User login
AATS Mitral Conclave Call for Abstracts & Videos
AATS invites you to submit your abstracts and videos to the 2017 Mitral Conclave.
AATS Mitral Conclave
April 27-28, 2017
New York, NY
Submission Deadline:
Sunday, January 8, 2017 @ 11.59 pm EST
Share:
AATS invites you to submit your abstracts and videos to the 2017 Mitral Conclave.
AATS Mitral Conclave
April 27-28, 2017
New York, NY
Submission Deadline:
Sunday, January 8, 2017 @ 11.59 pm EST
Share:
AATS invites you to submit your abstracts and videos to the 2017 Mitral Conclave.
AATS Mitral Conclave
April 27-28, 2017
New York, NY
Submission Deadline:
Sunday, January 8, 2017 @ 11.59 pm EST
Share:
Hinged-Knee External Fixator Used to Reduce and Maintain Subacute Tibiofemoral Coronal Subluxation
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
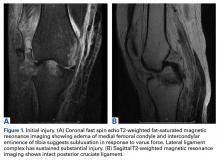
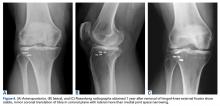
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
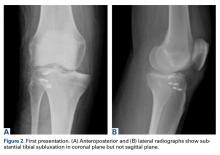
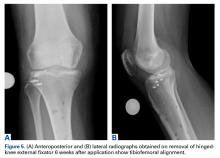
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
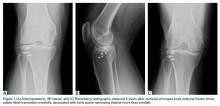
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
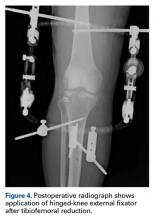
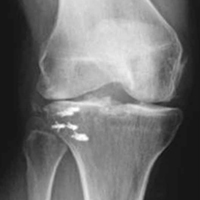
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
IV brivaracetam similarly well tolerated as oral formulation
HOUSTON – Pooled data for a recently approved antiepileptic drug show good safety and tolerability when it’s administered intravenously.
Intravenous brivaracetam, when given to 104 patients with epilepsy and 49 healthy volunteers, was generally well tolerated, though about one in three patients experienced somnolence, and one in four had some dizziness and fatigue. This adverse event profile was similar to that seen in clinical trials of the oral formulation of brivaracetam, Pavel Klein, MD, said in an interview during a poster session at the annual meeting of the American Epilepsy Society, though euphoria and dysgeusia were more common among healthy volunteers receiving intravenous brivaracetam.
“Looking at the side effects, the medication was well tolerated. … The main side effects were dizziness, somnolence, and fatigue, and by and large, these were mild or moderate. Nobody discontinued the medication because of side effects in the patient population,” said Dr. Klein, director of the Mid-Atlantic Epilepsy and Sleep Center, Bethesda, Md.
In the overall group of participants in all three studies, somnolence was seen in 30.1% of participants, dizziness in 15.7%, and fatigue in 15.0%. However, when compared with patients with epilepsy, healthy volunteers given brivaracetam 100 mg IV had a higher overall adverse event incidence, and notably more dizziness (35.5% versus 7.7%) and fatigue (29.0% versus 4.8%). One epilepsy patient in the phase III study (1/104; 1%) discontinued the study because of an adverse event.
Also, healthy volunteers, rather than patients, were more likely to experience euphoric mood and dysgeusia with intravenous as compared with oral brivaracetam.
The reason for these differences between the epilepsy and nonepilepsy groups is not known; however, wrote Dr. Klein and his coauthors, part of the difference might be “due to heterogeneous medical histories and concomitant medication use.”
Otherwise, intravenous brivaracetam administration was associated with about the same number of adverse events as when the drug was taken orally.
Brivaracetam, approved earlier in 2016, like levetiracetam, is a ligand for the synaptic vesicle protein 2a, the mechanism that’s thought to be responsible for the antiepileptic effect of this drug class. However, brivaracetam has a 15- to 30-fold higher affinity for its target than its older cousin. It’s approved in the United States as adjunctive therapy for focal seizures in individuals aged 16 and older with epilepsy. When administered intravenously, brivaracetam may be given as a bolus or an infusion over 15 minutes.
Dr. Klein serves on the speakers bureau of UCB Pharma, and has received research support from the company, which funded the study and assisted with poster preparation.
[email protected]
On Twitter @karioakes
HOUSTON – Pooled data for a recently approved antiepileptic drug show good safety and tolerability when it’s administered intravenously.
Intravenous brivaracetam, when given to 104 patients with epilepsy and 49 healthy volunteers, was generally well tolerated, though about one in three patients experienced somnolence, and one in four had some dizziness and fatigue. This adverse event profile was similar to that seen in clinical trials of the oral formulation of brivaracetam, Pavel Klein, MD, said in an interview during a poster session at the annual meeting of the American Epilepsy Society, though euphoria and dysgeusia were more common among healthy volunteers receiving intravenous brivaracetam.
“Looking at the side effects, the medication was well tolerated. … The main side effects were dizziness, somnolence, and fatigue, and by and large, these were mild or moderate. Nobody discontinued the medication because of side effects in the patient population,” said Dr. Klein, director of the Mid-Atlantic Epilepsy and Sleep Center, Bethesda, Md.
In the overall group of participants in all three studies, somnolence was seen in 30.1% of participants, dizziness in 15.7%, and fatigue in 15.0%. However, when compared with patients with epilepsy, healthy volunteers given brivaracetam 100 mg IV had a higher overall adverse event incidence, and notably more dizziness (35.5% versus 7.7%) and fatigue (29.0% versus 4.8%). One epilepsy patient in the phase III study (1/104; 1%) discontinued the study because of an adverse event.
Also, healthy volunteers, rather than patients, were more likely to experience euphoric mood and dysgeusia with intravenous as compared with oral brivaracetam.
The reason for these differences between the epilepsy and nonepilepsy groups is not known; however, wrote Dr. Klein and his coauthors, part of the difference might be “due to heterogeneous medical histories and concomitant medication use.”
Otherwise, intravenous brivaracetam administration was associated with about the same number of adverse events as when the drug was taken orally.
Brivaracetam, approved earlier in 2016, like levetiracetam, is a ligand for the synaptic vesicle protein 2a, the mechanism that’s thought to be responsible for the antiepileptic effect of this drug class. However, brivaracetam has a 15- to 30-fold higher affinity for its target than its older cousin. It’s approved in the United States as adjunctive therapy for focal seizures in individuals aged 16 and older with epilepsy. When administered intravenously, brivaracetam may be given as a bolus or an infusion over 15 minutes.
Dr. Klein serves on the speakers bureau of UCB Pharma, and has received research support from the company, which funded the study and assisted with poster preparation.
[email protected]
On Twitter @karioakes
HOUSTON – Pooled data for a recently approved antiepileptic drug show good safety and tolerability when it’s administered intravenously.
Intravenous brivaracetam, when given to 104 patients with epilepsy and 49 healthy volunteers, was generally well tolerated, though about one in three patients experienced somnolence, and one in four had some dizziness and fatigue. This adverse event profile was similar to that seen in clinical trials of the oral formulation of brivaracetam, Pavel Klein, MD, said in an interview during a poster session at the annual meeting of the American Epilepsy Society, though euphoria and dysgeusia were more common among healthy volunteers receiving intravenous brivaracetam.
“Looking at the side effects, the medication was well tolerated. … The main side effects were dizziness, somnolence, and fatigue, and by and large, these were mild or moderate. Nobody discontinued the medication because of side effects in the patient population,” said Dr. Klein, director of the Mid-Atlantic Epilepsy and Sleep Center, Bethesda, Md.
In the overall group of participants in all three studies, somnolence was seen in 30.1% of participants, dizziness in 15.7%, and fatigue in 15.0%. However, when compared with patients with epilepsy, healthy volunteers given brivaracetam 100 mg IV had a higher overall adverse event incidence, and notably more dizziness (35.5% versus 7.7%) and fatigue (29.0% versus 4.8%). One epilepsy patient in the phase III study (1/104; 1%) discontinued the study because of an adverse event.
Also, healthy volunteers, rather than patients, were more likely to experience euphoric mood and dysgeusia with intravenous as compared with oral brivaracetam.
The reason for these differences between the epilepsy and nonepilepsy groups is not known; however, wrote Dr. Klein and his coauthors, part of the difference might be “due to heterogeneous medical histories and concomitant medication use.”
Otherwise, intravenous brivaracetam administration was associated with about the same number of adverse events as when the drug was taken orally.
Brivaracetam, approved earlier in 2016, like levetiracetam, is a ligand for the synaptic vesicle protein 2a, the mechanism that’s thought to be responsible for the antiepileptic effect of this drug class. However, brivaracetam has a 15- to 30-fold higher affinity for its target than its older cousin. It’s approved in the United States as adjunctive therapy for focal seizures in individuals aged 16 and older with epilepsy. When administered intravenously, brivaracetam may be given as a bolus or an infusion over 15 minutes.
Dr. Klein serves on the speakers bureau of UCB Pharma, and has received research support from the company, which funded the study and assisted with poster preparation.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: Somnolence was the most frequent adverse event with intravenous brivaracetam, reported by 30.1% of all study participants.
Data source: Pooled data from clinical trials in 104 patients with focal or generalized seizures and 49 healthy controls.
Disclosures: Dr. Klein serves on the speakers bureau of UCB Pharma, and has received research support from the company, which funded the study and assisted with poster preparation.
Echocardiogram, exercise testing improve PAH prognostic accuracy
Adding echocardiography and cardiopulmonary exercise testing to baseline right heart catheterization improves prognostic accuracy in idiopathic pulmonary arterial hypertension, according to a prospective Italian study of 102 newly diagnosed patients.
A combination of low right ventricular fractional area change (RVFAC) on echocardiography and low oxygen pulse on cardiopulmonary exercise testing (CPET) “identifies patients at a particularly high risk of clinical deterioration.” Both are markers of right ventricular (RV) function, which is a major determinant of outcome in idiopathic pulmonary arterial hypertension [iPAH], said investigators led by Roberto Badagliacca, MD, of the Sapienza University of Rome (Chest. 2016 Aug 20. pii: S0012-3692(16)56052-8. doi: 10.1016/j.chest.2016.07.036).
PAH diagnosis requires right heart catheterization, and findings have long been known to predict PAH outcome. However, catheterization allows only “an indirect description of RV function,” the investigators said. Recent studies have shown that RV echocardiography and CPET improve the accuracy of heart failure prognosis, so the investigators wanted to see if they’d do the same for PAH.
Their results “strongly suggest that noninvasive measurements related to RV function obtained by combining resting echocardiography and CPET are of added value to right heart catheterization in the assessment of severity and prognostication of PAH,” they said.
During a mean follow-up of 528 days, 54 patients (53%) had clinical worsening, defined as a 15% reduction in 6-minute walk distance from baseline plus a worsening of functional class, nonelective PAH hospitalization, or death.
Baseline functional class and cardiac index proved to be independent predictors of clinical worsening. Adding echocardiographic and CPET variables independently improved prognostic power (area under the curve, 0.81 vs. 0.66; P = .005).
Compared with patients with high RVFAC and high oxygen pulse at baseline, patients with low RVFAC and low oxygen pulse had a 99.8 increase in the hazard ratio for clinical worsening, and those with high RVFAC and low oxygen had a 29.4 increase (P = .0001).
Several echocardiographic variables for RV function have previously been reported as independent predictors of PAH outcome. “The new finding here is that RVFAC outperformed other echocardiographic indices of systolic function,” the investigators wrote.
“As for peak oxygen pulse, this variable is thought to assess maximum [stroke volume],” assumed to be determined by RV function; MRI-determined stroke volume has been previously shown to be an important predictor of survival in PAH,” they said.
The mean age in the study was 52 years, mean functional class was 2.7, and mean 6-minute walk distance was 430 m; 62 subjects were women. The most relevant comorbidities were diabetes in 5 patients, hypercholesterolemia in 10, thyroid diseases in 6, and clinical depression in 7. Patients with severe tricuspid regurgitation or exercise-induced opening of the foramen ovale were excluded. However, a reanalysis including patients with exercise-induced right to left shunting showed the same independent predictors of PAH outcome.
After diagnosis, patients were treated with endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostanoids.
Dr. Badagliacca reported speaker and adviser fees from United Therapeutics, Dompe, GSK, and Bayer. His colleagues reported no conflicts of interest.
Adding echocardiography and cardiopulmonary exercise testing to baseline right heart catheterization improves prognostic accuracy in idiopathic pulmonary arterial hypertension, according to a prospective Italian study of 102 newly diagnosed patients.
A combination of low right ventricular fractional area change (RVFAC) on echocardiography and low oxygen pulse on cardiopulmonary exercise testing (CPET) “identifies patients at a particularly high risk of clinical deterioration.” Both are markers of right ventricular (RV) function, which is a major determinant of outcome in idiopathic pulmonary arterial hypertension [iPAH], said investigators led by Roberto Badagliacca, MD, of the Sapienza University of Rome (Chest. 2016 Aug 20. pii: S0012-3692(16)56052-8. doi: 10.1016/j.chest.2016.07.036).
PAH diagnosis requires right heart catheterization, and findings have long been known to predict PAH outcome. However, catheterization allows only “an indirect description of RV function,” the investigators said. Recent studies have shown that RV echocardiography and CPET improve the accuracy of heart failure prognosis, so the investigators wanted to see if they’d do the same for PAH.
