User login
Gene therapy exceeds expectations in β-thalassemia
The gene therapy LentiGlobin can reduce or eliminate transfusion dependence in patients with β-thalassemia, according to a pair of phase 1/2 studies.
Fifteen of the 22 patients in these trials were able to discontinue red blood cell (RBC) transfusions after receiving LentiGlobin.
In the 9 patients with severe transfusion-dependent β-thalassemia (TDT), LentiGlobin reduced the transfusion volume by 73%.
There were 5 adverse events (AEs) considered possibly or probably related to LentiGlobin, all them grade 1.
“These study results exceeded our expectations, with clinical benefit for nearly all patients . . . ,” said Alexis Thompson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois.
“Since we saw such positive results, we are now enrolling patients as young as 5 years old on a phase 3 trial of gene therapy for transfusion-dependent thalassemia.”
Dr Thompson and her colleagues reported results from the phase 1/2 trials—known as HGB-204 and HGB-205—in NEJM. The studies were sponsored by Bluebird Bio, the company developing LentiGlobin.
Patients in HGB-204
HGB-204 (also known as Northstar) is a multicenter study that was recently completed. It included 18 patients with TDT. They had a median age of 20 (range, 12 to 35) at baseline, and 72% were female. Seventy-eight percent were Asian, and 22% were white.
Eight patients had a β0/β0 genotype, 6 had a βE/β0 genotype, and 4 had other genotypes.
The patients’ median monthly transfusion volume for 2 years before study enrollment was 13.6 ml/kg (range, 10.4 to 21.8). The median age at which patients started regular transfusions was 3.5 years (range, 0 to 26.0). Six patients had undergone splenectomy.
Patients in HGB-205
HGB-205 is an ongoing study being conducted at a single site in France. It was designed to evaluate LentiGlobin in patients with TDT or severe sickle cell disease.
The NEJM paper includes 4 patients with TDT from this study. They had a median age of 18 (range, 16 to 19) at baseline, and half were female. Half were Asian, and the other half were white.
Three patients had a βE/β0 genotype. The remaining patient was homozygous for the IVS1-110 mutation and had a severe clinical presentation similar to that seen in β0/β0 genotypes.
The patients’ median monthly transfusion volume for 2 years before study enrollment was 15.2 ml/kg (range, 11.6 to 15.7). The median age at which patients started regular transfusions was 1.8 years (range, 0 to 14.0). Three patients had undergone splenectomy.
Treatment
For both studies, the researchers harvested hematopoietic stem and progenitor cells (mobilized with filgrastim and plerixafor) from the patients.
CD34+ cells were transduced ex vivo with LentiGlobin BB305 vector, which encodes adult hemoglobin (HbA) with a T87Q amino acid substitution (HbAT87Q).
The patients underwent myeloablative conditioning with busulfan, and the final LentiGlobin product was infused into patients after a 72-hour washout period.
In HGB-205 only, patients received enhanced RBC transfusions for at least 3 months before stem cell mobilization and harvest to maintain a hemoglobin level of more than 11.0 g/dL.
Safety
In HGB-204, there were 5 grade 1 AEs considered possibly or probably related to LentiGlobin. These included abdominal pain (n=2), dyspnea (n=1), hot flush (n=1), and non-cardiac chest pain (n=1).
There were 9 serious AEs, including 2 episodes of grade 3 veno-occlusive liver disease that were attributed to busulfan.
The remaining serious AEs were Klebsiella infection, cardiac ventricular thrombosis, cellulitis, hyperglycemia, and gastroenteritis (all grade 3), as well as device-related thrombosis and infectious diarrhea (both grade 2).
In HGB-205, there were no AEs considered possibly or probably related to LentiGlobin.
The 3 serious AEs were tooth infection and major depression (both grade 3), as well as pneumonia (grade 2).
Efficacy
The median time to neutrophil engraftment was 18.5 days (range, 14.0 to 30.0) in HGB-204 and 16.5 days (range, 14.0 to 29.0) in HGB-205.
The median time to platelet engraftment was 39.5 days (range, 19.0 to 191.0) in HGB-204 and 23.0 days (range, 20.0 to 26.0) in HGB-205.
In both studies, the median follow-up was 26 months (range, 15 to 42) after LentiGlobin infusion.
At last follow-up, all but 1 of the 13 patients with a non-β0/β0 genotype had stopped receiving RBC transfusions.
At the last study visit (12 to 36 months post-treatment), the median HbAT87Q level in these patients was 6.0 g/dL (range, 3.4 to 10.0), and the median total hemoglobin was 11.2 g/dL (range, 8.2 to 13.7).
In the 8 patients with a β0/β0 genotype and the 1 patient with 2 copies of the IVS1-110 mutation, the median annualized transfusion volume decreased by 73% after LentiGlobin infusion.
Two patients with a β0/β0 genotype were able to stop receiving RBC transfusions, as was the patient with 2 copies of the IVS1-110 mutation.
At their most recent study visit (12 months to 30 months), these 3 patients had median HbAT87Q levels of 8.2 g/dL, 6.8 g/dL, and 6.6 g/dL, respectively. Their median total hemoglobin levels were 9.0 g/dL, 10.2 g/dL, and 8.3 g/dL, respectively.
For the 6 patients with a β0/β0 genotype who continued to receive RBC transfusions, the median HbAT87Q level was 4.2 g/dL (range, 0.3 to 8.7) at the last study visit.
“There is room for improvement, as we’d like to see the elimination of dependency on transfusion even for patients with the most severe form of the disease,” said study author Philippe Leboulch, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“But there is also hope with protocol modifications we have introduced in our phase 3 trials.”
The gene therapy LentiGlobin can reduce or eliminate transfusion dependence in patients with β-thalassemia, according to a pair of phase 1/2 studies.
Fifteen of the 22 patients in these trials were able to discontinue red blood cell (RBC) transfusions after receiving LentiGlobin.
In the 9 patients with severe transfusion-dependent β-thalassemia (TDT), LentiGlobin reduced the transfusion volume by 73%.
There were 5 adverse events (AEs) considered possibly or probably related to LentiGlobin, all them grade 1.
“These study results exceeded our expectations, with clinical benefit for nearly all patients . . . ,” said Alexis Thompson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois.
“Since we saw such positive results, we are now enrolling patients as young as 5 years old on a phase 3 trial of gene therapy for transfusion-dependent thalassemia.”
Dr Thompson and her colleagues reported results from the phase 1/2 trials—known as HGB-204 and HGB-205—in NEJM. The studies were sponsored by Bluebird Bio, the company developing LentiGlobin.
Patients in HGB-204
HGB-204 (also known as Northstar) is a multicenter study that was recently completed. It included 18 patients with TDT. They had a median age of 20 (range, 12 to 35) at baseline, and 72% were female. Seventy-eight percent were Asian, and 22% were white.
Eight patients had a β0/β0 genotype, 6 had a βE/β0 genotype, and 4 had other genotypes.
The patients’ median monthly transfusion volume for 2 years before study enrollment was 13.6 ml/kg (range, 10.4 to 21.8). The median age at which patients started regular transfusions was 3.5 years (range, 0 to 26.0). Six patients had undergone splenectomy.
Patients in HGB-205
HGB-205 is an ongoing study being conducted at a single site in France. It was designed to evaluate LentiGlobin in patients with TDT or severe sickle cell disease.
The NEJM paper includes 4 patients with TDT from this study. They had a median age of 18 (range, 16 to 19) at baseline, and half were female. Half were Asian, and the other half were white.
Three patients had a βE/β0 genotype. The remaining patient was homozygous for the IVS1-110 mutation and had a severe clinical presentation similar to that seen in β0/β0 genotypes.
The patients’ median monthly transfusion volume for 2 years before study enrollment was 15.2 ml/kg (range, 11.6 to 15.7). The median age at which patients started regular transfusions was 1.8 years (range, 0 to 14.0). Three patients had undergone splenectomy.
Treatment
For both studies, the researchers harvested hematopoietic stem and progenitor cells (mobilized with filgrastim and plerixafor) from the patients.
CD34+ cells were transduced ex vivo with LentiGlobin BB305 vector, which encodes adult hemoglobin (HbA) with a T87Q amino acid substitution (HbAT87Q).
The patients underwent myeloablative conditioning with busulfan, and the final LentiGlobin product was infused into patients after a 72-hour washout period.
In HGB-205 only, patients received enhanced RBC transfusions for at least 3 months before stem cell mobilization and harvest to maintain a hemoglobin level of more than 11.0 g/dL.
Safety
In HGB-204, there were 5 grade 1 AEs considered possibly or probably related to LentiGlobin. These included abdominal pain (n=2), dyspnea (n=1), hot flush (n=1), and non-cardiac chest pain (n=1).
There were 9 serious AEs, including 2 episodes of grade 3 veno-occlusive liver disease that were attributed to busulfan.
The remaining serious AEs were Klebsiella infection, cardiac ventricular thrombosis, cellulitis, hyperglycemia, and gastroenteritis (all grade 3), as well as device-related thrombosis and infectious diarrhea (both grade 2).
In HGB-205, there were no AEs considered possibly or probably related to LentiGlobin.
The 3 serious AEs were tooth infection and major depression (both grade 3), as well as pneumonia (grade 2).
Efficacy
The median time to neutrophil engraftment was 18.5 days (range, 14.0 to 30.0) in HGB-204 and 16.5 days (range, 14.0 to 29.0) in HGB-205.
The median time to platelet engraftment was 39.5 days (range, 19.0 to 191.0) in HGB-204 and 23.0 days (range, 20.0 to 26.0) in HGB-205.
In both studies, the median follow-up was 26 months (range, 15 to 42) after LentiGlobin infusion.
At last follow-up, all but 1 of the 13 patients with a non-β0/β0 genotype had stopped receiving RBC transfusions.
At the last study visit (12 to 36 months post-treatment), the median HbAT87Q level in these patients was 6.0 g/dL (range, 3.4 to 10.0), and the median total hemoglobin was 11.2 g/dL (range, 8.2 to 13.7).
In the 8 patients with a β0/β0 genotype and the 1 patient with 2 copies of the IVS1-110 mutation, the median annualized transfusion volume decreased by 73% after LentiGlobin infusion.
Two patients with a β0/β0 genotype were able to stop receiving RBC transfusions, as was the patient with 2 copies of the IVS1-110 mutation.
At their most recent study visit (12 months to 30 months), these 3 patients had median HbAT87Q levels of 8.2 g/dL, 6.8 g/dL, and 6.6 g/dL, respectively. Their median total hemoglobin levels were 9.0 g/dL, 10.2 g/dL, and 8.3 g/dL, respectively.
For the 6 patients with a β0/β0 genotype who continued to receive RBC transfusions, the median HbAT87Q level was 4.2 g/dL (range, 0.3 to 8.7) at the last study visit.
“There is room for improvement, as we’d like to see the elimination of dependency on transfusion even for patients with the most severe form of the disease,” said study author Philippe Leboulch, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“But there is also hope with protocol modifications we have introduced in our phase 3 trials.”
The gene therapy LentiGlobin can reduce or eliminate transfusion dependence in patients with β-thalassemia, according to a pair of phase 1/2 studies.
Fifteen of the 22 patients in these trials were able to discontinue red blood cell (RBC) transfusions after receiving LentiGlobin.
In the 9 patients with severe transfusion-dependent β-thalassemia (TDT), LentiGlobin reduced the transfusion volume by 73%.
There were 5 adverse events (AEs) considered possibly or probably related to LentiGlobin, all them grade 1.
“These study results exceeded our expectations, with clinical benefit for nearly all patients . . . ,” said Alexis Thompson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois.
“Since we saw such positive results, we are now enrolling patients as young as 5 years old on a phase 3 trial of gene therapy for transfusion-dependent thalassemia.”
Dr Thompson and her colleagues reported results from the phase 1/2 trials—known as HGB-204 and HGB-205—in NEJM. The studies were sponsored by Bluebird Bio, the company developing LentiGlobin.
