User login
Confront youth opioid misuse head on
SAN FRANCISCO – Clinicians treating children should seek out and advocate for resources needed to treat opioid addiction rather than shying away from doing so because of a feeling of helplessness, Pamela Gonzalez, MD, said at the annual meeting of the American Academy of Pediatrics.
Opioid poisonings have nearly doubled among children and adolescents over the past decade and a half, a retrospective analysis of 13,052 national hospital discharge records found. Pediatric hospitalizations for opioid poisonings increased nearly twofold from 1997 to 2012. That is, the annual incidence of hospitalizations for opioid poisonings per 100,000 children aged 1-19 years rose from 1.40 to 3.71, an increase of 165% (P less than.001) (JAMA Pediatr. 2016 Oct 31. doi: 10.1001/jamapediatrics.2016.2154).
“Silence is deadly,” she said. “What’s going to stop this problem? Not being silent, not being quiet about it.
“I hear a lot of people still saying, ‘I don’t have enough resources; I don’t know where to send them to; what am I going to do?’ ” she said. “There are a lot of illnesses that we look for, that we get the diagnosis for, and the outcome may be supportive or may be a difficult conversation with the family, but just because at this point resources aren’t what we want them to be does not mean not to look.”
Understanding the problem
Dr. Gonzalez pointed out how accessible opioids are for children and adolescents. Most youth access prescription opioids for misuse or nonmedical use from legitimate prescriptions diverted from an intended use. The largest source of diverted medication is prescribing to adults, and the problem is worsened by the fact that some youth have an enhanced vulnerability to misuse or nonmedical use of opioids.
“Therapeutic use is still exposure,” she explained, citing a one-third increased risk of nonmedical use during ages 19-23 among youth who were prescribed opioids before 12th grade. Those prescribed opioids before their senior year also have a 2.7 times greater risk of using the opioids recreationally to get high (Pediatrics. 2015 Nov;136[5]:e1169-77).
The problem is exacerbated by the fact that patients at higher risk for substance use disorder also happen to be more likely to be prescribed chronic opioid therapy. Children and teens with preexisting psychiatric conditions have a 2.4 times greater risk of receiving long-term opioids than not receiving opioids at all, and they are 1.8 times more likely to receive long-term opioids than some opioids.
Prescription opioids have begun to replace heroin as the starting point on the path toward opioid use disorder, Dr. Gonzalez pointed out. A study in 2014 found that more than 80% of individuals who began taking opioids in the 1960s started with heroin, whereas 75% of users in the 2000s began their addiction with prescription opioids (JAMA Psychiatry. 2014;71[7]:821-6).
What pediatricians can do
“When our primary and secondary prevention efforts don’t work, we’re going to need to look at treatment options” for opioid use disorder, Dr. Gonzalez said. “Kids do better on some kind of medication than not.”
The most effective medications are buprenorphine and injectable naltrexone, but these are frequently unavailable to the adolescents who need them, she said. One way to begin saving lives is to increase the number of pediatricians who are trained and approved to provide buprenorphine to youth. Physicians can seek a waiver to be able to prescribe buprenorphine to youth with opioid use disorder and learn about treatment with naltrexone by taking an 8-hour online course that is free to AAP members at www.aap.org/mat.
She acknowledged that more resources are needed to address the problem of opioid misuse, something the surgeon general has made a priority as well, but that resource deficit should not be an excuse not to take action. Federal funding is available for states to treat opioid addiction, but some states, such as Minnesota, where Dr. Gonzalez works, may not qualify if there is “not enough of a problem.”
“If every state can’t get it to help with their treatment and prevention resources, that’s not enough money earmarked for it,” she said, “but we can advocate for it.”
At the same time, pediatricians can work toward prevention by screening for mental health symptoms and for substance use – two separate screenings – at every pediatric visit starting no later than age 11 years and at any visit where opioids are being prescribed. Further, before prescribing opioids to youth, doctors should weigh the need to reduce pain against the risks of future addiction to determine if opioids are really the best option for that patient.
Dr. Gonzalez concluded her plenary speech with a plea to her colleagues: “It begins with one pill, but the end begins with us. Every kid matters. We’re not going to save them all. We have to start with one kid at a time. We’re not going to save everybody, but one life for everybody in this room is a lot of kids. Help me save one life today.”
Dr. Gonzalez had no disclosures.
SAN FRANCISCO – Clinicians treating children should seek out and advocate for resources needed to treat opioid addiction rather than shying away from doing so because of a feeling of helplessness, Pamela Gonzalez, MD, said at the annual meeting of the American Academy of Pediatrics.
Opioid poisonings have nearly doubled among children and adolescents over the past decade and a half, a retrospective analysis of 13,052 national hospital discharge records found. Pediatric hospitalizations for opioid poisonings increased nearly twofold from 1997 to 2012. That is, the annual incidence of hospitalizations for opioid poisonings per 100,000 children aged 1-19 years rose from 1.40 to 3.71, an increase of 165% (P less than.001) (JAMA Pediatr. 2016 Oct 31. doi: 10.1001/jamapediatrics.2016.2154).
“Silence is deadly,” she said. “What’s going to stop this problem? Not being silent, not being quiet about it.
“I hear a lot of people still saying, ‘I don’t have enough resources; I don’t know where to send them to; what am I going to do?’ ” she said. “There are a lot of illnesses that we look for, that we get the diagnosis for, and the outcome may be supportive or may be a difficult conversation with the family, but just because at this point resources aren’t what we want them to be does not mean not to look.”
Understanding the problem
Dr. Gonzalez pointed out how accessible opioids are for children and adolescents. Most youth access prescription opioids for misuse or nonmedical use from legitimate prescriptions diverted from an intended use. The largest source of diverted medication is prescribing to adults, and the problem is worsened by the fact that some youth have an enhanced vulnerability to misuse or nonmedical use of opioids.
“Therapeutic use is still exposure,” she explained, citing a one-third increased risk of nonmedical use during ages 19-23 among youth who were prescribed opioids before 12th grade. Those prescribed opioids before their senior year also have a 2.7 times greater risk of using the opioids recreationally to get high (Pediatrics. 2015 Nov;136[5]:e1169-77).
The problem is exacerbated by the fact that patients at higher risk for substance use disorder also happen to be more likely to be prescribed chronic opioid therapy. Children and teens with preexisting psychiatric conditions have a 2.4 times greater risk of receiving long-term opioids than not receiving opioids at all, and they are 1.8 times more likely to receive long-term opioids than some opioids.
Prescription opioids have begun to replace heroin as the starting point on the path toward opioid use disorder, Dr. Gonzalez pointed out. A study in 2014 found that more than 80% of individuals who began taking opioids in the 1960s started with heroin, whereas 75% of users in the 2000s began their addiction with prescription opioids (JAMA Psychiatry. 2014;71[7]:821-6).
What pediatricians can do
“When our primary and secondary prevention efforts don’t work, we’re going to need to look at treatment options” for opioid use disorder, Dr. Gonzalez said. “Kids do better on some kind of medication than not.”
The most effective medications are buprenorphine and injectable naltrexone, but these are frequently unavailable to the adolescents who need them, she said. One way to begin saving lives is to increase the number of pediatricians who are trained and approved to provide buprenorphine to youth. Physicians can seek a waiver to be able to prescribe buprenorphine to youth with opioid use disorder and learn about treatment with naltrexone by taking an 8-hour online course that is free to AAP members at www.aap.org/mat.
She acknowledged that more resources are needed to address the problem of opioid misuse, something the surgeon general has made a priority as well, but that resource deficit should not be an excuse not to take action. Federal funding is available for states to treat opioid addiction, but some states, such as Minnesota, where Dr. Gonzalez works, may not qualify if there is “not enough of a problem.”
“If every state can’t get it to help with their treatment and prevention resources, that’s not enough money earmarked for it,” she said, “but we can advocate for it.”
At the same time, pediatricians can work toward prevention by screening for mental health symptoms and for substance use – two separate screenings – at every pediatric visit starting no later than age 11 years and at any visit where opioids are being prescribed. Further, before prescribing opioids to youth, doctors should weigh the need to reduce pain against the risks of future addiction to determine if opioids are really the best option for that patient.
Dr. Gonzalez concluded her plenary speech with a plea to her colleagues: “It begins with one pill, but the end begins with us. Every kid matters. We’re not going to save them all. We have to start with one kid at a time. We’re not going to save everybody, but one life for everybody in this room is a lot of kids. Help me save one life today.”
Dr. Gonzalez had no disclosures.
SAN FRANCISCO – Clinicians treating children should seek out and advocate for resources needed to treat opioid addiction rather than shying away from doing so because of a feeling of helplessness, Pamela Gonzalez, MD, said at the annual meeting of the American Academy of Pediatrics.
Opioid poisonings have nearly doubled among children and adolescents over the past decade and a half, a retrospective analysis of 13,052 national hospital discharge records found. Pediatric hospitalizations for opioid poisonings increased nearly twofold from 1997 to 2012. That is, the annual incidence of hospitalizations for opioid poisonings per 100,000 children aged 1-19 years rose from 1.40 to 3.71, an increase of 165% (P less than.001) (JAMA Pediatr. 2016 Oct 31. doi: 10.1001/jamapediatrics.2016.2154).
“Silence is deadly,” she said. “What’s going to stop this problem? Not being silent, not being quiet about it.
“I hear a lot of people still saying, ‘I don’t have enough resources; I don’t know where to send them to; what am I going to do?’ ” she said. “There are a lot of illnesses that we look for, that we get the diagnosis for, and the outcome may be supportive or may be a difficult conversation with the family, but just because at this point resources aren’t what we want them to be does not mean not to look.”
Understanding the problem
Dr. Gonzalez pointed out how accessible opioids are for children and adolescents. Most youth access prescription opioids for misuse or nonmedical use from legitimate prescriptions diverted from an intended use. The largest source of diverted medication is prescribing to adults, and the problem is worsened by the fact that some youth have an enhanced vulnerability to misuse or nonmedical use of opioids.
“Therapeutic use is still exposure,” she explained, citing a one-third increased risk of nonmedical use during ages 19-23 among youth who were prescribed opioids before 12th grade. Those prescribed opioids before their senior year also have a 2.7 times greater risk of using the opioids recreationally to get high (Pediatrics. 2015 Nov;136[5]:e1169-77).
The problem is exacerbated by the fact that patients at higher risk for substance use disorder also happen to be more likely to be prescribed chronic opioid therapy. Children and teens with preexisting psychiatric conditions have a 2.4 times greater risk of receiving long-term opioids than not receiving opioids at all, and they are 1.8 times more likely to receive long-term opioids than some opioids.
Prescription opioids have begun to replace heroin as the starting point on the path toward opioid use disorder, Dr. Gonzalez pointed out. A study in 2014 found that more than 80% of individuals who began taking opioids in the 1960s started with heroin, whereas 75% of users in the 2000s began their addiction with prescription opioids (JAMA Psychiatry. 2014;71[7]:821-6).
What pediatricians can do
“When our primary and secondary prevention efforts don’t work, we’re going to need to look at treatment options” for opioid use disorder, Dr. Gonzalez said. “Kids do better on some kind of medication than not.”
