User login
ASH: Hemophilia B gene therapy posts strong update
SAN DIEGO – Patients with hemophilia B who received a single 1-hour infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the 12% steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, said during a press briefing at the annual meeting of the American Society of Hematology. One patient infused himself once with factor IX after developing a suspected ankle bleed 2 days after treatment, Dr. High and her associates reported in the accompanying abstract.
This therapy works at a lower dose than previous factor IX gene transfer products and therefore has not caused the hepatotoxicity that halted their development, according to Dr. High, president and chief scientific officer of Spark Therapeutics, which makes SPK-9001. Two of nine patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels, but the immune response was halted by tapering doses of corticosteroids, and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor, she said.
Because the virus capsid breaks down over time, a transient immune response to it “is not really a safety issue, but is an efficacy issue,” Dr. High emphasized. “If it is not caught in time, and patients are not given steroids promptly, they can lose the donated gene. Therefore, quick recognition is key.” Patients who develop an immune response to the viral capsid show sharp declines in factor IX activity levels, rises in baseline AST and ALT, and mononuclear cell reactivity, she explained during an interview.
The current standard of care for hemophilia B involves the cost and treatment burden of intravenous factor IX injections given one to three times weekly. Previous work evaluated factor IX gene transfer mediated by adeno-associated virus, but long-term factor IX activity levels did not reach the trough levels typically achieved with long-acting factor IX prophylaxis. Simply escalating the vector dose did not work because the viral capsid triggered immune-mediated hepatotoxicity, Dr. High noted.
To develop a more efficient product that works at lower doses, she and her associates created a recombinant vector containing a bioengineered adeno-associated virus capsid and a DNA sequence with a promoter designed to drive hepatic expression of a highly active variant of factor IX. To test the product, researchers in Mississippi, Pennsylvania, and California enrolled men aged 18-52 years with a confirmed diagnosis of hemophilia B (no more than 2 IU/dL or 2% endogenous factor IX) who had received at least 50 days of exposure to factor IX products and averaged at least four bleeding events per year requiring factor IX treatment or prophylaxis. Patients had no measurable inhibitory antibodies but otherwise represented the “general hemophilia B population,” Dr. High said. Five of nine patients had multiple target joints, liver disease associated with hepatitis C virus infection, or both. Each patient received a 1-hour infusion of 5 x 1011 vector genomes per body weight and was followed for 7-52 weeks.
Among seven patients who, by Nov. 30, 2016, had surpassed the 12 weeks needed to reach steady state factor IX expression levels, median steady-state level was 30% (range, 13%-38%), Dr. High reported. “Now we can give one quarter the dose [of adeno-associated virus vector] that was given before, and its driving factor IX expression levels five to eight times higher,” she concluded. Results for the first seven treated patients prompted Food and Drug Administration to give the product orphan drug designation in July 2016. Plans for phase III trials are underway, and researchers also are planning to investigate this approach to gene therapy in hemophilia A, Dr. High said.
Spark Therapeutics Inc. and Pfizer sponsored the study. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
SAN DIEGO – Patients with hemophilia B who received a single 1-hour infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the 12% steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, said during a press briefing at the annual meeting of the American Society of Hematology. One patient infused himself once with factor IX after developing a suspected ankle bleed 2 days after treatment, Dr. High and her associates reported in the accompanying abstract.
This therapy works at a lower dose than previous factor IX gene transfer products and therefore has not caused the hepatotoxicity that halted their development, according to Dr. High, president and chief scientific officer of Spark Therapeutics, which makes SPK-9001. Two of nine patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels, but the immune response was halted by tapering doses of corticosteroids, and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor, she said.
Because the virus capsid breaks down over time, a transient immune response to it “is not really a safety issue, but is an efficacy issue,” Dr. High emphasized. “If it is not caught in time, and patients are not given steroids promptly, they can lose the donated gene. Therefore, quick recognition is key.” Patients who develop an immune response to the viral capsid show sharp declines in factor IX activity levels, rises in baseline AST and ALT, and mononuclear cell reactivity, she explained during an interview.
The current standard of care for hemophilia B involves the cost and treatment burden of intravenous factor IX injections given one to three times weekly. Previous work evaluated factor IX gene transfer mediated by adeno-associated virus, but long-term factor IX activity levels did not reach the trough levels typically achieved with long-acting factor IX prophylaxis. Simply escalating the vector dose did not work because the viral capsid triggered immune-mediated hepatotoxicity, Dr. High noted.
To develop a more efficient product that works at lower doses, she and her associates created a recombinant vector containing a bioengineered adeno-associated virus capsid and a DNA sequence with a promoter designed to drive hepatic expression of a highly active variant of factor IX. To test the product, researchers in Mississippi, Pennsylvania, and California enrolled men aged 18-52 years with a confirmed diagnosis of hemophilia B (no more than 2 IU/dL or 2% endogenous factor IX) who had received at least 50 days of exposure to factor IX products and averaged at least four bleeding events per year requiring factor IX treatment or prophylaxis. Patients had no measurable inhibitory antibodies but otherwise represented the “general hemophilia B population,” Dr. High said. Five of nine patients had multiple target joints, liver disease associated with hepatitis C virus infection, or both. Each patient received a 1-hour infusion of 5 x 1011 vector genomes per body weight and was followed for 7-52 weeks.
Among seven patients who, by Nov. 30, 2016, had surpassed the 12 weeks needed to reach steady state factor IX expression levels, median steady-state level was 30% (range, 13%-38%), Dr. High reported. “Now we can give one quarter the dose [of adeno-associated virus vector] that was given before, and its driving factor IX expression levels five to eight times higher,” she concluded. Results for the first seven treated patients prompted Food and Drug Administration to give the product orphan drug designation in July 2016. Plans for phase III trials are underway, and researchers also are planning to investigate this approach to gene therapy in hemophilia A, Dr. High said.
Spark Therapeutics Inc. and Pfizer sponsored the study. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
SAN DIEGO – Patients with hemophilia B who received a single 1-hour infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the 12% steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, said during a press briefing at the annual meeting of the American Society of Hematology. One patient infused himself once with factor IX after developing a suspected ankle bleed 2 days after treatment, Dr. High and her associates reported in the accompanying abstract.
This therapy works at a lower dose than previous factor IX gene transfer products and therefore has not caused the hepatotoxicity that halted their development, according to Dr. High, president and chief scientific officer of Spark Therapeutics, which makes SPK-9001. Two of nine patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels, but the immune response was halted by tapering doses of corticosteroids, and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor, she said.
Because the virus capsid breaks down over time, a transient immune response to it “is not really a safety issue, but is an efficacy issue,” Dr. High emphasized. “If it is not caught in time, and patients are not given steroids promptly, they can lose the donated gene. Therefore, quick recognition is key.” Patients who develop an immune response to the viral capsid show sharp declines in factor IX activity levels, rises in baseline AST and ALT, and mononuclear cell reactivity, she explained during an interview.
The current standard of care for hemophilia B involves the cost and treatment burden of intravenous factor IX injections given one to three times weekly. Previous work evaluated factor IX gene transfer mediated by adeno-associated virus, but long-term factor IX activity levels did not reach the trough levels typically achieved with long-acting factor IX prophylaxis. Simply escalating the vector dose did not work because the viral capsid triggered immune-mediated hepatotoxicity, Dr. High noted.
To develop a more efficient product that works at lower doses, she and her associates created a recombinant vector containing a bioengineered adeno-associated virus capsid and a DNA sequence with a promoter designed to drive hepatic expression of a highly active variant of factor IX. To test the product, researchers in Mississippi, Pennsylvania, and California enrolled men aged 18-52 years with a confirmed diagnosis of hemophilia B (no more than 2 IU/dL or 2% endogenous factor IX) who had received at least 50 days of exposure to factor IX products and averaged at least four bleeding events per year requiring factor IX treatment or prophylaxis. Patients had no measurable inhibitory antibodies but otherwise represented the “general hemophilia B population,” Dr. High said. Five of nine patients had multiple target joints, liver disease associated with hepatitis C virus infection, or both. Each patient received a 1-hour infusion of 5 x 1011 vector genomes per body weight and was followed for 7-52 weeks.
Among seven patients who, by Nov. 30, 2016, had surpassed the 12 weeks needed to reach steady state factor IX expression levels, median steady-state level was 30% (range, 13%-38%), Dr. High reported. “Now we can give one quarter the dose [of adeno-associated virus vector] that was given before, and its driving factor IX expression levels five to eight times higher,” she concluded. Results for the first seven treated patients prompted Food and Drug Administration to give the product orphan drug designation in July 2016. Plans for phase III trials are underway, and researchers also are planning to investigate this approach to gene therapy in hemophilia A, Dr. High said.
Spark Therapeutics Inc. and Pfizer sponsored the study. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
AT ASH 2016
Key clinical point: Gene therapy with SPK-9001 continues to post strong results in patients with moderate to severe hemophilia B.
Major finding: As of Nov. 30, median steady-state factor IX levels were 30% (range, 13% to 38%). Two of nine patients developed an immune response to the adeno-associated virus capsid that appears to have been halted with tapering doses of corticosteroids.
Data source: An ongoing phase I/II trial of SPK-9001, dosed at 5 x 1011 vector genomes (vg)/kg body weight.
Disclosures: Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
VIDEO: Hemophilia B gene therapy maintains factor IX levels averaging 28%
SAN DIEGO – Patients with hemophilia B who received a single infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, reported at the American Society of Hematology. There have been no confirmed bleeds, all patients remain off prophylactic factor IX, none have developed factor IX inhibitory antibodies, and Enzyme-Linked ImmunoSpot testing has uncovered no evidence of emergent reactivity to the gene product. Two patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels. Tapering doses of corticosteroids halted the immune response and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor.
Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. She discussed the trial in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Patients with hemophilia B who received a single infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, reported at the American Society of Hematology. There have been no confirmed bleeds, all patients remain off prophylactic factor IX, none have developed factor IX inhibitory antibodies, and Enzyme-Linked ImmunoSpot testing has uncovered no evidence of emergent reactivity to the gene product. Two patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels. Tapering doses of corticosteroids halted the immune response and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor.
Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. She discussed the trial in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Patients with hemophilia B who received a single infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, reported at the American Society of Hematology. There have been no confirmed bleeds, all patients remain off prophylactic factor IX, none have developed factor IX inhibitory antibodies, and Enzyme-Linked ImmunoSpot testing has uncovered no evidence of emergent reactivity to the gene product. Two patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels. Tapering doses of corticosteroids halted the immune response and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor.
Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. She discussed the trial in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
ASH: Novel microcapsules show promise in hemophilia A with inhibitory antibodies
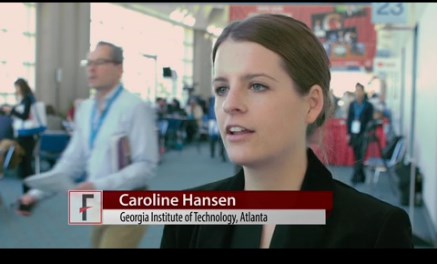
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in a model of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” Ms. Hansen said at a press briefing.
To create the microcapsules, the investigators deposited alternatingly charged layers of polyelectrolytes, poly-L-lysine, and poly-L-glutamic acid onto a calcium carbonate core covered with factor VIII and dextran. They added fibrinogen to the final polyelectrolyte layer and then chelated out the innermost core, leaving the dextran layer as a shield between factor VIII and the outside of the microcapsule. Initial in vitro experiments showed that the microcapsules adhered to platelets and were incorporated into fibrin networks when platelets were activated, Ms. Hansen reported. Because the microcapsules only ruptured upon platelet contraction, factor VIII was only delivered to actively forming clots as intended, she added.
As a next step, the researchers perfused recalcified whole blood and platelet-poor plasma into a collagen and tissue factor patch designed to mimic vascular injury, and then measured fibrin fluorescence on the patch. Microcapsules lacking dextran, fibrinogen, or loaded factor VIII did not work – a treated sample and a phosphate-buffered saline (PBS) control yielded statistically similar fibrin production. However, complete microcapsules loaded with 0.01 U/mL factor VIII produced four times more fibrin than systemic infusion of 0.05 U/mL factor VIII.
“These were really promising results but we want to take a step back and see if a clot would form in the presence of inhibitory antibodies,” Ms. Hansen said. Accordingly, they added factor VIII inhibitory antibody 2-76 into blood samples from healthy donors. The microcapsules triggered 2.7 times more fibrin production in this setting than systemic treatment did (P less than .05). “This increased efficacy is likely due to the microcapsule shielding effect on factor VIII, preventing exposure to inhibitory antibodies,” Ms. Hansen and her associates concluded in their abstract.
The investigators are now studying the extent to which the microcapsules induce thrombin production, and how agents such as blebbistatin, ROCK, and myosin affect platelet contraction force and the efficiency of the microcapsule.
Ms. Hansen had no disclosures.
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in a model of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” Ms. Hansen said at a press briefing.
To create the microcapsules, the investigators deposited alternatingly charged layers of polyelectrolytes, poly-L-lysine, and poly-L-glutamic acid onto a calcium carbonate core covered with factor VIII and dextran. They added fibrinogen to the final polyelectrolyte layer and then chelated out the innermost core, leaving the dextran layer as a shield between factor VIII and the outside of the microcapsule. Initial in vitro experiments showed that the microcapsules adhered to platelets and were incorporated into fibrin networks when platelets were activated, Ms. Hansen reported. Because the microcapsules only ruptured upon platelet contraction, factor VIII was only delivered to actively forming clots as intended, she added.
As a next step, the researchers perfused recalcified whole blood and platelet-poor plasma into a collagen and tissue factor patch designed to mimic vascular injury, and then measured fibrin fluorescence on the patch. Microcapsules lacking dextran, fibrinogen, or loaded factor VIII did not work – a treated sample and a phosphate-buffered saline (PBS) control yielded statistically similar fibrin production. However, complete microcapsules loaded with 0.01 U/mL factor VIII produced four times more fibrin than systemic infusion of 0.05 U/mL factor VIII.
“These were really promising results but we want to take a step back and see if a clot would form in the presence of inhibitory antibodies,” Ms. Hansen said. Accordingly, they added factor VIII inhibitory antibody 2-76 into blood samples from healthy donors. The microcapsules triggered 2.7 times more fibrin production in this setting than systemic treatment did (P less than .05). “This increased efficacy is likely due to the microcapsule shielding effect on factor VIII, preventing exposure to inhibitory antibodies,” Ms. Hansen and her associates concluded in their abstract.
The investigators are now studying the extent to which the microcapsules induce thrombin production, and how agents such as blebbistatin, ROCK, and myosin affect platelet contraction force and the efficiency of the microcapsule.
Ms. Hansen had no disclosures.
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in a model of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” Ms. Hansen said at a press briefing.
To create the microcapsules, the investigators deposited alternatingly charged layers of polyelectrolytes, poly-L-lysine, and poly-L-glutamic acid onto a calcium carbonate core covered with factor VIII and dextran. They added fibrinogen to the final polyelectrolyte layer and then chelated out the innermost core, leaving the dextran layer as a shield between factor VIII and the outside of the microcapsule. Initial in vitro experiments showed that the microcapsules adhered to platelets and were incorporated into fibrin networks when platelets were activated, Ms. Hansen reported. Because the microcapsules only ruptured upon platelet contraction, factor VIII was only delivered to actively forming clots as intended, she added.
As a next step, the researchers perfused recalcified whole blood and platelet-poor plasma into a collagen and tissue factor patch designed to mimic vascular injury, and then measured fibrin fluorescence on the patch. Microcapsules lacking dextran, fibrinogen, or loaded factor VIII did not work – a treated sample and a phosphate-buffered saline (PBS) control yielded statistically similar fibrin production. However, complete microcapsules loaded with 0.01 U/mL factor VIII produced four times more fibrin than systemic infusion of 0.05 U/mL factor VIII.
“These were really promising results but we want to take a step back and see if a clot would form in the presence of inhibitory antibodies,” Ms. Hansen said. Accordingly, they added factor VIII inhibitory antibody 2-76 into blood samples from healthy donors. The microcapsules triggered 2.7 times more fibrin production in this setting than systemic treatment did (P less than .05). “This increased efficacy is likely due to the microcapsule shielding effect on factor VIII, preventing exposure to inhibitory antibodies,” Ms. Hansen and her associates concluded in their abstract.
The investigators are now studying the extent to which the microcapsules induce thrombin production, and how agents such as blebbistatin, ROCK, and myosin affect platelet contraction force and the efficiency of the microcapsule.
Ms. Hansen had no disclosures.
AT ASH 2016
Key clinical point: Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in an in vitro model of hemophilia A with inhibitory antibodies.
Major finding: In an in vitro model of this disease state, the microcapsules triggered 2.7 times more fibrin production than systemic treatment with factor VIII (P less than .05).
Data source: A multicenter laboratory study.
Disclosures: Ms. Hansen had no relevant financial disclosures.
VIDEO: Novel microcapsules show promise in hemophilia A with inhibitory antibodies
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in models of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” said Ms. Hansen of Georgia Institute of Technology, Atlanta.
The microcapsules are designed to mechanically shield factor VIII from the immune system. When they reached a modeled site of vascular injury, they contracted and released factor VIII. Initial work showed that this approach triggered significantly more fibrin production in a developing clot than did systemic infusions of factor VIII.
Ms. Hansen had no disclosures. She discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in models of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” said Ms. Hansen of Georgia Institute of Technology, Atlanta.
The microcapsules are designed to mechanically shield factor VIII from the immune system. When they reached a modeled site of vascular injury, they contracted and released factor VIII. Initial work showed that this approach triggered significantly more fibrin production in a developing clot than did systemic infusions of factor VIII.
Ms. Hansen had no disclosures. She discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in models of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” said Ms. Hansen of Georgia Institute of Technology, Atlanta.
The microcapsules are designed to mechanically shield factor VIII from the immune system. When they reached a modeled site of vascular injury, they contracted and released factor VIII. Initial work showed that this approach triggered significantly more fibrin production in a developing clot than did systemic infusions of factor VIII.
Ms. Hansen had no disclosures. She discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in an in vitro model of hemophilia A with inhibitory antibodies.
Major finding: In an in vitro model, the microcapsules triggered 2.7 times more fibrin production than systemic treatment with factor VIII (P less than .05).
Data source: A multicenter laboratory study.
Disclosures: Ms. Hansen had no relevant financial disclosures.
Phase II trial: Drug reduces sickle cell ‘pain crises’
An industry-funded phase II trial has shown that high doses of the experimental drug crizanlizumab significantly reduced the number of dangerous “pain crises” in subjects with sickle cell disease.
The median per-year rate of pain crises was 45.3% lower among those who took the high dose of crizanlizumab, compared with the placebo group (P = .01) More than a third of the subjects who took the high dose reported no pain crises during the treatment phase, more than double the rate among the placebo group.
The trial findings were released at the annual meeting of the American Society of Hematology and published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1611770).
The American Society of Hematology estimates that 70,000-100,000 people in the United States have sickle cell anemia and some patients are treated with hydroxyurea (Hydrea) are available. According to background material provided in the trial report, however, hydroxyurea has limited value, and some patients still face the prospect of pain crises which can lead to end-organ damage, and early death.
