User login
Age, not infusion frequency, affects hemophilia prophylaxis adherence
ORLANDO – The frequency of prophylactic clotting factor infusions does not appear to adversely affect adherence to hemophilia therapy in children, but age seems to play a role in compliance to prescribed regimens, investigators in two separate studies report.
A study of pediatric patients with moderate to severe hemophilia A or B on bleeding prophylaxis showed no significant differences in adherence between patients receiving two clotting factor infusions per week and those receiving three or four per week, reported Karen Strike, a physiotherapist at McMaster Children’s Hospital in Hamilton, Ont., and colleagues.
“This study demonstrates that our patients have a very high degree of adherence, and it doesn’t appear to be related to infusion frequency,” she said in an interview at a scientific poster session at the World Federation of Hemophilia World Congress.
Although their sample size was small – just 23 patients – the median levels of adherence were high for both twice-weekly infusers (99.5%) and 3-4 times per week infusers (96%; P = .053).
Ms. Strike acknowledges that the borderline P value could be due to the small sample size instead of a lack of association. Additionally, because the patients are managed by a regional hemophilia treatment center, they tend to be more engaged and more likely to cooperate with clinician instructions, she said.
“Basically, patients are either going to do what’s prescribed or they’re not. What that actual prescription is doesn’t seem to matter. If they prescribe you twice a week or they prescribe me every other day, you’re going to be adherent to twice a week or you’re not.
The investigators have expanded their study to include adult patients on primary prophylaxis and to look at additional co-variates that might have an effect on adherence, including interactions with a health care team, geographical distance from a hemophilia treatment center, joint health status, and infusion delivery method (peripheral vs. port).
Age may be a factor
In a separate study, German investigators report that adherence appears to vary by age.
Wolfgang Miesbach, MD, from Goethe University Hospital in Frankfurt, Germany, and colleagues asked all members of the German hemophilia patient organization with moderate or severe hemophilia to fill out the VERITAS-PRO (Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis) questionnaire and compared scores across age groups.
Data were available on a total of 364 patients. The investigators found that among 131 children from birth to age 19, adherence to prescribed regimens was 100%. In contrast, the adherence rate among 189 adults aged 20-59 was 88.1%. After age 59, adherence rates began to improve as those 60 and older (44 patients) reported 93.9% adherence.
“Within the patients aged 20+, care by a hemophilia center was the only significant indicator for better adherence. The tendency of better adherence of patients aged greater than 60 compared to patients 20-59 may be explained by the significant association of the occurrence of pain with increasing age although a significant influence of pain on the adherence levels could not be demonstrated,” the investigators wrote.
Their findings point the way to possible interventions to facilitate adherence to prophylaxis among patients with hemophilia, they wrote.
Both studies were internally funded. The authors reported no relevant disclosures.
ORLANDO – The frequency of prophylactic clotting factor infusions does not appear to adversely affect adherence to hemophilia therapy in children, but age seems to play a role in compliance to prescribed regimens, investigators in two separate studies report.
A study of pediatric patients with moderate to severe hemophilia A or B on bleeding prophylaxis showed no significant differences in adherence between patients receiving two clotting factor infusions per week and those receiving three or four per week, reported Karen Strike, a physiotherapist at McMaster Children’s Hospital in Hamilton, Ont., and colleagues.
“This study demonstrates that our patients have a very high degree of adherence, and it doesn’t appear to be related to infusion frequency,” she said in an interview at a scientific poster session at the World Federation of Hemophilia World Congress.
Although their sample size was small – just 23 patients – the median levels of adherence were high for both twice-weekly infusers (99.5%) and 3-4 times per week infusers (96%; P = .053).
Ms. Strike acknowledges that the borderline P value could be due to the small sample size instead of a lack of association. Additionally, because the patients are managed by a regional hemophilia treatment center, they tend to be more engaged and more likely to cooperate with clinician instructions, she said.
“Basically, patients are either going to do what’s prescribed or they’re not. What that actual prescription is doesn’t seem to matter. If they prescribe you twice a week or they prescribe me every other day, you’re going to be adherent to twice a week or you’re not.
The investigators have expanded their study to include adult patients on primary prophylaxis and to look at additional co-variates that might have an effect on adherence, including interactions with a health care team, geographical distance from a hemophilia treatment center, joint health status, and infusion delivery method (peripheral vs. port).
Age may be a factor
In a separate study, German investigators report that adherence appears to vary by age.
Wolfgang Miesbach, MD, from Goethe University Hospital in Frankfurt, Germany, and colleagues asked all members of the German hemophilia patient organization with moderate or severe hemophilia to fill out the VERITAS-PRO (Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis) questionnaire and compared scores across age groups.
Data were available on a total of 364 patients. The investigators found that among 131 children from birth to age 19, adherence to prescribed regimens was 100%. In contrast, the adherence rate among 189 adults aged 20-59 was 88.1%. After age 59, adherence rates began to improve as those 60 and older (44 patients) reported 93.9% adherence.
“Within the patients aged 20+, care by a hemophilia center was the only significant indicator for better adherence. The tendency of better adherence of patients aged greater than 60 compared to patients 20-59 may be explained by the significant association of the occurrence of pain with increasing age although a significant influence of pain on the adherence levels could not be demonstrated,” the investigators wrote.
Their findings point the way to possible interventions to facilitate adherence to prophylaxis among patients with hemophilia, they wrote.
Both studies were internally funded. The authors reported no relevant disclosures.
ORLANDO – The frequency of prophylactic clotting factor infusions does not appear to adversely affect adherence to hemophilia therapy in children, but age seems to play a role in compliance to prescribed regimens, investigators in two separate studies report.
A study of pediatric patients with moderate to severe hemophilia A or B on bleeding prophylaxis showed no significant differences in adherence between patients receiving two clotting factor infusions per week and those receiving three or four per week, reported Karen Strike, a physiotherapist at McMaster Children’s Hospital in Hamilton, Ont., and colleagues.
“This study demonstrates that our patients have a very high degree of adherence, and it doesn’t appear to be related to infusion frequency,” she said in an interview at a scientific poster session at the World Federation of Hemophilia World Congress.
Although their sample size was small – just 23 patients – the median levels of adherence were high for both twice-weekly infusers (99.5%) and 3-4 times per week infusers (96%; P = .053).
Ms. Strike acknowledges that the borderline P value could be due to the small sample size instead of a lack of association. Additionally, because the patients are managed by a regional hemophilia treatment center, they tend to be more engaged and more likely to cooperate with clinician instructions, she said.
“Basically, patients are either going to do what’s prescribed or they’re not. What that actual prescription is doesn’t seem to matter. If they prescribe you twice a week or they prescribe me every other day, you’re going to be adherent to twice a week or you’re not.
The investigators have expanded their study to include adult patients on primary prophylaxis and to look at additional co-variates that might have an effect on adherence, including interactions with a health care team, geographical distance from a hemophilia treatment center, joint health status, and infusion delivery method (peripheral vs. port).
Age may be a factor
In a separate study, German investigators report that adherence appears to vary by age.
Wolfgang Miesbach, MD, from Goethe University Hospital in Frankfurt, Germany, and colleagues asked all members of the German hemophilia patient organization with moderate or severe hemophilia to fill out the VERITAS-PRO (Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis) questionnaire and compared scores across age groups.
Data were available on a total of 364 patients. The investigators found that among 131 children from birth to age 19, adherence to prescribed regimens was 100%. In contrast, the adherence rate among 189 adults aged 20-59 was 88.1%. After age 59, adherence rates began to improve as those 60 and older (44 patients) reported 93.9% adherence.
“Within the patients aged 20+, care by a hemophilia center was the only significant indicator for better adherence. The tendency of better adherence of patients aged greater than 60 compared to patients 20-59 may be explained by the significant association of the occurrence of pain with increasing age although a significant influence of pain on the adherence levels could not be demonstrated,” the investigators wrote.
Their findings point the way to possible interventions to facilitate adherence to prophylaxis among patients with hemophilia, they wrote.
Both studies were internally funded. The authors reported no relevant disclosures.
AT WFH 2016 WORLD CONGRESS
Key clinical point: Adherence to hemophilia prophylaxis regimens may be influenced by age but not frequency of infusions.
Major finding: Infusion frequency did not make a difference in adherence rates, but young and middle-aged adults reported lower adherence than did children or seniors.
Data source: A study of 23 pediatric hemophilia patients in Canada, and a separate study of 364 children and adults with moderate to severe hemophilia in Germany.
Disclosures: Both studies were internally funded. The authors reported no relevant disclosures.
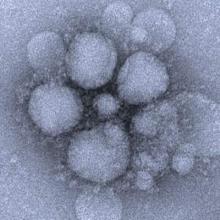
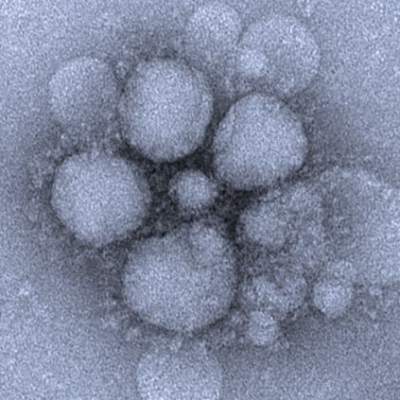
Blood viral RNA may indicate severity of MERS coronavirus clinical course
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: Checking for blood viral RNA at hospital admission may be a reliable indicator of the severity of MERS coronavirus infection clinical course.
Major finding: Blood viral RNA positivity at admission was associated with fever higher than 37.5 °C on the sampling date (P = .007), requirement for mechanical ventilation during the following clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and patient death (P = .025).
Data source: Prospective analysis of 21 patients with Middle East respiratory syndrome coronavirus (MERS-CoV).
Disclosures: The research was funded by the National Medical Center Research Institute.
HIV chemoprophylaxis shown effective in 15-year-olds
DURBAN, SOUTH AFRICA – Oral emtricitabine/tenofovir for pre-exposure prophylaxis against HIV acquisition in high-risk 15- to 17-year-old males proved safe and effective in the first clinical trial looking at the drug’s effects in a population so young, Sybil Hosek, PhD, reported at the 21st International AIDS Conference.
Based upon these encouraging findings, the drug’s manufacturer, Gilead Sciences, plans to file a request for the Food and Drug Administration to grant an expanded indication for emtricitabine/tenofovir (Truvada) for pre-exposure prophylaxis (PrEP) against HIV infection in teens as young as 15 years. The drug is currently approved for use only in patients aged 18 and up because there were no data in younger patients, said Dr. Hosek of John H. Stroger, Jr. Hospital, Chicago.
The prospect of an expanded indication in younger adolescents is most welcome news, she added.
“I really want to strongly, strongly, strongly say that adolescents need access to PrEP,” Dr. Hosek declared. “This is one of the best prevention options we’ve had in a long time.”
Co-investigator Craig M. Wilson, MD, concurred. “The epicenter of the HIV/AIDS epidemic in the U.S. is in 13- to 24-year-old males who have sex with males, particular MSM of color,” noted Dr. Wilson, professor of epidemiology, pediatrics, and director of the Sparkman Center for Global Health at the University of Alabama, Birmingham.
Dr. Hosek reported on 77 male teens ages 15-17 at high self-reported risk for HIV infection because of behaviors such as condomless anal intercourse with an HIV-positive or unknown-status partner. All 77 were negative for HIV at enrollment, which didn’t require parental permission. Prior to embarking on 48 months of once-daily, open-label emtricitabine/tenofovir for PrEP in this multicenter U.S. trial, they received personalized risk reduction, adherence, and behavior counseling. As part of the study protocol they had clinic visits monthly for the first 12 weeks. At that point the visits, which included testing for HIV and other STIs as well as measurement of blood drug levels as an indicator of adherence, were scaled back to once every 3 months.
The PrEP was safe and well tolerated. No one discontinued treatment because of side effects. The only adverse event of note was weight loss of 10%-19% in two patients. New STIs were diagnosed and treated in 12.3% of participants in the first 24 weeks of the study and in 10.6% in the next 24 weeks.
Three patients seroconverted during the 48-week study, for a hefty HIV infection rate of 6.41% per year. One of these patients never took the PrEP medication, the other two did so on and off but had no or very low blood levels of the drug at the time of seroconversion.
Adherence was a major issue, according to Dr. Hosek. She deemed adherence to be “really good” during the first 12 weeks of the study. During that period, the majority of participants had blood levels indicating they were taking their medication at least 4 days per week, providing high-level protection. More than 95% of subjects had detectable levels of drug, indicating they were at least trying to keep up with their medication schedule. However, once the clinic visits were scaled back from monthly to quarterly, adherence fell off drastically.
Audience member Carlos del Rio, MD, commented that he found the poor adherence over time to be really discouraging.
“The adolescent challenge is tremendous. All the studies show us that this group isn’t getting any protection. Are we trying to fit a square peg in a round hole? Is this something that’s just not going to happen, so we should look at alternatives such as long-acting injectables? It looks to me like we’re not going to get the adherence we need in adolescents with any of the things that are out there at this moment,” said Dr. del Rio, professor and chair of the department of global health and codirector of the center for AIDS research at Emory University in Atlanta.
Dr. Hosek replied that she found heartening the “outstanding” treatment adherence rate when patients were being seen monthly.
“Young people need more time,” Dr. Hosek observed. “And if they need that time from us, we have to give it to them. If they need to see us more frequently, if they need to text with us, if they need interim phone calls, a peer support group, an adherence club – whatever they need, if they want PrEP and they want to make it work, then we need to help them make it work. That’s our responsibility, to give them the time and attention they need.”
Loss of bone mineral density with PrEP
Dr. Wilson said an issue that bears watching, assuming a large increase in the use of emtricitabine/tenofovir for HIV PrEP in adolescents is in store in the near future, is drug-related loss in bone mineral density.
He presented data on changes in bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry with results assessed at a core laboratory every 6 months in a companion study to the one presented by Dr. Hosek, this one involving 72 high-HIV-risk patients aged 18-22 years on 48 weeks of open-label emtricitabine/tenofovir followed by 48 weeks off PrEP.
