User login
Newborn with multiple plaques
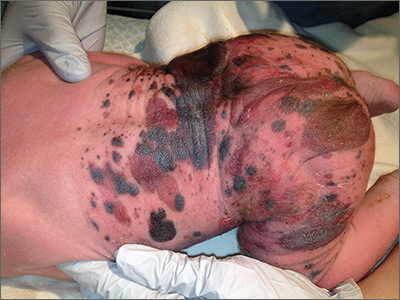
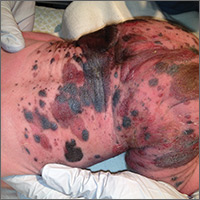
Giant congenital nevus was diagnosed in this patient. Congenital melanocytic nevi (CMN) are pigmented lesions that are present at birth and created by the abnormal migration of neural crest cells during embryogenesis. Nevi are categorized by size as small (<1.5 cm), medium (1.5-20 cm), large (>20 cm), or giant (>40 cm).
Congenital nevi tend to start out flat, with uniform pigmentation, but can become more variegated in texture and color as normal growth and development continue. Giant congenital nevi, which are rare, are likely to thicken, darken, and enlarge as the patient grows. Some nevi may develop very coarse or dark hair. CMN can cover any part of the body and occur independent of skin color and other ethnic factors.
CMN may present in almost any location and may be brown, black, pink, or purple in color. Café au lait macules, blue-gray spots, nevus of Ota, nevus spilus, and vascular malformations are part of the differential diagnosis for CMN and have individual location and color characteristics that set them apart clinically.
Patients with CMN are at increased risk for neurocutaneous melanosis (NCM; a melanocyte proliferation in the central nervous system) and melanoma. Magnetic resonance imaging (MRI) is helpful to exclude NCM. Treatment options for patients with large and giant CMN include early curettage, local excision, dermabrasion, and laser therapy. (There is, however, considerable debate about the value of surgery.) The newborn in the case underwent an MRI and the results were normal. At 4 months of age, he hadn’t developed any neurologic symptoms. The child’s nevi continue to grow and he sees his family physician for routine well-child visits and a dermatologist annually to monitor the nevi.
Adapted from: Karnes J, Griffin C. Large plaques on a baby boy. J Fam Pract. 2016;65:407-409.

Giant congenital nevus was diagnosed in this patient. Congenital melanocytic nevi (CMN) are pigmented lesions that are present at birth and created by the abnormal migration of neural crest cells during embryogenesis. Nevi are categorized by size as small (<1.5 cm), medium (1.5-20 cm), large (>20 cm), or giant (>40 cm).
Congenital nevi tend to start out flat, with uniform pigmentation, but can become more variegated in texture and color as normal growth and development continue. Giant congenital nevi, which are rare, are likely to thicken, darken, and enlarge as the patient grows. Some nevi may develop very coarse or dark hair. CMN can cover any part of the body and occur independent of skin color and other ethnic factors.
CMN may present in almost any location and may be brown, black, pink, or purple in color. Café au lait macules, blue-gray spots, nevus of Ota, nevus spilus, and vascular malformations are part of the differential diagnosis for CMN and have individual location and color characteristics that set them apart clinically.
Patients with CMN are at increased risk for neurocutaneous melanosis (NCM; a melanocyte proliferation in the central nervous system) and melanoma. Magnetic resonance imaging (MRI) is helpful to exclude NCM. Treatment options for patients with large and giant CMN include early curettage, local excision, dermabrasion, and laser therapy. (There is, however, considerable debate about the value of surgery.) The newborn in the case underwent an MRI and the results were normal. At 4 months of age, he hadn’t developed any neurologic symptoms. The child’s nevi continue to grow and he sees his family physician for routine well-child visits and a dermatologist annually to monitor the nevi.
Adapted from: Karnes J, Griffin C. Large plaques on a baby boy. J Fam Pract. 2016;65:407-409.

Giant congenital nevus was diagnosed in this patient. Congenital melanocytic nevi (CMN) are pigmented lesions that are present at birth and created by the abnormal migration of neural crest cells during embryogenesis. Nevi are categorized by size as small (<1.5 cm), medium (1.5-20 cm), large (>20 cm), or giant (>40 cm).
Congenital nevi tend to start out flat, with uniform pigmentation, but can become more variegated in texture and color as normal growth and development continue. Giant congenital nevi, which are rare, are likely to thicken, darken, and enlarge as the patient grows. Some nevi may develop very coarse or dark hair. CMN can cover any part of the body and occur independent of skin color and other ethnic factors.
CMN may present in almost any location and may be brown, black, pink, or purple in color. Café au lait macules, blue-gray spots, nevus of Ota, nevus spilus, and vascular malformations are part of the differential diagnosis for CMN and have individual location and color characteristics that set them apart clinically.
Patients with CMN are at increased risk for neurocutaneous melanosis (NCM; a melanocyte proliferation in the central nervous system) and melanoma. Magnetic resonance imaging (MRI) is helpful to exclude NCM. Treatment options for patients with large and giant CMN include early curettage, local excision, dermabrasion, and laser therapy. (There is, however, considerable debate about the value of surgery.) The newborn in the case underwent an MRI and the results were normal. At 4 months of age, he hadn’t developed any neurologic symptoms. The child’s nevi continue to grow and he sees his family physician for routine well-child visits and a dermatologist annually to monitor the nevi.
Adapted from: Karnes J, Griffin C. Large plaques on a baby boy. J Fam Pract. 2016;65:407-409.
Nivolumab helps some with advanced NSCLC reach 5-year mark
Some patients with previously treated advanced non-small cell lung cancer (NSCLC), a malignancy with a historically dim prognosis, survived at least 5 years after receiving the immune checkpoint inhibitor nivolumab (Opdivo) in an early phase 1 trial.
For 129 patients with NSCLC treated with nivolumab in the CA209-003 trial, the estimated 5 year overall survival (OS) was 16%. Twelve patients who did not receive any subsequent therapy following completion of nivolumab were alive with no evidence of disease at the 5-year follow-up mark, reported Scott Gettinger, MD, of the Yale Cancer Center in New Haven, Connecticut, and colleagues.
“Considering the historically low 5-year survival rate for patients with metastatic lung cancer, the estimated 5-year OS rate of 16% from the time of nivolumab treatment initiation observed in this cohort of heavily pretreated patients with advanced NSCLC constitutes a milestone in the advancement of lung cancer treatment,” they wrote in the Journal of Clinical Oncology. In the NSCLC cohort of the phase 1 dose-escalation and expansion study, 129 patients with heavily pretreated advanced NSCLC received nivolumab in doses of 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. The investigators previously reportedthat after a median follow-up of 39 weeks, the median overall survival across all three dose groups was 9.9 months. For 37 patients treated at the 3 mg/kg dose chosen for further development, the median 1-, 2-, and 3-year OS rates were 56%, 42%, and 27%, respectively.
In the current study, they followed the patients out to a minimum of 58.25 months. The median OS was 9.9 months, and the estimated 5-year OS rate, as noted before, was 16%. The 5-year OS rates for patients with squamous histology cancers was 16%, and the rate for patients with nonsquamous histology was 15%.
In all, 16 patients survived at least 5 years, with the longest follow-up out to 88.6 months. Two of the patients died before the database lock in November 2016, one from disease progression, and one from chronic obstructive pulmonary disease.
Among 10 long-term survivors who had quantifiable expression of the programmed death-1 ligand 1 (PD-L1), seven had at least 1% PD-L1 expression at baseline.
Of the 16 5-year survivors, 12 (75%) had a partial response to nivolumab according to RECIST (Response Evaluation Criteria in Solid Tumors), version 1. Two others had stable disease, and two had disease progression at the best response.
In all, nine of the 5-year survivors had completed the maximum 96 weeks of nivolumab, four had discontinued due to adverse events, and three had stopped because of disease progression.
“The findings from CA209-003 indicate some patients can derive long-term benefit from nivolumab treatment that is limited to 2 years; however, the question of optimal treatment duration remains to be formally addressed in a prospective controlled trial,” Dr. Gettinger and associates wrote.
The study was supported by Bristol-Myers Squibb and Ono Pharmaceuticals. Dr. Gettinger and multiple co-authors reported consulting/advisory roles and research funding with BMS and other relationships with multiple companies. Several co-authors are BMS employees.
SOURCE: : Getting S et al. J Clin Oncol. 2018 Mar 23 doi: 10.1200/JCO.2017.77.0412 .
Some patients with previously treated advanced non-small cell lung cancer (NSCLC), a malignancy with a historically dim prognosis, survived at least 5 years after receiving the immune checkpoint inhibitor nivolumab (Opdivo) in an early phase 1 trial.
For 129 patients with NSCLC treated with nivolumab in the CA209-003 trial, the estimated 5 year overall survival (OS) was 16%. Twelve patients who did not receive any subsequent therapy following completion of nivolumab were alive with no evidence of disease at the 5-year follow-up mark, reported Scott Gettinger, MD, of the Yale Cancer Center in New Haven, Connecticut, and colleagues.
“Considering the historically low 5-year survival rate for patients with metastatic lung cancer, the estimated 5-year OS rate of 16% from the time of nivolumab treatment initiation observed in this cohort of heavily pretreated patients with advanced NSCLC constitutes a milestone in the advancement of lung cancer treatment,” they wrote in the Journal of Clinical Oncology. In the NSCLC cohort of the phase 1 dose-escalation and expansion study, 129 patients with heavily pretreated advanced NSCLC received nivolumab in doses of 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. The investigators previously reportedthat after a median follow-up of 39 weeks, the median overall survival across all three dose groups was 9.9 months. For 37 patients treated at the 3 mg/kg dose chosen for further development, the median 1-, 2-, and 3-year OS rates were 56%, 42%, and 27%, respectively.
In the current study, they followed the patients out to a minimum of 58.25 months. The median OS was 9.9 months, and the estimated 5-year OS rate, as noted before, was 16%. The 5-year OS rates for patients with squamous histology cancers was 16%, and the rate for patients with nonsquamous histology was 15%.
In all, 16 patients survived at least 5 years, with the longest follow-up out to 88.6 months. Two of the patients died before the database lock in November 2016, one from disease progression, and one from chronic obstructive pulmonary disease.
Among 10 long-term survivors who had quantifiable expression of the programmed death-1 ligand 1 (PD-L1), seven had at least 1% PD-L1 expression at baseline.
Of the 16 5-year survivors, 12 (75%) had a partial response to nivolumab according to RECIST (Response Evaluation Criteria in Solid Tumors), version 1. Two others had stable disease, and two had disease progression at the best response.
In all, nine of the 5-year survivors had completed the maximum 96 weeks of nivolumab, four had discontinued due to adverse events, and three had stopped because of disease progression.
“The findings from CA209-003 indicate some patients can derive long-term benefit from nivolumab treatment that is limited to 2 years; however, the question of optimal treatment duration remains to be formally addressed in a prospective controlled trial,” Dr. Gettinger and associates wrote.
The study was supported by Bristol-Myers Squibb and Ono Pharmaceuticals. Dr. Gettinger and multiple co-authors reported consulting/advisory roles and research funding with BMS and other relationships with multiple companies. Several co-authors are BMS employees.
SOURCE: : Getting S et al. J Clin Oncol. 2018 Mar 23 doi: 10.1200/JCO.2017.77.0412 .
Some patients with previously treated advanced non-small cell lung cancer (NSCLC), a malignancy with a historically dim prognosis, survived at least 5 years after receiving the immune checkpoint inhibitor nivolumab (Opdivo) in an early phase 1 trial.
For 129 patients with NSCLC treated with nivolumab in the CA209-003 trial, the estimated 5 year overall survival (OS) was 16%. Twelve patients who did not receive any subsequent therapy following completion of nivolumab were alive with no evidence of disease at the 5-year follow-up mark, reported Scott Gettinger, MD, of the Yale Cancer Center in New Haven, Connecticut, and colleagues.
“Considering the historically low 5-year survival rate for patients with metastatic lung cancer, the estimated 5-year OS rate of 16% from the time of nivolumab treatment initiation observed in this cohort of heavily pretreated patients with advanced NSCLC constitutes a milestone in the advancement of lung cancer treatment,” they wrote in the Journal of Clinical Oncology. In the NSCLC cohort of the phase 1 dose-escalation and expansion study, 129 patients with heavily pretreated advanced NSCLC received nivolumab in doses of 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. The investigators previously reportedthat after a median follow-up of 39 weeks, the median overall survival across all three dose groups was 9.9 months. For 37 patients treated at the 3 mg/kg dose chosen for further development, the median 1-, 2-, and 3-year OS rates were 56%, 42%, and 27%, respectively.
In the current study, they followed the patients out to a minimum of 58.25 months. The median OS was 9.9 months, and the estimated 5-year OS rate, as noted before, was 16%. The 5-year OS rates for patients with squamous histology cancers was 16%, and the rate for patients with nonsquamous histology was 15%.
In all, 16 patients survived at least 5 years, with the longest follow-up out to 88.6 months. Two of the patients died before the database lock in November 2016, one from disease progression, and one from chronic obstructive pulmonary disease.
Among 10 long-term survivors who had quantifiable expression of the programmed death-1 ligand 1 (PD-L1), seven had at least 1% PD-L1 expression at baseline.
Of the 16 5-year survivors, 12 (75%) had a partial response to nivolumab according to RECIST (Response Evaluation Criteria in Solid Tumors), version 1. Two others had stable disease, and two had disease progression at the best response.
In all, nine of the 5-year survivors had completed the maximum 96 weeks of nivolumab, four had discontinued due to adverse events, and three had stopped because of disease progression.
“The findings from CA209-003 indicate some patients can derive long-term benefit from nivolumab treatment that is limited to 2 years; however, the question of optimal treatment duration remains to be formally addressed in a prospective controlled trial,” Dr. Gettinger and associates wrote.
The study was supported by Bristol-Myers Squibb and Ono Pharmaceuticals. Dr. Gettinger and multiple co-authors reported consulting/advisory roles and research funding with BMS and other relationships with multiple companies. Several co-authors are BMS employees.
SOURCE: : Getting S et al. J Clin Oncol. 2018 Mar 23 doi: 10.1200/JCO.2017.77.0412 .
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The programmed death-1 inhibitor nivolumab (Opdivo) is associated with long-term survival in a subset of patients with heavily pre-treated advanced non-small cell lung cancer (NSCLC).Major finding: The estimated 5-year overall survival rate was 16%.Study details: Follow-up study of 129 patients with NSCLC treated with nivolumab in a phase 1 study.
Disclosures: The study was supported by Bristol-Myers Squibb and Ono Pharmaceuticals. Dr. Gettinger and multiple co-authors reported consulting/advisory roles and research finding with BMS and other relationships with multiple companies. Several co-authors are BMS employees.
Source: Gettinger S et al. J Clin Oncol. 2018 Mar 23 doi: 10.1200/JCO.2017.77.0412.
Hot Threads in ACS Communities
Your colleagues have a lot to say to each other. Here are the top discussion threads in ACS Communities this week:
1. ABS Continuous Certification Program (General Surgery)
2. ACS Leadership Response to ABS Continuous Certification Program (General Surgery)
3. Update on ACS Violence Prevention Strategy (General Surgery)
4. FNA parathyroid? (Endocrine Surgery)
5. Risk reduction mastectomy with mastectomy for unilateral breast cancer (Breast Surgery)
6. First mammogram after treatment (Breast Surgery)
7. Transferring for “pt preference” (Rural Surgery)
8. Where will the Gen X surgeon work? (Young Fellows)
9. Hiatal hernias and the sleeve (Bariatric Surgery)
10. Level 3 trauma (Trauma Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org, click “Communities” on the blue bar, select “All Communities,” and then click the “Join” button next to the communities you’d like to join. If you have any questions, please send them to [email protected].
Your colleagues have a lot to say to each other. Here are the top discussion threads in ACS Communities this week:
1. ABS Continuous Certification Program (General Surgery)
2. ACS Leadership Response to ABS Continuous Certification Program (General Surgery)
3. Update on ACS Violence Prevention Strategy (General Surgery)
4. FNA parathyroid? (Endocrine Surgery)
5. Risk reduction mastectomy with mastectomy for unilateral breast cancer (Breast Surgery)
6. First mammogram after treatment (Breast Surgery)
7. Transferring for “pt preference” (Rural Surgery)
8. Where will the Gen X surgeon work? (Young Fellows)
9. Hiatal hernias and the sleeve (Bariatric Surgery)
10. Level 3 trauma (Trauma Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org, click “Communities” on the blue bar, select “All Communities,” and then click the “Join” button next to the communities you’d like to join. If you have any questions, please send them to [email protected].
Your colleagues have a lot to say to each other. Here are the top discussion threads in ACS Communities this week:
1. ABS Continuous Certification Program (General Surgery)
2. ACS Leadership Response to ABS Continuous Certification Program (General Surgery)
3. Update on ACS Violence Prevention Strategy (General Surgery)
4. FNA parathyroid? (Endocrine Surgery)
5. Risk reduction mastectomy with mastectomy for unilateral breast cancer (Breast Surgery)
6. First mammogram after treatment (Breast Surgery)
7. Transferring for “pt preference” (Rural Surgery)
8. Where will the Gen X surgeon work? (Young Fellows)
9. Hiatal hernias and the sleeve (Bariatric Surgery)
10. Level 3 trauma (Trauma Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org, click “Communities” on the blue bar, select “All Communities,” and then click the “Join” button next to the communities you’d like to join. If you have any questions, please send them to [email protected].
Reducing SNF Readmissions: At What Cost?
The landscape of postacute care in skilled nursing facilities (SNFs) in the United States is evolving. As the population ages, a growing number of elderly persons are being discharged to SNFs at an enormous cost and with clear evidence of disappointing outcomes. The reaction to these trends includes payment reforms that “bundle” hospital and postacute care, act as incentives to discourage SNFs, or penalize SNFs for undesired patient outcomes. Hospitalists are expected to increasingly feel the effect of these reforms.1
Thus, hospitals are demonstrating renewed interest in reducing readmissions from SNFs. In this issue of Journal of Hospital Medicine, Rosen and colleagues present the results of the Enhanced Care Program (ECP), a multicomponent intervention consisting of 9 nurse practitioners (NPs), a pharmacist, a pharmacy technician, a nurse educator, a program administrator, and a medical director.2 These providers are deployed to 8 SNFs around a large teaching hospital, providing direct clinical care as well as 24/7 call availability for enrolled patients, robust medication reconciliation, and monthly education for SNF nursing staff. A unique aspect of this model was that individual attending physicians in the associated SNFs could decide whether to enroll their patients in the model; patients not enrolled represented a contemporaneous control cohort. The authors found a nearly 30% reduction in the odds of 30-day readmission (OR 0.71 [0.60–0.85] after adjustment), which was robust to multiple sensitivity analyses, including a propensity-matched cohort comparison. The authors should be commended for working to mitigate these potential confounders, thereby strengthening their conclusions. Such a large reduction in readmissions reflects their high underlying prevalence (23% in the nonintervention cohort).
