User login
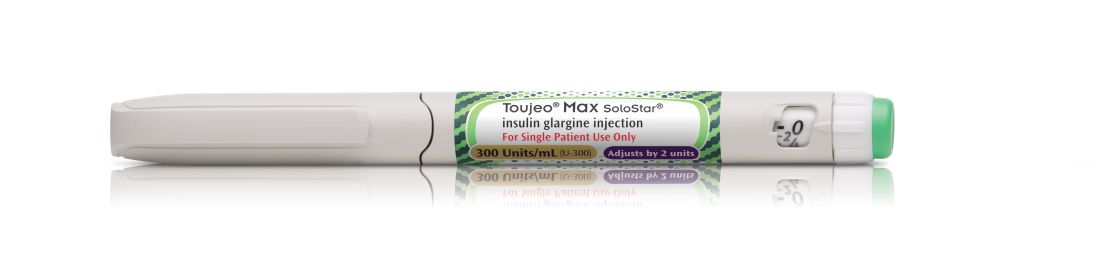
FDA approves highest capacity insulin pen
This will be the highest capacity long-acting insulin pen to be available commercially, according to a company press release.
The Max SoloStar pen holds more than 900 units of insulin glargine and can provide up to 160 units/mL in a single injection, which may reduce the number of injections needed to deliver the necessary dosage to adults. Another benefit of the higher capacity is that the device will require fewer refills and the associated copays, which could potentially lower costs for patients, depending on their insurance coverage.
The dosing instructions for insulin glargine vary depending on whether patients use the older SoloStar or the high capacity Max SoloStar. The SoloStar holds 450 units (1.5mL) of insulin glargine and delivers doses in 1 unit increments, delivering a maximum dose of 80 units per injection. The MaxSoloStar has twice the capacity of the original – 900 units (3 mL) of insulin glargine – and delivers doses in 2 unit increments up to a maximum 160 unit injection. This is recommended for patients who require at least 20 units per day. All insulin glargine injections should be administered subcutaneously in the abdomen, thigh, or deltoid at the same time each day.
The most common side effect of insulin products, such as insulin glargine, is hypoglycemia.
The Max SoloStar will be available in retail pharmacies throughout the United States in the third quarter of 2018.
This will be the highest capacity long-acting insulin pen to be available commercially, according to a company press release.
The Max SoloStar pen holds more than 900 units of insulin glargine and can provide up to 160 units/mL in a single injection, which may reduce the number of injections needed to deliver the necessary dosage to adults. Another benefit of the higher capacity is that the device will require fewer refills and the associated copays, which could potentially lower costs for patients, depending on their insurance coverage.
The dosing instructions for insulin glargine vary depending on whether patients use the older SoloStar or the high capacity Max SoloStar. The SoloStar holds 450 units (1.5mL) of insulin glargine and delivers doses in 1 unit increments, delivering a maximum dose of 80 units per injection. The MaxSoloStar has twice the capacity of the original – 900 units (3 mL) of insulin glargine – and delivers doses in 2 unit increments up to a maximum 160 unit injection. This is recommended for patients who require at least 20 units per day. All insulin glargine injections should be administered subcutaneously in the abdomen, thigh, or deltoid at the same time each day.
The most common side effect of insulin products, such as insulin glargine, is hypoglycemia.
The Max SoloStar will be available in retail pharmacies throughout the United States in the third quarter of 2018.
This will be the highest capacity long-acting insulin pen to be available commercially, according to a company press release.
The Max SoloStar pen holds more than 900 units of insulin glargine and can provide up to 160 units/mL in a single injection, which may reduce the number of injections needed to deliver the necessary dosage to adults. Another benefit of the higher capacity is that the device will require fewer refills and the associated copays, which could potentially lower costs for patients, depending on their insurance coverage.
The dosing instructions for insulin glargine vary depending on whether patients use the older SoloStar or the high capacity Max SoloStar. The SoloStar holds 450 units (1.5mL) of insulin glargine and delivers doses in 1 unit increments, delivering a maximum dose of 80 units per injection. The MaxSoloStar has twice the capacity of the original – 900 units (3 mL) of insulin glargine – and delivers doses in 2 unit increments up to a maximum 160 unit injection. This is recommended for patients who require at least 20 units per day. All insulin glargine injections should be administered subcutaneously in the abdomen, thigh, or deltoid at the same time each day.
The most common side effect of insulin products, such as insulin glargine, is hypoglycemia.
The Max SoloStar will be available in retail pharmacies throughout the United States in the third quarter of 2018.
Sessile serrated colon polyps may be detectable noninvasively
Sessile serrated polyps (SSPs), notorious for being difficult to detect and for their potential to become malignant colorectal tumors, appear to be caused by a single oncogenic mutation, a finding that could lead to better early detection of some colorectal cancers through noninvasive stool testing, investigators say.
Using a comprehensive battery of genomic testing and DNA methylation profiling, David Jones, PhD, of the Oklahoma Medical Research Foundation in Oklahoma City and his colleagues compared SSPs with familial adenomatous polyps (FAPs), and found that the V600E mutation in BRAF (V-Raf Murine Sarcoma Viral Oncogene Homolog B) was the sole cancer-causing mutation in SSPs.
They also found a distinct DNA methylation pattern unique to SSPs.
“These SSP-specific methylation patterns effectively distinguish SSP from adenomatous polyps, which could be important for both diagnosis and treatment. It also suggests that the BRAF-V600E mutation directly or indirectly results in the remodeling of the epigenome and that this may set a stage for tumor progression,” they wrote in the open-access journal PLOS One.
Approximately one-third of sporadic colorectal cancers, which account for about 95% of all colorectal malignancies, are thought to arise from premalignant serrated lesions, including SSPs, hyperplastic polyps, and traditional serrated adenomas, the authors noted.
Although SSPs and traditional serrated adenomas both have significant potential for malignant transformation, SSPs are much more common, making them important targets for research, diagnosis, and possible interventions.
“Previous surveys of cancer-associated mutations in SSP samples, through targeted analysis of limited known mutations, identified the BRAF-V600E as the key mutation in this disease. However, it is not clear whether other mutations in the same samples contribute to the etiology of this disease,” Dr. Jones and his associates wrote.
To better understand both the inherited (genetic) and acquired (epigenetic) basis for SSP and tumor development, the investigators used whole-exome sequencing, genome-wide mutation detection, and DNA methylation profiling on multiple samples of both SSPs and FAPs.
They performed exome sequencing on DNA extracted from SSP samples from six patients diagnosed with typical SSP-type colon polyps via colonoscopy and pathology. The samples included one each from five patients and three taken from different portions of the colon in one patient. In all of the samples, BRAF-V600E was the only common somatic mutation detected. In the patient from whom the three SSP samples were taken, the mutation was found in each polyp, but not in grossly uninvolved colon from the same patient.
The investigators next performed genome-wide DNA methylation profiling on 15 colon biopsy samples from 11 patients, including five SSPs, two traditional serrated adenomas, three FAPs, two carcinomas, one grossly uninvolved tissue sample, and two normal tissue samples. They found that the BRAF-V600E mutation correlated with a unique and reproducible DNA methylation signature.
They then determined that the DNA methylation signature that they identified is associated with specific markers for molecular characterization of SSPs, and that these markers showed an approximately 3- to 30-fold increase in methylation levels in only SSP samples.
Furthermore, they showed that the unique DNA methylation patterns they identified could be used to distinguish SPPs from adenomatous polyps, with better discrimination than parallel-gene expression profiling.
“The results presented here provide strong evidence that the BRAF-V600E mutation is the main cause of generation of SSP and SSP-specific DNA methylation pattern,” the investigators wrote in the study’s conclusion.
The study was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute. The authors declared no competing financial interests in the work.
SOURCE: Dehghanizadeh S et al. PLOS One 13(3): e0192499.
Sessile serrated polyps (SSPs), notorious for being difficult to detect and for their potential to become malignant colorectal tumors, appear to be caused by a single oncogenic mutation, a finding that could lead to better early detection of some colorectal cancers through noninvasive stool testing, investigators say.
Using a comprehensive battery of genomic testing and DNA methylation profiling, David Jones, PhD, of the Oklahoma Medical Research Foundation in Oklahoma City and his colleagues compared SSPs with familial adenomatous polyps (FAPs), and found that the V600E mutation in BRAF (V-Raf Murine Sarcoma Viral Oncogene Homolog B) was the sole cancer-causing mutation in SSPs.
