User login
Presenting Treatment Safety Data: Subjective Interpretations of Objective Information
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
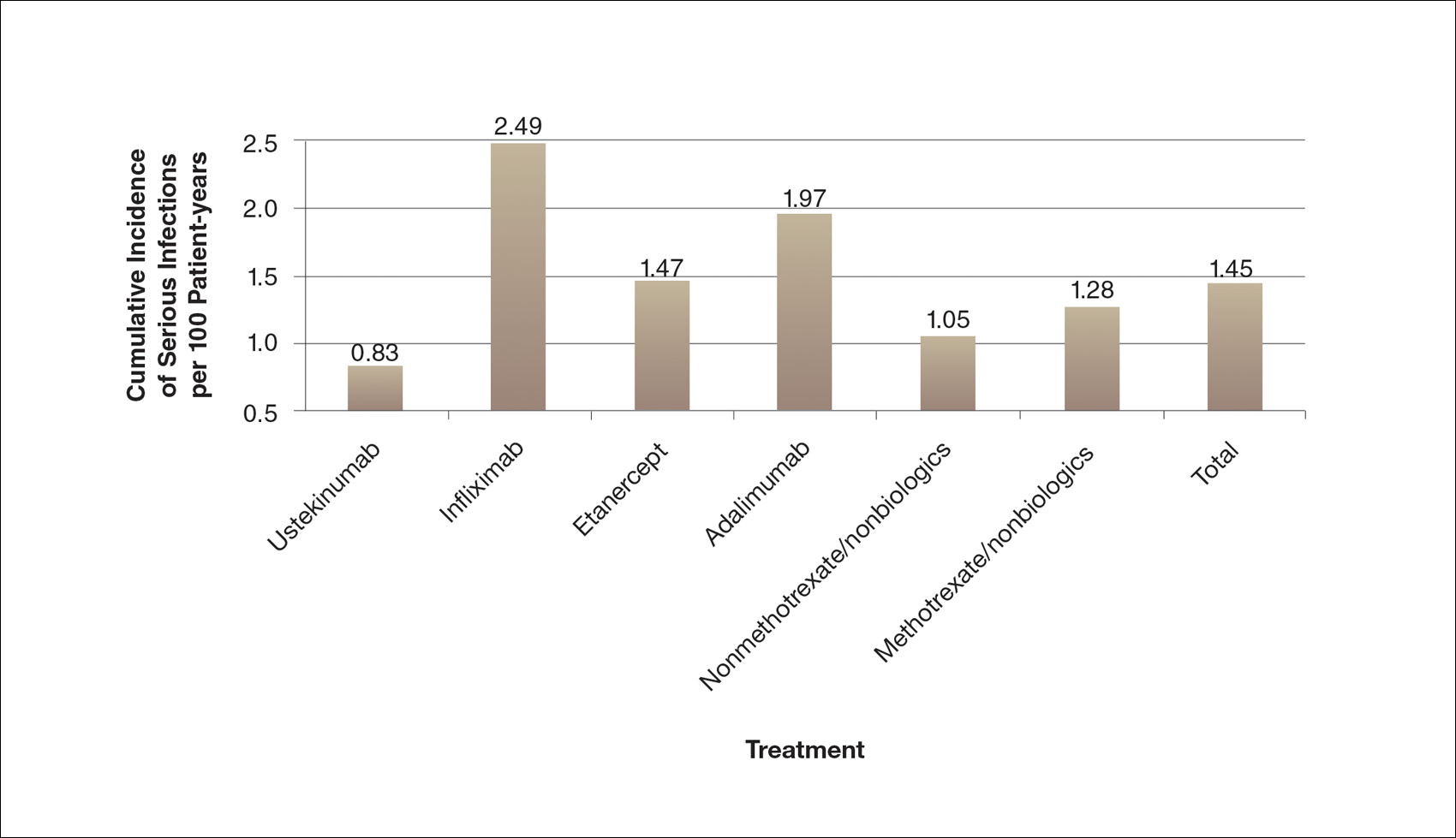
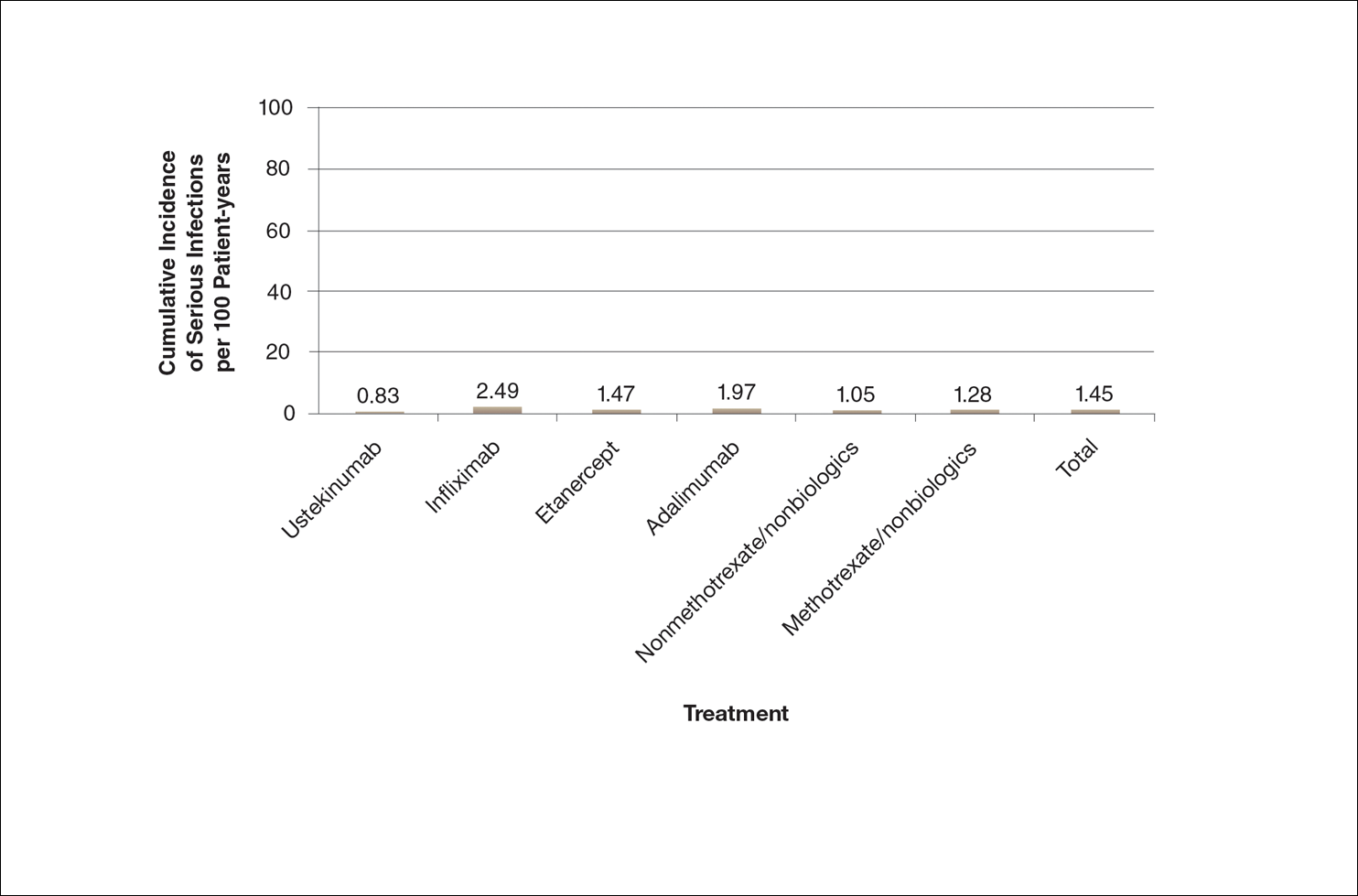
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.
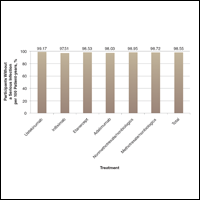
A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.
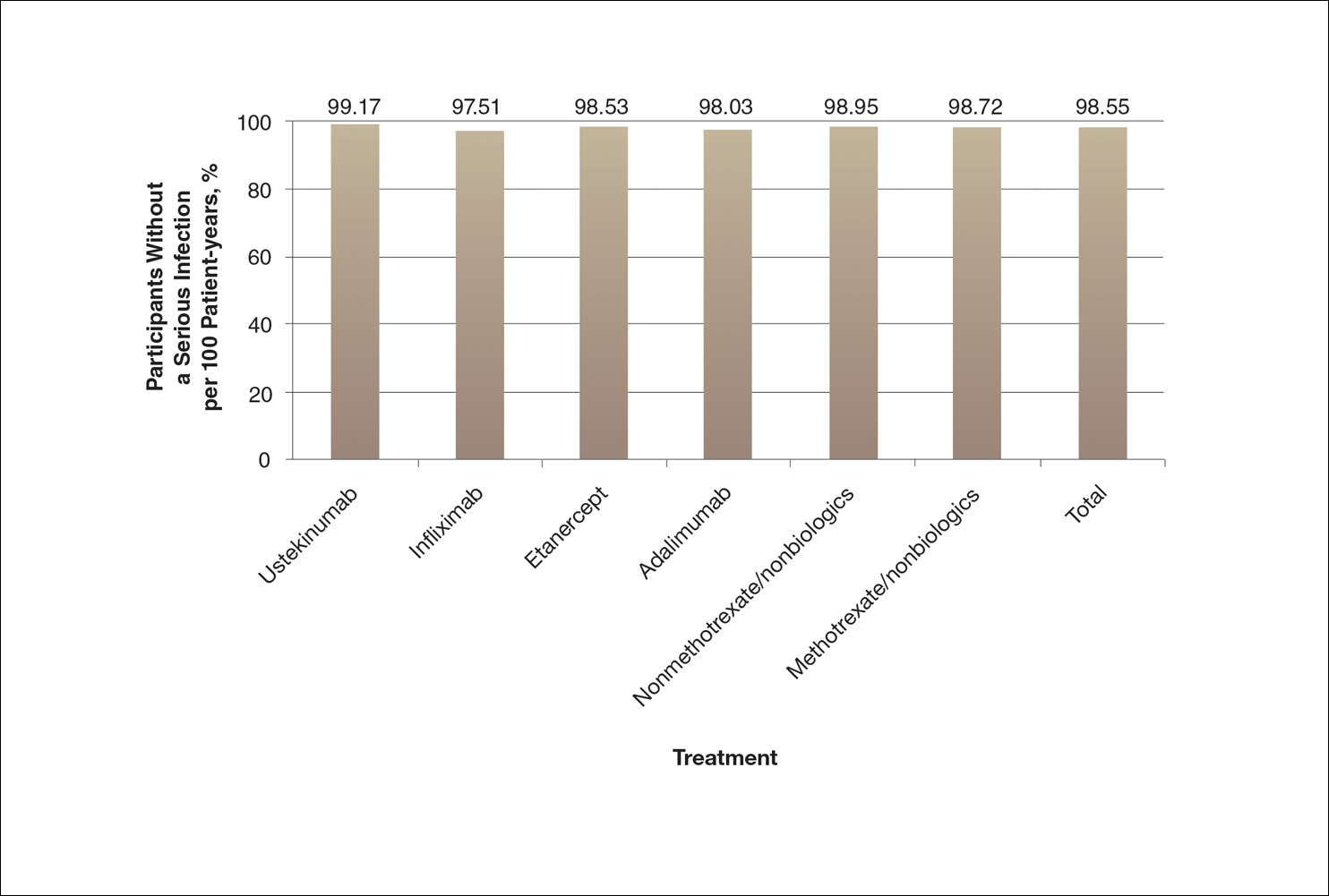
Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
Practice Points
- Physicians can guide patients’ perceptions of drug safety by the way safety data are presented.
- For patients who are concerned about rare treatment risks, presenting data on the patients who have not experienced adverse effects can be reassuring.
Psoriasis not consistently linked to adverse pregnancy outcomes
Psoriasis was not consistently associated with adverse pregnancy outcomes across nine studies in a systematic review of the literature, but four of the studies reported significant increases in at least one adverse outcome among women with psoriasis, according to a report in the British Journal of Dermatology.
Many women with psoriasis develop the disorder during their reproductive years, and more than 100,000 births to such patients are estimated to occur in the United States each year. Other autoimmune diseases are known to adversely affect pregnancy outcomes, but the issue has not been well studied among women with psoriasis, said Robert Bobotsis, a medical student at Western University, London (Ont.), and his associates.
They performed a systematic review of the literature to examine a possible link, but were only able to find nine fair- or good-quality studies involving a total of 4,756 pregnancies from which to extract data concerning a possible association. This small sample size may have been underpowered to detect the uncommon adverse pregnancy outcomes being assessed. Moreover, the investigators were unable to conduct a meta-analysis pooling the data because the effect measures were inconsistent across the nine studies, Mr. Bobotsis and his associates noted.
The review included a retrospective case series, a retrospective case control study, three retrospective cohort studies, two prospective cohort studies, one cross-sectional study, and one study combining prospective and retrospective cohorts. It “did not demonstrate an increased risk of poor outcomes in pregnant women with psoriasis” (Br J Dermatol. 2016 Jul 24;175:464-72).
However, four studies showed that compared with women who didn’t have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6. “Our results should be viewed as an opportunity to further research pregnancy outcomes in psoriasis,” the investigators said.
Psoriasis was not consistently associated with adverse pregnancy outcomes across nine studies in a systematic review of the literature, but four of the studies reported significant increases in at least one adverse outcome among women with psoriasis, according to a report in the British Journal of Dermatology.
Many women with psoriasis develop the disorder during their reproductive years, and more than 100,000 births to such patients are estimated to occur in the United States each year. Other autoimmune diseases are known to adversely affect pregnancy outcomes, but the issue has not been well studied among women with psoriasis, said Robert Bobotsis, a medical student at Western University, London (Ont.), and his associates.
They performed a systematic review of the literature to examine a possible link, but were only able to find nine fair- or good-quality studies involving a total of 4,756 pregnancies from which to extract data concerning a possible association. This small sample size may have been underpowered to detect the uncommon adverse pregnancy outcomes being assessed. Moreover, the investigators were unable to conduct a meta-analysis pooling the data because the effect measures were inconsistent across the nine studies, Mr. Bobotsis and his associates noted.
The review included a retrospective case series, a retrospective case control study, three retrospective cohort studies, two prospective cohort studies, one cross-sectional study, and one study combining prospective and retrospective cohorts. It “did not demonstrate an increased risk of poor outcomes in pregnant women with psoriasis” (Br J Dermatol. 2016 Jul 24;175:464-72).
However, four studies showed that compared with women who didn’t have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6. “Our results should be viewed as an opportunity to further research pregnancy outcomes in psoriasis,” the investigators said.
Psoriasis was not consistently associated with adverse pregnancy outcomes across nine studies in a systematic review of the literature, but four of the studies reported significant increases in at least one adverse outcome among women with psoriasis, according to a report in the British Journal of Dermatology.
Many women with psoriasis develop the disorder during their reproductive years, and more than 100,000 births to such patients are estimated to occur in the United States each year. Other autoimmune diseases are known to adversely affect pregnancy outcomes, but the issue has not been well studied among women with psoriasis, said Robert Bobotsis, a medical student at Western University, London (Ont.), and his associates.
They performed a systematic review of the literature to examine a possible link, but were only able to find nine fair- or good-quality studies involving a total of 4,756 pregnancies from which to extract data concerning a possible association. This small sample size may have been underpowered to detect the uncommon adverse pregnancy outcomes being assessed. Moreover, the investigators were unable to conduct a meta-analysis pooling the data because the effect measures were inconsistent across the nine studies, Mr. Bobotsis and his associates noted.
The review included a retrospective case series, a retrospective case control study, three retrospective cohort studies, two prospective cohort studies, one cross-sectional study, and one study combining prospective and retrospective cohorts. It “did not demonstrate an increased risk of poor outcomes in pregnant women with psoriasis” (Br J Dermatol. 2016 Jul 24;175:464-72).
However, four studies showed that compared with women who didn’t have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6. “Our results should be viewed as an opportunity to further research pregnancy outcomes in psoriasis,” the investigators said.
Key clinical point: Psoriasis is not consistently associated with adverse pregnancy outcomes across studies, but individual studies reported such links.
Major finding: Four of nine studies showed that compared with women who did not have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6.
Data source: A systematic review of nine reports in the literature concerning adverse outcomes in 4,756 pregnancies among women with psoriasis.
Disclosures: The authors reported that this work had no funding sources. Mr. Bobotsis reported having no relevant financial disclosures; two of his associates reported ties to AbbVie, Actelion, Amgen, Bio-K, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Roche, and Valeant.
Pediatricians partner with hospitals for value-based models
When Jason Vargas, MD, first moved to the Phoenix area 13 years ago, he found an atmosphere of distant relationships between general pediatricians like him, subspecialists, and hospitals. Getting patients a referral to a subspecialist could take months, and communication among providers was often weak, Dr. Vargas said.
Today, things are vastly different thanks in large part to the clinically integrated network of which Dr. Vargas and 950 area providers are a part.
Phoenix Children’s Care Network (PCCN), established in 2014, coordinates health care across multiple providers and settings in the Phoenix area, including half of all general pediatricians and 80% of pediatric subspecialists practicing in Maricopa County. The network is a value- and risk-based system that provides financial incentives to participating providers and health systems that meet established quality metrics. Patients have access to 950 providers within the network, including primary and specialty care sites of service, urgent care locations, surgery centers, and Phoenix Children’s Hospital.
Meanwhile, 2,000 miles away, another unique payment model is changing the way pediatric care is delivered in the Columbus, Ohio area. Partners For Kids (PFK) is a pediatric accountable care organization (ACO) that coordinates care between Nationwide Children’s Hospital and more than 1,000 doctors. Through its 20-year evolution, PFK has successfully assumed full financial and clinical risk for children under age 19 enrolled in Medicaid managed care. This means PFK is responsible for paying for the costs of all patient care, no matter how much or where that care occurs.
The two models illustrate how pediatricians are affiliating with value-centric networks while keeping their independence, said Timothy Johnson, senior vice president of pediatrics at Valence Health, a consulting firm that helps health providers transition to value-based care.
With MACRA (the Medicare Access and CHIP Reauthorization Act of 2015) “it’s going to be difficult for individual pediatricians to do what is required in a value-based medical system because they just don’t have the resources,” Mr. Johnson said in an interview. “That doesn’t mean they can’t be independent. It means they are going to have to band together in some way, whether with a health system, with other practices. It is extremely important for pediatricians to start thinking about how to do that.”
Going from splintered to unified
Like most communities, care delivery in the Phoenix area was relatively fractured prior to 2014. To bring everyone together, Phoenix Children’s started with community outreach and education.
Along with building trust among providers, project leaders had to overcome operational hurdles. This included creating a process for 110 practices to collectively negotiate contracts, operate under a new structure, and adhere to quality metrics, he said.
“Operationally, you have to take 110 different ways of doing things and try merge them into one common way as you develop these new contractual risk-based models,” he said. “At the same time, we had to transition people away from what they were used to as a purely fee-for-service model. It was a very big operational transition.”
To bolster engagement by community pediatricians, PCCN developed a physician-governance approach, assigning participating providers leadership responsibilities. Participating physicians then worked to create the benchmarks by which doctors are measured against. To date, provider performance is tracked against 14 primary care and 34 specialist metrics encompassing engagement, safety, quality, and transparency.
PCCN leaders also had to ensure that participating in such a network was beneficial for busy doctors, said Dr. Vargas, who is chair of the PCCN Network and Utilization committee and a member of the network’s board of managers.
Asking physicians to change their framework, track patient data, and meet metrics, all while potentially losing money if they fail to hit benchmarks is not the most popular proposition, he said. So PCCN created advantages for member doctors, such as nighttime pediatric triage, a negotiated discount for professional services, IT support, streamlined access to specialists, and more avenues to communicate with subspecialists.
“With so many schedules, professional, and academic pressures on our daily professional lives, we have wanted to make sure that there were practical value added benefits to members,” he said. “I think right now that the benefits outweigh the administrative burdens.”
A changing payer relationship
As a network, PCCN works with payers to assume the risk that insurers have historically taken. Payers continue to handle the administrative and billing side of the equation, while the network controls the medical management and care coordination of the patient population, Mr. Johnson said.
“We feel we can do it much more efficiently, much more effectively, and we feel it’s better care for the patient when we’re the one controlling that,” he said. “The insurance companies don’t disagree.”
The network partners with Medicaid and commercial payers and has a direct-to-employer agreement with a major employer in conjunction with an adult partner system/network. Early performance efforts by the PCCN have been rewarded by shared savings disbursements from two payers, according to PCCN officials. The network has also met or exceeded state Medicaid pediatric quality targets and consistently contained medical expenses below expected medical cost trends for its managed pediatric populations.
Building a population health model
For more than 2 decades, PFK in Ohio has taken a novel care delivery approach that has focused on value and community partnerships.
Back in 1994, Nationwide Children’s Hospital partnered with community pediatricians to create PFK, a physician/hospital organization with governance shared equally. Today, PFK has assumed full financial and clinical risk for pediatric managed Medicaid enrollees, and is the largest and oldest known pediatric ACO.
A key hurdle was collecting timely, complete, and accurate data for the patient population, Dr. Gleeson said, adding that working with data and understanding changing trends is an everyday challenge. Interacting with busy physicians and securing their time and cooperation also has been an obstacle.
“The lessons learned for us is that we really need to approach them understanding that there is a limited amount of time that practices can invest in infrastructure or invest in the processes of care,” he said. “We have to approach things knowing that [doctors] are going to struggle with the amount of time necessary to engage in large projects, so it needs to be chopped up into bite-sized pieces that they can consume on the run, so they can keep their practices running well.”
PFK efforts have paid off in terms of lowering costs and improving care. Between 2008 and 2013, PFK achieved lower cost growth than Medicaid fee-for-service programs and managed care plans in the Columbus, Ohio, area (Pediatrics. 2015 Mar;135[3];e582-9).
Fundamentally, the model has remained the same over the years, Dr. Gleeson said, but in 2005, PFK made the decision to expand and take responsibility for all the Medicaid-enrolled children in the region.
“It really gives a much broader field of view and perspective on patients in the region,” he said. “We know that they are all our responsibility so we take more of a population health type of approach, working with any physician who is caring for those children.”
Guidance for other practices
Dr. Gleeson encouraged other pediatricians interested in transitioning to value-based care to start by evaluating their data. Take a hard look at the quality of care you provide and begin to measure it, he said. For smaller practices, consider joining a larger group or network that will allow pediatricians to engage in collaborative work, he added.
Dr. Vargas stressed that change is coming whether pediatricians are prepared or not. Aligning with the right partners will be the difference between sinking or staying afloat in the value-based landscape.
“Payers are moving toward value-based models and it is not practical for the general pediatrician to be able to provide the infrastructure and professional resources necessary,” he said. “To maintain our professional livelihood as independent pediatricians, and to continue to provide the individually crafted, quality care our families are accustomed to, we will have to align ourselves with organizations that value the experience and insight of the independent pediatrician to deliver that care.”
[email protected]
On Twitter @legal_med
When Jason Vargas, MD, first moved to the Phoenix area 13 years ago, he found an atmosphere of distant relationships between general pediatricians like him, subspecialists, and hospitals. Getting patients a referral to a subspecialist could take months, and communication among providers was often weak, Dr. Vargas said.
Today, things are vastly different thanks in large part to the clinically integrated network of which Dr. Vargas and 950 area providers are a part.
Phoenix Children’s Care Network (PCCN), established in 2014, coordinates health care across multiple providers and settings in the Phoenix area, including half of all general pediatricians and 80% of pediatric subspecialists practicing in Maricopa County. The network is a value- and risk-based system that provides financial incentives to participating providers and health systems that meet established quality metrics. Patients have access to 950 providers within the network, including primary and specialty care sites of service, urgent care locations, surgery centers, and Phoenix Children’s Hospital.
Meanwhile, 2,000 miles away, another unique payment model is changing the way pediatric care is delivered in the Columbus, Ohio area. Partners For Kids (PFK) is a pediatric accountable care organization (ACO) that coordinates care between Nationwide Children’s Hospital and more than 1,000 doctors. Through its 20-year evolution, PFK has successfully assumed full financial and clinical risk for children under age 19 enrolled in Medicaid managed care. This means PFK is responsible for paying for the costs of all patient care, no matter how much or where that care occurs.
The two models illustrate how pediatricians are affiliating with value-centric networks while keeping their independence, said Timothy Johnson, senior vice president of pediatrics at Valence Health, a consulting firm that helps health providers transition to value-based care.
With MACRA (the Medicare Access and CHIP Reauthorization Act of 2015) “it’s going to be difficult for individual pediatricians to do what is required in a value-based medical system because they just don’t have the resources,” Mr. Johnson said in an interview. “That doesn’t mean they can’t be independent. It means they are going to have to band together in some way, whether with a health system, with other practices. It is extremely important for pediatricians to start thinking about how to do that.”
Going from splintered to unified
Like most communities, care delivery in the Phoenix area was relatively fractured prior to 2014. To bring everyone together, Phoenix Children’s started with community outreach and education.
Along with building trust among providers, project leaders had to overcome operational hurdles. This included creating a process for 110 practices to collectively negotiate contracts, operate under a new structure, and adhere to quality metrics, he said.
“Operationally, you have to take 110 different ways of doing things and try merge them into one common way as you develop these new contractual risk-based models,” he said. “At the same time, we had to transition people away from what they were used to as a purely fee-for-service model. It was a very big operational transition.”
To bolster engagement by community pediatricians, PCCN developed a physician-governance approach, assigning participating providers leadership responsibilities. Participating physicians then worked to create the benchmarks by which doctors are measured against. To date, provider performance is tracked against 14 primary care and 34 specialist metrics encompassing engagement, safety, quality, and transparency.
