User login
Factor IX product launched in US
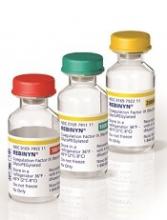
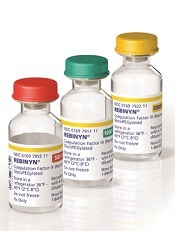
The recombinant, GlycoPEGylated coagulation factor IX product Rebinyn® is now available in the US for the treatment of patients with hemophilia B.
Last May, Rebinyn was approved by the US Food and Drug Administration for on-demand treatment and control of bleeding episodes as well as perioperative management of bleeding in adults and children with hemophilia B.
The product is not approved for routine prophylaxis or immune tolerance induction in hemophilia B patients.
The full prescribing information is available at www.Rebinyn.com.
Rebinyn is also approved for use in the European Union, where it is known as nonacog beta pegol or by the brand name Refixia.
There, the product is approved for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
Trial results
The US and European approvals of nonacog beta pegol (N9-GP) were based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in the phase 3 trials paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined. ![]()
The recombinant, GlycoPEGylated coagulation factor IX product Rebinyn® is now available in the US for the treatment of patients with hemophilia B.
Last May, Rebinyn was approved by the US Food and Drug Administration for on-demand treatment and control of bleeding episodes as well as perioperative management of bleeding in adults and children with hemophilia B.
The product is not approved for routine prophylaxis or immune tolerance induction in hemophilia B patients.
The full prescribing information is available at www.Rebinyn.com.
Rebinyn is also approved for use in the European Union, where it is known as nonacog beta pegol or by the brand name Refixia.
There, the product is approved for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
Trial results
The US and European approvals of nonacog beta pegol (N9-GP) were based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in the phase 3 trials paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined. ![]()
The recombinant, GlycoPEGylated coagulation factor IX product Rebinyn® is now available in the US for the treatment of patients with hemophilia B.
Last May, Rebinyn was approved by the US Food and Drug Administration for on-demand treatment and control of bleeding episodes as well as perioperative management of bleeding in adults and children with hemophilia B.
The product is not approved for routine prophylaxis or immune tolerance induction in hemophilia B patients.
The full prescribing information is available at www.Rebinyn.com.
Rebinyn is also approved for use in the European Union, where it is known as nonacog beta pegol or by the brand name Refixia.
There, the product is approved for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
Trial results
The US and European approvals of nonacog beta pegol (N9-GP) were based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in the phase 3 trials paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined. ![]()
Assay identifies actionable mutations in lymphoid malignancies
Researchers say hybrid capture sequencing is an accurate and sensitive method for identifying actionable gene mutations in lymphoid malignancies.
This method revealed potentially actionable mutations in 91% of patients studied, who had diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), or chronic lymphocytic leukemia (CLL).
The researchers therefore believe hybrid capture sequencing will bring the benefits of precision diagnosis and individualized therapy to patients with lymphoid malignancies.
“To realize the benefits of the most recent progress in cancer genomics, clinical implementation of precision medicine approaches is needed in the form of novel biomarker assays,” said study author Christian Steidl, MD, of the University of British Columbia in Vancouver, Canada.
“Fully implemented targeted sequencing-based assays in routine diagnostic pathology laboratories are currently lacking in lymphoid cancer care. Our findings demonstrate the feasibility and outline the clinical utility of integrating a lymphoma-specific pipeline into personalized cancer care.”
Dr Steidl and his colleagues reported these findings in The Journal of Molecular Diagnostics.
The researchers first compared capture hybridization and amplicon sequencing using samples from 8 patients with lymphoma. Fresh-frozen and formalin-fixed, paraffin-embedded tumor samples were sequenced using a panel of 20 lymphoma-specific genes.
The team found that capture hybridization provided “deep, more uniform coverage” and yielded “higher sensitivity for variant calling” than amplicon sequencing.
The researchers then developed a targeted sequencing pipeline using a 32-gene panel. The panel was developed with input from a group of 6 specialists who kept updating it based on the latest available information.
“This allows for continuous integration of additional gene features as our knowledge base improves,” Dr Steidl noted.
He and his colleagues then applied the hybrid capture sequencing assay and 32-gene panel to tissues from 219 patients—114 with FL, 76 with DLBCL, and 29 with CLL—who were treated in British Columbia between 2013 and 2016.
Results revealed at least one actionable mutation in 91% of the tumors. And the assay uncovered subtype-specific mutational profiles that were highly similar to published mutational profiles for FL, DLBCL, and CLL.
Furthermore, the assay had 93% concordance with whole-genome sequencing.
“Our developed assay harnesses the power of modern sequencing for clinical diagnostics purposes and potentially better deployment of novel treatments in lymphoid cancers,” Dr Steidl said. “We believe our study will help establish evidence-based approaches to decision making in lymphoid cancer care.”
“The next steps are to implement sequencing-based biomarker assays, such as reported in our study, in accredited pathology laboratories. Toward the goal of biomarker-driven clinical decision making, testing of potentially predictive biomarker assays is needed alongside clinical trials investigating novel cancer therapeutics.” ![]()
Researchers say hybrid capture sequencing is an accurate and sensitive method for identifying actionable gene mutations in lymphoid malignancies.
This method revealed potentially actionable mutations in 91% of patients studied, who had diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), or chronic lymphocytic leukemia (CLL).
The researchers therefore believe hybrid capture sequencing will bring the benefits of precision diagnosis and individualized therapy to patients with lymphoid malignancies.
“To realize the benefits of the most recent progress in cancer genomics, clinical implementation of precision medicine approaches is needed in the form of novel biomarker assays,” said study author Christian Steidl, MD, of the University of British Columbia in Vancouver, Canada.
“Fully implemented targeted sequencing-based assays in routine diagnostic pathology laboratories are currently lacking in lymphoid cancer care. Our findings demonstrate the feasibility and outline the clinical utility of integrating a lymphoma-specific pipeline into personalized cancer care.”
Dr Steidl and his colleagues reported these findings in The Journal of Molecular Diagnostics.
The researchers first compared capture hybridization and amplicon sequencing using samples from 8 patients with lymphoma. Fresh-frozen and formalin-fixed, paraffin-embedded tumor samples were sequenced using a panel of 20 lymphoma-specific genes.
The team found that capture hybridization provided “deep, more uniform coverage” and yielded “higher sensitivity for variant calling” than amplicon sequencing.
The researchers then developed a targeted sequencing pipeline using a 32-gene panel. The panel was developed with input from a group of 6 specialists who kept updating it based on the latest available information.
“This allows for continuous integration of additional gene features as our knowledge base improves,” Dr Steidl noted.
He and his colleagues then applied the hybrid capture sequencing assay and 32-gene panel to tissues from 219 patients—114 with FL, 76 with DLBCL, and 29 with CLL—who were treated in British Columbia between 2013 and 2016.
Results revealed at least one actionable mutation in 91% of the tumors. And the assay uncovered subtype-specific mutational profiles that were highly similar to published mutational profiles for FL, DLBCL, and CLL.
Furthermore, the assay had 93% concordance with whole-genome sequencing.
“Our developed assay harnesses the power of modern sequencing for clinical diagnostics purposes and potentially better deployment of novel treatments in lymphoid cancers,” Dr Steidl said. “We believe our study will help establish evidence-based approaches to decision making in lymphoid cancer care.”
“The next steps are to implement sequencing-based biomarker assays, such as reported in our study, in accredited pathology laboratories. Toward the goal of biomarker-driven clinical decision making, testing of potentially predictive biomarker assays is needed alongside clinical trials investigating novel cancer therapeutics.” ![]()
Researchers say hybrid capture sequencing is an accurate and sensitive method for identifying actionable gene mutations in lymphoid malignancies.
This method revealed potentially actionable mutations in 91% of patients studied, who had diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), or chronic lymphocytic leukemia (CLL).
The researchers therefore believe hybrid capture sequencing will bring the benefits of precision diagnosis and individualized therapy to patients with lymphoid malignancies.
“To realize the benefits of the most recent progress in cancer genomics, clinical implementation of precision medicine approaches is needed in the form of novel biomarker assays,” said study author Christian Steidl, MD, of the University of British Columbia in Vancouver, Canada.
“Fully implemented targeted sequencing-based assays in routine diagnostic pathology laboratories are currently lacking in lymphoid cancer care. Our findings demonstrate the feasibility and outline the clinical utility of integrating a lymphoma-specific pipeline into personalized cancer care.”
Dr Steidl and his colleagues reported these findings in The Journal of Molecular Diagnostics.
The researchers first compared capture hybridization and amplicon sequencing using samples from 8 patients with lymphoma. Fresh-frozen and formalin-fixed, paraffin-embedded tumor samples were sequenced using a panel of 20 lymphoma-specific genes.
The team found that capture hybridization provided “deep, more uniform coverage” and yielded “higher sensitivity for variant calling” than amplicon sequencing.
The researchers then developed a targeted sequencing pipeline using a 32-gene panel. The panel was developed with input from a group of 6 specialists who kept updating it based on the latest available information.
“This allows for continuous integration of additional gene features as our knowledge base improves,” Dr Steidl noted.
He and his colleagues then applied the hybrid capture sequencing assay and 32-gene panel to tissues from 219 patients—114 with FL, 76 with DLBCL, and 29 with CLL—who were treated in British Columbia between 2013 and 2016.
Results revealed at least one actionable mutation in 91% of the tumors. And the assay uncovered subtype-specific mutational profiles that were highly similar to published mutational profiles for FL, DLBCL, and CLL.
Furthermore, the assay had 93% concordance with whole-genome sequencing.
“Our developed assay harnesses the power of modern sequencing for clinical diagnostics purposes and potentially better deployment of novel treatments in lymphoid cancers,” Dr Steidl said. “We believe our study will help establish evidence-based approaches to decision making in lymphoid cancer care.”
“The next steps are to implement sequencing-based biomarker assays, such as reported in our study, in accredited pathology laboratories. Toward the goal of biomarker-driven clinical decision making, testing of potentially predictive biomarker assays is needed alongside clinical trials investigating novel cancer therapeutics.” ![]()
Azacitidine now available in China
Azacitidine for injection (Vidaza®) is now available in China.
The nucleoside metabolic inhibitor was approved in China to treat patients with intermediate-2/high-risk myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) with 20% to 30% bone marrow blasts, and chronic myelomonocytic leukemia (CMML).
Azacitidine for injection is marketed in China by BeiGene Ltd. under an exclusive license from Celgene Corporation.
“Vidaza is the only approved hypomethylating agent shown to prolong survival for patients with MDS and the first new treatment for MDS patients approved in China since 2009,” said John V. Oyler, founder, chief executive officer, and chairman of BeiGene.
“We are excited to announce that the first prescription was made in January 2018. From now on, Chinese patients can benefit from Vidaza in hospitals around China.”
Azacitidine was evaluated in a global phase 3 trial of patients with intermediate-2- and high-risk MDS, CMML, or AML (AZA-001). Results from this trial were published in The Lancet Oncology in 2009.
Patients were randomized to receive azacitidine plus best supportive care (BSC, n=179) or conventional care regimens plus BSC (105 to BSC alone, 49 to low-dose cytarabine, and 25 to chemotherapy with cytarabine and anthracycline).
Azacitidine was given subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days every 28 days until disease progression, relapse after response, or unacceptable toxicity.
The median overall survival was 24.5 months with azacitidine, compared to 15 months for patients treated with conventional care regimens.
There was a higher hematologic response rate in the azacitidine arm than the conventional care arm—29% and 12%, respectively.
