User login
Alcohol and CV Risk: Both Beneficial and Harmful Effects?
with evidence emerging that alcohol use may both increase and decrease the risk for CVD.
The answer may depend on the presence of circulating metabolites of alcohol, some of which may be beneficial while others may be harmful, new research suggests.
“We adopted an association analysis, looking at 60 metabolites produced during or after alcohol has been metabolized, to see whether those metabolites can link alcohol consumption with CVD,” senior author Jiantao Ma, PhD, MBBS, assistant professor, Division of Nutrition Epidemiology and Data Science, Friedman School, Tufts University, Boston, Massachusetts, said in an interview.
“We found that the relationship is quite complex, with some metabolites showing protective effects against CVD and others showing harmful effects,” said Dr. Ma. “This opens the door for future research because we think that these molecules can help [us] understand the mechanism of the relationship between alcohol and CVD.”
The study was published online in BMC Medicine.
J-Shaped Relationship?
Previous research has painted a confusing picture of the relationship between alcohol consumption and CVD. For example, some studies have suggested that moderate levels of drinking may be hazardous to cardiac health, while others have pointed to potential cardioprotective effects.
Nevertheless, “according to the latest ACC/AHA guidelines regarding alcohol consumption and its relationship to CVD, there is no level of alcohol use that is deemed safe and considered acceptable,” Saurabh Sharma, MD, program director, Internal Medicine Residency Program, and clinical assistant professor of cardiology, Geisinger Commonwealth School of Medicine, Scranton, Pennsylvania, said in an interview.
Older observational data suggested a “J-shaped” relationship between alcohol consumption and cardiovascular risk, such that a low to moderate amount might reduce risk, while higher amounts increase it, said Dr. Sharma, a member of the American College of Cardiology (ACC) Prevention of Cardiovascular Diseases Council.
“But it’s essential to note that these findings were based on observational studies. No randomized controlled trials have provided conclusive evidence supporting the idea that moderate alcohol consumption actively reduces cardiovascular risk,” he said.
The current study is also observational, but it shines a somewhat different spotlight on the subject by examining alcohol consumption–related metabolites, said Dr. Ma — that is, small molecules that are the intermediates or end-products of metabolism in many cellular processes.
Some recent research “shows that alcohol may be harmful or at least has no beneficial effect in CVD prevention,” he said. “Our motivation was to analyze the association using metabolites, genetics, and epigenetics, because we think that these molecules may help us understand some of the mechanisms that underlie the relationship between alcohol consumption and CVD, and partially answer the question of whether alcohol may be harmful or helpful.”
Caution Warranted
Although some previous studies have looked at metabolites, most analyzed alcohol consumption measured at a single time point, “which may not represent habitual or long-term alcohol consumption,” the researchers note.
The team used data derived from 2458 Framingham Heart Study Offspring participants (mean age, 56 ± 9.3 years at the fifth examination; 52% female), calculating the cumulative average alcohol consumption from total intake of beer, wine, and liquor over an average 20-year period. Most participants were overweight, close to one fifth were current smokers, and 636 developed CVD over the study period.
Participants were assessed every 4-8 years, with metabolites measured during the fifth examination.
Linear models were used to investigate the association of alcohol consumption with 211 plasma metabolites, adjusting for a variety of potential confounders, including age, sex, batch, smoking, diet, physical activity, body mass index, and familial relationship.
Sixty metabolites associated with cumulative average alcohol consumption were identified (P < .00024), after adjustment for confounders. Of these, 40 displayed positive associations with the cumulative average alcohol consumption, with the most significant metabolite being cholesteryl palmitoleate (CE16:1), a plasma cholesteryl ester involved with cholesterol metabolism.
One gram per day of higher alcohol consumption was associated with a higher-level CE16:1 in the blood (b = .023). Several other phosphatidylcholine metabolites were also positively associated with alcohol consumption.
On the other hand, 20 metabolites were negatively associated with alcohol consumption, with triacylglycerol 54:4 (TAG 54:4) displaying the most significant association (b = –.017).
The alcoholic beverages were not equal when it came to association with metabolites: 19 metabolites were significantly associated with the cumulative average consumption of beer, 30 with wine, and 32 with liquor. Seven were significantly associated with the cumulative consumption of all three types of drinks.
The researchers conducted survival analysis that identified 10 alcohol-associated metabolites associated with differential CVD risks, after adjusting for confounders. They also built two alcohol consumption–weighted metabolite scores using these 10 metabolites. After adjustment for confounders including CVD risk factors, the two scores had “comparable but opposite” associations with incident CVD, HR 1.11 (95% CI, 1.02-1.21) vs 0.88 (0.78-0.98; both P values = .02).
“We found that seven metabolites were harmful, while three were beneficial, “ Dr. Ma reported.
Dr. Ma cautioned that association “doesn’t represent causation.” On the basis of the findings, however, “we can hypothesize that if you drink a moderate amount of alcohol, you can either increase or decrease your risk of CVD.”
For people with cardiac conditions, “it would be [wise to be] cautious in recommending alcohol consumption,” he said. “For people without cardiac conditions, I would follow the recommendations of the AHA. If people don’t already drink alcohol, we don’t recommend that you start drinking it; and if you already drink, we’d recommend keeping it minimal.”
He cautioned that this is “only one study and we need more research if we are to generate a clearer message to the patient.” At present, perhaps the best message to patients is “to be cautious and warn them that there are potentially harmful effects,” he said.
Mendelian Randomization?
Dr. Sharma, who was not involved in the study, emphasized that it’s “crucial” to recognize that the study “does not alter the established understanding that any level of alcohol consumption poses harm to the heart,” and that “any amount of alcohol consumption has the potential to elevate triglyceride levels, thereby contributing to the increased risk of cardiovascular complications.”
Previously reported cardioprotective benefits “are likely influenced by confounding factors, such as lifestyle and sociodemographic elements,” he speculated.
He noted that observational studies “encounter challenges in disentangling the influence of factors like obesity, lack of exercise, and tobacco use” as well as reverse causality.
“To overcome these limitations, Mendelian randomization emerges as a robust method,” he suggested. “This approach utilizes measured genetic variations with known functions to investigate the causal effect of a modifiable exposure on disease within the framework of observational studies.”
Notably, certain studies using this approach, including one by Larsson and colleagues, and another by Biddinger and associates, “have provided valuable insights by establishing a clear and causal relationship between alcohol consumption and CVD,” he said.
The study was funded by the National Institute of Health’s National Institute on Alcohol Abuse and Alcoholism. Data collection in the Framingham Heart Study was supported by the National Heart, Lung, and Blood Institute. Dr. Ma and coauthors and Dr. Sharma disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
with evidence emerging that alcohol use may both increase and decrease the risk for CVD.
The answer may depend on the presence of circulating metabolites of alcohol, some of which may be beneficial while others may be harmful, new research suggests.
“We adopted an association analysis, looking at 60 metabolites produced during or after alcohol has been metabolized, to see whether those metabolites can link alcohol consumption with CVD,” senior author Jiantao Ma, PhD, MBBS, assistant professor, Division of Nutrition Epidemiology and Data Science, Friedman School, Tufts University, Boston, Massachusetts, said in an interview.
“We found that the relationship is quite complex, with some metabolites showing protective effects against CVD and others showing harmful effects,” said Dr. Ma. “This opens the door for future research because we think that these molecules can help [us] understand the mechanism of the relationship between alcohol and CVD.”
The study was published online in BMC Medicine.
J-Shaped Relationship?
Previous research has painted a confusing picture of the relationship between alcohol consumption and CVD. For example, some studies have suggested that moderate levels of drinking may be hazardous to cardiac health, while others have pointed to potential cardioprotective effects.
Nevertheless, “according to the latest ACC/AHA guidelines regarding alcohol consumption and its relationship to CVD, there is no level of alcohol use that is deemed safe and considered acceptable,” Saurabh Sharma, MD, program director, Internal Medicine Residency Program, and clinical assistant professor of cardiology, Geisinger Commonwealth School of Medicine, Scranton, Pennsylvania, said in an interview.
Older observational data suggested a “J-shaped” relationship between alcohol consumption and cardiovascular risk, such that a low to moderate amount might reduce risk, while higher amounts increase it, said Dr. Sharma, a member of the American College of Cardiology (ACC) Prevention of Cardiovascular Diseases Council.
“But it’s essential to note that these findings were based on observational studies. No randomized controlled trials have provided conclusive evidence supporting the idea that moderate alcohol consumption actively reduces cardiovascular risk,” he said.
The current study is also observational, but it shines a somewhat different spotlight on the subject by examining alcohol consumption–related metabolites, said Dr. Ma — that is, small molecules that are the intermediates or end-products of metabolism in many cellular processes.
Some recent research “shows that alcohol may be harmful or at least has no beneficial effect in CVD prevention,” he said. “Our motivation was to analyze the association using metabolites, genetics, and epigenetics, because we think that these molecules may help us understand some of the mechanisms that underlie the relationship between alcohol consumption and CVD, and partially answer the question of whether alcohol may be harmful or helpful.”
Caution Warranted
Although some previous studies have looked at metabolites, most analyzed alcohol consumption measured at a single time point, “which may not represent habitual or long-term alcohol consumption,” the researchers note.
The team used data derived from 2458 Framingham Heart Study Offspring participants (mean age, 56 ± 9.3 years at the fifth examination; 52% female), calculating the cumulative average alcohol consumption from total intake of beer, wine, and liquor over an average 20-year period. Most participants were overweight, close to one fifth were current smokers, and 636 developed CVD over the study period.
Participants were assessed every 4-8 years, with metabolites measured during the fifth examination.
Linear models were used to investigate the association of alcohol consumption with 211 plasma metabolites, adjusting for a variety of potential confounders, including age, sex, batch, smoking, diet, physical activity, body mass index, and familial relationship.
Sixty metabolites associated with cumulative average alcohol consumption were identified (P < .00024), after adjustment for confounders. Of these, 40 displayed positive associations with the cumulative average alcohol consumption, with the most significant metabolite being cholesteryl palmitoleate (CE16:1), a plasma cholesteryl ester involved with cholesterol metabolism.
One gram per day of higher alcohol consumption was associated with a higher-level CE16:1 in the blood (b = .023). Several other phosphatidylcholine metabolites were also positively associated with alcohol consumption.
On the other hand, 20 metabolites were negatively associated with alcohol consumption, with triacylglycerol 54:4 (TAG 54:4) displaying the most significant association (b = –.017).
The alcoholic beverages were not equal when it came to association with metabolites: 19 metabolites were significantly associated with the cumulative average consumption of beer, 30 with wine, and 32 with liquor. Seven were significantly associated with the cumulative consumption of all three types of drinks.
The researchers conducted survival analysis that identified 10 alcohol-associated metabolites associated with differential CVD risks, after adjusting for confounders. They also built two alcohol consumption–weighted metabolite scores using these 10 metabolites. After adjustment for confounders including CVD risk factors, the two scores had “comparable but opposite” associations with incident CVD, HR 1.11 (95% CI, 1.02-1.21) vs 0.88 (0.78-0.98; both P values = .02).
“We found that seven metabolites were harmful, while three were beneficial, “ Dr. Ma reported.
Dr. Ma cautioned that association “doesn’t represent causation.” On the basis of the findings, however, “we can hypothesize that if you drink a moderate amount of alcohol, you can either increase or decrease your risk of CVD.”
For people with cardiac conditions, “it would be [wise to be] cautious in recommending alcohol consumption,” he said. “For people without cardiac conditions, I would follow the recommendations of the AHA. If people don’t already drink alcohol, we don’t recommend that you start drinking it; and if you already drink, we’d recommend keeping it minimal.”
He cautioned that this is “only one study and we need more research if we are to generate a clearer message to the patient.” At present, perhaps the best message to patients is “to be cautious and warn them that there are potentially harmful effects,” he said.
Mendelian Randomization?
Dr. Sharma, who was not involved in the study, emphasized that it’s “crucial” to recognize that the study “does not alter the established understanding that any level of alcohol consumption poses harm to the heart,” and that “any amount of alcohol consumption has the potential to elevate triglyceride levels, thereby contributing to the increased risk of cardiovascular complications.”
Previously reported cardioprotective benefits “are likely influenced by confounding factors, such as lifestyle and sociodemographic elements,” he speculated.
He noted that observational studies “encounter challenges in disentangling the influence of factors like obesity, lack of exercise, and tobacco use” as well as reverse causality.
“To overcome these limitations, Mendelian randomization emerges as a robust method,” he suggested. “This approach utilizes measured genetic variations with known functions to investigate the causal effect of a modifiable exposure on disease within the framework of observational studies.”