The SUSTAIN trial focuses on pain crises, also known as vaso-occlusive and sickle cell crises, which can occur without warning when sickle cells block blood flow and decrease oxygen delivery.
Researchers led by Kenneth I. Ataga, MB, of the University of North Carolina, Chapel Hill, recruited 198 subjects who had sickle cell disease and who had experienced 2-10 pain crises related to their condition over the past year. They randomly assigned 67 subjects to receive a low 2.5-mg/kg dose of crizanlizumab (also known as SelG1), 66 to a high 5.0-mg/kg dose, and 65 to a placebo. Crizanlizumab is an antibody against the molecule P-selectin, whose up-regulation in certain cells and platelets is thought to contribute to vaso-occlusion and sickle cell pain crises.
All the doses were administered intravenously 14 times over a year at sites in Brazil, the United States, and Jamaica. Risk groups for sickle cell include people of African and South American descent, among groups.
The first two doses were loading doses given at 2-week intervals, and the rest were given at 4-week intervals.
Subjects were aged 16-63 years; the median age was 29 for the two crizanlizumab groups and 26 for the placebo group. The percentage of black subjects ranged from 90% to 94% in each group, and the percentage of female subjects ranged from 52% to 58%.
Some subjects, but not all, were taking hydroxyurea. If they were taking the drug, they needed to have been on it for at least 6 months prior to the trial, and at least the last 3 months at a steady dose. Those who didn’t take hydroxyurea weren’t allowed to start taking it.
The researchers found that the median number of pain crises per year was 1.63 in the high-dose group, 2.01 in the low-dose group, and 2.98 in the placebo group. That translates to a 45.3% lower rate for the high-dose group than placebo (P = .01) and a 32.6% lower rate for low-dose than placebo (P = .18).
A total of 36% of the subjects in the high-dose group had no pain crises during the treatment phase, compared with 18% and 17% in the low-dose and placebo groups, respectively.
In a per-protocol analysis of 125 subjects, the researchers found similar numbers for median pain crises and no pain crises with one exception: The rate of annual pain crises was only 8.3% lower for the low-dose group than the placebo (P = .13).
Overall, the researchers wrote, the rates of adverse and serious adverse events were “similar” among all the subjects regardless of their randomized group.
Five patients died during the trial: two from the high dose group, one in the low dose group, and two in the placebo group. Among serious adverse events, pyrexia and pneumonia occurred more frequently in at least one of the crizanlizumab groups than in the placebo group, but their levels were low at zero to three cases of each event in the three groups.
The researchers noted that they didn’t detect any antibody response against crizanlizumab. However, “longer follow-up and monitoring are necessary to ensure that late neutralizing antibodies do not emerge that might limit the ability to administer crizanlizumab on a long-term basis.”
The study was funded by Selexys Pharmaceuticals, which received grants from the National Heart, Lung, and Blood Institute and the Food and Drug Administration’s Orphan Products Grant Program. Dr. Ataga reports personal fees from Selexys Pharmaceuticals. The other authors report various disclosures or none. The complete list of disclosures is available at NEJM.org.
An industry-funded phase II trial has shown that high doses of the experimental drug crizanlizumab significantly reduced the number of dangerous “pain crises” in subjects with sickle cell disease.
The median per-year rate of pain crises was 45.3% lower among those who took the high dose of crizanlizumab, compared with the placebo group (P = .01) More than a third of the subjects who took the high dose reported no pain crises during the treatment phase, more than double the rate among the placebo group.
The trial findings were released at the annual meeting of the American Society of Hematology and published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1611770).
The American Society of Hematology estimates that 70,000-100,000 people in the United States have sickle cell anemia and some patients are treated with hydroxyurea (Hydrea) are available. According to background material provided in the trial report, however, hydroxyurea has limited value, and some patients still face the prospect of pain crises which can lead to end-organ damage, and early death.
The SUSTAIN trial focuses on pain crises, also known as vaso-occlusive and sickle cell crises, which can occur without warning when sickle cells block blood flow and decrease oxygen delivery.
Researchers led by Kenneth I. Ataga, MB, of the University of North Carolina, Chapel Hill, recruited 198 subjects who had sickle cell disease and who had experienced 2-10 pain crises related to their condition over the past year. They randomly assigned 67 subjects to receive a low 2.5-mg/kg dose of crizanlizumab (also known as SelG1), 66 to a high 5.0-mg/kg dose, and 65 to a placebo. Crizanlizumab is an antibody against the molecule P-selectin, whose up-regulation in certain cells and platelets is thought to contribute to vaso-occlusion and sickle cell pain crises.
All the doses were administered intravenously 14 times over a year at sites in Brazil, the United States, and Jamaica. Risk groups for sickle cell include people of African and South American descent, among groups.
The first two doses were loading doses given at 2-week intervals, and the rest were given at 4-week intervals.
Subjects were aged 16-63 years; the median age was 29 for the two crizanlizumab groups and 26 for the placebo group. The percentage of black subjects ranged from 90% to 94% in each group, and the percentage of female subjects ranged from 52% to 58%.
Some subjects, but not all, were taking hydroxyurea. If they were taking the drug, they needed to have been on it for at least 6 months prior to the trial, and at least the last 3 months at a steady dose. Those who didn’t take hydroxyurea weren’t allowed to start taking it.
The researchers found that the median number of pain crises per year was 1.63 in the high-dose group, 2.01 in the low-dose group, and 2.98 in the placebo group. That translates to a 45.3% lower rate for the high-dose group than placebo (P = .01) and a 32.6% lower rate for low-dose than placebo (P = .18).
A total of 36% of the subjects in the high-dose group had no pain crises during the treatment phase, compared with 18% and 17% in the low-dose and placebo groups, respectively.
In a per-protocol analysis of 125 subjects, the researchers found similar numbers for median pain crises and no pain crises with one exception: The rate of annual pain crises was only 8.3% lower for the low-dose group than the placebo (P = .13).
Overall, the researchers wrote, the rates of adverse and serious adverse events were “similar” among all the subjects regardless of their randomized group.
Five patients died during the trial: two from the high dose group, one in the low dose group, and two in the placebo group. Among serious adverse events, pyrexia and pneumonia occurred more frequently in at least one of the crizanlizumab groups than in the placebo group, but their levels were low at zero to three cases of each event in the three groups.
The researchers noted that they didn’t detect any antibody response against crizanlizumab. However, “longer follow-up and monitoring are necessary to ensure that late neutralizing antibodies do not emerge that might limit the ability to administer crizanlizumab on a long-term basis.”
The study was funded by Selexys Pharmaceuticals, which received grants from the National Heart, Lung, and Blood Institute and the Food and Drug Administration’s Orphan Products Grant Program. Dr. Ataga reports personal fees from Selexys Pharmaceuticals. The other authors report various disclosures or none. The complete list of disclosures is available at NEJM.org.
An industry-funded phase II trial has shown that high doses of the experimental drug crizanlizumab significantly reduced the number of dangerous “pain crises” in subjects with sickle cell disease.
The median per-year rate of pain crises was 45.3% lower among those who took the high dose of crizanlizumab, compared with the placebo group (P = .01) More than a third of the subjects who took the high dose reported no pain crises during the treatment phase, more than double the rate among the placebo group.
The trial findings were released at the annual meeting of the American Society of Hematology and published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1611770).
The American Society of Hematology estimates that 70,000-100,000 people in the United States have sickle cell anemia and some patients are treated with hydroxyurea (Hydrea) are available. According to background material provided in the trial report, however, hydroxyurea has limited value, and some patients still face the prospect of pain crises which can lead to end-organ damage, and early death.
The SUSTAIN trial focuses on pain crises, also known as vaso-occlusive and sickle cell crises, which can occur without warning when sickle cells block blood flow and decrease oxygen delivery.
Researchers led by Kenneth I. Ataga, MB, of the University of North Carolina, Chapel Hill, recruited 198 subjects who had sickle cell disease and who had experienced 2-10 pain crises related to their condition over the past year. They randomly assigned 67 subjects to receive a low 2.5-mg/kg dose of crizanlizumab (also known as SelG1), 66 to a high 5.0-mg/kg dose, and 65 to a placebo. Crizanlizumab is an antibody against the molecule P-selectin, whose up-regulation in certain cells and platelets is thought to contribute to vaso-occlusion and sickle cell pain crises.
All the doses were administered intravenously 14 times over a year at sites in Brazil, the United States, and Jamaica. Risk groups for sickle cell include people of African and South American descent, among groups.
The first two doses were loading doses given at 2-week intervals, and the rest were given at 4-week intervals.
Subjects were aged 16-63 years; the median age was 29 for the two crizanlizumab groups and 26 for the placebo group. The percentage of black subjects ranged from 90% to 94% in each group, and the percentage of female subjects ranged from 52% to 58%.
Some subjects, but not all, were taking hydroxyurea. If they were taking the drug, they needed to have been on it for at least 6 months prior to the trial, and at least the last 3 months at a steady dose. Those who didn’t take hydroxyurea weren’t allowed to start taking it.
The researchers found that the median number of pain crises per year was 1.63 in the high-dose group, 2.01 in the low-dose group, and 2.98 in the placebo group. That translates to a 45.3% lower rate for the high-dose group than placebo (P = .01) and a 32.6% lower rate for low-dose than placebo (P = .18).
A total of 36% of the subjects in the high-dose group had no pain crises during the treatment phase, compared with 18% and 17% in the low-dose and placebo groups, respectively.
In a per-protocol analysis of 125 subjects, the researchers found similar numbers for median pain crises and no pain crises with one exception: The rate of annual pain crises was only 8.3% lower for the low-dose group than the placebo (P = .13).
Overall, the researchers wrote, the rates of adverse and serious adverse events were “similar” among all the subjects regardless of their randomized group.
