User login
Laparoscopic nerve-sparing approach is effective in deep infiltrating endometriosis
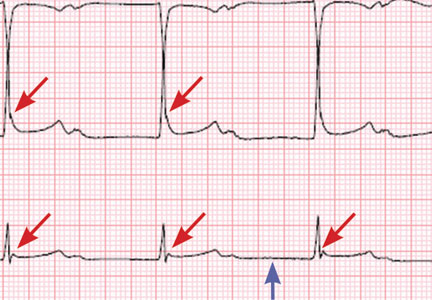
NATIONAL HARBOR, MD. – Laparoscopic retroperitoneal nerve-sparing surgery is a safe approach that relieves pain in women with deep infiltrating endometriosis, according to findings presented by Giovanni Roviglione, MD, at the AAGL Global Congress.
The prospective case series study with a single gynecologic surgeon in Verona, Italy, involved 382 women who had deep infiltrating endometriosis with sciatica and anogenital pain. All of the women had some level of nervous compression of somatic structures and infiltration of their fascial envelope.
The surgery involved whole decompression and partial neurolysis of nervous structures for most patients, while nearly 20% of women required complete neurolysis based on their level of infiltration. Most women (64%) had severe enough infiltration that a concomitant bowel resection was also necessary.
The surgeon performed a medial approach for deep pelvic endometriosis with rectal and/or parametrial involvement extending to the pelvic wall and somatic nerve, or a lateral approach for isolated endometriosis of the pelvic wall and somatic nerves.
At 6 months after surgery, all patients reported complete relief from pain. However, 77 women (20%) experienced postoperative neuritis, which was successfully treated with corticosteroids, antiepileptics, and opioids.
Endometriosis that extends into somatic nerves and the sacral roots is a common cause of pelvic pain, Dr. Roviglione said.
“This kind of endometriosis is resistant to opioids and drugs,” he said. The difficulty in treating deep infiltrating endometriosis is compounded by the often long delay in diagnosis, he added.
Using laparoscopy for neurolysis and decompression of somatic nerves affected by endometriosis is a “more accurate and effective treatment” for providing pain relief, Dr. Roviglione said. But laparoscopic retroperitoneal nerve-sparing surgery should be performed only by skilled neuroanatomy surgeons at referral centers because of the complex nature of the procedure, he noted.
Dr. Roviglione reported having no relevant financial disclosures.
SOURCE: Ceccaroni M et al. AAGL 2017 Abstract 166.
NATIONAL HARBOR, MD. – Laparoscopic retroperitoneal nerve-sparing surgery is a safe approach that relieves pain in women with deep infiltrating endometriosis, according to findings presented by Giovanni Roviglione, MD, at the AAGL Global Congress.
The prospective case series study with a single gynecologic surgeon in Verona, Italy, involved 382 women who had deep infiltrating endometriosis with sciatica and anogenital pain. All of the women had some level of nervous compression of somatic structures and infiltration of their fascial envelope.
The surgery involved whole decompression and partial neurolysis of nervous structures for most patients, while nearly 20% of women required complete neurolysis based on their level of infiltration. Most women (64%) had severe enough infiltration that a concomitant bowel resection was also necessary.
The surgeon performed a medial approach for deep pelvic endometriosis with rectal and/or parametrial involvement extending to the pelvic wall and somatic nerve, or a lateral approach for isolated endometriosis of the pelvic wall and somatic nerves.
At 6 months after surgery, all patients reported complete relief from pain. However, 77 women (20%) experienced postoperative neuritis, which was successfully treated with corticosteroids, antiepileptics, and opioids.
Endometriosis that extends into somatic nerves and the sacral roots is a common cause of pelvic pain, Dr. Roviglione said.
“This kind of endometriosis is resistant to opioids and drugs,” he said. The difficulty in treating deep infiltrating endometriosis is compounded by the often long delay in diagnosis, he added.
Using laparoscopy for neurolysis and decompression of somatic nerves affected by endometriosis is a “more accurate and effective treatment” for providing pain relief, Dr. Roviglione said. But laparoscopic retroperitoneal nerve-sparing surgery should be performed only by skilled neuroanatomy surgeons at referral centers because of the complex nature of the procedure, he noted.
Dr. Roviglione reported having no relevant financial disclosures.
SOURCE: Ceccaroni M et al. AAGL 2017 Abstract 166.
NATIONAL HARBOR, MD. – Laparoscopic retroperitoneal nerve-sparing surgery is a safe approach that relieves pain in women with deep infiltrating endometriosis, according to findings presented by Giovanni Roviglione, MD, at the AAGL Global Congress.
The prospective case series study with a single gynecologic surgeon in Verona, Italy, involved 382 women who had deep infiltrating endometriosis with sciatica and anogenital pain. All of the women had some level of nervous compression of somatic structures and infiltration of their fascial envelope.
The surgery involved whole decompression and partial neurolysis of nervous structures for most patients, while nearly 20% of women required complete neurolysis based on their level of infiltration. Most women (64%) had severe enough infiltration that a concomitant bowel resection was also necessary.
The surgeon performed a medial approach for deep pelvic endometriosis with rectal and/or parametrial involvement extending to the pelvic wall and somatic nerve, or a lateral approach for isolated endometriosis of the pelvic wall and somatic nerves.
At 6 months after surgery, all patients reported complete relief from pain. However, 77 women (20%) experienced postoperative neuritis, which was successfully treated with corticosteroids, antiepileptics, and opioids.
Endometriosis that extends into somatic nerves and the sacral roots is a common cause of pelvic pain, Dr. Roviglione said.
“This kind of endometriosis is resistant to opioids and drugs,” he said. The difficulty in treating deep infiltrating endometriosis is compounded by the often long delay in diagnosis, he added.
Using laparoscopy for neurolysis and decompression of somatic nerves affected by endometriosis is a “more accurate and effective treatment” for providing pain relief, Dr. Roviglione said. But laparoscopic retroperitoneal nerve-sparing surgery should be performed only by skilled neuroanatomy surgeons at referral centers because of the complex nature of the procedure, he noted.
Dr. Roviglione reported having no relevant financial disclosures.
SOURCE: Ceccaroni M et al. AAGL 2017 Abstract 166.
REPORTING FROM AAGL 2017
Key clinical point:
Major finding: All patients reported complete relief of neurologic symptoms at 6 months after surgery.
Study details: Single center, prospective case series of 382 women who underwent laparoscopic retroperitoneal nerve-sparing surgery to treat pain associated with deep infiltrating endometriosis.
Disclosures: Dr. Roviglione reported having no relevant financial disclosures.
Source: Ceccaroni M et al. AAGL 2017 Abstract 166.
Enzalutamide plus exemestane improves PFS in HR+ breast cancer subset
SAN ANTONIO – Enzalutamide added to exemestane improved progression-free survival (PFS) in patients with hormone receptor (HR)-positive advanced breast cancer, investigators reported.
Specifically, it improved outcomes in patients who had not received any prior endocrine therapy and who were positive for a gene signature-based biomarker indicating androgen receptor (AR) signaling.
Patients in this subset who were treated with combination enzalutamide and exemestane achieved a median PFS of 16.5 months, which was significantly higher than the 4 months observed with placebo and exemestane.
However, the addition of enzalutamide had no effect on PFS in the overall cohort or among patients who were biomarker positive but who had received prior endocrine therapy.
“The study met its primary endpoint in improving PFS in the enzalutamide plus exemestane-treated patients who were biomarker positive and HR positive with no prior endocrine therapy for advanced disease as compared [with] exemestane alone,” Denise A. Yardley, MD, of Tennessee Oncology, Nashville, said at the San Antonio Breast Cancer Symposium.
“The role of the AR in HR-positive breast cancer and the predictive value of the identified biomarker are still unclear and will require further studies and validation,” said Dr. Yardley.
Targeting AR is an active area of breast cancer research, as a majority of HR-positive tumors express the AR, as do a moderate number of HER2-positive tumors and almost a third of triple-negative breast cancers. AR signaling has also been associated with resistance to endocrine therapy. Aromatase inhibitors divert estrogen precursors to androgens and data from preclinical models have shown that enzalutamide blocked both estrogen- and androgen-mediated growth of HR+ cells.
Enzalutamide is an inhibitor of AR signaling that is currently used to treat patients with castration-resistant prostate cancer, and has demonstrated clinical activity and was well tolerated in patients with AR-positive advanced triple negative breast cancer, explained Dr. Yardley.
In this study, Dr. Yardley and her colleagues conducted a placebo-controlled phase 2 randomized trial that included 247 patients with HR+/HER2-normal advanced/metastatic breast cancer who were assigned to either 25 mg exemestane plus placebo or 50 mg exemestane and 160 mg enzalutamide daily.
The patients were divided into two parallel cohorts: those with no prior endocrine therapy (C1; n = 127) or those who had received one prior endocrine therapy for metastatic disease (C2; n = 120).
The primary endpoint was PFS in the intent-to-treat population and in the biomarker subgroup of each cohort. Secondary endpoints included the clinical benefit rate at 24 weeks, best overall response, and safety.
The authors found that the PFS in the intent-to-treat population did not significantly differ between those randomized to enzalutamide or placebo in either cohort. In cohort 1, the median PFS was 11.8 months in the enzalutamide arm and 5.8 months in the placebo arm (hazard ratio, 0.82; P = .3631), and in cohort 2, 3.6 months and 3.9 months, respectively (HR, 1.02; P = .9212).
However, statistically significant improvements in median PFS and clinical benefit rate at 24 weeks were observed only in the group with a positive biomarker who had not received any prior endocrine therapy. In cohort 1, the median PFS was 16.5 months in the enzalutamide arm vs. 4.3 months in the placebo arm (HR, 0.44, P = .0335). In cohort 2, median PFS did not significantly differ between groups (6.0 vs. 5.3 months; HR, 0.55; P = .1936).
The clinical response rate in cohort 1 of the biomarker positive group was 83% in the enzalutamide arm versus 38% in the placebo arm (P = .0012).
Adverse events with enzalutamide was similar to those previously reported, and the most common were nausea (39%) in cohort 1 and fatigue (37%) in cohort 2. Dose interruptions due to adverse events occurred in 21.0% and 25.0% of patients randomized to enzalutamide in cohorts 1 and 2 vs. 20.6% and 15.0% in the placebo group.
Dr. Yardley explained that the biomarker used in the study was identified on PAM50. “It was exploratory and proprietary,” she noted, adding that she is unable to share any further information about it at this time.
SOURCE: Yardley et al. SABCS Abstract GS4-07
SAN ANTONIO – Enzalutamide added to exemestane improved progression-free survival (PFS) in patients with hormone receptor (HR)-positive advanced breast cancer, investigators reported.
Specifically, it improved outcomes in patients who had not received any prior endocrine therapy and who were positive for a gene signature-based biomarker indicating androgen receptor (AR) signaling.
Patients in this subset who were treated with combination enzalutamide and exemestane achieved a median PFS of 16.5 months, which was significantly higher than the 4 months observed with placebo and exemestane.
However, the addition of enzalutamide had no effect on PFS in the overall cohort or among patients who were biomarker positive but who had received prior endocrine therapy.
“The study met its primary endpoint in improving PFS in the enzalutamide plus exemestane-treated patients who were biomarker positive and HR positive with no prior endocrine therapy for advanced disease as compared [with] exemestane alone,” Denise A. Yardley, MD, of Tennessee Oncology, Nashville, said at the San Antonio Breast Cancer Symposium.
“The role of the AR in HR-positive breast cancer and the predictive value of the identified biomarker are still unclear and will require further studies and validation,” said Dr. Yardley.
Targeting AR is an active area of breast cancer research, as a majority of HR-positive tumors express the AR, as do a moderate number of HER2-positive tumors and almost a third of triple-negative breast cancers. AR signaling has also been associated with resistance to endocrine therapy. Aromatase inhibitors divert estrogen precursors to androgens and data from preclinical models have shown that enzalutamide blocked both estrogen- and androgen-mediated growth of HR+ cells.
Enzalutamide is an inhibitor of AR signaling that is currently used to treat patients with castration-resistant prostate cancer, and has demonstrated clinical activity and was well tolerated in patients with AR-positive advanced triple negative breast cancer, explained Dr. Yardley.
In this study, Dr. Yardley and her colleagues conducted a placebo-controlled phase 2 randomized trial that included 247 patients with HR+/HER2-normal advanced/metastatic breast cancer who were assigned to either 25 mg exemestane plus placebo or 50 mg exemestane and 160 mg enzalutamide daily.
The patients were divided into two parallel cohorts: those with no prior endocrine therapy (C1; n = 127) or those who had received one prior endocrine therapy for metastatic disease (C2; n = 120).
The primary endpoint was PFS in the intent-to-treat population and in the biomarker subgroup of each cohort. Secondary endpoints included the clinical benefit rate at 24 weeks, best overall response, and safety.
The authors found that the PFS in the intent-to-treat population did not significantly differ between those randomized to enzalutamide or placebo in either cohort. In cohort 1, the median PFS was 11.8 months in the enzalutamide arm and 5.8 months in the placebo arm (hazard ratio, 0.82; P = .3631), and in cohort 2, 3.6 months and 3.9 months, respectively (HR, 1.02; P = .9212).
However, statistically significant improvements in median PFS and clinical benefit rate at 24 weeks were observed only in the group with a positive biomarker who had not received any prior endocrine therapy. In cohort 1, the median PFS was 16.5 months in the enzalutamide arm vs. 4.3 months in the placebo arm (HR, 0.44, P = .0335). In cohort 2, median PFS did not significantly differ between groups (6.0 vs. 5.3 months; HR, 0.55; P = .1936).
The clinical response rate in cohort 1 of the biomarker positive group was 83% in the enzalutamide arm versus 38% in the placebo arm (P = .0012).
Adverse events with enzalutamide was similar to those previously reported, and the most common were nausea (39%) in cohort 1 and fatigue (37%) in cohort 2. Dose interruptions due to adverse events occurred in 21.0% and 25.0% of patients randomized to enzalutamide in cohorts 1 and 2 vs. 20.6% and 15.0% in the placebo group.
Dr. Yardley explained that the biomarker used in the study was identified on PAM50. “It was exploratory and proprietary,” she noted, adding that she is unable to share any further information about it at this time.
SOURCE: Yardley et al. SABCS Abstract GS4-07
SAN ANTONIO – Enzalutamide added to exemestane improved progression-free survival (PFS) in patients with hormone receptor (HR)-positive advanced breast cancer, investigators reported.
Specifically, it improved outcomes in patients who had not received any prior endocrine therapy and who were positive for a gene signature-based biomarker indicating androgen receptor (AR) signaling.
Patients in this subset who were treated with combination enzalutamide and exemestane achieved a median PFS of 16.5 months, which was significantly higher than the 4 months observed with placebo and exemestane.
However, the addition of enzalutamide had no effect on PFS in the overall cohort or among patients who were biomarker positive but who had received prior endocrine therapy.
