User login
Oligoclonal Bands May Predict MS Relapses and Progression
NASHVILLE—Patients with multiple sclerosis (MS) and 10 or more oligoclonal bands (OCBs) in CSF may have significantly more clinical and radiographic relapses and clinical progression during short-term follow-up than those who have fewer OCBs, according to data described at the 2018 CMSC Annual Meeting. OCBs may have greater diagnostic weight in the future, and their quantity may be important to consider during the selection of disease-modifying therapies, said the investigators.
Data Suggest the Predictive Value of OCBs
OCBs in the CSF are a common laboratory abnormality in MS. More than 90% of patients with MS have this finding. Previous research has suggested that OCBs predict the likelihood of progressing from clinically isolated syndrome to clinically definite MS. When observed early in the disease course, OCBs indicate a worse prognosis. Reflecting this emerging research, the latest version of the McDonald Criteria for MS diagnosis has incorporated OCBs.
Yet the predictive value of OCBs has been incompletely explored, said Christopher Perrone, MD, a resident at the University of Pennsylvania in Philadelphia, and colleagues. “While most studies examine the presence or absence of OCBs with regard to prognosis, only a few small studies have investigated correlations between the number of OCBs on single disease metrics,” he added.
OCBs May Predict Need for Assistive Devices
In their retrospective study, Dr. Perrone and colleagues intended to examine relationships between the number of OCBs and markers of clinical and radiographic relapses and progression in two-year follow-up. They screened 1,270 patients receiving MS disease-modifying therapies for OCB testing. Further selection criteria included a diagnosis of relapsing-remitting MS and adherence to a DMT with two years of follow-up clinical visits and imaging. In all, 128 patients met the inclusion criteria.
The study’s primary outcome measures were clinical relapses (defined as the number of steroid prescriptions) and radiographic relapses (defined as the number of new lesions on MRI) at two-year follow-up. Secondary outcome measures were clinical progression (categorized as independent, cane, walker, or wheelchair) and radiographic progression (net changes in third ventricular width, lateral ventricular width, and cortical width). Unpaired, two-tailed t tests were used for comparative analyses.
At two years, the number of clinical relapses was significantly greater in patients with 10 or more OCBs, compared with patients with fewer than 10 OCBs. Similarly, patients with 10 or more OCBs were more likely to have radiographic relapses, with nearly twice the number of new lesions on MRI at two years, compared with patients with fewer than 10 OCBs. Although the investigators found no significant difference between groups at baseline in the use of an assistive device, within-subjects analysis demonstrated that the use of a new assistive device was more common for patients with 10 or more OCBs. While lateral ventricular width increased more in patients with 10 or more OCBs, changes in third ventricular width and cortical width were not significantly different between groups.
NASHVILLE—Patients with multiple sclerosis (MS) and 10 or more oligoclonal bands (OCBs) in CSF may have significantly more clinical and radiographic relapses and clinical progression during short-term follow-up than those who have fewer OCBs, according to data described at the 2018 CMSC Annual Meeting. OCBs may have greater diagnostic weight in the future, and their quantity may be important to consider during the selection of disease-modifying therapies, said the investigators.
Data Suggest the Predictive Value of OCBs
OCBs in the CSF are a common laboratory abnormality in MS. More than 90% of patients with MS have this finding. Previous research has suggested that OCBs predict the likelihood of progressing from clinically isolated syndrome to clinically definite MS. When observed early in the disease course, OCBs indicate a worse prognosis. Reflecting this emerging research, the latest version of the McDonald Criteria for MS diagnosis has incorporated OCBs.
Yet the predictive value of OCBs has been incompletely explored, said Christopher Perrone, MD, a resident at the University of Pennsylvania in Philadelphia, and colleagues. “While most studies examine the presence or absence of OCBs with regard to prognosis, only a few small studies have investigated correlations between the number of OCBs on single disease metrics,” he added.
OCBs May Predict Need for Assistive Devices
In their retrospective study, Dr. Perrone and colleagues intended to examine relationships between the number of OCBs and markers of clinical and radiographic relapses and progression in two-year follow-up. They screened 1,270 patients receiving MS disease-modifying therapies for OCB testing. Further selection criteria included a diagnosis of relapsing-remitting MS and adherence to a DMT with two years of follow-up clinical visits and imaging. In all, 128 patients met the inclusion criteria.
The study’s primary outcome measures were clinical relapses (defined as the number of steroid prescriptions) and radiographic relapses (defined as the number of new lesions on MRI) at two-year follow-up. Secondary outcome measures were clinical progression (categorized as independent, cane, walker, or wheelchair) and radiographic progression (net changes in third ventricular width, lateral ventricular width, and cortical width). Unpaired, two-tailed t tests were used for comparative analyses.
At two years, the number of clinical relapses was significantly greater in patients with 10 or more OCBs, compared with patients with fewer than 10 OCBs. Similarly, patients with 10 or more OCBs were more likely to have radiographic relapses, with nearly twice the number of new lesions on MRI at two years, compared with patients with fewer than 10 OCBs. Although the investigators found no significant difference between groups at baseline in the use of an assistive device, within-subjects analysis demonstrated that the use of a new assistive device was more common for patients with 10 or more OCBs. While lateral ventricular width increased more in patients with 10 or more OCBs, changes in third ventricular width and cortical width were not significantly different between groups.
NASHVILLE—Patients with multiple sclerosis (MS) and 10 or more oligoclonal bands (OCBs) in CSF may have significantly more clinical and radiographic relapses and clinical progression during short-term follow-up than those who have fewer OCBs, according to data described at the 2018 CMSC Annual Meeting. OCBs may have greater diagnostic weight in the future, and their quantity may be important to consider during the selection of disease-modifying therapies, said the investigators.
Data Suggest the Predictive Value of OCBs
OCBs in the CSF are a common laboratory abnormality in MS. More than 90% of patients with MS have this finding. Previous research has suggested that OCBs predict the likelihood of progressing from clinically isolated syndrome to clinically definite MS. When observed early in the disease course, OCBs indicate a worse prognosis. Reflecting this emerging research, the latest version of the McDonald Criteria for MS diagnosis has incorporated OCBs.
Yet the predictive value of OCBs has been incompletely explored, said Christopher Perrone, MD, a resident at the University of Pennsylvania in Philadelphia, and colleagues. “While most studies examine the presence or absence of OCBs with regard to prognosis, only a few small studies have investigated correlations between the number of OCBs on single disease metrics,” he added.
OCBs May Predict Need for Assistive Devices
In their retrospective study, Dr. Perrone and colleagues intended to examine relationships between the number of OCBs and markers of clinical and radiographic relapses and progression in two-year follow-up. They screened 1,270 patients receiving MS disease-modifying therapies for OCB testing. Further selection criteria included a diagnosis of relapsing-remitting MS and adherence to a DMT with two years of follow-up clinical visits and imaging. In all, 128 patients met the inclusion criteria.
The study’s primary outcome measures were clinical relapses (defined as the number of steroid prescriptions) and radiographic relapses (defined as the number of new lesions on MRI) at two-year follow-up. Secondary outcome measures were clinical progression (categorized as independent, cane, walker, or wheelchair) and radiographic progression (net changes in third ventricular width, lateral ventricular width, and cortical width). Unpaired, two-tailed t tests were used for comparative analyses.
At two years, the number of clinical relapses was significantly greater in patients with 10 or more OCBs, compared with patients with fewer than 10 OCBs. Similarly, patients with 10 or more OCBs were more likely to have radiographic relapses, with nearly twice the number of new lesions on MRI at two years, compared with patients with fewer than 10 OCBs. Although the investigators found no significant difference between groups at baseline in the use of an assistive device, within-subjects analysis demonstrated that the use of a new assistive device was more common for patients with 10 or more OCBs. While lateral ventricular width increased more in patients with 10 or more OCBs, changes in third ventricular width and cortical width were not significantly different between groups.
Rapid Foot-Tapping Task Distinguishes Between MS Subtypes
NASHVILLE—A rapid foot-tapping task can distinguish between healthy controls and people with multiple sclerosis (MS), as well as between MS subtypes, according to a study presented at the 2018 CMSC Annual Meeting. “The associations between foot-tap speed and mobility measures such as the Timed Up and Go [TUG] test and the 25-Foot Walk test [25FWT] suggest that rapid foot tapping may be a useful marker for tracking or predicting progression of mobility dysfunction in people with MS, regardless of their ability to ambulate,” said Sumire Sato, a graduate student in the Neuroscience and Behavior Program at the University of Massachusetts Amherst, and colleagues.
The TUG test and the 25FWT are common clinical measurements that require ambulation and are used to assess mobility in people with MS. Not all people with MS are ambulatory, however. Preliminary, unpublished work by Ms. Sato and colleagues suggests that while the TUG test and the 25FWT can distinguish individuals with MS from controls without MS, they cannot distinguish between nonprogressive (MS-NP) and progressive (MS-P) MS subtypes. “Therefore, there is a critical need to identify a sensitive and nonambulatory task that can distinguish MS subtypes,” said the investigators.
Ms. Sato and colleagues recruited 30 participants with MS-NP, 30 participants with MS-P, and 17 age- and sex-matched controls for a study to determine whether rapid foot tapping ability can distinguish people with MS from controls and between MS subtypes. Each participant wore inertial sensors (manufactured by APDM) on the foot to measure angular velocity and acceleration. Participants were instructed to tap one foot as fast as possible for 10 seconds. Participants performed three trials on each foot while seated with self-selected knee and ankle angle.
The researchers analyzed sensor data using a custom MATLAB program that identified taps as acceleration peaks that occurred after every other zero-crossing of angular velocity. They administered the TUG test and 25FWT to participants to compare mobility to rapid foot tapping. Analysis of variance was used to analyze main effects of group and to make pairwise comparisons between groups. Ms. Sato and colleagues evaluated associations between foot tap count and mobility measures in MS groups using Spearman’s rho.
The researchers observed a main effect of group for foot-tap count, such that tap count differed between controls and participants with MS-NP and MS-P. The tap count also differed between participants with MS-NP and those with MS-P. Foot-tap count was negatively correlated with the 25FWT and the TUG test, thus indicating an association between the slowing of tapping speed and mobility measures.
NASHVILLE—A rapid foot-tapping task can distinguish between healthy controls and people with multiple sclerosis (MS), as well as between MS subtypes, according to a study presented at the 2018 CMSC Annual Meeting. “The associations between foot-tap speed and mobility measures such as the Timed Up and Go [TUG] test and the 25-Foot Walk test [25FWT] suggest that rapid foot tapping may be a useful marker for tracking or predicting progression of mobility dysfunction in people with MS, regardless of their ability to ambulate,” said Sumire Sato, a graduate student in the Neuroscience and Behavior Program at the University of Massachusetts Amherst, and colleagues.
The TUG test and the 25FWT are common clinical measurements that require ambulation and are used to assess mobility in people with MS. Not all people with MS are ambulatory, however. Preliminary, unpublished work by Ms. Sato and colleagues suggests that while the TUG test and the 25FWT can distinguish individuals with MS from controls without MS, they cannot distinguish between nonprogressive (MS-NP) and progressive (MS-P) MS subtypes. “Therefore, there is a critical need to identify a sensitive and nonambulatory task that can distinguish MS subtypes,” said the investigators.
Ms. Sato and colleagues recruited 30 participants with MS-NP, 30 participants with MS-P, and 17 age- and sex-matched controls for a study to determine whether rapid foot tapping ability can distinguish people with MS from controls and between MS subtypes. Each participant wore inertial sensors (manufactured by APDM) on the foot to measure angular velocity and acceleration. Participants were instructed to tap one foot as fast as possible for 10 seconds. Participants performed three trials on each foot while seated with self-selected knee and ankle angle.
The researchers analyzed sensor data using a custom MATLAB program that identified taps as acceleration peaks that occurred after every other zero-crossing of angular velocity. They administered the TUG test and 25FWT to participants to compare mobility to rapid foot tapping. Analysis of variance was used to analyze main effects of group and to make pairwise comparisons between groups. Ms. Sato and colleagues evaluated associations between foot tap count and mobility measures in MS groups using Spearman’s rho.
The researchers observed a main effect of group for foot-tap count, such that tap count differed between controls and participants with MS-NP and MS-P. The tap count also differed between participants with MS-NP and those with MS-P. Foot-tap count was negatively correlated with the 25FWT and the TUG test, thus indicating an association between the slowing of tapping speed and mobility measures.
NASHVILLE—A rapid foot-tapping task can distinguish between healthy controls and people with multiple sclerosis (MS), as well as between MS subtypes, according to a study presented at the 2018 CMSC Annual Meeting. “The associations between foot-tap speed and mobility measures such as the Timed Up and Go [TUG] test and the 25-Foot Walk test [25FWT] suggest that rapid foot tapping may be a useful marker for tracking or predicting progression of mobility dysfunction in people with MS, regardless of their ability to ambulate,” said Sumire Sato, a graduate student in the Neuroscience and Behavior Program at the University of Massachusetts Amherst, and colleagues.
The TUG test and the 25FWT are common clinical measurements that require ambulation and are used to assess mobility in people with MS. Not all people with MS are ambulatory, however. Preliminary, unpublished work by Ms. Sato and colleagues suggests that while the TUG test and the 25FWT can distinguish individuals with MS from controls without MS, they cannot distinguish between nonprogressive (MS-NP) and progressive (MS-P) MS subtypes. “Therefore, there is a critical need to identify a sensitive and nonambulatory task that can distinguish MS subtypes,” said the investigators.
Ms. Sato and colleagues recruited 30 participants with MS-NP, 30 participants with MS-P, and 17 age- and sex-matched controls for a study to determine whether rapid foot tapping ability can distinguish people with MS from controls and between MS subtypes. Each participant wore inertial sensors (manufactured by APDM) on the foot to measure angular velocity and acceleration. Participants were instructed to tap one foot as fast as possible for 10 seconds. Participants performed three trials on each foot while seated with self-selected knee and ankle angle.
The researchers analyzed sensor data using a custom MATLAB program that identified taps as acceleration peaks that occurred after every other zero-crossing of angular velocity. They administered the TUG test and 25FWT to participants to compare mobility to rapid foot tapping. Analysis of variance was used to analyze main effects of group and to make pairwise comparisons between groups. Ms. Sato and colleagues evaluated associations between foot tap count and mobility measures in MS groups using Spearman’s rho.
The researchers observed a main effect of group for foot-tap count, such that tap count differed between controls and participants with MS-NP and MS-P. The tap count also differed between participants with MS-NP and those with MS-P. Foot-tap count was negatively correlated with the 25FWT and the TUG test, thus indicating an association between the slowing of tapping speed and mobility measures.
Value of alemtuzumab demonstrated in RRMS patients with prior IFNB-1a treatment
LOS ANGELES – Patients with multiple sclerosis in the CARE-MS II study who switched from interferon beta-1a therapy to the humanized monoclonal antibody alemtuzumab experienced continued reductions in brain volume loss and lesions on MRI through 5 years, according to follow-up data from the CARE-MS II extension study known as TOPAZ.
These outcomes support core findings from the CARE-MS II study, and suggest that alemtuzumab (Lemtrada) provides a unique treatment approach for patients with prior subcutaneous interferon beta-1a (IFNB-1a) treatment, Daniel Pelletier, MD, reported in a poster discussion session at the annual meeting of the American Academy of Neurology.
In CARE-MS II, relapsing-remitting multiple sclerosis patients with inadequate response to prior therapy experienced improvements in MRI lesions and brain volume loss with two courses of alemtuzumab versus IFNB-1a through 2 years, and in a 4-year extension in which participants discontinued subcutaneous IFNB-1a and initiated alemtuzumab at 12 mg/day, they experienced durable efficacy in the absence of continuous treatment, explained Dr. Pelletier, a professor of neurology at the University of Southern California, Los Angeles.