This report closely follows the evaluation of a similar program at the Cleveland Clinic called Connected Care Model (CCM), in which 4 physicians and 5 NPs or physician assistants provided care, including 24/7 call availability, in 7 associated SNFs.3 In a retrospective pre-post analysis comparing the 30-day readmission rates of these SNFs with those of others in the network, similar reductions in readmissions were observed. ECP and CCM represent important extensions of a much larger body of evidence, from the Evercare model4 to the Initiative to Reduce Avoidable Hospitalizations demonstration project, which suggests that adding NPs to nursing homes reduces hospitalizations.5
However, several factors have to be considered before disseminating ECP or CCM. First, other promising “proof of concept” quality improvement studies were not efficacious when rigorously tested in nursing homes.6 Second, these programs are representative of large academic medical centers, which may establish different relationships with different SNFs compared with smaller or less well-resourced hospitals. As the Initiative to Reduce Hospitalizations demonstrated, even a fundamentally similar intervention can have extremely different results depending on the nursing homes involved,5 and the science behind establishing effective hospital–SNF partnerships is still in its infancy.7 Third, both studies have significant methodological limitations, including most importantly that they are conducted within SNFs selected to be part of their hospitals’ network.
These significant early efforts also present an opportunity to reconsider the underlying assumption of these models: that adding more supervisory clinicians to SNFs is the right approach to reduce hospitalizations. Although adding resources is an attractive “plug and play” solution for many problems in healthcare delivery, placing only 1 NP in each of the 15,583 certified nursing facilities in the United States would employ fully 10% of the entire NP workforce. Amid rising concerns about costs related to our aging population, these interventions face substantial headwinds toward becoming the standard of care without demonstrating cost effectiveness. Furthermore, many SNF directors might suggest that hospitals and hospitalists working with them to address fundamental (but much more intransigent) problems in SNFs, such as high staff turnover, low concentration of highly skilled staff (RNs and MDs), regulatory burden, and hospitals using SNFs like stepdown units, could represent a generalizable and sustainable solution.
We realize that this argument is tricky for hospitalists because its underlying logic (care has become too complex, patients are too sick, and dedicated personnel are needed) also played a major role in establishing our existence. One possibility is that like hospitalists, NPs and a growing cadre of “SNFists” will become major drivers of quality improvement, education, and leadership locally at these facilities, thereby leading to sustainable change.8 Similarly, current conditions may drive recognition that a specific set of skills is required to function effectively in the SNF environment,9 just as we believe hospitalists need unique skills to excel in today’s hospital environment.
Studies such as that of Rosen et al. are valuable for JHM because they prompt us to recognize that we as hospitalists have much to share and learn from nursing homes and the dedicated practitioners who work there. In fact, we argue that few places in the healthcare system are more in need of innovation than hospital–nursing home relationships, and hospitalists do not just have a vested clinical interest; in many ways, we see a mirror of our own development as a “specialty.” We encourage hospitals and hospitalists to take up this challenge on behalf of some of the most vulnerable patients in our system during critical times in their care trajectory. As the Commission for Long-Term Care (www.ltccommission.org) wrote in its final report to Congress: “The need is great. The time to act is now.”
Disclosures
Dr. Burke is supported by a VA Health Services Research and Development (HSR&D) Career Development Award. All opinions are those of the authors and do not necessarily represent those of the Department of Veterans Affairs. Dr. Greysen has nothing to disclose.
1. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: Implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. 10.3810/hp.2012.02.958. PubMed
2. Rosen BT, Halbert RJ, Hart K, Diniz MA, Isonaka S, Black JT. The enhanced care program: Impact of a care transitions program on 30-day hospital readmissions for patients discharged from an acute care facility to skilled nursing facilities. J Hosp Med. 2018;13(4):229-235.
3. Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. 10.12788/jhm.2710. PubMed
4. Kane RL, Keckhafer G, Flood S, Bershadsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc. 2003;51(10):1427-1434. 10.1046/j.1532-5415.2003.51461.x. PubMed
5. Ingber MJ, Feng Z, Khatutsky G, et al. Initiative to reduce avoidable hospitalizations among nursing facility residents shows promising results. Health Aff Proj Hope. 2017;36(3):441-450. 10.1377/hlthaff.2016.1310. PubMed
6. Kane RL, Huckfeldt P, Tappen R, et al. Effects of an intervention to reduce hospitalizations from nursing homes: A randomized implementation trial of the INTERACT Program. JAMA Intern Med. 2017;177(9):1257-1264. 10.1001/jamainternmed.2017.2657. PubMed
7. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. 10.1111/jgs.13351. PubMed
8. Ryskina KL, Polsky D, Werner RM. Physicians and advanced practitioners specializing in nursing home care. JAMA. 2017;318(20):2040-2042. 10.1001/jama.2017.13378. PubMed
9. Gillespie SM, Levy CR, Katz PR. What exactly is an “SNF-ist?” JAMA Intern Med. 2018;178(1):153-154. 10.1001/jamainternmed.2017.7212. PubMed
The landscape of postacute care in skilled nursing facilities (SNFs) in the United States is evolving. As the population ages, a growing number of elderly persons are being discharged to SNFs at an enormous cost and with clear evidence of disappointing outcomes. The reaction to these trends includes payment reforms that “bundle” hospital and postacute care, act as incentives to discourage SNFs, or penalize SNFs for undesired patient outcomes. Hospitalists are expected to increasingly feel the effect of these reforms.1
Thus, hospitals are demonstrating renewed interest in reducing readmissions from SNFs. In this issue of Journal of Hospital Medicine, Rosen and colleagues present the results of the Enhanced Care Program (ECP), a multicomponent intervention consisting of 9 nurse practitioners (NPs), a pharmacist, a pharmacy technician, a nurse educator, a program administrator, and a medical director.2 These providers are deployed to 8 SNFs around a large teaching hospital, providing direct clinical care as well as 24/7 call availability for enrolled patients, robust medication reconciliation, and monthly education for SNF nursing staff. A unique aspect of this model was that individual attending physicians in the associated SNFs could decide whether to enroll their patients in the model; patients not enrolled represented a contemporaneous control cohort. The authors found a nearly 30% reduction in the odds of 30-day readmission (OR 0.71 [0.60–0.85] after adjustment), which was robust to multiple sensitivity analyses, including a propensity-matched cohort comparison. The authors should be commended for working to mitigate these potential confounders, thereby strengthening their conclusions. Such a large reduction in readmissions reflects their high underlying prevalence (23% in the nonintervention cohort).
This report closely follows the evaluation of a similar program at the Cleveland Clinic called Connected Care Model (CCM), in which 4 physicians and 5 NPs or physician assistants provided care, including 24/7 call availability, in 7 associated SNFs.3 In a retrospective pre-post analysis comparing the 30-day readmission rates of these SNFs with those of others in the network, similar reductions in readmissions were observed. ECP and CCM represent important extensions of a much larger body of evidence, from the Evercare model4 to the Initiative to Reduce Avoidable Hospitalizations demonstration project, which suggests that adding NPs to nursing homes reduces hospitalizations.5
However, several factors have to be considered before disseminating ECP or CCM. First, other promising “proof of concept” quality improvement studies were not efficacious when rigorously tested in nursing homes.6 Second, these programs are representative of large academic medical centers, which may establish different relationships with different SNFs compared with smaller or less well-resourced hospitals. As the Initiative to Reduce Hospitalizations demonstrated, even a fundamentally similar intervention can have extremely different results depending on the nursing homes involved,5 and the science behind establishing effective hospital–SNF partnerships is still in its infancy.7 Third, both studies have significant methodological limitations, including most importantly that they are conducted within SNFs selected to be part of their hospitals’ network.
These significant early efforts also present an opportunity to reconsider the underlying assumption of these models: that adding more supervisory clinicians to SNFs is the right approach to reduce hospitalizations. Although adding resources is an attractive “plug and play” solution for many problems in healthcare delivery, placing only 1 NP in each of the 15,583 certified nursing facilities in the United States would employ fully 10% of the entire NP workforce. Amid rising concerns about costs related to our aging population, these interventions face substantial headwinds toward becoming the standard of care without demonstrating cost effectiveness. Furthermore, many SNF directors might suggest that hospitals and hospitalists working with them to address fundamental (but much more intransigent) problems in SNFs, such as high staff turnover, low concentration of highly skilled staff (RNs and MDs), regulatory burden, and hospitals using SNFs like stepdown units, could represent a generalizable and sustainable solution.
We realize that this argument is tricky for hospitalists because its underlying logic (care has become too complex, patients are too sick, and dedicated personnel are needed) also played a major role in establishing our existence. One possibility is that like hospitalists, NPs and a growing cadre of “SNFists” will become major drivers of quality improvement, education, and leadership locally at these facilities, thereby leading to sustainable change.8 Similarly, current conditions may drive recognition that a specific set of skills is required to function effectively in the SNF environment,9 just as we believe hospitalists need unique skills to excel in today’s hospital environment.
Studies such as that of Rosen et al. are valuable for JHM because they prompt us to recognize that we as hospitalists have much to share and learn from nursing homes and the dedicated practitioners who work there. In fact, we argue that few places in the healthcare system are more in need of innovation than hospital–nursing home relationships, and hospitalists do not just have a vested clinical interest; in many ways, we see a mirror of our own development as a “specialty.” We encourage hospitals and hospitalists to take up this challenge on behalf of some of the most vulnerable patients in our system during critical times in their care trajectory. As the Commission for Long-Term Care (www.ltccommission.org) wrote in its final report to Congress: “The need is great. The time to act is now.”
Disclosures
Dr. Burke is supported by a VA Health Services Research and Development (HSR&D) Career Development Award. All opinions are those of the authors and do not necessarily represent those of the Department of Veterans Affairs. Dr. Greysen has nothing to disclose.
The landscape of postacute care in skilled nursing facilities (SNFs) in the United States is evolving. As the population ages, a growing number of elderly persons are being discharged to SNFs at an enormous cost and with clear evidence of disappointing outcomes. The reaction to these trends includes payment reforms that “bundle” hospital and postacute care, act as incentives to discourage SNFs, or penalize SNFs for undesired patient outcomes. Hospitalists are expected to increasingly feel the effect of these reforms.1
Thus, hospitals are demonstrating renewed interest in reducing readmissions from SNFs. In this issue of Journal of Hospital Medicine, Rosen and colleagues present the results of the Enhanced Care Program (ECP), a multicomponent intervention consisting of 9 nurse practitioners (NPs), a pharmacist, a pharmacy technician, a nurse educator, a program administrator, and a medical director.2 These providers are deployed to 8 SNFs around a large teaching hospital, providing direct clinical care as well as 24/7 call availability for enrolled patients, robust medication reconciliation, and monthly education for SNF nursing staff. A unique aspect of this model was that individual attending physicians in the associated SNFs could decide whether to enroll their patients in the model; patients not enrolled represented a contemporaneous control cohort. The authors found a nearly 30% reduction in the odds of 30-day readmission (OR 0.71 [0.60–0.85] after adjustment), which was robust to multiple sensitivity analyses, including a propensity-matched cohort comparison. The authors should be commended for working to mitigate these potential confounders, thereby strengthening their conclusions. Such a large reduction in readmissions reflects their high underlying prevalence (23% in the nonintervention cohort).
This report closely follows the evaluation of a similar program at the Cleveland Clinic called Connected Care Model (CCM), in which 4 physicians and 5 NPs or physician assistants provided care, including 24/7 call availability, in 7 associated SNFs.3 In a retrospective pre-post analysis comparing the 30-day readmission rates of these SNFs with those of others in the network, similar reductions in readmissions were observed. ECP and CCM represent important extensions of a much larger body of evidence, from the Evercare model4 to the Initiative to Reduce Avoidable Hospitalizations demonstration project, which suggests that adding NPs to nursing homes reduces hospitalizations.5
However, several factors have to be considered before disseminating ECP or CCM. First, other promising “proof of concept” quality improvement studies were not efficacious when rigorously tested in nursing homes.6 Second, these programs are representative of large academic medical centers, which may establish different relationships with different SNFs compared with smaller or less well-resourced hospitals. As the Initiative to Reduce Hospitalizations demonstrated, even a fundamentally similar intervention can have extremely different results depending on the nursing homes involved,5 and the science behind establishing effective hospital–SNF partnerships is still in its infancy.7 Third, both studies have significant methodological limitations, including most importantly that they are conducted within SNFs selected to be part of their hospitals’ network.
These significant early efforts also present an opportunity to reconsider the underlying assumption of these models: that adding more supervisory clinicians to SNFs is the right approach to reduce hospitalizations. Although adding resources is an attractive “plug and play” solution for many problems in healthcare delivery, placing only 1 NP in each of the 15,583 certified nursing facilities in the United States would employ fully 10% of the entire NP workforce. Amid rising concerns about costs related to our aging population, these interventions face substantial headwinds toward becoming the standard of care without demonstrating cost effectiveness. Furthermore, many SNF directors might suggest that hospitals and hospitalists working with them to address fundamental (but much more intransigent) problems in SNFs, such as high staff turnover, low concentration of highly skilled staff (RNs and MDs), regulatory burden, and hospitals using SNFs like stepdown units, could represent a generalizable and sustainable solution.
We realize that this argument is tricky for hospitalists because its underlying logic (care has become too complex, patients are too sick, and dedicated personnel are needed) also played a major role in establishing our existence. One possibility is that like hospitalists, NPs and a growing cadre of “SNFists” will become major drivers of quality improvement, education, and leadership locally at these facilities, thereby leading to sustainable change.8 Similarly, current conditions may drive recognition that a specific set of skills is required to function effectively in the SNF environment,9 just as we believe hospitalists need unique skills to excel in today’s hospital environment.
Studies such as that of Rosen et al. are valuable for JHM because they prompt us to recognize that we as hospitalists have much to share and learn from nursing homes and the dedicated practitioners who work there. In fact, we argue that few places in the healthcare system are more in need of innovation than hospital–nursing home relationships, and hospitalists do not just have a vested clinical interest; in many ways, we see a mirror of our own development as a “specialty.” We encourage hospitals and hospitalists to take up this challenge on behalf of some of the most vulnerable patients in our system during critical times in their care trajectory. As the Commission for Long-Term Care (www.ltccommission.org) wrote in its final report to Congress: “The need is great. The time to act is now.”
Disclosures
Dr. Burke is supported by a VA Health Services Research and Development (HSR&D) Career Development Award. All opinions are those of the authors and do not necessarily represent those of the Department of Veterans Affairs. Dr. Greysen has nothing to disclose.
1. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: Implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. 10.3810/hp.2012.02.958. PubMed
2. Rosen BT, Halbert RJ, Hart K, Diniz MA, Isonaka S, Black JT. The enhanced care program: Impact of a care transitions program on 30-day hospital readmissions for patients discharged from an acute care facility to skilled nursing facilities. J Hosp Med. 2018;13(4):229-235.
3. Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. 10.12788/jhm.2710. PubMed
4. Kane RL, Keckhafer G, Flood S, Bershadsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc. 2003;51(10):1427-1434. 10.1046/j.1532-5415.2003.51461.x. PubMed
5. Ingber MJ, Feng Z, Khatutsky G, et al. Initiative to reduce avoidable hospitalizations among nursing facility residents shows promising results. Health Aff Proj Hope. 2017;36(3):441-450. 10.1377/hlthaff.2016.1310. PubMed
6. Kane RL, Huckfeldt P, Tappen R, et al. Effects of an intervention to reduce hospitalizations from nursing homes: A randomized implementation trial of the INTERACT Program. JAMA Intern Med. 2017;177(9):1257-1264. 10.1001/jamainternmed.2017.2657. PubMed
7. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. 10.1111/jgs.13351. PubMed
8. Ryskina KL, Polsky D, Werner RM. Physicians and advanced practitioners specializing in nursing home care. JAMA. 2017;318(20):2040-2042. 10.1001/jama.2017.13378. PubMed
9. Gillespie SM, Levy CR, Katz PR. What exactly is an “SNF-ist?” JAMA Intern Med. 2018;178(1):153-154. 10.1001/jamainternmed.2017.7212. PubMed
1. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: Implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. 10.3810/hp.2012.02.958. PubMed
2. Rosen BT, Halbert RJ, Hart K, Diniz MA, Isonaka S, Black JT. The enhanced care program: Impact of a care transitions program on 30-day hospital readmissions for patients discharged from an acute care facility to skilled nursing facilities. J Hosp Med. 2018;13(4):229-235.
3. Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. 10.12788/jhm.2710. PubMed
4. Kane RL, Keckhafer G, Flood S, Bershadsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc. 2003;51(10):1427-1434. 10.1046/j.1532-5415.2003.51461.x. PubMed
5. Ingber MJ, Feng Z, Khatutsky G, et al. Initiative to reduce avoidable hospitalizations among nursing facility residents shows promising results. Health Aff Proj Hope. 2017;36(3):441-450. 10.1377/hlthaff.2016.1310. PubMed
6. Kane RL, Huckfeldt P, Tappen R, et al. Effects of an intervention to reduce hospitalizations from nursing homes: A randomized implementation trial of the INTERACT Program. JAMA Intern Med. 2017;177(9):1257-1264. 10.1001/jamainternmed.2017.2657. PubMed
7. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. 10.1111/jgs.13351. PubMed
8. Ryskina KL, Polsky D, Werner RM. Physicians and advanced practitioners specializing in nursing home care. JAMA. 2017;318(20):2040-2042. 10.1001/jama.2017.13378. PubMed
9. Gillespie SM, Levy CR, Katz PR. What exactly is an “SNF-ist?” JAMA Intern Med. 2018;178(1):153-154. 10.1001/jamainternmed.2017.7212. PubMed
© 2018 Society of Hospital Medicine
Declining androgen levels correlated with increased frailty
CHICAGO – according to a study presented at the annual meeting of the Endocrine Society.
The findings provide more evidence of the relationship between function and lower levels of androgens such as testosterone and introduce the possibility of using hormones to offset frailty in older patients.
“The decline in total and free [testosterone] and DHEA-S [dehydroepiandrosterone sulfate] was significantly associated with small deteriorations in physical function and worsening frailty,” according to presenter Frederick C. Wu, MD, an endocrinologist at the University of Manchester (England). “The present results are consistent with and support our hypothesis that the decline in these hormones can contribute to a worsening physical function and frailty in the elderly.”
Investigators gathered data on 2,278 men from eight centers across Europe to conduct an observational study of their physical functions.
Patients were all men, an average of 58 years old, and had an average body mass index of 27.6 kg/m2 and average free testosterone and DHEA-S levels at 16.9 nmol/L and 4.7 micromol/L, respectively.
At follow-up, which was on average conducted 4.4 years later, average age was 63 years, and average testosterone and DHEA-S levels had dropped to 287.3 nmol/L and 4 micromol/L respectively, which Dr. Wu described as a moderate drop.
Decreases of free testosterone or DHEA-S by one standard deviation – 86.8 nmol/L and 2.6 micromol/L, respectively – accounted for 11%-17% of the average rate of deterioration of physical function, according to Dr. Wu.
Patients with lower levels of free testosterone and DHEA-S experienced worsening 15-meter walk time, five chair-stands, physical quality of life, and overall worsening of frailty phenotypes at follow up.
Dr. Wu and his colleagues measured frailness in patients by looking for the presence of frailty phenotypes, which include slowness, sarcopenia, exhaustion, low activity, and weakness.
If one-two of these criteria were present, patients would be considered “prefrail,” and if three or more were present, patients would be deemed “frail.”
Patients experienced an average 2.5% increase in frailty per year during the time between baseline and follow up, Dr. Wu told attendees.
Investigators used the mean of 60 years old to adjust for age, Dr. Wu explained in response to a question from the audience; however, this may have been an overadjustment as free testosterone and DHEA-S are age dependent, Dr. Wu admitted.
