User login
Four-branched arch replacement gets acceptable results
NEW YORK – A total aortic arch replacement approach that uses a four-branched graft with antegrade cerebral perfusion can be done with low rates of in-hospital death and complications, a large series from two institutions in Japan showed.
Kenji Minatoya, MD, of the National Cerebral and Cardiovascular Center in Osaka, Japan, reported that his institution’s approach for total arch replacement (TAR) had an in-hospital death rate of 5.2%.
Dr. Minatoya and his colleagues started using four-branch TAR in the 1980s, switching from retrograde to antegrade cerebral perfusion to protect the brain later on. “The study purpose was to investigate the results of total arch replacement using the four-branch graft as a benchmark in the endovascular era,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
The study involved 1,005 cases of total arch replacement performed at Dr. Minatoya’s center and at Tokyo Medical University from 2001 to 2016.
The study population included a cohort of 152 people in their 80s. The in-hospital death rate in this group was 11.8%, Dr. Minatoya said. The over-80 group mostly underwent thoracic endovascular aortic repair (TEVAR) beginning in 2008, he said, but in recent years some had open total arch replacement operations.
The univariate analysis showed that chronic kidney disease, long operation times, long durations for coronary bypass and circulatory arrest, and extended time on mechanical ventilation were risk factors for in-hospital death in octogenarians, Dr. Minatoya said. The multivariate analysis showed that male gender along with extended mechanical ventilation were risk factors for in-hospital death in this group, he said.
The overall population included 252 emergent operations, 224 of which were for acute aortic dissections, Dr. Minatoya said. The in-hospital death rate was 4.5% for elective operations and 7.1% for emergent cases, he said. The death rate for isolated, elective total arch replacement was 3.4%.
Focusing on acute aortic dissections, Dr. Minatoya said, “We have adopted an aggressive strategy for entry-site resection, including total arch replacement, in patients with arch tears.” Almost 50% of patients with acute aortic dissection had total arch replacement, he said, with identical 4.9% rates for in-hospital mortality rate and permanent neurological deficit in this group.
The leading overall causes of in-hospital death were low-output syndrome (38.5%), sepsis (25%), respiratory failure (21%) and rupture of the residual aneurysm (9%), Dr. Minatoya said.
Fifteen patients (1.5%) underwent second operations for arch grafts, he said: 11 for pseudoaneurysm; three for hemolysis and one for infection. Other overall measures in the analysis were a permanent neurological dysfunction rate of 3.6%, a temporary neurological dysfunction rate of 6.4%, and no spinal cord complications. Overall 5-year survival was 80.7% and 10-year survival was 63.1%, Dr. Minatoya said.
A total of 311 patients had concomitant procedures. They included aortic valve operations (64); aortic root replacement (38); mitral valve replacement (13); and coronary artery bypass grafting (196).
The typical operation in the study population took about 8 hours, Dr. Minatoya said (482 minutes). Timing of key operative steps were cardiopulmonary time of 254 minutes, cardiac arrest time of 146 minutes, antegrade cerebral perfusion time of 160 minutes and lower-body circulatory arrest time of 62 minutes.
“Since the mean age was 70 years old, we think the survival rate was acceptable,” Dr. Minatoya said, regarding overall study results. Overall risk factors for in-hospital death were short stature, long pump time, chronic kidney disease, and age of 80 and up, he said. Short stature was a risk factor for permanent neurological deficit, and males over age 80 had a higher risk for total arch replacement.
“Total arch replacement using the four-branched graft with antegrade cerebral perfusion could be accomplished with acceptable early and late results,” Dr. Minatoya said. “The branched-arch TEVAR may be a good option for octogenarians and patients with chronic kidney disease.”
Dr. Minatoya had no financial relationships to disclose.
NEW YORK – A total aortic arch replacement approach that uses a four-branched graft with antegrade cerebral perfusion can be done with low rates of in-hospital death and complications, a large series from two institutions in Japan showed.
Kenji Minatoya, MD, of the National Cerebral and Cardiovascular Center in Osaka, Japan, reported that his institution’s approach for total arch replacement (TAR) had an in-hospital death rate of 5.2%.
Dr. Minatoya and his colleagues started using four-branch TAR in the 1980s, switching from retrograde to antegrade cerebral perfusion to protect the brain later on. “The study purpose was to investigate the results of total arch replacement using the four-branch graft as a benchmark in the endovascular era,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
The study involved 1,005 cases of total arch replacement performed at Dr. Minatoya’s center and at Tokyo Medical University from 2001 to 2016.
The study population included a cohort of 152 people in their 80s. The in-hospital death rate in this group was 11.8%, Dr. Minatoya said. The over-80 group mostly underwent thoracic endovascular aortic repair (TEVAR) beginning in 2008, he said, but in recent years some had open total arch replacement operations.
The univariate analysis showed that chronic kidney disease, long operation times, long durations for coronary bypass and circulatory arrest, and extended time on mechanical ventilation were risk factors for in-hospital death in octogenarians, Dr. Minatoya said. The multivariate analysis showed that male gender along with extended mechanical ventilation were risk factors for in-hospital death in this group, he said.
The overall population included 252 emergent operations, 224 of which were for acute aortic dissections, Dr. Minatoya said. The in-hospital death rate was 4.5% for elective operations and 7.1% for emergent cases, he said. The death rate for isolated, elective total arch replacement was 3.4%.
Focusing on acute aortic dissections, Dr. Minatoya said, “We have adopted an aggressive strategy for entry-site resection, including total arch replacement, in patients with arch tears.” Almost 50% of patients with acute aortic dissection had total arch replacement, he said, with identical 4.9% rates for in-hospital mortality rate and permanent neurological deficit in this group.
The leading overall causes of in-hospital death were low-output syndrome (38.5%), sepsis (25%), respiratory failure (21%) and rupture of the residual aneurysm (9%), Dr. Minatoya said.
Fifteen patients (1.5%) underwent second operations for arch grafts, he said: 11 for pseudoaneurysm; three for hemolysis and one for infection. Other overall measures in the analysis were a permanent neurological dysfunction rate of 3.6%, a temporary neurological dysfunction rate of 6.4%, and no spinal cord complications. Overall 5-year survival was 80.7% and 10-year survival was 63.1%, Dr. Minatoya said.
A total of 311 patients had concomitant procedures. They included aortic valve operations (64); aortic root replacement (38); mitral valve replacement (13); and coronary artery bypass grafting (196).
The typical operation in the study population took about 8 hours, Dr. Minatoya said (482 minutes). Timing of key operative steps were cardiopulmonary time of 254 minutes, cardiac arrest time of 146 minutes, antegrade cerebral perfusion time of 160 minutes and lower-body circulatory arrest time of 62 minutes.
“Since the mean age was 70 years old, we think the survival rate was acceptable,” Dr. Minatoya said, regarding overall study results. Overall risk factors for in-hospital death were short stature, long pump time, chronic kidney disease, and age of 80 and up, he said. Short stature was a risk factor for permanent neurological deficit, and males over age 80 had a higher risk for total arch replacement.
“Total arch replacement using the four-branched graft with antegrade cerebral perfusion could be accomplished with acceptable early and late results,” Dr. Minatoya said. “The branched-arch TEVAR may be a good option for octogenarians and patients with chronic kidney disease.”
Dr. Minatoya had no financial relationships to disclose.
NEW YORK – A total aortic arch replacement approach that uses a four-branched graft with antegrade cerebral perfusion can be done with low rates of in-hospital death and complications, a large series from two institutions in Japan showed.
Kenji Minatoya, MD, of the National Cerebral and Cardiovascular Center in Osaka, Japan, reported that his institution’s approach for total arch replacement (TAR) had an in-hospital death rate of 5.2%.
Dr. Minatoya and his colleagues started using four-branch TAR in the 1980s, switching from retrograde to antegrade cerebral perfusion to protect the brain later on. “The study purpose was to investigate the results of total arch replacement using the four-branch graft as a benchmark in the endovascular era,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
The study involved 1,005 cases of total arch replacement performed at Dr. Minatoya’s center and at Tokyo Medical University from 2001 to 2016.
The study population included a cohort of 152 people in their 80s. The in-hospital death rate in this group was 11.8%, Dr. Minatoya said. The over-80 group mostly underwent thoracic endovascular aortic repair (TEVAR) beginning in 2008, he said, but in recent years some had open total arch replacement operations.
The univariate analysis showed that chronic kidney disease, long operation times, long durations for coronary bypass and circulatory arrest, and extended time on mechanical ventilation were risk factors for in-hospital death in octogenarians, Dr. Minatoya said. The multivariate analysis showed that male gender along with extended mechanical ventilation were risk factors for in-hospital death in this group, he said.
The overall population included 252 emergent operations, 224 of which were for acute aortic dissections, Dr. Minatoya said. The in-hospital death rate was 4.5% for elective operations and 7.1% for emergent cases, he said. The death rate for isolated, elective total arch replacement was 3.4%.
Focusing on acute aortic dissections, Dr. Minatoya said, “We have adopted an aggressive strategy for entry-site resection, including total arch replacement, in patients with arch tears.” Almost 50% of patients with acute aortic dissection had total arch replacement, he said, with identical 4.9% rates for in-hospital mortality rate and permanent neurological deficit in this group.
The leading overall causes of in-hospital death were low-output syndrome (38.5%), sepsis (25%), respiratory failure (21%) and rupture of the residual aneurysm (9%), Dr. Minatoya said.
Fifteen patients (1.5%) underwent second operations for arch grafts, he said: 11 for pseudoaneurysm; three for hemolysis and one for infection. Other overall measures in the analysis were a permanent neurological dysfunction rate of 3.6%, a temporary neurological dysfunction rate of 6.4%, and no spinal cord complications. Overall 5-year survival was 80.7% and 10-year survival was 63.1%, Dr. Minatoya said.
