User login
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
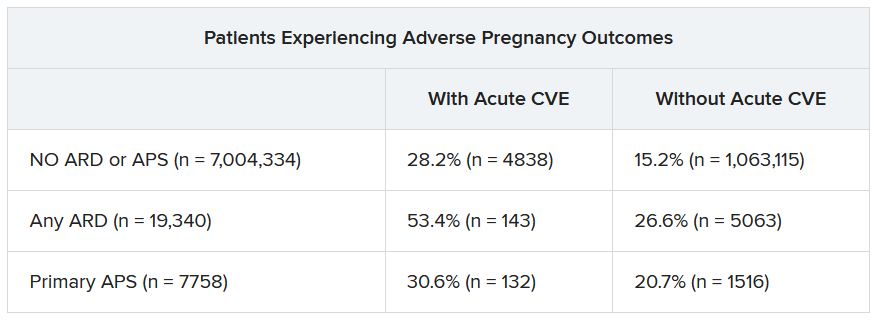
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Painless nodules on legs
A 34-YEAR-OLD MAN presented with a 6-month history of asymptomatic, progressively enlarging subcutaneous nodules over his bilateral lower legs. He denied any history of injury, and there was no bleeding or discharge. The patient had a history of Graves disease that had been treated with radioiodine therapy 2 years prior, followed by thyroxine replacement (150 mcg/d, 5 d/wk and 125 mcg/d, 2 d/wk). At the time of presentation, his thyroid function tests indicated subclinical hypothyroidism: free T4, 21.2 pmol/L (normal range, 11.8-24.6 pmol/L) and thyroid-stimulating hormone (TSH), 14.07 mIU/L (normal range, 0.27-4.2 mIU/L).
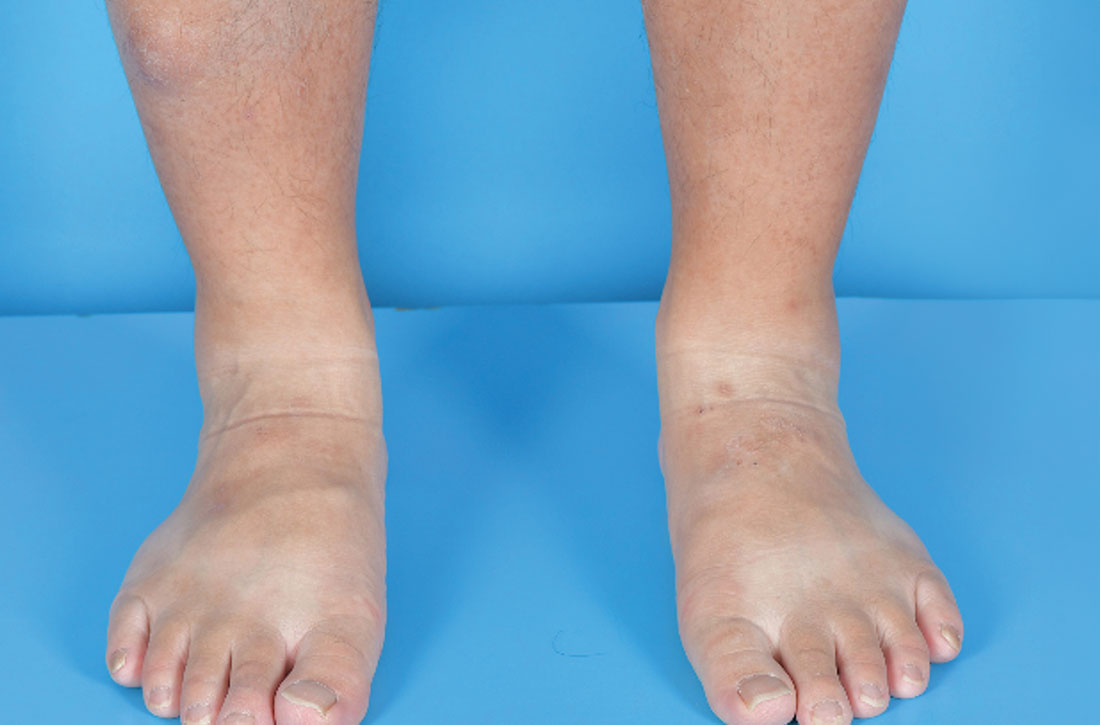
Examination revealed nontender, soft brown nodules over the bilateral shins, with minimal overlying lichenification (FIGURE 1). There was no peau d’orange (orange peel) appearance to suggest significant edema. A punch biopsy was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pretibial myxedema
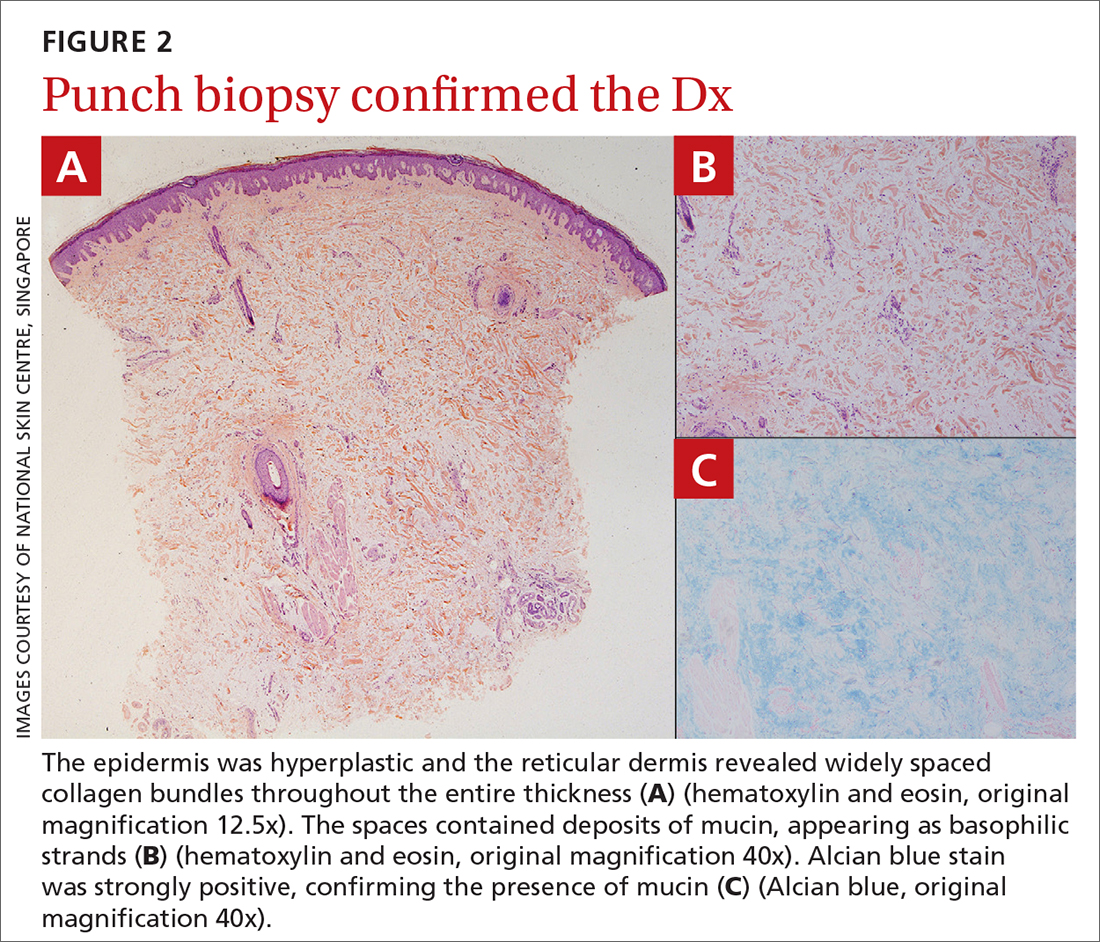
The patient’s history, paired with the results of the punch biopsy, were consistent with a diagnosis of pretibial myxedema, part of the triad of Graves disease along with thyroid ophthalmopathy and acropachy (soft-tissue swelling of the hands and clubbing of the fingers). Histopathologic findings revealed wide separation of collagen bundles throughout the entire reticular dermis without fibroplasia (FIGURE 2A). The spaces contained basophilic strands (FIGURE 2B), and the strands stained strongly positive on Alcian blue (FIGURE 2C), confirming the presence of dermal mucin. Widely separated collagen fibers and deposited mucin are indicative of pretibial myxedema. No granulomas or lymphoid proliferations were seen.
The pathogenesis of pretibial myxedema is widely postulated to be due to the stimulation of dermal fibroblasts by anti–TSH antibodies, causing overproduction of glycosaminoglycans and hyaluronic acid1 and obstructing lymphatic microcirculation, resulting in nonpitting edema.2
There are 5 distinct clinical variants of pretibial myxedema1,3:
- The diffuse form is the most common. It manifests on the lower leg with hard, nonpitting edema and cutaneous thickening.
- The plaque form manifests on the lower leg as well-demarcated erythematous or pigmented flat-topped lesions.