Investigators also incorporated a frailty index of 39 health deficits – 16 physical or cognitive, 11 comorbidities, and 12 clinical – measuring on a 0-1 scale in order to measure different levels of frailty.
While the link between these androgens and frailty are evident, the potential benefits of hormonal intervention in elderly men are still in the air and demand further study.
“The decline in androgen levels in the physiological range, because of the modest degree of change, is unlikely to be the single greatest cause of deterioration in the majority of aging men in the population,” said Dr. Wu. “Therefore, the possible therapeutic roles of androgens in improving physical health may be limited to a minority of men with very low levels of testosterone.”
Dr. Wu is on the advisory board of Bayer-Schering, Eli Lilly, and Besins Healthcare. Dr. Wu is a research supporter or consultant for Repros Therapeutics, Merck Serono, and Mereo Biopharma. Investigators reported no additional relevant financial disclosures.
SOURCE: Wu F C et al. ENDO 2018, Abstract OR15-1.
CHICAGO – according to a study presented at the annual meeting of the Endocrine Society.
The findings provide more evidence of the relationship between function and lower levels of androgens such as testosterone and introduce the possibility of using hormones to offset frailty in older patients.
“The decline in total and free [testosterone] and DHEA-S [dehydroepiandrosterone sulfate] was significantly associated with small deteriorations in physical function and worsening frailty,” according to presenter Frederick C. Wu, MD, an endocrinologist at the University of Manchester (England). “The present results are consistent with and support our hypothesis that the decline in these hormones can contribute to a worsening physical function and frailty in the elderly.”
Investigators gathered data on 2,278 men from eight centers across Europe to conduct an observational study of their physical functions.
Patients were all men, an average of 58 years old, and had an average body mass index of 27.6 kg/m2 and average free testosterone and DHEA-S levels at 16.9 nmol/L and 4.7 micromol/L, respectively.
At follow-up, which was on average conducted 4.4 years later, average age was 63 years, and average testosterone and DHEA-S levels had dropped to 287.3 nmol/L and 4 micromol/L respectively, which Dr. Wu described as a moderate drop.
Decreases of free testosterone or DHEA-S by one standard deviation – 86.8 nmol/L and 2.6 micromol/L, respectively – accounted for 11%-17% of the average rate of deterioration of physical function, according to Dr. Wu.
Patients with lower levels of free testosterone and DHEA-S experienced worsening 15-meter walk time, five chair-stands, physical quality of life, and overall worsening of frailty phenotypes at follow up.
Dr. Wu and his colleagues measured frailness in patients by looking for the presence of frailty phenotypes, which include slowness, sarcopenia, exhaustion, low activity, and weakness.
If one-two of these criteria were present, patients would be considered “prefrail,” and if three or more were present, patients would be deemed “frail.”
Patients experienced an average 2.5% increase in frailty per year during the time between baseline and follow up, Dr. Wu told attendees.
Investigators used the mean of 60 years old to adjust for age, Dr. Wu explained in response to a question from the audience; however, this may have been an overadjustment as free testosterone and DHEA-S are age dependent, Dr. Wu admitted.
Investigators also incorporated a frailty index of 39 health deficits – 16 physical or cognitive, 11 comorbidities, and 12 clinical – measuring on a 0-1 scale in order to measure different levels of frailty.
While the link between these androgens and frailty are evident, the potential benefits of hormonal intervention in elderly men are still in the air and demand further study.
“The decline in androgen levels in the physiological range, because of the modest degree of change, is unlikely to be the single greatest cause of deterioration in the majority of aging men in the population,” said Dr. Wu. “Therefore, the possible therapeutic roles of androgens in improving physical health may be limited to a minority of men with very low levels of testosterone.”
Dr. Wu is on the advisory board of Bayer-Schering, Eli Lilly, and Besins Healthcare. Dr. Wu is a research supporter or consultant for Repros Therapeutics, Merck Serono, and Mereo Biopharma. Investigators reported no additional relevant financial disclosures.
SOURCE: Wu F C et al. ENDO 2018, Abstract OR15-1.
CHICAGO – according to a study presented at the annual meeting of the Endocrine Society.
The findings provide more evidence of the relationship between function and lower levels of androgens such as testosterone and introduce the possibility of using hormones to offset frailty in older patients.
“The decline in total and free [testosterone] and DHEA-S [dehydroepiandrosterone sulfate] was significantly associated with small deteriorations in physical function and worsening frailty,” according to presenter Frederick C. Wu, MD, an endocrinologist at the University of Manchester (England). “The present results are consistent with and support our hypothesis that the decline in these hormones can contribute to a worsening physical function and frailty in the elderly.”
Investigators gathered data on 2,278 men from eight centers across Europe to conduct an observational study of their physical functions.
Patients were all men, an average of 58 years old, and had an average body mass index of 27.6 kg/m2 and average free testosterone and DHEA-S levels at 16.9 nmol/L and 4.7 micromol/L, respectively.
At follow-up, which was on average conducted 4.4 years later, average age was 63 years, and average testosterone and DHEA-S levels had dropped to 287.3 nmol/L and 4 micromol/L respectively, which Dr. Wu described as a moderate drop.
Decreases of free testosterone or DHEA-S by one standard deviation – 86.8 nmol/L and 2.6 micromol/L, respectively – accounted for 11%-17% of the average rate of deterioration of physical function, according to Dr. Wu.
Patients with lower levels of free testosterone and DHEA-S experienced worsening 15-meter walk time, five chair-stands, physical quality of life, and overall worsening of frailty phenotypes at follow up.
Dr. Wu and his colleagues measured frailness in patients by looking for the presence of frailty phenotypes, which include slowness, sarcopenia, exhaustion, low activity, and weakness.
If one-two of these criteria were present, patients would be considered “prefrail,” and if three or more were present, patients would be deemed “frail.”
Patients experienced an average 2.5% increase in frailty per year during the time between baseline and follow up, Dr. Wu told attendees.
Investigators used the mean of 60 years old to adjust for age, Dr. Wu explained in response to a question from the audience; however, this may have been an overadjustment as free testosterone and DHEA-S are age dependent, Dr. Wu admitted.
Investigators also incorporated a frailty index of 39 health deficits – 16 physical or cognitive, 11 comorbidities, and 12 clinical – measuring on a 0-1 scale in order to measure different levels of frailty.
While the link between these androgens and frailty are evident, the potential benefits of hormonal intervention in elderly men are still in the air and demand further study.
“The decline in androgen levels in the physiological range, because of the modest degree of change, is unlikely to be the single greatest cause of deterioration in the majority of aging men in the population,” said Dr. Wu. “Therefore, the possible therapeutic roles of androgens in improving physical health may be limited to a minority of men with very low levels of testosterone.”
Dr. Wu is on the advisory board of Bayer-Schering, Eli Lilly, and Besins Healthcare. Dr. Wu is a research supporter or consultant for Repros Therapeutics, Merck Serono, and Mereo Biopharma. Investigators reported no additional relevant financial disclosures.
SOURCE: Wu F C et al. ENDO 2018, Abstract OR15-1.
REPORTING FROM ENDO 2018
Key clinical point: Declining androgen levels correlates with lower physical function in elder men.
Major finding: Decline in testosterone by one standard deviation accounted for 11%-17% of the average population rate of physical function deterioration.
Data source: Prospective study of 2,278 men gathered from eight European centers.
Disclosures: Frederick C. Wu is on the advisory board of Bayer-Schering, Eli Lilly, and Besins Healthcare. Dr. Wu is a research supporter or consultant for Repros Therapeutics, Merck Serono, and Mereo Biopharma.
Source: Wu F C et al. ENDO 2018 OR15-1.
Hospitalist Value in an ACO World
The accountable care organization (ACO) concept, elucidated in 2006 as the development of partnerships between hospitals and physicians to coordinate and deliver efficient care,1 seeks to remove existing barriers to improving value.2 Some advocate this concept as a promising payment model that could successfully realign the current payment system to financially reward improvements in quality and efficiency that bend the cost curve.3,4 Hospitalists fit well with this philosophy. As the fastest growing medical specialty in the history of American medicine, from a couple of thousand hospitalists in the mid-1990s to more than 50,000, the remarkable progression of hospitalists has ostensibly been driven partially by hospitals’ efforts to improve the value equation through enhanced efficiency in inpatient care. Importantly, hospitalists probably provide care for more than half of all hospitalized Medicare beneficiaries and increasingly patients in skilled nursing facilities (ie, SNFists).5 Along with primary care physicians, hospitalists thus represent an essential group of physicians needed to transform care delivery.
RAPID GROWTH AND THE FUTURE OF ACOs
When the Affordable Care Act (ACA) established the Medicare Shared Savings Program (MSSP), ACOs leaped from being an intellectual concept1,2 into a pragmatic health system strategy.3,4 Following Medicare, various private health insurance plans and some state Medicaid programs entered into contracts with groups of healthcare providers (hospitals, physicians, or health systems) to serve as ACOs for their insured enrollees.6 Leavitt Partners’ ACO tracking database showed that the number of ACOs increased from 157 in March of 2012 to 782 in December of 2015.7
Until recently, the federal government’s commitment to having 50% of total Medicare spending via value-based payment models by 2018, coupled with endorsement from state Medicaid programs and commercial insurers, demonstrated strong support for continuation of ACOs. Unexpectedly on August 15, 2017, the Centers for Medicare & Medicaid Services (CMS) outlined a plan in its proposed rulemaking to cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment model, which were scheduled to commence on January 1, 2018. CMS also plans to scale back the mandatory Comprehensive Care for Joint Replacement (CCJR) bundled payment model from 67 selected geographic areas to 34. Although this proposed rulemaking created some equipoise in the healthcare industry regarding the future of value-based reimbursement approaches, cost containment and improved efficiency remain as major focuses of the federal government’s healthcare effort. Notably, CMS offers providers that are newly excluded from the CCJR model the opportunity to voluntarily participate in the program and is expected to increase opportunities for providers to participate in voluntary rather than large-scale mandatory episode payment model initiatives. In 2018, the agency also plans to develop new voluntary bundled payment models that will meet criteria to be considered an advanced alternative payment model for Quality Payment Program purposes.
Importantly, the value-based reimbursement movement was well underway before ACA legislation. Through ACA health reform, value-based reimbursement efforts were expanded through ACOs, bundled payments, value-based purchasing, the CMS Innovation Center and other initiatives. With health systems having an overflowing plate of activities, a wait-and-see attitude might seem reasonable at first. However, being unprepared for the inevitable shift to value-based reimbursement and reduced fee-for-service revenue places an organization at risk. A successful ACO requires system-level transformation, especially cultural and structural changes to achieve clinical integration. Being embedded in health system delivery, hospitalists can help shape a team-oriented culture and foster success in value-based payment models. This requires hospitalists to take a more active role in assessing and striking a balance between high-quality, cost-efficient care and financial risk inherent in ACO models.
WHAT HOSPITALISTS NEED TO KNOW ABOUT ACOs
The key to hospitalists fulfilling their value creation potential and becoming enablers for ACO success lies in developing a thorough understanding of the aspects of an ACO that promote efficient and effective care, while accounting for financial factors. Fundamentally, the ACO concept combines provider payment and delivery system reforms. Specifically, the definition of an ACO contains 3 factors: (1) a local healthcare organization (eg, hospital or multispecialty group of physicians) with a related set of providers that (2) can be held accountable for the cost and quality of care delivered to (3) a defined population. While the notion of accountability is not new, the locus of accountability is changed in the ACO model—emphasizing accountability at the level of actual care delivery with documentation of quality and cost outcomes. The ACO approach aims to address multiple, frequent, and recurring problems, including lack of financial incentives to improve quality and reduce cost, as well as the negative consequences of a pay-for-volume system—uncoordinated and fragmented care, overutilization of unnecessary tests and treatments, and poor patient experience all manifested as unwarranted geographic variation in practice patterns, clinical outcomes, and health spending. Participants in an ACO are rewarded financially if they can slow the growth of their patients’ healthcare costs while maintaining or improving the quality of care delivered. To succeed in this ACO world, hospitalists must assume greater prudence in the use of healthcare services while improving (or at a minimum, maintaining) patient outcomes, thus excising avoidable waste across the continuum of care.
More than half of ACOs include a hospital.8 However, whether hospital-led ACOs possess an advantage remains to be elucidated. Early reports indicated that physician-led ACOs saved more money.9,10 However, others argue that hospitals11 are better capitalized, have greater capacity for data sharing, and possess economies of scale that allow them to invest in more advanced technology, such as predictive modeling and/or simulation software. Such analytics can identify high-cost patients (ie, multiple comorbidities), super utilizers and populations lacking care, allowing ACOs to implement preventive measures to reduce unnecessary utilization. Recently released CMS MSSP 2016 performance data12 showed that nearly half (45%) of physician-only ACOs earned shared savings, whereas 23% of ACOs that include hospitals earned shared savings. However, among all the ACOs that achieved savings, ACO entities that include hospitals generated the highest amount of shared savings (eg, Advocate, Hackensack Alliance, Cleveland Clinic, and AMITA Health). Notably, hospital-led ACOs tend to have much larger beneficiary populations than physician-led ACOs, which may create a scenario of higher risk but higher potential reward.
HOW HOSPITALISTS CONTRIBUTE VALUE TO ACO SUCCESS
The emphasis on value over volume inherent in the development of ACOs occurs through employing care strategies implemented through changes in policies, and eventual structural and cultural changes. These changes require participating organizations to possess certain key competencies, including the following: 1) leadership that facilitates change; 2) organizational culture of teamwork; 3) collaborative relationships among providers; 4) information technology infrastructure for population management and care coordination; 5) infrastructure for monitoring, managing, and reporting quality; 6) ability to manage financial risk; 7) ability to receive and distribute payments or savings; and 8) resources for patient education and support.2,3,13-16 Table 1 summarizes the broad range of roles that hospitalists can serve in delivering care to ACO populations.17-19
Hospitalists’ active pursuit of nonclinical training and selection for administrative positions demonstrate their proclivity to provide these competencies. In addition to full-time clinician hospitalists, who can directly influence the delivery of high-value care to patients, hospitalists serve many other roles in hospitals and each can contribute differently based on their specialized expertise. Examples include the success of the Society of Hospital Medicine’s Leadership Academy; the acknowledged expertise of hospitalists in quality improvement (QI), informatics, teamwork, patient experience, care coordination and utilization; and advancement of hospitalists to senior leadership positions (eg, CQO, CMO, CEO). Given that nearly a third of healthcare expenditures are for hospital care,20 hospitalists are in a unique position to foster ACO competencies while impacting the quality of care episodes associated with an index hospital stay.
Importantly, hospitalists cannot act as gatekeepers to restrict care. Managed care organizations and health maintenance organizations use of this approach in the 1990s to limit access to services in order to reduce costs led to unacceptable outcomes and numerous malpractice lawsuits. ACOs should aspire to deliver the most cost-effective high-quality care, and their performance should be monitored to ensure that they provide recommended services and timely access. The Medicare ACO contract holds the provider accountable for meeting 34 different quality measures (Supplemental Table 1), and hospitalists can influence outcomes for the majority. Especially through hospital and health system QI initiatives, hospitalists can directly impact and share accountability for measures ranging from care coordination to implementation of evidence-based care (eg, ACE inhibitors and beta blockers for heart failure) to patient and family caregiver experience.
Aligned with Medicare ACO quality measures, 5 high-impact target areas were identified for ACOs21: (1) Prevention and wellness; (2) Chronic conditions/care management; (3) Reduced hospitalizations; (4) Care transitions across the fragmented system; and (5) Multispecialty care coordination of complex patients. One essential element of a successful ACO is the ability to implement evidence-based medical guidelines and/or practices across the continuum of care for selected targeted initiatives. Optimizing care coordination/continuum requires team-based care, and hospitalists already routinely collaborate with nurses, social workers, case managers, pharmacists, and other stakeholders such as dieticians and physical therapists on inpatient care. Hospitalists are also experienced in facilitating communication and improving integration and coordination efficiencies among primary care providers and specialists, and between hospital care and post-acute care, as they coordinate post-hospital care and follow-up. This provides an opportunity to lead health system care coordination efforts, especially for complex and/or high-risk patients.22,23 CMS MSSP 2016 performance data12 showed that ACOs achieving shared savings had a decline in inpatient expenditures and utilization across several facility types (hospital, SNF, rehabilitation, long term). Postacute care management is critical to earning shared savings; SNF and Home Health expenditures fell by 18.3% and 9.7%, respectively, on average. We believe that hospitalists can have more influence over these cost areas by influencing treatment of hospitalized patients in a timely manner, discharge coordination, and selection of appropriate disposition locations. Hospitalists also play an integral role in ensuring the hospital performs well on quality metrics, including 30-day readmissions, hospital acquired conditions, and patient satisfaction. Examples below document the effectiveness of hospitalists in this new ACO era.
Care Transitions/Coordination
Before the Hospital Readmission Reduction Program (HRRP) delineated in the ACA, hospitalists developed Project BOOST (Better Outcomes by Optimizing Care Transitions) to improve hospital discharge care transition. The evidence-based foundation of this project led CMS to list Project BOOST as an example program that can reduce readmissions.24 Through the dissemination and mentored implementation of Project BOOST to over 200 hospitals across the United States,25 hospitalists contributed to the marked reduction in hospital readmission occurring since 2010.26 Although hospital medicine began as a practice specific to the hospital setting, hospitalists’ skills generated growing demand for them in postacute facilities. SNF residents commonly come from hospitals postdischarge and suffer from multiple comorbidities and limitations in activities of daily living. Not surprisingly, SNF residents experience high rates of rehospitalizations.27 Hospitalists can serve as a bridge between hospitals and SNFs and optimize this transition process to yield improved outcomes. Industry experts endorse this approach.28 A recent study demonstrated a significant reduction in readmissions in 1 SNF (32.3% to 16.1%, odds ratio = 0.403, P < .001), by having a hospitalist-led team follow patients discharged from the hospital.29
Chronic Conditions Management/High-Risk Patients
Interest in patients with multiple chronic comorbidities and social issues intensifies as healthcare systems focus limited resources on these high-risk patients to prevent the unnecessary use of costly services.30,31 As health systems assume financial risk for health outcomes and costs of designated patient groups, they undertake efforts to understand the population they serve. Such efforts aim to identify patients with established high utilization patterns (or those at risk for high utilization). This knowledge enables targeted actions to provide access, treatment, and preventive interventions to avoid unneeded emergency and hospital services. Hospitalists commonly care for these patients and are positioned to lead the implementation of patient risk assessment and stratification, develop patient-centered care models across care settings, and act as a liaison with primary care. For frail elderly and seriously ill patients, the integration of hospitalists into palliative care provides several opportunities for improving the quality of care at the end of life.32 As patients and their family caregivers commonly do not address goals of care until faced with a life-threatening condition in the hospital, hospitalists represent ideal primary palliative care physicians to initiate these conversations.33 A hospitalist communicating with a patient and/or their family caregiver about alleviating symptoms and clarifying patients’ preferences for care often yields decreases in ineffective healthcare utilization and better patient outcomes. The hospitalists’ ability to communicate with other providers within the hospital setting also allows them to better coordinate interdisciplinary care and prevent unnecessary and ineffective treatments and procedures.
