User login
T-cell lymphoma therapies on the horizon
LA JOLLA, CALIF. – There are several biologic compounds in early clinical development for treatment of patients with T-cell lymphomas, including an antibody-drug conjugate, novel immune checkpoint inhibitor, and bi-specific antibody.
These investigational agents show promising single-agent activity and have the potential to improve clinical responses when combined with combination chemotherapy regimens or other treatments, Ahmed Sawas, MD, of the Center for Lymphoid Malignancies at Columbia University, New York, said at the annual T-cell Lymphoma Forum.
AGS67E: Antibody-drug conjugate
AGS67E is an antibody-drug conjugate targeted against CD37, a transmembrane protein preferentially expressed on malignant B cells, T cells, and acute myeloid leukemia cells. In a study published in 2015 in Molecular Cancer Therapeutics, investigators from Agensys (an affiliate of Astellas Pharma) reported that this compound bound to more than 80% of patient-derived T cells in vitro (Mol Cancer Ther. 2015;14[7]:1650-60).
In a phase 1 dose-escalation study reported at the 2017 International Conference on Malignant Lymphoma in Lugano, Switzerland, Dr. Sawas and his colleagues found that patients with B-cell and T-cell malignancies, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma, tolerated the drug well when it was delivered both with or without growth factor. Neutropenia was the most frequent adverse event and dose-limiting toxicity.
The drug showed single-agent activity in 16 of 53 patients with heavily pretreated non-Hodgkin lymphoma, including a partial response in one of two patients with cutaneous T-cell lymphoma, and partial responses in two of four patients with peripheral T-cell lymphoma. There were no complete responses at any of three dose levels of the drug, with or without growth factor.
One patient, a 75-year-old man with stage IVB mycosis fungoides who had disease progression on prior therapy with methotrexate, romidepsin, bendamustine, whole-body irradiation, liposomal doxorubicin, pralatrexate, and pembrolizumab experienced significant reduction in tumor burden and resolution of lymph node involvement after three 3-week cycles of therapy with AGS67E. The patient had a deepening of the response with additional cycles, and remained on therapy for 30 cycles until he experienced disease progression.
TTI-621: Tuck in, macrophages
TT1-621 is a molecule with two functions: It acts as an immune checkpoint inhibitor by blocking CD47, which binds to signal-regulatory protein alpha to produce an antiphagocytic or “do not eat” signal. TTI-621 does not, however, bind to CD47-positive erythrocytes.
In addition to blocking CD47 and the do-not-eat signal, TTI-621 delivers an activating signal to macrophages through Fc gamma receptors, telling them, in effect, “bon appétit.”
In a study presented at the 2017 annual meeting of the American Society of Hematology (Abstract 4076), investigators from City of Hope in Duarte, Calif., and other centers reported that a single direct intratumoral injection of TTI-621 was associated with significant antitumor activity in patients with relapsed or refractory mycosis fungoides and Sézary syndrome, with one of nine patients having a complete response in the injected lesion, and five having decreases in tumor size and/or circulating Sézary cells.
Patients appeared to tolerate this agent very well, with 1 of 18 having a grade 3 increase in white blood cell count. The most commonly reported side effects were fatigue, chills, decreased appetite, headache, injection site pain, and generalized pruritus, each occurring in 3 of the 18 patients.
TTI-621 injection was associated with rapid declines in Composite Assessment of Index Lesion Severity scores in dose-finding studies in patients with heavily pretreated cutaneous T-cell lymphoma, Dr. Sawas said.
AFM13: Two for the price of one
AFM13 is a bi-specific antibody that binds to CD30, which is expressed on anaplastic large cell lymphoma cells, as well as Reed-Sternberg cells of classical Hodgkin lymphoma. This antibody also engages CD16A-positive cells, resulting in lysis of CD30-positive tumor cells. It is a specific recruiter of natural killer cells, and does not bind to neutrophils.
In an early biologic effects study of this agent in CD30-positive lymphoid malignancies with cutaneous presentation, Dr. Sawas and his colleagues observed an early response and regression of cutaneous anaplastic large cell lymphoma lesions in a heavily pretreated patient, with progression occurring when the patient went off therapy, and tumors that diminished on reinitiation of therapy that sustained beyond a second discontinuation of therapy. This patient had measurable reductions in lymphoma burden on PET CT scans and improvements in cutaneous lesions. Dr. Sawas did not present safety data for this agent.
AGS67E studies are supported by Agensys. TTI-621 studies are supported by Trillium Therapeutics. The AFM13 study is supported by Columbia University, with Dr. Sawas listed as the sponsor. He did not report potential conflicts of interests. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – There are several biologic compounds in early clinical development for treatment of patients with T-cell lymphomas, including an antibody-drug conjugate, novel immune checkpoint inhibitor, and bi-specific antibody.
These investigational agents show promising single-agent activity and have the potential to improve clinical responses when combined with combination chemotherapy regimens or other treatments, Ahmed Sawas, MD, of the Center for Lymphoid Malignancies at Columbia University, New York, said at the annual T-cell Lymphoma Forum.
AGS67E: Antibody-drug conjugate
AGS67E is an antibody-drug conjugate targeted against CD37, a transmembrane protein preferentially expressed on malignant B cells, T cells, and acute myeloid leukemia cells. In a study published in 2015 in Molecular Cancer Therapeutics, investigators from Agensys (an affiliate of Astellas Pharma) reported that this compound bound to more than 80% of patient-derived T cells in vitro (Mol Cancer Ther. 2015;14[7]:1650-60).
In a phase 1 dose-escalation study reported at the 2017 International Conference on Malignant Lymphoma in Lugano, Switzerland, Dr. Sawas and his colleagues found that patients with B-cell and T-cell malignancies, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma, tolerated the drug well when it was delivered both with or without growth factor. Neutropenia was the most frequent adverse event and dose-limiting toxicity.
The drug showed single-agent activity in 16 of 53 patients with heavily pretreated non-Hodgkin lymphoma, including a partial response in one of two patients with cutaneous T-cell lymphoma, and partial responses in two of four patients with peripheral T-cell lymphoma. There were no complete responses at any of three dose levels of the drug, with or without growth factor.
One patient, a 75-year-old man with stage IVB mycosis fungoides who had disease progression on prior therapy with methotrexate, romidepsin, bendamustine, whole-body irradiation, liposomal doxorubicin, pralatrexate, and pembrolizumab experienced significant reduction in tumor burden and resolution of lymph node involvement after three 3-week cycles of therapy with AGS67E. The patient had a deepening of the response with additional cycles, and remained on therapy for 30 cycles until he experienced disease progression.
TTI-621: Tuck in, macrophages
TT1-621 is a molecule with two functions: It acts as an immune checkpoint inhibitor by blocking CD47, which binds to signal-regulatory protein alpha to produce an antiphagocytic or “do not eat” signal. TTI-621 does not, however, bind to CD47-positive erythrocytes.
In addition to blocking CD47 and the do-not-eat signal, TTI-621 delivers an activating signal to macrophages through Fc gamma receptors, telling them, in effect, “bon appétit.”
In a study presented at the 2017 annual meeting of the American Society of Hematology (Abstract 4076), investigators from City of Hope in Duarte, Calif., and other centers reported that a single direct intratumoral injection of TTI-621 was associated with significant antitumor activity in patients with relapsed or refractory mycosis fungoides and Sézary syndrome, with one of nine patients having a complete response in the injected lesion, and five having decreases in tumor size and/or circulating Sézary cells.
Patients appeared to tolerate this agent very well, with 1 of 18 having a grade 3 increase in white blood cell count. The most commonly reported side effects were fatigue, chills, decreased appetite, headache, injection site pain, and generalized pruritus, each occurring in 3 of the 18 patients.
TTI-621 injection was associated with rapid declines in Composite Assessment of Index Lesion Severity scores in dose-finding studies in patients with heavily pretreated cutaneous T-cell lymphoma, Dr. Sawas said.
AFM13: Two for the price of one
AFM13 is a bi-specific antibody that binds to CD30, which is expressed on anaplastic large cell lymphoma cells, as well as Reed-Sternberg cells of classical Hodgkin lymphoma. This antibody also engages CD16A-positive cells, resulting in lysis of CD30-positive tumor cells. It is a specific recruiter of natural killer cells, and does not bind to neutrophils.
In an early biologic effects study of this agent in CD30-positive lymphoid malignancies with cutaneous presentation, Dr. Sawas and his colleagues observed an early response and regression of cutaneous anaplastic large cell lymphoma lesions in a heavily pretreated patient, with progression occurring when the patient went off therapy, and tumors that diminished on reinitiation of therapy that sustained beyond a second discontinuation of therapy. This patient had measurable reductions in lymphoma burden on PET CT scans and improvements in cutaneous lesions. Dr. Sawas did not present safety data for this agent.
AGS67E studies are supported by Agensys. TTI-621 studies are supported by Trillium Therapeutics. The AFM13 study is supported by Columbia University, with Dr. Sawas listed as the sponsor. He did not report potential conflicts of interests. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – There are several biologic compounds in early clinical development for treatment of patients with T-cell lymphomas, including an antibody-drug conjugate, novel immune checkpoint inhibitor, and bi-specific antibody.
These investigational agents show promising single-agent activity and have the potential to improve clinical responses when combined with combination chemotherapy regimens or other treatments, Ahmed Sawas, MD, of the Center for Lymphoid Malignancies at Columbia University, New York, said at the annual T-cell Lymphoma Forum.
AGS67E: Antibody-drug conjugate
AGS67E is an antibody-drug conjugate targeted against CD37, a transmembrane protein preferentially expressed on malignant B cells, T cells, and acute myeloid leukemia cells. In a study published in 2015 in Molecular Cancer Therapeutics, investigators from Agensys (an affiliate of Astellas Pharma) reported that this compound bound to more than 80% of patient-derived T cells in vitro (Mol Cancer Ther. 2015;14[7]:1650-60).
In a phase 1 dose-escalation study reported at the 2017 International Conference on Malignant Lymphoma in Lugano, Switzerland, Dr. Sawas and his colleagues found that patients with B-cell and T-cell malignancies, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma, tolerated the drug well when it was delivered both with or without growth factor. Neutropenia was the most frequent adverse event and dose-limiting toxicity.
The drug showed single-agent activity in 16 of 53 patients with heavily pretreated non-Hodgkin lymphoma, including a partial response in one of two patients with cutaneous T-cell lymphoma, and partial responses in two of four patients with peripheral T-cell lymphoma. There were no complete responses at any of three dose levels of the drug, with or without growth factor.
One patient, a 75-year-old man with stage IVB mycosis fungoides who had disease progression on prior therapy with methotrexate, romidepsin, bendamustine, whole-body irradiation, liposomal doxorubicin, pralatrexate, and pembrolizumab experienced significant reduction in tumor burden and resolution of lymph node involvement after three 3-week cycles of therapy with AGS67E. The patient had a deepening of the response with additional cycles, and remained on therapy for 30 cycles until he experienced disease progression.
TTI-621: Tuck in, macrophages
TT1-621 is a molecule with two functions: It acts as an immune checkpoint inhibitor by blocking CD47, which binds to signal-regulatory protein alpha to produce an antiphagocytic or “do not eat” signal. TTI-621 does not, however, bind to CD47-positive erythrocytes.
In addition to blocking CD47 and the do-not-eat signal, TTI-621 delivers an activating signal to macrophages through Fc gamma receptors, telling them, in effect, “bon appétit.”
In a study presented at the 2017 annual meeting of the American Society of Hematology (Abstract 4076), investigators from City of Hope in Duarte, Calif., and other centers reported that a single direct intratumoral injection of TTI-621 was associated with significant antitumor activity in patients with relapsed or refractory mycosis fungoides and Sézary syndrome, with one of nine patients having a complete response in the injected lesion, and five having decreases in tumor size and/or circulating Sézary cells.
Patients appeared to tolerate this agent very well, with 1 of 18 having a grade 3 increase in white blood cell count. The most commonly reported side effects were fatigue, chills, decreased appetite, headache, injection site pain, and generalized pruritus, each occurring in 3 of the 18 patients.
TTI-621 injection was associated with rapid declines in Composite Assessment of Index Lesion Severity scores in dose-finding studies in patients with heavily pretreated cutaneous T-cell lymphoma, Dr. Sawas said.
AFM13: Two for the price of one
AFM13 is a bi-specific antibody that binds to CD30, which is expressed on anaplastic large cell lymphoma cells, as well as Reed-Sternberg cells of classical Hodgkin lymphoma. This antibody also engages CD16A-positive cells, resulting in lysis of CD30-positive tumor cells. It is a specific recruiter of natural killer cells, and does not bind to neutrophils.
In an early biologic effects study of this agent in CD30-positive lymphoid malignancies with cutaneous presentation, Dr. Sawas and his colleagues observed an early response and regression of cutaneous anaplastic large cell lymphoma lesions in a heavily pretreated patient, with progression occurring when the patient went off therapy, and tumors that diminished on reinitiation of therapy that sustained beyond a second discontinuation of therapy. This patient had measurable reductions in lymphoma burden on PET CT scans and improvements in cutaneous lesions. Dr. Sawas did not present safety data for this agent.
