User login
Light and heavy mesh deliver similar outcomes and QOL for lap inguinal repair
WASHINGTON – The weight of mesh used in laparoscopic inguinal hernia repairs was not a significant factor in postoperative outcomes and quality of life, in a large, long-term study.
“There are approximately 700,000 inguinal hernia repairs annually,” said Steve Groene, MD, of the Carolinas Medical Center, Charlotte, N.C. “The goal of our study was to utilize a large sample size of long-term follow-up and compare surgical and quality of life outcomes between light-weight and heavy-weight mesh in laparoscopic inguinal hernia repairs.”
The rates of postoperative complications such as surgical infection, urinary retention, and recurrence in the two groups were similar. Although the LW mesh group had a significantly higher rate of hematoma and seroma, that difference vanished with a multivariate analysis that accounted for confounding factors such as smoking, elective vs. emergent surgery, and surgical technique, Dr. Groene said at the annual clinical congress of the American College of Surgeons.
Quality of life (QOL) was measured with the Carolina Comfort Scale before surgery and at the 2-week, 1-month, 6-month, 12-month, 24-month, and 36-month follow-ups. The investigators looked at pain, movement limitation, and mesh sensation for outcomes and symptoms. There were no other statistically significant differences in QOL between the groups at any of the follow-up time points.
When asked during the discussion about the experience of the investigators in getting patients to continue through a 36-month follow-up, Dr. Groene said that “at 1 month, we were about 60%, [and] at 1 year about 40%; having about 40%-42% of people following up at 1 month is very good.”
Dr. Groene concluded that surgeons should continue to use the type of mesh they feel most comfortable with for laparoscopic inguinal hernia repair and expect to have similar outcomes.
He reported having no relevant financial disclosures.
WASHINGTON – The weight of mesh used in laparoscopic inguinal hernia repairs was not a significant factor in postoperative outcomes and quality of life, in a large, long-term study.
“There are approximately 700,000 inguinal hernia repairs annually,” said Steve Groene, MD, of the Carolinas Medical Center, Charlotte, N.C. “The goal of our study was to utilize a large sample size of long-term follow-up and compare surgical and quality of life outcomes between light-weight and heavy-weight mesh in laparoscopic inguinal hernia repairs.”
The rates of postoperative complications such as surgical infection, urinary retention, and recurrence in the two groups were similar. Although the LW mesh group had a significantly higher rate of hematoma and seroma, that difference vanished with a multivariate analysis that accounted for confounding factors such as smoking, elective vs. emergent surgery, and surgical technique, Dr. Groene said at the annual clinical congress of the American College of Surgeons.
Quality of life (QOL) was measured with the Carolina Comfort Scale before surgery and at the 2-week, 1-month, 6-month, 12-month, 24-month, and 36-month follow-ups. The investigators looked at pain, movement limitation, and mesh sensation for outcomes and symptoms. There were no other statistically significant differences in QOL between the groups at any of the follow-up time points.
When asked during the discussion about the experience of the investigators in getting patients to continue through a 36-month follow-up, Dr. Groene said that “at 1 month, we were about 60%, [and] at 1 year about 40%; having about 40%-42% of people following up at 1 month is very good.”
Dr. Groene concluded that surgeons should continue to use the type of mesh they feel most comfortable with for laparoscopic inguinal hernia repair and expect to have similar outcomes.
He reported having no relevant financial disclosures.
WASHINGTON – The weight of mesh used in laparoscopic inguinal hernia repairs was not a significant factor in postoperative outcomes and quality of life, in a large, long-term study.
“There are approximately 700,000 inguinal hernia repairs annually,” said Steve Groene, MD, of the Carolinas Medical Center, Charlotte, N.C. “The goal of our study was to utilize a large sample size of long-term follow-up and compare surgical and quality of life outcomes between light-weight and heavy-weight mesh in laparoscopic inguinal hernia repairs.”
The rates of postoperative complications such as surgical infection, urinary retention, and recurrence in the two groups were similar. Although the LW mesh group had a significantly higher rate of hematoma and seroma, that difference vanished with a multivariate analysis that accounted for confounding factors such as smoking, elective vs. emergent surgery, and surgical technique, Dr. Groene said at the annual clinical congress of the American College of Surgeons.
Quality of life (QOL) was measured with the Carolina Comfort Scale before surgery and at the 2-week, 1-month, 6-month, 12-month, 24-month, and 36-month follow-ups. The investigators looked at pain, movement limitation, and mesh sensation for outcomes and symptoms. There were no other statistically significant differences in QOL between the groups at any of the follow-up time points.
When asked during the discussion about the experience of the investigators in getting patients to continue through a 36-month follow-up, Dr. Groene said that “at 1 month, we were about 60%, [and] at 1 year about 40%; having about 40%-42% of people following up at 1 month is very good.”
Dr. Groene concluded that surgeons should continue to use the type of mesh they feel most comfortable with for laparoscopic inguinal hernia repair and expect to have similar outcomes.
He reported having no relevant financial disclosures.
FROM ACS CLINICAL CONGRESS
Key clinical point:
Major finding: For laparoscopic inguinal hernia repair, mesh weight was not a significant factor in postoperative complications or quality of life, as measured by the Carolinas Comfort Scale.
Data source: A prospective study of 1,270 laparoscopic inguinal hernia repair patients from a hernia-specific database.
Disclosures: Dr. Groene reported having no relevant financial disclosures.
Risk models for hernia recurrence don’t hold up to external validation
Five common variable selection strategies failed to produce a statistical model that accurately predicted ventral hernia recurrence in an investigation published in the Journal of Surgical Research.
The finding matters because those five techniques – expert opinion and various multivariate regression and bootstrapping strategies – have been widely used in previous studies to create risk scores for ventral hernia recurrence. The new study calls the value of existing scoring systems into question (J Surg Res. 2016 Nov;206[1]:159-67. doi: 10.1016/j.jss.2016.07.042).
The lack of external validation in many studies leads to medical findings that often can’t be confirmed by subsequent studies. It’s a problem that has contributed to skepticism about research results in both the medical community and the general public, they said.
“This study demonstrates the importance of true external validation on an external data set. Simply splitting a data set and validating [internally] does not appear to be an adequate assessment of predictive accuracy. … We recommend that future researchers consider using and presenting the results of multiple variable selection strategies [and] focus on presenting predictive accuracy on external data sets to validate their model,” the team concluded.
The original goal of the project was to identify the best predictors of ventral hernia recurrence since suggestions from past studies have varied. The team first used a prospective database of 790 ventral hernia repair patients to identify predictors of recurrence. Of that group, 526 patients – 173 (32.9%) of whom had a recurrence after a median follow-up of 20 months – were used to identify risk variables using expert opinion, selective stepwise regression, liberal stepwise regression, and bootstrapping with both restrictive and liberal internal resampling.
The team used the remaining 264 patients to confirm the findings. As in previous studies, internal validation worked: all five models had a Harrell’s C-statistic of about 0.76, which is considered reasonable, Dr. Holihan and her associates reported.
However, when the investigators applied their models to a second database of 1,225 patients followed for a median of 9 months – with 155 recurrences (12.7%) – they were not much better at predicting recurrence than a coin toss, with C-statistic values of about 0.56.
Some variables made the cut with all five selection techniques, including hernia type, wound class, and albumin levels, which are related to how well the wound heals. Other variables were significant in some selection strategies but not others, including smoking status, open versus laparoscopic approach, and mesh use.
At least for now, clinical intuition remains important for assessing rerupture risk, they said.
The National Institutes of Health funded the work. Author disclosures were not reported.
Five common variable selection strategies failed to produce a statistical model that accurately predicted ventral hernia recurrence in an investigation published in the Journal of Surgical Research.
The finding matters because those five techniques – expert opinion and various multivariate regression and bootstrapping strategies – have been widely used in previous studies to create risk scores for ventral hernia recurrence. The new study calls the value of existing scoring systems into question (J Surg Res. 2016 Nov;206[1]:159-67. doi: 10.1016/j.jss.2016.07.042).
The lack of external validation in many studies leads to medical findings that often can’t be confirmed by subsequent studies. It’s a problem that has contributed to skepticism about research results in both the medical community and the general public, they said.
“This study demonstrates the importance of true external validation on an external data set. Simply splitting a data set and validating [internally] does not appear to be an adequate assessment of predictive accuracy. … We recommend that future researchers consider using and presenting the results of multiple variable selection strategies [and] focus on presenting predictive accuracy on external data sets to validate their model,” the team concluded.