They also found a distinct DNA methylation pattern unique to SSPs.
“These SSP-specific methylation patterns effectively distinguish SSP from adenomatous polyps, which could be important for both diagnosis and treatment. It also suggests that the BRAF-V600E mutation directly or indirectly results in the remodeling of the epigenome and that this may set a stage for tumor progression,” they wrote in the open-access journal PLOS One.
Approximately one-third of sporadic colorectal cancers, which account for about 95% of all colorectal malignancies, are thought to arise from premalignant serrated lesions, including SSPs, hyperplastic polyps, and traditional serrated adenomas, the authors noted.
Although SSPs and traditional serrated adenomas both have significant potential for malignant transformation, SSPs are much more common, making them important targets for research, diagnosis, and possible interventions.
“Previous surveys of cancer-associated mutations in SSP samples, through targeted analysis of limited known mutations, identified the BRAF-V600E as the key mutation in this disease. However, it is not clear whether other mutations in the same samples contribute to the etiology of this disease,” Dr. Jones and his associates wrote.
To better understand both the inherited (genetic) and acquired (epigenetic) basis for SSP and tumor development, the investigators used whole-exome sequencing, genome-wide mutation detection, and DNA methylation profiling on multiple samples of both SSPs and FAPs.
They performed exome sequencing on DNA extracted from SSP samples from six patients diagnosed with typical SSP-type colon polyps via colonoscopy and pathology. The samples included one each from five patients and three taken from different portions of the colon in one patient. In all of the samples, BRAF-V600E was the only common somatic mutation detected. In the patient from whom the three SSP samples were taken, the mutation was found in each polyp, but not in grossly uninvolved colon from the same patient.
The investigators next performed genome-wide DNA methylation profiling on 15 colon biopsy samples from 11 patients, including five SSPs, two traditional serrated adenomas, three FAPs, two carcinomas, one grossly uninvolved tissue sample, and two normal tissue samples. They found that the BRAF-V600E mutation correlated with a unique and reproducible DNA methylation signature.
They then determined that the DNA methylation signature that they identified is associated with specific markers for molecular characterization of SSPs, and that these markers showed an approximately 3- to 30-fold increase in methylation levels in only SSP samples.
Furthermore, they showed that the unique DNA methylation patterns they identified could be used to distinguish SPPs from adenomatous polyps, with better discrimination than parallel-gene expression profiling.
“The results presented here provide strong evidence that the BRAF-V600E mutation is the main cause of generation of SSP and SSP-specific DNA methylation pattern,” the investigators wrote in the study’s conclusion.
The study was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute. The authors declared no competing financial interests in the work.
SOURCE: Dehghanizadeh S et al. PLOS One 13(3): e0192499.
Sessile serrated polyps (SSPs), notorious for being difficult to detect and for their potential to become malignant colorectal tumors, appear to be caused by a single oncogenic mutation, a finding that could lead to better early detection of some colorectal cancers through noninvasive stool testing, investigators say.
Using a comprehensive battery of genomic testing and DNA methylation profiling, David Jones, PhD, of the Oklahoma Medical Research Foundation in Oklahoma City and his colleagues compared SSPs with familial adenomatous polyps (FAPs), and found that the V600E mutation in BRAF (V-Raf Murine Sarcoma Viral Oncogene Homolog B) was the sole cancer-causing mutation in SSPs.
They also found a distinct DNA methylation pattern unique to SSPs.
“These SSP-specific methylation patterns effectively distinguish SSP from adenomatous polyps, which could be important for both diagnosis and treatment. It also suggests that the BRAF-V600E mutation directly or indirectly results in the remodeling of the epigenome and that this may set a stage for tumor progression,” they wrote in the open-access journal PLOS One.
Approximately one-third of sporadic colorectal cancers, which account for about 95% of all colorectal malignancies, are thought to arise from premalignant serrated lesions, including SSPs, hyperplastic polyps, and traditional serrated adenomas, the authors noted.
Although SSPs and traditional serrated adenomas both have significant potential for malignant transformation, SSPs are much more common, making them important targets for research, diagnosis, and possible interventions.
“Previous surveys of cancer-associated mutations in SSP samples, through targeted analysis of limited known mutations, identified the BRAF-V600E as the key mutation in this disease. However, it is not clear whether other mutations in the same samples contribute to the etiology of this disease,” Dr. Jones and his associates wrote.
To better understand both the inherited (genetic) and acquired (epigenetic) basis for SSP and tumor development, the investigators used whole-exome sequencing, genome-wide mutation detection, and DNA methylation profiling on multiple samples of both SSPs and FAPs.
They performed exome sequencing on DNA extracted from SSP samples from six patients diagnosed with typical SSP-type colon polyps via colonoscopy and pathology. The samples included one each from five patients and three taken from different portions of the colon in one patient. In all of the samples, BRAF-V600E was the only common somatic mutation detected. In the patient from whom the three SSP samples were taken, the mutation was found in each polyp, but not in grossly uninvolved colon from the same patient.
The investigators next performed genome-wide DNA methylation profiling on 15 colon biopsy samples from 11 patients, including five SSPs, two traditional serrated adenomas, three FAPs, two carcinomas, one grossly uninvolved tissue sample, and two normal tissue samples. They found that the BRAF-V600E mutation correlated with a unique and reproducible DNA methylation signature.
They then determined that the DNA methylation signature that they identified is associated with specific markers for molecular characterization of SSPs, and that these markers showed an approximately 3- to 30-fold increase in methylation levels in only SSP samples.
Furthermore, they showed that the unique DNA methylation patterns they identified could be used to distinguish SPPs from adenomatous polyps, with better discrimination than parallel-gene expression profiling.
“The results presented here provide strong evidence that the BRAF-V600E mutation is the main cause of generation of SSP and SSP-specific DNA methylation pattern,” the investigators wrote in the study’s conclusion.
The study was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute. The authors declared no competing financial interests in the work.
SOURCE: Dehghanizadeh S et al. PLOS One 13(3): e0192499.
PLOS ONE
Key clinical point: Sessile serrated polyps appear to arise from a single oncogenic mutation that can be detected noninvasively.
Major finding: A distinct DNA methylation signature can distinguish SSPs from adenomatous polyps.
Study details: Genomic and DNA methylation studies of biopsy samples from patients with SSPs and others with familial adenomatous polyps.
Disclosures: The study was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute. The authors declared no competing financial interests in the work.
Source: Dehghanizadeh S et al. PLOS One 13(3):e0192499.
Waning vaccine immunity linked to pertussis resurgence
researchers said.
In the March 28, 2018, edition of Science Translational Medicine, researchers reported on a study that used different models of transmission to explore what might be the cause of the steady increase in pertussis infections since the mid-1970s.
Using 16 years’ worth of detailed, age-stratified incidence data from Massachusetts, researchers found that the model which assumed a gradual waning in protection was the best fit for the observed patterns of pertussis incidence across the population.
This model suggested significant variability in how the level of protection changes over time, with a 10% risk of vaccine protection waning to zero within 10 years of completing routine vaccination and a 55% chance that the vaccine would confer lifelong protection.
“Crucially, we find that the vaccine is effective at reducing pathogen circulation but not so effective that eradication of this highly contagious bacterium should be possible without targeted booster campaigns,” wrote Dr. Matthieu Domenech de Cellès, PhD, of the Institut Pasteur at the University of Versailles (France) and his coauthors.
The model also considered the possibility that the whole-cell and acellular pertussis vaccines might show differences in immunity, which had been suggested as one explanation for the resurgence of the disease. However, the authors found little evidence of a marked epidemiological switch from the whole-cell to acellular vaccines, although their results did suggest the acellular vaccine has a moderately reduced efficacy.
“Our results suggest that the train of events leading to the resurgence of pertussis was set in motion well before the shift to the DTaP vaccine,” Dr. Domenech de Cellès and his associates said.
The model also pointed to big shifts in the age-specific immunological profile caused by introduction of vaccination, which led to a reduction in transmission and also a reduction in natural infections both in vaccinated and unvaccinated individuals.
This meant individuals who either did not get vaccinated as children or who did not gain immunity from vaccination were growing to adulthood without ever being exposed to natural infection.
“Concurrently, older cohorts, with their long-lived immunity derived from natural infections experienced during the prevaccine period, were gradually dying out,” the authors said. “The resulting rise in the number of susceptible adults sets the stage for the pertussis resurgence, especially among adults.”
Two authors were supported by the National Institutes of Health and by Models of Infectious Disease Agent Study–National Institute of General Medical Sciences. No conflicts of interest were declared.