AGS67E studies are supported by Agensys. TTI-621 studies are supported by Trillium Therapeutics. The AFM13 study is supported by Columbia University, with Dr. Sawas listed as the sponsor. He did not report potential conflicts of interests. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2018
Prazosin falls short for veterans’ PTSD-related sleep problems
The alpha-1 adrenergic receptor prazosin failed to improve recurring nightmares or sleep quality compared with placebo in veterans with PTSD in a 26-week randomized trial of 304 adult veterans.
In several previous randomized trials lasting fewer than 15 weeks, veterans with PTSD and recurring nightmares who received prazosin showed benefits, including improved sleep quality and PTSD symptoms, compared with placebo patients, wrote Murray A. Raskind, MD, of the Department of Veterans Affairs Puget Sound Health Care System, Seattle, and his colleagues.
In a study published in the New England Journal of Medicine, the researchers randomized 152 veterans with sleep problems and PTSD to prazosin and 152 to a placebo. The participants were recruited from 12 VA medical centers. The average age of the participants was 52 years, more than 96% were male, and about two-thirds were white. Demographics were similar between the two groups.
After 10 weeks and after 26 weeks, there were no significant differences between the two groups in changes from baseline measures of recurring nightmares, using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). Similarly, no significant differences appeared between the two groups based on Pittsburgh Sleep Quality Index scores.
“A possible explanation for these negative results is selection bias resulting from recruitment of patients who were mainly in clinically stable condition, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” reported Dr. Raskind and his colleagues.
The average maintenance dose of prazosin was 14.8 mg, compared with 16.4 mg in the placebo group; 187 male study participants reached the maximum dose of 20 mg/day (54% of the prazosin group and 70% of the placebo group).
After 10 weeks, no significant differences were found between the two groups in changes from baseline measures of “recurring distressing dreams,” using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). The between group difference was 0.2. In addition, no significant differences were found at 10 weeks in the average change from baseline Pittsburgh Sleep Quality Index scores.
Similarly, no significant differences appeared between the two groups at 26 weeks. “ since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” the researchers said.
On average, patients in the prazosin group had significantly greater decreases in blood pressure, compared with the placebo group. In addition, they had fewer reports of new or worsening suicidal ideation, compared with the placebo group (8% vs.15%).
“Given the concern about suicide among veterans, it is noteworthy that the specifically solicited adverse event of new or worsening suicidal ideation was less common in the prazosin group than in the placebo group, but the absolute number of events was small; this issue warrants further study,” the researchers said.
The study was limited by several factors, including the absence of screening for sleep apnea or sleep-disordered breathing, Dr. Raskind and his colleagues noted. However, the results suggest that “further studies with more refined characterization of autonomic nervous system activity and nocturnal behaviors are needed to determine whether there might be subgroups of veterans with PTSD who can benefit from prazosin.”
Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
SOURCE: Raskind MA et al. N Engl J Med. 2018 Feb 8;378:507-17. doi: 10.1056/NEJMoa1507598.
The alpha-1 adrenergic receptor prazosin failed to improve recurring nightmares or sleep quality compared with placebo in veterans with PTSD in a 26-week randomized trial of 304 adult veterans.
In several previous randomized trials lasting fewer than 15 weeks, veterans with PTSD and recurring nightmares who received prazosin showed benefits, including improved sleep quality and PTSD symptoms, compared with placebo patients, wrote Murray A. Raskind, MD, of the Department of Veterans Affairs Puget Sound Health Care System, Seattle, and his colleagues.
In a study published in the New England Journal of Medicine, the researchers randomized 152 veterans with sleep problems and PTSD to prazosin and 152 to a placebo. The participants were recruited from 12 VA medical centers. The average age of the participants was 52 years, more than 96% were male, and about two-thirds were white. Demographics were similar between the two groups.
After 10 weeks and after 26 weeks, there were no significant differences between the two groups in changes from baseline measures of recurring nightmares, using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). Similarly, no significant differences appeared between the two groups based on Pittsburgh Sleep Quality Index scores.
“A possible explanation for these negative results is selection bias resulting from recruitment of patients who were mainly in clinically stable condition, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” reported Dr. Raskind and his colleagues.
The average maintenance dose of prazosin was 14.8 mg, compared with 16.4 mg in the placebo group; 187 male study participants reached the maximum dose of 20 mg/day (54% of the prazosin group and 70% of the placebo group).
After 10 weeks, no significant differences were found between the two groups in changes from baseline measures of “recurring distressing dreams,” using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). The between group difference was 0.2. In addition, no significant differences were found at 10 weeks in the average change from baseline Pittsburgh Sleep Quality Index scores.
Similarly, no significant differences appeared between the two groups at 26 weeks. “ since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” the researchers said.
On average, patients in the prazosin group had significantly greater decreases in blood pressure, compared with the placebo group. In addition, they had fewer reports of new or worsening suicidal ideation, compared with the placebo group (8% vs.15%).
“Given the concern about suicide among veterans, it is noteworthy that the specifically solicited adverse event of new or worsening suicidal ideation was less common in the prazosin group than in the placebo group, but the absolute number of events was small; this issue warrants further study,” the researchers said.
The study was limited by several factors, including the absence of screening for sleep apnea or sleep-disordered breathing, Dr. Raskind and his colleagues noted. However, the results suggest that “further studies with more refined characterization of autonomic nervous system activity and nocturnal behaviors are needed to determine whether there might be subgroups of veterans with PTSD who can benefit from prazosin.”
Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
SOURCE: Raskind MA et al. N Engl J Med. 2018 Feb 8;378:507-17. doi: 10.1056/NEJMoa1507598.
The alpha-1 adrenergic receptor prazosin failed to improve recurring nightmares or sleep quality compared with placebo in veterans with PTSD in a 26-week randomized trial of 304 adult veterans.
In several previous randomized trials lasting fewer than 15 weeks, veterans with PTSD and recurring nightmares who received prazosin showed benefits, including improved sleep quality and PTSD symptoms, compared with placebo patients, wrote Murray A. Raskind, MD, of the Department of Veterans Affairs Puget Sound Health Care System, Seattle, and his colleagues.
In a study published in the New England Journal of Medicine, the researchers randomized 152 veterans with sleep problems and PTSD to prazosin and 152 to a placebo. The participants were recruited from 12 VA medical centers. The average age of the participants was 52 years, more than 96% were male, and about two-thirds were white. Demographics were similar between the two groups.
After 10 weeks and after 26 weeks, there were no significant differences between the two groups in changes from baseline measures of recurring nightmares, using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). Similarly, no significant differences appeared between the two groups based on Pittsburgh Sleep Quality Index scores.
“A possible explanation for these negative results is selection bias resulting from recruitment of patients who were mainly in clinically stable condition, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” reported Dr. Raskind and his colleagues.
The average maintenance dose of prazosin was 14.8 mg, compared with 16.4 mg in the placebo group; 187 male study participants reached the maximum dose of 20 mg/day (54% of the prazosin group and 70% of the placebo group).
After 10 weeks, no significant differences were found between the two groups in changes from baseline measures of “recurring distressing dreams,” using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). The between group difference was 0.2. In addition, no significant differences were found at 10 weeks in the average change from baseline Pittsburgh Sleep Quality Index scores.
Similarly, no significant differences appeared between the two groups at 26 weeks. “ since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” the researchers said.
On average, patients in the prazosin group had significantly greater decreases in blood pressure, compared with the placebo group. In addition, they had fewer reports of new or worsening suicidal ideation, compared with the placebo group (8% vs.15%).
“Given the concern about suicide among veterans, it is noteworthy that the specifically solicited adverse event of new or worsening suicidal ideation was less common in the prazosin group than in the placebo group, but the absolute number of events was small; this issue warrants further study,” the researchers said.
The study was limited by several factors, including the absence of screening for sleep apnea or sleep-disordered breathing, Dr. Raskind and his colleagues noted. However, the results suggest that “further studies with more refined characterization of autonomic nervous system activity and nocturnal behaviors are needed to determine whether there might be subgroups of veterans with PTSD who can benefit from prazosin.”
Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
SOURCE: Raskind MA et al. N Engl J Med. 2018 Feb 8;378:507-17. doi: 10.1056/NEJMoa1507598.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Prazosin had no apparent effect on recurrent distressing dreams or sleep quality in veterans with PTSD.
Major finding: The between-group difference in scores on a measure of “recurrent distressing dreams” between the prazosin and placebo groups was a nonsignificant 0.2.
Study details: The data come from a randomized trial of 304 military veterans with PTSD who reported frequent nightmares.
Disclosures: Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
Source: Raskind MA et al. N Engl J Med. 2018;378:507-17.
Panniculitis, Pancreatitis, and Polyarthritis: A Rare Clinical Syndrome
Pancreatic panniculitis is a rare disease contributing to widespread fat necrosis in patients with underlying pancreatic disorders. This entity was first described in 1883,1 but it was not until 1947 that it was reported in the English-language literature.2 Patients with pancreatitis infrequently develop extrapancreatic manifestations. It has been estimated that only 2% to 3% of patients worldwide with an underlying pancreatic disease develop cutaneous lesions.3 Patients who develop pancreatic panniculitis typically present with tender, edematous, erythematous to brown, subcutaneous nodules on the lower legs with the tendency for spontaneous ulceration. Lesions tend to exude a viscous, yellow-brown, oily substance that represents liquefactive necrosis of enzymatic fat in subcutaneous tissue. Cutaneous lesions may precede, occur simultaneously, or follow the development of an underlying pancreatic disorder. Rarely, patients may develop inflammatory arthritis secondary to intraosseous fat necrosis, completing the triad of findings diagnostic for panniculitis, pancreatitis, and polyarthritis (PPP) syndrome. Although the underlying pancreatic pathology may vary, roughly 80% of cases worldwide have acute/chronic pancreatitis or pancreatic carcinoma, most commonly acinar cell carcinoma.4-6 Less common pancreatic disorders include pancreatic pseudocyst, pancreatic divisum, and vascular pancreatic fistulas.7 Narváez et al8 found that of the 25 cases of PPP syndrome reported in the literature, 68% (17/25) were men, 32% (8/25) were women, 56% (14/25) were younger than 50 years, and 64% (16/25) had a history of prior or current alcohol abuse.
Case Report
A 68-year-old man with a history of hypertension, gastroesophageal reflux disease, chronic pancreatitis of unknown etiology, and arthritis presented to our clinic for evaluation of painful skin nodules on the lower legs of 8 months’ duration, in addition to joint pain and swelling of the metacarpophalangeal (MCP), metatarsophalangeal, and ankle joints. He had a history of numerous hospital admissions over the last 2 years for pancreatitis and was being managed by the rheumatology department for arthritic symptoms.
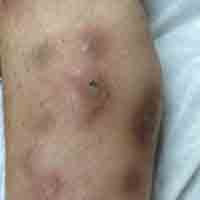
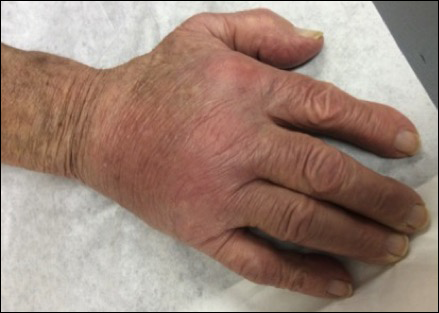
Physical examination revealed multiple 1- to 4-cm, ill-defined, erythematous to brown, subcutaneous nodules on the bilateral lower legs (Figure 1) and right inferomedial thigh that were tender to palpation. Marked erythema and edema of the MCP and metatarsophalangeal joints (Figure 2) and bilateral ankles were observed. Diffuse 2+ pitting edema was present in the bilateral lower extremities, along with areas of hyperpigmentation overlying resolving lesions.

Laboratory data revealed an elevated lipase level (>16,000 U/L [reference range, 31–186 U/L]), amylase level (>4700 U/L [reference range, 27–131 U/L]), erythrocyte sedimentation rate (94 mm/h [reference range, 0–20 mm/h]), and C-reactive protein level (93.5 mg/L [0.08–3.1 mg/L]). The patient had more than 6 episodes of recurrent idiopathic pancreatitis over the last 2 years, though symptoms of abdominal pain were minimal to nonexistent. Liver function tests and alcohol, calcium, and triglyceride levels all were within reference range. Rheumatoid factor and antinuclear antibodies were negative.
