User login
Time reveals benefit of CABG over PCI for left main disease
SAN DIEGO – In the long run, patients with left main coronary artery disease fare better if they undergo coronary artery bypass grafting (CABG) instead of percutaneous coronary intervention (PCI) with drug-eluting stents, suggest 10-year results of the MAIN-COMPARE trial. Findings were reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although CABG is the standard choice for revascularization in this patient population, PCI has been making inroads thanks to advances in stents, antithrombotic drugs, periprocedural management, and operator expertise, noted senior author Seung-Jung Park, MD, PhD, chairman of the Heart Institute at Asan Medical Center in Seoul and professor of medicine at University of Ulsan, South Korea. “Indeed, many studies showed that PCI using drug-eluting stents might be a good alternative for selected patients with left main coronary artery disease.”
Two large, randomized, controlled trials, EXCEL and NOBLE, have compared these treatment strategies and helped clarify outcomes at intermediate follow-up periods of 3-5 years. But long-term data, increasingly important as survival improves, are lacking.
Dr. Park reported the 10-year update of a prospective, observational cohort study that analyzed data from more than 2,000 patients with unprotected left main coronary artery disease in the MAIN-COMPARE registry, which captures revascularization procedures performed at 12 Korean cardiac centers.
In the entire cohort, about a fifth of the patients died, and roughly a fourth experienced a composite adverse outcome of death and cardiovascular events regardless of whether they received PCI or CABG, but the former yielded a rate of target vessel revascularization that was more than three times higher, according to results reported at the meeting and simultaneously published (J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012). Among the subset of patients treated in the more recent drug-eluting stent era, those who underwent PCI were more likely to die and to experience the composite outcome starting at the 5-year mark.
“Drug-eluting stents were associated with higher risks of death and serious composite outcomes compared to CABG after 5 years. The treatment benefit of CABG has diverged over time during continued follow-up,” Dr. Park noted. “The rate of target-vessel failure was consistently higher in the PCI group.”
“We used mainly first-generation drug-eluting stents,” he acknowledged. “However, many studies have demonstrated there is not too much difference between the first- and second-generation stents.”
Data worth the wait
In the same session, investigators reported the 10-year update of the European and U.S. randomized SYNTAX Extended Survival trial, called SYNTAXES. SYNTAX enrolled patients with three-vessel or left main coronary disease. That trial found no significant difference in survival between PCI with drug-eluting stents and CABG overall. In stratified analysis, mortality was higher with PCI among patients with three-vessel disease, but not among patients with left main disease.
Taken together, these trials help clarify the long-term comparative efficacy of PCI and begin to inform patient selection, according to press conference panelist Morton J. Kern, MD, a professor at the University of California, Irvine Medical Center.
“The fine subgroup analysis of who the best candidates are is still in question,” he elaborated. “The SYNTAXES study told us that surgery for left mains is still pretty good, and even though you can get good results with PCI, the event rates are higher in that three-vessel, high-SYNTAX score group, so we should be careful. Interventionalists need to know their limitations. I think that’s what both studies tell us, actually.”
Study details
The MAIN-COMPARE analyses were based on 2,240 patients with unprotected left main coronary artery disease (stenosis of more than 50% and no coronary artery bypass grafts to the left anterior descending or the left circumflex artery) treated during 2000-2006.
A total of 1,102 patients underwent PCI with stenting: 318 in the era of bare-metal stents and 784 in the era of drug-eluting stents, predominantly sirolimus-eluting stents. A total of 1,138 patients underwent CABG. The minimum follow-up was 10 years in all patients, with a median of 12 years.
In the entire study cohort, PCI and CABG yielded similar rates of death (21.1% vs. 23.2%) and the composite of death, Q-wave myocardial infarction, or stroke (23.8% vs. 26.3%), but the PCI patients had a significantly higher rate of target vessel revascularization (21.1% vs. 5.8%), according to data reported at the meeting, which was sponsored by the New York–based Cardiovascular Research Foundation.
In analyses using a propensity score weighting technique, results for the entire cohort were much the same. But on stratification, PCI with drug-eluting stents versus CABG yielded higher risks of death (hazard ratio, 1.35; P = .05) and the composite adverse outcome (HR, 1.46; P = .009) from 5 years onward, as well as a sharply higher risk of target vessel revascularization for the full duration of follow-up (HR, 5.82; P less than .001).
Dr. Park disclosed that he had no conflicts of interest. The study was supported by the Korean Society of Interventional Cardiology and the CardioVascular Research Foundation of South Korea.
SOURCE: Park SJ et al. J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012).
SAN DIEGO – In the long run, patients with left main coronary artery disease fare better if they undergo coronary artery bypass grafting (CABG) instead of percutaneous coronary intervention (PCI) with drug-eluting stents, suggest 10-year results of the MAIN-COMPARE trial. Findings were reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although CABG is the standard choice for revascularization in this patient population, PCI has been making inroads thanks to advances in stents, antithrombotic drugs, periprocedural management, and operator expertise, noted senior author Seung-Jung Park, MD, PhD, chairman of the Heart Institute at Asan Medical Center in Seoul and professor of medicine at University of Ulsan, South Korea. “Indeed, many studies showed that PCI using drug-eluting stents might be a good alternative for selected patients with left main coronary artery disease.”
Two large, randomized, controlled trials, EXCEL and NOBLE, have compared these treatment strategies and helped clarify outcomes at intermediate follow-up periods of 3-5 years. But long-term data, increasingly important as survival improves, are lacking.
Dr. Park reported the 10-year update of a prospective, observational cohort study that analyzed data from more than 2,000 patients with unprotected left main coronary artery disease in the MAIN-COMPARE registry, which captures revascularization procedures performed at 12 Korean cardiac centers.
In the entire cohort, about a fifth of the patients died, and roughly a fourth experienced a composite adverse outcome of death and cardiovascular events regardless of whether they received PCI or CABG, but the former yielded a rate of target vessel revascularization that was more than three times higher, according to results reported at the meeting and simultaneously published (J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012). Among the subset of patients treated in the more recent drug-eluting stent era, those who underwent PCI were more likely to die and to experience the composite outcome starting at the 5-year mark.
“Drug-eluting stents were associated with higher risks of death and serious composite outcomes compared to CABG after 5 years. The treatment benefit of CABG has diverged over time during continued follow-up,” Dr. Park noted. “The rate of target-vessel failure was consistently higher in the PCI group.”
“We used mainly first-generation drug-eluting stents,” he acknowledged. “However, many studies have demonstrated there is not too much difference between the first- and second-generation stents.”
Data worth the wait
In the same session, investigators reported the 10-year update of the European and U.S. randomized SYNTAX Extended Survival trial, called SYNTAXES. SYNTAX enrolled patients with three-vessel or left main coronary disease. That trial found no significant difference in survival between PCI with drug-eluting stents and CABG overall. In stratified analysis, mortality was higher with PCI among patients with three-vessel disease, but not among patients with left main disease.
Taken together, these trials help clarify the long-term comparative efficacy of PCI and begin to inform patient selection, according to press conference panelist Morton J. Kern, MD, a professor at the University of California, Irvine Medical Center.
“The fine subgroup analysis of who the best candidates are is still in question,” he elaborated. “The SYNTAXES study told us that surgery for left mains is still pretty good, and even though you can get good results with PCI, the event rates are higher in that three-vessel, high-SYNTAX score group, so we should be careful. Interventionalists need to know their limitations. I think that’s what both studies tell us, actually.”
Study details
The MAIN-COMPARE analyses were based on 2,240 patients with unprotected left main coronary artery disease (stenosis of more than 50% and no coronary artery bypass grafts to the left anterior descending or the left circumflex artery) treated during 2000-2006.
A total of 1,102 patients underwent PCI with stenting: 318 in the era of bare-metal stents and 784 in the era of drug-eluting stents, predominantly sirolimus-eluting stents. A total of 1,138 patients underwent CABG. The minimum follow-up was 10 years in all patients, with a median of 12 years.
In the entire study cohort, PCI and CABG yielded similar rates of death (21.1% vs. 23.2%) and the composite of death, Q-wave myocardial infarction, or stroke (23.8% vs. 26.3%), but the PCI patients had a significantly higher rate of target vessel revascularization (21.1% vs. 5.8%), according to data reported at the meeting, which was sponsored by the New York–based Cardiovascular Research Foundation.
In analyses using a propensity score weighting technique, results for the entire cohort were much the same. But on stratification, PCI with drug-eluting stents versus CABG yielded higher risks of death (hazard ratio, 1.35; P = .05) and the composite adverse outcome (HR, 1.46; P = .009) from 5 years onward, as well as a sharply higher risk of target vessel revascularization for the full duration of follow-up (HR, 5.82; P less than .001).
Dr. Park disclosed that he had no conflicts of interest. The study was supported by the Korean Society of Interventional Cardiology and the CardioVascular Research Foundation of South Korea.
SOURCE: Park SJ et al. J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012).
SAN DIEGO – In the long run, patients with left main coronary artery disease fare better if they undergo coronary artery bypass grafting (CABG) instead of percutaneous coronary intervention (PCI) with drug-eluting stents, suggest 10-year results of the MAIN-COMPARE trial. Findings were reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although CABG is the standard choice for revascularization in this patient population, PCI has been making inroads thanks to advances in stents, antithrombotic drugs, periprocedural management, and operator expertise, noted senior author Seung-Jung Park, MD, PhD, chairman of the Heart Institute at Asan Medical Center in Seoul and professor of medicine at University of Ulsan, South Korea. “Indeed, many studies showed that PCI using drug-eluting stents might be a good alternative for selected patients with left main coronary artery disease.”
Two large, randomized, controlled trials, EXCEL and NOBLE, have compared these treatment strategies and helped clarify outcomes at intermediate follow-up periods of 3-5 years. But long-term data, increasingly important as survival improves, are lacking.
Dr. Park reported the 10-year update of a prospective, observational cohort study that analyzed data from more than 2,000 patients with unprotected left main coronary artery disease in the MAIN-COMPARE registry, which captures revascularization procedures performed at 12 Korean cardiac centers.
In the entire cohort, about a fifth of the patients died, and roughly a fourth experienced a composite adverse outcome of death and cardiovascular events regardless of whether they received PCI or CABG, but the former yielded a rate of target vessel revascularization that was more than three times higher, according to results reported at the meeting and simultaneously published (J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012). Among the subset of patients treated in the more recent drug-eluting stent era, those who underwent PCI were more likely to die and to experience the composite outcome starting at the 5-year mark.
“Drug-eluting stents were associated with higher risks of death and serious composite outcomes compared to CABG after 5 years. The treatment benefit of CABG has diverged over time during continued follow-up,” Dr. Park noted. “The rate of target-vessel failure was consistently higher in the PCI group.”
“We used mainly first-generation drug-eluting stents,” he acknowledged. “However, many studies have demonstrated there is not too much difference between the first- and second-generation stents.”
Data worth the wait
In the same session, investigators reported the 10-year update of the European and U.S. randomized SYNTAX Extended Survival trial, called SYNTAXES. SYNTAX enrolled patients with three-vessel or left main coronary disease. That trial found no significant difference in survival between PCI with drug-eluting stents and CABG overall. In stratified analysis, mortality was higher with PCI among patients with three-vessel disease, but not among patients with left main disease.
Taken together, these trials help clarify the long-term comparative efficacy of PCI and begin to inform patient selection, according to press conference panelist Morton J. Kern, MD, a professor at the University of California, Irvine Medical Center.
“The fine subgroup analysis of who the best candidates are is still in question,” he elaborated. “The SYNTAXES study told us that surgery for left mains is still pretty good, and even though you can get good results with PCI, the event rates are higher in that three-vessel, high-SYNTAX score group, so we should be careful. Interventionalists need to know their limitations. I think that’s what both studies tell us, actually.”
Study details
The MAIN-COMPARE analyses were based on 2,240 patients with unprotected left main coronary artery disease (stenosis of more than 50% and no coronary artery bypass grafts to the left anterior descending or the left circumflex artery) treated during 2000-2006.
A total of 1,102 patients underwent PCI with stenting: 318 in the era of bare-metal stents and 784 in the era of drug-eluting stents, predominantly sirolimus-eluting stents. A total of 1,138 patients underwent CABG. The minimum follow-up was 10 years in all patients, with a median of 12 years.
In the entire study cohort, PCI and CABG yielded similar rates of death (21.1% vs. 23.2%) and the composite of death, Q-wave myocardial infarction, or stroke (23.8% vs. 26.3%), but the PCI patients had a significantly higher rate of target vessel revascularization (21.1% vs. 5.8%), according to data reported at the meeting, which was sponsored by the New York–based Cardiovascular Research Foundation.