A total of 311 patients had concomitant procedures. They included aortic valve operations (64); aortic root replacement (38); mitral valve replacement (13); and coronary artery bypass grafting (196).
The typical operation in the study population took about 8 hours, Dr. Minatoya said (482 minutes). Timing of key operative steps were cardiopulmonary time of 254 minutes, cardiac arrest time of 146 minutes, antegrade cerebral perfusion time of 160 minutes and lower-body circulatory arrest time of 62 minutes.
“Since the mean age was 70 years old, we think the survival rate was acceptable,” Dr. Minatoya said, regarding overall study results. Overall risk factors for in-hospital death were short stature, long pump time, chronic kidney disease, and age of 80 and up, he said. Short stature was a risk factor for permanent neurological deficit, and males over age 80 had a higher risk for total arch replacement.
“Total arch replacement using the four-branched graft with antegrade cerebral perfusion could be accomplished with acceptable early and late results,” Dr. Minatoya said. “The branched-arch TEVAR may be a good option for octogenarians and patients with chronic kidney disease.”
Dr. Minatoya had no financial relationships to disclose.
AT THE AATS AORTIC SYMPOSIUM 2016
Key clinical point: Four-branched total aortic arch replacement can achieve acceptable early and late results.
Major finding: Overall in-hospital mortality was 5.2% and 5-year survival was 80.7%.
Data source: Consecutive series of 1,005 patients who had total arch replacement between 2001 and 2016 at two centers in Japan.
Disclosures: Dr. Minatoya reported having no financial disclosures.
First female-to-male sexual transmission of Zika virus
A suspected case of sexual transmission of the Zika virus from a female to a male has occurred in New York City, according to the New York City Department of Health and Mental Hygiene (DOHMH).
“This case represents the first reported occurrence of female-to-male sexual transmission of Zika virus,” Alexander Davidson of the DOHMH and his coauthors stated in the CDC’s Morbidity and Mortality Weekly Report. “Current guidance to prevent sexual transmission of Zika virus is based on the assumption that transmission occurs from a male partner to a receptive partner.”
The woman, reportedly in her twenties and not pregnant at the time of infection, had traveled to a region experiencing high volumes of Zika virus transmission. Upon returning home, the woman engaged in condomless vaginal intercourse with her male partner, and subsequently developed symptoms consistent with a Zika virus infection. Three days after symptom onset, her primary care provider took blood and urine samples, from which a Zika virus infection was confirmed. (MMWR Morb Mortal Wkly Rep. 2016 Jul 15. doi: 10.15585/mmwr.mm6528e2)
A week after the sexual encounter, her partner – also in his twenties – began experiencing symptoms of Zika virus infection. Three days after the onset of his symptoms, he went to the same primary care provider as the woman. He confirmed that he had not traveled outside of the United States in the last year, had engaged in condomless vaginal sex with just one individual (the aforementioned female), had no blood on his penis to indicate vaginal bleeding or the presence of open lesions, and had no mosquito bites in the previous week.
“The timing and sequence of events support female-to-male Zika virus transmission through condomless vaginal intercourse,” the coauthors conclude, adding that “virus present in either vaginal fluids or menstrual blood might have been transmitted during exposure to her male partner’s urethral mucosa or undetected abrasions on his penis.”
Both the female and male were tested via real-time reverse transcription–polymerase chain reaction (rRT-PCR), with serum testing done via the Zika immunoglobulin M antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA). The rRT-PCR testing showed Zika virus RNA in the woman’s serum, despite being collected three days after the sexual encounter, meaning she was viremic at the time. Studies on nonhuman primates have shown that Zika virus RNA can remain present in vaginal fluid for up to a week, according to the report.
The CDC is cautioning pregnant women against travel to Zika-heavy areas, in particular the 2016 Summer Olympic Games in Rio de Janeiro. Health care providers who receive patients with Zika-like symptoms should ask if the patient has had sexual contact with someone who has traveled to an affected region, if the patient did not travel to such a region.
A suspected case of sexual transmission of the Zika virus from a female to a male has occurred in New York City, according to the New York City Department of Health and Mental Hygiene (DOHMH).
“This case represents the first reported occurrence of female-to-male sexual transmission of Zika virus,” Alexander Davidson of the DOHMH and his coauthors stated in the CDC’s Morbidity and Mortality Weekly Report. “Current guidance to prevent sexual transmission of Zika virus is based on the assumption that transmission occurs from a male partner to a receptive partner.”
The woman, reportedly in her twenties and not pregnant at the time of infection, had traveled to a region experiencing high volumes of Zika virus transmission. Upon returning home, the woman engaged in condomless vaginal intercourse with her male partner, and subsequently developed symptoms consistent with a Zika virus infection. Three days after symptom onset, her primary care provider took blood and urine samples, from which a Zika virus infection was confirmed. (MMWR Morb Mortal Wkly Rep. 2016 Jul 15. doi: 10.15585/mmwr.mm6528e2)
A week after the sexual encounter, her partner – also in his twenties – began experiencing symptoms of Zika virus infection. Three days after the onset of his symptoms, he went to the same primary care provider as the woman. He confirmed that he had not traveled outside of the United States in the last year, had engaged in condomless vaginal sex with just one individual (the aforementioned female), had no blood on his penis to indicate vaginal bleeding or the presence of open lesions, and had no mosquito bites in the previous week.
“The timing and sequence of events support female-to-male Zika virus transmission through condomless vaginal intercourse,” the coauthors conclude, adding that “virus present in either vaginal fluids or menstrual blood might have been transmitted during exposure to her male partner’s urethral mucosa or undetected abrasions on his penis.”
Both the female and male were tested via real-time reverse transcription–polymerase chain reaction (rRT-PCR), with serum testing done via the Zika immunoglobulin M antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA). The rRT-PCR testing showed Zika virus RNA in the woman’s serum, despite being collected three days after the sexual encounter, meaning she was viremic at the time. Studies on nonhuman primates have shown that Zika virus RNA can remain present in vaginal fluid for up to a week, according to the report.
The CDC is cautioning pregnant women against travel to Zika-heavy areas, in particular the 2016 Summer Olympic Games in Rio de Janeiro. Health care providers who receive patients with Zika-like symptoms should ask if the patient has had sexual contact with someone who has traveled to an affected region, if the patient did not travel to such a region.
A suspected case of sexual transmission of the Zika virus from a female to a male has occurred in New York City, according to the New York City Department of Health and Mental Hygiene (DOHMH).
“This case represents the first reported occurrence of female-to-male sexual transmission of Zika virus,” Alexander Davidson of the DOHMH and his coauthors stated in the CDC’s Morbidity and Mortality Weekly Report. “Current guidance to prevent sexual transmission of Zika virus is based on the assumption that transmission occurs from a male partner to a receptive partner.”
The woman, reportedly in her twenties and not pregnant at the time of infection, had traveled to a region experiencing high volumes of Zika virus transmission. Upon returning home, the woman engaged in condomless vaginal intercourse with her male partner, and subsequently developed symptoms consistent with a Zika virus infection. Three days after symptom onset, her primary care provider took blood and urine samples, from which a Zika virus infection was confirmed. (MMWR Morb Mortal Wkly Rep. 2016 Jul 15. doi: 10.15585/mmwr.mm6528e2)
A week after the sexual encounter, her partner – also in his twenties – began experiencing symptoms of Zika virus infection. Three days after the onset of his symptoms, he went to the same primary care provider as the woman. He confirmed that he had not traveled outside of the United States in the last year, had engaged in condomless vaginal sex with just one individual (the aforementioned female), had no blood on his penis to indicate vaginal bleeding or the presence of open lesions, and had no mosquito bites in the previous week.
“The timing and sequence of events support female-to-male Zika virus transmission through condomless vaginal intercourse,” the coauthors conclude, adding that “virus present in either vaginal fluids or menstrual blood might have been transmitted during exposure to her male partner’s urethral mucosa or undetected abrasions on his penis.”
Both the female and male were tested via real-time reverse transcription–polymerase chain reaction (rRT-PCR), with serum testing done via the Zika immunoglobulin M antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA). The rRT-PCR testing showed Zika virus RNA in the woman’s serum, despite being collected three days after the sexual encounter, meaning she was viremic at the time. Studies on nonhuman primates have shown that Zika virus RNA can remain present in vaginal fluid for up to a week, according to the report.
The CDC is cautioning pregnant women against travel to Zika-heavy areas, in particular the 2016 Summer Olympic Games in Rio de Janeiro. Health care providers who receive patients with Zika-like symptoms should ask if the patient has had sexual contact with someone who has traveled to an affected region, if the patient did not travel to such a region.
FROM MMWR
Pediatric autologous aortic repair built to last
NEW YORK – With more than 1 million adults living today with congenital aortic disease, cardiovascular surgeons must think of outcomes in terms of decades, not years, when performing aortic arch repair in newborns, infants, and children, according to Charles D. Fraser Jr., M.D.
To that end, an all-autologous approach to aortic arch repair is key in preserving problem-free aortic function in adulthood, said Dr. Fraser, surgeon-in-chief at Texas Children’s Hospital in Houston.
Dr. Fraser reported on his center’s experience with all-autologous aortic arch repair techniques. He reviewed the following five principles that guide aortic arch repair in newborns, infants, and children at Texas Children’s Hospital:
• Use of autologous tissue reconstruction and avoidance of prosthetic material.
• Concomitant intracardiac repair.
• Use of anatomic reconstruction.
• Optimization of ventriculoarterial coupling.
• Preservation of laryngeal nerve function.
