User login
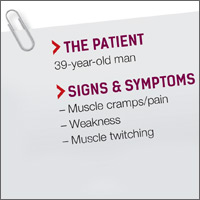
Muscle cramps/pain • weakness • muscle twitching • Dx?
THE CASE
A 39-year-old man who worked in construction presented to our clinic with complaints of muscle cramps and muscle pain that had been bothering him for several months. The cramps and pain started in both of his arms and subsequently became diffuse and generalized. He also reported an unintentional 15-pound weight loss.
His exam at that time was unremarkable. He was diagnosed with dehydration and cramping due to overexertion at work. A basic metabolic panel, hemogram, lipid panel, and thyroid stimulating hormone level were ordered. The patient’s triglyceride level, which was 227 mg/dL, was the only significant result (normal level: <150 mg/dL).
The patient’s symptoms continued to worsen until he returned to the clinic 6 months later, again complaining of muscle cramps and pain throughout his body. At that second visit, he also reported profound overall weakness and the development of diffuse muscle twitching, which his wife had observed while he was sleeping. As a result of these worrisome symptoms, he had become anxious and depressed.
A review of his medical record revealed a weight loss of about 20 pounds over the previous year. On exam, he had diffuse fasciculations in all the major muscle groups, including his tongue. The patient’s strength was 4/5 in all muscle groups. His deep tendon reflexes were 3+. He had a negative Babinski reflex (ie, he had downward facing toes with plantar stimulation), and cranial nerves II to XII were all intact. His rapid alternating movements and gait were slow.
THE DIAGNOSIS
Based on the exam, the primary diagnostic consideration for the patient was amyotrophic lateral sclerosis (ALS). Lab tests were ordered and revealed normal calcium and electrolyte levels, a normal erythrocyte sedimentation rate, a normal C-reactive protein level, and a negative test for acetylcholine receptor antibodies. However, the patient had an elevated creatine kinase level of 664 U/L (normal: 30-200 U/L). The patient was sent to a neuromuscular specialist, who identified signs of upper and lower motor neuron disease in all 4 of the patient’s extremities (he had foot drop that had not been present previously) and a very brisk jaw jerk. Along with the tongue fasciculations, the results of the specialist’s physical exam suggested ALS. Four-limb electromyography (EMG) showed widespread fasciculations and some large motor unit potentials and recruitment abnormalities, which were also consistent with ALS. It appeared that the patient’s weight loss was due to both muscle atrophy and the amount of calories burned from his constant twitching.
Extensive testing was done to rule out other potential causes of the patient’s symptoms, including magnetic resonance imaging (MRI) of the spine and brain (which was normal). In addition, the patient’s aldolase level and antineutrophil cytoplasmic antibodies were normal. The patient tested negative for human immunodeficiency virus and antibodies to double-stranded DNA. After serial neurologic exams, the final diagnosis of ALS was made.
DISCUSSION
ALS, also known as Lou Gehrig’s disease, is a degenerative motor neuron disease.1-3 The incidence in North America is 1.5 to 2.7 per 100,000 per year, and the prevalence is 2.7 to 7.4 per 100,000.4 The incidence of ALS increases with each decade of life, especially after age 40, and peaks at 74 years of age.4 The male to female ratio is 1:1.5-2.4 ALS affects upper and lower motor neurons and is progressive; however, the rate of progression and phenotype vary greatly between individuals.2 Most patients with ALS die within 2 to 5 years of onset.5
There is no specific test for ALS; the diagnosis is made clinically based on the revised El Escorial World Federation of Neurology criteria, also known as the Airlie House criteria.2,6,7 These criteria include evidence of lower motor neuron degeneration by clinical, electrophysiologic, or neuropathologic exam; evidence of upper motor neuron disease by clinical exam; progressive spread of symptoms or signs within a region or to other regions (by history or exam); and the absence of electrophysiologic, neuroimaging, or pathologic evidence of other disease processes that could explain the symptoms. If patients have evidence of upper and lower motor neuron disease, they should be reevaluated in 4 weeks to see if symptoms are improving or progressing.
Like our patient, many patients will have an elevated creatine kinase level (some with levels as high as 1000 U/L), and calcium may also be elevated because, rarely, ALS is associated with primary hyperparathyroidism.8 Electrophysiologic studies can be helpful in identifying active denervation of lower motor neurons.4,6,7
The differential diagnosis for ALS includes myasthenia gravis, inclusion-body myositis, multifocal motor neuropathy, benign fasciculations, hereditary spastic paraplegia, primary lateral sclerosis, post-polio progressive muscle atrophy, cervical spondylosis, and multiple sclerosis. A negative acetylcholine receptor antibody test will rule out myasthenia gravis, imaging of the spine can rule out cervical spondylosis, and electrophysiologic testing helps eliminate the other conditions (TABLE 14).
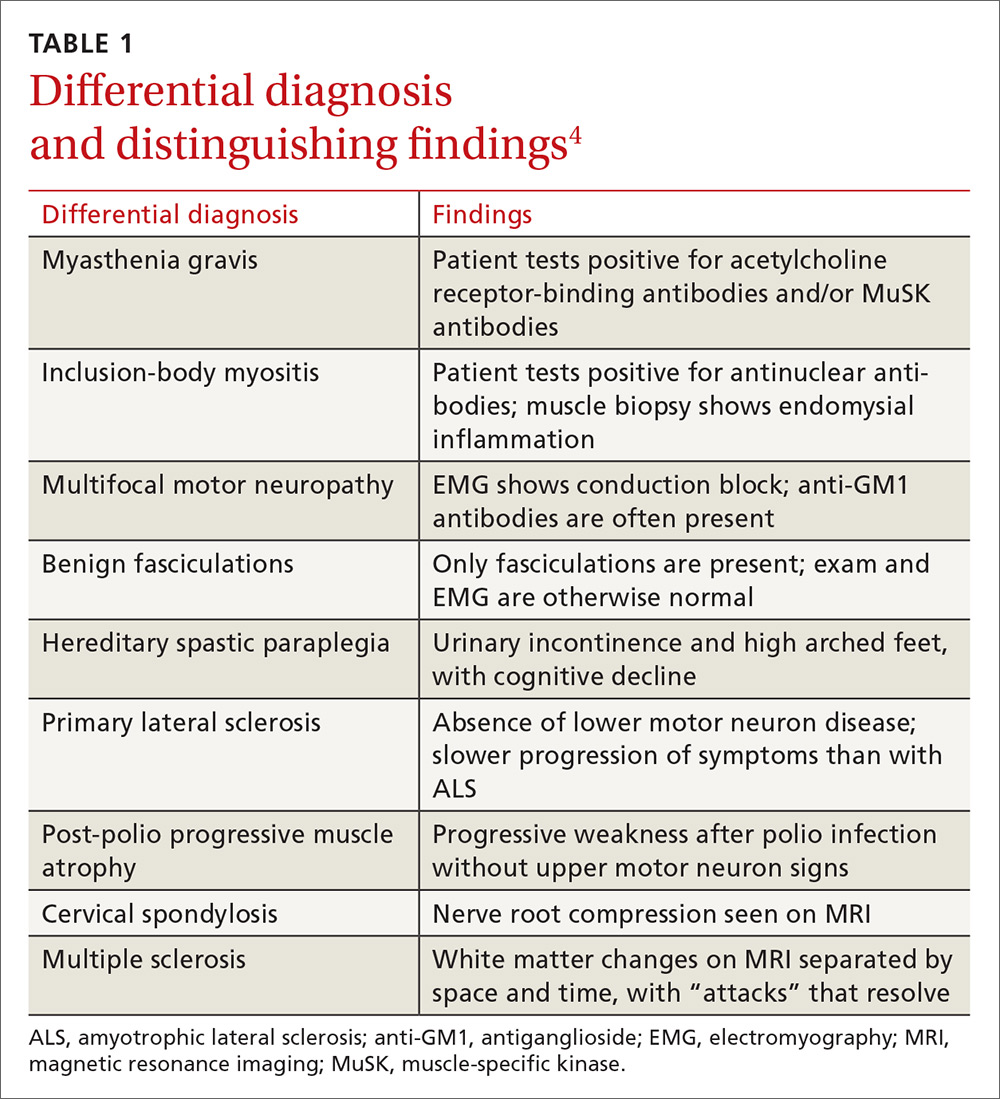
Treatment in specialty clinics can prolong survival
The mainstays of treatment are symptom management, multidisciplinary care (by physicians, physical/occupational/speech therapists, nutritionists, psychologists, psychotherapists, and genetic counselors), palliative care, and counseling about end-of-life issues for patients and family.1,5 Utilization of an ALS specialty clinic can provide access to all of these services and should be considered, as there is evidence that treatment in such clinics can prolong survival.5 The location of ALS specialty clinics can be found on the ALS Association’s Web site at http://www.alsa.org/community/.
Despite treatment, however, ALS is a progressive disease. The prognosis is poor, with a median survival of 2 to 5 years after diagnosis.9
The El Escorial World Federation of Neurology criteria for the diagnosis of ALS address how to treat the most common symptoms of ALS that occur as the disease progresses. These symptoms include dyspnea, muscle spasms, spasticity, sialorrhea, and pseudobulbar affect (TABLE 21,5).
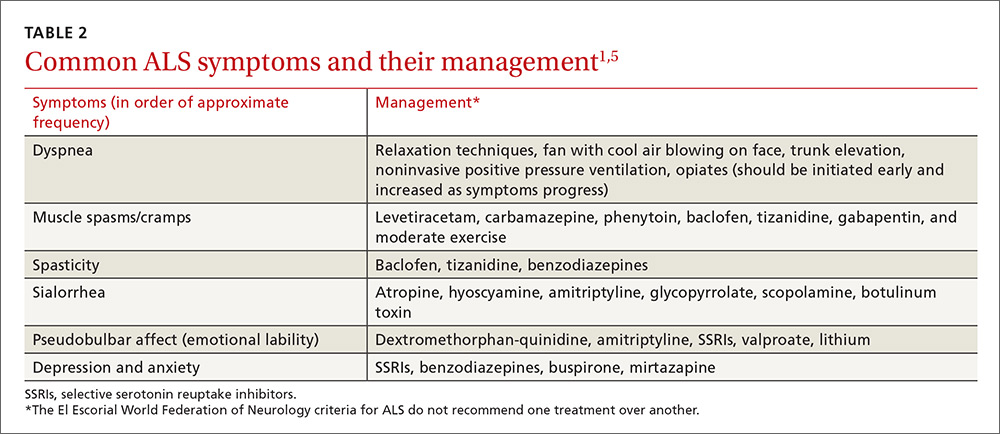
Our patient was started on baclofen 10 mg 3 times per day (titrated up as needed) for muscle spasms and cramps, which resulted in some improvement of his cramps, but no improvement in the spasms. He was also started on sertraline 50 mg for anxiety and depression. His overall weakness continued to progress, and we recommended that the patient get ankle-foot orthosis braces to help with the mobility impairment caused by foot drop.
We then referred him to an ALS specialty clinic recommended by the neuromuscular specialist. The patient is now enrolled in a clinical trial designed to test a cerebrospinal fluid marker for diagnosis and for a new drug aimed at symptom management.
THE TAKEAWAY
Muscle cramps and pain are early signs of ALS. Although ALS is uncommon, patients who present with muscle cramps and muscle pain should have a creatine kinase test ordered (which, if elevated, should prompt further investigation into ALS as the possible cause). Patients should also undergo a neurologic examination to seek evidence of upper and lower motor neuron disease. They should then be reevaluated in 4 weeks to see if symptoms are improving or progressing. If no improvement is seen and symptoms are progressive, a work-up for ALS should be considered.
The mainstay of treatment for patients with ALS is multidisciplinary symptom management and palliative care. Utilization of an ALS specialty clinic should also be recommended, as it can improve survival.5
1. Miller RG, Gelinas D, O’Connor P. Amyotrophic Lateral Sclerosis: American Academy of Neurology Press Quality of Life Guide Series. Demos Medical Publishing; 2004.
2. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643-657.
3. Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3-9.
4. Shaw PJ. ALS and other motor neuron diseases. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2015:chap 418.
5. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameter update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227-1233.
6. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124:96-107.
7. Brooks BR, Miller RG, Swash M, et al; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
8. Jackson CE, Amato AA, Bryan WW, et al. Primary hyperparathyroidism and ALS: is there a relation? Neurology. 1998;50:1795-1799.
9. Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833-841.
THE CASE
A 39-year-old man who worked in construction presented to our clinic with complaints of muscle cramps and muscle pain that had been bothering him for several months. The cramps and pain started in both of his arms and subsequently became diffuse and generalized. He also reported an unintentional 15-pound weight loss.
His exam at that time was unremarkable. He was diagnosed with dehydration and cramping due to overexertion at work. A basic metabolic panel, hemogram, lipid panel, and thyroid stimulating hormone level were ordered. The patient’s triglyceride level, which was 227 mg/dL, was the only significant result (normal level: <150 mg/dL).
The patient’s symptoms continued to worsen until he returned to the clinic 6 months later, again complaining of muscle cramps and pain throughout his body. At that second visit, he also reported profound overall weakness and the development of diffuse muscle twitching, which his wife had observed while he was sleeping. As a result of these worrisome symptoms, he had become anxious and depressed.
A review of his medical record revealed a weight loss of about 20 pounds over the previous year. On exam, he had diffuse fasciculations in all the major muscle groups, including his tongue. The patient’s strength was 4/5 in all muscle groups. His deep tendon reflexes were 3+. He had a negative Babinski reflex (ie, he had downward facing toes with plantar stimulation), and cranial nerves II to XII were all intact. His rapid alternating movements and gait were slow.
THE DIAGNOSIS
Based on the exam, the primary diagnostic consideration for the patient was amyotrophic lateral sclerosis (ALS). Lab tests were ordered and revealed normal calcium and electrolyte levels, a normal erythrocyte sedimentation rate, a normal C-reactive protein level, and a negative test for acetylcholine receptor antibodies. However, the patient had an elevated creatine kinase level of 664 U/L (normal: 30-200 U/L). The patient was sent to a neuromuscular specialist, who identified signs of upper and lower motor neuron disease in all 4 of the patient’s extremities (he had foot drop that had not been present previously) and a very brisk jaw jerk. Along with the tongue fasciculations, the results of the specialist’s physical exam suggested ALS. Four-limb electromyography (EMG) showed widespread fasciculations and some large motor unit potentials and recruitment abnormalities, which were also consistent with ALS. It appeared that the patient’s weight loss was due to both muscle atrophy and the amount of calories burned from his constant twitching.
Extensive testing was done to rule out other potential causes of the patient’s symptoms, including magnetic resonance imaging (MRI) of the spine and brain (which was normal). In addition, the patient’s aldolase level and antineutrophil cytoplasmic antibodies were normal. The patient tested negative for human immunodeficiency virus and antibodies to double-stranded DNA. After serial neurologic exams, the final diagnosis of ALS was made.
DISCUSSION
ALS, also known as Lou Gehrig’s disease, is a degenerative motor neuron disease.1-3 The incidence in North America is 1.5 to 2.7 per 100,000 per year, and the prevalence is 2.7 to 7.4 per 100,000.4 The incidence of ALS increases with each decade of life, especially after age 40, and peaks at 74 years of age.4 The male to female ratio is 1:1.5-2.4 ALS affects upper and lower motor neurons and is progressive; however, the rate of progression and phenotype vary greatly between individuals.2 Most patients with ALS die within 2 to 5 years of onset.5
There is no specific test for ALS; the diagnosis is made clinically based on the revised El Escorial World Federation of Neurology criteria, also known as the Airlie House criteria.2,6,7 These criteria include evidence of lower motor neuron degeneration by clinical, electrophysiologic, or neuropathologic exam; evidence of upper motor neuron disease by clinical exam; progressive spread of symptoms or signs within a region or to other regions (by history or exam); and the absence of electrophysiologic, neuroimaging, or pathologic evidence of other disease processes that could explain the symptoms. If patients have evidence of upper and lower motor neuron disease, they should be reevaluated in 4 weeks to see if symptoms are improving or progressing.
Like our patient, many patients will have an elevated creatine kinase level (some with levels as high as 1000 U/L), and calcium may also be elevated because, rarely, ALS is associated with primary hyperparathyroidism.8 Electrophysiologic studies can be helpful in identifying active denervation of lower motor neurons.4,6,7
The differential diagnosis for ALS includes myasthenia gravis, inclusion-body myositis, multifocal motor neuropathy, benign fasciculations, hereditary spastic paraplegia, primary lateral sclerosis, post-polio progressive muscle atrophy, cervical spondylosis, and multiple sclerosis. A negative acetylcholine receptor antibody test will rule out myasthenia gravis, imaging of the spine can rule out cervical spondylosis, and electrophysiologic testing helps eliminate the other conditions (TABLE 14).

Treatment in specialty clinics can prolong survival
The mainstays of treatment are symptom management, multidisciplinary care (by physicians, physical/occupational/speech therapists, nutritionists, psychologists, psychotherapists, and genetic counselors), palliative care, and counseling about end-of-life issues for patients and family.1,5 Utilization of an ALS specialty clinic can provide access to all of these services and should be considered, as there is evidence that treatment in such clinics can prolong survival.5 The location of ALS specialty clinics can be found on the ALS Association’s Web site at http://www.alsa.org/community/.
Despite treatment, however, ALS is a progressive disease. The prognosis is poor, with a median survival of 2 to 5 years after diagnosis.9
The El Escorial World Federation of Neurology criteria for the diagnosis of ALS address how to treat the most common symptoms of ALS that occur as the disease progresses. These symptoms include dyspnea, muscle spasms, spasticity, sialorrhea, and pseudobulbar affect (TABLE 21,5).

