User login
Dementia isn’t passed on via transfusion, team says

Photo by Elise Amendola
Results of a large, retrospective study suggest that neurological diseases are not transmitted via blood transfusion.
Previous studies have shown that such diseases can be induced in healthy laboratory animals through the injection of diseased brain tissue from humans.
This has caused concern that neurological diseases might be transmitted from human to human via blood transfusions.
However, a study published in Annals of Internal Medicine suggests such transmission does not occur.
“The results are unusually clear for such a complicated subject as this,” said study author Gustaf Edgren, PhD, of Karolinska Institutet in Stockholm, Sweden.
“We’ve been working with this question for a long time now and have found no indication that these diseases can be transmitted via transfusions.”
Dr Edgren and his colleagues conducted this study by analyzing data from 1,465,845 patients who received blood transfusions in Sweden or Denmark between 1968 and 2012.
The team used multivariable Cox regression models (taking into account sex, age, place of residence, blood group, number of transfusions, and time since first transfusion) to estimate hazard ratios for dementia of any type, Alzheimer’s disease, and Parkinson’s disease in patients who received transfusions from donors who were later diagnosed with any of these diseases, compared to patients who received blood from healthy donors.
In all, 2.9% of patients received a transfusion from a donor diagnosed with one of the aforementioned neurological diseases. And there was no evidence of disease transmission via transfusion.
The hazard ratio for dementia in transfusion recipients whose donors were diagnosed with dementia, compared to recipients of blood from healthy donors, was 1.04 (95% CI, 0.99 to 1.09).
The hazard ratios for Alzheimer’s disease and Parkinson’s disease were 0.99 (95% CI, 0.85 to 1.15) and 0.94 (95% CI, 0.78 to 1.14), respectively.
“Blood transfusions are extremely safe in the Western world today, but, even so, we are working continuously and proactively on identifying any overlooked risks,” Dr Edgren said.
“The Swedish-Danish database that we have built up and used in many similar studies clearly demonstrates the value of our vast health registries. This kind of study would have simply been extremely difficult anywhere else in the world.” ![]()

Photo by Elise Amendola
Results of a large, retrospective study suggest that neurological diseases are not transmitted via blood transfusion.
Previous studies have shown that such diseases can be induced in healthy laboratory animals through the injection of diseased brain tissue from humans.
This has caused concern that neurological diseases might be transmitted from human to human via blood transfusions.
However, a study published in Annals of Internal Medicine suggests such transmission does not occur.
“The results are unusually clear for such a complicated subject as this,” said study author Gustaf Edgren, PhD, of Karolinska Institutet in Stockholm, Sweden.
“We’ve been working with this question for a long time now and have found no indication that these diseases can be transmitted via transfusions.”
Dr Edgren and his colleagues conducted this study by analyzing data from 1,465,845 patients who received blood transfusions in Sweden or Denmark between 1968 and 2012.
The team used multivariable Cox regression models (taking into account sex, age, place of residence, blood group, number of transfusions, and time since first transfusion) to estimate hazard ratios for dementia of any type, Alzheimer’s disease, and Parkinson’s disease in patients who received transfusions from donors who were later diagnosed with any of these diseases, compared to patients who received blood from healthy donors.
In all, 2.9% of patients received a transfusion from a donor diagnosed with one of the aforementioned neurological diseases. And there was no evidence of disease transmission via transfusion.
The hazard ratio for dementia in transfusion recipients whose donors were diagnosed with dementia, compared to recipients of blood from healthy donors, was 1.04 (95% CI, 0.99 to 1.09).
The hazard ratios for Alzheimer’s disease and Parkinson’s disease were 0.99 (95% CI, 0.85 to 1.15) and 0.94 (95% CI, 0.78 to 1.14), respectively.
“Blood transfusions are extremely safe in the Western world today, but, even so, we are working continuously and proactively on identifying any overlooked risks,” Dr Edgren said.
“The Swedish-Danish database that we have built up and used in many similar studies clearly demonstrates the value of our vast health registries. This kind of study would have simply been extremely difficult anywhere else in the world.” ![]()

Photo by Elise Amendola
Results of a large, retrospective study suggest that neurological diseases are not transmitted via blood transfusion.
Previous studies have shown that such diseases can be induced in healthy laboratory animals through the injection of diseased brain tissue from humans.
This has caused concern that neurological diseases might be transmitted from human to human via blood transfusions.
However, a study published in Annals of Internal Medicine suggests such transmission does not occur.
“The results are unusually clear for such a complicated subject as this,” said study author Gustaf Edgren, PhD, of Karolinska Institutet in Stockholm, Sweden.
“We’ve been working with this question for a long time now and have found no indication that these diseases can be transmitted via transfusions.”
Dr Edgren and his colleagues conducted this study by analyzing data from 1,465,845 patients who received blood transfusions in Sweden or Denmark between 1968 and 2012.
The team used multivariable Cox regression models (taking into account sex, age, place of residence, blood group, number of transfusions, and time since first transfusion) to estimate hazard ratios for dementia of any type, Alzheimer’s disease, and Parkinson’s disease in patients who received transfusions from donors who were later diagnosed with any of these diseases, compared to patients who received blood from healthy donors.
In all, 2.9% of patients received a transfusion from a donor diagnosed with one of the aforementioned neurological diseases. And there was no evidence of disease transmission via transfusion.
The hazard ratio for dementia in transfusion recipients whose donors were diagnosed with dementia, compared to recipients of blood from healthy donors, was 1.04 (95% CI, 0.99 to 1.09).
The hazard ratios for Alzheimer’s disease and Parkinson’s disease were 0.99 (95% CI, 0.85 to 1.15) and 0.94 (95% CI, 0.78 to 1.14), respectively.
“Blood transfusions are extremely safe in the Western world today, but, even so, we are working continuously and proactively on identifying any overlooked risks,” Dr Edgren said.
“The Swedish-Danish database that we have built up and used in many similar studies clearly demonstrates the value of our vast health registries. This kind of study would have simply been extremely difficult anywhere else in the world.” ![]()
Artemisinin resistance confined to Asia, study shows

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
The first global mapping of artemisinin resistance indicates that resistance to the drug, which is used to treat Plasmodium falciparum malaria, is confined to Southeast Asia and has not yet spread to sub-Saharan Africa.