In the extension, patients could receive alemtuzumab retreatment as needed for relapse/MRI activity or receive other disease-modifying therapies at the investigator’s discretion. Patients completing the extension could enter the 5-year TOPAZ study for further evaluation, he said.
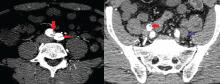
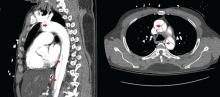
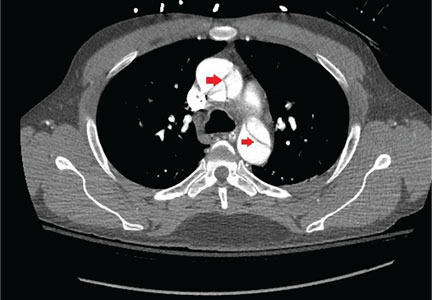
Of 119 patients who completed TOPAZ year 1, and thus had 5 years of follow-up after initiating alemtuzumab, 78% were free of new, gadolinium-enhancing lesions in IFNB-1a year 2; this increased significantly to 92% in post-alemtuzumab year 2, and remained high at 85%-89% in years 3-5. Additionally, 48% of patients were free of new/enlarging T2 lesions in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2 and remained high in years 3-5.
Further, 47% of the TOPAZ patients were MRI disease activity–free in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2, and remained high at 67%-72% in years 3-5.
“Perhaps for me what makes even more sense is looking at the yearly brain parenchymal fraction change,” Dr. Pelletier said, noting that the median annual reductions in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%, compared with –0.33% for subcutaneous IFNB-1a at year 2 in CARE-MS II. The median brain parenchymal fraction change from baseline to post-alemtuzumab year 5 was –1.40%.
In 61% of patients, no further treatment was given after the second course of alemtuzumab.
The findings suggest that alemtuzumab provides durable efficacy without continuous treatment, he concluded.
This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
SOURCE: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
LOS ANGELES – Patients with multiple sclerosis in the CARE-MS II study who switched from interferon beta-1a therapy to the humanized monoclonal antibody alemtuzumab experienced continued reductions in brain volume loss and lesions on MRI through 5 years, according to follow-up data from the CARE-MS II extension study known as TOPAZ.
These outcomes support core findings from the CARE-MS II study, and suggest that alemtuzumab (Lemtrada) provides a unique treatment approach for patients with prior subcutaneous interferon beta-1a (IFNB-1a) treatment, Daniel Pelletier, MD, reported in a poster discussion session at the annual meeting of the American Academy of Neurology.
In CARE-MS II, relapsing-remitting multiple sclerosis patients with inadequate response to prior therapy experienced improvements in MRI lesions and brain volume loss with two courses of alemtuzumab versus IFNB-1a through 2 years, and in a 4-year extension in which participants discontinued subcutaneous IFNB-1a and initiated alemtuzumab at 12 mg/day, they experienced durable efficacy in the absence of continuous treatment, explained Dr. Pelletier, a professor of neurology at the University of Southern California, Los Angeles.
In the extension, patients could receive alemtuzumab retreatment as needed for relapse/MRI activity or receive other disease-modifying therapies at the investigator’s discretion. Patients completing the extension could enter the 5-year TOPAZ study for further evaluation, he said.
Of 119 patients who completed TOPAZ year 1, and thus had 5 years of follow-up after initiating alemtuzumab, 78% were free of new, gadolinium-enhancing lesions in IFNB-1a year 2; this increased significantly to 92% in post-alemtuzumab year 2, and remained high at 85%-89% in years 3-5. Additionally, 48% of patients were free of new/enlarging T2 lesions in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2 and remained high in years 3-5.
Further, 47% of the TOPAZ patients were MRI disease activity–free in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2, and remained high at 67%-72% in years 3-5.
“Perhaps for me what makes even more sense is looking at the yearly brain parenchymal fraction change,” Dr. Pelletier said, noting that the median annual reductions in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%, compared with –0.33% for subcutaneous IFNB-1a at year 2 in CARE-MS II. The median brain parenchymal fraction change from baseline to post-alemtuzumab year 5 was –1.40%.
In 61% of patients, no further treatment was given after the second course of alemtuzumab.
The findings suggest that alemtuzumab provides durable efficacy without continuous treatment, he concluded.
This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
SOURCE: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
LOS ANGELES – Patients with multiple sclerosis in the CARE-MS II study who switched from interferon beta-1a therapy to the humanized monoclonal antibody alemtuzumab experienced continued reductions in brain volume loss and lesions on MRI through 5 years, according to follow-up data from the CARE-MS II extension study known as TOPAZ.
These outcomes support core findings from the CARE-MS II study, and suggest that alemtuzumab (Lemtrada) provides a unique treatment approach for patients with prior subcutaneous interferon beta-1a (IFNB-1a) treatment, Daniel Pelletier, MD, reported in a poster discussion session at the annual meeting of the American Academy of Neurology.
In CARE-MS II, relapsing-remitting multiple sclerosis patients with inadequate response to prior therapy experienced improvements in MRI lesions and brain volume loss with two courses of alemtuzumab versus IFNB-1a through 2 years, and in a 4-year extension in which participants discontinued subcutaneous IFNB-1a and initiated alemtuzumab at 12 mg/day, they experienced durable efficacy in the absence of continuous treatment, explained Dr. Pelletier, a professor of neurology at the University of Southern California, Los Angeles.
In the extension, patients could receive alemtuzumab retreatment as needed for relapse/MRI activity or receive other disease-modifying therapies at the investigator’s discretion. Patients completing the extension could enter the 5-year TOPAZ study for further evaluation, he said.
Of 119 patients who completed TOPAZ year 1, and thus had 5 years of follow-up after initiating alemtuzumab, 78% were free of new, gadolinium-enhancing lesions in IFNB-1a year 2; this increased significantly to 92% in post-alemtuzumab year 2, and remained high at 85%-89% in years 3-5. Additionally, 48% of patients were free of new/enlarging T2 lesions in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2 and remained high in years 3-5.
Further, 47% of the TOPAZ patients were MRI disease activity–free in IFNB-1a year 2; this increased significantly to 81% in post-alemtuzumab year 2, and remained high at 67%-72% in years 3-5.
“Perhaps for me what makes even more sense is looking at the yearly brain parenchymal fraction change,” Dr. Pelletier said, noting that the median annual reductions in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%, compared with –0.33% for subcutaneous IFNB-1a at year 2 in CARE-MS II. The median brain parenchymal fraction change from baseline to post-alemtuzumab year 5 was –1.40%.
In 61% of patients, no further treatment was given after the second course of alemtuzumab.
The findings suggest that alemtuzumab provides durable efficacy without continuous treatment, he concluded.
This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
SOURCE: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
REPORTING FROM AAN 2018
Key clinical point: Alemtuzumab appears to provide durable efficacy without continuous treatment.
Major finding: Brain parenchymal fraction reductions with alemtuzumab in years 1-5, respectively, were 0.02%, 0.04%, 0.15%, 0.14%, and 0.08%.
Study details: A total of 119 patients who completed CARE-MS II and its 4-year extension, as well as 1 year of the TOPAZ study.
Disclosures: This study was supported by Sanofi and Bayer Healthcare Pharmaceuticals. Dr. Pelletier has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Merck Serono, Novartis, Roche, and Sanofi, as well as research support from Biogen, Merck Serono, Novartis, Roche, and Sanofi.
Source: Pelletier D et al. Neurology. 2018 Apr 10. 90(15 Suppl.):P5.031.
Research on exercise in MS needs to build up some muscle
NASHVILLE, TENN. – Physical activity appears to have profound rehabilitative effects – both physical and cognitive – upon patients with multiple sclerosis, but the body of evidence remains largely based on small, sometimes problematic studies, Alan Thompson, MD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
There are compelling animal data that exercise promotes a number of beneficial neuronal changes that improve patient-reported outcomes, said Dr. Thompson, the Garfield Weston Professor of Clinical Neurology and Neurorehabilitation at University College London (England).
“A lot of animal work suggests that exercise can have a major impact on repair and recovery in neurons, synaptic signaling, dendritic branching, long-term potentiation,” and can beneficially affect inflammation and demyelination, he said. Besides the direct effect on nerves, exercise builds up muscle mass, strengthens connective tissue, improves movement, and reduces cardiovascular risk. “Exercise improves inactivity, but also may improve the underlying disease process,” he said. “The effect can be quite profound, and we are building a very good evidence base to support the use of exercise in MS.”
Unfortunately, the existing body of literature remains unimpressive, Dr. Thompson admitted. He compared research in physical activity to that of medicinal therapeutics. Disease-modifying therapeutics research is highly regulated, very well funded, adequately powered and replicated, and – once it shows positive results – receives substantial marketing and sales effort. “As a result, there can be a substantial impact on care.”
Research on rehabilitation and symptom management, with physical activity and other similar interventions, is not well funded, relies on diverse outcome measures, has small cohort numbers, and often is unreplicated. Even positive results “are just left to lie there,” he said. “Thus, it has a modest impact on care. I would like to see equal resources for both research areas.”
The recent surge in stroke rehabilitation is an excellent example of how academic focus can change practice for neurologic illness, he said. A 2017 research letter in Lancet Neurology described the current state of research on exercise in MS (Lancet Neurol. 2017;16[10];848-56). An accompanying editorial compared the MS literature to that in stroke (Lancet Neurol. 2017;16[10]:768-9).
During 1990-2005, there were almost no clinical studies in rehabilitation for stroke, Parkinson’s disease, spinal cord injury, and MS. Around 2005, things began to change in stroke, with close to 60 publications in just 1 year. During 2010-2015, the pace of research accelerated dramatically. Researchers, clinicians, and patients began to see the immediate and long-term benefits of early poststroke rehabilitation. These interventions have now been encoded in practice guidelines and are a core part of clinical care, Dr. Thompson said.
The picture in MS, Parkinson’s, and spinal cord injury remained almost unchanged, although there has been a very slight increase in these studies since 2010.
“We are way behind the stroke research,” Dr. Thompson said. “We need global collaboration to correct this.”
That may be coming. Dr. Thompson described a newly minted, multinational study sponsored by the Canadian Multiple Sclerosis Society. The four-armed “Improving Cognition in People with Progressive MS” study will determine whether cognitive rehabilitation and exercise are effective treatments for cognitive dysfunction in people with progressive MS. It seeks to enroll 360 patients in six countries. They will be randomized to a wait list, exercise plus cognitive rehabilitation, exercise only, or cognitive rehabilitation only.
The primary investigator is Anthony Feinstein, MBBCh, PhD, a psychiatrist at the University of Toronto.
Dr. Thompson had no disclosures relevant to his discussion.
NASHVILLE, TENN. – Physical activity appears to have profound rehabilitative effects – both physical and cognitive – upon patients with multiple sclerosis, but the body of evidence remains largely based on small, sometimes problematic studies, Alan Thompson, MD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
There are compelling animal data that exercise promotes a number of beneficial neuronal changes that improve patient-reported outcomes, said Dr. Thompson, the Garfield Weston Professor of Clinical Neurology and Neurorehabilitation at University College London (England).
“A lot of animal work suggests that exercise can have a major impact on repair and recovery in neurons, synaptic signaling, dendritic branching, long-term potentiation,” and can beneficially affect inflammation and demyelination, he said. Besides the direct effect on nerves, exercise builds up muscle mass, strengthens connective tissue, improves movement, and reduces cardiovascular risk. “Exercise improves inactivity, but also may improve the underlying disease process,” he said. “The effect can be quite profound, and we are building a very good evidence base to support the use of exercise in MS.”
Unfortunately, the existing body of literature remains unimpressive, Dr. Thompson admitted. He compared research in physical activity to that of medicinal therapeutics. Disease-modifying therapeutics research is highly regulated, very well funded, adequately powered and replicated, and – once it shows positive results – receives substantial marketing and sales effort. “As a result, there can be a substantial impact on care.”
Research on rehabilitation and symptom management, with physical activity and other similar interventions, is not well funded, relies on diverse outcome measures, has small cohort numbers, and often is unreplicated. Even positive results “are just left to lie there,” he said. “Thus, it has a modest impact on care. I would like to see equal resources for both research areas.”
The recent surge in stroke rehabilitation is an excellent example of how academic focus can change practice for neurologic illness, he said. A 2017 research letter in Lancet Neurology described the current state of research on exercise in MS (Lancet Neurol. 2017;16[10];848-56). An accompanying editorial compared the MS literature to that in stroke (Lancet Neurol. 2017;16[10]:768-9).
During 1990-2005, there were almost no clinical studies in rehabilitation for stroke, Parkinson’s disease, spinal cord injury, and MS. Around 2005, things began to change in stroke, with close to 60 publications in just 1 year. During 2010-2015, the pace of research accelerated dramatically. Researchers, clinicians, and patients began to see the immediate and long-term benefits of early poststroke rehabilitation. These interventions have now been encoded in practice guidelines and are a core part of clinical care, Dr. Thompson said.
The picture in MS, Parkinson’s, and spinal cord injury remained almost unchanged, although there has been a very slight increase in these studies since 2010.
“We are way behind the stroke research,” Dr. Thompson said. “We need global collaboration to correct this.”
That may be coming. Dr. Thompson described a newly minted, multinational study sponsored by the Canadian Multiple Sclerosis Society. The four-armed “Improving Cognition in People with Progressive MS” study will determine whether cognitive rehabilitation and exercise are effective treatments for cognitive dysfunction in people with progressive MS. It seeks to enroll 360 patients in six countries. They will be randomized to a wait list, exercise plus cognitive rehabilitation, exercise only, or cognitive rehabilitation only.
The primary investigator is Anthony Feinstein, MBBCh, PhD, a psychiatrist at the University of Toronto.
Dr. Thompson had no disclosures relevant to his discussion.
NASHVILLE, TENN. – Physical activity appears to have profound rehabilitative effects – both physical and cognitive – upon patients with multiple sclerosis, but the body of evidence remains largely based on small, sometimes problematic studies, Alan Thompson, MD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
There are compelling animal data that exercise promotes a number of beneficial neuronal changes that improve patient-reported outcomes, said Dr. Thompson, the Garfield Weston Professor of Clinical Neurology and Neurorehabilitation at University College London (England).
“A lot of animal work suggests that exercise can have a major impact on repair and recovery in neurons, synaptic signaling, dendritic branching, long-term potentiation,” and can beneficially affect inflammation and demyelination, he said. Besides the direct effect on nerves, exercise builds up muscle mass, strengthens connective tissue, improves movement, and reduces cardiovascular risk. “Exercise improves inactivity, but also may improve the underlying disease process,” he said. “The effect can be quite profound, and we are building a very good evidence base to support the use of exercise in MS.”
Unfortunately, the existing body of literature remains unimpressive, Dr. Thompson admitted. He compared research in physical activity to that of medicinal therapeutics. Disease-modifying therapeutics research is highly regulated, very well funded, adequately powered and replicated, and – once it shows positive results – receives substantial marketing and sales effort. “As a result, there can be a substantial impact on care.”
Research on rehabilitation and symptom management, with physical activity and other similar interventions, is not well funded, relies on diverse outcome measures, has small cohort numbers, and often is unreplicated. Even positive results “are just left to lie there,” he said. “Thus, it has a modest impact on care. I would like to see equal resources for both research areas.”
The recent surge in stroke rehabilitation is an excellent example of how academic focus can change practice for neurologic illness, he said. A 2017 research letter in Lancet Neurology described the current state of research on exercise in MS (Lancet Neurol. 2017;16[10];848-56). An accompanying editorial compared the MS literature to that in stroke (Lancet Neurol. 2017;16[10]:768-9).