PCCN leaders also had to ensure that participating in such a network was beneficial for busy doctors, said Dr. Vargas, who is chair of the PCCN Network and Utilization committee and a member of the network’s board of managers.
Asking physicians to change their framework, track patient data, and meet metrics, all while potentially losing money if they fail to hit benchmarks is not the most popular proposition, he said. So PCCN created advantages for member doctors, such as nighttime pediatric triage, a negotiated discount for professional services, IT support, streamlined access to specialists, and more avenues to communicate with subspecialists.
“With so many schedules, professional, and academic pressures on our daily professional lives, we have wanted to make sure that there were practical value added benefits to members,” he said. “I think right now that the benefits outweigh the administrative burdens.”
A changing payer relationship
As a network, PCCN works with payers to assume the risk that insurers have historically taken. Payers continue to handle the administrative and billing side of the equation, while the network controls the medical management and care coordination of the patient population, Mr. Johnson said.
“We feel we can do it much more efficiently, much more effectively, and we feel it’s better care for the patient when we’re the one controlling that,” he said. “The insurance companies don’t disagree.”
The network partners with Medicaid and commercial payers and has a direct-to-employer agreement with a major employer in conjunction with an adult partner system/network. Early performance efforts by the PCCN have been rewarded by shared savings disbursements from two payers, according to PCCN officials. The network has also met or exceeded state Medicaid pediatric quality targets and consistently contained medical expenses below expected medical cost trends for its managed pediatric populations.
Building a population health model
For more than 2 decades, PFK in Ohio has taken a novel care delivery approach that has focused on value and community partnerships.
Back in 1994, Nationwide Children’s Hospital partnered with community pediatricians to create PFK, a physician/hospital organization with governance shared equally. Today, PFK has assumed full financial and clinical risk for pediatric managed Medicaid enrollees, and is the largest and oldest known pediatric ACO.
A key hurdle was collecting timely, complete, and accurate data for the patient population, Dr. Gleeson said, adding that working with data and understanding changing trends is an everyday challenge. Interacting with busy physicians and securing their time and cooperation also has been an obstacle.
“The lessons learned for us is that we really need to approach them understanding that there is a limited amount of time that practices can invest in infrastructure or invest in the processes of care,” he said. “We have to approach things knowing that [doctors] are going to struggle with the amount of time necessary to engage in large projects, so it needs to be chopped up into bite-sized pieces that they can consume on the run, so they can keep their practices running well.”
PFK efforts have paid off in terms of lowering costs and improving care. Between 2008 and 2013, PFK achieved lower cost growth than Medicaid fee-for-service programs and managed care plans in the Columbus, Ohio, area (Pediatrics. 2015 Mar;135[3];e582-9).
Fundamentally, the model has remained the same over the years, Dr. Gleeson said, but in 2005, PFK made the decision to expand and take responsibility for all the Medicaid-enrolled children in the region.
“It really gives a much broader field of view and perspective on patients in the region,” he said. “We know that they are all our responsibility so we take more of a population health type of approach, working with any physician who is caring for those children.”
Guidance for other practices
Dr. Gleeson encouraged other pediatricians interested in transitioning to value-based care to start by evaluating their data. Take a hard look at the quality of care you provide and begin to measure it, he said. For smaller practices, consider joining a larger group or network that will allow pediatricians to engage in collaborative work, he added.
Dr. Vargas stressed that change is coming whether pediatricians are prepared or not. Aligning with the right partners will be the difference between sinking or staying afloat in the value-based landscape.
“Payers are moving toward value-based models and it is not practical for the general pediatrician to be able to provide the infrastructure and professional resources necessary,” he said. “To maintain our professional livelihood as independent pediatricians, and to continue to provide the individually crafted, quality care our families are accustomed to, we will have to align ourselves with organizations that value the experience and insight of the independent pediatrician to deliver that care.”
[email protected]
On Twitter @legal_med
When Jason Vargas, MD, first moved to the Phoenix area 13 years ago, he found an atmosphere of distant relationships between general pediatricians like him, subspecialists, and hospitals. Getting patients a referral to a subspecialist could take months, and communication among providers was often weak, Dr. Vargas said.
Today, things are vastly different thanks in large part to the clinically integrated network of which Dr. Vargas and 950 area providers are a part.
Phoenix Children’s Care Network (PCCN), established in 2014, coordinates health care across multiple providers and settings in the Phoenix area, including half of all general pediatricians and 80% of pediatric subspecialists practicing in Maricopa County. The network is a value- and risk-based system that provides financial incentives to participating providers and health systems that meet established quality metrics. Patients have access to 950 providers within the network, including primary and specialty care sites of service, urgent care locations, surgery centers, and Phoenix Children’s Hospital.
Meanwhile, 2,000 miles away, another unique payment model is changing the way pediatric care is delivered in the Columbus, Ohio area. Partners For Kids (PFK) is a pediatric accountable care organization (ACO) that coordinates care between Nationwide Children’s Hospital and more than 1,000 doctors. Through its 20-year evolution, PFK has successfully assumed full financial and clinical risk for children under age 19 enrolled in Medicaid managed care. This means PFK is responsible for paying for the costs of all patient care, no matter how much or where that care occurs.
The two models illustrate how pediatricians are affiliating with value-centric networks while keeping their independence, said Timothy Johnson, senior vice president of pediatrics at Valence Health, a consulting firm that helps health providers transition to value-based care.
With MACRA (the Medicare Access and CHIP Reauthorization Act of 2015) “it’s going to be difficult for individual pediatricians to do what is required in a value-based medical system because they just don’t have the resources,” Mr. Johnson said in an interview. “That doesn’t mean they can’t be independent. It means they are going to have to band together in some way, whether with a health system, with other practices. It is extremely important for pediatricians to start thinking about how to do that.”
Going from splintered to unified
Like most communities, care delivery in the Phoenix area was relatively fractured prior to 2014. To bring everyone together, Phoenix Children’s started with community outreach and education.
Along with building trust among providers, project leaders had to overcome operational hurdles. This included creating a process for 110 practices to collectively negotiate contracts, operate under a new structure, and adhere to quality metrics, he said.
“Operationally, you have to take 110 different ways of doing things and try merge them into one common way as you develop these new contractual risk-based models,” he said. “At the same time, we had to transition people away from what they were used to as a purely fee-for-service model. It was a very big operational transition.”
To bolster engagement by community pediatricians, PCCN developed a physician-governance approach, assigning participating providers leadership responsibilities. Participating physicians then worked to create the benchmarks by which doctors are measured against. To date, provider performance is tracked against 14 primary care and 34 specialist metrics encompassing engagement, safety, quality, and transparency.
PCCN leaders also had to ensure that participating in such a network was beneficial for busy doctors, said Dr. Vargas, who is chair of the PCCN Network and Utilization committee and a member of the network’s board of managers.
Asking physicians to change their framework, track patient data, and meet metrics, all while potentially losing money if they fail to hit benchmarks is not the most popular proposition, he said. So PCCN created advantages for member doctors, such as nighttime pediatric triage, a negotiated discount for professional services, IT support, streamlined access to specialists, and more avenues to communicate with subspecialists.
“With so many schedules, professional, and academic pressures on our daily professional lives, we have wanted to make sure that there were practical value added benefits to members,” he said. “I think right now that the benefits outweigh the administrative burdens.”
A changing payer relationship
As a network, PCCN works with payers to assume the risk that insurers have historically taken. Payers continue to handle the administrative and billing side of the equation, while the network controls the medical management and care coordination of the patient population, Mr. Johnson said.
“We feel we can do it much more efficiently, much more effectively, and we feel it’s better care for the patient when we’re the one controlling that,” he said. “The insurance companies don’t disagree.”
The network partners with Medicaid and commercial payers and has a direct-to-employer agreement with a major employer in conjunction with an adult partner system/network. Early performance efforts by the PCCN have been rewarded by shared savings disbursements from two payers, according to PCCN officials. The network has also met or exceeded state Medicaid pediatric quality targets and consistently contained medical expenses below expected medical cost trends for its managed pediatric populations.
Building a population health model
For more than 2 decades, PFK in Ohio has taken a novel care delivery approach that has focused on value and community partnerships.
Back in 1994, Nationwide Children’s Hospital partnered with community pediatricians to create PFK, a physician/hospital organization with governance shared equally. Today, PFK has assumed full financial and clinical risk for pediatric managed Medicaid enrollees, and is the largest and oldest known pediatric ACO.
A key hurdle was collecting timely, complete, and accurate data for the patient population, Dr. Gleeson said, adding that working with data and understanding changing trends is an everyday challenge. Interacting with busy physicians and securing their time and cooperation also has been an obstacle.
“The lessons learned for us is that we really need to approach them understanding that there is a limited amount of time that practices can invest in infrastructure or invest in the processes of care,” he said. “We have to approach things knowing that [doctors] are going to struggle with the amount of time necessary to engage in large projects, so it needs to be chopped up into bite-sized pieces that they can consume on the run, so they can keep their practices running well.”
PFK efforts have paid off in terms of lowering costs and improving care. Between 2008 and 2013, PFK achieved lower cost growth than Medicaid fee-for-service programs and managed care plans in the Columbus, Ohio, area (Pediatrics. 2015 Mar;135[3];e582-9).
Fundamentally, the model has remained the same over the years, Dr. Gleeson said, but in 2005, PFK made the decision to expand and take responsibility for all the Medicaid-enrolled children in the region.
“It really gives a much broader field of view and perspective on patients in the region,” he said. “We know that they are all our responsibility so we take more of a population health type of approach, working with any physician who is caring for those children.”
Guidance for other practices
Dr. Gleeson encouraged other pediatricians interested in transitioning to value-based care to start by evaluating their data. Take a hard look at the quality of care you provide and begin to measure it, he said. For smaller practices, consider joining a larger group or network that will allow pediatricians to engage in collaborative work, he added.
Dr. Vargas stressed that change is coming whether pediatricians are prepared or not. Aligning with the right partners will be the difference between sinking or staying afloat in the value-based landscape.
“Payers are moving toward value-based models and it is not practical for the general pediatrician to be able to provide the infrastructure and professional resources necessary,” he said. “To maintain our professional livelihood as independent pediatricians, and to continue to provide the individually crafted, quality care our families are accustomed to, we will have to align ourselves with organizations that value the experience and insight of the independent pediatrician to deliver that care.”
[email protected]
On Twitter @legal_med
Occupational Complexity May Protect Cognition in People at Risk for Alzheimer’s Disease
TORONTO—High levels of occupational complexity, specifically related to work with people, enable individuals to maintain normal cognition despite white matter pathology in the brain, according to research described at the Alzheimer’s Association International Conference. The results “could have potential implications for preventing or maybe delaying Alzheimer’s disease onset in the future,” said Elizabeth Boots, research specialist at Wisconsin Alzheimer’s Disease Research Center in Madison.
According to one estimate, about 30% of cognitively healthy elderly adults may have widespread Alzheimer’s disease pathology. Cognitive reserve may allow these individuals to perform at a normal level of cognition despite this pathology. Because many people spend the majority of their lives at work, Ms. Boots and colleagues chose to examine occupational complexity as a measure of cognitive reserve. The investigators also focused on white matter hyperintensities, which increase the risk for cognitive decline and are common in Alzheimer’s disease. “The objective of our study was to determine whether occupational complexity is associated with more white matter hyperintensities when participants are matched for cognitive function, which would support the cognitive reserve hypothesis,” said Ms. Boots.
Examining Cognitive Testing and Imaging
She and her colleagues, led by senior author Ozioma Okonkwo, PhD, Assistant Professor of Geriatrics at the University of Wisconsin School of Medicine in Madison, selected 284 participants in the Wisconsin Registry for Alzheimer’s Prevention, a group of approximately 1,500 cognitively healthy people with increased risk for Alzheimer’s disease because of parental family history. Participants underwent extensive cognitive testing, and the researchers looked at the average of four cognitive domains—verbal learning and memory, immediate memory, working memory, and speed and flexibility—to match individuals on cognitive function. Participants also underwent a brain scan for white matter hyperintensities.
In addition, study participants described as many as three occupations that they had performed, including the number of years spent on each occupation. Ms. Boots and her colleagues rated each job for three categories of occupational complexity (ie, complexity of work with data, people, and things). In the domain of work with people, for example, the researchers considered taking instructions as the least complex occupation, and mentoring the most complex. They weighted the scores by the number of years on the job and summed the scores to create a total occupational complexity measure.
Social Interaction May Be Crucial
Average age in the study cohort was 60, and 67% of participants were female. Study participants had an average of 16.67 years of education. When the investigators controlled the data for cognitive function, they found that higher levels of occupational complexity were associated with increased white matter hyperintensities in the brain. “Those with higher levels of occupational complexity are able to tolerate more white matter pathology in the brain and still perform at the same cognitive level as their peers,” said Ms. Boots. The association did not change when the researchers controlled for potential confounders such as education, socioeconomic status, and vascular risk. Furthermore, Ms. Boots and colleagues found that complexity of work with people, but not complexity of work with data or things, had the greatest effect on preserving cognitive performance.
The results support the cognitive reserve hypothesis and suggest that social interaction plays a unique role in cognitive reserve, according to Dr. Okonkwo. “These analyses underscore the importance of social engagement in the work setting for building resilience to Alzheimer’s disease,” he added. The Alzheimer’s Association, the NIH, and the Extendicare Foundation funded the study.
Suggested Reading
Boots EA, Schultz SA, Almeida RP, et al. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin
Lo RY, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord. 2013;27(4):343-350.
Pool LR, Weuve J, Wilson RS, et al. Occupational cognitive requirements and late-life cognitive aging. Neurology. 2016;86(15):1386-1392.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012; 11(11):1006-1012.
TORONTO—High levels of occupational complexity, specifically related to work with people, enable individuals to maintain normal cognition despite white matter pathology in the brain, according to research described at the Alzheimer’s Association International Conference. The results “could have potential implications for preventing or maybe delaying Alzheimer’s disease onset in the future,” said Elizabeth Boots, research specialist at Wisconsin Alzheimer’s Disease Research Center in Madison.
According to one estimate, about 30% of cognitively healthy elderly adults may have widespread Alzheimer’s disease pathology. Cognitive reserve may allow these individuals to perform at a normal level of cognition despite this pathology. Because many people spend the majority of their lives at work, Ms. Boots and colleagues chose to examine occupational complexity as a measure of cognitive reserve. The investigators also focused on white matter hyperintensities, which increase the risk for cognitive decline and are common in Alzheimer’s disease. “The objective of our study was to determine whether occupational complexity is associated with more white matter hyperintensities when participants are matched for cognitive function, which would support the cognitive reserve hypothesis,” said Ms. Boots.
