User login
Air pollution linked to childhood hypertension
Third-trimester exposure to airborne particulate matter (PM) smaller than 2.5 mcm in size (PM2.5) has been linked to higher levels of systolic blood pressure during childhood.
Mingyu Zhang of Johns Hopkins Bloomberg School of Public Health, Baltimore, and his associates found that the highest tertile exposure was tied to an increased likelihood of childhood elevated BP, defined as systolic blood pressure higher than the 90th percentile, compared with those in the lowest tertile (relative risk, 1.60; 95% confidence interval, 1.12-2.27).
Previous studies have shown a relationship between exposure to PM2.5and elevated BP in children and adults. Mouse models suggest that PM2.5 may interfere with in utero development of the cardiovascular system. One previous study found evidence that third-trimester exposure to PM2.5was linked to heightened BP in newborns, while a retrospective analysis showed no association with BP in adolescents.
They identified PM2.5 exposure by matching the mother’s residential address to the U.S. Environmental Protection Agency’s local air quality monitors, from which they extracted daily PM2.5 values.
For every 5 mcg/m3 increment in PM2.5 exposure, the relative risk for heightened BP in a child at a single visit was 1.46 (95% CI, 1.17-1.83). The relative risk increased after independent additions to the confounder model of birth weight (RR, 3.39; 95% CI, 0.63-6.15), gestational age (RR, 3.08; 95% CI, 0.33-5.82), and child body mass index (BMI) z score (RR, 2.75; 95% CI, 0.01-5.50).
A multivariable-adjusted cubic spline model revealed a significant increase in risk of elevated BP at a cutoff threshold of 13 mcg/m3 (RR, 1.80; 95% CI, 1.33-2.44).
Further analysis suggested that 35% of the association between exposure and elevated BP risk was mediated by birth weight and BMI z score during childhood. When these factors were added to the models, the association between PM exposure and BP risk was no longer significant.
Although the findings are intriguing, they cannot prove causation, according to the researchers.
The study was funded by the National Institutes of Health and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
SOURCE: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
A paper recently published by the same group outlines a possible mechanism by which PM2.5 might cause harm – specifically, an increase in placental intrauterine inflammation. The study adds evidence to the case that air pollution is linked to hypertension as it includes more economically disadvantaged subjects, children of color, preterm births, and small-for-gestational-age births. Overall, the study imaginatively made use of pediatric data in a disadvantaged urban setting to better understand how air pollution affects an important physiological outcome.
Diane Gold, MD, and Antonella Zanobetti, PhD, are at the Harvard T.H. Chan School of Public Health, Boston.
A paper recently published by the same group outlines a possible mechanism by which PM2.5 might cause harm – specifically, an increase in placental intrauterine inflammation. The study adds evidence to the case that air pollution is linked to hypertension as it includes more economically disadvantaged subjects, children of color, preterm births, and small-for-gestational-age births. Overall, the study imaginatively made use of pediatric data in a disadvantaged urban setting to better understand how air pollution affects an important physiological outcome.
Diane Gold, MD, and Antonella Zanobetti, PhD, are at the Harvard T.H. Chan School of Public Health, Boston.
A paper recently published by the same group outlines a possible mechanism by which PM2.5 might cause harm – specifically, an increase in placental intrauterine inflammation. The study adds evidence to the case that air pollution is linked to hypertension as it includes more economically disadvantaged subjects, children of color, preterm births, and small-for-gestational-age births. Overall, the study imaginatively made use of pediatric data in a disadvantaged urban setting to better understand how air pollution affects an important physiological outcome.
Diane Gold, MD, and Antonella Zanobetti, PhD, are at the Harvard T.H. Chan School of Public Health, Boston.
Third-trimester exposure to airborne particulate matter (PM) smaller than 2.5 mcm in size (PM2.5) has been linked to higher levels of systolic blood pressure during childhood.
Mingyu Zhang of Johns Hopkins Bloomberg School of Public Health, Baltimore, and his associates found that the highest tertile exposure was tied to an increased likelihood of childhood elevated BP, defined as systolic blood pressure higher than the 90th percentile, compared with those in the lowest tertile (relative risk, 1.60; 95% confidence interval, 1.12-2.27).
Previous studies have shown a relationship between exposure to PM2.5and elevated BP in children and adults. Mouse models suggest that PM2.5 may interfere with in utero development of the cardiovascular system. One previous study found evidence that third-trimester exposure to PM2.5was linked to heightened BP in newborns, while a retrospective analysis showed no association with BP in adolescents.
They identified PM2.5 exposure by matching the mother’s residential address to the U.S. Environmental Protection Agency’s local air quality monitors, from which they extracted daily PM2.5 values.
For every 5 mcg/m3 increment in PM2.5 exposure, the relative risk for heightened BP in a child at a single visit was 1.46 (95% CI, 1.17-1.83). The relative risk increased after independent additions to the confounder model of birth weight (RR, 3.39; 95% CI, 0.63-6.15), gestational age (RR, 3.08; 95% CI, 0.33-5.82), and child body mass index (BMI) z score (RR, 2.75; 95% CI, 0.01-5.50).
A multivariable-adjusted cubic spline model revealed a significant increase in risk of elevated BP at a cutoff threshold of 13 mcg/m3 (RR, 1.80; 95% CI, 1.33-2.44).
Further analysis suggested that 35% of the association between exposure and elevated BP risk was mediated by birth weight and BMI z score during childhood. When these factors were added to the models, the association between PM exposure and BP risk was no longer significant.
Although the findings are intriguing, they cannot prove causation, according to the researchers.
The study was funded by the National Institutes of Health and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
SOURCE: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
Third-trimester exposure to airborne particulate matter (PM) smaller than 2.5 mcm in size (PM2.5) has been linked to higher levels of systolic blood pressure during childhood.
Mingyu Zhang of Johns Hopkins Bloomberg School of Public Health, Baltimore, and his associates found that the highest tertile exposure was tied to an increased likelihood of childhood elevated BP, defined as systolic blood pressure higher than the 90th percentile, compared with those in the lowest tertile (relative risk, 1.60; 95% confidence interval, 1.12-2.27).
Previous studies have shown a relationship between exposure to PM2.5and elevated BP in children and adults. Mouse models suggest that PM2.5 may interfere with in utero development of the cardiovascular system. One previous study found evidence that third-trimester exposure to PM2.5was linked to heightened BP in newborns, while a retrospective analysis showed no association with BP in adolescents.
They identified PM2.5 exposure by matching the mother’s residential address to the U.S. Environmental Protection Agency’s local air quality monitors, from which they extracted daily PM2.5 values.
For every 5 mcg/m3 increment in PM2.5 exposure, the relative risk for heightened BP in a child at a single visit was 1.46 (95% CI, 1.17-1.83). The relative risk increased after independent additions to the confounder model of birth weight (RR, 3.39; 95% CI, 0.63-6.15), gestational age (RR, 3.08; 95% CI, 0.33-5.82), and child body mass index (BMI) z score (RR, 2.75; 95% CI, 0.01-5.50).
A multivariable-adjusted cubic spline model revealed a significant increase in risk of elevated BP at a cutoff threshold of 13 mcg/m3 (RR, 1.80; 95% CI, 1.33-2.44).
Further analysis suggested that 35% of the association between exposure and elevated BP risk was mediated by birth weight and BMI z score during childhood. When these factors were added to the models, the association between PM exposure and BP risk was no longer significant.
Although the findings are intriguing, they cannot prove causation, according to the researchers.
The study was funded by the National Institutes of Health and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
SOURCE: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
FROM HYPERTENSION
Key clinical point: Maternal air pollution exposure could be useful in screening and prevention of childhood hypertension.
Major finding: Each 5 µg/m3 exposure increment was associated with 46% increased odds of elevated BP.
Study details: Prospective study of 1,293 mothers.
Disclosures: The study was funded by the NIH and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
Source: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
Rapid Deterioration and Death Caused by Bilateral Phlegmasia Cerulea Dolens
Phlegmasia cerulea dolens (PCD), a life-threatening complication of deep venous thrombosis (DVT), is characterized by massive iliofemoral thrombus that extends to the collateral veins, leading to fluid sequestration and elevated compartment pressures that ultimately compromise arterial flow. Phlegmasia cerulea dolens can rapidly progress to compartment syndrome and gangrene.1,2 The affected limbs of patients with PCD can be hypoxic and appear purple in color due to substantial lack of blood flow, with diminished or absent pulses. Risk factors for PCD include malignancy, hypercoagulable states, venous stasis, contraceptive agents, inferior vena cava (IVC) filter, aneurysm, history of DVT, trauma, heparin-induced thrombocytopenia, femoral vein catheterization, antiphospholipid syndrome, or pregnancy.3-6 Failure to treat PCD early and aggressively carries an amputation rate of up to 50% and a mortality rate of up to 40%.4
We present the case of a patient with PCD, whose condition rapidly deteriorated despite prompt diagnosis and treatment.
Case
A 58-year-old woman presented to the ED with a 1-day history of back and leg pain and difficulty walking. When asked about the severity of her pain, she rated her leg pain at 10 on a scale of 0 to 10. The patient’s history was significant for DVT and pulmonary embolism (PE), for which a Greenfield IVC had been placed and for which she was on prophylactic warfarin therapy. The patient stated that she had been taken off warfarin several weeks prior to presentation in preparation for an elective colonoscopy and dental procedure, but had restarted the warfarin therapy 2 days prior to presentation. She had no history of diabetes mellitus or renal disease.
Initial vital signs at presentation were: blood pressure, 120/91 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min; and temperature, 96.6°F. Oxygen saturation was 100% on a nonrebreather mask.
On examination, the patient was alert and oriented to person, time, and place, but appeared dyspneic. An electrocardiogram revealed sinus tachycardia. On physical examination, lung sounds were clear to auscultation bilaterally with good air movement, and the abdomen was soft and nontender with normal bowel sounds. The dorsalis pedis and posterior tibial pulses were absent bilaterally, lower extremity capillary refill was 3 seconds, and the legs appeared mildly erythematous and cool to touch. No speech or neurological deficits were present.
Laboratory evaluation was remarkable for metabolic acidosis, venous pH, 7.11; bicarbonate, 11.7; partial pressure of carbon dioxide, 37.6; lactic acid, 8.8 mEq/L leukocytosis, 24,900 u/L; glucose, 296 mg/dL; creatinine, 2.41 mg/dL; and international normalized ratio, 1.36.
Before additional laboratory studies and imaging could be obtained, the patient developed altered mental status, hypotension, and paralysis of the lower extremities. She was orally intubated for airway protection and was given a total of 4 L of normal saline intravenously (IV) for hypotension and acidosis; sodium bicarbonate for metabolic acidosis; norepinephrine for hypotension; fentanyl for pain; and ondansetron for nausea. A central line and arterial line were placed for administering medication and hemodynamic monitoring.
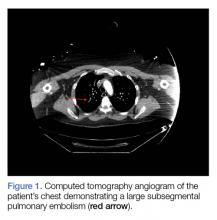
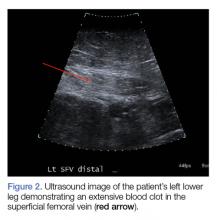
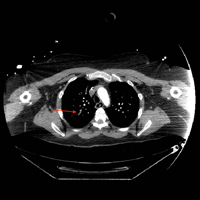
Computed tomography (CT) angiography of the chest, abdomen, and pelvis demonstrated multiple subsegmental bilateral PE with no arterial pathology (Figure 1). Beside ultrasound revealed extensive bilateral DVTs involving the superficial and common femoral veins (Figure 2). The patient’s bilateral DVTs, arterial compromise, and leg cyanosis led to the diagnosis of PCD.
Critical care and vascular surgery services were consulted, and the patient was admitted to the intensive care unit. Since the patient was too unstable to undergo thrombectomy, she was given IV tissue plasminogen activator. Despite aggressive pharmacological treatment, the patient’s condition continued to deteriorate. On hospital day 2, the patient’s family changed the patient’s code status to do-not-resuscitate/comfort-care only; she died shortly thereafter.
Discussion
This case illustrates the severity and complications of PCD and the rapidity with which this condition can deteriorate. At the time of ED presentation, the patient had already developed bilateral PCD, metabolic acidosis, and bilateral PE. Unfortunately, due to decreased venous return, decreased cardiac output, and severe shock, she quickly became unstable and progressed rapidly to multisystem organ failure leading to death.
Risk Factors
A prior patient history DVT and an IVC filter are both significant risk factors for the progression of DVT to PCD;3,6 however, in this case, IVC filter failed to prevent emboli from reaching the lungs. Extensive thrombi led to severely decreased venous return and cardiac output, causing life-threatening shock, ischemia, and metabolic acidosis. A lactic acid level taken on hospital day 2 was elevated at 19 mEq/L, demonstrating the severity, morbidity, and progression of PCD.
Signs and Symptoms
The three cardinal signs that lead to a clinical diagnosis of PCD are edema, pain, and violaceous discoloration or skin mottling.3 Although most commonly found in the lower extremity, PCD can occur in any limb due to occlusion of venous outflow.7 Unfortunately, a clinical diagnosis of PCD is not often made until the venous occlusion becomes severe enough to impair arterial flow and cause venous gangrene, tissue ischemia, shock, and death.8
Although IVC filters are designed to prevent life-threatening PE, there are risk factors associated with their use. Whether placed recently or decades prior, urgent investigation, such as immediate CT scan, should be undertaken in patients presenting with DVT-like symptoms who have a history of an IVC filter, to ensure the filter has not shifted from its original placement and is not occluding the IVC.
Conclusion
Phlegmasia cerulea dolens is an uncommon vascular emergency, but one that has a high-morbidity and high-mortality rate. This case demonstrates the importance of early diagnosis, aggressive treatment, and the severe complications that can develop in PCD.
There are cases in the literature where patients diagnosed with PCD had a successful outcome with pharmacological or surgical intervention such as thrombectomy. Treatment for PCD is most effective when instituted early in onset. As seen in our patient, the tendency for rapid deterioration in PCD can limit potentially lifesaving therapeutic options, decreasing the chances of a successful outcome. Emergency physicians, therefore, must be aware of the high-mortality rate associated with this disorder and the possibility of rapid progression from stable to critical condition.
1. Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59-69. doi:10.2147/JBM.S19009.
2. Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Ultrasound. 2007;35(7):401-404. doi:10.1002/jcu.20317.
3. Maiti A, Das A, Smith DT. Phlegmasia cerulean dolens. Postgrad Med J. 2016;pii: postgradmedj-2016-134185. doi:10.1136/postgradmedj-2016-134185.
4. Abdul W, Hickey B, Wilson C. Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency. BMJ Case Rep. 2016;2016:pii:bcr2016215078. doi:10.1136/bcr-2016-215078.
5. Onuoha CU. Phlegmasia cerulea dolens: A rare clinical presentation. Am J Med. 2015;128(9):e27-e28. doi:10.1016/j.amjmed.2015.04.009.
6. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5-14. doi:10.1177/1538574410388309.
7. Bagenal JD, Nasralla D. Bilateral phlegmasia cerulea dolens in an occluded inferior vena cava filter. BMJ Case Rep. 2013;pii: bcr2013009302. doi:10.1136/bcr-2013-009302.
8. Kiefer CS, Colletti JE. Phlegmasia cerulea dolens in a patient with an inferior vena cava filter. J Emerg Med. 2013;44(1):e95-e97. doi:10.1016/j.jemermed.2012.01.018.
Phlegmasia cerulea dolens (PCD), a life-threatening complication of deep venous thrombosis (DVT), is characterized by massive iliofemoral thrombus that extends to the collateral veins, leading to fluid sequestration and elevated compartment pressures that ultimately compromise arterial flow. Phlegmasia cerulea dolens can rapidly progress to compartment syndrome and gangrene.1,2 The affected limbs of patients with PCD can be hypoxic and appear purple in color due to substantial lack of blood flow, with diminished or absent pulses. Risk factors for PCD include malignancy, hypercoagulable states, venous stasis, contraceptive agents, inferior vena cava (IVC) filter, aneurysm, history of DVT, trauma, heparin-induced thrombocytopenia, femoral vein catheterization, antiphospholipid syndrome, or pregnancy.3-6 Failure to treat PCD early and aggressively carries an amputation rate of up to 50% and a mortality rate of up to 40%.4
We present the case of a patient with PCD, whose condition rapidly deteriorated despite prompt diagnosis and treatment.
Case
A 58-year-old woman presented to the ED with a 1-day history of back and leg pain and difficulty walking. When asked about the severity of her pain, she rated her leg pain at 10 on a scale of 0 to 10. The patient’s history was significant for DVT and pulmonary embolism (PE), for which a Greenfield IVC had been placed and for which she was on prophylactic warfarin therapy. The patient stated that she had been taken off warfarin several weeks prior to presentation in preparation for an elective colonoscopy and dental procedure, but had restarted the warfarin therapy 2 days prior to presentation. She had no history of diabetes mellitus or renal disease.
Initial vital signs at presentation were: blood pressure, 120/91 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min; and temperature, 96.6°F. Oxygen saturation was 100% on a nonrebreather mask.
On examination, the patient was alert and oriented to person, time, and place, but appeared dyspneic. An electrocardiogram revealed sinus tachycardia. On physical examination, lung sounds were clear to auscultation bilaterally with good air movement, and the abdomen was soft and nontender with normal bowel sounds. The dorsalis pedis and posterior tibial pulses were absent bilaterally, lower extremity capillary refill was 3 seconds, and the legs appeared mildly erythematous and cool to touch. No speech or neurological deficits were present.
Laboratory evaluation was remarkable for metabolic acidosis, venous pH, 7.11; bicarbonate, 11.7; partial pressure of carbon dioxide, 37.6; lactic acid, 8.8 mEq/L leukocytosis, 24,900 u/L; glucose, 296 mg/dL; creatinine, 2.41 mg/dL; and international normalized ratio, 1.36.
Before additional laboratory studies and imaging could be obtained, the patient developed altered mental status, hypotension, and paralysis of the lower extremities. She was orally intubated for airway protection and was given a total of 4 L of normal saline intravenously (IV) for hypotension and acidosis; sodium bicarbonate for metabolic acidosis; norepinephrine for hypotension; fentanyl for pain; and ondansetron for nausea. A central line and arterial line were placed for administering medication and hemodynamic monitoring.
Computed tomography (CT) angiography of the chest, abdomen, and pelvis demonstrated multiple subsegmental bilateral PE with no arterial pathology (Figure 1). Beside ultrasound revealed extensive bilateral DVTs involving the superficial and common femoral veins (Figure 2). The patient’s bilateral DVTs, arterial compromise, and leg cyanosis led to the diagnosis of PCD.
Critical care and vascular surgery services were consulted, and the patient was admitted to the intensive care unit. Since the patient was too unstable to undergo thrombectomy, she was given IV tissue plasminogen activator. Despite aggressive pharmacological treatment, the patient’s condition continued to deteriorate. On hospital day 2, the patient’s family changed the patient’s code status to do-not-resuscitate/comfort-care only; she died shortly thereafter.