- The nodular form, which our patient had, typically manifests on the lower leg as well-demarcated erythematous, pigmented, or skin-colored raised, solid lesions. There may be 1 lesion or several.
- The mixed form manifests as 2 or more of the other variants.
- The elephantiasic form is the rarest and the most severe. There are widespread swollen nodules and plaques on the lower legs and/or arms.
A rare, late manifestation
Although pathognomonic for Graves disease, pretibial myxedema is a late manifestation that occurs in less than 5% of these patients.4 The most common site of involvement is the pretibial region, although less common sites include the face, arms, shoulders, abdomen, pinna, and the location of previous scars.4
While pretibial myxedema usually is associated with hyperthyroidism, it can occur after treatment (as was the case here), while the patient is in a euthyroid or hypothyroid state. Radioiodine therapy has been reported to be a trigger for pretibial myxedema in 1 case report, although the pathophysiology is not fully understood.5
Continue to: More serious conditions must be ruled out
More serious conditions must be ruled out
The differential for painless nodules includes cutaneous lymphoma and atypical infections of fungal or mycobacterial etiology.
Cutaneous lymphoma that manifests with leg tumors includes primary cutaneous anaplastic CD30+ large cell lymphoma (PCALCL) and primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBL-LT). The former may occur in young patients, whereas the latter tends to manifest in the elderly. Biopsy shows a neoplastic proliferation of atypical lymphocytes within the dermis,6 differing from our case.
Atypical infections may be detected through bacterial, mycobacterial, or fungal cultures, and may be accompanied by elevated inflammatory markers or other systemic symptoms of the infection, setting it apart from pretibial myxedema.
Treatment is simple and noninvasive
Pretibial myxedema is usually asymptomatic, with minimal morbidity. The nodular variant may resolve spontaneously; thus, therapeutic management often is reserved for severe cases or for those with cosmetic concerns. Treatment options include mid- to high-potency topical corticosteroids with an occlusive dressing for 1 to 2 weeks (or until resolution) or an intralesional triamcinolone injection (5-10 mg/mL, single or monthly until resolution), compression stockings, and pneumatic compression.2
This patient was treated with a single intralesional injection of triamcinolone 10 mg/mL. The nodules resolved within a month.
