User login
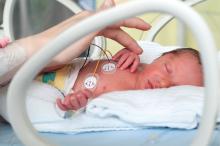
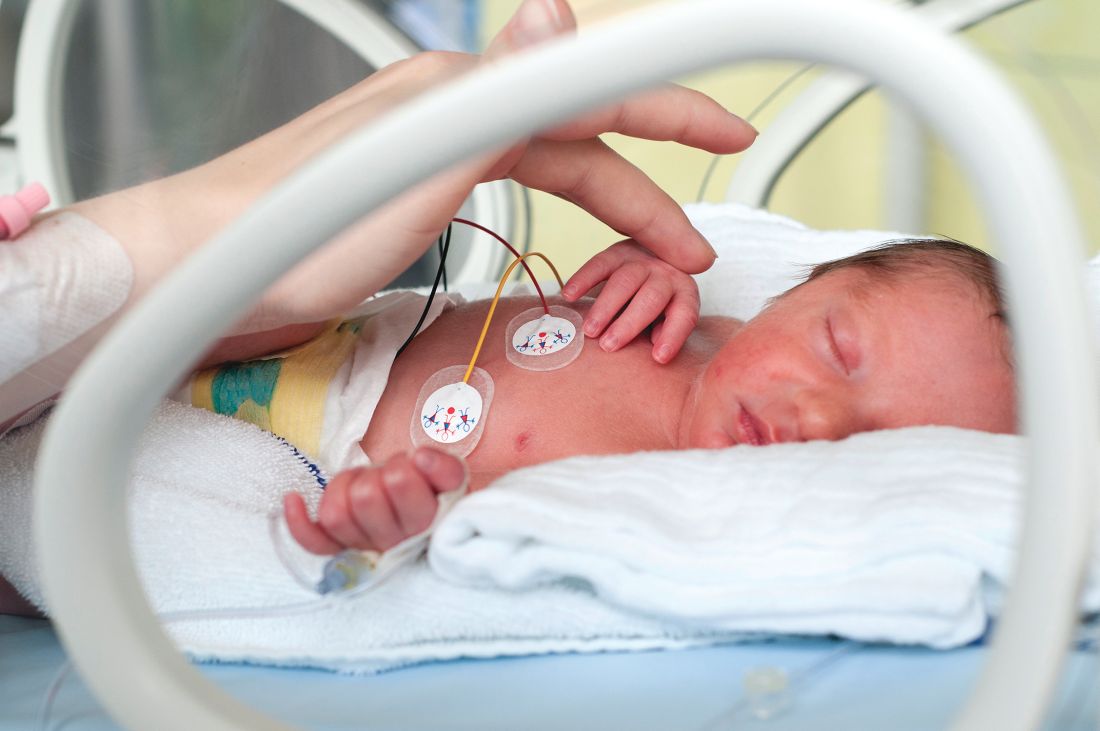
App found to improve quality of life for families of premature infants
TORONTO – Significant improvement in quality of life was observed in neonatal ICU families using the PreeMe+You app, preliminary results from a two-center study showed.
“NICU time is stressful,” one of the study authors, Abigail Whitney, said at the Pediatric Academic Societies annual meeting. “With the birth of a preterm infant, parents are often quickly transitioned into the role of becoming a parent much sooner and in much different circumstances than they might have anticipated. Parents have reported feelings of isolation, alienation, and insecurity in the parental role while in the NICU. Studies have shown that interventions that engage parents in their infant’s progress can decrease parental stress and anxiety, increase positive parent-infant interaction, and even reduce the infant’s length of stay. Also, with advancing technology there has been a push to find ways to use mobile technology to help parents balance engaging with their infant with the rest of their busy lives.”
In a study overseen by PreeMe+You’s chief medical expert, Bree Andrews, MD, MPH, Ms. Whitney and her associates administered the app to 48 families at either the University of Chicago Medicine Comer Children’s Hospital NICU or the Evanston Hospital NICU to assess readiness for using mobile technologies at the bedside. All families were recommended by a child life specialist who identified families who might be interested in using something like PreeMe+You. They excluded any families that were currently involved with child and family services, those with an infant younger than 7 days old, those whose child required escalation of care or upcoming surgeries, and those whose infant was over 37 weeks’ gestation.
First, the researchers briefed NICU staff about the study at charge nurse meetings, faculty meetings, and daily huddles for 2 weeks before first enrollment. “We did this knowing that parents might go to their nurses or doctors about how to answer specific questions within the app, or maybe want to learn more about a certain topic they learned from PreeMe+You,” Ms. Whitney said.
Data measurements included the PreeMe+You composite survey, which pulled questions from the Fragile Infant Parent Readiness Evaluation (FIPRE) and the NICU Parent Risk Evaluation and Engagement Model and Instrument (PREEMI). “We also included additional questions about technology use and capacity, as well as the PedsQL [Pediatric Quality of Life Inventory] Family Impact Module to assess parental quality of life throughout the study,” she said.
Over a period of 9 months, the researchers collected 153 quality of life measurements from 48 families. Of these, 48 occurred at enrollment, 23 occurred less than 1 week after enrollment, 30 occurred 1-2 weeks after enrollment, 28 occurred 3-4 weeks after enrollment, and 24 occurred 4 weeks or more after enrollment. By study closure, the researchers had follow-up data on 44 of the 48 families. The average gestational age at birth was 29.3 weeks, the average day of life at enrollment was 25.4, and the average birth weight was 1,280 grams.
On the app’s composite survey, 14.6% “agreed” and 79.2% “strongly agreed” that they were currently using a smart phone or tablet to look for information about preemies/NICU on the Internet, and about half “agreed” or “strongly agreed” (27.1% and 33.3%, respectively) that they spent more than 30 minutes per week looking up information about their NICU baby online. Nearly all families “agreed” or “strongly agreed” (14.6% and 85.4%) that they had a smart phone or tablet for Internet use in the NICU, and nearly all “agreed” or “strongly agreed” (33.3% and 62.5%) that having an app at the NICU bedside/home would be helpful. “This showed us that families were ready to use technology and interested in something like PreeMe+You at the bedside,” Ms. Whitney said.
At the time of study enrollment, 12 were in the purple stage, 8 were in the blue stage, 19 infants were in the orange stage, and 9 were in the yellow stage. Ms. Whitney reported that based on the PedsQL Family Impact Module, 35 of the 44 families showed increased quality of life functionality after participating in the study. This change was significant, with a P value of .001. Improvements were seen in the measure’s eight domains (physical, emotional, social, cognitive, communication, worry, daily activities, and family relationship functionality). “We saw increases across all of the domains based on how long the parents had been using the app,” Ms. Whitney said. “We found the biggest increase in quality of life in families of babies born less than 25 weeks’ gestational age, those born 25-26 weeks gestational age, those born 27-28 weeks gestational age, and those born 33-37 weeks gestational age. We are encouraged to see some of these quality of life changes in some of the earliest-born gestation babies because these are presumably the families that would have the longest time to go in the NICU and could benefit the most from using an app like PreeMe+You.”
She acknowledged certain limitations of the study, including the fact that it was conducted in two NICUs, “and we definitely need more comparisons to look at the natural trajectory of quality of life changes while families are in the NICU. Also, all of the families enrolled in our study had access to a research team that checked in with them weekly. In the real world, PreeMe+You would probably be self-guided.” Going forward, PreeMe+You plans to include additional features to give parents more self-guidance, making it easier for them to interact and partner with their baby’s medical team.
Funding for the study was provided by the Bucksbaum Institute for Clinical Excellence. Ms. Whitney was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
TORONTO – Significant improvement in quality of life was observed in neonatal ICU families using the PreeMe+You app, preliminary results from a two-center study showed.
“NICU time is stressful,” one of the study authors, Abigail Whitney, said at the Pediatric Academic Societies annual meeting. “With the birth of a preterm infant, parents are often quickly transitioned into the role of becoming a parent much sooner and in much different circumstances than they might have anticipated. Parents have reported feelings of isolation, alienation, and insecurity in the parental role while in the NICU. Studies have shown that interventions that engage parents in their infant’s progress can decrease parental stress and anxiety, increase positive parent-infant interaction, and even reduce the infant’s length of stay. Also, with advancing technology there has been a push to find ways to use mobile technology to help parents balance engaging with their infant with the rest of their busy lives.”
In a study overseen by PreeMe+You’s chief medical expert, Bree Andrews, MD, MPH, Ms. Whitney and her associates administered the app to 48 families at either the University of Chicago Medicine Comer Children’s Hospital NICU or the Evanston Hospital NICU to assess readiness for using mobile technologies at the bedside. All families were recommended by a child life specialist who identified families who might be interested in using something like PreeMe+You. They excluded any families that were currently involved with child and family services, those with an infant younger than 7 days old, those whose child required escalation of care or upcoming surgeries, and those whose infant was over 37 weeks’ gestation.
First, the researchers briefed NICU staff about the study at charge nurse meetings, faculty meetings, and daily huddles for 2 weeks before first enrollment. “We did this knowing that parents might go to their nurses or doctors about how to answer specific questions within the app, or maybe want to learn more about a certain topic they learned from PreeMe+You,” Ms. Whitney said.
Data measurements included the PreeMe+You composite survey, which pulled questions from the Fragile Infant Parent Readiness Evaluation (FIPRE) and the NICU Parent Risk Evaluation and Engagement Model and Instrument (PREEMI). “We also included additional questions about technology use and capacity, as well as the PedsQL [Pediatric Quality of Life Inventory] Family Impact Module to assess parental quality of life throughout the study,” she said.
Over a period of 9 months, the researchers collected 153 quality of life measurements from 48 families. Of these, 48 occurred at enrollment, 23 occurred less than 1 week after enrollment, 30 occurred 1-2 weeks after enrollment, 28 occurred 3-4 weeks after enrollment, and 24 occurred 4 weeks or more after enrollment. By study closure, the researchers had follow-up data on 44 of the 48 families. The average gestational age at birth was 29.3 weeks, the average day of life at enrollment was 25.4, and the average birth weight was 1,280 grams.
