User login
Shades of gray
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Use of a Small-Bore Needle Arthroscope to Diagnose Intra-Articular Knee Pathology: Comparison With Magnetic Resonance Imaging
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
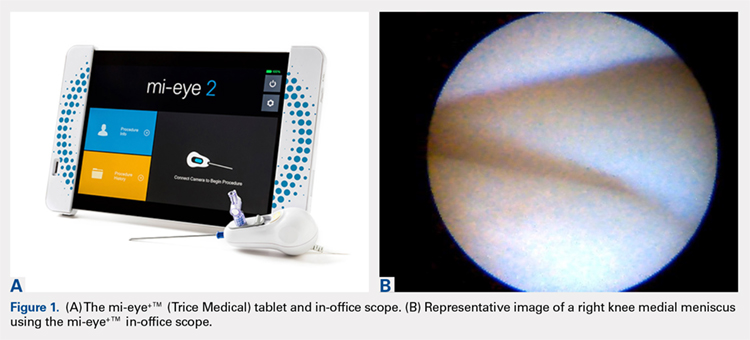
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).
The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
TAKE-HOME POINTS
- Small-bore needle arthroscopy is an effective way to diagnose intra-articular knee pathology.
- Small-bore needle arthroscopy is safe and easy to use with no complications reported in this series.
- Small-bore needle arthroscopy is a useful diagnostic tool in office settings.
- In this series, small-bore needle arthroscopy was more accurate than MRI to diagnose knee meniscal tears.
- In-office diagnostic arthroscopy can be used for other joints such as shoulder, elbow, and ankle.
Commentary—Serotonin Syndrome and Triptans
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Imaging methods for stroke thrombectomy eligibility yield similar results
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
REPORTING FROM ISC 2018
Key clinical point: DW-MRI is the gold standard for imaging, but CTP is more widely available.
Major finding: Rates of neurologic deterioration in hospital, symptomatic intracranial hemorrhage, and death related to stroke were similar regardless of whether CT or MR imaging was used to assess patients’ infarcts.
Data source: A subanalysis of the DAWN randomized, controlled trial (n = 206).
Disclosures: The DAWN study was funded by Stryker Neurovascular. Dr. Sila reported receiving honoraria from Medtronic.
Source: Sila C et al. ISC 2018, abstract LB11.
Hemophilia A drug heads toward approval in Europe
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
Obesity affects diagnosis of liver fibrosis with imaging techniques
, according to a study from the University of California, San Diego.
“This study demonstrates that BMI is a significant factor of discordancy between MRE and TE for the stage of significant fibrosis (2-4 vs. 0-1),” wrote Cyrielle Caussy, MD, and her colleagues (Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037). “Furthermore, this study showed that the grade of obesity is also a significant predictor of discordancy between MRE and TE because the discordance rate between MRE and TE increases with the increase in BMI.”
Dr. Caussy of the University of California, San Diego, and her colleagues had noted that MRE and TE had discordant findings in obese patients. To ascertain under what conditions TE and MRE produce the same readings, Dr. Caussy and her associates conducted a cross-sectional study of two cohorts with nonalcoholic fatty liver disease (NAFLD) who underwent contemporaneous MRE, TE, and liver biopsy. TE utilized both M and XL probes during imaging. The training cohort involved 119 adult patients undergoing NAFLD testing from October 2011 through January 2017. The validation cohort, consisting of 75 adults with NAFLD undergoing liver imaging from March 2010 through May 2013, was formed to validate the findings of the training cohort.
The study revealed that BMI was a significant predictor of the difference between MRE and TE results and made it difficult to assess the stage of liver fibrosis (2-4 vs. 0-1). After adjustment for age and sex, BMI accounted for a 5-unit increase of 1.694 (95% confidence interval, 1.145-2.507; P = .008). This was not a static relationship, and as BMI increased, so did the discordance between MRE and TE (P = .0309). Interestingly, the discordance rate was significantly higher in participants with BMIs greater than 35 kg/m2, compared with participants with BMIs below 35 (63.0% vs. 38.0%; P = .022), the investigators reported. In severely obese adults with liver disease caused mainly by NAFLD, the accuracy of MRE was higher than TE for diagnosing significant fibrosis and advanced fibrosis.
While the study revealed valuable information, it had both strengths and limitations. A strength of the study was the use of two cohorts, specifically the validation cohort. The use of the liver biopsy as a reference, which is the standard for assessing fibrosis, was also a strength of the study. A limitation was that the study was conducted at specialized, tertiary care centers using advanced imaging techniques that may not be available at other clinics. Additionally, the cohorts included a small number of patients with advanced fibrosis.
“The integration of the BMI in the screening strategy for the noninvasive detection of liver fibrosis in NAFLD should be considered, and this parameter would help to determine when MRE is not needed in future guidelines” wrote Dr. Caussy and her associates. “Further cost-effectiveness studies are necessary to evaluate the clinical utility of MRE, TE, and/or liver biopsy to develop optimal screening strategies for diagnosing NAFLD-associated fibrosis.”
Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
SOURCE: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
, according to a study from the University of California, San Diego.
“This study demonstrates that BMI is a significant factor of discordancy between MRE and TE for the stage of significant fibrosis (2-4 vs. 0-1),” wrote Cyrielle Caussy, MD, and her colleagues (Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037). “Furthermore, this study showed that the grade of obesity is also a significant predictor of discordancy between MRE and TE because the discordance rate between MRE and TE increases with the increase in BMI.”
Dr. Caussy of the University of California, San Diego, and her colleagues had noted that MRE and TE had discordant findings in obese patients. To ascertain under what conditions TE and MRE produce the same readings, Dr. Caussy and her associates conducted a cross-sectional study of two cohorts with nonalcoholic fatty liver disease (NAFLD) who underwent contemporaneous MRE, TE, and liver biopsy. TE utilized both M and XL probes during imaging. The training cohort involved 119 adult patients undergoing NAFLD testing from October 2011 through January 2017. The validation cohort, consisting of 75 adults with NAFLD undergoing liver imaging from March 2010 through May 2013, was formed to validate the findings of the training cohort.