Our patient was started on baclofen 10 mg 3 times per day (titrated up as needed) for muscle spasms and cramps, which resulted in some improvement of his cramps, but no improvement in the spasms. He was also started on sertraline 50 mg for anxiety and depression. His overall weakness continued to progress, and we recommended that the patient get ankle-foot orthosis braces to help with the mobility impairment caused by foot drop.
We then referred him to an ALS specialty clinic recommended by the neuromuscular specialist. The patient is now enrolled in a clinical trial designed to test a cerebrospinal fluid marker for diagnosis and for a new drug aimed at symptom management.
THE TAKEAWAY
Muscle cramps and pain are early signs of ALS. Although ALS is uncommon, patients who present with muscle cramps and muscle pain should have a creatine kinase test ordered (which, if elevated, should prompt further investigation into ALS as the possible cause). Patients should also undergo a neurologic examination to seek evidence of upper and lower motor neuron disease. They should then be reevaluated in 4 weeks to see if symptoms are improving or progressing. If no improvement is seen and symptoms are progressive, a work-up for ALS should be considered.
The mainstay of treatment for patients with ALS is multidisciplinary symptom management and palliative care. Utilization of an ALS specialty clinic should also be recommended, as it can improve survival.5
THE CASE
A 39-year-old man who worked in construction presented to our clinic with complaints of muscle cramps and muscle pain that had been bothering him for several months. The cramps and pain started in both of his arms and subsequently became diffuse and generalized. He also reported an unintentional 15-pound weight loss.
His exam at that time was unremarkable. He was diagnosed with dehydration and cramping due to overexertion at work. A basic metabolic panel, hemogram, lipid panel, and thyroid stimulating hormone level were ordered. The patient’s triglyceride level, which was 227 mg/dL, was the only significant result (normal level: <150 mg/dL).
The patient’s symptoms continued to worsen until he returned to the clinic 6 months later, again complaining of muscle cramps and pain throughout his body. At that second visit, he also reported profound overall weakness and the development of diffuse muscle twitching, which his wife had observed while he was sleeping. As a result of these worrisome symptoms, he had become anxious and depressed.
A review of his medical record revealed a weight loss of about 20 pounds over the previous year. On exam, he had diffuse fasciculations in all the major muscle groups, including his tongue. The patient’s strength was 4/5 in all muscle groups. His deep tendon reflexes were 3+. He had a negative Babinski reflex (ie, he had downward facing toes with plantar stimulation), and cranial nerves II to XII were all intact. His rapid alternating movements and gait were slow.
THE DIAGNOSIS
Based on the exam, the primary diagnostic consideration for the patient was amyotrophic lateral sclerosis (ALS). Lab tests were ordered and revealed normal calcium and electrolyte levels, a normal erythrocyte sedimentation rate, a normal C-reactive protein level, and a negative test for acetylcholine receptor antibodies. However, the patient had an elevated creatine kinase level of 664 U/L (normal: 30-200 U/L). The patient was sent to a neuromuscular specialist, who identified signs of upper and lower motor neuron disease in all 4 of the patient’s extremities (he had foot drop that had not been present previously) and a very brisk jaw jerk. Along with the tongue fasciculations, the results of the specialist’s physical exam suggested ALS. Four-limb electromyography (EMG) showed widespread fasciculations and some large motor unit potentials and recruitment abnormalities, which were also consistent with ALS. It appeared that the patient’s weight loss was due to both muscle atrophy and the amount of calories burned from his constant twitching.
Extensive testing was done to rule out other potential causes of the patient’s symptoms, including magnetic resonance imaging (MRI) of the spine and brain (which was normal). In addition, the patient’s aldolase level and antineutrophil cytoplasmic antibodies were normal. The patient tested negative for human immunodeficiency virus and antibodies to double-stranded DNA. After serial neurologic exams, the final diagnosis of ALS was made.
DISCUSSION
ALS, also known as Lou Gehrig’s disease, is a degenerative motor neuron disease.1-3 The incidence in North America is 1.5 to 2.7 per 100,000 per year, and the prevalence is 2.7 to 7.4 per 100,000.4 The incidence of ALS increases with each decade of life, especially after age 40, and peaks at 74 years of age.4 The male to female ratio is 1:1.5-2.4 ALS affects upper and lower motor neurons and is progressive; however, the rate of progression and phenotype vary greatly between individuals.2 Most patients with ALS die within 2 to 5 years of onset.5
There is no specific test for ALS; the diagnosis is made clinically based on the revised El Escorial World Federation of Neurology criteria, also known as the Airlie House criteria.2,6,7 These criteria include evidence of lower motor neuron degeneration by clinical, electrophysiologic, or neuropathologic exam; evidence of upper motor neuron disease by clinical exam; progressive spread of symptoms or signs within a region or to other regions (by history or exam); and the absence of electrophysiologic, neuroimaging, or pathologic evidence of other disease processes that could explain the symptoms. If patients have evidence of upper and lower motor neuron disease, they should be reevaluated in 4 weeks to see if symptoms are improving or progressing.
Like our patient, many patients will have an elevated creatine kinase level (some with levels as high as 1000 U/L), and calcium may also be elevated because, rarely, ALS is associated with primary hyperparathyroidism.8 Electrophysiologic studies can be helpful in identifying active denervation of lower motor neurons.4,6,7
The differential diagnosis for ALS includes myasthenia gravis, inclusion-body myositis, multifocal motor neuropathy, benign fasciculations, hereditary spastic paraplegia, primary lateral sclerosis, post-polio progressive muscle atrophy, cervical spondylosis, and multiple sclerosis. A negative acetylcholine receptor antibody test will rule out myasthenia gravis, imaging of the spine can rule out cervical spondylosis, and electrophysiologic testing helps eliminate the other conditions (TABLE 14).

Treatment in specialty clinics can prolong survival
The mainstays of treatment are symptom management, multidisciplinary care (by physicians, physical/occupational/speech therapists, nutritionists, psychologists, psychotherapists, and genetic counselors), palliative care, and counseling about end-of-life issues for patients and family.1,5 Utilization of an ALS specialty clinic can provide access to all of these services and should be considered, as there is evidence that treatment in such clinics can prolong survival.5 The location of ALS specialty clinics can be found on the ALS Association’s Web site at http://www.alsa.org/community/.
Despite treatment, however, ALS is a progressive disease. The prognosis is poor, with a median survival of 2 to 5 years after diagnosis.9
The El Escorial World Federation of Neurology criteria for the diagnosis of ALS address how to treat the most common symptoms of ALS that occur as the disease progresses. These symptoms include dyspnea, muscle spasms, spasticity, sialorrhea, and pseudobulbar affect (TABLE 21,5).

Our patient was started on baclofen 10 mg 3 times per day (titrated up as needed) for muscle spasms and cramps, which resulted in some improvement of his cramps, but no improvement in the spasms. He was also started on sertraline 50 mg for anxiety and depression. His overall weakness continued to progress, and we recommended that the patient get ankle-foot orthosis braces to help with the mobility impairment caused by foot drop.
We then referred him to an ALS specialty clinic recommended by the neuromuscular specialist. The patient is now enrolled in a clinical trial designed to test a cerebrospinal fluid marker for diagnosis and for a new drug aimed at symptom management.
THE TAKEAWAY
Muscle cramps and pain are early signs of ALS. Although ALS is uncommon, patients who present with muscle cramps and muscle pain should have a creatine kinase test ordered (which, if elevated, should prompt further investigation into ALS as the possible cause). Patients should also undergo a neurologic examination to seek evidence of upper and lower motor neuron disease. They should then be reevaluated in 4 weeks to see if symptoms are improving or progressing. If no improvement is seen and symptoms are progressive, a work-up for ALS should be considered.
The mainstay of treatment for patients with ALS is multidisciplinary symptom management and palliative care. Utilization of an ALS specialty clinic should also be recommended, as it can improve survival.5
1. Miller RG, Gelinas D, O’Connor P. Amyotrophic Lateral Sclerosis: American Academy of Neurology Press Quality of Life Guide Series. Demos Medical Publishing; 2004.
2. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643-657.
3. Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3-9.
4. Shaw PJ. ALS and other motor neuron diseases. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2015:chap 418.
5. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameter update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227-1233.
6. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124:96-107.
7. Brooks BR, Miller RG, Swash M, et al; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
8. Jackson CE, Amato AA, Bryan WW, et al. Primary hyperparathyroidism and ALS: is there a relation? Neurology. 1998;50:1795-1799.
9. Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833-841.
1. Miller RG, Gelinas D, O’Connor P. Amyotrophic Lateral Sclerosis: American Academy of Neurology Press Quality of Life Guide Series. Demos Medical Publishing; 2004.
2. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643-657.
3. Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3-9.
4. Shaw PJ. ALS and other motor neuron diseases. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2015:chap 418.
5. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameter update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227-1233.
6. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124:96-107.
7. Brooks BR, Miller RG, Swash M, et al; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
8. Jackson CE, Amato AA, Bryan WW, et al. Primary hyperparathyroidism and ALS: is there a relation? Neurology. 1998;50:1795-1799.
9. Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833-841.
Hemodialysis patient with finger ulcerations
A 62-year-old man with end-stage renal disease presented to our dermatology clinic with 2-month-old ulcerations on his distal left ring finger. He was on hemodialysis and had a radiocephalic arteriovenous fistula (AVF) on his left arm. He had been empirically treated elsewhere with oral trimethoprim-sulfamethoxazole for a presumed bacterial infection, without improvement. He was then treated for contact dermatitis with topical clobetasol, which led to ulcer expansion and worsening pain.
At our clinic, the patient reported intermittent pain in his finger and paresthesias during activity and dialysis, but no tenderness of the ulcers. He had atrophy of the intrinsic left hand muscles (his non-dominant hand) with associated weakness. Three weeks earlier, he’d received a blood transfusion for anemia. Afterward, the pain in his hand improved and the ulcers decreased in size.
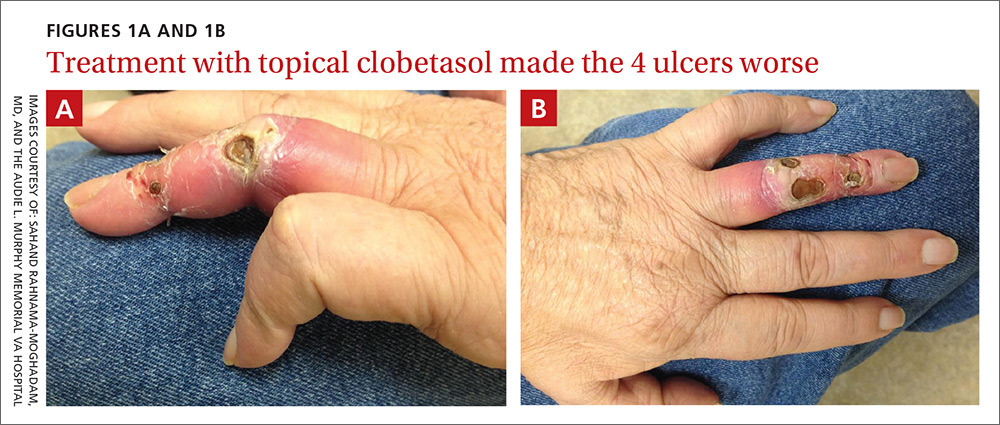
On exam, the AVF had a palpable thrill over the left forearm. The radial pulses were palpable bilaterally (2+) and the left ulnar artery was palpable, but diminished (1+). The patient’s left hand was cooler than the right (with a slight cyanotic hue and visible intrinsic muscle atrophy) and had decreased sensation to pain and temperature. Four ulcers with dry yellow eschar were located over the dorsal interphalangeal joints (FIGURES 1A AND 1B). They were essentially non-tender, but there was tenderness in the adjacent intact skin. There was violaceous blue edematous congestion noted on the fourth finger, and the distal phalange was constricted, giving it a “pseudoainhum” appearance.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Dialysis access steal syndrome
We suspected dialysis access steal syndrome (also known as AVF steal syndrome), so a duplex ultrasound was performed. The ultrasound was inconclusive. (We couldn’t confirm a limitation in blood flow, nor delineate anatomy.) So, we referred the patient for a thoracic and upper extremity angiogram.
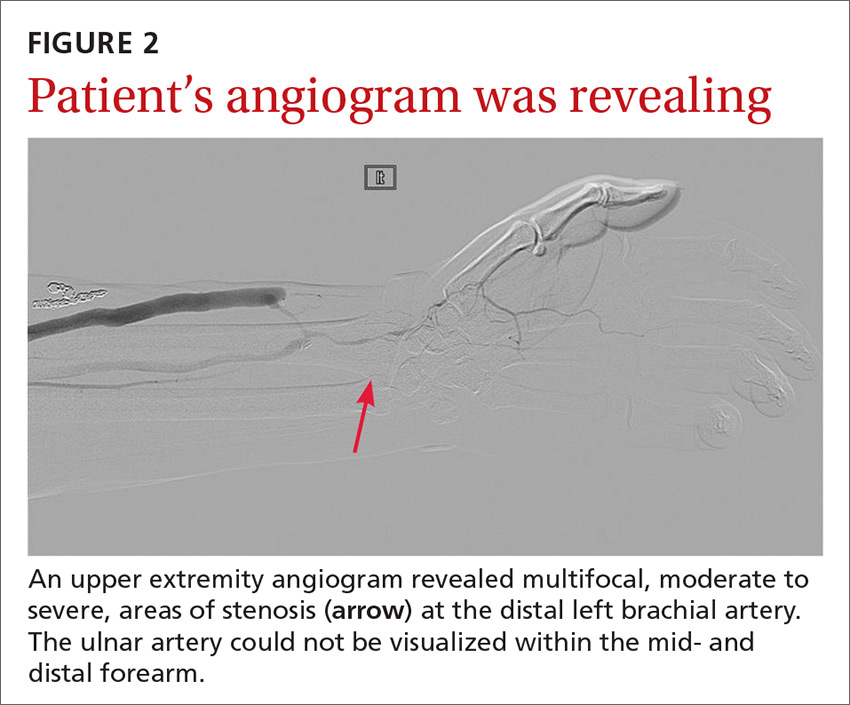
The angiogram demonstrated multifocal, moderate to severe, areas of stenosis at the distal left brachial artery. The radial artery was patent at the level of the wrist, but showed diffuse narrowing beyond the level of the arteriovenous (AV) anastomosis. There was only a faint palmar arch identified on the radial aspect of the hand with digital branches feeding the radial portion of the hand. In contrast, the ulnar artery was not seen within the mid- and distal forearm (FIGURE 2). Palmar branches to the ulnar half of the hand were not identified. The fistula itself didn’t show any stenosis.
Based on these findings, our suspicions of dialysis access steal syndrome were confirmed.
Dialysis access steal syndrome is caused by a significant decrease or reversal of blood flow in the arterial segment distal to the AVF or graft, which is induced by the low resistance of the fistula outflow. Patients with adequate collateral vasculature are able to compensate for the steal effect; however, patients with end-stage renal disease typically have preexisting vascular disease that increases the risk for vascular steal and, ultimately, demand ischemia after placement of an AVF.1 Interestingly, a steal effect occurs in 73% of patients after AVF construction, yet it is estimated that only 10% of patients demonstrating a steal phenomenon become symptomatic.2
In our patient’s case, the vaso-occlusive properties of topical steroids explain why the superpotent steroid (clobetasol) he was prescribed increased his pain and worsened the underlying problem.
A broad differential; a useful exam maneuver
The differential diagnosis of ulcers includes infection (mainly from bacterial or mycobacterial sources), trauma facilitated by neuropathy (neuropathic ulceration), vasculitis, and ischemia. When the history and physical exam suggest ischemic ulceration, then thromboembolism, thoracic outlet syndrome, vasculitis, atherosclerosis, and steal syndrome become more likely causes.
Signs and symptoms of ischemic steal syndrome are initially subtle and include extremity coolness, neurosensory changes, intrinsic muscle weakness, ulceration, and ultimately, gangrene of the affected extremity.3,4 A cold, numb, and/or painful hand during dialysis is another clue.3 Factors that increase the likelihood of the syndrome include age >60 years, female sex, and the presence of diabetes or peripheral artery disease.2,3,5,6
One physical exam maneuver that can help make the diagnosis of steal syndrome is manual occlusion of the AVF. If palpable distal pulses disappear when the AVF is patent and reappear when the fistula is occluded with downward pressure, then AVF steal syndrome is likely.4 Pain at rest, sensory loss, loss of pulse, and digital gangrene are emergency symptoms that warrant immediate surgical evaluation.3
Tests will confirm suspicions. Doppler ultrasound can be used to assess changes in the blood flow rate of the affected vessels when the AVF is patent vs when it is occluded. Similarly, pulse oximetry can be used with and without AVF occlusion to compare changes in oxygen saturation. The confirmatory diagnosis, however, is made via a fistulogram (angiography) with and without manual compression.6 Images taken after dye injection into the AVF show dramatic improvement of distal blood flow with AVF compression.
Treatment requires surgery
Severe steal-related ischemic manifestations that threaten the function and viability of digits require surgical treatment that is primarily directed toward improving distal blood flow and secondarily toward preserving hemodialysis access. Several surgical treatments are commonly used, including access ligation, banding, elongation, distal arterial ligation, and distal revascularization and interval ligation.2-5,7
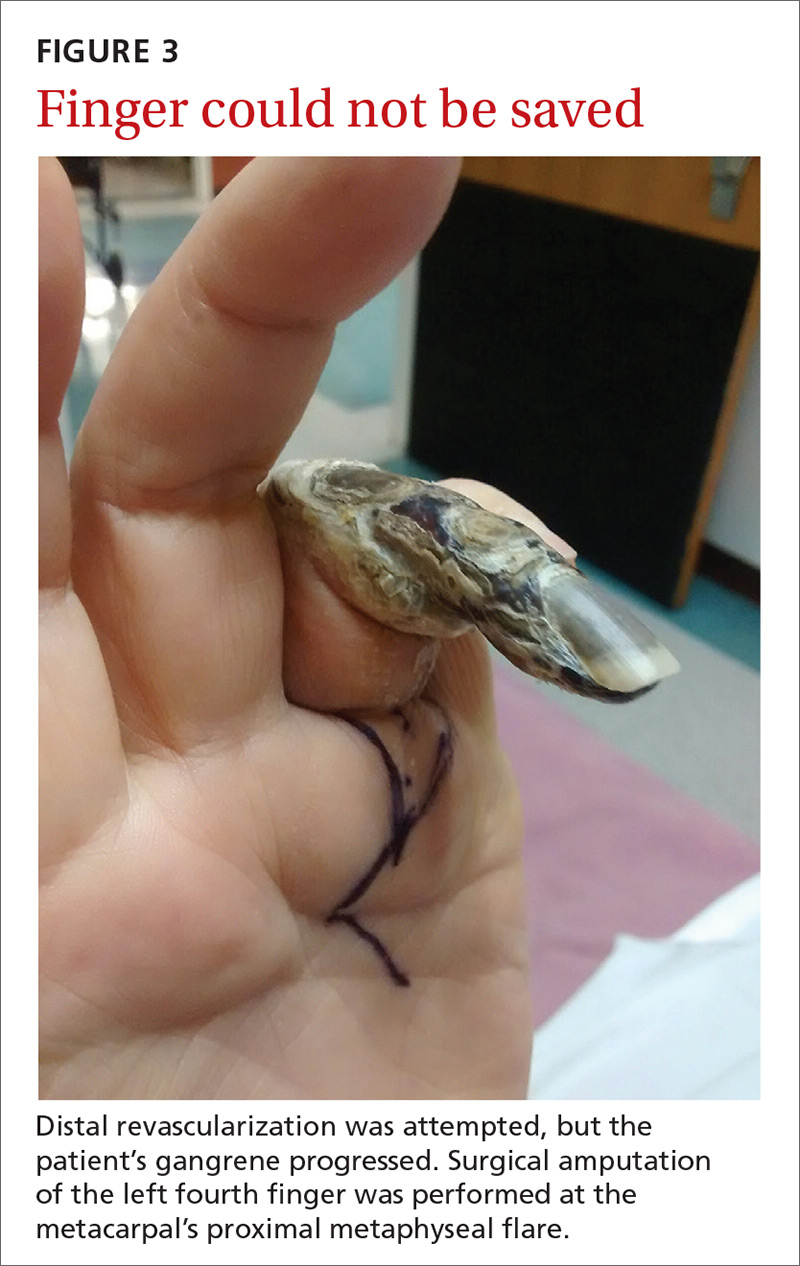
Our patient. Distal revascularization was attempted, but unfortunately, the patient’s gangrene was progressive (FIGURE 3) and surgical amputation of the left fourth finger was performed at the metacarpal’s proximal metaphyseal flare. The patient was transitioned to peritoneal dialysis to avoid further ischemia.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, Department of Dermatology, Indiana University, 545 Barnhill Drive, Indianapolis, IN 46202; [email protected].
1. Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
2. Puryear A, Villarreal S, Wells MJ, et al. JAAD grand rounds quiz. Hand ischemia in a hemodialysis patient. J Am Acad Dermatol. 2014;70:393-395.
3. Pelle MT, Miller OF 3rd. Dermatologic manifestations and management of vascular steal syndrome in hemodialysis patients with arteriovenous fistulas. Arch Dermatol. 2002;138:1296-1298.
4. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310.
5. Gupta N, Yuo TH, Konig G 4th, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162-167.
6. Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376.
7. Leake AE, Winger DG, Leers SA, et al. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61:754-760.
A 62-year-old man with end-stage renal disease presented to our dermatology clinic with 2-month-old ulcerations on his distal left ring finger. He was on hemodialysis and had a radiocephalic arteriovenous fistula (AVF) on his left arm. He had been empirically treated elsewhere with oral trimethoprim-sulfamethoxazole for a presumed bacterial infection, without improvement. He was then treated for contact dermatitis with topical clobetasol, which led to ulcer expansion and worsening pain.
At our clinic, the patient reported intermittent pain in his finger and paresthesias during activity and dialysis, but no tenderness of the ulcers. He had atrophy of the intrinsic left hand muscles (his non-dominant hand) with associated weakness. Three weeks earlier, he’d received a blood transfusion for anemia. Afterward, the pain in his hand improved and the ulcers decreased in size.
On exam, the AVF had a palpable thrill over the left forearm. The radial pulses were palpable bilaterally (2+) and the left ulnar artery was palpable, but diminished (1+). The patient’s left hand was cooler than the right (with a slight cyanotic hue and visible intrinsic muscle atrophy) and had decreased sensation to pain and temperature. Four ulcers with dry yellow eschar were located over the dorsal interphalangeal joints (FIGURES 1A AND 1B). They were essentially non-tender, but there was tenderness in the adjacent intact skin. There was violaceous blue edematous congestion noted on the fourth finger, and the distal phalange was constricted, giving it a “pseudoainhum” appearance.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Dialysis access steal syndrome
We suspected dialysis access steal syndrome (also known as AVF steal syndrome), so a duplex ultrasound was performed. The ultrasound was inconclusive. (We couldn’t confirm a limitation in blood flow, nor delineate anatomy.) So, we referred the patient for a thoracic and upper extremity angiogram.
The angiogram demonstrated multifocal, moderate to severe, areas of stenosis at the distal left brachial artery. The radial artery was patent at the level of the wrist, but showed diffuse narrowing beyond the level of the arteriovenous (AV) anastomosis. There was only a faint palmar arch identified on the radial aspect of the hand with digital branches feeding the radial portion of the hand. In contrast, the ulnar artery was not seen within the mid- and distal forearm (FIGURE 2). Palmar branches to the ulnar half of the hand were not identified. The fistula itself didn’t show any stenosis.

