User login
FDA approves certolizumab label update for pregnancy, breastfeeding
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
What’s in the way of you and new tech?
BOSTON – Bringing new technology to your practice is not as simple as flipping a switch, as attendees of the Thursday afternoon AGA Tech Summit session “Physician Perspective on Barriers to Incorporating New Technology” learned. The Tech Summit is sponsored by the AGA Center for GI Innovation and Technology.
“As physicians think about being a part of taking on new technology, there are varying perspectives, including the perspective they have about their patients and the perspective they have for themselves,” Richard Rothstein, MD, chair of the department of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. “However, there are other perspectives as well, like the perspectives of the hospital or the ambulatory endoscopy center in which they work.”
He presented an intriguing historical example. Within months of the first demonstration of anesthetized surgery in 1846, the use of ether and the machine to deliver it were spreading rapidly through hospitals in large U.S. cities. European adoption soon followed.
However, decades passed before there was wide acceptance of Lister’s ideas on carbolic acid as a surgical antiseptic.
“Why was one technology adopted early and one later? Incentives to adopt both went in the same direction – improved patient outcomes. Both were based on ideas that violated prior beliefs. Both were technically complex. But one combatted a visible and immediate problem: pain. The other combatted an invisible and unproven problem: germs. Both made life better for the patient – but only one made life better for the surgeon. And that one, anesthesia, was the one that was quickly adopted.”
Even today, clinicians are the main drivers of the adoption of novel medical technology. They fall into two general categories, Dr. Rothstein said: early adopters, who want to be the first to offer an exciting new procedure, and late adopters, who wait for more information and want all the issues of that technology to be sorted out before diving in.
Each one stands in the same circle, however, forced to evaluate the issues that come along with adopting new tech, including training, credentialing and insurance, facility support, and how the new tool or procedure might affect the entire clinical team
Facilities have to tussle with these issues, too, Dr. Rothstein said.
Administrations wonder, “‘Will I get paid for this? Will it displace something else that’s equally effective that could be making more money? What resources do I need to implement it? Will it impact malpractice insurance rates for clinicians who work at my facility?’”
Patient choice also plays into the matter. Third-party payers may or may not have cutting-edge tech on their payment ledger. The specter of a self-pay procedure, no matter how potentially effective, is an enormous deterrent for patients, especially when figuring in the possibility of footing the bill for any associated complications. And of course, new technology and procedures lack the deep pool of efficacy and safety data that established ones lean upon – another potential sticking point for both clinicians and patients, Dr. Rothstein said.
“There are a lot of great ideas out there, and a lot of innovative devices, but without addressing the barriers to adoption, the technology will never get to the targeted goal of delivering better care to our patients,” he said.
BOSTON – Bringing new technology to your practice is not as simple as flipping a switch, as attendees of the Thursday afternoon AGA Tech Summit session “Physician Perspective on Barriers to Incorporating New Technology” learned. The Tech Summit is sponsored by the AGA Center for GI Innovation and Technology.
“As physicians think about being a part of taking on new technology, there are varying perspectives, including the perspective they have about their patients and the perspective they have for themselves,” Richard Rothstein, MD, chair of the department of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. “However, there are other perspectives as well, like the perspectives of the hospital or the ambulatory endoscopy center in which they work.”
He presented an intriguing historical example. Within months of the first demonstration of anesthetized surgery in 1846, the use of ether and the machine to deliver it were spreading rapidly through hospitals in large U.S. cities. European adoption soon followed.
However, decades passed before there was wide acceptance of Lister’s ideas on carbolic acid as a surgical antiseptic.
“Why was one technology adopted early and one later? Incentives to adopt both went in the same direction – improved patient outcomes. Both were based on ideas that violated prior beliefs. Both were technically complex. But one combatted a visible and immediate problem: pain. The other combatted an invisible and unproven problem: germs. Both made life better for the patient – but only one made life better for the surgeon. And that one, anesthesia, was the one that was quickly adopted.”
Even today, clinicians are the main drivers of the adoption of novel medical technology. They fall into two general categories, Dr. Rothstein said: early adopters, who want to be the first to offer an exciting new procedure, and late adopters, who wait for more information and want all the issues of that technology to be sorted out before diving in.
Each one stands in the same circle, however, forced to evaluate the issues that come along with adopting new tech, including training, credentialing and insurance, facility support, and how the new tool or procedure might affect the entire clinical team
Facilities have to tussle with these issues, too, Dr. Rothstein said.