Patients in HGB-204
HGB-204 (also known as Northstar) is a multicenter study that was recently completed. It included 18 patients with TDT. They had a median age of 20 (range, 12 to 35) at baseline, and 72% were female. Seventy-eight percent were Asian, and 22% were white.
Eight patients had a β0/β0 genotype, 6 had a βE/β0 genotype, and 4 had other genotypes.
The patients’ median monthly transfusion volume for 2 years before study enrollment was 13.6 ml/kg (range, 10.4 to 21.8). The median age at which patients started regular transfusions was 3.5 years (range, 0 to 26.0). Six patients had undergone splenectomy.
Patients in HGB-205
HGB-205 is an ongoing study being conducted at a single site in France. It was designed to evaluate LentiGlobin in patients with TDT or severe sickle cell disease.
The NEJM paper includes 4 patients with TDT from this study. They had a median age of 18 (range, 16 to 19) at baseline, and half were female. Half were Asian, and the other half were white.
Three patients had a βE/β0 genotype. The remaining patient was homozygous for the IVS1-110 mutation and had a severe clinical presentation similar to that seen in β0/β0 genotypes.
The patients’ median monthly transfusion volume for 2 years before study enrollment was 15.2 ml/kg (range, 11.6 to 15.7). The median age at which patients started regular transfusions was 1.8 years (range, 0 to 14.0). Three patients had undergone splenectomy.
Treatment
For both studies, the researchers harvested hematopoietic stem and progenitor cells (mobilized with filgrastim and plerixafor) from the patients.
CD34+ cells were transduced ex vivo with LentiGlobin BB305 vector, which encodes adult hemoglobin (HbA) with a T87Q amino acid substitution (HbAT87Q).
The patients underwent myeloablative conditioning with busulfan, and the final LentiGlobin product was infused into patients after a 72-hour washout period.
In HGB-205 only, patients received enhanced RBC transfusions for at least 3 months before stem cell mobilization and harvest to maintain a hemoglobin level of more than 11.0 g/dL.
Safety
In HGB-204, there were 5 grade 1 AEs considered possibly or probably related to LentiGlobin. These included abdominal pain (n=2), dyspnea (n=1), hot flush (n=1), and non-cardiac chest pain (n=1).
There were 9 serious AEs, including 2 episodes of grade 3 veno-occlusive liver disease that were attributed to busulfan.
The remaining serious AEs were Klebsiella infection, cardiac ventricular thrombosis, cellulitis, hyperglycemia, and gastroenteritis (all grade 3), as well as device-related thrombosis and infectious diarrhea (both grade 2).
In HGB-205, there were no AEs considered possibly or probably related to LentiGlobin.
The 3 serious AEs were tooth infection and major depression (both grade 3), as well as pneumonia (grade 2).
Efficacy
The median time to neutrophil engraftment was 18.5 days (range, 14.0 to 30.0) in HGB-204 and 16.5 days (range, 14.0 to 29.0) in HGB-205.
The median time to platelet engraftment was 39.5 days (range, 19.0 to 191.0) in HGB-204 and 23.0 days (range, 20.0 to 26.0) in HGB-205.
In both studies, the median follow-up was 26 months (range, 15 to 42) after LentiGlobin infusion.
At last follow-up, all but 1 of the 13 patients with a non-β0/β0 genotype had stopped receiving RBC transfusions.
At the last study visit (12 to 36 months post-treatment), the median HbAT87Q level in these patients was 6.0 g/dL (range, 3.4 to 10.0), and the median total hemoglobin was 11.2 g/dL (range, 8.2 to 13.7).
In the 8 patients with a β0/β0 genotype and the 1 patient with 2 copies of the IVS1-110 mutation, the median annualized transfusion volume decreased by 73% after LentiGlobin infusion.
Two patients with a β0/β0 genotype were able to stop receiving RBC transfusions, as was the patient with 2 copies of the IVS1-110 mutation.
At their most recent study visit (12 months to 30 months), these 3 patients had median HbAT87Q levels of 8.2 g/dL, 6.8 g/dL, and 6.6 g/dL, respectively. Their median total hemoglobin levels were 9.0 g/dL, 10.2 g/dL, and 8.3 g/dL, respectively.
For the 6 patients with a β0/β0 genotype who continued to receive RBC transfusions, the median HbAT87Q level was 4.2 g/dL (range, 0.3 to 8.7) at the last study visit.
“There is room for improvement, as we’d like to see the elimination of dependency on transfusion even for patients with the most severe form of the disease,” said study author Philippe Leboulch, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“But there is also hope with protocol modifications we have introduced in our phase 3 trials.”
Idarucizumab receives full FDA approval
The US Food and Drug Administration (FDA) has granted full approval to idarucizumab (Praxbind®), the specific reversal agent for dabigatran etexilate mesylate (Pradaxa®).
Idarucizumab received accelerated approval from the FDA in October 2015.
Now, the drug has full approval for use in reversing dabigatran’s anticoagulant effects in patients who require emergency surgery/urgent procedures and those who experience life-threatening or uncontrolled bleeding.
When the FDA granted idarucizumab accelerated approval, continued approval of the drug was contingent upon results from the phase 3 RE-VERSE AD™ trial.
Final results from RE-VERSE AD were published in NEJM in July 2017.
The trial enrolled 503 patients who required dabigatran reversal. They were divided into 2 groups:
- Group A included 301 patients with uncontrolled or life-threatening bleeding complications (eg, intracranial hemorrhage or severe trauma after a car accident)
- Group B included 202 patients requiring an invasive procedure or an emergency surgery or intervention (eg, surgery for an open fracture after a fall).
The study’s primary endpoint was the degree of reversal of the anticoagulant effect of dabigatran achieved by idarucizumab within 4 hours. The median maximum percentage reversal was 100%, as assessed on the basis of either the diluted thrombin time or the ecarin clotting time.
In group A, 68% of evaluable patients (134/203) had confirmed bleeding cessation within 24 hours of idarucizumab administration. Their median time to hemostasis after idarucizumab administration was 2.5 hours. (The time to the cessation of bleeding could not be assessed in 98 patients with intracranial bleeding.)
For patients in group B, their required procedures began a median of 1.6 hours from idarucizumab administration. Hemostasis during the procedure was described as normal for 93.4% of the patients, mildly abnormal in 5.1%, and moderately abnormal in 1.5%.
At 90 days, thrombotic events had occurred in 6.3% of patients in group A and 7.4% of those in group B. The 90-day mortality rates were 18.8% and 18.9%, respectively.
The US Food and Drug Administration (FDA) has granted full approval to idarucizumab (Praxbind®), the specific reversal agent for dabigatran etexilate mesylate (Pradaxa®).
Idarucizumab received accelerated approval from the FDA in October 2015.
Now, the drug has full approval for use in reversing dabigatran’s anticoagulant effects in patients who require emergency surgery/urgent procedures and those who experience life-threatening or uncontrolled bleeding.
When the FDA granted idarucizumab accelerated approval, continued approval of the drug was contingent upon results from the phase 3 RE-VERSE AD™ trial.
Final results from RE-VERSE AD were published in NEJM in July 2017.
The trial enrolled 503 patients who required dabigatran reversal. They were divided into 2 groups:
- Group A included 301 patients with uncontrolled or life-threatening bleeding complications (eg, intracranial hemorrhage or severe trauma after a car accident)
- Group B included 202 patients requiring an invasive procedure or an emergency surgery or intervention (eg, surgery for an open fracture after a fall).
The study’s primary endpoint was the degree of reversal of the anticoagulant effect of dabigatran achieved by idarucizumab within 4 hours. The median maximum percentage reversal was 100%, as assessed on the basis of either the diluted thrombin time or the ecarin clotting time.
In group A, 68% of evaluable patients (134/203) had confirmed bleeding cessation within 24 hours of idarucizumab administration. Their median time to hemostasis after idarucizumab administration was 2.5 hours. (The time to the cessation of bleeding could not be assessed in 98 patients with intracranial bleeding.)
For patients in group B, their required procedures began a median of 1.6 hours from idarucizumab administration. Hemostasis during the procedure was described as normal for 93.4% of the patients, mildly abnormal in 5.1%, and moderately abnormal in 1.5%.
At 90 days, thrombotic events had occurred in 6.3% of patients in group A and 7.4% of those in group B. The 90-day mortality rates were 18.8% and 18.9%, respectively.
The US Food and Drug Administration (FDA) has granted full approval to idarucizumab (Praxbind®), the specific reversal agent for dabigatran etexilate mesylate (Pradaxa®).
Idarucizumab received accelerated approval from the FDA in October 2015.
Now, the drug has full approval for use in reversing dabigatran’s anticoagulant effects in patients who require emergency surgery/urgent procedures and those who experience life-threatening or uncontrolled bleeding.
When the FDA granted idarucizumab accelerated approval, continued approval of the drug was contingent upon results from the phase 3 RE-VERSE AD™ trial.
Final results from RE-VERSE AD were published in NEJM in July 2017.
The trial enrolled 503 patients who required dabigatran reversal. They were divided into 2 groups:
- Group A included 301 patients with uncontrolled or life-threatening bleeding complications (eg, intracranial hemorrhage or severe trauma after a car accident)
- Group B included 202 patients requiring an invasive procedure or an emergency surgery or intervention (eg, surgery for an open fracture after a fall).
The study’s primary endpoint was the degree of reversal of the anticoagulant effect of dabigatran achieved by idarucizumab within 4 hours. The median maximum percentage reversal was 100%, as assessed on the basis of either the diluted thrombin time or the ecarin clotting time.
In group A, 68% of evaluable patients (134/203) had confirmed bleeding cessation within 24 hours of idarucizumab administration. Their median time to hemostasis after idarucizumab administration was 2.5 hours. (The time to the cessation of bleeding could not be assessed in 98 patients with intracranial bleeding.)
For patients in group B, their required procedures began a median of 1.6 hours from idarucizumab administration. Hemostasis during the procedure was described as normal for 93.4% of the patients, mildly abnormal in 5.1%, and moderately abnormal in 1.5%.
At 90 days, thrombotic events had occurred in 6.3% of patients in group A and 7.4% of those in group B. The 90-day mortality rates were 18.8% and 18.9%, respectively.
Antibody has ‘potent’ effects against AML
CHICAGO—The bispecific antibody APVO436 has demonstrated robust T-cell activation with limited cytokine release in acute myeloid leukemia (AML), according to researchers.
APVO436 binds CD123 and CD3 to redirect T-cell cytotoxicity against CD123-expressing tumor cells.
Researchers found that APVO436 induced T-cell cytotoxicity in AML cells in vitro and in mouse models.
In addition, levels of several cytokines were lower in experiments with APVO436 than in experiments with a comparator antibody.
These findings were presented in a poster at the AACR Annual Meeting 2018 (abstract 1786).
The research was conducted by employees of Aptevo Therapeutics Inc., the company developing APVO436.
“We are especially excited about these latest data for APVO436, which continue to show robust T-cell engagement and cytotoxic activity with reduced levels of cytokine release,” said Jane Gross, PhD, senior vice president and chief scientific officer for Aptevo.
Dr Gross and her colleagues found that APVO436 binds human CD123 and CD3-expressing cells and has “potent” target-specific activity against CD123-expressing AML cell lines (Molm-13 and KG-1a).
In addition, APVO436 induced endogenous T-cell activation and proliferation, accompanied by depletion of CD123-expressing cells, in samples from AML patients and healthy donors.
T cells from these cultures (both AML and non-AML) were expanded and co-cultured with Molm-13 cells and APVO436 or a control antibody. Again, the researchers observed “potent” cytotoxic activity in the presence of APVO436.
Dr Gross and her colleagues also tested APVO436, co-administered with human T cells, in mice with established disseminated Molm-13 tumors. The treatment resulted in a “rapid and significant” reduction in skeletal tumor burden.
Finally, the team compared APVO436 with an Aptevo-generated version of MGD006, a CD123 x CD3 dual-affinity re-targeting molecule being developed by Macrogenics, Inc.
The researchers took purified T cells from healthy donors and cultured them with Molm-13 cells, as well as APVO436, Aptevo’s version of MGD006, and a control antibody.