1. Thammarucha S, Sudtikoonaseth P. Nodular pretibial myxedema with Graves’ disease: a case report. Thai J Dermatol. 2021;37:30-36.
2. Singla M, Gupta A. Nodular thyroid dermopathy: not a hallmark of Graves’ disease. Am J Med. 2019;132:e521-e522. doi: 10.1016/j.amjmed.2018.11.004
3. Lan C, Wang Y, Zeng X, et al. Morphological diversity of pretibial myxedema and its mechanism of evolving process and outcome: a retrospective study of 216 cases. J Thyroid Res. 2016:2016:265217
4. doi: 10.1155/2016/2652174 4. Patil MM, Kamalanathan S, Sahoo J, et al. Pretibial myxedema. QJM. 2015;108:985. doi: 10.1093/qjmed/hcv136
5. Harvey RD, Metcalfe RA, Morteo C, et al. Acute pre-tibial myxoedema following radioiodine therapy for thyrotoxic Graves’ disease. Clin Endocrinol (Oxf). 1995;42:657-660. doi: 10.1111/j.1365-2265.1995.tb02695.x
6. Schukow C, Ahmed A. Dermatopathology, cutaneous lymphomas. StatPearls [Internet]. Updated February 16, 2023. Accessed October 23, 2023. www.ncbi.nlm.nih.gov/books/NBK589703/
A 34-YEAR-OLD MAN presented with a 6-month history of asymptomatic, progressively enlarging subcutaneous nodules over his bilateral lower legs. He denied any history of injury, and there was no bleeding or discharge. The patient had a history of Graves disease that had been treated with radioiodine therapy 2 years prior, followed by thyroxine replacement (150 mcg/d, 5 d/wk and 125 mcg/d, 2 d/wk). At the time of presentation, his thyroid function tests indicated subclinical hypothyroidism: free T4, 21.2 pmol/L (normal range, 11.8-24.6 pmol/L) and thyroid-stimulating hormone (TSH), 14.07 mIU/L (normal range, 0.27-4.2 mIU/L).
Examination revealed nontender, soft brown nodules over the bilateral shins, with minimal overlying lichenification (FIGURE 1). There was no peau d’orange (orange peel) appearance to suggest significant edema. A punch biopsy was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pretibial myxedema
The patient’s history, paired with the results of the punch biopsy, were consistent with a diagnosis of pretibial myxedema, part of the triad of Graves disease along with thyroid ophthalmopathy and acropachy (soft-tissue swelling of the hands and clubbing of the fingers). Histopathologic findings revealed wide separation of collagen bundles throughout the entire reticular dermis without fibroplasia (FIGURE 2A). The spaces contained basophilic strands (FIGURE 2B), and the strands stained strongly positive on Alcian blue (FIGURE 2C), confirming the presence of dermal mucin. Widely separated collagen fibers and deposited mucin are indicative of pretibial myxedema. No granulomas or lymphoid proliferations were seen.

The pathogenesis of pretibial myxedema is widely postulated to be due to the stimulation of dermal fibroblasts by anti–TSH antibodies, causing overproduction of glycosaminoglycans and hyaluronic acid1 and obstructing lymphatic microcirculation, resulting in nonpitting edema.2
There are 5 distinct clinical variants of pretibial myxedema1,3:
- The diffuse form is the most common. It manifests on the lower leg with hard, nonpitting edema and cutaneous thickening.
- The plaque form manifests on the lower leg as well-demarcated erythematous or pigmented flat-topped lesions.
- The nodular form, which our patient had, typically manifests on the lower leg as well-demarcated erythematous, pigmented, or skin-colored raised, solid lesions. There may be 1 lesion or several.
- The mixed form manifests as 2 or more of the other variants.
- The elephantiasic form is the rarest and the most severe. There are widespread swollen nodules and plaques on the lower legs and/or arms.
A rare, late manifestation
Although pathognomonic for Graves disease, pretibial myxedema is a late manifestation that occurs in less than 5% of these patients.4 The most common site of involvement is the pretibial region, although less common sites include the face, arms, shoulders, abdomen, pinna, and the location of previous scars.4
While pretibial myxedema usually is associated with hyperthyroidism, it can occur after treatment (as was the case here), while the patient is in a euthyroid or hypothyroid state. Radioiodine therapy has been reported to be a trigger for pretibial myxedema in 1 case report, although the pathophysiology is not fully understood.5
Continue to: More serious conditions must be ruled out
More serious conditions must be ruled out
The differential for painless nodules includes cutaneous lymphoma and atypical infections of fungal or mycobacterial etiology.
Cutaneous lymphoma that manifests with leg tumors includes primary cutaneous anaplastic CD30+ large cell lymphoma (PCALCL) and primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBL-LT). The former may occur in young patients, whereas the latter tends to manifest in the elderly. Biopsy shows a neoplastic proliferation of atypical lymphocytes within the dermis,6 differing from our case.
Atypical infections may be detected through bacterial, mycobacterial, or fungal cultures, and may be accompanied by elevated inflammatory markers or other systemic symptoms of the infection, setting it apart from pretibial myxedema.
