User login
Ceramic Femoral Heads for All Patients? An Argument for Cost Containment in Hip Surgery
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
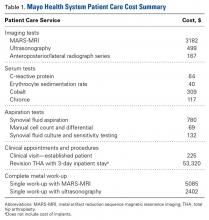
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
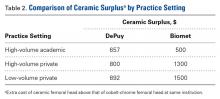
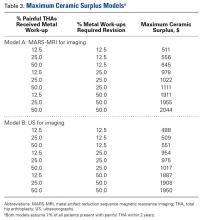
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Fluoxetine fails to slow progressive multiple sclerosis
LONDON – Contrary to expectation of a neuroprotective benefit, fluoxetine does not slow down the progressive phase of multiple sclerosis, according to the results of a randomized, double-blind, multicenter trial.
The first results of the FLUOX-PMS trial, reported by Melissa Cambron, MD, of University Hospital Brussels (Belgium), showed no statistically significant difference between fluoxetine and placebo for improving the primary endpoint of the time to confirmed disease progression.
“The progressive phase of MS remains an ill-understood part of the disease and it is a holy grail to find a drug that can stop this progression,” Dr. Cambron said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The rationale for looking at whether fluoxetine, a well-studied antidepressant drug, could be such a drug, was that it had several neuroprotective features – it has been shown to stimulate the release of brain-derived neurotrophic factor, stimulate metabolism in astrocytes, and lower glutamatergic toxicity, she said. All of these could potentially help prevent axonal degeneration.
The FLUOX-PMS (Fluoxetine in Progressive Multiple Sclerosis) trial (Trials. 2014;15:37) ran from 2012 to June 2016 and enrolled patients with primary progressive MS (PPMS) or secondary progressive MS (SPMS), as defined by the 2010 McDonald criteria. A total of 137 patients were enrolled, and 69 were randomized to treatment with fluoxetine 40 mg/day and 68 were randomized to placebo. Fluoxetine treatment was started at a dose of 20 mg and titrated to the full 40-mg dose by 12 weeks.
Patient demographics were mostly similar between the groups. Around 44% of patients in the fluoxetine and placebo groups were female; roughly 40% had PPMS and 60% had SPMS in both groups; the mean Expanded Disability Status Scale score was 5.2 in both groups; the mean age was 54 and 51 years, respectively; and the disease duration was between 18 and 20 years.
Dr. Cambron also reported that the trials’ secondary endpoints showed no advantage of using fluoxetine over placebo. The proportion of patients without sustained progression during the trial was similar among the fluoxetine- and placebo-treated patients, at a respective 69.6% and 61.8% (P = .434). The proportion of patients with a stable Hauser Ambulation Index was also similar (P = .371).
The primary and secondary endpoints were assessed every 3 months in the trial. Patients also underwent cognitive testing, completed the Beck Depression Inventory-II, and Modified Fatigue Impact Scale before treatment and at 48 and 108 weeks after treatment with fluoxetine or placebo. Brain MRI was also performed at baseline and at week 108. The results of these measurements have yet to be analyzed.
Although patients in the fluoxetine group versus the placebo arm experienced more side effects, there was no evidence of an excess of severe adverse events.
“Unfortunately, our study was inconclusive because we failed to show a statistical significant difference between the placebo arm and the fluoxetine group, although I’m convinced that there’s a trend that can certainly not be ignored,” Dr. Cambron maintained. “Probably there was not enough progression in the study and possibly the study duration was too short, she suggested, “but it remains challenging to study these patients for a long period of time, especially with a placebo-controlled design.”
The trial was funded by IWT, the Government Agency for Innovation by Science and Technology in Flanders (Belgium). Dr. Cambron had no relevant financial disclosures.
LONDON – Contrary to expectation of a neuroprotective benefit, fluoxetine does not slow down the progressive phase of multiple sclerosis, according to the results of a randomized, double-blind, multicenter trial.
The first results of the FLUOX-PMS trial, reported by Melissa Cambron, MD, of University Hospital Brussels (Belgium), showed no statistically significant difference between fluoxetine and placebo for improving the primary endpoint of the time to confirmed disease progression.
“The progressive phase of MS remains an ill-understood part of the disease and it is a holy grail to find a drug that can stop this progression,” Dr. Cambron said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The rationale for looking at whether fluoxetine, a well-studied antidepressant drug, could be such a drug, was that it had several neuroprotective features – it has been shown to stimulate the release of brain-derived neurotrophic factor, stimulate metabolism in astrocytes, and lower glutamatergic toxicity, she said. All of these could potentially help prevent axonal degeneration.
The FLUOX-PMS (Fluoxetine in Progressive Multiple Sclerosis) trial (Trials. 2014;15:37) ran from 2012 to June 2016 and enrolled patients with primary progressive MS (PPMS) or secondary progressive MS (SPMS), as defined by the 2010 McDonald criteria. A total of 137 patients were enrolled, and 69 were randomized to treatment with fluoxetine 40 mg/day and 68 were randomized to placebo. Fluoxetine treatment was started at a dose of 20 mg and titrated to the full 40-mg dose by 12 weeks.
Patient demographics were mostly similar between the groups. Around 44% of patients in the fluoxetine and placebo groups were female; roughly 40% had PPMS and 60% had SPMS in both groups; the mean Expanded Disability Status Scale score was 5.2 in both groups; the mean age was 54 and 51 years, respectively; and the disease duration was between 18 and 20 years.
Dr. Cambron also reported that the trials’ secondary endpoints showed no advantage of using fluoxetine over placebo. The proportion of patients without sustained progression during the trial was similar among the fluoxetine- and placebo-treated patients, at a respective 69.6% and 61.8% (P = .434). The proportion of patients with a stable Hauser Ambulation Index was also similar (P = .371).
The primary and secondary endpoints were assessed every 3 months in the trial. Patients also underwent cognitive testing, completed the Beck Depression Inventory-II, and Modified Fatigue Impact Scale before treatment and at 48 and 108 weeks after treatment with fluoxetine or placebo. Brain MRI was also performed at baseline and at week 108. The results of these measurements have yet to be analyzed.
Although patients in the fluoxetine group versus the placebo arm experienced more side effects, there was no evidence of an excess of severe adverse events.
“Unfortunately, our study was inconclusive because we failed to show a statistical significant difference between the placebo arm and the fluoxetine group, although I’m convinced that there’s a trend that can certainly not be ignored,” Dr. Cambron maintained. “Probably there was not enough progression in the study and possibly the study duration was too short, she suggested, “but it remains challenging to study these patients for a long period of time, especially with a placebo-controlled design.”
The trial was funded by IWT, the Government Agency for Innovation by Science and Technology in Flanders (Belgium). Dr. Cambron had no relevant financial disclosures.
LONDON – Contrary to expectation of a neuroprotective benefit, fluoxetine does not slow down the progressive phase of multiple sclerosis, according to the results of a randomized, double-blind, multicenter trial.
The first results of the FLUOX-PMS trial, reported by Melissa Cambron, MD, of University Hospital Brussels (Belgium), showed no statistically significant difference between fluoxetine and placebo for improving the primary endpoint of the time to confirmed disease progression.
“The progressive phase of MS remains an ill-understood part of the disease and it is a holy grail to find a drug that can stop this progression,” Dr. Cambron said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The rationale for looking at whether fluoxetine, a well-studied antidepressant drug, could be such a drug, was that it had several neuroprotective features – it has been shown to stimulate the release of brain-derived neurotrophic factor, stimulate metabolism in astrocytes, and lower glutamatergic toxicity, she said. All of these could potentially help prevent axonal degeneration.
The FLUOX-PMS (Fluoxetine in Progressive Multiple Sclerosis) trial (Trials. 2014;15:37) ran from 2012 to June 2016 and enrolled patients with primary progressive MS (PPMS) or secondary progressive MS (SPMS), as defined by the 2010 McDonald criteria. A total of 137 patients were enrolled, and 69 were randomized to treatment with fluoxetine 40 mg/day and 68 were randomized to placebo. Fluoxetine treatment was started at a dose of 20 mg and titrated to the full 40-mg dose by 12 weeks.
Patient demographics were mostly similar between the groups. Around 44% of patients in the fluoxetine and placebo groups were female; roughly 40% had PPMS and 60% had SPMS in both groups; the mean Expanded Disability Status Scale score was 5.2 in both groups; the mean age was 54 and 51 years, respectively; and the disease duration was between 18 and 20 years.
Dr. Cambron also reported that the trials’ secondary endpoints showed no advantage of using fluoxetine over placebo. The proportion of patients without sustained progression during the trial was similar among the fluoxetine- and placebo-treated patients, at a respective 69.6% and 61.8% (P = .434). The proportion of patients with a stable Hauser Ambulation Index was also similar (P = .371).
The primary and secondary endpoints were assessed every 3 months in the trial. Patients also underwent cognitive testing, completed the Beck Depression Inventory-II, and Modified Fatigue Impact Scale before treatment and at 48 and 108 weeks after treatment with fluoxetine or placebo. Brain MRI was also performed at baseline and at week 108. The results of these measurements have yet to be analyzed.
Although patients in the fluoxetine group versus the placebo arm experienced more side effects, there was no evidence of an excess of severe adverse events.
“Unfortunately, our study was inconclusive because we failed to show a statistical significant difference between the placebo arm and the fluoxetine group, although I’m convinced that there’s a trend that can certainly not be ignored,” Dr. Cambron maintained. “Probably there was not enough progression in the study and possibly the study duration was too short, she suggested, “but it remains challenging to study these patients for a long period of time, especially with a placebo-controlled design.”
The trial was funded by IWT, the Government Agency for Innovation by Science and Technology in Flanders (Belgium). Dr. Cambron had no relevant financial disclosures.
Key clinical point:
Major finding: There was no difference in the time to confirmed disease progression between fluoxetine- and placebo-treated patients (P = .07).
Data source: FLUOX-PMS, a multicenter, randomized, double-blind, placebo-controlled clinical study of 137 patients with primary or secondary progressive multiple sclerosis treated with fluoxetine or placebo.
Disclosures: The trial was funded by IWT, the Government Agency for Innovation by Science and Technology in Flanders (Belgium). Dr. Cambron had no relevant financial disclosures.
Zika virus shows no signs of slowing down
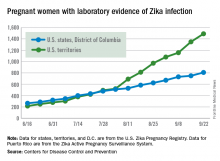
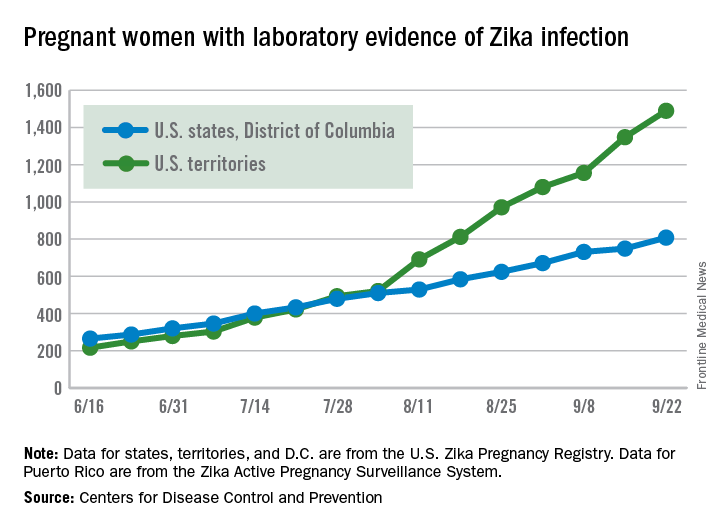
Zika virus transmission continues at a rapid clip as the number of new cases among pregnant women topped 200 again for the week ending Sept. 22, according to the Centers for Disease Control and Prevention.
After setting a new high of 210 the previous week, there were 201 new cases of pregnant women with laboratory-confirmed Zika infection for the week ending Sept. 22. There were 59 new cases in the 50 states and the District of Columbia and 142 new cases in the U.S. territories, the CDC reported Sept. 29. In the United States this year, there have been 2,298 reported cases of Zika-infected pregnant women: 808 in the states and D.C. and 1,490 in the territories.
Among all Americans, there were 2,559 new cases of Zika infection as of Sept. 28 – 267 in the states/D.C. and 2,292 in the territories – although Puerto Rico continues to retroactively report cases, which has been pushing the numbers higher in recent weeks. There have been 25,694 total cases of Zika infection in 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus transmission continues at a rapid clip as the number of new cases among pregnant women topped 200 again for the week ending Sept. 22, according to the Centers for Disease Control and Prevention.
After setting a new high of 210 the previous week, there were 201 new cases of pregnant women with laboratory-confirmed Zika infection for the week ending Sept. 22. There were 59 new cases in the 50 states and the District of Columbia and 142 new cases in the U.S. territories, the CDC reported Sept. 29. In the United States this year, there have been 2,298 reported cases of Zika-infected pregnant women: 808 in the states and D.C. and 1,490 in the territories.
Among all Americans, there were 2,559 new cases of Zika infection as of Sept. 28 – 267 in the states/D.C. and 2,292 in the territories – although Puerto Rico continues to retroactively report cases, which has been pushing the numbers higher in recent weeks. There have been 25,694 total cases of Zika infection in 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus transmission continues at a rapid clip as the number of new cases among pregnant women topped 200 again for the week ending Sept. 22, according to the Centers for Disease Control and Prevention.
After setting a new high of 210 the previous week, there were 201 new cases of pregnant women with laboratory-confirmed Zika infection for the week ending Sept. 22. There were 59 new cases in the 50 states and the District of Columbia and 142 new cases in the U.S. territories, the CDC reported Sept. 29. In the United States this year, there have been 2,298 reported cases of Zika-infected pregnant women: 808 in the states and D.C. and 1,490 in the territories.
Among all Americans, there were 2,559 new cases of Zika infection as of Sept. 28 – 267 in the states/D.C. and 2,292 in the territories – although Puerto Rico continues to retroactively report cases, which has been pushing the numbers higher in recent weeks. There have been 25,694 total cases of Zika infection in 2015-2016, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Sentinel lymph node technique in endometrial cancer, Part 2
As reviewed in Part 1, surgery is indicated for the staging and treatment of endometrial cancer. Lymph node status is one of the most important factors in determining prognosis and the need for adjuvant treatment. The extent of lymph node evaluation is controversial as full lymphadenectomy carries risks, including increased operative time, blood loss, nerve injury, and lymphedema.
Two trials have found no survival benefit from lymphadenectomy for endometrial cancer; however, other evidence suggests that women without known nodal status may be more likely to receive radiotherapy.1,2,3
Given these issues, the sentinel lymph node technique strikes a balance between the risks and benefits of lymph node evaluation in endometrial cancer.
Sentinel lymph nodes (SLN) are the first nodes to drain a tumor site, and thus, are typically the first to demonstrate occult malignancy. The use of the SLN technique as an alternative to complete lymphadenectomy in endometrial cancer has been well described, although its accuracy and the validity of its use are still debated.
