User login
Make a PEST of your psoriasis patients
KAUAI, HAWAII – for the rheumatologic disease once per year, advised Jashin J. Wu, MD. The PEST is a simple, validated, five-question yes/no screening tool. It’s geared towards nonrheumatologists who may not feel competent to diagnose psoriatic arthritis or who just don’t have time to do so. Three or more “yes” answers is deemed a positive result warranting consideration of referral to a rheumatologist, explained Dr. Wu, the director of the psoriasis clinic and director of dermatology research at Kaiser Permanente Los Angeles Medical Center.
The five PEST questions are:
- Have you ever had a swollen joint (or joints)?
- Has a doctor ever told you that you have arthritis?
- Do your fingernails or toenails have holes or pits?
- Have you had pain in your heel?
- Have you had a finger or toe that was completely swollen and painful for no apparent reason?
The PEST has been shown to have 92% sensitivity and 78% specificity for diagnosis of psoriatic arthritis (Clin Exp Rheumatol. 2009 May-Jun;27[3]:469-74).
Dr. Wu’s call for regular screening for psoriatic arthritis resonated with another psoriasis expert at the meeting, Craig L. Leonardi, MD.
“It’s our moral obligation to be on the lookout for that disease. Remember that patients who develop psoriatic arthritis usually have their skin disease for 10 years before they develop their first signs and symptoms of psoriatic arthritis. So that means they should be in the dermatologist’s office getting their skin treated as they start to have problems with their joints,” observed Dr. Leonardi, of Saint Louis University.
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – for the rheumatologic disease once per year, advised Jashin J. Wu, MD. The PEST is a simple, validated, five-question yes/no screening tool. It’s geared towards nonrheumatologists who may not feel competent to diagnose psoriatic arthritis or who just don’t have time to do so. Three or more “yes” answers is deemed a positive result warranting consideration of referral to a rheumatologist, explained Dr. Wu, the director of the psoriasis clinic and director of dermatology research at Kaiser Permanente Los Angeles Medical Center.
The five PEST questions are:
- Have you ever had a swollen joint (or joints)?
- Has a doctor ever told you that you have arthritis?
- Do your fingernails or toenails have holes or pits?
- Have you had pain in your heel?
- Have you had a finger or toe that was completely swollen and painful for no apparent reason?
The PEST has been shown to have 92% sensitivity and 78% specificity for diagnosis of psoriatic arthritis (Clin Exp Rheumatol. 2009 May-Jun;27[3]:469-74).
Dr. Wu’s call for regular screening for psoriatic arthritis resonated with another psoriasis expert at the meeting, Craig L. Leonardi, MD.
“It’s our moral obligation to be on the lookout for that disease. Remember that patients who develop psoriatic arthritis usually have their skin disease for 10 years before they develop their first signs and symptoms of psoriatic arthritis. So that means they should be in the dermatologist’s office getting their skin treated as they start to have problems with their joints,” observed Dr. Leonardi, of Saint Louis University.
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – for the rheumatologic disease once per year, advised Jashin J. Wu, MD. The PEST is a simple, validated, five-question yes/no screening tool. It’s geared towards nonrheumatologists who may not feel competent to diagnose psoriatic arthritis or who just don’t have time to do so. Three or more “yes” answers is deemed a positive result warranting consideration of referral to a rheumatologist, explained Dr. Wu, the director of the psoriasis clinic and director of dermatology research at Kaiser Permanente Los Angeles Medical Center.
The five PEST questions are:
- Have you ever had a swollen joint (or joints)?
- Has a doctor ever told you that you have arthritis?
- Do your fingernails or toenails have holes or pits?
- Have you had pain in your heel?
- Have you had a finger or toe that was completely swollen and painful for no apparent reason?
The PEST has been shown to have 92% sensitivity and 78% specificity for diagnosis of psoriatic arthritis (Clin Exp Rheumatol. 2009 May-Jun;27[3]:469-74).
Dr. Wu’s call for regular screening for psoriatic arthritis resonated with another psoriasis expert at the meeting, Craig L. Leonardi, MD.
“It’s our moral obligation to be on the lookout for that disease. Remember that patients who develop psoriatic arthritis usually have their skin disease for 10 years before they develop their first signs and symptoms of psoriatic arthritis. So that means they should be in the dermatologist’s office getting their skin treated as they start to have problems with their joints,” observed Dr. Leonardi, of Saint Louis University.
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Adult bronchiectasis, asthma therapy, frailty in ILD
Clinical Research
New guidelines for adult bronchiectasis
Clinically significant bronchiectasis is a combination of radiologic bronchial dilatation with clinical symptoms. Guidelines on management of adult bronchiectasis were recently published (Eur Respir J. 2017; Sep 10;50[3]).
For all adult patients with clinically significant bronchiectasis, the guidelines suggest standardized minimum testing with differential blood count, serum immunoglobulins, and testing for allergic bronchopulmonary aspergillosis with any further workup on an individual basis. Annual sputum surveillance is suggested for clinically stable adult patients; however, the evidence for this recommendation came from studies done on patients with cystic fibrosis.
Inhaled bronchodilators are suggested as the first-line treatment in symptomatic patients. Long-term antibiotics (greater than 3 months) are recommended in patients with greater than 3 exacerbations/year after optimizing airway clearance and disease-specific treatment. Pseudomonas aeruginosa infections are to be treated with inhaled antibiotics (colistin or gentamicin) (Charles SH, et al. Am J Respir Crit Care Med. 2014;189[8]975; Murray P, et al. Am J Respir Crit Care Med. 2011;183[4]:491; and nonpseudomonal infections are to be treated with macrolides (Conroy W, et al. Lancet. 2012;380[9842]:660; Altenburg J, et al. JAMA. 2013;309[12];1251), although interchangeable for intolerance. Sputum cultured early will guide therapy among poor responders. Long-term mucolytic agents are suggested in appropriate tolerating patients. Pulmonary rehabilitation for 6-8 weeks is strongly recommended in adult bronchiectasis with impaired exercise capacity. Surgical interventions for bronchiectasis are reserved for a small group of patients who have localized disease and high exacerbation rates despite maximal medical therapy. Inhaled corticosteroids are suggested not to be used in adult bronchiectasis. Guidelines recommend against the use of statins and recombinant human DNase as it increases exacerbations (Chest. 1998;113[5]:1329. The task force acknowledged the low quality of evidence for their recommendations requiring more research in the field of adult bronchiectasis.
Bharat Bajantri, MD
Fellow-in-Training Member
Airways Disorders
ICS/LABA combo therapy: black box warning removed
Publication of the Salmeterol Multicenter Asthma Research Trial (SMART) in 2006 caused panic among asthmatics and the physicians who treat them (Nelson et al. Chest. 2006;129[1]:15). The study suggested that the long-acting beta-2-agonist (LABA) salmeterol leads to an increased risk of asthma/respiratory-related deaths compared with placebo. This finding was more pronounced in the African American subpopulation. The study left many questions unanswered, including whether or not this risk is present when LABA therapy is combined with inhaled corticosteroids (ICS) (O’Byrne. Chest. 2006;129[1]:3). Subsequent meta-analyses confirmed the increased risk with LABA monotherapy but not with LABA/ICS (Salpeter et al. Ann Inter Med. 2006;144[12]: 904; Jaeschke et al. Am J Respir Crit Care Med. 2008;178[10]:1009). Still, a black box warning relating LABA to asthma-related death was applied to LABA/ICS products.
In 2011, the Food and Drug Administration (FDA) mandated large, randomized controlled trials be performed for LABA/ICS products to assess safety. These trials were recently completed, showing no difference in asthma-related deaths between LABA/ICS and ICS alone. There were 41, 297 patients across four trials, three included teenagers and adults (age ≥ 12) and one enrolled children (ages 4-11). These studies prompted the FDA to remove the black box warning from salmeterol/fluticasone, formoterol/budesonide, and formoterol/mometasone (https://wwwfdagov/downloads/Drugs/DrugSafety/UCM589997pdf).
Conclusion: Because LABA/ICS therapy is effective for asthma, most pulmonologists continue to prescribe it despite the SMART study results and FDA warning. In a practical sense, we don’t expect the FDA findings to radically change asthma care. Still, it seems we can finally put this question to rest – LABA/ICS is indeed safe for asthmatics. Most physicians will continue to avoid LABA monotherapy, and now that tiotropium is included in the 2017 GINA guidelines, it’s only matter of time before we’re debating whether LABA/long-acting muscarinic antagonist (LAMA) is safe for asthmatics.
Aaron Holley, MD, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Critical Care
Standardized handoffs in the ICU: room for improvement?
Transitions in patient care are commonplace in the ICU. But handoffs are particularly susceptible to error given the complexity of the patient population. Impacts of less-than-ideal handoffs likely include adverse events, delays in medical diagnosis and treatment, redundant communications, redundant activities such as additional procedures and tests, lower provider and patient satisfaction, higher costs, longer hospital stays, more hospital admissions, and less effective training for health -care providers. Yet, there is great heterogeneity in handoff practiced, and the impact of standardized handoffs in the ICU is unclear (Cochran A. JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5468. [Epub ahead of print]).
In a survey of over 600 academic intensivists, 55% of the participants stated that attending handoffs in the ICU should be standardized, yet, only 13% of those participating in handoffs reported using a standardized process (Lane-Fall M. Crit Care Med. 2016;44[4]690). Clinician miscommunication contributes to an estimated 250,000 deaths in US hospitals per year (Makary M. BMJ. 2016 May 3;353:i2139. doi: 10.1136/bmj.i2139). Standardized handoffs may improve outcomes in the ICU.
In many ICUs that do use standardized sign-out templates, higher clinician satisfaction and fewer unexpected patient events have been reported (Bavare AC. J Healthc Qual. 2015;37[5]:267; Nanchal R. BMJ Qual Saf. 2017;26[12]:987). In a recent randomized controlled trial, use of a standardized handoff curriculum in the ICU resulted in a significant 3% decrease in communication errors, without any change in the duration of the handoff. There also was a clinician-reported improvement in team communication and patient safety; but no changes in ICU length of stay, duration of mechanical ventilation, or number of re-intubations were noted (JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5440. [Epub ahead of print]).
Unfortunately, despite interest in improving patient handoffs, there are few tools to evaluate the effectiveness of different handoff strategies. Most studies report clinician perceptions rather than patient-centered outcomes. Further research is required to examine the optimal approach to handover communication. However, based on the available evidence, a standardized approach to handoffs is likely better than a nonstandardized format.
Shruti Gadre, MD
Fellow-in-Training Member
Christopher Carroll, MD, FCCP
Vice-Chair
Home-Based Mechanical Ventilation and Neuromuscular Disease
Update on two recent FDA-approved therapies for ALS and SMA
Amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) are neuromuscular diseases often deteriorating to progressive respiratory failure. Two medications received recent FDA approval and are now available in clinical practice – edaravone for ALS and nusinersen for SMA. We present a balanced overview of the favorable data along with realistic challenges.
Edaravone (Radicava) is the second FDA-approved medication for management of ALS (Riluzole was approved over 20 years ago). Edaravone is a free radical scavenger that reduces oxidative stress, resulting in a protective effect on neuronal cells. It originally showed promise in acute ischemic stroke in Japan and was subsequently studied for ALS. A phase 3 randomized, double-blind placebo-controlled study performed in Japan (Lancet Neurol. 2017;16[7]:505) compared ALSFRS-R scores of a specific subset of ALS patients receiving edaravone vs placebo. This study revealed that patients with early ALS (2 years duration or less) with rapid progression (ALSFRS-R score of 7.5 in 6 months) had a 33% decrease in their degree of progression (reducing their ALSFRS-R score to 5) in the edaravone group. Of note, there was also slowing in the decline of FVC, though not clinically significant. Although the drug was rapidly approved by the FDA, there are obvious challenges that must be recognized. First, it is unclear if patients will discern such a mild degree of slowing of disease progression. In addition, the annual cost may be prohibitive, and lifelong IV administration of the medication for 10 days every month may pose logistical barriers.