Treatment is simple and noninvasive
Pretibial myxedema is usually asymptomatic, with minimal morbidity. The nodular variant may resolve spontaneously; thus, therapeutic management often is reserved for severe cases or for those with cosmetic concerns. Treatment options include mid- to high-potency topical corticosteroids with an occlusive dressing for 1 to 2 weeks (or until resolution) or an intralesional triamcinolone injection (5-10 mg/mL, single or monthly until resolution), compression stockings, and pneumatic compression.2
This patient was treated with a single intralesional injection of triamcinolone 10 mg/mL. The nodules resolved within a month.
A 34-YEAR-OLD MAN presented with a 6-month history of asymptomatic, progressively enlarging subcutaneous nodules over his bilateral lower legs. He denied any history of injury, and there was no bleeding or discharge. The patient had a history of Graves disease that had been treated with radioiodine therapy 2 years prior, followed by thyroxine replacement (150 mcg/d, 5 d/wk and 125 mcg/d, 2 d/wk). At the time of presentation, his thyroid function tests indicated subclinical hypothyroidism: free T4, 21.2 pmol/L (normal range, 11.8-24.6 pmol/L) and thyroid-stimulating hormone (TSH), 14.07 mIU/L (normal range, 0.27-4.2 mIU/L).
Examination revealed nontender, soft brown nodules over the bilateral shins, with minimal overlying lichenification (FIGURE 1). There was no peau d’orange (orange peel) appearance to suggest significant edema. A punch biopsy was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pretibial myxedema
The patient’s history, paired with the results of the punch biopsy, were consistent with a diagnosis of pretibial myxedema, part of the triad of Graves disease along with thyroid ophthalmopathy and acropachy (soft-tissue swelling of the hands and clubbing of the fingers). Histopathologic findings revealed wide separation of collagen bundles throughout the entire reticular dermis without fibroplasia (FIGURE 2A). The spaces contained basophilic strands (FIGURE 2B), and the strands stained strongly positive on Alcian blue (FIGURE 2C), confirming the presence of dermal mucin. Widely separated collagen fibers and deposited mucin are indicative of pretibial myxedema. No granulomas or lymphoid proliferations were seen.

The pathogenesis of pretibial myxedema is widely postulated to be due to the stimulation of dermal fibroblasts by anti–TSH antibodies, causing overproduction of glycosaminoglycans and hyaluronic acid1 and obstructing lymphatic microcirculation, resulting in nonpitting edema.2
There are 5 distinct clinical variants of pretibial myxedema1,3:
- The diffuse form is the most common. It manifests on the lower leg with hard, nonpitting edema and cutaneous thickening.
- The plaque form manifests on the lower leg as well-demarcated erythematous or pigmented flat-topped lesions.
- The nodular form, which our patient had, typically manifests on the lower leg as well-demarcated erythematous, pigmented, or skin-colored raised, solid lesions. There may be 1 lesion or several.
- The mixed form manifests as 2 or more of the other variants.
- The elephantiasic form is the rarest and the most severe. There are widespread swollen nodules and plaques on the lower legs and/or arms.
A rare, late manifestation
Although pathognomonic for Graves disease, pretibial myxedema is a late manifestation that occurs in less than 5% of these patients.4 The most common site of involvement is the pretibial region, although less common sites include the face, arms, shoulders, abdomen, pinna, and the location of previous scars.4
While pretibial myxedema usually is associated with hyperthyroidism, it can occur after treatment (as was the case here), while the patient is in a euthyroid or hypothyroid state. Radioiodine therapy has been reported to be a trigger for pretibial myxedema in 1 case report, although the pathophysiology is not fully understood.5
Continue to: More serious conditions must be ruled out
More serious conditions must be ruled out
The differential for painless nodules includes cutaneous lymphoma and atypical infections of fungal or mycobacterial etiology.
Cutaneous lymphoma that manifests with leg tumors includes primary cutaneous anaplastic CD30+ large cell lymphoma (PCALCL) and primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBL-LT). The former may occur in young patients, whereas the latter tends to manifest in the elderly. Biopsy shows a neoplastic proliferation of atypical lymphocytes within the dermis,6 differing from our case.
Atypical infections may be detected through bacterial, mycobacterial, or fungal cultures, and may be accompanied by elevated inflammatory markers or other systemic symptoms of the infection, setting it apart from pretibial myxedema.
Treatment is simple and noninvasive
Pretibial myxedema is usually asymptomatic, with minimal morbidity. The nodular variant may resolve spontaneously; thus, therapeutic management often is reserved for severe cases or for those with cosmetic concerns. Treatment options include mid- to high-potency topical corticosteroids with an occlusive dressing for 1 to 2 weeks (or until resolution) or an intralesional triamcinolone injection (5-10 mg/mL, single or monthly until resolution), compression stockings, and pneumatic compression.2
This patient was treated with a single intralesional injection of triamcinolone 10 mg/mL. The nodules resolved within a month.
1. Thammarucha S, Sudtikoonaseth P. Nodular pretibial myxedema with Graves’ disease: a case report. Thai J Dermatol. 2021;37:30-36.