De-Implementation/Waste Reduction
The largest inefficiencies in healthcare noted in the National Academy of Medicine report, Demanding Value from Our Health Care (2012), are failure to deliver known beneficial therapies or providing unnecessary or nonevidenced based services that do not improve outcomes, but come with associated risk and cost.34 “De-implementation” of unnecessary diagnostic tests or ineffective or even harmful treatments by hospitalists represents a significant opportunity to reduce costs while maintaining or even improving the quality of care. The Society of Hospital Medicine joined the Choosing Wisely® campaign and made 5 recommendations in adult care as an explicit starting point for eliminating waste in the hospital in 2013.35 Since then, hospitalists have participated in multiple successful efforts to address overutilization of care; some published results include the following:
- decreased frequency of unnecessary common labs through a multifaceted hospitalist QI intervention;36
- reduced length of stay and cost by appropriate use of telemetry;37 and
- reduced unnecessary radiology testing by providing physicians with individualized audit and feedback reports.38
CONCLUSION
Hundreds of ACOs now exist across the US, formed by a variety of providers including hospitals, physician groups, and integrated delivery systems. Provider groups range in size from primary care-focused physician groups with a handful of offices to large, multistate integrated delivery systems with dozens of hospitals and hundreds of office locations. Evaluations of ACO outcomes reveal mixed results.9,39-53 Admittedly, assessments attempting to compare the magnitude of savings across ACO models are difficult given the variation in size, variability in specific efforts to influence utilization, and substantial turnover among participating beneficiaries.54 Nonetheless, a newly published Office of Inspector General report55 showed that most Medicare ACOs reduced spending and improved care quality (82% of the individual quality measures) over the first 3 years of the program, and savings increased with duration of an ACO program. The report also noted that considerable time and managerial resources are required to implement changes to improve quality and lower costs. While the political terrain ostensibly supports value-based care and the need to diminish the proportion of our nation’s gross domestic product dedicated to healthcare, health systems are navigating an environment that still largely rewards volume. Hospitalists may be ideal facilitators for this transitional period as they possess the clinical experience caring for complex patients with multiple comorbidities and quality improvement skills to lead efforts in this new ACO era.
Disclosures
The authors have nothing to disclose.
1. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff(Project Hope). 2007;26(1):w44-w57. PubMed
2. Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff(Project Hope). 2009;28(2):w219-w231. PubMed
3. McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff(Project Hope). 2010;29(5):982-990. PubMed
4. Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753-1756. PubMed
5. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
6. Kennedy K. Health Care Providers Embracing Cost-saving Groups. USA Today, July 24, 2011.
7. Leavitt Partners. Available at http://leavittpartners.com, April 2016.
8. Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals Participating In ACOs Tend To Be Large And Urban, Allowing Access To Capital And Data. Health Aff(Millwood). 2016;35(3):431-439. PubMed
9. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early Performance of Accountable Care Organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. PubMed
10. Muhlestein D, Saunders R, McClellan M. Medicare Accountable Care Organization Results For 2015: The Journey To Better Quality And Lower Costs Continues. In. Health Affairs Blog. Bethesda, MD 2016.
11. Chernew ME. New Health Care Symposium: Building An ACO---What Services Do You Need And How Are Physicians Impacted? In Health Affairs Blog. Bethesda, MD 2016.
12. Centers for Medicare & Medicaid Services. Performance Year 2016 Quality Performance and Financial Reconciliation Results for ACOs with 2012-2016 Start Dates. Available at https://strategichealthcare.net/wp-content/uploads/2017/10/CMS-Slides-on-ACOs.pdf. 2017.
13. Shortell SM, Casalino LP. Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA. 2010;303(17):1747-1748. PubMed
14. Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Project Hope). 2010;29(7):1293-1298. PubMed
15. Medicare Payment Advisory Commission. Accountable Care Organizations Payment Systems October 2015. Available at http://www.medpac.gov/documents/payment-basics/accountable-care-organization-payment-systems-15.pdf?sfvrsn=0.
16. American Hospital Association. 2010 Committee on Research. AHA Research Synthesis Report: Accountable Care Organization.
17. D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors That Distinguish High-Performing Accountable Care Organizations in the Medicare Shared Savings Program. Health Serv. Res. 2016. PubMed
18. Peiris D, Phipps-Taylor MC, Stachowski CA, et al. ACOs Holding Commercial Contracts Are Larger And More Efficient Than Noncommercial ACOs. Health Aff (Project Hope). 2016;35(10):1849-1856. PubMed
19. Ouayogode MH, Colla CH, Lewis VA. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare (Amsterdam, Netherlands). 2017;5(1-2):53-61. PubMed
20. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. In: Hyattsville, MD.2017. PubMed
21. Gbemudu JN. Larson BK, Van Citters AD, Kreindler SA, Nelson EC, Shortell SM, Fisher ES. Norton Healthcare: A Strong Payer–Provider Partnership for the Journey to Accountable Care. January 2012. Available at http://www.commonwealthfund.org/~/media/files/publications/case-study/2012/jan/1574_gbemudu_norton_case-study_01_12_2012.pdf.
22. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. PubMed
23. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J. Hosp. Med.. 2013;8(8):421-427. PubMed
24. Centers for Medicare and Medicaid Services. Solicitation for Applications: Community-based Care Transitions Program. Available at https://innovation.cms.gov/Files/Migrated-Medicare-Demonstration-x/CCTP-Solicitation.pdf. September 7, 2017.
25. Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90(3):303-310. PubMed
26. Williams MV, Li J, Hansen LO, et al. Project BOOST implementation: lessons learned. South Med J. 2014;107(7):455-465. PubMed
27. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761]. J Am Geriatr Soc. 2010;58(4):627-635. PubMed
28. Pittman D. SNFs: New Turf for Hospitalists? 2013. Available at https://www.medpagetoday.com/hospitalbasedmedicine/hospitalists/39401.
29. Petigara S, Krishnamurthy M, Livert D. Necessity is the mother of invention: an innovative hospitalist-resident initiative for improving quality and reducing readmissions from skilled nursing facilities. J Community Hosp Intern Med Perspect. 2017;7(2):66-69. PubMed
30. Silow-Carroll S, Edwards J. Early Adopters of the Accountable Care Model: A Field Report on Improvements in Health Care Delivery. New York, NY: The Commonwealth Fund;March 2013.
31. Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies;October 2013.
32. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
33. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
34. O’Kane M, Buto K, Alteras T, et. al. Demanding Value from Our Health Care: Motivating Patient Action to Reduce Waste in Health Care. Institute of Medicine of the National Academies. July 2012. https://nam.edu/wp-content/uploads/2015/06/VSRT-DemandingValue.pdf. Accessed Accessed June 18, 2017.
35. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
36. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. PubMed
37. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
38. Neeman N, Quinn K, Soni K, Mourad M, Sehgal NL. Reducing radiology use on an inpatient medical service: choosing wisely. JAMA Intern Med. 2012;172(20):1606-1608. PubMed
39. Abrams M, Nuzum R, Zezza M, Ryan J, Kiszla J, Guterman S. The Affordable Care Act’s Payment and Delivery System Reforms: A Progress Report at Five Years. Bipartisan Policy Center, May 2015. Available at http://www.commonwealthfund.org/publications/issue-briefs/2015/may/aca-payment-and-delivery-system-reforms-at-5-years.
40. Kocot SL, White R, Katikaneni P, McClellan MB. A More Complete Picture of Pioneer ACO Results. The Brookings Institution, October 13, 2014. Available at http://www.brookings.edu/blogs/up-front/posts/2014/10/09-pioneer-aco-results-mcclellan/#recent_rr/
41. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372(25):2451-2458. PubMed
42. Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med. 2016;176(8):1167-1175. PubMed
43. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Project Hope). 2014;33(1):95-102. PubMed
44. Fullerton CA, Henke RM, Crable E, Hohlbauch A, Cummings N. The Impact Of Medicare ACOs On Improving Integration And Coordination Of Physical And Behavioral Health Care. Health Aff (Project Hope). 2016;35(7):1257-1265. PubMed
45. Herrel LA, Norton EC, Hawken SR, Ye Z, Hollenbeck BK, Miller DC. Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer. 2016;122(17):2739-2746. PubMed
46. McConnell KJ, Renfro S, Chan BK, et al. Early Performance in Medicaid Accountable Care Organizations: A Comparison of Oregon and Colorado. JAMA Intern Med. 2017;177(4):538-545. PubMed
47. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer Accountable Care Organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
48. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center--a five-year self-assessment. N Engl J Med. 2015;372(21):1981-1983. PubMed
49. Rose S, Zaslavsky AM, McWilliams JM. Variation In Accountable Care Organization Spending And Sensitivity To Risk Adjustment: Implications For Benchmarking. Health affairs (Project Hope). 2016;35(3):440-448. PubMed
50. Shortell SM, Poon BY, Ramsay PP, et al. A Multilevel Analysis of Patient Engagement and Patient-Reported Outcomes in Primary Care Practices of Accountable Care Organizations. J Gen Intern Med. 2017;32(6):640-647. PubMed
51. Winblad U, Mor V, McHugh JP, Rahman M. ACO-Affiliated Hospitals Reduced Rehospitalizations From Skilled Nursing Facilities Faster Than Other Hospitals. Health Aff (Project Hope). 2017;36(1):67-73. PubMed
52. Zhang Y, Caines KJ, Powers CA. Evaluating the Effects of Pioneer Accountable Care Organizations on Medicare Part D Drug Spending and Utilization. Med Care. 2017;55(5):470-475. PubMed
53. Muhlestein D. Medicare ACOs: Mixed Initial Results and Cautious Optimism. Health Affairs Blog, February 4, 2014. Available at http://healthaffairs.org/blog/2014/02/04/medicare-acos-mixed-initial-results-and-cautious-optimism/.
54. Hsu J, Price M, Vogeli C, et al. Bending The Spending Curve By Altering Care Delivery Patterns: The Role Of Care Management Within A Pioneer ACO. Health Aff (Project Hope). 2017;36(5):876-884. PubMed
55. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential For Reducing Spending And Improving Quality. Office of Inspector General;August 2017.
The accountable care organization (ACO) concept, elucidated in 2006 as the development of partnerships between hospitals and physicians to coordinate and deliver efficient care,1 seeks to remove existing barriers to improving value.2 Some advocate this concept as a promising payment model that could successfully realign the current payment system to financially reward improvements in quality and efficiency that bend the cost curve.3,4 Hospitalists fit well with this philosophy. As the fastest growing medical specialty in the history of American medicine, from a couple of thousand hospitalists in the mid-1990s to more than 50,000, the remarkable progression of hospitalists has ostensibly been driven partially by hospitals’ efforts to improve the value equation through enhanced efficiency in inpatient care. Importantly, hospitalists probably provide care for more than half of all hospitalized Medicare beneficiaries and increasingly patients in skilled nursing facilities (ie, SNFists).5 Along with primary care physicians, hospitalists thus represent an essential group of physicians needed to transform care delivery.
RAPID GROWTH AND THE FUTURE OF ACOs
When the Affordable Care Act (ACA) established the Medicare Shared Savings Program (MSSP), ACOs leaped from being an intellectual concept1,2 into a pragmatic health system strategy.3,4 Following Medicare, various private health insurance plans and some state Medicaid programs entered into contracts with groups of healthcare providers (hospitals, physicians, or health systems) to serve as ACOs for their insured enrollees.6 Leavitt Partners’ ACO tracking database showed that the number of ACOs increased from 157 in March of 2012 to 782 in December of 2015.7
Until recently, the federal government’s commitment to having 50% of total Medicare spending via value-based payment models by 2018, coupled with endorsement from state Medicaid programs and commercial insurers, demonstrated strong support for continuation of ACOs. Unexpectedly on August 15, 2017, the Centers for Medicare & Medicaid Services (CMS) outlined a plan in its proposed rulemaking to cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment model, which were scheduled to commence on January 1, 2018. CMS also plans to scale back the mandatory Comprehensive Care for Joint Replacement (CCJR) bundled payment model from 67 selected geographic areas to 34. Although this proposed rulemaking created some equipoise in the healthcare industry regarding the future of value-based reimbursement approaches, cost containment and improved efficiency remain as major focuses of the federal government’s healthcare effort. Notably, CMS offers providers that are newly excluded from the CCJR model the opportunity to voluntarily participate in the program and is expected to increase opportunities for providers to participate in voluntary rather than large-scale mandatory episode payment model initiatives. In 2018, the agency also plans to develop new voluntary bundled payment models that will meet criteria to be considered an advanced alternative payment model for Quality Payment Program purposes.
Importantly, the value-based reimbursement movement was well underway before ACA legislation. Through ACA health reform, value-based reimbursement efforts were expanded through ACOs, bundled payments, value-based purchasing, the CMS Innovation Center and other initiatives. With health systems having an overflowing plate of activities, a wait-and-see attitude might seem reasonable at first. However, being unprepared for the inevitable shift to value-based reimbursement and reduced fee-for-service revenue places an organization at risk. A successful ACO requires system-level transformation, especially cultural and structural changes to achieve clinical integration. Being embedded in health system delivery, hospitalists can help shape a team-oriented culture and foster success in value-based payment models. This requires hospitalists to take a more active role in assessing and striking a balance between high-quality, cost-efficient care and financial risk inherent in ACO models.
WHAT HOSPITALISTS NEED TO KNOW ABOUT ACOs
The key to hospitalists fulfilling their value creation potential and becoming enablers for ACO success lies in developing a thorough understanding of the aspects of an ACO that promote efficient and effective care, while accounting for financial factors. Fundamentally, the ACO concept combines provider payment and delivery system reforms. Specifically, the definition of an ACO contains 3 factors: (1) a local healthcare organization (eg, hospital or multispecialty group of physicians) with a related set of providers that (2) can be held accountable for the cost and quality of care delivered to (3) a defined population. While the notion of accountability is not new, the locus of accountability is changed in the ACO model—emphasizing accountability at the level of actual care delivery with documentation of quality and cost outcomes. The ACO approach aims to address multiple, frequent, and recurring problems, including lack of financial incentives to improve quality and reduce cost, as well as the negative consequences of a pay-for-volume system—uncoordinated and fragmented care, overutilization of unnecessary tests and treatments, and poor patient experience all manifested as unwarranted geographic variation in practice patterns, clinical outcomes, and health spending. Participants in an ACO are rewarded financially if they can slow the growth of their patients’ healthcare costs while maintaining or improving the quality of care delivered. To succeed in this ACO world, hospitalists must assume greater prudence in the use of healthcare services while improving (or at a minimum, maintaining) patient outcomes, thus excising avoidable waste across the continuum of care.
More than half of ACOs include a hospital.8 However, whether hospital-led ACOs possess an advantage remains to be elucidated. Early reports indicated that physician-led ACOs saved more money.9,10 However, others argue that hospitals11 are better capitalized, have greater capacity for data sharing, and possess economies of scale that allow them to invest in more advanced technology, such as predictive modeling and/or simulation software. Such analytics can identify high-cost patients (ie, multiple comorbidities), super utilizers and populations lacking care, allowing ACOs to implement preventive measures to reduce unnecessary utilization. Recently released CMS MSSP 2016 performance data12 showed that nearly half (45%) of physician-only ACOs earned shared savings, whereas 23% of ACOs that include hospitals earned shared savings. However, among all the ACOs that achieved savings, ACO entities that include hospitals generated the highest amount of shared savings (eg, Advocate, Hackensack Alliance, Cleveland Clinic, and AMITA Health). Notably, hospital-led ACOs tend to have much larger beneficiary populations than physician-led ACOs, which may create a scenario of higher risk but higher potential reward.
HOW HOSPITALISTS CONTRIBUTE VALUE TO ACO SUCCESS
The emphasis on value over volume inherent in the development of ACOs occurs through employing care strategies implemented through changes in policies, and eventual structural and cultural changes. These changes require participating organizations to possess certain key competencies, including the following: 1) leadership that facilitates change; 2) organizational culture of teamwork; 3) collaborative relationships among providers; 4) information technology infrastructure for population management and care coordination; 5) infrastructure for monitoring, managing, and reporting quality; 6) ability to manage financial risk; 7) ability to receive and distribute payments or savings; and 8) resources for patient education and support.2,3,13-16 Table 1 summarizes the broad range of roles that hospitalists can serve in delivering care to ACO populations.17-19
Hospitalists’ active pursuit of nonclinical training and selection for administrative positions demonstrate their proclivity to provide these competencies. In addition to full-time clinician hospitalists, who can directly influence the delivery of high-value care to patients, hospitalists serve many other roles in hospitals and each can contribute differently based on their specialized expertise. Examples include the success of the Society of Hospital Medicine’s Leadership Academy; the acknowledged expertise of hospitalists in quality improvement (QI), informatics, teamwork, patient experience, care coordination and utilization; and advancement of hospitalists to senior leadership positions (eg, CQO, CMO, CEO). Given that nearly a third of healthcare expenditures are for hospital care,20 hospitalists are in a unique position to foster ACO competencies while impacting the quality of care episodes associated with an index hospital stay.
Importantly, hospitalists cannot act as gatekeepers to restrict care. Managed care organizations and health maintenance organizations use of this approach in the 1990s to limit access to services in order to reduce costs led to unacceptable outcomes and numerous malpractice lawsuits. ACOs should aspire to deliver the most cost-effective high-quality care, and their performance should be monitored to ensure that they provide recommended services and timely access. The Medicare ACO contract holds the provider accountable for meeting 34 different quality measures (Supplemental Table 1), and hospitalists can influence outcomes for the majority. Especially through hospital and health system QI initiatives, hospitalists can directly impact and share accountability for measures ranging from care coordination to implementation of evidence-based care (eg, ACE inhibitors and beta blockers for heart failure) to patient and family caregiver experience.
Aligned with Medicare ACO quality measures, 5 high-impact target areas were identified for ACOs21: (1) Prevention and wellness; (2) Chronic conditions/care management; (3) Reduced hospitalizations; (4) Care transitions across the fragmented system; and (5) Multispecialty care coordination of complex patients. One essential element of a successful ACO is the ability to implement evidence-based medical guidelines and/or practices across the continuum of care for selected targeted initiatives. Optimizing care coordination/continuum requires team-based care, and hospitalists already routinely collaborate with nurses, social workers, case managers, pharmacists, and other stakeholders such as dieticians and physical therapists on inpatient care. Hospitalists are also experienced in facilitating communication and improving integration and coordination efficiencies among primary care providers and specialists, and between hospital care and post-acute care, as they coordinate post-hospital care and follow-up. This provides an opportunity to lead health system care coordination efforts, especially for complex and/or high-risk patients.22,23 CMS MSSP 2016 performance data12 showed that ACOs achieving shared savings had a decline in inpatient expenditures and utilization across several facility types (hospital, SNF, rehabilitation, long term). Postacute care management is critical to earning shared savings; SNF and Home Health expenditures fell by 18.3% and 9.7%, respectively, on average. We believe that hospitalists can have more influence over these cost areas by influencing treatment of hospitalized patients in a timely manner, discharge coordination, and selection of appropriate disposition locations. Hospitalists also play an integral role in ensuring the hospital performs well on quality metrics, including 30-day readmissions, hospital acquired conditions, and patient satisfaction. Examples below document the effectiveness of hospitalists in this new ACO era.