The study revealed that BMI was a significant predictor of the difference between MRE and TE results and made it difficult to assess the stage of liver fibrosis (2-4 vs. 0-1). After adjustment for age and sex, BMI accounted for a 5-unit increase of 1.694 (95% confidence interval, 1.145-2.507; P = .008). This was not a static relationship, and as BMI increased, so did the discordance between MRE and TE (P = .0309). Interestingly, the discordance rate was significantly higher in participants with BMIs greater than 35 kg/m2, compared with participants with BMIs below 35 (63.0% vs. 38.0%; P = .022), the investigators reported. In severely obese adults with liver disease caused mainly by NAFLD, the accuracy of MRE was higher than TE for diagnosing significant fibrosis and advanced fibrosis.
While the study revealed valuable information, it had both strengths and limitations. A strength of the study was the use of two cohorts, specifically the validation cohort. The use of the liver biopsy as a reference, which is the standard for assessing fibrosis, was also a strength of the study. A limitation was that the study was conducted at specialized, tertiary care centers using advanced imaging techniques that may not be available at other clinics. Additionally, the cohorts included a small number of patients with advanced fibrosis.
“The integration of the BMI in the screening strategy for the noninvasive detection of liver fibrosis in NAFLD should be considered, and this parameter would help to determine when MRE is not needed in future guidelines” wrote Dr. Caussy and her associates. “Further cost-effectiveness studies are necessary to evaluate the clinical utility of MRE, TE, and/or liver biopsy to develop optimal screening strategies for diagnosing NAFLD-associated fibrosis.”
Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
SOURCE: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
, according to a study from the University of California, San Diego.
“This study demonstrates that BMI is a significant factor of discordancy between MRE and TE for the stage of significant fibrosis (2-4 vs. 0-1),” wrote Cyrielle Caussy, MD, and her colleagues (Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037). “Furthermore, this study showed that the grade of obesity is also a significant predictor of discordancy between MRE and TE because the discordance rate between MRE and TE increases with the increase in BMI.”
Dr. Caussy of the University of California, San Diego, and her colleagues had noted that MRE and TE had discordant findings in obese patients. To ascertain under what conditions TE and MRE produce the same readings, Dr. Caussy and her associates conducted a cross-sectional study of two cohorts with nonalcoholic fatty liver disease (NAFLD) who underwent contemporaneous MRE, TE, and liver biopsy. TE utilized both M and XL probes during imaging. The training cohort involved 119 adult patients undergoing NAFLD testing from October 2011 through January 2017. The validation cohort, consisting of 75 adults with NAFLD undergoing liver imaging from March 2010 through May 2013, was formed to validate the findings of the training cohort.
The study revealed that BMI was a significant predictor of the difference between MRE and TE results and made it difficult to assess the stage of liver fibrosis (2-4 vs. 0-1). After adjustment for age and sex, BMI accounted for a 5-unit increase of 1.694 (95% confidence interval, 1.145-2.507; P = .008). This was not a static relationship, and as BMI increased, so did the discordance between MRE and TE (P = .0309). Interestingly, the discordance rate was significantly higher in participants with BMIs greater than 35 kg/m2, compared with participants with BMIs below 35 (63.0% vs. 38.0%; P = .022), the investigators reported. In severely obese adults with liver disease caused mainly by NAFLD, the accuracy of MRE was higher than TE for diagnosing significant fibrosis and advanced fibrosis.
While the study revealed valuable information, it had both strengths and limitations. A strength of the study was the use of two cohorts, specifically the validation cohort. The use of the liver biopsy as a reference, which is the standard for assessing fibrosis, was also a strength of the study. A limitation was that the study was conducted at specialized, tertiary care centers using advanced imaging techniques that may not be available at other clinics. Additionally, the cohorts included a small number of patients with advanced fibrosis.
“The integration of the BMI in the screening strategy for the noninvasive detection of liver fibrosis in NAFLD should be considered, and this parameter would help to determine when MRE is not needed in future guidelines” wrote Dr. Caussy and her associates. “Further cost-effectiveness studies are necessary to evaluate the clinical utility of MRE, TE, and/or liver biopsy to develop optimal screening strategies for diagnosing NAFLD-associated fibrosis.”
Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
SOURCE: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Higher BMIs make it difficult to diagnose liver fibrosis with imaging techniques.
Major finding: The discordance rate between magnetic resonance elastography and transient elastrography was 43.7% in this study.
Study details: A cross-sectional study of two cohorts with nonalcoholic fatty liver disease patients who underwent contemporaneous MRE, TE, and liver biopsy; one with 119 adults, the other with 75.
Disclosures: Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
Source: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
AAN Recommends Exercise for People With MCI
Neurologists should recommend twice-weekly exercise to patients diagnosed with mild cognitive impairment (MCI) as part of an overall approach to management, according to a practice guideline update from the American Academy of Neurology (AAN). The Level B recommendation is based on six-month studies that suggest that such exercise possibly improves cognition. The update was published online ahead of print December 27, 2017, in Neurology.
“Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with MCI,” said Ronald Petersen, MD, PhD, Director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minnesota, and lead author of the update. “What is good for your heart can be good for your brain.”
The update also states that clinicians may recommend cognitive training for people with MCI (Level C). The evidence, however, is insufficient “to support or refute the use of any individual cognitive intervention strategy,” according to the guideline. “When various cognitive interventions are considered as a group, for patients with MCI, cognitive interventions may improve select measures of cognitive function.”
Document Updates 2001 Practice Parameter
The current practice guideline update revises the AAN’s 2001 practice parameter that provided recommendations for the diagnosis and treatment of MCI. Dr. Petersen and colleagues based the update on a systematic review of articles about MCI prevalence, prognosis, and treatment. They classified evidence according to AAN criteria and based recommendations on modified Delphi consensus.
The authors found that the prevalence of MCI is 6.7% for people between ages 60 and 64, 8.4% for people between ages 65 and 69, 10.1% for people between ages 70 and 74, 14.8% for people between ages 75 and 79, and 25.2% for people between ages 80 and 84. Approximately 15% of people with MCI who are older than 65 develop dementia during two years of follow-up.
No Evidence for Pharmacologic Treatment
Evidence does not support a symptomatic cognitive benefit in MCI for any pharmacologic or dietary agents, according to the authors. The FDA has not approved any medication for treating MCI. If clinicians offer cholinesterase inhibitors to their patients with MCI, they must first discuss the fact that the treatment is off label and not backed by empirical evidence, according to the update. Gastrointestinal symptoms and cardiac concerns are common side effects of cholinesterase inhibitors.