The viability of the SLN technique is predicated on the ability to achieve mapping of dye or tracer from the tumor to the first lymph node to drain the tumor. The lymphatic drainage of the endometrium is complex and unlike vulvar or breast cancer, endometrial cancer is less accessible for peritumoral injection. Several injection techniques have been described; cervical injection is the easiest to achieve and has been found to have similar or higher SLN detection than hysteroscopic or fundal injections.4,5
There are a number of techniques for SLN detection, each with unique benefits and risks. Visual identification of blue dye, most frequently isosulfan blue, is the “colorimetric method” and has been used most commonly with cervical injection for endometrial cancer. Injection of isosulfan blue does not require specialized equipment, however visualization in obese patients is inferior.6
Technetium sulfur colloid (Tc) is a radioactive tracer that can be detected by gamma probes. A preoperative lymphoscintigraphy and a handheld gamma probe are used to map lymphatics. This technique has limitations, including the additional time and coordination of procedures, as well as some evidence of poor correlation between lymphoscintigraphy and surgical SLN mapping.7
Indocyanine green (ICG) is a fluorescent dye that has excellent signal penetration and allows for real-time visual identification using near-infrared fluorescence imaging. The bilateral detection rate with ICG appears comparable or better than blue dye.8 Combinations of dye, either ICG plus Tc or Tc plus blue dye, may be also used to increase SLN detection.
The accuracy of the SLN technique is the cornerstone to its success. In a prospective multicenter study – Senti-Endo – patients with early-stage disease underwent pelvic SLN assessment with cervical injection of a combination of dyes followed by systematic pelvic node dissection. The overall negative predictive value was 97% with three patients who had positive lymph nodes that were not detected, all of whom had a type 2 endometrial cancer.9
With the uptake of the SLN technique, many institutions have protocols surrounding the technique to ensure appropriate SLN detection and evaluation. Physicians using this technique should adhere to protocols supported by National Comprehensive Cancer Network guidelines, taking care to remove any suspicious lymph nodes and perform a full side-specific lymphadenectomy if bilateral mapping is not achieved.
The extent of lymphadenectomy and application of the SLN technique in high-risk endometrial cancer remains controversial. These patients are at higher risk for unsuccessful mapping and isolated para-aortic metastasis. Retrospective series have suggested equivalent oncologic outcomes for women with high-grade cancers who have been staged by SLN biopsy, compared with selective or complete lymphadenectomy.10,11
We await the results of a large prospective trial in which patients undergo comprehensive lymphadenectomy in addition to SLN biopsy to assess the accuracy of the technique (NCT01673022).
Pathologic evaluation of SLNs is frequently done with ultrastaging, which describes additional sectioning and staining of the node. This technique frequently identifies isolated tumor cells and micrometastasis (collectively called low-volume disease) in addition to macrometastasis. The clinical and prognostic significance of low-volume disease is unknown and additional investigation is urgently needed to determine appropriate adjuvant therapy and follow-up for these patients.
The SLN technique is an acceptable approach to assess clinical stage I endometrial cancer. Physicians should consider adding the SLN biopsy to their routine staging techniques prior to exclusively adopting the new technique. They should take care to adhere to SLN algorithms and monitor outcomes.
References
1. J Natl Cancer Inst. 2008;100(23):1707-16.
2. Lancet. 2009 Jan;373(9658):125-36.
3. Am J Obstet Gynecol. 2011 Dec;205(6):562.e1–9.
4. Gynecol Oncol. 2013 Nov;131(2):299-303.
5. Int J Gynecol Cancer. 2013 Nov;23(9):1704-11.
6. Gynecol Oncol 2014 Aug;134(2):281-6.
7. Gynecol Oncol. 2009 Feb;112(2):348-352.
8. Gynecol Oncol. 2014 May;133(2):274-7.
9. Lancet Oncol. 2011 May;12(5):469-76.
10. Ann Surg Oncol. 2016 Jan;23(1):196-202.
11. Gynecol Oncol. 2016 Mar;140(3):394-9.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Sullivan is a clinical fellow in the division of gynecologic oncology at UNC, Chapel Hill. Dr. Rossi and Dr. Sullivan reported having no relevant financial disclosures.
As reviewed in Part 1, surgery is indicated for the staging and treatment of endometrial cancer. Lymph node status is one of the most important factors in determining prognosis and the need for adjuvant treatment. The extent of lymph node evaluation is controversial as full lymphadenectomy carries risks, including increased operative time, blood loss, nerve injury, and lymphedema.
Two trials have found no survival benefit from lymphadenectomy for endometrial cancer; however, other evidence suggests that women without known nodal status may be more likely to receive radiotherapy.1,2,3
Given these issues, the sentinel lymph node technique strikes a balance between the risks and benefits of lymph node evaluation in endometrial cancer.
Sentinel lymph nodes (SLN) are the first nodes to drain a tumor site, and thus, are typically the first to demonstrate occult malignancy. The use of the SLN technique as an alternative to complete lymphadenectomy in endometrial cancer has been well described, although its accuracy and the validity of its use are still debated.
The viability of the SLN technique is predicated on the ability to achieve mapping of dye or tracer from the tumor to the first lymph node to drain the tumor. The lymphatic drainage of the endometrium is complex and unlike vulvar or breast cancer, endometrial cancer is less accessible for peritumoral injection. Several injection techniques have been described; cervical injection is the easiest to achieve and has been found to have similar or higher SLN detection than hysteroscopic or fundal injections.4,5
There are a number of techniques for SLN detection, each with unique benefits and risks. Visual identification of blue dye, most frequently isosulfan blue, is the “colorimetric method” and has been used most commonly with cervical injection for endometrial cancer. Injection of isosulfan blue does not require specialized equipment, however visualization in obese patients is inferior.6
Technetium sulfur colloid (Tc) is a radioactive tracer that can be detected by gamma probes. A preoperative lymphoscintigraphy and a handheld gamma probe are used to map lymphatics. This technique has limitations, including the additional time and coordination of procedures, as well as some evidence of poor correlation between lymphoscintigraphy and surgical SLN mapping.7
Indocyanine green (ICG) is a fluorescent dye that has excellent signal penetration and allows for real-time visual identification using near-infrared fluorescence imaging. The bilateral detection rate with ICG appears comparable or better than blue dye.8 Combinations of dye, either ICG plus Tc or Tc plus blue dye, may be also used to increase SLN detection.
The accuracy of the SLN technique is the cornerstone to its success. In a prospective multicenter study – Senti-Endo – patients with early-stage disease underwent pelvic SLN assessment with cervical injection of a combination of dyes followed by systematic pelvic node dissection. The overall negative predictive value was 97% with three patients who had positive lymph nodes that were not detected, all of whom had a type 2 endometrial cancer.9
With the uptake of the SLN technique, many institutions have protocols surrounding the technique to ensure appropriate SLN detection and evaluation. Physicians using this technique should adhere to protocols supported by National Comprehensive Cancer Network guidelines, taking care to remove any suspicious lymph nodes and perform a full side-specific lymphadenectomy if bilateral mapping is not achieved.
The extent of lymphadenectomy and application of the SLN technique in high-risk endometrial cancer remains controversial. These patients are at higher risk for unsuccessful mapping and isolated para-aortic metastasis. Retrospective series have suggested equivalent oncologic outcomes for women with high-grade cancers who have been staged by SLN biopsy, compared with selective or complete lymphadenectomy.10,11
We await the results of a large prospective trial in which patients undergo comprehensive lymphadenectomy in addition to SLN biopsy to assess the accuracy of the technique (NCT01673022).
Pathologic evaluation of SLNs is frequently done with ultrastaging, which describes additional sectioning and staining of the node. This technique frequently identifies isolated tumor cells and micrometastasis (collectively called low-volume disease) in addition to macrometastasis. The clinical and prognostic significance of low-volume disease is unknown and additional investigation is urgently needed to determine appropriate adjuvant therapy and follow-up for these patients.
The SLN technique is an acceptable approach to assess clinical stage I endometrial cancer. Physicians should consider adding the SLN biopsy to their routine staging techniques prior to exclusively adopting the new technique. They should take care to adhere to SLN algorithms and monitor outcomes.
References
1. J Natl Cancer Inst. 2008;100(23):1707-16.
2. Lancet. 2009 Jan;373(9658):125-36.
3. Am J Obstet Gynecol. 2011 Dec;205(6):562.e1–9.
4. Gynecol Oncol. 2013 Nov;131(2):299-303.
5. Int J Gynecol Cancer. 2013 Nov;23(9):1704-11.
6. Gynecol Oncol 2014 Aug;134(2):281-6.
7. Gynecol Oncol. 2009 Feb;112(2):348-352.
8. Gynecol Oncol. 2014 May;133(2):274-7.
9. Lancet Oncol. 2011 May;12(5):469-76.
10. Ann Surg Oncol. 2016 Jan;23(1):196-202.
11. Gynecol Oncol. 2016 Mar;140(3):394-9.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Sullivan is a clinical fellow in the division of gynecologic oncology at UNC, Chapel Hill. Dr. Rossi and Dr. Sullivan reported having no relevant financial disclosures.
As reviewed in Part 1, surgery is indicated for the staging and treatment of endometrial cancer. Lymph node status is one of the most important factors in determining prognosis and the need for adjuvant treatment. The extent of lymph node evaluation is controversial as full lymphadenectomy carries risks, including increased operative time, blood loss, nerve injury, and lymphedema.
Two trials have found no survival benefit from lymphadenectomy for endometrial cancer; however, other evidence suggests that women without known nodal status may be more likely to receive radiotherapy.1,2,3
Given these issues, the sentinel lymph node technique strikes a balance between the risks and benefits of lymph node evaluation in endometrial cancer.
Sentinel lymph nodes (SLN) are the first nodes to drain a tumor site, and thus, are typically the first to demonstrate occult malignancy. The use of the SLN technique as an alternative to complete lymphadenectomy in endometrial cancer has been well described, although its accuracy and the validity of its use are still debated.
The viability of the SLN technique is predicated on the ability to achieve mapping of dye or tracer from the tumor to the first lymph node to drain the tumor. The lymphatic drainage of the endometrium is complex and unlike vulvar or breast cancer, endometrial cancer is less accessible for peritumoral injection. Several injection techniques have been described; cervical injection is the easiest to achieve and has been found to have similar or higher SLN detection than hysteroscopic or fundal injections.4,5
There are a number of techniques for SLN detection, each with unique benefits and risks. Visual identification of blue dye, most frequently isosulfan blue, is the “colorimetric method” and has been used most commonly with cervical injection for endometrial cancer. Injection of isosulfan blue does not require specialized equipment, however visualization in obese patients is inferior.6
Technetium sulfur colloid (Tc) is a radioactive tracer that can be detected by gamma probes. A preoperative lymphoscintigraphy and a handheld gamma probe are used to map lymphatics. This technique has limitations, including the additional time and coordination of procedures, as well as some evidence of poor correlation between lymphoscintigraphy and surgical SLN mapping.7
Indocyanine green (ICG) is a fluorescent dye that has excellent signal penetration and allows for real-time visual identification using near-infrared fluorescence imaging. The bilateral detection rate with ICG appears comparable or better than blue dye.8 Combinations of dye, either ICG plus Tc or Tc plus blue dye, may be also used to increase SLN detection.
The accuracy of the SLN technique is the cornerstone to its success. In a prospective multicenter study – Senti-Endo – patients with early-stage disease underwent pelvic SLN assessment with cervical injection of a combination of dyes followed by systematic pelvic node dissection. The overall negative predictive value was 97% with three patients who had positive lymph nodes that were not detected, all of whom had a type 2 endometrial cancer.9
With the uptake of the SLN technique, many institutions have protocols surrounding the technique to ensure appropriate SLN detection and evaluation. Physicians using this technique should adhere to protocols supported by National Comprehensive Cancer Network guidelines, taking care to remove any suspicious lymph nodes and perform a full side-specific lymphadenectomy if bilateral mapping is not achieved.
The extent of lymphadenectomy and application of the SLN technique in high-risk endometrial cancer remains controversial. These patients are at higher risk for unsuccessful mapping and isolated para-aortic metastasis. Retrospective series have suggested equivalent oncologic outcomes for women with high-grade cancers who have been staged by SLN biopsy, compared with selective or complete lymphadenectomy.10,11
We await the results of a large prospective trial in which patients undergo comprehensive lymphadenectomy in addition to SLN biopsy to assess the accuracy of the technique (NCT01673022).
Pathologic evaluation of SLNs is frequently done with ultrastaging, which describes additional sectioning and staining of the node. This technique frequently identifies isolated tumor cells and micrometastasis (collectively called low-volume disease) in addition to macrometastasis. The clinical and prognostic significance of low-volume disease is unknown and additional investigation is urgently needed to determine appropriate adjuvant therapy and follow-up for these patients.
The SLN technique is an acceptable approach to assess clinical stage I endometrial cancer. Physicians should consider adding the SLN biopsy to their routine staging techniques prior to exclusively adopting the new technique. They should take care to adhere to SLN algorithms and monitor outcomes.
References
1. J Natl Cancer Inst. 2008;100(23):1707-16.
2. Lancet. 2009 Jan;373(9658):125-36.
3. Am J Obstet Gynecol. 2011 Dec;205(6):562.e1–9.
4. Gynecol Oncol. 2013 Nov;131(2):299-303.
5. Int J Gynecol Cancer. 2013 Nov;23(9):1704-11.
6. Gynecol Oncol 2014 Aug;134(2):281-6.
7. Gynecol Oncol. 2009 Feb;112(2):348-352.
8. Gynecol Oncol. 2014 May;133(2):274-7.
9. Lancet Oncol. 2011 May;12(5):469-76.
10. Ann Surg Oncol. 2016 Jan;23(1):196-202.
11. Gynecol Oncol. 2016 Mar;140(3):394-9.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Sullivan is a clinical fellow in the division of gynecologic oncology at UNC, Chapel Hill. Dr. Rossi and Dr. Sullivan reported having no relevant financial disclosures.
Well-woman care: Reshaping the routine visit
From her vantage point in medical education, Christine M. Peterson, MD, is acutely aware that well-woman care is at a turning point.
“We’re at an interesting crossroads between tradition and evidence-based practice,” said Dr. Peterson, who practiced obstetrics and gynecology in the 1980s and now serves as associate professor of ob.gyn. and assistant dean for student affairs at the University of Virginia’s School of Medicine in Charlottesville.
“Even young trainees are aware of the traditions for the annual well-woman visit – the Pap, the pelvic, the breast exam,” she said. “But the evidence is pointing us in a different direction ... toward doing only those things that are effective for prevention and early detection of disease, or that have [demonstrated] value in one way or another.”
If all goes as planned at a national level, Dr. Peterson’s students who become ob.gyns., family physicians, and general internists will practice under a reshaped and well-defined umbrella of well-woman care – one that includes a broad array of insurer-covered screening and counseling services.
That umbrella already includes HPV testing, and screening and/or counseling on intimate partner violence, sexually transmitted infections, and HIV, in addition to contraception counseling. These preventive services were described in the Women’s Preventive Services Guidelines and adopted as covered benefits by the Department of Health & Human Services in 2011.