The original goal of the project was to identify the best predictors of ventral hernia recurrence since suggestions from past studies have varied. The team first used a prospective database of 790 ventral hernia repair patients to identify predictors of recurrence. Of that group, 526 patients – 173 (32.9%) of whom had a recurrence after a median follow-up of 20 months – were used to identify risk variables using expert opinion, selective stepwise regression, liberal stepwise regression, and bootstrapping with both restrictive and liberal internal resampling.
The team used the remaining 264 patients to confirm the findings. As in previous studies, internal validation worked: all five models had a Harrell’s C-statistic of about 0.76, which is considered reasonable, Dr. Holihan and her associates reported.
However, when the investigators applied their models to a second database of 1,225 patients followed for a median of 9 months – with 155 recurrences (12.7%) – they were not much better at predicting recurrence than a coin toss, with C-statistic values of about 0.56.
Some variables made the cut with all five selection techniques, including hernia type, wound class, and albumin levels, which are related to how well the wound heals. Other variables were significant in some selection strategies but not others, including smoking status, open versus laparoscopic approach, and mesh use.
At least for now, clinical intuition remains important for assessing rerupture risk, they said.
The National Institutes of Health funded the work. Author disclosures were not reported.
Five common variable selection strategies failed to produce a statistical model that accurately predicted ventral hernia recurrence in an investigation published in the Journal of Surgical Research.
The finding matters because those five techniques – expert opinion and various multivariate regression and bootstrapping strategies – have been widely used in previous studies to create risk scores for ventral hernia recurrence. The new study calls the value of existing scoring systems into question (J Surg Res. 2016 Nov;206[1]:159-67. doi: 10.1016/j.jss.2016.07.042).
The lack of external validation in many studies leads to medical findings that often can’t be confirmed by subsequent studies. It’s a problem that has contributed to skepticism about research results in both the medical community and the general public, they said.
“This study demonstrates the importance of true external validation on an external data set. Simply splitting a data set and validating [internally] does not appear to be an adequate assessment of predictive accuracy. … We recommend that future researchers consider using and presenting the results of multiple variable selection strategies [and] focus on presenting predictive accuracy on external data sets to validate their model,” the team concluded.
The original goal of the project was to identify the best predictors of ventral hernia recurrence since suggestions from past studies have varied. The team first used a prospective database of 790 ventral hernia repair patients to identify predictors of recurrence. Of that group, 526 patients – 173 (32.9%) of whom had a recurrence after a median follow-up of 20 months – were used to identify risk variables using expert opinion, selective stepwise regression, liberal stepwise regression, and bootstrapping with both restrictive and liberal internal resampling.
The team used the remaining 264 patients to confirm the findings. As in previous studies, internal validation worked: all five models had a Harrell’s C-statistic of about 0.76, which is considered reasonable, Dr. Holihan and her associates reported.
However, when the investigators applied their models to a second database of 1,225 patients followed for a median of 9 months – with 155 recurrences (12.7%) – they were not much better at predicting recurrence than a coin toss, with C-statistic values of about 0.56.
Some variables made the cut with all five selection techniques, including hernia type, wound class, and albumin levels, which are related to how well the wound heals. Other variables were significant in some selection strategies but not others, including smoking status, open versus laparoscopic approach, and mesh use.
At least for now, clinical intuition remains important for assessing rerupture risk, they said.
The National Institutes of Health funded the work. Author disclosures were not reported.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point:
Major finding: Risk models developed from the five strategies weren’t much better at predicting recurrence than a coin toss, with C-statistic values of about 0.56.
Data source: Analysis of two datasets containing a total of 2,015 ventral hernia repair patients.
Disclosures: The National Institutes of Health funded the work. Author disclosures were not reported.
Tenotomy, Tenodesis, Transfer: A Review of Treatment Options for Biceps-Labrum Complex Disease
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
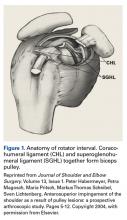
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
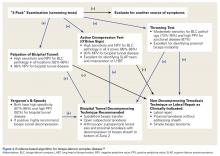
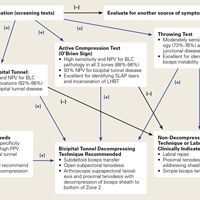
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
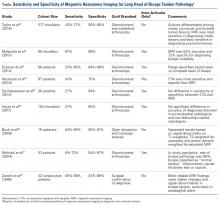
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
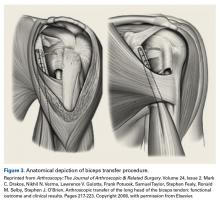
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
The Role of Hysteroscopy in Minimally Invasive Management of Intrauterine Health
Faculty/Faculty Disclosure
Linda D. Bradley, MD
Obstetrics, Gynecology and Women’s
Health Institute,
Cleveland Clinic,
Cleveland, Ohio, USA
Competing Interest and Financial Disclosures: Dr. Bradley reports that she has received grant/research/clinical trial support from Bayer Healthcare Pharmaceuticals Inc. She is a consultant and on the advisory board for Bayer Healthcare Pharmaceuticals Inc., Boston Scientific Corporation, and Smith & Nephew; she is on the advisory board for Patient-Centered Outcomes Research Institute; she is on the speakers' bureau for Smith & Nephew (Medtronic); she is on the scientific advisory panel for Karl Storz; and she is on the data safety and monitoring board for Gynesonics.
Faculty/Faculty Disclosure
Linda D. Bradley, MD
Obstetrics, Gynecology and Women’s
Health Institute,
Cleveland Clinic,
Cleveland, Ohio, USA
Competing Interest and Financial Disclosures: Dr. Bradley reports that she has received grant/research/clinical trial support from Bayer Healthcare Pharmaceuticals Inc. She is a consultant and on the advisory board for Bayer Healthcare Pharmaceuticals Inc., Boston Scientific Corporation, and Smith & Nephew; she is on the advisory board for Patient-Centered Outcomes Research Institute; she is on the speakers' bureau for Smith & Nephew (Medtronic); she is on the scientific advisory panel for Karl Storz; and she is on the data safety and monitoring board for Gynesonics.
Faculty/Faculty Disclosure
Linda D. Bradley, MD
Obstetrics, Gynecology and Women’s
Health Institute,
Cleveland Clinic,
Cleveland, Ohio, USA
Competing Interest and Financial Disclosures: Dr. Bradley reports that she has received grant/research/clinical trial support from Bayer Healthcare Pharmaceuticals Inc. She is a consultant and on the advisory board for Bayer Healthcare Pharmaceuticals Inc., Boston Scientific Corporation, and Smith & Nephew; she is on the advisory board for Patient-Centered Outcomes Research Institute; she is on the speakers' bureau for Smith & Nephew (Medtronic); she is on the scientific advisory panel for Karl Storz; and she is on the data safety and monitoring board for Gynesonics.
c-Myc could be key to alisertib potential in small-cell lung cancer
VIENNA – The investigational agent alisertib plus paclitaxel improved progression-free survival (PFS), compared with paclitaxel plus placebo, in a randomized, double-blind, phase II study of patients with relapsed or refractory small-cell lung cancer (SCLC).
The median PFS was 3.32 months with alisertib/paclitaxel and 2.17 months with paclitaxel/placebo, giving an overall 30% improvement with the combination (hazard ratio, 0.71; 95% confidence interval, 0.509-0.985; P = .038).
“c-Myc protein expression showed a strong association with improved PFS, despite the small number of evaluable patients,” study investigator Taofeek Owonikoko, MD, of Emory University, Atlanta, said at the World Conference on Lung Cancer.
Amplification and overexpression of c-Myc is a strong oncogenic driver in many cancers, he explained. Around 18%-31% of SCLCs overexpress the protein, and it is often found in patients with chemorefractory disease, he said.
In the study, longer PFS was achieved in patients treated with alisertib/paclitaxel than in those given placebo/paclitaxel if they were c-Myc positive (4.64 vs. 2.27 months; HR, 0.29). Conversely, patients who were c-Myc negative had a shorter PFS with the combination (3.32 vs. 5.16 months; HR, 11.8).
“A prospective study is needed to further validate the predictive value of c-Myc in the clinic,” Dr. Owonikoko said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
Alisertib is an aurora A kinase inhibitor that has already been shown to have antitumor activity in patients with solid tumors, including those with SCLC (Lancet Oncol. 2015;16[4]:395-405).
The current phase II study was designed to evaluate the efficacy and safety of alisertib in combination with paclitaxel, compared with placebo plus paclitaxel, in a larger population of patients with SCLC who had relapsed within 6 months or did not respond to standard first-line, platinum-based chemotherapy.