Assessment for MCI is appropriate for patients who complain of impaired memory or cognition, as well as those who present for a Medicare Annual Wellness Visit, according to the update. Clinicians should evaluate patients with MCI for risk factors that are potentially modifiable. Patients with MCI also should undergo serial assessments over time so that clinicians can monitor them for changes in cognitive status.
—Erik Greb
Suggested Reading
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551-2561.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Dec 27 [Epub ahead of print].
Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490.
Neurologists should recommend twice-weekly exercise to patients diagnosed with mild cognitive impairment (MCI) as part of an overall approach to management, according to a practice guideline update from the American Academy of Neurology (AAN). The Level B recommendation is based on six-month studies that suggest that such exercise possibly improves cognition. The update was published online ahead of print December 27, 2017, in Neurology.
“Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with MCI,” said Ronald Petersen, MD, PhD, Director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minnesota, and lead author of the update. “What is good for your heart can be good for your brain.”
The update also states that clinicians may recommend cognitive training for people with MCI (Level C). The evidence, however, is insufficient “to support or refute the use of any individual cognitive intervention strategy,” according to the guideline. “When various cognitive interventions are considered as a group, for patients with MCI, cognitive interventions may improve select measures of cognitive function.”
Document Updates 2001 Practice Parameter
The current practice guideline update revises the AAN’s 2001 practice parameter that provided recommendations for the diagnosis and treatment of MCI. Dr. Petersen and colleagues based the update on a systematic review of articles about MCI prevalence, prognosis, and treatment. They classified evidence according to AAN criteria and based recommendations on modified Delphi consensus.
The authors found that the prevalence of MCI is 6.7% for people between ages 60 and 64, 8.4% for people between ages 65 and 69, 10.1% for people between ages 70 and 74, 14.8% for people between ages 75 and 79, and 25.2% for people between ages 80 and 84. Approximately 15% of people with MCI who are older than 65 develop dementia during two years of follow-up.
No Evidence for Pharmacologic Treatment
Evidence does not support a symptomatic cognitive benefit in MCI for any pharmacologic or dietary agents, according to the authors. The FDA has not approved any medication for treating MCI. If clinicians offer cholinesterase inhibitors to their patients with MCI, they must first discuss the fact that the treatment is off label and not backed by empirical evidence, according to the update. Gastrointestinal symptoms and cardiac concerns are common side effects of cholinesterase inhibitors.
Assessment for MCI is appropriate for patients who complain of impaired memory or cognition, as well as those who present for a Medicare Annual Wellness Visit, according to the update. Clinicians should evaluate patients with MCI for risk factors that are potentially modifiable. Patients with MCI also should undergo serial assessments over time so that clinicians can monitor them for changes in cognitive status.
—Erik Greb
Suggested Reading
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551-2561.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Dec 27 [Epub ahead of print].
Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490.
Neurologists should recommend twice-weekly exercise to patients diagnosed with mild cognitive impairment (MCI) as part of an overall approach to management, according to a practice guideline update from the American Academy of Neurology (AAN). The Level B recommendation is based on six-month studies that suggest that such exercise possibly improves cognition. The update was published online ahead of print December 27, 2017, in Neurology.
“Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with MCI,” said Ronald Petersen, MD, PhD, Director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minnesota, and lead author of the update. “What is good for your heart can be good for your brain.”
The update also states that clinicians may recommend cognitive training for people with MCI (Level C). The evidence, however, is insufficient “to support or refute the use of any individual cognitive intervention strategy,” according to the guideline. “When various cognitive interventions are considered as a group, for patients with MCI, cognitive interventions may improve select measures of cognitive function.”
Document Updates 2001 Practice Parameter
The current practice guideline update revises the AAN’s 2001 practice parameter that provided recommendations for the diagnosis and treatment of MCI. Dr. Petersen and colleagues based the update on a systematic review of articles about MCI prevalence, prognosis, and treatment. They classified evidence according to AAN criteria and based recommendations on modified Delphi consensus.
The authors found that the prevalence of MCI is 6.7% for people between ages 60 and 64, 8.4% for people between ages 65 and 69, 10.1% for people between ages 70 and 74, 14.8% for people between ages 75 and 79, and 25.2% for people between ages 80 and 84. Approximately 15% of people with MCI who are older than 65 develop dementia during two years of follow-up.
No Evidence for Pharmacologic Treatment
Evidence does not support a symptomatic cognitive benefit in MCI for any pharmacologic or dietary agents, according to the authors. The FDA has not approved any medication for treating MCI. If clinicians offer cholinesterase inhibitors to their patients with MCI, they must first discuss the fact that the treatment is off label and not backed by empirical evidence, according to the update. Gastrointestinal symptoms and cardiac concerns are common side effects of cholinesterase inhibitors.
Assessment for MCI is appropriate for patients who complain of impaired memory or cognition, as well as those who present for a Medicare Annual Wellness Visit, according to the update. Clinicians should evaluate patients with MCI for risk factors that are potentially modifiable. Patients with MCI also should undergo serial assessments over time so that clinicians can monitor them for changes in cognitive status.
—Erik Greb
Suggested Reading
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551-2561.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Dec 27 [Epub ahead of print].
Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490.
The shrinking role of surgical aortic valve replacement
SNOWMASS, COLO. – Michael J. Mack, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
That’s assuming, hypothetically, that two ongoing randomized trials – PARTNER 3 and EVOLUT R – comparing TAVR (transcatheter aortic valve replacement) and SAVR (surgical aortic valve replacement) in low-surgical-risk patients have positive results. If so, TAVR will be proven noninferior to SAVR, as has already been convincingly shown in randomized trials conducted in high- and intermediate-surgical-risk patients.
Several factors are working against universal adoption of TAVR. There are unresolved concerns about TAVR’s durability, its issues with valve thrombosis, and its greater need for new pacemaker implantation relative to SAVR. Those questions are among the issues being addressed in nearly two dozen ongoing or upcoming TAVR trials, said Dr. Mack, director of the cardiovascular service line at Baylor Scott & White Health System, Houston.