Their results “strongly suggest that noninvasive measurements related to RV function obtained by combining resting echocardiography and CPET are of added value to right heart catheterization in the assessment of severity and prognostication of PAH,” they said.
During a mean follow-up of 528 days, 54 patients (53%) had clinical worsening, defined as a 15% reduction in 6-minute walk distance from baseline plus a worsening of functional class, nonelective PAH hospitalization, or death.
Baseline functional class and cardiac index proved to be independent predictors of clinical worsening. Adding echocardiographic and CPET variables independently improved prognostic power (area under the curve, 0.81 vs. 0.66; P = .005).
Compared with patients with high RVFAC and high oxygen pulse at baseline, patients with low RVFAC and low oxygen pulse had a 99.8 increase in the hazard ratio for clinical worsening, and those with high RVFAC and low oxygen had a 29.4 increase (P = .0001).
Several echocardiographic variables for RV function have previously been reported as independent predictors of PAH outcome. “The new finding here is that RVFAC outperformed other echocardiographic indices of systolic function,” the investigators wrote.
“As for peak oxygen pulse, this variable is thought to assess maximum [stroke volume],” assumed to be determined by RV function; MRI-determined stroke volume has been previously shown to be an important predictor of survival in PAH,” they said.
The mean age in the study was 52 years, mean functional class was 2.7, and mean 6-minute walk distance was 430 m; 62 subjects were women. The most relevant comorbidities were diabetes in 5 patients, hypercholesterolemia in 10, thyroid diseases in 6, and clinical depression in 7. Patients with severe tricuspid regurgitation or exercise-induced opening of the foramen ovale were excluded. However, a reanalysis including patients with exercise-induced right to left shunting showed the same independent predictors of PAH outcome.
After diagnosis, patients were treated with endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostanoids.
Dr. Badagliacca reported speaker and adviser fees from United Therapeutics, Dompe, GSK, and Bayer. His colleagues reported no conflicts of interest.
Adding echocardiography and cardiopulmonary exercise testing to baseline right heart catheterization improves prognostic accuracy in idiopathic pulmonary arterial hypertension, according to a prospective Italian study of 102 newly diagnosed patients.
A combination of low right ventricular fractional area change (RVFAC) on echocardiography and low oxygen pulse on cardiopulmonary exercise testing (CPET) “identifies patients at a particularly high risk of clinical deterioration.” Both are markers of right ventricular (RV) function, which is a major determinant of outcome in idiopathic pulmonary arterial hypertension [iPAH], said investigators led by Roberto Badagliacca, MD, of the Sapienza University of Rome (Chest. 2016 Aug 20. pii: S0012-3692(16)56052-8. doi: 10.1016/j.chest.2016.07.036).
PAH diagnosis requires right heart catheterization, and findings have long been known to predict PAH outcome. However, catheterization allows only “an indirect description of RV function,” the investigators said. Recent studies have shown that RV echocardiography and CPET improve the accuracy of heart failure prognosis, so the investigators wanted to see if they’d do the same for PAH.
Their results “strongly suggest that noninvasive measurements related to RV function obtained by combining resting echocardiography and CPET are of added value to right heart catheterization in the assessment of severity and prognostication of PAH,” they said.
During a mean follow-up of 528 days, 54 patients (53%) had clinical worsening, defined as a 15% reduction in 6-minute walk distance from baseline plus a worsening of functional class, nonelective PAH hospitalization, or death.
Baseline functional class and cardiac index proved to be independent predictors of clinical worsening. Adding echocardiographic and CPET variables independently improved prognostic power (area under the curve, 0.81 vs. 0.66; P = .005).
Compared with patients with high RVFAC and high oxygen pulse at baseline, patients with low RVFAC and low oxygen pulse had a 99.8 increase in the hazard ratio for clinical worsening, and those with high RVFAC and low oxygen had a 29.4 increase (P = .0001).
Several echocardiographic variables for RV function have previously been reported as independent predictors of PAH outcome. “The new finding here is that RVFAC outperformed other echocardiographic indices of systolic function,” the investigators wrote.
“As for peak oxygen pulse, this variable is thought to assess maximum [stroke volume],” assumed to be determined by RV function; MRI-determined stroke volume has been previously shown to be an important predictor of survival in PAH,” they said.
The mean age in the study was 52 years, mean functional class was 2.7, and mean 6-minute walk distance was 430 m; 62 subjects were women. The most relevant comorbidities were diabetes in 5 patients, hypercholesterolemia in 10, thyroid diseases in 6, and clinical depression in 7. Patients with severe tricuspid regurgitation or exercise-induced opening of the foramen ovale were excluded. However, a reanalysis including patients with exercise-induced right to left shunting showed the same independent predictors of PAH outcome.
After diagnosis, patients were treated with endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostanoids.
Dr. Badagliacca reported speaker and adviser fees from United Therapeutics, Dompe, GSK, and Bayer. His colleagues reported no conflicts of interest.
FROM CHEST
Key clinical point:
Major finding: Baseline functional class and cardiac index proved to be independent predictors of clinical worsening. Adding echocardiographic and CPET variables independently improved prognostic power (area under the curve, 0.81 vs. 0.66; P = .005).
Data source: A prospective Italian study of 102 newly diagnosed patients.
Disclosures: The lead investigator reported speaker and adviser fees from United Therapeutics, Dompe, GSK, and Bayer.
Strength of fibromyalgia as marker for seizures questioned
HOUSTON – The specificity of fibromyalgia as a marker for psychogenic nonepileptic seizures is less reliable than previously described, results from a large analysis showed.
“Fibromyalgia has been referred to as a reliable clinical indicator for differentiating psychogenic nonepileptic seizures from epilepsy, but there hasn’t been a lot of research looking into this idea,” Emily K. Acton said in an interview at the annual meeting of the American Epilepsy Society. In fact, the most recent study to investigate the association focused on a small patient cohort in a tertiary care epilepsy referral center and found that a diagnosis of fibromyalgia had a predictive value of 75% and a specificity of 99% for the diagnosis of psychogenic nonepileptic seizures (Epilepsy Behav. 2005; 6[2]:264-5).
Ms. Acton reported that of the 1,730 patients studied, 95 (5.5%) had a medical history of fibromyalgia. A majority of the 95 patients (95%) were female, and the mean age was 53 years. In addition, 43% of fibromyalgia patients had a nonparoxysmal, neurologic primary clinical diagnosis, most commonly chronic pain. She said that no differences were seen between fibromyalgia patients and case-matched controls in terms of age, race, marital status, or reason for referral. However, compared with case-matched controls, fibromyalgia patients were underrepresented in education (P = .02) and employment (P = .009).
Paroxysmal events were present in 57% of fibromyalgia patients and in 54% of case-matched controls. Among fibromyalgia patients with paroxysmal disorders, 11% had epileptic seizures, 74% had PNES, and 15% had physiologic nonepileptic events. Among matched controls, 37% had epileptic seizures, 51% had PNES, and 12% had physiologic nonepileptic events (P = .009).
After comparing the two groups of patients, the researchers determined that a historical diagnosis of fibromyalgia had a 61% sensitivity, 64% specificity, 74% positive predictive value, and 49% negative predictive value for the diagnosis of PNES. “Another interesting finding was that quite a few of our fibromyalgia patients felt they didn’t have fibromyalgia,” Ms. Action said. “Unprompted, during the course of their first visit, about 10% of this cohort mentioned to a physician that they were diagnosed with fibromyalgia, but they didn’t believe it.”
In their abstract, the researchers wrote that the study design “and a larger sample size involving mixed general neurology patients may account for the lesser association seen between fibromyalgia and PNES in this study.” They reported having no financial disclosures.
HOUSTON – The specificity of fibromyalgia as a marker for psychogenic nonepileptic seizures is less reliable than previously described, results from a large analysis showed.
“Fibromyalgia has been referred to as a reliable clinical indicator for differentiating psychogenic nonepileptic seizures from epilepsy, but there hasn’t been a lot of research looking into this idea,” Emily K. Acton said in an interview at the annual meeting of the American Epilepsy Society. In fact, the most recent study to investigate the association focused on a small patient cohort in a tertiary care epilepsy referral center and found that a diagnosis of fibromyalgia had a predictive value of 75% and a specificity of 99% for the diagnosis of psychogenic nonepileptic seizures (Epilepsy Behav. 2005; 6[2]:264-5).
Ms. Acton reported that of the 1,730 patients studied, 95 (5.5%) had a medical history of fibromyalgia. A majority of the 95 patients (95%) were female, and the mean age was 53 years. In addition, 43% of fibromyalgia patients had a nonparoxysmal, neurologic primary clinical diagnosis, most commonly chronic pain. She said that no differences were seen between fibromyalgia patients and case-matched controls in terms of age, race, marital status, or reason for referral. However, compared with case-matched controls, fibromyalgia patients were underrepresented in education (P = .02) and employment (P = .009).
Paroxysmal events were present in 57% of fibromyalgia patients and in 54% of case-matched controls. Among fibromyalgia patients with paroxysmal disorders, 11% had epileptic seizures, 74% had PNES, and 15% had physiologic nonepileptic events. Among matched controls, 37% had epileptic seizures, 51% had PNES, and 12% had physiologic nonepileptic events (P = .009).
After comparing the two groups of patients, the researchers determined that a historical diagnosis of fibromyalgia had a 61% sensitivity, 64% specificity, 74% positive predictive value, and 49% negative predictive value for the diagnosis of PNES. “Another interesting finding was that quite a few of our fibromyalgia patients felt they didn’t have fibromyalgia,” Ms. Action said. “Unprompted, during the course of their first visit, about 10% of this cohort mentioned to a physician that they were diagnosed with fibromyalgia, but they didn’t believe it.”
In their abstract, the researchers wrote that the study design “and a larger sample size involving mixed general neurology patients may account for the lesser association seen between fibromyalgia and PNES in this study.” They reported having no financial disclosures.
HOUSTON – The specificity of fibromyalgia as a marker for psychogenic nonepileptic seizures is less reliable than previously described, results from a large analysis showed.
“Fibromyalgia has been referred to as a reliable clinical indicator for differentiating psychogenic nonepileptic seizures from epilepsy, but there hasn’t been a lot of research looking into this idea,” Emily K. Acton said in an interview at the annual meeting of the American Epilepsy Society. In fact, the most recent study to investigate the association focused on a small patient cohort in a tertiary care epilepsy referral center and found that a diagnosis of fibromyalgia had a predictive value of 75% and a specificity of 99% for the diagnosis of psychogenic nonepileptic seizures (Epilepsy Behav. 2005; 6[2]:264-5).
Ms. Acton reported that of the 1,730 patients studied, 95 (5.5%) had a medical history of fibromyalgia. A majority of the 95 patients (95%) were female, and the mean age was 53 years. In addition, 43% of fibromyalgia patients had a nonparoxysmal, neurologic primary clinical diagnosis, most commonly chronic pain. She said that no differences were seen between fibromyalgia patients and case-matched controls in terms of age, race, marital status, or reason for referral. However, compared with case-matched controls, fibromyalgia patients were underrepresented in education (P = .02) and employment (P = .009).
Paroxysmal events were present in 57% of fibromyalgia patients and in 54% of case-matched controls. Among fibromyalgia patients with paroxysmal disorders, 11% had epileptic seizures, 74% had PNES, and 15% had physiologic nonepileptic events. Among matched controls, 37% had epileptic seizures, 51% had PNES, and 12% had physiologic nonepileptic events (P = .009).
After comparing the two groups of patients, the researchers determined that a historical diagnosis of fibromyalgia had a 61% sensitivity, 64% specificity, 74% positive predictive value, and 49% negative predictive value for the diagnosis of PNES. “Another interesting finding was that quite a few of our fibromyalgia patients felt they didn’t have fibromyalgia,” Ms. Action said. “Unprompted, during the course of their first visit, about 10% of this cohort mentioned to a physician that they were diagnosed with fibromyalgia, but they didn’t believe it.”
In their abstract, the researchers wrote that the study design “and a larger sample size involving mixed general neurology patients may account for the lesser association seen between fibromyalgia and PNES in this study.” They reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: The diagnosis of fibromyalgia had a 61% sensitivity, 64% specificity, and a 74% positive predictive value for the diagnosis of PNES.
Data source: A retrospective review of 1,730 consecutive patients seen by a single epileptologist at a neurology clinic over a 3-year period.
Disclosures: The researchers reported having no financial disclosures.
Increasing maternal vaccine uptake requires paradigm shift
ATLANTA – Both the influenza and the tetanus-diphtheria-acellular pertussis (Tdap) vaccines have been recommended during pregnancy for years, but uptake remains low.
The most recent national data from the Centers for Disease Control and Prevention show that the Tdap vaccination rate is about 14% before pregnancy and 10% during pregnancy. For influenza, the vaccination rate among pregnant women is about 50%, with 14% of women being vaccinated in the 6 months before pregnancy and 36% during pregnancy.
To get a handle on how ob.gyn. practices approach vaccination, Dr. O’Leary and his colleagues sent out a mail and Internet survey to 482 physicians from June through September 2015 and analyzed 353 responses.
Among the responders, 92% routinely assessed whether their pregnant patients had received the Tdap vaccine, and 98% routinely assessed whether pregnant patients had received the influenza vaccine. But only about half of the physicians (51%) assessed Tdap vaccination in nonpregnant patients, and 82% assessed influenza vaccine status in nonpregnant patients.