“The principles we developed at Texas Children’s Hospital we hope will translate into fewer of these patients that surgeons caring for adults with aortic disease will have to take care of later in life,” Dr. Fraser said at the meeting sponsored by the American Association for Thoracic Surgery. He reviewed cases in which he explained techniques he and his colleagues developed to address long-term outcomes.
The first challenge is to determine when to perform aortic repair in pediatric patients. “A question often asked is how small is too small when assessing the aortic arch in association with significant periductal coarctation?” he said. “Our rule of thumb has been that the arch diameter measured in millimeters should be at least the patient’s weight in kilograms plus one.” In other words, a 3-kg baby should have an aortic arch of at least 4 mm in diameter, he said.
He described the case of a 3.8-kg male baby on prostaglandin E1 who had aortic arch advancement repair and closure of atrial and ventricular septal defects at 8 days of age. The patient had an early origin of the left common carotid artery and a small proximal aortic arch. “This is the kind of patient in which we would do a complete aortic arch reconstruction, again with the autologous technique,” Dr. Fraser said.
In such a patient, Dr. Fraser and his colleagues at Texas Children’s Hospital support the circulation to the brain with antegrade cerebral perfusion, using transcranial Doppler and near-infrared spectroscopy to guide their profusion strategy, before putting the child on cardiac bypass and “profound” hypothermia. Careful planning before cannulation is important to perform the aortic transection at the correct level, he said
He also explained the ascending sliding arch aortoplasty, also known as the “Texas slide,” first described by E. Dean McKenzie, M.D., at Texas Children’s Hospital in 2011 (Ann. Thorac. Surg. 2011;91:805-10) This technique involves sliding a tongue-shaped piece of the ascending aorta underneath the aortic arch to construct an all-autologous repair.
“In patients with bicuspid aortic valves, we often observe that the ascending aorta is extremely elongated,” he said. “The idea is to take advantage of that and slide the ascending aorta completely up underneath the aortic arch and construct an all-autologous arch advancement type of repair.”
He presented the case of a 4-year-old boy with coarctation of the aorta in whom the Texas slide was indicated. “If this patient were treated with a simple coarctectomy, the patient would be subject to a life with a moderately hypoplastic aortic arch, and over the course of time, this could be problematic,” Dr. Fraser said. “The sliding reconstruction has relevance not only to the status of the aortic arch over the long term but it also has a profound effect on ventricular function.”
He noted a single-center, retrospective study from the United Kingdom that demonstrated that the quality of the aortic arch reconstruction, and the related opportunity for ventricular arterial coupling, directly correlate with long-term performance of the aortic arch in patients with hypoplastic left heart syndrome (J Thorac Cardiovasc Surg. 2014;148:1526-33).
“This is very important as part of the growing population of these patients who need long-term management, most of whom we’re anticipating managing not just for years, but for decades,” Dr. Fraser said.
He said he had no relevant financial disclosures.
NEW YORK – With more than 1 million adults living today with congenital aortic disease, cardiovascular surgeons must think of outcomes in terms of decades, not years, when performing aortic arch repair in newborns, infants, and children, according to Charles D. Fraser Jr., M.D.
To that end, an all-autologous approach to aortic arch repair is key in preserving problem-free aortic function in adulthood, said Dr. Fraser, surgeon-in-chief at Texas Children’s Hospital in Houston.
Dr. Fraser reported on his center’s experience with all-autologous aortic arch repair techniques. He reviewed the following five principles that guide aortic arch repair in newborns, infants, and children at Texas Children’s Hospital:
• Use of autologous tissue reconstruction and avoidance of prosthetic material.
• Concomitant intracardiac repair.
• Use of anatomic reconstruction.
• Optimization of ventriculoarterial coupling.
• Preservation of laryngeal nerve function.
“The principles we developed at Texas Children’s Hospital we hope will translate into fewer of these patients that surgeons caring for adults with aortic disease will have to take care of later in life,” Dr. Fraser said at the meeting sponsored by the American Association for Thoracic Surgery. He reviewed cases in which he explained techniques he and his colleagues developed to address long-term outcomes.
The first challenge is to determine when to perform aortic repair in pediatric patients. “A question often asked is how small is too small when assessing the aortic arch in association with significant periductal coarctation?” he said. “Our rule of thumb has been that the arch diameter measured in millimeters should be at least the patient’s weight in kilograms plus one.” In other words, a 3-kg baby should have an aortic arch of at least 4 mm in diameter, he said.
He described the case of a 3.8-kg male baby on prostaglandin E1 who had aortic arch advancement repair and closure of atrial and ventricular septal defects at 8 days of age. The patient had an early origin of the left common carotid artery and a small proximal aortic arch. “This is the kind of patient in which we would do a complete aortic arch reconstruction, again with the autologous technique,” Dr. Fraser said.
In such a patient, Dr. Fraser and his colleagues at Texas Children’s Hospital support the circulation to the brain with antegrade cerebral perfusion, using transcranial Doppler and near-infrared spectroscopy to guide their profusion strategy, before putting the child on cardiac bypass and “profound” hypothermia. Careful planning before cannulation is important to perform the aortic transection at the correct level, he said
He also explained the ascending sliding arch aortoplasty, also known as the “Texas slide,” first described by E. Dean McKenzie, M.D., at Texas Children’s Hospital in 2011 (Ann. Thorac. Surg. 2011;91:805-10) This technique involves sliding a tongue-shaped piece of the ascending aorta underneath the aortic arch to construct an all-autologous repair.
“In patients with bicuspid aortic valves, we often observe that the ascending aorta is extremely elongated,” he said. “The idea is to take advantage of that and slide the ascending aorta completely up underneath the aortic arch and construct an all-autologous arch advancement type of repair.”
He presented the case of a 4-year-old boy with coarctation of the aorta in whom the Texas slide was indicated. “If this patient were treated with a simple coarctectomy, the patient would be subject to a life with a moderately hypoplastic aortic arch, and over the course of time, this could be problematic,” Dr. Fraser said. “The sliding reconstruction has relevance not only to the status of the aortic arch over the long term but it also has a profound effect on ventricular function.”
He noted a single-center, retrospective study from the United Kingdom that demonstrated that the quality of the aortic arch reconstruction, and the related opportunity for ventricular arterial coupling, directly correlate with long-term performance of the aortic arch in patients with hypoplastic left heart syndrome (J Thorac Cardiovasc Surg. 2014;148:1526-33).
“This is very important as part of the growing population of these patients who need long-term management, most of whom we’re anticipating managing not just for years, but for decades,” Dr. Fraser said.
He said he had no relevant financial disclosures.
NEW YORK – With more than 1 million adults living today with congenital aortic disease, cardiovascular surgeons must think of outcomes in terms of decades, not years, when performing aortic arch repair in newborns, infants, and children, according to Charles D. Fraser Jr., M.D.
To that end, an all-autologous approach to aortic arch repair is key in preserving problem-free aortic function in adulthood, said Dr. Fraser, surgeon-in-chief at Texas Children’s Hospital in Houston.
Dr. Fraser reported on his center’s experience with all-autologous aortic arch repair techniques. He reviewed the following five principles that guide aortic arch repair in newborns, infants, and children at Texas Children’s Hospital:
• Use of autologous tissue reconstruction and avoidance of prosthetic material.
• Concomitant intracardiac repair.
• Use of anatomic reconstruction.
• Optimization of ventriculoarterial coupling.
• Preservation of laryngeal nerve function.
“The principles we developed at Texas Children’s Hospital we hope will translate into fewer of these patients that surgeons caring for adults with aortic disease will have to take care of later in life,” Dr. Fraser said at the meeting sponsored by the American Association for Thoracic Surgery. He reviewed cases in which he explained techniques he and his colleagues developed to address long-term outcomes.
The first challenge is to determine when to perform aortic repair in pediatric patients. “A question often asked is how small is too small when assessing the aortic arch in association with significant periductal coarctation?” he said. “Our rule of thumb has been that the arch diameter measured in millimeters should be at least the patient’s weight in kilograms plus one.” In other words, a 3-kg baby should have an aortic arch of at least 4 mm in diameter, he said.
He described the case of a 3.8-kg male baby on prostaglandin E1 who had aortic arch advancement repair and closure of atrial and ventricular septal defects at 8 days of age. The patient had an early origin of the left common carotid artery and a small proximal aortic arch. “This is the kind of patient in which we would do a complete aortic arch reconstruction, again with the autologous technique,” Dr. Fraser said.
In such a patient, Dr. Fraser and his colleagues at Texas Children’s Hospital support the circulation to the brain with antegrade cerebral perfusion, using transcranial Doppler and near-infrared spectroscopy to guide their profusion strategy, before putting the child on cardiac bypass and “profound” hypothermia. Careful planning before cannulation is important to perform the aortic transection at the correct level, he said
He also explained the ascending sliding arch aortoplasty, also known as the “Texas slide,” first described by E. Dean McKenzie, M.D., at Texas Children’s Hospital in 2011 (Ann. Thorac. Surg. 2011;91:805-10) This technique involves sliding a tongue-shaped piece of the ascending aorta underneath the aortic arch to construct an all-autologous repair.
“In patients with bicuspid aortic valves, we often observe that the ascending aorta is extremely elongated,” he said. “The idea is to take advantage of that and slide the ascending aorta completely up underneath the aortic arch and construct an all-autologous arch advancement type of repair.”
He presented the case of a 4-year-old boy with coarctation of the aorta in whom the Texas slide was indicated. “If this patient were treated with a simple coarctectomy, the patient would be subject to a life with a moderately hypoplastic aortic arch, and over the course of time, this could be problematic,” Dr. Fraser said. “The sliding reconstruction has relevance not only to the status of the aortic arch over the long term but it also has a profound effect on ventricular function.”
