User login
Do 30-day readmissions mean anything?
Hospitalists have been paying close attention to 30-day readmission figures since public reporting and payment programs embraced that number as an indicator of the quality of hospital care. But there is limited evidence to demonstrate 30-day readmission is really a meaningful interval of time, according to a recent study, “Rethinking Thirty-Day Hospital Readmissions: Shorter Intervals Might Be Better Indicators of Quality of Care.”
“I began to dig through the literature to find some sort of evidence to support this figure – I couldn’t find anything. In talking with quality experts, they all, more or less, believe that things that happen outside of 7 or 10 days are really out of the control of the clinician,” says lead author David L. Chin, PhD, of the Center for Healthcare Policy and Research at the University of California, Davis.
Dr. Chin and his team examined the 30-day risk of unplanned inpatient readmission at the hospital level for Medicare patients aged 65 and older in four states and for three conditions: acute myocardial infarction, heart failure, and pneumonia. Across states and diagnoses, the hospital-level quality signal captured in readmission risk was highest on the first day after discharge, and it declined quickly to its lowest level at day 7.
“The rapid decay in the quality signal suggests that most readmissions after the seventh-day postdischarge were explained by community- and household-level factors beyond hospitals’ control,” the authors concluded.
Dr. Chin said the study results show the 30-day measure is “a blunt instrument.”
“It isn’t really measuring anything that we’re supposed to be measuring,” he explains. “Essentially, 97% of the reasons a person comes back to the hospital is due to some other, non-hospital thing.”
He does not advocate for 7 days as the new standard, however.
“This is more intended to be a message that this is really not the right way of approaching [readmissions] to begin with,” he says. “I think we convincingly showed that it shouldn’t be 30 days, but we don’t really have a very good picture of what is driving readmission. Hospitals are getting dinged on these things that have happened that, really, they don’t have direct influence on.”
Reference
1. Chin DL, Bang H, Manickam RN, Romano PS. Rethinking thirty-day hospital readmissions: shorter intervals might be better indicators of quality of care. Health Aff (Millwood). 2016;35(10):1867-75.
Quality Improvement: Overuse as medical error
Hospitalists may recognize a culture of overuse at their hospitals, but how can they address it? That’s the question behind an HM16 abstract, “Occam’s Conference: Overuse as a Medical Error.”
“We wanted to change the culture of overuse here among the hospitalists and the house staff,” said lead author Hyung Cho, MD, director of quality and patient safety at the Icahn School of Medicine at Mount Sinai in New York City. “We wanted to frame it in a way that people can recognize and feel free to talk about and also give it the weight that it deserves. It’s a common thing that we all do: the chest x-ray or the EKG before a surgery, things like that.”
Seeing overuse as a medical error is a place to start.
“A framework in which overuse is considered a medical error would facilitate understanding of the drivers of overuse and systems factors that lead to it,” the authors wrote.
Dr. Cho and colleagues chose a monthly inpatient conference format, with all the relevant players gathered together.
“We also wanted to use the formula that Brandon Combs had with the ‘Do No Harm’ project, which is taking cases of overuse that actually lead to harm or a near miss. I think people respond to that as opposed to just talking about the cost, which people have a hard time actually figuring out,” Dr. Cho said.
The resulting Occam’s Conference provides a process to identify and discuss overuse as a medical error. It uses a fish-bone diagram to help analyze each case.
“That conversation needs to happen,” Dr. Cho said. “You realize that people are all on the same page, and if they’re not, they need to get on the same page and have an open dialogue.”
Reference
1. Cho HJ, Lutz C, Truong TTN, et al. Occam’s Conference: overuse as a medical error [abstract]. J Hosp Med. 2016;11(suppl1).
Practice Management: There’s an app for … end-of-life communications
Hospitalists wanting to help patients navigate end-of-life decisions or assist bereaved families in dealing with the death of a loved one have some new tools, according to The New York Times article, “Start-Ups for the End of Life.”
A start-up called Grace is intended to help its users deal with the myriad issues family members face after a death; it connects users with estate lawyers, financial planners, funeral homes, and caterers. Grace customers receive a list of tasks to complete before and after a death, and it includes relevant paperwork. The app also has staff ready to assist customers.
Currently, there’s little guidance available in this area, Alex Kruger, Grace’s cofounder and chief executive, and a licensed funeral director, told the New York Times: “At Grace we say, ‘Here are the 17 things you need to do this week’ and you can check them off as you do them. Here’s what you do the week before someone dies, when they die and then two weeks later.”
Another start-up mentioned in the article that could be relevant to hospitalists and their patients is called Parting. It provides an online directory of funeral homes searchable by ZIP code so users can quickly compare prices, services, and locations.
Reference
1. Zimmerman E. “Start-ups for the end of life,” New York Times, Nov. 22, 2016.
Quality Improvement: How to create a high-value culture
Hospitalists today are overseeing the health system’s movement toward an emphasis on value over volume.
“As leaders begin to strategize the best ways to spur transformation, our team realized that checklists and algorithms alone would likely not be enough to create sustained change in divisions and practices across the country,” said Reshma Gupta, MD, MSHPM, of the Robert Wood Johnson Clinical Scholars Program in the department of medicine at the University of California, Los Angeles. She’s the lead author of the recent study, “Development of a High-Value Care Culture Survey: A Modified Delphi Process and Psychometric Evaluation.”“Patient safety culture surveys have previously been used to drive care improvements, but no comparable survey of high-value care culture currently exists,” the authors wrote. “We aimed to develop a High-Value Care Culture Survey (HVCCS) for use by health care leaders and training programs to target future improvements in value-based care.”
Researchers conducted a two-phase, national modified Delphi process among 28 physicians and nurse experts with diverse backgrounds. They then administered a cross-sectional survey at two large academic medical centers among 162 internal medicine residents and 91 hospitalists for psychometric evaluation.
Four factors emerged with strong reliability:
• Leadership and health system messaging.
• Data transparency and access.
• Comfort with cost conversations.
• Blame-free environment.
The HVCCS can assist hospitalists and administrators in identifying tangible areas to target, Dr. Gupta said. The instrument was found to have good reliability and appears to correlate with an important patient outcome metric: the Centers for Medicare & Medicaid Services value-based purchasing score that has determined hospital reimbursement in recent years.
Now that this instrument has been created, the next step is for hospitals to begin using it. Dr. Gupta recognizes hospital medicine already has been a national leader in efforts to promote value-based care.
“With hospitalists leading many operational or research efforts in this area,” she says, “it will be vital to measure and address culture change. We believe this tool can aid them in their efforts to shape the story of value promotion at their institutions.”
Reference
Gupta R, Moriates C, Harrison JD, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation [published online ahead of print Oct. 26, 2016]. BMJ Qual Saf. doi: 10.1136/bmjqs-2016-005612.
Quick Byte: Cleaning robotic surgical instruments
Robotic surgical instruments can retain some contamination even after cleaning, a new study suggests. Over 21 months, the researchers assessed protein residue on robotic and standard surgical instruments that were cleaned according to manufacturers’ instructions. The cleanings were 99.1% effective on the standard instruments but 97.6% effective on the robotic instruments, suggesting complete eradication of surface contaminants from robotic surgical instruments may not be possible with the current cleaning procedures.
Reference
1. Preidt R. “Robotic surgical tools tough to keep clean,” HealthDay News, Nov. 1, 2016.
Suzanne Bopp is a freelance writer in New Jersey.
Hospitalists have been paying close attention to 30-day readmission figures since public reporting and payment programs embraced that number as an indicator of the quality of hospital care. But there is limited evidence to demonstrate 30-day readmission is really a meaningful interval of time, according to a recent study, “Rethinking Thirty-Day Hospital Readmissions: Shorter Intervals Might Be Better Indicators of Quality of Care.”
“I began to dig through the literature to find some sort of evidence to support this figure – I couldn’t find anything. In talking with quality experts, they all, more or less, believe that things that happen outside of 7 or 10 days are really out of the control of the clinician,” says lead author David L. Chin, PhD, of the Center for Healthcare Policy and Research at the University of California, Davis.
Dr. Chin and his team examined the 30-day risk of unplanned inpatient readmission at the hospital level for Medicare patients aged 65 and older in four states and for three conditions: acute myocardial infarction, heart failure, and pneumonia. Across states and diagnoses, the hospital-level quality signal captured in readmission risk was highest on the first day after discharge, and it declined quickly to its lowest level at day 7.
“The rapid decay in the quality signal suggests that most readmissions after the seventh-day postdischarge were explained by community- and household-level factors beyond hospitals’ control,” the authors concluded.
Dr. Chin said the study results show the 30-day measure is “a blunt instrument.”
“It isn’t really measuring anything that we’re supposed to be measuring,” he explains. “Essentially, 97% of the reasons a person comes back to the hospital is due to some other, non-hospital thing.”
He does not advocate for 7 days as the new standard, however.
“This is more intended to be a message that this is really not the right way of approaching [readmissions] to begin with,” he says. “I think we convincingly showed that it shouldn’t be 30 days, but we don’t really have a very good picture of what is driving readmission. Hospitals are getting dinged on these things that have happened that, really, they don’t have direct influence on.”
Reference
1. Chin DL, Bang H, Manickam RN, Romano PS. Rethinking thirty-day hospital readmissions: shorter intervals might be better indicators of quality of care. Health Aff (Millwood). 2016;35(10):1867-75.
Quality Improvement: Overuse as medical error
Hospitalists may recognize a culture of overuse at their hospitals, but how can they address it? That’s the question behind an HM16 abstract, “Occam’s Conference: Overuse as a Medical Error.”
“We wanted to change the culture of overuse here among the hospitalists and the house staff,” said lead author Hyung Cho, MD, director of quality and patient safety at the Icahn School of Medicine at Mount Sinai in New York City. “We wanted to frame it in a way that people can recognize and feel free to talk about and also give it the weight that it deserves. It’s a common thing that we all do: the chest x-ray or the EKG before a surgery, things like that.”
Seeing overuse as a medical error is a place to start.
“A framework in which overuse is considered a medical error would facilitate understanding of the drivers of overuse and systems factors that lead to it,” the authors wrote.
Dr. Cho and colleagues chose a monthly inpatient conference format, with all the relevant players gathered together.
“We also wanted to use the formula that Brandon Combs had with the ‘Do No Harm’ project, which is taking cases of overuse that actually lead to harm or a near miss. I think people respond to that as opposed to just talking about the cost, which people have a hard time actually figuring out,” Dr. Cho said.
The resulting Occam’s Conference provides a process to identify and discuss overuse as a medical error. It uses a fish-bone diagram to help analyze each case.