For the human papillomavirus (HPV) vaccine, ob.gyns. were more likely to ask their nonpregnant patients about the vaccine. A total of 46% of providers routinely assessed whether their pregnant patients had received it, while 92% assessed whether their nonpregnant patients needed or had received the HPV vaccine.
The numbers were lower when it came to actually administering the vaccines. Just over three-quarters of providers routinely administered the Tdap vaccine, and 85% routinely administered the influenza vaccine to their pregnant patients.
For their nonpregnant patients, 55% routinely administered Tdap, 70% routinely administered the flu vaccine, and 82% routinely administered the HPV vaccine.
Ob.gyns. were most likely to have standing orders in place for influenza vaccine for their pregnant patients, with 66% of providers reporting that they had these orders in place, compared with 51% for nonpregnant patients. Standing orders were less likely for Tdap vaccine administration (39% for pregnant patients and 37% for nonpregnant patients).
Barriers
Reimbursement-related issues topped the reasons that ob.gyns. found it burdensome to stock and administer vaccines. The most commonly reported barrier – cited by 54% of the respondents – was lack of adequate reimbursement for purchasing vaccines, and 30% of physicians cited this as a major barrier. Similarly, lack of adequate reimbursement for administration of the vaccine was listed as a major barrier for a quarter of the respondents and a moderate barrier by 21% of the respondents.
A quarter of physicians also cited difficulty determining if a patient’s insurance would reimburse for a vaccine as a major barrier.
Other barriers included having too little time for vaccination during visits when other preventive services took precedence, having patients who refused vaccines because of safety concerns, the burden of storing, ordering, and tracking vaccines, and difficulty determining whether a patient had already received a particular vaccine.
Fewer than 2% of ob.gyns., however, reported uncertainty about a particular vaccine’s effectiveness or safety in pregnant women as a barrier.
“Physician attitudinal barriers are nonexistent,” Dr. O’Leary said. “The perceived barriers were primarily financial, but logistical and patient attitudinal barriers were also important.”
Testing interventions
While the barriers to routine vaccine administration are clear, the solutions are less obvious. A recently reported intensive intervention to increase the uptake of maternal vaccines in ob.gyn. practices had only modest success in increasing Tdap vaccination and no significant impact on administration of the influenza vaccine.
“Immunization delivery in the ob.gyn. setting may present different challenges than more traditional settings for adult vaccination, such as family medicine or internal medicine offices,” Dr. O’Leary said.
The study involved eight ob.gyn. practices in Colorado and ran from August 2011 through March 2014, a period during which the Advisory Committee for Immunization Practices recommended that Tdap vaccination be given in every pregnancy.
Four ob.gyn. practices – one rural and three urban – were randomly assigned to usual care while the other four – two rural and two urban – were randomly assigned to the intervention. The practices were balanced in terms of their number of providers, the proportion of Medicaid patients they served, the number of deliveries per month, and an immunization delivery score at baseline.
The researchers assessed receipt of influenza vaccines among women pregnant during the previous influenza season and receipt of the Tdap vaccine among women at at least 34 weeks’ gestation. There were 13,324 patients in the control arm and 12,103 patients in the intervention arm.
The multimodal intervention involved seven components:
1. Designating immunization champions at each practice.
2. Assisting with vaccine purchasing and management.
3. Historical vaccination documentation training.
4. Implementing standing orders for both vaccines.
5. Chart review and feedback.
6. Patient/staff education materials and training.
7. Frequent contact with the project team, at least once a month during the study period.
At baseline, the rate of Tdap vaccination among pregnant women was 3% in the intervention clinics and 11% in the control clinics. During year 2, following the intervention, 38% of women at the intervention clinics and 34% of the women at the control clinics had received the Tdap vaccine. Those increases translated to a four times greater likelihood of getting the Tdap vaccine among women at clinics who underwent the intervention (risk ratio, 3.9; 95% confidence interval, 1.1-13.3).
Influenza vaccine uptake also increased collectively at the clinics, from 19% at intervention clinics and 18% at control clinics at baseline, to 21% at intervention clinics and 25% at control clinics a year later. But there was no significant difference in uptake between the intervention and control clinics.
An additional qualitative component of the study involved hour-long interviews with staff members from six of the clinics to assess specific components of the intervention, such as implementing standing orders for each vaccine.
“Prior to establishing standing orders at practices, the responsibility for assessing immunization history and eligibility had fallen to the medical providers,” Dr. O’Leary said. “By establishing standing orders for immunizations, providers and staff reported overall improved immunization delivery to their patient population.”
But barriers existed for standing orders as well, including patient reluctance to receive the vaccine without first discussing it with her physician.
The qualitative interviews also revealed that some nurses may have felt anxious about administering vaccines to pregnant women until they received vaccine education. Overall, staff education and implementation of standing orders were well received at the intervention practices.
“Adding immunization questions to standard intake forms was an efficient and effective method to collect immunization history that fit into already established patient check-in processes,” Dr. O’Leary said.
Standing order templates could also be customized to each practice’s processes, and the process of the staff reviewing these templates often led to consensus about how to integrate the orders into routine care, according to Dr. O’Leary.
“To increase the uptake of vaccinations in pregnancy, all ob.gyns. need to stock and administer influenza and Tdap vaccines,” Dr. O’Leary said. “And if ob.gyns. are to play a significant role as vaccinators of nonpregnant women, a paradigm shift is required.”
Both studies were funded by the CDC. Dr. O’Leary reported having no relevant financial disclosures, but one of the coinvestigators in the intervention study reported financial relationships with Merck and Pfizer.
ATLANTA – Both the influenza and the tetanus-diphtheria-acellular pertussis (Tdap) vaccines have been recommended during pregnancy for years, but uptake remains low.
The most recent national data from the Centers for Disease Control and Prevention show that the Tdap vaccination rate is about 14% before pregnancy and 10% during pregnancy. For influenza, the vaccination rate among pregnant women is about 50%, with 14% of women being vaccinated in the 6 months before pregnancy and 36% during pregnancy.
To get a handle on how ob.gyn. practices approach vaccination, Dr. O’Leary and his colleagues sent out a mail and Internet survey to 482 physicians from June through September 2015 and analyzed 353 responses.
Among the responders, 92% routinely assessed whether their pregnant patients had received the Tdap vaccine, and 98% routinely assessed whether pregnant patients had received the influenza vaccine. But only about half of the physicians (51%) assessed Tdap vaccination in nonpregnant patients, and 82% assessed influenza vaccine status in nonpregnant patients.
For the human papillomavirus (HPV) vaccine, ob.gyns. were more likely to ask their nonpregnant patients about the vaccine. A total of 46% of providers routinely assessed whether their pregnant patients had received it, while 92% assessed whether their nonpregnant patients needed or had received the HPV vaccine.
The numbers were lower when it came to actually administering the vaccines. Just over three-quarters of providers routinely administered the Tdap vaccine, and 85% routinely administered the influenza vaccine to their pregnant patients.
For their nonpregnant patients, 55% routinely administered Tdap, 70% routinely administered the flu vaccine, and 82% routinely administered the HPV vaccine.
Ob.gyns. were most likely to have standing orders in place for influenza vaccine for their pregnant patients, with 66% of providers reporting that they had these orders in place, compared with 51% for nonpregnant patients. Standing orders were less likely for Tdap vaccine administration (39% for pregnant patients and 37% for nonpregnant patients).
Barriers
Reimbursement-related issues topped the reasons that ob.gyns. found it burdensome to stock and administer vaccines. The most commonly reported barrier – cited by 54% of the respondents – was lack of adequate reimbursement for purchasing vaccines, and 30% of physicians cited this as a major barrier. Similarly, lack of adequate reimbursement for administration of the vaccine was listed as a major barrier for a quarter of the respondents and a moderate barrier by 21% of the respondents.
A quarter of physicians also cited difficulty determining if a patient’s insurance would reimburse for a vaccine as a major barrier.
Other barriers included having too little time for vaccination during visits when other preventive services took precedence, having patients who refused vaccines because of safety concerns, the burden of storing, ordering, and tracking vaccines, and difficulty determining whether a patient had already received a particular vaccine.
Fewer than 2% of ob.gyns., however, reported uncertainty about a particular vaccine’s effectiveness or safety in pregnant women as a barrier.
“Physician attitudinal barriers are nonexistent,” Dr. O’Leary said. “The perceived barriers were primarily financial, but logistical and patient attitudinal barriers were also important.”
Testing interventions
While the barriers to routine vaccine administration are clear, the solutions are less obvious. A recently reported intensive intervention to increase the uptake of maternal vaccines in ob.gyn. practices had only modest success in increasing Tdap vaccination and no significant impact on administration of the influenza vaccine.
“Immunization delivery in the ob.gyn. setting may present different challenges than more traditional settings for adult vaccination, such as family medicine or internal medicine offices,” Dr. O’Leary said.
The study involved eight ob.gyn. practices in Colorado and ran from August 2011 through March 2014, a period during which the Advisory Committee for Immunization Practices recommended that Tdap vaccination be given in every pregnancy.
Four ob.gyn. practices – one rural and three urban – were randomly assigned to usual care while the other four – two rural and two urban – were randomly assigned to the intervention. The practices were balanced in terms of their number of providers, the proportion of Medicaid patients they served, the number of deliveries per month, and an immunization delivery score at baseline.
The researchers assessed receipt of influenza vaccines among women pregnant during the previous influenza season and receipt of the Tdap vaccine among women at at least 34 weeks’ gestation. There were 13,324 patients in the control arm and 12,103 patients in the intervention arm.
The multimodal intervention involved seven components:
1. Designating immunization champions at each practice.
2. Assisting with vaccine purchasing and management.
3. Historical vaccination documentation training.
4. Implementing standing orders for both vaccines.
5. Chart review and feedback.
6. Patient/staff education materials and training.
7. Frequent contact with the project team, at least once a month during the study period.
At baseline, the rate of Tdap vaccination among pregnant women was 3% in the intervention clinics and 11% in the control clinics. During year 2, following the intervention, 38% of women at the intervention clinics and 34% of the women at the control clinics had received the Tdap vaccine. Those increases translated to a four times greater likelihood of getting the Tdap vaccine among women at clinics who underwent the intervention (risk ratio, 3.9; 95% confidence interval, 1.1-13.3).
Influenza vaccine uptake also increased collectively at the clinics, from 19% at intervention clinics and 18% at control clinics at baseline, to 21% at intervention clinics and 25% at control clinics a year later. But there was no significant difference in uptake between the intervention and control clinics.
An additional qualitative component of the study involved hour-long interviews with staff members from six of the clinics to assess specific components of the intervention, such as implementing standing orders for each vaccine.
“Prior to establishing standing orders at practices, the responsibility for assessing immunization history and eligibility had fallen to the medical providers,” Dr. O’Leary said. “By establishing standing orders for immunizations, providers and staff reported overall improved immunization delivery to their patient population.”
But barriers existed for standing orders as well, including patient reluctance to receive the vaccine without first discussing it with her physician.
The qualitative interviews also revealed that some nurses may have felt anxious about administering vaccines to pregnant women until they received vaccine education. Overall, staff education and implementation of standing orders were well received at the intervention practices.
“Adding immunization questions to standard intake forms was an efficient and effective method to collect immunization history that fit into already established patient check-in processes,” Dr. O’Leary said.
Standing order templates could also be customized to each practice’s processes, and the process of the staff reviewing these templates often led to consensus about how to integrate the orders into routine care, according to Dr. O’Leary.
“To increase the uptake of vaccinations in pregnancy, all ob.gyns. need to stock and administer influenza and Tdap vaccines,” Dr. O’Leary said. “And if ob.gyns. are to play a significant role as vaccinators of nonpregnant women, a paradigm shift is required.”
Both studies were funded by the CDC. Dr. O’Leary reported having no relevant financial disclosures, but one of the coinvestigators in the intervention study reported financial relationships with Merck and Pfizer.
ATLANTA – Both the influenza and the tetanus-diphtheria-acellular pertussis (Tdap) vaccines have been recommended during pregnancy for years, but uptake remains low.
The most recent national data from the Centers for Disease Control and Prevention show that the Tdap vaccination rate is about 14% before pregnancy and 10% during pregnancy. For influenza, the vaccination rate among pregnant women is about 50%, with 14% of women being vaccinated in the 6 months before pregnancy and 36% during pregnancy.
To get a handle on how ob.gyn. practices approach vaccination, Dr. O’Leary and his colleagues sent out a mail and Internet survey to 482 physicians from June through September 2015 and analyzed 353 responses.
Among the responders, 92% routinely assessed whether their pregnant patients had received the Tdap vaccine, and 98% routinely assessed whether pregnant patients had received the influenza vaccine. But only about half of the physicians (51%) assessed Tdap vaccination in nonpregnant patients, and 82% assessed influenza vaccine status in nonpregnant patients.
For the human papillomavirus (HPV) vaccine, ob.gyns. were more likely to ask their nonpregnant patients about the vaccine. A total of 46% of providers routinely assessed whether their pregnant patients had received it, while 92% assessed whether their nonpregnant patients needed or had received the HPV vaccine.
The numbers were lower when it came to actually administering the vaccines. Just over three-quarters of providers routinely administered the Tdap vaccine, and 85% routinely administered the influenza vaccine to their pregnant patients.
For their nonpregnant patients, 55% routinely administered Tdap, 70% routinely administered the flu vaccine, and 82% routinely administered the HPV vaccine.