Consistent with what’s been seen in studies of adults on emtricitabine/tenofovir, statistically significant decreases in mean Z-scores adjusted for age, sex, and race were seen at the hip and lumbar spine in this younger population between baseline and week 48 of PrEP. The reductions in BMD were in the range of 0.1-0.2 standard deviation. That’s noteworthy because up until age 20, people are supposed to be accruing bone mineralization, he observed.
During the subsequent 48 weeks off-PrEP patients showed evidence of partial but not full remineralization.
“There’s nothing here to indicate we should stop using PrEP in this age group, but given that we’d like to see high-risk young patients remain on therapy for longer than in this 48-week study, I think it would be smart to get longer-term exposure data to ensure that we still believe it’s safe,” the pediatrician commented.
Reassuringly, there is no evidence of an increase in fractures or complaints of bone pain in any studies of HIV-positive patients on tenofovir, he observed.
Because it’s unrealistic to expect to be able to routinely do serial DEXA scans in young patients on emtricitabine/tenofovir once PrEP is ramped up to the scale HIV specialists are hoping for, Dr. Wilson said he and his coinvestigators are now looking at potential biomarkers of clinically significant bone loss in young patients on chemoprophylaxis.
Dr. Wilson drew attention to the disturbingly high HIV seroconversion rate of 7.2% per year following discontinuation of PrEP after 48 weeks.
“Remember, this is a population that had already gone through extensive counseling, behavioral interventions, and personalized prevention and adherence support during the 48 weeks they were on the study drug, so they had been informed as to what the risks were. Yet we still end up with one of the highest seroconversion rates observed in any PrEP study. That tells us we still have a lot of work to do in these particular young populations,” according to Dr. Wilson.
These clinical trials of PrEP in 15- to 17- and 18- to 22-year-olds were carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. Dr. Husek and Dr. Wilson reported having no financial conflicts.
DURBAN, SOUTH AFRICA – Oral emtricitabine/tenofovir for pre-exposure prophylaxis against HIV acquisition in high-risk 15- to 17-year-old males proved safe and effective in the first clinical trial looking at the drug’s effects in a population so young, Sybil Hosek, PhD, reported at the 21st International AIDS Conference.
Based upon these encouraging findings, the drug’s manufacturer, Gilead Sciences, plans to file a request for the Food and Drug Administration to grant an expanded indication for emtricitabine/tenofovir (Truvada) for pre-exposure prophylaxis (PrEP) against HIV infection in teens as young as 15 years. The drug is currently approved for use only in patients aged 18 and up because there were no data in younger patients, said Dr. Hosek of John H. Stroger, Jr. Hospital, Chicago.
The prospect of an expanded indication in younger adolescents is most welcome news, she added.
“I really want to strongly, strongly, strongly say that adolescents need access to PrEP,” Dr. Hosek declared. “This is one of the best prevention options we’ve had in a long time.”
Co-investigator Craig M. Wilson, MD, concurred. “The epicenter of the HIV/AIDS epidemic in the U.S. is in 13- to 24-year-old males who have sex with males, particular MSM of color,” noted Dr. Wilson, professor of epidemiology, pediatrics, and director of the Sparkman Center for Global Health at the University of Alabama, Birmingham.
Dr. Hosek reported on 77 male teens ages 15-17 at high self-reported risk for HIV infection because of behaviors such as condomless anal intercourse with an HIV-positive or unknown-status partner. All 77 were negative for HIV at enrollment, which didn’t require parental permission. Prior to embarking on 48 months of once-daily, open-label emtricitabine/tenofovir for PrEP in this multicenter U.S. trial, they received personalized risk reduction, adherence, and behavior counseling. As part of the study protocol they had clinic visits monthly for the first 12 weeks. At that point the visits, which included testing for HIV and other STIs as well as measurement of blood drug levels as an indicator of adherence, were scaled back to once every 3 months.
The PrEP was safe and well tolerated. No one discontinued treatment because of side effects. The only adverse event of note was weight loss of 10%-19% in two patients. New STIs were diagnosed and treated in 12.3% of participants in the first 24 weeks of the study and in 10.6% in the next 24 weeks.
Three patients seroconverted during the 48-week study, for a hefty HIV infection rate of 6.41% per year. One of these patients never took the PrEP medication, the other two did so on and off but had no or very low blood levels of the drug at the time of seroconversion.
Adherence was a major issue, according to Dr. Hosek. She deemed adherence to be “really good” during the first 12 weeks of the study. During that period, the majority of participants had blood levels indicating they were taking their medication at least 4 days per week, providing high-level protection. More than 95% of subjects had detectable levels of drug, indicating they were at least trying to keep up with their medication schedule. However, once the clinic visits were scaled back from monthly to quarterly, adherence fell off drastically.
Audience member Carlos del Rio, MD, commented that he found the poor adherence over time to be really discouraging.
“The adolescent challenge is tremendous. All the studies show us that this group isn’t getting any protection. Are we trying to fit a square peg in a round hole? Is this something that’s just not going to happen, so we should look at alternatives such as long-acting injectables? It looks to me like we’re not going to get the adherence we need in adolescents with any of the things that are out there at this moment,” said Dr. del Rio, professor and chair of the department of global health and codirector of the center for AIDS research at Emory University in Atlanta.
Dr. Hosek replied that she found heartening the “outstanding” treatment adherence rate when patients were being seen monthly.
“Young people need more time,” Dr. Hosek observed. “And if they need that time from us, we have to give it to them. If they need to see us more frequently, if they need to text with us, if they need interim phone calls, a peer support group, an adherence club – whatever they need, if they want PrEP and they want to make it work, then we need to help them make it work. That’s our responsibility, to give them the time and attention they need.”
Loss of bone mineral density with PrEP
Dr. Wilson said an issue that bears watching, assuming a large increase in the use of emtricitabine/tenofovir for HIV PrEP in adolescents is in store in the near future, is drug-related loss in bone mineral density.
He presented data on changes in bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry with results assessed at a core laboratory every 6 months in a companion study to the one presented by Dr. Hosek, this one involving 72 high-HIV-risk patients aged 18-22 years on 48 weeks of open-label emtricitabine/tenofovir followed by 48 weeks off PrEP.
Consistent with what’s been seen in studies of adults on emtricitabine/tenofovir, statistically significant decreases in mean Z-scores adjusted for age, sex, and race were seen at the hip and lumbar spine in this younger population between baseline and week 48 of PrEP. The reductions in BMD were in the range of 0.1-0.2 standard deviation. That’s noteworthy because up until age 20, people are supposed to be accruing bone mineralization, he observed.
During the subsequent 48 weeks off-PrEP patients showed evidence of partial but not full remineralization.
“There’s nothing here to indicate we should stop using PrEP in this age group, but given that we’d like to see high-risk young patients remain on therapy for longer than in this 48-week study, I think it would be smart to get longer-term exposure data to ensure that we still believe it’s safe,” the pediatrician commented.
Reassuringly, there is no evidence of an increase in fractures or complaints of bone pain in any studies of HIV-positive patients on tenofovir, he observed.
Because it’s unrealistic to expect to be able to routinely do serial DEXA scans in young patients on emtricitabine/tenofovir once PrEP is ramped up to the scale HIV specialists are hoping for, Dr. Wilson said he and his coinvestigators are now looking at potential biomarkers of clinically significant bone loss in young patients on chemoprophylaxis.
Dr. Wilson drew attention to the disturbingly high HIV seroconversion rate of 7.2% per year following discontinuation of PrEP after 48 weeks.
“Remember, this is a population that had already gone through extensive counseling, behavioral interventions, and personalized prevention and adherence support during the 48 weeks they were on the study drug, so they had been informed as to what the risks were. Yet we still end up with one of the highest seroconversion rates observed in any PrEP study. That tells us we still have a lot of work to do in these particular young populations,” according to Dr. Wilson.
These clinical trials of PrEP in 15- to 17- and 18- to 22-year-olds were carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. Dr. Husek and Dr. Wilson reported having no financial conflicts.
DURBAN, SOUTH AFRICA – Oral emtricitabine/tenofovir for pre-exposure prophylaxis against HIV acquisition in high-risk 15- to 17-year-old males proved safe and effective in the first clinical trial looking at the drug’s effects in a population so young, Sybil Hosek, PhD, reported at the 21st International AIDS Conference.
Based upon these encouraging findings, the drug’s manufacturer, Gilead Sciences, plans to file a request for the Food and Drug Administration to grant an expanded indication for emtricitabine/tenofovir (Truvada) for pre-exposure prophylaxis (PrEP) against HIV infection in teens as young as 15 years. The drug is currently approved for use only in patients aged 18 and up because there were no data in younger patients, said Dr. Hosek of John H. Stroger, Jr. Hospital, Chicago.
The prospect of an expanded indication in younger adolescents is most welcome news, she added.
“I really want to strongly, strongly, strongly say that adolescents need access to PrEP,” Dr. Hosek declared. “This is one of the best prevention options we’ve had in a long time.”
Co-investigator Craig M. Wilson, MD, concurred. “The epicenter of the HIV/AIDS epidemic in the U.S. is in 13- to 24-year-old males who have sex with males, particular MSM of color,” noted Dr. Wilson, professor of epidemiology, pediatrics, and director of the Sparkman Center for Global Health at the University of Alabama, Birmingham.
Dr. Hosek reported on 77 male teens ages 15-17 at high self-reported risk for HIV infection because of behaviors such as condomless anal intercourse with an HIV-positive or unknown-status partner. All 77 were negative for HIV at enrollment, which didn’t require parental permission. Prior to embarking on 48 months of once-daily, open-label emtricitabine/tenofovir for PrEP in this multicenter U.S. trial, they received personalized risk reduction, adherence, and behavior counseling. As part of the study protocol they had clinic visits monthly for the first 12 weeks. At that point the visits, which included testing for HIV and other STIs as well as measurement of blood drug levels as an indicator of adherence, were scaled back to once every 3 months.
The PrEP was safe and well tolerated. No one discontinued treatment because of side effects. The only adverse event of note was weight loss of 10%-19% in two patients. New STIs were diagnosed and treated in 12.3% of participants in the first 24 weeks of the study and in 10.6% in the next 24 weeks.
Three patients seroconverted during the 48-week study, for a hefty HIV infection rate of 6.41% per year. One of these patients never took the PrEP medication, the other two did so on and off but had no or very low blood levels of the drug at the time of seroconversion.
Adherence was a major issue, according to Dr. Hosek. She deemed adherence to be “really good” during the first 12 weeks of the study. During that period, the majority of participants had blood levels indicating they were taking their medication at least 4 days per week, providing high-level protection. More than 95% of subjects had detectable levels of drug, indicating they were at least trying to keep up with their medication schedule. However, once the clinic visits were scaled back from monthly to quarterly, adherence fell off drastically.
Audience member Carlos del Rio, MD, commented that he found the poor adherence over time to be really discouraging.
“The adolescent challenge is tremendous. All the studies show us that this group isn’t getting any protection. Are we trying to fit a square peg in a round hole? Is this something that’s just not going to happen, so we should look at alternatives such as long-acting injectables? It looks to me like we’re not going to get the adherence we need in adolescents with any of the things that are out there at this moment,” said Dr. del Rio, professor and chair of the department of global health and codirector of the center for AIDS research at Emory University in Atlanta.
Dr. Hosek replied that she found heartening the “outstanding” treatment adherence rate when patients were being seen monthly.
“Young people need more time,” Dr. Hosek observed. “And if they need that time from us, we have to give it to them. If they need to see us more frequently, if they need to text with us, if they need interim phone calls, a peer support group, an adherence club – whatever they need, if they want PrEP and they want to make it work, then we need to help them make it work. That’s our responsibility, to give them the time and attention they need.”
Loss of bone mineral density with PrEP
Dr. Wilson said an issue that bears watching, assuming a large increase in the use of emtricitabine/tenofovir for HIV PrEP in adolescents is in store in the near future, is drug-related loss in bone mineral density.
He presented data on changes in bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry with results assessed at a core laboratory every 6 months in a companion study to the one presented by Dr. Hosek, this one involving 72 high-HIV-risk patients aged 18-22 years on 48 weeks of open-label emtricitabine/tenofovir followed by 48 weeks off PrEP.
Consistent with what’s been seen in studies of adults on emtricitabine/tenofovir, statistically significant decreases in mean Z-scores adjusted for age, sex, and race were seen at the hip and lumbar spine in this younger population between baseline and week 48 of PrEP. The reductions in BMD were in the range of 0.1-0.2 standard deviation. That’s noteworthy because up until age 20, people are supposed to be accruing bone mineralization, he observed.
During the subsequent 48 weeks off-PrEP patients showed evidence of partial but not full remineralization.
“There’s nothing here to indicate we should stop using PrEP in this age group, but given that we’d like to see high-risk young patients remain on therapy for longer than in this 48-week study, I think it would be smart to get longer-term exposure data to ensure that we still believe it’s safe,” the pediatrician commented.
Reassuringly, there is no evidence of an increase in fractures or complaints of bone pain in any studies of HIV-positive patients on tenofovir, he observed.
Because it’s unrealistic to expect to be able to routinely do serial DEXA scans in young patients on emtricitabine/tenofovir once PrEP is ramped up to the scale HIV specialists are hoping for, Dr. Wilson said he and his coinvestigators are now looking at potential biomarkers of clinically significant bone loss in young patients on chemoprophylaxis.
Dr. Wilson drew attention to the disturbingly high HIV seroconversion rate of 7.2% per year following discontinuation of PrEP after 48 weeks.
“Remember, this is a population that had already gone through extensive counseling, behavioral interventions, and personalized prevention and adherence support during the 48 weeks they were on the study drug, so they had been informed as to what the risks were. Yet we still end up with one of the highest seroconversion rates observed in any PrEP study. That tells us we still have a lot of work to do in these particular young populations,” according to Dr. Wilson.
These clinical trials of PrEP in 15- to 17- and 18- to 22-year-olds were carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. Dr. Husek and Dr. Wilson reported having no financial conflicts.