Nusinersen (Spinraza) is the first FDA-approved therapeutic medication for spinal muscular atrophy (SMA). SMA is a hereditary neuromuscular disorder leading to degeneration of motor neuron cells and ultimately diffuse muscle weakness and often respiratory failure. Nusinersen is an antisense oligonucleotide that modifies splicing of the SMN-2 gene to increase production of normal, full-length SMN protein, which is deficient in SMA. The ENDEAR trial (Finkel RS, et al. N Engl J Med. 2017;377[18]:1723) was a phase 3, multicenter, double-blind study that enrolled SMA infants to receive nusinersen vs sham. Infants who received treatment had improvements in motor milestones (41% vs 0%) and less permanent-assisted ventilation or death in the nusinersen group (39% vs 68%), a 47% reduction in risk of death. The therapy is safe and tolerable, although there is reported risk of bleeding abnormalities, renal toxicity, and constipation. Administered intrathecally, there is a series of four loading doses, followed by maintenance doses every 4 months – presumably lifelong. Although FDA approved all three SMA subtypes, the eventual impact is uncertain, especially in cases of advanced muscle weakness. There are realistic challenges: the high cost ($125,000/dose), limited longitudinal evidence, technical administration, and limited access.
Pulmonologists should be aware of both medications as new therapeutic options for ALS and SMA; however, the long-term impact is yet to be determined.
Ashraf Elsayegh, MD, FCCP
Steering Committee Member
Won Y. Lee, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Frailty as a measure of disease activity in ILD
Frailty is a systemic geriatric syndrome characterized by age-related accumulation of physiologic deficits across several systems with an attenuated response to biological stress. Considering that interstitial lung disease (ILD), particularly, idiopathic pulmonary fibrosis (IPF), is a disease of the aging population, frailty is an emerging area of clinical interest. The biological pathways driving the association of frailty with worse prognosis are complex but hinge on cellular senescence, systemic inflammation, and sarcopenia.
There is a high prevalence of frailty in adults with chronic lung diseases and is associated with worse prognosis. The current literature, though, is mostly derived from patients with COPD. Frailty measured using the 42-item patient-reported frailty index is associated with dyspnea severity in patients with fibrotic ILD (Milne et al. Respirology. 2017;22[4]:728) and systemic sclerosis-associated ILD (Guler et al. Respir Med. 2017 Aug;129:1-7. doi: 10.1016/j.rmed.2017.05.012. Epub 2017 May 25.). The SHARE-Frailty and the Edmonton Frail Scale instruments utilized to measure frailty in the University of Alabama at Birmingham IPF cohort detected a high-percentage of frail and pre-frail patients (Luckhardt et al. Am J Respir Crit Care Med. 2017;195:A7012). However, there are differences in targeted domains between the various frailty instruments, and this could affect the identification of the frailty syndrome in patients.
Frailty as a measure of disease activity and progression is not currently employed in clinical trials for ILD, primarily due to lack of standardized tools for this patient population. Future studies designed to utilize the frailty syndrome as outcome measures may further our understanding of the clinical manifestations and underlying mechanisms, as well as identify potential therapeutic interventions for patients with ILD.
Tejaswini Kulkarni MD, MPH
Fellow-in-Training Member
Clinical Research
New guidelines for adult bronchiectasis
Clinically significant bronchiectasis is a combination of radiologic bronchial dilatation with clinical symptoms. Guidelines on management of adult bronchiectasis were recently published (Eur Respir J. 2017; Sep 10;50[3]).
For all adult patients with clinically significant bronchiectasis, the guidelines suggest standardized minimum testing with differential blood count, serum immunoglobulins, and testing for allergic bronchopulmonary aspergillosis with any further workup on an individual basis. Annual sputum surveillance is suggested for clinically stable adult patients; however, the evidence for this recommendation came from studies done on patients with cystic fibrosis.
Inhaled bronchodilators are suggested as the first-line treatment in symptomatic patients. Long-term antibiotics (greater than 3 months) are recommended in patients with greater than 3 exacerbations/year after optimizing airway clearance and disease-specific treatment. Pseudomonas aeruginosa infections are to be treated with inhaled antibiotics (colistin or gentamicin) (Charles SH, et al. Am J Respir Crit Care Med. 2014;189[8]975; Murray P, et al. Am J Respir Crit Care Med. 2011;183[4]:491; and nonpseudomonal infections are to be treated with macrolides (Conroy W, et al. Lancet. 2012;380[9842]:660; Altenburg J, et al. JAMA. 2013;309[12];1251), although interchangeable for intolerance. Sputum cultured early will guide therapy among poor responders. Long-term mucolytic agents are suggested in appropriate tolerating patients. Pulmonary rehabilitation for 6-8 weeks is strongly recommended in adult bronchiectasis with impaired exercise capacity. Surgical interventions for bronchiectasis are reserved for a small group of patients who have localized disease and high exacerbation rates despite maximal medical therapy. Inhaled corticosteroids are suggested not to be used in adult bronchiectasis. Guidelines recommend against the use of statins and recombinant human DNase as it increases exacerbations (Chest. 1998;113[5]:1329. The task force acknowledged the low quality of evidence for their recommendations requiring more research in the field of adult bronchiectasis.
Bharat Bajantri, MD
Fellow-in-Training Member
Airways Disorders
ICS/LABA combo therapy: black box warning removed
Publication of the Salmeterol Multicenter Asthma Research Trial (SMART) in 2006 caused panic among asthmatics and the physicians who treat them (Nelson et al. Chest. 2006;129[1]:15). The study suggested that the long-acting beta-2-agonist (LABA) salmeterol leads to an increased risk of asthma/respiratory-related deaths compared with placebo. This finding was more pronounced in the African American subpopulation. The study left many questions unanswered, including whether or not this risk is present when LABA therapy is combined with inhaled corticosteroids (ICS) (O’Byrne. Chest. 2006;129[1]:3). Subsequent meta-analyses confirmed the increased risk with LABA monotherapy but not with LABA/ICS (Salpeter et al. Ann Inter Med. 2006;144[12]: 904; Jaeschke et al. Am J Respir Crit Care Med. 2008;178[10]:1009). Still, a black box warning relating LABA to asthma-related death was applied to LABA/ICS products.
In 2011, the Food and Drug Administration (FDA) mandated large, randomized controlled trials be performed for LABA/ICS products to assess safety. These trials were recently completed, showing no difference in asthma-related deaths between LABA/ICS and ICS alone. There were 41, 297 patients across four trials, three included teenagers and adults (age ≥ 12) and one enrolled children (ages 4-11). These studies prompted the FDA to remove the black box warning from salmeterol/fluticasone, formoterol/budesonide, and formoterol/mometasone (https://wwwfdagov/downloads/Drugs/DrugSafety/UCM589997pdf).
Conclusion: Because LABA/ICS therapy is effective for asthma, most pulmonologists continue to prescribe it despite the SMART study results and FDA warning. In a practical sense, we don’t expect the FDA findings to radically change asthma care. Still, it seems we can finally put this question to rest – LABA/ICS is indeed safe for asthmatics. Most physicians will continue to avoid LABA monotherapy, and now that tiotropium is included in the 2017 GINA guidelines, it’s only matter of time before we’re debating whether LABA/long-acting muscarinic antagonist (LAMA) is safe for asthmatics.
Aaron Holley, MD, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Critical Care
Standardized handoffs in the ICU: room for improvement?
Transitions in patient care are commonplace in the ICU. But handoffs are particularly susceptible to error given the complexity of the patient population. Impacts of less-than-ideal handoffs likely include adverse events, delays in medical diagnosis and treatment, redundant communications, redundant activities such as additional procedures and tests, lower provider and patient satisfaction, higher costs, longer hospital stays, more hospital admissions, and less effective training for health -care providers. Yet, there is great heterogeneity in handoff practiced, and the impact of standardized handoffs in the ICU is unclear (Cochran A. JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5468. [Epub ahead of print]).
In a survey of over 600 academic intensivists, 55% of the participants stated that attending handoffs in the ICU should be standardized, yet, only 13% of those participating in handoffs reported using a standardized process (Lane-Fall M. Crit Care Med. 2016;44[4]690). Clinician miscommunication contributes to an estimated 250,000 deaths in US hospitals per year (Makary M. BMJ. 2016 May 3;353:i2139. doi: 10.1136/bmj.i2139). Standardized handoffs may improve outcomes in the ICU.
In many ICUs that do use standardized sign-out templates, higher clinician satisfaction and fewer unexpected patient events have been reported (Bavare AC. J Healthc Qual. 2015;37[5]:267; Nanchal R. BMJ Qual Saf. 2017;26[12]:987). In a recent randomized controlled trial, use of a standardized handoff curriculum in the ICU resulted in a significant 3% decrease in communication errors, without any change in the duration of the handoff. There also was a clinician-reported improvement in team communication and patient safety; but no changes in ICU length of stay, duration of mechanical ventilation, or number of re-intubations were noted (JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5440. [Epub ahead of print]).
Unfortunately, despite interest in improving patient handoffs, there are few tools to evaluate the effectiveness of different handoff strategies. Most studies report clinician perceptions rather than patient-centered outcomes. Further research is required to examine the optimal approach to handover communication. However, based on the available evidence, a standardized approach to handoffs is likely better than a nonstandardized format.
Shruti Gadre, MD
Fellow-in-Training Member
Christopher Carroll, MD, FCCP
Vice-Chair
Home-Based Mechanical Ventilation and Neuromuscular Disease
Update on two recent FDA-approved therapies for ALS and SMA
Amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) are neuromuscular diseases often deteriorating to progressive respiratory failure. Two medications received recent FDA approval and are now available in clinical practice – edaravone for ALS and nusinersen for SMA. We present a balanced overview of the favorable data along with realistic challenges.
Edaravone (Radicava) is the second FDA-approved medication for management of ALS (Riluzole was approved over 20 years ago). Edaravone is a free radical scavenger that reduces oxidative stress, resulting in a protective effect on neuronal cells. It originally showed promise in acute ischemic stroke in Japan and was subsequently studied for ALS. A phase 3 randomized, double-blind placebo-controlled study performed in Japan (Lancet Neurol. 2017;16[7]:505) compared ALSFRS-R scores of a specific subset of ALS patients receiving edaravone vs placebo. This study revealed that patients with early ALS (2 years duration or less) with rapid progression (ALSFRS-R score of 7.5 in 6 months) had a 33% decrease in their degree of progression (reducing their ALSFRS-R score to 5) in the edaravone group. Of note, there was also slowing in the decline of FVC, though not clinically significant. Although the drug was rapidly approved by the FDA, there are obvious challenges that must be recognized. First, it is unclear if patients will discern such a mild degree of slowing of disease progression. In addition, the annual cost may be prohibitive, and lifelong IV administration of the medication for 10 days every month may pose logistical barriers.
Nusinersen (Spinraza) is the first FDA-approved therapeutic medication for spinal muscular atrophy (SMA). SMA is a hereditary neuromuscular disorder leading to degeneration of motor neuron cells and ultimately diffuse muscle weakness and often respiratory failure. Nusinersen is an antisense oligonucleotide that modifies splicing of the SMN-2 gene to increase production of normal, full-length SMN protein, which is deficient in SMA. The ENDEAR trial (Finkel RS, et al. N Engl J Med. 2017;377[18]:1723) was a phase 3, multicenter, double-blind study that enrolled SMA infants to receive nusinersen vs sham. Infants who received treatment had improvements in motor milestones (41% vs 0%) and less permanent-assisted ventilation or death in the nusinersen group (39% vs 68%), a 47% reduction in risk of death. The therapy is safe and tolerable, although there is reported risk of bleeding abnormalities, renal toxicity, and constipation. Administered intrathecally, there is a series of four loading doses, followed by maintenance doses every 4 months – presumably lifelong. Although FDA approved all three SMA subtypes, the eventual impact is uncertain, especially in cases of advanced muscle weakness. There are realistic challenges: the high cost ($125,000/dose), limited longitudinal evidence, technical administration, and limited access.
Pulmonologists should be aware of both medications as new therapeutic options for ALS and SMA; however, the long-term impact is yet to be determined.
Ashraf Elsayegh, MD, FCCP
Steering Committee Member
Won Y. Lee, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Frailty as a measure of disease activity in ILD
Frailty is a systemic geriatric syndrome characterized by age-related accumulation of physiologic deficits across several systems with an attenuated response to biological stress. Considering that interstitial lung disease (ILD), particularly, idiopathic pulmonary fibrosis (IPF), is a disease of the aging population, frailty is an emerging area of clinical interest. The biological pathways driving the association of frailty with worse prognosis are complex but hinge on cellular senescence, systemic inflammation, and sarcopenia.