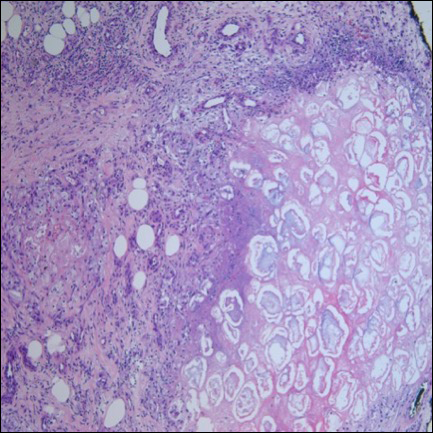
Ultrasonography showed no evidence of cholelithiasis. Computed tomography of the abdomen and pelvis demonstrated a 1.8×1.4-cm hypodense lesion within the pancreatic head with calcifications and mild proximal pancreatic ductal dilatation (Figure 3). However, multiple magnetic resonance cholangiopancreatography examinations and endoscopic ultrasounds with fine-needle aspiration specimens were performed, all negative for malignancy. Computed tomography of the left ankle demonstrated evidence of bony cortical destruction in the lateral aspect of the posterior calcaneus. Bone biopsy specimens demonstrated mild chronic inflammation with no evidence of osteomyelitis. A serum uric acid level was found to be 4.4 mg/dL (reference range, 4.0–8.0 mg/dL) and a joint aspirate demonstrated turbid fluid with lipoid material and no evidence of crystals or organisms on culture. Furthermore, a 4-mm punch biopsy of a nodule on the right leg revealed extensive lobular and septal liquefactive adipocyte necrosis with scattered neutrophils and lymphocytes (Figure 4). Aggregates of fine granular basophilic material were observed with prominent adipocyte degeneration and calcification.

Symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) along with intralesional, topical, and oral corticosteroids had proven ineffective in the management of this patient. He was subsequently referred to the surgery department for a pancreaticoduodenectomy (Whipple procedure) with notable improvement in pancreatic enzyme levels, lower leg subcutaneous nodules, and arthritis weeks after surgery.
Comment
A triad of pancreatic panniculitis, pancreatitis, and polyarthritis characterizes a rare entity known as PPP syndrome. Pancreatic panniculitis is a rare form of subcutaneous lobular fat necrosis associated with various underlying pancreatic disorders. Approximately 0.3% to 3.0% of patients with an underlying pancreatic disorder are affected with pancreatic panniculitis.9 Pancreatic panniculitis has been found in roughly 2% to 3% of patients with acute or chronic pancreatitis and pancreatic carcinoma, most commonly the acinar cell type.10 Narváez et al8 reported that nearly two-thirds of patients diagnosed with PPP syndrome have minimal to absent abdominal symptoms that often lead to misdiagnosis and affect the overall prognosis of patients with pancreatic disease. Any delay in the diagnosis of PPP syndrome leads to a worse prognosis, with a mortality rate reported to be approximately 24%.8 Potts et al5 provided a review of 27 patients with pancreatic panniculitis in which all 8 patients with pancreatic carcinoma and 42% (8/19) of patients with pancreatitis died.
Pancreatic panniculitis in the setting of PPP syndrome commonly presents with erythematous to brown, exquisitely tender, edematous, subcutaneous nodules on the lower legs. Lesions can range in size from several millimeters to 5 cm. The subcutaneous nodules may spontaneously ulcerate and exude oily viscous material from the liquefactive necrosis of adipocytes. In approximately 40% of patients, skin lesions are the presenting feature.11 Lesions typically resolve only after the pancreatic inflammation regresses, leaving behind atrophic hyperpigmented scars.3 Other presenting symptoms may include joint pain, pitting edema, and subcutaneous nodules, which can precede the diagnosis by up to 9 months.
The exact pathogenesis of PPP syndrome remains unclear. The most widely recognized hypothesis suggests that pancreatic enzymes (eg, trypsin, amylase, lipase, phospholipase A) released from the damaged pancreas are transported through the bloodstream to distant visceral and soft tissue sites, leading to lipolysis and inflammation to the surrounding subcutis and bone marrow.3 Ferrari et al12 reported this effect as a product of the accumulation of high levels of free fatty acids within the joint space by the action of lipolytic pancreatic enzymes on adipose cell membranes, resulting in acute arthritis.
Histopathologic findings of pancreatic panniculitis vary based on the acuity of the disease. Acute lesions typically demonstrate lobular and septal panniculitis. Szymanski and Bluefarb13 described the pathognomonic histologic findings of focal liquefactive necrosis and anucleate necrotic adipocytes surrounded by a shadowy and thickened cell membrane signifying the characteristic ghost cells. Fine basophilic material also may be seen intermixed with the necrotic adipocytes, representing saponified calcium. A brisk inflammatory infiltrate involving lymphocytes, macrophages, and neutrophils tends to surround the areas of necrotic adipocytes. Chronic lesions often demonstrate a paucity of fat necrosis and ghost cells and more granulomatous infiltrate. Langerhans giant cells, macrophages, and lymphocytes predominate in the subcutaneous fat.
Laboratory findings associated with pancreatic panniculitis may include elevated serum amylase, lipase, and/or trypsin levels. Not all the enzymes have to be elevated simultaneously. On occasion, one enzyme may be within reference range while the others are elevated. Rarely, patients may have an elevated lipase level with no signs of underlying pancreatic disease, which demonstrates that panniculitis does not correlate with the enzyme levels. In all cases of suspected pancreatic panniculitis, a complete laboratory workup is recommended including lipase, amylase, and trypsin serum levels. Eosinophilia may be a prominent finding in patients with pancreatic panniculitis and tends to occur in association with an underlying pancreatic carcinoma. Patients with pancreatic panniculitis associated with pancreatic carcinoma tend to have more severe, diffuse, and persistent subcutaneous nodules that often are refractory to treatment with frequent recurrence. A rare constellation of findings known as Schmid triad is comprised of panniculitis, polyarthritis, and eosinophilia and typically portends a poor prognosis secondary to an underlying pancreatic tumor.14 Cutaneous nodules may predate the diagnosis of pancreatic carcinoma by several months, thus signifying the need for a high index of suspicion in patients with lower leg subcutaneous nodules.
Joint disease most commonly involves the ankles, knees, wrists, and MCP joints.5,6,11 It has been suggested that arthritic symptoms are from periarticular fat necrosis or a direct extension from the necrotic subcutaneous tissue to the adjacent joint space.15 Dahl et al3 reported the composition of joint effusion fluid in 3 patients with PPP syndrome. The aspirate in all 3 patients contained viscous yellow material similar to the necrotic adipose tissue seen draining from subcutaneous nodules. Joint aspirate analysis demonstrated increased concentration of free fatty acids in the joint fluid consistent with severe lipolysis.3
The PPP syndrome acronym may be misleading to physicians, as arthritis is not always polyarticular. Dahl et al3 reported that monoarticular or oligoarticular arthritic symptoms were present in 56% of patients studied. In rare cases, the arthritic symptoms antedated the diagnosis of clinically asymptomatic pancreatic disease. Arthritis can be either symmetric or asymmetric and infrequently follows a chronic course, leading to radiographic lytic lesions and symptoms that often are unresponsive to conventional therapy.16
Treatment of PPP syndrome is largely supportive, with a focus on correcting the underlying pancreatic disease. It is imperative to identify any complicating factors contributing to high levels of circulating pancreatic enzymes. Pseudocysts must be addressed if discovered in these patients, as they often perpetuate the substantial release of pancreatic enzymes into the serum, leading to characteristic subcutaneous fat necrosis and arthritis. Sepsis also is a concern, likely secondary to bacterial colonization of the ulcerated subcutaneous nodules and compromised skin barrier. Nonsteroidal anti-inflammatory drugs and corticosteroids have been used for symptomatic relief but usually are ineffective and have not been shown to reduce the duration of the disease.12,16 Octreotide has been utilized and may potentially reduce pancreatic enzyme secretion leading to improvement in cutaneous and musculoskeletal lesions.17 Plasmapheresis has been used as an adjuvant treatment in patients with persistent hyperamylasemia and hyperlipasemia, but reports are anecdotal. Often reserved for severe disease, cholecystectomy, pancreatic duct removal, and pancreaticoduodenectomy have demonstrated success in the management of chronic pancreatitis and panniculitis. Dahl et al3 reported 2 cases in which cholecystectomy was performed with complete resolution of the skin and pancreatic disease. Our patient was initially treated symptomatically with NSAIDs and corticosteroids but there was no clinical response. The patient eventually underwent a pancreaticoduodenectomy 9 months after the onset of symptoms with complete resolution of joint pain and swelling, greater than 50% resolution of his lower leg subcutaneous nodules, and remarkable reduction in amylase and lipase levels on 1-month follow-up.
Conclusion
Panniculitis, pancreatitis, and polyarthritis syndrome is a rare diagnosis characterized by a triad of pancreatic panniculitis, pancreatitis, and polyarthritis. Adjuvant therapies for PPP syndrome, such as NSAIDs, corticosteroids, plasmapheresis, and octreotide, have been used with equivocal results, but definitive treatment requires correction of the primary pancreatic disorder. More importantly, many pancreatic diseases can cause pancreatic panniculitis, but extensive, refractory, or ulcerated cases could be an early indicator of an occult pancreatic malignancy and should prompt early evaluation with a multidisciplinary approach. This approach should incorporate management from dermatology, internal medicine, rheumatology, gastroenterology, surgery, and primary care.
- Chiari H. Uber die Sogenannte Fettnekrose. Prag Med Wochenschr. 1883;8:285-286, 299-301.
- Blauvelt H. Case of acute pancreatitis with subcutaneous fat necrosis. Br J Surg. 1946;34:207-208.
- Dahl PR, Su D, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
- Mullen GT, Caperton EM Jr, Crespin SR, et al. Arthritis and skin lesions resembling erythema nodosum in pancreatic disease. Ann Intern Med. 1968;68:75-87.
- Potts DE, Mass MF, Iseman MD. Syndrome and pancreatic disease, subcutaneous fat necrosis and polyserositis: case report and review of literature. Am J Med. 1975;58:417-423.
- Sorensen EV. Subcutaneous fat necrosis in pancreatic disease: a review and two new case reports. J Clin Gastroenterol. 1988;10:71-75.
- García-Romero D, Vanaclocha F. Pancreatic panniculitis. Dermatol Clin. 2008;26:465-470.
- Narváez J, Bianchi M, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum. 2010;39:417-423.
- Rongioetti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
- Poelman SM, Nguyen K. Pancreatic panniculitis associated with acinar cell pancreatic carcinoma. J Cutan Med Surg. 2008;12:38-42.
- Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975:111:506-510.
- Ferrari R, Wendelboe M, Ford PM, et al. Pancreatitis arthritis with periarticular fat necrosis. J Rheumatol. 1993;20:1436-1437.
- Szymanski FJ, Bluefarb SM. Nodular fat necrosis and pancreatic diseases. Arch Dermatol. 1961;83:224-229.
- Beltraminelly HS, Buechner SA, Hausermann P. Pancreatic panniculitis in a patient with an acinar cell cystadenocarcinoma of the pancreas. Dermatology. 2004;208:265-267.
- Burns WA, Matthews MJ, Hamosh M, et al. Lipase-secreting acinar cell carcinoma of the pancreas with polyarthropathy: a light and electron microscopic, histochemical, and biochemical study. Cancer. 1974;33:1002-1009.
- Baron M, Paltiel H, Lander P. Aseptic necrosis of the talus and calcaneal insufficiency fractures in a patient with pancreatitis, subcutaneous fat necrosis, and arthritis. Arthritis Rheum. 1984;27:1309-1313.
- Zundler S, Erber R, Agaimy A, et al. Pancreatic panniculitis in a patient with pancreatic-type acinar cell carcinoma of the liver—case report and review of literature. BMC Cancer. 2016;16:130.
Pancreatic panniculitis is a rare disease contributing to widespread fat necrosis in patients with underlying pancreatic disorders. This entity was first described in 1883,1 but it was not until 1947 that it was reported in the English-language literature.2 Patients with pancreatitis infrequently develop extrapancreatic manifestations. It has been estimated that only 2% to 3% of patients worldwide with an underlying pancreatic disease develop cutaneous lesions.3 Patients who develop pancreatic panniculitis typically present with tender, edematous, erythematous to brown, subcutaneous nodules on the lower legs with the tendency for spontaneous ulceration. Lesions tend to exude a viscous, yellow-brown, oily substance that represents liquefactive necrosis of enzymatic fat in subcutaneous tissue. Cutaneous lesions may precede, occur simultaneously, or follow the development of an underlying pancreatic disorder. Rarely, patients may develop inflammatory arthritis secondary to intraosseous fat necrosis, completing the triad of findings diagnostic for panniculitis, pancreatitis, and polyarthritis (PPP) syndrome. Although the underlying pancreatic pathology may vary, roughly 80% of cases worldwide have acute/chronic pancreatitis or pancreatic carcinoma, most commonly acinar cell carcinoma.4-6 Less common pancreatic disorders include pancreatic pseudocyst, pancreatic divisum, and vascular pancreatic fistulas.7 Narváez et al8 found that of the 25 cases of PPP syndrome reported in the literature, 68% (17/25) were men, 32% (8/25) were women, 56% (14/25) were younger than 50 years, and 64% (16/25) had a history of prior or current alcohol abuse.