Notably, certain studies using this approach, including one by Larsson and colleagues, and another by Biddinger and associates, “have provided valuable insights by establishing a clear and causal relationship between alcohol consumption and CVD,” he said.
The study was funded by the National Institute of Health’s National Institute on Alcohol Abuse and Alcoholism. Data collection in the Framingham Heart Study was supported by the National Heart, Lung, and Blood Institute. Dr. Ma and coauthors and Dr. Sharma disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
with evidence emerging that alcohol use may both increase and decrease the risk for CVD.
The answer may depend on the presence of circulating metabolites of alcohol, some of which may be beneficial while others may be harmful, new research suggests.
“We adopted an association analysis, looking at 60 metabolites produced during or after alcohol has been metabolized, to see whether those metabolites can link alcohol consumption with CVD,” senior author Jiantao Ma, PhD, MBBS, assistant professor, Division of Nutrition Epidemiology and Data Science, Friedman School, Tufts University, Boston, Massachusetts, said in an interview.
“We found that the relationship is quite complex, with some metabolites showing protective effects against CVD and others showing harmful effects,” said Dr. Ma. “This opens the door for future research because we think that these molecules can help [us] understand the mechanism of the relationship between alcohol and CVD.”
The study was published online in BMC Medicine.
J-Shaped Relationship?
Previous research has painted a confusing picture of the relationship between alcohol consumption and CVD. For example, some studies have suggested that moderate levels of drinking may be hazardous to cardiac health, while others have pointed to potential cardioprotective effects.
Nevertheless, “according to the latest ACC/AHA guidelines regarding alcohol consumption and its relationship to CVD, there is no level of alcohol use that is deemed safe and considered acceptable,” Saurabh Sharma, MD, program director, Internal Medicine Residency Program, and clinical assistant professor of cardiology, Geisinger Commonwealth School of Medicine, Scranton, Pennsylvania, said in an interview.
Older observational data suggested a “J-shaped” relationship between alcohol consumption and cardiovascular risk, such that a low to moderate amount might reduce risk, while higher amounts increase it, said Dr. Sharma, a member of the American College of Cardiology (ACC) Prevention of Cardiovascular Diseases Council.
“But it’s essential to note that these findings were based on observational studies. No randomized controlled trials have provided conclusive evidence supporting the idea that moderate alcohol consumption actively reduces cardiovascular risk,” he said.
The current study is also observational, but it shines a somewhat different spotlight on the subject by examining alcohol consumption–related metabolites, said Dr. Ma — that is, small molecules that are the intermediates or end-products of metabolism in many cellular processes.
Some recent research “shows that alcohol may be harmful or at least has no beneficial effect in CVD prevention,” he said. “Our motivation was to analyze the association using metabolites, genetics, and epigenetics, because we think that these molecules may help us understand some of the mechanisms that underlie the relationship between alcohol consumption and CVD, and partially answer the question of whether alcohol may be harmful or helpful.”
Caution Warranted
Although some previous studies have looked at metabolites, most analyzed alcohol consumption measured at a single time point, “which may not represent habitual or long-term alcohol consumption,” the researchers note.
The team used data derived from 2458 Framingham Heart Study Offspring participants (mean age, 56 ± 9.3 years at the fifth examination; 52% female), calculating the cumulative average alcohol consumption from total intake of beer, wine, and liquor over an average 20-year period. Most participants were overweight, close to one fifth were current smokers, and 636 developed CVD over the study period.
Participants were assessed every 4-8 years, with metabolites measured during the fifth examination.
Linear models were used to investigate the association of alcohol consumption with 211 plasma metabolites, adjusting for a variety of potential confounders, including age, sex, batch, smoking, diet, physical activity, body mass index, and familial relationship.
Sixty metabolites associated with cumulative average alcohol consumption were identified (P < .00024), after adjustment for confounders. Of these, 40 displayed positive associations with the cumulative average alcohol consumption, with the most significant metabolite being cholesteryl palmitoleate (CE16:1), a plasma cholesteryl ester involved with cholesterol metabolism.
One gram per day of higher alcohol consumption was associated with a higher-level CE16:1 in the blood (b = .023). Several other phosphatidylcholine metabolites were also positively associated with alcohol consumption.
On the other hand, 20 metabolites were negatively associated with alcohol consumption, with triacylglycerol 54:4 (TAG 54:4) displaying the most significant association (b = –.017).
The alcoholic beverages were not equal when it came to association with metabolites: 19 metabolites were significantly associated with the cumulative average consumption of beer, 30 with wine, and 32 with liquor. Seven were significantly associated with the cumulative consumption of all three types of drinks.
The researchers conducted survival analysis that identified 10 alcohol-associated metabolites associated with differential CVD risks, after adjusting for confounders. They also built two alcohol consumption–weighted metabolite scores using these 10 metabolites. After adjustment for confounders including CVD risk factors, the two scores had “comparable but opposite” associations with incident CVD, HR 1.11 (95% CI, 1.02-1.21) vs 0.88 (0.78-0.98; both P values = .02).
“We found that seven metabolites were harmful, while three were beneficial, “ Dr. Ma reported.
Dr. Ma cautioned that association “doesn’t represent causation.” On the basis of the findings, however, “we can hypothesize that if you drink a moderate amount of alcohol, you can either increase or decrease your risk of CVD.”
For people with cardiac conditions, “it would be [wise to be] cautious in recommending alcohol consumption,” he said. “For people without cardiac conditions, I would follow the recommendations of the AHA. If people don’t already drink alcohol, we don’t recommend that you start drinking it; and if you already drink, we’d recommend keeping it minimal.”
He cautioned that this is “only one study and we need more research if we are to generate a clearer message to the patient.” At present, perhaps the best message to patients is “to be cautious and warn them that there are potentially harmful effects,” he said.
Mendelian Randomization?
Dr. Sharma, who was not involved in the study, emphasized that it’s “crucial” to recognize that the study “does not alter the established understanding that any level of alcohol consumption poses harm to the heart,” and that “any amount of alcohol consumption has the potential to elevate triglyceride levels, thereby contributing to the increased risk of cardiovascular complications.”
Previously reported cardioprotective benefits “are likely influenced by confounding factors, such as lifestyle and sociodemographic elements,” he speculated.
He noted that observational studies “encounter challenges in disentangling the influence of factors like obesity, lack of exercise, and tobacco use” as well as reverse causality.
“To overcome these limitations, Mendelian randomization emerges as a robust method,” he suggested. “This approach utilizes measured genetic variations with known functions to investigate the causal effect of a modifiable exposure on disease within the framework of observational studies.”
Notably, certain studies using this approach, including one by Larsson and colleagues, and another by Biddinger and associates, “have provided valuable insights by establishing a clear and causal relationship between alcohol consumption and CVD,” he said.
The study was funded by the National Institute of Health’s National Institute on Alcohol Abuse and Alcoholism. Data collection in the Framingham Heart Study was supported by the National Heart, Lung, and Blood Institute. Dr. Ma and coauthors and Dr. Sharma disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM BMC MEDICINE
Updates on Investigational Treatments for HR-Positive, HER2-Negative Breast Cancer
Results from TROPION-Breast01, EMBER, and OPERA were recently presented at ESMO Breast Cancer 2023.
A number of exciting updates on systemic therapies for the treatment of hormone receptor (HR)-positive, HER2-negative breast cancer were presented at the European Society for Medical Oncology (ESMO) Breast Cancer 2023, including novel endocrine agents and antibody-drug conjugates (ADC). We have highlighted 3 key studies, including the phase III study of datopotamab deruxtecan (Dato-DXd), the new trophoblast cell surface antigen 2 (TROP2)-directed ADC; the phase I study of imlunestrant, a selective estrogen receptor degrader (SERD); and phase I/II data evaluating OP-1250, a small molecule oral complete estrogen receptor antagonist (CERAN) and SERD.
TROPION-Breast01: Dato-DXd Improves Progression-Free Survival Compared With Systemic Chemotherapy
Study synopsis
Dato-DXd, an investigational TROP2 ADC, resulted in significantly improved progression-free survival (PFS) when compared with investigator’s choice chemotherapy (ICC) in individuals with inoperable or metastatic HR-positive, HER2-low or HER2-negative breast cancer, according to a randomized phase III trial.
Participants in the study had progressed on or were not eligible for endocrine therapy and had received 1 or 2 prior lines of systemic chemotherapy. Patients were randomized to receive either 6 mg/kg of Dato-DXd once every 3 weeks (n=365; median age 56), or ICC with eribulin, vinorelbine, capecitabine, or gemcitabine (n=367; median age 54) until progression or unacceptable toxicity. Blinded independent review assessed PFS and overall survival. Among the results:
In the blinded independent review, PFS was 6.9 months for Dato-DXd and 4.9 months for ICC (HR 0.63 [95% CI: 0.52, 0.76]; p<0.0001)
At 6 months, 53% of participants receiving Dato-DXd achieved PFS, compared with 39% in the systemic chemotherapy contingent
In the Dato-DXd group, treatment-related adverse events led to dose reductions in 23% and discontinuation in 3% of patients
In the systemic chemotherapy cohort, the dose reduction and discontinuation rates were 32% and 3%, respectively
At the time data were reported at ESMO, overall survival data were not mature but trending favorably for Dato-DXd
The investigators concluded that Dato-DXd is a promising novel treatment option for individuals with inoperable or metastatic HR-positive, HER2-low or HER2-negative breast cancer who have received prior chemotherapy.
EMBER: Imlunestrant Alone or With a Kinase Inhibitor: Early Safety and Efficacy Results Are Encouraging
Study synopsis
The SERD imlunestrant—used either alone or combined with a kinase inhibitor—showed favorable efficacy in individuals with estrogen receptor (ER)-positive, HER2-negative advanced breast cancer, according to the first set of clinical data reported from the phase 1a/b EMBER study.
Key eligibility criteria for phase 1b enrollment included prior sensitivity to endocrine therapy, ≤2 prior therapies, and a PIK3CA mutation (alpelisib arm only). Prior therapies included endocrine therapy (100%), CDK4/6 inhibitors (100%), hormonal therapy with fulvestrant (35%), and chemotherapy (17%). At baseline, 46% of patients had visceral disease and 46% had an ESR1 mutation. Participants received imlunestrant alone (n=114) or with the kinase inhibitors everolimus (n=42) or alpelisib (n=21). Investigators assessed each regimen’s safety profile, as well as the objective response rate and clinical benefit rate.
The safety profile of each regimen was similar to those seen with everolimus and alpelisib alone. No cardiac or ocular toxicities were observed. Regarding grade ≥3 treatment-related adverse events:
The imlunestrant alone group experienced fatigue (2%) and neutropenia (2%)
The imlunestrant + everolimus group experienced hypertriglyceridemia (5%) and aspartate aminotransferase increase (5%)
The imlunestrant + alpelisib cohort experienced rash (43%) and hyperglycemia (10%).
In the imlunestrant alone group, 2% of individuals had their doses reduced due to adverse events; none discontinued treatment
In the imlunestrant + everolimus cohort, 12% of patients experienced dose reduction due to everolimus and 2% due to both medications; 2% discontinued treatment due to everolimus
In the imlunestrant + alpelisib cohort, 24% of patients experienced dose reduction due to alpelisib and 14% due to both medications; 29% discontinued treatment due to alpelisib
Regarding efficacy:
The objective response rates in the imlunestrant alone, imlunestrant + everolimus, and imlunestrant + alpelisib groups were 9%, 21%, and 50%, respectively
The clinical benefit rates in the imlunestrant alone, imlunestrant + everolimus, and imlunestrant + alpelisib groups were 42%, 62%, and 62%, respectively
Investigators concluded that imlunestrant used alone or in combination with 1 of the 2 kinase inhibitors demonstrated robust efficacy in individuals with pretreated, ER-positive, HER2-negative advanced breast cancer.
OPERA: OP-1250 Paired With a CDK4/6 Inhibitor: Anti-Tumor Activity With No Dose-Limiting Toxicities
Study synopsis
OP-1250, a CERAN and SERD, continues to show promising results when paired with a CDK4/6 inhibitor. The combination of OP-1250 and the CDK4/6 inhibitor palbociclib appears to be well tolerated and has a similar safety profile to each drug when used alone, according to a phase I/II study involving 20 individuals with pretreated ER-positive, HER2-negative breast cancer.