2. Singla M, Gupta A. Nodular thyroid dermopathy: not a hallmark of Graves’ disease. Am J Med. 2019;132:e521-e522. doi: 10.1016/j.amjmed.2018.11.004
3. Lan C, Wang Y, Zeng X, et al. Morphological diversity of pretibial myxedema and its mechanism of evolving process and outcome: a retrospective study of 216 cases. J Thyroid Res. 2016:2016:265217
4. doi: 10.1155/2016/2652174 4. Patil MM, Kamalanathan S, Sahoo J, et al. Pretibial myxedema. QJM. 2015;108:985. doi: 10.1093/qjmed/hcv136
5. Harvey RD, Metcalfe RA, Morteo C, et al. Acute pre-tibial myxoedema following radioiodine therapy for thyrotoxic Graves’ disease. Clin Endocrinol (Oxf). 1995;42:657-660. doi: 10.1111/j.1365-2265.1995.tb02695.x
6. Schukow C, Ahmed A. Dermatopathology, cutaneous lymphomas. StatPearls [Internet]. Updated February 16, 2023. Accessed October 23, 2023. www.ncbi.nlm.nih.gov/books/NBK589703/
1. Thammarucha S, Sudtikoonaseth P. Nodular pretibial myxedema with Graves’ disease: a case report. Thai J Dermatol. 2021;37:30-36.
2. Singla M, Gupta A. Nodular thyroid dermopathy: not a hallmark of Graves’ disease. Am J Med. 2019;132:e521-e522. doi: 10.1016/j.amjmed.2018.11.004
3. Lan C, Wang Y, Zeng X, et al. Morphological diversity of pretibial myxedema and its mechanism of evolving process and outcome: a retrospective study of 216 cases. J Thyroid Res. 2016:2016:265217
4. doi: 10.1155/2016/2652174 4. Patil MM, Kamalanathan S, Sahoo J, et al. Pretibial myxedema. QJM. 2015;108:985. doi: 10.1093/qjmed/hcv136
5. Harvey RD, Metcalfe RA, Morteo C, et al. Acute pre-tibial myxoedema following radioiodine therapy for thyrotoxic Graves’ disease. Clin Endocrinol (Oxf). 1995;42:657-660. doi: 10.1111/j.1365-2265.1995.tb02695.x
6. Schukow C, Ahmed A. Dermatopathology, cutaneous lymphomas. StatPearls [Internet]. Updated February 16, 2023. Accessed October 23, 2023. www.ncbi.nlm.nih.gov/books/NBK589703/
Alpha-gal syndrome: Red meat is ‘just the beginning,’ expert says
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Obinutuzumab promotes renal preservation in lupus nephritis
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
Salt intake associated with increased type 2 diabetes risk
TOPLINE:
, even after adjustment for confounding factors.
METHODOLOGY:
- Researchers identified 402,982 participants in the UK Biobank from March 2006 to October 2010 who had completed a questionnaire about the frequency with which they added salt to food and who did not have diabetes, chronic kidney disease, cancer, or cardiovascular disease at baseline.
- Urine samples were collected at baseline, sodium and potassium levels were measured, and 24-hour sodium excretion was estimated.
- Investigators followed participants from baseline to diagnosis of diabetes, death, or the censoring date (May 23, 2021), whichever occurred first. Information on T2D events were collected through medical history linkage to data on hospital admissions, questionnaire, and the death register.
TAKEAWAY:
- During a mean follow-up of 11.9 years, 13,120 incident cases of T2D were documented.
- Compared with people who reported “never/rarely” adding salt to food, the sex- and age-adjusted hazard ratios (HRs) for developing T2D were 1.20, 1.32, and 1.86 for those who reported “sometimes,” “usually,” and “always” adding salt, respectively (P-trend < .001).
- After further adjustment for the Townsend deprivation index, education level, income, smoking, drinking, physical activity, and high cholesterol, the association was attenuated but remained significant, with HRs of 1.11, 1.18, and 1.28 for “sometimes,” “usually,” and “always” responses, respectively (P-trend < .001).
- After full adjustment, there was also a dose-dependent relationship across quintiles of urinary sodium and higher T2D risk, with HRs of 1 (reference), 1.12, 1.17, 1.28, and 1.34 for quintiles 2-5, respectively (P-trend < .001).
- Body fat percentage and body fat mass significantly mediated the association of adding salt with T2D, by estimated effects of 37.9% and 39.9%, respectively (both P < .001).
IN PRACTICE:
“These findings provide support that reduction of adding salt to foods may act as a potential behavioral intervention approach for preventing T2D. Future clinical trials are needed to further validate our findings,” the authors wrote.
SOURCE:
The study by Xuan Wang, MD, PhD, department of epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, and colleagues was published in the November 2023 issue of Mayo Clinic Proceedings.
LIMITATIONS:
The researchers could not completely exclude the possibility that high frequency of adding salt to foods is a marker for an unhealthy lifestyle. Self-reported frequency of adding salt to food might be subject to information bias and did not provide quantitative information on total sodium intake. In addition, participants were mainly of European descent, making application of the findings to other ethnic groups unclear; the observational design meant researchers could not rule out residual confounding; and information on addition of salt to food was available only at baseline, so potential changes in salt consumption during follow-up could not be considered.
DISCLOSURES:
The study was supported by grants from the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the Fogarty International Center; and Tulane Research Centers of Excellence Awards. The authors reported no potential competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
, even after adjustment for confounding factors.
METHODOLOGY:
- Researchers identified 402,982 participants in the UK Biobank from March 2006 to October 2010 who had completed a questionnaire about the frequency with which they added salt to food and who did not have diabetes, chronic kidney disease, cancer, or cardiovascular disease at baseline.
- Urine samples were collected at baseline, sodium and potassium levels were measured, and 24-hour sodium excretion was estimated.
- Investigators followed participants from baseline to diagnosis of diabetes, death, or the censoring date (May 23, 2021), whichever occurred first. Information on T2D events were collected through medical history linkage to data on hospital admissions, questionnaire, and the death register.
TAKEAWAY:
- During a mean follow-up of 11.9 years, 13,120 incident cases of T2D were documented.