During 1990-2005, there were almost no clinical studies in rehabilitation for stroke, Parkinson’s disease, spinal cord injury, and MS. Around 2005, things began to change in stroke, with close to 60 publications in just 1 year. During 2010-2015, the pace of research accelerated dramatically. Researchers, clinicians, and patients began to see the immediate and long-term benefits of early poststroke rehabilitation. These interventions have now been encoded in practice guidelines and are a core part of clinical care, Dr. Thompson said.
The picture in MS, Parkinson’s, and spinal cord injury remained almost unchanged, although there has been a very slight increase in these studies since 2010.
“We are way behind the stroke research,” Dr. Thompson said. “We need global collaboration to correct this.”
That may be coming. Dr. Thompson described a newly minted, multinational study sponsored by the Canadian Multiple Sclerosis Society. The four-armed “Improving Cognition in People with Progressive MS” study will determine whether cognitive rehabilitation and exercise are effective treatments for cognitive dysfunction in people with progressive MS. It seeks to enroll 360 patients in six countries. They will be randomized to a wait list, exercise plus cognitive rehabilitation, exercise only, or cognitive rehabilitation only.
The primary investigator is Anthony Feinstein, MBBCh, PhD, a psychiatrist at the University of Toronto.
Dr. Thompson had no disclosures relevant to his discussion.
REPORTING FROM THE CMSC ANNUAL MEETING
In CRC patients, chemo yields more toxicities in women than in men
Compared with men, women receiving fluorouracil-based chemotherapy for colorectal cancer had higher rates of treatment-emergent adverse events, a retrospective analysis shows.
Women had statistically significant and clinically relevant increased risks of multiple hematologic and nonhematologic toxicities in the analysis, which was based on data from the PETACC-3 trial conducted by the EORTC Gastrointestinal Group.
The findings suggest that drug targets may be different between women and men, as may be the optimal doses needed to hit those targets with acceptable levels of adverse events, said Valerie Cristina, MD, of Lausanne (Switzerland) University Hospital, and her coinvestigators.
“In an age of personalized medicine, and also considering growing knowledge about sex-related differences in molecular profiles and disease biology, the potential effect of sex on efficacy and toxic effects of systemic treatments in oncology deserves more awareness and further investigation,” the researchers wrote. The report was published in JAMA Oncology.
Patients in this retrospective study had stage II-III colorectal cancer and had received treatment with either adjuvant fluorouracil/leucovorin or leucovorin, fluorouracil, and irinotecan (FOLFIRI). Of 2,974 patients, 1,656 (55.7%) were men and 1,318 (44.3%) were women.
The primary analysis in the study was a comparison by sex of treatment-emergent adverse events of any grade. The investigators found women had significantly higher rates of all-grade alopecia, anemia, cholinergic syndrome, constipation, cramping, lethargy, leukopenia, nausea, neutropenia, stomatitis, and vomiting.
Significant differences were reported for hematologic adverse events such as leukopenia of any grade, which was seen in 49.6% of women and 38.9% of men (P less than .001), and nonhematologic adverse events, such as nausea of any grade, seen in 61.8% of women and 53.7% of men (P less than .001).
More serious toxicities (grade 3 or 4) occurring significantly more often in women were alopecia, anemia, diarrhea, leukopenia, nausea, neutropenia, and stomatitis, according to the report.
Treatment with FOLFIRI was associated with higher rates of toxicity overall, and numerically increased differences in incidence between women and men, the investigators said. They noted that incidence of grade 3 or 4 alopecia, diarrhea, lethargy, and stomatitis were all significantly higher among FOLFIRI-treated women.
This was the largest systematic analysis of sex-related differences in adverse effects related to standard fluorouracil with or without irinotecan, according to the researchers, who noted that a previous study had identified female sex as a risk factor for irinotecan-induced neutropenia.
Dr. Cristina had no conflicts of interest to disclose. Coauthors reported disclosures related to Amgen, Bayer, Boehringer, Bristol-Myers Squibb, Celgene, Ipsen, Lilly, Merck, Merck KgA, Novartis, Pfizer, Roche, Sanofi, Servier, and Shire.
SOURCE: Cristina V et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1080.
Compared with men, women receiving fluorouracil-based chemotherapy for colorectal cancer had higher rates of treatment-emergent adverse events, a retrospective analysis shows.
Women had statistically significant and clinically relevant increased risks of multiple hematologic and nonhematologic toxicities in the analysis, which was based on data from the PETACC-3 trial conducted by the EORTC Gastrointestinal Group.
The findings suggest that drug targets may be different between women and men, as may be the optimal doses needed to hit those targets with acceptable levels of adverse events, said Valerie Cristina, MD, of Lausanne (Switzerland) University Hospital, and her coinvestigators.
“In an age of personalized medicine, and also considering growing knowledge about sex-related differences in molecular profiles and disease biology, the potential effect of sex on efficacy and toxic effects of systemic treatments in oncology deserves more awareness and further investigation,” the researchers wrote. The report was published in JAMA Oncology.
Patients in this retrospective study had stage II-III colorectal cancer and had received treatment with either adjuvant fluorouracil/leucovorin or leucovorin, fluorouracil, and irinotecan (FOLFIRI). Of 2,974 patients, 1,656 (55.7%) were men and 1,318 (44.3%) were women.
The primary analysis in the study was a comparison by sex of treatment-emergent adverse events of any grade. The investigators found women had significantly higher rates of all-grade alopecia, anemia, cholinergic syndrome, constipation, cramping, lethargy, leukopenia, nausea, neutropenia, stomatitis, and vomiting.
Significant differences were reported for hematologic adverse events such as leukopenia of any grade, which was seen in 49.6% of women and 38.9% of men (P less than .001), and nonhematologic adverse events, such as nausea of any grade, seen in 61.8% of women and 53.7% of men (P less than .001).
More serious toxicities (grade 3 or 4) occurring significantly more often in women were alopecia, anemia, diarrhea, leukopenia, nausea, neutropenia, and stomatitis, according to the report.
Treatment with FOLFIRI was associated with higher rates of toxicity overall, and numerically increased differences in incidence between women and men, the investigators said. They noted that incidence of grade 3 or 4 alopecia, diarrhea, lethargy, and stomatitis were all significantly higher among FOLFIRI-treated women.
This was the largest systematic analysis of sex-related differences in adverse effects related to standard fluorouracil with or without irinotecan, according to the researchers, who noted that a previous study had identified female sex as a risk factor for irinotecan-induced neutropenia.
Dr. Cristina had no conflicts of interest to disclose. Coauthors reported disclosures related to Amgen, Bayer, Boehringer, Bristol-Myers Squibb, Celgene, Ipsen, Lilly, Merck, Merck KgA, Novartis, Pfizer, Roche, Sanofi, Servier, and Shire.
SOURCE: Cristina V et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1080.
Compared with men, women receiving fluorouracil-based chemotherapy for colorectal cancer had higher rates of treatment-emergent adverse events, a retrospective analysis shows.
Women had statistically significant and clinically relevant increased risks of multiple hematologic and nonhematologic toxicities in the analysis, which was based on data from the PETACC-3 trial conducted by the EORTC Gastrointestinal Group.
The findings suggest that drug targets may be different between women and men, as may be the optimal doses needed to hit those targets with acceptable levels of adverse events, said Valerie Cristina, MD, of Lausanne (Switzerland) University Hospital, and her coinvestigators.
“In an age of personalized medicine, and also considering growing knowledge about sex-related differences in molecular profiles and disease biology, the potential effect of sex on efficacy and toxic effects of systemic treatments in oncology deserves more awareness and further investigation,” the researchers wrote. The report was published in JAMA Oncology.
Patients in this retrospective study had stage II-III colorectal cancer and had received treatment with either adjuvant fluorouracil/leucovorin or leucovorin, fluorouracil, and irinotecan (FOLFIRI). Of 2,974 patients, 1,656 (55.7%) were men and 1,318 (44.3%) were women.
The primary analysis in the study was a comparison by sex of treatment-emergent adverse events of any grade. The investigators found women had significantly higher rates of all-grade alopecia, anemia, cholinergic syndrome, constipation, cramping, lethargy, leukopenia, nausea, neutropenia, stomatitis, and vomiting.
Significant differences were reported for hematologic adverse events such as leukopenia of any grade, which was seen in 49.6% of women and 38.9% of men (P less than .001), and nonhematologic adverse events, such as nausea of any grade, seen in 61.8% of women and 53.7% of men (P less than .001).
More serious toxicities (grade 3 or 4) occurring significantly more often in women were alopecia, anemia, diarrhea, leukopenia, nausea, neutropenia, and stomatitis, according to the report.
Treatment with FOLFIRI was associated with higher rates of toxicity overall, and numerically increased differences in incidence between women and men, the investigators said. They noted that incidence of grade 3 or 4 alopecia, diarrhea, lethargy, and stomatitis were all significantly higher among FOLFIRI-treated women.
This was the largest systematic analysis of sex-related differences in adverse effects related to standard fluorouracil with or without irinotecan, according to the researchers, who noted that a previous study had identified female sex as a risk factor for irinotecan-induced neutropenia.
Dr. Cristina had no conflicts of interest to disclose. Coauthors reported disclosures related to Amgen, Bayer, Boehringer, Bristol-Myers Squibb, Celgene, Ipsen, Lilly, Merck, Merck KgA, Novartis, Pfizer, Roche, Sanofi, Servier, and Shire.
SOURCE: Cristina V et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1080.
FROM JAMA ONCOLOGY
Key clinical point: Risks of adverse events were significantly higher in women treated with fluorouracil with or without irinotecan.
Major finding: Compared with men, women had significantly higher rates of all-grade alopecia, anemia, cholinergic syndrome, constipation, cramping, lethargy, leukopenia, nausea, neutropenia, stomatitis, and vomiting.
Study details: A retrospective analysis of treatment-emergent adverse events for 2,974 participants in the PETACC-3 trial conducted by the EORTC Gastrointestinal Group.
Disclosures: The authors reported disclosures related to Amgen, Bayer, Boehringer, Bristol-Myers Squibb, Celgene, Ipsen, Lilly, Merck, Merck KgA, Novartis, Pfizer, Roche, Sanofi, Servier, and Shire.
Source: Cristina V et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1080.
Gyn surgeons’ EndoMarch empowers patients
Empowering women through a grassroots approach is what Camran Nezhat, MD, a gynecologic surgeon in Palo Alto, Calif., had in mind when he founded the Worldwide Endometriosis March, or EndoMarch, some years ago. In March 2018, the 5th annual international day of marches and calls to action took place across at least eight U.S. cities and dozens of locations across Europe, Africa, the Middle East, and Asia.
Dr. Nezhat founded the 501(c)(3) public charity nonprofit along with his brothers, Farr Nezhat, MD, and Ceana Nezhat, MD; his niece Azadeh Nezhat, MD; and Barbara Page, a graduate of the University of California, Berkeley, who was working in his practice at the time.
“We’d published so much on the disease [in the medical literature], we didn’t know what else to do ... to help these women. We practice in one of the most advanced cultures for medical care ... and yet women come to us who’ve been told it’s all in their heads, or that they have PID [pelvic inflammatory disease] or depression,” Dr. Camran Nezhat said. “We’d get together and talk about this ... and we thought about how not much changed [with civil rights] in this country until people marched and took matters into their own hands.”
A final catalyst was a lengthy account and reflection on the history of endometriosis that the Nezhat brothers wrote, titled “Endometriosis: Ancient disease, ancient treatments” (Fertil Steril. 2012;98[6 Suppl]:S1-62). They dedicated their research to their mother, who suffered from endometriosis during her life in Iran and who inspired them to pursue medicine and become gynecologic surgeons.
Each year’s EndoMarch events are organized by EndoMarch chapters that are run by volunteers, many of whom have used the annual events to network and fuel year-round advocacy. Chapters have played important roles, for instance, in a national, government-sponsored awareness campaign launched in 2016 in France to alert the public through ads at bus stops and on TV and other media that pain during menstruation is “not natural” and may be a sign of endometriosis.
In Australia, EndoMarch advocates also helped drive plans in December 2017 to create a federally funded “national action plan” for endometriosis. In announcing the plan, Australian health minister Greg Hunt apologized, saying that the disease should have been acknowledged and acted upon “long ago.”
Empowering women through a grassroots approach is what Camran Nezhat, MD, a gynecologic surgeon in Palo Alto, Calif., had in mind when he founded the Worldwide Endometriosis March, or EndoMarch, some years ago. In March 2018, the 5th annual international day of marches and calls to action took place across at least eight U.S. cities and dozens of locations across Europe, Africa, the Middle East, and Asia.
Dr. Nezhat founded the 501(c)(3) public charity nonprofit along with his brothers, Farr Nezhat, MD, and Ceana Nezhat, MD; his niece Azadeh Nezhat, MD; and Barbara Page, a graduate of the University of California, Berkeley, who was working in his practice at the time.
“We’d published so much on the disease [in the medical literature], we didn’t know what else to do ... to help these women. We practice in one of the most advanced cultures for medical care ... and yet women come to us who’ve been told it’s all in their heads, or that they have PID [pelvic inflammatory disease] or depression,” Dr. Camran Nezhat said. “We’d get together and talk about this ... and we thought about how not much changed [with civil rights] in this country until people marched and took matters into their own hands.”
A final catalyst was a lengthy account and reflection on the history of endometriosis that the Nezhat brothers wrote, titled “Endometriosis: Ancient disease, ancient treatments” (Fertil Steril. 2012;98[6 Suppl]:S1-62). They dedicated their research to their mother, who suffered from endometriosis during her life in Iran and who inspired them to pursue medicine and become gynecologic surgeons.
Each year’s EndoMarch events are organized by EndoMarch chapters that are run by volunteers, many of whom have used the annual events to network and fuel year-round advocacy. Chapters have played important roles, for instance, in a national, government-sponsored awareness campaign launched in 2016 in France to alert the public through ads at bus stops and on TV and other media that pain during menstruation is “not natural” and may be a sign of endometriosis.
In Australia, EndoMarch advocates also helped drive plans in December 2017 to create a federally funded “national action plan” for endometriosis. In announcing the plan, Australian health minister Greg Hunt apologized, saying that the disease should have been acknowledged and acted upon “long ago.”
Empowering women through a grassroots approach is what Camran Nezhat, MD, a gynecologic surgeon in Palo Alto, Calif., had in mind when he founded the Worldwide Endometriosis March, or EndoMarch, some years ago. In March 2018, the 5th annual international day of marches and calls to action took place across at least eight U.S. cities and dozens of locations across Europe, Africa, the Middle East, and Asia.
Dr. Nezhat founded the 501(c)(3) public charity nonprofit along with his brothers, Farr Nezhat, MD, and Ceana Nezhat, MD; his niece Azadeh Nezhat, MD; and Barbara Page, a graduate of the University of California, Berkeley, who was working in his practice at the time.
“We’d published so much on the disease [in the medical literature], we didn’t know what else to do ... to help these women. We practice in one of the most advanced cultures for medical care ... and yet women come to us who’ve been told it’s all in their heads, or that they have PID [pelvic inflammatory disease] or depression,” Dr. Camran Nezhat said. “We’d get together and talk about this ... and we thought about how not much changed [with civil rights] in this country until people marched and took matters into their own hands.”
A final catalyst was a lengthy account and reflection on the history of endometriosis that the Nezhat brothers wrote, titled “Endometriosis: Ancient disease, ancient treatments” (Fertil Steril. 2012;98[6 Suppl]:S1-62). They dedicated their research to their mother, who suffered from endometriosis during her life in Iran and who inspired them to pursue medicine and become gynecologic surgeons.
Each year’s EndoMarch events are organized by EndoMarch chapters that are run by volunteers, many of whom have used the annual events to network and fuel year-round advocacy. Chapters have played important roles, for instance, in a national, government-sponsored awareness campaign launched in 2016 in France to alert the public through ads at bus stops and on TV and other media that pain during menstruation is “not natural” and may be a sign of endometriosis.