On the app’s composite survey, 14.6% “agreed” and 79.2% “strongly agreed” that they were currently using a smart phone or tablet to look for information about preemies/NICU on the Internet, and about half “agreed” or “strongly agreed” (27.1% and 33.3%, respectively) that they spent more than 30 minutes per week looking up information about their NICU baby online. Nearly all families “agreed” or “strongly agreed” (14.6% and 85.4%) that they had a smart phone or tablet for Internet use in the NICU, and nearly all “agreed” or “strongly agreed” (33.3% and 62.5%) that having an app at the NICU bedside/home would be helpful. “This showed us that families were ready to use technology and interested in something like PreeMe+You at the bedside,” Ms. Whitney said.
At the time of study enrollment, 12 were in the purple stage, 8 were in the blue stage, 19 infants were in the orange stage, and 9 were in the yellow stage. Ms. Whitney reported that based on the PedsQL Family Impact Module, 35 of the 44 families showed increased quality of life functionality after participating in the study. This change was significant, with a P value of .001. Improvements were seen in the measure’s eight domains (physical, emotional, social, cognitive, communication, worry, daily activities, and family relationship functionality). “We saw increases across all of the domains based on how long the parents had been using the app,” Ms. Whitney said. “We found the biggest increase in quality of life in families of babies born less than 25 weeks’ gestational age, those born 25-26 weeks gestational age, those born 27-28 weeks gestational age, and those born 33-37 weeks gestational age. We are encouraged to see some of these quality of life changes in some of the earliest-born gestation babies because these are presumably the families that would have the longest time to go in the NICU and could benefit the most from using an app like PreeMe+You.”
She acknowledged certain limitations of the study, including the fact that it was conducted in two NICUs, “and we definitely need more comparisons to look at the natural trajectory of quality of life changes while families are in the NICU. Also, all of the families enrolled in our study had access to a research team that checked in with them weekly. In the real world, PreeMe+You would probably be self-guided.” Going forward, PreeMe+You plans to include additional features to give parents more self-guidance, making it easier for them to interact and partner with their baby’s medical team.
Funding for the study was provided by the Bucksbaum Institute for Clinical Excellence. Ms. Whitney was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
TORONTO – Significant improvement in quality of life was observed in neonatal ICU families using the PreeMe+You app, preliminary results from a two-center study showed.
“NICU time is stressful,” one of the study authors, Abigail Whitney, said at the Pediatric Academic Societies annual meeting. “With the birth of a preterm infant, parents are often quickly transitioned into the role of becoming a parent much sooner and in much different circumstances than they might have anticipated. Parents have reported feelings of isolation, alienation, and insecurity in the parental role while in the NICU. Studies have shown that interventions that engage parents in their infant’s progress can decrease parental stress and anxiety, increase positive parent-infant interaction, and even reduce the infant’s length of stay. Also, with advancing technology there has been a push to find ways to use mobile technology to help parents balance engaging with their infant with the rest of their busy lives.”
In a study overseen by PreeMe+You’s chief medical expert, Bree Andrews, MD, MPH, Ms. Whitney and her associates administered the app to 48 families at either the University of Chicago Medicine Comer Children’s Hospital NICU or the Evanston Hospital NICU to assess readiness for using mobile technologies at the bedside. All families were recommended by a child life specialist who identified families who might be interested in using something like PreeMe+You. They excluded any families that were currently involved with child and family services, those with an infant younger than 7 days old, those whose child required escalation of care or upcoming surgeries, and those whose infant was over 37 weeks’ gestation.
First, the researchers briefed NICU staff about the study at charge nurse meetings, faculty meetings, and daily huddles for 2 weeks before first enrollment. “We did this knowing that parents might go to their nurses or doctors about how to answer specific questions within the app, or maybe want to learn more about a certain topic they learned from PreeMe+You,” Ms. Whitney said.
Data measurements included the PreeMe+You composite survey, which pulled questions from the Fragile Infant Parent Readiness Evaluation (FIPRE) and the NICU Parent Risk Evaluation and Engagement Model and Instrument (PREEMI). “We also included additional questions about technology use and capacity, as well as the PedsQL [Pediatric Quality of Life Inventory] Family Impact Module to assess parental quality of life throughout the study,” she said.
Over a period of 9 months, the researchers collected 153 quality of life measurements from 48 families. Of these, 48 occurred at enrollment, 23 occurred less than 1 week after enrollment, 30 occurred 1-2 weeks after enrollment, 28 occurred 3-4 weeks after enrollment, and 24 occurred 4 weeks or more after enrollment. By study closure, the researchers had follow-up data on 44 of the 48 families. The average gestational age at birth was 29.3 weeks, the average day of life at enrollment was 25.4, and the average birth weight was 1,280 grams.
On the app’s composite survey, 14.6% “agreed” and 79.2% “strongly agreed” that they were currently using a smart phone or tablet to look for information about preemies/NICU on the Internet, and about half “agreed” or “strongly agreed” (27.1% and 33.3%, respectively) that they spent more than 30 minutes per week looking up information about their NICU baby online. Nearly all families “agreed” or “strongly agreed” (14.6% and 85.4%) that they had a smart phone or tablet for Internet use in the NICU, and nearly all “agreed” or “strongly agreed” (33.3% and 62.5%) that having an app at the NICU bedside/home would be helpful. “This showed us that families were ready to use technology and interested in something like PreeMe+You at the bedside,” Ms. Whitney said.
At the time of study enrollment, 12 were in the purple stage, 8 were in the blue stage, 19 infants were in the orange stage, and 9 were in the yellow stage. Ms. Whitney reported that based on the PedsQL Family Impact Module, 35 of the 44 families showed increased quality of life functionality after participating in the study. This change was significant, with a P value of .001. Improvements were seen in the measure’s eight domains (physical, emotional, social, cognitive, communication, worry, daily activities, and family relationship functionality). “We saw increases across all of the domains based on how long the parents had been using the app,” Ms. Whitney said. “We found the biggest increase in quality of life in families of babies born less than 25 weeks’ gestational age, those born 25-26 weeks gestational age, those born 27-28 weeks gestational age, and those born 33-37 weeks gestational age. We are encouraged to see some of these quality of life changes in some of the earliest-born gestation babies because these are presumably the families that would have the longest time to go in the NICU and could benefit the most from using an app like PreeMe+You.”
She acknowledged certain limitations of the study, including the fact that it was conducted in two NICUs, “and we definitely need more comparisons to look at the natural trajectory of quality of life changes while families are in the NICU. Also, all of the families enrolled in our study had access to a research team that checked in with them weekly. In the real world, PreeMe+You would probably be self-guided.” Going forward, PreeMe+You plans to include additional features to give parents more self-guidance, making it easier for them to interact and partner with their baby’s medical team.
Funding for the study was provided by the Bucksbaum Institute for Clinical Excellence. Ms. Whitney was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
REPORTING FROM PAS 2018
Key clinical point:
Major finding: In all, 35 of the 44 families showed increased quality of life functionality, based on the PedsQL Family Impact Module (P = .001).
Study details: A two-center study of 44 families with premature infants intended to assess readiness for using mobile technologies at the bedside.
Disclosures: Funding for the study was provided by the Bucksbaum Institute for Clinical Excellence. Ms. Whitney was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
CDC concerned about multidrug-resistant Shigella
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
FDA approves long-acting ESA for dialysis-related anemia in children, adolescents
The patients aged 5-17 years with chronic kidney disease whose hemoglobin had been stabilized with an erythropoiesis-stimulating agent (ESA).
Efficacy was based partly on how well target hemoglobin levels were maintained in this trial, but also on extrapolation from results of trials in adults. The safety profile in these pediatric patients was consistent with those previously observed in adults. Mircera is manufactured by Vifor Pharma.
More information on the approval of Mircera in this population can be found in the FDA release. The prescribing information for Mircera, initially approved in 2007, has also been updated.
The patients aged 5-17 years with chronic kidney disease whose hemoglobin had been stabilized with an erythropoiesis-stimulating agent (ESA).
Efficacy was based partly on how well target hemoglobin levels were maintained in this trial, but also on extrapolation from results of trials in adults. The safety profile in these pediatric patients was consistent with those previously observed in adults. Mircera is manufactured by Vifor Pharma.
More information on the approval of Mircera in this population can be found in the FDA release. The prescribing information for Mircera, initially approved in 2007, has also been updated.
The patients aged 5-17 years with chronic kidney disease whose hemoglobin had been stabilized with an erythropoiesis-stimulating agent (ESA).
Efficacy was based partly on how well target hemoglobin levels were maintained in this trial, but also on extrapolation from results of trials in adults. The safety profile in these pediatric patients was consistent with those previously observed in adults. Mircera is manufactured by Vifor Pharma.
More information on the approval of Mircera in this population can be found in the FDA release. The prescribing information for Mircera, initially approved in 2007, has also been updated.
Pemphigus remission rate tops 80% with rituximab
ORLANDO – , and could be approved for the indication soon.
With approval pending, “rituximab is quickly emerging as frontline therapy” for pemphigus, so “we should begin to prepare to answer our patients’ questions. It’s likely they will be interested in its use,” said Carolyn Kushner, a medical student and dermatology research fellow at the University of Pennsylvania, Philadelphia. Rituximab manufacturer Genentech announced the priority review for this indication in a Feb. 2018 press release.
“We get a lot of questions in the clinic,” she said at the International Investigative Dermatology meeting. Patients with pemphigus want to know how well rituximab will work, and if they’ll be able to go off other medications. They wonder if it’s safe, and when they’ll need to be retreated. The goal of the study was to provide information for both clinicians and patients regarding what to expect from the treatment.