Ob.gyns. were most likely to have standing orders in place for influenza vaccine for their pregnant patients, with 66% of providers reporting that they had these orders in place, compared with 51% for nonpregnant patients. Standing orders were less likely for Tdap vaccine administration (39% for pregnant patients and 37% for nonpregnant patients).
Barriers
Reimbursement-related issues topped the reasons that ob.gyns. found it burdensome to stock and administer vaccines. The most commonly reported barrier – cited by 54% of the respondents – was lack of adequate reimbursement for purchasing vaccines, and 30% of physicians cited this as a major barrier. Similarly, lack of adequate reimbursement for administration of the vaccine was listed as a major barrier for a quarter of the respondents and a moderate barrier by 21% of the respondents.
A quarter of physicians also cited difficulty determining if a patient’s insurance would reimburse for a vaccine as a major barrier.
Other barriers included having too little time for vaccination during visits when other preventive services took precedence, having patients who refused vaccines because of safety concerns, the burden of storing, ordering, and tracking vaccines, and difficulty determining whether a patient had already received a particular vaccine.
Fewer than 2% of ob.gyns., however, reported uncertainty about a particular vaccine’s effectiveness or safety in pregnant women as a barrier.
“Physician attitudinal barriers are nonexistent,” Dr. O’Leary said. “The perceived barriers were primarily financial, but logistical and patient attitudinal barriers were also important.”
Testing interventions
While the barriers to routine vaccine administration are clear, the solutions are less obvious. A recently reported intensive intervention to increase the uptake of maternal vaccines in ob.gyn. practices had only modest success in increasing Tdap vaccination and no significant impact on administration of the influenza vaccine.
“Immunization delivery in the ob.gyn. setting may present different challenges than more traditional settings for adult vaccination, such as family medicine or internal medicine offices,” Dr. O’Leary said.
The study involved eight ob.gyn. practices in Colorado and ran from August 2011 through March 2014, a period during which the Advisory Committee for Immunization Practices recommended that Tdap vaccination be given in every pregnancy.
Four ob.gyn. practices – one rural and three urban – were randomly assigned to usual care while the other four – two rural and two urban – were randomly assigned to the intervention. The practices were balanced in terms of their number of providers, the proportion of Medicaid patients they served, the number of deliveries per month, and an immunization delivery score at baseline.
The researchers assessed receipt of influenza vaccines among women pregnant during the previous influenza season and receipt of the Tdap vaccine among women at at least 34 weeks’ gestation. There were 13,324 patients in the control arm and 12,103 patients in the intervention arm.
The multimodal intervention involved seven components:
1. Designating immunization champions at each practice.
2. Assisting with vaccine purchasing and management.
3. Historical vaccination documentation training.
4. Implementing standing orders for both vaccines.
5. Chart review and feedback.
6. Patient/staff education materials and training.
7. Frequent contact with the project team, at least once a month during the study period.
At baseline, the rate of Tdap vaccination among pregnant women was 3% in the intervention clinics and 11% in the control clinics. During year 2, following the intervention, 38% of women at the intervention clinics and 34% of the women at the control clinics had received the Tdap vaccine. Those increases translated to a four times greater likelihood of getting the Tdap vaccine among women at clinics who underwent the intervention (risk ratio, 3.9; 95% confidence interval, 1.1-13.3).
Influenza vaccine uptake also increased collectively at the clinics, from 19% at intervention clinics and 18% at control clinics at baseline, to 21% at intervention clinics and 25% at control clinics a year later. But there was no significant difference in uptake between the intervention and control clinics.
An additional qualitative component of the study involved hour-long interviews with staff members from six of the clinics to assess specific components of the intervention, such as implementing standing orders for each vaccine.
“Prior to establishing standing orders at practices, the responsibility for assessing immunization history and eligibility had fallen to the medical providers,” Dr. O’Leary said. “By establishing standing orders for immunizations, providers and staff reported overall improved immunization delivery to their patient population.”
But barriers existed for standing orders as well, including patient reluctance to receive the vaccine without first discussing it with her physician.
The qualitative interviews also revealed that some nurses may have felt anxious about administering vaccines to pregnant women until they received vaccine education. Overall, staff education and implementation of standing orders were well received at the intervention practices.
“Adding immunization questions to standard intake forms was an efficient and effective method to collect immunization history that fit into already established patient check-in processes,” Dr. O’Leary said.
Standing order templates could also be customized to each practice’s processes, and the process of the staff reviewing these templates often led to consensus about how to integrate the orders into routine care, according to Dr. O’Leary.
“To increase the uptake of vaccinations in pregnancy, all ob.gyns. need to stock and administer influenza and Tdap vaccines,” Dr. O’Leary said. “And if ob.gyns. are to play a significant role as vaccinators of nonpregnant women, a paradigm shift is required.”
Both studies were funded by the CDC. Dr. O’Leary reported having no relevant financial disclosures, but one of the coinvestigators in the intervention study reported financial relationships with Merck and Pfizer.
AT THE NATIONAL IMMUNIZATION CONFERENCE
Tips for Working with Difficult Doctors
As a hospitalist, caring for critically ill or injured patients can be stressful and demanding. Working with difficult doctors, those who exhibit intimidating and disruptive behaviors such as verbal outbursts and physical threats as well as passive activities such as refusing to perform assigned tasks, can make the work environment even more challenging.1 Some docs are routinely reluctant—or refuse—to answer questions or return phone calls or pages. Some communicate in condescending language or voice intonation; some are brutally impatient.1
The most difficult doctors to work with are those who are not aligned with the hospital’s or treatment team’s goals and those who aren’t open to feedback and coaching, says Rob Zipper, MD, MMM, SFHM, regional chief medical officer of Sound Physicians, based in Tacoma, Wash.
“If physicians are aware of a practice’s guidelines and goals but simply won’t comply with them, it makes it harder on everyone else who is pulling the ship in the same direction,” he says.
Unruly physicians don’t just annoy their coworkers. According to a sentinel event alert from The Joint Commission, they can:
- foster medical errors;
- contribute to poor patient satisfaction;
- contribute to preventable adverse outcomes;
- increase the cost of care;
- undermine team effectiveness; and
- cause qualified clinicians, administrators, and managers to seek new positions in more professional environments.1
“These issues are all connected,” says Stephen R. Nichols, MD, chief of clinical operations performance at the Schumacher Group in Brownwood, Texas. “Disruptive behaviors create mitigated communications and dissatisfaction among staff, which bleeds over into other aspects that are involved secondarily.”
Stephen M. Paskoff, Esq., president and CEO of ELI in Atlanta, can attest to the most severe consequences of bad behavior on patient care.
At one institution, a surgeon’s disruptive behavior lead to a coworker forgetting to perform a procedure and a patient dying.2 In another incident, the emergency department stopped calling on a medical subspecialist who was predictably abusive. The subspecialist knew how to treat a specific patient with an unusual intervention. Since the specialist was not consulted initially, the patient ended up in the intensive care unit.2
One bad hospitalist can bring down the reputation of an entire team.
“Many programs are incentivized based on medical staff and primary-care physicians’ perceptions of their care, so there are direct and indirect consequences,” Dr. Zipper says.
The bottom line, says Felix Aguirre, MD, SFHM, vice president of medical affairs at IPC Healthcare in North Hollywood, Calif., is that it only takes one bad experience to tarnish a group, but it takes many positive experiences to erase the damage.
The Roots of Evil
Intimidating and disruptive behavior stems from both individual and systemic factors. Care providers who exhibit characteristics such as self-centeredness, immaturity, or defensiveness can be more prone to unprofessional behavior. They can lack interpersonal, coping, or conflict-management skills.1
Systemic factors are marked by pressures related to increased productivity demands, cost-containment requirements, embedded hierarchies, and fear of litigation in the healthcare environment. These pressures can be further exacerbated by changes to or differences in the authority, autonomy, empowerment, and roles or values of professionals on the healthcare team as well as by the continual daily changes in shifts, rotations, and interdepartmental support staff. This dynamic creates challenges for interprofessional communication and development of trust among team members.1
According to The Joint Commission, intimidating and disruptive behaviors are often manifested by healthcare professionals in positions of power.1 But other members of the care team can be problematic as well.
“In my experience, conflicts usually revolve around different perspectives and objectives, even if both parties are acting respectfully,” Dr. Zipper says. “Sometimes, however, providers or other care team members are tired or stressed and don’t behave professionally.”
Paskoff, who has more than 40 years of experience in healthcare-related workplace issues, including serving as an investigator for the U.S. Equal Employment Opportunity Commission, says some doctors learn bad behaviors from their mentors and that behaviors can be passed down through generations because they are tolerated.
“When I asked one physician who had outstanding training and an outstanding technical reputation how he became abusive, he said, ‘I learned from the best.’” Paskoff was actually able to track the doctor’s training to the late 1800s and physicians who were known for similar behaviors.
Confronting Those Who Misbehave
Dr. Zipper says physicians should confront behavioral issues directly.
“I will typically discuss a complaint with a doctor privately, and ask him or her what happened without being accusatory,” he says. “I try to provide as much concrete and objective information as I can. The doctor needs to know that you are trying to help him or her succeed. That said, if something is clearly bad behavior, feedback should be direct and include a statement such as, ‘This is not how we behave in this practice.’”
At times, it may not be possible to discuss an emergent matter, such as during a code blue.
“However, I will often ask if anyone on the code team has any ideas or concerns before ending the code,” Dr. Nichols says. “Then after the critical time has passed, it is important to debrief and reconnect with the team, especially the less-experienced members who may have lingering concerns.”
For many employees, however, it is difficult to report disruptive behaviors. This is due to a fear of retaliation and the stigma associated with “blowing the whistle” on a colleague as well as a general reluctance to confront an intimidator.1
If an employee cannot muster the courage to confront a disruptive coworker or if the issue isn’t resolved by talking with the difficult individual, an employee should be a good citizen and report bad behavior to the appropriate hospital authority in a timely manner, says A. Kevin Troutman, Esq., a partner at Fisher Phillips in Houston and a former healthcare human resources executive.
Hospitals accredited by The Joint Commission are required to create a code of conduct that defines disruptive and inappropriate behaviors. In addition, leaders must create and implement a process for managing these behaviors.1
Helping Difficult Doctors
After a physician or another employee has been called out for bad behavior, steps need to be taken to correct the problem. Robert Fuller, Esq., an attorney with Nelson Hardiman, LLP, in Los Angeles, has found a positive-oriented intervention called “the 3-Ds”—which stands for diagnose, design, and do—that has been a successful tool for achieving positive change. The strategy involves a supervisor and employee mutually developing a worksheet to diagnose the problem. Next, they design a remediation and improvement plan. Finally, they implement the plan and specify dates to achieve certain milestones. Coworkers should be informed of the plan and be urged to support it.
“Make it clear that the positive aspect of this plan turns to progressive discipline, including termination, if the employee doesn’t improve or abandons the plan of action,” Fuller says. In most cases, troublemakers will make a sincere effort to control disruptive tendencies.
Troutman suggests enlisting the assistance of a respected peer.
“Have a senior-level doctor help the noncompliant physician understand why his or her behavior creates problems for everyone, including the doctor himself,” he says. “Also, consider connecting compensation and other rewards to job performance, which encompasses good behavior and good citizenship within the organization. Make expectations and consequences clear.”
If an employee has a recent change in behavior, ask if there is a reason.
“It is my experience that sudden changes in behaviors are often the result of a personal or clinical issue, so it is important and humane to make certain that there is not some other cause for the change before assuming someone is simply being disruptive or difficult,” Dr. Nichols says.
Many healthcare institutions are now setting up centers of professionalism. Paskoff reports that The Center for Professionalism and Peer Support (CPPS) was created in 2008 at Brigham and Women’s Hospital in Boston to educate the hospital community regarding professionalism and manage unprofessional behavior.3 CPPS has established standards of behavior and a framework to deal with difficult behaviors.
“An employee is told what he or she is doing wrong, receives counseling, and is given resources to improve,” he explains. “If an employee doesn’t improve, he or she is told that the behavior won’t be tolerated.”
Dismissing Bad Employees
After addressing the specifics of unacceptable behavior and explaining the consequences of repeating it, leadership should monitor subsequent conduct and provide feedback.
“If the employee commits other violations or behaves badly, promptly address the misconduct again and make it clear that further such actions will not be tolerated,” Troutman says. “Expect immediate and sustained improvement and compliance. Be consistent, and if bad conduct continues after an opportunity to improve, do not prolong anyone’s suffering. Instead, terminate the disruptive employee. When you do, make the reasons clear.”
Karen Appold is a medical writer in Pennsylvania.
References
- Behaviors that undermine a culture of safety. The Joint Commission website. Accessed April 17, 2015.
- Whittemore AD, New England Society for Vascular Surgery. The impact of professionalism on safe surgical care. J Vasc Surg. 2007;45(2):415-419.
- Shapiro J, Whittemore A, Tsen LC. Instituting a culture of professionalism: the establishment of a center for professionalism and peer support. Jt Comm J Qual Patient Saf. 2014;40(4):168-177.
