User login
Patients with HR-positive breast cancer can safely use ART
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Less is more for axillary surgery in early breast cancer
SAN ANTONIO — than do those who have more extensive surgery, according to findings from a large meta-analysis.
Less extensive surgery also reduced patients’ risk for lymphedema, according to research (abstract GS02-05) presented at the San Antonio Breast Cancer Symposium.
These results, which included data from more than 20,000 women, may “reassure” patients and clinicians that more extensive axillary lymph node dissection “does not improve outcomes in many women with early-stage breast cancer,” said Andrea V. Barrio, MD, a breast surgeon at Memorial Sloan Kettering Cancer Center, New York City, who was not involved in the study.
Gurdeep S. Mannu, DPhil, of the University of Oxford, United Kingdom, who presented the findings at SABCS, explained that the optimal surgical management of the axilla remains uncertain in this patient population.
To better understand the long-term risks and benefits of more vs less aggressive axillary surgery in early breast cancer, Dr. Mannu and colleagues performed a meta-analysis of 29 randomized trials conducted over six decades, which included data on 20,285 women. The trials compared more vs less extensive axillary surgery as well as axillary surgery vs axillary radiotherapy.
In trials comparing more vs less extensive axillary surgery, researchers found that 83% of locoregional recurrences occurred in the breast or in multiple sites/unspecified locations, and the remaining 17% occurred in isolated axilla or other local recurrences, such as in the supraclavicular fossa or internal mammary chain.
Those with recurrences in the breast or multiple sites/unspecified locations did not benefit from more extensive surgery, demonstrating similar recurrence rates (RR) (RR for breast, 1.13; 95% CI, 0.92-1.40; RR for other, 0.89; 95% CI, 0.67-1.18).
The group with recurrences in isolated axilla or other local recurrences tended to do better with more extensive surgery (RR, 0.43 and 0.41, respectively).
Overall though, after a median follow-up of 10 years, differences in locoregional recurrence rates at any site did not differ among patients who had more vs less extensive axillary surgery (RR, 0.91; P = .22). This finding held even when restricting the analysis to women with node-positive disease/unknown nodal status (RR, 1.00; P = .98) and for node-negative women (RR, 0.88; P = .15).
Dr. Mannu and colleagues observed similar findings for distant recurrence, breast cancer mortality, and death from any cause.
“But where there was quite a striking difference was in morbidity,” said Dr. Mannu.
To examine rates of lymphedema — the surgical complication that has been “one of the main motivations” for the deescalation trials of the past few decades — the researchers focused on more recent trials, which “are most relevant to women treated today,” Dr. Mannu explained.
These showed that more extensive axillary surgery was associated with almost 2.5-times the rate of lymphedema compared with less extensive treatment (odds ratio [OR], 2.43).
Finally, the team compared axillary dissection with axillary radiotherapy across five trials and found no significant differences in the treatment approaches in terms of locoregional occurrence, distant recurrence, breast cancer mortality, and death from any cause.
However, once again, a notable difference in rates of lymphedema occurred, with axillary dissection associated with higher rates compared with radiotherapy (OR, 1.79).
This is “probably the largest meta-analysis comparing more vs less axillary surgery,” Dr. Barrio said in an interview.
“When we have one or two positive sentinel nodes, anywhere from 30%-50% of women will have additional positive lymph nodes that we’re not removing” with less extensive surgery, she explained. This study shows that, even then, this “doesn’t seem to impact on survival.”
This is “likely related to better medical treatment and radiation techniques that can treat that disease just as well as big surgery, but with less lymphedema,” she added.
Nevertheless, Dr. Barrio believes that there are “situations where we still feel that axillary lymph node dissection is important: in women with advanced cancer, like inflammatory breast cancer, and in women who’ve received chemotherapy upfront, then had surgery, and still have positive nodes after the chemo.”
The study was funded by Cancer Research UK, British Heart Foundation, Medical Research Council.
No relevant financial relationships have been declared.
A version of this article appeared on Medscape.com.
SAN ANTONIO — than do those who have more extensive surgery, according to findings from a large meta-analysis.
Less extensive surgery also reduced patients’ risk for lymphedema, according to research (abstract GS02-05) presented at the San Antonio Breast Cancer Symposium.
These results, which included data from more than 20,000 women, may “reassure” patients and clinicians that more extensive axillary lymph node dissection “does not improve outcomes in many women with early-stage breast cancer,” said Andrea V. Barrio, MD, a breast surgeon at Memorial Sloan Kettering Cancer Center, New York City, who was not involved in the study.
Gurdeep S. Mannu, DPhil, of the University of Oxford, United Kingdom, who presented the findings at SABCS, explained that the optimal surgical management of the axilla remains uncertain in this patient population.
