User login
Research Career Development Travel Awards
The SVS Foundation has announced a new award opportunity, the Research Career Development Travel Awards, designed to support early-career vascular surgeons with an interest in basic science, translational or clinical research.
Applications for these awards are due Aug. 8.
In addition to $1,500 in financial support to attend an established research career development course, awardees also will be assigned an SVS research mentor to provide guidance and discuss academic career advancement, including other available resources and opportunities.
Learn more at the link above.
The SVS Foundation has announced a new award opportunity, the Research Career Development Travel Awards, designed to support early-career vascular surgeons with an interest in basic science, translational or clinical research.
Applications for these awards are due Aug. 8.
In addition to $1,500 in financial support to attend an established research career development course, awardees also will be assigned an SVS research mentor to provide guidance and discuss academic career advancement, including other available resources and opportunities.
Learn more at the link above.
The SVS Foundation has announced a new award opportunity, the Research Career Development Travel Awards, designed to support early-career vascular surgeons with an interest in basic science, translational or clinical research.
Applications for these awards are due Aug. 8.
In addition to $1,500 in financial support to attend an established research career development course, awardees also will be assigned an SVS research mentor to provide guidance and discuss academic career advancement, including other available resources and opportunities.
Learn more at the link above.
Prevalence of Iron Deficiency in Heart Failure Patients, Study Says
NEW YORK - About a third of heart failure patients have anemia and most also have iron deficiency, according to UK researchers.
This observational analysis, Dr. John G.F. Cleland told Reuters Health by email, "shows that iron deficiency is very common in patients with heart failure and often leads to anemia and that the prevalence of both iron deficiency and anemia are both highly sensitive to the criteria used to define them."
In a June 29 online paper in JAMA Cardiology, Dr. Cleland, of the University of Hull, and colleagues report that they came to this conclusion after studying data on more than 4,400 patients seen at a local clinic over a 10-year period. All were referred because of suspected heart failure, and their median age was 73 years.
Data collected included hemoglobin, serum iron, transferrin saturation, and serum ferritin concentrations.
Overall, 1,237 patients (27.8%) had anemia, with a higher prevalence (33.3%) in patients who met the criteria for heart failure with or without left ventricular systolic dysfunction (LVSD).
Depending on the definition used, iron deficiency was present in 270 (43.2%) to 425 (68.0%) patients with anemia. This was the case in 260 (14.7%) to 624 (35.3%) of those without anemia.
Lower concentrations of hemoglobin (hazard ratio 0.92) and serum iron (HR 0.98) were independently associated with higher all-cause and cardiovascular mortality in multivariable analyses.
Moreover, said Dr. Cleland, "Serum iron and transferrin saturation were highly correlated (raising the question of the need to measure both) and both were strongly related to anemia. In contrast, serum ferritin, the most widely (supposed) measure of iron deficiency, was more strongly related to measures of inflammation than anemia."
"Lower concentrations of hemoglobin and serum iron and lower transferrin saturation," he stressed, "were associated with a higher mortality. In contrast, lower serum ferritin was associated with a better prognosis, probably because it is more a measure of inflammation than of iron deficiency."
Dr. Cleland concluded, "Serum ferritin is a poor measure of iron deficiency in patients with heart failure. Many patients with normal serum ferritin (defined by many as greater than 100 ng/mL) have iron deficiency. Clinical trials of intravenous iron for patients with heart failure should be aware of this issue to ensure they enroll appropriate patients."
The National Heart Service, Vifor Pharma, and Amgen supported this research. Dr. Cleland reported research support from Vifor and Amgen.
SOURCE: http://bit.ly/29ukoX4
JAMA Cardiol 2016.
NEW YORK - About a third of heart failure patients have anemia and most also have iron deficiency, according to UK researchers.
This observational analysis, Dr. John G.F. Cleland told Reuters Health by email, "shows that iron deficiency is very common in patients with heart failure and often leads to anemia and that the prevalence of both iron deficiency and anemia are both highly sensitive to the criteria used to define them."
In a June 29 online paper in JAMA Cardiology, Dr. Cleland, of the University of Hull, and colleagues report that they came to this conclusion after studying data on more than 4,400 patients seen at a local clinic over a 10-year period. All were referred because of suspected heart failure, and their median age was 73 years.
Data collected included hemoglobin, serum iron, transferrin saturation, and serum ferritin concentrations.
Overall, 1,237 patients (27.8%) had anemia, with a higher prevalence (33.3%) in patients who met the criteria for heart failure with or without left ventricular systolic dysfunction (LVSD).
Depending on the definition used, iron deficiency was present in 270 (43.2%) to 425 (68.0%) patients with anemia. This was the case in 260 (14.7%) to 624 (35.3%) of those without anemia.
Lower concentrations of hemoglobin (hazard ratio 0.92) and serum iron (HR 0.98) were independently associated with higher all-cause and cardiovascular mortality in multivariable analyses.
Moreover, said Dr. Cleland, "Serum iron and transferrin saturation were highly correlated (raising the question of the need to measure both) and both were strongly related to anemia. In contrast, serum ferritin, the most widely (supposed) measure of iron deficiency, was more strongly related to measures of inflammation than anemia."
"Lower concentrations of hemoglobin and serum iron and lower transferrin saturation," he stressed, "were associated with a higher mortality. In contrast, lower serum ferritin was associated with a better prognosis, probably because it is more a measure of inflammation than of iron deficiency."
Dr. Cleland concluded, "Serum ferritin is a poor measure of iron deficiency in patients with heart failure. Many patients with normal serum ferritin (defined by many as greater than 100 ng/mL) have iron deficiency. Clinical trials of intravenous iron for patients with heart failure should be aware of this issue to ensure they enroll appropriate patients."
The National Heart Service, Vifor Pharma, and Amgen supported this research. Dr. Cleland reported research support from Vifor and Amgen.
SOURCE: http://bit.ly/29ukoX4
JAMA Cardiol 2016.
NEW YORK - About a third of heart failure patients have anemia and most also have iron deficiency, according to UK researchers.
This observational analysis, Dr. John G.F. Cleland told Reuters Health by email, "shows that iron deficiency is very common in patients with heart failure and often leads to anemia and that the prevalence of both iron deficiency and anemia are both highly sensitive to the criteria used to define them."
In a June 29 online paper in JAMA Cardiology, Dr. Cleland, of the University of Hull, and colleagues report that they came to this conclusion after studying data on more than 4,400 patients seen at a local clinic over a 10-year period. All were referred because of suspected heart failure, and their median age was 73 years.
Data collected included hemoglobin, serum iron, transferrin saturation, and serum ferritin concentrations.
Overall, 1,237 patients (27.8%) had anemia, with a higher prevalence (33.3%) in patients who met the criteria for heart failure with or without left ventricular systolic dysfunction (LVSD).
Depending on the definition used, iron deficiency was present in 270 (43.2%) to 425 (68.0%) patients with anemia. This was the case in 260 (14.7%) to 624 (35.3%) of those without anemia.
Lower concentrations of hemoglobin (hazard ratio 0.92) and serum iron (HR 0.98) were independently associated with higher all-cause and cardiovascular mortality in multivariable analyses.
Moreover, said Dr. Cleland, "Serum iron and transferrin saturation were highly correlated (raising the question of the need to measure both) and both were strongly related to anemia. In contrast, serum ferritin, the most widely (supposed) measure of iron deficiency, was more strongly related to measures of inflammation than anemia."
"Lower concentrations of hemoglobin and serum iron and lower transferrin saturation," he stressed, "were associated with a higher mortality. In contrast, lower serum ferritin was associated with a better prognosis, probably because it is more a measure of inflammation than of iron deficiency."
Dr. Cleland concluded, "Serum ferritin is a poor measure of iron deficiency in patients with heart failure. Many patients with normal serum ferritin (defined by many as greater than 100 ng/mL) have iron deficiency. Clinical trials of intravenous iron for patients with heart failure should be aware of this issue to ensure they enroll appropriate patients."
The National Heart Service, Vifor Pharma, and Amgen supported this research. Dr. Cleland reported research support from Vifor and Amgen.
SOURCE: http://bit.ly/29ukoX4
JAMA Cardiol 2016.
Study Links Severe Childhood Eczema to Sedentary Behaviors
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Study links severe childhood eczema to sedentary behaviors
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A large national study linked severe atopic dermatitis to sedentary behaviors and screen time.
Major finding: Compared with children without eczema, those with severe disease were about 60% less likely to exercise at least once a week (OR, 0.39).
Data source: An analysis of data for 131,783 children from the National Survey of Children’s Health.
Disclosures: The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
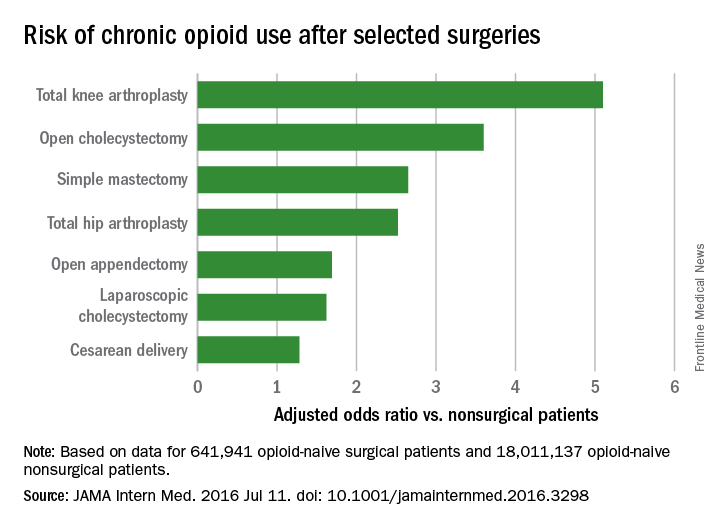
Common surgeries linked to chronic opioid use among opioid-naive patients
Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories, according to an insurance claims analysis from Stanford (Calif.) University.