There is a high prevalence of frailty in adults with chronic lung diseases and is associated with worse prognosis. The current literature, though, is mostly derived from patients with COPD. Frailty measured using the 42-item patient-reported frailty index is associated with dyspnea severity in patients with fibrotic ILD (Milne et al. Respirology. 2017;22[4]:728) and systemic sclerosis-associated ILD (Guler et al. Respir Med. 2017 Aug;129:1-7. doi: 10.1016/j.rmed.2017.05.012. Epub 2017 May 25.). The SHARE-Frailty and the Edmonton Frail Scale instruments utilized to measure frailty in the University of Alabama at Birmingham IPF cohort detected a high-percentage of frail and pre-frail patients (Luckhardt et al. Am J Respir Crit Care Med. 2017;195:A7012). However, there are differences in targeted domains between the various frailty instruments, and this could affect the identification of the frailty syndrome in patients.
Frailty as a measure of disease activity and progression is not currently employed in clinical trials for ILD, primarily due to lack of standardized tools for this patient population. Future studies designed to utilize the frailty syndrome as outcome measures may further our understanding of the clinical manifestations and underlying mechanisms, as well as identify potential therapeutic interventions for patients with ILD.
Tejaswini Kulkarni MD, MPH
Fellow-in-Training Member
Clinical Research
New guidelines for adult bronchiectasis
Clinically significant bronchiectasis is a combination of radiologic bronchial dilatation with clinical symptoms. Guidelines on management of adult bronchiectasis were recently published (Eur Respir J. 2017; Sep 10;50[3]).
For all adult patients with clinically significant bronchiectasis, the guidelines suggest standardized minimum testing with differential blood count, serum immunoglobulins, and testing for allergic bronchopulmonary aspergillosis with any further workup on an individual basis. Annual sputum surveillance is suggested for clinically stable adult patients; however, the evidence for this recommendation came from studies done on patients with cystic fibrosis.
Inhaled bronchodilators are suggested as the first-line treatment in symptomatic patients. Long-term antibiotics (greater than 3 months) are recommended in patients with greater than 3 exacerbations/year after optimizing airway clearance and disease-specific treatment. Pseudomonas aeruginosa infections are to be treated with inhaled antibiotics (colistin or gentamicin) (Charles SH, et al. Am J Respir Crit Care Med. 2014;189[8]975; Murray P, et al. Am J Respir Crit Care Med. 2011;183[4]:491; and nonpseudomonal infections are to be treated with macrolides (Conroy W, et al. Lancet. 2012;380[9842]:660; Altenburg J, et al. JAMA. 2013;309[12];1251), although interchangeable for intolerance. Sputum cultured early will guide therapy among poor responders. Long-term mucolytic agents are suggested in appropriate tolerating patients. Pulmonary rehabilitation for 6-8 weeks is strongly recommended in adult bronchiectasis with impaired exercise capacity. Surgical interventions for bronchiectasis are reserved for a small group of patients who have localized disease and high exacerbation rates despite maximal medical therapy. Inhaled corticosteroids are suggested not to be used in adult bronchiectasis. Guidelines recommend against the use of statins and recombinant human DNase as it increases exacerbations (Chest. 1998;113[5]:1329. The task force acknowledged the low quality of evidence for their recommendations requiring more research in the field of adult bronchiectasis.
Bharat Bajantri, MD
Fellow-in-Training Member
Airways Disorders
ICS/LABA combo therapy: black box warning removed
Publication of the Salmeterol Multicenter Asthma Research Trial (SMART) in 2006 caused panic among asthmatics and the physicians who treat them (Nelson et al. Chest. 2006;129[1]:15). The study suggested that the long-acting beta-2-agonist (LABA) salmeterol leads to an increased risk of asthma/respiratory-related deaths compared with placebo. This finding was more pronounced in the African American subpopulation. The study left many questions unanswered, including whether or not this risk is present when LABA therapy is combined with inhaled corticosteroids (ICS) (O’Byrne. Chest. 2006;129[1]:3). Subsequent meta-analyses confirmed the increased risk with LABA monotherapy but not with LABA/ICS (Salpeter et al. Ann Inter Med. 2006;144[12]: 904; Jaeschke et al. Am J Respir Crit Care Med. 2008;178[10]:1009). Still, a black box warning relating LABA to asthma-related death was applied to LABA/ICS products.
In 2011, the Food and Drug Administration (FDA) mandated large, randomized controlled trials be performed for LABA/ICS products to assess safety. These trials were recently completed, showing no difference in asthma-related deaths between LABA/ICS and ICS alone. There were 41, 297 patients across four trials, three included teenagers and adults (age ≥ 12) and one enrolled children (ages 4-11). These studies prompted the FDA to remove the black box warning from salmeterol/fluticasone, formoterol/budesonide, and formoterol/mometasone (https://wwwfdagov/downloads/Drugs/DrugSafety/UCM589997pdf).
Conclusion: Because LABA/ICS therapy is effective for asthma, most pulmonologists continue to prescribe it despite the SMART study results and FDA warning. In a practical sense, we don’t expect the FDA findings to radically change asthma care. Still, it seems we can finally put this question to rest – LABA/ICS is indeed safe for asthmatics. Most physicians will continue to avoid LABA monotherapy, and now that tiotropium is included in the 2017 GINA guidelines, it’s only matter of time before we’re debating whether LABA/long-acting muscarinic antagonist (LAMA) is safe for asthmatics.
Aaron Holley, MD, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Critical Care
Standardized handoffs in the ICU: room for improvement?
Transitions in patient care are commonplace in the ICU. But handoffs are particularly susceptible to error given the complexity of the patient population. Impacts of less-than-ideal handoffs likely include adverse events, delays in medical diagnosis and treatment, redundant communications, redundant activities such as additional procedures and tests, lower provider and patient satisfaction, higher costs, longer hospital stays, more hospital admissions, and less effective training for health -care providers. Yet, there is great heterogeneity in handoff practiced, and the impact of standardized handoffs in the ICU is unclear (Cochran A. JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5468. [Epub ahead of print]).
In a survey of over 600 academic intensivists, 55% of the participants stated that attending handoffs in the ICU should be standardized, yet, only 13% of those participating in handoffs reported using a standardized process (Lane-Fall M. Crit Care Med. 2016;44[4]690). Clinician miscommunication contributes to an estimated 250,000 deaths in US hospitals per year (Makary M. BMJ. 2016 May 3;353:i2139. doi: 10.1136/bmj.i2139). Standardized handoffs may improve outcomes in the ICU.
In many ICUs that do use standardized sign-out templates, higher clinician satisfaction and fewer unexpected patient events have been reported (Bavare AC. J Healthc Qual. 2015;37[5]:267; Nanchal R. BMJ Qual Saf. 2017;26[12]:987). In a recent randomized controlled trial, use of a standardized handoff curriculum in the ICU resulted in a significant 3% decrease in communication errors, without any change in the duration of the handoff. There also was a clinician-reported improvement in team communication and patient safety; but no changes in ICU length of stay, duration of mechanical ventilation, or number of re-intubations were noted (JAMA Surg. 2018 Jan 3. doi: 10.1001/jamasurg.2017.5440. [Epub ahead of print]).
Unfortunately, despite interest in improving patient handoffs, there are few tools to evaluate the effectiveness of different handoff strategies. Most studies report clinician perceptions rather than patient-centered outcomes. Further research is required to examine the optimal approach to handover communication. However, based on the available evidence, a standardized approach to handoffs is likely better than a nonstandardized format.
Shruti Gadre, MD
Fellow-in-Training Member
Christopher Carroll, MD, FCCP
Vice-Chair
Home-Based Mechanical Ventilation and Neuromuscular Disease
Update on two recent FDA-approved therapies for ALS and SMA
Amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) are neuromuscular diseases often deteriorating to progressive respiratory failure. Two medications received recent FDA approval and are now available in clinical practice – edaravone for ALS and nusinersen for SMA. We present a balanced overview of the favorable data along with realistic challenges.
Edaravone (Radicava) is the second FDA-approved medication for management of ALS (Riluzole was approved over 20 years ago). Edaravone is a free radical scavenger that reduces oxidative stress, resulting in a protective effect on neuronal cells. It originally showed promise in acute ischemic stroke in Japan and was subsequently studied for ALS. A phase 3 randomized, double-blind placebo-controlled study performed in Japan (Lancet Neurol. 2017;16[7]:505) compared ALSFRS-R scores of a specific subset of ALS patients receiving edaravone vs placebo. This study revealed that patients with early ALS (2 years duration or less) with rapid progression (ALSFRS-R score of 7.5 in 6 months) had a 33% decrease in their degree of progression (reducing their ALSFRS-R score to 5) in the edaravone group. Of note, there was also slowing in the decline of FVC, though not clinically significant. Although the drug was rapidly approved by the FDA, there are obvious challenges that must be recognized. First, it is unclear if patients will discern such a mild degree of slowing of disease progression. In addition, the annual cost may be prohibitive, and lifelong IV administration of the medication for 10 days every month may pose logistical barriers.
Nusinersen (Spinraza) is the first FDA-approved therapeutic medication for spinal muscular atrophy (SMA). SMA is a hereditary neuromuscular disorder leading to degeneration of motor neuron cells and ultimately diffuse muscle weakness and often respiratory failure. Nusinersen is an antisense oligonucleotide that modifies splicing of the SMN-2 gene to increase production of normal, full-length SMN protein, which is deficient in SMA. The ENDEAR trial (Finkel RS, et al. N Engl J Med. 2017;377[18]:1723) was a phase 3, multicenter, double-blind study that enrolled SMA infants to receive nusinersen vs sham. Infants who received treatment had improvements in motor milestones (41% vs 0%) and less permanent-assisted ventilation or death in the nusinersen group (39% vs 68%), a 47% reduction in risk of death. The therapy is safe and tolerable, although there is reported risk of bleeding abnormalities, renal toxicity, and constipation. Administered intrathecally, there is a series of four loading doses, followed by maintenance doses every 4 months – presumably lifelong. Although FDA approved all three SMA subtypes, the eventual impact is uncertain, especially in cases of advanced muscle weakness. There are realistic challenges: the high cost ($125,000/dose), limited longitudinal evidence, technical administration, and limited access.
Pulmonologists should be aware of both medications as new therapeutic options for ALS and SMA; however, the long-term impact is yet to be determined.
Ashraf Elsayegh, MD, FCCP
Steering Committee Member
Won Y. Lee, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Frailty as a measure of disease activity in ILD
Frailty is a systemic geriatric syndrome characterized by age-related accumulation of physiologic deficits across several systems with an attenuated response to biological stress. Considering that interstitial lung disease (ILD), particularly, idiopathic pulmonary fibrosis (IPF), is a disease of the aging population, frailty is an emerging area of clinical interest. The biological pathways driving the association of frailty with worse prognosis are complex but hinge on cellular senescence, systemic inflammation, and sarcopenia.
There is a high prevalence of frailty in adults with chronic lung diseases and is associated with worse prognosis. The current literature, though, is mostly derived from patients with COPD. Frailty measured using the 42-item patient-reported frailty index is associated with dyspnea severity in patients with fibrotic ILD (Milne et al. Respirology. 2017;22[4]:728) and systemic sclerosis-associated ILD (Guler et al. Respir Med. 2017 Aug;129:1-7. doi: 10.1016/j.rmed.2017.05.012. Epub 2017 May 25.). The SHARE-Frailty and the Edmonton Frail Scale instruments utilized to measure frailty in the University of Alabama at Birmingham IPF cohort detected a high-percentage of frail and pre-frail patients (Luckhardt et al. Am J Respir Crit Care Med. 2017;195:A7012). However, there are differences in targeted domains between the various frailty instruments, and this could affect the identification of the frailty syndrome in patients.
Frailty as a measure of disease activity and progression is not currently employed in clinical trials for ILD, primarily due to lack of standardized tools for this patient population. Future studies designed to utilize the frailty syndrome as outcome measures may further our understanding of the clinical manifestations and underlying mechanisms, as well as identify potential therapeutic interventions for patients with ILD.