Overall, 54 patients (48%) achieved a complete response off therapy (CROT) after their first treatment cycle, meaning they had no new lesions for at least 2 months off of all systemic and topical treatments. The median time to a complete response was 7.4 months, and the median time to relapse was 20.9 months after the first infusion. An additional 15 patients (13%) had a complete remission with minimal therapy after one cycle.
In short, “61% of patients achieved complete healing of their skin after one cycle,” Ms. Kushner said. The number rose to 82% (93 patients) when those who had more than one cycle were included. The maximum in the study was seven. Among all patients, the median time from the first to second rituximab dose was 25.1 months.
When age, sex, and disease duration were controlled for, patients who received lymphoma dosing – 375 mg/m2 weekly for 4 weeks – were 2.7 times more likely to achieve CROT than those on the rheumatoid arthritis dosing, two 1,000 mg IV infusions 2 weeks apart (P = .037). “We almost never use RA dosing now,” she said.
The odds of success also increased with age, with patients 45 years and older 3.5 to almost 7 times more likely to achieve CROT than younger patients, also a statistically significant finding.
There were four serious adverse events across 155 cycles of the lymphoma regimen, and one with 90 cycles of arthritis dosing, all infectious and none fatal. Ms. Kushner cautioned the true rate was probably higher, since their review data might have missed some cases.
Race, sex, and disease duration had no significant effect on response rates. About 60% of the patients were women.
Rituximab, approved in 1997, is a CD20-directed cytolytic antibody.
There was no industry funding for the work, and Ms. Kushner didn’t have any disclosures.
SOURCE: Kushner CJ et al. IID 2018, Abstract 552.
ORLANDO – , and could be approved for the indication soon.
With approval pending, “rituximab is quickly emerging as frontline therapy” for pemphigus, so “we should begin to prepare to answer our patients’ questions. It’s likely they will be interested in its use,” said Carolyn Kushner, a medical student and dermatology research fellow at the University of Pennsylvania, Philadelphia. Rituximab manufacturer Genentech announced the priority review for this indication in a Feb. 2018 press release.
“We get a lot of questions in the clinic,” she said at the International Investigative Dermatology meeting. Patients with pemphigus want to know how well rituximab will work, and if they’ll be able to go off other medications. They wonder if it’s safe, and when they’ll need to be retreated. The goal of the study was to provide information for both clinicians and patients regarding what to expect from the treatment.
Overall, 54 patients (48%) achieved a complete response off therapy (CROT) after their first treatment cycle, meaning they had no new lesions for at least 2 months off of all systemic and topical treatments. The median time to a complete response was 7.4 months, and the median time to relapse was 20.9 months after the first infusion. An additional 15 patients (13%) had a complete remission with minimal therapy after one cycle.
In short, “61% of patients achieved complete healing of their skin after one cycle,” Ms. Kushner said. The number rose to 82% (93 patients) when those who had more than one cycle were included. The maximum in the study was seven. Among all patients, the median time from the first to second rituximab dose was 25.1 months.
When age, sex, and disease duration were controlled for, patients who received lymphoma dosing – 375 mg/m2 weekly for 4 weeks – were 2.7 times more likely to achieve CROT than those on the rheumatoid arthritis dosing, two 1,000 mg IV infusions 2 weeks apart (P = .037). “We almost never use RA dosing now,” she said.
The odds of success also increased with age, with patients 45 years and older 3.5 to almost 7 times more likely to achieve CROT than younger patients, also a statistically significant finding.
There were four serious adverse events across 155 cycles of the lymphoma regimen, and one with 90 cycles of arthritis dosing, all infectious and none fatal. Ms. Kushner cautioned the true rate was probably higher, since their review data might have missed some cases.
Race, sex, and disease duration had no significant effect on response rates. About 60% of the patients were women.
Rituximab, approved in 1997, is a CD20-directed cytolytic antibody.
There was no industry funding for the work, and Ms. Kushner didn’t have any disclosures.
SOURCE: Kushner CJ et al. IID 2018, Abstract 552.
ORLANDO – , and could be approved for the indication soon.
With approval pending, “rituximab is quickly emerging as frontline therapy” for pemphigus, so “we should begin to prepare to answer our patients’ questions. It’s likely they will be interested in its use,” said Carolyn Kushner, a medical student and dermatology research fellow at the University of Pennsylvania, Philadelphia. Rituximab manufacturer Genentech announced the priority review for this indication in a Feb. 2018 press release.
“We get a lot of questions in the clinic,” she said at the International Investigative Dermatology meeting. Patients with pemphigus want to know how well rituximab will work, and if they’ll be able to go off other medications. They wonder if it’s safe, and when they’ll need to be retreated. The goal of the study was to provide information for both clinicians and patients regarding what to expect from the treatment.
Overall, 54 patients (48%) achieved a complete response off therapy (CROT) after their first treatment cycle, meaning they had no new lesions for at least 2 months off of all systemic and topical treatments. The median time to a complete response was 7.4 months, and the median time to relapse was 20.9 months after the first infusion. An additional 15 patients (13%) had a complete remission with minimal therapy after one cycle.
In short, “61% of patients achieved complete healing of their skin after one cycle,” Ms. Kushner said. The number rose to 82% (93 patients) when those who had more than one cycle were included. The maximum in the study was seven. Among all patients, the median time from the first to second rituximab dose was 25.1 months.
When age, sex, and disease duration were controlled for, patients who received lymphoma dosing – 375 mg/m2 weekly for 4 weeks – were 2.7 times more likely to achieve CROT than those on the rheumatoid arthritis dosing, two 1,000 mg IV infusions 2 weeks apart (P = .037). “We almost never use RA dosing now,” she said.
The odds of success also increased with age, with patients 45 years and older 3.5 to almost 7 times more likely to achieve CROT than younger patients, also a statistically significant finding.
There were four serious adverse events across 155 cycles of the lymphoma regimen, and one with 90 cycles of arthritis dosing, all infectious and none fatal. Ms. Kushner cautioned the true rate was probably higher, since their review data might have missed some cases.
Race, sex, and disease duration had no significant effect on response rates. About 60% of the patients were women.
Rituximab, approved in 1997, is a CD20-directed cytolytic antibody.
There was no industry funding for the work, and Ms. Kushner didn’t have any disclosures.
SOURCE: Kushner CJ et al. IID 2018, Abstract 552.
REPORTING FROM IID 2018
Key clinical point: Rituximab puts the majority of pemphigus patients in remission, with a median time between doses of about 2 years.
Major finding: Sixty-one percent of patients achieved complete healing of their skin after one cycle, increasing to 82% when those who had more than one cycle were included.
Study details: A single-center review of 113 patients
Disclosures: There was no industry funding, and the lead investigator had no disclosures.
Source: Kushner CJ et al. IID 2018, Abstract 552.
Botox, PVCs, and statins
Transcatheter aortic valves outlast surgical valves in the NOTION study presented at EuroPCR. From Heart Rhythm 2018, a registry study suggests myocarditis is the culprit in many cases of frequent premature ventricular contractions, and one set of Botox shots into intracardiac fat pads cut atrial fibrillation for up to 3 years. Also this week, many patients with NAFLD and abnormal alanine aminotransferase should be taking a statin but aren’t, and why statins reduce the risk of lethal prostate cancer.
Listen to Cardiocast weekly for all the latest cardiology news.
Transcatheter aortic valves outlast surgical valves in the NOTION study presented at EuroPCR. From Heart Rhythm 2018, a registry study suggests myocarditis is the culprit in many cases of frequent premature ventricular contractions, and one set of Botox shots into intracardiac fat pads cut atrial fibrillation for up to 3 years. Also this week, many patients with NAFLD and abnormal alanine aminotransferase should be taking a statin but aren’t, and why statins reduce the risk of lethal prostate cancer.
Listen to Cardiocast weekly for all the latest cardiology news.
Transcatheter aortic valves outlast surgical valves in the NOTION study presented at EuroPCR. From Heart Rhythm 2018, a registry study suggests myocarditis is the culprit in many cases of frequent premature ventricular contractions, and one set of Botox shots into intracardiac fat pads cut atrial fibrillation for up to 3 years. Also this week, many patients with NAFLD and abnormal alanine aminotransferase should be taking a statin but aren’t, and why statins reduce the risk of lethal prostate cancer.
Listen to Cardiocast weekly for all the latest cardiology news.
Long-term follow-up most important for hydroxychloroquine retinal screening
LIVERPOOL, ENGLAND – , but long-term follow-up is much more important, according to data presented at the British Society for Rheumatology annual conference.
In just one specialist rheumatology center in England, which treats more than 8,000 patients annually, the cost of the first year’s optical coherence tomography (OCT) assessment would be more than $60,000. Additional costs would be incurred to screen those who had been on the drug for more than 5 years ,who were known to be at greater risk of hydroxychloroquine-induced retinopathy. This is within the National Health Service in England where the cost of a single OCT scan is around $70; in the private health sector, the cost of one test can be as high as $400.
Indeed, of 887 hydroxychloroquine users identified, 44% had at least one risk factor for hydroxychloroquine-induced retinopathy. These included being older than 60 years of age (30% of all users), having renal (10%) or hepatic (2%) impairment, retinal disease at baseline (8%), or using high (more than 6.5 mg/kg) doses of the drug based on their actual (9%) or ideal (4%) body weight.
“The retinal toxicity of hydroxychloroquine is a bit of a hot topic at the moment,” Dr. Yates said at the conference. While the drug has been around for years and used successfully to treat many patients with rheumatoid arthritis and systemic lupus erythematosus (SLE), a known side effect is retinal toxicity.