“The study met its primary endpoint in improving PFS in the enzalutamide plus exemestane-treated patients who were biomarker positive and HR positive with no prior endocrine therapy for advanced disease as compared [with] exemestane alone,” Denise A. Yardley, MD, of Tennessee Oncology, Nashville, said at the San Antonio Breast Cancer Symposium.
“The role of the AR in HR-positive breast cancer and the predictive value of the identified biomarker are still unclear and will require further studies and validation,” said Dr. Yardley.
Targeting AR is an active area of breast cancer research, as a majority of HR-positive tumors express the AR, as do a moderate number of HER2-positive tumors and almost a third of triple-negative breast cancers. AR signaling has also been associated with resistance to endocrine therapy. Aromatase inhibitors divert estrogen precursors to androgens and data from preclinical models have shown that enzalutamide blocked both estrogen- and androgen-mediated growth of HR+ cells.
Enzalutamide is an inhibitor of AR signaling that is currently used to treat patients with castration-resistant prostate cancer, and has demonstrated clinical activity and was well tolerated in patients with AR-positive advanced triple negative breast cancer, explained Dr. Yardley.
In this study, Dr. Yardley and her colleagues conducted a placebo-controlled phase 2 randomized trial that included 247 patients with HR+/HER2-normal advanced/metastatic breast cancer who were assigned to either 25 mg exemestane plus placebo or 50 mg exemestane and 160 mg enzalutamide daily.
The patients were divided into two parallel cohorts: those with no prior endocrine therapy (C1; n = 127) or those who had received one prior endocrine therapy for metastatic disease (C2; n = 120).
The primary endpoint was PFS in the intent-to-treat population and in the biomarker subgroup of each cohort. Secondary endpoints included the clinical benefit rate at 24 weeks, best overall response, and safety.
The authors found that the PFS in the intent-to-treat population did not significantly differ between those randomized to enzalutamide or placebo in either cohort. In cohort 1, the median PFS was 11.8 months in the enzalutamide arm and 5.8 months in the placebo arm (hazard ratio, 0.82; P = .3631), and in cohort 2, 3.6 months and 3.9 months, respectively (HR, 1.02; P = .9212).
However, statistically significant improvements in median PFS and clinical benefit rate at 24 weeks were observed only in the group with a positive biomarker who had not received any prior endocrine therapy. In cohort 1, the median PFS was 16.5 months in the enzalutamide arm vs. 4.3 months in the placebo arm (HR, 0.44, P = .0335). In cohort 2, median PFS did not significantly differ between groups (6.0 vs. 5.3 months; HR, 0.55; P = .1936).
The clinical response rate in cohort 1 of the biomarker positive group was 83% in the enzalutamide arm versus 38% in the placebo arm (P = .0012).
Adverse events with enzalutamide was similar to those previously reported, and the most common were nausea (39%) in cohort 1 and fatigue (37%) in cohort 2. Dose interruptions due to adverse events occurred in 21.0% and 25.0% of patients randomized to enzalutamide in cohorts 1 and 2 vs. 20.6% and 15.0% in the placebo group.
Dr. Yardley explained that the biomarker used in the study was identified on PAM50. “It was exploratory and proprietary,” she noted, adding that she is unable to share any further information about it at this time.
SOURCE: Yardley et al. SABCS Abstract GS4-07
REPORTING FROM SABCS 2017
Key clinical point: Enzalutamide added to exemestane improves progression-free survival in hormone receptor–positive advanced breast cancer patients with a biomarker indicating androgen receptor signaling.
Major finding: In this subset of patients, combination therapy improved PFS: 16.5 months vs. 4.3 months for the placebo arm (HR 0.44, P = .0335).
Data source: A placebo-controlled phase 2 randomized trial comprising 247 patients with HR+/HER2-normal advanced/metastatic breast cancer.
Disclosures: Study funding was not disclosed.
Source: Yardley et al. SABCS Abstract GS4-07.
FDA bans 24 ingredients from OTC health care antiseptic products
in hospital settings and other health care situations outside the hospital, the U.S. Food and Drug Administration announced in a final rule.
The affected products include health care personnel hand washes and hand rubs, surgical hand scrubs and hand rubs, and patient antiseptic skin preparations. The final rule was published Dec. 20 in the Federal Register and becomes effective in December 2018.
The agency determined that a deferral is warranted for six health care antiseptic active ingredients – benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine – to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients.
“The FDA expects that this information may help better inform us on antiseptic resistance and antibiotic cross-resistance in the health care setting,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Importantly, this doesn’t mean that products containing these six ingredients are ineffective or unsafe. These antiseptic products remain an important resource in health care settings. Personnel should continue to use these products consistent with infection control guidelines while the additional data are gathered.”
No additional data was provided for another 24 products, which were deemed not generally recognized as safe and effective. The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: human safety studies, nonclinical safety studies (developmental and reproductive toxicity studies and carcinogenicity studies), data to characterize potential hormonal effects, and data to evaluate the development of antimicrobial resistance, the final rule states.
The FDA noted that manufacturers started to remove nearly all of these 24 active ingredients from their products following a 2015 proposed rule. Triclosan is currently being used in available products.
The active ingredients affected are chlorhexidine gluconate; cloflucarban; fluorosalan; hexachlorophene; hexylresorcinol; iodophors (iodine-containing ingredients including iodine complex [ammonium ether sulfate and polyoxyethylene sorbitan monolaurate], iodine complex [phosphate ester of alkylaryloxy polyethylene glycol], iodine tincture USP, iodine topical solution USP, nonylphenoxypoly [ethyleneoxy] ethanoliodine, poloxamer–iodine complex, undecoylium chloride iodine complex); mercufenol chloride; methylbenzethonium chloride; phenol; secondary amyltricresols; sodium oxychlorosene; tribromsalan; triclocarban; triclosan; triple dye; combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative; and combination of mercufenol chloride and secondary amyltricresols in 50% alcohol.
If manufacturers want to use one or more of these 24 active ingredients in future OTC health care antiseptic drug products, those products will be considered new drugs for which a new drug application approval will be required, the agency said.
The rule does not affect health care antiseptics that are currently marketed under new drug applications and abbreviated new drug applications.
FDA’s action follows a similar final rule published Sept. 6, 2016, which removed triclosan and 18 other active ingredients from consumer antiseptic products.
in hospital settings and other health care situations outside the hospital, the U.S. Food and Drug Administration announced in a final rule.
The affected products include health care personnel hand washes and hand rubs, surgical hand scrubs and hand rubs, and patient antiseptic skin preparations. The final rule was published Dec. 20 in the Federal Register and becomes effective in December 2018.
The agency determined that a deferral is warranted for six health care antiseptic active ingredients – benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine – to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients.
“The FDA expects that this information may help better inform us on antiseptic resistance and antibiotic cross-resistance in the health care setting,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Importantly, this doesn’t mean that products containing these six ingredients are ineffective or unsafe. These antiseptic products remain an important resource in health care settings. Personnel should continue to use these products consistent with infection control guidelines while the additional data are gathered.”
No additional data was provided for another 24 products, which were deemed not generally recognized as safe and effective. The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: human safety studies, nonclinical safety studies (developmental and reproductive toxicity studies and carcinogenicity studies), data to characterize potential hormonal effects, and data to evaluate the development of antimicrobial resistance, the final rule states.
The FDA noted that manufacturers started to remove nearly all of these 24 active ingredients from their products following a 2015 proposed rule. Triclosan is currently being used in available products.
The active ingredients affected are chlorhexidine gluconate; cloflucarban; fluorosalan; hexachlorophene; hexylresorcinol; iodophors (iodine-containing ingredients including iodine complex [ammonium ether sulfate and polyoxyethylene sorbitan monolaurate], iodine complex [phosphate ester of alkylaryloxy polyethylene glycol], iodine tincture USP, iodine topical solution USP, nonylphenoxypoly [ethyleneoxy] ethanoliodine, poloxamer–iodine complex, undecoylium chloride iodine complex); mercufenol chloride; methylbenzethonium chloride; phenol; secondary amyltricresols; sodium oxychlorosene; tribromsalan; triclocarban; triclosan; triple dye; combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative; and combination of mercufenol chloride and secondary amyltricresols in 50% alcohol.
If manufacturers want to use one or more of these 24 active ingredients in future OTC health care antiseptic drug products, those products will be considered new drugs for which a new drug application approval will be required, the agency said.
The rule does not affect health care antiseptics that are currently marketed under new drug applications and abbreviated new drug applications.
FDA’s action follows a similar final rule published Sept. 6, 2016, which removed triclosan and 18 other active ingredients from consumer antiseptic products.
in hospital settings and other health care situations outside the hospital, the U.S. Food and Drug Administration announced in a final rule.
The affected products include health care personnel hand washes and hand rubs, surgical hand scrubs and hand rubs, and patient antiseptic skin preparations. The final rule was published Dec. 20 in the Federal Register and becomes effective in December 2018.
The agency determined that a deferral is warranted for six health care antiseptic active ingredients – benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine – to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients.
“The FDA expects that this information may help better inform us on antiseptic resistance and antibiotic cross-resistance in the health care setting,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Importantly, this doesn’t mean that products containing these six ingredients are ineffective or unsafe. These antiseptic products remain an important resource in health care settings. Personnel should continue to use these products consistent with infection control guidelines while the additional data are gathered.”
No additional data was provided for another 24 products, which were deemed not generally recognized as safe and effective. The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: human safety studies, nonclinical safety studies (developmental and reproductive toxicity studies and carcinogenicity studies), data to characterize potential hormonal effects, and data to evaluate the development of antimicrobial resistance, the final rule states.
The FDA noted that manufacturers started to remove nearly all of these 24 active ingredients from their products following a 2015 proposed rule. Triclosan is currently being used in available products.
The active ingredients affected are chlorhexidine gluconate; cloflucarban; fluorosalan; hexachlorophene; hexylresorcinol; iodophors (iodine-containing ingredients including iodine complex [ammonium ether sulfate and polyoxyethylene sorbitan monolaurate], iodine complex [phosphate ester of alkylaryloxy polyethylene glycol], iodine tincture USP, iodine topical solution USP, nonylphenoxypoly [ethyleneoxy] ethanoliodine, poloxamer–iodine complex, undecoylium chloride iodine complex); mercufenol chloride; methylbenzethonium chloride; phenol; secondary amyltricresols; sodium oxychlorosene; tribromsalan; triclocarban; triclosan; triple dye; combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative; and combination of mercufenol chloride and secondary amyltricresols in 50% alcohol.
If manufacturers want to use one or more of these 24 active ingredients in future OTC health care antiseptic drug products, those products will be considered new drugs for which a new drug application approval will be required, the agency said.
The rule does not affect health care antiseptics that are currently marketed under new drug applications and abbreviated new drug applications.
FDA’s action follows a similar final rule published Sept. 6, 2016, which removed triclosan and 18 other active ingredients from consumer antiseptic products.
Preventing cardiovascular disease in older adults: One size does not fit all
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
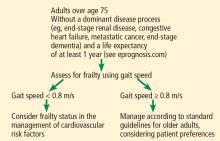
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
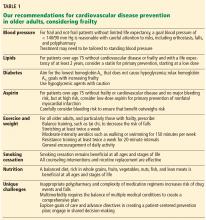
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
When assessing and attempting to modify the risk of cardiovascular disease in older patients, physicians should consider incorporating the concept of frailty. The balance of risk and benefit may differ considerably for 2 patients of the same age if one is fit and the other is frail. Because the aging population is a diverse group, a one-size-fits-all approach to cardiovascular disease prevention and risk-factor management is not appropriate.
A GROWING, DIVERSE GROUP
The number of older adults with multiple cardiovascular risk factors is increasing as life expectancy improves. US residents who are age 65 today can expect to live to an average age of 84 (men) or 87 (women).1
However, the range of life expectancy for people reaching these advanced ages is wide, and chronologic age is no longer sufficient to determine a patient’s risk profile. Furthermore, the prevalence of cardiovascular disease rises with age, and age itself is the strongest predictor of cardiovascular risk.2
Current risk calculators have not been validated in people over age 80,2 making them inadequate for use in older patients. Age alone cannot identify who will benefit from preventive strategies, except in situations when a dominant disease such as metastatic cancer, end-stage renal disease, end-stage dementia, or end-stage heart failure is expected to lead to mortality within a year. Guidelines for treating common risk factors such as elevated cholesterol3 in the general population have generally not focused on adults over 75 or recognized their diversity in health status.4 In order to generate an individualized prescription for cardiovascular disease prevention for older adults, issues such as frailty, cognitive and functional status, disability, and comorbidity must be considered.
WHAT IS FRAILTY?
Clinicians have recognized frailty for decades, but to date there remains a debate on how to define it.
Clegg et al5 described frailty as “a state of increased vulnerability to poor resolution of homeostasis after a stressor event,”5 a definition generally agreed upon, as frailty predicts both poor health outcomes and death.
Indeed, in a prospective study of 5,317 men and women ranging in age from 65 to 101, those identified as frail at baseline were 6 times more likely to have died 3 years later (mortality rates 18% vs 3%), and the difference persisted at 7 years.6 After adjusting for comorbidities, those identified as frail were also more likely to fall, develop limitations in mobility or activities of daily living, or be hospitalized.
The two current leading theories of frailty were defined by Fried et al6 and by Rockwood and Mitnitski.7
Fried et al6 have operationalized frailty as a “physical phenotype,” defined as 3 or more of the following:
- Unintentional weight loss of 10 pounds in the past year
- Self-reported exhaustion
- Weakness as measured by grip strength
- Slow walking speed
- Decreased physical activity.6
Rockwood and Mitnitski7 define frailty as an accumulation of health-related deficits over time. They recommend that 30 to 40 possible deficits that cover a variety of health systems be included such as cognition, mood, function, and comorbidity. These are added and divided by the total possible number of variables to generate a score between 0 and 1.8
The difficulty in defining frailty has led to varying estimates of its prevalence, ranging from 25% to 50% in adults over 65 who have cardiovascular disease.9
CAUSE AND CONSEQUENCE OF CARDIOVASCULAR DISEASE
Studies have highlighted the bidirectional connection between frailty and cardiovascular disease.10 Frailty may predict cardiovascular disease, while cardiovascular disease is associated with an increased risk of incident frailty.9,11
Frail adults with cardiovascular disease have a higher risk of poor outcomes, even after correcting for age, comorbidities, disability, and disease severity. For example, frailty is associated with a twofold higher mortality rate in individuals with cardiovascular disease.9
A prospective cohort study12 of 3,895 middle-aged men and women demonstrated that those with an elevated cardiovascular risk score were at increased risk of frailty over 10 years (odds ratio [OR] 1.35, 95% confidence interval [CI] 1.21–1.51) and incident cardiovascular events (OR 1.36, 95% CI 1.15–1.61). This suggests that modification of cardiovascular risk factors earlier in life may reduce the risk of subsequently becoming frail.