Care Transitions/Coordination
Before the Hospital Readmission Reduction Program (HRRP) delineated in the ACA, hospitalists developed Project BOOST (Better Outcomes by Optimizing Care Transitions) to improve hospital discharge care transition. The evidence-based foundation of this project led CMS to list Project BOOST as an example program that can reduce readmissions.24 Through the dissemination and mentored implementation of Project BOOST to over 200 hospitals across the United States,25 hospitalists contributed to the marked reduction in hospital readmission occurring since 2010.26 Although hospital medicine began as a practice specific to the hospital setting, hospitalists’ skills generated growing demand for them in postacute facilities. SNF residents commonly come from hospitals postdischarge and suffer from multiple comorbidities and limitations in activities of daily living. Not surprisingly, SNF residents experience high rates of rehospitalizations.27 Hospitalists can serve as a bridge between hospitals and SNFs and optimize this transition process to yield improved outcomes. Industry experts endorse this approach.28 A recent study demonstrated a significant reduction in readmissions in 1 SNF (32.3% to 16.1%, odds ratio = 0.403, P < .001), by having a hospitalist-led team follow patients discharged from the hospital.29
Chronic Conditions Management/High-Risk Patients
Interest in patients with multiple chronic comorbidities and social issues intensifies as healthcare systems focus limited resources on these high-risk patients to prevent the unnecessary use of costly services.30,31 As health systems assume financial risk for health outcomes and costs of designated patient groups, they undertake efforts to understand the population they serve. Such efforts aim to identify patients with established high utilization patterns (or those at risk for high utilization). This knowledge enables targeted actions to provide access, treatment, and preventive interventions to avoid unneeded emergency and hospital services. Hospitalists commonly care for these patients and are positioned to lead the implementation of patient risk assessment and stratification, develop patient-centered care models across care settings, and act as a liaison with primary care. For frail elderly and seriously ill patients, the integration of hospitalists into palliative care provides several opportunities for improving the quality of care at the end of life.32 As patients and their family caregivers commonly do not address goals of care until faced with a life-threatening condition in the hospital, hospitalists represent ideal primary palliative care physicians to initiate these conversations.33 A hospitalist communicating with a patient and/or their family caregiver about alleviating symptoms and clarifying patients’ preferences for care often yields decreases in ineffective healthcare utilization and better patient outcomes. The hospitalists’ ability to communicate with other providers within the hospital setting also allows them to better coordinate interdisciplinary care and prevent unnecessary and ineffective treatments and procedures.
De-Implementation/Waste Reduction
The largest inefficiencies in healthcare noted in the National Academy of Medicine report, Demanding Value from Our Health Care (2012), are failure to deliver known beneficial therapies or providing unnecessary or nonevidenced based services that do not improve outcomes, but come with associated risk and cost.34 “De-implementation” of unnecessary diagnostic tests or ineffective or even harmful treatments by hospitalists represents a significant opportunity to reduce costs while maintaining or even improving the quality of care. The Society of Hospital Medicine joined the Choosing Wisely® campaign and made 5 recommendations in adult care as an explicit starting point for eliminating waste in the hospital in 2013.35 Since then, hospitalists have participated in multiple successful efforts to address overutilization of care; some published results include the following:
- decreased frequency of unnecessary common labs through a multifaceted hospitalist QI intervention;36
- reduced length of stay and cost by appropriate use of telemetry;37 and
- reduced unnecessary radiology testing by providing physicians with individualized audit and feedback reports.38
CONCLUSION
Hundreds of ACOs now exist across the US, formed by a variety of providers including hospitals, physician groups, and integrated delivery systems. Provider groups range in size from primary care-focused physician groups with a handful of offices to large, multistate integrated delivery systems with dozens of hospitals and hundreds of office locations. Evaluations of ACO outcomes reveal mixed results.9,39-53 Admittedly, assessments attempting to compare the magnitude of savings across ACO models are difficult given the variation in size, variability in specific efforts to influence utilization, and substantial turnover among participating beneficiaries.54 Nonetheless, a newly published Office of Inspector General report55 showed that most Medicare ACOs reduced spending and improved care quality (82% of the individual quality measures) over the first 3 years of the program, and savings increased with duration of an ACO program. The report also noted that considerable time and managerial resources are required to implement changes to improve quality and lower costs. While the political terrain ostensibly supports value-based care and the need to diminish the proportion of our nation’s gross domestic product dedicated to healthcare, health systems are navigating an environment that still largely rewards volume. Hospitalists may be ideal facilitators for this transitional period as they possess the clinical experience caring for complex patients with multiple comorbidities and quality improvement skills to lead efforts in this new ACO era.
Disclosures
The authors have nothing to disclose.
The accountable care organization (ACO) concept, elucidated in 2006 as the development of partnerships between hospitals and physicians to coordinate and deliver efficient care,1 seeks to remove existing barriers to improving value.2 Some advocate this concept as a promising payment model that could successfully realign the current payment system to financially reward improvements in quality and efficiency that bend the cost curve.3,4 Hospitalists fit well with this philosophy. As the fastest growing medical specialty in the history of American medicine, from a couple of thousand hospitalists in the mid-1990s to more than 50,000, the remarkable progression of hospitalists has ostensibly been driven partially by hospitals’ efforts to improve the value equation through enhanced efficiency in inpatient care. Importantly, hospitalists probably provide care for more than half of all hospitalized Medicare beneficiaries and increasingly patients in skilled nursing facilities (ie, SNFists).5 Along with primary care physicians, hospitalists thus represent an essential group of physicians needed to transform care delivery.
RAPID GROWTH AND THE FUTURE OF ACOs
When the Affordable Care Act (ACA) established the Medicare Shared Savings Program (MSSP), ACOs leaped from being an intellectual concept1,2 into a pragmatic health system strategy.3,4 Following Medicare, various private health insurance plans and some state Medicaid programs entered into contracts with groups of healthcare providers (hospitals, physicians, or health systems) to serve as ACOs for their insured enrollees.6 Leavitt Partners’ ACO tracking database showed that the number of ACOs increased from 157 in March of 2012 to 782 in December of 2015.7
Until recently, the federal government’s commitment to having 50% of total Medicare spending via value-based payment models by 2018, coupled with endorsement from state Medicaid programs and commercial insurers, demonstrated strong support for continuation of ACOs. Unexpectedly on August 15, 2017, the Centers for Medicare & Medicaid Services (CMS) outlined a plan in its proposed rulemaking to cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment model, which were scheduled to commence on January 1, 2018. CMS also plans to scale back the mandatory Comprehensive Care for Joint Replacement (CCJR) bundled payment model from 67 selected geographic areas to 34. Although this proposed rulemaking created some equipoise in the healthcare industry regarding the future of value-based reimbursement approaches, cost containment and improved efficiency remain as major focuses of the federal government’s healthcare effort. Notably, CMS offers providers that are newly excluded from the CCJR model the opportunity to voluntarily participate in the program and is expected to increase opportunities for providers to participate in voluntary rather than large-scale mandatory episode payment model initiatives. In 2018, the agency also plans to develop new voluntary bundled payment models that will meet criteria to be considered an advanced alternative payment model for Quality Payment Program purposes.
Importantly, the value-based reimbursement movement was well underway before ACA legislation. Through ACA health reform, value-based reimbursement efforts were expanded through ACOs, bundled payments, value-based purchasing, the CMS Innovation Center and other initiatives. With health systems having an overflowing plate of activities, a wait-and-see attitude might seem reasonable at first. However, being unprepared for the inevitable shift to value-based reimbursement and reduced fee-for-service revenue places an organization at risk. A successful ACO requires system-level transformation, especially cultural and structural changes to achieve clinical integration. Being embedded in health system delivery, hospitalists can help shape a team-oriented culture and foster success in value-based payment models. This requires hospitalists to take a more active role in assessing and striking a balance between high-quality, cost-efficient care and financial risk inherent in ACO models.
WHAT HOSPITALISTS NEED TO KNOW ABOUT ACOs
The key to hospitalists fulfilling their value creation potential and becoming enablers for ACO success lies in developing a thorough understanding of the aspects of an ACO that promote efficient and effective care, while accounting for financial factors. Fundamentally, the ACO concept combines provider payment and delivery system reforms. Specifically, the definition of an ACO contains 3 factors: (1) a local healthcare organization (eg, hospital or multispecialty group of physicians) with a related set of providers that (2) can be held accountable for the cost and quality of care delivered to (3) a defined population. While the notion of accountability is not new, the locus of accountability is changed in the ACO model—emphasizing accountability at the level of actual care delivery with documentation of quality and cost outcomes. The ACO approach aims to address multiple, frequent, and recurring problems, including lack of financial incentives to improve quality and reduce cost, as well as the negative consequences of a pay-for-volume system—uncoordinated and fragmented care, overutilization of unnecessary tests and treatments, and poor patient experience all manifested as unwarranted geographic variation in practice patterns, clinical outcomes, and health spending. Participants in an ACO are rewarded financially if they can slow the growth of their patients’ healthcare costs while maintaining or improving the quality of care delivered. To succeed in this ACO world, hospitalists must assume greater prudence in the use of healthcare services while improving (or at a minimum, maintaining) patient outcomes, thus excising avoidable waste across the continuum of care.
More than half of ACOs include a hospital.8 However, whether hospital-led ACOs possess an advantage remains to be elucidated. Early reports indicated that physician-led ACOs saved more money.9,10 However, others argue that hospitals11 are better capitalized, have greater capacity for data sharing, and possess economies of scale that allow them to invest in more advanced technology, such as predictive modeling and/or simulation software. Such analytics can identify high-cost patients (ie, multiple comorbidities), super utilizers and populations lacking care, allowing ACOs to implement preventive measures to reduce unnecessary utilization. Recently released CMS MSSP 2016 performance data12 showed that nearly half (45%) of physician-only ACOs earned shared savings, whereas 23% of ACOs that include hospitals earned shared savings. However, among all the ACOs that achieved savings, ACO entities that include hospitals generated the highest amount of shared savings (eg, Advocate, Hackensack Alliance, Cleveland Clinic, and AMITA Health). Notably, hospital-led ACOs tend to have much larger beneficiary populations than physician-led ACOs, which may create a scenario of higher risk but higher potential reward.
HOW HOSPITALISTS CONTRIBUTE VALUE TO ACO SUCCESS
The emphasis on value over volume inherent in the development of ACOs occurs through employing care strategies implemented through changes in policies, and eventual structural and cultural changes. These changes require participating organizations to possess certain key competencies, including the following: 1) leadership that facilitates change; 2) organizational culture of teamwork; 3) collaborative relationships among providers; 4) information technology infrastructure for population management and care coordination; 5) infrastructure for monitoring, managing, and reporting quality; 6) ability to manage financial risk; 7) ability to receive and distribute payments or savings; and 8) resources for patient education and support.2,3,13-16 Table 1 summarizes the broad range of roles that hospitalists can serve in delivering care to ACO populations.17-19
Hospitalists’ active pursuit of nonclinical training and selection for administrative positions demonstrate their proclivity to provide these competencies. In addition to full-time clinician hospitalists, who can directly influence the delivery of high-value care to patients, hospitalists serve many other roles in hospitals and each can contribute differently based on their specialized expertise. Examples include the success of the Society of Hospital Medicine’s Leadership Academy; the acknowledged expertise of hospitalists in quality improvement (QI), informatics, teamwork, patient experience, care coordination and utilization; and advancement of hospitalists to senior leadership positions (eg, CQO, CMO, CEO). Given that nearly a third of healthcare expenditures are for hospital care,20 hospitalists are in a unique position to foster ACO competencies while impacting the quality of care episodes associated with an index hospital stay.
Importantly, hospitalists cannot act as gatekeepers to restrict care. Managed care organizations and health maintenance organizations use of this approach in the 1990s to limit access to services in order to reduce costs led to unacceptable outcomes and numerous malpractice lawsuits. ACOs should aspire to deliver the most cost-effective high-quality care, and their performance should be monitored to ensure that they provide recommended services and timely access. The Medicare ACO contract holds the provider accountable for meeting 34 different quality measures (Supplemental Table 1), and hospitalists can influence outcomes for the majority. Especially through hospital and health system QI initiatives, hospitalists can directly impact and share accountability for measures ranging from care coordination to implementation of evidence-based care (eg, ACE inhibitors and beta blockers for heart failure) to patient and family caregiver experience.
Aligned with Medicare ACO quality measures, 5 high-impact target areas were identified for ACOs21: (1) Prevention and wellness; (2) Chronic conditions/care management; (3) Reduced hospitalizations; (4) Care transitions across the fragmented system; and (5) Multispecialty care coordination of complex patients. One essential element of a successful ACO is the ability to implement evidence-based medical guidelines and/or practices across the continuum of care for selected targeted initiatives. Optimizing care coordination/continuum requires team-based care, and hospitalists already routinely collaborate with nurses, social workers, case managers, pharmacists, and other stakeholders such as dieticians and physical therapists on inpatient care. Hospitalists are also experienced in facilitating communication and improving integration and coordination efficiencies among primary care providers and specialists, and between hospital care and post-acute care, as they coordinate post-hospital care and follow-up. This provides an opportunity to lead health system care coordination efforts, especially for complex and/or high-risk patients.22,23 CMS MSSP 2016 performance data12 showed that ACOs achieving shared savings had a decline in inpatient expenditures and utilization across several facility types (hospital, SNF, rehabilitation, long term). Postacute care management is critical to earning shared savings; SNF and Home Health expenditures fell by 18.3% and 9.7%, respectively, on average. We believe that hospitalists can have more influence over these cost areas by influencing treatment of hospitalized patients in a timely manner, discharge coordination, and selection of appropriate disposition locations. Hospitalists also play an integral role in ensuring the hospital performs well on quality metrics, including 30-day readmissions, hospital acquired conditions, and patient satisfaction. Examples below document the effectiveness of hospitalists in this new ACO era.
Care Transitions/Coordination
Before the Hospital Readmission Reduction Program (HRRP) delineated in the ACA, hospitalists developed Project BOOST (Better Outcomes by Optimizing Care Transitions) to improve hospital discharge care transition. The evidence-based foundation of this project led CMS to list Project BOOST as an example program that can reduce readmissions.24 Through the dissemination and mentored implementation of Project BOOST to over 200 hospitals across the United States,25 hospitalists contributed to the marked reduction in hospital readmission occurring since 2010.26 Although hospital medicine began as a practice specific to the hospital setting, hospitalists’ skills generated growing demand for them in postacute facilities. SNF residents commonly come from hospitals postdischarge and suffer from multiple comorbidities and limitations in activities of daily living. Not surprisingly, SNF residents experience high rates of rehospitalizations.27 Hospitalists can serve as a bridge between hospitals and SNFs and optimize this transition process to yield improved outcomes. Industry experts endorse this approach.28 A recent study demonstrated a significant reduction in readmissions in 1 SNF (32.3% to 16.1%, odds ratio = 0.403, P < .001), by having a hospitalist-led team follow patients discharged from the hospital.29
Chronic Conditions Management/High-Risk Patients
Interest in patients with multiple chronic comorbidities and social issues intensifies as healthcare systems focus limited resources on these high-risk patients to prevent the unnecessary use of costly services.30,31 As health systems assume financial risk for health outcomes and costs of designated patient groups, they undertake efforts to understand the population they serve. Such efforts aim to identify patients with established high utilization patterns (or those at risk for high utilization). This knowledge enables targeted actions to provide access, treatment, and preventive interventions to avoid unneeded emergency and hospital services. Hospitalists commonly care for these patients and are positioned to lead the implementation of patient risk assessment and stratification, develop patient-centered care models across care settings, and act as a liaison with primary care. For frail elderly and seriously ill patients, the integration of hospitalists into palliative care provides several opportunities for improving the quality of care at the end of life.32 As patients and their family caregivers commonly do not address goals of care until faced with a life-threatening condition in the hospital, hospitalists represent ideal primary palliative care physicians to initiate these conversations.33 A hospitalist communicating with a patient and/or their family caregiver about alleviating symptoms and clarifying patients’ preferences for care often yields decreases in ineffective healthcare utilization and better patient outcomes. The hospitalists’ ability to communicate with other providers within the hospital setting also allows them to better coordinate interdisciplinary care and prevent unnecessary and ineffective treatments and procedures.
De-Implementation/Waste Reduction
The largest inefficiencies in healthcare noted in the National Academy of Medicine report, Demanding Value from Our Health Care (2012), are failure to deliver known beneficial therapies or providing unnecessary or nonevidenced based services that do not improve outcomes, but come with associated risk and cost.34 “De-implementation” of unnecessary diagnostic tests or ineffective or even harmful treatments by hospitalists represents a significant opportunity to reduce costs while maintaining or even improving the quality of care. The Society of Hospital Medicine joined the Choosing Wisely® campaign and made 5 recommendations in adult care as an explicit starting point for eliminating waste in the hospital in 2013.35 Since then, hospitalists have participated in multiple successful efforts to address overutilization of care; some published results include the following:
- decreased frequency of unnecessary common labs through a multifaceted hospitalist QI intervention;36
- reduced length of stay and cost by appropriate use of telemetry;37 and
- reduced unnecessary radiology testing by providing physicians with individualized audit and feedback reports.38
CONCLUSION
Hundreds of ACOs now exist across the US, formed by a variety of providers including hospitals, physician groups, and integrated delivery systems. Provider groups range in size from primary care-focused physician groups with a handful of offices to large, multistate integrated delivery systems with dozens of hospitals and hundreds of office locations. Evaluations of ACO outcomes reveal mixed results.9,39-53 Admittedly, assessments attempting to compare the magnitude of savings across ACO models are difficult given the variation in size, variability in specific efforts to influence utilization, and substantial turnover among participating beneficiaries.54 Nonetheless, a newly published Office of Inspector General report55 showed that most Medicare ACOs reduced spending and improved care quality (82% of the individual quality measures) over the first 3 years of the program, and savings increased with duration of an ACO program. The report also noted that considerable time and managerial resources are required to implement changes to improve quality and lower costs. While the political terrain ostensibly supports value-based care and the need to diminish the proportion of our nation’s gross domestic product dedicated to healthcare, health systems are navigating an environment that still largely rewards volume. Hospitalists may be ideal facilitators for this transitional period as they possess the clinical experience caring for complex patients with multiple comorbidities and quality improvement skills to lead efforts in this new ACO era.
Disclosures
The authors have nothing to disclose.
1. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff(Project Hope). 2007;26(1):w44-w57. PubMed
2. Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff(Project Hope). 2009;28(2):w219-w231. PubMed
3. McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff(Project Hope). 2010;29(5):982-990. PubMed
4. Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753-1756. PubMed
5. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
6. Kennedy K. Health Care Providers Embracing Cost-saving Groups. USA Today, July 24, 2011.
7. Leavitt Partners. Available at http://leavittpartners.com, April 2016.
8. Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals Participating In ACOs Tend To Be Large And Urban, Allowing Access To Capital And Data. Health Aff(Millwood). 2016;35(3):431-439. PubMed
9. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early Performance of Accountable Care Organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. PubMed
10. Muhlestein D, Saunders R, McClellan M. Medicare Accountable Care Organization Results For 2015: The Journey To Better Quality And Lower Costs Continues. In. Health Affairs Blog. Bethesda, MD 2016.
11. Chernew ME. New Health Care Symposium: Building An ACO---What Services Do You Need And How Are Physicians Impacted? In Health Affairs Blog. Bethesda, MD 2016.