Administrations wonder, “‘Will I get paid for this? Will it displace something else that’s equally effective that could be making more money? What resources do I need to implement it? Will it impact malpractice insurance rates for clinicians who work at my facility?’”
Patient choice also plays into the matter. Third-party payers may or may not have cutting-edge tech on their payment ledger. The specter of a self-pay procedure, no matter how potentially effective, is an enormous deterrent for patients, especially when figuring in the possibility of footing the bill for any associated complications. And of course, new technology and procedures lack the deep pool of efficacy and safety data that established ones lean upon – another potential sticking point for both clinicians and patients, Dr. Rothstein said.
“There are a lot of great ideas out there, and a lot of innovative devices, but without addressing the barriers to adoption, the technology will never get to the targeted goal of delivering better care to our patients,” he said.
BOSTON – Bringing new technology to your practice is not as simple as flipping a switch, as attendees of the Thursday afternoon AGA Tech Summit session “Physician Perspective on Barriers to Incorporating New Technology” learned. The Tech Summit is sponsored by the AGA Center for GI Innovation and Technology.
“As physicians think about being a part of taking on new technology, there are varying perspectives, including the perspective they have about their patients and the perspective they have for themselves,” Richard Rothstein, MD, chair of the department of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. “However, there are other perspectives as well, like the perspectives of the hospital or the ambulatory endoscopy center in which they work.”
He presented an intriguing historical example. Within months of the first demonstration of anesthetized surgery in 1846, the use of ether and the machine to deliver it were spreading rapidly through hospitals in large U.S. cities. European adoption soon followed.
However, decades passed before there was wide acceptance of Lister’s ideas on carbolic acid as a surgical antiseptic.
“Why was one technology adopted early and one later? Incentives to adopt both went in the same direction – improved patient outcomes. Both were based on ideas that violated prior beliefs. Both were technically complex. But one combatted a visible and immediate problem: pain. The other combatted an invisible and unproven problem: germs. Both made life better for the patient – but only one made life better for the surgeon. And that one, anesthesia, was the one that was quickly adopted.”
Even today, clinicians are the main drivers of the adoption of novel medical technology. They fall into two general categories, Dr. Rothstein said: early adopters, who want to be the first to offer an exciting new procedure, and late adopters, who wait for more information and want all the issues of that technology to be sorted out before diving in.
Each one stands in the same circle, however, forced to evaluate the issues that come along with adopting new tech, including training, credentialing and insurance, facility support, and how the new tool or procedure might affect the entire clinical team
Facilities have to tussle with these issues, too, Dr. Rothstein said.
Administrations wonder, “‘Will I get paid for this? Will it displace something else that’s equally effective that could be making more money? What resources do I need to implement it? Will it impact malpractice insurance rates for clinicians who work at my facility?’”
Patient choice also plays into the matter. Third-party payers may or may not have cutting-edge tech on their payment ledger. The specter of a self-pay procedure, no matter how potentially effective, is an enormous deterrent for patients, especially when figuring in the possibility of footing the bill for any associated complications. And of course, new technology and procedures lack the deep pool of efficacy and safety data that established ones lean upon – another potential sticking point for both clinicians and patients, Dr. Rothstein said.
“There are a lot of great ideas out there, and a lot of innovative devices, but without addressing the barriers to adoption, the technology will never get to the targeted goal of delivering better care to our patients,” he said.
REPORTING FROM 2018 AGA TECH SUMMIT
Experimental voxtalisib shows mixed results in phase 2 study
Voxtalisib, an investigational agent that targets both mTOR and multiple isoforms of PI3K, showed “promising” efficacy with acceptable safety in patients with relapsed or refractory follicular lymphoma (FL), results of a phase 2 trial indicate.
Among 46 patients with FL, the overall response rate was 41.3%, including five (10.9%) complete responses. The median progression-free survival in this group was 58 weeks, reported Jennifer R. Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and her colleagues.
“The observed activity of voxtalisib in relapsed or refractory follicular lymphoma, notable for inducing complete responses in 10.9% of patients, warrants further study,” the investigators wrote in a study published in the Lancet Haematology.
Efficacy of the drug was limited, however, against aggressive malignancies, including mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Voxtalisib (XL765) is a potent inhibitor of all four class I PI3Ks, as well as a less robust inhibitor of the mammalian target of rapamycin (mTOR). In contrast, idelalisib (Zydelig) – which is approved by the Food and Drug Administration for treatment of relapsed/refractory FL or for CLL, in combination with rituximab – inhibits only the delta isoform of PI3K, and does not have marked anti–mTOR properties.