The researchers reviewed opioid prescribing in the first postop year – excluding the first 90 days – for 641,941 patients and compared that information with opioid prescribing for 18,011,137 adult patients who did not have surgery. None of the subjects had filled an opioid prescription in the previous year (JAMA Intern Med. 2016 Jul 11. doi: 10.1001/jamainternmed.2016.3298).
Chronic opioid use, defined as filling at least 120 days of opioid prescriptions within the first year of surgery, ranged up to 1.41% for total knee replacement, versus 0.136% in the nonsurgical controls. After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; total hip replacement and simple mastectomy almost threefold; and laparoscopic cholecystectomy and open appendectomy almost twofold. Cesarean delivery increased the risk of chronic use by 28%.
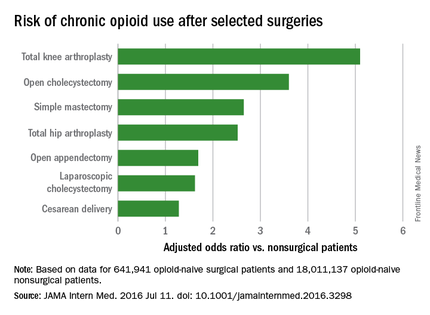
With the exception of knee and hip replacements, “these procedures are not indicated to relieve pain and are not thought to place patients at risk for long-term pain ... Our results suggest that primary care clinicians and surgeons should monitor opioid use closely in the postsurgical period,” wrote Eric C. Sun, MD, PhD, of the department of anesthesiology, perioperative and pain medicine at Stanford (Calif.) University, and his colleagues.
Preoperative antidepressants and benzodiazepines carried about the same risk of chronic use as alcohol abuse (odds ratio 1.83; P less than .001), while drug abuse history increased the risk even more (OR 3.15; P less than .001). Male sex, age over 50 years, and history of depression were also associated with chronic use on multivariate analysis. Meanwhile, transurethral prostatectomy, laparoscopic appendectomy, functional endoscopic sinus surgery, and cataract surgery did not increase chronic use risk.
“Surgical patients, particularly those at higher risk for chronic opioid use, may benefit from techniques to reduce the risk such as multimodal analgesia and regional anesthesia, particularly in light of literature suggesting that these interventions may improve other perioperative outcomes ... Patients may also benefit from other preoperative and postoperative interventions, such as evidence-based psychobehavioral pain management skills,” the investigators said.
It wasn’t clear until now that even opioid-naive patients are at risk for opioid problems after surgery. Stanford’s investigation is not the first to link surgery and opioid abuse, but previous studies tended to focus on patients with preexisting use and more painful operations.
The study included prescriptions for oral and patch fentanyl, hydrocodone, oral hydromorphone, methadone, morphine, oxymorphone, and oxycodone. Hydrocodone cough remedies and acetaminophen/codeine analgesics were excluded.
Nonsurgical patients tended to be younger than their surgical peers (mean 42 vs. 44 years) and more likely to be male (49% vs. 26%).
The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories, according to an insurance claims analysis from Stanford (Calif.) University.
The researchers reviewed opioid prescribing in the first postop year – excluding the first 90 days – for 641,941 patients and compared that information with opioid prescribing for 18,011,137 adult patients who did not have surgery. None of the subjects had filled an opioid prescription in the previous year (JAMA Intern Med. 2016 Jul 11. doi: 10.1001/jamainternmed.2016.3298).
Chronic opioid use, defined as filling at least 120 days of opioid prescriptions within the first year of surgery, ranged up to 1.41% for total knee replacement, versus 0.136% in the nonsurgical controls. After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; total hip replacement and simple mastectomy almost threefold; and laparoscopic cholecystectomy and open appendectomy almost twofold. Cesarean delivery increased the risk of chronic use by 28%.

With the exception of knee and hip replacements, “these procedures are not indicated to relieve pain and are not thought to place patients at risk for long-term pain ... Our results suggest that primary care clinicians and surgeons should monitor opioid use closely in the postsurgical period,” wrote Eric C. Sun, MD, PhD, of the department of anesthesiology, perioperative and pain medicine at Stanford (Calif.) University, and his colleagues.
Preoperative antidepressants and benzodiazepines carried about the same risk of chronic use as alcohol abuse (odds ratio 1.83; P less than .001), while drug abuse history increased the risk even more (OR 3.15; P less than .001). Male sex, age over 50 years, and history of depression were also associated with chronic use on multivariate analysis. Meanwhile, transurethral prostatectomy, laparoscopic appendectomy, functional endoscopic sinus surgery, and cataract surgery did not increase chronic use risk.
“Surgical patients, particularly those at higher risk for chronic opioid use, may benefit from techniques to reduce the risk such as multimodal analgesia and regional anesthesia, particularly in light of literature suggesting that these interventions may improve other perioperative outcomes ... Patients may also benefit from other preoperative and postoperative interventions, such as evidence-based psychobehavioral pain management skills,” the investigators said.
It wasn’t clear until now that even opioid-naive patients are at risk for opioid problems after surgery. Stanford’s investigation is not the first to link surgery and opioid abuse, but previous studies tended to focus on patients with preexisting use and more painful operations.
The study included prescriptions for oral and patch fentanyl, hydrocodone, oral hydromorphone, methadone, morphine, oxymorphone, and oxycodone. Hydrocodone cough remedies and acetaminophen/codeine analgesics were excluded.
Nonsurgical patients tended to be younger than their surgical peers (mean 42 vs. 44 years) and more likely to be male (49% vs. 26%).
The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories, according to an insurance claims analysis from Stanford (Calif.) University.
The researchers reviewed opioid prescribing in the first postop year – excluding the first 90 days – for 641,941 patients and compared that information with opioid prescribing for 18,011,137 adult patients who did not have surgery. None of the subjects had filled an opioid prescription in the previous year (JAMA Intern Med. 2016 Jul 11. doi: 10.1001/jamainternmed.2016.3298).
Chronic opioid use, defined as filling at least 120 days of opioid prescriptions within the first year of surgery, ranged up to 1.41% for total knee replacement, versus 0.136% in the nonsurgical controls. After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; total hip replacement and simple mastectomy almost threefold; and laparoscopic cholecystectomy and open appendectomy almost twofold. Cesarean delivery increased the risk of chronic use by 28%.

With the exception of knee and hip replacements, “these procedures are not indicated to relieve pain and are not thought to place patients at risk for long-term pain ... Our results suggest that primary care clinicians and surgeons should monitor opioid use closely in the postsurgical period,” wrote Eric C. Sun, MD, PhD, of the department of anesthesiology, perioperative and pain medicine at Stanford (Calif.) University, and his colleagues.
Preoperative antidepressants and benzodiazepines carried about the same risk of chronic use as alcohol abuse (odds ratio 1.83; P less than .001), while drug abuse history increased the risk even more (OR 3.15; P less than .001). Male sex, age over 50 years, and history of depression were also associated with chronic use on multivariate analysis. Meanwhile, transurethral prostatectomy, laparoscopic appendectomy, functional endoscopic sinus surgery, and cataract surgery did not increase chronic use risk.
“Surgical patients, particularly those at higher risk for chronic opioid use, may benefit from techniques to reduce the risk such as multimodal analgesia and regional anesthesia, particularly in light of literature suggesting that these interventions may improve other perioperative outcomes ... Patients may also benefit from other preoperative and postoperative interventions, such as evidence-based psychobehavioral pain management skills,” the investigators said.
It wasn’t clear until now that even opioid-naive patients are at risk for opioid problems after surgery. Stanford’s investigation is not the first to link surgery and opioid abuse, but previous studies tended to focus on patients with preexisting use and more painful operations.
The study included prescriptions for oral and patch fentanyl, hydrocodone, oral hydromorphone, methadone, morphine, oxymorphone, and oxycodone. Hydrocodone cough remedies and acetaminophen/codeine analgesics were excluded.
Nonsurgical patients tended to be younger than their surgical peers (mean 42 vs. 44 years) and more likely to be male (49% vs. 26%).
The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
FROM JAMA INTERNAL MEDICINE
Key clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
4 cases involving intraoperative injuries to adjacent organs
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar. Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM: The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE: Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT: An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Bowel perforation during myomectomy: $200,000 verdictA 44-year-old woman underwent hysteroscopic myomectomy. During the procedure the ObGyn realized that he had perforated the uterus. He switched to a laparoscopic procedure, found a 3-cm uterine tear, and converted to laparotomy to repair the injury. The postsurgical pathology report revealed multiple colon fragments.
Three days after surgery, the patient became ill and was found to have a bowel injury. She underwent bowel resection with colostomy and, a year later, colostomy reversal. She sustained abdominal scarring.
PATIENT'S CLAIM: The ObGyn was negligent in performing the myomectomy. He should have identified the bowel injury intraoperatively. When the pathology report indicated multiple colon fragments, he should have investigated rather than wait for the patient to develop symptoms.
PHYSICIAN'S DEFENSE: Uterine and colon injuries are known complications of the procedure and can occur within the standard of care. The ObGyn intraoperatively inspected the organs adjacent to the uterus but there was no evident injury. The patient’s postsurgical treatment was proper.
VERDICT: A $200,000 Illinois verdict was returned.
Injured ureter allegedly not treatedA 42-year-old woman underwent hysterectomy on December 6. Postoperatively, she reported increasing dysuria with pain and fever. On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient. The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM: The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE: Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT: The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.

During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar. Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM: The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE: Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT: An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Bowel perforation during myomectomy: $200,000 verdictA 44-year-old woman underwent hysteroscopic myomectomy. During the procedure the ObGyn realized that he had perforated the uterus. He switched to a laparoscopic procedure, found a 3-cm uterine tear, and converted to laparotomy to repair the injury. The postsurgical pathology report revealed multiple colon fragments.