Participants had advanced or metastatic ER-positive, HER2-negative breast cancer that progressed on ≤1 lines of endocrine therapy. Fourteen participants had received prior CDK4/6 inhibitor therapy, including 11 who were previously treated with palbociclib. Patients received escalating doses of OP-1250 with 125 mg of palbociclib orally daily for 21 of 28 days. OP-1250 doses were 30 mg (n=3), 60 mg (n=3), 90 mg (n=3), and 120 mg (n=11). Investigators assessed pharmacokinetics, drug-drug interactions, safety, and efficacy. Among the results observed to date:
Grade 3 neutropenia occurred in 55% of participants
There were no grade 4 treatment-related adverse events and no dose-limiting toxicities
OP-1250 exposure yielded similar results to what was seen in the previous monotherapy study
Palbociclib exposure was comparable to published monotherapy data when combined with OP-1250 for all dosages
Investigators observed antitumor activity, including partial responses
Researchers concluded that OP-1250 does not affect the pharmacokinetics of palbociclib, and there do not appear to be drug-drug interactions. Tumor response to this combination was encouraging and requires continued investigation.
Conclusions
These 3 studies presented at ESMO 2023 highlight exciting novel therapies for the treatment of HR-positive, HER2-low, and HER2-negative metastatic breast cancer. The EMBER and OPERA updates provide support for the safety and efficacy of these novel endocrine agents in combination with kinase inhibitors and CDK4/6 inhibitors, respectively, in patients with endocrine-sensitive disease, while the TROPION-01 study demonstrates the encouraging efficacy and safety of a second TROP-2-directed ADC in a more heavily pretreated population.
Results from TROPION-Breast01, EMBER, and OPERA were recently presented at ESMO Breast Cancer 2023.
A number of exciting updates on systemic therapies for the treatment of hormone receptor (HR)-positive, HER2-negative breast cancer were presented at the European Society for Medical Oncology (ESMO) Breast Cancer 2023, including novel endocrine agents and antibody-drug conjugates (ADC). We have highlighted 3 key studies, including the phase III study of datopotamab deruxtecan (Dato-DXd), the new trophoblast cell surface antigen 2 (TROP2)-directed ADC; the phase I study of imlunestrant, a selective estrogen receptor degrader (SERD); and phase I/II data evaluating OP-1250, a small molecule oral complete estrogen receptor antagonist (CERAN) and SERD.
TROPION-Breast01: Dato-DXd Improves Progression-Free Survival Compared With Systemic Chemotherapy
Study synopsis
Dato-DXd, an investigational TROP2 ADC, resulted in significantly improved progression-free survival (PFS) when compared with investigator’s choice chemotherapy (ICC) in individuals with inoperable or metastatic HR-positive, HER2-low or HER2-negative breast cancer, according to a randomized phase III trial.
Participants in the study had progressed on or were not eligible for endocrine therapy and had received 1 or 2 prior lines of systemic chemotherapy. Patients were randomized to receive either 6 mg/kg of Dato-DXd once every 3 weeks (n=365; median age 56), or ICC with eribulin, vinorelbine, capecitabine, or gemcitabine (n=367; median age 54) until progression or unacceptable toxicity. Blinded independent review assessed PFS and overall survival. Among the results:
In the blinded independent review, PFS was 6.9 months for Dato-DXd and 4.9 months for ICC (HR 0.63 [95% CI: 0.52, 0.76]; p<0.0001)
At 6 months, 53% of participants receiving Dato-DXd achieved PFS, compared with 39% in the systemic chemotherapy contingent
In the Dato-DXd group, treatment-related adverse events led to dose reductions in 23% and discontinuation in 3% of patients
In the systemic chemotherapy cohort, the dose reduction and discontinuation rates were 32% and 3%, respectively
At the time data were reported at ESMO, overall survival data were not mature but trending favorably for Dato-DXd
The investigators concluded that Dato-DXd is a promising novel treatment option for individuals with inoperable or metastatic HR-positive, HER2-low or HER2-negative breast cancer who have received prior chemotherapy.
EMBER: Imlunestrant Alone or With a Kinase Inhibitor: Early Safety and Efficacy Results Are Encouraging
Study synopsis
The SERD imlunestrant—used either alone or combined with a kinase inhibitor—showed favorable efficacy in individuals with estrogen receptor (ER)-positive, HER2-negative advanced breast cancer, according to the first set of clinical data reported from the phase 1a/b EMBER study.
Key eligibility criteria for phase 1b enrollment included prior sensitivity to endocrine therapy, ≤2 prior therapies, and a PIK3CA mutation (alpelisib arm only). Prior therapies included endocrine therapy (100%), CDK4/6 inhibitors (100%), hormonal therapy with fulvestrant (35%), and chemotherapy (17%). At baseline, 46% of patients had visceral disease and 46% had an ESR1 mutation. Participants received imlunestrant alone (n=114) or with the kinase inhibitors everolimus (n=42) or alpelisib (n=21). Investigators assessed each regimen’s safety profile, as well as the objective response rate and clinical benefit rate.
The safety profile of each regimen was similar to those seen with everolimus and alpelisib alone. No cardiac or ocular toxicities were observed. Regarding grade ≥3 treatment-related adverse events:
The imlunestrant alone group experienced fatigue (2%) and neutropenia (2%)
The imlunestrant + everolimus group experienced hypertriglyceridemia (5%) and aspartate aminotransferase increase (5%)
The imlunestrant + alpelisib cohort experienced rash (43%) and hyperglycemia (10%).
In the imlunestrant alone group, 2% of individuals had their doses reduced due to adverse events; none discontinued treatment
In the imlunestrant + everolimus cohort, 12% of patients experienced dose reduction due to everolimus and 2% due to both medications; 2% discontinued treatment due to everolimus
In the imlunestrant + alpelisib cohort, 24% of patients experienced dose reduction due to alpelisib and 14% due to both medications; 29% discontinued treatment due to alpelisib
Regarding efficacy:
The objective response rates in the imlunestrant alone, imlunestrant + everolimus, and imlunestrant + alpelisib groups were 9%, 21%, and 50%, respectively
The clinical benefit rates in the imlunestrant alone, imlunestrant + everolimus, and imlunestrant + alpelisib groups were 42%, 62%, and 62%, respectively
Investigators concluded that imlunestrant used alone or in combination with 1 of the 2 kinase inhibitors demonstrated robust efficacy in individuals with pretreated, ER-positive, HER2-negative advanced breast cancer.
OPERA: OP-1250 Paired With a CDK4/6 Inhibitor: Anti-Tumor Activity With No Dose-Limiting Toxicities
Study synopsis
OP-1250, a CERAN and SERD, continues to show promising results when paired with a CDK4/6 inhibitor. The combination of OP-1250 and the CDK4/6 inhibitor palbociclib appears to be well tolerated and has a similar safety profile to each drug when used alone, according to a phase I/II study involving 20 individuals with pretreated ER-positive, HER2-negative breast cancer.
Participants had advanced or metastatic ER-positive, HER2-negative breast cancer that progressed on ≤1 lines of endocrine therapy. Fourteen participants had received prior CDK4/6 inhibitor therapy, including 11 who were previously treated with palbociclib. Patients received escalating doses of OP-1250 with 125 mg of palbociclib orally daily for 21 of 28 days. OP-1250 doses were 30 mg (n=3), 60 mg (n=3), 90 mg (n=3), and 120 mg (n=11). Investigators assessed pharmacokinetics, drug-drug interactions, safety, and efficacy. Among the results observed to date:
Grade 3 neutropenia occurred in 55% of participants
There were no grade 4 treatment-related adverse events and no dose-limiting toxicities
OP-1250 exposure yielded similar results to what was seen in the previous monotherapy study
Palbociclib exposure was comparable to published monotherapy data when combined with OP-1250 for all dosages
Investigators observed antitumor activity, including partial responses
Researchers concluded that OP-1250 does not affect the pharmacokinetics of palbociclib, and there do not appear to be drug-drug interactions. Tumor response to this combination was encouraging and requires continued investigation.
Conclusions
These 3 studies presented at ESMO 2023 highlight exciting novel therapies for the treatment of HR-positive, HER2-low, and HER2-negative metastatic breast cancer. The EMBER and OPERA updates provide support for the safety and efficacy of these novel endocrine agents in combination with kinase inhibitors and CDK4/6 inhibitors, respectively, in patients with endocrine-sensitive disease, while the TROPION-01 study demonstrates the encouraging efficacy and safety of a second TROP-2-directed ADC in a more heavily pretreated population.
Results from TROPION-Breast01, EMBER, and OPERA were recently presented at ESMO Breast Cancer 2023.
A number of exciting updates on systemic therapies for the treatment of hormone receptor (HR)-positive, HER2-negative breast cancer were presented at the European Society for Medical Oncology (ESMO) Breast Cancer 2023, including novel endocrine agents and antibody-drug conjugates (ADC). We have highlighted 3 key studies, including the phase III study of datopotamab deruxtecan (Dato-DXd), the new trophoblast cell surface antigen 2 (TROP2)-directed ADC; the phase I study of imlunestrant, a selective estrogen receptor degrader (SERD); and phase I/II data evaluating OP-1250, a small molecule oral complete estrogen receptor antagonist (CERAN) and SERD.
TROPION-Breast01: Dato-DXd Improves Progression-Free Survival Compared With Systemic Chemotherapy
Study synopsis
Dato-DXd, an investigational TROP2 ADC, resulted in significantly improved progression-free survival (PFS) when compared with investigator’s choice chemotherapy (ICC) in individuals with inoperable or metastatic HR-positive, HER2-low or HER2-negative breast cancer, according to a randomized phase III trial.
Participants in the study had progressed on or were not eligible for endocrine therapy and had received 1 or 2 prior lines of systemic chemotherapy. Patients were randomized to receive either 6 mg/kg of Dato-DXd once every 3 weeks (n=365; median age 56), or ICC with eribulin, vinorelbine, capecitabine, or gemcitabine (n=367; median age 54) until progression or unacceptable toxicity. Blinded independent review assessed PFS and overall survival. Among the results:
In the blinded independent review, PFS was 6.9 months for Dato-DXd and 4.9 months for ICC (HR 0.63 [95% CI: 0.52, 0.76]; p<0.0001)
At 6 months, 53% of participants receiving Dato-DXd achieved PFS, compared with 39% in the systemic chemotherapy contingent
In the Dato-DXd group, treatment-related adverse events led to dose reductions in 23% and discontinuation in 3% of patients
In the systemic chemotherapy cohort, the dose reduction and discontinuation rates were 32% and 3%, respectively
At the time data were reported at ESMO, overall survival data were not mature but trending favorably for Dato-DXd
The investigators concluded that Dato-DXd is a promising novel treatment option for individuals with inoperable or metastatic HR-positive, HER2-low or HER2-negative breast cancer who have received prior chemotherapy.
EMBER: Imlunestrant Alone or With a Kinase Inhibitor: Early Safety and Efficacy Results Are Encouraging
Study synopsis
The SERD imlunestrant—used either alone or combined with a kinase inhibitor—showed favorable efficacy in individuals with estrogen receptor (ER)-positive, HER2-negative advanced breast cancer, according to the first set of clinical data reported from the phase 1a/b EMBER study.
Key eligibility criteria for phase 1b enrollment included prior sensitivity to endocrine therapy, ≤2 prior therapies, and a PIK3CA mutation (alpelisib arm only). Prior therapies included endocrine therapy (100%), CDK4/6 inhibitors (100%), hormonal therapy with fulvestrant (35%), and chemotherapy (17%). At baseline, 46% of patients had visceral disease and 46% had an ESR1 mutation. Participants received imlunestrant alone (n=114) or with the kinase inhibitors everolimus (n=42) or alpelisib (n=21). Investigators assessed each regimen’s safety profile, as well as the objective response rate and clinical benefit rate.
The safety profile of each regimen was similar to those seen with everolimus and alpelisib alone. No cardiac or ocular toxicities were observed. Regarding grade ≥3 treatment-related adverse events:
The imlunestrant alone group experienced fatigue (2%) and neutropenia (2%)
The imlunestrant + everolimus group experienced hypertriglyceridemia (5%) and aspartate aminotransferase increase (5%)
The imlunestrant + alpelisib cohort experienced rash (43%) and hyperglycemia (10%).