In the azacitidine group, 45% of patients who were dependent on red blood cell transfusions at baseline became transfusion independent, compared with 11% in the conventional care group.
Forty-six percent of patients in the azacitidine arm and 63% in the conventional care arm died.
Grade 3/4 hematologic toxicity (in the azacitidine and conventional care arms, respectively) included neutropenia (91% and 76%), thrombocytopenia (85% and 80%), and anemia (57% and 68%). ![]()
Azacitidine for injection (Vidaza®) is now available in China.
The nucleoside metabolic inhibitor was approved in China to treat patients with intermediate-2/high-risk myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) with 20% to 30% bone marrow blasts, and chronic myelomonocytic leukemia (CMML).
Azacitidine for injection is marketed in China by BeiGene Ltd. under an exclusive license from Celgene Corporation.
“Vidaza is the only approved hypomethylating agent shown to prolong survival for patients with MDS and the first new treatment for MDS patients approved in China since 2009,” said John V. Oyler, founder, chief executive officer, and chairman of BeiGene.
“We are excited to announce that the first prescription was made in January 2018. From now on, Chinese patients can benefit from Vidaza in hospitals around China.”
Azacitidine was evaluated in a global phase 3 trial of patients with intermediate-2- and high-risk MDS, CMML, or AML (AZA-001). Results from this trial were published in The Lancet Oncology in 2009.
Patients were randomized to receive azacitidine plus best supportive care (BSC, n=179) or conventional care regimens plus BSC (105 to BSC alone, 49 to low-dose cytarabine, and 25 to chemotherapy with cytarabine and anthracycline).
Azacitidine was given subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days every 28 days until disease progression, relapse after response, or unacceptable toxicity.
The median overall survival was 24.5 months with azacitidine, compared to 15 months for patients treated with conventional care regimens.
There was a higher hematologic response rate in the azacitidine arm than the conventional care arm—29% and 12%, respectively.
In the azacitidine group, 45% of patients who were dependent on red blood cell transfusions at baseline became transfusion independent, compared with 11% in the conventional care group.
Forty-six percent of patients in the azacitidine arm and 63% in the conventional care arm died.
Grade 3/4 hematologic toxicity (in the azacitidine and conventional care arms, respectively) included neutropenia (91% and 76%), thrombocytopenia (85% and 80%), and anemia (57% and 68%). ![]()
Azacitidine for injection (Vidaza®) is now available in China.
The nucleoside metabolic inhibitor was approved in China to treat patients with intermediate-2/high-risk myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) with 20% to 30% bone marrow blasts, and chronic myelomonocytic leukemia (CMML).
Azacitidine for injection is marketed in China by BeiGene Ltd. under an exclusive license from Celgene Corporation.
“Vidaza is the only approved hypomethylating agent shown to prolong survival for patients with MDS and the first new treatment for MDS patients approved in China since 2009,” said John V. Oyler, founder, chief executive officer, and chairman of BeiGene.
“We are excited to announce that the first prescription was made in January 2018. From now on, Chinese patients can benefit from Vidaza in hospitals around China.”
Azacitidine was evaluated in a global phase 3 trial of patients with intermediate-2- and high-risk MDS, CMML, or AML (AZA-001). Results from this trial were published in The Lancet Oncology in 2009.
Patients were randomized to receive azacitidine plus best supportive care (BSC, n=179) or conventional care regimens plus BSC (105 to BSC alone, 49 to low-dose cytarabine, and 25 to chemotherapy with cytarabine and anthracycline).
Azacitidine was given subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days every 28 days until disease progression, relapse after response, or unacceptable toxicity.
The median overall survival was 24.5 months with azacitidine, compared to 15 months for patients treated with conventional care regimens.
There was a higher hematologic response rate in the azacitidine arm than the conventional care arm—29% and 12%, respectively.
In the azacitidine group, 45% of patients who were dependent on red blood cell transfusions at baseline became transfusion independent, compared with 11% in the conventional care group.
Forty-six percent of patients in the azacitidine arm and 63% in the conventional care arm died.
Grade 3/4 hematologic toxicity (in the azacitidine and conventional care arms, respectively) included neutropenia (91% and 76%), thrombocytopenia (85% and 80%), and anemia (57% and 68%). ![]()
Abstract: Is Adherence to the American College of Chest Physicians Recommended Anticoagulation Treatment Duration Associated With Different Outcomes Among Patients With Venous Thromboembolism?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Spyropoulos, A.C., et al, Clin Appl Thromb Hemost 23(6):532, September 2017
BACKGROUND: The American College of Chest Physicians (ACCP) recommends three months or at least six months of oral anticoagulant therapy, depending on history and risk factors, to prevent recurrent venous thromboembolism (VTE) events.
METHODS: This retrospective study coordinated at Hofstra School of Medicine evaluated adherence to the ACCP guidelines and associated clinical and economic outcomes. A US health care claims database identified 81,827 adults (median age 56 years; 52% male) with at least one claim for deep vein thrombosis or pulmonary embolism in 2008-2014, use of anticoagulation therapy after the index event, and continuous insurance coverage for one year before and after the event. Patients were classified as adherent (n=60,550; 74%) or nonadherent (n=21,277) with the ACCP minimum treatment durations. The primary study outcomes were VTE recurrence, all-cause and bleeding-related hospitalizations, and medical costs.
RESULTS: Most patients (94%) received warfarin. Nonadherent patients had significantly more VTE recurrences, resource use, bleeding complications, and costs during follow-up. On multivariate analysis controlling for patient characteristics, adherent patients had lower risks of all-cause hospitalization (adjusted odds ratio [AOR] 0.85; 95% CI 0.82-0.88; p<0.0001), bleeding-related hospitalization (AOR 0.74; 95% CI 0.69-0.78; p<0.0001), and VTE recurrence (AOR 0.92; 95% CI 0.88-0.97; p=0.0014), as well as lower total health care costs (mean difference -$2121; p=0.0003), VTE-related costs (-$2294; p<0.0001), and bleeding-related costs (-$248; p<0.0001).
CONCLUSIONS: In this large study, compliance with ACCP anticoagulant guidelines was associated with a reduction in VTE morbidity and health care costs. 16 references ([email protected] – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Spyropoulos, A.C., et al, Clin Appl Thromb Hemost 23(6):532, September 2017
BACKGROUND: The American College of Chest Physicians (ACCP) recommends three months or at least six months of oral anticoagulant therapy, depending on history and risk factors, to prevent recurrent venous thromboembolism (VTE) events.
METHODS: This retrospective study coordinated at Hofstra School of Medicine evaluated adherence to the ACCP guidelines and associated clinical and economic outcomes. A US health care claims database identified 81,827 adults (median age 56 years; 52% male) with at least one claim for deep vein thrombosis or pulmonary embolism in 2008-2014, use of anticoagulation therapy after the index event, and continuous insurance coverage for one year before and after the event. Patients were classified as adherent (n=60,550; 74%) or nonadherent (n=21,277) with the ACCP minimum treatment durations. The primary study outcomes were VTE recurrence, all-cause and bleeding-related hospitalizations, and medical costs.
RESULTS: Most patients (94%) received warfarin. Nonadherent patients had significantly more VTE recurrences, resource use, bleeding complications, and costs during follow-up. On multivariate analysis controlling for patient characteristics, adherent patients had lower risks of all-cause hospitalization (adjusted odds ratio [AOR] 0.85; 95% CI 0.82-0.88; p<0.0001), bleeding-related hospitalization (AOR 0.74; 95% CI 0.69-0.78; p<0.0001), and VTE recurrence (AOR 0.92; 95% CI 0.88-0.97; p=0.0014), as well as lower total health care costs (mean difference -$2121; p=0.0003), VTE-related costs (-$2294; p<0.0001), and bleeding-related costs (-$248; p<0.0001).
CONCLUSIONS: In this large study, compliance with ACCP anticoagulant guidelines was associated with a reduction in VTE morbidity and health care costs. 16 references ([email protected] – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Spyropoulos, A.C., et al, Clin Appl Thromb Hemost 23(6):532, September 2017
BACKGROUND: The American College of Chest Physicians (ACCP) recommends three months or at least six months of oral anticoagulant therapy, depending on history and risk factors, to prevent recurrent venous thromboembolism (VTE) events.
METHODS: This retrospective study coordinated at Hofstra School of Medicine evaluated adherence to the ACCP guidelines and associated clinical and economic outcomes. A US health care claims database identified 81,827 adults (median age 56 years; 52% male) with at least one claim for deep vein thrombosis or pulmonary embolism in 2008-2014, use of anticoagulation therapy after the index event, and continuous insurance coverage for one year before and after the event. Patients were classified as adherent (n=60,550; 74%) or nonadherent (n=21,277) with the ACCP minimum treatment durations. The primary study outcomes were VTE recurrence, all-cause and bleeding-related hospitalizations, and medical costs.
RESULTS: Most patients (94%) received warfarin. Nonadherent patients had significantly more VTE recurrences, resource use, bleeding complications, and costs during follow-up. On multivariate analysis controlling for patient characteristics, adherent patients had lower risks of all-cause hospitalization (adjusted odds ratio [AOR] 0.85; 95% CI 0.82-0.88; p<0.0001), bleeding-related hospitalization (AOR 0.74; 95% CI 0.69-0.78; p<0.0001), and VTE recurrence (AOR 0.92; 95% CI 0.88-0.97; p=0.0014), as well as lower total health care costs (mean difference -$2121; p=0.0003), VTE-related costs (-$2294; p<0.0001), and bleeding-related costs (-$248; p<0.0001).
CONCLUSIONS: In this large study, compliance with ACCP anticoagulant guidelines was associated with a reduction in VTE morbidity and health care costs. 16 references ([email protected] – no reprints)
Learn more about the Primary Care Medical Abstracts and podcasts, for which you can earn up to 9 CME credits per month.
Copyright © The Center for Medical Education
Adjuvant chemo halves DFS events in upper-tract urothelial cancer
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
SAN FRANCISCO – Patients who have undergone nephro-ureterectomy for upper-tract urothelial cancer (UTUC) fare much better if they are given adjuvant chemotherapy, according to the first results of the POUT trial reported at the 2018 Genitourinary Cancers Symposium.
Standard treatment for this rare cancer is radical nephro-ureterectomy followed by surveillance, noted lead author Alison Jane Birtle, MD, MRCP, FRCR, a consultant clinical oncologist at the Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom. Evidence has been insufficient to recommend adjuvant therapy.
“We know that UTUC shares a similar etiology with bladder cancer, where there is strong evidence for chemotherapy,” she said. “Trials of adjuvant chemotherapy in bladder cancer have been challenging because of cystectomy being a much more morbid operation. So adjuvant chemotherapy after nephro-ureterectomy should be much easier to give because of the less morbid procedure.”
The POUT (Peri-Operative Chemotherapy Versus sUrveillance in Upper Tract Urothelial Cancer, NCT0199397) investigators enrolled in the trial 261 patients from 57 UK centers who had undergone radical nephro-ureterectomy for urothelial cancer of the renal pelvis or ureter. Patients were randomized evenly to receive surveillance or adjuvant platinum-based combination chemotherapy, with specific platinum (cisplatin or carboplatin) based on glomerular filtration rate (GFR).
Trial enrollment was stopped early, after a median follow-up of 19.3 months, because of efficacy of chemotherapy, Dr. Birtle reported at the symposium, which was sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Main results showed that risks of both disease-free survival events and metastasis-free survival events were 51% lower for the chemotherapy group as compared with the surveillance group. Overall survival showed a trend toward benefit as well.
Not surprisingly, grade 3 or worse adverse events during treatment were about four times more common with chemotherapy, but the treatment was overall feasible and safe, even though the majority of patients were older than 60 years.