Three days after surgery, the patient became ill and was found to have a bowel injury. She underwent bowel resection with colostomy and, a year later, colostomy reversal. She sustained abdominal scarring.
PATIENT'S CLAIM: The ObGyn was negligent in performing the myomectomy. He should have identified the bowel injury intraoperatively. When the pathology report indicated multiple colon fragments, he should have investigated rather than wait for the patient to develop symptoms.
PHYSICIAN'S DEFENSE: Uterine and colon injuries are known complications of the procedure and can occur within the standard of care. The ObGyn intraoperatively inspected the organs adjacent to the uterus but there was no evident injury. The patient’s postsurgical treatment was proper.
VERDICT: A $200,000 Illinois verdict was returned.
Injured ureter allegedly not treatedA 42-year-old woman underwent hysterectomy on December 6. Postoperatively, she reported increasing dysuria with pain and fever. On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient. The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM: The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE: Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT: The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.

During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar. Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM: The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE: Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT: An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Bowel perforation during myomectomy: $200,000 verdictA 44-year-old woman underwent hysteroscopic myomectomy. During the procedure the ObGyn realized that he had perforated the uterus. He switched to a laparoscopic procedure, found a 3-cm uterine tear, and converted to laparotomy to repair the injury. The postsurgical pathology report revealed multiple colon fragments.
Three days after surgery, the patient became ill and was found to have a bowel injury. She underwent bowel resection with colostomy and, a year later, colostomy reversal. She sustained abdominal scarring.
PATIENT'S CLAIM: The ObGyn was negligent in performing the myomectomy. He should have identified the bowel injury intraoperatively. When the pathology report indicated multiple colon fragments, he should have investigated rather than wait for the patient to develop symptoms.
PHYSICIAN'S DEFENSE: Uterine and colon injuries are known complications of the procedure and can occur within the standard of care. The ObGyn intraoperatively inspected the organs adjacent to the uterus but there was no evident injury. The patient’s postsurgical treatment was proper.
VERDICT: A $200,000 Illinois verdict was returned.
Injured ureter allegedly not treatedA 42-year-old woman underwent hysterectomy on December 6. Postoperatively, she reported increasing dysuria with pain and fever. On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient. The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM: The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE: Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT: The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
Was FHR properly monitored?
PARENT'S CLAIM: The pregnancy was at high risk because of the mother’s hypertension. The ObGyns misread the FHR at triage and discontinued FHR monitoring too early. If continuous FHR monitoring had occurred, fetal distress would have been detected earlier, resulting in a better outcome for the baby.
PHYSICIAN'S DEFENSE: There were no signs of fetal distress when the FHR monitoring was discontinued. Placental abruption is an acute event that cannot be predicted.
VERDICT: A Missouri defense verdict was returned.
Should the ObGyn have come to the hospital earlier?At 39 weeks’ gestation, a mother presented to the hospital for induction of labor. A FHR monitor was immediately placed. That evening, the ObGyn, who was not at the hospital, was notified that the mother had an elevated temperature and that the fetus was experiencing tachycardia. The ObGyn prescribed antibiotics, and the fever subsided. After an hour, the patient was fully dilated and started to push under the nurse’s supervision. Twenty minutes later, the ObGyn was notified that the fetus was experiencing variable decelerations. The ObGyn arrived in 30 minutes and ordered cesarean delivery. The baby was born 24 minutes later.
Ten hours after birth, the baby began to have seizures. He was transferred to another hospital and remained in the neonatal intensive care unit for 15 days. The child received a diagnosis of cerebral palsy.
PARENT'S CLAIM: The ObGyn was negligent in not coming to the hospital when the mother was feverish and the baby tachycardic. The baby experienced an acute hypoxic ischemic injury; an earlier cesarean delivery would have avoided brain injury.
PHYSICIAN'S DEFENSE: There was no breach in the standard of care. The infant did not meet all the criteria for an acute hypoxic ischemic injury. Based on a computed tomography scan taken after seizures began, the infant’s brain injury most likely occurred hours before birth.
VERDICT: A Virginia defense verdict was returned.
These cases were selected by the editors of OBG Management from "Medical Malpractice Verdicts, Settlements, & Experts," with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PARENT'S CLAIM: The pregnancy was at high risk because of the mother’s hypertension. The ObGyns misread the FHR at triage and discontinued FHR monitoring too early. If continuous FHR monitoring had occurred, fetal distress would have been detected earlier, resulting in a better outcome for the baby.
PHYSICIAN'S DEFENSE: There were no signs of fetal distress when the FHR monitoring was discontinued. Placental abruption is an acute event that cannot be predicted.
VERDICT: A Missouri defense verdict was returned.
Should the ObGyn have come to the hospital earlier?At 39 weeks’ gestation, a mother presented to the hospital for induction of labor. A FHR monitor was immediately placed. That evening, the ObGyn, who was not at the hospital, was notified that the mother had an elevated temperature and that the fetus was experiencing tachycardia. The ObGyn prescribed antibiotics, and the fever subsided. After an hour, the patient was fully dilated and started to push under the nurse’s supervision. Twenty minutes later, the ObGyn was notified that the fetus was experiencing variable decelerations. The ObGyn arrived in 30 minutes and ordered cesarean delivery. The baby was born 24 minutes later.
Ten hours after birth, the baby began to have seizures. He was transferred to another hospital and remained in the neonatal intensive care unit for 15 days. The child received a diagnosis of cerebral palsy.
PARENT'S CLAIM: The ObGyn was negligent in not coming to the hospital when the mother was feverish and the baby tachycardic. The baby experienced an acute hypoxic ischemic injury; an earlier cesarean delivery would have avoided brain injury.
PHYSICIAN'S DEFENSE: There was no breach in the standard of care. The infant did not meet all the criteria for an acute hypoxic ischemic injury. Based on a computed tomography scan taken after seizures began, the infant’s brain injury most likely occurred hours before birth.
VERDICT: A Virginia defense verdict was returned.
These cases were selected by the editors of OBG Management from "Medical Malpractice Verdicts, Settlements, & Experts," with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PARENT'S CLAIM: The pregnancy was at high risk because of the mother’s hypertension. The ObGyns misread the FHR at triage and discontinued FHR monitoring too early. If continuous FHR monitoring had occurred, fetal distress would have been detected earlier, resulting in a better outcome for the baby.
PHYSICIAN'S DEFENSE: There were no signs of fetal distress when the FHR monitoring was discontinued. Placental abruption is an acute event that cannot be predicted.
VERDICT: A Missouri defense verdict was returned.
Should the ObGyn have come to the hospital earlier?At 39 weeks’ gestation, a mother presented to the hospital for induction of labor. A FHR monitor was immediately placed. That evening, the ObGyn, who was not at the hospital, was notified that the mother had an elevated temperature and that the fetus was experiencing tachycardia. The ObGyn prescribed antibiotics, and the fever subsided. After an hour, the patient was fully dilated and started to push under the nurse’s supervision. Twenty minutes later, the ObGyn was notified that the fetus was experiencing variable decelerations. The ObGyn arrived in 30 minutes and ordered cesarean delivery. The baby was born 24 minutes later.
Ten hours after birth, the baby began to have seizures. He was transferred to another hospital and remained in the neonatal intensive care unit for 15 days. The child received a diagnosis of cerebral palsy.
PARENT'S CLAIM: The ObGyn was negligent in not coming to the hospital when the mother was feverish and the baby tachycardic. The baby experienced an acute hypoxic ischemic injury; an earlier cesarean delivery would have avoided brain injury.
PHYSICIAN'S DEFENSE: There was no breach in the standard of care. The infant did not meet all the criteria for an acute hypoxic ischemic injury. Based on a computed tomography scan taken after seizures began, the infant’s brain injury most likely occurred hours before birth.
VERDICT: A Virginia defense verdict was returned.
These cases were selected by the editors of OBG Management from "Medical Malpractice Verdicts, Settlements, & Experts," with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Study shows no benefit with brief immobilization after IUI
HELSINKI, FINLAND – A brief period of immobilization after intrauterine insemination did not improve pregnancy rates and was actually associated with a slight reduction in the pregnancy rate in a large single-center, randomized controlled trial.
The findings conflict with those of some prior smaller studies and contradict a widely held belief in the benefit of immobilization, which is usually carried out while the patient is in a supine position with the knees raised, Joukje van Rijswijk, MD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
In 479 patients with idiopathic or mild male subfertility and an indication for IUI who were randomized to 15 minutes of immobilization following IUI (950 cycles) or immediate mobilization (984 cycles), the cumulative ongoing pregnancy rate per couple was 32.2% and 40.3% in the groups, respectively (odds ratio, 0.70). The difference between the groups was not statistically significant, said Dr. van Rijswijk of VU University Medical Center, Amsterdam, The Netherlands.
Randomization in the study was stratified for the diagnosis of idiopathic or mild male subfertility. After adjustment for duration of subfertility, the difference between the group still did not reach statistical significance (odds ratio, 0.72), Dr. van Rijswijk said.
IUI is an established treatment for idiopathic and mild male subfertility, and while several factors are associated with pregnancy outcomes, the role of direct mobilization has remained controversial. Two recent studies showed a beneficial effect but were of questionable quality. For example, one of the studies found that 10 and 15 minutes of immobilization, vs. 5 minutes, had a beneficial effect on pregnancy rates, but the results were based on just one treatment cycle and “not on the more real-world context of multiple cycles,” according to an ESHRE press release.
The responsible mechanism for the benefit of immobilization remains unclear, Dr. van Rijswijk said, explaining that it is known from other studies that sperm cells can reach the fallopian tube 5 minutes after intravaginal insemination and can survive for several days in the womb.
“Why should bed rest affect that? There’s no biological explanation for a positive effect of immobilization,” she said.