The investigators conducted an open-label, nonrandomized trial of voxtalisib in 30 centers in the United States, Belgium, France, Germany, the Netherlands, and Australia.
Adults 18 years or older with relapsed or refractory MCL, FL, DLBCL or CLL/SLL with Eastern Cooperative Oncology Group performance status of 2 or less were enrolled. All patients received voxtalisib 50 mg orally twice daily in 28-day continuous dosing cycles until progression or unacceptable toxicity.
All patients who received more the 4 weeks of treatment and had both a baseline and one or more on-treatment tumor assessments were included in the efficacy analysis. Patients with lymphoma had received a median of three prior lines of therapy, and those with CLL had received a median of four prior lines.
The overall response rate in the entire study population was 18.3% (30 patients), including 22 partial and 8 complete responses. ORR rates were as follows:
- FL: 41.3% (19 of 46 patients).
- MCL: 11.9% (5 of 42 patients).
- DLBCL: 4.9% (2 of 41 patients).
- CLL/SLL: 11.4% (4 of 35 patients).
The safety analysis, which included all 167 patients enrolled, was consistent with that of previous studies of voxtalisib, the investigators said. The most frequently reported adverse events of any grade or type were diarrhea in 35% of patients, fatigue in 32%, nausea in 27%, pyrexia in 26%, cough 24%, and decreased appetite in 21%.
Grade 3 or greater adverse events include anemia in 12%, and pneumonia and thrombocytopenia in 8% each. Slightly more than half of all patients (58.1%) had a serious adverse event.
The investigators noted that voxtalisib’s short plasma half-life may explain the drug’s lack of efficacy against the aggressive lymphomas and CLL/SLL. Longer-acting formulations of the drug or more frequent dosing might address this problem, although the latter solution could be challenging for patients to follow, the investigators acknowledged.
In light of the results, no further studies of voxtalisib in CLL are planned, thought investigation of the drug alone or in combination with chemoimmunotherapy is warranted for patients with follicular lymphoma, the investigators wrote.
The study was funded by Sanofi. Dr. Brown reported consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
SOURCE: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
Voxtalisib, an investigational agent that targets both mTOR and multiple isoforms of PI3K, showed “promising” efficacy with acceptable safety in patients with relapsed or refractory follicular lymphoma (FL), results of a phase 2 trial indicate.
Among 46 patients with FL, the overall response rate was 41.3%, including five (10.9%) complete responses. The median progression-free survival in this group was 58 weeks, reported Jennifer R. Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and her colleagues.
“The observed activity of voxtalisib in relapsed or refractory follicular lymphoma, notable for inducing complete responses in 10.9% of patients, warrants further study,” the investigators wrote in a study published in the Lancet Haematology.
Efficacy of the drug was limited, however, against aggressive malignancies, including mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Voxtalisib (XL765) is a potent inhibitor of all four class I PI3Ks, as well as a less robust inhibitor of the mammalian target of rapamycin (mTOR). In contrast, idelalisib (Zydelig) – which is approved by the Food and Drug Administration for treatment of relapsed/refractory FL or for CLL, in combination with rituximab – inhibits only the delta isoform of PI3K, and does not have marked anti–mTOR properties.
The investigators conducted an open-label, nonrandomized trial of voxtalisib in 30 centers in the United States, Belgium, France, Germany, the Netherlands, and Australia.
Adults 18 years or older with relapsed or refractory MCL, FL, DLBCL or CLL/SLL with Eastern Cooperative Oncology Group performance status of 2 or less were enrolled. All patients received voxtalisib 50 mg orally twice daily in 28-day continuous dosing cycles until progression or unacceptable toxicity.
All patients who received more the 4 weeks of treatment and had both a baseline and one or more on-treatment tumor assessments were included in the efficacy analysis. Patients with lymphoma had received a median of three prior lines of therapy, and those with CLL had received a median of four prior lines.
The overall response rate in the entire study population was 18.3% (30 patients), including 22 partial and 8 complete responses. ORR rates were as follows:
- FL: 41.3% (19 of 46 patients).
- MCL: 11.9% (5 of 42 patients).
- DLBCL: 4.9% (2 of 41 patients).
- CLL/SLL: 11.4% (4 of 35 patients).
