User login
SVS Booth will be in the Exhibit Hall
SVS members who are attending the Vascular Annual Meeting can get in-person help with any application issues, or, indeed any membership services concerns. The SVS Booth, #1015, in the Exhibit Hall will be staffed both Thursday and Friday, June 21-22. SVS staff members will be able to provide information on a wide range of topics, including:
• Member services, including
- The benefits of belonging
- Dues
- Membership applications
- Log-in issues
- Contact information updates – don’t fall off the contacts list!
• The Affinity Program of expanded benefits
• Educational products, including the fourth edition of the Vascular Education Self-Assessment Program (VESAP), the SVS coding Class, the SVS Vascular Research Initiatives Conference and the Rutherford’s Vascular Surgery book.
• The Journal of Vascular Surgery publications (offering JVS socks!)
• The SVS Patient Safety Organization Vascular Quality Initiative
• Quality and clinical practice guideline initiatives (including printed copies of four of the latest SVS guidelines)
• The Mobile App or VAM Planner
• The SVS Foundation and the SVS Political Action Committee; members can make a donation to either or both
• Scan the QR code as part of the Exhibit Hall Scavenger Hunt
Enter to win a prize: Those who drop by can be entered into a drawing for three prizes: an SVS Membership waiver for next year, free VAM 2019 registration and VAM on Demand from the ’18 meeting. In addition, while supplies last, visitors also can pick up a memento of their visit.
SVS members who are attending the Vascular Annual Meeting can get in-person help with any application issues, or, indeed any membership services concerns. The SVS Booth, #1015, in the Exhibit Hall will be staffed both Thursday and Friday, June 21-22. SVS staff members will be able to provide information on a wide range of topics, including:
• Member services, including
- The benefits of belonging
- Dues
- Membership applications
- Log-in issues
- Contact information updates – don’t fall off the contacts list!
• The Affinity Program of expanded benefits
• Educational products, including the fourth edition of the Vascular Education Self-Assessment Program (VESAP), the SVS coding Class, the SVS Vascular Research Initiatives Conference and the Rutherford’s Vascular Surgery book.
• The Journal of Vascular Surgery publications (offering JVS socks!)
• The SVS Patient Safety Organization Vascular Quality Initiative
• Quality and clinical practice guideline initiatives (including printed copies of four of the latest SVS guidelines)
• The Mobile App or VAM Planner
• The SVS Foundation and the SVS Political Action Committee; members can make a donation to either or both
• Scan the QR code as part of the Exhibit Hall Scavenger Hunt
Enter to win a prize: Those who drop by can be entered into a drawing for three prizes: an SVS Membership waiver for next year, free VAM 2019 registration and VAM on Demand from the ’18 meeting. In addition, while supplies last, visitors also can pick up a memento of their visit.
SVS members who are attending the Vascular Annual Meeting can get in-person help with any application issues, or, indeed any membership services concerns. The SVS Booth, #1015, in the Exhibit Hall will be staffed both Thursday and Friday, June 21-22. SVS staff members will be able to provide information on a wide range of topics, including:
• Member services, including
- The benefits of belonging
- Dues
- Membership applications
- Log-in issues
- Contact information updates – don’t fall off the contacts list!
• The Affinity Program of expanded benefits
• Educational products, including the fourth edition of the Vascular Education Self-Assessment Program (VESAP), the SVS coding Class, the SVS Vascular Research Initiatives Conference and the Rutherford’s Vascular Surgery book.
• The Journal of Vascular Surgery publications (offering JVS socks!)
• The SVS Patient Safety Organization Vascular Quality Initiative
• Quality and clinical practice guideline initiatives (including printed copies of four of the latest SVS guidelines)
• The Mobile App or VAM Planner
• The SVS Foundation and the SVS Political Action Committee; members can make a donation to either or both
• Scan the QR code as part of the Exhibit Hall Scavenger Hunt
Enter to win a prize: Those who drop by can be entered into a drawing for three prizes: an SVS Membership waiver for next year, free VAM 2019 registration and VAM on Demand from the ’18 meeting. In addition, while supplies last, visitors also can pick up a memento of their visit.
A Look into the Future at the Crawford Forum
Will the demand be sufficient for the supply of the vascular surgery work force in the United States? That topic, along with potential solutions, will be front and center at the E. Stanley Crawford Critical Issues Forum from 10:30 a.m. to 12 p.m. See HCC, Ballroom A/B.
Will the demand be sufficient for the supply of the vascular surgery work force in the United States? That topic, along with potential solutions, will be front and center at the E. Stanley Crawford Critical Issues Forum from 10:30 a.m. to 12 p.m. See HCC, Ballroom A/B.
Will the demand be sufficient for the supply of the vascular surgery work force in the United States? That topic, along with potential solutions, will be front and center at the E. Stanley Crawford Critical Issues Forum from 10:30 a.m. to 12 p.m. See HCC, Ballroom A/B.
PAD Patients at Risk for High Opioid Use
National efforts have intensified to reduce opioid prescriptions because of the opioid crisis. However, little is known about the relationship between peripheral arterial disease (PAD) and high-risk opioid use, according to Nathan K. Itoga, MD, of Stanford University, Calif. “As a vascular surgery resident, I wanted to know how I could do my part in reducing opioid prescriptions. However, I didn’t know if vascular patients were at risk for high opioid use. The impetus for this study was the lack of studies regarding opioid use in patients with vascular disease,” said Dr. Itoga.
Dr. Itoga will present a study in Thursday’s Scientific Session 2 that he and his colleagues performed to evaluate the relationship between PAD and high opioid use and to assess whether PAD treatment impacts high opioid use.
The researchers used the 2007-2015 Truven Marketscan database, a deidentified national private insurance claims database, to identify patients with 2 ICD-9 diagnosis codes of PAD 2 months apart with at least 2 years of continuous enrollment. Critical limb ischemia (CLI) was defined as rest pain, ulcers, or gangrene.
“Our primary outcome was high opioid use, defined as 2 opioid prescriptions within a 1-year period,” said Dr. Itoga. Opioid prescriptions were excluded if filled within 90 days of a PAD-related procedure, as identified by CPT codes for lower extremity open/endovascular revascularization or amputation. A total of 182,186 patients with PAD met the inclusion criteria, 27.1% of whom had CLI. The mean follow-up was 5.29 years. An average of 24.4% of patients with PAD met the high opioid use criteria in any given calendar year, with a significant decreasing trend for patients meeting criteria beginning in 2010 (P less than .01), said Dr. Itoga. Among the high opioid users, 26.0% continued to meet the criteria for 5 years. High opioid use was found to be significantly more common for patients with CLI (32.2%) compared to PAD patients without CLI (vs. 21.4%). During years of high opioid use, an average of 5.9 yearly prescriptions was filled.
According to multivariate analysis, illicit drug use and back pain were the strongest significant predictors of high opioid use (P less than .001).
A new diagnosis of PAD significantly increased the incidence of high opioid use (21.3% before PAD diagnosis vs. 26.9% after diagnosis, P less than .01). This association with a new diagnosis of CLI increased high opioid use from 27.5% before CLI diagnosis to 37.7% after CLI diagnosis (P less than .01), highlighting the increased risk in this patient population. A total of 45,028 patients (24.7%) underwent 88,229 PAD-related procedures. After exclusion of periprocedural opioid prescriptions (18% of all opioid prescriptions), the yearly percentage of high opioid users increased from 25.6% pretreatment to 29.2% post treatment, also a significant difference (P less than .01).
“Our research shows that patients with PAD are at increased risk for high opioid use, with nearly one-quarter meeting described criteria. CLI additionally increases opioid utilization, and treatment of PAD does not appear to decrease high opioid use,” he said. “In addition to heightened awareness and active opioid management, our findings warrant further investigation into causes and deterrence of high-risk opioid use,” Dr. Itoga concluded.
Thursday, June 21, 2018
1:30 - 3:00 p.m.
HCC, Ballroom A/B
S2: Scientific Session 2
National efforts have intensified to reduce opioid prescriptions because of the opioid crisis. However, little is known about the relationship between peripheral arterial disease (PAD) and high-risk opioid use, according to Nathan K. Itoga, MD, of Stanford University, Calif. “As a vascular surgery resident, I wanted to know how I could do my part in reducing opioid prescriptions. However, I didn’t know if vascular patients were at risk for high opioid use. The impetus for this study was the lack of studies regarding opioid use in patients with vascular disease,” said Dr. Itoga.
Dr. Itoga will present a study in Thursday’s Scientific Session 2 that he and his colleagues performed to evaluate the relationship between PAD and high opioid use and to assess whether PAD treatment impacts high opioid use.
The researchers used the 2007-2015 Truven Marketscan database, a deidentified national private insurance claims database, to identify patients with 2 ICD-9 diagnosis codes of PAD 2 months apart with at least 2 years of continuous enrollment. Critical limb ischemia (CLI) was defined as rest pain, ulcers, or gangrene.