SOURCE: Domenech de Cellès M et al. Sci Transl Med. 2018 Mar 28;10:eaaj1748.
researchers said.
In the March 28, 2018, edition of Science Translational Medicine, researchers reported on a study that used different models of transmission to explore what might be the cause of the steady increase in pertussis infections since the mid-1970s.
Using 16 years’ worth of detailed, age-stratified incidence data from Massachusetts, researchers found that the model which assumed a gradual waning in protection was the best fit for the observed patterns of pertussis incidence across the population.
This model suggested significant variability in how the level of protection changes over time, with a 10% risk of vaccine protection waning to zero within 10 years of completing routine vaccination and a 55% chance that the vaccine would confer lifelong protection.
“Crucially, we find that the vaccine is effective at reducing pathogen circulation but not so effective that eradication of this highly contagious bacterium should be possible without targeted booster campaigns,” wrote Dr. Matthieu Domenech de Cellès, PhD, of the Institut Pasteur at the University of Versailles (France) and his coauthors.
The model also considered the possibility that the whole-cell and acellular pertussis vaccines might show differences in immunity, which had been suggested as one explanation for the resurgence of the disease. However, the authors found little evidence of a marked epidemiological switch from the whole-cell to acellular vaccines, although their results did suggest the acellular vaccine has a moderately reduced efficacy.
“Our results suggest that the train of events leading to the resurgence of pertussis was set in motion well before the shift to the DTaP vaccine,” Dr. Domenech de Cellès and his associates said.
The model also pointed to big shifts in the age-specific immunological profile caused by introduction of vaccination, which led to a reduction in transmission and also a reduction in natural infections both in vaccinated and unvaccinated individuals.
This meant individuals who either did not get vaccinated as children or who did not gain immunity from vaccination were growing to adulthood without ever being exposed to natural infection.
“Concurrently, older cohorts, with their long-lived immunity derived from natural infections experienced during the prevaccine period, were gradually dying out,” the authors said. “The resulting rise in the number of susceptible adults sets the stage for the pertussis resurgence, especially among adults.”
Two authors were supported by the National Institutes of Health and by Models of Infectious Disease Agent Study–National Institute of General Medical Sciences. No conflicts of interest were declared.
SOURCE: Domenech de Cellès M et al. Sci Transl Med. 2018 Mar 28;10:eaaj1748.
researchers said.
In the March 28, 2018, edition of Science Translational Medicine, researchers reported on a study that used different models of transmission to explore what might be the cause of the steady increase in pertussis infections since the mid-1970s.
Using 16 years’ worth of detailed, age-stratified incidence data from Massachusetts, researchers found that the model which assumed a gradual waning in protection was the best fit for the observed patterns of pertussis incidence across the population.
This model suggested significant variability in how the level of protection changes over time, with a 10% risk of vaccine protection waning to zero within 10 years of completing routine vaccination and a 55% chance that the vaccine would confer lifelong protection.
“Crucially, we find that the vaccine is effective at reducing pathogen circulation but not so effective that eradication of this highly contagious bacterium should be possible without targeted booster campaigns,” wrote Dr. Matthieu Domenech de Cellès, PhD, of the Institut Pasteur at the University of Versailles (France) and his coauthors.
The model also considered the possibility that the whole-cell and acellular pertussis vaccines might show differences in immunity, which had been suggested as one explanation for the resurgence of the disease. However, the authors found little evidence of a marked epidemiological switch from the whole-cell to acellular vaccines, although their results did suggest the acellular vaccine has a moderately reduced efficacy.
“Our results suggest that the train of events leading to the resurgence of pertussis was set in motion well before the shift to the DTaP vaccine,” Dr. Domenech de Cellès and his associates said.
The model also pointed to big shifts in the age-specific immunological profile caused by introduction of vaccination, which led to a reduction in transmission and also a reduction in natural infections both in vaccinated and unvaccinated individuals.
This meant individuals who either did not get vaccinated as children or who did not gain immunity from vaccination were growing to adulthood without ever being exposed to natural infection.
“Concurrently, older cohorts, with their long-lived immunity derived from natural infections experienced during the prevaccine period, were gradually dying out,” the authors said. “The resulting rise in the number of susceptible adults sets the stage for the pertussis resurgence, especially among adults.”
Two authors were supported by the National Institutes of Health and by Models of Infectious Disease Agent Study–National Institute of General Medical Sciences. No conflicts of interest were declared.
SOURCE: Domenech de Cellès M et al. Sci Transl Med. 2018 Mar 28;10:eaaj1748.
FROM SCIENCE TRANSLATIONAL MEDICINE
VIDEO: Nanotechnology is making a mark in gastroenterology
BOSTON – Nanotechnology, though small in scale, is making a big difference in gastroenterology. Nanoparticles can deliver therapeutic compounds or enable other diagnostic tools, said Vadim Backman, PhD, the Walter Dill Scott Professor of Biomedical Engineering at Northwestern University, Chicago, in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. Nanotechnology can treat disease by reprogramming gene expression or gene regulation. Nanoparticle formulations are FDA approved now for treatment of esophageal, colon, and pancreatic cancers, said Dr. Backman in a video interview, but the ability of nanotechnology to reprogram biological processes at the genetic level has researchers looking at treating inflammatory diseases and regenerating tissues.
BOSTON – Nanotechnology, though small in scale, is making a big difference in gastroenterology. Nanoparticles can deliver therapeutic compounds or enable other diagnostic tools, said Vadim Backman, PhD, the Walter Dill Scott Professor of Biomedical Engineering at Northwestern University, Chicago, in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. Nanotechnology can treat disease by reprogramming gene expression or gene regulation. Nanoparticle formulations are FDA approved now for treatment of esophageal, colon, and pancreatic cancers, said Dr. Backman in a video interview, but the ability of nanotechnology to reprogram biological processes at the genetic level has researchers looking at treating inflammatory diseases and regenerating tissues.
BOSTON – Nanotechnology, though small in scale, is making a big difference in gastroenterology. Nanoparticles can deliver therapeutic compounds or enable other diagnostic tools, said Vadim Backman, PhD, the Walter Dill Scott Professor of Biomedical Engineering at Northwestern University, Chicago, in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. Nanotechnology can treat disease by reprogramming gene expression or gene regulation. Nanoparticle formulations are FDA approved now for treatment of esophageal, colon, and pancreatic cancers, said Dr. Backman in a video interview, but the ability of nanotechnology to reprogram biological processes at the genetic level has researchers looking at treating inflammatory diseases and regenerating tissues.
FROM THE 2018 AGA TECH SUMMIT
VIDEO: Adipogenic genes upregulated in high-BMI sucralose users
CHICAGO – , there was significant upregulation of genes that promote intracellular glucose transport. Genes known to be adipogenic and those governing sweet taste receptors also were significantly upregulated with sucralose exposure.
“Effects of sucralose are particularly more detrimental in obese individuals who are prediabetic or diabetic, rather than nonobese consumers of low-calorie sweetener,” said Sabyasachi Sen, MD, during a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
These new findings, together with in vitro examination of human adipose-derived mesenchymal stromal cells (MSCs) exposed to sucralose, are helping solve the puzzle of how a sweetener that delivers no energy may contribute to metabolic derangement, said Dr. Sen, professor of endocrinology at George Washington University in Washington.
Dr. Sen and his collaborators first exposed the MSCs to concentrations of sucralose ranging from 0 mM to 0.2 mM – a physiologic level for high sucralose consumers – to the supraphysiologic concentration of 1 mM.
The adipogenic genes CEBPa and FABP4 were upregulated in the sucralose-exposed MSCs, which also showed more intracellular fat droplet accumulation. Reactive oxygen species increased in the MSCs in a dose-dependent fashion as well, said Dr. Sen in a video interview.
All of this upregulation, said Dr. Sen, was pushing the MSCs toward becoming fat cells. “At the same time, we saw that there are certain genes that were upregulating that were allowing more glucose to enter the cell.” The increase in reactive oxygen species paralleled what was seen in a similar model that used glucose rather than sucralose, he said.
The investigators then took subcutaneous fat biopsies from four normal-weight individuals (body mass index, 23.4-24.8 kg/m2), and from 14 obese individuals (BMI, 32-64 kg/m2). Each group had sucralose users and nonusers. Using mRNA gene expression profiles, they saw that glucose transporter genes, adipogenic genes, and antioxidant genes were upregulated among sucralose consumers with obesity, significantly more than for the normal-weight participants.