12. Centers for Medicare & Medicaid Services. Performance Year 2016 Quality Performance and Financial Reconciliation Results for ACOs with 2012-2016 Start Dates. Available at https://strategichealthcare.net/wp-content/uploads/2017/10/CMS-Slides-on-ACOs.pdf. 2017.
13. Shortell SM, Casalino LP. Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA. 2010;303(17):1747-1748. PubMed
14. Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Project Hope). 2010;29(7):1293-1298. PubMed
15. Medicare Payment Advisory Commission. Accountable Care Organizations Payment Systems October 2015. Available at http://www.medpac.gov/documents/payment-basics/accountable-care-organization-payment-systems-15.pdf?sfvrsn=0.
16. American Hospital Association. 2010 Committee on Research. AHA Research Synthesis Report: Accountable Care Organization.
17. D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors That Distinguish High-Performing Accountable Care Organizations in the Medicare Shared Savings Program. Health Serv. Res. 2016. PubMed
18. Peiris D, Phipps-Taylor MC, Stachowski CA, et al. ACOs Holding Commercial Contracts Are Larger And More Efficient Than Noncommercial ACOs. Health Aff (Project Hope). 2016;35(10):1849-1856. PubMed
19. Ouayogode MH, Colla CH, Lewis VA. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare (Amsterdam, Netherlands). 2017;5(1-2):53-61. PubMed
20. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. In: Hyattsville, MD.2017. PubMed
21. Gbemudu JN. Larson BK, Van Citters AD, Kreindler SA, Nelson EC, Shortell SM, Fisher ES. Norton Healthcare: A Strong Payer–Provider Partnership for the Journey to Accountable Care. January 2012. Available at http://www.commonwealthfund.org/~/media/files/publications/case-study/2012/jan/1574_gbemudu_norton_case-study_01_12_2012.pdf.
22. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. PubMed
23. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J. Hosp. Med.. 2013;8(8):421-427. PubMed
24. Centers for Medicare and Medicaid Services. Solicitation for Applications: Community-based Care Transitions Program. Available at https://innovation.cms.gov/Files/Migrated-Medicare-Demonstration-x/CCTP-Solicitation.pdf. September 7, 2017.
25. Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90(3):303-310. PubMed
26. Williams MV, Li J, Hansen LO, et al. Project BOOST implementation: lessons learned. South Med J. 2014;107(7):455-465. PubMed
27. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761]. J Am Geriatr Soc. 2010;58(4):627-635. PubMed
28. Pittman D. SNFs: New Turf for Hospitalists? 2013. Available at https://www.medpagetoday.com/hospitalbasedmedicine/hospitalists/39401.
29. Petigara S, Krishnamurthy M, Livert D. Necessity is the mother of invention: an innovative hospitalist-resident initiative for improving quality and reducing readmissions from skilled nursing facilities. J Community Hosp Intern Med Perspect. 2017;7(2):66-69. PubMed
30. Silow-Carroll S, Edwards J. Early Adopters of the Accountable Care Model: A Field Report on Improvements in Health Care Delivery. New York, NY: The Commonwealth Fund;March 2013.
31. Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies;October 2013.
32. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
33. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
34. O’Kane M, Buto K, Alteras T, et. al. Demanding Value from Our Health Care: Motivating Patient Action to Reduce Waste in Health Care. Institute of Medicine of the National Academies. July 2012. https://nam.edu/wp-content/uploads/2015/06/VSRT-DemandingValue.pdf. Accessed Accessed June 18, 2017.
35. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
36. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. PubMed
37. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
38. Neeman N, Quinn K, Soni K, Mourad M, Sehgal NL. Reducing radiology use on an inpatient medical service: choosing wisely. JAMA Intern Med. 2012;172(20):1606-1608. PubMed
39. Abrams M, Nuzum R, Zezza M, Ryan J, Kiszla J, Guterman S. The Affordable Care Act’s Payment and Delivery System Reforms: A Progress Report at Five Years. Bipartisan Policy Center, May 2015. Available at http://www.commonwealthfund.org/publications/issue-briefs/2015/may/aca-payment-and-delivery-system-reforms-at-5-years.
40. Kocot SL, White R, Katikaneni P, McClellan MB. A More Complete Picture of Pioneer ACO Results. The Brookings Institution, October 13, 2014. Available at http://www.brookings.edu/blogs/up-front/posts/2014/10/09-pioneer-aco-results-mcclellan/#recent_rr/
41. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372(25):2451-2458. PubMed
42. Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med. 2016;176(8):1167-1175. PubMed
43. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Project Hope). 2014;33(1):95-102. PubMed
44. Fullerton CA, Henke RM, Crable E, Hohlbauch A, Cummings N. The Impact Of Medicare ACOs On Improving Integration And Coordination Of Physical And Behavioral Health Care. Health Aff (Project Hope). 2016;35(7):1257-1265. PubMed
45. Herrel LA, Norton EC, Hawken SR, Ye Z, Hollenbeck BK, Miller DC. Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer. 2016;122(17):2739-2746. PubMed
46. McConnell KJ, Renfro S, Chan BK, et al. Early Performance in Medicaid Accountable Care Organizations: A Comparison of Oregon and Colorado. JAMA Intern Med. 2017;177(4):538-545. PubMed
47. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer Accountable Care Organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
48. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center--a five-year self-assessment. N Engl J Med. 2015;372(21):1981-1983. PubMed
49. Rose S, Zaslavsky AM, McWilliams JM. Variation In Accountable Care Organization Spending And Sensitivity To Risk Adjustment: Implications For Benchmarking. Health affairs (Project Hope). 2016;35(3):440-448. PubMed
50. Shortell SM, Poon BY, Ramsay PP, et al. A Multilevel Analysis of Patient Engagement and Patient-Reported Outcomes in Primary Care Practices of Accountable Care Organizations. J Gen Intern Med. 2017;32(6):640-647. PubMed
51. Winblad U, Mor V, McHugh JP, Rahman M. ACO-Affiliated Hospitals Reduced Rehospitalizations From Skilled Nursing Facilities Faster Than Other Hospitals. Health Aff (Project Hope). 2017;36(1):67-73. PubMed
52. Zhang Y, Caines KJ, Powers CA. Evaluating the Effects of Pioneer Accountable Care Organizations on Medicare Part D Drug Spending and Utilization. Med Care. 2017;55(5):470-475. PubMed
53. Muhlestein D. Medicare ACOs: Mixed Initial Results and Cautious Optimism. Health Affairs Blog, February 4, 2014. Available at http://healthaffairs.org/blog/2014/02/04/medicare-acos-mixed-initial-results-and-cautious-optimism/.
54. Hsu J, Price M, Vogeli C, et al. Bending The Spending Curve By Altering Care Delivery Patterns: The Role Of Care Management Within A Pioneer ACO. Health Aff (Project Hope). 2017;36(5):876-884. PubMed
55. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential For Reducing Spending And Improving Quality. Office of Inspector General;August 2017.
1. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff(Project Hope). 2007;26(1):w44-w57. PubMed
2. Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff(Project Hope). 2009;28(2):w219-w231. PubMed
3. McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff(Project Hope). 2010;29(5):982-990. PubMed
4. Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753-1756. PubMed
5. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
6. Kennedy K. Health Care Providers Embracing Cost-saving Groups. USA Today, July 24, 2011.
7. Leavitt Partners. Available at http://leavittpartners.com, April 2016.
8. Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals Participating In ACOs Tend To Be Large And Urban, Allowing Access To Capital And Data. Health Aff(Millwood). 2016;35(3):431-439. PubMed
9. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early Performance of Accountable Care Organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. PubMed
10. Muhlestein D, Saunders R, McClellan M. Medicare Accountable Care Organization Results For 2015: The Journey To Better Quality And Lower Costs Continues. In. Health Affairs Blog. Bethesda, MD 2016.
11. Chernew ME. New Health Care Symposium: Building An ACO---What Services Do You Need And How Are Physicians Impacted? In Health Affairs Blog. Bethesda, MD 2016.
12. Centers for Medicare & Medicaid Services. Performance Year 2016 Quality Performance and Financial Reconciliation Results for ACOs with 2012-2016 Start Dates. Available at https://strategichealthcare.net/wp-content/uploads/2017/10/CMS-Slides-on-ACOs.pdf. 2017.
13. Shortell SM, Casalino LP. Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA. 2010;303(17):1747-1748. PubMed
14. Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Project Hope). 2010;29(7):1293-1298. PubMed
15. Medicare Payment Advisory Commission. Accountable Care Organizations Payment Systems October 2015. Available at http://www.medpac.gov/documents/payment-basics/accountable-care-organization-payment-systems-15.pdf?sfvrsn=0.
16. American Hospital Association. 2010 Committee on Research. AHA Research Synthesis Report: Accountable Care Organization.
17. D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors That Distinguish High-Performing Accountable Care Organizations in the Medicare Shared Savings Program. Health Serv. Res. 2016. PubMed
18. Peiris D, Phipps-Taylor MC, Stachowski CA, et al. ACOs Holding Commercial Contracts Are Larger And More Efficient Than Noncommercial ACOs. Health Aff (Project Hope). 2016;35(10):1849-1856. PubMed
19. Ouayogode MH, Colla CH, Lewis VA. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare (Amsterdam, Netherlands). 2017;5(1-2):53-61. PubMed
20. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. In: Hyattsville, MD.2017. PubMed
21. Gbemudu JN. Larson BK, Van Citters AD, Kreindler SA, Nelson EC, Shortell SM, Fisher ES. Norton Healthcare: A Strong Payer–Provider Partnership for the Journey to Accountable Care. January 2012. Available at http://www.commonwealthfund.org/~/media/files/publications/case-study/2012/jan/1574_gbemudu_norton_case-study_01_12_2012.pdf.
22. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. PubMed
23. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J. Hosp. Med.. 2013;8(8):421-427. PubMed
24. Centers for Medicare and Medicaid Services. Solicitation for Applications: Community-based Care Transitions Program. Available at https://innovation.cms.gov/Files/Migrated-Medicare-Demonstration-x/CCTP-Solicitation.pdf. September 7, 2017.
25. Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90(3):303-310. PubMed
26. Williams MV, Li J, Hansen LO, et al. Project BOOST implementation: lessons learned. South Med J. 2014;107(7):455-465. PubMed
27. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761]. J Am Geriatr Soc. 2010;58(4):627-635. PubMed
28. Pittman D. SNFs: New Turf for Hospitalists? 2013. Available at https://www.medpagetoday.com/hospitalbasedmedicine/hospitalists/39401.
29. Petigara S, Krishnamurthy M, Livert D. Necessity is the mother of invention: an innovative hospitalist-resident initiative for improving quality and reducing readmissions from skilled nursing facilities. J Community Hosp Intern Med Perspect. 2017;7(2):66-69. PubMed
30. Silow-Carroll S, Edwards J. Early Adopters of the Accountable Care Model: A Field Report on Improvements in Health Care Delivery. New York, NY: The Commonwealth Fund;March 2013.
31. Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies;October 2013.
32. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
33. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
34. O’Kane M, Buto K, Alteras T, et. al. Demanding Value from Our Health Care: Motivating Patient Action to Reduce Waste in Health Care. Institute of Medicine of the National Academies. July 2012. https://nam.edu/wp-content/uploads/2015/06/VSRT-DemandingValue.pdf. Accessed Accessed June 18, 2017.
35. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
36. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. PubMed
37. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
38. Neeman N, Quinn K, Soni K, Mourad M, Sehgal NL. Reducing radiology use on an inpatient medical service: choosing wisely. JAMA Intern Med. 2012;172(20):1606-1608. PubMed
39. Abrams M, Nuzum R, Zezza M, Ryan J, Kiszla J, Guterman S. The Affordable Care Act’s Payment and Delivery System Reforms: A Progress Report at Five Years. Bipartisan Policy Center, May 2015. Available at http://www.commonwealthfund.org/publications/issue-briefs/2015/may/aca-payment-and-delivery-system-reforms-at-5-years.
40. Kocot SL, White R, Katikaneni P, McClellan MB. A More Complete Picture of Pioneer ACO Results. The Brookings Institution, October 13, 2014. Available at http://www.brookings.edu/blogs/up-front/posts/2014/10/09-pioneer-aco-results-mcclellan/#recent_rr/
41. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372(25):2451-2458. PubMed
42. Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med. 2016;176(8):1167-1175. PubMed
43. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Project Hope). 2014;33(1):95-102. PubMed
44. Fullerton CA, Henke RM, Crable E, Hohlbauch A, Cummings N. The Impact Of Medicare ACOs On Improving Integration And Coordination Of Physical And Behavioral Health Care. Health Aff (Project Hope). 2016;35(7):1257-1265. PubMed
45. Herrel LA, Norton EC, Hawken SR, Ye Z, Hollenbeck BK, Miller DC. Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer. 2016;122(17):2739-2746. PubMed
46. McConnell KJ, Renfro S, Chan BK, et al. Early Performance in Medicaid Accountable Care Organizations: A Comparison of Oregon and Colorado. JAMA Intern Med. 2017;177(4):538-545. PubMed
47. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer Accountable Care Organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
48. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center--a five-year self-assessment. N Engl J Med. 2015;372(21):1981-1983. PubMed
49. Rose S, Zaslavsky AM, McWilliams JM. Variation In Accountable Care Organization Spending And Sensitivity To Risk Adjustment: Implications For Benchmarking. Health affairs (Project Hope). 2016;35(3):440-448. PubMed
50. Shortell SM, Poon BY, Ramsay PP, et al. A Multilevel Analysis of Patient Engagement and Patient-Reported Outcomes in Primary Care Practices of Accountable Care Organizations. J Gen Intern Med. 2017;32(6):640-647. PubMed
51. Winblad U, Mor V, McHugh JP, Rahman M. ACO-Affiliated Hospitals Reduced Rehospitalizations From Skilled Nursing Facilities Faster Than Other Hospitals. Health Aff (Project Hope). 2017;36(1):67-73. PubMed
52. Zhang Y, Caines KJ, Powers CA. Evaluating the Effects of Pioneer Accountable Care Organizations on Medicare Part D Drug Spending and Utilization. Med Care. 2017;55(5):470-475. PubMed
53. Muhlestein D. Medicare ACOs: Mixed Initial Results and Cautious Optimism. Health Affairs Blog, February 4, 2014. Available at http://healthaffairs.org/blog/2014/02/04/medicare-acos-mixed-initial-results-and-cautious-optimism/.
54. Hsu J, Price M, Vogeli C, et al. Bending The Spending Curve By Altering Care Delivery Patterns: The Role Of Care Management Within A Pioneer ACO. Health Aff (Project Hope). 2017;36(5):876-884. PubMed
55. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential For Reducing Spending And Improving Quality. Office of Inspector General;August 2017.
© Society of Hospital Medicine
VIDEO: Women living with HIV have more myocardial steatosis, reduced diastolic function
CHICAGO – Median intramyocardial triglyceride content was nearly four times higher in a group of middle-age women living with HIV, compared with peers without the infection, according to a recent study that also found an association between high myocardial lipids and lower diastolic function.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For women without HIV, the value was 0.13% (95% CI, 0.11-0.23; P = .004). Further, left atrial passive ejection fraction was significantly lower among women living with HIV, compared with those without HIV (28% vs. 38%, P = .02), said Mabel Toribio, MD, speaking at the annual meeting of the Endocrine Society.
“Probably the most important aspect is that we found an inverse relationship between the intramyocardial triglyceride content and the diastolic function; the higher the intracardiac lipid content of the women living with HIV, the worse their cardiac function,” Dr. Toribio said in an interview. She and her colleagues at Massachusetts General Hospital, Boston, where she is a clinical investigator, found a Spearman’s rank coefficient of –0.51 for the correlation (P = .03)
“The reason that this is important is that individuals with HIV do have an increased risk of heart failure,” said Dr. Toribio. People living with HIV have a hazard ratio for heart failure that ranges from about 1.2 to 1.7, she said.
For women living with HIV with heart failure, about 70% have heart failure with preserved ejection fraction (HFpEF), which is associated with diastolic dysfunction. “In women with HIV, this has been relatively understudied, and one of the mechanisms we were looking into is myocardial steatosis, where we have increased intramyocardial lipid content,” said Dr. Toribio.
“I think, certainly, our work has a lot of clinical implications,” said Dr. Toribio, noting that there are no therapies that improve survival after a diagnosis of HFpEF. In a population with increased rates of diastolic dysfunction, “It’s imperative that we understand the mechanism of this disease process in women living with HIV,” she said.
Intramyocardial lipid content was a reasonable line of inquiry, since it’s known that people living with HIV have increased deposition of fat in various organ systems, including the liver, skeletal muscle, and the heart, said Dr. Toribio. Both HIV and antiretroviral therapy can contribute to ectopic fat deposition, she said.
Women with (n = 18) and without (n = 6) HIV were matched according to age, body mass index (BMI), history of hypertension, and smoking status, said Dr. Toribio. For women with HIV, they had to be on stable antiretroviral therapy for at least 3 months and have no interruption in therapy greater than 2 weeks over the 3 months preceding enrollment.
The study excluded women who had known preexisting heart failure, diabetes, or atherosclerotic cardiovascular disease. Participants also could not be taking lipid-lowering agents or anti-inflammatory medications.
Participants were about 52 years old on average, and had a mean BMI of a little over 30 kg/m2. Lipid values did not differ significantly between groups, except that triglycerides were a mean 107 mg/dL in women living with HIV, compared with 69 mg/dL for women without HIV (P = .01).
Of the women living with HIV, 7/18 (38.5%) were white, the same number were black, and 2 were Hispanic. Three of six women without HIV were white, two were black, and one was Hispanic; racial and ethnic differences between the groups were not statistically significant overall.
Magnetic resonance spectroscopy was used to assess intramyocardial triglyceride levels, measured at the interventricular septum, a region where there’s little overlying pericardial fat.
“We found that the women living with HIV have an increased intramyocardial triglyceride content compared to women without HIV. And notably, we sought to see if there was any relationship between circulating triglyceride levels or body mass index, and there actually was no relationship between intramyocardial triglyceride content and these factors,” said Dr. Toribio in a video interview.
Next steps include two studies, said Dr. Toribio. The first is investigating whether statin therapy improves myocardial steatosis and heart function over time in women living with HIV. The second, involving the same population, is a pilot study to see if growth hormone releasing hormone – which is known to lessen visceral adiposity in people living with HIV – can reduce intramyocardial steatosis and boost cardiac function, she said.
Dr. Toribio reported no financial disclosures. The study was supported by funding from the National Institutes of Health.
SOURCE: Toribio M et al. ENDO 2018, Abstract OR11-2.
CHICAGO – Median intramyocardial triglyceride content was nearly four times higher in a group of middle-age women living with HIV, compared with peers without the infection, according to a recent study that also found an association between high myocardial lipids and lower diastolic function.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For women without HIV, the value was 0.13% (95% CI, 0.11-0.23; P = .004). Further, left atrial passive ejection fraction was significantly lower among women living with HIV, compared with those without HIV (28% vs. 38%, P = .02), said Mabel Toribio, MD, speaking at the annual meeting of the Endocrine Society.
“Probably the most important aspect is that we found an inverse relationship between the intramyocardial triglyceride content and the diastolic function; the higher the intracardiac lipid content of the women living with HIV, the worse their cardiac function,” Dr. Toribio said in an interview. She and her colleagues at Massachusetts General Hospital, Boston, where she is a clinical investigator, found a Spearman’s rank coefficient of –0.51 for the correlation (P = .03)
“The reason that this is important is that individuals with HIV do have an increased risk of heart failure,” said Dr. Toribio. People living with HIV have a hazard ratio for heart failure that ranges from about 1.2 to 1.7, she said.