Case Report
A 68-year-old man with a history of hypertension, gastroesophageal reflux disease, chronic pancreatitis of unknown etiology, and arthritis presented to our clinic for evaluation of painful skin nodules on the lower legs of 8 months’ duration, in addition to joint pain and swelling of the metacarpophalangeal (MCP), metatarsophalangeal, and ankle joints. He had a history of numerous hospital admissions over the last 2 years for pancreatitis and was being managed by the rheumatology department for arthritic symptoms.
Physical examination revealed multiple 1- to 4-cm, ill-defined, erythematous to brown, subcutaneous nodules on the bilateral lower legs (Figure 1) and right inferomedial thigh that were tender to palpation. Marked erythema and edema of the MCP and metatarsophalangeal joints (Figure 2) and bilateral ankles were observed. Diffuse 2+ pitting edema was present in the bilateral lower extremities, along with areas of hyperpigmentation overlying resolving lesions.


Laboratory data revealed an elevated lipase level (>16,000 U/L [reference range, 31–186 U/L]), amylase level (>4700 U/L [reference range, 27–131 U/L]), erythrocyte sedimentation rate (94 mm/h [reference range, 0–20 mm/h]), and C-reactive protein level (93.5 mg/L [0.08–3.1 mg/L]). The patient had more than 6 episodes of recurrent idiopathic pancreatitis over the last 2 years, though symptoms of abdominal pain were minimal to nonexistent. Liver function tests and alcohol, calcium, and triglyceride levels all were within reference range. Rheumatoid factor and antinuclear antibodies were negative.
Ultrasonography showed no evidence of cholelithiasis. Computed tomography of the abdomen and pelvis demonstrated a 1.8×1.4-cm hypodense lesion within the pancreatic head with calcifications and mild proximal pancreatic ductal dilatation (Figure 3). However, multiple magnetic resonance cholangiopancreatography examinations and endoscopic ultrasounds with fine-needle aspiration specimens were performed, all negative for malignancy. Computed tomography of the left ankle demonstrated evidence of bony cortical destruction in the lateral aspect of the posterior calcaneus. Bone biopsy specimens demonstrated mild chronic inflammation with no evidence of osteomyelitis. A serum uric acid level was found to be 4.4 mg/dL (reference range, 4.0–8.0 mg/dL) and a joint aspirate demonstrated turbid fluid with lipoid material and no evidence of crystals or organisms on culture. Furthermore, a 4-mm punch biopsy of a nodule on the right leg revealed extensive lobular and septal liquefactive adipocyte necrosis with scattered neutrophils and lymphocytes (Figure 4). Aggregates of fine granular basophilic material were observed with prominent adipocyte degeneration and calcification.


Symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) along with intralesional, topical, and oral corticosteroids had proven ineffective in the management of this patient. He was subsequently referred to the surgery department for a pancreaticoduodenectomy (Whipple procedure) with notable improvement in pancreatic enzyme levels, lower leg subcutaneous nodules, and arthritis weeks after surgery.
Comment
A triad of pancreatic panniculitis, pancreatitis, and polyarthritis characterizes a rare entity known as PPP syndrome. Pancreatic panniculitis is a rare form of subcutaneous lobular fat necrosis associated with various underlying pancreatic disorders. Approximately 0.3% to 3.0% of patients with an underlying pancreatic disorder are affected with pancreatic panniculitis.9 Pancreatic panniculitis has been found in roughly 2% to 3% of patients with acute or chronic pancreatitis and pancreatic carcinoma, most commonly the acinar cell type.10 Narváez et al8 reported that nearly two-thirds of patients diagnosed with PPP syndrome have minimal to absent abdominal symptoms that often lead to misdiagnosis and affect the overall prognosis of patients with pancreatic disease. Any delay in the diagnosis of PPP syndrome leads to a worse prognosis, with a mortality rate reported to be approximately 24%.8 Potts et al5 provided a review of 27 patients with pancreatic panniculitis in which all 8 patients with pancreatic carcinoma and 42% (8/19) of patients with pancreatitis died.
Pancreatic panniculitis in the setting of PPP syndrome commonly presents with erythematous to brown, exquisitely tender, edematous, subcutaneous nodules on the lower legs. Lesions can range in size from several millimeters to 5 cm. The subcutaneous nodules may spontaneously ulcerate and exude oily viscous material from the liquefactive necrosis of adipocytes. In approximately 40% of patients, skin lesions are the presenting feature.11 Lesions typically resolve only after the pancreatic inflammation regresses, leaving behind atrophic hyperpigmented scars.3 Other presenting symptoms may include joint pain, pitting edema, and subcutaneous nodules, which can precede the diagnosis by up to 9 months.
The exact pathogenesis of PPP syndrome remains unclear. The most widely recognized hypothesis suggests that pancreatic enzymes (eg, trypsin, amylase, lipase, phospholipase A) released from the damaged pancreas are transported through the bloodstream to distant visceral and soft tissue sites, leading to lipolysis and inflammation to the surrounding subcutis and bone marrow.3 Ferrari et al12 reported this effect as a product of the accumulation of high levels of free fatty acids within the joint space by the action of lipolytic pancreatic enzymes on adipose cell membranes, resulting in acute arthritis.
Histopathologic findings of pancreatic panniculitis vary based on the acuity of the disease. Acute lesions typically demonstrate lobular and septal panniculitis. Szymanski and Bluefarb13 described the pathognomonic histologic findings of focal liquefactive necrosis and anucleate necrotic adipocytes surrounded by a shadowy and thickened cell membrane signifying the characteristic ghost cells. Fine basophilic material also may be seen intermixed with the necrotic adipocytes, representing saponified calcium. A brisk inflammatory infiltrate involving lymphocytes, macrophages, and neutrophils tends to surround the areas of necrotic adipocytes. Chronic lesions often demonstrate a paucity of fat necrosis and ghost cells and more granulomatous infiltrate. Langerhans giant cells, macrophages, and lymphocytes predominate in the subcutaneous fat.
Laboratory findings associated with pancreatic panniculitis may include elevated serum amylase, lipase, and/or trypsin levels. Not all the enzymes have to be elevated simultaneously. On occasion, one enzyme may be within reference range while the others are elevated. Rarely, patients may have an elevated lipase level with no signs of underlying pancreatic disease, which demonstrates that panniculitis does not correlate with the enzyme levels. In all cases of suspected pancreatic panniculitis, a complete laboratory workup is recommended including lipase, amylase, and trypsin serum levels. Eosinophilia may be a prominent finding in patients with pancreatic panniculitis and tends to occur in association with an underlying pancreatic carcinoma. Patients with pancreatic panniculitis associated with pancreatic carcinoma tend to have more severe, diffuse, and persistent subcutaneous nodules that often are refractory to treatment with frequent recurrence. A rare constellation of findings known as Schmid triad is comprised of panniculitis, polyarthritis, and eosinophilia and typically portends a poor prognosis secondary to an underlying pancreatic tumor.14 Cutaneous nodules may predate the diagnosis of pancreatic carcinoma by several months, thus signifying the need for a high index of suspicion in patients with lower leg subcutaneous nodules.
Joint disease most commonly involves the ankles, knees, wrists, and MCP joints.5,6,11 It has been suggested that arthritic symptoms are from periarticular fat necrosis or a direct extension from the necrotic subcutaneous tissue to the adjacent joint space.15 Dahl et al3 reported the composition of joint effusion fluid in 3 patients with PPP syndrome. The aspirate in all 3 patients contained viscous yellow material similar to the necrotic adipose tissue seen draining from subcutaneous nodules. Joint aspirate analysis demonstrated increased concentration of free fatty acids in the joint fluid consistent with severe lipolysis.3
The PPP syndrome acronym may be misleading to physicians, as arthritis is not always polyarticular. Dahl et al3 reported that monoarticular or oligoarticular arthritic symptoms were present in 56% of patients studied. In rare cases, the arthritic symptoms antedated the diagnosis of clinically asymptomatic pancreatic disease. Arthritis can be either symmetric or asymmetric and infrequently follows a chronic course, leading to radiographic lytic lesions and symptoms that often are unresponsive to conventional therapy.16
Treatment of PPP syndrome is largely supportive, with a focus on correcting the underlying pancreatic disease. It is imperative to identify any complicating factors contributing to high levels of circulating pancreatic enzymes. Pseudocysts must be addressed if discovered in these patients, as they often perpetuate the substantial release of pancreatic enzymes into the serum, leading to characteristic subcutaneous fat necrosis and arthritis. Sepsis also is a concern, likely secondary to bacterial colonization of the ulcerated subcutaneous nodules and compromised skin barrier. Nonsteroidal anti-inflammatory drugs and corticosteroids have been used for symptomatic relief but usually are ineffective and have not been shown to reduce the duration of the disease.12,16 Octreotide has been utilized and may potentially reduce pancreatic enzyme secretion leading to improvement in cutaneous and musculoskeletal lesions.17 Plasmapheresis has been used as an adjuvant treatment in patients with persistent hyperamylasemia and hyperlipasemia, but reports are anecdotal. Often reserved for severe disease, cholecystectomy, pancreatic duct removal, and pancreaticoduodenectomy have demonstrated success in the management of chronic pancreatitis and panniculitis. Dahl et al3 reported 2 cases in which cholecystectomy was performed with complete resolution of the skin and pancreatic disease. Our patient was initially treated symptomatically with NSAIDs and corticosteroids but there was no clinical response. The patient eventually underwent a pancreaticoduodenectomy 9 months after the onset of symptoms with complete resolution of joint pain and swelling, greater than 50% resolution of his lower leg subcutaneous nodules, and remarkable reduction in amylase and lipase levels on 1-month follow-up.
Conclusion
Panniculitis, pancreatitis, and polyarthritis syndrome is a rare diagnosis characterized by a triad of pancreatic panniculitis, pancreatitis, and polyarthritis. Adjuvant therapies for PPP syndrome, such as NSAIDs, corticosteroids, plasmapheresis, and octreotide, have been used with equivocal results, but definitive treatment requires correction of the primary pancreatic disorder. More importantly, many pancreatic diseases can cause pancreatic panniculitis, but extensive, refractory, or ulcerated cases could be an early indicator of an occult pancreatic malignancy and should prompt early evaluation with a multidisciplinary approach. This approach should incorporate management from dermatology, internal medicine, rheumatology, gastroenterology, surgery, and primary care.
Pancreatic panniculitis is a rare disease contributing to widespread fat necrosis in patients with underlying pancreatic disorders. This entity was first described in 1883,1 but it was not until 1947 that it was reported in the English-language literature.2 Patients with pancreatitis infrequently develop extrapancreatic manifestations. It has been estimated that only 2% to 3% of patients worldwide with an underlying pancreatic disease develop cutaneous lesions.3 Patients who develop pancreatic panniculitis typically present with tender, edematous, erythematous to brown, subcutaneous nodules on the lower legs with the tendency for spontaneous ulceration. Lesions tend to exude a viscous, yellow-brown, oily substance that represents liquefactive necrosis of enzymatic fat in subcutaneous tissue. Cutaneous lesions may precede, occur simultaneously, or follow the development of an underlying pancreatic disorder. Rarely, patients may develop inflammatory arthritis secondary to intraosseous fat necrosis, completing the triad of findings diagnostic for panniculitis, pancreatitis, and polyarthritis (PPP) syndrome. Although the underlying pancreatic pathology may vary, roughly 80% of cases worldwide have acute/chronic pancreatitis or pancreatic carcinoma, most commonly acinar cell carcinoma.4-6 Less common pancreatic disorders include pancreatic pseudocyst, pancreatic divisum, and vascular pancreatic fistulas.7 Narváez et al8 found that of the 25 cases of PPP syndrome reported in the literature, 68% (17/25) were men, 32% (8/25) were women, 56% (14/25) were younger than 50 years, and 64% (16/25) had a history of prior or current alcohol abuse.
Case Report
A 68-year-old man with a history of hypertension, gastroesophageal reflux disease, chronic pancreatitis of unknown etiology, and arthritis presented to our clinic for evaluation of painful skin nodules on the lower legs of 8 months’ duration, in addition to joint pain and swelling of the metacarpophalangeal (MCP), metatarsophalangeal, and ankle joints. He had a history of numerous hospital admissions over the last 2 years for pancreatitis and was being managed by the rheumatology department for arthritic symptoms.
Physical examination revealed multiple 1- to 4-cm, ill-defined, erythematous to brown, subcutaneous nodules on the bilateral lower legs (Figure 1) and right inferomedial thigh that were tender to palpation. Marked erythema and edema of the MCP and metatarsophalangeal joints (Figure 2) and bilateral ankles were observed. Diffuse 2+ pitting edema was present in the bilateral lower extremities, along with areas of hyperpigmentation overlying resolving lesions.


Laboratory data revealed an elevated lipase level (>16,000 U/L [reference range, 31–186 U/L]), amylase level (>4700 U/L [reference range, 27–131 U/L]), erythrocyte sedimentation rate (94 mm/h [reference range, 0–20 mm/h]), and C-reactive protein level (93.5 mg/L [0.08–3.1 mg/L]). The patient had more than 6 episodes of recurrent idiopathic pancreatitis over the last 2 years, though symptoms of abdominal pain were minimal to nonexistent. Liver function tests and alcohol, calcium, and triglyceride levels all were within reference range. Rheumatoid factor and antinuclear antibodies were negative.