He noted a single-center, retrospective study from the United Kingdom that demonstrated that the quality of the aortic arch reconstruction, and the related opportunity for ventricular arterial coupling, directly correlate with long-term performance of the aortic arch in patients with hypoplastic left heart syndrome (J Thorac Cardiovasc Surg. 2014;148:1526-33).
“This is very important as part of the growing population of these patients who need long-term management, most of whom we’re anticipating managing not just for years, but for decades,” Dr. Fraser said.
He said he had no relevant financial disclosures.
AT AATS AORTIC SYMPOSIUM 2016
Key clinical point: Surgeons must think of outcomes for operations to correct aortic arch disease in children in the context of decades, not years.
Major finding: Five principles should guide autologous aortic arch repair in newborns, infants, and children.
Data source: Case studies from Texas Children’s Hospital.
Disclosures: Dr. Fraser reported having no relevant financial disclosures.
Prevention of postop GI disorders may reduce ‘failed discharges’ for laparoscopic hernia surgery
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).
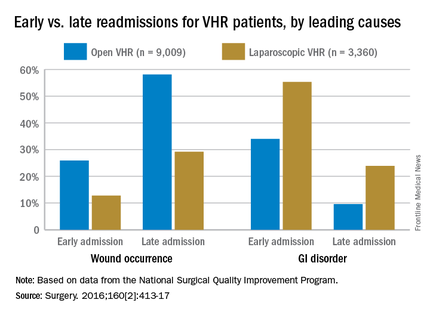
Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).

Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).

Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
FROM SURGERY
Key clinical point: Postoperative complications and likelihood of readmission differ according to the type of ventral hernia repair procedure (open or laparoscopic).
Major finding: Thirty-day readmissions occurred among 6.9% of patients who had laparoscopic surgery, compared with 9.2% of open-procedure patients; gastrointestinal disorders were more common in early readmissions but wound occurrences were more common in late readmissions.
Data source: Retrospective cohort study of 12,369 ventral hernia repair patients (open and laparoscopic) in 2012.
Disclosures: Funding source not disclosed. Authors did not report any relevant financial disclosures.
Protective hypothermia during arch surgery lacked benefit, study shows
NEW YORK – Deep hypothermia may affect long-term survival in individuals who have aortic arch surgery with antegrade cerebral perfusion (ACP), but not short-term outcomes in terms of death and major morbidities, according to a Baylor College of Medicine study.
The study evaluated outcomes of 544 consecutive patients who had proximal and total aortic arch surgery and received ACP for more than 30 minutes over a 10-year period, said lead investigator Ourania Preventza, MD, of the division of cardiothoracic surgery at the college in Houston. The researchers compared results of three different hypothermia levels: deep hypothermia at 14.1°-20° C; low-moderate at 20.1°-23.9° C; and high-moderate at 24°-28° C. The study also classified ACP time in two levels: 31-45 minutes for 238 patients (43.8%); and 45 minutes or more in 306 patients (56.3%).
“The different temperature levels did not significantly affect the short-term mortality and major morbidity rates,” Dr. Preventza said. “Reoperation for bleeding was associated with lower temperature (14.1°-20° C). The long-term survival rate in patients who underwent proximal arch surgery involving ACP for more than 30 minutes and use of moderate hypothermia (20.1°-28° C) were actually improved.”
While the outcomes showed small variations between the three groups, with deep hypothermia being associated with a higher percentage of adverse outcomes, Dr. Preventza said the differences were not statistically significant. The overall operative mortality rate was 12.5% (68 patients): 15.5% (18 patients) in the deep-hypothermia group; 11.8% (31 patients) in the low-moderate group; and 11.5% (19 patients) in the high-moderate group (P = 0.54).
The patients who underwent deep hypothermia were more likely to receive unilateral ACP, and those who underwent moderate hypothermia were more likely to have bilateral ACP, Dr. Preventza said at the meeting sponsored by the American Association for Thoracic Surgery. The deep-hypothermia group had higher transfusion rates, but, again, the researchers did not consider this variation to be statistically significant.
In the deep-hypothermia group, 20.9% of patients had a reoperation for bleeding, compared with 11.3% in the overall group and 7.7% and 10.2% in the low- and high-moderate groups, respectively, Dr. Preventza reported. Multivariate analysis revealed that higher temperature was associated with less bleeding, with an odds ratio of 0.61 (P = 0.015).
Deep hypothermia was related to statistically significant differences in the rates of permanent stroke and permanent neurologic events in the univariate analysis only, Dr. Preventza said: 6.3% and 7.2%, respectively, in the overall analysis vs. 12.2% for both events in the deep-hypothermia group. In the propensity score analysis, the rates of permanent stroke and permanent neurologic events in the moderate-hypothermia group were 7.6% and 8.5%, respectively, vs. 11.3% for both events in the deep-hypothermia group, a nonsignificant difference.
“With regard to permanent stoke and permanent neurological events, the multivariate analysis showed that preoperatively a neurologic deficit as well as acute type I aortic dissection were associated with adverse neurological events,” she said.
“However,” Dr. Preventza added, “the surprising thing is that when we looked at long-term survival for the entire cohort, we saw that the patients with moderate hypothermia did better.”
Kaplan-Meier analysis for the propensity pairs showed that the probability of survival at 8 years was 55.3% for the deep-hypothermia group vs. 68.5% for the moderate-hypothermia group.
The approach the Baylor researchers used involved cannulating the axillary or innominate artery in most patients before administering ACP, although a few patients had femoral or direct aortic cannulation, Dr. Preventza said. For bilateral ACP, the researchers delivered cerebral perfusion via a 9-French Pruitt balloon-tip catheter (LeMaitre Vascular) in the left common carotid artery. To protect the brain, they administered perfusion at 8-12 cc/kg per min and maintained a perfusion pressure of 50-70 mm Hg, as measured via the radial arterial line and guided by near-infrared spectroscopy.
Dr. Preventza had no relevant disclosures.
NEW YORK – Deep hypothermia may affect long-term survival in individuals who have aortic arch surgery with antegrade cerebral perfusion (ACP), but not short-term outcomes in terms of death and major morbidities, according to a Baylor College of Medicine study.
The study evaluated outcomes of 544 consecutive patients who had proximal and total aortic arch surgery and received ACP for more than 30 minutes over a 10-year period, said lead investigator Ourania Preventza, MD, of the division of cardiothoracic surgery at the college in Houston. The researchers compared results of three different hypothermia levels: deep hypothermia at 14.1°-20° C; low-moderate at 20.1°-23.9° C; and high-moderate at 24°-28° C. The study also classified ACP time in two levels: 31-45 minutes for 238 patients (43.8%); and 45 minutes or more in 306 patients (56.3%).
“The different temperature levels did not significantly affect the short-term mortality and major morbidity rates,” Dr. Preventza said. “Reoperation for bleeding was associated with lower temperature (14.1°-20° C). The long-term survival rate in patients who underwent proximal arch surgery involving ACP for more than 30 minutes and use of moderate hypothermia (20.1°-28° C) were actually improved.”
While the outcomes showed small variations between the three groups, with deep hypothermia being associated with a higher percentage of adverse outcomes, Dr. Preventza said the differences were not statistically significant. The overall operative mortality rate was 12.5% (68 patients): 15.5% (18 patients) in the deep-hypothermia group; 11.8% (31 patients) in the low-moderate group; and 11.5% (19 patients) in the high-moderate group (P = 0.54).
The patients who underwent deep hypothermia were more likely to receive unilateral ACP, and those who underwent moderate hypothermia were more likely to have bilateral ACP, Dr. Preventza said at the meeting sponsored by the American Association for Thoracic Surgery. The deep-hypothermia group had higher transfusion rates, but, again, the researchers did not consider this variation to be statistically significant.
In the deep-hypothermia group, 20.9% of patients had a reoperation for bleeding, compared with 11.3% in the overall group and 7.7% and 10.2% in the low- and high-moderate groups, respectively, Dr. Preventza reported. Multivariate analysis revealed that higher temperature was associated with less bleeding, with an odds ratio of 0.61 (P = 0.015).
Deep hypothermia was related to statistically significant differences in the rates of permanent stroke and permanent neurologic events in the univariate analysis only, Dr. Preventza said: 6.3% and 7.2%, respectively, in the overall analysis vs. 12.2% for both events in the deep-hypothermia group. In the propensity score analysis, the rates of permanent stroke and permanent neurologic events in the moderate-hypothermia group were 7.6% and 8.5%, respectively, vs. 11.3% for both events in the deep-hypothermia group, a nonsignificant difference.
“With regard to permanent stoke and permanent neurological events, the multivariate analysis showed that preoperatively a neurologic deficit as well as acute type I aortic dissection were associated with adverse neurological events,” she said.
“However,” Dr. Preventza added, “the surprising thing is that when we looked at long-term survival for the entire cohort, we saw that the patients with moderate hypothermia did better.”
Kaplan-Meier analysis for the propensity pairs showed that the probability of survival at 8 years was 55.3% for the deep-hypothermia group vs. 68.5% for the moderate-hypothermia group.
The approach the Baylor researchers used involved cannulating the axillary or innominate artery in most patients before administering ACP, although a few patients had femoral or direct aortic cannulation, Dr. Preventza said. For bilateral ACP, the researchers delivered cerebral perfusion via a 9-French Pruitt balloon-tip catheter (LeMaitre Vascular) in the left common carotid artery. To protect the brain, they administered perfusion at 8-12 cc/kg per min and maintained a perfusion pressure of 50-70 mm Hg, as measured via the radial arterial line and guided by near-infrared spectroscopy.