In analyses using a propensity score weighting technique, results for the entire cohort were much the same. But on stratification, PCI with drug-eluting stents versus CABG yielded higher risks of death (hazard ratio, 1.35; P = .05) and the composite adverse outcome (HR, 1.46; P = .009) from 5 years onward, as well as a sharply higher risk of target vessel revascularization for the full duration of follow-up (HR, 5.82; P less than .001).
Dr. Park disclosed that he had no conflicts of interest. The study was supported by the Korean Society of Interventional Cardiology and the CardioVascular Research Foundation of South Korea.
SOURCE: Park SJ et al. J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012).
REPORTING FROM TCT 2018
Key clinical point: CABG had an edge over PCI with drug-eluting stents in patients with left main disease that became evident with longer follow-up.
Major finding: Compared with CABG, PCI with drug-eluting stents carried higher risks of death (hazard ratio, 1.35; P = .05) and a composite adverse outcome (HR, 1.46; P = .009) from 5 years onward.
Study details: Ten-year follow-up of a multicenter prospective cohort study of 2,240 patients with unprotected left main coronary artery disease who underwent either PCI with stenting or CABG (MAIN-COMPARE study).
Disclosures: Dr. Park disclosed that he had no conflicts of interest. The study was supported by the Korean Society of Interventional Cardiology and the CardioVascular Research Foundation of South Korea.
Source: Park S-J et al. J Am Coll Cardiol. 2018 Sep 14. doi: 10.1016/j.jacc.2018.09.012.
What Would I Tell My Intern-Year Self?
The training path to dermatology can seem interminable. From getting good grades in college to seeking out the “right” extracurricular activities and cramming for the MCAT, just getting into medical school was a huge challenge. In medical school, you may recognize the same chaos as you begin to prepare for US Medical Licensing Examination Step 1, try to volunteer, and publish original research. Dermatology is undeniably a competitive specialty. The 2018 data released by the National Resident Match Program (also called The Match) showed that only 83% of 412 US seniors who applied were matched to dermatology.1 The average Step 1 score for those who matched was 249 versus 241 for those who did not match. In addition, they had an average of 5.2 research experiences, 9.1 volunteer experiences, and 49.1 were members of Alpha Omega Alpha.1
After studying and working to meet these targets, it is not surprising that the transition to residency is a big change. As a dermatology preliminary intern, or“prelim,” our experience differs compared to other specialties, as other interns are jumping into their area of practice right away.
During my intern year, I had a tremendous amount of anxiety about 2 things: (1) being a subpar medical intern and (2) being unprepared for the beginning of my dermatology residency. This anxiety drove me to read a tremendous amount of medical and dermatological literature in an effort to do everything. Although hindsight is always 20/20, I will share some thoughts of my own as well as some from friends and colleagues.
First, enjoy intern year. I know that may sound ridiculous, but there were many aspects of intern year that I loved! When your pager beeps, it’s for YOU! You are no longer a subintern, running every decision past your intern or explaining your student status to the patients! Proudly introduce yourself as Dr. So-and-So. You earned it! I loved the camaraderie of working with my co-interns and senior residents. Going through the challenges of intern year together is a deep bonding experience, and I absolutely made lifelong friendships. It also does not hurt that I met my boyfriend (now husband), which has changed my life in a big way.
When it comes to learning internal medicine, pediatrics, or surgery (depending on your intern year), prepare for rounds, read about your patients, and pay attention in Grand Rounds. You can even consider taking the dermatologic cases that may be on your team, just for fun. I am always grateful for my internal medicine knowledge when managing complex medical dermatology patients and rounding on our consultation service on the wards. However, do not burden yourself with excessive studying. Enjoy your time off: spend it with family and friends or rediscover a hobby that has been neglected while you have been working toward your achievements.
When it comes to learning dermatology, do not rush it! You have 3 years and a ton of studying ahead of you! You will learn all of it. When July 1 of your first year of dermatology finally starts, immerse yourself in this new world:
- Attend conferences. Even if they are on topics you might not be interested in—from cosmetics to psoriasis—they provide a real-world perspective and often have great lecturers sharing their knowledge.
- Get involved. There are many dermatologic societies to take part in, and dues are waived or reduced when you sign up as a resident. Many of them provide great resources from study materials to journals, and they are always a great way to network when there are events.
- Volunteer. Many of the dermatologic societies sponsor volunteer events such as skin cancer screenings. It can be a fun way to network while also giving back to the community.
- Spend time figuring out what you really enjoy. This step may seem self-evident, but after many years of fulfilling the necessary criteria to get into medical school and residency, it can be habitual to start fulfilling the same criteria all over again. Explore all aspects of dermatology and see what truly interests you. Consider how you expect your life after residency to be and think what learning opportunities might be helpful down the road. Reach out to attendings you would like to work with, both in dermatology and in other specialties. I personally enjoyed working in wound and oncology clinics, learning how other specialties approach clinical dilemmas that we see in dermatology.
As I embark on my final year of dermatology residency, I am truly grateful for the wisdom that has been shared with me on this journey. Many people have provided key pieces of information that have helped shape my training and my plans for the future, and I hope that sharing it will help others!
- National Resident Matching Program, Charting Outcomes in the Match: U.S. Allopathic Seniors, 2018. Washington, DC: National Resident Matching Program; 2018. http://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed September 20, 2018.
The training path to dermatology can seem interminable. From getting good grades in college to seeking out the “right” extracurricular activities and cramming for the MCAT, just getting into medical school was a huge challenge. In medical school, you may recognize the same chaos as you begin to prepare for US Medical Licensing Examination Step 1, try to volunteer, and publish original research. Dermatology is undeniably a competitive specialty. The 2018 data released by the National Resident Match Program (also called The Match) showed that only 83% of 412 US seniors who applied were matched to dermatology.1 The average Step 1 score for those who matched was 249 versus 241 for those who did not match. In addition, they had an average of 5.2 research experiences, 9.1 volunteer experiences, and 49.1 were members of Alpha Omega Alpha.1
After studying and working to meet these targets, it is not surprising that the transition to residency is a big change. As a dermatology preliminary intern, or“prelim,” our experience differs compared to other specialties, as other interns are jumping into their area of practice right away.
During my intern year, I had a tremendous amount of anxiety about 2 things: (1) being a subpar medical intern and (2) being unprepared for the beginning of my dermatology residency. This anxiety drove me to read a tremendous amount of medical and dermatological literature in an effort to do everything. Although hindsight is always 20/20, I will share some thoughts of my own as well as some from friends and colleagues.
First, enjoy intern year. I know that may sound ridiculous, but there were many aspects of intern year that I loved! When your pager beeps, it’s for YOU! You are no longer a subintern, running every decision past your intern or explaining your student status to the patients! Proudly introduce yourself as Dr. So-and-So. You earned it! I loved the camaraderie of working with my co-interns and senior residents. Going through the challenges of intern year together is a deep bonding experience, and I absolutely made lifelong friendships. It also does not hurt that I met my boyfriend (now husband), which has changed my life in a big way.
When it comes to learning internal medicine, pediatrics, or surgery (depending on your intern year), prepare for rounds, read about your patients, and pay attention in Grand Rounds. You can even consider taking the dermatologic cases that may be on your team, just for fun. I am always grateful for my internal medicine knowledge when managing complex medical dermatology patients and rounding on our consultation service on the wards. However, do not burden yourself with excessive studying. Enjoy your time off: spend it with family and friends or rediscover a hobby that has been neglected while you have been working toward your achievements.
When it comes to learning dermatology, do not rush it! You have 3 years and a ton of studying ahead of you! You will learn all of it. When July 1 of your first year of dermatology finally starts, immerse yourself in this new world:
- Attend conferences. Even if they are on topics you might not be interested in—from cosmetics to psoriasis—they provide a real-world perspective and often have great lecturers sharing their knowledge.
- Get involved. There are many dermatologic societies to take part in, and dues are waived or reduced when you sign up as a resident. Many of them provide great resources from study materials to journals, and they are always a great way to network when there are events.
- Volunteer. Many of the dermatologic societies sponsor volunteer events such as skin cancer screenings. It can be a fun way to network while also giving back to the community.
- Spend time figuring out what you really enjoy. This step may seem self-evident, but after many years of fulfilling the necessary criteria to get into medical school and residency, it can be habitual to start fulfilling the same criteria all over again. Explore all aspects of dermatology and see what truly interests you. Consider how you expect your life after residency to be and think what learning opportunities might be helpful down the road. Reach out to attendings you would like to work with, both in dermatology and in other specialties. I personally enjoyed working in wound and oncology clinics, learning how other specialties approach clinical dilemmas that we see in dermatology.
As I embark on my final year of dermatology residency, I am truly grateful for the wisdom that has been shared with me on this journey. Many people have provided key pieces of information that have helped shape my training and my plans for the future, and I hope that sharing it will help others!
The training path to dermatology can seem interminable. From getting good grades in college to seeking out the “right” extracurricular activities and cramming for the MCAT, just getting into medical school was a huge challenge. In medical school, you may recognize the same chaos as you begin to prepare for US Medical Licensing Examination Step 1, try to volunteer, and publish original research. Dermatology is undeniably a competitive specialty. The 2018 data released by the National Resident Match Program (also called The Match) showed that only 83% of 412 US seniors who applied were matched to dermatology.1 The average Step 1 score for those who matched was 249 versus 241 for those who did not match. In addition, they had an average of 5.2 research experiences, 9.1 volunteer experiences, and 49.1 were members of Alpha Omega Alpha.1
After studying and working to meet these targets, it is not surprising that the transition to residency is a big change. As a dermatology preliminary intern, or“prelim,” our experience differs compared to other specialties, as other interns are jumping into their area of practice right away.
During my intern year, I had a tremendous amount of anxiety about 2 things: (1) being a subpar medical intern and (2) being unprepared for the beginning of my dermatology residency. This anxiety drove me to read a tremendous amount of medical and dermatological literature in an effort to do everything. Although hindsight is always 20/20, I will share some thoughts of my own as well as some from friends and colleagues.
First, enjoy intern year. I know that may sound ridiculous, but there were many aspects of intern year that I loved! When your pager beeps, it’s for YOU! You are no longer a subintern, running every decision past your intern or explaining your student status to the patients! Proudly introduce yourself as Dr. So-and-So. You earned it! I loved the camaraderie of working with my co-interns and senior residents. Going through the challenges of intern year together is a deep bonding experience, and I absolutely made lifelong friendships. It also does not hurt that I met my boyfriend (now husband), which has changed my life in a big way.
When it comes to learning internal medicine, pediatrics, or surgery (depending on your intern year), prepare for rounds, read about your patients, and pay attention in Grand Rounds. You can even consider taking the dermatologic cases that may be on your team, just for fun. I am always grateful for my internal medicine knowledge when managing complex medical dermatology patients and rounding on our consultation service on the wards. However, do not burden yourself with excessive studying. Enjoy your time off: spend it with family and friends or rediscover a hobby that has been neglected while you have been working toward your achievements.
When it comes to learning dermatology, do not rush it! You have 3 years and a ton of studying ahead of you! You will learn all of it. When July 1 of your first year of dermatology finally starts, immerse yourself in this new world:
- Attend conferences. Even if they are on topics you might not be interested in—from cosmetics to psoriasis—they provide a real-world perspective and often have great lecturers sharing their knowledge.
- Get involved. There are many dermatologic societies to take part in, and dues are waived or reduced when you sign up as a resident. Many of them provide great resources from study materials to journals, and they are always a great way to network when there are events.
- Volunteer. Many of the dermatologic societies sponsor volunteer events such as skin cancer screenings. It can be a fun way to network while also giving back to the community.
- Spend time figuring out what you really enjoy. This step may seem self-evident, but after many years of fulfilling the necessary criteria to get into medical school and residency, it can be habitual to start fulfilling the same criteria all over again. Explore all aspects of dermatology and see what truly interests you. Consider how you expect your life after residency to be and think what learning opportunities might be helpful down the road. Reach out to attendings you would like to work with, both in dermatology and in other specialties. I personally enjoyed working in wound and oncology clinics, learning how other specialties approach clinical dilemmas that we see in dermatology.
As I embark on my final year of dermatology residency, I am truly grateful for the wisdom that has been shared with me on this journey. Many people have provided key pieces of information that have helped shape my training and my plans for the future, and I hope that sharing it will help others!
- National Resident Matching Program, Charting Outcomes in the Match: U.S. Allopathic Seniors, 2018. Washington, DC: National Resident Matching Program; 2018. http://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed September 20, 2018.
- National Resident Matching Program, Charting Outcomes in the Match: U.S. Allopathic Seniors, 2018. Washington, DC: National Resident Matching Program; 2018. http://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed September 20, 2018.
Weight-loss drug lorcaserin’s glycemic effects revealed
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
REPORTING FROM EASD 2018
Key clinical point: Lorcaserin is an adjunctive treatment to lifestyle modification for chronic weight management that may improve metabolic health.
