User login
Minimum 5-Year Follow-up of Articular Surface Replacement Acetabular Components Used in Total Hip Arthroplasty
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
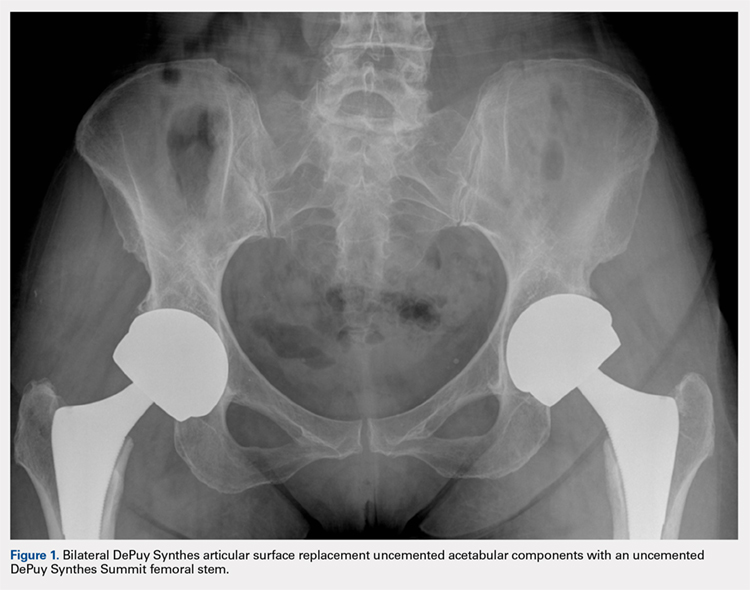
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.
All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
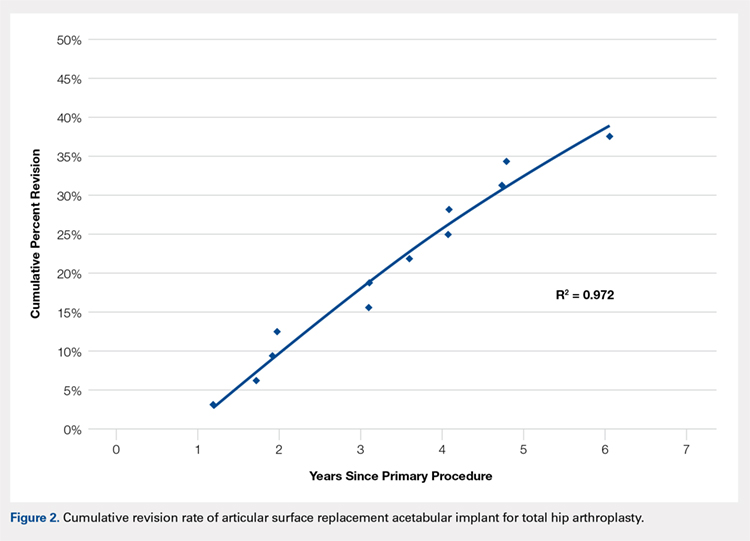
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).
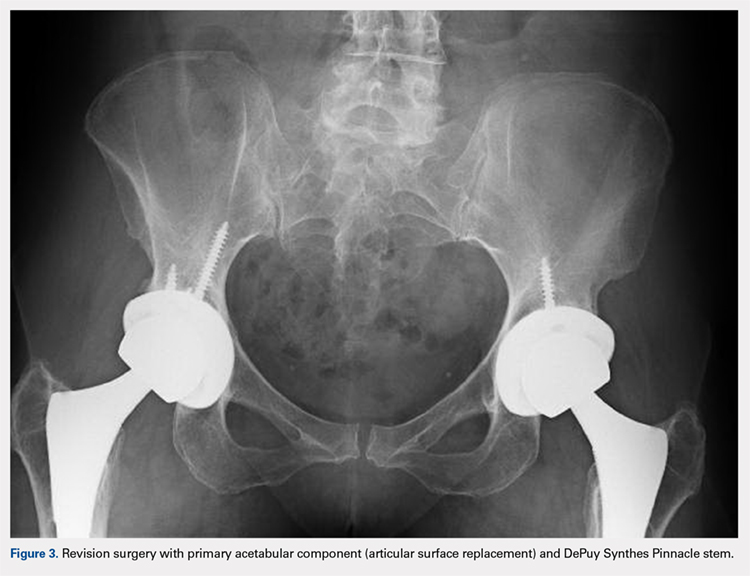
Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.
The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
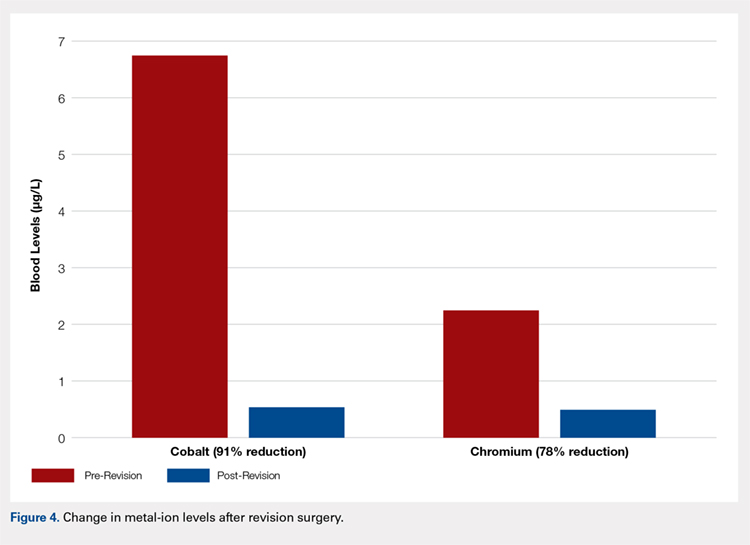
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |
Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.

All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).

Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.

The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |

Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.

All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).

Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.

The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |

Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
TAKE-HOME POINTS
- High rate of failure of DePuy Synthes ASR™ XL Acetabular hip system used in THA, approaching 34.4% at 5 years.
- Mean time to revision was 3.1 years with pain being the most common indication for revision surgery.
- Age, gender, acetabular component abduction angle, acetabular size, and serum cobalt or chromium levels were not associated with increased rate of failure.
- Serum cobalt and chromium levels decreased significantly within 6 months of revision surgery.
- Close clinical surveillance and laboratory monitoring of patients is required.
Pembrolizumab does not surpass paclitaxel for gastric cancer
BARCELONA – The immune checkpoint inhibitor pembrolizumab (Keytruda) did not significantly improve overall survival of advanced/metastatic gastric or gastroesophageal junction cancer compared with paclitaxel, the results of the KEYNOTE-061 study show.
Among 395 patients with gastric or gastroesophageal junction (GEJ) cancer who had expression of the programmed death ligand 1 (PD-L1) on 1% or more of their tumor cells, lymphocytes, and macrophages, median overall survival (OS) for patients treated with pembrolizumab was 9.1 months, compared with 8.3 months. This translated into a hazard ratio (HR) for death in the pembrolizumab arm of 0.82, but with the 95% confidence interval crossing 1.00, and a P value (.04205) that did not meet the prespecified threshold for significance (P equal to or less than .0135).
Despite the failure of the trial to reach its primary endpoint, “these results may support further research to identify patients likely to benefit from pembrolizumab monotherapy and ongoing development of pembrolizumab-based combination therapy for gastric cancer,” he said at the European Society of Medical Oncology World Congress on Gastrointestinal Cancer.
Pembrolizumab is approved by the Food and Drug Administration for the treatment of patients with recurrent locally advanced or metastatic gastric or GEJ adenocarcinoma with tumors confirmed to carry PD-L1 for whom two or more prior lines of therapy had failed.
The approval was based on results of the nonrandomized, open label KEYNOTE-059 trial, which enrolled 259 patients with gastric or GEJ adenocarcinoma that progressed on at least two prior systemic treatments for advanced disease. Of the enrollees, 143 patients had tumors with a PD-L1 Combined Positive Score (CPS) of 1 or greater. The primary trial outcome, the objective response rate for these 143 patients, was 13.3% (95% confidence interval; 8.2-20), with a complete response rate of 1.4% and a partial response rate of 11.9%. The duration of response ranged from at least 2.8 months to at least 19.4 months.
The drug is also approved for unresectable of metastatic gastric tumors with high levels of microsatellite instability that progressed on prior therapy.
The KEYNOTE-061 study was designed to test the proposition that pembrolizumab could improve on paclitaxel for treatment of patients with adenocarcinoma of the stomach or GEJ that was metastatic or locally advanced and unresectable, and for which first-line therapy with a platinum agent and fluoropyrimidine had failed.
A total of 592 patients from Europe, Israel, North America, Asia, and Australia were enrolled and randomly assigned to receive either pembrolizumab 200 mg every 3 weeks for 35 cycles, or paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 4-week cycle. Each treatment was continued until the maximum number of cycles (for pembrolizumab) or until confirmed disease progression, intolerable toxicity, patient withdrawal, or investigator’s decision.
As noted, OS for patients with a CPS score of 1 or greater, the primary endpoint, did not differ between treatment arms, but there was a numerical tilt that appeared to be in favor of pembrolizumab.
For example, the 12-month OS rates were 39.8% in the pembrolizumab groups vs. 27.1% in the paclitaxel group, and respective 18-month OS rates were 25.7% and 14.8%.
In an analysis of protocol-specified subgroups, there were general trends slightly favoring the checkpoint inhibitor over the taxane, but the only significant difference was among patients with GEJ cancers as the primary tumor location (HR, 0.61, 95% confidence interval, 0.41-0.90).
Pembrolizumab offered a small but significant survival advantage among patients with Eastern Cooperative Oncology Group performance status of 0, with a median OS of 12.3 months vs. 9.3 months (HR, 0.69, 95% CI, 0.49-0.97).
Analyses of PFS and OS by CPS score groups (less than 1, 1-10, or 10 and higher) showed no significant differences, however.
Treatment-related adverse events were more frequent with paclitaxel (84.1% vs. 52.7% of patients on pembrolizumab), but pembrolizumab was associated with more treatment-related deaths (three vs. one). Two of the deaths in the pembrolizumab arm were immune mediated. Grade 3 or greater adverse events occurred in 14.3% vs. 34.8%, respectively.
In the question-and-response following Dr. Shitara’s presentation, session comoderator David Cunningham, MD, of Royal Marsden NHS Foundation Trust in Sutton, England, asked why the KEYNOTE-061 investigators did not pit pembrolizumab against the combination of paclitaxel and ramucirumab (Cyramza) “since that’s what many people would use in this situation.”
Dr. Shitara replied that ramucirumab was not available or accepted as an option in many countries when the trial was first planned in 2014. He added that paclitaxel and ramucirumab should be the control arm for clinical trials going forward.
SOURCE: Shitara K. et al. ESMO World Congress on Gastrointestinal Cancer 2018. Abstract LBA-005.
BARCELONA – The immune checkpoint inhibitor pembrolizumab (Keytruda) did not significantly improve overall survival of advanced/metastatic gastric or gastroesophageal junction cancer compared with paclitaxel, the results of the KEYNOTE-061 study show.