Biologic mechanisms that may explain the connection between frailty and cardiovascular disease include derangements in inflammatory, hematologic, and endocrine pathways. People who are found to be clinically frail are more likely to have insulin resistance and elevated biomarkers such as C-reactive protein, D-dimer, and factor VIII.13 The inflammatory cytokine interleukin 6 is suggested as a common link between inflammation and thrombosis, perhaps contributing to the connection between cardiovascular disease and frailty. Many of these biomarkers have been linked to the pathophysiologic changes of aging, so-called “inflamm-aging” or immunosenescence, including sarcopenia, osteoporosis, and cardiovascular disease.14
ASSESSING FRAILTY IN THE CLINIC
For adults over age 70, frailty assessment is an important first step in managing cardiovascular disease risk.15 Frailty status will better identify those at risk of adverse outcomes in the short term and those who are most likely to benefit from long-term cardiovascular preventive strategies. Additionally, incorporating frailty assessment into traditional risk factor evaluation may permit appropriate intervention and prevention of a potentially modifiable risk factor.
Gait speed is a quick, easy, inexpensive, and sensitive way to assess frailty status, with excellent inter-rater and test-retest reliability, even in those with cognitive impairment.16 Slow gait speed predicts limitations in mobility, limitations in activities of daily living, and death.8,17
In a prospective study18 of 1,567 men and women, mean age 74, slow gait speed was the strongest predictor of subsequent cardiovascular events.18
Gait speed is usually measured over a distance of 4 meters (13.1 feet),17 and the patient is asked to walk comfortably in an unobstructed, marked area. An assistive walking device can be used if needed. If possible, this is repeated once after a brief recovery period, and the average is recorded.
The FRAIL scale19,20 is a simple, validated questionnaire that combines the Fried and Rockwood concepts of frailty and can be given over the phone or to patients in a waiting room. One point is given for each of the following, and people who have 3 or more are considered frail:
- Fatigue
- Resistance (inability to climb 1 flight of stairs)
- Ambulation (inability to walk 1 block)
- Illnesses (having more than 5)
- Loss of more than 5% of body weight.
Other measures of physical function such as grip strength (using a dynamometer), the Timed Up and Go test (assessing the ability to get up from a chair and walk a short distance), and Short Physical Performance Battery (assessing balance, chair stands, and walking speed) can be used to screen for frailty, but are more time-intensive than gait speed alone, and so are not always practical to use in a busy clinic.21
MANAGEMENT OF RISK FACTORS
Management of cardiovascular risk factors is best individualized as outlined below.
LOWERING HIGH BLOOD PRESSURE
The incidence of ischemic heart disease and stroke increases with age across all levels of elevated systolic and diastolic blood pressure.22 Hypertension is also associated with increased risk of cognitive decline. However, a J-shaped relationship has been observed in older adults, with increased cardiovascular events for both low and elevated blood pressure, although the clinical relevance remains controversial.23
Odden et al24 performed an observational study and found that high blood pressure was associated with an increased mortality rate in older adults with normal gait speed, while in those with slow gait speed, high blood pressure neither harmed nor helped. Those who could not walk 6 meters appeared to benefit from higher blood pressure.
HYVET (the Hypertension in the Very Elderly Trial),25 a randomized controlled trial in 3,845 community-dwelling people age 80 or older with sustained systolic blood pressure higher than 160 mm Hg, found a significant reduction in rates of stroke and all-cause mortality (relative risk [RR] 0.76, P = .007) in the treatment arm using indapamide with perindopril if necessary to reach a target blood pressure of 150/80 mm Hg.
Frailty was not assessed during the trial; however, in a reanalysis, the results did not change in those identified as frail using a Rockwood frailty index (a count of health-related deficits accumulated over the lifespan).26
SPRINT (the Systolic Blood Pressure Intervention Trial)27 randomized participants age 50 and older with systolic blood pressure of 130 to 180 mm Hg and at increased risk of cardiovascular disease to intensive treatment (goal systolic blood pressure ≤ 120 mm Hg) or standard treatment (goal systolic blood pressure ≤ 140 mm Hg). In a prespecified subgroup of 2,636 participants over age 75 (mean age 80), hazard ratios and 95% confidence intervals for adverse outcomes with intensive treatment were:
- Major cardiovascular events: HR 0.66, 95% CI 0.51–0.85
- Death: HR 0.67, 95% CI 0.49–0.91.
Over 3 years of treatment this translated into a number needed to treat of 27 to prevent 1 cardiovascular event and 41 to prevent 1 death.
Within this subgroup, the benefit was similar regardless of level of frailty (measured both by a Rockwood frailty index and by gait speed).
However, the incidence of serious adverse treatment effects such as hypotension, orthostasis, electrolyte abnormalities, and acute kidney injury was higher with intensive treatment in the frail group. Although the difference was not statistically significant, it is cause for caution. Further, the exclusion criteria (history of diabetes, heart failure, dementia, stroke, weight loss of > 10%, nursing home residence) make it difficult to generalize the SPRINT findings to the general aging population.27
Tinetti et al28 performed an observational study using a nationally representative sample of older adults. They found that receiving any antihypertensive therapy was associated with an increased risk of falls with serious adverse outcomes. The risks of adverse events related to antihypertensive therapy increased with age.
Recommendations on hypertension
Managing hypertension in frail patients at risk of cardiovascular disease requires balancing the benefits vs the risks of treatment, such as polypharmacy, falls, and orthostatic hypotension.
The Eighth Joint National Committee suggests a blood pressure goal of less than 150/90 mm Hg for all adults over age 60, and less than 140/90 mm Hg for those with a history of cardiovascular disease or diabetes.29
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on hypertension, recently released, recommend a new blood pressure target of <120/<80 as normal, with 120–129/<80 considered elevated, 130–139/80–89 stage 1 hypertension, and ≥140/≥90 as stage 2 hypertension.30 An important caveat to these guidelines is the recommendation to measure blood pressure accurately and with accurate technique, which is often not possible in many busy clinics. These guidelines are intended to apply to older adults as well, with a note that those with multiple morbidities and limited life expectancy will benefit from a shared decision that incorporates patient preferences and clinical judgment. Little guidance is given on how to incorporate frailty, although note is made that older adults who reside in assisted living facilities and nursing homes have not been represented in randomized controlled trials.30
American Diabetes Association guidelines on hypertension in patients with diabetes recommend considering functional status, frailty, and life expectancy to decide on a blood pressure goal of either 140/90 mm Hg (if fit) or 150/90 mm Hg (if frail). They do not specify how to diagnose frailty.31
Canadian guidelines say that in those with advanced frailty (ie, entirely dependent for personal care and activities of daily living) and short life expectancy (months), it is reasonable to liberalize the systolic blood pressure goal to 160 to 190 mm Hg.32
Our recommendations. In both frail and nonfrail individuals without a limited life expectancy, it is reasonable to aim for a blood pressure of at least less than 140/90 mm Hg. For those at increased risk of cardiovascular disease and able to tolerate treatment, careful lowering to 130/80 mm Hg may be considered, with close attention to side effects.
Treatment should start with the lowest possible dose, be titrated slowly, and may need to be tailored to standing blood pressure to avoid orthostatic hypotension.
Home blood pressure measurements may be beneficial in monitoring treatment.
MANAGING LIPIDS
For those over age 75, data on efficacy of statins are mixed due to the small number of older adults enrolled in randomized controlled trials of these drugs. To our knowledge, no statin trial has examined the role of frailty.
The PROSPER trial (Prospective Study of Pravastatin in the Elderly at Risk)33 randomized 5,804 patients ages 70 to 82 to receive either pravastatin or placebo. Overall, the incidence of a composite end point of major cardiovascular events was 15% lower with active treatment (P = .014). However, the mean age was 75, which does little to address the paucity of evidence for those over age 75; follow-up time was only 3 years, and subgroup analysis did not show benefit in those who did not have a history of cardiovascular disease or in women.
The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)34 randomized 5,695 people over age 70 without cardiovascular disease to receive either rosuvastatin or placebo. Exploratory analysis showed a significant 39% reduction in all-cause mortality and major cardiovascular events with active treatment (HR 0.61, 95% CI 0.46–0.82). Over 5 years of treatment, this translates to a number needed to treat of 19 to prevent 1 major cardiovascular event and 29 to prevent 1 cardiovascular death.
The benefit of statins for primary prevention in these trials began to be apparent 2 years after treatment was initiated.
The Women’s Health Initiative,35 an observational study, found no difference in incident frailty in women older than 65 taking statins for 3 years compared with those who did not take statins
Odden et al36 found that although statin use is generally well tolerated, the risks of statin-associated functional and cognitive decline may outweigh the benefits in those older than 75. The ongoing Statin in Reducing Events in the Elderly (STAREE) trial may shed light on this issue.
Recommendations on lipid management
The ACC/AHA,3 in their 2013 guidelines, do not recommend routine statin treatment for primary prevention in those over age 75, given a lack of evidence from randomized controlled trials. For secondary prevention, ie, for those who have a history of atherosclerotic cardiovascular disease, they recommend moderate-intensity statin therapy in this age group.
Our recommendations. For patients over age 75 without cardiovascular disease or frailty and with a life expectancy of at least 2 years, consider offering a statin for primary prevention of cardiovascular disease as part of shared decision-making.
In those with known cardiovascular disease, it is reasonable to continue statin therapy except in situations where the life expectancy is less than 6 months.37
Although moderate- or high-intensity statin therapy is recommended in current guidelines, for many older adults it is prudent to consider the lowest tolerable dose to improve adherence, with close monitoring for side effects such as myalgia and weakness.
TYPE 2 DIABETES
Evidence suggests that tight glycemic control in type 2 diabetes is harmful for adults ages 55 to 79 and does not provide clear benefits for cardiovascular risk reduction, and controlling hemoglobin A1c to less than 6.0% is associated with increased mortality in older adults.38
The American Diabetes Association31 and the American Geriatrics Society39 recommend hemoglobin A1c goals of:
- 7.5% or less for older adults with 3 or more coexisting chronic illnesses requiring medical intervention (eg, arthritis, hypertension, and heart failure) and with intact cognition and function
- 8.0% or less for those identified as frail, or with multiple chronic illnesses or moderate cognitive or functional impairment
- 8.5% or 9.0% or less for those with very complex comorbidities, in long-term care, or with end-stage chronic illnesses (eg, end-stage heart failure), or with moderate to severe cognitive or functional limitation.
These guidelines do not endorse a specific frailty assessment, although the references allude to the Fried phenotype criteria, which include gait speed. An update from the American Diabetes Association provides a patient-centered approach to tailoring treatment regimens, taking into consideration the risk of hypoglycemia for each class of drugs, side effects, and cost.40
Our recommendations. Hyperglycemia remains a risk factor for cardiovascular disease in older adults and increases the risk of many geriatric conditions including delirium, dementia, frailty, and functional decline. The goal in individualizing hemoglobin A1c goals should be to avoid both hyper- and hypoglycemia.
Sulfonylureas and insulins should be used with caution, as they have the highest associated incidence of hypoglycemia of the diabetes medications.
ASPIRIN
For secondary prevention in older adults with a history of cardiovascular disease, pooled trials have consistently demonstrated a long-term benefit for aspirin use that exceeds bleeding risks, although age and frailty status were not considered.41
Aspirin for primary prevention?
The evidence for aspirin for primary prevention in older adults is mixed. Meta-analysis suggests a modest decrease in risk of nonfatal myocardial infarction but no appreciable effects on nonfatal stroke and cardiovascular death.42
The Japanese Primary Prevention Project,43 a randomized trial of low-dose aspirin for primary prevention of cardiovascular disease in adults ages 60 to 85, showed no reduction in major cardiovascular events. However, the event rate was lower than expected, the crossover rates were high, the incidence of hemorrhagic strokes was higher than in Western studies, and the trial may have been underpowered to detect the benefits of aspirin.
The US Preventive Services Task Force44 in 2016 noted that among individuals with a 10-year cardiovascular disease risk of 10% or higher based on the ACC/AHA pooled cohort equation,3 the greatest benefit of aspirin was in those ages 50 to 59. In this age group, 225 nonfatal myocardial infarctions and 84 nonfatal strokes were prevented per 10,000 men treated, with a net gain of 333 life-years. Similar findings were noted in women.
However, in those ages 60 to 69, the risks of harm begin to rise and the benefit of starting daily aspirin necessitates individualized clinical decision-making, with particular attention to bleeding risk and life expectancy.44
In those age 70 and older, data on benefit and harm are mixed. The bleeding risk of aspirin increases with age, predominantly due to gastrointestinal bleeding.44
The ongoing Aspirin in Reducing Events in Elderly trial will add to the evidence.
Aspirin recommendations for primary prevention
The American Geriatrics Society Beers Criteria do not routinely recommend aspirin use for primary prevention in those over age 80, even in those with diabetes.45
Our recommendations. In adults over age 75 who are not frail but are identified as being at moderate to high risk of cardiovascular disease using either the ACC/AHA calculator or any other risk estimator, and without a limited life expectancy, we believe it is reasonable to consider low-dose aspirin (75–100 mg daily) for primary prevention. However, there must be careful consideration particularly for those at risk of major bleeding. One approach to consider would be the addition of a proton pump inhibitor along with aspirin, though this requires further study.46
For those who have been on aspirin for primary prevention and are now older than age 80 without an adverse bleeding event, it is reasonable to stop aspirin, although risks and benefits of discontinuing aspirin should be discussed with the patient as part of shared decision-making.
In frail individuals the risks of aspirin therapy likely outweigh any benefit for primary prevention, and aspirin cannot be routinely recommended.
EXERCISE AND WEIGHT MANAGEMENT
A low body mass index is often associated with frailty, and weight loss may be a marker of underlying illness, which increases the risk of poor outcomes. However, those with an elevated body mass index and increased adiposity are in fact more likely to be frail (using the Fried physical phenotype definition) than those with a low body mass index,47 due in part to unrecognized sarcopenic obesity, ie, replacement of lean muscle with fat.
Physical activity is currently the only intervention known to improve frailty.5
Physical activity and a balanced diet are just as important in older adults, including those with reduced functional ability and multiple comorbid conditions, as in younger individuals.
A trial in frail long-term care residents (mean age 87) found that high-intensity resistance training improved muscle strength and mobility.48 The addition of a nutritional supplement with or without exercise did not affect frailty status. In community-dwelling older adults, physical activity has also been shown to improve sarcopenia and reduce falls and hip fractures.49
Progressive resistance training has been shown to improve strength and gait speed even in those with dementia.50
Tai chi has shown promising results in reducing falls and improving balance and function in both community-dwelling older adults and those in assisted living.51,52
Exercise recommendations
The US Department of Health and Human Services53 issued physical activity guidelines in 2008 with specific recommendations for older adults that include flexibility and balance training, which have been shown to reduce falls, in addition to aerobic activities and strength training.
Our recommendations. For all older adults, particularly those who are frail, we recommend a regimen of general daily activity, balance training such as tai chi, moderate-intensity aerobics such as cycling, resistance training such as using light weights, and stretching. Sessions lasting as little as 10 minutes are beneficial.