Discussion
This case illustrates the severity and complications of PCD and the rapidity with which this condition can deteriorate. At the time of ED presentation, the patient had already developed bilateral PCD, metabolic acidosis, and bilateral PE. Unfortunately, due to decreased venous return, decreased cardiac output, and severe shock, she quickly became unstable and progressed rapidly to multisystem organ failure leading to death.
Risk Factors
A prior patient history DVT and an IVC filter are both significant risk factors for the progression of DVT to PCD;3,6 however, in this case, IVC filter failed to prevent emboli from reaching the lungs. Extensive thrombi led to severely decreased venous return and cardiac output, causing life-threatening shock, ischemia, and metabolic acidosis. A lactic acid level taken on hospital day 2 was elevated at 19 mEq/L, demonstrating the severity, morbidity, and progression of PCD.
Signs and Symptoms
The three cardinal signs that lead to a clinical diagnosis of PCD are edema, pain, and violaceous discoloration or skin mottling.3 Although most commonly found in the lower extremity, PCD can occur in any limb due to occlusion of venous outflow.7 Unfortunately, a clinical diagnosis of PCD is not often made until the venous occlusion becomes severe enough to impair arterial flow and cause venous gangrene, tissue ischemia, shock, and death.8
Although IVC filters are designed to prevent life-threatening PE, there are risk factors associated with their use. Whether placed recently or decades prior, urgent investigation, such as immediate CT scan, should be undertaken in patients presenting with DVT-like symptoms who have a history of an IVC filter, to ensure the filter has not shifted from its original placement and is not occluding the IVC.
Conclusion
Phlegmasia cerulea dolens is an uncommon vascular emergency, but one that has a high-morbidity and high-mortality rate. This case demonstrates the importance of early diagnosis, aggressive treatment, and the severe complications that can develop in PCD.
There are cases in the literature where patients diagnosed with PCD had a successful outcome with pharmacological or surgical intervention such as thrombectomy. Treatment for PCD is most effective when instituted early in onset. As seen in our patient, the tendency for rapid deterioration in PCD can limit potentially lifesaving therapeutic options, decreasing the chances of a successful outcome. Emergency physicians, therefore, must be aware of the high-mortality rate associated with this disorder and the possibility of rapid progression from stable to critical condition.
Phlegmasia cerulea dolens (PCD), a life-threatening complication of deep venous thrombosis (DVT), is characterized by massive iliofemoral thrombus that extends to the collateral veins, leading to fluid sequestration and elevated compartment pressures that ultimately compromise arterial flow. Phlegmasia cerulea dolens can rapidly progress to compartment syndrome and gangrene.1,2 The affected limbs of patients with PCD can be hypoxic and appear purple in color due to substantial lack of blood flow, with diminished or absent pulses. Risk factors for PCD include malignancy, hypercoagulable states, venous stasis, contraceptive agents, inferior vena cava (IVC) filter, aneurysm, history of DVT, trauma, heparin-induced thrombocytopenia, femoral vein catheterization, antiphospholipid syndrome, or pregnancy.3-6 Failure to treat PCD early and aggressively carries an amputation rate of up to 50% and a mortality rate of up to 40%.4
We present the case of a patient with PCD, whose condition rapidly deteriorated despite prompt diagnosis and treatment.
Case
A 58-year-old woman presented to the ED with a 1-day history of back and leg pain and difficulty walking. When asked about the severity of her pain, she rated her leg pain at 10 on a scale of 0 to 10. The patient’s history was significant for DVT and pulmonary embolism (PE), for which a Greenfield IVC had been placed and for which she was on prophylactic warfarin therapy. The patient stated that she had been taken off warfarin several weeks prior to presentation in preparation for an elective colonoscopy and dental procedure, but had restarted the warfarin therapy 2 days prior to presentation. She had no history of diabetes mellitus or renal disease.
Initial vital signs at presentation were: blood pressure, 120/91 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min; and temperature, 96.6°F. Oxygen saturation was 100% on a nonrebreather mask.
On examination, the patient was alert and oriented to person, time, and place, but appeared dyspneic. An electrocardiogram revealed sinus tachycardia. On physical examination, lung sounds were clear to auscultation bilaterally with good air movement, and the abdomen was soft and nontender with normal bowel sounds. The dorsalis pedis and posterior tibial pulses were absent bilaterally, lower extremity capillary refill was 3 seconds, and the legs appeared mildly erythematous and cool to touch. No speech or neurological deficits were present.
Laboratory evaluation was remarkable for metabolic acidosis, venous pH, 7.11; bicarbonate, 11.7; partial pressure of carbon dioxide, 37.6; lactic acid, 8.8 mEq/L leukocytosis, 24,900 u/L; glucose, 296 mg/dL; creatinine, 2.41 mg/dL; and international normalized ratio, 1.36.
Before additional laboratory studies and imaging could be obtained, the patient developed altered mental status, hypotension, and paralysis of the lower extremities. She was orally intubated for airway protection and was given a total of 4 L of normal saline intravenously (IV) for hypotension and acidosis; sodium bicarbonate for metabolic acidosis; norepinephrine for hypotension; fentanyl for pain; and ondansetron for nausea. A central line and arterial line were placed for administering medication and hemodynamic monitoring.
Computed tomography (CT) angiography of the chest, abdomen, and pelvis demonstrated multiple subsegmental bilateral PE with no arterial pathology (Figure 1). Beside ultrasound revealed extensive bilateral DVTs involving the superficial and common femoral veins (Figure 2). The patient’s bilateral DVTs, arterial compromise, and leg cyanosis led to the diagnosis of PCD.
Critical care and vascular surgery services were consulted, and the patient was admitted to the intensive care unit. Since the patient was too unstable to undergo thrombectomy, she was given IV tissue plasminogen activator. Despite aggressive pharmacological treatment, the patient’s condition continued to deteriorate. On hospital day 2, the patient’s family changed the patient’s code status to do-not-resuscitate/comfort-care only; she died shortly thereafter.
Discussion
This case illustrates the severity and complications of PCD and the rapidity with which this condition can deteriorate. At the time of ED presentation, the patient had already developed bilateral PCD, metabolic acidosis, and bilateral PE. Unfortunately, due to decreased venous return, decreased cardiac output, and severe shock, she quickly became unstable and progressed rapidly to multisystem organ failure leading to death.
Risk Factors
A prior patient history DVT and an IVC filter are both significant risk factors for the progression of DVT to PCD;3,6 however, in this case, IVC filter failed to prevent emboli from reaching the lungs. Extensive thrombi led to severely decreased venous return and cardiac output, causing life-threatening shock, ischemia, and metabolic acidosis. A lactic acid level taken on hospital day 2 was elevated at 19 mEq/L, demonstrating the severity, morbidity, and progression of PCD.
Signs and Symptoms
The three cardinal signs that lead to a clinical diagnosis of PCD are edema, pain, and violaceous discoloration or skin mottling.3 Although most commonly found in the lower extremity, PCD can occur in any limb due to occlusion of venous outflow.7 Unfortunately, a clinical diagnosis of PCD is not often made until the venous occlusion becomes severe enough to impair arterial flow and cause venous gangrene, tissue ischemia, shock, and death.8
Although IVC filters are designed to prevent life-threatening PE, there are risk factors associated with their use. Whether placed recently or decades prior, urgent investigation, such as immediate CT scan, should be undertaken in patients presenting with DVT-like symptoms who have a history of an IVC filter, to ensure the filter has not shifted from its original placement and is not occluding the IVC.
Conclusion
Phlegmasia cerulea dolens is an uncommon vascular emergency, but one that has a high-morbidity and high-mortality rate. This case demonstrates the importance of early diagnosis, aggressive treatment, and the severe complications that can develop in PCD.
There are cases in the literature where patients diagnosed with PCD had a successful outcome with pharmacological or surgical intervention such as thrombectomy. Treatment for PCD is most effective when instituted early in onset. As seen in our patient, the tendency for rapid deterioration in PCD can limit potentially lifesaving therapeutic options, decreasing the chances of a successful outcome. Emergency physicians, therefore, must be aware of the high-mortality rate associated with this disorder and the possibility of rapid progression from stable to critical condition.
1. Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59-69. doi:10.2147/JBM.S19009.
2. Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Ultrasound. 2007;35(7):401-404. doi:10.1002/jcu.20317.
3. Maiti A, Das A, Smith DT. Phlegmasia cerulean dolens. Postgrad Med J. 2016;pii: postgradmedj-2016-134185. doi:10.1136/postgradmedj-2016-134185.
4. Abdul W, Hickey B, Wilson C. Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency. BMJ Case Rep. 2016;2016:pii:bcr2016215078. doi:10.1136/bcr-2016-215078.
5. Onuoha CU. Phlegmasia cerulea dolens: A rare clinical presentation. Am J Med. 2015;128(9):e27-e28. doi:10.1016/j.amjmed.2015.04.009.
6. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5-14. doi:10.1177/1538574410388309.
7. Bagenal JD, Nasralla D. Bilateral phlegmasia cerulea dolens in an occluded inferior vena cava filter. BMJ Case Rep. 2013;pii: bcr2013009302. doi:10.1136/bcr-2013-009302.
8. Kiefer CS, Colletti JE. Phlegmasia cerulea dolens in a patient with an inferior vena cava filter. J Emerg Med. 2013;44(1):e95-e97. doi:10.1016/j.jemermed.2012.01.018.
1. Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59-69. doi:10.2147/JBM.S19009.
2. Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Ultrasound. 2007;35(7):401-404. doi:10.1002/jcu.20317.
3. Maiti A, Das A, Smith DT. Phlegmasia cerulean dolens. Postgrad Med J. 2016;pii: postgradmedj-2016-134185. doi:10.1136/postgradmedj-2016-134185.
4. Abdul W, Hickey B, Wilson C. Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency. BMJ Case Rep. 2016;2016:pii:bcr2016215078. doi:10.1136/bcr-2016-215078.
5. Onuoha CU. Phlegmasia cerulea dolens: A rare clinical presentation. Am J Med. 2015;128(9):e27-e28. doi:10.1016/j.amjmed.2015.04.009.
6. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5-14. doi:10.1177/1538574410388309.
7. Bagenal JD, Nasralla D. Bilateral phlegmasia cerulea dolens in an occluded inferior vena cava filter. BMJ Case Rep. 2013;pii: bcr2013009302. doi:10.1136/bcr-2013-009302.
8. Kiefer CS, Colletti JE. Phlegmasia cerulea dolens in a patient with an inferior vena cava filter. J Emerg Med. 2013;44(1):e95-e97. doi:10.1016/j.jemermed.2012.01.018.
Bell Palsy Mimics
Facial paralysis is a common medical complaint—one that has fascinated ancient and contemporary physicians alike.1 An idiopathic facial nerve paresis involving the lower motor neuron was described in 1821 by Sir Charles Bell. This entity became known as a Bell’s palsy, the hallmark of which was weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. However, not all facial paralysis is due to Bell’s palsy.
We present a case of a patient with a Bell’s palsy mimic to facilitate and guide the differential diagnosis and distinguish conditions from the classical presentation that Bell first described to the more concerning symptoms that may not be immediately obvious. Our case further underscores the importance of performing a thorough assessment to determine the presence of other neurological findings.
Case
A 61-year-old woman presented to the ED for evaluation of right facial droop and sensation of “room spinning.” The patient stated both symptoms began approximately 36 hours prior to presentation, upon awakening.
The patient denied any headache, neck or chest pain, extremity numbness, or weakness, but stated that she felt like she was going to fall toward her right side whenever she attempted to walk. The patient’s medical history was significant for hypertension, for which she was taking losartan. Her surgical history was notable for a left oophorectomy secondary to an ovarian cyst. Regarding the social history, the patient admitted to smoking 90 packs of cigarettes per year, but denied alcohol or illicit drug use.
Upon arrival at the ED, the patient’s vital signs were: blood pressure, 164/86 mm Hg: pulse, 89 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.6°F. Oxygen saturation was 98% on room air.
Physical examination revealed the patient had a right facial droop consistent with right facial palsy. She was unable to wrinkle her right forehead or fully close her right eye. There were no field cuts on confrontation. The patient’s speech was noticeable for a mild dysarthria. The motor examination revealed mild weakness of the left upper extremity and impaired right facial sensation. There were no rashes noted on the face, head, or ears. The patient had slightly impaired hearing in the right ear, which was new in onset. The remainder of the physical examination was unremarkable.
Although the patient exhibited the classic signs of Bell’s palsy, including complete paralysis of the muscles of one side of the face, inability to wrinkle the muscle of the right forehead, and inability to fully close the right eye, she also had concerning symptoms of vertigo, dysarthria, and contralateral upper extremity weakness.
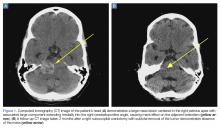
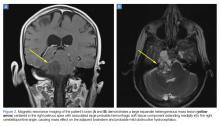
A computed tomography (CT) scan of the head was ordered, which revealed a large mass lesion centered in the right petrous apex, with an associated large component extending medially into the right cerebellopontine angle (CPA) that caused a mass effect on the adjacent brainstem (Figures 1a and 1b).
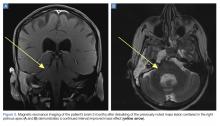
Upon these findings, the patient was transferred to another facility for neurosurgical evaluation. Magnetic resonance imaging (MRI) studies performed at the receiving hospital demonstrated a large expansile heterogeneous mass lesion centered in the right petrous apex with an associated large, probable hemorrhagic soft-tissue component extending medially into the right CPA, causing a mass effect on the adjacent brainstem and mild obstructive hydrocephalus (Figures 2a and 2b).
The patient was given dexamethasone 10 mg intravenously and taken to the operating room for a right suboccipital craniotomy with subtotal tumor removal. Intraoperative high-voltage stimulation of the fifth to eighth cranial nerves showed no response, indicating significant impairment.
While there were no intraoperative complications, the patient had significant postoperative dysphagia and resultant aspiration. A tracheostomy and percutaneous endoscopic gastrostomy tube were subsequently placed. Results of a biopsy taken during surgery identified an atypical meningioma. The patient remained in the hospital for 4 weeks, after which she was discharged to a long-term care (LTC) and rehabilitation facility.
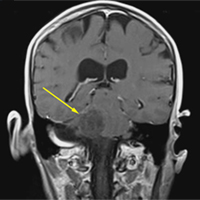
A repeat CT scan taken 2 months after surgery demonstrated absence of the previously identified large mass (Figure 1b). Three months after discharge from the LTC-rehabilitation facility, MRI of the brain showed continued interval improvement of the previously noted mass centered in the right petrous apex (Figures 3a and 3b).
Discussion
Accounts of facial paralysis and facial nerve disorders have been noted throughout history and include accounts of the condition by Hippocrates.1 Bell’s palsy was named after surgeon Sir Charles Bell, who described a peripheral-nerve paralysis of the facial nerve in 1821. Bell’s work helped to elucidate the anatomy and functional role of the facial nerve.1,2
Signs and Symptoms
The classic presentation of Bell’s palsy is weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. The eyelid on the affected side generally does not close, which can result in ocular irritation due to ineffective lubrication.
A scoring system has been developed by House and Brackmann which grades the degree impairment based on such characteristics as facial muscle function and eye closure.3,4 Approximately 96% of patients with a Bell’s palsy will improve to a House-Brackmann score of 2 or better within 1 year from diagnosis,5 and 85% of patients with Bell’s palsy will show at least some improvement within 3 weeks of onset (Table).2 Although the classic description of Bell’s palsy notes the condition as idiopathic, there is an increasing body of evidence in the literature showing a link to herpes simplex virus 1.5-7
Ramsey-Hunt Syndrome
The relationship between Bell’s palsy and Ramsey-Hunt syndrome is complex and controversial. Ramsey-Hunt syndrome is a constellation of possible complications from varicella-virus infection. Symptoms of Ramsey-Hunt syndrome include facial paralysis, tinnitus, hearing loss, vertigo, hyperacusis (increased sensitivity to certain frequencies and volume ranges of sound), and decreased ocular tearing.8 Due to the nature of symptoms associated with Ramsey-Hunt syndrome, it is apparent that the condition involves more than the seventh cranial nerve. In fact, studies have shown that Ramsey-Hunt syndrome can affect the fifth, sixth, eighth, and ninth cranial nerves.8
Ramsey-Hunt syndrome, which can present in the absence of cutaneous rash (referred to as zoster sine herpete), is estimated to occur in 8% to 20% of unilateral facial nerve palsies in adult patients.8,9 Regardless of the etiology of Bell’s palsy, a review of the literature makes it clear that facial nerve paralysis is not synonymous with Bell’s palsy.10 In one example, Yetter et al10 describe the case of a patient who, though initially diagnosed with Bell’s palsy, ultimately was found to have a facial palsy due to a parotid gland malignancy.
Likewise, Stomeo11 describes a case of a patient with facial paralysis and profound ipsilateral hearing loss who ultimately was found to have a mucoepithelial carcinoma of the parotid gland. In their report, the authors note that approximately 80% of facial nerve paralysis is due to Bell’s palsy, while 5% is due to malignancy.
In another report, Clemis12 describes a case in which a patient who initially was diagnosed with Bell’s palsy eventually was found to have an adenoid cystic carcinoma of the parotid. Thus, the authors appropriately emphasize in their report that “all that palsies is not Bell’s.”
Differential Diagnosis
Historical factors, including timing and duration of symptom onset, help to distinguish a Bell’s palsy from other disorders that can mimic this condition. In their study, Brach VanSwewaringen13 highlight the fact that “not all facial paralysis is Bell’s palsy.” In their review, the authors describe clues to help distinguish conditions that mimic Bell’s palsy. For example, maximal weakness from Bell’s Palsy typically occurs within 3 to 7 days from symptom onset, and that a more gradual onset of symptoms, with slow or negligible improvement over 6 to 12 months, is more indicative of a space-occupying lesion than Bell’s palsy.13It is, however, important to note that although the patient in our case had a central lesion, she experienced an acute onset of symptoms.
The presence of additional symptoms may also suggest an alternative diagnosis. Brach and VanSwearingen13 further noted that symptoms associated with the eighth nerve, such as vertigo, tinnitus, and hearing loss may be found in patients with a CPA tumor. In patients with larger tumors, ninth and 10th nerve symptoms, including the impaired hearing noted in our patient, may be present. Some patients with ninth and 10th nerve symptoms may perceive a sense of facial numbness, but actual sensory changes in the facial nerve distribution are unlikely in Bell’s palsy. Gustatory changes, however, are consistent with Bell’s palsy.
Ear pain is consistent with Bell’s palsy and is a signal to be vigilant for the possible emergence of an ear rash, which would suggest the diagnosis of herpes zoster oticus along the trajectory of Ramsey-Hunt syndrome. Facial pain in the area of the facial nerve is inconsistent with Bell’s palsy, while hyperacusis is consistent with Bell’s palsy. Hearing loss is an eighth nerve symptom that is inconsistent with Bell’s palsy.