Both APVO436 and Aptevo’s version of MGD006 were effective at stimulating a tumor-directed immune response, inducing comparable levels of T-cell activation, proliferation, and cytotoxicity.
However, APVO436 induced lower levels of several cytokines—including IFNγ, IL-2, IL-6, and TNFα.
“Importantly, IFNγ, IL-6, and TNFα are considered to be the most relevant cytokines responsible for dosing toxicities observed in clinical studies with T-cell engaging molecules, which suggests that APVO436 could offer the potential for reduced toxicities compared to other CD123 x CD3 T-cell engagers at comparable or higher doses,” Dr Gross said.
She added that Aptevo is planning to launch a phase 1 trial of APVO436 in patients with AML and myelodysplastic syndromes later this year.
CHICAGO—The bispecific antibody APVO436 has demonstrated robust T-cell activation with limited cytokine release in acute myeloid leukemia (AML), according to researchers.
APVO436 binds CD123 and CD3 to redirect T-cell cytotoxicity against CD123-expressing tumor cells.
Researchers found that APVO436 induced T-cell cytotoxicity in AML cells in vitro and in mouse models.
In addition, levels of several cytokines were lower in experiments with APVO436 than in experiments with a comparator antibody.
These findings were presented in a poster at the AACR Annual Meeting 2018 (abstract 1786).
The research was conducted by employees of Aptevo Therapeutics Inc., the company developing APVO436.
“We are especially excited about these latest data for APVO436, which continue to show robust T-cell engagement and cytotoxic activity with reduced levels of cytokine release,” said Jane Gross, PhD, senior vice president and chief scientific officer for Aptevo.
Dr Gross and her colleagues found that APVO436 binds human CD123 and CD3-expressing cells and has “potent” target-specific activity against CD123-expressing AML cell lines (Molm-13 and KG-1a).
In addition, APVO436 induced endogenous T-cell activation and proliferation, accompanied by depletion of CD123-expressing cells, in samples from AML patients and healthy donors.
T cells from these cultures (both AML and non-AML) were expanded and co-cultured with Molm-13 cells and APVO436 or a control antibody. Again, the researchers observed “potent” cytotoxic activity in the presence of APVO436.
Dr Gross and her colleagues also tested APVO436, co-administered with human T cells, in mice with established disseminated Molm-13 tumors. The treatment resulted in a “rapid and significant” reduction in skeletal tumor burden.
Finally, the team compared APVO436 with an Aptevo-generated version of MGD006, a CD123 x CD3 dual-affinity re-targeting molecule being developed by Macrogenics, Inc.
The researchers took purified T cells from healthy donors and cultured them with Molm-13 cells, as well as APVO436, Aptevo’s version of MGD006, and a control antibody.
Both APVO436 and Aptevo’s version of MGD006 were effective at stimulating a tumor-directed immune response, inducing comparable levels of T-cell activation, proliferation, and cytotoxicity.
However, APVO436 induced lower levels of several cytokines—including IFNγ, IL-2, IL-6, and TNFα.
“Importantly, IFNγ, IL-6, and TNFα are considered to be the most relevant cytokines responsible for dosing toxicities observed in clinical studies with T-cell engaging molecules, which suggests that APVO436 could offer the potential for reduced toxicities compared to other CD123 x CD3 T-cell engagers at comparable or higher doses,” Dr Gross said.
She added that Aptevo is planning to launch a phase 1 trial of APVO436 in patients with AML and myelodysplastic syndromes later this year.
CHICAGO—The bispecific antibody APVO436 has demonstrated robust T-cell activation with limited cytokine release in acute myeloid leukemia (AML), according to researchers.
APVO436 binds CD123 and CD3 to redirect T-cell cytotoxicity against CD123-expressing tumor cells.
Researchers found that APVO436 induced T-cell cytotoxicity in AML cells in vitro and in mouse models.
In addition, levels of several cytokines were lower in experiments with APVO436 than in experiments with a comparator antibody.
These findings were presented in a poster at the AACR Annual Meeting 2018 (abstract 1786).
The research was conducted by employees of Aptevo Therapeutics Inc., the company developing APVO436.
“We are especially excited about these latest data for APVO436, which continue to show robust T-cell engagement and cytotoxic activity with reduced levels of cytokine release,” said Jane Gross, PhD, senior vice president and chief scientific officer for Aptevo.
Dr Gross and her colleagues found that APVO436 binds human CD123 and CD3-expressing cells and has “potent” target-specific activity against CD123-expressing AML cell lines (Molm-13 and KG-1a).
In addition, APVO436 induced endogenous T-cell activation and proliferation, accompanied by depletion of CD123-expressing cells, in samples from AML patients and healthy donors.
T cells from these cultures (both AML and non-AML) were expanded and co-cultured with Molm-13 cells and APVO436 or a control antibody. Again, the researchers observed “potent” cytotoxic activity in the presence of APVO436.
Dr Gross and her colleagues also tested APVO436, co-administered with human T cells, in mice with established disseminated Molm-13 tumors. The treatment resulted in a “rapid and significant” reduction in skeletal tumor burden.
Finally, the team compared APVO436 with an Aptevo-generated version of MGD006, a CD123 x CD3 dual-affinity re-targeting molecule being developed by Macrogenics, Inc.
The researchers took purified T cells from healthy donors and cultured them with Molm-13 cells, as well as APVO436, Aptevo’s version of MGD006, and a control antibody.
Both APVO436 and Aptevo’s version of MGD006 were effective at stimulating a tumor-directed immune response, inducing comparable levels of T-cell activation, proliferation, and cytotoxicity.
However, APVO436 induced lower levels of several cytokines—including IFNγ, IL-2, IL-6, and TNFα.
“Importantly, IFNγ, IL-6, and TNFα are considered to be the most relevant cytokines responsible for dosing toxicities observed in clinical studies with T-cell engaging molecules, which suggests that APVO436 could offer the potential for reduced toxicities compared to other CD123 x CD3 T-cell engagers at comparable or higher doses,” Dr Gross said.
She added that Aptevo is planning to launch a phase 1 trial of APVO436 in patients with AML and myelodysplastic syndromes later this year.
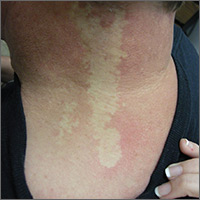
Light skin on back of neck
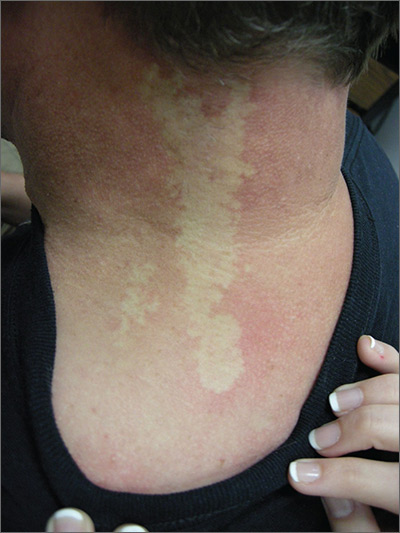
The FP diagnosed nevus anemicus in this patient.
Nevus anemicus is a congenital hypopigmented macule or patch that is stable in relative size and distribution. It occurs as a result of localized hypersensitivity to catecholamines and not as a result of a decrease in melanocytes. The localized hypersensitivity to catecholamines causes the affected area to stay lighter than the surrounding skin. On diascopy (pressure with a glass slide), the skin is indistinguishable from the surrounding skin.
There is no treatment for this benign condition, and it is not associated with any underlying malignancy or other health problem.
Photo courtesy of the University of Texas Health, San Antonio and text courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, Chumley H. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP diagnosed nevus anemicus in this patient.
Nevus anemicus is a congenital hypopigmented macule or patch that is stable in relative size and distribution. It occurs as a result of localized hypersensitivity to catecholamines and not as a result of a decrease in melanocytes. The localized hypersensitivity to catecholamines causes the affected area to stay lighter than the surrounding skin. On diascopy (pressure with a glass slide), the skin is indistinguishable from the surrounding skin.
There is no treatment for this benign condition, and it is not associated with any underlying malignancy or other health problem.
Photo courtesy of the University of Texas Health, San Antonio and text courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, Chumley H. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP diagnosed nevus anemicus in this patient.
Nevus anemicus is a congenital hypopigmented macule or patch that is stable in relative size and distribution. It occurs as a result of localized hypersensitivity to catecholamines and not as a result of a decrease in melanocytes. The localized hypersensitivity to catecholamines causes the affected area to stay lighter than the surrounding skin. On diascopy (pressure with a glass slide), the skin is indistinguishable from the surrounding skin.
There is no treatment for this benign condition, and it is not associated with any underlying malignancy or other health problem.
Photo courtesy of the University of Texas Health, San Antonio and text courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, Chumley H. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
No increased intussusception risk from rotavirus vaccine in Africa
Neither the first nor second dose of the monovalent rotavirus vaccine increased the risk of intussusception in the 3 weeks after immunization, a recent study found.
“This finding contrasts with previous studies in high- and upper-middle-income countries, in which an association with intussusception was found,” Jacqueline E. Tate, PhD, of the Centers for Disease Control and Prevention, and her associates reported in the New England Journal of Medicine.
“Given these large health benefits, the absence of increased risk of intussusception after RV1 [monovalent Rotarix vaccine] administration in our study is reassuring,” the authors wrote.
An African Intussusception Surveillance Network at 29 hospitals in Ethiopia, Ghana, Kenya, Malawi, Tanzania, Zambia and Zimbabwe enrolled 1,060 infants younger than age 12 months who experienced intussusception during Feb. 2012-Dec. 2016.
The researchers excluded infants without confirmed record of rotavirus vaccination status or who developed intussusception symptoms when younger than 28 days or older than 245 days. A little more than a third of the remaining 717 infants (36%) were from Ghana, more than half (61%) were male, and their median age was 25 weeks. Only 2% of the children had never received breast milk before developing intussusception symptoms.
The researchers used vaccine cards and clinic records to determine the rotavirus vaccination status for those 717 children with intussusception. The majority of the children (84%) had received both doses of the monovalent rotavirus vaccine. A total of 6% had received only one dose, and 10% received none. Five children received at least one rotavirus vaccine dose after having had intussusception already.
One intussusception case occurred within the first 7 days after the first vaccine dose, and five cases occurred within a week of the second dose. These incidences were no higher than was the background rate of intussusception, so no increased risk of intussusception in the week after either dose was identified, the researchers said.
The relative incidence of intussusception for dose one during days 1-7 was 0.25 (95% confidence interval, less than .001-1.16), and the relative incidence of intussusception for dose two during days 1-7 was 0.76 (95% CI, 0.16-1.87), Dr. Tate and her associates said.
Incidence of intussusception during the period 8-21 days after vaccination included 6 cases after the first dose and 16 cases after the second dose. Intussusception risk in this extended postvaccination period also was no higher than background risk.
“No clustering of cases occurred in any of the risk windows (1-7 days, 8-21 days, or 1-21 days) after receipt of either dose of RV1,” the authors reported.
They offered several possible reasons why no increased intussusception risk with rotavirus vaccination occurred in these countries despite studies in middle- and high-income countries showing an increased risk.
“First, although the exact mechanism is not known, intussusception may be related to intestinal replication of the orally administered, live-vaccine rotavirus strain,” Dr. Tate and her colleagues wrote. “Because oral rotavirus vaccines are less efficacious and shedding of vaccine virus – a potential marker of vaccine replication – is less frequently detected in low-income countries than in high- and middle-income countries, rotavirus vaccination might also be associated with a lower intussusception risk in low-income countries.”
Coadministration of rotavirus vaccination with the first dose of oral polio vaccine, which can reduce the rotavirus vaccine’s immunogenicity, also may play a role. Further, the children in this study were vaccinated against rotavirus at age 6- and 10-weeks-old – earlier than the 8 and 16 weeks in middle- and high-income countries – and intussusception is less common under 2 months old, potentially reducing likelihood of an association. Diet, breastfeeding practices, microbiome, maternal antibody levels, or other factors also may be at play.