- Compared with people who reported “never/rarely” adding salt to food, the sex- and age-adjusted hazard ratios (HRs) for developing T2D were 1.20, 1.32, and 1.86 for those who reported “sometimes,” “usually,” and “always” adding salt, respectively (P-trend < .001).
- After further adjustment for the Townsend deprivation index, education level, income, smoking, drinking, physical activity, and high cholesterol, the association was attenuated but remained significant, with HRs of 1.11, 1.18, and 1.28 for “sometimes,” “usually,” and “always” responses, respectively (P-trend < .001).
- After full adjustment, there was also a dose-dependent relationship across quintiles of urinary sodium and higher T2D risk, with HRs of 1 (reference), 1.12, 1.17, 1.28, and 1.34 for quintiles 2-5, respectively (P-trend < .001).
- Body fat percentage and body fat mass significantly mediated the association of adding salt with T2D, by estimated effects of 37.9% and 39.9%, respectively (both P < .001).
IN PRACTICE:
“These findings provide support that reduction of adding salt to foods may act as a potential behavioral intervention approach for preventing T2D. Future clinical trials are needed to further validate our findings,” the authors wrote.
SOURCE:
The study by Xuan Wang, MD, PhD, department of epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, and colleagues was published in the November 2023 issue of Mayo Clinic Proceedings.
LIMITATIONS:
The researchers could not completely exclude the possibility that high frequency of adding salt to foods is a marker for an unhealthy lifestyle. Self-reported frequency of adding salt to food might be subject to information bias and did not provide quantitative information on total sodium intake. In addition, participants were mainly of European descent, making application of the findings to other ethnic groups unclear; the observational design meant researchers could not rule out residual confounding; and information on addition of salt to food was available only at baseline, so potential changes in salt consumption during follow-up could not be considered.
DISCLOSURES:
The study was supported by grants from the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the Fogarty International Center; and Tulane Research Centers of Excellence Awards. The authors reported no potential competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
, even after adjustment for confounding factors.
METHODOLOGY:
- Researchers identified 402,982 participants in the UK Biobank from March 2006 to October 2010 who had completed a questionnaire about the frequency with which they added salt to food and who did not have diabetes, chronic kidney disease, cancer, or cardiovascular disease at baseline.
- Urine samples were collected at baseline, sodium and potassium levels were measured, and 24-hour sodium excretion was estimated.
- Investigators followed participants from baseline to diagnosis of diabetes, death, or the censoring date (May 23, 2021), whichever occurred first. Information on T2D events were collected through medical history linkage to data on hospital admissions, questionnaire, and the death register.
TAKEAWAY:
- During a mean follow-up of 11.9 years, 13,120 incident cases of T2D were documented.
- Compared with people who reported “never/rarely” adding salt to food, the sex- and age-adjusted hazard ratios (HRs) for developing T2D were 1.20, 1.32, and 1.86 for those who reported “sometimes,” “usually,” and “always” adding salt, respectively (P-trend < .001).
- After further adjustment for the Townsend deprivation index, education level, income, smoking, drinking, physical activity, and high cholesterol, the association was attenuated but remained significant, with HRs of 1.11, 1.18, and 1.28 for “sometimes,” “usually,” and “always” responses, respectively (P-trend < .001).
- After full adjustment, there was also a dose-dependent relationship across quintiles of urinary sodium and higher T2D risk, with HRs of 1 (reference), 1.12, 1.17, 1.28, and 1.34 for quintiles 2-5, respectively (P-trend < .001).
- Body fat percentage and body fat mass significantly mediated the association of adding salt with T2D, by estimated effects of 37.9% and 39.9%, respectively (both P < .001).
IN PRACTICE:
“These findings provide support that reduction of adding salt to foods may act as a potential behavioral intervention approach for preventing T2D. Future clinical trials are needed to further validate our findings,” the authors wrote.
SOURCE:
The study by Xuan Wang, MD, PhD, department of epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, and colleagues was published in the November 2023 issue of Mayo Clinic Proceedings.
LIMITATIONS:
The researchers could not completely exclude the possibility that high frequency of adding salt to foods is a marker for an unhealthy lifestyle. Self-reported frequency of adding salt to food might be subject to information bias and did not provide quantitative information on total sodium intake. In addition, participants were mainly of European descent, making application of the findings to other ethnic groups unclear; the observational design meant researchers could not rule out residual confounding; and information on addition of salt to food was available only at baseline, so potential changes in salt consumption during follow-up could not be considered.
DISCLOSURES:
The study was supported by grants from the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the Fogarty International Center; and Tulane Research Centers of Excellence Awards. The authors reported no potential competing interests.
A version of this article appeared on Medscape.com.
Sustained reductions in Lp(a) achieved with novel siRNA drug
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Short steroid taper tested with tocilizumab for giant cell arteritis
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
Dropping aspirin cuts bleeding in LVAD patients: ARIES-HM3
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
AT AHA 2023
AAD updates guidelines for managing AD with phototherapy and systemic therapies
.
The guidelines cover approved and off-label uses of systemic therapies and phototherapy, including new treatments that have become available since the last guidelines were published almost a decade ago. These include biologics and oral Janus kinase (JAK) inhibitors, as well as older oral or injectable immunomodulators and antimetabolites, oral antibiotics, antihistamines, and phosphodiesterase-4 inhibitors. The guidelines rate the existing evidence as “strong” for dupilumab, tralokinumab, abrocitinib, baricitinib, and upadacitinib. They also conditionally recommend phototherapy, as well as cyclosporine, methotrexate, azathioprine, and mycophenolate, but recommend against the use of systemic corticosteroids.