Among 395 patients with gastric or gastroesophageal junction (GEJ) cancer who had expression of the programmed death ligand 1 (PD-L1) on 1% or more of their tumor cells, lymphocytes, and macrophages, median overall survival (OS) for patients treated with pembrolizumab was 9.1 months, compared with 8.3 months. This translated into a hazard ratio (HR) for death in the pembrolizumab arm of 0.82, but with the 95% confidence interval crossing 1.00, and a P value (.04205) that did not meet the prespecified threshold for significance (P equal to or less than .0135).
Despite the failure of the trial to reach its primary endpoint, “these results may support further research to identify patients likely to benefit from pembrolizumab monotherapy and ongoing development of pembrolizumab-based combination therapy for gastric cancer,” he said at the European Society of Medical Oncology World Congress on Gastrointestinal Cancer.
Pembrolizumab is approved by the Food and Drug Administration for the treatment of patients with recurrent locally advanced or metastatic gastric or GEJ adenocarcinoma with tumors confirmed to carry PD-L1 for whom two or more prior lines of therapy had failed.
The approval was based on results of the nonrandomized, open label KEYNOTE-059 trial, which enrolled 259 patients with gastric or GEJ adenocarcinoma that progressed on at least two prior systemic treatments for advanced disease. Of the enrollees, 143 patients had tumors with a PD-L1 Combined Positive Score (CPS) of 1 or greater. The primary trial outcome, the objective response rate for these 143 patients, was 13.3% (95% confidence interval; 8.2-20), with a complete response rate of 1.4% and a partial response rate of 11.9%. The duration of response ranged from at least 2.8 months to at least 19.4 months.
The drug is also approved for unresectable of metastatic gastric tumors with high levels of microsatellite instability that progressed on prior therapy.
The KEYNOTE-061 study was designed to test the proposition that pembrolizumab could improve on paclitaxel for treatment of patients with adenocarcinoma of the stomach or GEJ that was metastatic or locally advanced and unresectable, and for which first-line therapy with a platinum agent and fluoropyrimidine had failed.
A total of 592 patients from Europe, Israel, North America, Asia, and Australia were enrolled and randomly assigned to receive either pembrolizumab 200 mg every 3 weeks for 35 cycles, or paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 4-week cycle. Each treatment was continued until the maximum number of cycles (for pembrolizumab) or until confirmed disease progression, intolerable toxicity, patient withdrawal, or investigator’s decision.
As noted, OS for patients with a CPS score of 1 or greater, the primary endpoint, did not differ between treatment arms, but there was a numerical tilt that appeared to be in favor of pembrolizumab.
For example, the 12-month OS rates were 39.8% in the pembrolizumab groups vs. 27.1% in the paclitaxel group, and respective 18-month OS rates were 25.7% and 14.8%.
In an analysis of protocol-specified subgroups, there were general trends slightly favoring the checkpoint inhibitor over the taxane, but the only significant difference was among patients with GEJ cancers as the primary tumor location (HR, 0.61, 95% confidence interval, 0.41-0.90).
Pembrolizumab offered a small but significant survival advantage among patients with Eastern Cooperative Oncology Group performance status of 0, with a median OS of 12.3 months vs. 9.3 months (HR, 0.69, 95% CI, 0.49-0.97).
Analyses of PFS and OS by CPS score groups (less than 1, 1-10, or 10 and higher) showed no significant differences, however.
Treatment-related adverse events were more frequent with paclitaxel (84.1% vs. 52.7% of patients on pembrolizumab), but pembrolizumab was associated with more treatment-related deaths (three vs. one). Two of the deaths in the pembrolizumab arm were immune mediated. Grade 3 or greater adverse events occurred in 14.3% vs. 34.8%, respectively.
In the question-and-response following Dr. Shitara’s presentation, session comoderator David Cunningham, MD, of Royal Marsden NHS Foundation Trust in Sutton, England, asked why the KEYNOTE-061 investigators did not pit pembrolizumab against the combination of paclitaxel and ramucirumab (Cyramza) “since that’s what many people would use in this situation.”
Dr. Shitara replied that ramucirumab was not available or accepted as an option in many countries when the trial was first planned in 2014. He added that paclitaxel and ramucirumab should be the control arm for clinical trials going forward.
SOURCE: Shitara K. et al. ESMO World Congress on Gastrointestinal Cancer 2018. Abstract LBA-005.
BARCELONA – The immune checkpoint inhibitor pembrolizumab (Keytruda) did not significantly improve overall survival of advanced/metastatic gastric or gastroesophageal junction cancer compared with paclitaxel, the results of the KEYNOTE-061 study show.
Among 395 patients with gastric or gastroesophageal junction (GEJ) cancer who had expression of the programmed death ligand 1 (PD-L1) on 1% or more of their tumor cells, lymphocytes, and macrophages, median overall survival (OS) for patients treated with pembrolizumab was 9.1 months, compared with 8.3 months. This translated into a hazard ratio (HR) for death in the pembrolizumab arm of 0.82, but with the 95% confidence interval crossing 1.00, and a P value (.04205) that did not meet the prespecified threshold for significance (P equal to or less than .0135).
Despite the failure of the trial to reach its primary endpoint, “these results may support further research to identify patients likely to benefit from pembrolizumab monotherapy and ongoing development of pembrolizumab-based combination therapy for gastric cancer,” he said at the European Society of Medical Oncology World Congress on Gastrointestinal Cancer.
Pembrolizumab is approved by the Food and Drug Administration for the treatment of patients with recurrent locally advanced or metastatic gastric or GEJ adenocarcinoma with tumors confirmed to carry PD-L1 for whom two or more prior lines of therapy had failed.
The approval was based on results of the nonrandomized, open label KEYNOTE-059 trial, which enrolled 259 patients with gastric or GEJ adenocarcinoma that progressed on at least two prior systemic treatments for advanced disease. Of the enrollees, 143 patients had tumors with a PD-L1 Combined Positive Score (CPS) of 1 or greater. The primary trial outcome, the objective response rate for these 143 patients, was 13.3% (95% confidence interval; 8.2-20), with a complete response rate of 1.4% and a partial response rate of 11.9%. The duration of response ranged from at least 2.8 months to at least 19.4 months.
The drug is also approved for unresectable of metastatic gastric tumors with high levels of microsatellite instability that progressed on prior therapy.
The KEYNOTE-061 study was designed to test the proposition that pembrolizumab could improve on paclitaxel for treatment of patients with adenocarcinoma of the stomach or GEJ that was metastatic or locally advanced and unresectable, and for which first-line therapy with a platinum agent and fluoropyrimidine had failed.
A total of 592 patients from Europe, Israel, North America, Asia, and Australia were enrolled and randomly assigned to receive either pembrolizumab 200 mg every 3 weeks for 35 cycles, or paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 4-week cycle. Each treatment was continued until the maximum number of cycles (for pembrolizumab) or until confirmed disease progression, intolerable toxicity, patient withdrawal, or investigator’s decision.
As noted, OS for patients with a CPS score of 1 or greater, the primary endpoint, did not differ between treatment arms, but there was a numerical tilt that appeared to be in favor of pembrolizumab.
For example, the 12-month OS rates were 39.8% in the pembrolizumab groups vs. 27.1% in the paclitaxel group, and respective 18-month OS rates were 25.7% and 14.8%.
In an analysis of protocol-specified subgroups, there were general trends slightly favoring the checkpoint inhibitor over the taxane, but the only significant difference was among patients with GEJ cancers as the primary tumor location (HR, 0.61, 95% confidence interval, 0.41-0.90).
Pembrolizumab offered a small but significant survival advantage among patients with Eastern Cooperative Oncology Group performance status of 0, with a median OS of 12.3 months vs. 9.3 months (HR, 0.69, 95% CI, 0.49-0.97).
Analyses of PFS and OS by CPS score groups (less than 1, 1-10, or 10 and higher) showed no significant differences, however.
Treatment-related adverse events were more frequent with paclitaxel (84.1% vs. 52.7% of patients on pembrolizumab), but pembrolizumab was associated with more treatment-related deaths (three vs. one). Two of the deaths in the pembrolizumab arm were immune mediated. Grade 3 or greater adverse events occurred in 14.3% vs. 34.8%, respectively.
In the question-and-response following Dr. Shitara’s presentation, session comoderator David Cunningham, MD, of Royal Marsden NHS Foundation Trust in Sutton, England, asked why the KEYNOTE-061 investigators did not pit pembrolizumab against the combination of paclitaxel and ramucirumab (Cyramza) “since that’s what many people would use in this situation.”
Dr. Shitara replied that ramucirumab was not available or accepted as an option in many countries when the trial was first planned in 2014. He added that paclitaxel and ramucirumab should be the control arm for clinical trials going forward.
SOURCE: Shitara K. et al. ESMO World Congress on Gastrointestinal Cancer 2018. Abstract LBA-005.
REPORTING FROM ESMO GI 2018
Key clinical point: KEYNOTE-061 did not meet its primary endpoint of a significant survival advantage with pembrolizumab over paclitaxel.
Major finding: There was no significant difference in overall survival between patients with gastric or gastroesophageal junction cancers treated with pembrolizumab or paclitaxel.
Study details: Phase 3 randomized open-label trial in 592 patients with advanced or metastatic and unresectable tumors of the stomach of GEJ.
Disclosures: The trial was supported by Merck. Dr. Shitara has disclosed honoraria from Abbvie, Novartis and Yakult, consulting or advising with Astellas, BMS. Lilly, Ono Pharmaceutical, Pfizer, and Takeda, and institutional research funding from other companies. Dr. Cunningham has disclosed institutional research finding from Merck Serono and others.
Source: Shitara K et al. ESMO World Congress on Gastrointestinal Cancer 2018. Abstract LBA-005.
Ramucirumab improves HCC survival after sorafenib failure
BARCELONA – For patients with hepatocellular carcinoma (HCC) with elevated alpha-fetoprotein (AFP) levels who have disease progression following first-line sorafenib (Nexavar), the antiangiogenic agent ramucirumab was associated with a modest but significant improvement in overall survival, compared with placebo, a pooled analysis of clinical trial data showed.
Among 316 patients assigned to receive ramucirumab in the phase 3 REACH and REACH-2 trials, median overall survival was 8.1 months, compared with 5.0 months for placebo, an absolute difference of 3.1 months that translated into a hazard ratio favoring ramucirumab of 0.694 (P = .0002), reported Andrew X. Zhu, MD, PhD of the Massachusetts General Hospital Cancer Center in Boston.
Ramucirumab (Cyramza) is a human IgG1 monoclonal antibody directed against ligand activation of the vascular endothelial growth factor receptor 2.
The REACH trial did not meet its primary endpoint of an improvement in overall survival with ramucirumab in an intention-to-treat population. (Lancet Oncol. 2015 Jul;16(7):859-70). REACH-2, however, results of which were presented at the 2018 annual meeting of the American Society of Clinical Oncology (ASCO 2018, Abstract 4003), met its primary endpoint of overall survival with ramucirumab, with a median overall survival of 8.5 months versus 7.3 months for placebo (HR, 0.71; P = .0199) in patients with AFP levels of 400 ng/mL or greater who had experienced disease progression after first-line sorafenib.