And more change is on the way. In March, the American College of Obstetricians and Gynecologists (ACOG) launched the Women’s Preventive Services Initiative (WPSI) – a broad coalition tasked with recommending updates to the 2011 guidelines and developing new recommendations for the scope and implementation of women’s preventive health care services.
The effort is funded through a 5-year cooperative agreement with the Health Resources & Services Administration (HRSA). It’s similar to the American Academy of Pediatrics’ approach in developing the HRSA-supported Bright Futures guidelines almost 25 years ago. Under the Affordable Care Act, all HRSA-recommended preventive services must be covered by most private insurers without patient cost sharing.
“Over the years, we’ve gone from an emphasis on the Pap and pelvic to well-woman care that assesses the whole woman,” said Jeanne A. Conry, MD, assistant physician-in-chief at the Permanente Medical Group in Roseville, Calif., and a past president of ACOG.
Ob.gyns. have long provided preventive care, but today more than ever before, Dr. Conry said, “my emphasis is on helping women to get well and stay well.”
The evolution
Fifty years ago, in 1966, use of the Pap test was being widely promoted, modern mammography techniques were on the cusp of advancement, and ob.gyns. were prescribing the first birth control pill approved by the Food and Drug Administration. ACOG’s main practice guidance book, “Standards for Obstetric-Gynecologic Hospital Services,” addressed the general physical examination but otherwise focused on obstetrics and reproductive health.
Thirty years later, women’s health care as described in the first edition of ACOG’s “Guidelines for Women’s Health Care” (1996) had grown to include distinct categories of “primary and preventive care” and “evaluation and counseling” that were separate from gynecologic services and broken down by age.
What was referred to as the “women’s health exam” through the 1990s gradually took on the “well-woman” label in the 2000s. Ob.gyns. were encouraged to address a growing range of preventive issues, but the annual Pap test remained a focus and, in many ways, drove women’s visits.
“Women would say, ‘I’m going for my Pap,’” Dr. Conry said.
Most recently, new technology and evidence-based reviews have changed the framework for well-woman visits to one with longer intervals for cervical cancer screening (every 3-5 years) and a move away from performing internal pelvic exams in all women (ACOG recommends pelvic exams annually for patients aged 21 and older but advises shared decision making for complete pelvic exams in asymptomatic patients).
Recent evidence reviews have also added some uncertainty about the role of annual breast exams in all women, as well as the role of breast self-exams, particularly for women not at high risk.
Maintaining patient relationships
One of the biggest and most immediate challenges for ob.gyns in the face of changing guidelines lies in maintaining the physician-patient relationship and “continuing communication” about the importance of regular well-woman visits, said Jill Rabin, MD, cochief of the division of ambulatory care, women’s health programs–prenatal care assistance program services at Northwell Health, New Hyde Park, N.Y.
“We want patients to understand that, even though they no longer need the Pap every year, they still should see us for good, comprehensive care ... that we can help them achieve their health care goals,” she said.
How often well-woman visits should occur has been a subject of much discussion. The HRSA-supported preventive services guidelines call for well-woman preventive care annually but note that “several visits may be needed to obtain all the necessary recommended services.”
And, in its first set of draft recommendations for HRSA, WPSI offered clarifications, saying there’s a need for “at least one annual preventive care visit for women beginning in adolescence and continuing across the lifespan to ensure that women obtain recommended preventive services” as determined by age and risk factors.
The draft recommendations, aimed at reviewing and updating the 2011 HRSA-sponsored guidelines, will be finalized by the end of 2016. WPSI will submit additional recommendations over the next 4 years.
Carol S. Weisman, PhD, a sociologist and health services researcher who sat on the Institute of Medicine committee that wrote the Women’s Preventive Services Guidelines, said the IOM’s recommendation for well-women visits – “in plural” form – recognizes “that historically many women have patched together their well-woman care from multiple providers, getting some of their preventive care from their generalist, and some from their ob.gyn.”
What’s more, the current list of preventive services covered under the Affordable Care Act is “enormous” – too long to address in one visit for many patients, said Dr. Weisman of the Penn State Center for Women’s Health Research in Hershey.
In addition to the women’s preventive services, the Affordable Care Act requires plans to cover services recommended with a grade A or B rating by the U.S. Preventive Services Task Force (counseling and screening for cancer, cardiovascular disease, and more) as well as vaccines recommended by the Advisory Committee on Immunization Practices, and HRSA’s Bright Futures services (for adolescents as well as children).
Beyond gynecology
How much further ob.gyns. will reach outside the gynecologic realm to offer additional preventive care services is an open question, but it’s likely to be based mainly on comfort levels, sources said.
“The changing needs of the gynecologic visit enable us to spend more time on other things,” said Hal C. Lawrence III, MD, ACOG’s executive vice president and chief executive officer. “But within the specialty, there’s going to be variation as to what level of expanded services ob.gyns. provide. Some will provide a lot of what women need, others not as much.”
Heather Johnson, MD, practices with a large ob.gyn. group in Chevy Chase, Md. and provides well-woman care largely to women in their 50s and 60s, whose children she delivered. She’s comfortable, she said, with screening for and treating osteoporosis and mild depression, for instance. She regularly performs lipid testing but refers out for management of high cholesterol levels or high blood pressure.
“I encourage all my patients to have a primary care physician of record,” she said, “but I still am happy to discuss the issues with them.”
What’s key, according to Dr. Lawrence and Dr. Conry, is coordination.
“We know there will be different individuals providing different components [of well-woman care],” Dr. Conry said. “I may see a woman for various things. Then she may go to her internist. But we should be able to collaborate to hit all the preventive health goals for her.”
Dr. Conry emphasized that the ob.gyn.’s expertise in reproductive health is critical to well-woman care planning, especially given medicine’s growing knowledge of how obstetric health and pregnancy complications can have long-term impacts on cardiovascular disease and other conditions. “It’s important for all providers to realize this,” she said.
Primary care status?
Intertwined with the future of well-women care is the issue of primary care status for ob.gyns. ACOG continues to advocate for ob.gyns. to be listed as primary care providers and part of primary care payment policies. Leaders are also pushing for projects on ob.gyn.–led medical homes.
At the same time, ACOG has been taking a broad collaborative approach to shaping well-woman care, aiming to develop comprehensive, age-specific recommendations for use by any provider who cares for adolescent girls and women.
Representatives of the American Academy of Family Physicians, the American College of Physicians, and the National Association of Nurse Practitioners in Women’s Health were involved in a Well-Woman Task Force that Dr. Conry appointed while serving as ACOG president in 2013-2014. These organizations now sit on WPSI’s advisory panel with ACOG.
Representatives from the American Academy of Pediatrics have also been close partners, as ACOG officials view the HRSA-sponsored Bright Futures guidelines (which includes the “Periodicity Schedule”) as a potential model for well-woman care. The guidelines are comprehensive, well-organized, and user-friendly, ACOG officials said.
At the University of Virginia, in the meantime, Dr. Peterson is arming the next generation of ob.gyns. with the skills needed for a team-based approach to well-woman visits. She said nurse practitioners and physician assistants will provide much more of the education and “more of the truly individualized conversations with patients” that will increasingly be part of well-woman care.
This is already happening. All of the nurse practitioners in Dr. Johnson’s group practice perform well-woman visits, “referring to the gynecologists for complicated gyn problems and out to the patient’s primary care physician for complicated medical problems,” Dr. Johnson said.
Even as the tools and recommendations for women’s preventive care become more evidence-based, the scope of well-woman visits will be based on risk factors, shared decision making, and other issues, Dr. Peterson said. “We will be telling patients, this is your path for your well-woman care,” she said.
Throughout 2016, Ob.Gyn. News is celebrating its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and whether the practice environment is better or worse.
From her vantage point in medical education, Christine M. Peterson, MD, is acutely aware that well-woman care is at a turning point.
“We’re at an interesting crossroads between tradition and evidence-based practice,” said Dr. Peterson, who practiced obstetrics and gynecology in the 1980s and now serves as associate professor of ob.gyn. and assistant dean for student affairs at the University of Virginia’s School of Medicine in Charlottesville.
“Even young trainees are aware of the traditions for the annual well-woman visit – the Pap, the pelvic, the breast exam,” she said. “But the evidence is pointing us in a different direction ... toward doing only those things that are effective for prevention and early detection of disease, or that have [demonstrated] value in one way or another.”
If all goes as planned at a national level, Dr. Peterson’s students who become ob.gyns., family physicians, and general internists will practice under a reshaped and well-defined umbrella of well-woman care – one that includes a broad array of insurer-covered screening and counseling services.
That umbrella already includes HPV testing, and screening and/or counseling on intimate partner violence, sexually transmitted infections, and HIV, in addition to contraception counseling. These preventive services were described in the Women’s Preventive Services Guidelines and adopted as covered benefits by the Department of Health & Human Services in 2011.
And more change is on the way. In March, the American College of Obstetricians and Gynecologists (ACOG) launched the Women’s Preventive Services Initiative (WPSI) – a broad coalition tasked with recommending updates to the 2011 guidelines and developing new recommendations for the scope and implementation of women’s preventive health care services.
The effort is funded through a 5-year cooperative agreement with the Health Resources & Services Administration (HRSA). It’s similar to the American Academy of Pediatrics’ approach in developing the HRSA-supported Bright Futures guidelines almost 25 years ago. Under the Affordable Care Act, all HRSA-recommended preventive services must be covered by most private insurers without patient cost sharing.
“Over the years, we’ve gone from an emphasis on the Pap and pelvic to well-woman care that assesses the whole woman,” said Jeanne A. Conry, MD, assistant physician-in-chief at the Permanente Medical Group in Roseville, Calif., and a past president of ACOG.
Ob.gyns. have long provided preventive care, but today more than ever before, Dr. Conry said, “my emphasis is on helping women to get well and stay well.”
The evolution
Fifty years ago, in 1966, use of the Pap test was being widely promoted, modern mammography techniques were on the cusp of advancement, and ob.gyns. were prescribing the first birth control pill approved by the Food and Drug Administration. ACOG’s main practice guidance book, “Standards for Obstetric-Gynecologic Hospital Services,” addressed the general physical examination but otherwise focused on obstetrics and reproductive health.
Thirty years later, women’s health care as described in the first edition of ACOG’s “Guidelines for Women’s Health Care” (1996) had grown to include distinct categories of “primary and preventive care” and “evaluation and counseling” that were separate from gynecologic services and broken down by age.
What was referred to as the “women’s health exam” through the 1990s gradually took on the “well-woman” label in the 2000s. Ob.gyns. were encouraged to address a growing range of preventive issues, but the annual Pap test remained a focus and, in many ways, drove women’s visits.
“Women would say, ‘I’m going for my Pap,’” Dr. Conry said.
Most recently, new technology and evidence-based reviews have changed the framework for well-woman visits to one with longer intervals for cervical cancer screening (every 3-5 years) and a move away from performing internal pelvic exams in all women (ACOG recommends pelvic exams annually for patients aged 21 and older but advises shared decision making for complete pelvic exams in asymptomatic patients).
Recent evidence reviews have also added some uncertainty about the role of annual breast exams in all women, as well as the role of breast self-exams, particularly for women not at high risk.
Maintaining patient relationships
One of the biggest and most immediate challenges for ob.gyns in the face of changing guidelines lies in maintaining the physician-patient relationship and “continuing communication” about the importance of regular well-woman visits, said Jill Rabin, MD, cochief of the division of ambulatory care, women’s health programs–prenatal care assistance program services at Northwell Health, New Hyde Park, N.Y.
“We want patients to understand that, even though they no longer need the Pap every year, they still should see us for good, comprehensive care ... that we can help them achieve their health care goals,” she said.
How often well-woman visits should occur has been a subject of much discussion. The HRSA-supported preventive services guidelines call for well-woman preventive care annually but note that “several visits may be needed to obtain all the necessary recommended services.”
And, in its first set of draft recommendations for HRSA, WPSI offered clarifications, saying there’s a need for “at least one annual preventive care visit for women beginning in adolescence and continuing across the lifespan to ensure that women obtain recommended preventive services” as determined by age and risk factors.
The draft recommendations, aimed at reviewing and updating the 2011 HRSA-sponsored guidelines, will be finalized by the end of 2016. WPSI will submit additional recommendations over the next 4 years.
Carol S. Weisman, PhD, a sociologist and health services researcher who sat on the Institute of Medicine committee that wrote the Women’s Preventive Services Guidelines, said the IOM’s recommendation for well-women visits – “in plural” form – recognizes “that historically many women have patched together their well-woman care from multiple providers, getting some of their preventive care from their generalist, and some from their ob.gyn.”
What’s more, the current list of preventive services covered under the Affordable Care Act is “enormous” – too long to address in one visit for many patients, said Dr. Weisman of the Penn State Center for Women’s Health Research in Hershey.
In addition to the women’s preventive services, the Affordable Care Act requires plans to cover services recommended with a grade A or B rating by the U.S. Preventive Services Task Force (counseling and screening for cancer, cardiovascular disease, and more) as well as vaccines recommended by the Advisory Committee on Immunization Practices, and HRSA’s Bright Futures services (for adolescents as well as children).
Beyond gynecology
How much further ob.gyns. will reach outside the gynecologic realm to offer additional preventive care services is an open question, but it’s likely to be based mainly on comfort levels, sources said.
“The changing needs of the gynecologic visit enable us to spend more time on other things,” said Hal C. Lawrence III, MD, ACOG’s executive vice president and chief executive officer. “But within the specialty, there’s going to be variation as to what level of expanded services ob.gyns. provide. Some will provide a lot of what women need, others not as much.”
Heather Johnson, MD, practices with a large ob.gyn. group in Chevy Chase, Md. and provides well-woman care largely to women in their 50s and 60s, whose children she delivered. She’s comfortable, she said, with screening for and treating osteoporosis and mild depression, for instance. She regularly performs lipid testing but refers out for management of high cholesterol levels or high blood pressure.
“I encourage all my patients to have a primary care physician of record,” she said, “but I still am happy to discuss the issues with them.”
What’s key, according to Dr. Lawrence and Dr. Conry, is coordination.
“We know there will be different individuals providing different components [of well-woman care],” Dr. Conry said. “I may see a woman for various things. Then she may go to her internist. But we should be able to collaborate to hit all the preventive health goals for her.”
Dr. Conry emphasized that the ob.gyn.’s expertise in reproductive health is critical to well-woman care planning, especially given medicine’s growing knowledge of how obstetric health and pregnancy complications can have long-term impacts on cardiovascular disease and other conditions. “It’s important for all providers to realize this,” she said.
Primary care status?
Intertwined with the future of well-women care is the issue of primary care status for ob.gyns. ACOG continues to advocate for ob.gyns. to be listed as primary care providers and part of primary care payment policies. Leaders are also pushing for projects on ob.gyn.–led medical homes.
At the same time, ACOG has been taking a broad collaborative approach to shaping well-woman care, aiming to develop comprehensive, age-specific recommendations for use by any provider who cares for adolescent girls and women.