Major finding: A total of 8.5% of lorcaserin-treated individuals with prediabetes versus 10.3% of placebo-treated individuals developed incident type 2 diabetes mellitus at 1 year (hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038).
Study details: A randomized, double-blind, placebo-controlled trial of 12,000 overweight or obese individuals with established cardiovascular disease, established or no type 2 diabetes mellitus, and other cardiovascular risk factors.
Disclosures: The study was designed by the Thrombolysis in Myocardial Infarction Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
Sources: Bohula May EA et al. Lancet. 2018. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018;379:1107-17; Sattar N. EASD 2018, Session S33.
For most veterans with PTSD, helping others is a lifeline
I am a former military psychiatrist who has published extensively about posttraumatic stress disorder and other psychological effects of war. Thus, I got sent the news clips many times about a potential candidate for mayor of Kansas City leaving the race to care for himself, his depression, and posttraumatic stress disorder symptoms.
Like many of our readers who are physicians, I have a very mixed response to the former candidate’s news.
On the one hand, kudos to him that he has decided to 1) get the help he says he needs, and 2) go public. On the other hand, I really wish that he did not have to drop out of the race to do so.
There are some parallels with leaving for severe physical illness, such as getting chemotherapy for cancer. However, for example, when Gov. Larry Hogan of Maryland received treatment for his cancer, he stayed in office.
Why can you stay in a race or office with cancer or heart disease but not with the very common psychiatric and treatable condition of PTSD?
I certainly do not know all the reasons the candidate for Kansas City mayor made this decision. He said he is encouraging other veterans to follow his example and get treatment for PTSD. He also alluded to suicidal ideation.
This got me thinking about the concept of needing to leave work to take care of yourself – a decision that is often lauded as both noble and wise. I will not opine much on nobility, other than saying it is always noble to help fellow veterans. Maybe his decision to go public will help other veterans. Hard to say. But I can on opine on wisdom, based on many years of working with veterans with PTSD. I almost always advise them to keep their jobs, if at all possible.
Taking time off from a job you care for actually might increase suicidal thoughts. That is due to less structure in the day, less socialization, and fewer feelings of self-worth. A consequent lack of funds might not help. I have long called holding a good job one of the best mental health interventions, superior to medicine and therapy alone (OK – I am being doctrinaire; there are no placebo-controlled, double blind trials on the topic. But I am also serious.)
In general, Why is it work or saving oneself? In my opinion, work helps to save oneself. Helping others, for most veterans, is a lifeline.
I wonder why he should have to drop out of work to receive treatment. Perhaps he was placed in a residential Veterans Affairs program, which often are 30-60 days long. It is notoriously hard to maintain a job during such treatment.
I believe that we should structure our PTSD therapy so that one can both work and receive appropriate treatment. We need war veterans, with or without PTSD, to run for office. And win.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
I am a former military psychiatrist who has published extensively about posttraumatic stress disorder and other psychological effects of war. Thus, I got sent the news clips many times about a potential candidate for mayor of Kansas City leaving the race to care for himself, his depression, and posttraumatic stress disorder symptoms.
Like many of our readers who are physicians, I have a very mixed response to the former candidate’s news.
On the one hand, kudos to him that he has decided to 1) get the help he says he needs, and 2) go public. On the other hand, I really wish that he did not have to drop out of the race to do so.
There are some parallels with leaving for severe physical illness, such as getting chemotherapy for cancer. However, for example, when Gov. Larry Hogan of Maryland received treatment for his cancer, he stayed in office.
Why can you stay in a race or office with cancer or heart disease but not with the very common psychiatric and treatable condition of PTSD?
I certainly do not know all the reasons the candidate for Kansas City mayor made this decision. He said he is encouraging other veterans to follow his example and get treatment for PTSD. He also alluded to suicidal ideation.
This got me thinking about the concept of needing to leave work to take care of yourself – a decision that is often lauded as both noble and wise. I will not opine much on nobility, other than saying it is always noble to help fellow veterans. Maybe his decision to go public will help other veterans. Hard to say. But I can on opine on wisdom, based on many years of working with veterans with PTSD. I almost always advise them to keep their jobs, if at all possible.
Taking time off from a job you care for actually might increase suicidal thoughts. That is due to less structure in the day, less socialization, and fewer feelings of self-worth. A consequent lack of funds might not help. I have long called holding a good job one of the best mental health interventions, superior to medicine and therapy alone (OK – I am being doctrinaire; there are no placebo-controlled, double blind trials on the topic. But I am also serious.)
In general, Why is it work or saving oneself? In my opinion, work helps to save oneself. Helping others, for most veterans, is a lifeline.
I wonder why he should have to drop out of work to receive treatment. Perhaps he was placed in a residential Veterans Affairs program, which often are 30-60 days long. It is notoriously hard to maintain a job during such treatment.
I believe that we should structure our PTSD therapy so that one can both work and receive appropriate treatment. We need war veterans, with or without PTSD, to run for office. And win.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
I am a former military psychiatrist who has published extensively about posttraumatic stress disorder and other psychological effects of war. Thus, I got sent the news clips many times about a potential candidate for mayor of Kansas City leaving the race to care for himself, his depression, and posttraumatic stress disorder symptoms.
Like many of our readers who are physicians, I have a very mixed response to the former candidate’s news.
On the one hand, kudos to him that he has decided to 1) get the help he says he needs, and 2) go public. On the other hand, I really wish that he did not have to drop out of the race to do so.
There are some parallels with leaving for severe physical illness, such as getting chemotherapy for cancer. However, for example, when Gov. Larry Hogan of Maryland received treatment for his cancer, he stayed in office.
Why can you stay in a race or office with cancer or heart disease but not with the very common psychiatric and treatable condition of PTSD?
I certainly do not know all the reasons the candidate for Kansas City mayor made this decision. He said he is encouraging other veterans to follow his example and get treatment for PTSD. He also alluded to suicidal ideation.
This got me thinking about the concept of needing to leave work to take care of yourself – a decision that is often lauded as both noble and wise. I will not opine much on nobility, other than saying it is always noble to help fellow veterans. Maybe his decision to go public will help other veterans. Hard to say. But I can on opine on wisdom, based on many years of working with veterans with PTSD. I almost always advise them to keep their jobs, if at all possible.
Taking time off from a job you care for actually might increase suicidal thoughts. That is due to less structure in the day, less socialization, and fewer feelings of self-worth. A consequent lack of funds might not help. I have long called holding a good job one of the best mental health interventions, superior to medicine and therapy alone (OK – I am being doctrinaire; there are no placebo-controlled, double blind trials on the topic. But I am also serious.)
In general, Why is it work or saving oneself? In my opinion, work helps to save oneself. Helping others, for most veterans, is a lifeline.
I wonder why he should have to drop out of work to receive treatment. Perhaps he was placed in a residential Veterans Affairs program, which often are 30-60 days long. It is notoriously hard to maintain a job during such treatment.
I believe that we should structure our PTSD therapy so that one can both work and receive appropriate treatment. We need war veterans, with or without PTSD, to run for office. And win.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
A change in ‘incident to’ billing
Also today, stepping down to oral ciprofloxacin looks safe in gram-negative bloodstream infections, a nasal cannula device may be an option for severe COPD, and brexanolone injection quickly improves postpartum depression.
Also today, stepping down to oral ciprofloxacin looks safe in gram-negative bloodstream infections, a nasal cannula device may be an option for severe COPD, and brexanolone injection quickly improves postpartum depression.
Also today, stepping down to oral ciprofloxacin looks safe in gram-negative bloodstream infections, a nasal cannula device may be an option for severe COPD, and brexanolone injection quickly improves postpartum depression.
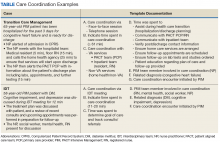
Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Chronic diseases affect a substantial proportion of the US population, with 25% of adults diagnosed with 2 or more chronic health conditions.1 In 2010, 2 chronic diseases, heart disease and cancer, accounted for nearly 48% of deaths.2 Due to the significant public heath burden, strategies to improve chronic disease management have attracted a great deal of focus.3,4 Within increasingly complex health care delivery systems, policy makers are promoting care coordination (CC) as a tool to reduce fragmented care for patients with multiple comorbidities, improve patient experience and quality of care, and decrease costs and risks for error.3-8
Background
The Agency for Healthcare Research and Quality (AHRQ) defines care coordination as “deliberately organizing patient care activities and sharing information among all of the participants concerned with a patient’s care to achieve safer and more effective care.”5 Nationally, large scale investments have expanded health care models that provide team-based CC, such as patient-centered medical homes, known as patient-aligned care teams (PACTs) within the Department of Veterans Affairs (VA), accountable care organizations, and other complex care management programs.9-12 Additionally, incentives that reimburse for CC, such as Medicare’s chronic care management and transition care management billing codes, also are emerging.13,14
While there is significant interest and investment in promoting CC, little data about the specific activities and time required to provide necessary CC exist, which limits the ability of health care teams to optimize CC delivery.6 Understanding the components of CC has implications for human resource allocation, labor mapping, reimbursement, staff training, and optimizing collaborative networks for health care systems, which may improve the quality of CC and health outcomes for patients. To date, few tools exist that can be used to identify and track the CC services delivered by interdisciplinary teams within and outside of the health care setting.
This article describes the development and preliminary results of the implementation of a CC Template that was created in the VA Computerized Patient Record System (CPRS) to identify and track the components of CC services, delivered by a multidisciplinary team, as part of a quality improvement (QI) pilot project. Through use of the template, the team sought a formative understanding of the following questions: (1) Is it feasible to use the CC Template during routine workflow? (2) What specific types of CC services are provided by the team? (3) How much time does it take to perform these activities? (4) Who is the team collaborating with inside and outside of the health care setting and how are they communicating? (5) Given new reimbursement incentives, can the provision of CC be standardized and documented for broad applicability?
In complex systems, where coordination is needed among primary, specialty, hospital, emergency, and nonclinical care settings, a tool such as the CC Template offers a sustainable and replicable way to standardize documentation and knowledge about CC components. This foundational information can be used to optimize team structure, training, and resource allocation, to improve the quality of CC and to link elements of CC with clinical and operational outcomes.
Pact Intensive Management
Despite the implementation of PACT within VA, patients with complex medical conditions combined with socioeconomic stressors, mental health comorbidities, and low health literacy are at high risk for preventable hospitalizations and acute care utilization.17,18 Due to unmet needs that are beyond what PACTs are able to deliver, these high-risk patients may benefit from additional services to coordinate care within and outside the VA health care system, as suggest by the Extended Chronic Care Model.19-21
In 2014, the Office of Primary Care Services sponsored a QI initiative at 5 VA demonstration sites to develop PACT Intensive Management (PIM) interventions targeting patients at high risk for hospitalization and acute care utilization within VA. The PIM program design is based on work described previously, with patients identified for enrollment based on 90-day hospitalization risk ≥ 90th percentile, based on a VA risk modeling tool, and an acute care episode in the previous 6 months. 19 A common component of all PIM programs is the provision of intensive care management and CC by an interdisciplinary team working in conjunction with PACT. The CC Template was developed to assist in documenting and rigorously understanding the implementation of CC by the PIM team.
Local Setting
The Atlanta VA Medical Center (AVAMC) was chosen as one of the PIM demonstration sites. The Atlanta PIM team identified and enrolled a random sample of eligible, high-risk patients from 1 community-based outpatient clinic (CBOC) in an urban location with 7,524 unique patients. Between September 2014 and September 2016, 300 patients were identified, and 86 patients agreed to participate in the PIM program.
In the CC Template pilot, the Atlanta PIM team included 2 nurse practitioners (NP), 2 social workers (SW), and 1 telehealth registered nurse (RN). Upon enrollment, members of the PIM team conducted comprehensive home assessments and offered intensive care management for medical, social, and behavioral needs. The main pillars of care management offered to high-risk patients were based on previous work done both inside and outside VA and included home visits, telephone-based disease management, co-attending appointments with patients, transition care management, and interdisciplinary team meetings with a focus on care coordination between PACT and all services required by patients.11,19
The Atlanta PIM team performed a variety of tasks to coordinate care for enrolled patients that included simple, 1-step tasks, such as chart reviews, and multistep, complex tasks that required the expertise of multiple team members (Figure 1).
Additionally, inconsistency in delivery of CC between PIM team members was noted. For example, there was significant variability in CC services provided by different team members in the provision of transition care management (TCM) and coordinating care from hospitalization back to home. Some PIM staff coordinated care and communicated with the patient, hospital team, home-care service, and primary-care team, while other staff only reviewed the chart and placed orders in CPRS. Additionally, much of the CC work was documented in administrative notes that did not trigger workload credit. This made it difficult to show how to appropriately labor map PIM staff or how staff were spending their time caring for patients.