Results of the effort, known as the KARMA study, were published in NEJM.
The study builds on the 2014 discovery that the K13 gene is the major determinant of P falciparum’s resistance to artemisinin.
Researchers studied the diversity of the K13 gene in 14,037 blood samples taken from P falciparum-infected patients in 59 malaria-endemic countries—72% in Africa, 19% in Asia, 8% in Latin America, and 1% in Oceania. All samples were collected after 2012.
The researchers identified 108 nonsynonymous K13 mutations. In Asia, 36.5% of the mutations were distributed within 2 areas—Cambodia-Vietnam-Laos and western Thailand-Myanmar-China—with no overlap.
In samples from Africa, the researchers identified nonsynonymous K13 mutations that were not associated with artemisinin resistance, including the most frequent mutation found in Africa, A578S.
“We suspect that only a small number of mutations appear to be associated with resistance, which should facilitate global monitoring of resistance to artemisinin,” said study author Odile Mercereau-Puijalon, PhD, of the Institut Pasteur in Paris, France.
“Until now, scientists have not had the tools to be properly informed about the nature of resistance to antimalarial drugs in key affected regions such as sub-Saharan Africa,” added Didier Ménard, PhD, of the Institut Pasteur in Phnom Penh, Cambodia.
“We now have the capacity, thanks to molecular markers, to be able to trace—at a global level and virtually in real-time—resistance to antimalarial drugs. We must ensure that we use this technology to keep us a step ahead of the parasite.” ![]()

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
The first global mapping of artemisinin resistance indicates that resistance to the drug, which is used to treat Plasmodium falciparum malaria, is confined to Southeast Asia and has not yet spread to sub-Saharan Africa.
Results of the effort, known as the KARMA study, were published in NEJM.
The study builds on the 2014 discovery that the K13 gene is the major determinant of P falciparum’s resistance to artemisinin.
Researchers studied the diversity of the K13 gene in 14,037 blood samples taken from P falciparum-infected patients in 59 malaria-endemic countries—72% in Africa, 19% in Asia, 8% in Latin America, and 1% in Oceania. All samples were collected after 2012.
The researchers identified 108 nonsynonymous K13 mutations. In Asia, 36.5% of the mutations were distributed within 2 areas—Cambodia-Vietnam-Laos and western Thailand-Myanmar-China—with no overlap.
In samples from Africa, the researchers identified nonsynonymous K13 mutations that were not associated with artemisinin resistance, including the most frequent mutation found in Africa, A578S.
“We suspect that only a small number of mutations appear to be associated with resistance, which should facilitate global monitoring of resistance to artemisinin,” said study author Odile Mercereau-Puijalon, PhD, of the Institut Pasteur in Paris, France.
“Until now, scientists have not had the tools to be properly informed about the nature of resistance to antimalarial drugs in key affected regions such as sub-Saharan Africa,” added Didier Ménard, PhD, of the Institut Pasteur in Phnom Penh, Cambodia.
“We now have the capacity, thanks to molecular markers, to be able to trace—at a global level and virtually in real-time—resistance to antimalarial drugs. We must ensure that we use this technology to keep us a step ahead of the parasite.” ![]()

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
The first global mapping of artemisinin resistance indicates that resistance to the drug, which is used to treat Plasmodium falciparum malaria, is confined to Southeast Asia and has not yet spread to sub-Saharan Africa.
Results of the effort, known as the KARMA study, were published in NEJM.
The study builds on the 2014 discovery that the K13 gene is the major determinant of P falciparum’s resistance to artemisinin.
Researchers studied the diversity of the K13 gene in 14,037 blood samples taken from P falciparum-infected patients in 59 malaria-endemic countries—72% in Africa, 19% in Asia, 8% in Latin America, and 1% in Oceania. All samples were collected after 2012.
The researchers identified 108 nonsynonymous K13 mutations. In Asia, 36.5% of the mutations were distributed within 2 areas—Cambodia-Vietnam-Laos and western Thailand-Myanmar-China—with no overlap.
In samples from Africa, the researchers identified nonsynonymous K13 mutations that were not associated with artemisinin resistance, including the most frequent mutation found in Africa, A578S.
“We suspect that only a small number of mutations appear to be associated with resistance, which should facilitate global monitoring of resistance to artemisinin,” said study author Odile Mercereau-Puijalon, PhD, of the Institut Pasteur in Paris, France.
“Until now, scientists have not had the tools to be properly informed about the nature of resistance to antimalarial drugs in key affected regions such as sub-Saharan Africa,” added Didier Ménard, PhD, of the Institut Pasteur in Phnom Penh, Cambodia.
“We now have the capacity, thanks to molecular markers, to be able to trace—at a global level and virtually in real-time—resistance to antimalarial drugs. We must ensure that we use this technology to keep us a step ahead of the parasite.” ![]()
Agreements may constrain publication of trial results

for a clinical trial
Photo by Esther Dyson
Publication agreements between industry and academic investigators involved in clinical trials are not often reported in the publications themselves, according to a study published in PLOS Medicine.
In most of the agreements studied, industry had the right to reject or review manuscripts before publication.
Therefore, according to researchers, publication agreements may compromise the scientific evidence base established by randomized clinical trials.
Matthias Briel, MD, of the University Hospital Basel in Switzerland, and his colleagues sought to understand how publication agreements might constrain the publication of trial results.
The researchers examined publication agreements in 647 randomized trial protocols approved from 2000 to 2003 by 6 research ethics committees in Switzerland, Canada, and Germany, as well as the 388 corresponding journal publications.
The team found that 71% of protocols mentioned an agreement on publication rights between industry and academic investigators.
In 86% of those agreements, industry retained the right to disapprove or at least review manuscripts before publication.
And 74% of the agreements documented in protocols were not mentioned in corresponding journal articles.
The researchers noted that half of the included journal articles were published before 2008, leaving open the possibility that these findings do not reflect current practice.