Tejaswini Kulkarni MD, MPH
Fellow-in-Training Member
Postoperative pulmonary complications of cardiac surgery
Cardiac surgery patients are sicker today than in previous decades due to an aging population and a rising complexity in medical care. There is an increasing reliance on noncardiac surgeons to care for these patients. The optimal postoperative providers and structure of the ICU where patients are cared for remain unclear, but what is irrefutable is patients’ increased postoperative morbidity. Pulmonary complications are a leading cause of morbidity in these patients, occurring in up to one-fifth of cases (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Common pulmonary complications of cardiac surgery are listed in Table 1. Those complications, captured by The Society of Thoracic Surgeons (STS) Cardiac Surgery Database, include receiving ventilation longer than 24 hours, pneumonia, pulmonary embolism, and pleural effusion requiring drainage (The Society of Thoracic Surgeons. STS National Database. https://www.sts.org/registries-research-center/sts-national-database. Accessed January 9, 2018).
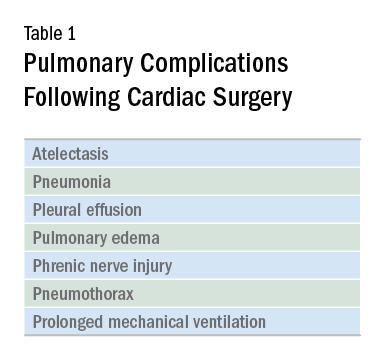
It should come as no surprise that cardiac surgery can have pronounced effects on lung function. The anesthetic agents, chest wall alteration, and direct lung manipulation can all affect pulmonary parameters. Functional residual capacity (FRC) can decrease by up to 20% with anesthesia (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531), and the thoracic manipulation and alteration of rib cage mechanics with a classic median sternotomy approach can lead to decreases in forced vital capacity (FVC) and expiratory volume in the first second of forced expiration (FEV1) that can last for months after surgery. Use of the cardiopulmonary bypass circuit can also lead to bronchoconstriction. These changes in pulmonary function are less pronounced in alternative surgical approaches, such as partial sternotomies (Weissman C. Seminars in Cardiothoracic and Vascular Anesthesia: Pulmonary Complications After Cardiac Surgery. Glen Head, NY: Westminister Publications; 2004).
The most frequent pulmonary consequence of cardiac surgery is atelectasis, seen on postoperative chest radiographs in approximately 50% to 90% of patients (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Induction, apnea during cardiopulmonary bypass, manual compression of the lungs for surgical exposure, internal mammary harvesting, and pleurotomy can lead to atelectasis in the intraoperative setting while weak cough, poor inspiratory efforts, interstitial edema, and immobility further contribute postoperatively (Weissman 2004). While frequently seen, clinically significant pulmonary consequences from this radiographic finding alone are rare (Weissman 2004).
Pleural effusions are seen on immediate postoperative chest radiographs in the majority of patients. Additionally, 10% to 40% of patients develop pleural effusions 2 to 3 weeks after surgery secondary to postpericardiotomy syndrome. While some effusions require drainage and further intervention (eg, hemothorax), most effusions require no specific treatment and resolve over time (Weissman 2004).
The prevalence of pneumonia following cardiac surgery varies based on differences in study populations and diagnostic criteria, but it remains an important source of morbidity and mortality. In one series, postoperative pneumonia occurred in 3.1% of patients, with higher rates observed in patients who were older, had worse left ventricular ejection fraction, had COPD, experienced longer bypass times, and received more red blood cell transfusions in the operating room (Allou N, et al. Crit Care Med. 2014;42[5]:1150). A meta-analysis found that an average of 6.37% of patients developed ventilator-associated pneumonia (VAP), and this rose to 35.2% in those receiving ventilation for greater than 48 hours. Those who developed VAP had an odds ratio of dying of 15.18 (95% CI 5.81-39.68) compared with those who did not (He S, et al. J Thorac Cardiovasc Surg. 2014;148[6]:3148).
A small proportion of patients go on to develop ARDS. While relatively uncommon, ARDS carries a high mortality rate. Many possible etiologies for ARDS in cardiac surgery patients have been proposed, including an inflammatory response related to the cardiopulmonary bypass circuit, reperfusion injury secondary to reduced pulmonary blood flow during bypass, protamine administration, transfusion, hypothermia, and lack of ventilation during bypass (Weissman 2004); (Stephens RS, et al. Ann Thorac Surg. 2013;95[3]:1122). Type of surgery may also play a role, as patients who undergo aortic surgery are at an even greater risk (Stephens 2013). As with other cases of ARDS, treatment is supportive: low tidal volume ventilation and careful management of fluid balance, as well as paralysis, prone positioning, and consideration for extracorporeal membrane oxygenation (ECMO), as appropriate (Stephens 2013).
Therapies to prevent postoperative pulmonary complications have included early extubation, aggressive pain control, deep breathing, physical therapy, early mobilization, and noninvasive ventilation in the form of CPAP and intermittent positive pressure breathing. A meta-analysis of 18 trials looking at the use of various forms of prophylactic postoperative physiotherapy did not show a difference in any measured clinical outcome (Pasquina P, Walder B. Br Med J. 2003;327[7428]:1379).
However, the heterogeneity, short follow-up, and low quality of included studies made it difficult to draw meaningful conclusions on the benefit or lack thereof for these therapies. More recent studies have shown promise for chest physiotherapy started several weeks prior to elective coronary bypass graft surgery and extended CPAP via nasal CPAP mask immediately following extubation (Hulzebos EH. JAMA. 2006;296[15]:1851), (Stephens 2013).
Ongoing areas for improvement include further clarification and standardization of best practices for postcardiac surgery patients, including blood product transfusion, optimal tidal volumes for surgical and postsurgical ventilation, timing of extubation, and the use of preventive therapies in the pre- and postsurgical periods. As providers who care for these patients, understanding how we can improve their postoperative pulmonary recovery will allow us to enhance our patient’s experience.
Dr. Noel is a Critical Care Fellow, Cooper Medical School of Rowan University, Camden, New Jersey.
Cardiac surgery patients are sicker today than in previous decades due to an aging population and a rising complexity in medical care. There is an increasing reliance on noncardiac surgeons to care for these patients. The optimal postoperative providers and structure of the ICU where patients are cared for remain unclear, but what is irrefutable is patients’ increased postoperative morbidity. Pulmonary complications are a leading cause of morbidity in these patients, occurring in up to one-fifth of cases (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Common pulmonary complications of cardiac surgery are listed in Table 1. Those complications, captured by The Society of Thoracic Surgeons (STS) Cardiac Surgery Database, include receiving ventilation longer than 24 hours, pneumonia, pulmonary embolism, and pleural effusion requiring drainage (The Society of Thoracic Surgeons. STS National Database. https://www.sts.org/registries-research-center/sts-national-database. Accessed January 9, 2018).

It should come as no surprise that cardiac surgery can have pronounced effects on lung function. The anesthetic agents, chest wall alteration, and direct lung manipulation can all affect pulmonary parameters. Functional residual capacity (FRC) can decrease by up to 20% with anesthesia (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531), and the thoracic manipulation and alteration of rib cage mechanics with a classic median sternotomy approach can lead to decreases in forced vital capacity (FVC) and expiratory volume in the first second of forced expiration (FEV1) that can last for months after surgery. Use of the cardiopulmonary bypass circuit can also lead to bronchoconstriction. These changes in pulmonary function are less pronounced in alternative surgical approaches, such as partial sternotomies (Weissman C. Seminars in Cardiothoracic and Vascular Anesthesia: Pulmonary Complications After Cardiac Surgery. Glen Head, NY: Westminister Publications; 2004).
The most frequent pulmonary consequence of cardiac surgery is atelectasis, seen on postoperative chest radiographs in approximately 50% to 90% of patients (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Induction, apnea during cardiopulmonary bypass, manual compression of the lungs for surgical exposure, internal mammary harvesting, and pleurotomy can lead to atelectasis in the intraoperative setting while weak cough, poor inspiratory efforts, interstitial edema, and immobility further contribute postoperatively (Weissman 2004). While frequently seen, clinically significant pulmonary consequences from this radiographic finding alone are rare (Weissman 2004).
Pleural effusions are seen on immediate postoperative chest radiographs in the majority of patients. Additionally, 10% to 40% of patients develop pleural effusions 2 to 3 weeks after surgery secondary to postpericardiotomy syndrome. While some effusions require drainage and further intervention (eg, hemothorax), most effusions require no specific treatment and resolve over time (Weissman 2004).
The prevalence of pneumonia following cardiac surgery varies based on differences in study populations and diagnostic criteria, but it remains an important source of morbidity and mortality. In one series, postoperative pneumonia occurred in 3.1% of patients, with higher rates observed in patients who were older, had worse left ventricular ejection fraction, had COPD, experienced longer bypass times, and received more red blood cell transfusions in the operating room (Allou N, et al. Crit Care Med. 2014;42[5]:1150). A meta-analysis found that an average of 6.37% of patients developed ventilator-associated pneumonia (VAP), and this rose to 35.2% in those receiving ventilation for greater than 48 hours. Those who developed VAP had an odds ratio of dying of 15.18 (95% CI 5.81-39.68) compared with those who did not (He S, et al. J Thorac Cardiovasc Surg. 2014;148[6]:3148).
A small proportion of patients go on to develop ARDS. While relatively uncommon, ARDS carries a high mortality rate. Many possible etiologies for ARDS in cardiac surgery patients have been proposed, including an inflammatory response related to the cardiopulmonary bypass circuit, reperfusion injury secondary to reduced pulmonary blood flow during bypass, protamine administration, transfusion, hypothermia, and lack of ventilation during bypass (Weissman 2004); (Stephens RS, et al. Ann Thorac Surg. 2013;95[3]:1122). Type of surgery may also play a role, as patients who undergo aortic surgery are at an even greater risk (Stephens 2013). As with other cases of ARDS, treatment is supportive: low tidal volume ventilation and careful management of fluid balance, as well as paralysis, prone positioning, and consideration for extracorporeal membrane oxygenation (ECMO), as appropriate (Stephens 2013).
Therapies to prevent postoperative pulmonary complications have included early extubation, aggressive pain control, deep breathing, physical therapy, early mobilization, and noninvasive ventilation in the form of CPAP and intermittent positive pressure breathing. A meta-analysis of 18 trials looking at the use of various forms of prophylactic postoperative physiotherapy did not show a difference in any measured clinical outcome (Pasquina P, Walder B. Br Med J. 2003;327[7428]:1379).
However, the heterogeneity, short follow-up, and low quality of included studies made it difficult to draw meaningful conclusions on the benefit or lack thereof for these therapies. More recent studies have shown promise for chest physiotherapy started several weeks prior to elective coronary bypass graft surgery and extended CPAP via nasal CPAP mask immediately following extubation (Hulzebos EH. JAMA. 2006;296[15]:1851), (Stephens 2013).
Ongoing areas for improvement include further clarification and standardization of best practices for postcardiac surgery patients, including blood product transfusion, optimal tidal volumes for surgical and postsurgical ventilation, timing of extubation, and the use of preventive therapies in the pre- and postsurgical periods. As providers who care for these patients, understanding how we can improve their postoperative pulmonary recovery will allow us to enhance our patient’s experience.
Dr. Noel is a Critical Care Fellow, Cooper Medical School of Rowan University, Camden, New Jersey.
Cardiac surgery patients are sicker today than in previous decades due to an aging population and a rising complexity in medical care. There is an increasing reliance on noncardiac surgeons to care for these patients. The optimal postoperative providers and structure of the ICU where patients are cared for remain unclear, but what is irrefutable is patients’ increased postoperative morbidity. Pulmonary complications are a leading cause of morbidity in these patients, occurring in up to one-fifth of cases (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Common pulmonary complications of cardiac surgery are listed in Table 1. Those complications, captured by The Society of Thoracic Surgeons (STS) Cardiac Surgery Database, include receiving ventilation longer than 24 hours, pneumonia, pulmonary embolism, and pleural effusion requiring drainage (The Society of Thoracic Surgeons. STS National Database. https://www.sts.org/registries-research-center/sts-national-database. Accessed January 9, 2018).