To better understand the long-term risks and benefits of more vs less aggressive axillary surgery in early breast cancer, Dr. Mannu and colleagues performed a meta-analysis of 29 randomized trials conducted over six decades, which included data on 20,285 women. The trials compared more vs less extensive axillary surgery as well as axillary surgery vs axillary radiotherapy.
In trials comparing more vs less extensive axillary surgery, researchers found that 83% of locoregional recurrences occurred in the breast or in multiple sites/unspecified locations, and the remaining 17% occurred in isolated axilla or other local recurrences, such as in the supraclavicular fossa or internal mammary chain.
Those with recurrences in the breast or multiple sites/unspecified locations did not benefit from more extensive surgery, demonstrating similar recurrence rates (RR) (RR for breast, 1.13; 95% CI, 0.92-1.40; RR for other, 0.89; 95% CI, 0.67-1.18).
The group with recurrences in isolated axilla or other local recurrences tended to do better with more extensive surgery (RR, 0.43 and 0.41, respectively).
Overall though, after a median follow-up of 10 years, differences in locoregional recurrence rates at any site did not differ among patients who had more vs less extensive axillary surgery (RR, 0.91; P = .22). This finding held even when restricting the analysis to women with node-positive disease/unknown nodal status (RR, 1.00; P = .98) and for node-negative women (RR, 0.88; P = .15).
Dr. Mannu and colleagues observed similar findings for distant recurrence, breast cancer mortality, and death from any cause.
“But where there was quite a striking difference was in morbidity,” said Dr. Mannu.
To examine rates of lymphedema — the surgical complication that has been “one of the main motivations” for the deescalation trials of the past few decades — the researchers focused on more recent trials, which “are most relevant to women treated today,” Dr. Mannu explained.
These showed that more extensive axillary surgery was associated with almost 2.5-times the rate of lymphedema compared with less extensive treatment (odds ratio [OR], 2.43).
Finally, the team compared axillary dissection with axillary radiotherapy across five trials and found no significant differences in the treatment approaches in terms of locoregional occurrence, distant recurrence, breast cancer mortality, and death from any cause.
However, once again, a notable difference in rates of lymphedema occurred, with axillary dissection associated with higher rates compared with radiotherapy (OR, 1.79).
This is “probably the largest meta-analysis comparing more vs less axillary surgery,” Dr. Barrio said in an interview.
“When we have one or two positive sentinel nodes, anywhere from 30%-50% of women will have additional positive lymph nodes that we’re not removing” with less extensive surgery, she explained. This study shows that, even then, this “doesn’t seem to impact on survival.”
This is “likely related to better medical treatment and radiation techniques that can treat that disease just as well as big surgery, but with less lymphedema,” she added.
Nevertheless, Dr. Barrio believes that there are “situations where we still feel that axillary lymph node dissection is important: in women with advanced cancer, like inflammatory breast cancer, and in women who’ve received chemotherapy upfront, then had surgery, and still have positive nodes after the chemo.”
The study was funded by Cancer Research UK, British Heart Foundation, Medical Research Council.
No relevant financial relationships have been declared.
A version of this article appeared on Medscape.com.
SAN ANTONIO — than do those who have more extensive surgery, according to findings from a large meta-analysis.
Less extensive surgery also reduced patients’ risk for lymphedema, according to research (abstract GS02-05) presented at the San Antonio Breast Cancer Symposium.
These results, which included data from more than 20,000 women, may “reassure” patients and clinicians that more extensive axillary lymph node dissection “does not improve outcomes in many women with early-stage breast cancer,” said Andrea V. Barrio, MD, a breast surgeon at Memorial Sloan Kettering Cancer Center, New York City, who was not involved in the study.
Gurdeep S. Mannu, DPhil, of the University of Oxford, United Kingdom, who presented the findings at SABCS, explained that the optimal surgical management of the axilla remains uncertain in this patient population.
To better understand the long-term risks and benefits of more vs less aggressive axillary surgery in early breast cancer, Dr. Mannu and colleagues performed a meta-analysis of 29 randomized trials conducted over six decades, which included data on 20,285 women. The trials compared more vs less extensive axillary surgery as well as axillary surgery vs axillary radiotherapy.
In trials comparing more vs less extensive axillary surgery, researchers found that 83% of locoregional recurrences occurred in the breast or in multiple sites/unspecified locations, and the remaining 17% occurred in isolated axilla or other local recurrences, such as in the supraclavicular fossa or internal mammary chain.
Those with recurrences in the breast or multiple sites/unspecified locations did not benefit from more extensive surgery, demonstrating similar recurrence rates (RR) (RR for breast, 1.13; 95% CI, 0.92-1.40; RR for other, 0.89; 95% CI, 0.67-1.18).
The group with recurrences in isolated axilla or other local recurrences tended to do better with more extensive surgery (RR, 0.43 and 0.41, respectively).