Ultrasonography showed no evidence of cholelithiasis. Computed tomography of the abdomen and pelvis demonstrated a 1.8×1.4-cm hypodense lesion within the pancreatic head with calcifications and mild proximal pancreatic ductal dilatation (Figure 3). However, multiple magnetic resonance cholangiopancreatography examinations and endoscopic ultrasounds with fine-needle aspiration specimens were performed, all negative for malignancy. Computed tomography of the left ankle demonstrated evidence of bony cortical destruction in the lateral aspect of the posterior calcaneus. Bone biopsy specimens demonstrated mild chronic inflammation with no evidence of osteomyelitis. A serum uric acid level was found to be 4.4 mg/dL (reference range, 4.0–8.0 mg/dL) and a joint aspirate demonstrated turbid fluid with lipoid material and no evidence of crystals or organisms on culture. Furthermore, a 4-mm punch biopsy of a nodule on the right leg revealed extensive lobular and septal liquefactive adipocyte necrosis with scattered neutrophils and lymphocytes (Figure 4). Aggregates of fine granular basophilic material were observed with prominent adipocyte degeneration and calcification.


Symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) along with intralesional, topical, and oral corticosteroids had proven ineffective in the management of this patient. He was subsequently referred to the surgery department for a pancreaticoduodenectomy (Whipple procedure) with notable improvement in pancreatic enzyme levels, lower leg subcutaneous nodules, and arthritis weeks after surgery.
Comment
A triad of pancreatic panniculitis, pancreatitis, and polyarthritis characterizes a rare entity known as PPP syndrome. Pancreatic panniculitis is a rare form of subcutaneous lobular fat necrosis associated with various underlying pancreatic disorders. Approximately 0.3% to 3.0% of patients with an underlying pancreatic disorder are affected with pancreatic panniculitis.9 Pancreatic panniculitis has been found in roughly 2% to 3% of patients with acute or chronic pancreatitis and pancreatic carcinoma, most commonly the acinar cell type.10 Narváez et al8 reported that nearly two-thirds of patients diagnosed with PPP syndrome have minimal to absent abdominal symptoms that often lead to misdiagnosis and affect the overall prognosis of patients with pancreatic disease. Any delay in the diagnosis of PPP syndrome leads to a worse prognosis, with a mortality rate reported to be approximately 24%.8 Potts et al5 provided a review of 27 patients with pancreatic panniculitis in which all 8 patients with pancreatic carcinoma and 42% (8/19) of patients with pancreatitis died.
Pancreatic panniculitis in the setting of PPP syndrome commonly presents with erythematous to brown, exquisitely tender, edematous, subcutaneous nodules on the lower legs. Lesions can range in size from several millimeters to 5 cm. The subcutaneous nodules may spontaneously ulcerate and exude oily viscous material from the liquefactive necrosis of adipocytes. In approximately 40% of patients, skin lesions are the presenting feature.11 Lesions typically resolve only after the pancreatic inflammation regresses, leaving behind atrophic hyperpigmented scars.3 Other presenting symptoms may include joint pain, pitting edema, and subcutaneous nodules, which can precede the diagnosis by up to 9 months.
The exact pathogenesis of PPP syndrome remains unclear. The most widely recognized hypothesis suggests that pancreatic enzymes (eg, trypsin, amylase, lipase, phospholipase A) released from the damaged pancreas are transported through the bloodstream to distant visceral and soft tissue sites, leading to lipolysis and inflammation to the surrounding subcutis and bone marrow.3 Ferrari et al12 reported this effect as a product of the accumulation of high levels of free fatty acids within the joint space by the action of lipolytic pancreatic enzymes on adipose cell membranes, resulting in acute arthritis.
Histopathologic findings of pancreatic panniculitis vary based on the acuity of the disease. Acute lesions typically demonstrate lobular and septal panniculitis. Szymanski and Bluefarb13 described the pathognomonic histologic findings of focal liquefactive necrosis and anucleate necrotic adipocytes surrounded by a shadowy and thickened cell membrane signifying the characteristic ghost cells. Fine basophilic material also may be seen intermixed with the necrotic adipocytes, representing saponified calcium. A brisk inflammatory infiltrate involving lymphocytes, macrophages, and neutrophils tends to surround the areas of necrotic adipocytes. Chronic lesions often demonstrate a paucity of fat necrosis and ghost cells and more granulomatous infiltrate. Langerhans giant cells, macrophages, and lymphocytes predominate in the subcutaneous fat.
Laboratory findings associated with pancreatic panniculitis may include elevated serum amylase, lipase, and/or trypsin levels. Not all the enzymes have to be elevated simultaneously. On occasion, one enzyme may be within reference range while the others are elevated. Rarely, patients may have an elevated lipase level with no signs of underlying pancreatic disease, which demonstrates that panniculitis does not correlate with the enzyme levels. In all cases of suspected pancreatic panniculitis, a complete laboratory workup is recommended including lipase, amylase, and trypsin serum levels. Eosinophilia may be a prominent finding in patients with pancreatic panniculitis and tends to occur in association with an underlying pancreatic carcinoma. Patients with pancreatic panniculitis associated with pancreatic carcinoma tend to have more severe, diffuse, and persistent subcutaneous nodules that often are refractory to treatment with frequent recurrence. A rare constellation of findings known as Schmid triad is comprised of panniculitis, polyarthritis, and eosinophilia and typically portends a poor prognosis secondary to an underlying pancreatic tumor.14 Cutaneous nodules may predate the diagnosis of pancreatic carcinoma by several months, thus signifying the need for a high index of suspicion in patients with lower leg subcutaneous nodules.
Joint disease most commonly involves the ankles, knees, wrists, and MCP joints.5,6,11 It has been suggested that arthritic symptoms are from periarticular fat necrosis or a direct extension from the necrotic subcutaneous tissue to the adjacent joint space.15 Dahl et al3 reported the composition of joint effusion fluid in 3 patients with PPP syndrome. The aspirate in all 3 patients contained viscous yellow material similar to the necrotic adipose tissue seen draining from subcutaneous nodules. Joint aspirate analysis demonstrated increased concentration of free fatty acids in the joint fluid consistent with severe lipolysis.3
The PPP syndrome acronym may be misleading to physicians, as arthritis is not always polyarticular. Dahl et al3 reported that monoarticular or oligoarticular arthritic symptoms were present in 56% of patients studied. In rare cases, the arthritic symptoms antedated the diagnosis of clinically asymptomatic pancreatic disease. Arthritis can be either symmetric or asymmetric and infrequently follows a chronic course, leading to radiographic lytic lesions and symptoms that often are unresponsive to conventional therapy.16
Treatment of PPP syndrome is largely supportive, with a focus on correcting the underlying pancreatic disease. It is imperative to identify any complicating factors contributing to high levels of circulating pancreatic enzymes. Pseudocysts must be addressed if discovered in these patients, as they often perpetuate the substantial release of pancreatic enzymes into the serum, leading to characteristic subcutaneous fat necrosis and arthritis. Sepsis also is a concern, likely secondary to bacterial colonization of the ulcerated subcutaneous nodules and compromised skin barrier. Nonsteroidal anti-inflammatory drugs and corticosteroids have been used for symptomatic relief but usually are ineffective and have not been shown to reduce the duration of the disease.12,16 Octreotide has been utilized and may potentially reduce pancreatic enzyme secretion leading to improvement in cutaneous and musculoskeletal lesions.17 Plasmapheresis has been used as an adjuvant treatment in patients with persistent hyperamylasemia and hyperlipasemia, but reports are anecdotal. Often reserved for severe disease, cholecystectomy, pancreatic duct removal, and pancreaticoduodenectomy have demonstrated success in the management of chronic pancreatitis and panniculitis. Dahl et al3 reported 2 cases in which cholecystectomy was performed with complete resolution of the skin and pancreatic disease. Our patient was initially treated symptomatically with NSAIDs and corticosteroids but there was no clinical response. The patient eventually underwent a pancreaticoduodenectomy 9 months after the onset of symptoms with complete resolution of joint pain and swelling, greater than 50% resolution of his lower leg subcutaneous nodules, and remarkable reduction in amylase and lipase levels on 1-month follow-up.
Conclusion
Panniculitis, pancreatitis, and polyarthritis syndrome is a rare diagnosis characterized by a triad of pancreatic panniculitis, pancreatitis, and polyarthritis. Adjuvant therapies for PPP syndrome, such as NSAIDs, corticosteroids, plasmapheresis, and octreotide, have been used with equivocal results, but definitive treatment requires correction of the primary pancreatic disorder. More importantly, many pancreatic diseases can cause pancreatic panniculitis, but extensive, refractory, or ulcerated cases could be an early indicator of an occult pancreatic malignancy and should prompt early evaluation with a multidisciplinary approach. This approach should incorporate management from dermatology, internal medicine, rheumatology, gastroenterology, surgery, and primary care.
- Chiari H. Uber die Sogenannte Fettnekrose. Prag Med Wochenschr. 1883;8:285-286, 299-301.
- Blauvelt H. Case of acute pancreatitis with subcutaneous fat necrosis. Br J Surg. 1946;34:207-208.
- Dahl PR, Su D, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
- Mullen GT, Caperton EM Jr, Crespin SR, et al. Arthritis and skin lesions resembling erythema nodosum in pancreatic disease. Ann Intern Med. 1968;68:75-87.
- Potts DE, Mass MF, Iseman MD. Syndrome and pancreatic disease, subcutaneous fat necrosis and polyserositis: case report and review of literature. Am J Med. 1975;58:417-423.
- Sorensen EV. Subcutaneous fat necrosis in pancreatic disease: a review and two new case reports. J Clin Gastroenterol. 1988;10:71-75.
- García-Romero D, Vanaclocha F. Pancreatic panniculitis. Dermatol Clin. 2008;26:465-470.
- Narváez J, Bianchi M, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum. 2010;39:417-423.
- Rongioetti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
- Poelman SM, Nguyen K. Pancreatic panniculitis associated with acinar cell pancreatic carcinoma. J Cutan Med Surg. 2008;12:38-42.
- Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975:111:506-510.
- Ferrari R, Wendelboe M, Ford PM, et al. Pancreatitis arthritis with periarticular fat necrosis. J Rheumatol. 1993;20:1436-1437.
- Szymanski FJ, Bluefarb SM. Nodular fat necrosis and pancreatic diseases. Arch Dermatol. 1961;83:224-229.
- Beltraminelly HS, Buechner SA, Hausermann P. Pancreatic panniculitis in a patient with an acinar cell cystadenocarcinoma of the pancreas. Dermatology. 2004;208:265-267.
- Burns WA, Matthews MJ, Hamosh M, et al. Lipase-secreting acinar cell carcinoma of the pancreas with polyarthropathy: a light and electron microscopic, histochemical, and biochemical study. Cancer. 1974;33:1002-1009.
- Baron M, Paltiel H, Lander P. Aseptic necrosis of the talus and calcaneal insufficiency fractures in a patient with pancreatitis, subcutaneous fat necrosis, and arthritis. Arthritis Rheum. 1984;27:1309-1313.
- Zundler S, Erber R, Agaimy A, et al. Pancreatic panniculitis in a patient with pancreatic-type acinar cell carcinoma of the liver—case report and review of literature. BMC Cancer. 2016;16:130.
- Chiari H. Uber die Sogenannte Fettnekrose. Prag Med Wochenschr. 1883;8:285-286, 299-301.
- Blauvelt H. Case of acute pancreatitis with subcutaneous fat necrosis. Br J Surg. 1946;34:207-208.
- Dahl PR, Su D, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
- Mullen GT, Caperton EM Jr, Crespin SR, et al. Arthritis and skin lesions resembling erythema nodosum in pancreatic disease. Ann Intern Med. 1968;68:75-87.
- Potts DE, Mass MF, Iseman MD. Syndrome and pancreatic disease, subcutaneous fat necrosis and polyserositis: case report and review of literature. Am J Med. 1975;58:417-423.
- Sorensen EV. Subcutaneous fat necrosis in pancreatic disease: a review and two new case reports. J Clin Gastroenterol. 1988;10:71-75.
- García-Romero D, Vanaclocha F. Pancreatic panniculitis. Dermatol Clin. 2008;26:465-470.
- Narváez J, Bianchi M, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum. 2010;39:417-423.
- Rongioetti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
- Poelman SM, Nguyen K. Pancreatic panniculitis associated with acinar cell pancreatic carcinoma. J Cutan Med Surg. 2008;12:38-42.
- Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975:111:506-510.
- Ferrari R, Wendelboe M, Ford PM, et al. Pancreatitis arthritis with periarticular fat necrosis. J Rheumatol. 1993;20:1436-1437.
- Szymanski FJ, Bluefarb SM. Nodular fat necrosis and pancreatic diseases. Arch Dermatol. 1961;83:224-229.