“That conversation needs to happen,” Dr. Cho said. “You realize that people are all on the same page, and if they’re not, they need to get on the same page and have an open dialogue.”
Reference
1. Cho HJ, Lutz C, Truong TTN, et al. Occam’s Conference: overuse as a medical error [abstract]. J Hosp Med. 2016;11(suppl1).
Practice Management: There’s an app for … end-of-life communications
Hospitalists wanting to help patients navigate end-of-life decisions or assist bereaved families in dealing with the death of a loved one have some new tools, according to The New York Times article, “Start-Ups for the End of Life.”
A start-up called Grace is intended to help its users deal with the myriad issues family members face after a death; it connects users with estate lawyers, financial planners, funeral homes, and caterers. Grace customers receive a list of tasks to complete before and after a death, and it includes relevant paperwork. The app also has staff ready to assist customers.
Currently, there’s little guidance available in this area, Alex Kruger, Grace’s cofounder and chief executive, and a licensed funeral director, told the New York Times: “At Grace we say, ‘Here are the 17 things you need to do this week’ and you can check them off as you do them. Here’s what you do the week before someone dies, when they die and then two weeks later.”
Another start-up mentioned in the article that could be relevant to hospitalists and their patients is called Parting. It provides an online directory of funeral homes searchable by ZIP code so users can quickly compare prices, services, and locations.
Reference
1. Zimmerman E. “Start-ups for the end of life,” New York Times, Nov. 22, 2016.
Quality Improvement: How to create a high-value culture
Hospitalists today are overseeing the health system’s movement toward an emphasis on value over volume.
“As leaders begin to strategize the best ways to spur transformation, our team realized that checklists and algorithms alone would likely not be enough to create sustained change in divisions and practices across the country,” said Reshma Gupta, MD, MSHPM, of the Robert Wood Johnson Clinical Scholars Program in the department of medicine at the University of California, Los Angeles. She’s the lead author of the recent study, “Development of a High-Value Care Culture Survey: A Modified Delphi Process and Psychometric Evaluation.”“Patient safety culture surveys have previously been used to drive care improvements, but no comparable survey of high-value care culture currently exists,” the authors wrote. “We aimed to develop a High-Value Care Culture Survey (HVCCS) for use by health care leaders and training programs to target future improvements in value-based care.”
Researchers conducted a two-phase, national modified Delphi process among 28 physicians and nurse experts with diverse backgrounds. They then administered a cross-sectional survey at two large academic medical centers among 162 internal medicine residents and 91 hospitalists for psychometric evaluation.
Four factors emerged with strong reliability:
• Leadership and health system messaging.
• Data transparency and access.
• Comfort with cost conversations.
• Blame-free environment.
The HVCCS can assist hospitalists and administrators in identifying tangible areas to target, Dr. Gupta said. The instrument was found to have good reliability and appears to correlate with an important patient outcome metric: the Centers for Medicare & Medicaid Services value-based purchasing score that has determined hospital reimbursement in recent years.
Now that this instrument has been created, the next step is for hospitals to begin using it. Dr. Gupta recognizes hospital medicine already has been a national leader in efforts to promote value-based care.
“With hospitalists leading many operational or research efforts in this area,” she says, “it will be vital to measure and address culture change. We believe this tool can aid them in their efforts to shape the story of value promotion at their institutions.”
Reference
Gupta R, Moriates C, Harrison JD, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation [published online ahead of print Oct. 26, 2016]. BMJ Qual Saf. doi: 10.1136/bmjqs-2016-005612.
Quick Byte: Cleaning robotic surgical instruments
Robotic surgical instruments can retain some contamination even after cleaning, a new study suggests. Over 21 months, the researchers assessed protein residue on robotic and standard surgical instruments that were cleaned according to manufacturers’ instructions. The cleanings were 99.1% effective on the standard instruments but 97.6% effective on the robotic instruments, suggesting complete eradication of surface contaminants from robotic surgical instruments may not be possible with the current cleaning procedures.
Reference
1. Preidt R. “Robotic surgical tools tough to keep clean,” HealthDay News, Nov. 1, 2016.
Suzanne Bopp is a freelance writer in New Jersey.
Hospitalists have been paying close attention to 30-day readmission figures since public reporting and payment programs embraced that number as an indicator of the quality of hospital care. But there is limited evidence to demonstrate 30-day readmission is really a meaningful interval of time, according to a recent study, “Rethinking Thirty-Day Hospital Readmissions: Shorter Intervals Might Be Better Indicators of Quality of Care.”
“I began to dig through the literature to find some sort of evidence to support this figure – I couldn’t find anything. In talking with quality experts, they all, more or less, believe that things that happen outside of 7 or 10 days are really out of the control of the clinician,” says lead author David L. Chin, PhD, of the Center for Healthcare Policy and Research at the University of California, Davis.
Dr. Chin and his team examined the 30-day risk of unplanned inpatient readmission at the hospital level for Medicare patients aged 65 and older in four states and for three conditions: acute myocardial infarction, heart failure, and pneumonia. Across states and diagnoses, the hospital-level quality signal captured in readmission risk was highest on the first day after discharge, and it declined quickly to its lowest level at day 7.
“The rapid decay in the quality signal suggests that most readmissions after the seventh-day postdischarge were explained by community- and household-level factors beyond hospitals’ control,” the authors concluded.
Dr. Chin said the study results show the 30-day measure is “a blunt instrument.”
“It isn’t really measuring anything that we’re supposed to be measuring,” he explains. “Essentially, 97% of the reasons a person comes back to the hospital is due to some other, non-hospital thing.”
He does not advocate for 7 days as the new standard, however.
“This is more intended to be a message that this is really not the right way of approaching [readmissions] to begin with,” he says. “I think we convincingly showed that it shouldn’t be 30 days, but we don’t really have a very good picture of what is driving readmission. Hospitals are getting dinged on these things that have happened that, really, they don’t have direct influence on.”
Reference
1. Chin DL, Bang H, Manickam RN, Romano PS. Rethinking thirty-day hospital readmissions: shorter intervals might be better indicators of quality of care. Health Aff (Millwood). 2016;35(10):1867-75.
Quality Improvement: Overuse as medical error
Hospitalists may recognize a culture of overuse at their hospitals, but how can they address it? That’s the question behind an HM16 abstract, “Occam’s Conference: Overuse as a Medical Error.”
“We wanted to change the culture of overuse here among the hospitalists and the house staff,” said lead author Hyung Cho, MD, director of quality and patient safety at the Icahn School of Medicine at Mount Sinai in New York City. “We wanted to frame it in a way that people can recognize and feel free to talk about and also give it the weight that it deserves. It’s a common thing that we all do: the chest x-ray or the EKG before a surgery, things like that.”
Seeing overuse as a medical error is a place to start.
“A framework in which overuse is considered a medical error would facilitate understanding of the drivers of overuse and systems factors that lead to it,” the authors wrote.
Dr. Cho and colleagues chose a monthly inpatient conference format, with all the relevant players gathered together.
“We also wanted to use the formula that Brandon Combs had with the ‘Do No Harm’ project, which is taking cases of overuse that actually lead to harm or a near miss. I think people respond to that as opposed to just talking about the cost, which people have a hard time actually figuring out,” Dr. Cho said.
The resulting Occam’s Conference provides a process to identify and discuss overuse as a medical error. It uses a fish-bone diagram to help analyze each case.
“That conversation needs to happen,” Dr. Cho said. “You realize that people are all on the same page, and if they’re not, they need to get on the same page and have an open dialogue.”
Reference
1. Cho HJ, Lutz C, Truong TTN, et al. Occam’s Conference: overuse as a medical error [abstract]. J Hosp Med. 2016;11(suppl1).
Practice Management: There’s an app for … end-of-life communications
Hospitalists wanting to help patients navigate end-of-life decisions or assist bereaved families in dealing with the death of a loved one have some new tools, according to The New York Times article, “Start-Ups for the End of Life.”
A start-up called Grace is intended to help its users deal with the myriad issues family members face after a death; it connects users with estate lawyers, financial planners, funeral homes, and caterers. Grace customers receive a list of tasks to complete before and after a death, and it includes relevant paperwork. The app also has staff ready to assist customers.
Currently, there’s little guidance available in this area, Alex Kruger, Grace’s cofounder and chief executive, and a licensed funeral director, told the New York Times: “At Grace we say, ‘Here are the 17 things you need to do this week’ and you can check them off as you do them. Here’s what you do the week before someone dies, when they die and then two weeks later.”
Another start-up mentioned in the article that could be relevant to hospitalists and their patients is called Parting. It provides an online directory of funeral homes searchable by ZIP code so users can quickly compare prices, services, and locations.
Reference
1. Zimmerman E. “Start-ups for the end of life,” New York Times, Nov. 22, 2016.
Quality Improvement: How to create a high-value culture
Hospitalists today are overseeing the health system’s movement toward an emphasis on value over volume.
“As leaders begin to strategize the best ways to spur transformation, our team realized that checklists and algorithms alone would likely not be enough to create sustained change in divisions and practices across the country,” said Reshma Gupta, MD, MSHPM, of the Robert Wood Johnson Clinical Scholars Program in the department of medicine at the University of California, Los Angeles. She’s the lead author of the recent study, “Development of a High-Value Care Culture Survey: A Modified Delphi Process and Psychometric Evaluation.”“Patient safety culture surveys have previously been used to drive care improvements, but no comparable survey of high-value care culture currently exists,” the authors wrote. “We aimed to develop a High-Value Care Culture Survey (HVCCS) for use by health care leaders and training programs to target future improvements in value-based care.”
Researchers conducted a two-phase, national modified Delphi process among 28 physicians and nurse experts with diverse backgrounds. They then administered a cross-sectional survey at two large academic medical centers among 162 internal medicine residents and 91 hospitalists for psychometric evaluation.
Four factors emerged with strong reliability:
• Leadership and health system messaging.
• Data transparency and access.
• Comfort with cost conversations.
• Blame-free environment.
The HVCCS can assist hospitalists and administrators in identifying tangible areas to target, Dr. Gupta said. The instrument was found to have good reliability and appears to correlate with an important patient outcome metric: the Centers for Medicare & Medicaid Services value-based purchasing score that has determined hospital reimbursement in recent years.
Now that this instrument has been created, the next step is for hospitals to begin using it. Dr. Gupta recognizes hospital medicine already has been a national leader in efforts to promote value-based care.
“With hospitalists leading many operational or research efforts in this area,” she says, “it will be vital to measure and address culture change. We believe this tool can aid them in their efforts to shape the story of value promotion at their institutions.”