The most effective medications are buprenorphine and injectable naltrexone, but these are frequently unavailable to the adolescents who need them, she said. One way to begin saving lives is to increase the number of pediatricians who are trained and approved to provide buprenorphine to youth. Physicians can seek a waiver to be able to prescribe buprenorphine to youth with opioid use disorder and learn about treatment with naltrexone by taking an 8-hour online course that is free to AAP members at www.aap.org/mat.
She acknowledged that more resources are needed to address the problem of opioid misuse, something the surgeon general has made a priority as well, but that resource deficit should not be an excuse not to take action. Federal funding is available for states to treat opioid addiction, but some states, such as Minnesota, where Dr. Gonzalez works, may not qualify if there is “not enough of a problem.”
“If every state can’t get it to help with their treatment and prevention resources, that’s not enough money earmarked for it,” she said, “but we can advocate for it.”
At the same time, pediatricians can work toward prevention by screening for mental health symptoms and for substance use – two separate screenings – at every pediatric visit starting no later than age 11 years and at any visit where opioids are being prescribed. Further, before prescribing opioids to youth, doctors should weigh the need to reduce pain against the risks of future addiction to determine if opioids are really the best option for that patient.
Dr. Gonzalez concluded her plenary speech with a plea to her colleagues: “It begins with one pill, but the end begins with us. Every kid matters. We’re not going to save them all. We have to start with one kid at a time. We’re not going to save everybody, but one life for everybody in this room is a lot of kids. Help me save one life today.”
Dr. Gonzalez had no disclosures.
EXPERT ANALYSIS FROM AAP 16
Instability After Reverse Total Shoulder Arthroplasty: Which Patients Dislocate?
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
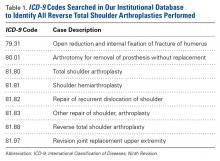
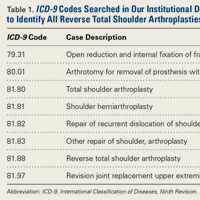
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
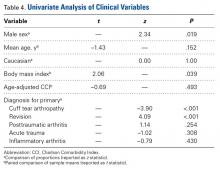
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
VIDEO: ACR recommendations for glucocorticoid-induced osteoporosis unveiled
WASHINGTON – New American College of Rheumatology recommendations for glucocorticoid-induced osteoporosis prevention and treatment include refinements in risk assessment and treatment.
“These are draft recommendations not yet accepted by ACR,” said Lenore M. Buckley, MD, of Yale University, New Haven, Conn. “They are intended to be dynamic, because risk factors change for patients over time,” she added.
The draft recommendations build upon the 2010 ACR recommendations.
“About 1% of the United States population is on glucocorticoid treatment. Fracture is the most common adverse event, and trabecular bone in the spine is the most vulnerable,” Dr. Buckley explained in her presentation of the recommendations at the annual meeting of the American College of Rheumatology. “The risk of glucocorticoid (GC)-induced fracture is related to dose level and cumulative dose.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The draft recommendations were developed via GRADE (Grading of Assessment, Development, and Evaluation) methodology by a core team of internists, rheumatologists, a GRADE expert, a voting panel, and an expert panel.
Recommendations for risk assessment
Risk assessment for GC-induced osteoporosis is individualized. “You need to know the patient in front of you. Fracture risk is not just related to GC use, but also to bone mass, age, and race. Older age, female gender, Caucasian race all increase risk, and these factors need to be brought in to assessment,” Dr. Buckley explained.
For men and women over age 40, the Fracture Risk Assessment Tool (FRAX), which calculates the 10-year fracture risk in adults over age 40, should be used for risk assessment, incorporating GC use as a risk factor, she said.
“Adjust risk of FRAX according to dose of glucocorticoid. For 2.5-7.5 mg/day, the FRAX risk is fine, but if the patient is on higher doses, adjust the FRAX accordingly,” she said.
FRAX is not valid for women and men under age 40, she continued. A new recommendation is the inclusion of a moderate risk group based on very low bone mass score (Z score less than –3 below the standard deviation of the mean) and/or rapid bone loss (greater than 10% in 1 year). Patients who had prior GC-associated fracture under 40 years are considered high risk.
A thorough history and physical exam are necessary for all patients, and risk assessment should be done within 6 months of GC initiation. Physical exam should be repeated annually, and bone mineral density (BMD) should be assessed every 2-3 years for patients who continue on GC.
Treatment
The proposed treatment recommendations were not age dependent. Patients with moderate to high risk should be treated, in descending order, with oral bisphosphonates, intravenous bisphosphonates, teriparatide (Forteo), and denosumab (Prolia). The order of preference for these treatments was based on cost, efficacy, toxicity, and patient preference.
All patients should take calcium and vitamin D, regardless of risk level.
The proposed recommendations also addressed four special groups considered at significant risk: women of childbearing potential, organ transplant recipients, children, and people on very high doses of GC (greater than 30 mg/day, cumulative dose of 5 g/year).
“For women of childbearing potential, there are emerging data suggesting that bisphosphonates are safe. For this group, consider oral bisphosphonate and teriparatide as a second choice. Animal data suggest that IV bisphosphonates and denosumab are harmful to the fetus,” Dr. Buckley said.
For organ transplant recipients, general recommendations can be followed with two provisions: Kidney transplant recipients should have a work-up for metabolic bone disease, and denosumab should not be used in people on multiple immunosuppressants.
Optimize calcium and vitamin D for children, and treat with oral bisphosphonate, Dr. Buckley continued. If oral bisphosphonates are contraindicated, IV bisphosphonates can be used.
Patients on very high GC dose should be treated with oral bisphosphonates if they are age 30 or older.
Osteoporosis medications can be discontinued in low-risk patients who stop taking GC, but should be continued for those at moderate to high risk.
Patients who benefit from osteoporosis medications but remain at moderate to high risk at the end of 3 years should continue GC treatment.
The authors and sponsors had no relevant financial disclosures.
WASHINGTON – New American College of Rheumatology recommendations for glucocorticoid-induced osteoporosis prevention and treatment include refinements in risk assessment and treatment.
“These are draft recommendations not yet accepted by ACR,” said Lenore M. Buckley, MD, of Yale University, New Haven, Conn. “They are intended to be dynamic, because risk factors change for patients over time,” she added.
The draft recommendations build upon the 2010 ACR recommendations.
“About 1% of the United States population is on glucocorticoid treatment. Fracture is the most common adverse event, and trabecular bone in the spine is the most vulnerable,” Dr. Buckley explained in her presentation of the recommendations at the annual meeting of the American College of Rheumatology. “The risk of glucocorticoid (GC)-induced fracture is related to dose level and cumulative dose.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The draft recommendations were developed via GRADE (Grading of Assessment, Development, and Evaluation) methodology by a core team of internists, rheumatologists, a GRADE expert, a voting panel, and an expert panel.
Recommendations for risk assessment
Risk assessment for GC-induced osteoporosis is individualized. “You need to know the patient in front of you. Fracture risk is not just related to GC use, but also to bone mass, age, and race. Older age, female gender, Caucasian race all increase risk, and these factors need to be brought in to assessment,” Dr. Buckley explained.
For men and women over age 40, the Fracture Risk Assessment Tool (FRAX), which calculates the 10-year fracture risk in adults over age 40, should be used for risk assessment, incorporating GC use as a risk factor, she said.
“Adjust risk of FRAX according to dose of glucocorticoid. For 2.5-7.5 mg/day, the FRAX risk is fine, but if the patient is on higher doses, adjust the FRAX accordingly,” she said.
FRAX is not valid for women and men under age 40, she continued. A new recommendation is the inclusion of a moderate risk group based on very low bone mass score (Z score less than –3 below the standard deviation of the mean) and/or rapid bone loss (greater than 10% in 1 year). Patients who had prior GC-associated fracture under 40 years are considered high risk.
A thorough history and physical exam are necessary for all patients, and risk assessment should be done within 6 months of GC initiation. Physical exam should be repeated annually, and bone mineral density (BMD) should be assessed every 2-3 years for patients who continue on GC.
Treatment
The proposed treatment recommendations were not age dependent. Patients with moderate to high risk should be treated, in descending order, with oral bisphosphonates, intravenous bisphosphonates, teriparatide (Forteo), and denosumab (Prolia). The order of preference for these treatments was based on cost, efficacy, toxicity, and patient preference.
All patients should take calcium and vitamin D, regardless of risk level.
The proposed recommendations also addressed four special groups considered at significant risk: women of childbearing potential, organ transplant recipients, children, and people on very high doses of GC (greater than 30 mg/day, cumulative dose of 5 g/year).
“For women of childbearing potential, there are emerging data suggesting that bisphosphonates are safe. For this group, consider oral bisphosphonate and teriparatide as a second choice. Animal data suggest that IV bisphosphonates and denosumab are harmful to the fetus,” Dr. Buckley said.
For organ transplant recipients, general recommendations can be followed with two provisions: Kidney transplant recipients should have a work-up for metabolic bone disease, and denosumab should not be used in people on multiple immunosuppressants.
Optimize calcium and vitamin D for children, and treat with oral bisphosphonate, Dr. Buckley continued. If oral bisphosphonates are contraindicated, IV bisphosphonates can be used.
Patients on very high GC dose should be treated with oral bisphosphonates if they are age 30 or older.
Osteoporosis medications can be discontinued in low-risk patients who stop taking GC, but should be continued for those at moderate to high risk.
Patients who benefit from osteoporosis medications but remain at moderate to high risk at the end of 3 years should continue GC treatment.
The authors and sponsors had no relevant financial disclosures.
WASHINGTON – New American College of Rheumatology recommendations for glucocorticoid-induced osteoporosis prevention and treatment include refinements in risk assessment and treatment.
“These are draft recommendations not yet accepted by ACR,” said Lenore M. Buckley, MD, of Yale University, New Haven, Conn. “They are intended to be dynamic, because risk factors change for patients over time,” she added.
The draft recommendations build upon the 2010 ACR recommendations.
“About 1% of the United States population is on glucocorticoid treatment. Fracture is the most common adverse event, and trabecular bone in the spine is the most vulnerable,” Dr. Buckley explained in her presentation of the recommendations at the annual meeting of the American College of Rheumatology. “The risk of glucocorticoid (GC)-induced fracture is related to dose level and cumulative dose.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The draft recommendations were developed via GRADE (Grading of Assessment, Development, and Evaluation) methodology by a core team of internists, rheumatologists, a GRADE expert, a voting panel, and an expert panel.
Recommendations for risk assessment
Risk assessment for GC-induced osteoporosis is individualized. “You need to know the patient in front of you. Fracture risk is not just related to GC use, but also to bone mass, age, and race. Older age, female gender, Caucasian race all increase risk, and these factors need to be brought in to assessment,” Dr. Buckley explained.
For men and women over age 40, the Fracture Risk Assessment Tool (FRAX), which calculates the 10-year fracture risk in adults over age 40, should be used for risk assessment, incorporating GC use as a risk factor, she said.
“Adjust risk of FRAX according to dose of glucocorticoid. For 2.5-7.5 mg/day, the FRAX risk is fine, but if the patient is on higher doses, adjust the FRAX accordingly,” she said.