The pattern, said Dr. Sen, was strikingly similar to what had been seen with the MSC-sucralose exposure findings. “The upregulation that we saw in the petri dish could now be seen in the human fat samples,” he said.
“We think that the sucralose is … allowing more glucose to enter the cell,” said Dr. Sen. “We think that we actually have figured out a mechanism.” He and his colleagues next plan to tag glucose molecules to follow what actually happens as they enter cells in the presence of sucralose.
When Dr. Sen’s patients ask whether they should switch to low-calorie sweetened beverages, he answers with an emphatic “no.” “I say, ‘It’s not going to do you any good, because it still may allow glucose to enter the cells … you’re going to come back to the same status quo’ ” in the context of obesity and insulin resistance, he said.
Dr. Sen reported that he has no relevant disclosures.
SOURCE: Sen S et al. ENDO 2018, Abstract SUN-071.
CHICAGO – , there was significant upregulation of genes that promote intracellular glucose transport. Genes known to be adipogenic and those governing sweet taste receptors also were significantly upregulated with sucralose exposure.
“Effects of sucralose are particularly more detrimental in obese individuals who are prediabetic or diabetic, rather than nonobese consumers of low-calorie sweetener,” said Sabyasachi Sen, MD, during a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
These new findings, together with in vitro examination of human adipose-derived mesenchymal stromal cells (MSCs) exposed to sucralose, are helping solve the puzzle of how a sweetener that delivers no energy may contribute to metabolic derangement, said Dr. Sen, professor of endocrinology at George Washington University in Washington.
Dr. Sen and his collaborators first exposed the MSCs to concentrations of sucralose ranging from 0 mM to 0.2 mM – a physiologic level for high sucralose consumers – to the supraphysiologic concentration of 1 mM.
The adipogenic genes CEBPa and FABP4 were upregulated in the sucralose-exposed MSCs, which also showed more intracellular fat droplet accumulation. Reactive oxygen species increased in the MSCs in a dose-dependent fashion as well, said Dr. Sen in a video interview.
All of this upregulation, said Dr. Sen, was pushing the MSCs toward becoming fat cells. “At the same time, we saw that there are certain genes that were upregulating that were allowing more glucose to enter the cell.” The increase in reactive oxygen species paralleled what was seen in a similar model that used glucose rather than sucralose, he said.
The investigators then took subcutaneous fat biopsies from four normal-weight individuals (body mass index, 23.4-24.8 kg/m2), and from 14 obese individuals (BMI, 32-64 kg/m2). Each group had sucralose users and nonusers. Using mRNA gene expression profiles, they saw that glucose transporter genes, adipogenic genes, and antioxidant genes were upregulated among sucralose consumers with obesity, significantly more than for the normal-weight participants.
The pattern, said Dr. Sen, was strikingly similar to what had been seen with the MSC-sucralose exposure findings. “The upregulation that we saw in the petri dish could now be seen in the human fat samples,” he said.
“We think that the sucralose is … allowing more glucose to enter the cell,” said Dr. Sen. “We think that we actually have figured out a mechanism.” He and his colleagues next plan to tag glucose molecules to follow what actually happens as they enter cells in the presence of sucralose.
When Dr. Sen’s patients ask whether they should switch to low-calorie sweetened beverages, he answers with an emphatic “no.” “I say, ‘It’s not going to do you any good, because it still may allow glucose to enter the cells … you’re going to come back to the same status quo’ ” in the context of obesity and insulin resistance, he said.
Dr. Sen reported that he has no relevant disclosures.
SOURCE: Sen S et al. ENDO 2018, Abstract SUN-071.
CHICAGO – , there was significant upregulation of genes that promote intracellular glucose transport. Genes known to be adipogenic and those governing sweet taste receptors also were significantly upregulated with sucralose exposure.
“Effects of sucralose are particularly more detrimental in obese individuals who are prediabetic or diabetic, rather than nonobese consumers of low-calorie sweetener,” said Sabyasachi Sen, MD, during a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
These new findings, together with in vitro examination of human adipose-derived mesenchymal stromal cells (MSCs) exposed to sucralose, are helping solve the puzzle of how a sweetener that delivers no energy may contribute to metabolic derangement, said Dr. Sen, professor of endocrinology at George Washington University in Washington.
Dr. Sen and his collaborators first exposed the MSCs to concentrations of sucralose ranging from 0 mM to 0.2 mM – a physiologic level for high sucralose consumers – to the supraphysiologic concentration of 1 mM.
The adipogenic genes CEBPa and FABP4 were upregulated in the sucralose-exposed MSCs, which also showed more intracellular fat droplet accumulation. Reactive oxygen species increased in the MSCs in a dose-dependent fashion as well, said Dr. Sen in a video interview.
All of this upregulation, said Dr. Sen, was pushing the MSCs toward becoming fat cells. “At the same time, we saw that there are certain genes that were upregulating that were allowing more glucose to enter the cell.” The increase in reactive oxygen species paralleled what was seen in a similar model that used glucose rather than sucralose, he said.
The investigators then took subcutaneous fat biopsies from four normal-weight individuals (body mass index, 23.4-24.8 kg/m2), and from 14 obese individuals (BMI, 32-64 kg/m2). Each group had sucralose users and nonusers. Using mRNA gene expression profiles, they saw that glucose transporter genes, adipogenic genes, and antioxidant genes were upregulated among sucralose consumers with obesity, significantly more than for the normal-weight participants.
The pattern, said Dr. Sen, was strikingly similar to what had been seen with the MSC-sucralose exposure findings. “The upregulation that we saw in the petri dish could now be seen in the human fat samples,” he said.
“We think that the sucralose is … allowing more glucose to enter the cell,” said Dr. Sen. “We think that we actually have figured out a mechanism.” He and his colleagues next plan to tag glucose molecules to follow what actually happens as they enter cells in the presence of sucralose.
When Dr. Sen’s patients ask whether they should switch to low-calorie sweetened beverages, he answers with an emphatic “no.” “I say, ‘It’s not going to do you any good, because it still may allow glucose to enter the cells … you’re going to come back to the same status quo’ ” in the context of obesity and insulin resistance, he said.
Dr. Sen reported that he has no relevant disclosures.
SOURCE: Sen S et al. ENDO 2018, Abstract SUN-071.
REPORTING FROM ENDO 2018
Insomnia – going beyond sleep hygiene
Difficulties with sleep are prevalent and significant across the developmental spectrum. Not only does poor sleep affect daytime functioning in relation to mood, focus, appetite, and emotional regulation, but ineffective bedtime routines can cause significant distress for youth and caregivers, as well. The American Academy of Sleep Medicine describes insomnia as “repeated difficulty with sleep initiation, duration, consolidation, or quality that occurs despite age-appropriate time and opportunity for sleep and results in daytime functional impairment for the child and/or family.’’1
Pediatric providers likely are familiar already with initial steps in the evaluation and treatment of insomnia. The emphasis here is assessment and intervention approaches beyond the foundational use of sleep hygiene recommendations.
In working with a patient such as Katie who comes laden with diagnoses and medications, stepping back to reconsider the assessment is an important starting point. Problems related to sleep are rife in psychiatric conditions, from depression, anxiety, and PTSD to bipolar disorder, ADHD, and autism.2
Next is see if there are external factors engendering insomnia. Sleep hygiene focuses on these, but sometimes recent stressors or familial conflict are overlooked, which may be linchpins to improving sleep patterns. Commonly prescribed medications (steroids, bupropion, and stimulants) and intoxication or withdrawal symptoms from substance use can contribute to wakefulness and deserve consideration. It can be useful to track sleep for a while to identify contributing factors, impediments to sleep, and ineffective patterns (see tools at sleepfoundation.org or the free app CBT-I Coach).
After assessment, the bulk of the evidence for pediatric insomnia is for behavioral treatments, mostly for infants and young children. This may be familiar territory, and it offers a good time to assess the level of motivation. Are the patient and family aware of how insomnia affects their lives on a day-to-day basis and is this problem a priority?
For adolescents who are convinced of the life-changing properties of a good night’s sleep, cognitive-behavioral therapy for insomnia (CBT-i) is developing a strong evidence base for insomnia in adolescents.3 CBT-i adds to the usual interventions for addressing insomnia in infants and young children by additionally training adolescents relaxation techniques, by addressing cognitive distortions about sleep, and by actually restricting sleep. This last technique involves initially reducing the amount of sleep in order to build a tight association between sleep and the bedroom, improve sleep efficiency, and increase sleep drive.