Examining Cognitive Testing and Imaging
She and her colleagues, led by senior author Ozioma Okonkwo, PhD, Assistant Professor of Geriatrics at the University of Wisconsin School of Medicine in Madison, selected 284 participants in the Wisconsin Registry for Alzheimer’s Prevention, a group of approximately 1,500 cognitively healthy people with increased risk for Alzheimer’s disease because of parental family history. Participants underwent extensive cognitive testing, and the researchers looked at the average of four cognitive domains—verbal learning and memory, immediate memory, working memory, and speed and flexibility—to match individuals on cognitive function. Participants also underwent a brain scan for white matter hyperintensities.
In addition, study participants described as many as three occupations that they had performed, including the number of years spent on each occupation. Ms. Boots and her colleagues rated each job for three categories of occupational complexity (ie, complexity of work with data, people, and things). In the domain of work with people, for example, the researchers considered taking instructions as the least complex occupation, and mentoring the most complex. They weighted the scores by the number of years on the job and summed the scores to create a total occupational complexity measure.
Social Interaction May Be Crucial
Average age in the study cohort was 60, and 67% of participants were female. Study participants had an average of 16.67 years of education. When the investigators controlled the data for cognitive function, they found that higher levels of occupational complexity were associated with increased white matter hyperintensities in the brain. “Those with higher levels of occupational complexity are able to tolerate more white matter pathology in the brain and still perform at the same cognitive level as their peers,” said Ms. Boots. The association did not change when the researchers controlled for potential confounders such as education, socioeconomic status, and vascular risk. Furthermore, Ms. Boots and colleagues found that complexity of work with people, but not complexity of work with data or things, had the greatest effect on preserving cognitive performance.
The results support the cognitive reserve hypothesis and suggest that social interaction plays a unique role in cognitive reserve, according to Dr. Okonkwo. “These analyses underscore the importance of social engagement in the work setting for building resilience to Alzheimer’s disease,” he added. The Alzheimer’s Association, the NIH, and the Extendicare Foundation funded the study.
Suggested Reading
Boots EA, Schultz SA, Almeida RP, et al. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin
Lo RY, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord. 2013;27(4):343-350.
Pool LR, Weuve J, Wilson RS, et al. Occupational cognitive requirements and late-life cognitive aging. Neurology. 2016;86(15):1386-1392.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012; 11(11):1006-1012.
TORONTO—High levels of occupational complexity, specifically related to work with people, enable individuals to maintain normal cognition despite white matter pathology in the brain, according to research described at the Alzheimer’s Association International Conference. The results “could have potential implications for preventing or maybe delaying Alzheimer’s disease onset in the future,” said Elizabeth Boots, research specialist at Wisconsin Alzheimer’s Disease Research Center in Madison.
According to one estimate, about 30% of cognitively healthy elderly adults may have widespread Alzheimer’s disease pathology. Cognitive reserve may allow these individuals to perform at a normal level of cognition despite this pathology. Because many people spend the majority of their lives at work, Ms. Boots and colleagues chose to examine occupational complexity as a measure of cognitive reserve. The investigators also focused on white matter hyperintensities, which increase the risk for cognitive decline and are common in Alzheimer’s disease. “The objective of our study was to determine whether occupational complexity is associated with more white matter hyperintensities when participants are matched for cognitive function, which would support the cognitive reserve hypothesis,” said Ms. Boots.
Examining Cognitive Testing and Imaging
She and her colleagues, led by senior author Ozioma Okonkwo, PhD, Assistant Professor of Geriatrics at the University of Wisconsin School of Medicine in Madison, selected 284 participants in the Wisconsin Registry for Alzheimer’s Prevention, a group of approximately 1,500 cognitively healthy people with increased risk for Alzheimer’s disease because of parental family history. Participants underwent extensive cognitive testing, and the researchers looked at the average of four cognitive domains—verbal learning and memory, immediate memory, working memory, and speed and flexibility—to match individuals on cognitive function. Participants also underwent a brain scan for white matter hyperintensities.
In addition, study participants described as many as three occupations that they had performed, including the number of years spent on each occupation. Ms. Boots and her colleagues rated each job for three categories of occupational complexity (ie, complexity of work with data, people, and things). In the domain of work with people, for example, the researchers considered taking instructions as the least complex occupation, and mentoring the most complex. They weighted the scores by the number of years on the job and summed the scores to create a total occupational complexity measure.
Social Interaction May Be Crucial
Average age in the study cohort was 60, and 67% of participants were female. Study participants had an average of 16.67 years of education. When the investigators controlled the data for cognitive function, they found that higher levels of occupational complexity were associated with increased white matter hyperintensities in the brain. “Those with higher levels of occupational complexity are able to tolerate more white matter pathology in the brain and still perform at the same cognitive level as their peers,” said Ms. Boots. The association did not change when the researchers controlled for potential confounders such as education, socioeconomic status, and vascular risk. Furthermore, Ms. Boots and colleagues found that complexity of work with people, but not complexity of work with data or things, had the greatest effect on preserving cognitive performance.
The results support the cognitive reserve hypothesis and suggest that social interaction plays a unique role in cognitive reserve, according to Dr. Okonkwo. “These analyses underscore the importance of social engagement in the work setting for building resilience to Alzheimer’s disease,” he added. The Alzheimer’s Association, the NIH, and the Extendicare Foundation funded the study.
Suggested Reading
Boots EA, Schultz SA, Almeida RP, et al. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin
Lo RY, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord. 2013;27(4):343-350.
Pool LR, Weuve J, Wilson RS, et al. Occupational cognitive requirements and late-life cognitive aging. Neurology. 2016;86(15):1386-1392.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012; 11(11):1006-1012.
Bullous Pemphigoid Associated With a Lymphoepithelial Cyst of the Pancreas
Bullous pemphigoid (BP) is an acquired, autoimmune, subepidermal blistering disease that is more common in elderly patients.1 An association with internal neoplasms and BP has been established; however, there is debate regarding the precise nature of the relationship.2 Several gastrointestinal tract tumors have been associated with BP, including adenocarcinoma of the colon, adenosquamous cell carcinoma and adenocarcinoma of the stomach, adenocarcinoma of the rectum, and liver and bile duct malignancies.3-5 Association with pancreatic neoplasms (eg, carcinoma of the pancreas) rarely has been reported.5-7 We present an unusual case of a lymphoepithelial cyst of the pancreas in a patient with BP.
Case Report
A 67-year-old man presented with erythematous crusted plaques and pink scars over the chest, back, arms, and legs (Figure 1). A 1.5-cm tense bullous lesion was observed on the right knee. The patient’s medical history was notable for biopsy-proven BP of 8 months’ duration as well as diabetes mellitus and hypothyroidism. The patient was being followed by his surgeon for a 1.9-cm soft-tissue lesion in the pancreatic tail and was awaiting surgical excision at the time of the current presentation. The pancreatic lesion was discovered incidentally on magnetic resonance imaging performed following urologic concerns. At the current presentation, the patient’s medications included nifedipine, hydralazine, metoprolol, torsemide, aspirin, levothyroxine, atorvastatin, insulin lispro, and insulin glargine. He had previously been treated for BP with prednisone at a maximum dosage of 60 mg daily, clobetasol propionate cream 0.05%, and mupirocin ointment 2% without improvement. Because of substantial weight gain and poorly controlled diabetes, prednisone was discontinued.
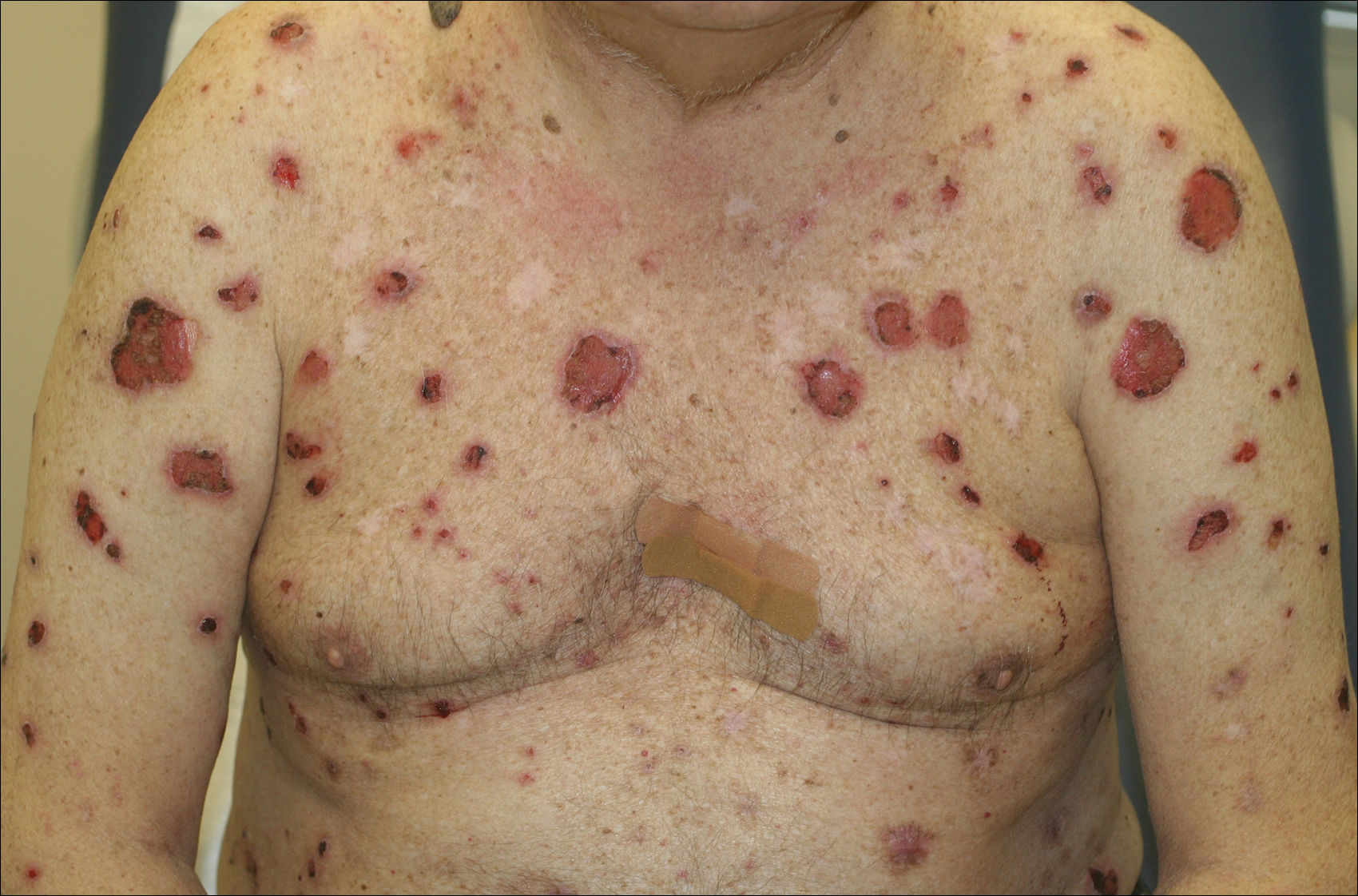
Bullous pemphigoid had been diagnosed histopathologically by a prior dermatologist. Hematoxylin and eosin staining demonstrated a subepidermal separation with eosinophils within the perivascular infiltrate (Figure 2). Direct immunofluorescence was noted in a linear pattern at the dermoepidermal junction with IgG and C3. Bullous pemphigoid antigen antibodies 1 and 2 were obtained via enzyme-linked immunosorbent assay with a positive BP-1 antigen antibody of 19 U/mL (positive, >15 U/mL) and a normal BP-2 antigen antibody of less than 9 U/mL (reference range, <9 U/mL). The glucagon level was elevated at 245 pg/mL (reference range, ≤134 pg/mL).
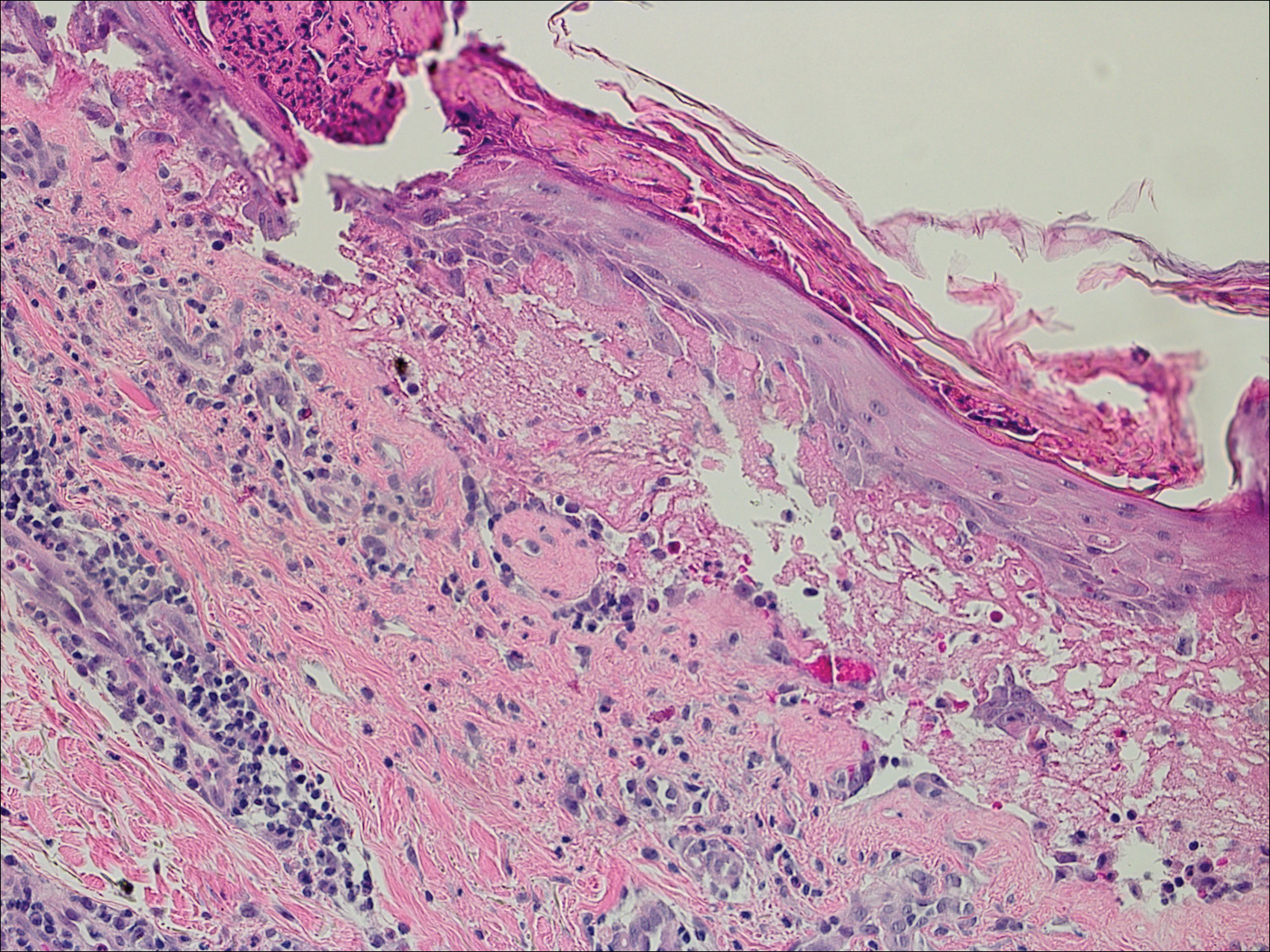
The patient was prescribed minocycline 100 mg twice daily and niacinamide 500 mg 3 times daily. Topical treatment with clobetasol and mupirocin was continued. One month later, the patient returned with an increase in disease activity. Changes to his therapeutic regimen were deferred until after excision of the pancreatic lesion based on the decision not to start immunosuppressive therapy until the precise nature of the pancreatic lesion was determined.
The patient underwent excision of the pancreatic lesion approximately 3 months later, which proved to be a benign lymphoepithelial cyst of the pancreas. Histology of the cyst consisted of dense fibrous tissue with a squamous epithelial lining focally infiltrated by lymphocytes (Figure 3A). Immunoperoxidase staining of the cyst revealed focal linear areas of C3d staining along the basement membrane of the stratified squamous epithelium (Figure 3B).
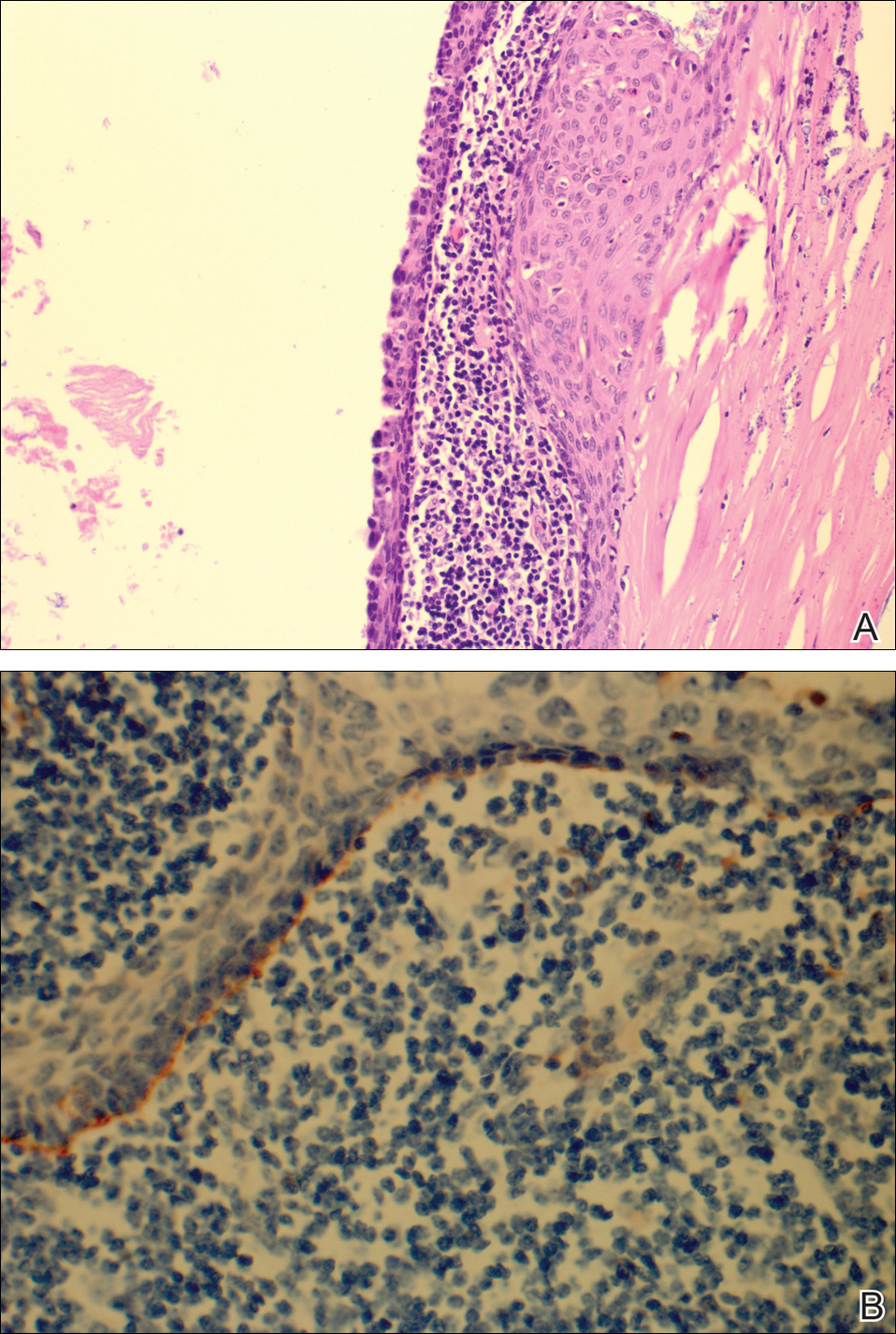
The patient stated that his skin started to improve virtually immediately following the excision without systemic treatment for BP. On follow-up examination 3 weeks postoperatively, no bullae were observed and there was a notable decrease in erythematous crusted plaques (Figure 4).
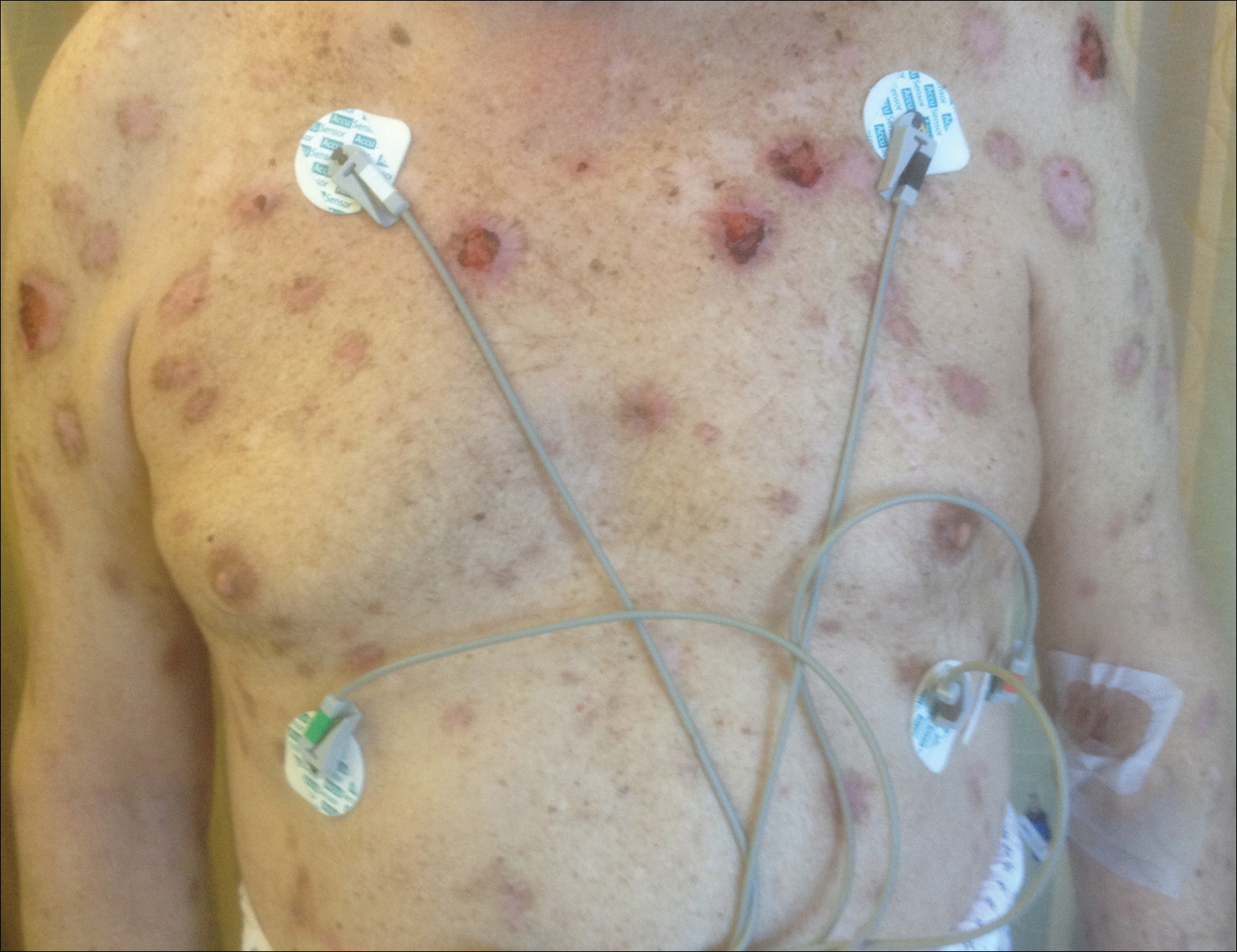
Comment
Paraneoplastic BP has been documented; however, lymphoepithelial cysts of the pancreas in association with BP are rare. We propose that the lymphoepithelial cyst of the pancreas provided the immunologic stimulus for the development of cutaneous BP based on the observation that our patient’s condition remarkably improved with resection of the tumor.
There are fewer than 100 cases of lymphoepithelial cysts of the pancreas reported in the literature.8 The histologic appearance is consistent with a true cyst exhibiting a well-differentiated stratified squamous epithelium, often with keratinization, surrounded by lymphoid tissue. These tumors are most commonly seen in middle-aged men and are frequently found incidentally,8-10 as was the case with our patient. Although histologically similar, lymphoepithelial cysts of the pancreas are considered distinct from lymphoepithelial cysts of the parotid gland or head and neck region.10 Lymphoepithelial cysts of the pancreas are unrelated to elevated glucagon levels; it is likely that our patient’s glucagon levels were associated with his history of diabetes.11
The diagnosis of BP is characteristically confirmed by direct immunofluorescence. Although it was performed for our patient’s cutaneous lesions, it was not obtained for the lymphoepithelial cyst of the pancreas. Once the diagnosis of the lymphoepithelial cyst of the pancreas was established, as direct immunofluorescence could not be performed in formalin-fixed tissue, immunoperoxidase staining with C3d was obtained. C3 has a well-established role in activation of complement and as a marker in BP. Deposition of C3d is a result of deactivation of C3b, a cleavage product of C3. In a study of 6 autoimmune blistering disorders that included 32 patients with BP, Pfaltz et al12 found positive immunoperoxidase staining for C3d in 31 of 32 patients, which translated to a sensitivity of 97%, a positive predictive value of 100%, and a negative predictive value of 98% among the blistering diseases being studied. Similarly, Magro and Dyrsen13 had positive staining of C3d in 17 of 17 (100%) patients with BP.
In theory, any process that involves deposition of C3 should be positive for C3d on immunoperoxidase staining. Other dermatologic inflammatory conditions stain positively with C3d, such as systemic lupus erythematosus, discoid lupus erythematosus, subacute cutaneous lupus erythematosus, and dermatomysositis.13 The staining for these diseases correlates with the site of the associated inflammatory component seen on hematoxylin and eosin staining. The staining of C3d along the basement membrane of stratified squamous epithelium in the lymphoepithelial cyst of the pancreas seen in our patient closely resembles the staining seen in cutaneous BP.
A proposed mechanism for BP in our patient would be exposure of BP-1 antigen in the pancreatic cyst leading to antibody recognition and C3 deposition along the basement membrane in the cyst, as evidenced by C3d immunoperoxidase staining. The IgG and C3 deposition along the cutaneous basement membrane would then represent a systemic response to the antigen exposure in the cyst. Thus, the lymphoepithelial cyst provided the immunologic stimulus for the development of the cutaneous BP. This theory is based on the observation of our patient’s rapid improvement without a change in his treatment regimen immediately after surgical excision of the cyst.
Despite the plausibility of our hypothesis, several questions remain regarding the validity of our assumptions. Although sensitive for C3 deposition, C3d immunoperoxidase staining is not specific for BP. If the proposed mechanism for causation is true, one might have expected that a subepithelial cleft along the basement membrane of the pancreatic cyst would be observed, which was not seen. A repeat BP antigen antibody was not obtained, which would have been helpful in determining if there was clearance of the antibody that would have correlated with the clinical resolution of the BP lesions.
Conclusion
Our case suggests that paraneoplastic BP is a genuine entity. Indeed, the primary tumor itself may be the immunologic stimulus in the development of BP. Recalcitrant BP should raise the question of a neoplastic process that is exposing the BP antigen. If a thorough review of systems accompanied by corroborating laboratory studies suggests a neoplastic process, the suspect lesion should be further evaluated and surgically excised if clinically indicated. Further evaluation of neoplasms with advanced staining methods may aid in establishing the causative nature of tumors in the development of BP.
Acknowledgments
We are grateful to John Stanley, MD, and Aimee Payne, MD (both from Philadelphia, Pennsylvania), for theirinsights into this case.
- Charneux J, Lorin J, Vitry F, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:286-291.
- Patel M, Sniha AA, Gilbert E. Bullous pemphigoid associated with renal cell carcinoma and invasive squamous cell carcinoma. J Drugs Dermatol. 2012;11:234-238.
- Song HJ, Han SH, Hong WK, et al. Paraneoplastic bullous pemphigoid: clinical disease activity correlated with enzyme-linked immunosorbent assay index for NC16A domain of BP180. J Dermatol. 2009;36:66-68.
- Muramatsu T, Iida T, Tada H, et al. Bullous pemphigoid associated with internal malignancies: identification of 180-kDa antigen by Western immunoblotting. Br J Dermatol. 1996;135:782-784.
- Ogawa H, Sakuma M, Morioka S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995;9:136-141.
- Boyd RV. Pemphigoid and carcinoma of the pancreas. Br Med J. 1964;1:1092.
- Eustace S, Morrow G, O’Loughlin S, et al. The role of computed tomography and sonography in acute bullous pemphigoid. Ir J Med Sci. 1993;162:401-404.
- Clemente G, Sarno G, De Rose AM, et al. Lymphoepithelial cyst of the pancreas: case report and review of the literature. Acta Gastroenterol Belg. 2011;74:343-346.
- Frezza E, Wachtel MS. Lymphoepithelial cyst of the pancreas tail. case report and review of the literature. JOP. 2008;9:46-49.
- Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis and clinical implications. Arch Pathol Lab Med. 2009;133:423-438.
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4-12.
- Pfaltz K, Mertz K, Rose C, et al. C3d immunohistochemistry on formalin-fixed tissue is a valuable tool in the diagnosis of bullous pemphigoid of the skin. J Cutan Pathol. 2010;37:654-658.
- Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59:822-833.
Bullous pemphigoid (BP) is an acquired, autoimmune, subepidermal blistering disease that is more common in elderly patients.1 An association with internal neoplasms and BP has been established; however, there is debate regarding the precise nature of the relationship.2 Several gastrointestinal tract tumors have been associated with BP, including adenocarcinoma of the colon, adenosquamous cell carcinoma and adenocarcinoma of the stomach, adenocarcinoma of the rectum, and liver and bile duct malignancies.3-5 Association with pancreatic neoplasms (eg, carcinoma of the pancreas) rarely has been reported.5-7 We present an unusual case of a lymphoepithelial cyst of the pancreas in a patient with BP.
Case Report
A 67-year-old man presented with erythematous crusted plaques and pink scars over the chest, back, arms, and legs (Figure 1). A 1.5-cm tense bullous lesion was observed on the right knee. The patient’s medical history was notable for biopsy-proven BP of 8 months’ duration as well as diabetes mellitus and hypothyroidism. The patient was being followed by his surgeon for a 1.9-cm soft-tissue lesion in the pancreatic tail and was awaiting surgical excision at the time of the current presentation. The pancreatic lesion was discovered incidentally on magnetic resonance imaging performed following urologic concerns. At the current presentation, the patient’s medications included nifedipine, hydralazine, metoprolol, torsemide, aspirin, levothyroxine, atorvastatin, insulin lispro, and insulin glargine. He had previously been treated for BP with prednisone at a maximum dosage of 60 mg daily, clobetasol propionate cream 0.05%, and mupirocin ointment 2% without improvement. Because of substantial weight gain and poorly controlled diabetes, prednisone was discontinued.