Dr. Preventza had no relevant disclosures.
NEW YORK – Deep hypothermia may affect long-term survival in individuals who have aortic arch surgery with antegrade cerebral perfusion (ACP), but not short-term outcomes in terms of death and major morbidities, according to a Baylor College of Medicine study.
The study evaluated outcomes of 544 consecutive patients who had proximal and total aortic arch surgery and received ACP for more than 30 minutes over a 10-year period, said lead investigator Ourania Preventza, MD, of the division of cardiothoracic surgery at the college in Houston. The researchers compared results of three different hypothermia levels: deep hypothermia at 14.1°-20° C; low-moderate at 20.1°-23.9° C; and high-moderate at 24°-28° C. The study also classified ACP time in two levels: 31-45 minutes for 238 patients (43.8%); and 45 minutes or more in 306 patients (56.3%).
“The different temperature levels did not significantly affect the short-term mortality and major morbidity rates,” Dr. Preventza said. “Reoperation for bleeding was associated with lower temperature (14.1°-20° C). The long-term survival rate in patients who underwent proximal arch surgery involving ACP for more than 30 minutes and use of moderate hypothermia (20.1°-28° C) were actually improved.”
While the outcomes showed small variations between the three groups, with deep hypothermia being associated with a higher percentage of adverse outcomes, Dr. Preventza said the differences were not statistically significant. The overall operative mortality rate was 12.5% (68 patients): 15.5% (18 patients) in the deep-hypothermia group; 11.8% (31 patients) in the low-moderate group; and 11.5% (19 patients) in the high-moderate group (P = 0.54).
The patients who underwent deep hypothermia were more likely to receive unilateral ACP, and those who underwent moderate hypothermia were more likely to have bilateral ACP, Dr. Preventza said at the meeting sponsored by the American Association for Thoracic Surgery. The deep-hypothermia group had higher transfusion rates, but, again, the researchers did not consider this variation to be statistically significant.
In the deep-hypothermia group, 20.9% of patients had a reoperation for bleeding, compared with 11.3% in the overall group and 7.7% and 10.2% in the low- and high-moderate groups, respectively, Dr. Preventza reported. Multivariate analysis revealed that higher temperature was associated with less bleeding, with an odds ratio of 0.61 (P = 0.015).
Deep hypothermia was related to statistically significant differences in the rates of permanent stroke and permanent neurologic events in the univariate analysis only, Dr. Preventza said: 6.3% and 7.2%, respectively, in the overall analysis vs. 12.2% for both events in the deep-hypothermia group. In the propensity score analysis, the rates of permanent stroke and permanent neurologic events in the moderate-hypothermia group were 7.6% and 8.5%, respectively, vs. 11.3% for both events in the deep-hypothermia group, a nonsignificant difference.
“With regard to permanent stoke and permanent neurological events, the multivariate analysis showed that preoperatively a neurologic deficit as well as acute type I aortic dissection were associated with adverse neurological events,” she said.
“However,” Dr. Preventza added, “the surprising thing is that when we looked at long-term survival for the entire cohort, we saw that the patients with moderate hypothermia did better.”
Kaplan-Meier analysis for the propensity pairs showed that the probability of survival at 8 years was 55.3% for the deep-hypothermia group vs. 68.5% for the moderate-hypothermia group.
The approach the Baylor researchers used involved cannulating the axillary or innominate artery in most patients before administering ACP, although a few patients had femoral or direct aortic cannulation, Dr. Preventza said. For bilateral ACP, the researchers delivered cerebral perfusion via a 9-French Pruitt balloon-tip catheter (LeMaitre Vascular) in the left common carotid artery. To protect the brain, they administered perfusion at 8-12 cc/kg per min and maintained a perfusion pressure of 50-70 mm Hg, as measured via the radial arterial line and guided by near-infrared spectroscopy.
Dr. Preventza had no relevant disclosures.
AT AATS AORTIC SYMPOSIUM 2016
Key clinical point: Differing temperatures of hypothermia did not affect death or morbidity in patients who had aortic arch surgery with more than 30 minutes of antegrade cerebral perfusion.
Major finding: The overall operative death rate in the study was 12.4% with no statistically significant differences between three different hypothermia groups.
Data source: Series of 510 consecutive patients who had proximal and total arch surgery and received antegrade cerebral perfusion for more than 30 minutes over a 10-year period.
Disclosures: Dr. Preventza reported having no financial disclosures.
Congress sends opioid legislation to the President
Compromise legislation to address the opioid epidemic has overwhelmingly passed both houses of Congress and been sent to President Obama, who has signaled that he will sign it begrudgingly.
“We continue to believe this bill falls far short,” according to a statement from the White House. “That’s why the administration strongly supported Democratic efforts to add $920 million in funding for states to provide treatment for Americans struggling with opioid addiction. ... While the president will sign this bill once it reaches his desk because some action is better than none, he won’t stop fighting to secure the resources this public health crisis demands.”
The final version of the Comprehensive Addiction and Recovery Act (S. 534) passed the House by a 407-5 vote and the Senate by a 92-2 vote.
The legislation allows the Health & Human Services Department to make training and education grants to address opioid addiction, as well as grants to address opioid abuse locally. HHS may also make grants to states to help pay for opioid overdose–reversal programs. It also provides $50 million over 5 years to improve state-level prescription drug-monitoring programs.
S. 524 also allows the Department of Justice to develop programs to combat opioid abuse in a number of ways. Grants would fund alternatives to incarceration programs, train first responders in using opioid overdose–reversal medications, create more prescription take-back programs, and investigative activities related to unlawful distribution of opioids.
The bill also expands access to medication-assisted treatment of opioid abuse/addiction by extending prescribing authority to certain specially trained nurse practitioners and physician assistants. It also allows Medicare Parts C and D to develop safe prescribing and dispensing programs, such as “lock-in” programs that require beneficiaries at risk for substance abuse to use a single designated source for opioid prescriptions. The legislation also has a number of provisions aimed specifically at the Department of Veterans Affairs.
In a statement, the American College of Physicians highlighted a few additional provisions that it supported, including the development of a federal task force to identify best practices for pain management and prescribing pain medication; expanding grants to education physician and other stakeholders on the risks of opioid misuse; improvements to the Prescription Drug Monitoring Program; increased availability of opioid overdose–reversal medication; and expansion of the use of partial fills for opioid prescriptions.
“This legislation takes important steps to address the growing crisis and to provide patients with greater access to care and treatment they need to deal with substance use disorders,” Nitin Damle, MD, ACP president, said in a statement.
Compromise legislation to address the opioid epidemic has overwhelmingly passed both houses of Congress and been sent to President Obama, who has signaled that he will sign it begrudgingly.
“We continue to believe this bill falls far short,” according to a statement from the White House. “That’s why the administration strongly supported Democratic efforts to add $920 million in funding for states to provide treatment for Americans struggling with opioid addiction. ... While the president will sign this bill once it reaches his desk because some action is better than none, he won’t stop fighting to secure the resources this public health crisis demands.”
The final version of the Comprehensive Addiction and Recovery Act (S. 534) passed the House by a 407-5 vote and the Senate by a 92-2 vote.
The legislation allows the Health & Human Services Department to make training and education grants to address opioid addiction, as well as grants to address opioid abuse locally. HHS may also make grants to states to help pay for opioid overdose–reversal programs. It also provides $50 million over 5 years to improve state-level prescription drug-monitoring programs.
S. 524 also allows the Department of Justice to develop programs to combat opioid abuse in a number of ways. Grants would fund alternatives to incarceration programs, train first responders in using opioid overdose–reversal medications, create more prescription take-back programs, and investigative activities related to unlawful distribution of opioids.
The bill also expands access to medication-assisted treatment of opioid abuse/addiction by extending prescribing authority to certain specially trained nurse practitioners and physician assistants. It also allows Medicare Parts C and D to develop safe prescribing and dispensing programs, such as “lock-in” programs that require beneficiaries at risk for substance abuse to use a single designated source for opioid prescriptions. The legislation also has a number of provisions aimed specifically at the Department of Veterans Affairs.
In a statement, the American College of Physicians highlighted a few additional provisions that it supported, including the development of a federal task force to identify best practices for pain management and prescribing pain medication; expanding grants to education physician and other stakeholders on the risks of opioid misuse; improvements to the Prescription Drug Monitoring Program; increased availability of opioid overdose–reversal medication; and expansion of the use of partial fills for opioid prescriptions.
“This legislation takes important steps to address the growing crisis and to provide patients with greater access to care and treatment they need to deal with substance use disorders,” Nitin Damle, MD, ACP president, said in a statement.
Compromise legislation to address the opioid epidemic has overwhelmingly passed both houses of Congress and been sent to President Obama, who has signaled that he will sign it begrudgingly.
“We continue to believe this bill falls far short,” according to a statement from the White House. “That’s why the administration strongly supported Democratic efforts to add $920 million in funding for states to provide treatment for Americans struggling with opioid addiction. ... While the president will sign this bill once it reaches his desk because some action is better than none, he won’t stop fighting to secure the resources this public health crisis demands.”
The final version of the Comprehensive Addiction and Recovery Act (S. 534) passed the House by a 407-5 vote and the Senate by a 92-2 vote.
The legislation allows the Health & Human Services Department to make training and education grants to address opioid addiction, as well as grants to address opioid abuse locally. HHS may also make grants to states to help pay for opioid overdose–reversal programs. It also provides $50 million over 5 years to improve state-level prescription drug-monitoring programs.
S. 524 also allows the Department of Justice to develop programs to combat opioid abuse in a number of ways. Grants would fund alternatives to incarceration programs, train first responders in using opioid overdose–reversal medications, create more prescription take-back programs, and investigative activities related to unlawful distribution of opioids.