Gait speed can be monitored in the clinic to assess improvement in function over time.
SMOKING CESSATION
Although rates of smoking are decreasing, smoking remains one of the most important cardiovascular risk factors. Smoking has been associated with increased risk of frailty and significantly increased risk of death compared with never smoking.54 Smoking cessation is beneficial even for those who quit later in life.
The US Department of Health and Human Services in 2008 released an update on tobacco use and dependence,55 with specific attention to the benefit of smoking cessation for older adults.
All counseling interventions have been shown to be effective in older adults, as has nicotine replacement. Newer medications such as varenicline should be used with caution, as the risk of side effects is higher in older patients.
NUTRITION
Samieri et al,56 in an observational study of 10,670 nurses, found that those adhering to Mediterranean-style diets during midlife had 46% increased odds of healthy aging.
The PREDIMED study (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet)57 in adults ages 55 to 80 showed the Mediterranean diet supplemented with olive oil and nuts reduced the incidence of major cardiovascular disease.
Leon-Munoz et al.58 A prospective study of 1,815 community-dwelling older adults followed for 3.5 years in Spain demonstrated that adhering to a Mediterranean diet was associated with a lower incidence of frailty (P = .002) and a lower risk of slow gait speed (OR 0.53, 95% CI 0.35–0.79). Interestingly, this study also found a protective association between fish and fruit consumption and frailty.
Our recommendations. A well-balanced, diverse diet rich in whole grains, fruits, vegetables, nuts, fish, and healthy fats (polyunsaturated fatty acids), with a moderate amount of lean meats, is recommended to prevent heart disease. However, poor dental health may limit the ability of older individuals to adhere to such diets, and modifications may be needed. Additionally, age-related changes in taste and smell may contribute to poor nutrition and unintended weight loss.59 Involving a nutritionist and social worker in the patient care team should be considered especially as poor nutrition may be a sign of cognitive impairment, functional decline, and frailty.
SPECIAL CONSIDERATIONS
Special considerations when managing cardiovascular risk in the older adult include polypharmacy, multimorbidity, quality of life, and the patient’s personal preferences.
Polypharmacy, defined as taking more than 5 medications, is associated with an increased risk of adverse drug events, falls, fractures, decreased adherence, and “prescribing cascade”— prescribing more drugs to treat side effects of the first drug (eg, adding hypertensive medications to treat hypertension induced by nonsteroidal anti-inflammatory drugs).60 This is particularly important when considering adding additional medications. If a statin will be the 20th pill, it may be less beneficial and more likely to lead to additional adverse effects than if it is the fifth medication.
Patient preferences are critically important, particularly when adding or removing medications. Interventions should include a detailed medication review for appropriate prescribing and deprescribing, referral to a pharmacist, and engaging the patient’s support system.
Multimorbidity. Many older individuals have multiple chronic illnesses. The interaction of multiple conditions must be considered in creating a comprehensive plan, including prognosis, patient preference, available evidence, treatment interactions, and risks and benefits.
Quality of life. Outlook on life and choices made regarding prolongation vs quality of life may be different for the older patient than the younger patient.
Personal preferences. Although interventions such as high-intensity statins for a robust 85-year-old may be appropriate, the individual can choose to forgo any treatment. It is important to explore the patient’s goals of care and advanced directives as part of shared decision-making when building a patient-centered prevention plan.61
ONE SIZE DOES NOT FIT ALL
The heterogeneity of aging rules out a one-size-fits-all recommendation for cardiovascular disease prevention and management of cardiovascular risk factors in older adults.
There is significant overlap between cardiovascular risk status and frailty.
Incorporating frailty into the creation of a cardiovascular risk prescription can aid in the development of an individualized care plan for the prevention of cardiovascular disease in the aging population.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
- Social Security Administration (SSA). Calculators: life expectancy. www.ssa.gov/planners/lifeexpectancy.html. Accessed December 8, 2017.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934.
- Rich MW, Chyun DA, Skolnick AH, et al; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016; 133:2103–2122.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–762.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621.
- Woods NF, LaCroix AZ, Gray SL, et al; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–1330.
- Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013; 99:737–742.
- Walston J, McBurnie MA, Newman A, et al; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–2341.
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80:219–227.
- Morley JE. Frailty fantasia. J Am Med Dir Assoc 2017; 18:813–815.
- Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011; 52:e67–e70.
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009; 13:881–889.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12:29–37.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging 2012;16:601–608.
- Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135:e894–e918.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015 9;13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. Nov 13 [Epub ahead of print].)
- American Diabetes Association. 11. Older adults. Diabetes Care 2017; 40(suppl 1):S99–S104.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496, W174.
- LaCroix AZ, Gray SL, Aragaki A, et al; Women’s Health Initiative. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2008; 63:369–375.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175:691–700.
- Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34:1329–1336.
- Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60:2342–2356.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149.
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002; 324:71–86.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312:2510–2520.
- Bibbins-Domingo K; US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:836–845.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499.
- Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–641.
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330:1769–1775.
- Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015; 175:703–711.
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. J Am Geriatr Soc 2012; 60:8–15.
- Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health 2016; 106:2026–2031.
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 2014; 62:1484–1489.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging 2009; 13:468–472.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35:158–176.
- Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013; 159:584–591.
- Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369:676–677.
- Leon-Munoz LM, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 2014; 15:899–903.
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226:1441–1443.
- Merel SE, Paauw DS. Common drug side effects and drug-drug interactions in elderly adults in primary care. J Am Geriatr Soc 2017 Mar 21. Epub ahead of print.
- Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA 2009; 302:195–197.
KEY POINTS
- With the aging of the population, individualized prevention strategies must incorporate geriatric syndromes such as frailty.
- However, current guidelines and available evidence for cardiovascular disease prevention strategies have not incorporated frailty or make no recommendation at all for those over age 75.
- Four-meter gait speed, a simple measure of physical function and a proxy for frailty, can be used clinically to diagnose frailty.
The enemy of good
“But no perfection is so absolute,
That some impurity doth not pollute.”
– William Shakespeare
While lounging in the ivory tower of academia, we frequently find ourselves condemning the peasantry who fail to grasp limitations of clinical trials. We deride the ignorant masses who insist on flaunting the inclusion criteria of the latest study and extrapolate the results to the ineligible patient sitting in front of them. The disrespect heaped on the “referring doctor” for treating their patients in the absence of evidence from prospective, randomized, multi-institutional trials is routine in conference rooms across the National Cancer Institute’s designated cancer centers.
The introduction of third-party insurers decades ago warped the economics of health care to the point that modern patients generally expect to pay little or nothing for pretty much any medical intervention. As a result, physicians tend to prescribe treatments without regard to cost. Predictably, this results in a steady increase in costs, which have now become unsustainable for our nation. Those rising costs have resulted in many proposals for control, including the Affordable Care Act and the efforts to reverse it. Many see a single payer, government administered system as the only viable way forward.
No matter the final system our society settles on, it will have to account for the almost miraculous results from modern therapeutics, which seem to be announced more and more frequently.
As I write this column, the annual meeting of the American Society of Hematology is being held in Atlanta. The presentations recount studies of new agents alone, or in combination, that report unprecedented response and survival rates. In particular, cellular immunotherapy with chimeric antigen receptor T cells (CAR T cells) has captured the attention of physicians, patients, and investors. Simultaneously – and recognizing this revolution in oncologic therapeutics – the New England Journal of Medicine prepublished two papers presenting the results of CAR T-cell therapy for diffuse large B-cell lymphoma (DLBCL). The results are impressive, and earlier this year, the Food and Drug Administration approved axicabtagene ciloleucel for the treatment of relapsed or refractory DLBCL based on these data. An approval for tisagenlecleucel exists for the treatment of B-cell acute lymphoblastic leukemia, but approval for DLBCL is likely forthcoming, too.
These are wonderful developments. Patients with incurable lymphoma may now be offered potentially curative treatment. Hematology News has covered the development of these treatments closely.
Yet, there is a glaring problem that has also attracted attention: CAR T-cell therapy is incredibly expensive. The potentially mitigating effect of competing products on cost will be canceled by the demand, as well as by geographic scarcity, because only certain large centers will provide this treatment. Remember that the price of imatinib went up over time even though competitors entered the market.
Entering CAR T-cell treatments into the nation’s formulary for some patients will lead to rising premiums for all patients. Disturbingly, CAR T cells are just a treatment for hematology patients at present. What about the equally impressive new – and expensive – technologies in cardiology, neurology, surgery, and every other medical subspecialty? Our system is already struggling to accommodate rapidly rising costs as our population ages and demands more and more medical care.
Many believe that our society will ultimately require strict controls on access to these expensive treatments. While the idea of rationing care is abhorrent to clinicians, “evidence-based” restrictions to access appear not to be. For example, rituximab is effective for immune thrombocytopenic purpura (ITP), but is not FDA approved for it. Despite the restriction, rituximab is frequently used for ITP and generally reimbursed. Venetoclax is a useful agent for patients in relapse of chronic lymphocytic leukemia, but the FDA only approved it for those harboring a deletion of 17p. While insurers seem willing to reimburse the use of rituximab for ITP, they balk at covering venetoclax for off-label indications. More recently, and more ominously for the implications, the FDA approval for tisagenlecleucel in the treatment of B-cell acute lymphoblastic leukemia only extends to those up to age 25. That could mean a 26-year-old in relapse after an allogeneic transplant would be denied coverage for potentially curative CAR T-cell therapy.
The federal government is not the only bureaucracy with a financial interest in limiting access to expensive treatments. Commercial insurers have a fiduciary duty to their shareholders, not to the patients who consume their services. They employ thousands, among them physicians, tasked with reviewing our treatment recommendations to determine whether treatments will be paid for, often citing FDA approvals. Preauthorization for coverage results in innumerable treatment delays and added administrative costs that frustrate us and anger our patients. The insurers defend this incessant obstructionism by claiming they are protecting patients from unnecessary or unhelpful care. Like the FDA, they invoke our own penchant for evidence-based medicine or declare that some care pathway is the ultimate arbiter of truth in coverage determination. Therein lies the danger.
Where do you suppose the evidence and care pathways the FDA and insurers rely on come from? They come from academics like many of this publication’s readers. We gladly provide them with the data needed to restrict care. Through published studies in “major” journals, consensus guidelines promulgated through national organizations, and care pathways generated by our own institutions, we provide the fodder that feeds the regulatory apparatus that decides whose care is approved and paid for. As Walt Kelly’s Pogo stated in 1970, “We have met the enemy and he is us.”
In the interest of science and in the interest of safety – but mostly in the interest of ensuring regulatory approval – clinical trials of new agents often restrict eligibility. Our group recently found that randomized trials routinely exclude patients for rather arbitrary organ dysfunction (Leukemia. 2017 Aug;31[8]:1808-15).
Another recent study concluded, “Current oncology clinical trials stipulate many inclusion and exclusion criteria that specifically define the patient population under study. Although eligibility criteria are needed to define the study population and improve safety, overly restrictive eligibility criteria limit participation in clinical trials, cause the study population to be unrepresentative of the general population of patients with cancer, and limit patient access to new treatments.” (J Clin Oncol. 2017 Nov 20;35[33]:3745-52).
Federal and private agencies will necessarily become stricter in their interpretations of studies and policies in order to control costs. They will happily cite the data we produce in order to do so. For the vast majority of patients who do not meet stringent inclusion criteria, access to new treatments may well be denied. To ensure that patients are provided with the best and most economical care, I am an advocate for evidence-based medicine and care pathways to standardize practice. However, I am progressively more wary of their potential to restrict the availability of innovative remedies to our patients who are not fortunate enough to meet exacting inclusion criteria. Faced with complex patients for whom no study applies, our colleagues in the fields who feed us need flexibility to provide the best care for their patients. Those of us in the ivory tower who determine such inclusion criteria should not let perfect be the enemy of good and must do everything we can to help them and our patients.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
“But no perfection is so absolute,
That some impurity doth not pollute.”
– William Shakespeare
While lounging in the ivory tower of academia, we frequently find ourselves condemning the peasantry who fail to grasp limitations of clinical trials. We deride the ignorant masses who insist on flaunting the inclusion criteria of the latest study and extrapolate the results to the ineligible patient sitting in front of them. The disrespect heaped on the “referring doctor” for treating their patients in the absence of evidence from prospective, randomized, multi-institutional trials is routine in conference rooms across the National Cancer Institute’s designated cancer centers.
The introduction of third-party insurers decades ago warped the economics of health care to the point that modern patients generally expect to pay little or nothing for pretty much any medical intervention. As a result, physicians tend to prescribe treatments without regard to cost. Predictably, this results in a steady increase in costs, which have now become unsustainable for our nation. Those rising costs have resulted in many proposals for control, including the Affordable Care Act and the efforts to reverse it. Many see a single payer, government administered system as the only viable way forward.
No matter the final system our society settles on, it will have to account for the almost miraculous results from modern therapeutics, which seem to be announced more and more frequently.
As I write this column, the annual meeting of the American Society of Hematology is being held in Atlanta. The presentations recount studies of new agents alone, or in combination, that report unprecedented response and survival rates. In particular, cellular immunotherapy with chimeric antigen receptor T cells (CAR T cells) has captured the attention of physicians, patients, and investors. Simultaneously – and recognizing this revolution in oncologic therapeutics – the New England Journal of Medicine prepublished two papers presenting the results of CAR T-cell therapy for diffuse large B-cell lymphoma (DLBCL). The results are impressive, and earlier this year, the Food and Drug Administration approved axicabtagene ciloleucel for the treatment of relapsed or refractory DLBCL based on these data. An approval for tisagenlecleucel exists for the treatment of B-cell acute lymphoblastic leukemia, but approval for DLBCL is likely forthcoming, too.
These are wonderful developments. Patients with incurable lymphoma may now be offered potentially curative treatment. Hematology News has covered the development of these treatments closely.
Yet, there is a glaring problem that has also attracted attention: CAR T-cell therapy is incredibly expensive. The potentially mitigating effect of competing products on cost will be canceled by the demand, as well as by geographic scarcity, because only certain large centers will provide this treatment. Remember that the price of imatinib went up over time even though competitors entered the market.
Entering CAR T-cell treatments into the nation’s formulary for some patients will lead to rising premiums for all patients. Disturbingly, CAR T cells are just a treatment for hematology patients at present. What about the equally impressive new – and expensive – technologies in cardiology, neurology, surgery, and every other medical subspecialty? Our system is already struggling to accommodate rapidly rising costs as our population ages and demands more and more medical care.
Many believe that our society will ultimately require strict controls on access to these expensive treatments. While the idea of rationing care is abhorrent to clinicians, “evidence-based” restrictions to access appear not to be. For example, rituximab is effective for immune thrombocytopenic purpura (ITP), but is not FDA approved for it. Despite the restriction, rituximab is frequently used for ITP and generally reimbursed. Venetoclax is a useful agent for patients in relapse of chronic lymphocytic leukemia, but the FDA only approved it for those harboring a deletion of 17p. While insurers seem willing to reimburse the use of rituximab for ITP, they balk at covering venetoclax for off-label indications. More recently, and more ominously for the implications, the FDA approval for tisagenlecleucel in the treatment of B-cell acute lymphoblastic leukemia only extends to those up to age 25. That could mean a 26-year-old in relapse after an allogeneic transplant would be denied coverage for potentially curative CAR T-cell therapy.