It should come as no surprise that cardiac surgery can have pronounced effects on lung function. The anesthetic agents, chest wall alteration, and direct lung manipulation can all affect pulmonary parameters. Functional residual capacity (FRC) can decrease by up to 20% with anesthesia (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531), and the thoracic manipulation and alteration of rib cage mechanics with a classic median sternotomy approach can lead to decreases in forced vital capacity (FVC) and expiratory volume in the first second of forced expiration (FEV1) that can last for months after surgery. Use of the cardiopulmonary bypass circuit can also lead to bronchoconstriction. These changes in pulmonary function are less pronounced in alternative surgical approaches, such as partial sternotomies (Weissman C. Seminars in Cardiothoracic and Vascular Anesthesia: Pulmonary Complications After Cardiac Surgery. Glen Head, NY: Westminister Publications; 2004).
The most frequent pulmonary consequence of cardiac surgery is atelectasis, seen on postoperative chest radiographs in approximately 50% to 90% of patients (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Induction, apnea during cardiopulmonary bypass, manual compression of the lungs for surgical exposure, internal mammary harvesting, and pleurotomy can lead to atelectasis in the intraoperative setting while weak cough, poor inspiratory efforts, interstitial edema, and immobility further contribute postoperatively (Weissman 2004). While frequently seen, clinically significant pulmonary consequences from this radiographic finding alone are rare (Weissman 2004).
Pleural effusions are seen on immediate postoperative chest radiographs in the majority of patients. Additionally, 10% to 40% of patients develop pleural effusions 2 to 3 weeks after surgery secondary to postpericardiotomy syndrome. While some effusions require drainage and further intervention (eg, hemothorax), most effusions require no specific treatment and resolve over time (Weissman 2004).
The prevalence of pneumonia following cardiac surgery varies based on differences in study populations and diagnostic criteria, but it remains an important source of morbidity and mortality. In one series, postoperative pneumonia occurred in 3.1% of patients, with higher rates observed in patients who were older, had worse left ventricular ejection fraction, had COPD, experienced longer bypass times, and received more red blood cell transfusions in the operating room (Allou N, et al. Crit Care Med. 2014;42[5]:1150). A meta-analysis found that an average of 6.37% of patients developed ventilator-associated pneumonia (VAP), and this rose to 35.2% in those receiving ventilation for greater than 48 hours. Those who developed VAP had an odds ratio of dying of 15.18 (95% CI 5.81-39.68) compared with those who did not (He S, et al. J Thorac Cardiovasc Surg. 2014;148[6]:3148).
A small proportion of patients go on to develop ARDS. While relatively uncommon, ARDS carries a high mortality rate. Many possible etiologies for ARDS in cardiac surgery patients have been proposed, including an inflammatory response related to the cardiopulmonary bypass circuit, reperfusion injury secondary to reduced pulmonary blood flow during bypass, protamine administration, transfusion, hypothermia, and lack of ventilation during bypass (Weissman 2004); (Stephens RS, et al. Ann Thorac Surg. 2013;95[3]:1122). Type of surgery may also play a role, as patients who undergo aortic surgery are at an even greater risk (Stephens 2013). As with other cases of ARDS, treatment is supportive: low tidal volume ventilation and careful management of fluid balance, as well as paralysis, prone positioning, and consideration for extracorporeal membrane oxygenation (ECMO), as appropriate (Stephens 2013).
Therapies to prevent postoperative pulmonary complications have included early extubation, aggressive pain control, deep breathing, physical therapy, early mobilization, and noninvasive ventilation in the form of CPAP and intermittent positive pressure breathing. A meta-analysis of 18 trials looking at the use of various forms of prophylactic postoperative physiotherapy did not show a difference in any measured clinical outcome (Pasquina P, Walder B. Br Med J. 2003;327[7428]:1379).
However, the heterogeneity, short follow-up, and low quality of included studies made it difficult to draw meaningful conclusions on the benefit or lack thereof for these therapies. More recent studies have shown promise for chest physiotherapy started several weeks prior to elective coronary bypass graft surgery and extended CPAP via nasal CPAP mask immediately following extubation (Hulzebos EH. JAMA. 2006;296[15]:1851), (Stephens 2013).
Ongoing areas for improvement include further clarification and standardization of best practices for postcardiac surgery patients, including blood product transfusion, optimal tidal volumes for surgical and postsurgical ventilation, timing of extubation, and the use of preventive therapies in the pre- and postsurgical periods. As providers who care for these patients, understanding how we can improve their postoperative pulmonary recovery will allow us to enhance our patient’s experience.
Dr. Noel is a Critical Care Fellow, Cooper Medical School of Rowan University, Camden, New Jersey.
Ambulatory BP monitoring shows hypertension prevalence 1 year after preeclampsia
as it misses forms of hypertension commonly experienced in that population, according to a study published Feb. 5 in Hypertension.
Researchers at Erasmus University, Rotterdam, the Netherlands, conducted a retrospective cohort study of 200 women who underwent 24-hour ambulatory BP monitoring and office BP measurement at a 1-year follow-up for delivery with severe preeclampsia. Measurements were taken between 9 months and 15 months after delivery.
“Current clinical guidelines on the prevention of [cardiovascular disease] and stroke after a hypertensive pregnancy disorder lack advice on [ambulatory BP monitoring] after delivery. We think that [ambulatory BP monitoring] should be offered to all women who experienced severe preeclampsia for more accurate BP assessment,” wrote Laura Benschop, MD, and her coauthors.
SOURCE: Benschop L et al. Hypertension. 2018 Feb;71:491-8.
as it misses forms of hypertension commonly experienced in that population, according to a study published Feb. 5 in Hypertension.
Researchers at Erasmus University, Rotterdam, the Netherlands, conducted a retrospective cohort study of 200 women who underwent 24-hour ambulatory BP monitoring and office BP measurement at a 1-year follow-up for delivery with severe preeclampsia. Measurements were taken between 9 months and 15 months after delivery.
“Current clinical guidelines on the prevention of [cardiovascular disease] and stroke after a hypertensive pregnancy disorder lack advice on [ambulatory BP monitoring] after delivery. We think that [ambulatory BP monitoring] should be offered to all women who experienced severe preeclampsia for more accurate BP assessment,” wrote Laura Benschop, MD, and her coauthors.
SOURCE: Benschop L et al. Hypertension. 2018 Feb;71:491-8.
as it misses forms of hypertension commonly experienced in that population, according to a study published Feb. 5 in Hypertension.
Researchers at Erasmus University, Rotterdam, the Netherlands, conducted a retrospective cohort study of 200 women who underwent 24-hour ambulatory BP monitoring and office BP measurement at a 1-year follow-up for delivery with severe preeclampsia. Measurements were taken between 9 months and 15 months after delivery.
“Current clinical guidelines on the prevention of [cardiovascular disease] and stroke after a hypertensive pregnancy disorder lack advice on [ambulatory BP monitoring] after delivery. We think that [ambulatory BP monitoring] should be offered to all women who experienced severe preeclampsia for more accurate BP assessment,” wrote Laura Benschop, MD, and her coauthors.
SOURCE: Benschop L et al. Hypertension. 2018 Feb;71:491-8.
FROM HYPERTENSION
On Diagnosing Sepsis
Two years ago, a panel appointed by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, referred to as a consensus conference, proposed a new definition for sepsis and new diagnostic criteria for sepsis and septic shock, known as Sepsis-3 (Singer M, et al. JAMA. 2016;315[8]:801). The panel proposed that sepsis be defined as life-threatening organ dysfunction due to a dysregulated host response to infection. Upon reflection, one could see that what we had called definitions of sepsis, severe sepsis, and septic shock for over 2 decades actually represented diagnostic criteria more than concise definitions. In that regard, a concise definition is a useful addition in the tool kit for training all health-care professionals to recognize sepsis and to treat it early and aggressively.
However, the diagnostic criteria leave something to be desired, in terms of both practicality and sensitivity for detecting patients whose infection has made them seriously ill. Those who participate in quality improvement efforts in their own hospitals will recognize that to promote change and to achieve a goal of better, higher quality care, it is important to remove obstacles in the system and to structure it so that doing the right thing is easier than not doing it. For sepsis, the first step in the process, recognizing that sepsis is present, has always been complex enough that it has been the bane of the enterprise. As many as two-thirds of patients with sepsis presenting to the ED with severe sepsis never receive that diagnosis while in the hospital. (Deis AS, et al. Chest. 2018;153[1]:39). As any sepsis core measure coordinator can attest, diagnostic criteria that are readily visible on retrospective examination are often unnoticed or misinterpreted in real time.
The crux of this issue is that the very entity of sepsis is not a definite thing but a not-quite-focused idea. Much is known of pathophysiologic features that seem to be important, but there is no one unifying pathologic condition. Contrast that with another critical illness, myocardial infarction. The very name states the unifying pathology. Our predecessors were able to work backward from an understanding that acute blockage of a small artery led to ischemia and infarction, in order to identify methods to detect it while it is happening—measuring enzymes and evaluating an ECG. For sepsis, we don’t even understand why patients are sick or why they die. There is a complex interaction of inflammation, microcirculatory thrombosis, mitochondrial dysfunction, immune suppression, but there is no one combination of those things that is yet understood in a way that lends itself to diagnostic testing. The best we can say is that the patient reacted to their infection in a way that was detrimental to their own body’s functioning. Rather than recognizing a few symptoms and sending a confirmatory test, with sepsis, we must tote up the signs and symptoms in the domains of recognizing infection and recognizing organ dysfunction, then determine whether they are present in sufficient amounts; it is an exercise that requires mental discipline.
If the diagnostic criteria we use, whether Sepsis-1, 2, or 3, are all gross descriptions of complex internal interactions that are not specific, then the syndrome that any of these criteria identifies is also not specific for anything particular. It falls to the medical community, as a whole, to determine exactly what it is that we desire a given syndrome to be indicative of. The Sepsis-3 authors decided that the appropriate syndrome should predict death or prolonged ICU stay. They used several large data sets to develop and validate infection-associated variables that would have good predictive ability for that outcome, and they compared what they found with sepsis by the Sepsis-1 definition, infection plus SIRS (Seymour C, et al. JAMA. 2016;315[8]:762). Infection + SIRS is a strawman in this comparison, because they tested its predictive ability for the outcome against that of the Sequential Organ Failure Assessment (SOFA) and the Logistic Organ Dysfunction Score (LODS). These two scoring systems were developed as severity of injury scales and validated as mortality predictors; the higher the score, the likelier mortality, whereas SIRS clearly contains no information about organ dysfunction. The comparator of interest for this outcome is actually severe sepsis, infection plus SIRS plus organ dysfunction.
Although the criteria the Sepsis-3 investigators used for defining patients with suspected infection were novel and reasonable, we lack additional important information about the patients they studied. They did not report the spectrum of treatments for sepsis in their cohort, whether early or late, adequate or inadequate, so it is impossible to determine whether the criteria address patients who are undertreated, patients who are treated late, patients who will die regardless of adequate therapy, or some combination. In other words, there is no way to tell whether patients who were recognized early in their course via Sepsis-1 criteria and treated aggressively and effectively may have avoided shock, ICU admission, and death. It is, of course, the business of physicians and nurses to help patients avoid exactly those things. Multiple studies have now demonstrated that SIRS criteria are more sensitive than SOFA-based screens, specifically qSOFA, for identifying infection with organ dysfunction, and that qSOFA is more specific for mortality (Serafim, et al. Chest. 2017; http://dx.doi.org/10.1016/j.chest.2017.12.015).
In contrast, the Sepsis-1 authors proposed infection plus SIRS as a sensitive screening tool that could warn of the possibility of an associated organ dysfunction (Sprung, et al. Crit Care Med. 2017;45[9]:1564). Previous to the Sepsis-1 conference, Bone and colleagues had defined the sepsis syndrome, which incorporated both SIRS and organ dysfunction (Bone, et al. Crit Care Med. 1989;17[5]:389). It was the collective insight of the Sepsis-1 participants to recognize that SIRS induced by infection could be a harbinger of organ failure. The Sepsis-3 authors believe that SIRS is a “normal and adaptive” part of infection and that it is “not useful” in the diagnosis of sepsis. That analysis neglects a couple of important things about SIRS. First, numerous studies demonstrate that infection with SIRS is associated with a mortality rate of 7% to 9%, which is by no means trivial (Rangel-Frausto MS, et al. JAMA. 1995;273[2]:117). Second, the components of SIRS have been recognized as representative of serious illness for millennia; the assertion that the Sepsis-1 definitions are not evidence-based is mistaken and discounts the collective experience of the medical profession.