The safety analysis, which included all 167 patients enrolled, was consistent with that of previous studies of voxtalisib, the investigators said. The most frequently reported adverse events of any grade or type were diarrhea in 35% of patients, fatigue in 32%, nausea in 27%, pyrexia in 26%, cough 24%, and decreased appetite in 21%.
Grade 3 or greater adverse events include anemia in 12%, and pneumonia and thrombocytopenia in 8% each. Slightly more than half of all patients (58.1%) had a serious adverse event.
The investigators noted that voxtalisib’s short plasma half-life may explain the drug’s lack of efficacy against the aggressive lymphomas and CLL/SLL. Longer-acting formulations of the drug or more frequent dosing might address this problem, although the latter solution could be challenging for patients to follow, the investigators acknowledged.
In light of the results, no further studies of voxtalisib in CLL are planned, thought investigation of the drug alone or in combination with chemoimmunotherapy is warranted for patients with follicular lymphoma, the investigators wrote.
The study was funded by Sanofi. Dr. Brown reported consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
SOURCE: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
Voxtalisib, an investigational agent that targets both mTOR and multiple isoforms of PI3K, showed “promising” efficacy with acceptable safety in patients with relapsed or refractory follicular lymphoma (FL), results of a phase 2 trial indicate.
Among 46 patients with FL, the overall response rate was 41.3%, including five (10.9%) complete responses. The median progression-free survival in this group was 58 weeks, reported Jennifer R. Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and her colleagues.
“The observed activity of voxtalisib in relapsed or refractory follicular lymphoma, notable for inducing complete responses in 10.9% of patients, warrants further study,” the investigators wrote in a study published in the Lancet Haematology.
Efficacy of the drug was limited, however, against aggressive malignancies, including mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Voxtalisib (XL765) is a potent inhibitor of all four class I PI3Ks, as well as a less robust inhibitor of the mammalian target of rapamycin (mTOR). In contrast, idelalisib (Zydelig) – which is approved by the Food and Drug Administration for treatment of relapsed/refractory FL or for CLL, in combination with rituximab – inhibits only the delta isoform of PI3K, and does not have marked anti–mTOR properties.
The investigators conducted an open-label, nonrandomized trial of voxtalisib in 30 centers in the United States, Belgium, France, Germany, the Netherlands, and Australia.
Adults 18 years or older with relapsed or refractory MCL, FL, DLBCL or CLL/SLL with Eastern Cooperative Oncology Group performance status of 2 or less were enrolled. All patients received voxtalisib 50 mg orally twice daily in 28-day continuous dosing cycles until progression or unacceptable toxicity.
All patients who received more the 4 weeks of treatment and had both a baseline and one or more on-treatment tumor assessments were included in the efficacy analysis. Patients with lymphoma had received a median of three prior lines of therapy, and those with CLL had received a median of four prior lines.
The overall response rate in the entire study population was 18.3% (30 patients), including 22 partial and 8 complete responses. ORR rates were as follows:
- FL: 41.3% (19 of 46 patients).
- MCL: 11.9% (5 of 42 patients).
- DLBCL: 4.9% (2 of 41 patients).
- CLL/SLL: 11.4% (4 of 35 patients).
The safety analysis, which included all 167 patients enrolled, was consistent with that of previous studies of voxtalisib, the investigators said. The most frequently reported adverse events of any grade or type were diarrhea in 35% of patients, fatigue in 32%, nausea in 27%, pyrexia in 26%, cough 24%, and decreased appetite in 21%.
Grade 3 or greater adverse events include anemia in 12%, and pneumonia and thrombocytopenia in 8% each. Slightly more than half of all patients (58.1%) had a serious adverse event.
The investigators noted that voxtalisib’s short plasma half-life may explain the drug’s lack of efficacy against the aggressive lymphomas and CLL/SLL. Longer-acting formulations of the drug or more frequent dosing might address this problem, although the latter solution could be challenging for patients to follow, the investigators acknowledged.
In light of the results, no further studies of voxtalisib in CLL are planned, thought investigation of the drug alone or in combination with chemoimmunotherapy is warranted for patients with follicular lymphoma, the investigators wrote.
The study was funded by Sanofi. Dr. Brown reported consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
SOURCE: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The overall response rate in patients with relapsed/refractory FL was 41.3%.
Study details: Open-label, nonrandomized trial in 167 patients from 30 centers in six countries.