Based on these findings, our suspicions of dialysis access steal syndrome were confirmed.
Dialysis access steal syndrome is caused by a significant decrease or reversal of blood flow in the arterial segment distal to the AVF or graft, which is induced by the low resistance of the fistula outflow. Patients with adequate collateral vasculature are able to compensate for the steal effect; however, patients with end-stage renal disease typically have preexisting vascular disease that increases the risk for vascular steal and, ultimately, demand ischemia after placement of an AVF.1 Interestingly, a steal effect occurs in 73% of patients after AVF construction, yet it is estimated that only 10% of patients demonstrating a steal phenomenon become symptomatic.2
In our patient’s case, the vaso-occlusive properties of topical steroids explain why the superpotent steroid (clobetasol) he was prescribed increased his pain and worsened the underlying problem.
A broad differential; a useful exam maneuver
The differential diagnosis of ulcers includes infection (mainly from bacterial or mycobacterial sources), trauma facilitated by neuropathy (neuropathic ulceration), vasculitis, and ischemia. When the history and physical exam suggest ischemic ulceration, then thromboembolism, thoracic outlet syndrome, vasculitis, atherosclerosis, and steal syndrome become more likely causes.
Signs and symptoms of ischemic steal syndrome are initially subtle and include extremity coolness, neurosensory changes, intrinsic muscle weakness, ulceration, and ultimately, gangrene of the affected extremity.3,4 A cold, numb, and/or painful hand during dialysis is another clue.3 Factors that increase the likelihood of the syndrome include age >60 years, female sex, and the presence of diabetes or peripheral artery disease.2,3,5,6
One physical exam maneuver that can help make the diagnosis of steal syndrome is manual occlusion of the AVF. If palpable distal pulses disappear when the AVF is patent and reappear when the fistula is occluded with downward pressure, then AVF steal syndrome is likely.4 Pain at rest, sensory loss, loss of pulse, and digital gangrene are emergency symptoms that warrant immediate surgical evaluation.3
Tests will confirm suspicions. Doppler ultrasound can be used to assess changes in the blood flow rate of the affected vessels when the AVF is patent vs when it is occluded. Similarly, pulse oximetry can be used with and without AVF occlusion to compare changes in oxygen saturation. The confirmatory diagnosis, however, is made via a fistulogram (angiography) with and without manual compression.6 Images taken after dye injection into the AVF show dramatic improvement of distal blood flow with AVF compression.
Treatment requires surgery
Severe steal-related ischemic manifestations that threaten the function and viability of digits require surgical treatment that is primarily directed toward improving distal blood flow and secondarily toward preserving hemodialysis access. Several surgical treatments are commonly used, including access ligation, banding, elongation, distal arterial ligation, and distal revascularization and interval ligation.2-5,7
Our patient. Distal revascularization was attempted, but unfortunately, the patient’s gangrene was progressive (FIGURE 3) and surgical amputation of the left fourth finger was performed at the metacarpal’s proximal metaphyseal flare. The patient was transitioned to peritoneal dialysis to avoid further ischemia.

CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, Department of Dermatology, Indiana University, 545 Barnhill Drive, Indianapolis, IN 46202; [email protected].
A 62-year-old man with end-stage renal disease presented to our dermatology clinic with 2-month-old ulcerations on his distal left ring finger. He was on hemodialysis and had a radiocephalic arteriovenous fistula (AVF) on his left arm. He had been empirically treated elsewhere with oral trimethoprim-sulfamethoxazole for a presumed bacterial infection, without improvement. He was then treated for contact dermatitis with topical clobetasol, which led to ulcer expansion and worsening pain.
At our clinic, the patient reported intermittent pain in his finger and paresthesias during activity and dialysis, but no tenderness of the ulcers. He had atrophy of the intrinsic left hand muscles (his non-dominant hand) with associated weakness. Three weeks earlier, he’d received a blood transfusion for anemia. Afterward, the pain in his hand improved and the ulcers decreased in size.
On exam, the AVF had a palpable thrill over the left forearm. The radial pulses were palpable bilaterally (2+) and the left ulnar artery was palpable, but diminished (1+). The patient’s left hand was cooler than the right (with a slight cyanotic hue and visible intrinsic muscle atrophy) and had decreased sensation to pain and temperature. Four ulcers with dry yellow eschar were located over the dorsal interphalangeal joints (FIGURES 1A AND 1B). They were essentially non-tender, but there was tenderness in the adjacent intact skin. There was violaceous blue edematous congestion noted on the fourth finger, and the distal phalange was constricted, giving it a “pseudoainhum” appearance.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Dialysis access steal syndrome
We suspected dialysis access steal syndrome (also known as AVF steal syndrome), so a duplex ultrasound was performed. The ultrasound was inconclusive. (We couldn’t confirm a limitation in blood flow, nor delineate anatomy.) So, we referred the patient for a thoracic and upper extremity angiogram.
The angiogram demonstrated multifocal, moderate to severe, areas of stenosis at the distal left brachial artery. The radial artery was patent at the level of the wrist, but showed diffuse narrowing beyond the level of the arteriovenous (AV) anastomosis. There was only a faint palmar arch identified on the radial aspect of the hand with digital branches feeding the radial portion of the hand. In contrast, the ulnar artery was not seen within the mid- and distal forearm (FIGURE 2). Palmar branches to the ulnar half of the hand were not identified. The fistula itself didn’t show any stenosis.

Based on these findings, our suspicions of dialysis access steal syndrome were confirmed.
Dialysis access steal syndrome is caused by a significant decrease or reversal of blood flow in the arterial segment distal to the AVF or graft, which is induced by the low resistance of the fistula outflow. Patients with adequate collateral vasculature are able to compensate for the steal effect; however, patients with end-stage renal disease typically have preexisting vascular disease that increases the risk for vascular steal and, ultimately, demand ischemia after placement of an AVF.1 Interestingly, a steal effect occurs in 73% of patients after AVF construction, yet it is estimated that only 10% of patients demonstrating a steal phenomenon become symptomatic.2
In our patient’s case, the vaso-occlusive properties of topical steroids explain why the superpotent steroid (clobetasol) he was prescribed increased his pain and worsened the underlying problem.
A broad differential; a useful exam maneuver
The differential diagnosis of ulcers includes infection (mainly from bacterial or mycobacterial sources), trauma facilitated by neuropathy (neuropathic ulceration), vasculitis, and ischemia. When the history and physical exam suggest ischemic ulceration, then thromboembolism, thoracic outlet syndrome, vasculitis, atherosclerosis, and steal syndrome become more likely causes.
Signs and symptoms of ischemic steal syndrome are initially subtle and include extremity coolness, neurosensory changes, intrinsic muscle weakness, ulceration, and ultimately, gangrene of the affected extremity.3,4 A cold, numb, and/or painful hand during dialysis is another clue.3 Factors that increase the likelihood of the syndrome include age >60 years, female sex, and the presence of diabetes or peripheral artery disease.2,3,5,6
One physical exam maneuver that can help make the diagnosis of steal syndrome is manual occlusion of the AVF. If palpable distal pulses disappear when the AVF is patent and reappear when the fistula is occluded with downward pressure, then AVF steal syndrome is likely.4 Pain at rest, sensory loss, loss of pulse, and digital gangrene are emergency symptoms that warrant immediate surgical evaluation.3
Tests will confirm suspicions. Doppler ultrasound can be used to assess changes in the blood flow rate of the affected vessels when the AVF is patent vs when it is occluded. Similarly, pulse oximetry can be used with and without AVF occlusion to compare changes in oxygen saturation. The confirmatory diagnosis, however, is made via a fistulogram (angiography) with and without manual compression.6 Images taken after dye injection into the AVF show dramatic improvement of distal blood flow with AVF compression.
Treatment requires surgery
Severe steal-related ischemic manifestations that threaten the function and viability of digits require surgical treatment that is primarily directed toward improving distal blood flow and secondarily toward preserving hemodialysis access. Several surgical treatments are commonly used, including access ligation, banding, elongation, distal arterial ligation, and distal revascularization and interval ligation.2-5,7
Our patient. Distal revascularization was attempted, but unfortunately, the patient’s gangrene was progressive (FIGURE 3) and surgical amputation of the left fourth finger was performed at the metacarpal’s proximal metaphyseal flare. The patient was transitioned to peritoneal dialysis to avoid further ischemia.

CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, Department of Dermatology, Indiana University, 545 Barnhill Drive, Indianapolis, IN 46202; [email protected].
1. Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
2. Puryear A, Villarreal S, Wells MJ, et al. JAAD grand rounds quiz. Hand ischemia in a hemodialysis patient. J Am Acad Dermatol. 2014;70:393-395.
3. Pelle MT, Miller OF 3rd. Dermatologic manifestations and management of vascular steal syndrome in hemodialysis patients with arteriovenous fistulas. Arch Dermatol. 2002;138:1296-1298.
4. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310.
5. Gupta N, Yuo TH, Konig G 4th, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162-167.
6. Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376.
7. Leake AE, Winger DG, Leers SA, et al. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61:754-760.
1. Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
2. Puryear A, Villarreal S, Wells MJ, et al. JAAD grand rounds quiz. Hand ischemia in a hemodialysis patient. J Am Acad Dermatol. 2014;70:393-395.
3. Pelle MT, Miller OF 3rd. Dermatologic manifestations and management of vascular steal syndrome in hemodialysis patients with arteriovenous fistulas. Arch Dermatol. 2002;138:1296-1298.
4. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310.
5. Gupta N, Yuo TH, Konig G 4th, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162-167.
6. Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376.
7. Leake AE, Winger DG, Leers SA, et al. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61:754-760.
Can mobile technology improve weight loss in overweight and obese patients?
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).
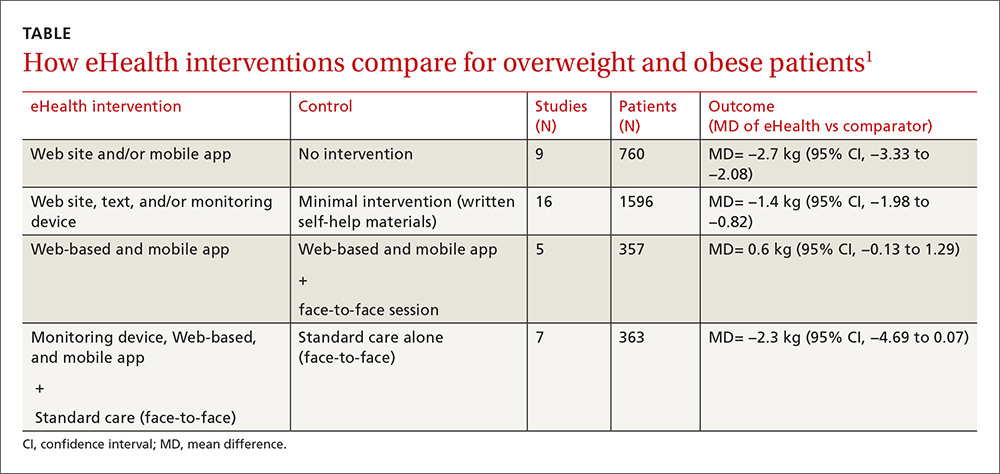
Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).

Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).

Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes, this technology can help in the short term. Mobile technology compared with minimal or no intervention increases short-term (<6 months) weight loss (1.4 to 2.7 kg) in overweight and obese patients (strength of recommendation [SOR]: A, meta-analysis of good-quality studies and randomized controlled trials [RCTs]).
Interventions that combine nonelectronic measures with mobile technology increase weight loss more effectively (3.7 kg) than no intervention (SOR: A, meta-analysis of good-quality studies and RCTs).
Using mobile technology shows no significant benefits for weight loss after 12 months (SOR: A, multiple good-quality RCTs).
It’s time to screen for bullying
When I became a family physician (FP), it never crossed my mind that I would one day be asking school-aged children about bullying. Not so much because bullying didn’t exist, but because I wasn’t aware of the pervasiveness and seriousness of the problem and because there were no professional recommendations to do so.
That said, my family had some first-hand experience with the issue: One of my children was bullied in grade school. When my wife found out, she promptly visited the 2 boys’ homes and told them and their parents that the behavior would stop or else! (She may have used more colorful language.) And it did stop. But times have changed, and so has the nature of bullying, which can now extend beyond the hallway to an entire school body in seconds with a few taps on a cell phone. And the adverse consequences can be significant, as described by McClowry and colleagues.
The prevalence of bullying is discouragingly high, estimated to be about 20% in national surveys.1 Because bullying occurs so frequently, public health, community-based, and school-based approaches, rather than one-on-one office-based interventions, are likely to have the greatest overall impact on decreasing bullying. Randomized trials bear this out, showing that prevention programs in schools can effectively reduce the behavior.2,3
What is our responsibility as FPs? Screening is a reasonable first step, even in the absence of randomized trials demonstrating benefit. Because there have been no physician office-based trials of screening or interventions for bullying, we must rely on “expert opinion” at this time, with no assurance that what we do will actually help children. Absence of proof of benefit, however, does not mean absence of benefit, and doing nothing will definitely not help anyone. The authors recommend a single screening question: "Are you being bullied?"—especially for children who are at higher risk, such as those with disabilities/special health needs, LGBTQ+ status, and who are under- or overweight.
Clearly we need research to know which interventions truly help these children/adolescents and their parents. In the meantime, however, identifying the problem and offering emotional support are unlikely to harm—and may help. Opening the lines of communication, connecting children and their parents with available community resources, and supporting anti-bullying programs in your schools are additional ways we can make a difference today.
1. Kann L, McManus T, Harris WA, et al. Youth Risk Behavior Surveillance System – United States, 2015. MMWR Morb Mortal Wkly. 2016;65:1-174.
2. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
3. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
When I became a family physician (FP), it never crossed my mind that I would one day be asking school-aged children about bullying. Not so much because bullying didn’t exist, but because I wasn’t aware of the pervasiveness and seriousness of the problem and because there were no professional recommendations to do so.
That said, my family had some first-hand experience with the issue: One of my children was bullied in grade school. When my wife found out, she promptly visited the 2 boys’ homes and told them and their parents that the behavior would stop or else! (She may have used more colorful language.) And it did stop. But times have changed, and so has the nature of bullying, which can now extend beyond the hallway to an entire school body in seconds with a few taps on a cell phone. And the adverse consequences can be significant, as described by McClowry and colleagues.
The prevalence of bullying is discouragingly high, estimated to be about 20% in national surveys.1 Because bullying occurs so frequently, public health, community-based, and school-based approaches, rather than one-on-one office-based interventions, are likely to have the greatest overall impact on decreasing bullying. Randomized trials bear this out, showing that prevention programs in schools can effectively reduce the behavior.2,3
What is our responsibility as FPs? Screening is a reasonable first step, even in the absence of randomized trials demonstrating benefit. Because there have been no physician office-based trials of screening or interventions for bullying, we must rely on “expert opinion” at this time, with no assurance that what we do will actually help children. Absence of proof of benefit, however, does not mean absence of benefit, and doing nothing will definitely not help anyone. The authors recommend a single screening question: "Are you being bullied?"—especially for children who are at higher risk, such as those with disabilities/special health needs, LGBTQ+ status, and who are under- or overweight.
Clearly we need research to know which interventions truly help these children/adolescents and their parents. In the meantime, however, identifying the problem and offering emotional support are unlikely to harm—and may help. Opening the lines of communication, connecting children and their parents with available community resources, and supporting anti-bullying programs in your schools are additional ways we can make a difference today.
When I became a family physician (FP), it never crossed my mind that I would one day be asking school-aged children about bullying. Not so much because bullying didn’t exist, but because I wasn’t aware of the pervasiveness and seriousness of the problem and because there were no professional recommendations to do so.
That said, my family had some first-hand experience with the issue: One of my children was bullied in grade school. When my wife found out, she promptly visited the 2 boys’ homes and told them and their parents that the behavior would stop or else! (She may have used more colorful language.) And it did stop. But times have changed, and so has the nature of bullying, which can now extend beyond the hallway to an entire school body in seconds with a few taps on a cell phone. And the adverse consequences can be significant, as described by McClowry and colleagues.
The prevalence of bullying is discouragingly high, estimated to be about 20% in national surveys.1 Because bullying occurs so frequently, public health, community-based, and school-based approaches, rather than one-on-one office-based interventions, are likely to have the greatest overall impact on decreasing bullying. Randomized trials bear this out, showing that prevention programs in schools can effectively reduce the behavior.2,3
What is our responsibility as FPs? Screening is a reasonable first step, even in the absence of randomized trials demonstrating benefit. Because there have been no physician office-based trials of screening or interventions for bullying, we must rely on “expert opinion” at this time, with no assurance that what we do will actually help children. Absence of proof of benefit, however, does not mean absence of benefit, and doing nothing will definitely not help anyone. The authors recommend a single screening question: "Are you being bullied?"—especially for children who are at higher risk, such as those with disabilities/special health needs, LGBTQ+ status, and who are under- or overweight.
Clearly we need research to know which interventions truly help these children/adolescents and their parents. In the meantime, however, identifying the problem and offering emotional support are unlikely to harm—and may help. Opening the lines of communication, connecting children and their parents with available community resources, and supporting anti-bullying programs in your schools are additional ways we can make a difference today.
1. Kann L, McManus T, Harris WA, et al. Youth Risk Behavior Surveillance System – United States, 2015. MMWR Morb Mortal Wkly. 2016;65:1-174.
2. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
3. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
1. Kann L, McManus T, Harris WA, et al. Youth Risk Behavior Surveillance System – United States, 2015. MMWR Morb Mortal Wkly. 2016;65:1-174.
2. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
3. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
Recurrent UTIs in women: How you can refine your care
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21
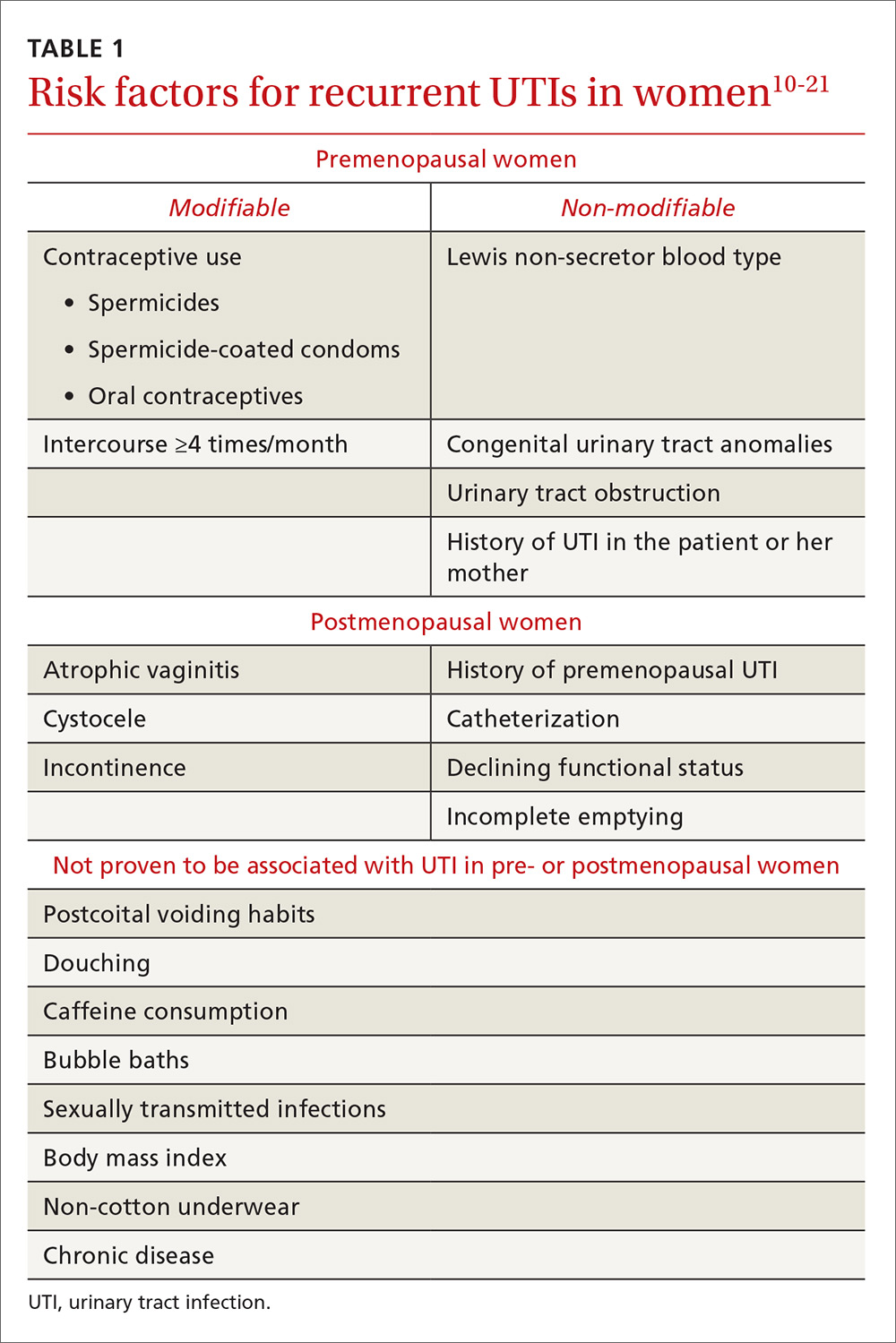
Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18
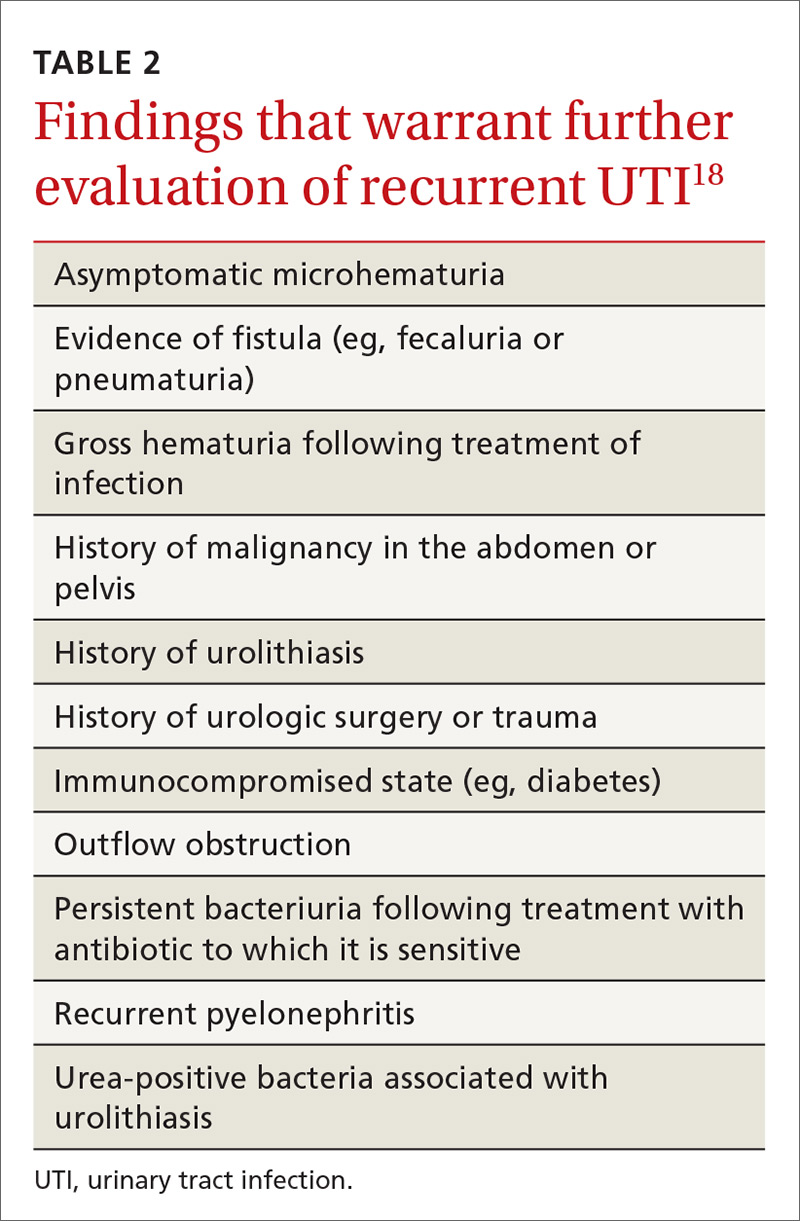
Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.
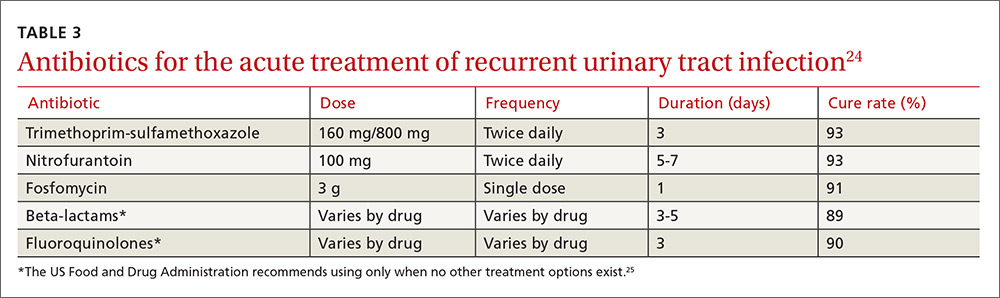
Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18 Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21

Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.

Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18 Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21

Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.

Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18 Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
PRACTICE RECOMMENDATIONS
› Avoid routine use of cystoscopy and imaging when evaluating women with recurrent urinary tract infection (UTI).
› Keep in mind that 3- to 5-day courses of antibiotics (nitrofurantoin, trimethoprim- sulfamethoxazole, fosfomycin, or beta-lactams) for UTIs are as effective as longer courses, and are associated with better compliance and fewer adverse effects.
› Assure patients considering prophylaxis for recurrent UTI that either continuous or postcoital antibiotics are effective.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Adult ADHD: Addressing a unique set of challenges
Attention-deficit/hyperactivity disorder (ADHD) in adults brings with it unique challenges, not the least of which are arriving at a proper diagnosis and ensuring that any psychostimulant drugs that you prescribe are not misused. A number of conditions such as anxiety, bipolar disorder, and substance abuse can mimic some of the symptoms of ADHD, and diagnostic criteria for the condition in adults changed with the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Furthermore, for many of the estimated 4.4% of adults who have ADHD,1 psychostimulants provide necessary and effective treatment, but misuse and diversion of these agents are real concerns. In fact, recent research reveals that these issues are more common than previously thought.2 Data suggest that the prevalence of misuse and diversion of ADHD medication is 5% to 10% among high school students and 5% to 35% among college students.2,3
This is not meant to indicate that adults diagnosed with ADHD should go untreated. In fact, adults with ADHD often struggle in their professional and family lives because they do not receive the treatment they need.
Rather, family physicians should take certain steps, first to diagnose ADHD correctly, and then to ascertain and maintain correct use of psychostimulants and other treatments among their adult patient populations. Read on for several practical strategies.
Criteria for adult Dx differ from those in children
ADHD, a common behavioral disorder that often, but not always, begins in childhood, is characterized by deficits in paying attention, difficulty controlling impulses, and marked hyperactivity. Diagnosis of ADHD is based on the DSM-5 criteria and supplemented with historical data and clinical observations.4 Using self-report measures may also aid in the diagnosis, and psychological testing may be required for some individuals when the clinical presentation is unclear.
With the DSM-5 changes (TABLE 14), the diagnosis of ADHD in adults (people ≥18 years) requires fewer symptoms than the diagnosis of ADHD in children; just 5 symptoms from either of the 2 categories of diagnostic criteria are sufficient now, whereas 6 symptoms were required previously and still are required to make the diagnosis in young people. People may present with the inattentive profile (5 or more symptoms of inattention), the hyperactive-impulsive profile (5 or more hyperactive-impulsive symptoms), or a combination of the 2 (5 or more symptoms of inattention plus 5 or more symptoms of hyperactivity-impulsivity for a total of 10 or more symptoms). While children are more likely to present with the combined type of ADHD, adults of any age are more likely to present with the inattentive type.4
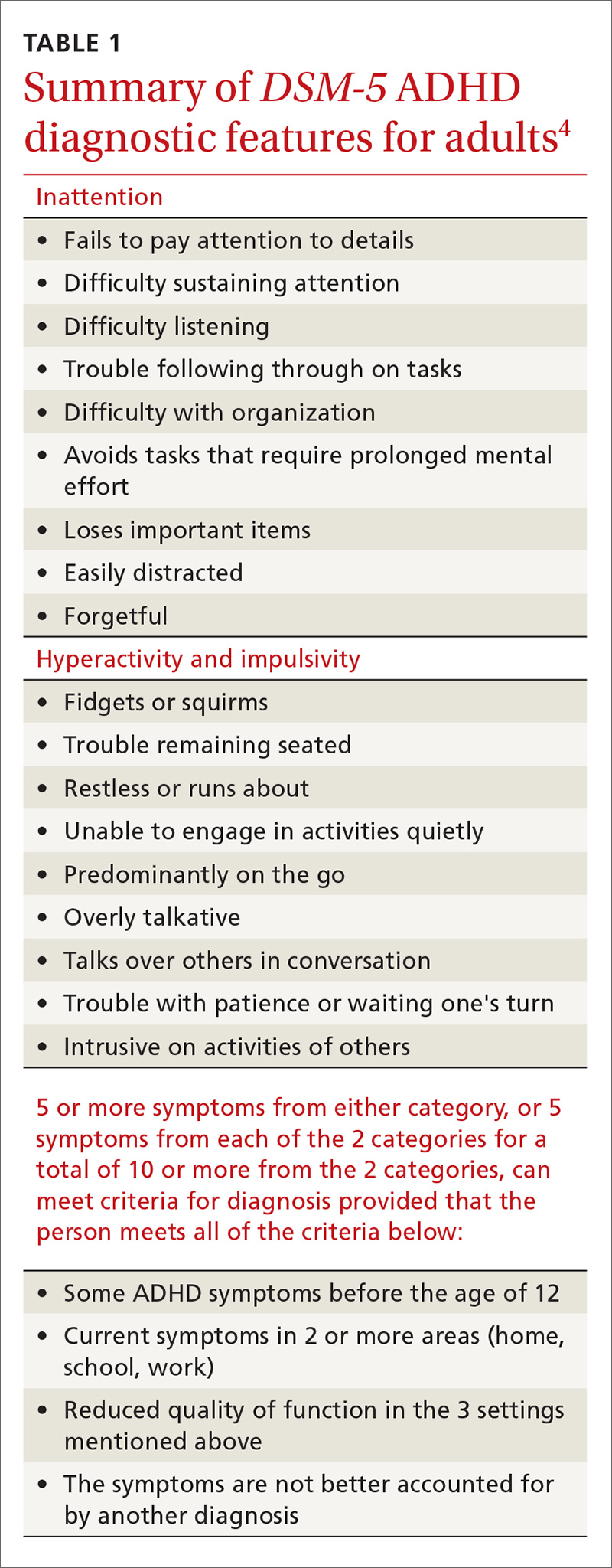
In addition, patients must meet the diagnostic criteria for ADHD for at least 6 months, have had some of the symptoms prior to age 12, and the symptoms must cause significant impairment in 2 or more environments (eg, home, work, school). When the diagnosis is unclear, it is important to obtain collateral information from the family, school, or workplace. The requirement regarding symptomatology before age 12 indicates the need for a review of the patient’s educational history. Research reveals that many adults with ADHD struggled in school and were considered “underachievers” as students.5
Common complaints and characteristics. Previous studies have shown the following to be common complaints and characteristics of adult patients diagnosed with ADHD:5
- difficulty meeting time limits
- vocational struggles, such as frequent job changes or nonpromotion at work
- anger issues
- addiction
- relationship/social strain
- comprehension problems, and
- a family history of ADHD.
Common correlates include low socioeconomic status, driving violations, frequent injuries, legal problems, alcohol and/or tobacco use, and self-reported maladjustment.5 People treated for ADHD have a comorbid DSM diagnosis 81% of the time with the most likely diagnoses being substance abuse, depression, and anxiety.6
Adult-onset ADHD? Even though the DSM-5 criteria for an ADHD diagnosis in adulthood require that some ADHD symptoms were manifest prior to age 12, recent longitudinal research on ADHD in Brazil and the United Kingdom reveals that a large portion of people who meet the criteria for ADHD in adulthood did not meet the criteria as children. The researchers in these studies proposed that there may be a form of ADHD that manifests later in life, a so-called “adult-onset ADHD.”7 While this information is something for clinicians to consider, further research is needed to justify a paradigm shift in how ADHD is diagnosed.
Self-rating measures can offer clarification. Whether or not history-gathering leaves the diagnosis murky, self-rating measures can be valuable in rounding out the clinical picture and alerting clinicians to any inconsistencies in symptoms.8 Four common ADHD self-rating measures are provided in TABLE 2. As one example, the Adult ADHD Self-Report Scale (ASRS) Symptom Checklist is a valuable ADHD screening tool that is free of charge and takes only 5 to 10 minutes.8 Other self-report measures require a similar amount of time, but are not available on a complimentary basis.
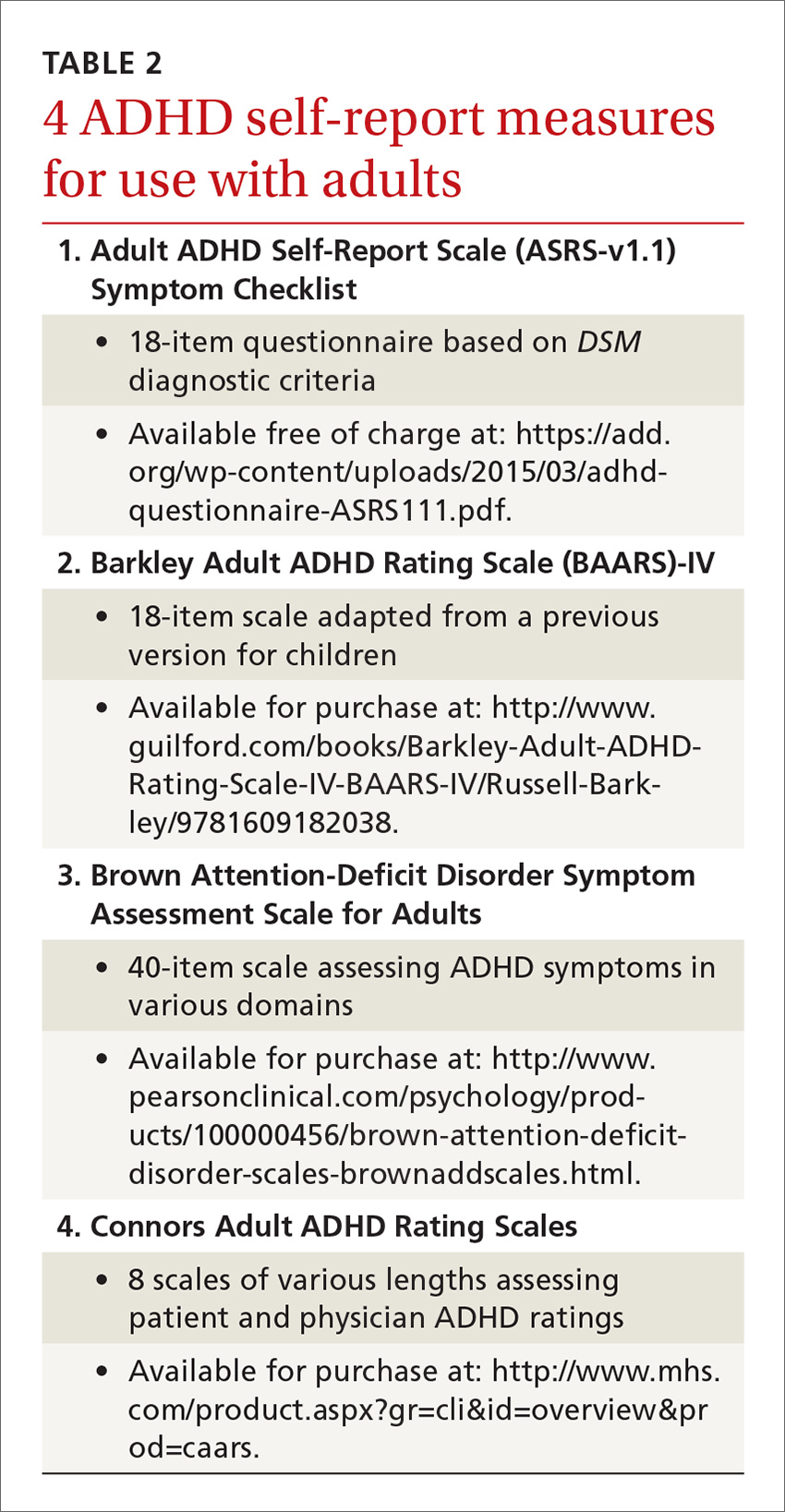
Psychological testing. Some adults who seem to have symptoms of ADHD may require a referral for psychological testing. These may be patients who present with complicated cases or whose histories and/or findings do not consistently indicate an ADHD diagnosis. In such cases, psychological testing can fill in the holes and provide a more complete picture of the patient’s neurocognitive abilities and deficits.9,10
Psychostimulant treatment: Opt for longer-acting agents
The standard treatment for ADHD is a psychostimulant. One controlled trial, for example, of a mixed amphetamine salts compound (Adderall) found that the compound effectively treated ADHD symptoms (hyperactivity, impulsivity, inattention) in adults and was well tolerated.11 While far fewer studies have been performed in adult vs youth populations, those that have been conducted in adults indicate that psychostimulants are largely safe and efficacious. In fact, the study mentioned above found that 70% of patients with ADHD ages 18 and older reported improvement of symptoms while on a short-acting psychostimulant, as compared to 7% who reported improvement on placebo.11
Similarly, a meta-analysis of 1991 participants in 11 studies found significant improvement in patients who received medication vs placebo, with stimulant medications demonstrating greater efficacy than non-stimulant treatments for ADHD.12 In general, psychostimulant treatment for adults is similar to that for children; the only difference is that adults tend to be more forthcoming with information regarding how the treatment is working and what adjustments might be needed.
Longer-acting stimulants (ie, extended release) tend to be preferred by patients to short-acting ones because they typically provide adequate control of symptoms over a longer period of time and thus may be taken less frequently.13 Also, the potential for abuse of psychostimulant medication tends to be lower with the longer-acting, extended-release formulations.14 A shorter acting formulation may be preferred if a patient has a specific window of time when their ADHD symptoms impact them. For example, a patient may request a short-acting form of medication for afternoons if he or she has to attend many business meetings at that time of day. A relatively new category is the intermediate-acting psychostimulants. For more on specific psychostimulants, see Table 3.15
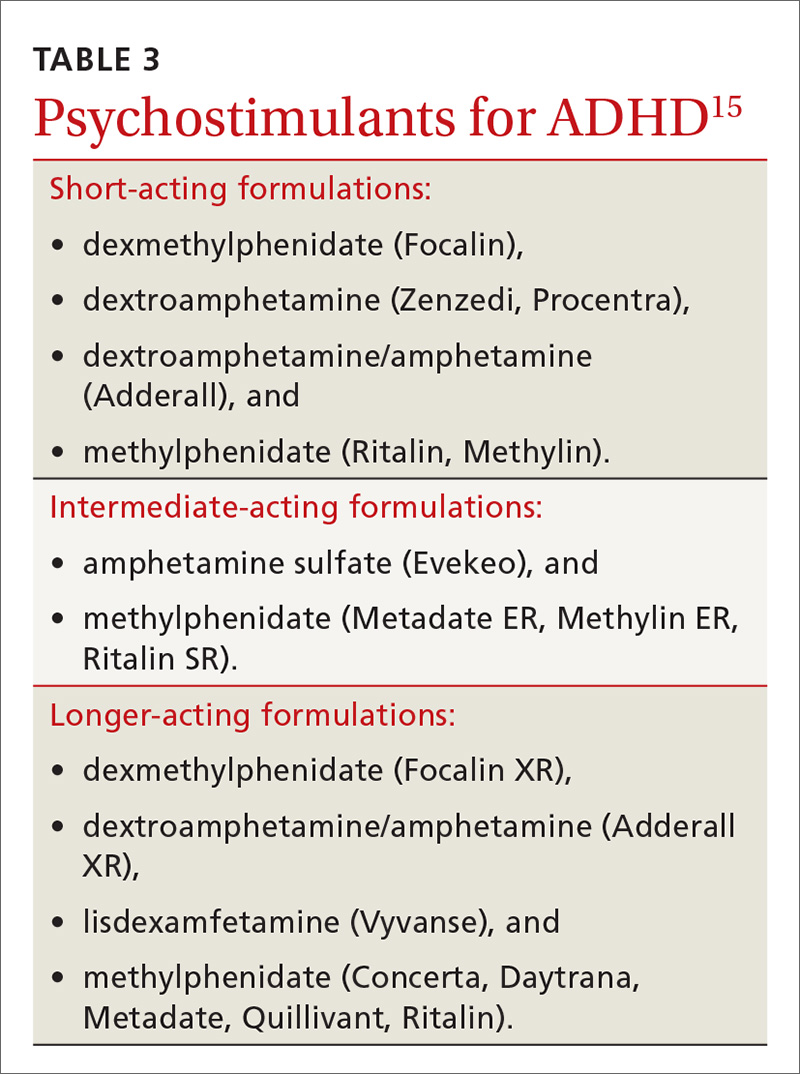
Adverse effects lead many to discontinue treatment
Regardless of the length of action of the psychostimulant, studies show that about 30% of adults (and, incidentally, 10% to 30% of children) discontinue treatment due to uncontrolled/unwanted symptoms or adverse effects.16 These include decreased appetite, headache, insomnia, abdominal pain, and irritable mood.17 If you are prescribing a psychostimulant for an adult with ADHD, it is important to tell the patient that if the effects become intolerable, adjustments can be made, such as tweaking dosages, switching to a different medication, or adding an adjunctive therapy such as cognitive behavioral therapy. Keep in mind, too, that if a medication is to be discontinued, tapering is suggested for most psychostimulants; patients should take a lower (eg, half) dose for about a week prior to complete discontinuation. People who have difficulties with a number of treatments for ADHD should be reevaluated in a year to see if circumstances have changed.5
Combatting misuse and diversion
Perhaps the most controversial issues surrounding the treatment of adults with ADHD are abuse and diversion of psychostimulants. Abuse generally refers to misuse of the drug by the person prescribed the agent, whereas diversion refers to use of the drug by people for whom the drug was not intended—with or without the prescribee’s knowledge.3 Although rare, chronic abuse of psychostimulants can lead to serious problems such as aggression, suicidal thoughts/behaviors, psychosis, and mania.5
While earlier studies tended to downplay the likelihood of diversion, recent research indicates that physicians should not underestimate the possibility. In fact, a previous article in this journal about student athletes with ADHD (http://bit.ly/2k1a6TL) indicated that psychostimulants have “great potential for misuse” and that recently there has been “a surge in nonprescription stimulant use among adolescents and young adults.”17 The authors of the article concluded, however, that while physicians should be aware of the potential for misuse, fear should not preclude treatment.17
A national multi-cohort study of 4572 US high school seniors who had used psychostimulants either medically or nonmedically indicated that while one in 6 high school seniors had been exposed to psychostimulants, about half were appropriately exposed through prescription use, while the other half was not. The researchers also reported that current nonmedical users of psychostimulants and those with a history of nonmedical use had a greater risk of substance use and abuse when compared to medical users of psychostimulants.18
Young men are at higher risk for diversion. In a nationwide survey of a sample of adults ages 18 to 49 years who had a prescription for psychostimulant medication in the past month, 17% admitted diverting their medication.
In another study, 483 students ages 17 to 19 years were followed for one year and interviewed frequently regarding their use of medications.20 The researchers reported that the lifetime prevalence of diversion of any medication in those students was around 36%. They also found that of those who diverted medication, 62% diverted ADHD medications at least once. These were most commonly diverted by sharing (34%) and selling (9%) the psychostimulants. Interview analysis revealed that those students who diverted were more likely to have used illicit drugs and to have had conduct problems. The authors advised “vigilance regarding…stimulant medications for young adults.”
Psychostimulant prescriptions do not cause substance abuse. Nevertheless, a salient point in the literature is that there is no causal relationship between psychostimulant prescriptions that are properly prescribed for people who have ADHD and substance abuse. One study that followed young people with ADHD for 8 years into adulthood revealed that psychostimulant treatment did not make adolescents or young adults any more or less likely to abuse drugs.21 The study also found that alcohol use was common in young adults whether they were diagnosed with ADHD or not. Nonetheless, family physicians should urge those taking psychostimulants to refrain from alcohol use or at least to drink in moderation.
The bottom line is that adults diagnosed with ADHD carry about the same risk of substance abuse as the general population if they are effectively treated for their presenting attention problems.22 If they are taking a psychostimulant, however, they have access to a controlled substance, unlike most of their cohorts. So it's important to teach patients with ADHD that for safety and legal reasons, they should not share or sell their stimulant medication to anyone.
Minimize the risk for abuse, diversion using these strategies
As with any drug regimen, it is important to monitor the patient’s response to treatment and minimize adverse effects and outcomes. When the drug is a psychostimulant for adults diagnosed with ADHD, it’s also important to minimize the risk for abuse and diversion. The following steps can help:
- Obtain a signed controlled substance agreement.23 This agreement between the physician and the patient usually outlines such specifics as frequency of office visits, circumstances surrounding medication refills, urine drug monitoring, and pill counts. (For more on the specifics of a controlled substance agreement, see "Key points of a controlled substance agreement.")
- Schedule frequent follow-up appointments with open communication about abuse and diversion.20,23 The age-old adage, “Start low, go slow,” applies to stimulant medications for ADHD. Medication dosage may vary and necessitate titration depending on the person’s weight and tolerance. At the onset of treatment, frequent office visits allow the physician to gauge treatment response and the patient’s commitment to therapy.
- Review your state’s prescription drug monitoring program.24 It is imperative that providers check their state’s medical board rules for prescribing controlled medications to ensure practice compliance. As diversion rates of controlled medications have risen in this country, most states have established monitoring systems through their pharmacy boards.24 Although the names of the programs vary, these prescription drug monitoring programs provide information on any medication prescribed. This allows the prescribing physician to ensure patient compliance and ascertain that no other controlled medications are being prescribed that could interfere with treatment. (For more information about state prescription drug monitoring programs, see https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.)
- Perform random urine drug screenings (UDS).20,23 An important strategy for ensuring adherence to the treatment plan and the controlled substance contract is UDS. Explain to patients that this is a way of making sure they are taking the medication exactly as prescribed. If the UDS indicates that the patient has not been taking the medication, then the provider should intervene by either restricting or discontinuing the controlled substance to prevent or counteract potential diversion. Similarly, if a higher dose is requested by a patient, the provider can closely monitor the situation to determine whether the additional drug is actually being taken and whether the dose is optimal. (See JFP’s October 2016 “3 in 3” video on urine drug testing at: http://bit.ly/2iDnfgD.)
- Employ a team-based, multimodal approach.25 A referral to a mental health professional and multimodal treatment are often recommended in the literature as best practices.25 Behavioral therapies are a cornerstone of treatment in adults with ADHD and often serve as important adjuncts to pharmacotherapy. Also, a referral provides a second professional opinion about the patient’s motivations, adherence, and response to treatment.
Trained cognitive behavioral therapists (eg, psychologists, counselors) can be helpful with treatment for ADHD.23 Therapists can be useful in setting goals for the patient regarding adherence, organization, impulse control, and social skills training. Therapists may wish to involve the family in treatment, depending on the nature of the patient’s presenting issues.
SIDEBAR
Key points of a controlled substance agreementThe primary purpose of a controlled substance agreement is to provide clarity for the provider and the patient regarding the use of controlled medications. The document is meant to prevent potential problems and confusion down the road. There are generally 3 parts:
- a doctor/patient agreement
- information about medications
- patient consent to utilize controlled substances that the provider believes would be beneficial.
Patients are typically told of the potential value of controlled medications in helping them and are warned about the potential for problems should the medications be used in ways other than intended. While wording may differ, patients are generally asked to agree to variations of the following 10 guidelines:
- I will talk with my doctor before using more than the prescribed amount of the medicine or discontinuing its use.
- I will tell my doctor if new medications are prescribed by another provider.
- I will tell the doctor if I become pregnant, so that any necessary medication adjustments can be made.
- If I abuse this drug, I understand that the doctor may need to stop treatment.
- I will uphold the visit schedule to the office/clinic according to guidelines for controlled substances (eg, every 90 days).
- I will refrain from using illicit drugs including marijuana and excessive quantities of alcohol.
- I will refrain from sharing, trading, or selling controlled substances.
- I will submit to regular urine drug screens as requested by the doctor.
- I understand that a failed drug screen may mean discontinuation of treatment.
- I will be forthright and honest about how the treatment is going, adverse effects, and how I am taking the medication.
Adapted from: https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf.
Don’t tempt fate. As with any controlled medication, safe storage of psychostimulants is paramount. Patients should be urged to keep their medication in a locked box or cupboard that is accessible to only the adult for whom the drug is prescribed. Prior research cautions that open access to controlled substances can lead to larger issues with abuse and diversion, particularly when adolescents are in the home.26
Consider atomoxetine. Research has also demonstrated that the non-stimulant medication atomoxetine has some benefit in the treatment of ADHD.12 Unlike psychostimulants that act on the neurotransmitter dopamine, atomoxetine acts on the neurotransmitter norepinephrine. This different mechanism of action results in a lower potential for abuse and diversion.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected].
1. Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
2. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64-81.
3. Novak SP, Kroutil LA, Williams RL, et al. The nonmedical use of prescription ADHD medications: results from a national internet panel. Subst Abuse Treat Prev Policy. 2007;2:32.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
5. Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389-403.
6. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621-1627.
7. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry.
8. van de Glind G, van den Brink W, Koeter MWJ, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132:587-596.
9. Gualtieri CT, Johnson LG. ADHD: Is objective diagnosis possible? Psychiatry. 2005;2:44-53.
10. Perrin AE, Jotwani VM. Addressing the unique issues of student athletes with ADHD. J Fam Pract. 2014;63:E1-E9.
11. Spencer T, Biederman J, Wilens T, et al. Efficacy of mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775-782.
12. Mészáros A, Czobor P, Bálint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-1147.
13. Weiss M, Shingler T, Capone NM. Medication satisfaction among adults with ADHD: long term results from the Quality of Life, Effectiveness, Safety and Tolerability (Qu.S.T) study. New Orleans: Program and Abstracts of the 19th US Psychiatric and Mental Health Congress, Abstract 120, 2006.
14. Lopez FA, Leroux JR.
15. Felt BT, Biermann B, Christner JG, et al. Diagnosis and management of ADHD in children. Am Fam Physician. 2014;90:456-464.
16. Spencer T, Biederman J, Wilens T. Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:373-383.
17. Withrow LM, Hash PA, Holten KB. Managing ADHD in children: are you doing enough? J Fam Pract. 2011;60:E1-E3.
18. McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52:1272-1280.
19. Aldridge AP, Kroutil LA, Cowell AJ, et al. Medication costs to private insurers of diversion of medications for attention-deficit hyperactivity disorder. Pharmacoeconomics. 2011;29:621-635.
20. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
21. Molina BSG, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250-263.
22. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143.
23. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85:890-896.
24. Cepeda MS, Fife D, Berwaerts J, et al. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41:226-229.
25. Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-880.
26. Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medication in the home. J Adolesc Health. 2013;53:260-264.
Attention-deficit/hyperactivity disorder (ADHD) in adults brings with it unique challenges, not the least of which are arriving at a proper diagnosis and ensuring that any psychostimulant drugs that you prescribe are not misused. A number of conditions such as anxiety, bipolar disorder, and substance abuse can mimic some of the symptoms of ADHD, and diagnostic criteria for the condition in adults changed with the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Furthermore, for many of the estimated 4.4% of adults who have ADHD,1 psychostimulants provide necessary and effective treatment, but misuse and diversion of these agents are real concerns. In fact, recent research reveals that these issues are more common than previously thought.2 Data suggest that the prevalence of misuse and diversion of ADHD medication is 5% to 10% among high school students and 5% to 35% among college students.2,3
This is not meant to indicate that adults diagnosed with ADHD should go untreated. In fact, adults with ADHD often struggle in their professional and family lives because they do not receive the treatment they need.
Rather, family physicians should take certain steps, first to diagnose ADHD correctly, and then to ascertain and maintain correct use of psychostimulants and other treatments among their adult patient populations. Read on for several practical strategies.
Criteria for adult Dx differ from those in children
ADHD, a common behavioral disorder that often, but not always, begins in childhood, is characterized by deficits in paying attention, difficulty controlling impulses, and marked hyperactivity. Diagnosis of ADHD is based on the DSM-5 criteria and supplemented with historical data and clinical observations.4 Using self-report measures may also aid in the diagnosis, and psychological testing may be required for some individuals when the clinical presentation is unclear.
With the DSM-5 changes (TABLE 14), the diagnosis of ADHD in adults (people ≥18 years) requires fewer symptoms than the diagnosis of ADHD in children; just 5 symptoms from either of the 2 categories of diagnostic criteria are sufficient now, whereas 6 symptoms were required previously and still are required to make the diagnosis in young people. People may present with the inattentive profile (5 or more symptoms of inattention), the hyperactive-impulsive profile (5 or more hyperactive-impulsive symptoms), or a combination of the 2 (5 or more symptoms of inattention plus 5 or more symptoms of hyperactivity-impulsivity for a total of 10 or more symptoms). While children are more likely to present with the combined type of ADHD, adults of any age are more likely to present with the inattentive type.4