“Our primary outcome was high opioid use, defined as 2 opioid prescriptions within a 1-year period,” said Dr. Itoga. Opioid prescriptions were excluded if filled within 90 days of a PAD-related procedure, as identified by CPT codes for lower extremity open/endovascular revascularization or amputation. A total of 182,186 patients with PAD met the inclusion criteria, 27.1% of whom had CLI. The mean follow-up was 5.29 years. An average of 24.4% of patients with PAD met the high opioid use criteria in any given calendar year, with a significant decreasing trend for patients meeting criteria beginning in 2010 (P less than .01), said Dr. Itoga. Among the high opioid users, 26.0% continued to meet the criteria for 5 years. High opioid use was found to be significantly more common for patients with CLI (32.2%) compared to PAD patients without CLI (vs. 21.4%). During years of high opioid use, an average of 5.9 yearly prescriptions was filled.
According to multivariate analysis, illicit drug use and back pain were the strongest significant predictors of high opioid use (P less than .001).
A new diagnosis of PAD significantly increased the incidence of high opioid use (21.3% before PAD diagnosis vs. 26.9% after diagnosis, P less than .01). This association with a new diagnosis of CLI increased high opioid use from 27.5% before CLI diagnosis to 37.7% after CLI diagnosis (P less than .01), highlighting the increased risk in this patient population. A total of 45,028 patients (24.7%) underwent 88,229 PAD-related procedures. After exclusion of periprocedural opioid prescriptions (18% of all opioid prescriptions), the yearly percentage of high opioid users increased from 25.6% pretreatment to 29.2% post treatment, also a significant difference (P less than .01).
“Our research shows that patients with PAD are at increased risk for high opioid use, with nearly one-quarter meeting described criteria. CLI additionally increases opioid utilization, and treatment of PAD does not appear to decrease high opioid use,” he said. “In addition to heightened awareness and active opioid management, our findings warrant further investigation into causes and deterrence of high-risk opioid use,” Dr. Itoga concluded.
Thursday, June 21, 2018
1:30 - 3:00 p.m.
HCC, Ballroom A/B
S2: Scientific Session 2
National efforts have intensified to reduce opioid prescriptions because of the opioid crisis. However, little is known about the relationship between peripheral arterial disease (PAD) and high-risk opioid use, according to Nathan K. Itoga, MD, of Stanford University, Calif. “As a vascular surgery resident, I wanted to know how I could do my part in reducing opioid prescriptions. However, I didn’t know if vascular patients were at risk for high opioid use. The impetus for this study was the lack of studies regarding opioid use in patients with vascular disease,” said Dr. Itoga.
Dr. Itoga will present a study in Thursday’s Scientific Session 2 that he and his colleagues performed to evaluate the relationship between PAD and high opioid use and to assess whether PAD treatment impacts high opioid use.
The researchers used the 2007-2015 Truven Marketscan database, a deidentified national private insurance claims database, to identify patients with 2 ICD-9 diagnosis codes of PAD 2 months apart with at least 2 years of continuous enrollment. Critical limb ischemia (CLI) was defined as rest pain, ulcers, or gangrene.
“Our primary outcome was high opioid use, defined as 2 opioid prescriptions within a 1-year period,” said Dr. Itoga. Opioid prescriptions were excluded if filled within 90 days of a PAD-related procedure, as identified by CPT codes for lower extremity open/endovascular revascularization or amputation. A total of 182,186 patients with PAD met the inclusion criteria, 27.1% of whom had CLI. The mean follow-up was 5.29 years. An average of 24.4% of patients with PAD met the high opioid use criteria in any given calendar year, with a significant decreasing trend for patients meeting criteria beginning in 2010 (P less than .01), said Dr. Itoga. Among the high opioid users, 26.0% continued to meet the criteria for 5 years. High opioid use was found to be significantly more common for patients with CLI (32.2%) compared to PAD patients without CLI (vs. 21.4%). During years of high opioid use, an average of 5.9 yearly prescriptions was filled.
According to multivariate analysis, illicit drug use and back pain were the strongest significant predictors of high opioid use (P less than .001).
A new diagnosis of PAD significantly increased the incidence of high opioid use (21.3% before PAD diagnosis vs. 26.9% after diagnosis, P less than .01). This association with a new diagnosis of CLI increased high opioid use from 27.5% before CLI diagnosis to 37.7% after CLI diagnosis (P less than .01), highlighting the increased risk in this patient population. A total of 45,028 patients (24.7%) underwent 88,229 PAD-related procedures. After exclusion of periprocedural opioid prescriptions (18% of all opioid prescriptions), the yearly percentage of high opioid users increased from 25.6% pretreatment to 29.2% post treatment, also a significant difference (P less than .01).
“Our research shows that patients with PAD are at increased risk for high opioid use, with nearly one-quarter meeting described criteria. CLI additionally increases opioid utilization, and treatment of PAD does not appear to decrease high opioid use,” he said. “In addition to heightened awareness and active opioid management, our findings warrant further investigation into causes and deterrence of high-risk opioid use,” Dr. Itoga concluded.
Thursday, June 21, 2018
1:30 - 3:00 p.m.
HCC, Ballroom A/B
S2: Scientific Session 2
SVS von Liebig Forum: Complex Aortic Endografting
Innovative approaches to minimally invasive repair of the aorta will be featured during Thursday morning’s William J. von Liebig Forum.
“This year, the forum’s focus is on complex aortic endografting for a wide variety of aortic diseases, predominantly thoracoabdominal aortic aneurysms and aortic dissections,” said session co-moderator Matthew Eagleton, MD, chief of the division of vascular and endovascular surgery at Massachusetts General Hospital in Boston. “Surgeons who treat aortic disease will learn about new devices and new techniques that they will want to incorporate into their practice. They’ll also learn whether some of the techniques and procedures they are currently employing are still worth doing or may have unforeseen risks. Should they change their practices and approaches to patients with complex aortic disease, or should they continue down the road and recognize that it’s safe for patients?” The session will feature eight abstracts from vascular surgeons in the United States and Europe that will accentuate some of the newer, advanced technology, said session co-moderator R. Clement Darling III, MD, chief of the division of vascular surgery at Albany Medical Center Hospital in New York.
“To minimize the complications of complex aortic aneurysm repair, endovascular technology has to be evaluated, and we need to know what works, what doesn’t, and what are the long-term outcomes,” he said. “These presentations will help us understand the best techniques and the best current and future technology in percutaneous repair of complex aneurysms from the aortic arch down to the abdominal aorta.”
The program kicks off with a talk by Emanuel R. Tenorio, MD, PhD, of the Mayo Clinic about a prospective, nonrandomized study to evaluate cone beam computed tomography for technical assessment of standard and complex endovascular aortic repair. In other presentations, Tilo Kölbel, MD, PhD, of the University Heart Center Hamburg in Germany will discuss a single-center experience with a double-branched aortic arch endograft, technology not yet available in the United States. A U.S. national consortium, organized to better assess outcomes for endovascular therapy for complex aortic disease, will discuss their work with target artery outcomes after branched and fenestrated endovascular repair of pararenal and thoracoabdominal aortic aneurysms in the U.S. IDE (investigational device exemption) experience, and a group from Bologna, Italy, will discuss the risk of aneurysm rupture and target visceral vessel occlusion during the lead period of custom-made fenestrated/branched endografts.
Additional talks will go over percutaneous large-bore axillary artery access techniques for complicated EVAR (endovascular aneurysm repair) and current guidelines and indications for repair of abdominal aortic aneurysms.
One concern for much of this new technology is durability, Dr. Darling said. Jason Hurd, MD, of the University of Washington, Seattle, will discuss the long-term durability of a physician-modified endograft.
“We in the U.S. don’t have access to some of the technology that’s available worldwide, and there are physicians out there who do modifications of their own endografts,” Dr. Eagleton said. “This study happens to look at a prospectively maintained database from a physician-sponsored IDE study to specifically look at outcomes with regard to physician modification. The results of this are very important for us to know – is this something we can do for patients [who] need it, or is this something we should just shy away from completely? We don’t know the answer.”
Added Dr. Darling, “This presentation will allow us to see not only the current and future technology to be used but also how well it works over time. We are lucky to be involved in the evolution of minimally invasive technology for the repair of complex aortic pathology.”
Overall, said Dr. Darling, “This forum will outline the advances that have been made and some of the techniques that can be used to treat patients. Continued evaluation of the outcomes, applications and limitations of technology will help us take better care of patients.”
Thursday
8:30 – 10 a.m.
HCC, Ballroom A/B
S1: William J. von Liebig Forum
Innovative approaches to minimally invasive repair of the aorta will be featured during Thursday morning’s William J. von Liebig Forum.
“This year, the forum’s focus is on complex aortic endografting for a wide variety of aortic diseases, predominantly thoracoabdominal aortic aneurysms and aortic dissections,” said session co-moderator Matthew Eagleton, MD, chief of the division of vascular and endovascular surgery at Massachusetts General Hospital in Boston. “Surgeons who treat aortic disease will learn about new devices and new techniques that they will want to incorporate into their practice. They’ll also learn whether some of the techniques and procedures they are currently employing are still worth doing or may have unforeseen risks. Should they change their practices and approaches to patients with complex aortic disease, or should they continue down the road and recognize that it’s safe for patients?” The session will feature eight abstracts from vascular surgeons in the United States and Europe that will accentuate some of the newer, advanced technology, said session co-moderator R. Clement Darling III, MD, chief of the division of vascular surgery at Albany Medical Center Hospital in New York.