The bill also expands access to medication-assisted treatment of opioid abuse/addiction by extending prescribing authority to certain specially trained nurse practitioners and physician assistants. It also allows Medicare Parts C and D to develop safe prescribing and dispensing programs, such as “lock-in” programs that require beneficiaries at risk for substance abuse to use a single designated source for opioid prescriptions. The legislation also has a number of provisions aimed specifically at the Department of Veterans Affairs.
In a statement, the American College of Physicians highlighted a few additional provisions that it supported, including the development of a federal task force to identify best practices for pain management and prescribing pain medication; expanding grants to education physician and other stakeholders on the risks of opioid misuse; improvements to the Prescription Drug Monitoring Program; increased availability of opioid overdose–reversal medication; and expansion of the use of partial fills for opioid prescriptions.
“This legislation takes important steps to address the growing crisis and to provide patients with greater access to care and treatment they need to deal with substance use disorders,” Nitin Damle, MD, ACP president, said in a statement.
Switch may increase supply of HSCs from cord blood

Photo courtesy of NHS
Researchers have discovered a genetic switch that could increase the supply of hematopoietic stem cells (HSCs) derived from cord blood, according to a paper published in Cell Stem Cell.
However, experiments in mice suggest that flipping the switch may also spur the development of myeloproliferative disease.
The researchers explained that, when an HSC divides, it produces multipotent progenitor (MPP) cells immediately downstream that retain the ability to differentiate but have lost the ability to self-renew.
By analyzing murine and human models of hematopoiesis, the team discovered that a microRNA, miR-125a, controls self-renewal and is normally switched on in HSCs but turned off in MPPs.
“Our work shows that if we artificially throw the switch on in those downstream cells [MPPs], we can endow them with stemness, and they basically become stem cells and can be maintained over the long term,” said study author John Dick, PhD, of Princess Margaret Cancer Centre at University Health Network in Toronto, Ontario, Canada.
Specifically, Dr Dick and his colleagues found that overexpression of miR-125a induced stem cell potential in both human MPPs and an as-yet-unidentified population within the CD34+38+ committed progenitor compartment.
Overexpression of miR-125a had a similar effect in murine MPPs. When the researchers transplanted these MPPs into recipient mice, the cells exhibited increased self-renewal and generated high levels of multi-lineage reconstitution for up to 16 weeks after transplant.
Unfortunately, when the miR-125a-overexpressed MPPs were transplanted into secondary and tertiary recipient mice, the animals developed symptoms of a myeloproliferative disease.
The researchers said this result is in line with findings from previous studies and has been shown to be dependent upon sustained expression of miR-125a and dosage.
The current study suggested that the miR-125a-induced myeloproliferative disease occurs, in part, as a function of replicative stress.
The researchers therefore speculated that, with careful titration of miR-125a levels, it may be possible to reap the beneficial self-renewal effects of miR-125a without inducing myeloproliferative disease.
In fact, the team hopes that, in the future, approaches combining miR-125a-modified MPPs with existing small-molecule compounds will enable the expansion of cord blood units for transplant. ![]()

Photo courtesy of NHS
Researchers have discovered a genetic switch that could increase the supply of hematopoietic stem cells (HSCs) derived from cord blood, according to a paper published in Cell Stem Cell.
However, experiments in mice suggest that flipping the switch may also spur the development of myeloproliferative disease.
The researchers explained that, when an HSC divides, it produces multipotent progenitor (MPP) cells immediately downstream that retain the ability to differentiate but have lost the ability to self-renew.
By analyzing murine and human models of hematopoiesis, the team discovered that a microRNA, miR-125a, controls self-renewal and is normally switched on in HSCs but turned off in MPPs.
“Our work shows that if we artificially throw the switch on in those downstream cells [MPPs], we can endow them with stemness, and they basically become stem cells and can be maintained over the long term,” said study author John Dick, PhD, of Princess Margaret Cancer Centre at University Health Network in Toronto, Ontario, Canada.
Specifically, Dr Dick and his colleagues found that overexpression of miR-125a induced stem cell potential in both human MPPs and an as-yet-unidentified population within the CD34+38+ committed progenitor compartment.
Overexpression of miR-125a had a similar effect in murine MPPs. When the researchers transplanted these MPPs into recipient mice, the cells exhibited increased self-renewal and generated high levels of multi-lineage reconstitution for up to 16 weeks after transplant.
Unfortunately, when the miR-125a-overexpressed MPPs were transplanted into secondary and tertiary recipient mice, the animals developed symptoms of a myeloproliferative disease.
The researchers said this result is in line with findings from previous studies and has been shown to be dependent upon sustained expression of miR-125a and dosage.
The current study suggested that the miR-125a-induced myeloproliferative disease occurs, in part, as a function of replicative stress.
The researchers therefore speculated that, with careful titration of miR-125a levels, it may be possible to reap the beneficial self-renewal effects of miR-125a without inducing myeloproliferative disease.
In fact, the team hopes that, in the future, approaches combining miR-125a-modified MPPs with existing small-molecule compounds will enable the expansion of cord blood units for transplant. ![]()

Photo courtesy of NHS
Researchers have discovered a genetic switch that could increase the supply of hematopoietic stem cells (HSCs) derived from cord blood, according to a paper published in Cell Stem Cell.
However, experiments in mice suggest that flipping the switch may also spur the development of myeloproliferative disease.
The researchers explained that, when an HSC divides, it produces multipotent progenitor (MPP) cells immediately downstream that retain the ability to differentiate but have lost the ability to self-renew.
By analyzing murine and human models of hematopoiesis, the team discovered that a microRNA, miR-125a, controls self-renewal and is normally switched on in HSCs but turned off in MPPs.
“Our work shows that if we artificially throw the switch on in those downstream cells [MPPs], we can endow them with stemness, and they basically become stem cells and can be maintained over the long term,” said study author John Dick, PhD, of Princess Margaret Cancer Centre at University Health Network in Toronto, Ontario, Canada.
Specifically, Dr Dick and his colleagues found that overexpression of miR-125a induced stem cell potential in both human MPPs and an as-yet-unidentified population within the CD34+38+ committed progenitor compartment.
Overexpression of miR-125a had a similar effect in murine MPPs. When the researchers transplanted these MPPs into recipient mice, the cells exhibited increased self-renewal and generated high levels of multi-lineage reconstitution for up to 16 weeks after transplant.
Unfortunately, when the miR-125a-overexpressed MPPs were transplanted into secondary and tertiary recipient mice, the animals developed symptoms of a myeloproliferative disease.
The researchers said this result is in line with findings from previous studies and has been shown to be dependent upon sustained expression of miR-125a and dosage.
The current study suggested that the miR-125a-induced myeloproliferative disease occurs, in part, as a function of replicative stress.
The researchers therefore speculated that, with careful titration of miR-125a levels, it may be possible to reap the beneficial self-renewal effects of miR-125a without inducing myeloproliferative disease.
In fact, the team hopes that, in the future, approaches combining miR-125a-modified MPPs with existing small-molecule compounds will enable the expansion of cord blood units for transplant. ![]()
Cancer patients and docs disagree about prognosis

patient and her father
Photo by Rhoda Baer
In a survey of advanced cancer patients and their oncologists, differing opinions about prognosis were common.
And the vast majority of patients didn’t know their doctors held different opinions about how long the patients might live.
Results of the survey were published in JAMA Oncology.
“We’ve discovered 2 important things happening between oncologists and patients with advanced cancer,” said study author Ronald M. Epstein, MD, of the University of Rochester Medical Center in Rochester, New York.
“First, some patients might know the doctor’s prognosis estimate, but the patient chooses to disagree, often because they believe other sources. And, second, some patients think that their doctor agrees with their opinion about prognosis but, in fact, the doctor doesn’t.”
Dr Epstein and his colleagues surveyed 236 patients with stage 3 or 4 cancer. According to medical evidence, fewer than 5% of these patients would be expected to live for 5 years.
The 38 oncologists who treated these patients were also surveyed. The doctors were asked,“What do you believe are the chances that this patient will live for 2 years or more?” And the patients were asked, “What do you believe are the chances that you will live for 2 years or more?”
Additional survey questions gauged whether patients knew their prognosis opinions differed from their doctors and to what extent treatment options were discussed in the context of life expectancy.
Among the 236 patients, 68% rated their survival prognosis differently than their oncologists, and 89% of these patients did not realize their opinions differed from their oncologists. In nearly all cases (96%), the patients were more optimistic than their doctors.
“Of course, it’s only possible for doctors to provide a ball-park estimate about life expectancy, and some people do beat the odds,” Dr Epstein noted. “But when a patient with very advanced cancer says that he has a 90% to 100% chance of being alive in 2 years and his oncologist believes that chance is more like 10%, there’s a problem.”
The challenge, according to Dr Epstein and his colleagues, is that talking about a cancer prognosis is not a straightforward exchange of information. It occurs in the context of fear, confusion, and uncertainty.
The researchers said prognosis should be addressed in several conversations about personal values and treatment goals. When doctor-patient communication is poor, it can result in mutual regret about end-of-life circumstances.
For example, nearly all of the patients surveyed said they wanted to be involved in treatment decisions. And 70% said they preferred supportive care at the end of their lives as opposed to aggressive therapy. However, as the researchers pointed out, making an informed decision requires knowing when death is approaching.
“When people think they’ll live a very long time with cancer, despite evidence to the contrary, they may end up taking more aggressive chemotherapy and agreeing to be placed on ventilators or dialysis, paradoxically reducing their quality of life, keeping them from enjoying time with family, and sometimes even shortening their lives,” Dr Epstein said. “So it’s very important for doctors and patients to be on the same page.” ![]()

patient and her father
Photo by Rhoda Baer
In a survey of advanced cancer patients and their oncologists, differing opinions about prognosis were common.