Representatives of the American Academy of Family Physicians, the American College of Physicians, and the National Association of Nurse Practitioners in Women’s Health were involved in a Well-Woman Task Force that Dr. Conry appointed while serving as ACOG president in 2013-2014. These organizations now sit on WPSI’s advisory panel with ACOG.
Representatives from the American Academy of Pediatrics have also been close partners, as ACOG officials view the HRSA-sponsored Bright Futures guidelines (which includes the “Periodicity Schedule”) as a potential model for well-woman care. The guidelines are comprehensive, well-organized, and user-friendly, ACOG officials said.
At the University of Virginia, in the meantime, Dr. Peterson is arming the next generation of ob.gyns. with the skills needed for a team-based approach to well-woman visits. She said nurse practitioners and physician assistants will provide much more of the education and “more of the truly individualized conversations with patients” that will increasingly be part of well-woman care.
This is already happening. All of the nurse practitioners in Dr. Johnson’s group practice perform well-woman visits, “referring to the gynecologists for complicated gyn problems and out to the patient’s primary care physician for complicated medical problems,” Dr. Johnson said.
Even as the tools and recommendations for women’s preventive care become more evidence-based, the scope of well-woman visits will be based on risk factors, shared decision making, and other issues, Dr. Peterson said. “We will be telling patients, this is your path for your well-woman care,” she said.
Throughout 2016, Ob.Gyn. News is celebrating its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and whether the practice environment is better or worse.
From her vantage point in medical education, Christine M. Peterson, MD, is acutely aware that well-woman care is at a turning point.
“We’re at an interesting crossroads between tradition and evidence-based practice,” said Dr. Peterson, who practiced obstetrics and gynecology in the 1980s and now serves as associate professor of ob.gyn. and assistant dean for student affairs at the University of Virginia’s School of Medicine in Charlottesville.
“Even young trainees are aware of the traditions for the annual well-woman visit – the Pap, the pelvic, the breast exam,” she said. “But the evidence is pointing us in a different direction ... toward doing only those things that are effective for prevention and early detection of disease, or that have [demonstrated] value in one way or another.”
If all goes as planned at a national level, Dr. Peterson’s students who become ob.gyns., family physicians, and general internists will practice under a reshaped and well-defined umbrella of well-woman care – one that includes a broad array of insurer-covered screening and counseling services.
That umbrella already includes HPV testing, and screening and/or counseling on intimate partner violence, sexually transmitted infections, and HIV, in addition to contraception counseling. These preventive services were described in the Women’s Preventive Services Guidelines and adopted as covered benefits by the Department of Health & Human Services in 2011.
And more change is on the way. In March, the American College of Obstetricians and Gynecologists (ACOG) launched the Women’s Preventive Services Initiative (WPSI) – a broad coalition tasked with recommending updates to the 2011 guidelines and developing new recommendations for the scope and implementation of women’s preventive health care services.
The effort is funded through a 5-year cooperative agreement with the Health Resources & Services Administration (HRSA). It’s similar to the American Academy of Pediatrics’ approach in developing the HRSA-supported Bright Futures guidelines almost 25 years ago. Under the Affordable Care Act, all HRSA-recommended preventive services must be covered by most private insurers without patient cost sharing.
“Over the years, we’ve gone from an emphasis on the Pap and pelvic to well-woman care that assesses the whole woman,” said Jeanne A. Conry, MD, assistant physician-in-chief at the Permanente Medical Group in Roseville, Calif., and a past president of ACOG.
Ob.gyns. have long provided preventive care, but today more than ever before, Dr. Conry said, “my emphasis is on helping women to get well and stay well.”
The evolution
Fifty years ago, in 1966, use of the Pap test was being widely promoted, modern mammography techniques were on the cusp of advancement, and ob.gyns. were prescribing the first birth control pill approved by the Food and Drug Administration. ACOG’s main practice guidance book, “Standards for Obstetric-Gynecologic Hospital Services,” addressed the general physical examination but otherwise focused on obstetrics and reproductive health.
Thirty years later, women’s health care as described in the first edition of ACOG’s “Guidelines for Women’s Health Care” (1996) had grown to include distinct categories of “primary and preventive care” and “evaluation and counseling” that were separate from gynecologic services and broken down by age.
What was referred to as the “women’s health exam” through the 1990s gradually took on the “well-woman” label in the 2000s. Ob.gyns. were encouraged to address a growing range of preventive issues, but the annual Pap test remained a focus and, in many ways, drove women’s visits.
“Women would say, ‘I’m going for my Pap,’” Dr. Conry said.
Most recently, new technology and evidence-based reviews have changed the framework for well-woman visits to one with longer intervals for cervical cancer screening (every 3-5 years) and a move away from performing internal pelvic exams in all women (ACOG recommends pelvic exams annually for patients aged 21 and older but advises shared decision making for complete pelvic exams in asymptomatic patients).
Recent evidence reviews have also added some uncertainty about the role of annual breast exams in all women, as well as the role of breast self-exams, particularly for women not at high risk.
Maintaining patient relationships
One of the biggest and most immediate challenges for ob.gyns in the face of changing guidelines lies in maintaining the physician-patient relationship and “continuing communication” about the importance of regular well-woman visits, said Jill Rabin, MD, cochief of the division of ambulatory care, women’s health programs–prenatal care assistance program services at Northwell Health, New Hyde Park, N.Y.
“We want patients to understand that, even though they no longer need the Pap every year, they still should see us for good, comprehensive care ... that we can help them achieve their health care goals,” she said.
How often well-woman visits should occur has been a subject of much discussion. The HRSA-supported preventive services guidelines call for well-woman preventive care annually but note that “several visits may be needed to obtain all the necessary recommended services.”
And, in its first set of draft recommendations for HRSA, WPSI offered clarifications, saying there’s a need for “at least one annual preventive care visit for women beginning in adolescence and continuing across the lifespan to ensure that women obtain recommended preventive services” as determined by age and risk factors.
The draft recommendations, aimed at reviewing and updating the 2011 HRSA-sponsored guidelines, will be finalized by the end of 2016. WPSI will submit additional recommendations over the next 4 years.
Carol S. Weisman, PhD, a sociologist and health services researcher who sat on the Institute of Medicine committee that wrote the Women’s Preventive Services Guidelines, said the IOM’s recommendation for well-women visits – “in plural” form – recognizes “that historically many women have patched together their well-woman care from multiple providers, getting some of their preventive care from their generalist, and some from their ob.gyn.”
What’s more, the current list of preventive services covered under the Affordable Care Act is “enormous” – too long to address in one visit for many patients, said Dr. Weisman of the Penn State Center for Women’s Health Research in Hershey.
In addition to the women’s preventive services, the Affordable Care Act requires plans to cover services recommended with a grade A or B rating by the U.S. Preventive Services Task Force (counseling and screening for cancer, cardiovascular disease, and more) as well as vaccines recommended by the Advisory Committee on Immunization Practices, and HRSA’s Bright Futures services (for adolescents as well as children).
Beyond gynecology
How much further ob.gyns. will reach outside the gynecologic realm to offer additional preventive care services is an open question, but it’s likely to be based mainly on comfort levels, sources said.
“The changing needs of the gynecologic visit enable us to spend more time on other things,” said Hal C. Lawrence III, MD, ACOG’s executive vice president and chief executive officer. “But within the specialty, there’s going to be variation as to what level of expanded services ob.gyns. provide. Some will provide a lot of what women need, others not as much.”
Heather Johnson, MD, practices with a large ob.gyn. group in Chevy Chase, Md. and provides well-woman care largely to women in their 50s and 60s, whose children she delivered. She’s comfortable, she said, with screening for and treating osteoporosis and mild depression, for instance. She regularly performs lipid testing but refers out for management of high cholesterol levels or high blood pressure.
“I encourage all my patients to have a primary care physician of record,” she said, “but I still am happy to discuss the issues with them.”
What’s key, according to Dr. Lawrence and Dr. Conry, is coordination.
“We know there will be different individuals providing different components [of well-woman care],” Dr. Conry said. “I may see a woman for various things. Then she may go to her internist. But we should be able to collaborate to hit all the preventive health goals for her.”
Dr. Conry emphasized that the ob.gyn.’s expertise in reproductive health is critical to well-woman care planning, especially given medicine’s growing knowledge of how obstetric health and pregnancy complications can have long-term impacts on cardiovascular disease and other conditions. “It’s important for all providers to realize this,” she said.
Primary care status?
Intertwined with the future of well-women care is the issue of primary care status for ob.gyns. ACOG continues to advocate for ob.gyns. to be listed as primary care providers and part of primary care payment policies. Leaders are also pushing for projects on ob.gyn.–led medical homes.
At the same time, ACOG has been taking a broad collaborative approach to shaping well-woman care, aiming to develop comprehensive, age-specific recommendations for use by any provider who cares for adolescent girls and women.
Representatives of the American Academy of Family Physicians, the American College of Physicians, and the National Association of Nurse Practitioners in Women’s Health were involved in a Well-Woman Task Force that Dr. Conry appointed while serving as ACOG president in 2013-2014. These organizations now sit on WPSI’s advisory panel with ACOG.
Representatives from the American Academy of Pediatrics have also been close partners, as ACOG officials view the HRSA-sponsored Bright Futures guidelines (which includes the “Periodicity Schedule”) as a potential model for well-woman care. The guidelines are comprehensive, well-organized, and user-friendly, ACOG officials said.
At the University of Virginia, in the meantime, Dr. Peterson is arming the next generation of ob.gyns. with the skills needed for a team-based approach to well-woman visits. She said nurse practitioners and physician assistants will provide much more of the education and “more of the truly individualized conversations with patients” that will increasingly be part of well-woman care.
This is already happening. All of the nurse practitioners in Dr. Johnson’s group practice perform well-woman visits, “referring to the gynecologists for complicated gyn problems and out to the patient’s primary care physician for complicated medical problems,” Dr. Johnson said.
Even as the tools and recommendations for women’s preventive care become more evidence-based, the scope of well-woman visits will be based on risk factors, shared decision making, and other issues, Dr. Peterson said. “We will be telling patients, this is your path for your well-woman care,” she said.
Throughout 2016, Ob.Gyn. News is celebrating its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and whether the practice environment is better or worse.
U.S. Falls Short on Protecting Against Vehicular Deaths
The U.S. has more motor vehicle crash deaths than do other high-income countries. Iin fact, the rate in the U.S. is roughly double the nearest countries in line: 10 deaths per 100,000 people, vs 5 to 6 in New Zealand, Canada, and France.
About one-third of deaths are due to drunk driving, and speeding contributes to another third. More than 9,500 of the deaths were due to passengers not using seat belts, car seats, or booster seats. Seat belts saved 12,500 plus lives in 2013, the CDC says, but about half of drivers or passengers who died in crashes weren’t buckled up. By contrast, 99% of drivers and passengers use front seat belts in France, and the average of the 19 countries studied is 94%—vs 87% in the U.S.
Although U.S. crash deaths fell 31% between 2000 and 2013, losing 90 people a day to crashes is still far too high, says the CDC. According to a Vital Signs report, more than 18,000 of the 32,000 lives lost each year could be saved if the U.S. took some safety tips from the other countries, the report says. Enforcing seat belt laws that cover everyone in every seat would be a good step, for instance. As would redefining blood alcohol concentration (BAC) limits—all the comparison countries use BAC levels at 0.02% to 0.05%, vs 0.08% in the U.S., Canada, and the United Kingdom. The report also urges using advanced engineering and technology, such as ignition interlocks for people convicted of drunk driving.
In the meantime, the CDC says, health care providers can help by reminding patients about using a seat belt on every trip, no matter how short; counseling parents on age- and size-appropriate seats for children; talking to patients about the dangers of impaired driving and “distracted” driving (eg, using cell phones or texting); and giving parents and caregivers of teens resources on safe teen driving.
The U.S. has more motor vehicle crash deaths than do other high-income countries. Iin fact, the rate in the U.S. is roughly double the nearest countries in line: 10 deaths per 100,000 people, vs 5 to 6 in New Zealand, Canada, and France.
About one-third of deaths are due to drunk driving, and speeding contributes to another third. More than 9,500 of the deaths were due to passengers not using seat belts, car seats, or booster seats. Seat belts saved 12,500 plus lives in 2013, the CDC says, but about half of drivers or passengers who died in crashes weren’t buckled up. By contrast, 99% of drivers and passengers use front seat belts in France, and the average of the 19 countries studied is 94%—vs 87% in the U.S.
Although U.S. crash deaths fell 31% between 2000 and 2013, losing 90 people a day to crashes is still far too high, says the CDC. According to a Vital Signs report, more than 18,000 of the 32,000 lives lost each year could be saved if the U.S. took some safety tips from the other countries, the report says. Enforcing seat belt laws that cover everyone in every seat would be a good step, for instance. As would redefining blood alcohol concentration (BAC) limits—all the comparison countries use BAC levels at 0.02% to 0.05%, vs 0.08% in the U.S., Canada, and the United Kingdom. The report also urges using advanced engineering and technology, such as ignition interlocks for people convicted of drunk driving.
In the meantime, the CDC says, health care providers can help by reminding patients about using a seat belt on every trip, no matter how short; counseling parents on age- and size-appropriate seats for children; talking to patients about the dangers of impaired driving and “distracted” driving (eg, using cell phones or texting); and giving parents and caregivers of teens resources on safe teen driving.
The U.S. has more motor vehicle crash deaths than do other high-income countries. Iin fact, the rate in the U.S. is roughly double the nearest countries in line: 10 deaths per 100,000 people, vs 5 to 6 in New Zealand, Canada, and France.
About one-third of deaths are due to drunk driving, and speeding contributes to another third. More than 9,500 of the deaths were due to passengers not using seat belts, car seats, or booster seats. Seat belts saved 12,500 plus lives in 2013, the CDC says, but about half of drivers or passengers who died in crashes weren’t buckled up. By contrast, 99% of drivers and passengers use front seat belts in France, and the average of the 19 countries studied is 94%—vs 87% in the U.S.
Although U.S. crash deaths fell 31% between 2000 and 2013, losing 90 people a day to crashes is still far too high, says the CDC. According to a Vital Signs report, more than 18,000 of the 32,000 lives lost each year could be saved if the U.S. took some safety tips from the other countries, the report says. Enforcing seat belt laws that cover everyone in every seat would be a good step, for instance. As would redefining blood alcohol concentration (BAC) limits—all the comparison countries use BAC levels at 0.02% to 0.05%, vs 0.08% in the U.S., Canada, and the United Kingdom. The report also urges using advanced engineering and technology, such as ignition interlocks for people convicted of drunk driving.
In the meantime, the CDC says, health care providers can help by reminding patients about using a seat belt on every trip, no matter how short; counseling parents on age- and size-appropriate seats for children; talking to patients about the dangers of impaired driving and “distracted” driving (eg, using cell phones or texting); and giving parents and caregivers of teens resources on safe teen driving.
Intraocular Lens Offers Better Vision to Patients With Cataracts
More than half of all Americans have a cataract or have had cataract surgery by age 80. Almost 4 million cataract surgeries are performed each year.
The mainstay of treatment has been monofocal lenses that improve distance vision. However, the FDA has just approved the first intraocular lens (IOL) to provide extended depth-of-focus, which improves sharpness of vision at near, intermediate, and far distances.
