelihood of having a healthy cardiovascular system and decreased alcohol consumption.
The epidemiology of migraine and findings like those in this study prompt the question: What is migraine good for?
Amy A. Gelfand, MD , of the University of California, San Francisco, and Elizabeth Loder, MD , MPH, of Harvard Medical School in Boston made these comments in an editorial accompanying Dr. Fagherazzi’s study. They disclosed a number of financial relationships with companies marketing treatments for migraine.
Although it has been noted for some time in the clinical setting, researchers are still unsure why there is an inverse association between active migraine and type 2 diabetes mellitus, as noted by Fagherazzi et al. in a recent study.
One explanation is the presence of calcitonin gene–related peptide in both animal models of energy metabolism and the pathophysiology of migraine. It is possible that insulin resistance and hyperglycemia damage the sensory neurons that produce the peptide. If these damaged nerves are soothed, migraine may resolve.
Other silver linings associated with active migraine include an increased likelihood of having a healthy cardiovascular system and decreased alcohol consumption.
The epidemiology of migraine and findings like those in this study prompt the question: What is migraine good for?
Amy A. Gelfand, MD , of the University of California, San Francisco, and Elizabeth Loder, MD , MPH, of Harvard Medical School in Boston made these comments in an editorial accompanying Dr. Fagherazzi’s study. They disclosed a number of financial relationships with companies marketing treatments for migraine.
Women with active migraines are less likely to have type 2 diabetes mellitus (T2DM) and show a decrease in migraine symptoms prior to diagnosis of T2DM, indicating an inverse relationship between hyperglycemia, hyperinsulinism, and migraines, according to recent research published in JAMA Neurology.
“Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease,” Guy Fagherazzi, PhD, at the Center for Research in Epidemiology and Population Health at the Gustave Roussy Institute in Villejuif, France, and his colleagues wrote in their study. “Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.”
The researchers used data from the prospective Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study, initiated in 1990 and identified 74,247 women (mean age, 61 years old) with self-reported migraine in a 2002 follow-up questionnaire who had 10-year follow-up data during 2004-2014. The women in the cohort were born during 1925-1950 and completed biennial questionnaires about their health, including migraine status and medications, since 1992. The participants were divided into groups based on no migraine (49,199 participants), active migraine (7,839 participants), or prior migraine history (17,209 participants), and patients with T2DM at baseline were excluded.
Dr. Fagherazzi and his colleagues found 2,372 cases of type 2 diabetes over the follow-up period. Women who had active migraine status were less likely to have T2DM (hazard ratio, 0.80; 95% confidence interval, 0.67-0.96) than were the participants who did not have migraines, and this inverse association persisted after the researchers adjusted for factors such as myocardial infarction, education level, family history of diabetes, body mass index, smoking status, hypertension, physical activity, oral contraceptive use, menopausal status, menopausal hormone therapy, handedness, antimigraine preparations, and other prescribed migraine drugs (HR, 0.70; 95% CI, 0.58-0.85).
In the participants who developed T2DM, the researchers also found that there was a decrease in the prevalence of active migraine in the 24 years prior to T2DM diagnosis from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) after adjusting for T2DM risk factors, which was then followed by an up to 22-year plateau in migraine prevalence of 11% for these participants.
“The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations,” Dr. Fagherazzi and his colleagues wrote. “The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.”
The researchers noted limitations in the study, such as self-reported migraine by participants in the cohort, exclusion of non–pharmacologically treated T2DM cases, observational nature of the study, and homogenized population in the E3N cohort consisting of mainly women in menopause who were teachers and belonged to the same health insurance plan.
This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the “Mutuelle Générale de l’Education Nationale,” European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided BMJ with editorial services.
SOURCE: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
Women with active migraines are less likely to have type 2 diabetes mellitus (T2DM) and show a decrease in migraine symptoms prior to diagnosis of T2DM, indicating an inverse relationship between hyperglycemia, hyperinsulinism, and migraines, according to recent research published in JAMA Neurology.
“Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease,” Guy Fagherazzi, PhD, at the Center for Research in Epidemiology and Population Health at the Gustave Roussy Institute in Villejuif, France, and his colleagues wrote in their study. “Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.”
The researchers used data from the prospective Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study, initiated in 1990 and identified 74,247 women (mean age, 61 years old) with self-reported migraine in a 2002 follow-up questionnaire who had 10-year follow-up data during 2004-2014. The women in the cohort were born during 1925-1950 and completed biennial questionnaires about their health, including migraine status and medications, since 1992. The participants were divided into groups based on no migraine (49,199 participants), active migraine (7,839 participants), or prior migraine history (17,209 participants), and patients with T2DM at baseline were excluded.
Dr. Fagherazzi and his colleagues found 2,372 cases of type 2 diabetes over the follow-up period. Women who had active migraine status were less likely to have T2DM (hazard ratio, 0.80; 95% confidence interval, 0.67-0.96) than were the participants who did not have migraines, and this inverse association persisted after the researchers adjusted for factors such as myocardial infarction, education level, family history of diabetes, body mass index, smoking status, hypertension, physical activity, oral contraceptive use, menopausal status, menopausal hormone therapy, handedness, antimigraine preparations, and other prescribed migraine drugs (HR, 0.70; 95% CI, 0.58-0.85).
In the participants who developed T2DM, the researchers also found that there was a decrease in the prevalence of active migraine in the 24 years prior to T2DM diagnosis from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) after adjusting for T2DM risk factors, which was then followed by an up to 22-year plateau in migraine prevalence of 11% for these participants.
“The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations,” Dr. Fagherazzi and his colleagues wrote. “The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.”
The researchers noted limitations in the study, such as self-reported migraine by participants in the cohort, exclusion of non–pharmacologically treated T2DM cases, observational nature of the study, and homogenized population in the E3N cohort consisting of mainly women in menopause who were teachers and belonged to the same health insurance plan.
This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the “Mutuelle Générale de l’Education Nationale,” European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided BMJ with editorial services.
SOURCE: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
FROM JAMA NEUROLOGY
Key clinical point: There was an inverse association between active migraine and type 2 diabetes mellitus in women over 10 years of follow-up.
Major finding: Compared with women who had no history of active migraine, women with active migraine had a lower risk of developing type 2 diabetes (univariate hazard ratio, 0.80; 95% confidence interval, 0.67-0.96).
Study details: Results from a prospective, population-based study of 74,247 women with active migraines in the E3N cohort study in France.
Disclosures: This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the Mutuelle Générale de l’Education Nationale, European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided the BMJ with editorial services.
Source: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.