The pooled analysis Dr. Zhu presented was designed to provide a better assessment of the efficacy and safety of ramucirumab as a second-line agent in this high-risk population.
In REACH, 250 patients with advanced HCC and AFP 400 ng/mL or greater were enrolled and randomized on a 1:1 basis to ramucirumab or placebo. In REACH 2, patients were randomized on a 2:1 basis to either ramucirumab 8 mg/kg intravenously every 2 weeks (the same dose as in REACH) or placebo. The pooled analysis included 316 patients on ramucirumab and 226 placebo-treated controls.
In each trial, treatment was continued until disease progression or unacceptable toxicity. The primary endpoint was overall survival, and secondary endpoints were progression-free survival, overall response rate, safety, and patient-reported outcomes.
Overall survival in the pooled analysis was as noted before. The benefits of ramucirumab were significantly better for men, for patients younger than 65 years, for patients of white and Asian race, for those with non–hepatitis B or C etiology of liver disease, and in patients with extrahepatic metastases. In addition, those without microvascular invasion, patients with Barcelona Clinic Liver Cancer score C versus B, those with excellent baseline performance status, those who received prior locoregional therapy, and patients who discontinued sorafenib because of disease progression also saw benefit from ramucirumab.
“Ramucirumab’s overall survival treatment benefit was consistent and robust across all subgroups. Sensitivity analyses, include random effect models, were consistent,” Dr. Zhu said.
The median progression-free survival with ramucirumab was 2.8 months, compared with 1.5 months for placebo (P less than .0001). The overall response rate, including all complete and partial responses, was 5.4% for ramucirumab versus 0.9% for placebo (P = .0064). The respective disease control rates, including patients with stable disease, were 56.3% versus 37.2% (P less than .0001).
Adverse events of special interest included grade 3 or greater liver injury or failure in 19.9% of patients on ramucirumab versus 26.5% on placebo, bleeding and/or hemorrhagic events in 4.7% versus 6.7%, and hypertension in 12.7% versus 3.6%.
The REACH trials were supported by Eli Lilly. Dr. Zhu disclosed consulting/advising for Eli Lilly and others, and institutional research from Eli Lilly and others.
SOURCE: Zhu AX et al. ESMO GI 2018, Abstract LBA-001.
BARCELONA – For patients with hepatocellular carcinoma (HCC) with elevated alpha-fetoprotein (AFP) levels who have disease progression following first-line sorafenib (Nexavar), the antiangiogenic agent ramucirumab was associated with a modest but significant improvement in overall survival, compared with placebo, a pooled analysis of clinical trial data showed.
Among 316 patients assigned to receive ramucirumab in the phase 3 REACH and REACH-2 trials, median overall survival was 8.1 months, compared with 5.0 months for placebo, an absolute difference of 3.1 months that translated into a hazard ratio favoring ramucirumab of 0.694 (P = .0002), reported Andrew X. Zhu, MD, PhD of the Massachusetts General Hospital Cancer Center in Boston.
Ramucirumab (Cyramza) is a human IgG1 monoclonal antibody directed against ligand activation of the vascular endothelial growth factor receptor 2.
The REACH trial did not meet its primary endpoint of an improvement in overall survival with ramucirumab in an intention-to-treat population. (Lancet Oncol. 2015 Jul;16(7):859-70). REACH-2, however, results of which were presented at the 2018 annual meeting of the American Society of Clinical Oncology (ASCO 2018, Abstract 4003), met its primary endpoint of overall survival with ramucirumab, with a median overall survival of 8.5 months versus 7.3 months for placebo (HR, 0.71; P = .0199) in patients with AFP levels of 400 ng/mL or greater who had experienced disease progression after first-line sorafenib.
The pooled analysis Dr. Zhu presented was designed to provide a better assessment of the efficacy and safety of ramucirumab as a second-line agent in this high-risk population.
In REACH, 250 patients with advanced HCC and AFP 400 ng/mL or greater were enrolled and randomized on a 1:1 basis to ramucirumab or placebo. In REACH 2, patients were randomized on a 2:1 basis to either ramucirumab 8 mg/kg intravenously every 2 weeks (the same dose as in REACH) or placebo. The pooled analysis included 316 patients on ramucirumab and 226 placebo-treated controls.
In each trial, treatment was continued until disease progression or unacceptable toxicity. The primary endpoint was overall survival, and secondary endpoints were progression-free survival, overall response rate, safety, and patient-reported outcomes.
Overall survival in the pooled analysis was as noted before. The benefits of ramucirumab were significantly better for men, for patients younger than 65 years, for patients of white and Asian race, for those with non–hepatitis B or C etiology of liver disease, and in patients with extrahepatic metastases. In addition, those without microvascular invasion, patients with Barcelona Clinic Liver Cancer score C versus B, those with excellent baseline performance status, those who received prior locoregional therapy, and patients who discontinued sorafenib because of disease progression also saw benefit from ramucirumab.
“Ramucirumab’s overall survival treatment benefit was consistent and robust across all subgroups. Sensitivity analyses, include random effect models, were consistent,” Dr. Zhu said.
The median progression-free survival with ramucirumab was 2.8 months, compared with 1.5 months for placebo (P less than .0001). The overall response rate, including all complete and partial responses, was 5.4% for ramucirumab versus 0.9% for placebo (P = .0064). The respective disease control rates, including patients with stable disease, were 56.3% versus 37.2% (P less than .0001).
Adverse events of special interest included grade 3 or greater liver injury or failure in 19.9% of patients on ramucirumab versus 26.5% on placebo, bleeding and/or hemorrhagic events in 4.7% versus 6.7%, and hypertension in 12.7% versus 3.6%.
The REACH trials were supported by Eli Lilly. Dr. Zhu disclosed consulting/advising for Eli Lilly and others, and institutional research from Eli Lilly and others.
SOURCE: Zhu AX et al. ESMO GI 2018, Abstract LBA-001.
BARCELONA – For patients with hepatocellular carcinoma (HCC) with elevated alpha-fetoprotein (AFP) levels who have disease progression following first-line sorafenib (Nexavar), the antiangiogenic agent ramucirumab was associated with a modest but significant improvement in overall survival, compared with placebo, a pooled analysis of clinical trial data showed.
Among 316 patients assigned to receive ramucirumab in the phase 3 REACH and REACH-2 trials, median overall survival was 8.1 months, compared with 5.0 months for placebo, an absolute difference of 3.1 months that translated into a hazard ratio favoring ramucirumab of 0.694 (P = .0002), reported Andrew X. Zhu, MD, PhD of the Massachusetts General Hospital Cancer Center in Boston.
Ramucirumab (Cyramza) is a human IgG1 monoclonal antibody directed against ligand activation of the vascular endothelial growth factor receptor 2.
The REACH trial did not meet its primary endpoint of an improvement in overall survival with ramucirumab in an intention-to-treat population. (Lancet Oncol. 2015 Jul;16(7):859-70). REACH-2, however, results of which were presented at the 2018 annual meeting of the American Society of Clinical Oncology (ASCO 2018, Abstract 4003), met its primary endpoint of overall survival with ramucirumab, with a median overall survival of 8.5 months versus 7.3 months for placebo (HR, 0.71; P = .0199) in patients with AFP levels of 400 ng/mL or greater who had experienced disease progression after first-line sorafenib.
The pooled analysis Dr. Zhu presented was designed to provide a better assessment of the efficacy and safety of ramucirumab as a second-line agent in this high-risk population.
In REACH, 250 patients with advanced HCC and AFP 400 ng/mL or greater were enrolled and randomized on a 1:1 basis to ramucirumab or placebo. In REACH 2, patients were randomized on a 2:1 basis to either ramucirumab 8 mg/kg intravenously every 2 weeks (the same dose as in REACH) or placebo. The pooled analysis included 316 patients on ramucirumab and 226 placebo-treated controls.
In each trial, treatment was continued until disease progression or unacceptable toxicity. The primary endpoint was overall survival, and secondary endpoints were progression-free survival, overall response rate, safety, and patient-reported outcomes.
Overall survival in the pooled analysis was as noted before. The benefits of ramucirumab were significantly better for men, for patients younger than 65 years, for patients of white and Asian race, for those with non–hepatitis B or C etiology of liver disease, and in patients with extrahepatic metastases. In addition, those without microvascular invasion, patients with Barcelona Clinic Liver Cancer score C versus B, those with excellent baseline performance status, those who received prior locoregional therapy, and patients who discontinued sorafenib because of disease progression also saw benefit from ramucirumab.
“Ramucirumab’s overall survival treatment benefit was consistent and robust across all subgroups. Sensitivity analyses, include random effect models, were consistent,” Dr. Zhu said.
The median progression-free survival with ramucirumab was 2.8 months, compared with 1.5 months for placebo (P less than .0001). The overall response rate, including all complete and partial responses, was 5.4% for ramucirumab versus 0.9% for placebo (P = .0064). The respective disease control rates, including patients with stable disease, were 56.3% versus 37.2% (P less than .0001).
Adverse events of special interest included grade 3 or greater liver injury or failure in 19.9% of patients on ramucirumab versus 26.5% on placebo, bleeding and/or hemorrhagic events in 4.7% versus 6.7%, and hypertension in 12.7% versus 3.6%.
The REACH trials were supported by Eli Lilly. Dr. Zhu disclosed consulting/advising for Eli Lilly and others, and institutional research from Eli Lilly and others.
SOURCE: Zhu AX et al. ESMO GI 2018, Abstract LBA-001.
REPORTING FROM ESMO GI 2018
Key clinical point: Second-line therapy with a vascular endothelial growth factor receptor 2 inhibitor modestly improved survival of patients with advanced hepatocellular carcinoma that progressed after first-line sorafenib.
Major finding: Median overall survival was 8.1 months with ramucirumab versus 5 months for placebo.
Study details: An analysis of pooled data from two phase 3 clinical trials with a total of 316 patients treated with ramucirumab and 226 treated with placebo.
Disclosures: The REACH trials were supported by Eli Lilly. Dr. Zhu disclosed consulting/advising for Eli Lilly and others, and institutional research from Eli Lilly and others.
Source: Zhu AX et al. ESMO GI 2018, Abstract LBA-001.
Biomarker duo rapidly identifies serious bacterial infections
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.
In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.

In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.

In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: The area under the curve combining sensitivity and specificity was 93%.
Study details: This was a prospective, observational, single-center, clinician-blinded study of 657 patients admitted to a pediatric ICU with symptoms of systemic inflammatory response syndrome.
Disclosures: The study was funded by the U.K. National Institute for Health Research. The presenter reported having no relevant financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
Mindfulness skill can help in parenting
Behavioral parent management training (PMT), which teaches parents concrete skills to increase their attention to positive behavior and to plan for their response to undesired behavior, has abundant evidence for success for many challenging child behaviors. But sometimes parents have a hard time managing their own emotional responses in the often highly triggering situation of family conflict. Mindfulness has the potential to provide a complement to the PMT skills. Studies are beginning to explore these possibilities.