Finally, SIRS is criticized on the basis of being nonspecific. “If I climb a flight of stairs, I get SIRS.” This is clearly a true statement. In fact, one could propose that the name could more accurately be Systemic Stress Response Syndrome, though “scissors” is certainly less catchy than “sirs” when one says it aloud. However, the critique neglects an important concept, encapsulated in Bayes’ Theorem. The value of any positive test result is largely dependent on the prevalence of the disease being tested for in the population being tested. It is unlikely that the prevalence of sepsis is very high among patients whose SIRS is induced by climbing a flight of stairs. On the other hand, tachycardia and tachypnea in a patient who is indulging in no activity while lying on a bed feeling miserable should prompt a search for both the infection that could be causing it and the organ dysfunction that could be associated with it. The specificity of SIRS derives from the population in which it is witnessed, and its sensitivity is to be respected.
To quote a friend, the remarkable CEO of a small Kansas hospital, “If a patient with an infection feels bad enough that they climb up on that gurney and place themselves at our mercy, we owe it to them to prove why they don’t have sepsis, rather than why they do.”
Editor’s Comment
The progress made in the last several years emphasizes the importance of early identification and aggressive treatment of sepsis. The Third International Consensus Definitions (Sepsis-3) have sparked great controversy in the sepsis community, because they delay the recognition of sepsis until organ damage occurs. In this Critical Care Commentary, Dr. Steven Q. Simpson asserts with solid arguments that the use of a screening tool with higher specificity for mortality, at the expense of sensitivity, is not a step in the right direction. Moving away from criteria that have been widely adopted in clinical trials and quality improvement initiatives throughout the world can be a setback in the battle to improve sepsis outcomes. Until prospectively validated criteria that allow earlier identification of sepsis are developed, there is no compelling reason for change.
Angel Coz, MD, FCCP
Section Editor
Dr. Simpson is Professor, Interim Director; Division of Pulmonary and Critical Care Medicine, University of Kansas, Kansas City, Kansas.
Two years ago, a panel appointed by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, referred to as a consensus conference, proposed a new definition for sepsis and new diagnostic criteria for sepsis and septic shock, known as Sepsis-3 (Singer M, et al. JAMA. 2016;315[8]:801). The panel proposed that sepsis be defined as life-threatening organ dysfunction due to a dysregulated host response to infection. Upon reflection, one could see that what we had called definitions of sepsis, severe sepsis, and septic shock for over 2 decades actually represented diagnostic criteria more than concise definitions. In that regard, a concise definition is a useful addition in the tool kit for training all health-care professionals to recognize sepsis and to treat it early and aggressively.
However, the diagnostic criteria leave something to be desired, in terms of both practicality and sensitivity for detecting patients whose infection has made them seriously ill. Those who participate in quality improvement efforts in their own hospitals will recognize that to promote change and to achieve a goal of better, higher quality care, it is important to remove obstacles in the system and to structure it so that doing the right thing is easier than not doing it. For sepsis, the first step in the process, recognizing that sepsis is present, has always been complex enough that it has been the bane of the enterprise. As many as two-thirds of patients with sepsis presenting to the ED with severe sepsis never receive that diagnosis while in the hospital. (Deis AS, et al. Chest. 2018;153[1]:39). As any sepsis core measure coordinator can attest, diagnostic criteria that are readily visible on retrospective examination are often unnoticed or misinterpreted in real time.
The crux of this issue is that the very entity of sepsis is not a definite thing but a not-quite-focused idea. Much is known of pathophysiologic features that seem to be important, but there is no one unifying pathologic condition. Contrast that with another critical illness, myocardial infarction. The very name states the unifying pathology. Our predecessors were able to work backward from an understanding that acute blockage of a small artery led to ischemia and infarction, in order to identify methods to detect it while it is happening—measuring enzymes and evaluating an ECG. For sepsis, we don’t even understand why patients are sick or why they die. There is a complex interaction of inflammation, microcirculatory thrombosis, mitochondrial dysfunction, immune suppression, but there is no one combination of those things that is yet understood in a way that lends itself to diagnostic testing. The best we can say is that the patient reacted to their infection in a way that was detrimental to their own body’s functioning. Rather than recognizing a few symptoms and sending a confirmatory test, with sepsis, we must tote up the signs and symptoms in the domains of recognizing infection and recognizing organ dysfunction, then determine whether they are present in sufficient amounts; it is an exercise that requires mental discipline.
If the diagnostic criteria we use, whether Sepsis-1, 2, or 3, are all gross descriptions of complex internal interactions that are not specific, then the syndrome that any of these criteria identifies is also not specific for anything particular. It falls to the medical community, as a whole, to determine exactly what it is that we desire a given syndrome to be indicative of. The Sepsis-3 authors decided that the appropriate syndrome should predict death or prolonged ICU stay. They used several large data sets to develop and validate infection-associated variables that would have good predictive ability for that outcome, and they compared what they found with sepsis by the Sepsis-1 definition, infection plus SIRS (Seymour C, et al. JAMA. 2016;315[8]:762). Infection + SIRS is a strawman in this comparison, because they tested its predictive ability for the outcome against that of the Sequential Organ Failure Assessment (SOFA) and the Logistic Organ Dysfunction Score (LODS). These two scoring systems were developed as severity of injury scales and validated as mortality predictors; the higher the score, the likelier mortality, whereas SIRS clearly contains no information about organ dysfunction. The comparator of interest for this outcome is actually severe sepsis, infection plus SIRS plus organ dysfunction.
Although the criteria the Sepsis-3 investigators used for defining patients with suspected infection were novel and reasonable, we lack additional important information about the patients they studied. They did not report the spectrum of treatments for sepsis in their cohort, whether early or late, adequate or inadequate, so it is impossible to determine whether the criteria address patients who are undertreated, patients who are treated late, patients who will die regardless of adequate therapy, or some combination. In other words, there is no way to tell whether patients who were recognized early in their course via Sepsis-1 criteria and treated aggressively and effectively may have avoided shock, ICU admission, and death. It is, of course, the business of physicians and nurses to help patients avoid exactly those things. Multiple studies have now demonstrated that SIRS criteria are more sensitive than SOFA-based screens, specifically qSOFA, for identifying infection with organ dysfunction, and that qSOFA is more specific for mortality (Serafim, et al. Chest. 2017; http://dx.doi.org/10.1016/j.chest.2017.12.015).
In contrast, the Sepsis-1 authors proposed infection plus SIRS as a sensitive screening tool that could warn of the possibility of an associated organ dysfunction (Sprung, et al. Crit Care Med. 2017;45[9]:1564). Previous to the Sepsis-1 conference, Bone and colleagues had defined the sepsis syndrome, which incorporated both SIRS and organ dysfunction (Bone, et al. Crit Care Med. 1989;17[5]:389). It was the collective insight of the Sepsis-1 participants to recognize that SIRS induced by infection could be a harbinger of organ failure. The Sepsis-3 authors believe that SIRS is a “normal and adaptive” part of infection and that it is “not useful” in the diagnosis of sepsis. That analysis neglects a couple of important things about SIRS. First, numerous studies demonstrate that infection with SIRS is associated with a mortality rate of 7% to 9%, which is by no means trivial (Rangel-Frausto MS, et al. JAMA. 1995;273[2]:117). Second, the components of SIRS have been recognized as representative of serious illness for millennia; the assertion that the Sepsis-1 definitions are not evidence-based is mistaken and discounts the collective experience of the medical profession.
Finally, SIRS is criticized on the basis of being nonspecific. “If I climb a flight of stairs, I get SIRS.” This is clearly a true statement. In fact, one could propose that the name could more accurately be Systemic Stress Response Syndrome, though “scissors” is certainly less catchy than “sirs” when one says it aloud. However, the critique neglects an important concept, encapsulated in Bayes’ Theorem. The value of any positive test result is largely dependent on the prevalence of the disease being tested for in the population being tested. It is unlikely that the prevalence of sepsis is very high among patients whose SIRS is induced by climbing a flight of stairs. On the other hand, tachycardia and tachypnea in a patient who is indulging in no activity while lying on a bed feeling miserable should prompt a search for both the infection that could be causing it and the organ dysfunction that could be associated with it. The specificity of SIRS derives from the population in which it is witnessed, and its sensitivity is to be respected.
To quote a friend, the remarkable CEO of a small Kansas hospital, “If a patient with an infection feels bad enough that they climb up on that gurney and place themselves at our mercy, we owe it to them to prove why they don’t have sepsis, rather than why they do.”
Editor’s Comment
The progress made in the last several years emphasizes the importance of early identification and aggressive treatment of sepsis. The Third International Consensus Definitions (Sepsis-3) have sparked great controversy in the sepsis community, because they delay the recognition of sepsis until organ damage occurs. In this Critical Care Commentary, Dr. Steven Q. Simpson asserts with solid arguments that the use of a screening tool with higher specificity for mortality, at the expense of sensitivity, is not a step in the right direction. Moving away from criteria that have been widely adopted in clinical trials and quality improvement initiatives throughout the world can be a setback in the battle to improve sepsis outcomes. Until prospectively validated criteria that allow earlier identification of sepsis are developed, there is no compelling reason for change.
Angel Coz, MD, FCCP
Section Editor
Dr. Simpson is Professor, Interim Director; Division of Pulmonary and Critical Care Medicine, University of Kansas, Kansas City, Kansas.
Two years ago, a panel appointed by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, referred to as a consensus conference, proposed a new definition for sepsis and new diagnostic criteria for sepsis and septic shock, known as Sepsis-3 (Singer M, et al. JAMA. 2016;315[8]:801). The panel proposed that sepsis be defined as life-threatening organ dysfunction due to a dysregulated host response to infection. Upon reflection, one could see that what we had called definitions of sepsis, severe sepsis, and septic shock for over 2 decades actually represented diagnostic criteria more than concise definitions. In that regard, a concise definition is a useful addition in the tool kit for training all health-care professionals to recognize sepsis and to treat it early and aggressively.
However, the diagnostic criteria leave something to be desired, in terms of both practicality and sensitivity for detecting patients whose infection has made them seriously ill. Those who participate in quality improvement efforts in their own hospitals will recognize that to promote change and to achieve a goal of better, higher quality care, it is important to remove obstacles in the system and to structure it so that doing the right thing is easier than not doing it. For sepsis, the first step in the process, recognizing that sepsis is present, has always been complex enough that it has been the bane of the enterprise. As many as two-thirds of patients with sepsis presenting to the ED with severe sepsis never receive that diagnosis while in the hospital. (Deis AS, et al. Chest. 2018;153[1]:39). As any sepsis core measure coordinator can attest, diagnostic criteria that are readily visible on retrospective examination are often unnoticed or misinterpreted in real time.
The crux of this issue is that the very entity of sepsis is not a definite thing but a not-quite-focused idea. Much is known of pathophysiologic features that seem to be important, but there is no one unifying pathologic condition. Contrast that with another critical illness, myocardial infarction. The very name states the unifying pathology. Our predecessors were able to work backward from an understanding that acute blockage of a small artery led to ischemia and infarction, in order to identify methods to detect it while it is happening—measuring enzymes and evaluating an ECG. For sepsis, we don’t even understand why patients are sick or why they die. There is a complex interaction of inflammation, microcirculatory thrombosis, mitochondrial dysfunction, immune suppression, but there is no one combination of those things that is yet understood in a way that lends itself to diagnostic testing. The best we can say is that the patient reacted to their infection in a way that was detrimental to their own body’s functioning. Rather than recognizing a few symptoms and sending a confirmatory test, with sepsis, we must tote up the signs and symptoms in the domains of recognizing infection and recognizing organ dysfunction, then determine whether they are present in sufficient amounts; it is an exercise that requires mental discipline.