Overall though, after a median follow-up of 10 years, differences in locoregional recurrence rates at any site did not differ among patients who had more vs less extensive axillary surgery (RR, 0.91; P = .22). This finding held even when restricting the analysis to women with node-positive disease/unknown nodal status (RR, 1.00; P = .98) and for node-negative women (RR, 0.88; P = .15).
Dr. Mannu and colleagues observed similar findings for distant recurrence, breast cancer mortality, and death from any cause.
“But where there was quite a striking difference was in morbidity,” said Dr. Mannu.
To examine rates of lymphedema — the surgical complication that has been “one of the main motivations” for the deescalation trials of the past few decades — the researchers focused on more recent trials, which “are most relevant to women treated today,” Dr. Mannu explained.
These showed that more extensive axillary surgery was associated with almost 2.5-times the rate of lymphedema compared with less extensive treatment (odds ratio [OR], 2.43).
Finally, the team compared axillary dissection with axillary radiotherapy across five trials and found no significant differences in the treatment approaches in terms of locoregional occurrence, distant recurrence, breast cancer mortality, and death from any cause.
However, once again, a notable difference in rates of lymphedema occurred, with axillary dissection associated with higher rates compared with radiotherapy (OR, 1.79).
This is “probably the largest meta-analysis comparing more vs less axillary surgery,” Dr. Barrio said in an interview.
“When we have one or two positive sentinel nodes, anywhere from 30%-50% of women will have additional positive lymph nodes that we’re not removing” with less extensive surgery, she explained. This study shows that, even then, this “doesn’t seem to impact on survival.”
This is “likely related to better medical treatment and radiation techniques that can treat that disease just as well as big surgery, but with less lymphedema,” she added.
Nevertheless, Dr. Barrio believes that there are “situations where we still feel that axillary lymph node dissection is important: in women with advanced cancer, like inflammatory breast cancer, and in women who’ve received chemotherapy upfront, then had surgery, and still have positive nodes after the chemo.”
The study was funded by Cancer Research UK, British Heart Foundation, Medical Research Council.
No relevant financial relationships have been declared.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Supercharge your medical practice with ChatGPT: Here’s why you should upgrade
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
What if a single GLP-1 shot could last for months?
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
Few with inflammatory breast cancer get guideline-based care
SAN ANTONIO — Yet, a retrospective study of patients with inflammatory breast carcinoma shows that the majority of patients don’t receive it.
The study also showed that overall survival was lowest for Black women who didn’t receive guideline-concordant care, said Brian Diskin, MD, with the Division of Breast Surgery, Memorial Sloan Kettering Cancer Center, New York City, here at the San Antonio Breast Cancer Symposium.
The results highlight the importance of adhering to guidelines in inflammatory breast carcinoma and suggest that improving the rates among Black patients “may help to mitigate racial disparities and survival,” Dr.Diskin told the conference.
Inflammatory breast carcinoma is an aggressive form of breast cancer associated with worse survival outcomes compared with other subtypes of breast cancer. Yet, it’s unclear how often and consistently guideline-concordant care — defined as treatment with neoadjuvant chemotherapy followed by modified radical mastectomy without immediate reconstruction, and postmastectomy radiotherapy — is received and what factors play a role in receiving recommended care.
To investigate, Dr. Diskin and colleagues identified 6945 women from the National Cancer Database with nonmetastatic inflammatory breast cancer treated from 2010-2018. Guideline-concordant care was defined as trimodality treatment administered in the correct sequence, with neoadjuvant chemotherapy started within 60 days of diagnosis.
Most patients (88%) did not start neoadjuvant chemotherapy within 60 days of diagnosis.
Black and Asian patients were less likely than were White patients to start chemotherapy within 60 days (odds ratio [OR] 0.54 and 0.51, respectively; P < .001), while patients with Medicare or private insurance were more likely to receive chemotherapy within 60 days of diagnosis than uninsured patients (OR 1.37 and 1.87, respectively; P < .001).
Roughly half of all patients didn’t receive appropriate surgical treatment (modified radical mastectomy without immediate reconstruction and postmastectomy radiotherapy).
Overall, only about one third of the cohort received guideline-concordant treatment, Dr. Diskin reported.
Patients aged 60-69 were more likely than were patients aged 40-49 to receive guideline-concordant treatment (odds ratio [OR], 1.24; P < .001), as were patients with a higher clinical nodal burden (OR, 1.34 for N1; OR, 1.28 for N2; OR, 1.15 for N3 vs N0; P < .001 for N1 and N2).
Patients treated between 2014 and 2018 were less likely to receive guideline-concordant treatment than patients treated between 2010 and 2013 (OR, 0.63; P <.001).
Receiving guideline-concordant care and being privately insured were both positively associated with improved overall survival (OR, 0.75 and 0.62, respectively; P < .001). Conversely, triple-negative subtype and Black race were associated with worse overall survival (HR, 1.6 and 1.4, respectively; P < .001).