Treatment was given in 28-day cycles, with patients randomized to the combination receiving alisertib at an oral, 40-mg, twice-daily dose on days 1-3, 8-10, and 15-17 and paclitaxel administered intravenously at a dose of 60 mg/m2 IV on days 1, 8, 15. Patients randomized to the control arm received a matched placebo plus paclitaxel given on the same days but at a dose of 80 mg/m2.
Overall, 178 patients were randomized, with a mean age of 62 years, and just over half (57%) were men.
Numerically higher objective response rates (22% vs. 18%), disease control rates (77 vs. 67%), and overall survival (6.87 vs. 5.58 months) also were seen with the combination over paclitaxel alone, although they did not achieve statistical significance.
Additional toxicities were observed with the combination treatment versus paclitaxel. Many of these were to be expected, Dr. Owonikoko said. Almost all (99% vs. 96%) of patients reported some type of adverse event, of which 75% and 51% were grade 3 or higher, respectively. Common any-grade adverse effects were diarrhea (59% vs. 20%), neutropenia (49% vs. 8%), anemia (44% vs. 20%), and fatigue (44% vs. 33%).
These toxicities, however, did not appear to affect patients’ quality of life, Dr. Owonikoko said, as comparable changes in quality-of-life scores using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 instrument were observed. There was even an indication that patients taking alisertib/paclitaxel might have improved lung-related symptoms, such as shortness of breath and worsening cough.
The treatment of SCLC, particularly in the second-line setting, remains challenging, observed the invited discussant for the trial Jens Benn Sørensen, MD, of the Finsen Centre at Rigshospitalet, Copenhagen.
“There have been no major improvements in the last decade and there are no targeted agents approved for use,” Dr. Sørensen said. Although that is not for lack of trying, he qualified.
When first-line therapies fail, the guideline-recommended option is to put patients back on platinum-based chemotherapy if they were sensitive to such chemotherapy. If not, then topotecan is often an appropriate, reasonably tolerated option.
“The clinical relevance of second-line paclitaxel/alisertib in all comers is questionable,” he said. The PFS improvement of 1.2 months in this phase II study seems “mostly at the same level” when comparing trials of second-line, single-agent topotecan.
“However, the use of c-Myc as a predictor for efficacy seems very promising and should clearly be explored,” Dr. Sørensen noted.
He also suggested that it might be interesting to look into the combination of topotecan and alisertib versus topotecan alone in c-Myc-positive patients.
The study was funded by Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company. Dr. Owonikoko disclosed consulting for Takeda and several other pharmaceutical companies. Dr. Sørensen did not have disclosures.
VIENNA – The investigational agent alisertib plus paclitaxel improved progression-free survival (PFS), compared with paclitaxel plus placebo, in a randomized, double-blind, phase II study of patients with relapsed or refractory small-cell lung cancer (SCLC).
The median PFS was 3.32 months with alisertib/paclitaxel and 2.17 months with paclitaxel/placebo, giving an overall 30% improvement with the combination (hazard ratio, 0.71; 95% confidence interval, 0.509-0.985; P = .038).
“c-Myc protein expression showed a strong association with improved PFS, despite the small number of evaluable patients,” study investigator Taofeek Owonikoko, MD, of Emory University, Atlanta, said at the World Conference on Lung Cancer.
Amplification and overexpression of c-Myc is a strong oncogenic driver in many cancers, he explained. Around 18%-31% of SCLCs overexpress the protein, and it is often found in patients with chemorefractory disease, he said.
In the study, longer PFS was achieved in patients treated with alisertib/paclitaxel than in those given placebo/paclitaxel if they were c-Myc positive (4.64 vs. 2.27 months; HR, 0.29). Conversely, patients who were c-Myc negative had a shorter PFS with the combination (3.32 vs. 5.16 months; HR, 11.8).
“A prospective study is needed to further validate the predictive value of c-Myc in the clinic,” Dr. Owonikoko said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
Alisertib is an aurora A kinase inhibitor that has already been shown to have antitumor activity in patients with solid tumors, including those with SCLC (Lancet Oncol. 2015;16[4]:395-405).
The current phase II study was designed to evaluate the efficacy and safety of alisertib in combination with paclitaxel, compared with placebo plus paclitaxel, in a larger population of patients with SCLC who had relapsed within 6 months or did not respond to standard first-line, platinum-based chemotherapy.
Treatment was given in 28-day cycles, with patients randomized to the combination receiving alisertib at an oral, 40-mg, twice-daily dose on days 1-3, 8-10, and 15-17 and paclitaxel administered intravenously at a dose of 60 mg/m2 IV on days 1, 8, 15. Patients randomized to the control arm received a matched placebo plus paclitaxel given on the same days but at a dose of 80 mg/m2.
Overall, 178 patients were randomized, with a mean age of 62 years, and just over half (57%) were men.
Numerically higher objective response rates (22% vs. 18%), disease control rates (77 vs. 67%), and overall survival (6.87 vs. 5.58 months) also were seen with the combination over paclitaxel alone, although they did not achieve statistical significance.
Additional toxicities were observed with the combination treatment versus paclitaxel. Many of these were to be expected, Dr. Owonikoko said. Almost all (99% vs. 96%) of patients reported some type of adverse event, of which 75% and 51% were grade 3 or higher, respectively. Common any-grade adverse effects were diarrhea (59% vs. 20%), neutropenia (49% vs. 8%), anemia (44% vs. 20%), and fatigue (44% vs. 33%).
These toxicities, however, did not appear to affect patients’ quality of life, Dr. Owonikoko said, as comparable changes in quality-of-life scores using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 instrument were observed. There was even an indication that patients taking alisertib/paclitaxel might have improved lung-related symptoms, such as shortness of breath and worsening cough.
The treatment of SCLC, particularly in the second-line setting, remains challenging, observed the invited discussant for the trial Jens Benn Sørensen, MD, of the Finsen Centre at Rigshospitalet, Copenhagen.
“There have been no major improvements in the last decade and there are no targeted agents approved for use,” Dr. Sørensen said. Although that is not for lack of trying, he qualified.
When first-line therapies fail, the guideline-recommended option is to put patients back on platinum-based chemotherapy if they were sensitive to such chemotherapy. If not, then topotecan is often an appropriate, reasonably tolerated option.
“The clinical relevance of second-line paclitaxel/alisertib in all comers is questionable,” he said. The PFS improvement of 1.2 months in this phase II study seems “mostly at the same level” when comparing trials of second-line, single-agent topotecan.
“However, the use of c-Myc as a predictor for efficacy seems very promising and should clearly be explored,” Dr. Sørensen noted.
He also suggested that it might be interesting to look into the combination of topotecan and alisertib versus topotecan alone in c-Myc-positive patients.
The study was funded by Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company. Dr. Owonikoko disclosed consulting for Takeda and several other pharmaceutical companies. Dr. Sørensen did not have disclosures.
VIENNA – The investigational agent alisertib plus paclitaxel improved progression-free survival (PFS), compared with paclitaxel plus placebo, in a randomized, double-blind, phase II study of patients with relapsed or refractory small-cell lung cancer (SCLC).
The median PFS was 3.32 months with alisertib/paclitaxel and 2.17 months with paclitaxel/placebo, giving an overall 30% improvement with the combination (hazard ratio, 0.71; 95% confidence interval, 0.509-0.985; P = .038).
“c-Myc protein expression showed a strong association with improved PFS, despite the small number of evaluable patients,” study investigator Taofeek Owonikoko, MD, of Emory University, Atlanta, said at the World Conference on Lung Cancer.
Amplification and overexpression of c-Myc is a strong oncogenic driver in many cancers, he explained. Around 18%-31% of SCLCs overexpress the protein, and it is often found in patients with chemorefractory disease, he said.
In the study, longer PFS was achieved in patients treated with alisertib/paclitaxel than in those given placebo/paclitaxel if they were c-Myc positive (4.64 vs. 2.27 months; HR, 0.29). Conversely, patients who were c-Myc negative had a shorter PFS with the combination (3.32 vs. 5.16 months; HR, 11.8).
“A prospective study is needed to further validate the predictive value of c-Myc in the clinic,” Dr. Owonikoko said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
Alisertib is an aurora A kinase inhibitor that has already been shown to have antitumor activity in patients with solid tumors, including those with SCLC (Lancet Oncol. 2015;16[4]:395-405).
The current phase II study was designed to evaluate the efficacy and safety of alisertib in combination with paclitaxel, compared with placebo plus paclitaxel, in a larger population of patients with SCLC who had relapsed within 6 months or did not respond to standard first-line, platinum-based chemotherapy.
Treatment was given in 28-day cycles, with patients randomized to the combination receiving alisertib at an oral, 40-mg, twice-daily dose on days 1-3, 8-10, and 15-17 and paclitaxel administered intravenously at a dose of 60 mg/m2 IV on days 1, 8, 15. Patients randomized to the control arm received a matched placebo plus paclitaxel given on the same days but at a dose of 80 mg/m2.