The research was funded by the Gavi Alliance through the CDC Foundation. Dr. Cunliffe and Dr. Lopman have received personal fees from GlaxoSmithKline and Takeda Pharmaceutical, respectively. The other authors had no disclosures.
SOURCE: Tate JE et al. N Engl J Med. 2018;378:1521-8.
Neither the first nor second dose of the monovalent rotavirus vaccine increased the risk of intussusception in the 3 weeks after immunization, a recent study found.
“This finding contrasts with previous studies in high- and upper-middle-income countries, in which an association with intussusception was found,” Jacqueline E. Tate, PhD, of the Centers for Disease Control and Prevention, and her associates reported in the New England Journal of Medicine.
“Given these large health benefits, the absence of increased risk of intussusception after RV1 [monovalent Rotarix vaccine] administration in our study is reassuring,” the authors wrote.
An African Intussusception Surveillance Network at 29 hospitals in Ethiopia, Ghana, Kenya, Malawi, Tanzania, Zambia and Zimbabwe enrolled 1,060 infants younger than age 12 months who experienced intussusception during Feb. 2012-Dec. 2016.
The researchers excluded infants without confirmed record of rotavirus vaccination status or who developed intussusception symptoms when younger than 28 days or older than 245 days. A little more than a third of the remaining 717 infants (36%) were from Ghana, more than half (61%) were male, and their median age was 25 weeks. Only 2% of the children had never received breast milk before developing intussusception symptoms.
The researchers used vaccine cards and clinic records to determine the rotavirus vaccination status for those 717 children with intussusception. The majority of the children (84%) had received both doses of the monovalent rotavirus vaccine. A total of 6% had received only one dose, and 10% received none. Five children received at least one rotavirus vaccine dose after having had intussusception already.
One intussusception case occurred within the first 7 days after the first vaccine dose, and five cases occurred within a week of the second dose. These incidences were no higher than was the background rate of intussusception, so no increased risk of intussusception in the week after either dose was identified, the researchers said.
The relative incidence of intussusception for dose one during days 1-7 was 0.25 (95% confidence interval, less than .001-1.16), and the relative incidence of intussusception for dose two during days 1-7 was 0.76 (95% CI, 0.16-1.87), Dr. Tate and her associates said.
Incidence of intussusception during the period 8-21 days after vaccination included 6 cases after the first dose and 16 cases after the second dose. Intussusception risk in this extended postvaccination period also was no higher than background risk.
“No clustering of cases occurred in any of the risk windows (1-7 days, 8-21 days, or 1-21 days) after receipt of either dose of RV1,” the authors reported.
They offered several possible reasons why no increased intussusception risk with rotavirus vaccination occurred in these countries despite studies in middle- and high-income countries showing an increased risk.
“First, although the exact mechanism is not known, intussusception may be related to intestinal replication of the orally administered, live-vaccine rotavirus strain,” Dr. Tate and her colleagues wrote. “Because oral rotavirus vaccines are less efficacious and shedding of vaccine virus – a potential marker of vaccine replication – is less frequently detected in low-income countries than in high- and middle-income countries, rotavirus vaccination might also be associated with a lower intussusception risk in low-income countries.”
Coadministration of rotavirus vaccination with the first dose of oral polio vaccine, which can reduce the rotavirus vaccine’s immunogenicity, also may play a role. Further, the children in this study were vaccinated against rotavirus at age 6- and 10-weeks-old – earlier than the 8 and 16 weeks in middle- and high-income countries – and intussusception is less common under 2 months old, potentially reducing likelihood of an association. Diet, breastfeeding practices, microbiome, maternal antibody levels, or other factors also may be at play.
The research was funded by the Gavi Alliance through the CDC Foundation. Dr. Cunliffe and Dr. Lopman have received personal fees from GlaxoSmithKline and Takeda Pharmaceutical, respectively. The other authors had no disclosures.
SOURCE: Tate JE et al. N Engl J Med. 2018;378:1521-8.
Neither the first nor second dose of the monovalent rotavirus vaccine increased the risk of intussusception in the 3 weeks after immunization, a recent study found.
“This finding contrasts with previous studies in high- and upper-middle-income countries, in which an association with intussusception was found,” Jacqueline E. Tate, PhD, of the Centers for Disease Control and Prevention, and her associates reported in the New England Journal of Medicine.
“Given these large health benefits, the absence of increased risk of intussusception after RV1 [monovalent Rotarix vaccine] administration in our study is reassuring,” the authors wrote.
An African Intussusception Surveillance Network at 29 hospitals in Ethiopia, Ghana, Kenya, Malawi, Tanzania, Zambia and Zimbabwe enrolled 1,060 infants younger than age 12 months who experienced intussusception during Feb. 2012-Dec. 2016.
The researchers excluded infants without confirmed record of rotavirus vaccination status or who developed intussusception symptoms when younger than 28 days or older than 245 days. A little more than a third of the remaining 717 infants (36%) were from Ghana, more than half (61%) were male, and their median age was 25 weeks. Only 2% of the children had never received breast milk before developing intussusception symptoms.
The researchers used vaccine cards and clinic records to determine the rotavirus vaccination status for those 717 children with intussusception. The majority of the children (84%) had received both doses of the monovalent rotavirus vaccine. A total of 6% had received only one dose, and 10% received none. Five children received at least one rotavirus vaccine dose after having had intussusception already.
One intussusception case occurred within the first 7 days after the first vaccine dose, and five cases occurred within a week of the second dose. These incidences were no higher than was the background rate of intussusception, so no increased risk of intussusception in the week after either dose was identified, the researchers said.
The relative incidence of intussusception for dose one during days 1-7 was 0.25 (95% confidence interval, less than .001-1.16), and the relative incidence of intussusception for dose two during days 1-7 was 0.76 (95% CI, 0.16-1.87), Dr. Tate and her associates said.
Incidence of intussusception during the period 8-21 days after vaccination included 6 cases after the first dose and 16 cases after the second dose. Intussusception risk in this extended postvaccination period also was no higher than background risk.
“No clustering of cases occurred in any of the risk windows (1-7 days, 8-21 days, or 1-21 days) after receipt of either dose of RV1,” the authors reported.
They offered several possible reasons why no increased intussusception risk with rotavirus vaccination occurred in these countries despite studies in middle- and high-income countries showing an increased risk.
“First, although the exact mechanism is not known, intussusception may be related to intestinal replication of the orally administered, live-vaccine rotavirus strain,” Dr. Tate and her colleagues wrote. “Because oral rotavirus vaccines are less efficacious and shedding of vaccine virus – a potential marker of vaccine replication – is less frequently detected in low-income countries than in high- and middle-income countries, rotavirus vaccination might also be associated with a lower intussusception risk in low-income countries.”
Coadministration of rotavirus vaccination with the first dose of oral polio vaccine, which can reduce the rotavirus vaccine’s immunogenicity, also may play a role. Further, the children in this study were vaccinated against rotavirus at age 6- and 10-weeks-old – earlier than the 8 and 16 weeks in middle- and high-income countries – and intussusception is less common under 2 months old, potentially reducing likelihood of an association. Diet, breastfeeding practices, microbiome, maternal antibody levels, or other factors also may be at play.
The research was funded by the Gavi Alliance through the CDC Foundation. Dr. Cunliffe and Dr. Lopman have received personal fees from GlaxoSmithKline and Takeda Pharmaceutical, respectively. The other authors had no disclosures.
SOURCE: Tate JE et al. N Engl J Med. 2018;378:1521-8.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The relative incidence of intussusception for dose one during days 1-7 was 0.25 (95% confidence interval, less than .001-1.16), and the relative incidence of intussusception for dose two during days 1-7 was 0.76 (95% CI, 0.16-1.87).
Data source: The findings are based on a self-controlled case-series study involving 717 infants with intussusception and confirmed status of rotavirus vaccination, from Ethiopia, Ghana, Kenya, Malawi, Tanzania, Zambia, and Zimbabwe.
Disclosures: The research was funded by the Gavi Alliance through the CDC Foundation. Dr. Cunliffe and Dr. Lopman have received personal fees from GlaxoSmithKline and Takeda Pharmaceutical, respectively. The other authors had no disclosures.
Source: Tate JE et al. N Engl J Med. 2018;378:1521-8.
24-hour ambulatory BP measurements strongly predict mortality
Ambulatory measurements of blood pressure more strongly predicted all-cause and cardiovascular mortality than did BP measured in the clinic, according to analysis of a large patient registry in Spain.
The results also showed an increased risk of death associated with white coat hypertension and an even stronger association between death and masked hypertension. They were published in the New England Journal of Medicine.
Previous investigations had found that 24-hour ambulatory BP measurements were better predictors of patient outcomes than those obtained in the clinic or at home, but those investigations were small or population based.“In these studies, the number of clinical outcomes was limited, which reduced the ability to assess the predictive value of clinic blood pressure data as compared with ambulatory data,” reported José R. Banegas, MD, of the department of preventive medicine and public health at the Autonomous University of Madrid and his colleagues.
To better define the prognostic value of 24-hour ambulatory blood pressure measurement, Dr. Banegas and his colleagues looked at data on a large cohort of primary care patients in the Spanish Ambulatory Blood Pressure Registry. Their analysis included 63,910 adults recruited to the registry during 2004-2014.
Patients had blood pressure measurements taken in the clinic according to standard procedures. Afterward, they had ambulatory blood pressure monitoring that used an automated device programmed to record BP every 20 minutes during the day and every 30 minutes at night.
They found that overall clinic and ambulatory blood pressure measurements had a relatively similar magnitude of association with all-cause and cardiovascular mortality.
However, clinic systolic pressure lost its predictive power for all-cause mortality after adjustment for 24-hour ambulatory systolic pressure. The hazard ratio for all-cause mortality dropped from 1.54 before the adjustment to 1.02 after the adjustment, Dr. Banegas and his colleagues reported.
By contrast, ambulatory systolic pressure kept its predictive value after accounting for clinical systolic pressure, with a hazard ratio for all-cause mortality of 1.58 before and after the adjustment, they said in the report.
The strongest association with all-cause mortality was found in patients with masked hypertension – normal clinic readings but elevated ambulatory readings. The hazard ratio for all-cause mortality in that group was 2.83 when adjusted for clinic blood pressure, with similar findings reported for cardiovascular mortality.
White coat hypertension was also associated with increased risk of mortality. The finding of elevated clinic BP and normal 24-hour ambulatory BP had a hazard ratio of 1.79 for all-cause mortality after adjustment for clinic BP, results showed.
“In our study, white coat hypertension was not benign, which may be due in part to the higher mean blood pressure over 24 hours in these patients (119.9/71.9 mm Hg vs. 116.6/70.6 mm Hg in normotensive patients; P less than .001) or to their metabolic phenotype,” the investigators wrote.
Lacer Laboratories, the Spanish Society of Hypertension, and some European government agencies supported the study. Dr. Banegas reported grants from Fondo de Investigación Sanitaria and personal fees from Lacer. Coauthors reported disclosures related to Vascular Dynamics USA, Relypsa USA, Novartis Pharma USA, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, Lacer Laboratories Spain, and others.
SOURCE: Banegas JR et al. N Engl J Med. 2018;378:1509-20.
The investigation by Dr. Banegas and colleagues confirms that ambulatory blood pressure monitoring is useful for assessing blood pressure, the most important and treatable factor contributing to death and disability.
The registry study addresses several clinically relevant issues. In particular, ambulatory blood pressure measures more strongly predicted all-cause and cardiovascular mortality as compared with blood pressure measured in the clinic.
Moreover, the highest hazard ratio of death was seen in patients with masked hypertension, or those with normal clinic-measured blood pressure but elevated ambulatory measurements.
Finally, patients with white coat hypertension (elevated clinic but normal ambulatory blood pressure) had a risk of cardiovascular death twice as high as patients with normal clinic and ambulatory values.
The ominous effect of white coat hypertension has been noted by others, and it is probably related to the increasing magnitude (that is, the difference between clinic blood pressure and ambulatory blood pressure) to white coat hypertension with age.
Ambulatory blood pressure monitoring equipment has evolved and is much lighter than in the past, making it more acceptable to patients.