The federal government is not the only bureaucracy with a financial interest in limiting access to expensive treatments. Commercial insurers have a fiduciary duty to their shareholders, not to the patients who consume their services. They employ thousands, among them physicians, tasked with reviewing our treatment recommendations to determine whether treatments will be paid for, often citing FDA approvals. Preauthorization for coverage results in innumerable treatment delays and added administrative costs that frustrate us and anger our patients. The insurers defend this incessant obstructionism by claiming they are protecting patients from unnecessary or unhelpful care. Like the FDA, they invoke our own penchant for evidence-based medicine or declare that some care pathway is the ultimate arbiter of truth in coverage determination. Therein lies the danger.
Where do you suppose the evidence and care pathways the FDA and insurers rely on come from? They come from academics like many of this publication’s readers. We gladly provide them with the data needed to restrict care. Through published studies in “major” journals, consensus guidelines promulgated through national organizations, and care pathways generated by our own institutions, we provide the fodder that feeds the regulatory apparatus that decides whose care is approved and paid for. As Walt Kelly’s Pogo stated in 1970, “We have met the enemy and he is us.”
In the interest of science and in the interest of safety – but mostly in the interest of ensuring regulatory approval – clinical trials of new agents often restrict eligibility. Our group recently found that randomized trials routinely exclude patients for rather arbitrary organ dysfunction (Leukemia. 2017 Aug;31[8]:1808-15).
Another recent study concluded, “Current oncology clinical trials stipulate many inclusion and exclusion criteria that specifically define the patient population under study. Although eligibility criteria are needed to define the study population and improve safety, overly restrictive eligibility criteria limit participation in clinical trials, cause the study population to be unrepresentative of the general population of patients with cancer, and limit patient access to new treatments.” (J Clin Oncol. 2017 Nov 20;35[33]:3745-52).
Federal and private agencies will necessarily become stricter in their interpretations of studies and policies in order to control costs. They will happily cite the data we produce in order to do so. For the vast majority of patients who do not meet stringent inclusion criteria, access to new treatments may well be denied. To ensure that patients are provided with the best and most economical care, I am an advocate for evidence-based medicine and care pathways to standardize practice. However, I am progressively more wary of their potential to restrict the availability of innovative remedies to our patients who are not fortunate enough to meet exacting inclusion criteria. Faced with complex patients for whom no study applies, our colleagues in the fields who feed us need flexibility to provide the best care for their patients. Those of us in the ivory tower who determine such inclusion criteria should not let perfect be the enemy of good and must do everything we can to help them and our patients.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
“But no perfection is so absolute,
That some impurity doth not pollute.”
– William Shakespeare
While lounging in the ivory tower of academia, we frequently find ourselves condemning the peasantry who fail to grasp limitations of clinical trials. We deride the ignorant masses who insist on flaunting the inclusion criteria of the latest study and extrapolate the results to the ineligible patient sitting in front of them. The disrespect heaped on the “referring doctor” for treating their patients in the absence of evidence from prospective, randomized, multi-institutional trials is routine in conference rooms across the National Cancer Institute’s designated cancer centers.
The introduction of third-party insurers decades ago warped the economics of health care to the point that modern patients generally expect to pay little or nothing for pretty much any medical intervention. As a result, physicians tend to prescribe treatments without regard to cost. Predictably, this results in a steady increase in costs, which have now become unsustainable for our nation. Those rising costs have resulted in many proposals for control, including the Affordable Care Act and the efforts to reverse it. Many see a single payer, government administered system as the only viable way forward.
No matter the final system our society settles on, it will have to account for the almost miraculous results from modern therapeutics, which seem to be announced more and more frequently.
As I write this column, the annual meeting of the American Society of Hematology is being held in Atlanta. The presentations recount studies of new agents alone, or in combination, that report unprecedented response and survival rates. In particular, cellular immunotherapy with chimeric antigen receptor T cells (CAR T cells) has captured the attention of physicians, patients, and investors. Simultaneously – and recognizing this revolution in oncologic therapeutics – the New England Journal of Medicine prepublished two papers presenting the results of CAR T-cell therapy for diffuse large B-cell lymphoma (DLBCL). The results are impressive, and earlier this year, the Food and Drug Administration approved axicabtagene ciloleucel for the treatment of relapsed or refractory DLBCL based on these data. An approval for tisagenlecleucel exists for the treatment of B-cell acute lymphoblastic leukemia, but approval for DLBCL is likely forthcoming, too.
These are wonderful developments. Patients with incurable lymphoma may now be offered potentially curative treatment. Hematology News has covered the development of these treatments closely.
Yet, there is a glaring problem that has also attracted attention: CAR T-cell therapy is incredibly expensive. The potentially mitigating effect of competing products on cost will be canceled by the demand, as well as by geographic scarcity, because only certain large centers will provide this treatment. Remember that the price of imatinib went up over time even though competitors entered the market.
Entering CAR T-cell treatments into the nation’s formulary for some patients will lead to rising premiums for all patients. Disturbingly, CAR T cells are just a treatment for hematology patients at present. What about the equally impressive new – and expensive – technologies in cardiology, neurology, surgery, and every other medical subspecialty? Our system is already struggling to accommodate rapidly rising costs as our population ages and demands more and more medical care.
Many believe that our society will ultimately require strict controls on access to these expensive treatments. While the idea of rationing care is abhorrent to clinicians, “evidence-based” restrictions to access appear not to be. For example, rituximab is effective for immune thrombocytopenic purpura (ITP), but is not FDA approved for it. Despite the restriction, rituximab is frequently used for ITP and generally reimbursed. Venetoclax is a useful agent for patients in relapse of chronic lymphocytic leukemia, but the FDA only approved it for those harboring a deletion of 17p. While insurers seem willing to reimburse the use of rituximab for ITP, they balk at covering venetoclax for off-label indications. More recently, and more ominously for the implications, the FDA approval for tisagenlecleucel in the treatment of B-cell acute lymphoblastic leukemia only extends to those up to age 25. That could mean a 26-year-old in relapse after an allogeneic transplant would be denied coverage for potentially curative CAR T-cell therapy.
The federal government is not the only bureaucracy with a financial interest in limiting access to expensive treatments. Commercial insurers have a fiduciary duty to their shareholders, not to the patients who consume their services. They employ thousands, among them physicians, tasked with reviewing our treatment recommendations to determine whether treatments will be paid for, often citing FDA approvals. Preauthorization for coverage results in innumerable treatment delays and added administrative costs that frustrate us and anger our patients. The insurers defend this incessant obstructionism by claiming they are protecting patients from unnecessary or unhelpful care. Like the FDA, they invoke our own penchant for evidence-based medicine or declare that some care pathway is the ultimate arbiter of truth in coverage determination. Therein lies the danger.
Where do you suppose the evidence and care pathways the FDA and insurers rely on come from? They come from academics like many of this publication’s readers. We gladly provide them with the data needed to restrict care. Through published studies in “major” journals, consensus guidelines promulgated through national organizations, and care pathways generated by our own institutions, we provide the fodder that feeds the regulatory apparatus that decides whose care is approved and paid for. As Walt Kelly’s Pogo stated in 1970, “We have met the enemy and he is us.”
In the interest of science and in the interest of safety – but mostly in the interest of ensuring regulatory approval – clinical trials of new agents often restrict eligibility. Our group recently found that randomized trials routinely exclude patients for rather arbitrary organ dysfunction (Leukemia. 2017 Aug;31[8]:1808-15).
Another recent study concluded, “Current oncology clinical trials stipulate many inclusion and exclusion criteria that specifically define the patient population under study. Although eligibility criteria are needed to define the study population and improve safety, overly restrictive eligibility criteria limit participation in clinical trials, cause the study population to be unrepresentative of the general population of patients with cancer, and limit patient access to new treatments.” (J Clin Oncol. 2017 Nov 20;35[33]:3745-52).
Federal and private agencies will necessarily become stricter in their interpretations of studies and policies in order to control costs. They will happily cite the data we produce in order to do so. For the vast majority of patients who do not meet stringent inclusion criteria, access to new treatments may well be denied. To ensure that patients are provided with the best and most economical care, I am an advocate for evidence-based medicine and care pathways to standardize practice. However, I am progressively more wary of their potential to restrict the availability of innovative remedies to our patients who are not fortunate enough to meet exacting inclusion criteria. Faced with complex patients for whom no study applies, our colleagues in the fields who feed us need flexibility to provide the best care for their patients. Those of us in the ivory tower who determine such inclusion criteria should not let perfect be the enemy of good and must do everything we can to help them and our patients.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Frailty and cardiovascular disease: A two-way street?
Despite a marked increase in awareness in recent years surrounding the prevalence and prognosis of frailty in our aging population and its association with cardiovascular disease, itself highly prevalent in elderly cohorts, the exact pathobiological links between the 2 conditions have not been fully elucidated. As a consequence, this has led to difficulty not only in accurately defining cardiovascular risk in vulnerable elderly patients, but also in adequately mitigating against it.
It is well accepted that cardiovascular disease, whether clinical or subclinical, is associated with an increased risk of developing the frail phenotype.1,2 Frailty, in turn, has been consistently identified as a universal marker of adverse outcomes in patients at risk of, and in patients with already manifest, cardiovascular disease.2,3 However, whether or not frailty is its own unique risk factor for cardiovascular disease, independent of co-associated risk markers, or is merely a downstream byproduct indicating a more advanced disease state, has yet to be determined. Furthermore, the question of whether modification of frail status may impact the development and progression of cardiovascular disease has not yet been established.
The article by Orkaby et al4 in this issue delves deeper into this question by looking specifically at the interaction between frailty and standard risk factors as they relate to the prevention of cardiovascular disease.
NEEDED: A UNIVERSAL DEFINITION OF FRAILTY
It is important to acknowledge up front that before we can truly examine frailty as a novel risk entity in the assessment and management of cardiovascular risk in older-age patients, we need to agree on an accepted, validated definition of the phenotype as it relates to this population. As acknowledged by Orkaby et al,4 lack of such a standardized definition has resulted in highly variable estimates of the prevalence of frailty, ranging from 6.9% in a community-dwelling population in the original Cardiovascular Health Study to as high as 50% in older adults with manifest cardiovascular disease.1,2
The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, reproducible definition that can then be used in real time by the clinician to determine the absolute presence or absence of the phenotype, much like hypertension or diabetes. Whether this optimal tool will turn out to be the traditional or modified version of the Fried Scale,1 an alternative multicomponent measure such as the Deficit Index,5 or even the increasingly popular single-item measures such as gait speed or grip strength, remains to be determined.
Exact choice of tool is perhaps less important than the singular adoption of a universal method that can then be rigorously tried and tested in multicenter studies. Given the bulk of data to date for the original Fried phenotype and its development in an older-age community setting with a typical prevalence of cardiovascular risk factors, the Fried Scale appears a particularly suitable tool to use for this domain of disease prevention. Single-item spin-off measures from this phenotype, including gait speed, may also be useful for their increased feasibility and practicality in certain situations.
A TWO-WAY STREET
Given what we know about the pathophysiological, immunological, and inflammatory processes underlying advancing age that have also been implicated in both frailty and cardiovascular disease syndromes, how can we determine if frailty truly is an independent risk factor for cardiovascular disease or merely an epiphenomenon of the aging process?
We do know that older age is not a prerequisite for frailty, as is evident in studies of the phenotype in middle-aged (and younger) patients with advanced heart failure.6 We also know not only that frail populations have a higher age-adjusted prevalence of cardiovascular risk factors including diabetes and hypertension,1 but also that community-dwellers with prefrailty (as defined in studies using the Fried criteria as 1 or 2 vs 3 present criteria) at baseline have a significantly increased risk of developing incident cardiovascular disease compared with those defined as nonfrail, even after adjustment for traditional risk factors and other biomarkers.3 Exploring the differences between these subgroups at baseline revealed that prefrailty was significantly associated with several subclinical insults that may serve as adverse vascular mediators, including insulin resistance, elevated inflammatory markers, and central adiposity.3
A substudy of the Cardiovascular Health Study also found that in over 1,200 participants without a prior history of a cardiovascular event, the presence of frailty was associated with multiple noninvasive measures of subclinical cardiovascular disease, including electrocardiographic and echocardiographic markers of left ventricular hypertrophy, carotid stenosis, and silent cerebrovascular infarcts on magnetic resonance imaging.7
These findings support a mechanistic link between evolving stages of frailty and a gradient of progressive cardiovascular risk, with a multifaceted dysregulation of metabolic processes known to underpin the pathogenesis of the frailty phenotype likely also triggering risk pathways (altered insulin metabolism, inflammation) involved in incident cardiovascular disease. Although the exact pathobiological pathways underlying these complex interlinked relationships between aging, frailty, and cardiovascular disease have yet to be fully elucidated, awareness of the bidirectional relationship between both morbid conditions highlights the absolute importance of modifying risk factors and subclinical conditions that are common to both.
CAN RISK BE MODIFIED IN FRAIL ADULTS?
Orkaby et al4 nicely lay out the guidelines for standard cardiovascular risk factor modification viewed in light of what is currently known—or not known—about how these recommendations should be interpreted for the older, frail, at-risk population. It is important to note at the outset that clinical trial data both inclusive of this population and incorporating the up-front assessment of frailty to predefine frail-or-not subgroups are sparse, and thereby evidence for how to optimize cardiovascular disease prevention in this important cohort is largely based on smaller observational studies and expert consensus.
Hypertension
However, important subanalyses derived from 2 large randomized controlled trials (Hypertension in the Very Elderly Trial [HYVET] and Systolic Blood Pressure Intervention Trial [SPRINT]) looking specifically at the impact of frail status on blood pressure treatment targets and related outcomes in elderly adults have recently been published.8,9 Notably, both studies showed the beneficial outcomes of more intensive treatment (to 150/80 mm Hg or 120 mm Hg systolic, respectively) persisted in those characterized as frail (via Rockwood frailty index or slow gait speed).8,9 Importantly, in the SPRINT analysis, higher event rates were seen with increasing frailty in both treatment groups; across each frailty stratum, absolute event rates were lower for the intensive treatment arm.9 These results were evident without a significant difference in the overall rate of serious adverse events9 or withdrawal rates8 between treatment groups.
Hypertension is the primary domain in which up-to-date clinical trial data have shown benefit for continued aggressive treatment for cardiovascular disease prevention regardless of the presence of frailty. Despite these data, in the real world, the “eyeball” frailty test often leads us to err on the side of caution regarding blood pressure management in the frail older adult. Certainly, the use of antihypertensive therapy in this population requires balanced consideration of the risk for adverse effects; the SPRINT analysis also found higher absolute rates of hypotension, falls, and acute kidney injury in the more intensively treated group.9 These adverse effects may be ameliorated not necessarily by modifying the target goal that is required, but by employing alternative strategies in achieving this goal, such as starting with lower doses, uptitrating more slowly, and monitoring with more frequent laboratory testing.