The Tecnis Symfony Extended Range of Vision IOL has been available in Europe since 2014. At the 2014 American Academy of Ophthalmology meeting, US cataract surgeon Mark Packer, MD, called the new lens “an exciting development.”
The lens is designed to correct both chromatic aberration (inability to focus due to competing wavelengths of light passing through the lens at different angles) and spherical aberration (lack of focus due to the shape of the lens). Clinical studies have demonstrated a low incidence of dysphotopsias such as halo and glare, which can impede night vision and driving. However, the FDA cautions that some patients experience visual halos, glare, or starbursts; some may experience worsening of or blurred vision, bleeding, or infection; and the device may cause reduced contrast sensitivity that worsens under poor visibility conditions.
More than 50 countries have approved the IOL has been approved, and has been widely studied with data from clinical studies involving more than 2,000 eyes, according to the manufacturer, Abbott Laboratories. FDA approval was based on a review of results from a study comparing 148 cataract patients implanted with the Tecnis Symfony Extended Range of Vision IOL and 151 patients implanted with a monofocal IOL.
Both groups of patients had comparable results for good distance vision. Of the patients in the Tecnis Symfony group, 77% had good vision (20/25) without glasses at intermediate distance, compared with 34% of those in the monofocal group. At near distances, patients with the Tecnis Symfony IOL could read 2 additional, progressively smaller lines on a standard eye chart, compared with those in the monofocal group.
In clinical trials for Tecnis IOLs, adverse events occurred at rates between 1.6% and 3.3%, including macular edema, endophthalmitis, and anterior lens tissue ongrowth. However, the events were not related to the lenses, the manufacturer says.
The FDA approval includes a version of the lens for people with astigmatism. The new lens is available in 4 toric models.
More than half of all Americans have a cataract or have had cataract surgery by age 80. Almost 4 million cataract surgeries are performed each year.
The mainstay of treatment has been monofocal lenses that improve distance vision. However, the FDA has just approved the first intraocular lens (IOL) to provide extended depth-of-focus, which improves sharpness of vision at near, intermediate, and far distances.
The Tecnis Symfony Extended Range of Vision IOL has been available in Europe since 2014. At the 2014 American Academy of Ophthalmology meeting, US cataract surgeon Mark Packer, MD, called the new lens “an exciting development.”
The lens is designed to correct both chromatic aberration (inability to focus due to competing wavelengths of light passing through the lens at different angles) and spherical aberration (lack of focus due to the shape of the lens). Clinical studies have demonstrated a low incidence of dysphotopsias such as halo and glare, which can impede night vision and driving. However, the FDA cautions that some patients experience visual halos, glare, or starbursts; some may experience worsening of or blurred vision, bleeding, or infection; and the device may cause reduced contrast sensitivity that worsens under poor visibility conditions.
More than 50 countries have approved the IOL has been approved, and has been widely studied with data from clinical studies involving more than 2,000 eyes, according to the manufacturer, Abbott Laboratories. FDA approval was based on a review of results from a study comparing 148 cataract patients implanted with the Tecnis Symfony Extended Range of Vision IOL and 151 patients implanted with a monofocal IOL.
Both groups of patients had comparable results for good distance vision. Of the patients in the Tecnis Symfony group, 77% had good vision (20/25) without glasses at intermediate distance, compared with 34% of those in the monofocal group. At near distances, patients with the Tecnis Symfony IOL could read 2 additional, progressively smaller lines on a standard eye chart, compared with those in the monofocal group.
In clinical trials for Tecnis IOLs, adverse events occurred at rates between 1.6% and 3.3%, including macular edema, endophthalmitis, and anterior lens tissue ongrowth. However, the events were not related to the lenses, the manufacturer says.
The FDA approval includes a version of the lens for people with astigmatism. The new lens is available in 4 toric models.
More than half of all Americans have a cataract or have had cataract surgery by age 80. Almost 4 million cataract surgeries are performed each year.
The mainstay of treatment has been monofocal lenses that improve distance vision. However, the FDA has just approved the first intraocular lens (IOL) to provide extended depth-of-focus, which improves sharpness of vision at near, intermediate, and far distances.
The Tecnis Symfony Extended Range of Vision IOL has been available in Europe since 2014. At the 2014 American Academy of Ophthalmology meeting, US cataract surgeon Mark Packer, MD, called the new lens “an exciting development.”
The lens is designed to correct both chromatic aberration (inability to focus due to competing wavelengths of light passing through the lens at different angles) and spherical aberration (lack of focus due to the shape of the lens). Clinical studies have demonstrated a low incidence of dysphotopsias such as halo and glare, which can impede night vision and driving. However, the FDA cautions that some patients experience visual halos, glare, or starbursts; some may experience worsening of or blurred vision, bleeding, or infection; and the device may cause reduced contrast sensitivity that worsens under poor visibility conditions.
More than 50 countries have approved the IOL has been approved, and has been widely studied with data from clinical studies involving more than 2,000 eyes, according to the manufacturer, Abbott Laboratories. FDA approval was based on a review of results from a study comparing 148 cataract patients implanted with the Tecnis Symfony Extended Range of Vision IOL and 151 patients implanted with a monofocal IOL.
Both groups of patients had comparable results for good distance vision. Of the patients in the Tecnis Symfony group, 77% had good vision (20/25) without glasses at intermediate distance, compared with 34% of those in the monofocal group. At near distances, patients with the Tecnis Symfony IOL could read 2 additional, progressively smaller lines on a standard eye chart, compared with those in the monofocal group.
In clinical trials for Tecnis IOLs, adverse events occurred at rates between 1.6% and 3.3%, including macular edema, endophthalmitis, and anterior lens tissue ongrowth. However, the events were not related to the lenses, the manufacturer says.
The FDA approval includes a version of the lens for people with astigmatism. The new lens is available in 4 toric models.
Updated Guideline for Acute Diarrheal Infection
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Risk-Assessment Models Are Unreliable Predictors of Venous Thromboembolism
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Veteran Perceptions, Interest, and Use of Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) are health and wellness practices that are outside conventional allopathic medicine. In the U.S., the popularity of CAM has grown, and patients often use CAM to treat pain, insomnia, anxiety, and depression.1-5 Veterans also have been increasingly adding CAM to conventional medicine, although limited studies exist on veteran use and attitudes toward CAM.6-8
Recently, the VA has increased its CAM services, offering different treatments at various VA facilities where CAM is most commonly used to treat anxiety, posttraumatic stress disorder (PTSD), depression, and back pain.9 Some veterans also seek CAM services outside the VA.6,8 Across studies of veterans and the broader population, having more years of education and higher income and being middle-aged, female, and white were associated with greater CAM use.1,3,6-8
Some CAM practices, such as acupuncture, require a practitioner’s regular and direct involvement. Other, independent CAM practices can be taught in classes, individual sessions, or through self-instructional multimedia. Once learned, these practices can be done independently, allowing for easier and less costly access. Independent CAM practices, such as yoga, meditation, breathing exercises, qigong, and tai chi promote general wellness or treat a particular ailment.
Although results have been mixed, several studies support independent CAM practices for treatment and symptom relief. For example, yoga improves symptoms in neurologic and psychiatric disorders, lessens pain, and helps decrease anxiety and depression and improve self-efficacy.10-13 Qigong can improve hypertensionand self-efficacy.14,15
This study examines veterans’ attitudes and beliefs about CAM, which can affect their interest and use of CAM services within and outside the VA. The focus is exclusively on independent CAM practices. At the time of the study, the availability of more direct CAM practices, such as acupuncture, was limited at many VA sites, and independently practiced techniques often require fewer resources and, therefore, could be adapted more easily. Subsequent references to CAM in this study refer only to independent CAM practices.
The current study surveyed veterans in New Jersey in multiple VA clinics and non-VA peer-counseling settings as part of an implementation study of a veteran-centric DVD called the STAR (Simple Tools to Aid and Restore) Well-Kit (SWK), which serves as a veteran introduction to CAM.16 Before watching the DVD, veterans were asked to fill out a baseline survey about their knowledge, attitudes, beliefs, and experiences with CAM as well as answer screening and demographic questions.
The authors describe the findings of the baseline survey to inform how to best implement CAM more broadly throughout VA. They expected that knowledge, attitudes, beliefs, and experiences with CAM would vary by clinical setting and respondent characteristics and hypothesized that psychological factors would be related to interest in CAM. Finally, barriers and facilitators of use of CAM are reported to inform policies to promote veteran access to CAM.
Methods
This cross-sectional analysis of the baseline SWK surveys had no inclusion or exclusion criteria because participation was anonymous. Recipients received a packet that instructed them to complete a previewing survey, watch the DVD, and complete a postviewing survey about the DVD. Surveys were returned in person or by postage-paid envelopes. No follow-up reminders were provided. This study examines data from only the previewing survey, and all further references to the veteran presurvey refers to it as the survey.
Study sites were the outpatient services of the VA New Jersey Health Care System (VANJHCS) and a non-VHA New Jersey veteran peer-counseling office. VANJHCS, which enrolls patients from northern and central New Jersey, offers health care services at 2 campuses and 9 outpatient clinics. Waivers of informed consent were approved by the VANJHCS Institutional Review Board and Research and Development Committee given the anonymous and low-risk nature of the research.
Participant Recruitment
The survey was distributed at 4 settings selected with a focus on ambulatory services and a goal of ensuring participant diversity in age, deployment experience, and mental and physical health conditions. At 3 settings, surveys were distributed using 3 methods: by a researcher; left for pickup in waiting rooms; or by selected health care providers at their discretion in the context of routine clinical visits. The VANJHCS settings were outpatient mental-health clinics, outpatient primary-care settings, and outpatient transition-unit clinics for recent combat veterans. The fourth setting was a community veteran peer-support organization staffed by veterans and included events held at the organization’s offices, veteran informational and health fairs in the community, and outreach events at college campuses. In this setting, veteran peers distributed the SWK at their discretion; they were given suggested talking points for distribution.
Survey Data Collection
Veterans filled out baseline surveys before viewing the SWK DVD. The surveys were anonymous but coded with a number to allow for tracking by setting and dissemination method. The surveys asked for demographic and health information and experience with and interest in CAM techniques. To minimize respondent burden, the authors focused on the most critical domains as summarized in the background section (demographics; health status and symptoms, including pain; self-efficacy; mental health conditions; knowledge, attitudes, and beliefs about CAM).
Demographic Information
Age range was assessed to avoid collecting identifying information. The questionnaire also included gender, military era/deployment, employment status, and race and ethnicity.
- Self-Rated Health (SF1). Self-rated health was assessed with a widely used single-item question that correlates highly with actual overall health and with function and quality of life.17,18 Respondents were asked to rate their health as excellent (5), very good, good, fair, or poor (1).
- Pain Screen (PEG 3-item scale). This 3-item screen has shown reliability and validity and is comparable to longer pain questionnaires.19 Respondents were asked to rate 3 measures of their pain and its consequences on a scale of 0 (no pain or no interference from pain) to 10 (worst pain or interference). Responses were averaged to determine pain score.
- PTSD Screen. This 4-item PTSD screen was developed for primary care and is widely used in VA settings.20 For each item, respondents were asked to check off whether they have had specific PTSD symptoms within the past month. The screen was considered positive with 3 of 4 affirmative responses.
- Anxiety and Depression Screen (PHQ-4). This 4-item scale combines the brief 2-item scales for screening anxiety and depression in primary care.21 For each depression or anxiety symptom, respondents selected from “not at all,” (1) “several days ”(2), “more days than not,” (3) and “nearly every day.” (4) For each 2-item screen, a sum of 5 or more indicated a positive screen.
- Self-Efficacy for Health Management (modified). The original 6-item self-efficacy screen was developed to test self-efficacy in managing chronic disease.22 Since not all participants in the current study were expected to have a chronic disease, the questions were modified to address more general self-efficacy for health management. Although the scale had not been adapted in this way or validated with this change, other authors have similarly adapted it to address specific chronic diseases with satisfactory results.23,24 For each item, respondents were asked to rate their confidence in their ability to manage aspects of their health on a scale of 1 (not at all confident) to 10 (very confident). Participants could also check “not applicable” for items that did not apply to their health concerns, and these items were not counted in the average score.
- Familiarity With and Interest in CAM. The authors developed a checklist to assess whether participants had heard of, tried, or were practicing the 4 CAM techniques featured in the SWK and to gauge their interest in learning about them (ie, meditation/guided imagery, breathing exercises, yoga, tai chi or qigong). For each technique, respondents selected that they have “never heard of,” “heard of but never tried,” “have done this in the past,” or “are currently doing.” For some analyses, the first 2 and last 2 options were combined to determine whether respondents had done each practice. They were also asked to check off whether they would like to learn more about the practice and whether they would like to try it with an instructor and/or try it on their own. For some analyses, each technique was looked at separately, whereas for others, the 4 techniques were combined to determine whether they had tried or were currently doing any of them.
- Barriers to Practice. The authors developed a checklist of 10 barriers to practicing CAM techniques based on research but with adjustments to the specific practices and population under investigation.25 The checklist included an open-end response to allow respondents to add barriers. The barrier list was a checklist and not a validated scale.
- Perceived Benefits of CAM. The authors developed 2 questions to assess the perceived benefits of these techniques on functionality and overall wellness, rated on a Likert scale from 1 (no benefits) to 10 (very much).
Statistical Analysis
Survey instruments were scored according to generally accepted and published practices. Item-level analysis was performed to identify missing responses and describe the sample. Summary statistics were reported. Pearson product moment correlation was used to detect associations between continuous variables. Analysis of variance (ANOVA) was used to detect associations between dichotomous and continuous variables. Chi-square tests were used to detect associations between categorical variables, specifically looking at clinically meaningful differences between veterans who had experience with or interest in trying independent CAM practices and those who did not. Linear regression analysis was used to determine significant associations between participant characteristics and the belief that independent CAM practices would be helpful with daily function.
Results
The response rate for returning surveys was low (n = 134; 18.2%). Surveys distributed by peers in the community setting had the highest response rate (38%), followed by surveys distributed in primary care (23%).
Due to the anonymous nature of the survey, information on nonresponder characteristics was not available. Respondents covered a range of ages, with 64% of respondents aged ≥ 50 years. Respondents were men (88%) and white (49%) or African American (40%). Fifty-five percent screened positive for at least 1 mental health condition (PTSD, depression, or anxiety). The average self-rated health was 2.9 on a scale of 1 (poor health) to 5 (excellent health). Gender, age range, race, and deployment status were comparable with New Jersey VA veteran demographics.26
Table 1 shows veteran experience and interest in CAM practices. More than half of veterans who returned the survey reported doing either a CAM practice or having done 1 (n = 82; 61%). Many also reported interest in trying at least 1 practice (n = 73; 55%) or learning more about at least 1 practice (n = 71; 53%) either on their own or with an instructor. More veterans indicated they would prefer to try the techniques with an instructor (n = 61; 46%) rather than on their own (n = 26; 19%). Chi-square testing showed that interest and experience with CAM were not significantly associated with specific demographic characteristics.