Overall, 178 patients were randomized, with a mean age of 62 years, and just over half (57%) were men.
Numerically higher objective response rates (22% vs. 18%), disease control rates (77 vs. 67%), and overall survival (6.87 vs. 5.58 months) also were seen with the combination over paclitaxel alone, although they did not achieve statistical significance.
Additional toxicities were observed with the combination treatment versus paclitaxel. Many of these were to be expected, Dr. Owonikoko said. Almost all (99% vs. 96%) of patients reported some type of adverse event, of which 75% and 51% were grade 3 or higher, respectively. Common any-grade adverse effects were diarrhea (59% vs. 20%), neutropenia (49% vs. 8%), anemia (44% vs. 20%), and fatigue (44% vs. 33%).
These toxicities, however, did not appear to affect patients’ quality of life, Dr. Owonikoko said, as comparable changes in quality-of-life scores using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 instrument were observed. There was even an indication that patients taking alisertib/paclitaxel might have improved lung-related symptoms, such as shortness of breath and worsening cough.
The treatment of SCLC, particularly in the second-line setting, remains challenging, observed the invited discussant for the trial Jens Benn Sørensen, MD, of the Finsen Centre at Rigshospitalet, Copenhagen.
“There have been no major improvements in the last decade and there are no targeted agents approved for use,” Dr. Sørensen said. Although that is not for lack of trying, he qualified.
When first-line therapies fail, the guideline-recommended option is to put patients back on platinum-based chemotherapy if they were sensitive to such chemotherapy. If not, then topotecan is often an appropriate, reasonably tolerated option.
“The clinical relevance of second-line paclitaxel/alisertib in all comers is questionable,” he said. The PFS improvement of 1.2 months in this phase II study seems “mostly at the same level” when comparing trials of second-line, single-agent topotecan.
“However, the use of c-Myc as a predictor for efficacy seems very promising and should clearly be explored,” Dr. Sørensen noted.
He also suggested that it might be interesting to look into the combination of topotecan and alisertib versus topotecan alone in c-Myc-positive patients.
The study was funded by Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company. Dr. Owonikoko disclosed consulting for Takeda and several other pharmaceutical companies. Dr. Sørensen did not have disclosures.
AT WCLC 2016
Key clinical point: Alisertib/paclitaxel is an investigational combination that was tested for the treatment of relapsed or refractory small-cell lung cancer (SCLC).
Major finding: Improved progression-free survival was seen with the combination versus paclitaxel alone (3.2 vs. 2.17 months, P less than .038).
Data source: Randomized, double-blind, multicenter, phase II study of alisertib/paclitaxel versus placebo/paclitaxel in 178 patients with relapsed or refractory SCLC.
Disclosures: The study was funded by Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company. Dr. Owonikoko disclosed consulting for Takeda and several other pharmaceutical companies. Dr. Sørensen had no conflicts of interest to disclose.
Simulation Training, Coaching, and Cue Cards Improve Delirium Care
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
Miguel Angel Villagra Brings Management Skills to The Hospitalist's Volunteer Editorial Advisory Board
Some have called this the “Year of the Hospitalist,” as it’s the 20th anniversary of the New England Journal of Medicine paper by Dr. Robert Wachter and Dr. Lee Goldman that first used the term “hospitalist” to describe physicians who care for hospitalized patients.
But the paper was more than just that to Miguel Angel Villagra, MD.
He saw it four years ago while training in internal medicine at Texas Tech University Health Sciences Center in El Paso, Texas.
“I was very intrigued,” Dr. Villagra says. “I asked a few of my mentors. They were very skeptical on following a hospitalist career, [but] I saw opportunities for improvement and professional growth in the field, so I decided to jump in. And after four years, I don’t regret my decision of becoming a full-time hospitalist.”
The field doesn’t regret it either. Dr. Villagra was promoted last fall to hospitalist department program medical director at White River Medical Center in Batesville, Ark. And this year, he was named one of eight new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: Why did you choose a career in medicine?
Answer: At age 11 and after an emergent appendectomy, I decided that I wanted to become a physician. That was one of the best decisions of my life. It is a great combination of art and science, and you get to help people in difficult moments of their life.
Q: Tell us more about your background.
A: I went to medical school in my country of origin at Universidad Autonóma de Nicaragua of Managua, and I did an internal medicine residency at Hospital Militar Escuela Dr. Alejandro Dávila Bolaños. I came to the U.S. for internal medicine residency training at Texas Tech of El Paso. I enjoy learning new skills and this power of knowledge that can help your patients in desperate moments. Most of my challenges during my training involved how to manage stress and sleep deprivation.
Q: Did you have a mentor during your training or early career? If so, who was the mentor, and what were most important lessons you learned from them?
A: Dr. Jorge Cuadra [from Hospital Militar in Nicaragua] and Dr. Manuel Rivera [from Texas Tech], both pulmonologists. They taught me that medicine is a changing field that requires everyday reading. You never end learning new things and approaches. Taking full advantage of your interaction with your patients always improves your clinical skills.
Q: What do you like most about working as a hospitalist?
A: It is an evolving field; we are still trying to “figure it out.” That creates challenges but also opportunities for growth and career development, [for example], how to tackle the readmission problem, how to improve quality at lower cost while keeping patient satisfaction, how to face the burnout challenge and improve physician engagement, just to name a few.
Q: What do you dislike most?
A: In the beginning of my career as a hospitalist, I was exposed constantly to high patient loads that were more than I should have. I also dislike the difficulties at times of electronic medical records. You have to spend excessive time sitting in front of a monitor.
Q: You note the challenges the field of HM is facing. How exciting is it to hopefully be part of the solutions?
A: I feel pumped having been part of this amazing movement of hospital medicine. I think we are leading the change from the acute-care setting front line, helping to take better care of our patients. The current healthcare changes create multiple challenges and, along with that, endless opportunities for professional growth and career development.
Q: You’ve said you see being a chief quality officer in the future. Why? What appeals about those C-suite positions?
A: I think that physicians as leaders are in a great position to drive the change within a healthcare organization toward high-value care. We are at the front line, at the bedside taking care of patients. That gives us firsthand information on what needs to be done. With appropriate training, we can be the executives the institution needs. When I started my role as medical director, initially I focused mainly on managing the group, but rapidly I was involved in several quality projects and academic activities. And soon I realized that I can have a broader impact on what I was doing, going beyond the bedside where you try to offer the best care possible for your patients to an organizational level of change.
Q: How has your journey from Nicaragua to the U.S. shaped you, and how has it shaped the way you practice medicine?
A: Certainly it shaped what I am today, coming from a country that struggles with poverty. During medical school, you lack advances in technology and depend mainly on your desire to excel and be better for the benefit of your patients. You build strong clinical skills from history to physical exam. When you move to the U.S. and have access to so many technological advances, from new diagnostic tests to top-of the-line imaging studies, you combine the best of both worlds, and [that] makes you a better physician. I am very proud of my heritage, and definitely I wouldn’t change anything on my path thus far. I believe the more you overcome difficulties and adversities, the more you appreciate what you accomplished.
Q: As a group leader, why is it important for you to continue seeing patients?
A: We lead our teams by example, and that requires treating patients. I am also a clinician, and I love my profession, so I don’t foresee myself only in an administrative role. Finding the sweet spot of clinician-administrative time is very difficult, and I am still working on it.
Q: What’s the best advice you ever received?
A: Read and learn every day, be good to people, and also dream big.
Q: What’s the worst advice you ever received?
A: Never get married. I didn’t listen.
Richard Quinn is a freelance writer in New Jersey.
Some have called this the “Year of the Hospitalist,” as it’s the 20th anniversary of the New England Journal of Medicine paper by Dr. Robert Wachter and Dr. Lee Goldman that first used the term “hospitalist” to describe physicians who care for hospitalized patients.
But the paper was more than just that to Miguel Angel Villagra, MD.
He saw it four years ago while training in internal medicine at Texas Tech University Health Sciences Center in El Paso, Texas.
“I was very intrigued,” Dr. Villagra says. “I asked a few of my mentors. They were very skeptical on following a hospitalist career, [but] I saw opportunities for improvement and professional growth in the field, so I decided to jump in. And after four years, I don’t regret my decision of becoming a full-time hospitalist.”
The field doesn’t regret it either. Dr. Villagra was promoted last fall to hospitalist department program medical director at White River Medical Center in Batesville, Ark. And this year, he was named one of eight new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: Why did you choose a career in medicine?