“To minimize the complications of complex aortic aneurysm repair, endovascular technology has to be evaluated, and we need to know what works, what doesn’t, and what are the long-term outcomes,” he said. “These presentations will help us understand the best techniques and the best current and future technology in percutaneous repair of complex aneurysms from the aortic arch down to the abdominal aorta.”
The program kicks off with a talk by Emanuel R. Tenorio, MD, PhD, of the Mayo Clinic about a prospective, nonrandomized study to evaluate cone beam computed tomography for technical assessment of standard and complex endovascular aortic repair. In other presentations, Tilo Kölbel, MD, PhD, of the University Heart Center Hamburg in Germany will discuss a single-center experience with a double-branched aortic arch endograft, technology not yet available in the United States. A U.S. national consortium, organized to better assess outcomes for endovascular therapy for complex aortic disease, will discuss their work with target artery outcomes after branched and fenestrated endovascular repair of pararenal and thoracoabdominal aortic aneurysms in the U.S. IDE (investigational device exemption) experience, and a group from Bologna, Italy, will discuss the risk of aneurysm rupture and target visceral vessel occlusion during the lead period of custom-made fenestrated/branched endografts.
Additional talks will go over percutaneous large-bore axillary artery access techniques for complicated EVAR (endovascular aneurysm repair) and current guidelines and indications for repair of abdominal aortic aneurysms.
One concern for much of this new technology is durability, Dr. Darling said. Jason Hurd, MD, of the University of Washington, Seattle, will discuss the long-term durability of a physician-modified endograft.
“We in the U.S. don’t have access to some of the technology that’s available worldwide, and there are physicians out there who do modifications of their own endografts,” Dr. Eagleton said. “This study happens to look at a prospectively maintained database from a physician-sponsored IDE study to specifically look at outcomes with regard to physician modification. The results of this are very important for us to know – is this something we can do for patients [who] need it, or is this something we should just shy away from completely? We don’t know the answer.”
Added Dr. Darling, “This presentation will allow us to see not only the current and future technology to be used but also how well it works over time. We are lucky to be involved in the evolution of minimally invasive technology for the repair of complex aortic pathology.”
Overall, said Dr. Darling, “This forum will outline the advances that have been made and some of the techniques that can be used to treat patients. Continued evaluation of the outcomes, applications and limitations of technology will help us take better care of patients.”
Thursday
8:30 – 10 a.m.
HCC, Ballroom A/B
S1: William J. von Liebig Forum
Innovative approaches to minimally invasive repair of the aorta will be featured during Thursday morning’s William J. von Liebig Forum.
“This year, the forum’s focus is on complex aortic endografting for a wide variety of aortic diseases, predominantly thoracoabdominal aortic aneurysms and aortic dissections,” said session co-moderator Matthew Eagleton, MD, chief of the division of vascular and endovascular surgery at Massachusetts General Hospital in Boston. “Surgeons who treat aortic disease will learn about new devices and new techniques that they will want to incorporate into their practice. They’ll also learn whether some of the techniques and procedures they are currently employing are still worth doing or may have unforeseen risks. Should they change their practices and approaches to patients with complex aortic disease, or should they continue down the road and recognize that it’s safe for patients?” The session will feature eight abstracts from vascular surgeons in the United States and Europe that will accentuate some of the newer, advanced technology, said session co-moderator R. Clement Darling III, MD, chief of the division of vascular surgery at Albany Medical Center Hospital in New York.
“To minimize the complications of complex aortic aneurysm repair, endovascular technology has to be evaluated, and we need to know what works, what doesn’t, and what are the long-term outcomes,” he said. “These presentations will help us understand the best techniques and the best current and future technology in percutaneous repair of complex aneurysms from the aortic arch down to the abdominal aorta.”
The program kicks off with a talk by Emanuel R. Tenorio, MD, PhD, of the Mayo Clinic about a prospective, nonrandomized study to evaluate cone beam computed tomography for technical assessment of standard and complex endovascular aortic repair. In other presentations, Tilo Kölbel, MD, PhD, of the University Heart Center Hamburg in Germany will discuss a single-center experience with a double-branched aortic arch endograft, technology not yet available in the United States. A U.S. national consortium, organized to better assess outcomes for endovascular therapy for complex aortic disease, will discuss their work with target artery outcomes after branched and fenestrated endovascular repair of pararenal and thoracoabdominal aortic aneurysms in the U.S. IDE (investigational device exemption) experience, and a group from Bologna, Italy, will discuss the risk of aneurysm rupture and target visceral vessel occlusion during the lead period of custom-made fenestrated/branched endografts.
Additional talks will go over percutaneous large-bore axillary artery access techniques for complicated EVAR (endovascular aneurysm repair) and current guidelines and indications for repair of abdominal aortic aneurysms.
One concern for much of this new technology is durability, Dr. Darling said. Jason Hurd, MD, of the University of Washington, Seattle, will discuss the long-term durability of a physician-modified endograft.
“We in the U.S. don’t have access to some of the technology that’s available worldwide, and there are physicians out there who do modifications of their own endografts,” Dr. Eagleton said. “This study happens to look at a prospectively maintained database from a physician-sponsored IDE study to specifically look at outcomes with regard to physician modification. The results of this are very important for us to know – is this something we can do for patients [who] need it, or is this something we should just shy away from completely? We don’t know the answer.”
Added Dr. Darling, “This presentation will allow us to see not only the current and future technology to be used but also how well it works over time. We are lucky to be involved in the evolution of minimally invasive technology for the repair of complex aortic pathology.”
Overall, said Dr. Darling, “This forum will outline the advances that have been made and some of the techniques that can be used to treat patients. Continued evaluation of the outcomes, applications and limitations of technology will help us take better care of patients.”
Thursday
8:30 – 10 a.m.
HCC, Ballroom A/B
S1: William J. von Liebig Forum
Resident Research Award Highlights Diet-Based Strategy to Prevent Vein Graft Disease
This year’s SVS Foundation’s Resident Research Award is being presented to Kaspar M. Trocha, MD, for his research on vein graft disease. As a member of the laboratories of C. Keith Ozaki, MD, and James Mitchell, MD, along with co-first author Peter Kip, MD, and microsurgeon Ming Tao, MD, the group studies the effects of food intake immediately before surgery on vascular adaptations.
Dietary restriction (reducing food intake without malnutrition) has been a topic of special interest in the science community for decades, and restricting calories in the long term has been shown to extend life and health span in a variety of species. It can also protect from overexuberant responses to trauma and ischemia-reperfusion.
Dr. Mitchell found that the benefits from long-term food restriction could be acquired as rapidly as in a few days, pointing to a potential for implementing dietary restriction recommendations prior to elective surgery.
The response to stress benefits seen following brief dietary interventions are mediated by the gaseous signaling molecule hydrogen sulfide (H2S). H2S is strongly upregulated by dietary restriction and this “rotten egg” gas seems to be protective for the cardiovascular system and has been demonstrated to be involved in vasodilation, inflammation, and atherogenesis, according to the researchers. The group’s experiments thus tested short-term protein restriction (a more feasible dietary approach for patients that observed to induce H2S) in a microsurgical mouse vein graft model.
The team discovered that cutting all protein from the animal diet for just 1 week before surgery led to increased levels of the enzyme cystathionine-gamma-lyase (CGL) that makes H2S, higher levels of the protective gaseous molecule, less early vein graft inflammation, and less eventual occlusive vein graft disease even though the animals were returned to their usual high-fat diet postop. The group also confirmed these results by constructing a new mouse strain that overexpresses CGL, and these mice were protected from vein graft disease. Conversely, blocking this enzyme negated all the beneficial effects of the dietary restriction.
According to Dr. Trocha and his colleagues, “short-term pre-operative protein restriction and manipulation of H2S stand as novel, economical approaches to enhance vein graft durability and perhaps even lessen peri-operative complications, and we are in the early stages of testing this strategy in vascular surgery patients.”
Dr. Trocha’s research was supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant and Dr. Ozaki’s NIH and American Heart Association grants. The Resident Research Award is given to one individual each year as determined by the SVS Research and Education Committee with the intent of motivating physicians early in their training to pursue their interest in research that explores the biology of vascular disease and potential translational therapies. The recognition includes plenary presentation at the Vascular Annual Meeting, a $5,000 award and a 1-year complimentary subscription to the Journal of Vascular Surgery.
This year’s SVS Foundation’s Resident Research Award is being presented to Kaspar M. Trocha, MD, for his research on vein graft disease. As a member of the laboratories of C. Keith Ozaki, MD, and James Mitchell, MD, along with co-first author Peter Kip, MD, and microsurgeon Ming Tao, MD, the group studies the effects of food intake immediately before surgery on vascular adaptations.
Dietary restriction (reducing food intake without malnutrition) has been a topic of special interest in the science community for decades, and restricting calories in the long term has been shown to extend life and health span in a variety of species. It can also protect from overexuberant responses to trauma and ischemia-reperfusion.
Dr. Mitchell found that the benefits from long-term food restriction could be acquired as rapidly as in a few days, pointing to a potential for implementing dietary restriction recommendations prior to elective surgery.