The guidelines update the AAD’s 2014 recommendations for managing AD in adults with phototherapy and systemic therapies. “At that time, prednisone – universally agreed to be the least appropriate chronic therapy for AD – was the only Food and Drug Administration–approved agent,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the guidelines, told this news organization. “This was the driver.”
The latest guidelines were published online in the Journal of the American Academy of Dermatology.
Broad evidence review
Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at the Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic evidence review of phototherapy such as narrowband and broadband UVB and systemic therapies, including biologics such as dupilumab and tralokinumab, JAK inhibitors such as upadacitinib and abrocitinib, and immunosuppressants such as methotrexate and azathioprine.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
Recommendations, future studies
Of the 11 evidence-based recommendations of therapies for adults with AD refractory to topical medications, the work group ranks 5 as “strong” based on the evidence and the rest as “conditional.” “Strong” implies the benefits clearly outweigh risks and burdens, they apply to most patients in most circumstances, and they fall under good clinical practice. “Conditional” means the benefits and risks are closely balanced for most patients, “but the appropriate action may different depending on the patient or other stakeholder values,” the authors wrote.
In their remarks about phototherapy, the work group noted that most published literature on the topic “reports on the efficacy and safety of narrow band UVB. Wherever possible, use a light source that minimizes the potential for harm under the supervision of a qualified clinician.”
In their remarks about cyclosporine, they noted that evidence suggests an initial dose of 3 mg/kg per day to 5 mg/kg per day is effective, but that the Food and Drug Administration has not approved cyclosporine for use in AD. “The FDA has approved limited-term use (up to 1 year) in psoriasis,” they wrote. “Comorbidities or drug interactions that may exacerbate toxicity make this intervention inappropriate for select patients.” The work group noted that significant research gaps remain in phototherapy, especially trials that compare different phototherapy modalities and those that compare phototherapy with other AD treatment strategies.
“Larger clinical trials would also be helpful for cyclosporine, methotrexate, azathioprine, and mycophenolate to improve the certainty of evidence for those medications,” they added. “Furthermore, formal cost-effectiveness analyses comparing older to newer treatments are needed.”
They recommended the inclusion of active comparator arms in randomized, controlled trials as new systemic therapies continue to be developed and tested.
The work group ranked the level of evidence they reviewed for the therapies from very low to moderate. No therapy was judged to have high evidence. They also cited the short duration of most randomized controlled trials of phototherapy.
Using the guidelines in clinical care
According to Dr. Davis, the topic of which agent if any should be considered “first line” generated robust discussion among the work group members.
“When there are not robust head-to-head trials – and there are not – it is often opinion that governs this decision, and opinion should not, when possible, govern a guideline,” Dr. Davis said. “Accordingly, we determined based upon the evidence agents – plural – that deserve to be considered ‘first line’ but not a single agent.”
In her opinion, the top three considerations regarding use of systemic therapy for AD relate to patient selection and shared decision making. One, standard therapy has failed. Two, diagnosis is assured. And three, “steroid phobia should be considered,” and patients should be “fully informed of risks and benefits of both treating and not treating,” she said.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Micreos. Dr. Davis reported having no relevant disclosures. Other work group members reported having financial disclosures with many pharmaceutical companies. The study was supported by internal funds from the American Academy of Dermatology.
.
The guidelines cover approved and off-label uses of systemic therapies and phototherapy, including new treatments that have become available since the last guidelines were published almost a decade ago. These include biologics and oral Janus kinase (JAK) inhibitors, as well as older oral or injectable immunomodulators and antimetabolites, oral antibiotics, antihistamines, and phosphodiesterase-4 inhibitors. The guidelines rate the existing evidence as “strong” for dupilumab, tralokinumab, abrocitinib, baricitinib, and upadacitinib. They also conditionally recommend phototherapy, as well as cyclosporine, methotrexate, azathioprine, and mycophenolate, but recommend against the use of systemic corticosteroids.
The guidelines update the AAD’s 2014 recommendations for managing AD in adults with phototherapy and systemic therapies. “At that time, prednisone – universally agreed to be the least appropriate chronic therapy for AD – was the only Food and Drug Administration–approved agent,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the guidelines, told this news organization. “This was the driver.”
The latest guidelines were published online in the Journal of the American Academy of Dermatology.
Broad evidence review
Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at the Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic evidence review of phototherapy such as narrowband and broadband UVB and systemic therapies, including biologics such as dupilumab and tralokinumab, JAK inhibitors such as upadacitinib and abrocitinib, and immunosuppressants such as methotrexate and azathioprine.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
Recommendations, future studies
Of the 11 evidence-based recommendations of therapies for adults with AD refractory to topical medications, the work group ranks 5 as “strong” based on the evidence and the rest as “conditional.” “Strong” implies the benefits clearly outweigh risks and burdens, they apply to most patients in most circumstances, and they fall under good clinical practice. “Conditional” means the benefits and risks are closely balanced for most patients, “but the appropriate action may different depending on the patient or other stakeholder values,” the authors wrote.
In their remarks about phototherapy, the work group noted that most published literature on the topic “reports on the efficacy and safety of narrow band UVB. Wherever possible, use a light source that minimizes the potential for harm under the supervision of a qualified clinician.”
In their remarks about cyclosporine, they noted that evidence suggests an initial dose of 3 mg/kg per day to 5 mg/kg per day is effective, but that the Food and Drug Administration has not approved cyclosporine for use in AD. “The FDA has approved limited-term use (up to 1 year) in psoriasis,” they wrote. “Comorbidities or drug interactions that may exacerbate toxicity make this intervention inappropriate for select patients.” The work group noted that significant research gaps remain in phototherapy, especially trials that compare different phototherapy modalities and those that compare phototherapy with other AD treatment strategies.