In the imlunestrant alone group, 2% of individuals had their doses reduced due to adverse events; none discontinued treatment
In the imlunestrant + everolimus cohort, 12% of patients experienced dose reduction due to everolimus and 2% due to both medications; 2% discontinued treatment due to everolimus
In the imlunestrant + alpelisib cohort, 24% of patients experienced dose reduction due to alpelisib and 14% due to both medications; 29% discontinued treatment due to alpelisib
Regarding efficacy:
The objective response rates in the imlunestrant alone, imlunestrant + everolimus, and imlunestrant + alpelisib groups were 9%, 21%, and 50%, respectively
The clinical benefit rates in the imlunestrant alone, imlunestrant + everolimus, and imlunestrant + alpelisib groups were 42%, 62%, and 62%, respectively
Investigators concluded that imlunestrant used alone or in combination with 1 of the 2 kinase inhibitors demonstrated robust efficacy in individuals with pretreated, ER-positive, HER2-negative advanced breast cancer.
OPERA: OP-1250 Paired With a CDK4/6 Inhibitor: Anti-Tumor Activity With No Dose-Limiting Toxicities
Study synopsis
OP-1250, a CERAN and SERD, continues to show promising results when paired with a CDK4/6 inhibitor. The combination of OP-1250 and the CDK4/6 inhibitor palbociclib appears to be well tolerated and has a similar safety profile to each drug when used alone, according to a phase I/II study involving 20 individuals with pretreated ER-positive, HER2-negative breast cancer.
Participants had advanced or metastatic ER-positive, HER2-negative breast cancer that progressed on ≤1 lines of endocrine therapy. Fourteen participants had received prior CDK4/6 inhibitor therapy, including 11 who were previously treated with palbociclib. Patients received escalating doses of OP-1250 with 125 mg of palbociclib orally daily for 21 of 28 days. OP-1250 doses were 30 mg (n=3), 60 mg (n=3), 90 mg (n=3), and 120 mg (n=11). Investigators assessed pharmacokinetics, drug-drug interactions, safety, and efficacy. Among the results observed to date:
Grade 3 neutropenia occurred in 55% of participants
There were no grade 4 treatment-related adverse events and no dose-limiting toxicities
OP-1250 exposure yielded similar results to what was seen in the previous monotherapy study
Palbociclib exposure was comparable to published monotherapy data when combined with OP-1250 for all dosages
Investigators observed antitumor activity, including partial responses
Researchers concluded that OP-1250 does not affect the pharmacokinetics of palbociclib, and there do not appear to be drug-drug interactions. Tumor response to this combination was encouraging and requires continued investigation.
Conclusions
These 3 studies presented at ESMO 2023 highlight exciting novel therapies for the treatment of HR-positive, HER2-low, and HER2-negative metastatic breast cancer. The EMBER and OPERA updates provide support for the safety and efficacy of these novel endocrine agents in combination with kinase inhibitors and CDK4/6 inhibitors, respectively, in patients with endocrine-sensitive disease, while the TROPION-01 study demonstrates the encouraging efficacy and safety of a second TROP-2-directed ADC in a more heavily pretreated population.
Bariatric surgery tied to less pregnancy weight gain
TOPLINE:
Pregnancy weight gain is lower in women with a history of gastric bypass or sleeve gastrectomy than in those without such a history, especially when the interval between surgery and conception is shorter, new data suggest.
METHODOLOGY:
- Using Swedish national registers, researchers investigated the association of pregnancy weight gain with history in 12,776 pregnancies — 6388 in women with a history of bariatric surgery and 6388 in women without such a history.
- Pregnancies were propensity score matched to patients’ early-pregnancy body mass index (BMI), prepregnancy diabetes, , smoking status, education, height, country of birth, and delivery year.
- Post-gastric bypass pregnancies were matched to post-sleeve gastrectomy pregnancies using the same matching strategy.
- Time from surgery to conception was also assessed.
TAKEAWAY:
- Across all early-pregnancy BMI strata, women with a history of bariatric surgery had lower pregnancy weight gain than matched controls.
- The magnitude of difference was largest for women with normal weight or overweight early-pregnancy BMI status (adjusted mean difference in z score, −0.33), which then decreased stepwise within the subclasses (−0.21, −0.16, and −0.08 for obesity classes I, II, and III, respectively).
- Pregnancy weight gain did not differ by surgery type, but lower pregnancy weight gain was associated with a shorter surgery-to-conception interval (particularly within 1 year) or lower surgery-to-conception weight loss.
IN PRACTICE:
“The highest proportion of weight gain below the recommendations was found among women with a normal weight status. Hence, clinical attention to women with history of bariatric surgery and a normal weight status in early pregnancy might be warranted,” the authors advised.
SOURCE:
The study, with the first author Huiling Xu, MD, MSc, Karolinska Institutet, Stockholm, Sweden, was published online in JAMA Network Open.
LIMITATIONS:
Despite rigorous matching, residual confounding was possible. The sample size was limited for some subgroups, possibly affecting statistical power. Although the study provides an overview of pregnancy outcomes within surgery-to-conception interval and pregnancy weight gain z scores, a more in-depth investigation is needed to understand the associations among bariatric surgery, pregnancy weight gain, and pregnancy outcomes.
DISCLOSURES:
Research for this study was supported by the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Pregnancy weight gain is lower in women with a history of gastric bypass or sleeve gastrectomy than in those without such a history, especially when the interval between surgery and conception is shorter, new data suggest.
METHODOLOGY:
- Using Swedish national registers, researchers investigated the association of pregnancy weight gain with history in 12,776 pregnancies — 6388 in women with a history of bariatric surgery and 6388 in women without such a history.
- Pregnancies were propensity score matched to patients’ early-pregnancy body mass index (BMI), prepregnancy diabetes, , smoking status, education, height, country of birth, and delivery year.
- Post-gastric bypass pregnancies were matched to post-sleeve gastrectomy pregnancies using the same matching strategy.
- Time from surgery to conception was also assessed.
TAKEAWAY:
- Across all early-pregnancy BMI strata, women with a history of bariatric surgery had lower pregnancy weight gain than matched controls.
- The magnitude of difference was largest for women with normal weight or overweight early-pregnancy BMI status (adjusted mean difference in z score, −0.33), which then decreased stepwise within the subclasses (−0.21, −0.16, and −0.08 for obesity classes I, II, and III, respectively).
- Pregnancy weight gain did not differ by surgery type, but lower pregnancy weight gain was associated with a shorter surgery-to-conception interval (particularly within 1 year) or lower surgery-to-conception weight loss.
IN PRACTICE:
“The highest proportion of weight gain below the recommendations was found among women with a normal weight status. Hence, clinical attention to women with history of bariatric surgery and a normal weight status in early pregnancy might be warranted,” the authors advised.
SOURCE:
The study, with the first author Huiling Xu, MD, MSc, Karolinska Institutet, Stockholm, Sweden, was published online in JAMA Network Open.
LIMITATIONS:
Despite rigorous matching, residual confounding was possible. The sample size was limited for some subgroups, possibly affecting statistical power. Although the study provides an overview of pregnancy outcomes within surgery-to-conception interval and pregnancy weight gain z scores, a more in-depth investigation is needed to understand the associations among bariatric surgery, pregnancy weight gain, and pregnancy outcomes.
DISCLOSURES:
Research for this study was supported by the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Pregnancy weight gain is lower in women with a history of gastric bypass or sleeve gastrectomy than in those without such a history, especially when the interval between surgery and conception is shorter, new data suggest.
METHODOLOGY:
- Using Swedish national registers, researchers investigated the association of pregnancy weight gain with history in 12,776 pregnancies — 6388 in women with a history of bariatric surgery and 6388 in women without such a history.
- Pregnancies were propensity score matched to patients’ early-pregnancy body mass index (BMI), prepregnancy diabetes, , smoking status, education, height, country of birth, and delivery year.
- Post-gastric bypass pregnancies were matched to post-sleeve gastrectomy pregnancies using the same matching strategy.
- Time from surgery to conception was also assessed.
TAKEAWAY:
- Across all early-pregnancy BMI strata, women with a history of bariatric surgery had lower pregnancy weight gain than matched controls.
- The magnitude of difference was largest for women with normal weight or overweight early-pregnancy BMI status (adjusted mean difference in z score, −0.33), which then decreased stepwise within the subclasses (−0.21, −0.16, and −0.08 for obesity classes I, II, and III, respectively).
- Pregnancy weight gain did not differ by surgery type, but lower pregnancy weight gain was associated with a shorter surgery-to-conception interval (particularly within 1 year) or lower surgery-to-conception weight loss.
IN PRACTICE:
“The highest proportion of weight gain below the recommendations was found among women with a normal weight status. Hence, clinical attention to women with history of bariatric surgery and a normal weight status in early pregnancy might be warranted,” the authors advised.
SOURCE:
The study, with the first author Huiling Xu, MD, MSc, Karolinska Institutet, Stockholm, Sweden, was published online in JAMA Network Open.
LIMITATIONS:
Despite rigorous matching, residual confounding was possible. The sample size was limited for some subgroups, possibly affecting statistical power. Although the study provides an overview of pregnancy outcomes within surgery-to-conception interval and pregnancy weight gain z scores, a more in-depth investigation is needed to understand the associations among bariatric surgery, pregnancy weight gain, and pregnancy outcomes.
DISCLOSURES:
Research for this study was supported by the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Debate grows over facility fees as lawmakers urge greater transparency
Can the US healthcare system learn something about how to operate from car dealerships? Lawrence Kosinski, MD, MBA, a governing board member of American Gastroenterological Association (AGA), believes so.
There’s growing concern in the United States about the lack of clarity surrounding facility fees, which are intended to cover costs of maintaining medical facilities. Dr. Kosinski thinks that Congress should look into the transparency mandate it created for car prices as a model for how to address this.
A 1958 federal law set the stage for the consumer-friendly breakdown of costs and relevant performance data that anyone who has bought a new vehicle in the United States would recognize.
“You look at that and you know exactly what you are paying for,” Dr. Kosinski told this news organization. “In healthcare, we need something like that.”
Novel solutions like Dr. Kosinski’s will be increasingly necessary, as lawmakers on the state and federal level have begun to set their sights on tackling this issue.
The Biden administration in July expressed concern about an increased use of facility fees for healthcare provided at doctors’ offices, saying these additional costs often surprise consumers. House Energy and Commerce Chairwoman Cathy McMorris Rodgers (R-WA) also raised this issue several times this year, including at a May meeting about pending legislation on price transparency for health services, where she mentioned the case of a man who underwent eye surgery in Maine.
“His bill included three separate facility fees totaling $7800 and professional fees totaling $6200,” Ms. Rodgers said. “Why are three facility fees necessary for 1 hour of surgery in one O.R.?”
AGA’s Dr. Kosinski said facility fees cover the additional costs hospitals and clinics face in providing even routine treatments for some patients. For example, colonoscopy for a patient with a body mass index of 50 would pose special challenges for the anesthesiologist.
These factors need to be considered in setting policies on facility fees, he said. But there is no reason hospitals and other sites of medical care can’t make the information about facility fees easy for patients to find and understand, Dr. Kosinski said.
“I’m struggling to see a reason why we can’t be more transparent,” he said.
Big Battles Ahead
There are two connected battles ahead regarding facility fees: Efforts to restrict these additional charges for many medical services and fights over the need for greater transparency in general about health costs.
Senate Health, Education, Labor and Pensions Chairman Bernie Sanders (I-VT) is seeking to broadly restrict facility fees through his pending Primary Care and Health Workforce Act (S. 2840). The measure would block hospitals from charging health plans facility fees for many evaluation, management, and telehealth services.
The American Hospital Association (AHA) opposes it. They argue that the current payment approach rightly accounts for the added costs incurred when hospitals treat patients who are more likely to be ill or have chronic conditions than those seen in independent practices.
AHA said hospitals also need to maintain standby capacity for natural and man-made disasters, public health emergencies, and unexpected traumatic events. In September, AHA launched a television ad campaign to oppose any drive toward site-neutral policies. AHA says reducing the extra payments could cause more hospitals to shut their doors.
But there’s persistent interest in site-neutral payment, the term describing when the same reimbursement is given for care regardless of setting. This would lower pay for hospitals.
Among those pressing for change is an umbrella group of medical organizations known as the Alliance for Site Neutral Payment Reform. Its members include the American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Physicians, Community Oncology Alliance, and Digestive Health Physicians Association.
And on November 9, Sen. Maggie Hassan (D-NH) argued for eventually including a site-neutral Medicare provision to a major healthcare package that the Senate Finance Committee is putting together.
Sen. Hassan is seeking to end what she called the “the practice of charging patients unfair hospital facility fees for care provided in the off-campus outpatient setting, like at a regular doctor’s office.”
Senate Finance Chairman Ron Wyden (D-OR) and the ranking Republican on the committee, Sen. Mike Crapo (R-ID), told Sen. Hassan they intended to work with her to see if this issue could be addressed in the pending legislative package.