AT AIDS 2016
Key clinical point: The licensed indication for daily emtricitabine/tenofovir for prevention of HIV infection might be expanded to include high-risk patients ages 15 and older based upon new study results.
Major finding: Emtricitabine/tenofovir was safe and well-tolerated for pre-exposure prophylaxis against HIV acquisition in teen males ages 15-17; however, adherence was a problem.
Data source: This prospective, open-label study included 77 male 15- to 17-year-olds at high risk for HIV infection who were placed on daily oral emtricitabine/tenofovir for chemoprophylaxis for 48 weeks.
Disclosures: The study was carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. The presenter reported having no financial conflicts.
Spinal stimulator devices becoming more versatile
LAKE BUENA VISTA, FLA. – Innovation in the design of spinal cord stimulators for control of chronic pain syndromes includes wireless devices to eliminate surgical implantation of the battery and devices with high frequency conduction to reduce paresthesias, according to an update at the Pain Care for Primary Care meeting.
“Paresthesias have been poorly tolerated by some patients, but this effect was not observed in a trial with high frequency stimulation,” explained Paul J. Cristo, MD, of the department of anesthesiology and critical care medicine, Johns Hopkins University, Baltimore.
Spinal cord stimulation for chronic pain control is not a new concept. The first device received Food and Drug Administration approval in 1989. Typically, these devices involve implantation of electrodes in the epidural space to deliver a low level of electricity that for many, but not all, individuals alleviates pain. With traditional devices, a battery and programmable generator is implanted in the buttock or abdomen to supply impulses.
Innovations since their introduction have included incremental reductions in the size of the generator and battery, facilitating implantation, and batteries that function longer or can be recharged. Some batteries now deliver stimulation for 10 or more years before they must be surgically replaced. In addition, several manufacturers now market devices that do not interfere with MRI.
The mechanism by which these devices relieve pain remains incompletely understood, according to Dr. Cristo. He cited animal studies that support several mechanisms, such as stimulation of A alpha and A beta afferent fibers to interrupt pain signals and release of serotonin and norepinephrine to inhibit pain transmission.
High-frequency stimulation
Up until last year, all devices provided neurostimulation through low frequency pulses. One of the major adverse effects has been a tingling sensation that some patients tolerate poorly. The first device to deliver high frequency pulses was approved by the FDA last year. According to Dr. Cristo, the advantage of high frequency energy is that it eliminates the risk of paresthesias.
In the registration trial with the high frequency device, which is now marketed under the name Senza by the Nevro Company, 198 chronic pain patients were randomized to an experimental or traditional device (Anesthesiology. 2015 Oct;123[4]:851-60). With the novel device, the spinal cord stimulation was delivered at a frequency of 10 kHz. Conventional spinal stimulators operate at frequencies of less than 100 Hz.
At 3 months, the proportion of patients classified as responders was greater for both back pain (84.5% vs. 43.8%) and leg pain (83.1% vs. 55.5%) when the novel and traditional devices were compared, but paresthesias, which were commonly reported on the traditional device, were not reported at all with the high frequency device.
Wireless stimulators
Wireless spinal stimulators are another relatively recent innovation with the potential to expand the proportion of chronic pain patients who may benefit from these devices. The first such device, marketed by Stimwave under the brand name Freedom SCS System, with a sister device for peripheral nerve stimulation called the StimQ PNS System, still requires implantation of a wire to deliver electrical stimulation, but the power source is external.
“The patient wears a belt that is equipped with a wireless programming device,” Dr. Cristo explained. He suggested that some may find the belt cumbersome, but a less invasive procedure is particularly attractive for patients with multiple comorbidities that make them poor candidates for device implantation.
Candidates for spinal cord stimulation are typically offered a trial to gauge response and tolerability before the device is implanted, according to Dr. Cristo. He said that pain relief of 50% or greater is generally considered a criterion for long-term benefit, but clinicians should also consider favorable effects on other outcomes, such as quality of sleep or mood.
“For some patients who do not respond to first-line options, spinal cord stimulation can substantially reduce pain levels,” Dr. Cristo said. “I use these in my own practice most commonly for failed back pain syndrome, pelvic pain, and complex regional pain syndromes.”
Although spinal cord stimulation is not first-line, Dr. Cristo also cautioned that success appears to be better when this treatment is initiated within a reasonable time frame after chronic pain has been diagnosed.
“Specifically, there is an 85% success rate if the pain has been diagnosed within 2 years. If we wait more than 15 years after the chronic pain has been diagnosed, the success rate drops to 8%,” reported Dr. Cristo, citing a published analysis.
For primary care physicians with chronic pain patients, Dr. Cristo said, “I think they should be aware of this option, because it can be effective, but they should also be aware of the time frame in which it is most likely to offer relief.”
Dr. Cristo reports financial relationships with Algiarty, Grünenthal, Quest Diagnostics, and Recro Pharma. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned the same company.
LAKE BUENA VISTA, FLA. – Innovation in the design of spinal cord stimulators for control of chronic pain syndromes includes wireless devices to eliminate surgical implantation of the battery and devices with high frequency conduction to reduce paresthesias, according to an update at the Pain Care for Primary Care meeting.
“Paresthesias have been poorly tolerated by some patients, but this effect was not observed in a trial with high frequency stimulation,” explained Paul J. Cristo, MD, of the department of anesthesiology and critical care medicine, Johns Hopkins University, Baltimore.
Spinal cord stimulation for chronic pain control is not a new concept. The first device received Food and Drug Administration approval in 1989. Typically, these devices involve implantation of electrodes in the epidural space to deliver a low level of electricity that for many, but not all, individuals alleviates pain. With traditional devices, a battery and programmable generator is implanted in the buttock or abdomen to supply impulses.
Innovations since their introduction have included incremental reductions in the size of the generator and battery, facilitating implantation, and batteries that function longer or can be recharged. Some batteries now deliver stimulation for 10 or more years before they must be surgically replaced. In addition, several manufacturers now market devices that do not interfere with MRI.
The mechanism by which these devices relieve pain remains incompletely understood, according to Dr. Cristo. He cited animal studies that support several mechanisms, such as stimulation of A alpha and A beta afferent fibers to interrupt pain signals and release of serotonin and norepinephrine to inhibit pain transmission.
High-frequency stimulation
Up until last year, all devices provided neurostimulation through low frequency pulses. One of the major adverse effects has been a tingling sensation that some patients tolerate poorly. The first device to deliver high frequency pulses was approved by the FDA last year. According to Dr. Cristo, the advantage of high frequency energy is that it eliminates the risk of paresthesias.
In the registration trial with the high frequency device, which is now marketed under the name Senza by the Nevro Company, 198 chronic pain patients were randomized to an experimental or traditional device (Anesthesiology. 2015 Oct;123[4]:851-60). With the novel device, the spinal cord stimulation was delivered at a frequency of 10 kHz. Conventional spinal stimulators operate at frequencies of less than 100 Hz.
At 3 months, the proportion of patients classified as responders was greater for both back pain (84.5% vs. 43.8%) and leg pain (83.1% vs. 55.5%) when the novel and traditional devices were compared, but paresthesias, which were commonly reported on the traditional device, were not reported at all with the high frequency device.
Wireless stimulators
Wireless spinal stimulators are another relatively recent innovation with the potential to expand the proportion of chronic pain patients who may benefit from these devices. The first such device, marketed by Stimwave under the brand name Freedom SCS System, with a sister device for peripheral nerve stimulation called the StimQ PNS System, still requires implantation of a wire to deliver electrical stimulation, but the power source is external.
“The patient wears a belt that is equipped with a wireless programming device,” Dr. Cristo explained. He suggested that some may find the belt cumbersome, but a less invasive procedure is particularly attractive for patients with multiple comorbidities that make them poor candidates for device implantation.
Candidates for spinal cord stimulation are typically offered a trial to gauge response and tolerability before the device is implanted, according to Dr. Cristo. He said that pain relief of 50% or greater is generally considered a criterion for long-term benefit, but clinicians should also consider favorable effects on other outcomes, such as quality of sleep or mood.
“For some patients who do not respond to first-line options, spinal cord stimulation can substantially reduce pain levels,” Dr. Cristo said. “I use these in my own practice most commonly for failed back pain syndrome, pelvic pain, and complex regional pain syndromes.”
Although spinal cord stimulation is not first-line, Dr. Cristo also cautioned that success appears to be better when this treatment is initiated within a reasonable time frame after chronic pain has been diagnosed.
“Specifically, there is an 85% success rate if the pain has been diagnosed within 2 years. If we wait more than 15 years after the chronic pain has been diagnosed, the success rate drops to 8%,” reported Dr. Cristo, citing a published analysis.
For primary care physicians with chronic pain patients, Dr. Cristo said, “I think they should be aware of this option, because it can be effective, but they should also be aware of the time frame in which it is most likely to offer relief.”
Dr. Cristo reports financial relationships with Algiarty, Grünenthal, Quest Diagnostics, and Recro Pharma. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned the same company.
LAKE BUENA VISTA, FLA. – Innovation in the design of spinal cord stimulators for control of chronic pain syndromes includes wireless devices to eliminate surgical implantation of the battery and devices with high frequency conduction to reduce paresthesias, according to an update at the Pain Care for Primary Care meeting.
“Paresthesias have been poorly tolerated by some patients, but this effect was not observed in a trial with high frequency stimulation,” explained Paul J. Cristo, MD, of the department of anesthesiology and critical care medicine, Johns Hopkins University, Baltimore.
Spinal cord stimulation for chronic pain control is not a new concept. The first device received Food and Drug Administration approval in 1989. Typically, these devices involve implantation of electrodes in the epidural space to deliver a low level of electricity that for many, but not all, individuals alleviates pain. With traditional devices, a battery and programmable generator is implanted in the buttock or abdomen to supply impulses.
Innovations since their introduction have included incremental reductions in the size of the generator and battery, facilitating implantation, and batteries that function longer or can be recharged. Some batteries now deliver stimulation for 10 or more years before they must be surgically replaced. In addition, several manufacturers now market devices that do not interfere with MRI.
The mechanism by which these devices relieve pain remains incompletely understood, according to Dr. Cristo. He cited animal studies that support several mechanisms, such as stimulation of A alpha and A beta afferent fibers to interrupt pain signals and release of serotonin and norepinephrine to inhibit pain transmission.
High-frequency stimulation
Up until last year, all devices provided neurostimulation through low frequency pulses. One of the major adverse effects has been a tingling sensation that some patients tolerate poorly. The first device to deliver high frequency pulses was approved by the FDA last year. According to Dr. Cristo, the advantage of high frequency energy is that it eliminates the risk of paresthesias.
In the registration trial with the high frequency device, which is now marketed under the name Senza by the Nevro Company, 198 chronic pain patients were randomized to an experimental or traditional device (Anesthesiology. 2015 Oct;123[4]:851-60). With the novel device, the spinal cord stimulation was delivered at a frequency of 10 kHz. Conventional spinal stimulators operate at frequencies of less than 100 Hz.
At 3 months, the proportion of patients classified as responders was greater for both back pain (84.5% vs. 43.8%) and leg pain (83.1% vs. 55.5%) when the novel and traditional devices were compared, but paresthesias, which were commonly reported on the traditional device, were not reported at all with the high frequency device.
Wireless stimulators
Wireless spinal stimulators are another relatively recent innovation with the potential to expand the proportion of chronic pain patients who may benefit from these devices. The first such device, marketed by Stimwave under the brand name Freedom SCS System, with a sister device for peripheral nerve stimulation called the StimQ PNS System, still requires implantation of a wire to deliver electrical stimulation, but the power source is external.
“The patient wears a belt that is equipped with a wireless programming device,” Dr. Cristo explained. He suggested that some may find the belt cumbersome, but a less invasive procedure is particularly attractive for patients with multiple comorbidities that make them poor candidates for device implantation.
Candidates for spinal cord stimulation are typically offered a trial to gauge response and tolerability before the device is implanted, according to Dr. Cristo. He said that pain relief of 50% or greater is generally considered a criterion for long-term benefit, but clinicians should also consider favorable effects on other outcomes, such as quality of sleep or mood.
“For some patients who do not respond to first-line options, spinal cord stimulation can substantially reduce pain levels,” Dr. Cristo said. “I use these in my own practice most commonly for failed back pain syndrome, pelvic pain, and complex regional pain syndromes.”
Although spinal cord stimulation is not first-line, Dr. Cristo also cautioned that success appears to be better when this treatment is initiated within a reasonable time frame after chronic pain has been diagnosed.
“Specifically, there is an 85% success rate if the pain has been diagnosed within 2 years. If we wait more than 15 years after the chronic pain has been diagnosed, the success rate drops to 8%,” reported Dr. Cristo, citing a published analysis.
For primary care physicians with chronic pain patients, Dr. Cristo said, “I think they should be aware of this option, because it can be effective, but they should also be aware of the time frame in which it is most likely to offer relief.”
Dr. Cristo reports financial relationships with Algiarty, Grünenthal, Quest Diagnostics, and Recro Pharma. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned the same company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
10 Strategies for Delivering a Great Presentation
It’s noon on Tuesday, and James, a new PGY-2 resident, begins his presentation on COPD. After five minutes, you notice half of the residents playing Words with Friends, the “ortho-bound” medical student talking with a buddy in the back, and the attendings looking on with innate skepticism.
Your talk on atrial fibrillation is next month, and just watching James brings on palpitations of your own. So what do you do?
Introduction
Public speaking is a near certainty for most of us regardless of training stage. A well-executed presentation establishes the clinician as an institutional authority, adroitly educating anyone around you.
So how can you deliver that killer update on atrial fibrillation? Here, we provide you with 10 tips for preparing and delivering a great presentation.
Preparation
1. Consider the audience and what they already know. No matter how interesting we think we are, if we don’t present with the audience’s needs in mind, we might as well be talking to an empty room. Consider what the audience may or may not know about the topic; this allows you to decide whether to give a comprehensive didactic on atrial fibrillation for trainees or an anticoagulation update for cardiologists. Great presenters survey their audience early on with a question such as, “How many of you here know the results of the AFFIRM trial?” This allows you to make small alterations to meet the needs of your audience.