“Based on these results, adjuvant platinum-based chemotherapy should be considered a new standard of care in these patients,” Dr. Birtle maintained. “The next thing is how are we going to move this data forward? We are looking at the successor study at the moment, which is currently in development…[POUT] has been a triumph of UK urologists really getting behind it, but to do a further study, it would be great to look at international collaboration.”
Optimal timing of chemotherapy, before or after surgery, remains an open question, according session co-chair Jeanny B. Aragon-Ching, MD, a medical oncologist with the Inova Medical Group, Fairfax, Virginia.
“This trial offers high-level evidence for consideration of adjuvant chemotherapy in those who are unable to receive neoadjuvant chemotherapy in UTUC, although it is still important to evaluate what the prospective neoadjuvant chemotherapy data in UTUC would show,” she said in an interview. “Ongoing studies include the ECOG 8141 study (clinicaltrials.gov NCT02412670), but certainly, several retrospective trials showed benefit of neoadjuvant chemotherapy in UTUC.”
Other viewpoints
“It’s fantastic that we finally have data in upper-tract cancer. However, I worry about how this data is going to be interpreted, and I argue that it should not be considered the standard of care,” session attendee Matthew Campbell, MD, of the MD Anderson Cancer Center, Houston, commented during a question and answer period.
“I believe that this is showing that chemotherapy is very important in this disease, and if anything, it should be moved into the neoadjuvant setting, where more patients will be cisplatin candidates. Though there is challenge with staging upper-tract disease, at MD Anderson, we consider patients with high-grade disease or hydronephrosis to be at high enough risk to consider for neoadjuvant chemotherapy, and that’s been our approach.”
“When we looked at developing POUT, there was a big debate about whether it should be a neoadjuvant or adjuvant study,” Dr. Birtle replied. UK oncologists expressed concern that not all patients have histologic confirmation of UTUC preoperatively; therefore, chemotherapy could lead to overtreatment for some.
“We plan to go back and look at the CT urograms and the diagnostic imaging done prior to surgery just to see if we can be 100% confident in our diagnostic accuracy preoperatively, to see if we would be more confident with a neoadjuvant study,” she added. The investigators have also reviewed data from the British Association of Urological Surgeons on the postoperative dip in GFR. “It didn’t really seem from that 2015 data that there was a huge unmet need for patients postoperatively, where we would have missed them had we not treated them preoperatively,” she said.
On a related note, session attendee Surena Matin, MD, also of MD Anderson, asked “How many patients were potentially precluded because of their GFR, and do you have any data regarding response rates in the carboplatin arm?”
The main reason for exclusion was ineligible pathology and not GFR, according to Dr. Birtle. “This goes back to the previous question of can you be certain that a patient has locally advanced disease prior to nephro-ureterectomy? About 60% of patients who were thought to have muscle-invasive disease ultimately on nephro-ureterectomy didn’t.”
Confidence intervals for chemotherapy benefit in the subgroup given carboplatin were wide and overlapped unity, but still favoring benefit and falling within the overall treatment effect, she said.
Session attendee Joaquim Bellmunt, MD, Dana-Farber Cancer Institute, Boston, noted that the chemotherapy benefit was not significant in that subgroup and also in the subgroups with lymph node–positive disease and with positive margins. “I think that saying … chemotherapy is for everybody based on this trial” is incorrect, he asserted. “It may be good just to tone down the message that this is the new standard of care.” He further questioned the trial’s early stopping, noting that continuing would have provided more information in these patients.
Those subgroups were small, so analyses were underpowered to definitively rule out chemotherapy benefit, according to Dr. Birtle. The investigators had intensive discussion about the recommendation to stop early, because of a goal to determine overall survival impact. Ultimately, “when we saw the data in terms of disease-free survival and metastasis-free survival, the magnitude of the effect was so big that we felt it was uncomfortable and unethical not to offer patients treatment,” she said.
Study details
Patients in POUT’s chemotherapy arm received four cycles of chemotherapy—gemcitabine-cisplatin if their GFR was 50 mL/min or higher, or gemcitabine-carboplatin if their GFR was 30-49 mL/min—starting within 90 days of nephro-ureterectomy.
Of note, approximately 40% of all patients in the trial were aged 70 years or older, including the 5% who were aged 80 years or older. “This is very reassuring for a study of adjuvant platinum-based chemotherapy,” Dr. Birtle commented. Fully 71.2% of the chemotherapy patients received all four planned cycles.
In an intention-to-treat analysis, risk of disease-free survival events (death from any cause, metastasis, or any ureteric or renal bed recurrence) was sharply reduced with chemotherapy versus surveillance (hazard ratio, 0.49; P=.001). The proportion of patients event free at 2 years was 71% in the chemotherapy group and 54% in the surveillance group. Benefit was generally similar across subgroups, and findings were much the same after adjustment for nodal involvement, microscopic margin status, and planned chemotherapy regimen (hazard ratio, 0.47; P=.001).
Risk of metastasis-free survival events was also sharply lower with chemotherapy (hazard ratio, 0.49; P=.002), with a proportion event free at 2 years of 74% in the chemotherapy group and 60% in the surveillance group. These findings were also much the same after adjustment for the above factors (hazard ratio, 0.47; P=.002).
Overall survival tended to be better with chemotherapy than with surveillance (hazard ratio, 0.55), but data are still immature for this endpoint.
The rate of grade 3 or worse adverse events during the treatment period was 53.2% with chemotherapy and 13.5% with surveillance; events with chemotherapy were as expected, with neutropenia, thrombocytopenia, and gastrointestinal events predominating. The rate of febrile neutropenia was 5.7% with gemcitabine-cisplatin and 7.8% with gemcitabine-carboplatin, but there were no neutropenic deaths.
The rate of grade 3 or worse adverse events for the entire trial period was 62.1% with chemotherapy and 24.8% with surveillance.
Data on nephrotoxicity are still being evaluated, according to Dr. Birtle. Only seven patients who started on gemcitabine-cisplatin had to switch to gemcitabine-carboplatin because their GFR fell.
In a related translational study, the investigators are evaluating both the baseline CT urograms and the resected tumors to identify prognostic and predictive markers, she said.
Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
SOURCE: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point:
Major finding: Compared with surveillance, adjuvant chemotherapy halved risk of disease-free survival events (hazard ratio, 0.49; P=.001).
Data source: A phase 3 randomized trial of adjuvant platinum-based chemotherapy versus surveillance in 261 patients with upper-tract urothelial cancer (POUT trial).
Disclosures: Dr. Birtle disclosed that she receives honoraria from Roche, Janssen, Astellas, and Bayer, and is a consultant to Sanofi-Aventis. The trial was funded by the UK Clinical Trials Awards and Advisory Committee.
Source: Birtle A et al. Genitourinary Cancers Symposium. Abstract 407.
An intervention to track fetal movement attempts to prevent stillbirth
DALLAS – A hospital-wide intervention designed to prevent stillbirth by focusing on reduced fetal movement failed to do so – but did result in significant increases in labor induction and cesarean sections.
After the interventions was implemented, hospitals achieved a stillbirth rate of 4 per 1,000 – not significantly different than the 4.4 per 1,000 rate in the controls, Jane Norman, MD, reported at the meeting sponsored by the Society for Maternal-Fetal Medicine. On the other hand, the risk of a C-section increased by 9% and the risk of a labor induction by 8%, said Prof. Norman of the University of Edinburgh.
The results are disappointing, but consistent with most of the literature on this topic, she said.
“There is very good evidence that, if you ask women to count kicks, it doesn’t prevent stillbirth,” said Dr. Norman, referring to a 2015 Cochrane review. That paper, which examined five studies comprising more than 71,000 women, found that rates of stillbirth were similar between those who employed kick counts and those who did not.
Still, said Dr. Norman, there is evidence that stillbirth often is preceded by a period of reduced fetal movement. And a 2009 quality improvement project conducted in Oslo has driven enthusiasm for the idea of implementing some form of maternal and provider awareness of this area of obstetric care.
Stillbirth rates fell about 2% after the participating hospitals provided written information to women about fetal activity and reduced fetal movement, including an invitation to monitor fetal movements and formalized clinical guidelines for management of reduced fetal movement.
“There is a huge interest in the United Kingdom right now in using reduced fetal movement as an alert to act on to prevent stillbirth,” Dr. Norman said. “The National Health Service has recommended that we talk with women and clinicians about reduced fetal movement,” and try to incorporate it into clinical care.
Dr. Norman and her team wanted to emulate the Oslo experience, with a target of a 30% reduction in stillbirth rate.
The AFFIRM trial employed a clinical management guideline and patient education handout to raise awareness of reduced fetal movement as a trigger to investigate fetal well-being. The study used hospital records from 37 institutions in the United Kingdom and Ireland, which sequentially implemented the package. Hospitals were grouped into eight clusters. The trial began in January 2014. The first cluster began the intervention in mid-March; a new cluster came online every 3 months thereafter, until April 2016. Each cluster used its own baseline data as control.
The leaflet educated women about what fetal movements should feel like in all stages of pregnancy and what to expect from normal fetal movement. It encouraged them to report any lessening or cessation of fetal movement to their health care provider without delay.
The clinical guideline was matched to different gestational stages, but generally advocated cardiotocography and scans to estimate amniotic fluid volume and fetal size in cases of reduced movement with a low threshold for early delivery if there were abnormal findings at 37 weeks or later.
The final sample comprised 385,582 births, with 157,692 in the control period and 227,860 in the intervention period. The mean age of women was 30 years; 50% were white, 50% were overweight or obese, and 15% smoked. About 40% were nulliparous.
In the control arm, there were 691 stillbirths at 24 weeks’ gestational age or older (4.4/1,000). In the intervention arm, there were 921 events (4.06/1,000). The 10% risk reduction was not statistically significant.
The investigators also examined stillbirths occurring at 22 weeks or older, 28 weeks or older, and 37 weeks or older. Again, there were no significant between-group differences. Nor was there a difference in the perinatal mortality rate (0.68% vs. 0.62%).
There were, however, differences in interventions. The risk of a C-section increased by 5% (25.5% vs. 28.5%; adjusted odds ratio, 1.05), and the risk of induction by 9% (33.6% vs. 39.8%; AOR, 1.09). Most of the induction risk was driven by an 11% increase in induction risk at 39 weeks or older. The team also found a 5% increase in the chance of an elective delivery at 39 weeks or older (45.2% vs. 52.4%; AOR, 1.05)
There was a corresponding significant 10% decrease in the chance of spontaneous vaginal delivery (59.8% vs. 57.4%; AOR, 0.90).
Although there was no overall increase in the risk of a neonatal ICU admission, there was a significant 12% increase in the risk of a neonatal ICU admission of at least 48 hours (6.2% vs. 6.7%; AOR, 1.12). There were no other significant differences in neonatal outcomes (gestational age, size at birth, preterm delivery).
“We also looked at the potential impact if we implemented this intervention on a population of 10,000 pregnancies,” Dr. Norman said. “We would have 5 fewer stillbirths – but potentially anywhere from 11 fewer to 3 more. However, we would have 162 more cesarean deliveries and 108 more inductions.”
The AFFIRM trial was sponsored by the University of Edinburgh and the National Health Service. Dr. Norman had no financial disclosures.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2018;218,S603.
DALLAS – A hospital-wide intervention designed to prevent stillbirth by focusing on reduced fetal movement failed to do so – but did result in significant increases in labor induction and cesarean sections.