Traditionally, retinopathy has been quoted as being a relatively rare side effect, affecting around 0.5%-2% of the treated population. Recent data (JAMA Ophthalmol. 2014;132[12]:1453-60) suggest, however, that is probably a vast underestimate, with 7.5% of patients taking hydroxychloroquine for more than 5 years likely to be affected, as are up to 20% of those taking the drug for up to 20 years of treatment.
Dr. Yates and associates wanted to assess the burden of hydroxychloroquine use at their center and look at the risk factors and impact of the recent screening guidelines issued by the British Society for Rheumatology (Rheumatology [Oxford]. 2017;56[6]:865-8) in 2017 and by the Royal College of Ophthalmologists in 2018. These state that patients should have a formal baseline ophthalmic examination, ideally including OCT, within 6-12 months of starting therapy and an annual eye assessment with repeat OCT thereafter for the following 5 years; the ophthalmology guidelines recommending annual screening for the duration of therapy.
One criticism of increased screening for retinal toxicity in routine practice is consultants saying that they see only a handful of cases during their career, Dr. Yates observed. However, if you consider that in an average rheumatology department there are five consultants and 900 patients on hydroxychloroquine, 500 patients take the drug for 5 years or longer, 2% are picked up with non-OCT screening, that amounts to around two cases per year over a 5- to 10-year period. “So that fits with the narrative of only having seen a handful of cases pre-OCT,” Dr. Yates reasoned.
“I believe that this is a real problem, but I’m afraid this is the tip of the iceberg,” commented Caroline Gordon, MD, after her presentation. “We’ve been screening our patients in Birmingham now for about 5 years and we are definitely finding a significant number of patients with hydroxychloroquine toxicity who can be picked up with OCT and visual fields screening.”
Dr. Gordon, professor of rheumatology at the University of Birmingham (England) and a consultant rheumatologist for the University Hospitals NHS Foundation Trust and the Sandwell & West Birmingham Hospitals NHS Trust, helps look after one of the largest cohorts of patients with SLE in the United Kingdom.
A baseline eye examination has always been recommended, Dr. Gordon said, but she suggested that this could remain in the realm of the opticians with further assessment and referral as needed.
“I’m not convinced, from the work we’ve done, that there is any value in the baseline OCT,” Dr. Gordon said, “because we never find anything on the baseline OCT that we didn’t already expect from the opticians’ assessment.”
It is the long-term (longer than10 years) follow-up that needs to be the focus, rather than the initial period, she stressed, as the highest risk appears to be in patients who have been taking the drug for 15 years or longer. Prior to this, different types of retinopathy can occur that are actually attributable to the underlying disease and are not related hydroxychloroquine. Of course, patients on higher doses of hydroxychloroquine may need closer monitoring early on, “as they are at risk,” she acknowledged.
Dr. Gordon suggested that the guidelines as they currently stand may not be that useful for real-life practice. Following them could result in a large amount of money being spent on early tests that are perhaps not necessary.
“What we do need to do is focus on the patients who’ve been on treatment long term,” she said.
SOURCE: Yates M et al. Rheumatology. 2018;57(Suppl. 3):key075.188.
LIVERPOOL, ENGLAND – , but long-term follow-up is much more important, according to data presented at the British Society for Rheumatology annual conference.
In just one specialist rheumatology center in England, which treats more than 8,000 patients annually, the cost of the first year’s optical coherence tomography (OCT) assessment would be more than $60,000. Additional costs would be incurred to screen those who had been on the drug for more than 5 years ,who were known to be at greater risk of hydroxychloroquine-induced retinopathy. This is within the National Health Service in England where the cost of a single OCT scan is around $70; in the private health sector, the cost of one test can be as high as $400.
Indeed, of 887 hydroxychloroquine users identified, 44% had at least one risk factor for hydroxychloroquine-induced retinopathy. These included being older than 60 years of age (30% of all users), having renal (10%) or hepatic (2%) impairment, retinal disease at baseline (8%), or using high (more than 6.5 mg/kg) doses of the drug based on their actual (9%) or ideal (4%) body weight.
“The retinal toxicity of hydroxychloroquine is a bit of a hot topic at the moment,” Dr. Yates said at the conference. While the drug has been around for years and used successfully to treat many patients with rheumatoid arthritis and systemic lupus erythematosus (SLE), a known side effect is retinal toxicity.
Traditionally, retinopathy has been quoted as being a relatively rare side effect, affecting around 0.5%-2% of the treated population. Recent data (JAMA Ophthalmol. 2014;132[12]:1453-60) suggest, however, that is probably a vast underestimate, with 7.5% of patients taking hydroxychloroquine for more than 5 years likely to be affected, as are up to 20% of those taking the drug for up to 20 years of treatment.
Dr. Yates and associates wanted to assess the burden of hydroxychloroquine use at their center and look at the risk factors and impact of the recent screening guidelines issued by the British Society for Rheumatology (Rheumatology [Oxford]. 2017;56[6]:865-8) in 2017 and by the Royal College of Ophthalmologists in 2018. These state that patients should have a formal baseline ophthalmic examination, ideally including OCT, within 6-12 months of starting therapy and an annual eye assessment with repeat OCT thereafter for the following 5 years; the ophthalmology guidelines recommending annual screening for the duration of therapy.
One criticism of increased screening for retinal toxicity in routine practice is consultants saying that they see only a handful of cases during their career, Dr. Yates observed. However, if you consider that in an average rheumatology department there are five consultants and 900 patients on hydroxychloroquine, 500 patients take the drug for 5 years or longer, 2% are picked up with non-OCT screening, that amounts to around two cases per year over a 5- to 10-year period. “So that fits with the narrative of only having seen a handful of cases pre-OCT,” Dr. Yates reasoned.
“I believe that this is a real problem, but I’m afraid this is the tip of the iceberg,” commented Caroline Gordon, MD, after her presentation. “We’ve been screening our patients in Birmingham now for about 5 years and we are definitely finding a significant number of patients with hydroxychloroquine toxicity who can be picked up with OCT and visual fields screening.”
Dr. Gordon, professor of rheumatology at the University of Birmingham (England) and a consultant rheumatologist for the University Hospitals NHS Foundation Trust and the Sandwell & West Birmingham Hospitals NHS Trust, helps look after one of the largest cohorts of patients with SLE in the United Kingdom.
A baseline eye examination has always been recommended, Dr. Gordon said, but she suggested that this could remain in the realm of the opticians with further assessment and referral as needed.
“I’m not convinced, from the work we’ve done, that there is any value in the baseline OCT,” Dr. Gordon said, “because we never find anything on the baseline OCT that we didn’t already expect from the opticians’ assessment.”
It is the long-term (longer than10 years) follow-up that needs to be the focus, rather than the initial period, she stressed, as the highest risk appears to be in patients who have been taking the drug for 15 years or longer. Prior to this, different types of retinopathy can occur that are actually attributable to the underlying disease and are not related hydroxychloroquine. Of course, patients on higher doses of hydroxychloroquine may need closer monitoring early on, “as they are at risk,” she acknowledged.
Dr. Gordon suggested that the guidelines as they currently stand may not be that useful for real-life practice. Following them could result in a large amount of money being spent on early tests that are perhaps not necessary.
“What we do need to do is focus on the patients who’ve been on treatment long term,” she said.
SOURCE: Yates M et al. Rheumatology. 2018;57(Suppl. 3):key075.188.
LIVERPOOL, ENGLAND – , but long-term follow-up is much more important, according to data presented at the British Society for Rheumatology annual conference.
In just one specialist rheumatology center in England, which treats more than 8,000 patients annually, the cost of the first year’s optical coherence tomography (OCT) assessment would be more than $60,000. Additional costs would be incurred to screen those who had been on the drug for more than 5 years ,who were known to be at greater risk of hydroxychloroquine-induced retinopathy. This is within the National Health Service in England where the cost of a single OCT scan is around $70; in the private health sector, the cost of one test can be as high as $400.
Indeed, of 887 hydroxychloroquine users identified, 44% had at least one risk factor for hydroxychloroquine-induced retinopathy. These included being older than 60 years of age (30% of all users), having renal (10%) or hepatic (2%) impairment, retinal disease at baseline (8%), or using high (more than 6.5 mg/kg) doses of the drug based on their actual (9%) or ideal (4%) body weight.
“The retinal toxicity of hydroxychloroquine is a bit of a hot topic at the moment,” Dr. Yates said at the conference. While the drug has been around for years and used successfully to treat many patients with rheumatoid arthritis and systemic lupus erythematosus (SLE), a known side effect is retinal toxicity.
Traditionally, retinopathy has been quoted as being a relatively rare side effect, affecting around 0.5%-2% of the treated population. Recent data (JAMA Ophthalmol. 2014;132[12]:1453-60) suggest, however, that is probably a vast underestimate, with 7.5% of patients taking hydroxychloroquine for more than 5 years likely to be affected, as are up to 20% of those taking the drug for up to 20 years of treatment.
Dr. Yates and associates wanted to assess the burden of hydroxychloroquine use at their center and look at the risk factors and impact of the recent screening guidelines issued by the British Society for Rheumatology (Rheumatology [Oxford]. 2017;56[6]:865-8) in 2017 and by the Royal College of Ophthalmologists in 2018. These state that patients should have a formal baseline ophthalmic examination, ideally including OCT, within 6-12 months of starting therapy and an annual eye assessment with repeat OCT thereafter for the following 5 years; the ophthalmology guidelines recommending annual screening for the duration of therapy.
One criticism of increased screening for retinal toxicity in routine practice is consultants saying that they see only a handful of cases during their career, Dr. Yates observed. However, if you consider that in an average rheumatology department there are five consultants and 900 patients on hydroxychloroquine, 500 patients take the drug for 5 years or longer, 2% are picked up with non-OCT screening, that amounts to around two cases per year over a 5- to 10-year period. “So that fits with the narrative of only having seen a handful of cases pre-OCT,” Dr. Yates reasoned.