Case summary
Zoe is a bright 5-year-old who has been “strong willed” and shown intense emotional responses since early in life. The usual 2-year-old temper tantrums increased over time. She has outbursts of yelling, kicking, and hitting, especially with transitions. Her parents tried behavioral parent training, but found it frustrating. If Zoe has been yelling and hitting earlier in the day, her mother feels hurt and angry and can’t bring herself to pay warm attention when Zoe is doing better. When Zoe refuses to pick up her room, her father is flooded with thoughts about his own father hitting him for the slightest disrespect. He thinks that he is a bad, weak father, and sometimes “sees red” and ends up yelling at Zoe instead of putting into place a calm consequence.
Discussion
Mindfulness is defined by Jon Kabat-Zinn as “paying attention in a particular way – on purpose, in the present moment, and nonjudgmentally.” A central feature of mindfulness is strengthening the ability to focus our attention. We learn to pay attention to aspects of the present moment, be that breathing, the sensations in our body, or the experiences of our senses. Often the first skill in behavioral training methods is getting parents to pay attention to their children by participating in child-led play or spending attentive time with older children. This means attending to what the child is doing or talking about rather than jumping in and taking over with suggestions, instructions, or judgments. This meshes very well with this central aspect of mindfulness.
As we practice paying attention, we observe that the mind naturally jumps around from what we mean to be attending to, to a host of distractions, worries, plans, memories, thoughts, and emotions. Mindfulness encourages practitioners to notice these thoughts, to avoid criticizing or judging oneself for becoming involved with these, but instead gently lead the mind back to what you had intended to focus on. This observation of the mind’s activity gives the mindfulness practitioner a bit of space from the thought or emotion itself. We are encouraged to name the thought or emotional processes we notice: “I am worrying, I am planning, I am remembering.”
In the heat of a difficult moment with the child, parents often are flooded with intense emotions (such as anger, fear, anxiety, panic, despair) and thoughts (such as “If my child keeps acting this way he is going to go to jail when he grows up,” “I am a terrible parent,” “Why is my child doing this to me?” or “He is just like his father”). These emotions and thoughts can drive intense, impulsive responses from the parents. As they practice mindfulness, they can gain the ability to observe themselves having these thoughts; observe harsh judgments of themselves or their children or their partners; have some space from them; and realize they may change in a few minutes or realize they may be painful but don’t necessarily have to spur impulsive action. In that moment, parents can give themselves time and space to think through possible actions, and then choose one.
From a behavioral parenting standpoint, we know that parents and humans often react intensely to negative behaviors and inadvertently make them worse with intense emotional reactivity. We want parents to have a plan about how they will respond, to remain calm in the moment, and then put the plan in place. Mindfulness may enhance parents’ ability to notice their own responses and have the space to remember what the plan was and then put it into place. It also can give them space to consider what the child might be experiencing and respond in light of this awareness. This ability does require a significant amount of mindfulness practice.
The combination of mindfulness and parenting is just beginning to be studied in research trials using a range of study designs. Some of these programs have looked at the effect of mindfulness courses, especially mindfulness-based stress reduction without any specific parenting content or indices of parent stress and child behavior. Others have looked at programs which add mindfulness to standard behavioral parenting programs, and still others are specific mindfulness/parenting programs. So far, many of these studies are quasi-experimental in nature. A recent systematic review by Townshend et al. found seven randomized controlled trials of low to moderate quality with some suggestion of ability to decrease parental stress and ADHD symptoms (JBI Database System Rev Implement Rep. 2016 Mar;14[3]:139-80). There is a clear need for randomized controlled trials with larger sample sizes.
While we may not have specific, highly evidence-based mindful parenting programs available, individuals with experience in yoga, meditation, mindfulness, dialectical behavioral therapy, and acceptance and commitment therapy can be encouraged to bring these skills to bear as parents.
Zoe’s parents had pursued outside mindfulness programs. Mindfulness concepts were brought into a standard parenting program. Her parents were encouraged to engage in child-led play with Zoe in a mindful way, fully attending to her actions and experience. Zoe’s parents also were encouraged to observe their own emotional reactions and thoughts in stressful moments and to take a breathing space before taking action.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
Resources
“Mindful Parenting” (New York: Norton & Co., 2015).
“Integrating mindfulness with parent training: Effects of the mindfulness-enhanced strengthening families program” (Dev Psychol. 2015;51[1]:26-35).
“Everyday blessings: The inner work of mindful parenting,” (New York: Hyperion, 1997).
Behavioral parent management training (PMT), which teaches parents concrete skills to increase their attention to positive behavior and to plan for their response to undesired behavior, has abundant evidence for success for many challenging child behaviors. But sometimes parents have a hard time managing their own emotional responses in the often highly triggering situation of family conflict. Mindfulness has the potential to provide a complement to the PMT skills. Studies are beginning to explore these possibilities.
Case summary
Zoe is a bright 5-year-old who has been “strong willed” and shown intense emotional responses since early in life. The usual 2-year-old temper tantrums increased over time. She has outbursts of yelling, kicking, and hitting, especially with transitions. Her parents tried behavioral parent training, but found it frustrating. If Zoe has been yelling and hitting earlier in the day, her mother feels hurt and angry and can’t bring herself to pay warm attention when Zoe is doing better. When Zoe refuses to pick up her room, her father is flooded with thoughts about his own father hitting him for the slightest disrespect. He thinks that he is a bad, weak father, and sometimes “sees red” and ends up yelling at Zoe instead of putting into place a calm consequence.
Discussion
Mindfulness is defined by Jon Kabat-Zinn as “paying attention in a particular way – on purpose, in the present moment, and nonjudgmentally.” A central feature of mindfulness is strengthening the ability to focus our attention. We learn to pay attention to aspects of the present moment, be that breathing, the sensations in our body, or the experiences of our senses. Often the first skill in behavioral training methods is getting parents to pay attention to their children by participating in child-led play or spending attentive time with older children. This means attending to what the child is doing or talking about rather than jumping in and taking over with suggestions, instructions, or judgments. This meshes very well with this central aspect of mindfulness.
As we practice paying attention, we observe that the mind naturally jumps around from what we mean to be attending to, to a host of distractions, worries, plans, memories, thoughts, and emotions. Mindfulness encourages practitioners to notice these thoughts, to avoid criticizing or judging oneself for becoming involved with these, but instead gently lead the mind back to what you had intended to focus on. This observation of the mind’s activity gives the mindfulness practitioner a bit of space from the thought or emotion itself. We are encouraged to name the thought or emotional processes we notice: “I am worrying, I am planning, I am remembering.”
In the heat of a difficult moment with the child, parents often are flooded with intense emotions (such as anger, fear, anxiety, panic, despair) and thoughts (such as “If my child keeps acting this way he is going to go to jail when he grows up,” “I am a terrible parent,” “Why is my child doing this to me?” or “He is just like his father”). These emotions and thoughts can drive intense, impulsive responses from the parents. As they practice mindfulness, they can gain the ability to observe themselves having these thoughts; observe harsh judgments of themselves or their children or their partners; have some space from them; and realize they may change in a few minutes or realize they may be painful but don’t necessarily have to spur impulsive action. In that moment, parents can give themselves time and space to think through possible actions, and then choose one.
From a behavioral parenting standpoint, we know that parents and humans often react intensely to negative behaviors and inadvertently make them worse with intense emotional reactivity. We want parents to have a plan about how they will respond, to remain calm in the moment, and then put the plan in place. Mindfulness may enhance parents’ ability to notice their own responses and have the space to remember what the plan was and then put it into place. It also can give them space to consider what the child might be experiencing and respond in light of this awareness. This ability does require a significant amount of mindfulness practice.
The combination of mindfulness and parenting is just beginning to be studied in research trials using a range of study designs. Some of these programs have looked at the effect of mindfulness courses, especially mindfulness-based stress reduction without any specific parenting content or indices of parent stress and child behavior. Others have looked at programs which add mindfulness to standard behavioral parenting programs, and still others are specific mindfulness/parenting programs. So far, many of these studies are quasi-experimental in nature. A recent systematic review by Townshend et al. found seven randomized controlled trials of low to moderate quality with some suggestion of ability to decrease parental stress and ADHD symptoms (JBI Database System Rev Implement Rep. 2016 Mar;14[3]:139-80). There is a clear need for randomized controlled trials with larger sample sizes.
While we may not have specific, highly evidence-based mindful parenting programs available, individuals with experience in yoga, meditation, mindfulness, dialectical behavioral therapy, and acceptance and commitment therapy can be encouraged to bring these skills to bear as parents.
Zoe’s parents had pursued outside mindfulness programs. Mindfulness concepts were brought into a standard parenting program. Her parents were encouraged to engage in child-led play with Zoe in a mindful way, fully attending to her actions and experience. Zoe’s parents also were encouraged to observe their own emotional reactions and thoughts in stressful moments and to take a breathing space before taking action.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
Resources
“Mindful Parenting” (New York: Norton & Co., 2015).
“Integrating mindfulness with parent training: Effects of the mindfulness-enhanced strengthening families program” (Dev Psychol. 2015;51[1]:26-35).
“Everyday blessings: The inner work of mindful parenting,” (New York: Hyperion, 1997).
Behavioral parent management training (PMT), which teaches parents concrete skills to increase their attention to positive behavior and to plan for their response to undesired behavior, has abundant evidence for success for many challenging child behaviors. But sometimes parents have a hard time managing their own emotional responses in the often highly triggering situation of family conflict. Mindfulness has the potential to provide a complement to the PMT skills. Studies are beginning to explore these possibilities.
Case summary
Zoe is a bright 5-year-old who has been “strong willed” and shown intense emotional responses since early in life. The usual 2-year-old temper tantrums increased over time. She has outbursts of yelling, kicking, and hitting, especially with transitions. Her parents tried behavioral parent training, but found it frustrating. If Zoe has been yelling and hitting earlier in the day, her mother feels hurt and angry and can’t bring herself to pay warm attention when Zoe is doing better. When Zoe refuses to pick up her room, her father is flooded with thoughts about his own father hitting him for the slightest disrespect. He thinks that he is a bad, weak father, and sometimes “sees red” and ends up yelling at Zoe instead of putting into place a calm consequence.
Discussion
Mindfulness is defined by Jon Kabat-Zinn as “paying attention in a particular way – on purpose, in the present moment, and nonjudgmentally.” A central feature of mindfulness is strengthening the ability to focus our attention. We learn to pay attention to aspects of the present moment, be that breathing, the sensations in our body, or the experiences of our senses. Often the first skill in behavioral training methods is getting parents to pay attention to their children by participating in child-led play or spending attentive time with older children. This means attending to what the child is doing or talking about rather than jumping in and taking over with suggestions, instructions, or judgments. This meshes very well with this central aspect of mindfulness.