Nonetheless, the team said the findings suggest that more transparency on publication constraints is warranted. ![]()

for a clinical trial
Photo by Esther Dyson
Publication agreements between industry and academic investigators involved in clinical trials are not often reported in the publications themselves, according to a study published in PLOS Medicine.
In most of the agreements studied, industry had the right to reject or review manuscripts before publication.
Therefore, according to researchers, publication agreements may compromise the scientific evidence base established by randomized clinical trials.
Matthias Briel, MD, of the University Hospital Basel in Switzerland, and his colleagues sought to understand how publication agreements might constrain the publication of trial results.
The researchers examined publication agreements in 647 randomized trial protocols approved from 2000 to 2003 by 6 research ethics committees in Switzerland, Canada, and Germany, as well as the 388 corresponding journal publications.
The team found that 71% of protocols mentioned an agreement on publication rights between industry and academic investigators.
In 86% of those agreements, industry retained the right to disapprove or at least review manuscripts before publication.
And 74% of the agreements documented in protocols were not mentioned in corresponding journal articles.
The researchers noted that half of the included journal articles were published before 2008, leaving open the possibility that these findings do not reflect current practice.
Nonetheless, the team said the findings suggest that more transparency on publication constraints is warranted. ![]()

for a clinical trial
Photo by Esther Dyson
Publication agreements between industry and academic investigators involved in clinical trials are not often reported in the publications themselves, according to a study published in PLOS Medicine.
In most of the agreements studied, industry had the right to reject or review manuscripts before publication.
Therefore, according to researchers, publication agreements may compromise the scientific evidence base established by randomized clinical trials.
Matthias Briel, MD, of the University Hospital Basel in Switzerland, and his colleagues sought to understand how publication agreements might constrain the publication of trial results.
The researchers examined publication agreements in 647 randomized trial protocols approved from 2000 to 2003 by 6 research ethics committees in Switzerland, Canada, and Germany, as well as the 388 corresponding journal publications.
The team found that 71% of protocols mentioned an agreement on publication rights between industry and academic investigators.
In 86% of those agreements, industry retained the right to disapprove or at least review manuscripts before publication.
And 74% of the agreements documented in protocols were not mentioned in corresponding journal articles.
The researchers noted that half of the included journal articles were published before 2008, leaving open the possibility that these findings do not reflect current practice.
Nonetheless, the team said the findings suggest that more transparency on publication constraints is warranted. ![]()
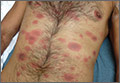
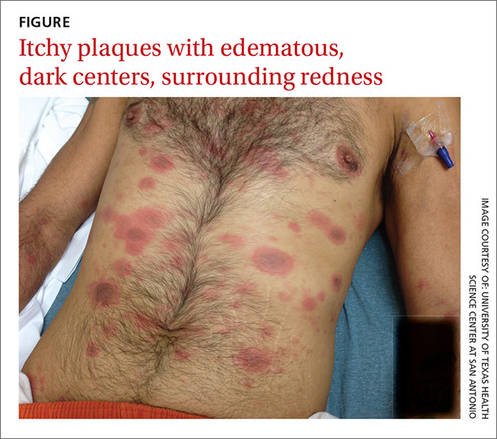
Generalized rash follows ankle ulceration
A 31-year-old incarcerated man sought care for one crusted ulcer and one adjacent open ulcer with granulation tissue on his left malleolus. The ulcers were caused by chronic venous insufficiency—the result of previous trauma to the ankle. Concerned that the ulcers would become infected, the physician prescribed one double-strength tablet twice a day of trimethoprim-sulfamethoxazole (TMP-SMX). The patient took 2 doses of the antibiotic and one dose of naproxen.
When the patient awoke the next morning, he had a generalized skin eruption on his chin, trunk, buttocks, glans penis, and extremities (FIGURE). The rash began as red edematous plaques that became itchy and painful with dark, violaceous dusky centers surrounded by redness. The patient was treated with topical hydrocortisone 2.5% twice a day and oral diphenhydramine 25 mg followed by 50 mg, but the rash didn’t improve.
The patient was transported to the local emergency department where physicians noted that the patient had about 30 to 40 well-demarcated papules and plaques of various sizes that were haphazardly located over the patient’s chin, chest, back, upper and lower extremities, and genitalia. There was one lesion on the chest with central vesiculation. There were no lesions on the mucous membranes of his eyes, ears, nose, mouth, or anus.
The patient, whose vital signs were within normal limits, was empirically treated with one dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. Lab work revealed no elevation in his white blood cell count, creatinine, liver function enzymes, or C-reactive protein.
The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. He noted that during the previous episode, the lesions were located on the exact same areas of his glans penis and chin.
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated fixed-drug eruption
The diagnosis was based on the morphologic characteristics of the eruption and the patient’s history of similar lesions that appeared in the exact same initial locations (chin and glans penis) following previous treatment with TMP-SMX.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with our patient).
The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.1
The diagnosis is usually made based on a history of similar lesions recurring at the same location in response to a specific drug2 and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. To confirm a fixed-drug eruption in the case of clinical equipoise, a skin biopsy may be performed.
Classic histologic findings of a fixed-drug eruption include:
- band-like lichenoid lymphocytic infiltrates with vacuolar changes at the dermoepidermal junction,
- mixed cellular infiltrates, including eosinophils, throughout the dermis and occasional superficial and deep mixed cellular perivascular infiltrates, and
- abundant melanophages suggesting pigment incontinence.
There are several reports of similar TMP-SMX–induced generalized fixed-drug eruptions in the literature.3 One study of 64 cases of fixed-drug eruption found that TMP-SMX was the most common offender, causing 75% of fixed-drug eruption cases; naproxen sodium came in second with 12.5%.3 Other common culprits include the antipyretic metamizole and other pyrazolone derivatives such as tetracycline, metronidazole, ciprofloxacin, and phenytoin sodium.4 There is evidence supporting a correlation between the offending drug and the subsequent site of reaction; TMP-SMX is associated with mucosal junction and genital involvement.4,5 This finding may aid physicians in the investigation of provoking agents.
Distinguish fixed-drug eruptions from serious bullous diseases
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches. Fixed-drug eruptions can be distinguished by the lack of simultaneous involvement of 2 mucosal surfaces, lack of generalized desquamation, and normal vital signs and lab values, including white blood cell count and erythrocyte sedimentation rate/C-reactive protein.