Here are the patients Dr. Mack predicts will likely stick with SAVR for the foreseeable future:
- Younger patients. Many younger, low-surgical-risk patients will be concerned about TAVR valves’ durability and the current higher pacemaker rate.
“We have some idea of what durability is in the surgical population. What we usually quote is 12-15 years. There has been no major signal of early valve deterioration in TAVR, but there aren’t yet a significant number of patients alive for more than 5 years. Be that as it may, no significant concerns yet, but the other shoe hasn’t dropped,” the cardiothoracic surgeon observed.
Both PARTNER 3 and the EVOLUT R low-risk trial will follow participants annually for 10 years, eventually providing a robust, combined 2,000-patient database to assess comparative device durability.
The 30-day rates of permanent pacemaker implantation in TAVR recipients without a preexisting pacemaker is in the 12%-16% range with the newer-generation Evolut R and Sapien 3 valves. The indication is often new-onset left bundle branch block or complete heart block.
“I don’t think the increased pacemaker incidence is a huge problem now, but with the younger population I think it’s going to be more of an issue,” according to Dr. Mack. “It’s one thing to need a pacemaker if you’re 80-85 years old, but it’s totally different in somebody who’s 55 or 65 that has 30-35 years to live. Pacemaker leads are not benign in that population.”
“On the other hand, there is a thought that younger patients have less intrinsic conduction system disease. It may be that they are less prone to needing a new pacemaker afterward,” he added.
TAVR valve leaflet thrombosis is a concern. While a change in transvalvular gradient on echocardiography provides a signal for this complication, the actual diagnosis of valve thrombosis requires four-dimensional CT. Much better data on the true incidence and clinical ramifications of valve thrombosis in both TAVR and SAVR are coming. For example, the PARTNER 3 and EVOLUT R LR trials will each include 400-patient substudies with four-dimensional CT aimed at answering questions about valve thrombosis. The GALILEO trial, involving 1,520 TAVR patients, is designed to determine whether low-dose rivaroxaban prevents valve thrombosis and periprocedural embolization. Results of this randomized trial are due in about a year. Similarly, the 1,509-patient randomized ATLANTIS trial is looking at the effects of apixaban versus warfarin versus dual or single antiplatelet therapy.
If long-term anticoagulation in TAVR patients is found to be beneficial, that may steer some patients toward SAVR, especially if they are physically active or at high bleeding risk.
- Aortic stenosis patients with a high Syntax score and multivessel coronary artery disease. This group is best served by concomitant SAVR and coronary artery bypass grafting surgery.
- Patients with low or intermediate surgical risk with multivalve disease. “We know that patients with moderate to severe mitral regurgitation and/or moderate to severe tricuspid regurgitation do not do well after TAVR,” the surgeon said.
- Rheumatic valve disease patients.
- Patients who present at a site that offers SAVR only. “There are about 1,150 cardiac surgery programs in the United States and about 560 TAVR programs. There is concern that patients who don’t come in to a center that offers TAVR will not be offered TAVR as an option. I think there’s probably some truth to that,” Dr. Mack said.
- Patients going to sites without a well-functioning collaborative heart team. “In institutions where surgeons and cardiologists don’t get along, turf is much more of an issue, and surgeons are going to hold on to those patients more closely,” he observed.
- Patient’s with certain preferences. “All other things being equal, the patient is always going to choose the less invasive option. However, we do see some patients – I think it’s about 1 out of 10 – who express a preference to stay with the tried and true surgery,” according to Dr. Mack.
He reported receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
SNOWMASS, COLO. – Michael J. Mack, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
That’s assuming, hypothetically, that two ongoing randomized trials – PARTNER 3 and EVOLUT R – comparing TAVR (transcatheter aortic valve replacement) and SAVR (surgical aortic valve replacement) in low-surgical-risk patients have positive results. If so, TAVR will be proven noninferior to SAVR, as has already been convincingly shown in randomized trials conducted in high- and intermediate-surgical-risk patients.
Several factors are working against universal adoption of TAVR. There are unresolved concerns about TAVR’s durability, its issues with valve thrombosis, and its greater need for new pacemaker implantation relative to SAVR. Those questions are among the issues being addressed in nearly two dozen ongoing or upcoming TAVR trials, said Dr. Mack, director of the cardiovascular service line at Baylor Scott & White Health System, Houston.
Here are the patients Dr. Mack predicts will likely stick with SAVR for the foreseeable future:
- Younger patients. Many younger, low-surgical-risk patients will be concerned about TAVR valves’ durability and the current higher pacemaker rate.
“We have some idea of what durability is in the surgical population. What we usually quote is 12-15 years. There has been no major signal of early valve deterioration in TAVR, but there aren’t yet a significant number of patients alive for more than 5 years. Be that as it may, no significant concerns yet, but the other shoe hasn’t dropped,” the cardiothoracic surgeon observed.
Both PARTNER 3 and the EVOLUT R low-risk trial will follow participants annually for 10 years, eventually providing a robust, combined 2,000-patient database to assess comparative device durability.
The 30-day rates of permanent pacemaker implantation in TAVR recipients without a preexisting pacemaker is in the 12%-16% range with the newer-generation Evolut R and Sapien 3 valves. The indication is often new-onset left bundle branch block or complete heart block.
“I don’t think the increased pacemaker incidence is a huge problem now, but with the younger population I think it’s going to be more of an issue,” according to Dr. Mack. “It’s one thing to need a pacemaker if you’re 80-85 years old, but it’s totally different in somebody who’s 55 or 65 that has 30-35 years to live. Pacemaker leads are not benign in that population.”
“On the other hand, there is a thought that younger patients have less intrinsic conduction system disease. It may be that they are less prone to needing a new pacemaker afterward,” he added.
TAVR valve leaflet thrombosis is a concern. While a change in transvalvular gradient on echocardiography provides a signal for this complication, the actual diagnosis of valve thrombosis requires four-dimensional CT. Much better data on the true incidence and clinical ramifications of valve thrombosis in both TAVR and SAVR are coming. For example, the PARTNER 3 and EVOLUT R LR trials will each include 400-patient substudies with four-dimensional CT aimed at answering questions about valve thrombosis. The GALILEO trial, involving 1,520 TAVR patients, is designed to determine whether low-dose rivaroxaban prevents valve thrombosis and periprocedural embolization. Results of this randomized trial are due in about a year. Similarly, the 1,509-patient randomized ATLANTIS trial is looking at the effects of apixaban versus warfarin versus dual or single antiplatelet therapy.