If the diagnostic criteria we use, whether Sepsis-1, 2, or 3, are all gross descriptions of complex internal interactions that are not specific, then the syndrome that any of these criteria identifies is also not specific for anything particular. It falls to the medical community, as a whole, to determine exactly what it is that we desire a given syndrome to be indicative of. The Sepsis-3 authors decided that the appropriate syndrome should predict death or prolonged ICU stay. They used several large data sets to develop and validate infection-associated variables that would have good predictive ability for that outcome, and they compared what they found with sepsis by the Sepsis-1 definition, infection plus SIRS (Seymour C, et al. JAMA. 2016;315[8]:762). Infection + SIRS is a strawman in this comparison, because they tested its predictive ability for the outcome against that of the Sequential Organ Failure Assessment (SOFA) and the Logistic Organ Dysfunction Score (LODS). These two scoring systems were developed as severity of injury scales and validated as mortality predictors; the higher the score, the likelier mortality, whereas SIRS clearly contains no information about organ dysfunction. The comparator of interest for this outcome is actually severe sepsis, infection plus SIRS plus organ dysfunction.
Although the criteria the Sepsis-3 investigators used for defining patients with suspected infection were novel and reasonable, we lack additional important information about the patients they studied. They did not report the spectrum of treatments for sepsis in their cohort, whether early or late, adequate or inadequate, so it is impossible to determine whether the criteria address patients who are undertreated, patients who are treated late, patients who will die regardless of adequate therapy, or some combination. In other words, there is no way to tell whether patients who were recognized early in their course via Sepsis-1 criteria and treated aggressively and effectively may have avoided shock, ICU admission, and death. It is, of course, the business of physicians and nurses to help patients avoid exactly those things. Multiple studies have now demonstrated that SIRS criteria are more sensitive than SOFA-based screens, specifically qSOFA, for identifying infection with organ dysfunction, and that qSOFA is more specific for mortality (Serafim, et al. Chest. 2017; http://dx.doi.org/10.1016/j.chest.2017.12.015).
In contrast, the Sepsis-1 authors proposed infection plus SIRS as a sensitive screening tool that could warn of the possibility of an associated organ dysfunction (Sprung, et al. Crit Care Med. 2017;45[9]:1564). Previous to the Sepsis-1 conference, Bone and colleagues had defined the sepsis syndrome, which incorporated both SIRS and organ dysfunction (Bone, et al. Crit Care Med. 1989;17[5]:389). It was the collective insight of the Sepsis-1 participants to recognize that SIRS induced by infection could be a harbinger of organ failure. The Sepsis-3 authors believe that SIRS is a “normal and adaptive” part of infection and that it is “not useful” in the diagnosis of sepsis. That analysis neglects a couple of important things about SIRS. First, numerous studies demonstrate that infection with SIRS is associated with a mortality rate of 7% to 9%, which is by no means trivial (Rangel-Frausto MS, et al. JAMA. 1995;273[2]:117). Second, the components of SIRS have been recognized as representative of serious illness for millennia; the assertion that the Sepsis-1 definitions are not evidence-based is mistaken and discounts the collective experience of the medical profession.
Finally, SIRS is criticized on the basis of being nonspecific. “If I climb a flight of stairs, I get SIRS.” This is clearly a true statement. In fact, one could propose that the name could more accurately be Systemic Stress Response Syndrome, though “scissors” is certainly less catchy than “sirs” when one says it aloud. However, the critique neglects an important concept, encapsulated in Bayes’ Theorem. The value of any positive test result is largely dependent on the prevalence of the disease being tested for in the population being tested. It is unlikely that the prevalence of sepsis is very high among patients whose SIRS is induced by climbing a flight of stairs. On the other hand, tachycardia and tachypnea in a patient who is indulging in no activity while lying on a bed feeling miserable should prompt a search for both the infection that could be causing it and the organ dysfunction that could be associated with it. The specificity of SIRS derives from the population in which it is witnessed, and its sensitivity is to be respected.
To quote a friend, the remarkable CEO of a small Kansas hospital, “If a patient with an infection feels bad enough that they climb up on that gurney and place themselves at our mercy, we owe it to them to prove why they don’t have sepsis, rather than why they do.”
Editor’s Comment
The progress made in the last several years emphasizes the importance of early identification and aggressive treatment of sepsis. The Third International Consensus Definitions (Sepsis-3) have sparked great controversy in the sepsis community, because they delay the recognition of sepsis until organ damage occurs. In this Critical Care Commentary, Dr. Steven Q. Simpson asserts with solid arguments that the use of a screening tool with higher specificity for mortality, at the expense of sensitivity, is not a step in the right direction. Moving away from criteria that have been widely adopted in clinical trials and quality improvement initiatives throughout the world can be a setback in the battle to improve sepsis outcomes. Until prospectively validated criteria that allow earlier identification of sepsis are developed, there is no compelling reason for change.
Angel Coz, MD, FCCP
Section Editor
Dr. Simpson is Professor, Interim Director; Division of Pulmonary and Critical Care Medicine, University of Kansas, Kansas City, Kansas.
Shop the CHEST Store
Have you shopped at the CHEST Store lately? We’ve recently updated our merchandise to include several new items perfect for staff gifts or for yourself. Along with our old standbys, including lab coats and scrubs, we now feature CHEST-branded scarves, knit caps (perfect for winter), and insulated hot and cold drink mugs. And don’t forget, most items are customizable for a small, additional fee. For the more education-inclined, the store also features the best in CHEST print, including CHEST SEEKTM volumes, Coding for Chest Medicine, patient education guides, and much more.
Products can be ordered online, and most items are sold on-site at our live learning and board review courses and at the CHEST Annual Meeting. If you can’t purchase in person, visit the store at chestnet.org/store to stock up.
Have you shopped at the CHEST Store lately? We’ve recently updated our merchandise to include several new items perfect for staff gifts or for yourself. Along with our old standbys, including lab coats and scrubs, we now feature CHEST-branded scarves, knit caps (perfect for winter), and insulated hot and cold drink mugs. And don’t forget, most items are customizable for a small, additional fee. For the more education-inclined, the store also features the best in CHEST print, including CHEST SEEKTM volumes, Coding for Chest Medicine, patient education guides, and much more.
Products can be ordered online, and most items are sold on-site at our live learning and board review courses and at the CHEST Annual Meeting. If you can’t purchase in person, visit the store at chestnet.org/store to stock up.
Have you shopped at the CHEST Store lately? We’ve recently updated our merchandise to include several new items perfect for staff gifts or for yourself. Along with our old standbys, including lab coats and scrubs, we now feature CHEST-branded scarves, knit caps (perfect for winter), and insulated hot and cold drink mugs. And don’t forget, most items are customizable for a small, additional fee. For the more education-inclined, the store also features the best in CHEST print, including CHEST SEEKTM volumes, Coding for Chest Medicine, patient education guides, and much more.
Products can be ordered online, and most items are sold on-site at our live learning and board review courses and at the CHEST Annual Meeting. If you can’t purchase in person, visit the store at chestnet.org/store to stock up.
In Memoriam
CHEST has been informed of the following members’ deaths.
We extend our sincere condolences.
Nagesh V Salian, MD, FCCP (2016)
Ted A Calinog, MD, FCCP (2017)
Azam Ansari, MD (2017)
Arthur E. Schmidt, MD, FCCP (2017)
CHEST has been informed of the following members’ deaths.
We extend our sincere condolences.
Nagesh V Salian, MD, FCCP (2016)
Ted A Calinog, MD, FCCP (2017)
Azam Ansari, MD (2017)
Arthur E. Schmidt, MD, FCCP (2017)
CHEST has been informed of the following members’ deaths.
We extend our sincere condolences.
Nagesh V Salian, MD, FCCP (2016)
Ted A Calinog, MD, FCCP (2017)
Azam Ansari, MD (2017)
Arthur E. Schmidt, MD, FCCP (2017)
This Month in the Journal CHEST® Editor’s Picks
Giants In Chest Medicine Professor Nan-shan Zhong, MD.
By Wei-jie Guan.
Original Research
Long-term Use of Inhaled Corticosteroids in COPD and the Risk of Fracture. By Dr. A. V. Gonzalez, et al.
Cardiac Troponin Values in Patients With Acute Coronary Syndrome and Sleep Apnea: A Pilot Study. By Dr. A. Sánchez-de-la-Torre, et al.
CA-125 in Disease Progression and Treatment of Lymphangioleiomyomatosis. By Dr. C. G. Glasgow, et al.
Evidence-Based Medicine
Cough Due to TB and Other Chronic Infections: CHEST Guideline and Expert Panel Report. By Dr. S. K. Field, et al, on behalf of the CHEST Expert Cough Panel.
Giants In Chest Medicine Professor Nan-shan Zhong, MD.
By Wei-jie Guan.
Original Research
Long-term Use of Inhaled Corticosteroids in COPD and the Risk of Fracture. By Dr. A. V. Gonzalez, et al.
Cardiac Troponin Values in Patients With Acute Coronary Syndrome and Sleep Apnea: A Pilot Study. By Dr. A. Sánchez-de-la-Torre, et al.
CA-125 in Disease Progression and Treatment of Lymphangioleiomyomatosis. By Dr. C. G. Glasgow, et al.
Evidence-Based Medicine
Cough Due to TB and Other Chronic Infections: CHEST Guideline and Expert Panel Report. By Dr. S. K. Field, et al, on behalf of the CHEST Expert Cough Panel.
Giants In Chest Medicine Professor Nan-shan Zhong, MD.
By Wei-jie Guan.
Original Research
Long-term Use of Inhaled Corticosteroids in COPD and the Risk of Fracture. By Dr. A. V. Gonzalez, et al.
Cardiac Troponin Values in Patients With Acute Coronary Syndrome and Sleep Apnea: A Pilot Study. By Dr. A. Sánchez-de-la-Torre, et al.
CA-125 in Disease Progression and Treatment of Lymphangioleiomyomatosis. By Dr. C. G. Glasgow, et al.
Evidence-Based Medicine
Cough Due to TB and Other Chronic Infections: CHEST Guideline and Expert Panel Report. By Dr. S. K. Field, et al, on behalf of the CHEST Expert Cough Panel.
VIDEO: Painless PDT and other AK tricks
KAUAI, HAWAII – Imiquimod on the lip can be extremely painful, but it works wonders for actinic cheilitis, sometimes with only a few treatments.
Also, and how long patients stay under the lamp, advised Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
These are just a few of the tips Dr. Rosen shared in a presentation about AKs at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In a video interview after his talk, he went into detail about the use of imiquimod for actinic cheilitis – AKs of the lip – and painless PDT, as well as how to discuss AKs with patients – and a quick, clever way to help distinguish AKs from squamous cell carcinoma.
Dr. Rosen is an adviser to Aclaris, Cipher, Pfizer, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Imiquimod on the lip can be extremely painful, but it works wonders for actinic cheilitis, sometimes with only a few treatments.
Also, and how long patients stay under the lamp, advised Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
These are just a few of the tips Dr. Rosen shared in a presentation about AKs at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In a video interview after his talk, he went into detail about the use of imiquimod for actinic cheilitis – AKs of the lip – and painless PDT, as well as how to discuss AKs with patients – and a quick, clever way to help distinguish AKs from squamous cell carcinoma.
Dr. Rosen is an adviser to Aclaris, Cipher, Pfizer, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Imiquimod on the lip can be extremely painful, but it works wonders for actinic cheilitis, sometimes with only a few treatments.
Also, and how long patients stay under the lamp, advised Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
These are just a few of the tips Dr. Rosen shared in a presentation about AKs at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In a video interview after his talk, he went into detail about the use of imiquimod for actinic cheilitis – AKs of the lip – and painless PDT, as well as how to discuss AKs with patients – and a quick, clever way to help distinguish AKs from squamous cell carcinoma.
Dr. Rosen is an adviser to Aclaris, Cipher, Pfizer, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Sequence of regorafenib, cetuximab matters in later-line CRC therapy
SAN FRANCISCO – The order in which targeted therapies are given to patients with previously treated metastatic colorectal cancer can have a marked impact on overall survival, according to results of the REVERCE trial reported at the 2018 GI Cancers Symposium.
Investigators led by Kohei Shitara, MD, National Cancer Center Hospital East, Kashiwa, Japan, enrolled in the multicenter, phase 2, randomized trial 101 patients with mainly KRAS wild-type metastatic colorectal cancer who had previously received fluoropyrimidine, oxaliplatin, and irinotecan, but not any therapy targeting epidermal growth factor receptor (EGFR).
Main results at a median follow-up of 29.0 months showed that median overall survival was almost 6 months longer when regorafenib was given first as compared with when cetuximab was given first, a difference translating to a 39% reduction in risk of death with the former sequence.