Similarly, there are physical examination findings that can help distinguish a true Bell’s palsy from a mimic. Changes in tear production are consistent with Bell’s palsy, but imbalance and disequilibrium are not.14
As previously noted, the patient in this case had difficulty walking and felt as if she was falling toward her right side.
One way to organize the causes of facial paralysis has been proposed by Adour et al.15 In this system, etiologies are listed as either acute paralysis or chronic, progressive paralysis. Acute paralysis (ie, the sudden onset of symptoms with maximal severity within 2 weeks), of which Bell’s palsy is the most common, can be seen in cases of polyneuritis.
A new case of Bell’s palsy has been estimated to occur in the United States every 10 minutes.8 Guillain-Barré syndrome and Lyme disease are also in this category, as is Ramsey-Hunt syndrome. Patients with Lyme disease may have a history of a tick bite or rash.14
Trauma can also cause acute facial nerve paralysis (eg, blunt trauma-associated facial fracture, penetrating trauma, birth trauma). Unilateral central facial weakness can have a neurological cause, such as a lesion to the contralateral cortex, subcortical white matter, or internal capsule.2,15 Otitis media can sometimes cause facial paralysis.16 A cholesteatoma can cause acute facial paralysis.2 Malignancies cause 5% of all cases of facial paralysis. Primary parotid tumors of various types are in this category. Metastatic disease from breast, lung, skin, colon, and kidney may cause facial paralysis. As our case illustrates, CPA tumors can cause facial paralysis.15 It is important to also note that a patient can have both a Bell’s palsy and a concurrent disease. There are a number of case reports in the literature that describe acute onset of facial paralysis as a presenting symptom of malignancy.17 In addition, there are cases wherein a neurological finding on imaging, such as an acoustic neuroma, was presumed to be the cause of facial paralysis, yet the patient’s symptoms resolved in a manner consistent with Bell’s palsy.18
For example, Lagman et al19 described a patient in which a CPA lipoma was presumed to be the cause of the facial paralysis, but the eventual outcome showed the lipoma to have been an incidentaloma.
Conclusion
This case demonstrates a presenting symptom of facial palsy and the presence of a CPA tumor. The presence of vertigo along with other historical and physical examination findings inconsistent with Bell’s palsy prompted the CT scan of the head. A review of the literature suggests a number of important findings in patients with facial palsy to assist the clinician in distinguishing true Bell’s palsy from other diseases that can mimic this condition. This case serves as a reminder of the need to perform a thorough and diligent workup to determine the presence or absence of other neurologic findings prior to closing on the diagnosis of Bell’s palsy.
1. Glicenstein J. Ann Chir Plast Esthet. 2015;60(5):347-362. doi:10.1016/j.anplas.2015.05.007.
2. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
3. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi:10.1177/019459988509300202.
4. Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140(2):154-158. doi:10.1016/j.otohns.2008.11.021.
5. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34(2):159-164. doi:10.1016/j.anl.2006.09.005.
6. Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72(5):398-401, 405.
7. Gilden DH. Clinical practice. Bell’s palsy. N Engl J Med. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120.
8. Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35:Suppl:S62-S64.
9. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55(5):708-710.
10. Yetter MF, Ogren FP, Moore GF, Yonkers AJ. Bell’s palsy: a facial nerve paralysis diagnosis of exclusion. Nebr Med J. 1990;75(5):109-116.
11. Stomeo F. Possibilities of diagnostic errors in paralysis of the 7th cranial nerve. Acta Otorhinolaryngol Ital. 1989;9(6):629-633.
12. Clemis JD. All that palsies is not Bell’s: Bell’s palsy due to adenoid cystic carcinoma of the parotid. Am J Otol. 1991;12(5):397.
13. Brach JS, VanSwearingen JM. Not all facial paralysis is Bell’s palsy: a case report. Arch Phys Med Rehabil. 1999;80(7):857-859.
14. Albers JR, Tamang S. Common questions about Bell palsy. Am Fam Physician. 2014;89(3):209-212.
15. Adour KK, Hilsinger RL Jr, Callan EJ. Facial paralysis and Bell’s palsy: a protocol for differential diagnosis. Am J Otol. 1985;Suppl:68-73.
16. Morrow MJ. Bell’s palsy and herpes zoster. Curr Treat Options Neurol. 2000;2(5):407-416.
17. Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339-342. doi:10.1016/j.amjoto.2009.04.003.
18. Kaushal A, Curran WJ Jr. For whom the Bell’s palsy tolls? Am J Clin Oncol. 2009;32(4):450-451. doi:10.1097/01.coc.0000239141.22916.22.
19. Lagman C, Choy W, Lee SJ, et al. A Case of Bell’s palsy with an incidental finding of a cerebellopontine angle lipoma. Cureus. 2016;8(8):e747. doi:10.7759/cureus.747.
Facial paralysis is a common medical complaint—one that has fascinated ancient and contemporary physicians alike.1 An idiopathic facial nerve paresis involving the lower motor neuron was described in 1821 by Sir Charles Bell. This entity became known as a Bell’s palsy, the hallmark of which was weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. However, not all facial paralysis is due to Bell’s palsy.
We present a case of a patient with a Bell’s palsy mimic to facilitate and guide the differential diagnosis and distinguish conditions from the classical presentation that Bell first described to the more concerning symptoms that may not be immediately obvious. Our case further underscores the importance of performing a thorough assessment to determine the presence of other neurological findings.
Case
A 61-year-old woman presented to the ED for evaluation of right facial droop and sensation of “room spinning.” The patient stated both symptoms began approximately 36 hours prior to presentation, upon awakening.
The patient denied any headache, neck or chest pain, extremity numbness, or weakness, but stated that she felt like she was going to fall toward her right side whenever she attempted to walk. The patient’s medical history was significant for hypertension, for which she was taking losartan. Her surgical history was notable for a left oophorectomy secondary to an ovarian cyst. Regarding the social history, the patient admitted to smoking 90 packs of cigarettes per year, but denied alcohol or illicit drug use.
Upon arrival at the ED, the patient’s vital signs were: blood pressure, 164/86 mm Hg: pulse, 89 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.6°F. Oxygen saturation was 98% on room air.
Physical examination revealed the patient had a right facial droop consistent with right facial palsy. She was unable to wrinkle her right forehead or fully close her right eye. There were no field cuts on confrontation. The patient’s speech was noticeable for a mild dysarthria. The motor examination revealed mild weakness of the left upper extremity and impaired right facial sensation. There were no rashes noted on the face, head, or ears. The patient had slightly impaired hearing in the right ear, which was new in onset. The remainder of the physical examination was unremarkable.
Although the patient exhibited the classic signs of Bell’s palsy, including complete paralysis of the muscles of one side of the face, inability to wrinkle the muscle of the right forehead, and inability to fully close the right eye, she also had concerning symptoms of vertigo, dysarthria, and contralateral upper extremity weakness.
A computed tomography (CT) scan of the head was ordered, which revealed a large mass lesion centered in the right petrous apex, with an associated large component extending medially into the right cerebellopontine angle (CPA) that caused a mass effect on the adjacent brainstem (Figures 1a and 1b).
Upon these findings, the patient was transferred to another facility for neurosurgical evaluation. Magnetic resonance imaging (MRI) studies performed at the receiving hospital demonstrated a large expansile heterogeneous mass lesion centered in the right petrous apex with an associated large, probable hemorrhagic soft-tissue component extending medially into the right CPA, causing a mass effect on the adjacent brainstem and mild obstructive hydrocephalus (Figures 2a and 2b).
The patient was given dexamethasone 10 mg intravenously and taken to the operating room for a right suboccipital craniotomy with subtotal tumor removal. Intraoperative high-voltage stimulation of the fifth to eighth cranial nerves showed no response, indicating significant impairment.
While there were no intraoperative complications, the patient had significant postoperative dysphagia and resultant aspiration. A tracheostomy and percutaneous endoscopic gastrostomy tube were subsequently placed. Results of a biopsy taken during surgery identified an atypical meningioma. The patient remained in the hospital for 4 weeks, after which she was discharged to a long-term care (LTC) and rehabilitation facility.
A repeat CT scan taken 2 months after surgery demonstrated absence of the previously identified large mass (Figure 1b). Three months after discharge from the LTC-rehabilitation facility, MRI of the brain showed continued interval improvement of the previously noted mass centered in the right petrous apex (Figures 3a and 3b).
Discussion
Accounts of facial paralysis and facial nerve disorders have been noted throughout history and include accounts of the condition by Hippocrates.1 Bell’s palsy was named after surgeon Sir Charles Bell, who described a peripheral-nerve paralysis of the facial nerve in 1821. Bell’s work helped to elucidate the anatomy and functional role of the facial nerve.1,2
Signs and Symptoms
The classic presentation of Bell’s palsy is weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. The eyelid on the affected side generally does not close, which can result in ocular irritation due to ineffective lubrication.
A scoring system has been developed by House and Brackmann which grades the degree impairment based on such characteristics as facial muscle function and eye closure.3,4 Approximately 96% of patients with a Bell’s palsy will improve to a House-Brackmann score of 2 or better within 1 year from diagnosis,5 and 85% of patients with Bell’s palsy will show at least some improvement within 3 weeks of onset (Table).2 Although the classic description of Bell’s palsy notes the condition as idiopathic, there is an increasing body of evidence in the literature showing a link to herpes simplex virus 1.5-7
Ramsey-Hunt Syndrome
The relationship between Bell’s palsy and Ramsey-Hunt syndrome is complex and controversial. Ramsey-Hunt syndrome is a constellation of possible complications from varicella-virus infection. Symptoms of Ramsey-Hunt syndrome include facial paralysis, tinnitus, hearing loss, vertigo, hyperacusis (increased sensitivity to certain frequencies and volume ranges of sound), and decreased ocular tearing.8 Due to the nature of symptoms associated with Ramsey-Hunt syndrome, it is apparent that the condition involves more than the seventh cranial nerve. In fact, studies have shown that Ramsey-Hunt syndrome can affect the fifth, sixth, eighth, and ninth cranial nerves.8
Ramsey-Hunt syndrome, which can present in the absence of cutaneous rash (referred to as zoster sine herpete), is estimated to occur in 8% to 20% of unilateral facial nerve palsies in adult patients.8,9 Regardless of the etiology of Bell’s palsy, a review of the literature makes it clear that facial nerve paralysis is not synonymous with Bell’s palsy.10 In one example, Yetter et al10 describe the case of a patient who, though initially diagnosed with Bell’s palsy, ultimately was found to have a facial palsy due to a parotid gland malignancy.
Likewise, Stomeo11 describes a case of a patient with facial paralysis and profound ipsilateral hearing loss who ultimately was found to have a mucoepithelial carcinoma of the parotid gland. In their report, the authors note that approximately 80% of facial nerve paralysis is due to Bell’s palsy, while 5% is due to malignancy.
In another report, Clemis12 describes a case in which a patient who initially was diagnosed with Bell’s palsy eventually was found to have an adenoid cystic carcinoma of the parotid. Thus, the authors appropriately emphasize in their report that “all that palsies is not Bell’s.”
Differential Diagnosis
Historical factors, including timing and duration of symptom onset, help to distinguish a Bell’s palsy from other disorders that can mimic this condition. In their study, Brach VanSwewaringen13 highlight the fact that “not all facial paralysis is Bell’s palsy.” In their review, the authors describe clues to help distinguish conditions that mimic Bell’s palsy. For example, maximal weakness from Bell’s Palsy typically occurs within 3 to 7 days from symptom onset, and that a more gradual onset of symptoms, with slow or negligible improvement over 6 to 12 months, is more indicative of a space-occupying lesion than Bell’s palsy.13It is, however, important to note that although the patient in our case had a central lesion, she experienced an acute onset of symptoms.
The presence of additional symptoms may also suggest an alternative diagnosis. Brach and VanSwearingen13 further noted that symptoms associated with the eighth nerve, such as vertigo, tinnitus, and hearing loss may be found in patients with a CPA tumor. In patients with larger tumors, ninth and 10th nerve symptoms, including the impaired hearing noted in our patient, may be present. Some patients with ninth and 10th nerve symptoms may perceive a sense of facial numbness, but actual sensory changes in the facial nerve distribution are unlikely in Bell’s palsy. Gustatory changes, however, are consistent with Bell’s palsy.
Ear pain is consistent with Bell’s palsy and is a signal to be vigilant for the possible emergence of an ear rash, which would suggest the diagnosis of herpes zoster oticus along the trajectory of Ramsey-Hunt syndrome. Facial pain in the area of the facial nerve is inconsistent with Bell’s palsy, while hyperacusis is consistent with Bell’s palsy. Hearing loss is an eighth nerve symptom that is inconsistent with Bell’s palsy.
Similarly, there are physical examination findings that can help distinguish a true Bell’s palsy from a mimic. Changes in tear production are consistent with Bell’s palsy, but imbalance and disequilibrium are not.14
As previously noted, the patient in this case had difficulty walking and felt as if she was falling toward her right side.
One way to organize the causes of facial paralysis has been proposed by Adour et al.15 In this system, etiologies are listed as either acute paralysis or chronic, progressive paralysis. Acute paralysis (ie, the sudden onset of symptoms with maximal severity within 2 weeks), of which Bell’s palsy is the most common, can be seen in cases of polyneuritis.
A new case of Bell’s palsy has been estimated to occur in the United States every 10 minutes.8 Guillain-Barré syndrome and Lyme disease are also in this category, as is Ramsey-Hunt syndrome. Patients with Lyme disease may have a history of a tick bite or rash.14
Trauma can also cause acute facial nerve paralysis (eg, blunt trauma-associated facial fracture, penetrating trauma, birth trauma). Unilateral central facial weakness can have a neurological cause, such as a lesion to the contralateral cortex, subcortical white matter, or internal capsule.2,15 Otitis media can sometimes cause facial paralysis.16 A cholesteatoma can cause acute facial paralysis.2 Malignancies cause 5% of all cases of facial paralysis. Primary parotid tumors of various types are in this category. Metastatic disease from breast, lung, skin, colon, and kidney may cause facial paralysis. As our case illustrates, CPA tumors can cause facial paralysis.15 It is important to also note that a patient can have both a Bell’s palsy and a concurrent disease. There are a number of case reports in the literature that describe acute onset of facial paralysis as a presenting symptom of malignancy.17 In addition, there are cases wherein a neurological finding on imaging, such as an acoustic neuroma, was presumed to be the cause of facial paralysis, yet the patient’s symptoms resolved in a manner consistent with Bell’s palsy.18
For example, Lagman et al19 described a patient in which a CPA lipoma was presumed to be the cause of the facial paralysis, but the eventual outcome showed the lipoma to have been an incidentaloma.
Conclusion
This case demonstrates a presenting symptom of facial palsy and the presence of a CPA tumor. The presence of vertigo along with other historical and physical examination findings inconsistent with Bell’s palsy prompted the CT scan of the head. A review of the literature suggests a number of important findings in patients with facial palsy to assist the clinician in distinguishing true Bell’s palsy from other diseases that can mimic this condition. This case serves as a reminder of the need to perform a thorough and diligent workup to determine the presence or absence of other neurologic findings prior to closing on the diagnosis of Bell’s palsy.
Facial paralysis is a common medical complaint—one that has fascinated ancient and contemporary physicians alike.1 An idiopathic facial nerve paresis involving the lower motor neuron was described in 1821 by Sir Charles Bell. This entity became known as a Bell’s palsy, the hallmark of which was weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. However, not all facial paralysis is due to Bell’s palsy.
We present a case of a patient with a Bell’s palsy mimic to facilitate and guide the differential diagnosis and distinguish conditions from the classical presentation that Bell first described to the more concerning symptoms that may not be immediately obvious. Our case further underscores the importance of performing a thorough assessment to determine the presence of other neurological findings.
Case
A 61-year-old woman presented to the ED for evaluation of right facial droop and sensation of “room spinning.” The patient stated both symptoms began approximately 36 hours prior to presentation, upon awakening.
The patient denied any headache, neck or chest pain, extremity numbness, or weakness, but stated that she felt like she was going to fall toward her right side whenever she attempted to walk. The patient’s medical history was significant for hypertension, for which she was taking losartan. Her surgical history was notable for a left oophorectomy secondary to an ovarian cyst. Regarding the social history, the patient admitted to smoking 90 packs of cigarettes per year, but denied alcohol or illicit drug use.
Upon arrival at the ED, the patient’s vital signs were: blood pressure, 164/86 mm Hg: pulse, 89 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.6°F. Oxygen saturation was 98% on room air.
Physical examination revealed the patient had a right facial droop consistent with right facial palsy. She was unable to wrinkle her right forehead or fully close her right eye. There were no field cuts on confrontation. The patient’s speech was noticeable for a mild dysarthria. The motor examination revealed mild weakness of the left upper extremity and impaired right facial sensation. There were no rashes noted on the face, head, or ears. The patient had slightly impaired hearing in the right ear, which was new in onset. The remainder of the physical examination was unremarkable.
Although the patient exhibited the classic signs of Bell’s palsy, including complete paralysis of the muscles of one side of the face, inability to wrinkle the muscle of the right forehead, and inability to fully close the right eye, she also had concerning symptoms of vertigo, dysarthria, and contralateral upper extremity weakness.
A computed tomography (CT) scan of the head was ordered, which revealed a large mass lesion centered in the right petrous apex, with an associated large component extending medially into the right cerebellopontine angle (CPA) that caused a mass effect on the adjacent brainstem (Figures 1a and 1b).
Upon these findings, the patient was transferred to another facility for neurosurgical evaluation. Magnetic resonance imaging (MRI) studies performed at the receiving hospital demonstrated a large expansile heterogeneous mass lesion centered in the right petrous apex with an associated large, probable hemorrhagic soft-tissue component extending medially into the right CPA, causing a mass effect on the adjacent brainstem and mild obstructive hydrocephalus (Figures 2a and 2b).
The patient was given dexamethasone 10 mg intravenously and taken to the operating room for a right suboccipital craniotomy with subtotal tumor removal. Intraoperative high-voltage stimulation of the fifth to eighth cranial nerves showed no response, indicating significant impairment.
While there were no intraoperative complications, the patient had significant postoperative dysphagia and resultant aspiration. A tracheostomy and percutaneous endoscopic gastrostomy tube were subsequently placed. Results of a biopsy taken during surgery identified an atypical meningioma. The patient remained in the hospital for 4 weeks, after which she was discharged to a long-term care (LTC) and rehabilitation facility.
A repeat CT scan taken 2 months after surgery demonstrated absence of the previously identified large mass (Figure 1b). Three months after discharge from the LTC-rehabilitation facility, MRI of the brain showed continued interval improvement of the previously noted mass centered in the right petrous apex (Figures 3a and 3b).