“I believe that this is a real problem, but I’m afraid this is the tip of the iceberg,” commented Caroline Gordon, MD, after her presentation. “We’ve been screening our patients in Birmingham now for about 5 years and we are definitely finding a significant number of patients with hydroxychloroquine toxicity who can be picked up with OCT and visual fields screening.”
Dr. Gordon, professor of rheumatology at the University of Birmingham (England) and a consultant rheumatologist for the University Hospitals NHS Foundation Trust and the Sandwell & West Birmingham Hospitals NHS Trust, helps look after one of the largest cohorts of patients with SLE in the United Kingdom.
A baseline eye examination has always been recommended, Dr. Gordon said, but she suggested that this could remain in the realm of the opticians with further assessment and referral as needed.
“I’m not convinced, from the work we’ve done, that there is any value in the baseline OCT,” Dr. Gordon said, “because we never find anything on the baseline OCT that we didn’t already expect from the opticians’ assessment.”
It is the long-term (longer than10 years) follow-up that needs to be the focus, rather than the initial period, she stressed, as the highest risk appears to be in patients who have been taking the drug for 15 years or longer. Prior to this, different types of retinopathy can occur that are actually attributable to the underlying disease and are not related hydroxychloroquine. Of course, patients on higher doses of hydroxychloroquine may need closer monitoring early on, “as they are at risk,” she acknowledged.
Dr. Gordon suggested that the guidelines as they currently stand may not be that useful for real-life practice. Following them could result in a large amount of money being spent on early tests that are perhaps not necessary.
“What we do need to do is focus on the patients who’ve been on treatment long term,” she said.
SOURCE: Yates M et al. Rheumatology. 2018;57(Suppl. 3):key075.188.
REPORTING FROM BSR 2018
Key clinical point: Long-term follow up is important for assessing hydroxychloroquine toxicity.
Major finding: 44% of patients had at least one risk factor for hydroxychloroquine-induced retinopathy after more than 5 years of treatment.
Study details: Electronic record review of 887 patients treated with hydroxychloroquine for about 5 years in a large tertiary rheumatology service.
Disclosures: Dr. Yates had nothing to disclose.
Source: Yates M et al. Rheumatology. 2018;57(Suppl. 3):key075.312.
Raised LDL cholesterol, hsCRP tied to polymyalgia rheumatica, GCA
LIVERPOOL, ENGLAND – The presence of traditional cardiovascular risk factors may precede the development of polymyalgia rheumatica and giant cell arteritis.
Data from the EPIC-Norfolk study, reported at the British Society for Rheumatology annual conference, showed that raised LDL cholesterol was associated with the onset of polymyalgia rheumatica (PMR) and that high sensitivity C-reactive protein (hsCRP) was associated with giant cell arteritis (GCA).
“There’s been an association between vascular disease and PMR and GCA reported, but the way cardiovascular disease has been defined has been based on rather late endpoints, such as angina, myocardial infarction, peripheral vascular disease, and ischemia,” said Max Yates, MBBS, MRCP, in an interview.
“So, what we wanted to do was look at underlying risk factors for those diseases and see how they play in, in terms of the timing of the diagnosis of PMR and GCA,” he explained. Dr. Yates, who is a National Institute for Health Research clinical lecturer in rheumatology at the University of East Anglia, Norwich, England, noted that this was probably the first prospective study to look at clinical and laboratory parameters for vascular disease prior to the onset of these diseases.
Previously, French researchers suggested that there might be a link between hypertension and subsequent PMR, but that was a descriptive study published over 30 years ago, Dr. Yates said. “There was another case-control study from the Mayo Clinic where they said that smoking was associated with incidence GCA,” he added. “So most of the work has been retrospective, case-control studies.”
The EPIC (European Prospective Investigation of Cancer)-Norfolk study is a large, prospective, community-based cohort study that, as its name might suggest, was originally set up to look at risk factors for cancer. Since then it has broadened to enable the study of risk factors for a whole host of other conditions.
More than 30,000 people aged 40-70 years were recruited into the study during 1993-1997, and 25,600 people (440,237 at-risk person-years) who had the necessary baseline and follow-up data were included in the current analysis performed by Dr. Yates and associates.
A total of 395 cases of PMR and 118 cases of GCA were identified using current classification criteria. Those with PMR were diagnosed at a mean age of 73.6 years and those with GCA at a mean age of 74.1 years. For both conditions, about three-quarters of patients were women.
The investigators then looked back at the patients’ original recruitment data in terms of their cardiovascular risk factors, which included their blood pressure readings; body mass index; smoking status; presence of diabetes; hsCRP; and LDL cholesterol, triglycerides, and HDL cholesterol levels.
“Ultimately, these traditional cardiovascular risk factors are present early on, prior to PMR and GCA,” Dr. Yates said.
What this means is that perhaps clinicians need to be more aware of managing these risk factors aggressively, he suggested, but therein lies a problem. “It’s obviously very difficult, early on, before anyone’s developed any disease, to target these risk factors, and you have to balance the risk and benefit for individuals.”
GCA is a “pretty rare” disease whereas PMR is “quite common,” Dr. Yates said, “but we probably need to target these risk factors as soon as people are diagnosed with these conditions, to try to prevent the cardiovascular morbidity that is seen.”
These data might also help explain the underlying etiology and why there is an increased risk of vascular disease seen in populations of patients with inflammatory arthritides.
Dr. Yates had no conflicts of interest to disclose.
SOURCE: Yates M et al. Rheumatology. 2018 Apr;57[Suppl. 3]:key075.312.
LIVERPOOL, ENGLAND – The presence of traditional cardiovascular risk factors may precede the development of polymyalgia rheumatica and giant cell arteritis.
Data from the EPIC-Norfolk study, reported at the British Society for Rheumatology annual conference, showed that raised LDL cholesterol was associated with the onset of polymyalgia rheumatica (PMR) and that high sensitivity C-reactive protein (hsCRP) was associated with giant cell arteritis (GCA).
“There’s been an association between vascular disease and PMR and GCA reported, but the way cardiovascular disease has been defined has been based on rather late endpoints, such as angina, myocardial infarction, peripheral vascular disease, and ischemia,” said Max Yates, MBBS, MRCP, in an interview.
“So, what we wanted to do was look at underlying risk factors for those diseases and see how they play in, in terms of the timing of the diagnosis of PMR and GCA,” he explained. Dr. Yates, who is a National Institute for Health Research clinical lecturer in rheumatology at the University of East Anglia, Norwich, England, noted that this was probably the first prospective study to look at clinical and laboratory parameters for vascular disease prior to the onset of these diseases.
Previously, French researchers suggested that there might be a link between hypertension and subsequent PMR, but that was a descriptive study published over 30 years ago, Dr. Yates said. “There was another case-control study from the Mayo Clinic where they said that smoking was associated with incidence GCA,” he added. “So most of the work has been retrospective, case-control studies.”
The EPIC (European Prospective Investigation of Cancer)-Norfolk study is a large, prospective, community-based cohort study that, as its name might suggest, was originally set up to look at risk factors for cancer. Since then it has broadened to enable the study of risk factors for a whole host of other conditions.
More than 30,000 people aged 40-70 years were recruited into the study during 1993-1997, and 25,600 people (440,237 at-risk person-years) who had the necessary baseline and follow-up data were included in the current analysis performed by Dr. Yates and associates.
A total of 395 cases of PMR and 118 cases of GCA were identified using current classification criteria. Those with PMR were diagnosed at a mean age of 73.6 years and those with GCA at a mean age of 74.1 years. For both conditions, about three-quarters of patients were women.
The investigators then looked back at the patients’ original recruitment data in terms of their cardiovascular risk factors, which included their blood pressure readings; body mass index; smoking status; presence of diabetes; hsCRP; and LDL cholesterol, triglycerides, and HDL cholesterol levels.
“Ultimately, these traditional cardiovascular risk factors are present early on, prior to PMR and GCA,” Dr. Yates said.
What this means is that perhaps clinicians need to be more aware of managing these risk factors aggressively, he suggested, but therein lies a problem. “It’s obviously very difficult, early on, before anyone’s developed any disease, to target these risk factors, and you have to balance the risk and benefit for individuals.”
GCA is a “pretty rare” disease whereas PMR is “quite common,” Dr. Yates said, “but we probably need to target these risk factors as soon as people are diagnosed with these conditions, to try to prevent the cardiovascular morbidity that is seen.”
These data might also help explain the underlying etiology and why there is an increased risk of vascular disease seen in populations of patients with inflammatory arthritides.
Dr. Yates had no conflicts of interest to disclose.
SOURCE: Yates M et al. Rheumatology. 2018 Apr;57[Suppl. 3]:key075.312.
LIVERPOOL, ENGLAND – The presence of traditional cardiovascular risk factors may precede the development of polymyalgia rheumatica and giant cell arteritis.
Data from the EPIC-Norfolk study, reported at the British Society for Rheumatology annual conference, showed that raised LDL cholesterol was associated with the onset of polymyalgia rheumatica (PMR) and that high sensitivity C-reactive protein (hsCRP) was associated with giant cell arteritis (GCA).
“There’s been an association between vascular disease and PMR and GCA reported, but the way cardiovascular disease has been defined has been based on rather late endpoints, such as angina, myocardial infarction, peripheral vascular disease, and ischemia,” said Max Yates, MBBS, MRCP, in an interview.
“So, what we wanted to do was look at underlying risk factors for those diseases and see how they play in, in terms of the timing of the diagnosis of PMR and GCA,” he explained. Dr. Yates, who is a National Institute for Health Research clinical lecturer in rheumatology at the University of East Anglia, Norwich, England, noted that this was probably the first prospective study to look at clinical and laboratory parameters for vascular disease prior to the onset of these diseases.