In general, medications are considered when other appropriate interventions have proven inadequate. There is very little evidence for using pharmacologic interventions for pediatric insomnia, so even if a medication is selected, behavioral approaches should remain a mainstay.4 Patients and caregivers should agree to specific short-term goals ahead of time when using sleep medicine, given the limited effectiveness and recommended short duration of use. Many medications change sleep architecture, and none have been clearly shown to sustainably improve sleep quality or quantity or reduce daytime symptoms of insomnia.
Prescribing guidelines for insomnia suggest selecting an agent matched to the symptoms and relevant to any comorbidities. Melatonin may be most helpful in shifting the sleep phase rather than for direct hypnotic effects; thus adolescents or patients with ADHD whose sleep schedule has naturally shifted later may benefit from a small dose of melatonin (1-3 mg) several hours before bedtime to prime their system. Beware that melatonin is not regulated by the Food and Drug Administration and animal studies have shown significant alterations of the gonadal hormone axis, although this has not been examined in human trials. Alpha-2 agonists – such as clonidine and guanfacine – may be helpful for sleep initiation, especially in populations with comorbid ADHD, aggression, or tics, where these medications might be otherwise indicated. Prazosin, an alpha-1 antagonist, has some limited evidence as a treatment for nightmares and PTSD symptoms, so it may be a good choice for children with trauma-related hypervigilance.
In patients with depression, low doses of trazodone (12.5-50 mg) or mirtazapine (7.5-15 mg) may be effective. Although short-acting benzodiazepines may be useful in the short-term, particularly for sleep-onset difficulties, they generally are not recommended because of the risks of abuse, diversion, withdrawal, cognitive side effects, disinhibition, development of tolerance, and contraindication with such comorbidities as sleep apnea. However, the benzodiazepine receptor agonists such as zaleplon, zolpidem, and eszopiclone, while lacking evidence in the pediatric population, may be worthwhile considerations as their varying half-lives allow for specificity in treating sleep-onset vs. sleep-maintenance problems. Caregivers should be warned about the potential for sleepwalking or other complex sleep-related behaviors with this class of medicines.
Avoid tricyclic antidepressants because of the potential for anticholinergic effects and cardiotoxicity. Atypical antipsychotics generally are not worth the risk of serious and rapid side effects associated with this class of medications, which include metabolic syndrome.
The assessment and treatment of pediatric insomnia may require several visits to complete. But, given growing knowledge of how much sleep contributes to learning, longevity, and well-being, and the consequences of sleep deprivation with regard to safety, irritability, poor concentration, disordered metabolism and appetite, etc., the potential benefits seem well worth the time.
Dr. Rosenfeld is assistant professor of psychiatry at Vermont Center for Children, Youth & Families, at the University of Vermont Medical Center, and the University of Vermont, Burlington. He has received honorarium from Oakstone Publishing for contributing board review course content on human development.
References
1. International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd edition. (Westchester: American Academy of Sleep Medicine, 2005).
2. Child Adolesc Psychiatr Clin N Am. 2009 Oct;18(4):979-1000
3. J Child Psychol Psychiatry. 2017, Oct 20. doi: 10.1111/jcpp.12834.
4. Child Adolesc Psychiatric Clin N Am. 2009, Oct;18(4):1001-16.
Difficulties with sleep are prevalent and significant across the developmental spectrum. Not only does poor sleep affect daytime functioning in relation to mood, focus, appetite, and emotional regulation, but ineffective bedtime routines can cause significant distress for youth and caregivers, as well. The American Academy of Sleep Medicine describes insomnia as “repeated difficulty with sleep initiation, duration, consolidation, or quality that occurs despite age-appropriate time and opportunity for sleep and results in daytime functional impairment for the child and/or family.’’1
Pediatric providers likely are familiar already with initial steps in the evaluation and treatment of insomnia. The emphasis here is assessment and intervention approaches beyond the foundational use of sleep hygiene recommendations.
In working with a patient such as Katie who comes laden with diagnoses and medications, stepping back to reconsider the assessment is an important starting point. Problems related to sleep are rife in psychiatric conditions, from depression, anxiety, and PTSD to bipolar disorder, ADHD, and autism.2
Next is see if there are external factors engendering insomnia. Sleep hygiene focuses on these, but sometimes recent stressors or familial conflict are overlooked, which may be linchpins to improving sleep patterns. Commonly prescribed medications (steroids, bupropion, and stimulants) and intoxication or withdrawal symptoms from substance use can contribute to wakefulness and deserve consideration. It can be useful to track sleep for a while to identify contributing factors, impediments to sleep, and ineffective patterns (see tools at sleepfoundation.org or the free app CBT-I Coach).
After assessment, the bulk of the evidence for pediatric insomnia is for behavioral treatments, mostly for infants and young children. This may be familiar territory, and it offers a good time to assess the level of motivation. Are the patient and family aware of how insomnia affects their lives on a day-to-day basis and is this problem a priority?
For adolescents who are convinced of the life-changing properties of a good night’s sleep, cognitive-behavioral therapy for insomnia (CBT-i) is developing a strong evidence base for insomnia in adolescents.3 CBT-i adds to the usual interventions for addressing insomnia in infants and young children by additionally training adolescents relaxation techniques, by addressing cognitive distortions about sleep, and by actually restricting sleep. This last technique involves initially reducing the amount of sleep in order to build a tight association between sleep and the bedroom, improve sleep efficiency, and increase sleep drive.
In general, medications are considered when other appropriate interventions have proven inadequate. There is very little evidence for using pharmacologic interventions for pediatric insomnia, so even if a medication is selected, behavioral approaches should remain a mainstay.4 Patients and caregivers should agree to specific short-term goals ahead of time when using sleep medicine, given the limited effectiveness and recommended short duration of use. Many medications change sleep architecture, and none have been clearly shown to sustainably improve sleep quality or quantity or reduce daytime symptoms of insomnia.
Prescribing guidelines for insomnia suggest selecting an agent matched to the symptoms and relevant to any comorbidities. Melatonin may be most helpful in shifting the sleep phase rather than for direct hypnotic effects; thus adolescents or patients with ADHD whose sleep schedule has naturally shifted later may benefit from a small dose of melatonin (1-3 mg) several hours before bedtime to prime their system. Beware that melatonin is not regulated by the Food and Drug Administration and animal studies have shown significant alterations of the gonadal hormone axis, although this has not been examined in human trials. Alpha-2 agonists – such as clonidine and guanfacine – may be helpful for sleep initiation, especially in populations with comorbid ADHD, aggression, or tics, where these medications might be otherwise indicated. Prazosin, an alpha-1 antagonist, has some limited evidence as a treatment for nightmares and PTSD symptoms, so it may be a good choice for children with trauma-related hypervigilance.
In patients with depression, low doses of trazodone (12.5-50 mg) or mirtazapine (7.5-15 mg) may be effective. Although short-acting benzodiazepines may be useful in the short-term, particularly for sleep-onset difficulties, they generally are not recommended because of the risks of abuse, diversion, withdrawal, cognitive side effects, disinhibition, development of tolerance, and contraindication with such comorbidities as sleep apnea. However, the benzodiazepine receptor agonists such as zaleplon, zolpidem, and eszopiclone, while lacking evidence in the pediatric population, may be worthwhile considerations as their varying half-lives allow for specificity in treating sleep-onset vs. sleep-maintenance problems. Caregivers should be warned about the potential for sleepwalking or other complex sleep-related behaviors with this class of medicines.
Avoid tricyclic antidepressants because of the potential for anticholinergic effects and cardiotoxicity. Atypical antipsychotics generally are not worth the risk of serious and rapid side effects associated with this class of medications, which include metabolic syndrome.
The assessment and treatment of pediatric insomnia may require several visits to complete. But, given growing knowledge of how much sleep contributes to learning, longevity, and well-being, and the consequences of sleep deprivation with regard to safety, irritability, poor concentration, disordered metabolism and appetite, etc., the potential benefits seem well worth the time.
Dr. Rosenfeld is assistant professor of psychiatry at Vermont Center for Children, Youth & Families, at the University of Vermont Medical Center, and the University of Vermont, Burlington. He has received honorarium from Oakstone Publishing for contributing board review course content on human development.
References
1. International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd edition. (Westchester: American Academy of Sleep Medicine, 2005).