Five patients died during the trial: two from the high dose group, one in the low dose group, and two in the placebo group. Among serious adverse events, pyrexia and pneumonia occurred more frequently in at least one of the crizanlizumab groups than in the placebo group, but their levels were low at zero to three cases of each event in the three groups.
The researchers noted that they didn’t detect any antibody response against crizanlizumab. However, “longer follow-up and monitoring are necessary to ensure that late neutralizing antibodies do not emerge that might limit the ability to administer crizanlizumab on a long-term basis.”
The study was funded by Selexys Pharmaceuticals, which received grants from the National Heart, Lung, and Blood Institute and the Food and Drug Administration’s Orphan Products Grant Program. Dr. Ataga reports personal fees from Selexys Pharmaceuticals. The other authors report various disclosures or none. The complete list of disclosures is available at NEJM.org.
FROM ASH 2016
Key clinical point: High-dose crizanlizumab significantly lowers, but does not eliminate, dangerous ‘pain crises’ that strike sickle cell patients.
Major finding: Patients who took high-dose crizanlizumab had a median of 1.63 pain crises a year versus 2.98 for the placebo group. (P = .01)
Data source: A phase II, 12-month, multicenter, double-blind, randomized, placebo-controlled study of 198 patients with sickle cell disease; 129 subjects completed the trial.
Disclosures: The study was funded by Selexys Pharmaceuticals, which received grants from the National Heart, Lung, and Blood Institute and the FDA’s Orphan Products Grant Program. Dr. Ataga reports personal fees from Selexys Pharmaceuticals. The other authors report various disclosures or none. The complete list of disclosures is available at NEJM.org.
Empagliflozin first antidiabetes drug to gain cardioprotective indication
Empagliflozin is the first antidiabetes medication to be approved for reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease.
The Food and Drug Administration granted the new indication based on the EMPA-REG OUTCOME study, a postmarketing analysis that found that empagliflozin (Jardiance, Boehringer-Ingelheim) reduced the risk of cardiovascular death by 38% when added to standard-of-care type 2 diabetes therapy.
When empagliflozin was approved for type 2 diabetes in 2014, the FDA required an additional postmarketing study to examine its cardiovascular safety. The 48-month, open-label EMPA-REG enrolled more than 7,000 patients who had type 2 diabetes and a high risk of cardiovascular disease.
The study’s big surprise, however, was not empagliflozin’s safety, but its striking cardioprotective qualities. It reduced by 14% the risk of the primary endpoint, a composite of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (N Engl J Med. 2015;373:2117-28).
When examined as individual outcomes in a secondary analysis, empagliflozin significantly reduced the risk of cardiovascular death by 38%. However, risk reductions on the other endpoints were not significant. Nevertheless, experts called empagliflozin’s cardiovascular benefit a potential game-changer for the clinical challenge of managing patients with both disorders.
But the drug barely squeaked by its June FDA approval hearing for the cardioprotective indication, receiving a split 12-11 endorsement from the Endocrinologic and Metabolic Drugs Advisory Committee. The major sticking point was that EMPA-REG was a test of empagliflozin’s cardiovascular safety, not its efficacy, and that cardiovascular death was not a prespecified endpoint.
Although there were no significant cardiovascular safety issues, empagliflozin has been associated with hypotension, serious urinary tract infection, acute kidney injury, and genital infections.
“Cardiovascular disease is a leading cause of death in adults with type 2 diabetes mellitus,” Jean-Marc Guettier, MD, director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, wrote in a press statement. “Availability of antidiabetes therapies that can help people live longer by reducing the risk of cardiovascular death is an important advance for adults with type 2 diabetes.”
[email protected]
On Twitter @alz_gal
Empagliflozin is the first antidiabetes medication to be approved for reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease.
The Food and Drug Administration granted the new indication based on the EMPA-REG OUTCOME study, a postmarketing analysis that found that empagliflozin (Jardiance, Boehringer-Ingelheim) reduced the risk of cardiovascular death by 38% when added to standard-of-care type 2 diabetes therapy.
When empagliflozin was approved for type 2 diabetes in 2014, the FDA required an additional postmarketing study to examine its cardiovascular safety. The 48-month, open-label EMPA-REG enrolled more than 7,000 patients who had type 2 diabetes and a high risk of cardiovascular disease.
The study’s big surprise, however, was not empagliflozin’s safety, but its striking cardioprotective qualities. It reduced by 14% the risk of the primary endpoint, a composite of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (N Engl J Med. 2015;373:2117-28).
When examined as individual outcomes in a secondary analysis, empagliflozin significantly reduced the risk of cardiovascular death by 38%. However, risk reductions on the other endpoints were not significant. Nevertheless, experts called empagliflozin’s cardiovascular benefit a potential game-changer for the clinical challenge of managing patients with both disorders.
But the drug barely squeaked by its June FDA approval hearing for the cardioprotective indication, receiving a split 12-11 endorsement from the Endocrinologic and Metabolic Drugs Advisory Committee. The major sticking point was that EMPA-REG was a test of empagliflozin’s cardiovascular safety, not its efficacy, and that cardiovascular death was not a prespecified endpoint.
Although there were no significant cardiovascular safety issues, empagliflozin has been associated with hypotension, serious urinary tract infection, acute kidney injury, and genital infections.
“Cardiovascular disease is a leading cause of death in adults with type 2 diabetes mellitus,” Jean-Marc Guettier, MD, director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, wrote in a press statement. “Availability of antidiabetes therapies that can help people live longer by reducing the risk of cardiovascular death is an important advance for adults with type 2 diabetes.”
[email protected]
On Twitter @alz_gal
Empagliflozin is the first antidiabetes medication to be approved for reducing the risk of cardiovascular death in patients with type 2 diabetes and concomitant cardiovascular disease.
The Food and Drug Administration granted the new indication based on the EMPA-REG OUTCOME study, a postmarketing analysis that found that empagliflozin (Jardiance, Boehringer-Ingelheim) reduced the risk of cardiovascular death by 38% when added to standard-of-care type 2 diabetes therapy.
When empagliflozin was approved for type 2 diabetes in 2014, the FDA required an additional postmarketing study to examine its cardiovascular safety. The 48-month, open-label EMPA-REG enrolled more than 7,000 patients who had type 2 diabetes and a high risk of cardiovascular disease.
The study’s big surprise, however, was not empagliflozin’s safety, but its striking cardioprotective qualities. It reduced by 14% the risk of the primary endpoint, a composite of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (N Engl J Med. 2015;373:2117-28).
When examined as individual outcomes in a secondary analysis, empagliflozin significantly reduced the risk of cardiovascular death by 38%. However, risk reductions on the other endpoints were not significant. Nevertheless, experts called empagliflozin’s cardiovascular benefit a potential game-changer for the clinical challenge of managing patients with both disorders.
But the drug barely squeaked by its June FDA approval hearing for the cardioprotective indication, receiving a split 12-11 endorsement from the Endocrinologic and Metabolic Drugs Advisory Committee. The major sticking point was that EMPA-REG was a test of empagliflozin’s cardiovascular safety, not its efficacy, and that cardiovascular death was not a prespecified endpoint.
Although there were no significant cardiovascular safety issues, empagliflozin has been associated with hypotension, serious urinary tract infection, acute kidney injury, and genital infections.
“Cardiovascular disease is a leading cause of death in adults with type 2 diabetes mellitus,” Jean-Marc Guettier, MD, director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, wrote in a press statement. “Availability of antidiabetes therapies that can help people live longer by reducing the risk of cardiovascular death is an important advance for adults with type 2 diabetes.”
[email protected]
On Twitter @alz_gal
U.S. okay looms for third drug-coated PAD balloon
WASHINGTON – Good pivotal-trial performance of a drug-coated balloon for treating superficial femoral and popliteal artery stenoses raised the prospect that it might soon be the third drug-coated balloon on the U.S. market, creating an opportunity for lower prices and competitive improvements for an increasingly used device.
“Having another drug-coated balloon would be useful for several reasons,” commented William A. Gray, MD, during the Transcatheter Cardiovascular Therapeutics annual meeting. The competition should mean lower cost, and accumulating reports on performance might identify a specific drug-coated balloon as most effective. Drug-coated balloons for peripheral artery stenoses “have been introduced over the past 2 years, with a significant increase in use during that time. It’s still not a majority of patients, but it’s increasing,” said Dr. Gray, chief of the division of cardiovascular disease at Main Line Health and president of Main Line Health’s Lankenau Heart Institute in Wynnewood, Pa.
The ILLUMENATE pivotal trial enrolled 300 patients at 43 centers in the United States and Europe. Patients had Rutherford 2, 3 or 4 disease, and averaged about 69 years old. More than 60% had class 3 disease and another 30% had class 2 disease.
The study’s primary safety endpoint was freedom from device- or procedure-related death to 30 days, and freedom from clinically drived target lesion revascularization at 12 months, a 92% rate in the 200 patients who had PTA with the Stellarex drug-coated balloon and 83% in the 100 controls who had PTA with an uncoated balloon. This statistically significant eight percentage point difference met the prespecified criteria for safety superiority.
The two drug-coated balloons already approved for U.S. use are the Lutonix and the IN.PACT Admiral.
“All the drug-coated balloons have worked well. It’s pretty exciting to see them work. It will be interesting to compare them against each other. We need side-by-side comparisons,” commented Craig M. Walker, MD, an interventional cardiologist in Houma, La. and a discussant for Dr. Lyden’s report.