Answer: At age 11 and after an emergent appendectomy, I decided that I wanted to become a physician. That was one of the best decisions of my life. It is a great combination of art and science, and you get to help people in difficult moments of their life.
Q: Tell us more about your background.
A: I went to medical school in my country of origin at Universidad Autonóma de Nicaragua of Managua, and I did an internal medicine residency at Hospital Militar Escuela Dr. Alejandro Dávila Bolaños. I came to the U.S. for internal medicine residency training at Texas Tech of El Paso. I enjoy learning new skills and this power of knowledge that can help your patients in desperate moments. Most of my challenges during my training involved how to manage stress and sleep deprivation.
Q: Did you have a mentor during your training or early career? If so, who was the mentor, and what were most important lessons you learned from them?
A: Dr. Jorge Cuadra [from Hospital Militar in Nicaragua] and Dr. Manuel Rivera [from Texas Tech], both pulmonologists. They taught me that medicine is a changing field that requires everyday reading. You never end learning new things and approaches. Taking full advantage of your interaction with your patients always improves your clinical skills.
Q: What do you like most about working as a hospitalist?
A: It is an evolving field; we are still trying to “figure it out.” That creates challenges but also opportunities for growth and career development, [for example], how to tackle the readmission problem, how to improve quality at lower cost while keeping patient satisfaction, how to face the burnout challenge and improve physician engagement, just to name a few.
Q: What do you dislike most?
A: In the beginning of my career as a hospitalist, I was exposed constantly to high patient loads that were more than I should have. I also dislike the difficulties at times of electronic medical records. You have to spend excessive time sitting in front of a monitor.
Q: You note the challenges the field of HM is facing. How exciting is it to hopefully be part of the solutions?
A: I feel pumped having been part of this amazing movement of hospital medicine. I think we are leading the change from the acute-care setting front line, helping to take better care of our patients. The current healthcare changes create multiple challenges and, along with that, endless opportunities for professional growth and career development.
Q: You’ve said you see being a chief quality officer in the future. Why? What appeals about those C-suite positions?
A: I think that physicians as leaders are in a great position to drive the change within a healthcare organization toward high-value care. We are at the front line, at the bedside taking care of patients. That gives us firsthand information on what needs to be done. With appropriate training, we can be the executives the institution needs. When I started my role as medical director, initially I focused mainly on managing the group, but rapidly I was involved in several quality projects and academic activities. And soon I realized that I can have a broader impact on what I was doing, going beyond the bedside where you try to offer the best care possible for your patients to an organizational level of change.
Q: How has your journey from Nicaragua to the U.S. shaped you, and how has it shaped the way you practice medicine?
A: Certainly it shaped what I am today, coming from a country that struggles with poverty. During medical school, you lack advances in technology and depend mainly on your desire to excel and be better for the benefit of your patients. You build strong clinical skills from history to physical exam. When you move to the U.S. and have access to so many technological advances, from new diagnostic tests to top-of the-line imaging studies, you combine the best of both worlds, and [that] makes you a better physician. I am very proud of my heritage, and definitely I wouldn’t change anything on my path thus far. I believe the more you overcome difficulties and adversities, the more you appreciate what you accomplished.
Q: As a group leader, why is it important for you to continue seeing patients?
A: We lead our teams by example, and that requires treating patients. I am also a clinician, and I love my profession, so I don’t foresee myself only in an administrative role. Finding the sweet spot of clinician-administrative time is very difficult, and I am still working on it.
Q: What’s the best advice you ever received?
A: Read and learn every day, be good to people, and also dream big.
Q: What’s the worst advice you ever received?
A: Never get married. I didn’t listen.
Richard Quinn is a freelance writer in New Jersey.
Some have called this the “Year of the Hospitalist,” as it’s the 20th anniversary of the New England Journal of Medicine paper by Dr. Robert Wachter and Dr. Lee Goldman that first used the term “hospitalist” to describe physicians who care for hospitalized patients.
But the paper was more than just that to Miguel Angel Villagra, MD.
He saw it four years ago while training in internal medicine at Texas Tech University Health Sciences Center in El Paso, Texas.
“I was very intrigued,” Dr. Villagra says. “I asked a few of my mentors. They were very skeptical on following a hospitalist career, [but] I saw opportunities for improvement and professional growth in the field, so I decided to jump in. And after four years, I don’t regret my decision of becoming a full-time hospitalist.”
The field doesn’t regret it either. Dr. Villagra was promoted last fall to hospitalist department program medical director at White River Medical Center in Batesville, Ark. And this year, he was named one of eight new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: Why did you choose a career in medicine?
Answer: At age 11 and after an emergent appendectomy, I decided that I wanted to become a physician. That was one of the best decisions of my life. It is a great combination of art and science, and you get to help people in difficult moments of their life.
Q: Tell us more about your background.
A: I went to medical school in my country of origin at Universidad Autonóma de Nicaragua of Managua, and I did an internal medicine residency at Hospital Militar Escuela Dr. Alejandro Dávila Bolaños. I came to the U.S. for internal medicine residency training at Texas Tech of El Paso. I enjoy learning new skills and this power of knowledge that can help your patients in desperate moments. Most of my challenges during my training involved how to manage stress and sleep deprivation.
Q: Did you have a mentor during your training or early career? If so, who was the mentor, and what were most important lessons you learned from them?
A: Dr. Jorge Cuadra [from Hospital Militar in Nicaragua] and Dr. Manuel Rivera [from Texas Tech], both pulmonologists. They taught me that medicine is a changing field that requires everyday reading. You never end learning new things and approaches. Taking full advantage of your interaction with your patients always improves your clinical skills.
Q: What do you like most about working as a hospitalist?
A: It is an evolving field; we are still trying to “figure it out.” That creates challenges but also opportunities for growth and career development, [for example], how to tackle the readmission problem, how to improve quality at lower cost while keeping patient satisfaction, how to face the burnout challenge and improve physician engagement, just to name a few.
Q: What do you dislike most?
A: In the beginning of my career as a hospitalist, I was exposed constantly to high patient loads that were more than I should have. I also dislike the difficulties at times of electronic medical records. You have to spend excessive time sitting in front of a monitor.
Q: You note the challenges the field of HM is facing. How exciting is it to hopefully be part of the solutions?
A: I feel pumped having been part of this amazing movement of hospital medicine. I think we are leading the change from the acute-care setting front line, helping to take better care of our patients. The current healthcare changes create multiple challenges and, along with that, endless opportunities for professional growth and career development.
Q: You’ve said you see being a chief quality officer in the future. Why? What appeals about those C-suite positions?
A: I think that physicians as leaders are in a great position to drive the change within a healthcare organization toward high-value care. We are at the front line, at the bedside taking care of patients. That gives us firsthand information on what needs to be done. With appropriate training, we can be the executives the institution needs. When I started my role as medical director, initially I focused mainly on managing the group, but rapidly I was involved in several quality projects and academic activities. And soon I realized that I can have a broader impact on what I was doing, going beyond the bedside where you try to offer the best care possible for your patients to an organizational level of change.
Q: How has your journey from Nicaragua to the U.S. shaped you, and how has it shaped the way you practice medicine?
A: Certainly it shaped what I am today, coming from a country that struggles with poverty. During medical school, you lack advances in technology and depend mainly on your desire to excel and be better for the benefit of your patients. You build strong clinical skills from history to physical exam. When you move to the U.S. and have access to so many technological advances, from new diagnostic tests to top-of the-line imaging studies, you combine the best of both worlds, and [that] makes you a better physician. I am very proud of my heritage, and definitely I wouldn’t change anything on my path thus far. I believe the more you overcome difficulties and adversities, the more you appreciate what you accomplished.
Q: As a group leader, why is it important for you to continue seeing patients?
A: We lead our teams by example, and that requires treating patients. I am also a clinician, and I love my profession, so I don’t foresee myself only in an administrative role. Finding the sweet spot of clinician-administrative time is very difficult, and I am still working on it.
Q: What’s the best advice you ever received?
A: Read and learn every day, be good to people, and also dream big.
Q: What’s the worst advice you ever received?
A: Never get married. I didn’t listen.
Richard Quinn is a freelance writer in New Jersey.
How NPs and PAs Work with HM Groups
While the role of nurse practitioners (NPs) and physician assistants (PAs) in hospital medicine is far from uniform, data from the 2016 State of Hospital Medicine Report show that a majority of hospital medicine groups utilize NPs or PAs. Over the past decade, the percentage of hospital medicine groups that include NPs or PAs has grown from about 20% in 20051 to 65% in 2015.2
I suspect a large part of this growth is fueled by demand continuing to outstrip supply for hospitalist physicians and continued increases in hospitalist salaries. In academic institutions, restrictions in house staff duty hours over the past decade no doubt also contributed to the growth of NP and PA utilization.