- Beltraminelly HS, Buechner SA, Hausermann P. Pancreatic panniculitis in a patient with an acinar cell cystadenocarcinoma of the pancreas. Dermatology. 2004;208:265-267.
- Burns WA, Matthews MJ, Hamosh M, et al. Lipase-secreting acinar cell carcinoma of the pancreas with polyarthropathy: a light and electron microscopic, histochemical, and biochemical study. Cancer. 1974;33:1002-1009.
- Baron M, Paltiel H, Lander P. Aseptic necrosis of the talus and calcaneal insufficiency fractures in a patient with pancreatitis, subcutaneous fat necrosis, and arthritis. Arthritis Rheum. 1984;27:1309-1313.
- Zundler S, Erber R, Agaimy A, et al. Pancreatic panniculitis in a patient with pancreatic-type acinar cell carcinoma of the liver—case report and review of literature. BMC Cancer. 2016;16:130.
Practice Points
- Recognition of skin lesions in a patient with a history of pancreatitis may represent a rare entity known as pancreatic panniculitis.
- Panniculitis, pancreatitis, and polyarthritis (PPP) syndrome is a rare diagnosis characterized by a triad of pancreatic panniculitis, pancreatitis, and polyarthritis.
- A rare constellation of findings known as Schmid triad is comprised of panniculitis, polyarthritis, and eosinophilia and typically portends a poor prognosis secondary to an underlying pancreatic tumor.
- These findings should prompt early evaluation with a multidisciplinary approach.
COPD in Primary Care: Key Considerations for Optimized Management
Click Here to Read the Supplement
This supplement provides an overview of 4 key topics critical to the effective management of COPD in primary care. The articles in this supplement are:
Dyspnea and Hyperinflation in COPD: Impact on Physical Activity
by Nathaniel Marchetti, DO; and Alan Kaplan, MD
Anxiety and Depression in COPD: Recognition and Management
by Abebaw Mengistu Yohannes, PhD; Alan Kaplan, MD; and Nicola A. Hanania, MD, MS
Considerations for Optimal Inhaler Device Selection in COPD
by Rajiv Dhand, MD; Tricia Cavanaugh, MD; and Neil Skolnik, MD
Treatment Options for Stable COPD: Current Recommendations and Unmet Needs
by Barbara Yawn, MD, MSc, FAAFP; and Victor Kim, MD
Click Here to Read the Supplement
Click Here to Read the Supplement
This supplement provides an overview of 4 key topics critical to the effective management of COPD in primary care. The articles in this supplement are:
Dyspnea and Hyperinflation in COPD: Impact on Physical Activity
by Nathaniel Marchetti, DO; and Alan Kaplan, MD
Anxiety and Depression in COPD: Recognition and Management
by Abebaw Mengistu Yohannes, PhD; Alan Kaplan, MD; and Nicola A. Hanania, MD, MS
Considerations for Optimal Inhaler Device Selection in COPD
by Rajiv Dhand, MD; Tricia Cavanaugh, MD; and Neil Skolnik, MD
Treatment Options for Stable COPD: Current Recommendations and Unmet Needs
by Barbara Yawn, MD, MSc, FAAFP; and Victor Kim, MD
Click Here to Read the Supplement
Click Here to Read the Supplement
This supplement provides an overview of 4 key topics critical to the effective management of COPD in primary care. The articles in this supplement are:
Dyspnea and Hyperinflation in COPD: Impact on Physical Activity
by Nathaniel Marchetti, DO; and Alan Kaplan, MD
Anxiety and Depression in COPD: Recognition and Management
by Abebaw Mengistu Yohannes, PhD; Alan Kaplan, MD; and Nicola A. Hanania, MD, MS
Considerations for Optimal Inhaler Device Selection in COPD
by Rajiv Dhand, MD; Tricia Cavanaugh, MD; and Neil Skolnik, MD
Treatment Options for Stable COPD: Current Recommendations and Unmet Needs
by Barbara Yawn, MD, MSc, FAAFP; and Victor Kim, MD
Click Here to Read the Supplement
Congress extends CHIP, funds opioid crisis response following temporary shutdown
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Growing old with HIV: What’s likely, and how can physicians help?
Individuals infected with HIV require early intervention with safe and effective antiretroviral therapy beyond the standard care required for successful aging. The long-term residual inflammation associated with fully suppressive ART must also be addressed, according to a review in the International Journal of Infectious Diseases.
The review was prompted by the increasing number of aging persons with HIV in the population, due to the advent of life-protecting drug treatments.
Gerome V. Escota, MD, and his colleagues at Washington University, St. Louis, assessed the factors faced by the normally aging population and then added an assessment of the path to successful aging given the unique aspects of the HIV infected population (Int J Infect Dis. 2018; 66:56-64).
For example, in San Francisco, 58% of the people with HIV were over 50 years of age by 2014, according to the authors, and those numbers will continue to increase. Such patients will not only be suffering the effects of the normal aging process, but also the potential burden of long-term antiretroviral drug use on their systems.
In addition, even the use of highly-effective antiretroviral therapy does not completely eliminate the inflammatory markers among HIV-infected individuals, and such markers have been associated with increased risk of cardiovascular and other problems. Of particular concern: “It remains unknown whether age-associated inflammation will aggravate residual HIV-associated inflammation in these patients over time,” the authors wrote.
Exacerbated comorbidities that may be a risk among the aging population infected with HIV include cardiovascular disease, osteoporosis, malignancies, chronic liver and kidney disease, and HIV-related neurocognitive disease.
The review was not sponsored and the authors reported that they had no disclosures.
SOURCE: Escota, J V et al. Int J Infect Dis. 2018;66:56-64.
Individuals infected with HIV require early intervention with safe and effective antiretroviral therapy beyond the standard care required for successful aging. The long-term residual inflammation associated with fully suppressive ART must also be addressed, according to a review in the International Journal of Infectious Diseases.
The review was prompted by the increasing number of aging persons with HIV in the population, due to the advent of life-protecting drug treatments.
Gerome V. Escota, MD, and his colleagues at Washington University, St. Louis, assessed the factors faced by the normally aging population and then added an assessment of the path to successful aging given the unique aspects of the HIV infected population (Int J Infect Dis. 2018; 66:56-64).
For example, in San Francisco, 58% of the people with HIV were over 50 years of age by 2014, according to the authors, and those numbers will continue to increase. Such patients will not only be suffering the effects of the normal aging process, but also the potential burden of long-term antiretroviral drug use on their systems.
In addition, even the use of highly-effective antiretroviral therapy does not completely eliminate the inflammatory markers among HIV-infected individuals, and such markers have been associated with increased risk of cardiovascular and other problems. Of particular concern: “It remains unknown whether age-associated inflammation will aggravate residual HIV-associated inflammation in these patients over time,” the authors wrote.
Exacerbated comorbidities that may be a risk among the aging population infected with HIV include cardiovascular disease, osteoporosis, malignancies, chronic liver and kidney disease, and HIV-related neurocognitive disease.
The review was not sponsored and the authors reported that they had no disclosures.
SOURCE: Escota, J V et al. Int J Infect Dis. 2018;66:56-64.
Individuals infected with HIV require early intervention with safe and effective antiretroviral therapy beyond the standard care required for successful aging. The long-term residual inflammation associated with fully suppressive ART must also be addressed, according to a review in the International Journal of Infectious Diseases.
The review was prompted by the increasing number of aging persons with HIV in the population, due to the advent of life-protecting drug treatments.
Gerome V. Escota, MD, and his colleagues at Washington University, St. Louis, assessed the factors faced by the normally aging population and then added an assessment of the path to successful aging given the unique aspects of the HIV infected population (Int J Infect Dis. 2018; 66:56-64).
For example, in San Francisco, 58% of the people with HIV were over 50 years of age by 2014, according to the authors, and those numbers will continue to increase. Such patients will not only be suffering the effects of the normal aging process, but also the potential burden of long-term antiretroviral drug use on their systems.
In addition, even the use of highly-effective antiretroviral therapy does not completely eliminate the inflammatory markers among HIV-infected individuals, and such markers have been associated with increased risk of cardiovascular and other problems. Of particular concern: “It remains unknown whether age-associated inflammation will aggravate residual HIV-associated inflammation in these patients over time,” the authors wrote.
Exacerbated comorbidities that may be a risk among the aging population infected with HIV include cardiovascular disease, osteoporosis, malignancies, chronic liver and kidney disease, and HIV-related neurocognitive disease.
The review was not sponsored and the authors reported that they had no disclosures.
SOURCE: Escota, J V et al. Int J Infect Dis. 2018;66:56-64.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Key clinical point: Significant medical monitoring and intervention may be required to enable successful aging in the HIV-infected population.
Major finding: Aggressive use of antiretroviral therapy, primary prevention, and early diagnosis and treatment of comorbidities are key.
Study details: Literature review.
Disclosures: The review was not sponsored and the authors reported that they had no disclosures.
Source: Escota, J V et al. Int J Infect Dis. 2018;66:56-64.
A Veteran With Alcohol Use Disorder and Acute Pancreatitis
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
Case Presentation. A 23-year-old male U.S. Army veteran with a history of alcohol use disorder and posttraumatic stress disorder (PTSD) presented to the VA Boston Healthcare System (VABHS) West Roxbury campus emergency department (ED) with epigastric abdominal pain in the setting of consuming alcohol. The patient had served in the infantry in Afghanistan during Operation Enduring Freedom. He consumed up to 12 alcoholic drinks per day (both beer and hard liquor) for the past 3 years and had been hospitalized 3 times previously; twice for alcohol detoxification and once for PTSD. He is a former tobacco smoker with fewer than 5 pack-years, he uses marijuana often and does not use IV drugs. In the ED, his physical examination was notable for a heart rate of 130 beats per minute and blood pressure of 161/111 mm Hg. He was alert and oriented and had a mild tremor. The patient was diaphoretic with dry mucous membranes, tenderness to palpation in the epigastrium, and abdominal guarding. A computed tomography (CT) scan of the abdomen revealed acute pancreatitis without necrosis. The patient received 1 L of normal saline and was admitted to the medical ward for presumed alcoholic pancreatitis.
► Rahul Ganatra, MD, MPH, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center. Dr. Weber, we care for many young people who drink more than they should and almost none of them end up with alcoholic pancreatitis. What are the relevant risk factors that make individuals like this patient more susceptible to alcoholic pancreatitis?
►Horst Christian Weber, MD, Gastroenterology Service, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. While we don’t have a good understanding of the precise mechanism of alcoholic pancreatitis, we do know that in the U.S., alcohol consumption is responsible for about one-third of all cases.1 Acute pancreatitis in general may present with a wide range of disease severity. It is the most common cause of gastrointestinal-related hospitalization,2 and the mortality of hospital inpatients with pancreatitis is about 5%.3,4 Therefore, acute pancreatitis represents a prevalent condition with a critical impact on morbidity and mortality. Alcoholic pancreatitis typically occurs after many years of heavy alcohol use, not after a single drinking
► Dr. Ganatra. At this point, the chemistry laboratory paged the admitting resident with the notification that the patient’s blood was grossly lipemic. Ultracentrifugation was performed to separate the lipid layer and his laboratory values result (Table). Notable abnormalities included polycythemia with a hemoglobin of 17.4 g/dL, hyponatremia with a sodium of 129 mmol/L, normal renal function, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (AST 258 IU/L and ALT 153 IU/L, respectively), hyperbilirubinemia with a total bilirubin of 2.7 mg/dL, and a serum alcohol level of 147 mg/dL. Due to anticipated requirement for a higher level of care, the patient was transferred to the Medical Intensive Care Unit (MICU).
Dr. Breu, can you help us interpret this patient’s numerous laboratory abnormalities? Without yet having the triglyceride level available, how does the fact that the patient’s blood was lipemic affect our interpretation of his labs? What further workup is warranted?
► Anthony Breu, MD, Medical Service, VABHS, Assistant Professor of Medicine, Harvard Medical School. First, the positive alcohol level confirms a recent ingestion. Second, he has elevated transaminases with the AST greater than the ALT, which is consistent with alcoholic liver disease. While the initial assumption is that this patient has alcohol-induced pancreatitis, the elevations in bilirubin and alkaline phosphatase may suggest gallstone pancreatitis, and the lipemic appearing serum could suggest triglyceride-mediated pancreatitis. If the patient does have elevated triglyceride levels, the sodium level may indicate pseudohyponatremia, a laboratory artifact seen if a dilution step is used. To further evaluate the patient, I would obtain a triglyceride level and a right upper quadrant ultrasound. Direct ion-selective electrode analysis of the sodium level can be done with a device used to measure blood gases to exclude pseudohyponatremia.
► Dr. Ganatra. A right upper quadrant ultrasound was obtained in the MICU, which showed hepatic steatosis and hepatomegaly to 19 cm, but no evidence of biliary obstruction by stones or sludge. The common bile duct measured 3.2 mm in diameter. A triglyceride level returned above assay at > 3,392 mg/dL. A review of the medical record revealed a triglyceride level of 105 mg/dL 16 months prior. The Gastroenterology Department was consulted.