The ILLUMENATE Pivotal trial was funded by Spectranetics, the company that is developing the Stellarex drug-coated balloon. Dr. Lyden has been a consultant to Spectranetics and to Biomet, Endologix, and TVA Medical. He received research support from Spectranetics and several other companies. Dr. Gray has been a consultant to Abbott Vascular, Boston Scientific, Cook, Medtronic, and Shockwave. He has received research support from Gore and Intact Vascular. Dr. Walker has been a consultant to Spectranetics as well as to Abbott Vascular, Bard, Boston Scientific, Cook, Gore, and Medtronic.
[email protected]
On Twitter @mitchelzoler
It’s good to have competition among various models of drug-eluting balloons because it will help drive costs down and help drive additional improvements in device design. We win by having a third good drug-coated balloon option available.
Drug-coated balloons are increasingly used in routine U.S. practice. A recent report showed that one of the drug-coated balloons already on the U.S. market outperformed balloon angioplasty out to 3 years of follow-up. Drug-coated balloons hold an advantage over stents by leaving nothing behind. Another attraction of drug-coated balloons is that they can potentially be used as an adjunct to additional interventions for complex lesions, such as atherectomy.
So far, we have not seen a clear winner for safety and efficacy among the two drug-coated balloons already on the U.S. market and this new drug-coated balloon, which may soon be the third option for U.S. practice. But there is no single class effect from these drug-coated balloons; they must be evaluated individually.
D. Christopher Metzger, MD, is an interventional cardiologist and director of cardiac and peripheral vascular catheterization labs at the Wellmont CVA Heart Institute in Kingsport, Tenn. He has been a consultant to and received honoraria from Abbott Vascular, Bard, and Medtronic. He made these comments in an interview.
It’s good to have competition among various models of drug-eluting balloons because it will help drive costs down and help drive additional improvements in device design. We win by having a third good drug-coated balloon option available.
Drug-coated balloons are increasingly used in routine U.S. practice. A recent report showed that one of the drug-coated balloons already on the U.S. market outperformed balloon angioplasty out to 3 years of follow-up. Drug-coated balloons hold an advantage over stents by leaving nothing behind. Another attraction of drug-coated balloons is that they can potentially be used as an adjunct to additional interventions for complex lesions, such as atherectomy.
So far, we have not seen a clear winner for safety and efficacy among the two drug-coated balloons already on the U.S. market and this new drug-coated balloon, which may soon be the third option for U.S. practice. But there is no single class effect from these drug-coated balloons; they must be evaluated individually.
D. Christopher Metzger, MD, is an interventional cardiologist and director of cardiac and peripheral vascular catheterization labs at the Wellmont CVA Heart Institute in Kingsport, Tenn. He has been a consultant to and received honoraria from Abbott Vascular, Bard, and Medtronic. He made these comments in an interview.
It’s good to have competition among various models of drug-eluting balloons because it will help drive costs down and help drive additional improvements in device design. We win by having a third good drug-coated balloon option available.
Drug-coated balloons are increasingly used in routine U.S. practice. A recent report showed that one of the drug-coated balloons already on the U.S. market outperformed balloon angioplasty out to 3 years of follow-up. Drug-coated balloons hold an advantage over stents by leaving nothing behind. Another attraction of drug-coated balloons is that they can potentially be used as an adjunct to additional interventions for complex lesions, such as atherectomy.
So far, we have not seen a clear winner for safety and efficacy among the two drug-coated balloons already on the U.S. market and this new drug-coated balloon, which may soon be the third option for U.S. practice. But there is no single class effect from these drug-coated balloons; they must be evaluated individually.
D. Christopher Metzger, MD, is an interventional cardiologist and director of cardiac and peripheral vascular catheterization labs at the Wellmont CVA Heart Institute in Kingsport, Tenn. He has been a consultant to and received honoraria from Abbott Vascular, Bard, and Medtronic. He made these comments in an interview.
WASHINGTON – Good pivotal-trial performance of a drug-coated balloon for treating superficial femoral and popliteal artery stenoses raised the prospect that it might soon be the third drug-coated balloon on the U.S. market, creating an opportunity for lower prices and competitive improvements for an increasingly used device.
“Having another drug-coated balloon would be useful for several reasons,” commented William A. Gray, MD, during the Transcatheter Cardiovascular Therapeutics annual meeting. The competition should mean lower cost, and accumulating reports on performance might identify a specific drug-coated balloon as most effective. Drug-coated balloons for peripheral artery stenoses “have been introduced over the past 2 years, with a significant increase in use during that time. It’s still not a majority of patients, but it’s increasing,” said Dr. Gray, chief of the division of cardiovascular disease at Main Line Health and president of Main Line Health’s Lankenau Heart Institute in Wynnewood, Pa.
The ILLUMENATE pivotal trial enrolled 300 patients at 43 centers in the United States and Europe. Patients had Rutherford 2, 3 or 4 disease, and averaged about 69 years old. More than 60% had class 3 disease and another 30% had class 2 disease.
The study’s primary safety endpoint was freedom from device- or procedure-related death to 30 days, and freedom from clinically drived target lesion revascularization at 12 months, a 92% rate in the 200 patients who had PTA with the Stellarex drug-coated balloon and 83% in the 100 controls who had PTA with an uncoated balloon. This statistically significant eight percentage point difference met the prespecified criteria for safety superiority.
The two drug-coated balloons already approved for U.S. use are the Lutonix and the IN.PACT Admiral.
“All the drug-coated balloons have worked well. It’s pretty exciting to see them work. It will be interesting to compare them against each other. We need side-by-side comparisons,” commented Craig M. Walker, MD, an interventional cardiologist in Houma, La. and a discussant for Dr. Lyden’s report.
The ILLUMENATE Pivotal trial was funded by Spectranetics, the company that is developing the Stellarex drug-coated balloon. Dr. Lyden has been a consultant to Spectranetics and to Biomet, Endologix, and TVA Medical. He received research support from Spectranetics and several other companies. Dr. Gray has been a consultant to Abbott Vascular, Boston Scientific, Cook, Medtronic, and Shockwave. He has received research support from Gore and Intact Vascular. Dr. Walker has been a consultant to Spectranetics as well as to Abbott Vascular, Bard, Boston Scientific, Cook, Gore, and Medtronic.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Good pivotal-trial performance of a drug-coated balloon for treating superficial femoral and popliteal artery stenoses raised the prospect that it might soon be the third drug-coated balloon on the U.S. market, creating an opportunity for lower prices and competitive improvements for an increasingly used device.
“Having another drug-coated balloon would be useful for several reasons,” commented William A. Gray, MD, during the Transcatheter Cardiovascular Therapeutics annual meeting. The competition should mean lower cost, and accumulating reports on performance might identify a specific drug-coated balloon as most effective. Drug-coated balloons for peripheral artery stenoses “have been introduced over the past 2 years, with a significant increase in use during that time. It’s still not a majority of patients, but it’s increasing,” said Dr. Gray, chief of the division of cardiovascular disease at Main Line Health and president of Main Line Health’s Lankenau Heart Institute in Wynnewood, Pa.
The ILLUMENATE pivotal trial enrolled 300 patients at 43 centers in the United States and Europe. Patients had Rutherford 2, 3 or 4 disease, and averaged about 69 years old. More than 60% had class 3 disease and another 30% had class 2 disease.
The study’s primary safety endpoint was freedom from device- or procedure-related death to 30 days, and freedom from clinically drived target lesion revascularization at 12 months, a 92% rate in the 200 patients who had PTA with the Stellarex drug-coated balloon and 83% in the 100 controls who had PTA with an uncoated balloon. This statistically significant eight percentage point difference met the prespecified criteria for safety superiority.
The two drug-coated balloons already approved for U.S. use are the Lutonix and the IN.PACT Admiral.
“All the drug-coated balloons have worked well. It’s pretty exciting to see them work. It will be interesting to compare them against each other. We need side-by-side comparisons,” commented Craig M. Walker, MD, an interventional cardiologist in Houma, La. and a discussant for Dr. Lyden’s report.
The ILLUMENATE Pivotal trial was funded by Spectranetics, the company that is developing the Stellarex drug-coated balloon. Dr. Lyden has been a consultant to Spectranetics and to Biomet, Endologix, and TVA Medical. He received research support from Spectranetics and several other companies. Dr. Gray has been a consultant to Abbott Vascular, Boston Scientific, Cook, Medtronic, and Shockwave. He has received research support from Gore and Intact Vascular. Dr. Walker has been a consultant to Spectranetics as well as to Abbott Vascular, Bard, Boston Scientific, Cook, Gore, and Medtronic.
[email protected]
On Twitter @mitchelzoler
Key clinical point:
Major finding: The primary efficacy endpoint occurred in 76% of patients in the drug-coated balloon arm and 58% of controls.
Data source: The ILLUMENATE pivotal trial, which enrolled 300 patients at 63 U.S. and European centers.
Disclosures: The ILLUMENATE pivotal trial was funded by Spectranetics, the company that is developing the Stellarex drug-coated balloon. Dr. Lyden has been a consultant to Spectranetics and to Biomet, Endologix, and TVA Medical. He received research support from Spectranetics and several other companies. Dr. Gray has been a consultant to Abbott Vascular, Boston Scientific, Cook, Medtronic, and Shockwave. He has received research support from Gore and Intact Vascular. Dr. Walker has been a consultant to Spectranetics as well as to Abbott Vascular, Bard, Boston Scientific, Cook, Gore, and Medtronic.