Previously, French researchers suggested that there might be a link between hypertension and subsequent PMR, but that was a descriptive study published over 30 years ago, Dr. Yates said. “There was another case-control study from the Mayo Clinic where they said that smoking was associated with incidence GCA,” he added. “So most of the work has been retrospective, case-control studies.”
The EPIC (European Prospective Investigation of Cancer)-Norfolk study is a large, prospective, community-based cohort study that, as its name might suggest, was originally set up to look at risk factors for cancer. Since then it has broadened to enable the study of risk factors for a whole host of other conditions.
More than 30,000 people aged 40-70 years were recruited into the study during 1993-1997, and 25,600 people (440,237 at-risk person-years) who had the necessary baseline and follow-up data were included in the current analysis performed by Dr. Yates and associates.
A total of 395 cases of PMR and 118 cases of GCA were identified using current classification criteria. Those with PMR were diagnosed at a mean age of 73.6 years and those with GCA at a mean age of 74.1 years. For both conditions, about three-quarters of patients were women.
The investigators then looked back at the patients’ original recruitment data in terms of their cardiovascular risk factors, which included their blood pressure readings; body mass index; smoking status; presence of diabetes; hsCRP; and LDL cholesterol, triglycerides, and HDL cholesterol levels.
“Ultimately, these traditional cardiovascular risk factors are present early on, prior to PMR and GCA,” Dr. Yates said.
What this means is that perhaps clinicians need to be more aware of managing these risk factors aggressively, he suggested, but therein lies a problem. “It’s obviously very difficult, early on, before anyone’s developed any disease, to target these risk factors, and you have to balance the risk and benefit for individuals.”
GCA is a “pretty rare” disease whereas PMR is “quite common,” Dr. Yates said, “but we probably need to target these risk factors as soon as people are diagnosed with these conditions, to try to prevent the cardiovascular morbidity that is seen.”
These data might also help explain the underlying etiology and why there is an increased risk of vascular disease seen in populations of patients with inflammatory arthritides.
Dr. Yates had no conflicts of interest to disclose.
SOURCE: Yates M et al. Rheumatology. 2018 Apr;57[Suppl. 3]:key075.312.
REPORTING FROM BSR 2018
Key clinical point: The presence of traditional cardiovascular risk factors may precede the development of polymyalgia rheumatica (PMR) and giant cell arteritis (GCA).
Major finding: Raised LDL cholesterol was linked with the onset of PMR (subhazard ratio, 1.29) and raised hsCRP was associated with GCA (SHR, 1.85).
Study details: Data from the EPIC-Norfolk study: 385 cases of PMR and 118 cases of GCA identified from a population of more than 25,000 subjects.
Disclosures: Dr. Yates had no conflicts of interest to disclose.
Source: Yates M et al. Rheumatology. 2018 Apr;57[Suppl. 3]:key075.312.
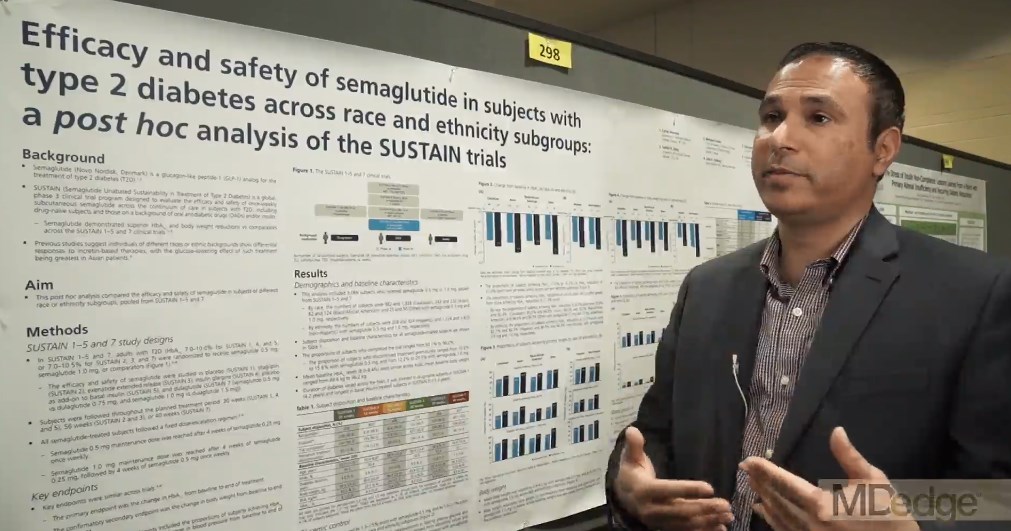
Semaglutide drops HbA1c, weight, across ethnicities
BOSTON – studied in a series of clinical trials; the efficacy did not come at the cost of frequent hypoglycemia or other serious adverse events, according to a pooled subgroup analysis of the SUSTAIN trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trials investigated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in the treatment of T2DM. Cyrus V. Desouza, MBBS, presented results of a post hoc analysis of racial and ethnic subgroups, drawing on SUSTAIN trials 1-5 and 7 (SUSTAIN 6 had a different design, focusing on cardiovascular outcomes).
“The trials incorporated patients on the whole spectrum of diabetes, starting from people who are newly diagnosed ... all the way to patients who were on a combination of oral antidiabetic drugs plus insulin,” Dr. Desouza explained in an interview at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
The mean time since diagnosis in the SUSTAIN trials varied from 4.2 years in SUSTAIN 1 to 13.3 years in SUSTAIN 5. Dr. Desouza and his colleagues pooled data from the six trials to conduct the subgroup analyses.
Patients in the intervention arms of all trials received once weekly subcutaneous semaglutide, at a dose of either 0.5 mg or 1.0 mg, according to Dr. Desouza, professor of diabetes, endocrinology, and metabolism and Schultz Professor of Diabetes Research, Diabetes, Endocrinology, and Metabolism at the University of Nebraska, Lincoln.
In all, data from 3,066 patients were available. In the racial analysis, 982 low- and 1,328 high-dose semaglutide recipients were white, 243 and 232 were Asian, 82 and 124 were African American, and 25 and 50 identified as “other,” respectively.
An analysis by ethnicity found that 208 low- and 324 high-dose recipients were Hispanic.
At baseline in all trials, mean hemoglobin A1c levels were similar, ranging from 8% to 8.4%; weights at baseline were a mean 89.6 kg to 96.2 kg across the trials.
The range of reductions in HbA1c was similar across racial and ethnic groups. “If you look at the proportion of patients who actually achieved an A1c below 7[%], it’s pretty impressive – it’s between 70% to 80%.” Between 50% and 60% of patients reached an HbA1c less than 6.5%, said Dr. Desouza.
Looking at the data another way, 62.2%-72.4% of patients saw an HbA1c reduction of at least 1% on low-dose semaglutide; the range across ethnicities was 74.2%-87.1% on high-dose semaglutide. Dr. Desouza said that the sample sizes weren’t large enough to calculate statistical significance for these subgroup differences.
“But I think what is impressive is that over 50% of patients in all the races and ethnicities were able to achieve a 5% body weight loss, which is metabolically significant in terms of improving outcomes,” he said. “I think that’s a really important fact.” A smaller proportion – around 20% – lost at least 10% of body weight, mostly on high-dose semaglutide.
Severe hypoglycemia, as defined by American Diabetes Association classification, was very rare across trials, except that 4.7% of African Americans saw this adverse event on high-dose semaglutide. Incidence in other subgroups, at either dose, ranged from 0% to 2.4%.
Otherwise, the medication was generally well tolerated, though gastrointestinal side effects were seen. “Asian people have a little higher GI side effects – up to 50% of Asians did develop GI side effects, and between 10% and 13% of Asians had to stop medication due to side effects,” said Dr. Desouza. “So I think that would be the one caveat in terms of tolerance that we did learn.”
The SUSTAIN trials were sponsored by Novo Nordisk. Dr. Desouza has received consulting fees for Novo Nordisk and has received grant support from several other pharmaceutical companies. Two coauthors are Novo Nordisk employees.
SOURCE: Desouza C et al. AACE 2018, Abstract 298
BOSTON – studied in a series of clinical trials; the efficacy did not come at the cost of frequent hypoglycemia or other serious adverse events, according to a pooled subgroup analysis of the SUSTAIN trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trials investigated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in the treatment of T2DM. Cyrus V. Desouza, MBBS, presented results of a post hoc analysis of racial and ethnic subgroups, drawing on SUSTAIN trials 1-5 and 7 (SUSTAIN 6 had a different design, focusing on cardiovascular outcomes).
“The trials incorporated patients on the whole spectrum of diabetes, starting from people who are newly diagnosed ... all the way to patients who were on a combination of oral antidiabetic drugs plus insulin,” Dr. Desouza explained in an interview at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
The mean time since diagnosis in the SUSTAIN trials varied from 4.2 years in SUSTAIN 1 to 13.3 years in SUSTAIN 5. Dr. Desouza and his colleagues pooled data from the six trials to conduct the subgroup analyses.
Patients in the intervention arms of all trials received once weekly subcutaneous semaglutide, at a dose of either 0.5 mg or 1.0 mg, according to Dr. Desouza, professor of diabetes, endocrinology, and metabolism and Schultz Professor of Diabetes Research, Diabetes, Endocrinology, and Metabolism at the University of Nebraska, Lincoln.
In all, data from 3,066 patients were available. In the racial analysis, 982 low- and 1,328 high-dose semaglutide recipients were white, 243 and 232 were Asian, 82 and 124 were African American, and 25 and 50 identified as “other,” respectively.
An analysis by ethnicity found that 208 low- and 324 high-dose recipients were Hispanic.
At baseline in all trials, mean hemoglobin A1c levels were similar, ranging from 8% to 8.4%; weights at baseline were a mean 89.6 kg to 96.2 kg across the trials.