“In our opinion, immobilization after IUI has no positive effect on pregnancy rates, and there is no reason why patients should stay immobilized after treatment,” she concluded, adding that the findings are “sufficiently strong to render the recommendation for bed rest obsolete.”
As for whether immobilization is also unwarranted for natural conception, Dr. van Rijswijk said the two insemination techniques are too different, thus the findings are not generalizable.
She reported having no relevant financial disclosures. The trial was funded by the VU University Medical Center.
HELSINKI, FINLAND – A brief period of immobilization after intrauterine insemination did not improve pregnancy rates and was actually associated with a slight reduction in the pregnancy rate in a large single-center, randomized controlled trial.
The findings conflict with those of some prior smaller studies and contradict a widely held belief in the benefit of immobilization, which is usually carried out while the patient is in a supine position with the knees raised, Joukje van Rijswijk, MD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
In 479 patients with idiopathic or mild male subfertility and an indication for IUI who were randomized to 15 minutes of immobilization following IUI (950 cycles) or immediate mobilization (984 cycles), the cumulative ongoing pregnancy rate per couple was 32.2% and 40.3% in the groups, respectively (odds ratio, 0.70). The difference between the groups was not statistically significant, said Dr. van Rijswijk of VU University Medical Center, Amsterdam, The Netherlands.
Randomization in the study was stratified for the diagnosis of idiopathic or mild male subfertility. After adjustment for duration of subfertility, the difference between the group still did not reach statistical significance (odds ratio, 0.72), Dr. van Rijswijk said.
IUI is an established treatment for idiopathic and mild male subfertility, and while several factors are associated with pregnancy outcomes, the role of direct mobilization has remained controversial. Two recent studies showed a beneficial effect but were of questionable quality. For example, one of the studies found that 10 and 15 minutes of immobilization, vs. 5 minutes, had a beneficial effect on pregnancy rates, but the results were based on just one treatment cycle and “not on the more real-world context of multiple cycles,” according to an ESHRE press release.
The responsible mechanism for the benefit of immobilization remains unclear, Dr. van Rijswijk said, explaining that it is known from other studies that sperm cells can reach the fallopian tube 5 minutes after intravaginal insemination and can survive for several days in the womb.
“Why should bed rest affect that? There’s no biological explanation for a positive effect of immobilization,” she said.
“In our opinion, immobilization after IUI has no positive effect on pregnancy rates, and there is no reason why patients should stay immobilized after treatment,” she concluded, adding that the findings are “sufficiently strong to render the recommendation for bed rest obsolete.”
As for whether immobilization is also unwarranted for natural conception, Dr. van Rijswijk said the two insemination techniques are too different, thus the findings are not generalizable.
She reported having no relevant financial disclosures. The trial was funded by the VU University Medical Center.
HELSINKI, FINLAND – A brief period of immobilization after intrauterine insemination did not improve pregnancy rates and was actually associated with a slight reduction in the pregnancy rate in a large single-center, randomized controlled trial.
The findings conflict with those of some prior smaller studies and contradict a widely held belief in the benefit of immobilization, which is usually carried out while the patient is in a supine position with the knees raised, Joukje van Rijswijk, MD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
In 479 patients with idiopathic or mild male subfertility and an indication for IUI who were randomized to 15 minutes of immobilization following IUI (950 cycles) or immediate mobilization (984 cycles), the cumulative ongoing pregnancy rate per couple was 32.2% and 40.3% in the groups, respectively (odds ratio, 0.70). The difference between the groups was not statistically significant, said Dr. van Rijswijk of VU University Medical Center, Amsterdam, The Netherlands.
Randomization in the study was stratified for the diagnosis of idiopathic or mild male subfertility. After adjustment for duration of subfertility, the difference between the group still did not reach statistical significance (odds ratio, 0.72), Dr. van Rijswijk said.
IUI is an established treatment for idiopathic and mild male subfertility, and while several factors are associated with pregnancy outcomes, the role of direct mobilization has remained controversial. Two recent studies showed a beneficial effect but were of questionable quality. For example, one of the studies found that 10 and 15 minutes of immobilization, vs. 5 minutes, had a beneficial effect on pregnancy rates, but the results were based on just one treatment cycle and “not on the more real-world context of multiple cycles,” according to an ESHRE press release.
The responsible mechanism for the benefit of immobilization remains unclear, Dr. van Rijswijk said, explaining that it is known from other studies that sperm cells can reach the fallopian tube 5 minutes after intravaginal insemination and can survive for several days in the womb.
“Why should bed rest affect that? There’s no biological explanation for a positive effect of immobilization,” she said.
“In our opinion, immobilization after IUI has no positive effect on pregnancy rates, and there is no reason why patients should stay immobilized after treatment,” she concluded, adding that the findings are “sufficiently strong to render the recommendation for bed rest obsolete.”
As for whether immobilization is also unwarranted for natural conception, Dr. van Rijswijk said the two insemination techniques are too different, thus the findings are not generalizable.
She reported having no relevant financial disclosures. The trial was funded by the VU University Medical Center.
AT ESHRE 2016
Key clinical point: Brief immobilization after intrauterine insemination did not improve pregnancy rates.
Major finding: The cumulative ongoing pregnancy rate per couple was 32.2% in the immobilization group, compared with 40.3% in the mobilization group (odds ratio, 0.70).
Data source: A single center, randomized controlled trial of 479 patients and 1,934 IUI cycles.
Disclosures: Dr. van Rijswijk reported having no financial disclosures. The trial was funded by the VU University Medical Center.
Tuberculous Cellulitis: Diseases Behind Cellulitislike Erythema
Local tender erythema is a typical manifestation of cellulitis, which is commonly seen by dermatologists; however, cutaneous manifestations of other diseases may bear resemblance to the more banal cellulitis. We present the case of a patient with tuberculous cellulitis, a rare variant of cutaneous tuberculosis.
Case Report
An 89-year-old man presented to a local primary care physician with a fever (temperature, 38°C). Infectious disease was suspected. Antibiotic therapy with oral cefaclor and intravenous cefotiam hydrochloride was started, but the patient’s fever did not subside. Six days after initiation of treatment, he was referred to our dermatology department for evaluation of a painful erythematous rash on the left thigh that had suddenly appeared. The patient had a history of pulmonary tuberculosis 71 years prior. He also underwent surgical treatment of pancreatic cancer 14 years prior. Additionally, he had chronic kidney disease (CKD) and polymyalgia rheumatica, which was currently being treated with oral prednisolone 5 mg once daily.
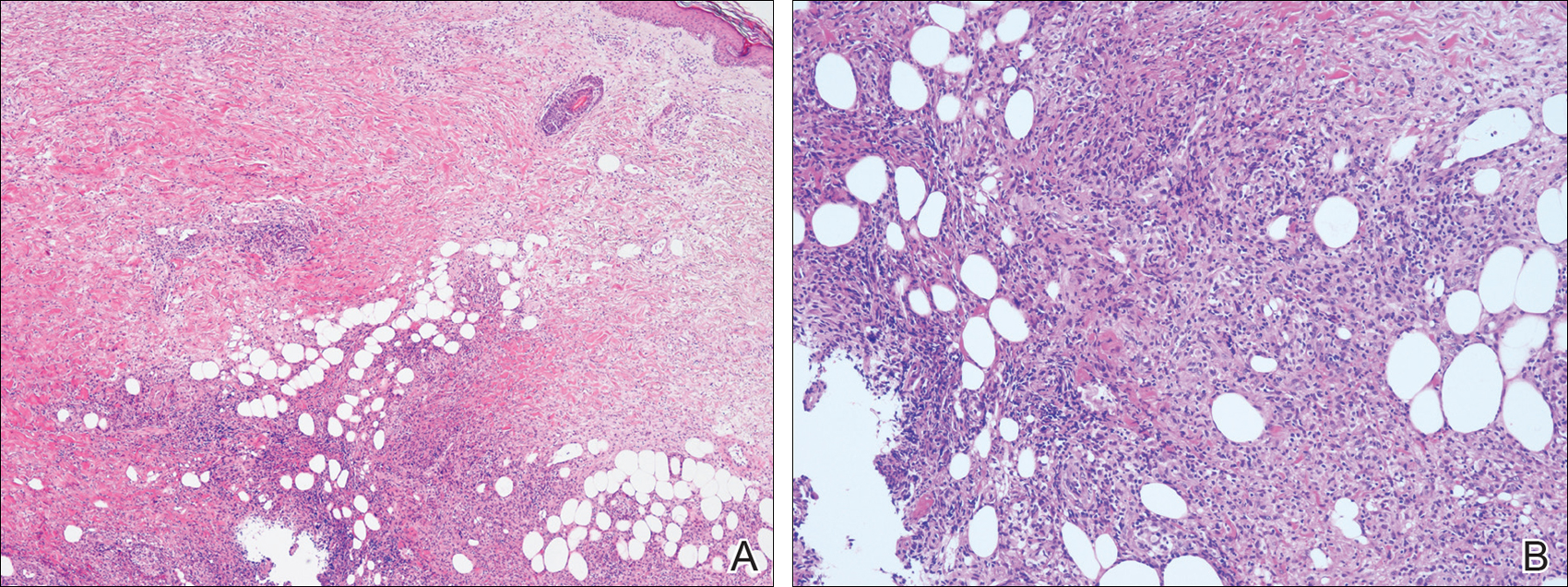
Physical examination revealed a hot and tender erythematous plaque on the left thigh (Figure 1). The edge of the lesion was not well defined and there was no regional lymphadenopathy.

A complete blood cell count revealed anemia (white blood cell count, 8070/μL [reference range, 4000–9,000/μL]; neutrophils, 77.1% [reference range, 44%–74%]; lymphocytes, 13.8% [reference range, 20%–50%]; hemoglobin, 9.3 g/dL [reference range, 13.0–17.0 g/dL]; and platelet count, 329×103/μL [reference range, 150–400×103/μL]). The C-reactive protein level was 7.3 mg/dL (reference range, 0.08–0.3 mg/dL). The creatinine level was 2.93 mg/dL (reference range, 0.6–1.2 mg/dL). There were no signs of liver dysfunction.