Thyme
Native to the western Mediterranean, Thymus vulgaris (one of approximately 300 Thymus species) is a small bush used for centuries as a spice and in medicine, particularly to treat bronchitis.1Thymus species are among the wild and cultivated species used in traditional medicine in Bosnia and Herzegovina for various indications, including skin disorders.2 Thyme essential oil is a natural compound generally recognized as safe by the Food and Drug Administration, with demonstrated antibacterial, antifungal, and antispasmodic activities.3,4 Several other biologic activities have been associated with the polyphenol-rich herb, many of which have dermatologic implications. Notably, the essential oil of thyme and thymol, a key constituent of thyme, are known to act as skin sensitizers and allergens.5
Photoprotective activity
Recently, Sun et al. showed that UVB-induced skin damage was attenuated by treating hairless mice (HR-1) with T. vulgaris, as indicated by reduced matrix metalloproteinases and elevated collagen synthesis. In cultured normal human dermal fibroblasts, the investigators found that T. vulgaris blocked UVB-induced reactive oxygen species and lactate dehydrogenase, and dose-dependently yielded increases in glutathione, NAD(P)H: quinone oxidoreductase 1, and heme oxygenase-1. Further, the botanical significantly reduced UVB-induced phosphorylation of mitogen-activated protein kinases. The investigators concluded that T. vulgaris has potential for use in preventing skin damage caused by UV radiation–induced oxidative stress.6
Thyme also was demonstrated by Cornaghi et al. in 2016 to exert a protective effect on normal human skin explants obtained from seven young healthy women that were treated 1 hour before UVB irradiation.7
In 2015, Calò et al. evaluated the protective effects of a dry extract from T. vulgaris and its primary synthetic constituent thymol against UVA- and UVB-induced oxidative and genotoxic damage in the keratinocyte cell line NCTC 2544. Both thymol and T. vulgaris suppressed reactive oxygen species production in UVA- and UVB-treated cells, but lowered malondialdehyde synthesis only in cells treated with UVA.8
Antioxidant activity
In 2007, Wei and Shibamoto reported that thyme essential oil mixed with clove oil exhibited over a 90% inhibitory effect against the formation of malondialdehyde. They speculated that the presence of thymol and eugenol might account for the strong antioxidant activity displayed by the thyme/clove leaf combination.9 The investigators previously observed antioxidant activities exhibited by volatile extracts isolated from thyme (as well as various other herbs and spices) using aldehyde/carboxylic acid as well as conjugated diene assays.10 The antioxidant activity of thyme also was demonstrated by Miura et al. using the oil stability index method.11
Antimicrobial activity
In 2011, Sienkiewicz et al. reported that the oil of T. vulgaris displayed potent activity against clinical bacterial strains of Staphylococcus, Enterococcus, Escherichia, and Pseudomonas genera. In addition, thyme essential oil exhibited efficacy against tested antibiotic-resistant strains of bacteria.12 The following year, Sienkiewicz et al. assessed the antimicrobial activity of thyme essential oil against clinical multidrug-resistant strains of Staphylococcus, Enterococcus, Escherichia, and Pseudomonas, finding that it potently suppressed the growth of each.13
Potential cutaneous indications: atopic dermatitis, leishmaniasis, eczema, hair growth
In 2015, Seo and Jeong showed that lavender oil, thyme oil, and a blend of the two were all effective in reducing the symptoms of atopic dermatitis in mice. The researchers suggested that developing treatments with these oils for human patients with atopic dermatitis is warranted.14
Nilforoushzadeh et al. found in 2008 that herbal extracts of T. vulgaris and Achillea millefolium (yarrow), as well as propolis hydroalcoholic extracts, were effective in treating cutaneous leishmaniasis in mice and recommended the study of these extracts alone or in combination in human trials.15
A two-arm, randomized, double-blind, placebo-controlled trial conducted by Shimelis et al. in 2012 evaluated the efficacy of a 3% thyme essential oil antifungal cream and a 10% chamomile extract cream in the treatment of eczemalike lesions. Complete healing was achieved in 10 patients (66.5%) treated with the thyme cream, compared with four patients (28.5%) in the placebo group. Although no significant differences were observed between the active chamomile group and placebo, an appreciable number of subjects improved or healed. The investigators concluded that their findings from this small study suggest that, while more research is needed, a 3% thyme essential oil cream appears to be an inexpensive and readily available option to treat mild to moderate cutaneous conditions, including fungal infections, pityriasis alba, and eczema.16
In 2013, Rastegar et al. found that the combination of herbal extracts (including thyme) and platelet-rich plasma induced significant proliferation of human dermal papilla cells by regulating extracellular signal-regulated kinase (ERK) and Akt (protein kinase B). They concluded that their findings suggest the potential for developing combination therapies intended to improve hair growth.17
Insect repellent activity
In a 2016 study by Gutiérrez et al., the essential oil of T. vulgaris was found to be effective against Pediculus humanus capitis (head lice) adults and eggs. The researchers concluded that T. vulgaris achieves a strong knockdown and mortality rate in adult head lice and toxicity in the eggs after 21 minutes of application at a low concentration.18
In a small 1999 study by Barnard of the repellency to Aedes aegypti and Anopheles albimanus of various concentrations and combinations of five essential oils (Bourbon geranium, cedarwood, clove, peppermint, and thyme) applied to human skin, thyme and clove oils were found to be the most effective mosquito repellents. The author noted that thyme oil (as well as clove and peppermint oils) can irritate the skin and the odor of thyme and clove oils, at concentrations of 25%, or more were deemed unacceptable by the two participants in the study.19
Three years later, Choi et al. found that the essential oil of T. vulgaris also repelled adult mosquitoes (Culex pipiens pallens) on hairless mice and displayed potent repellent activity.20
Melanoma
In 2005, Carrera et al. reported a case of long-term complete remission of cutaneous melanoma metastases in a 73-year-old white woman who consumed a dried thyme herbal tea and thyme topical applications in compresses.4 An association between thyme and melanoma has not been reported in the subsequent literature.
Conclusion
Thyme has a long history of culinary and medical uses. Its antimicrobial and antioxidant activity are well documented. While there is reason to consider the potential applications of thyme for dermatologic conditions, much more research is necessary to determine its viability for such purposes.
References
1. An Illustrated Guide to 101 Medicinal Herbs: Their History, Use, Recommended Dosages, and Cautions (Loveland, Colo.: Interweave Press, 1998, pp. 198-9).