A subset of fixed-drug eruption, generalized bullous fixed-drug eruption (which has been defined as blistering on >10% of the body’s surface area at 3 different anatomic sites), may be particularly hard to distinguish from SJS and TEN. Generalized bullous fixed-drug eruption generally has a shorter latency period than SJS or TEN (usually <3 days compared to 7-10 days) and has less mucosal involvement.6
Symptomatic therapy includes antihistamines, glucocorticoid ointment
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like our patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days. Be advised, however, that these therapies are based on case report level data.2
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
Our patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption.
He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
CORRESPONDENCE
Jackie Bucher, MD, 7733 Louis Pasteur Drive Apt. 209, San Antonio, TX 78229; [email protected].
1. Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
2. Wolff K, Johnson RA. Dermatology and internal medicine: fixed drug eruption. In: Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill; 2009:566-568.
3. Ozkaya-Bayazit E, Bayazit H, Ozarmagan G. Drug related clinical pattern in fixed drug eruption. Eur J Dermatol. 2000;10:288-291.
4. Sharma VK, Dhar S, Gill AN. Drug related involvement of specific sites in fixed eruptions: a statistical evaluation. J Dermatol. 1996;23:530-534.
5. Thankappan TP, Zachariah J. Drug-specific clinical pattern in fixed drug eruptions. Int J Dermatol. 1991;30:867-870.
6. Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
A 31-year-old incarcerated man sought care for one crusted ulcer and one adjacent open ulcer with granulation tissue on his left malleolus. The ulcers were caused by chronic venous insufficiency—the result of previous trauma to the ankle. Concerned that the ulcers would become infected, the physician prescribed one double-strength tablet twice a day of trimethoprim-sulfamethoxazole (TMP-SMX). The patient took 2 doses of the antibiotic and one dose of naproxen.
When the patient awoke the next morning, he had a generalized skin eruption on his chin, trunk, buttocks, glans penis, and extremities (FIGURE). The rash began as red edematous plaques that became itchy and painful with dark, violaceous dusky centers surrounded by redness. The patient was treated with topical hydrocortisone 2.5% twice a day and oral diphenhydramine 25 mg followed by 50 mg, but the rash didn’t improve.
The patient was transported to the local emergency department where physicians noted that the patient had about 30 to 40 well-demarcated papules and plaques of various sizes that were haphazardly located over the patient’s chin, chest, back, upper and lower extremities, and genitalia. There was one lesion on the chest with central vesiculation. There were no lesions on the mucous membranes of his eyes, ears, nose, mouth, or anus.
The patient, whose vital signs were within normal limits, was empirically treated with one dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. Lab work revealed no elevation in his white blood cell count, creatinine, liver function enzymes, or C-reactive protein.
The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. He noted that during the previous episode, the lesions were located on the exact same areas of his glans penis and chin.

HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated fixed-drug eruption
The diagnosis was based on the morphologic characteristics of the eruption and the patient’s history of similar lesions that appeared in the exact same initial locations (chin and glans penis) following previous treatment with TMP-SMX.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with our patient).
The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.1
The diagnosis is usually made based on a history of similar lesions recurring at the same location in response to a specific drug2 and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. To confirm a fixed-drug eruption in the case of clinical equipoise, a skin biopsy may be performed.
Classic histologic findings of a fixed-drug eruption include:
- band-like lichenoid lymphocytic infiltrates with vacuolar changes at the dermoepidermal junction,
- mixed cellular infiltrates, including eosinophils, throughout the dermis and occasional superficial and deep mixed cellular perivascular infiltrates, and
- abundant melanophages suggesting pigment incontinence.
There are several reports of similar TMP-SMX–induced generalized fixed-drug eruptions in the literature.3 One study of 64 cases of fixed-drug eruption found that TMP-SMX was the most common offender, causing 75% of fixed-drug eruption cases; naproxen sodium came in second with 12.5%.3 Other common culprits include the antipyretic metamizole and other pyrazolone derivatives such as tetracycline, metronidazole, ciprofloxacin, and phenytoin sodium.4 There is evidence supporting a correlation between the offending drug and the subsequent site of reaction; TMP-SMX is associated with mucosal junction and genital involvement.4,5 This finding may aid physicians in the investigation of provoking agents.
Distinguish fixed-drug eruptions from serious bullous diseases
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches. Fixed-drug eruptions can be distinguished by the lack of simultaneous involvement of 2 mucosal surfaces, lack of generalized desquamation, and normal vital signs and lab values, including white blood cell count and erythrocyte sedimentation rate/C-reactive protein.
A subset of fixed-drug eruption, generalized bullous fixed-drug eruption (which has been defined as blistering on >10% of the body’s surface area at 3 different anatomic sites), may be particularly hard to distinguish from SJS and TEN. Generalized bullous fixed-drug eruption generally has a shorter latency period than SJS or TEN (usually <3 days compared to 7-10 days) and has less mucosal involvement.6
Symptomatic therapy includes antihistamines, glucocorticoid ointment
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like our patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days. Be advised, however, that these therapies are based on case report level data.2
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
Our patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption.
He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
CORRESPONDENCE
Jackie Bucher, MD, 7733 Louis Pasteur Drive Apt. 209, San Antonio, TX 78229; [email protected].
A 31-year-old incarcerated man sought care for one crusted ulcer and one adjacent open ulcer with granulation tissue on his left malleolus. The ulcers were caused by chronic venous insufficiency—the result of previous trauma to the ankle. Concerned that the ulcers would become infected, the physician prescribed one double-strength tablet twice a day of trimethoprim-sulfamethoxazole (TMP-SMX). The patient took 2 doses of the antibiotic and one dose of naproxen.
When the patient awoke the next morning, he had a generalized skin eruption on his chin, trunk, buttocks, glans penis, and extremities (FIGURE). The rash began as red edematous plaques that became itchy and painful with dark, violaceous dusky centers surrounded by redness. The patient was treated with topical hydrocortisone 2.5% twice a day and oral diphenhydramine 25 mg followed by 50 mg, but the rash didn’t improve.