After the interventions was implemented, hospitals achieved a stillbirth rate of 4 per 1,000 – not significantly different than the 4.4 per 1,000 rate in the controls, Jane Norman, MD, reported at the meeting sponsored by the Society for Maternal-Fetal Medicine. On the other hand, the risk of a C-section increased by 9% and the risk of a labor induction by 8%, said Prof. Norman of the University of Edinburgh.
The results are disappointing, but consistent with most of the literature on this topic, she said.
“There is very good evidence that, if you ask women to count kicks, it doesn’t prevent stillbirth,” said Dr. Norman, referring to a 2015 Cochrane review. That paper, which examined five studies comprising more than 71,000 women, found that rates of stillbirth were similar between those who employed kick counts and those who did not.
Still, said Dr. Norman, there is evidence that stillbirth often is preceded by a period of reduced fetal movement. And a 2009 quality improvement project conducted in Oslo has driven enthusiasm for the idea of implementing some form of maternal and provider awareness of this area of obstetric care.
Stillbirth rates fell about 2% after the participating hospitals provided written information to women about fetal activity and reduced fetal movement, including an invitation to monitor fetal movements and formalized clinical guidelines for management of reduced fetal movement.
“There is a huge interest in the United Kingdom right now in using reduced fetal movement as an alert to act on to prevent stillbirth,” Dr. Norman said. “The National Health Service has recommended that we talk with women and clinicians about reduced fetal movement,” and try to incorporate it into clinical care.
Dr. Norman and her team wanted to emulate the Oslo experience, with a target of a 30% reduction in stillbirth rate.
The AFFIRM trial employed a clinical management guideline and patient education handout to raise awareness of reduced fetal movement as a trigger to investigate fetal well-being. The study used hospital records from 37 institutions in the United Kingdom and Ireland, which sequentially implemented the package. Hospitals were grouped into eight clusters. The trial began in January 2014. The first cluster began the intervention in mid-March; a new cluster came online every 3 months thereafter, until April 2016. Each cluster used its own baseline data as control.
The leaflet educated women about what fetal movements should feel like in all stages of pregnancy and what to expect from normal fetal movement. It encouraged them to report any lessening or cessation of fetal movement to their health care provider without delay.
The clinical guideline was matched to different gestational stages, but generally advocated cardiotocography and scans to estimate amniotic fluid volume and fetal size in cases of reduced movement with a low threshold for early delivery if there were abnormal findings at 37 weeks or later.
The final sample comprised 385,582 births, with 157,692 in the control period and 227,860 in the intervention period. The mean age of women was 30 years; 50% were white, 50% were overweight or obese, and 15% smoked. About 40% were nulliparous.
In the control arm, there were 691 stillbirths at 24 weeks’ gestational age or older (4.4/1,000). In the intervention arm, there were 921 events (4.06/1,000). The 10% risk reduction was not statistically significant.
The investigators also examined stillbirths occurring at 22 weeks or older, 28 weeks or older, and 37 weeks or older. Again, there were no significant between-group differences. Nor was there a difference in the perinatal mortality rate (0.68% vs. 0.62%).
There were, however, differences in interventions. The risk of a C-section increased by 5% (25.5% vs. 28.5%; adjusted odds ratio, 1.05), and the risk of induction by 9% (33.6% vs. 39.8%; AOR, 1.09). Most of the induction risk was driven by an 11% increase in induction risk at 39 weeks or older. The team also found a 5% increase in the chance of an elective delivery at 39 weeks or older (45.2% vs. 52.4%; AOR, 1.05)
There was a corresponding significant 10% decrease in the chance of spontaneous vaginal delivery (59.8% vs. 57.4%; AOR, 0.90).
Although there was no overall increase in the risk of a neonatal ICU admission, there was a significant 12% increase in the risk of a neonatal ICU admission of at least 48 hours (6.2% vs. 6.7%; AOR, 1.12). There were no other significant differences in neonatal outcomes (gestational age, size at birth, preterm delivery).
“We also looked at the potential impact if we implemented this intervention on a population of 10,000 pregnancies,” Dr. Norman said. “We would have 5 fewer stillbirths – but potentially anywhere from 11 fewer to 3 more. However, we would have 162 more cesarean deliveries and 108 more inductions.”
The AFFIRM trial was sponsored by the University of Edinburgh and the National Health Service. Dr. Norman had no financial disclosures.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2018;218,S603.
DALLAS – A hospital-wide intervention designed to prevent stillbirth by focusing on reduced fetal movement failed to do so – but did result in significant increases in labor induction and cesarean sections.
After the interventions was implemented, hospitals achieved a stillbirth rate of 4 per 1,000 – not significantly different than the 4.4 per 1,000 rate in the controls, Jane Norman, MD, reported at the meeting sponsored by the Society for Maternal-Fetal Medicine. On the other hand, the risk of a C-section increased by 9% and the risk of a labor induction by 8%, said Prof. Norman of the University of Edinburgh.
The results are disappointing, but consistent with most of the literature on this topic, she said.
“There is very good evidence that, if you ask women to count kicks, it doesn’t prevent stillbirth,” said Dr. Norman, referring to a 2015 Cochrane review. That paper, which examined five studies comprising more than 71,000 women, found that rates of stillbirth were similar between those who employed kick counts and those who did not.
Still, said Dr. Norman, there is evidence that stillbirth often is preceded by a period of reduced fetal movement. And a 2009 quality improvement project conducted in Oslo has driven enthusiasm for the idea of implementing some form of maternal and provider awareness of this area of obstetric care.
Stillbirth rates fell about 2% after the participating hospitals provided written information to women about fetal activity and reduced fetal movement, including an invitation to monitor fetal movements and formalized clinical guidelines for management of reduced fetal movement.
“There is a huge interest in the United Kingdom right now in using reduced fetal movement as an alert to act on to prevent stillbirth,” Dr. Norman said. “The National Health Service has recommended that we talk with women and clinicians about reduced fetal movement,” and try to incorporate it into clinical care.
Dr. Norman and her team wanted to emulate the Oslo experience, with a target of a 30% reduction in stillbirth rate.
The AFFIRM trial employed a clinical management guideline and patient education handout to raise awareness of reduced fetal movement as a trigger to investigate fetal well-being. The study used hospital records from 37 institutions in the United Kingdom and Ireland, which sequentially implemented the package. Hospitals were grouped into eight clusters. The trial began in January 2014. The first cluster began the intervention in mid-March; a new cluster came online every 3 months thereafter, until April 2016. Each cluster used its own baseline data as control.
The leaflet educated women about what fetal movements should feel like in all stages of pregnancy and what to expect from normal fetal movement. It encouraged them to report any lessening or cessation of fetal movement to their health care provider without delay.
The clinical guideline was matched to different gestational stages, but generally advocated cardiotocography and scans to estimate amniotic fluid volume and fetal size in cases of reduced movement with a low threshold for early delivery if there were abnormal findings at 37 weeks or later.
The final sample comprised 385,582 births, with 157,692 in the control period and 227,860 in the intervention period. The mean age of women was 30 years; 50% were white, 50% were overweight or obese, and 15% smoked. About 40% were nulliparous.
In the control arm, there were 691 stillbirths at 24 weeks’ gestational age or older (4.4/1,000). In the intervention arm, there were 921 events (4.06/1,000). The 10% risk reduction was not statistically significant.
The investigators also examined stillbirths occurring at 22 weeks or older, 28 weeks or older, and 37 weeks or older. Again, there were no significant between-group differences. Nor was there a difference in the perinatal mortality rate (0.68% vs. 0.62%).
There were, however, differences in interventions. The risk of a C-section increased by 5% (25.5% vs. 28.5%; adjusted odds ratio, 1.05), and the risk of induction by 9% (33.6% vs. 39.8%; AOR, 1.09). Most of the induction risk was driven by an 11% increase in induction risk at 39 weeks or older. The team also found a 5% increase in the chance of an elective delivery at 39 weeks or older (45.2% vs. 52.4%; AOR, 1.05)
There was a corresponding significant 10% decrease in the chance of spontaneous vaginal delivery (59.8% vs. 57.4%; AOR, 0.90).
Although there was no overall increase in the risk of a neonatal ICU admission, there was a significant 12% increase in the risk of a neonatal ICU admission of at least 48 hours (6.2% vs. 6.7%; AOR, 1.12). There were no other significant differences in neonatal outcomes (gestational age, size at birth, preterm delivery).
“We also looked at the potential impact if we implemented this intervention on a population of 10,000 pregnancies,” Dr. Norman said. “We would have 5 fewer stillbirths – but potentially anywhere from 11 fewer to 3 more. However, we would have 162 more cesarean deliveries and 108 more inductions.”
The AFFIRM trial was sponsored by the University of Edinburgh and the National Health Service. Dr. Norman had no financial disclosures.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2018;218,S603.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Reduced fetal movements as an alert for investigation didn’t reduce stillbirth rates.
Major finding: Hospitals achieved a stillbirth rate of 4/1,000 – not significantly different than the 4.4/1,000 rate before the intervention.
Study details: The randomized, cluster trial comprised 37 hospitals in the United Kingdom and Ireland.
Disclosures: The University of Edinburgh and the National Health Service sponsored the trial. Dr. Norman had no financial disclosures.
Source: Norman JE et al. Am J Obstet Gynecol. 2018;218,S603.
ACR sounds more welcoming tone in new biosimilars position paper
The American College of Rheumatology has shifted from a more cautious stance toward the use of biosimilars in clinical practice to now recommend in a new position statement that health care providers incorporate biosimilars, where appropriate, into treatment regimens for their patients living with rheumatic diseases.
“Now that biosimilars have been used successfully in Europe, with rigorously acquired data supporting their broader use, and , S. Louis Bridges Jr., MD, PhD, chair of the ACR Committee on Research, wrote with seven other authors of the position statement in Arthritis & Rheumatology.
The ACR position statement addresses the issues of immunogenicity, extrapolation of indications, interchangeability, substitution, switching, and cost surrounding biosimilars.
The position statement’s authors said they expect switching and nonmedical substitution to become as common in the United States as it is in the rest of the world. They do not anticipate efficacy and safety issues for biosimilars based on available data regarding switching between reference products and biosimilars and their understanding of product drift.
“However, we encourage vigorous postmarketing surveillance of both biosimilars and their reference products as we enter the era where patients may undergo multiple switches as a result of insurance company and [pharmacy benefits manager] formulary preferences,” they said.
Immunogenicity
Concerns about immunogenicity for biosimilars approved in the United States have mostly been well addressed through studies showing similar frequencies of binding and neutralizing antidrug antibodies (ADA) in biosimilars and their reference products. Furthermore, no safety signals between biosimilars and their reference products have been observed that suggest a differential effect of ADA on efficacy, safety, or patient outcomes, the authors said. But they noted that “if immunogenicity findings are to be extrapolated from one disease to additional indications, the subjects being studied should be those most likely to develop ADA, such as subjects not receiving concomitant immunosuppressive medications.” The results of comparative immunogenicity studies carried out to date also indicate that “a patient who develops ADA to a reference drug with resultant loss of clinical response should not be switched to its biosimilar.”
Again, the authors said postmarketing pharmacovigilance using observational registry data would be critical to assessing the effect of switching on immunogenicity.
Extrapolation of indications
The extrapolation of biosimilars to reference product indications for which the biosimilar was not assessed in clinical trials continues to “be an area of uneasiness” among clinicians “who are surprised to find” that a biosimilar can be approved for inflammatory bowel disease in the absence of clinical trials in the relevant patient populations, the authors said. In geographic areas where it is not mandatory to use biosimilars, this lack of confidence in extrapolation of indications may limit their acceptance, the authors wrote, but data from studies such as NOR-SWITCH and DANBIO have provided reassuring evidence to support regulated extrapolation of indication for biosimilars.