2. J Ethnopharmacol. 2010 Aug 19;131(1):33-55.
3. J Food Sci. 2014 May;79(5):M903-10.
4. J Am Acad Dermatol. 2005 Apr;52(4):713-5.
5. Nat Neurosci. 2006 May;9(5):628-35.
6. J Cell Mol Med. 2016 Sep 19. doi: 10.1111/jcmm.12968. [Epub ahead of print]
7. Cells Tissues Organs. 2016;201(3):180-92.
8. Mutat Res Genet Toxicol Environ Mutagen. 2015 Sep;791:30-7.
9. Cutan Ocul Toxicol. 2007;26(3):227-33.
10. J Agric Food Chem. 2002 Aug 14;50(17):4947-52.
11. J Agric Food Chem. 2002 Mar 27;50(7):1845-51.
12. Med Chem. 2011 Nov;7(6):674-89.
13. Microb Drug Resist. 2012 Apr;18(2):137-48.
14. J Korean Acad Nurs. 2015 Jun;45(3):367-77.
15. J Vector Borne Dis. 2008 Dec;45(4):301-6.
16. Int J Dermatol. 2012 Jul;51(7):790-5.
17. J Cosmet Dermatol. 2013 Jun;12(2):116-22.
18. Parasitol Res. 2016 Feb;115(2):633-41.
19. J Med Entomol. 1999 Sep;36(5):625-9.
20. J Am Mosq Control Assoc. 2002 Dec;18(4):348-51.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Native to the western Mediterranean, Thymus vulgaris (one of approximately 300 Thymus species) is a small bush used for centuries as a spice and in medicine, particularly to treat bronchitis.1Thymus species are among the wild and cultivated species used in traditional medicine in Bosnia and Herzegovina for various indications, including skin disorders.2 Thyme essential oil is a natural compound generally recognized as safe by the Food and Drug Administration, with demonstrated antibacterial, antifungal, and antispasmodic activities.3,4 Several other biologic activities have been associated with the polyphenol-rich herb, many of which have dermatologic implications. Notably, the essential oil of thyme and thymol, a key constituent of thyme, are known to act as skin sensitizers and allergens.5
Photoprotective activity
Recently, Sun et al. showed that UVB-induced skin damage was attenuated by treating hairless mice (HR-1) with T. vulgaris, as indicated by reduced matrix metalloproteinases and elevated collagen synthesis. In cultured normal human dermal fibroblasts, the investigators found that T. vulgaris blocked UVB-induced reactive oxygen species and lactate dehydrogenase, and dose-dependently yielded increases in glutathione, NAD(P)H: quinone oxidoreductase 1, and heme oxygenase-1. Further, the botanical significantly reduced UVB-induced phosphorylation of mitogen-activated protein kinases. The investigators concluded that T. vulgaris has potential for use in preventing skin damage caused by UV radiation–induced oxidative stress.6
Thyme also was demonstrated by Cornaghi et al. in 2016 to exert a protective effect on normal human skin explants obtained from seven young healthy women that were treated 1 hour before UVB irradiation.7
In 2015, Calò et al. evaluated the protective effects of a dry extract from T. vulgaris and its primary synthetic constituent thymol against UVA- and UVB-induced oxidative and genotoxic damage in the keratinocyte cell line NCTC 2544. Both thymol and T. vulgaris suppressed reactive oxygen species production in UVA- and UVB-treated cells, but lowered malondialdehyde synthesis only in cells treated with UVA.8
Antioxidant activity
In 2007, Wei and Shibamoto reported that thyme essential oil mixed with clove oil exhibited over a 90% inhibitory effect against the formation of malondialdehyde. They speculated that the presence of thymol and eugenol might account for the strong antioxidant activity displayed by the thyme/clove leaf combination.9 The investigators previously observed antioxidant activities exhibited by volatile extracts isolated from thyme (as well as various other herbs and spices) using aldehyde/carboxylic acid as well as conjugated diene assays.10 The antioxidant activity of thyme also was demonstrated by Miura et al. using the oil stability index method.11
Antimicrobial activity
In 2011, Sienkiewicz et al. reported that the oil of T. vulgaris displayed potent activity against clinical bacterial strains of Staphylococcus, Enterococcus, Escherichia, and Pseudomonas genera. In addition, thyme essential oil exhibited efficacy against tested antibiotic-resistant strains of bacteria.12 The following year, Sienkiewicz et al. assessed the antimicrobial activity of thyme essential oil against clinical multidrug-resistant strains of Staphylococcus, Enterococcus, Escherichia, and Pseudomonas, finding that it potently suppressed the growth of each.13
Potential cutaneous indications: atopic dermatitis, leishmaniasis, eczema, hair growth
In 2015, Seo and Jeong showed that lavender oil, thyme oil, and a blend of the two were all effective in reducing the symptoms of atopic dermatitis in mice. The researchers suggested that developing treatments with these oils for human patients with atopic dermatitis is warranted.14
Nilforoushzadeh et al. found in 2008 that herbal extracts of T. vulgaris and Achillea millefolium (yarrow), as well as propolis hydroalcoholic extracts, were effective in treating cutaneous leishmaniasis in mice and recommended the study of these extracts alone or in combination in human trials.15
A two-arm, randomized, double-blind, placebo-controlled trial conducted by Shimelis et al. in 2012 evaluated the efficacy of a 3% thyme essential oil antifungal cream and a 10% chamomile extract cream in the treatment of eczemalike lesions. Complete healing was achieved in 10 patients (66.5%) treated with the thyme cream, compared with four patients (28.5%) in the placebo group. Although no significant differences were observed between the active chamomile group and placebo, an appreciable number of subjects improved or healed. The investigators concluded that their findings from this small study suggest that, while more research is needed, a 3% thyme essential oil cream appears to be an inexpensive and readily available option to treat mild to moderate cutaneous conditions, including fungal infections, pityriasis alba, and eczema.16
In 2013, Rastegar et al. found that the combination of herbal extracts (including thyme) and platelet-rich plasma induced significant proliferation of human dermal papilla cells by regulating extracellular signal-regulated kinase (ERK) and Akt (protein kinase B). They concluded that their findings suggest the potential for developing combination therapies intended to improve hair growth.17
Insect repellent activity
In a 2016 study by Gutiérrez et al., the essential oil of T. vulgaris was found to be effective against Pediculus humanus capitis (head lice) adults and eggs. The researchers concluded that T. vulgaris achieves a strong knockdown and mortality rate in adult head lice and toxicity in the eggs after 21 minutes of application at a low concentration.18
In a small 1999 study by Barnard of the repellency to Aedes aegypti and Anopheles albimanus of various concentrations and combinations of five essential oils (Bourbon geranium, cedarwood, clove, peppermint, and thyme) applied to human skin, thyme and clove oils were found to be the most effective mosquito repellents. The author noted that thyme oil (as well as clove and peppermint oils) can irritate the skin and the odor of thyme and clove oils, at concentrations of 25%, or more were deemed unacceptable by the two participants in the study.19
Three years later, Choi et al. found that the essential oil of T. vulgaris also repelled adult mosquitoes (Culex pipiens pallens) on hairless mice and displayed potent repellent activity.20
Melanoma
In 2005, Carrera et al. reported a case of long-term complete remission of cutaneous melanoma metastases in a 73-year-old white woman who consumed a dried thyme herbal tea and thyme topical applications in compresses.4 An association between thyme and melanoma has not been reported in the subsequent literature.
Conclusion
Thyme has a long history of culinary and medical uses. Its antimicrobial and antioxidant activity are well documented. While there is reason to consider the potential applications of thyme for dermatologic conditions, much more research is necessary to determine its viability for such purposes.
References
1. An Illustrated Guide to 101 Medicinal Herbs: Their History, Use, Recommended Dosages, and Cautions (Loveland, Colo.: Interweave Press, 1998, pp. 198-9).
2. J Ethnopharmacol. 2010 Aug 19;131(1):33-55.
3. J Food Sci. 2014 May;79(5):M903-10.
4. J Am Acad Dermatol. 2005 Apr;52(4):713-5.
5. Nat Neurosci. 2006 May;9(5):628-35.
6. J Cell Mol Med. 2016 Sep 19. doi: 10.1111/jcmm.12968. [Epub ahead of print]
7. Cells Tissues Organs. 2016;201(3):180-92.
8. Mutat Res Genet Toxicol Environ Mutagen. 2015 Sep;791:30-7.
9. Cutan Ocul Toxicol. 2007;26(3):227-33.
10. J Agric Food Chem. 2002 Aug 14;50(17):4947-52.
11. J Agric Food Chem. 2002 Mar 27;50(7):1845-51.
12. Med Chem. 2011 Nov;7(6):674-89.
13. Microb Drug Resist. 2012 Apr;18(2):137-48.
14. J Korean Acad Nurs. 2015 Jun;45(3):367-77.
15. J Vector Borne Dis. 2008 Dec;45(4):301-6.
16. Int J Dermatol. 2012 Jul;51(7):790-5.
17. J Cosmet Dermatol. 2013 Jun;12(2):116-22.
18. Parasitol Res. 2016 Feb;115(2):633-41.
19. J Med Entomol. 1999 Sep;36(5):625-9.
20. J Am Mosq Control Assoc. 2002 Dec;18(4):348-51.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Native to the western Mediterranean, Thymus vulgaris (one of approximately 300 Thymus species) is a small bush used for centuries as a spice and in medicine, particularly to treat bronchitis.1Thymus species are among the wild and cultivated species used in traditional medicine in Bosnia and Herzegovina for various indications, including skin disorders.2 Thyme essential oil is a natural compound generally recognized as safe by the Food and Drug Administration, with demonstrated antibacterial, antifungal, and antispasmodic activities.3,4 Several other biologic activities have been associated with the polyphenol-rich herb, many of which have dermatologic implications. Notably, the essential oil of thyme and thymol, a key constituent of thyme, are known to act as skin sensitizers and allergens.5
Photoprotective activity
Recently, Sun et al. showed that UVB-induced skin damage was attenuated by treating hairless mice (HR-1) with T. vulgaris, as indicated by reduced matrix metalloproteinases and elevated collagen synthesis. In cultured normal human dermal fibroblasts, the investigators found that T. vulgaris blocked UVB-induced reactive oxygen species and lactate dehydrogenase, and dose-dependently yielded increases in glutathione, NAD(P)H: quinone oxidoreductase 1, and heme oxygenase-1. Further, the botanical significantly reduced UVB-induced phosphorylation of mitogen-activated protein kinases. The investigators concluded that T. vulgaris has potential for use in preventing skin damage caused by UV radiation–induced oxidative stress.6
Thyme also was demonstrated by Cornaghi et al. in 2016 to exert a protective effect on normal human skin explants obtained from seven young healthy women that were treated 1 hour before UVB irradiation.7
In 2015, Calò et al. evaluated the protective effects of a dry extract from T. vulgaris and its primary synthetic constituent thymol against UVA- and UVB-induced oxidative and genotoxic damage in the keratinocyte cell line NCTC 2544. Both thymol and T. vulgaris suppressed reactive oxygen species production in UVA- and UVB-treated cells, but lowered malondialdehyde synthesis only in cells treated with UVA.8
Antioxidant activity
In 2007, Wei and Shibamoto reported that thyme essential oil mixed with clove oil exhibited over a 90% inhibitory effect against the formation of malondialdehyde. They speculated that the presence of thymol and eugenol might account for the strong antioxidant activity displayed by the thyme/clove leaf combination.9 The investigators previously observed antioxidant activities exhibited by volatile extracts isolated from thyme (as well as various other herbs and spices) using aldehyde/carboxylic acid as well as conjugated diene assays.10 The antioxidant activity of thyme also was demonstrated by Miura et al. using the oil stability index method.11
Antimicrobial activity
In 2011, Sienkiewicz et al. reported that the oil of T. vulgaris displayed potent activity against clinical bacterial strains of Staphylococcus, Enterococcus, Escherichia, and Pseudomonas genera. In addition, thyme essential oil exhibited efficacy against tested antibiotic-resistant strains of bacteria.12 The following year, Sienkiewicz et al. assessed the antimicrobial activity of thyme essential oil against clinical multidrug-resistant strains of Staphylococcus, Enterococcus, Escherichia, and Pseudomonas, finding that it potently suppressed the growth of each.13
Potential cutaneous indications: atopic dermatitis, leishmaniasis, eczema, hair growth
In 2015, Seo and Jeong showed that lavender oil, thyme oil, and a blend of the two were all effective in reducing the symptoms of atopic dermatitis in mice. The researchers suggested that developing treatments with these oils for human patients with atopic dermatitis is warranted.14
Nilforoushzadeh et al. found in 2008 that herbal extracts of T. vulgaris and Achillea millefolium (yarrow), as well as propolis hydroalcoholic extracts, were effective in treating cutaneous leishmaniasis in mice and recommended the study of these extracts alone or in combination in human trials.15
A two-arm, randomized, double-blind, placebo-controlled trial conducted by Shimelis et al. in 2012 evaluated the efficacy of a 3% thyme essential oil antifungal cream and a 10% chamomile extract cream in the treatment of eczemalike lesions. Complete healing was achieved in 10 patients (66.5%) treated with the thyme cream, compared with four patients (28.5%) in the placebo group. Although no significant differences were observed between the active chamomile group and placebo, an appreciable number of subjects improved or healed. The investigators concluded that their findings from this small study suggest that, while more research is needed, a 3% thyme essential oil cream appears to be an inexpensive and readily available option to treat mild to moderate cutaneous conditions, including fungal infections, pityriasis alba, and eczema.16
In 2013, Rastegar et al. found that the combination of herbal extracts (including thyme) and platelet-rich plasma induced significant proliferation of human dermal papilla cells by regulating extracellular signal-regulated kinase (ERK) and Akt (protein kinase B). They concluded that their findings suggest the potential for developing combination therapies intended to improve hair growth.17
Insect repellent activity
In a 2016 study by Gutiérrez et al., the essential oil of T. vulgaris was found to be effective against Pediculus humanus capitis (head lice) adults and eggs. The researchers concluded that T. vulgaris achieves a strong knockdown and mortality rate in adult head lice and toxicity in the eggs after 21 minutes of application at a low concentration.18
In a small 1999 study by Barnard of the repellency to Aedes aegypti and Anopheles albimanus of various concentrations and combinations of five essential oils (Bourbon geranium, cedarwood, clove, peppermint, and thyme) applied to human skin, thyme and clove oils were found to be the most effective mosquito repellents. The author noted that thyme oil (as well as clove and peppermint oils) can irritate the skin and the odor of thyme and clove oils, at concentrations of 25%, or more were deemed unacceptable by the two participants in the study.19
Three years later, Choi et al. found that the essential oil of T. vulgaris also repelled adult mosquitoes (Culex pipiens pallens) on hairless mice and displayed potent repellent activity.20
Melanoma
In 2005, Carrera et al. reported a case of long-term complete remission of cutaneous melanoma metastases in a 73-year-old white woman who consumed a dried thyme herbal tea and thyme topical applications in compresses.4 An association between thyme and melanoma has not been reported in the subsequent literature.