In order to standardize documentation of the interdisciplinary CC activities performed by the PIM team and account for staff time, the Atlanta PIM team decided to develop a CPRS CC Template. The objective of the CC Template was to facilitate documentation of CC activities in the EHR, describe the types of CC activities performed by PIM team members, and track the time to perform these activities for patients with various chronic diseases.
Template Design and Implementation
The original design of the template was informed by the Atlanta PIM team after several informal focus groups and process mapping of CC pathways in the fall of 2015. The participants were all members of the Atlanta PIM team, 2 primary care physicians working with PIM, an AVAMC documentation specialist and a clinical applications coordinator (CAC) assigned to work with PIM. The major themes that arose during the brainstorming discussion were that the template should: (1) be feasible to use during their daily clinic workflow; (2) improve documentation of CC; and (3) have value for spread to other VA sites. Discussion centered on creating a CC Template versatile enough to:
- Decrease the number of steps for documenting CC;
- Consist only of check boxes, with very little need for free text, with the option to enter narrative free text after template completion;
- Document time spent in aggregate for completing complex CC encounters;
- Document various types of CC work and modes of communication;
- Allow for use by all PIM staff;
- Identify all team members that participated in the CC encounter to reduce redundant documentation by multiple staff;
- Adapt to different clinic sites based on the varied disciplines participating in other locations;
- Use evidence-based checklists to help standardize delivery of CC for certain activities such as TCM; and
- Extract data without extensive chart reviews to inform current CC and future QI work.
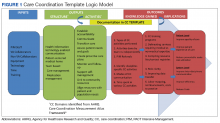
Following the brainstorming sessions, the authors performed a literature review to identify and integrate CC best practices. The AHRQ Care Coordination Atlas served as the main resource in the design of the logic model that depicted the delivery and subsequent documentation of high-quality, evidence-based CC in the CC Template (Figure 2).6
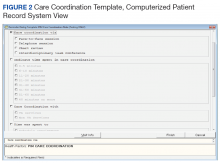
After reaching consensus about the key components of the CC Template, the CAC created a pilot version (Figure 3). All of the elements within the CC Template allowed for data abstraction from the VA Corporate Data Warehouse (CDW) via discrete data elements known as health factors.
Over the course of implementation, the team became more enthusiastic about using the CC template to document previously unrecognized CC workload. Because the CC Template only was used to document CC workload and excluded encounters for clinical evaluation and management, specific notes were created and linked with the CC Template for optimal capture of encounters.
All components of the template were mandatory to eliminate the possibility of missing data. The Atlanta PIM site principal investigator developed a multicomponent training designed to increase support for the template by describing its value and to mitigate the potential for variability in how data are captured. Training included a face-to-face session with the team to review the template and work through sample CC cases. Additionally, a training manual with clear operational definitions and examples of how to complete each element of the CC Template was disseminated. The training was subsequently conducted with the San Francisco VA Medical Center PIM team, a spread site, via video conference. The spread site offered significant feedback on clarifying the training documents and adapting the CC template for their distinct care team structure. This feedback was incorporated into the final CC Template design to increase adaptability.
Implementation Evaluation
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)framework served as the basis for evaluation of CC Template implementation. The RE-AIM framework is well established and able to evaluate the implementation and potential successful spread of new programs.23,24 Using RE-AIM, the authors planned to analyze data to explore the reach effectiveness, adoption, implementation, and maintenance of the CC Template use while providing complex care management for high-risk patients.
All data for the evaluation was extracted from the CDW by a data analyst and stored on a secure server. A statistical process control (SPC) chart was used to analyze the implementation process to assess variation in template use.
Results
After implementation, 35 weeks of CC Template pilot data were analyzed from June 1, 2015 to January 5, 2016. The PIM team completed 393 CC Templates over this collection period. After week 23, the CC template was linked to specific CC notes automatically. From weeks 23 to 35 an average of 20.3 CC Templates were completed per week by the team. The RE-AIM was used to assess the implementation of the CC Template.
Reach was determined by the number of patients enrolled in PIM with CC Template documentation. Of patients enrolled in Atlanta PIM, 90.1% had ≥ 1 CC encounter documented by the CC Template; 74.4% of Atlanta PIM patients had ≥ 1 CC encounter documented; 15.5% of patients had > 10 CC encounters documented; and 1 patient had > 25 CC encounters documented by the CC template.
Effectiveness for describing CC activities was captured through data from CC Template. The CC Template documentation by the PIM team showed that 79.4% of CC encounters were < 20 minutes, and 9.9% of encounters were > 61 minutes. Telephone communication was involved in 50.4% of CC encounters, and 24% required multiple modes of communication such as face-to-face, instant messenger, chart-based communication. Care coordination during hospitalization and discharge accounted for 5.9% of template use. Of the CC encounters documenting hospital transitions, 94.4% documented communication with the inpatient team, 58.3% documented coordination with social support, and only 11.1% documented communication with primary care teams. Improving communication with PACT teams after hospital discharge was identified as a future QI project based on these data. The PIM team initiated 83.2% of CC encounters.
Adoption was determined by the use of the CC Template by the team. All 5 team members used the CC template to document at least 1 CC encounter.
Implementation allowed for improvement based on feedback from the PIM team. Mean completion of CC Templates rose from 10.9 per week to 20.3 per week after automatically linking the CC Template to specific CC notes. (Figure 4)
Maintenance was monitored over the course of the pilot. Consistent use of the CC Template over 35 full work weeks of data collection was seen, and mean utilization per week nearly doubled in the latter half of the pilot period.
Because several elements were added to the CC Template over the course of the pilot period, our ability to analyze the data for descriptive statistics about the types of CC services, related diagnoses, collaborators, and PIM staff involved in CC encounters was limited.
Discussion
Though all components of CC encounters could not be assessed during the pilot phase due to continuous improvement of the CC Template, the authors showed that it is feasible to use this tool to document and describe granular details about team-based CC. Pilot data from AVAMC show that the use of the CC Template standardized team CC documentation in a busy clinic setting provided data about the complexity of coordination activities and duration of CC activities. It also informed future CC QI projects, such as improving communication with primary care during the hospital discharge process.
Future evaluation of CC Template data can be used to (1) describe types of CC activities for high-risk PIM patients; (2) quantify the time required to complete CC activities to assist with staff labor mapping; (3) describe staff roles and referrals needed to complete specific CC activities inside and outside VA; (4) describe modes of communication between PIM and collaborators; (5) relate patient demographics and associated diagnoses with quantity of CC encounters; and (6) quantify frequency and time frame of CC after hospitalizations and ED care and subsequent impact on repeat hospitalizations and ED visits. Future research also can explore the link between CC activities and effort with clinical and patient-reported outcomes.
Social network analysis could be used with CC Template data to understand the network of referrals and collaborators involved in the care of a CC team’s patients. This type of analysis would assist teams to strengthen and formalize ties with collaborators as appropriate. For example, if data show that the team frequently collaborates with the cardiology clinic for a large subset of its patients, they may consider creating a CC agreement with formalized modes of communication that would streamline collaboration.
In order to improve the quality of the CC Template and to assess factors that may lead to sustainable use in clinical practice, qualitative assessment through survey, interview, or usability testing with staff would be beneficial to identify strategies to increase its adoption among clinical providers. This type of assessment will add knowledge about the CC Template implementation process, including contextual barriers or facilitators, feasibility of use during day-to-day operations, versatility of template use within construct of team-based care, and overall satisfaction with the template.
Limitations
Though the CC Template offers a large amount of data about the components of CC delivery, the information is based on self-report by staff. Training to ensure that all team members are documenting in the same manner is crucial to maintain the internal validity of the data. The template is limited to the fields currently developed, and future research could explore additional data elements that are critical to include based on feedback from VA staff.
Conclusion
To our knowledge, this VA medical center CC Template is the first tool described in the literature that standardizes and captures data about CC components in the EHR. This pilot data show that the template is feasible for use in a busy clinic setting and can streamline the process for capturing CC data that may otherwise not be documented.
During the pilot phase, the CC template allowed the PIM team to identify a small subset of patients within the PIM complex management who have a high level of CC needs. By identifying these patients, further work can be done to understand the specific needs of these higher utilizers and the types of CC activities required to assist them so that resources can be directed appropriately to that smaller subset. Telephone CC accounted for a large proportion of delivery, which has implications for ensuring that staff have access to mobile phones and EHR capability to document this additional workload. The PIM staff maintained use of the template throughout the pilot period and increased documentation when the CC Template was easily accessible and already linked to their CPRS notes, suggesting that in future implementation, ensuring that the template is linked to notes in use by the care team will be important for successful spread.
Additionally, CC Template data identified gaps in high-quality, evidence-based CC that can be addressed in real time, for example during the discharge process. Data from the CC Template showed that only 11.1% of CC encounters had documentation of communication between the PIM and primary care teams during transitions from hospital to home. Improving communication with PACT teams after hospital discharge was identified as a future PIM QI project based on these data. By improving documentation of CC in the EHR, the resulting information is foundational for future work that can improve the quality of team-based CC; plan staffing, team composition, and labor mapping; determine the cost of CC activities and improve reimbursement in certain settings; and assess outcomes of CC.
This tool has potential for application beyond the PIM team in the VA. The CC Template and training manual is scalable to any setting with team-based CC, including PACT, homeless programs, palliative care, Mental Health Intensive Case Management (MHICM) programs, nurse navigator programs, and other complex care delivery models involving care coordinators. Future study of its implementation and data may inform initiatives to develop ongoing team-based care coordination programs.
Acknowledgments
The authors thank the following colleagues for their input and support: Florence Longchamp, RN, Clinical Applications Coordinator at the Atlanta VA Medical Center without whom the CC Template would not have been created; the Atlanta and San Francisco VA PIM teams for their thoughtful comments and enthusiastic embrace of the CC Template; and the PIM National Evaluation Center for their support of this QI project. PACT Intensive Management demonstration sites are funded by the VA Office of Patient Care Services. During the implementation of the CC Template pilot and the preparation of this paper, the primary author was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Advanced Fellowships, VA Quality Scholars Program.
1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
2. Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated May 3, 2017. Accessed August 8, 2018.
3. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
4. US Department of Health and Human Services. Healthy people 2010: general data issues. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_general_data_issues.pdf. Published 2010. Accessed August 1, 2018.
5. McDonald KM, Sunderam V, Bravata DM, et al. Closing the quality gap: a critical analysis of quality improvement strategies, Vol 7: care coordination. Agency for Healthcare Research and Quality. https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. Published June 2007. Accessed August 1, 2018.
6. McDonald KM, Schultz E, Albin L, et al. Care coordination measures atlas. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed August 2, 2018.
7. Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating care across diseases, settings, and clinicians: a key role for the generalist in practice. Ann Intern Med. 2005;142(8):700-708.
8. Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15(5):329-336.
9. National Committee for Quality Assurance. The future of patient-centered medical homes: foundation for a better health care system. https://www.ncqa.org/Portals/0/Public%20Policy/2014%20PDFS/The_Future_of_PCMH.pdf. Accessed August 2, 2018.
10. US Department of Veterans Affairs, Veterans Health Administration. Patient Aligned Care Team (PACT) Handbook. VHA Handbook 1101.10:1–65. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2977. Updated May 26, 2017. Accessed August 2, 2018.
11. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
12. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19.
13. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Chronic care management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Published December 2016. Accessed August 2, 2018.
14. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Frequently asked questions about billing the Medicare physician fee schedule for transitional care management services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-TCMS.pdf. Published March 17, 2016. Accessed August 2, 2018.
15. Antonelli RC, Stille CJ, Antonelli DM. Care coordination for children and youth with special health care needs: a descriptive, multisite study of activities, personnel costs, and outcomes. Pediatrics. 2008;122(1):e209-e216.
16. Antonelli RC, Antonelli DM. Providing a medical home: the cost of care coordination services in a community-based, general pediatric practice. Pediatrics. 2004;113( suppl 5 ):1522-1528.
17. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
18. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an intensive management patient-aligned care team (ImPACT). J Gen Intern Med. 2014;29(suppl 4):S861-S869.
20. Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166-175.
21. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73-82.
22. US Department of Health & Human Services, Agency for Healthcare Research and Quality. Re-engineered discharge (RED) toolkit. http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html. Updated May 2017. Accessed August 3, 2018.
23. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327.
24. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-e46.