As we practice paying attention, we observe that the mind naturally jumps around from what we mean to be attending to, to a host of distractions, worries, plans, memories, thoughts, and emotions. Mindfulness encourages practitioners to notice these thoughts, to avoid criticizing or judging oneself for becoming involved with these, but instead gently lead the mind back to what you had intended to focus on. This observation of the mind’s activity gives the mindfulness practitioner a bit of space from the thought or emotion itself. We are encouraged to name the thought or emotional processes we notice: “I am worrying, I am planning, I am remembering.”
In the heat of a difficult moment with the child, parents often are flooded with intense emotions (such as anger, fear, anxiety, panic, despair) and thoughts (such as “If my child keeps acting this way he is going to go to jail when he grows up,” “I am a terrible parent,” “Why is my child doing this to me?” or “He is just like his father”). These emotions and thoughts can drive intense, impulsive responses from the parents. As they practice mindfulness, they can gain the ability to observe themselves having these thoughts; observe harsh judgments of themselves or their children or their partners; have some space from them; and realize they may change in a few minutes or realize they may be painful but don’t necessarily have to spur impulsive action. In that moment, parents can give themselves time and space to think through possible actions, and then choose one.
From a behavioral parenting standpoint, we know that parents and humans often react intensely to negative behaviors and inadvertently make them worse with intense emotional reactivity. We want parents to have a plan about how they will respond, to remain calm in the moment, and then put the plan in place. Mindfulness may enhance parents’ ability to notice their own responses and have the space to remember what the plan was and then put it into place. It also can give them space to consider what the child might be experiencing and respond in light of this awareness. This ability does require a significant amount of mindfulness practice.
The combination of mindfulness and parenting is just beginning to be studied in research trials using a range of study designs. Some of these programs have looked at the effect of mindfulness courses, especially mindfulness-based stress reduction without any specific parenting content or indices of parent stress and child behavior. Others have looked at programs which add mindfulness to standard behavioral parenting programs, and still others are specific mindfulness/parenting programs. So far, many of these studies are quasi-experimental in nature. A recent systematic review by Townshend et al. found seven randomized controlled trials of low to moderate quality with some suggestion of ability to decrease parental stress and ADHD symptoms (JBI Database System Rev Implement Rep. 2016 Mar;14[3]:139-80). There is a clear need for randomized controlled trials with larger sample sizes.
While we may not have specific, highly evidence-based mindful parenting programs available, individuals with experience in yoga, meditation, mindfulness, dialectical behavioral therapy, and acceptance and commitment therapy can be encouraged to bring these skills to bear as parents.
Zoe’s parents had pursued outside mindfulness programs. Mindfulness concepts were brought into a standard parenting program. Her parents were encouraged to engage in child-led play with Zoe in a mindful way, fully attending to her actions and experience. Zoe’s parents also were encouraged to observe their own emotional reactions and thoughts in stressful moments and to take a breathing space before taking action.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
Resources
“Mindful Parenting” (New York: Norton & Co., 2015).
“Integrating mindfulness with parent training: Effects of the mindfulness-enhanced strengthening families program” (Dev Psychol. 2015;51[1]:26-35).
“Everyday blessings: The inner work of mindful parenting,” (New York: Hyperion, 1997).
Pediatric Dermatology Consult - July 2018
Frequently misdiagnosed, streptococcal intertrigo more commonly affects infants and toddlers but is rarely reported, especially compared with other Streptococcus pyogenes infections, including impetigo, erysipelas, and cellulitis.1
Intertrigo, meaning “between” (inter) and “to rub” (terere) in Latin, describes any skin disorder involving two opposing skin surfaces that touch or rub to cause friction.2 The continuous chaffing, coupled with moisture trapped within the skin folds, leads to irritation and maceration, which provides an ideal environment for pathogens to thrive. Thus, frictional dermatitides that arise may become secondarily infected with one or more microorganisms, such as Candida albicans, Staphylococcus aureus, Streptococcus pyogenes, and even organisms less commonly associated with cutaneous infection, such as Proteus mirabilis.3
Streptococcal intertrigo may affect any intertriginous area, but most commonly it affects the folds of the neck; this is likely because of the combination of the deep folds that develop in shorter, infantile necks and the moisture from drool and saliva that pools in the area.5,6 In addition to these cervical folds, other intertriginous areas commonly are affected, including the inguinal, axillary, popliteal, posterior auricular, perianal, and genital folds.
Perianal streptococcal disease may present in a similar manner as streptococcal intertrigo, manifesting as well-demarcated, beefy red plaques in the skin folds around the anus and, in females, frequently perivaginally.7 Unlike streptococcal intertrigo, perianal streptococcal disease is often characterized by pain, pruritus, and fissuring of the involved area.8 It is associated with pharyngeal colonization of group A beta-hemolytic streptococci.7
Diagnosis is straight forward and may be confirmed by a positive streptococcal rapid antigen test of swab specimens of one or more surfaces of affected skin or by culture from a skin swab yielding growth of the organism.1,5 Skin biopsy is not necessary. If the index of suspicion for candida is high, a potassium hydroxide preparation and culture may be performed. Checking serum anti-DNase B antibodies, antistreptolysin O, and pharyngeal cultures is often unrevealing.9 A urinalysis may be performed to assess for poststreptococcal glomerulonephritis if the patient later develops facial or orbital edema, hypertension, hematuria, or lethargy.9
Treatment consists of systemic antistreptococcal therapy; oral amoxicillin and penicillin frequently have been used.9 Moisture in the area should be reduced with application of absorptive powders and physical barriers, such as zinc oxide, after gentle cleansing of the area.5
Other diagnoses to consider when evaluating dermatitides affecting skin folds include: other infectious causes, which may be ruled out by fungal or bacterial culture; inverse psoriasis, which will frequently demonstrate scale; atopic dermatitis, which will be pruritic with history of atopy; irritant or contact dermatitis, which will often have correlating clinical history; seborrheic dermatitis, which will often involve greasiness and scale; and less commonly, acrodermatitis enteropathica, which will be accompanied by diarrhea and hair loss.2,9 Scabies also may be on the differential if the patient endorses severe pruritus with close contacts with similar symptoms.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university. They had no conflicts of interest or disclosures to report.
References
1. Pediatr Dermatol. 2014 Mar-Apr;31(2):e71-2.
2. Clin Dermatol. 2011 Mar-Apr;29(2):173-9.
3. Pediatrics. 2003 Dec;112(6 pt 1):1427-9.
4. BMJ Case Rep. 2018 Mar 20. doi: 10.1136/bcr-2018-224179.
5. Pediatr Infect Dis J. 2012 Aug;31(8):872-3.
6. J Pediatr. 2015 May;166(5):1318.
7. J Pediatr. 2015 Sep;167(3):687-93.e1-2.
8. Pediatrics in Review. 1991;12(8):248-55.
9. J Pediatr. 2017 May;184:230-1.e1.
Frequently misdiagnosed, streptococcal intertrigo more commonly affects infants and toddlers but is rarely reported, especially compared with other Streptococcus pyogenes infections, including impetigo, erysipelas, and cellulitis.1
Intertrigo, meaning “between” (inter) and “to rub” (terere) in Latin, describes any skin disorder involving two opposing skin surfaces that touch or rub to cause friction.2 The continuous chaffing, coupled with moisture trapped within the skin folds, leads to irritation and maceration, which provides an ideal environment for pathogens to thrive. Thus, frictional dermatitides that arise may become secondarily infected with one or more microorganisms, such as Candida albicans, Staphylococcus aureus, Streptococcus pyogenes, and even organisms less commonly associated with cutaneous infection, such as Proteus mirabilis.3
Streptococcal intertrigo may affect any intertriginous area, but most commonly it affects the folds of the neck; this is likely because of the combination of the deep folds that develop in shorter, infantile necks and the moisture from drool and saliva that pools in the area.5,6 In addition to these cervical folds, other intertriginous areas commonly are affected, including the inguinal, axillary, popliteal, posterior auricular, perianal, and genital folds.
Perianal streptococcal disease may present in a similar manner as streptococcal intertrigo, manifesting as well-demarcated, beefy red plaques in the skin folds around the anus and, in females, frequently perivaginally.7 Unlike streptococcal intertrigo, perianal streptococcal disease is often characterized by pain, pruritus, and fissuring of the involved area.8 It is associated with pharyngeal colonization of group A beta-hemolytic streptococci.7
Diagnosis is straight forward and may be confirmed by a positive streptococcal rapid antigen test of swab specimens of one or more surfaces of affected skin or by culture from a skin swab yielding growth of the organism.1,5 Skin biopsy is not necessary. If the index of suspicion for candida is high, a potassium hydroxide preparation and culture may be performed. Checking serum anti-DNase B antibodies, antistreptolysin O, and pharyngeal cultures is often unrevealing.9 A urinalysis may be performed to assess for poststreptococcal glomerulonephritis if the patient later develops facial or orbital edema, hypertension, hematuria, or lethargy.9
Treatment consists of systemic antistreptococcal therapy; oral amoxicillin and penicillin frequently have been used.9 Moisture in the area should be reduced with application of absorptive powders and physical barriers, such as zinc oxide, after gentle cleansing of the area.5
Other diagnoses to consider when evaluating dermatitides affecting skin folds include: other infectious causes, which may be ruled out by fungal or bacterial culture; inverse psoriasis, which will frequently demonstrate scale; atopic dermatitis, which will be pruritic with history of atopy; irritant or contact dermatitis, which will often have correlating clinical history; seborrheic dermatitis, which will often involve greasiness and scale; and less commonly, acrodermatitis enteropathica, which will be accompanied by diarrhea and hair loss.2,9 Scabies also may be on the differential if the patient endorses severe pruritus with close contacts with similar symptoms.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university. They had no conflicts of interest or disclosures to report.
References
1. Pediatr Dermatol. 2014 Mar-Apr;31(2):e71-2.
2. Clin Dermatol. 2011 Mar-Apr;29(2):173-9.
3. Pediatrics. 2003 Dec;112(6 pt 1):1427-9.
4. BMJ Case Rep. 2018 Mar 20. doi: 10.1136/bcr-2018-224179.
5. Pediatr Infect Dis J. 2012 Aug;31(8):872-3.
6. J Pediatr. 2015 May;166(5):1318.
7. J Pediatr. 2015 Sep;167(3):687-93.e1-2.
8. Pediatrics in Review. 1991;12(8):248-55.
9. J Pediatr. 2017 May;184:230-1.e1.
Frequently misdiagnosed, streptococcal intertrigo more commonly affects infants and toddlers but is rarely reported, especially compared with other Streptococcus pyogenes infections, including impetigo, erysipelas, and cellulitis.1
Intertrigo, meaning “between” (inter) and “to rub” (terere) in Latin, describes any skin disorder involving two opposing skin surfaces that touch or rub to cause friction.2 The continuous chaffing, coupled with moisture trapped within the skin folds, leads to irritation and maceration, which provides an ideal environment for pathogens to thrive. Thus, frictional dermatitides that arise may become secondarily infected with one or more microorganisms, such as Candida albicans, Staphylococcus aureus, Streptococcus pyogenes, and even organisms less commonly associated with cutaneous infection, such as Proteus mirabilis.3
Streptococcal intertrigo may affect any intertriginous area, but most commonly it affects the folds of the neck; this is likely because of the combination of the deep folds that develop in shorter, infantile necks and the moisture from drool and saliva that pools in the area.5,6 In addition to these cervical folds, other intertriginous areas commonly are affected, including the inguinal, axillary, popliteal, posterior auricular, perianal, and genital folds.