With more patients undergoing ambulatory blood pressure monitoring, several countries established ambulatory monitoring registries, such as the Spanish registry evaluated in this study.
Ultimately, one hopes the results of this registry study would serve as one more spur to providers and device manufacturers to initiate a registry in the United States.
Raymond R. Townsend, MD, is from the University of Pennsylvania, Philadelphia. These comments are based on his editorial that appeared in the New England Journal of Medicine . Dr. Townsend reported disclosures related to Medtronic, AXIO, and CLARUS Therapeutics, among others.
The investigation by Dr. Banegas and colleagues confirms that ambulatory blood pressure monitoring is useful for assessing blood pressure, the most important and treatable factor contributing to death and disability.
The registry study addresses several clinically relevant issues. In particular, ambulatory blood pressure measures more strongly predicted all-cause and cardiovascular mortality as compared with blood pressure measured in the clinic.
Moreover, the highest hazard ratio of death was seen in patients with masked hypertension, or those with normal clinic-measured blood pressure but elevated ambulatory measurements.
Finally, patients with white coat hypertension (elevated clinic but normal ambulatory blood pressure) had a risk of cardiovascular death twice as high as patients with normal clinic and ambulatory values.
The ominous effect of white coat hypertension has been noted by others, and it is probably related to the increasing magnitude (that is, the difference between clinic blood pressure and ambulatory blood pressure) to white coat hypertension with age.
Ambulatory blood pressure monitoring equipment has evolved and is much lighter than in the past, making it more acceptable to patients.
With more patients undergoing ambulatory blood pressure monitoring, several countries established ambulatory monitoring registries, such as the Spanish registry evaluated in this study.
Ultimately, one hopes the results of this registry study would serve as one more spur to providers and device manufacturers to initiate a registry in the United States.
Raymond R. Townsend, MD, is from the University of Pennsylvania, Philadelphia. These comments are based on his editorial that appeared in the New England Journal of Medicine . Dr. Townsend reported disclosures related to Medtronic, AXIO, and CLARUS Therapeutics, among others.
The investigation by Dr. Banegas and colleagues confirms that ambulatory blood pressure monitoring is useful for assessing blood pressure, the most important and treatable factor contributing to death and disability.
The registry study addresses several clinically relevant issues. In particular, ambulatory blood pressure measures more strongly predicted all-cause and cardiovascular mortality as compared with blood pressure measured in the clinic.
Moreover, the highest hazard ratio of death was seen in patients with masked hypertension, or those with normal clinic-measured blood pressure but elevated ambulatory measurements.
Finally, patients with white coat hypertension (elevated clinic but normal ambulatory blood pressure) had a risk of cardiovascular death twice as high as patients with normal clinic and ambulatory values.
The ominous effect of white coat hypertension has been noted by others, and it is probably related to the increasing magnitude (that is, the difference between clinic blood pressure and ambulatory blood pressure) to white coat hypertension with age.
Ambulatory blood pressure monitoring equipment has evolved and is much lighter than in the past, making it more acceptable to patients.
With more patients undergoing ambulatory blood pressure monitoring, several countries established ambulatory monitoring registries, such as the Spanish registry evaluated in this study.
Ultimately, one hopes the results of this registry study would serve as one more spur to providers and device manufacturers to initiate a registry in the United States.
Raymond R. Townsend, MD, is from the University of Pennsylvania, Philadelphia. These comments are based on his editorial that appeared in the New England Journal of Medicine . Dr. Townsend reported disclosures related to Medtronic, AXIO, and CLARUS Therapeutics, among others.
Ambulatory measurements of blood pressure more strongly predicted all-cause and cardiovascular mortality than did BP measured in the clinic, according to analysis of a large patient registry in Spain.
The results also showed an increased risk of death associated with white coat hypertension and an even stronger association between death and masked hypertension. They were published in the New England Journal of Medicine.
Previous investigations had found that 24-hour ambulatory BP measurements were better predictors of patient outcomes than those obtained in the clinic or at home, but those investigations were small or population based.“In these studies, the number of clinical outcomes was limited, which reduced the ability to assess the predictive value of clinic blood pressure data as compared with ambulatory data,” reported José R. Banegas, MD, of the department of preventive medicine and public health at the Autonomous University of Madrid and his colleagues.
To better define the prognostic value of 24-hour ambulatory blood pressure measurement, Dr. Banegas and his colleagues looked at data on a large cohort of primary care patients in the Spanish Ambulatory Blood Pressure Registry. Their analysis included 63,910 adults recruited to the registry during 2004-2014.
Patients had blood pressure measurements taken in the clinic according to standard procedures. Afterward, they had ambulatory blood pressure monitoring that used an automated device programmed to record BP every 20 minutes during the day and every 30 minutes at night.
They found that overall clinic and ambulatory blood pressure measurements had a relatively similar magnitude of association with all-cause and cardiovascular mortality.
However, clinic systolic pressure lost its predictive power for all-cause mortality after adjustment for 24-hour ambulatory systolic pressure. The hazard ratio for all-cause mortality dropped from 1.54 before the adjustment to 1.02 after the adjustment, Dr. Banegas and his colleagues reported.
By contrast, ambulatory systolic pressure kept its predictive value after accounting for clinical systolic pressure, with a hazard ratio for all-cause mortality of 1.58 before and after the adjustment, they said in the report.
The strongest association with all-cause mortality was found in patients with masked hypertension – normal clinic readings but elevated ambulatory readings. The hazard ratio for all-cause mortality in that group was 2.83 when adjusted for clinic blood pressure, with similar findings reported for cardiovascular mortality.
White coat hypertension was also associated with increased risk of mortality. The finding of elevated clinic BP and normal 24-hour ambulatory BP had a hazard ratio of 1.79 for all-cause mortality after adjustment for clinic BP, results showed.
“In our study, white coat hypertension was not benign, which may be due in part to the higher mean blood pressure over 24 hours in these patients (119.9/71.9 mm Hg vs. 116.6/70.6 mm Hg in normotensive patients; P less than .001) or to their metabolic phenotype,” the investigators wrote.
Lacer Laboratories, the Spanish Society of Hypertension, and some European government agencies supported the study. Dr. Banegas reported grants from Fondo de Investigación Sanitaria and personal fees from Lacer. Coauthors reported disclosures related to Vascular Dynamics USA, Relypsa USA, Novartis Pharma USA, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, Lacer Laboratories Spain, and others.
SOURCE: Banegas JR et al. N Engl J Med. 2018;378:1509-20.
Ambulatory measurements of blood pressure more strongly predicted all-cause and cardiovascular mortality than did BP measured in the clinic, according to analysis of a large patient registry in Spain.
The results also showed an increased risk of death associated with white coat hypertension and an even stronger association between death and masked hypertension. They were published in the New England Journal of Medicine.
Previous investigations had found that 24-hour ambulatory BP measurements were better predictors of patient outcomes than those obtained in the clinic or at home, but those investigations were small or population based.“In these studies, the number of clinical outcomes was limited, which reduced the ability to assess the predictive value of clinic blood pressure data as compared with ambulatory data,” reported José R. Banegas, MD, of the department of preventive medicine and public health at the Autonomous University of Madrid and his colleagues.
To better define the prognostic value of 24-hour ambulatory blood pressure measurement, Dr. Banegas and his colleagues looked at data on a large cohort of primary care patients in the Spanish Ambulatory Blood Pressure Registry. Their analysis included 63,910 adults recruited to the registry during 2004-2014.
Patients had blood pressure measurements taken in the clinic according to standard procedures. Afterward, they had ambulatory blood pressure monitoring that used an automated device programmed to record BP every 20 minutes during the day and every 30 minutes at night.
They found that overall clinic and ambulatory blood pressure measurements had a relatively similar magnitude of association with all-cause and cardiovascular mortality.
However, clinic systolic pressure lost its predictive power for all-cause mortality after adjustment for 24-hour ambulatory systolic pressure. The hazard ratio for all-cause mortality dropped from 1.54 before the adjustment to 1.02 after the adjustment, Dr. Banegas and his colleagues reported.
By contrast, ambulatory systolic pressure kept its predictive value after accounting for clinical systolic pressure, with a hazard ratio for all-cause mortality of 1.58 before and after the adjustment, they said in the report.
The strongest association with all-cause mortality was found in patients with masked hypertension – normal clinic readings but elevated ambulatory readings. The hazard ratio for all-cause mortality in that group was 2.83 when adjusted for clinic blood pressure, with similar findings reported for cardiovascular mortality.
White coat hypertension was also associated with increased risk of mortality. The finding of elevated clinic BP and normal 24-hour ambulatory BP had a hazard ratio of 1.79 for all-cause mortality after adjustment for clinic BP, results showed.
“In our study, white coat hypertension was not benign, which may be due in part to the higher mean blood pressure over 24 hours in these patients (119.9/71.9 mm Hg vs. 116.6/70.6 mm Hg in normotensive patients; P less than .001) or to their metabolic phenotype,” the investigators wrote.
Lacer Laboratories, the Spanish Society of Hypertension, and some European government agencies supported the study. Dr. Banegas reported grants from Fondo de Investigación Sanitaria and personal fees from Lacer. Coauthors reported disclosures related to Vascular Dynamics USA, Relypsa USA, Novartis Pharma USA, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, Lacer Laboratories Spain, and others.
SOURCE: Banegas JR et al. N Engl J Med. 2018;378:1509-20.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Modeling showed a stronger association between ambulatory systolic pressure and all-cause mortality (adjusted HR, 1.58 per 1-SD pressure increase) than between clinic systolic pressure and all-cause mortality (adjusted HR, 1.02).
Study details: Retrospective analysis of mortality from a cohort of 63,910 adults recruited to a registry in Spain during 2004-2014.
Disclosures: Lacer Laboratories, the Spanish Society of Hypertension, and some European government agencies supported the study. Dr. Banegas reported grants from Fondo de Investigación Sanitaria and personal fees from Lacer. Coauthors reported disclosures related to Vascular Dynamics USA, Relypsa USA, Novartis Pharma USA, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, Lacer Laboratories Spain, and others.
Source: Banegas JR et al. N Engl J Med 2018;378:1509-20.
Doctors call for a pause to rethink MIPS measures
A “time-out” is needed to reevaluate how quality measures are used as part of Medicare’s Merit-based Incentive Payment System (MIPS), according to officials at the American College of Physicians.
according to ACP criteria.
The quality measures were assessed regarding importance, appropriate care, clinical evidence base, measure specifications, and measure feasibility and applicability.
“We also determined that the proportion of the measures that had been developed by the National Committee for Quality Assurance [NCQA] or endorsed by the National Quality Forum [NQF] that were rated as valid by our method,” Dr. MacLean and colleagues wrote. “As compared with measures that were not endorsed by these organizations, greater percentages of NCQA-developed and NQF-endorsed measures were deemed valid [59% and 48%, respectively, vs. 27% for nonendorsed measures], and smaller percentages were deemed not valid [7% and 22% vs. 49% for nonendorsed measures].”
The lack of measures that were found to be valid for primary care is frustrating for doctors and could cause harm to patients, according to the authors.
“We need a time-out during which to assess and revise our approach to physician performance measurement,” they wrote.
The ACP recommends that “physicians with expertise in clinical medicine and research develop measures using clinically relevant methodology,” President Jack Ende said in a statement. “Performance measures should be fully integrated into care delivery so they can help address the most pressing performance gaps and direct quality improvement.”
The time-out call comes amidst differing opinions on how to proceed with the MIPS track of the Quality Payment Program. The Medicare Payment Advisory Commission has recommended to Congress that MIPS be repealed and replaced, while health care experts and physician associations believe the program should stay the course, given the investments that have been made already to accommodate the program’s reporting requirements.
SOURCE: MacLean CH et al. New Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMp1802595.
A “time-out” is needed to reevaluate how quality measures are used as part of Medicare’s Merit-based Incentive Payment System (MIPS), according to officials at the American College of Physicians.
according to ACP criteria.
The quality measures were assessed regarding importance, appropriate care, clinical evidence base, measure specifications, and measure feasibility and applicability.