A blood culture was negative. A purified protein derivative (tuberculin) skin test was negative (6×7 mm [reference range, ≤9 mm). A chest computed tomography (CT) scan showed small centrilobular nodules that had not changed in number or size since evaluation 3 months prior.
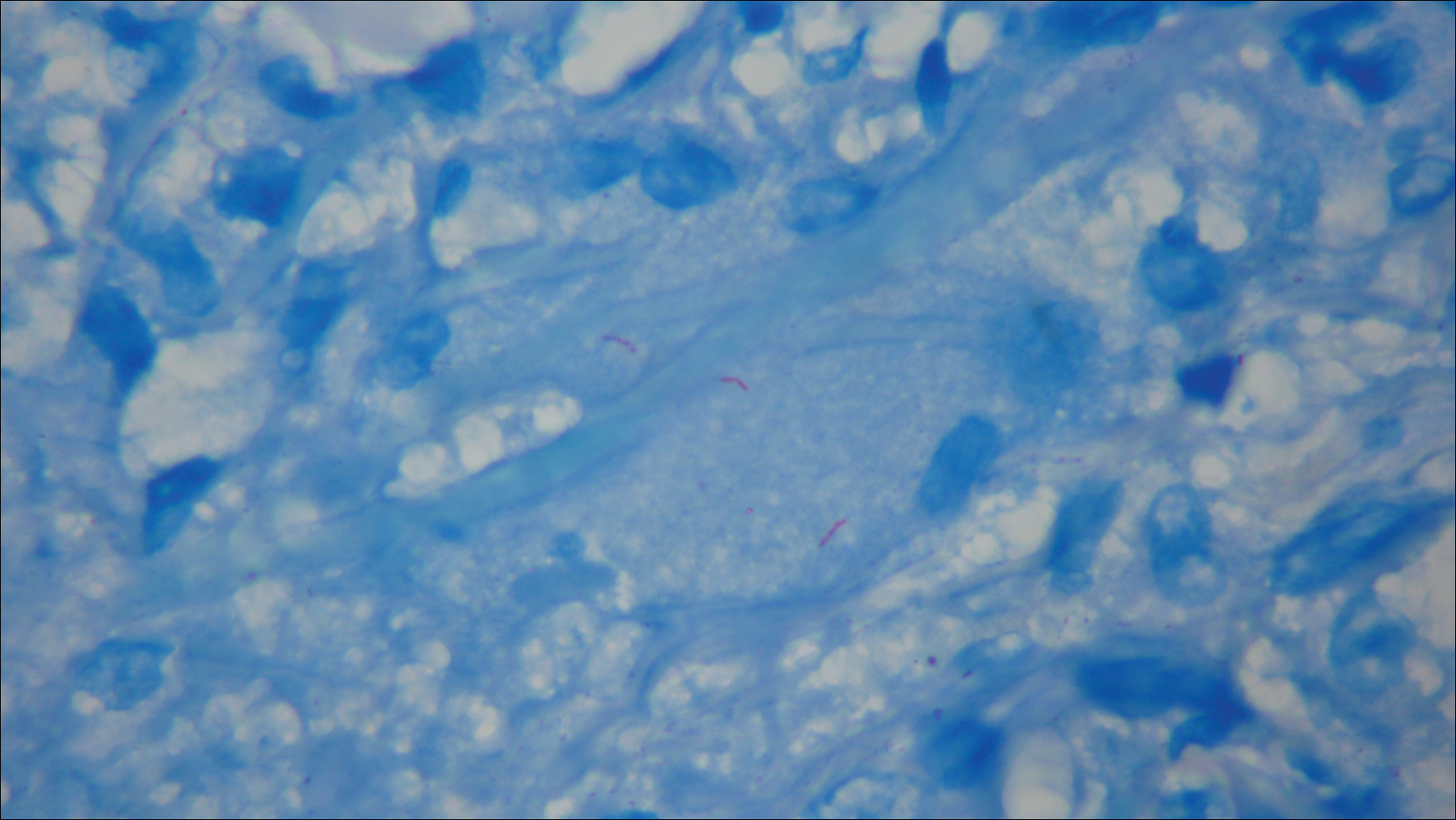
The antibiotics were changed to meropenem hydrate 0.5 g and clindamycin 300 mg twice daily for presumed bacterial cellulitis, then meropenem hydrate 1 g and clindamycin 600 mg daily, but there was still no improvement after about 1 week. Therefore, a skin biopsy was performed on the left thigh. The specimen showed epithelioid cell granulomas throughout the dermis and subcutis (Figure 2). Ziehl-Neelsen stain revealed numerous acid-fast bacilli (Figure 3). Polymerase chain reaction was positive for Mycobacterium tuberculosis in the skin biopsy specimen and gastric fluid. Additionally, M tuberculosis was isolated from the skin biopsy specimen, gastric fluid, and sputum culture. After the series of treatments described above, a remarkable increase in nodule size and number was observed in a follow-up chest CT scan compared with the prior examination. These pulmonary lesions showed bronchogenic spread.
A diagnosis of tuberculous cellulitis with pulmonary tuberculosis was made. Treatment with isoniazid 200 mg once daily, rifampin 300 mg once daily, and ethambutol 500 mg once every other day was started; the dosages were reduced from the standard dose due to the patient’s CKD.1 Four days after initiation of these medications, the patient was transferred to a hospital specifically for the treatment of tuberculosis. Approximately 8 months after treatment with isoniazid, rifampin, and ethambutol, M tuberculosis could not be detected in the sputum and a chest CT revealed that the pulmonary lesions were remarkably improved. However, polymerase chain reaction of the skin biopsy specimen was still positive for M tuberculosis. It was determined that debridement of the skin lesion was needed, but the patient died from complications of deteriorating CKD 10 months after the initiation of the antituberculosis medications.
Comment
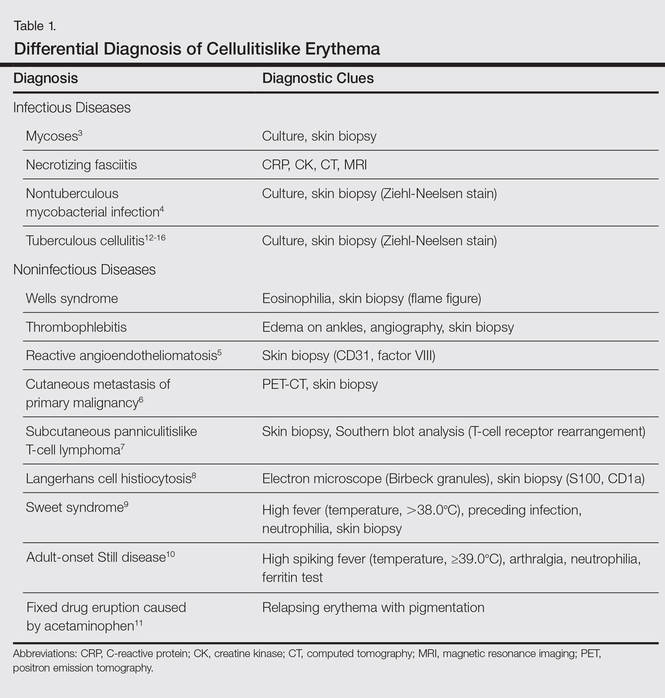
Cellulitis is a suppurative inflammation involving the subcutis.2 Local tender erythema, malaise, chills, and fever may be present at the onset. Cellulitis is commonly seen by dermatologists, and it is well known that other infectious diseases such as necrotizing fasciitis, cutaneous and subcutaneous mycoses,3 and nontuberculous mycobacterial infections4 sometimes present as cellulitislike skin lesions. Moreover, noninfectious diseases, such as Wells syndrome, thrombophlebitis, reactive angioendotheliomatosis,5 cutaneous metastasis of a primary malignancy,6 subcutaneous panniculitislike T-cell lymphoma,7 Langerhans cell histiocytosis,8 Sweet syndrome,9 adult-onset Still disease,10 and fixed drug eruption caused by acetaminophen11 should be excluded. These differential diagnoses and diagnostic clues of cellulitislike erythema are summarized in Table 1.3-16
Cutaneous tuberculosis presenting as cellulitis, so-called tuberculous cellulitis, also is characterized as a clinical mimicker of cellulitis. On the other hand, histologically, it has features of cutaneous tuberculosis (eg, necrotic granuloma).12,14,15 Tuberculous cellulitis is rare and therefore may often be misdiagnosed even in highly endemic areas. We summarized the clinical information of 5 well-documented cases of tuberculous cellulitis along with the current case in Table 2.12-16 All of these cases had an associated disease and involved patients who were currently taking oral corticosteroids. If a patient undergoing immunosuppressive therapy develops cellulitislike erythema, tuberculous cellulitis should be considered in the differential diagnosis.

Cutaneous tuberculosis generally is classified into 4 types according to the mechanism of disease acquisition: (1) inoculation from an exogenous source, (2) endogenous cutaneous spread contiguously or by autoinoculation, (3) hematogenous spread to the skin, and (4) tuberculids. In our case, it was suspected that the cellulitislike erythema may have been caused by hematogenous spread from pulmonary tuberculosis. Considering that negative reactions to purified protein derivative (tuberculin) skin tests often are observed in cases of miliary tuberculosis (widespread dissemination of M tuberculosis to 2 or more organs via hematogenous spread), we suspected that our patient could proceed to miliary tuberculosis; in fact, a case was reported in which miliary tuberculosis emerged approximately 3 weeks after the onset of erythema,13 as observed in the present case. Therefore, erythema in the setting of tuberculosis may be a predictor of miliary tuberculosis. The types of cutaneous lesions caused by tuberculosis infection also are dependent on multiple host factors.2 Cutaneous tuberculosis with an atypical clinical appearance has become more common because of the increasing number of immunocompromised patients.17
In addition, most cases of cutaneous tuberculosis are not associated with pain. Generally, tuberculous cellulitis also causes nontender erythematous plaques or nodules.2 However, in some cases of tuberculous cellulitis, including our case, tender skin lesions have been reported.12-14 Therefore, this symptom is not a sensitive factor for differential diagnosis.