Reference
Gupta R, Moriates C, Harrison JD, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation [published online ahead of print Oct. 26, 2016]. BMJ Qual Saf. doi: 10.1136/bmjqs-2016-005612.
Quick Byte: Cleaning robotic surgical instruments
Robotic surgical instruments can retain some contamination even after cleaning, a new study suggests. Over 21 months, the researchers assessed protein residue on robotic and standard surgical instruments that were cleaned according to manufacturers’ instructions. The cleanings were 99.1% effective on the standard instruments but 97.6% effective on the robotic instruments, suggesting complete eradication of surface contaminants from robotic surgical instruments may not be possible with the current cleaning procedures.
Reference
1. Preidt R. “Robotic surgical tools tough to keep clean,” HealthDay News, Nov. 1, 2016.
Suzanne Bopp is a freelance writer in New Jersey.
Sneak Peek: The Hospital Leader blog
Editor’s note: First published on The Hospital Leader blog under the title, “How I Realized QI Could Be a Dirty Word.”
With the recent election, there has been a new recognition of the various “bubbles” we all seem to be living in. It reminds me of the parable I like to often mention, popularized by the late great writer David Foster Wallace: Two fish were swimming along when an older fish swam by, nodded his head at them and said, “Mornin’ boys, how’s the water?” The two young fish nod back and swim for a bit, then one turns to the other and says, “What the hell is water?”
Imagine the hard reality that hit me when I read this quote from a resident: “Truly, the first thing I think of when I hear [QI] is going to make more work for residents.”
Wait – is QI actually a dirty word for other residents and physicians?
The quote comes from an Academic Medicine study titled “ ‘It Feels Like a Lot of Extra Work’: Resident Attitudes About Quality Improvement and Implications for an Effective Learning Health Care System.” I read on, and it got worse.
“This hasn’t really made any difference to the patients. Like this checklist we do on rounds, like I don’t know. Maybe it has.”
And, by far, most concerning: “There’s like the central line protocols … If you suspect that anybody has any type of bacteremia, you don’t do a blood culture; you just do a urine culture and pull the lines … we just don’t even test for it because the quality improvement then like marks you off.”
Wow.
That is some harsh truth about unintended consequences right there. (Also, apparently us kids of the 1990s still say “like” a lot, which is, like, not very professional and also like kinda grating.)
The residents in this study were from the University of Utah, Salt Lake City – an institution I frequently– and publicly – admire for their incredible progress on systematically introducing value improvement into their practice.
What can we do?
Read the full post at hospitalleader.org.
Also on The Hospital Leader…
- THIS Is What Teamwork Looks Like, by Danielle Scheurer, MD, MSCR, SFHM
- The Medicaid Overhaul and How Hospitals and Their Providers Could Be Hardest Hit, by Brad Flansbaum, DO, MPH, MHM
- Count Me – and My Intuition – In, by Tracy Cardin, ACNP-BC, SFHM
Editor’s note: First published on The Hospital Leader blog under the title, “How I Realized QI Could Be a Dirty Word.”
With the recent election, there has been a new recognition of the various “bubbles” we all seem to be living in. It reminds me of the parable I like to often mention, popularized by the late great writer David Foster Wallace: Two fish were swimming along when an older fish swam by, nodded his head at them and said, “Mornin’ boys, how’s the water?” The two young fish nod back and swim for a bit, then one turns to the other and says, “What the hell is water?”
Imagine the hard reality that hit me when I read this quote from a resident: “Truly, the first thing I think of when I hear [QI] is going to make more work for residents.”
Wait – is QI actually a dirty word for other residents and physicians?
The quote comes from an Academic Medicine study titled “ ‘It Feels Like a Lot of Extra Work’: Resident Attitudes About Quality Improvement and Implications for an Effective Learning Health Care System.” I read on, and it got worse.
“This hasn’t really made any difference to the patients. Like this checklist we do on rounds, like I don’t know. Maybe it has.”
And, by far, most concerning: “There’s like the central line protocols … If you suspect that anybody has any type of bacteremia, you don’t do a blood culture; you just do a urine culture and pull the lines … we just don’t even test for it because the quality improvement then like marks you off.”
Wow.
That is some harsh truth about unintended consequences right there. (Also, apparently us kids of the 1990s still say “like” a lot, which is, like, not very professional and also like kinda grating.)
The residents in this study were from the University of Utah, Salt Lake City – an institution I frequently– and publicly – admire for their incredible progress on systematically introducing value improvement into their practice.
What can we do?
Read the full post at hospitalleader.org.
Also on The Hospital Leader…
- THIS Is What Teamwork Looks Like, by Danielle Scheurer, MD, MSCR, SFHM
- The Medicaid Overhaul and How Hospitals and Their Providers Could Be Hardest Hit, by Brad Flansbaum, DO, MPH, MHM
- Count Me – and My Intuition – In, by Tracy Cardin, ACNP-BC, SFHM
Editor’s note: First published on The Hospital Leader blog under the title, “How I Realized QI Could Be a Dirty Word.”
With the recent election, there has been a new recognition of the various “bubbles” we all seem to be living in. It reminds me of the parable I like to often mention, popularized by the late great writer David Foster Wallace: Two fish were swimming along when an older fish swam by, nodded his head at them and said, “Mornin’ boys, how’s the water?” The two young fish nod back and swim for a bit, then one turns to the other and says, “What the hell is water?”
Imagine the hard reality that hit me when I read this quote from a resident: “Truly, the first thing I think of when I hear [QI] is going to make more work for residents.”
Wait – is QI actually a dirty word for other residents and physicians?
The quote comes from an Academic Medicine study titled “ ‘It Feels Like a Lot of Extra Work’: Resident Attitudes About Quality Improvement and Implications for an Effective Learning Health Care System.” I read on, and it got worse.
“This hasn’t really made any difference to the patients. Like this checklist we do on rounds, like I don’t know. Maybe it has.”
And, by far, most concerning: “There’s like the central line protocols … If you suspect that anybody has any type of bacteremia, you don’t do a blood culture; you just do a urine culture and pull the lines … we just don’t even test for it because the quality improvement then like marks you off.”
Wow.
That is some harsh truth about unintended consequences right there. (Also, apparently us kids of the 1990s still say “like” a lot, which is, like, not very professional and also like kinda grating.)
The residents in this study were from the University of Utah, Salt Lake City – an institution I frequently– and publicly – admire for their incredible progress on systematically introducing value improvement into their practice.
What can we do?
Read the full post at hospitalleader.org.
Also on The Hospital Leader…
- THIS Is What Teamwork Looks Like, by Danielle Scheurer, MD, MSCR, SFHM
- The Medicaid Overhaul and How Hospitals and Their Providers Could Be Hardest Hit, by Brad Flansbaum, DO, MPH, MHM
- Count Me – and My Intuition – In, by Tracy Cardin, ACNP-BC, SFHM
Pediatric Dermatology Consult - February 2017
Diagnosing CARP
BY CATALINA MATIZ, MD, AND ANDREA WALDMAN, MD
Frontline Medical News
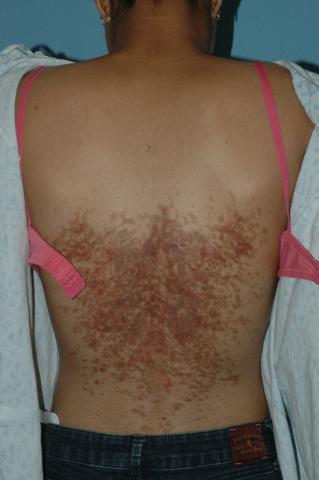
The distribution and morphology of our patient’s cutaneous eruption was highly suggestive of confluent and reticulated papillomatosis (CARP) of Gougerot and Carteaud, a rare dermatologic disorder characterized by epidermal changes (hyperkeratosis, scaling) and hyperpigmentation. The lesions classically begin as brown or red hyperpigmented hyperkeratotic or verrucous papules that spread centrifugally, coalescing into confluent plaques with reticulation at the periphery.1 CARP also may manifest as shiny papules or atrophic macules.1 Reports of scaly papules with rippling also are documented in the literature.2 Characteristically, the lesions frequently involve the inframammary region and the posterior trunk. Other less common sites of presentation include the abdomen, neck, axilla, face, and shoulders.1
Pathogenically, CARP results from disordered keratinization of the epidermis and increased melanosomes within the stratum corneum. The condition is chronic in nature and often intermittent, with a remitting-relapsing course. CARP is limited to cutaneous involvement, with no systemic manifestations. The lesions are typically asymptomatic; however, some individuals complain of mild pruritus.1 Primarily seen in adolescent patients, this dermatitis afflicts females twice as often as males, and occurs in all races.1
Several hypotheses concerning the etiology of CARP have been suggested in the literature; however, the definitive pathophysiology remains unknown. Suggested causes include defective keratinization, and infectious, endocrine, genetic, and environmental etiologies.3 In light of its clinical similarity to tinea versicolor and occasional clinical response to antifungal therapies, Malassezia has been postulated as a potential etiology for CARP. Potassium hydroxide examinations of the skin for fungal species are negative in most patients, and thus, this suggestion remains highly controversial.1 More convincing infectious culprits implicated include bacterial species such as Rhodococcus and Dietzia, papillomatosis previously isolated from skin scrapings of CARP patients. This is further supported by the successful treatment of CARP with antibiotic therapy.4 A highly controversial association between CARP and endocrine abnormalities such as insulin resistance and thyroid disease is well documented in the literature. Acanthosis nigricans and obesity may present concurrently with CARP as well; however, in most cases this association does not occur.3
The diagnosis of CARP is primarily clinical, formulated based on the presence of cutaneous lesions with the characteristic distribution and morphology. Biopsy and histopathology often are utilized to exclude other diagnoses. Davis et al. previously proposed the following five diagnostic criteria for CARP: involvement of the upper trunk and neck, clinical findings of scaly brown macules and patches (at least a portion of which appear reticulated and papillomatous), negative fungal staining of lesions, lack of response to antifungal therapy, and excellent response to minocycline therapy.5 These diagnostic criteria were validated by a retrospective analysis of CARP patients in Singapore.6
An extensive variety of cutaneous conditions bearing a resemblance to CARP were considered in the differential diagnosis, including acanthosis nigricans (AN), tinea versicolor, Darier disease, terra firma-forme dermatosis, prurigo pigmentosa, flagellate dermatosis, and dyskeratosis congenita.1 A common challenge for practitioners is distinguishing CARP from similar dermatoses, particularly AN. Differentiating AN from CARP cannot be accomplished based on the history of insulin resistance or increased body mass index alone, as these comorbidities may coexist with CARP.3 The clinical presence of reticulation peripherally, combined with a positive response to minocycline or azithromycin therapy, distinguish CARP from AN in most cases.