Chronic diseases affect a substantial proportion of the US population, with 25% of adults diagnosed with 2 or more chronic health conditions.1 In 2010, 2 chronic diseases, heart disease and cancer, accounted for nearly 48% of deaths.2 Due to the significant public heath burden, strategies to improve chronic disease management have attracted a great deal of focus.3,4 Within increasingly complex health care delivery systems, policy makers are promoting care coordination (CC) as a tool to reduce fragmented care for patients with multiple comorbidities, improve patient experience and quality of care, and decrease costs and risks for error.3-8
Background
The Agency for Healthcare Research and Quality (AHRQ) defines care coordination as “deliberately organizing patient care activities and sharing information among all of the participants concerned with a patient’s care to achieve safer and more effective care.”5 Nationally, large scale investments have expanded health care models that provide team-based CC, such as patient-centered medical homes, known as patient-aligned care teams (PACTs) within the Department of Veterans Affairs (VA), accountable care organizations, and other complex care management programs.9-12 Additionally, incentives that reimburse for CC, such as Medicare’s chronic care management and transition care management billing codes, also are emerging.13,14
While there is significant interest and investment in promoting CC, little data about the specific activities and time required to provide necessary CC exist, which limits the ability of health care teams to optimize CC delivery.6 Understanding the components of CC has implications for human resource allocation, labor mapping, reimbursement, staff training, and optimizing collaborative networks for health care systems, which may improve the quality of CC and health outcomes for patients. To date, few tools exist that can be used to identify and track the CC services delivered by interdisciplinary teams within and outside of the health care setting.
This article describes the development and preliminary results of the implementation of a CC Template that was created in the VA Computerized Patient Record System (CPRS) to identify and track the components of CC services, delivered by a multidisciplinary team, as part of a quality improvement (QI) pilot project. Through use of the template, the team sought a formative understanding of the following questions: (1) Is it feasible to use the CC Template during routine workflow? (2) What specific types of CC services are provided by the team? (3) How much time does it take to perform these activities? (4) Who is the team collaborating with inside and outside of the health care setting and how are they communicating? (5) Given new reimbursement incentives, can the provision of CC be standardized and documented for broad applicability?
In complex systems, where coordination is needed among primary, specialty, hospital, emergency, and nonclinical care settings, a tool such as the CC Template offers a sustainable and replicable way to standardize documentation and knowledge about CC components. This foundational information can be used to optimize team structure, training, and resource allocation, to improve the quality of CC and to link elements of CC with clinical and operational outcomes.
Pact Intensive Management
Despite the implementation of PACT within VA, patients with complex medical conditions combined with socioeconomic stressors, mental health comorbidities, and low health literacy are at high risk for preventable hospitalizations and acute care utilization.17,18 Due to unmet needs that are beyond what PACTs are able to deliver, these high-risk patients may benefit from additional services to coordinate care within and outside the VA health care system, as suggest by the Extended Chronic Care Model.19-21
In 2014, the Office of Primary Care Services sponsored a QI initiative at 5 VA demonstration sites to develop PACT Intensive Management (PIM) interventions targeting patients at high risk for hospitalization and acute care utilization within VA. The PIM program design is based on work described previously, with patients identified for enrollment based on 90-day hospitalization risk ≥ 90th percentile, based on a VA risk modeling tool, and an acute care episode in the previous 6 months. 19 A common component of all PIM programs is the provision of intensive care management and CC by an interdisciplinary team working in conjunction with PACT. The CC Template was developed to assist in documenting and rigorously understanding the implementation of CC by the PIM team.
Local Setting
The Atlanta VA Medical Center (AVAMC) was chosen as one of the PIM demonstration sites. The Atlanta PIM team identified and enrolled a random sample of eligible, high-risk patients from 1 community-based outpatient clinic (CBOC) in an urban location with 7,524 unique patients. Between September 2014 and September 2016, 300 patients were identified, and 86 patients agreed to participate in the PIM program.
In the CC Template pilot, the Atlanta PIM team included 2 nurse practitioners (NP), 2 social workers (SW), and 1 telehealth registered nurse (RN). Upon enrollment, members of the PIM team conducted comprehensive home assessments and offered intensive care management for medical, social, and behavioral needs. The main pillars of care management offered to high-risk patients were based on previous work done both inside and outside VA and included home visits, telephone-based disease management, co-attending appointments with patients, transition care management, and interdisciplinary team meetings with a focus on care coordination between PACT and all services required by patients.11,19
The Atlanta PIM team performed a variety of tasks to coordinate care for enrolled patients that included simple, 1-step tasks, such as chart reviews, and multistep, complex tasks that required the expertise of multiple team members (Figure 1).
Additionally, inconsistency in delivery of CC between PIM team members was noted. For example, there was significant variability in CC services provided by different team members in the provision of transition care management (TCM) and coordinating care from hospitalization back to home. Some PIM staff coordinated care and communicated with the patient, hospital team, home-care service, and primary-care team, while other staff only reviewed the chart and placed orders in CPRS. Additionally, much of the CC work was documented in administrative notes that did not trigger workload credit. This made it difficult to show how to appropriately labor map PIM staff or how staff were spending their time caring for patients.
In order to standardize documentation of the interdisciplinary CC activities performed by the PIM team and account for staff time, the Atlanta PIM team decided to develop a CPRS CC Template. The objective of the CC Template was to facilitate documentation of CC activities in the EHR, describe the types of CC activities performed by PIM team members, and track the time to perform these activities for patients with various chronic diseases.
Template Design and Implementation
The original design of the template was informed by the Atlanta PIM team after several informal focus groups and process mapping of CC pathways in the fall of 2015. The participants were all members of the Atlanta PIM team, 2 primary care physicians working with PIM, an AVAMC documentation specialist and a clinical applications coordinator (CAC) assigned to work with PIM. The major themes that arose during the brainstorming discussion were that the template should: (1) be feasible to use during their daily clinic workflow; (2) improve documentation of CC; and (3) have value for spread to other VA sites. Discussion centered on creating a CC Template versatile enough to:
- Decrease the number of steps for documenting CC;
- Consist only of check boxes, with very little need for free text, with the option to enter narrative free text after template completion;
- Document time spent in aggregate for completing complex CC encounters;
- Document various types of CC work and modes of communication;
- Allow for use by all PIM staff;
- Identify all team members that participated in the CC encounter to reduce redundant documentation by multiple staff;
- Adapt to different clinic sites based on the varied disciplines participating in other locations;
- Use evidence-based checklists to help standardize delivery of CC for certain activities such as TCM; and
- Extract data without extensive chart reviews to inform current CC and future QI work.
Following the brainstorming sessions, the authors performed a literature review to identify and integrate CC best practices. The AHRQ Care Coordination Atlas served as the main resource in the design of the logic model that depicted the delivery and subsequent documentation of high-quality, evidence-based CC in the CC Template (Figure 2).6
After reaching consensus about the key components of the CC Template, the CAC created a pilot version (Figure 3). All of the elements within the CC Template allowed for data abstraction from the VA Corporate Data Warehouse (CDW) via discrete data elements known as health factors.
Over the course of implementation, the team became more enthusiastic about using the CC template to document previously unrecognized CC workload. Because the CC Template only was used to document CC workload and excluded encounters for clinical evaluation and management, specific notes were created and linked with the CC Template for optimal capture of encounters.
All components of the template were mandatory to eliminate the possibility of missing data. The Atlanta PIM site principal investigator developed a multicomponent training designed to increase support for the template by describing its value and to mitigate the potential for variability in how data are captured. Training included a face-to-face session with the team to review the template and work through sample CC cases. Additionally, a training manual with clear operational definitions and examples of how to complete each element of the CC Template was disseminated. The training was subsequently conducted with the San Francisco VA Medical Center PIM team, a spread site, via video conference. The spread site offered significant feedback on clarifying the training documents and adapting the CC template for their distinct care team structure. This feedback was incorporated into the final CC Template design to increase adaptability.
Implementation Evaluation
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)framework served as the basis for evaluation of CC Template implementation. The RE-AIM framework is well established and able to evaluate the implementation and potential successful spread of new programs.23,24 Using RE-AIM, the authors planned to analyze data to explore the reach effectiveness, adoption, implementation, and maintenance of the CC Template use while providing complex care management for high-risk patients.
All data for the evaluation was extracted from the CDW by a data analyst and stored on a secure server. A statistical process control (SPC) chart was used to analyze the implementation process to assess variation in template use.
Results
After implementation, 35 weeks of CC Template pilot data were analyzed from June 1, 2015 to January 5, 2016. The PIM team completed 393 CC Templates over this collection period. After week 23, the CC template was linked to specific CC notes automatically. From weeks 23 to 35 an average of 20.3 CC Templates were completed per week by the team. The RE-AIM was used to assess the implementation of the CC Template.
Reach was determined by the number of patients enrolled in PIM with CC Template documentation. Of patients enrolled in Atlanta PIM, 90.1% had ≥ 1 CC encounter documented by the CC Template; 74.4% of Atlanta PIM patients had ≥ 1 CC encounter documented; 15.5% of patients had > 10 CC encounters documented; and 1 patient had > 25 CC encounters documented by the CC template.
Effectiveness for describing CC activities was captured through data from CC Template. The CC Template documentation by the PIM team showed that 79.4% of CC encounters were < 20 minutes, and 9.9% of encounters were > 61 minutes. Telephone communication was involved in 50.4% of CC encounters, and 24% required multiple modes of communication such as face-to-face, instant messenger, chart-based communication. Care coordination during hospitalization and discharge accounted for 5.9% of template use. Of the CC encounters documenting hospital transitions, 94.4% documented communication with the inpatient team, 58.3% documented coordination with social support, and only 11.1% documented communication with primary care teams. Improving communication with PACT teams after hospital discharge was identified as a future QI project based on these data. The PIM team initiated 83.2% of CC encounters.
Adoption was determined by the use of the CC Template by the team. All 5 team members used the CC template to document at least 1 CC encounter.
Implementation allowed for improvement based on feedback from the PIM team. Mean completion of CC Templates rose from 10.9 per week to 20.3 per week after automatically linking the CC Template to specific CC notes. (Figure 4)
Maintenance was monitored over the course of the pilot. Consistent use of the CC Template over 35 full work weeks of data collection was seen, and mean utilization per week nearly doubled in the latter half of the pilot period.
Because several elements were added to the CC Template over the course of the pilot period, our ability to analyze the data for descriptive statistics about the types of CC services, related diagnoses, collaborators, and PIM staff involved in CC encounters was limited.
Discussion
Though all components of CC encounters could not be assessed during the pilot phase due to continuous improvement of the CC Template, the authors showed that it is feasible to use this tool to document and describe granular details about team-based CC. Pilot data from AVAMC show that the use of the CC Template standardized team CC documentation in a busy clinic setting provided data about the complexity of coordination activities and duration of CC activities. It also informed future CC QI projects, such as improving communication with primary care during the hospital discharge process.
Future evaluation of CC Template data can be used to (1) describe types of CC activities for high-risk PIM patients; (2) quantify the time required to complete CC activities to assist with staff labor mapping; (3) describe staff roles and referrals needed to complete specific CC activities inside and outside VA; (4) describe modes of communication between PIM and collaborators; (5) relate patient demographics and associated diagnoses with quantity of CC encounters; and (6) quantify frequency and time frame of CC after hospitalizations and ED care and subsequent impact on repeat hospitalizations and ED visits. Future research also can explore the link between CC activities and effort with clinical and patient-reported outcomes.
Social network analysis could be used with CC Template data to understand the network of referrals and collaborators involved in the care of a CC team’s patients. This type of analysis would assist teams to strengthen and formalize ties with collaborators as appropriate. For example, if data show that the team frequently collaborates with the cardiology clinic for a large subset of its patients, they may consider creating a CC agreement with formalized modes of communication that would streamline collaboration.
In order to improve the quality of the CC Template and to assess factors that may lead to sustainable use in clinical practice, qualitative assessment through survey, interview, or usability testing with staff would be beneficial to identify strategies to increase its adoption among clinical providers. This type of assessment will add knowledge about the CC Template implementation process, including contextual barriers or facilitators, feasibility of use during day-to-day operations, versatility of template use within construct of team-based care, and overall satisfaction with the template.
Limitations
Though the CC Template offers a large amount of data about the components of CC delivery, the information is based on self-report by staff. Training to ensure that all team members are documenting in the same manner is crucial to maintain the internal validity of the data. The template is limited to the fields currently developed, and future research could explore additional data elements that are critical to include based on feedback from VA staff.
Conclusion
To our knowledge, this VA medical center CC Template is the first tool described in the literature that standardizes and captures data about CC components in the EHR. This pilot data show that the template is feasible for use in a busy clinic setting and can streamline the process for capturing CC data that may otherwise not be documented.
During the pilot phase, the CC template allowed the PIM team to identify a small subset of patients within the PIM complex management who have a high level of CC needs. By identifying these patients, further work can be done to understand the specific needs of these higher utilizers and the types of CC activities required to assist them so that resources can be directed appropriately to that smaller subset. Telephone CC accounted for a large proportion of delivery, which has implications for ensuring that staff have access to mobile phones and EHR capability to document this additional workload. The PIM staff maintained use of the template throughout the pilot period and increased documentation when the CC Template was easily accessible and already linked to their CPRS notes, suggesting that in future implementation, ensuring that the template is linked to notes in use by the care team will be important for successful spread.