If long-term anticoagulation in TAVR patients is found to be beneficial, that may steer some patients toward SAVR, especially if they are physically active or at high bleeding risk.
- Aortic stenosis patients with a high Syntax score and multivessel coronary artery disease. This group is best served by concomitant SAVR and coronary artery bypass grafting surgery.
- Patients with low or intermediate surgical risk with multivalve disease. “We know that patients with moderate to severe mitral regurgitation and/or moderate to severe tricuspid regurgitation do not do well after TAVR,” the surgeon said.
- Rheumatic valve disease patients.
- Patients who present at a site that offers SAVR only. “There are about 1,150 cardiac surgery programs in the United States and about 560 TAVR programs. There is concern that patients who don’t come in to a center that offers TAVR will not be offered TAVR as an option. I think there’s probably some truth to that,” Dr. Mack said.
- Patients going to sites without a well-functioning collaborative heart team. “In institutions where surgeons and cardiologists don’t get along, turf is much more of an issue, and surgeons are going to hold on to those patients more closely,” he observed.
- Patient’s with certain preferences. “All other things being equal, the patient is always going to choose the less invasive option. However, we do see some patients – I think it’s about 1 out of 10 – who express a preference to stay with the tried and true surgery,” according to Dr. Mack.
He reported receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
SNOWMASS, COLO. – Michael J. Mack, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
That’s assuming, hypothetically, that two ongoing randomized trials – PARTNER 3 and EVOLUT R – comparing TAVR (transcatheter aortic valve replacement) and SAVR (surgical aortic valve replacement) in low-surgical-risk patients have positive results. If so, TAVR will be proven noninferior to SAVR, as has already been convincingly shown in randomized trials conducted in high- and intermediate-surgical-risk patients.
Several factors are working against universal adoption of TAVR. There are unresolved concerns about TAVR’s durability, its issues with valve thrombosis, and its greater need for new pacemaker implantation relative to SAVR. Those questions are among the issues being addressed in nearly two dozen ongoing or upcoming TAVR trials, said Dr. Mack, director of the cardiovascular service line at Baylor Scott & White Health System, Houston.
Here are the patients Dr. Mack predicts will likely stick with SAVR for the foreseeable future:
- Younger patients. Many younger, low-surgical-risk patients will be concerned about TAVR valves’ durability and the current higher pacemaker rate.
“We have some idea of what durability is in the surgical population. What we usually quote is 12-15 years. There has been no major signal of early valve deterioration in TAVR, but there aren’t yet a significant number of patients alive for more than 5 years. Be that as it may, no significant concerns yet, but the other shoe hasn’t dropped,” the cardiothoracic surgeon observed.
Both PARTNER 3 and the EVOLUT R low-risk trial will follow participants annually for 10 years, eventually providing a robust, combined 2,000-patient database to assess comparative device durability.
The 30-day rates of permanent pacemaker implantation in TAVR recipients without a preexisting pacemaker is in the 12%-16% range with the newer-generation Evolut R and Sapien 3 valves. The indication is often new-onset left bundle branch block or complete heart block.
“I don’t think the increased pacemaker incidence is a huge problem now, but with the younger population I think it’s going to be more of an issue,” according to Dr. Mack. “It’s one thing to need a pacemaker if you’re 80-85 years old, but it’s totally different in somebody who’s 55 or 65 that has 30-35 years to live. Pacemaker leads are not benign in that population.”
“On the other hand, there is a thought that younger patients have less intrinsic conduction system disease. It may be that they are less prone to needing a new pacemaker afterward,” he added.
TAVR valve leaflet thrombosis is a concern. While a change in transvalvular gradient on echocardiography provides a signal for this complication, the actual diagnosis of valve thrombosis requires four-dimensional CT. Much better data on the true incidence and clinical ramifications of valve thrombosis in both TAVR and SAVR are coming. For example, the PARTNER 3 and EVOLUT R LR trials will each include 400-patient substudies with four-dimensional CT aimed at answering questions about valve thrombosis. The GALILEO trial, involving 1,520 TAVR patients, is designed to determine whether low-dose rivaroxaban prevents valve thrombosis and periprocedural embolization. Results of this randomized trial are due in about a year. Similarly, the 1,509-patient randomized ATLANTIS trial is looking at the effects of apixaban versus warfarin versus dual or single antiplatelet therapy.
If long-term anticoagulation in TAVR patients is found to be beneficial, that may steer some patients toward SAVR, especially if they are physically active or at high bleeding risk.
- Aortic stenosis patients with a high Syntax score and multivessel coronary artery disease. This group is best served by concomitant SAVR and coronary artery bypass grafting surgery.
- Patients with low or intermediate surgical risk with multivalve disease. “We know that patients with moderate to severe mitral regurgitation and/or moderate to severe tricuspid regurgitation do not do well after TAVR,” the surgeon said.
- Rheumatic valve disease patients.
- Patients who present at a site that offers SAVR only. “There are about 1,150 cardiac surgery programs in the United States and about 560 TAVR programs. There is concern that patients who don’t come in to a center that offers TAVR will not be offered TAVR as an option. I think there’s probably some truth to that,” Dr. Mack said.
- Patients going to sites without a well-functioning collaborative heart team. “In institutions where surgeons and cardiologists don’t get along, turf is much more of an issue, and surgeons are going to hold on to those patients more closely,” he observed.
- Patient’s with certain preferences. “All other things being equal, the patient is always going to choose the less invasive option. However, we do see some patients – I think it’s about 1 out of 10 – who express a preference to stay with the tried and true surgery,” according to Dr. Mack.
He reported receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Low Serum Caffeine Level Could Indicate Early Parkinson’s Disease
Low serum caffeine and caffeine metabolite levels after an overnight fast may be a sensitive way to detect Parkinson’s disease, according to the results of a case–control study published online ahead of print January 3 in Neurology.