We suggest that tuberculous cellulitis should always be included in the differential diagnosis of a cellulitislike rash with or without pain if the skin lesion is not improved despite antibiotic therapy.
- Daido-Horiuchi Y, Kikuchi Y, Kobayashi S, et al. Tuberculous cellulitis in a patient with chronic kidney disease and polymyalgia rheumatica. Intern Med. 2012;51:3203-3206.
- James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders Elsevier; 2011:322-329.
- Schupbach CW, Wheeler CE Jr, Briggaman RA, et al. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112:1734-1740.
- Hsu PY, Yang YH, Hsiao CH, et al. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J Formos Med Assoc. 2002;101:581-584.
- Aguayo-Leiva I, Vano-Galván S, Salguero I, et al. Reactive angioendotheliomatosis in a patient with myelodysplastic syndrome presenting as a cellulitis-like plaque. Eur J Dermatol. 2009;19:182-183.
- Yang HI, Lee MC, Kuo TT, et al. Cellulitis-like cutaneous metastasis of uterine cervical carcinoma. J Am Acad Dermatol. 2007;56:S26-S28.
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitis-like T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106:S55-S59.
- Sharma PK, Sabhnani S, Bhardwaj M, et al. Acral, pure cutaneous, self-healing, late-onset, cellulitis-like Langerhans cell histiocytosis. J Cutan Med Surg. 2009;13:43-47.
- Tercedor J, Ródenas JM, Henraz MT, et al. Facial cellulitis-like Sweet’s syndrome in acute myelogenous leukemia. Int J Dermatol. 1992;31:598-599.
- Inaoki M, Nishijima C, Kumada S, et al. Adult-onset Still disease with a cellulitis-like eruption. Eur J Dermatol. 2009;19:80-81.
- Prabhu MM, Prabhu S, Mishra P, et al. Cellulitis-like fixed drug eruption attributed to paracetamol (acetaminophen). Dermatol Online J. 2005;11:24.
- Lee NH, Choi EH, Lee WS, et al. Tuberculous cellulitis. Clin Exp Dermatol. 2000;25:222-223.
- Kim JE, Ko JY, Bae SC, et al. Tuberculous cellulitis as a manifestation of miliary tuberculosis in a patient with malignancy-associated dermatomyositis. J Am Acad Dermatol. 2011;65:450-452.
- Chin PW, Koh CK, Wong KT. Cutaneous tuberculosis mimicking cellulitis in an immunosuppressed patient. Singapore Med J. 1999;40:44-45.
- Seyahi N, Apaydin S, Kahveci A, et al. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transpl Infect Dis. 2005;7:80-85.
- Kato G, Watanabe K, Shibuya Y, et al. A case of cutaneous tuberculosis with cellulitis-like appearance [in Japanese]. Rinsho Hifuka (Jpn J Clin Dermatol). 2010;64:1055-1059.
- Fariña MC, Gegundez MI, Piqué E, et al. Cutaneous tuberculosis: a clinical, histopathologic, and bacteriologic study. J Am Acad Dermatol. 1995;33:433-440.
Local tender erythema is a typical manifestation of cellulitis, which is commonly seen by dermatologists; however, cutaneous manifestations of other diseases may bear resemblance to the more banal cellulitis. We present the case of a patient with tuberculous cellulitis, a rare variant of cutaneous tuberculosis.
Case Report
An 89-year-old man presented to a local primary care physician with a fever (temperature, 38°C). Infectious disease was suspected. Antibiotic therapy with oral cefaclor and intravenous cefotiam hydrochloride was started, but the patient’s fever did not subside. Six days after initiation of treatment, he was referred to our dermatology department for evaluation of a painful erythematous rash on the left thigh that had suddenly appeared. The patient had a history of pulmonary tuberculosis 71 years prior. He also underwent surgical treatment of pancreatic cancer 14 years prior. Additionally, he had chronic kidney disease (CKD) and polymyalgia rheumatica, which was currently being treated with oral prednisolone 5 mg once daily.
Physical examination revealed a hot and tender erythematous plaque on the left thigh (Figure 1). The edge of the lesion was not well defined and there was no regional lymphadenopathy.

A complete blood cell count revealed anemia (white blood cell count, 8070/μL [reference range, 4000–9,000/μL]; neutrophils, 77.1% [reference range, 44%–74%]; lymphocytes, 13.8% [reference range, 20%–50%]; hemoglobin, 9.3 g/dL [reference range, 13.0–17.0 g/dL]; and platelet count, 329×103/μL [reference range, 150–400×103/μL]). The C-reactive protein level was 7.3 mg/dL (reference range, 0.08–0.3 mg/dL). The creatinine level was 2.93 mg/dL (reference range, 0.6–1.2 mg/dL). There were no signs of liver dysfunction.
A blood culture was negative. A purified protein derivative (tuberculin) skin test was negative (6×7 mm [reference range, ≤9 mm). A chest computed tomography (CT) scan showed small centrilobular nodules that had not changed in number or size since evaluation 3 months prior.
The antibiotics were changed to meropenem hydrate 0.5 g and clindamycin 300 mg twice daily for presumed bacterial cellulitis, then meropenem hydrate 1 g and clindamycin 600 mg daily, but there was still no improvement after about 1 week. Therefore, a skin biopsy was performed on the left thigh. The specimen showed epithelioid cell granulomas throughout the dermis and subcutis (Figure 2). Ziehl-Neelsen stain revealed numerous acid-fast bacilli (Figure 3). Polymerase chain reaction was positive for Mycobacterium tuberculosis in the skin biopsy specimen and gastric fluid. Additionally, M tuberculosis was isolated from the skin biopsy specimen, gastric fluid, and sputum culture. After the series of treatments described above, a remarkable increase in nodule size and number was observed in a follow-up chest CT scan compared with the prior examination. These pulmonary lesions showed bronchogenic spread.


A diagnosis of tuberculous cellulitis with pulmonary tuberculosis was made. Treatment with isoniazid 200 mg once daily, rifampin 300 mg once daily, and ethambutol 500 mg once every other day was started; the dosages were reduced from the standard dose due to the patient’s CKD.1 Four days after initiation of these medications, the patient was transferred to a hospital specifically for the treatment of tuberculosis. Approximately 8 months after treatment with isoniazid, rifampin, and ethambutol, M tuberculosis could not be detected in the sputum and a chest CT revealed that the pulmonary lesions were remarkably improved. However, polymerase chain reaction of the skin biopsy specimen was still positive for M tuberculosis. It was determined that debridement of the skin lesion was needed, but the patient died from complications of deteriorating CKD 10 months after the initiation of the antituberculosis medications.
Comment
Cellulitis is a suppurative inflammation involving the subcutis.2 Local tender erythema, malaise, chills, and fever may be present at the onset. Cellulitis is commonly seen by dermatologists, and it is well known that other infectious diseases such as necrotizing fasciitis, cutaneous and subcutaneous mycoses,3 and nontuberculous mycobacterial infections4 sometimes present as cellulitislike skin lesions. Moreover, noninfectious diseases, such as Wells syndrome, thrombophlebitis, reactive angioendotheliomatosis,5 cutaneous metastasis of a primary malignancy,6 subcutaneous panniculitislike T-cell lymphoma,7 Langerhans cell histiocytosis,8 Sweet syndrome,9 adult-onset Still disease,10 and fixed drug eruption caused by acetaminophen11 should be excluded. These differential diagnoses and diagnostic clues of cellulitislike erythema are summarized in Table 1.3-16

Cutaneous tuberculosis presenting as cellulitis, so-called tuberculous cellulitis, also is characterized as a clinical mimicker of cellulitis. On the other hand, histologically, it has features of cutaneous tuberculosis (eg, necrotic granuloma).12,14,15 Tuberculous cellulitis is rare and therefore may often be misdiagnosed even in highly endemic areas. We summarized the clinical information of 5 well-documented cases of tuberculous cellulitis along with the current case in Table 2.12-16 All of these cases had an associated disease and involved patients who were currently taking oral corticosteroids. If a patient undergoing immunosuppressive therapy develops cellulitislike erythema, tuberculous cellulitis should be considered in the differential diagnosis.

Cutaneous tuberculosis generally is classified into 4 types according to the mechanism of disease acquisition: (1) inoculation from an exogenous source, (2) endogenous cutaneous spread contiguously or by autoinoculation, (3) hematogenous spread to the skin, and (4) tuberculids. In our case, it was suspected that the cellulitislike erythema may have been caused by hematogenous spread from pulmonary tuberculosis. Considering that negative reactions to purified protein derivative (tuberculin) skin tests often are observed in cases of miliary tuberculosis (widespread dissemination of M tuberculosis to 2 or more organs via hematogenous spread), we suspected that our patient could proceed to miliary tuberculosis; in fact, a case was reported in which miliary tuberculosis emerged approximately 3 weeks after the onset of erythema,13 as observed in the present case. Therefore, erythema in the setting of tuberculosis may be a predictor of miliary tuberculosis. The types of cutaneous lesions caused by tuberculosis infection also are dependent on multiple host factors.2 Cutaneous tuberculosis with an atypical clinical appearance has become more common because of the increasing number of immunocompromised patients.17
In addition, most cases of cutaneous tuberculosis are not associated with pain. Generally, tuberculous cellulitis also causes nontender erythematous plaques or nodules.2 However, in some cases of tuberculous cellulitis, including our case, tender skin lesions have been reported.12-14 Therefore, this symptom is not a sensitive factor for differential diagnosis.