Bullous pemphigoid had been diagnosed histopathologically by a prior dermatologist. Hematoxylin and eosin staining demonstrated a subepidermal separation with eosinophils within the perivascular infiltrate (Figure 2). Direct immunofluorescence was noted in a linear pattern at the dermoepidermal junction with IgG and C3. Bullous pemphigoid antigen antibodies 1 and 2 were obtained via enzyme-linked immunosorbent assay with a positive BP-1 antigen antibody of 19 U/mL (positive, >15 U/mL) and a normal BP-2 antigen antibody of less than 9 U/mL (reference range, <9 U/mL). The glucagon level was elevated at 245 pg/mL (reference range, ≤134 pg/mL).

The patient was prescribed minocycline 100 mg twice daily and niacinamide 500 mg 3 times daily. Topical treatment with clobetasol and mupirocin was continued. One month later, the patient returned with an increase in disease activity. Changes to his therapeutic regimen were deferred until after excision of the pancreatic lesion based on the decision not to start immunosuppressive therapy until the precise nature of the pancreatic lesion was determined.
The patient underwent excision of the pancreatic lesion approximately 3 months later, which proved to be a benign lymphoepithelial cyst of the pancreas. Histology of the cyst consisted of dense fibrous tissue with a squamous epithelial lining focally infiltrated by lymphocytes (Figure 3A). Immunoperoxidase staining of the cyst revealed focal linear areas of C3d staining along the basement membrane of the stratified squamous epithelium (Figure 3B).