In the study, levels of caffeine and its metabolites were lower in patients with Parkinson’s disease and motor dysfunction, compared with those without motor dysfunction. The investigators detected no differences in serum levels of caffeine metabolites between patients with mild Parkinson’s disease and those with severe Parkinson’s disease, said Motoki Fujimaki, MD, of Juntendo University School of Medicine in Tokyo, and colleagues.
A Single-Center Study
Previous research had shown that people drinking four or more cups of coffee per day had a greater than fivefold reduction in the risk of developing Parkinson’s disease. Mouse models of Parkinson’s disease showed that caffeine and two of its metabolites have a neuroprotective effect. Those results suggested that serum caffeine might be useful as a blood marker for Parkinson’s disease.
To test that idea, Dr. Fujimaki and associates recruited 31 healthy controls (18 women) and 108 patients with Parkinson’s disease but no dementia (50 women). The control group’s mean caffeine intake of 115.81 mg/day was similar to that of patients with Parkinson’s disease (107.50 mg/day).
Serum caffeine levels measured after an overnight fast showed that a cutoff of 33.04 pmol/10 µL identified Parkinson’s disease with an area under the curve (AUC) of 0.78 (sensitivity, 76.9%; specificity, 74.2%). Inclusion of the primary caffeine metabolites theophylline, theobromine, and paraxanthine increased the AUC to 0.87. When the researchers included all 11 measurable metabolites, the AUC increased further to 0.98.
Genetic analyses revealed no significant differences in the frequencies of caffeine metabolism–associated genetic variants between patients and controls.
The study was limited by the fact that it was conducted at a single university hospital, and the patient population did not include many severe cases. The association should also be studied in other Parkinson’s disease patient populations, according to the authors.
Did Treatment Effects Influence the Findings?
A key question that the study raises is what caused the decrease in serum concentration found in patients with Parkinson’s disease, said David G. Munoz, MD, of the Department of Laboratory Medicine and Pathobiology at the University of Toronto, and Shinsuke Fujioka, MD, of the Department of Neurology at Fukuoka University in Japan, in an accompanying editorial. Almost all of the patients were receiving treatment, which could have affected serum levels, they added. The researchers looked for, but did not find, an association between serum caffeine metabolite levels and levodopa equivalent doses.
“The validity of the study depends on whether caffeine metabolism may be affected by treatment,” said Drs. Munoz and Fujioka. “To demonstrate the utility of caffeine metabolites unequivocally, a future study will have to reproduce these results in patients with untreated Parkinson’s disease or subjects at high risk of Parkinson’s disease, such as those with prodromal signs of Parkinson’s disease.”
—Jim Kling
Suggested Reading
Fujimaki M, Saiki S, Li Y, et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology. 2018 Jan 3 [Epub ahead of print].
Munoz DG, Fujioka S. Caffeine and Parkinson disease: A possible diagnostic and pathogenic breakthrough. Neurology. 2018 Jan 3 [Epub ahead of print].
Low serum caffeine and caffeine metabolite levels after an overnight fast may be a sensitive way to detect Parkinson’s disease, according to the results of a case–control study published online ahead of print January 3 in Neurology.
In the study, levels of caffeine and its metabolites were lower in patients with Parkinson’s disease and motor dysfunction, compared with those without motor dysfunction. The investigators detected no differences in serum levels of caffeine metabolites between patients with mild Parkinson’s disease and those with severe Parkinson’s disease, said Motoki Fujimaki, MD, of Juntendo University School of Medicine in Tokyo, and colleagues.
A Single-Center Study
Previous research had shown that people drinking four or more cups of coffee per day had a greater than fivefold reduction in the risk of developing Parkinson’s disease. Mouse models of Parkinson’s disease showed that caffeine and two of its metabolites have a neuroprotective effect. Those results suggested that serum caffeine might be useful as a blood marker for Parkinson’s disease.
To test that idea, Dr. Fujimaki and associates recruited 31 healthy controls (18 women) and 108 patients with Parkinson’s disease but no dementia (50 women). The control group’s mean caffeine intake of 115.81 mg/day was similar to that of patients with Parkinson’s disease (107.50 mg/day).
Serum caffeine levels measured after an overnight fast showed that a cutoff of 33.04 pmol/10 µL identified Parkinson’s disease with an area under the curve (AUC) of 0.78 (sensitivity, 76.9%; specificity, 74.2%). Inclusion of the primary caffeine metabolites theophylline, theobromine, and paraxanthine increased the AUC to 0.87. When the researchers included all 11 measurable metabolites, the AUC increased further to 0.98.
Genetic analyses revealed no significant differences in the frequencies of caffeine metabolism–associated genetic variants between patients and controls.
The study was limited by the fact that it was conducted at a single university hospital, and the patient population did not include many severe cases. The association should also be studied in other Parkinson’s disease patient populations, according to the authors.
Did Treatment Effects Influence the Findings?
A key question that the study raises is what caused the decrease in serum concentration found in patients with Parkinson’s disease, said David G. Munoz, MD, of the Department of Laboratory Medicine and Pathobiology at the University of Toronto, and Shinsuke Fujioka, MD, of the Department of Neurology at Fukuoka University in Japan, in an accompanying editorial. Almost all of the patients were receiving treatment, which could have affected serum levels, they added. The researchers looked for, but did not find, an association between serum caffeine metabolite levels and levodopa equivalent doses.
“The validity of the study depends on whether caffeine metabolism may be affected by treatment,” said Drs. Munoz and Fujioka. “To demonstrate the utility of caffeine metabolites unequivocally, a future study will have to reproduce these results in patients with untreated Parkinson’s disease or subjects at high risk of Parkinson’s disease, such as those with prodromal signs of Parkinson’s disease.”
—Jim Kling
Suggested Reading
Fujimaki M, Saiki S, Li Y, et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology. 2018 Jan 3 [Epub ahead of print].
Munoz DG, Fujioka S. Caffeine and Parkinson disease: A possible diagnostic and pathogenic breakthrough. Neurology. 2018 Jan 3 [Epub ahead of print].
Low serum caffeine and caffeine metabolite levels after an overnight fast may be a sensitive way to detect Parkinson’s disease, according to the results of a case–control study published online ahead of print January 3 in Neurology.