We suggest that tuberculous cellulitis should always be included in the differential diagnosis of a cellulitislike rash with or without pain if the skin lesion is not improved despite antibiotic therapy.
Local tender erythema is a typical manifestation of cellulitis, which is commonly seen by dermatologists; however, cutaneous manifestations of other diseases may bear resemblance to the more banal cellulitis. We present the case of a patient with tuberculous cellulitis, a rare variant of cutaneous tuberculosis.
Case Report
An 89-year-old man presented to a local primary care physician with a fever (temperature, 38°C). Infectious disease was suspected. Antibiotic therapy with oral cefaclor and intravenous cefotiam hydrochloride was started, but the patient’s fever did not subside. Six days after initiation of treatment, he was referred to our dermatology department for evaluation of a painful erythematous rash on the left thigh that had suddenly appeared. The patient had a history of pulmonary tuberculosis 71 years prior. He also underwent surgical treatment of pancreatic cancer 14 years prior. Additionally, he had chronic kidney disease (CKD) and polymyalgia rheumatica, which was currently being treated with oral prednisolone 5 mg once daily.
Physical examination revealed a hot and tender erythematous plaque on the left thigh (Figure 1). The edge of the lesion was not well defined and there was no regional lymphadenopathy.

A complete blood cell count revealed anemia (white blood cell count, 8070/μL [reference range, 4000–9,000/μL]; neutrophils, 77.1% [reference range, 44%–74%]; lymphocytes, 13.8% [reference range, 20%–50%]; hemoglobin, 9.3 g/dL [reference range, 13.0–17.0 g/dL]; and platelet count, 329×103/μL [reference range, 150–400×103/μL]). The C-reactive protein level was 7.3 mg/dL (reference range, 0.08–0.3 mg/dL). The creatinine level was 2.93 mg/dL (reference range, 0.6–1.2 mg/dL). There were no signs of liver dysfunction.
A blood culture was negative. A purified protein derivative (tuberculin) skin test was negative (6×7 mm [reference range, ≤9 mm). A chest computed tomography (CT) scan showed small centrilobular nodules that had not changed in number or size since evaluation 3 months prior.
The antibiotics were changed to meropenem hydrate 0.5 g and clindamycin 300 mg twice daily for presumed bacterial cellulitis, then meropenem hydrate 1 g and clindamycin 600 mg daily, but there was still no improvement after about 1 week. Therefore, a skin biopsy was performed on the left thigh. The specimen showed epithelioid cell granulomas throughout the dermis and subcutis (Figure 2). Ziehl-Neelsen stain revealed numerous acid-fast bacilli (Figure 3). Polymerase chain reaction was positive for Mycobacterium tuberculosis in the skin biopsy specimen and gastric fluid. Additionally, M tuberculosis was isolated from the skin biopsy specimen, gastric fluid, and sputum culture. After the series of treatments described above, a remarkable increase in nodule size and number was observed in a follow-up chest CT scan compared with the prior examination. These pulmonary lesions showed bronchogenic spread.


A diagnosis of tuberculous cellulitis with pulmonary tuberculosis was made. Treatment with isoniazid 200 mg once daily, rifampin 300 mg once daily, and ethambutol 500 mg once every other day was started; the dosages were reduced from the standard dose due to the patient’s CKD.1 Four days after initiation of these medications, the patient was transferred to a hospital specifically for the treatment of tuberculosis. Approximately 8 months after treatment with isoniazid, rifampin, and ethambutol, M tuberculosis could not be detected in the sputum and a chest CT revealed that the pulmonary lesions were remarkably improved. However, polymerase chain reaction of the skin biopsy specimen was still positive for M tuberculosis. It was determined that debridement of the skin lesion was needed, but the patient died from complications of deteriorating CKD 10 months after the initiation of the antituberculosis medications.
Comment
Cellulitis is a suppurative inflammation involving the subcutis.2 Local tender erythema, malaise, chills, and fever may be present at the onset. Cellulitis is commonly seen by dermatologists, and it is well known that other infectious diseases such as necrotizing fasciitis, cutaneous and subcutaneous mycoses,3 and nontuberculous mycobacterial infections4 sometimes present as cellulitislike skin lesions. Moreover, noninfectious diseases, such as Wells syndrome, thrombophlebitis, reactive angioendotheliomatosis,5 cutaneous metastasis of a primary malignancy,6 subcutaneous panniculitislike T-cell lymphoma,7 Langerhans cell histiocytosis,8 Sweet syndrome,9 adult-onset Still disease,10 and fixed drug eruption caused by acetaminophen11 should be excluded. These differential diagnoses and diagnostic clues of cellulitislike erythema are summarized in Table 1.3-16

Cutaneous tuberculosis presenting as cellulitis, so-called tuberculous cellulitis, also is characterized as a clinical mimicker of cellulitis. On the other hand, histologically, it has features of cutaneous tuberculosis (eg, necrotic granuloma).12,14,15 Tuberculous cellulitis is rare and therefore may often be misdiagnosed even in highly endemic areas. We summarized the clinical information of 5 well-documented cases of tuberculous cellulitis along with the current case in Table 2.12-16 All of these cases had an associated disease and involved patients who were currently taking oral corticosteroids. If a patient undergoing immunosuppressive therapy develops cellulitislike erythema, tuberculous cellulitis should be considered in the differential diagnosis.

Cutaneous tuberculosis generally is classified into 4 types according to the mechanism of disease acquisition: (1) inoculation from an exogenous source, (2) endogenous cutaneous spread contiguously or by autoinoculation, (3) hematogenous spread to the skin, and (4) tuberculids. In our case, it was suspected that the cellulitislike erythema may have been caused by hematogenous spread from pulmonary tuberculosis. Considering that negative reactions to purified protein derivative (tuberculin) skin tests often are observed in cases of miliary tuberculosis (widespread dissemination of M tuberculosis to 2 or more organs via hematogenous spread), we suspected that our patient could proceed to miliary tuberculosis; in fact, a case was reported in which miliary tuberculosis emerged approximately 3 weeks after the onset of erythema,13 as observed in the present case. Therefore, erythema in the setting of tuberculosis may be a predictor of miliary tuberculosis. The types of cutaneous lesions caused by tuberculosis infection also are dependent on multiple host factors.2 Cutaneous tuberculosis with an atypical clinical appearance has become more common because of the increasing number of immunocompromised patients.17
In addition, most cases of cutaneous tuberculosis are not associated with pain. Generally, tuberculous cellulitis also causes nontender erythematous plaques or nodules.2 However, in some cases of tuberculous cellulitis, including our case, tender skin lesions have been reported.12-14 Therefore, this symptom is not a sensitive factor for differential diagnosis.
We suggest that tuberculous cellulitis should always be included in the differential diagnosis of a cellulitislike rash with or without pain if the skin lesion is not improved despite antibiotic therapy.
- Daido-Horiuchi Y, Kikuchi Y, Kobayashi S, et al. Tuberculous cellulitis in a patient with chronic kidney disease and polymyalgia rheumatica. Intern Med. 2012;51:3203-3206.
- James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders Elsevier; 2011:322-329.
- Schupbach CW, Wheeler CE Jr, Briggaman RA, et al. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112:1734-1740.
- Hsu PY, Yang YH, Hsiao CH, et al. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J Formos Med Assoc. 2002;101:581-584.
- Aguayo-Leiva I, Vano-Galván S, Salguero I, et al. Reactive angioendotheliomatosis in a patient with myelodysplastic syndrome presenting as a cellulitis-like plaque. Eur J Dermatol. 2009;19:182-183.
- Yang HI, Lee MC, Kuo TT, et al. Cellulitis-like cutaneous metastasis of uterine cervical carcinoma. J Am Acad Dermatol. 2007;56:S26-S28.
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitis-like T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106:S55-S59.
- Sharma PK, Sabhnani S, Bhardwaj M, et al. Acral, pure cutaneous, self-healing, late-onset, cellulitis-like Langerhans cell histiocytosis. J Cutan Med Surg. 2009;13:43-47.
- Tercedor J, Ródenas JM, Henraz MT, et al. Facial cellulitis-like Sweet’s syndrome in acute myelogenous leukemia. Int J Dermatol. 1992;31:598-599.
- Inaoki M, Nishijima C, Kumada S, et al. Adult-onset Still disease with a cellulitis-like eruption. Eur J Dermatol. 2009;19:80-81.
- Prabhu MM, Prabhu S, Mishra P, et al. Cellulitis-like fixed drug eruption attributed to paracetamol (acetaminophen). Dermatol Online J. 2005;11:24.
- Lee NH, Choi EH, Lee WS, et al. Tuberculous cellulitis. Clin Exp Dermatol. 2000;25:222-223.
- Kim JE, Ko JY, Bae SC, et al. Tuberculous cellulitis as a manifestation of miliary tuberculosis in a patient with malignancy-associated dermatomyositis. J Am Acad Dermatol. 2011;65:450-452.
- Chin PW, Koh CK, Wong KT. Cutaneous tuberculosis mimicking cellulitis in an immunosuppressed patient. Singapore Med J. 1999;40:44-45.
- Seyahi N, Apaydin S, Kahveci A, et al. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transpl Infect Dis. 2005;7:80-85.
- Kato G, Watanabe K, Shibuya Y, et al. A case of cutaneous tuberculosis with cellulitis-like appearance [in Japanese]. Rinsho Hifuka (Jpn J Clin Dermatol). 2010;64:1055-1059.
- Fariña MC, Gegundez MI, Piqué E, et al. Cutaneous tuberculosis: a clinical, histopathologic, and bacteriologic study. J Am Acad Dermatol. 1995;33:433-440.