Several barriers to CAM practice were frequently cited (Table 2). The 2 most commonly endorsed barriers were veterans who wanted to try the techniques but needed more guidance (n = 62; 46%) and heard of CAM but never thought to try them (n = 43; 32%). Only a small percentage of veterans indicated that they did not think the practice would help (n = 13; 10%) or were concerned that it might hurt them (n = 11; 8%).
There were several significant bivariate associations (Table 3), although overall r2 values were low. More severe pain was associated with a weaker belief that the techniques could benefit overall wellness (r2 = – .19; P = .04) and help daily functioning (r2= – .27; P < .01). Higher health-related self-efficacy was associated with a stronger belief in the techniques’ effectiveness for overall wellness (r2= .30; P < .01) and daily function (r2 = .35; P < .01). Higher self-rated health was associated with stronger belief in effectiveness for overall wellness (r2 = .20; P = .02) and daily function (r2= .23; P < .01). One-way ANOVAs found no significant associations between belief in the techniques’ effectiveness for wellness or for daily activities (for which statistics are presented here) and positive screens for PTSD (F1,116 = 3.04; P = .08), depression (F1,116 = 2.06; P = .15), anxiety (F1,122 = 1.41; P = .23), or any of the 3 combined (F1,116 = 3.74; P = .06). None of the health factors was associated with veteran interest in trying a technique or with a history of trying at least 1 technique.
Of the multivariate linear regression models examining associations between veteran characteristics and responses to CAM, only 1 was significant (Table 4). Of all the factors in the model, only self-efficacy was significantly associated with the belief that CAM can improve daily function. Pain moderated this relationship; those with higher pain levels believed CAM could help with daily function only if they also had high self-efficacy (interaction term β = 0.27; SE = 0.03). For example, veterans with no pain (pain score 1 on a scale of 1-10) had a β = .07 (SE .13, P = .28), whereas those with the highest pain level (10) had a β = .92 (SE = .24, P = .001).
Discussion
The authors report 3 main findings from this study: Personal characteristics are not associated with experience, interest in, or belief in the efficacy of CAM; despite a large proportion reporting experience with CAM, veterans reported several barriers to using CAM; and the level of pain reported moderated the relationship between health-related self-efficacy and the belief that CAM will help with daily function.
Determining which personal characteristics are associated with CAM perceptions may indicate who is willing to try CAM techniques and who may require additional education or support. Although the authors hypothesized a difference in experience, interest, and belief of efficacy according to patient characteristics, these differences were not demonstrated. Some published research supports an association between white race, female sex, and middle or younger age and use of CAM, but this sample of veterans did not confirm these associations.1,3,6-8
The lack of associations may be related to selection bias, reflected in the relatively high report of baseline use of CAM. Nevertheless, this finding implies that clinicians should not make assumptions about an individual’s experience with CAM or interest in trying a modality. From a policy perspective, the VHA should consider a broad-based approach targeting a general audience or multiple segmented audiences to increase awareness and a trial of CAM for veterans.
Barriers should be considered when introducing CAM into routine clinical care. The current study revealed several important barriers to veterans accessing or trying CAM techniques, including need for guidance, the lack of awareness or access, and cost. The VHA services are often provided at low or no cost to eligible veterans, likely mitigating the cost barrier to a great extent. However, being able to easily access instruction in CAM modalities in a timely manner may be just as important. The authors detected a preference among respondents for classes to learn CAM (46%) vs independently (19%), supported by the commonly endorsed barrier to trying CAM of “I want in-person instruction but can’t find it.” Offering CAM modalities that can be taught in a group or individual setting and later practiced independently may be an appropriate approach to introduce CAM techniques to the largest number of people and encourage uptake. This approach can maximize access while satisfying many veterans’ preferences for in-person instruction. This leverage of skilled practitioner time could be extended for some modalities through remote telehealth participation or on-demand instruction, such as online videos or DVDs, including the SWK.
Chronic pain can be a challenge for patients and clinicians to manage, so the role of CAM in pain management is growing.2,27 The study’s findings suggest that motivating veterans with chronic pain to try CAM may take extra effort by the clinician. Multivariate linear regression modeling showed that respondents with higher pain levels believed CAM could help with their function only if they also had high health-related self-efficacy, whereas those with low pain scores reported this belief even with low self-efficacy. Thus, strong self-efficacy may overpower doubts about CAM that accompany having pain. Conversely, high reported pain levels may reduce self-efficacy and lead to doubts about the benefit of CAM.
In one study, the belief that lifestyle contributes to illness predicted CAM use, which is similar to this study’s finding that health-related self-efficacy predicted CAM use.8 Several other studies examined CAM use and self-efficacy, although usually not self-efficacy for general health management. To promote experimentation with CAM, patients with chronic pain may require interventions targeted to increase self-efficacy related to CAM.
Of the 733 surveys distributed to veterans, 134 (18%) responses were received. More than 60% of the respondents had tried a CAM technique, higher rates than reported by most other CAM utilization studies: U.S. prevalence studies range from 29% to 42% of respondents having tried some CAM technique, and studies of veterans or military personnel range from 37% to 50% having tried CAM.3,5-7 Because these studies asked about CAM generally or about specific practices that do not fully overlap with the independent CAM practices evaluated in the current study, it is difficult to assess how the experiences of the current sample compare with those populations.
Another study asked veterans with multiple sclerosis whether they were interested in trying CAM practices, and 40% responded “yes,” which is similar to 55% in the current study.6 It is possible the rate of experience with CAM is higher in the current study due to self-selection of respondents who were interested in the SWK. Another factor may be that some veterans were recruited from VA mental health clinics where independent CAM practices are more frequently offered.9 It is also possible that there are regional differences in CAM use; this study took place at a single facility in the northeast U.S., although subsequent phases of the SWK project involved more widespread national dissemination, to be reported in the future.
Limitations
Self-selection and low response rate are limitations in this study. Despite the low response rate, the demographic information of the sample generally resembles the population of veterans at VANJHCS for age, sex, era, health status, and presence of mental health problems.24 Of note, the authors received responses from a wide range of veterans in terms of age, military era, and care setting, including some veterans who do not use the VA. However, data are lacking for nonresponders, and the possibility remains that survey respondents self-selected and were more interested in or experienced with CAM than were nonrespondents. Regardless, many findings, including barriers to CAM and the interaction of pain and self-efficacy, are internally valid and are important to consider even if the sample is not representative of the veteran population.
Conclusion
No studies have focused on veteran use of independent CAM practices as defined for this study. These techniques (eg, meditation, qigong) may promote wellness and relieve common symptoms in veterans. The authors’ results suggest that a broad interest in independent CAM practices among veterans exists. The VA and other health care settings should consider implementing classes in these modalities, especially as their reach may be greater than other CAM modalities requiring one-on-one practitioner-patient interaction. Even with broader availability, patients with chronic pain may require extra attention and context to improve or overcome low health-related self-efficacy, maximizing their likelihood of engaging in CAM. This possibility needs to be explored.
Acknowledgments
Funding for this research was provided by the Veterans Affairs Office of Patient Centered Care and Cultural Transformation, which was not involved in the study design or production of the manuscript. The authors also acknowledge the work of Anna Rusiewicz, PhD, in developing the STAR Well-Kit that was disseminated during this study.
1. Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135(5):344-351.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
3. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45-56.
4. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252.
5. Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
6. Campbell DG, Turner AP, Williams RM, et al. Complementary and alternative medicine use in veterans with multiple sclerosis: prevalence and demographic associations. J Rehabil Res Dev. 2006;43(1):99-110.
7. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704.
8. McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med. 2006;6:34.
9. Ezeji-Okoye SC, Kotar TM, Smeeding SJ, Durfee JM. State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results. Fed Pract. 2013;30(11):14-19.
10. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4).
11. Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1-9.
12. Lee SW, Mancuso CA, Charlson ME. Prospective study of new participants in a community-based mind-body training program. J Gen Intern Med. 2004;19(7):760-765.
13. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677-687.
14. Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114(7):777-786.
15. Lee MS, Lim HJ, Lee MS. Impact of qigong exercise on self-efficacy and other cognitive perceptual variables in patients with essential hypertension. J Altern Complement Med. 2004;10(4):675-680.
16. U.S. Department of Veterans Affairs, War Related Illness & Injury Study Center. STAR Well-Kit. http://www.warrelatedillness.va.gov/WARRELATEDILLNESS/education/STAR/index.asp. Updated September 18, 2015. Accessed July 6, 2016.
17. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):P107-P116.
18. Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344-S353.
19. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738.
20. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9(1):9-14.
21. Kroenke K. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621.
22. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256-262.
23. Kim MT, Han HR, Song HJ, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educ. 2009;35(6):986-994.
24. Webel AR, Okonsky J. Psychometric properties of a Symptom Management Self-Efficacy Scale for women living with HIV/AIDS. J Pain Symptom Manage. 2011;41(3):549-557.
25. Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
26. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. http://www.va.gov/vetdata. Updated June 3, 2016. Accessed July 6, 2016.
27. Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299-309.
Complementary and alternative medicine (CAM) are health and wellness practices that are outside conventional allopathic medicine. In the U.S., the popularity of CAM has grown, and patients often use CAM to treat pain, insomnia, anxiety, and depression.1-5 Veterans also have been increasingly adding CAM to conventional medicine, although limited studies exist on veteran use and attitudes toward CAM.6-8
Recently, the VA has increased its CAM services, offering different treatments at various VA facilities where CAM is most commonly used to treat anxiety, posttraumatic stress disorder (PTSD), depression, and back pain.9 Some veterans also seek CAM services outside the VA.6,8 Across studies of veterans and the broader population, having more years of education and higher income and being middle-aged, female, and white were associated with greater CAM use.1,3,6-8
Some CAM practices, such as acupuncture, require a practitioner’s regular and direct involvement. Other, independent CAM practices can be taught in classes, individual sessions, or through self-instructional multimedia. Once learned, these practices can be done independently, allowing for easier and less costly access. Independent CAM practices, such as yoga, meditation, breathing exercises, qigong, and tai chi promote general wellness or treat a particular ailment.
Although results have been mixed, several studies support independent CAM practices for treatment and symptom relief. For example, yoga improves symptoms in neurologic and psychiatric disorders, lessens pain, and helps decrease anxiety and depression and improve self-efficacy.10-13 Qigong can improve hypertensionand self-efficacy.14,15
This study examines veterans’ attitudes and beliefs about CAM, which can affect their interest and use of CAM services within and outside the VA. The focus is exclusively on independent CAM practices. At the time of the study, the availability of more direct CAM practices, such as acupuncture, was limited at many VA sites, and independently practiced techniques often require fewer resources and, therefore, could be adapted more easily. Subsequent references to CAM in this study refer only to independent CAM practices.
The current study surveyed veterans in New Jersey in multiple VA clinics and non-VA peer-counseling settings as part of an implementation study of a veteran-centric DVD called the STAR (Simple Tools to Aid and Restore) Well-Kit (SWK), which serves as a veteran introduction to CAM.16 Before watching the DVD, veterans were asked to fill out a baseline survey about their knowledge, attitudes, beliefs, and experiences with CAM as well as answer screening and demographic questions.
The authors describe the findings of the baseline survey to inform how to best implement CAM more broadly throughout VA. They expected that knowledge, attitudes, beliefs, and experiences with CAM would vary by clinical setting and respondent characteristics and hypothesized that psychological factors would be related to interest in CAM. Finally, barriers and facilitators of use of CAM are reported to inform policies to promote veteran access to CAM.
Methods
This cross-sectional analysis of the baseline SWK surveys had no inclusion or exclusion criteria because participation was anonymous. Recipients received a packet that instructed them to complete a previewing survey, watch the DVD, and complete a postviewing survey about the DVD. Surveys were returned in person or by postage-paid envelopes. No follow-up reminders were provided. This study examines data from only the previewing survey, and all further references to the veteran presurvey refers to it as the survey.
Study sites were the outpatient services of the VA New Jersey Health Care System (VANJHCS) and a non-VHA New Jersey veteran peer-counseling office. VANJHCS, which enrolls patients from northern and central New Jersey, offers health care services at 2 campuses and 9 outpatient clinics. Waivers of informed consent were approved by the VANJHCS Institutional Review Board and Research and Development Committee given the anonymous and low-risk nature of the research.
Participant Recruitment
The survey was distributed at 4 settings selected with a focus on ambulatory services and a goal of ensuring participant diversity in age, deployment experience, and mental and physical health conditions. At 3 settings, surveys were distributed using 3 methods: by a researcher; left for pickup in waiting rooms; or by selected health care providers at their discretion in the context of routine clinical visits. The VANJHCS settings were outpatient mental-health clinics, outpatient primary-care settings, and outpatient transition-unit clinics for recent combat veterans. The fourth setting was a community veteran peer-support organization staffed by veterans and included events held at the organization’s offices, veteran informational and health fairs in the community, and outreach events at college campuses. In this setting, veteran peers distributed the SWK at their discretion; they were given suggested talking points for distribution.
Survey Data Collection
Veterans filled out baseline surveys before viewing the SWK DVD. The surveys were anonymous but coded with a number to allow for tracking by setting and dissemination method. The surveys asked for demographic and health information and experience with and interest in CAM techniques. To minimize respondent burden, the authors focused on the most critical domains as summarized in the background section (demographics; health status and symptoms, including pain; self-efficacy; mental health conditions; knowledge, attitudes, and beliefs about CAM).
Demographic Information
Age range was assessed to avoid collecting identifying information. The questionnaire also included gender, military era/deployment, employment status, and race and ethnicity.
- Self-Rated Health (SF1). Self-rated health was assessed with a widely used single-item question that correlates highly with actual overall health and with function and quality of life.17,18 Respondents were asked to rate their health as excellent (5), very good, good, fair, or poor (1).
- Pain Screen (PEG 3-item scale). This 3-item screen has shown reliability and validity and is comparable to longer pain questionnaires.19 Respondents were asked to rate 3 measures of their pain and its consequences on a scale of 0 (no pain or no interference from pain) to 10 (worst pain or interference). Responses were averaged to determine pain score.
- PTSD Screen. This 4-item PTSD screen was developed for primary care and is widely used in VA settings.20 For each item, respondents were asked to check off whether they have had specific PTSD symptoms within the past month. The screen was considered positive with 3 of 4 affirmative responses.
- Anxiety and Depression Screen (PHQ-4). This 4-item scale combines the brief 2-item scales for screening anxiety and depression in primary care.21 For each depression or anxiety symptom, respondents selected from “not at all,” (1) “several days ”(2), “more days than not,” (3) and “nearly every day.” (4) For each 2-item screen, a sum of 5 or more indicated a positive screen.
- Self-Efficacy for Health Management (modified). The original 6-item self-efficacy screen was developed to test self-efficacy in managing chronic disease.22 Since not all participants in the current study were expected to have a chronic disease, the questions were modified to address more general self-efficacy for health management. Although the scale had not been adapted in this way or validated with this change, other authors have similarly adapted it to address specific chronic diseases with satisfactory results.23,24 For each item, respondents were asked to rate their confidence in their ability to manage aspects of their health on a scale of 1 (not at all confident) to 10 (very confident). Participants could also check “not applicable” for items that did not apply to their health concerns, and these items were not counted in the average score.