“These results suggest that overall survival was longer with regorafenib as the first treatment followed by cetuximab than that of the current standard sequence,” Dr. Shitara said. “Efficacy was observed in almost all subgroups.”
Progression-free survival on the first treatment did not differ significantly by agent, but on the second treatment, it was almost 3.5 months longer with cetuximab. “This might contribute to the overall survival difference,” he proposed.
The specific biologic mechanisms driving the differing efficacy of the two sequences are still unclear, but one possibility is that regorafenib downregulates MAP kinase and certain other signaling molecules, sensitizing cells to subsequent anti-EGFR therapy, Dr. Shitara said. “Biomarker analyses are still ongoing. We hope that further biomarker analysis will clarify the reason for the survival difference.”
The data did not reveal any unexpected safety signals. Although quality of life scores were lower with regorafenib during each treatment period, scores for all time on treatment were similar for the two sequences.
Clinical implications
“The amount of difference in overall survival [between sequences] was pretty striking. These were patients in second line and third line,” commented session chair Michael J. Hall, MD, of Fox Chase Cancer Center, Philadelphia.
“This trial adds excitement to the idea that there is still a lot of work to be done in sequencing of therapies and also our understanding of how induction of molecular events by one therapy, for instance, the cetuximab leading to RAS mutations, then affects how the downstream therapies work,” Dr. Hall maintained. “So maybe we need to think more intelligently about that.”
Study details
The REVERCE investigators planned to enroll 180 patients in the trial but stopped after 101 patients because of slow accrual. Most of those studied had previously received bevacizumab.
About 70% of patients in the regorafenib-first group needed a dose reduction at some point, compared with about 30% in the cetuximab-first group, a difference likely related to the longer duration of treatment for the former, Dr. Shitara speculated.
Median overall survival was 17.4 months when regorafenib was given first and 11.6 months when cetuximab was give first (hazard ratio, 0.61; P = .029), according to data reported at the symposium, which was sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
In subgroup analyses, the regorafenib-first strategy yielded significantly better overall survival among patients with left-sided tumors (HR, 0.51; P = .011), but not among those with right-sided tumors (HR, 0.88).
The regorafenib-first strategy had similar benefit when restricted to patients with RAS/RAF wild-type tumors (18.2 vs. 12.7 months; HR, 0.60; P = .036).
Analysis of blood samples collected after the first agent indicated that 26% of patients had emergence of a RAS mutation after receiving cetuximab. However, these patients did not appear to have worse overall survival, according to Dr. Shitara.
Progression-free survival on the first agent was 2.4 months in the regorafenib-first group and 4.2 months in the cetuximab-first group, a nonsignificant difference. In contrast, for the second treatment, it was 5.2 months in the regorafenib-first group and just 1.8 months in the cetuximab-first group (HR, 0.29; P less than .0001).
Dr. Shitara disclosed that he receives honoraria from Abbvie, Novartis, Ono Pharmaceutical, and Yakult; has a consulting or advisory role with Astellas Pharma, Bristol-Myers Squibb, Lilly, Pfizer, and Takeda; and that his institution receives research funding from Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Lilly, MSD, Taiho Pharmaceutical, and Yakult. The trial was funded by Bayer Yahin.
SOURCE: Shitara et al. GI Cancers Symposium Abstract 557
SAN FRANCISCO – The order in which targeted therapies are given to patients with previously treated metastatic colorectal cancer can have a marked impact on overall survival, according to results of the REVERCE trial reported at the 2018 GI Cancers Symposium.
Investigators led by Kohei Shitara, MD, National Cancer Center Hospital East, Kashiwa, Japan, enrolled in the multicenter, phase 2, randomized trial 101 patients with mainly KRAS wild-type metastatic colorectal cancer who had previously received fluoropyrimidine, oxaliplatin, and irinotecan, but not any therapy targeting epidermal growth factor receptor (EGFR).
Main results at a median follow-up of 29.0 months showed that median overall survival was almost 6 months longer when regorafenib was given first as compared with when cetuximab was given first, a difference translating to a 39% reduction in risk of death with the former sequence.
“These results suggest that overall survival was longer with regorafenib as the first treatment followed by cetuximab than that of the current standard sequence,” Dr. Shitara said. “Efficacy was observed in almost all subgroups.”
Progression-free survival on the first treatment did not differ significantly by agent, but on the second treatment, it was almost 3.5 months longer with cetuximab. “This might contribute to the overall survival difference,” he proposed.
The specific biologic mechanisms driving the differing efficacy of the two sequences are still unclear, but one possibility is that regorafenib downregulates MAP kinase and certain other signaling molecules, sensitizing cells to subsequent anti-EGFR therapy, Dr. Shitara said. “Biomarker analyses are still ongoing. We hope that further biomarker analysis will clarify the reason for the survival difference.”
The data did not reveal any unexpected safety signals. Although quality of life scores were lower with regorafenib during each treatment period, scores for all time on treatment were similar for the two sequences.
Clinical implications
“The amount of difference in overall survival [between sequences] was pretty striking. These were patients in second line and third line,” commented session chair Michael J. Hall, MD, of Fox Chase Cancer Center, Philadelphia.
“This trial adds excitement to the idea that there is still a lot of work to be done in sequencing of therapies and also our understanding of how induction of molecular events by one therapy, for instance, the cetuximab leading to RAS mutations, then affects how the downstream therapies work,” Dr. Hall maintained. “So maybe we need to think more intelligently about that.”
Study details
The REVERCE investigators planned to enroll 180 patients in the trial but stopped after 101 patients because of slow accrual. Most of those studied had previously received bevacizumab.
About 70% of patients in the regorafenib-first group needed a dose reduction at some point, compared with about 30% in the cetuximab-first group, a difference likely related to the longer duration of treatment for the former, Dr. Shitara speculated.
Median overall survival was 17.4 months when regorafenib was given first and 11.6 months when cetuximab was give first (hazard ratio, 0.61; P = .029), according to data reported at the symposium, which was sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
In subgroup analyses, the regorafenib-first strategy yielded significantly better overall survival among patients with left-sided tumors (HR, 0.51; P = .011), but not among those with right-sided tumors (HR, 0.88).
The regorafenib-first strategy had similar benefit when restricted to patients with RAS/RAF wild-type tumors (18.2 vs. 12.7 months; HR, 0.60; P = .036).
Analysis of blood samples collected after the first agent indicated that 26% of patients had emergence of a RAS mutation after receiving cetuximab. However, these patients did not appear to have worse overall survival, according to Dr. Shitara.
Progression-free survival on the first agent was 2.4 months in the regorafenib-first group and 4.2 months in the cetuximab-first group, a nonsignificant difference. In contrast, for the second treatment, it was 5.2 months in the regorafenib-first group and just 1.8 months in the cetuximab-first group (HR, 0.29; P less than .0001).
Dr. Shitara disclosed that he receives honoraria from Abbvie, Novartis, Ono Pharmaceutical, and Yakult; has a consulting or advisory role with Astellas Pharma, Bristol-Myers Squibb, Lilly, Pfizer, and Takeda; and that his institution receives research funding from Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Lilly, MSD, Taiho Pharmaceutical, and Yakult. The trial was funded by Bayer Yahin.
SOURCE: Shitara et al. GI Cancers Symposium Abstract 557
SAN FRANCISCO – The order in which targeted therapies are given to patients with previously treated metastatic colorectal cancer can have a marked impact on overall survival, according to results of the REVERCE trial reported at the 2018 GI Cancers Symposium.
Investigators led by Kohei Shitara, MD, National Cancer Center Hospital East, Kashiwa, Japan, enrolled in the multicenter, phase 2, randomized trial 101 patients with mainly KRAS wild-type metastatic colorectal cancer who had previously received fluoropyrimidine, oxaliplatin, and irinotecan, but not any therapy targeting epidermal growth factor receptor (EGFR).
Main results at a median follow-up of 29.0 months showed that median overall survival was almost 6 months longer when regorafenib was given first as compared with when cetuximab was given first, a difference translating to a 39% reduction in risk of death with the former sequence.
“These results suggest that overall survival was longer with regorafenib as the first treatment followed by cetuximab than that of the current standard sequence,” Dr. Shitara said. “Efficacy was observed in almost all subgroups.”
Progression-free survival on the first treatment did not differ significantly by agent, but on the second treatment, it was almost 3.5 months longer with cetuximab. “This might contribute to the overall survival difference,” he proposed.
The specific biologic mechanisms driving the differing efficacy of the two sequences are still unclear, but one possibility is that regorafenib downregulates MAP kinase and certain other signaling molecules, sensitizing cells to subsequent anti-EGFR therapy, Dr. Shitara said. “Biomarker analyses are still ongoing. We hope that further biomarker analysis will clarify the reason for the survival difference.”
The data did not reveal any unexpected safety signals. Although quality of life scores were lower with regorafenib during each treatment period, scores for all time on treatment were similar for the two sequences.
Clinical implications
“The amount of difference in overall survival [between sequences] was pretty striking. These were patients in second line and third line,” commented session chair Michael J. Hall, MD, of Fox Chase Cancer Center, Philadelphia.
“This trial adds excitement to the idea that there is still a lot of work to be done in sequencing of therapies and also our understanding of how induction of molecular events by one therapy, for instance, the cetuximab leading to RAS mutations, then affects how the downstream therapies work,” Dr. Hall maintained. “So maybe we need to think more intelligently about that.”
Study details
The REVERCE investigators planned to enroll 180 patients in the trial but stopped after 101 patients because of slow accrual. Most of those studied had previously received bevacizumab.
About 70% of patients in the regorafenib-first group needed a dose reduction at some point, compared with about 30% in the cetuximab-first group, a difference likely related to the longer duration of treatment for the former, Dr. Shitara speculated.
Median overall survival was 17.4 months when regorafenib was given first and 11.6 months when cetuximab was give first (hazard ratio, 0.61; P = .029), according to data reported at the symposium, which was sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
In subgroup analyses, the regorafenib-first strategy yielded significantly better overall survival among patients with left-sided tumors (HR, 0.51; P = .011), but not among those with right-sided tumors (HR, 0.88).
The regorafenib-first strategy had similar benefit when restricted to patients with RAS/RAF wild-type tumors (18.2 vs. 12.7 months; HR, 0.60; P = .036).
Analysis of blood samples collected after the first agent indicated that 26% of patients had emergence of a RAS mutation after receiving cetuximab. However, these patients did not appear to have worse overall survival, according to Dr. Shitara.
Progression-free survival on the first agent was 2.4 months in the regorafenib-first group and 4.2 months in the cetuximab-first group, a nonsignificant difference. In contrast, for the second treatment, it was 5.2 months in the regorafenib-first group and just 1.8 months in the cetuximab-first group (HR, 0.29; P less than .0001).
Dr. Shitara disclosed that he receives honoraria from Abbvie, Novartis, Ono Pharmaceutical, and Yakult; has a consulting or advisory role with Astellas Pharma, Bristol-Myers Squibb, Lilly, Pfizer, and Takeda; and that his institution receives research funding from Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Lilly, MSD, Taiho Pharmaceutical, and Yakult. The trial was funded by Bayer Yahin.
SOURCE: Shitara et al. GI Cancers Symposium Abstract 557
REPORTING FROM THE 2018 GI CANCERS SYMPOSIUM
Key clinical point:
Major finding: Median overall survival was 17.4 months when regorafenib was given first and 11.6 months when cetuximab was given first (HR, 0.61; P = .029).
Data source: A multicenter, randomized, phase 2 trial of regorafenib followed by cetuximab, versus the reverse sequence, among 101 patients with metastatic colorectal cancer previously treated with fluoropyrimidine, oxaliplatin, and irinotecan (REVERCE trial).
Disclosures: Dr. Shitara disclosed that he receives honoraria from Abbvie, Novartis, Ono Pharmaceutical, and Yakult; has a consulting or advisory role with Astellas Pharma, Bristol-Myers Squibb, Lilly, Pfizer, and Takeda; and that his institution receives research funding from Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Lilly, MSD, Taiho Pharmaceutical, and Yakult. The trial was funded by Bayer Yahin.
Source: Shitara et al. GI Cancers Symposium Abstract 557