A 2015 budget deal marked the last time Congress took a major step to address the higher cost of services provided in hospital-owned facilities.
Lawmakers then were scrambling to find cuts to offset spending in what became the 2015 Bipartisan Budget Act. This law established site-neutral payments under Medicare for services received at off-campus outpatient departments but exempted hospitals that already ran these kinds of operations or had advanced plans to create them.
Lawmakers are well aware of the potential savings from site-neutral policies and could look in time again to use them as part of a future budget deal.
In fact, in June, Sen. Hassan and Sens. Mike Braun (R-IN) and John Kennedy (R-LA) introduced a bill meant to basically end the exemption given in the 2015 deal to existing hospital outpatient departments, which has allowed higher Medicare payments. In a press release, Braun estimated that their proposed site-neutral change could save taxpayers $40 billion over a decade.
As Debate Continues, States Are Moving Ahead With Changes
Consumer activists have won a few battles this year at the state level about facility fees.
In July, Maine Gov. Janet Mills, a Democrat, signed a law that requires medical organizations to report facility fees to the state, which will share them publicly. Facility fees can pop up after a patient has received an insurance company estimate of the out-of-pocket costs for care.
“Patients receive bills bloated by healthcare providers that overcharge for services and insurance companies that deny claims without explanation,” the Portland Press Herald reported in a 2022 story. “And with little clout to fight back or even negotiate, feeling helpless, they often give up and pay, worn down by a system that is as time-consuming as it is obtuse.”
In May, Colorado enacted a law that will require patient notification about facility fees at many hospitals in the state.
In June, Connecticut expanded its law regarding facility fees and prohibited them for certain routine outpatient healthcare services. A statement from Gov. Ned Lamont’s office said the original intent of these facility fees was to ensure hospitals could maintain the around-the-clock care needed for inpatient and emergency care.
“However, these fees have been increasingly applied to services such as diagnostic testing and other routine services,” the statement said.
But there have been setbacks as well for those seeking to curb facilities.
The Texas Hospital Association (THA) in May said its advocacy defeated a pair of state bills, House bill 1692 and Senate bill 1275, that sought to limit facility fees for outpatient services.
In rallying opposition to these bills, THA said the loss of facility fees would threaten care for patients. Facility fees help cover costs “beyond the doctor’s bill,” such as “lab technicians, interpreters, medical records, security personnel, janitorial staff, and others,” THA said.
More Patients Shopping?
It’s unclear when — or if — Congress and other states will take major steps to reduce additional payments to hospitals for outpatient care.
But the increased use of high deductibles in health plans is driving more consumers to try to understand all of the costs of medical procedures ahead of time and, thus, drawing attention to facility fees, said Charlie Byrge, the chief operating officer of MDsave.
The average annual deductible levels for an individual increased by 3.0% to $2004 from 2020 to 2021 and for a family plan by 3.9% to $3868, according to a federal report. Some people have higher deductibles, exceeding $5000, Mr. Byrge said.
“That’s creating an opportunity for firms that can connect physicians directly with patients who will pay part or all of the costs of a treatment out of pocket,” he told this news organization.
Doctors and hospitals work with MDsave to charge preset prices for certain services, such as colonoscopies and mammograms. Consumers then can shop online to see if they can save. For example, in Nashville, Tennessee, where MDsave is based, the cost of a colonoscopy through MDsave is $2334, about half of the $4714 national average, according to the firm’s website.
This model for pricing routine medical care is akin to those used for other products and services, where companies decide ahead of time what to charge, he said.
“You don’t buy an airline ticket from Southwest or United or Delta and then there’s a bill after the fact because the price of gas went up a little bit on your flight,” Mr. Byrge said.
This will drive more competition among hospitals and clinics, in places where there are several sites of care in a region, Mr. Byrge said. But there are advantages for physicians and hospitals from the MDsave approach, he said.
“They know they’re getting paid upfront. They’re not going through the delays and headaches of the insurance reimbursement process. There are no denials. It’s just an upfront payment, and I think that’s what we’re starting to see the market really moving toward,” he said.
A version of this article appeared on Medscape.com.
Can the US healthcare system learn something about how to operate from car dealerships? Lawrence Kosinski, MD, MBA, a governing board member of American Gastroenterological Association (AGA), believes so.
There’s growing concern in the United States about the lack of clarity surrounding facility fees, which are intended to cover costs of maintaining medical facilities. Dr. Kosinski thinks that Congress should look into the transparency mandate it created for car prices as a model for how to address this.
A 1958 federal law set the stage for the consumer-friendly breakdown of costs and relevant performance data that anyone who has bought a new vehicle in the United States would recognize.
“You look at that and you know exactly what you are paying for,” Dr. Kosinski told this news organization. “In healthcare, we need something like that.”
Novel solutions like Dr. Kosinski’s will be increasingly necessary, as lawmakers on the state and federal level have begun to set their sights on tackling this issue.
The Biden administration in July expressed concern about an increased use of facility fees for healthcare provided at doctors’ offices, saying these additional costs often surprise consumers. House Energy and Commerce Chairwoman Cathy McMorris Rodgers (R-WA) also raised this issue several times this year, including at a May meeting about pending legislation on price transparency for health services, where she mentioned the case of a man who underwent eye surgery in Maine.
“His bill included three separate facility fees totaling $7800 and professional fees totaling $6200,” Ms. Rodgers said. “Why are three facility fees necessary for 1 hour of surgery in one O.R.?”
AGA’s Dr. Kosinski said facility fees cover the additional costs hospitals and clinics face in providing even routine treatments for some patients. For example, colonoscopy for a patient with a body mass index of 50 would pose special challenges for the anesthesiologist.
These factors need to be considered in setting policies on facility fees, he said. But there is no reason hospitals and other sites of medical care can’t make the information about facility fees easy for patients to find and understand, Dr. Kosinski said.
“I’m struggling to see a reason why we can’t be more transparent,” he said.
Big Battles Ahead
There are two connected battles ahead regarding facility fees: Efforts to restrict these additional charges for many medical services and fights over the need for greater transparency in general about health costs.
Senate Health, Education, Labor and Pensions Chairman Bernie Sanders (I-VT) is seeking to broadly restrict facility fees through his pending Primary Care and Health Workforce Act (S. 2840). The measure would block hospitals from charging health plans facility fees for many evaluation, management, and telehealth services.
The American Hospital Association (AHA) opposes it. They argue that the current payment approach rightly accounts for the added costs incurred when hospitals treat patients who are more likely to be ill or have chronic conditions than those seen in independent practices.
AHA said hospitals also need to maintain standby capacity for natural and man-made disasters, public health emergencies, and unexpected traumatic events. In September, AHA launched a television ad campaign to oppose any drive toward site-neutral policies. AHA says reducing the extra payments could cause more hospitals to shut their doors.
But there’s persistent interest in site-neutral payment, the term describing when the same reimbursement is given for care regardless of setting. This would lower pay for hospitals.
Among those pressing for change is an umbrella group of medical organizations known as the Alliance for Site Neutral Payment Reform. Its members include the American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Physicians, Community Oncology Alliance, and Digestive Health Physicians Association.
And on November 9, Sen. Maggie Hassan (D-NH) argued for eventually including a site-neutral Medicare provision to a major healthcare package that the Senate Finance Committee is putting together.
Sen. Hassan is seeking to end what she called the “the practice of charging patients unfair hospital facility fees for care provided in the off-campus outpatient setting, like at a regular doctor’s office.”
Senate Finance Chairman Ron Wyden (D-OR) and the ranking Republican on the committee, Sen. Mike Crapo (R-ID), told Sen. Hassan they intended to work with her to see if this issue could be addressed in the pending legislative package.
A 2015 budget deal marked the last time Congress took a major step to address the higher cost of services provided in hospital-owned facilities.
Lawmakers then were scrambling to find cuts to offset spending in what became the 2015 Bipartisan Budget Act. This law established site-neutral payments under Medicare for services received at off-campus outpatient departments but exempted hospitals that already ran these kinds of operations or had advanced plans to create them.
Lawmakers are well aware of the potential savings from site-neutral policies and could look in time again to use them as part of a future budget deal.
In fact, in June, Sen. Hassan and Sens. Mike Braun (R-IN) and John Kennedy (R-LA) introduced a bill meant to basically end the exemption given in the 2015 deal to existing hospital outpatient departments, which has allowed higher Medicare payments. In a press release, Braun estimated that their proposed site-neutral change could save taxpayers $40 billion over a decade.
As Debate Continues, States Are Moving Ahead With Changes
Consumer activists have won a few battles this year at the state level about facility fees.
In July, Maine Gov. Janet Mills, a Democrat, signed a law that requires medical organizations to report facility fees to the state, which will share them publicly. Facility fees can pop up after a patient has received an insurance company estimate of the out-of-pocket costs for care.
“Patients receive bills bloated by healthcare providers that overcharge for services and insurance companies that deny claims without explanation,” the Portland Press Herald reported in a 2022 story. “And with little clout to fight back or even negotiate, feeling helpless, they often give up and pay, worn down by a system that is as time-consuming as it is obtuse.”
In May, Colorado enacted a law that will require patient notification about facility fees at many hospitals in the state.
In June, Connecticut expanded its law regarding facility fees and prohibited them for certain routine outpatient healthcare services. A statement from Gov. Ned Lamont’s office said the original intent of these facility fees was to ensure hospitals could maintain the around-the-clock care needed for inpatient and emergency care.
“However, these fees have been increasingly applied to services such as diagnostic testing and other routine services,” the statement said.
But there have been setbacks as well for those seeking to curb facilities.
The Texas Hospital Association (THA) in May said its advocacy defeated a pair of state bills, House bill 1692 and Senate bill 1275, that sought to limit facility fees for outpatient services.
In rallying opposition to these bills, THA said the loss of facility fees would threaten care for patients. Facility fees help cover costs “beyond the doctor’s bill,” such as “lab technicians, interpreters, medical records, security personnel, janitorial staff, and others,” THA said.
More Patients Shopping?
It’s unclear when — or if — Congress and other states will take major steps to reduce additional payments to hospitals for outpatient care.
But the increased use of high deductibles in health plans is driving more consumers to try to understand all of the costs of medical procedures ahead of time and, thus, drawing attention to facility fees, said Charlie Byrge, the chief operating officer of MDsave.
The average annual deductible levels for an individual increased by 3.0% to $2004 from 2020 to 2021 and for a family plan by 3.9% to $3868, according to a federal report. Some people have higher deductibles, exceeding $5000, Mr. Byrge said.
“That’s creating an opportunity for firms that can connect physicians directly with patients who will pay part or all of the costs of a treatment out of pocket,” he told this news organization.
Doctors and hospitals work with MDsave to charge preset prices for certain services, such as colonoscopies and mammograms. Consumers then can shop online to see if they can save. For example, in Nashville, Tennessee, where MDsave is based, the cost of a colonoscopy through MDsave is $2334, about half of the $4714 national average, according to the firm’s website.
This model for pricing routine medical care is akin to those used for other products and services, where companies decide ahead of time what to charge, he said.
“You don’t buy an airline ticket from Southwest or United or Delta and then there’s a bill after the fact because the price of gas went up a little bit on your flight,” Mr. Byrge said.
This will drive more competition among hospitals and clinics, in places where there are several sites of care in a region, Mr. Byrge said. But there are advantages for physicians and hospitals from the MDsave approach, he said.
“They know they’re getting paid upfront. They’re not going through the delays and headaches of the insurance reimbursement process. There are no denials. It’s just an upfront payment, and I think that’s what we’re starting to see the market really moving toward,” he said.
A version of this article appeared on Medscape.com.
Can the US healthcare system learn something about how to operate from car dealerships? Lawrence Kosinski, MD, MBA, a governing board member of American Gastroenterological Association (AGA), believes so.
There’s growing concern in the United States about the lack of clarity surrounding facility fees, which are intended to cover costs of maintaining medical facilities. Dr. Kosinski thinks that Congress should look into the transparency mandate it created for car prices as a model for how to address this.
A 1958 federal law set the stage for the consumer-friendly breakdown of costs and relevant performance data that anyone who has bought a new vehicle in the United States would recognize.
“You look at that and you know exactly what you are paying for,” Dr. Kosinski told this news organization. “In healthcare, we need something like that.”
Novel solutions like Dr. Kosinski’s will be increasingly necessary, as lawmakers on the state and federal level have begun to set their sights on tackling this issue.