In addition, patients must meet the diagnostic criteria for ADHD for at least 6 months, have had some of the symptoms prior to age 12, and the symptoms must cause significant impairment in 2 or more environments (eg, home, work, school). When the diagnosis is unclear, it is important to obtain collateral information from the family, school, or workplace. The requirement regarding symptomatology before age 12 indicates the need for a review of the patient’s educational history. Research reveals that many adults with ADHD struggled in school and were considered “underachievers” as students.5
Common complaints and characteristics. Previous studies have shown the following to be common complaints and characteristics of adult patients diagnosed with ADHD:5
- difficulty meeting time limits
- vocational struggles, such as frequent job changes or nonpromotion at work
- anger issues
- addiction
- relationship/social strain
- comprehension problems, and
- a family history of ADHD.
Common correlates include low socioeconomic status, driving violations, frequent injuries, legal problems, alcohol and/or tobacco use, and self-reported maladjustment.5 People treated for ADHD have a comorbid DSM diagnosis 81% of the time with the most likely diagnoses being substance abuse, depression, and anxiety.6
Adult-onset ADHD? Even though the DSM-5 criteria for an ADHD diagnosis in adulthood require that some ADHD symptoms were manifest prior to age 12, recent longitudinal research on ADHD in Brazil and the United Kingdom reveals that a large portion of people who meet the criteria for ADHD in adulthood did not meet the criteria as children. The researchers in these studies proposed that there may be a form of ADHD that manifests later in life, a so-called “adult-onset ADHD.”7 While this information is something for clinicians to consider, further research is needed to justify a paradigm shift in how ADHD is diagnosed.
Self-rating measures can offer clarification. Whether or not history-gathering leaves the diagnosis murky, self-rating measures can be valuable in rounding out the clinical picture and alerting clinicians to any inconsistencies in symptoms.8 Four common ADHD self-rating measures are provided in TABLE 2. As one example, the Adult ADHD Self-Report Scale (ASRS) Symptom Checklist is a valuable ADHD screening tool that is free of charge and takes only 5 to 10 minutes.8 Other self-report measures require a similar amount of time, but are not available on a complimentary basis.

Psychological testing. Some adults who seem to have symptoms of ADHD may require a referral for psychological testing. These may be patients who present with complicated cases or whose histories and/or findings do not consistently indicate an ADHD diagnosis. In such cases, psychological testing can fill in the holes and provide a more complete picture of the patient’s neurocognitive abilities and deficits.9,10
Psychostimulant treatment: Opt for longer-acting agents
The standard treatment for ADHD is a psychostimulant. One controlled trial, for example, of a mixed amphetamine salts compound (Adderall) found that the compound effectively treated ADHD symptoms (hyperactivity, impulsivity, inattention) in adults and was well tolerated.11 While far fewer studies have been performed in adult vs youth populations, those that have been conducted in adults indicate that psychostimulants are largely safe and efficacious. In fact, the study mentioned above found that 70% of patients with ADHD ages 18 and older reported improvement of symptoms while on a short-acting psychostimulant, as compared to 7% who reported improvement on placebo.11
Similarly, a meta-analysis of 1991 participants in 11 studies found significant improvement in patients who received medication vs placebo, with stimulant medications demonstrating greater efficacy than non-stimulant treatments for ADHD.12 In general, psychostimulant treatment for adults is similar to that for children; the only difference is that adults tend to be more forthcoming with information regarding how the treatment is working and what adjustments might be needed.
Longer-acting stimulants (ie, extended release) tend to be preferred by patients to short-acting ones because they typically provide adequate control of symptoms over a longer period of time and thus may be taken less frequently.13 Also, the potential for abuse of psychostimulant medication tends to be lower with the longer-acting, extended-release formulations.14 A shorter acting formulation may be preferred if a patient has a specific window of time when their ADHD symptoms impact them. For example, a patient may request a short-acting form of medication for afternoons if he or she has to attend many business meetings at that time of day. A relatively new category is the intermediate-acting psychostimulants. For more on specific psychostimulants, see Table 3.15

Adverse effects lead many to discontinue treatment
Regardless of the length of action of the psychostimulant, studies show that about 30% of adults (and, incidentally, 10% to 30% of children) discontinue treatment due to uncontrolled/unwanted symptoms or adverse effects.16 These include decreased appetite, headache, insomnia, abdominal pain, and irritable mood.17 If you are prescribing a psychostimulant for an adult with ADHD, it is important to tell the patient that if the effects become intolerable, adjustments can be made, such as tweaking dosages, switching to a different medication, or adding an adjunctive therapy such as cognitive behavioral therapy. Keep in mind, too, that if a medication is to be discontinued, tapering is suggested for most psychostimulants; patients should take a lower (eg, half) dose for about a week prior to complete discontinuation. People who have difficulties with a number of treatments for ADHD should be reevaluated in a year to see if circumstances have changed.5
Combatting misuse and diversion
Perhaps the most controversial issues surrounding the treatment of adults with ADHD are abuse and diversion of psychostimulants. Abuse generally refers to misuse of the drug by the person prescribed the agent, whereas diversion refers to use of the drug by people for whom the drug was not intended—with or without the prescribee’s knowledge.3 Although rare, chronic abuse of psychostimulants can lead to serious problems such as aggression, suicidal thoughts/behaviors, psychosis, and mania.5
While earlier studies tended to downplay the likelihood of diversion, recent research indicates that physicians should not underestimate the possibility. In fact, a previous article in this journal about student athletes with ADHD (http://bit.ly/2k1a6TL) indicated that psychostimulants have “great potential for misuse” and that recently there has been “a surge in nonprescription stimulant use among adolescents and young adults.”17 The authors of the article concluded, however, that while physicians should be aware of the potential for misuse, fear should not preclude treatment.17
A national multi-cohort study of 4572 US high school seniors who had used psychostimulants either medically or nonmedically indicated that while one in 6 high school seniors had been exposed to psychostimulants, about half were appropriately exposed through prescription use, while the other half was not. The researchers also reported that current nonmedical users of psychostimulants and those with a history of nonmedical use had a greater risk of substance use and abuse when compared to medical users of psychostimulants.18
Young men are at higher risk for diversion. In a nationwide survey of a sample of adults ages 18 to 49 years who had a prescription for psychostimulant medication in the past month, 17% admitted diverting their medication.
In another study, 483 students ages 17 to 19 years were followed for one year and interviewed frequently regarding their use of medications.20 The researchers reported that the lifetime prevalence of diversion of any medication in those students was around 36%. They also found that of those who diverted medication, 62% diverted ADHD medications at least once. These were most commonly diverted by sharing (34%) and selling (9%) the psychostimulants. Interview analysis revealed that those students who diverted were more likely to have used illicit drugs and to have had conduct problems. The authors advised “vigilance regarding…stimulant medications for young adults.”
Psychostimulant prescriptions do not cause substance abuse. Nevertheless, a salient point in the literature is that there is no causal relationship between psychostimulant prescriptions that are properly prescribed for people who have ADHD and substance abuse. One study that followed young people with ADHD for 8 years into adulthood revealed that psychostimulant treatment did not make adolescents or young adults any more or less likely to abuse drugs.21 The study also found that alcohol use was common in young adults whether they were diagnosed with ADHD or not. Nonetheless, family physicians should urge those taking psychostimulants to refrain from alcohol use or at least to drink in moderation.
The bottom line is that adults diagnosed with ADHD carry about the same risk of substance abuse as the general population if they are effectively treated for their presenting attention problems.22 If they are taking a psychostimulant, however, they have access to a controlled substance, unlike most of their cohorts. So it's important to teach patients with ADHD that for safety and legal reasons, they should not share or sell their stimulant medication to anyone.
Minimize the risk for abuse, diversion using these strategies
As with any drug regimen, it is important to monitor the patient’s response to treatment and minimize adverse effects and outcomes. When the drug is a psychostimulant for adults diagnosed with ADHD, it’s also important to minimize the risk for abuse and diversion. The following steps can help:
- Obtain a signed controlled substance agreement.23 This agreement between the physician and the patient usually outlines such specifics as frequency of office visits, circumstances surrounding medication refills, urine drug monitoring, and pill counts. (For more on the specifics of a controlled substance agreement, see "Key points of a controlled substance agreement.")
- Schedule frequent follow-up appointments with open communication about abuse and diversion.20,23 The age-old adage, “Start low, go slow,” applies to stimulant medications for ADHD. Medication dosage may vary and necessitate titration depending on the person’s weight and tolerance. At the onset of treatment, frequent office visits allow the physician to gauge treatment response and the patient’s commitment to therapy.
- Review your state’s prescription drug monitoring program.24 It is imperative that providers check their state’s medical board rules for prescribing controlled medications to ensure practice compliance. As diversion rates of controlled medications have risen in this country, most states have established monitoring systems through their pharmacy boards.24 Although the names of the programs vary, these prescription drug monitoring programs provide information on any medication prescribed. This allows the prescribing physician to ensure patient compliance and ascertain that no other controlled medications are being prescribed that could interfere with treatment. (For more information about state prescription drug monitoring programs, see https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.)
- Perform random urine drug screenings (UDS).20,23 An important strategy for ensuring adherence to the treatment plan and the controlled substance contract is UDS. Explain to patients that this is a way of making sure they are taking the medication exactly as prescribed. If the UDS indicates that the patient has not been taking the medication, then the provider should intervene by either restricting or discontinuing the controlled substance to prevent or counteract potential diversion. Similarly, if a higher dose is requested by a patient, the provider can closely monitor the situation to determine whether the additional drug is actually being taken and whether the dose is optimal. (See JFP’s October 2016 “3 in 3” video on urine drug testing at: http://bit.ly/2iDnfgD.)
- Employ a team-based, multimodal approach.25 A referral to a mental health professional and multimodal treatment are often recommended in the literature as best practices.25 Behavioral therapies are a cornerstone of treatment in adults with ADHD and often serve as important adjuncts to pharmacotherapy. Also, a referral provides a second professional opinion about the patient’s motivations, adherence, and response to treatment.
Trained cognitive behavioral therapists (eg, psychologists, counselors) can be helpful with treatment for ADHD.23 Therapists can be useful in setting goals for the patient regarding adherence, organization, impulse control, and social skills training. Therapists may wish to involve the family in treatment, depending on the nature of the patient’s presenting issues.
SIDEBAR
Key points of a controlled substance agreementThe primary purpose of a controlled substance agreement is to provide clarity for the provider and the patient regarding the use of controlled medications. The document is meant to prevent potential problems and confusion down the road. There are generally 3 parts:
- a doctor/patient agreement
- information about medications
- patient consent to utilize controlled substances that the provider believes would be beneficial.
Patients are typically told of the potential value of controlled medications in helping them and are warned about the potential for problems should the medications be used in ways other than intended. While wording may differ, patients are generally asked to agree to variations of the following 10 guidelines:
- I will talk with my doctor before using more than the prescribed amount of the medicine or discontinuing its use.
- I will tell my doctor if new medications are prescribed by another provider.
- I will tell the doctor if I become pregnant, so that any necessary medication adjustments can be made.
- If I abuse this drug, I understand that the doctor may need to stop treatment.
- I will uphold the visit schedule to the office/clinic according to guidelines for controlled substances (eg, every 90 days).
- I will refrain from using illicit drugs including marijuana and excessive quantities of alcohol.
- I will refrain from sharing, trading, or selling controlled substances.
- I will submit to regular urine drug screens as requested by the doctor.
- I understand that a failed drug screen may mean discontinuation of treatment.
- I will be forthright and honest about how the treatment is going, adverse effects, and how I am taking the medication.
Adapted from: https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf.
Don’t tempt fate. As with any controlled medication, safe storage of psychostimulants is paramount. Patients should be urged to keep their medication in a locked box or cupboard that is accessible to only the adult for whom the drug is prescribed. Prior research cautions that open access to controlled substances can lead to larger issues with abuse and diversion, particularly when adolescents are in the home.26
Consider atomoxetine. Research has also demonstrated that the non-stimulant medication atomoxetine has some benefit in the treatment of ADHD.12 Unlike psychostimulants that act on the neurotransmitter dopamine, atomoxetine acts on the neurotransmitter norepinephrine. This different mechanism of action results in a lower potential for abuse and diversion.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected].
Attention-deficit/hyperactivity disorder (ADHD) in adults brings with it unique challenges, not the least of which are arriving at a proper diagnosis and ensuring that any psychostimulant drugs that you prescribe are not misused. A number of conditions such as anxiety, bipolar disorder, and substance abuse can mimic some of the symptoms of ADHD, and diagnostic criteria for the condition in adults changed with the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Furthermore, for many of the estimated 4.4% of adults who have ADHD,1 psychostimulants provide necessary and effective treatment, but misuse and diversion of these agents are real concerns. In fact, recent research reveals that these issues are more common than previously thought.2 Data suggest that the prevalence of misuse and diversion of ADHD medication is 5% to 10% among high school students and 5% to 35% among college students.2,3
This is not meant to indicate that adults diagnosed with ADHD should go untreated. In fact, adults with ADHD often struggle in their professional and family lives because they do not receive the treatment they need.
Rather, family physicians should take certain steps, first to diagnose ADHD correctly, and then to ascertain and maintain correct use of psychostimulants and other treatments among their adult patient populations. Read on for several practical strategies.
Criteria for adult Dx differ from those in children
ADHD, a common behavioral disorder that often, but not always, begins in childhood, is characterized by deficits in paying attention, difficulty controlling impulses, and marked hyperactivity. Diagnosis of ADHD is based on the DSM-5 criteria and supplemented with historical data and clinical observations.4 Using self-report measures may also aid in the diagnosis, and psychological testing may be required for some individuals when the clinical presentation is unclear.
With the DSM-5 changes (TABLE 14), the diagnosis of ADHD in adults (people ≥18 years) requires fewer symptoms than the diagnosis of ADHD in children; just 5 symptoms from either of the 2 categories of diagnostic criteria are sufficient now, whereas 6 symptoms were required previously and still are required to make the diagnosis in young people. People may present with the inattentive profile (5 or more symptoms of inattention), the hyperactive-impulsive profile (5 or more hyperactive-impulsive symptoms), or a combination of the 2 (5 or more symptoms of inattention plus 5 or more symptoms of hyperactivity-impulsivity for a total of 10 or more symptoms). While children are more likely to present with the combined type of ADHD, adults of any age are more likely to present with the inattentive type.4

In addition, patients must meet the diagnostic criteria for ADHD for at least 6 months, have had some of the symptoms prior to age 12, and the symptoms must cause significant impairment in 2 or more environments (eg, home, work, school). When the diagnosis is unclear, it is important to obtain collateral information from the family, school, or workplace. The requirement regarding symptomatology before age 12 indicates the need for a review of the patient’s educational history. Research reveals that many adults with ADHD struggled in school and were considered “underachievers” as students.5
Common complaints and characteristics. Previous studies have shown the following to be common complaints and characteristics of adult patients diagnosed with ADHD:5
- difficulty meeting time limits
- vocational struggles, such as frequent job changes or nonpromotion at work
- anger issues
- addiction
- relationship/social strain
- comprehension problems, and
- a family history of ADHD.
Common correlates include low socioeconomic status, driving violations, frequent injuries, legal problems, alcohol and/or tobacco use, and self-reported maladjustment.5 People treated for ADHD have a comorbid DSM diagnosis 81% of the time with the most likely diagnoses being substance abuse, depression, and anxiety.6
Adult-onset ADHD? Even though the DSM-5 criteria for an ADHD diagnosis in adulthood require that some ADHD symptoms were manifest prior to age 12, recent longitudinal research on ADHD in Brazil and the United Kingdom reveals that a large portion of people who meet the criteria for ADHD in adulthood did not meet the criteria as children. The researchers in these studies proposed that there may be a form of ADHD that manifests later in life, a so-called “adult-onset ADHD.”7 While this information is something for clinicians to consider, further research is needed to justify a paradigm shift in how ADHD is diagnosed.
Self-rating measures can offer clarification. Whether or not history-gathering leaves the diagnosis murky, self-rating measures can be valuable in rounding out the clinical picture and alerting clinicians to any inconsistencies in symptoms.8 Four common ADHD self-rating measures are provided in TABLE 2. As one example, the Adult ADHD Self-Report Scale (ASRS) Symptom Checklist is a valuable ADHD screening tool that is free of charge and takes only 5 to 10 minutes.8 Other self-report measures require a similar amount of time, but are not available on a complimentary basis.