“We also determined that the proportion of the measures that had been developed by the National Committee for Quality Assurance [NCQA] or endorsed by the National Quality Forum [NQF] that were rated as valid by our method,” Dr. MacLean and colleagues wrote. “As compared with measures that were not endorsed by these organizations, greater percentages of NCQA-developed and NQF-endorsed measures were deemed valid [59% and 48%, respectively, vs. 27% for nonendorsed measures], and smaller percentages were deemed not valid [7% and 22% vs. 49% for nonendorsed measures].”
The lack of measures that were found to be valid for primary care is frustrating for doctors and could cause harm to patients, according to the authors.
“We need a time-out during which to assess and revise our approach to physician performance measurement,” they wrote.
The ACP recommends that “physicians with expertise in clinical medicine and research develop measures using clinically relevant methodology,” President Jack Ende said in a statement. “Performance measures should be fully integrated into care delivery so they can help address the most pressing performance gaps and direct quality improvement.”
The time-out call comes amidst differing opinions on how to proceed with the MIPS track of the Quality Payment Program. The Medicare Payment Advisory Commission has recommended to Congress that MIPS be repealed and replaced, while health care experts and physician associations believe the program should stay the course, given the investments that have been made already to accommodate the program’s reporting requirements.
SOURCE: MacLean CH et al. New Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMp1802595.
A “time-out” is needed to reevaluate how quality measures are used as part of Medicare’s Merit-based Incentive Payment System (MIPS), according to officials at the American College of Physicians.
according to ACP criteria.
The quality measures were assessed regarding importance, appropriate care, clinical evidence base, measure specifications, and measure feasibility and applicability.
“We also determined that the proportion of the measures that had been developed by the National Committee for Quality Assurance [NCQA] or endorsed by the National Quality Forum [NQF] that were rated as valid by our method,” Dr. MacLean and colleagues wrote. “As compared with measures that were not endorsed by these organizations, greater percentages of NCQA-developed and NQF-endorsed measures were deemed valid [59% and 48%, respectively, vs. 27% for nonendorsed measures], and smaller percentages were deemed not valid [7% and 22% vs. 49% for nonendorsed measures].”
The lack of measures that were found to be valid for primary care is frustrating for doctors and could cause harm to patients, according to the authors.
“We need a time-out during which to assess and revise our approach to physician performance measurement,” they wrote.
The ACP recommends that “physicians with expertise in clinical medicine and research develop measures using clinically relevant methodology,” President Jack Ende said in a statement. “Performance measures should be fully integrated into care delivery so they can help address the most pressing performance gaps and direct quality improvement.”
The time-out call comes amidst differing opinions on how to proceed with the MIPS track of the Quality Payment Program. The Medicare Payment Advisory Commission has recommended to Congress that MIPS be repealed and replaced, while health care experts and physician associations believe the program should stay the course, given the investments that have been made already to accommodate the program’s reporting requirements.
SOURCE: MacLean CH et al. New Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMp1802595.
FROM NEW ENGLAND JOURNAL OF MEDICINE
JADAS appears best for measuring various aspects of JIA activity
Applying clinical Juvenile Arthritis Disease Activity Score criteria to identify clinically inactive disease in patients with juvenile idiopathic arthritis (JIA) resulted in patients with better long-term functional activity and psychosocial health outcomes, compared with patients whose disease state was assessed using Wallace’s preliminary criteria, according to recent research from the multicenter Childhood Arthritis Prospective Study.
“A challenge is in understanding how best to apply these results in the clinical setting,” wrote Stephanie J.W. Shoop-Worrall of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) and her coauthors. “As achievement of CID [clinically inactive disease] according to cJADAS10 [clinical Juvenile Arthritis Disease Activity Score assessed in 10 joints] was associated with equivalent or superior outcomes to Wallace’s preliminary criteria and it is more feasible to complete in clinical practice, due to containing only three routinely collected components, one could argue that this is likely to be a superior treatment target for application in clinical practice.”
Investigators applied the cJADAS10 to determine MDA, while CID was assessed using both cJADAS10 and Wallace’s preliminary criteria at 1-year follow-up. Children were categorized based on whether they achieved CID according to cJADAS10 only, whether they did so according to Wallace’s preliminary criteria only, whether they did so according to both cJADAS10 and Wallace’s preliminary criteria, or whether they did not achieve CID according to either; they also were categorized based on whether they achieved MDA but not CID according to cJADAS10. Researchers examined patient function, limited joints, and psychosocial health, as well as annual pain between 1-year and 5-year follow-up. The results were recently published in Arthritis & Rheumatology.
“Wallace’s preliminary criteria includes five components, observed or measured by a physician, which must all be absent or in the normal range, but do not include an assessment by the patient or their proxy,” Ms. Shoop-Worrall and her colleagues wrote. “In contrast, the JADAS and cJADAS include fewer overall components, meaning they may be easier to complete in a routine clinical setting, but do include a patient or proxy subjective assessment of patient wellbeing.”
Of the patients analyzed, 68% had oligoarthritis, while 27% had RF-negative and 5% had RF-positive polyarticular JIA. Over half (56%) of patients did not achieve CID, while 21% had achieved CID through both definitions. A further 23% of patients achieved CID in only one definition – 16% of patients according to cJADAS10 and 7% of patients according to Wallace’s preliminary criteria. Patients who achieved CID had significantly increased odds of having no limited joints according to either cJADAS10 (odds ratio, 3.9; 95% confidence interval, 2.5-6.3) or Wallace’s preliminary criteria (OR, 7.5; 95% CI, 2.9-19.2). Patients had better Child Health Questionnaire psychosocial scores when they achieved CID according to either cJADAS10 (coefficient, 5.3; 95% CI, 0.5-10.1) or both cJADAS10 and Wallace’s preliminary criteria (coefficient, 5.5; 95% CI, 2.2-9.5).
When patients’ function was assessed using the Childhood Health Assessment questionnaire, they had significantly increased odds of having no disability recorded when they achieved CID with either cJADAS10 (OR, 4.5; 95% CI, 2.2-9.5) or both criteria (OR 5.2; 95% CI, 2.7-9.9). Patients assessed with Wallace’s preliminary criteria had “no better Child Health Questionnaire psychosocial scores or Childhood Health Assessment questionnaire scores than those with active disease at 1 year.” Most patients who achieved CID also achieved MDA, but 10% of patients reached MDA without achieving CID.
The researchers noted that reliance on either cJADAS10 or Wallace’s preliminary criteria alone may miss data needed for clinical treatment. Data obtained from cJADAS10 may lead to additional psychological and physiotherapy treatments not seen in Wallace’s preliminary criteria; however, “relying solely on Wallace’s preliminary criteria may guide immunosuppressive therapy very well, but may ignore other symptoms relevant to the patient.”
“As the two scores differ in their components one could argue that they are not capturing the same construct,” Ms. Shoop-Worrall and her associates wrote. “Wallace’s preliminary criteria capture more objective measures of inflammation, whilst the cJADAS10, through inclusion of a patient well-being measure, may also capture other noninflammatory components of the disease, such as chronic pain and fatigue, not captured by Wallace’s preliminary criteria.”
The authors reported having no relevant financial disclosures for this study.
SOURCE: Shoop‐Worrall SJW et al. Arthritis Rheumatol. 2018 April 12. doi: 10.1002/art.40519.
Applying clinical Juvenile Arthritis Disease Activity Score criteria to identify clinically inactive disease in patients with juvenile idiopathic arthritis (JIA) resulted in patients with better long-term functional activity and psychosocial health outcomes, compared with patients whose disease state was assessed using Wallace’s preliminary criteria, according to recent research from the multicenter Childhood Arthritis Prospective Study.
“A challenge is in understanding how best to apply these results in the clinical setting,” wrote Stephanie J.W. Shoop-Worrall of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) and her coauthors. “As achievement of CID [clinically inactive disease] according to cJADAS10 [clinical Juvenile Arthritis Disease Activity Score assessed in 10 joints] was associated with equivalent or superior outcomes to Wallace’s preliminary criteria and it is more feasible to complete in clinical practice, due to containing only three routinely collected components, one could argue that this is likely to be a superior treatment target for application in clinical practice.”
Investigators applied the cJADAS10 to determine MDA, while CID was assessed using both cJADAS10 and Wallace’s preliminary criteria at 1-year follow-up. Children were categorized based on whether they achieved CID according to cJADAS10 only, whether they did so according to Wallace’s preliminary criteria only, whether they did so according to both cJADAS10 and Wallace’s preliminary criteria, or whether they did not achieve CID according to either; they also were categorized based on whether they achieved MDA but not CID according to cJADAS10. Researchers examined patient function, limited joints, and psychosocial health, as well as annual pain between 1-year and 5-year follow-up. The results were recently published in Arthritis & Rheumatology.
“Wallace’s preliminary criteria includes five components, observed or measured by a physician, which must all be absent or in the normal range, but do not include an assessment by the patient or their proxy,” Ms. Shoop-Worrall and her colleagues wrote. “In contrast, the JADAS and cJADAS include fewer overall components, meaning they may be easier to complete in a routine clinical setting, but do include a patient or proxy subjective assessment of patient wellbeing.”
Of the patients analyzed, 68% had oligoarthritis, while 27% had RF-negative and 5% had RF-positive polyarticular JIA. Over half (56%) of patients did not achieve CID, while 21% had achieved CID through both definitions. A further 23% of patients achieved CID in only one definition – 16% of patients according to cJADAS10 and 7% of patients according to Wallace’s preliminary criteria. Patients who achieved CID had significantly increased odds of having no limited joints according to either cJADAS10 (odds ratio, 3.9; 95% confidence interval, 2.5-6.3) or Wallace’s preliminary criteria (OR, 7.5; 95% CI, 2.9-19.2). Patients had better Child Health Questionnaire psychosocial scores when they achieved CID according to either cJADAS10 (coefficient, 5.3; 95% CI, 0.5-10.1) or both cJADAS10 and Wallace’s preliminary criteria (coefficient, 5.5; 95% CI, 2.2-9.5).
When patients’ function was assessed using the Childhood Health Assessment questionnaire, they had significantly increased odds of having no disability recorded when they achieved CID with either cJADAS10 (OR, 4.5; 95% CI, 2.2-9.5) or both criteria (OR 5.2; 95% CI, 2.7-9.9). Patients assessed with Wallace’s preliminary criteria had “no better Child Health Questionnaire psychosocial scores or Childhood Health Assessment questionnaire scores than those with active disease at 1 year.” Most patients who achieved CID also achieved MDA, but 10% of patients reached MDA without achieving CID.
The researchers noted that reliance on either cJADAS10 or Wallace’s preliminary criteria alone may miss data needed for clinical treatment. Data obtained from cJADAS10 may lead to additional psychological and physiotherapy treatments not seen in Wallace’s preliminary criteria; however, “relying solely on Wallace’s preliminary criteria may guide immunosuppressive therapy very well, but may ignore other symptoms relevant to the patient.”
“As the two scores differ in their components one could argue that they are not capturing the same construct,” Ms. Shoop-Worrall and her associates wrote. “Wallace’s preliminary criteria capture more objective measures of inflammation, whilst the cJADAS10, through inclusion of a patient well-being measure, may also capture other noninflammatory components of the disease, such as chronic pain and fatigue, not captured by Wallace’s preliminary criteria.”
The authors reported having no relevant financial disclosures for this study.
SOURCE: Shoop‐Worrall SJW et al. Arthritis Rheumatol. 2018 April 12. doi: 10.1002/art.40519.
Applying clinical Juvenile Arthritis Disease Activity Score criteria to identify clinically inactive disease in patients with juvenile idiopathic arthritis (JIA) resulted in patients with better long-term functional activity and psychosocial health outcomes, compared with patients whose disease state was assessed using Wallace’s preliminary criteria, according to recent research from the multicenter Childhood Arthritis Prospective Study.
“A challenge is in understanding how best to apply these results in the clinical setting,” wrote Stephanie J.W. Shoop-Worrall of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) and her coauthors. “As achievement of CID [clinically inactive disease] according to cJADAS10 [clinical Juvenile Arthritis Disease Activity Score assessed in 10 joints] was associated with equivalent or superior outcomes to Wallace’s preliminary criteria and it is more feasible to complete in clinical practice, due to containing only three routinely collected components, one could argue that this is likely to be a superior treatment target for application in clinical practice.”