For women living with HIV with heart failure, about 70% have heart failure with preserved ejection fraction (HFpEF), which is associated with diastolic dysfunction. “In women with HIV, this has been relatively understudied, and one of the mechanisms we were looking into is myocardial steatosis, where we have increased intramyocardial lipid content,” said Dr. Toribio.
“I think, certainly, our work has a lot of clinical implications,” said Dr. Toribio, noting that there are no therapies that improve survival after a diagnosis of HFpEF. In a population with increased rates of diastolic dysfunction, “It’s imperative that we understand the mechanism of this disease process in women living with HIV,” she said.
Intramyocardial lipid content was a reasonable line of inquiry, since it’s known that people living with HIV have increased deposition of fat in various organ systems, including the liver, skeletal muscle, and the heart, said Dr. Toribio. Both HIV and antiretroviral therapy can contribute to ectopic fat deposition, she said.
Women with (n = 18) and without (n = 6) HIV were matched according to age, body mass index (BMI), history of hypertension, and smoking status, said Dr. Toribio. For women with HIV, they had to be on stable antiretroviral therapy for at least 3 months and have no interruption in therapy greater than 2 weeks over the 3 months preceding enrollment.
The study excluded women who had known preexisting heart failure, diabetes, or atherosclerotic cardiovascular disease. Participants also could not be taking lipid-lowering agents or anti-inflammatory medications.
Participants were about 52 years old on average, and had a mean BMI of a little over 30 kg/m2. Lipid values did not differ significantly between groups, except that triglycerides were a mean 107 mg/dL in women living with HIV, compared with 69 mg/dL for women without HIV (P = .01).
Of the women living with HIV, 7/18 (38.5%) were white, the same number were black, and 2 were Hispanic. Three of six women without HIV were white, two were black, and one was Hispanic; racial and ethnic differences between the groups were not statistically significant overall.
Magnetic resonance spectroscopy was used to assess intramyocardial triglyceride levels, measured at the interventricular septum, a region where there’s little overlying pericardial fat.
“We found that the women living with HIV have an increased intramyocardial triglyceride content compared to women without HIV. And notably, we sought to see if there was any relationship between circulating triglyceride levels or body mass index, and there actually was no relationship between intramyocardial triglyceride content and these factors,” said Dr. Toribio in a video interview.
Next steps include two studies, said Dr. Toribio. The first is investigating whether statin therapy improves myocardial steatosis and heart function over time in women living with HIV. The second, involving the same population, is a pilot study to see if growth hormone releasing hormone – which is known to lessen visceral adiposity in people living with HIV – can reduce intramyocardial steatosis and boost cardiac function, she said.
Dr. Toribio reported no financial disclosures. The study was supported by funding from the National Institutes of Health.
SOURCE: Toribio M et al. ENDO 2018, Abstract OR11-2.
CHICAGO – Median intramyocardial triglyceride content was nearly four times higher in a group of middle-age women living with HIV, compared with peers without the infection, according to a recent study that also found an association between high myocardial lipids and lower diastolic function.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For women without HIV, the value was 0.13% (95% CI, 0.11-0.23; P = .004). Further, left atrial passive ejection fraction was significantly lower among women living with HIV, compared with those without HIV (28% vs. 38%, P = .02), said Mabel Toribio, MD, speaking at the annual meeting of the Endocrine Society.
“Probably the most important aspect is that we found an inverse relationship between the intramyocardial triglyceride content and the diastolic function; the higher the intracardiac lipid content of the women living with HIV, the worse their cardiac function,” Dr. Toribio said in an interview. She and her colleagues at Massachusetts General Hospital, Boston, where she is a clinical investigator, found a Spearman’s rank coefficient of –0.51 for the correlation (P = .03)
“The reason that this is important is that individuals with HIV do have an increased risk of heart failure,” said Dr. Toribio. People living with HIV have a hazard ratio for heart failure that ranges from about 1.2 to 1.7, she said.
For women living with HIV with heart failure, about 70% have heart failure with preserved ejection fraction (HFpEF), which is associated with diastolic dysfunction. “In women with HIV, this has been relatively understudied, and one of the mechanisms we were looking into is myocardial steatosis, where we have increased intramyocardial lipid content,” said Dr. Toribio.
“I think, certainly, our work has a lot of clinical implications,” said Dr. Toribio, noting that there are no therapies that improve survival after a diagnosis of HFpEF. In a population with increased rates of diastolic dysfunction, “It’s imperative that we understand the mechanism of this disease process in women living with HIV,” she said.
Intramyocardial lipid content was a reasonable line of inquiry, since it’s known that people living with HIV have increased deposition of fat in various organ systems, including the liver, skeletal muscle, and the heart, said Dr. Toribio. Both HIV and antiretroviral therapy can contribute to ectopic fat deposition, she said.
Women with (n = 18) and without (n = 6) HIV were matched according to age, body mass index (BMI), history of hypertension, and smoking status, said Dr. Toribio. For women with HIV, they had to be on stable antiretroviral therapy for at least 3 months and have no interruption in therapy greater than 2 weeks over the 3 months preceding enrollment.
The study excluded women who had known preexisting heart failure, diabetes, or atherosclerotic cardiovascular disease. Participants also could not be taking lipid-lowering agents or anti-inflammatory medications.
Participants were about 52 years old on average, and had a mean BMI of a little over 30 kg/m2. Lipid values did not differ significantly between groups, except that triglycerides were a mean 107 mg/dL in women living with HIV, compared with 69 mg/dL for women without HIV (P = .01).
Of the women living with HIV, 7/18 (38.5%) were white, the same number were black, and 2 were Hispanic. Three of six women without HIV were white, two were black, and one was Hispanic; racial and ethnic differences between the groups were not statistically significant overall.
Magnetic resonance spectroscopy was used to assess intramyocardial triglyceride levels, measured at the interventricular septum, a region where there’s little overlying pericardial fat.
“We found that the women living with HIV have an increased intramyocardial triglyceride content compared to women without HIV. And notably, we sought to see if there was any relationship between circulating triglyceride levels or body mass index, and there actually was no relationship between intramyocardial triglyceride content and these factors,” said Dr. Toribio in a video interview.
Next steps include two studies, said Dr. Toribio. The first is investigating whether statin therapy improves myocardial steatosis and heart function over time in women living with HIV. The second, involving the same population, is a pilot study to see if growth hormone releasing hormone – which is known to lessen visceral adiposity in people living with HIV – can reduce intramyocardial steatosis and boost cardiac function, she said.
Dr. Toribio reported no financial disclosures. The study was supported by funding from the National Institutes of Health.
SOURCE: Toribio M et al. ENDO 2018, Abstract OR11-2.
REPORTING FROM ENDO 2018
Predictors of Long-Term Opioid Use After Opioid Initiation at Discharge From Medical and Surgical Hospitalizations
While patients may be newly exposed to opioids during medical and surgical hospitalization and the prescription of opioids at discharge is common,1-5 prescribers of opioids at discharge may not intend to initiate long-term opioid (LTO) use. By understanding the frequency of progression to LTO use, hospitalists can better balance postdischarge pain treatment and the risk for unintended LTO initiation.
Estimates of LTO use rates following hospital discharge in selected populations1,2,4-6 have varied depending on the population studied and the method of defining LTO use.7 Rates of LTO use following incident opioid prescription have not been directly compared at medical versus surgical discharge or compared with initiation in the ambulatory setting. We present the rates of LTO use following incident opioid exposure at surgical discharge and medical discharge and identify the factors associated with LTO use following surgical and medical discharge.
METHODS
Data Sources
Veterans Health Administration (VHA) data were obtained through the Austin Information Technology Center for fiscal years (FYs) 2003 through 2012 (Austin, Texas). Decision support system national data extracts were used to identify prescription-dispensing events, and inpatient and outpatient medical SAS data sets were used to identify diagnostic codes. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Veterans Affairs (VA) Health Care System Research and Development Committee.
Patients
We included all patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-year opioid-free period.7 Patients with broadly accepted indications for LTO use (eg, metastatic cancer, palliative care, or opioid-dependence treatment) were excluded.7
Opioid Exposure
We included all outpatient prescription fills for noninjectable dosage forms of butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, and tramadol. Consistent with the Centers for Disease Control and Prevention and VA/Department of Defense guidelines, LTO use was defined conceptually as regular use for >90 days. Operationalizing this definition to pharmacy refill data was established by using a cabinet supply methodology,7 which allows for the construction of episodes of continuous medication therapy by estimating the medication supply available to a patient for each day during a defined period based on the pattern of observed refills. LTO use was defined as an episode of continuous opioid supply for >90 days and beginning within 30 days of the initial prescription. While some studies have defined LTO use based on onset within 1 year following surgery,5 the requirement for onset within 30 days of initiation was applied to more strongly tie the association of developing LTO use with the discharge event and minimize various forms of bias that are introduced with extended follow-up periods.
Clinical Characteristics
Patients were classified as being medical discharges, surgical discharges, or outpatient initiators. Patients with an opioid index date within 2 days following discharge were designated based on discharge bed section; additionally, if patients had a surgical bed section during hospitalization, they were assigned as surgical discharges. Demographic, diagnosis, and medication exposure variables that were previously associated with LTO use were selected.8,9 Substance use disorder, chronic pain, anxiety disorder, and depressive disorder were based on International Classification of Diseases, 9th Revision (ICD-9) codes in the preceding year. The use of concurrent benzodiazepines, skeletal muscle relaxants, and antidepressants were determined at opioid initiation.10 Rural or urban residence was assigned by using the Rural-Urban Commuting Area Codes system and mapped with the zip code of a veteran’s residence.11
Analysis
Bivariate and multivariable relationships were determined by using logistic regression. The multivariable model considered all pairwise interaction terms between inpatient service (surgery versus medicine) and each of the variables in the model. Statistically significant interaction terms (P < .05) were retained, and all others were omitted from the final model. The main effects for variables that were involved in a significant interaction term were not reported in the final multivariable model; instead, we created fully specified multivariable models for surgery service and medicine service and reported odds ratios (ORs) for the main effects. All analyses were conducted by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.
CONCLUSION
The initiation of LTO use at discharge is more common in veterans who are discharged from medical than surgical hospitalizations, likely reflecting differences in the patient population, pain conditions, and discharge prescribing decisions. While patient characteristics are associated with LTO use, the strongest associations are with increasing index dose and days’ supply; both represent potentially modifiable prescriber behaviors. These findings support policy changes and other efforts to minimize dose and days supplied when short-term use is intended as a means to address the current opioid epidemic.
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development (Dr. Mosher and Dr. Hofmeyer), and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412) and a Career Development Award (CDA 10-017; Dr. Lund).
Disclosures
The authors report no conflict of interest in regard to this study. The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted by using SAS version 9.2 (SAS Institute Inc, Cary, NC). This manuscript is not under review elsewhere, and there is no prior publication of the manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Healthcare System Research and Development Committee.
1. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med. 2016;31(5):478-485. PubMed
2. Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369-1376. PubMed
3. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. PubMed
4. Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075-1080.
5. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
6. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. PubMed
7. Mosher HJ, Richardson KK, Lund BC. The 1-Year Treatment Course of New Opioid Recipients in Veterans Health Administration. Pain Med. 2016. [Epub ahead of print]. PubMed
8. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332-339. PubMed
9. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. PubMed
10. Mosher HJ, Richardson KK, Lund BC. Sedative Prescriptions Are Common at Opioid Initiation: An Observational Study in the Veterans Health Administration. Pain Med. 2017. [Epub ahead of print]. PubMed
11. Lund BC, Abrams TE, Bernardy NC, Alexander B, Friedman MJ. Benzodiazepine prescribing variation and clinical uncertainty in treating posttraumatic stress disorder. Psychiatr Serv. 2013;64(1):21-27. PubMed
12. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. PubMed
13. Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiol Scand. 2016;60(1):128-137. PubMed
14. Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21-27. PubMed
15. Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain. 2017;18(11):1374-1383. PubMed
While patients may be newly exposed to opioids during medical and surgical hospitalization and the prescription of opioids at discharge is common,1-5 prescribers of opioids at discharge may not intend to initiate long-term opioid (LTO) use. By understanding the frequency of progression to LTO use, hospitalists can better balance postdischarge pain treatment and the risk for unintended LTO initiation.
Estimates of LTO use rates following hospital discharge in selected populations1,2,4-6 have varied depending on the population studied and the method of defining LTO use.7 Rates of LTO use following incident opioid prescription have not been directly compared at medical versus surgical discharge or compared with initiation in the ambulatory setting. We present the rates of LTO use following incident opioid exposure at surgical discharge and medical discharge and identify the factors associated with LTO use following surgical and medical discharge.
METHODS
Data Sources
Veterans Health Administration (VHA) data were obtained through the Austin Information Technology Center for fiscal years (FYs) 2003 through 2012 (Austin, Texas). Decision support system national data extracts were used to identify prescription-dispensing events, and inpatient and outpatient medical SAS data sets were used to identify diagnostic codes. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Veterans Affairs (VA) Health Care System Research and Development Committee.
Patients
We included all patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-year opioid-free period.7 Patients with broadly accepted indications for LTO use (eg, metastatic cancer, palliative care, or opioid-dependence treatment) were excluded.7
Opioid Exposure
We included all outpatient prescription fills for noninjectable dosage forms of butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, and tramadol. Consistent with the Centers for Disease Control and Prevention and VA/Department of Defense guidelines, LTO use was defined conceptually as regular use for >90 days. Operationalizing this definition to pharmacy refill data was established by using a cabinet supply methodology,7 which allows for the construction of episodes of continuous medication therapy by estimating the medication supply available to a patient for each day during a defined period based on the pattern of observed refills. LTO use was defined as an episode of continuous opioid supply for >90 days and beginning within 30 days of the initial prescription. While some studies have defined LTO use based on onset within 1 year following surgery,5 the requirement for onset within 30 days of initiation was applied to more strongly tie the association of developing LTO use with the discharge event and minimize various forms of bias that are introduced with extended follow-up periods.
Clinical Characteristics
Patients were classified as being medical discharges, surgical discharges, or outpatient initiators. Patients with an opioid index date within 2 days following discharge were designated based on discharge bed section; additionally, if patients had a surgical bed section during hospitalization, they were assigned as surgical discharges. Demographic, diagnosis, and medication exposure variables that were previously associated with LTO use were selected.8,9 Substance use disorder, chronic pain, anxiety disorder, and depressive disorder were based on International Classification of Diseases, 9th Revision (ICD-9) codes in the preceding year. The use of concurrent benzodiazepines, skeletal muscle relaxants, and antidepressants were determined at opioid initiation.10 Rural or urban residence was assigned by using the Rural-Urban Commuting Area Codes system and mapped with the zip code of a veteran’s residence.11
Analysis
Bivariate and multivariable relationships were determined by using logistic regression. The multivariable model considered all pairwise interaction terms between inpatient service (surgery versus medicine) and each of the variables in the model. Statistically significant interaction terms (P < .05) were retained, and all others were omitted from the final model. The main effects for variables that were involved in a significant interaction term were not reported in the final multivariable model; instead, we created fully specified multivariable models for surgery service and medicine service and reported odds ratios (ORs) for the main effects. All analyses were conducted by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.
CONCLUSION
The initiation of LTO use at discharge is more common in veterans who are discharged from medical than surgical hospitalizations, likely reflecting differences in the patient population, pain conditions, and discharge prescribing decisions. While patient characteristics are associated with LTO use, the strongest associations are with increasing index dose and days’ supply; both represent potentially modifiable prescriber behaviors. These findings support policy changes and other efforts to minimize dose and days supplied when short-term use is intended as a means to address the current opioid epidemic.
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development (Dr. Mosher and Dr. Hofmeyer), and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412) and a Career Development Award (CDA 10-017; Dr. Lund).
Disclosures
The authors report no conflict of interest in regard to this study. The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted by using SAS version 9.2 (SAS Institute Inc, Cary, NC). This manuscript is not under review elsewhere, and there is no prior publication of the manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Healthcare System Research and Development Committee.
While patients may be newly exposed to opioids during medical and surgical hospitalization and the prescription of opioids at discharge is common,1-5 prescribers of opioids at discharge may not intend to initiate long-term opioid (LTO) use. By understanding the frequency of progression to LTO use, hospitalists can better balance postdischarge pain treatment and the risk for unintended LTO initiation.
Estimates of LTO use rates following hospital discharge in selected populations1,2,4-6 have varied depending on the population studied and the method of defining LTO use.7 Rates of LTO use following incident opioid prescription have not been directly compared at medical versus surgical discharge or compared with initiation in the ambulatory setting. We present the rates of LTO use following incident opioid exposure at surgical discharge and medical discharge and identify the factors associated with LTO use following surgical and medical discharge.
METHODS
Data Sources
Veterans Health Administration (VHA) data were obtained through the Austin Information Technology Center for fiscal years (FYs) 2003 through 2012 (Austin, Texas). Decision support system national data extracts were used to identify prescription-dispensing events, and inpatient and outpatient medical SAS data sets were used to identify diagnostic codes. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Veterans Affairs (VA) Health Care System Research and Development Committee.
Patients
We included all patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-year opioid-free period.7 Patients with broadly accepted indications for LTO use (eg, metastatic cancer, palliative care, or opioid-dependence treatment) were excluded.7
Opioid Exposure
We included all outpatient prescription fills for noninjectable dosage forms of butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, and tramadol. Consistent with the Centers for Disease Control and Prevention and VA/Department of Defense guidelines, LTO use was defined conceptually as regular use for >90 days. Operationalizing this definition to pharmacy refill data was established by using a cabinet supply methodology,7 which allows for the construction of episodes of continuous medication therapy by estimating the medication supply available to a patient for each day during a defined period based on the pattern of observed refills. LTO use was defined as an episode of continuous opioid supply for >90 days and beginning within 30 days of the initial prescription. While some studies have defined LTO use based on onset within 1 year following surgery,5 the requirement for onset within 30 days of initiation was applied to more strongly tie the association of developing LTO use with the discharge event and minimize various forms of bias that are introduced with extended follow-up periods.
Clinical Characteristics
Patients were classified as being medical discharges, surgical discharges, or outpatient initiators. Patients with an opioid index date within 2 days following discharge were designated based on discharge bed section; additionally, if patients had a surgical bed section during hospitalization, they were assigned as surgical discharges. Demographic, diagnosis, and medication exposure variables that were previously associated with LTO use were selected.8,9 Substance use disorder, chronic pain, anxiety disorder, and depressive disorder were based on International Classification of Diseases, 9th Revision (ICD-9) codes in the preceding year. The use of concurrent benzodiazepines, skeletal muscle relaxants, and antidepressants were determined at opioid initiation.10 Rural or urban residence was assigned by using the Rural-Urban Commuting Area Codes system and mapped with the zip code of a veteran’s residence.11
Analysis
Bivariate and multivariable relationships were determined by using logistic regression. The multivariable model considered all pairwise interaction terms between inpatient service (surgery versus medicine) and each of the variables in the model. Statistically significant interaction terms (P < .05) were retained, and all others were omitted from the final model. The main effects for variables that were involved in a significant interaction term were not reported in the final multivariable model; instead, we created fully specified multivariable models for surgery service and medicine service and reported odds ratios (ORs) for the main effects. All analyses were conducted by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.