In the study, levels of caffeine and its metabolites were lower in patients with Parkinson’s disease and motor dysfunction, compared with those without motor dysfunction. The investigators detected no differences in serum levels of caffeine metabolites between patients with mild Parkinson’s disease and those with severe Parkinson’s disease, said Motoki Fujimaki, MD, of Juntendo University School of Medicine in Tokyo, and colleagues.
A Single-Center Study
Previous research had shown that people drinking four or more cups of coffee per day had a greater than fivefold reduction in the risk of developing Parkinson’s disease. Mouse models of Parkinson’s disease showed that caffeine and two of its metabolites have a neuroprotective effect. Those results suggested that serum caffeine might be useful as a blood marker for Parkinson’s disease.
To test that idea, Dr. Fujimaki and associates recruited 31 healthy controls (18 women) and 108 patients with Parkinson’s disease but no dementia (50 women). The control group’s mean caffeine intake of 115.81 mg/day was similar to that of patients with Parkinson’s disease (107.50 mg/day).
Serum caffeine levels measured after an overnight fast showed that a cutoff of 33.04 pmol/10 µL identified Parkinson’s disease with an area under the curve (AUC) of 0.78 (sensitivity, 76.9%; specificity, 74.2%). Inclusion of the primary caffeine metabolites theophylline, theobromine, and paraxanthine increased the AUC to 0.87. When the researchers included all 11 measurable metabolites, the AUC increased further to 0.98.
Genetic analyses revealed no significant differences in the frequencies of caffeine metabolism–associated genetic variants between patients and controls.
The study was limited by the fact that it was conducted at a single university hospital, and the patient population did not include many severe cases. The association should also be studied in other Parkinson’s disease patient populations, according to the authors.
Did Treatment Effects Influence the Findings?
A key question that the study raises is what caused the decrease in serum concentration found in patients with Parkinson’s disease, said David G. Munoz, MD, of the Department of Laboratory Medicine and Pathobiology at the University of Toronto, and Shinsuke Fujioka, MD, of the Department of Neurology at Fukuoka University in Japan, in an accompanying editorial. Almost all of the patients were receiving treatment, which could have affected serum levels, they added. The researchers looked for, but did not find, an association between serum caffeine metabolite levels and levodopa equivalent doses.
“The validity of the study depends on whether caffeine metabolism may be affected by treatment,” said Drs. Munoz and Fujioka. “To demonstrate the utility of caffeine metabolites unequivocally, a future study will have to reproduce these results in patients with untreated Parkinson’s disease or subjects at high risk of Parkinson’s disease, such as those with prodromal signs of Parkinson’s disease.”
—Jim Kling
Suggested Reading
Fujimaki M, Saiki S, Li Y, et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology. 2018 Jan 3 [Epub ahead of print].
Munoz DG, Fujioka S. Caffeine and Parkinson disease: A possible diagnostic and pathogenic breakthrough. Neurology. 2018 Jan 3 [Epub ahead of print].
Embracing Life’s Simple 7 slashes PAD risk
ANAHEIM, CALIF. – Adherence to the American Heart Association’s widely publicized “Life’s Simple 7” program addressing key modifiable cardiovascular health factors substantially reduces the risk of developing peripheral arterial disease, Parveen Garg, MD, said at the American Heart Association scientific sessions.
This is new evidence-supported information. Until this new analysis from the landmark ARIC (Atherosclerosis Risk in Communities) study, the relationship between Life’s Simple 7 and peripheral arterial disease (PAD) hadn’t been studied. It’s a relationship worthy of examination, considering that more than 8 million Americans have PAD, and nearly 40% of them don’t have concomitant coronary or cerebrovascular disease, which raised the question of whether Life’s Simple 7 applied to PAD risk, noted Dr. Garg of the University of Southern California, Los Angeles.
ARIC is a National Heart, Lung, and Blood Institute–sponsored prospective study of nearly 16,000 black or white individuals who were middle-aged at enrollment and have been followed for more than 2 decades. Dr. Garg’s analysis focused on 12,865 participants who were free of CHD, heart failure, prior stroke, and PAD at baseline, and have been followed for a median of 24 years.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for ideal, 1 for intermediate, and 0 for poor. The composite Life’s Simple 7 score is rated optimal at 10-14 points, average at 5-9, and inadequate at 0-4.
During follow-up, 3.4% of ARIC participants developed PAD sufficiently severe to involve hospitalization. The incidence rate was 5.2 cases per 1,000 person-years for the 1,008 subjects categorized as having an inadequate Life’s Simple 7 score, 1.1/1,000 person-years for the 8,395 people in the average category, and just 0.4 cases/1,000 person-years for the 3,462 individuals in the optimal Life’s Simple 7 group.
Compared with subjects in the inadequate category, those in the average group were 56% less likely to develop PAD. Those in the optimal Life’s Simple 7 category had an 86% reduction in risk.
For each of the seven components of Life’s Simple 7 a person scored ideally in, the risk of incident PAD was reduced by 28% in a multivariate analysis fully adjusted for demographics, alcohol consumption, aspirin use, study site, left ventricular hypertrophy, and other potential confounders.
The inverse relationship between Life’s Simple 7 score and PAD risk was stronger in women than men. However, the association didn’t differ by race.
Dr. Garg noted that his study undoubtedly underestimates the true incidence of PAD in the ARIC population, since a hospital diagnosis was required. Also, to date he and his coinvestigators have only analyzed the results in terms of baseline Life’s Simple 7 score. It would be useful to also document the impact of change in the score over time.
Session moderator David C. Goff Jr., MD, observed, “This is very consistent with evidence in CHD that people who are in ideal cardiovascular health status have about an 80%-90% lower risk of cardiovascular mortality and a 70%-80% reduction in risk of total mortality compared with people who are in poor cardiovascular health status.”
“This study really does provide additional evidence that if we could get more people into the ideal cardiovascular health range, we’d probably see less atherosclerotic cardiovascular disease in general,” added Dr. Goff, who is director of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute.
Dr. Garg reported having no financial conflicts of interest.
ANAHEIM, CALIF. – Adherence to the American Heart Association’s widely publicized “Life’s Simple 7” program addressing key modifiable cardiovascular health factors substantially reduces the risk of developing peripheral arterial disease, Parveen Garg, MD, said at the American Heart Association scientific sessions.