- Daido-Horiuchi Y, Kikuchi Y, Kobayashi S, et al. Tuberculous cellulitis in a patient with chronic kidney disease and polymyalgia rheumatica. Intern Med. 2012;51:3203-3206.
- James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders Elsevier; 2011:322-329.
- Schupbach CW, Wheeler CE Jr, Briggaman RA, et al. Cutaneous manifestations of disseminated cryptococcosis. Arch Dermatol. 1976;112:1734-1740.
- Hsu PY, Yang YH, Hsiao CH, et al. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J Formos Med Assoc. 2002;101:581-584.
- Aguayo-Leiva I, Vano-Galván S, Salguero I, et al. Reactive angioendotheliomatosis in a patient with myelodysplastic syndrome presenting as a cellulitis-like plaque. Eur J Dermatol. 2009;19:182-183.
- Yang HI, Lee MC, Kuo TT, et al. Cellulitis-like cutaneous metastasis of uterine cervical carcinoma. J Am Acad Dermatol. 2007;56:S26-S28.
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitis-like T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106:S55-S59.
- Sharma PK, Sabhnani S, Bhardwaj M, et al. Acral, pure cutaneous, self-healing, late-onset, cellulitis-like Langerhans cell histiocytosis. J Cutan Med Surg. 2009;13:43-47.
- Tercedor J, Ródenas JM, Henraz MT, et al. Facial cellulitis-like Sweet’s syndrome in acute myelogenous leukemia. Int J Dermatol. 1992;31:598-599.
- Inaoki M, Nishijima C, Kumada S, et al. Adult-onset Still disease with a cellulitis-like eruption. Eur J Dermatol. 2009;19:80-81.
- Prabhu MM, Prabhu S, Mishra P, et al. Cellulitis-like fixed drug eruption attributed to paracetamol (acetaminophen). Dermatol Online J. 2005;11:24.
- Lee NH, Choi EH, Lee WS, et al. Tuberculous cellulitis. Clin Exp Dermatol. 2000;25:222-223.
- Kim JE, Ko JY, Bae SC, et al. Tuberculous cellulitis as a manifestation of miliary tuberculosis in a patient with malignancy-associated dermatomyositis. J Am Acad Dermatol. 2011;65:450-452.
- Chin PW, Koh CK, Wong KT. Cutaneous tuberculosis mimicking cellulitis in an immunosuppressed patient. Singapore Med J. 1999;40:44-45.
- Seyahi N, Apaydin S, Kahveci A, et al. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transpl Infect Dis. 2005;7:80-85.
- Kato G, Watanabe K, Shibuya Y, et al. A case of cutaneous tuberculosis with cellulitis-like appearance [in Japanese]. Rinsho Hifuka (Jpn J Clin Dermatol). 2010;64:1055-1059.
- Fariña MC, Gegundez MI, Piqué E, et al. Cutaneous tuberculosis: a clinical, histopathologic, and bacteriologic study. J Am Acad Dermatol. 1995;33:433-440.
Mobile phone messaging had little impact on missed dermatology visits
SCOTTSDALE, ARIZ. – Although cost-effective, a mobile phone appointment reminder service only minimally increased attendance rates at dermatology outpatient clinics, according to a large longitudinal study.
“There was a small, statistically significant increase in attendance at the adult dermatology clinic, but there was very little effect at satellite and specialty dermatology clinics,” said Dr. Noori Kim of Johns Hopkins University in Baltimore.
Baseline attendance rates were high, exceeding 80%, so perhaps mobile phone appointment reminders have little effect in that setting, she said. In addition, most reminders were by phone call, not text, which could have limited their efficacy if patients did not answer the phone or listen to their voicemail, Dr. Kim added during an oral presentation at the annual meeting of the Society for Investigative Dermatology.
When patients miss medical appointments, it’s usually because they forget them. In this study, the first to assess mobile phone appointment reminders in dermatology, the investigators compared daily attendance at Johns Hopkins outpatient dermatology clinics before and after implementing an automated mobile phone appointment reminder system. The baseline time period without the service spanned four months in 2014, while the comparison period with the system covered the same four-month period a year later.
Patients kept 90% of 11,455 dermatology appointments scheduled during the pre-service period. A year later, the attendance rate was nearly identical, at 89%. Likewise, there were no statistically significant changes in attendance at Johns Hopkins specialty, satellite, pediatric dermatology, and pediatric laser clinics.
In contrast, attendance at the adult dermatology clinic rose by about three percentage points after the service was implemented, from 81% (2,530 visits attended of 3,141 scheduled) to 84% (2,965 visits attended of 3,533 scheduled), and the difference was statistically significant.
“About 88% of reminders were answered across sites, with little variance. The cost was about $5,500 for a 17-month period,” Dr. Kim said. She noted that 71% of patients opted into the service at the adult clinic with the increased attendance rate, compared with about 30% of patients at the other clinics that showed no statistically significant increase in attendance rates.
The “strong continuity already present between patients and providers” and high baseline attendance rates might have limited any effects of mobile phone messaging at these other clinics, she said. Attendance rates also did not change significantly at three Johns Hopkins dermatology clinics that never implemented mobile phone reminders, she noted.
Dr. Kim reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Although cost-effective, a mobile phone appointment reminder service only minimally increased attendance rates at dermatology outpatient clinics, according to a large longitudinal study.
“There was a small, statistically significant increase in attendance at the adult dermatology clinic, but there was very little effect at satellite and specialty dermatology clinics,” said Dr. Noori Kim of Johns Hopkins University in Baltimore.
Baseline attendance rates were high, exceeding 80%, so perhaps mobile phone appointment reminders have little effect in that setting, she said. In addition, most reminders were by phone call, not text, which could have limited their efficacy if patients did not answer the phone or listen to their voicemail, Dr. Kim added during an oral presentation at the annual meeting of the Society for Investigative Dermatology.
When patients miss medical appointments, it’s usually because they forget them. In this study, the first to assess mobile phone appointment reminders in dermatology, the investigators compared daily attendance at Johns Hopkins outpatient dermatology clinics before and after implementing an automated mobile phone appointment reminder system. The baseline time period without the service spanned four months in 2014, while the comparison period with the system covered the same four-month period a year later.
Patients kept 90% of 11,455 dermatology appointments scheduled during the pre-service period. A year later, the attendance rate was nearly identical, at 89%. Likewise, there were no statistically significant changes in attendance at Johns Hopkins specialty, satellite, pediatric dermatology, and pediatric laser clinics.
In contrast, attendance at the adult dermatology clinic rose by about three percentage points after the service was implemented, from 81% (2,530 visits attended of 3,141 scheduled) to 84% (2,965 visits attended of 3,533 scheduled), and the difference was statistically significant.
“About 88% of reminders were answered across sites, with little variance. The cost was about $5,500 for a 17-month period,” Dr. Kim said. She noted that 71% of patients opted into the service at the adult clinic with the increased attendance rate, compared with about 30% of patients at the other clinics that showed no statistically significant increase in attendance rates.
The “strong continuity already present between patients and providers” and high baseline attendance rates might have limited any effects of mobile phone messaging at these other clinics, she said. Attendance rates also did not change significantly at three Johns Hopkins dermatology clinics that never implemented mobile phone reminders, she noted.
Dr. Kim reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Although cost-effective, a mobile phone appointment reminder service only minimally increased attendance rates at dermatology outpatient clinics, according to a large longitudinal study.
“There was a small, statistically significant increase in attendance at the adult dermatology clinic, but there was very little effect at satellite and specialty dermatology clinics,” said Dr. Noori Kim of Johns Hopkins University in Baltimore.
Baseline attendance rates were high, exceeding 80%, so perhaps mobile phone appointment reminders have little effect in that setting, she said. In addition, most reminders were by phone call, not text, which could have limited their efficacy if patients did not answer the phone or listen to their voicemail, Dr. Kim added during an oral presentation at the annual meeting of the Society for Investigative Dermatology.
When patients miss medical appointments, it’s usually because they forget them. In this study, the first to assess mobile phone appointment reminders in dermatology, the investigators compared daily attendance at Johns Hopkins outpatient dermatology clinics before and after implementing an automated mobile phone appointment reminder system. The baseline time period without the service spanned four months in 2014, while the comparison period with the system covered the same four-month period a year later.
Patients kept 90% of 11,455 dermatology appointments scheduled during the pre-service period. A year later, the attendance rate was nearly identical, at 89%. Likewise, there were no statistically significant changes in attendance at Johns Hopkins specialty, satellite, pediatric dermatology, and pediatric laser clinics.
In contrast, attendance at the adult dermatology clinic rose by about three percentage points after the service was implemented, from 81% (2,530 visits attended of 3,141 scheduled) to 84% (2,965 visits attended of 3,533 scheduled), and the difference was statistically significant.
“About 88% of reminders were answered across sites, with little variance. The cost was about $5,500 for a 17-month period,” Dr. Kim said. She noted that 71% of patients opted into the service at the adult clinic with the increased attendance rate, compared with about 30% of patients at the other clinics that showed no statistically significant increase in attendance rates.
The “strong continuity already present between patients and providers” and high baseline attendance rates might have limited any effects of mobile phone messaging at these other clinics, she said. Attendance rates also did not change significantly at three Johns Hopkins dermatology clinics that never implemented mobile phone reminders, she noted.
Dr. Kim reported no funding sources and had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A mobile phone messaging appointment reminder service had little effect on attendance rates at outpatient dermatology clinics.
Major finding: Attendance rates did not change overall or at most individual clinics, although attendance at the general adult dermatology clinic rose significantly by three percentage points.
Data source: A retrospective study of attendance rates at Johns Hopkins outpatient dermatology clinics before and after implementation of the messaging service.
Disclosures: Dr. Kim reported no funding sources and had no disclosures.