Psychological testing. Some adults who seem to have symptoms of ADHD may require a referral for psychological testing. These may be patients who present with complicated cases or whose histories and/or findings do not consistently indicate an ADHD diagnosis. In such cases, psychological testing can fill in the holes and provide a more complete picture of the patient’s neurocognitive abilities and deficits.9,10
Psychostimulant treatment: Opt for longer-acting agents
The standard treatment for ADHD is a psychostimulant. One controlled trial, for example, of a mixed amphetamine salts compound (Adderall) found that the compound effectively treated ADHD symptoms (hyperactivity, impulsivity, inattention) in adults and was well tolerated.11 While far fewer studies have been performed in adult vs youth populations, those that have been conducted in adults indicate that psychostimulants are largely safe and efficacious. In fact, the study mentioned above found that 70% of patients with ADHD ages 18 and older reported improvement of symptoms while on a short-acting psychostimulant, as compared to 7% who reported improvement on placebo.11
Similarly, a meta-analysis of 1991 participants in 11 studies found significant improvement in patients who received medication vs placebo, with stimulant medications demonstrating greater efficacy than non-stimulant treatments for ADHD.12 In general, psychostimulant treatment for adults is similar to that for children; the only difference is that adults tend to be more forthcoming with information regarding how the treatment is working and what adjustments might be needed.
Longer-acting stimulants (ie, extended release) tend to be preferred by patients to short-acting ones because they typically provide adequate control of symptoms over a longer period of time and thus may be taken less frequently.13 Also, the potential for abuse of psychostimulant medication tends to be lower with the longer-acting, extended-release formulations.14 A shorter acting formulation may be preferred if a patient has a specific window of time when their ADHD symptoms impact them. For example, a patient may request a short-acting form of medication for afternoons if he or she has to attend many business meetings at that time of day. A relatively new category is the intermediate-acting psychostimulants. For more on specific psychostimulants, see Table 3.15