However, timely receipt of guideline-concordant care for Black patients with triple-negative disease did lead to improved overall survival. Among recipients of guideline-based care with triple-negative disease, there was no racial disparity in overall survival.
Study discussant Kathryn Hudson, MD, director of survivorship and medical oncologist at Texas Oncology, Austin, said it’s important to note that Black women have a 4% lower incidence of breast cancer than do White women but a 40% higher breast cancer death rate.
“This study is important because it confirms that those who receive guideline-based care have better outcomes and that Black women have worse survival in [inflammatory breast cancer],” Dr. Hudson said.
The finding that Black and Asian women in the study were less likely to have timely neoadjuvant chemotherapy, “likely reflects worse access to care, and this may play a role in why Black women had worse outcomes,” she added.
Dr. Hudson said she found it “surprising” that only about one third of patients received guideline-concordant care.
In her view, “the take-home message is that improving guideline-concordant will improve outcomes for all patients with inflammatory breast cancer. And it’s really important, as a next step, to examine the barriers to guideline-concordant care in inflammatory breast cancer and continue to understand the reasons for worse [rates of] survival of Black women.”
Dr. Diskin has disclosed no relevant financial relationships. Dr. Hudson has received honoraria from the Menarini Group and Gilead.
A version of this article appeared on Medscape.com.
SAN ANTONIO — Yet, a retrospective study of patients with inflammatory breast carcinoma shows that the majority of patients don’t receive it.
The study also showed that overall survival was lowest for Black women who didn’t receive guideline-concordant care, said Brian Diskin, MD, with the Division of Breast Surgery, Memorial Sloan Kettering Cancer Center, New York City, here at the San Antonio Breast Cancer Symposium.
The results highlight the importance of adhering to guidelines in inflammatory breast carcinoma and suggest that improving the rates among Black patients “may help to mitigate racial disparities and survival,” Dr.Diskin told the conference.
Inflammatory breast carcinoma is an aggressive form of breast cancer associated with worse survival outcomes compared with other subtypes of breast cancer. Yet, it’s unclear how often and consistently guideline-concordant care — defined as treatment with neoadjuvant chemotherapy followed by modified radical mastectomy without immediate reconstruction, and postmastectomy radiotherapy — is received and what factors play a role in receiving recommended care.
To investigate, Dr. Diskin and colleagues identified 6945 women from the National Cancer Database with nonmetastatic inflammatory breast cancer treated from 2010-2018. Guideline-concordant care was defined as trimodality treatment administered in the correct sequence, with neoadjuvant chemotherapy started within 60 days of diagnosis.
Most patients (88%) did not start neoadjuvant chemotherapy within 60 days of diagnosis.
Black and Asian patients were less likely than were White patients to start chemotherapy within 60 days (odds ratio [OR] 0.54 and 0.51, respectively; P < .001), while patients with Medicare or private insurance were more likely to receive chemotherapy within 60 days of diagnosis than uninsured patients (OR 1.37 and 1.87, respectively; P < .001).
Roughly half of all patients didn’t receive appropriate surgical treatment (modified radical mastectomy without immediate reconstruction and postmastectomy radiotherapy).
Overall, only about one third of the cohort received guideline-concordant treatment, Dr. Diskin reported.
Patients aged 60-69 were more likely than were patients aged 40-49 to receive guideline-concordant treatment (odds ratio [OR], 1.24; P < .001), as were patients with a higher clinical nodal burden (OR, 1.34 for N1; OR, 1.28 for N2; OR, 1.15 for N3 vs N0; P < .001 for N1 and N2).
Patients treated between 2014 and 2018 were less likely to receive guideline-concordant treatment than patients treated between 2010 and 2013 (OR, 0.63; P <.001).
Receiving guideline-concordant care and being privately insured were both positively associated with improved overall survival (OR, 0.75 and 0.62, respectively; P < .001). Conversely, triple-negative subtype and Black race were associated with worse overall survival (HR, 1.6 and 1.4, respectively; P < .001).
However, timely receipt of guideline-concordant care for Black patients with triple-negative disease did lead to improved overall survival. Among recipients of guideline-based care with triple-negative disease, there was no racial disparity in overall survival.
Study discussant Kathryn Hudson, MD, director of survivorship and medical oncologist at Texas Oncology, Austin, said it’s important to note that Black women have a 4% lower incidence of breast cancer than do White women but a 40% higher breast cancer death rate.
“This study is important because it confirms that those who receive guideline-based care have better outcomes and that Black women have worse survival in [inflammatory breast cancer],” Dr. Hudson said.
The finding that Black and Asian women in the study were less likely to have timely neoadjuvant chemotherapy, “likely reflects worse access to care, and this may play a role in why Black women had worse outcomes,” she added.
Dr. Hudson said she found it “surprising” that only about one third of patients received guideline-concordant care.