Currently, consensus guidelines in Canada have recommended liberalizing blood pressure treatment goals in those with “advanced frailty” associated with a shorter life expectancy.10
Dyslipidemia
Regarding the other major vascular risk factors, trials looking at the role of frailty in the targeted treatment of hyperlipidemia with statins in older patients for primary prevention of cardiovascular disease are lacking, although the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial showed a significant positive benefit for statin therapy in adults over age 70 (number needed to treat of 19 to prevent 1 major cardiovascular event, and 29 to prevent 1 cardiovascular death).11 This again may be counterbalanced by the purported increased risk of cognitive and potential adverse functional effects of statins in this age group; however, trial data specific to frail status or not is required to truly assess the benefit-risk ratio in this population.
Hyperglycemia
Meanwhile, recent clinical trials looking at the impact of age, functional impairment, and burden of comorbidities (rather than specific frailty measures) on glucose-lowering targets and cardiovascular outcomes have failed to show a benefit from intensive glycemic control strategies, leading guideline societies to endorse less-stringent hemoglobin A1c goals in this population.12 Given the well-documented association between hyperglycemia and cardiovascular disease, as well as the purported dysregulated glucose metabolism underlying the frail phenotype, it is important that future trials looking at optimal hemoglobin A1c targets incorporate the presence or absence of frailty to better inform specific recommendations for this population.
ONE SIZE MAY NOT FIT ALL
Overall, if both prefrailty and frailty are independent risk factors for, and a consequence of, clinical cardiovascular disease, it is worth bearing in mind that the modification of “intensive” or best practice therapies based on qualitatively assessed frailty may actually contribute to the problem. With best intentions, the negative impact of frailty on cardiovascular outcomes may be augmented by automatically assuming it to reflect a need for “therapy-light.” The adverse downstream consequences of inadequately treated cardiovascular risk factors are not in doubt, and it is important as the role of frailty becomes an increasingly recognized cofactor in the management of older adults with these risk factors that the vicious cycle underlying both syndromes is kept in mind, in order to avoid frailty becoming a harbinger of undertreatment in older, geriatric populations.
What is clear is that more prospective clinical trial data in this population are urgently needed in order to better delineate the exact interactions between frail status and these risk factors and the potential downstream consequences, using prespecified and robust frailty assessment methods.
Perhaps frailty should be seen as a series of stages rather than simply as a binary “there or not there” biomarker; through initial and established stages of the syndrome, which have been independently associated with both clinical events and subclinical surrogates of cardiovascular disease, risk factors should continue to be treated aggressively and according to best available evidence. However, as guideline societies are already beginning to endorse as highlighted above, once the phenotype becomes tethered with a certain threshold burden of comorbidity, cognitive or functional impairment, or more end-stage disease status, then goals for cardiovascular disease prevention may need to be readdressed and modified. If frailty is truly confirmed as a cardiovascular disease equivalent, not only appropriately treating associated cardiovascular risk factors but also seeking therapies that actively target the frailty syndrome itself should be an important goal of future studies seeking to impact the development of both clinical and subclinical cardiovascular disease in this population.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Orkaby AR, Onuma O, Qazi S, Gaziano JM, Driver JA. Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med 2018; 85:55–64.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
- Joyce E. Frailty in advanced heart failure. Heart Fail Clin 2016; 12:363–374.
- Newman AB, Gottdiener JS, McBurnie MA, et al; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–M166.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154:554–559.
Despite a marked increase in awareness in recent years surrounding the prevalence and prognosis of frailty in our aging population and its association with cardiovascular disease, itself highly prevalent in elderly cohorts, the exact pathobiological links between the 2 conditions have not been fully elucidated. As a consequence, this has led to difficulty not only in accurately defining cardiovascular risk in vulnerable elderly patients, but also in adequately mitigating against it.
It is well accepted that cardiovascular disease, whether clinical or subclinical, is associated with an increased risk of developing the frail phenotype.1,2 Frailty, in turn, has been consistently identified as a universal marker of adverse outcomes in patients at risk of, and in patients with already manifest, cardiovascular disease.2,3 However, whether or not frailty is its own unique risk factor for cardiovascular disease, independent of co-associated risk markers, or is merely a downstream byproduct indicating a more advanced disease state, has yet to be determined. Furthermore, the question of whether modification of frail status may impact the development and progression of cardiovascular disease has not yet been established.
The article by Orkaby et al4 in this issue delves deeper into this question by looking specifically at the interaction between frailty and standard risk factors as they relate to the prevention of cardiovascular disease.
NEEDED: A UNIVERSAL DEFINITION OF FRAILTY
It is important to acknowledge up front that before we can truly examine frailty as a novel risk entity in the assessment and management of cardiovascular risk in older-age patients, we need to agree on an accepted, validated definition of the phenotype as it relates to this population. As acknowledged by Orkaby et al,4 lack of such a standardized definition has resulted in highly variable estimates of the prevalence of frailty, ranging from 6.9% in a community-dwelling population in the original Cardiovascular Health Study to as high as 50% in older adults with manifest cardiovascular disease.1,2
The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, reproducible definition that can then be used in real time by the clinician to determine the absolute presence or absence of the phenotype, much like hypertension or diabetes. Whether this optimal tool will turn out to be the traditional or modified version of the Fried Scale,1 an alternative multicomponent measure such as the Deficit Index,5 or even the increasingly popular single-item measures such as gait speed or grip strength, remains to be determined.
Exact choice of tool is perhaps less important than the singular adoption of a universal method that can then be rigorously tried and tested in multicenter studies. Given the bulk of data to date for the original Fried phenotype and its development in an older-age community setting with a typical prevalence of cardiovascular risk factors, the Fried Scale appears a particularly suitable tool to use for this domain of disease prevention. Single-item spin-off measures from this phenotype, including gait speed, may also be useful for their increased feasibility and practicality in certain situations.
A TWO-WAY STREET
Given what we know about the pathophysiological, immunological, and inflammatory processes underlying advancing age that have also been implicated in both frailty and cardiovascular disease syndromes, how can we determine if frailty truly is an independent risk factor for cardiovascular disease or merely an epiphenomenon of the aging process?
We do know that older age is not a prerequisite for frailty, as is evident in studies of the phenotype in middle-aged (and younger) patients with advanced heart failure.6 We also know not only that frail populations have a higher age-adjusted prevalence of cardiovascular risk factors including diabetes and hypertension,1 but also that community-dwellers with prefrailty (as defined in studies using the Fried criteria as 1 or 2 vs 3 present criteria) at baseline have a significantly increased risk of developing incident cardiovascular disease compared with those defined as nonfrail, even after adjustment for traditional risk factors and other biomarkers.3 Exploring the differences between these subgroups at baseline revealed that prefrailty was significantly associated with several subclinical insults that may serve as adverse vascular mediators, including insulin resistance, elevated inflammatory markers, and central adiposity.3
A substudy of the Cardiovascular Health Study also found that in over 1,200 participants without a prior history of a cardiovascular event, the presence of frailty was associated with multiple noninvasive measures of subclinical cardiovascular disease, including electrocardiographic and echocardiographic markers of left ventricular hypertrophy, carotid stenosis, and silent cerebrovascular infarcts on magnetic resonance imaging.7
These findings support a mechanistic link between evolving stages of frailty and a gradient of progressive cardiovascular risk, with a multifaceted dysregulation of metabolic processes known to underpin the pathogenesis of the frailty phenotype likely also triggering risk pathways (altered insulin metabolism, inflammation) involved in incident cardiovascular disease. Although the exact pathobiological pathways underlying these complex interlinked relationships between aging, frailty, and cardiovascular disease have yet to be fully elucidated, awareness of the bidirectional relationship between both morbid conditions highlights the absolute importance of modifying risk factors and subclinical conditions that are common to both.
CAN RISK BE MODIFIED IN FRAIL ADULTS?
Orkaby et al4 nicely lay out the guidelines for standard cardiovascular risk factor modification viewed in light of what is currently known—or not known—about how these recommendations should be interpreted for the older, frail, at-risk population. It is important to note at the outset that clinical trial data both inclusive of this population and incorporating the up-front assessment of frailty to predefine frail-or-not subgroups are sparse, and thereby evidence for how to optimize cardiovascular disease prevention in this important cohort is largely based on smaller observational studies and expert consensus.
Hypertension
However, important subanalyses derived from 2 large randomized controlled trials (Hypertension in the Very Elderly Trial [HYVET] and Systolic Blood Pressure Intervention Trial [SPRINT]) looking specifically at the impact of frail status on blood pressure treatment targets and related outcomes in elderly adults have recently been published.8,9 Notably, both studies showed the beneficial outcomes of more intensive treatment (to 150/80 mm Hg or 120 mm Hg systolic, respectively) persisted in those characterized as frail (via Rockwood frailty index or slow gait speed).8,9 Importantly, in the SPRINT analysis, higher event rates were seen with increasing frailty in both treatment groups; across each frailty stratum, absolute event rates were lower for the intensive treatment arm.9 These results were evident without a significant difference in the overall rate of serious adverse events9 or withdrawal rates8 between treatment groups.
Hypertension is the primary domain in which up-to-date clinical trial data have shown benefit for continued aggressive treatment for cardiovascular disease prevention regardless of the presence of frailty. Despite these data, in the real world, the “eyeball” frailty test often leads us to err on the side of caution regarding blood pressure management in the frail older adult. Certainly, the use of antihypertensive therapy in this population requires balanced consideration of the risk for adverse effects; the SPRINT analysis also found higher absolute rates of hypotension, falls, and acute kidney injury in the more intensively treated group.9 These adverse effects may be ameliorated not necessarily by modifying the target goal that is required, but by employing alternative strategies in achieving this goal, such as starting with lower doses, uptitrating more slowly, and monitoring with more frequent laboratory testing.
Currently, consensus guidelines in Canada have recommended liberalizing blood pressure treatment goals in those with “advanced frailty” associated with a shorter life expectancy.10
Dyslipidemia
Regarding the other major vascular risk factors, trials looking at the role of frailty in the targeted treatment of hyperlipidemia with statins in older patients for primary prevention of cardiovascular disease are lacking, although the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial showed a significant positive benefit for statin therapy in adults over age 70 (number needed to treat of 19 to prevent 1 major cardiovascular event, and 29 to prevent 1 cardiovascular death).11 This again may be counterbalanced by the purported increased risk of cognitive and potential adverse functional effects of statins in this age group; however, trial data specific to frail status or not is required to truly assess the benefit-risk ratio in this population.
Hyperglycemia
Meanwhile, recent clinical trials looking at the impact of age, functional impairment, and burden of comorbidities (rather than specific frailty measures) on glucose-lowering targets and cardiovascular outcomes have failed to show a benefit from intensive glycemic control strategies, leading guideline societies to endorse less-stringent hemoglobin A1c goals in this population.12 Given the well-documented association between hyperglycemia and cardiovascular disease, as well as the purported dysregulated glucose metabolism underlying the frail phenotype, it is important that future trials looking at optimal hemoglobin A1c targets incorporate the presence or absence of frailty to better inform specific recommendations for this population.
ONE SIZE MAY NOT FIT ALL
Overall, if both prefrailty and frailty are independent risk factors for, and a consequence of, clinical cardiovascular disease, it is worth bearing in mind that the modification of “intensive” or best practice therapies based on qualitatively assessed frailty may actually contribute to the problem. With best intentions, the negative impact of frailty on cardiovascular outcomes may be augmented by automatically assuming it to reflect a need for “therapy-light.” The adverse downstream consequences of inadequately treated cardiovascular risk factors are not in doubt, and it is important as the role of frailty becomes an increasingly recognized cofactor in the management of older adults with these risk factors that the vicious cycle underlying both syndromes is kept in mind, in order to avoid frailty becoming a harbinger of undertreatment in older, geriatric populations.
What is clear is that more prospective clinical trial data in this population are urgently needed in order to better delineate the exact interactions between frail status and these risk factors and the potential downstream consequences, using prespecified and robust frailty assessment methods.
Perhaps frailty should be seen as a series of stages rather than simply as a binary “there or not there” biomarker; through initial and established stages of the syndrome, which have been independently associated with both clinical events and subclinical surrogates of cardiovascular disease, risk factors should continue to be treated aggressively and according to best available evidence. However, as guideline societies are already beginning to endorse as highlighted above, once the phenotype becomes tethered with a certain threshold burden of comorbidity, cognitive or functional impairment, or more end-stage disease status, then goals for cardiovascular disease prevention may need to be readdressed and modified. If frailty is truly confirmed as a cardiovascular disease equivalent, not only appropriately treating associated cardiovascular risk factors but also seeking therapies that actively target the frailty syndrome itself should be an important goal of future studies seeking to impact the development of both clinical and subclinical cardiovascular disease in this population.
Despite a marked increase in awareness in recent years surrounding the prevalence and prognosis of frailty in our aging population and its association with cardiovascular disease, itself highly prevalent in elderly cohorts, the exact pathobiological links between the 2 conditions have not been fully elucidated. As a consequence, this has led to difficulty not only in accurately defining cardiovascular risk in vulnerable elderly patients, but also in adequately mitigating against it.
It is well accepted that cardiovascular disease, whether clinical or subclinical, is associated with an increased risk of developing the frail phenotype.1,2 Frailty, in turn, has been consistently identified as a universal marker of adverse outcomes in patients at risk of, and in patients with already manifest, cardiovascular disease.2,3 However, whether or not frailty is its own unique risk factor for cardiovascular disease, independent of co-associated risk markers, or is merely a downstream byproduct indicating a more advanced disease state, has yet to be determined. Furthermore, the question of whether modification of frail status may impact the development and progression of cardiovascular disease has not yet been established.
The article by Orkaby et al4 in this issue delves deeper into this question by looking specifically at the interaction between frailty and standard risk factors as they relate to the prevention of cardiovascular disease.
NEEDED: A UNIVERSAL DEFINITION OF FRAILTY
It is important to acknowledge up front that before we can truly examine frailty as a novel risk entity in the assessment and management of cardiovascular risk in older-age patients, we need to agree on an accepted, validated definition of the phenotype as it relates to this population. As acknowledged by Orkaby et al,4 lack of such a standardized definition has resulted in highly variable estimates of the prevalence of frailty, ranging from 6.9% in a community-dwelling population in the original Cardiovascular Health Study to as high as 50% in older adults with manifest cardiovascular disease.1,2
The ideal frailty assessment tool should be a simple, quantitative, objective, and universally accepted method, capable of providing a consistent, valid, reproducible definition that can then be used in real time by the clinician to determine the absolute presence or absence of the phenotype, much like hypertension or diabetes. Whether this optimal tool will turn out to be the traditional or modified version of the Fried Scale,1 an alternative multicomponent measure such as the Deficit Index,5 or even the increasingly popular single-item measures such as gait speed or grip strength, remains to be determined.