- Familiarity With and Interest in CAM. The authors developed a checklist to assess whether participants had heard of, tried, or were practicing the 4 CAM techniques featured in the SWK and to gauge their interest in learning about them (ie, meditation/guided imagery, breathing exercises, yoga, tai chi or qigong). For each technique, respondents selected that they have “never heard of,” “heard of but never tried,” “have done this in the past,” or “are currently doing.” For some analyses, the first 2 and last 2 options were combined to determine whether respondents had done each practice. They were also asked to check off whether they would like to learn more about the practice and whether they would like to try it with an instructor and/or try it on their own. For some analyses, each technique was looked at separately, whereas for others, the 4 techniques were combined to determine whether they had tried or were currently doing any of them.
- Barriers to Practice. The authors developed a checklist of 10 barriers to practicing CAM techniques based on research but with adjustments to the specific practices and population under investigation.25 The checklist included an open-end response to allow respondents to add barriers. The barrier list was a checklist and not a validated scale.
- Perceived Benefits of CAM. The authors developed 2 questions to assess the perceived benefits of these techniques on functionality and overall wellness, rated on a Likert scale from 1 (no benefits) to 10 (very much).
Statistical Analysis
Survey instruments were scored according to generally accepted and published practices. Item-level analysis was performed to identify missing responses and describe the sample. Summary statistics were reported. Pearson product moment correlation was used to detect associations between continuous variables. Analysis of variance (ANOVA) was used to detect associations between dichotomous and continuous variables. Chi-square tests were used to detect associations between categorical variables, specifically looking at clinically meaningful differences between veterans who had experience with or interest in trying independent CAM practices and those who did not. Linear regression analysis was used to determine significant associations between participant characteristics and the belief that independent CAM practices would be helpful with daily function.
Results
The response rate for returning surveys was low (n = 134; 18.2%). Surveys distributed by peers in the community setting had the highest response rate (38%), followed by surveys distributed in primary care (23%).
Due to the anonymous nature of the survey, information on nonresponder characteristics was not available. Respondents covered a range of ages, with 64% of respondents aged ≥ 50 years. Respondents were men (88%) and white (49%) or African American (40%). Fifty-five percent screened positive for at least 1 mental health condition (PTSD, depression, or anxiety). The average self-rated health was 2.9 on a scale of 1 (poor health) to 5 (excellent health). Gender, age range, race, and deployment status were comparable with New Jersey VA veteran demographics.26
Table 1 shows veteran experience and interest in CAM practices. More than half of veterans who returned the survey reported doing either a CAM practice or having done 1 (n = 82; 61%). Many also reported interest in trying at least 1 practice (n = 73; 55%) or learning more about at least 1 practice (n = 71; 53%) either on their own or with an instructor. More veterans indicated they would prefer to try the techniques with an instructor (n = 61; 46%) rather than on their own (n = 26; 19%). Chi-square testing showed that interest and experience with CAM were not significantly associated with specific demographic characteristics.
Several barriers to CAM practice were frequently cited (Table 2). The 2 most commonly endorsed barriers were veterans who wanted to try the techniques but needed more guidance (n = 62; 46%) and heard of CAM but never thought to try them (n = 43; 32%). Only a small percentage of veterans indicated that they did not think the practice would help (n = 13; 10%) or were concerned that it might hurt them (n = 11; 8%).
There were several significant bivariate associations (Table 3), although overall r2 values were low. More severe pain was associated with a weaker belief that the techniques could benefit overall wellness (r2 = – .19; P = .04) and help daily functioning (r2= – .27; P < .01). Higher health-related self-efficacy was associated with a stronger belief in the techniques’ effectiveness for overall wellness (r2= .30; P < .01) and daily function (r2 = .35; P < .01). Higher self-rated health was associated with stronger belief in effectiveness for overall wellness (r2 = .20; P = .02) and daily function (r2= .23; P < .01). One-way ANOVAs found no significant associations between belief in the techniques’ effectiveness for wellness or for daily activities (for which statistics are presented here) and positive screens for PTSD (F1,116 = 3.04; P = .08), depression (F1,116 = 2.06; P = .15), anxiety (F1,122 = 1.41; P = .23), or any of the 3 combined (F1,116 = 3.74; P = .06). None of the health factors was associated with veteran interest in trying a technique or with a history of trying at least 1 technique.
Of the multivariate linear regression models examining associations between veteran characteristics and responses to CAM, only 1 was significant (Table 4). Of all the factors in the model, only self-efficacy was significantly associated with the belief that CAM can improve daily function. Pain moderated this relationship; those with higher pain levels believed CAM could help with daily function only if they also had high self-efficacy (interaction term β = 0.27; SE = 0.03). For example, veterans with no pain (pain score 1 on a scale of 1-10) had a β = .07 (SE .13, P = .28), whereas those with the highest pain level (10) had a β = .92 (SE = .24, P = .001).
Discussion
The authors report 3 main findings from this study: Personal characteristics are not associated with experience, interest in, or belief in the efficacy of CAM; despite a large proportion reporting experience with CAM, veterans reported several barriers to using CAM; and the level of pain reported moderated the relationship between health-related self-efficacy and the belief that CAM will help with daily function.
Determining which personal characteristics are associated with CAM perceptions may indicate who is willing to try CAM techniques and who may require additional education or support. Although the authors hypothesized a difference in experience, interest, and belief of efficacy according to patient characteristics, these differences were not demonstrated. Some published research supports an association between white race, female sex, and middle or younger age and use of CAM, but this sample of veterans did not confirm these associations.1,3,6-8
The lack of associations may be related to selection bias, reflected in the relatively high report of baseline use of CAM. Nevertheless, this finding implies that clinicians should not make assumptions about an individual’s experience with CAM or interest in trying a modality. From a policy perspective, the VHA should consider a broad-based approach targeting a general audience or multiple segmented audiences to increase awareness and a trial of CAM for veterans.
Barriers should be considered when introducing CAM into routine clinical care. The current study revealed several important barriers to veterans accessing or trying CAM techniques, including need for guidance, the lack of awareness or access, and cost. The VHA services are often provided at low or no cost to eligible veterans, likely mitigating the cost barrier to a great extent. However, being able to easily access instruction in CAM modalities in a timely manner may be just as important. The authors detected a preference among respondents for classes to learn CAM (46%) vs independently (19%), supported by the commonly endorsed barrier to trying CAM of “I want in-person instruction but can’t find it.” Offering CAM modalities that can be taught in a group or individual setting and later practiced independently may be an appropriate approach to introduce CAM techniques to the largest number of people and encourage uptake. This approach can maximize access while satisfying many veterans’ preferences for in-person instruction. This leverage of skilled practitioner time could be extended for some modalities through remote telehealth participation or on-demand instruction, such as online videos or DVDs, including the SWK.
Chronic pain can be a challenge for patients and clinicians to manage, so the role of CAM in pain management is growing.2,27 The study’s findings suggest that motivating veterans with chronic pain to try CAM may take extra effort by the clinician. Multivariate linear regression modeling showed that respondents with higher pain levels believed CAM could help with their function only if they also had high health-related self-efficacy, whereas those with low pain scores reported this belief even with low self-efficacy. Thus, strong self-efficacy may overpower doubts about CAM that accompany having pain. Conversely, high reported pain levels may reduce self-efficacy and lead to doubts about the benefit of CAM.
In one study, the belief that lifestyle contributes to illness predicted CAM use, which is similar to this study’s finding that health-related self-efficacy predicted CAM use.8 Several other studies examined CAM use and self-efficacy, although usually not self-efficacy for general health management. To promote experimentation with CAM, patients with chronic pain may require interventions targeted to increase self-efficacy related to CAM.
Of the 733 surveys distributed to veterans, 134 (18%) responses were received. More than 60% of the respondents had tried a CAM technique, higher rates than reported by most other CAM utilization studies: U.S. prevalence studies range from 29% to 42% of respondents having tried some CAM technique, and studies of veterans or military personnel range from 37% to 50% having tried CAM.3,5-7 Because these studies asked about CAM generally or about specific practices that do not fully overlap with the independent CAM practices evaluated in the current study, it is difficult to assess how the experiences of the current sample compare with those populations.
Another study asked veterans with multiple sclerosis whether they were interested in trying CAM practices, and 40% responded “yes,” which is similar to 55% in the current study.6 It is possible the rate of experience with CAM is higher in the current study due to self-selection of respondents who were interested in the SWK. Another factor may be that some veterans were recruited from VA mental health clinics where independent CAM practices are more frequently offered.9 It is also possible that there are regional differences in CAM use; this study took place at a single facility in the northeast U.S., although subsequent phases of the SWK project involved more widespread national dissemination, to be reported in the future.
Limitations
Self-selection and low response rate are limitations in this study. Despite the low response rate, the demographic information of the sample generally resembles the population of veterans at VANJHCS for age, sex, era, health status, and presence of mental health problems.24 Of note, the authors received responses from a wide range of veterans in terms of age, military era, and care setting, including some veterans who do not use the VA. However, data are lacking for nonresponders, and the possibility remains that survey respondents self-selected and were more interested in or experienced with CAM than were nonrespondents. Regardless, many findings, including barriers to CAM and the interaction of pain and self-efficacy, are internally valid and are important to consider even if the sample is not representative of the veteran population.
Conclusion
No studies have focused on veteran use of independent CAM practices as defined for this study. These techniques (eg, meditation, qigong) may promote wellness and relieve common symptoms in veterans. The authors’ results suggest that a broad interest in independent CAM practices among veterans exists. The VA and other health care settings should consider implementing classes in these modalities, especially as their reach may be greater than other CAM modalities requiring one-on-one practitioner-patient interaction. Even with broader availability, patients with chronic pain may require extra attention and context to improve or overcome low health-related self-efficacy, maximizing their likelihood of engaging in CAM. This possibility needs to be explored.
Acknowledgments
Funding for this research was provided by the Veterans Affairs Office of Patient Centered Care and Cultural Transformation, which was not involved in the study design or production of the manuscript. The authors also acknowledge the work of Anna Rusiewicz, PhD, in developing the STAR Well-Kit that was disseminated during this study.
Complementary and alternative medicine (CAM) are health and wellness practices that are outside conventional allopathic medicine. In the U.S., the popularity of CAM has grown, and patients often use CAM to treat pain, insomnia, anxiety, and depression.1-5 Veterans also have been increasingly adding CAM to conventional medicine, although limited studies exist on veteran use and attitudes toward CAM.6-8
Recently, the VA has increased its CAM services, offering different treatments at various VA facilities where CAM is most commonly used to treat anxiety, posttraumatic stress disorder (PTSD), depression, and back pain.9 Some veterans also seek CAM services outside the VA.6,8 Across studies of veterans and the broader population, having more years of education and higher income and being middle-aged, female, and white were associated with greater CAM use.1,3,6-8
Some CAM practices, such as acupuncture, require a practitioner’s regular and direct involvement. Other, independent CAM practices can be taught in classes, individual sessions, or through self-instructional multimedia. Once learned, these practices can be done independently, allowing for easier and less costly access. Independent CAM practices, such as yoga, meditation, breathing exercises, qigong, and tai chi promote general wellness or treat a particular ailment.
Although results have been mixed, several studies support independent CAM practices for treatment and symptom relief. For example, yoga improves symptoms in neurologic and psychiatric disorders, lessens pain, and helps decrease anxiety and depression and improve self-efficacy.10-13 Qigong can improve hypertensionand self-efficacy.14,15
This study examines veterans’ attitudes and beliefs about CAM, which can affect their interest and use of CAM services within and outside the VA. The focus is exclusively on independent CAM practices. At the time of the study, the availability of more direct CAM practices, such as acupuncture, was limited at many VA sites, and independently practiced techniques often require fewer resources and, therefore, could be adapted more easily. Subsequent references to CAM in this study refer only to independent CAM practices.
The current study surveyed veterans in New Jersey in multiple VA clinics and non-VA peer-counseling settings as part of an implementation study of a veteran-centric DVD called the STAR (Simple Tools to Aid and Restore) Well-Kit (SWK), which serves as a veteran introduction to CAM.16 Before watching the DVD, veterans were asked to fill out a baseline survey about their knowledge, attitudes, beliefs, and experiences with CAM as well as answer screening and demographic questions.
The authors describe the findings of the baseline survey to inform how to best implement CAM more broadly throughout VA. They expected that knowledge, attitudes, beliefs, and experiences with CAM would vary by clinical setting and respondent characteristics and hypothesized that psychological factors would be related to interest in CAM. Finally, barriers and facilitators of use of CAM are reported to inform policies to promote veteran access to CAM.
Methods
This cross-sectional analysis of the baseline SWK surveys had no inclusion or exclusion criteria because participation was anonymous. Recipients received a packet that instructed them to complete a previewing survey, watch the DVD, and complete a postviewing survey about the DVD. Surveys were returned in person or by postage-paid envelopes. No follow-up reminders were provided. This study examines data from only the previewing survey, and all further references to the veteran presurvey refers to it as the survey.
Study sites were the outpatient services of the VA New Jersey Health Care System (VANJHCS) and a non-VHA New Jersey veteran peer-counseling office. VANJHCS, which enrolls patients from northern and central New Jersey, offers health care services at 2 campuses and 9 outpatient clinics. Waivers of informed consent were approved by the VANJHCS Institutional Review Board and Research and Development Committee given the anonymous and low-risk nature of the research.
Participant Recruitment
The survey was distributed at 4 settings selected with a focus on ambulatory services and a goal of ensuring participant diversity in age, deployment experience, and mental and physical health conditions. At 3 settings, surveys were distributed using 3 methods: by a researcher; left for pickup in waiting rooms; or by selected health care providers at their discretion in the context of routine clinical visits. The VANJHCS settings were outpatient mental-health clinics, outpatient primary-care settings, and outpatient transition-unit clinics for recent combat veterans. The fourth setting was a community veteran peer-support organization staffed by veterans and included events held at the organization’s offices, veteran informational and health fairs in the community, and outreach events at college campuses. In this setting, veteran peers distributed the SWK at their discretion; they were given suggested talking points for distribution.
Survey Data Collection
Veterans filled out baseline surveys before viewing the SWK DVD. The surveys were anonymous but coded with a number to allow for tracking by setting and dissemination method. The surveys asked for demographic and health information and experience with and interest in CAM techniques. To minimize respondent burden, the authors focused on the most critical domains as summarized in the background section (demographics; health status and symptoms, including pain; self-efficacy; mental health conditions; knowledge, attitudes, and beliefs about CAM).
Demographic Information
Age range was assessed to avoid collecting identifying information. The questionnaire also included gender, military era/deployment, employment status, and race and ethnicity.
- Self-Rated Health (SF1). Self-rated health was assessed with a widely used single-item question that correlates highly with actual overall health and with function and quality of life.17,18 Respondents were asked to rate their health as excellent (5), very good, good, fair, or poor (1).