The Biden administration in July expressed concern about an increased use of facility fees for healthcare provided at doctors’ offices, saying these additional costs often surprise consumers. House Energy and Commerce Chairwoman Cathy McMorris Rodgers (R-WA) also raised this issue several times this year, including at a May meeting about pending legislation on price transparency for health services, where she mentioned the case of a man who underwent eye surgery in Maine.
“His bill included three separate facility fees totaling $7800 and professional fees totaling $6200,” Ms. Rodgers said. “Why are three facility fees necessary for 1 hour of surgery in one O.R.?”
AGA’s Dr. Kosinski said facility fees cover the additional costs hospitals and clinics face in providing even routine treatments for some patients. For example, colonoscopy for a patient with a body mass index of 50 would pose special challenges for the anesthesiologist.
These factors need to be considered in setting policies on facility fees, he said. But there is no reason hospitals and other sites of medical care can’t make the information about facility fees easy for patients to find and understand, Dr. Kosinski said.
“I’m struggling to see a reason why we can’t be more transparent,” he said.
Big Battles Ahead
There are two connected battles ahead regarding facility fees: Efforts to restrict these additional charges for many medical services and fights over the need for greater transparency in general about health costs.
Senate Health, Education, Labor and Pensions Chairman Bernie Sanders (I-VT) is seeking to broadly restrict facility fees through his pending Primary Care and Health Workforce Act (S. 2840). The measure would block hospitals from charging health plans facility fees for many evaluation, management, and telehealth services.
The American Hospital Association (AHA) opposes it. They argue that the current payment approach rightly accounts for the added costs incurred when hospitals treat patients who are more likely to be ill or have chronic conditions than those seen in independent practices.
AHA said hospitals also need to maintain standby capacity for natural and man-made disasters, public health emergencies, and unexpected traumatic events. In September, AHA launched a television ad campaign to oppose any drive toward site-neutral policies. AHA says reducing the extra payments could cause more hospitals to shut their doors.
But there’s persistent interest in site-neutral payment, the term describing when the same reimbursement is given for care regardless of setting. This would lower pay for hospitals.
Among those pressing for change is an umbrella group of medical organizations known as the Alliance for Site Neutral Payment Reform. Its members include the American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Physicians, Community Oncology Alliance, and Digestive Health Physicians Association.
And on November 9, Sen. Maggie Hassan (D-NH) argued for eventually including a site-neutral Medicare provision to a major healthcare package that the Senate Finance Committee is putting together.
Sen. Hassan is seeking to end what she called the “the practice of charging patients unfair hospital facility fees for care provided in the off-campus outpatient setting, like at a regular doctor’s office.”
Senate Finance Chairman Ron Wyden (D-OR) and the ranking Republican on the committee, Sen. Mike Crapo (R-ID), told Sen. Hassan they intended to work with her to see if this issue could be addressed in the pending legislative package.
A 2015 budget deal marked the last time Congress took a major step to address the higher cost of services provided in hospital-owned facilities.
Lawmakers then were scrambling to find cuts to offset spending in what became the 2015 Bipartisan Budget Act. This law established site-neutral payments under Medicare for services received at off-campus outpatient departments but exempted hospitals that already ran these kinds of operations or had advanced plans to create them.
Lawmakers are well aware of the potential savings from site-neutral policies and could look in time again to use them as part of a future budget deal.
In fact, in June, Sen. Hassan and Sens. Mike Braun (R-IN) and John Kennedy (R-LA) introduced a bill meant to basically end the exemption given in the 2015 deal to existing hospital outpatient departments, which has allowed higher Medicare payments. In a press release, Braun estimated that their proposed site-neutral change could save taxpayers $40 billion over a decade.
As Debate Continues, States Are Moving Ahead With Changes
Consumer activists have won a few battles this year at the state level about facility fees.
In July, Maine Gov. Janet Mills, a Democrat, signed a law that requires medical organizations to report facility fees to the state, which will share them publicly. Facility fees can pop up after a patient has received an insurance company estimate of the out-of-pocket costs for care.
“Patients receive bills bloated by healthcare providers that overcharge for services and insurance companies that deny claims without explanation,” the Portland Press Herald reported in a 2022 story. “And with little clout to fight back or even negotiate, feeling helpless, they often give up and pay, worn down by a system that is as time-consuming as it is obtuse.”
In May, Colorado enacted a law that will require patient notification about facility fees at many hospitals in the state.
In June, Connecticut expanded its law regarding facility fees and prohibited them for certain routine outpatient healthcare services. A statement from Gov. Ned Lamont’s office said the original intent of these facility fees was to ensure hospitals could maintain the around-the-clock care needed for inpatient and emergency care.
“However, these fees have been increasingly applied to services such as diagnostic testing and other routine services,” the statement said.
But there have been setbacks as well for those seeking to curb facilities.
The Texas Hospital Association (THA) in May said its advocacy defeated a pair of state bills, House bill 1692 and Senate bill 1275, that sought to limit facility fees for outpatient services.
In rallying opposition to these bills, THA said the loss of facility fees would threaten care for patients. Facility fees help cover costs “beyond the doctor’s bill,” such as “lab technicians, interpreters, medical records, security personnel, janitorial staff, and others,” THA said.
More Patients Shopping?
It’s unclear when — or if — Congress and other states will take major steps to reduce additional payments to hospitals for outpatient care.
But the increased use of high deductibles in health plans is driving more consumers to try to understand all of the costs of medical procedures ahead of time and, thus, drawing attention to facility fees, said Charlie Byrge, the chief operating officer of MDsave.
The average annual deductible levels for an individual increased by 3.0% to $2004 from 2020 to 2021 and for a family plan by 3.9% to $3868, according to a federal report. Some people have higher deductibles, exceeding $5000, Mr. Byrge said.
“That’s creating an opportunity for firms that can connect physicians directly with patients who will pay part or all of the costs of a treatment out of pocket,” he told this news organization.
Doctors and hospitals work with MDsave to charge preset prices for certain services, such as colonoscopies and mammograms. Consumers then can shop online to see if they can save. For example, in Nashville, Tennessee, where MDsave is based, the cost of a colonoscopy through MDsave is $2334, about half of the $4714 national average, according to the firm’s website.
This model for pricing routine medical care is akin to those used for other products and services, where companies decide ahead of time what to charge, he said.
“You don’t buy an airline ticket from Southwest or United or Delta and then there’s a bill after the fact because the price of gas went up a little bit on your flight,” Mr. Byrge said.
This will drive more competition among hospitals and clinics, in places where there are several sites of care in a region, Mr. Byrge said. But there are advantages for physicians and hospitals from the MDsave approach, he said.
“They know they’re getting paid upfront. They’re not going through the delays and headaches of the insurance reimbursement process. There are no denials. It’s just an upfront payment, and I think that’s what we’re starting to see the market really moving toward,” he said.
A version of this article appeared on Medscape.com.
Tax Questions Frequently Asked by Physicians
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.
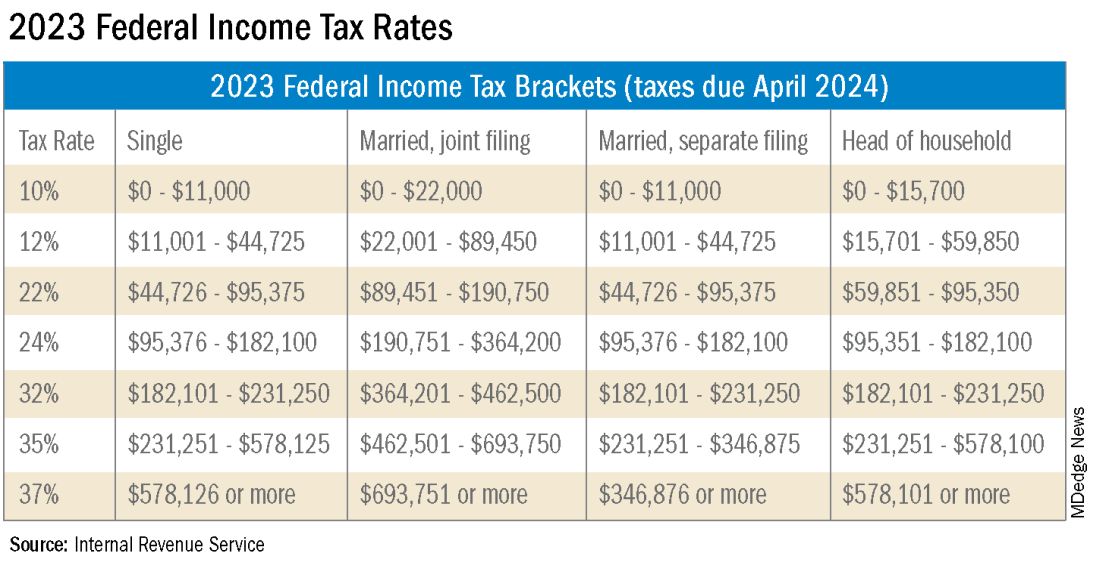
FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.

FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.

FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Roflumilast foam gets nod as new option for seborrheic dermatitis
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
Survival-Toxicity Trade-off With T-DM1 in HER+ Breast Cancer
SAN ANTONIO — in older patients with advanced human epidermal growth factor receptor 2–positive (HER2+) breast cancer, although toxicity is much lower, results from the HERB TEA study show.
Overall, the standard-of-care triple regimen of monoclonal antibodies pertuzumab and trastuzumab plus docetaxel remains the “first-line treatment for HER2-positive advanced breast cancer, regardless of age,” said study author Akihiko Shimomura, MD, PhD, who presented the findings (abstract RF02-04) on December 7 at the San Antonio Breast Cancer Symposium.
However, he noted that the standard-of-care regimen appears to be “intolerable mentally and physically” in those older than 65 years, and “impairs” quality of life.
Therefore a “new standard treatment with less toxicity and noninferior efficacy for older patients is needed,” said Dr. Shimomura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, Tokyo.
Dr. Shimomura and colleagues recruited patients aged 65 years or older with advanced HER2+ breast cancer who had received no prior chemotherapy for metastatic breast cancer and had a good performance status.
Patients were randomly assigned to either pertuzumab and trastuzumab plus docetaxel or T-DM1 until disease progression. The planned sample size was 250 patients, but the study was terminated after 148 participants were recruited because an interim analysis showed that T-DM1 failed to show noninferiority.
Among 75 patients assigned to the standard-of-care regimen, the mean age was 71 years, with 64% aged 65-74 years. Sixty-five percent had stage IV disease, and 35% had relapsed. These baseline characteristics were similar among the 73 patients given T-DM1.
At the data cutoff of June 15, 2023, the median progression-free survival was comparable between the two groups, at 15.6 months with the triple therapy vs 11.3 months with T-DM1 (hazard ratio [HR], 1.358; P =.1236).
There was also no significant difference in overall survival between the two groups (HR, 1.263; P =.95322).
However, T-DM1 failed to meet its primary endpoint of noninferiority to pertuzumab and trastuzumab plus docetaxel, defined as a hazard ratio for overall survival of 1.35.
Nevertheless, T-DM1 was associated with significantly less toxicity than the standard-of care-regimen, with rates of grade 3 or worse adverse events of 36.1% vs 56.8%, Shimomura reported.
The most common hematologic adverse events with the triple therapy were leukopenia (34.2%) and neutropenia (52.0%), whereas thrombocytopenia was the most common event with T-DM1 (16.7%).
Liver toxicities were also increased with the antibody-drug conjugate, whereas fatigue, diarrhea, and appetite loss were more frequently seen with the standard-of-care regimen.
Although T-DM1 did not achieve noninferiority, given its lower toxicity profile, a “detailed analysis, including geriatric assessment, is needed to identify the patient population for whom T-DM1 may be used as first line treatment,” said Shimomura.
Virginia Kaklamani, MD, codirector of the SABCS and leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center, Texas, said in an interview that the trial shows T-DM1 could be “a good alternative to our first line therapy in HER2+ metastatic breast cancer” for some patients.
“It is, however, unlikely to change the standard of care due to several changes in the field including the results from the KATHERINE trial and the DESTINY-Breast trials,” she said.
The study was funded by the Japanese National Cancer Center. Dr. Shimomura declares relationships with Daiichi Sankyo, Pfizer, AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., MSD Co. Ltd, Eisai Co. Ltd, Gilead Sciences, and Taiho Pharmaceutical Co. Ltd.
A version of this article appeared on Medscape.com.
SAN ANTONIO — in older patients with advanced human epidermal growth factor receptor 2–positive (HER2+) breast cancer, although toxicity is much lower, results from the HERB TEA study show.