The patient was transported to the local emergency department where physicians noted that the patient had about 30 to 40 well-demarcated papules and plaques of various sizes that were haphazardly located over the patient’s chin, chest, back, upper and lower extremities, and genitalia. There was one lesion on the chest with central vesiculation. There were no lesions on the mucous membranes of his eyes, ears, nose, mouth, or anus.
The patient, whose vital signs were within normal limits, was empirically treated with one dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. Lab work revealed no elevation in his white blood cell count, creatinine, liver function enzymes, or C-reactive protein.
The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. He noted that during the previous episode, the lesions were located on the exact same areas of his glans penis and chin.

HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated fixed-drug eruption
The diagnosis was based on the morphologic characteristics of the eruption and the patient’s history of similar lesions that appeared in the exact same initial locations (chin and glans penis) following previous treatment with TMP-SMX.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with our patient).
The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.1
The diagnosis is usually made based on a history of similar lesions recurring at the same location in response to a specific drug2 and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. To confirm a fixed-drug eruption in the case of clinical equipoise, a skin biopsy may be performed.
Classic histologic findings of a fixed-drug eruption include:
- band-like lichenoid lymphocytic infiltrates with vacuolar changes at the dermoepidermal junction,
- mixed cellular infiltrates, including eosinophils, throughout the dermis and occasional superficial and deep mixed cellular perivascular infiltrates, and
- abundant melanophages suggesting pigment incontinence.
There are several reports of similar TMP-SMX–induced generalized fixed-drug eruptions in the literature.3 One study of 64 cases of fixed-drug eruption found that TMP-SMX was the most common offender, causing 75% of fixed-drug eruption cases; naproxen sodium came in second with 12.5%.3 Other common culprits include the antipyretic metamizole and other pyrazolone derivatives such as tetracycline, metronidazole, ciprofloxacin, and phenytoin sodium.4 There is evidence supporting a correlation between the offending drug and the subsequent site of reaction; TMP-SMX is associated with mucosal junction and genital involvement.4,5 This finding may aid physicians in the investigation of provoking agents.
Distinguish fixed-drug eruptions from serious bullous diseases
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches. Fixed-drug eruptions can be distinguished by the lack of simultaneous involvement of 2 mucosal surfaces, lack of generalized desquamation, and normal vital signs and lab values, including white blood cell count and erythrocyte sedimentation rate/C-reactive protein.
A subset of fixed-drug eruption, generalized bullous fixed-drug eruption (which has been defined as blistering on >10% of the body’s surface area at 3 different anatomic sites), may be particularly hard to distinguish from SJS and TEN. Generalized bullous fixed-drug eruption generally has a shorter latency period than SJS or TEN (usually <3 days compared to 7-10 days) and has less mucosal involvement.6
Symptomatic therapy includes antihistamines, glucocorticoid ointment
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like our patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days. Be advised, however, that these therapies are based on case report level data.2
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
Our patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption.
He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
CORRESPONDENCE
Jackie Bucher, MD, 7733 Louis Pasteur Drive Apt. 209, San Antonio, TX 78229; [email protected].
1. Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
2. Wolff K, Johnson RA. Dermatology and internal medicine: fixed drug eruption. In: Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill; 2009:566-568.
3. Ozkaya-Bayazit E, Bayazit H, Ozarmagan G. Drug related clinical pattern in fixed drug eruption. Eur J Dermatol. 2000;10:288-291.
4. Sharma VK, Dhar S, Gill AN. Drug related involvement of specific sites in fixed eruptions: a statistical evaluation. J Dermatol. 1996;23:530-534.
5. Thankappan TP, Zachariah J. Drug-specific clinical pattern in fixed drug eruptions. Int J Dermatol. 1991;30:867-870.
6. Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
1. Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
2. Wolff K, Johnson RA. Dermatology and internal medicine: fixed drug eruption. In: Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York: McGraw-Hill; 2009:566-568.
3. Ozkaya-Bayazit E, Bayazit H, Ozarmagan G. Drug related clinical pattern in fixed drug eruption. Eur J Dermatol. 2000;10:288-291.
4. Sharma VK, Dhar S, Gill AN. Drug related involvement of specific sites in fixed eruptions: a statistical evaluation. J Dermatol. 1996;23:530-534.
5. Thankappan TP, Zachariah J. Drug-specific clinical pattern in fixed drug eruptions. Int J Dermatol. 1991;30:867-870.
6. Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
USPSTF update: Screening for abnormal blood glucose, diabetes
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
FDA approves Epclusa for patients with chronic hepatitis C
Adult patients with chronic hepatitis C virus (HCV) both with and without cirrhosis can now be prescribed Epclusa. The Food and Drug Administration approved the fixed-dose combination tablet is the first treatment that controls genotypes 1-6 of HCV.
“This approval offers a management and treatment option for a wider scope of patients with chronic hepatitis C,” said Edward Cox, MD, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Epclusa, which contains sofosbuvir and velpatasvir, is manufactured by Gilead Sciences, located in Foster City, Calif. Before approval, the drug was evaluated for 12 weeks in three phase III clinical trials that included 1,558 patients diagnosed without cirrhosis and patients with compensated cirrhosis. Twelve weeks after finishing treatment, 95%-99% of patients who received Epclusa had no signs of infection. Epclusa cured the condition, which means no sign of the virus was detected in any of the patients’ blood. In addition, 267 patients were studied for the safety and efficacy of Epclusa, 87 of whom took Epclusa in combination with ribavirin for 12 weeks, and 94% of these patients had no virus detected in the blood 12 weeks after finishing treatment.
Side effects of Epclusa include headache and fatigue. Epclusa also carries a warning not to use with certain drugs that may reduce the effective amount of Epclusa.
Adult patients with chronic hepatitis C virus (HCV) both with and without cirrhosis can now be prescribed Epclusa. The Food and Drug Administration approved the fixed-dose combination tablet is the first treatment that controls genotypes 1-6 of HCV.