- Pain Screen (PEG 3-item scale). This 3-item screen has shown reliability and validity and is comparable to longer pain questionnaires.19 Respondents were asked to rate 3 measures of their pain and its consequences on a scale of 0 (no pain or no interference from pain) to 10 (worst pain or interference). Responses were averaged to determine pain score.
- PTSD Screen. This 4-item PTSD screen was developed for primary care and is widely used in VA settings.20 For each item, respondents were asked to check off whether they have had specific PTSD symptoms within the past month. The screen was considered positive with 3 of 4 affirmative responses.
- Anxiety and Depression Screen (PHQ-4). This 4-item scale combines the brief 2-item scales for screening anxiety and depression in primary care.21 For each depression or anxiety symptom, respondents selected from “not at all,” (1) “several days ”(2), “more days than not,” (3) and “nearly every day.” (4) For each 2-item screen, a sum of 5 or more indicated a positive screen.
- Self-Efficacy for Health Management (modified). The original 6-item self-efficacy screen was developed to test self-efficacy in managing chronic disease.22 Since not all participants in the current study were expected to have a chronic disease, the questions were modified to address more general self-efficacy for health management. Although the scale had not been adapted in this way or validated with this change, other authors have similarly adapted it to address specific chronic diseases with satisfactory results.23,24 For each item, respondents were asked to rate their confidence in their ability to manage aspects of their health on a scale of 1 (not at all confident) to 10 (very confident). Participants could also check “not applicable” for items that did not apply to their health concerns, and these items were not counted in the average score.
- Familiarity With and Interest in CAM. The authors developed a checklist to assess whether participants had heard of, tried, or were practicing the 4 CAM techniques featured in the SWK and to gauge their interest in learning about them (ie, meditation/guided imagery, breathing exercises, yoga, tai chi or qigong). For each technique, respondents selected that they have “never heard of,” “heard of but never tried,” “have done this in the past,” or “are currently doing.” For some analyses, the first 2 and last 2 options were combined to determine whether respondents had done each practice. They were also asked to check off whether they would like to learn more about the practice and whether they would like to try it with an instructor and/or try it on their own. For some analyses, each technique was looked at separately, whereas for others, the 4 techniques were combined to determine whether they had tried or were currently doing any of them.
- Barriers to Practice. The authors developed a checklist of 10 barriers to practicing CAM techniques based on research but with adjustments to the specific practices and population under investigation.25 The checklist included an open-end response to allow respondents to add barriers. The barrier list was a checklist and not a validated scale.
- Perceived Benefits of CAM. The authors developed 2 questions to assess the perceived benefits of these techniques on functionality and overall wellness, rated on a Likert scale from 1 (no benefits) to 10 (very much).
Statistical Analysis
Survey instruments were scored according to generally accepted and published practices. Item-level analysis was performed to identify missing responses and describe the sample. Summary statistics were reported. Pearson product moment correlation was used to detect associations between continuous variables. Analysis of variance (ANOVA) was used to detect associations between dichotomous and continuous variables. Chi-square tests were used to detect associations between categorical variables, specifically looking at clinically meaningful differences between veterans who had experience with or interest in trying independent CAM practices and those who did not. Linear regression analysis was used to determine significant associations between participant characteristics and the belief that independent CAM practices would be helpful with daily function.
Results
The response rate for returning surveys was low (n = 134; 18.2%). Surveys distributed by peers in the community setting had the highest response rate (38%), followed by surveys distributed in primary care (23%).
Due to the anonymous nature of the survey, information on nonresponder characteristics was not available. Respondents covered a range of ages, with 64% of respondents aged ≥ 50 years. Respondents were men (88%) and white (49%) or African American (40%). Fifty-five percent screened positive for at least 1 mental health condition (PTSD, depression, or anxiety). The average self-rated health was 2.9 on a scale of 1 (poor health) to 5 (excellent health). Gender, age range, race, and deployment status were comparable with New Jersey VA veteran demographics.26
Table 1 shows veteran experience and interest in CAM practices. More than half of veterans who returned the survey reported doing either a CAM practice or having done 1 (n = 82; 61%). Many also reported interest in trying at least 1 practice (n = 73; 55%) or learning more about at least 1 practice (n = 71; 53%) either on their own or with an instructor. More veterans indicated they would prefer to try the techniques with an instructor (n = 61; 46%) rather than on their own (n = 26; 19%). Chi-square testing showed that interest and experience with CAM were not significantly associated with specific demographic characteristics.
Several barriers to CAM practice were frequently cited (Table 2). The 2 most commonly endorsed barriers were veterans who wanted to try the techniques but needed more guidance (n = 62; 46%) and heard of CAM but never thought to try them (n = 43; 32%). Only a small percentage of veterans indicated that they did not think the practice would help (n = 13; 10%) or were concerned that it might hurt them (n = 11; 8%).
There were several significant bivariate associations (Table 3), although overall r2 values were low. More severe pain was associated with a weaker belief that the techniques could benefit overall wellness (r2 = – .19; P = .04) and help daily functioning (r2= – .27; P < .01). Higher health-related self-efficacy was associated with a stronger belief in the techniques’ effectiveness for overall wellness (r2= .30; P < .01) and daily function (r2 = .35; P < .01). Higher self-rated health was associated with stronger belief in effectiveness for overall wellness (r2 = .20; P = .02) and daily function (r2= .23; P < .01). One-way ANOVAs found no significant associations between belief in the techniques’ effectiveness for wellness or for daily activities (for which statistics are presented here) and positive screens for PTSD (F1,116 = 3.04; P = .08), depression (F1,116 = 2.06; P = .15), anxiety (F1,122 = 1.41; P = .23), or any of the 3 combined (F1,116 = 3.74; P = .06). None of the health factors was associated with veteran interest in trying a technique or with a history of trying at least 1 technique.
Of the multivariate linear regression models examining associations between veteran characteristics and responses to CAM, only 1 was significant (Table 4). Of all the factors in the model, only self-efficacy was significantly associated with the belief that CAM can improve daily function. Pain moderated this relationship; those with higher pain levels believed CAM could help with daily function only if they also had high self-efficacy (interaction term β = 0.27; SE = 0.03). For example, veterans with no pain (pain score 1 on a scale of 1-10) had a β = .07 (SE .13, P = .28), whereas those with the highest pain level (10) had a β = .92 (SE = .24, P = .001).
Discussion
The authors report 3 main findings from this study: Personal characteristics are not associated with experience, interest in, or belief in the efficacy of CAM; despite a large proportion reporting experience with CAM, veterans reported several barriers to using CAM; and the level of pain reported moderated the relationship between health-related self-efficacy and the belief that CAM will help with daily function.
Determining which personal characteristics are associated with CAM perceptions may indicate who is willing to try CAM techniques and who may require additional education or support. Although the authors hypothesized a difference in experience, interest, and belief of efficacy according to patient characteristics, these differences were not demonstrated. Some published research supports an association between white race, female sex, and middle or younger age and use of CAM, but this sample of veterans did not confirm these associations.1,3,6-8
The lack of associations may be related to selection bias, reflected in the relatively high report of baseline use of CAM. Nevertheless, this finding implies that clinicians should not make assumptions about an individual’s experience with CAM or interest in trying a modality. From a policy perspective, the VHA should consider a broad-based approach targeting a general audience or multiple segmented audiences to increase awareness and a trial of CAM for veterans.
Barriers should be considered when introducing CAM into routine clinical care. The current study revealed several important barriers to veterans accessing or trying CAM techniques, including need for guidance, the lack of awareness or access, and cost. The VHA services are often provided at low or no cost to eligible veterans, likely mitigating the cost barrier to a great extent. However, being able to easily access instruction in CAM modalities in a timely manner may be just as important. The authors detected a preference among respondents for classes to learn CAM (46%) vs independently (19%), supported by the commonly endorsed barrier to trying CAM of “I want in-person instruction but can’t find it.” Offering CAM modalities that can be taught in a group or individual setting and later practiced independently may be an appropriate approach to introduce CAM techniques to the largest number of people and encourage uptake. This approach can maximize access while satisfying many veterans’ preferences for in-person instruction. This leverage of skilled practitioner time could be extended for some modalities through remote telehealth participation or on-demand instruction, such as online videos or DVDs, including the SWK.
Chronic pain can be a challenge for patients and clinicians to manage, so the role of CAM in pain management is growing.2,27 The study’s findings suggest that motivating veterans with chronic pain to try CAM may take extra effort by the clinician. Multivariate linear regression modeling showed that respondents with higher pain levels believed CAM could help with their function only if they also had high health-related self-efficacy, whereas those with low pain scores reported this belief even with low self-efficacy. Thus, strong self-efficacy may overpower doubts about CAM that accompany having pain. Conversely, high reported pain levels may reduce self-efficacy and lead to doubts about the benefit of CAM.
In one study, the belief that lifestyle contributes to illness predicted CAM use, which is similar to this study’s finding that health-related self-efficacy predicted CAM use.8 Several other studies examined CAM use and self-efficacy, although usually not self-efficacy for general health management. To promote experimentation with CAM, patients with chronic pain may require interventions targeted to increase self-efficacy related to CAM.
Of the 733 surveys distributed to veterans, 134 (18%) responses were received. More than 60% of the respondents had tried a CAM technique, higher rates than reported by most other CAM utilization studies: U.S. prevalence studies range from 29% to 42% of respondents having tried some CAM technique, and studies of veterans or military personnel range from 37% to 50% having tried CAM.3,5-7 Because these studies asked about CAM generally or about specific practices that do not fully overlap with the independent CAM practices evaluated in the current study, it is difficult to assess how the experiences of the current sample compare with those populations.
Another study asked veterans with multiple sclerosis whether they were interested in trying CAM practices, and 40% responded “yes,” which is similar to 55% in the current study.6 It is possible the rate of experience with CAM is higher in the current study due to self-selection of respondents who were interested in the SWK. Another factor may be that some veterans were recruited from VA mental health clinics where independent CAM practices are more frequently offered.9 It is also possible that there are regional differences in CAM use; this study took place at a single facility in the northeast U.S., although subsequent phases of the SWK project involved more widespread national dissemination, to be reported in the future.
Limitations
Self-selection and low response rate are limitations in this study. Despite the low response rate, the demographic information of the sample generally resembles the population of veterans at VANJHCS for age, sex, era, health status, and presence of mental health problems.24 Of note, the authors received responses from a wide range of veterans in terms of age, military era, and care setting, including some veterans who do not use the VA. However, data are lacking for nonresponders, and the possibility remains that survey respondents self-selected and were more interested in or experienced with CAM than were nonrespondents. Regardless, many findings, including barriers to CAM and the interaction of pain and self-efficacy, are internally valid and are important to consider even if the sample is not representative of the veteran population.
Conclusion
No studies have focused on veteran use of independent CAM practices as defined for this study. These techniques (eg, meditation, qigong) may promote wellness and relieve common symptoms in veterans. The authors’ results suggest that a broad interest in independent CAM practices among veterans exists. The VA and other health care settings should consider implementing classes in these modalities, especially as their reach may be greater than other CAM modalities requiring one-on-one practitioner-patient interaction. Even with broader availability, patients with chronic pain may require extra attention and context to improve or overcome low health-related self-efficacy, maximizing their likelihood of engaging in CAM. This possibility needs to be explored.
Acknowledgments
Funding for this research was provided by the Veterans Affairs Office of Patient Centered Care and Cultural Transformation, which was not involved in the study design or production of the manuscript. The authors also acknowledge the work of Anna Rusiewicz, PhD, in developing the STAR Well-Kit that was disseminated during this study.
1. Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135(5):344-351.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
3. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45-56.
4. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252.
5. Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
6. Campbell DG, Turner AP, Williams RM, et al. Complementary and alternative medicine use in veterans with multiple sclerosis: prevalence and demographic associations. J Rehabil Res Dev. 2006;43(1):99-110.
7. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704.
8. McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med. 2006;6:34.
9. Ezeji-Okoye SC, Kotar TM, Smeeding SJ, Durfee JM. State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results. Fed Pract. 2013;30(11):14-19.
10. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4).
11. Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1-9.
12. Lee SW, Mancuso CA, Charlson ME. Prospective study of new participants in a community-based mind-body training program. J Gen Intern Med. 2004;19(7):760-765.
13. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677-687.
14. Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114(7):777-786.
15. Lee MS, Lim HJ, Lee MS. Impact of qigong exercise on self-efficacy and other cognitive perceptual variables in patients with essential hypertension. J Altern Complement Med. 2004;10(4):675-680.
16. U.S. Department of Veterans Affairs, War Related Illness & Injury Study Center. STAR Well-Kit. http://www.warrelatedillness.va.gov/WARRELATEDILLNESS/education/STAR/index.asp. Updated September 18, 2015. Accessed July 6, 2016.
17. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):P107-P116.
18. Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344-S353.
19. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738.
20. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9(1):9-14.
21. Kroenke K. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621.
22. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256-262.
23. Kim MT, Han HR, Song HJ, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educ. 2009;35(6):986-994.
24. Webel AR, Okonsky J. Psychometric properties of a Symptom Management Self-Efficacy Scale for women living with HIV/AIDS. J Pain Symptom Manage. 2011;41(3):549-557.
25. Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
26. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. http://www.va.gov/vetdata. Updated June 3, 2016. Accessed July 6, 2016.
27. Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299-309.
1. Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135(5):344-351.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
3. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45-56.
4. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252.
5. Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
6. Campbell DG, Turner AP, Williams RM, et al. Complementary and alternative medicine use in veterans with multiple sclerosis: prevalence and demographic associations. J Rehabil Res Dev. 2006;43(1):99-110.
7. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704.
8. McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med. 2006;6:34.
9. Ezeji-Okoye SC, Kotar TM, Smeeding SJ, Durfee JM. State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results. Fed Pract. 2013;30(11):14-19.
10. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4).
11. Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1-9.
12. Lee SW, Mancuso CA, Charlson ME. Prospective study of new participants in a community-based mind-body training program. J Gen Intern Med. 2004;19(7):760-765.
13. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677-687.
14. Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114(7):777-786.
15. Lee MS, Lim HJ, Lee MS. Impact of qigong exercise on self-efficacy and other cognitive perceptual variables in patients with essential hypertension. J Altern Complement Med. 2004;10(4):675-680.
16. U.S. Department of Veterans Affairs, War Related Illness & Injury Study Center. STAR Well-Kit. http://www.warrelatedillness.va.gov/WARRELATEDILLNESS/education/STAR/index.asp. Updated September 18, 2015. Accessed July 6, 2016.
17. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):P107-P116.
18. Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344-S353.
19. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738.
20. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9(1):9-14.
21. Kroenke K. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621.
22. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256-262.
23. Kim MT, Han HR, Song HJ, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educ. 2009;35(6):986-994.
24. Webel AR, Okonsky J. Psychometric properties of a Symptom Management Self-Efficacy Scale for women living with HIV/AIDS. J Pain Symptom Manage. 2011;41(3):549-557.
25. Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
26. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. http://www.va.gov/vetdata. Updated June 3, 2016. Accessed July 6, 2016.
27. Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299-309.