The response to stress benefits seen following brief dietary interventions are mediated by the gaseous signaling molecule hydrogen sulfide (H2S). H2S is strongly upregulated by dietary restriction and this “rotten egg” gas seems to be protective for the cardiovascular system and has been demonstrated to be involved in vasodilation, inflammation, and atherogenesis, according to the researchers. The group’s experiments thus tested short-term protein restriction (a more feasible dietary approach for patients that observed to induce H2S) in a microsurgical mouse vein graft model.
The team discovered that cutting all protein from the animal diet for just 1 week before surgery led to increased levels of the enzyme cystathionine-gamma-lyase (CGL) that makes H2S, higher levels of the protective gaseous molecule, less early vein graft inflammation, and less eventual occlusive vein graft disease even though the animals were returned to their usual high-fat diet postop. The group also confirmed these results by constructing a new mouse strain that overexpresses CGL, and these mice were protected from vein graft disease. Conversely, blocking this enzyme negated all the beneficial effects of the dietary restriction.
According to Dr. Trocha and his colleagues, “short-term pre-operative protein restriction and manipulation of H2S stand as novel, economical approaches to enhance vein graft durability and perhaps even lessen peri-operative complications, and we are in the early stages of testing this strategy in vascular surgery patients.”
Dr. Trocha’s research was supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant and Dr. Ozaki’s NIH and American Heart Association grants. The Resident Research Award is given to one individual each year as determined by the SVS Research and Education Committee with the intent of motivating physicians early in their training to pursue their interest in research that explores the biology of vascular disease and potential translational therapies. The recognition includes plenary presentation at the Vascular Annual Meeting, a $5,000 award and a 1-year complimentary subscription to the Journal of Vascular Surgery.
This year’s SVS Foundation’s Resident Research Award is being presented to Kaspar M. Trocha, MD, for his research on vein graft disease. As a member of the laboratories of C. Keith Ozaki, MD, and James Mitchell, MD, along with co-first author Peter Kip, MD, and microsurgeon Ming Tao, MD, the group studies the effects of food intake immediately before surgery on vascular adaptations.
Dietary restriction (reducing food intake without malnutrition) has been a topic of special interest in the science community for decades, and restricting calories in the long term has been shown to extend life and health span in a variety of species. It can also protect from overexuberant responses to trauma and ischemia-reperfusion.
Dr. Mitchell found that the benefits from long-term food restriction could be acquired as rapidly as in a few days, pointing to a potential for implementing dietary restriction recommendations prior to elective surgery.
The response to stress benefits seen following brief dietary interventions are mediated by the gaseous signaling molecule hydrogen sulfide (H2S). H2S is strongly upregulated by dietary restriction and this “rotten egg” gas seems to be protective for the cardiovascular system and has been demonstrated to be involved in vasodilation, inflammation, and atherogenesis, according to the researchers. The group’s experiments thus tested short-term protein restriction (a more feasible dietary approach for patients that observed to induce H2S) in a microsurgical mouse vein graft model.
The team discovered that cutting all protein from the animal diet for just 1 week before surgery led to increased levels of the enzyme cystathionine-gamma-lyase (CGL) that makes H2S, higher levels of the protective gaseous molecule, less early vein graft inflammation, and less eventual occlusive vein graft disease even though the animals were returned to their usual high-fat diet postop. The group also confirmed these results by constructing a new mouse strain that overexpresses CGL, and these mice were protected from vein graft disease. Conversely, blocking this enzyme negated all the beneficial effects of the dietary restriction.
According to Dr. Trocha and his colleagues, “short-term pre-operative protein restriction and manipulation of H2S stand as novel, economical approaches to enhance vein graft durability and perhaps even lessen peri-operative complications, and we are in the early stages of testing this strategy in vascular surgery patients.”
Dr. Trocha’s research was supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant and Dr. Ozaki’s NIH and American Heart Association grants. The Resident Research Award is given to one individual each year as determined by the SVS Research and Education Committee with the intent of motivating physicians early in their training to pursue their interest in research that explores the biology of vascular disease and potential translational therapies. The recognition includes plenary presentation at the Vascular Annual Meeting, a $5,000 award and a 1-year complimentary subscription to the Journal of Vascular Surgery.
Private Facebook chats show promise as depression, cannabis use intervention
SAN DIEGO – A pair of small studies suggest that cyber-based interventions with private online meeting areas – like those available on Facebook – could help lift depression and encourage less marijuana use in young people with cannabis use disorder.
“Even individuals who are not looking to quit their use of cannabis have some degree of ambivalence about the extent of their use of it,” said study researcher Suzette Glasner, PhD, in an interview. “When given tools to explore the consequences of their use and change if they want to, they do find reasons and effective ways to change or reduce their use – even if they don’t quit completely.”
Research shows that nearly one-third of cannabis users in 2012-2013 showed signs of cannabis use disorder (JAMA Psychiatry. 2015 Dec; 72[12]:1235-42).
For the first study, researchers recruited 26 participants who were diagnosed with major depressive disorder and cannabis use disorder (all had used marijuana on at least 40 of the previous 90 days). After a brief in-person intervention session, they received 9 therapy sessions via computer and brief weekly check-ins by clinicians over 10 weeks.
The average age of the participants was 29, and 54% were women. They reported that their average depression severity fell from 13 to 6 at 14-week follow-up, based on the Patient Health Questionnaire-9 scale. Cannabis use also declined.
For the separate Facebook study, an additional 18 participants received access to a private Facebook group that included daily posts reinforcing the program. The average age of this group was 27, and 56% were women.
The posts asked questions about topics such as “your fears about cutting back” and feelings that are “triggers” for cannabis use. One post directed users to details about mindfulness activities, and another asked users to describe on a scale of 1-10 how important it is for them to cut back.
Participants responded with praise for the private group, offering comments such as “the Facebook group is really helpful and offers a lot of support,” “it’s nice throughout the day to have uplifting Facebook notifications,” and “I like the tips, the positive feedback and the feeling that we’re in it together, not alone.”
Dr. Glasner said. “The Facebook approach enables those addicted to cannabis, for whom motivation for many things – including socializing – may be lacking, to utilize social support, without having to leave the comfort of their home or wherever they might isolate when they’re using cannabis heavily.”
She added that “this social support may be inspiring, motivating, and reassuring to them,” letting them know that they’re not alone in experiencing an addiction whose existence is sometimes doubted.
Dr. Glasner said she next plans a large randomized trial to evaluate the efficacy of the Facebook intervention and its mechanisms of action.
After her presentation at the meeting, Dr. Glasner was asked about the ongoing concerns regarding privacy on Facebook. “When I funded and started this study, it was nearly 2 years ago, and it wasn’t as large of a concern,” she said. “We may need to look to utilize a social media platform that may not be Facebook: ‘We have one to support you, but it’s not Facebook.’”
In a related presentation at the meeting, another researcher reported success using 80 interactive texts over 4 weeks to reach out to participants in an intervention programs targeting cannabis use disorder.
The National Institute on Drug Abuse funded the study. Dr. Glasner is author of The Addiction Recovery Skills Workbook: Changing Addictive Behaviors Using CBT, Mindfulness, and Motivational Interviewing Techniques (Oakland, Calif.: New Harbinger Publications, 2015). The authors reported no relevant disclosures.
SAN DIEGO – A pair of small studies suggest that cyber-based interventions with private online meeting areas – like those available on Facebook – could help lift depression and encourage less marijuana use in young people with cannabis use disorder.
“Even individuals who are not looking to quit their use of cannabis have some degree of ambivalence about the extent of their use of it,” said study researcher Suzette Glasner, PhD, in an interview. “When given tools to explore the consequences of their use and change if they want to, they do find reasons and effective ways to change or reduce their use – even if they don’t quit completely.”
Research shows that nearly one-third of cannabis users in 2012-2013 showed signs of cannabis use disorder (JAMA Psychiatry. 2015 Dec; 72[12]:1235-42).
For the first study, researchers recruited 26 participants who were diagnosed with major depressive disorder and cannabis use disorder (all had used marijuana on at least 40 of the previous 90 days). After a brief in-person intervention session, they received 9 therapy sessions via computer and brief weekly check-ins by clinicians over 10 weeks.
The average age of the participants was 29, and 54% were women. They reported that their average depression severity fell from 13 to 6 at 14-week follow-up, based on the Patient Health Questionnaire-9 scale. Cannabis use also declined.
For the separate Facebook study, an additional 18 participants received access to a private Facebook group that included daily posts reinforcing the program. The average age of this group was 27, and 56% were women.
The posts asked questions about topics such as “your fears about cutting back” and feelings that are “triggers” for cannabis use. One post directed users to details about mindfulness activities, and another asked users to describe on a scale of 1-10 how important it is for them to cut back.
Participants responded with praise for the private group, offering comments such as “the Facebook group is really helpful and offers a lot of support,” “it’s nice throughout the day to have uplifting Facebook notifications,” and “I like the tips, the positive feedback and the feeling that we’re in it together, not alone.”
Dr. Glasner said. “The Facebook approach enables those addicted to cannabis, for whom motivation for many things – including socializing – may be lacking, to utilize social support, without having to leave the comfort of their home or wherever they might isolate when they’re using cannabis heavily.”
She added that “this social support may be inspiring, motivating, and reassuring to them,” letting them know that they’re not alone in experiencing an addiction whose existence is sometimes doubted.
Dr. Glasner said she next plans a large randomized trial to evaluate the efficacy of the Facebook intervention and its mechanisms of action.