CONCLUSION
The initiation of LTO use at discharge is more common in veterans who are discharged from medical than surgical hospitalizations, likely reflecting differences in the patient population, pain conditions, and discharge prescribing decisions. While patient characteristics are associated with LTO use, the strongest associations are with increasing index dose and days’ supply; both represent potentially modifiable prescriber behaviors. These findings support policy changes and other efforts to minimize dose and days supplied when short-term use is intended as a means to address the current opioid epidemic.
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development (Dr. Mosher and Dr. Hofmeyer), and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412) and a Career Development Award (CDA 10-017; Dr. Lund).
Disclosures
The authors report no conflict of interest in regard to this study. The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted by using SAS version 9.2 (SAS Institute Inc, Cary, NC). This manuscript is not under review elsewhere, and there is no prior publication of the manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Healthcare System Research and Development Committee.
1. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med. 2016;31(5):478-485. PubMed
2. Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369-1376. PubMed
3. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. PubMed
4. Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075-1080.
5. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
6. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. PubMed
7. Mosher HJ, Richardson KK, Lund BC. The 1-Year Treatment Course of New Opioid Recipients in Veterans Health Administration. Pain Med. 2016. [Epub ahead of print]. PubMed
8. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332-339. PubMed
9. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. PubMed
10. Mosher HJ, Richardson KK, Lund BC. Sedative Prescriptions Are Common at Opioid Initiation: An Observational Study in the Veterans Health Administration. Pain Med. 2017. [Epub ahead of print]. PubMed
11. Lund BC, Abrams TE, Bernardy NC, Alexander B, Friedman MJ. Benzodiazepine prescribing variation and clinical uncertainty in treating posttraumatic stress disorder. Psychiatr Serv. 2013;64(1):21-27. PubMed
12. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. PubMed
13. Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiol Scand. 2016;60(1):128-137. PubMed
14. Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21-27. PubMed
15. Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain. 2017;18(11):1374-1383. PubMed
1. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med. 2016;31(5):478-485. PubMed
2. Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369-1376. PubMed
3. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. PubMed
4. Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075-1080.
5. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
6. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. PubMed
7. Mosher HJ, Richardson KK, Lund BC. The 1-Year Treatment Course of New Opioid Recipients in Veterans Health Administration. Pain Med. 2016. [Epub ahead of print]. PubMed
8. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332-339. PubMed
9. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. PubMed
10. Mosher HJ, Richardson KK, Lund BC. Sedative Prescriptions Are Common at Opioid Initiation: An Observational Study in the Veterans Health Administration. Pain Med. 2017. [Epub ahead of print]. PubMed
11. Lund BC, Abrams TE, Bernardy NC, Alexander B, Friedman MJ. Benzodiazepine prescribing variation and clinical uncertainty in treating posttraumatic stress disorder. Psychiatr Serv. 2013;64(1):21-27. PubMed
12. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. PubMed
13. Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiol Scand. 2016;60(1):128-137. PubMed
14. Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21-27. PubMed
15. Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain. 2017;18(11):1374-1383. PubMed
Engaging skeptical parents
While every day seems to bring extraordinary new advances in science – robotic surgery, individually targeted medications, and even gene therapy – there are many people who currently approach the science of medicine with skepticism.
While it is the right of legally competent adults in a free society to chose how best to care for their own health, to explore holistic or alternative therapies, or avoid medicine altogether, it is more complex when they are skeptical of accepted medical practice in managing the health of their children. For those parents who trust you enough to bring their children to you for care but remain skeptical of vaccines or other treatments, you have an opportunity to work with that trust and engage in a discussion so that they might reconsider their position on valuable and even life-saving treatments for their children.
In each of these cases, launching into an enthusiastic explanation of the advanced statistics that underpin your recommendation is unlikely to bridge the gap. Instead, you want to start with these parents by being curious. Resist the urge to tell, and listen instead. What is their understanding of the problem you are treating or preventing? What have they heard or read about the treatment or test in question? What do they most fear is going to happen to their child if they do or do not accept your recommendation? Are there specific events (with their child or with the health care system) that have informed this fear?
Respectfully listening to their experiences, thoughts, and feelings goes a long way toward building a trusting alliance. It can help overcome feelings of distrust or defensiveness around authority figures. And it models the thoughtful, respectful give and take that are essential to a healthy collaboration between pediatrician and parents.
Once you have information about what they think and some about how they think and make decisions, you then can offer your perspective. “You are the expert on your child, what I bring to this equation is experience with (this problem) and with assessing the scientific evidence that guides treatments in medicine. It is true that treatments often change as we learn more, but here is what the evidence currently supports.”
After learning something about how they think, you might offer more data or more warm acknowledgment of how difficult it can be to make medical decisions for your children with imperfect information. Be humble while also being accurate about your level of confidence in a recommendation. Humility is important because it is easy for parents to feel insecure and condescended to. You understand their greatest fear, now let them know what your greatest worry is for their child should they forgo a recommended treatment. Explaining all of this with humility and warmth makes it more likely that the parents will take in the facts you are trying to share with them and not be derailed by suspicion, defensiveness, or insecurity.
Make building an alliance with the parents your top priority. This does not mean that you do not offer your best recommendation for their child. Rather, it means that, if they still decline recommended treatment, you treat them with respect and invest your time in explaining what they should be watching or monitoring their child for without recommended treatment. Building trust is a long game. If you patiently stick with parents even when it’s not easy, they may be ready to trust you with a subsequent decision when the stakes are even higher.
Of course, all this thoughtful communication takes a lot of time! You may learn to block off more time for certain families. It also can be helpful to have these conversations as a team. If you and your nurse or social worker can meet with parents together, then some of the listening and learning can be done by the nurse or social worker alone, so that everyone’s time might be managed more efficiently. And managing skeptical parents as a team also can help to prevent frustration or burnout. It will not always succeed, but in some cases, your investment will pay off in a trusting alliance, mutual respect, and healthy patients.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
While every day seems to bring extraordinary new advances in science – robotic surgery, individually targeted medications, and even gene therapy – there are many people who currently approach the science of medicine with skepticism.
While it is the right of legally competent adults in a free society to chose how best to care for their own health, to explore holistic or alternative therapies, or avoid medicine altogether, it is more complex when they are skeptical of accepted medical practice in managing the health of their children. For those parents who trust you enough to bring their children to you for care but remain skeptical of vaccines or other treatments, you have an opportunity to work with that trust and engage in a discussion so that they might reconsider their position on valuable and even life-saving treatments for their children.
In each of these cases, launching into an enthusiastic explanation of the advanced statistics that underpin your recommendation is unlikely to bridge the gap. Instead, you want to start with these parents by being curious. Resist the urge to tell, and listen instead. What is their understanding of the problem you are treating or preventing? What have they heard or read about the treatment or test in question? What do they most fear is going to happen to their child if they do or do not accept your recommendation? Are there specific events (with their child or with the health care system) that have informed this fear?
Respectfully listening to their experiences, thoughts, and feelings goes a long way toward building a trusting alliance. It can help overcome feelings of distrust or defensiveness around authority figures. And it models the thoughtful, respectful give and take that are essential to a healthy collaboration between pediatrician and parents.
Once you have information about what they think and some about how they think and make decisions, you then can offer your perspective. “You are the expert on your child, what I bring to this equation is experience with (this problem) and with assessing the scientific evidence that guides treatments in medicine. It is true that treatments often change as we learn more, but here is what the evidence currently supports.”
After learning something about how they think, you might offer more data or more warm acknowledgment of how difficult it can be to make medical decisions for your children with imperfect information. Be humble while also being accurate about your level of confidence in a recommendation. Humility is important because it is easy for parents to feel insecure and condescended to. You understand their greatest fear, now let them know what your greatest worry is for their child should they forgo a recommended treatment. Explaining all of this with humility and warmth makes it more likely that the parents will take in the facts you are trying to share with them and not be derailed by suspicion, defensiveness, or insecurity.
Make building an alliance with the parents your top priority. This does not mean that you do not offer your best recommendation for their child. Rather, it means that, if they still decline recommended treatment, you treat them with respect and invest your time in explaining what they should be watching or monitoring their child for without recommended treatment. Building trust is a long game. If you patiently stick with parents even when it’s not easy, they may be ready to trust you with a subsequent decision when the stakes are even higher.
Of course, all this thoughtful communication takes a lot of time! You may learn to block off more time for certain families. It also can be helpful to have these conversations as a team. If you and your nurse or social worker can meet with parents together, then some of the listening and learning can be done by the nurse or social worker alone, so that everyone’s time might be managed more efficiently. And managing skeptical parents as a team also can help to prevent frustration or burnout. It will not always succeed, but in some cases, your investment will pay off in a trusting alliance, mutual respect, and healthy patients.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
While every day seems to bring extraordinary new advances in science – robotic surgery, individually targeted medications, and even gene therapy – there are many people who currently approach the science of medicine with skepticism.
While it is the right of legally competent adults in a free society to chose how best to care for their own health, to explore holistic or alternative therapies, or avoid medicine altogether, it is more complex when they are skeptical of accepted medical practice in managing the health of their children. For those parents who trust you enough to bring their children to you for care but remain skeptical of vaccines or other treatments, you have an opportunity to work with that trust and engage in a discussion so that they might reconsider their position on valuable and even life-saving treatments for their children.
In each of these cases, launching into an enthusiastic explanation of the advanced statistics that underpin your recommendation is unlikely to bridge the gap. Instead, you want to start with these parents by being curious. Resist the urge to tell, and listen instead. What is their understanding of the problem you are treating or preventing? What have they heard or read about the treatment or test in question? What do they most fear is going to happen to their child if they do or do not accept your recommendation? Are there specific events (with their child or with the health care system) that have informed this fear?
Respectfully listening to their experiences, thoughts, and feelings goes a long way toward building a trusting alliance. It can help overcome feelings of distrust or defensiveness around authority figures. And it models the thoughtful, respectful give and take that are essential to a healthy collaboration between pediatrician and parents.
Once you have information about what they think and some about how they think and make decisions, you then can offer your perspective. “You are the expert on your child, what I bring to this equation is experience with (this problem) and with assessing the scientific evidence that guides treatments in medicine. It is true that treatments often change as we learn more, but here is what the evidence currently supports.”
After learning something about how they think, you might offer more data or more warm acknowledgment of how difficult it can be to make medical decisions for your children with imperfect information. Be humble while also being accurate about your level of confidence in a recommendation. Humility is important because it is easy for parents to feel insecure and condescended to. You understand their greatest fear, now let them know what your greatest worry is for their child should they forgo a recommended treatment. Explaining all of this with humility and warmth makes it more likely that the parents will take in the facts you are trying to share with them and not be derailed by suspicion, defensiveness, or insecurity.
Make building an alliance with the parents your top priority. This does not mean that you do not offer your best recommendation for their child. Rather, it means that, if they still decline recommended treatment, you treat them with respect and invest your time in explaining what they should be watching or monitoring their child for without recommended treatment. Building trust is a long game. If you patiently stick with parents even when it’s not easy, they may be ready to trust you with a subsequent decision when the stakes are even higher.
Of course, all this thoughtful communication takes a lot of time! You may learn to block off more time for certain families. It also can be helpful to have these conversations as a team. If you and your nurse or social worker can meet with parents together, then some of the listening and learning can be done by the nurse or social worker alone, so that everyone’s time might be managed more efficiently. And managing skeptical parents as a team also can help to prevent frustration or burnout. It will not always succeed, but in some cases, your investment will pay off in a trusting alliance, mutual respect, and healthy patients.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
Oral SGLT-2 inhibitor reduced liver fat in diabetics with NAFLD
CHICAGO – and improved ALT in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus, according to a study presented at the annual meeting of the Endocrine Society.
As insulin resistance is the mechanism for NAFLD development, this new addition to the list of drugs on offer to patients with diabetes could help decrease the chance of developing metabolic syndrome and cardiovascular disease.
“SGLT-2 inhibitors are newer antidiabetic agents that reduce blood glucose by promoting urinary glucose excretion,” said presenter Mohammad Shafi Kuchay, MD, DM, an endocrinologist at Medanta The Medicity, Gurugram, India. “NAFLD, which also increases the risk of type 2 diabetes, often responds to strategies that improve hyperglycemia.”
Dr. Kuchay and fellow investigators conducted a small, 20-week randomized controlled trial of 42 patients with type 2 diabetes and NAFLD.
Patients in the test group were mostly male and on average 50 years old, with baseline AST, ALT, and gamma-glutamyltransferase scores of 44.6 U/L, 64.3 U/L, and 65.8 U/L, respectively. Those randomized to the control group had similar characteristics.
After adding 10 mg of empagliflozin to their diabetes regimen, liver fat density in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001). The drop stands in sharp contrast to the control group, which decreased from 16.4% to 15.5% (P = .054). Measurement of liver fat density was made by MRI-derived proton density fat fraction (MRI-PDFF). This method has higher sensitivity for detecting changes in liver fat, compared with histology, explained Dr. Kuchay.
When broken down by individual liver fat, 25% of patients in the control group increased in liver fat, 50% had no significant change, and 25% decreased in liver fat, according to Dr. Kuchay.
In comparison, 77% of patients in the empagliflozin group had a decrease in liver fat, 23% had no change, and no patients saw an increase in liver fat.
When comparing levels of hemoglobin A1c between the two groups, both had a similarly significant reduction of around 2%, which Dr. Kuchay attributes to deliberate intervention by investigators.
Further studies will need to be conducted regarding the long-term effects of this treatment; however, using SGLT-2 to reduce liver fat could be a boon to preventing more serious liver diseases, concluded Dr. Kuchay.
“There are studies in which liver fat reduction led to improvement in inflammation and fibrosis,” said Dr. Kuchay in response to a question from the audience. “Because liver fat accumulation is the first inhibitor in the pathogenesis of more severe forms of liver disease, reducing liver fat should help improve patient outcomes.”
Dr. Kuchay reported no relevant financial disclosures.
Source: M. Kuchay et al. ENDO 2018, Abstract OR27-2.
CHICAGO – and improved ALT in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus, according to a study presented at the annual meeting of the Endocrine Society.
As insulin resistance is the mechanism for NAFLD development, this new addition to the list of drugs on offer to patients with diabetes could help decrease the chance of developing metabolic syndrome and cardiovascular disease.
“SGLT-2 inhibitors are newer antidiabetic agents that reduce blood glucose by promoting urinary glucose excretion,” said presenter Mohammad Shafi Kuchay, MD, DM, an endocrinologist at Medanta The Medicity, Gurugram, India. “NAFLD, which also increases the risk of type 2 diabetes, often responds to strategies that improve hyperglycemia.”
Dr. Kuchay and fellow investigators conducted a small, 20-week randomized controlled trial of 42 patients with type 2 diabetes and NAFLD.
Patients in the test group were mostly male and on average 50 years old, with baseline AST, ALT, and gamma-glutamyltransferase scores of 44.6 U/L, 64.3 U/L, and 65.8 U/L, respectively. Those randomized to the control group had similar characteristics.
After adding 10 mg of empagliflozin to their diabetes regimen, liver fat density in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001). The drop stands in sharp contrast to the control group, which decreased from 16.4% to 15.5% (P = .054). Measurement of liver fat density was made by MRI-derived proton density fat fraction (MRI-PDFF). This method has higher sensitivity for detecting changes in liver fat, compared with histology, explained Dr. Kuchay.
When broken down by individual liver fat, 25% of patients in the control group increased in liver fat, 50% had no significant change, and 25% decreased in liver fat, according to Dr. Kuchay.
In comparison, 77% of patients in the empagliflozin group had a decrease in liver fat, 23% had no change, and no patients saw an increase in liver fat.
When comparing levels of hemoglobin A1c between the two groups, both had a similarly significant reduction of around 2%, which Dr. Kuchay attributes to deliberate intervention by investigators.
Further studies will need to be conducted regarding the long-term effects of this treatment; however, using SGLT-2 to reduce liver fat could be a boon to preventing more serious liver diseases, concluded Dr. Kuchay.
“There are studies in which liver fat reduction led to improvement in inflammation and fibrosis,” said Dr. Kuchay in response to a question from the audience. “Because liver fat accumulation is the first inhibitor in the pathogenesis of more severe forms of liver disease, reducing liver fat should help improve patient outcomes.”
Dr. Kuchay reported no relevant financial disclosures.
Source: M. Kuchay et al. ENDO 2018, Abstract OR27-2.
CHICAGO – and improved ALT in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus, according to a study presented at the annual meeting of the Endocrine Society.
As insulin resistance is the mechanism for NAFLD development, this new addition to the list of drugs on offer to patients with diabetes could help decrease the chance of developing metabolic syndrome and cardiovascular disease.
“SGLT-2 inhibitors are newer antidiabetic agents that reduce blood glucose by promoting urinary glucose excretion,” said presenter Mohammad Shafi Kuchay, MD, DM, an endocrinologist at Medanta The Medicity, Gurugram, India. “NAFLD, which also increases the risk of type 2 diabetes, often responds to strategies that improve hyperglycemia.”
Dr. Kuchay and fellow investigators conducted a small, 20-week randomized controlled trial of 42 patients with type 2 diabetes and NAFLD.
Patients in the test group were mostly male and on average 50 years old, with baseline AST, ALT, and gamma-glutamyltransferase scores of 44.6 U/L, 64.3 U/L, and 65.8 U/L, respectively. Those randomized to the control group had similar characteristics.
After adding 10 mg of empagliflozin to their diabetes regimen, liver fat density in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001). The drop stands in sharp contrast to the control group, which decreased from 16.4% to 15.5% (P = .054). Measurement of liver fat density was made by MRI-derived proton density fat fraction (MRI-PDFF). This method has higher sensitivity for detecting changes in liver fat, compared with histology, explained Dr. Kuchay.
When broken down by individual liver fat, 25% of patients in the control group increased in liver fat, 50% had no significant change, and 25% decreased in liver fat, according to Dr. Kuchay.
In comparison, 77% of patients in the empagliflozin group had a decrease in liver fat, 23% had no change, and no patients saw an increase in liver fat.
When comparing levels of hemoglobin A1c between the two groups, both had a similarly significant reduction of around 2%, which Dr. Kuchay attributes to deliberate intervention by investigators.
Further studies will need to be conducted regarding the long-term effects of this treatment; however, using SGLT-2 to reduce liver fat could be a boon to preventing more serious liver diseases, concluded Dr. Kuchay.
“There are studies in which liver fat reduction led to improvement in inflammation and fibrosis,” said Dr. Kuchay in response to a question from the audience. “Because liver fat accumulation is the first inhibitor in the pathogenesis of more severe forms of liver disease, reducing liver fat should help improve patient outcomes.”
Dr. Kuchay reported no relevant financial disclosures.
Source: M. Kuchay et al. ENDO 2018, Abstract OR27-2.
REPORTING FROM ENDO 2018
Key clinical point: Empagliflozin reduced liver fat in patients with NAFLD and type 2 diabetes.
Major finding: MRI-PDFF in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001), compared with control patients, who saw a decrease from 19.4% to 15.5% (P = .057)
Data source: Prospective, randomized, controlled trial of 60 patients with type 2 diabetes and NAFLD.
Disclosures: Dr. Kuchay reported no relevant financial disclosures.
Source: Kuchay M et al. ENDO 2018, Abstract OR27-2.