2. Visualize the stage and setting. Understanding the stage helps you anticipate and address barriers to learning. Imagine for a moment the difference in these two scenarios: a discussion of hyponatremia with a group of medical students at 4 p.m. in a dark room versus a discussion on principles of atrial fibrillation management at 11 a.m. in an auditorium. Both require interaction, although an auditorium-based presentation requires testing your audio-visual equipment in advance.
3. Determine your objectives. To determine your objectives, begin with the end in mind. If you were to visualize your audience members at the end of the talk, what would they know (knowledge), be able to do (behavior), or have a new outlook on (attitude)? The objectives will determine the content you deliver and the activities for learning. For a one-hour presentation, identifying three to five objectives is a good rule of thumb.
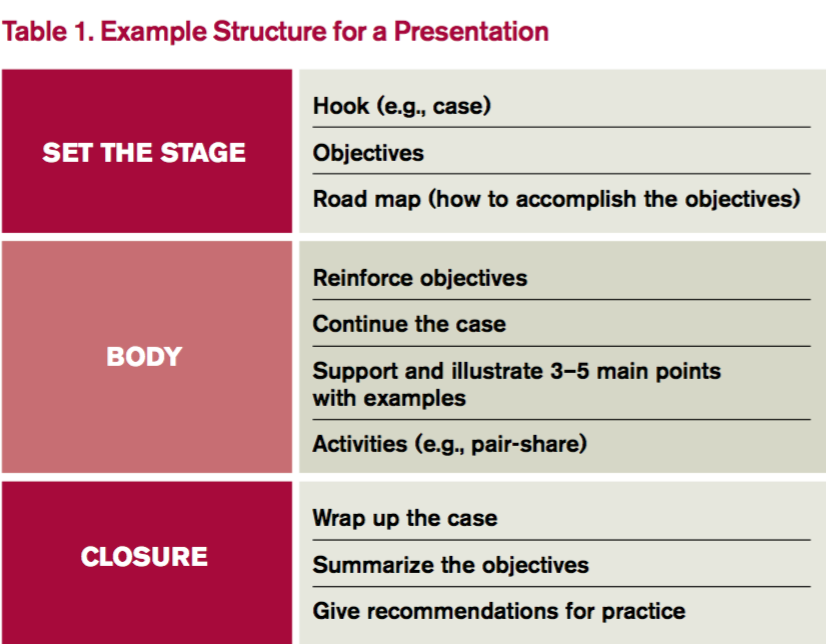
4. Build your presentation. Whether using PowerPoint, Prezi, or a white board, “build” the presentation from the objectives. Table 1 outlines one example format; Figure 1 outlines some best practices of PowerPoint.
Humans evolved to interpret visual imagery, not read text, so try to use pictures instead of bullet points. Consider first building slides with text and then using an internet search engine to convert words to pictures. For example, “atrial rate 200 bpm” is better displayed with an actual ECG.
5. Practice. Practicing helps you become more comfortable with the content itself as well as how to present that content. If you can, practice with a colleague and receive feedback to sharpen your material. No time to spare? Practice the introduction and any major point that you want to get across. Audiences decide within the first five minutes whether your talk is worth listening to before pulling out their cellphones to open up Facebook.
Delivery
1. Confront nervousness. Many of us become nervous when speaking in front of an audience. To address this, it’s perfectly reasonable to rehearse a presentation at home or in a quiet call room ahead of time. If you feel extremely nervous, breathe deeply for five- to 10-second intervals. During the presentation itself, find friendly or familiar faces in the audience and look them in the eyes as you speak. This eases nerves and improves your technique.
2. Hook your audience. The purpose of the hook is to “grab” the attention of the audience. The best presenters intrigue the audience with a story or problem at the outset and use the presentation to address that problem. Consider the differences in these openings:
- “As of 2010, atrial fibrillation has affected 33.5 million Americans each year, with a reported prevalence of stroke of 2.8% to 24.2%.”
- “Sarah is a 67-year-old woman with a history of atrial fibrillation who loved to play the piano until she experienced a stroke, paralysis of her left arm, and the end of her career as a pianist. Today, I’m going to teach you how to reduce the risk of stroke in your patients with atrial fibrillation.”
Then as the presentation proceeds, develop the case to keep the audience thinking about their differential diagnosis or management strategy.
3. Speak clearly. We all use fillers such as “uh” and “um” without noticing. To learn to speak well, practice as much as possible and ask for feedback on your diction. Consider watching TED Talks, short clips of fascinating stories whose presenters are highly coached in public speaking. Use specific statements to key in the audience on important points, such as, “If you remember anything from this talk, I want you to remember …”
Remember, too, that public speaking requires enthusiasm. There’s nothing worse than beginning with, “I know that you all have heard about atrial fibrillation 500 times, so let’s just get through this.” The energy of the audience reflects the energy of the speaker.
4. Facilitate learning. Don’t do all of the talking; in fact, let the audience talk for you. For audience members to learn, they must engage with the material. Use a question/answer forum such as polleverywhere.com, where the audience responds in real time. Alternatively, pose a scenario to discuss using a pair-share technique. For a talk on atrial fibrillation, give direction to “turn to a neighbor and discuss anticoagulation for Mr. Jones, a 66-year-old man with cirrhosis, CVA, and hypertension admitted with atrial fibrillation.” Debrief this activity to solicit thoughts from the audience and then address the scenario.
5. Break the glass. Don’t hide behind the podium! “Breaking the glass” means stepping away from the podium to create an experience more akin to a dialogue. Remember, the audience is interested in hearing what you have to say—otherwise, they would have read about atrial fibrillation from UpToDate. Stepping away from the podium breaks the expected monotony and can help burn nervous energy.
Bottom Line
A fantastic presentation requires preparation and a thoughtful delivery. Spend the time to prepare. After all, that upcoming presentation on atrial fibrillation is only one month away. It will arrive sooner than you think. TH
Dr. Rendon is associate program director and Dr. Roesch is an assistant professor in the Division of Hospital Medicine at the University of New Mexico Hospital in Albuquerque. They are co-directors of the medical student clinical reasoning course. Both are members of SHM’s Physicians in Training Committee.
References
- Anderson C. How to give a killer presentation. Harvard Business Review. June 2013.
- Covey C. The 7 Habits of Highly Effective People. Franklin Covey Co.; 2004.
- Ganz L. Epidemiology of and risk factors for atrial fibrillation. Updated October 15, 2015.
- Sharpe B. How to give a great talk. Presented as part of SHM national conference; 2014; Las Vegas.
- Skeff K, Stratos G. Methods for Teaching Medicine. Philadelphia: ACP Press; 2010.
It’s noon on Tuesday, and James, a new PGY-2 resident, begins his presentation on COPD. After five minutes, you notice half of the residents playing Words with Friends, the “ortho-bound” medical student talking with a buddy in the back, and the attendings looking on with innate skepticism.
Your talk on atrial fibrillation is next month, and just watching James brings on palpitations of your own. So what do you do?
Introduction
Public speaking is a near certainty for most of us regardless of training stage. A well-executed presentation establishes the clinician as an institutional authority, adroitly educating anyone around you.
So how can you deliver that killer update on atrial fibrillation? Here, we provide you with 10 tips for preparing and delivering a great presentation.
Preparation
1. Consider the audience and what they already know. No matter how interesting we think we are, if we don’t present with the audience’s needs in mind, we might as well be talking to an empty room. Consider what the audience may or may not know about the topic; this allows you to decide whether to give a comprehensive didactic on atrial fibrillation for trainees or an anticoagulation update for cardiologists. Great presenters survey their audience early on with a question such as, “How many of you here know the results of the AFFIRM trial?” This allows you to make small alterations to meet the needs of your audience.
2. Visualize the stage and setting. Understanding the stage helps you anticipate and address barriers to learning. Imagine for a moment the difference in these two scenarios: a discussion of hyponatremia with a group of medical students at 4 p.m. in a dark room versus a discussion on principles of atrial fibrillation management at 11 a.m. in an auditorium. Both require interaction, although an auditorium-based presentation requires testing your audio-visual equipment in advance.
3. Determine your objectives. To determine your objectives, begin with the end in mind. If you were to visualize your audience members at the end of the talk, what would they know (knowledge), be able to do (behavior), or have a new outlook on (attitude)? The objectives will determine the content you deliver and the activities for learning. For a one-hour presentation, identifying three to five objectives is a good rule of thumb.
4. Build your presentation. Whether using PowerPoint, Prezi, or a white board, “build” the presentation from the objectives. Table 1 outlines one example format; Figure 1 outlines some best practices of PowerPoint.
Humans evolved to interpret visual imagery, not read text, so try to use pictures instead of bullet points. Consider first building slides with text and then using an internet search engine to convert words to pictures. For example, “atrial rate 200 bpm” is better displayed with an actual ECG.
5. Practice. Practicing helps you become more comfortable with the content itself as well as how to present that content. If you can, practice with a colleague and receive feedback to sharpen your material. No time to spare? Practice the introduction and any major point that you want to get across. Audiences decide within the first five minutes whether your talk is worth listening to before pulling out their cellphones to open up Facebook.
Delivery
1. Confront nervousness. Many of us become nervous when speaking in front of an audience. To address this, it’s perfectly reasonable to rehearse a presentation at home or in a quiet call room ahead of time. If you feel extremely nervous, breathe deeply for five- to 10-second intervals. During the presentation itself, find friendly or familiar faces in the audience and look them in the eyes as you speak. This eases nerves and improves your technique.
2. Hook your audience. The purpose of the hook is to “grab” the attention of the audience. The best presenters intrigue the audience with a story or problem at the outset and use the presentation to address that problem. Consider the differences in these openings:
- “As of 2010, atrial fibrillation has affected 33.5 million Americans each year, with a reported prevalence of stroke of 2.8% to 24.2%.”
- “Sarah is a 67-year-old woman with a history of atrial fibrillation who loved to play the piano until she experienced a stroke, paralysis of her left arm, and the end of her career as a pianist. Today, I’m going to teach you how to reduce the risk of stroke in your patients with atrial fibrillation.”
Then as the presentation proceeds, develop the case to keep the audience thinking about their differential diagnosis or management strategy.
3. Speak clearly. We all use fillers such as “uh” and “um” without noticing. To learn to speak well, practice as much as possible and ask for feedback on your diction. Consider watching TED Talks, short clips of fascinating stories whose presenters are highly coached in public speaking. Use specific statements to key in the audience on important points, such as, “If you remember anything from this talk, I want you to remember …”
Remember, too, that public speaking requires enthusiasm. There’s nothing worse than beginning with, “I know that you all have heard about atrial fibrillation 500 times, so let’s just get through this.” The energy of the audience reflects the energy of the speaker.
4. Facilitate learning. Don’t do all of the talking; in fact, let the audience talk for you. For audience members to learn, they must engage with the material. Use a question/answer forum such as polleverywhere.com, where the audience responds in real time. Alternatively, pose a scenario to discuss using a pair-share technique. For a talk on atrial fibrillation, give direction to “turn to a neighbor and discuss anticoagulation for Mr. Jones, a 66-year-old man with cirrhosis, CVA, and hypertension admitted with atrial fibrillation.” Debrief this activity to solicit thoughts from the audience and then address the scenario.
5. Break the glass. Don’t hide behind the podium! “Breaking the glass” means stepping away from the podium to create an experience more akin to a dialogue. Remember, the audience is interested in hearing what you have to say—otherwise, they would have read about atrial fibrillation from UpToDate. Stepping away from the podium breaks the expected monotony and can help burn nervous energy.
Bottom Line
A fantastic presentation requires preparation and a thoughtful delivery. Spend the time to prepare. After all, that upcoming presentation on atrial fibrillation is only one month away. It will arrive sooner than you think. TH
Dr. Rendon is associate program director and Dr. Roesch is an assistant professor in the Division of Hospital Medicine at the University of New Mexico Hospital in Albuquerque. They are co-directors of the medical student clinical reasoning course. Both are members of SHM’s Physicians in Training Committee.
References
- Anderson C. How to give a killer presentation. Harvard Business Review. June 2013.
- Covey C. The 7 Habits of Highly Effective People. Franklin Covey Co.; 2004.
- Ganz L. Epidemiology of and risk factors for atrial fibrillation. Updated October 15, 2015.
- Sharpe B. How to give a great talk. Presented as part of SHM national conference; 2014; Las Vegas.
- Skeff K, Stratos G. Methods for Teaching Medicine. Philadelphia: ACP Press; 2010.
It’s noon on Tuesday, and James, a new PGY-2 resident, begins his presentation on COPD. After five minutes, you notice half of the residents playing Words with Friends, the “ortho-bound” medical student talking with a buddy in the back, and the attendings looking on with innate skepticism.
Your talk on atrial fibrillation is next month, and just watching James brings on palpitations of your own. So what do you do?
Introduction
Public speaking is a near certainty for most of us regardless of training stage. A well-executed presentation establishes the clinician as an institutional authority, adroitly educating anyone around you.
So how can you deliver that killer update on atrial fibrillation? Here, we provide you with 10 tips for preparing and delivering a great presentation.
Preparation
1. Consider the audience and what they already know. No matter how interesting we think we are, if we don’t present with the audience’s needs in mind, we might as well be talking to an empty room. Consider what the audience may or may not know about the topic; this allows you to decide whether to give a comprehensive didactic on atrial fibrillation for trainees or an anticoagulation update for cardiologists. Great presenters survey their audience early on with a question such as, “How many of you here know the results of the AFFIRM trial?” This allows you to make small alterations to meet the needs of your audience.
2. Visualize the stage and setting. Understanding the stage helps you anticipate and address barriers to learning. Imagine for a moment the difference in these two scenarios: a discussion of hyponatremia with a group of medical students at 4 p.m. in a dark room versus a discussion on principles of atrial fibrillation management at 11 a.m. in an auditorium. Both require interaction, although an auditorium-based presentation requires testing your audio-visual equipment in advance.