Other disorders commonly presenting in a similar distribution to CARP were further excluded based on the morphologic appearance of the lesions and further testing. Tinea versicolor was unlikely, because of the lack of response to antifungal agents and the absence of organisms on KOH examination. Furthermore, the morphologic characteristics of our patient’s lesions were inconsistent with the typical appearance of tinea versicolor – hypopigmented or hyperpigmented patches with overlying scale. Reticulated hyperpigmentation without papules or plaques may occur in dyskeratosis congenita.1 If the patient presents with reticulated hyperpigmentation in addition to pruritus, prurigo pigmentosa may be considered in the differential.4 The presentation of slightly verrucous “dirtlike” plaques refractory to cleansing with soap and water alone, should prompt the consideration of terra firma-forme dermatosis. Terra firma-forme dermatosis, a benign disorder of keratinization, may be confirmed at the bedside upon the removal of the plaques with a 70% alcohol swab.7 Flagellate erythema, characterized by the presence of linear streaks of erythema or brown pigmentation, often presents in a similar distribution to CARP. This condition occurs mostly in individuals treated with bleomycin or other chemotherapeutic agents, and spontaneously resolves.1 Darier disease classically presents as hyperkeratotic papules coalescing into plaques in seborrheic areas, and may manifest in the same distribution as CARP. Palmoplantar pits and nail changes (blue-red striations and distal V-shaped nicking) may be present in the former.1
A variety of treatments for CARP are documented in the literature, including antibiotic therapy with minocycline or azithromycin, topical salicylic acid, urea or lactic acid–containing emollients, and oral or topical retinoids.1,3 A substantial proportion of patients respond to oral minocycline (100 mg twice daily). At the time of initial evaluation, our patient was prescribed a 6-week course of systemic minocycline (100 mg twice daily), with subsequent improvement.
References
1. Am J Clin Dermatol. 2006;7(5):305-13.
2. Postepy Dermatol Alergol. 2014 Oct;31(5):335-7.
4. Br J Dermatol. 2006 Feb;154(2):287-93.
5. Ann Dermatol. 2014 Jun;26(3):409-10.
6. Am J Clin Dermatol. 2015 Apr;16(2):131-6.
7. Clinical Experiment Dermatol. 2012;37(4):446-7.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego, University of California, San Diego. Dr. Waldman is a clinical research fellow at the hospital. Dr. Matiz and Dr. Waldman said they had no relevant financial disclosures.
Diagnosing CARP
BY CATALINA MATIZ, MD, AND ANDREA WALDMAN, MD
Frontline Medical News
The distribution and morphology of our patient’s cutaneous eruption was highly suggestive of confluent and reticulated papillomatosis (CARP) of Gougerot and Carteaud, a rare dermatologic disorder characterized by epidermal changes (hyperkeratosis, scaling) and hyperpigmentation. The lesions classically begin as brown or red hyperpigmented hyperkeratotic or verrucous papules that spread centrifugally, coalescing into confluent plaques with reticulation at the periphery.1 CARP also may manifest as shiny papules or atrophic macules.1 Reports of scaly papules with rippling also are documented in the literature.2 Characteristically, the lesions frequently involve the inframammary region and the posterior trunk. Other less common sites of presentation include the abdomen, neck, axilla, face, and shoulders.1
Pathogenically, CARP results from disordered keratinization of the epidermis and increased melanosomes within the stratum corneum. The condition is chronic in nature and often intermittent, with a remitting-relapsing course. CARP is limited to cutaneous involvement, with no systemic manifestations. The lesions are typically asymptomatic; however, some individuals complain of mild pruritus.1 Primarily seen in adolescent patients, this dermatitis afflicts females twice as often as males, and occurs in all races.1
Several hypotheses concerning the etiology of CARP have been suggested in the literature; however, the definitive pathophysiology remains unknown. Suggested causes include defective keratinization, and infectious, endocrine, genetic, and environmental etiologies.3 In light of its clinical similarity to tinea versicolor and occasional clinical response to antifungal therapies, Malassezia has been postulated as a potential etiology for CARP. Potassium hydroxide examinations of the skin for fungal species are negative in most patients, and thus, this suggestion remains highly controversial.1 More convincing infectious culprits implicated include bacterial species such as Rhodococcus and Dietzia, papillomatosis previously isolated from skin scrapings of CARP patients. This is further supported by the successful treatment of CARP with antibiotic therapy.4 A highly controversial association between CARP and endocrine abnormalities such as insulin resistance and thyroid disease is well documented in the literature. Acanthosis nigricans and obesity may present concurrently with CARP as well; however, in most cases this association does not occur.3
The diagnosis of CARP is primarily clinical, formulated based on the presence of cutaneous lesions with the characteristic distribution and morphology. Biopsy and histopathology often are utilized to exclude other diagnoses. Davis et al. previously proposed the following five diagnostic criteria for CARP: involvement of the upper trunk and neck, clinical findings of scaly brown macules and patches (at least a portion of which appear reticulated and papillomatous), negative fungal staining of lesions, lack of response to antifungal therapy, and excellent response to minocycline therapy.5 These diagnostic criteria were validated by a retrospective analysis of CARP patients in Singapore.6
An extensive variety of cutaneous conditions bearing a resemblance to CARP were considered in the differential diagnosis, including acanthosis nigricans (AN), tinea versicolor, Darier disease, terra firma-forme dermatosis, prurigo pigmentosa, flagellate dermatosis, and dyskeratosis congenita.1 A common challenge for practitioners is distinguishing CARP from similar dermatoses, particularly AN. Differentiating AN from CARP cannot be accomplished based on the history of insulin resistance or increased body mass index alone, as these comorbidities may coexist with CARP.3 The clinical presence of reticulation peripherally, combined with a positive response to minocycline or azithromycin therapy, distinguish CARP from AN in most cases.
Other disorders commonly presenting in a similar distribution to CARP were further excluded based on the morphologic appearance of the lesions and further testing. Tinea versicolor was unlikely, because of the lack of response to antifungal agents and the absence of organisms on KOH examination. Furthermore, the morphologic characteristics of our patient’s lesions were inconsistent with the typical appearance of tinea versicolor – hypopigmented or hyperpigmented patches with overlying scale. Reticulated hyperpigmentation without papules or plaques may occur in dyskeratosis congenita.1 If the patient presents with reticulated hyperpigmentation in addition to pruritus, prurigo pigmentosa may be considered in the differential.4 The presentation of slightly verrucous “dirtlike” plaques refractory to cleansing with soap and water alone, should prompt the consideration of terra firma-forme dermatosis. Terra firma-forme dermatosis, a benign disorder of keratinization, may be confirmed at the bedside upon the removal of the plaques with a 70% alcohol swab.7 Flagellate erythema, characterized by the presence of linear streaks of erythema or brown pigmentation, often presents in a similar distribution to CARP. This condition occurs mostly in individuals treated with bleomycin or other chemotherapeutic agents, and spontaneously resolves.1 Darier disease classically presents as hyperkeratotic papules coalescing into plaques in seborrheic areas, and may manifest in the same distribution as CARP. Palmoplantar pits and nail changes (blue-red striations and distal V-shaped nicking) may be present in the former.1
A variety of treatments for CARP are documented in the literature, including antibiotic therapy with minocycline or azithromycin, topical salicylic acid, urea or lactic acid–containing emollients, and oral or topical retinoids.1,3 A substantial proportion of patients respond to oral minocycline (100 mg twice daily). At the time of initial evaluation, our patient was prescribed a 6-week course of systemic minocycline (100 mg twice daily), with subsequent improvement.
References
1. Am J Clin Dermatol. 2006;7(5):305-13.
2. Postepy Dermatol Alergol. 2014 Oct;31(5):335-7.
4. Br J Dermatol. 2006 Feb;154(2):287-93.
5. Ann Dermatol. 2014 Jun;26(3):409-10.
6. Am J Clin Dermatol. 2015 Apr;16(2):131-6.
7. Clinical Experiment Dermatol. 2012;37(4):446-7.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego, University of California, San Diego. Dr. Waldman is a clinical research fellow at the hospital. Dr. Matiz and Dr. Waldman said they had no relevant financial disclosures.
Diagnosing CARP
BY CATALINA MATIZ, MD, AND ANDREA WALDMAN, MD
Frontline Medical News
The distribution and morphology of our patient’s cutaneous eruption was highly suggestive of confluent and reticulated papillomatosis (CARP) of Gougerot and Carteaud, a rare dermatologic disorder characterized by epidermal changes (hyperkeratosis, scaling) and hyperpigmentation. The lesions classically begin as brown or red hyperpigmented hyperkeratotic or verrucous papules that spread centrifugally, coalescing into confluent plaques with reticulation at the periphery.1 CARP also may manifest as shiny papules or atrophic macules.1 Reports of scaly papules with rippling also are documented in the literature.2 Characteristically, the lesions frequently involve the inframammary region and the posterior trunk. Other less common sites of presentation include the abdomen, neck, axilla, face, and shoulders.1
Pathogenically, CARP results from disordered keratinization of the epidermis and increased melanosomes within the stratum corneum. The condition is chronic in nature and often intermittent, with a remitting-relapsing course. CARP is limited to cutaneous involvement, with no systemic manifestations. The lesions are typically asymptomatic; however, some individuals complain of mild pruritus.1 Primarily seen in adolescent patients, this dermatitis afflicts females twice as often as males, and occurs in all races.1
Several hypotheses concerning the etiology of CARP have been suggested in the literature; however, the definitive pathophysiology remains unknown. Suggested causes include defective keratinization, and infectious, endocrine, genetic, and environmental etiologies.3 In light of its clinical similarity to tinea versicolor and occasional clinical response to antifungal therapies, Malassezia has been postulated as a potential etiology for CARP. Potassium hydroxide examinations of the skin for fungal species are negative in most patients, and thus, this suggestion remains highly controversial.1 More convincing infectious culprits implicated include bacterial species such as Rhodococcus and Dietzia, papillomatosis previously isolated from skin scrapings of CARP patients. This is further supported by the successful treatment of CARP with antibiotic therapy.4 A highly controversial association between CARP and endocrine abnormalities such as insulin resistance and thyroid disease is well documented in the literature. Acanthosis nigricans and obesity may present concurrently with CARP as well; however, in most cases this association does not occur.3
The diagnosis of CARP is primarily clinical, formulated based on the presence of cutaneous lesions with the characteristic distribution and morphology. Biopsy and histopathology often are utilized to exclude other diagnoses. Davis et al. previously proposed the following five diagnostic criteria for CARP: involvement of the upper trunk and neck, clinical findings of scaly brown macules and patches (at least a portion of which appear reticulated and papillomatous), negative fungal staining of lesions, lack of response to antifungal therapy, and excellent response to minocycline therapy.5 These diagnostic criteria were validated by a retrospective analysis of CARP patients in Singapore.6
An extensive variety of cutaneous conditions bearing a resemblance to CARP were considered in the differential diagnosis, including acanthosis nigricans (AN), tinea versicolor, Darier disease, terra firma-forme dermatosis, prurigo pigmentosa, flagellate dermatosis, and dyskeratosis congenita.1 A common challenge for practitioners is distinguishing CARP from similar dermatoses, particularly AN. Differentiating AN from CARP cannot be accomplished based on the history of insulin resistance or increased body mass index alone, as these comorbidities may coexist with CARP.3 The clinical presence of reticulation peripherally, combined with a positive response to minocycline or azithromycin therapy, distinguish CARP from AN in most cases.