As a hospitalist, caring for critically ill or injured patients can be stressful and demanding. Working with difficult doctors, those who exhibit intimidating and disruptive behaviors such as verbal outbursts and physical threats as well as passive activities such as refusing to perform assigned tasks, can make the work environment even more challenging.1 Some docs are routinely reluctant—or refuse—to answer questions or return phone calls or pages. Some communicate in condescending language or voice intonation; some are brutally impatient.1
The most difficult doctors to work with are those who are not aligned with the hospital’s or treatment team’s goals and those who aren’t open to feedback and coaching, says Rob Zipper, MD, MMM, SFHM, regional chief medical officer of Sound Physicians, based in Tacoma, Wash.
“If physicians are aware of a practice’s guidelines and goals but simply won’t comply with them, it makes it harder on everyone else who is pulling the ship in the same direction,” he says.
Unruly physicians don’t just annoy their coworkers. According to a sentinel event alert from The Joint Commission, they can:
- foster medical errors;
- contribute to poor patient satisfaction;
- contribute to preventable adverse outcomes;
- increase the cost of care;
- undermine team effectiveness; and
- cause qualified clinicians, administrators, and managers to seek new positions in more professional environments.1
“These issues are all connected,” says Stephen R. Nichols, MD, chief of clinical operations performance at the Schumacher Group in Brownwood, Texas. “Disruptive behaviors create mitigated communications and dissatisfaction among staff, which bleeds over into other aspects that are involved secondarily.”
Stephen M. Paskoff, Esq., president and CEO of ELI in Atlanta, can attest to the most severe consequences of bad behavior on patient care.
At one institution, a surgeon’s disruptive behavior lead to a coworker forgetting to perform a procedure and a patient dying.2 In another incident, the emergency department stopped calling on a medical subspecialist who was predictably abusive. The subspecialist knew how to treat a specific patient with an unusual intervention. Since the specialist was not consulted initially, the patient ended up in the intensive care unit.2
One bad hospitalist can bring down the reputation of an entire team.
“Many programs are incentivized based on medical staff and primary-care physicians’ perceptions of their care, so there are direct and indirect consequences,” Dr. Zipper says.
The bottom line, says Felix Aguirre, MD, SFHM, vice president of medical affairs at IPC Healthcare in North Hollywood, Calif., is that it only takes one bad experience to tarnish a group, but it takes many positive experiences to erase the damage.
The Roots of Evil
Intimidating and disruptive behavior stems from both individual and systemic factors. Care providers who exhibit characteristics such as self-centeredness, immaturity, or defensiveness can be more prone to unprofessional behavior. They can lack interpersonal, coping, or conflict-management skills.1
Systemic factors are marked by pressures related to increased productivity demands, cost-containment requirements, embedded hierarchies, and fear of litigation in the healthcare environment. These pressures can be further exacerbated by changes to or differences in the authority, autonomy, empowerment, and roles or values of professionals on the healthcare team as well as by the continual daily changes in shifts, rotations, and interdepartmental support staff. This dynamic creates challenges for interprofessional communication and development of trust among team members.1
According to The Joint Commission, intimidating and disruptive behaviors are often manifested by healthcare professionals in positions of power.1 But other members of the care team can be problematic as well.
“In my experience, conflicts usually revolve around different perspectives and objectives, even if both parties are acting respectfully,” Dr. Zipper says. “Sometimes, however, providers or other care team members are tired or stressed and don’t behave professionally.”
Paskoff, who has more than 40 years of experience in healthcare-related workplace issues, including serving as an investigator for the U.S. Equal Employment Opportunity Commission, says some doctors learn bad behaviors from their mentors and that behaviors can be passed down through generations because they are tolerated.
“When I asked one physician who had outstanding training and an outstanding technical reputation how he became abusive, he said, ‘I learned from the best.’” Paskoff was actually able to track the doctor’s training to the late 1800s and physicians who were known for similar behaviors.
Confronting Those Who Misbehave
Dr. Zipper says physicians should confront behavioral issues directly.
“I will typically discuss a complaint with a doctor privately, and ask him or her what happened without being accusatory,” he says. “I try to provide as much concrete and objective information as I can. The doctor needs to know that you are trying to help him or her succeed. That said, if something is clearly bad behavior, feedback should be direct and include a statement such as, ‘This is not how we behave in this practice.’”
At times, it may not be possible to discuss an emergent matter, such as during a code blue.
“However, I will often ask if anyone on the code team has any ideas or concerns before ending the code,” Dr. Nichols says. “Then after the critical time has passed, it is important to debrief and reconnect with the team, especially the less-experienced members who may have lingering concerns.”
For many employees, however, it is difficult to report disruptive behaviors. This is due to a fear of retaliation and the stigma associated with “blowing the whistle” on a colleague as well as a general reluctance to confront an intimidator.1
If an employee cannot muster the courage to confront a disruptive coworker or if the issue isn’t resolved by talking with the difficult individual, an employee should be a good citizen and report bad behavior to the appropriate hospital authority in a timely manner, says A. Kevin Troutman, Esq., a partner at Fisher Phillips in Houston and a former healthcare human resources executive.
Hospitals accredited by The Joint Commission are required to create a code of conduct that defines disruptive and inappropriate behaviors. In addition, leaders must create and implement a process for managing these behaviors.1
Helping Difficult Doctors
After a physician or another employee has been called out for bad behavior, steps need to be taken to correct the problem. Robert Fuller, Esq., an attorney with Nelson Hardiman, LLP, in Los Angeles, has found a positive-oriented intervention called “the 3-Ds”—which stands for diagnose, design, and do—that has been a successful tool for achieving positive change. The strategy involves a supervisor and employee mutually developing a worksheet to diagnose the problem. Next, they design a remediation and improvement plan. Finally, they implement the plan and specify dates to achieve certain milestones. Coworkers should be informed of the plan and be urged to support it.
“Make it clear that the positive aspect of this plan turns to progressive discipline, including termination, if the employee doesn’t improve or abandons the plan of action,” Fuller says. In most cases, troublemakers will make a sincere effort to control disruptive tendencies.
Troutman suggests enlisting the assistance of a respected peer.
“Have a senior-level doctor help the noncompliant physician understand why his or her behavior creates problems for everyone, including the doctor himself,” he says. “Also, consider connecting compensation and other rewards to job performance, which encompasses good behavior and good citizenship within the organization. Make expectations and consequences clear.”
If an employee has a recent change in behavior, ask if there is a reason.
“It is my experience that sudden changes in behaviors are often the result of a personal or clinical issue, so it is important and humane to make certain that there is not some other cause for the change before assuming someone is simply being disruptive or difficult,” Dr. Nichols says.
Many healthcare institutions are now setting up centers of professionalism. Paskoff reports that The Center for Professionalism and Peer Support (CPPS) was created in 2008 at Brigham and Women’s Hospital in Boston to educate the hospital community regarding professionalism and manage unprofessional behavior.3 CPPS has established standards of behavior and a framework to deal with difficult behaviors.
“An employee is told what he or she is doing wrong, receives counseling, and is given resources to improve,” he explains. “If an employee doesn’t improve, he or she is told that the behavior won’t be tolerated.”
Dismissing Bad Employees
After addressing the specifics of unacceptable behavior and explaining the consequences of repeating it, leadership should monitor subsequent conduct and provide feedback.
“If the employee commits other violations or behaves badly, promptly address the misconduct again and make it clear that further such actions will not be tolerated,” Troutman says. “Expect immediate and sustained improvement and compliance. Be consistent, and if bad conduct continues after an opportunity to improve, do not prolong anyone’s suffering. Instead, terminate the disruptive employee. When you do, make the reasons clear.”
Karen Appold is a medical writer in Pennsylvania.
References
- Behaviors that undermine a culture of safety. The Joint Commission website. Accessed April 17, 2015.
- Whittemore AD, New England Society for Vascular Surgery. The impact of professionalism on safe surgical care. J Vasc Surg. 2007;45(2):415-419.
- Shapiro J, Whittemore A, Tsen LC. Instituting a culture of professionalism: the establishment of a center for professionalism and peer support. Jt Comm J Qual Patient Saf. 2014;40(4):168-177.
As a hospitalist, caring for critically ill or injured patients can be stressful and demanding. Working with difficult doctors, those who exhibit intimidating and disruptive behaviors such as verbal outbursts and physical threats as well as passive activities such as refusing to perform assigned tasks, can make the work environment even more challenging.1 Some docs are routinely reluctant—or refuse—to answer questions or return phone calls or pages. Some communicate in condescending language or voice intonation; some are brutally impatient.1
The most difficult doctors to work with are those who are not aligned with the hospital’s or treatment team’s goals and those who aren’t open to feedback and coaching, says Rob Zipper, MD, MMM, SFHM, regional chief medical officer of Sound Physicians, based in Tacoma, Wash.
“If physicians are aware of a practice’s guidelines and goals but simply won’t comply with them, it makes it harder on everyone else who is pulling the ship in the same direction,” he says.
Unruly physicians don’t just annoy their coworkers. According to a sentinel event alert from The Joint Commission, they can:
- foster medical errors;
- contribute to poor patient satisfaction;
- contribute to preventable adverse outcomes;
- increase the cost of care;
- undermine team effectiveness; and
- cause qualified clinicians, administrators, and managers to seek new positions in more professional environments.1
“These issues are all connected,” says Stephen R. Nichols, MD, chief of clinical operations performance at the Schumacher Group in Brownwood, Texas. “Disruptive behaviors create mitigated communications and dissatisfaction among staff, which bleeds over into other aspects that are involved secondarily.”
Stephen M. Paskoff, Esq., president and CEO of ELI in Atlanta, can attest to the most severe consequences of bad behavior on patient care.
At one institution, a surgeon’s disruptive behavior lead to a coworker forgetting to perform a procedure and a patient dying.2 In another incident, the emergency department stopped calling on a medical subspecialist who was predictably abusive. The subspecialist knew how to treat a specific patient with an unusual intervention. Since the specialist was not consulted initially, the patient ended up in the intensive care unit.2
One bad hospitalist can bring down the reputation of an entire team.
“Many programs are incentivized based on medical staff and primary-care physicians’ perceptions of their care, so there are direct and indirect consequences,” Dr. Zipper says.
The bottom line, says Felix Aguirre, MD, SFHM, vice president of medical affairs at IPC Healthcare in North Hollywood, Calif., is that it only takes one bad experience to tarnish a group, but it takes many positive experiences to erase the damage.
The Roots of Evil
Intimidating and disruptive behavior stems from both individual and systemic factors. Care providers who exhibit characteristics such as self-centeredness, immaturity, or defensiveness can be more prone to unprofessional behavior. They can lack interpersonal, coping, or conflict-management skills.1
Systemic factors are marked by pressures related to increased productivity demands, cost-containment requirements, embedded hierarchies, and fear of litigation in the healthcare environment. These pressures can be further exacerbated by changes to or differences in the authority, autonomy, empowerment, and roles or values of professionals on the healthcare team as well as by the continual daily changes in shifts, rotations, and interdepartmental support staff. This dynamic creates challenges for interprofessional communication and development of trust among team members.1
According to The Joint Commission, intimidating and disruptive behaviors are often manifested by healthcare professionals in positions of power.1 But other members of the care team can be problematic as well.
“In my experience, conflicts usually revolve around different perspectives and objectives, even if both parties are acting respectfully,” Dr. Zipper says. “Sometimes, however, providers or other care team members are tired or stressed and don’t behave professionally.”
Paskoff, who has more than 40 years of experience in healthcare-related workplace issues, including serving as an investigator for the U.S. Equal Employment Opportunity Commission, says some doctors learn bad behaviors from their mentors and that behaviors can be passed down through generations because they are tolerated.
“When I asked one physician who had outstanding training and an outstanding technical reputation how he became abusive, he said, ‘I learned from the best.’” Paskoff was actually able to track the doctor’s training to the late 1800s and physicians who were known for similar behaviors.
Confronting Those Who Misbehave
Dr. Zipper says physicians should confront behavioral issues directly.
“I will typically discuss a complaint with a doctor privately, and ask him or her what happened without being accusatory,” he says. “I try to provide as much concrete and objective information as I can. The doctor needs to know that you are trying to help him or her succeed. That said, if something is clearly bad behavior, feedback should be direct and include a statement such as, ‘This is not how we behave in this practice.’”
At times, it may not be possible to discuss an emergent matter, such as during a code blue.
“However, I will often ask if anyone on the code team has any ideas or concerns before ending the code,” Dr. Nichols says. “Then after the critical time has passed, it is important to debrief and reconnect with the team, especially the less-experienced members who may have lingering concerns.”
For many employees, however, it is difficult to report disruptive behaviors. This is due to a fear of retaliation and the stigma associated with “blowing the whistle” on a colleague as well as a general reluctance to confront an intimidator.1
If an employee cannot muster the courage to confront a disruptive coworker or if the issue isn’t resolved by talking with the difficult individual, an employee should be a good citizen and report bad behavior to the appropriate hospital authority in a timely manner, says A. Kevin Troutman, Esq., a partner at Fisher Phillips in Houston and a former healthcare human resources executive.
Hospitals accredited by The Joint Commission are required to create a code of conduct that defines disruptive and inappropriate behaviors. In addition, leaders must create and implement a process for managing these behaviors.1
Helping Difficult Doctors
After a physician or another employee has been called out for bad behavior, steps need to be taken to correct the problem. Robert Fuller, Esq., an attorney with Nelson Hardiman, LLP, in Los Angeles, has found a positive-oriented intervention called “the 3-Ds”—which stands for diagnose, design, and do—that has been a successful tool for achieving positive change. The strategy involves a supervisor and employee mutually developing a worksheet to diagnose the problem. Next, they design a remediation and improvement plan. Finally, they implement the plan and specify dates to achieve certain milestones. Coworkers should be informed of the plan and be urged to support it.