“Larger clinical trials would also be helpful for cyclosporine, methotrexate, azathioprine, and mycophenolate to improve the certainty of evidence for those medications,” they added. “Furthermore, formal cost-effectiveness analyses comparing older to newer treatments are needed.”
They recommended the inclusion of active comparator arms in randomized, controlled trials as new systemic therapies continue to be developed and tested.
The work group ranked the level of evidence they reviewed for the therapies from very low to moderate. No therapy was judged to have high evidence. They also cited the short duration of most randomized controlled trials of phototherapy.
Using the guidelines in clinical care
According to Dr. Davis, the topic of which agent if any should be considered “first line” generated robust discussion among the work group members.
“When there are not robust head-to-head trials – and there are not – it is often opinion that governs this decision, and opinion should not, when possible, govern a guideline,” Dr. Davis said. “Accordingly, we determined based upon the evidence agents – plural – that deserve to be considered ‘first line’ but not a single agent.”
In her opinion, the top three considerations regarding use of systemic therapy for AD relate to patient selection and shared decision making. One, standard therapy has failed. Two, diagnosis is assured. And three, “steroid phobia should be considered,” and patients should be “fully informed of risks and benefits of both treating and not treating,” she said.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Micreos. Dr. Davis reported having no relevant disclosures. Other work group members reported having financial disclosures with many pharmaceutical companies. The study was supported by internal funds from the American Academy of Dermatology.
.
The guidelines cover approved and off-label uses of systemic therapies and phototherapy, including new treatments that have become available since the last guidelines were published almost a decade ago. These include biologics and oral Janus kinase (JAK) inhibitors, as well as older oral or injectable immunomodulators and antimetabolites, oral antibiotics, antihistamines, and phosphodiesterase-4 inhibitors. The guidelines rate the existing evidence as “strong” for dupilumab, tralokinumab, abrocitinib, baricitinib, and upadacitinib. They also conditionally recommend phototherapy, as well as cyclosporine, methotrexate, azathioprine, and mycophenolate, but recommend against the use of systemic corticosteroids.
The guidelines update the AAD’s 2014 recommendations for managing AD in adults with phototherapy and systemic therapies. “At that time, prednisone – universally agreed to be the least appropriate chronic therapy for AD – was the only Food and Drug Administration–approved agent,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the guidelines, told this news organization. “This was the driver.”
The latest guidelines were published online in the Journal of the American Academy of Dermatology.
Broad evidence review
Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at the Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic evidence review of phototherapy such as narrowband and broadband UVB and systemic therapies, including biologics such as dupilumab and tralokinumab, JAK inhibitors such as upadacitinib and abrocitinib, and immunosuppressants such as methotrexate and azathioprine.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
Recommendations, future studies
Of the 11 evidence-based recommendations of therapies for adults with AD refractory to topical medications, the work group ranks 5 as “strong” based on the evidence and the rest as “conditional.” “Strong” implies the benefits clearly outweigh risks and burdens, they apply to most patients in most circumstances, and they fall under good clinical practice. “Conditional” means the benefits and risks are closely balanced for most patients, “but the appropriate action may different depending on the patient or other stakeholder values,” the authors wrote.
In their remarks about phototherapy, the work group noted that most published literature on the topic “reports on the efficacy and safety of narrow band UVB. Wherever possible, use a light source that minimizes the potential for harm under the supervision of a qualified clinician.”
In their remarks about cyclosporine, they noted that evidence suggests an initial dose of 3 mg/kg per day to 5 mg/kg per day is effective, but that the Food and Drug Administration has not approved cyclosporine for use in AD. “The FDA has approved limited-term use (up to 1 year) in psoriasis,” they wrote. “Comorbidities or drug interactions that may exacerbate toxicity make this intervention inappropriate for select patients.” The work group noted that significant research gaps remain in phototherapy, especially trials that compare different phototherapy modalities and those that compare phototherapy with other AD treatment strategies.
“Larger clinical trials would also be helpful for cyclosporine, methotrexate, azathioprine, and mycophenolate to improve the certainty of evidence for those medications,” they added. “Furthermore, formal cost-effectiveness analyses comparing older to newer treatments are needed.”
They recommended the inclusion of active comparator arms in randomized, controlled trials as new systemic therapies continue to be developed and tested.
The work group ranked the level of evidence they reviewed for the therapies from very low to moderate. No therapy was judged to have high evidence. They also cited the short duration of most randomized controlled trials of phototherapy.
Using the guidelines in clinical care
According to Dr. Davis, the topic of which agent if any should be considered “first line” generated robust discussion among the work group members.
“When there are not robust head-to-head trials – and there are not – it is often opinion that governs this decision, and opinion should not, when possible, govern a guideline,” Dr. Davis said. “Accordingly, we determined based upon the evidence agents – plural – that deserve to be considered ‘first line’ but not a single agent.”
In her opinion, the top three considerations regarding use of systemic therapy for AD relate to patient selection and shared decision making. One, standard therapy has failed. Two, diagnosis is assured. And three, “steroid phobia should be considered,” and patients should be “fully informed of risks and benefits of both treating and not treating,” she said.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Micreos. Dr. Davis reported having no relevant disclosures. Other work group members reported having financial disclosures with many pharmaceutical companies. The study was supported by internal funds from the American Academy of Dermatology.
FROM JAMA DERMATOLOGY