Other disorders commonly presenting in a similar distribution to CARP were further excluded based on the morphologic appearance of the lesions and further testing. Tinea versicolor was unlikely, because of the lack of response to antifungal agents and the absence of organisms on KOH examination. Furthermore, the morphologic characteristics of our patient’s lesions were inconsistent with the typical appearance of tinea versicolor – hypopigmented or hyperpigmented patches with overlying scale. Reticulated hyperpigmentation without papules or plaques may occur in dyskeratosis congenita.1 If the patient presents with reticulated hyperpigmentation in addition to pruritus, prurigo pigmentosa may be considered in the differential.4 The presentation of slightly verrucous “dirtlike” plaques refractory to cleansing with soap and water alone, should prompt the consideration of terra firma-forme dermatosis. Terra firma-forme dermatosis, a benign disorder of keratinization, may be confirmed at the bedside upon the removal of the plaques with a 70% alcohol swab.7 Flagellate erythema, characterized by the presence of linear streaks of erythema or brown pigmentation, often presents in a similar distribution to CARP. This condition occurs mostly in individuals treated with bleomycin or other chemotherapeutic agents, and spontaneously resolves.1 Darier disease classically presents as hyperkeratotic papules coalescing into plaques in seborrheic areas, and may manifest in the same distribution as CARP. Palmoplantar pits and nail changes (blue-red striations and distal V-shaped nicking) may be present in the former.1
A variety of treatments for CARP are documented in the literature, including antibiotic therapy with minocycline or azithromycin, topical salicylic acid, urea or lactic acid–containing emollients, and oral or topical retinoids.1,3 A substantial proportion of patients respond to oral minocycline (100 mg twice daily). At the time of initial evaluation, our patient was prescribed a 6-week course of systemic minocycline (100 mg twice daily), with subsequent improvement.
References
1. Am J Clin Dermatol. 2006;7(5):305-13.
2. Postepy Dermatol Alergol. 2014 Oct;31(5):335-7.
4. Br J Dermatol. 2006 Feb;154(2):287-93.
5. Ann Dermatol. 2014 Jun;26(3):409-10.
6. Am J Clin Dermatol. 2015 Apr;16(2):131-6.
7. Clinical Experiment Dermatol. 2012;37(4):446-7.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego, University of California, San Diego. Dr. Waldman is a clinical research fellow at the hospital. Dr. Matiz and Dr. Waldman said they had no relevant financial disclosures.
Clinical presentation
A 14-year-old previously healthy female presents to the dermatology clinic for evaluation of a persistent dermatitis on the trunk and abdomen. The patient reports the development of two distinct lesions covering the back and abdomen 6 months prior to initial presentation. The patient denies pruritus, pain, burning, or xerosis. Shortly after the lesions erupted, the pediatrician diagnosed her with tinea versicolor. The patient’s rash failed to improve over the following 4 months, despite the intermittent use of clotrimazole 1% cream and ketoconazole 2% cream. The patient denies any recent travel or pets residing in the home. The family history is noncontributory.
Physical exam
The patient is a well-appearing nonobese teenage female who is in no acute distress, with normal affect. On skin examination, there are confluent tan-brown papules coalescing into plaques involving the back and abdomen, with a reticulated appearance around the periphery of the plaques. There are no other lesions present on the cutaneous surface. The patient is afebrile, and vital signs are within normal limits.
AGA Clinical Practice Update: Clostridium difficile in IBD
Inflammatory bowel disease (IBD) increases the risk and severity of Clostridium difficile infection (CDI) while CDI tends to complicate and worsen the clinical course of IBD, experts note in a clinical practice update.
Thus, it is crucial that clinicians pursue stool testing for toxigenic C. difficile infection whenever a patient with IBD presents with a colitis flare, regardless of the recent antibiotic history, wrote Sahil Khanna, MBBS, of the Mayo Clinic, Rochester, Minn., and his associates (Clin Gastroenterol Hepatol. 2016 Feb. doi: 10.1016/j.cgh.2016.10.024). Clinicians should also test for recurrent CDI if symptoms of colitis persist or return after antibiotic therapy for CDI, they emphasized.
CDI can present atypically in IBD. Underlying colitis leads to colonic dysbiosis and loss of resistance to bacterial colonization, which permits CDI to develop even when patients have not recently received antibiotics. Patients with IBD also tend to develop CDI starting at younger ages, more often acquire it from community settings, and may lack the typical colonoscopic features of CDI. Simple colonization of C. difficile without infection also is more common in patients with IBD than in those without IBD, the experts note.
The authors contradict guidelines from both the American College of Gastroenterology and Infectious Diseases Society of America by recommending consideration of vancomycin over metronidazole for treatment of CDI. Not only are C. difficile treatment failures with metronidazole rising, but vancomycin was more effective than was metronidazole in a recent post hoc analysis (Clin Infect Dis. 2014;59[3]:345-54) of two large multicenter phase III trials. Furthermore, another phase III trial (N Engl J Med. 2011;364:422-31) found vancomycin noninferior to fidaxomicin for CDI.
The experts recommend “strong consideration” of hospitalization if patients with IBD and CDI present with profuse diarrhea, severe abdominal pain, a markedly increased peripheral blood leukocyte count, or other signs and symptoms of sepsis. Aggressive monitoring and treatment are especially important because it can be difficult to distinguish an IBD flare, which merits immunosuppression, from superimposed CDI, which might exacerbate the underlying infection, they noted. Few studies are available to help guide the decision about when to intensify steroids and other immunosuppressives in IBD patients with acute CDI. Thus, the experts suggest delaying this step until after starting therapy for CDI, but note that this decision should be individualized pending more robust data.
The authors emphasized the potential role of fecal microbiota transplantation (FMT), which has been shown to be very effective in both immunocompetent patients with CDI and those who are immunosuppressed, including because of IBD therapies. They recommend considering referral for FMT as early as the first recurrence of CDI in patients with IBD, particularly because of the strong safety and efficacy profile of FMT, the risk of complications from CDI in IBD patients, and scarce data on antibiotic therapy for recurrent CDI in the setting of IBD.
Dr. Khanna disclosed consulting relationships with Rebiotix. and Summit Pharmaceuticals. Senior author Ciaran P. Kelly, MD, disclosed serving as a consultant to Merck, Seres Therapeutics, Summit Pharmaceuticals, and Takeda Pharmaceuticals. The third author, Andrea Shin, MD, had no relevant disclosures.
Inflammatory bowel disease (IBD) increases the risk and severity of Clostridium difficile infection (CDI) while CDI tends to complicate and worsen the clinical course of IBD, experts note in a clinical practice update.
Thus, it is crucial that clinicians pursue stool testing for toxigenic C. difficile infection whenever a patient with IBD presents with a colitis flare, regardless of the recent antibiotic history, wrote Sahil Khanna, MBBS, of the Mayo Clinic, Rochester, Minn., and his associates (Clin Gastroenterol Hepatol. 2016 Feb. doi: 10.1016/j.cgh.2016.10.024). Clinicians should also test for recurrent CDI if symptoms of colitis persist or return after antibiotic therapy for CDI, they emphasized.
CDI can present atypically in IBD. Underlying colitis leads to colonic dysbiosis and loss of resistance to bacterial colonization, which permits CDI to develop even when patients have not recently received antibiotics. Patients with IBD also tend to develop CDI starting at younger ages, more often acquire it from community settings, and may lack the typical colonoscopic features of CDI. Simple colonization of C. difficile without infection also is more common in patients with IBD than in those without IBD, the experts note.
The authors contradict guidelines from both the American College of Gastroenterology and Infectious Diseases Society of America by recommending consideration of vancomycin over metronidazole for treatment of CDI. Not only are C. difficile treatment failures with metronidazole rising, but vancomycin was more effective than was metronidazole in a recent post hoc analysis (Clin Infect Dis. 2014;59[3]:345-54) of two large multicenter phase III trials. Furthermore, another phase III trial (N Engl J Med. 2011;364:422-31) found vancomycin noninferior to fidaxomicin for CDI.
The experts recommend “strong consideration” of hospitalization if patients with IBD and CDI present with profuse diarrhea, severe abdominal pain, a markedly increased peripheral blood leukocyte count, or other signs and symptoms of sepsis. Aggressive monitoring and treatment are especially important because it can be difficult to distinguish an IBD flare, which merits immunosuppression, from superimposed CDI, which might exacerbate the underlying infection, they noted. Few studies are available to help guide the decision about when to intensify steroids and other immunosuppressives in IBD patients with acute CDI. Thus, the experts suggest delaying this step until after starting therapy for CDI, but note that this decision should be individualized pending more robust data.
The authors emphasized the potential role of fecal microbiota transplantation (FMT), which has been shown to be very effective in both immunocompetent patients with CDI and those who are immunosuppressed, including because of IBD therapies. They recommend considering referral for FMT as early as the first recurrence of CDI in patients with IBD, particularly because of the strong safety and efficacy profile of FMT, the risk of complications from CDI in IBD patients, and scarce data on antibiotic therapy for recurrent CDI in the setting of IBD.
Dr. Khanna disclosed consulting relationships with Rebiotix. and Summit Pharmaceuticals. Senior author Ciaran P. Kelly, MD, disclosed serving as a consultant to Merck, Seres Therapeutics, Summit Pharmaceuticals, and Takeda Pharmaceuticals. The third author, Andrea Shin, MD, had no relevant disclosures.