After her presentation at the meeting, Dr. Glasner was asked about the ongoing concerns regarding privacy on Facebook. “When I funded and started this study, it was nearly 2 years ago, and it wasn’t as large of a concern,” she said. “We may need to look to utilize a social media platform that may not be Facebook: ‘We have one to support you, but it’s not Facebook.’”
In a related presentation at the meeting, another researcher reported success using 80 interactive texts over 4 weeks to reach out to participants in an intervention programs targeting cannabis use disorder.
The National Institute on Drug Abuse funded the study. Dr. Glasner is author of The Addiction Recovery Skills Workbook: Changing Addictive Behaviors Using CBT, Mindfulness, and Motivational Interviewing Techniques (Oakland, Calif.: New Harbinger Publications, 2015). The authors reported no relevant disclosures.
SAN DIEGO – A pair of small studies suggest that cyber-based interventions with private online meeting areas – like those available on Facebook – could help lift depression and encourage less marijuana use in young people with cannabis use disorder.
“Even individuals who are not looking to quit their use of cannabis have some degree of ambivalence about the extent of their use of it,” said study researcher Suzette Glasner, PhD, in an interview. “When given tools to explore the consequences of their use and change if they want to, they do find reasons and effective ways to change or reduce their use – even if they don’t quit completely.”
Research shows that nearly one-third of cannabis users in 2012-2013 showed signs of cannabis use disorder (JAMA Psychiatry. 2015 Dec; 72[12]:1235-42).
For the first study, researchers recruited 26 participants who were diagnosed with major depressive disorder and cannabis use disorder (all had used marijuana on at least 40 of the previous 90 days). After a brief in-person intervention session, they received 9 therapy sessions via computer and brief weekly check-ins by clinicians over 10 weeks.
The average age of the participants was 29, and 54% were women. They reported that their average depression severity fell from 13 to 6 at 14-week follow-up, based on the Patient Health Questionnaire-9 scale. Cannabis use also declined.
For the separate Facebook study, an additional 18 participants received access to a private Facebook group that included daily posts reinforcing the program. The average age of this group was 27, and 56% were women.
The posts asked questions about topics such as “your fears about cutting back” and feelings that are “triggers” for cannabis use. One post directed users to details about mindfulness activities, and another asked users to describe on a scale of 1-10 how important it is for them to cut back.
Participants responded with praise for the private group, offering comments such as “the Facebook group is really helpful and offers a lot of support,” “it’s nice throughout the day to have uplifting Facebook notifications,” and “I like the tips, the positive feedback and the feeling that we’re in it together, not alone.”
Dr. Glasner said. “The Facebook approach enables those addicted to cannabis, for whom motivation for many things – including socializing – may be lacking, to utilize social support, without having to leave the comfort of their home or wherever they might isolate when they’re using cannabis heavily.”
She added that “this social support may be inspiring, motivating, and reassuring to them,” letting them know that they’re not alone in experiencing an addiction whose existence is sometimes doubted.
Dr. Glasner said she next plans a large randomized trial to evaluate the efficacy of the Facebook intervention and its mechanisms of action.
After her presentation at the meeting, Dr. Glasner was asked about the ongoing concerns regarding privacy on Facebook. “When I funded and started this study, it was nearly 2 years ago, and it wasn’t as large of a concern,” she said. “We may need to look to utilize a social media platform that may not be Facebook: ‘We have one to support you, but it’s not Facebook.’”
In a related presentation at the meeting, another researcher reported success using 80 interactive texts over 4 weeks to reach out to participants in an intervention programs targeting cannabis use disorder.
The National Institute on Drug Abuse funded the study. Dr. Glasner is author of The Addiction Recovery Skills Workbook: Changing Addictive Behaviors Using CBT, Mindfulness, and Motivational Interviewing Techniques (Oakland, Calif.: New Harbinger Publications, 2015). The authors reported no relevant disclosures.
REPORTING FROM CPDD 2018
Postmenopausal estrogen use down since 2006
according to a commercial database with prescription claims for more than 12 million women.
The prevalence of prescriptions for noncontraceptive oral estrogen dropped from 83 per 1,000 women in 2007 to 42 per 1,000 in 2015 for women aged 50 years and older, Joel L. Weissfeld, MD, MPH, of the Office of Surveillance and Epidemiology at the Center for Drug Evaluation and Research at the Food and Drug Administration, and his associates reported based on data from IQVIA Real World Data Adjudicated Claims – U.S. Database.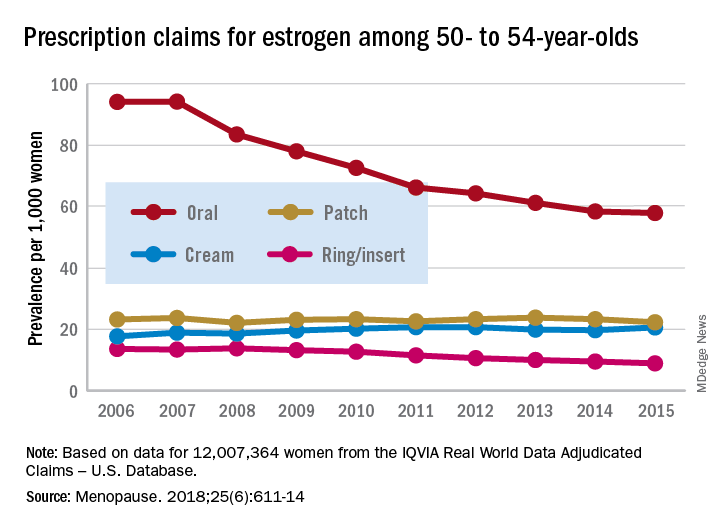
Prescriptions for vaginal forms (creams, rings, and inserts) rose from 27 per 1,000 women in 2006 to 42 per 1,000 in 2011 but declined to 35 per 1,000 by 2017, the investigators reported in Menopause.
For women aged 50-54 years, use of vaginal rings and inserts was steady for 2 years and then dropped every year after 2009, with prevalence lower in 2017 than in 2006. Vaginal ring/insert use increased for the first 2 years among those aged 55-59 years and for the first 5 years for those aged 60-64 years, but then each group started a fairly rapid and ongoing decline that had 2017 levels below those in 2006. Among women aged 65 years and older, however, prevalence of ring/insert use rose for most of the study period, and the declines left prevalences for 2015 above those for 2006, Dr. Weissfeld and his associates wrote.
The data source couldn’t provide reasons for use of vaginal rings or inserts, but the researchers noted that it’s possible that use for vasomotor symptoms “predominated in younger (aged 50-59 years) women closer in age to the onset of menopause. We presume that use for [vulvar and vaginal atrophy] predominated in women 60-65 years of age and older, women who are more distant in age from onset of menopause.”
Trends for use of transdermal patches also varied by age group but with smaller levels of difference. Use among women aged 50-54 years and 55-59 years fluctuated but showed no overall change. Those aged 60-64 years had a gradual decline over the study period, those aged 70-74 years had an initial increase in 2007 and then a decline, and those aged 75 years or older had an increase that lasted until 2011 before use started to fall, they wrote.
SOURCE: Weissfeld JL et al. Menopause. 2018;25(6):611-14.
according to a commercial database with prescription claims for more than 12 million women.
The prevalence of prescriptions for noncontraceptive oral estrogen dropped from 83 per 1,000 women in 2007 to 42 per 1,000 in 2015 for women aged 50 years and older, Joel L. Weissfeld, MD, MPH, of the Office of Surveillance and Epidemiology at the Center for Drug Evaluation and Research at the Food and Drug Administration, and his associates reported based on data from IQVIA Real World Data Adjudicated Claims – U.S. Database.
Prescriptions for vaginal forms (creams, rings, and inserts) rose from 27 per 1,000 women in 2006 to 42 per 1,000 in 2011 but declined to 35 per 1,000 by 2017, the investigators reported in Menopause.
For women aged 50-54 years, use of vaginal rings and inserts was steady for 2 years and then dropped every year after 2009, with prevalence lower in 2017 than in 2006. Vaginal ring/insert use increased for the first 2 years among those aged 55-59 years and for the first 5 years for those aged 60-64 years, but then each group started a fairly rapid and ongoing decline that had 2017 levels below those in 2006. Among women aged 65 years and older, however, prevalence of ring/insert use rose for most of the study period, and the declines left prevalences for 2015 above those for 2006, Dr. Weissfeld and his associates wrote.
The data source couldn’t provide reasons for use of vaginal rings or inserts, but the researchers noted that it’s possible that use for vasomotor symptoms “predominated in younger (aged 50-59 years) women closer in age to the onset of menopause. We presume that use for [vulvar and vaginal atrophy] predominated in women 60-65 years of age and older, women who are more distant in age from onset of menopause.”
Trends for use of transdermal patches also varied by age group but with smaller levels of difference. Use among women aged 50-54 years and 55-59 years fluctuated but showed no overall change. Those aged 60-64 years had a gradual decline over the study period, those aged 70-74 years had an initial increase in 2007 and then a decline, and those aged 75 years or older had an increase that lasted until 2011 before use started to fall, they wrote.
SOURCE: Weissfeld JL et al. Menopause. 2018;25(6):611-14.
according to a commercial database with prescription claims for more than 12 million women.