The range of reductions in HbA1c was similar across racial and ethnic groups. “If you look at the proportion of patients who actually achieved an A1c below 7[%], it’s pretty impressive – it’s between 70% to 80%.” Between 50% and 60% of patients reached an HbA1c less than 6.5%, said Dr. Desouza.
Looking at the data another way, 62.2%-72.4% of patients saw an HbA1c reduction of at least 1% on low-dose semaglutide; the range across ethnicities was 74.2%-87.1% on high-dose semaglutide. Dr. Desouza said that the sample sizes weren’t large enough to calculate statistical significance for these subgroup differences.
“But I think what is impressive is that over 50% of patients in all the races and ethnicities were able to achieve a 5% body weight loss, which is metabolically significant in terms of improving outcomes,” he said. “I think that’s a really important fact.” A smaller proportion – around 20% – lost at least 10% of body weight, mostly on high-dose semaglutide.
Severe hypoglycemia, as defined by American Diabetes Association classification, was very rare across trials, except that 4.7% of African Americans saw this adverse event on high-dose semaglutide. Incidence in other subgroups, at either dose, ranged from 0% to 2.4%.
Otherwise, the medication was generally well tolerated, though gastrointestinal side effects were seen. “Asian people have a little higher GI side effects – up to 50% of Asians did develop GI side effects, and between 10% and 13% of Asians had to stop medication due to side effects,” said Dr. Desouza. “So I think that would be the one caveat in terms of tolerance that we did learn.”
The SUSTAIN trials were sponsored by Novo Nordisk. Dr. Desouza has received consulting fees for Novo Nordisk and has received grant support from several other pharmaceutical companies. Two coauthors are Novo Nordisk employees.
SOURCE: Desouza C et al. AACE 2018, Abstract 298
BOSTON – studied in a series of clinical trials; the efficacy did not come at the cost of frequent hypoglycemia or other serious adverse events, according to a pooled subgroup analysis of the SUSTAIN trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trials investigated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in the treatment of T2DM. Cyrus V. Desouza, MBBS, presented results of a post hoc analysis of racial and ethnic subgroups, drawing on SUSTAIN trials 1-5 and 7 (SUSTAIN 6 had a different design, focusing on cardiovascular outcomes).
“The trials incorporated patients on the whole spectrum of diabetes, starting from people who are newly diagnosed ... all the way to patients who were on a combination of oral antidiabetic drugs plus insulin,” Dr. Desouza explained in an interview at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
The mean time since diagnosis in the SUSTAIN trials varied from 4.2 years in SUSTAIN 1 to 13.3 years in SUSTAIN 5. Dr. Desouza and his colleagues pooled data from the six trials to conduct the subgroup analyses.
Patients in the intervention arms of all trials received once weekly subcutaneous semaglutide, at a dose of either 0.5 mg or 1.0 mg, according to Dr. Desouza, professor of diabetes, endocrinology, and metabolism and Schultz Professor of Diabetes Research, Diabetes, Endocrinology, and Metabolism at the University of Nebraska, Lincoln.
In all, data from 3,066 patients were available. In the racial analysis, 982 low- and 1,328 high-dose semaglutide recipients were white, 243 and 232 were Asian, 82 and 124 were African American, and 25 and 50 identified as “other,” respectively.
An analysis by ethnicity found that 208 low- and 324 high-dose recipients were Hispanic.
At baseline in all trials, mean hemoglobin A1c levels were similar, ranging from 8% to 8.4%; weights at baseline were a mean 89.6 kg to 96.2 kg across the trials.
The range of reductions in HbA1c was similar across racial and ethnic groups. “If you look at the proportion of patients who actually achieved an A1c below 7[%], it’s pretty impressive – it’s between 70% to 80%.” Between 50% and 60% of patients reached an HbA1c less than 6.5%, said Dr. Desouza.
Looking at the data another way, 62.2%-72.4% of patients saw an HbA1c reduction of at least 1% on low-dose semaglutide; the range across ethnicities was 74.2%-87.1% on high-dose semaglutide. Dr. Desouza said that the sample sizes weren’t large enough to calculate statistical significance for these subgroup differences.
“But I think what is impressive is that over 50% of patients in all the races and ethnicities were able to achieve a 5% body weight loss, which is metabolically significant in terms of improving outcomes,” he said. “I think that’s a really important fact.” A smaller proportion – around 20% – lost at least 10% of body weight, mostly on high-dose semaglutide.
Severe hypoglycemia, as defined by American Diabetes Association classification, was very rare across trials, except that 4.7% of African Americans saw this adverse event on high-dose semaglutide. Incidence in other subgroups, at either dose, ranged from 0% to 2.4%.
Otherwise, the medication was generally well tolerated, though gastrointestinal side effects were seen. “Asian people have a little higher GI side effects – up to 50% of Asians did develop GI side effects, and between 10% and 13% of Asians had to stop medication due to side effects,” said Dr. Desouza. “So I think that would be the one caveat in terms of tolerance that we did learn.”
The SUSTAIN trials were sponsored by Novo Nordisk. Dr. Desouza has received consulting fees for Novo Nordisk and has received grant support from several other pharmaceutical companies. Two coauthors are Novo Nordisk employees.
SOURCE: Desouza C et al. AACE 2018, Abstract 298
REPORTING FROM AACE 2018
Suicides up 30%; risk factors go beyond diagnosed disorders
About 45,000 individuals in the United States took their own lives in 2016, and about half of them had no known mental health diagnosis at the time of death, based on data from the Centers for Disease Control and Prevention. Suicide rates rose by approximately 30% across all age groups up to age 75 years.
“Suicide is preventable; that’s why it is important to understand all the factors,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in a June 7 teleconference announcing the findings. Although mental health conditions often are seen as the cause of suicide, the results highlight the need to address other factors, including relationship problems, substance abuse, trouble with life transitions, and financial difficulties.
In a Vital Signs report published June 7, a team of CDC researchers led by Deborah M. Stone, ScD, reviewed data from suicide rates by state from 1999-2016. To examine the circumstances of suicide among individuals with and without mental health conditions, the researchers also reviewed data from the CDC’s National Violent Death Reporting System for 2015, which included 27 states.
Although rates increased among all age groups,.
Overall, 54% of the suicides in 2016 had no mental health diagnosis. Compared with those with a mental health diagnosis, those without a diagnosis were more likely to be male, part of an ethnic minority, and to have a history of homicide. In addition, those without known mental health conditions were more likely to have served in the military.
The most common causes of suicide were firearms, hanging/suffocation/strangulation, and poisoning.
Individuals without known mental health conditions were significantly more likely than those with mental health con-ditions to have used firearms (55% vs. 41%) and significantly less likely to die from hanging/suffocation/strangulation (27% vs. 31%) or poisoning (10% vs. 20%) in adjusted models, the researchers noted.
“If we only look at this as a mental health issue, we won’t make the progress that we need,” Dr. Schuchat said. She urged health professionals, community organizations, government organizations, and the public at large to learn to rec-ognize warning signs and factors that can lead to suicide.
To help achieve the national goal of a 20% reduction in the annual suicide rate by 2025, the CDC has developed a technical package of recommendations for policies, prevention strategies, and resources aimed at communities and states.
In addition, “Health care providers have an important role to play” to prevent those at risk for suicide from falling through the cracks, Dr. Schuchat said. She noted the importance of protocols for patient safety and support, and she stressed that health providers should be especially vigilant during times of life transition such as changes in relationship stages, leaving for college, retirement, financial insecurity, or the loss of a loved one.
“We don’t think we can just leave this to the mental health discipline,” Dr. Schuchat noted. “Preventing suicide takes everyone; everyone in the community can help by learning the warning signs,” she said.
[email protected]
SOURCE: Stone D et al. MMWR. 2018 Jun 7; 67(22):617-24
About 45,000 individuals in the United States took their own lives in 2016, and about half of them had no known mental health diagnosis at the time of death, based on data from the Centers for Disease Control and Prevention. Suicide rates rose by approximately 30% across all age groups up to age 75 years.
“Suicide is preventable; that’s why it is important to understand all the factors,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in a June 7 teleconference announcing the findings. Although mental health conditions often are seen as the cause of suicide, the results highlight the need to address other factors, including relationship problems, substance abuse, trouble with life transitions, and financial difficulties.
In a Vital Signs report published June 7, a team of CDC researchers led by Deborah M. Stone, ScD, reviewed data from suicide rates by state from 1999-2016. To examine the circumstances of suicide among individuals with and without mental health conditions, the researchers also reviewed data from the CDC’s National Violent Death Reporting System for 2015, which included 27 states.
Although rates increased among all age groups,.
Overall, 54% of the suicides in 2016 had no mental health diagnosis. Compared with those with a mental health diagnosis, those without a diagnosis were more likely to be male, part of an ethnic minority, and to have a history of homicide. In addition, those without known mental health conditions were more likely to have served in the military.
The most common causes of suicide were firearms, hanging/suffocation/strangulation, and poisoning.
Individuals without known mental health conditions were significantly more likely than those with mental health con-ditions to have used firearms (55% vs. 41%) and significantly less likely to die from hanging/suffocation/strangulation (27% vs. 31%) or poisoning (10% vs. 20%) in adjusted models, the researchers noted.
“If we only look at this as a mental health issue, we won’t make the progress that we need,” Dr. Schuchat said. She urged health professionals, community organizations, government organizations, and the public at large to learn to rec-ognize warning signs and factors that can lead to suicide.
To help achieve the national goal of a 20% reduction in the annual suicide rate by 2025, the CDC has developed a technical package of recommendations for policies, prevention strategies, and resources aimed at communities and states.
In addition, “Health care providers have an important role to play” to prevent those at risk for suicide from falling through the cracks, Dr. Schuchat said. She noted the importance of protocols for patient safety and support, and she stressed that health providers should be especially vigilant during times of life transition such as changes in relationship stages, leaving for college, retirement, financial insecurity, or the loss of a loved one.