Overall, the standard-of-care triple regimen of monoclonal antibodies pertuzumab and trastuzumab plus docetaxel remains the “first-line treatment for HER2-positive advanced breast cancer, regardless of age,” said study author Akihiko Shimomura, MD, PhD, who presented the findings (abstract RF02-04) on December 7 at the San Antonio Breast Cancer Symposium.
However, he noted that the standard-of-care regimen appears to be “intolerable mentally and physically” in those older than 65 years, and “impairs” quality of life.
Therefore a “new standard treatment with less toxicity and noninferior efficacy for older patients is needed,” said Dr. Shimomura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, Tokyo.
Dr. Shimomura and colleagues recruited patients aged 65 years or older with advanced HER2+ breast cancer who had received no prior chemotherapy for metastatic breast cancer and had a good performance status.
Patients were randomly assigned to either pertuzumab and trastuzumab plus docetaxel or T-DM1 until disease progression. The planned sample size was 250 patients, but the study was terminated after 148 participants were recruited because an interim analysis showed that T-DM1 failed to show noninferiority.
Among 75 patients assigned to the standard-of-care regimen, the mean age was 71 years, with 64% aged 65-74 years. Sixty-five percent had stage IV disease, and 35% had relapsed. These baseline characteristics were similar among the 73 patients given T-DM1.
At the data cutoff of June 15, 2023, the median progression-free survival was comparable between the two groups, at 15.6 months with the triple therapy vs 11.3 months with T-DM1 (hazard ratio [HR], 1.358; P =.1236).
There was also no significant difference in overall survival between the two groups (HR, 1.263; P =.95322).
However, T-DM1 failed to meet its primary endpoint of noninferiority to pertuzumab and trastuzumab plus docetaxel, defined as a hazard ratio for overall survival of 1.35.
Nevertheless, T-DM1 was associated with significantly less toxicity than the standard-of care-regimen, with rates of grade 3 or worse adverse events of 36.1% vs 56.8%, Shimomura reported.
The most common hematologic adverse events with the triple therapy were leukopenia (34.2%) and neutropenia (52.0%), whereas thrombocytopenia was the most common event with T-DM1 (16.7%).
Liver toxicities were also increased with the antibody-drug conjugate, whereas fatigue, diarrhea, and appetite loss were more frequently seen with the standard-of-care regimen.
Although T-DM1 did not achieve noninferiority, given its lower toxicity profile, a “detailed analysis, including geriatric assessment, is needed to identify the patient population for whom T-DM1 may be used as first line treatment,” said Shimomura.
Virginia Kaklamani, MD, codirector of the SABCS and leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center, Texas, said in an interview that the trial shows T-DM1 could be “a good alternative to our first line therapy in HER2+ metastatic breast cancer” for some patients.
“It is, however, unlikely to change the standard of care due to several changes in the field including the results from the KATHERINE trial and the DESTINY-Breast trials,” she said.
The study was funded by the Japanese National Cancer Center. Dr. Shimomura declares relationships with Daiichi Sankyo, Pfizer, AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., MSD Co. Ltd, Eisai Co. Ltd, Gilead Sciences, and Taiho Pharmaceutical Co. Ltd.
A version of this article appeared on Medscape.com.
SAN ANTONIO — in older patients with advanced human epidermal growth factor receptor 2–positive (HER2+) breast cancer, although toxicity is much lower, results from the HERB TEA study show.
Overall, the standard-of-care triple regimen of monoclonal antibodies pertuzumab and trastuzumab plus docetaxel remains the “first-line treatment for HER2-positive advanced breast cancer, regardless of age,” said study author Akihiko Shimomura, MD, PhD, who presented the findings (abstract RF02-04) on December 7 at the San Antonio Breast Cancer Symposium.
However, he noted that the standard-of-care regimen appears to be “intolerable mentally and physically” in those older than 65 years, and “impairs” quality of life.
Therefore a “new standard treatment with less toxicity and noninferior efficacy for older patients is needed,” said Dr. Shimomura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, Tokyo.
Dr. Shimomura and colleagues recruited patients aged 65 years or older with advanced HER2+ breast cancer who had received no prior chemotherapy for metastatic breast cancer and had a good performance status.
Patients were randomly assigned to either pertuzumab and trastuzumab plus docetaxel or T-DM1 until disease progression. The planned sample size was 250 patients, but the study was terminated after 148 participants were recruited because an interim analysis showed that T-DM1 failed to show noninferiority.
Among 75 patients assigned to the standard-of-care regimen, the mean age was 71 years, with 64% aged 65-74 years. Sixty-five percent had stage IV disease, and 35% had relapsed. These baseline characteristics were similar among the 73 patients given T-DM1.
At the data cutoff of June 15, 2023, the median progression-free survival was comparable between the two groups, at 15.6 months with the triple therapy vs 11.3 months with T-DM1 (hazard ratio [HR], 1.358; P =.1236).
There was also no significant difference in overall survival between the two groups (HR, 1.263; P =.95322).
However, T-DM1 failed to meet its primary endpoint of noninferiority to pertuzumab and trastuzumab plus docetaxel, defined as a hazard ratio for overall survival of 1.35.
Nevertheless, T-DM1 was associated with significantly less toxicity than the standard-of care-regimen, with rates of grade 3 or worse adverse events of 36.1% vs 56.8%, Shimomura reported.
The most common hematologic adverse events with the triple therapy were leukopenia (34.2%) and neutropenia (52.0%), whereas thrombocytopenia was the most common event with T-DM1 (16.7%).
Liver toxicities were also increased with the antibody-drug conjugate, whereas fatigue, diarrhea, and appetite loss were more frequently seen with the standard-of-care regimen.
Although T-DM1 did not achieve noninferiority, given its lower toxicity profile, a “detailed analysis, including geriatric assessment, is needed to identify the patient population for whom T-DM1 may be used as first line treatment,” said Shimomura.
Virginia Kaklamani, MD, codirector of the SABCS and leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center, Texas, said in an interview that the trial shows T-DM1 could be “a good alternative to our first line therapy in HER2+ metastatic breast cancer” for some patients.
“It is, however, unlikely to change the standard of care due to several changes in the field including the results from the KATHERINE trial and the DESTINY-Breast trials,” she said.
The study was funded by the Japanese National Cancer Center. Dr. Shimomura declares relationships with Daiichi Sankyo, Pfizer, AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., MSD Co. Ltd, Eisai Co. Ltd, Gilead Sciences, and Taiho Pharmaceutical Co. Ltd.
A version of this article appeared on Medscape.com.
AT SABCS 2023
Deciphering the usefulness of probiotics
The idea of the use of probiotics has a history going back more than a century when Russian scientist, Elie Metchnikoff, theorized that lactic acid bacteria may offer health benefits as well as promote longevity. In the early 1900s, intestinal disorders were frequently treated with nonpathogenic bacteria to replace gut microbes.
Today, the market is flooded with products from foods to prescription medications containing probiotics that extol their health benefits. It has been estimated that the global market for probiotics is more than $32 billion dollars annually and is expected to increase 8% per year.
As family doctors, patients come to us with many questions about the use of probiotics. Look online or on store shelves — there are so many types, doses, and brands of probiotics it is hard to decipher which are worth using. We older doctors never received much education about them.
Earlier this year, the World Gastroenterology Organization (WGO) developed recommendations around the use of probiotics and defined them as “live microbes that have been shown in controlled human studies to impart a health benefit.” Their recommendation is to use the strains that have been shown to be beneficial for the condition they claim to help and have been shown to do so in controlled studies. The dosage advised should be that shown to be useful in studies.
While this is an easy statement to make, it is much less so in clinical practice. The guidelines do a good job breaking down the conditions they help and the strains that have shown to be beneficial for specific conditions.
There have been claims that probiotics have been shown to be beneficial in colorectal cancer. While there have been some studies to show that they can improve markers associated with colorectal cancer, there are no data that probiotics actually do much in terms of prevention. Eating a healthy diet is more helpful here.
One area where probiotics have been shown to be beneficial is in the prevention of antibiotic-associated diarrhea. This makes sense since we know that antibiotics can kill the “good bacteria” lining the gut wall and probiotics work to replace them. Other conditions where these agents have been shown to be beneficial include radiation-induced diarrhea, acute diarrhea, irritable bowel syndrome, and colic in breast-fed infants.
The guideline contains good evidence of where and which types of probiotics are useful and it is good to look at the charts in the paper to see the specific strains recommended. It also contains an extensive reference section, and as primary care physicians, it is imperative that we educate ourselves on these agents.
While probiotics are typically sold as supplements, we should not dismiss them summarily. It is easy to do that when supplemental products are marketed and sold unethically with no clinical evidence of benefit. We need to remember that just because something is a supplement doesn’t necessarily mean that it was not studied.
Family physicians need to be able to educate their patients and answer their questions. When we don’t have the answers, we need to find them. Any time our patient doesn’t get good information from us, they will probably go to the Internet and get bad advice from someone else.
There is much ongoing research about the gut microbiome and the bacteria that can be found in the gut. Researchers are looking into the “gut-brain” axis but there is not much good evidence of this link yet. There is no evidence that probiotics can cure Alzheimer’s disease or Parkinsonism. The future may reveal different stories, but for now, we need to follow the evidence we have available.
There are many outlandish claims about what the gut microbiome is responsible for and can do for health. It is easy to have a knee-jerk reaction when anyone brings it up in conversation. We need to arm ourselves with the evidence. We are stewards of the health and safety of our patients.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
The idea of the use of probiotics has a history going back more than a century when Russian scientist, Elie Metchnikoff, theorized that lactic acid bacteria may offer health benefits as well as promote longevity. In the early 1900s, intestinal disorders were frequently treated with nonpathogenic bacteria to replace gut microbes.
Today, the market is flooded with products from foods to prescription medications containing probiotics that extol their health benefits. It has been estimated that the global market for probiotics is more than $32 billion dollars annually and is expected to increase 8% per year.
As family doctors, patients come to us with many questions about the use of probiotics. Look online or on store shelves — there are so many types, doses, and brands of probiotics it is hard to decipher which are worth using. We older doctors never received much education about them.
Earlier this year, the World Gastroenterology Organization (WGO) developed recommendations around the use of probiotics and defined them as “live microbes that have been shown in controlled human studies to impart a health benefit.” Their recommendation is to use the strains that have been shown to be beneficial for the condition they claim to help and have been shown to do so in controlled studies. The dosage advised should be that shown to be useful in studies.
While this is an easy statement to make, it is much less so in clinical practice. The guidelines do a good job breaking down the conditions they help and the strains that have shown to be beneficial for specific conditions.
There have been claims that probiotics have been shown to be beneficial in colorectal cancer. While there have been some studies to show that they can improve markers associated with colorectal cancer, there are no data that probiotics actually do much in terms of prevention. Eating a healthy diet is more helpful here.
One area where probiotics have been shown to be beneficial is in the prevention of antibiotic-associated diarrhea. This makes sense since we know that antibiotics can kill the “good bacteria” lining the gut wall and probiotics work to replace them. Other conditions where these agents have been shown to be beneficial include radiation-induced diarrhea, acute diarrhea, irritable bowel syndrome, and colic in breast-fed infants.
The guideline contains good evidence of where and which types of probiotics are useful and it is good to look at the charts in the paper to see the specific strains recommended. It also contains an extensive reference section, and as primary care physicians, it is imperative that we educate ourselves on these agents.
While probiotics are typically sold as supplements, we should not dismiss them summarily. It is easy to do that when supplemental products are marketed and sold unethically with no clinical evidence of benefit. We need to remember that just because something is a supplement doesn’t necessarily mean that it was not studied.
Family physicians need to be able to educate their patients and answer their questions. When we don’t have the answers, we need to find them. Any time our patient doesn’t get good information from us, they will probably go to the Internet and get bad advice from someone else.
There is much ongoing research about the gut microbiome and the bacteria that can be found in the gut. Researchers are looking into the “gut-brain” axis but there is not much good evidence of this link yet. There is no evidence that probiotics can cure Alzheimer’s disease or Parkinsonism. The future may reveal different stories, but for now, we need to follow the evidence we have available.
There are many outlandish claims about what the gut microbiome is responsible for and can do for health. It is easy to have a knee-jerk reaction when anyone brings it up in conversation. We need to arm ourselves with the evidence. We are stewards of the health and safety of our patients.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
The idea of the use of probiotics has a history going back more than a century when Russian scientist, Elie Metchnikoff, theorized that lactic acid bacteria may offer health benefits as well as promote longevity. In the early 1900s, intestinal disorders were frequently treated with nonpathogenic bacteria to replace gut microbes.