In her view, “the take-home message is that improving guideline-concordant will improve outcomes for all patients with inflammatory breast cancer. And it’s really important, as a next step, to examine the barriers to guideline-concordant care in inflammatory breast cancer and continue to understand the reasons for worse [rates of] survival of Black women.”
Dr. Diskin has disclosed no relevant financial relationships. Dr. Hudson has received honoraria from the Menarini Group and Gilead.
A version of this article appeared on Medscape.com.
SAN ANTONIO — Yet, a retrospective study of patients with inflammatory breast carcinoma shows that the majority of patients don’t receive it.
The study also showed that overall survival was lowest for Black women who didn’t receive guideline-concordant care, said Brian Diskin, MD, with the Division of Breast Surgery, Memorial Sloan Kettering Cancer Center, New York City, here at the San Antonio Breast Cancer Symposium.
The results highlight the importance of adhering to guidelines in inflammatory breast carcinoma and suggest that improving the rates among Black patients “may help to mitigate racial disparities and survival,” Dr.Diskin told the conference.
Inflammatory breast carcinoma is an aggressive form of breast cancer associated with worse survival outcomes compared with other subtypes of breast cancer. Yet, it’s unclear how often and consistently guideline-concordant care — defined as treatment with neoadjuvant chemotherapy followed by modified radical mastectomy without immediate reconstruction, and postmastectomy radiotherapy — is received and what factors play a role in receiving recommended care.
To investigate, Dr. Diskin and colleagues identified 6945 women from the National Cancer Database with nonmetastatic inflammatory breast cancer treated from 2010-2018. Guideline-concordant care was defined as trimodality treatment administered in the correct sequence, with neoadjuvant chemotherapy started within 60 days of diagnosis.
Most patients (88%) did not start neoadjuvant chemotherapy within 60 days of diagnosis.
Black and Asian patients were less likely than were White patients to start chemotherapy within 60 days (odds ratio [OR] 0.54 and 0.51, respectively; P < .001), while patients with Medicare or private insurance were more likely to receive chemotherapy within 60 days of diagnosis than uninsured patients (OR 1.37 and 1.87, respectively; P < .001).
Roughly half of all patients didn’t receive appropriate surgical treatment (modified radical mastectomy without immediate reconstruction and postmastectomy radiotherapy).
Overall, only about one third of the cohort received guideline-concordant treatment, Dr. Diskin reported.
Patients aged 60-69 were more likely than were patients aged 40-49 to receive guideline-concordant treatment (odds ratio [OR], 1.24; P < .001), as were patients with a higher clinical nodal burden (OR, 1.34 for N1; OR, 1.28 for N2; OR, 1.15 for N3 vs N0; P < .001 for N1 and N2).
Patients treated between 2014 and 2018 were less likely to receive guideline-concordant treatment than patients treated between 2010 and 2013 (OR, 0.63; P <.001).
Receiving guideline-concordant care and being privately insured were both positively associated with improved overall survival (OR, 0.75 and 0.62, respectively; P < .001). Conversely, triple-negative subtype and Black race were associated with worse overall survival (HR, 1.6 and 1.4, respectively; P < .001).
However, timely receipt of guideline-concordant care for Black patients with triple-negative disease did lead to improved overall survival. Among recipients of guideline-based care with triple-negative disease, there was no racial disparity in overall survival.
Study discussant Kathryn Hudson, MD, director of survivorship and medical oncologist at Texas Oncology, Austin, said it’s important to note that Black women have a 4% lower incidence of breast cancer than do White women but a 40% higher breast cancer death rate.
“This study is important because it confirms that those who receive guideline-based care have better outcomes and that Black women have worse survival in [inflammatory breast cancer],” Dr. Hudson said.
The finding that Black and Asian women in the study were less likely to have timely neoadjuvant chemotherapy, “likely reflects worse access to care, and this may play a role in why Black women had worse outcomes,” she added.
Dr. Hudson said she found it “surprising” that only about one third of patients received guideline-concordant care.
In her view, “the take-home message is that improving guideline-concordant will improve outcomes for all patients with inflammatory breast cancer. And it’s really important, as a next step, to examine the barriers to guideline-concordant care in inflammatory breast cancer and continue to understand the reasons for worse [rates of] survival of Black women.”
Dr. Diskin has disclosed no relevant financial relationships. Dr. Hudson has received honoraria from the Menarini Group and Gilead.
A version of this article appeared on Medscape.com.