In Australia, EndoMarch advocates also helped drive plans in December 2017 to create a federally funded “national action plan” for endometriosis. In announcing the plan, Australian health minister Greg Hunt apologized, saying that the disease should have been acknowledged and acted upon “long ago.”
The push is on to recognize endometriosis in adolescents
Meg Hayden, RN, a school nurse in Oxford, Miss., used to be a labor and delivery nurse and considers herself more attuned to women’s health issues than other school nurses are. Still, a new educational initiative on endometriosis that stresses that menstrual pain is not normal – and that teenagers are not too young to have endometriosis – has helped her “connect the dots.”
“It’s a good reminder for me to look at patterns” and advise those girls who have repeated episodes of pelvic pain and other symptoms to “keep a diary” and to seek care, Ms. Hayden said.
They are demanding that serious diagnostic delays be rectified – that disease symptoms be better recognized by gynecologists, pediatricians, and other primary care physicians – and then, that the disease be better managed.
Some of the advocacy groups have petitioned the American College of Obstetricians and Gynecologists to involve patients and endometriosis experts in creating new standards of care. And at press time, activist Shannon Cohn, who developed the School Nurse initiative after producing a documentary film titled Endo What?, was working with Sen. Orrin Hatch (R-Utah) and Sen. Elizabeth Warren (D-Mass.) on finalizing plans for a national public service announcement campaign. (Sen. Hatch wrote an opinion piece for CNN in late March describing his granddaughter’s experience with the disease and calling the widespread prevalence of the disease – and the lack of any long-term treatment options – “nothing short of a public health emergency.”)
Estimates vary, but the average interval between presentation of symptoms and definitive diagnosis of endometriosis by laparoscopy (and usually) biopsy is commonly reported as 7-10 years. The disease can cause incapacitating pain, missed days of school and work, and increasing morbidities over time, including infertility and organ damage both inside and outside the pelvic cavity. A majority of women with endometriosis – two-thirds, according to one survey of more than 4,300 women with surgically diagnosed disease (Fertil Steril. 2009;91:32-9) – report first experiencing symptoms during adolescence.
Yet, too often, adolescents believe or are told that “periods are supposed to hurt,” and other symptoms of the disease – such as gastrointestinal symptoms – are overlooked.
“If we can diagnose endometriosis in its early stages, we could prevent a lifetime of pain and suffering, and decrease rates of infertility ... hopefully stopping disease progression before it does damage,” said Marc R. Laufer, MD, chief of gynecology at Boston Children’s Hospital and professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, also in Boston. “If we don’t, we’re missing a huge opportunity because we know that endometriosis affects 10% of women.”
Atypical symptoms and presentation
Endometriosis is an enigmatic disease. It traditionally has been associated with retrograde menstruation, but today, there are more nuanced and likely overlapping theories of etiology. Identified in girls even prior to the onset of menses, the disease is generally believed to be progressive, but perhaps not all the time. Patients with significant amounts of disease may have tremendous pain or they may have very little discomfort.
While adolescents can have advanced endometriosis, most have early-stage disease, experts say. Still, adolescence offers its own complexities. Preteen and teen patients with endometriosis tend to present more often with atypical symptoms and with much more subtle and variable laparoscopic findings than do adult patients. Dr. Laufer reported more than 20 years ago that only 9.7% of 46 girls presented classically with dysmenorrhea. In 63%, pain was both acyclic and cyclic, and in 28%, pain was acyclic only (J Pediatr Adolesc Gynecol. 1997;10:199-202).
In a more recent report on adolescents treated by gynecologic surgeon Ceana Nezhat, MD, 64% had dysmenorrhea, 44% had menorrhagia, 60% had abnormal or irregular uterine bleeding, 56% had at least one gastrointestinal symptom, and 52% had at least one genitourinary symptom. The girls had seen a mean of three physicians, including psychiatrists and orthopedic surgeons, and had received diagnoses of pelvic inflammatory disease, irritable bowel syndrome, dysmenorrhea, appendicitis, ovarian cysts, and musculoskeletal pain (JSLS. 2015;19:e2015.00019). Notably, 56% had a family history of endometriosis, Dr. Nezhat, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, and his colleagues found.
To address levels of pain, Dr. Laufer usually asks young women if they feel they’re at a disadvantage to other young women or to men. This opens the door to learning more about school absences, missed activities, and decreased quality of life. Pain, he emphasizes, is only part of the picture. “It’s also about fatigue and energy levels, social interaction, depression, sexual function if they’re sexually active, body image issues, and bowel and bladder functionality.”
If the new generation of school nurse programs and other educational initiatives are successful, teens will increasingly come to appointments with notes in hand. Ms. Hayden counsels students on what to discuss with the doctor. And high school students in New York who have been educated through the Endometriosis Foundation of America’s 5-year-old ENPOWR Project for endometriosis education are urged to keep a journal or use a symptom tracker app if they are experiencing pain or other symptoms associated with endometriosis.
“We tell them that, with a record, you can show that the second week of every month I’m in terrible pain, for instance, or I’ve fainted twice in the last month, or here’s when my nausea is really aggressive,” said Nina Baker, outreach coordinator for the foundation. “We’re very honest about how often this is dismissed ... and we assure them that by no means are you wrong about your pain.”
ENPOWR lessons have been taught in more than 165 schools thus far (mostly in health classes in New York schools and largely by foundation-trained educators), and a recently developed online package of educational materials for schools – the Endo EduKit – is expanding the foundation’s geographical reach to other states. Students are encouraged during the training to see a gynecologist if they’re concerned about endometriosis, Ms. Baker said.
In Mississippi, Ms. Hayden suggests that younger high-schoolers see their pediatrician, but after that, “I feel like they should go to the gynecologist.” (ACOG recommends a first visit to the gynecologist between the ages of 13 and 15 for anticipatory guidance.) The year-old School Nurse Initiative has sent toolkits, posters, and DVD copies of the “Endo What?” film to nurses in 652 schools thus far. “Our goal,” said Ms. Cohn, a lawyer, filmmaker, and an endometriosis patient, “is to educate every school nurse in middle and high schools across the country.”
Treatment dilemmas
The first-line treatment for dysmenorrhea and for suspected endometriosis in adolescents has long been empiric treatment with NSAIDs and oral contraceptive pills. Experts commonly recommend today that combined oral contraceptive pills (COCPs) be started cyclically and then changed to continuous dosing if necessary with the goal of inducing amenorrhea.
If symptoms are not well controlled within 3-6 months of compliant medication management with COCPs and NSAIDs and endometriosis is suspected, then laparoscopy by a physician who is familiar with adolescent endometriosis and can simultaneously diagnose and treat the disease should be considered, according to Dr. Laufer and several other experts in pediatric and adolescent gynecology who spoke with Ob.Gyn. News.
“If someone still has pain on one COCP, then switching to another COCP is not going to solve the problem – there is no study that shows that one pill is better than another,” Dr. Laufer said.
Yet extra months and sometimes years of pill-switching and empiric therapy with other medications – rather than surgical evaluation, diagnosis, and treatment – is not uncommon. “Usually, by the time a patient comes to me, they’ve already been on multiple birth control pills, they’ve failed NSAIDs, and they’ve often tried other medications as well,” such as progestins and gonadotropin-releasing hormone agonists, said Iris Kerin Orbuch, MD, director of the Advanced Gynecologic Laparoscopy Centers in New York and Los Angeles.
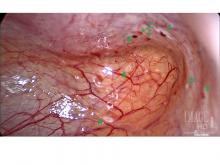
Some also have had diagnostic laparoscopies and been wrongly told that nothing is wrong. Endometriosis is “not all powder-burn lesions and chocolate cysts, which is what we’re taught in medical school,” she said. “It can have many appearances, especially in teens and adolescents. It can be clear vesicles, white, fibrotic, yellow, blue, and brown ... and quite commonly there can simply be areas of increased vascularity. I only learned this in my fellowship.”
Dr. Orbuch, who routinely treats adolescents with endometriosis, takes a holistic approach to the disease that includes working with patients – often before surgery and in partnership with other providers – to downregulate the central nervous system and to alleviate pelvic floor dysfunction that often develops secondary to the disease. When she does operate and finds endometriosis, she performs excisional surgery, in contrast with ablative techniques such as cauterization or desiccation that are used by many physicians.
Treatment of endometriosis is rife with dilemmas, controversies, and shortcomings. Medical treatments can improve pain, but as ACOG’s current Practice Bulletin (No. 114) points out, recurrence rates are high after medication is discontinued – and there is concern among some experts that hormone therapy may not keep the disease from progressing. In adolescents, there is concern about the significant side effects of gonadotropin-releasing hormone agonists, which are sometimes chosen if COCPs and NSAIDs fail to relieve symptoms. COCPs themselves may be problematic, causing premature closure of growth plates.
And when it comes to surgical treatment, there’s often sharp debate over which operative approaches are best for endometriosis. Advocates of excision – including many of the patient advocacy groups – say that ablation too often causes scar tissue and leaves behind disease, leading to recurrent symptoms and multiple surgeries. Critics of excisional surgery express concern about excision-induced adhesions and scar tissue, and about some excisional surgery being too “radical,” particularly when it is performed for earlier-stage disease in adolescents. Research is limited, comprised largely of small retrospective reports and single-institution cohort studies.
Meredith Loveless, MD, a pediatric and adolescent gynecologist who chairs ACOG’s Committee on Adolescent Health Care, is leading the development of a new ACOG committee opinion on dysmenorrhea and endometriosis in adolescents. The laparoscopic appearance of endometriosis in young patients and the need “for fertility preservation as a priority” in surgery will be among the points discussed in ACOG’s upcoming guidance, she said.
“Somebody who manages adult endometriosis and who does extremely aggressive surgical work may actually be harming an adolescent rather than helping them,” said Dr. Loveless of the Norton Children’s Hospital in Louisville, Ky. (Dr. Loveless has also worked with the American Academy of Pediatrics and notes that the academy provides education on dysmenorrhea and endometriosis as part of its national conference.)
Nicole Donnellan, MD, of the University of Pittsburgh Magee–Womens Hospital, said that fertility preservation is always a goal – and is possible – regardless of age. “A lot of us who are advanced laparoscopic surgeons are passionate about excision because (with other approaches) you’re not fully exploring the extent of the disease – what’s behind the superficial things you see,” she said. “Whether you’re 38 and wanting to preserve your fertility, or whether you’re 18, I’m still going to use the same approach. I want to make sure you have a functioning tube, ovaries, and uterus.”
Ken R. Sinervo, MD, medical director of the Center for Endometriosis Care in Atlanta, which has followed patients postsurgically for an average of 7-8 years, said adhesions can occur "whether you're ablating the disease or excising it," and that in his excisional surgeries, he successfully prevents adhesion formation with the use of various intraoperative adhesion barriers as well as bioregenerative medicine to facilitate healing. The key to avoiding repeat surgeries is to "remove all the disease that is present," he emphasized, adding that the "great majority of young patients will have peritoneal disease and very little ovarian involvement."*
ACOG under fire
Dr. Sinervo and Dr. Orbuch are among the gynecologic surgeons, other providers, and patients who have signed a petition to ACOG urging it to involve both educated patients and expert, multidisciplinary endometriosis providers in improving their guidance and policies on endometriosis to facilitate earlier diagnosis and more effective treatment.
The petition was organized by advocate Casey Berna in July and supported by more than a half-dozen endometriosis advocacy groups; in early May, it had almost 8,700 signatures. Ms. Berna also co-organized a demonstration outside ACOG headquarters on April 5-6 as leaders were reviewing practice bulletins and deciding which need revision – and a virtual protest (#WeMatterACOG) – to push for better guidelines.
Ms. Berna, Ms. Cohn, and others have also expressed concern that ob.gyns.’ management of endometriosis – and the development of guidelines – is colored by financial conflicts of interest. The petition, moreover, calls upon ACOG to help create coding specific for excision surgery; currently, because of the lack of reimbursement, many surgeons operate out of network and patients struggle with treatment costs.
In a statement issued in response to the protests, ACOG chief executive officer and executive vice president Hal Lawrence, MD, said that “ACOG is aware of the sensitivities and concerns surrounding timely and accurate diagnosis and treatment of endometriosis. We are always working diligently to review all the available literature and ensure that our guidance to members is accurate and up to date. It’s our aim that [diagnosis and care] are both evidence based and patient centered. To that end, we recognize that patient voices and advocacy are an important part of ensuring we are meeting these high standards.”
In an interview before the protests, Dr. Lawrence said the Committee on Practice Bulletins–Gynecology will revise its guidelines on the management of endometriosis, which were last revised in 2010 and reaffirmed in 2016. He said that he had spoken at length with Ms. Berna on the phone and had passed on a file of research and other materials to the Committee for their consideration.
On April 5, ACOG also joined the American Society for Reproductive Medicine and seven other organizations in sending a letter to the U.S. Senate and House calling for more research on and attention to the disease. NIH research dollars for the disease have dropped from $16 million in 2010 to $7 million in 2018, and “there are too few treatment options available to patients,” the letter says. “We urge you to [prioritize endometriosis] as an important women’s health issue.”
*This article was updated June 5, 2018. An earlier version of this article misstated Dr. Ken R. Sinervo’s name.
Meg Hayden, RN, a school nurse in Oxford, Miss., used to be a labor and delivery nurse and considers herself more attuned to women’s health issues than other school nurses are. Still, a new educational initiative on endometriosis that stresses that menstrual pain is not normal – and that teenagers are not too young to have endometriosis – has helped her “connect the dots.”
“It’s a good reminder for me to look at patterns” and advise those girls who have repeated episodes of pelvic pain and other symptoms to “keep a diary” and to seek care, Ms. Hayden said.
They are demanding that serious diagnostic delays be rectified – that disease symptoms be better recognized by gynecologists, pediatricians, and other primary care physicians – and then, that the disease be better managed.
Some of the advocacy groups have petitioned the American College of Obstetricians and Gynecologists to involve patients and endometriosis experts in creating new standards of care. And at press time, activist Shannon Cohn, who developed the School Nurse initiative after producing a documentary film titled Endo What?, was working with Sen. Orrin Hatch (R-Utah) and Sen. Elizabeth Warren (D-Mass.) on finalizing plans for a national public service announcement campaign. (Sen. Hatch wrote an opinion piece for CNN in late March describing his granddaughter’s experience with the disease and calling the widespread prevalence of the disease – and the lack of any long-term treatment options – “nothing short of a public health emergency.”)
Estimates vary, but the average interval between presentation of symptoms and definitive diagnosis of endometriosis by laparoscopy (and usually) biopsy is commonly reported as 7-10 years. The disease can cause incapacitating pain, missed days of school and work, and increasing morbidities over time, including infertility and organ damage both inside and outside the pelvic cavity. A majority of women with endometriosis – two-thirds, according to one survey of more than 4,300 women with surgically diagnosed disease (Fertil Steril. 2009;91:32-9) – report first experiencing symptoms during adolescence.
Yet, too often, adolescents believe or are told that “periods are supposed to hurt,” and other symptoms of the disease – such as gastrointestinal symptoms – are overlooked.
“If we can diagnose endometriosis in its early stages, we could prevent a lifetime of pain and suffering, and decrease rates of infertility ... hopefully stopping disease progression before it does damage,” said Marc R. Laufer, MD, chief of gynecology at Boston Children’s Hospital and professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, also in Boston. “If we don’t, we’re missing a huge opportunity because we know that endometriosis affects 10% of women.”
Atypical symptoms and presentation
Endometriosis is an enigmatic disease. It traditionally has been associated with retrograde menstruation, but today, there are more nuanced and likely overlapping theories of etiology. Identified in girls even prior to the onset of menses, the disease is generally believed to be progressive, but perhaps not all the time. Patients with significant amounts of disease may have tremendous pain or they may have very little discomfort.