3. Determine your objectives. To determine your objectives, begin with the end in mind. If you were to visualize your audience members at the end of the talk, what would they know (knowledge), be able to do (behavior), or have a new outlook on (attitude)? The objectives will determine the content you deliver and the activities for learning. For a one-hour presentation, identifying three to five objectives is a good rule of thumb.
4. Build your presentation. Whether using PowerPoint, Prezi, or a white board, “build” the presentation from the objectives. Table 1 outlines one example format; Figure 1 outlines some best practices of PowerPoint.
Humans evolved to interpret visual imagery, not read text, so try to use pictures instead of bullet points. Consider first building slides with text and then using an internet search engine to convert words to pictures. For example, “atrial rate 200 bpm” is better displayed with an actual ECG.
5. Practice. Practicing helps you become more comfortable with the content itself as well as how to present that content. If you can, practice with a colleague and receive feedback to sharpen your material. No time to spare? Practice the introduction and any major point that you want to get across. Audiences decide within the first five minutes whether your talk is worth listening to before pulling out their cellphones to open up Facebook.
Delivery
1. Confront nervousness. Many of us become nervous when speaking in front of an audience. To address this, it’s perfectly reasonable to rehearse a presentation at home or in a quiet call room ahead of time. If you feel extremely nervous, breathe deeply for five- to 10-second intervals. During the presentation itself, find friendly or familiar faces in the audience and look them in the eyes as you speak. This eases nerves and improves your technique.
2. Hook your audience. The purpose of the hook is to “grab” the attention of the audience. The best presenters intrigue the audience with a story or problem at the outset and use the presentation to address that problem. Consider the differences in these openings:
- “As of 2010, atrial fibrillation has affected 33.5 million Americans each year, with a reported prevalence of stroke of 2.8% to 24.2%.”
- “Sarah is a 67-year-old woman with a history of atrial fibrillation who loved to play the piano until she experienced a stroke, paralysis of her left arm, and the end of her career as a pianist. Today, I’m going to teach you how to reduce the risk of stroke in your patients with atrial fibrillation.”
Then as the presentation proceeds, develop the case to keep the audience thinking about their differential diagnosis or management strategy.
3. Speak clearly. We all use fillers such as “uh” and “um” without noticing. To learn to speak well, practice as much as possible and ask for feedback on your diction. Consider watching TED Talks, short clips of fascinating stories whose presenters are highly coached in public speaking. Use specific statements to key in the audience on important points, such as, “If you remember anything from this talk, I want you to remember …”
Remember, too, that public speaking requires enthusiasm. There’s nothing worse than beginning with, “I know that you all have heard about atrial fibrillation 500 times, so let’s just get through this.” The energy of the audience reflects the energy of the speaker.
4. Facilitate learning. Don’t do all of the talking; in fact, let the audience talk for you. For audience members to learn, they must engage with the material. Use a question/answer forum such as polleverywhere.com, where the audience responds in real time. Alternatively, pose a scenario to discuss using a pair-share technique. For a talk on atrial fibrillation, give direction to “turn to a neighbor and discuss anticoagulation for Mr. Jones, a 66-year-old man with cirrhosis, CVA, and hypertension admitted with atrial fibrillation.” Debrief this activity to solicit thoughts from the audience and then address the scenario.
5. Break the glass. Don’t hide behind the podium! “Breaking the glass” means stepping away from the podium to create an experience more akin to a dialogue. Remember, the audience is interested in hearing what you have to say—otherwise, they would have read about atrial fibrillation from UpToDate. Stepping away from the podium breaks the expected monotony and can help burn nervous energy.
Bottom Line
A fantastic presentation requires preparation and a thoughtful delivery. Spend the time to prepare. After all, that upcoming presentation on atrial fibrillation is only one month away. It will arrive sooner than you think. TH
Dr. Rendon is associate program director and Dr. Roesch is an assistant professor in the Division of Hospital Medicine at the University of New Mexico Hospital in Albuquerque. They are co-directors of the medical student clinical reasoning course. Both are members of SHM’s Physicians in Training Committee.
References
- Anderson C. How to give a killer presentation. Harvard Business Review. June 2013.
- Covey C. The 7 Habits of Highly Effective People. Franklin Covey Co.; 2004.
- Ganz L. Epidemiology of and risk factors for atrial fibrillation. Updated October 15, 2015.
- Sharpe B. How to give a great talk. Presented as part of SHM national conference; 2014; Las Vegas.
- Skeff K, Stratos G. Methods for Teaching Medicine. Philadelphia: ACP Press; 2010.
Products granted orphan designation for use in HSCT
The European Commission has granted orphan drug designation for the T-cell therapy product candidate BPX-501 and the small molecule rimiducid.
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate the T cells in the event of toxicity.
Rimiducid is used to activate the CaspaCIDe safety switch, which consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway.
The goal of this therapy is to allow physicians to more safely perform haploidentical hematopoietic stem cell transplant (haplo-HSCT).
Haplo-HSCT recipients receive BPX-501 to speed immune reconstitution and provide control over viral infections. And rimiducid is used to eliminate BPX-501 alloreactive T cells if severe graft-vs-host disease (GVHD) occurs.
If a patient develops severe GVHD, rimiducid is used to trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About orphan designation
Orphan drug designation from the European Commission provides regulatory and financial incentives for companies to develop and market therapies that treat serious or life-threatening conditions that affect no more than 5 in 10,000 people in the European Union (EU), and where no treatment is currently approved.
In addition to a 10-year period of marketing exclusivity in the EU upon product approval, orphan drug designation provides fee waivers, protocol assistance, and marketing authorization under the centralized procedure granting approval in all EU countries.
BPX-501/rimiducid development
BPX-501 and rimiducid are being developed by Bellicum Pharmaceuticals.
The company has met with regulatory authorities in Europe to discuss the potential approval pathway for BPX-501 and rimiducid for the treatment of immunodeficiency and GVHD following haplo-HSCT in pediatric patients with leukemias, lymphomas, and rare inherited blood diseases who do not have a matched donor.
These discussions have resulted in an initial agreement regarding the company’s development plans, subject to further refinement in a formal protocol assistance process that is available for orphan drug products.
Based on regulatory discussions, Bellicum believes that data from the European arm of its BP-004 trial, with a 6-month follow-up time and expanded to enroll additional patients, could form the basis of marketing authorization applications for BPX-501 and rimiducid.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has agreed that review and approval under “exceptional circumstances” may be suitable, recognizing that a randomized trial may not be feasible in the pediatric setting. In place of a randomized trial, Bellicum intends to collect data from a concurrent observational study of allogeneic HSCT outcomes in the pediatric setting.
The European Medicines Agency can grant early market authorization to orphan drug products under exceptional circumstances. Exceptional circumstances can be granted for medicines that treat very rare diseases or where controlled studies are impractical or not consistent with accepted principles of medical ethics.
BP-004 trial
BP-004 is a phase 1/2 dose-escalation trial of BPX-501 and rimiducid in pediatric patients with malignant and nonmalignant diseases. Interim results from this trial were reported in 2 presentations at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation in April 2016.
One presentation involved patients with acute leukemia who received BPX-501 after haplo-HSCT. At a median follow-up of 7 months, 16 of the 17 patients were alive and disease-free. There were several cases of GVHD, but nearly all were resolved.
The other presentation covered patients with nonmalignant disorders who received BPX-501 after haplo-HSCT. At a median follow-up of 7 months, all 24 patients studied were still alive and disease-free. The incidence of GVHD was considered “very low.” ![]()
The European Commission has granted orphan drug designation for the T-cell therapy product candidate BPX-501 and the small molecule rimiducid.
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate the T cells in the event of toxicity.
Rimiducid is used to activate the CaspaCIDe safety switch, which consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway.
The goal of this therapy is to allow physicians to more safely perform haploidentical hematopoietic stem cell transplant (haplo-HSCT).
Haplo-HSCT recipients receive BPX-501 to speed immune reconstitution and provide control over viral infections. And rimiducid is used to eliminate BPX-501 alloreactive T cells if severe graft-vs-host disease (GVHD) occurs.
If a patient develops severe GVHD, rimiducid is used to trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About orphan designation
Orphan drug designation from the European Commission provides regulatory and financial incentives for companies to develop and market therapies that treat serious or life-threatening conditions that affect no more than 5 in 10,000 people in the European Union (EU), and where no treatment is currently approved.
In addition to a 10-year period of marketing exclusivity in the EU upon product approval, orphan drug designation provides fee waivers, protocol assistance, and marketing authorization under the centralized procedure granting approval in all EU countries.
BPX-501/rimiducid development
BPX-501 and rimiducid are being developed by Bellicum Pharmaceuticals.
The company has met with regulatory authorities in Europe to discuss the potential approval pathway for BPX-501 and rimiducid for the treatment of immunodeficiency and GVHD following haplo-HSCT in pediatric patients with leukemias, lymphomas, and rare inherited blood diseases who do not have a matched donor.
These discussions have resulted in an initial agreement regarding the company’s development plans, subject to further refinement in a formal protocol assistance process that is available for orphan drug products.
Based on regulatory discussions, Bellicum believes that data from the European arm of its BP-004 trial, with a 6-month follow-up time and expanded to enroll additional patients, could form the basis of marketing authorization applications for BPX-501 and rimiducid.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has agreed that review and approval under “exceptional circumstances” may be suitable, recognizing that a randomized trial may not be feasible in the pediatric setting. In place of a randomized trial, Bellicum intends to collect data from a concurrent observational study of allogeneic HSCT outcomes in the pediatric setting.
The European Medicines Agency can grant early market authorization to orphan drug products under exceptional circumstances. Exceptional circumstances can be granted for medicines that treat very rare diseases or where controlled studies are impractical or not consistent with accepted principles of medical ethics.
BP-004 trial
BP-004 is a phase 1/2 dose-escalation trial of BPX-501 and rimiducid in pediatric patients with malignant and nonmalignant diseases. Interim results from this trial were reported in 2 presentations at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation in April 2016.
One presentation involved patients with acute leukemia who received BPX-501 after haplo-HSCT. At a median follow-up of 7 months, 16 of the 17 patients were alive and disease-free. There were several cases of GVHD, but nearly all were resolved.
The other presentation covered patients with nonmalignant disorders who received BPX-501 after haplo-HSCT. At a median follow-up of 7 months, all 24 patients studied were still alive and disease-free. The incidence of GVHD was considered “very low.” ![]()
The European Commission has granted orphan drug designation for the T-cell therapy product candidate BPX-501 and the small molecule rimiducid.
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate the T cells in the event of toxicity.
Rimiducid is used to activate the CaspaCIDe safety switch, which consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway.
The goal of this therapy is to allow physicians to more safely perform haploidentical hematopoietic stem cell transplant (haplo-HSCT).
Haplo-HSCT recipients receive BPX-501 to speed immune reconstitution and provide control over viral infections. And rimiducid is used to eliminate BPX-501 alloreactive T cells if severe graft-vs-host disease (GVHD) occurs.
If a patient develops severe GVHD, rimiducid is used to trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About orphan designation
Orphan drug designation from the European Commission provides regulatory and financial incentives for companies to develop and market therapies that treat serious or life-threatening conditions that affect no more than 5 in 10,000 people in the European Union (EU), and where no treatment is currently approved.
In addition to a 10-year period of marketing exclusivity in the EU upon product approval, orphan drug designation provides fee waivers, protocol assistance, and marketing authorization under the centralized procedure granting approval in all EU countries.
BPX-501/rimiducid development
BPX-501 and rimiducid are being developed by Bellicum Pharmaceuticals.
The company has met with regulatory authorities in Europe to discuss the potential approval pathway for BPX-501 and rimiducid for the treatment of immunodeficiency and GVHD following haplo-HSCT in pediatric patients with leukemias, lymphomas, and rare inherited blood diseases who do not have a matched donor.
These discussions have resulted in an initial agreement regarding the company’s development plans, subject to further refinement in a formal protocol assistance process that is available for orphan drug products.
Based on regulatory discussions, Bellicum believes that data from the European arm of its BP-004 trial, with a 6-month follow-up time and expanded to enroll additional patients, could form the basis of marketing authorization applications for BPX-501 and rimiducid.
The European Medicines Agency’s Committee for Medicinal Products for Human Use has agreed that review and approval under “exceptional circumstances” may be suitable, recognizing that a randomized trial may not be feasible in the pediatric setting. In place of a randomized trial, Bellicum intends to collect data from a concurrent observational study of allogeneic HSCT outcomes in the pediatric setting.
The European Medicines Agency can grant early market authorization to orphan drug products under exceptional circumstances. Exceptional circumstances can be granted for medicines that treat very rare diseases or where controlled studies are impractical or not consistent with accepted principles of medical ethics.
BP-004 trial
BP-004 is a phase 1/2 dose-escalation trial of BPX-501 and rimiducid in pediatric patients with malignant and nonmalignant diseases. Interim results from this trial were reported in 2 presentations at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation in April 2016.
One presentation involved patients with acute leukemia who received BPX-501 after haplo-HSCT. At a median follow-up of 7 months, 16 of the 17 patients were alive and disease-free. There were several cases of GVHD, but nearly all were resolved.
The other presentation covered patients with nonmalignant disorders who received BPX-501 after haplo-HSCT. At a median follow-up of 7 months, all 24 patients studied were still alive and disease-free. The incidence of GVHD was considered “very low.” ![]()
Core curriculum for opioid prescribing preempts certification
LAKE BUENA VISTA, FLA. – If the Food and Drug Administration moves to require some form of certification to prescribe opioids, an educational program based on the FDA’s own risk evaluation and mitigation strategy (REMS) report of 2012 is already in place to fulfill that purpose, according to a pain specialist who explained the principles of this program at Pain Care for Primary Care.
Some form of certification process for opioid prescribing has been discussed for many years, explained David E. J. Bazzo, MD, clinical professor of family medicine at the University of California, San Diego, but “there has been renewed interest as a result of the continuing public health crisis regarding opioid overdoses.”