The prevalence of prescriptions for noncontraceptive oral estrogen dropped from 83 per 1,000 women in 2007 to 42 per 1,000 in 2015 for women aged 50 years and older, Joel L. Weissfeld, MD, MPH, of the Office of Surveillance and Epidemiology at the Center for Drug Evaluation and Research at the Food and Drug Administration, and his associates reported based on data from IQVIA Real World Data Adjudicated Claims – U.S. Database.
Prescriptions for vaginal forms (creams, rings, and inserts) rose from 27 per 1,000 women in 2006 to 42 per 1,000 in 2011 but declined to 35 per 1,000 by 2017, the investigators reported in Menopause.
For women aged 50-54 years, use of vaginal rings and inserts was steady for 2 years and then dropped every year after 2009, with prevalence lower in 2017 than in 2006. Vaginal ring/insert use increased for the first 2 years among those aged 55-59 years and for the first 5 years for those aged 60-64 years, but then each group started a fairly rapid and ongoing decline that had 2017 levels below those in 2006. Among women aged 65 years and older, however, prevalence of ring/insert use rose for most of the study period, and the declines left prevalences for 2015 above those for 2006, Dr. Weissfeld and his associates wrote.
The data source couldn’t provide reasons for use of vaginal rings or inserts, but the researchers noted that it’s possible that use for vasomotor symptoms “predominated in younger (aged 50-59 years) women closer in age to the onset of menopause. We presume that use for [vulvar and vaginal atrophy] predominated in women 60-65 years of age and older, women who are more distant in age from onset of menopause.”
Trends for use of transdermal patches also varied by age group but with smaller levels of difference. Use among women aged 50-54 years and 55-59 years fluctuated but showed no overall change. Those aged 60-64 years had a gradual decline over the study period, those aged 70-74 years had an initial increase in 2007 and then a decline, and those aged 75 years or older had an increase that lasted until 2011 before use started to fall, they wrote.
SOURCE: Weissfeld JL et al. Menopause. 2018;25(6):611-14.
FROM MENOPAUSE
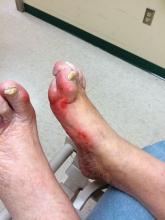
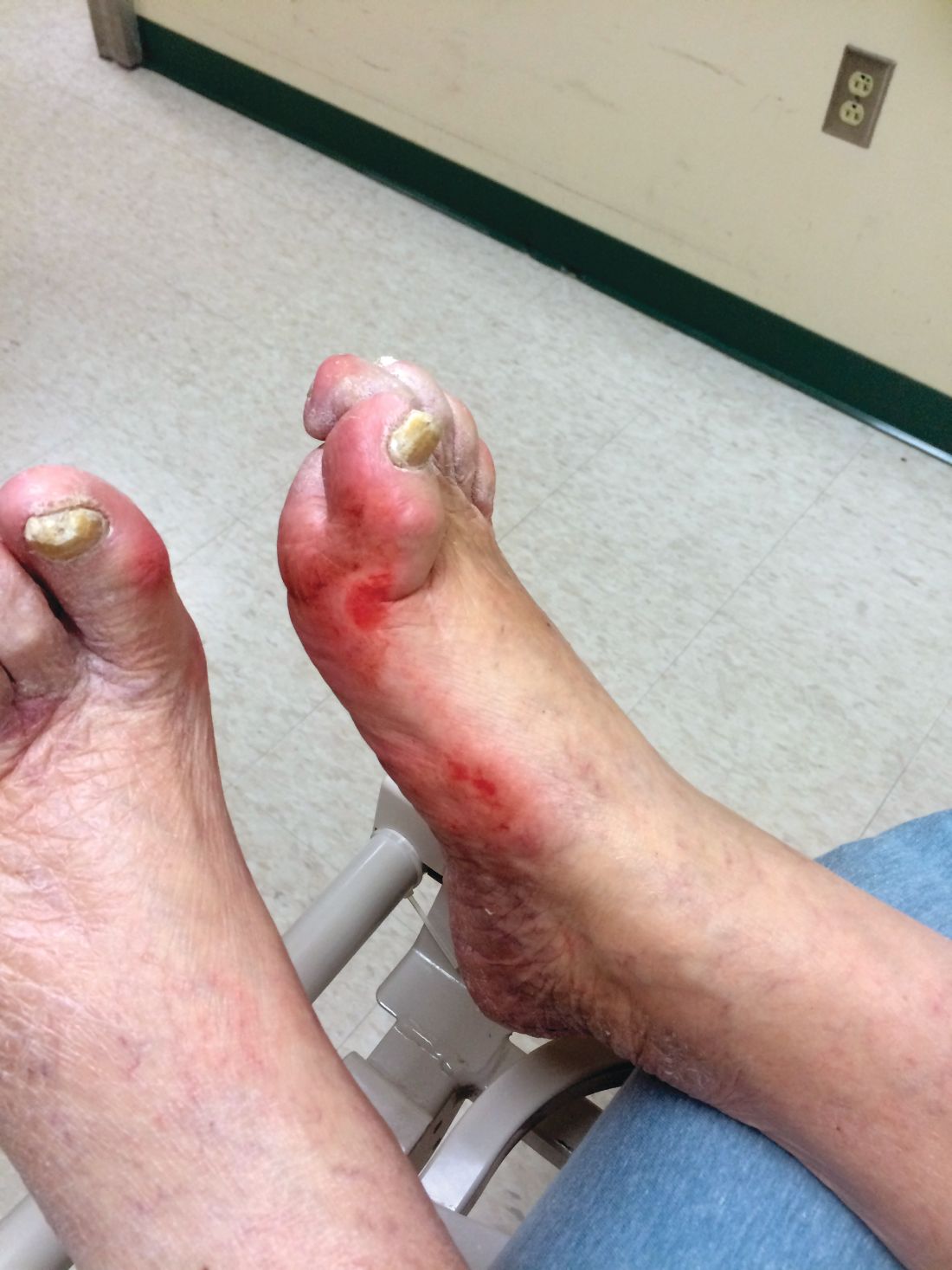
Diabetic foot ulcer healing is predictable by WIfI stage scores
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: The Wound, Ischemia, and foot Infection (WIfI) classification of diabetic foot ulcers provides a predictable primary outcome for wound healing at 1 year.
Major finding: Wound healing probability at 1 year was 94.1% for WIfI stage 1 wounds and 67.4% for stage 4 wounds.
Study details: A single-location, multidisciplinary-setting, retrospective study of 709 WIfI stage 1-4 wounds presented by 310 diabetic foot ulcer patients.
Disclosures: The authors reported no conflicts of interest.
Source: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Are we using the right metrics to measure cesarean rates?
St. Joseph Hospital in Orange, California, like most institutions performing deliveries in 2016, started releasing metrics internally before subsequently releasing them to the public. Data for the first 9 months of 2016 were released. As I am often an outlier, I was gratified to see that I ranked 1st in the vaginal birth after cesarean delivery (VBAC) rate at 36.8% and 4th at 15.9% for my cesarean delivery (CD) rate in the low-risk nulliparous term singleton vertex (NTSV) population.
I have been an avid proponent of VBAC since 1984 when one of the fathers of modern obstetric care, Edward J. Quilligan, MD, presented the benefits and safety of VBAC at our institution.
Experiences that may alter a reported rate
I list here a few circumstances of a CD on maternal request:
- A primagravida with a 10-cm nonphysiologic, nonmalignant ovarian cyst at term elects a primary CD with ovarian cystectomy.
- A woman who is concerned about pelvic organ prolapse and urinary incontinence later in life requests a CD. After all, normal babies do not weigh 5 and 6 lb anymore.
- An elderly primagravida with an in vitro fertilization pregnancy requests a CD.
Should these experiences adversely affect a physician’s statistics? Personally, I don’t think so. Is the morbidity and mortality from a CD really all that much higher than a normal spontaneous vaginal delivery (NSVD)? Granted, the cost is more. But are we really helping all our patients by insisting on a NSVD? Thousands of people have medically indicated and elective surgery in the United States each day.
Of course, these data points depend on the denominator (the number of deliveries attributed to each ObGyn). Those with a contradictory opinion will say that this evens out over time. I dispute that claim. This might be closer to being true for the ObGyn with the highest number, say, 134 in the NTSV denominator versus someone with a low number, such as 4. For VBAC, the denominator range at our institution was 1 to 115 cases.
Rethinking my position
Two recent cases have caused me to rethink my position on using VBAC and CD rates to evaluate ObGyns.
Uterine rupture
A 31-year-old G3P1 woman at 39 6/7 weeks’ gestation was admitted in early labor for a VBAC. She had undergone a CD with her first baby because of fetal intolerance to labor. Her prenatal course was complicated by white-coat hypertension, but I monitored her blood pressure at home and it had been normal. She took aspirin 81 mg during the pregnancy. The fetus was not reactive to a nonstress test on the day of admission.
That evening, amniotomy results showed clear fluid. I placed an intrauterine pressure catheter. The patient’s labor progressed well during the night, she received an epidural anesthetic, and labor was augmented with intravenous oxytocin. She progressed to complete dilation. I was notified of severe, prolonged, variable fetal heart-rate decelerations.