However, since extrapolation of indications also applies to pediatric patients who often metabolize drugs faster than adults, the position paper says that “it may be important” to conduct pharmacodynamic and pharmacokinetic studies in children as well as postmarketing surveillance “since potential immunogenicity may be of particular importance in these younger patients with chronic diseases who might encounter several biological agents during their lifetime.”
Substitution, interchangeability, and switching
The “interchangeability” regulatory pathway in the United States that would allow substitution at the point of dispensing has not been finalized, but most states have enacted, or are in the process of enacting, legislation to regulate the practice, the statement says.
While substitution describes a change made by someone other than the prescriber, the authors note that switching defines the “intentional change initiated by a health care provider in partnership with the patient” for economic or medical reasons. Switching has been studied most often in open-label extension studies of biosimilar clinic trials and has shown no loss of efficacy or increase in adverse events.
Dr. Fleischmann contended that substitution, extrapolation, and interchangeability of biosimilars in clinical practice remain gray areas. For example, in a clinical trial, patients switching from a reference product to a biosimilar may show equivalency of clinical response and adverse events. “But as rheumatologists, we don’t treat groups of patients; we treat individual patients and here the results may be different,” he wrote in Arthritis & Rheumatology.
While the white paper appropriately points out that interchangeability among multiple biosimilars is a question that should be answered in postmarketing registries, Dr. Fleischmann noted that no interchangeability study has been reported, even though the FDA has issued guidance on how a study should be done.
“Although interchangeability may be safe and effective in many patients, until the results of such a study are available and properly analyzed, it is only conjecture that interchangeability is appropriate and safe,” he said.
Costs
The white paper acknowledged that the “only anticipated advantage” of a biosimilar over its reference product was lower cost, since both drugs should be therapeutically equivalent. “The degree to which the availability of biosimilars in the U.S. will drive down the cost of biologic therapy, and who will benefit from any cost reductions remains to be seen,” the authors wrote.
“To incentivize the use of biosimilars,” the authors suggested that “commercial and government insurance programs could harmonize drug prices with patients’ out-of-pocket costs and provider reimbursement. Currently, however, patients with commercial insurance are likely to have similar copayments for both biosimilars and originator biologics because of [pharmacy benefits manager]– or plan-mandated patient cost sharing. Also, patients’ out-of-pocket costs for biosimilars in the Medicare Part D (self-administered drug) program likely will be higher than for originator biologics because of a flaw that maintains, rather than reduces, biosimilar patient cost sharing in the coverage gap (also known as the “donut hole”) until 2020.”
It is not at all clear that a biosimilar would be cheaper for the individual patient, Dr. Fleischmann said. “It may be cheaper to the pharmacy benefit management firm, but this may not really help patient access to these medications. It is also not clear that nonmedical substitution will be effective in every patient nor has it been demonstrated that extrapolation is effective,” he wrote.
No disclosures were listed for the authors of the position statement. Dr. Fleischmann is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, Sanofi Aventis, and UCB.
SOURCES: Bridges S et al. Arthritis Rheumatol. 2018 Feb 7. doi: 10.1002/art.40388; and Fleischmann R. Arthritis Rheumatol. 2018 Feb 7. doi: 10.1002/art.40402
The American College of Rheumatology has shifted from a more cautious stance toward the use of biosimilars in clinical practice to now recommend in a new position statement that health care providers incorporate biosimilars, where appropriate, into treatment regimens for their patients living with rheumatic diseases.
“Now that biosimilars have been used successfully in Europe, with rigorously acquired data supporting their broader use, and , S. Louis Bridges Jr., MD, PhD, chair of the ACR Committee on Research, wrote with seven other authors of the position statement in Arthritis & Rheumatology.
The ACR position statement addresses the issues of immunogenicity, extrapolation of indications, interchangeability, substitution, switching, and cost surrounding biosimilars.
The position statement’s authors said they expect switching and nonmedical substitution to become as common in the United States as it is in the rest of the world. They do not anticipate efficacy and safety issues for biosimilars based on available data regarding switching between reference products and biosimilars and their understanding of product drift.
“However, we encourage vigorous postmarketing surveillance of both biosimilars and their reference products as we enter the era where patients may undergo multiple switches as a result of insurance company and [pharmacy benefits manager] formulary preferences,” they said.
Immunogenicity
Concerns about immunogenicity for biosimilars approved in the United States have mostly been well addressed through studies showing similar frequencies of binding and neutralizing antidrug antibodies (ADA) in biosimilars and their reference products. Furthermore, no safety signals between biosimilars and their reference products have been observed that suggest a differential effect of ADA on efficacy, safety, or patient outcomes, the authors said. But they noted that “if immunogenicity findings are to be extrapolated from one disease to additional indications, the subjects being studied should be those most likely to develop ADA, such as subjects not receiving concomitant immunosuppressive medications.” The results of comparative immunogenicity studies carried out to date also indicate that “a patient who develops ADA to a reference drug with resultant loss of clinical response should not be switched to its biosimilar.”
Again, the authors said postmarketing pharmacovigilance using observational registry data would be critical to assessing the effect of switching on immunogenicity.
Extrapolation of indications
The extrapolation of biosimilars to reference product indications for which the biosimilar was not assessed in clinical trials continues to “be an area of uneasiness” among clinicians “who are surprised to find” that a biosimilar can be approved for inflammatory bowel disease in the absence of clinical trials in the relevant patient populations, the authors said. In geographic areas where it is not mandatory to use biosimilars, this lack of confidence in extrapolation of indications may limit their acceptance, the authors wrote, but data from studies such as NOR-SWITCH and DANBIO have provided reassuring evidence to support regulated extrapolation of indication for biosimilars.
However, since extrapolation of indications also applies to pediatric patients who often metabolize drugs faster than adults, the position paper says that “it may be important” to conduct pharmacodynamic and pharmacokinetic studies in children as well as postmarketing surveillance “since potential immunogenicity may be of particular importance in these younger patients with chronic diseases who might encounter several biological agents during their lifetime.”
Substitution, interchangeability, and switching
The “interchangeability” regulatory pathway in the United States that would allow substitution at the point of dispensing has not been finalized, but most states have enacted, or are in the process of enacting, legislation to regulate the practice, the statement says.
While substitution describes a change made by someone other than the prescriber, the authors note that switching defines the “intentional change initiated by a health care provider in partnership with the patient” for economic or medical reasons. Switching has been studied most often in open-label extension studies of biosimilar clinic trials and has shown no loss of efficacy or increase in adverse events.
Dr. Fleischmann contended that substitution, extrapolation, and interchangeability of biosimilars in clinical practice remain gray areas. For example, in a clinical trial, patients switching from a reference product to a biosimilar may show equivalency of clinical response and adverse events. “But as rheumatologists, we don’t treat groups of patients; we treat individual patients and here the results may be different,” he wrote in Arthritis & Rheumatology.
While the white paper appropriately points out that interchangeability among multiple biosimilars is a question that should be answered in postmarketing registries, Dr. Fleischmann noted that no interchangeability study has been reported, even though the FDA has issued guidance on how a study should be done.
“Although interchangeability may be safe and effective in many patients, until the results of such a study are available and properly analyzed, it is only conjecture that interchangeability is appropriate and safe,” he said.
Costs
The white paper acknowledged that the “only anticipated advantage” of a biosimilar over its reference product was lower cost, since both drugs should be therapeutically equivalent. “The degree to which the availability of biosimilars in the U.S. will drive down the cost of biologic therapy, and who will benefit from any cost reductions remains to be seen,” the authors wrote.
“To incentivize the use of biosimilars,” the authors suggested that “commercial and government insurance programs could harmonize drug prices with patients’ out-of-pocket costs and provider reimbursement. Currently, however, patients with commercial insurance are likely to have similar copayments for both biosimilars and originator biologics because of [pharmacy benefits manager]– or plan-mandated patient cost sharing. Also, patients’ out-of-pocket costs for biosimilars in the Medicare Part D (self-administered drug) program likely will be higher than for originator biologics because of a flaw that maintains, rather than reduces, biosimilar patient cost sharing in the coverage gap (also known as the “donut hole”) until 2020.”
It is not at all clear that a biosimilar would be cheaper for the individual patient, Dr. Fleischmann said. “It may be cheaper to the pharmacy benefit management firm, but this may not really help patient access to these medications. It is also not clear that nonmedical substitution will be effective in every patient nor has it been demonstrated that extrapolation is effective,” he wrote.
No disclosures were listed for the authors of the position statement. Dr. Fleischmann is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, Sanofi Aventis, and UCB.
SOURCES: Bridges S et al. Arthritis Rheumatol. 2018 Feb 7. doi: 10.1002/art.40388; and Fleischmann R. Arthritis Rheumatol. 2018 Feb 7. doi: 10.1002/art.40402
The American College of Rheumatology has shifted from a more cautious stance toward the use of biosimilars in clinical practice to now recommend in a new position statement that health care providers incorporate biosimilars, where appropriate, into treatment regimens for their patients living with rheumatic diseases.
“Now that biosimilars have been used successfully in Europe, with rigorously acquired data supporting their broader use, and , S. Louis Bridges Jr., MD, PhD, chair of the ACR Committee on Research, wrote with seven other authors of the position statement in Arthritis & Rheumatology.
The ACR position statement addresses the issues of immunogenicity, extrapolation of indications, interchangeability, substitution, switching, and cost surrounding biosimilars.
The position statement’s authors said they expect switching and nonmedical substitution to become as common in the United States as it is in the rest of the world. They do not anticipate efficacy and safety issues for biosimilars based on available data regarding switching between reference products and biosimilars and their understanding of product drift.
“However, we encourage vigorous postmarketing surveillance of both biosimilars and their reference products as we enter the era where patients may undergo multiple switches as a result of insurance company and [pharmacy benefits manager] formulary preferences,” they said.
Immunogenicity
Concerns about immunogenicity for biosimilars approved in the United States have mostly been well addressed through studies showing similar frequencies of binding and neutralizing antidrug antibodies (ADA) in biosimilars and their reference products. Furthermore, no safety signals between biosimilars and their reference products have been observed that suggest a differential effect of ADA on efficacy, safety, or patient outcomes, the authors said. But they noted that “if immunogenicity findings are to be extrapolated from one disease to additional indications, the subjects being studied should be those most likely to develop ADA, such as subjects not receiving concomitant immunosuppressive medications.” The results of comparative immunogenicity studies carried out to date also indicate that “a patient who develops ADA to a reference drug with resultant loss of clinical response should not be switched to its biosimilar.”
Again, the authors said postmarketing pharmacovigilance using observational registry data would be critical to assessing the effect of switching on immunogenicity.
Extrapolation of indications
The extrapolation of biosimilars to reference product indications for which the biosimilar was not assessed in clinical trials continues to “be an area of uneasiness” among clinicians “who are surprised to find” that a biosimilar can be approved for inflammatory bowel disease in the absence of clinical trials in the relevant patient populations, the authors said. In geographic areas where it is not mandatory to use biosimilars, this lack of confidence in extrapolation of indications may limit their acceptance, the authors wrote, but data from studies such as NOR-SWITCH and DANBIO have provided reassuring evidence to support regulated extrapolation of indication for biosimilars.
However, since extrapolation of indications also applies to pediatric patients who often metabolize drugs faster than adults, the position paper says that “it may be important” to conduct pharmacodynamic and pharmacokinetic studies in children as well as postmarketing surveillance “since potential immunogenicity may be of particular importance in these younger patients with chronic diseases who might encounter several biological agents during their lifetime.”