FRAX is not valid for women and men under age 40, she continued. A new recommendation is the inclusion of a moderate risk group based on very low bone mass score (Z score less than –3 below the standard deviation of the mean) and/or rapid bone loss (greater than 10% in 1 year). Patients who had prior GC-associated fracture under 40 years are considered high risk.
A thorough history and physical exam are necessary for all patients, and risk assessment should be done within 6 months of GC initiation. Physical exam should be repeated annually, and bone mineral density (BMD) should be assessed every 2-3 years for patients who continue on GC.
Treatment
The proposed treatment recommendations were not age dependent. Patients with moderate to high risk should be treated, in descending order, with oral bisphosphonates, intravenous bisphosphonates, teriparatide (Forteo), and denosumab (Prolia). The order of preference for these treatments was based on cost, efficacy, toxicity, and patient preference.
All patients should take calcium and vitamin D, regardless of risk level.
The proposed recommendations also addressed four special groups considered at significant risk: women of childbearing potential, organ transplant recipients, children, and people on very high doses of GC (greater than 30 mg/day, cumulative dose of 5 g/year).
“For women of childbearing potential, there are emerging data suggesting that bisphosphonates are safe. For this group, consider oral bisphosphonate and teriparatide as a second choice. Animal data suggest that IV bisphosphonates and denosumab are harmful to the fetus,” Dr. Buckley said.
For organ transplant recipients, general recommendations can be followed with two provisions: Kidney transplant recipients should have a work-up for metabolic bone disease, and denosumab should not be used in people on multiple immunosuppressants.
Optimize calcium and vitamin D for children, and treat with oral bisphosphonate, Dr. Buckley continued. If oral bisphosphonates are contraindicated, IV bisphosphonates can be used.
Patients on very high GC dose should be treated with oral bisphosphonates if they are age 30 or older.
Osteoporosis medications can be discontinued in low-risk patients who stop taking GC, but should be continued for those at moderate to high risk.
Patients who benefit from osteoporosis medications but remain at moderate to high risk at the end of 3 years should continue GC treatment.
The authors and sponsors had no relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Drug produces mixed results in myelofibrosis

Two phase 3 trials have shown mixed results in myelofibrosis (MF) patients receiving the JAK inhibitor momelotinib, according to Gilead Sciences, Inc., the company developing the drug.
In the SIMPLIFY-1 study, momelotinib proved non-inferior to ruxolitinib when it came to the study’s primary endpoint but not its key secondary
endpoint.
In the SIMPLIFY-2 trial, momelotinib was not superior to best available therapy (BAT) with regard to the primary endpoint.
However, there were differences in favor of momelotinib when it came to some secondary endpoints.
“The results from both the SIMPLIFY-1 and SIMPLIFY-2 studies indicate that momelotinib provides some treatment benefit, including benefit on anemia-related endpoints,” said Norbert Bischofberger, PhD, executive vice president of research and development and chief scientific officer at Gilead Sciences, Inc.
“We plan to discuss these results with regulatory authorities to determine the next steps.”
About the studies
The SIMPLIFY studies are randomized, phase 3 trials designed to evaluate momelotinib in patients with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF. The trials have the same primary and secondary endpoints.
The primary efficacy endpoint is splenic response rate at week 24 (SRR24), defined as the proportion of patients achieving a ≥ 35% reduction in spleen volume at week 24, as measured by MRI or CT scan.
Secondary endpoints include:
- Response rate in total symptom score (TSS) at week 24, defined as the proportion of patients achieving ≥ 50% reduction in symptoms, as measured by the modified Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score diary
- The proportion of patients who are transfusion-independent at week 24, defined as no red blood cell transfusion and no hemoglobin level below 8 g/dL in the prior 12 weeks
- The proportion who are transfusion-dependent at week 24, defined as at least 4 units of red blood cell transfusion or hemoglobin level below 8 g/dL in the prior 8 weeks
- The rate of red blood cell transfusion through week 24.
SIMPLIFY-1 trial
In SIMPLIFY-1, a double-blind, active-controlled study, 432 MF patients who had not previously been treated with a JAK inhibitor were randomized (1:1) to receive momelotinib or ruxolitinib for 24 weeks.
The study achieved its pre-specified primary endpoint of non-inferiority to ruxolitinib for SRR24. The incidence of SRR24 was 26.5% in the momelotinib arm and 29.0% in the ruxolitinib arm (95% CI: -11.2% to +5.6%; P=0.011).
However, non-inferiority was not achieved for the key secondary endpoint of response rate in TSS.
Greater improvements in all 3 anemia-related secondary endpoints—transfusion independence, transfusion dependence, and transfusion rate—were observed in patients receiving momelotinib compared to ruxolitinib.
However, because the TSS response rate did not meet the non-inferiority test, formal sequential statistical testing was not undertaken for these 3 secondary endpoints.
During 24 weeks of treatment in SIMPLIFY-1, the most frequent adverse events in patients receiving momelotinib were thrombocytopenia, diarrhea, headache, dizziness, and nausea.
The most frequent adverse events in patients receiving ruxolitinib were anemia, thrombocytopenia, diarrhea, headache, and dizziness.
Ten percent of patients receiving momelotinib reported peripheral neuropathy (any grade), compared to 5% of ruxolitinib-treated patients. There was no grade 3 or higher peripheral neuropathy in momelotinib-treated patients, but there was 1 case in the ruxolitinib arm.
SIMPLIFY-2 trial
In SIMPLIFY-2, 156 patients previously treated with, but not refractory to, ruxolitinib were randomized (2:1) to receive momelotinib or BAT for 24 weeks.
Eighty-eight percent of patients randomized to the BAT arm continued to receive ruxolitinib. The remainder of patients received chemotherapy, interferon, corticosteroids, other therapies, or some combination thereof.
The study’s primary endpoint was not met. Momelotinib did not prove superior to BAT with regard to SRR24. The incidence of SRR24 was 6.7% in the momelotinib arm and 5.8% in the BAT arm (95% CI: -8.9% to +10.2%; P=0.90).
Differences in favor of momelotinib were observed for the secondary endpoints of TSS and transfusion independence. However, formal sequential statistical testing was not undertaken because the primary superiority endpoint was not achieved.
Gilead did not release safety data from this trial. The company said detailed results from both SIMPLIFY studies will be submitted for presentation at upcoming scientific conferences. ![]()

Two phase 3 trials have shown mixed results in myelofibrosis (MF) patients receiving the JAK inhibitor momelotinib, according to Gilead Sciences, Inc., the company developing the drug.
In the SIMPLIFY-1 study, momelotinib proved non-inferior to ruxolitinib when it came to the study’s primary endpoint but not its key secondary
endpoint.
In the SIMPLIFY-2 trial, momelotinib was not superior to best available therapy (BAT) with regard to the primary endpoint.
However, there were differences in favor of momelotinib when it came to some secondary endpoints.
“The results from both the SIMPLIFY-1 and SIMPLIFY-2 studies indicate that momelotinib provides some treatment benefit, including benefit on anemia-related endpoints,” said Norbert Bischofberger, PhD, executive vice president of research and development and chief scientific officer at Gilead Sciences, Inc.
“We plan to discuss these results with regulatory authorities to determine the next steps.”
About the studies
The SIMPLIFY studies are randomized, phase 3 trials designed to evaluate momelotinib in patients with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF. The trials have the same primary and secondary endpoints.
The primary efficacy endpoint is splenic response rate at week 24 (SRR24), defined as the proportion of patients achieving a ≥ 35% reduction in spleen volume at week 24, as measured by MRI or CT scan.
Secondary endpoints include:
- Response rate in total symptom score (TSS) at week 24, defined as the proportion of patients achieving ≥ 50% reduction in symptoms, as measured by the modified Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score diary
- The proportion of patients who are transfusion-independent at week 24, defined as no red blood cell transfusion and no hemoglobin level below 8 g/dL in the prior 12 weeks
- The proportion who are transfusion-dependent at week 24, defined as at least 4 units of red blood cell transfusion or hemoglobin level below 8 g/dL in the prior 8 weeks
- The rate of red blood cell transfusion through week 24.
SIMPLIFY-1 trial
In SIMPLIFY-1, a double-blind, active-controlled study, 432 MF patients who had not previously been treated with a JAK inhibitor were randomized (1:1) to receive momelotinib or ruxolitinib for 24 weeks.
The study achieved its pre-specified primary endpoint of non-inferiority to ruxolitinib for SRR24. The incidence of SRR24 was 26.5% in the momelotinib arm and 29.0% in the ruxolitinib arm (95% CI: -11.2% to +5.6%; P=0.011).
However, non-inferiority was not achieved for the key secondary endpoint of response rate in TSS.
Greater improvements in all 3 anemia-related secondary endpoints—transfusion independence, transfusion dependence, and transfusion rate—were observed in patients receiving momelotinib compared to ruxolitinib.
However, because the TSS response rate did not meet the non-inferiority test, formal sequential statistical testing was not undertaken for these 3 secondary endpoints.
During 24 weeks of treatment in SIMPLIFY-1, the most frequent adverse events in patients receiving momelotinib were thrombocytopenia, diarrhea, headache, dizziness, and nausea.
The most frequent adverse events in patients receiving ruxolitinib were anemia, thrombocytopenia, diarrhea, headache, and dizziness.
Ten percent of patients receiving momelotinib reported peripheral neuropathy (any grade), compared to 5% of ruxolitinib-treated patients. There was no grade 3 or higher peripheral neuropathy in momelotinib-treated patients, but there was 1 case in the ruxolitinib arm.
SIMPLIFY-2 trial
In SIMPLIFY-2, 156 patients previously treated with, but not refractory to, ruxolitinib were randomized (2:1) to receive momelotinib or BAT for 24 weeks.
Eighty-eight percent of patients randomized to the BAT arm continued to receive ruxolitinib. The remainder of patients received chemotherapy, interferon, corticosteroids, other therapies, or some combination thereof.
The study’s primary endpoint was not met. Momelotinib did not prove superior to BAT with regard to SRR24. The incidence of SRR24 was 6.7% in the momelotinib arm and 5.8% in the BAT arm (95% CI: -8.9% to +10.2%; P=0.90).
Differences in favor of momelotinib were observed for the secondary endpoints of TSS and transfusion independence. However, formal sequential statistical testing was not undertaken because the primary superiority endpoint was not achieved.
Gilead did not release safety data from this trial. The company said detailed results from both SIMPLIFY studies will be submitted for presentation at upcoming scientific conferences. ![]()

Two phase 3 trials have shown mixed results in myelofibrosis (MF) patients receiving the JAK inhibitor momelotinib, according to Gilead Sciences, Inc., the company developing the drug.
In the SIMPLIFY-1 study, momelotinib proved non-inferior to ruxolitinib when it came to the study’s primary endpoint but not its key secondary
endpoint.
In the SIMPLIFY-2 trial, momelotinib was not superior to best available therapy (BAT) with regard to the primary endpoint.
However, there were differences in favor of momelotinib when it came to some secondary endpoints.