Inflammatory bowel disease (IBD) increases the risk and severity of Clostridium difficile infection (CDI) while CDI tends to complicate and worsen the clinical course of IBD, experts note in a clinical practice update.
Thus, it is crucial that clinicians pursue stool testing for toxigenic C. difficile infection whenever a patient with IBD presents with a colitis flare, regardless of the recent antibiotic history, wrote Sahil Khanna, MBBS, of the Mayo Clinic, Rochester, Minn., and his associates (Clin Gastroenterol Hepatol. 2016 Feb. doi: 10.1016/j.cgh.2016.10.024). Clinicians should also test for recurrent CDI if symptoms of colitis persist or return after antibiotic therapy for CDI, they emphasized.
CDI can present atypically in IBD. Underlying colitis leads to colonic dysbiosis and loss of resistance to bacterial colonization, which permits CDI to develop even when patients have not recently received antibiotics. Patients with IBD also tend to develop CDI starting at younger ages, more often acquire it from community settings, and may lack the typical colonoscopic features of CDI. Simple colonization of C. difficile without infection also is more common in patients with IBD than in those without IBD, the experts note.
The authors contradict guidelines from both the American College of Gastroenterology and Infectious Diseases Society of America by recommending consideration of vancomycin over metronidazole for treatment of CDI. Not only are C. difficile treatment failures with metronidazole rising, but vancomycin was more effective than was metronidazole in a recent post hoc analysis (Clin Infect Dis. 2014;59[3]:345-54) of two large multicenter phase III trials. Furthermore, another phase III trial (N Engl J Med. 2011;364:422-31) found vancomycin noninferior to fidaxomicin for CDI.
The experts recommend “strong consideration” of hospitalization if patients with IBD and CDI present with profuse diarrhea, severe abdominal pain, a markedly increased peripheral blood leukocyte count, or other signs and symptoms of sepsis. Aggressive monitoring and treatment are especially important because it can be difficult to distinguish an IBD flare, which merits immunosuppression, from superimposed CDI, which might exacerbate the underlying infection, they noted. Few studies are available to help guide the decision about when to intensify steroids and other immunosuppressives in IBD patients with acute CDI. Thus, the experts suggest delaying this step until after starting therapy for CDI, but note that this decision should be individualized pending more robust data.
The authors emphasized the potential role of fecal microbiota transplantation (FMT), which has been shown to be very effective in both immunocompetent patients with CDI and those who are immunosuppressed, including because of IBD therapies. They recommend considering referral for FMT as early as the first recurrence of CDI in patients with IBD, particularly because of the strong safety and efficacy profile of FMT, the risk of complications from CDI in IBD patients, and scarce data on antibiotic therapy for recurrent CDI in the setting of IBD.
Dr. Khanna disclosed consulting relationships with Rebiotix. and Summit Pharmaceuticals. Senior author Ciaran P. Kelly, MD, disclosed serving as a consultant to Merck, Seres Therapeutics, Summit Pharmaceuticals, and Takeda Pharmaceuticals. The third author, Andrea Shin, MD, had no relevant disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA Guideline: Acute liver failure
Physicians should avoid routinely testing patients with acute liver failure for Wilson’s disease unless there is “high clinical suspicion” for the disorder, according to a new guideline from the AGA Institute.
Wilson’s disease so rarely accompanies acute liver failure that a positive test will have low predictive value, Steven L. Flamm, MD, of Northwestern University, Chicago, and his associates wrote in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.12.026). Diagnosing Wilson’s disease also is unlikely to change treatment “because liver transplantation is the ultimate outcome,” they emphasize.
The guideline grades seven recommendations as “conditional” based on “very-low” quality evidence. These include the statement on Wilson’s disease testing, plus suggestions to test and treat patients with ALF for herpes simplex virus (HSV) infection, to test pregnant patients for hepatitis E virus infection, and to perform autoantibody testing for autoimmune hepatitis. Case series report only about a 1% prevalence of HSV infection in ALF, and there is little information on diagnostic accuracy or treatment in this setting, the guidelines state. Although acyclovir is relatively safe and inexpensive, data on efficacy is limited to “a suggestion on a case-report level that patients with acute hepatitis secondary to HSV do better with treatment than without.”
The guideline also conditionally recommends against routine testing for varicella zoster virus infection and routine liver biopsy in ALF. The authors note only about 10 case reports of varicella zoster–associated ALF and few data on how liver biopsy results in ALF alter treatment plan, outcome, or the choice to seek liver transplantation. The experts do recommend prognostic scoring with Model for End-Stage Liver Disease, which pooled analyses have found to be more sensitive than King’s College Criteria, they wrote.
The guideline conditionally recommends against empirically treating elevated intracranial pressure in ALF, on the basis of five randomized trials that found no overall mortality benefit of moderate hypothermia, hypertonic saline, L-ornithine, L-aspartate, intravenous mannitol, or hyperventilation.
The experts cite insufficient evidence to recommend using N-acetyl cysteine in patients whose ALF is not associated with acetaminophen exposure. Likewise, they find inadequate data to make any recommendation about using extracorporeal liver support systems outside of the setting of clinical trials. Although such systems can “potentially” buy time for patients to either spontaneously recover without transplant or survive longer on the transplantation list, three systematic reviews found “no clear effect on mortality,” and randomized trials reported either null results or a “marginally significant survival benefit” in the face of steep costs and potentially significant toxicities, the authors emphasize.
None of the experts had relevant financial disclosures.
Physicians should avoid routinely testing patients with acute liver failure for Wilson’s disease unless there is “high clinical suspicion” for the disorder, according to a new guideline from the AGA Institute.
Wilson’s disease so rarely accompanies acute liver failure that a positive test will have low predictive value, Steven L. Flamm, MD, of Northwestern University, Chicago, and his associates wrote in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.12.026). Diagnosing Wilson’s disease also is unlikely to change treatment “because liver transplantation is the ultimate outcome,” they emphasize.
The guideline grades seven recommendations as “conditional” based on “very-low” quality evidence. These include the statement on Wilson’s disease testing, plus suggestions to test and treat patients with ALF for herpes simplex virus (HSV) infection, to test pregnant patients for hepatitis E virus infection, and to perform autoantibody testing for autoimmune hepatitis. Case series report only about a 1% prevalence of HSV infection in ALF, and there is little information on diagnostic accuracy or treatment in this setting, the guidelines state. Although acyclovir is relatively safe and inexpensive, data on efficacy is limited to “a suggestion on a case-report level that patients with acute hepatitis secondary to HSV do better with treatment than without.”
The guideline also conditionally recommends against routine testing for varicella zoster virus infection and routine liver biopsy in ALF. The authors note only about 10 case reports of varicella zoster–associated ALF and few data on how liver biopsy results in ALF alter treatment plan, outcome, or the choice to seek liver transplantation. The experts do recommend prognostic scoring with Model for End-Stage Liver Disease, which pooled analyses have found to be more sensitive than King’s College Criteria, they wrote.
The guideline conditionally recommends against empirically treating elevated intracranial pressure in ALF, on the basis of five randomized trials that found no overall mortality benefit of moderate hypothermia, hypertonic saline, L-ornithine, L-aspartate, intravenous mannitol, or hyperventilation.
The experts cite insufficient evidence to recommend using N-acetyl cysteine in patients whose ALF is not associated with acetaminophen exposure. Likewise, they find inadequate data to make any recommendation about using extracorporeal liver support systems outside of the setting of clinical trials. Although such systems can “potentially” buy time for patients to either spontaneously recover without transplant or survive longer on the transplantation list, three systematic reviews found “no clear effect on mortality,” and randomized trials reported either null results or a “marginally significant survival benefit” in the face of steep costs and potentially significant toxicities, the authors emphasize.
None of the experts had relevant financial disclosures.
Physicians should avoid routinely testing patients with acute liver failure for Wilson’s disease unless there is “high clinical suspicion” for the disorder, according to a new guideline from the AGA Institute.
Wilson’s disease so rarely accompanies acute liver failure that a positive test will have low predictive value, Steven L. Flamm, MD, of Northwestern University, Chicago, and his associates wrote in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.12.026). Diagnosing Wilson’s disease also is unlikely to change treatment “because liver transplantation is the ultimate outcome,” they emphasize.
The guideline grades seven recommendations as “conditional” based on “very-low” quality evidence. These include the statement on Wilson’s disease testing, plus suggestions to test and treat patients with ALF for herpes simplex virus (HSV) infection, to test pregnant patients for hepatitis E virus infection, and to perform autoantibody testing for autoimmune hepatitis. Case series report only about a 1% prevalence of HSV infection in ALF, and there is little information on diagnostic accuracy or treatment in this setting, the guidelines state. Although acyclovir is relatively safe and inexpensive, data on efficacy is limited to “a suggestion on a case-report level that patients with acute hepatitis secondary to HSV do better with treatment than without.”
The guideline also conditionally recommends against routine testing for varicella zoster virus infection and routine liver biopsy in ALF. The authors note only about 10 case reports of varicella zoster–associated ALF and few data on how liver biopsy results in ALF alter treatment plan, outcome, or the choice to seek liver transplantation. The experts do recommend prognostic scoring with Model for End-Stage Liver Disease, which pooled analyses have found to be more sensitive than King’s College Criteria, they wrote.
The guideline conditionally recommends against empirically treating elevated intracranial pressure in ALF, on the basis of five randomized trials that found no overall mortality benefit of moderate hypothermia, hypertonic saline, L-ornithine, L-aspartate, intravenous mannitol, or hyperventilation.
The experts cite insufficient evidence to recommend using N-acetyl cysteine in patients whose ALF is not associated with acetaminophen exposure. Likewise, they find inadequate data to make any recommendation about using extracorporeal liver support systems outside of the setting of clinical trials. Although such systems can “potentially” buy time for patients to either spontaneously recover without transplant or survive longer on the transplantation list, three systematic reviews found “no clear effect on mortality,” and randomized trials reported either null results or a “marginally significant survival benefit” in the face of steep costs and potentially significant toxicities, the authors emphasize.
None of the experts had relevant financial disclosures.
FROM GASTROENTEROLOGY
Trump nominates Neil Gorsuch as 9th Supreme Court justice
President Donald Trump has chosen Neil Gorsuch, a conservative judge who presides over Denver’s 10th Circuit as his nominee for U.S. Supreme Court justice. A federal judge for 10 years, Judge Gorsuch is a long-time comrade of deceased Supreme Court Justice Antonin Scalia with a strong record of supporting religious freedom and less government control.