The Laborist who evaluated the patient recommended an emergency CD. I came immediately to Labor and Delivery and performed a CD with delivery of a 7 lb 4 oz infant whose Apgars were 2, 5, and 8 at 1, 5, and 10 minutes, respectively. Arterial cord blood gas tests revealed: pH, 6.94; pCO2, 95 mm Hg; pO2, 19.9 mm Hg; HCO3, 19.9 mmol/L; and base excess (BE), –14.4 mmol/L. Venous cord blood gas tests revealed: pH, 7.25; pCO2, 45 mm Hg; pO2, 35 mm Hg; HCO3, 19.2 mmol/L; BE, −8.0 mmol/L. The cord blood gases revealed that the baby was becoming compromised, but was delivered in time to avoid complications.
After advocating and performing many successful VBACs for 33 years, this was my first uterine rupture.
The uterus had ruptured in the lower segment from the mid-portion extending inferolaterally on the right side and was hemorrhaging. I successfully repaired the rupture. Maternal quantitative blood loss was 1,020 mL.
The baby initially was apneic and was limp. He required continuous positive airway pressure (CPAP) and positive pressure ventilation in the operating room. The baby was transferred to the neonatal intensive care unit (NICU), recovered well, and was discharged home with the mother on the 4th day of life.
Commentary: Why should this necessary, emergency CD count against me on my core measure rate? Although I have advocated for VBACs for 33 years, perhaps they aren’t so safe. After this experience, I do not ever want to have to deal with a ruptured uterus, a compromised baby, and maternal hemorrhage again.
Read Dr. Kanofsky’s solution to using this metric.
Depressed baby
A 24-year-old G1P0 woman at 39 weeks’ gestation was admitted for induction of labor because of mild pregnancy-induced hypertension. Her prenatal course was complicated by Class A1 gestational diabetes mellitus, which was untreated due to compliance issues, Group B streptococcus, and cholelithiasis. Clinically, I suspected she was going to have a large (9 lb) baby. An ultrasound to estimate fetal weight at 37 2/7 weeks’ gestation showed the fetus at 3.937 kg. I was concerned, but, because the mother was 5 ft 5 in tall and weighed 282 lbs, I thought it was reasonable for her to attempt a NSVD.
Induction and labor progressed normally. Her labor curve decelerated at an anterior lip, but subsequently stage 2 progressed normally and lasted 2 hrs. Her temperature was elevated in stage 2 to 100.00F. The fetal heart rate tracings were reassuring.
Immediately after delivery of the fetal vertex, a turtleneck sign was seen and shoulder dystocia occurred. A Wood’s maneuver was performed in both directions, the nurse applied suprapubic pressure, and the infant was delivered. A loose nuchal cord x2 was reduced. The infant was apneic and had no tone. She was taken to the warmer, given oxygen, suctioned, and stimulated until the NICU team arrived. Her Apgar scores were 2, 5, and 9 at 1, 5, and 10 minutes, respectively. The birthweight was 9 lb 0 oz.
A depressed baby of this magnitude was certainly not expected from the FHR tracing or the shoulder dystocia. Venous cord gas evaluation revealed pH, 7.16; pCO2, 57 mm Hg; pO2, 17 mm Hg; HCO3, 20.2 mmol/L; and BE, –19.1 mmol/L.
The baby recovered quickly in the labor and delivery recovery room, went to the NICU on CPAP, subsequently transitioned to room air, and was discharged on the 4th day of life with her mother.
Commentary: Did I do the best I could for this mother and baby? In hindsight, I should have performed a CD because of my concerns for a large fetus. The “retrospectoscope” always makes cases more clear! Note that, if I had performed an elective CD for fetal macrosomia, it would have counted against me on this metric. Prior to labor, if I thought an elective CD was the right approach to this patient, and was providing the best care I could for this mother and fetus, why should it count against me?
Is there a solution?
With my newfound concerns, it is my opinion that VBAC and CD/NTSV rates may not be the correct things to use as quality metric measures without some additional qualifying information.
Better metrics of quality and safety that might be more helpful to measure include:
- Prophylactic oxytocin after delivery of the baby’s anterior shoulder
- Since “6 is the new 4,” in order to increase the NTSV rate, we could measure1:
- patients admitted before active labor
- patients receiving an epidural before active labor.
- Since NTSV is a goal, measure the number of patients in an advanced stage of labor whose labor pattern has become dysfunctional, no interventions are taken, and who subsequently deliver by primary CD.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 687: Approaches to limit intervention during labor and birth. Obstet Gynecol. 2017;129(2):e20–e28.
St. Joseph Hospital in Orange, California, like most institutions performing deliveries in 2016, started releasing metrics internally before subsequently releasing them to the public. Data for the first 9 months of 2016 were released. As I am often an outlier, I was gratified to see that I ranked 1st in the vaginal birth after cesarean delivery (VBAC) rate at 36.8% and 4th at 15.9% for my cesarean delivery (CD) rate in the low-risk nulliparous term singleton vertex (NTSV) population.
I have been an avid proponent of VBAC since 1984 when one of the fathers of modern obstetric care, Edward J. Quilligan, MD, presented the benefits and safety of VBAC at our institution.
Experiences that may alter a reported rate
I list here a few circumstances of a CD on maternal request:
- A primagravida with a 10-cm nonphysiologic, nonmalignant ovarian cyst at term elects a primary CD with ovarian cystectomy.
- A woman who is concerned about pelvic organ prolapse and urinary incontinence later in life requests a CD. After all, normal babies do not weigh 5 and 6 lb anymore.
- An elderly primagravida with an in vitro fertilization pregnancy requests a CD.
Should these experiences adversely affect a physician’s statistics? Personally, I don’t think so. Is the morbidity and mortality from a CD really all that much higher than a normal spontaneous vaginal delivery (NSVD)? Granted, the cost is more. But are we really helping all our patients by insisting on a NSVD? Thousands of people have medically indicated and elective surgery in the United States each day.
Of course, these data points depend on the denominator (the number of deliveries attributed to each ObGyn). Those with a contradictory opinion will say that this evens out over time. I dispute that claim. This might be closer to being true for the ObGyn with the highest number, say, 134 in the NTSV denominator versus someone with a low number, such as 4. For VBAC, the denominator range at our institution was 1 to 115 cases.
Rethinking my position
Two recent cases have caused me to rethink my position on using VBAC and CD rates to evaluate ObGyns.
Uterine rupture
A 31-year-old G3P1 woman at 39 6/7 weeks’ gestation was admitted in early labor for a VBAC. She had undergone a CD with her first baby because of fetal intolerance to labor. Her prenatal course was complicated by white-coat hypertension, but I monitored her blood pressure at home and it had been normal. She took aspirin 81 mg during the pregnancy. The fetus was not reactive to a nonstress test on the day of admission.
That evening, amniotomy results showed clear fluid. I placed an intrauterine pressure catheter. The patient’s labor progressed well during the night, she received an epidural anesthetic, and labor was augmented with intravenous oxytocin. She progressed to complete dilation. I was notified of severe, prolonged, variable fetal heart-rate decelerations.
The Laborist who evaluated the patient recommended an emergency CD. I came immediately to Labor and Delivery and performed a CD with delivery of a 7 lb 4 oz infant whose Apgars were 2, 5, and 8 at 1, 5, and 10 minutes, respectively. Arterial cord blood gas tests revealed: pH, 6.94; pCO2, 95 mm Hg; pO2, 19.9 mm Hg; HCO3, 19.9 mmol/L; and base excess (BE), –14.4 mmol/L. Venous cord blood gas tests revealed: pH, 7.25; pCO2, 45 mm Hg; pO2, 35 mm Hg; HCO3, 19.2 mmol/L; BE, −8.0 mmol/L. The cord blood gases revealed that the baby was becoming compromised, but was delivered in time to avoid complications.
After advocating and performing many successful VBACs for 33 years, this was my first uterine rupture.
The uterus had ruptured in the lower segment from the mid-portion extending inferolaterally on the right side and was hemorrhaging. I successfully repaired the rupture. Maternal quantitative blood loss was 1,020 mL.
The baby initially was apneic and was limp. He required continuous positive airway pressure (CPAP) and positive pressure ventilation in the operating room. The baby was transferred to the neonatal intensive care unit (NICU), recovered well, and was discharged home with the mother on the 4th day of life.
Commentary: Why should this necessary, emergency CD count against me on my core measure rate? Although I have advocated for VBACs for 33 years, perhaps they aren’t so safe. After this experience, I do not ever want to have to deal with a ruptured uterus, a compromised baby, and maternal hemorrhage again.
Read Dr. Kanofsky’s solution to using this metric.
Depressed baby
A 24-year-old G1P0 woman at 39 weeks’ gestation was admitted for induction of labor because of mild pregnancy-induced hypertension. Her prenatal course was complicated by Class A1 gestational diabetes mellitus, which was untreated due to compliance issues, Group B streptococcus, and cholelithiasis. Clinically, I suspected she was going to have a large (9 lb) baby. An ultrasound to estimate fetal weight at 37 2/7 weeks’ gestation showed the fetus at 3.937 kg. I was concerned, but, because the mother was 5 ft 5 in tall and weighed 282 lbs, I thought it was reasonable for her to attempt a NSVD.