Perianal streptococcal disease may present in a similar manner as streptococcal intertrigo, manifesting as well-demarcated, beefy red plaques in the skin folds around the anus and, in females, frequently perivaginally.7 Unlike streptococcal intertrigo, perianal streptococcal disease is often characterized by pain, pruritus, and fissuring of the involved area.8 It is associated with pharyngeal colonization of group A beta-hemolytic streptococci.7
Diagnosis is straight forward and may be confirmed by a positive streptococcal rapid antigen test of swab specimens of one or more surfaces of affected skin or by culture from a skin swab yielding growth of the organism.1,5 Skin biopsy is not necessary. If the index of suspicion for candida is high, a potassium hydroxide preparation and culture may be performed. Checking serum anti-DNase B antibodies, antistreptolysin O, and pharyngeal cultures is often unrevealing.9 A urinalysis may be performed to assess for poststreptococcal glomerulonephritis if the patient later develops facial or orbital edema, hypertension, hematuria, or lethargy.9
Treatment consists of systemic antistreptococcal therapy; oral amoxicillin and penicillin frequently have been used.9 Moisture in the area should be reduced with application of absorptive powders and physical barriers, such as zinc oxide, after gentle cleansing of the area.5
Other diagnoses to consider when evaluating dermatitides affecting skin folds include: other infectious causes, which may be ruled out by fungal or bacterial culture; inverse psoriasis, which will frequently demonstrate scale; atopic dermatitis, which will be pruritic with history of atopy; irritant or contact dermatitis, which will often have correlating clinical history; seborrheic dermatitis, which will often involve greasiness and scale; and less commonly, acrodermatitis enteropathica, which will be accompanied by diarrhea and hair loss.2,9 Scabies also may be on the differential if the patient endorses severe pruritus with close contacts with similar symptoms.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university. They had no conflicts of interest or disclosures to report.
References
1. Pediatr Dermatol. 2014 Mar-Apr;31(2):e71-2.
2. Clin Dermatol. 2011 Mar-Apr;29(2):173-9.
3. Pediatrics. 2003 Dec;112(6 pt 1):1427-9.
4. BMJ Case Rep. 2018 Mar 20. doi: 10.1136/bcr-2018-224179.
5. Pediatr Infect Dis J. 2012 Aug;31(8):872-3.
6. J Pediatr. 2015 May;166(5):1318.
7. J Pediatr. 2015 Sep;167(3):687-93.e1-2.
8. Pediatrics in Review. 1991;12(8):248-55.
9. J Pediatr. 2017 May;184:230-1.e1.
An 8-week-old male with a history of cradle cap presented for a second evaluation of an erythematous rash on the neck that started 1.5 weeks before, and it had since worsened. The parents note that their infant has been more irritable, but they otherwise deny any fever, diarrhea, constipation, or decrease in oral intake.
The patient’s first evaluation had been 3 days prior; nystatin cream was prescribed, and the parents applied it twice a day but without improvement to the rash. The patient also had a rash behind the ears bilaterally, which was treated with hydrocortisone 2.5% ointment with some improvement
On physical exam, the central neck is covered by a bright, beefy red, erythematous plaque with distinct borders and strong odor. There is faint scale and superficial desquamation between the skin folds. There are no surrounding papules or pustules. The patient’s chin is moist with drool. In the postauricular skin folds bilaterally, there are fainter but still erythematous plaques with mild scale.
Serum troponin predicts cardiovascular death in early arthritis
LIVERPOOL, ENGLAND – Serum levels of the cardiac biomarker troponin might prove useful for assessing the risk of death from cardiovascular causes in patients with inflammatory arthritis, according to study findings presented at the British Society for Rheumatology annual conference.
“In this analysis we have shown that baseline troponin levels predict cardiovascular death in inflammatory arthritis, and this association is independent of the traditional risk factors, inflammation, and disease characteristics at baseline,” said study author Sarah Skeoch, MBChB, who works at the Arthritis Research UK Centre for Epidemiology in the division of musculoskeletal and dermatological sciences at the University of Manchester (England).
Furthermore, the association remained in patients who had rheumatoid arthritis classified according to the 2010 American College of Rheumatology and European League Against Rheumatism criteria (overall adjusted HR, 2.25) and in those without prior cardiovascular disease at baseline (HR, 1.63).
Individuals with inflammatory arthritis are known to have an increased risk of developing cardiovascular problems versus the general population, but current prediction models using traditional risk factors do not fully account for the increased risk seen in patients with inflammatory arthritis, Dr. Skeoch explained.
“There has been some work looking at troponin in inflammatory arthritis already,” she said, with “higher levels observed versus age- and sex-matched controls, and associations have been shown with traditional risk factors.” There has also been a link to C-reactive protein levels and disease activity, and there has also been an association with coronary stenosis on CT scans. The aim of the current study was to see if there was any link to cardiovascular events and death.
A total of 1,023 patients who had been recruited into NOAR between 2000 and 2009 were studied. NOAR is an inception cohort study that includes patients with a history of two or more swollen joints for 4 weeks or more and has been running for almost 30 years. At baseline serum samples are taken and a variety of assessments made, including cardiovascular risk factors.
The study population was mostly female (66%), aged a median of 56 years, and had symptoms for a median of 10.6 months. Around half were seropositive for rheumatoid factor, anti–citrullinated protein antibodies, or both. The median baseline disease activity score in 28 joints (DAS28) was 3.73, and 61% met ACR/EULAR 2010 criteria for RA.
Baseline serum samples were analyzed using a chemiluminescent assay to determine hs-TnI levels, with the median being 6.3 pg/mL. All patients had detectable hs-TnI levels, and 2.6% had levels exceeding 26.1 pg/mL, which is the level associated with having had an acute myocardial infarction. Almost 4% had a previous cardiovascular event, and 7% had diabetes. One in five were current smokers, and roughly 18% had hypertension. The investigators adjusted for all of these factors in the multivariate analyses.
The median follow up was 11.2 years, totaling 11,237 person-years, and during that time 158 deaths occurred, of which 27 were due to ischemic events. The median time from inclusion in NOAR to death was 7.4 years.
When levels of hs-TnI were separated into tertiles, a 12.5-fold increased risk was observed when comparing patients in the highest (more than 7.7 pg/mL) to lowest tertiles (less than 5.2 pg/mL).
“The magnitude of risk between the highest and the lowest tertile was much greater than observed in the general population,” Dr. Skeoch said, and although not directly comparable, she said the hazard ratios were 12.5 and 1.67, “which again suggests that troponin may be an effective tool or addition to the risk prediction models in inflammatory arthritis.”
Unlike some biomarkers, assays to assess troponin are already available in the clinic, Dr. Skeoch commented, “so if further work by us and other groups do suggest a role for troponin, this could be translated fairly rapidly into clinical practice.”
Further research needs to look at why troponin is raised and what is its relationship to other risk factors. “There is a strong association with traditional risk factors such as lipids, so it would stand to reason that managing those risk factors, as well as lifestyle factors, would have a positive impact,” Dr. Skeoch suggested.
The NOAR register is funded by Arthritis Research UK and the U.K. National Institute for Health Research. Dr. Skeoch and her coauthors had no relevant financial conflicts of interest.
SOURCE: Skeoch S et al. BSR 2018. Rheumatology. 2018;57[Suppl. 3]:key075.192.
LIVERPOOL, ENGLAND – Serum levels of the cardiac biomarker troponin might prove useful for assessing the risk of death from cardiovascular causes in patients with inflammatory arthritis, according to study findings presented at the British Society for Rheumatology annual conference.
“In this analysis we have shown that baseline troponin levels predict cardiovascular death in inflammatory arthritis, and this association is independent of the traditional risk factors, inflammation, and disease characteristics at baseline,” said study author Sarah Skeoch, MBChB, who works at the Arthritis Research UK Centre for Epidemiology in the division of musculoskeletal and dermatological sciences at the University of Manchester (England).
Furthermore, the association remained in patients who had rheumatoid arthritis classified according to the 2010 American College of Rheumatology and European League Against Rheumatism criteria (overall adjusted HR, 2.25) and in those without prior cardiovascular disease at baseline (HR, 1.63).
Individuals with inflammatory arthritis are known to have an increased risk of developing cardiovascular problems versus the general population, but current prediction models using traditional risk factors do not fully account for the increased risk seen in patients with inflammatory arthritis, Dr. Skeoch explained.
“There has been some work looking at troponin in inflammatory arthritis already,” she said, with “higher levels observed versus age- and sex-matched controls, and associations have been shown with traditional risk factors.” There has also been a link to C-reactive protein levels and disease activity, and there has also been an association with coronary stenosis on CT scans. The aim of the current study was to see if there was any link to cardiovascular events and death.
A total of 1,023 patients who had been recruited into NOAR between 2000 and 2009 were studied. NOAR is an inception cohort study that includes patients with a history of two or more swollen joints for 4 weeks or more and has been running for almost 30 years. At baseline serum samples are taken and a variety of assessments made, including cardiovascular risk factors.
The study population was mostly female (66%), aged a median of 56 years, and had symptoms for a median of 10.6 months. Around half were seropositive for rheumatoid factor, anti–citrullinated protein antibodies, or both. The median baseline disease activity score in 28 joints (DAS28) was 3.73, and 61% met ACR/EULAR 2010 criteria for RA.
Baseline serum samples were analyzed using a chemiluminescent assay to determine hs-TnI levels, with the median being 6.3 pg/mL. All patients had detectable hs-TnI levels, and 2.6% had levels exceeding 26.1 pg/mL, which is the level associated with having had an acute myocardial infarction. Almost 4% had a previous cardiovascular event, and 7% had diabetes. One in five were current smokers, and roughly 18% had hypertension. The investigators adjusted for all of these factors in the multivariate analyses.
The median follow up was 11.2 years, totaling 11,237 person-years, and during that time 158 deaths occurred, of which 27 were due to ischemic events. The median time from inclusion in NOAR to death was 7.4 years.
When levels of hs-TnI were separated into tertiles, a 12.5-fold increased risk was observed when comparing patients in the highest (more than 7.7 pg/mL) to lowest tertiles (less than 5.2 pg/mL).
“The magnitude of risk between the highest and the lowest tertile was much greater than observed in the general population,” Dr. Skeoch said, and although not directly comparable, she said the hazard ratios were 12.5 and 1.67, “which again suggests that troponin may be an effective tool or addition to the risk prediction models in inflammatory arthritis.”
Unlike some biomarkers, assays to assess troponin are already available in the clinic, Dr. Skeoch commented, “so if further work by us and other groups do suggest a role for troponin, this could be translated fairly rapidly into clinical practice.”