Discussion
Accounts of facial paralysis and facial nerve disorders have been noted throughout history and include accounts of the condition by Hippocrates.1 Bell’s palsy was named after surgeon Sir Charles Bell, who described a peripheral-nerve paralysis of the facial nerve in 1821. Bell’s work helped to elucidate the anatomy and functional role of the facial nerve.1,2
Signs and Symptoms
The classic presentation of Bell’s palsy is weakness or complete paralysis of the muscles of one side of the face, with no sparing of the muscles of the forehead. The eyelid on the affected side generally does not close, which can result in ocular irritation due to ineffective lubrication.
A scoring system has been developed by House and Brackmann which grades the degree impairment based on such characteristics as facial muscle function and eye closure.3,4 Approximately 96% of patients with a Bell’s palsy will improve to a House-Brackmann score of 2 or better within 1 year from diagnosis,5 and 85% of patients with Bell’s palsy will show at least some improvement within 3 weeks of onset (Table).2 Although the classic description of Bell’s palsy notes the condition as idiopathic, there is an increasing body of evidence in the literature showing a link to herpes simplex virus 1.5-7
Ramsey-Hunt Syndrome
The relationship between Bell’s palsy and Ramsey-Hunt syndrome is complex and controversial. Ramsey-Hunt syndrome is a constellation of possible complications from varicella-virus infection. Symptoms of Ramsey-Hunt syndrome include facial paralysis, tinnitus, hearing loss, vertigo, hyperacusis (increased sensitivity to certain frequencies and volume ranges of sound), and decreased ocular tearing.8 Due to the nature of symptoms associated with Ramsey-Hunt syndrome, it is apparent that the condition involves more than the seventh cranial nerve. In fact, studies have shown that Ramsey-Hunt syndrome can affect the fifth, sixth, eighth, and ninth cranial nerves.8
Ramsey-Hunt syndrome, which can present in the absence of cutaneous rash (referred to as zoster sine herpete), is estimated to occur in 8% to 20% of unilateral facial nerve palsies in adult patients.8,9 Regardless of the etiology of Bell’s palsy, a review of the literature makes it clear that facial nerve paralysis is not synonymous with Bell’s palsy.10 In one example, Yetter et al10 describe the case of a patient who, though initially diagnosed with Bell’s palsy, ultimately was found to have a facial palsy due to a parotid gland malignancy.
Likewise, Stomeo11 describes a case of a patient with facial paralysis and profound ipsilateral hearing loss who ultimately was found to have a mucoepithelial carcinoma of the parotid gland. In their report, the authors note that approximately 80% of facial nerve paralysis is due to Bell’s palsy, while 5% is due to malignancy.
In another report, Clemis12 describes a case in which a patient who initially was diagnosed with Bell’s palsy eventually was found to have an adenoid cystic carcinoma of the parotid. Thus, the authors appropriately emphasize in their report that “all that palsies is not Bell’s.”
Differential Diagnosis
Historical factors, including timing and duration of symptom onset, help to distinguish a Bell’s palsy from other disorders that can mimic this condition. In their study, Brach VanSwewaringen13 highlight the fact that “not all facial paralysis is Bell’s palsy.” In their review, the authors describe clues to help distinguish conditions that mimic Bell’s palsy. For example, maximal weakness from Bell’s Palsy typically occurs within 3 to 7 days from symptom onset, and that a more gradual onset of symptoms, with slow or negligible improvement over 6 to 12 months, is more indicative of a space-occupying lesion than Bell’s palsy.13It is, however, important to note that although the patient in our case had a central lesion, she experienced an acute onset of symptoms.
The presence of additional symptoms may also suggest an alternative diagnosis. Brach and VanSwearingen13 further noted that symptoms associated with the eighth nerve, such as vertigo, tinnitus, and hearing loss may be found in patients with a CPA tumor. In patients with larger tumors, ninth and 10th nerve symptoms, including the impaired hearing noted in our patient, may be present. Some patients with ninth and 10th nerve symptoms may perceive a sense of facial numbness, but actual sensory changes in the facial nerve distribution are unlikely in Bell’s palsy. Gustatory changes, however, are consistent with Bell’s palsy.
Ear pain is consistent with Bell’s palsy and is a signal to be vigilant for the possible emergence of an ear rash, which would suggest the diagnosis of herpes zoster oticus along the trajectory of Ramsey-Hunt syndrome. Facial pain in the area of the facial nerve is inconsistent with Bell’s palsy, while hyperacusis is consistent with Bell’s palsy. Hearing loss is an eighth nerve symptom that is inconsistent with Bell’s palsy.
Similarly, there are physical examination findings that can help distinguish a true Bell’s palsy from a mimic. Changes in tear production are consistent with Bell’s palsy, but imbalance and disequilibrium are not.14
As previously noted, the patient in this case had difficulty walking and felt as if she was falling toward her right side.
One way to organize the causes of facial paralysis has been proposed by Adour et al.15 In this system, etiologies are listed as either acute paralysis or chronic, progressive paralysis. Acute paralysis (ie, the sudden onset of symptoms with maximal severity within 2 weeks), of which Bell’s palsy is the most common, can be seen in cases of polyneuritis.
A new case of Bell’s palsy has been estimated to occur in the United States every 10 minutes.8 Guillain-Barré syndrome and Lyme disease are also in this category, as is Ramsey-Hunt syndrome. Patients with Lyme disease may have a history of a tick bite or rash.14
Trauma can also cause acute facial nerve paralysis (eg, blunt trauma-associated facial fracture, penetrating trauma, birth trauma). Unilateral central facial weakness can have a neurological cause, such as a lesion to the contralateral cortex, subcortical white matter, or internal capsule.2,15 Otitis media can sometimes cause facial paralysis.16 A cholesteatoma can cause acute facial paralysis.2 Malignancies cause 5% of all cases of facial paralysis. Primary parotid tumors of various types are in this category. Metastatic disease from breast, lung, skin, colon, and kidney may cause facial paralysis. As our case illustrates, CPA tumors can cause facial paralysis.15 It is important to also note that a patient can have both a Bell’s palsy and a concurrent disease. There are a number of case reports in the literature that describe acute onset of facial paralysis as a presenting symptom of malignancy.17 In addition, there are cases wherein a neurological finding on imaging, such as an acoustic neuroma, was presumed to be the cause of facial paralysis, yet the patient’s symptoms resolved in a manner consistent with Bell’s palsy.18
For example, Lagman et al19 described a patient in which a CPA lipoma was presumed to be the cause of the facial paralysis, but the eventual outcome showed the lipoma to have been an incidentaloma.
Conclusion
This case demonstrates a presenting symptom of facial palsy and the presence of a CPA tumor. The presence of vertigo along with other historical and physical examination findings inconsistent with Bell’s palsy prompted the CT scan of the head. A review of the literature suggests a number of important findings in patients with facial palsy to assist the clinician in distinguishing true Bell’s palsy from other diseases that can mimic this condition. This case serves as a reminder of the need to perform a thorough and diligent workup to determine the presence or absence of other neurologic findings prior to closing on the diagnosis of Bell’s palsy.
1. Glicenstein J. Ann Chir Plast Esthet. 2015;60(5):347-362. doi:10.1016/j.anplas.2015.05.007.
2. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
3. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi:10.1177/019459988509300202.
4. Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140(2):154-158. doi:10.1016/j.otohns.2008.11.021.
5. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34(2):159-164. doi:10.1016/j.anl.2006.09.005.
6. Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72(5):398-401, 405.
7. Gilden DH. Clinical practice. Bell’s palsy. N Engl J Med. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120.
8. Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35:Suppl:S62-S64.
9. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55(5):708-710.
10. Yetter MF, Ogren FP, Moore GF, Yonkers AJ. Bell’s palsy: a facial nerve paralysis diagnosis of exclusion. Nebr Med J. 1990;75(5):109-116.
11. Stomeo F. Possibilities of diagnostic errors in paralysis of the 7th cranial nerve. Acta Otorhinolaryngol Ital. 1989;9(6):629-633.
12. Clemis JD. All that palsies is not Bell’s: Bell’s palsy due to adenoid cystic carcinoma of the parotid. Am J Otol. 1991;12(5):397.
13. Brach JS, VanSwearingen JM. Not all facial paralysis is Bell’s palsy: a case report. Arch Phys Med Rehabil. 1999;80(7):857-859.
14. Albers JR, Tamang S. Common questions about Bell palsy. Am Fam Physician. 2014;89(3):209-212.
15. Adour KK, Hilsinger RL Jr, Callan EJ. Facial paralysis and Bell’s palsy: a protocol for differential diagnosis. Am J Otol. 1985;Suppl:68-73.
16. Morrow MJ. Bell’s palsy and herpes zoster. Curr Treat Options Neurol. 2000;2(5):407-416.
17. Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339-342. doi:10.1016/j.amjoto.2009.04.003.
18. Kaushal A, Curran WJ Jr. For whom the Bell’s palsy tolls? Am J Clin Oncol. 2009;32(4):450-451. doi:10.1097/01.coc.0000239141.22916.22.
19. Lagman C, Choy W, Lee SJ, et al. A Case of Bell’s palsy with an incidental finding of a cerebellopontine angle lipoma. Cureus. 2016;8(8):e747. doi:10.7759/cureus.747.
1. Glicenstein J. Ann Chir Plast Esthet. 2015;60(5):347-362. doi:10.1016/j.anplas.2015.05.007.
2. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. Am Fam Physician. 2007;76(7):997-1002.
3. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147. doi:10.1177/019459988509300202.
4. Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009;140(2):154-158. doi:10.1016/j.otohns.2008.11.021.
5. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34(2):159-164. doi:10.1016/j.anl.2006.09.005.
6. Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72(5):398-401, 405.
7. Gilden DH. Clinical practice. Bell’s palsy. N Engl J Med. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120.
8. Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35:Suppl:S62-S64.
9. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55(5):708-710.
10. Yetter MF, Ogren FP, Moore GF, Yonkers AJ. Bell’s palsy: a facial nerve paralysis diagnosis of exclusion. Nebr Med J. 1990;75(5):109-116.
11. Stomeo F. Possibilities of diagnostic errors in paralysis of the 7th cranial nerve. Acta Otorhinolaryngol Ital. 1989;9(6):629-633.
12. Clemis JD. All that palsies is not Bell’s: Bell’s palsy due to adenoid cystic carcinoma of the parotid. Am J Otol. 1991;12(5):397.
13. Brach JS, VanSwearingen JM. Not all facial paralysis is Bell’s palsy: a case report. Arch Phys Med Rehabil. 1999;80(7):857-859.
14. Albers JR, Tamang S. Common questions about Bell palsy. Am Fam Physician. 2014;89(3):209-212.
15. Adour KK, Hilsinger RL Jr, Callan EJ. Facial paralysis and Bell’s palsy: a protocol for differential diagnosis. Am J Otol. 1985;Suppl:68-73.
16. Morrow MJ. Bell’s palsy and herpes zoster. Curr Treat Options Neurol. 2000;2(5):407-416.
17. Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339-342. doi:10.1016/j.amjoto.2009.04.003.
18. Kaushal A, Curran WJ Jr. For whom the Bell’s palsy tolls? Am J Clin Oncol. 2009;32(4):450-451. doi:10.1097/01.coc.0000239141.22916.22.
19. Lagman C, Choy W, Lee SJ, et al. A Case of Bell’s palsy with an incidental finding of a cerebellopontine angle lipoma. Cureus. 2016;8(8):e747. doi:10.7759/cureus.747.
2017 notches up some landmark approvals
With advances in the understanding of cellular pathways, molecular genetics, and immunology, new drugs for cancer are being released at an increasing rate. A variety of novel agents have recently become available for use, generating excitement for patients and oncologists. Keeping track of all of these new agents is increasingly challenging. This brief review will summarize some of the newest drugs, their indications, and benefits (see related article).
Therapies by tumor
Breast cancer
CDK4/6 inhibitors. The CDK4/6 inhibitor palbociclib was approved in 2015 for the treatment of estrogen-positive, HER2-negative advanced breast cancer, and this year, two more drugs in this class – ribociclib and abemaciclib – were approved for the treatment of hormone receptor–positive breast cancer.
Ribociclib (Kisqali) 600 mg daily (3 weeks on, 1 week off) is approved for use in combination with an aromatase inhibitor. In the study on which the approval was based, there was a response rate of 53% for patients in the study group, compared with 37% for those who received aromatase inhibitor alone (progression-free survival (PFS), not reached vs 14.7 months for single-agent aromatase inhibitor).1 The occurrence of neutropenia seemed to be similar to that in patients receiving palbociclib. However, unlike with palbociclib, ribociclib requires ECG monitoring for QTc prolongation as well as monitoring of liver function tests.
Abemaciclib (Verzenio) has been approved in combination with fulvestrant as well as a monotherapy.2 PFS was 16.4 months for abemaciclib (150 mg bid in combination with fulvestrant), compared with 9.3 months for fulvestrant alone, with corresponding response rates of 48% and 21%. As monotherapy, abemaciclib 200 mg bid had a response rate of 20% with a duration of response of 8.6 months.
Tyrosine kinase inhibitors. The tyrosine kinase inhibitor neratinib (Nerlynx) was approved for extended adjuvant treatment of HER2-positive breast cancer after 1 year of adjuvant trastuzumab.3 Given at 240 mg (6 tablets) daily for a year, compared with a no-treatment control arm, it demonstrated an improvement in invasive disease-free survival (DFS) at 2 years from 91.9% to 94.2%, with no difference in overall survival yet noted. It is associated with diarrhea and also requires hepatic function monitoring.
Acute myelogenous leukemia
Multiple new agents were recently approved for use in acute myelogenous leukemia (AML), after decades of slow advance in new drug development.
Midostaurin (Rydapt) is an FLT3 inhibitor approved for use in combination with daunorubicin and Ara-C (cytosine arabinoside) for newly diagnosed AML with FLT3 mutations, which occur in about 30% of AML patients.4 It is given orally on days 8-21 at 50 mg bid with induction and consolidation.
In the study on which the approval was based, there was a 10% improvement in overall survival for this subset of AML patients who have a typically a worse prognosis. Event-free survival in patients in the study group was 8.2 months, compared with 3 months in the control arm patients, who did not receive the agent. The drug was also approved for aggressive systemic mastocytosis.
Enasidenib (Idhifa) has been approved for AML with an IDH2 mutation in the refractory/relapsed settings.5IDH2 mutations are present in about 20% of patients with AML. Given orally at 100 mg daily as a single agent, enasidenib was associated with a 19% complete remission rate. Patients need to be monitored for differentiation syndrome, somewhat similar to what is seen with ATRA with acute promyelocytic leukemia.
Liposomal daunorubicin and cytarabine (Vyxeos) was approved for newly diagnosed therapy- or myelodysplasia-related AML.6 This novel liposomal formulation combines two standard agents and is given intravenously on days 1, 3 and 5 over 90 minutes as daunorubicin 44 mg/m2 and cytarabine 100 mg/ m2. (For a second induction and in lower dose on consolidation cycles, it is given only on days 1 and 3). The liposomal formulation achieved a superior complete response rate compared with the standard 7+3 daunorubicin plus cytarabine regimen (38% vs 26%, respectively) and longer overall survival (9.6 versus 5.9 months) in these generally poor prognosis subsets.
Gemtuzumab ozogamicin (Mylotarg) was initially approved in 2000 but withdrawn from use in 2010 after trials failed to confirm benefit and demonstrated safety concerns. It has now been re-released in a lower dose and schedule from its original label.7 This immunoconjugate of an anti-CD33 bound to calicheamicin is approved for CD33-positive AML. Given at 3 mg/m2 on days 1, 4, and 7 in combination with standard daunorubicin–cytarabine induction chemotherapy, it improved event-free survival from 9.5 to 17.3 months. When administered as a single agent (6 mg/m2 on day 1 and 3 mg/m2 on day 8) in patients who were unable or unwilling to tolerate standard chemotherapy, it improved overall survival (4.9 months versus 3.6 months for best supportive care). As a single agent in relapsed AML, given at 3 mg/m2 days 1, 4, and 7 and followed by cytarabine consolidation, it was associated with a 26% complete response rate, with a median relapse-free survival of 11.6 months.
Ovarian/fallopian tube cancers
PARP inhibitors. For patients with ovarian/fallopian tube cancer, there are new indications and agents for PARP inhibition, including for patients with BRCA mutations (both somatic and germline) and those without BRCA mutations.
Olaparib (Lynparza) was previously approved only in a fourth-line setting for germline BRCA-mutated patients with advanced ovarian cancer, with a response rate of 34% with a median duration of 7.9 months. Given at 300 mg orally bid, it is now approved for use in maintenance in recurrence after response to platinum-based chemotherapy after 2 or more lines of therapy regardless of BRCA status. In this setting, progression-free survival increased to 8.4 months, compared with 4.8 months for placebo.8
Rubicarib (Rubraca) is approved for BRCA-mutated patients (either germline or somatic) with advanced ovarian cancer after two or more lines of chemotherapy.9 At 600 mg orally bid, results from phase 2 trials noted a 54% response rate, with a median duration of 9.2 months.
Niraparib (Zejula) is approved for use in maintenance in recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancers after platinum-based chemotherapy.10 In patients with germline BRCA mutations, niraparib at 300 mg orally daily resulted in a PFS of 21 months, compared with 5.5 months with placebo; PFS in patients with nongermline BRCA mutations was 9.3 versus 3.9 months, respectively.
Non-small cell lung cancer with EML-4 alk translocation
Crizotinib (Xalkori) has been the mainstay for treatment of for EML4-alk translocated non-small cell lung cancer. However, alectinib (Alcensa), previously for predominantly second-line use, seems more active than crizotinib in the first-line setting, particularly in the treatment and prevention of CNS metastases.
In addition, brigantinib (Alunbrig) has been approved for patients who are intolerant/refractory to crizotinib.11 At 90 mg once daily for 7 days, then escalating to 180 mg daily, it was noted to have a 50% response rate in crizotinib failures, including in the CNS.
Ceritinib (Zykadia) was approved at 750 mg once daily for EML4 alk positive NSCLC.12 In first line it had a response rate of 73% (versus 27% for chemotherapy) with a remission duration of 23.9 months (versus 11.1 months for chemotherapy).
Therapies by drug class
PD-1/PD-L1 antibodies
Anti-PD-1 antibodies nivolumab (Opdivo) and pembrolizumab (Keytruda) are widely used for a range of tumor types. Newer approvals for pembrolizumab are for adenocarcinoma of the stomach/gastro-esophageal junction with at least 1% PD-L1 expression, and in any tumor demonstrated to be MSI-high. Newer indications for nivolumab are for bladder cancer, MSI-high colon cancer, and for hepatoma previously treated with sorafenib. The anti-PD-L1 antibody atezolizumab (Tencentriq) is now approved for platinum-resistant metastatic lung cancer, in addition to platinum-ineligible and platinum-resistant urothelial cancer.
Avelumab (Bavencio) is an anti-PD-L1 approved for both Merkel cell and previously treated urothelial cancers at a dose of 10 mg/kg every 2 weeks.13 It demonstrated a 33% response rate for Merkel cell and a 16% response rate for urothelial cancer.