“This approval offers a management and treatment option for a wider scope of patients with chronic hepatitis C,” said Edward Cox, MD, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Epclusa, which contains sofosbuvir and velpatasvir, is manufactured by Gilead Sciences, located in Foster City, Calif. Before approval, the drug was evaluated for 12 weeks in three phase III clinical trials that included 1,558 patients diagnosed without cirrhosis and patients with compensated cirrhosis. Twelve weeks after finishing treatment, 95%-99% of patients who received Epclusa had no signs of infection. Epclusa cured the condition, which means no sign of the virus was detected in any of the patients’ blood. In addition, 267 patients were studied for the safety and efficacy of Epclusa, 87 of whom took Epclusa in combination with ribavirin for 12 weeks, and 94% of these patients had no virus detected in the blood 12 weeks after finishing treatment.
Side effects of Epclusa include headache and fatigue. Epclusa also carries a warning not to use with certain drugs that may reduce the effective amount of Epclusa.
Adult patients with chronic hepatitis C virus (HCV) both with and without cirrhosis can now be prescribed Epclusa. The Food and Drug Administration approved the fixed-dose combination tablet is the first treatment that controls genotypes 1-6 of HCV.
“This approval offers a management and treatment option for a wider scope of patients with chronic hepatitis C,” said Edward Cox, MD, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Epclusa, which contains sofosbuvir and velpatasvir, is manufactured by Gilead Sciences, located in Foster City, Calif. Before approval, the drug was evaluated for 12 weeks in three phase III clinical trials that included 1,558 patients diagnosed without cirrhosis and patients with compensated cirrhosis. Twelve weeks after finishing treatment, 95%-99% of patients who received Epclusa had no signs of infection. Epclusa cured the condition, which means no sign of the virus was detected in any of the patients’ blood. In addition, 267 patients were studied for the safety and efficacy of Epclusa, 87 of whom took Epclusa in combination with ribavirin for 12 weeks, and 94% of these patients had no virus detected in the blood 12 weeks after finishing treatment.
Side effects of Epclusa include headache and fatigue. Epclusa also carries a warning not to use with certain drugs that may reduce the effective amount of Epclusa.
Sylvia Lucas, MD, PhD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
When Do Efficacy Outcomes in Clinical Trials Correlate With Clinical Relevance? Analysis of Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel in Moderate to Severe Acne Vulgaris
Acne vulgaris (AV) is a common skin disease that usually presents in adolescence and can persist into adulthood. Some cases may start in adulthood, especially in women. Acne vulgaris remains a challenge to treat successfully, both in teenagers and adults. Unrealistic expectations that therapy will rapidly clear and sustain clearance of AV completely can lead to incomplete adherence or complete cessation of treatment.1-4 Local tolerability reactions also may decrease adherence to topical medications. Suboptimal adherence to medications for AV is one of the major reasons for treatment failure.5 Acne vulgaris can strongly influence psychological well-being and self-esteem.6 In general, severe AV causes more psychological distress, but the adverse emotional impact of AV can be independent of its severity.7
An effective relationship between the patient and his/her physician and staff is believed to be important in setting realistic expectations, optimizing adherence, and achieving a positive therapeutic outcome. One component related to setting reasonable expectations is the discussion about when the patient may begin to visibly perceive that the treatment regimen is working. This article evaluates the time course of a clinically meaningful response using pivotal trial data with clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel for treatment of AV.
Are data available that evaluate the time course of a clinically relevant response to treatment of AV?
Unfortunately, data on what might be perceived as a clinically meaningful improvement in AV and how long it might take to achieve this treatment effect are limited. A meta-analysis of more than 4000 patients with moderate to severe AV suggested that a 10% to 20% difference in acne lesion counts from baseline as compared to a subsequent designated time point was clinically relevant.8 A review of 24 comparative studies of patients with mild to moderate AV used a primary outcome parameter of a 25% reduction in mean inflammatory lesion count to evaluate time to onset of action (TOA) to achieve a clinically meaningful benefit.9 This outcome was based on a previously identified threshold of clinical relevance and the authors’ clinical experience in a patient population with milder AV. In this same analysis, a difference of greater than 4 days between the active group and the vehicle group was considered to be relevant to the patient.9
A faster onset of visible improvement as perceived by the patient should be more desirable and is likely to improve treatment adherence, as long as it is not counterbalanced by an increase in adverse events.
What is meant by TOA?
Time to onset of action refers to the duration required to achieve a 25% mean lesion count reduction from baseline, which is believed to correlate with the time point at which many patients would be able to perceive visible improvement when viewing their full face. Therefore, TOA represents an attempt to correlate data that is quantitative (based on lesion count reduction) with what is likely to be the average time that a patient may qualitatively observe an initial visible improvement in their AV. This concept may be useful as a tool when communicating with AV patients but should not be used in a way that will overpromise and underdeliver; rather, it is a guide for discussion with the patient and with a parent or guardian when applicable.
Consistent with the comparative AV study analysis that evaluated TOA, a linear course of lesion reductions between the provided time intervals was assumed. In this linear model, the TOA was calculated using the 2 extracted lesion count values between which the 25% lesion reduction was achieved as well as their corresponding given time points.9 Differences between the results in the active and vehicle study arms were calculated for a number of determinants.
How was pivotal trial data with clindamycin-BP 3.75% gel used to assess TOA?