Exact choice of tool is perhaps less important than the singular adoption of a universal method that can then be rigorously tried and tested in multicenter studies. Given the bulk of data to date for the original Fried phenotype and its development in an older-age community setting with a typical prevalence of cardiovascular risk factors, the Fried Scale appears a particularly suitable tool to use for this domain of disease prevention. Single-item spin-off measures from this phenotype, including gait speed, may also be useful for their increased feasibility and practicality in certain situations.
A TWO-WAY STREET
Given what we know about the pathophysiological, immunological, and inflammatory processes underlying advancing age that have also been implicated in both frailty and cardiovascular disease syndromes, how can we determine if frailty truly is an independent risk factor for cardiovascular disease or merely an epiphenomenon of the aging process?
We do know that older age is not a prerequisite for frailty, as is evident in studies of the phenotype in middle-aged (and younger) patients with advanced heart failure.6 We also know not only that frail populations have a higher age-adjusted prevalence of cardiovascular risk factors including diabetes and hypertension,1 but also that community-dwellers with prefrailty (as defined in studies using the Fried criteria as 1 or 2 vs 3 present criteria) at baseline have a significantly increased risk of developing incident cardiovascular disease compared with those defined as nonfrail, even after adjustment for traditional risk factors and other biomarkers.3 Exploring the differences between these subgroups at baseline revealed that prefrailty was significantly associated with several subclinical insults that may serve as adverse vascular mediators, including insulin resistance, elevated inflammatory markers, and central adiposity.3
A substudy of the Cardiovascular Health Study also found that in over 1,200 participants without a prior history of a cardiovascular event, the presence of frailty was associated with multiple noninvasive measures of subclinical cardiovascular disease, including electrocardiographic and echocardiographic markers of left ventricular hypertrophy, carotid stenosis, and silent cerebrovascular infarcts on magnetic resonance imaging.7
These findings support a mechanistic link between evolving stages of frailty and a gradient of progressive cardiovascular risk, with a multifaceted dysregulation of metabolic processes known to underpin the pathogenesis of the frailty phenotype likely also triggering risk pathways (altered insulin metabolism, inflammation) involved in incident cardiovascular disease. Although the exact pathobiological pathways underlying these complex interlinked relationships between aging, frailty, and cardiovascular disease have yet to be fully elucidated, awareness of the bidirectional relationship between both morbid conditions highlights the absolute importance of modifying risk factors and subclinical conditions that are common to both.
CAN RISK BE MODIFIED IN FRAIL ADULTS?
Orkaby et al4 nicely lay out the guidelines for standard cardiovascular risk factor modification viewed in light of what is currently known—or not known—about how these recommendations should be interpreted for the older, frail, at-risk population. It is important to note at the outset that clinical trial data both inclusive of this population and incorporating the up-front assessment of frailty to predefine frail-or-not subgroups are sparse, and thereby evidence for how to optimize cardiovascular disease prevention in this important cohort is largely based on smaller observational studies and expert consensus.
Hypertension
However, important subanalyses derived from 2 large randomized controlled trials (Hypertension in the Very Elderly Trial [HYVET] and Systolic Blood Pressure Intervention Trial [SPRINT]) looking specifically at the impact of frail status on blood pressure treatment targets and related outcomes in elderly adults have recently been published.8,9 Notably, both studies showed the beneficial outcomes of more intensive treatment (to 150/80 mm Hg or 120 mm Hg systolic, respectively) persisted in those characterized as frail (via Rockwood frailty index or slow gait speed).8,9 Importantly, in the SPRINT analysis, higher event rates were seen with increasing frailty in both treatment groups; across each frailty stratum, absolute event rates were lower for the intensive treatment arm.9 These results were evident without a significant difference in the overall rate of serious adverse events9 or withdrawal rates8 between treatment groups.
Hypertension is the primary domain in which up-to-date clinical trial data have shown benefit for continued aggressive treatment for cardiovascular disease prevention regardless of the presence of frailty. Despite these data, in the real world, the “eyeball” frailty test often leads us to err on the side of caution regarding blood pressure management in the frail older adult. Certainly, the use of antihypertensive therapy in this population requires balanced consideration of the risk for adverse effects; the SPRINT analysis also found higher absolute rates of hypotension, falls, and acute kidney injury in the more intensively treated group.9 These adverse effects may be ameliorated not necessarily by modifying the target goal that is required, but by employing alternative strategies in achieving this goal, such as starting with lower doses, uptitrating more slowly, and monitoring with more frequent laboratory testing.
Currently, consensus guidelines in Canada have recommended liberalizing blood pressure treatment goals in those with “advanced frailty” associated with a shorter life expectancy.10
Dyslipidemia
Regarding the other major vascular risk factors, trials looking at the role of frailty in the targeted treatment of hyperlipidemia with statins in older patients for primary prevention of cardiovascular disease are lacking, although the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial showed a significant positive benefit for statin therapy in adults over age 70 (number needed to treat of 19 to prevent 1 major cardiovascular event, and 29 to prevent 1 cardiovascular death).11 This again may be counterbalanced by the purported increased risk of cognitive and potential adverse functional effects of statins in this age group; however, trial data specific to frail status or not is required to truly assess the benefit-risk ratio in this population.
Hyperglycemia
Meanwhile, recent clinical trials looking at the impact of age, functional impairment, and burden of comorbidities (rather than specific frailty measures) on glucose-lowering targets and cardiovascular outcomes have failed to show a benefit from intensive glycemic control strategies, leading guideline societies to endorse less-stringent hemoglobin A1c goals in this population.12 Given the well-documented association between hyperglycemia and cardiovascular disease, as well as the purported dysregulated glucose metabolism underlying the frail phenotype, it is important that future trials looking at optimal hemoglobin A1c targets incorporate the presence or absence of frailty to better inform specific recommendations for this population.
ONE SIZE MAY NOT FIT ALL
Overall, if both prefrailty and frailty are independent risk factors for, and a consequence of, clinical cardiovascular disease, it is worth bearing in mind that the modification of “intensive” or best practice therapies based on qualitatively assessed frailty may actually contribute to the problem. With best intentions, the negative impact of frailty on cardiovascular outcomes may be augmented by automatically assuming it to reflect a need for “therapy-light.” The adverse downstream consequences of inadequately treated cardiovascular risk factors are not in doubt, and it is important as the role of frailty becomes an increasingly recognized cofactor in the management of older adults with these risk factors that the vicious cycle underlying both syndromes is kept in mind, in order to avoid frailty becoming a harbinger of undertreatment in older, geriatric populations.
What is clear is that more prospective clinical trial data in this population are urgently needed in order to better delineate the exact interactions between frail status and these risk factors and the potential downstream consequences, using prespecified and robust frailty assessment methods.
Perhaps frailty should be seen as a series of stages rather than simply as a binary “there or not there” biomarker; through initial and established stages of the syndrome, which have been independently associated with both clinical events and subclinical surrogates of cardiovascular disease, risk factors should continue to be treated aggressively and according to best available evidence. However, as guideline societies are already beginning to endorse as highlighted above, once the phenotype becomes tethered with a certain threshold burden of comorbidity, cognitive or functional impairment, or more end-stage disease status, then goals for cardiovascular disease prevention may need to be readdressed and modified. If frailty is truly confirmed as a cardiovascular disease equivalent, not only appropriately treating associated cardiovascular risk factors but also seeking therapies that actively target the frailty syndrome itself should be an important goal of future studies seeking to impact the development of both clinical and subclinical cardiovascular disease in this population.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Orkaby AR, Onuma O, Qazi S, Gaziano JM, Driver JA. Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med 2018; 85:55–64.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
- Joyce E. Frailty in advanced heart failure. Heart Fail Clin 2016; 12:363–374.
- Newman AB, Gottdiener JS, McBurnie MA, et al; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–M166.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154:554–559.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63:747–762.
- Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–983.
- Orkaby AR, Onuma O, Qazi S, Gaziano JM, Driver JA. Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med 2018; 85:55–64.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24.
- Joyce E. Frailty in advanced heart failure. Heart Fail Clin 2016; 12:363–374.
- Newman AB, Gottdiener JS, McBurnie MA, et al; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–M166.
- Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78.
- Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682.
- Mallery LH, Allen M, Fleming I, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014; 81:427–437.
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010; 152:488–496.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154:554–559.
Hypothermia and severe first-degree heart block
A 96-year-old woman with hypertension, diabetes, and dementia was found unresponsive in her nursing home and was transferred to the hospital.
At presentation to the hospital, her blood pressure was 76/43 mm Hg, heart rate 42 beats per minute, rectal temperature 31.6°C (88.8°F), and blood glucose 36 mg/dL.
Causes of secondary hypothermia were sought. Blood and urine cultures were negative. Computed tomography of the head showed no acute intracranial abnormalities. Tests for adrenal insufficiency and hypothyroidism were negative.
HYPOTHERMIA AND THE ECG
Hypothermia can produce a number of changes on the ECG. At the start of hypothermia, a stress reaction is induced, resulting in sinus tachycardia. But when the temperature goes below 32°C, sinus bradycardia ensues,1 resulting in various degrees of heart block.2 In our patient, a severely prolonged PR interval resulted in first-degree heart block.
Other findings on ECG associated with hypothermia include atrial fibrillation, widening of the P and T waves, prolonging of the QT interval, and widening of the QRS interval. Progressive widening of the QRS interval can predispose to ventricular fibrillation.1,3
An Osborn or J wave is a wave found between the end of the QRS and the beginning of the ST segment and is usually seen on the inferior and lateral precordial leads. It is found in as many as 80% of patients when the body temperature is below 30°C.1,3,4
Although Osborn waves are a common finding in hypothermia, they are also seen in electrolyte imbalances such as hypercalcemia and in central nervous system diseases.5,6 Hypothermia-associated changes on ECG are usually readily reversible with rewarming.1
TAKE-HOME MESSAGES
The ECG should always be interpreted in the proper clinical context and, whenever possible, compared with a previous ECG. It is prudent to always consider potentially reversible triggers of hypothermia other than environmental exposure such as hypothyroidism, infection, adrenal insufficiency, ketoacidosis, medication side effects, and alcohol use.
Hypothermia, especially in elderly patients with multiple comorbidities, can lead to bradycardia and varying degrees of heart block.
- Alhaddad IA, Khalil M, Brown EJ Jr. Osborn waves of hypothermia. Circulation 2000; 101:E233–E244.
- Bashour TT, Gualberto A, Ryan C. Atrioventricular block in accidental hypothermia—a case report. Angiology 1989; 40:63–66.
- Okada M, Nishimura F, Yoshino H, Kimura M, Ogino T. The J wave in accidental hypothermia. J Electrocardiol 1983; 16:23–28.
- Kukla P, Baranchuk A, Jastrzebski M, Zabojszcz M, Bryniarski L. Electrocardiographic landmarks of hypothermia. Kardiol Pol 2013; 71:1188–1189.
- Maruyama M, Kobayashi Y, Kodani E, et al. Osborn waves: history and significance. Indian Pacing Electrophysiol J 2004; 4:33–39.
- Sheikh AM, Hurst JW. Osborn waves in the electrocardiogram, hypothermia not due to exposure, and death due to diabetic ketoacidosis. Clin Cardiol 2003; 26:555–560.
A 96-year-old woman with hypertension, diabetes, and dementia was found unresponsive in her nursing home and was transferred to the hospital.
At presentation to the hospital, her blood pressure was 76/43 mm Hg, heart rate 42 beats per minute, rectal temperature 31.6°C (88.8°F), and blood glucose 36 mg/dL.
Causes of secondary hypothermia were sought. Blood and urine cultures were negative. Computed tomography of the head showed no acute intracranial abnormalities. Tests for adrenal insufficiency and hypothyroidism were negative.
HYPOTHERMIA AND THE ECG
Hypothermia can produce a number of changes on the ECG. At the start of hypothermia, a stress reaction is induced, resulting in sinus tachycardia. But when the temperature goes below 32°C, sinus bradycardia ensues,1 resulting in various degrees of heart block.2 In our patient, a severely prolonged PR interval resulted in first-degree heart block.
Other findings on ECG associated with hypothermia include atrial fibrillation, widening of the P and T waves, prolonging of the QT interval, and widening of the QRS interval. Progressive widening of the QRS interval can predispose to ventricular fibrillation.1,3
An Osborn or J wave is a wave found between the end of the QRS and the beginning of the ST segment and is usually seen on the inferior and lateral precordial leads. It is found in as many as 80% of patients when the body temperature is below 30°C.1,3,4
Although Osborn waves are a common finding in hypothermia, they are also seen in electrolyte imbalances such as hypercalcemia and in central nervous system diseases.5,6 Hypothermia-associated changes on ECG are usually readily reversible with rewarming.1
TAKE-HOME MESSAGES
The ECG should always be interpreted in the proper clinical context and, whenever possible, compared with a previous ECG. It is prudent to always consider potentially reversible triggers of hypothermia other than environmental exposure such as hypothyroidism, infection, adrenal insufficiency, ketoacidosis, medication side effects, and alcohol use.
Hypothermia, especially in elderly patients with multiple comorbidities, can lead to bradycardia and varying degrees of heart block.
A 96-year-old woman with hypertension, diabetes, and dementia was found unresponsive in her nursing home and was transferred to the hospital.
At presentation to the hospital, her blood pressure was 76/43 mm Hg, heart rate 42 beats per minute, rectal temperature 31.6°C (88.8°F), and blood glucose 36 mg/dL.
Causes of secondary hypothermia were sought. Blood and urine cultures were negative. Computed tomography of the head showed no acute intracranial abnormalities. Tests for adrenal insufficiency and hypothyroidism were negative.
HYPOTHERMIA AND THE ECG
Hypothermia can produce a number of changes on the ECG. At the start of hypothermia, a stress reaction is induced, resulting in sinus tachycardia. But when the temperature goes below 32°C, sinus bradycardia ensues,1 resulting in various degrees of heart block.2 In our patient, a severely prolonged PR interval resulted in first-degree heart block.
Other findings on ECG associated with hypothermia include atrial fibrillation, widening of the P and T waves, prolonging of the QT interval, and widening of the QRS interval. Progressive widening of the QRS interval can predispose to ventricular fibrillation.1,3
An Osborn or J wave is a wave found between the end of the QRS and the beginning of the ST segment and is usually seen on the inferior and lateral precordial leads. It is found in as many as 80% of patients when the body temperature is below 30°C.1,3,4
Although Osborn waves are a common finding in hypothermia, they are also seen in electrolyte imbalances such as hypercalcemia and in central nervous system diseases.5,6 Hypothermia-associated changes on ECG are usually readily reversible with rewarming.1
TAKE-HOME MESSAGES
The ECG should always be interpreted in the proper clinical context and, whenever possible, compared with a previous ECG. It is prudent to always consider potentially reversible triggers of hypothermia other than environmental exposure such as hypothyroidism, infection, adrenal insufficiency, ketoacidosis, medication side effects, and alcohol use.