Substitution, interchangeability, and switching
The “interchangeability” regulatory pathway in the United States that would allow substitution at the point of dispensing has not been finalized, but most states have enacted, or are in the process of enacting, legislation to regulate the practice, the statement says.
While substitution describes a change made by someone other than the prescriber, the authors note that switching defines the “intentional change initiated by a health care provider in partnership with the patient” for economic or medical reasons. Switching has been studied most often in open-label extension studies of biosimilar clinic trials and has shown no loss of efficacy or increase in adverse events.
Dr. Fleischmann contended that substitution, extrapolation, and interchangeability of biosimilars in clinical practice remain gray areas. For example, in a clinical trial, patients switching from a reference product to a biosimilar may show equivalency of clinical response and adverse events. “But as rheumatologists, we don’t treat groups of patients; we treat individual patients and here the results may be different,” he wrote in Arthritis & Rheumatology.
While the white paper appropriately points out that interchangeability among multiple biosimilars is a question that should be answered in postmarketing registries, Dr. Fleischmann noted that no interchangeability study has been reported, even though the FDA has issued guidance on how a study should be done.
“Although interchangeability may be safe and effective in many patients, until the results of such a study are available and properly analyzed, it is only conjecture that interchangeability is appropriate and safe,” he said.
Costs
The white paper acknowledged that the “only anticipated advantage” of a biosimilar over its reference product was lower cost, since both drugs should be therapeutically equivalent. “The degree to which the availability of biosimilars in the U.S. will drive down the cost of biologic therapy, and who will benefit from any cost reductions remains to be seen,” the authors wrote.
“To incentivize the use of biosimilars,” the authors suggested that “commercial and government insurance programs could harmonize drug prices with patients’ out-of-pocket costs and provider reimbursement. Currently, however, patients with commercial insurance are likely to have similar copayments for both biosimilars and originator biologics because of [pharmacy benefits manager]– or plan-mandated patient cost sharing. Also, patients’ out-of-pocket costs for biosimilars in the Medicare Part D (self-administered drug) program likely will be higher than for originator biologics because of a flaw that maintains, rather than reduces, biosimilar patient cost sharing in the coverage gap (also known as the “donut hole”) until 2020.”
It is not at all clear that a biosimilar would be cheaper for the individual patient, Dr. Fleischmann said. “It may be cheaper to the pharmacy benefit management firm, but this may not really help patient access to these medications. It is also not clear that nonmedical substitution will be effective in every patient nor has it been demonstrated that extrapolation is effective,” he wrote.
No disclosures were listed for the authors of the position statement. Dr. Fleischmann is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, Sanofi Aventis, and UCB.
SOURCES: Bridges S et al. Arthritis Rheumatol. 2018 Feb 7. doi: 10.1002/art.40388; and Fleischmann R. Arthritis Rheumatol. 2018 Feb 7. doi: 10.1002/art.40402
FROM ARTHRITIS & RHEUMATOLOGY
VIDEO: Off-label dupilumab finding a home in pediatric AD
KAUAI, HAWAII – Pediatric dermatologists aren’t waiting for Food and Drug Administration approval to try dupilumab (Dupixent) for their patients with severe atopic dermatitis.
It’s not approved in children, but the possibility of good control without the side effects of cyclosporine and other alternatives is too much to resist. A phase 2, company-sponsored study reported Eczema Area and Severity Index score improvements of up to 76% in pediatric patients treated with dupilumab, an interleukin-4 and IL-13 signaling blocker approved in 2017 for moderate to severe AD in adults.
Large pediatric trials are pending, but with results like that, “many of us just feel if it was our own kid, and we could get dupilumab, we would like to do that first,” said Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego.
It’s not just dupilumab that’s causing excitement. With almost 20 biologics in the pipeline, eczema seems poised to undergo a revolution in treatment much like psoriasis has in recent years.
Dr. Eichenfield explained (Eucrisa), a topical nonsteroidal phosphodiesterase-4 inhibitor approved for mild to moderate AD for children and adults ages two and older in December 2016, which doesn’t seem to have the duration limits of steroids, he said in an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Treatment of pediatric AD is “going to be a very different picture over the next few years,” he said.
Dr. Eichenfield is a consultant or investigator for many companies, including Regeneron/Sanofi, Genentech, Novartis, Pfizer, Lilly, and Allergan.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Pediatric dermatologists aren’t waiting for Food and Drug Administration approval to try dupilumab (Dupixent) for their patients with severe atopic dermatitis.
It’s not approved in children, but the possibility of good control without the side effects of cyclosporine and other alternatives is too much to resist. A phase 2, company-sponsored study reported Eczema Area and Severity Index score improvements of up to 76% in pediatric patients treated with dupilumab, an interleukin-4 and IL-13 signaling blocker approved in 2017 for moderate to severe AD in adults.
Large pediatric trials are pending, but with results like that, “many of us just feel if it was our own kid, and we could get dupilumab, we would like to do that first,” said Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego.
It’s not just dupilumab that’s causing excitement. With almost 20 biologics in the pipeline, eczema seems poised to undergo a revolution in treatment much like psoriasis has in recent years.
Dr. Eichenfield explained (Eucrisa), a topical nonsteroidal phosphodiesterase-4 inhibitor approved for mild to moderate AD for children and adults ages two and older in December 2016, which doesn’t seem to have the duration limits of steroids, he said in an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Treatment of pediatric AD is “going to be a very different picture over the next few years,” he said.
Dr. Eichenfield is a consultant or investigator for many companies, including Regeneron/Sanofi, Genentech, Novartis, Pfizer, Lilly, and Allergan.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Pediatric dermatologists aren’t waiting for Food and Drug Administration approval to try dupilumab (Dupixent) for their patients with severe atopic dermatitis.
It’s not approved in children, but the possibility of good control without the side effects of cyclosporine and other alternatives is too much to resist. A phase 2, company-sponsored study reported Eczema Area and Severity Index score improvements of up to 76% in pediatric patients treated with dupilumab, an interleukin-4 and IL-13 signaling blocker approved in 2017 for moderate to severe AD in adults.
Large pediatric trials are pending, but with results like that, “many of us just feel if it was our own kid, and we could get dupilumab, we would like to do that first,” said Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego.
It’s not just dupilumab that’s causing excitement. With almost 20 biologics in the pipeline, eczema seems poised to undergo a revolution in treatment much like psoriasis has in recent years.
Dr. Eichenfield explained (Eucrisa), a topical nonsteroidal phosphodiesterase-4 inhibitor approved for mild to moderate AD for children and adults ages two and older in December 2016, which doesn’t seem to have the duration limits of steroids, he said in an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Treatment of pediatric AD is “going to be a very different picture over the next few years,” he said.
Dr. Eichenfield is a consultant or investigator for many companies, including Regeneron/Sanofi, Genentech, Novartis, Pfizer, Lilly, and Allergan.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Adding docetaxel to hormone therapy for advanced prostate cancer nets QOL benefit
SAN FRANCISCO – Adding docetaxel to first-line long-term hormone therapy for advanced prostate cancer, whether metastatic or not, improves quality of life, reduces need for subsequent therapy, and is cost effective, suggests a new analysis of data from the STAMPEDE trial.
Hormone therapy alone has historically been standard of care for this disease, lead study author Nicholas D. James, MD, PhD, a professor of clinical oncology at the University of Birmingham, England, noted in a press briefing in advance of the 2018 Genitourinary Cancers Symposium.
The new analysis focused on health economic and quality of life outcomes. Results of a model based largely on STAMPEDE data showed that adding docetaxel to first-line hormone therapy yielded an increase of about 0.4 quality-adjusted life-years among men with nonmetastatic disease and about 0.5 quality-adjusted life-years among men with metastatic disease.
Adding docetaxel actually led to a small reduction in total lifetime costs, on the order of several hundred pounds sterling, for patients with nonmetastatic disease, because the drug costs were more than offset by reductions in costs associated with adverse outcomes. On the other hand, adding docetaxel increased total costs by a fairly modest amount, roughly 3,000 pounds sterling, for patients with metastatic disease, but that increase was due in large part to costs associated with prolonged survival.
“Upfront docetaxel gives you a gain in quality-adjusted life-years in all subgroups,” Dr. James said. “Analysis suggests a high degree of certainty relating to the quality-adjusted life-year gain associated with docetaxel.”
“The results therefore support the existing health care policy in the U.K. and elsewhere across the world in newly diagnosed metastatic patients. You’ve got a pretty modest cost for a survival gain and a quality gain,” he maintained. “But very importantly, we think these results extend the applicability to nonmetastatic patients because at the patient level there is very clear evidence of a quality-adjusted life-year gain, and the modeling also predicts an eventual survival gain. And at the provider level, it’s a very cost-effective use of resources. For a very modest upfront docetaxel cost, it actually is cost saving in the nonmetastatic patient because of the subsequent downstream [averted events], things like spinal cord compression.”
Full results of the analysis, including model-predicted overall survival, will be reported at the symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Clinical implications
“In this analysis of data from the STAMPEDE trial, we see that docetaxel, a chemotherapy drug that has been in our armamentarium for prostate cancer for over a decade, may be applied in earlier and earlier settings in the disease,” commented ASCO expert and moderator of the press briefing, Sumanta K. Pal, MD. “Dr. James has previously reported the survival gains associated with this drug, but today adds the dimension of demonstrating improved quality of life and potentially cost-effectiveness as well.”
Results can be compared against those seen with abiraterone (Zytiga), an oral hormonal therapy that has shown similar benefit in these same patient populations, he said. “Abiraterone may offer the benefit of improved tolerability over a short course versus chemotherapy, but does require a much more extensive duration of use and further mandates concomitant use of prednisone.”
“Understanding the cost and quality of life associated with abiraterone in this setting may help adjudicate between giving this drug or docetaxel for the patients that Dr. James describes,” concluded Dr. Pal, codirector of the Kidney Cancer Program at City of Hope, Duarte, Calif.
Study details
The ongoing multi-arm STAMPEDE randomized trial has thus far enrolled more than 9,000 men with advanced (nonmetastatic and metastatic) prostate cancer and evaluated 10 treatments for the disease. About 60% of enrolled men have metastases.
Dr. James and colleagues developed a state transition model to reflect the natural history of patients entering the trial. Rate of progression and quality of life data (assessed with the EuroQol EQ-5D tool) were ascertained from the trial; costs came from both the trial and the literature.
Results indicated that, despite the adverse quality of life impacts of receiving chemotherapy and of living longer with metastatic disease, adding docetaxel to hormone therapy ultimately increased quality-adjusted life-years, with similar benefit seen in both nonmetastatic and metastatic disease.
“It was very interesting to us that, in the nonmetastatic patients, the quality-adjusted life-year gain was almost of the same magnitude, despite there being no evidence yet in STAMPEDE follow-up of a definite survival benefit in this group,” Dr. James noted. In these patients, chemotherapy has an upfront “quality of life penalty,” but that is offset by the later quality of life gains from delaying relapses (and thus time to further treatment) and, when nonmetastatic disease becomes metastatic, reducing events such as fractures and spinal cord compression.
“These later gains essentially wipe out the quality of life cost, if you like, from docetaxel, giving you a net gain almost as big in magnitude as the gain in metastatic patients,” he summarized. “We think this is a really important new finding.”
In a cost breakdown, adding docetaxel to hormone therapy increased drug costs in patients with both nonmetastatic and metastatic disease. Costs for end of life care, adverse events, and monitoring changed little in either group
Adding docetaxel increased costs for management by about 1,000 pounds sterling in patients with nonmetastatic disease (likely related to the chemotherapy administration) and about 2,000 pounds sterling in patients with metastatic disease (likely related to chemotherapy administration and longer survival).