Durvalumab (Imfinzi) is another anti PD-L1 antibody approved at 10 mg/kg every 2 weeks for previously treated urothelial cancer with a 17% response rate (RR: PD-L1 high, 26%; low, 4%).14
PI3K kinase inhibitors
Copanlisib (Aliqopa) is a PI3K inhibitor approved for relapsed follicular lymphoma in patients who have progressed after two previous lines of therapy.15 It is a 60-mg, 1-hour infusion given on days 1, 8, and 15 every 28 days. In a phase 2 tria
BTK inhibitors
Acalabruitnib (Calquence) is approved for adults with previously treated mantle cell lymphoma. In a phase 2 trial at 100 mg orally bid, it achieved an 80% overall and 40% complete response rate.16 These response rates are higher than were seen for ibrutinib in its original phase 2 trial. The spectrum of toxicities seems similar to ibruitinib and includes bleeding, cytopenias, infection, and atrial fibrillation.
CD19 CAR-T cells
Perhaps the most exciting and novel new agents are genetically engineered autologous T cells. Tisagenlecleucel (Kymriah), a chimeric antigen receptor T cell (CART) that targets CD19 is approved for refractory B cell precursor acute lymphoblastic leukemia (in patients under 25 years) where the complete response rate was 83% (including patients with incomplete blood count recovery).17
Axicabtagene ciloleucel (aci-cel; Yescarta), also CD19-directed CART, is approved for adults with relapsed or refractory non-Hodgkin lymphoma after two lines of previous therapy (specifically large-cell lymphoma, primary mediastinal large B-cell lymphoma, and transformed follicular lymphoma). Response rate was 72% (complete, 51%; partial, 21%), with a median duration of response of 9.2 months.18
1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
2. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
3. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367-377.
4. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464.
5. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017 Aug 10;130(6):722-731.
6. Lancet JE, Rizzieri D, Schiller GJ, et al. Overall survival (OS) with CPX-351 versus 7+3 in older adults with newly diagnosed, therapy-related acute myeloid leukemia (tAML): subgroup analysis of a phase III study. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.7035. Published May 2017. Accessed November 20, 2017.
7. Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. http://www.bloodjournal.org/content/early/2017/10/11/blood-2017-09-797712?sso-checked=true. September 2017. Accessed November 20, 2017.
8. Kim G, Ison G, McKee AE, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257-4261.
9. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
10. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
11. Kim DW, Tiseo M, Ahn MJ, Reckamp KL, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498.
12. Soria J-C, Tan DSW, MD, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917-929.
13. Apolo AB, Infante JR, Balmanoukian A et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117-2124.
14. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-3125.
15. Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169-2178.
16. Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-016-0250-9. Published March 9, 2016. Accessed November 20, 2017.
17. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517.
18. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295.
With advances in the understanding of cellular pathways, molecular genetics, and immunology, new drugs for cancer are being released at an increasing rate. A variety of novel agents have recently become available for use, generating excitement for patients and oncologists. Keeping track of all of these new agents is increasingly challenging. This brief review will summarize some of the newest drugs, their indications, and benefits (see related article).
Therapies by tumor
Breast cancer
CDK4/6 inhibitors. The CDK4/6 inhibitor palbociclib was approved in 2015 for the treatment of estrogen-positive, HER2-negative advanced breast cancer, and this year, two more drugs in this class – ribociclib and abemaciclib – were approved for the treatment of hormone receptor–positive breast cancer.
Ribociclib (Kisqali) 600 mg daily (3 weeks on, 1 week off) is approved for use in combination with an aromatase inhibitor. In the study on which the approval was based, there was a response rate of 53% for patients in the study group, compared with 37% for those who received aromatase inhibitor alone (progression-free survival (PFS), not reached vs 14.7 months for single-agent aromatase inhibitor).1 The occurrence of neutropenia seemed to be similar to that in patients receiving palbociclib. However, unlike with palbociclib, ribociclib requires ECG monitoring for QTc prolongation as well as monitoring of liver function tests.
Abemaciclib (Verzenio) has been approved in combination with fulvestrant as well as a monotherapy.2 PFS was 16.4 months for abemaciclib (150 mg bid in combination with fulvestrant), compared with 9.3 months for fulvestrant alone, with corresponding response rates of 48% and 21%. As monotherapy, abemaciclib 200 mg bid had a response rate of 20% with a duration of response of 8.6 months.
Tyrosine kinase inhibitors. The tyrosine kinase inhibitor neratinib (Nerlynx) was approved for extended adjuvant treatment of HER2-positive breast cancer after 1 year of adjuvant trastuzumab.3 Given at 240 mg (6 tablets) daily for a year, compared with a no-treatment control arm, it demonstrated an improvement in invasive disease-free survival (DFS) at 2 years from 91.9% to 94.2%, with no difference in overall survival yet noted. It is associated with diarrhea and also requires hepatic function monitoring.
Acute myelogenous leukemia
Multiple new agents were recently approved for use in acute myelogenous leukemia (AML), after decades of slow advance in new drug development.
Midostaurin (Rydapt) is an FLT3 inhibitor approved for use in combination with daunorubicin and Ara-C (cytosine arabinoside) for newly diagnosed AML with FLT3 mutations, which occur in about 30% of AML patients.4 It is given orally on days 8-21 at 50 mg bid with induction and consolidation.
In the study on which the approval was based, there was a 10% improvement in overall survival for this subset of AML patients who have a typically a worse prognosis. Event-free survival in patients in the study group was 8.2 months, compared with 3 months in the control arm patients, who did not receive the agent. The drug was also approved for aggressive systemic mastocytosis.
Enasidenib (Idhifa) has been approved for AML with an IDH2 mutation in the refractory/relapsed settings.5IDH2 mutations are present in about 20% of patients with AML. Given orally at 100 mg daily as a single agent, enasidenib was associated with a 19% complete remission rate. Patients need to be monitored for differentiation syndrome, somewhat similar to what is seen with ATRA with acute promyelocytic leukemia.
Liposomal daunorubicin and cytarabine (Vyxeos) was approved for newly diagnosed therapy- or myelodysplasia-related AML.6 This novel liposomal formulation combines two standard agents and is given intravenously on days 1, 3 and 5 over 90 minutes as daunorubicin 44 mg/m2 and cytarabine 100 mg/ m2. (For a second induction and in lower dose on consolidation cycles, it is given only on days 1 and 3). The liposomal formulation achieved a superior complete response rate compared with the standard 7+3 daunorubicin plus cytarabine regimen (38% vs 26%, respectively) and longer overall survival (9.6 versus 5.9 months) in these generally poor prognosis subsets.
Gemtuzumab ozogamicin (Mylotarg) was initially approved in 2000 but withdrawn from use in 2010 after trials failed to confirm benefit and demonstrated safety concerns. It has now been re-released in a lower dose and schedule from its original label.7 This immunoconjugate of an anti-CD33 bound to calicheamicin is approved for CD33-positive AML. Given at 3 mg/m2 on days 1, 4, and 7 in combination with standard daunorubicin–cytarabine induction chemotherapy, it improved event-free survival from 9.5 to 17.3 months. When administered as a single agent (6 mg/m2 on day 1 and 3 mg/m2 on day 8) in patients who were unable or unwilling to tolerate standard chemotherapy, it improved overall survival (4.9 months versus 3.6 months for best supportive care). As a single agent in relapsed AML, given at 3 mg/m2 days 1, 4, and 7 and followed by cytarabine consolidation, it was associated with a 26% complete response rate, with a median relapse-free survival of 11.6 months.
Ovarian/fallopian tube cancers
PARP inhibitors. For patients with ovarian/fallopian tube cancer, there are new indications and agents for PARP inhibition, including for patients with BRCA mutations (both somatic and germline) and those without BRCA mutations.
Olaparib (Lynparza) was previously approved only in a fourth-line setting for germline BRCA-mutated patients with advanced ovarian cancer, with a response rate of 34% with a median duration of 7.9 months. Given at 300 mg orally bid, it is now approved for use in maintenance in recurrence after response to platinum-based chemotherapy after 2 or more lines of therapy regardless of BRCA status. In this setting, progression-free survival increased to 8.4 months, compared with 4.8 months for placebo.8
Rubicarib (Rubraca) is approved for BRCA-mutated patients (either germline or somatic) with advanced ovarian cancer after two or more lines of chemotherapy.9 At 600 mg orally bid, results from phase 2 trials noted a 54% response rate, with a median duration of 9.2 months.
Niraparib (Zejula) is approved for use in maintenance in recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancers after platinum-based chemotherapy.10 In patients with germline BRCA mutations, niraparib at 300 mg orally daily resulted in a PFS of 21 months, compared with 5.5 months with placebo; PFS in patients with nongermline BRCA mutations was 9.3 versus 3.9 months, respectively.
Non-small cell lung cancer with EML-4 alk translocation
Crizotinib (Xalkori) has been the mainstay for treatment of for EML4-alk translocated non-small cell lung cancer. However, alectinib (Alcensa), previously for predominantly second-line use, seems more active than crizotinib in the first-line setting, particularly in the treatment and prevention of CNS metastases.
In addition, brigantinib (Alunbrig) has been approved for patients who are intolerant/refractory to crizotinib.11 At 90 mg once daily for 7 days, then escalating to 180 mg daily, it was noted to have a 50% response rate in crizotinib failures, including in the CNS.
Ceritinib (Zykadia) was approved at 750 mg once daily for EML4 alk positive NSCLC.12 In first line it had a response rate of 73% (versus 27% for chemotherapy) with a remission duration of 23.9 months (versus 11.1 months for chemotherapy).
Therapies by drug class
PD-1/PD-L1 antibodies
Anti-PD-1 antibodies nivolumab (Opdivo) and pembrolizumab (Keytruda) are widely used for a range of tumor types. Newer approvals for pembrolizumab are for adenocarcinoma of the stomach/gastro-esophageal junction with at least 1% PD-L1 expression, and in any tumor demonstrated to be MSI-high. Newer indications for nivolumab are for bladder cancer, MSI-high colon cancer, and for hepatoma previously treated with sorafenib. The anti-PD-L1 antibody atezolizumab (Tencentriq) is now approved for platinum-resistant metastatic lung cancer, in addition to platinum-ineligible and platinum-resistant urothelial cancer.
Avelumab (Bavencio) is an anti-PD-L1 approved for both Merkel cell and previously treated urothelial cancers at a dose of 10 mg/kg every 2 weeks.13 It demonstrated a 33% response rate for Merkel cell and a 16% response rate for urothelial cancer.
Durvalumab (Imfinzi) is another anti PD-L1 antibody approved at 10 mg/kg every 2 weeks for previously treated urothelial cancer with a 17% response rate (RR: PD-L1 high, 26%; low, 4%).14
PI3K kinase inhibitors
Copanlisib (Aliqopa) is a PI3K inhibitor approved for relapsed follicular lymphoma in patients who have progressed after two previous lines of therapy.15 It is a 60-mg, 1-hour infusion given on days 1, 8, and 15 every 28 days. In a phase 2 tria
BTK inhibitors
Acalabruitnib (Calquence) is approved for adults with previously treated mantle cell lymphoma. In a phase 2 trial at 100 mg orally bid, it achieved an 80% overall and 40% complete response rate.16 These response rates are higher than were seen for ibrutinib in its original phase 2 trial. The spectrum of toxicities seems similar to ibruitinib and includes bleeding, cytopenias, infection, and atrial fibrillation.
CD19 CAR-T cells
Perhaps the most exciting and novel new agents are genetically engineered autologous T cells. Tisagenlecleucel (Kymriah), a chimeric antigen receptor T cell (CART) that targets CD19 is approved for refractory B cell precursor acute lymphoblastic leukemia (in patients under 25 years) where the complete response rate was 83% (including patients with incomplete blood count recovery).17
Axicabtagene ciloleucel (aci-cel; Yescarta), also CD19-directed CART, is approved for adults with relapsed or refractory non-Hodgkin lymphoma after two lines of previous therapy (specifically large-cell lymphoma, primary mediastinal large B-cell lymphoma, and transformed follicular lymphoma). Response rate was 72% (complete, 51%; partial, 21%), with a median duration of response of 9.2 months.18
With advances in the understanding of cellular pathways, molecular genetics, and immunology, new drugs for cancer are being released at an increasing rate. A variety of novel agents have recently become available for use, generating excitement for patients and oncologists. Keeping track of all of these new agents is increasingly challenging. This brief review will summarize some of the newest drugs, their indications, and benefits (see related article).
Therapies by tumor
Breast cancer
CDK4/6 inhibitors. The CDK4/6 inhibitor palbociclib was approved in 2015 for the treatment of estrogen-positive, HER2-negative advanced breast cancer, and this year, two more drugs in this class – ribociclib and abemaciclib – were approved for the treatment of hormone receptor–positive breast cancer.
Ribociclib (Kisqali) 600 mg daily (3 weeks on, 1 week off) is approved for use in combination with an aromatase inhibitor. In the study on which the approval was based, there was a response rate of 53% for patients in the study group, compared with 37% for those who received aromatase inhibitor alone (progression-free survival (PFS), not reached vs 14.7 months for single-agent aromatase inhibitor).1 The occurrence of neutropenia seemed to be similar to that in patients receiving palbociclib. However, unlike with palbociclib, ribociclib requires ECG monitoring for QTc prolongation as well as monitoring of liver function tests.
Abemaciclib (Verzenio) has been approved in combination with fulvestrant as well as a monotherapy.2 PFS was 16.4 months for abemaciclib (150 mg bid in combination with fulvestrant), compared with 9.3 months for fulvestrant alone, with corresponding response rates of 48% and 21%. As monotherapy, abemaciclib 200 mg bid had a response rate of 20% with a duration of response of 8.6 months.
Tyrosine kinase inhibitors. The tyrosine kinase inhibitor neratinib (Nerlynx) was approved for extended adjuvant treatment of HER2-positive breast cancer after 1 year of adjuvant trastuzumab.3 Given at 240 mg (6 tablets) daily for a year, compared with a no-treatment control arm, it demonstrated an improvement in invasive disease-free survival (DFS) at 2 years from 91.9% to 94.2%, with no difference in overall survival yet noted. It is associated with diarrhea and also requires hepatic function monitoring.
Acute myelogenous leukemia
Multiple new agents were recently approved for use in acute myelogenous leukemia (AML), after decades of slow advance in new drug development.
Midostaurin (Rydapt) is an FLT3 inhibitor approved for use in combination with daunorubicin and Ara-C (cytosine arabinoside) for newly diagnosed AML with FLT3 mutations, which occur in about 30% of AML patients.4 It is given orally on days 8-21 at 50 mg bid with induction and consolidation.
In the study on which the approval was based, there was a 10% improvement in overall survival for this subset of AML patients who have a typically a worse prognosis. Event-free survival in patients in the study group was 8.2 months, compared with 3 months in the control arm patients, who did not receive the agent. The drug was also approved for aggressive systemic mastocytosis.
Enasidenib (Idhifa) has been approved for AML with an IDH2 mutation in the refractory/relapsed settings.5IDH2 mutations are present in about 20% of patients with AML. Given orally at 100 mg daily as a single agent, enasidenib was associated with a 19% complete remission rate. Patients need to be monitored for differentiation syndrome, somewhat similar to what is seen with ATRA with acute promyelocytic leukemia.
Liposomal daunorubicin and cytarabine (Vyxeos) was approved for newly diagnosed therapy- or myelodysplasia-related AML.6 This novel liposomal formulation combines two standard agents and is given intravenously on days 1, 3 and 5 over 90 minutes as daunorubicin 44 mg/m2 and cytarabine 100 mg/ m2. (For a second induction and in lower dose on consolidation cycles, it is given only on days 1 and 3). The liposomal formulation achieved a superior complete response rate compared with the standard 7+3 daunorubicin plus cytarabine regimen (38% vs 26%, respectively) and longer overall survival (9.6 versus 5.9 months) in these generally poor prognosis subsets.
Gemtuzumab ozogamicin (Mylotarg) was initially approved in 2000 but withdrawn from use in 2010 after trials failed to confirm benefit and demonstrated safety concerns. It has now been re-released in a lower dose and schedule from its original label.7 This immunoconjugate of an anti-CD33 bound to calicheamicin is approved for CD33-positive AML. Given at 3 mg/m2 on days 1, 4, and 7 in combination with standard daunorubicin–cytarabine induction chemotherapy, it improved event-free survival from 9.5 to 17.3 months. When administered as a single agent (6 mg/m2 on day 1 and 3 mg/m2 on day 8) in patients who were unable or unwilling to tolerate standard chemotherapy, it improved overall survival (4.9 months versus 3.6 months for best supportive care). As a single agent in relapsed AML, given at 3 mg/m2 days 1, 4, and 7 and followed by cytarabine consolidation, it was associated with a 26% complete response rate, with a median relapse-free survival of 11.6 months.
Ovarian/fallopian tube cancers
PARP inhibitors. For patients with ovarian/fallopian tube cancer, there are new indications and agents for PARP inhibition, including for patients with BRCA mutations (both somatic and germline) and those without BRCA mutations.
Olaparib (Lynparza) was previously approved only in a fourth-line setting for germline BRCA-mutated patients with advanced ovarian cancer, with a response rate of 34% with a median duration of 7.9 months. Given at 300 mg orally bid, it is now approved for use in maintenance in recurrence after response to platinum-based chemotherapy after 2 or more lines of therapy regardless of BRCA status. In this setting, progression-free survival increased to 8.4 months, compared with 4.8 months for placebo.8
Rubicarib (Rubraca) is approved for BRCA-mutated patients (either germline or somatic) with advanced ovarian cancer after two or more lines of chemotherapy.9 At 600 mg orally bid, results from phase 2 trials noted a 54% response rate, with a median duration of 9.2 months.
Niraparib (Zejula) is approved for use in maintenance in recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancers after platinum-based chemotherapy.10 In patients with germline BRCA mutations, niraparib at 300 mg orally daily resulted in a PFS of 21 months, compared with 5.5 months with placebo; PFS in patients with nongermline BRCA mutations was 9.3 versus 3.9 months, respectively.
Non-small cell lung cancer with EML-4 alk translocation
Crizotinib (Xalkori) has been the mainstay for treatment of for EML4-alk translocated non-small cell lung cancer. However, alectinib (Alcensa), previously for predominantly second-line use, seems more active than crizotinib in the first-line setting, particularly in the treatment and prevention of CNS metastases.
In addition, brigantinib (Alunbrig) has been approved for patients who are intolerant/refractory to crizotinib.11 At 90 mg once daily for 7 days, then escalating to 180 mg daily, it was noted to have a 50% response rate in crizotinib failures, including in the CNS.
Ceritinib (Zykadia) was approved at 750 mg once daily for EML4 alk positive NSCLC.12 In first line it had a response rate of 73% (versus 27% for chemotherapy) with a remission duration of 23.9 months (versus 11.1 months for chemotherapy).