How Should HM Groups Use NPs and PAs?
In addition to obtaining group buy-in prior to deploying NPs or PAs in a hospital medicine group, a thoughtful consideration about the role of NPs or PAs in providing care is perhaps most important. Even with careful planning, groups should expect implementation of an NP or PA model to require lead-in and training time.
The fact that NPs and PAs have been adopted by so many groups suggests that practices have found increased value; however, models vary widely. In some practices, NPs or PAs work side by side with hospitalists, assisting with documentation, meeting with patients and families, and implementing an agreed-upon management plan. In this model, NPs and PAs may also add value by helping with time-consuming discharges, freeing hospitalists to attend to other patients and thus improving productivity, bed flow, and perhaps also job sustainability.
Other groups have found niche roles for NPs or PAs, including but not limited to providing cross-coverage, performing procedures, triaging admissions, staffing observation units, and developing expertise in a specific clinical area such as consultative medicine or orthopedics.
How to Bill
Depending on the model adopted, groups must also decide whether it is advantageous for NPs or PAs to bill for services independently versus as shared services under the supervising physician’s provider number. The Centers for Medicare & Medicaid Services reimburses NPs and PAs who bill independently at 85% of the physician rate. Nonetheless, some groups may find it more cost-effective for NPs or PAs to bill independently given the substantial salary differential compared to hospitalists. Data published in the 2016 State of Hospital Medicine Report show that NPs and PAs bill independently 47.9% of the time (see Figure 1).
At the University of California, San Diego, we’ve found that our PAs added the most value when working one-on-one with a single hospitalist rather than with multiple physicians. Specifically, our PAs have added to the capacity of our non-teaching service, typically implementing a plan of care developed cooperatively with the hospitalist but also managing a select cohort of patients independently during times of excess volume. By increasing capacity, our PAs have also allowed us to avoid calling in a backup hospitalist during high census periods.
References
- Society of Hospital Medicine. 2006 State of Hospital Medicine Report.
- 2016 State of Hospital Medicine Report. Society of Hospital Medicine website. Accessed October 23, 2016.
While the role of nurse practitioners (NPs) and physician assistants (PAs) in hospital medicine is far from uniform, data from the 2016 State of Hospital Medicine Report show that a majority of hospital medicine groups utilize NPs or PAs. Over the past decade, the percentage of hospital medicine groups that include NPs or PAs has grown from about 20% in 20051 to 65% in 2015.2
I suspect a large part of this growth is fueled by demand continuing to outstrip supply for hospitalist physicians and continued increases in hospitalist salaries. In academic institutions, restrictions in house staff duty hours over the past decade no doubt also contributed to the growth of NP and PA utilization.
How Should HM Groups Use NPs and PAs?
In addition to obtaining group buy-in prior to deploying NPs or PAs in a hospital medicine group, a thoughtful consideration about the role of NPs or PAs in providing care is perhaps most important. Even with careful planning, groups should expect implementation of an NP or PA model to require lead-in and training time.
The fact that NPs and PAs have been adopted by so many groups suggests that practices have found increased value; however, models vary widely. In some practices, NPs or PAs work side by side with hospitalists, assisting with documentation, meeting with patients and families, and implementing an agreed-upon management plan. In this model, NPs and PAs may also add value by helping with time-consuming discharges, freeing hospitalists to attend to other patients and thus improving productivity, bed flow, and perhaps also job sustainability.
Other groups have found niche roles for NPs or PAs, including but not limited to providing cross-coverage, performing procedures, triaging admissions, staffing observation units, and developing expertise in a specific clinical area such as consultative medicine or orthopedics.
How to Bill
Depending on the model adopted, groups must also decide whether it is advantageous for NPs or PAs to bill for services independently versus as shared services under the supervising physician’s provider number. The Centers for Medicare & Medicaid Services reimburses NPs and PAs who bill independently at 85% of the physician rate. Nonetheless, some groups may find it more cost-effective for NPs or PAs to bill independently given the substantial salary differential compared to hospitalists. Data published in the 2016 State of Hospital Medicine Report show that NPs and PAs bill independently 47.9% of the time (see Figure 1).
At the University of California, San Diego, we’ve found that our PAs added the most value when working one-on-one with a single hospitalist rather than with multiple physicians. Specifically, our PAs have added to the capacity of our non-teaching service, typically implementing a plan of care developed cooperatively with the hospitalist but also managing a select cohort of patients independently during times of excess volume. By increasing capacity, our PAs have also allowed us to avoid calling in a backup hospitalist during high census periods.
References
- Society of Hospital Medicine. 2006 State of Hospital Medicine Report.
- 2016 State of Hospital Medicine Report. Society of Hospital Medicine website. Accessed October 23, 2016.
While the role of nurse practitioners (NPs) and physician assistants (PAs) in hospital medicine is far from uniform, data from the 2016 State of Hospital Medicine Report show that a majority of hospital medicine groups utilize NPs or PAs. Over the past decade, the percentage of hospital medicine groups that include NPs or PAs has grown from about 20% in 20051 to 65% in 2015.2
I suspect a large part of this growth is fueled by demand continuing to outstrip supply for hospitalist physicians and continued increases in hospitalist salaries. In academic institutions, restrictions in house staff duty hours over the past decade no doubt also contributed to the growth of NP and PA utilization.
How Should HM Groups Use NPs and PAs?
In addition to obtaining group buy-in prior to deploying NPs or PAs in a hospital medicine group, a thoughtful consideration about the role of NPs or PAs in providing care is perhaps most important. Even with careful planning, groups should expect implementation of an NP or PA model to require lead-in and training time.
The fact that NPs and PAs have been adopted by so many groups suggests that practices have found increased value; however, models vary widely. In some practices, NPs or PAs work side by side with hospitalists, assisting with documentation, meeting with patients and families, and implementing an agreed-upon management plan. In this model, NPs and PAs may also add value by helping with time-consuming discharges, freeing hospitalists to attend to other patients and thus improving productivity, bed flow, and perhaps also job sustainability.
Other groups have found niche roles for NPs or PAs, including but not limited to providing cross-coverage, performing procedures, triaging admissions, staffing observation units, and developing expertise in a specific clinical area such as consultative medicine or orthopedics.
How to Bill
Depending on the model adopted, groups must also decide whether it is advantageous for NPs or PAs to bill for services independently versus as shared services under the supervising physician’s provider number. The Centers for Medicare & Medicaid Services reimburses NPs and PAs who bill independently at 85% of the physician rate. Nonetheless, some groups may find it more cost-effective for NPs or PAs to bill independently given the substantial salary differential compared to hospitalists. Data published in the 2016 State of Hospital Medicine Report show that NPs and PAs bill independently 47.9% of the time (see Figure 1).
At the University of California, San Diego, we’ve found that our PAs added the most value when working one-on-one with a single hospitalist rather than with multiple physicians. Specifically, our PAs have added to the capacity of our non-teaching service, typically implementing a plan of care developed cooperatively with the hospitalist but also managing a select cohort of patients independently during times of excess volume. By increasing capacity, our PAs have also allowed us to avoid calling in a backup hospitalist during high census periods.
References
- Society of Hospital Medicine. 2006 State of Hospital Medicine Report.
- 2016 State of Hospital Medicine Report. Society of Hospital Medicine website. Accessed October 23, 2016.
Novel interferon appears safer than HU in PV

Photo by Zak Hubbard
SAN DIEGO—Results of the PROUD-PV trial suggest ropeginterferon alfa-2b is safer than hydroxyurea (HU) for patients with polycythemia vera (PV).
In this phase 3 trial, ropeginterferon alfa-2b demonstrated non-inferiority to HU with regard to complete hematologic response (CHR).
Ropeginterferon alfa-2b also had a significantly better overall safety profile.
Unlike the patients who received HU, none of the patients on ropeginterferon alfa-2b developed secondary malignancies.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, presented these results at the 2016 ASH Annual Meeting (abstract 475). The PROUD-PV study was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Gisslinger noted that interferons have been successful in treating PV since the 1980s, although toxicities contribute to discontinuation rates of approximately 25%. Still, interferons are the only known drugs with the potential for disease modification by specific targeting of the malignant clone.
Ropeginterferon alfa-2b is a long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties that allow for administration once every 2 weeks.
The goal of PROUD-PV was to determine how this drug stacks up against HU in both treatment-naive and HU-pretreated patients with PV.
“Our results from the first and largest, prospective, controlled trial of an interferon in polycythemia vera confirm previously reported efficacy,” Dr Gisslinger said.