Additionally, CC Template data identified gaps in high-quality, evidence-based CC that can be addressed in real time, for example during the discharge process. Data from the CC Template showed that only 11.1% of CC encounters had documentation of communication between the PIM and primary care teams during transitions from hospital to home. Improving communication with PACT teams after hospital discharge was identified as a future PIM QI project based on these data. By improving documentation of CC in the EHR, the resulting information is foundational for future work that can improve the quality of team-based CC; plan staffing, team composition, and labor mapping; determine the cost of CC activities and improve reimbursement in certain settings; and assess outcomes of CC.
This tool has potential for application beyond the PIM team in the VA. The CC Template and training manual is scalable to any setting with team-based CC, including PACT, homeless programs, palliative care, Mental Health Intensive Case Management (MHICM) programs, nurse navigator programs, and other complex care delivery models involving care coordinators. Future study of its implementation and data may inform initiatives to develop ongoing team-based care coordination programs.
Acknowledgments
The authors thank the following colleagues for their input and support: Florence Longchamp, RN, Clinical Applications Coordinator at the Atlanta VA Medical Center without whom the CC Template would not have been created; the Atlanta and San Francisco VA PIM teams for their thoughtful comments and enthusiastic embrace of the CC Template; and the PIM National Evaluation Center for their support of this QI project. PACT Intensive Management demonstration sites are funded by the VA Office of Patient Care Services. During the implementation of the CC Template pilot and the preparation of this paper, the primary author was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Advanced Fellowships, VA Quality Scholars Program.
Chronic diseases affect a substantial proportion of the US population, with 25% of adults diagnosed with 2 or more chronic health conditions.1 In 2010, 2 chronic diseases, heart disease and cancer, accounted for nearly 48% of deaths.2 Due to the significant public heath burden, strategies to improve chronic disease management have attracted a great deal of focus.3,4 Within increasingly complex health care delivery systems, policy makers are promoting care coordination (CC) as a tool to reduce fragmented care for patients with multiple comorbidities, improve patient experience and quality of care, and decrease costs and risks for error.3-8
Background
The Agency for Healthcare Research and Quality (AHRQ) defines care coordination as “deliberately organizing patient care activities and sharing information among all of the participants concerned with a patient’s care to achieve safer and more effective care.”5 Nationally, large scale investments have expanded health care models that provide team-based CC, such as patient-centered medical homes, known as patient-aligned care teams (PACTs) within the Department of Veterans Affairs (VA), accountable care organizations, and other complex care management programs.9-12 Additionally, incentives that reimburse for CC, such as Medicare’s chronic care management and transition care management billing codes, also are emerging.13,14
While there is significant interest and investment in promoting CC, little data about the specific activities and time required to provide necessary CC exist, which limits the ability of health care teams to optimize CC delivery.6 Understanding the components of CC has implications for human resource allocation, labor mapping, reimbursement, staff training, and optimizing collaborative networks for health care systems, which may improve the quality of CC and health outcomes for patients. To date, few tools exist that can be used to identify and track the CC services delivered by interdisciplinary teams within and outside of the health care setting.
This article describes the development and preliminary results of the implementation of a CC Template that was created in the VA Computerized Patient Record System (CPRS) to identify and track the components of CC services, delivered by a multidisciplinary team, as part of a quality improvement (QI) pilot project. Through use of the template, the team sought a formative understanding of the following questions: (1) Is it feasible to use the CC Template during routine workflow? (2) What specific types of CC services are provided by the team? (3) How much time does it take to perform these activities? (4) Who is the team collaborating with inside and outside of the health care setting and how are they communicating? (5) Given new reimbursement incentives, can the provision of CC be standardized and documented for broad applicability?
In complex systems, where coordination is needed among primary, specialty, hospital, emergency, and nonclinical care settings, a tool such as the CC Template offers a sustainable and replicable way to standardize documentation and knowledge about CC components. This foundational information can be used to optimize team structure, training, and resource allocation, to improve the quality of CC and to link elements of CC with clinical and operational outcomes.
Pact Intensive Management
Despite the implementation of PACT within VA, patients with complex medical conditions combined with socioeconomic stressors, mental health comorbidities, and low health literacy are at high risk for preventable hospitalizations and acute care utilization.17,18 Due to unmet needs that are beyond what PACTs are able to deliver, these high-risk patients may benefit from additional services to coordinate care within and outside the VA health care system, as suggest by the Extended Chronic Care Model.19-21
In 2014, the Office of Primary Care Services sponsored a QI initiative at 5 VA demonstration sites to develop PACT Intensive Management (PIM) interventions targeting patients at high risk for hospitalization and acute care utilization within VA. The PIM program design is based on work described previously, with patients identified for enrollment based on 90-day hospitalization risk ≥ 90th percentile, based on a VA risk modeling tool, and an acute care episode in the previous 6 months. 19 A common component of all PIM programs is the provision of intensive care management and CC by an interdisciplinary team working in conjunction with PACT. The CC Template was developed to assist in documenting and rigorously understanding the implementation of CC by the PIM team.
Local Setting
The Atlanta VA Medical Center (AVAMC) was chosen as one of the PIM demonstration sites. The Atlanta PIM team identified and enrolled a random sample of eligible, high-risk patients from 1 community-based outpatient clinic (CBOC) in an urban location with 7,524 unique patients. Between September 2014 and September 2016, 300 patients were identified, and 86 patients agreed to participate in the PIM program.
In the CC Template pilot, the Atlanta PIM team included 2 nurse practitioners (NP), 2 social workers (SW), and 1 telehealth registered nurse (RN). Upon enrollment, members of the PIM team conducted comprehensive home assessments and offered intensive care management for medical, social, and behavioral needs. The main pillars of care management offered to high-risk patients were based on previous work done both inside and outside VA and included home visits, telephone-based disease management, co-attending appointments with patients, transition care management, and interdisciplinary team meetings with a focus on care coordination between PACT and all services required by patients.11,19
The Atlanta PIM team performed a variety of tasks to coordinate care for enrolled patients that included simple, 1-step tasks, such as chart reviews, and multistep, complex tasks that required the expertise of multiple team members (Figure 1).
Additionally, inconsistency in delivery of CC between PIM team members was noted. For example, there was significant variability in CC services provided by different team members in the provision of transition care management (TCM) and coordinating care from hospitalization back to home. Some PIM staff coordinated care and communicated with the patient, hospital team, home-care service, and primary-care team, while other staff only reviewed the chart and placed orders in CPRS. Additionally, much of the CC work was documented in administrative notes that did not trigger workload credit. This made it difficult to show how to appropriately labor map PIM staff or how staff were spending their time caring for patients.
In order to standardize documentation of the interdisciplinary CC activities performed by the PIM team and account for staff time, the Atlanta PIM team decided to develop a CPRS CC Template. The objective of the CC Template was to facilitate documentation of CC activities in the EHR, describe the types of CC activities performed by PIM team members, and track the time to perform these activities for patients with various chronic diseases.
Template Design and Implementation
The original design of the template was informed by the Atlanta PIM team after several informal focus groups and process mapping of CC pathways in the fall of 2015. The participants were all members of the Atlanta PIM team, 2 primary care physicians working with PIM, an AVAMC documentation specialist and a clinical applications coordinator (CAC) assigned to work with PIM. The major themes that arose during the brainstorming discussion were that the template should: (1) be feasible to use during their daily clinic workflow; (2) improve documentation of CC; and (3) have value for spread to other VA sites. Discussion centered on creating a CC Template versatile enough to:
- Decrease the number of steps for documenting CC;
- Consist only of check boxes, with very little need for free text, with the option to enter narrative free text after template completion;
- Document time spent in aggregate for completing complex CC encounters;
- Document various types of CC work and modes of communication;
- Allow for use by all PIM staff;
- Identify all team members that participated in the CC encounter to reduce redundant documentation by multiple staff;
- Adapt to different clinic sites based on the varied disciplines participating in other locations;
- Use evidence-based checklists to help standardize delivery of CC for certain activities such as TCM; and
- Extract data without extensive chart reviews to inform current CC and future QI work.
Following the brainstorming sessions, the authors performed a literature review to identify and integrate CC best practices. The AHRQ Care Coordination Atlas served as the main resource in the design of the logic model that depicted the delivery and subsequent documentation of high-quality, evidence-based CC in the CC Template (Figure 2).6
After reaching consensus about the key components of the CC Template, the CAC created a pilot version (Figure 3). All of the elements within the CC Template allowed for data abstraction from the VA Corporate Data Warehouse (CDW) via discrete data elements known as health factors.
Over the course of implementation, the team became more enthusiastic about using the CC template to document previously unrecognized CC workload. Because the CC Template only was used to document CC workload and excluded encounters for clinical evaluation and management, specific notes were created and linked with the CC Template for optimal capture of encounters.
All components of the template were mandatory to eliminate the possibility of missing data. The Atlanta PIM site principal investigator developed a multicomponent training designed to increase support for the template by describing its value and to mitigate the potential for variability in how data are captured. Training included a face-to-face session with the team to review the template and work through sample CC cases. Additionally, a training manual with clear operational definitions and examples of how to complete each element of the CC Template was disseminated. The training was subsequently conducted with the San Francisco VA Medical Center PIM team, a spread site, via video conference. The spread site offered significant feedback on clarifying the training documents and adapting the CC template for their distinct care team structure. This feedback was incorporated into the final CC Template design to increase adaptability.
Implementation Evaluation
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)framework served as the basis for evaluation of CC Template implementation. The RE-AIM framework is well established and able to evaluate the implementation and potential successful spread of new programs.23,24 Using RE-AIM, the authors planned to analyze data to explore the reach effectiveness, adoption, implementation, and maintenance of the CC Template use while providing complex care management for high-risk patients.
All data for the evaluation was extracted from the CDW by a data analyst and stored on a secure server. A statistical process control (SPC) chart was used to analyze the implementation process to assess variation in template use.
Results
After implementation, 35 weeks of CC Template pilot data were analyzed from June 1, 2015 to January 5, 2016. The PIM team completed 393 CC Templates over this collection period. After week 23, the CC template was linked to specific CC notes automatically. From weeks 23 to 35 an average of 20.3 CC Templates were completed per week by the team. The RE-AIM was used to assess the implementation of the CC Template.
Reach was determined by the number of patients enrolled in PIM with CC Template documentation. Of patients enrolled in Atlanta PIM, 90.1% had ≥ 1 CC encounter documented by the CC Template; 74.4% of Atlanta PIM patients had ≥ 1 CC encounter documented; 15.5% of patients had > 10 CC encounters documented; and 1 patient had > 25 CC encounters documented by the CC template.
Effectiveness for describing CC activities was captured through data from CC Template. The CC Template documentation by the PIM team showed that 79.4% of CC encounters were < 20 minutes, and 9.9% of encounters were > 61 minutes. Telephone communication was involved in 50.4% of CC encounters, and 24% required multiple modes of communication such as face-to-face, instant messenger, chart-based communication. Care coordination during hospitalization and discharge accounted for 5.9% of template use. Of the CC encounters documenting hospital transitions, 94.4% documented communication with the inpatient team, 58.3% documented coordination with social support, and only 11.1% documented communication with primary care teams. Improving communication with PACT teams after hospital discharge was identified as a future QI project based on these data. The PIM team initiated 83.2% of CC encounters.
Adoption was determined by the use of the CC Template by the team. All 5 team members used the CC template to document at least 1 CC encounter.
Implementation allowed for improvement based on feedback from the PIM team. Mean completion of CC Templates rose from 10.9 per week to 20.3 per week after automatically linking the CC Template to specific CC notes. (Figure 4)
Maintenance was monitored over the course of the pilot. Consistent use of the CC Template over 35 full work weeks of data collection was seen, and mean utilization per week nearly doubled in the latter half of the pilot period.
Because several elements were added to the CC Template over the course of the pilot period, our ability to analyze the data for descriptive statistics about the types of CC services, related diagnoses, collaborators, and PIM staff involved in CC encounters was limited.
Discussion
Though all components of CC encounters could not be assessed during the pilot phase due to continuous improvement of the CC Template, the authors showed that it is feasible to use this tool to document and describe granular details about team-based CC. Pilot data from AVAMC show that the use of the CC Template standardized team CC documentation in a busy clinic setting provided data about the complexity of coordination activities and duration of CC activities. It also informed future CC QI projects, such as improving communication with primary care during the hospital discharge process.
Future evaluation of CC Template data can be used to (1) describe types of CC activities for high-risk PIM patients; (2) quantify the time required to complete CC activities to assist with staff labor mapping; (3) describe staff roles and referrals needed to complete specific CC activities inside and outside VA; (4) describe modes of communication between PIM and collaborators; (5) relate patient demographics and associated diagnoses with quantity of CC encounters; and (6) quantify frequency and time frame of CC after hospitalizations and ED care and subsequent impact on repeat hospitalizations and ED visits. Future research also can explore the link between CC activities and effort with clinical and patient-reported outcomes.