“Make it clear that the positive aspect of this plan turns to progressive discipline, including termination, if the employee doesn’t improve or abandons the plan of action,” Fuller says. In most cases, troublemakers will make a sincere effort to control disruptive tendencies.
Troutman suggests enlisting the assistance of a respected peer.
“Have a senior-level doctor help the noncompliant physician understand why his or her behavior creates problems for everyone, including the doctor himself,” he says. “Also, consider connecting compensation and other rewards to job performance, which encompasses good behavior and good citizenship within the organization. Make expectations and consequences clear.”
If an employee has a recent change in behavior, ask if there is a reason.
“It is my experience that sudden changes in behaviors are often the result of a personal or clinical issue, so it is important and humane to make certain that there is not some other cause for the change before assuming someone is simply being disruptive or difficult,” Dr. Nichols says.
Many healthcare institutions are now setting up centers of professionalism. Paskoff reports that The Center for Professionalism and Peer Support (CPPS) was created in 2008 at Brigham and Women’s Hospital in Boston to educate the hospital community regarding professionalism and manage unprofessional behavior.3 CPPS has established standards of behavior and a framework to deal with difficult behaviors.
“An employee is told what he or she is doing wrong, receives counseling, and is given resources to improve,” he explains. “If an employee doesn’t improve, he or she is told that the behavior won’t be tolerated.”
Dismissing Bad Employees
After addressing the specifics of unacceptable behavior and explaining the consequences of repeating it, leadership should monitor subsequent conduct and provide feedback.
“If the employee commits other violations or behaves badly, promptly address the misconduct again and make it clear that further such actions will not be tolerated,” Troutman says. “Expect immediate and sustained improvement and compliance. Be consistent, and if bad conduct continues after an opportunity to improve, do not prolong anyone’s suffering. Instead, terminate the disruptive employee. When you do, make the reasons clear.”
Karen Appold is a medical writer in Pennsylvania.
References
- Behaviors that undermine a culture of safety. The Joint Commission website. Accessed April 17, 2015.
- Whittemore AD, New England Society for Vascular Surgery. The impact of professionalism on safe surgical care. J Vasc Surg. 2007;45(2):415-419.
- Shapiro J, Whittemore A, Tsen LC. Instituting a culture of professionalism: the establishment of a center for professionalism and peer support. Jt Comm J Qual Patient Saf. 2014;40(4):168-177.
Pacritinib bests BAT despite study truncation

SAN DIEGO—The JAK2/FLT3 inhibitor pacritinib significantly reduces spleen volume and symptoms in patients with myelofibrosis and low platelet counts,
compared to best available therapy (BAT), according to results of the PERSIST-2 trial.
In this phase 3 trial, BAT included the JAK1/2 inhibitor ruxolitinib. And pacritinib demonstrated benefits over BAT despite a truncated trial.
The US Food and Drug Administration (FDA) placed PERSIST-2 on clinical hold in February 2016 due to concerns over interim survival results, bleeding, and cardiovascular events.
Patients randomized at least 22 weeks prior to the clinical hold contributed data to the week 24 endpoint, the results of which were presented at the 2016 ASH Annual Meeting.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, delivered the results as a late-breaking abstract (LBA-5).
Ruxolitinib is FDA-approved to treat myelofibrosis, but it is associated with dose-limiting cytopenias and is not indicated for patients with platelet counts less than 50,000/μL.
The earlier PERSIST-1 trial demonstrated sustained spleen volume reduction (SVR) and symptom control with pacritinib therapy regardless of baseline platelet count, but it did not include ruxolitinib in the BAT arm.
PERSIST-2 study design
Patients were randomized on a 1:1:1 basis to pacritinib at 400 mg once daily (PAC QD), pacritinib at 200 mg twice daily (PAC BID), or BAT, including ruxolitinib.
Patients had to have primary or secondary myelofibrosis and platelet counts of 100,000/μL or less. Patients were allowed to have had prior JAK2 inhibitors.
Crossover from BAT was allowed after progression at any time or at week 24.
The study had 2 primary endpoints: percent of patients achieving a 35% or greater SVR and the percent of patients achieving a 50% or more reduction in total symptom score (TSS) by MPN-SAF TSS 2.0.
The primary study objective was the efficacy of pooled QD and BID pacritinib compared to BAT. The secondary objective was the efficacy of QD or BD separately compared to BAT.
Patient demographics
The study randomized 311 patients, and 221 were included in the intent-to-treat efficacy population.
The efficacy population consisted of all patients randomized prior to September 7, 2015, which allowed their data to be included in the week 24 endpoint analysis prior to the clinical hold.
The PAC QD arm consisted of 75 patients with a median age of 69 (range, 39-85). Seventy-one percent were 65 or older, and about half were male.
About three-quarters had an ECOG performance status of 0-1, 61% had primary myelofibrosis, 21% had primary polycythemia vera (PPV), and 17% had primary essential thrombocythemia (PET). About half were DIPSS Intermediate-2 risk, 80% were JAK2V617F positive, and 51% had a platelet count less than 50,000/μL.
The PAC BID arm consisted of 74 patients with a median age of 67 (range, 39-85). Sixty-two percent were 65 or older, and 65% were male.
Eighty-eight percent had an ECOG performance status of 0-1, 74% had primary myelofibrosis, 19% had PPV, and 7% had PET. About half were DIPSS Intermediate-2 risk, 80% were JAK2V617F positive, and 42% had a platelet count less than 50,000/μL.
The BAT arm consisted of 72 patients with a median age of 69 (range, 32-83). Seventy-one percent were 65 or older, and about half were male.
Three-quarters had an ECOG performance status of 0-1, 60% had primary myelofibrosis, 22% had PPV, and 18% had PET. About half were DIPSS Intermediate-2 risk, 71% were JAK2V617F positive, and 44% had a platelet count less than 50,000/µL.
Prior ruxolitinib therapy was consistent across the arms—41.3% (PAC QD), 42% (PAC BID), and 46% (BAT).
“Now, it’s important to note,” Dr Mascarenhas said, “that the most common BAT was ruxolitinib, 45%, and hydroxyurea, 19%. And 19% of BAT patients actually had no treatment, watch and wait. This highlights the fact that this is an area where there is really no other viable therapeutic option for these patients.”
Efficacy
Pacritinib-treated patients had significantly greater spleen reduction from baseline to week 24 than BAT-treated patients, with 18% (QD+BID), 15% (QD), and 22% (BID) achieving 35% or more SVR compared to 3% in the BAT arm.
Pacritinib-treated patients also experienced greater TSS reduction, with 25% (QD+BID), 17% (QD), and 32% (BID) reporting 50% or more reduction in TSS compared to 14% in the BAT arm. However, only the PAC BID arm was significantly different from BAT.
SVR in all subgroups—age, gender, JAK2 mutation status, prior treatment, platelet count, hemoglobin, peripheral blasts, and white blood cell count—demonstrated superiority for pacritinib.
Median changes in individual symptom scores were also better in the pacritinib arms than in the BAT arm in almost every category—tiredness, early satiety, abdominal discomfort, inactivity, night sweats, bone pain, and pain under ribs on the left side.
Pruritus was the only category in which BAT was superior, and that was compared to the QD arm and not the BID arm.
The majority of patients who stopped pacritinib therapy were taken off due to the clinical hold.
There were no significant differences between the groups in overall survival. Hazard ratios for overall survival (95% confidence intervals) were 0.68 (0.30, 1.53) for PAC BID vs BAT, 1.18 (0.57, 2.44) for PAC QD vs BAT, and 0.61 (0.27, 1.35) for PAC BID vs QD.
PAC BID maintained this survival advantage compared with BAT across nearly all demographic and myelofibrosis-associated risk factors.
Patients who were red blood cell transfusion-dependent at baseline experienced a statistically significant decrease in transfusion frequency from baseline to week 24 in both the QD and BID pacritinib arms compared with BAT.
And thrombocytopenia was not a significant factor for patients who were receiving pacritinib and had platelet counts less than 50,000/μL.
Toxicity
The most common treatment-emergent adverse events (AEs) associated with pacritinib were diarrhea, nausea, vomiting, anemia, and thrombocytopenia. They were generally less frequent for BID compared with QD administration.
The most common serious adverse events (SAEs)—occurring in 5% of patients or more in any arms—were anemia (5%, 8%, and 3%), thrombocytopenia (2%, 6%, and 2%), pneumonia (5%, 6%, and 4%), and acute renal failure (5%, 2%, and 2%) in the QD, BID, and BAT arms, respectively.
SAEs of interest included congestive heart failure, atrial fibrillation, cardiac arrest, epistaxis, and subdural hematoma, which occurred in 3% or fewer patients in any arm.
“They [the SAEs] were relatively infrequent, and there was not a clear signal of toxicity,” Dr Mascarenhas said.
Deaths
Deaths in the intent-to-treat evaluable population were censored at the time of full clinical hold.
For the entire enrolled population, 15/104 deaths occurred in the QD arm, 10/107 in the BID arm, and 14/100 in the BAT arm.
After the full clinical hold, 7, 10, and 6 deaths occurred in the QD, BID, and BAT arms, respectively.
Seven of 20 patients died after crossover to pacritinib. Five were due to AEs—3 cardiac, 1 bleeding, and 1 other.
“It’s important to note that progression of disease was the leading cause of death in the PAC BID arm,” Dr Mascarenhas noted. “This is after the patients stopped the drug [when the trial was on clinical hold].”
Conclusions
Despite study truncation, pacritinib (QD+BID) was significantly more effective than BAT for SVR (P=0.001) and trended toward improved TSS (P=0.079).
Pacritinib BID appeared more effective than QD versus BAT for SVR and TSS, and pacritinib BID appeared to have a better benefit/risk profile than BAT.
“This is a well-tolerated drug in many respects,” Dr Mascarenhas said. “This is a patient population that is quite ill, low platelets, poor outcome, and they did pretty well.”
When asked about the future of pacritinib, Dr Mascarenhas said he believes the benefit-to-risk ratio is in favor of the drug.
“Pacritinib offers patients in this vulnerable situation an opportunity for symptom relief, basically spleen and symptoms,” he said. “So I think this is a drug that will hopefully move forward.”
Dr Mascarenhas disclosed research funding from CTI Biopharma, the sponsor of the trial. ![]()

SAN DIEGO—The JAK2/FLT3 inhibitor pacritinib significantly reduces spleen volume and symptoms in patients with myelofibrosis and low platelet counts,
compared to best available therapy (BAT), according to results of the PERSIST-2 trial.
In this phase 3 trial, BAT included the JAK1/2 inhibitor ruxolitinib. And pacritinib demonstrated benefits over BAT despite a truncated trial.
The US Food and Drug Administration (FDA) placed PERSIST-2 on clinical hold in February 2016 due to concerns over interim survival results, bleeding, and cardiovascular events.
Patients randomized at least 22 weeks prior to the clinical hold contributed data to the week 24 endpoint, the results of which were presented at the 2016 ASH Annual Meeting.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, delivered the results as a late-breaking abstract (LBA-5).
Ruxolitinib is FDA-approved to treat myelofibrosis, but it is associated with dose-limiting cytopenias and is not indicated for patients with platelet counts less than 50,000/μL.
The earlier PERSIST-1 trial demonstrated sustained spleen volume reduction (SVR) and symptom control with pacritinib therapy regardless of baseline platelet count, but it did not include ruxolitinib in the BAT arm.
PERSIST-2 study design
Patients were randomized on a 1:1:1 basis to pacritinib at 400 mg once daily (PAC QD), pacritinib at 200 mg twice daily (PAC BID), or BAT, including ruxolitinib.
Patients had to have primary or secondary myelofibrosis and platelet counts of 100,000/μL or less. Patients were allowed to have had prior JAK2 inhibitors.
Crossover from BAT was allowed after progression at any time or at week 24.
The study had 2 primary endpoints: percent of patients achieving a 35% or greater SVR and the percent of patients achieving a 50% or more reduction in total symptom score (TSS) by MPN-SAF TSS 2.0.
The primary study objective was the efficacy of pooled QD and BID pacritinib compared to BAT. The secondary objective was the efficacy of QD or BD separately compared to BAT.
Patient demographics
The study randomized 311 patients, and 221 were included in the intent-to-treat efficacy population.
The efficacy population consisted of all patients randomized prior to September 7, 2015, which allowed their data to be included in the week 24 endpoint analysis prior to the clinical hold.
The PAC QD arm consisted of 75 patients with a median age of 69 (range, 39-85). Seventy-one percent were 65 or older, and about half were male.
About three-quarters had an ECOG performance status of 0-1, 61% had primary myelofibrosis, 21% had primary polycythemia vera (PPV), and 17% had primary essential thrombocythemia (PET). About half were DIPSS Intermediate-2 risk, 80% were JAK2V617F positive, and 51% had a platelet count less than 50,000/μL.
The PAC BID arm consisted of 74 patients with a median age of 67 (range, 39-85). Sixty-two percent were 65 or older, and 65% were male.
Eighty-eight percent had an ECOG performance status of 0-1, 74% had primary myelofibrosis, 19% had PPV, and 7% had PET. About half were DIPSS Intermediate-2 risk, 80% were JAK2V617F positive, and 42% had a platelet count less than 50,000/μL.