The core curriculum provided by the Collaborative for REMS Education (CO*RE) curriculum is based on clinical competencies to meet the requirements of the FDA REMS blueprint, which was issued in July 2012. The focus of REMS is on opioids with an extended-release (ER) or long-acting (LA) formulation. It is not the only such program available, but Dr. Bazzo noted that CO*RE was actually initiated in 2010 and provided some of the basis for the FDA REMS that followed 2 years later.
The CO*RE program, which can be completed at no cost, is available online. Those who complete the program are provided with a completer certificate, which Dr. Bazzo expects to be honored by the FDA if it does decide to require proof of competency. The program involves the participation of 13 organizations, such as the American Pain Society (APS), the American Society of Addiction Medicine (ASAM), and the American College of Emergency Physicians (ACEP).
There is a substantial possibility that the FDA will move to require competency for opioid prescribers. At a recent joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee in which modifications in the REMS were discussed, the majority of those on both advisory committees endorsed mandatory training.
“Why is education so important? It is because we are in a super tough position,” Dr. Bazzo explained. He suggested that physicians need effective agents for controlling pain, but the mortality and morbidity associated with opioids has steadily increased despite the FDA REMS.
“Deaths due to drug abuse or poisoning are just the beginning. For every death, there are 10 treatment admissions for abuse, and 130 people who are addicted,” said Dr. Bazzo, citing data from the Centers for Disease Control and Prevention (CDC).
In addition to an introduction, which outlines the goals of appropriate opioid prescribing, the CO*RE curriculum consists of six units. The first focuses on assessing candidates for opioid therapy, which includes instructions on history taking and documenting the findings. Tools for assessing risks posed by opioids, particularly for abuse, are also outlined.
The second unit describes best practices for initiating therapy, modifying doses, and discontinuing opioids, the third unit provides information on monitoring adherence and aberrant behavior in patients, and the fourth unit provides information about how to counsel patients about what constitutes appropriate use of opioids and the risks of inappropriate use. The fifth unit is largely an overview of key information in the previous four units and the sixth unit outlines the specific features of currently available opioids.
“The bottom line for the program overall is learning how to balance risks and benefits,” Dr. Bazzo explained. He suggested that many clinicians do not fully grasp that opioids are not stand-alone medications but a tool within a more comprehensive strategy for improving function.
“When we give analgesics for chronic pain, the goal is not to eliminate pain. If this is your goal, you will fail 99.9999% of the time. The goal is to improve function,” Dr. Bazzo said. He characterized treatment of chronic pain “as a team sport” that involves a collaboration between specialists and patients to achieve specific endpoints.
“It is a little like treating hypertension. You adjust medications until you get to the goal,” Dr. Bazzo said. This involves defining the goal before the treatment is initiated and then documenting progress toward that goal to guide treatment strategies.
The CO*RE REMS program is consistent with the CDC Guideline for Prescribing Opioids for Chronic Pain (MMWR. 65[1];1–49). It is part of a nationwide effort to reduce deaths related to opioid use.
“The problems with opioids can be greatly reduced if clinicians recognize and adhere to best practices,” Dr. Bazzo maintained.
Dr. Bazzo reports no potential financial conflicts of interest. The meeting was held by the APS and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
LAKE BUENA VISTA, FLA. – If the Food and Drug Administration moves to require some form of certification to prescribe opioids, an educational program based on the FDA’s own risk evaluation and mitigation strategy (REMS) report of 2012 is already in place to fulfill that purpose, according to a pain specialist who explained the principles of this program at Pain Care for Primary Care.
Some form of certification process for opioid prescribing has been discussed for many years, explained David E. J. Bazzo, MD, clinical professor of family medicine at the University of California, San Diego, but “there has been renewed interest as a result of the continuing public health crisis regarding opioid overdoses.”
The core curriculum provided by the Collaborative for REMS Education (CO*RE) curriculum is based on clinical competencies to meet the requirements of the FDA REMS blueprint, which was issued in July 2012. The focus of REMS is on opioids with an extended-release (ER) or long-acting (LA) formulation. It is not the only such program available, but Dr. Bazzo noted that CO*RE was actually initiated in 2010 and provided some of the basis for the FDA REMS that followed 2 years later.
The CO*RE program, which can be completed at no cost, is available online. Those who complete the program are provided with a completer certificate, which Dr. Bazzo expects to be honored by the FDA if it does decide to require proof of competency. The program involves the participation of 13 organizations, such as the American Pain Society (APS), the American Society of Addiction Medicine (ASAM), and the American College of Emergency Physicians (ACEP).
There is a substantial possibility that the FDA will move to require competency for opioid prescribers. At a recent joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee in which modifications in the REMS were discussed, the majority of those on both advisory committees endorsed mandatory training.
“Why is education so important? It is because we are in a super tough position,” Dr. Bazzo explained. He suggested that physicians need effective agents for controlling pain, but the mortality and morbidity associated with opioids has steadily increased despite the FDA REMS.
“Deaths due to drug abuse or poisoning are just the beginning. For every death, there are 10 treatment admissions for abuse, and 130 people who are addicted,” said Dr. Bazzo, citing data from the Centers for Disease Control and Prevention (CDC).
In addition to an introduction, which outlines the goals of appropriate opioid prescribing, the CO*RE curriculum consists of six units. The first focuses on assessing candidates for opioid therapy, which includes instructions on history taking and documenting the findings. Tools for assessing risks posed by opioids, particularly for abuse, are also outlined.
The second unit describes best practices for initiating therapy, modifying doses, and discontinuing opioids, the third unit provides information on monitoring adherence and aberrant behavior in patients, and the fourth unit provides information about how to counsel patients about what constitutes appropriate use of opioids and the risks of inappropriate use. The fifth unit is largely an overview of key information in the previous four units and the sixth unit outlines the specific features of currently available opioids.
“The bottom line for the program overall is learning how to balance risks and benefits,” Dr. Bazzo explained. He suggested that many clinicians do not fully grasp that opioids are not stand-alone medications but a tool within a more comprehensive strategy for improving function.
“When we give analgesics for chronic pain, the goal is not to eliminate pain. If this is your goal, you will fail 99.9999% of the time. The goal is to improve function,” Dr. Bazzo said. He characterized treatment of chronic pain “as a team sport” that involves a collaboration between specialists and patients to achieve specific endpoints.
“It is a little like treating hypertension. You adjust medications until you get to the goal,” Dr. Bazzo said. This involves defining the goal before the treatment is initiated and then documenting progress toward that goal to guide treatment strategies.
The CO*RE REMS program is consistent with the CDC Guideline for Prescribing Opioids for Chronic Pain (MMWR. 65[1];1–49). It is part of a nationwide effort to reduce deaths related to opioid use.
“The problems with opioids can be greatly reduced if clinicians recognize and adhere to best practices,” Dr. Bazzo maintained.
Dr. Bazzo reports no potential financial conflicts of interest. The meeting was held by the APS and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
LAKE BUENA VISTA, FLA. – If the Food and Drug Administration moves to require some form of certification to prescribe opioids, an educational program based on the FDA’s own risk evaluation and mitigation strategy (REMS) report of 2012 is already in place to fulfill that purpose, according to a pain specialist who explained the principles of this program at Pain Care for Primary Care.
Some form of certification process for opioid prescribing has been discussed for many years, explained David E. J. Bazzo, MD, clinical professor of family medicine at the University of California, San Diego, but “there has been renewed interest as a result of the continuing public health crisis regarding opioid overdoses.”
The core curriculum provided by the Collaborative for REMS Education (CO*RE) curriculum is based on clinical competencies to meet the requirements of the FDA REMS blueprint, which was issued in July 2012. The focus of REMS is on opioids with an extended-release (ER) or long-acting (LA) formulation. It is not the only such program available, but Dr. Bazzo noted that CO*RE was actually initiated in 2010 and provided some of the basis for the FDA REMS that followed 2 years later.
The CO*RE program, which can be completed at no cost, is available online. Those who complete the program are provided with a completer certificate, which Dr. Bazzo expects to be honored by the FDA if it does decide to require proof of competency. The program involves the participation of 13 organizations, such as the American Pain Society (APS), the American Society of Addiction Medicine (ASAM), and the American College of Emergency Physicians (ACEP).
There is a substantial possibility that the FDA will move to require competency for opioid prescribers. At a recent joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee in which modifications in the REMS were discussed, the majority of those on both advisory committees endorsed mandatory training.
“Why is education so important? It is because we are in a super tough position,” Dr. Bazzo explained. He suggested that physicians need effective agents for controlling pain, but the mortality and morbidity associated with opioids has steadily increased despite the FDA REMS.
“Deaths due to drug abuse or poisoning are just the beginning. For every death, there are 10 treatment admissions for abuse, and 130 people who are addicted,” said Dr. Bazzo, citing data from the Centers for Disease Control and Prevention (CDC).
In addition to an introduction, which outlines the goals of appropriate opioid prescribing, the CO*RE curriculum consists of six units. The first focuses on assessing candidates for opioid therapy, which includes instructions on history taking and documenting the findings. Tools for assessing risks posed by opioids, particularly for abuse, are also outlined.
The second unit describes best practices for initiating therapy, modifying doses, and discontinuing opioids, the third unit provides information on monitoring adherence and aberrant behavior in patients, and the fourth unit provides information about how to counsel patients about what constitutes appropriate use of opioids and the risks of inappropriate use. The fifth unit is largely an overview of key information in the previous four units and the sixth unit outlines the specific features of currently available opioids.
“The bottom line for the program overall is learning how to balance risks and benefits,” Dr. Bazzo explained. He suggested that many clinicians do not fully grasp that opioids are not stand-alone medications but a tool within a more comprehensive strategy for improving function.
“When we give analgesics for chronic pain, the goal is not to eliminate pain. If this is your goal, you will fail 99.9999% of the time. The goal is to improve function,” Dr. Bazzo said. He characterized treatment of chronic pain “as a team sport” that involves a collaboration between specialists and patients to achieve specific endpoints.
“It is a little like treating hypertension. You adjust medications until you get to the goal,” Dr. Bazzo said. This involves defining the goal before the treatment is initiated and then documenting progress toward that goal to guide treatment strategies.
The CO*RE REMS program is consistent with the CDC Guideline for Prescribing Opioids for Chronic Pain (MMWR. 65[1];1–49). It is part of a nationwide effort to reduce deaths related to opioid use.
“The problems with opioids can be greatly reduced if clinicians recognize and adhere to best practices,” Dr. Bazzo maintained.
Dr. Bazzo reports no potential financial conflicts of interest. The meeting was held by the APS and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
For Neuropathic Pain, Marijuana Yields Modest Benefits
LAKE BUENA VISTA, FLA. – As medical marijuana penetrates mainstream medical practice in the United States and elsewhere, awareness of the potential benefits, potential risks, and local laws governing its use for treatment of chronic pain gain importance, according to a specialist reviewing available data at Pain Care for Primary Care.
For many specific types of chronic pain, more data are needed to judge the benefit-to-risk ratio of marijuana relative to other options, but there are reasonable data suggesting both acceptable safety and meaningful efficacy of this analgesic in neuropathic pain, according to Mark A. Ware, MBBS, associate professor in the departments of anesthesia and family medicine, McGill University, Montreal.
In neuropathic pain, the evidence includes at least five randomized trials, according to Dr. Ware. In a recently published review article for which Dr. Ware served as senior author, the degree of neuropathic pain reductions were characterized as being on an order similar to those achieved with opioids and anticonvulsants (J Pain. 2016 Jun;17[6]:654-68). In one study, the number needed to treat (NNT) for a 50% pain reduction was just 2.
In Canada, marijuana is now available for at least some medical uses in every province. In the United States, 23 states have passed laws permitting clinical use of marijuana, according to Dr. Ware. He suggested that legalization of marijuana has fueled a growing acceptance of marijuana as a treatment option whether or not it is prescribed. For this reason, it’s necessary to examine the objective evidence to provide appropriate counseling.
“I think we are past the point where this option can simply be ignored,” Dr. Ware said. Even if they do not intend to prescribe marijuana for chronic pain, “clinicians should become familiar with the evidence regarding benefit and safety as well as the laws regarding its use.” In a study of long-term safety led by Dr. Ware, a standardized cannabis product containing 12.5% tetrahydrocannabinol was dispensed to 215 current or prior users of marijuana with a non-cancer chronic pain syndrome (J Pain. 2015 Dec;16[12]:1233-42). Followed for 1 year, adverse events in this group were compared with 216 control patients who also had chronic pain but were not using cannabis.
The odds ratio (OR) of non-serious adverse events in the categories of respiratory disorders (OR, 1.77) infectious disorders (OR, 1.51), nervous system disorders (OR, 1.77), and psychiatric disorders (OR, 2.74) were all significantly higher in the group treated with cannabis, but almost all were judged to be of mild to moderate severity. There was no significant difference in the risk of serious AEs. Dr. Ware also reported there was no difference between the cannabis group and controls for neurocognitive testing at baseline, 6 months, or the end of 1 year.
Pain control was also monitored over the course of the study. According to Dr. Ware, average pain scores in the cannabis group fell modestly but consistently over the course of the study. Over the same period, the pain scores rose slightly in the control group.
There is a long list of unanswered questions regarding effective use of marijuana in the control of chronic pain, he said. For example, Dr. Ware noted that the optimal composition of cannabinoids has yet to be determined. He noted that more than one of the complex constituents may contribute to pain control, and these constituents are not necessarily the same as those most favored by recreational users seeking a euphoric “high.”
There is also a long list of unanswered questions about safety. Dr. Ware reviewed some evidence that inhaled vaporized marijuana may be safer than traditional smoked marijuana due to a reduced exposure to toxins, but he suggested more rigorous studies are needed to generate objective data that can better quantify the benefit-to-risk of this and other methods of marijuana delivery.
Despite unanswered questions, marijuana is widely available and likely to be considered by patients for chronic pain whether or not it is recommended by physicians. It is for this reason that clinicians need to become familiar with both its potential risks and benefits.
“It will be helpful to patients if you can provide them practical and accurate information about what is and is not known about this treatment,” Dr. Ware suggested.