“The observed safety and tolerability profile of ropeginterferon appears to be superior compared to previously reported data of interferon treatment. The unique disease-modification capability of interferon and its potential to improve progression-free survival hold promise for long-term benefit for patients.”
Patients and treatment
PROUD-PV enrolled 254 patients, and they were randomized to receive ropeginterferon alfa-2b (n=127) or HU (n=127). In both arms, 100% of patients were Caucasian, slightly more than half were female, and the median age was 60 (overall range, 21-85).
The median disease duration was 1.9 months in the ropeginterferon alfa-2b arm and 3.6 months in the HU arm. Thirty-seven percent (n=47) of patients in each arm had previously received HU.
The mean hematocrit was about 50% in both arms, the median spleen length was about 13 cm, about 90% of patients had a normal/slightly enlarged spleen, and the mean JAK2V617F burden was slightly more than 40%.
The median plateau dose was 450 µg in the ropeginterferon alfa-2b arm and 1250 mg in the HU arm.
A quarter (25.2%) of patients had dose reductions due to adverse events (AEs) in the ropeginterferon alfa-2b arm, as did 51.2% of patients in the HU arm. The 12-month discontinuation rate was 16.5% in the ropeginterferon alfa-2b arm and 12.6% in the HU arm.
Response
The study’s primary objective was to demonstrate non-inferiority of ropeginterferon alfa-2b compared to HU. For this, the researchers used the 12-month CHR rate. CHR was defined as normalization of red blood cell, white blood cell, and platelet counts (without phlebotomy).
At 12 months, in the intent-to-treat population, the CHR rate was 43.1% in the ropeginterferon alfa-2b arm and 45.6% in the HU arm (P=0.0028). In the per-protocol population, the CHR rate was 44.3% and 46.5%, respectively (P=0.0036).
The researchers therefore concluded that non-inferiority was demonstrated.
The study’s pre-specified primary endpoint was actually a composite of CHR and spleen length normality. However, this was confounded by the fact that the patients’ median spleen length was almost normal at baseline and the observed change was not clinically relevant.
In the intent-to-treat-population, CHR with spleen normality occurred in 21.3% of patients in the ropeginterferon alfa-2b arm and 27.6% of patients in the HU arm (P=0.2233).
Safety
The incidence of AEs was 81.9% in the ropeginterferon alfa-2b arm and 87.4% in the HU arm. The incidence of grade 3 AEs was 16.5% and 20.5%, respectively. And the incidence of treatment-related AEs was 59.6% and 75.6%, respectively (P<0.05).
There was a significantly higher incidence (P<0.01) of the following AEs in the HU arm than the ropeginterferon alfa-2b arm: anemia (24.4% vs 6.3%), leukopenia (21.3% vs 8.7%), thrombocytopenia (28.3% vs 15.0%), and nausea (11.8% vs 2.4%).
There was no significant difference in the incidence of fatigue—13.4% in the HU arm and 12.6% in the ropeginterferon alfa-2b arm.
Patients in the ropeginterferon alfa-2b arm had a significantly higher incidence of gamma-glutamyl transferase increase—14.2% vs 0.8% in the HU arm (P<0.01).
Patients in the ropeginterferon alfa-2b arm also had a higher—but non-significant—incidence of endocrine disorders (3.1% vs 0.8%), psychiatric disorders (1.6% vs 0%), cardiac/vascular disorders (3.1% vs 1.6%), and tissue disorders (1.6% vs 0%).
None of the patients in the ropeginterferon alfa-2b arm developed secondary related malignancies. In the HU arm, however, there were 2 cases of acute leukemia, 2 cases of basal cell carcinoma, and 1 case of malignant melanoma. (This includes data from the ongoing follow-up trial CONTINUATION-PV.)
Drug development
AOP Orphan Pharmaceuticals AG said that, in the coming months, it will submit data from PROUD-PV and the ongoing follow-up trial, CONTINUATION-PV, to obtain European marketing authorization for ropeginterferon alfa-2b.
PharmaEssentia plans to submit the same data to the US Food and Drug Administration.
PharmaEssentia discovered ropeginterferon alfa-2b and has licensed the rights for development and commercialization of the drug in myeloproliferative neoplasms to AOP Orphan Pharmaceuticals AG in Europe, the Commonwealth of Independent States, and Middle Eastern markets. ![]()
*Information presented at the meeting differs from the abstract.

Photo by Zak Hubbard
SAN DIEGO—Results of the PROUD-PV trial suggest ropeginterferon alfa-2b is safer than hydroxyurea (HU) for patients with polycythemia vera (PV).
In this phase 3 trial, ropeginterferon alfa-2b demonstrated non-inferiority to HU with regard to complete hematologic response (CHR).
Ropeginterferon alfa-2b also had a significantly better overall safety profile.
Unlike the patients who received HU, none of the patients on ropeginterferon alfa-2b developed secondary malignancies.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, presented these results at the 2016 ASH Annual Meeting (abstract 475). The PROUD-PV study was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Gisslinger noted that interferons have been successful in treating PV since the 1980s, although toxicities contribute to discontinuation rates of approximately 25%. Still, interferons are the only known drugs with the potential for disease modification by specific targeting of the malignant clone.
Ropeginterferon alfa-2b is a long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties that allow for administration once every 2 weeks.
The goal of PROUD-PV was to determine how this drug stacks up against HU in both treatment-naive and HU-pretreated patients with PV.
“Our results from the first and largest, prospective, controlled trial of an interferon in polycythemia vera confirm previously reported efficacy,” Dr Gisslinger said.
“The observed safety and tolerability profile of ropeginterferon appears to be superior compared to previously reported data of interferon treatment. The unique disease-modification capability of interferon and its potential to improve progression-free survival hold promise for long-term benefit for patients.”
Patients and treatment
PROUD-PV enrolled 254 patients, and they were randomized to receive ropeginterferon alfa-2b (n=127) or HU (n=127). In both arms, 100% of patients were Caucasian, slightly more than half were female, and the median age was 60 (overall range, 21-85).
The median disease duration was 1.9 months in the ropeginterferon alfa-2b arm and 3.6 months in the HU arm. Thirty-seven percent (n=47) of patients in each arm had previously received HU.
The mean hematocrit was about 50% in both arms, the median spleen length was about 13 cm, about 90% of patients had a normal/slightly enlarged spleen, and the mean JAK2V617F burden was slightly more than 40%.
The median plateau dose was 450 µg in the ropeginterferon alfa-2b arm and 1250 mg in the HU arm.
A quarter (25.2%) of patients had dose reductions due to adverse events (AEs) in the ropeginterferon alfa-2b arm, as did 51.2% of patients in the HU arm. The 12-month discontinuation rate was 16.5% in the ropeginterferon alfa-2b arm and 12.6% in the HU arm.
Response
The study’s primary objective was to demonstrate non-inferiority of ropeginterferon alfa-2b compared to HU. For this, the researchers used the 12-month CHR rate. CHR was defined as normalization of red blood cell, white blood cell, and platelet counts (without phlebotomy).
At 12 months, in the intent-to-treat population, the CHR rate was 43.1% in the ropeginterferon alfa-2b arm and 45.6% in the HU arm (P=0.0028). In the per-protocol population, the CHR rate was 44.3% and 46.5%, respectively (P=0.0036).
The researchers therefore concluded that non-inferiority was demonstrated.
The study’s pre-specified primary endpoint was actually a composite of CHR and spleen length normality. However, this was confounded by the fact that the patients’ median spleen length was almost normal at baseline and the observed change was not clinically relevant.
In the intent-to-treat-population, CHR with spleen normality occurred in 21.3% of patients in the ropeginterferon alfa-2b arm and 27.6% of patients in the HU arm (P=0.2233).
Safety
The incidence of AEs was 81.9% in the ropeginterferon alfa-2b arm and 87.4% in the HU arm. The incidence of grade 3 AEs was 16.5% and 20.5%, respectively. And the incidence of treatment-related AEs was 59.6% and 75.6%, respectively (P<0.05).
There was a significantly higher incidence (P<0.01) of the following AEs in the HU arm than the ropeginterferon alfa-2b arm: anemia (24.4% vs 6.3%), leukopenia (21.3% vs 8.7%), thrombocytopenia (28.3% vs 15.0%), and nausea (11.8% vs 2.4%).
There was no significant difference in the incidence of fatigue—13.4% in the HU arm and 12.6% in the ropeginterferon alfa-2b arm.
Patients in the ropeginterferon alfa-2b arm had a significantly higher incidence of gamma-glutamyl transferase increase—14.2% vs 0.8% in the HU arm (P<0.01).