“The results from both the SIMPLIFY-1 and SIMPLIFY-2 studies indicate that momelotinib provides some treatment benefit, including benefit on anemia-related endpoints,” said Norbert Bischofberger, PhD, executive vice president of research and development and chief scientific officer at Gilead Sciences, Inc.
“We plan to discuss these results with regulatory authorities to determine the next steps.”
About the studies
The SIMPLIFY studies are randomized, phase 3 trials designed to evaluate momelotinib in patients with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF. The trials have the same primary and secondary endpoints.
The primary efficacy endpoint is splenic response rate at week 24 (SRR24), defined as the proportion of patients achieving a ≥ 35% reduction in spleen volume at week 24, as measured by MRI or CT scan.
Secondary endpoints include:
- Response rate in total symptom score (TSS) at week 24, defined as the proportion of patients achieving ≥ 50% reduction in symptoms, as measured by the modified Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score diary
- The proportion of patients who are transfusion-independent at week 24, defined as no red blood cell transfusion and no hemoglobin level below 8 g/dL in the prior 12 weeks
- The proportion who are transfusion-dependent at week 24, defined as at least 4 units of red blood cell transfusion or hemoglobin level below 8 g/dL in the prior 8 weeks
- The rate of red blood cell transfusion through week 24.
SIMPLIFY-1 trial
In SIMPLIFY-1, a double-blind, active-controlled study, 432 MF patients who had not previously been treated with a JAK inhibitor were randomized (1:1) to receive momelotinib or ruxolitinib for 24 weeks.
The study achieved its pre-specified primary endpoint of non-inferiority to ruxolitinib for SRR24. The incidence of SRR24 was 26.5% in the momelotinib arm and 29.0% in the ruxolitinib arm (95% CI: -11.2% to +5.6%; P=0.011).
However, non-inferiority was not achieved for the key secondary endpoint of response rate in TSS.
Greater improvements in all 3 anemia-related secondary endpoints—transfusion independence, transfusion dependence, and transfusion rate—were observed in patients receiving momelotinib compared to ruxolitinib.
However, because the TSS response rate did not meet the non-inferiority test, formal sequential statistical testing was not undertaken for these 3 secondary endpoints.
During 24 weeks of treatment in SIMPLIFY-1, the most frequent adverse events in patients receiving momelotinib were thrombocytopenia, diarrhea, headache, dizziness, and nausea.
The most frequent adverse events in patients receiving ruxolitinib were anemia, thrombocytopenia, diarrhea, headache, and dizziness.
Ten percent of patients receiving momelotinib reported peripheral neuropathy (any grade), compared to 5% of ruxolitinib-treated patients. There was no grade 3 or higher peripheral neuropathy in momelotinib-treated patients, but there was 1 case in the ruxolitinib arm.
SIMPLIFY-2 trial
In SIMPLIFY-2, 156 patients previously treated with, but not refractory to, ruxolitinib were randomized (2:1) to receive momelotinib or BAT for 24 weeks.
Eighty-eight percent of patients randomized to the BAT arm continued to receive ruxolitinib. The remainder of patients received chemotherapy, interferon, corticosteroids, other therapies, or some combination thereof.
The study’s primary endpoint was not met. Momelotinib did not prove superior to BAT with regard to SRR24. The incidence of SRR24 was 6.7% in the momelotinib arm and 5.8% in the BAT arm (95% CI: -8.9% to +10.2%; P=0.90).
Differences in favor of momelotinib were observed for the secondary endpoints of TSS and transfusion independence. However, formal sequential statistical testing was not undertaken because the primary superiority endpoint was not achieved.
Gilead did not release safety data from this trial. The company said detailed results from both SIMPLIFY studies will be submitted for presentation at upcoming scientific conferences. ![]()
Tips for Hospitalists on Solving Difficult Situations
At Bay Area Medical Center in Marinette, Wis., the time had come to start talking about an elderly woman’s end-of-life care.
Her hospitalist thought that those discussions should take place with the patient present, but the woman’s family felt otherwise and made this known to the hospitalist, who stood his ground.
Eventually, the family told a nurse that they wanted to fire the physician. But the only other hospitalist on shift didn’t want to take the patient.
As case managers and hospital administrators tried to wrap their heads around the situation, it became clear: They didn’t really know what to do.
Could the patient fire a physician? Was the second physician obligated to take what he knew from the outset would be a difficult case? What if nobody wanted to take care of this patient?
“There was no black-and-white to this,” says Robin Dequaine, director of medical staff services at the hospital, who was involved in the case.
Some “difficult patient” scenarios are fairly straightforward. A patient is violent? Enact your security measures. An addict wants narcotics? Don’t give them.
But there are other situations that enter murkier territory: What if a patient makes inappropriate or abusive remarks? How much should a hospitalist put up with? What if a patient’s request for treatment might not be the hospitalist’s first choice but could be seen as reasonable? Is the patient’s request accommodated? And what about those firings?
Hospitalists, administrators, and patient advocates say these tense situations with patients involving firings, or would-be firings, while not a daily occurrence, are actually fairly common.1 Getting to the root of the problem is essential. And as with so much in healthcare, good communication is the absolute crux of it all, they say.
“These are almost all communication issues,” says John Bulger, DO, MBA, FACP, SFHM, chief medical officer at Geisinger Health Plan in Danville, Pa., who has had a long career as a hospitalist and administrator handling and trying to resolve these situations. “They’re all [about] the way the hospitalist and the team is relating to the patient.”
Jackie O’Doherty, a private patient advocate who practices in New Jersey and New York across a gamut of hospital types, has a similar view.
“For me, the biggest problem, period, against hospitalists, doctors, everybody in the hospital, is communication—the lack of it,” she says. “Their communication skills are really poor.”
Patients accustomed to choice in the outpatient setting might not handle it well when they don’t have an established relationship with their hospitalist, says John Vazquez, MD, associate director for the Emory University School of Medicine’s Division of Hospital Medicine in Atlanta.
But the system, he says, “does not allow for, unfortunately, that much patient choice.”
End-of-life Discussion at a Small Hospital
Dequaine says the staff at Bay Area Medical Center was caught flat-footed with the case of the family not wanting end-of-life care discussed with their elderly mother.
“The doctor felt very confident that he was in a position that he could have that discussion in front of the patient,” she says.
At the 99-bed center, there were just two hospitalists, who were also employees of the hospital, on shift. And the communication channels involving the medical director of hospital medicine, a case manager, and the chief nursing executive were not well-controlled, Dequaine says.
“It didn’t go up the ladder correctly,” she says. “Too many people got involved, not knowing that somebody else was already involved.”
The second hospitalist at first said he would take the case, but later Dequaine learned that he changed his mind.
“He knew his care would be no different, and we were very, very busy, so they both had a high census already,” Dequaine says.
A third physician reluctantly took over until the issue subsided. And the family still brings the patient to the hospital for care.
Ultimately, the center adopted a new policy that doesn’t guarantee a patient a new doctor, only that the hospital will have frank discussions to try to resolve the issue and then try to arrange for a transfer if the situation can’t be resolved.
“The goal is not to get rid of the patient or to force them to keep the provider,” Dequaine says. “The goal is to resolve it in a mutually satisfactory way.”
A Patient Demands a Contraindicated Medication
A middle-aged woman with Crohn’s disease was hospitalized at Emory with an infection. The woman, worried about her disease flaring, wanted to keep getting her immunosuppressant, but the hospitalist suspended it because she needed to fight off the infection. The patient became upset. At a point when the hospitalist wasn’t in the room, the woman insisted to a nurse that she get her medication. The nurse called a doctor who was on call, but that doctor wouldn’t give the immunosuppressant either.
The patient began to think she wasn’t being listened to. Dr. Vazquez went in to see the patient and apologized for the misunderstanding.
“I went back into the room and explained here’s why I’m doing it: ‘I totally understand where you’re coming from; you don’t want your disease to be out of control. I appreciate that. What I’m worried about is killing you if we give you an immunosuppressant at the wrong time,’” Dr. Vazquez says.
Dr. Vazquez has underscored at his center how important it is for the physicians to be consulted and go back into the room when patients want to fire them, even though the expedient step might be to just bring in a new doctor. At previous centers, he says, it wouldn’t be unusual for the director to get a call from a nurse, who would say, “Yeah, they want to fire this physician, so let me know who’s going to see the patient.”
But simply switching doctors, he cautions, is like saying, “I agree with you we have incompetent doctors here, so we’re going to remove that doctor and I’m going to put a doctor on who actually knows what they’re doing.”
When doctors try to resolve the issues, good things tend to happen, Dr. Vazquez says.
“There’s generally a large amount of appreciation that someone comes back into the room and says, ‘We want to do this right.’”
Of course, there are times when, if tension remains after such discussions, patient care might be better served by a swap. At large centers, that might be possible, Dr. Bulger of Geisinger says.
“If the patient doesn’t tell the doctor something because he or she doesn’t like the doctor, then the doctor’s decisions are made on partial information—that’s the issue,” he says.
O’Doherty, the patient advocate, says that if patients frustrated with poor communication actually fired physicians as often as they would like, there would be more firings.
“Patients don’t like firing the doctors because they don’t want to be the patient who everybody doesn’t like,” she says. “They’re afraid that if they argue or disagree or ask too many questions, that they’re not going to get the care they need. And the family is afraid of that as well, especially in the older population. They think doctors are like God, they hold your life in their hands. So they don’t want to really question doctors.”
She says patients don’t necessarily need a particular finesse or expert bedside manner. In many cases, she says, it’s “just giving the information.”
A Patient Demands Pain Medication
Martin Austin, MD, SFHM, recently cared for a patient with chronic headaches. The patient asked for higher doses of pain medication, insinuating that she might turn to heroin if denied.
“I was trying to make the argument that I kind of disagreed with that but, ‘I respect your opinion,’” says Dr. Austin, medical director at the Gwinnett Medical Center Inpatient Medical Group in Georgia. “We came to a negotiation about how long we would use narcotics acutely until her other acute issues were over, but then we would try to get her away from narcotics.”
A good approach, he says, is to “outline to the patient why you’re doing what you’re doing. We try not to pick battles and give the patient some degree of control if it’s not contraindicated.”
But sometimes there can be no negotiating these kinds of requests, he says.
“Sometimes we’ll just say, ‘Look, it’s not a good thing for you to continue on this medication. You’re showing side effects, you’re sedated. … We think that the risk outweighs the benefit in this case,” he says.
A Patient Feels Left in the Dark
One patient at Emory wanted to fire his hospitalist because he wouldn’t tell him what was on his CT scan.
Dr. Vazquez held a discussion between the patient and the doctor. If not for the seriousness of the patient’s condition (he had tremors and neurological concerns), it would have been almost comical.
The patient had asked, “What’s on my scan?” The patient interpreted the doctor’s response, “It’s negative,” to mean that he wasn’t being told something about the scan.
Dr. Vazquez realized that the patient had felt dismissed.
“He was a sick gentleman,” Dr. Vazquez says. “And what he wanted to hear was, ‘Look, the great news is your CT scan looks good. There’s not an anatomical abnormality. It’s not a tumor. It’s not a big bleed. … That’s great news, but I, as a physician, I am concerned about you. You’re sick. We’ve got to really figure out what’s going on with you.’… He wanted a pat on the back, and that’s all it took.”
After that, the patient no longer wanted to fire the hospitalist.
Verbal Abuse
One case at Gwinnett involves a hospitalist who was quite shy and easily intimidated and was not comfortable with a patient.
“They were struggling with a patient who was very difficult and very angry and a little abusive,” Dr. Austin says. “This doctor was really suffering psychically from this whole thing, and we switched.” Another doctor, who would not be thrown by the situation, took over the case. And Dr. Austin says he had great respect for the first doctor’s request to hand over the case.
“They needed a different personality,” he says. “It worked out beautifully. The patient and the doctor got along much better. The doctor was firm with the patient but respectful, and the other doctor felt relieved. And the [original] doctor is great with patients who need a lot of emotional support, probably better than the other doctor. So that worked out really well.”
It might be a challenge during a busy day, but it’s helpful to step back and see the situation as a whole, Dr. Bulger says. Sometimes, hospitalists can get flustered when patients are not acting rationally. But there’s usually a good reason they’re acting that way, he says.
“The patient is sick. And if it’s the patient’s family, they’re stressed by the fact that the patient’s sick. So you really need to take a step back and understand that.” TH
Thomas R. Collins is a freelance writer based in West Palm Beach, Fla.
Reference
- Centor R. Can I fire my hospitalist? SGIM Forum. 32(5):112-13.
At Bay Area Medical Center in Marinette, Wis., the time had come to start talking about an elderly woman’s end-of-life care.
Her hospitalist thought that those discussions should take place with the patient present, but the woman’s family felt otherwise and made this known to the hospitalist, who stood his ground.
Eventually, the family told a nurse that they wanted to fire the physician. But the only other hospitalist on shift didn’t want to take the patient.
As case managers and hospital administrators tried to wrap their heads around the situation, it became clear: They didn’t really know what to do.
Could the patient fire a physician? Was the second physician obligated to take what he knew from the outset would be a difficult case? What if nobody wanted to take care of this patient?
“There was no black-and-white to this,” says Robin Dequaine, director of medical staff services at the hospital, who was involved in the case.
Some “difficult patient” scenarios are fairly straightforward. A patient is violent? Enact your security measures. An addict wants narcotics? Don’t give them.
But there are other situations that enter murkier territory: What if a patient makes inappropriate or abusive remarks? How much should a hospitalist put up with? What if a patient’s request for treatment might not be the hospitalist’s first choice but could be seen as reasonable? Is the patient’s request accommodated? And what about those firings?
Hospitalists, administrators, and patient advocates say these tense situations with patients involving firings, or would-be firings, while not a daily occurrence, are actually fairly common.1 Getting to the root of the problem is essential. And as with so much in healthcare, good communication is the absolute crux of it all, they say.
“These are almost all communication issues,” says John Bulger, DO, MBA, FACP, SFHM, chief medical officer at Geisinger Health Plan in Danville, Pa., who has had a long career as a hospitalist and administrator handling and trying to resolve these situations. “They’re all [about] the way the hospitalist and the team is relating to the patient.”
Jackie O’Doherty, a private patient advocate who practices in New Jersey and New York across a gamut of hospital types, has a similar view.
“For me, the biggest problem, period, against hospitalists, doctors, everybody in the hospital, is communication—the lack of it,” she says. “Their communication skills are really poor.”
Patients accustomed to choice in the outpatient setting might not handle it well when they don’t have an established relationship with their hospitalist, says John Vazquez, MD, associate director for the Emory University School of Medicine’s Division of Hospital Medicine in Atlanta.
But the system, he says, “does not allow for, unfortunately, that much patient choice.”
End-of-life Discussion at a Small Hospital
Dequaine says the staff at Bay Area Medical Center was caught flat-footed with the case of the family not wanting end-of-life care discussed with their elderly mother.
“The doctor felt very confident that he was in a position that he could have that discussion in front of the patient,” she says.
At the 99-bed center, there were just two hospitalists, who were also employees of the hospital, on shift. And the communication channels involving the medical director of hospital medicine, a case manager, and the chief nursing executive were not well-controlled, Dequaine says.
“It didn’t go up the ladder correctly,” she says. “Too many people got involved, not knowing that somebody else was already involved.”
The second hospitalist at first said he would take the case, but later Dequaine learned that he changed his mind.
“He knew his care would be no different, and we were very, very busy, so they both had a high census already,” Dequaine says.
A third physician reluctantly took over until the issue subsided. And the family still brings the patient to the hospital for care.
Ultimately, the center adopted a new policy that doesn’t guarantee a patient a new doctor, only that the hospital will have frank discussions to try to resolve the issue and then try to arrange for a transfer if the situation can’t be resolved.
“The goal is not to get rid of the patient or to force them to keep the provider,” Dequaine says. “The goal is to resolve it in a mutually satisfactory way.”
A Patient Demands a Contraindicated Medication
A middle-aged woman with Crohn’s disease was hospitalized at Emory with an infection. The woman, worried about her disease flaring, wanted to keep getting her immunosuppressant, but the hospitalist suspended it because she needed to fight off the infection. The patient became upset. At a point when the hospitalist wasn’t in the room, the woman insisted to a nurse that she get her medication. The nurse called a doctor who was on call, but that doctor wouldn’t give the immunosuppressant either.
The patient began to think she wasn’t being listened to. Dr. Vazquez went in to see the patient and apologized for the misunderstanding.
“I went back into the room and explained here’s why I’m doing it: ‘I totally understand where you’re coming from; you don’t want your disease to be out of control. I appreciate that. What I’m worried about is killing you if we give you an immunosuppressant at the wrong time,’” Dr. Vazquez says.
Dr. Vazquez has underscored at his center how important it is for the physicians to be consulted and go back into the room when patients want to fire them, even though the expedient step might be to just bring in a new doctor. At previous centers, he says, it wouldn’t be unusual for the director to get a call from a nurse, who would say, “Yeah, they want to fire this physician, so let me know who’s going to see the patient.”
But simply switching doctors, he cautions, is like saying, “I agree with you we have incompetent doctors here, so we’re going to remove that doctor and I’m going to put a doctor on who actually knows what they’re doing.”
When doctors try to resolve the issues, good things tend to happen, Dr. Vazquez says.
“There’s generally a large amount of appreciation that someone comes back into the room and says, ‘We want to do this right.’”
Of course, there are times when, if tension remains after such discussions, patient care might be better served by a swap. At large centers, that might be possible, Dr. Bulger of Geisinger says.
“If the patient doesn’t tell the doctor something because he or she doesn’t like the doctor, then the doctor’s decisions are made on partial information—that’s the issue,” he says.
O’Doherty, the patient advocate, says that if patients frustrated with poor communication actually fired physicians as often as they would like, there would be more firings.
“Patients don’t like firing the doctors because they don’t want to be the patient who everybody doesn’t like,” she says. “They’re afraid that if they argue or disagree or ask too many questions, that they’re not going to get the care they need. And the family is afraid of that as well, especially in the older population. They think doctors are like God, they hold your life in their hands. So they don’t want to really question doctors.”
She says patients don’t necessarily need a particular finesse or expert bedside manner. In many cases, she says, it’s “just giving the information.”
A Patient Demands Pain Medication
Martin Austin, MD, SFHM, recently cared for a patient with chronic headaches. The patient asked for higher doses of pain medication, insinuating that she might turn to heroin if denied.
“I was trying to make the argument that I kind of disagreed with that but, ‘I respect your opinion,’” says Dr. Austin, medical director at the Gwinnett Medical Center Inpatient Medical Group in Georgia. “We came to a negotiation about how long we would use narcotics acutely until her other acute issues were over, but then we would try to get her away from narcotics.”
A good approach, he says, is to “outline to the patient why you’re doing what you’re doing. We try not to pick battles and give the patient some degree of control if it’s not contraindicated.”
But sometimes there can be no negotiating these kinds of requests, he says.
“Sometimes we’ll just say, ‘Look, it’s not a good thing for you to continue on this medication. You’re showing side effects, you’re sedated. … We think that the risk outweighs the benefit in this case,” he says.
A Patient Feels Left in the Dark
One patient at Emory wanted to fire his hospitalist because he wouldn’t tell him what was on his CT scan.
Dr. Vazquez held a discussion between the patient and the doctor. If not for the seriousness of the patient’s condition (he had tremors and neurological concerns), it would have been almost comical.
The patient had asked, “What’s on my scan?” The patient interpreted the doctor’s response, “It’s negative,” to mean that he wasn’t being told something about the scan.
Dr. Vazquez realized that the patient had felt dismissed.
“He was a sick gentleman,” Dr. Vazquez says. “And what he wanted to hear was, ‘Look, the great news is your CT scan looks good. There’s not an anatomical abnormality. It’s not a tumor. It’s not a big bleed. … That’s great news, but I, as a physician, I am concerned about you. You’re sick. We’ve got to really figure out what’s going on with you.’… He wanted a pat on the back, and that’s all it took.”
After that, the patient no longer wanted to fire the hospitalist.
Verbal Abuse
One case at Gwinnett involves a hospitalist who was quite shy and easily intimidated and was not comfortable with a patient.
“They were struggling with a patient who was very difficult and very angry and a little abusive,” Dr. Austin says. “This doctor was really suffering psychically from this whole thing, and we switched.” Another doctor, who would not be thrown by the situation, took over the case. And Dr. Austin says he had great respect for the first doctor’s request to hand over the case.
“They needed a different personality,” he says. “It worked out beautifully. The patient and the doctor got along much better. The doctor was firm with the patient but respectful, and the other doctor felt relieved. And the [original] doctor is great with patients who need a lot of emotional support, probably better than the other doctor. So that worked out really well.”
It might be a challenge during a busy day, but it’s helpful to step back and see the situation as a whole, Dr. Bulger says. Sometimes, hospitalists can get flustered when patients are not acting rationally. But there’s usually a good reason they’re acting that way, he says.
“The patient is sick. And if it’s the patient’s family, they’re stressed by the fact that the patient’s sick. So you really need to take a step back and understand that.” TH
Thomas R. Collins is a freelance writer based in West Palm Beach, Fla.
Reference
- Centor R. Can I fire my hospitalist? SGIM Forum. 32(5):112-13.
At Bay Area Medical Center in Marinette, Wis., the time had come to start talking about an elderly woman’s end-of-life care.
Her hospitalist thought that those discussions should take place with the patient present, but the woman’s family felt otherwise and made this known to the hospitalist, who stood his ground.
Eventually, the family told a nurse that they wanted to fire the physician. But the only other hospitalist on shift didn’t want to take the patient.
As case managers and hospital administrators tried to wrap their heads around the situation, it became clear: They didn’t really know what to do.
Could the patient fire a physician? Was the second physician obligated to take what he knew from the outset would be a difficult case? What if nobody wanted to take care of this patient?
“There was no black-and-white to this,” says Robin Dequaine, director of medical staff services at the hospital, who was involved in the case.
Some “difficult patient” scenarios are fairly straightforward. A patient is violent? Enact your security measures. An addict wants narcotics? Don’t give them.
But there are other situations that enter murkier territory: What if a patient makes inappropriate or abusive remarks? How much should a hospitalist put up with? What if a patient’s request for treatment might not be the hospitalist’s first choice but could be seen as reasonable? Is the patient’s request accommodated? And what about those firings?
Hospitalists, administrators, and patient advocates say these tense situations with patients involving firings, or would-be firings, while not a daily occurrence, are actually fairly common.1 Getting to the root of the problem is essential. And as with so much in healthcare, good communication is the absolute crux of it all, they say.
“These are almost all communication issues,” says John Bulger, DO, MBA, FACP, SFHM, chief medical officer at Geisinger Health Plan in Danville, Pa., who has had a long career as a hospitalist and administrator handling and trying to resolve these situations. “They’re all [about] the way the hospitalist and the team is relating to the patient.”
Jackie O’Doherty, a private patient advocate who practices in New Jersey and New York across a gamut of hospital types, has a similar view.
“For me, the biggest problem, period, against hospitalists, doctors, everybody in the hospital, is communication—the lack of it,” she says. “Their communication skills are really poor.”
Patients accustomed to choice in the outpatient setting might not handle it well when they don’t have an established relationship with their hospitalist, says John Vazquez, MD, associate director for the Emory University School of Medicine’s Division of Hospital Medicine in Atlanta.
But the system, he says, “does not allow for, unfortunately, that much patient choice.”
End-of-life Discussion at a Small Hospital
Dequaine says the staff at Bay Area Medical Center was caught flat-footed with the case of the family not wanting end-of-life care discussed with their elderly mother.
“The doctor felt very confident that he was in a position that he could have that discussion in front of the patient,” she says.
At the 99-bed center, there were just two hospitalists, who were also employees of the hospital, on shift. And the communication channels involving the medical director of hospital medicine, a case manager, and the chief nursing executive were not well-controlled, Dequaine says.
“It didn’t go up the ladder correctly,” she says. “Too many people got involved, not knowing that somebody else was already involved.”
The second hospitalist at first said he would take the case, but later Dequaine learned that he changed his mind.
“He knew his care would be no different, and we were very, very busy, so they both had a high census already,” Dequaine says.
A third physician reluctantly took over until the issue subsided. And the family still brings the patient to the hospital for care.
Ultimately, the center adopted a new policy that doesn’t guarantee a patient a new doctor, only that the hospital will have frank discussions to try to resolve the issue and then try to arrange for a transfer if the situation can’t be resolved.
“The goal is not to get rid of the patient or to force them to keep the provider,” Dequaine says. “The goal is to resolve it in a mutually satisfactory way.”
A Patient Demands a Contraindicated Medication
A middle-aged woman with Crohn’s disease was hospitalized at Emory with an infection. The woman, worried about her disease flaring, wanted to keep getting her immunosuppressant, but the hospitalist suspended it because she needed to fight off the infection. The patient became upset. At a point when the hospitalist wasn’t in the room, the woman insisted to a nurse that she get her medication. The nurse called a doctor who was on call, but that doctor wouldn’t give the immunosuppressant either.
The patient began to think she wasn’t being listened to. Dr. Vazquez went in to see the patient and apologized for the misunderstanding.
“I went back into the room and explained here’s why I’m doing it: ‘I totally understand where you’re coming from; you don’t want your disease to be out of control. I appreciate that. What I’m worried about is killing you if we give you an immunosuppressant at the wrong time,’” Dr. Vazquez says.
Dr. Vazquez has underscored at his center how important it is for the physicians to be consulted and go back into the room when patients want to fire them, even though the expedient step might be to just bring in a new doctor. At previous centers, he says, it wouldn’t be unusual for the director to get a call from a nurse, who would say, “Yeah, they want to fire this physician, so let me know who’s going to see the patient.”
But simply switching doctors, he cautions, is like saying, “I agree with you we have incompetent doctors here, so we’re going to remove that doctor and I’m going to put a doctor on who actually knows what they’re doing.”
When doctors try to resolve the issues, good things tend to happen, Dr. Vazquez says.
“There’s generally a large amount of appreciation that someone comes back into the room and says, ‘We want to do this right.’”
Of course, there are times when, if tension remains after such discussions, patient care might be better served by a swap. At large centers, that might be possible, Dr. Bulger of Geisinger says.
“If the patient doesn’t tell the doctor something because he or she doesn’t like the doctor, then the doctor’s decisions are made on partial information—that’s the issue,” he says.
O’Doherty, the patient advocate, says that if patients frustrated with poor communication actually fired physicians as often as they would like, there would be more firings.
“Patients don’t like firing the doctors because they don’t want to be the patient who everybody doesn’t like,” she says. “They’re afraid that if they argue or disagree or ask too many questions, that they’re not going to get the care they need. And the family is afraid of that as well, especially in the older population. They think doctors are like God, they hold your life in their hands. So they don’t want to really question doctors.”
She says patients don’t necessarily need a particular finesse or expert bedside manner. In many cases, she says, it’s “just giving the information.”
A Patient Demands Pain Medication
Martin Austin, MD, SFHM, recently cared for a patient with chronic headaches. The patient asked for higher doses of pain medication, insinuating that she might turn to heroin if denied.
“I was trying to make the argument that I kind of disagreed with that but, ‘I respect your opinion,’” says Dr. Austin, medical director at the Gwinnett Medical Center Inpatient Medical Group in Georgia. “We came to a negotiation about how long we would use narcotics acutely until her other acute issues were over, but then we would try to get her away from narcotics.”
A good approach, he says, is to “outline to the patient why you’re doing what you’re doing. We try not to pick battles and give the patient some degree of control if it’s not contraindicated.”
But sometimes there can be no negotiating these kinds of requests, he says.
“Sometimes we’ll just say, ‘Look, it’s not a good thing for you to continue on this medication. You’re showing side effects, you’re sedated. … We think that the risk outweighs the benefit in this case,” he says.
A Patient Feels Left in the Dark
One patient at Emory wanted to fire his hospitalist because he wouldn’t tell him what was on his CT scan.
Dr. Vazquez held a discussion between the patient and the doctor. If not for the seriousness of the patient’s condition (he had tremors and neurological concerns), it would have been almost comical.
The patient had asked, “What’s on my scan?” The patient interpreted the doctor’s response, “It’s negative,” to mean that he wasn’t being told something about the scan.
Dr. Vazquez realized that the patient had felt dismissed.
“He was a sick gentleman,” Dr. Vazquez says. “And what he wanted to hear was, ‘Look, the great news is your CT scan looks good. There’s not an anatomical abnormality. It’s not a tumor. It’s not a big bleed. … That’s great news, but I, as a physician, I am concerned about you. You’re sick. We’ve got to really figure out what’s going on with you.’… He wanted a pat on the back, and that’s all it took.”
After that, the patient no longer wanted to fire the hospitalist.
Verbal Abuse
One case at Gwinnett involves a hospitalist who was quite shy and easily intimidated and was not comfortable with a patient.
“They were struggling with a patient who was very difficult and very angry and a little abusive,” Dr. Austin says. “This doctor was really suffering psychically from this whole thing, and we switched.” Another doctor, who would not be thrown by the situation, took over the case. And Dr. Austin says he had great respect for the first doctor’s request to hand over the case.
“They needed a different personality,” he says. “It worked out beautifully. The patient and the doctor got along much better. The doctor was firm with the patient but respectful, and the other doctor felt relieved. And the [original] doctor is great with patients who need a lot of emotional support, probably better than the other doctor. So that worked out really well.”
It might be a challenge during a busy day, but it’s helpful to step back and see the situation as a whole, Dr. Bulger says. Sometimes, hospitalists can get flustered when patients are not acting rationally. But there’s usually a good reason they’re acting that way, he says.
“The patient is sick. And if it’s the patient’s family, they’re stressed by the fact that the patient’s sick. So you really need to take a step back and understand that.” TH
Thomas R. Collins is a freelance writer based in West Palm Beach, Fla.
Reference
- Centor R. Can I fire my hospitalist? SGIM Forum. 32(5):112-13.
AACN releases updated resource on VTE
caring for a patient in
an intensive care unit
The American Association of Critical-Care Nurses (AACN) has released updated resources aimed at helping nurses prevent serious complications facing critically ill patients.
The resources, or “practice alerts,” address venous thromboembolism (VTE), delirium, and catheter-associated urinary tract infections (CAUTIs).
Each alert outlines the scope of the problem, summarizes the expected nursing practice, and provides supporting evidence and research.
These documents are available on the AACN website.
The VTE practice alert, “Preventing Venous Thromboembolism in Adults,” notes that VTE affects approximately 900,000 adult patients in the US annually and results in an estimated 300,000 deaths. Furthermore, VTE prevalence is predicted to more than double in the next 35 years.
The document also reviews risk factors for VTE and methods of VTE prophylaxis, including medications and compression devices.
The CAUTI practice alert, “Prevention of Catheter-Associated Urinary Tract Infections in Adults,” notes that urinary tract infections are the most common healthcare-associated infection, and prolonged indwelling catheterization is the major risk factor for CAUTIs.
The document outlines preliminary and ongoing assessment, documentation, and adherence to infection control protocols.
The delirium practice alert, “Assessment and Management of Delirium Across the Life Span,” states that delirium affects up to 80% of critically ill patients in the US, with associated annual costs between $4 billion and $16 billion.
The document reviews risk factors for pediatric and adult patients and the use of validated tools to assess for delirium.
According to AACN, each practice alert is supported by authoritative evidence and seeks to ensure excellence in practice along with promotion of a safe and humane work environment.
Topics address both nursing and interprofessional activities of importance for patients in acute and critical care environments. Some alerts include additional resources for staff education and performance-improvement activities. ![]()

caring for a patient in
an intensive care unit
The American Association of Critical-Care Nurses (AACN) has released updated resources aimed at helping nurses prevent serious complications facing critically ill patients.
The resources, or “practice alerts,” address venous thromboembolism (VTE), delirium, and catheter-associated urinary tract infections (CAUTIs).
Each alert outlines the scope of the problem, summarizes the expected nursing practice, and provides supporting evidence and research.
These documents are available on the AACN website.
The VTE practice alert, “Preventing Venous Thromboembolism in Adults,” notes that VTE affects approximately 900,000 adult patients in the US annually and results in an estimated 300,000 deaths. Furthermore, VTE prevalence is predicted to more than double in the next 35 years.
The document also reviews risk factors for VTE and methods of VTE prophylaxis, including medications and compression devices.
The CAUTI practice alert, “Prevention of Catheter-Associated Urinary Tract Infections in Adults,” notes that urinary tract infections are the most common healthcare-associated infection, and prolonged indwelling catheterization is the major risk factor for CAUTIs.
The document outlines preliminary and ongoing assessment, documentation, and adherence to infection control protocols.
The delirium practice alert, “Assessment and Management of Delirium Across the Life Span,” states that delirium affects up to 80% of critically ill patients in the US, with associated annual costs between $4 billion and $16 billion.
The document reviews risk factors for pediatric and adult patients and the use of validated tools to assess for delirium.
According to AACN, each practice alert is supported by authoritative evidence and seeks to ensure excellence in practice along with promotion of a safe and humane work environment.
Topics address both nursing and interprofessional activities of importance for patients in acute and critical care environments. Some alerts include additional resources for staff education and performance-improvement activities. ![]()

caring for a patient in
an intensive care unit
The American Association of Critical-Care Nurses (AACN) has released updated resources aimed at helping nurses prevent serious complications facing critically ill patients.
The resources, or “practice alerts,” address venous thromboembolism (VTE), delirium, and catheter-associated urinary tract infections (CAUTIs).
Each alert outlines the scope of the problem, summarizes the expected nursing practice, and provides supporting evidence and research.
These documents are available on the AACN website.
The VTE practice alert, “Preventing Venous Thromboembolism in Adults,” notes that VTE affects approximately 900,000 adult patients in the US annually and results in an estimated 300,000 deaths. Furthermore, VTE prevalence is predicted to more than double in the next 35 years.
The document also reviews risk factors for VTE and methods of VTE prophylaxis, including medications and compression devices.
The CAUTI practice alert, “Prevention of Catheter-Associated Urinary Tract Infections in Adults,” notes that urinary tract infections are the most common healthcare-associated infection, and prolonged indwelling catheterization is the major risk factor for CAUTIs.
The document outlines preliminary and ongoing assessment, documentation, and adherence to infection control protocols.
The delirium practice alert, “Assessment and Management of Delirium Across the Life Span,” states that delirium affects up to 80% of critically ill patients in the US, with associated annual costs between $4 billion and $16 billion.
The document reviews risk factors for pediatric and adult patients and the use of validated tools to assess for delirium.
According to AACN, each practice alert is supported by authoritative evidence and seeks to ensure excellence in practice along with promotion of a safe and humane work environment.
Topics address both nursing and interprofessional activities of importance for patients in acute and critical care environments. Some alerts include additional resources for staff education and performance-improvement activities. ![]()
Eligible patients aren’t receiving anticoagulants

NEW ORLEANS—Many US patients who are candidates for treatment with oral anticoagulants (OACs) are not actually receiving these drugs, a large study suggests.
Investigators analyzed information on nearly 1.6 million hospital admissions of patients with atrial fibrillation who were candidates for OAC treatment according to guideline recommendations.
The data showed that only 46% of these patients actually received an OAC at discharge.
“This low rate of OAC use in hospitalized patients highlights an important opportunity to improve care in atrial fibrillation patients,” said Sean Pokorney, MD, of Duke University School of Medicine in Durham, North Carolina.
Dr Pokorney and his colleagues presented this research at the American Heart Association Scientific Sessions (abstract 17636).
The study was supported by Janssen Scientific Affairs, and some of the study’s investigators reported financial relationships with Janssen.
The investigators analyzed data on 1,579,456 hospital admissions across the US, occurring between January 2010 and June 2015, in which patients were treated for atrial fibrillation. The information was taken from the Premier Healthcare Database, which includes data for 1 in 5 hospital discharges in the US.
The patients analyzed were at least 40 years old and stayed in the hospital for at least 1 day. They also had a CHA2DS2-VASc stroke risk score of 2 or higher and were therefore candidates for treatment with an OAC, according to guideline recommendations from the American Heart Association and American College of Cardiology.
The CHA2DS2-VASc stroke risk score considers several factors, including age, sex, and history of congestive heart failure, stroke, diabetes, hypertension, and vascular disease.
“[I]n certain cases, it may not be safe for patients with a high stroke risk score to take blood thinners because of complications that could arise,” Dr Pokorney noted. “Still, we think 50% is too low and that there are thousands of preventable strokes happening in the United States each year because of the low rates of OAC usage.”
Dr Pokorney noted that use of OACs hovered just below 50% across several subgroups in the study.
OAC use by subgroup
The proportion of OAC use was:
- 46% overall
- 47% for patients with prior stroke
- 45% for females
- 46% for non-whites
- 47% for patients with hypertension
- 49% for those with diabetes
- 45% for patients with chronic kidney disease
- 35% for those with dementia
- 38% for patients with a history of falls
- 47% for those younger than 55
- 50% for ages 55-64 and 65-74
- 49% for ages 75-84
- 38% for patients 85 and older.
“This study identified a gap in care and is a critical first step in raising questions about how we can optimize the OAC decision-making process that atrial fibrillation patients and their providers are engaging in during a hospital stay and at the point of discharge,” Dr Pokorney said.
Barriers to OAC use
Dr Pokorney and his colleagues hope to conduct further research to determine what barriers to OAC use might exist. Dr Pokorney said possible barriers could include:
- A lack of understanding about atrial fibrillation and the risk of stroke or fear of using OACs among patients
- Knowledge deficits about stroke prevention or overemphasis of the risks of OACs among healthcare providers
- A view that OAC use is an outpatient issue, rather than an inpatient issue, among healthcare providers and systems.
Study limitations
The data showed whether patients were provided an OAC during their hospital stays. For the purposes of the study, the investigators assumed that those inpatients who were on an OAC within 24 hours of hospital discharge were also prescribed an OAC upon discharge.
However, there was no way to verify that a prescription was indeed made or filled after discharge. Additionally, although the investigators tried to exclude patients who were not candidates for OACs because of the risk of complications, there is the possibility that some remained in the study’s sample. ![]()

NEW ORLEANS—Many US patients who are candidates for treatment with oral anticoagulants (OACs) are not actually receiving these drugs, a large study suggests.
Investigators analyzed information on nearly 1.6 million hospital admissions of patients with atrial fibrillation who were candidates for OAC treatment according to guideline recommendations.
The data showed that only 46% of these patients actually received an OAC at discharge.
“This low rate of OAC use in hospitalized patients highlights an important opportunity to improve care in atrial fibrillation patients,” said Sean Pokorney, MD, of Duke University School of Medicine in Durham, North Carolina.
Dr Pokorney and his colleagues presented this research at the American Heart Association Scientific Sessions (abstract 17636).
The study was supported by Janssen Scientific Affairs, and some of the study’s investigators reported financial relationships with Janssen.
The investigators analyzed data on 1,579,456 hospital admissions across the US, occurring between January 2010 and June 2015, in which patients were treated for atrial fibrillation. The information was taken from the Premier Healthcare Database, which includes data for 1 in 5 hospital discharges in the US.
The patients analyzed were at least 40 years old and stayed in the hospital for at least 1 day. They also had a CHA2DS2-VASc stroke risk score of 2 or higher and were therefore candidates for treatment with an OAC, according to guideline recommendations from the American Heart Association and American College of Cardiology.
The CHA2DS2-VASc stroke risk score considers several factors, including age, sex, and history of congestive heart failure, stroke, diabetes, hypertension, and vascular disease.
“[I]n certain cases, it may not be safe for patients with a high stroke risk score to take blood thinners because of complications that could arise,” Dr Pokorney noted. “Still, we think 50% is too low and that there are thousands of preventable strokes happening in the United States each year because of the low rates of OAC usage.”
Dr Pokorney noted that use of OACs hovered just below 50% across several subgroups in the study.
OAC use by subgroup
The proportion of OAC use was:
- 46% overall
- 47% for patients with prior stroke
- 45% for females
- 46% for non-whites
- 47% for patients with hypertension
- 49% for those with diabetes
- 45% for patients with chronic kidney disease
- 35% for those with dementia
- 38% for patients with a history of falls
- 47% for those younger than 55
- 50% for ages 55-64 and 65-74
- 49% for ages 75-84
- 38% for patients 85 and older.
“This study identified a gap in care and is a critical first step in raising questions about how we can optimize the OAC decision-making process that atrial fibrillation patients and their providers are engaging in during a hospital stay and at the point of discharge,” Dr Pokorney said.
Barriers to OAC use
Dr Pokorney and his colleagues hope to conduct further research to determine what barriers to OAC use might exist. Dr Pokorney said possible barriers could include:
- A lack of understanding about atrial fibrillation and the risk of stroke or fear of using OACs among patients
- Knowledge deficits about stroke prevention or overemphasis of the risks of OACs among healthcare providers
- A view that OAC use is an outpatient issue, rather than an inpatient issue, among healthcare providers and systems.
Study limitations
The data showed whether patients were provided an OAC during their hospital stays. For the purposes of the study, the investigators assumed that those inpatients who were on an OAC within 24 hours of hospital discharge were also prescribed an OAC upon discharge.
However, there was no way to verify that a prescription was indeed made or filled after discharge. Additionally, although the investigators tried to exclude patients who were not candidates for OACs because of the risk of complications, there is the possibility that some remained in the study’s sample. ![]()

NEW ORLEANS—Many US patients who are candidates for treatment with oral anticoagulants (OACs) are not actually receiving these drugs, a large study suggests.
Investigators analyzed information on nearly 1.6 million hospital admissions of patients with atrial fibrillation who were candidates for OAC treatment according to guideline recommendations.
The data showed that only 46% of these patients actually received an OAC at discharge.
“This low rate of OAC use in hospitalized patients highlights an important opportunity to improve care in atrial fibrillation patients,” said Sean Pokorney, MD, of Duke University School of Medicine in Durham, North Carolina.
Dr Pokorney and his colleagues presented this research at the American Heart Association Scientific Sessions (abstract 17636).
The study was supported by Janssen Scientific Affairs, and some of the study’s investigators reported financial relationships with Janssen.
The investigators analyzed data on 1,579,456 hospital admissions across the US, occurring between January 2010 and June 2015, in which patients were treated for atrial fibrillation. The information was taken from the Premier Healthcare Database, which includes data for 1 in 5 hospital discharges in the US.
The patients analyzed were at least 40 years old and stayed in the hospital for at least 1 day. They also had a CHA2DS2-VASc stroke risk score of 2 or higher and were therefore candidates for treatment with an OAC, according to guideline recommendations from the American Heart Association and American College of Cardiology.
The CHA2DS2-VASc stroke risk score considers several factors, including age, sex, and history of congestive heart failure, stroke, diabetes, hypertension, and vascular disease.
“[I]n certain cases, it may not be safe for patients with a high stroke risk score to take blood thinners because of complications that could arise,” Dr Pokorney noted. “Still, we think 50% is too low and that there are thousands of preventable strokes happening in the United States each year because of the low rates of OAC usage.”
Dr Pokorney noted that use of OACs hovered just below 50% across several subgroups in the study.
OAC use by subgroup
The proportion of OAC use was:
- 46% overall
- 47% for patients with prior stroke
- 45% for females
- 46% for non-whites
- 47% for patients with hypertension
- 49% for those with diabetes
- 45% for patients with chronic kidney disease
- 35% for those with dementia
- 38% for patients with a history of falls
- 47% for those younger than 55
- 50% for ages 55-64 and 65-74
- 49% for ages 75-84
- 38% for patients 85 and older.
“This study identified a gap in care and is a critical first step in raising questions about how we can optimize the OAC decision-making process that atrial fibrillation patients and their providers are engaging in during a hospital stay and at the point of discharge,” Dr Pokorney said.
Barriers to OAC use
Dr Pokorney and his colleagues hope to conduct further research to determine what barriers to OAC use might exist. Dr Pokorney said possible barriers could include:
- A lack of understanding about atrial fibrillation and the risk of stroke or fear of using OACs among patients
- Knowledge deficits about stroke prevention or overemphasis of the risks of OACs among healthcare providers
- A view that OAC use is an outpatient issue, rather than an inpatient issue, among healthcare providers and systems.
Study limitations
The data showed whether patients were provided an OAC during their hospital stays. For the purposes of the study, the investigators assumed that those inpatients who were on an OAC within 24 hours of hospital discharge were also prescribed an OAC upon discharge.
However, there was no way to verify that a prescription was indeed made or filled after discharge. Additionally, although the investigators tried to exclude patients who were not candidates for OACs because of the risk of complications, there is the possibility that some remained in the study’s sample. ![]()
Asthma in 2016
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
New patient INFO resource available on IBD and pregnancy
AGA has expanded the Patient INFO Center with a new resource that covers the common questions from patients with IBD who are looking to conceive. This easy-to-access and easy-to-read fact sheet covers frequently asked questions from both women and men, and includes information on staying on medications while trying to get pregnant, precautions pregnant women should be aware of, and much more.
The resource guide was written with Sunanda V. Kane, MD, from Mayo Clinic, Rochester, Minn. View, download, print, or share the IBD & Pregnancy FAQ fact sheet with your IBD patients today.
Visit the Patient INFO Center for all patient education on IBD and more.
AGA has expanded the Patient INFO Center with a new resource that covers the common questions from patients with IBD who are looking to conceive. This easy-to-access and easy-to-read fact sheet covers frequently asked questions from both women and men, and includes information on staying on medications while trying to get pregnant, precautions pregnant women should be aware of, and much more.
The resource guide was written with Sunanda V. Kane, MD, from Mayo Clinic, Rochester, Minn. View, download, print, or share the IBD & Pregnancy FAQ fact sheet with your IBD patients today.
Visit the Patient INFO Center for all patient education on IBD and more.
AGA has expanded the Patient INFO Center with a new resource that covers the common questions from patients with IBD who are looking to conceive. This easy-to-access and easy-to-read fact sheet covers frequently asked questions from both women and men, and includes information on staying on medications while trying to get pregnant, precautions pregnant women should be aware of, and much more.
The resource guide was written with Sunanda V. Kane, MD, from Mayo Clinic, Rochester, Minn. View, download, print, or share the IBD & Pregnancy FAQ fact sheet with your IBD patients today.
Visit the Patient INFO Center for all patient education on IBD and more.
VIDEO: PBC patients with compensated cirrhosis fare well on obeticholic acid
BOSTON – Patients with primary biliary cholangitis (PBC) who have compensated cirrhosis fared just as well on obeticholic acid (OCA) as did PBC patients without cirrhosis, according to an analysis of data from POISE, the pivotal clinical trial for approval of OCA for PBC.
The POISE trial included 36 individuals with PBC and compensated cirrhosis, since cirrhosis “is an endpoint for virtually all liver diseases,” John Vierling, MD, said in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To see how this group fared, Dr. Vierling and his coinvestigators performed a post hoc analysis of the POISE data to examine OCA’s safety and efficacy for patients with compensated cirrhosis. Patients with decompensated cirrhosis were not included in the trial.
Dr. Vierling, chief of hepatology at Baylor College of Medicine, Houston, noted that investigators worked hard to set the bar high for inclusion in the group with cirrhosis, to achieve very high specificity. “We did this by using very stringent criteria of liver biopsy, or transient elastography adjusted for a very high range of kilopascals required to diagnose cirrhosis in cholestatic patients,” he said. To be included, patients also had to have elevated total bilirubin levels and a baseline alkaline phosphatase level greater than five times the upper limit of normal.
Statistically, the patients were evenly distributed across the placebo arm and the two treatment arms, one of which dosed OCA at 10 mg/day; the other treatment arm had flexible dosing at 5-10 mg/day.
The POISE trial used a composite primary efficacy endpoint of achieving an alkaline phosphatase (ALP) less than 1.67 times the upper limit of normal, with total bilirubin within normal limits, and at least a 15% reduction in ALP.
“Significantly more OCA-treated patients with cirrhosis achieved the primary composite endpoint compared to placebo,” Dr. Vierling and his coauthors wrote in a poster presented at the annual meeting for the American Association for the Study of Liver Diseases. The difference was individually significant for all three values that made up the composite primary endpoint as well.
Secondary endpoints included gamma-glutamyltransferase, alanine aminotrasferase, and aspartate aminotransferase, all of which were significantly reduced among patients taking OCA. Patients on placebo saw these values rise over the time period of the study.
There were no new safety signals seen in the post hoc analysis of the group with cirrhosis that were not seen in the trial at large, said Dr. Vierling. Two individuals in the subgroup dropped out of the trial because of pruritis, a similar proportion to that seen in the full trial population.
The drug’s manufacturer, Intercept Pharmaceuticals, is working with the Food and Drug Administration to establish appropriate doses and intervals for obeticholic acid so it may be used safely in individuals with decompensated cirrhosis, said Dr. Vierling.
Obeticholic acid, a farnesoid-X receptor agonist, is an approved agent to use as add-on therapy to ursodeoxycholic acid (UDCA), or as monotherapy for patients who can’t tolerate UDCA.
Dr. Vierling disclosed financial relationships with Intercept Pharmaceuticals and with several other pharmaceutical companies. The study was funded by Intercept Pharmaceuticals.
[email protected]
On Twitter @karioakes
BOSTON – Patients with primary biliary cholangitis (PBC) who have compensated cirrhosis fared just as well on obeticholic acid (OCA) as did PBC patients without cirrhosis, according to an analysis of data from POISE, the pivotal clinical trial for approval of OCA for PBC.
The POISE trial included 36 individuals with PBC and compensated cirrhosis, since cirrhosis “is an endpoint for virtually all liver diseases,” John Vierling, MD, said in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To see how this group fared, Dr. Vierling and his coinvestigators performed a post hoc analysis of the POISE data to examine OCA’s safety and efficacy for patients with compensated cirrhosis. Patients with decompensated cirrhosis were not included in the trial.
Dr. Vierling, chief of hepatology at Baylor College of Medicine, Houston, noted that investigators worked hard to set the bar high for inclusion in the group with cirrhosis, to achieve very high specificity. “We did this by using very stringent criteria of liver biopsy, or transient elastography adjusted for a very high range of kilopascals required to diagnose cirrhosis in cholestatic patients,” he said. To be included, patients also had to have elevated total bilirubin levels and a baseline alkaline phosphatase level greater than five times the upper limit of normal.
Statistically, the patients were evenly distributed across the placebo arm and the two treatment arms, one of which dosed OCA at 10 mg/day; the other treatment arm had flexible dosing at 5-10 mg/day.
The POISE trial used a composite primary efficacy endpoint of achieving an alkaline phosphatase (ALP) less than 1.67 times the upper limit of normal, with total bilirubin within normal limits, and at least a 15% reduction in ALP.
“Significantly more OCA-treated patients with cirrhosis achieved the primary composite endpoint compared to placebo,” Dr. Vierling and his coauthors wrote in a poster presented at the annual meeting for the American Association for the Study of Liver Diseases. The difference was individually significant for all three values that made up the composite primary endpoint as well.
Secondary endpoints included gamma-glutamyltransferase, alanine aminotrasferase, and aspartate aminotransferase, all of which were significantly reduced among patients taking OCA. Patients on placebo saw these values rise over the time period of the study.
There were no new safety signals seen in the post hoc analysis of the group with cirrhosis that were not seen in the trial at large, said Dr. Vierling. Two individuals in the subgroup dropped out of the trial because of pruritis, a similar proportion to that seen in the full trial population.
The drug’s manufacturer, Intercept Pharmaceuticals, is working with the Food and Drug Administration to establish appropriate doses and intervals for obeticholic acid so it may be used safely in individuals with decompensated cirrhosis, said Dr. Vierling.
Obeticholic acid, a farnesoid-X receptor agonist, is an approved agent to use as add-on therapy to ursodeoxycholic acid (UDCA), or as monotherapy for patients who can’t tolerate UDCA.
Dr. Vierling disclosed financial relationships with Intercept Pharmaceuticals and with several other pharmaceutical companies. The study was funded by Intercept Pharmaceuticals.
[email protected]
On Twitter @karioakes
BOSTON – Patients with primary biliary cholangitis (PBC) who have compensated cirrhosis fared just as well on obeticholic acid (OCA) as did PBC patients without cirrhosis, according to an analysis of data from POISE, the pivotal clinical trial for approval of OCA for PBC.
The POISE trial included 36 individuals with PBC and compensated cirrhosis, since cirrhosis “is an endpoint for virtually all liver diseases,” John Vierling, MD, said in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To see how this group fared, Dr. Vierling and his coinvestigators performed a post hoc analysis of the POISE data to examine OCA’s safety and efficacy for patients with compensated cirrhosis. Patients with decompensated cirrhosis were not included in the trial.
Dr. Vierling, chief of hepatology at Baylor College of Medicine, Houston, noted that investigators worked hard to set the bar high for inclusion in the group with cirrhosis, to achieve very high specificity. “We did this by using very stringent criteria of liver biopsy, or transient elastography adjusted for a very high range of kilopascals required to diagnose cirrhosis in cholestatic patients,” he said. To be included, patients also had to have elevated total bilirubin levels and a baseline alkaline phosphatase level greater than five times the upper limit of normal.
Statistically, the patients were evenly distributed across the placebo arm and the two treatment arms, one of which dosed OCA at 10 mg/day; the other treatment arm had flexible dosing at 5-10 mg/day.
The POISE trial used a composite primary efficacy endpoint of achieving an alkaline phosphatase (ALP) less than 1.67 times the upper limit of normal, with total bilirubin within normal limits, and at least a 15% reduction in ALP.
“Significantly more OCA-treated patients with cirrhosis achieved the primary composite endpoint compared to placebo,” Dr. Vierling and his coauthors wrote in a poster presented at the annual meeting for the American Association for the Study of Liver Diseases. The difference was individually significant for all three values that made up the composite primary endpoint as well.
Secondary endpoints included gamma-glutamyltransferase, alanine aminotrasferase, and aspartate aminotransferase, all of which were significantly reduced among patients taking OCA. Patients on placebo saw these values rise over the time period of the study.
There were no new safety signals seen in the post hoc analysis of the group with cirrhosis that were not seen in the trial at large, said Dr. Vierling. Two individuals in the subgroup dropped out of the trial because of pruritis, a similar proportion to that seen in the full trial population.
The drug’s manufacturer, Intercept Pharmaceuticals, is working with the Food and Drug Administration to establish appropriate doses and intervals for obeticholic acid so it may be used safely in individuals with decompensated cirrhosis, said Dr. Vierling.
Obeticholic acid, a farnesoid-X receptor agonist, is an approved agent to use as add-on therapy to ursodeoxycholic acid (UDCA), or as monotherapy for patients who can’t tolerate UDCA.
Dr. Vierling disclosed financial relationships with Intercept Pharmaceuticals and with several other pharmaceutical companies. The study was funded by Intercept Pharmaceuticals.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE LIVER MEETING