AT SABCS 2023
What’s Eating You? Update on the Sticktight Flea (Echidnophaga gallinacea)
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
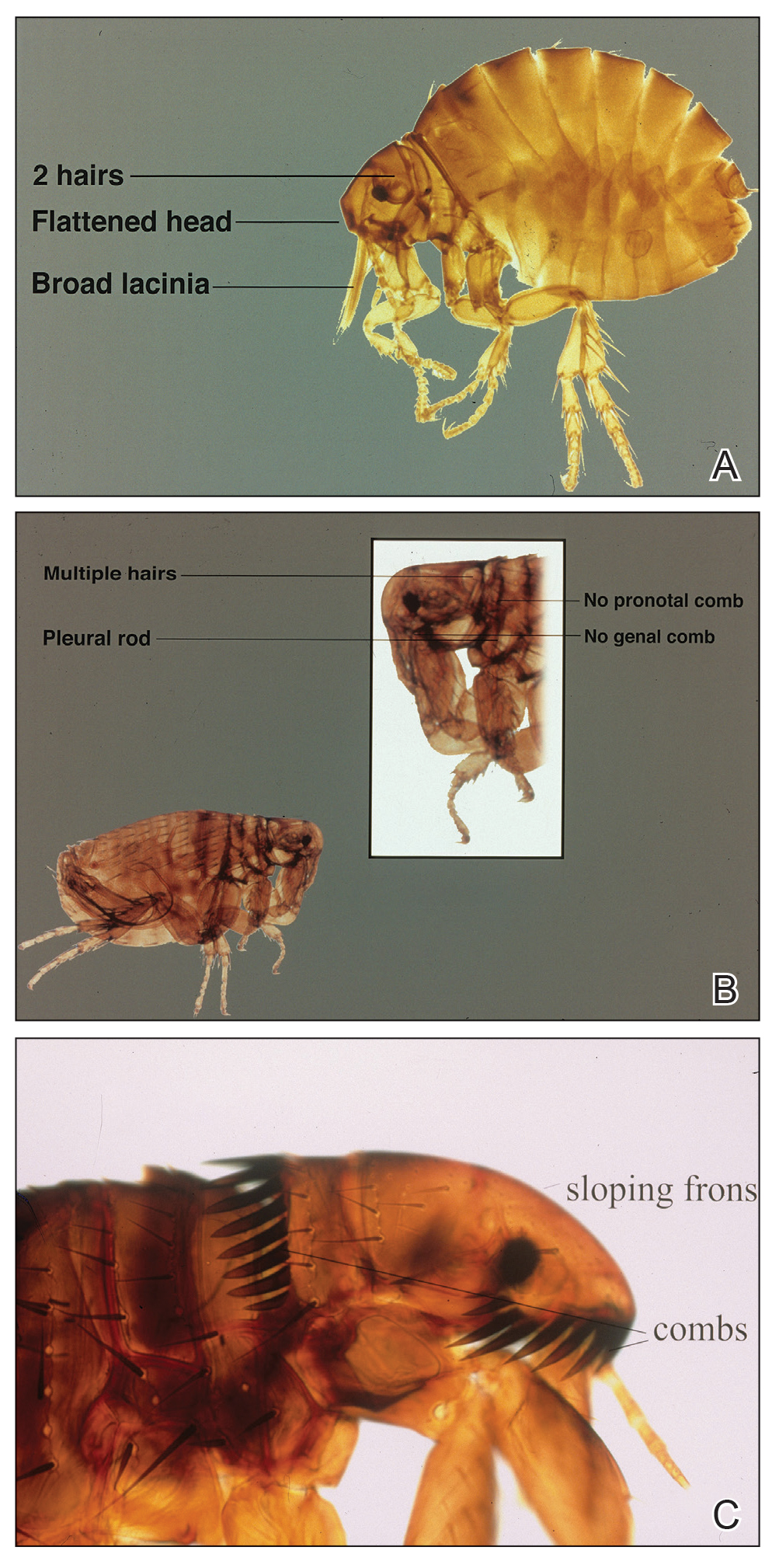
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8
Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Practice Points
- The sticktight flea (Echidnophaga gallinacea) attaches to its host by embedding its head in the skin for days at a time.
- Unlike other fleas that bite and run, the sticktight flea can be identified dermoscopically.
- The sticktight flea serves as a vector for plague as a carrier of Yersinia pestis, rickettsial infections, and other diseases.