Further research needs to look at why troponin is raised and what is its relationship to other risk factors. “There is a strong association with traditional risk factors such as lipids, so it would stand to reason that managing those risk factors, as well as lifestyle factors, would have a positive impact,” Dr. Skeoch suggested.
The NOAR register is funded by Arthritis Research UK and the U.K. National Institute for Health Research. Dr. Skeoch and her coauthors had no relevant financial conflicts of interest.
SOURCE: Skeoch S et al. BSR 2018. Rheumatology. 2018;57[Suppl. 3]:key075.192.
LIVERPOOL, ENGLAND – Serum levels of the cardiac biomarker troponin might prove useful for assessing the risk of death from cardiovascular causes in patients with inflammatory arthritis, according to study findings presented at the British Society for Rheumatology annual conference.
“In this analysis we have shown that baseline troponin levels predict cardiovascular death in inflammatory arthritis, and this association is independent of the traditional risk factors, inflammation, and disease characteristics at baseline,” said study author Sarah Skeoch, MBChB, who works at the Arthritis Research UK Centre for Epidemiology in the division of musculoskeletal and dermatological sciences at the University of Manchester (England).
Furthermore, the association remained in patients who had rheumatoid arthritis classified according to the 2010 American College of Rheumatology and European League Against Rheumatism criteria (overall adjusted HR, 2.25) and in those without prior cardiovascular disease at baseline (HR, 1.63).
Individuals with inflammatory arthritis are known to have an increased risk of developing cardiovascular problems versus the general population, but current prediction models using traditional risk factors do not fully account for the increased risk seen in patients with inflammatory arthritis, Dr. Skeoch explained.
“There has been some work looking at troponin in inflammatory arthritis already,” she said, with “higher levels observed versus age- and sex-matched controls, and associations have been shown with traditional risk factors.” There has also been a link to C-reactive protein levels and disease activity, and there has also been an association with coronary stenosis on CT scans. The aim of the current study was to see if there was any link to cardiovascular events and death.
A total of 1,023 patients who had been recruited into NOAR between 2000 and 2009 were studied. NOAR is an inception cohort study that includes patients with a history of two or more swollen joints for 4 weeks or more and has been running for almost 30 years. At baseline serum samples are taken and a variety of assessments made, including cardiovascular risk factors.
The study population was mostly female (66%), aged a median of 56 years, and had symptoms for a median of 10.6 months. Around half were seropositive for rheumatoid factor, anti–citrullinated protein antibodies, or both. The median baseline disease activity score in 28 joints (DAS28) was 3.73, and 61% met ACR/EULAR 2010 criteria for RA.
Baseline serum samples were analyzed using a chemiluminescent assay to determine hs-TnI levels, with the median being 6.3 pg/mL. All patients had detectable hs-TnI levels, and 2.6% had levels exceeding 26.1 pg/mL, which is the level associated with having had an acute myocardial infarction. Almost 4% had a previous cardiovascular event, and 7% had diabetes. One in five were current smokers, and roughly 18% had hypertension. The investigators adjusted for all of these factors in the multivariate analyses.
The median follow up was 11.2 years, totaling 11,237 person-years, and during that time 158 deaths occurred, of which 27 were due to ischemic events. The median time from inclusion in NOAR to death was 7.4 years.
When levels of hs-TnI were separated into tertiles, a 12.5-fold increased risk was observed when comparing patients in the highest (more than 7.7 pg/mL) to lowest tertiles (less than 5.2 pg/mL).
“The magnitude of risk between the highest and the lowest tertile was much greater than observed in the general population,” Dr. Skeoch said, and although not directly comparable, she said the hazard ratios were 12.5 and 1.67, “which again suggests that troponin may be an effective tool or addition to the risk prediction models in inflammatory arthritis.”
Unlike some biomarkers, assays to assess troponin are already available in the clinic, Dr. Skeoch commented, “so if further work by us and other groups do suggest a role for troponin, this could be translated fairly rapidly into clinical practice.”
Further research needs to look at why troponin is raised and what is its relationship to other risk factors. “There is a strong association with traditional risk factors such as lipids, so it would stand to reason that managing those risk factors, as well as lifestyle factors, would have a positive impact,” Dr. Skeoch suggested.
The NOAR register is funded by Arthritis Research UK and the U.K. National Institute for Health Research. Dr. Skeoch and her coauthors had no relevant financial conflicts of interest.
SOURCE: Skeoch S et al. BSR 2018. Rheumatology. 2018;57[Suppl. 3]:key075.192.
REPORTING FROM BSR 2018
Key clinical point: Cardiovascular mortality was predicted by baseline levels of high-sensitivity troponin I.
Major finding: For every log unit increase in hs-TnI at baseline, there was an increase in cardiovascular mortality (HR, 2.16).
Study details: Analysis of data on 1,023 patients with inflammatory arthritis listed in the Norfolk Arthritis Register.
Disclosures: Dr. Skeoch and coauthors had no relevant financial conflicts of interest.
Source: Skeoch S et al. BSR 2018. Rheumatology. 2018;57[Suppl. 3]:key075.192.
Tralokinumab appears safe and effective for atopic dermatitis, in phase 2b study
Treatment with symptoms in patients with moderate to severe atopic dermatitis (AD), in a recent phase 2b study published in the Journal of Allergy and Clinical Immunology.
The randomized, double-blind, placebo-controlled, dose-ranging study assigned 204 patients to receive placebo or 45 mg, 150 mg, or 300 mg of tralokinumab administered subcutaneously every second week for 12 weeks. The groups had similar demographics and disease characteristics. The patients were aged 15-75 years and had Eczema Area and Severity Index (EASI) scores of 12 or more and an Investigator Global Assessment (IGA) score of 3 or higher. The coprimary endpoints were change in EASI from baseline to week 12 and the percentage of patients with either 0 (clear) or 1 (almost clear) on the IGA scale.
The higher dosages of tralokinumab showed the greatest adjusted mean differences in EASI scores: reductions of 4.36 for the 150-mg group (P = .03) and 4.94 for the 300-mg group (P = .01), compared with placebo. The changes in the 300-mg group were apparent as early as 4 weeks into treatment and were maintained beyond the 12-week mark. The greatest differences, compared with placebo, in IGA were seen in the 300-mg group as well.
Furthermore, patients who had high levels of biomarkers associated with IL-13 showed greater improvements than those seen in the intention-to-treat population at large. By week 12, patient-reported pruritus was also improved, and there were improvements in Dermatology Quality of Life Index (which did not persist past 12 weeks).
Most treatment-emergent adverse events were considered only mild or moderate, and the few more serious events were deemed unrelated to the study drug. The most common adverse events were upper respiratory infections and headaches.
“Participants entering the study had not achieved an adequate response to stable topical glucocorticoids during the 2-week run-in period and, therefore, represent a population with moderate to severe AD and major unmet treatment needs,” the investigators wrote. “The clinically meaningful benefits observed by combining tralokinumab treatment with topical glucocorticoids suggests that tralokinumab could demonstrate improvements in participants whose symptoms cannot be effectively controlled by topical glucocorticoids alone.”
The study was funded by MedImmune, a member of the AstraZeneca Group. Five authors were or are employees of the company; the three remaining authors had disclosures related to numerous pharmaceutical companies, including two with disclosures that included MedImmune.
SOURCE: Wollenberg A et al. J Allergy Clin Immunol. 2018 Jun 12. doi: 10.1016/j.jaci.2018.05.029.
Treatment with symptoms in patients with moderate to severe atopic dermatitis (AD), in a recent phase 2b study published in the Journal of Allergy and Clinical Immunology.
The randomized, double-blind, placebo-controlled, dose-ranging study assigned 204 patients to receive placebo or 45 mg, 150 mg, or 300 mg of tralokinumab administered subcutaneously every second week for 12 weeks. The groups had similar demographics and disease characteristics. The patients were aged 15-75 years and had Eczema Area and Severity Index (EASI) scores of 12 or more and an Investigator Global Assessment (IGA) score of 3 or higher. The coprimary endpoints were change in EASI from baseline to week 12 and the percentage of patients with either 0 (clear) or 1 (almost clear) on the IGA scale.
The higher dosages of tralokinumab showed the greatest adjusted mean differences in EASI scores: reductions of 4.36 for the 150-mg group (P = .03) and 4.94 for the 300-mg group (P = .01), compared with placebo. The changes in the 300-mg group were apparent as early as 4 weeks into treatment and were maintained beyond the 12-week mark. The greatest differences, compared with placebo, in IGA were seen in the 300-mg group as well.
Furthermore, patients who had high levels of biomarkers associated with IL-13 showed greater improvements than those seen in the intention-to-treat population at large. By week 12, patient-reported pruritus was also improved, and there were improvements in Dermatology Quality of Life Index (which did not persist past 12 weeks).
Most treatment-emergent adverse events were considered only mild or moderate, and the few more serious events were deemed unrelated to the study drug. The most common adverse events were upper respiratory infections and headaches.
“Participants entering the study had not achieved an adequate response to stable topical glucocorticoids during the 2-week run-in period and, therefore, represent a population with moderate to severe AD and major unmet treatment needs,” the investigators wrote. “The clinically meaningful benefits observed by combining tralokinumab treatment with topical glucocorticoids suggests that tralokinumab could demonstrate improvements in participants whose symptoms cannot be effectively controlled by topical glucocorticoids alone.”
The study was funded by MedImmune, a member of the AstraZeneca Group. Five authors were or are employees of the company; the three remaining authors had disclosures related to numerous pharmaceutical companies, including two with disclosures that included MedImmune.
SOURCE: Wollenberg A et al. J Allergy Clin Immunol. 2018 Jun 12. doi: 10.1016/j.jaci.2018.05.029.
Treatment with symptoms in patients with moderate to severe atopic dermatitis (AD), in a recent phase 2b study published in the Journal of Allergy and Clinical Immunology.
The randomized, double-blind, placebo-controlled, dose-ranging study assigned 204 patients to receive placebo or 45 mg, 150 mg, or 300 mg of tralokinumab administered subcutaneously every second week for 12 weeks. The groups had similar demographics and disease characteristics. The patients were aged 15-75 years and had Eczema Area and Severity Index (EASI) scores of 12 or more and an Investigator Global Assessment (IGA) score of 3 or higher. The coprimary endpoints were change in EASI from baseline to week 12 and the percentage of patients with either 0 (clear) or 1 (almost clear) on the IGA scale.
The higher dosages of tralokinumab showed the greatest adjusted mean differences in EASI scores: reductions of 4.36 for the 150-mg group (P = .03) and 4.94 for the 300-mg group (P = .01), compared with placebo. The changes in the 300-mg group were apparent as early as 4 weeks into treatment and were maintained beyond the 12-week mark. The greatest differences, compared with placebo, in IGA were seen in the 300-mg group as well.
Furthermore, patients who had high levels of biomarkers associated with IL-13 showed greater improvements than those seen in the intention-to-treat population at large. By week 12, patient-reported pruritus was also improved, and there were improvements in Dermatology Quality of Life Index (which did not persist past 12 weeks).