Therapies by drug class
PD-1/PD-L1 antibodies
Anti-PD-1 antibodies nivolumab (Opdivo) and pembrolizumab (Keytruda) are widely used for a range of tumor types. Newer approvals for pembrolizumab are for adenocarcinoma of the stomach/gastro-esophageal junction with at least 1% PD-L1 expression, and in any tumor demonstrated to be MSI-high. Newer indications for nivolumab are for bladder cancer, MSI-high colon cancer, and for hepatoma previously treated with sorafenib. The anti-PD-L1 antibody atezolizumab (Tencentriq) is now approved for platinum-resistant metastatic lung cancer, in addition to platinum-ineligible and platinum-resistant urothelial cancer.
Avelumab (Bavencio) is an anti-PD-L1 approved for both Merkel cell and previously treated urothelial cancers at a dose of 10 mg/kg every 2 weeks.13 It demonstrated a 33% response rate for Merkel cell and a 16% response rate for urothelial cancer.
Durvalumab (Imfinzi) is another anti PD-L1 antibody approved at 10 mg/kg every 2 weeks for previously treated urothelial cancer with a 17% response rate (RR: PD-L1 high, 26%; low, 4%).14
PI3K kinase inhibitors
Copanlisib (Aliqopa) is a PI3K inhibitor approved for relapsed follicular lymphoma in patients who have progressed after two previous lines of therapy.15 It is a 60-mg, 1-hour infusion given on days 1, 8, and 15 every 28 days. In a phase 2 tria
BTK inhibitors
Acalabruitnib (Calquence) is approved for adults with previously treated mantle cell lymphoma. In a phase 2 trial at 100 mg orally bid, it achieved an 80% overall and 40% complete response rate.16 These response rates are higher than were seen for ibrutinib in its original phase 2 trial. The spectrum of toxicities seems similar to ibruitinib and includes bleeding, cytopenias, infection, and atrial fibrillation.
CD19 CAR-T cells
Perhaps the most exciting and novel new agents are genetically engineered autologous T cells. Tisagenlecleucel (Kymriah), a chimeric antigen receptor T cell (CART) that targets CD19 is approved for refractory B cell precursor acute lymphoblastic leukemia (in patients under 25 years) where the complete response rate was 83% (including patients with incomplete blood count recovery).17
Axicabtagene ciloleucel (aci-cel; Yescarta), also CD19-directed CART, is approved for adults with relapsed or refractory non-Hodgkin lymphoma after two lines of previous therapy (specifically large-cell lymphoma, primary mediastinal large B-cell lymphoma, and transformed follicular lymphoma). Response rate was 72% (complete, 51%; partial, 21%), with a median duration of response of 9.2 months.18
1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
2. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
3. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367-377.
4. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464.
5. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017 Aug 10;130(6):722-731.
6. Lancet JE, Rizzieri D, Schiller GJ, et al. Overall survival (OS) with CPX-351 versus 7+3 in older adults with newly diagnosed, therapy-related acute myeloid leukemia (tAML): subgroup analysis of a phase III study. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.7035. Published May 2017. Accessed November 20, 2017.
7. Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. http://www.bloodjournal.org/content/early/2017/10/11/blood-2017-09-797712?sso-checked=true. September 2017. Accessed November 20, 2017.
8. Kim G, Ison G, McKee AE, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257-4261.
9. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
10. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
11. Kim DW, Tiseo M, Ahn MJ, Reckamp KL, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498.
12. Soria J-C, Tan DSW, MD, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917-929.
13. Apolo AB, Infante JR, Balmanoukian A et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117-2124.
14. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-3125.
15. Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169-2178.
16. Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-016-0250-9. Published March 9, 2016. Accessed November 20, 2017.
17. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517.
18. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295.
1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
2. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
3. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367-377.
4. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464.
5. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017 Aug 10;130(6):722-731.
6. Lancet JE, Rizzieri D, Schiller GJ, et al. Overall survival (OS) with CPX-351 versus 7+3 in older adults with newly diagnosed, therapy-related acute myeloid leukemia (tAML): subgroup analysis of a phase III study. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.7035. Published May 2017. Accessed November 20, 2017.
7. Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. http://www.bloodjournal.org/content/early/2017/10/11/blood-2017-09-797712?sso-checked=true. September 2017. Accessed November 20, 2017.
8. Kim G, Ison G, McKee AE, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257-4261.
9. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
10. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
11. Kim DW, Tiseo M, Ahn MJ, Reckamp KL, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498.
12. Soria J-C, Tan DSW, MD, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917-929.
13. Apolo AB, Infante JR, Balmanoukian A et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117-2124.
14. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-3125.
15. Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169-2178.
16. Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-016-0250-9. Published March 9, 2016. Accessed November 20, 2017.
17. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517.
18. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295.
Rivaroxaban vs dalteparin for VTE in cancer
Rivaroxaban is a feasible alternative to low-molecular-weight heparin (LMWH) for the treatment of venous thromboembolism (VTE) in cancer patients, according to researchers.
In the SELECT-D trial, cancer patients with VTE received 6 months of treatment with rivaroxaban or the LMWH dalteparin.
Patients who received rivaroxaban had a lower rate of VTE recurrence but higher rates of bleeding than those who received dalteparin.
These results were published in the Journal of Clinical Oncology. The research was supported by Bayer AG.
The trial enrolled 406 patients who had cancer and VTE. Sixty-nine percent of patients were receiving cancer treatment (83% chemotherapy) at baseline, and 58% had metastases.
Patients were randomized to receive dalteparin or rivaroxaban for 6 months. Dalteparin was given at 200 IU/kg daily for the first month, then at 150 IU/kg daily for months 2 to 6. Rivaroxaban was given at 15 mg twice daily for 3 weeks, then at 20 mg once daily for the rest of the treatment period.
The 6-month cumulative VTE recurrence rate was 11% in the dalteparin arm and 4% in the rivaroxaban arm (hazard ratio [HR]=0.43; 95% CI, 0.19 to 0.99).
The 6-month cumulative rate of major bleeding was 4% in the dalteparin arm and 6% in the rivaroxaban arm (HR=1.83; 95% CI, 0.68 to 4.96).
And the 6-month cumulative rate of clinically relevant non-major bleeding was 4% in the dalteparin arm and 13% in the rivaroxaban arm (HR=3.76; 95% CI, 1.63 to 8.69).
The researchers said these results suggest rivaroxaban can be an alternative to LMWH in cancer patients.
“Clinicians were already adopting [rivaroxaban] into practice for non-cancer patients, and now they have data from this study to indicate that this form of treatment is an alternative option for many cancer patients who have a clot,” said study author Annie Young, SRN, PhD, of Warwick Medical School at University of Warwick in Coventry, UK.
“We now need to be sitting down with each one of our cancer patients with VTE, discussing their preference alongside looking at all their clinical details—including whether the cancer lesion is still there, what other medications are being taken, and what other conditions the patient has—so that we can choose the optimal VTE treatment for each patient.”
Rivaroxaban is a feasible alternative to low-molecular-weight heparin (LMWH) for the treatment of venous thromboembolism (VTE) in cancer patients, according to researchers.
In the SELECT-D trial, cancer patients with VTE received 6 months of treatment with rivaroxaban or the LMWH dalteparin.
Patients who received rivaroxaban had a lower rate of VTE recurrence but higher rates of bleeding than those who received dalteparin.
These results were published in the Journal of Clinical Oncology. The research was supported by Bayer AG.
The trial enrolled 406 patients who had cancer and VTE. Sixty-nine percent of patients were receiving cancer treatment (83% chemotherapy) at baseline, and 58% had metastases.
Patients were randomized to receive dalteparin or rivaroxaban for 6 months. Dalteparin was given at 200 IU/kg daily for the first month, then at 150 IU/kg daily for months 2 to 6. Rivaroxaban was given at 15 mg twice daily for 3 weeks, then at 20 mg once daily for the rest of the treatment period.
The 6-month cumulative VTE recurrence rate was 11% in the dalteparin arm and 4% in the rivaroxaban arm (hazard ratio [HR]=0.43; 95% CI, 0.19 to 0.99).
The 6-month cumulative rate of major bleeding was 4% in the dalteparin arm and 6% in the rivaroxaban arm (HR=1.83; 95% CI, 0.68 to 4.96).
And the 6-month cumulative rate of clinically relevant non-major bleeding was 4% in the dalteparin arm and 13% in the rivaroxaban arm (HR=3.76; 95% CI, 1.63 to 8.69).
The researchers said these results suggest rivaroxaban can be an alternative to LMWH in cancer patients.
“Clinicians were already adopting [rivaroxaban] into practice for non-cancer patients, and now they have data from this study to indicate that this form of treatment is an alternative option for many cancer patients who have a clot,” said study author Annie Young, SRN, PhD, of Warwick Medical School at University of Warwick in Coventry, UK.
“We now need to be sitting down with each one of our cancer patients with VTE, discussing their preference alongside looking at all their clinical details—including whether the cancer lesion is still there, what other medications are being taken, and what other conditions the patient has—so that we can choose the optimal VTE treatment for each patient.”
Rivaroxaban is a feasible alternative to low-molecular-weight heparin (LMWH) for the treatment of venous thromboembolism (VTE) in cancer patients, according to researchers.
In the SELECT-D trial, cancer patients with VTE received 6 months of treatment with rivaroxaban or the LMWH dalteparin.
Patients who received rivaroxaban had a lower rate of VTE recurrence but higher rates of bleeding than those who received dalteparin.
These results were published in the Journal of Clinical Oncology. The research was supported by Bayer AG.
The trial enrolled 406 patients who had cancer and VTE. Sixty-nine percent of patients were receiving cancer treatment (83% chemotherapy) at baseline, and 58% had metastases.
Patients were randomized to receive dalteparin or rivaroxaban for 6 months. Dalteparin was given at 200 IU/kg daily for the first month, then at 150 IU/kg daily for months 2 to 6. Rivaroxaban was given at 15 mg twice daily for 3 weeks, then at 20 mg once daily for the rest of the treatment period.
The 6-month cumulative VTE recurrence rate was 11% in the dalteparin arm and 4% in the rivaroxaban arm (hazard ratio [HR]=0.43; 95% CI, 0.19 to 0.99).
The 6-month cumulative rate of major bleeding was 4% in the dalteparin arm and 6% in the rivaroxaban arm (HR=1.83; 95% CI, 0.68 to 4.96).
And the 6-month cumulative rate of clinically relevant non-major bleeding was 4% in the dalteparin arm and 13% in the rivaroxaban arm (HR=3.76; 95% CI, 1.63 to 8.69).
The researchers said these results suggest rivaroxaban can be an alternative to LMWH in cancer patients.
“Clinicians were already adopting [rivaroxaban] into practice for non-cancer patients, and now they have data from this study to indicate that this form of treatment is an alternative option for many cancer patients who have a clot,” said study author Annie Young, SRN, PhD, of Warwick Medical School at University of Warwick in Coventry, UK.
“We now need to be sitting down with each one of our cancer patients with VTE, discussing their preference alongside looking at all their clinical details—including whether the cancer lesion is still there, what other medications are being taken, and what other conditions the patient has—so that we can choose the optimal VTE treatment for each patient.”
Parents smoke less tobacco, more cannabis at home
Children are increasingly less likely to be exposed to secondhand cigarette smoke in the home, but cannabis seems to be picking up some of the slack, according to Renee D. Goodwin, PhD, and her associates.
Using a study population of 169,259 adults with data available from the National Survey on Drug Use and Health, the researchers found that from 2002 to 2015, the prevalence of parents with children in the home who were also current smokers dropped from 27.6% to 20.2%. By contrast, the rate of cannabis usage increased from 4.9% to 6.8% over the same time period. Overall, the rate of children living in a home with secondhand smoke fell from 29.7% to 23.5%.
“Public health efforts that have shown success in decreasing exposure to STS [secondhand tobacco smoke] in the home may be complicated by increased use of other smoked products, such as cannabis. Parents may benefit from education about protecting children from marijuana products, paraphernalia, waste, and smoke,” the investigators concluded.
SOURCE: Goodwin RD et al. Pediatrics. 2018 May 14. doi: 10.1542/peds.2017-3506.
Children are increasingly less likely to be exposed to secondhand cigarette smoke in the home, but cannabis seems to be picking up some of the slack, according to Renee D. Goodwin, PhD, and her associates.
Using a study population of 169,259 adults with data available from the National Survey on Drug Use and Health, the researchers found that from 2002 to 2015, the prevalence of parents with children in the home who were also current smokers dropped from 27.6% to 20.2%. By contrast, the rate of cannabis usage increased from 4.9% to 6.8% over the same time period. Overall, the rate of children living in a home with secondhand smoke fell from 29.7% to 23.5%.
“Public health efforts that have shown success in decreasing exposure to STS [secondhand tobacco smoke] in the home may be complicated by increased use of other smoked products, such as cannabis. Parents may benefit from education about protecting children from marijuana products, paraphernalia, waste, and smoke,” the investigators concluded.
SOURCE: Goodwin RD et al. Pediatrics. 2018 May 14. doi: 10.1542/peds.2017-3506.
Children are increasingly less likely to be exposed to secondhand cigarette smoke in the home, but cannabis seems to be picking up some of the slack, according to Renee D. Goodwin, PhD, and her associates.
Using a study population of 169,259 adults with data available from the National Survey on Drug Use and Health, the researchers found that from 2002 to 2015, the prevalence of parents with children in the home who were also current smokers dropped from 27.6% to 20.2%. By contrast, the rate of cannabis usage increased from 4.9% to 6.8% over the same time period. Overall, the rate of children living in a home with secondhand smoke fell from 29.7% to 23.5%.
“Public health efforts that have shown success in decreasing exposure to STS [secondhand tobacco smoke] in the home may be complicated by increased use of other smoked products, such as cannabis. Parents may benefit from education about protecting children from marijuana products, paraphernalia, waste, and smoke,” the investigators concluded.
SOURCE: Goodwin RD et al. Pediatrics. 2018 May 14. doi: 10.1542/peds.2017-3506.
FROM PEDIATRICS
VIDEO: The effect of removing pregnancy drug category labeling
AUSTIN, TEX. – In a randomized survey, the Food and Drug Administration’s previous letter-category labeling of drugs for pregnant women made prescribers more likely to prescribe appropriate medication than the new labeling standards without the letters.
The FDA removed the letter categories A, B, C, D, and X in 2014 in the belief “that a narrative structure for pregnancy labeling is best able to capture and convey the potential risks of drug exposure based on animal or human data, or both,” as stated in the FDA ruling.
“The [old] FDA categories are actually based upon the evidence related to clinical trials or animal trials and known risks to the fetus or the mother. The categorizations really reflect the evidence that we have or the absence of evidence as to whether medications can be safely used during pregnancy,” Dr. Robinson explained in a video interview. But letters can be perceived as overall “grades,” even though they aren’t.
The researchers sought to evaluate the effect of the letters’ removal by surveying doctors at two centers in New York City and two annual specialty meetings from October 2015 to May 2016.
The survey “included demographic information, followed by four clinical vignettes. Each vignette described a pregnant woman and presented an indication for prescribing a particular drug which was FDA approved. Each vignette was followed by detailed drug information as found in the FDA-approved package insert with the new [Pregnancy and Lactation Labeling Rule] content and formatting,” Dr. Robinson said at the meeting.
The 162 survey respondents estimated their likelihood of prescribing given the information in the vignette. The respondents were randomized to see the letter category or not. Category X (positive evidence of risk that clearly outweighs potential benefits) was not included. For all four remaining categories, the respondents who were shown the letter category were more likely to prescribe. Category B was significantly affected in a mixed linear model, and both categories B and C were significantly affected in a multivariate model.
“The new system of revised labeling may be more ambiguous and present new challenges for clinical decision making that could result in decreased access to medications for pregnant women,” Dr. Robinson said.
Dr. Robinson and her coauthors reported no relevant disclosures.
SOURCE: Robinson A et al. ACOG 2018. Abstract OP5.
AUSTIN, TEX. – In a randomized survey, the Food and Drug Administration’s previous letter-category labeling of drugs for pregnant women made prescribers more likely to prescribe appropriate medication than the new labeling standards without the letters.
The FDA removed the letter categories A, B, C, D, and X in 2014 in the belief “that a narrative structure for pregnancy labeling is best able to capture and convey the potential risks of drug exposure based on animal or human data, or both,” as stated in the FDA ruling.
“The [old] FDA categories are actually based upon the evidence related to clinical trials or animal trials and known risks to the fetus or the mother. The categorizations really reflect the evidence that we have or the absence of evidence as to whether medications can be safely used during pregnancy,” Dr. Robinson explained in a video interview. But letters can be perceived as overall “grades,” even though they aren’t.
The researchers sought to evaluate the effect of the letters’ removal by surveying doctors at two centers in New York City and two annual specialty meetings from October 2015 to May 2016.
The survey “included demographic information, followed by four clinical vignettes. Each vignette described a pregnant woman and presented an indication for prescribing a particular drug which was FDA approved. Each vignette was followed by detailed drug information as found in the FDA-approved package insert with the new [Pregnancy and Lactation Labeling Rule] content and formatting,” Dr. Robinson said at the meeting.
The 162 survey respondents estimated their likelihood of prescribing given the information in the vignette. The respondents were randomized to see the letter category or not. Category X (positive evidence of risk that clearly outweighs potential benefits) was not included. For all four remaining categories, the respondents who were shown the letter category were more likely to prescribe. Category B was significantly affected in a mixed linear model, and both categories B and C were significantly affected in a multivariate model.
“The new system of revised labeling may be more ambiguous and present new challenges for clinical decision making that could result in decreased access to medications for pregnant women,” Dr. Robinson said.
Dr. Robinson and her coauthors reported no relevant disclosures.
SOURCE: Robinson A et al. ACOG 2018. Abstract OP5.
AUSTIN, TEX. – In a randomized survey, the Food and Drug Administration’s previous letter-category labeling of drugs for pregnant women made prescribers more likely to prescribe appropriate medication than the new labeling standards without the letters.
The FDA removed the letter categories A, B, C, D, and X in 2014 in the belief “that a narrative structure for pregnancy labeling is best able to capture and convey the potential risks of drug exposure based on animal or human data, or both,” as stated in the FDA ruling.
“The [old] FDA categories are actually based upon the evidence related to clinical trials or animal trials and known risks to the fetus or the mother. The categorizations really reflect the evidence that we have or the absence of evidence as to whether medications can be safely used during pregnancy,” Dr. Robinson explained in a video interview. But letters can be perceived as overall “grades,” even though they aren’t.
The researchers sought to evaluate the effect of the letters’ removal by surveying doctors at two centers in New York City and two annual specialty meetings from October 2015 to May 2016.
The survey “included demographic information, followed by four clinical vignettes. Each vignette described a pregnant woman and presented an indication for prescribing a particular drug which was FDA approved. Each vignette was followed by detailed drug information as found in the FDA-approved package insert with the new [Pregnancy and Lactation Labeling Rule] content and formatting,” Dr. Robinson said at the meeting.