Disclosures: The study was funded by Sanofi. Dr. Brown disclosed consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
Source: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
VIDEO: Digital health technologies are here to be embraced
BOSTON – One of the innovations in gastroenterology that is used on a day-to-day basis is digital technology, said Sri Komanduri, MD, in a video interview at the 2018 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Everything from Internet rate-your-doctor sites to bowel prep apps and EHRs qualify as digital health technology, said Dr. Komanduri, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago and vice chair of the AGA Center for GI Innovation and Technology.
Digital technology can facilitate endoscopy procedures, help patients communicate with their doctors about chronic conditions, and help patients better understand their illness. The role of the physician is to embrace those technologies that improve the quality of care.
BOSTON – One of the innovations in gastroenterology that is used on a day-to-day basis is digital technology, said Sri Komanduri, MD, in a video interview at the 2018 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Everything from Internet rate-your-doctor sites to bowel prep apps and EHRs qualify as digital health technology, said Dr. Komanduri, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago and vice chair of the AGA Center for GI Innovation and Technology.
Digital technology can facilitate endoscopy procedures, help patients communicate with their doctors about chronic conditions, and help patients better understand their illness. The role of the physician is to embrace those technologies that improve the quality of care.
BOSTON – One of the innovations in gastroenterology that is used on a day-to-day basis is digital technology, said Sri Komanduri, MD, in a video interview at the 2018 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Everything from Internet rate-your-doctor sites to bowel prep apps and EHRs qualify as digital health technology, said Dr. Komanduri, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago and vice chair of the AGA Center for GI Innovation and Technology.
Digital technology can facilitate endoscopy procedures, help patients communicate with their doctors about chronic conditions, and help patients better understand their illness. The role of the physician is to embrace those technologies that improve the quality of care.
FROM THE 2018 AGA TECH SUMMIT
Update on AGA-Medtronic Research & Development Pilot Award in Technology
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
REPORTING FROM 2018 AGA TECH SUMMIT
‘Right to try’ bill passes House
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Same-day discharge for hysterectomy
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies
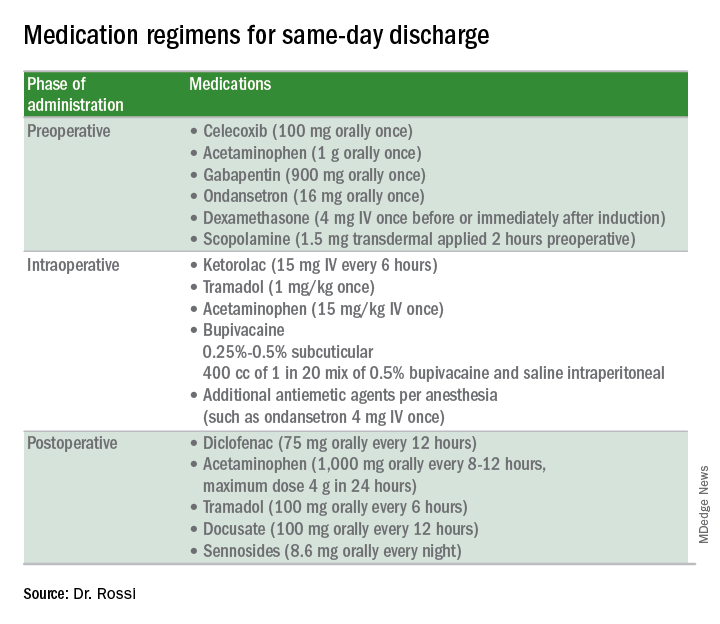
Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
FDA approves IL-23 antagonist for plaque psoriasis
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
Think methotrexate for granulomatous mastitis
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM RWCS 2018
Key clinical point:
Major finding: At 3 months, 17 of 19 patients showed improvement on methotrexate at 15 mg/week, and at 12 months, 12 of 15 had experienced disease resolution on 20 mg/week.
Study details: A single-center retrospective review of 19 patients with granulomatous mastitis.
Disclosures: The presenter reported having no financial conflicts of interest regarding her presentation.
Tardive dyskinesia is theme of awards competition for early career psychiatrists
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at [email protected]. Winners will be announced by Aug. 10, 2018. For additional information, write to [email protected] or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at [email protected]. Winners will be announced by Aug. 10, 2018. For additional information, write to [email protected] or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at [email protected]. Winners will be announced by Aug. 10, 2018. For additional information, write to [email protected] or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.