And the vast majority of patients didn’t know their doctors held different opinions about how long the patients might live.
Results of the survey were published in JAMA Oncology.
“We’ve discovered 2 important things happening between oncologists and patients with advanced cancer,” said study author Ronald M. Epstein, MD, of the University of Rochester Medical Center in Rochester, New York.
“First, some patients might know the doctor’s prognosis estimate, but the patient chooses to disagree, often because they believe other sources. And, second, some patients think that their doctor agrees with their opinion about prognosis but, in fact, the doctor doesn’t.”
Dr Epstein and his colleagues surveyed 236 patients with stage 3 or 4 cancer. According to medical evidence, fewer than 5% of these patients would be expected to live for 5 years.
The 38 oncologists who treated these patients were also surveyed. The doctors were asked,“What do you believe are the chances that this patient will live for 2 years or more?” And the patients were asked, “What do you believe are the chances that you will live for 2 years or more?”
Additional survey questions gauged whether patients knew their prognosis opinions differed from their doctors and to what extent treatment options were discussed in the context of life expectancy.
Among the 236 patients, 68% rated their survival prognosis differently than their oncologists, and 89% of these patients did not realize their opinions differed from their oncologists. In nearly all cases (96%), the patients were more optimistic than their doctors.
“Of course, it’s only possible for doctors to provide a ball-park estimate about life expectancy, and some people do beat the odds,” Dr Epstein noted. “But when a patient with very advanced cancer says that he has a 90% to 100% chance of being alive in 2 years and his oncologist believes that chance is more like 10%, there’s a problem.”
The challenge, according to Dr Epstein and his colleagues, is that talking about a cancer prognosis is not a straightforward exchange of information. It occurs in the context of fear, confusion, and uncertainty.
The researchers said prognosis should be addressed in several conversations about personal values and treatment goals. When doctor-patient communication is poor, it can result in mutual regret about end-of-life circumstances.
For example, nearly all of the patients surveyed said they wanted to be involved in treatment decisions. And 70% said they preferred supportive care at the end of their lives as opposed to aggressive therapy. However, as the researchers pointed out, making an informed decision requires knowing when death is approaching.
“When people think they’ll live a very long time with cancer, despite evidence to the contrary, they may end up taking more aggressive chemotherapy and agreeing to be placed on ventilators or dialysis, paradoxically reducing their quality of life, keeping them from enjoying time with family, and sometimes even shortening their lives,” Dr Epstein said. “So it’s very important for doctors and patients to be on the same page.” ![]()

patient and her father
Photo by Rhoda Baer
In a survey of advanced cancer patients and their oncologists, differing opinions about prognosis were common.
And the vast majority of patients didn’t know their doctors held different opinions about how long the patients might live.
Results of the survey were published in JAMA Oncology.
“We’ve discovered 2 important things happening between oncologists and patients with advanced cancer,” said study author Ronald M. Epstein, MD, of the University of Rochester Medical Center in Rochester, New York.
“First, some patients might know the doctor’s prognosis estimate, but the patient chooses to disagree, often because they believe other sources. And, second, some patients think that their doctor agrees with their opinion about prognosis but, in fact, the doctor doesn’t.”
Dr Epstein and his colleagues surveyed 236 patients with stage 3 or 4 cancer. According to medical evidence, fewer than 5% of these patients would be expected to live for 5 years.
The 38 oncologists who treated these patients were also surveyed. The doctors were asked,“What do you believe are the chances that this patient will live for 2 years or more?” And the patients were asked, “What do you believe are the chances that you will live for 2 years or more?”
Additional survey questions gauged whether patients knew their prognosis opinions differed from their doctors and to what extent treatment options were discussed in the context of life expectancy.
Among the 236 patients, 68% rated their survival prognosis differently than their oncologists, and 89% of these patients did not realize their opinions differed from their oncologists. In nearly all cases (96%), the patients were more optimistic than their doctors.
“Of course, it’s only possible for doctors to provide a ball-park estimate about life expectancy, and some people do beat the odds,” Dr Epstein noted. “But when a patient with very advanced cancer says that he has a 90% to 100% chance of being alive in 2 years and his oncologist believes that chance is more like 10%, there’s a problem.”
The challenge, according to Dr Epstein and his colleagues, is that talking about a cancer prognosis is not a straightforward exchange of information. It occurs in the context of fear, confusion, and uncertainty.
The researchers said prognosis should be addressed in several conversations about personal values and treatment goals. When doctor-patient communication is poor, it can result in mutual regret about end-of-life circumstances.
For example, nearly all of the patients surveyed said they wanted to be involved in treatment decisions. And 70% said they preferred supportive care at the end of their lives as opposed to aggressive therapy. However, as the researchers pointed out, making an informed decision requires knowing when death is approaching.
“When people think they’ll live a very long time with cancer, despite evidence to the contrary, they may end up taking more aggressive chemotherapy and agreeing to be placed on ventilators or dialysis, paradoxically reducing their quality of life, keeping them from enjoying time with family, and sometimes even shortening their lives,” Dr Epstein said. “So it’s very important for doctors and patients to be on the same page.” ![]()
Weight loss lowers levels of cancer-associated proteins

A study of more than 400 women suggests that losing weight can reduce levels of cancer-promoting proteins in the blood.
Overweight or obese women who lost weight over a 12-month period—through diet alone or both diet and exercise—significantly lowered their levels of proteins that play a role in angiogenesis.
Researchers say this finding suggests that losing weight might help reduce the risk of developing certain cancers.
“We know that being overweight and having a sedentary lifestyle is associated with an increase in risk for developing certain types of cancer,” said Catherine Duggan, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“However, we don’t know exactly why. We wanted to investigate how levels of some biomarkers associated with angiogenesis were altered when overweight, sedentary, postmenopausal women enrolled in a research study lost weight and/or became physically active over the course of a year.”
Dr Duggan and her colleagues described this investigation in Cancer Research.
The team studied 439 women who were postmenopausal and overweight or obese but were otherwise healthy and ranged in age from 50 to 75.
The women were randomized to 1 of 4 study arms:
- A diet arm, in which women restricted their calorie intake to no more than 2000 kcal per day that included less than 30% of fat calories
- An aerobic exercise arm, in which women performed 45 minutes of moderate to vigorous exercise 5 days a week
- A combined diet and exercise arm
- A control arm.
The researchers collected blood samples at baseline and at 12 months, measuring levels of the angiogenesis-related proteins VEGF, PAI-1, and PEDF.
They also measured weight loss at 12 months and found that women in all 3 intervention arms had a significantly higher mean weight loss than women in the control arm.
The mean weight loss was 0.8% of body weight for women in the control arm, 2.4% for women in the exercise arm (P=0.03), 8.5% for women in the diet arm (P<0.001), and 10.8% for women in the diet and exercise arm (P<0.001).
Compared with women in the control arm, those in the diet-only arm and the diet and exercise arm had significantly lower levels of the angiogenesis-related proteins at 12 months. However, such effects were not apparent among women in the exercise-only arm.
Specifically, women in the diet and exercise arm had a significantly greater reduction in PAI-1 at 12 months than women in the control arm (-19.3% and +3.48%, respectively, P<0.0001).
Women in the diet-only arm and the diet and exercise arm had significantly greater reductions in PEDF than controls (-9.20%, -9.90%, and +0.18%, respectively, both P<0.0001).
And women in the diet-only arm (-8.25%, P=0.0005) and the diet and exercise arm (-9.98%, P<0.0001) had significantly greater reductions in VEGF than controls (-1.21%).
The researchers also observed a linear trend in the reductions. So the more weight loss the women experienced, the greater the reduction in angiogenesis-related protein levels.
“Our study shows that weight loss is a safe and effective method of improving the angiogenic profile in healthy individuals,” Dr Duggan said. “We were surprised by the magnitude of change in these biomarkers with weight loss.”
“While we can’t say for certain that reducing the circulating levels of angiogenic factors through weight loss would impact the growth of tumors, it is possible that they might be associated with a less favorable milieu for tumor growth and proliferation.”
Dr Duggan and her colleagues said limitations of this study include the fact that the researchers only measured 3 angiogenic factors and did not measure them in adipose or other tissues. ![]()

A study of more than 400 women suggests that losing weight can reduce levels of cancer-promoting proteins in the blood.
Overweight or obese women who lost weight over a 12-month period—through diet alone or both diet and exercise—significantly lowered their levels of proteins that play a role in angiogenesis.
Researchers say this finding suggests that losing weight might help reduce the risk of developing certain cancers.
“We know that being overweight and having a sedentary lifestyle is associated with an increase in risk for developing certain types of cancer,” said Catherine Duggan, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“However, we don’t know exactly why. We wanted to investigate how levels of some biomarkers associated with angiogenesis were altered when overweight, sedentary, postmenopausal women enrolled in a research study lost weight and/or became physically active over the course of a year.”
Dr Duggan and her colleagues described this investigation in Cancer Research.
The team studied 439 women who were postmenopausal and overweight or obese but were otherwise healthy and ranged in age from 50 to 75.
The women were randomized to 1 of 4 study arms:
- A diet arm, in which women restricted their calorie intake to no more than 2000 kcal per day that included less than 30% of fat calories
- An aerobic exercise arm, in which women performed 45 minutes of moderate to vigorous exercise 5 days a week
- A combined diet and exercise arm
- A control arm.
The researchers collected blood samples at baseline and at 12 months, measuring levels of the angiogenesis-related proteins VEGF, PAI-1, and PEDF.