The 162 survey respondents estimated their likelihood of prescribing given the information in the vignette. The respondents were randomized to see the letter category or not. Category X (positive evidence of risk that clearly outweighs potential benefits) was not included. For all four remaining categories, the respondents who were shown the letter category were more likely to prescribe. Category B was significantly affected in a mixed linear model, and both categories B and C were significantly affected in a multivariate model.
“The new system of revised labeling may be more ambiguous and present new challenges for clinical decision making that could result in decreased access to medications for pregnant women,” Dr. Robinson said.
Dr. Robinson and her coauthors reported no relevant disclosures.
SOURCE: Robinson A et al. ACOG 2018. Abstract OP5.
REPORTING FROM ACOG 2018
VIDEO: Promoting upright and mobile labor could save over $700 million yearly
AUSTIN, TEX. – Encouraging an upright position and allowing mobility during labor is a cost-effective intervention that could save hundreds of millions of dollars while preventing cesarean deliveries, uterine rupture, and maternal deaths, according to a recent cost-effectiveness study.
Alyssa Hersh, a medical student at Oregon Health & Sciences University, Portland, developed the analysis using an innovative model that examines the costs associated not just with the first delivery, but also the probable next delivery.
“Our model was dependent on the ability to reduce cesareans and also reduce labor times,” said Ms. Hersh in a video interview. “So this reduction in cesareans allowed women to avoid having an increased risk of uterine rupture, of emergent hysterectomy, and other downstream consequences.”
The “two-delivery model” takes into account the average number of births per woman in the United States, “such that the risks, benefits, and costs are framed within the public health perspective of the average U.S. childrearing woman’s entire reproductive course,” she and her coauthors wrote in the poster accompanying the presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
This model captures the downstream effects of a first cesarean delivery on the next delivery, for example, providing a more realistic picture of the true costs of cesarean delivery for a nulliparous female.
Some of the known benefits of being upright and mobile during labor, Ms. Hersh said, include shortened labor and reduced risk for cesarean delivery. Cost-effectiveness of this approach for low-risk women, she said, had not been fully explored.
For the analysis, Ms. Hersh and her colleagues used a theoretical cohort of 1.8 million women, approximating the number of nulliparous term deliveries in the United States each year. They used rates of cesarean delivery for women laboring in upright and recumbent positions that were drawn from the literature, but lower than national averages: 7.8% of recumbent women and 5.4% of upright women went on to cesarean delivery in the model used by the investigators.
The outcomes tracked in the analysis included cesarean delivery, uterine rupture, hysterectomy attributed to uterine rupture, costs, and quality-adjusted life years (QALYs). All of the outcomes were tracked for the index pregnancy and the second pregnancy.
Ms. Hersh and her coinvestigators found that in the theoretical cohort, “laboring upright led to 64,890 fewer cesarean deliveries, 15 fewer maternal deaths, 113 fewer uterine ruptures, and 30 fewer hysterectomies.”
These reductions were associated with a savings for this cohort of $785 million, and an increase in QALYs of 2,142.
Using Monte Carlo simulation techniques to ascertain the effect of varying cesarean rates and other components of the model, Ms. Hersh and her colleagues found that the model remained cost-effective even with variation in all of the inputs.
“Laboring upright is a no-cost intervention that leads to improved outcomes, decreased costs, and increased QALYs during a woman’s first and second deliveries,” wrote Ms. Hersh and her associates. “This model argues for increasing systems factors that support women to be upright and mobile during labor, and in doing so, promoting improved health for our patients.”
Said Ms. Hersh, “This is an easy way for hospitals to adopt policies that can enable women to have improved outcomes.”
Ms. Hersh and her colleagues had no relevant financial disclosures.
SOURCE: Hersh A et al. ACOG 2018. Abstract 34C.
AUSTIN, TEX. – Encouraging an upright position and allowing mobility during labor is a cost-effective intervention that could save hundreds of millions of dollars while preventing cesarean deliveries, uterine rupture, and maternal deaths, according to a recent cost-effectiveness study.
Alyssa Hersh, a medical student at Oregon Health & Sciences University, Portland, developed the analysis using an innovative model that examines the costs associated not just with the first delivery, but also the probable next delivery.
“Our model was dependent on the ability to reduce cesareans and also reduce labor times,” said Ms. Hersh in a video interview. “So this reduction in cesareans allowed women to avoid having an increased risk of uterine rupture, of emergent hysterectomy, and other downstream consequences.”
The “two-delivery model” takes into account the average number of births per woman in the United States, “such that the risks, benefits, and costs are framed within the public health perspective of the average U.S. childrearing woman’s entire reproductive course,” she and her coauthors wrote in the poster accompanying the presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
This model captures the downstream effects of a first cesarean delivery on the next delivery, for example, providing a more realistic picture of the true costs of cesarean delivery for a nulliparous female.
Some of the known benefits of being upright and mobile during labor, Ms. Hersh said, include shortened labor and reduced risk for cesarean delivery. Cost-effectiveness of this approach for low-risk women, she said, had not been fully explored.
For the analysis, Ms. Hersh and her colleagues used a theoretical cohort of 1.8 million women, approximating the number of nulliparous term deliveries in the United States each year. They used rates of cesarean delivery for women laboring in upright and recumbent positions that were drawn from the literature, but lower than national averages: 7.8% of recumbent women and 5.4% of upright women went on to cesarean delivery in the model used by the investigators.
The outcomes tracked in the analysis included cesarean delivery, uterine rupture, hysterectomy attributed to uterine rupture, costs, and quality-adjusted life years (QALYs). All of the outcomes were tracked for the index pregnancy and the second pregnancy.
Ms. Hersh and her coinvestigators found that in the theoretical cohort, “laboring upright led to 64,890 fewer cesarean deliveries, 15 fewer maternal deaths, 113 fewer uterine ruptures, and 30 fewer hysterectomies.”
These reductions were associated with a savings for this cohort of $785 million, and an increase in QALYs of 2,142.
Using Monte Carlo simulation techniques to ascertain the effect of varying cesarean rates and other components of the model, Ms. Hersh and her colleagues found that the model remained cost-effective even with variation in all of the inputs.
“Laboring upright is a no-cost intervention that leads to improved outcomes, decreased costs, and increased QALYs during a woman’s first and second deliveries,” wrote Ms. Hersh and her associates. “This model argues for increasing systems factors that support women to be upright and mobile during labor, and in doing so, promoting improved health for our patients.”
Said Ms. Hersh, “This is an easy way for hospitals to adopt policies that can enable women to have improved outcomes.”
Ms. Hersh and her colleagues had no relevant financial disclosures.
SOURCE: Hersh A et al. ACOG 2018. Abstract 34C.
AUSTIN, TEX. – Encouraging an upright position and allowing mobility during labor is a cost-effective intervention that could save hundreds of millions of dollars while preventing cesarean deliveries, uterine rupture, and maternal deaths, according to a recent cost-effectiveness study.
Alyssa Hersh, a medical student at Oregon Health & Sciences University, Portland, developed the analysis using an innovative model that examines the costs associated not just with the first delivery, but also the probable next delivery.
“Our model was dependent on the ability to reduce cesareans and also reduce labor times,” said Ms. Hersh in a video interview. “So this reduction in cesareans allowed women to avoid having an increased risk of uterine rupture, of emergent hysterectomy, and other downstream consequences.”
The “two-delivery model” takes into account the average number of births per woman in the United States, “such that the risks, benefits, and costs are framed within the public health perspective of the average U.S. childrearing woman’s entire reproductive course,” she and her coauthors wrote in the poster accompanying the presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
This model captures the downstream effects of a first cesarean delivery on the next delivery, for example, providing a more realistic picture of the true costs of cesarean delivery for a nulliparous female.
Some of the known benefits of being upright and mobile during labor, Ms. Hersh said, include shortened labor and reduced risk for cesarean delivery. Cost-effectiveness of this approach for low-risk women, she said, had not been fully explored.
For the analysis, Ms. Hersh and her colleagues used a theoretical cohort of 1.8 million women, approximating the number of nulliparous term deliveries in the United States each year. They used rates of cesarean delivery for women laboring in upright and recumbent positions that were drawn from the literature, but lower than national averages: 7.8% of recumbent women and 5.4% of upright women went on to cesarean delivery in the model used by the investigators.
The outcomes tracked in the analysis included cesarean delivery, uterine rupture, hysterectomy attributed to uterine rupture, costs, and quality-adjusted life years (QALYs). All of the outcomes were tracked for the index pregnancy and the second pregnancy.
Ms. Hersh and her coinvestigators found that in the theoretical cohort, “laboring upright led to 64,890 fewer cesarean deliveries, 15 fewer maternal deaths, 113 fewer uterine ruptures, and 30 fewer hysterectomies.”
These reductions were associated with a savings for this cohort of $785 million, and an increase in QALYs of 2,142.
Using Monte Carlo simulation techniques to ascertain the effect of varying cesarean rates and other components of the model, Ms. Hersh and her colleagues found that the model remained cost-effective even with variation in all of the inputs.
“Laboring upright is a no-cost intervention that leads to improved outcomes, decreased costs, and increased QALYs during a woman’s first and second deliveries,” wrote Ms. Hersh and her associates. “This model argues for increasing systems factors that support women to be upright and mobile during labor, and in doing so, promoting improved health for our patients.”
Said Ms. Hersh, “This is an easy way for hospitals to adopt policies that can enable women to have improved outcomes.”
Ms. Hersh and her colleagues had no relevant financial disclosures.
SOURCE: Hersh A et al. ACOG 2018. Abstract 34C.
REPORTING FROM ACOG 2018
To detect subclinical AF, insertable monitor is unequaled
ORLANDO – with other intermittent ambulatory monitoring strategies, in a secondary analysis from the REVEAL AF study.
“If you really want to look for atrial fibrillation because your concern is that the patient has a high-risk profile, and if you saw it you would anticoagulate it and maybe prophylactically use rate control, nothing beats the implanted monitor,” James A. Reiffel, MD, said at the annual meeting of the American College of Cardiology.
As previously reported (JAMA Cardiol. 2017 Oct 1;2[10]:1120-7), the primary outcome – an AF episode lasting for at least 6 minutes – occurred during the first 18 months of the study in 29% of participants. By 30 months, it was 40%. At ACC 2018, Dr. Reiffel presented a new analysis looking at how the insertable cardiac monitor (ICM) would have stacked up against other device-based strategies aimed at detecting silent AF, including a 30-day implantable memory loop, daily transtelephonic ECG monitoring, and one-time or periodic 24- or 48-hour Holter monitoring.
To accomplish this, he and his coinvestigators conducted modeling studies harnessing the REVEAL AF continuous monitoring data. They looked at how many of the real-world patients found to have AF in the study would have been identified had they instead undergone a one-time recording period lasting 1, 2, 7, 14, or 30 days beginning at the time they would have received their ICM. They also looked at the yield of repeated monitoring strategies, including monthly or quarterly 24- or 48-hour Holter monitoring sessions. They repeated the various simulated monitoring strategies 10,000 times each in order to beef up the sample size and stability of the results.
It was no contest, according to Dr. Reiffel, professor of clinical medicine at Columbia University in New York.
That’s because the median time to AF detection in REVEAL AF was 123 days. Thus, any monitoring strategy of 30 days duration or less was doomed to be of comparatively low yield. Indeed, the 12-month AF incidence rate as detected by ICM in REVEAL AF was 27.1%, compared with 1.1%-13.5% for the various modeled monitoring strategies.
Among patients who met the primary endpoint in REVEAL AF, 10.2% had one or more AF episodes lasting 24 hours or more. So a significant proportion of the asymptomatic episodes of AF were not brief.
The take-home lesson of this analysis is straightforward, he said. “While the incidence of screen-detected atrial fibrillation is dependent upon the population screened, it is also strongly dependent upon the duration and intensity of monitoring.”
Session cochair Jeanne E. Poole, MD, observed that while the new REVEAL AF analysis is informative, it leaves unanswered the big questions regarding the clinical importance of these silent episodes of subclinical device-detected AF. That is, are these episodes associated with significantly increased stroke risk, and if so are they just another nonmodifiable risk marker, or are they a risk factor that can be dampened via oral anticoagulation, like symptomatic AF? said Dr. Poole, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle.
“My own belief is that they are both a risk marker and a risk factor that contributes to stroke,” Dr. Reiffel replied.
He noted that there are two major ongoing clinical trials evaluating the impact of oral anticoagulation in patients with ICM-detected AF. The 3,400-patient German multicenter Non–vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes (NOAH–AFNET 6 ) trial is testing whether oral anticoagulation with edoxaban (Savaysa) is superior to aspirin or no antithrombotic therapy for prevention of stroke or cardiovascular death. And the 4,000-patient Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESIA) trial is randomizing patients to apixaban (Eliquis) or aspirin.
“We’ll know within the next 3-4 years whether patients with high-risk profiles for atrial fibrillation but no clinically manifest atrial fibrillation should in fact be detected and should in fact be anticoagulated if atrial fibrillation is detected. Putting on my ‘Carnac The Magnificent’ hat [made famous by Johnny Carson on the ‘Tonight Show’], I predict the answer to both of those questions is likely to be yes,” the cardiologist added.
He noted that the Medtronic ICM, which is one-third the size of a AAA battery, is implanted via a small incision, which is then covered by a adhesive bandage.
“There’s no major surgical technique involved in putting [int ICM] in,” he said.
The REVEAL AF study was sponsored by Medtronic. Dr. Reiffel reported serving as a consultant to the company.
SOURCE: Reiffel JA et al. ACC 2018, Abstract 900-08.
ORLANDO – with other intermittent ambulatory monitoring strategies, in a secondary analysis from the REVEAL AF study.
“If you really want to look for atrial fibrillation because your concern is that the patient has a high-risk profile, and if you saw it you would anticoagulate it and maybe prophylactically use rate control, nothing beats the implanted monitor,” James A. Reiffel, MD, said at the annual meeting of the American College of Cardiology.
As previously reported (JAMA Cardiol. 2017 Oct 1;2[10]:1120-7), the primary outcome – an AF episode lasting for at least 6 minutes – occurred during the first 18 months of the study in 29% of participants. By 30 months, it was 40%. At ACC 2018, Dr. Reiffel presented a new analysis looking at how the insertable cardiac monitor (ICM) would have stacked up against other device-based strategies aimed at detecting silent AF, including a 30-day implantable memory loop, daily transtelephonic ECG monitoring, and one-time or periodic 24- or 48-hour Holter monitoring.
To accomplish this, he and his coinvestigators conducted modeling studies harnessing the REVEAL AF continuous monitoring data. They looked at how many of the real-world patients found to have AF in the study would have been identified had they instead undergone a one-time recording period lasting 1, 2, 7, 14, or 30 days beginning at the time they would have received their ICM. They also looked at the yield of repeated monitoring strategies, including monthly or quarterly 24- or 48-hour Holter monitoring sessions. They repeated the various simulated monitoring strategies 10,000 times each in order to beef up the sample size and stability of the results.
It was no contest, according to Dr. Reiffel, professor of clinical medicine at Columbia University in New York.
That’s because the median time to AF detection in REVEAL AF was 123 days. Thus, any monitoring strategy of 30 days duration or less was doomed to be of comparatively low yield. Indeed, the 12-month AF incidence rate as detected by ICM in REVEAL AF was 27.1%, compared with 1.1%-13.5% for the various modeled monitoring strategies.
Among patients who met the primary endpoint in REVEAL AF, 10.2% had one or more AF episodes lasting 24 hours or more. So a significant proportion of the asymptomatic episodes of AF were not brief.
The take-home lesson of this analysis is straightforward, he said. “While the incidence of screen-detected atrial fibrillation is dependent upon the population screened, it is also strongly dependent upon the duration and intensity of monitoring.”
Session cochair Jeanne E. Poole, MD, observed that while the new REVEAL AF analysis is informative, it leaves unanswered the big questions regarding the clinical importance of these silent episodes of subclinical device-detected AF. That is, are these episodes associated with significantly increased stroke risk, and if so are they just another nonmodifiable risk marker, or are they a risk factor that can be dampened via oral anticoagulation, like symptomatic AF? said Dr. Poole, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle.
“My own belief is that they are both a risk marker and a risk factor that contributes to stroke,” Dr. Reiffel replied.
He noted that there are two major ongoing clinical trials evaluating the impact of oral anticoagulation in patients with ICM-detected AF. The 3,400-patient German multicenter Non–vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes (NOAH–AFNET 6 ) trial is testing whether oral anticoagulation with edoxaban (Savaysa) is superior to aspirin or no antithrombotic therapy for prevention of stroke or cardiovascular death. And the 4,000-patient Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESIA) trial is randomizing patients to apixaban (Eliquis) or aspirin.
“We’ll know within the next 3-4 years whether patients with high-risk profiles for atrial fibrillation but no clinically manifest atrial fibrillation should in fact be detected and should in fact be anticoagulated if atrial fibrillation is detected. Putting on my ‘Carnac The Magnificent’ hat [made famous by Johnny Carson on the ‘Tonight Show’], I predict the answer to both of those questions is likely to be yes,” the cardiologist added.
He noted that the Medtronic ICM, which is one-third the size of a AAA battery, is implanted via a small incision, which is then covered by a adhesive bandage.
“There’s no major surgical technique involved in putting [int ICM] in,” he said.
The REVEAL AF study was sponsored by Medtronic. Dr. Reiffel reported serving as a consultant to the company.
SOURCE: Reiffel JA et al. ACC 2018, Abstract 900-08.
ORLANDO – with other intermittent ambulatory monitoring strategies, in a secondary analysis from the REVEAL AF study.
“If you really want to look for atrial fibrillation because your concern is that the patient has a high-risk profile, and if you saw it you would anticoagulate it and maybe prophylactically use rate control, nothing beats the implanted monitor,” James A. Reiffel, MD, said at the annual meeting of the American College of Cardiology.
As previously reported (JAMA Cardiol. 2017 Oct 1;2[10]:1120-7), the primary outcome – an AF episode lasting for at least 6 minutes – occurred during the first 18 months of the study in 29% of participants. By 30 months, it was 40%. At ACC 2018, Dr. Reiffel presented a new analysis looking at how the insertable cardiac monitor (ICM) would have stacked up against other device-based strategies aimed at detecting silent AF, including a 30-day implantable memory loop, daily transtelephonic ECG monitoring, and one-time or periodic 24- or 48-hour Holter monitoring.
To accomplish this, he and his coinvestigators conducted modeling studies harnessing the REVEAL AF continuous monitoring data. They looked at how many of the real-world patients found to have AF in the study would have been identified had they instead undergone a one-time recording period lasting 1, 2, 7, 14, or 30 days beginning at the time they would have received their ICM. They also looked at the yield of repeated monitoring strategies, including monthly or quarterly 24- or 48-hour Holter monitoring sessions. They repeated the various simulated monitoring strategies 10,000 times each in order to beef up the sample size and stability of the results.