The BAT arm consisted of 72 patients with a median age of 69 (range, 32-83). Seventy-one percent were 65 or older, and about half were male.
Three-quarters had an ECOG performance status of 0-1, 60% had primary myelofibrosis, 22% had PPV, and 18% had PET. About half were DIPSS Intermediate-2 risk, 71% were JAK2V617F positive, and 44% had a platelet count less than 50,000/µL.
Prior ruxolitinib therapy was consistent across the arms—41.3% (PAC QD), 42% (PAC BID), and 46% (BAT).
“Now, it’s important to note,” Dr Mascarenhas said, “that the most common BAT was ruxolitinib, 45%, and hydroxyurea, 19%. And 19% of BAT patients actually had no treatment, watch and wait. This highlights the fact that this is an area where there is really no other viable therapeutic option for these patients.”
Efficacy
Pacritinib-treated patients had significantly greater spleen reduction from baseline to week 24 than BAT-treated patients, with 18% (QD+BID), 15% (QD), and 22% (BID) achieving 35% or more SVR compared to 3% in the BAT arm.
Pacritinib-treated patients also experienced greater TSS reduction, with 25% (QD+BID), 17% (QD), and 32% (BID) reporting 50% or more reduction in TSS compared to 14% in the BAT arm. However, only the PAC BID arm was significantly different from BAT.
SVR in all subgroups—age, gender, JAK2 mutation status, prior treatment, platelet count, hemoglobin, peripheral blasts, and white blood cell count—demonstrated superiority for pacritinib.
Median changes in individual symptom scores were also better in the pacritinib arms than in the BAT arm in almost every category—tiredness, early satiety, abdominal discomfort, inactivity, night sweats, bone pain, and pain under ribs on the left side.
Pruritus was the only category in which BAT was superior, and that was compared to the QD arm and not the BID arm.
The majority of patients who stopped pacritinib therapy were taken off due to the clinical hold.
There were no significant differences between the groups in overall survival. Hazard ratios for overall survival (95% confidence intervals) were 0.68 (0.30, 1.53) for PAC BID vs BAT, 1.18 (0.57, 2.44) for PAC QD vs BAT, and 0.61 (0.27, 1.35) for PAC BID vs QD.
PAC BID maintained this survival advantage compared with BAT across nearly all demographic and myelofibrosis-associated risk factors.
Patients who were red blood cell transfusion-dependent at baseline experienced a statistically significant decrease in transfusion frequency from baseline to week 24 in both the QD and BID pacritinib arms compared with BAT.
And thrombocytopenia was not a significant factor for patients who were receiving pacritinib and had platelet counts less than 50,000/μL.
Toxicity
The most common treatment-emergent adverse events (AEs) associated with pacritinib were diarrhea, nausea, vomiting, anemia, and thrombocytopenia. They were generally less frequent for BID compared with QD administration.
The most common serious adverse events (SAEs)—occurring in 5% of patients or more in any arms—were anemia (5%, 8%, and 3%), thrombocytopenia (2%, 6%, and 2%), pneumonia (5%, 6%, and 4%), and acute renal failure (5%, 2%, and 2%) in the QD, BID, and BAT arms, respectively.
SAEs of interest included congestive heart failure, atrial fibrillation, cardiac arrest, epistaxis, and subdural hematoma, which occurred in 3% or fewer patients in any arm.
“They [the SAEs] were relatively infrequent, and there was not a clear signal of toxicity,” Dr Mascarenhas said.
Deaths
Deaths in the intent-to-treat evaluable population were censored at the time of full clinical hold.
For the entire enrolled population, 15/104 deaths occurred in the QD arm, 10/107 in the BID arm, and 14/100 in the BAT arm.
After the full clinical hold, 7, 10, and 6 deaths occurred in the QD, BID, and BAT arms, respectively.
Seven of 20 patients died after crossover to pacritinib. Five were due to AEs—3 cardiac, 1 bleeding, and 1 other.
“It’s important to note that progression of disease was the leading cause of death in the PAC BID arm,” Dr Mascarenhas noted. “This is after the patients stopped the drug [when the trial was on clinical hold].”
Conclusions
Despite study truncation, pacritinib (QD+BID) was significantly more effective than BAT for SVR (P=0.001) and trended toward improved TSS (P=0.079).
Pacritinib BID appeared more effective than QD versus BAT for SVR and TSS, and pacritinib BID appeared to have a better benefit/risk profile than BAT.
“This is a well-tolerated drug in many respects,” Dr Mascarenhas said. “This is a patient population that is quite ill, low platelets, poor outcome, and they did pretty well.”
When asked about the future of pacritinib, Dr Mascarenhas said he believes the benefit-to-risk ratio is in favor of the drug.
“Pacritinib offers patients in this vulnerable situation an opportunity for symptom relief, basically spleen and symptoms,” he said. “So I think this is a drug that will hopefully move forward.”
Dr Mascarenhas disclosed research funding from CTI Biopharma, the sponsor of the trial. ![]()

SAN DIEGO—The JAK2/FLT3 inhibitor pacritinib significantly reduces spleen volume and symptoms in patients with myelofibrosis and low platelet counts,
compared to best available therapy (BAT), according to results of the PERSIST-2 trial.
In this phase 3 trial, BAT included the JAK1/2 inhibitor ruxolitinib. And pacritinib demonstrated benefits over BAT despite a truncated trial.
The US Food and Drug Administration (FDA) placed PERSIST-2 on clinical hold in February 2016 due to concerns over interim survival results, bleeding, and cardiovascular events.
Patients randomized at least 22 weeks prior to the clinical hold contributed data to the week 24 endpoint, the results of which were presented at the 2016 ASH Annual Meeting.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, delivered the results as a late-breaking abstract (LBA-5).
Ruxolitinib is FDA-approved to treat myelofibrosis, but it is associated with dose-limiting cytopenias and is not indicated for patients with platelet counts less than 50,000/μL.
The earlier PERSIST-1 trial demonstrated sustained spleen volume reduction (SVR) and symptom control with pacritinib therapy regardless of baseline platelet count, but it did not include ruxolitinib in the BAT arm.
PERSIST-2 study design
Patients were randomized on a 1:1:1 basis to pacritinib at 400 mg once daily (PAC QD), pacritinib at 200 mg twice daily (PAC BID), or BAT, including ruxolitinib.
Patients had to have primary or secondary myelofibrosis and platelet counts of 100,000/μL or less. Patients were allowed to have had prior JAK2 inhibitors.
Crossover from BAT was allowed after progression at any time or at week 24.
The study had 2 primary endpoints: percent of patients achieving a 35% or greater SVR and the percent of patients achieving a 50% or more reduction in total symptom score (TSS) by MPN-SAF TSS 2.0.
The primary study objective was the efficacy of pooled QD and BID pacritinib compared to BAT. The secondary objective was the efficacy of QD or BD separately compared to BAT.
Patient demographics
The study randomized 311 patients, and 221 were included in the intent-to-treat efficacy population.
The efficacy population consisted of all patients randomized prior to September 7, 2015, which allowed their data to be included in the week 24 endpoint analysis prior to the clinical hold.
The PAC QD arm consisted of 75 patients with a median age of 69 (range, 39-85). Seventy-one percent were 65 or older, and about half were male.
About three-quarters had an ECOG performance status of 0-1, 61% had primary myelofibrosis, 21% had primary polycythemia vera (PPV), and 17% had primary essential thrombocythemia (PET). About half were DIPSS Intermediate-2 risk, 80% were JAK2V617F positive, and 51% had a platelet count less than 50,000/μL.
The PAC BID arm consisted of 74 patients with a median age of 67 (range, 39-85). Sixty-two percent were 65 or older, and 65% were male.
Eighty-eight percent had an ECOG performance status of 0-1, 74% had primary myelofibrosis, 19% had PPV, and 7% had PET. About half were DIPSS Intermediate-2 risk, 80% were JAK2V617F positive, and 42% had a platelet count less than 50,000/μL.
The BAT arm consisted of 72 patients with a median age of 69 (range, 32-83). Seventy-one percent were 65 or older, and about half were male.
Three-quarters had an ECOG performance status of 0-1, 60% had primary myelofibrosis, 22% had PPV, and 18% had PET. About half were DIPSS Intermediate-2 risk, 71% were JAK2V617F positive, and 44% had a platelet count less than 50,000/µL.
Prior ruxolitinib therapy was consistent across the arms—41.3% (PAC QD), 42% (PAC BID), and 46% (BAT).
“Now, it’s important to note,” Dr Mascarenhas said, “that the most common BAT was ruxolitinib, 45%, and hydroxyurea, 19%. And 19% of BAT patients actually had no treatment, watch and wait. This highlights the fact that this is an area where there is really no other viable therapeutic option for these patients.”
Efficacy
Pacritinib-treated patients had significantly greater spleen reduction from baseline to week 24 than BAT-treated patients, with 18% (QD+BID), 15% (QD), and 22% (BID) achieving 35% or more SVR compared to 3% in the BAT arm.
Pacritinib-treated patients also experienced greater TSS reduction, with 25% (QD+BID), 17% (QD), and 32% (BID) reporting 50% or more reduction in TSS compared to 14% in the BAT arm. However, only the PAC BID arm was significantly different from BAT.
SVR in all subgroups—age, gender, JAK2 mutation status, prior treatment, platelet count, hemoglobin, peripheral blasts, and white blood cell count—demonstrated superiority for pacritinib.
Median changes in individual symptom scores were also better in the pacritinib arms than in the BAT arm in almost every category—tiredness, early satiety, abdominal discomfort, inactivity, night sweats, bone pain, and pain under ribs on the left side.
Pruritus was the only category in which BAT was superior, and that was compared to the QD arm and not the BID arm.
The majority of patients who stopped pacritinib therapy were taken off due to the clinical hold.
There were no significant differences between the groups in overall survival. Hazard ratios for overall survival (95% confidence intervals) were 0.68 (0.30, 1.53) for PAC BID vs BAT, 1.18 (0.57, 2.44) for PAC QD vs BAT, and 0.61 (0.27, 1.35) for PAC BID vs QD.
PAC BID maintained this survival advantage compared with BAT across nearly all demographic and myelofibrosis-associated risk factors.
Patients who were red blood cell transfusion-dependent at baseline experienced a statistically significant decrease in transfusion frequency from baseline to week 24 in both the QD and BID pacritinib arms compared with BAT.
And thrombocytopenia was not a significant factor for patients who were receiving pacritinib and had platelet counts less than 50,000/μL.
Toxicity
The most common treatment-emergent adverse events (AEs) associated with pacritinib were diarrhea, nausea, vomiting, anemia, and thrombocytopenia. They were generally less frequent for BID compared with QD administration.
The most common serious adverse events (SAEs)—occurring in 5% of patients or more in any arms—were anemia (5%, 8%, and 3%), thrombocytopenia (2%, 6%, and 2%), pneumonia (5%, 6%, and 4%), and acute renal failure (5%, 2%, and 2%) in the QD, BID, and BAT arms, respectively.
SAEs of interest included congestive heart failure, atrial fibrillation, cardiac arrest, epistaxis, and subdural hematoma, which occurred in 3% or fewer patients in any arm.
“They [the SAEs] were relatively infrequent, and there was not a clear signal of toxicity,” Dr Mascarenhas said.
Deaths
Deaths in the intent-to-treat evaluable population were censored at the time of full clinical hold.
For the entire enrolled population, 15/104 deaths occurred in the QD arm, 10/107 in the BID arm, and 14/100 in the BAT arm.
After the full clinical hold, 7, 10, and 6 deaths occurred in the QD, BID, and BAT arms, respectively.
Seven of 20 patients died after crossover to pacritinib. Five were due to AEs—3 cardiac, 1 bleeding, and 1 other.
“It’s important to note that progression of disease was the leading cause of death in the PAC BID arm,” Dr Mascarenhas noted. “This is after the patients stopped the drug [when the trial was on clinical hold].”
Conclusions
Despite study truncation, pacritinib (QD+BID) was significantly more effective than BAT for SVR (P=0.001) and trended toward improved TSS (P=0.079).
Pacritinib BID appeared more effective than QD versus BAT for SVR and TSS, and pacritinib BID appeared to have a better benefit/risk profile than BAT.
“This is a well-tolerated drug in many respects,” Dr Mascarenhas said. “This is a patient population that is quite ill, low platelets, poor outcome, and they did pretty well.”
When asked about the future of pacritinib, Dr Mascarenhas said he believes the benefit-to-risk ratio is in favor of the drug.
“Pacritinib offers patients in this vulnerable situation an opportunity for symptom relief, basically spleen and symptoms,” he said. “So I think this is a drug that will hopefully move forward.”
Dr Mascarenhas disclosed research funding from CTI Biopharma, the sponsor of the trial. ![]()
Blood products unharmed by drone trips

products attached to a
S900-model drone.
Photo courtesy of
Johns Hopkins Medicine
A proof-of-concept study suggests that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
Researchers say these findings, published in Transfusion, add to evidence that remotely piloted drones are an effective, safe, and timely way to quickly get blood products to remote accident or natural catastrophe sites, or other time-sensitive destinations.
“For rural areas that lack access to nearby clinics, or that may lack the infrastructure for collecting blood products or transporting them on their own, drones can provide that access,” said study author Timothy Amukele, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Drones also can help in urban centers to improve distribution of blood products and the quality of care, he added.
Dr Amukele and his colleagues previously studied the impact of drone transportation on the chemical, hematological, and microbial makeup of drone-flown blood samples and found that none were negatively affected.