They also measured weight loss at 12 months and found that women in all 3 intervention arms had a significantly higher mean weight loss than women in the control arm.
The mean weight loss was 0.8% of body weight for women in the control arm, 2.4% for women in the exercise arm (P=0.03), 8.5% for women in the diet arm (P<0.001), and 10.8% for women in the diet and exercise arm (P<0.001).
Compared with women in the control arm, those in the diet-only arm and the diet and exercise arm had significantly lower levels of the angiogenesis-related proteins at 12 months. However, such effects were not apparent among women in the exercise-only arm.
Specifically, women in the diet and exercise arm had a significantly greater reduction in PAI-1 at 12 months than women in the control arm (-19.3% and +3.48%, respectively, P<0.0001).
Women in the diet-only arm and the diet and exercise arm had significantly greater reductions in PEDF than controls (-9.20%, -9.90%, and +0.18%, respectively, both P<0.0001).
And women in the diet-only arm (-8.25%, P=0.0005) and the diet and exercise arm (-9.98%, P<0.0001) had significantly greater reductions in VEGF than controls (-1.21%).
The researchers also observed a linear trend in the reductions. So the more weight loss the women experienced, the greater the reduction in angiogenesis-related protein levels.
“Our study shows that weight loss is a safe and effective method of improving the angiogenic profile in healthy individuals,” Dr Duggan said. “We were surprised by the magnitude of change in these biomarkers with weight loss.”
“While we can’t say for certain that reducing the circulating levels of angiogenic factors through weight loss would impact the growth of tumors, it is possible that they might be associated with a less favorable milieu for tumor growth and proliferation.”
Dr Duggan and her colleagues said limitations of this study include the fact that the researchers only measured 3 angiogenic factors and did not measure them in adipose or other tissues. ![]()

A study of more than 400 women suggests that losing weight can reduce levels of cancer-promoting proteins in the blood.
Overweight or obese women who lost weight over a 12-month period—through diet alone or both diet and exercise—significantly lowered their levels of proteins that play a role in angiogenesis.
Researchers say this finding suggests that losing weight might help reduce the risk of developing certain cancers.
“We know that being overweight and having a sedentary lifestyle is associated with an increase in risk for developing certain types of cancer,” said Catherine Duggan, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“However, we don’t know exactly why. We wanted to investigate how levels of some biomarkers associated with angiogenesis were altered when overweight, sedentary, postmenopausal women enrolled in a research study lost weight and/or became physically active over the course of a year.”
Dr Duggan and her colleagues described this investigation in Cancer Research.
The team studied 439 women who were postmenopausal and overweight or obese but were otherwise healthy and ranged in age from 50 to 75.
The women were randomized to 1 of 4 study arms:
- A diet arm, in which women restricted their calorie intake to no more than 2000 kcal per day that included less than 30% of fat calories
- An aerobic exercise arm, in which women performed 45 minutes of moderate to vigorous exercise 5 days a week
- A combined diet and exercise arm
- A control arm.
The researchers collected blood samples at baseline and at 12 months, measuring levels of the angiogenesis-related proteins VEGF, PAI-1, and PEDF.
They also measured weight loss at 12 months and found that women in all 3 intervention arms had a significantly higher mean weight loss than women in the control arm.
The mean weight loss was 0.8% of body weight for women in the control arm, 2.4% for women in the exercise arm (P=0.03), 8.5% for women in the diet arm (P<0.001), and 10.8% for women in the diet and exercise arm (P<0.001).
Compared with women in the control arm, those in the diet-only arm and the diet and exercise arm had significantly lower levels of the angiogenesis-related proteins at 12 months. However, such effects were not apparent among women in the exercise-only arm.
Specifically, women in the diet and exercise arm had a significantly greater reduction in PAI-1 at 12 months than women in the control arm (-19.3% and +3.48%, respectively, P<0.0001).
Women in the diet-only arm and the diet and exercise arm had significantly greater reductions in PEDF than controls (-9.20%, -9.90%, and +0.18%, respectively, both P<0.0001).
And women in the diet-only arm (-8.25%, P=0.0005) and the diet and exercise arm (-9.98%, P<0.0001) had significantly greater reductions in VEGF than controls (-1.21%).
The researchers also observed a linear trend in the reductions. So the more weight loss the women experienced, the greater the reduction in angiogenesis-related protein levels.
“Our study shows that weight loss is a safe and effective method of improving the angiogenic profile in healthy individuals,” Dr Duggan said. “We were surprised by the magnitude of change in these biomarkers with weight loss.”
“While we can’t say for certain that reducing the circulating levels of angiogenic factors through weight loss would impact the growth of tumors, it is possible that they might be associated with a less favorable milieu for tumor growth and proliferation.”
Dr Duggan and her colleagues said limitations of this study include the fact that the researchers only measured 3 angiogenic factors and did not measure them in adipose or other tissues. ![]()
Predicting outcomes in AML patients

Photo by Darren Baker
An international competition has produced models that can help predict outcomes in patients with acute myeloid leukemia (AML), according to researchers.
For the competition, known as the DREAM 9 challenge, 31 teams of computational researchers attempted to predict outcomes using data from hundreds of patients with AML.
DREAM, which stands for Dialogue for Reverse Engineering Assessment and Methods, is a platform for crowd-sourced studies that focus on developing computational tools to solve biomedical problems.
Essentially, it’s a competition that serves as a large, long-standing, international scientific collaboration.
“We used DREAM as a way to get general insight into making more accurate predictive models of clinical outcomes,” said Amina Qutub, PhD, of Rice University in Houston, Texas.
She and her colleagues described this effort in PLOS Computational Biology.
For the DREAM 9 challenge, each team was presented with training data from 191 AML patients, which included demographic information, such as age and gender, and more complex proteomic and phosphoprotein data that describes signaling protein pathways believed to play a role in AML.
The teams were also presented with a test set of 100 AML patients and were asked to predict response to therapy, remission duration, or overall survival for these patients.
The top-performing models—by Team EvoMed of Arizona State University and Team Chipmunks of the Ontario Institute for Cancer Research—were able to predict patient response to therapy with an accuracy of close to 80%.
Both of these models were impacted by the perturbation of PIK3CA and NPM1, which singles out these proteins as candidates for further study, according to researchers.
Another discovery resulting from this competition was that, overall, the 31 models were not as effective for predicting outcomes in patients classified as “resistant to therapy” than for responsive patients.
The median model prediction accuracy was 42% for resistant patients and 73% for responsive patients. ![]()

Photo by Darren Baker
An international competition has produced models that can help predict outcomes in patients with acute myeloid leukemia (AML), according to researchers.
For the competition, known as the DREAM 9 challenge, 31 teams of computational researchers attempted to predict outcomes using data from hundreds of patients with AML.
DREAM, which stands for Dialogue for Reverse Engineering Assessment and Methods, is a platform for crowd-sourced studies that focus on developing computational tools to solve biomedical problems.
Essentially, it’s a competition that serves as a large, long-standing, international scientific collaboration.
“We used DREAM as a way to get general insight into making more accurate predictive models of clinical outcomes,” said Amina Qutub, PhD, of Rice University in Houston, Texas.
She and her colleagues described this effort in PLOS Computational Biology.
For the DREAM 9 challenge, each team was presented with training data from 191 AML patients, which included demographic information, such as age and gender, and more complex proteomic and phosphoprotein data that describes signaling protein pathways believed to play a role in AML.
The teams were also presented with a test set of 100 AML patients and were asked to predict response to therapy, remission duration, or overall survival for these patients.
The top-performing models—by Team EvoMed of Arizona State University and Team Chipmunks of the Ontario Institute for Cancer Research—were able to predict patient response to therapy with an accuracy of close to 80%.
Both of these models were impacted by the perturbation of PIK3CA and NPM1, which singles out these proteins as candidates for further study, according to researchers.
Another discovery resulting from this competition was that, overall, the 31 models were not as effective for predicting outcomes in patients classified as “resistant to therapy” than for responsive patients.
The median model prediction accuracy was 42% for resistant patients and 73% for responsive patients. ![]()

Photo by Darren Baker
An international competition has produced models that can help predict outcomes in patients with acute myeloid leukemia (AML), according to researchers.
For the competition, known as the DREAM 9 challenge, 31 teams of computational researchers attempted to predict outcomes using data from hundreds of patients with AML.
DREAM, which stands for Dialogue for Reverse Engineering Assessment and Methods, is a platform for crowd-sourced studies that focus on developing computational tools to solve biomedical problems.
Essentially, it’s a competition that serves as a large, long-standing, international scientific collaboration.
“We used DREAM as a way to get general insight into making more accurate predictive models of clinical outcomes,” said Amina Qutub, PhD, of Rice University in Houston, Texas.
She and her colleagues described this effort in PLOS Computational Biology.
For the DREAM 9 challenge, each team was presented with training data from 191 AML patients, which included demographic information, such as age and gender, and more complex proteomic and phosphoprotein data that describes signaling protein pathways believed to play a role in AML.
The teams were also presented with a test set of 100 AML patients and were asked to predict response to therapy, remission duration, or overall survival for these patients.
The top-performing models—by Team EvoMed of Arizona State University and Team Chipmunks of the Ontario Institute for Cancer Research—were able to predict patient response to therapy with an accuracy of close to 80%.
Both of these models were impacted by the perturbation of PIK3CA and NPM1, which singles out these proteins as candidates for further study, according to researchers.
Another discovery resulting from this competition was that, overall, the 31 models were not as effective for predicting outcomes in patients classified as “resistant to therapy” than for responsive patients.
The median model prediction accuracy was 42% for resistant patients and 73% for responsive patients. ![]()