It was no contest, according to Dr. Reiffel, professor of clinical medicine at Columbia University in New York.
That’s because the median time to AF detection in REVEAL AF was 123 days. Thus, any monitoring strategy of 30 days duration or less was doomed to be of comparatively low yield. Indeed, the 12-month AF incidence rate as detected by ICM in REVEAL AF was 27.1%, compared with 1.1%-13.5% for the various modeled monitoring strategies.
Among patients who met the primary endpoint in REVEAL AF, 10.2% had one or more AF episodes lasting 24 hours or more. So a significant proportion of the asymptomatic episodes of AF were not brief.
The take-home lesson of this analysis is straightforward, he said. “While the incidence of screen-detected atrial fibrillation is dependent upon the population screened, it is also strongly dependent upon the duration and intensity of monitoring.”
Session cochair Jeanne E. Poole, MD, observed that while the new REVEAL AF analysis is informative, it leaves unanswered the big questions regarding the clinical importance of these silent episodes of subclinical device-detected AF. That is, are these episodes associated with significantly increased stroke risk, and if so are they just another nonmodifiable risk marker, or are they a risk factor that can be dampened via oral anticoagulation, like symptomatic AF? said Dr. Poole, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle.
“My own belief is that they are both a risk marker and a risk factor that contributes to stroke,” Dr. Reiffel replied.
He noted that there are two major ongoing clinical trials evaluating the impact of oral anticoagulation in patients with ICM-detected AF. The 3,400-patient German multicenter Non–vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes (NOAH–AFNET 6 ) trial is testing whether oral anticoagulation with edoxaban (Savaysa) is superior to aspirin or no antithrombotic therapy for prevention of stroke or cardiovascular death. And the 4,000-patient Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESIA) trial is randomizing patients to apixaban (Eliquis) or aspirin.
“We’ll know within the next 3-4 years whether patients with high-risk profiles for atrial fibrillation but no clinically manifest atrial fibrillation should in fact be detected and should in fact be anticoagulated if atrial fibrillation is detected. Putting on my ‘Carnac The Magnificent’ hat [made famous by Johnny Carson on the ‘Tonight Show’], I predict the answer to both of those questions is likely to be yes,” the cardiologist added.
He noted that the Medtronic ICM, which is one-third the size of a AAA battery, is implanted via a small incision, which is then covered by a adhesive bandage.
“There’s no major surgical technique involved in putting [int ICM] in,” he said.
The REVEAL AF study was sponsored by Medtronic. Dr. Reiffel reported serving as a consultant to the company.
SOURCE: Reiffel JA et al. ACC 2018, Abstract 900-08.
REPORTING FROM ACC 2018
Key clinical point: Most patients at high risk for previously unidentified atrial fibrillation whose arrhythmia is detected by means of an insertable continuous ECG monitor would go unidentified by any other detection strategy.
Major finding: The median time to insertable cardiac monitor–based detection of a first episode of silent AF of at least 6 minutes length was 123 days.
Study details: This was a multicenter, prospective, single-arm study of 385 patients at high risk for AF who received an insertable cardiac monitor and were followed for 30 months.
Disclosures: The REVEAL AF study was sponsored by Medtronic. The presenter reported serving as a consultant to the company.
Source: Reiffel JA et al. ACC 2018, Abstract 900-08.
VIDEO: Three questions with Aaron B. Caughey, MD
AUSTIN, TEX. – This interview was conducted at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. It has been edited for length and clarity.
OB.GYN. NEWS: Here at ACOG, there were two studies from your research group at Oregon Health & Sciences University, Portland, that examined the cost-effectiveness of pregnancy interventions using a “two-delivery” model. Could you explain a little more about what that is, and how you arrived at this model?
DR. CAUGHEY: We’ve been working on decision analytics and cost-effectiveness studies of a number of ways to approach pregnancy, whether it be complicated pregnancies or uncomplicated normal pregnancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
One of the things that we think is really important to think about is the impact of management and outcomes in the current pregnancy, and how it might affect future pregnancies.
So, for example, if you have a vaginal delivery this time, that makes you multiparous with a prior vaginal delivery next time; that is kind of the goal in pregnancy. It just makes all future labor and delivery experiences so much easier. Whereas, if you have a prior C-section, we all know that now you’re high risk. Everybody gets nervous about you. Are you going to get a trial of labor? Can you find a hospital that will do a trial of labor? And there are all these downstream implications of the delivery in the first pregnancy.
If you think about it, there are a number of ways that we can manage the first pregnancy. There are a number of risk factors for increasing risk for cesarean delivery. And we can include those in models that are considered in downstream pregnancies. And so we’ve been doing that increasingly.
When we think about our vaginal birth after cesarean models, we had two presentations that considered that. One was how we manage the maternal position in labor, and the other was how we use doulas in labor. You think, well gosh, we’re spending money on this doula in this pregnancy – that’s a certain expenditure. Is it worth it?
Part of “Is it worth it?” is not just the current pregnancy, but the downstream pregnancy as well.
OB.GYN. NEWS: What has sparked your interest in looking at obstetric care delivery in this way?
DR. CAUGHEY: I did my PhD in health economics. I began it as a 3rd-year maternal fetal medicine fellow and during my early career as an assistant professor at the University of California, San Francisco.
A lot of people think that economics in general is kind of about finance. Actually, microeconomics in particular is about the allocation of scarce resources to optimize utility – utility as general wellbeing or happiness.
So people will say that this thing, or that intervention, is cost effective, and often what they think they mean is, “It saves money.” But most things don’t save money. Most things in health care cost money. There are a few things in health care that do save money – vaccinations, contraception. Contraception actually saves money – but most things cost money. We have to spend money to get something right.
And the way we do that in health economics is that we think we’re going to get some happiness, some utility, some better outcomes from the money we’re putting in. One of the things that we don’t do very well as a species is think about these downstream outcomes.
In our models, when we think about morbidity and mortality we’re incorporating two and three pregnancy models. Think about what happens in the future, and then think about if we do something – if we spend money on an induction of labor or having a doula or something like that, is it worth it for what we get?
Antenatal testing, for example. Or, is it worth it for you to be testing people with diabetes once a week? Twice a week? More? Less? Part of it is figuring out what you’ll get for it. The measurement of what you get for it is called quality-adjusted life years, and that’s the measure of happiness multiplied times life expectancy.
We incorporate that in standard ways to build these models, to help us make decisions around best practices. Now, the economics piece of it probably matters. We’re the richest country that’s probably ever going to be – not just ever has been but probably ever going to be – the way we’re using up scarce resources to beat the band.
Yet, we still have an issue of allocation. We have people that have less; we have enormous disparities, whether it be racial and ethnic disparities, or socioeconomic disparities. And so we need to figure out ways to be more efficient and allocate those scarce resources properly to the outcomes that will be the best.
I think that’s what we’re working on: creating models to think about how to allocate those scarce resources.
OB.GYN. NEWS: How can a busy obstetrician think about the work you’re doing and incorporate it into her practice on a day-to-day basis?
DR. CAUGHEY: As an economist, I want to step back from time to time and think about public health and allocation of scarce resources.
But as a busy practicing clinician, I don’t necessarily want you consciously to think about cost. There are people who will push back on this and say, “Oh no, we should always be cost conscious.” Actually, what I really want you to do is incorporate best practices into the care of the patient at that moment, and do the thing that improves her outcomes best at that moment.
What we want to do, instead, is design systems that will properly incentivize. Incentivize doesn’t mean you think, “Oh gosh, if I do this thing I’m going to get an extra dollar.” It means subconsciously that those incentives are there, and those incentives don’t have to just be about dollars. Often in our field, they’re about time – what takes less time and more time to do something. So if we provide little extra roadblocks, then you’re more likely to go the other way and do something else.
For example: the hard stop. We did all this research in the 2000s to show that you probably shouldn’t deliver babies just for fun before 39 weeks’ gestational age. There should be an indication. If we don’t allow any hard stop, if we don’t block the pathway, then patients are pushing us, they’re uncomfortable, they’re like, “Just deliver me.” So we said, “No, no, no, we’re going to block this. In fact, we’re actually going to provide a hard stop reimbursement-wise. And medical directors of hospitals are going to have to preapprove.” And so that provides a blockade, and makes it easier just not to do it.
So I think that’s what we want: At the bedside and in your office, we want clinicians to still just be really good doctors. But then, to get involved and help design systems to incentivize us to do the right things.
Aaron B. Caughey, MD, PhD, is professor and chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health & Science University, Portland, and is a member of the U.S. Preventive Services Task Force. He reported that he had no relevant financial disclosures.
AUSTIN, TEX. – This interview was conducted at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. It has been edited for length and clarity.
OB.GYN. NEWS: Here at ACOG, there were two studies from your research group at Oregon Health & Sciences University, Portland, that examined the cost-effectiveness of pregnancy interventions using a “two-delivery” model. Could you explain a little more about what that is, and how you arrived at this model?
DR. CAUGHEY: We’ve been working on decision analytics and cost-effectiveness studies of a number of ways to approach pregnancy, whether it be complicated pregnancies or uncomplicated normal pregnancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
One of the things that we think is really important to think about is the impact of management and outcomes in the current pregnancy, and how it might affect future pregnancies.
So, for example, if you have a vaginal delivery this time, that makes you multiparous with a prior vaginal delivery next time; that is kind of the goal in pregnancy. It just makes all future labor and delivery experiences so much easier. Whereas, if you have a prior C-section, we all know that now you’re high risk. Everybody gets nervous about you. Are you going to get a trial of labor? Can you find a hospital that will do a trial of labor? And there are all these downstream implications of the delivery in the first pregnancy.
If you think about it, there are a number of ways that we can manage the first pregnancy. There are a number of risk factors for increasing risk for cesarean delivery. And we can include those in models that are considered in downstream pregnancies. And so we’ve been doing that increasingly.
When we think about our vaginal birth after cesarean models, we had two presentations that considered that. One was how we manage the maternal position in labor, and the other was how we use doulas in labor. You think, well gosh, we’re spending money on this doula in this pregnancy – that’s a certain expenditure. Is it worth it?
Part of “Is it worth it?” is not just the current pregnancy, but the downstream pregnancy as well.
OB.GYN. NEWS: What has sparked your interest in looking at obstetric care delivery in this way?
DR. CAUGHEY: I did my PhD in health economics. I began it as a 3rd-year maternal fetal medicine fellow and during my early career as an assistant professor at the University of California, San Francisco.
A lot of people think that economics in general is kind of about finance. Actually, microeconomics in particular is about the allocation of scarce resources to optimize utility – utility as general wellbeing or happiness.
So people will say that this thing, or that intervention, is cost effective, and often what they think they mean is, “It saves money.” But most things don’t save money. Most things in health care cost money. There are a few things in health care that do save money – vaccinations, contraception. Contraception actually saves money – but most things cost money. We have to spend money to get something right.
And the way we do that in health economics is that we think we’re going to get some happiness, some utility, some better outcomes from the money we’re putting in. One of the things that we don’t do very well as a species is think about these downstream outcomes.
In our models, when we think about morbidity and mortality we’re incorporating two and three pregnancy models. Think about what happens in the future, and then think about if we do something – if we spend money on an induction of labor or having a doula or something like that, is it worth it for what we get?
Antenatal testing, for example. Or, is it worth it for you to be testing people with diabetes once a week? Twice a week? More? Less? Part of it is figuring out what you’ll get for it. The measurement of what you get for it is called quality-adjusted life years, and that’s the measure of happiness multiplied times life expectancy.
We incorporate that in standard ways to build these models, to help us make decisions around best practices. Now, the economics piece of it probably matters. We’re the richest country that’s probably ever going to be – not just ever has been but probably ever going to be – the way we’re using up scarce resources to beat the band.
Yet, we still have an issue of allocation. We have people that have less; we have enormous disparities, whether it be racial and ethnic disparities, or socioeconomic disparities. And so we need to figure out ways to be more efficient and allocate those scarce resources properly to the outcomes that will be the best.
I think that’s what we’re working on: creating models to think about how to allocate those scarce resources.
OB.GYN. NEWS: How can a busy obstetrician think about the work you’re doing and incorporate it into her practice on a day-to-day basis?
DR. CAUGHEY: As an economist, I want to step back from time to time and think about public health and allocation of scarce resources.
But as a busy practicing clinician, I don’t necessarily want you consciously to think about cost. There are people who will push back on this and say, “Oh no, we should always be cost conscious.” Actually, what I really want you to do is incorporate best practices into the care of the patient at that moment, and do the thing that improves her outcomes best at that moment.
What we want to do, instead, is design systems that will properly incentivize. Incentivize doesn’t mean you think, “Oh gosh, if I do this thing I’m going to get an extra dollar.” It means subconsciously that those incentives are there, and those incentives don’t have to just be about dollars. Often in our field, they’re about time – what takes less time and more time to do something. So if we provide little extra roadblocks, then you’re more likely to go the other way and do something else.
For example: the hard stop. We did all this research in the 2000s to show that you probably shouldn’t deliver babies just for fun before 39 weeks’ gestational age. There should be an indication. If we don’t allow any hard stop, if we don’t block the pathway, then patients are pushing us, they’re uncomfortable, they’re like, “Just deliver me.” So we said, “No, no, no, we’re going to block this. In fact, we’re actually going to provide a hard stop reimbursement-wise. And medical directors of hospitals are going to have to preapprove.” And so that provides a blockade, and makes it easier just not to do it.
So I think that’s what we want: At the bedside and in your office, we want clinicians to still just be really good doctors. But then, to get involved and help design systems to incentivize us to do the right things.
Aaron B. Caughey, MD, PhD, is professor and chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health & Science University, Portland, and is a member of the U.S. Preventive Services Task Force. He reported that he had no relevant financial disclosures.
AUSTIN, TEX. – This interview was conducted at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. It has been edited for length and clarity.
OB.GYN. NEWS: Here at ACOG, there were two studies from your research group at Oregon Health & Sciences University, Portland, that examined the cost-effectiveness of pregnancy interventions using a “two-delivery” model. Could you explain a little more about what that is, and how you arrived at this model?
DR. CAUGHEY: We’ve been working on decision analytics and cost-effectiveness studies of a number of ways to approach pregnancy, whether it be complicated pregnancies or uncomplicated normal pregnancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
One of the things that we think is really important to think about is the impact of management and outcomes in the current pregnancy, and how it might affect future pregnancies.
So, for example, if you have a vaginal delivery this time, that makes you multiparous with a prior vaginal delivery next time; that is kind of the goal in pregnancy. It just makes all future labor and delivery experiences so much easier. Whereas, if you have a prior C-section, we all know that now you’re high risk. Everybody gets nervous about you. Are you going to get a trial of labor? Can you find a hospital that will do a trial of labor? And there are all these downstream implications of the delivery in the first pregnancy.
If you think about it, there are a number of ways that we can manage the first pregnancy. There are a number of risk factors for increasing risk for cesarean delivery. And we can include those in models that are considered in downstream pregnancies. And so we’ve been doing that increasingly.
When we think about our vaginal birth after cesarean models, we had two presentations that considered that. One was how we manage the maternal position in labor, and the other was how we use doulas in labor. You think, well gosh, we’re spending money on this doula in this pregnancy – that’s a certain expenditure. Is it worth it?
Part of “Is it worth it?” is not just the current pregnancy, but the downstream pregnancy as well.
OB.GYN. NEWS: What has sparked your interest in looking at obstetric care delivery in this way?
DR. CAUGHEY: I did my PhD in health economics. I began it as a 3rd-year maternal fetal medicine fellow and during my early career as an assistant professor at the University of California, San Francisco.
A lot of people think that economics in general is kind of about finance. Actually, microeconomics in particular is about the allocation of scarce resources to optimize utility – utility as general wellbeing or happiness.
So people will say that this thing, or that intervention, is cost effective, and often what they think they mean is, “It saves money.” But most things don’t save money. Most things in health care cost money. There are a few things in health care that do save money – vaccinations, contraception. Contraception actually saves money – but most things cost money. We have to spend money to get something right.
And the way we do that in health economics is that we think we’re going to get some happiness, some utility, some better outcomes from the money we’re putting in. One of the things that we don’t do very well as a species is think about these downstream outcomes.
In our models, when we think about morbidity and mortality we’re incorporating two and three pregnancy models. Think about what happens in the future, and then think about if we do something – if we spend money on an induction of labor or having a doula or something like that, is it worth it for what we get?
Antenatal testing, for example. Or, is it worth it for you to be testing people with diabetes once a week? Twice a week? More? Less? Part of it is figuring out what you’ll get for it. The measurement of what you get for it is called quality-adjusted life years, and that’s the measure of happiness multiplied times life expectancy.
We incorporate that in standard ways to build these models, to help us make decisions around best practices. Now, the economics piece of it probably matters. We’re the richest country that’s probably ever going to be – not just ever has been but probably ever going to be – the way we’re using up scarce resources to beat the band.
Yet, we still have an issue of allocation. We have people that have less; we have enormous disparities, whether it be racial and ethnic disparities, or socioeconomic disparities. And so we need to figure out ways to be more efficient and allocate those scarce resources properly to the outcomes that will be the best.
I think that’s what we’re working on: creating models to think about how to allocate those scarce resources.
OB.GYN. NEWS: How can a busy obstetrician think about the work you’re doing and incorporate it into her practice on a day-to-day basis?
DR. CAUGHEY: As an economist, I want to step back from time to time and think about public health and allocation of scarce resources.
But as a busy practicing clinician, I don’t necessarily want you consciously to think about cost. There are people who will push back on this and say, “Oh no, we should always be cost conscious.” Actually, what I really want you to do is incorporate best practices into the care of the patient at that moment, and do the thing that improves her outcomes best at that moment.
What we want to do, instead, is design systems that will properly incentivize. Incentivize doesn’t mean you think, “Oh gosh, if I do this thing I’m going to get an extra dollar.” It means subconsciously that those incentives are there, and those incentives don’t have to just be about dollars. Often in our field, they’re about time – what takes less time and more time to do something. So if we provide little extra roadblocks, then you’re more likely to go the other way and do something else.
For example: the hard stop. We did all this research in the 2000s to show that you probably shouldn’t deliver babies just for fun before 39 weeks’ gestational age. There should be an indication. If we don’t allow any hard stop, if we don’t block the pathway, then patients are pushing us, they’re uncomfortable, they’re like, “Just deliver me.” So we said, “No, no, no, we’re going to block this. In fact, we’re actually going to provide a hard stop reimbursement-wise. And medical directors of hospitals are going to have to preapprove.” And so that provides a blockade, and makes it easier just not to do it.
So I think that’s what we want: At the bedside and in your office, we want clinicians to still just be really good doctors. But then, to get involved and help design systems to incentivize us to do the right things.
Aaron B. Caughey, MD, PhD, is professor and chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health & Science University, Portland, and is a member of the U.S. Preventive Services Task Force. He reported that he had no relevant financial disclosures.
REPORTING FROM ACOG 2018