2. Child Adolesc Psychiatr Clin N Am. 2009 Oct;18(4):979-1000
3. J Child Psychol Psychiatry. 2017, Oct 20. doi: 10.1111/jcpp.12834.
4. Child Adolesc Psychiatric Clin N Am. 2009, Oct;18(4):1001-16.
Difficulties with sleep are prevalent and significant across the developmental spectrum. Not only does poor sleep affect daytime functioning in relation to mood, focus, appetite, and emotional regulation, but ineffective bedtime routines can cause significant distress for youth and caregivers, as well. The American Academy of Sleep Medicine describes insomnia as “repeated difficulty with sleep initiation, duration, consolidation, or quality that occurs despite age-appropriate time and opportunity for sleep and results in daytime functional impairment for the child and/or family.’’1
Pediatric providers likely are familiar already with initial steps in the evaluation and treatment of insomnia. The emphasis here is assessment and intervention approaches beyond the foundational use of sleep hygiene recommendations.
In working with a patient such as Katie who comes laden with diagnoses and medications, stepping back to reconsider the assessment is an important starting point. Problems related to sleep are rife in psychiatric conditions, from depression, anxiety, and PTSD to bipolar disorder, ADHD, and autism.2
Next is see if there are external factors engendering insomnia. Sleep hygiene focuses on these, but sometimes recent stressors or familial conflict are overlooked, which may be linchpins to improving sleep patterns. Commonly prescribed medications (steroids, bupropion, and stimulants) and intoxication or withdrawal symptoms from substance use can contribute to wakefulness and deserve consideration. It can be useful to track sleep for a while to identify contributing factors, impediments to sleep, and ineffective patterns (see tools at sleepfoundation.org or the free app CBT-I Coach).
After assessment, the bulk of the evidence for pediatric insomnia is for behavioral treatments, mostly for infants and young children. This may be familiar territory, and it offers a good time to assess the level of motivation. Are the patient and family aware of how insomnia affects their lives on a day-to-day basis and is this problem a priority?
For adolescents who are convinced of the life-changing properties of a good night’s sleep, cognitive-behavioral therapy for insomnia (CBT-i) is developing a strong evidence base for insomnia in adolescents.3 CBT-i adds to the usual interventions for addressing insomnia in infants and young children by additionally training adolescents relaxation techniques, by addressing cognitive distortions about sleep, and by actually restricting sleep. This last technique involves initially reducing the amount of sleep in order to build a tight association between sleep and the bedroom, improve sleep efficiency, and increase sleep drive.
In general, medications are considered when other appropriate interventions have proven inadequate. There is very little evidence for using pharmacologic interventions for pediatric insomnia, so even if a medication is selected, behavioral approaches should remain a mainstay.4 Patients and caregivers should agree to specific short-term goals ahead of time when using sleep medicine, given the limited effectiveness and recommended short duration of use. Many medications change sleep architecture, and none have been clearly shown to sustainably improve sleep quality or quantity or reduce daytime symptoms of insomnia.
Prescribing guidelines for insomnia suggest selecting an agent matched to the symptoms and relevant to any comorbidities. Melatonin may be most helpful in shifting the sleep phase rather than for direct hypnotic effects; thus adolescents or patients with ADHD whose sleep schedule has naturally shifted later may benefit from a small dose of melatonin (1-3 mg) several hours before bedtime to prime their system. Beware that melatonin is not regulated by the Food and Drug Administration and animal studies have shown significant alterations of the gonadal hormone axis, although this has not been examined in human trials. Alpha-2 agonists – such as clonidine and guanfacine – may be helpful for sleep initiation, especially in populations with comorbid ADHD, aggression, or tics, where these medications might be otherwise indicated. Prazosin, an alpha-1 antagonist, has some limited evidence as a treatment for nightmares and PTSD symptoms, so it may be a good choice for children with trauma-related hypervigilance.
In patients with depression, low doses of trazodone (12.5-50 mg) or mirtazapine (7.5-15 mg) may be effective. Although short-acting benzodiazepines may be useful in the short-term, particularly for sleep-onset difficulties, they generally are not recommended because of the risks of abuse, diversion, withdrawal, cognitive side effects, disinhibition, development of tolerance, and contraindication with such comorbidities as sleep apnea. However, the benzodiazepine receptor agonists such as zaleplon, zolpidem, and eszopiclone, while lacking evidence in the pediatric population, may be worthwhile considerations as their varying half-lives allow for specificity in treating sleep-onset vs. sleep-maintenance problems. Caregivers should be warned about the potential for sleepwalking or other complex sleep-related behaviors with this class of medicines.
Avoid tricyclic antidepressants because of the potential for anticholinergic effects and cardiotoxicity. Atypical antipsychotics generally are not worth the risk of serious and rapid side effects associated with this class of medications, which include metabolic syndrome.
The assessment and treatment of pediatric insomnia may require several visits to complete. But, given growing knowledge of how much sleep contributes to learning, longevity, and well-being, and the consequences of sleep deprivation with regard to safety, irritability, poor concentration, disordered metabolism and appetite, etc., the potential benefits seem well worth the time.
Dr. Rosenfeld is assistant professor of psychiatry at Vermont Center for Children, Youth & Families, at the University of Vermont Medical Center, and the University of Vermont, Burlington. He has received honorarium from Oakstone Publishing for contributing board review course content on human development.
References
1. International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd edition. (Westchester: American Academy of Sleep Medicine, 2005).
2. Child Adolesc Psychiatr Clin N Am. 2009 Oct;18(4):979-1000
3. J Child Psychol Psychiatry. 2017, Oct 20. doi: 10.1111/jcpp.12834.
4. Child Adolesc Psychiatric Clin N Am. 2009, Oct;18(4):1001-16.
Dexcom G6 gets FDA nod
from the Food and Drug Administration.
The Dexcom G6 is about 28% smaller than its predecessor, the G5, can be worn for up to 10 days – 43% longer than the G5 – and doesn’t require any finger-stick calibrations or treatment decisions. It’s the first FDA-approved integrated continuous glucose monitoring (iCGM) system that can link electronically to other compatible devices, including automated insulin dosing systems, insulin pumps, blood glucose meters, and other electronic devices used for diabetes management, the FDA said in a press statement. Its revamped sensor doesn’t interact with acetaminophen – another distinct advantage over the G5.
The device will be commercially available sometime this year, the Dexcom website noted.
The device also set a new premarketing review standard for CGM’s, which can now utilize the less-burdensome 510(k) clearance pathway. Until now, they have been treated as the highest-risk Class III medical devices.
According to the FDA statement, the agency “…recognized this as an opportunity to reduce the regulatory burden for this type of device by establishing criteria that would classify these as ‘moderate risk,’ class II medical devices with special controls.”
G6 was approved through this new pathway, dedicated to novel, low-to-moderate-risk devices that are not “substantially equivalent” to an already legally marketed device, the press statement said.
The FDA evaluated data from two clinical studies of the Dexcom G6, which included 324 adults and children aged 2 years and older with diabetes. Both studies included multiple clinical visits within a 10-day period where system readings were compared to a laboratory test method that measures blood glucose values. No serious adverse events were reported during the studies.
from the Food and Drug Administration.
The Dexcom G6 is about 28% smaller than its predecessor, the G5, can be worn for up to 10 days – 43% longer than the G5 – and doesn’t require any finger-stick calibrations or treatment decisions. It’s the first FDA-approved integrated continuous glucose monitoring (iCGM) system that can link electronically to other compatible devices, including automated insulin dosing systems, insulin pumps, blood glucose meters, and other electronic devices used for diabetes management, the FDA said in a press statement. Its revamped sensor doesn’t interact with acetaminophen – another distinct advantage over the G5.
The device will be commercially available sometime this year, the Dexcom website noted.
The device also set a new premarketing review standard for CGM’s, which can now utilize the less-burdensome 510(k) clearance pathway. Until now, they have been treated as the highest-risk Class III medical devices.
According to the FDA statement, the agency “…recognized this as an opportunity to reduce the regulatory burden for this type of device by establishing criteria that would classify these as ‘moderate risk,’ class II medical devices with special controls.”
G6 was approved through this new pathway, dedicated to novel, low-to-moderate-risk devices that are not “substantially equivalent” to an already legally marketed device, the press statement said.
The FDA evaluated data from two clinical studies of the Dexcom G6, which included 324 adults and children aged 2 years and older with diabetes. Both studies included multiple clinical visits within a 10-day period where system readings were compared to a laboratory test method that measures blood glucose values. No serious adverse events were reported during the studies.
from the Food and Drug Administration.