Dr. Ware reported a financial relationship with CanniMed. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
LAKE BUENA VISTA, FLA. – As medical marijuana penetrates mainstream medical practice in the United States and elsewhere, awareness of the potential benefits, potential risks, and local laws governing its use for treatment of chronic pain gain importance, according to a specialist reviewing available data at Pain Care for Primary Care.
For many specific types of chronic pain, more data are needed to judge the benefit-to-risk ratio of marijuana relative to other options, but there are reasonable data suggesting both acceptable safety and meaningful efficacy of this analgesic in neuropathic pain, according to Mark A. Ware, MBBS, associate professor in the departments of anesthesia and family medicine, McGill University, Montreal.
In neuropathic pain, the evidence includes at least five randomized trials, according to Dr. Ware. In a recently published review article for which Dr. Ware served as senior author, the degree of neuropathic pain reductions were characterized as being on an order similar to those achieved with opioids and anticonvulsants (J Pain. 2016 Jun;17[6]:654-68). In one study, the number needed to treat (NNT) for a 50% pain reduction was just 2.
In Canada, marijuana is now available for at least some medical uses in every province. In the United States, 23 states have passed laws permitting clinical use of marijuana, according to Dr. Ware. He suggested that legalization of marijuana has fueled a growing acceptance of marijuana as a treatment option whether or not it is prescribed. For this reason, it’s necessary to examine the objective evidence to provide appropriate counseling.
“I think we are past the point where this option can simply be ignored,” Dr. Ware said. Even if they do not intend to prescribe marijuana for chronic pain, “clinicians should become familiar with the evidence regarding benefit and safety as well as the laws regarding its use.” In a study of long-term safety led by Dr. Ware, a standardized cannabis product containing 12.5% tetrahydrocannabinol was dispensed to 215 current or prior users of marijuana with a non-cancer chronic pain syndrome (J Pain. 2015 Dec;16[12]:1233-42). Followed for 1 year, adverse events in this group were compared with 216 control patients who also had chronic pain but were not using cannabis.
The odds ratio (OR) of non-serious adverse events in the categories of respiratory disorders (OR, 1.77) infectious disorders (OR, 1.51), nervous system disorders (OR, 1.77), and psychiatric disorders (OR, 2.74) were all significantly higher in the group treated with cannabis, but almost all were judged to be of mild to moderate severity. There was no significant difference in the risk of serious AEs. Dr. Ware also reported there was no difference between the cannabis group and controls for neurocognitive testing at baseline, 6 months, or the end of 1 year.
Pain control was also monitored over the course of the study. According to Dr. Ware, average pain scores in the cannabis group fell modestly but consistently over the course of the study. Over the same period, the pain scores rose slightly in the control group.
There is a long list of unanswered questions regarding effective use of marijuana in the control of chronic pain, he said. For example, Dr. Ware noted that the optimal composition of cannabinoids has yet to be determined. He noted that more than one of the complex constituents may contribute to pain control, and these constituents are not necessarily the same as those most favored by recreational users seeking a euphoric “high.”
There is also a long list of unanswered questions about safety. Dr. Ware reviewed some evidence that inhaled vaporized marijuana may be safer than traditional smoked marijuana due to a reduced exposure to toxins, but he suggested more rigorous studies are needed to generate objective data that can better quantify the benefit-to-risk of this and other methods of marijuana delivery.
Despite unanswered questions, marijuana is widely available and likely to be considered by patients for chronic pain whether or not it is recommended by physicians. It is for this reason that clinicians need to become familiar with both its potential risks and benefits.
“It will be helpful to patients if you can provide them practical and accurate information about what is and is not known about this treatment,” Dr. Ware suggested.
Dr. Ware reported a financial relationship with CanniMed. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
LAKE BUENA VISTA, FLA. – As medical marijuana penetrates mainstream medical practice in the United States and elsewhere, awareness of the potential benefits, potential risks, and local laws governing its use for treatment of chronic pain gain importance, according to a specialist reviewing available data at Pain Care for Primary Care.
For many specific types of chronic pain, more data are needed to judge the benefit-to-risk ratio of marijuana relative to other options, but there are reasonable data suggesting both acceptable safety and meaningful efficacy of this analgesic in neuropathic pain, according to Mark A. Ware, MBBS, associate professor in the departments of anesthesia and family medicine, McGill University, Montreal.
In neuropathic pain, the evidence includes at least five randomized trials, according to Dr. Ware. In a recently published review article for which Dr. Ware served as senior author, the degree of neuropathic pain reductions were characterized as being on an order similar to those achieved with opioids and anticonvulsants (J Pain. 2016 Jun;17[6]:654-68). In one study, the number needed to treat (NNT) for a 50% pain reduction was just 2.
In Canada, marijuana is now available for at least some medical uses in every province. In the United States, 23 states have passed laws permitting clinical use of marijuana, according to Dr. Ware. He suggested that legalization of marijuana has fueled a growing acceptance of marijuana as a treatment option whether or not it is prescribed. For this reason, it’s necessary to examine the objective evidence to provide appropriate counseling.
“I think we are past the point where this option can simply be ignored,” Dr. Ware said. Even if they do not intend to prescribe marijuana for chronic pain, “clinicians should become familiar with the evidence regarding benefit and safety as well as the laws regarding its use.” In a study of long-term safety led by Dr. Ware, a standardized cannabis product containing 12.5% tetrahydrocannabinol was dispensed to 215 current or prior users of marijuana with a non-cancer chronic pain syndrome (J Pain. 2015 Dec;16[12]:1233-42). Followed for 1 year, adverse events in this group were compared with 216 control patients who also had chronic pain but were not using cannabis.
The odds ratio (OR) of non-serious adverse events in the categories of respiratory disorders (OR, 1.77) infectious disorders (OR, 1.51), nervous system disorders (OR, 1.77), and psychiatric disorders (OR, 2.74) were all significantly higher in the group treated with cannabis, but almost all were judged to be of mild to moderate severity. There was no significant difference in the risk of serious AEs. Dr. Ware also reported there was no difference between the cannabis group and controls for neurocognitive testing at baseline, 6 months, or the end of 1 year.
Pain control was also monitored over the course of the study. According to Dr. Ware, average pain scores in the cannabis group fell modestly but consistently over the course of the study. Over the same period, the pain scores rose slightly in the control group.
There is a long list of unanswered questions regarding effective use of marijuana in the control of chronic pain, he said. For example, Dr. Ware noted that the optimal composition of cannabinoids has yet to be determined. He noted that more than one of the complex constituents may contribute to pain control, and these constituents are not necessarily the same as those most favored by recreational users seeking a euphoric “high.”
There is also a long list of unanswered questions about safety. Dr. Ware reviewed some evidence that inhaled vaporized marijuana may be safer than traditional smoked marijuana due to a reduced exposure to toxins, but he suggested more rigorous studies are needed to generate objective data that can better quantify the benefit-to-risk of this and other methods of marijuana delivery.
Despite unanswered questions, marijuana is widely available and likely to be considered by patients for chronic pain whether or not it is recommended by physicians. It is for this reason that clinicians need to become familiar with both its potential risks and benefits.
“It will be helpful to patients if you can provide them practical and accurate information about what is and is not known about this treatment,” Dr. Ware suggested.
Dr. Ware reported a financial relationship with CanniMed. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
For neuropathic pain, marijuana yields modest benefits
LAKE BUENA VISTA, FLA. – As medical marijuana penetrates mainstream medical practice in the United States and elsewhere, awareness of the potential benefits, potential risks, and local laws governing its use for treatment of chronic pain gain importance, according to a specialist reviewing available data at Pain Care for Primary Care.
For many specific types of chronic pain, more data are needed to judge the benefit-to-risk ratio of marijuana relative to other options, but there are reasonable data suggesting both acceptable safety and meaningful efficacy of this analgesic in neuropathic pain, according to Mark A. Ware, MBBS, associate professor in the departments of anesthesia and family medicine, McGill University, Montreal.
In neuropathic pain, the evidence includes at least five randomized trials, according to Dr. Ware. In a recently published review article for which Dr. Ware served as senior author, the degree of neuropathic pain reductions were characterized as being on an order similar to those achieved with opioids and anticonvulsants (J Pain. 2016 Jun;17[6]:654-68). In one study, the number needed to treat (NNT) for a 50% pain reduction was just 2.
In Canada, marijuana is now available for at least some medical uses in every province. In the United States, 23 states have passed laws permitting clinical use of marijuana, according to Dr. Ware. He suggested that legalization of marijuana has fueled a growing acceptance of marijuana as a treatment option whether or not it is prescribed. For this reason, it’s necessary to examine the objective evidence to provide appropriate counseling.
“I think we are past the point where this option can simply be ignored,” Dr. Ware said. Even if they do not intend to prescribe marijuana for chronic pain, “clinicians should become familiar with the evidence regarding benefit and safety as well as the laws regarding its use.” In a study of long-term safety led by Dr. Ware, a standardized cannabis product containing 12.5% tetrahydrocannabinol was dispensed to 215 current or prior users of marijuana with a non-cancer chronic pain syndrome (J Pain. 2015 Dec;16[12]:1233-42). Followed for 1 year, adverse events in this group were compared with 216 control patients who also had chronic pain but were not using cannabis.
The odds ratio (OR) of non-serious adverse events in the categories of respiratory disorders (OR, 1.77) infectious disorders (OR, 1.51), nervous system disorders (OR, 1.77), and psychiatric disorders (OR, 2.74) were all significantly higher in the group treated with cannabis, but almost all were judged to be of mild to moderate severity. There was no significant difference in the risk of serious AEs. Dr. Ware also reported there was no difference between the cannabis group and controls for neurocognitive testing at baseline, 6 months, or the end of 1 year.
Pain control was also monitored over the course of the study. According to Dr. Ware, average pain scores in the cannabis group fell modestly but consistently over the course of the study. Over the same period, the pain scores rose slightly in the control group.
There is a long list of unanswered questions regarding effective use of marijuana in the control of chronic pain, he said. For example, Dr. Ware noted that the optimal composition of cannabinoids has yet to be determined. He noted that more than one of the complex constituents may contribute to pain control, and these constituents are not necessarily the same as those most favored by recreational users seeking a euphoric “high.”
There is also a long list of unanswered questions about safety. Dr. Ware reviewed some evidence that inhaled vaporized marijuana may be safer than traditional smoked marijuana due to a reduced exposure to toxins, but he suggested more rigorous studies are needed to generate objective data that can better quantify the benefit-to-risk of this and other methods of marijuana delivery.
Despite unanswered questions, marijuana is widely available and likely to be considered by patients for chronic pain whether or not it is recommended by physicians. It is for this reason that clinicians need to become familiar with both its potential risks and benefits.
“It will be helpful to patients if you can provide them practical and accurate information about what is and is not known about this treatment,” Dr. Ware suggested.
Dr. Ware reported a financial relationship with CanniMed. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
LAKE BUENA VISTA, FLA. – As medical marijuana penetrates mainstream medical practice in the United States and elsewhere, awareness of the potential benefits, potential risks, and local laws governing its use for treatment of chronic pain gain importance, according to a specialist reviewing available data at Pain Care for Primary Care.
For many specific types of chronic pain, more data are needed to judge the benefit-to-risk ratio of marijuana relative to other options, but there are reasonable data suggesting both acceptable safety and meaningful efficacy of this analgesic in neuropathic pain, according to Mark A. Ware, MBBS, associate professor in the departments of anesthesia and family medicine, McGill University, Montreal.
In neuropathic pain, the evidence includes at least five randomized trials, according to Dr. Ware. In a recently published review article for which Dr. Ware served as senior author, the degree of neuropathic pain reductions were characterized as being on an order similar to those achieved with opioids and anticonvulsants (J Pain. 2016 Jun;17[6]:654-68). In one study, the number needed to treat (NNT) for a 50% pain reduction was just 2.
In Canada, marijuana is now available for at least some medical uses in every province. In the United States, 23 states have passed laws permitting clinical use of marijuana, according to Dr. Ware. He suggested that legalization of marijuana has fueled a growing acceptance of marijuana as a treatment option whether or not it is prescribed. For this reason, it’s necessary to examine the objective evidence to provide appropriate counseling.
“I think we are past the point where this option can simply be ignored,” Dr. Ware said. Even if they do not intend to prescribe marijuana for chronic pain, “clinicians should become familiar with the evidence regarding benefit and safety as well as the laws regarding its use.” In a study of long-term safety led by Dr. Ware, a standardized cannabis product containing 12.5% tetrahydrocannabinol was dispensed to 215 current or prior users of marijuana with a non-cancer chronic pain syndrome (J Pain. 2015 Dec;16[12]:1233-42). Followed for 1 year, adverse events in this group were compared with 216 control patients who also had chronic pain but were not using cannabis.
The odds ratio (OR) of non-serious adverse events in the categories of respiratory disorders (OR, 1.77) infectious disorders (OR, 1.51), nervous system disorders (OR, 1.77), and psychiatric disorders (OR, 2.74) were all significantly higher in the group treated with cannabis, but almost all were judged to be of mild to moderate severity. There was no significant difference in the risk of serious AEs. Dr. Ware also reported there was no difference between the cannabis group and controls for neurocognitive testing at baseline, 6 months, or the end of 1 year.
Pain control was also monitored over the course of the study. According to Dr. Ware, average pain scores in the cannabis group fell modestly but consistently over the course of the study. Over the same period, the pain scores rose slightly in the control group.
There is a long list of unanswered questions regarding effective use of marijuana in the control of chronic pain, he said. For example, Dr. Ware noted that the optimal composition of cannabinoids has yet to be determined. He noted that more than one of the complex constituents may contribute to pain control, and these constituents are not necessarily the same as those most favored by recreational users seeking a euphoric “high.”
There is also a long list of unanswered questions about safety. Dr. Ware reviewed some evidence that inhaled vaporized marijuana may be safer than traditional smoked marijuana due to a reduced exposure to toxins, but he suggested more rigorous studies are needed to generate objective data that can better quantify the benefit-to-risk of this and other methods of marijuana delivery.