At a White House East Room ceremony on Jan. 31, Judge Gorsuch said he was honored and humbled by President’s Trump’s nomination and that he looked forward to answering questions during his Senate nomination hearing.
President Trump hailed Judge Gorsuch’s credentials as the “best he has ever seen,” and called him more than qualified to take the reins as the next Supreme Court justice.
“He has an extraordinary resume, as good as it gets,” President Trump said. “The qualifications of Judge Gorsuch are beyond dispute. He is a man of our country and a man who our country needs – and needs badly – to uphold the rule of law and the rule of justice.”
Judge Gorsuch, 49,was appointed to the 10th U.S. Circuit Court of Appeals in Denver, by President George W. Bush. His nomination was confirmed unanimously in the Senate. He holds a doctoral degree from Oxford University, (England), a law degree from Harvard Law School, Cambridge, Mass., and an undergraduate degree from Columbia University, New York. Judge Gorsuch began his legal career as a law clerk to Supreme Court justices Byron R. White and Anthony M. Kennedy, as well as to Judge David B. Sentelle of the U.S. Court of Appeals for the D.C. Circuit. Prior to his judicial appointment, Judge Gorsuch served as principal deputy associate attorney general at the Justice Department under George W. Bush.

A Senate confirmation hearing had not been announced at press time. Judge Gorsuch has pledged to work with both parties during the hearing to answer questions and alleviate any concerns.
“I look forward to speaking with members from both sides of the aisle,” he said. “I consider the United States Senate the greatest deliberative body in the world, and I respect the important role the Constitution affords it in the confirmation of our judges.”
Justice Antonin Scalia died suddenly on Feb. 13, 2016, leaving the high court with only eight members. Then-President Barack Obama nominated Judge Merrick Garland of the U.S. Court of Appeals for the District of Columbia Circuit to fill Scalia’s seat. However, the Republican-controlled Senate blocked Judge Garland from ever having a hearing and his nomination was never fully considered.
[email protected]
On Twitter @legal_med
President Donald Trump has chosen Neil Gorsuch, a conservative judge who presides over Denver’s 10th Circuit as his nominee for U.S. Supreme Court justice. A federal judge for 10 years, Judge Gorsuch is a long-time comrade of deceased Supreme Court Justice Antonin Scalia with a strong record of supporting religious freedom and less government control.
At a White House East Room ceremony on Jan. 31, Judge Gorsuch said he was honored and humbled by President’s Trump’s nomination and that he looked forward to answering questions during his Senate nomination hearing.
President Trump hailed Judge Gorsuch’s credentials as the “best he has ever seen,” and called him more than qualified to take the reins as the next Supreme Court justice.
“He has an extraordinary resume, as good as it gets,” President Trump said. “The qualifications of Judge Gorsuch are beyond dispute. He is a man of our country and a man who our country needs – and needs badly – to uphold the rule of law and the rule of justice.”
Judge Gorsuch, 49,was appointed to the 10th U.S. Circuit Court of Appeals in Denver, by President George W. Bush. His nomination was confirmed unanimously in the Senate. He holds a doctoral degree from Oxford University, (England), a law degree from Harvard Law School, Cambridge, Mass., and an undergraduate degree from Columbia University, New York. Judge Gorsuch began his legal career as a law clerk to Supreme Court justices Byron R. White and Anthony M. Kennedy, as well as to Judge David B. Sentelle of the U.S. Court of Appeals for the D.C. Circuit. Prior to his judicial appointment, Judge Gorsuch served as principal deputy associate attorney general at the Justice Department under George W. Bush.

A Senate confirmation hearing had not been announced at press time. Judge Gorsuch has pledged to work with both parties during the hearing to answer questions and alleviate any concerns.
“I look forward to speaking with members from both sides of the aisle,” he said. “I consider the United States Senate the greatest deliberative body in the world, and I respect the important role the Constitution affords it in the confirmation of our judges.”
Justice Antonin Scalia died suddenly on Feb. 13, 2016, leaving the high court with only eight members. Then-President Barack Obama nominated Judge Merrick Garland of the U.S. Court of Appeals for the District of Columbia Circuit to fill Scalia’s seat. However, the Republican-controlled Senate blocked Judge Garland from ever having a hearing and his nomination was never fully considered.
[email protected]
On Twitter @legal_med
President Donald Trump has chosen Neil Gorsuch, a conservative judge who presides over Denver’s 10th Circuit as his nominee for U.S. Supreme Court justice. A federal judge for 10 years, Judge Gorsuch is a long-time comrade of deceased Supreme Court Justice Antonin Scalia with a strong record of supporting religious freedom and less government control.
At a White House East Room ceremony on Jan. 31, Judge Gorsuch said he was honored and humbled by President’s Trump’s nomination and that he looked forward to answering questions during his Senate nomination hearing.
President Trump hailed Judge Gorsuch’s credentials as the “best he has ever seen,” and called him more than qualified to take the reins as the next Supreme Court justice.
“He has an extraordinary resume, as good as it gets,” President Trump said. “The qualifications of Judge Gorsuch are beyond dispute. He is a man of our country and a man who our country needs – and needs badly – to uphold the rule of law and the rule of justice.”
Judge Gorsuch, 49,was appointed to the 10th U.S. Circuit Court of Appeals in Denver, by President George W. Bush. His nomination was confirmed unanimously in the Senate. He holds a doctoral degree from Oxford University, (England), a law degree from Harvard Law School, Cambridge, Mass., and an undergraduate degree from Columbia University, New York. Judge Gorsuch began his legal career as a law clerk to Supreme Court justices Byron R. White and Anthony M. Kennedy, as well as to Judge David B. Sentelle of the U.S. Court of Appeals for the D.C. Circuit. Prior to his judicial appointment, Judge Gorsuch served as principal deputy associate attorney general at the Justice Department under George W. Bush.

A Senate confirmation hearing had not been announced at press time. Judge Gorsuch has pledged to work with both parties during the hearing to answer questions and alleviate any concerns.
“I look forward to speaking with members from both sides of the aisle,” he said. “I consider the United States Senate the greatest deliberative body in the world, and I respect the important role the Constitution affords it in the confirmation of our judges.”
Justice Antonin Scalia died suddenly on Feb. 13, 2016, leaving the high court with only eight members. Then-President Barack Obama nominated Judge Merrick Garland of the U.S. Court of Appeals for the District of Columbia Circuit to fill Scalia’s seat. However, the Republican-controlled Senate blocked Judge Garland from ever having a hearing and his nomination was never fully considered.
[email protected]
On Twitter @legal_med
VIDEO: Don’t miss reservoirs when treating recurrent onychomycosis
WAILEA, HAWAII – Patients attribute every nail problem to nail fungus, but part of the problem with nails is concomitant tinea pedis, tinea corporis, and other reservoirs of infection, according to Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego.
Failure to locate and treat these other reservoirs can lead to recurrence of the nail infection, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“If we aren’t treating the skin as well as the nails, it is creating a reservoir effect that reaccumulates in the nail itself,” he said. Similarly, the nails can serve as a reservoir of infection for the skin.
“That makes it all the more important” to treat both skin and nails and “treat through the disease state,” he commented in a video interview. Cost can influence treatment decisions, and there is a role for both topical and systemic therapy, he said. However, “if there’s not adequate testing or if there’s not a good proof of the diagnosis, we are just losing out no matter what we use,” he cautioned.
Dr. Bhatia disclosed relationships with companies including Actavis, Aqua, Allergan, Anacor, Bayer, Biofrontera, Biopharmx, Dermira, Dusa, Exceltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novartis, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Patients attribute every nail problem to nail fungus, but part of the problem with nails is concomitant tinea pedis, tinea corporis, and other reservoirs of infection, according to Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego.
Failure to locate and treat these other reservoirs can lead to recurrence of the nail infection, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“If we aren’t treating the skin as well as the nails, it is creating a reservoir effect that reaccumulates in the nail itself,” he said. Similarly, the nails can serve as a reservoir of infection for the skin.
“That makes it all the more important” to treat both skin and nails and “treat through the disease state,” he commented in a video interview. Cost can influence treatment decisions, and there is a role for both topical and systemic therapy, he said. However, “if there’s not adequate testing or if there’s not a good proof of the diagnosis, we are just losing out no matter what we use,” he cautioned.
Dr. Bhatia disclosed relationships with companies including Actavis, Aqua, Allergan, Anacor, Bayer, Biofrontera, Biopharmx, Dermira, Dusa, Exceltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novartis, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Patients attribute every nail problem to nail fungus, but part of the problem with nails is concomitant tinea pedis, tinea corporis, and other reservoirs of infection, according to Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego.
Failure to locate and treat these other reservoirs can lead to recurrence of the nail infection, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“If we aren’t treating the skin as well as the nails, it is creating a reservoir effect that reaccumulates in the nail itself,” he said. Similarly, the nails can serve as a reservoir of infection for the skin.
“That makes it all the more important” to treat both skin and nails and “treat through the disease state,” he commented in a video interview. Cost can influence treatment decisions, and there is a role for both topical and systemic therapy, he said. However, “if there’s not adequate testing or if there’s not a good proof of the diagnosis, we are just losing out no matter what we use,” he cautioned.
Dr. Bhatia disclosed relationships with companies including Actavis, Aqua, Allergan, Anacor, Bayer, Biofrontera, Biopharmx, Dermira, Dusa, Exceltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novartis, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Dems force delay in vote for HHS secretary
Democrats on the Senate Finance Committee forced a delay action that would have moved the nomination of Rep. Tom Price (R-Ga.) to the Senate floor for consideration, citing ongoing concerns with stock purchases made by Rep. Price.
The vote on Rep. Price’s nomination to serve as secretary of Health and Human Services was scheduled for Jan. 31, but all committee Democrats boycotted the executive session, forcing the delay. Committee rules state that at least 13 members, including at least 1 voting member from the minority party, must be present for a vote to proceed. The 26-member panel is made up of 14 Republicans and 12 Democrats.
“I asked Congressman Price [at his confirmation hearing] directly if he got an exclusive discount on stock in an Australian biomedical firm, and he said no,” Sen. Ron Wyden (D-Ore.), the committee’s leading Democrat, said in a statement. “From the committee’s investigation to company documents to the company official’s own words, the evidence tells a different story. It looks more and more like Congressman Price got special access to a special deal.
“The Finance Committee needs to continue following its bipartisan vetting process that has been upheld for more than 20 years. This is about getting answers to questions, plain and simple. Ethics laws are not optional, and nominees do not have a right to treat disclosures like a shell game,” Sen Wyden said.