Investigators applied the cJADAS10 to determine MDA, while CID was assessed using both cJADAS10 and Wallace’s preliminary criteria at 1-year follow-up. Children were categorized based on whether they achieved CID according to cJADAS10 only, whether they did so according to Wallace’s preliminary criteria only, whether they did so according to both cJADAS10 and Wallace’s preliminary criteria, or whether they did not achieve CID according to either; they also were categorized based on whether they achieved MDA but not CID according to cJADAS10. Researchers examined patient function, limited joints, and psychosocial health, as well as annual pain between 1-year and 5-year follow-up. The results were recently published in Arthritis & Rheumatology.
“Wallace’s preliminary criteria includes five components, observed or measured by a physician, which must all be absent or in the normal range, but do not include an assessment by the patient or their proxy,” Ms. Shoop-Worrall and her colleagues wrote. “In contrast, the JADAS and cJADAS include fewer overall components, meaning they may be easier to complete in a routine clinical setting, but do include a patient or proxy subjective assessment of patient wellbeing.”
Of the patients analyzed, 68% had oligoarthritis, while 27% had RF-negative and 5% had RF-positive polyarticular JIA. Over half (56%) of patients did not achieve CID, while 21% had achieved CID through both definitions. A further 23% of patients achieved CID in only one definition – 16% of patients according to cJADAS10 and 7% of patients according to Wallace’s preliminary criteria. Patients who achieved CID had significantly increased odds of having no limited joints according to either cJADAS10 (odds ratio, 3.9; 95% confidence interval, 2.5-6.3) or Wallace’s preliminary criteria (OR, 7.5; 95% CI, 2.9-19.2). Patients had better Child Health Questionnaire psychosocial scores when they achieved CID according to either cJADAS10 (coefficient, 5.3; 95% CI, 0.5-10.1) or both cJADAS10 and Wallace’s preliminary criteria (coefficient, 5.5; 95% CI, 2.2-9.5).
When patients’ function was assessed using the Childhood Health Assessment questionnaire, they had significantly increased odds of having no disability recorded when they achieved CID with either cJADAS10 (OR, 4.5; 95% CI, 2.2-9.5) or both criteria (OR 5.2; 95% CI, 2.7-9.9). Patients assessed with Wallace’s preliminary criteria had “no better Child Health Questionnaire psychosocial scores or Childhood Health Assessment questionnaire scores than those with active disease at 1 year.” Most patients who achieved CID also achieved MDA, but 10% of patients reached MDA without achieving CID.
The researchers noted that reliance on either cJADAS10 or Wallace’s preliminary criteria alone may miss data needed for clinical treatment. Data obtained from cJADAS10 may lead to additional psychological and physiotherapy treatments not seen in Wallace’s preliminary criteria; however, “relying solely on Wallace’s preliminary criteria may guide immunosuppressive therapy very well, but may ignore other symptoms relevant to the patient.”
“As the two scores differ in their components one could argue that they are not capturing the same construct,” Ms. Shoop-Worrall and her associates wrote. “Wallace’s preliminary criteria capture more objective measures of inflammation, whilst the cJADAS10, through inclusion of a patient well-being measure, may also capture other noninflammatory components of the disease, such as chronic pain and fatigue, not captured by Wallace’s preliminary criteria.”
The authors reported having no relevant financial disclosures for this study.
SOURCE: Shoop‐Worrall SJW et al. Arthritis Rheumatol. 2018 April 12. doi: 10.1002/art.40519.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Defining CID state using clinical JADAS criteria as a treatment target improved functional ability and psychosocial health, compared with that of patients defined using Wallace’s preliminary criteria.
Major finding: At 1-year follow-up, 16% of children achieved CID with cJADAS10 only and 7% with Wallace’s preliminary criteria only, while 21% of patients achieved CID when both definitions were used.
Story details: A prospective inception cohort of 832 children from eight pediatric and adolescent rheumatology centers in the United Kingdom recruited to the Childhood Arthritis Prospective Study during 2001-2011.
Disclosures: The authors reported having no financial disclosures.
Source: Shoop‐Worrall SJW et al. Arthritis Rheumatol. 2018 April 12. doi: 10.1002/art.40519.
Checkpoint inhibition after HiDAC shows promise in AML
NEWPORT BEACH, CALIF. – Adding pembrolizumab after high-dose cytarabine in patients with relapsed or refractory acute myeloid leukemia (AML) appears safe and feasible and shows promising efficacy, according to early results from a multicenter phase 2 study.
The overall response rate to this novel treatment approach in 19 evaluable patients (of 20 enrolled to date) was 42%, with 7 patients (37%) achieving a complete response or complete response with incomplete blood count recovery, and 1 (5%) achieving a partial response, reported Joshua Zeidner, MD, in a poster at the Acute Leukemia Forum of Hemedicus.
Those who went on to allogeneic stem cell transplant included three patients in complete response and one of the five who received pembrolizumab maintenance. That patient on pembrolizumab maintenance went to transplant after two cycles, three others on it relapsed after a median duration of 2.8 months in complete response, and one initially achieved a partial response and had stable disease for a “pretty remarkable” 12 cycles before progressing, he said.
Preliminary analyses in the first six patients, including three with complete response and three nonresponders, showed increased posttreatment diversity of the T-cell receptor repertoire versus baseline in peripheral blood CD8+ T cells in the complete response patients, compared with nonresponders. This suggests that T-cell diversity at baseline is a promising biomarker for programmed death-1 (PD-1) blockade response, Dr. Zeidner noted.
PD-1 suppresses immune activation, and the PD-1 pathway is exploited by AML cells to evade immune surveillance, Dr. Zeidner explained. PD-1 blockade has been shown to have antileukemic effects in vivo, there is expression of multiple coinhibitory receptors in AML patients at the time of diagnosis (that persists in refractory AML), and the ligand for PD-1 is up-regulated on AML blasts, particularly in relapsed/refractory disease, he added.
He and his colleagues hypothesized that targeting PD-1 with pembrolizumab after high-dose cytarabine (HiDAC) salvage chemotherapy would stimulate a T cell–mediated antileukemic immune response and lead to improved efficacy in patients with relapsed or refractory AML.
The ongoing study, with planned enrollment of 37 patients, includes patients aged 18-70 years with relapsed/refractory AML (median age of first 20 enrolled is 54 years). Those under age 60 years receive 2 g/m2 of intravenous HiDAC every 12 hours on days 1-5, and those over age 60 years receive 1.5 g/m2 every 12 hours on days 1-5. In both age groups, this is followed by intravenous pembrolizumab given at 200 mg on day 14.
Overall responders receive maintenance phase intravenous pembrolizumab at 200 mg every 3 weeks for up to 2 years until relapse or progression. Patients are allowed to proceed to stem cell transplant before or after the maintenance phase.
A number of correlative studies, including serial peripheral blood and bone marrow flow cytometry and T-cell receptor clonality studies, also are being conducted to look for predictive biomarkers of response, Dr. Zeidner said.
The study population to date is a relatively young, very-high-risk subgroup of AML patients; European LeukemiaNet-2017 genetic risk status was favorable in only 3 of the first 20 patients (15%), intermediate in 7 (35%), and adverse in 10 (50%). Treatment has been well tolerated; toxicities have been rare and manageable. “Overall, there have been no unexpected toxicities,” he said.
The most common overall toxicities included febrile neutropenia in 70% of patients; transaminitis (including one grade 3 case) in 50%, hyperbilirubinemia in 35%, and fatigue, increased alkaline phosphatase, and rash in 25% each.
Novel therapies for relapsed/refractory AML are urgently needed, and these early results demonstrated the safety and feasibility of adding pembrolizumab after HiDAC chemotherapy in relapsed/refractory AML patients. The preliminary finding that broadening of the immune repertoire, which may occur via increasing T-cell responses beyond endogenous viral responses, was associated with complete response was particularly encouraging, Dr. Zeidner said. The investigators hope to determine predictors of response to immune checkpoint blockade in AML through a comprehensive immune biomarker discovery approach, he added.
This study was funded by Merck. Dr. Zeidner reported having no financial disclosures. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
SOURCE: Zeidner J et al. ALF 2018, Poster Session.
NEWPORT BEACH, CALIF. – Adding pembrolizumab after high-dose cytarabine in patients with relapsed or refractory acute myeloid leukemia (AML) appears safe and feasible and shows promising efficacy, according to early results from a multicenter phase 2 study.
The overall response rate to this novel treatment approach in 19 evaluable patients (of 20 enrolled to date) was 42%, with 7 patients (37%) achieving a complete response or complete response with incomplete blood count recovery, and 1 (5%) achieving a partial response, reported Joshua Zeidner, MD, in a poster at the Acute Leukemia Forum of Hemedicus.
Those who went on to allogeneic stem cell transplant included three patients in complete response and one of the five who received pembrolizumab maintenance. That patient on pembrolizumab maintenance went to transplant after two cycles, three others on it relapsed after a median duration of 2.8 months in complete response, and one initially achieved a partial response and had stable disease for a “pretty remarkable” 12 cycles before progressing, he said.
Preliminary analyses in the first six patients, including three with complete response and three nonresponders, showed increased posttreatment diversity of the T-cell receptor repertoire versus baseline in peripheral blood CD8+ T cells in the complete response patients, compared with nonresponders. This suggests that T-cell diversity at baseline is a promising biomarker for programmed death-1 (PD-1) blockade response, Dr. Zeidner noted.
PD-1 suppresses immune activation, and the PD-1 pathway is exploited by AML cells to evade immune surveillance, Dr. Zeidner explained. PD-1 blockade has been shown to have antileukemic effects in vivo, there is expression of multiple coinhibitory receptors in AML patients at the time of diagnosis (that persists in refractory AML), and the ligand for PD-1 is up-regulated on AML blasts, particularly in relapsed/refractory disease, he added.
He and his colleagues hypothesized that targeting PD-1 with pembrolizumab after high-dose cytarabine (HiDAC) salvage chemotherapy would stimulate a T cell–mediated antileukemic immune response and lead to improved efficacy in patients with relapsed or refractory AML.
The ongoing study, with planned enrollment of 37 patients, includes patients aged 18-70 years with relapsed/refractory AML (median age of first 20 enrolled is 54 years). Those under age 60 years receive 2 g/m2 of intravenous HiDAC every 12 hours on days 1-5, and those over age 60 years receive 1.5 g/m2 every 12 hours on days 1-5. In both age groups, this is followed by intravenous pembrolizumab given at 200 mg on day 14.
Overall responders receive maintenance phase intravenous pembrolizumab at 200 mg every 3 weeks for up to 2 years until relapse or progression. Patients are allowed to proceed to stem cell transplant before or after the maintenance phase.
A number of correlative studies, including serial peripheral blood and bone marrow flow cytometry and T-cell receptor clonality studies, also are being conducted to look for predictive biomarkers of response, Dr. Zeidner said.
The study population to date is a relatively young, very-high-risk subgroup of AML patients; European LeukemiaNet-2017 genetic risk status was favorable in only 3 of the first 20 patients (15%), intermediate in 7 (35%), and adverse in 10 (50%). Treatment has been well tolerated; toxicities have been rare and manageable. “Overall, there have been no unexpected toxicities,” he said.
The most common overall toxicities included febrile neutropenia in 70% of patients; transaminitis (including one grade 3 case) in 50%, hyperbilirubinemia in 35%, and fatigue, increased alkaline phosphatase, and rash in 25% each.
Novel therapies for relapsed/refractory AML are urgently needed, and these early results demonstrated the safety and feasibility of adding pembrolizumab after HiDAC chemotherapy in relapsed/refractory AML patients. The preliminary finding that broadening of the immune repertoire, which may occur via increasing T-cell responses beyond endogenous viral responses, was associated with complete response was particularly encouraging, Dr. Zeidner said. The investigators hope to determine predictors of response to immune checkpoint blockade in AML through a comprehensive immune biomarker discovery approach, he added.