While adolescents can have advanced endometriosis, most have early-stage disease, experts say. Still, adolescence offers its own complexities. Preteen and teen patients with endometriosis tend to present more often with atypical symptoms and with much more subtle and variable laparoscopic findings than do adult patients. Dr. Laufer reported more than 20 years ago that only 9.7% of 46 girls presented classically with dysmenorrhea. In 63%, pain was both acyclic and cyclic, and in 28%, pain was acyclic only (J Pediatr Adolesc Gynecol. 1997;10:199-202).
In a more recent report on adolescents treated by gynecologic surgeon Ceana Nezhat, MD, 64% had dysmenorrhea, 44% had menorrhagia, 60% had abnormal or irregular uterine bleeding, 56% had at least one gastrointestinal symptom, and 52% had at least one genitourinary symptom. The girls had seen a mean of three physicians, including psychiatrists and orthopedic surgeons, and had received diagnoses of pelvic inflammatory disease, irritable bowel syndrome, dysmenorrhea, appendicitis, ovarian cysts, and musculoskeletal pain (JSLS. 2015;19:e2015.00019). Notably, 56% had a family history of endometriosis, Dr. Nezhat, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, and his colleagues found.
To address levels of pain, Dr. Laufer usually asks young women if they feel they’re at a disadvantage to other young women or to men. This opens the door to learning more about school absences, missed activities, and decreased quality of life. Pain, he emphasizes, is only part of the picture. “It’s also about fatigue and energy levels, social interaction, depression, sexual function if they’re sexually active, body image issues, and bowel and bladder functionality.”
If the new generation of school nurse programs and other educational initiatives are successful, teens will increasingly come to appointments with notes in hand. Ms. Hayden counsels students on what to discuss with the doctor. And high school students in New York who have been educated through the Endometriosis Foundation of America’s 5-year-old ENPOWR Project for endometriosis education are urged to keep a journal or use a symptom tracker app if they are experiencing pain or other symptoms associated with endometriosis.
“We tell them that, with a record, you can show that the second week of every month I’m in terrible pain, for instance, or I’ve fainted twice in the last month, or here’s when my nausea is really aggressive,” said Nina Baker, outreach coordinator for the foundation. “We’re very honest about how often this is dismissed ... and we assure them that by no means are you wrong about your pain.”
ENPOWR lessons have been taught in more than 165 schools thus far (mostly in health classes in New York schools and largely by foundation-trained educators), and a recently developed online package of educational materials for schools – the Endo EduKit – is expanding the foundation’s geographical reach to other states. Students are encouraged during the training to see a gynecologist if they’re concerned about endometriosis, Ms. Baker said.
In Mississippi, Ms. Hayden suggests that younger high-schoolers see their pediatrician, but after that, “I feel like they should go to the gynecologist.” (ACOG recommends a first visit to the gynecologist between the ages of 13 and 15 for anticipatory guidance.) The year-old School Nurse Initiative has sent toolkits, posters, and DVD copies of the “Endo What?” film to nurses in 652 schools thus far. “Our goal,” said Ms. Cohn, a lawyer, filmmaker, and an endometriosis patient, “is to educate every school nurse in middle and high schools across the country.”
Treatment dilemmas
The first-line treatment for dysmenorrhea and for suspected endometriosis in adolescents has long been empiric treatment with NSAIDs and oral contraceptive pills. Experts commonly recommend today that combined oral contraceptive pills (COCPs) be started cyclically and then changed to continuous dosing if necessary with the goal of inducing amenorrhea.
If symptoms are not well controlled within 3-6 months of compliant medication management with COCPs and NSAIDs and endometriosis is suspected, then laparoscopy by a physician who is familiar with adolescent endometriosis and can simultaneously diagnose and treat the disease should be considered, according to Dr. Laufer and several other experts in pediatric and adolescent gynecology who spoke with Ob.Gyn. News.
“If someone still has pain on one COCP, then switching to another COCP is not going to solve the problem – there is no study that shows that one pill is better than another,” Dr. Laufer said.
Yet extra months and sometimes years of pill-switching and empiric therapy with other medications – rather than surgical evaluation, diagnosis, and treatment – is not uncommon. “Usually, by the time a patient comes to me, they’ve already been on multiple birth control pills, they’ve failed NSAIDs, and they’ve often tried other medications as well,” such as progestins and gonadotropin-releasing hormone agonists, said Iris Kerin Orbuch, MD, director of the Advanced Gynecologic Laparoscopy Centers in New York and Los Angeles.
Some also have had diagnostic laparoscopies and been wrongly told that nothing is wrong. Endometriosis is “not all powder-burn lesions and chocolate cysts, which is what we’re taught in medical school,” she said. “It can have many appearances, especially in teens and adolescents. It can be clear vesicles, white, fibrotic, yellow, blue, and brown ... and quite commonly there can simply be areas of increased vascularity. I only learned this in my fellowship.”
Dr. Orbuch, who routinely treats adolescents with endometriosis, takes a holistic approach to the disease that includes working with patients – often before surgery and in partnership with other providers – to downregulate the central nervous system and to alleviate pelvic floor dysfunction that often develops secondary to the disease. When she does operate and finds endometriosis, she performs excisional surgery, in contrast with ablative techniques such as cauterization or desiccation that are used by many physicians.
Treatment of endometriosis is rife with dilemmas, controversies, and shortcomings. Medical treatments can improve pain, but as ACOG’s current Practice Bulletin (No. 114) points out, recurrence rates are high after medication is discontinued – and there is concern among some experts that hormone therapy may not keep the disease from progressing. In adolescents, there is concern about the significant side effects of gonadotropin-releasing hormone agonists, which are sometimes chosen if COCPs and NSAIDs fail to relieve symptoms. COCPs themselves may be problematic, causing premature closure of growth plates.
And when it comes to surgical treatment, there’s often sharp debate over which operative approaches are best for endometriosis. Advocates of excision – including many of the patient advocacy groups – say that ablation too often causes scar tissue and leaves behind disease, leading to recurrent symptoms and multiple surgeries. Critics of excisional surgery express concern about excision-induced adhesions and scar tissue, and about some excisional surgery being too “radical,” particularly when it is performed for earlier-stage disease in adolescents. Research is limited, comprised largely of small retrospective reports and single-institution cohort studies.
Meredith Loveless, MD, a pediatric and adolescent gynecologist who chairs ACOG’s Committee on Adolescent Health Care, is leading the development of a new ACOG committee opinion on dysmenorrhea and endometriosis in adolescents. The laparoscopic appearance of endometriosis in young patients and the need “for fertility preservation as a priority” in surgery will be among the points discussed in ACOG’s upcoming guidance, she said.
“Somebody who manages adult endometriosis and who does extremely aggressive surgical work may actually be harming an adolescent rather than helping them,” said Dr. Loveless of the Norton Children’s Hospital in Louisville, Ky. (Dr. Loveless has also worked with the American Academy of Pediatrics and notes that the academy provides education on dysmenorrhea and endometriosis as part of its national conference.)
Nicole Donnellan, MD, of the University of Pittsburgh Magee–Womens Hospital, said that fertility preservation is always a goal – and is possible – regardless of age. “A lot of us who are advanced laparoscopic surgeons are passionate about excision because (with other approaches) you’re not fully exploring the extent of the disease – what’s behind the superficial things you see,” she said. “Whether you’re 38 and wanting to preserve your fertility, or whether you’re 18, I’m still going to use the same approach. I want to make sure you have a functioning tube, ovaries, and uterus.”
Ken R. Sinervo, MD, medical director of the Center for Endometriosis Care in Atlanta, which has followed patients postsurgically for an average of 7-8 years, said adhesions can occur "whether you're ablating the disease or excising it," and that in his excisional surgeries, he successfully prevents adhesion formation with the use of various intraoperative adhesion barriers as well as bioregenerative medicine to facilitate healing. The key to avoiding repeat surgeries is to "remove all the disease that is present," he emphasized, adding that the "great majority of young patients will have peritoneal disease and very little ovarian involvement."*
ACOG under fire
Dr. Sinervo and Dr. Orbuch are among the gynecologic surgeons, other providers, and patients who have signed a petition to ACOG urging it to involve both educated patients and expert, multidisciplinary endometriosis providers in improving their guidance and policies on endometriosis to facilitate earlier diagnosis and more effective treatment.
The petition was organized by advocate Casey Berna in July and supported by more than a half-dozen endometriosis advocacy groups; in early May, it had almost 8,700 signatures. Ms. Berna also co-organized a demonstration outside ACOG headquarters on April 5-6 as leaders were reviewing practice bulletins and deciding which need revision – and a virtual protest (#WeMatterACOG) – to push for better guidelines.
Ms. Berna, Ms. Cohn, and others have also expressed concern that ob.gyns.’ management of endometriosis – and the development of guidelines – is colored by financial conflicts of interest. The petition, moreover, calls upon ACOG to help create coding specific for excision surgery; currently, because of the lack of reimbursement, many surgeons operate out of network and patients struggle with treatment costs.
In a statement issued in response to the protests, ACOG chief executive officer and executive vice president Hal Lawrence, MD, said that “ACOG is aware of the sensitivities and concerns surrounding timely and accurate diagnosis and treatment of endometriosis. We are always working diligently to review all the available literature and ensure that our guidance to members is accurate and up to date. It’s our aim that [diagnosis and care] are both evidence based and patient centered. To that end, we recognize that patient voices and advocacy are an important part of ensuring we are meeting these high standards.”
In an interview before the protests, Dr. Lawrence said the Committee on Practice Bulletins–Gynecology will revise its guidelines on the management of endometriosis, which were last revised in 2010 and reaffirmed in 2016. He said that he had spoken at length with Ms. Berna on the phone and had passed on a file of research and other materials to the Committee for their consideration.
On April 5, ACOG also joined the American Society for Reproductive Medicine and seven other organizations in sending a letter to the U.S. Senate and House calling for more research on and attention to the disease. NIH research dollars for the disease have dropped from $16 million in 2010 to $7 million in 2018, and “there are too few treatment options available to patients,” the letter says. “We urge you to [prioritize endometriosis] as an important women’s health issue.”
*This article was updated June 5, 2018. An earlier version of this article misstated Dr. Ken R. Sinervo’s name.
Meg Hayden, RN, a school nurse in Oxford, Miss., used to be a labor and delivery nurse and considers herself more attuned to women’s health issues than other school nurses are. Still, a new educational initiative on endometriosis that stresses that menstrual pain is not normal – and that teenagers are not too young to have endometriosis – has helped her “connect the dots.”
“It’s a good reminder for me to look at patterns” and advise those girls who have repeated episodes of pelvic pain and other symptoms to “keep a diary” and to seek care, Ms. Hayden said.
They are demanding that serious diagnostic delays be rectified – that disease symptoms be better recognized by gynecologists, pediatricians, and other primary care physicians – and then, that the disease be better managed.
Some of the advocacy groups have petitioned the American College of Obstetricians and Gynecologists to involve patients and endometriosis experts in creating new standards of care. And at press time, activist Shannon Cohn, who developed the School Nurse initiative after producing a documentary film titled Endo What?, was working with Sen. Orrin Hatch (R-Utah) and Sen. Elizabeth Warren (D-Mass.) on finalizing plans for a national public service announcement campaign. (Sen. Hatch wrote an opinion piece for CNN in late March describing his granddaughter’s experience with the disease and calling the widespread prevalence of the disease – and the lack of any long-term treatment options – “nothing short of a public health emergency.”)
Estimates vary, but the average interval between presentation of symptoms and definitive diagnosis of endometriosis by laparoscopy (and usually) biopsy is commonly reported as 7-10 years. The disease can cause incapacitating pain, missed days of school and work, and increasing morbidities over time, including infertility and organ damage both inside and outside the pelvic cavity. A majority of women with endometriosis – two-thirds, according to one survey of more than 4,300 women with surgically diagnosed disease (Fertil Steril. 2009;91:32-9) – report first experiencing symptoms during adolescence.
Yet, too often, adolescents believe or are told that “periods are supposed to hurt,” and other symptoms of the disease – such as gastrointestinal symptoms – are overlooked.
“If we can diagnose endometriosis in its early stages, we could prevent a lifetime of pain and suffering, and decrease rates of infertility ... hopefully stopping disease progression before it does damage,” said Marc R. Laufer, MD, chief of gynecology at Boston Children’s Hospital and professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, also in Boston. “If we don’t, we’re missing a huge opportunity because we know that endometriosis affects 10% of women.”
Atypical symptoms and presentation
Endometriosis is an enigmatic disease. It traditionally has been associated with retrograde menstruation, but today, there are more nuanced and likely overlapping theories of etiology. Identified in girls even prior to the onset of menses, the disease is generally believed to be progressive, but perhaps not all the time. Patients with significant amounts of disease may have tremendous pain or they may have very little discomfort.
While adolescents can have advanced endometriosis, most have early-stage disease, experts say. Still, adolescence offers its own complexities. Preteen and teen patients with endometriosis tend to present more often with atypical symptoms and with much more subtle and variable laparoscopic findings than do adult patients. Dr. Laufer reported more than 20 years ago that only 9.7% of 46 girls presented classically with dysmenorrhea. In 63%, pain was both acyclic and cyclic, and in 28%, pain was acyclic only (J Pediatr Adolesc Gynecol. 1997;10:199-202).
In a more recent report on adolescents treated by gynecologic surgeon Ceana Nezhat, MD, 64% had dysmenorrhea, 44% had menorrhagia, 60% had abnormal or irregular uterine bleeding, 56% had at least one gastrointestinal symptom, and 52% had at least one genitourinary symptom. The girls had seen a mean of three physicians, including psychiatrists and orthopedic surgeons, and had received diagnoses of pelvic inflammatory disease, irritable bowel syndrome, dysmenorrhea, appendicitis, ovarian cysts, and musculoskeletal pain (JSLS. 2015;19:e2015.00019). Notably, 56% had a family history of endometriosis, Dr. Nezhat, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, and his colleagues found.
To address levels of pain, Dr. Laufer usually asks young women if they feel they’re at a disadvantage to other young women or to men. This opens the door to learning more about school absences, missed activities, and decreased quality of life. Pain, he emphasizes, is only part of the picture. “It’s also about fatigue and energy levels, social interaction, depression, sexual function if they’re sexually active, body image issues, and bowel and bladder functionality.”
If the new generation of school nurse programs and other educational initiatives are successful, teens will increasingly come to appointments with notes in hand. Ms. Hayden counsels students on what to discuss with the doctor. And high school students in New York who have been educated through the Endometriosis Foundation of America’s 5-year-old ENPOWR Project for endometriosis education are urged to keep a journal or use a symptom tracker app if they are experiencing pain or other symptoms associated with endometriosis.
“We tell them that, with a record, you can show that the second week of every month I’m in terrible pain, for instance, or I’ve fainted twice in the last month, or here’s when my nausea is really aggressive,” said Nina Baker, outreach coordinator for the foundation. “We’re very honest about how often this is dismissed ... and we assure them that by no means are you wrong about your pain.”
ENPOWR lessons have been taught in more than 165 schools thus far (mostly in health classes in New York schools and largely by foundation-trained educators), and a recently developed online package of educational materials for schools – the Endo EduKit – is expanding the foundation’s geographical reach to other states. Students are encouraged during the training to see a gynecologist if they’re concerned about endometriosis, Ms. Baker said.
In Mississippi, Ms. Hayden suggests that younger high-schoolers see their pediatrician, but after that, “I feel like they should go to the gynecologist.” (ACOG recommends a first visit to the gynecologist between the ages of 13 and 15 for anticipatory guidance.) The year-old School Nurse Initiative has sent toolkits, posters, and DVD copies of the “Endo What?” film to nurses in 652 schools thus far. “Our goal,” said Ms. Cohn, a lawyer, filmmaker, and an endometriosis patient, “is to educate every school nurse in middle and high schools across the country.”