The patient stated that his skin started to improve virtually immediately following the excision without systemic treatment for BP. On follow-up examination 3 weeks postoperatively, no bullae were observed and there was a notable decrease in erythematous crusted plaques (Figure 4).

Comment
Paraneoplastic BP has been documented; however, lymphoepithelial cysts of the pancreas in association with BP are rare. We propose that the lymphoepithelial cyst of the pancreas provided the immunologic stimulus for the development of cutaneous BP based on the observation that our patient’s condition remarkably improved with resection of the tumor.
There are fewer than 100 cases of lymphoepithelial cysts of the pancreas reported in the literature.8 The histologic appearance is consistent with a true cyst exhibiting a well-differentiated stratified squamous epithelium, often with keratinization, surrounded by lymphoid tissue. These tumors are most commonly seen in middle-aged men and are frequently found incidentally,8-10 as was the case with our patient. Although histologically similar, lymphoepithelial cysts of the pancreas are considered distinct from lymphoepithelial cysts of the parotid gland or head and neck region.10 Lymphoepithelial cysts of the pancreas are unrelated to elevated glucagon levels; it is likely that our patient’s glucagon levels were associated with his history of diabetes.11
The diagnosis of BP is characteristically confirmed by direct immunofluorescence. Although it was performed for our patient’s cutaneous lesions, it was not obtained for the lymphoepithelial cyst of the pancreas. Once the diagnosis of the lymphoepithelial cyst of the pancreas was established, as direct immunofluorescence could not be performed in formalin-fixed tissue, immunoperoxidase staining with C3d was obtained. C3 has a well-established role in activation of complement and as a marker in BP. Deposition of C3d is a result of deactivation of C3b, a cleavage product of C3. In a study of 6 autoimmune blistering disorders that included 32 patients with BP, Pfaltz et al12 found positive immunoperoxidase staining for C3d in 31 of 32 patients, which translated to a sensitivity of 97%, a positive predictive value of 100%, and a negative predictive value of 98% among the blistering diseases being studied. Similarly, Magro and Dyrsen13 had positive staining of C3d in 17 of 17 (100%) patients with BP.
In theory, any process that involves deposition of C3 should be positive for C3d on immunoperoxidase staining. Other dermatologic inflammatory conditions stain positively with C3d, such as systemic lupus erythematosus, discoid lupus erythematosus, subacute cutaneous lupus erythematosus, and dermatomysositis.13 The staining for these diseases correlates with the site of the associated inflammatory component seen on hematoxylin and eosin staining. The staining of C3d along the basement membrane of stratified squamous epithelium in the lymphoepithelial cyst of the pancreas seen in our patient closely resembles the staining seen in cutaneous BP.
A proposed mechanism for BP in our patient would be exposure of BP-1 antigen in the pancreatic cyst leading to antibody recognition and C3 deposition along the basement membrane in the cyst, as evidenced by C3d immunoperoxidase staining. The IgG and C3 deposition along the cutaneous basement membrane would then represent a systemic response to the antigen exposure in the cyst. Thus, the lymphoepithelial cyst provided the immunologic stimulus for the development of the cutaneous BP. This theory is based on the observation of our patient’s rapid improvement without a change in his treatment regimen immediately after surgical excision of the cyst.
Despite the plausibility of our hypothesis, several questions remain regarding the validity of our assumptions. Although sensitive for C3 deposition, C3d immunoperoxidase staining is not specific for BP. If the proposed mechanism for causation is true, one might have expected that a subepithelial cleft along the basement membrane of the pancreatic cyst would be observed, which was not seen. A repeat BP antigen antibody was not obtained, which would have been helpful in determining if there was clearance of the antibody that would have correlated with the clinical resolution of the BP lesions.
Conclusion
Our case suggests that paraneoplastic BP is a genuine entity. Indeed, the primary tumor itself may be the immunologic stimulus in the development of BP. Recalcitrant BP should raise the question of a neoplastic process that is exposing the BP antigen. If a thorough review of systems accompanied by corroborating laboratory studies suggests a neoplastic process, the suspect lesion should be further evaluated and surgically excised if clinically indicated. Further evaluation of neoplasms with advanced staining methods may aid in establishing the causative nature of tumors in the development of BP.
Acknowledgments
We are grateful to John Stanley, MD, and Aimee Payne, MD (both from Philadelphia, Pennsylvania), for theirinsights into this case.
Bullous pemphigoid (BP) is an acquired, autoimmune, subepidermal blistering disease that is more common in elderly patients.1 An association with internal neoplasms and BP has been established; however, there is debate regarding the precise nature of the relationship.2 Several gastrointestinal tract tumors have been associated with BP, including adenocarcinoma of the colon, adenosquamous cell carcinoma and adenocarcinoma of the stomach, adenocarcinoma of the rectum, and liver and bile duct malignancies.3-5 Association with pancreatic neoplasms (eg, carcinoma of the pancreas) rarely has been reported.5-7 We present an unusual case of a lymphoepithelial cyst of the pancreas in a patient with BP.
Case Report
A 67-year-old man presented with erythematous crusted plaques and pink scars over the chest, back, arms, and legs (Figure 1). A 1.5-cm tense bullous lesion was observed on the right knee. The patient’s medical history was notable for biopsy-proven BP of 8 months’ duration as well as diabetes mellitus and hypothyroidism. The patient was being followed by his surgeon for a 1.9-cm soft-tissue lesion in the pancreatic tail and was awaiting surgical excision at the time of the current presentation. The pancreatic lesion was discovered incidentally on magnetic resonance imaging performed following urologic concerns. At the current presentation, the patient’s medications included nifedipine, hydralazine, metoprolol, torsemide, aspirin, levothyroxine, atorvastatin, insulin lispro, and insulin glargine. He had previously been treated for BP with prednisone at a maximum dosage of 60 mg daily, clobetasol propionate cream 0.05%, and mupirocin ointment 2% without improvement. Because of substantial weight gain and poorly controlled diabetes, prednisone was discontinued.

Bullous pemphigoid had been diagnosed histopathologically by a prior dermatologist. Hematoxylin and eosin staining demonstrated a subepidermal separation with eosinophils within the perivascular infiltrate (Figure 2). Direct immunofluorescence was noted in a linear pattern at the dermoepidermal junction with IgG and C3. Bullous pemphigoid antigen antibodies 1 and 2 were obtained via enzyme-linked immunosorbent assay with a positive BP-1 antigen antibody of 19 U/mL (positive, >15 U/mL) and a normal BP-2 antigen antibody of less than 9 U/mL (reference range, <9 U/mL). The glucagon level was elevated at 245 pg/mL (reference range, ≤134 pg/mL).

The patient was prescribed minocycline 100 mg twice daily and niacinamide 500 mg 3 times daily. Topical treatment with clobetasol and mupirocin was continued. One month later, the patient returned with an increase in disease activity. Changes to his therapeutic regimen were deferred until after excision of the pancreatic lesion based on the decision not to start immunosuppressive therapy until the precise nature of the pancreatic lesion was determined.
The patient underwent excision of the pancreatic lesion approximately 3 months later, which proved to be a benign lymphoepithelial cyst of the pancreas. Histology of the cyst consisted of dense fibrous tissue with a squamous epithelial lining focally infiltrated by lymphocytes (Figure 3A). Immunoperoxidase staining of the cyst revealed focal linear areas of C3d staining along the basement membrane of the stratified squamous epithelium (Figure 3B).

The patient stated that his skin started to improve virtually immediately following the excision without systemic treatment for BP. On follow-up examination 3 weeks postoperatively, no bullae were observed and there was a notable decrease in erythematous crusted plaques (Figure 4).