Electronic Health Records — Recent Survey Results
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Toward a better framework for postmarketing reproductive safety surveillance of medications
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
How to prescribe Zepbound
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 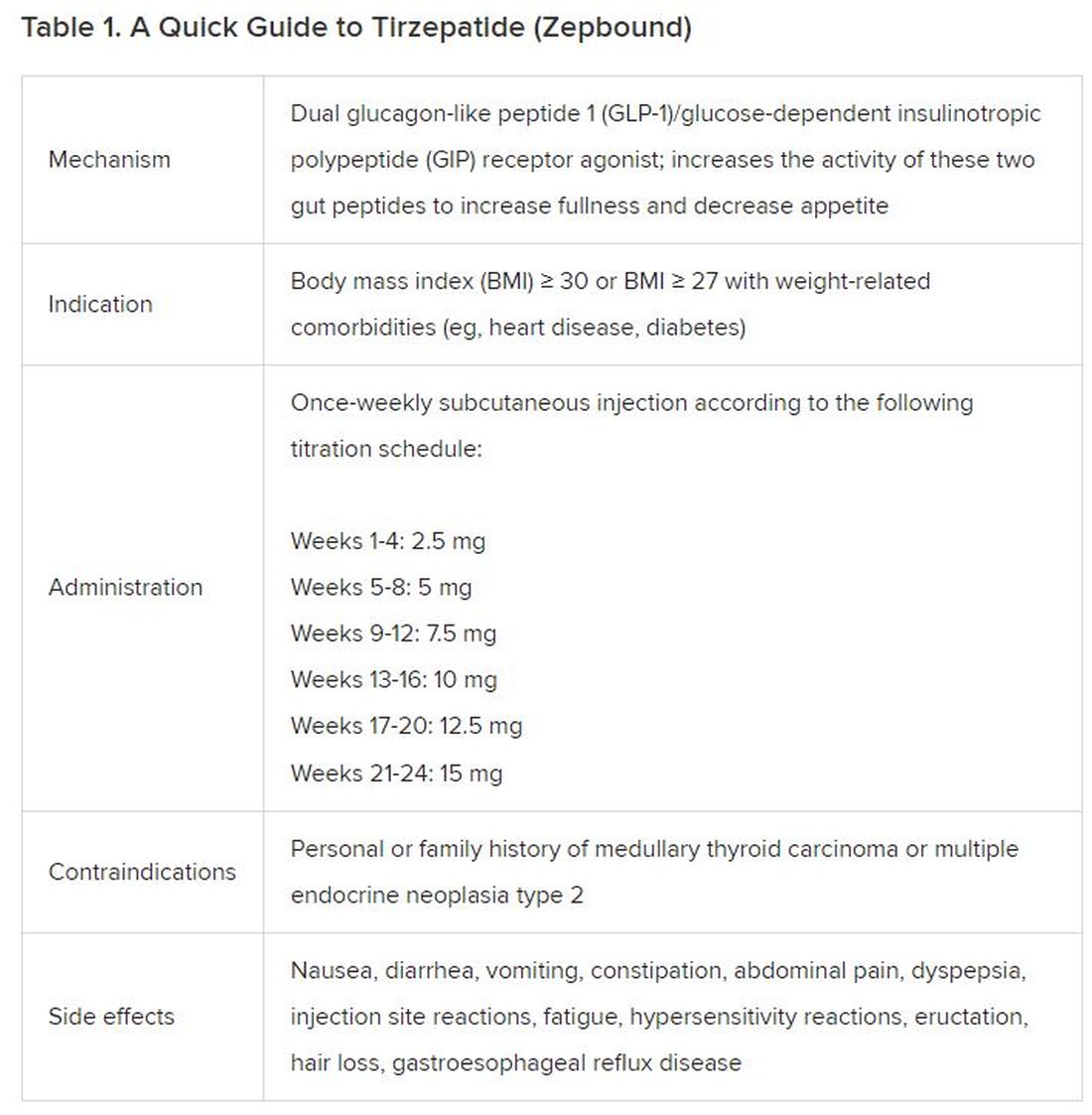
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).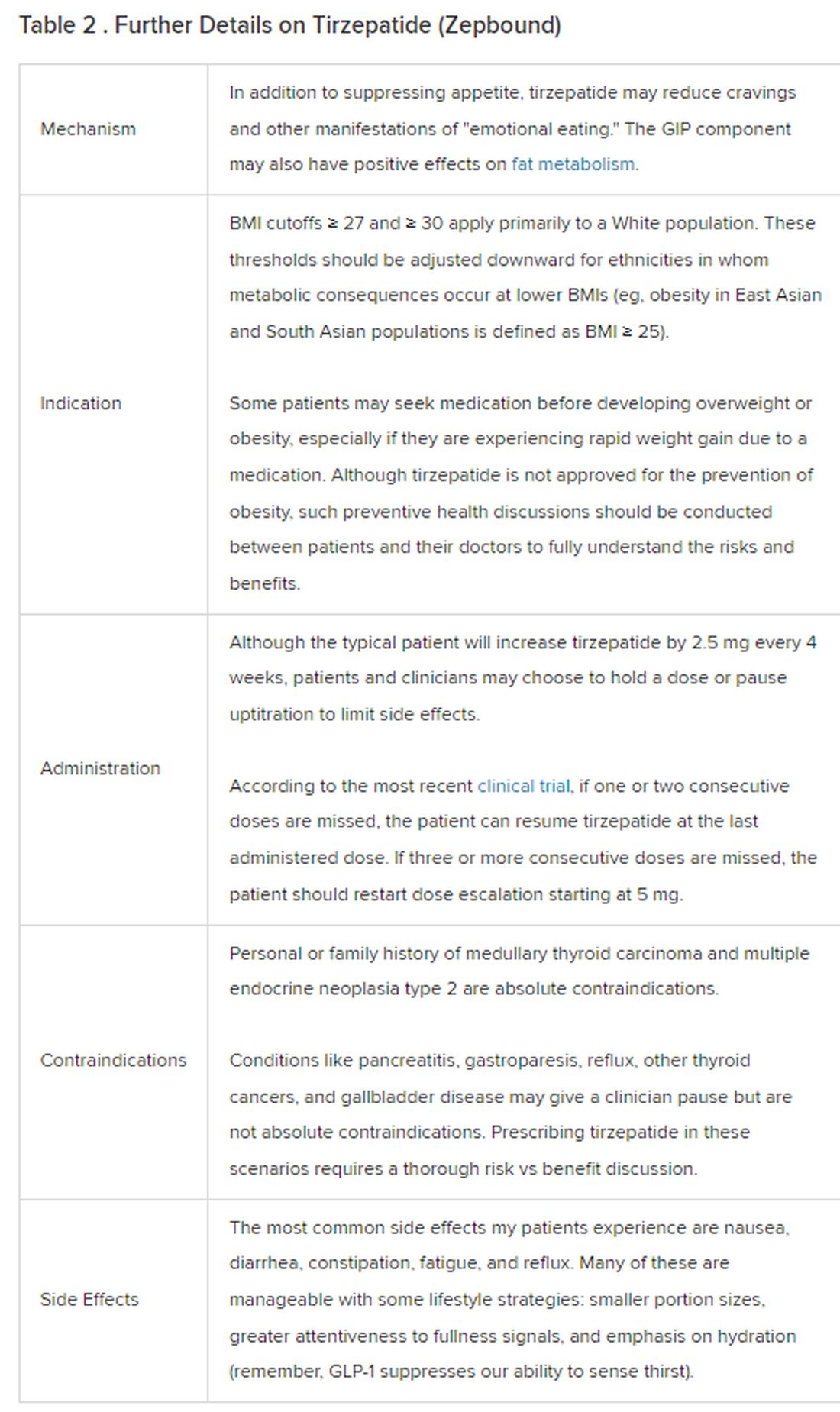
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
Abdominal distention and pain
Given the patient's symptomatology, laboratory studies, and the histopathology and immunophenotyping of the polypoid lesions in the transverse colon, this patient is diagnosed with advanced mantle cell lymphoma (MCL). The gastroenterologist shares the findings with the patient, and over the next several days, a multidisciplinary team forms to guide the patient through potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all non-Hodgkin lymphomas are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. One of the most frequent areas for extra-nodal MCL presentation is the gastrointestinal tract. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, which are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test, also help diagnose MCL. Gastrointestinal involvement of MCL typically presents as lymphoid polyposis on colonoscopy imaging and can appear in the colon, ileum, stomach, and duodenum.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options and associated adverse events, as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, when used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's symptomatology, laboratory studies, and the histopathology and immunophenotyping of the polypoid lesions in the transverse colon, this patient is diagnosed with advanced mantle cell lymphoma (MCL). The gastroenterologist shares the findings with the patient, and over the next several days, a multidisciplinary team forms to guide the patient through potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all non-Hodgkin lymphomas are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. One of the most frequent areas for extra-nodal MCL presentation is the gastrointestinal tract. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, which are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test, also help diagnose MCL. Gastrointestinal involvement of MCL typically presents as lymphoid polyposis on colonoscopy imaging and can appear in the colon, ileum, stomach, and duodenum.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options and associated adverse events, as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, when used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's symptomatology, laboratory studies, and the histopathology and immunophenotyping of the polypoid lesions in the transverse colon, this patient is diagnosed with advanced mantle cell lymphoma (MCL). The gastroenterologist shares the findings with the patient, and over the next several days, a multidisciplinary team forms to guide the patient through potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all non-Hodgkin lymphomas are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. One of the most frequent areas for extra-nodal MCL presentation is the gastrointestinal tract. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, which are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test, also help diagnose MCL. Gastrointestinal involvement of MCL typically presents as lymphoid polyposis on colonoscopy imaging and can appear in the colon, ileum, stomach, and duodenum.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options and associated adverse events, as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, when used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 60-year-old man presents to his primary care physician with weight loss, constipation, and abdominal distention and pain as well as fatigue and night sweats that have lasted for several months. The physician orders a complete blood count with differential and an ultrasound of the abdomen. Lab studies reveal anemia and cytopenias; ultrasound reveals hepatosplenomegaly and abdominal lymphadenopathy. The physician refers the patient to gastroenterology; he undergoes a colonoscopy. Multiple polypoid lesions are found throughout the transverse colon. Immunophenotyping shows CD5 and CD20 expression but a lack of CD23 and CD10 expression; cyclin D1 is overexpressed. Additional blood studies show lymphocytosis > 4000/μL, elevated lactate dehydrogenase levels, abnormal liver function tests, and a negative result on Coombs test.