Treatment dilemmas
The first-line treatment for dysmenorrhea and for suspected endometriosis in adolescents has long been empiric treatment with NSAIDs and oral contraceptive pills. Experts commonly recommend today that combined oral contraceptive pills (COCPs) be started cyclically and then changed to continuous dosing if necessary with the goal of inducing amenorrhea.
If symptoms are not well controlled within 3-6 months of compliant medication management with COCPs and NSAIDs and endometriosis is suspected, then laparoscopy by a physician who is familiar with adolescent endometriosis and can simultaneously diagnose and treat the disease should be considered, according to Dr. Laufer and several other experts in pediatric and adolescent gynecology who spoke with Ob.Gyn. News.
“If someone still has pain on one COCP, then switching to another COCP is not going to solve the problem – there is no study that shows that one pill is better than another,” Dr. Laufer said.
Yet extra months and sometimes years of pill-switching and empiric therapy with other medications – rather than surgical evaluation, diagnosis, and treatment – is not uncommon. “Usually, by the time a patient comes to me, they’ve already been on multiple birth control pills, they’ve failed NSAIDs, and they’ve often tried other medications as well,” such as progestins and gonadotropin-releasing hormone agonists, said Iris Kerin Orbuch, MD, director of the Advanced Gynecologic Laparoscopy Centers in New York and Los Angeles.
Some also have had diagnostic laparoscopies and been wrongly told that nothing is wrong. Endometriosis is “not all powder-burn lesions and chocolate cysts, which is what we’re taught in medical school,” she said. “It can have many appearances, especially in teens and adolescents. It can be clear vesicles, white, fibrotic, yellow, blue, and brown ... and quite commonly there can simply be areas of increased vascularity. I only learned this in my fellowship.”
Dr. Orbuch, who routinely treats adolescents with endometriosis, takes a holistic approach to the disease that includes working with patients – often before surgery and in partnership with other providers – to downregulate the central nervous system and to alleviate pelvic floor dysfunction that often develops secondary to the disease. When she does operate and finds endometriosis, she performs excisional surgery, in contrast with ablative techniques such as cauterization or desiccation that are used by many physicians.
Treatment of endometriosis is rife with dilemmas, controversies, and shortcomings. Medical treatments can improve pain, but as ACOG’s current Practice Bulletin (No. 114) points out, recurrence rates are high after medication is discontinued – and there is concern among some experts that hormone therapy may not keep the disease from progressing. In adolescents, there is concern about the significant side effects of gonadotropin-releasing hormone agonists, which are sometimes chosen if COCPs and NSAIDs fail to relieve symptoms. COCPs themselves may be problematic, causing premature closure of growth plates.
And when it comes to surgical treatment, there’s often sharp debate over which operative approaches are best for endometriosis. Advocates of excision – including many of the patient advocacy groups – say that ablation too often causes scar tissue and leaves behind disease, leading to recurrent symptoms and multiple surgeries. Critics of excisional surgery express concern about excision-induced adhesions and scar tissue, and about some excisional surgery being too “radical,” particularly when it is performed for earlier-stage disease in adolescents. Research is limited, comprised largely of small retrospective reports and single-institution cohort studies.
Meredith Loveless, MD, a pediatric and adolescent gynecologist who chairs ACOG’s Committee on Adolescent Health Care, is leading the development of a new ACOG committee opinion on dysmenorrhea and endometriosis in adolescents. The laparoscopic appearance of endometriosis in young patients and the need “for fertility preservation as a priority” in surgery will be among the points discussed in ACOG’s upcoming guidance, she said.
“Somebody who manages adult endometriosis and who does extremely aggressive surgical work may actually be harming an adolescent rather than helping them,” said Dr. Loveless of the Norton Children’s Hospital in Louisville, Ky. (Dr. Loveless has also worked with the American Academy of Pediatrics and notes that the academy provides education on dysmenorrhea and endometriosis as part of its national conference.)
Nicole Donnellan, MD, of the University of Pittsburgh Magee–Womens Hospital, said that fertility preservation is always a goal – and is possible – regardless of age. “A lot of us who are advanced laparoscopic surgeons are passionate about excision because (with other approaches) you’re not fully exploring the extent of the disease – what’s behind the superficial things you see,” she said. “Whether you’re 38 and wanting to preserve your fertility, or whether you’re 18, I’m still going to use the same approach. I want to make sure you have a functioning tube, ovaries, and uterus.”
Ken R. Sinervo, MD, medical director of the Center for Endometriosis Care in Atlanta, which has followed patients postsurgically for an average of 7-8 years, said adhesions can occur "whether you're ablating the disease or excising it," and that in his excisional surgeries, he successfully prevents adhesion formation with the use of various intraoperative adhesion barriers as well as bioregenerative medicine to facilitate healing. The key to avoiding repeat surgeries is to "remove all the disease that is present," he emphasized, adding that the "great majority of young patients will have peritoneal disease and very little ovarian involvement."*
ACOG under fire
Dr. Sinervo and Dr. Orbuch are among the gynecologic surgeons, other providers, and patients who have signed a petition to ACOG urging it to involve both educated patients and expert, multidisciplinary endometriosis providers in improving their guidance and policies on endometriosis to facilitate earlier diagnosis and more effective treatment.
The petition was organized by advocate Casey Berna in July and supported by more than a half-dozen endometriosis advocacy groups; in early May, it had almost 8,700 signatures. Ms. Berna also co-organized a demonstration outside ACOG headquarters on April 5-6 as leaders were reviewing practice bulletins and deciding which need revision – and a virtual protest (#WeMatterACOG) – to push for better guidelines.
Ms. Berna, Ms. Cohn, and others have also expressed concern that ob.gyns.’ management of endometriosis – and the development of guidelines – is colored by financial conflicts of interest. The petition, moreover, calls upon ACOG to help create coding specific for excision surgery; currently, because of the lack of reimbursement, many surgeons operate out of network and patients struggle with treatment costs.
In a statement issued in response to the protests, ACOG chief executive officer and executive vice president Hal Lawrence, MD, said that “ACOG is aware of the sensitivities and concerns surrounding timely and accurate diagnosis and treatment of endometriosis. We are always working diligently to review all the available literature and ensure that our guidance to members is accurate and up to date. It’s our aim that [diagnosis and care] are both evidence based and patient centered. To that end, we recognize that patient voices and advocacy are an important part of ensuring we are meeting these high standards.”
In an interview before the protests, Dr. Lawrence said the Committee on Practice Bulletins–Gynecology will revise its guidelines on the management of endometriosis, which were last revised in 2010 and reaffirmed in 2016. He said that he had spoken at length with Ms. Berna on the phone and had passed on a file of research and other materials to the Committee for their consideration.
On April 5, ACOG also joined the American Society for Reproductive Medicine and seven other organizations in sending a letter to the U.S. Senate and House calling for more research on and attention to the disease. NIH research dollars for the disease have dropped from $16 million in 2010 to $7 million in 2018, and “there are too few treatment options available to patients,” the letter says. “We urge you to [prioritize endometriosis] as an important women’s health issue.”
*This article was updated June 5, 2018. An earlier version of this article misstated Dr. Ken R. Sinervo’s name.
Thoracic aortic aneurysm: How to counsel, when to refer
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
KEY POINTS
- Screening and referral depend on clinical context. A size-based model to determine screening, referral, follow-up, and management serves most cases but should be modified in the context of connective tissue disease or family history of aneurysm and dissection.
- Medical management involves strict blood pressure and heart rate control with beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Activity modifications should be tailored to the individual, although extreme isometric exercises and heavy lifting should be discouraged.
- Patients with TAA should be followed up annually, unless the patient is presenting for initial evaluation or significant changes are seen with dedicated imaging.
Aortic dissection presenting as ischemic limb
A 40-year-old man with a history of hypertension and alcohol abuse presented with acute onset of mild chest tightness, left leg pain, and increasing agitation, which prevented us from obtaining additional meaningful information from him.
On admission, his heart rate was 120 beats per minute, blood pressure 211/122 mm Hg, respiratory rate 18 per minute, and oxygen saturation 92% on room air. Given his history of alcohol abuse, we checked his blood ethanol level, which was less than 0.01%, well below the legal limit for intoxication.
We gave the patient intravenous lorazepam for possible alcohol withdrawal and started labetalol by intravenous infusion to lower his blood pressure.
On physical examination, his left lower extremity was cold and without pulses, including the femoral pulse. Suspecting acute arterial thrombosis, we ordered immediate computed tomographic (CT) angiography of the abdomen and pelvis with left lower extremity runoff. The images showed dissection of the abdominal aorta with extension to both the left and right common iliac arteries and the origin of the right external iliac artery. There was resultant occlusion of the left external iliac artery (Figure 1).
Immediate CT angiography of the chest was then performed, which revealed dissection of the thoracic aorta as well, starting superior to the aortic valve annulus and involving the ascending aorta, aortic arch, and the entire descending thoracic aorta (Figure 2).
The patient underwent emergency surgical repair of the aortic root, ascending aorta, and aortic arch. Residual dissection of the descending aorta was managed conservatively with blood pressure control using intravenous labetalol initially, which was then switched to oral carvedilol, and the pulses returned in his left lower extremity. He had an unremarkable postoperative recovery and was discharged after 1 week.
AORTIC DISSECTION AND MALPERFUSION SYNDROME
Aortic dissection is most often associated with acute onset of sharp chest pain and upper back pain. On rare occasions, it can have an atypical presentation such as stroke, paraplegia, mesenteric ischemia, or lower limb malperfusion.1
Extension of aortic dissection into the iliac and femoral arteries can cause impaired or absent blood flow to the lower extremity. These pulse deficits are a part of limb malperfusion syndrome. Symptoms of malperfusion syndrome vary greatly and depend on the vessels involved. Malperfusion of the branches of the aortic arch can result in stroke or altered sensorium. Compromise of intra-abdominal vessels due to dissection can involve the mesenteric bed, the renal arteries, or both, resulting in laboratory derangements such as lactic acidosis and renal failure.
How aortic dissection and malperfusion syndrome occur
Over time, shear forces on the aortic wall result in degeneration of the tunica intima and media. Dissection occurs when deterioration of the intima causes propagation of blood through a cleavage plane into the outer portion of the diseased media, forming a false lumen.
Anterograde or retrograde progression of dissection depends on the balance of the pressure gradient between true and false lumens.2 With every systolic ventricular contraction, a fluid and pressure wave travels down both lumens (true and false). However, the pressure gradient between the false and true lumens allows the more pliable intimal flap to bulge into the true lumen and ostia of branch vessels, resulting in static or dynamic obstruction.
Static obstruction occurs when the false lumen projects completely into the branch vessel and there is resultant thrombosis. As the name implies, dynamic obstruction is intermittent and is responsible for 80% of the cases of malperfusion syndrome.3 Dynamic obstruction has 2 distinct mechanisms: hypoperfusion through the true lumen due to impaired flow, and prolapse of the false lumen into a branch vessel.
Factors that exacerbate hypoperfusion through the true lumen and make obliteration by the false lumen more likely include large circumference of the dissected aorta, rapid heart rate, and high systolic pressure.4 Therefore, it is important to control the heart rate and blood pressure using beta-blockers in cases of aortic dissection with malperfusion syndrome. This treatment may resolve the dynamic obstruction through expansion and resumption of perfusion through the true lumen.5
MANAGEMENT OF MALPERFUSION SYNDROME
Aortic dissection can be classified as either Stanford type A (involving the ascending aorta) or type B (involving the descending aorta). Type B dissection associated with malperfusion syndrome is termed “complicated” type B aortic dissection. Our patient had both Stanford type A and complicated type B aortic dissection.
Unlike type A aortic dissection, which requires definitive open surgical repair, complicated type B aortic dissection occasionally responds to medical management alone. A plausible explanation for resolution of limb malperfusion with optimal blood pressure control is expansion of the true lumen and obliteration of the false lumen, as was likely the case in our patient.
In most cases, however, limb malperfusion persists despite optimal medical management. In such patients, endovascular graft stenting or open surgical repair may be needed. Open surgical repair procedures like bypass grafting or surgical fenestration are associated with significant rates of mortality and morbidity.5 Therefore, an endovascular approach rather than conventional surgical repair for complicated type B aortic dissection is advocated after optimal medical management.6 Endovascular repair also promotes favorable aortic remodeling without the morbidity associated with open surgical repair.
- Namana V, Balasubramanian R, Kariyanna PT, Sarasam R, Namana S, Shetty V. Aortic dissection with hemopericardium and thrombosed left common iliac artery presenting as acute limb ischemia: a case report and review. Am J Med Case Rep 2015; 3(10):338–343. doi:10.12691/ajmcr-3-10-9
- Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med 2016; 21(3):264–273. doi:10.1177/1358863X15625371
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications—principles and results. J Vasc Interv Radiol 1997; 8(4):605–625. pmid:9232578
- Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214(1):99–106. doi:10.1148/radiology.214.1.r00ja3499
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014; 3(4):351–367. doi:10.3978/j.issn.2225-319X.2014.07.05
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340(20):1546–1552. doi:10.1056/NEJM199905203402004
A 40-year-old man with a history of hypertension and alcohol abuse presented with acute onset of mild chest tightness, left leg pain, and increasing agitation, which prevented us from obtaining additional meaningful information from him.
On admission, his heart rate was 120 beats per minute, blood pressure 211/122 mm Hg, respiratory rate 18 per minute, and oxygen saturation 92% on room air. Given his history of alcohol abuse, we checked his blood ethanol level, which was less than 0.01%, well below the legal limit for intoxication.
We gave the patient intravenous lorazepam for possible alcohol withdrawal and started labetalol by intravenous infusion to lower his blood pressure.
On physical examination, his left lower extremity was cold and without pulses, including the femoral pulse. Suspecting acute arterial thrombosis, we ordered immediate computed tomographic (CT) angiography of the abdomen and pelvis with left lower extremity runoff. The images showed dissection of the abdominal aorta with extension to both the left and right common iliac arteries and the origin of the right external iliac artery. There was resultant occlusion of the left external iliac artery (Figure 1).
Immediate CT angiography of the chest was then performed, which revealed dissection of the thoracic aorta as well, starting superior to the aortic valve annulus and involving the ascending aorta, aortic arch, and the entire descending thoracic aorta (Figure 2).
The patient underwent emergency surgical repair of the aortic root, ascending aorta, and aortic arch. Residual dissection of the descending aorta was managed conservatively with blood pressure control using intravenous labetalol initially, which was then switched to oral carvedilol, and the pulses returned in his left lower extremity. He had an unremarkable postoperative recovery and was discharged after 1 week.
AORTIC DISSECTION AND MALPERFUSION SYNDROME
Aortic dissection is most often associated with acute onset of sharp chest pain and upper back pain. On rare occasions, it can have an atypical presentation such as stroke, paraplegia, mesenteric ischemia, or lower limb malperfusion.1
Extension of aortic dissection into the iliac and femoral arteries can cause impaired or absent blood flow to the lower extremity. These pulse deficits are a part of limb malperfusion syndrome. Symptoms of malperfusion syndrome vary greatly and depend on the vessels involved. Malperfusion of the branches of the aortic arch can result in stroke or altered sensorium. Compromise of intra-abdominal vessels due to dissection can involve the mesenteric bed, the renal arteries, or both, resulting in laboratory derangements such as lactic acidosis and renal failure.
How aortic dissection and malperfusion syndrome occur
Over time, shear forces on the aortic wall result in degeneration of the tunica intima and media. Dissection occurs when deterioration of the intima causes propagation of blood through a cleavage plane into the outer portion of the diseased media, forming a false lumen.