Dr. Weber, we now have 2 etiologies for pancreatitis in this patient: alcohol and hypertriglyceridemia. How do each cause pancreatitis? Is it possible to determine in this case which one is the more likely driver?
► Dr. Weber. The mechanism for alcohol-induced pancreatitis is not fully known, but there are several hypotheses. One is that alcohol may increase the synthesis or activation of pancreatic digestive enzymes.6 Another is that metabolites of alcohol are directly toxic to the pancreas.6 Based on the epidemiologic observation that alcoholic pancreatitis usually happens in long-standing users, all we can say is that it is not very likely to be the effect of an acute insult. For hypertriglyceridemic pancreatitis, we believe the injury is due to the toxic effect of free fatty acids in the pancreas liberated by lipolysis of triglycerides by pancreatic lipases. Higher triglycerides are associated with higher risk, suggesting a dose-response relationship: This risk is not greatly increased until triglycerides exceed 500 mg/dL; above 1,000 mg/dL, the risk is about 5%, and above 2,000 mg/dL, the risk is between 10% and 20%.7 In summary, we cannot really determine whether the alcohol or the triglycerides are the main cause of his pancreatitis, but given his markedly elevated triglycerides, he should be treated for hypertriglyceridemic pancreatitis.
►Dr. Ganatra. Dr. Breu, regardless of the underlying etiology, this patient requires treatment. What does the literature suggest as the best course of action regarding crystalloid administration in patients with acute pancreatitis?
►Dr. Breu. There are 2 issues to discuss regarding IV fluids in acute pancreatitis: choice of crystalloid and rate of administration. For the choice of IV fluid, lactated Ringer solution (LR) may be preferred over normal saline (NS). There are both pathophysiologic and evidence-based rationales for this choice. As Dr. Weber alluded to, trypsinogen activation is an important step in the pathogenesis of acute pancreatitis and requires a low pH compartment. As most clinicians have experienced, NS may cause a metabolic acidosis; however, the use of LR may mitigate this. A 2011 randomized clinical trial showed that patients who received LR had less systemic inflammatory response syndrome (SIRS) and lower C-reactive protein (CRP) levels at 24 hours compared with patients who received NS.8 While these are surrogate outcomes, they, along with the theoretical basis, suggest LR is preferred.
Regarding rate, the key is fast and early.9 In my experience, internists often underdose IV rehydration within the first 12 to 24 hours, fail to change the rate based on clinical response, and leave patients on high rates too long. In a patient like this, a rate of 350 cc/h is a reasonable place to start. But, one must reassess response (ie, ensure there is a decrease in hematocrit and/or blood urea nitrogen) every 6 hours and increase the rate as needed. After the first 24 to 48 hours have passed, the rate should be lowered.
►Dr. Ganatra. The patient received 2 mg of IV hydromorphone and a 2 L bolus of LR. This was followed by a continuous infusion of LR at 200 cc/h. Dr. Weber, apart from the standard therapies for pancreatitis, what are our treatment options in hypertriglyceridemic pancreatitis?
►Dr. Weber. In the acute setting, IV insulin with or without dextrose is the most extensively studied therapy. Insulin rapidly decreases triglyceride levels by activating lipoprotein lipase and inhibiting hormone- sensitive lipase. The net effect is reduction in serum triglycerides available to be hydrolyzed to free fatty acids in the pancreas.7 For severe cases (ie, where acute pancreatitis is accompanied by hypocalcemia, lactic acidosis or a markedly elevated lipase), apheresis with therapeutic plasma exchange to more rapidly reduce triglyceride concentration is the preferred therapy. The goal is to reduce triglycerides to levels
►Dr. Ganatra. Due to the possibility that the patient would require apheresis, which was not available at the VABHS West Roxbury campus, the patient was transferred to an affiliate hospital. The patient was started on 10% dextrose at 300 cc/h and an IV insulin infusion. His triglycerides fell to < 500 mg/dL over the subsequent 48 hours, and ultimately, apheresis was not required. Enteral nutrition by nasogastric (NG) tube was initiated on hospital day 6. The patient’s hospital course was notable for acute respiratory distress syndrome that required intubation for 7 days, hyperbilirubinemia (with a peak bilirubin of 10.5 mg/dL), acute kidney injury (with a peak creatinine 4.7 mg/dL), fever without an identified infectious source, alcohol withdrawal syndrome that required phenobarbital, and delirium. Nine days later, he was transferred back to the VABHS West Roxbury campus. His condition stabilized, and he was transferred to the medical floor. On hospital day 14, the patient’s mental status improved, and he began tolerating oral nutrition.
Dr. Breu, over the years, the standard of care regarding when to start enteral nutrition in pancreatitis has changed considerably. This patient received enteral nutrition via NG tube but also had periods of being NPO (nothing by mouth) for up to 6 days. What is the current best practice for timing of initiating enteral nutrition in acute pancreatitis?
►Dr. Breu. It is true that the standard of care has changed and continues to evolve. Many decades ago, patients with acute pancreatitis would routinely undergo NG tube suction to reduce delivery of gastric contents to the duodenum, thereby decreasing pancreas activation, allowing it to rest.11 The NG tube also allowed for decompression of any ileus that had formed. Beginning in the 1970s, several clinical trials were performed, showing that NG tube suction was no better than simply making the patient NPO.12,13 More recently, we have begun to move toward earlier feeding. Again, there is a pathophysiologic rationale (bowel rest is associated with intestinal atrophy, predisposing to bacterial translocation and resulting infectious complications) and increasing evidence supporting this practice.9 Even in severe pancreatitis, hunger may be used to initiate oral intake.14
►Dr. Ganatra. On hospital day 16, the patient developed sudden-onset right-sided back and flank pain, and his hemoglobin dropped to 6.1 mg/dL, which required transfusion of packed red blood cells. He remained afebrile and hemodynamically stable. Dr. Weber, what are the major complications of acute pancreatitis, and when should we suspect them? Should we be worried about complications of pancreatitis in this patient?
►Dr. Weber. Organ failure in the acute setting can occur due to activation of cytokine cascades and the systemic inflammatory response syndrome and is described by clinical and radiologic criteria called the Atlanta Classification.15 Apart from organ failure, the most serious complications of acute pancreatitis are necrosis of pancreatic tissue leading to walled-off pancreatic necrosis and the formation of peripancreatic fluid collections and pseudocysts, which occur in about 15% of patients with acute pancreatitis. These complications are serious because they can become infected, which portends a higher mortality and in some cases require surgical resection.
Other complications of acute pancreatitis include pseudoaneurysm formation, which is when a vessel bleeds into a pancreatic pseudocyst, and thromboses of the splenic, portal, or mesenteric veins. Thrombotic complications may occur in up to half of patients with pancreatic necrosis but are uncommon without some degree of necrosis.16 No necrosis was noted on this patient’s initial CT scan, so the probability of thrombosis is low. Also, as it takes several weeks for pseudocyst formation to occur, a bleeding pseudoaneurysm is unlikely at this early stage. Therefore, a complication of pancreatitis is unlikely in this patient, and evaluation for other causes of abdominal pain should be considered.
►Dr. Ganatra. A noncontrast CT of the abdomen and pelvis was obtained and revealed no evidence of complications or other acute pathology. His pain was managed conservatively, and hemoglobin remained stable. Over the next 5 days, the patient’s symptoms gradually resolved, his oral intake improved, and he was discharged home on gemfibrozil 600 mg twice daily 19 days after admission. He declined psychiatry follow-up for his PTSD, and after discharge he did not keep his scheduled gastroenterology (GI) follow-up appointment. Four months later, the patient presented again with epigastric abdominal pain similar to his initial presentation. The patient had resumed drinking, stating that “alcohol is the only thing that helps [with the PTSD].” He had not been taking the gemfibrozil. He was admitted with a recurrent episode of pancreatitis; however, his triglycerides on admission were 119 mg/dL.
Dr. Weber, this patient’s triglycerides declined rapidly over a period of just 4 months with questionable adherence to gemfibrozil. However, he was admitted again with another episode of pancreatitis, this time in the setting of alcohol use alone without markedly elevated triglycerides. What do we know about recurrence risk for pancreatitis? Are some etiologies of pancreatitis more likely to present with recurrent attacks than are others?
►Dr. Weber. The rate of recurrence following an episode of acute pancreatitis varies according to the cause, but in general, about 20% to 30% of patients will experience a recurrence, and 5% to 10% will go on to develop chronic pancreatitis.17 Alcoholic pancreatitis does carry a higher risk of recurrence than pancreatitis due to other causes; the risk is as high as 50%. Not surprisingly, recurrence of acute pancreatitis increases risk for development of chronic pancreatitis. As this patient is a smoker, it is worth noting that smoking potentiates pancreatic damage from alcohol and increases the risk for both recurrent and chronic pancreatitis.5
►Dr. Ganatra. The patient was treated with IV hydromorphone and IV LR at 350 cc/h. Oral nutrition was begun immediately. He manifested no organ dysfunction, and his symptoms improved over the course of 48 hours. He was discharged home with psychiatry and GI follow-up scheduled. Dr. Breu and Dr. Weber, how should we counsel this patient to reduce his risk of recurrent attacks of pancreatitis in the future, and what options do we have for pharmacotherapy to decrease his risk?
►Dr. Breu. I’ll let Dr. Weber comment on mitigating the risk of hypertriglyceride-induced pancreatitis and reserve my comments to pharmacotherapy in alcohol use disorder. This patient may be a candidate for naltrexone therapy, either in oral or intramuscular formulations. Both have been showed to reduce the risk of returning to heavy drinking and may be particularly beneficial in those with a family history.18,19 Acamprosate is also an option.
►Dr. Weber. Data on recurrence risk in hypertriglyceridemic pancreatitis are limited, but there are case reports suggesting that a fatty diet and alcohol use are implicated in recurrence.20 I would counsel the patient on lifestyle modifications that are known to reduce this risk. I agree with Dr. Breu that devoting our efforts to helping him reduce or eliminate his alcohol consumption is the single most important thing we can do to reduce his risk for recurrent attacks. Since the patient reports that he drinks alcohol in order to cope with his PTSD, establishing care with a mental health provider to address this is of the utmost importance. In addition, smoking cessation and promoting medication adherence with gemfibrozil will also reduce risk for future episodes, but continued alcohol use is his strongest risk factor.
►Dr. Ganatra. After discharge, the patient engaged with outpatient psychiatry and GI. He still reports feeling that alcohol is the only thing that alleviates his PTSD and anxiety symptoms. He is not currently interested in pharmacotherapy for cessation of alcohol use.
Acknowledgments
The authors thank Ivana Jankovic, MD, Matthew Lewis Chase, MD
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
1. Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27(4):286-290.
2. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3.
3. Cavallini G, Frulloni L, Bassi C, et al; ProInf-AISP Study Group. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36(3):205-211.
4. Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
5. Hartwig W, Werner J, Ryschich E, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21(3):272-278.
6. Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421-7427.
7. Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195-203.
8. Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-717.e1.
9. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415; 1416.
10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
11. Nardi GL. Pancreatitis. N Engl J Med. 1963;268(19):1065-1067.
12. Naeije R, Salingret E, Clumeck N, De Troyer A, Devis G. Is nasogastric suction necessary in acute pancreatitis? Br Med J. 1978;2(6138):659-660.
13. Levant JA, Secrist DM, Resin H, Sturdevant RA, Guth PH. Nasogastric suction in the treatment of alcoholic pancreatitis: a controlled study. JAMA. 1974;229(1):51-52.
14. Zhao XL, Zhu SF, Xue GJ, et al. Early oral refeeding based on hunger in moderate and severe acute pancreatitis: a prospective controlled, randomized clinical trial. Nutrition. 2015;31(1):171-175.
15. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
16. Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854-862.
17. Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096-1103.
18. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900.
19. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617-1625.
20. Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13(1):96-99.
MDedge Daily News: Our surprising fetal alcohol problem
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many more children may have fetal alcohol syndrome, how menopause affects rheumatoid arthritis, Does mindset matter in inflammatory bowel disease? And aspirin could blunt stroke risk after pre-eclampsia.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many more children may have fetal alcohol syndrome, how menopause affects rheumatoid arthritis, Does mindset matter in inflammatory bowel disease? And aspirin could blunt stroke risk after pre-eclampsia.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many more children may have fetal alcohol syndrome, how menopause affects rheumatoid arthritis, Does mindset matter in inflammatory bowel disease? And aspirin could blunt stroke risk after pre-eclampsia.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
HIV Treatment: OK to Take a Break?
Antiretroviral therapy (ART) keeps viral load of HIV at undetectable levels. The key is taking it daily. But it may be possible for patients to temporarily stop ART without long-lasting or irreversible damage to the immune system.
That approach is called analytical treatment interruption (ATI), and it is the subject of a study by NIH researchers at the National Institute of Allergy and Infectious Diseases. They analyzed blood samples from 10 volunteers who participated in a clinical trial that evaluated whether infusions of a broadly neutralizing antibody could control HIV in the absence of ART.