Despite unanswered questions, marijuana is widely available and likely to be considered by patients for chronic pain whether or not it is recommended by physicians. It is for this reason that clinicians need to become familiar with both its potential risks and benefits.
“It will be helpful to patients if you can provide them practical and accurate information about what is and is not known about this treatment,” Dr. Ware suggested.
Dr. Ware reported a financial relationship with CanniMed. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
LAKE BUENA VISTA, FLA. – As medical marijuana penetrates mainstream medical practice in the United States and elsewhere, awareness of the potential benefits, potential risks, and local laws governing its use for treatment of chronic pain gain importance, according to a specialist reviewing available data at Pain Care for Primary Care.
For many specific types of chronic pain, more data are needed to judge the benefit-to-risk ratio of marijuana relative to other options, but there are reasonable data suggesting both acceptable safety and meaningful efficacy of this analgesic in neuropathic pain, according to Mark A. Ware, MBBS, associate professor in the departments of anesthesia and family medicine, McGill University, Montreal.
In neuropathic pain, the evidence includes at least five randomized trials, according to Dr. Ware. In a recently published review article for which Dr. Ware served as senior author, the degree of neuropathic pain reductions were characterized as being on an order similar to those achieved with opioids and anticonvulsants (J Pain. 2016 Jun;17[6]:654-68). In one study, the number needed to treat (NNT) for a 50% pain reduction was just 2.
In Canada, marijuana is now available for at least some medical uses in every province. In the United States, 23 states have passed laws permitting clinical use of marijuana, according to Dr. Ware. He suggested that legalization of marijuana has fueled a growing acceptance of marijuana as a treatment option whether or not it is prescribed. For this reason, it’s necessary to examine the objective evidence to provide appropriate counseling.
“I think we are past the point where this option can simply be ignored,” Dr. Ware said. Even if they do not intend to prescribe marijuana for chronic pain, “clinicians should become familiar with the evidence regarding benefit and safety as well as the laws regarding its use.” In a study of long-term safety led by Dr. Ware, a standardized cannabis product containing 12.5% tetrahydrocannabinol was dispensed to 215 current or prior users of marijuana with a non-cancer chronic pain syndrome (J Pain. 2015 Dec;16[12]:1233-42). Followed for 1 year, adverse events in this group were compared with 216 control patients who also had chronic pain but were not using cannabis.
The odds ratio (OR) of non-serious adverse events in the categories of respiratory disorders (OR, 1.77) infectious disorders (OR, 1.51), nervous system disorders (OR, 1.77), and psychiatric disorders (OR, 2.74) were all significantly higher in the group treated with cannabis, but almost all were judged to be of mild to moderate severity. There was no significant difference in the risk of serious AEs. Dr. Ware also reported there was no difference between the cannabis group and controls for neurocognitive testing at baseline, 6 months, or the end of 1 year.
Pain control was also monitored over the course of the study. According to Dr. Ware, average pain scores in the cannabis group fell modestly but consistently over the course of the study. Over the same period, the pain scores rose slightly in the control group.
There is a long list of unanswered questions regarding effective use of marijuana in the control of chronic pain, he said. For example, Dr. Ware noted that the optimal composition of cannabinoids has yet to be determined. He noted that more than one of the complex constituents may contribute to pain control, and these constituents are not necessarily the same as those most favored by recreational users seeking a euphoric “high.”
There is also a long list of unanswered questions about safety. Dr. Ware reviewed some evidence that inhaled vaporized marijuana may be safer than traditional smoked marijuana due to a reduced exposure to toxins, but he suggested more rigorous studies are needed to generate objective data that can better quantify the benefit-to-risk of this and other methods of marijuana delivery.
Despite unanswered questions, marijuana is widely available and likely to be considered by patients for chronic pain whether or not it is recommended by physicians. It is for this reason that clinicians need to become familiar with both its potential risks and benefits.
“It will be helpful to patients if you can provide them practical and accurate information about what is and is not known about this treatment,” Dr. Ware suggested.
Dr. Ware reported a financial relationship with CanniMed. The meeting was held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
HIV chemoprophylaxis in U.S. up 738% in recent 3-year period
DURBAN, SOUTH AFRICA – The number of Americans using oral emtricitabine/tenofovir (Truvada) to prevent HIV infection jumped by 738% during a recent 3-year period since the drug’s 2012 marketing approval. However, uptake in certain at-risk populations leaves much room for improvement, Scott McCallister, MD, said at the 21st International AIDS Conference.
“While there are encouraging signs in using Truvada for pre-exposure prophylaxis across the country as a whole, there are certainly barriers that must be addressed in women, those under age 25, and in certain regions of the U.S. where lifetime risk of HIV acquisition is high,” said Dr. McCallister, senior director for clinical research at Gilead Sciences in Foster City, Calif.
He presented key findings from a national survey of 80% of retail pharmacies that showed that more than three-quarters of the 79,684 individuals who started on emtricitabine/tenofovir for HIV chemoprophylaxis during the fourth quarter of 2012 through the end of 2015 were men. Just 11% of the men and 28% of women taking the drug for pre-exposure prophylaxis (PrEP) were under age 25, a particularly high-risk group.
Fifty-one percent of all patients on emtricitabine/tenofovir for PrEP resided in five states: Texas, New York, Florida, Illinois, and California. Yet that doesn’t reflect the distribution of the HIV epidemic. A 2016 Centers for Disease and Prevention report concluded that the highest lifetime risk of HIV infection occurs in eight southeastern states and the District of Columbia, as well as Texas and New York.
“Some of the states with the highest lifetime risks of HIV diagnosis are lagging behind and have low numbers for Truvada for PrEP,” Dr. McCallister observed.
The Centers for Disease Control and Prevention estimates that while the lifetime risk of HIV is 1 in 99 in the United States overall, it’s 1 in 48 among black or Hispanic men, 1 in 2 for black men who have sex with men, and 1 in 4 for Hispanic MSM. Moreover, the CDC has also reported that 44% of all new HIV diagnoses occur in African Americans. And while Dr. McCallister said it’s difficult to analyze race/ethnicity in the Gilead survey because the data were de-identified, it’s his impression from other studies and talking with clinicians that PrEP users are disproportionately white.
Asked why prescribing of emtricitabine/tenofovir to date for PrEP in men far outpaces that for women, Dr. McCallister offered a theory: “Based on conversations I’ve had with community-based physicians who are avid prescribers, I think it’s a matter of men having a greater comfort level in terms of using PrEP because their clinicians and the MSM communities are more knowledgeable about PrEP. Perhaps there are fewer touch points for women to see knowledgeable clinicians. They are probably most often going to an ob.gyn. or a primary care physician who may have slightly less knowledge of PrEP than the HIV specialists or sexual health clinics where many MSMs are going.”
He noted that in addition to the randomized trial evidence of safety and efficacy that won Food and Drug Administration approval of emtricitabine/tenofovir for HIV PrEP, the real-world experience accrued in 32 demonstration projects with a total of more than 7,000 person-years of follow-up showed an HIV seroconversion rate of 0.95% per year in patients on the daily oral medication.
Dr. McCallister is an employee of Gilead Sciences, which sponsored the national PrEP survey and markets emtricitabine/tenofovir.
DURBAN, SOUTH AFRICA – The number of Americans using oral emtricitabine/tenofovir (Truvada) to prevent HIV infection jumped by 738% during a recent 3-year period since the drug’s 2012 marketing approval. However, uptake in certain at-risk populations leaves much room for improvement, Scott McCallister, MD, said at the 21st International AIDS Conference.
“While there are encouraging signs in using Truvada for pre-exposure prophylaxis across the country as a whole, there are certainly barriers that must be addressed in women, those under age 25, and in certain regions of the U.S. where lifetime risk of HIV acquisition is high,” said Dr. McCallister, senior director for clinical research at Gilead Sciences in Foster City, Calif.
He presented key findings from a national survey of 80% of retail pharmacies that showed that more than three-quarters of the 79,684 individuals who started on emtricitabine/tenofovir for HIV chemoprophylaxis during the fourth quarter of 2012 through the end of 2015 were men. Just 11% of the men and 28% of women taking the drug for pre-exposure prophylaxis (PrEP) were under age 25, a particularly high-risk group.
Fifty-one percent of all patients on emtricitabine/tenofovir for PrEP resided in five states: Texas, New York, Florida, Illinois, and California. Yet that doesn’t reflect the distribution of the HIV epidemic. A 2016 Centers for Disease and Prevention report concluded that the highest lifetime risk of HIV infection occurs in eight southeastern states and the District of Columbia, as well as Texas and New York.
“Some of the states with the highest lifetime risks of HIV diagnosis are lagging behind and have low numbers for Truvada for PrEP,” Dr. McCallister observed.
The Centers for Disease Control and Prevention estimates that while the lifetime risk of HIV is 1 in 99 in the United States overall, it’s 1 in 48 among black or Hispanic men, 1 in 2 for black men who have sex with men, and 1 in 4 for Hispanic MSM. Moreover, the CDC has also reported that 44% of all new HIV diagnoses occur in African Americans. And while Dr. McCallister said it’s difficult to analyze race/ethnicity in the Gilead survey because the data were de-identified, it’s his impression from other studies and talking with clinicians that PrEP users are disproportionately white.
Asked why prescribing of emtricitabine/tenofovir to date for PrEP in men far outpaces that for women, Dr. McCallister offered a theory: “Based on conversations I’ve had with community-based physicians who are avid prescribers, I think it’s a matter of men having a greater comfort level in terms of using PrEP because their clinicians and the MSM communities are more knowledgeable about PrEP. Perhaps there are fewer touch points for women to see knowledgeable clinicians. They are probably most often going to an ob.gyn. or a primary care physician who may have slightly less knowledge of PrEP than the HIV specialists or sexual health clinics where many MSMs are going.”
He noted that in addition to the randomized trial evidence of safety and efficacy that won Food and Drug Administration approval of emtricitabine/tenofovir for HIV PrEP, the real-world experience accrued in 32 demonstration projects with a total of more than 7,000 person-years of follow-up showed an HIV seroconversion rate of 0.95% per year in patients on the daily oral medication.
Dr. McCallister is an employee of Gilead Sciences, which sponsored the national PrEP survey and markets emtricitabine/tenofovir.
DURBAN, SOUTH AFRICA – The number of Americans using oral emtricitabine/tenofovir (Truvada) to prevent HIV infection jumped by 738% during a recent 3-year period since the drug’s 2012 marketing approval. However, uptake in certain at-risk populations leaves much room for improvement, Scott McCallister, MD, said at the 21st International AIDS Conference.
“While there are encouraging signs in using Truvada for pre-exposure prophylaxis across the country as a whole, there are certainly barriers that must be addressed in women, those under age 25, and in certain regions of the U.S. where lifetime risk of HIV acquisition is high,” said Dr. McCallister, senior director for clinical research at Gilead Sciences in Foster City, Calif.
He presented key findings from a national survey of 80% of retail pharmacies that showed that more than three-quarters of the 79,684 individuals who started on emtricitabine/tenofovir for HIV chemoprophylaxis during the fourth quarter of 2012 through the end of 2015 were men. Just 11% of the men and 28% of women taking the drug for pre-exposure prophylaxis (PrEP) were under age 25, a particularly high-risk group.
Fifty-one percent of all patients on emtricitabine/tenofovir for PrEP resided in five states: Texas, New York, Florida, Illinois, and California. Yet that doesn’t reflect the distribution of the HIV epidemic. A 2016 Centers for Disease and Prevention report concluded that the highest lifetime risk of HIV infection occurs in eight southeastern states and the District of Columbia, as well as Texas and New York.
“Some of the states with the highest lifetime risks of HIV diagnosis are lagging behind and have low numbers for Truvada for PrEP,” Dr. McCallister observed.
The Centers for Disease Control and Prevention estimates that while the lifetime risk of HIV is 1 in 99 in the United States overall, it’s 1 in 48 among black or Hispanic men, 1 in 2 for black men who have sex with men, and 1 in 4 for Hispanic MSM. Moreover, the CDC has also reported that 44% of all new HIV diagnoses occur in African Americans. And while Dr. McCallister said it’s difficult to analyze race/ethnicity in the Gilead survey because the data were de-identified, it’s his impression from other studies and talking with clinicians that PrEP users are disproportionately white.
Asked why prescribing of emtricitabine/tenofovir to date for PrEP in men far outpaces that for women, Dr. McCallister offered a theory: “Based on conversations I’ve had with community-based physicians who are avid prescribers, I think it’s a matter of men having a greater comfort level in terms of using PrEP because their clinicians and the MSM communities are more knowledgeable about PrEP. Perhaps there are fewer touch points for women to see knowledgeable clinicians. They are probably most often going to an ob.gyn. or a primary care physician who may have slightly less knowledge of PrEP than the HIV specialists or sexual health clinics where many MSMs are going.”
He noted that in addition to the randomized trial evidence of safety and efficacy that won Food and Drug Administration approval of emtricitabine/tenofovir for HIV PrEP, the real-world experience accrued in 32 demonstration projects with a total of more than 7,000 person-years of follow-up showed an HIV seroconversion rate of 0.95% per year in patients on the daily oral medication.
Dr. McCallister is an employee of Gilead Sciences, which sponsored the national PrEP survey and markets emtricitabine/tenofovir.
AT AIDS 2016
Key clinical point: More than 79,000 Americans started taking emtricitabine/tenofovir for prevention of HIV infection in a recent 3-year period.
Major finding: Adoption of HIV chemoprophylaxis via daily oral emtricitabine/tenofovir has risen steeply of late in the United States, although not in close accord with the epidemiology of the HIV epidemic.
Data source: This national survey of patients starting on emtricitabine/tenofovir to prevent HIV infection was based upon de-identified data obtained from 80% of U.S. retail pharmacies for fourth quarter 2012 through 2015.
Disclosures: The national survey of HIV pre-exposure prophylaxis was sponsored by Gilead Sciences, which markets emtricitabine/tenofovir (Truvada). The presenter is a company employee.