Anterograde or retrograde progression of dissection depends on the balance of the pressure gradient between true and false lumens.2 With every systolic ventricular contraction, a fluid and pressure wave travels down both lumens (true and false). However, the pressure gradient between the false and true lumens allows the more pliable intimal flap to bulge into the true lumen and ostia of branch vessels, resulting in static or dynamic obstruction.
Static obstruction occurs when the false lumen projects completely into the branch vessel and there is resultant thrombosis. As the name implies, dynamic obstruction is intermittent and is responsible for 80% of the cases of malperfusion syndrome.3 Dynamic obstruction has 2 distinct mechanisms: hypoperfusion through the true lumen due to impaired flow, and prolapse of the false lumen into a branch vessel.
Factors that exacerbate hypoperfusion through the true lumen and make obliteration by the false lumen more likely include large circumference of the dissected aorta, rapid heart rate, and high systolic pressure.4 Therefore, it is important to control the heart rate and blood pressure using beta-blockers in cases of aortic dissection with malperfusion syndrome. This treatment may resolve the dynamic obstruction through expansion and resumption of perfusion through the true lumen.5
MANAGEMENT OF MALPERFUSION SYNDROME
Aortic dissection can be classified as either Stanford type A (involving the ascending aorta) or type B (involving the descending aorta). Type B dissection associated with malperfusion syndrome is termed “complicated” type B aortic dissection. Our patient had both Stanford type A and complicated type B aortic dissection.
Unlike type A aortic dissection, which requires definitive open surgical repair, complicated type B aortic dissection occasionally responds to medical management alone. A plausible explanation for resolution of limb malperfusion with optimal blood pressure control is expansion of the true lumen and obliteration of the false lumen, as was likely the case in our patient.
In most cases, however, limb malperfusion persists despite optimal medical management. In such patients, endovascular graft stenting or open surgical repair may be needed. Open surgical repair procedures like bypass grafting or surgical fenestration are associated with significant rates of mortality and morbidity.5 Therefore, an endovascular approach rather than conventional surgical repair for complicated type B aortic dissection is advocated after optimal medical management.6 Endovascular repair also promotes favorable aortic remodeling without the morbidity associated with open surgical repair.
A 40-year-old man with a history of hypertension and alcohol abuse presented with acute onset of mild chest tightness, left leg pain, and increasing agitation, which prevented us from obtaining additional meaningful information from him.
On admission, his heart rate was 120 beats per minute, blood pressure 211/122 mm Hg, respiratory rate 18 per minute, and oxygen saturation 92% on room air. Given his history of alcohol abuse, we checked his blood ethanol level, which was less than 0.01%, well below the legal limit for intoxication.
We gave the patient intravenous lorazepam for possible alcohol withdrawal and started labetalol by intravenous infusion to lower his blood pressure.
On physical examination, his left lower extremity was cold and without pulses, including the femoral pulse. Suspecting acute arterial thrombosis, we ordered immediate computed tomographic (CT) angiography of the abdomen and pelvis with left lower extremity runoff. The images showed dissection of the abdominal aorta with extension to both the left and right common iliac arteries and the origin of the right external iliac artery. There was resultant occlusion of the left external iliac artery (Figure 1).
Immediate CT angiography of the chest was then performed, which revealed dissection of the thoracic aorta as well, starting superior to the aortic valve annulus and involving the ascending aorta, aortic arch, and the entire descending thoracic aorta (Figure 2).
The patient underwent emergency surgical repair of the aortic root, ascending aorta, and aortic arch. Residual dissection of the descending aorta was managed conservatively with blood pressure control using intravenous labetalol initially, which was then switched to oral carvedilol, and the pulses returned in his left lower extremity. He had an unremarkable postoperative recovery and was discharged after 1 week.
AORTIC DISSECTION AND MALPERFUSION SYNDROME
Aortic dissection is most often associated with acute onset of sharp chest pain and upper back pain. On rare occasions, it can have an atypical presentation such as stroke, paraplegia, mesenteric ischemia, or lower limb malperfusion.1
Extension of aortic dissection into the iliac and femoral arteries can cause impaired or absent blood flow to the lower extremity. These pulse deficits are a part of limb malperfusion syndrome. Symptoms of malperfusion syndrome vary greatly and depend on the vessels involved. Malperfusion of the branches of the aortic arch can result in stroke or altered sensorium. Compromise of intra-abdominal vessels due to dissection can involve the mesenteric bed, the renal arteries, or both, resulting in laboratory derangements such as lactic acidosis and renal failure.
How aortic dissection and malperfusion syndrome occur
Over time, shear forces on the aortic wall result in degeneration of the tunica intima and media. Dissection occurs when deterioration of the intima causes propagation of blood through a cleavage plane into the outer portion of the diseased media, forming a false lumen.
Anterograde or retrograde progression of dissection depends on the balance of the pressure gradient between true and false lumens.2 With every systolic ventricular contraction, a fluid and pressure wave travels down both lumens (true and false). However, the pressure gradient between the false and true lumens allows the more pliable intimal flap to bulge into the true lumen and ostia of branch vessels, resulting in static or dynamic obstruction.
Static obstruction occurs when the false lumen projects completely into the branch vessel and there is resultant thrombosis. As the name implies, dynamic obstruction is intermittent and is responsible for 80% of the cases of malperfusion syndrome.3 Dynamic obstruction has 2 distinct mechanisms: hypoperfusion through the true lumen due to impaired flow, and prolapse of the false lumen into a branch vessel.
Factors that exacerbate hypoperfusion through the true lumen and make obliteration by the false lumen more likely include large circumference of the dissected aorta, rapid heart rate, and high systolic pressure.4 Therefore, it is important to control the heart rate and blood pressure using beta-blockers in cases of aortic dissection with malperfusion syndrome. This treatment may resolve the dynamic obstruction through expansion and resumption of perfusion through the true lumen.5
MANAGEMENT OF MALPERFUSION SYNDROME
Aortic dissection can be classified as either Stanford type A (involving the ascending aorta) or type B (involving the descending aorta). Type B dissection associated with malperfusion syndrome is termed “complicated” type B aortic dissection. Our patient had both Stanford type A and complicated type B aortic dissection.
Unlike type A aortic dissection, which requires definitive open surgical repair, complicated type B aortic dissection occasionally responds to medical management alone. A plausible explanation for resolution of limb malperfusion with optimal blood pressure control is expansion of the true lumen and obliteration of the false lumen, as was likely the case in our patient.
In most cases, however, limb malperfusion persists despite optimal medical management. In such patients, endovascular graft stenting or open surgical repair may be needed. Open surgical repair procedures like bypass grafting or surgical fenestration are associated with significant rates of mortality and morbidity.5 Therefore, an endovascular approach rather than conventional surgical repair for complicated type B aortic dissection is advocated after optimal medical management.6 Endovascular repair also promotes favorable aortic remodeling without the morbidity associated with open surgical repair.
- Namana V, Balasubramanian R, Kariyanna PT, Sarasam R, Namana S, Shetty V. Aortic dissection with hemopericardium and thrombosed left common iliac artery presenting as acute limb ischemia: a case report and review. Am J Med Case Rep 2015; 3(10):338–343. doi:10.12691/ajmcr-3-10-9
- Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med 2016; 21(3):264–273. doi:10.1177/1358863X15625371
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications—principles and results. J Vasc Interv Radiol 1997; 8(4):605–625. pmid:9232578
- Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214(1):99–106. doi:10.1148/radiology.214.1.r00ja3499
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014; 3(4):351–367. doi:10.3978/j.issn.2225-319X.2014.07.05
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340(20):1546–1552. doi:10.1056/NEJM199905203402004
- Namana V, Balasubramanian R, Kariyanna PT, Sarasam R, Namana S, Shetty V. Aortic dissection with hemopericardium and thrombosed left common iliac artery presenting as acute limb ischemia: a case report and review. Am J Med Case Rep 2015; 3(10):338–343. doi:10.12691/ajmcr-3-10-9
- Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med 2016; 21(3):264–273. doi:10.1177/1358863X15625371
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications—principles and results. J Vasc Interv Radiol 1997; 8(4):605–625. pmid:9232578
- Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214(1):99–106. doi:10.1148/radiology.214.1.r00ja3499
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014; 3(4):351–367. doi:10.3978/j.issn.2225-319X.2014.07.05
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340(20):1546–1552. doi:10.1056/NEJM199905203402004
Is Pap testing still needed after hysterectomy?
A 50-year-old woman presents for a new patient visit. She underwent vaginal hysterectomy for menorrhagia 4 years ago, with removal of the uterus and cervix. Tissue studies at that time were negative for dysplasia. Her previous physician performed routine Papanicolaou (Pap) tests, and she asks you to continue this screening. How do you counsel her about Pap testing after hysterectomy for benign disease?
SCREENING GUIDELINES
Introduced in 1941, the Pap test is an example of a successful screening tool, improving detection of early cervical cancer and reducing rates of morbidity and death due to cervical cancer. Early stages of cervical cancer are the most curable.1
Screening in women who have a cervix
In 2012, the US Preventive Services Task Force (USPSTF) updated its 2003 recommendations for cervical cancer screening.1 In the same year, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology published a consensus guideline.2 This was followed by publication of a guideline from the American College of Obstetricians and Gynecologists.3 These guidelines all recommend Pap testing for cervical cancer every 3 years in women ages 21 to 65. In women ages 30 to 65, the screening interval can be lengthened to every 5 years if the patient undergoes cotesting for human papillomavirus (HPV). These recommendations apply only to women with a cervix.
No screening after hysterectomy for benign indications
Women who undergo hysterectomy with complete removal of the cervix for benign indications, ie, for reasons other than malignancy, are no longer at risk of cervical cancer. Pap testing could still detect vaginal cancer, but vaginal cancer is rare and screening for it is not indicated. The USPSTF 2003 and 2012 guidelines recommend not performing Pap testing in women who had had a hysterectomy for benign indications.1
Vaginal cancer is rare
Although cervical and vaginal cancers share risk factors, vaginal cancer accounts for only 0.3% of all invasive cancers and 1% to 2% of all gynecologic malignancies in the United States.4
A review of 39 population-based cancer registries from 1998 to 2003 found the incidence rate for in situ vaginal cancer to be 0.18 per 100,000 women, and the incidence rate for invasive vaginal cancer was 0.69 per 100,000. Rates were higher in older women and in certain ethnic and racial groups, including black and Hispanic women.4
When the cervix is removed during hysterectomy for a benign indication, the patient’s risk of vaginal cancer or its precursors is extremely low. Pearce et al5 reviewed Pap tests obtained from the vaginal cuff in 6,265 women who had undergone hysterectomy for benign disease. Their 2-year study reviewed 9,610 vaginal Pap tests, and in only 5 women was vaginal intraepithelial neoplasia type I or II found, and none of the 5 had biopsy-proven vaginal cancer. Only 1.1% of all Pap tests were abnormal. The authors concluded that the positive predictive value for detecting vaginal cancer was 0%.5
A retrospective study by Piscitelli et al6 in 1995 looked back 10 years and found an extremely low incidence of vaginal dysplasia in women who had undergone hysterectomy for a benign indication. Their findings, coupled with the high rate of false-positive tests, do not support cytologic screening of the vagina after hysterectomy for a benign indication. The data also suggested that 633 tests would need to be performed to diagnose 1 case of vaginal dysplasia.6 Other studies have also reported a low yield of vaginal cuff cytologic testing after hysterectomy for benign disease.
Therefore, given the low prevalence of disease and the lack of evidence of benefit of screening after hysterectomy for benign indications, Pap testing of the vaginal cuff is not recommended in these patients.7
Screening for women at high risk after hysterectomy
For women with a history of grade 2 or 3 cervical intraepithelial neoplasia who have undergone hysterectomy, there are only limited data on subsequent disease risk.
Wiener et al8 followed 193 post-hysterectomy patients who had a history of cervical intraepithelial neoplasia with Pap testing annually for more than 10 years for a total of 2,800 years of follow-up. The estimated incidence of abnormal cytology (0.7/1,000) was higher than in the general population.8
Thus, for these women and for others at high risk who have undergone hysterectomy and have a previous diagnosis of cervical cancer, who had been exposed to diethylstilbestrol, or who are immunocompromised, Pap testing to screen for cancer in the vaginal cuff is recommended, as they are at higher risk of dysplasia at the vaginal cuff.2
PRACTICE TRENDS, AREAS FOR IMPROVEMENT
Despite recommendations against screening, many providers continue this non-evidence-based practice.4
The 2000–2013 National Health Interview Survey of women age 20 or older who had undergone hysterectomy asked about their most recent Pap test by self-report. Women were excluded if they had a history of cervical cancer, if they had had a Pap test for another health problem, or if the result of the recent Pap test was not known. In 2000, nearly half (49.1%) of the respondents said they had received a Pap test in the previous year; in 2013, the percentage undergoing testing was down to 32.1%, but testing was unnecessary in 22.1%. Screening was largely due to clinician recommendations, but it was initiated by patients without clinician recommendations in about one-fourth of cases.9 Lack of knowledge of the revised 2012 guidelines was cited as the primary reason for unnecessary screening.10
A study of provider attitudes toward the cancer screening guidelines cited several reasons for nonadherence: patient concern about the guidelines; quality metrics that are incongruent with the guidelines; provider disagreement with the guidelines; risk of malpractice litigation; and lack of time to discuss the guidelines with patients.11
As the healthcare landscape changes to team-based care, the clinician and the entire healthcare team should educate patients about the role of vaginal cancer screening after hysterectomy for benign reasons. Given the limited time clinicians have with patients during an office visit, innovative tools and systems outside the office are needed to educate patients about the risks and benefits of screening.11 And notices in the electronic medical record may help busy clinicians keep up with current guidelines.10
THE CLINICAL BOTTOM LINE
Pap testing to screen for vaginal cancer in women who have undergone hysterectomy for a benign indication is an example of more testing, not better care. Evidence is lacking to justify this test in women who are not at high risk of cervical cancer. To reduce the overuse of cytology screening tests, providers need to stay informed about evidence-based best practices and and to pass this information along to patients.
We should focus our resources on HPV vaccination and outreach to increase screening efforts in geographic areas with low rates of Pap testing rather than provide unnecessary Pap testing for women who have undergone hysterectomy for a benign indication.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156(11):880–891, W312. doi:10.7326/0003-4819-156-12-201206190-00424
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120(5):1222–1238. doi:10.1097/AOG.0b013e318277c92a
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(10 suppl):2873–2882. doi:10.1002/cncr.23757
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335(21):1559–1562. doi:10.1056/NEJM199611213352103
- Piscitelli JT, Bastian LA, Wilkes A, Simel DL. Cytologic screening after hysterectomy for benign disease. Am J Obstet Gynecol 1995;173(2):424–432. pmid:7645617
- Stokes-Lampard H, Wilson S, Waddell C, Ryan A, Holder R, Kehoe S. Vaginal vault smears after hysterectomy for reasons other than malignancy: a systematic review of the literature. BJOG 2006; 113(12):1354–1365. doi:10.1111/j.1471-0528.2006.01099.x
- Wiener JJ, Sweetnam PM, Jones JM. Long term follow up of women after hysterectomy with a history of pre-invasive cancer of the cervix. Br J Obstet Gynaecol 1992; 99(11):907–910. pmid:1450141
- Guo F, Kuo YF. Roles of health care providers and patients in initiation of unnecessary Papanicolaou testing after total hysterectomy. Am J Public Health 2016; 106(11):2005–2011. doi:10.2105/AJPH.2016.303360
- Teoh DG, Marriott AE, Isaksson Vogel R, et al. Adherence to the 2012 national cervical cancer screening guidelines: a pilot study. Am J Obstet Gynecol 2015; 212(1):62.e1–e9. doi:10.1016/j.ajog.2014.06.057
- Haas JS, Sprague BL, Klabunde CN, et al; PROSPR (Population-based Research Optimizing Screening through Personalized Regimens) Consortium. Provider attitudes and screening practices following changes in breast and cervical cancer screening guidelines. J Gen Intern Med 2016; 31(1):52–59. doi:10.1007/s11606-015-3449-5
A 50-year-old woman presents for a new patient visit. She underwent vaginal hysterectomy for menorrhagia 4 years ago, with removal of the uterus and cervix. Tissue studies at that time were negative for dysplasia. Her previous physician performed routine Papanicolaou (Pap) tests, and she asks you to continue this screening. How do you counsel her about Pap testing after hysterectomy for benign disease?