At the confirmation hearing, Rep. Price, who is a retired orthopedic surgeon, asserted that he did not violate any ethics laws related to his purchase of Innate stock. He reaffirmed those thoughts in written answers to questions from committee members, stating that throughout “my time as a member of the U.S. House of Representatives, I have abided by and adhered to all ethics and conflicts of interest rules applicable to me.”
Committee Chairman Orrin Hatch (R-Utah) criticized Democrats for their boycott.
The committee was also scheduled to vote on Steve Mnuchin, President Trump’s nominee for Secretary of the Treasury. “Assuming that they can’t support these two, then they can vote against them,” Sen. Hatch said during the open session. “That’s what really an honest approach to this matter would be.”
At press time, a vote on Rep. Price has not been rescheduled, but Sen. Hatch noted that “I am hopeful when we schedule this again, they’ll be here.”
Democrats on the Senate Finance Committee forced a delay action that would have moved the nomination of Rep. Tom Price (R-Ga.) to the Senate floor for consideration, citing ongoing concerns with stock purchases made by Rep. Price.
The vote on Rep. Price’s nomination to serve as secretary of Health and Human Services was scheduled for Jan. 31, but all committee Democrats boycotted the executive session, forcing the delay. Committee rules state that at least 13 members, including at least 1 voting member from the minority party, must be present for a vote to proceed. The 26-member panel is made up of 14 Republicans and 12 Democrats.
“I asked Congressman Price [at his confirmation hearing] directly if he got an exclusive discount on stock in an Australian biomedical firm, and he said no,” Sen. Ron Wyden (D-Ore.), the committee’s leading Democrat, said in a statement. “From the committee’s investigation to company documents to the company official’s own words, the evidence tells a different story. It looks more and more like Congressman Price got special access to a special deal.
“The Finance Committee needs to continue following its bipartisan vetting process that has been upheld for more than 20 years. This is about getting answers to questions, plain and simple. Ethics laws are not optional, and nominees do not have a right to treat disclosures like a shell game,” Sen Wyden said.
At the confirmation hearing, Rep. Price, who is a retired orthopedic surgeon, asserted that he did not violate any ethics laws related to his purchase of Innate stock. He reaffirmed those thoughts in written answers to questions from committee members, stating that throughout “my time as a member of the U.S. House of Representatives, I have abided by and adhered to all ethics and conflicts of interest rules applicable to me.”
Committee Chairman Orrin Hatch (R-Utah) criticized Democrats for their boycott.
The committee was also scheduled to vote on Steve Mnuchin, President Trump’s nominee for Secretary of the Treasury. “Assuming that they can’t support these two, then they can vote against them,” Sen. Hatch said during the open session. “That’s what really an honest approach to this matter would be.”
At press time, a vote on Rep. Price has not been rescheduled, but Sen. Hatch noted that “I am hopeful when we schedule this again, they’ll be here.”
Democrats on the Senate Finance Committee forced a delay action that would have moved the nomination of Rep. Tom Price (R-Ga.) to the Senate floor for consideration, citing ongoing concerns with stock purchases made by Rep. Price.
The vote on Rep. Price’s nomination to serve as secretary of Health and Human Services was scheduled for Jan. 31, but all committee Democrats boycotted the executive session, forcing the delay. Committee rules state that at least 13 members, including at least 1 voting member from the minority party, must be present for a vote to proceed. The 26-member panel is made up of 14 Republicans and 12 Democrats.
“I asked Congressman Price [at his confirmation hearing] directly if he got an exclusive discount on stock in an Australian biomedical firm, and he said no,” Sen. Ron Wyden (D-Ore.), the committee’s leading Democrat, said in a statement. “From the committee’s investigation to company documents to the company official’s own words, the evidence tells a different story. It looks more and more like Congressman Price got special access to a special deal.
“The Finance Committee needs to continue following its bipartisan vetting process that has been upheld for more than 20 years. This is about getting answers to questions, plain and simple. Ethics laws are not optional, and nominees do not have a right to treat disclosures like a shell game,” Sen Wyden said.
At the confirmation hearing, Rep. Price, who is a retired orthopedic surgeon, asserted that he did not violate any ethics laws related to his purchase of Innate stock. He reaffirmed those thoughts in written answers to questions from committee members, stating that throughout “my time as a member of the U.S. House of Representatives, I have abided by and adhered to all ethics and conflicts of interest rules applicable to me.”
Committee Chairman Orrin Hatch (R-Utah) criticized Democrats for their boycott.
The committee was also scheduled to vote on Steve Mnuchin, President Trump’s nominee for Secretary of the Treasury. “Assuming that they can’t support these two, then they can vote against them,” Sen. Hatch said during the open session. “That’s what really an honest approach to this matter would be.”
At press time, a vote on Rep. Price has not been rescheduled, but Sen. Hatch noted that “I am hopeful when we schedule this again, they’ll be here.”
VIDEO: Consider PPIs as a cause of cutaneous reactions
WAILEA, HAWAII – Any proton pump inhibitor (PPI) has the potential to cause skin reactions, so it is important to ask patients about their use, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
If patients are going to react to a PPI, they usually will do so within 3 or 4 months of starting treatment, rather than in the first week or so of treatment, Dr. Jackson said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Skin reactions to PPIs are often maculopapular, with a flat and a raised component that can be nonspecific, Dr. Jackson noted.
Interestingly, he added, many times patients can switch to a different PPI and not get a skin reaction. However, there are some patients who develop a lupuslike reaction on the skin, and in these cases, there tends to be cross reactivity, “so they couldn’t switch to a different PPI and be risk-free” of the same reaction, he noted.
Dr. Jackson disclosed financial relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Any proton pump inhibitor (PPI) has the potential to cause skin reactions, so it is important to ask patients about their use, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
If patients are going to react to a PPI, they usually will do so within 3 or 4 months of starting treatment, rather than in the first week or so of treatment, Dr. Jackson said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Skin reactions to PPIs are often maculopapular, with a flat and a raised component that can be nonspecific, Dr. Jackson noted.
Interestingly, he added, many times patients can switch to a different PPI and not get a skin reaction. However, there are some patients who develop a lupuslike reaction on the skin, and in these cases, there tends to be cross reactivity, “so they couldn’t switch to a different PPI and be risk-free” of the same reaction, he noted.
Dr. Jackson disclosed financial relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Any proton pump inhibitor (PPI) has the potential to cause skin reactions, so it is important to ask patients about their use, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
If patients are going to react to a PPI, they usually will do so within 3 or 4 months of starting treatment, rather than in the first week or so of treatment, Dr. Jackson said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Skin reactions to PPIs are often maculopapular, with a flat and a raised component that can be nonspecific, Dr. Jackson noted.
Interestingly, he added, many times patients can switch to a different PPI and not get a skin reaction. However, there are some patients who develop a lupuslike reaction on the skin, and in these cases, there tends to be cross reactivity, “so they couldn’t switch to a different PPI and be risk-free” of the same reaction, he noted.
Dr. Jackson disclosed financial relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Experts offer patch testing tips for AD patients
WAILEA, HAWAII – Many atopic dermatitis patients with refractory disease may have also developed allergic contact dermatitis, according to Jonathan Silverberg, MD, of Northwestern University in Chicago.
Clinically, there is often overlap between AD and allergic contact dermatitis, said Dr. Silverberg, who was involved in the development of recent consensus guidelines on when to do patch testing in the setting of AD.
For many patients with severe disease, “sometimes when we patch test, we can find a relevant allergen for the patient to avoid, [and] within a few months, their disease just goes down a notch, gets much better, and really starts to respond to topical and more conservative approaches,” Dr. Silverberg said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Or patients may respond well to AD treatment on certain parts of the body, but there may be other areas that don’t respond as well, suggesting a possible component of allergic contact dermatitis, he added
The guidelines sought to sort out scenarios “where it makes sense to patch test” and to provide direction on best practices, noted Dr. Silverberg, who is director of the Northwestern Medicine Multidisciplinary Eczema Center, and director of the patch testing clinic, Northwestern Memorial Hospital, Chicago.
He disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, GlaxoSmithKline, Lilly, MedImmune-AstraZeneca, Pfizer, Procter & Gamble, Puricore, and Regeneron-Sanofi.SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Many atopic dermatitis patients with refractory disease may have also developed allergic contact dermatitis, according to Jonathan Silverberg, MD, of Northwestern University in Chicago.
Clinically, there is often overlap between AD and allergic contact dermatitis, said Dr. Silverberg, who was involved in the development of recent consensus guidelines on when to do patch testing in the setting of AD.
For many patients with severe disease, “sometimes when we patch test, we can find a relevant allergen for the patient to avoid, [and] within a few months, their disease just goes down a notch, gets much better, and really starts to respond to topical and more conservative approaches,” Dr. Silverberg said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Or patients may respond well to AD treatment on certain parts of the body, but there may be other areas that don’t respond as well, suggesting a possible component of allergic contact dermatitis, he added
The guidelines sought to sort out scenarios “where it makes sense to patch test” and to provide direction on best practices, noted Dr. Silverberg, who is director of the Northwestern Medicine Multidisciplinary Eczema Center, and director of the patch testing clinic, Northwestern Memorial Hospital, Chicago.
He disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, GlaxoSmithKline, Lilly, MedImmune-AstraZeneca, Pfizer, Procter & Gamble, Puricore, and Regeneron-Sanofi.SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Many atopic dermatitis patients with refractory disease may have also developed allergic contact dermatitis, according to Jonathan Silverberg, MD, of Northwestern University in Chicago.
Clinically, there is often overlap between AD and allergic contact dermatitis, said Dr. Silverberg, who was involved in the development of recent consensus guidelines on when to do patch testing in the setting of AD.
For many patients with severe disease, “sometimes when we patch test, we can find a relevant allergen for the patient to avoid, [and] within a few months, their disease just goes down a notch, gets much better, and really starts to respond to topical and more conservative approaches,” Dr. Silverberg said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Or patients may respond well to AD treatment on certain parts of the body, but there may be other areas that don’t respond as well, suggesting a possible component of allergic contact dermatitis, he added
The guidelines sought to sort out scenarios “where it makes sense to patch test” and to provide direction on best practices, noted Dr. Silverberg, who is director of the Northwestern Medicine Multidisciplinary Eczema Center, and director of the patch testing clinic, Northwestern Memorial Hospital, Chicago.
He disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, GlaxoSmithKline, Lilly, MedImmune-AstraZeneca, Pfizer, Procter & Gamble, Puricore, and Regeneron-Sanofi.SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR