User login
AHA: Midlife hypertension linked with cognitive impairment
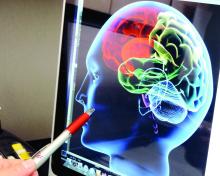
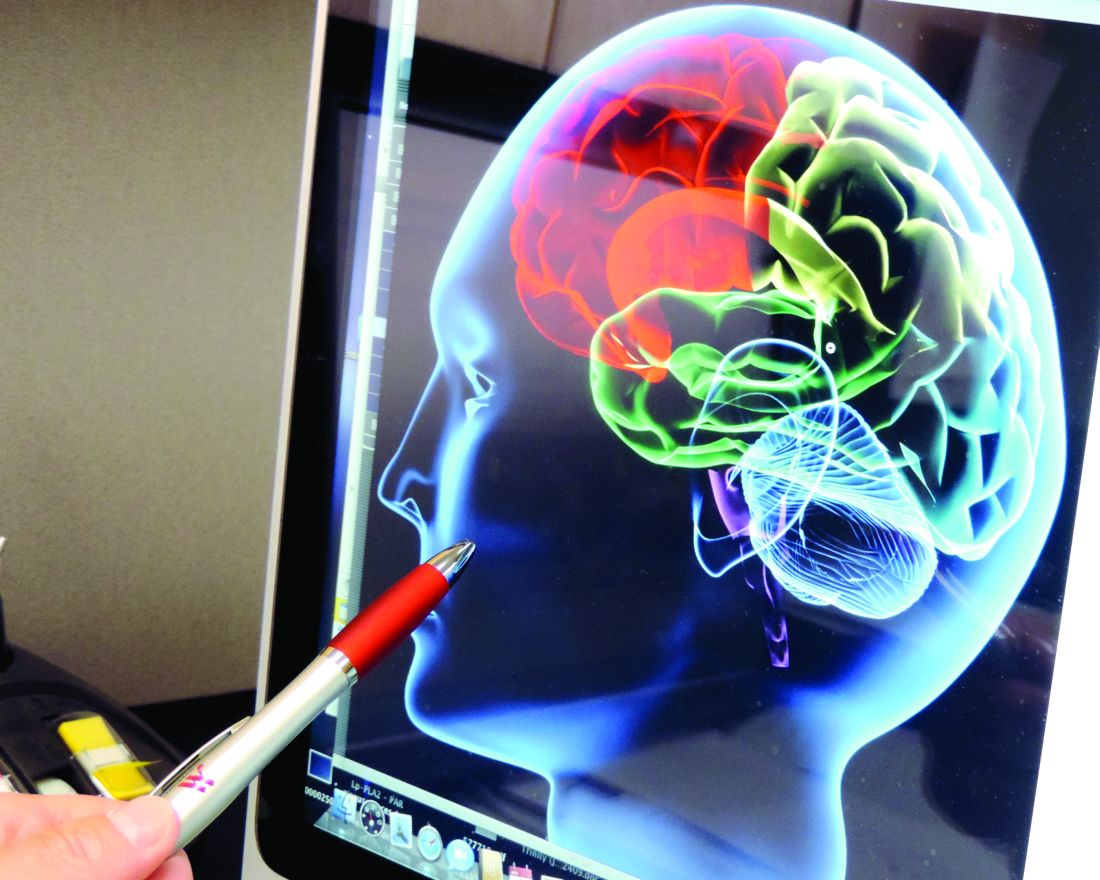
Unchecked hypertension has “devastating” long-term implications for cognitive health, the American Heart Association concluded in a scientific statement.
“Hypertension disrupts the structure and function of cerebral blood vessels, leads to ischemic damage of white matter regions critical for cognitive function, and may promote Alzheimer pathology,” Costantino Iadecola, MD, of Weill Medical College of Cornell University, wrote online with his associates in Hypertension. There is especially strong evidence that hypertension in middle age leads to cognitive dysfunction later in life, although “the cognitive impact of late-life hypertension is less clear,” they said.
The AHA statement summarizes the current evidence on hypertension, cognitive impairment, and the cognitive effects of hypertension therapy. Observational studies linked hypertension to cumulative cerebrovascular damage over the course of life, but clinical trials did not clearly show that antihypertensive drugs can prevent or reverse cognitive decline, the authors reported. In lieu of making evidence-based recommendations, they emphasized “personalized treatment of hypertension, taking into account age, sex, APOE [apolipoprotein E] genotype, metabolic traits, [and] comorbidities” (Hypertension. 2016 Oct 10. doi: 10.1161/HYP.0000000000000053).
The authors wrote on behalf of the American Heart Association Councils on Hypertension, Clinical Cardiology, Cardiovascular Disease in the Young, Cardiovascular and Stroke Nursing, and Quality of Care and Outcomes Research, and Stroke. Dr. Iadecola and nine coauthors had no relevant financial disclosures. The remaining three coauthors disclosed research funding from the National Institutes of Health, Lilly, TauRX, and Biogen, and one of these coauthors also disclosed consulting or advisory board work for Lundbeck Pharmaceuticals and the Dominantly Inherited Alzheimer Network Trials Unit.
Unchecked hypertension has “devastating” long-term implications for cognitive health, the American Heart Association concluded in a scientific statement.
“Hypertension disrupts the structure and function of cerebral blood vessels, leads to ischemic damage of white matter regions critical for cognitive function, and may promote Alzheimer pathology,” Costantino Iadecola, MD, of Weill Medical College of Cornell University, wrote online with his associates in Hypertension. There is especially strong evidence that hypertension in middle age leads to cognitive dysfunction later in life, although “the cognitive impact of late-life hypertension is less clear,” they said.
The AHA statement summarizes the current evidence on hypertension, cognitive impairment, and the cognitive effects of hypertension therapy. Observational studies linked hypertension to cumulative cerebrovascular damage over the course of life, but clinical trials did not clearly show that antihypertensive drugs can prevent or reverse cognitive decline, the authors reported. In lieu of making evidence-based recommendations, they emphasized “personalized treatment of hypertension, taking into account age, sex, APOE [apolipoprotein E] genotype, metabolic traits, [and] comorbidities” (Hypertension. 2016 Oct 10. doi: 10.1161/HYP.0000000000000053).
The authors wrote on behalf of the American Heart Association Councils on Hypertension, Clinical Cardiology, Cardiovascular Disease in the Young, Cardiovascular and Stroke Nursing, and Quality of Care and Outcomes Research, and Stroke. Dr. Iadecola and nine coauthors had no relevant financial disclosures. The remaining three coauthors disclosed research funding from the National Institutes of Health, Lilly, TauRX, and Biogen, and one of these coauthors also disclosed consulting or advisory board work for Lundbeck Pharmaceuticals and the Dominantly Inherited Alzheimer Network Trials Unit.
Unchecked hypertension has “devastating” long-term implications for cognitive health, the American Heart Association concluded in a scientific statement.
“Hypertension disrupts the structure and function of cerebral blood vessels, leads to ischemic damage of white matter regions critical for cognitive function, and may promote Alzheimer pathology,” Costantino Iadecola, MD, of Weill Medical College of Cornell University, wrote online with his associates in Hypertension. There is especially strong evidence that hypertension in middle age leads to cognitive dysfunction later in life, although “the cognitive impact of late-life hypertension is less clear,” they said.
The AHA statement summarizes the current evidence on hypertension, cognitive impairment, and the cognitive effects of hypertension therapy. Observational studies linked hypertension to cumulative cerebrovascular damage over the course of life, but clinical trials did not clearly show that antihypertensive drugs can prevent or reverse cognitive decline, the authors reported. In lieu of making evidence-based recommendations, they emphasized “personalized treatment of hypertension, taking into account age, sex, APOE [apolipoprotein E] genotype, metabolic traits, [and] comorbidities” (Hypertension. 2016 Oct 10. doi: 10.1161/HYP.0000000000000053).
The authors wrote on behalf of the American Heart Association Councils on Hypertension, Clinical Cardiology, Cardiovascular Disease in the Young, Cardiovascular and Stroke Nursing, and Quality of Care and Outcomes Research, and Stroke. Dr. Iadecola and nine coauthors had no relevant financial disclosures. The remaining three coauthors disclosed research funding from the National Institutes of Health, Lilly, TauRX, and Biogen, and one of these coauthors also disclosed consulting or advisory board work for Lundbeck Pharmaceuticals and the Dominantly Inherited Alzheimer Network Trials Unit.
Benefits of lebrikizumab in asthma inconsistent
LONDON – Two large phase III trials with lebrikizumab, a monoclonal antibody that blocks the activity of interleukin-13 (IL-13), produced inconsistent evidence of benefit in patients with uncontrolled asthma, according to a presentation of both sets of data at the annual congress of the European Respiratory Society.
The two phase III studies compared lebrikizumab in doses of 37.5 mg and 125 mg to placebo, each administered subcutaneously every 4 weeks. The results were mixed not only for the primary endpoint, which was a reduction in the rate of exacerbations, but also for several secondary outcomes.
Evenly randomized into three treatment arms, 1,081 patients were enrolled in LAVOLTA I and 1,081 in LAVOLTA II. For entry, patients were required to have a forced expiratory volume in 1 second (FEV1) between 40% and 80% of predicted. Lebrikizumab was added on top of stable background therapy. For the efficacy analyses, patients were divided into those who were biomarker high, defined as having levels of periostin greater than 50 ng/mL or blood eosinophils greater than 300 mcg/L, or biomarker low, defined as having lower levels of periostin and blood eosinophils.
Over the 52-week study period in the biomarker high patients enrolled in LAVOLTA I, the rate of exacerbations was 51% lower among those randomized to the 37.5 mg dose (P less than .001) and 30% lower (P = .0232) among those randomized to the 125-mg dose relative to placebo. In the biomarker low group, a statistically significant 45% reduction in exacerbations was achieved with the 37.5 mg dose relative to placebo (P = .0318), but a 28% reduction in exacerbations, which was achieved in patients taking the 125-mg dose, was not statistically significant.
In the biomarker high patients enrolled in LAVOLTA II, both the 37.5-mg and the 125-mg doses reduced the rate of exacerbations by 26% relative to placebo, but neither reduction reached statistical significance. In the biomarker low patients, the 37.5-mg dose was associated with a 46% increase in exacerbations and the 125-mg dose was associated with a 6% increase in exacerbations relative to placebo.
In LAVOLTA I, lung function (as measured by FEV1) improved in biomarker high patients in both active treatment arms relative to placebo. The relative advantage of taking lebrikizumab was seen only in the biomarker low patients who took the 125-mg dose.
In LAVOLTA II, a similar advantage was observed in biomarker high patients who took both doses of lebrikizumab over biomarker high patients who took the placebo. There were no significant differences in outcomes observed between any treatment arms in the biomarker low group of LAVOLTA II.
Changes in other secondary endpoints were also inconsistent between the two studies. For example, the time to first exacerbation was significantly increased by lebrikizumab in LAVOLTA I but not in LAVOLTA II. Although use of rescue medications was significantly lower in patients receiving lebrikizumab in both studies, the rate of urgent health care utilization, such as emergency department visits, was lower in patients who took lebrikizumab in LAVOLTA 1, but not in those patients who too the drug in LAVOLTA II.
One consistency between the two studies’ outcomes was a lack of “meaningful improvements” having occurred in patients’ quality of life, as measured by the Asthma Quality of Life Questionnaire and the 5-item Asthma Control Questionnaire, according to Dr. Hanania.
The only difference in the occurrences of events associated with impaired immune function observed between any of the groups was a slight increase in the rate of herpes zoster among those treated with lebrikizumab relative to placebo (1.1% vs. 0.3%), Dr. Hanania reported. The only death that occurred in either study involved a patient in a placebo group. The rates of other side effects were comparable among patients in the active treatment and placebo groups.
An improvement in exacerbations would be consistent with the purported mechanism of lebrikizumab, because IL-13 is believed to induce expression of periostin, which, in turn, is thought to mediate bronchial hyper-responsiveness. In previously completed phase II trials, lebrikizumab was associated with a significant reduction in the rate of exacerbations over 24 weeks among patients with moderate to severe asthma (Thorax. 2015;80:748-56).
Asked in the discussion period whether the results of LAVOLTA I and II signaled the end of lebrikizumab studies in asthma control, Dr. Hanania deferred the question to Roche, which is developing this agent.
He noted that additional studies in a more select group of patients may be warranted and that lebrikizumab is still being evaluated for use in other lung diseases, such as idiopathic pulmonary fibrosis.
Dr. Hanania reports financial relationships with Forest, GlaxoSmithKline, Novartis, Pfizer, and Roche.
LONDON – Two large phase III trials with lebrikizumab, a monoclonal antibody that blocks the activity of interleukin-13 (IL-13), produced inconsistent evidence of benefit in patients with uncontrolled asthma, according to a presentation of both sets of data at the annual congress of the European Respiratory Society.
The two phase III studies compared lebrikizumab in doses of 37.5 mg and 125 mg to placebo, each administered subcutaneously every 4 weeks. The results were mixed not only for the primary endpoint, which was a reduction in the rate of exacerbations, but also for several secondary outcomes.
Evenly randomized into three treatment arms, 1,081 patients were enrolled in LAVOLTA I and 1,081 in LAVOLTA II. For entry, patients were required to have a forced expiratory volume in 1 second (FEV1) between 40% and 80% of predicted. Lebrikizumab was added on top of stable background therapy. For the efficacy analyses, patients were divided into those who were biomarker high, defined as having levels of periostin greater than 50 ng/mL or blood eosinophils greater than 300 mcg/L, or biomarker low, defined as having lower levels of periostin and blood eosinophils.
Over the 52-week study period in the biomarker high patients enrolled in LAVOLTA I, the rate of exacerbations was 51% lower among those randomized to the 37.5 mg dose (P less than .001) and 30% lower (P = .0232) among those randomized to the 125-mg dose relative to placebo. In the biomarker low group, a statistically significant 45% reduction in exacerbations was achieved with the 37.5 mg dose relative to placebo (P = .0318), but a 28% reduction in exacerbations, which was achieved in patients taking the 125-mg dose, was not statistically significant.
In the biomarker high patients enrolled in LAVOLTA II, both the 37.5-mg and the 125-mg doses reduced the rate of exacerbations by 26% relative to placebo, but neither reduction reached statistical significance. In the biomarker low patients, the 37.5-mg dose was associated with a 46% increase in exacerbations and the 125-mg dose was associated with a 6% increase in exacerbations relative to placebo.
In LAVOLTA I, lung function (as measured by FEV1) improved in biomarker high patients in both active treatment arms relative to placebo. The relative advantage of taking lebrikizumab was seen only in the biomarker low patients who took the 125-mg dose.
In LAVOLTA II, a similar advantage was observed in biomarker high patients who took both doses of lebrikizumab over biomarker high patients who took the placebo. There were no significant differences in outcomes observed between any treatment arms in the biomarker low group of LAVOLTA II.
Changes in other secondary endpoints were also inconsistent between the two studies. For example, the time to first exacerbation was significantly increased by lebrikizumab in LAVOLTA I but not in LAVOLTA II. Although use of rescue medications was significantly lower in patients receiving lebrikizumab in both studies, the rate of urgent health care utilization, such as emergency department visits, was lower in patients who took lebrikizumab in LAVOLTA 1, but not in those patients who too the drug in LAVOLTA II.
One consistency between the two studies’ outcomes was a lack of “meaningful improvements” having occurred in patients’ quality of life, as measured by the Asthma Quality of Life Questionnaire and the 5-item Asthma Control Questionnaire, according to Dr. Hanania.
The only difference in the occurrences of events associated with impaired immune function observed between any of the groups was a slight increase in the rate of herpes zoster among those treated with lebrikizumab relative to placebo (1.1% vs. 0.3%), Dr. Hanania reported. The only death that occurred in either study involved a patient in a placebo group. The rates of other side effects were comparable among patients in the active treatment and placebo groups.
An improvement in exacerbations would be consistent with the purported mechanism of lebrikizumab, because IL-13 is believed to induce expression of periostin, which, in turn, is thought to mediate bronchial hyper-responsiveness. In previously completed phase II trials, lebrikizumab was associated with a significant reduction in the rate of exacerbations over 24 weeks among patients with moderate to severe asthma (Thorax. 2015;80:748-56).
Asked in the discussion period whether the results of LAVOLTA I and II signaled the end of lebrikizumab studies in asthma control, Dr. Hanania deferred the question to Roche, which is developing this agent.
He noted that additional studies in a more select group of patients may be warranted and that lebrikizumab is still being evaluated for use in other lung diseases, such as idiopathic pulmonary fibrosis.
Dr. Hanania reports financial relationships with Forest, GlaxoSmithKline, Novartis, Pfizer, and Roche.
LONDON – Two large phase III trials with lebrikizumab, a monoclonal antibody that blocks the activity of interleukin-13 (IL-13), produced inconsistent evidence of benefit in patients with uncontrolled asthma, according to a presentation of both sets of data at the annual congress of the European Respiratory Society.
The two phase III studies compared lebrikizumab in doses of 37.5 mg and 125 mg to placebo, each administered subcutaneously every 4 weeks. The results were mixed not only for the primary endpoint, which was a reduction in the rate of exacerbations, but also for several secondary outcomes.
Evenly randomized into three treatment arms, 1,081 patients were enrolled in LAVOLTA I and 1,081 in LAVOLTA II. For entry, patients were required to have a forced expiratory volume in 1 second (FEV1) between 40% and 80% of predicted. Lebrikizumab was added on top of stable background therapy. For the efficacy analyses, patients were divided into those who were biomarker high, defined as having levels of periostin greater than 50 ng/mL or blood eosinophils greater than 300 mcg/L, or biomarker low, defined as having lower levels of periostin and blood eosinophils.
Over the 52-week study period in the biomarker high patients enrolled in LAVOLTA I, the rate of exacerbations was 51% lower among those randomized to the 37.5 mg dose (P less than .001) and 30% lower (P = .0232) among those randomized to the 125-mg dose relative to placebo. In the biomarker low group, a statistically significant 45% reduction in exacerbations was achieved with the 37.5 mg dose relative to placebo (P = .0318), but a 28% reduction in exacerbations, which was achieved in patients taking the 125-mg dose, was not statistically significant.
In the biomarker high patients enrolled in LAVOLTA II, both the 37.5-mg and the 125-mg doses reduced the rate of exacerbations by 26% relative to placebo, but neither reduction reached statistical significance. In the biomarker low patients, the 37.5-mg dose was associated with a 46% increase in exacerbations and the 125-mg dose was associated with a 6% increase in exacerbations relative to placebo.
In LAVOLTA I, lung function (as measured by FEV1) improved in biomarker high patients in both active treatment arms relative to placebo. The relative advantage of taking lebrikizumab was seen only in the biomarker low patients who took the 125-mg dose.
In LAVOLTA II, a similar advantage was observed in biomarker high patients who took both doses of lebrikizumab over biomarker high patients who took the placebo. There were no significant differences in outcomes observed between any treatment arms in the biomarker low group of LAVOLTA II.
Changes in other secondary endpoints were also inconsistent between the two studies. For example, the time to first exacerbation was significantly increased by lebrikizumab in LAVOLTA I but not in LAVOLTA II. Although use of rescue medications was significantly lower in patients receiving lebrikizumab in both studies, the rate of urgent health care utilization, such as emergency department visits, was lower in patients who took lebrikizumab in LAVOLTA 1, but not in those patients who too the drug in LAVOLTA II.
One consistency between the two studies’ outcomes was a lack of “meaningful improvements” having occurred in patients’ quality of life, as measured by the Asthma Quality of Life Questionnaire and the 5-item Asthma Control Questionnaire, according to Dr. Hanania.
The only difference in the occurrences of events associated with impaired immune function observed between any of the groups was a slight increase in the rate of herpes zoster among those treated with lebrikizumab relative to placebo (1.1% vs. 0.3%), Dr. Hanania reported. The only death that occurred in either study involved a patient in a placebo group. The rates of other side effects were comparable among patients in the active treatment and placebo groups.
An improvement in exacerbations would be consistent with the purported mechanism of lebrikizumab, because IL-13 is believed to induce expression of periostin, which, in turn, is thought to mediate bronchial hyper-responsiveness. In previously completed phase II trials, lebrikizumab was associated with a significant reduction in the rate of exacerbations over 24 weeks among patients with moderate to severe asthma (Thorax. 2015;80:748-56).
Asked in the discussion period whether the results of LAVOLTA I and II signaled the end of lebrikizumab studies in asthma control, Dr. Hanania deferred the question to Roche, which is developing this agent.
He noted that additional studies in a more select group of patients may be warranted and that lebrikizumab is still being evaluated for use in other lung diseases, such as idiopathic pulmonary fibrosis.
Dr. Hanania reports financial relationships with Forest, GlaxoSmithKline, Novartis, Pfizer, and Roche.
AT THE ERS CONGRESS 2016
Key clinical point: The primary efficacy endpoint was achieved in only one of two phase III trials of lebrikizumab for treatment of uncontrolled asthma.
Major finding: For lebrikizumab, which targets IL-13, the inconsistency of significant benefit clouds its future as an asthma treatment.
Data source: A placebo-controlled multicenter phase III program.
Disclosures: Dr. Hanania reports financial relationships with Forest, GlaxoSmithKline, Novartis, Pfizer, and Roche.
Patients with stage 1 NSCLC more likely to die of other causes in short term
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with resected stage 1 non-small cell lung cancer are more likely to die of other causes in the short term. Major finding: The non–cancer-specific cumulative incidence of death was higher than CID from lung cancer for up to 1.5 years after resection.
Data source: A single-center competing risk analysis of 2,186 patients who underwent curative-intent resection for stage 1 non–small cell lung cancer.
Disclosures: Senior author Prasad Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Preperitoneal pelvic packing benefits subset of pelvic fracture patients
WAIKOLOA, HAWAII – Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage caused by unstable pelvic fractures, results from a long-term single-center study showed.
“Despite advances in care of the critically injured patient, mortality rates for patients with hemodynamic instability due to pelvic fractures remains greater than 30%,” Clay Cothren Burlew, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “The majority of trauma centers in the United States use angioembolization for hemorrhage control. While angioembolization is effective in controlling arterial sources of hemorrhage, which constitutes about 15% of pelvic bleeding, it does not address the venous or bony sources of hemorrhage within the pelvis. Additionally, time to arterial hemorrhage control using angioembolization usually takes several hours to accomplish, even at the most advanced level I trauma centers.”
For the current study, the researchers hypothesized that pelvic packing would result in a shorter time to intervention and lower mortality, compared with other pelvic fracture management strategies.
Since September 2004 all patients at the Denver Health Medical Center with persistent hemodynamic instability and a pelvic fracture underwent pelvic packing. Indication for packing is a persistent systolic blood pressure of less than 90 mm Hg in the initial resuscitation period despite the transfusion of two units of red blood cells. “Initial stabilization of the pelvis is performed in the emergency department with a pelvic sheet or a binder,” Dr. Burlew explained. “Skeletal fixation of the pelvis with an external fixator or a pelvic C clamp is done concurrently with preperitoneal pelvic packing in the operating room. Angiography is performed for ongoing pelvic bleeding, defined as greater than four units of red blood cells after the patients’ coagulopathy is corrected; immediate postpacking angiography is performed for ongoing hemodynamic instability despite packing and external fixation.”
Over a period of 11 years, 2,293 patients were admitted to Denver Health Medical Center with pelvic fractures. Of these, 128 patients in refractory shock underwent pelvic packing. More than half of the patients (70%) were men, their mean age was 44 years, and their mean Injury Severity Score was 48. The most common mechanism of injury was motor vehicle collision, followed by auto/pedestrian accidents and motorcycle collision. Pelvic fracture patterns included every classification, with anterior posterior compression (APC) III and lateral compression (LC) II patterns predominating. Of these, 18 patients had open pelvic fractures. More than half of patients (70%) had an extremity injury, 65% had a thoracic injury, 63% had an abdominal injury, 43% had a traumatic brain injury, and 38% had a spine injury.
The mean systolic blood pressure of patients was 74 mm Hg and their mean heart rate was 120 beats per minute in the emergency department. Their mean base deficit was 12. However, 32% of patients did not have an arterial blood gas reported during this time frame. When the researchers compared 13% of patients who underwent postpacking angioembolization with those who did not require angioembolization after pelvic packing, they observed no significant differences in age, injury severity score, presenting systolic BP, presenting systolic BP/base deficit, or ED transfusions. The only difference that reached statistical significance was a lower heart rate in the ED in the postpacking angioembolization group, compared with the preperitoneal pelvic packing alone group (110 vs. 121 beats per minute). “We also realized that the angioembolization group received more red blood cells and fresh frozen plasma prior to ICU admission, as well as in the subsequent 24 hours,” Dr. Burlew said.
The majority of patients (84%) had a single packing of the preperitoneal space, while 20 patients underwent repacking when returned to the operating room; all occurred prior to July 2011. “At this time point we determined that there was an increased infection rate with repacking of the pelvis,” Dr. Burlew said. “There were 15 pelvic space infections. Four occurred in those with open fractures or perineal degloving, four developed in those with associated bladder injuries, and seven pelvic space infections occurred in patients without an open fracture.” The infection rate was 6% among patients with a single packing of the pelvis, compared with an infection rate of 45% among those who underwent repacking. “This emphasizes the need for local control of small bleeders when the pelvis in unpacked, using electrocautery and topical hemostatic agents,” she said.
Dr. Burlew went on to note that comparison of two prospective observational study groups with differing management schema may provide salient information. “The AAST multicenter study was an evaluation of the modern-day care of pelvic fracture patients from 11 different centers,” she said. “In that study, the mortality rate among patients presenting in shock who did not undergo pelvic packing was 32%. The mortality rate in our series was 21%, with a relative risk reduction that was statistically significant.” She concluded that pelvic packing “has a faster time to intervention for pelvic fracture–related hemorrhage. Arterial bleed was only present in 13% of patients, rendering angiography of limited utility. Pelvic packing should be utilized for pelvic fracture–related bleeding in the patient who remains hemodynamically unstable despite red blood cell transfusion.”
One of the study authors, Ernest E. Moore, MD, disclosed that he has received research funding from Haemonetics, TEM, and Prytime Medical Devices, and Charles J. Fox, MD, is on the clinical advisory board for Prytime Medical Devices. Dr. Burlew reported having no financial disclosures.
WAIKOLOA, HAWAII – Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage caused by unstable pelvic fractures, results from a long-term single-center study showed.
“Despite advances in care of the critically injured patient, mortality rates for patients with hemodynamic instability due to pelvic fractures remains greater than 30%,” Clay Cothren Burlew, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “The majority of trauma centers in the United States use angioembolization for hemorrhage control. While angioembolization is effective in controlling arterial sources of hemorrhage, which constitutes about 15% of pelvic bleeding, it does not address the venous or bony sources of hemorrhage within the pelvis. Additionally, time to arterial hemorrhage control using angioembolization usually takes several hours to accomplish, even at the most advanced level I trauma centers.”
For the current study, the researchers hypothesized that pelvic packing would result in a shorter time to intervention and lower mortality, compared with other pelvic fracture management strategies.
Since September 2004 all patients at the Denver Health Medical Center with persistent hemodynamic instability and a pelvic fracture underwent pelvic packing. Indication for packing is a persistent systolic blood pressure of less than 90 mm Hg in the initial resuscitation period despite the transfusion of two units of red blood cells. “Initial stabilization of the pelvis is performed in the emergency department with a pelvic sheet or a binder,” Dr. Burlew explained. “Skeletal fixation of the pelvis with an external fixator or a pelvic C clamp is done concurrently with preperitoneal pelvic packing in the operating room. Angiography is performed for ongoing pelvic bleeding, defined as greater than four units of red blood cells after the patients’ coagulopathy is corrected; immediate postpacking angiography is performed for ongoing hemodynamic instability despite packing and external fixation.”
Over a period of 11 years, 2,293 patients were admitted to Denver Health Medical Center with pelvic fractures. Of these, 128 patients in refractory shock underwent pelvic packing. More than half of the patients (70%) were men, their mean age was 44 years, and their mean Injury Severity Score was 48. The most common mechanism of injury was motor vehicle collision, followed by auto/pedestrian accidents and motorcycle collision. Pelvic fracture patterns included every classification, with anterior posterior compression (APC) III and lateral compression (LC) II patterns predominating. Of these, 18 patients had open pelvic fractures. More than half of patients (70%) had an extremity injury, 65% had a thoracic injury, 63% had an abdominal injury, 43% had a traumatic brain injury, and 38% had a spine injury.
The mean systolic blood pressure of patients was 74 mm Hg and their mean heart rate was 120 beats per minute in the emergency department. Their mean base deficit was 12. However, 32% of patients did not have an arterial blood gas reported during this time frame. When the researchers compared 13% of patients who underwent postpacking angioembolization with those who did not require angioembolization after pelvic packing, they observed no significant differences in age, injury severity score, presenting systolic BP, presenting systolic BP/base deficit, or ED transfusions. The only difference that reached statistical significance was a lower heart rate in the ED in the postpacking angioembolization group, compared with the preperitoneal pelvic packing alone group (110 vs. 121 beats per minute). “We also realized that the angioembolization group received more red blood cells and fresh frozen plasma prior to ICU admission, as well as in the subsequent 24 hours,” Dr. Burlew said.
The majority of patients (84%) had a single packing of the preperitoneal space, while 20 patients underwent repacking when returned to the operating room; all occurred prior to July 2011. “At this time point we determined that there was an increased infection rate with repacking of the pelvis,” Dr. Burlew said. “There were 15 pelvic space infections. Four occurred in those with open fractures or perineal degloving, four developed in those with associated bladder injuries, and seven pelvic space infections occurred in patients without an open fracture.” The infection rate was 6% among patients with a single packing of the pelvis, compared with an infection rate of 45% among those who underwent repacking. “This emphasizes the need for local control of small bleeders when the pelvis in unpacked, using electrocautery and topical hemostatic agents,” she said.
Dr. Burlew went on to note that comparison of two prospective observational study groups with differing management schema may provide salient information. “The AAST multicenter study was an evaluation of the modern-day care of pelvic fracture patients from 11 different centers,” she said. “In that study, the mortality rate among patients presenting in shock who did not undergo pelvic packing was 32%. The mortality rate in our series was 21%, with a relative risk reduction that was statistically significant.” She concluded that pelvic packing “has a faster time to intervention for pelvic fracture–related hemorrhage. Arterial bleed was only present in 13% of patients, rendering angiography of limited utility. Pelvic packing should be utilized for pelvic fracture–related bleeding in the patient who remains hemodynamically unstable despite red blood cell transfusion.”
One of the study authors, Ernest E. Moore, MD, disclosed that he has received research funding from Haemonetics, TEM, and Prytime Medical Devices, and Charles J. Fox, MD, is on the clinical advisory board for Prytime Medical Devices. Dr. Burlew reported having no financial disclosures.
WAIKOLOA, HAWAII – Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage caused by unstable pelvic fractures, results from a long-term single-center study showed.
“Despite advances in care of the critically injured patient, mortality rates for patients with hemodynamic instability due to pelvic fractures remains greater than 30%,” Clay Cothren Burlew, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “The majority of trauma centers in the United States use angioembolization for hemorrhage control. While angioembolization is effective in controlling arterial sources of hemorrhage, which constitutes about 15% of pelvic bleeding, it does not address the venous or bony sources of hemorrhage within the pelvis. Additionally, time to arterial hemorrhage control using angioembolization usually takes several hours to accomplish, even at the most advanced level I trauma centers.”
For the current study, the researchers hypothesized that pelvic packing would result in a shorter time to intervention and lower mortality, compared with other pelvic fracture management strategies.
Since September 2004 all patients at the Denver Health Medical Center with persistent hemodynamic instability and a pelvic fracture underwent pelvic packing. Indication for packing is a persistent systolic blood pressure of less than 90 mm Hg in the initial resuscitation period despite the transfusion of two units of red blood cells. “Initial stabilization of the pelvis is performed in the emergency department with a pelvic sheet or a binder,” Dr. Burlew explained. “Skeletal fixation of the pelvis with an external fixator or a pelvic C clamp is done concurrently with preperitoneal pelvic packing in the operating room. Angiography is performed for ongoing pelvic bleeding, defined as greater than four units of red blood cells after the patients’ coagulopathy is corrected; immediate postpacking angiography is performed for ongoing hemodynamic instability despite packing and external fixation.”
Over a period of 11 years, 2,293 patients were admitted to Denver Health Medical Center with pelvic fractures. Of these, 128 patients in refractory shock underwent pelvic packing. More than half of the patients (70%) were men, their mean age was 44 years, and their mean Injury Severity Score was 48. The most common mechanism of injury was motor vehicle collision, followed by auto/pedestrian accidents and motorcycle collision. Pelvic fracture patterns included every classification, with anterior posterior compression (APC) III and lateral compression (LC) II patterns predominating. Of these, 18 patients had open pelvic fractures. More than half of patients (70%) had an extremity injury, 65% had a thoracic injury, 63% had an abdominal injury, 43% had a traumatic brain injury, and 38% had a spine injury.
The mean systolic blood pressure of patients was 74 mm Hg and their mean heart rate was 120 beats per minute in the emergency department. Their mean base deficit was 12. However, 32% of patients did not have an arterial blood gas reported during this time frame. When the researchers compared 13% of patients who underwent postpacking angioembolization with those who did not require angioembolization after pelvic packing, they observed no significant differences in age, injury severity score, presenting systolic BP, presenting systolic BP/base deficit, or ED transfusions. The only difference that reached statistical significance was a lower heart rate in the ED in the postpacking angioembolization group, compared with the preperitoneal pelvic packing alone group (110 vs. 121 beats per minute). “We also realized that the angioembolization group received more red blood cells and fresh frozen plasma prior to ICU admission, as well as in the subsequent 24 hours,” Dr. Burlew said.
The majority of patients (84%) had a single packing of the preperitoneal space, while 20 patients underwent repacking when returned to the operating room; all occurred prior to July 2011. “At this time point we determined that there was an increased infection rate with repacking of the pelvis,” Dr. Burlew said. “There were 15 pelvic space infections. Four occurred in those with open fractures or perineal degloving, four developed in those with associated bladder injuries, and seven pelvic space infections occurred in patients without an open fracture.” The infection rate was 6% among patients with a single packing of the pelvis, compared with an infection rate of 45% among those who underwent repacking. “This emphasizes the need for local control of small bleeders when the pelvis in unpacked, using electrocautery and topical hemostatic agents,” she said.
Dr. Burlew went on to note that comparison of two prospective observational study groups with differing management schema may provide salient information. “The AAST multicenter study was an evaluation of the modern-day care of pelvic fracture patients from 11 different centers,” she said. “In that study, the mortality rate among patients presenting in shock who did not undergo pelvic packing was 32%. The mortality rate in our series was 21%, with a relative risk reduction that was statistically significant.” She concluded that pelvic packing “has a faster time to intervention for pelvic fracture–related hemorrhage. Arterial bleed was only present in 13% of patients, rendering angiography of limited utility. Pelvic packing should be utilized for pelvic fracture–related bleeding in the patient who remains hemodynamically unstable despite red blood cell transfusion.”
One of the study authors, Ernest E. Moore, MD, disclosed that he has received research funding from Haemonetics, TEM, and Prytime Medical Devices, and Charles J. Fox, MD, is on the clinical advisory board for Prytime Medical Devices. Dr. Burlew reported having no financial disclosures.
Key clinical point:
Major finding: Of the 128 patients who underwent preperitoneal pelvic packing for life-threatening hemorrhage caused by unstable pelvic fractures, 27 died, for a mortality rate of 21%, which is significantly lower than other analyses in the medical literature.
Data source: An 11-year study of 2,293 patients who were admitted to Denver Health Medical Center with pelvic fractures.
Disclosures: One of the study authors, Ernest E. Moore, MD, disclosed that he has received research funding from Haemonetics, TEM, and Prytime Medical Devices, and Charles J. Fox, MD, is on the clinical advisory board for Prytime Medical Devices. Dr. Burlew reported having no financial disclosures.
RA prevention trials seek to stop clinical onset of disease
Investigators across the United States and Europe hope to answer questions surrounding the feasibility of preventing rheumatoid arthritis in a series of proof-of-concept clinical trials that are enrolling patients with various risk factors for the disease. At least five trials have begun – with one already completed – to prevent RA with disease-modifying antirheumatic drugs, including a U.S. trial with hydroxychloroquine and European trials involving methotrexate, rituximab, and abatacept. Another trial with atorvastatin is also underway.
All of the trials involve participants with different risk factors for RA and may thereby provide a range of answers about where the most promising areas for disease prevention lie.
Hydroxychloroquine
Perhaps the broadest in scope of all the studies investigating the prevention of RA is the StopRA (Strategy to Prevent the Onset of Clinically Apparent Rheumatoid Arthritis) trial, which is randomizing 200 subjects with an anticyclic citrullinated peptide 3 (anti-CCP3) that’s greater than twice the normal level, with or without arthralgia, to either hydroxychloroquine (HCQ) 200-400 mg/day or placebo for 1 year, followed by 2 years of follow-up. Pilot data indicated that a level of 40 U/mL or higher of anti-CCP3, which is twice the normal level for positivity, gave a 50% chance of developing RA in the next 3 years regardless of symptoms, family history, or other factors.
The trial’s focus on people with an elevated level of anti-CCP3 limits it to just seropositive individuals, specifically those who are anti-citrullinated protein antibody (ACPA)-positive who constitute about 60%-70% of RA patients with established RA.
The investigators chose to use HCQ because it is a safe and well-tolerated medication for RA, and it may have mechanisms of action that may work particularly well in the early development of RA by blocking inflammation and epitope spreading, Dr. Deane said. “While hydroxychloroquine might not be powerful enough to stop active RA, we hypothesize that it’s just the right drug to block key processes early in its development,” Dr. Deane said in an interview, noting that HCQ is also used to block disease flares in palindromic rheumatism and diminish autoantibody responses in systemic lupus erythematosus.
The StopRA trial is being performed by the Autoimmunity Centers of Excellence, a network sponsored by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases. Enrollment began at 18 U.S. sites in April 2016 with screening of first-degree relatives of patients with RA, community-based screening at health fairs, and finding CCP positive individuals without inflammatory arthritis in rheumatology clinics. The investigators estimate that they will need to screen 20,000 individuals to enroll 200 into the study. Dr. Deane said that he and his coinvestigators hope to use the variables that will arise from the diverse population to later determine subgroups who may respond best to the intervention. “We were concerned that if we made everybody too similar we wouldn’t be able to analyze whether symptoms or other factors mattered or not in who responded to the intervention. It may also allow us to catch some people at an early phase of their autoimmunity and others at a later phase and to determine for future studies when is the best time to intervene in preclinical RA with HCQ.”
It’s possible that HCQ will just delay RA rather than prevent it, Dr. Deane said, perhaps indicating that they may find that HCQ needs to be used longer. In any case, by the time the trial is completed in 2020 the investigators hope to gain rich natural history data to determine how to proceed in future trials, whether they use higher doses, longer-term dosing, or try a different target. In addition, the infrastructure of study sites established in StopRA should be incredibly valuable for future prevention studies.
“I envision a future where everybody is screened for RA risk using good blood tests and then given the opportunity for prevention,” first involving high-risk groups such as family members of people with RA, and then ultimately opening up to population-based screening because 90% of RA occurs in individuals without family history of the disease, Dr. Deane said. An analogy for this might be cardiovascular disease, he said, where we screen the cholesterol level of patients before they have had a heart attack, and if it’s above a certain threshold we prescribe a statin, healthy eating, and exercise in an attempt to prevent cardiovascular events. In the same way, it might be possible to screen people for biomarker risk factors and prescribe a drug and/or a lifestyle change to prevent RA or even prevent many of the other rheumatic diseases including lupus and Sjögren’s syndrome.
Methotrexate
The Dutch TREAT EARLIER (Treat Early Arthralgia to Reverse or Limit Impending Exacerbation to Rheumatoid Arthritis) trial is unique among the RA prevention trials for its inclusion of patients with clinically suspect arthralgia and subclinical inflammation on MRI in the hand or foot without any requirement for seropositivity. The trial’s 200 participants are randomized to methotrexate or placebo for 1 year. All subjects receive an initial 120-mg intramuscular injection of methylprednisolone. The primary outcome measured at 2 years is the frequency of clinically detectable arthritis fulfilling the 2010 European League Against Rheumatism/American College of Rheumatology criteria for RA or of unclassified arthritis with a swollen joint count of more than two joints, both persisting for at least 4 weeks. The trial, which is investigator initiated without industry sponsorship, has been enrolling patients for about 1.5 years and will need about that much more time to complete it.
“We do this because we don’t want to confine ourselves to autoantibody-positive patients; we also want to include the autoantibody-negative patients at risk for RA. That’s relevant because in the past, autoantibody-positive patients had more severe disease and more severe joint destruction, but clinically relevant joint destruction doesn’t develop anymore [because of effective treatment], and the problem is more that RA is still a chronic disease. Both ACPA-positive and -negative RA are chronic diseases.”
All the patients included in the trial are considered to be at risk for RA by rheumatologists because of their arthralgia. Because of the clinically relevant population, the results of TREAT EARLIER might be generalized more easily to daily practice, she said in an interview.
Abatacept
The APIPPRA (Arthritis Prevention in the Preclinical Phase of Rheumatoid Arthritis with Abatacept) trial hopes to find out whether RA or clinical synovitis can be prevented with abatacept (Orencia) 125 mg weekly by comparing it against placebo over a 12-month period in 206 British and Dutch patients with arthralgia who are rheumatoid factor (RF) and ACPA-positive. Subjects who are RF negative, but who carry high levels of serum ACPA defined as three times the upper limit of normal may be included. There will be a 1-year follow-up after the intervention period to monitor patients for arthritis.
The ARIAA (Abatacept Reversing Subclinical Inflammation as Measured by MRI in ACPA Positive Arthralgia) trial is randomizing 98 German patients with ACPA-positive arthralgia to 6 months of abatacept 125 mg weekly for 6 months vs. placebo, followed by a 12-month follow-up period. The primary outcome of the double-blind trial is the proportion of patients with an improvement of acute inflammation characterized as improvement of synovitis or osteitis in the MRI of the dominant hand after 6 months of treatment. The proportion of patients who develop RA based on ACR/EULAR 2010 criteria at 6-month intervals is one of a multitude of secondary outcomes that will be measured. Individuals in the study must test positive for ACPA (with or without RF); have joint pain in the hand, feet, knee, shoulder, or elbow for at least 6 weeks prior to inclusion or in past history; and synovitis or osteitis present on MRI of the dominant hand at baseline.
The two abatacept trials, both of which are expected to be completed in 2018, are sponsored by abatacept’s manufacturer, Bristol-Myers Squibb.
Rituximab
Results from the randomized, placebo-controlled PRAIRI (Prevention of RA by Rituximab) trial that were reported at the annual European Congress of Rheumatology in June showed that a single, intravenous infusion of 1,000 mg of rituximab (Rituxan) given to people with arthralgia and a high risk for developing rheumatoid arthritis did not prevent RA, but the treatment did appear to delay the development of RA. During follow-up, 16 of the 40 people in the placebo group (40%) developed RA after a median of 12 months, and 14 of the 41 in the treated arm (34%) developed RA after a median of 17 months. A Kaplan-Meier survival analysis found that the development of RA in 25% of people occurred at about 12 months in the placebo arm, whereas in the intervention arm it did not occur until 24 months.
At three Dutch centers, the PRAIRI trial investigators enrolled people with arthralgia who had never been diagnosed with arthritis, had never used a disease-modifying antirheumatic drug, and had at least one of these two risk factors: a serum level of IgM rheumatoid factor of more than 12.5 IU/mL and a serum level of anticitrullinated peptide antibodies of more than 25 IU/mL. Enrolled participants also needed to have at least one of the following: a serum level of C-reactive protein greater than 3 mg/L, an erythrocyte sedimentation rate of greater than 28 mm/hr, and evidence of subclinical synovitis identified by either ultrasound or MRI.
Atorvastatin
The STAPRA (Statins to Prevent Rheumatoid Arthritis) trial is randomizing 220 people to double-blind treatment with either atorvastatin 40 mg or placebo for 3 years to determine whether the combined lipid-lowering and anti-inflammatory effects of statin therapy may be able to prevent the development of clinical arthritis in people at increased risk for RA. The participants must test positive for RF and ACPA or a high ACPA titer of three times the cut-off value. Arthralgia is not required, and participants must not have current clinically apparent synovitis. The main endpoint is the development of clinical arthritis as confirmed by a rheumatologist in the study. The trial is scheduled to be completed in 2020.
Investigators across the United States and Europe hope to answer questions surrounding the feasibility of preventing rheumatoid arthritis in a series of proof-of-concept clinical trials that are enrolling patients with various risk factors for the disease. At least five trials have begun – with one already completed – to prevent RA with disease-modifying antirheumatic drugs, including a U.S. trial with hydroxychloroquine and European trials involving methotrexate, rituximab, and abatacept. Another trial with atorvastatin is also underway.
All of the trials involve participants with different risk factors for RA and may thereby provide a range of answers about where the most promising areas for disease prevention lie.
Hydroxychloroquine
Perhaps the broadest in scope of all the studies investigating the prevention of RA is the StopRA (Strategy to Prevent the Onset of Clinically Apparent Rheumatoid Arthritis) trial, which is randomizing 200 subjects with an anticyclic citrullinated peptide 3 (anti-CCP3) that’s greater than twice the normal level, with or without arthralgia, to either hydroxychloroquine (HCQ) 200-400 mg/day or placebo for 1 year, followed by 2 years of follow-up. Pilot data indicated that a level of 40 U/mL or higher of anti-CCP3, which is twice the normal level for positivity, gave a 50% chance of developing RA in the next 3 years regardless of symptoms, family history, or other factors.
The trial’s focus on people with an elevated level of anti-CCP3 limits it to just seropositive individuals, specifically those who are anti-citrullinated protein antibody (ACPA)-positive who constitute about 60%-70% of RA patients with established RA.
The investigators chose to use HCQ because it is a safe and well-tolerated medication for RA, and it may have mechanisms of action that may work particularly well in the early development of RA by blocking inflammation and epitope spreading, Dr. Deane said. “While hydroxychloroquine might not be powerful enough to stop active RA, we hypothesize that it’s just the right drug to block key processes early in its development,” Dr. Deane said in an interview, noting that HCQ is also used to block disease flares in palindromic rheumatism and diminish autoantibody responses in systemic lupus erythematosus.
The StopRA trial is being performed by the Autoimmunity Centers of Excellence, a network sponsored by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases. Enrollment began at 18 U.S. sites in April 2016 with screening of first-degree relatives of patients with RA, community-based screening at health fairs, and finding CCP positive individuals without inflammatory arthritis in rheumatology clinics. The investigators estimate that they will need to screen 20,000 individuals to enroll 200 into the study. Dr. Deane said that he and his coinvestigators hope to use the variables that will arise from the diverse population to later determine subgroups who may respond best to the intervention. “We were concerned that if we made everybody too similar we wouldn’t be able to analyze whether symptoms or other factors mattered or not in who responded to the intervention. It may also allow us to catch some people at an early phase of their autoimmunity and others at a later phase and to determine for future studies when is the best time to intervene in preclinical RA with HCQ.”
It’s possible that HCQ will just delay RA rather than prevent it, Dr. Deane said, perhaps indicating that they may find that HCQ needs to be used longer. In any case, by the time the trial is completed in 2020 the investigators hope to gain rich natural history data to determine how to proceed in future trials, whether they use higher doses, longer-term dosing, or try a different target. In addition, the infrastructure of study sites established in StopRA should be incredibly valuable for future prevention studies.
“I envision a future where everybody is screened for RA risk using good blood tests and then given the opportunity for prevention,” first involving high-risk groups such as family members of people with RA, and then ultimately opening up to population-based screening because 90% of RA occurs in individuals without family history of the disease, Dr. Deane said. An analogy for this might be cardiovascular disease, he said, where we screen the cholesterol level of patients before they have had a heart attack, and if it’s above a certain threshold we prescribe a statin, healthy eating, and exercise in an attempt to prevent cardiovascular events. In the same way, it might be possible to screen people for biomarker risk factors and prescribe a drug and/or a lifestyle change to prevent RA or even prevent many of the other rheumatic diseases including lupus and Sjögren’s syndrome.
Methotrexate
The Dutch TREAT EARLIER (Treat Early Arthralgia to Reverse or Limit Impending Exacerbation to Rheumatoid Arthritis) trial is unique among the RA prevention trials for its inclusion of patients with clinically suspect arthralgia and subclinical inflammation on MRI in the hand or foot without any requirement for seropositivity. The trial’s 200 participants are randomized to methotrexate or placebo for 1 year. All subjects receive an initial 120-mg intramuscular injection of methylprednisolone. The primary outcome measured at 2 years is the frequency of clinically detectable arthritis fulfilling the 2010 European League Against Rheumatism/American College of Rheumatology criteria for RA or of unclassified arthritis with a swollen joint count of more than two joints, both persisting for at least 4 weeks. The trial, which is investigator initiated without industry sponsorship, has been enrolling patients for about 1.5 years and will need about that much more time to complete it.
“We do this because we don’t want to confine ourselves to autoantibody-positive patients; we also want to include the autoantibody-negative patients at risk for RA. That’s relevant because in the past, autoantibody-positive patients had more severe disease and more severe joint destruction, but clinically relevant joint destruction doesn’t develop anymore [because of effective treatment], and the problem is more that RA is still a chronic disease. Both ACPA-positive and -negative RA are chronic diseases.”
All the patients included in the trial are considered to be at risk for RA by rheumatologists because of their arthralgia. Because of the clinically relevant population, the results of TREAT EARLIER might be generalized more easily to daily practice, she said in an interview.
Abatacept
The APIPPRA (Arthritis Prevention in the Preclinical Phase of Rheumatoid Arthritis with Abatacept) trial hopes to find out whether RA or clinical synovitis can be prevented with abatacept (Orencia) 125 mg weekly by comparing it against placebo over a 12-month period in 206 British and Dutch patients with arthralgia who are rheumatoid factor (RF) and ACPA-positive. Subjects who are RF negative, but who carry high levels of serum ACPA defined as three times the upper limit of normal may be included. There will be a 1-year follow-up after the intervention period to monitor patients for arthritis.
The ARIAA (Abatacept Reversing Subclinical Inflammation as Measured by MRI in ACPA Positive Arthralgia) trial is randomizing 98 German patients with ACPA-positive arthralgia to 6 months of abatacept 125 mg weekly for 6 months vs. placebo, followed by a 12-month follow-up period. The primary outcome of the double-blind trial is the proportion of patients with an improvement of acute inflammation characterized as improvement of synovitis or osteitis in the MRI of the dominant hand after 6 months of treatment. The proportion of patients who develop RA based on ACR/EULAR 2010 criteria at 6-month intervals is one of a multitude of secondary outcomes that will be measured. Individuals in the study must test positive for ACPA (with or without RF); have joint pain in the hand, feet, knee, shoulder, or elbow for at least 6 weeks prior to inclusion or in past history; and synovitis or osteitis present on MRI of the dominant hand at baseline.
The two abatacept trials, both of which are expected to be completed in 2018, are sponsored by abatacept’s manufacturer, Bristol-Myers Squibb.
Rituximab
Results from the randomized, placebo-controlled PRAIRI (Prevention of RA by Rituximab) trial that were reported at the annual European Congress of Rheumatology in June showed that a single, intravenous infusion of 1,000 mg of rituximab (Rituxan) given to people with arthralgia and a high risk for developing rheumatoid arthritis did not prevent RA, but the treatment did appear to delay the development of RA. During follow-up, 16 of the 40 people in the placebo group (40%) developed RA after a median of 12 months, and 14 of the 41 in the treated arm (34%) developed RA after a median of 17 months. A Kaplan-Meier survival analysis found that the development of RA in 25% of people occurred at about 12 months in the placebo arm, whereas in the intervention arm it did not occur until 24 months.
At three Dutch centers, the PRAIRI trial investigators enrolled people with arthralgia who had never been diagnosed with arthritis, had never used a disease-modifying antirheumatic drug, and had at least one of these two risk factors: a serum level of IgM rheumatoid factor of more than 12.5 IU/mL and a serum level of anticitrullinated peptide antibodies of more than 25 IU/mL. Enrolled participants also needed to have at least one of the following: a serum level of C-reactive protein greater than 3 mg/L, an erythrocyte sedimentation rate of greater than 28 mm/hr, and evidence of subclinical synovitis identified by either ultrasound or MRI.
Atorvastatin
The STAPRA (Statins to Prevent Rheumatoid Arthritis) trial is randomizing 220 people to double-blind treatment with either atorvastatin 40 mg or placebo for 3 years to determine whether the combined lipid-lowering and anti-inflammatory effects of statin therapy may be able to prevent the development of clinical arthritis in people at increased risk for RA. The participants must test positive for RF and ACPA or a high ACPA titer of three times the cut-off value. Arthralgia is not required, and participants must not have current clinically apparent synovitis. The main endpoint is the development of clinical arthritis as confirmed by a rheumatologist in the study. The trial is scheduled to be completed in 2020.
Investigators across the United States and Europe hope to answer questions surrounding the feasibility of preventing rheumatoid arthritis in a series of proof-of-concept clinical trials that are enrolling patients with various risk factors for the disease. At least five trials have begun – with one already completed – to prevent RA with disease-modifying antirheumatic drugs, including a U.S. trial with hydroxychloroquine and European trials involving methotrexate, rituximab, and abatacept. Another trial with atorvastatin is also underway.
All of the trials involve participants with different risk factors for RA and may thereby provide a range of answers about where the most promising areas for disease prevention lie.
Hydroxychloroquine
Perhaps the broadest in scope of all the studies investigating the prevention of RA is the StopRA (Strategy to Prevent the Onset of Clinically Apparent Rheumatoid Arthritis) trial, which is randomizing 200 subjects with an anticyclic citrullinated peptide 3 (anti-CCP3) that’s greater than twice the normal level, with or without arthralgia, to either hydroxychloroquine (HCQ) 200-400 mg/day or placebo for 1 year, followed by 2 years of follow-up. Pilot data indicated that a level of 40 U/mL or higher of anti-CCP3, which is twice the normal level for positivity, gave a 50% chance of developing RA in the next 3 years regardless of symptoms, family history, or other factors.
The trial’s focus on people with an elevated level of anti-CCP3 limits it to just seropositive individuals, specifically those who are anti-citrullinated protein antibody (ACPA)-positive who constitute about 60%-70% of RA patients with established RA.
The investigators chose to use HCQ because it is a safe and well-tolerated medication for RA, and it may have mechanisms of action that may work particularly well in the early development of RA by blocking inflammation and epitope spreading, Dr. Deane said. “While hydroxychloroquine might not be powerful enough to stop active RA, we hypothesize that it’s just the right drug to block key processes early in its development,” Dr. Deane said in an interview, noting that HCQ is also used to block disease flares in palindromic rheumatism and diminish autoantibody responses in systemic lupus erythematosus.
The StopRA trial is being performed by the Autoimmunity Centers of Excellence, a network sponsored by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases. Enrollment began at 18 U.S. sites in April 2016 with screening of first-degree relatives of patients with RA, community-based screening at health fairs, and finding CCP positive individuals without inflammatory arthritis in rheumatology clinics. The investigators estimate that they will need to screen 20,000 individuals to enroll 200 into the study. Dr. Deane said that he and his coinvestigators hope to use the variables that will arise from the diverse population to later determine subgroups who may respond best to the intervention. “We were concerned that if we made everybody too similar we wouldn’t be able to analyze whether symptoms or other factors mattered or not in who responded to the intervention. It may also allow us to catch some people at an early phase of their autoimmunity and others at a later phase and to determine for future studies when is the best time to intervene in preclinical RA with HCQ.”
It’s possible that HCQ will just delay RA rather than prevent it, Dr. Deane said, perhaps indicating that they may find that HCQ needs to be used longer. In any case, by the time the trial is completed in 2020 the investigators hope to gain rich natural history data to determine how to proceed in future trials, whether they use higher doses, longer-term dosing, or try a different target. In addition, the infrastructure of study sites established in StopRA should be incredibly valuable for future prevention studies.
“I envision a future where everybody is screened for RA risk using good blood tests and then given the opportunity for prevention,” first involving high-risk groups such as family members of people with RA, and then ultimately opening up to population-based screening because 90% of RA occurs in individuals without family history of the disease, Dr. Deane said. An analogy for this might be cardiovascular disease, he said, where we screen the cholesterol level of patients before they have had a heart attack, and if it’s above a certain threshold we prescribe a statin, healthy eating, and exercise in an attempt to prevent cardiovascular events. In the same way, it might be possible to screen people for biomarker risk factors and prescribe a drug and/or a lifestyle change to prevent RA or even prevent many of the other rheumatic diseases including lupus and Sjögren’s syndrome.
Methotrexate
The Dutch TREAT EARLIER (Treat Early Arthralgia to Reverse or Limit Impending Exacerbation to Rheumatoid Arthritis) trial is unique among the RA prevention trials for its inclusion of patients with clinically suspect arthralgia and subclinical inflammation on MRI in the hand or foot without any requirement for seropositivity. The trial’s 200 participants are randomized to methotrexate or placebo for 1 year. All subjects receive an initial 120-mg intramuscular injection of methylprednisolone. The primary outcome measured at 2 years is the frequency of clinically detectable arthritis fulfilling the 2010 European League Against Rheumatism/American College of Rheumatology criteria for RA or of unclassified arthritis with a swollen joint count of more than two joints, both persisting for at least 4 weeks. The trial, which is investigator initiated without industry sponsorship, has been enrolling patients for about 1.5 years and will need about that much more time to complete it.
“We do this because we don’t want to confine ourselves to autoantibody-positive patients; we also want to include the autoantibody-negative patients at risk for RA. That’s relevant because in the past, autoantibody-positive patients had more severe disease and more severe joint destruction, but clinically relevant joint destruction doesn’t develop anymore [because of effective treatment], and the problem is more that RA is still a chronic disease. Both ACPA-positive and -negative RA are chronic diseases.”
All the patients included in the trial are considered to be at risk for RA by rheumatologists because of their arthralgia. Because of the clinically relevant population, the results of TREAT EARLIER might be generalized more easily to daily practice, she said in an interview.
Abatacept
The APIPPRA (Arthritis Prevention in the Preclinical Phase of Rheumatoid Arthritis with Abatacept) trial hopes to find out whether RA or clinical synovitis can be prevented with abatacept (Orencia) 125 mg weekly by comparing it against placebo over a 12-month period in 206 British and Dutch patients with arthralgia who are rheumatoid factor (RF) and ACPA-positive. Subjects who are RF negative, but who carry high levels of serum ACPA defined as three times the upper limit of normal may be included. There will be a 1-year follow-up after the intervention period to monitor patients for arthritis.
The ARIAA (Abatacept Reversing Subclinical Inflammation as Measured by MRI in ACPA Positive Arthralgia) trial is randomizing 98 German patients with ACPA-positive arthralgia to 6 months of abatacept 125 mg weekly for 6 months vs. placebo, followed by a 12-month follow-up period. The primary outcome of the double-blind trial is the proportion of patients with an improvement of acute inflammation characterized as improvement of synovitis or osteitis in the MRI of the dominant hand after 6 months of treatment. The proportion of patients who develop RA based on ACR/EULAR 2010 criteria at 6-month intervals is one of a multitude of secondary outcomes that will be measured. Individuals in the study must test positive for ACPA (with or without RF); have joint pain in the hand, feet, knee, shoulder, or elbow for at least 6 weeks prior to inclusion or in past history; and synovitis or osteitis present on MRI of the dominant hand at baseline.
The two abatacept trials, both of which are expected to be completed in 2018, are sponsored by abatacept’s manufacturer, Bristol-Myers Squibb.
Rituximab
Results from the randomized, placebo-controlled PRAIRI (Prevention of RA by Rituximab) trial that were reported at the annual European Congress of Rheumatology in June showed that a single, intravenous infusion of 1,000 mg of rituximab (Rituxan) given to people with arthralgia and a high risk for developing rheumatoid arthritis did not prevent RA, but the treatment did appear to delay the development of RA. During follow-up, 16 of the 40 people in the placebo group (40%) developed RA after a median of 12 months, and 14 of the 41 in the treated arm (34%) developed RA after a median of 17 months. A Kaplan-Meier survival analysis found that the development of RA in 25% of people occurred at about 12 months in the placebo arm, whereas in the intervention arm it did not occur until 24 months.
At three Dutch centers, the PRAIRI trial investigators enrolled people with arthralgia who had never been diagnosed with arthritis, had never used a disease-modifying antirheumatic drug, and had at least one of these two risk factors: a serum level of IgM rheumatoid factor of more than 12.5 IU/mL and a serum level of anticitrullinated peptide antibodies of more than 25 IU/mL. Enrolled participants also needed to have at least one of the following: a serum level of C-reactive protein greater than 3 mg/L, an erythrocyte sedimentation rate of greater than 28 mm/hr, and evidence of subclinical synovitis identified by either ultrasound or MRI.
Atorvastatin
The STAPRA (Statins to Prevent Rheumatoid Arthritis) trial is randomizing 220 people to double-blind treatment with either atorvastatin 40 mg or placebo for 3 years to determine whether the combined lipid-lowering and anti-inflammatory effects of statin therapy may be able to prevent the development of clinical arthritis in people at increased risk for RA. The participants must test positive for RF and ACPA or a high ACPA titer of three times the cut-off value. Arthralgia is not required, and participants must not have current clinically apparent synovitis. The main endpoint is the development of clinical arthritis as confirmed by a rheumatologist in the study. The trial is scheduled to be completed in 2020.
CT evidence of emphysema in ever smokers predicts activity limitation
LONDON – Recent evidence of ever smokers having measurable deficits in activity even when lung function is preserved has been further expanded by findings suggesting a different phenotype for patients in this group with radiologic evidence of emphysema, according to data from SPIROMICS (Subpopulations and Intermediate Outcome Measures In COPD Study).
The most recent data were presented at the annual congress of the European Respiratory Society.
Earlier this year, published data drawn from SPIROMICS demonstrated that current and former smokers with preserved lung function (forced expiratory volume in 1 second:forced vital capacity greater than or equal to 0.70) had activity limitations, respiratory symptoms, and exacerbations even though they did not meet the current definition of chronic obstructive pulmonary disease (COPD) (N Engl J Med. 2016;374:1811-21). In the new analysis, further distinctions could be made for patients in this subgroup who also had emphysema on computed tomography (CT).
“Among smokers with preserved lung function, emphysema on CT was associated with reduced activity levels and desaturation on the 6-minute walk test (6MWT),” reported Christian M. Lo Cascio, MD, Columbia University, New York. Compared with smokers with preserved lung function without CT evidence of emphysema, the patients with emphysema on CT did not have more symptoms or exacerbations.
“These and prior findings suggest two distinct but overlapping phenotypes in ever smokers with preserved lung function: an airway disease with respiratory symptoms and frequent exacerbations and emphysema characterized by activity limitation and increased mortality,” said Dr. Lo Cascio.
SPIROMICS, an observational study supported by the National Heart, Lung, and Blood Institute, has enrolled current and former smokers at six centers in the United States to prospectively analyze biomarker, genetic, and clinical data. In the previously published analysis, the focus was on the difference between 849 ever smokers with preserved lung function and 963 patients with mild to moderate COPD. In the data presented by Dr. Lo Cascio, 901 SPIROMICS patients with preserved lung function were analyzed with the focus on the difference between the 66 (7%) who had radiologic evidence of emphysema and the 835 (93%) who had undergone CT indicating that they did not have emphysema.
“Our hypothesis was that the subgroup of patients with emphysema on CT would have lower activity levels, greater desaturation of at least 4% on the 6MWT, more respiratory symptoms, and more exacerbations,” Dr. Lo Cascio reported.
The hypothesis was only half correct. As measured on the activity component of the St. Georges Respiratory Questionnaire, there was about a 10-point (P less than .001) greater reduction in reported activity levels among those with emphysema on CT relative to those without. In addition, the odds ratio (OR) for significant oxygen desaturation, defined as a 4% fall in oxygen saturation of hemoglobin during the 6MWD, was approximately two times greater (P less than .001) for the patients with CT evidence of emphysema.
There were no significant differences between the two groups in walking distance on the 6MWD. Both sets of patients had the same rate of exacerbations and scores on the symptom component of the St. Georges Respiratory Questionnaire, which captures cough, phlegm, wheezing, shortness of breath, and symptom-free days.
Overall, when ever smokers with preserved lung function were subdivided into those with and without CT evidence of emphysema, several similarities were found between the two groups. For those with CT evidence of emphysema and those with no CT evidence of emphysema, mean pack-years of smoking were 43 years and 40 years, respectively, and average body mass indexes were 28.1 and 29.1, respectively. The percentage of patients who had a COPD Assessment Test score of greater than or equal to 10 was 61% among those with CT evidence of emphysema, vs. 50% among those patients without CT evidence of emphysema.
When asked if looking for emphysema on CT in ever smokers with preserved lung function might have clinical utility, Dr. Lo Cascio suggested that it is not yet clear how this information might change management. These data strengthen other evidence from SPIROMICS that ever smokers who do not meet the definition of COPD already have a significant disease burden, he said.
Dr. Lo Cascio reports no relevant financial relationships.
LONDON – Recent evidence of ever smokers having measurable deficits in activity even when lung function is preserved has been further expanded by findings suggesting a different phenotype for patients in this group with radiologic evidence of emphysema, according to data from SPIROMICS (Subpopulations and Intermediate Outcome Measures In COPD Study).
The most recent data were presented at the annual congress of the European Respiratory Society.
Earlier this year, published data drawn from SPIROMICS demonstrated that current and former smokers with preserved lung function (forced expiratory volume in 1 second:forced vital capacity greater than or equal to 0.70) had activity limitations, respiratory symptoms, and exacerbations even though they did not meet the current definition of chronic obstructive pulmonary disease (COPD) (N Engl J Med. 2016;374:1811-21). In the new analysis, further distinctions could be made for patients in this subgroup who also had emphysema on computed tomography (CT).
“Among smokers with preserved lung function, emphysema on CT was associated with reduced activity levels and desaturation on the 6-minute walk test (6MWT),” reported Christian M. Lo Cascio, MD, Columbia University, New York. Compared with smokers with preserved lung function without CT evidence of emphysema, the patients with emphysema on CT did not have more symptoms or exacerbations.
“These and prior findings suggest two distinct but overlapping phenotypes in ever smokers with preserved lung function: an airway disease with respiratory symptoms and frequent exacerbations and emphysema characterized by activity limitation and increased mortality,” said Dr. Lo Cascio.
SPIROMICS, an observational study supported by the National Heart, Lung, and Blood Institute, has enrolled current and former smokers at six centers in the United States to prospectively analyze biomarker, genetic, and clinical data. In the previously published analysis, the focus was on the difference between 849 ever smokers with preserved lung function and 963 patients with mild to moderate COPD. In the data presented by Dr. Lo Cascio, 901 SPIROMICS patients with preserved lung function were analyzed with the focus on the difference between the 66 (7%) who had radiologic evidence of emphysema and the 835 (93%) who had undergone CT indicating that they did not have emphysema.
“Our hypothesis was that the subgroup of patients with emphysema on CT would have lower activity levels, greater desaturation of at least 4% on the 6MWT, more respiratory symptoms, and more exacerbations,” Dr. Lo Cascio reported.
The hypothesis was only half correct. As measured on the activity component of the St. Georges Respiratory Questionnaire, there was about a 10-point (P less than .001) greater reduction in reported activity levels among those with emphysema on CT relative to those without. In addition, the odds ratio (OR) for significant oxygen desaturation, defined as a 4% fall in oxygen saturation of hemoglobin during the 6MWD, was approximately two times greater (P less than .001) for the patients with CT evidence of emphysema.
There were no significant differences between the two groups in walking distance on the 6MWD. Both sets of patients had the same rate of exacerbations and scores on the symptom component of the St. Georges Respiratory Questionnaire, which captures cough, phlegm, wheezing, shortness of breath, and symptom-free days.
Overall, when ever smokers with preserved lung function were subdivided into those with and without CT evidence of emphysema, several similarities were found between the two groups. For those with CT evidence of emphysema and those with no CT evidence of emphysema, mean pack-years of smoking were 43 years and 40 years, respectively, and average body mass indexes were 28.1 and 29.1, respectively. The percentage of patients who had a COPD Assessment Test score of greater than or equal to 10 was 61% among those with CT evidence of emphysema, vs. 50% among those patients without CT evidence of emphysema.
When asked if looking for emphysema on CT in ever smokers with preserved lung function might have clinical utility, Dr. Lo Cascio suggested that it is not yet clear how this information might change management. These data strengthen other evidence from SPIROMICS that ever smokers who do not meet the definition of COPD already have a significant disease burden, he said.
Dr. Lo Cascio reports no relevant financial relationships.
LONDON – Recent evidence of ever smokers having measurable deficits in activity even when lung function is preserved has been further expanded by findings suggesting a different phenotype for patients in this group with radiologic evidence of emphysema, according to data from SPIROMICS (Subpopulations and Intermediate Outcome Measures In COPD Study).
The most recent data were presented at the annual congress of the European Respiratory Society.
Earlier this year, published data drawn from SPIROMICS demonstrated that current and former smokers with preserved lung function (forced expiratory volume in 1 second:forced vital capacity greater than or equal to 0.70) had activity limitations, respiratory symptoms, and exacerbations even though they did not meet the current definition of chronic obstructive pulmonary disease (COPD) (N Engl J Med. 2016;374:1811-21). In the new analysis, further distinctions could be made for patients in this subgroup who also had emphysema on computed tomography (CT).
“Among smokers with preserved lung function, emphysema on CT was associated with reduced activity levels and desaturation on the 6-minute walk test (6MWT),” reported Christian M. Lo Cascio, MD, Columbia University, New York. Compared with smokers with preserved lung function without CT evidence of emphysema, the patients with emphysema on CT did not have more symptoms or exacerbations.
“These and prior findings suggest two distinct but overlapping phenotypes in ever smokers with preserved lung function: an airway disease with respiratory symptoms and frequent exacerbations and emphysema characterized by activity limitation and increased mortality,” said Dr. Lo Cascio.
SPIROMICS, an observational study supported by the National Heart, Lung, and Blood Institute, has enrolled current and former smokers at six centers in the United States to prospectively analyze biomarker, genetic, and clinical data. In the previously published analysis, the focus was on the difference between 849 ever smokers with preserved lung function and 963 patients with mild to moderate COPD. In the data presented by Dr. Lo Cascio, 901 SPIROMICS patients with preserved lung function were analyzed with the focus on the difference between the 66 (7%) who had radiologic evidence of emphysema and the 835 (93%) who had undergone CT indicating that they did not have emphysema.
“Our hypothesis was that the subgroup of patients with emphysema on CT would have lower activity levels, greater desaturation of at least 4% on the 6MWT, more respiratory symptoms, and more exacerbations,” Dr. Lo Cascio reported.
The hypothesis was only half correct. As measured on the activity component of the St. Georges Respiratory Questionnaire, there was about a 10-point (P less than .001) greater reduction in reported activity levels among those with emphysema on CT relative to those without. In addition, the odds ratio (OR) for significant oxygen desaturation, defined as a 4% fall in oxygen saturation of hemoglobin during the 6MWD, was approximately two times greater (P less than .001) for the patients with CT evidence of emphysema.
There were no significant differences between the two groups in walking distance on the 6MWD. Both sets of patients had the same rate of exacerbations and scores on the symptom component of the St. Georges Respiratory Questionnaire, which captures cough, phlegm, wheezing, shortness of breath, and symptom-free days.
Overall, when ever smokers with preserved lung function were subdivided into those with and without CT evidence of emphysema, several similarities were found between the two groups. For those with CT evidence of emphysema and those with no CT evidence of emphysema, mean pack-years of smoking were 43 years and 40 years, respectively, and average body mass indexes were 28.1 and 29.1, respectively. The percentage of patients who had a COPD Assessment Test score of greater than or equal to 10 was 61% among those with CT evidence of emphysema, vs. 50% among those patients without CT evidence of emphysema.
When asked if looking for emphysema on CT in ever smokers with preserved lung function might have clinical utility, Dr. Lo Cascio suggested that it is not yet clear how this information might change management. These data strengthen other evidence from SPIROMICS that ever smokers who do not meet the definition of COPD already have a significant disease burden, he said.
Dr. Lo Cascio reports no relevant financial relationships.
AT THE ERS CONGRESS 2016
Key clinical point: Among ever smokers with preserved lung function, CT evidence of emphysema identifies a group with even greater functional impairment.
Major finding: Despite preserved lung function in both groups, physical activity and desaturation on exercise was greater (P less than .001) in patients with emphysema.
Data source: Subpopulation analysis of prospective registry study.
Disclosures: Dr. Lo Cascio reports no relevant financial relationships.
Cariprazine shows efficacy for schizophrenia’s negative symptoms
VIENNA – Cariprazine appears to improve predominant negative symptoms of schizophrenia as well as functional status in affected patients, István Bitter, MD, PhD, DSci reported at the annual congress of the European College of Neuropsychopharmacology.
Cariprazine, a dopamine D3/D2 receptor partial agonist, was approved in the United States in 2015 as Vraylar for adults with bipolar disorder or schizophrenia. The drug remains investigational in Europe.
“There is very clearly an unmet need for the treatment of deficit schizophrenia, or schizophrenia with predominant and persistent negative symptoms,” the psychiatrist observed.
The strong efficacy signal for cariprazine in patients with predominant negative symptoms was identified in a post hoc analysis of a randomized, double-blind, phase III head-to-head comparison of cariprazine versus risperidone (Risperdal) in 461 affected adults. Dr. Bitter was a coinvestigator in the 26-week multicenter European study, which was preceded by a 4-week lead-in phase in which participants were uptitrated to a target dose of cariprazine at 4.5 mg/day or risperidone at 4 mg/day.
The primary endpoint in the post hoc analysis was change from baseline on the Positive and Negative Symptoms Factor Score for Negative Symptoms (PANSS-FSNS). A significant difference in favor of cariprazine was seen by week 14, and the gap steadily enlarged thereafter through the remainder of the study. The difference was significant in five of the seven items on the PANSS-FSNS: blunted affect, emotional withdrawal, poor rapport, active social avoidance, and passive/apathetic social withdrawal. Cariprazine also outperformed risperidone on the other two elements of the scale – motor retardation, and lack of spontaneity and flow of conversation – but in those domains, the difference didn’t reach statistical significance.
Patients randomized to cariprazine also fared well on secondary endpoints. They displayed a significantly greater overall improvement in Marder factor scores over 26 weeks than the risperidone-treated group. This advantage was driven by a strong, statistically significant difference in the domain of difficulty in abstract thinking. However, the cariprazine group also showed nonsignificant trends for greater improvement in conceptual disorganization, mannerisms and posturing, disorientation, poor attention, and disturbed volition.
As evidence that cariprazine was exerting specific benefit on negative symptoms, Dr. Bitter cited the fact that the drug showed no significant difference from risperidone in change from baseline on the Marder factor scores for uncontrolled hostility/excitement and anxiety/depression, nor in the Calgary Depression Rating Scale for Schizophrenia.
Regulatory agencies in the United States and Europe have made it clear that, for a drug to obtain an indication for treatment of predominant negative symptoms of schizophrenia, it also has to show evidence of improved patient social function. The cariprazine group showed a significantly greater improvement on the PANSS-FSNS Personal and Social Performance Score. This advantage over risperidone-treated patients reached statistical significance at week 10 and broadened thereafter until study conclusion. Improvements in the subdomain scores for self-care, socially useful activities, and personal and social relationships led the way.
These positive results for cariprazine represent a sharp departure from the psychiatric field’s long-standing history of failed therapeutic attempts to improve negative symptoms. A comprehensive 2015 meta-analysis of all 168 randomized, placebo-controlled studies of various potential treatments for predominant negative symptoms of schizophrenia published through 2013 concluded that none of them provided evidence of clinically meaningful improvement (Schizophr Bull. 2015 Jul;41[4]:892-9).
Dr. Bitter observed that, since that discouraging meta-analysis, several additional novel agents for treatment of predominant negative symptoms of schizophrenia that showed “fantastic” promise in phase II studies subsequently went down in flames in advanced phase III trials. Among those were D-serine as an add-on to second-generation antipsychotics; encenicline, an alpha-7 nicotinic acetylcholine receptor antagonist; pomaglumetad methionil, a selective agonist for glutamate receptor subtypes mGluR2 and mGluR3; and bitopertin, a glycine reuptake inhibitor.
“This is basically a very negative message, that there is not much we can do about negative symptoms. But depending on who you ask, you can get a little bit more optimistic picture,” Dr. Bitter said.
That’s because there are considerable differences among the studies both in how negative symptoms are defined as well as in the measurement tools employed. For example, the Scale for Assessment of Negative Symptoms (SANS) includes items which aren’t strictly speaking part of the negative symptoms concept, so it yields skewed results. The Brief Psychiatric Rating Scale is a relic that has been abandoned by younger psychiatrists. So at present, the PANS-FSNS is the best available tool, and a reasonable common sense definition of predominant negative symptoms of schizophrenia is that, in an affected patient, the PANSS negative subscale score is higher than the positive one, he continued.
It’s noteworthy that none of the multitude of failed drugs for negative symptoms of schizophrenia target activity of dopamine-3 receptors. But cariprazine does.
“There is hope that dopamine-3 receptors might play a role in schizophrenia, especially in negative symptoms. According to experts, they are much older by a couple of million years than the D2 receptors. They don’t operate like D2. In fact, their function is not very clear. In animal studies, though, they help with cognition,” Dr. Bitter said.
“These cariprazine results open the door to some hope for the future,” commented Dr. Ferrara, professor of psychiatry at the University of Naples, Italy.
Even so, she added in an interview, most clinicians aren’t ready to handle a drug with an indication for treatment of negative symptoms. “We are not ready as clinicians to rate negative symptoms in our patients, or in many, many cases to even recognize them. In my opinion, several promising drugs failed because of the fact that the evaluations were not very well carried out in spite of training, but also because, so far, the assessment instruments have not been the best you could think of. Clinicians need training, and the assessment instruments need to be refined. We need instruments that provide more in-depth evaluation of the different aspects of negative symptoms and that are also more appealing to clinicians,” according to the psychiatrist.
She agreed with Dr. Bitter that, for now, the PANSS-FSNS is the best available assessment tool.
“The SANS is not a good tool for negative symptoms. We are all sure about that. We can and should do better,” Dr. Ferrara said.
The cariprazine study was funded by Gedeon Richter and Allergen. Dr. Bitter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
VIENNA – Cariprazine appears to improve predominant negative symptoms of schizophrenia as well as functional status in affected patients, István Bitter, MD, PhD, DSci reported at the annual congress of the European College of Neuropsychopharmacology.
Cariprazine, a dopamine D3/D2 receptor partial agonist, was approved in the United States in 2015 as Vraylar for adults with bipolar disorder or schizophrenia. The drug remains investigational in Europe.
“There is very clearly an unmet need for the treatment of deficit schizophrenia, or schizophrenia with predominant and persistent negative symptoms,” the psychiatrist observed.
The strong efficacy signal for cariprazine in patients with predominant negative symptoms was identified in a post hoc analysis of a randomized, double-blind, phase III head-to-head comparison of cariprazine versus risperidone (Risperdal) in 461 affected adults. Dr. Bitter was a coinvestigator in the 26-week multicenter European study, which was preceded by a 4-week lead-in phase in which participants were uptitrated to a target dose of cariprazine at 4.5 mg/day or risperidone at 4 mg/day.
The primary endpoint in the post hoc analysis was change from baseline on the Positive and Negative Symptoms Factor Score for Negative Symptoms (PANSS-FSNS). A significant difference in favor of cariprazine was seen by week 14, and the gap steadily enlarged thereafter through the remainder of the study. The difference was significant in five of the seven items on the PANSS-FSNS: blunted affect, emotional withdrawal, poor rapport, active social avoidance, and passive/apathetic social withdrawal. Cariprazine also outperformed risperidone on the other two elements of the scale – motor retardation, and lack of spontaneity and flow of conversation – but in those domains, the difference didn’t reach statistical significance.
Patients randomized to cariprazine also fared well on secondary endpoints. They displayed a significantly greater overall improvement in Marder factor scores over 26 weeks than the risperidone-treated group. This advantage was driven by a strong, statistically significant difference in the domain of difficulty in abstract thinking. However, the cariprazine group also showed nonsignificant trends for greater improvement in conceptual disorganization, mannerisms and posturing, disorientation, poor attention, and disturbed volition.
As evidence that cariprazine was exerting specific benefit on negative symptoms, Dr. Bitter cited the fact that the drug showed no significant difference from risperidone in change from baseline on the Marder factor scores for uncontrolled hostility/excitement and anxiety/depression, nor in the Calgary Depression Rating Scale for Schizophrenia.
Regulatory agencies in the United States and Europe have made it clear that, for a drug to obtain an indication for treatment of predominant negative symptoms of schizophrenia, it also has to show evidence of improved patient social function. The cariprazine group showed a significantly greater improvement on the PANSS-FSNS Personal and Social Performance Score. This advantage over risperidone-treated patients reached statistical significance at week 10 and broadened thereafter until study conclusion. Improvements in the subdomain scores for self-care, socially useful activities, and personal and social relationships led the way.
These positive results for cariprazine represent a sharp departure from the psychiatric field’s long-standing history of failed therapeutic attempts to improve negative symptoms. A comprehensive 2015 meta-analysis of all 168 randomized, placebo-controlled studies of various potential treatments for predominant negative symptoms of schizophrenia published through 2013 concluded that none of them provided evidence of clinically meaningful improvement (Schizophr Bull. 2015 Jul;41[4]:892-9).
Dr. Bitter observed that, since that discouraging meta-analysis, several additional novel agents for treatment of predominant negative symptoms of schizophrenia that showed “fantastic” promise in phase II studies subsequently went down in flames in advanced phase III trials. Among those were D-serine as an add-on to second-generation antipsychotics; encenicline, an alpha-7 nicotinic acetylcholine receptor antagonist; pomaglumetad methionil, a selective agonist for glutamate receptor subtypes mGluR2 and mGluR3; and bitopertin, a glycine reuptake inhibitor.
“This is basically a very negative message, that there is not much we can do about negative symptoms. But depending on who you ask, you can get a little bit more optimistic picture,” Dr. Bitter said.
That’s because there are considerable differences among the studies both in how negative symptoms are defined as well as in the measurement tools employed. For example, the Scale for Assessment of Negative Symptoms (SANS) includes items which aren’t strictly speaking part of the negative symptoms concept, so it yields skewed results. The Brief Psychiatric Rating Scale is a relic that has been abandoned by younger psychiatrists. So at present, the PANS-FSNS is the best available tool, and a reasonable common sense definition of predominant negative symptoms of schizophrenia is that, in an affected patient, the PANSS negative subscale score is higher than the positive one, he continued.
It’s noteworthy that none of the multitude of failed drugs for negative symptoms of schizophrenia target activity of dopamine-3 receptors. But cariprazine does.
“There is hope that dopamine-3 receptors might play a role in schizophrenia, especially in negative symptoms. According to experts, they are much older by a couple of million years than the D2 receptors. They don’t operate like D2. In fact, their function is not very clear. In animal studies, though, they help with cognition,” Dr. Bitter said.
“These cariprazine results open the door to some hope for the future,” commented Dr. Ferrara, professor of psychiatry at the University of Naples, Italy.
Even so, she added in an interview, most clinicians aren’t ready to handle a drug with an indication for treatment of negative symptoms. “We are not ready as clinicians to rate negative symptoms in our patients, or in many, many cases to even recognize them. In my opinion, several promising drugs failed because of the fact that the evaluations were not very well carried out in spite of training, but also because, so far, the assessment instruments have not been the best you could think of. Clinicians need training, and the assessment instruments need to be refined. We need instruments that provide more in-depth evaluation of the different aspects of negative symptoms and that are also more appealing to clinicians,” according to the psychiatrist.
She agreed with Dr. Bitter that, for now, the PANSS-FSNS is the best available assessment tool.
“The SANS is not a good tool for negative symptoms. We are all sure about that. We can and should do better,” Dr. Ferrara said.
The cariprazine study was funded by Gedeon Richter and Allergen. Dr. Bitter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
VIENNA – Cariprazine appears to improve predominant negative symptoms of schizophrenia as well as functional status in affected patients, István Bitter, MD, PhD, DSci reported at the annual congress of the European College of Neuropsychopharmacology.
Cariprazine, a dopamine D3/D2 receptor partial agonist, was approved in the United States in 2015 as Vraylar for adults with bipolar disorder or schizophrenia. The drug remains investigational in Europe.
“There is very clearly an unmet need for the treatment of deficit schizophrenia, or schizophrenia with predominant and persistent negative symptoms,” the psychiatrist observed.
The strong efficacy signal for cariprazine in patients with predominant negative symptoms was identified in a post hoc analysis of a randomized, double-blind, phase III head-to-head comparison of cariprazine versus risperidone (Risperdal) in 461 affected adults. Dr. Bitter was a coinvestigator in the 26-week multicenter European study, which was preceded by a 4-week lead-in phase in which participants were uptitrated to a target dose of cariprazine at 4.5 mg/day or risperidone at 4 mg/day.
The primary endpoint in the post hoc analysis was change from baseline on the Positive and Negative Symptoms Factor Score for Negative Symptoms (PANSS-FSNS). A significant difference in favor of cariprazine was seen by week 14, and the gap steadily enlarged thereafter through the remainder of the study. The difference was significant in five of the seven items on the PANSS-FSNS: blunted affect, emotional withdrawal, poor rapport, active social avoidance, and passive/apathetic social withdrawal. Cariprazine also outperformed risperidone on the other two elements of the scale – motor retardation, and lack of spontaneity and flow of conversation – but in those domains, the difference didn’t reach statistical significance.
Patients randomized to cariprazine also fared well on secondary endpoints. They displayed a significantly greater overall improvement in Marder factor scores over 26 weeks than the risperidone-treated group. This advantage was driven by a strong, statistically significant difference in the domain of difficulty in abstract thinking. However, the cariprazine group also showed nonsignificant trends for greater improvement in conceptual disorganization, mannerisms and posturing, disorientation, poor attention, and disturbed volition.
As evidence that cariprazine was exerting specific benefit on negative symptoms, Dr. Bitter cited the fact that the drug showed no significant difference from risperidone in change from baseline on the Marder factor scores for uncontrolled hostility/excitement and anxiety/depression, nor in the Calgary Depression Rating Scale for Schizophrenia.
Regulatory agencies in the United States and Europe have made it clear that, for a drug to obtain an indication for treatment of predominant negative symptoms of schizophrenia, it also has to show evidence of improved patient social function. The cariprazine group showed a significantly greater improvement on the PANSS-FSNS Personal and Social Performance Score. This advantage over risperidone-treated patients reached statistical significance at week 10 and broadened thereafter until study conclusion. Improvements in the subdomain scores for self-care, socially useful activities, and personal and social relationships led the way.
These positive results for cariprazine represent a sharp departure from the psychiatric field’s long-standing history of failed therapeutic attempts to improve negative symptoms. A comprehensive 2015 meta-analysis of all 168 randomized, placebo-controlled studies of various potential treatments for predominant negative symptoms of schizophrenia published through 2013 concluded that none of them provided evidence of clinically meaningful improvement (Schizophr Bull. 2015 Jul;41[4]:892-9).
Dr. Bitter observed that, since that discouraging meta-analysis, several additional novel agents for treatment of predominant negative symptoms of schizophrenia that showed “fantastic” promise in phase II studies subsequently went down in flames in advanced phase III trials. Among those were D-serine as an add-on to second-generation antipsychotics; encenicline, an alpha-7 nicotinic acetylcholine receptor antagonist; pomaglumetad methionil, a selective agonist for glutamate receptor subtypes mGluR2 and mGluR3; and bitopertin, a glycine reuptake inhibitor.
“This is basically a very negative message, that there is not much we can do about negative symptoms. But depending on who you ask, you can get a little bit more optimistic picture,” Dr. Bitter said.
That’s because there are considerable differences among the studies both in how negative symptoms are defined as well as in the measurement tools employed. For example, the Scale for Assessment of Negative Symptoms (SANS) includes items which aren’t strictly speaking part of the negative symptoms concept, so it yields skewed results. The Brief Psychiatric Rating Scale is a relic that has been abandoned by younger psychiatrists. So at present, the PANS-FSNS is the best available tool, and a reasonable common sense definition of predominant negative symptoms of schizophrenia is that, in an affected patient, the PANSS negative subscale score is higher than the positive one, he continued.
It’s noteworthy that none of the multitude of failed drugs for negative symptoms of schizophrenia target activity of dopamine-3 receptors. But cariprazine does.
“There is hope that dopamine-3 receptors might play a role in schizophrenia, especially in negative symptoms. According to experts, they are much older by a couple of million years than the D2 receptors. They don’t operate like D2. In fact, their function is not very clear. In animal studies, though, they help with cognition,” Dr. Bitter said.
“These cariprazine results open the door to some hope for the future,” commented Dr. Ferrara, professor of psychiatry at the University of Naples, Italy.
Even so, she added in an interview, most clinicians aren’t ready to handle a drug with an indication for treatment of negative symptoms. “We are not ready as clinicians to rate negative symptoms in our patients, or in many, many cases to even recognize them. In my opinion, several promising drugs failed because of the fact that the evaluations were not very well carried out in spite of training, but also because, so far, the assessment instruments have not been the best you could think of. Clinicians need training, and the assessment instruments need to be refined. We need instruments that provide more in-depth evaluation of the different aspects of negative symptoms and that are also more appealing to clinicians,” according to the psychiatrist.
She agreed with Dr. Bitter that, for now, the PANSS-FSNS is the best available assessment tool.
“The SANS is not a good tool for negative symptoms. We are all sure about that. We can and should do better,” Dr. Ferrara said.
The cariprazine study was funded by Gedeon Richter and Allergen. Dr. Bitter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Cariprazine resulted in significantly greater improvement than risperidone in negative symptoms of schizophrenia as well as in social functioning.
Data source: This was a post hoc analysis of a 28-week, randomized, multicenter, phase III head-to-head comparative trial of cariprazine versus risperidone in 461 schizophrenia patients with predominant negative symptoms.
Disclosures: The study was funded by Gedeon Richter and Allergen. The presenter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
What’s Next in Federal Health Care?
The largest gathering of federal health care leadership is less than 2 months away. The AMSUS annual meeting will be held November 29 to December 2, 2016 at the Gaylord National in National Harbor, Maryland. “This meeting is future focused, VADM Michael L. Cowan, MD, executive director of AMSUS recently told Federal Practitioner. “We have leaders from across federal medicine, and they are going to tell you what they are doing and where their organizations are headed. It’s very exciting.”
Keynote speakers include VA Under Secretary for Health David J. Shulkin, MD; United States Surgeon General VADM Vivek H. Murthy, MD, MBA; Deputy Secretary of Defense Robert O. Work; and Director Defense Health Agency VADM Raquel Bono, MD.
The Military Health System will be developing 1 of the 6 education tracks, which will focus on the development and maintenance of a high reliability health care organization. The meeting also will focus on clinical best practices, psychological health and suicide prevention, alternatives to opioids for pain management, advances in blood products, and women in combat.
According to VADM Cowan, the meeting also will highlight some of the advances in the U.S. Air Force’s critical care evacuation system. A transport isolation unit will be on display, which includes a sealed patient care module with positive airway circulation designed to protect patients with infectious diseases and their care providers.
The meeting will provided up to 20 hours of continuing education credit. For more information and to register, visit http://www.amsusmeetings.org.
The largest gathering of federal health care leadership is less than 2 months away. The AMSUS annual meeting will be held November 29 to December 2, 2016 at the Gaylord National in National Harbor, Maryland. “This meeting is future focused, VADM Michael L. Cowan, MD, executive director of AMSUS recently told Federal Practitioner. “We have leaders from across federal medicine, and they are going to tell you what they are doing and where their organizations are headed. It’s very exciting.”
Keynote speakers include VA Under Secretary for Health David J. Shulkin, MD; United States Surgeon General VADM Vivek H. Murthy, MD, MBA; Deputy Secretary of Defense Robert O. Work; and Director Defense Health Agency VADM Raquel Bono, MD.
The Military Health System will be developing 1 of the 6 education tracks, which will focus on the development and maintenance of a high reliability health care organization. The meeting also will focus on clinical best practices, psychological health and suicide prevention, alternatives to opioids for pain management, advances in blood products, and women in combat.
According to VADM Cowan, the meeting also will highlight some of the advances in the U.S. Air Force’s critical care evacuation system. A transport isolation unit will be on display, which includes a sealed patient care module with positive airway circulation designed to protect patients with infectious diseases and their care providers.
The meeting will provided up to 20 hours of continuing education credit. For more information and to register, visit http://www.amsusmeetings.org.
The largest gathering of federal health care leadership is less than 2 months away. The AMSUS annual meeting will be held November 29 to December 2, 2016 at the Gaylord National in National Harbor, Maryland. “This meeting is future focused, VADM Michael L. Cowan, MD, executive director of AMSUS recently told Federal Practitioner. “We have leaders from across federal medicine, and they are going to tell you what they are doing and where their organizations are headed. It’s very exciting.”
Keynote speakers include VA Under Secretary for Health David J. Shulkin, MD; United States Surgeon General VADM Vivek H. Murthy, MD, MBA; Deputy Secretary of Defense Robert O. Work; and Director Defense Health Agency VADM Raquel Bono, MD.
The Military Health System will be developing 1 of the 6 education tracks, which will focus on the development and maintenance of a high reliability health care organization. The meeting also will focus on clinical best practices, psychological health and suicide prevention, alternatives to opioids for pain management, advances in blood products, and women in combat.
According to VADM Cowan, the meeting also will highlight some of the advances in the U.S. Air Force’s critical care evacuation system. A transport isolation unit will be on display, which includes a sealed patient care module with positive airway circulation designed to protect patients with infectious diseases and their care providers.
The meeting will provided up to 20 hours of continuing education credit. For more information and to register, visit http://www.amsusmeetings.org.
Inhaled antibiotic regimen reduces bronchiectasis exacerbations
LONDON – In patients with non–cystic fibrosis bronchiectasis (NCFB), use of inhaled ciprofloxacin on a schedule of 14 days on and 14 days off significantly reduced the rate of exacerbations, in a multicenter placebo-controlled trial. The results were presented at the annual congress of the European Respiratory Society.
“At last, an antibiotic intervention trial [in NCFB] with a positive outcome,” reported Anthony De Soyza, MBChB, PhD, senior lecturer in respiratory medicine, Newcastle (England) University. Dr. De Soyza was the study’s principal investigator.
In this study, called RESPIRE 1, the researchers conducted two placebo-controlled arms simultaneously. In one, patients randomized to inhaled ciprofloxacin or placebo took their assigned treatment twice daily for 14 days on and then 14 days off. In the other, patients took their assigned treatment of ciprofloxacin or placebo twice daily, but for 28 days on and then 28 days off. The on-off schedules were maintained for 48 weeks. The ratio of randomization of active therapy to placebo was 2:1.
There were two co-primary endpoints. One was the number of exacerbations over the 48 weeks of follow-up. Exacerbations were strictly defined as the worsening of at least three respiratory symptoms (dyspnea, wheezing, cough, increased sputum volume over 24 hours, or increased sputum purulence) plus fever with malaise or fatigue, and systemic antibiotic use. The other endpoint was the time to first exacerbation.
When the 111 patients randomized to the 14-day on-off regimen of inhaled ciprofloxacin were compared to 49 placebo patients, the mean number of exacerbations over 48 weeks of follow-up was 0.6 in the active treatment group, versus 1.0 in the placebo group (adjusted hazard ratio of 0.61, P = .0061), according to Dr. De Soyza.
The mean time to first exacerbation approached 1 year in those randomized to the 14-day on-off schedule of inhaled ciprofloxacin. In the two placebo arms (pooled for this analysis), the mean time to first exacerbation was 186 days. This difference was highly significant (HR = 0.53; P = .0005).
On the 28-day schedule, for 174 patients, there was a trend for a delay in the time to first exacerbation (HR 0.73; P = .0650) for those randomized to inhaled ciprofloxacin relative to placebo. The difference in the mean number of exacerbations between patients in the experimental group and the placebo group did not approach significance, which Dr. De Soyza said may have been caused by a lack of protection from antibiotics during the off period.
Ciprofloxacin, which was delivered in a dose of 32.5 mg in a proprietary dry powder inhalation device, was well tolerated. The overall rate of adverse events was similar across the study arms. The rates of discontinuation for adverse events in the 14-day and 28-day arms of ciprofloxacin were 12.5% and 10.6%, respectively. These rates were numerically lower than the 13.9% rate of adverse events that occurred among the patients receiving placebos.
According to previously published data cited by Dr. De Soyza, the proportion of NCFB patients with chronic lung infections is 70%, and approximately 40% of these patients have at least two exacerbations per year. In patients with at least two exacerbations per year, the 4-year mortality rate is 15%, Dr. De Soyza reported.
“There is an urgent medical need for better therapeutic strategies to improve outcome in patients with this disease,” he emphasized.
In this particular study, enrolled patients had relatively advanced NCFB. About 30% had a baseline forced expiratory volume in one second of less than 50% of predicted, about 55% had experienced two or more exacerbations in the past 12 months, 20% had been hospitalized in the last year, and more than 15% were on long-term oral macrolides, Dr. De Soyza reported.
These data will be rendered even more compelling if the similarly designed and nearly completed RESPIRE 2 trial produces similar results, he said. If both studies demonstrate protection from exacerbations, they might lead to “a paradigm shift” in the treatment of NCFB, he added.
In the context of the limited strategies currently available for NCFB, “the punch line is that this is a really exciting outcome,” he said.
Dr. De Soyza has financial relationships with AstraZeneca, Bayer, Chiesi, Forrest Labs, GlaxoSmithKline, Novartis, and Teva.
LONDON – In patients with non–cystic fibrosis bronchiectasis (NCFB), use of inhaled ciprofloxacin on a schedule of 14 days on and 14 days off significantly reduced the rate of exacerbations, in a multicenter placebo-controlled trial. The results were presented at the annual congress of the European Respiratory Society.
“At last, an antibiotic intervention trial [in NCFB] with a positive outcome,” reported Anthony De Soyza, MBChB, PhD, senior lecturer in respiratory medicine, Newcastle (England) University. Dr. De Soyza was the study’s principal investigator.
In this study, called RESPIRE 1, the researchers conducted two placebo-controlled arms simultaneously. In one, patients randomized to inhaled ciprofloxacin or placebo took their assigned treatment twice daily for 14 days on and then 14 days off. In the other, patients took their assigned treatment of ciprofloxacin or placebo twice daily, but for 28 days on and then 28 days off. The on-off schedules were maintained for 48 weeks. The ratio of randomization of active therapy to placebo was 2:1.
There were two co-primary endpoints. One was the number of exacerbations over the 48 weeks of follow-up. Exacerbations were strictly defined as the worsening of at least three respiratory symptoms (dyspnea, wheezing, cough, increased sputum volume over 24 hours, or increased sputum purulence) plus fever with malaise or fatigue, and systemic antibiotic use. The other endpoint was the time to first exacerbation.
When the 111 patients randomized to the 14-day on-off regimen of inhaled ciprofloxacin were compared to 49 placebo patients, the mean number of exacerbations over 48 weeks of follow-up was 0.6 in the active treatment group, versus 1.0 in the placebo group (adjusted hazard ratio of 0.61, P = .0061), according to Dr. De Soyza.
The mean time to first exacerbation approached 1 year in those randomized to the 14-day on-off schedule of inhaled ciprofloxacin. In the two placebo arms (pooled for this analysis), the mean time to first exacerbation was 186 days. This difference was highly significant (HR = 0.53; P = .0005).
On the 28-day schedule, for 174 patients, there was a trend for a delay in the time to first exacerbation (HR 0.73; P = .0650) for those randomized to inhaled ciprofloxacin relative to placebo. The difference in the mean number of exacerbations between patients in the experimental group and the placebo group did not approach significance, which Dr. De Soyza said may have been caused by a lack of protection from antibiotics during the off period.
Ciprofloxacin, which was delivered in a dose of 32.5 mg in a proprietary dry powder inhalation device, was well tolerated. The overall rate of adverse events was similar across the study arms. The rates of discontinuation for adverse events in the 14-day and 28-day arms of ciprofloxacin were 12.5% and 10.6%, respectively. These rates were numerically lower than the 13.9% rate of adverse events that occurred among the patients receiving placebos.
According to previously published data cited by Dr. De Soyza, the proportion of NCFB patients with chronic lung infections is 70%, and approximately 40% of these patients have at least two exacerbations per year. In patients with at least two exacerbations per year, the 4-year mortality rate is 15%, Dr. De Soyza reported.
“There is an urgent medical need for better therapeutic strategies to improve outcome in patients with this disease,” he emphasized.
In this particular study, enrolled patients had relatively advanced NCFB. About 30% had a baseline forced expiratory volume in one second of less than 50% of predicted, about 55% had experienced two or more exacerbations in the past 12 months, 20% had been hospitalized in the last year, and more than 15% were on long-term oral macrolides, Dr. De Soyza reported.
These data will be rendered even more compelling if the similarly designed and nearly completed RESPIRE 2 trial produces similar results, he said. If both studies demonstrate protection from exacerbations, they might lead to “a paradigm shift” in the treatment of NCFB, he added.
In the context of the limited strategies currently available for NCFB, “the punch line is that this is a really exciting outcome,” he said.
Dr. De Soyza has financial relationships with AstraZeneca, Bayer, Chiesi, Forrest Labs, GlaxoSmithKline, Novartis, and Teva.
LONDON – In patients with non–cystic fibrosis bronchiectasis (NCFB), use of inhaled ciprofloxacin on a schedule of 14 days on and 14 days off significantly reduced the rate of exacerbations, in a multicenter placebo-controlled trial. The results were presented at the annual congress of the European Respiratory Society.
“At last, an antibiotic intervention trial [in NCFB] with a positive outcome,” reported Anthony De Soyza, MBChB, PhD, senior lecturer in respiratory medicine, Newcastle (England) University. Dr. De Soyza was the study’s principal investigator.
In this study, called RESPIRE 1, the researchers conducted two placebo-controlled arms simultaneously. In one, patients randomized to inhaled ciprofloxacin or placebo took their assigned treatment twice daily for 14 days on and then 14 days off. In the other, patients took their assigned treatment of ciprofloxacin or placebo twice daily, but for 28 days on and then 28 days off. The on-off schedules were maintained for 48 weeks. The ratio of randomization of active therapy to placebo was 2:1.
There were two co-primary endpoints. One was the number of exacerbations over the 48 weeks of follow-up. Exacerbations were strictly defined as the worsening of at least three respiratory symptoms (dyspnea, wheezing, cough, increased sputum volume over 24 hours, or increased sputum purulence) plus fever with malaise or fatigue, and systemic antibiotic use. The other endpoint was the time to first exacerbation.
When the 111 patients randomized to the 14-day on-off regimen of inhaled ciprofloxacin were compared to 49 placebo patients, the mean number of exacerbations over 48 weeks of follow-up was 0.6 in the active treatment group, versus 1.0 in the placebo group (adjusted hazard ratio of 0.61, P = .0061), according to Dr. De Soyza.
The mean time to first exacerbation approached 1 year in those randomized to the 14-day on-off schedule of inhaled ciprofloxacin. In the two placebo arms (pooled for this analysis), the mean time to first exacerbation was 186 days. This difference was highly significant (HR = 0.53; P = .0005).
On the 28-day schedule, for 174 patients, there was a trend for a delay in the time to first exacerbation (HR 0.73; P = .0650) for those randomized to inhaled ciprofloxacin relative to placebo. The difference in the mean number of exacerbations between patients in the experimental group and the placebo group did not approach significance, which Dr. De Soyza said may have been caused by a lack of protection from antibiotics during the off period.
Ciprofloxacin, which was delivered in a dose of 32.5 mg in a proprietary dry powder inhalation device, was well tolerated. The overall rate of adverse events was similar across the study arms. The rates of discontinuation for adverse events in the 14-day and 28-day arms of ciprofloxacin were 12.5% and 10.6%, respectively. These rates were numerically lower than the 13.9% rate of adverse events that occurred among the patients receiving placebos.
According to previously published data cited by Dr. De Soyza, the proportion of NCFB patients with chronic lung infections is 70%, and approximately 40% of these patients have at least two exacerbations per year. In patients with at least two exacerbations per year, the 4-year mortality rate is 15%, Dr. De Soyza reported.
“There is an urgent medical need for better therapeutic strategies to improve outcome in patients with this disease,” he emphasized.
In this particular study, enrolled patients had relatively advanced NCFB. About 30% had a baseline forced expiratory volume in one second of less than 50% of predicted, about 55% had experienced two or more exacerbations in the past 12 months, 20% had been hospitalized in the last year, and more than 15% were on long-term oral macrolides, Dr. De Soyza reported.
These data will be rendered even more compelling if the similarly designed and nearly completed RESPIRE 2 trial produces similar results, he said. If both studies demonstrate protection from exacerbations, they might lead to “a paradigm shift” in the treatment of NCFB, he added.
In the context of the limited strategies currently available for NCFB, “the punch line is that this is a really exciting outcome,” he said.
Dr. De Soyza has financial relationships with AstraZeneca, Bayer, Chiesi, Forrest Labs, GlaxoSmithKline, Novartis, and Teva.
AT THE ERS CONGRESS 2016
Key clinical point: An every-14-day regimen of inhaled ciprofloxacin reduced the number of and the rate of exacerbations in non–cystic fibrosis bronchiectasis patients.
Major finding: The rate of exacerbations was reduced by 39% over a 48-week period in those randomized to the inhaled antibiotic relative to placebo.
Data source: A multicenter double-blind, placebo-controlled trial.
Disclosures: Dr. De Soyza has financial relationships with AstraZeneca, Bayer, Chiesi, Forrest Labs, GlaxoSmithKline, Novartis, and Teva.
Healing for Veteran Survivors of Sexual Trauma
According to a VA national screening program, about 1 in 4 female veterans has experienced a sexual assault of some type. Sexual trauma can lead to major depressive disorder (MDD) (some research suggests that 1 in 3 rape victims will have at least 1 period of MDD during their lives) and posttraumatic stress disorder (PTSD) with repeated thoughts of the assault, memories, nightmares, and increased arousal (eg, difficulty sleeping and concentrating).
Related: Update on Sexual Assault in the Military
The Warrior Renew treatment program can make a big difference to survivors of assault—particularly veterans, according to findings from recent studies. Warrior Renew, developed by Lori Katz, PhD, was designed specifically to address the “unique aspects” of military sexual trauma for both men and women. The program offers an integrated curriculum that helps participants develop coping skills for improving sleep; reducing anxiety, triggers, anger/resentment, and grief; resolving self-blame; and improving communication. By targeting trauma-related perceptions and feelings that may replicate themselves in relationships, the program also helps participants build a more positive self-perception and optimistic vision for the future, Katz says.
The program format consists of 90-minute “core” classes 4 days a week, adjunctive therapy classes (self-care, art therapy, yoga, relaxation), and recreational outings. Unlike other treatments for trauma, Warrior Renew does not have an exposure component. Instead, it’s grounded in the principles of holographic reprocessing (HR), an evidence-based treatment that helps participants identify emotional themes (such as feeling endangered) and interpersonal patterns. Participants also are taught skills for affect management and self-soothing, such as a technique of “cleansing breath, observation, positive self-talk, and explanation” (COPE) to calm the excitatory system.
Dr. Katz’s outcome studies have shown promising results, and most participants show “reliable” and sustained clinical change in anxiety, depression and posttraumatic negative cognitions, as well as significant increases in self-esteem, optimism, and satisfaction with life.
Related: Sexual Trauma in the Military
Katz also led a study to examine the change in “attachment style” in program graduates—that is, to find out whether they could learn to form healthier relationships. In this study, 62 veterans graduated the program over more than 2 years. Of those, 95% had been diagnosed with PTSD, 57% reported being in recovery from substance abuse, and 45% had considered suicide. Nearly all reported chronic medical conditions. The participants took the Relationship Scales Questionnaire and Brief Symptom Inventory pre- and posttreatment.
The graduates reported significant decreases in “fearful” and “dismissive” insecure attachment as perceived in relationships, with significant increases in “secure” attachment. Improved scores were significantly correlated with reported levels of symptoms of anxiety and depression.
A fourth study, still in review, evaluated the treatment protocol delivered in an outpatient therapy group for survivors of military sexual trauma at a VA medical center. Participants met twice a week to discuss topics such as coping with feelings, sleep and nightmares, and remembering trauma. Again, findings revealed significant reductions in symptoms of anxiety, depression, posttraumatic negative thinking, and PTSD.
Related: Recovering From Military Sexual Trauma
According to Dr. Katz, relating to others with less fear and avoidance while feeling more secure may translate into more engagement in activities and social interactions, supporting an “upward spiral” in healing.
According to a VA national screening program, about 1 in 4 female veterans has experienced a sexual assault of some type. Sexual trauma can lead to major depressive disorder (MDD) (some research suggests that 1 in 3 rape victims will have at least 1 period of MDD during their lives) and posttraumatic stress disorder (PTSD) with repeated thoughts of the assault, memories, nightmares, and increased arousal (eg, difficulty sleeping and concentrating).
Related: Update on Sexual Assault in the Military
The Warrior Renew treatment program can make a big difference to survivors of assault—particularly veterans, according to findings from recent studies. Warrior Renew, developed by Lori Katz, PhD, was designed specifically to address the “unique aspects” of military sexual trauma for both men and women. The program offers an integrated curriculum that helps participants develop coping skills for improving sleep; reducing anxiety, triggers, anger/resentment, and grief; resolving self-blame; and improving communication. By targeting trauma-related perceptions and feelings that may replicate themselves in relationships, the program also helps participants build a more positive self-perception and optimistic vision for the future, Katz says.
The program format consists of 90-minute “core” classes 4 days a week, adjunctive therapy classes (self-care, art therapy, yoga, relaxation), and recreational outings. Unlike other treatments for trauma, Warrior Renew does not have an exposure component. Instead, it’s grounded in the principles of holographic reprocessing (HR), an evidence-based treatment that helps participants identify emotional themes (such as feeling endangered) and interpersonal patterns. Participants also are taught skills for affect management and self-soothing, such as a technique of “cleansing breath, observation, positive self-talk, and explanation” (COPE) to calm the excitatory system.
Dr. Katz’s outcome studies have shown promising results, and most participants show “reliable” and sustained clinical change in anxiety, depression and posttraumatic negative cognitions, as well as significant increases in self-esteem, optimism, and satisfaction with life.
Related: Sexual Trauma in the Military
Katz also led a study to examine the change in “attachment style” in program graduates—that is, to find out whether they could learn to form healthier relationships. In this study, 62 veterans graduated the program over more than 2 years. Of those, 95% had been diagnosed with PTSD, 57% reported being in recovery from substance abuse, and 45% had considered suicide. Nearly all reported chronic medical conditions. The participants took the Relationship Scales Questionnaire and Brief Symptom Inventory pre- and posttreatment.
The graduates reported significant decreases in “fearful” and “dismissive” insecure attachment as perceived in relationships, with significant increases in “secure” attachment. Improved scores were significantly correlated with reported levels of symptoms of anxiety and depression.
A fourth study, still in review, evaluated the treatment protocol delivered in an outpatient therapy group for survivors of military sexual trauma at a VA medical center. Participants met twice a week to discuss topics such as coping with feelings, sleep and nightmares, and remembering trauma. Again, findings revealed significant reductions in symptoms of anxiety, depression, posttraumatic negative thinking, and PTSD.
Related: Recovering From Military Sexual Trauma
According to Dr. Katz, relating to others with less fear and avoidance while feeling more secure may translate into more engagement in activities and social interactions, supporting an “upward spiral” in healing.
According to a VA national screening program, about 1 in 4 female veterans has experienced a sexual assault of some type. Sexual trauma can lead to major depressive disorder (MDD) (some research suggests that 1 in 3 rape victims will have at least 1 period of MDD during their lives) and posttraumatic stress disorder (PTSD) with repeated thoughts of the assault, memories, nightmares, and increased arousal (eg, difficulty sleeping and concentrating).
Related: Update on Sexual Assault in the Military
The Warrior Renew treatment program can make a big difference to survivors of assault—particularly veterans, according to findings from recent studies. Warrior Renew, developed by Lori Katz, PhD, was designed specifically to address the “unique aspects” of military sexual trauma for both men and women. The program offers an integrated curriculum that helps participants develop coping skills for improving sleep; reducing anxiety, triggers, anger/resentment, and grief; resolving self-blame; and improving communication. By targeting trauma-related perceptions and feelings that may replicate themselves in relationships, the program also helps participants build a more positive self-perception and optimistic vision for the future, Katz says.
The program format consists of 90-minute “core” classes 4 days a week, adjunctive therapy classes (self-care, art therapy, yoga, relaxation), and recreational outings. Unlike other treatments for trauma, Warrior Renew does not have an exposure component. Instead, it’s grounded in the principles of holographic reprocessing (HR), an evidence-based treatment that helps participants identify emotional themes (such as feeling endangered) and interpersonal patterns. Participants also are taught skills for affect management and self-soothing, such as a technique of “cleansing breath, observation, positive self-talk, and explanation” (COPE) to calm the excitatory system.
Dr. Katz’s outcome studies have shown promising results, and most participants show “reliable” and sustained clinical change in anxiety, depression and posttraumatic negative cognitions, as well as significant increases in self-esteem, optimism, and satisfaction with life.
Related: Sexual Trauma in the Military
Katz also led a study to examine the change in “attachment style” in program graduates—that is, to find out whether they could learn to form healthier relationships. In this study, 62 veterans graduated the program over more than 2 years. Of those, 95% had been diagnosed with PTSD, 57% reported being in recovery from substance abuse, and 45% had considered suicide. Nearly all reported chronic medical conditions. The participants took the Relationship Scales Questionnaire and Brief Symptom Inventory pre- and posttreatment.
The graduates reported significant decreases in “fearful” and “dismissive” insecure attachment as perceived in relationships, with significant increases in “secure” attachment. Improved scores were significantly correlated with reported levels of symptoms of anxiety and depression.
A fourth study, still in review, evaluated the treatment protocol delivered in an outpatient therapy group for survivors of military sexual trauma at a VA medical center. Participants met twice a week to discuss topics such as coping with feelings, sleep and nightmares, and remembering trauma. Again, findings revealed significant reductions in symptoms of anxiety, depression, posttraumatic negative thinking, and PTSD.
Related: Recovering From Military Sexual Trauma
According to Dr. Katz, relating to others with less fear and avoidance while feeling more secure may translate into more engagement in activities and social interactions, supporting an “upward spiral” in healing.