SCREENING GUIDELINES
Introduced in 1941, the Pap test is an example of a successful screening tool, improving detection of early cervical cancer and reducing rates of morbidity and death due to cervical cancer. Early stages of cervical cancer are the most curable.1
Screening in women who have a cervix
In 2012, the US Preventive Services Task Force (USPSTF) updated its 2003 recommendations for cervical cancer screening.1 In the same year, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology published a consensus guideline.2 This was followed by publication of a guideline from the American College of Obstetricians and Gynecologists.3 These guidelines all recommend Pap testing for cervical cancer every 3 years in women ages 21 to 65. In women ages 30 to 65, the screening interval can be lengthened to every 5 years if the patient undergoes cotesting for human papillomavirus (HPV). These recommendations apply only to women with a cervix.
No screening after hysterectomy for benign indications
Women who undergo hysterectomy with complete removal of the cervix for benign indications, ie, for reasons other than malignancy, are no longer at risk of cervical cancer. Pap testing could still detect vaginal cancer, but vaginal cancer is rare and screening for it is not indicated. The USPSTF 2003 and 2012 guidelines recommend not performing Pap testing in women who had had a hysterectomy for benign indications.1
Vaginal cancer is rare
Although cervical and vaginal cancers share risk factors, vaginal cancer accounts for only 0.3% of all invasive cancers and 1% to 2% of all gynecologic malignancies in the United States.4
A review of 39 population-based cancer registries from 1998 to 2003 found the incidence rate for in situ vaginal cancer to be 0.18 per 100,000 women, and the incidence rate for invasive vaginal cancer was 0.69 per 100,000. Rates were higher in older women and in certain ethnic and racial groups, including black and Hispanic women.4
When the cervix is removed during hysterectomy for a benign indication, the patient’s risk of vaginal cancer or its precursors is extremely low. Pearce et al5 reviewed Pap tests obtained from the vaginal cuff in 6,265 women who had undergone hysterectomy for benign disease. Their 2-year study reviewed 9,610 vaginal Pap tests, and in only 5 women was vaginal intraepithelial neoplasia type I or II found, and none of the 5 had biopsy-proven vaginal cancer. Only 1.1% of all Pap tests were abnormal. The authors concluded that the positive predictive value for detecting vaginal cancer was 0%.5
A retrospective study by Piscitelli et al6 in 1995 looked back 10 years and found an extremely low incidence of vaginal dysplasia in women who had undergone hysterectomy for a benign indication. Their findings, coupled with the high rate of false-positive tests, do not support cytologic screening of the vagina after hysterectomy for a benign indication. The data also suggested that 633 tests would need to be performed to diagnose 1 case of vaginal dysplasia.6 Other studies have also reported a low yield of vaginal cuff cytologic testing after hysterectomy for benign disease.
Therefore, given the low prevalence of disease and the lack of evidence of benefit of screening after hysterectomy for benign indications, Pap testing of the vaginal cuff is not recommended in these patients.7
Screening for women at high risk after hysterectomy
For women with a history of grade 2 or 3 cervical intraepithelial neoplasia who have undergone hysterectomy, there are only limited data on subsequent disease risk.
Wiener et al8 followed 193 post-hysterectomy patients who had a history of cervical intraepithelial neoplasia with Pap testing annually for more than 10 years for a total of 2,800 years of follow-up. The estimated incidence of abnormal cytology (0.7/1,000) was higher than in the general population.8
Thus, for these women and for others at high risk who have undergone hysterectomy and have a previous diagnosis of cervical cancer, who had been exposed to diethylstilbestrol, or who are immunocompromised, Pap testing to screen for cancer in the vaginal cuff is recommended, as they are at higher risk of dysplasia at the vaginal cuff.2
PRACTICE TRENDS, AREAS FOR IMPROVEMENT
Despite recommendations against screening, many providers continue this non-evidence-based practice.4
The 2000–2013 National Health Interview Survey of women age 20 or older who had undergone hysterectomy asked about their most recent Pap test by self-report. Women were excluded if they had a history of cervical cancer, if they had had a Pap test for another health problem, or if the result of the recent Pap test was not known. In 2000, nearly half (49.1%) of the respondents said they had received a Pap test in the previous year; in 2013, the percentage undergoing testing was down to 32.1%, but testing was unnecessary in 22.1%. Screening was largely due to clinician recommendations, but it was initiated by patients without clinician recommendations in about one-fourth of cases.9 Lack of knowledge of the revised 2012 guidelines was cited as the primary reason for unnecessary screening.10
A study of provider attitudes toward the cancer screening guidelines cited several reasons for nonadherence: patient concern about the guidelines; quality metrics that are incongruent with the guidelines; provider disagreement with the guidelines; risk of malpractice litigation; and lack of time to discuss the guidelines with patients.11
As the healthcare landscape changes to team-based care, the clinician and the entire healthcare team should educate patients about the role of vaginal cancer screening after hysterectomy for benign reasons. Given the limited time clinicians have with patients during an office visit, innovative tools and systems outside the office are needed to educate patients about the risks and benefits of screening.11 And notices in the electronic medical record may help busy clinicians keep up with current guidelines.10
THE CLINICAL BOTTOM LINE
Pap testing to screen for vaginal cancer in women who have undergone hysterectomy for a benign indication is an example of more testing, not better care. Evidence is lacking to justify this test in women who are not at high risk of cervical cancer. To reduce the overuse of cytology screening tests, providers need to stay informed about evidence-based best practices and and to pass this information along to patients.
We should focus our resources on HPV vaccination and outreach to increase screening efforts in geographic areas with low rates of Pap testing rather than provide unnecessary Pap testing for women who have undergone hysterectomy for a benign indication.
A 50-year-old woman presents for a new patient visit. She underwent vaginal hysterectomy for menorrhagia 4 years ago, with removal of the uterus and cervix. Tissue studies at that time were negative for dysplasia. Her previous physician performed routine Papanicolaou (Pap) tests, and she asks you to continue this screening. How do you counsel her about Pap testing after hysterectomy for benign disease?
SCREENING GUIDELINES
Introduced in 1941, the Pap test is an example of a successful screening tool, improving detection of early cervical cancer and reducing rates of morbidity and death due to cervical cancer. Early stages of cervical cancer are the most curable.1
Screening in women who have a cervix
In 2012, the US Preventive Services Task Force (USPSTF) updated its 2003 recommendations for cervical cancer screening.1 In the same year, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology published a consensus guideline.2 This was followed by publication of a guideline from the American College of Obstetricians and Gynecologists.3 These guidelines all recommend Pap testing for cervical cancer every 3 years in women ages 21 to 65. In women ages 30 to 65, the screening interval can be lengthened to every 5 years if the patient undergoes cotesting for human papillomavirus (HPV). These recommendations apply only to women with a cervix.
No screening after hysterectomy for benign indications
Women who undergo hysterectomy with complete removal of the cervix for benign indications, ie, for reasons other than malignancy, are no longer at risk of cervical cancer. Pap testing could still detect vaginal cancer, but vaginal cancer is rare and screening for it is not indicated. The USPSTF 2003 and 2012 guidelines recommend not performing Pap testing in women who had had a hysterectomy for benign indications.1
Vaginal cancer is rare
Although cervical and vaginal cancers share risk factors, vaginal cancer accounts for only 0.3% of all invasive cancers and 1% to 2% of all gynecologic malignancies in the United States.4
A review of 39 population-based cancer registries from 1998 to 2003 found the incidence rate for in situ vaginal cancer to be 0.18 per 100,000 women, and the incidence rate for invasive vaginal cancer was 0.69 per 100,000. Rates were higher in older women and in certain ethnic and racial groups, including black and Hispanic women.4
When the cervix is removed during hysterectomy for a benign indication, the patient’s risk of vaginal cancer or its precursors is extremely low. Pearce et al5 reviewed Pap tests obtained from the vaginal cuff in 6,265 women who had undergone hysterectomy for benign disease. Their 2-year study reviewed 9,610 vaginal Pap tests, and in only 5 women was vaginal intraepithelial neoplasia type I or II found, and none of the 5 had biopsy-proven vaginal cancer. Only 1.1% of all Pap tests were abnormal. The authors concluded that the positive predictive value for detecting vaginal cancer was 0%.5
A retrospective study by Piscitelli et al6 in 1995 looked back 10 years and found an extremely low incidence of vaginal dysplasia in women who had undergone hysterectomy for a benign indication. Their findings, coupled with the high rate of false-positive tests, do not support cytologic screening of the vagina after hysterectomy for a benign indication. The data also suggested that 633 tests would need to be performed to diagnose 1 case of vaginal dysplasia.6 Other studies have also reported a low yield of vaginal cuff cytologic testing after hysterectomy for benign disease.
Therefore, given the low prevalence of disease and the lack of evidence of benefit of screening after hysterectomy for benign indications, Pap testing of the vaginal cuff is not recommended in these patients.7
Screening for women at high risk after hysterectomy
For women with a history of grade 2 or 3 cervical intraepithelial neoplasia who have undergone hysterectomy, there are only limited data on subsequent disease risk.
Wiener et al8 followed 193 post-hysterectomy patients who had a history of cervical intraepithelial neoplasia with Pap testing annually for more than 10 years for a total of 2,800 years of follow-up. The estimated incidence of abnormal cytology (0.7/1,000) was higher than in the general population.8
Thus, for these women and for others at high risk who have undergone hysterectomy and have a previous diagnosis of cervical cancer, who had been exposed to diethylstilbestrol, or who are immunocompromised, Pap testing to screen for cancer in the vaginal cuff is recommended, as they are at higher risk of dysplasia at the vaginal cuff.2
PRACTICE TRENDS, AREAS FOR IMPROVEMENT
Despite recommendations against screening, many providers continue this non-evidence-based practice.4
The 2000–2013 National Health Interview Survey of women age 20 or older who had undergone hysterectomy asked about their most recent Pap test by self-report. Women were excluded if they had a history of cervical cancer, if they had had a Pap test for another health problem, or if the result of the recent Pap test was not known. In 2000, nearly half (49.1%) of the respondents said they had received a Pap test in the previous year; in 2013, the percentage undergoing testing was down to 32.1%, but testing was unnecessary in 22.1%. Screening was largely due to clinician recommendations, but it was initiated by patients without clinician recommendations in about one-fourth of cases.9 Lack of knowledge of the revised 2012 guidelines was cited as the primary reason for unnecessary screening.10
A study of provider attitudes toward the cancer screening guidelines cited several reasons for nonadherence: patient concern about the guidelines; quality metrics that are incongruent with the guidelines; provider disagreement with the guidelines; risk of malpractice litigation; and lack of time to discuss the guidelines with patients.11
As the healthcare landscape changes to team-based care, the clinician and the entire healthcare team should educate patients about the role of vaginal cancer screening after hysterectomy for benign reasons. Given the limited time clinicians have with patients during an office visit, innovative tools and systems outside the office are needed to educate patients about the risks and benefits of screening.11 And notices in the electronic medical record may help busy clinicians keep up with current guidelines.10
THE CLINICAL BOTTOM LINE
Pap testing to screen for vaginal cancer in women who have undergone hysterectomy for a benign indication is an example of more testing, not better care. Evidence is lacking to justify this test in women who are not at high risk of cervical cancer. To reduce the overuse of cytology screening tests, providers need to stay informed about evidence-based best practices and and to pass this information along to patients.
We should focus our resources on HPV vaccination and outreach to increase screening efforts in geographic areas with low rates of Pap testing rather than provide unnecessary Pap testing for women who have undergone hysterectomy for a benign indication.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156(11):880–891, W312. doi:10.7326/0003-4819-156-12-201206190-00424
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120(5):1222–1238. doi:10.1097/AOG.0b013e318277c92a
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(10 suppl):2873–2882. doi:10.1002/cncr.23757
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335(21):1559–1562. doi:10.1056/NEJM199611213352103
- Piscitelli JT, Bastian LA, Wilkes A, Simel DL. Cytologic screening after hysterectomy for benign disease. Am J Obstet Gynecol 1995;173(2):424–432. pmid:7645617
- Stokes-Lampard H, Wilson S, Waddell C, Ryan A, Holder R, Kehoe S. Vaginal vault smears after hysterectomy for reasons other than malignancy: a systematic review of the literature. BJOG 2006; 113(12):1354–1365. doi:10.1111/j.1471-0528.2006.01099.x
- Wiener JJ, Sweetnam PM, Jones JM. Long term follow up of women after hysterectomy with a history of pre-invasive cancer of the cervix. Br J Obstet Gynaecol 1992; 99(11):907–910. pmid:1450141
- Guo F, Kuo YF. Roles of health care providers and patients in initiation of unnecessary Papanicolaou testing after total hysterectomy. Am J Public Health 2016; 106(11):2005–2011. doi:10.2105/AJPH.2016.303360
- Teoh DG, Marriott AE, Isaksson Vogel R, et al. Adherence to the 2012 national cervical cancer screening guidelines: a pilot study. Am J Obstet Gynecol 2015; 212(1):62.e1–e9. doi:10.1016/j.ajog.2014.06.057
- Haas JS, Sprague BL, Klabunde CN, et al; PROSPR (Population-based Research Optimizing Screening through Personalized Regimens) Consortium. Provider attitudes and screening practices following changes in breast and cervical cancer screening guidelines. J Gen Intern Med 2016; 31(1):52–59. doi:10.1007/s11606-015-3449-5
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156(11):880–891, W312. doi:10.7326/0003-4819-156-12-201206190-00424
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120(5):1222–1238. doi:10.1097/AOG.0b013e318277c92a
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(10 suppl):2873–2882. doi:10.1002/cncr.23757
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335(21):1559–1562. doi:10.1056/NEJM199611213352103
- Piscitelli JT, Bastian LA, Wilkes A, Simel DL. Cytologic screening after hysterectomy for benign disease. Am J Obstet Gynecol 1995;173(2):424–432. pmid:7645617
- Stokes-Lampard H, Wilson S, Waddell C, Ryan A, Holder R, Kehoe S. Vaginal vault smears after hysterectomy for reasons other than malignancy: a systematic review of the literature. BJOG 2006; 113(12):1354–1365. doi:10.1111/j.1471-0528.2006.01099.x
- Wiener JJ, Sweetnam PM, Jones JM. Long term follow up of women after hysterectomy with a history of pre-invasive cancer of the cervix. Br J Obstet Gynaecol 1992; 99(11):907–910. pmid:1450141
- Guo F, Kuo YF. Roles of health care providers and patients in initiation of unnecessary Papanicolaou testing after total hysterectomy. Am J Public Health 2016; 106(11):2005–2011. doi:10.2105/AJPH.2016.303360
- Teoh DG, Marriott AE, Isaksson Vogel R, et al. Adherence to the 2012 national cervical cancer screening guidelines: a pilot study. Am J Obstet Gynecol 2015; 212(1):62.e1–e9. doi:10.1016/j.ajog.2014.06.057
- Haas JS, Sprague BL, Klabunde CN, et al; PROSPR (Population-based Research Optimizing Screening through Personalized Regimens) Consortium. Provider attitudes and screening practices following changes in breast and cervical cancer screening guidelines. J Gen Intern Med 2016; 31(1):52–59. doi:10.1007/s11606-015-3449-5