Hypothermia, especially in elderly patients with multiple comorbidities, can lead to bradycardia and varying degrees of heart block.
- Alhaddad IA, Khalil M, Brown EJ Jr. Osborn waves of hypothermia. Circulation 2000; 101:E233–E244.
- Bashour TT, Gualberto A, Ryan C. Atrioventricular block in accidental hypothermia—a case report. Angiology 1989; 40:63–66.
- Okada M, Nishimura F, Yoshino H, Kimura M, Ogino T. The J wave in accidental hypothermia. J Electrocardiol 1983; 16:23–28.
- Kukla P, Baranchuk A, Jastrzebski M, Zabojszcz M, Bryniarski L. Electrocardiographic landmarks of hypothermia. Kardiol Pol 2013; 71:1188–1189.
- Maruyama M, Kobayashi Y, Kodani E, et al. Osborn waves: history and significance. Indian Pacing Electrophysiol J 2004; 4:33–39.
- Sheikh AM, Hurst JW. Osborn waves in the electrocardiogram, hypothermia not due to exposure, and death due to diabetic ketoacidosis. Clin Cardiol 2003; 26:555–560.
- Alhaddad IA, Khalil M, Brown EJ Jr. Osborn waves of hypothermia. Circulation 2000; 101:E233–E244.
- Bashour TT, Gualberto A, Ryan C. Atrioventricular block in accidental hypothermia—a case report. Angiology 1989; 40:63–66.
- Okada M, Nishimura F, Yoshino H, Kimura M, Ogino T. The J wave in accidental hypothermia. J Electrocardiol 1983; 16:23–28.
- Kukla P, Baranchuk A, Jastrzebski M, Zabojszcz M, Bryniarski L. Electrocardiographic landmarks of hypothermia. Kardiol Pol 2013; 71:1188–1189.
- Maruyama M, Kobayashi Y, Kodani E, et al. Osborn waves: history and significance. Indian Pacing Electrophysiol J 2004; 4:33–39.
- Sheikh AM, Hurst JW. Osborn waves in the electrocardiogram, hypothermia not due to exposure, and death due to diabetic ketoacidosis. Clin Cardiol 2003; 26:555–560.
BTK inhibitor zanubrutinib active in non-Hodgkin lymphomas
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
REPORTING FROM ASH 2017
Key clinical point: Monotherapy with the BTK inhibitor zanubrutinib (BGB-3111) was active and well tolerated in patients with a variety of non-Hodgkin lymphoma (NHL) subtypes.
Major finding: Response rates ranged from 31% to 88% depending on the lymphoma subtype.
Data source: Preliminary results of an open-label, multicenter, phase 1b study including 99 patients with relapsed or refractory diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, or marginal zone lymphoma.
Disclosures: Zanubrutinib is a product of BeiGene. Constantine S. Tam, MBBS, MD, reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
Source: Tam C et al. ASH 2017, Abstract 152.
Dysmorphic red blood cell formation
A 23-year-old woman presented with hematuria. Her blood pressure was normal, and she had no rash, joint pain, or other symptoms. Urinalysis was positive for proteinuria and hematuria, and urinary sediment analysis showed dysmorphic red blood cells (RBCs) and red cell casts, leading to a diagnosis of glomerulonephritis. She had proteinuria of 1.2 g/24 hours. Laboratory tests for systemic diseases were negative. Renal biopsy study revealed stage III immunoglobulin A (IgA) nephropathy.
GLOMERULAR HEMATURIA
Glomerular hematuria may represent an immune-mediated injury to the glomerular capillary wall, but it can also be present in noninflammatory glomerulopathies.1
The type of dysmorphic RBCs (crenated or misshapen cells, acanthocytes) may be of diagnostic importance. In particular, dysmorphic red cells alone may be predictive of only renal bleeding, while acanthocytes (ring-shaped RBCs with vesicle-shaped protrusions best seen on phase-contrast microscopy) appear to be most predictive of glomerular disease.2 For example, in 1 study,3 the presence of acanthocytes comprising at least 5% of excreted RBCs had a sensitivity of 52% for glomerular disease and a specificity of 98%.3
- Collar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int 2001; 59:2069–2072.
- Fogazzi GB, Ponticelli C, Ritz E. The Urinary Sediment: An Integrated View. 2nd ed. Oxford: Oxford University Press; 1999:30.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Fogazzi GB. The Urinary Sediment: An Integrated View. 3rd ed. France: Elsevier; 2010.
- Briner VA, Reinhart WH. In vitro production of ‘glomerular red cells’: role of pH and osmolality. Nephron 1990; 56:13–18.
- Schramek P, Moritsch A, Haschkowitz H, Binder BR, Maier M. In vitro generation of dysmorphic erythrocytes. Kidney Int 1989; 36:72–77.
- Pollock C, Liu PL, Györy AZ, et al. Dysmorphism of urinary red blood cells—value in diagnosis. Kidney Int 1989; 36:1045–1049.
- Shichiri M, Hosoda K, Nishio Y, et al. Red-cell-volume distribution curves in diagnosis of glomerular and non-glomerular haematuria. Lancet 1988; 1:908–911.
A 23-year-old woman presented with hematuria. Her blood pressure was normal, and she had no rash, joint pain, or other symptoms. Urinalysis was positive for proteinuria and hematuria, and urinary sediment analysis showed dysmorphic red blood cells (RBCs) and red cell casts, leading to a diagnosis of glomerulonephritis. She had proteinuria of 1.2 g/24 hours. Laboratory tests for systemic diseases were negative. Renal biopsy study revealed stage III immunoglobulin A (IgA) nephropathy.
GLOMERULAR HEMATURIA
Glomerular hematuria may represent an immune-mediated injury to the glomerular capillary wall, but it can also be present in noninflammatory glomerulopathies.1
The type of dysmorphic RBCs (crenated or misshapen cells, acanthocytes) may be of diagnostic importance. In particular, dysmorphic red cells alone may be predictive of only renal bleeding, while acanthocytes (ring-shaped RBCs with vesicle-shaped protrusions best seen on phase-contrast microscopy) appear to be most predictive of glomerular disease.2 For example, in 1 study,3 the presence of acanthocytes comprising at least 5% of excreted RBCs had a sensitivity of 52% for glomerular disease and a specificity of 98%.3
A 23-year-old woman presented with hematuria. Her blood pressure was normal, and she had no rash, joint pain, or other symptoms. Urinalysis was positive for proteinuria and hematuria, and urinary sediment analysis showed dysmorphic red blood cells (RBCs) and red cell casts, leading to a diagnosis of glomerulonephritis. She had proteinuria of 1.2 g/24 hours. Laboratory tests for systemic diseases were negative. Renal biopsy study revealed stage III immunoglobulin A (IgA) nephropathy.
GLOMERULAR HEMATURIA
Glomerular hematuria may represent an immune-mediated injury to the glomerular capillary wall, but it can also be present in noninflammatory glomerulopathies.1
The type of dysmorphic RBCs (crenated or misshapen cells, acanthocytes) may be of diagnostic importance. In particular, dysmorphic red cells alone may be predictive of only renal bleeding, while acanthocytes (ring-shaped RBCs with vesicle-shaped protrusions best seen on phase-contrast microscopy) appear to be most predictive of glomerular disease.2 For example, in 1 study,3 the presence of acanthocytes comprising at least 5% of excreted RBCs had a sensitivity of 52% for glomerular disease and a specificity of 98%.3
- Collar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int 2001; 59:2069–2072.
- Fogazzi GB, Ponticelli C, Ritz E. The Urinary Sediment: An Integrated View. 2nd ed. Oxford: Oxford University Press; 1999:30.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Fogazzi GB. The Urinary Sediment: An Integrated View. 3rd ed. France: Elsevier; 2010.
- Briner VA, Reinhart WH. In vitro production of ‘glomerular red cells’: role of pH and osmolality. Nephron 1990; 56:13–18.
- Schramek P, Moritsch A, Haschkowitz H, Binder BR, Maier M. In vitro generation of dysmorphic erythrocytes. Kidney Int 1989; 36:72–77.
- Pollock C, Liu PL, Györy AZ, et al. Dysmorphism of urinary red blood cells—value in diagnosis. Kidney Int 1989; 36:1045–1049.
- Shichiri M, Hosoda K, Nishio Y, et al. Red-cell-volume distribution curves in diagnosis of glomerular and non-glomerular haematuria. Lancet 1988; 1:908–911.
- Collar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int 2001; 59:2069–2072.
- Fogazzi GB, Ponticelli C, Ritz E. The Urinary Sediment: An Integrated View. 2nd ed. Oxford: Oxford University Press; 1999:30.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Fogazzi GB. The Urinary Sediment: An Integrated View. 3rd ed. France: Elsevier; 2010.
- Briner VA, Reinhart WH. In vitro production of ‘glomerular red cells’: role of pH and osmolality. Nephron 1990; 56:13–18.
- Schramek P, Moritsch A, Haschkowitz H, Binder BR, Maier M. In vitro generation of dysmorphic erythrocytes. Kidney Int 1989; 36:72–77.
- Pollock C, Liu PL, Györy AZ, et al. Dysmorphism of urinary red blood cells—value in diagnosis. Kidney Int 1989; 36:1045–1049.
- Shichiri M, Hosoda K, Nishio Y, et al. Red-cell-volume distribution curves in diagnosis of glomerular and non-glomerular haematuria. Lancet 1988; 1:908–911.
Quality in urine microscopy: The eyes of the beholder
The urine is the window to the kidney.This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron,1,2 the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations.1 At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.1
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen1 in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.1,3 In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.4
DYSMORPHIC RED BLOOD CELLS: TYPES AND SIGNIFICANCE
URINE MICROSCOPY: THE NEPHROLOGIST’S ROLE
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images. More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (eg, the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79% of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology.7,8 However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia.9 Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.10 At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25% of cases and a concrete change in management in 12% of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al11 showed that nephrologists identified the cause of acute kidney injury correctly 90% of the time when they performed their own urine microscopy, but this dropped to 23% when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69% when a report of another nephrologist’s microscopy findings was used.11
APPLYING RESULTS TO THE PATIENT
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int 1996; 50:1058–1068.
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015; 53(suppl 2):s1453–s1464.
- Harley G. The Urine and Its Derangements. London: J and A Churchill, 1872:178–179.
- Birch DF, Fairley K. Hematuria: glomerular or non-glomerular? Lancet 1979; 314:845–846.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Daza JL, De Rosa M, De Rosa G. Dysmorphic red blood cells. Cleve Clin J Med 2018; 85:12–13.
- Jauhar S. The demise of the physical exam. N Engl J Med 2006; 354:548–551.
- Mangione S. When the tail wags the dog: clinical skills in the age of technology. Cleve Clin J Med 2017; 84:278–280.
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008; 3:1615–1619.
- Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010; 5:402–408.
- Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005; 46:820–829.
Additional Reading
Fogazzi GB, Garigali G, Pirovano B, Muratore MT, Raimondi S, Berti S. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007; 45:407–412.
Fogazzi GB, Secchiero S. The role of nephrologists in teaching urinary sediment examination. Am J Kidney Dis 2006; 47:713.
Fogazzi GB, Verdesca S, Garigali G. Urinalysis: core curriculum 2008. Am J Kidney Dis 2008; 51:1052–1067.
The urine is the window to the kidney.This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron,1,2 the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations.1 At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.1
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen1 in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.1,3 In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.4
DYSMORPHIC RED BLOOD CELLS: TYPES AND SIGNIFICANCE
URINE MICROSCOPY: THE NEPHROLOGIST’S ROLE
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images. More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (eg, the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79% of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology.7,8 However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia.9 Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.10 At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25% of cases and a concrete change in management in 12% of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al11 showed that nephrologists identified the cause of acute kidney injury correctly 90% of the time when they performed their own urine microscopy, but this dropped to 23% when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69% when a report of another nephrologist’s microscopy findings was used.11
APPLYING RESULTS TO THE PATIENT
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
The urine is the window to the kidney.This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron,1,2 the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations.1 At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.1
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen1 in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.1,3 In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.4
DYSMORPHIC RED BLOOD CELLS: TYPES AND SIGNIFICANCE
URINE MICROSCOPY: THE NEPHROLOGIST’S ROLE
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images. More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (eg, the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79% of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology.7,8 However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia.9 Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.10 At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25% of cases and a concrete change in management in 12% of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al11 showed that nephrologists identified the cause of acute kidney injury correctly 90% of the time when they performed their own urine microscopy, but this dropped to 23% when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69% when a report of another nephrologist’s microscopy findings was used.11
APPLYING RESULTS TO THE PATIENT
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int 1996; 50:1058–1068.
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015; 53(suppl 2):s1453–s1464.
- Harley G. The Urine and Its Derangements. London: J and A Churchill, 1872:178–179.
- Birch DF, Fairley K. Hematuria: glomerular or non-glomerular? Lancet 1979; 314:845–846.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Daza JL, De Rosa M, De Rosa G. Dysmorphic red blood cells. Cleve Clin J Med 2018; 85:12–13.
- Jauhar S. The demise of the physical exam. N Engl J Med 2006; 354:548–551.
- Mangione S. When the tail wags the dog: clinical skills in the age of technology. Cleve Clin J Med 2017; 84:278–280.
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008; 3:1615–1619.
- Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010; 5:402–408.
- Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005; 46:820–829.
Additional Reading
Fogazzi GB, Garigali G, Pirovano B, Muratore MT, Raimondi S, Berti S. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007; 45:407–412.
Fogazzi GB, Secchiero S. The role of nephrologists in teaching urinary sediment examination. Am J Kidney Dis 2006; 47:713.
Fogazzi GB, Verdesca S, Garigali G. Urinalysis: core curriculum 2008. Am J Kidney Dis 2008; 51:1052–1067.
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int 1996; 50:1058–1068.
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015; 53(suppl 2):s1453–s1464.
- Harley G. The Urine and Its Derangements. London: J and A Churchill, 1872:178–179.
- Birch DF, Fairley K. Hematuria: glomerular or non-glomerular? Lancet 1979; 314:845–846.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Daza JL, De Rosa M, De Rosa G. Dysmorphic red blood cells. Cleve Clin J Med 2018; 85:12–13.
- Jauhar S. The demise of the physical exam. N Engl J Med 2006; 354:548–551.
- Mangione S. When the tail wags the dog: clinical skills in the age of technology. Cleve Clin J Med 2017; 84:278–280.
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008; 3:1615–1619.
- Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010; 5:402–408.
- Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005; 46:820–829.
Additional Reading
Fogazzi GB, Garigali G, Pirovano B, Muratore MT, Raimondi S, Berti S. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007; 45:407–412.
Fogazzi GB, Secchiero S. The role of nephrologists in teaching urinary sediment examination. Am J Kidney Dis 2006; 47:713.
Fogazzi GB, Verdesca S, Garigali G. Urinalysis: core curriculum 2008. Am J Kidney Dis 2008; 51:1052–1067.