Social network analysis could be used with CC Template data to understand the network of referrals and collaborators involved in the care of a CC team’s patients. This type of analysis would assist teams to strengthen and formalize ties with collaborators as appropriate. For example, if data show that the team frequently collaborates with the cardiology clinic for a large subset of its patients, they may consider creating a CC agreement with formalized modes of communication that would streamline collaboration.
In order to improve the quality of the CC Template and to assess factors that may lead to sustainable use in clinical practice, qualitative assessment through survey, interview, or usability testing with staff would be beneficial to identify strategies to increase its adoption among clinical providers. This type of assessment will add knowledge about the CC Template implementation process, including contextual barriers or facilitators, feasibility of use during day-to-day operations, versatility of template use within construct of team-based care, and overall satisfaction with the template.
Limitations
Though the CC Template offers a large amount of data about the components of CC delivery, the information is based on self-report by staff. Training to ensure that all team members are documenting in the same manner is crucial to maintain the internal validity of the data. The template is limited to the fields currently developed, and future research could explore additional data elements that are critical to include based on feedback from VA staff.
Conclusion
To our knowledge, this VA medical center CC Template is the first tool described in the literature that standardizes and captures data about CC components in the EHR. This pilot data show that the template is feasible for use in a busy clinic setting and can streamline the process for capturing CC data that may otherwise not be documented.
During the pilot phase, the CC template allowed the PIM team to identify a small subset of patients within the PIM complex management who have a high level of CC needs. By identifying these patients, further work can be done to understand the specific needs of these higher utilizers and the types of CC activities required to assist them so that resources can be directed appropriately to that smaller subset. Telephone CC accounted for a large proportion of delivery, which has implications for ensuring that staff have access to mobile phones and EHR capability to document this additional workload. The PIM staff maintained use of the template throughout the pilot period and increased documentation when the CC Template was easily accessible and already linked to their CPRS notes, suggesting that in future implementation, ensuring that the template is linked to notes in use by the care team will be important for successful spread.
Additionally, CC Template data identified gaps in high-quality, evidence-based CC that can be addressed in real time, for example during the discharge process. Data from the CC Template showed that only 11.1% of CC encounters had documentation of communication between the PIM and primary care teams during transitions from hospital to home. Improving communication with PACT teams after hospital discharge was identified as a future PIM QI project based on these data. By improving documentation of CC in the EHR, the resulting information is foundational for future work that can improve the quality of team-based CC; plan staffing, team composition, and labor mapping; determine the cost of CC activities and improve reimbursement in certain settings; and assess outcomes of CC.
This tool has potential for application beyond the PIM team in the VA. The CC Template and training manual is scalable to any setting with team-based CC, including PACT, homeless programs, palliative care, Mental Health Intensive Case Management (MHICM) programs, nurse navigator programs, and other complex care delivery models involving care coordinators. Future study of its implementation and data may inform initiatives to develop ongoing team-based care coordination programs.
Acknowledgments
The authors thank the following colleagues for their input and support: Florence Longchamp, RN, Clinical Applications Coordinator at the Atlanta VA Medical Center without whom the CC Template would not have been created; the Atlanta and San Francisco VA PIM teams for their thoughtful comments and enthusiastic embrace of the CC Template; and the PIM National Evaluation Center for their support of this QI project. PACT Intensive Management demonstration sites are funded by the VA Office of Patient Care Services. During the implementation of the CC Template pilot and the preparation of this paper, the primary author was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Advanced Fellowships, VA Quality Scholars Program.
1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
2. Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated May 3, 2017. Accessed August 8, 2018.
3. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
4. US Department of Health and Human Services. Healthy people 2010: general data issues. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_general_data_issues.pdf. Published 2010. Accessed August 1, 2018.
5. McDonald KM, Sunderam V, Bravata DM, et al. Closing the quality gap: a critical analysis of quality improvement strategies, Vol 7: care coordination. Agency for Healthcare Research and Quality. https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. Published June 2007. Accessed August 1, 2018.
6. McDonald KM, Schultz E, Albin L, et al. Care coordination measures atlas. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed August 2, 2018.
7. Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating care across diseases, settings, and clinicians: a key role for the generalist in practice. Ann Intern Med. 2005;142(8):700-708.
8. Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15(5):329-336.
9. National Committee for Quality Assurance. The future of patient-centered medical homes: foundation for a better health care system. https://www.ncqa.org/Portals/0/Public%20Policy/2014%20PDFS/The_Future_of_PCMH.pdf. Accessed August 2, 2018.
10. US Department of Veterans Affairs, Veterans Health Administration. Patient Aligned Care Team (PACT) Handbook. VHA Handbook 1101.10:1–65. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2977. Updated May 26, 2017. Accessed August 2, 2018.
11. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
12. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19.
13. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Chronic care management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Published December 2016. Accessed August 2, 2018.
14. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Frequently asked questions about billing the Medicare physician fee schedule for transitional care management services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-TCMS.pdf. Published March 17, 2016. Accessed August 2, 2018.
15. Antonelli RC, Stille CJ, Antonelli DM. Care coordination for children and youth with special health care needs: a descriptive, multisite study of activities, personnel costs, and outcomes. Pediatrics. 2008;122(1):e209-e216.
16. Antonelli RC, Antonelli DM. Providing a medical home: the cost of care coordination services in a community-based, general pediatric practice. Pediatrics. 2004;113( suppl 5 ):1522-1528.
17. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
18. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an intensive management patient-aligned care team (ImPACT). J Gen Intern Med. 2014;29(suppl 4):S861-S869.
20. Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166-175.
21. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73-82.
22. US Department of Health & Human Services, Agency for Healthcare Research and Quality. Re-engineered discharge (RED) toolkit. http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html. Updated May 2017. Accessed August 3, 2018.
23. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327.
24. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-e46.
1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
2. Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated May 3, 2017. Accessed August 8, 2018.
3. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
4. US Department of Health and Human Services. Healthy people 2010: general data issues. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_general_data_issues.pdf. Published 2010. Accessed August 1, 2018.
5. McDonald KM, Sunderam V, Bravata DM, et al. Closing the quality gap: a critical analysis of quality improvement strategies, Vol 7: care coordination. Agency for Healthcare Research and Quality. https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. Published June 2007. Accessed August 1, 2018.
6. McDonald KM, Schultz E, Albin L, et al. Care coordination measures atlas. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed August 2, 2018.
7. Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating care across diseases, settings, and clinicians: a key role for the generalist in practice. Ann Intern Med. 2005;142(8):700-708.
8. Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15(5):329-336.
9. National Committee for Quality Assurance. The future of patient-centered medical homes: foundation for a better health care system. https://www.ncqa.org/Portals/0/Public%20Policy/2014%20PDFS/The_Future_of_PCMH.pdf. Accessed August 2, 2018.
10. US Department of Veterans Affairs, Veterans Health Administration. Patient Aligned Care Team (PACT) Handbook. VHA Handbook 1101.10:1–65. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2977. Updated May 26, 2017. Accessed August 2, 2018.
11. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
12. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19.
13. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Chronic care management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Published December 2016. Accessed August 2, 2018.
14. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Frequently asked questions about billing the Medicare physician fee schedule for transitional care management services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-TCMS.pdf. Published March 17, 2016. Accessed August 2, 2018.
15. Antonelli RC, Stille CJ, Antonelli DM. Care coordination for children and youth with special health care needs: a descriptive, multisite study of activities, personnel costs, and outcomes. Pediatrics. 2008;122(1):e209-e216.
16. Antonelli RC, Antonelli DM. Providing a medical home: the cost of care coordination services in a community-based, general pediatric practice. Pediatrics. 2004;113( suppl 5 ):1522-1528.
17. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
18. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an intensive management patient-aligned care team (ImPACT). J Gen Intern Med. 2014;29(suppl 4):S861-S869.
20. Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166-175.
21. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73-82.
22. US Department of Health & Human Services, Agency for Healthcare Research and Quality. Re-engineered discharge (RED) toolkit. http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html. Updated May 2017. Accessed August 3, 2018.
23. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327.
24. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-e46.
Team reports success with mobile platelet collection
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
FDA issues draft guidance on MRD
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
Questions surround MRD assessment in MM
DUBROVNIK, CROATIA—Clinical trials are needed to answer the many questions related to minimal residual disease (MRD) assessment in multiple myeloma (MM), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
MM patients are increasingly assessed for MRD, which is a strong prognostic factor and surrogate for overall survival, according to Toni Valković, MD, PhD, of University Hospital Center Rijeka in Croatia.
However, Dr. Valković said MRD assessment has not become a part of routine clinical practice, perhaps because we haven’t determined the best way to utilize MRD assessment in MM patients.
The optimal sensitivity threshold, technique, and timing of MRD assessment are not known, and it isn’t clear how MRD should be used to guide treatment.
What we know
Dr. Valković cited studies showing that MRD negativity is associated with superior survival in MM1, and, when MRD negativity is achieved, high-risk cytogenetics, age, and previous treatment regimens appear to have no further impact on prognosis.2
Dr. Valković went on to explain the benefits and detriments of multiparametric flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment.3
NGS requires a baseline patient sample, but MFC does not. More cells are required with MFC than with NGS (>5 million vs. <1 million).
With MFC, samples must be processed within 24 to 48 hours, whereas, with NGS, fresh or stored samples can be used. MFC can be done in a few hours, while NGS can take several days.
Despite these differences, both methods provide similar levels of sensitivity for detecting MRD (≥1 in 105).
Dr. Valković also noted that MRD should be evaluated outside the bone marrow as well, which can be done with positron emission tomography-computed tomography (PET-CT).
Research has shown that patients who are MRD-negative according to both MFC and PET-CT have better outcomes than patients who are MRD-positive by MFC, PET-CT, or both.4
What we don’t know
Though he compared MFC and NGS, Dr. Valković said we don’t know the optimal technique for assessing MRD in the bone marrow.
Another uncertainty is the optimal sensitivity threshold. In the POLLUX study5, researchers found that a threshold of 10-4 resulted in lots of patients with MRD negativity, but some of these were false-negatives.
So although 10-4 proved inaccurate, it isn’t clear if the optimal threshold is 10-5 or 10-6, Dr. Valković said.
Likewise, it isn’t clear if PET-CT is the optimal method for evaluating MRD outside the bone marrow.
In a study published in 2017, PET produced false-negatives in MM patients.6 In 11% of patients (26/227), there was evidence of disease with diffusion-weighted magnetic resonance imaging with background signal suppression, but there was no apparent disease with PET. The researchers said low expression of hexokinase-2 was associated with a false-negative PET result.
Finally, Dr. Valković said we don’t know how best to use MRD to tailor therapy in MM patients. He posed the following questions:
- If patients don’t achieve MRD negativity, should they continue on the therapy?
- If MRD-negative patients become MRD-positive, should they begin therapy immediately, or should treatment be put off until a biochemical or clinical relapse?
- Should MRD status be used to determine the number of treatment cycles a patient receives, the timing of transplant, or when to begin and end maintenance therapy?
“There are a lot of issues and unanswered questions related to the optimal techniques for the evaluation of MRD and their sensitivity, the timing for MRD assessment during and after therapy, and its role in the treatment decisions, which should be answered in future clinical studies,” Dr. Valković concluded.
He did not declare any conflicts of interest.
1. Munshi NC et al. JAMA Oncol. 2017;3(1):28-35. doi:10.1001/jamaoncol.2016.3160
2. Paiva B et al. Blood. 2016 Jun 23;127(25):3165-74. doi: 10.1182/blood-2016-03-705319
3. Kumar S et al. Lancet Oncol. 2016; 17 (8):e328-46 doi: https://doi.org/10.1016/S1470-2045(16)30206-6
4. Fernandez RA et al. Blood. 2017; 130:3098
5. Dimopoulos MA et al. Haematologica. 2018 Sep 20. pii: haematol.2018.194282. doi: 10.3324/haematol.2018.194282
6. Rasche L et al. Blood. 2017 Jul 6;130(1):30-34. doi: 10.1182/blood-2017-03-774422
DUBROVNIK, CROATIA—Clinical trials are needed to answer the many questions related to minimal residual disease (MRD) assessment in multiple myeloma (MM), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
MM patients are increasingly assessed for MRD, which is a strong prognostic factor and surrogate for overall survival, according to Toni Valković, MD, PhD, of University Hospital Center Rijeka in Croatia.
However, Dr. Valković said MRD assessment has not become a part of routine clinical practice, perhaps because we haven’t determined the best way to utilize MRD assessment in MM patients.
The optimal sensitivity threshold, technique, and timing of MRD assessment are not known, and it isn’t clear how MRD should be used to guide treatment.