This study was funded by Merck. Dr. Zeidner reported having no financial disclosures. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
SOURCE: Zeidner J et al. ALF 2018, Poster Session.
NEWPORT BEACH, CALIF. – Adding pembrolizumab after high-dose cytarabine in patients with relapsed or refractory acute myeloid leukemia (AML) appears safe and feasible and shows promising efficacy, according to early results from a multicenter phase 2 study.
The overall response rate to this novel treatment approach in 19 evaluable patients (of 20 enrolled to date) was 42%, with 7 patients (37%) achieving a complete response or complete response with incomplete blood count recovery, and 1 (5%) achieving a partial response, reported Joshua Zeidner, MD, in a poster at the Acute Leukemia Forum of Hemedicus.
Those who went on to allogeneic stem cell transplant included three patients in complete response and one of the five who received pembrolizumab maintenance. That patient on pembrolizumab maintenance went to transplant after two cycles, three others on it relapsed after a median duration of 2.8 months in complete response, and one initially achieved a partial response and had stable disease for a “pretty remarkable” 12 cycles before progressing, he said.
Preliminary analyses in the first six patients, including three with complete response and three nonresponders, showed increased posttreatment diversity of the T-cell receptor repertoire versus baseline in peripheral blood CD8+ T cells in the complete response patients, compared with nonresponders. This suggests that T-cell diversity at baseline is a promising biomarker for programmed death-1 (PD-1) blockade response, Dr. Zeidner noted.
PD-1 suppresses immune activation, and the PD-1 pathway is exploited by AML cells to evade immune surveillance, Dr. Zeidner explained. PD-1 blockade has been shown to have antileukemic effects in vivo, there is expression of multiple coinhibitory receptors in AML patients at the time of diagnosis (that persists in refractory AML), and the ligand for PD-1 is up-regulated on AML blasts, particularly in relapsed/refractory disease, he added.
He and his colleagues hypothesized that targeting PD-1 with pembrolizumab after high-dose cytarabine (HiDAC) salvage chemotherapy would stimulate a T cell–mediated antileukemic immune response and lead to improved efficacy in patients with relapsed or refractory AML.
The ongoing study, with planned enrollment of 37 patients, includes patients aged 18-70 years with relapsed/refractory AML (median age of first 20 enrolled is 54 years). Those under age 60 years receive 2 g/m2 of intravenous HiDAC every 12 hours on days 1-5, and those over age 60 years receive 1.5 g/m2 every 12 hours on days 1-5. In both age groups, this is followed by intravenous pembrolizumab given at 200 mg on day 14.
Overall responders receive maintenance phase intravenous pembrolizumab at 200 mg every 3 weeks for up to 2 years until relapse or progression. Patients are allowed to proceed to stem cell transplant before or after the maintenance phase.
A number of correlative studies, including serial peripheral blood and bone marrow flow cytometry and T-cell receptor clonality studies, also are being conducted to look for predictive biomarkers of response, Dr. Zeidner said.
The study population to date is a relatively young, very-high-risk subgroup of AML patients; European LeukemiaNet-2017 genetic risk status was favorable in only 3 of the first 20 patients (15%), intermediate in 7 (35%), and adverse in 10 (50%). Treatment has been well tolerated; toxicities have been rare and manageable. “Overall, there have been no unexpected toxicities,” he said.
The most common overall toxicities included febrile neutropenia in 70% of patients; transaminitis (including one grade 3 case) in 50%, hyperbilirubinemia in 35%, and fatigue, increased alkaline phosphatase, and rash in 25% each.
Novel therapies for relapsed/refractory AML are urgently needed, and these early results demonstrated the safety and feasibility of adding pembrolizumab after HiDAC chemotherapy in relapsed/refractory AML patients. The preliminary finding that broadening of the immune repertoire, which may occur via increasing T-cell responses beyond endogenous viral responses, was associated with complete response was particularly encouraging, Dr. Zeidner said. The investigators hope to determine predictors of response to immune checkpoint blockade in AML through a comprehensive immune biomarker discovery approach, he added.
This study was funded by Merck. Dr. Zeidner reported having no financial disclosures. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
SOURCE: Zeidner J et al. ALF 2018, Poster Session.
REPORTING FROM ALF 2018
Key clinical point:
Major finding: The overall response rate was 42%; 37% of patients achieved complete response or CR with incomplete blood count recovery, and 5% achieved partial response.
Study details: A multicenter, phase 2 study with early results from the first 20 patients enrolled.
Disclosures: This study was funded by Merck. Dr. Zeidner reported having no financial disclosures. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Source: Zeidner J et al. ALF 2018, Poster Session.
Study: Delays in publishing oncology data near 1 year
A new study highlights a marked delay between completion of clinical trials and when the results reach practicing clinicians.
The study, published online in JAMA Oncology, found that even for the most pressing findings, the median publication of final clinical data is nearly 1 year.
Lindor Qunaj of Brown University, Providence, R.I., and colleagues measured the time frame between when eight large pharmaceutical companies issued press releases announcing completed phase 3 oncology clinical trials and the public sharing of the results either on ClinicalTrials.gov or a peer-reviewed biomedical journal. Collectively, the eight companies accounted for 72% of all oncology sales in 2015. The press releases were posted between January 2011 and June 2016. Investigators found the median time from press release to publication of data was 300 days.
Clinical trials with negative results had median delays of more than 100 days longer than positive results, the investigators found. Of the 100 press releases studied, only 31% included the magnitude of study findings. Most press releases (66%) reported outcomes of studies involving drugs already approved by the Food and Drug Administration for some indication. Press releases regarding preapproval drugs were less likely to contain quantitative reports of effect sizes. The majority of final clinical results (90%) were either posted, published, or both, within 2 years. No difference existed in the median delay between trials studying approved drugs compared with unapproved drugs.
Mr. Qunaj and associates noted that their analysis emphasizes the ongoing criticism of the speed at which pharmaceutical companies publish findings from clinical trials. The authors suggested that pharmaceutical companies, publishers, and journals work to minimize such delays. For instance, they might consider preprinting, the practice of publishing “draft” findings prior to, or in tandem with, peer review. Through preprints, companies could post all relevant study data, including measured outcomes and toxic effects, as well as study protocol, Mr. Qunaj and associates wrote. In addition, the authors suggested that publishers continue to consider innovative efforts to “responsibly accelerate the peer review process, or to further shorten the time between manuscript acceptance and data availability.”
“We do foresee possible objections from pharmaceutical companies, scientific journals, and medical societies,” the authors wrote. “Embargoed, timed data releases are a potent tool for marketing study findings and drawing traffic to the journal or meeting where the results are presented. However, these interests are narrow, while the needs of science more generally, and the patients with the conditions that these companies have studied, matter more.”
A coauthor, Peter Bach, MD, received personal fees and grants from numerous sources. No other disclosures were reported.
SOURCE: Qunaj L et al. JAMA Oncol. 2018 Apr 12. doi:10.1001/jamaoncol.2018.0264.
A new study highlights a marked delay between completion of clinical trials and when the results reach practicing clinicians.
The study, published online in JAMA Oncology, found that even for the most pressing findings, the median publication of final clinical data is nearly 1 year.
Lindor Qunaj of Brown University, Providence, R.I., and colleagues measured the time frame between when eight large pharmaceutical companies issued press releases announcing completed phase 3 oncology clinical trials and the public sharing of the results either on ClinicalTrials.gov or a peer-reviewed biomedical journal. Collectively, the eight companies accounted for 72% of all oncology sales in 2015. The press releases were posted between January 2011 and June 2016. Investigators found the median time from press release to publication of data was 300 days.
Clinical trials with negative results had median delays of more than 100 days longer than positive results, the investigators found. Of the 100 press releases studied, only 31% included the magnitude of study findings. Most press releases (66%) reported outcomes of studies involving drugs already approved by the Food and Drug Administration for some indication. Press releases regarding preapproval drugs were less likely to contain quantitative reports of effect sizes. The majority of final clinical results (90%) were either posted, published, or both, within 2 years. No difference existed in the median delay between trials studying approved drugs compared with unapproved drugs.
Mr. Qunaj and associates noted that their analysis emphasizes the ongoing criticism of the speed at which pharmaceutical companies publish findings from clinical trials. The authors suggested that pharmaceutical companies, publishers, and journals work to minimize such delays. For instance, they might consider preprinting, the practice of publishing “draft” findings prior to, or in tandem with, peer review. Through preprints, companies could post all relevant study data, including measured outcomes and toxic effects, as well as study protocol, Mr. Qunaj and associates wrote. In addition, the authors suggested that publishers continue to consider innovative efforts to “responsibly accelerate the peer review process, or to further shorten the time between manuscript acceptance and data availability.”
“We do foresee possible objections from pharmaceutical companies, scientific journals, and medical societies,” the authors wrote. “Embargoed, timed data releases are a potent tool for marketing study findings and drawing traffic to the journal or meeting where the results are presented. However, these interests are narrow, while the needs of science more generally, and the patients with the conditions that these companies have studied, matter more.”
A coauthor, Peter Bach, MD, received personal fees and grants from numerous sources. No other disclosures were reported.
SOURCE: Qunaj L et al. JAMA Oncol. 2018 Apr 12. doi:10.1001/jamaoncol.2018.0264.
A new study highlights a marked delay between completion of clinical trials and when the results reach practicing clinicians.
The study, published online in JAMA Oncology, found that even for the most pressing findings, the median publication of final clinical data is nearly 1 year.
Lindor Qunaj of Brown University, Providence, R.I., and colleagues measured the time frame between when eight large pharmaceutical companies issued press releases announcing completed phase 3 oncology clinical trials and the public sharing of the results either on ClinicalTrials.gov or a peer-reviewed biomedical journal. Collectively, the eight companies accounted for 72% of all oncology sales in 2015. The press releases were posted between January 2011 and June 2016. Investigators found the median time from press release to publication of data was 300 days.
Clinical trials with negative results had median delays of more than 100 days longer than positive results, the investigators found. Of the 100 press releases studied, only 31% included the magnitude of study findings. Most press releases (66%) reported outcomes of studies involving drugs already approved by the Food and Drug Administration for some indication. Press releases regarding preapproval drugs were less likely to contain quantitative reports of effect sizes. The majority of final clinical results (90%) were either posted, published, or both, within 2 years. No difference existed in the median delay between trials studying approved drugs compared with unapproved drugs.
Mr. Qunaj and associates noted that their analysis emphasizes the ongoing criticism of the speed at which pharmaceutical companies publish findings from clinical trials. The authors suggested that pharmaceutical companies, publishers, and journals work to minimize such delays. For instance, they might consider preprinting, the practice of publishing “draft” findings prior to, or in tandem with, peer review. Through preprints, companies could post all relevant study data, including measured outcomes and toxic effects, as well as study protocol, Mr. Qunaj and associates wrote. In addition, the authors suggested that publishers continue to consider innovative efforts to “responsibly accelerate the peer review process, or to further shorten the time between manuscript acceptance and data availability.”
“We do foresee possible objections from pharmaceutical companies, scientific journals, and medical societies,” the authors wrote. “Embargoed, timed data releases are a potent tool for marketing study findings and drawing traffic to the journal or meeting where the results are presented. However, these interests are narrow, while the needs of science more generally, and the patients with the conditions that these companies have studied, matter more.”
A coauthor, Peter Bach, MD, received personal fees and grants from numerous sources. No other disclosures were reported.
SOURCE: Qunaj L et al. JAMA Oncol. 2018 Apr 12. doi:10.1001/jamaoncol.2018.0264.
FROM JAMA ONCOLOGY
Key clinical point: A marked delay exists between clinical trial completion and publication of final clinical trial data.
Major finding: The median time between a press release announcing trial completion and publication of full data was 300 days.
Study details: A study of 100 press releases posted by eight pharmaceutical companies that accounted for the majority of oncology sales in 2015.
Disclosures: A coauthor, Peter Bach, MD, received personal fees and grants from numerous sources. No other disclosures were reported.
Source: Qunaj L et al. JAMA Oncol. 2018 Apr 12. doi:10.1001/jamaoncol.2018.0264.