Today, the market is flooded with products from foods to prescription medications containing probiotics that extol their health benefits. It has been estimated that the global market for probiotics is more than $32 billion dollars annually and is expected to increase 8% per year.
As family doctors, patients come to us with many questions about the use of probiotics. Look online or on store shelves — there are so many types, doses, and brands of probiotics it is hard to decipher which are worth using. We older doctors never received much education about them.
Earlier this year, the World Gastroenterology Organization (WGO) developed recommendations around the use of probiotics and defined them as “live microbes that have been shown in controlled human studies to impart a health benefit.” Their recommendation is to use the strains that have been shown to be beneficial for the condition they claim to help and have been shown to do so in controlled studies. The dosage advised should be that shown to be useful in studies.
While this is an easy statement to make, it is much less so in clinical practice. The guidelines do a good job breaking down the conditions they help and the strains that have shown to be beneficial for specific conditions.
There have been claims that probiotics have been shown to be beneficial in colorectal cancer. While there have been some studies to show that they can improve markers associated with colorectal cancer, there are no data that probiotics actually do much in terms of prevention. Eating a healthy diet is more helpful here.
One area where probiotics have been shown to be beneficial is in the prevention of antibiotic-associated diarrhea. This makes sense since we know that antibiotics can kill the “good bacteria” lining the gut wall and probiotics work to replace them. Other conditions where these agents have been shown to be beneficial include radiation-induced diarrhea, acute diarrhea, irritable bowel syndrome, and colic in breast-fed infants.
The guideline contains good evidence of where and which types of probiotics are useful and it is good to look at the charts in the paper to see the specific strains recommended. It also contains an extensive reference section, and as primary care physicians, it is imperative that we educate ourselves on these agents.
While probiotics are typically sold as supplements, we should not dismiss them summarily. It is easy to do that when supplemental products are marketed and sold unethically with no clinical evidence of benefit. We need to remember that just because something is a supplement doesn’t necessarily mean that it was not studied.
Family physicians need to be able to educate their patients and answer their questions. When we don’t have the answers, we need to find them. Any time our patient doesn’t get good information from us, they will probably go to the Internet and get bad advice from someone else.
There is much ongoing research about the gut microbiome and the bacteria that can be found in the gut. Researchers are looking into the “gut-brain” axis but there is not much good evidence of this link yet. There is no evidence that probiotics can cure Alzheimer’s disease or Parkinsonism. The future may reveal different stories, but for now, we need to follow the evidence we have available.
There are many outlandish claims about what the gut microbiome is responsible for and can do for health. It is easy to have a knee-jerk reaction when anyone brings it up in conversation. We need to arm ourselves with the evidence. We are stewards of the health and safety of our patients.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
Pregnancy safe after BRCA-mutated breast cancer
SAN ANTONIO — New research provides some reassuring news for young women hoping to become pregnant after a diagnosis of BRCA-mutated breast cancer.
said Jame Abraham, MD, chair of Hematology & Medical Oncology at the Cleveland Clinic, who was not involved in the research.
The analysis, presented at the San Antonio Breast Cancer Symposium, revealed no issue with women becoming pregnant and carrying a healthy baby to term and reported no sign of worse disease outcomes among BRCA carriers following diagnosis and treatment.
“The final and most important conclusion from our study is that conceiving after proper breast cancer treatment and follow-up should not be contraindicated anymore in young BRCA carriers,” a message of particular importance for oncofertility counseling, lead investigator Matteo Lambertini, MD, a breast cancer oncologist at the University of Genova, Italy, said during his SABCS presentation.
The study was published December 7 in JAMA to coincide with his presentation.
Although pregnancy after breast cancer is generally considered safe, limited data exist for BRCA carriers in particular, Dr. Lambertini said.
The current analysis represents the largest look into the matter to date. The study included 4732 young women from across the globe who had been diagnosed with stage I-III invasive breast cancer. These women, all BRCA carriers, were 40 years or younger (median age at diagnosis, 35 years).
The team compared outcomes between 659 patients who had at least one pregnancy over a median follow-up of almost 8 years with 4073 women who did not become pregnant.
Dr. Lambertini and colleagues reported a median time of 3.5 years from breast cancer diagnosis to conception. Overall, about 1 in 5 young BRCA carriers (22%) conceived within 10 years after their breast cancer diagnosis. Of the 80% of patients with a completed pregnancy, 91% delivered at term and only 4 infants (0.9%) had documented congenital anomalies.
In short, “the rate of pregnancy, fetal, and obstetric complications was low and in line with the expectations in a population of women with similar age and no history of breast cancer,” Dr. Lambertini said. The team cautioned, however, that the data was extracted from oncology medical records, which might have underreported maternal and fetal outcomes.
Disease-free survival was similar among women who became pregnant and those who did not after breast cancer (adjusted HR, 0.99; 95% CI, 0.81-1.20).
When looking at the specific BRCA gene, differences did emerge. BRCA1 carriers had better disease-free survival after pregnancy (aHR, 0.80), while BRCA2 carriers appeared to have worse disease-free survival after pregnancy (aHR, 1.55).
For reasons that remain unclear, the researchers also found that BRCA1 carriers who got pregnant had significantly better breast cancer-specific survival (aHR, 0.59; P < .01) and overall survival (aHR, 0.58; P < .01). These women tended to have HR-negative breast cancer, which the authors also found was associated with improved survival after pregnancy (aHR, 0.76).
It’s possible, the team posited, that hormone receptor status played a role in the observed survival benefit. It’s also possible that these women were healthier overall.
The overall survival advantage, however, did not extend to BRCA2 carriers, who tended to have hormone receptor-positive disease. Hormone receptor-positive status did not appear to have a significant impact on survival (aHR, 1.30; 95% CI, 0.95-1.76).
“While the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers, “ the investigators wrote.
The study was funded by the Italian Association for Cancer Research, Gilead, and others. Investigators had numerous ties to industry, including Dr. Lambertini, who is an adviser and speaker for Roche, Pfizer, Novartis, and others. The full list of disclosures can be found with the original article.
A version of this article appeared on Medscape.com.
SAN ANTONIO — New research provides some reassuring news for young women hoping to become pregnant after a diagnosis of BRCA-mutated breast cancer.
said Jame Abraham, MD, chair of Hematology & Medical Oncology at the Cleveland Clinic, who was not involved in the research.
The analysis, presented at the San Antonio Breast Cancer Symposium, revealed no issue with women becoming pregnant and carrying a healthy baby to term and reported no sign of worse disease outcomes among BRCA carriers following diagnosis and treatment.
“The final and most important conclusion from our study is that conceiving after proper breast cancer treatment and follow-up should not be contraindicated anymore in young BRCA carriers,” a message of particular importance for oncofertility counseling, lead investigator Matteo Lambertini, MD, a breast cancer oncologist at the University of Genova, Italy, said during his SABCS presentation.
The study was published December 7 in JAMA to coincide with his presentation.
Although pregnancy after breast cancer is generally considered safe, limited data exist for BRCA carriers in particular, Dr. Lambertini said.
The current analysis represents the largest look into the matter to date. The study included 4732 young women from across the globe who had been diagnosed with stage I-III invasive breast cancer. These women, all BRCA carriers, were 40 years or younger (median age at diagnosis, 35 years).
The team compared outcomes between 659 patients who had at least one pregnancy over a median follow-up of almost 8 years with 4073 women who did not become pregnant.
Dr. Lambertini and colleagues reported a median time of 3.5 years from breast cancer diagnosis to conception. Overall, about 1 in 5 young BRCA carriers (22%) conceived within 10 years after their breast cancer diagnosis. Of the 80% of patients with a completed pregnancy, 91% delivered at term and only 4 infants (0.9%) had documented congenital anomalies.
In short, “the rate of pregnancy, fetal, and obstetric complications was low and in line with the expectations in a population of women with similar age and no history of breast cancer,” Dr. Lambertini said. The team cautioned, however, that the data was extracted from oncology medical records, which might have underreported maternal and fetal outcomes.
Disease-free survival was similar among women who became pregnant and those who did not after breast cancer (adjusted HR, 0.99; 95% CI, 0.81-1.20).
When looking at the specific BRCA gene, differences did emerge. BRCA1 carriers had better disease-free survival after pregnancy (aHR, 0.80), while BRCA2 carriers appeared to have worse disease-free survival after pregnancy (aHR, 1.55).
For reasons that remain unclear, the researchers also found that BRCA1 carriers who got pregnant had significantly better breast cancer-specific survival (aHR, 0.59; P < .01) and overall survival (aHR, 0.58; P < .01). These women tended to have HR-negative breast cancer, which the authors also found was associated with improved survival after pregnancy (aHR, 0.76).
It’s possible, the team posited, that hormone receptor status played a role in the observed survival benefit. It’s also possible that these women were healthier overall.
The overall survival advantage, however, did not extend to BRCA2 carriers, who tended to have hormone receptor-positive disease. Hormone receptor-positive status did not appear to have a significant impact on survival (aHR, 1.30; 95% CI, 0.95-1.76).
“While the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers, “ the investigators wrote.
The study was funded by the Italian Association for Cancer Research, Gilead, and others. Investigators had numerous ties to industry, including Dr. Lambertini, who is an adviser and speaker for Roche, Pfizer, Novartis, and others. The full list of disclosures can be found with the original article.
A version of this article appeared on Medscape.com.
SAN ANTONIO — New research provides some reassuring news for young women hoping to become pregnant after a diagnosis of BRCA-mutated breast cancer.
said Jame Abraham, MD, chair of Hematology & Medical Oncology at the Cleveland Clinic, who was not involved in the research.
The analysis, presented at the San Antonio Breast Cancer Symposium, revealed no issue with women becoming pregnant and carrying a healthy baby to term and reported no sign of worse disease outcomes among BRCA carriers following diagnosis and treatment.
“The final and most important conclusion from our study is that conceiving after proper breast cancer treatment and follow-up should not be contraindicated anymore in young BRCA carriers,” a message of particular importance for oncofertility counseling, lead investigator Matteo Lambertini, MD, a breast cancer oncologist at the University of Genova, Italy, said during his SABCS presentation.
The study was published December 7 in JAMA to coincide with his presentation.
Although pregnancy after breast cancer is generally considered safe, limited data exist for BRCA carriers in particular, Dr. Lambertini said.
The current analysis represents the largest look into the matter to date. The study included 4732 young women from across the globe who had been diagnosed with stage I-III invasive breast cancer. These women, all BRCA carriers, were 40 years or younger (median age at diagnosis, 35 years).
The team compared outcomes between 659 patients who had at least one pregnancy over a median follow-up of almost 8 years with 4073 women who did not become pregnant.
Dr. Lambertini and colleagues reported a median time of 3.5 years from breast cancer diagnosis to conception. Overall, about 1 in 5 young BRCA carriers (22%) conceived within 10 years after their breast cancer diagnosis. Of the 80% of patients with a completed pregnancy, 91% delivered at term and only 4 infants (0.9%) had documented congenital anomalies.
In short, “the rate of pregnancy, fetal, and obstetric complications was low and in line with the expectations in a population of women with similar age and no history of breast cancer,” Dr. Lambertini said. The team cautioned, however, that the data was extracted from oncology medical records, which might have underreported maternal and fetal outcomes.
Disease-free survival was similar among women who became pregnant and those who did not after breast cancer (adjusted HR, 0.99; 95% CI, 0.81-1.20).
When looking at the specific BRCA gene, differences did emerge. BRCA1 carriers had better disease-free survival after pregnancy (aHR, 0.80), while BRCA2 carriers appeared to have worse disease-free survival after pregnancy (aHR, 1.55).
For reasons that remain unclear, the researchers also found that BRCA1 carriers who got pregnant had significantly better breast cancer-specific survival (aHR, 0.59; P < .01) and overall survival (aHR, 0.58; P < .01). These women tended to have HR-negative breast cancer, which the authors also found was associated with improved survival after pregnancy (aHR, 0.76).
It’s possible, the team posited, that hormone receptor status played a role in the observed survival benefit. It’s also possible that these women were healthier overall.
The overall survival advantage, however, did not extend to BRCA2 carriers, who tended to have hormone receptor-positive disease. Hormone receptor-positive status did not appear to have a significant impact on survival (aHR, 1.30; 95% CI, 0.95-1.76).
“While the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers, “ the investigators wrote.
The study was funded by the Italian Association for Cancer Research, Gilead, and others. Investigators had numerous ties to industry, including Dr. Lambertini, who is an adviser and speaker for Roche, Pfizer, Novartis, and others. The full list of disclosures can be found with the original article.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Caring for LGBTQ+ Patients with IBD
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.
Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.