The Dexcom G6 is about 28% smaller than its predecessor, the G5, can be worn for up to 10 days – 43% longer than the G5 – and doesn’t require any finger-stick calibrations or treatment decisions. It’s the first FDA-approved integrated continuous glucose monitoring (iCGM) system that can link electronically to other compatible devices, including automated insulin dosing systems, insulin pumps, blood glucose meters, and other electronic devices used for diabetes management, the FDA said in a press statement. Its revamped sensor doesn’t interact with acetaminophen – another distinct advantage over the G5.
The device will be commercially available sometime this year, the Dexcom website noted.
The device also set a new premarketing review standard for CGM’s, which can now utilize the less-burdensome 510(k) clearance pathway. Until now, they have been treated as the highest-risk Class III medical devices.
According to the FDA statement, the agency “…recognized this as an opportunity to reduce the regulatory burden for this type of device by establishing criteria that would classify these as ‘moderate risk,’ class II medical devices with special controls.”
G6 was approved through this new pathway, dedicated to novel, low-to-moderate-risk devices that are not “substantially equivalent” to an already legally marketed device, the press statement said.
The FDA evaluated data from two clinical studies of the Dexcom G6, which included 324 adults and children aged 2 years and older with diabetes. Both studies included multiple clinical visits within a 10-day period where system readings were compared to a laboratory test method that measures blood glucose values. No serious adverse events were reported during the studies.
The Prevention and Treatment of Femoral Trial Head Loss in Total Hip Arthroplasty
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
TAKE-HOME POINTS
- Femoral head trial loss is a complication that can occur during THA.
- This event can be a source of avoidable morbidity.
- Preventative measures can be taken to avoid this complication.
- If preventative measures fail, retrieval of the femoral trial head can be performed.
- A thorough understanding of preventative and retrieval methods is essential for surgeons that perform THA.
VIDEO: Digital innovation, consumer point of view can solve clinical problems
BOSTON – Carla E. Small, MBA, senior director of innovation at Boston Children’s Hospital, addressed digital health care in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. It is really just providing health care to patients in a digital world, she said, adding that she prefers the term “tech-enabled health care.” One up-and-coming example of this is the use of Alexa-type voice devices to do work and solve small clinical problems. Her program did a nationwide survey of pediatricians and found that half were interested in using voice technology. Artificial intelligence is another area of technology that Boston Children’s is using – in particular, they have created algorithms that help pediatricians analyze brain scans of young children, because so few pediatricians are trained in this area. The innovation program also has taken the digital world to pediatric patients in a program called Health Voyager in which children can take a virtual journey through their own diseased intestinal system.
BOSTON – Carla E. Small, MBA, senior director of innovation at Boston Children’s Hospital, addressed digital health care in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. It is really just providing health care to patients in a digital world, she said, adding that she prefers the term “tech-enabled health care.” One up-and-coming example of this is the use of Alexa-type voice devices to do work and solve small clinical problems. Her program did a nationwide survey of pediatricians and found that half were interested in using voice technology. Artificial intelligence is another area of technology that Boston Children’s is using – in particular, they have created algorithms that help pediatricians analyze brain scans of young children, because so few pediatricians are trained in this area. The innovation program also has taken the digital world to pediatric patients in a program called Health Voyager in which children can take a virtual journey through their own diseased intestinal system.
BOSTON – Carla E. Small, MBA, senior director of innovation at Boston Children’s Hospital, addressed digital health care in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. It is really just providing health care to patients in a digital world, she said, adding that she prefers the term “tech-enabled health care.” One up-and-coming example of this is the use of Alexa-type voice devices to do work and solve small clinical problems. Her program did a nationwide survey of pediatricians and found that half were interested in using voice technology. Artificial intelligence is another area of technology that Boston Children’s is using – in particular, they have created algorithms that help pediatricians analyze brain scans of young children, because so few pediatricians are trained in this area. The innovation program also has taken the digital world to pediatric patients in a program called Health Voyager in which children can take a virtual journey through their own diseased intestinal system.
FROM THE 20I8 AGA TECH SUMMIT
Thousands mistakenly enrolled during state’s Medicaid expansion, feds find
California signed up an estimated 450,000 people under Medicaid expansion who may not have been eligible for coverage, according to a report by the Health & Human Services’ chief watchdog.
In a Feb. 21 report, the HHS’s inspector general estimated that California spent $738.2 million on 366,078 expansion beneficiaries who were ineligible. It spent an additional $416.5 million for 79,055 expansion enrollees who were “potentially” ineligible, auditors found.
Auditors said nearly 90% of the $1.15 billion in questionable payments involved federal money, while the rest came from the state’s Medicaid program, known as Medi-Cal. They examined a 6-month period from Oct. 1, 2014, to March 31, 2015, when Medicaid payments of $6.2 billion were made related to 1.9 million newly eligible enrollees.
There were limitations to the California review, however. The audit extrapolated from a sample of 150 beneficiaries. The authors reported a 90% confidence level in their results – whereas 95% would be more common. That meant that the number of those ineligible could have been as low as 260,000 or as high as 630,000.
“If HHS has a strong reason to believe that California is systematically making enrollment errors, it would be helpful to show that in a more robust analysis,” said Ben Ippolito, a health care economist at the American Enterprise Institute, a conservative think tank. “The federal government should ensure that states are being good stewards of federal money.”
Nonetheless, the audit highlighted weaknesses in California’s Medicaid program, the largest in the nation with 13.4 million enrollees and an annual budget topping $100 billion, counting federal and state money. Medicaid covers one in three Californians.
The inspector general found deficiencies in the state’s computer system for verifying eligibility and discovered errors by caseworkers. The Medicaid payments cited in the report covered people in the state’s fee-for-service system, managed-care plans, drug treatment programs, and those receiving mental health services.
California’s Department of Health Care Services, which runs Medi-Cal, said in a statement that it agreed with nearly all of the auditors’ recommendations and that the agency “has taken steps to address all of the findings.”
In a written response to the inspector general, California officials said several computer upgrades were made after the audit period and before publication of the report that should improve the accuracy of eligibility decisions.
Among the 150 expansion enrollees analyzed in detail, 75%, or 112, were deemed eligible for the Medicaid program in California. Auditors discovered a variety of problems with the other 38 enrollees.
During the audit period, 12 enrollees in the sample group had incomes above 138% of the federal poverty level, making them ineligible financially for public assistance, according to the report.
In other instances, beneficiaries were already enrolled in Medicare, the federal health insurance for people 65 and older or who have severe disabilities, and did not qualify for Medi-Cal. One woman indicated she didn’t want Medi-Cal but was enrolled anyway.
In 2014, the state struggled to clear a massive backlog of Medi-Cal applications, which reached about 900,000 at one point. Many people complained about being mistakenly rejected for coverage or that their applications were lost in the state or county computer systems.
California was one of 31 states to expand Medicaid under the 2010 Affordable Care Act. The health law established a higher federal reimbursement for these newly eligible patients, primarily low-income adults without children. After expansion started in 2014, the HHS inspector general’s office began reviewing whether states were determining eligibility correctly and spending taxpayer dollars appropriately.
In a similar audit released in January, the inspector general estimated that New York spent $26.2 million in federal Medicaid money on 47,271 expansion enrollees who were ineligible for coverage. (The sample size there was 130 enrollees.) Overall, New York had far fewer expansion enrollees and related spending, compared with California.
Audits of other states’ records are planned
“It is inevitable that in a big rollout of new eligibility for any public program there are going to be glitches in implementation,” said Kathy Hempstead, a health-policy expert and senior adviser at the Robert Wood Johnson Foundation. “The inspector general wants to make sure that states are being sufficiently careful.”
Nationwide, Medicaid, the state-federal health insurance program designed for the poor, is the country’s largest health insurance program, covering 74 million Americans. In the past year, Republican efforts to reduce Medicaid funding and enrollment have sparked intense political debates and loud protests over the size and scope of the public program.
The federal government footed the entire cost of Medicaid expansion during the first three years, instead of taking the usual approach of splitting the costs with states. Now, states are picking up more of the bill. Their share of the costs will grow to 10 percent by 2020.
The California audit didn’t request a specific repayment from the state, but the findings were sent to the Centers for Medicare & Medicaid Services for review. CMS officials didn’t return a request for comment.
Donald White, a spokesman for the inspector general’s office, said the agency stood by the report’s findings and declined to comment further.
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
California signed up an estimated 450,000 people under Medicaid expansion who may not have been eligible for coverage, according to a report by the Health & Human Services’ chief watchdog.