What we know
Dr. Valković cited studies showing that MRD negativity is associated with superior survival in MM1, and, when MRD negativity is achieved, high-risk cytogenetics, age, and previous treatment regimens appear to have no further impact on prognosis.2
Dr. Valković went on to explain the benefits and detriments of multiparametric flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment.3
NGS requires a baseline patient sample, but MFC does not. More cells are required with MFC than with NGS (>5 million vs. <1 million).
With MFC, samples must be processed within 24 to 48 hours, whereas, with NGS, fresh or stored samples can be used. MFC can be done in a few hours, while NGS can take several days.
Despite these differences, both methods provide similar levels of sensitivity for detecting MRD (≥1 in 105).
Dr. Valković also noted that MRD should be evaluated outside the bone marrow as well, which can be done with positron emission tomography-computed tomography (PET-CT).
Research has shown that patients who are MRD-negative according to both MFC and PET-CT have better outcomes than patients who are MRD-positive by MFC, PET-CT, or both.4
What we don’t know
Though he compared MFC and NGS, Dr. Valković said we don’t know the optimal technique for assessing MRD in the bone marrow.
Another uncertainty is the optimal sensitivity threshold. In the POLLUX study5, researchers found that a threshold of 10-4 resulted in lots of patients with MRD negativity, but some of these were false-negatives.
So although 10-4 proved inaccurate, it isn’t clear if the optimal threshold is 10-5 or 10-6, Dr. Valković said.
Likewise, it isn’t clear if PET-CT is the optimal method for evaluating MRD outside the bone marrow.
In a study published in 2017, PET produced false-negatives in MM patients.6 In 11% of patients (26/227), there was evidence of disease with diffusion-weighted magnetic resonance imaging with background signal suppression, but there was no apparent disease with PET. The researchers said low expression of hexokinase-2 was associated with a false-negative PET result.
Finally, Dr. Valković said we don’t know how best to use MRD to tailor therapy in MM patients. He posed the following questions:
- If patients don’t achieve MRD negativity, should they continue on the therapy?
- If MRD-negative patients become MRD-positive, should they begin therapy immediately, or should treatment be put off until a biochemical or clinical relapse?
- Should MRD status be used to determine the number of treatment cycles a patient receives, the timing of transplant, or when to begin and end maintenance therapy?
“There are a lot of issues and unanswered questions related to the optimal techniques for the evaluation of MRD and their sensitivity, the timing for MRD assessment during and after therapy, and its role in the treatment decisions, which should be answered in future clinical studies,” Dr. Valković concluded.
He did not declare any conflicts of interest.
1. Munshi NC et al. JAMA Oncol. 2017;3(1):28-35. doi:10.1001/jamaoncol.2016.3160
2. Paiva B et al. Blood. 2016 Jun 23;127(25):3165-74. doi: 10.1182/blood-2016-03-705319
3. Kumar S et al. Lancet Oncol. 2016; 17 (8):e328-46 doi: https://doi.org/10.1016/S1470-2045(16)30206-6
4. Fernandez RA et al. Blood. 2017; 130:3098
5. Dimopoulos MA et al. Haematologica. 2018 Sep 20. pii: haematol.2018.194282. doi: 10.3324/haematol.2018.194282
6. Rasche L et al. Blood. 2017 Jul 6;130(1):30-34. doi: 10.1182/blood-2017-03-774422
DUBROVNIK, CROATIA—Clinical trials are needed to answer the many questions related to minimal residual disease (MRD) assessment in multiple myeloma (MM), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
MM patients are increasingly assessed for MRD, which is a strong prognostic factor and surrogate for overall survival, according to Toni Valković, MD, PhD, of University Hospital Center Rijeka in Croatia.
However, Dr. Valković said MRD assessment has not become a part of routine clinical practice, perhaps because we haven’t determined the best way to utilize MRD assessment in MM patients.
The optimal sensitivity threshold, technique, and timing of MRD assessment are not known, and it isn’t clear how MRD should be used to guide treatment.
What we know
Dr. Valković cited studies showing that MRD negativity is associated with superior survival in MM1, and, when MRD negativity is achieved, high-risk cytogenetics, age, and previous treatment regimens appear to have no further impact on prognosis.2
Dr. Valković went on to explain the benefits and detriments of multiparametric flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment.3
NGS requires a baseline patient sample, but MFC does not. More cells are required with MFC than with NGS (>5 million vs. <1 million).
With MFC, samples must be processed within 24 to 48 hours, whereas, with NGS, fresh or stored samples can be used. MFC can be done in a few hours, while NGS can take several days.
Despite these differences, both methods provide similar levels of sensitivity for detecting MRD (≥1 in 105).
Dr. Valković also noted that MRD should be evaluated outside the bone marrow as well, which can be done with positron emission tomography-computed tomography (PET-CT).
Research has shown that patients who are MRD-negative according to both MFC and PET-CT have better outcomes than patients who are MRD-positive by MFC, PET-CT, or both.4
What we don’t know
Though he compared MFC and NGS, Dr. Valković said we don’t know the optimal technique for assessing MRD in the bone marrow.
Another uncertainty is the optimal sensitivity threshold. In the POLLUX study5, researchers found that a threshold of 10-4 resulted in lots of patients with MRD negativity, but some of these were false-negatives.
So although 10-4 proved inaccurate, it isn’t clear if the optimal threshold is 10-5 or 10-6, Dr. Valković said.
Likewise, it isn’t clear if PET-CT is the optimal method for evaluating MRD outside the bone marrow.
In a study published in 2017, PET produced false-negatives in MM patients.6 In 11% of patients (26/227), there was evidence of disease with diffusion-weighted magnetic resonance imaging with background signal suppression, but there was no apparent disease with PET. The researchers said low expression of hexokinase-2 was associated with a false-negative PET result.
Finally, Dr. Valković said we don’t know how best to use MRD to tailor therapy in MM patients. He posed the following questions:
- If patients don’t achieve MRD negativity, should they continue on the therapy?
- If MRD-negative patients become MRD-positive, should they begin therapy immediately, or should treatment be put off until a biochemical or clinical relapse?
- Should MRD status be used to determine the number of treatment cycles a patient receives, the timing of transplant, or when to begin and end maintenance therapy?
“There are a lot of issues and unanswered questions related to the optimal techniques for the evaluation of MRD and their sensitivity, the timing for MRD assessment during and after therapy, and its role in the treatment decisions, which should be answered in future clinical studies,” Dr. Valković concluded.
He did not declare any conflicts of interest.
1. Munshi NC et al. JAMA Oncol. 2017;3(1):28-35. doi:10.1001/jamaoncol.2016.3160
2. Paiva B et al. Blood. 2016 Jun 23;127(25):3165-74. doi: 10.1182/blood-2016-03-705319
3. Kumar S et al. Lancet Oncol. 2016; 17 (8):e328-46 doi: https://doi.org/10.1016/S1470-2045(16)30206-6
4. Fernandez RA et al. Blood. 2017; 130:3098
5. Dimopoulos MA et al. Haematologica. 2018 Sep 20. pii: haematol.2018.194282. doi: 10.3324/haematol.2018.194282
6. Rasche L et al. Blood. 2017 Jul 6;130(1):30-34. doi: 10.1182/blood-2017-03-774422
CTPA overused in veterans with suspected PE
SAN ANTONIO—The recommended approach to evaluating suspected pulmonary embolism (PE) is “greatly underutilized” in the Veterans Health Administration system, according to a speaker at CHEST 2018.
A survey showed that, contrary to guideline recommendations, most Veterans Affairs sites did not require incorporation of a clinical decision rule (CDR) and D-dimer prior to the ordering of computed tomographic pulmonary angiography (CTPA) for suspected PE.
Therefore, CTPA was overused.
Nancy Hsu, MD, a pulmonologist in Los Angeles, California, discussed this finding at the meeting.
She noted that CTPA has become the imaging modality of choice for evaluating suspected PE, but it is overused and potentially avoidable in one-third of cases.
“In the 10 years following the advent of CTPA use, there was a 14-fold increase in usage, but there was no change in mortality,” Dr. Hsu said. “This is consistent with overdiagnosis.”
Indiscriminate use of CTPA results in unnecessary and avoidable radiation exposure, contrast-related reactions, and treatment-related bleeding, Dr. Hsu noted.
She and a colleague discovered CTPA overuse in the Veterans Health Administration system by conducting a survey of stakeholders at 18 Veterans Integrated Service Networks and 143 medical centers.
A total of 120 fully completed questionnaires were analyzed. Most respondents (63%) were chief physicians, and 80% had 11 or more years of experience.
Most respondents (85%) said CDR with or without D-dimer was not required before ordering CTPA. Less than 7% of respondents said they required both CDR and D-dimer before CTPA.
The biggest barrier to optimal practice may be the fear of having a patient who “falls through the cracks” based on false-negative CDR and D-dimer data, according to Dr. Hsu.
On the other hand, judicious use of CTPA likely avoids negative sequelae related to radiation, contrast exposure, and treatment-related bleeding, she said.
Dr. Hsu and her colleague, Guy Soo Hoo, MD, said they had no relationships relevant to this research.
SAN ANTONIO—The recommended approach to evaluating suspected pulmonary embolism (PE) is “greatly underutilized” in the Veterans Health Administration system, according to a speaker at CHEST 2018.
A survey showed that, contrary to guideline recommendations, most Veterans Affairs sites did not require incorporation of a clinical decision rule (CDR) and D-dimer prior to the ordering of computed tomographic pulmonary angiography (CTPA) for suspected PE.
Therefore, CTPA was overused.
Nancy Hsu, MD, a pulmonologist in Los Angeles, California, discussed this finding at the meeting.
She noted that CTPA has become the imaging modality of choice for evaluating suspected PE, but it is overused and potentially avoidable in one-third of cases.
“In the 10 years following the advent of CTPA use, there was a 14-fold increase in usage, but there was no change in mortality,” Dr. Hsu said. “This is consistent with overdiagnosis.”
Indiscriminate use of CTPA results in unnecessary and avoidable radiation exposure, contrast-related reactions, and treatment-related bleeding, Dr. Hsu noted.
She and a colleague discovered CTPA overuse in the Veterans Health Administration system by conducting a survey of stakeholders at 18 Veterans Integrated Service Networks and 143 medical centers.
A total of 120 fully completed questionnaires were analyzed. Most respondents (63%) were chief physicians, and 80% had 11 or more years of experience.
Most respondents (85%) said CDR with or without D-dimer was not required before ordering CTPA. Less than 7% of respondents said they required both CDR and D-dimer before CTPA.
The biggest barrier to optimal practice may be the fear of having a patient who “falls through the cracks” based on false-negative CDR and D-dimer data, according to Dr. Hsu.
On the other hand, judicious use of CTPA likely avoids negative sequelae related to radiation, contrast exposure, and treatment-related bleeding, she said.
Dr. Hsu and her colleague, Guy Soo Hoo, MD, said they had no relationships relevant to this research.
SAN ANTONIO—The recommended approach to evaluating suspected pulmonary embolism (PE) is “greatly underutilized” in the Veterans Health Administration system, according to a speaker at CHEST 2018.
A survey showed that, contrary to guideline recommendations, most Veterans Affairs sites did not require incorporation of a clinical decision rule (CDR) and D-dimer prior to the ordering of computed tomographic pulmonary angiography (CTPA) for suspected PE.
Therefore, CTPA was overused.
Nancy Hsu, MD, a pulmonologist in Los Angeles, California, discussed this finding at the meeting.
She noted that CTPA has become the imaging modality of choice for evaluating suspected PE, but it is overused and potentially avoidable in one-third of cases.
“In the 10 years following the advent of CTPA use, there was a 14-fold increase in usage, but there was no change in mortality,” Dr. Hsu said. “This is consistent with overdiagnosis.”
Indiscriminate use of CTPA results in unnecessary and avoidable radiation exposure, contrast-related reactions, and treatment-related bleeding, Dr. Hsu noted.
She and a colleague discovered CTPA overuse in the Veterans Health Administration system by conducting a survey of stakeholders at 18 Veterans Integrated Service Networks and 143 medical centers.
A total of 120 fully completed questionnaires were analyzed. Most respondents (63%) were chief physicians, and 80% had 11 or more years of experience.
Most respondents (85%) said CDR with or without D-dimer was not required before ordering CTPA. Less than 7% of respondents said they required both CDR and D-dimer before CTPA.
The biggest barrier to optimal practice may be the fear of having a patient who “falls through the cracks” based on false-negative CDR and D-dimer data, according to Dr. Hsu.
On the other hand, judicious use of CTPA likely avoids negative sequelae related to radiation, contrast exposure, and treatment-related bleeding, she said.
Dr. Hsu and her colleague, Guy Soo Hoo, MD, said they had no relationships relevant to this research.