Of special note, with addition of docetaxel, costs for other life-prolonging therapies – abiraterone, enzalutamide (Xtandi), radium (Xofigo), and similar treatments – fell by about 2,500 pounds sterling in nonmetastatic disease and 1,000 pounds sterling in metastatic disease. These savings are attributable to a shorter period of time spent in the relapsed state for patients who receive docetaxel, Dr. James explained. And savings are much greater for patients with nonmetastatic disease because they spend a much shorter time post relapse, given that the extended failure-free survival with docetaxel does not fully translate to extended overall survival.
When it comes to total lifetime costs, “basically, your downstream savings almost completely wipe out, even in the metastatic patients, the upfront costs,” he summarized.
Dr. James disclosed that he receives honoraria from Sanofi/Aventis, Janssen, Astellas Medivation, Bayer Health, and Merck Sharp & Dohme; has a consulting or advisory role with Janssen, Sanofi/Aventis, Merck Sharp & Dohme, Roche, Astellas Medivation, and Bayer; and is on the speakers’ bureau for Bayer, Janssen, Roche, and Ipsen; in addition, his institution receives research funding from Janssen, Astellas Medivation, Bayer, Sanofi/Aventis, and Roche. The study was funded by Cancer Research UK and the Medical Research Council, United Kingdom.
SOURCE: James ND et al. GUCS Abstract 162.
SAN FRANCISCO – Adding docetaxel to first-line long-term hormone therapy for advanced prostate cancer, whether metastatic or not, improves quality of life, reduces need for subsequent therapy, and is cost effective, suggests a new analysis of data from the STAMPEDE trial.
Hormone therapy alone has historically been standard of care for this disease, lead study author Nicholas D. James, MD, PhD, a professor of clinical oncology at the University of Birmingham, England, noted in a press briefing in advance of the 2018 Genitourinary Cancers Symposium.
The new analysis focused on health economic and quality of life outcomes. Results of a model based largely on STAMPEDE data showed that adding docetaxel to first-line hormone therapy yielded an increase of about 0.4 quality-adjusted life-years among men with nonmetastatic disease and about 0.5 quality-adjusted life-years among men with metastatic disease.
Adding docetaxel actually led to a small reduction in total lifetime costs, on the order of several hundred pounds sterling, for patients with nonmetastatic disease, because the drug costs were more than offset by reductions in costs associated with adverse outcomes. On the other hand, adding docetaxel increased total costs by a fairly modest amount, roughly 3,000 pounds sterling, for patients with metastatic disease, but that increase was due in large part to costs associated with prolonged survival.
“Upfront docetaxel gives you a gain in quality-adjusted life-years in all subgroups,” Dr. James said. “Analysis suggests a high degree of certainty relating to the quality-adjusted life-year gain associated with docetaxel.”
“The results therefore support the existing health care policy in the U.K. and elsewhere across the world in newly diagnosed metastatic patients. You’ve got a pretty modest cost for a survival gain and a quality gain,” he maintained. “But very importantly, we think these results extend the applicability to nonmetastatic patients because at the patient level there is very clear evidence of a quality-adjusted life-year gain, and the modeling also predicts an eventual survival gain. And at the provider level, it’s a very cost-effective use of resources. For a very modest upfront docetaxel cost, it actually is cost saving in the nonmetastatic patient because of the subsequent downstream [averted events], things like spinal cord compression.”
Full results of the analysis, including model-predicted overall survival, will be reported at the symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Clinical implications
“In this analysis of data from the STAMPEDE trial, we see that docetaxel, a chemotherapy drug that has been in our armamentarium for prostate cancer for over a decade, may be applied in earlier and earlier settings in the disease,” commented ASCO expert and moderator of the press briefing, Sumanta K. Pal, MD. “Dr. James has previously reported the survival gains associated with this drug, but today adds the dimension of demonstrating improved quality of life and potentially cost-effectiveness as well.”
Results can be compared against those seen with abiraterone (Zytiga), an oral hormonal therapy that has shown similar benefit in these same patient populations, he said. “Abiraterone may offer the benefit of improved tolerability over a short course versus chemotherapy, but does require a much more extensive duration of use and further mandates concomitant use of prednisone.”
“Understanding the cost and quality of life associated with abiraterone in this setting may help adjudicate between giving this drug or docetaxel for the patients that Dr. James describes,” concluded Dr. Pal, codirector of the Kidney Cancer Program at City of Hope, Duarte, Calif.
Study details
The ongoing multi-arm STAMPEDE randomized trial has thus far enrolled more than 9,000 men with advanced (nonmetastatic and metastatic) prostate cancer and evaluated 10 treatments for the disease. About 60% of enrolled men have metastases.
Dr. James and colleagues developed a state transition model to reflect the natural history of patients entering the trial. Rate of progression and quality of life data (assessed with the EuroQol EQ-5D tool) were ascertained from the trial; costs came from both the trial and the literature.
Results indicated that, despite the adverse quality of life impacts of receiving chemotherapy and of living longer with metastatic disease, adding docetaxel to hormone therapy ultimately increased quality-adjusted life-years, with similar benefit seen in both nonmetastatic and metastatic disease.
“It was very interesting to us that, in the nonmetastatic patients, the quality-adjusted life-year gain was almost of the same magnitude, despite there being no evidence yet in STAMPEDE follow-up of a definite survival benefit in this group,” Dr. James noted. In these patients, chemotherapy has an upfront “quality of life penalty,” but that is offset by the later quality of life gains from delaying relapses (and thus time to further treatment) and, when nonmetastatic disease becomes metastatic, reducing events such as fractures and spinal cord compression.
“These later gains essentially wipe out the quality of life cost, if you like, from docetaxel, giving you a net gain almost as big in magnitude as the gain in metastatic patients,” he summarized. “We think this is a really important new finding.”
In a cost breakdown, adding docetaxel to hormone therapy increased drug costs in patients with both nonmetastatic and metastatic disease. Costs for end of life care, adverse events, and monitoring changed little in either group
Adding docetaxel increased costs for management by about 1,000 pounds sterling in patients with nonmetastatic disease (likely related to the chemotherapy administration) and about 2,000 pounds sterling in patients with metastatic disease (likely related to chemotherapy administration and longer survival).
Of special note, with addition of docetaxel, costs for other life-prolonging therapies – abiraterone, enzalutamide (Xtandi), radium (Xofigo), and similar treatments – fell by about 2,500 pounds sterling in nonmetastatic disease and 1,000 pounds sterling in metastatic disease. These savings are attributable to a shorter period of time spent in the relapsed state for patients who receive docetaxel, Dr. James explained. And savings are much greater for patients with nonmetastatic disease because they spend a much shorter time post relapse, given that the extended failure-free survival with docetaxel does not fully translate to extended overall survival.
When it comes to total lifetime costs, “basically, your downstream savings almost completely wipe out, even in the metastatic patients, the upfront costs,” he summarized.
Dr. James disclosed that he receives honoraria from Sanofi/Aventis, Janssen, Astellas Medivation, Bayer Health, and Merck Sharp & Dohme; has a consulting or advisory role with Janssen, Sanofi/Aventis, Merck Sharp & Dohme, Roche, Astellas Medivation, and Bayer; and is on the speakers’ bureau for Bayer, Janssen, Roche, and Ipsen; in addition, his institution receives research funding from Janssen, Astellas Medivation, Bayer, Sanofi/Aventis, and Roche. The study was funded by Cancer Research UK and the Medical Research Council, United Kingdom.
SOURCE: James ND et al. GUCS Abstract 162.
SAN FRANCISCO – Adding docetaxel to first-line long-term hormone therapy for advanced prostate cancer, whether metastatic or not, improves quality of life, reduces need for subsequent therapy, and is cost effective, suggests a new analysis of data from the STAMPEDE trial.
Hormone therapy alone has historically been standard of care for this disease, lead study author Nicholas D. James, MD, PhD, a professor of clinical oncology at the University of Birmingham, England, noted in a press briefing in advance of the 2018 Genitourinary Cancers Symposium.
The new analysis focused on health economic and quality of life outcomes. Results of a model based largely on STAMPEDE data showed that adding docetaxel to first-line hormone therapy yielded an increase of about 0.4 quality-adjusted life-years among men with nonmetastatic disease and about 0.5 quality-adjusted life-years among men with metastatic disease.
Adding docetaxel actually led to a small reduction in total lifetime costs, on the order of several hundred pounds sterling, for patients with nonmetastatic disease, because the drug costs were more than offset by reductions in costs associated with adverse outcomes. On the other hand, adding docetaxel increased total costs by a fairly modest amount, roughly 3,000 pounds sterling, for patients with metastatic disease, but that increase was due in large part to costs associated with prolonged survival.
“Upfront docetaxel gives you a gain in quality-adjusted life-years in all subgroups,” Dr. James said. “Analysis suggests a high degree of certainty relating to the quality-adjusted life-year gain associated with docetaxel.”
“The results therefore support the existing health care policy in the U.K. and elsewhere across the world in newly diagnosed metastatic patients. You’ve got a pretty modest cost for a survival gain and a quality gain,” he maintained. “But very importantly, we think these results extend the applicability to nonmetastatic patients because at the patient level there is very clear evidence of a quality-adjusted life-year gain, and the modeling also predicts an eventual survival gain. And at the provider level, it’s a very cost-effective use of resources. For a very modest upfront docetaxel cost, it actually is cost saving in the nonmetastatic patient because of the subsequent downstream [averted events], things like spinal cord compression.”
Full results of the analysis, including model-predicted overall survival, will be reported at the symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Clinical implications
“In this analysis of data from the STAMPEDE trial, we see that docetaxel, a chemotherapy drug that has been in our armamentarium for prostate cancer for over a decade, may be applied in earlier and earlier settings in the disease,” commented ASCO expert and moderator of the press briefing, Sumanta K. Pal, MD. “Dr. James has previously reported the survival gains associated with this drug, but today adds the dimension of demonstrating improved quality of life and potentially cost-effectiveness as well.”
Results can be compared against those seen with abiraterone (Zytiga), an oral hormonal therapy that has shown similar benefit in these same patient populations, he said. “Abiraterone may offer the benefit of improved tolerability over a short course versus chemotherapy, but does require a much more extensive duration of use and further mandates concomitant use of prednisone.”
“Understanding the cost and quality of life associated with abiraterone in this setting may help adjudicate between giving this drug or docetaxel for the patients that Dr. James describes,” concluded Dr. Pal, codirector of the Kidney Cancer Program at City of Hope, Duarte, Calif.
Study details
The ongoing multi-arm STAMPEDE randomized trial has thus far enrolled more than 9,000 men with advanced (nonmetastatic and metastatic) prostate cancer and evaluated 10 treatments for the disease. About 60% of enrolled men have metastases.
Dr. James and colleagues developed a state transition model to reflect the natural history of patients entering the trial. Rate of progression and quality of life data (assessed with the EuroQol EQ-5D tool) were ascertained from the trial; costs came from both the trial and the literature.
Results indicated that, despite the adverse quality of life impacts of receiving chemotherapy and of living longer with metastatic disease, adding docetaxel to hormone therapy ultimately increased quality-adjusted life-years, with similar benefit seen in both nonmetastatic and metastatic disease.
“It was very interesting to us that, in the nonmetastatic patients, the quality-adjusted life-year gain was almost of the same magnitude, despite there being no evidence yet in STAMPEDE follow-up of a definite survival benefit in this group,” Dr. James noted. In these patients, chemotherapy has an upfront “quality of life penalty,” but that is offset by the later quality of life gains from delaying relapses (and thus time to further treatment) and, when nonmetastatic disease becomes metastatic, reducing events such as fractures and spinal cord compression.