Conclusion
Thyme has a long history of culinary and medical uses. Its antimicrobial and antioxidant activity are well documented. While there is reason to consider the potential applications of thyme for dermatologic conditions, much more research is necessary to determine its viability for such purposes.
References
1. An Illustrated Guide to 101 Medicinal Herbs: Their History, Use, Recommended Dosages, and Cautions (Loveland, Colo.: Interweave Press, 1998, pp. 198-9).
2. J Ethnopharmacol. 2010 Aug 19;131(1):33-55.
3. J Food Sci. 2014 May;79(5):M903-10.
4. J Am Acad Dermatol. 2005 Apr;52(4):713-5.
5. Nat Neurosci. 2006 May;9(5):628-35.
6. J Cell Mol Med. 2016 Sep 19. doi: 10.1111/jcmm.12968. [Epub ahead of print]
7. Cells Tissues Organs. 2016;201(3):180-92.
8. Mutat Res Genet Toxicol Environ Mutagen. 2015 Sep;791:30-7.
9. Cutan Ocul Toxicol. 2007;26(3):227-33.
10. J Agric Food Chem. 2002 Aug 14;50(17):4947-52.
11. J Agric Food Chem. 2002 Mar 27;50(7):1845-51.
12. Med Chem. 2011 Nov;7(6):674-89.
13. Microb Drug Resist. 2012 Apr;18(2):137-48.
14. J Korean Acad Nurs. 2015 Jun;45(3):367-77.
15. J Vector Borne Dis. 2008 Dec;45(4):301-6.
16. Int J Dermatol. 2012 Jul;51(7):790-5.
17. J Cosmet Dermatol. 2013 Jun;12(2):116-22.
18. Parasitol Res. 2016 Feb;115(2):633-41.
19. J Med Entomol. 1999 Sep;36(5):625-9.
20. J Am Mosq Control Assoc. 2002 Dec;18(4):348-51.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Sofosbuvir, daclatasvir combo best treatment for HCV cryoglobulinemia vasculitis
WASHINGTON – A combined regimen of sofosbuvir and daclatasvir is the best option to treat patients with hepatitis C virus infections experiencing cryoglobulinemia vasculitis, according to the findings of a new study presented at the annual meeting of the American College of Rheumatology.
“The HCV cryoglobulinemia vasculitis is a very important vasculitis because it represents 5% of chronically infected HCV patients in the world,” explained David Saadoun, MD, of Sorbonne Universities, Paris. “It’s sometimes a life-threatening vasculitis because patients may develop inflammation [so] there’s a need for very active and well-tolerated treatment.”
The primary endpoint – complete response to treatment at the end of the regimen – was achieved in 91% of subjects by the end of 24 weeks. Furthermore, 50% of patients experienced complete immunological response, defined as the complete clearance of cryoglobulin, within 24 weeks. At 12 weeks, average cryoglobulin levels decreased from 0.36 ± 0.12 to 0.10 ± 0.08 g/L, (P = .019), while average aminotransferase levels decreased from 57.6 ± 7.1 to 20.4 ± 2.0 IU/mL, (P less than .01).
But perhaps most significant, according to Dr. Saadoun, is that less than 5% of subjects required any additional treatment via immunosuppressants, such as steroids or rituximab. Average HCV viral loads dropped from 5.6 to 1.18 IU/mL at week 4 (P less than .01), with similarly sustained results through to week 12, indicating good virological responses. No serious adverse events were reported by any subjects throughout the trial period.
“The limitation is that there are quite a few patients, because it is only 35 patients this time, [and] that it’s a prospective, open-label study with no comparators,” Dr. Saadoun explained, adding that, in terms of further research, “[any] new study would focus on the way to avoid rituximab and steroid use in these patients, and to also have more patients treated with this regimen.”
No funding source was disclosed for this study. Dr. Saadoun did not report any relevant financial disclosures.
WASHINGTON – A combined regimen of sofosbuvir and daclatasvir is the best option to treat patients with hepatitis C virus infections experiencing cryoglobulinemia vasculitis, according to the findings of a new study presented at the annual meeting of the American College of Rheumatology.
“The HCV cryoglobulinemia vasculitis is a very important vasculitis because it represents 5% of chronically infected HCV patients in the world,” explained David Saadoun, MD, of Sorbonne Universities, Paris. “It’s sometimes a life-threatening vasculitis because patients may develop inflammation [so] there’s a need for very active and well-tolerated treatment.”
The primary endpoint – complete response to treatment at the end of the regimen – was achieved in 91% of subjects by the end of 24 weeks. Furthermore, 50% of patients experienced complete immunological response, defined as the complete clearance of cryoglobulin, within 24 weeks. At 12 weeks, average cryoglobulin levels decreased from 0.36 ± 0.12 to 0.10 ± 0.08 g/L, (P = .019), while average aminotransferase levels decreased from 57.6 ± 7.1 to 20.4 ± 2.0 IU/mL, (P less than .01).
But perhaps most significant, according to Dr. Saadoun, is that less than 5% of subjects required any additional treatment via immunosuppressants, such as steroids or rituximab. Average HCV viral loads dropped from 5.6 to 1.18 IU/mL at week 4 (P less than .01), with similarly sustained results through to week 12, indicating good virological responses. No serious adverse events were reported by any subjects throughout the trial period.
“The limitation is that there are quite a few patients, because it is only 35 patients this time, [and] that it’s a prospective, open-label study with no comparators,” Dr. Saadoun explained, adding that, in terms of further research, “[any] new study would focus on the way to avoid rituximab and steroid use in these patients, and to also have more patients treated with this regimen.”
No funding source was disclosed for this study. Dr. Saadoun did not report any relevant financial disclosures.
WASHINGTON – A combined regimen of sofosbuvir and daclatasvir is the best option to treat patients with hepatitis C virus infections experiencing cryoglobulinemia vasculitis, according to the findings of a new study presented at the annual meeting of the American College of Rheumatology.
“The HCV cryoglobulinemia vasculitis is a very important vasculitis because it represents 5% of chronically infected HCV patients in the world,” explained David Saadoun, MD, of Sorbonne Universities, Paris. “It’s sometimes a life-threatening vasculitis because patients may develop inflammation [so] there’s a need for very active and well-tolerated treatment.”
The primary endpoint – complete response to treatment at the end of the regimen – was achieved in 91% of subjects by the end of 24 weeks. Furthermore, 50% of patients experienced complete immunological response, defined as the complete clearance of cryoglobulin, within 24 weeks. At 12 weeks, average cryoglobulin levels decreased from 0.36 ± 0.12 to 0.10 ± 0.08 g/L, (P = .019), while average aminotransferase levels decreased from 57.6 ± 7.1 to 20.4 ± 2.0 IU/mL, (P less than .01).
But perhaps most significant, according to Dr. Saadoun, is that less than 5% of subjects required any additional treatment via immunosuppressants, such as steroids or rituximab. Average HCV viral loads dropped from 5.6 to 1.18 IU/mL at week 4 (P less than .01), with similarly sustained results through to week 12, indicating good virological responses. No serious adverse events were reported by any subjects throughout the trial period.
“The limitation is that there are quite a few patients, because it is only 35 patients this time, [and] that it’s a prospective, open-label study with no comparators,” Dr. Saadoun explained, adding that, in terms of further research, “[any] new study would focus on the way to avoid rituximab and steroid use in these patients, and to also have more patients treated with this regimen.”
No funding source was disclosed for this study. Dr. Saadoun did not report any relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Of 35 patients, 32 (91%) achieved complete clinical response in 6 months, with less than 5% requiring the use of additional immunosuppressants and none experiencing serious adverse events.
Data source: A prospective, open-label study of 35 patients with cryoglobulinemia vasculitis brought on by HCV infection.
Disclosures: Dr. Saadoun did not report any relevant financial disclosures.
Fecal calprotectin tops CRP as Crohn’s marker
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: A fecal calprotectin cutoff of 215 mcg/g identified mucosal healing with 82.8% sensitivity, 71.4% specificity, and an AUC of 0.81.
Data source: Review of 89 Crohn’s patients
Disclosures: The investigators had no conflicts of interest.