Most treatment-emergent adverse events were considered only mild or moderate, and the few more serious events were deemed unrelated to the study drug. The most common adverse events were upper respiratory infections and headaches.
“Participants entering the study had not achieved an adequate response to stable topical glucocorticoids during the 2-week run-in period and, therefore, represent a population with moderate to severe AD and major unmet treatment needs,” the investigators wrote. “The clinically meaningful benefits observed by combining tralokinumab treatment with topical glucocorticoids suggests that tralokinumab could demonstrate improvements in participants whose symptoms cannot be effectively controlled by topical glucocorticoids alone.”
The study was funded by MedImmune, a member of the AstraZeneca Group. Five authors were or are employees of the company; the three remaining authors had disclosures related to numerous pharmaceutical companies, including two with disclosures that included MedImmune.
SOURCE: Wollenberg A et al. J Allergy Clin Immunol. 2018 Jun 12. doi: 10.1016/j.jaci.2018.05.029.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Administration eases way for small businesses to buy insurance in bulk
Small employers will more easily be able to band together to buy health insurance under rules issued June 19 by the Trump administration, but the change could raise premiums for plans sold through the Affordable Care Act’s (ACA’s) online marketplaces, analysts say.
The move loosens restrictions on so-called association health plans, allowing more businesses, including sole proprietors, to join forces to buy health coverage in bulk for their workers.
By effectively shifting small-business coverage into the large-group market, it exempts such plans from ACA requirements for 10 “essential” health benefits, such as mental health care and prescription drug coverage, prompting warnings of “junk insurance” from consumer advocates.
Supporters say the new Labor Department rules, which the government estimated could create health plans covering as many as 11 million people, will lead to more affordable choices for some employers.
When it comes to health insurance, “the regulatory burden on small businesses should certainly not be more than that on large companies,” Labor Secretary Alexander Acosta told reporters June 19.
Existing rules limit association plans to groups of employers in the same industry in the same region.
The new regulations eliminate the geographical restriction for similar employers, allowing, for example, family-owned auto-repair shops in multiple states to offer one big health plan, said Christopher Condeluci, a health benefits lawyer and former Senate Finance Committee aide.
The rules, to be implemented in stages into next year, also allow companies in different industries in the same region to form a group to offer coverage – even if the only reason is to provide health insurance.
Like other coverage under the ACA, association insurance plans will still be required to cover preexisting illnesses.
Analysts warn that, because these changes will likely siphon away employers with relatively healthy consumers from ACA coverage into less-expensive trade-association plans, the result could be higher costs in the online marketplaces.
“If you have a group that is healthier than average, you might get a better rate from one of these plans, and your broker is going to come and say, ‘Hey, I can get you a better deal,’ ” said Dan Mendelson, president of Avalere Health, a consulting firm.
That would mean that, on balance, consumers insured through ACA small-group and individual plans could be older, sicker, and more expensive, adding to years of erosion of the ACA marketplaces engineered by Republicans hostile to the law.
Loosening rules for association plans would lead to 3.2 million people leaving the ACA plans by 2022 and raising premiums for those remaining in individual markets by 3.5%, Avalere calculated this year.
America’s Health Insurance Plans, the largest medical insurance trade group, issued a statement saying the regulation “may lead to higher premiums” in ACA insurance and “could result in fewer insured Americans.”
Unlike ACA plans, association coverage does not have to include benefits across the broad “essential” categories, including hospitalization and emergency care.
The National Association of Insurance Commissioners previously warned that such plans “threaten the stability of the small group market” and “provide inadequate benefits and insufficient protection to consumers.”
The American Academy of Actuaries has expressed similar concerns.
Business groups praised the change, proposed in draft form earlier this year.
“We’ve been advocating for association health plans for almost 20 years, and we’re pleased to see the department moving aggressively forward,” said David French, senior vice president of government relations for the National Retail Federation.
Association plans have been around for decades, although enrollment has been more limited since the ACA’s passage. While some of the plans have worked well for their members, others have a checkered history.
In April, for example, Massachusetts regulators settled with Kansas-based Unified Life Insurance Company, which agreed to pay $2.8 million to resolve allegations that it engaged in deceptive practices, such as claiming it covered services that it did not.
The coverage “was sold across state lines and was issued through a third-party association,” according to a release from the Massachusetts attorney general’s office.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Small employers will more easily be able to band together to buy health insurance under rules issued June 19 by the Trump administration, but the change could raise premiums for plans sold through the Affordable Care Act’s (ACA’s) online marketplaces, analysts say.
The move loosens restrictions on so-called association health plans, allowing more businesses, including sole proprietors, to join forces to buy health coverage in bulk for their workers.
By effectively shifting small-business coverage into the large-group market, it exempts such plans from ACA requirements for 10 “essential” health benefits, such as mental health care and prescription drug coverage, prompting warnings of “junk insurance” from consumer advocates.
Supporters say the new Labor Department rules, which the government estimated could create health plans covering as many as 11 million people, will lead to more affordable choices for some employers.
When it comes to health insurance, “the regulatory burden on small businesses should certainly not be more than that on large companies,” Labor Secretary Alexander Acosta told reporters June 19.
Existing rules limit association plans to groups of employers in the same industry in the same region.
The new regulations eliminate the geographical restriction for similar employers, allowing, for example, family-owned auto-repair shops in multiple states to offer one big health plan, said Christopher Condeluci, a health benefits lawyer and former Senate Finance Committee aide.
The rules, to be implemented in stages into next year, also allow companies in different industries in the same region to form a group to offer coverage – even if the only reason is to provide health insurance.
Like other coverage under the ACA, association insurance plans will still be required to cover preexisting illnesses.
Analysts warn that, because these changes will likely siphon away employers with relatively healthy consumers from ACA coverage into less-expensive trade-association plans, the result could be higher costs in the online marketplaces.
“If you have a group that is healthier than average, you might get a better rate from one of these plans, and your broker is going to come and say, ‘Hey, I can get you a better deal,’ ” said Dan Mendelson, president of Avalere Health, a consulting firm.
That would mean that, on balance, consumers insured through ACA small-group and individual plans could be older, sicker, and more expensive, adding to years of erosion of the ACA marketplaces engineered by Republicans hostile to the law.
Loosening rules for association plans would lead to 3.2 million people leaving the ACA plans by 2022 and raising premiums for those remaining in individual markets by 3.5%, Avalere calculated this year.
America’s Health Insurance Plans, the largest medical insurance trade group, issued a statement saying the regulation “may lead to higher premiums” in ACA insurance and “could result in fewer insured Americans.”
Unlike ACA plans, association coverage does not have to include benefits across the broad “essential” categories, including hospitalization and emergency care.
The National Association of Insurance Commissioners previously warned that such plans “threaten the stability of the small group market” and “provide inadequate benefits and insufficient protection to consumers.”
The American Academy of Actuaries has expressed similar concerns.
Business groups praised the change, proposed in draft form earlier this year.
“We’ve been advocating for association health plans for almost 20 years, and we’re pleased to see the department moving aggressively forward,” said David French, senior vice president of government relations for the National Retail Federation.
Association plans have been around for decades, although enrollment has been more limited since the ACA’s passage. While some of the plans have worked well for their members, others have a checkered history.
In April, for example, Massachusetts regulators settled with Kansas-based Unified Life Insurance Company, which agreed to pay $2.8 million to resolve allegations that it engaged in deceptive practices, such as claiming it covered services that it did not.
The coverage “was sold across state lines and was issued through a third-party association,” according to a release from the Massachusetts attorney general’s office.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Small employers will more easily be able to band together to buy health insurance under rules issued June 19 by the Trump administration, but the change could raise premiums for plans sold through the Affordable Care Act’s (ACA’s) online marketplaces, analysts say.
The move loosens restrictions on so-called association health plans, allowing more businesses, including sole proprietors, to join forces to buy health coverage in bulk for their workers.
By effectively shifting small-business coverage into the large-group market, it exempts such plans from ACA requirements for 10 “essential” health benefits, such as mental health care and prescription drug coverage, prompting warnings of “junk insurance” from consumer advocates.
Supporters say the new Labor Department rules, which the government estimated could create health plans covering as many as 11 million people, will lead to more affordable choices for some employers.
When it comes to health insurance, “the regulatory burden on small businesses should certainly not be more than that on large companies,” Labor Secretary Alexander Acosta told reporters June 19.
Existing rules limit association plans to groups of employers in the same industry in the same region.
The new regulations eliminate the geographical restriction for similar employers, allowing, for example, family-owned auto-repair shops in multiple states to offer one big health plan, said Christopher Condeluci, a health benefits lawyer and former Senate Finance Committee aide.
The rules, to be implemented in stages into next year, also allow companies in different industries in the same region to form a group to offer coverage – even if the only reason is to provide health insurance.
Like other coverage under the ACA, association insurance plans will still be required to cover preexisting illnesses.
Analysts warn that, because these changes will likely siphon away employers with relatively healthy consumers from ACA coverage into less-expensive trade-association plans, the result could be higher costs in the online marketplaces.
“If you have a group that is healthier than average, you might get a better rate from one of these plans, and your broker is going to come and say, ‘Hey, I can get you a better deal,’ ” said Dan Mendelson, president of Avalere Health, a consulting firm.
That would mean that, on balance, consumers insured through ACA small-group and individual plans could be older, sicker, and more expensive, adding to years of erosion of the ACA marketplaces engineered by Republicans hostile to the law.
Loosening rules for association plans would lead to 3.2 million people leaving the ACA plans by 2022 and raising premiums for those remaining in individual markets by 3.5%, Avalere calculated this year.
America’s Health Insurance Plans, the largest medical insurance trade group, issued a statement saying the regulation “may lead to higher premiums” in ACA insurance and “could result in fewer insured Americans.”
Unlike ACA plans, association coverage does not have to include benefits across the broad “essential” categories, including hospitalization and emergency care.
The National Association of Insurance Commissioners previously warned that such plans “threaten the stability of the small group market” and “provide inadequate benefits and insufficient protection to consumers.”
The American Academy of Actuaries has expressed similar concerns.
Business groups praised the change, proposed in draft form earlier this year.
“We’ve been advocating for association health plans for almost 20 years, and we’re pleased to see the department moving aggressively forward,” said David French, senior vice president of government relations for the National Retail Federation.
Association plans have been around for decades, although enrollment has been more limited since the ACA’s passage. While some of the plans have worked well for their members, others have a checkered history.
In April, for example, Massachusetts regulators settled with Kansas-based Unified Life Insurance Company, which agreed to pay $2.8 million to resolve allegations that it engaged in deceptive practices, such as claiming it covered services that it did not.
The coverage “was sold across state lines and was issued through a third-party association,” according to a release from the Massachusetts attorney general’s office.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Parents say cancer prevention is the best reason to give HPV vaccine
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Key clinical point: Among the reasons health care providers give parents for adolescent HPV vaccination, cancer prevention may be the best.
Major finding: Cancer prevention ranked highest (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
Study details: An analysis of 1,177 responses from parents of adolescents obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey.
Disclosures: The study was funded by the National Cancer Institute. Study authors had no potential conflicts of interest to disclose.
Source: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.