“These later gains essentially wipe out the quality of life cost, if you like, from docetaxel, giving you a net gain almost as big in magnitude as the gain in metastatic patients,” he summarized. “We think this is a really important new finding.”
In a cost breakdown, adding docetaxel to hormone therapy increased drug costs in patients with both nonmetastatic and metastatic disease. Costs for end of life care, adverse events, and monitoring changed little in either group
Adding docetaxel increased costs for management by about 1,000 pounds sterling in patients with nonmetastatic disease (likely related to the chemotherapy administration) and about 2,000 pounds sterling in patients with metastatic disease (likely related to chemotherapy administration and longer survival).
Of special note, with addition of docetaxel, costs for other life-prolonging therapies – abiraterone, enzalutamide (Xtandi), radium (Xofigo), and similar treatments – fell by about 2,500 pounds sterling in nonmetastatic disease and 1,000 pounds sterling in metastatic disease. These savings are attributable to a shorter period of time spent in the relapsed state for patients who receive docetaxel, Dr. James explained. And savings are much greater for patients with nonmetastatic disease because they spend a much shorter time post relapse, given that the extended failure-free survival with docetaxel does not fully translate to extended overall survival.
When it comes to total lifetime costs, “basically, your downstream savings almost completely wipe out, even in the metastatic patients, the upfront costs,” he summarized.
Dr. James disclosed that he receives honoraria from Sanofi/Aventis, Janssen, Astellas Medivation, Bayer Health, and Merck Sharp & Dohme; has a consulting or advisory role with Janssen, Sanofi/Aventis, Merck Sharp & Dohme, Roche, Astellas Medivation, and Bayer; and is on the speakers’ bureau for Bayer, Janssen, Roche, and Ipsen; in addition, his institution receives research funding from Janssen, Astellas Medivation, Bayer, Sanofi/Aventis, and Roche. The study was funded by Cancer Research UK and the Medical Research Council, United Kingdom.
SOURCE: James ND et al. GUCS Abstract 162.
REPORTING FROM GUCS 2018
Key clinical point:
Major finding: Compared with hormone therapy alone, adding docetaxel increased quality-adjusted life-years by about 0.4 among men with nonmetastatic disease and 0.5 among men with metastatic disease.
Data source: A health economic and quality of life analysis of data from a randomized controlled trial among 9,000 men with advanced (nonmetastatic and metastatic) prostate cancer starting first-line hormone therapy (STAMPEDE trial).
Disclosures: Dr. James disclosed that he receives honoraria from Sanofi/Aventis, Janssen, Astellas Medivation, Bayer Health, and Merck Sharp & Dohme; has a consulting or advisory role with Janssen, Sanofi/Aventis, Merck Sharp & Dohme, Roche, Astellas Medivation, and Bayer; and is on the speakers’ bureau for Bayer, Janssen, Roche, and Ipsen; in addition, his institution receives research funding from Janssen, Astellas Medivation, Bayer, Sanofi/Aventis, and Roche. The study was funded by Cancer Research UK and the Medical Research Council, UK.
Source: James ND et al. GUCS Abstract 162.
Singing Praises, Naming Names
Being a peer-reviewed journal, Federal Practitioner relies on the dedicated efforts of a great number of unsung (and uncompensated) people, and we would like to recognize these efforts. This journal exists because of the large body of federal health care providers who devote their time and energy to sharing best practices, case studies, literature reviews, and original research. An even larger number of men and women review the many submissions, check the research, and provide essential feedback to our authors. By design, this army of reviewers remains anonymous, but that does not diminish their importance.
Although it would be impossible to adequately thank our reviewers, authors, and other contributors, sufficiently, we are trying. We are delighted to induct some of the most engaged members of the Federal Practitioner community to the Editorial Advisory Association (EAA). The EAA helps guide the journal to ensure it remains focused on the essential issues that confront federal health care providers. Federal Practitioner strives for continuous improvement and is focused on enhancing the breadth, depth, and quality of the online and print content. The EAA plays an important role in that process, and we thank all the current EAA and new members for their guidance.
New EAA Members
Susanne G. Barnett, PharmD, BCPS, has directed the Pharmacy Notes column for Federal Practitioner since 2015. Dr. Barnett is an associate professor at the University of Wisconsin-Madison School of Pharmacy and a clinical pharmacist at the William S. Middleton Memorial Veterans Hospital. She has focused on antimicrobial stewardship and infectious diseases.
Anthony Breu, MD, conceived and directs the VA Boston Medical Forum series
Maggie Chartier, PsyD, MPH, has authored multiple articles for Federal Practitioner and provided guidance and direction for the 2017 and (forthcoming) 2018 editions of the public pathogens/infectious diseases special issues. Dr. Chartier is the deputy director for the HIV, Hepatitis, and Related Conditions Program in the VHA Office of Specialty Care Services and an assistant clinical professor at the University of California, San Francisco.
Marcia Johnson, DNP, FNP-BC, has been a highly active author and peer reviewer. Dr. Johnson has been a nurse practitioner at the VA for 18 years, and currently provides primary care at the Clermont CBOC in Florida. She previously served as the hepatitis C research coordinator at Philadelphia VAMC and practiced at the Orlando VAMC spinal cord injury clinic.
William Rodríguez-Cintrón, MD, is another active peer reviewer and a prolific contributor. Dr. Rodríguez-Cintrón is chief of the pulmonary, critical care and sleep medicine departments at the VA Caribbean Healthcare System in Puerto Rico.
Col. (Ret) Mona Pearl Treyball, PhD, MS, RN, USAF, also has been a dedicated peer reviewer. Col. Treyball is professor and specialty director of the
Federal Practitioner would like to thank all of the current and new members of the EAA for their continued support. All of the journal’s successes are built on their dedication and commitment. Federal Practitioner encourages all the members of the federal health care community to become more involved, whether as a peer reviewer, author, or by responding to our content in print, online, or via social media. Your feedback and involvement makes this journal better.
Being a peer-reviewed journal, Federal Practitioner relies on the dedicated efforts of a great number of unsung (and uncompensated) people, and we would like to recognize these efforts. This journal exists because of the large body of federal health care providers who devote their time and energy to sharing best practices, case studies, literature reviews, and original research. An even larger number of men and women review the many submissions, check the research, and provide essential feedback to our authors. By design, this army of reviewers remains anonymous, but that does not diminish their importance.
Although it would be impossible to adequately thank our reviewers, authors, and other contributors, sufficiently, we are trying. We are delighted to induct some of the most engaged members of the Federal Practitioner community to the Editorial Advisory Association (EAA). The EAA helps guide the journal to ensure it remains focused on the essential issues that confront federal health care providers. Federal Practitioner strives for continuous improvement and is focused on enhancing the breadth, depth, and quality of the online and print content. The EAA plays an important role in that process, and we thank all the current EAA and new members for their guidance.
New EAA Members
Susanne G. Barnett, PharmD, BCPS, has directed the Pharmacy Notes column for Federal Practitioner since 2015. Dr. Barnett is an associate professor at the University of Wisconsin-Madison School of Pharmacy and a clinical pharmacist at the William S. Middleton Memorial Veterans Hospital. She has focused on antimicrobial stewardship and infectious diseases.
Anthony Breu, MD, conceived and directs the VA Boston Medical Forum series
Maggie Chartier, PsyD, MPH, has authored multiple articles for Federal Practitioner and provided guidance and direction for the 2017 and (forthcoming) 2018 editions of the public pathogens/infectious diseases special issues. Dr. Chartier is the deputy director for the HIV, Hepatitis, and Related Conditions Program in the VHA Office of Specialty Care Services and an assistant clinical professor at the University of California, San Francisco.
Marcia Johnson, DNP, FNP-BC, has been a highly active author and peer reviewer. Dr. Johnson has been a nurse practitioner at the VA for 18 years, and currently provides primary care at the Clermont CBOC in Florida. She previously served as the hepatitis C research coordinator at Philadelphia VAMC and practiced at the Orlando VAMC spinal cord injury clinic.
William Rodríguez-Cintrón, MD, is another active peer reviewer and a prolific contributor. Dr. Rodríguez-Cintrón is chief of the pulmonary, critical care and sleep medicine departments at the VA Caribbean Healthcare System in Puerto Rico.
Col. (Ret) Mona Pearl Treyball, PhD, MS, RN, USAF, also has been a dedicated peer reviewer. Col. Treyball is professor and specialty director of the
Federal Practitioner would like to thank all of the current and new members of the EAA for their continued support. All of the journal’s successes are built on their dedication and commitment. Federal Practitioner encourages all the members of the federal health care community to become more involved, whether as a peer reviewer, author, or by responding to our content in print, online, or via social media. Your feedback and involvement makes this journal better.
Being a peer-reviewed journal, Federal Practitioner relies on the dedicated efforts of a great number of unsung (and uncompensated) people, and we would like to recognize these efforts. This journal exists because of the large body of federal health care providers who devote their time and energy to sharing best practices, case studies, literature reviews, and original research. An even larger number of men and women review the many submissions, check the research, and provide essential feedback to our authors. By design, this army of reviewers remains anonymous, but that does not diminish their importance.
Although it would be impossible to adequately thank our reviewers, authors, and other contributors, sufficiently, we are trying. We are delighted to induct some of the most engaged members of the Federal Practitioner community to the Editorial Advisory Association (EAA). The EAA helps guide the journal to ensure it remains focused on the essential issues that confront federal health care providers. Federal Practitioner strives for continuous improvement and is focused on enhancing the breadth, depth, and quality of the online and print content. The EAA plays an important role in that process, and we thank all the current EAA and new members for their guidance.
New EAA Members
Susanne G. Barnett, PharmD, BCPS, has directed the Pharmacy Notes column for Federal Practitioner since 2015. Dr. Barnett is an associate professor at the University of Wisconsin-Madison School of Pharmacy and a clinical pharmacist at the William S. Middleton Memorial Veterans Hospital. She has focused on antimicrobial stewardship and infectious diseases.
Anthony Breu, MD, conceived and directs the VA Boston Medical Forum series
Maggie Chartier, PsyD, MPH, has authored multiple articles for Federal Practitioner and provided guidance and direction for the 2017 and (forthcoming) 2018 editions of the public pathogens/infectious diseases special issues. Dr. Chartier is the deputy director for the HIV, Hepatitis, and Related Conditions Program in the VHA Office of Specialty Care Services and an assistant clinical professor at the University of California, San Francisco.
Marcia Johnson, DNP, FNP-BC, has been a highly active author and peer reviewer. Dr. Johnson has been a nurse practitioner at the VA for 18 years, and currently provides primary care at the Clermont CBOC in Florida. She previously served as the hepatitis C research coordinator at Philadelphia VAMC and practiced at the Orlando VAMC spinal cord injury clinic.
William Rodríguez-Cintrón, MD, is another active peer reviewer and a prolific contributor. Dr. Rodríguez-Cintrón is chief of the pulmonary, critical care and sleep medicine departments at the VA Caribbean Healthcare System in Puerto Rico.
Col. (Ret) Mona Pearl Treyball, PhD, MS, RN, USAF, also has been a dedicated peer reviewer. Col. Treyball is professor and specialty director of the
Federal Practitioner would like to thank all of the current and new members of the EAA for their continued support. All of the journal’s successes are built on their dedication and commitment. Federal Practitioner encourages all the members of the federal health care community to become more involved, whether as a peer reviewer, author, or by responding to our content in print, online, or via social media. Your feedback and involvement makes this journal better.