Induction and labor progressed normally. Her labor curve decelerated at an anterior lip, but subsequently stage 2 progressed normally and lasted 2 hrs. Her temperature was elevated in stage 2 to 100.00F. The fetal heart rate tracings were reassuring.
Immediately after delivery of the fetal vertex, a turtleneck sign was seen and shoulder dystocia occurred. A Wood’s maneuver was performed in both directions, the nurse applied suprapubic pressure, and the infant was delivered. A loose nuchal cord x2 was reduced. The infant was apneic and had no tone. She was taken to the warmer, given oxygen, suctioned, and stimulated until the NICU team arrived. Her Apgar scores were 2, 5, and 9 at 1, 5, and 10 minutes, respectively. The birthweight was 9 lb 0 oz.
A depressed baby of this magnitude was certainly not expected from the FHR tracing or the shoulder dystocia. Venous cord gas evaluation revealed pH, 7.16; pCO2, 57 mm Hg; pO2, 17 mm Hg; HCO3, 20.2 mmol/L; and BE, –19.1 mmol/L.
The baby recovered quickly in the labor and delivery recovery room, went to the NICU on CPAP, subsequently transitioned to room air, and was discharged on the 4th day of life with her mother.
Commentary: Did I do the best I could for this mother and baby? In hindsight, I should have performed a CD because of my concerns for a large fetus. The “retrospectoscope” always makes cases more clear! Note that, if I had performed an elective CD for fetal macrosomia, it would have counted against me on this metric. Prior to labor, if I thought an elective CD was the right approach to this patient, and was providing the best care I could for this mother and fetus, why should it count against me?
Is there a solution?
With my newfound concerns, it is my opinion that VBAC and CD/NTSV rates may not be the correct things to use as quality metric measures without some additional qualifying information.
Better metrics of quality and safety that might be more helpful to measure include:
- Prophylactic oxytocin after delivery of the baby’s anterior shoulder
- Since “6 is the new 4,” in order to increase the NTSV rate, we could measure1:
- patients admitted before active labor
- patients receiving an epidural before active labor.
- Since NTSV is a goal, measure the number of patients in an advanced stage of labor whose labor pattern has become dysfunctional, no interventions are taken, and who subsequently deliver by primary CD.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
St. Joseph Hospital in Orange, California, like most institutions performing deliveries in 2016, started releasing metrics internally before subsequently releasing them to the public. Data for the first 9 months of 2016 were released. As I am often an outlier, I was gratified to see that I ranked 1st in the vaginal birth after cesarean delivery (VBAC) rate at 36.8% and 4th at 15.9% for my cesarean delivery (CD) rate in the low-risk nulliparous term singleton vertex (NTSV) population.
I have been an avid proponent of VBAC since 1984 when one of the fathers of modern obstetric care, Edward J. Quilligan, MD, presented the benefits and safety of VBAC at our institution.
Experiences that may alter a reported rate
I list here a few circumstances of a CD on maternal request:
- A primagravida with a 10-cm nonphysiologic, nonmalignant ovarian cyst at term elects a primary CD with ovarian cystectomy.
- A woman who is concerned about pelvic organ prolapse and urinary incontinence later in life requests a CD. After all, normal babies do not weigh 5 and 6 lb anymore.
- An elderly primagravida with an in vitro fertilization pregnancy requests a CD.
Should these experiences adversely affect a physician’s statistics? Personally, I don’t think so. Is the morbidity and mortality from a CD really all that much higher than a normal spontaneous vaginal delivery (NSVD)? Granted, the cost is more. But are we really helping all our patients by insisting on a NSVD? Thousands of people have medically indicated and elective surgery in the United States each day.
Of course, these data points depend on the denominator (the number of deliveries attributed to each ObGyn). Those with a contradictory opinion will say that this evens out over time. I dispute that claim. This might be closer to being true for the ObGyn with the highest number, say, 134 in the NTSV denominator versus someone with a low number, such as 4. For VBAC, the denominator range at our institution was 1 to 115 cases.
Rethinking my position
Two recent cases have caused me to rethink my position on using VBAC and CD rates to evaluate ObGyns.
Uterine rupture
A 31-year-old G3P1 woman at 39 6/7 weeks’ gestation was admitted in early labor for a VBAC. She had undergone a CD with her first baby because of fetal intolerance to labor. Her prenatal course was complicated by white-coat hypertension, but I monitored her blood pressure at home and it had been normal. She took aspirin 81 mg during the pregnancy. The fetus was not reactive to a nonstress test on the day of admission.
That evening, amniotomy results showed clear fluid. I placed an intrauterine pressure catheter. The patient’s labor progressed well during the night, she received an epidural anesthetic, and labor was augmented with intravenous oxytocin. She progressed to complete dilation. I was notified of severe, prolonged, variable fetal heart-rate decelerations.
The Laborist who evaluated the patient recommended an emergency CD. I came immediately to Labor and Delivery and performed a CD with delivery of a 7 lb 4 oz infant whose Apgars were 2, 5, and 8 at 1, 5, and 10 minutes, respectively. Arterial cord blood gas tests revealed: pH, 6.94; pCO2, 95 mm Hg; pO2, 19.9 mm Hg; HCO3, 19.9 mmol/L; and base excess (BE), –14.4 mmol/L. Venous cord blood gas tests revealed: pH, 7.25; pCO2, 45 mm Hg; pO2, 35 mm Hg; HCO3, 19.2 mmol/L; BE, −8.0 mmol/L. The cord blood gases revealed that the baby was becoming compromised, but was delivered in time to avoid complications.
After advocating and performing many successful VBACs for 33 years, this was my first uterine rupture.
The uterus had ruptured in the lower segment from the mid-portion extending inferolaterally on the right side and was hemorrhaging. I successfully repaired the rupture. Maternal quantitative blood loss was 1,020 mL.
The baby initially was apneic and was limp. He required continuous positive airway pressure (CPAP) and positive pressure ventilation in the operating room. The baby was transferred to the neonatal intensive care unit (NICU), recovered well, and was discharged home with the mother on the 4th day of life.
Commentary: Why should this necessary, emergency CD count against me on my core measure rate? Although I have advocated for VBACs for 33 years, perhaps they aren’t so safe. After this experience, I do not ever want to have to deal with a ruptured uterus, a compromised baby, and maternal hemorrhage again.
Read Dr. Kanofsky’s solution to using this metric.
Depressed baby
A 24-year-old G1P0 woman at 39 weeks’ gestation was admitted for induction of labor because of mild pregnancy-induced hypertension. Her prenatal course was complicated by Class A1 gestational diabetes mellitus, which was untreated due to compliance issues, Group B streptococcus, and cholelithiasis. Clinically, I suspected she was going to have a large (9 lb) baby. An ultrasound to estimate fetal weight at 37 2/7 weeks’ gestation showed the fetus at 3.937 kg. I was concerned, but, because the mother was 5 ft 5 in tall and weighed 282 lbs, I thought it was reasonable for her to attempt a NSVD.
Induction and labor progressed normally. Her labor curve decelerated at an anterior lip, but subsequently stage 2 progressed normally and lasted 2 hrs. Her temperature was elevated in stage 2 to 100.00F. The fetal heart rate tracings were reassuring.
Immediately after delivery of the fetal vertex, a turtleneck sign was seen and shoulder dystocia occurred. A Wood’s maneuver was performed in both directions, the nurse applied suprapubic pressure, and the infant was delivered. A loose nuchal cord x2 was reduced. The infant was apneic and had no tone. She was taken to the warmer, given oxygen, suctioned, and stimulated until the NICU team arrived. Her Apgar scores were 2, 5, and 9 at 1, 5, and 10 minutes, respectively. The birthweight was 9 lb 0 oz.
A depressed baby of this magnitude was certainly not expected from the FHR tracing or the shoulder dystocia. Venous cord gas evaluation revealed pH, 7.16; pCO2, 57 mm Hg; pO2, 17 mm Hg; HCO3, 20.2 mmol/L; and BE, –19.1 mmol/L.
The baby recovered quickly in the labor and delivery recovery room, went to the NICU on CPAP, subsequently transitioned to room air, and was discharged on the 4th day of life with her mother.
Commentary: Did I do the best I could for this mother and baby? In hindsight, I should have performed a CD because of my concerns for a large fetus. The “retrospectoscope” always makes cases more clear! Note that, if I had performed an elective CD for fetal macrosomia, it would have counted against me on this metric. Prior to labor, if I thought an elective CD was the right approach to this patient, and was providing the best care I could for this mother and fetus, why should it count against me?
Is there a solution?
With my newfound concerns, it is my opinion that VBAC and CD/NTSV rates may not be the correct things to use as quality metric measures without some additional qualifying information.
Better metrics of quality and safety that might be more helpful to measure include:
- Prophylactic oxytocin after delivery of the baby’s anterior shoulder
- Since “6 is the new 4,” in order to increase the NTSV rate, we could measure1:
- patients admitted before active labor
- patients receiving an epidural before active labor.
- Since NTSV is a goal, measure the number of patients in an advanced stage of labor whose labor pattern has become dysfunctional, no interventions are taken, and who subsequently deliver by primary CD.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 687: Approaches to limit intervention during labor and birth. Obstet Gynecol. 2017;129(2):e20–e28.
- Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 687: Approaches to limit intervention during labor and birth. Obstet Gynecol. 2017;129(2):e20–e28.