During the trial, participants temporarily stopped taking ART, resuming 22 to 115 days after stopping. In that hiatus, HIV reservoirs expanded and viral load increased. The researchers also observed abnormalities in the participants’ immune cells. However, 6 to 12 months after the participants resumed ART, the size of the HIV reservoirs and the immune parameters returned to the pre-ATI levels.
These findings support the use of ATI in clinical trials of therapeutic strategies aimed at achieving sustained ART-free remission, the researchers say. They are now conducting a clinical trial to monitor the impact of short-term ATI on a variety of parameters in people living with HIV.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-study-supports-use-short-term-hiv-treatment-interruption-clinical-trials. Published January 11, 2018. Accessed February 8, 2018.
Antiretroviral therapy (ART) keeps viral load of HIV at undetectable levels. The key is taking it daily. But it may be possible for patients to temporarily stop ART without long-lasting or irreversible damage to the immune system.
That approach is called analytical treatment interruption (ATI), and it is the subject of a study by NIH researchers at the National Institute of Allergy and Infectious Diseases. They analyzed blood samples from 10 volunteers who participated in a clinical trial that evaluated whether infusions of a broadly neutralizing antibody could control HIV in the absence of ART.
During the trial, participants temporarily stopped taking ART, resuming 22 to 115 days after stopping. In that hiatus, HIV reservoirs expanded and viral load increased. The researchers also observed abnormalities in the participants’ immune cells. However, 6 to 12 months after the participants resumed ART, the size of the HIV reservoirs and the immune parameters returned to the pre-ATI levels.
These findings support the use of ATI in clinical trials of therapeutic strategies aimed at achieving sustained ART-free remission, the researchers say. They are now conducting a clinical trial to monitor the impact of short-term ATI on a variety of parameters in people living with HIV.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-study-supports-use-short-term-hiv-treatment-interruption-clinical-trials. Published January 11, 2018. Accessed February 8, 2018.
Antiretroviral therapy (ART) keeps viral load of HIV at undetectable levels. The key is taking it daily. But it may be possible for patients to temporarily stop ART without long-lasting or irreversible damage to the immune system.
That approach is called analytical treatment interruption (ATI), and it is the subject of a study by NIH researchers at the National Institute of Allergy and Infectious Diseases. They analyzed blood samples from 10 volunteers who participated in a clinical trial that evaluated whether infusions of a broadly neutralizing antibody could control HIV in the absence of ART.
During the trial, participants temporarily stopped taking ART, resuming 22 to 115 days after stopping. In that hiatus, HIV reservoirs expanded and viral load increased. The researchers also observed abnormalities in the participants’ immune cells. However, 6 to 12 months after the participants resumed ART, the size of the HIV reservoirs and the immune parameters returned to the pre-ATI levels.
These findings support the use of ATI in clinical trials of therapeutic strategies aimed at achieving sustained ART-free remission, the researchers say. They are now conducting a clinical trial to monitor the impact of short-term ATI on a variety of parameters in people living with HIV.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-study-supports-use-short-term-hiv-treatment-interruption-clinical-trials. Published January 11, 2018. Accessed February 8, 2018.
Drug may be option for B- and T-cell lymphomas
LA JOLLA, CA—The EZH1/2 inhibitor DS-3201b could be a novel therapeutic option for non-Hodgkin lymphoma (NHL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
DS-3201b was considered well tolerated in a phase 1 study of Japanese patients with relapsed/refractory NHL.
In addition, DS-3201b demonstrated activity against B- and T-cell lymphomas, producing an overall response rate of 59%.
Kunihiro Tsukasaki, MD, PhD, of Saitama Medical University in Moroyama, Saitama, Japan, presented these results at the meeting.
The trial was sponsored by Daiichi Sankyo Co., Ltd.
Dr Tsukasaki presented data on 18 patients with relapsed/refractory NHL.
The 12 B-cell lymphoma patients had follicular lymphoma (n=5), diffuse large B-cell lymphoma (n=3), MALT lymphoma (n=2), nodal marginal zone lymphoma (n=1), and lymphoplasmacytic lymphoma (n=1).
The 6 patients with T-cell lymphoma had peripheral T-cell lymphoma not otherwise specified (n=2), angioimmunoblastic T-cell lymphoma (n=2), and adult T-cell leukemia/lymphoma (n=2).
The patients’ median age was 67 (range, 44-75), and 10 were female. All patients had an ECOG performance status of 0 (72%) or 1 (28%).
Patients had a median of 2 prior chemotherapy regimens (range, 1-8).
For this study, they received DS-3201b at 150 mg (n=7), 200 mg (n=9), or 300 mg (n=2). They received the drug once daily in 28-day cycles until they progressed or experienced unacceptable toxicity.
DLTs and AEs
Dose-limiting toxicities (DLTs) were evaluated in cycle 1. All 18 patients were evaluable for DLT assessment.
There were 4 treatment-emergent adverse events (AEs) that met the definition of DLTs:
- 3 cases of grade 4 platelet count decrease (n=1 at 200 mg, n=2 at 300 mg)
- 1 case of grade 3 anemia requiring blood transfusion (at 300 mg).
All 4 DLTs led to treatment interruption.
There were 5 serious AEs reported in 3 patients. Only one of these—pneumocystis jiroveci pneumonia—was considered related to DS-3201b.
Hematologic AEs included decreases in platelets (grade 1-4), lymphocytes (grade 1-4), neutrophils (grade 2-4), and white blood cells (grade 2-3), as well as anemia (grade 1-3).
Other AEs (all grade 1/2) included dysgeusia, alopecia, diarrhea, decreased appetite, alanine aminotransferase increase, aspartate aminotransferase increase, nasopharyngitis, rash, and dry skin.
No deaths had been reported as of the data cutoff last November.
Responses
Seventeen patients were evaluable for response.
The overall response rate was 59%, with 1 patient achieving a complete response (CR) and 9 achieving a partial response (PR). Four patients had stable disease (SD), and 3 progressed.
Among the T-cell lymphoma patients, 1 had a CR, 4 had PRs, and 1 progressed. The complete responder had angioimmunoblastic T-cell lymphoma, and the patient who progressed had adult T-cell leukemia/lymphoma.
Among the B-cell lymphoma patients, 5 had PRs, 4 had SD, and 2 progressed.
Dr Tsukasaki said DS-3201b has demonstrated early clinical activity and therefore has the potential to be a novel therapeutic option for B-cell and T-cell lymphomas. However, further evaluation is warranted to determine the optimal dosing regimen and target diseases. ![]()
LA JOLLA, CA—The EZH1/2 inhibitor DS-3201b could be a novel therapeutic option for non-Hodgkin lymphoma (NHL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
DS-3201b was considered well tolerated in a phase 1 study of Japanese patients with relapsed/refractory NHL.
In addition, DS-3201b demonstrated activity against B- and T-cell lymphomas, producing an overall response rate of 59%.
Kunihiro Tsukasaki, MD, PhD, of Saitama Medical University in Moroyama, Saitama, Japan, presented these results at the meeting.
The trial was sponsored by Daiichi Sankyo Co., Ltd.
Dr Tsukasaki presented data on 18 patients with relapsed/refractory NHL.
The 12 B-cell lymphoma patients had follicular lymphoma (n=5), diffuse large B-cell lymphoma (n=3), MALT lymphoma (n=2), nodal marginal zone lymphoma (n=1), and lymphoplasmacytic lymphoma (n=1).
The 6 patients with T-cell lymphoma had peripheral T-cell lymphoma not otherwise specified (n=2), angioimmunoblastic T-cell lymphoma (n=2), and adult T-cell leukemia/lymphoma (n=2).
The patients’ median age was 67 (range, 44-75), and 10 were female. All patients had an ECOG performance status of 0 (72%) or 1 (28%).
Patients had a median of 2 prior chemotherapy regimens (range, 1-8).
For this study, they received DS-3201b at 150 mg (n=7), 200 mg (n=9), or 300 mg (n=2). They received the drug once daily in 28-day cycles until they progressed or experienced unacceptable toxicity.
DLTs and AEs
Dose-limiting toxicities (DLTs) were evaluated in cycle 1. All 18 patients were evaluable for DLT assessment.
There were 4 treatment-emergent adverse events (AEs) that met the definition of DLTs:
- 3 cases of grade 4 platelet count decrease (n=1 at 200 mg, n=2 at 300 mg)
- 1 case of grade 3 anemia requiring blood transfusion (at 300 mg).
All 4 DLTs led to treatment interruption.
There were 5 serious AEs reported in 3 patients. Only one of these—pneumocystis jiroveci pneumonia—was considered related to DS-3201b.
Hematologic AEs included decreases in platelets (grade 1-4), lymphocytes (grade 1-4), neutrophils (grade 2-4), and white blood cells (grade 2-3), as well as anemia (grade 1-3).
Other AEs (all grade 1/2) included dysgeusia, alopecia, diarrhea, decreased appetite, alanine aminotransferase increase, aspartate aminotransferase increase, nasopharyngitis, rash, and dry skin.
No deaths had been reported as of the data cutoff last November.
Responses
Seventeen patients were evaluable for response.
The overall response rate was 59%, with 1 patient achieving a complete response (CR) and 9 achieving a partial response (PR). Four patients had stable disease (SD), and 3 progressed.
Among the T-cell lymphoma patients, 1 had a CR, 4 had PRs, and 1 progressed. The complete responder had angioimmunoblastic T-cell lymphoma, and the patient who progressed had adult T-cell leukemia/lymphoma.
Among the B-cell lymphoma patients, 5 had PRs, 4 had SD, and 2 progressed.
Dr Tsukasaki said DS-3201b has demonstrated early clinical activity and therefore has the potential to be a novel therapeutic option for B-cell and T-cell lymphomas. However, further evaluation is warranted to determine the optimal dosing regimen and target diseases. ![]()
LA JOLLA, CA—The EZH1/2 inhibitor DS-3201b could be a novel therapeutic option for non-Hodgkin lymphoma (NHL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
DS-3201b was considered well tolerated in a phase 1 study of Japanese patients with relapsed/refractory NHL.
In addition, DS-3201b demonstrated activity against B- and T-cell lymphomas, producing an overall response rate of 59%.
Kunihiro Tsukasaki, MD, PhD, of Saitama Medical University in Moroyama, Saitama, Japan, presented these results at the meeting.
The trial was sponsored by Daiichi Sankyo Co., Ltd.
Dr Tsukasaki presented data on 18 patients with relapsed/refractory NHL.
The 12 B-cell lymphoma patients had follicular lymphoma (n=5), diffuse large B-cell lymphoma (n=3), MALT lymphoma (n=2), nodal marginal zone lymphoma (n=1), and lymphoplasmacytic lymphoma (n=1).
The 6 patients with T-cell lymphoma had peripheral T-cell lymphoma not otherwise specified (n=2), angioimmunoblastic T-cell lymphoma (n=2), and adult T-cell leukemia/lymphoma (n=2).
The patients’ median age was 67 (range, 44-75), and 10 were female. All patients had an ECOG performance status of 0 (72%) or 1 (28%).
Patients had a median of 2 prior chemotherapy regimens (range, 1-8).
For this study, they received DS-3201b at 150 mg (n=7), 200 mg (n=9), or 300 mg (n=2). They received the drug once daily in 28-day cycles until they progressed or experienced unacceptable toxicity.
DLTs and AEs
Dose-limiting toxicities (DLTs) were evaluated in cycle 1. All 18 patients were evaluable for DLT assessment.
There were 4 treatment-emergent adverse events (AEs) that met the definition of DLTs:
- 3 cases of grade 4 platelet count decrease (n=1 at 200 mg, n=2 at 300 mg)
- 1 case of grade 3 anemia requiring blood transfusion (at 300 mg).
All 4 DLTs led to treatment interruption.
There were 5 serious AEs reported in 3 patients. Only one of these—pneumocystis jiroveci pneumonia—was considered related to DS-3201b.
Hematologic AEs included decreases in platelets (grade 1-4), lymphocytes (grade 1-4), neutrophils (grade 2-4), and white blood cells (grade 2-3), as well as anemia (grade 1-3).
Other AEs (all grade 1/2) included dysgeusia, alopecia, diarrhea, decreased appetite, alanine aminotransferase increase, aspartate aminotransferase increase, nasopharyngitis, rash, and dry skin.
No deaths had been reported as of the data cutoff last November.
Responses
Seventeen patients were evaluable for response.
The overall response rate was 59%, with 1 patient achieving a complete response (CR) and 9 achieving a partial response (PR). Four patients had stable disease (SD), and 3 progressed.
Among the T-cell lymphoma patients, 1 had a CR, 4 had PRs, and 1 progressed. The complete responder had angioimmunoblastic T-cell lymphoma, and the patient who progressed had adult T-cell leukemia/lymphoma.
Among the B-cell lymphoma patients, 5 had PRs, 4 had SD, and 2 progressed.
Dr Tsukasaki said DS-3201b has demonstrated early clinical activity and therefore has the potential to be a novel therapeutic option for B-cell and T-cell lymphomas. However, further evaluation is warranted to determine the optimal dosing regimen and target diseases. ![]()