Patients in the ropeginterferon alfa-2b arm also had a higher—but non-significant—incidence of endocrine disorders (3.1% vs 0.8%), psychiatric disorders (1.6% vs 0%), cardiac/vascular disorders (3.1% vs 1.6%), and tissue disorders (1.6% vs 0%).
None of the patients in the ropeginterferon alfa-2b arm developed secondary related malignancies. In the HU arm, however, there were 2 cases of acute leukemia, 2 cases of basal cell carcinoma, and 1 case of malignant melanoma. (This includes data from the ongoing follow-up trial CONTINUATION-PV.)
Drug development
AOP Orphan Pharmaceuticals AG said that, in the coming months, it will submit data from PROUD-PV and the ongoing follow-up trial, CONTINUATION-PV, to obtain European marketing authorization for ropeginterferon alfa-2b.
PharmaEssentia plans to submit the same data to the US Food and Drug Administration.
PharmaEssentia discovered ropeginterferon alfa-2b and has licensed the rights for development and commercialization of the drug in myeloproliferative neoplasms to AOP Orphan Pharmaceuticals AG in Europe, the Commonwealth of Independent States, and Middle Eastern markets. ![]()
*Information presented at the meeting differs from the abstract.

Photo by Zak Hubbard
SAN DIEGO—Results of the PROUD-PV trial suggest ropeginterferon alfa-2b is safer than hydroxyurea (HU) for patients with polycythemia vera (PV).
In this phase 3 trial, ropeginterferon alfa-2b demonstrated non-inferiority to HU with regard to complete hematologic response (CHR).
Ropeginterferon alfa-2b also had a significantly better overall safety profile.
Unlike the patients who received HU, none of the patients on ropeginterferon alfa-2b developed secondary malignancies.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, presented these results at the 2016 ASH Annual Meeting (abstract 475). The PROUD-PV study was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Gisslinger noted that interferons have been successful in treating PV since the 1980s, although toxicities contribute to discontinuation rates of approximately 25%. Still, interferons are the only known drugs with the potential for disease modification by specific targeting of the malignant clone.
Ropeginterferon alfa-2b is a long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties that allow for administration once every 2 weeks.
The goal of PROUD-PV was to determine how this drug stacks up against HU in both treatment-naive and HU-pretreated patients with PV.
“Our results from the first and largest, prospective, controlled trial of an interferon in polycythemia vera confirm previously reported efficacy,” Dr Gisslinger said.
“The observed safety and tolerability profile of ropeginterferon appears to be superior compared to previously reported data of interferon treatment. The unique disease-modification capability of interferon and its potential to improve progression-free survival hold promise for long-term benefit for patients.”
Patients and treatment
PROUD-PV enrolled 254 patients, and they were randomized to receive ropeginterferon alfa-2b (n=127) or HU (n=127). In both arms, 100% of patients were Caucasian, slightly more than half were female, and the median age was 60 (overall range, 21-85).
The median disease duration was 1.9 months in the ropeginterferon alfa-2b arm and 3.6 months in the HU arm. Thirty-seven percent (n=47) of patients in each arm had previously received HU.
The mean hematocrit was about 50% in both arms, the median spleen length was about 13 cm, about 90% of patients had a normal/slightly enlarged spleen, and the mean JAK2V617F burden was slightly more than 40%.
The median plateau dose was 450 µg in the ropeginterferon alfa-2b arm and 1250 mg in the HU arm.
A quarter (25.2%) of patients had dose reductions due to adverse events (AEs) in the ropeginterferon alfa-2b arm, as did 51.2% of patients in the HU arm. The 12-month discontinuation rate was 16.5% in the ropeginterferon alfa-2b arm and 12.6% in the HU arm.
Response
The study’s primary objective was to demonstrate non-inferiority of ropeginterferon alfa-2b compared to HU. For this, the researchers used the 12-month CHR rate. CHR was defined as normalization of red blood cell, white blood cell, and platelet counts (without phlebotomy).
At 12 months, in the intent-to-treat population, the CHR rate was 43.1% in the ropeginterferon alfa-2b arm and 45.6% in the HU arm (P=0.0028). In the per-protocol population, the CHR rate was 44.3% and 46.5%, respectively (P=0.0036).
The researchers therefore concluded that non-inferiority was demonstrated.
The study’s pre-specified primary endpoint was actually a composite of CHR and spleen length normality. However, this was confounded by the fact that the patients’ median spleen length was almost normal at baseline and the observed change was not clinically relevant.
In the intent-to-treat-population, CHR with spleen normality occurred in 21.3% of patients in the ropeginterferon alfa-2b arm and 27.6% of patients in the HU arm (P=0.2233).
Safety
The incidence of AEs was 81.9% in the ropeginterferon alfa-2b arm and 87.4% in the HU arm. The incidence of grade 3 AEs was 16.5% and 20.5%, respectively. And the incidence of treatment-related AEs was 59.6% and 75.6%, respectively (P<0.05).
There was a significantly higher incidence (P<0.01) of the following AEs in the HU arm than the ropeginterferon alfa-2b arm: anemia (24.4% vs 6.3%), leukopenia (21.3% vs 8.7%), thrombocytopenia (28.3% vs 15.0%), and nausea (11.8% vs 2.4%).
There was no significant difference in the incidence of fatigue—13.4% in the HU arm and 12.6% in the ropeginterferon alfa-2b arm.
Patients in the ropeginterferon alfa-2b arm had a significantly higher incidence of gamma-glutamyl transferase increase—14.2% vs 0.8% in the HU arm (P<0.01).
Patients in the ropeginterferon alfa-2b arm also had a higher—but non-significant—incidence of endocrine disorders (3.1% vs 0.8%), psychiatric disorders (1.6% vs 0%), cardiac/vascular disorders (3.1% vs 1.6%), and tissue disorders (1.6% vs 0%).
None of the patients in the ropeginterferon alfa-2b arm developed secondary related malignancies. In the HU arm, however, there were 2 cases of acute leukemia, 2 cases of basal cell carcinoma, and 1 case of malignant melanoma. (This includes data from the ongoing follow-up trial CONTINUATION-PV.)
Drug development
AOP Orphan Pharmaceuticals AG said that, in the coming months, it will submit data from PROUD-PV and the ongoing follow-up trial, CONTINUATION-PV, to obtain European marketing authorization for ropeginterferon alfa-2b.
PharmaEssentia plans to submit the same data to the US Food and Drug Administration.
PharmaEssentia discovered ropeginterferon alfa-2b and has licensed the rights for development and commercialization of the drug in myeloproliferative neoplasms to AOP Orphan Pharmaceuticals AG in Europe, the Commonwealth of Independent States, and Middle Eastern markets. ![]()
*Information presented at the meeting differs from the abstract.
Company terminates study of drug for MM

multiple myeloma
BioInvent International has decided to terminate its phase 2 trial of the antibody BI-505 in patients with multiple myeloma (MM).
The decision follows a review and discussion with the US Food and Drug Administration (FDA), which put the trial on full clinical hold in November due to an adverse cardiopulmonary event.
The trial was designed to determine if BI-505 could deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
The termination of this trial may not mean the end of BI-505. BioInvent is currently in discussions with the FDA about the potential to develop the drug for use in other patient populations.
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
The development strategy for BI-505 has been focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
BI-505 has orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency.
Results of a phase 1 trial of BI-505 in MM patients were published in Clinical Cancer Research in June 2015. ![]()

multiple myeloma
BioInvent International has decided to terminate its phase 2 trial of the antibody BI-505 in patients with multiple myeloma (MM).
The decision follows a review and discussion with the US Food and Drug Administration (FDA), which put the trial on full clinical hold in November due to an adverse cardiopulmonary event.
The trial was designed to determine if BI-505 could deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
The termination of this trial may not mean the end of BI-505. BioInvent is currently in discussions with the FDA about the potential to develop the drug for use in other patient populations.
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
The development strategy for BI-505 has been focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
BI-505 has orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency.
Results of a phase 1 trial of BI-505 in MM patients were published in Clinical Cancer Research in June 2015. ![]()

multiple myeloma
BioInvent International has decided to terminate its phase 2 trial of the antibody BI-505 in patients with multiple myeloma (MM).
The decision follows a review and discussion with the US Food and Drug Administration (FDA), which put the trial on full clinical hold in November due to an adverse cardiopulmonary event.
The trial was designed to determine if BI-505 could deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
The termination of this trial may not mean the end of BI-505. BioInvent is currently in discussions with the FDA about the potential to develop the drug for use in other patient populations.
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
The development strategy for BI-505 has been focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
BI-505 has orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency.
Results of a phase 1 trial of BI-505 in MM patients were published in Clinical Cancer Research in June 2015. ![]()