This is new evidence-supported information. Until this new analysis from the landmark ARIC (Atherosclerosis Risk in Communities) study, the relationship between Life’s Simple 7 and peripheral arterial disease (PAD) hadn’t been studied. It’s a relationship worthy of examination, considering that more than 8 million Americans have PAD, and nearly 40% of them don’t have concomitant coronary or cerebrovascular disease, which raised the question of whether Life’s Simple 7 applied to PAD risk, noted Dr. Garg of the University of Southern California, Los Angeles.
ARIC is a National Heart, Lung, and Blood Institute–sponsored prospective study of nearly 16,000 black or white individuals who were middle-aged at enrollment and have been followed for more than 2 decades. Dr. Garg’s analysis focused on 12,865 participants who were free of CHD, heart failure, prior stroke, and PAD at baseline, and have been followed for a median of 24 years.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for ideal, 1 for intermediate, and 0 for poor. The composite Life’s Simple 7 score is rated optimal at 10-14 points, average at 5-9, and inadequate at 0-4.
During follow-up, 3.4% of ARIC participants developed PAD sufficiently severe to involve hospitalization. The incidence rate was 5.2 cases per 1,000 person-years for the 1,008 subjects categorized as having an inadequate Life’s Simple 7 score, 1.1/1,000 person-years for the 8,395 people in the average category, and just 0.4 cases/1,000 person-years for the 3,462 individuals in the optimal Life’s Simple 7 group.
Compared with subjects in the inadequate category, those in the average group were 56% less likely to develop PAD. Those in the optimal Life’s Simple 7 category had an 86% reduction in risk.
For each of the seven components of Life’s Simple 7 a person scored ideally in, the risk of incident PAD was reduced by 28% in a multivariate analysis fully adjusted for demographics, alcohol consumption, aspirin use, study site, left ventricular hypertrophy, and other potential confounders.
The inverse relationship between Life’s Simple 7 score and PAD risk was stronger in women than men. However, the association didn’t differ by race.
Dr. Garg noted that his study undoubtedly underestimates the true incidence of PAD in the ARIC population, since a hospital diagnosis was required. Also, to date he and his coinvestigators have only analyzed the results in terms of baseline Life’s Simple 7 score. It would be useful to also document the impact of change in the score over time.
Session moderator David C. Goff Jr., MD, observed, “This is very consistent with evidence in CHD that people who are in ideal cardiovascular health status have about an 80%-90% lower risk of cardiovascular mortality and a 70%-80% reduction in risk of total mortality compared with people who are in poor cardiovascular health status.”
“This study really does provide additional evidence that if we could get more people into the ideal cardiovascular health range, we’d probably see less atherosclerotic cardiovascular disease in general,” added Dr. Goff, who is director of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute.
Dr. Garg reported having no financial conflicts of interest.
ANAHEIM, CALIF. – Adherence to the American Heart Association’s widely publicized “Life’s Simple 7” program addressing key modifiable cardiovascular health factors substantially reduces the risk of developing peripheral arterial disease, Parveen Garg, MD, said at the American Heart Association scientific sessions.
This is new evidence-supported information. Until this new analysis from the landmark ARIC (Atherosclerosis Risk in Communities) study, the relationship between Life’s Simple 7 and peripheral arterial disease (PAD) hadn’t been studied. It’s a relationship worthy of examination, considering that more than 8 million Americans have PAD, and nearly 40% of them don’t have concomitant coronary or cerebrovascular disease, which raised the question of whether Life’s Simple 7 applied to PAD risk, noted Dr. Garg of the University of Southern California, Los Angeles.
ARIC is a National Heart, Lung, and Blood Institute–sponsored prospective study of nearly 16,000 black or white individuals who were middle-aged at enrollment and have been followed for more than 2 decades. Dr. Garg’s analysis focused on 12,865 participants who were free of CHD, heart failure, prior stroke, and PAD at baseline, and have been followed for a median of 24 years.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for ideal, 1 for intermediate, and 0 for poor. The composite Life’s Simple 7 score is rated optimal at 10-14 points, average at 5-9, and inadequate at 0-4.
During follow-up, 3.4% of ARIC participants developed PAD sufficiently severe to involve hospitalization. The incidence rate was 5.2 cases per 1,000 person-years for the 1,008 subjects categorized as having an inadequate Life’s Simple 7 score, 1.1/1,000 person-years for the 8,395 people in the average category, and just 0.4 cases/1,000 person-years for the 3,462 individuals in the optimal Life’s Simple 7 group.
Compared with subjects in the inadequate category, those in the average group were 56% less likely to develop PAD. Those in the optimal Life’s Simple 7 category had an 86% reduction in risk.
For each of the seven components of Life’s Simple 7 a person scored ideally in, the risk of incident PAD was reduced by 28% in a multivariate analysis fully adjusted for demographics, alcohol consumption, aspirin use, study site, left ventricular hypertrophy, and other potential confounders.
The inverse relationship between Life’s Simple 7 score and PAD risk was stronger in women than men. However, the association didn’t differ by race.
Dr. Garg noted that his study undoubtedly underestimates the true incidence of PAD in the ARIC population, since a hospital diagnosis was required. Also, to date he and his coinvestigators have only analyzed the results in terms of baseline Life’s Simple 7 score. It would be useful to also document the impact of change in the score over time.
Session moderator David C. Goff Jr., MD, observed, “This is very consistent with evidence in CHD that people who are in ideal cardiovascular health status have about an 80%-90% lower risk of cardiovascular mortality and a 70%-80% reduction in risk of total mortality compared with people who are in poor cardiovascular health status.”
“This study really does provide additional evidence that if we could get more people into the ideal cardiovascular health range, we’d probably see less atherosclerotic cardiovascular disease in general,” added Dr. Goff, who is director of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute.
Dr. Garg reported having no financial conflicts of interest.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The Life’s Simple 7 public health program points the way to reduced risk of PAD.
Major finding: Being in the top tertile of cardiovascular health by the American Heart Association’s Life’s Simple 7 metric is associated with an 86% lower risk of developing PAD than for those in poor cardiovascular health.
Study details: This biracial prospective observational study includes nearly 16,000 white and black Americans.
Disclosures: The ARIC study is funded by the NHLBI. The presenter reported having no financial conflicts.