Study design, methods
In the new study, the team examined the effects of drone transportation on larger amounts of blood products used for transfusion, which have significantly more complex handling, transport, and storage requirements compared to blood samples for laboratory testing.
The researchers purchased 6 units of red blood cells (RBCs), 6 units of platelets, and 6 units of unthawed plasma from the American Red Cross. They then packed the units into a 5-quart cooler, 2 to 3 units at a time, in keeping with weight restrictions for the transport drone.
The cooler was then attached to a commercial S900-model drone. This particular drone model comes equipped with a camera mount, which the team removed and replaced with the cooler.
For each test, the drone was flown by remote control a distance of approximately 13 to 20 kilometers (8 to 12 miles) while 100 meters (328 feet) above ground. This flight took up to 26.5 minutes.
The researchers designed the test to maintain temperature for the RBCs, platelets, and plasma units. They used wet ice, pre-calibrated thermal packs, and dry ice for each type of blood product, respectively.
Temperature monitoring was constant, keeping with transport and storage requirements for blood components.
The team conducted the tests in an unpopulated area, and a certified, ground-based pilot flew the drone.
Following flight, all units were transported to The Johns Hopkins Hospital and compared to blood products that had not taken a drone trip.
Results
Dr Amukele and his colleagues checked the RBCs for signs of hemolysis. They checked the platelets for changes in pH, the number of platelets, and mean platelet volume. And they checked the plasma units for evidence of air bubbles, which would indicate thawing.
The researchers found no evidence of hemolysis in the control RBCs or the RBCs that had taken the drone flight.
There was no significant difference in pH, platelet counts, or mean platelet volume between control and drone-flown platelets.
And there was no apparent change in the size or shape of air bubbles in the plasma units before and after drone flight.
However, the researchers did find that, for all flown units, there was a decrease in temperature of between 1.5°C and 4°C during the course of the flight. They said the cause of this decrease was probably the ambient temperature in the case of the platelet units, the wet ice in the case of the RBCs, and the dry ice in the case of the plasma.
The team also found an up to 2°C difference between individual flights for both the RBCs and the plasma units. They said this difference is likely due to the differences in the amounts of wet and dry ice placed in the cooler.
The researchers are planning further and larger studies in the US and overseas, and they hope to test methods of active cooling, such as programming a cooler to maintain a specific temperature.
“My vision is that, in the future, when a first responder arrives to the scene of an accident, he or she can test the victim’s blood type right on the spot and send for a drone to bring the correct blood product,” Dr Amukele said.
Funding for this study was provided by Peter Kovler of the Blum-Kovler Foundation. ![]()

products attached to a
S900-model drone.
Photo courtesy of
Johns Hopkins Medicine
A proof-of-concept study suggests that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
Researchers say these findings, published in Transfusion, add to evidence that remotely piloted drones are an effective, safe, and timely way to quickly get blood products to remote accident or natural catastrophe sites, or other time-sensitive destinations.
“For rural areas that lack access to nearby clinics, or that may lack the infrastructure for collecting blood products or transporting them on their own, drones can provide that access,” said study author Timothy Amukele, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Drones also can help in urban centers to improve distribution of blood products and the quality of care, he added.
Dr Amukele and his colleagues previously studied the impact of drone transportation on the chemical, hematological, and microbial makeup of drone-flown blood samples and found that none were negatively affected.
Study design, methods
In the new study, the team examined the effects of drone transportation on larger amounts of blood products used for transfusion, which have significantly more complex handling, transport, and storage requirements compared to blood samples for laboratory testing.
The researchers purchased 6 units of red blood cells (RBCs), 6 units of platelets, and 6 units of unthawed plasma from the American Red Cross. They then packed the units into a 5-quart cooler, 2 to 3 units at a time, in keeping with weight restrictions for the transport drone.
The cooler was then attached to a commercial S900-model drone. This particular drone model comes equipped with a camera mount, which the team removed and replaced with the cooler.
For each test, the drone was flown by remote control a distance of approximately 13 to 20 kilometers (8 to 12 miles) while 100 meters (328 feet) above ground. This flight took up to 26.5 minutes.
The researchers designed the test to maintain temperature for the RBCs, platelets, and plasma units. They used wet ice, pre-calibrated thermal packs, and dry ice for each type of blood product, respectively.
Temperature monitoring was constant, keeping with transport and storage requirements for blood components.
The team conducted the tests in an unpopulated area, and a certified, ground-based pilot flew the drone.
Following flight, all units were transported to The Johns Hopkins Hospital and compared to blood products that had not taken a drone trip.
Results
Dr Amukele and his colleagues checked the RBCs for signs of hemolysis. They checked the platelets for changes in pH, the number of platelets, and mean platelet volume. And they checked the plasma units for evidence of air bubbles, which would indicate thawing.
The researchers found no evidence of hemolysis in the control RBCs or the RBCs that had taken the drone flight.
There was no significant difference in pH, platelet counts, or mean platelet volume between control and drone-flown platelets.
And there was no apparent change in the size or shape of air bubbles in the plasma units before and after drone flight.
However, the researchers did find that, for all flown units, there was a decrease in temperature of between 1.5°C and 4°C during the course of the flight. They said the cause of this decrease was probably the ambient temperature in the case of the platelet units, the wet ice in the case of the RBCs, and the dry ice in the case of the plasma.
The team also found an up to 2°C difference between individual flights for both the RBCs and the plasma units. They said this difference is likely due to the differences in the amounts of wet and dry ice placed in the cooler.
The researchers are planning further and larger studies in the US and overseas, and they hope to test methods of active cooling, such as programming a cooler to maintain a specific temperature.
“My vision is that, in the future, when a first responder arrives to the scene of an accident, he or she can test the victim’s blood type right on the spot and send for a drone to bring the correct blood product,” Dr Amukele said.
Funding for this study was provided by Peter Kovler of the Blum-Kovler Foundation. ![]()

products attached to a
S900-model drone.
Photo courtesy of
Johns Hopkins Medicine
A proof-of-concept study suggests that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
Researchers say these findings, published in Transfusion, add to evidence that remotely piloted drones are an effective, safe, and timely way to quickly get blood products to remote accident or natural catastrophe sites, or other time-sensitive destinations.
“For rural areas that lack access to nearby clinics, or that may lack the infrastructure for collecting blood products or transporting them on their own, drones can provide that access,” said study author Timothy Amukele, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Drones also can help in urban centers to improve distribution of blood products and the quality of care, he added.
Dr Amukele and his colleagues previously studied the impact of drone transportation on the chemical, hematological, and microbial makeup of drone-flown blood samples and found that none were negatively affected.
Study design, methods
In the new study, the team examined the effects of drone transportation on larger amounts of blood products used for transfusion, which have significantly more complex handling, transport, and storage requirements compared to blood samples for laboratory testing.
The researchers purchased 6 units of red blood cells (RBCs), 6 units of platelets, and 6 units of unthawed plasma from the American Red Cross. They then packed the units into a 5-quart cooler, 2 to 3 units at a time, in keeping with weight restrictions for the transport drone.
The cooler was then attached to a commercial S900-model drone. This particular drone model comes equipped with a camera mount, which the team removed and replaced with the cooler.
For each test, the drone was flown by remote control a distance of approximately 13 to 20 kilometers (8 to 12 miles) while 100 meters (328 feet) above ground. This flight took up to 26.5 minutes.
The researchers designed the test to maintain temperature for the RBCs, platelets, and plasma units. They used wet ice, pre-calibrated thermal packs, and dry ice for each type of blood product, respectively.
Temperature monitoring was constant, keeping with transport and storage requirements for blood components.
The team conducted the tests in an unpopulated area, and a certified, ground-based pilot flew the drone.
Following flight, all units were transported to The Johns Hopkins Hospital and compared to blood products that had not taken a drone trip.
Results
Dr Amukele and his colleagues checked the RBCs for signs of hemolysis. They checked the platelets for changes in pH, the number of platelets, and mean platelet volume. And they checked the plasma units for evidence of air bubbles, which would indicate thawing.
The researchers found no evidence of hemolysis in the control RBCs or the RBCs that had taken the drone flight.
There was no significant difference in pH, platelet counts, or mean platelet volume between control and drone-flown platelets.
And there was no apparent change in the size or shape of air bubbles in the plasma units before and after drone flight.
However, the researchers did find that, for all flown units, there was a decrease in temperature of between 1.5°C and 4°C during the course of the flight. They said the cause of this decrease was probably the ambient temperature in the case of the platelet units, the wet ice in the case of the RBCs, and the dry ice in the case of the plasma.
The team also found an up to 2°C difference between individual flights for both the RBCs and the plasma units. They said this difference is likely due to the differences in the amounts of wet and dry ice placed in the cooler.
The researchers are planning further and larger studies in the US and overseas, and they hope to test methods of active cooling, such as programming a cooler to maintain a specific temperature.
“My vision is that, in the future, when a first responder arrives to the scene of an accident, he or she can test the victim’s blood type right on the spot and send for a drone to bring the correct blood product,” Dr Amukele said.
Funding for this study was provided by Peter Kovler of the Blum-Kovler Foundation. ![]()
EC grants venetoclax conditional approval for CLL

(US version, Venclexta)
Photo courtesy of Abbvie
The European Commission (EC) has granted conditional marketing authorization for the oral BCL-2 inhibitor venetoclax (Venclyxto™) to treat certain patients with chronic lymphocytic leukemia (CLL).
The drug is now approved as monotherapy to treat adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed a B-cell receptor pathway inhibitor.
Venetoclax is also approved as monotherapy to treat CLL in the absence of 17p deletion or TP53 mutation in adults who have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
Venetoclax is the first BCL-2 inhibitor authorized for use in Europe.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Phase 2 trials
Venetoclax has produced high objective response rates (ORR) in two phase 2 trials of CLL patients.
In one of these trials, researchers tested venetoclax in 107 patients with previously treated CLL and 17p deletion. The results were published in The Lancet Oncology in June.
The ORR in this trial was 79%. At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival.
The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%, and the incidence of serious adverse events was 55%.
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the second trial, researchers tested venetoclax in 64 patients with CLL who had failed treatment with ibrutinib and/or idelalisib. Results from this trial were presented at the 2016 ASH Annual Meeting.
The ORR was 67%. At 11.8 months of follow-up, the median duration of response, progression-free survival, and overall survival had not been reached. The estimated 12-month progression-free survival was 80%.
The incidence of adverse events was 100%, and the incidence of serious adverse events was 53%. No clinical TLS was observed, but 1 patient met Howard criteria for laboratory TLS. ![]()

(US version, Venclexta)
Photo courtesy of Abbvie
The European Commission (EC) has granted conditional marketing authorization for the oral BCL-2 inhibitor venetoclax (Venclyxto™) to treat certain patients with chronic lymphocytic leukemia (CLL).
The drug is now approved as monotherapy to treat adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed a B-cell receptor pathway inhibitor.
Venetoclax is also approved as monotherapy to treat CLL in the absence of 17p deletion or TP53 mutation in adults who have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
Venetoclax is the first BCL-2 inhibitor authorized for use in Europe.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Phase 2 trials
Venetoclax has produced high objective response rates (ORR) in two phase 2 trials of CLL patients.
In one of these trials, researchers tested venetoclax in 107 patients with previously treated CLL and 17p deletion. The results were published in The Lancet Oncology in June.
The ORR in this trial was 79%. At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival.
The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%, and the incidence of serious adverse events was 55%.
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the second trial, researchers tested venetoclax in 64 patients with CLL who had failed treatment with ibrutinib and/or idelalisib. Results from this trial were presented at the 2016 ASH Annual Meeting.
The ORR was 67%. At 11.8 months of follow-up, the median duration of response, progression-free survival, and overall survival had not been reached. The estimated 12-month progression-free survival was 80%.
The incidence of adverse events was 100%, and the incidence of serious adverse events was 53%. No clinical TLS was observed, but 1 patient met Howard criteria for laboratory TLS. ![]()

(US version, Venclexta)
Photo courtesy of Abbvie
The European Commission (EC) has granted conditional marketing authorization for the oral BCL-2 inhibitor venetoclax (Venclyxto™) to treat certain patients with chronic lymphocytic leukemia (CLL).
The drug is now approved as monotherapy to treat adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed a B-cell receptor pathway inhibitor.
Venetoclax is also approved as monotherapy to treat CLL in the absence of 17p deletion or TP53 mutation in adults who have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
Venetoclax is the first BCL-2 inhibitor authorized for use in Europe.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Phase 2 trials
Venetoclax has produced high objective response rates (ORR) in two phase 2 trials of CLL patients.
In one of these trials, researchers tested venetoclax in 107 patients with previously treated CLL and 17p deletion. The results were published in The Lancet Oncology in June.
The ORR in this trial was 79%. At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival.
The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%, and the incidence of serious adverse events was 55%.
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the second trial, researchers tested venetoclax in 64 patients with CLL who had failed treatment with ibrutinib and/or idelalisib. Results from this trial were presented at the 2016 ASH Annual Meeting.
The ORR was 67%. At 11.8 months of follow-up, the median duration of response, progression-free survival, and overall survival had not been reached. The estimated 12-month progression-free survival was 80%.
The incidence of adverse events was 100%, and the incidence of serious adverse events was 53%. No clinical TLS was observed, but 1 patient met Howard criteria for laboratory TLS. ![]()