Adverse effects lead many to discontinue treatment
Regardless of the length of action of the psychostimulant, studies show that about 30% of adults (and, incidentally, 10% to 30% of children) discontinue treatment due to uncontrolled/unwanted symptoms or adverse effects.16 These include decreased appetite, headache, insomnia, abdominal pain, and irritable mood.17 If you are prescribing a psychostimulant for an adult with ADHD, it is important to tell the patient that if the effects become intolerable, adjustments can be made, such as tweaking dosages, switching to a different medication, or adding an adjunctive therapy such as cognitive behavioral therapy. Keep in mind, too, that if a medication is to be discontinued, tapering is suggested for most psychostimulants; patients should take a lower (eg, half) dose for about a week prior to complete discontinuation. People who have difficulties with a number of treatments for ADHD should be reevaluated in a year to see if circumstances have changed.5
Combatting misuse and diversion
Perhaps the most controversial issues surrounding the treatment of adults with ADHD are abuse and diversion of psychostimulants. Abuse generally refers to misuse of the drug by the person prescribed the agent, whereas diversion refers to use of the drug by people for whom the drug was not intended—with or without the prescribee’s knowledge.3 Although rare, chronic abuse of psychostimulants can lead to serious problems such as aggression, suicidal thoughts/behaviors, psychosis, and mania.5
While earlier studies tended to downplay the likelihood of diversion, recent research indicates that physicians should not underestimate the possibility. In fact, a previous article in this journal about student athletes with ADHD (http://bit.ly/2k1a6TL) indicated that psychostimulants have “great potential for misuse” and that recently there has been “a surge in nonprescription stimulant use among adolescents and young adults.”17 The authors of the article concluded, however, that while physicians should be aware of the potential for misuse, fear should not preclude treatment.17
A national multi-cohort study of 4572 US high school seniors who had used psychostimulants either medically or nonmedically indicated that while one in 6 high school seniors had been exposed to psychostimulants, about half were appropriately exposed through prescription use, while the other half was not. The researchers also reported that current nonmedical users of psychostimulants and those with a history of nonmedical use had a greater risk of substance use and abuse when compared to medical users of psychostimulants.18
Young men are at higher risk for diversion. In a nationwide survey of a sample of adults ages 18 to 49 years who had a prescription for psychostimulant medication in the past month, 17% admitted diverting their medication.
In another study, 483 students ages 17 to 19 years were followed for one year and interviewed frequently regarding their use of medications.20 The researchers reported that the lifetime prevalence of diversion of any medication in those students was around 36%. They also found that of those who diverted medication, 62% diverted ADHD medications at least once. These were most commonly diverted by sharing (34%) and selling (9%) the psychostimulants. Interview analysis revealed that those students who diverted were more likely to have used illicit drugs and to have had conduct problems. The authors advised “vigilance regarding…stimulant medications for young adults.”
Psychostimulant prescriptions do not cause substance abuse. Nevertheless, a salient point in the literature is that there is no causal relationship between psychostimulant prescriptions that are properly prescribed for people who have ADHD and substance abuse. One study that followed young people with ADHD for 8 years into adulthood revealed that psychostimulant treatment did not make adolescents or young adults any more or less likely to abuse drugs.21 The study also found that alcohol use was common in young adults whether they were diagnosed with ADHD or not. Nonetheless, family physicians should urge those taking psychostimulants to refrain from alcohol use or at least to drink in moderation.
The bottom line is that adults diagnosed with ADHD carry about the same risk of substance abuse as the general population if they are effectively treated for their presenting attention problems.22 If they are taking a psychostimulant, however, they have access to a controlled substance, unlike most of their cohorts. So it's important to teach patients with ADHD that for safety and legal reasons, they should not share or sell their stimulant medication to anyone.
Minimize the risk for abuse, diversion using these strategies
As with any drug regimen, it is important to monitor the patient’s response to treatment and minimize adverse effects and outcomes. When the drug is a psychostimulant for adults diagnosed with ADHD, it’s also important to minimize the risk for abuse and diversion. The following steps can help:
- Obtain a signed controlled substance agreement.23 This agreement between the physician and the patient usually outlines such specifics as frequency of office visits, circumstances surrounding medication refills, urine drug monitoring, and pill counts. (For more on the specifics of a controlled substance agreement, see "Key points of a controlled substance agreement.")
- Schedule frequent follow-up appointments with open communication about abuse and diversion.20,23 The age-old adage, “Start low, go slow,” applies to stimulant medications for ADHD. Medication dosage may vary and necessitate titration depending on the person’s weight and tolerance. At the onset of treatment, frequent office visits allow the physician to gauge treatment response and the patient’s commitment to therapy.
- Review your state’s prescription drug monitoring program.24 It is imperative that providers check their state’s medical board rules for prescribing controlled medications to ensure practice compliance. As diversion rates of controlled medications have risen in this country, most states have established monitoring systems through their pharmacy boards.24 Although the names of the programs vary, these prescription drug monitoring programs provide information on any medication prescribed. This allows the prescribing physician to ensure patient compliance and ascertain that no other controlled medications are being prescribed that could interfere with treatment. (For more information about state prescription drug monitoring programs, see https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.)
- Perform random urine drug screenings (UDS).20,23 An important strategy for ensuring adherence to the treatment plan and the controlled substance contract is UDS. Explain to patients that this is a way of making sure they are taking the medication exactly as prescribed. If the UDS indicates that the patient has not been taking the medication, then the provider should intervene by either restricting or discontinuing the controlled substance to prevent or counteract potential diversion. Similarly, if a higher dose is requested by a patient, the provider can closely monitor the situation to determine whether the additional drug is actually being taken and whether the dose is optimal. (See JFP’s October 2016 “3 in 3” video on urine drug testing at: http://bit.ly/2iDnfgD.)
- Employ a team-based, multimodal approach.25 A referral to a mental health professional and multimodal treatment are often recommended in the literature as best practices.25 Behavioral therapies are a cornerstone of treatment in adults with ADHD and often serve as important adjuncts to pharmacotherapy. Also, a referral provides a second professional opinion about the patient’s motivations, adherence, and response to treatment.
Trained cognitive behavioral therapists (eg, psychologists, counselors) can be helpful with treatment for ADHD.23 Therapists can be useful in setting goals for the patient regarding adherence, organization, impulse control, and social skills training. Therapists may wish to involve the family in treatment, depending on the nature of the patient’s presenting issues.
SIDEBAR
Key points of a controlled substance agreementThe primary purpose of a controlled substance agreement is to provide clarity for the provider and the patient regarding the use of controlled medications. The document is meant to prevent potential problems and confusion down the road. There are generally 3 parts:
- a doctor/patient agreement
- information about medications
- patient consent to utilize controlled substances that the provider believes would be beneficial.
Patients are typically told of the potential value of controlled medications in helping them and are warned about the potential for problems should the medications be used in ways other than intended. While wording may differ, patients are generally asked to agree to variations of the following 10 guidelines:
- I will talk with my doctor before using more than the prescribed amount of the medicine or discontinuing its use.
- I will tell my doctor if new medications are prescribed by another provider.
- I will tell the doctor if I become pregnant, so that any necessary medication adjustments can be made.
- If I abuse this drug, I understand that the doctor may need to stop treatment.
- I will uphold the visit schedule to the office/clinic according to guidelines for controlled substances (eg, every 90 days).
- I will refrain from using illicit drugs including marijuana and excessive quantities of alcohol.
- I will refrain from sharing, trading, or selling controlled substances.
- I will submit to regular urine drug screens as requested by the doctor.
- I understand that a failed drug screen may mean discontinuation of treatment.
- I will be forthright and honest about how the treatment is going, adverse effects, and how I am taking the medication.
Adapted from: https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf.
Don’t tempt fate. As with any controlled medication, safe storage of psychostimulants is paramount. Patients should be urged to keep their medication in a locked box or cupboard that is accessible to only the adult for whom the drug is prescribed. Prior research cautions that open access to controlled substances can lead to larger issues with abuse and diversion, particularly when adolescents are in the home.26
Consider atomoxetine. Research has also demonstrated that the non-stimulant medication atomoxetine has some benefit in the treatment of ADHD.12 Unlike psychostimulants that act on the neurotransmitter dopamine, atomoxetine acts on the neurotransmitter norepinephrine. This different mechanism of action results in a lower potential for abuse and diversion.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected].
1. Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
2. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64-81.
3. Novak SP, Kroutil LA, Williams RL, et al. The nonmedical use of prescription ADHD medications: results from a national internet panel. Subst Abuse Treat Prev Policy. 2007;2:32.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
5. Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389-403.
6. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621-1627.
7. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry.
8. van de Glind G, van den Brink W, Koeter MWJ, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132:587-596.
9. Gualtieri CT, Johnson LG. ADHD: Is objective diagnosis possible? Psychiatry. 2005;2:44-53.
10. Perrin AE, Jotwani VM. Addressing the unique issues of student athletes with ADHD. J Fam Pract. 2014;63:E1-E9.
11. Spencer T, Biederman J, Wilens T, et al. Efficacy of mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775-782.
12. Mészáros A, Czobor P, Bálint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-1147.
13. Weiss M, Shingler T, Capone NM. Medication satisfaction among adults with ADHD: long term results from the Quality of Life, Effectiveness, Safety and Tolerability (Qu.S.T) study. New Orleans: Program and Abstracts of the 19th US Psychiatric and Mental Health Congress, Abstract 120, 2006.
14. Lopez FA, Leroux JR.
15. Felt BT, Biermann B, Christner JG, et al. Diagnosis and management of ADHD in children. Am Fam Physician. 2014;90:456-464.
16. Spencer T, Biederman J, Wilens T. Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:373-383.
17. Withrow LM, Hash PA, Holten KB. Managing ADHD in children: are you doing enough? J Fam Pract. 2011;60:E1-E3.
18. McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52:1272-1280.
19. Aldridge AP, Kroutil LA, Cowell AJ, et al. Medication costs to private insurers of diversion of medications for attention-deficit hyperactivity disorder. Pharmacoeconomics. 2011;29:621-635.
20. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
21. Molina BSG, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250-263.
22. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143.
23. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85:890-896.
24. Cepeda MS, Fife D, Berwaerts J, et al. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41:226-229.
25. Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-880.
26. Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medication in the home. J Adolesc Health. 2013;53:260-264.
1. Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
2. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64-81.
3. Novak SP, Kroutil LA, Williams RL, et al. The nonmedical use of prescription ADHD medications: results from a national internet panel. Subst Abuse Treat Prev Policy. 2007;2:32.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
5. Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389-403.
6. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621-1627.
7. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry.
8. van de Glind G, van den Brink W, Koeter MWJ, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132:587-596.
9. Gualtieri CT, Johnson LG. ADHD: Is objective diagnosis possible? Psychiatry. 2005;2:44-53.
10. Perrin AE, Jotwani VM. Addressing the unique issues of student athletes with ADHD. J Fam Pract. 2014;63:E1-E9.
11. Spencer T, Biederman J, Wilens T, et al. Efficacy of mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775-782.
12. Mészáros A, Czobor P, Bálint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-1147.
13. Weiss M, Shingler T, Capone NM. Medication satisfaction among adults with ADHD: long term results from the Quality of Life, Effectiveness, Safety and Tolerability (Qu.S.T) study. New Orleans: Program and Abstracts of the 19th US Psychiatric and Mental Health Congress, Abstract 120, 2006.
14. Lopez FA, Leroux JR.
15. Felt BT, Biermann B, Christner JG, et al. Diagnosis and management of ADHD in children. Am Fam Physician. 2014;90:456-464.
16. Spencer T, Biederman J, Wilens T. Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:373-383.
17. Withrow LM, Hash PA, Holten KB. Managing ADHD in children: are you doing enough? J Fam Pract. 2011;60:E1-E3.
18. McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52:1272-1280.
19. Aldridge AP, Kroutil LA, Cowell AJ, et al. Medication costs to private insurers of diversion of medications for attention-deficit hyperactivity disorder. Pharmacoeconomics. 2011;29:621-635.
20. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
21. Molina BSG, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250-263.
22. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143.
23. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85:890-896.
24. Cepeda MS, Fife D, Berwaerts J, et al. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41:226-229.
25. Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-880.
26. Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medication in the home. J Adolesc Health. 2013;53:260-264.
PRACTICE RECOMMENDATIONS
› Be sure to take steps, which include utilization of a self-report measure—to correctly diagnose attention-deficit/hyperactivity disorder (ADHD) in adult patients before beginning treatment.
› Consider prescribing stimulant medications, such as the short-acting dextroamphetamine/amphetamine or the long-acting lisdexamfetamine, for adults with ADHD.
› Don't underestimate the problems of misuse and diversion among patients taking psychostimulant medications, particularly among younger men.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What FPs should know about pharmacogenetic testing
Turnover rate for hospitalist groups trending downward
According to the 2016 State of Hospital Medicine Report based on 2015 data, the median physician turnover rate for hospital medicine groups (HMGs) serving adults only is 6.9%, lower compared with results from prior surveys. Particularly, turnover in 2010 was more than double the current rate (see Figure 1). This steady decline over the years is intriguing, yet encouraging, since hospital medicine is well known for its high turnover compared to other specialties.
Similarly, results from State of Hospital Medicine surveys also reveal a consistent trend for groups with no turnover. As expected, lower turnover rate usually parallels with higher percentage of groups with no turnover. This year, 40.2% of hospitalist groups reported no physician turnover at all, continuing the upward trend from 2014 (38.1%) and 2012 (36%). It is speculating that these groups are not just simply fortunate, but rather work zealously to build a strong internal culture within the group and proactively create a shared vision, values, accountability, and career goals. 
Sources in search of why providers leave a practice and advice on specific strategies to retain them are abundant. To secure retention, at a minimum, employers, leaders, or administrators should pay close attention to such basic factors as work schedules, workload, and compensation – and even consider using national and regional data from the State of Hospital Medicine Report for benchmarking to remain attractive and competitive in the market. Low or no turnover rate indicates workforce stability and program credibility, and allows cost saving as the overall estimated cost of turnover (losing a provider and hiring another one) ranges from $400,000 to $600,000 per provider.1
The turnover data further delineates differences based on academic status, Medicare Indirect Medical Education (IME) program status, and geographic region. For instance, the academic groups consistently report a higher turnover rate, compared with the nonacademic groups. The latter mirrors the overall decreasing trend of physician turnover. Non-teaching hospitals also score significantly higher on the number of groups with no turnover (42% as opposed to 24%-27% for teaching hospitals). Geographically, HMGs in the South and Midwest regions of the United States are the winners this year, with more than 50% of the groups reporting no turnover at all.
Specific information regarding turnover for nurse practitioners and physician assistants (NPs/PAs) can also be found in the report. This rate has been increasing slightly compared with the past, with a subsequent drop in the percentage of groups reporting no turnover. Yet, the overall percentage with no turnover for NPs/PAs remains impressively high at 62%.
The turnover rates for HMGs serving both adults and children, and groups serving children only, appear somewhat similar to those of groups serving adults only, though we cannot reliably analyze the data, elucidate significant differences, or detect any meaningful trends from these two groups because of insufficient numbers of responders.
The downward trend of hospitalist turnover found in SHM’s 2016 State of Hospital Medicine Report is reassuring, indicative of a higher retention rate and an extended stability for many programs. Although some hospitalists continue to shop around, most leaders and employers of HMGs work endlessly to strengthen their programs in hope to minimize turnover. The promising data likely reflect such effort. Hopefully, this trend will continue when the next State of Hospital Medicine report comes out.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
Reference
1. Frenz, D (2016). The staggering costs of physician turnover. Today’s Hospitalist.
According to the 2016 State of Hospital Medicine Report based on 2015 data, the median physician turnover rate for hospital medicine groups (HMGs) serving adults only is 6.9%, lower compared with results from prior surveys. Particularly, turnover in 2010 was more than double the current rate (see Figure 1). This steady decline over the years is intriguing, yet encouraging, since hospital medicine is well known for its high turnover compared to other specialties.
Similarly, results from State of Hospital Medicine surveys also reveal a consistent trend for groups with no turnover. As expected, lower turnover rate usually parallels with higher percentage of groups with no turnover. This year, 40.2% of hospitalist groups reported no physician turnover at all, continuing the upward trend from 2014 (38.1%) and 2012 (36%). It is speculating that these groups are not just simply fortunate, but rather work zealously to build a strong internal culture within the group and proactively create a shared vision, values, accountability, and career goals. 
Sources in search of why providers leave a practice and advice on specific strategies to retain them are abundant. To secure retention, at a minimum, employers, leaders, or administrators should pay close attention to such basic factors as work schedules, workload, and compensation – and even consider using national and regional data from the State of Hospital Medicine Report for benchmarking to remain attractive and competitive in the market. Low or no turnover rate indicates workforce stability and program credibility, and allows cost saving as the overall estimated cost of turnover (losing a provider and hiring another one) ranges from $400,000 to $600,000 per provider.1
The turnover data further delineates differences based on academic status, Medicare Indirect Medical Education (IME) program status, and geographic region. For instance, the academic groups consistently report a higher turnover rate, compared with the nonacademic groups. The latter mirrors the overall decreasing trend of physician turnover. Non-teaching hospitals also score significantly higher on the number of groups with no turnover (42% as opposed to 24%-27% for teaching hospitals). Geographically, HMGs in the South and Midwest regions of the United States are the winners this year, with more than 50% of the groups reporting no turnover at all.
Specific information regarding turnover for nurse practitioners and physician assistants (NPs/PAs) can also be found in the report. This rate has been increasing slightly compared with the past, with a subsequent drop in the percentage of groups reporting no turnover. Yet, the overall percentage with no turnover for NPs/PAs remains impressively high at 62%.
The turnover rates for HMGs serving both adults and children, and groups serving children only, appear somewhat similar to those of groups serving adults only, though we cannot reliably analyze the data, elucidate significant differences, or detect any meaningful trends from these two groups because of insufficient numbers of responders.
The downward trend of hospitalist turnover found in SHM’s 2016 State of Hospital Medicine Report is reassuring, indicative of a higher retention rate and an extended stability for many programs. Although some hospitalists continue to shop around, most leaders and employers of HMGs work endlessly to strengthen their programs in hope to minimize turnover. The promising data likely reflect such effort. Hopefully, this trend will continue when the next State of Hospital Medicine report comes out.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
Reference
1. Frenz, D (2016). The staggering costs of physician turnover. Today’s Hospitalist.
According to the 2016 State of Hospital Medicine Report based on 2015 data, the median physician turnover rate for hospital medicine groups (HMGs) serving adults only is 6.9%, lower compared with results from prior surveys. Particularly, turnover in 2010 was more than double the current rate (see Figure 1). This steady decline over the years is intriguing, yet encouraging, since hospital medicine is well known for its high turnover compared to other specialties.
Similarly, results from State of Hospital Medicine surveys also reveal a consistent trend for groups with no turnover. As expected, lower turnover rate usually parallels with higher percentage of groups with no turnover. This year, 40.2% of hospitalist groups reported no physician turnover at all, continuing the upward trend from 2014 (38.1%) and 2012 (36%). It is speculating that these groups are not just simply fortunate, but rather work zealously to build a strong internal culture within the group and proactively create a shared vision, values, accountability, and career goals. 
Sources in search of why providers leave a practice and advice on specific strategies to retain them are abundant. To secure retention, at a minimum, employers, leaders, or administrators should pay close attention to such basic factors as work schedules, workload, and compensation – and even consider using national and regional data from the State of Hospital Medicine Report for benchmarking to remain attractive and competitive in the market. Low or no turnover rate indicates workforce stability and program credibility, and allows cost saving as the overall estimated cost of turnover (losing a provider and hiring another one) ranges from $400,000 to $600,000 per provider.1
The turnover data further delineates differences based on academic status, Medicare Indirect Medical Education (IME) program status, and geographic region. For instance, the academic groups consistently report a higher turnover rate, compared with the nonacademic groups. The latter mirrors the overall decreasing trend of physician turnover. Non-teaching hospitals also score significantly higher on the number of groups with no turnover (42% as opposed to 24%-27% for teaching hospitals). Geographically, HMGs in the South and Midwest regions of the United States are the winners this year, with more than 50% of the groups reporting no turnover at all.
Specific information regarding turnover for nurse practitioners and physician assistants (NPs/PAs) can also be found in the report. This rate has been increasing slightly compared with the past, with a subsequent drop in the percentage of groups reporting no turnover. Yet, the overall percentage with no turnover for NPs/PAs remains impressively high at 62%.
The turnover rates for HMGs serving both adults and children, and groups serving children only, appear somewhat similar to those of groups serving adults only, though we cannot reliably analyze the data, elucidate significant differences, or detect any meaningful trends from these two groups because of insufficient numbers of responders.
The downward trend of hospitalist turnover found in SHM’s 2016 State of Hospital Medicine Report is reassuring, indicative of a higher retention rate and an extended stability for many programs. Although some hospitalists continue to shop around, most leaders and employers of HMGs work endlessly to strengthen their programs in hope to minimize turnover. The promising data likely reflect such effort. Hopefully, this trend will continue when the next State of Hospital Medicine report comes out.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
Reference
1. Frenz, D (2016). The staggering costs of physician turnover. Today’s Hospitalist.
Taking the hammer to sickle pain crises
Clinical question: Are any novel medicines designed to interrupt the pathophysiology of vaso-occlusion available to reduce the frequency and severity of pain crises in sickle cell anemia?
Background: Hypoxemia-dependent HbS polymerization is just the initiating step in a complex cascade of events leading to sickle pain crises. Animal studies have suggested that blockade of P-selectin, an adhesion molecule expressed by endothelium, blunts the aggregation of sickled erythrocytes, leukocytes and activated platelets and may reduce progression to vaso-occlusive crises.
Study Design: Multicenter, double-blinded, randomized placebo-controlled trial.
Setting: International (60 study sites in 3 countries) from August 2013 to January 2015.
Synopsis: Crizanlizumab is a humanized monoclonal antibody directed against P-selectin. A total of 198 patients aged 16-65 with HbSS, HbSC, HbSbeta+ and select other genotypes experiencing 2-10 pain crises in the 12 months prior to trial were block randomized to receive high-dose crizanlizumab, low-dose crizanlizumab, or placebo. Roughly 62% of enrolled patients in all three arms were already taking hydroxyurea – for which dose changes during trial period were forbidden. Patients not already on hydroxyurea could not initiate treatment during the trial period. The primary outcome was annualized rate of sickle pain crises (defined as pain without other demonstrable cause requiring medical facility visit and treatment with oral or parenteral narcotics or NSAIDs), including acute chest, hepatic or splenic sequestration crises and priapism. Secondary outcomes were annualized rates of hospital days, rates of uncomplicated crises, time to first and second crises, rates of acute chest syndrome and Brief Pain Inventory questionnaire. Primary outcome data were processed via intention-to-treat analysis.
For high-dose crizanlizumab, there were a median 1.63 crises per year compared to 2.98 in the placebo group, representing a 45.3% lower rate (P = .01). The protective effect of crizanlizumab was more pronounced in the per-protocol analysis (1.04 crises per year for the high dose group). In the subgroup of patients on concomitant hydroxyurea, the median annualized crisis rate among high dose versus placebo was 2.43 compared to 3.58, representing a 32.1% lower rate. In the non-hydroxyurea subgroup, the median annualized crisis rate for high dose versus placebo was 1.0 vs. 2.0, representing a 50% lower rate. Secondary endpoints similarly trended in favor of crizanlizumab in a dose-dependent fashion, although statistical significance was mixed.
Bottom line: Crizanlizumab, a humanized monoclonal antibody that blocks the action of the endothelial adhesion molecule P-selectin, is promising as a novel medication to reduce frequency of vaso-occlusive crises in patients with HbS-related disease.
Citation: Ataga KI, Kutler A, Kanter J, et al. Crizanlizumab for prevention of pain crises in sickle cell disease. N Engl J Med. 2016 Dec. 3. doi: 10.1056/NEJMoa1611770.
Clinical question: Are any novel medicines designed to interrupt the pathophysiology of vaso-occlusion available to reduce the frequency and severity of pain crises in sickle cell anemia?
Background: Hypoxemia-dependent HbS polymerization is just the initiating step in a complex cascade of events leading to sickle pain crises. Animal studies have suggested that blockade of P-selectin, an adhesion molecule expressed by endothelium, blunts the aggregation of sickled erythrocytes, leukocytes and activated platelets and may reduce progression to vaso-occlusive crises.
Study Design: Multicenter, double-blinded, randomized placebo-controlled trial.
Setting: International (60 study sites in 3 countries) from August 2013 to January 2015.
Synopsis: Crizanlizumab is a humanized monoclonal antibody directed against P-selectin. A total of 198 patients aged 16-65 with HbSS, HbSC, HbSbeta+ and select other genotypes experiencing 2-10 pain crises in the 12 months prior to trial were block randomized to receive high-dose crizanlizumab, low-dose crizanlizumab, or placebo. Roughly 62% of enrolled patients in all three arms were already taking hydroxyurea – for which dose changes during trial period were forbidden. Patients not already on hydroxyurea could not initiate treatment during the trial period. The primary outcome was annualized rate of sickle pain crises (defined as pain without other demonstrable cause requiring medical facility visit and treatment with oral or parenteral narcotics or NSAIDs), including acute chest, hepatic or splenic sequestration crises and priapism. Secondary outcomes were annualized rates of hospital days, rates of uncomplicated crises, time to first and second crises, rates of acute chest syndrome and Brief Pain Inventory questionnaire. Primary outcome data were processed via intention-to-treat analysis.
For high-dose crizanlizumab, there were a median 1.63 crises per year compared to 2.98 in the placebo group, representing a 45.3% lower rate (P = .01). The protective effect of crizanlizumab was more pronounced in the per-protocol analysis (1.04 crises per year for the high dose group). In the subgroup of patients on concomitant hydroxyurea, the median annualized crisis rate among high dose versus placebo was 2.43 compared to 3.58, representing a 32.1% lower rate. In the non-hydroxyurea subgroup, the median annualized crisis rate for high dose versus placebo was 1.0 vs. 2.0, representing a 50% lower rate. Secondary endpoints similarly trended in favor of crizanlizumab in a dose-dependent fashion, although statistical significance was mixed.
Bottom line: Crizanlizumab, a humanized monoclonal antibody that blocks the action of the endothelial adhesion molecule P-selectin, is promising as a novel medication to reduce frequency of vaso-occlusive crises in patients with HbS-related disease.
Citation: Ataga KI, Kutler A, Kanter J, et al. Crizanlizumab for prevention of pain crises in sickle cell disease. N Engl J Med. 2016 Dec. 3. doi: 10.1056/NEJMoa1611770.
Clinical question: Are any novel medicines designed to interrupt the pathophysiology of vaso-occlusion available to reduce the frequency and severity of pain crises in sickle cell anemia?
Background: Hypoxemia-dependent HbS polymerization is just the initiating step in a complex cascade of events leading to sickle pain crises. Animal studies have suggested that blockade of P-selectin, an adhesion molecule expressed by endothelium, blunts the aggregation of sickled erythrocytes, leukocytes and activated platelets and may reduce progression to vaso-occlusive crises.
Study Design: Multicenter, double-blinded, randomized placebo-controlled trial.
Setting: International (60 study sites in 3 countries) from August 2013 to January 2015.
Synopsis: Crizanlizumab is a humanized monoclonal antibody directed against P-selectin. A total of 198 patients aged 16-65 with HbSS, HbSC, HbSbeta+ and select other genotypes experiencing 2-10 pain crises in the 12 months prior to trial were block randomized to receive high-dose crizanlizumab, low-dose crizanlizumab, or placebo. Roughly 62% of enrolled patients in all three arms were already taking hydroxyurea – for which dose changes during trial period were forbidden. Patients not already on hydroxyurea could not initiate treatment during the trial period. The primary outcome was annualized rate of sickle pain crises (defined as pain without other demonstrable cause requiring medical facility visit and treatment with oral or parenteral narcotics or NSAIDs), including acute chest, hepatic or splenic sequestration crises and priapism. Secondary outcomes were annualized rates of hospital days, rates of uncomplicated crises, time to first and second crises, rates of acute chest syndrome and Brief Pain Inventory questionnaire. Primary outcome data were processed via intention-to-treat analysis.
For high-dose crizanlizumab, there were a median 1.63 crises per year compared to 2.98 in the placebo group, representing a 45.3% lower rate (P = .01). The protective effect of crizanlizumab was more pronounced in the per-protocol analysis (1.04 crises per year for the high dose group). In the subgroup of patients on concomitant hydroxyurea, the median annualized crisis rate among high dose versus placebo was 2.43 compared to 3.58, representing a 32.1% lower rate. In the non-hydroxyurea subgroup, the median annualized crisis rate for high dose versus placebo was 1.0 vs. 2.0, representing a 50% lower rate. Secondary endpoints similarly trended in favor of crizanlizumab in a dose-dependent fashion, although statistical significance was mixed.
Bottom line: Crizanlizumab, a humanized monoclonal antibody that blocks the action of the endothelial adhesion molecule P-selectin, is promising as a novel medication to reduce frequency of vaso-occlusive crises in patients with HbS-related disease.
Citation: Ataga KI, Kutler A, Kanter J, et al. Crizanlizumab for prevention of pain crises in sickle cell disease. N Engl J Med. 2016 Dec. 3. doi: 10.1056/NEJMoa1611770.
DVT prophylaxis not needed after casting
Clinical question: Is DVT prophylaxis necessary with lower leg casting or after knee arthroscopy?
Background: Patients who undergo immobilization from casting or knee arthroscopic surgery are thought to be at increased risk for venous thromboembolism (VTE). Most patients who undergo orthopedic procedures receive thromboprophylaxis but controversy exists surrounding its use in low limb casting or knee arthroscopic surgery. Prior studies did not establish consensus because of methodologic weaknesses.
Study design: Two parallel randomized-controlled, open-label trials with blinded outcome (POD-KAST for knee arthroscopy and POD-CAST for casting lower leg.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: Patients were randomly assigned to receive either a prophylactic dose of low-molecular-weight heparin (either for the 8 days after arthroscopy or for the duration of casting-related immobilization) or no anticoagulant therapy. The primary outcomes were the cumulative incidences of symptomatic venous thromboembolism (VTE) and major bleeding within 3 months after the procedure. For the 1,543 patients undergoing knee arthroscopy, VTE occurred in 0.7% of the treatment group and 0.4% of the control group (relative risk, 1.6; 95% confidence interval [CI], 0.4-6.8; absolute difference in risk, 0.3 percentage points; 95% CI, −0.6 to 1.2) and major bleeding was seen in 0.1% of both groups.
For the 1,519 patients undergoing knee arthroscopy, VTE occurred in 1.4% of the treatment group and 1.8% of the control group (relative risk, 0.8; 95% CI, 0.3-1.7; absolute difference in risk, −0.4 percentage points; 95% CI, −1.8 to 1.0) and no episodes of major bleeding were reported in either group.
Bottom line: VTE prophylaxis either after arthroscopy or during immobilization from casting did not prevent DVT.
Citations: Van Adrichem RA, Nemeth B, Algra A, et al. Thromboprophylaxis after knee arthroscopy and low-leg casting. N Engl J Med. 2016 Dec 3. doi: 10.1056/NEJMoa1613303.
Dr. Cerceo is assistant professor in the division of hospital medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: Is DVT prophylaxis necessary with lower leg casting or after knee arthroscopy?
Background: Patients who undergo immobilization from casting or knee arthroscopic surgery are thought to be at increased risk for venous thromboembolism (VTE). Most patients who undergo orthopedic procedures receive thromboprophylaxis but controversy exists surrounding its use in low limb casting or knee arthroscopic surgery. Prior studies did not establish consensus because of methodologic weaknesses.
Study design: Two parallel randomized-controlled, open-label trials with blinded outcome (POD-KAST for knee arthroscopy and POD-CAST for casting lower leg.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: Patients were randomly assigned to receive either a prophylactic dose of low-molecular-weight heparin (either for the 8 days after arthroscopy or for the duration of casting-related immobilization) or no anticoagulant therapy. The primary outcomes were the cumulative incidences of symptomatic venous thromboembolism (VTE) and major bleeding within 3 months after the procedure. For the 1,543 patients undergoing knee arthroscopy, VTE occurred in 0.7% of the treatment group and 0.4% of the control group (relative risk, 1.6; 95% confidence interval [CI], 0.4-6.8; absolute difference in risk, 0.3 percentage points; 95% CI, −0.6 to 1.2) and major bleeding was seen in 0.1% of both groups.
For the 1,519 patients undergoing knee arthroscopy, VTE occurred in 1.4% of the treatment group and 1.8% of the control group (relative risk, 0.8; 95% CI, 0.3-1.7; absolute difference in risk, −0.4 percentage points; 95% CI, −1.8 to 1.0) and no episodes of major bleeding were reported in either group.
Bottom line: VTE prophylaxis either after arthroscopy or during immobilization from casting did not prevent DVT.
Citations: Van Adrichem RA, Nemeth B, Algra A, et al. Thromboprophylaxis after knee arthroscopy and low-leg casting. N Engl J Med. 2016 Dec 3. doi: 10.1056/NEJMoa1613303.
Dr. Cerceo is assistant professor in the division of hospital medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: Is DVT prophylaxis necessary with lower leg casting or after knee arthroscopy?
Background: Patients who undergo immobilization from casting or knee arthroscopic surgery are thought to be at increased risk for venous thromboembolism (VTE). Most patients who undergo orthopedic procedures receive thromboprophylaxis but controversy exists surrounding its use in low limb casting or knee arthroscopic surgery. Prior studies did not establish consensus because of methodologic weaknesses.
Study design: Two parallel randomized-controlled, open-label trials with blinded outcome (POD-KAST for knee arthroscopy and POD-CAST for casting lower leg.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: Patients were randomly assigned to receive either a prophylactic dose of low-molecular-weight heparin (either for the 8 days after arthroscopy or for the duration of casting-related immobilization) or no anticoagulant therapy. The primary outcomes were the cumulative incidences of symptomatic venous thromboembolism (VTE) and major bleeding within 3 months after the procedure. For the 1,543 patients undergoing knee arthroscopy, VTE occurred in 0.7% of the treatment group and 0.4% of the control group (relative risk, 1.6; 95% confidence interval [CI], 0.4-6.8; absolute difference in risk, 0.3 percentage points; 95% CI, −0.6 to 1.2) and major bleeding was seen in 0.1% of both groups.
For the 1,519 patients undergoing knee arthroscopy, VTE occurred in 1.4% of the treatment group and 1.8% of the control group (relative risk, 0.8; 95% CI, 0.3-1.7; absolute difference in risk, −0.4 percentage points; 95% CI, −1.8 to 1.0) and no episodes of major bleeding were reported in either group.
Bottom line: VTE prophylaxis either after arthroscopy or during immobilization from casting did not prevent DVT.
Citations: Van Adrichem RA, Nemeth B, Algra A, et al. Thromboprophylaxis after knee arthroscopy and low-leg casting. N Engl J Med. 2016 Dec 3. doi: 10.1056/NEJMoa1613303.
Dr. Cerceo is assistant professor in the division of hospital medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.