“We don’t think we can just leave this to the mental health discipline,” Dr. Schuchat noted. “Preventing suicide takes everyone; everyone in the community can help by learning the warning signs,” she said.
[email protected]
SOURCE: Stone D et al. MMWR. 2018 Jun 7; 67(22):617-24
About 45,000 individuals in the United States took their own lives in 2016, and about half of them had no known mental health diagnosis at the time of death, based on data from the Centers for Disease Control and Prevention. Suicide rates rose by approximately 30% across all age groups up to age 75 years.
“Suicide is preventable; that’s why it is important to understand all the factors,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in a June 7 teleconference announcing the findings. Although mental health conditions often are seen as the cause of suicide, the results highlight the need to address other factors, including relationship problems, substance abuse, trouble with life transitions, and financial difficulties.
In a Vital Signs report published June 7, a team of CDC researchers led by Deborah M. Stone, ScD, reviewed data from suicide rates by state from 1999-2016. To examine the circumstances of suicide among individuals with and without mental health conditions, the researchers also reviewed data from the CDC’s National Violent Death Reporting System for 2015, which included 27 states.
Although rates increased among all age groups,.
Overall, 54% of the suicides in 2016 had no mental health diagnosis. Compared with those with a mental health diagnosis, those without a diagnosis were more likely to be male, part of an ethnic minority, and to have a history of homicide. In addition, those without known mental health conditions were more likely to have served in the military.
The most common causes of suicide were firearms, hanging/suffocation/strangulation, and poisoning.
Individuals without known mental health conditions were significantly more likely than those with mental health con-ditions to have used firearms (55% vs. 41%) and significantly less likely to die from hanging/suffocation/strangulation (27% vs. 31%) or poisoning (10% vs. 20%) in adjusted models, the researchers noted.
“If we only look at this as a mental health issue, we won’t make the progress that we need,” Dr. Schuchat said. She urged health professionals, community organizations, government organizations, and the public at large to learn to rec-ognize warning signs and factors that can lead to suicide.
To help achieve the national goal of a 20% reduction in the annual suicide rate by 2025, the CDC has developed a technical package of recommendations for policies, prevention strategies, and resources aimed at communities and states.
In addition, “Health care providers have an important role to play” to prevent those at risk for suicide from falling through the cracks, Dr. Schuchat said. She noted the importance of protocols for patient safety and support, and she stressed that health providers should be especially vigilant during times of life transition such as changes in relationship stages, leaving for college, retirement, financial insecurity, or the loss of a loved one.
“We don’t think we can just leave this to the mental health discipline,” Dr. Schuchat noted. “Preventing suicide takes everyone; everyone in the community can help by learning the warning signs,” she said.
[email protected]
SOURCE: Stone D et al. MMWR. 2018 Jun 7; 67(22):617-24
COA files lawsuit over budget sequestration
Part B drugs administered in the physician’s office are generally reimbursed at average sales price (ASP) plus 6%, a rate set in statute by the Medicare Modernization Act of 2003 (MMA). However, the Budget Control Act of 2011 put a 2% sequestration on the federal budget, effective April 1, 2013, and extended numerous times after, most recently in the Bipartisan Budget Act of 2018. This has the effect of lowering Part B drug reimbursement to ASP + 4.3%.
“We are filing to see an injunction against the United States Department of Health and Human Services and the Office of Management and Budget [OMB] because we have exhausted all possibilities in stopping what is an unconstitutional application of the 2% sequester cut to Part B drug reimbursement,” COA President Jeff Vacirca, MD, and Executive Director Ted Okon said in a May 30 letter to HHS Secretary Alex Azar alerting him to the legal action, which was filed on May 31.
Dr. Vacirca and Mr. Okon noted that they have met with “numerous HHS and OMB officials” to explain why they believe the sequestration is unconstitutional. In the court filing, they argue that the sequestration has “invaded the legislative sphere by effectively amending the MMA” and COA argues that the executive branch “cannot alter duly enacted legislation under the guise of executing the laws.
“Putting that aside, the Balanced Budget Act provides the ability for the Executive Branch to sequester up to 2% of funds for a [Budget Control Act] sequestration for Medicare Part B ‘services’ but it does not provide the same authority for Part B drugs and does not contain any express language indicating an intent that a sequestration can be applied to alter the MMA’s statutory formula.”
Despite raising this objection, “the response we have received at HHS and OMB reflects more on the current Administration’s views on the policy of Medicare Part B drug reimbursement and not on correcting the prior Administration’s unconstitutional application of the sequester,” the letter states. “As a result, we are left with no other option but to pursue legal action, something we have clearly communicated to both HHS and OMB on several occasions, as a last resort.”
COA notes in its 2018 Community Oncology Practice Report that since the sequester went into effect in 2013, around 135 independent community cancer clinics have closed and about 190 clinics have been acquired by hospitals.
“Shifting cancer care to the outpatient hospital setting costs all taxpayers, not just Medicare beneficiaries,” the letter states. “The actuarial firm Milliman estimated the shift from 2004 to 2014 to have cost Medicare $2 billion in just one year (2014) and, as a result, an estimated $500 million in the same year to senior beneficiaries responsible for the 20% coinsurance.”
Part B drugs administered in the physician’s office are generally reimbursed at average sales price (ASP) plus 6%, a rate set in statute by the Medicare Modernization Act of 2003 (MMA). However, the Budget Control Act of 2011 put a 2% sequestration on the federal budget, effective April 1, 2013, and extended numerous times after, most recently in the Bipartisan Budget Act of 2018. This has the effect of lowering Part B drug reimbursement to ASP + 4.3%.
“We are filing to see an injunction against the United States Department of Health and Human Services and the Office of Management and Budget [OMB] because we have exhausted all possibilities in stopping what is an unconstitutional application of the 2% sequester cut to Part B drug reimbursement,” COA President Jeff Vacirca, MD, and Executive Director Ted Okon said in a May 30 letter to HHS Secretary Alex Azar alerting him to the legal action, which was filed on May 31.
Dr. Vacirca and Mr. Okon noted that they have met with “numerous HHS and OMB officials” to explain why they believe the sequestration is unconstitutional. In the court filing, they argue that the sequestration has “invaded the legislative sphere by effectively amending the MMA” and COA argues that the executive branch “cannot alter duly enacted legislation under the guise of executing the laws.
“Putting that aside, the Balanced Budget Act provides the ability for the Executive Branch to sequester up to 2% of funds for a [Budget Control Act] sequestration for Medicare Part B ‘services’ but it does not provide the same authority for Part B drugs and does not contain any express language indicating an intent that a sequestration can be applied to alter the MMA’s statutory formula.”
Despite raising this objection, “the response we have received at HHS and OMB reflects more on the current Administration’s views on the policy of Medicare Part B drug reimbursement and not on correcting the prior Administration’s unconstitutional application of the sequester,” the letter states. “As a result, we are left with no other option but to pursue legal action, something we have clearly communicated to both HHS and OMB on several occasions, as a last resort.”
COA notes in its 2018 Community Oncology Practice Report that since the sequester went into effect in 2013, around 135 independent community cancer clinics have closed and about 190 clinics have been acquired by hospitals.
“Shifting cancer care to the outpatient hospital setting costs all taxpayers, not just Medicare beneficiaries,” the letter states. “The actuarial firm Milliman estimated the shift from 2004 to 2014 to have cost Medicare $2 billion in just one year (2014) and, as a result, an estimated $500 million in the same year to senior beneficiaries responsible for the 20% coinsurance.”
Part B drugs administered in the physician’s office are generally reimbursed at average sales price (ASP) plus 6%, a rate set in statute by the Medicare Modernization Act of 2003 (MMA). However, the Budget Control Act of 2011 put a 2% sequestration on the federal budget, effective April 1, 2013, and extended numerous times after, most recently in the Bipartisan Budget Act of 2018. This has the effect of lowering Part B drug reimbursement to ASP + 4.3%.
“We are filing to see an injunction against the United States Department of Health and Human Services and the Office of Management and Budget [OMB] because we have exhausted all possibilities in stopping what is an unconstitutional application of the 2% sequester cut to Part B drug reimbursement,” COA President Jeff Vacirca, MD, and Executive Director Ted Okon said in a May 30 letter to HHS Secretary Alex Azar alerting him to the legal action, which was filed on May 31.
Dr. Vacirca and Mr. Okon noted that they have met with “numerous HHS and OMB officials” to explain why they believe the sequestration is unconstitutional. In the court filing, they argue that the sequestration has “invaded the legislative sphere by effectively amending the MMA” and COA argues that the executive branch “cannot alter duly enacted legislation under the guise of executing the laws.
“Putting that aside, the Balanced Budget Act provides the ability for the Executive Branch to sequester up to 2% of funds for a [Budget Control Act] sequestration for Medicare Part B ‘services’ but it does not provide the same authority for Part B drugs and does not contain any express language indicating an intent that a sequestration can be applied to alter the MMA’s statutory formula.”
Despite raising this objection, “the response we have received at HHS and OMB reflects more on the current Administration’s views on the policy of Medicare Part B drug reimbursement and not on correcting the prior Administration’s unconstitutional application of the sequester,” the letter states. “As a result, we are left with no other option but to pursue legal action, something we have clearly communicated to both HHS and OMB on several occasions, as a last resort.”
COA notes in its 2018 Community Oncology Practice Report that since the sequester went into effect in 2013, around 135 independent community cancer clinics have closed and about 190 clinics have been acquired by hospitals.
“Shifting cancer care to the outpatient hospital setting costs all taxpayers, not just Medicare beneficiaries,” the letter states. “The actuarial firm Milliman estimated the shift from 2004 to 2014 to have cost Medicare $2 billion in just one year (2014) and, as a result, an estimated $500 million in the same year to senior beneficiaries responsible for the 20% coinsurance.”