Comment
Paraneoplastic BP has been documented; however, lymphoepithelial cysts of the pancreas in association with BP are rare. We propose that the lymphoepithelial cyst of the pancreas provided the immunologic stimulus for the development of cutaneous BP based on the observation that our patient’s condition remarkably improved with resection of the tumor.
There are fewer than 100 cases of lymphoepithelial cysts of the pancreas reported in the literature.8 The histologic appearance is consistent with a true cyst exhibiting a well-differentiated stratified squamous epithelium, often with keratinization, surrounded by lymphoid tissue. These tumors are most commonly seen in middle-aged men and are frequently found incidentally,8-10 as was the case with our patient. Although histologically similar, lymphoepithelial cysts of the pancreas are considered distinct from lymphoepithelial cysts of the parotid gland or head and neck region.10 Lymphoepithelial cysts of the pancreas are unrelated to elevated glucagon levels; it is likely that our patient’s glucagon levels were associated with his history of diabetes.11
The diagnosis of BP is characteristically confirmed by direct immunofluorescence. Although it was performed for our patient’s cutaneous lesions, it was not obtained for the lymphoepithelial cyst of the pancreas. Once the diagnosis of the lymphoepithelial cyst of the pancreas was established, as direct immunofluorescence could not be performed in formalin-fixed tissue, immunoperoxidase staining with C3d was obtained. C3 has a well-established role in activation of complement and as a marker in BP. Deposition of C3d is a result of deactivation of C3b, a cleavage product of C3. In a study of 6 autoimmune blistering disorders that included 32 patients with BP, Pfaltz et al12 found positive immunoperoxidase staining for C3d in 31 of 32 patients, which translated to a sensitivity of 97%, a positive predictive value of 100%, and a negative predictive value of 98% among the blistering diseases being studied. Similarly, Magro and Dyrsen13 had positive staining of C3d in 17 of 17 (100%) patients with BP.
In theory, any process that involves deposition of C3 should be positive for C3d on immunoperoxidase staining. Other dermatologic inflammatory conditions stain positively with C3d, such as systemic lupus erythematosus, discoid lupus erythematosus, subacute cutaneous lupus erythematosus, and dermatomysositis.13 The staining for these diseases correlates with the site of the associated inflammatory component seen on hematoxylin and eosin staining. The staining of C3d along the basement membrane of stratified squamous epithelium in the lymphoepithelial cyst of the pancreas seen in our patient closely resembles the staining seen in cutaneous BP.
A proposed mechanism for BP in our patient would be exposure of BP-1 antigen in the pancreatic cyst leading to antibody recognition and C3 deposition along the basement membrane in the cyst, as evidenced by C3d immunoperoxidase staining. The IgG and C3 deposition along the cutaneous basement membrane would then represent a systemic response to the antigen exposure in the cyst. Thus, the lymphoepithelial cyst provided the immunologic stimulus for the development of the cutaneous BP. This theory is based on the observation of our patient’s rapid improvement without a change in his treatment regimen immediately after surgical excision of the cyst.
Despite the plausibility of our hypothesis, several questions remain regarding the validity of our assumptions. Although sensitive for C3 deposition, C3d immunoperoxidase staining is not specific for BP. If the proposed mechanism for causation is true, one might have expected that a subepithelial cleft along the basement membrane of the pancreatic cyst would be observed, which was not seen. A repeat BP antigen antibody was not obtained, which would have been helpful in determining if there was clearance of the antibody that would have correlated with the clinical resolution of the BP lesions.
Conclusion
Our case suggests that paraneoplastic BP is a genuine entity. Indeed, the primary tumor itself may be the immunologic stimulus in the development of BP. Recalcitrant BP should raise the question of a neoplastic process that is exposing the BP antigen. If a thorough review of systems accompanied by corroborating laboratory studies suggests a neoplastic process, the suspect lesion should be further evaluated and surgically excised if clinically indicated. Further evaluation of neoplasms with advanced staining methods may aid in establishing the causative nature of tumors in the development of BP.
Acknowledgments
We are grateful to John Stanley, MD, and Aimee Payne, MD (both from Philadelphia, Pennsylvania), for theirinsights into this case.
- Charneux J, Lorin J, Vitry F, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:286-291.
- Patel M, Sniha AA, Gilbert E. Bullous pemphigoid associated with renal cell carcinoma and invasive squamous cell carcinoma. J Drugs Dermatol. 2012;11:234-238.
- Song HJ, Han SH, Hong WK, et al. Paraneoplastic bullous pemphigoid: clinical disease activity correlated with enzyme-linked immunosorbent assay index for NC16A domain of BP180. J Dermatol. 2009;36:66-68.
- Muramatsu T, Iida T, Tada H, et al. Bullous pemphigoid associated with internal malignancies: identification of 180-kDa antigen by Western immunoblotting. Br J Dermatol. 1996;135:782-784.
- Ogawa H, Sakuma M, Morioka S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995;9:136-141.
- Boyd RV. Pemphigoid and carcinoma of the pancreas. Br Med J. 1964;1:1092.
- Eustace S, Morrow G, O’Loughlin S, et al. The role of computed tomography and sonography in acute bullous pemphigoid. Ir J Med Sci. 1993;162:401-404.
- Clemente G, Sarno G, De Rose AM, et al. Lymphoepithelial cyst of the pancreas: case report and review of the literature. Acta Gastroenterol Belg. 2011;74:343-346.
- Frezza E, Wachtel MS. Lymphoepithelial cyst of the pancreas tail. case report and review of the literature. JOP. 2008;9:46-49.
- Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis and clinical implications. Arch Pathol Lab Med. 2009;133:423-438.
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4-12.
- Pfaltz K, Mertz K, Rose C, et al. C3d immunohistochemistry on formalin-fixed tissue is a valuable tool in the diagnosis of bullous pemphigoid of the skin. J Cutan Pathol. 2010;37:654-658.
- Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59:822-833.
- Charneux J, Lorin J, Vitry F, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:286-291.
- Patel M, Sniha AA, Gilbert E. Bullous pemphigoid associated with renal cell carcinoma and invasive squamous cell carcinoma. J Drugs Dermatol. 2012;11:234-238.
- Song HJ, Han SH, Hong WK, et al. Paraneoplastic bullous pemphigoid: clinical disease activity correlated with enzyme-linked immunosorbent assay index for NC16A domain of BP180. J Dermatol. 2009;36:66-68.
- Muramatsu T, Iida T, Tada H, et al. Bullous pemphigoid associated with internal malignancies: identification of 180-kDa antigen by Western immunoblotting. Br J Dermatol. 1996;135:782-784.
- Ogawa H, Sakuma M, Morioka S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995;9:136-141.
- Boyd RV. Pemphigoid and carcinoma of the pancreas. Br Med J. 1964;1:1092.
- Eustace S, Morrow G, O’Loughlin S, et al. The role of computed tomography and sonography in acute bullous pemphigoid. Ir J Med Sci. 1993;162:401-404.
- Clemente G, Sarno G, De Rose AM, et al. Lymphoepithelial cyst of the pancreas: case report and review of the literature. Acta Gastroenterol Belg. 2011;74:343-346.
- Frezza E, Wachtel MS. Lymphoepithelial cyst of the pancreas tail. case report and review of the literature. JOP. 2008;9:46-49.
- Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis and clinical implications. Arch Pathol Lab Med. 2009;133:423-438.
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4-12.
- Pfaltz K, Mertz K, Rose C, et al. C3d immunohistochemistry on formalin-fixed tissue is a valuable tool in the diagnosis of bullous pemphigoid of the skin. J Cutan Pathol. 2010;37:654-658.
- Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59:822-833.
Here on Earth
We live inundated with promises that technology will solve our most challenging problems, yet we are regularly disappointed when it does not. New technological solutions seem to appear daily, and we feel like we are falling behind if we do not jump to join the people who are implementing, selling, or imposing new solutions. Often these solutions are offered before the problem is even fully understood, and no assessment has been made to determine if the solution actually helps to solve the challenge identified. With 80% of us now having transitioned to EHRs, we know full well their benefits as well as their pitfalls. While we have mostly accommodated to electronic documentation, we are now at the point where we are beginning to explore some of the most exciting potential benefits of our EHRs – population health, enhanced data on medication adherence, and improved patient communication. As we look at this next stage of growth, we are reminded of a lesson from an old joke:
A rabbi dies and goes to heaven. When he gets there he is given an old robe and a wooden walking stick and is told to get in line to the entrance to heaven. While the rabbi waits in the long line, a taxi driver walks up and is greeted by a group of angels blowing their horns announcing his arrival. One of the angels walks over to the driver and gives him a flowing white satin robe and a golden walking stick. Another angel then escorts him to the front of the line.
The angel turned toward him, smiled, and shook his head. “Yes, yes,” the angel replied, “We know all that. But, here in heaven we care about results, not intent. While you gave your sermons, people slept. When the cab driver drove, people prayed.”
As we look ahead to the next generation of electronic health records, there are going to be many creative ideas of how to use them to help patients improve their health and take care of their diseases. One of the more notable new technologies over the last 5 years is the development of wearable health devices. Innovations like the Apple Watch, Fitbit, and other wearables allow us to track our activity and diet, and encourage us to behave better. They do this by providing constant feedback on how we are doing, and they offer the ability to use social groups to encourage sustained behavioral change. Some devices tell us regularly how far we have walked while others let us know when we have been sitting too long. As we input information about diet, the devices and their associated apps give us feedback on how we are adhering to our dietary goals. Some even allow data to be funneled into the EHR so that physicians can review the behavioral changes and track patient progress. The challenge that arises is that the technology itself is so fascinating and so filled with promise that it is easy to forget what is most important: ensuring it works not just to keep us engaged and busy but also to help us accomplish the real goals we have defined for its use.
Wearable technology is now the most recent and dramatic example of how the excitement over technology may be outpacing its utility. Most of us have tried (or have patients, friends and family who have tried) wearable technology solutions to track and encourage behavioral change. A recent article published in JAMA looked at more than 400 individuals randomized to a standard behavioral weight-loss intervention vs. a technology-enhanced weight loss intervention using a wearable device over 24 months. It was fairly obvious that the group with the wearable device would do better, and have improved fitness and more weight loss. It was obvious … except that is not what happened. Both groups improved equally in fitness, and the standard intervention group lost significantly more weight over 24 months than did the wearable technology group.
There are many reasons that this might have happened. It may be that the idea of this quick feedback loop is in itself flawed, or it may be that the devices and/or the dietary input is simply imprecise, causing people to think that they are doing better than they really are (and then modifying their behavior in the wrong direction). Whatever the explanation, seeing those results, I think again of the moral handed down though generations by that old joke – that here on earth we need to care less about intent and more about results.
Reference
Jakicic JM, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss The IDEA Randomized Clinical Trial. JAMA. 2016;316[11]:1161-71. doi: 10.1001/jama.2016.12858
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
We live inundated with promises that technology will solve our most challenging problems, yet we are regularly disappointed when it does not. New technological solutions seem to appear daily, and we feel like we are falling behind if we do not jump to join the people who are implementing, selling, or imposing new solutions. Often these solutions are offered before the problem is even fully understood, and no assessment has been made to determine if the solution actually helps to solve the challenge identified. With 80% of us now having transitioned to EHRs, we know full well their benefits as well as their pitfalls. While we have mostly accommodated to electronic documentation, we are now at the point where we are beginning to explore some of the most exciting potential benefits of our EHRs – population health, enhanced data on medication adherence, and improved patient communication. As we look at this next stage of growth, we are reminded of a lesson from an old joke:
A rabbi dies and goes to heaven. When he gets there he is given an old robe and a wooden walking stick and is told to get in line to the entrance to heaven. While the rabbi waits in the long line, a taxi driver walks up and is greeted by a group of angels blowing their horns announcing his arrival. One of the angels walks over to the driver and gives him a flowing white satin robe and a golden walking stick. Another angel then escorts him to the front of the line.
The angel turned toward him, smiled, and shook his head. “Yes, yes,” the angel replied, “We know all that. But, here in heaven we care about results, not intent. While you gave your sermons, people slept. When the cab driver drove, people prayed.”
As we look ahead to the next generation of electronic health records, there are going to be many creative ideas of how to use them to help patients improve their health and take care of their diseases. One of the more notable new technologies over the last 5 years is the development of wearable health devices. Innovations like the Apple Watch, Fitbit, and other wearables allow us to track our activity and diet, and encourage us to behave better. They do this by providing constant feedback on how we are doing, and they offer the ability to use social groups to encourage sustained behavioral change. Some devices tell us regularly how far we have walked while others let us know when we have been sitting too long. As we input information about diet, the devices and their associated apps give us feedback on how we are adhering to our dietary goals. Some even allow data to be funneled into the EHR so that physicians can review the behavioral changes and track patient progress. The challenge that arises is that the technology itself is so fascinating and so filled with promise that it is easy to forget what is most important: ensuring it works not just to keep us engaged and busy but also to help us accomplish the real goals we have defined for its use.
Wearable technology is now the most recent and dramatic example of how the excitement over technology may be outpacing its utility. Most of us have tried (or have patients, friends and family who have tried) wearable technology solutions to track and encourage behavioral change. A recent article published in JAMA looked at more than 400 individuals randomized to a standard behavioral weight-loss intervention vs. a technology-enhanced weight loss intervention using a wearable device over 24 months. It was fairly obvious that the group with the wearable device would do better, and have improved fitness and more weight loss. It was obvious … except that is not what happened. Both groups improved equally in fitness, and the standard intervention group lost significantly more weight over 24 months than did the wearable technology group.
There are many reasons that this might have happened. It may be that the idea of this quick feedback loop is in itself flawed, or it may be that the devices and/or the dietary input is simply imprecise, causing people to think that they are doing better than they really are (and then modifying their behavior in the wrong direction). Whatever the explanation, seeing those results, I think again of the moral handed down though generations by that old joke – that here on earth we need to care less about intent and more about results.
Reference
Jakicic JM, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss The IDEA Randomized Clinical Trial. JAMA. 2016;316[11]:1161-71. doi: 10.1001/jama.2016.12858
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
We live inundated with promises that technology will solve our most challenging problems, yet we are regularly disappointed when it does not. New technological solutions seem to appear daily, and we feel like we are falling behind if we do not jump to join the people who are implementing, selling, or imposing new solutions. Often these solutions are offered before the problem is even fully understood, and no assessment has been made to determine if the solution actually helps to solve the challenge identified. With 80% of us now having transitioned to EHRs, we know full well their benefits as well as their pitfalls. While we have mostly accommodated to electronic documentation, we are now at the point where we are beginning to explore some of the most exciting potential benefits of our EHRs – population health, enhanced data on medication adherence, and improved patient communication. As we look at this next stage of growth, we are reminded of a lesson from an old joke:
A rabbi dies and goes to heaven. When he gets there he is given an old robe and a wooden walking stick and is told to get in line to the entrance to heaven. While the rabbi waits in the long line, a taxi driver walks up and is greeted by a group of angels blowing their horns announcing his arrival. One of the angels walks over to the driver and gives him a flowing white satin robe and a golden walking stick. Another angel then escorts him to the front of the line.
The angel turned toward him, smiled, and shook his head. “Yes, yes,” the angel replied, “We know all that. But, here in heaven we care about results, not intent. While you gave your sermons, people slept. When the cab driver drove, people prayed.”
As we look ahead to the next generation of electronic health records, there are going to be many creative ideas of how to use them to help patients improve their health and take care of their diseases. One of the more notable new technologies over the last 5 years is the development of wearable health devices. Innovations like the Apple Watch, Fitbit, and other wearables allow us to track our activity and diet, and encourage us to behave better. They do this by providing constant feedback on how we are doing, and they offer the ability to use social groups to encourage sustained behavioral change. Some devices tell us regularly how far we have walked while others let us know when we have been sitting too long. As we input information about diet, the devices and their associated apps give us feedback on how we are adhering to our dietary goals. Some even allow data to be funneled into the EHR so that physicians can review the behavioral changes and track patient progress. The challenge that arises is that the technology itself is so fascinating and so filled with promise that it is easy to forget what is most important: ensuring it works not just to keep us engaged and busy but also to help us accomplish the real goals we have defined for its use.
Wearable technology is now the most recent and dramatic example of how the excitement over technology may be outpacing its utility. Most of us have tried (or have patients, friends and family who have tried) wearable technology solutions to track and encourage behavioral change. A recent article published in JAMA looked at more than 400 individuals randomized to a standard behavioral weight-loss intervention vs. a technology-enhanced weight loss intervention using a wearable device over 24 months. It was fairly obvious that the group with the wearable device would do better, and have improved fitness and more weight loss. It was obvious … except that is not what happened. Both groups improved equally in fitness, and the standard intervention group lost significantly more weight over 24 months than did the wearable technology group.
There are many reasons that this might have happened. It may be that the idea of this quick feedback loop is in itself flawed, or it may be that the devices and/or the dietary input is simply imprecise, causing people to think that they are doing better than they really are (and then modifying their behavior in the wrong direction). Whatever the explanation, seeing those results, I think again of the moral handed down though generations by that old joke – that here on earth we need to care less about intent and more about results.
Reference
Jakicic JM, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss The IDEA Randomized Clinical Trial. JAMA. 2016;316[11]:1161-71. doi: 10.1001/jama.2016.12858
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
Congenital Zika syndrome includes range of neurologic abnormalities
Researchers have proposed the term “congenital Zika syndrome” to cover the range of severe damage and developmental abnormalities – including microcephaly – caused by Zika virus infection.
In the Oct. 3 online edition of JAMA Neurology, Adriana Suely de Oliveira Melo, MD, PhD, of the Instituto de Pesquisa Professor Amorim Neto in Campina Grande, Brazil, and her coauthors report on 11 babies with congenital Zika virus infection who were followed from gestation to 6 months of age.
Researchers observed hypoplasia of the cerebellar vermis and cerebellum in nine patients, while MRI and CT imaging also found that all patients showed callosal hypoplasia, reduced cerebral volume, abnormal cortical development, and subcortical calcifications.
Four of the infants showed gyral disorganization, five showed evidence of pachygyria, and two had lissencephaly (JAMA Neurol. 2016 Oct 3. doi: 10.1001/jamaneurol.2016.3720).
“Although there was variable damage resulting from brain lesions associated with [Zika virus] congenital infection, a common pattern of brain atrophy and changes associated with disturbances in neuronal migration were observed,” the authors wrote. “Some patients presented with mild brain atrophy and calcifications, and others presented with more severe malformations, such as the absence of the thalamus and lissencephaly.”
Three of the infants died after delivery, representing a fatality rate of 27.3%. All three were found to have akinesia deformation sequence or arthrogryposis. One of the three pregnancies also involved polyhydramnios, and the infant was delivered at 36 weeks because of severe maternal respiratory distress.
All but one of the pregnant women had reported a skin rash at a median of 9.5 weeks in the pregnancy, suggesting Zika virus infection was acquired early.
Postmortem tissue analysis of two of the infants who died found Zika virus genome in the brain, cerebellum, spinal cord, and lung; a higher viral load in the tissue of one of the infants was associated with more severe brain damage.
Overall, nine patients tested positive for Zika virus using real-time reverse-transcription polymerase chain
reactions during gestation and/or after birth, while two patients only had serologic evidence of infection.
“It was interesting to note that the viral sequences amplified from patients 1 and 7 after birth gained a new substitution, V23I, which is located in the envelope domain I and may be implicated in viral tropism to different tissues.”
The study was supported by Consellho Nacional de Desenvolvimento e Pesquisa, Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Prefeitura Municipal de Campina Grande. No conflicts of interest were declared.
Many unanswered questions remain about Zika virus: How frequently does asymptomatic infection or second- and third-trimester infection lead to CNS disease? What are the long-term sequelae of intrauterine Zika virus infection? What is the reason for the substantial size, severity, and unexpected complications of the recent Zika virus outbreak in the Americas, compared with what has been seen with this virus in the past? And a broader question: How many CNS birth defects presently of unclear cause will be found to be virus induced?
It would be valuable to have adult and pediatric neurologists network with the U.S. Centers for Disease Control and Prevention to establish a surveillance system that could track Zika virus–induced Guillain-Barré syndrome and CNS disease. This would facilitate the identification and characterization of disorders, the formation of a registry, and the mounting of comprehensive epidemiological studies.
Dr. Raymond P. Roos is with the Department of Neurology at the University of Chicago. These comments are adapted from an accompanying editorial (JAMA Neurol. 2016 Oct 3. doi: 10.1001/jamaneurol.2016.3677). No conflicts of interest were declared.
Many unanswered questions remain about Zika virus: How frequently does asymptomatic infection or second- and third-trimester infection lead to CNS disease? What are the long-term sequelae of intrauterine Zika virus infection? What is the reason for the substantial size, severity, and unexpected complications of the recent Zika virus outbreak in the Americas, compared with what has been seen with this virus in the past? And a broader question: How many CNS birth defects presently of unclear cause will be found to be virus induced?
It would be valuable to have adult and pediatric neurologists network with the U.S. Centers for Disease Control and Prevention to establish a surveillance system that could track Zika virus–induced Guillain-Barré syndrome and CNS disease. This would facilitate the identification and characterization of disorders, the formation of a registry, and the mounting of comprehensive epidemiological studies.
Dr. Raymond P. Roos is with the Department of Neurology at the University of Chicago. These comments are adapted from an accompanying editorial (JAMA Neurol. 2016 Oct 3. doi: 10.1001/jamaneurol.2016.3677). No conflicts of interest were declared.
Many unanswered questions remain about Zika virus: How frequently does asymptomatic infection or second- and third-trimester infection lead to CNS disease? What are the long-term sequelae of intrauterine Zika virus infection? What is the reason for the substantial size, severity, and unexpected complications of the recent Zika virus outbreak in the Americas, compared with what has been seen with this virus in the past? And a broader question: How many CNS birth defects presently of unclear cause will be found to be virus induced?
It would be valuable to have adult and pediatric neurologists network with the U.S. Centers for Disease Control and Prevention to establish a surveillance system that could track Zika virus–induced Guillain-Barré syndrome and CNS disease. This would facilitate the identification and characterization of disorders, the formation of a registry, and the mounting of comprehensive epidemiological studies.
Dr. Raymond P. Roos is with the Department of Neurology at the University of Chicago. These comments are adapted from an accompanying editorial (JAMA Neurol. 2016 Oct 3. doi: 10.1001/jamaneurol.2016.3677). No conflicts of interest were declared.
Researchers have proposed the term “congenital Zika syndrome” to cover the range of severe damage and developmental abnormalities – including microcephaly – caused by Zika virus infection.
In the Oct. 3 online edition of JAMA Neurology, Adriana Suely de Oliveira Melo, MD, PhD, of the Instituto de Pesquisa Professor Amorim Neto in Campina Grande, Brazil, and her coauthors report on 11 babies with congenital Zika virus infection who were followed from gestation to 6 months of age.
Researchers observed hypoplasia of the cerebellar vermis and cerebellum in nine patients, while MRI and CT imaging also found that all patients showed callosal hypoplasia, reduced cerebral volume, abnormal cortical development, and subcortical calcifications.
Four of the infants showed gyral disorganization, five showed evidence of pachygyria, and two had lissencephaly (JAMA Neurol. 2016 Oct 3. doi: 10.1001/jamaneurol.2016.3720).
“Although there was variable damage resulting from brain lesions associated with [Zika virus] congenital infection, a common pattern of brain atrophy and changes associated with disturbances in neuronal migration were observed,” the authors wrote. “Some patients presented with mild brain atrophy and calcifications, and others presented with more severe malformations, such as the absence of the thalamus and lissencephaly.”
Three of the infants died after delivery, representing a fatality rate of 27.3%. All three were found to have akinesia deformation sequence or arthrogryposis. One of the three pregnancies also involved polyhydramnios, and the infant was delivered at 36 weeks because of severe maternal respiratory distress.
All but one of the pregnant women had reported a skin rash at a median of 9.5 weeks in the pregnancy, suggesting Zika virus infection was acquired early.
Postmortem tissue analysis of two of the infants who died found Zika virus genome in the brain, cerebellum, spinal cord, and lung; a higher viral load in the tissue of one of the infants was associated with more severe brain damage.
Overall, nine patients tested positive for Zika virus using real-time reverse-transcription polymerase chain
reactions during gestation and/or after birth, while two patients only had serologic evidence of infection.
“It was interesting to note that the viral sequences amplified from patients 1 and 7 after birth gained a new substitution, V23I, which is located in the envelope domain I and may be implicated in viral tropism to different tissues.”
The study was supported by Consellho Nacional de Desenvolvimento e Pesquisa, Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Prefeitura Municipal de Campina Grande. No conflicts of interest were declared.
Researchers have proposed the term “congenital Zika syndrome” to cover the range of severe damage and developmental abnormalities – including microcephaly – caused by Zika virus infection.
In the Oct. 3 online edition of JAMA Neurology, Adriana Suely de Oliveira Melo, MD, PhD, of the Instituto de Pesquisa Professor Amorim Neto in Campina Grande, Brazil, and her coauthors report on 11 babies with congenital Zika virus infection who were followed from gestation to 6 months of age.
Researchers observed hypoplasia of the cerebellar vermis and cerebellum in nine patients, while MRI and CT imaging also found that all patients showed callosal hypoplasia, reduced cerebral volume, abnormal cortical development, and subcortical calcifications.
Four of the infants showed gyral disorganization, five showed evidence of pachygyria, and two had lissencephaly (JAMA Neurol. 2016 Oct 3. doi: 10.1001/jamaneurol.2016.3720).
“Although there was variable damage resulting from brain lesions associated with [Zika virus] congenital infection, a common pattern of brain atrophy and changes associated with disturbances in neuronal migration were observed,” the authors wrote. “Some patients presented with mild brain atrophy and calcifications, and others presented with more severe malformations, such as the absence of the thalamus and lissencephaly.”
Three of the infants died after delivery, representing a fatality rate of 27.3%. All three were found to have akinesia deformation sequence or arthrogryposis. One of the three pregnancies also involved polyhydramnios, and the infant was delivered at 36 weeks because of severe maternal respiratory distress.
All but one of the pregnant women had reported a skin rash at a median of 9.5 weeks in the pregnancy, suggesting Zika virus infection was acquired early.
Postmortem tissue analysis of two of the infants who died found Zika virus genome in the brain, cerebellum, spinal cord, and lung; a higher viral load in the tissue of one of the infants was associated with more severe brain damage.
Overall, nine patients tested positive for Zika virus using real-time reverse-transcription polymerase chain
reactions during gestation and/or after birth, while two patients only had serologic evidence of infection.
“It was interesting to note that the viral sequences amplified from patients 1 and 7 after birth gained a new substitution, V23I, which is located in the envelope domain I and may be implicated in viral tropism to different tissues.”
The study was supported by Consellho Nacional de Desenvolvimento e Pesquisa, Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Prefeitura Municipal de Campina Grande. No conflicts of interest were declared.
Key clinical point:
Major finding: Congenital Zika syndrome is associated with microcephaly, reduced cerebral volume, cerebellar hypoplasia, lissencephaly with hydrocephalus, and fetal akinesia deformation sequence.
Data source: Prospective study of 11 Zika-affected infants followed from gestation to 6 months of age.
Disclosures: The study was supported by Consellho Nacional de Desenvolvimento e Pesquisa, Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Prefeitura Municipal de Campina Grande. No conflicts of interest were declared.
Patient-Reported Outcomes of Azelaic Acid Foam 15% for Patients With Papulopustular Rosacea: Secondary Efficacy Results From a Randomized, Controlled, Double-blind, Phase 3 Trial
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.

Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).
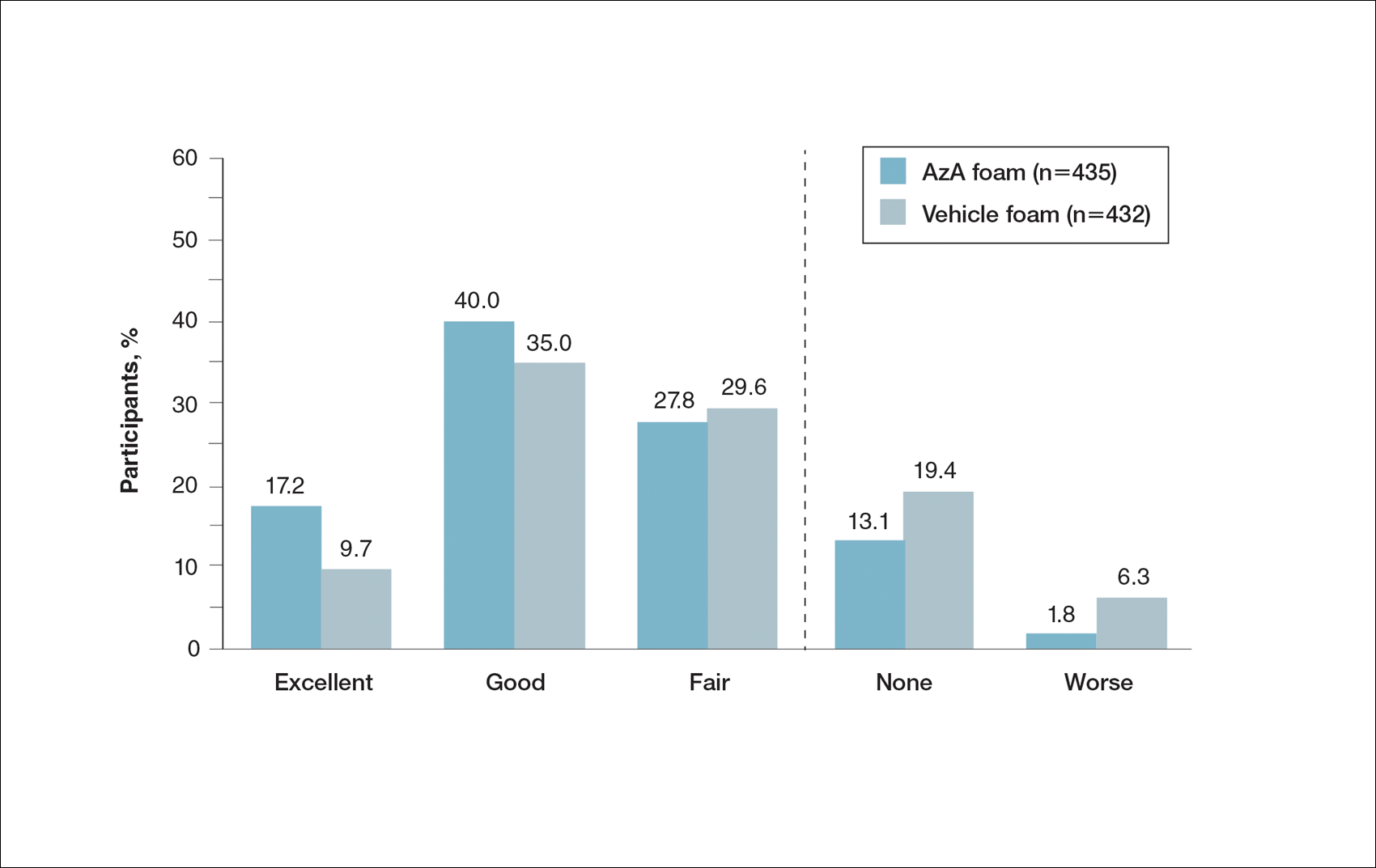
Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).
At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.
Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.

Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).

Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).

At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.

Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.

Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).

Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).

At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.

Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
Practice Points
- Patient perceptions of treatment are an important consideration in developing topical therapeutic strategies for papulopustular rosacea.
- A novel hydrophilic foam formulation of azelaic acid (AzA) provided substantial benefits in patient-reported measures of treatment response and quality of life.
- Patients reported high levels of satisfaction with the usability, tolerability, and practicability of AzA foam.
- The positive treatment experience described by patients parallels investigator-reported measures of clinical efficacy reported elsewhere.
Hypofractionated RT safe, convenient in low-risk prostate cancer
BOSTON – Shortening the radiation dosing schedule by 3 weeks in men with early-stage, low-risk prostate cancer does not appear to result in worse outcomes or diminished quality of life, according to investigators in the NRG Oncology/RTOG 0415 trial.
Efficacy results from the trial, reported at the annual meeting of the American Society for Radiation Oncology, showed that a radiation schedule of 70 Gy in 28 fractions delivered over 5.6 weeks was not inferior to 73.8 Gy delivered in 41 fractions over 8.2 weeks in terms of disease-free, progression-free, or overall survival.
“The International Atomic Energy [Agency] in its prostate cancer guidelines still lists hypofractionated radiation therapy as investigational, but I think we are at the stage where there is a ton of evidence, and this is really no leap of faith anymore that this can become the standard of care,” said Deborah W. Bruner, PhD, of Emory University and the Winship Cancer Institute in Atlanta.
In the treatment of patients with low-risk prostate cancer who opt for therapy over active surveillance, it is essential that “we treat them with the absolute minimum amount of treatment, with minimum side effects, and with minimum cost,” she said at a plenary session.
As reported by the investigators in 2015, there were small but significant increases in clinician-reported adverse gastrointestinal and genitourinary adverse events in the hypofractionation arm compared with the conventional fractionation arm in the trial, prompting the researchers to see whether patients were actually experiencing what clinicians thought they were seeing.
The investigators examined health-related quality of life and symptoms using the Expanded Prostate Index Composite (EPIC instrument).
They used a 50-item patient-reported outcomes questionnaire using a scale of 0 (no problem) to 4 (big problem), with responses transferred to a 0-100 scale. The questionnaire asked about symptoms in four domains: bowel, urinary, sexual, and hormonal. Patients filled out the questionnaire at baseline and at 6 and 12 months of follow-up.
At 1 year, there were no changes from baseline in hormonal scores in either study arm, but sexual function scores declined by approximately 15 points among patients treated with conventional fractionation and by 11 points among patients treated with hypofractionation (between-group difference nonsignificant).
There was a small decline in urinary scores in both arms, but this difference was not significant.
Patients treated with hypofractionation had a statistically larger decline in bowel scores compared with patients treated with conventional fractionation, a 1.8-point difference, but this difference did not translate into a clinically significant difference; that is, patients themselves could not detect a significant decline in bowel function, Dr. Bruner said.
The invited discussant, Ronald Chen, MD, MPH, director of the comparative effectiveness research program at the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, agreed that a difference of only 1.8 points on a 0-100 scale, while technically significant, is clinically meaningless.
“So now we have the complete story and a better understanding of perhaps the value of hypofractionation versus standard fractionation from the patient’s perspective,” he said.
He also emphasized that “quality of life is a central component of any value framework in cancer care, and we must hear the patient’s voice. For clinical trials to truly inform patient decision making and allow patients to assess the value of their treatment options, quality of life must be studied.”
BOSTON – Shortening the radiation dosing schedule by 3 weeks in men with early-stage, low-risk prostate cancer does not appear to result in worse outcomes or diminished quality of life, according to investigators in the NRG Oncology/RTOG 0415 trial.
Efficacy results from the trial, reported at the annual meeting of the American Society for Radiation Oncology, showed that a radiation schedule of 70 Gy in 28 fractions delivered over 5.6 weeks was not inferior to 73.8 Gy delivered in 41 fractions over 8.2 weeks in terms of disease-free, progression-free, or overall survival.
“The International Atomic Energy [Agency] in its prostate cancer guidelines still lists hypofractionated radiation therapy as investigational, but I think we are at the stage where there is a ton of evidence, and this is really no leap of faith anymore that this can become the standard of care,” said Deborah W. Bruner, PhD, of Emory University and the Winship Cancer Institute in Atlanta.
In the treatment of patients with low-risk prostate cancer who opt for therapy over active surveillance, it is essential that “we treat them with the absolute minimum amount of treatment, with minimum side effects, and with minimum cost,” she said at a plenary session.
As reported by the investigators in 2015, there were small but significant increases in clinician-reported adverse gastrointestinal and genitourinary adverse events in the hypofractionation arm compared with the conventional fractionation arm in the trial, prompting the researchers to see whether patients were actually experiencing what clinicians thought they were seeing.
The investigators examined health-related quality of life and symptoms using the Expanded Prostate Index Composite (EPIC instrument).
They used a 50-item patient-reported outcomes questionnaire using a scale of 0 (no problem) to 4 (big problem), with responses transferred to a 0-100 scale. The questionnaire asked about symptoms in four domains: bowel, urinary, sexual, and hormonal. Patients filled out the questionnaire at baseline and at 6 and 12 months of follow-up.
At 1 year, there were no changes from baseline in hormonal scores in either study arm, but sexual function scores declined by approximately 15 points among patients treated with conventional fractionation and by 11 points among patients treated with hypofractionation (between-group difference nonsignificant).
There was a small decline in urinary scores in both arms, but this difference was not significant.
Patients treated with hypofractionation had a statistically larger decline in bowel scores compared with patients treated with conventional fractionation, a 1.8-point difference, but this difference did not translate into a clinically significant difference; that is, patients themselves could not detect a significant decline in bowel function, Dr. Bruner said.
The invited discussant, Ronald Chen, MD, MPH, director of the comparative effectiveness research program at the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, agreed that a difference of only 1.8 points on a 0-100 scale, while technically significant, is clinically meaningless.
“So now we have the complete story and a better understanding of perhaps the value of hypofractionation versus standard fractionation from the patient’s perspective,” he said.
He also emphasized that “quality of life is a central component of any value framework in cancer care, and we must hear the patient’s voice. For clinical trials to truly inform patient decision making and allow patients to assess the value of their treatment options, quality of life must be studied.”
BOSTON – Shortening the radiation dosing schedule by 3 weeks in men with early-stage, low-risk prostate cancer does not appear to result in worse outcomes or diminished quality of life, according to investigators in the NRG Oncology/RTOG 0415 trial.
Efficacy results from the trial, reported at the annual meeting of the American Society for Radiation Oncology, showed that a radiation schedule of 70 Gy in 28 fractions delivered over 5.6 weeks was not inferior to 73.8 Gy delivered in 41 fractions over 8.2 weeks in terms of disease-free, progression-free, or overall survival.
“The International Atomic Energy [Agency] in its prostate cancer guidelines still lists hypofractionated radiation therapy as investigational, but I think we are at the stage where there is a ton of evidence, and this is really no leap of faith anymore that this can become the standard of care,” said Deborah W. Bruner, PhD, of Emory University and the Winship Cancer Institute in Atlanta.
In the treatment of patients with low-risk prostate cancer who opt for therapy over active surveillance, it is essential that “we treat them with the absolute minimum amount of treatment, with minimum side effects, and with minimum cost,” she said at a plenary session.
As reported by the investigators in 2015, there were small but significant increases in clinician-reported adverse gastrointestinal and genitourinary adverse events in the hypofractionation arm compared with the conventional fractionation arm in the trial, prompting the researchers to see whether patients were actually experiencing what clinicians thought they were seeing.
The investigators examined health-related quality of life and symptoms using the Expanded Prostate Index Composite (EPIC instrument).
They used a 50-item patient-reported outcomes questionnaire using a scale of 0 (no problem) to 4 (big problem), with responses transferred to a 0-100 scale. The questionnaire asked about symptoms in four domains: bowel, urinary, sexual, and hormonal. Patients filled out the questionnaire at baseline and at 6 and 12 months of follow-up.
At 1 year, there were no changes from baseline in hormonal scores in either study arm, but sexual function scores declined by approximately 15 points among patients treated with conventional fractionation and by 11 points among patients treated with hypofractionation (between-group difference nonsignificant).
There was a small decline in urinary scores in both arms, but this difference was not significant.
Patients treated with hypofractionation had a statistically larger decline in bowel scores compared with patients treated with conventional fractionation, a 1.8-point difference, but this difference did not translate into a clinically significant difference; that is, patients themselves could not detect a significant decline in bowel function, Dr. Bruner said.
The invited discussant, Ronald Chen, MD, MPH, director of the comparative effectiveness research program at the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, agreed that a difference of only 1.8 points on a 0-100 scale, while technically significant, is clinically meaningless.
“So now we have the complete story and a better understanding of perhaps the value of hypofractionation versus standard fractionation from the patient’s perspective,” he said.
He also emphasized that “quality of life is a central component of any value framework in cancer care, and we must hear the patient’s voice. For clinical trials to truly inform patient decision making and allow patients to assess the value of their treatment options, quality of life must be studied.”
Key clinical point: There were no clinically significant differences in survival or patient-reported outcomes between hypofractionated or conventional radiation schedules for patients with low-risk prostate cancer.
Major finding: There was statistically but not clinically significant difference of 1.8 out of 100 patients on a quality-of-life scale.
Data source: Analysis of patient-reported QoL in the NRG//RTOG 0415 trial.
Disclosures: The National Cancer Institute supported the study. Dr. Bruner and Dr. Chen reported having no conflicts of interest.
Pruritic Papules on the Scalp and Arms
Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that occurs mostly in adults with a male predilection. The disease clinically favors the head and neck. Patients commonly present with pruritic papules that often are grouped, alopecia, and frequent secondary bacterial infections. Less commonly patients present with acneiform lesions and mucinorrhea. Patients often experience more pruritus in FMF than in classic MF, which can provide a good means of assessing disease activity. Disease-specific 5-year survival is approximately 70% to 80%, which is worse than classic plaque-stage MF and similar to tumor-stage MF.1
Treatment of FMF differs from classic MF in that the lesions are less responsive to skin-targeted therapies due to the perifollicular nature of dermal infiltrates. Superficial skin lesions can be treated with psoralen plus UVA (PUVA) therapy. Other options include PUVA in combination with interferon alfa or retinoids and local radiotherapy for solitary thick tumors; however, in patients who have more infiltrative skin lesions or had PUVA therapy that failed, total skin electron beam therapy may be required.2
On histologic examination, there typically is perivascular and periadnexal localization of dermal infiltrates with varied involvement of the follicular epithelium and damage to hair follicles by atypical small, medium, and large hyperchromatic lymphocytes with cerebriform nuclei. Mucinous degeneration of the follicular epithelium can be seen, as highlighted on Alcian blue staining, and a mixed infiltrate of eosinophils and plasma cells often is present (quiz image and Figure 1). Frequent sparing of the epidermis is noteworthy.2-4 In most cases, the neoplastic T lymphocytes are characterized by a CD3+CD4+CD8-immunophenotype as is seen in classic MF. Sometimes an admixture of CD30+ blast cells is seen.1
Histologic differential considerations for FMF include eosinophilic pustular folliculitis (EPF), primary follicular mucinosis, lupus erythematosus, and pityrosporum folliculitis.
Eosinophilic pustular folliculitis has several clinical subtypes, such as classic Ofuji disease and immunosuppression-associated EPF secondary to human immunodeficiency virus. Histologically, EPF is characterized by spongiosis of the hair follicle epithelium with exocytosis of a mixed infiltrate of lymphocytes and eosinophils extending from the sebaceous gland and its duct to the infundibulum with formation of hallmark eosinophilic pustules (Figure 2). Infiltration of neutrophils in inflamed lesions generally is seen. Eosinophilic pustular folliculitis is an important differential for FMF, as follicular mucinosis has been observed in lesions of EPF.5 Both EPF and FMF can exhibit eosinophils and lymphocytes in the upper dermis, spongiosis of the hair follicle epithelium, and mucinous degeneration of follicles,6 though lymphocytic atypia and relatively fewer eosinophils are suggestive of the latter.
Primary follicular mucinosis (PFM) tends to occur as a solitary lesion in younger female patients in contrast to the multiple lesions that typically appear in older male patients with FMF. Histologically, PFM usually manifests as large, cystic, mucin-filled spaces and polyclonal perivascular and periadnexal lymphocytic infiltrate without notable cellular atypia or epidermotropism (Figure 3). Because follicular mucinosis is a common feature of FMF, its distinction from PFM can be challenging and often is aided by the absence of cellular atypia and relatively mild lymphocytic infiltrate in the latter.7
Cutaneous lupus erythematosus with its characteristic folliculocentric lymphocytic infiltration and associated dermal mucin also qualifies as a potential differential possibility for FMF; however, the perivascular and periadnexal pattern of lymphocytic infiltration as well as the localization of mucin to the reticular dermal interstitium8,9 are key histopathologic distinctions (Figure 4). Furthermore, although the histologic presentation of lupus erythematosus can be variable, it also classically shows interface dermatitis, basement membrane thickening, and follicular plugging.
Pityrosporum folliculitis is the most common cause of fungal folliculitis and is caused by the Malassezia species. On histology, there typically is an unremarkable epithelium with plugged follicles and suppurative folliculitis. Serial sections of the biopsy specimen often are required to identify dilated, follicle-containing, budding yeast cells (Figure 5). Organisms are located predominantly within the infundibulum and orifice of follicular lumen, are positive for periodic acid-Schiff, and are diastase resistant.10
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis. a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198.
- DeBloom J 2nd, Severson J, Gaspari A, et al. Follicular mycosis fungoides: a case report and review of the literature. J Cutan Pathol. 2001;28:318-324.
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423.
- Lee JY, Tsai YM, Sheu HM. Ofuji's disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol. 2003;30:307-313.
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19.
- Vincent JG, Chan MP. Specificity of dermal mucin in the diagnosis of lupus erythematosus: comparison with other dermatitides and normal skin. J Cutan Pathol. 2015;42:722-729.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
- Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39:9-25.
Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that occurs mostly in adults with a male predilection. The disease clinically favors the head and neck. Patients commonly present with pruritic papules that often are grouped, alopecia, and frequent secondary bacterial infections. Less commonly patients present with acneiform lesions and mucinorrhea. Patients often experience more pruritus in FMF than in classic MF, which can provide a good means of assessing disease activity. Disease-specific 5-year survival is approximately 70% to 80%, which is worse than classic plaque-stage MF and similar to tumor-stage MF.1
Treatment of FMF differs from classic MF in that the lesions are less responsive to skin-targeted therapies due to the perifollicular nature of dermal infiltrates. Superficial skin lesions can be treated with psoralen plus UVA (PUVA) therapy. Other options include PUVA in combination with interferon alfa or retinoids and local radiotherapy for solitary thick tumors; however, in patients who have more infiltrative skin lesions or had PUVA therapy that failed, total skin electron beam therapy may be required.2
On histologic examination, there typically is perivascular and periadnexal localization of dermal infiltrates with varied involvement of the follicular epithelium and damage to hair follicles by atypical small, medium, and large hyperchromatic lymphocytes with cerebriform nuclei. Mucinous degeneration of the follicular epithelium can be seen, as highlighted on Alcian blue staining, and a mixed infiltrate of eosinophils and plasma cells often is present (quiz image and Figure 1). Frequent sparing of the epidermis is noteworthy.2-4 In most cases, the neoplastic T lymphocytes are characterized by a CD3+CD4+CD8-immunophenotype as is seen in classic MF. Sometimes an admixture of CD30+ blast cells is seen.1

Histologic differential considerations for FMF include eosinophilic pustular folliculitis (EPF), primary follicular mucinosis, lupus erythematosus, and pityrosporum folliculitis.
Eosinophilic pustular folliculitis has several clinical subtypes, such as classic Ofuji disease and immunosuppression-associated EPF secondary to human immunodeficiency virus. Histologically, EPF is characterized by spongiosis of the hair follicle epithelium with exocytosis of a mixed infiltrate of lymphocytes and eosinophils extending from the sebaceous gland and its duct to the infundibulum with formation of hallmark eosinophilic pustules (Figure 2). Infiltration of neutrophils in inflamed lesions generally is seen. Eosinophilic pustular folliculitis is an important differential for FMF, as follicular mucinosis has been observed in lesions of EPF.5 Both EPF and FMF can exhibit eosinophils and lymphocytes in the upper dermis, spongiosis of the hair follicle epithelium, and mucinous degeneration of follicles,6 though lymphocytic atypia and relatively fewer eosinophils are suggestive of the latter.

Primary follicular mucinosis (PFM) tends to occur as a solitary lesion in younger female patients in contrast to the multiple lesions that typically appear in older male patients with FMF. Histologically, PFM usually manifests as large, cystic, mucin-filled spaces and polyclonal perivascular and periadnexal lymphocytic infiltrate without notable cellular atypia or epidermotropism (Figure 3). Because follicular mucinosis is a common feature of FMF, its distinction from PFM can be challenging and often is aided by the absence of cellular atypia and relatively mild lymphocytic infiltrate in the latter.7

Cutaneous lupus erythematosus with its characteristic folliculocentric lymphocytic infiltration and associated dermal mucin also qualifies as a potential differential possibility for FMF; however, the perivascular and periadnexal pattern of lymphocytic infiltration as well as the localization of mucin to the reticular dermal interstitium8,9 are key histopathologic distinctions (Figure 4). Furthermore, although the histologic presentation of lupus erythematosus can be variable, it also classically shows interface dermatitis, basement membrane thickening, and follicular plugging.

Pityrosporum folliculitis is the most common cause of fungal folliculitis and is caused by the Malassezia species. On histology, there typically is an unremarkable epithelium with plugged follicles and suppurative folliculitis. Serial sections of the biopsy specimen often are required to identify dilated, follicle-containing, budding yeast cells (Figure 5). Organisms are located predominantly within the infundibulum and orifice of follicular lumen, are positive for periodic acid-Schiff, and are diastase resistant.10

Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that occurs mostly in adults with a male predilection. The disease clinically favors the head and neck. Patients commonly present with pruritic papules that often are grouped, alopecia, and frequent secondary bacterial infections. Less commonly patients present with acneiform lesions and mucinorrhea. Patients often experience more pruritus in FMF than in classic MF, which can provide a good means of assessing disease activity. Disease-specific 5-year survival is approximately 70% to 80%, which is worse than classic plaque-stage MF and similar to tumor-stage MF.1
Treatment of FMF differs from classic MF in that the lesions are less responsive to skin-targeted therapies due to the perifollicular nature of dermal infiltrates. Superficial skin lesions can be treated with psoralen plus UVA (PUVA) therapy. Other options include PUVA in combination with interferon alfa or retinoids and local radiotherapy for solitary thick tumors; however, in patients who have more infiltrative skin lesions or had PUVA therapy that failed, total skin electron beam therapy may be required.2
On histologic examination, there typically is perivascular and periadnexal localization of dermal infiltrates with varied involvement of the follicular epithelium and damage to hair follicles by atypical small, medium, and large hyperchromatic lymphocytes with cerebriform nuclei. Mucinous degeneration of the follicular epithelium can be seen, as highlighted on Alcian blue staining, and a mixed infiltrate of eosinophils and plasma cells often is present (quiz image and Figure 1). Frequent sparing of the epidermis is noteworthy.2-4 In most cases, the neoplastic T lymphocytes are characterized by a CD3+CD4+CD8-immunophenotype as is seen in classic MF. Sometimes an admixture of CD30+ blast cells is seen.1

Histologic differential considerations for FMF include eosinophilic pustular folliculitis (EPF), primary follicular mucinosis, lupus erythematosus, and pityrosporum folliculitis.
Eosinophilic pustular folliculitis has several clinical subtypes, such as classic Ofuji disease and immunosuppression-associated EPF secondary to human immunodeficiency virus. Histologically, EPF is characterized by spongiosis of the hair follicle epithelium with exocytosis of a mixed infiltrate of lymphocytes and eosinophils extending from the sebaceous gland and its duct to the infundibulum with formation of hallmark eosinophilic pustules (Figure 2). Infiltration of neutrophils in inflamed lesions generally is seen. Eosinophilic pustular folliculitis is an important differential for FMF, as follicular mucinosis has been observed in lesions of EPF.5 Both EPF and FMF can exhibit eosinophils and lymphocytes in the upper dermis, spongiosis of the hair follicle epithelium, and mucinous degeneration of follicles,6 though lymphocytic atypia and relatively fewer eosinophils are suggestive of the latter.

Primary follicular mucinosis (PFM) tends to occur as a solitary lesion in younger female patients in contrast to the multiple lesions that typically appear in older male patients with FMF. Histologically, PFM usually manifests as large, cystic, mucin-filled spaces and polyclonal perivascular and periadnexal lymphocytic infiltrate without notable cellular atypia or epidermotropism (Figure 3). Because follicular mucinosis is a common feature of FMF, its distinction from PFM can be challenging and often is aided by the absence of cellular atypia and relatively mild lymphocytic infiltrate in the latter.7

Cutaneous lupus erythematosus with its characteristic folliculocentric lymphocytic infiltration and associated dermal mucin also qualifies as a potential differential possibility for FMF; however, the perivascular and periadnexal pattern of lymphocytic infiltration as well as the localization of mucin to the reticular dermal interstitium8,9 are key histopathologic distinctions (Figure 4). Furthermore, although the histologic presentation of lupus erythematosus can be variable, it also classically shows interface dermatitis, basement membrane thickening, and follicular plugging.

Pityrosporum folliculitis is the most common cause of fungal folliculitis and is caused by the Malassezia species. On histology, there typically is an unremarkable epithelium with plugged follicles and suppurative folliculitis. Serial sections of the biopsy specimen often are required to identify dilated, follicle-containing, budding yeast cells (Figure 5). Organisms are located predominantly within the infundibulum and orifice of follicular lumen, are positive for periodic acid-Schiff, and are diastase resistant.10

- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis. a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198.
- DeBloom J 2nd, Severson J, Gaspari A, et al. Follicular mycosis fungoides: a case report and review of the literature. J Cutan Pathol. 2001;28:318-324.
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423.
- Lee JY, Tsai YM, Sheu HM. Ofuji's disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol. 2003;30:307-313.
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19.
- Vincent JG, Chan MP. Specificity of dermal mucin in the diagnosis of lupus erythematosus: comparison with other dermatitides and normal skin. J Cutan Pathol. 2015;42:722-729.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
- Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39:9-25.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis. a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198.
- DeBloom J 2nd, Severson J, Gaspari A, et al. Follicular mycosis fungoides: a case report and review of the literature. J Cutan Pathol. 2001;28:318-324.
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423.
- Lee JY, Tsai YM, Sheu HM. Ofuji's disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol. 2003;30:307-313.
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19.
- Vincent JG, Chan MP. Specificity of dermal mucin in the diagnosis of lupus erythematosus: comparison with other dermatitides and normal skin. J Cutan Pathol. 2015;42:722-729.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996;135:355-362.
- Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013;39:9-25.
A 60-year-old man presented with a 3-month history of itchy bumps on the scalp and arms. He also noticed some patches of hair loss in these areas. He had no history of other skin conditions and was otherwise healthy with no other medical comorbidities.