In a Feb. 21 report, the HHS’s inspector general estimated that California spent $738.2 million on 366,078 expansion beneficiaries who were ineligible. It spent an additional $416.5 million for 79,055 expansion enrollees who were “potentially” ineligible, auditors found.
Auditors said nearly 90% of the $1.15 billion in questionable payments involved federal money, while the rest came from the state’s Medicaid program, known as Medi-Cal. They examined a 6-month period from Oct. 1, 2014, to March 31, 2015, when Medicaid payments of $6.2 billion were made related to 1.9 million newly eligible enrollees.
There were limitations to the California review, however. The audit extrapolated from a sample of 150 beneficiaries. The authors reported a 90% confidence level in their results – whereas 95% would be more common. That meant that the number of those ineligible could have been as low as 260,000 or as high as 630,000.
“If HHS has a strong reason to believe that California is systematically making enrollment errors, it would be helpful to show that in a more robust analysis,” said Ben Ippolito, a health care economist at the American Enterprise Institute, a conservative think tank. “The federal government should ensure that states are being good stewards of federal money.”
Nonetheless, the audit highlighted weaknesses in California’s Medicaid program, the largest in the nation with 13.4 million enrollees and an annual budget topping $100 billion, counting federal and state money. Medicaid covers one in three Californians.
The inspector general found deficiencies in the state’s computer system for verifying eligibility and discovered errors by caseworkers. The Medicaid payments cited in the report covered people in the state’s fee-for-service system, managed-care plans, drug treatment programs, and those receiving mental health services.
California’s Department of Health Care Services, which runs Medi-Cal, said in a statement that it agreed with nearly all of the auditors’ recommendations and that the agency “has taken steps to address all of the findings.”
In a written response to the inspector general, California officials said several computer upgrades were made after the audit period and before publication of the report that should improve the accuracy of eligibility decisions.
Among the 150 expansion enrollees analyzed in detail, 75%, or 112, were deemed eligible for the Medicaid program in California. Auditors discovered a variety of problems with the other 38 enrollees.
During the audit period, 12 enrollees in the sample group had incomes above 138% of the federal poverty level, making them ineligible financially for public assistance, according to the report.
In other instances, beneficiaries were already enrolled in Medicare, the federal health insurance for people 65 and older or who have severe disabilities, and did not qualify for Medi-Cal. One woman indicated she didn’t want Medi-Cal but was enrolled anyway.
In 2014, the state struggled to clear a massive backlog of Medi-Cal applications, which reached about 900,000 at one point. Many people complained about being mistakenly rejected for coverage or that their applications were lost in the state or county computer systems.
California was one of 31 states to expand Medicaid under the 2010 Affordable Care Act. The health law established a higher federal reimbursement for these newly eligible patients, primarily low-income adults without children. After expansion started in 2014, the HHS inspector general’s office began reviewing whether states were determining eligibility correctly and spending taxpayer dollars appropriately.
In a similar audit released in January, the inspector general estimated that New York spent $26.2 million in federal Medicaid money on 47,271 expansion enrollees who were ineligible for coverage. (The sample size there was 130 enrollees.) Overall, New York had far fewer expansion enrollees and related spending, compared with California.
Audits of other states’ records are planned
“It is inevitable that in a big rollout of new eligibility for any public program there are going to be glitches in implementation,” said Kathy Hempstead, a health-policy expert and senior adviser at the Robert Wood Johnson Foundation. “The inspector general wants to make sure that states are being sufficiently careful.”
Nationwide, Medicaid, the state-federal health insurance program designed for the poor, is the country’s largest health insurance program, covering 74 million Americans. In the past year, Republican efforts to reduce Medicaid funding and enrollment have sparked intense political debates and loud protests over the size and scope of the public program.
The federal government footed the entire cost of Medicaid expansion during the first three years, instead of taking the usual approach of splitting the costs with states. Now, states are picking up more of the bill. Their share of the costs will grow to 10 percent by 2020.
The California audit didn’t request a specific repayment from the state, but the findings were sent to the Centers for Medicare & Medicaid Services for review. CMS officials didn’t return a request for comment.
Donald White, a spokesman for the inspector general’s office, said the agency stood by the report’s findings and declined to comment further.
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
California signed up an estimated 450,000 people under Medicaid expansion who may not have been eligible for coverage, according to a report by the Health & Human Services’ chief watchdog.
In a Feb. 21 report, the HHS’s inspector general estimated that California spent $738.2 million on 366,078 expansion beneficiaries who were ineligible. It spent an additional $416.5 million for 79,055 expansion enrollees who were “potentially” ineligible, auditors found.
Auditors said nearly 90% of the $1.15 billion in questionable payments involved federal money, while the rest came from the state’s Medicaid program, known as Medi-Cal. They examined a 6-month period from Oct. 1, 2014, to March 31, 2015, when Medicaid payments of $6.2 billion were made related to 1.9 million newly eligible enrollees.
There were limitations to the California review, however. The audit extrapolated from a sample of 150 beneficiaries. The authors reported a 90% confidence level in their results – whereas 95% would be more common. That meant that the number of those ineligible could have been as low as 260,000 or as high as 630,000.
“If HHS has a strong reason to believe that California is systematically making enrollment errors, it would be helpful to show that in a more robust analysis,” said Ben Ippolito, a health care economist at the American Enterprise Institute, a conservative think tank. “The federal government should ensure that states are being good stewards of federal money.”
Nonetheless, the audit highlighted weaknesses in California’s Medicaid program, the largest in the nation with 13.4 million enrollees and an annual budget topping $100 billion, counting federal and state money. Medicaid covers one in three Californians.
The inspector general found deficiencies in the state’s computer system for verifying eligibility and discovered errors by caseworkers. The Medicaid payments cited in the report covered people in the state’s fee-for-service system, managed-care plans, drug treatment programs, and those receiving mental health services.
California’s Department of Health Care Services, which runs Medi-Cal, said in a statement that it agreed with nearly all of the auditors’ recommendations and that the agency “has taken steps to address all of the findings.”
In a written response to the inspector general, California officials said several computer upgrades were made after the audit period and before publication of the report that should improve the accuracy of eligibility decisions.
Among the 150 expansion enrollees analyzed in detail, 75%, or 112, were deemed eligible for the Medicaid program in California. Auditors discovered a variety of problems with the other 38 enrollees.
During the audit period, 12 enrollees in the sample group had incomes above 138% of the federal poverty level, making them ineligible financially for public assistance, according to the report.
In other instances, beneficiaries were already enrolled in Medicare, the federal health insurance for people 65 and older or who have severe disabilities, and did not qualify for Medi-Cal. One woman indicated she didn’t want Medi-Cal but was enrolled anyway.
In 2014, the state struggled to clear a massive backlog of Medi-Cal applications, which reached about 900,000 at one point. Many people complained about being mistakenly rejected for coverage or that their applications were lost in the state or county computer systems.
California was one of 31 states to expand Medicaid under the 2010 Affordable Care Act. The health law established a higher federal reimbursement for these newly eligible patients, primarily low-income adults without children. After expansion started in 2014, the HHS inspector general’s office began reviewing whether states were determining eligibility correctly and spending taxpayer dollars appropriately.
In a similar audit released in January, the inspector general estimated that New York spent $26.2 million in federal Medicaid money on 47,271 expansion enrollees who were ineligible for coverage. (The sample size there was 130 enrollees.) Overall, New York had far fewer expansion enrollees and related spending, compared with California.
Audits of other states’ records are planned
“It is inevitable that in a big rollout of new eligibility for any public program there are going to be glitches in implementation,” said Kathy Hempstead, a health-policy expert and senior adviser at the Robert Wood Johnson Foundation. “The inspector general wants to make sure that states are being sufficiently careful.”
Nationwide, Medicaid, the state-federal health insurance program designed for the poor, is the country’s largest health insurance program, covering 74 million Americans. In the past year, Republican efforts to reduce Medicaid funding and enrollment have sparked intense political debates and loud protests over the size and scope of the public program.
The federal government footed the entire cost of Medicaid expansion during the first three years, instead of taking the usual approach of splitting the costs with states. Now, states are picking up more of the bill. Their share of the costs will grow to 10 percent by 2020.
The California audit didn’t request a specific repayment from the state, but the findings were sent to the Centers for Medicare & Medicaid Services for review. CMS officials didn’t return a request for comment.
Donald White, a spokesman for the inspector general’s office, said the agency stood by the report’s findings and declined to comment further.
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.