A total of 498 patients with moderate to severe AV were randomized (1:1) to receive clindamycin-BP 3.75% gel or vehicle in a multicenter, double-blind, controlled, 12-week, 2-arm study.10 Before randomization, patients were stratified by acne severity based on a static Evaluator’s Global Severity Score (EGSS) ranging from 0 (clear) to 5 (very severe). Specifically, moderate AV (EGSS of 3) was described as predominantly noninflammatory lesions with evidence of multiple inflammatory lesions; several to many comedones, papules, and pustules; and no more than 1 small nodulocystic lesion. Severe AV (EGSS of 4) was characterized by inflammatory lesions; numerous comedones, papules, and pustules; and possibly a few nodulocystic lesions.10
Male and female patients aged 12 to 40 years with moderate to severe AV—defined as 20 to 40 inflammatory lesions (papules, pustules, nodules), 20 to 100 noninflammatory lesions (comedones), and no more than 2 nodules—were included in the study. Standard washout periods were required for patients using prior prescription and over-the-counter acne treatments.10
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
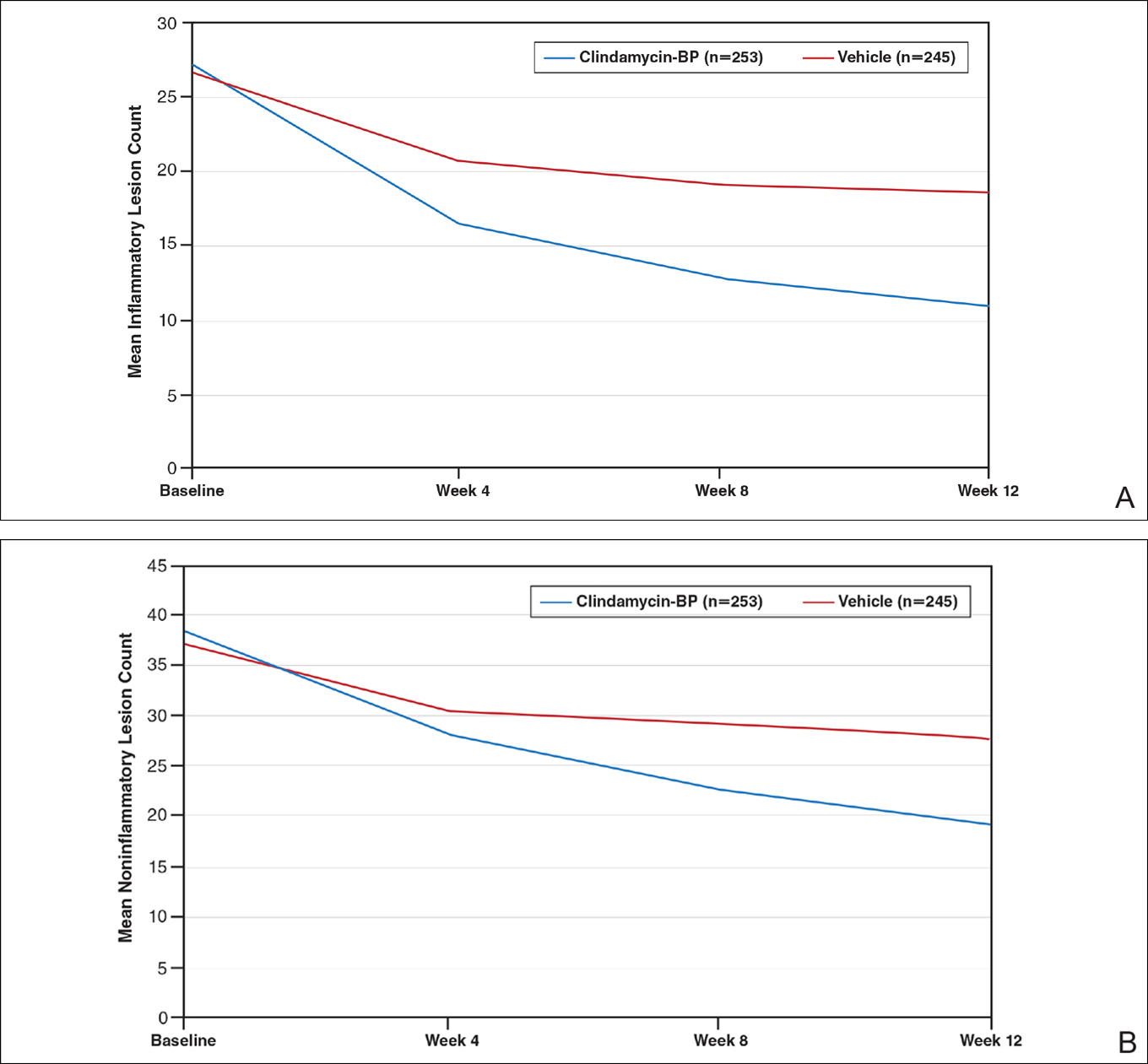
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
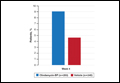
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10
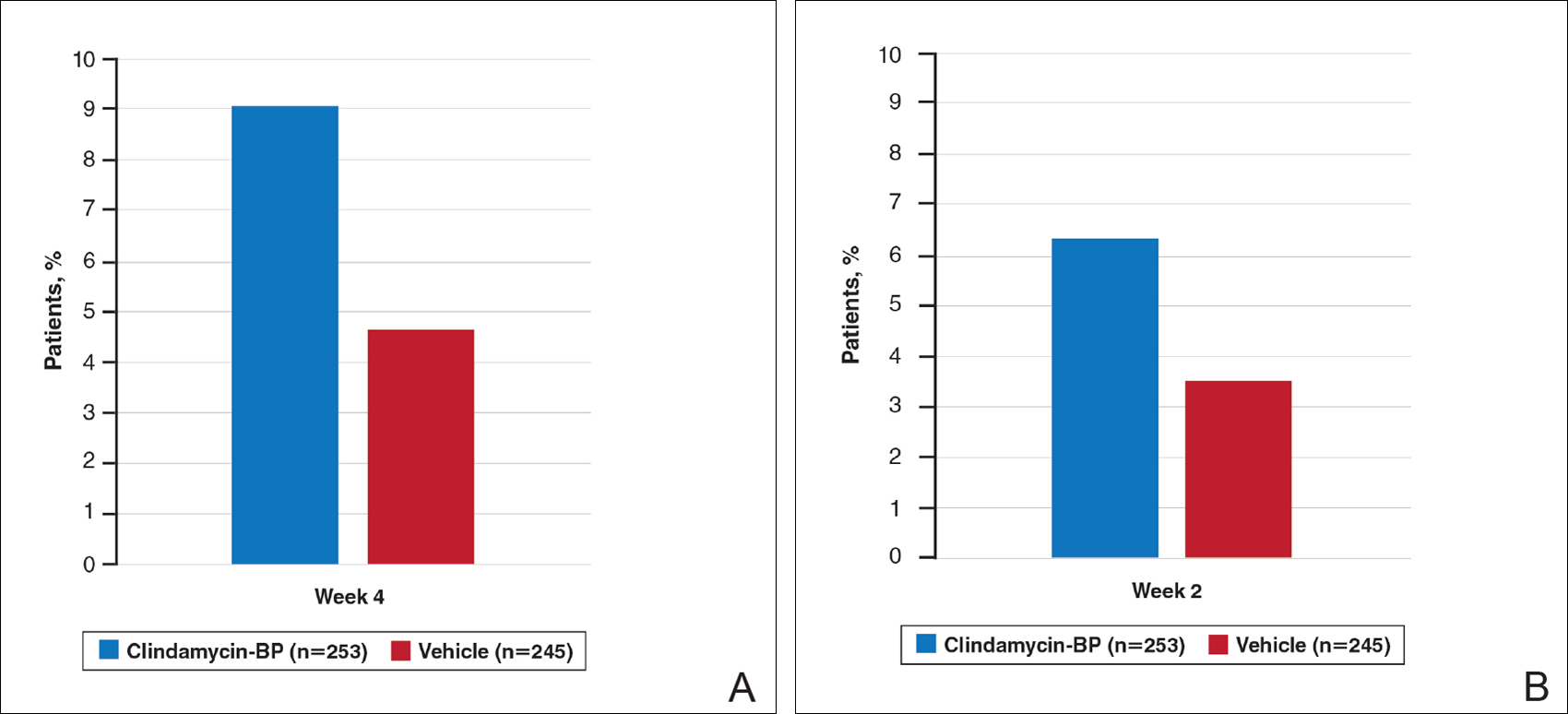
This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).
Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13
There are limitations with this analysis. First, it is not possible to assess the contributions from each of the monads to the efficacy of clindamycin-BP 3.75% gel or TOA. Also, the data extraction method used assumes a linear progression model during the provided time points and was used to provide some comparison with calculations for other combination products.9 Although no strong deviations from the linear model are likely, calculations of TOA using other methodologies may give different results. The definition of a clinically meaningful benefit, defined here as a 25% reduction in the mean lesion count, has been used as a guide, but it has not been validated in clinical practice. It also is important to recognize that the initial visible perception of improvement of AV is likely to differ based on interpatient variability; that is, how different individuals perceive improvement. It also may be affected by differences in baseline severity of AV among different patients. Additionally, the TOA reflects an average duration of time, so it should not be described to patients as a suggestion of when they will definitely see visible improvement in their AV.
Conclusion
Unrealistic expectations of acne therapy or poor tolerability can lead to low adherence and poor clinical outcomes.1-4 The data on TOA reported here suggests that a clinically meaningful benefit with clindamycin-BP 3.75% gel may be seen in some patients within 2 to 3 weeks and maybe sooner in females or those with milder disease; however, longer durations may be required in some patients. This information can help clinicians and their staff in providing reasonable expectations and stress the importance of encouraging patients about the need to adhere to treatment.
Acknowledgments
The author thanks Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for publication support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis. The author did not receive funding or any form of compensation for authorship of this publication.
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(suppl 1):1-14.
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15:87-94.
- Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223:174-181.
- Zauli S, Caracciolo S, Borghi A, et al. Which factors influence quality of life in acne patients? J Eur Acad Dermatol Venereol. 2014;28:46-50.
- Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124-130.
- Gerlinger C, Stadtler G, Gotzelmann R, et al. A noninferiority margin for acne lesion counts. Drug Inf J. 2008;42:607-615.
- Jacobs A, Starke G, Rosumeck S, et al. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170:557-564.
- Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:611-617.
- Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
- Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160-166.
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
Acne vulgaris (AV) is a common skin disease that usually presents in adolescence and can persist into adulthood. Some cases may start in adulthood, especially in women. Acne vulgaris remains a challenge to treat successfully, both in teenagers and adults. Unrealistic expectations that therapy will rapidly clear and sustain clearance of AV completely can lead to incomplete adherence or complete cessation of treatment.1-4 Local tolerability reactions also may decrease adherence to topical medications. Suboptimal adherence to medications for AV is one of the major reasons for treatment failure.5 Acne vulgaris can strongly influence psychological well-being and self-esteem.6 In general, severe AV causes more psychological distress, but the adverse emotional impact of AV can be independent of its severity.7
An effective relationship between the patient and his/her physician and staff is believed to be important in setting realistic expectations, optimizing adherence, and achieving a positive therapeutic outcome. One component related to setting reasonable expectations is the discussion about when the patient may begin to visibly perceive that the treatment regimen is working. This article evaluates the time course of a clinically meaningful response using pivotal trial data with clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel for treatment of AV.
Are data available that evaluate the time course of a clinically relevant response to treatment of AV?
Unfortunately, data on what might be perceived as a clinically meaningful improvement in AV and how long it might take to achieve this treatment effect are limited. A meta-analysis of more than 4000 patients with moderate to severe AV suggested that a 10% to 20% difference in acne lesion counts from baseline as compared to a subsequent designated time point was clinically relevant.8 A review of 24 comparative studies of patients with mild to moderate AV used a primary outcome parameter of a 25% reduction in mean inflammatory lesion count to evaluate time to onset of action (TOA) to achieve a clinically meaningful benefit.9 This outcome was based on a previously identified threshold of clinical relevance and the authors’ clinical experience in a patient population with milder AV. In this same analysis, a difference of greater than 4 days between the active group and the vehicle group was considered to be relevant to the patient.9
A faster onset of visible improvement as perceived by the patient should be more desirable and is likely to improve treatment adherence, as long as it is not counterbalanced by an increase in adverse events.
What is meant by TOA?
Time to onset of action refers to the duration required to achieve a 25% mean lesion count reduction from baseline, which is believed to correlate with the time point at which many patients would be able to perceive visible improvement when viewing their full face. Therefore, TOA represents an attempt to correlate data that is quantitative (based on lesion count reduction) with what is likely to be the average time that a patient may qualitatively observe an initial visible improvement in their AV. This concept may be useful as a tool when communicating with AV patients but should not be used in a way that will overpromise and underdeliver; rather, it is a guide for discussion with the patient and with a parent or guardian when applicable.
Consistent with the comparative AV study analysis that evaluated TOA, a linear course of lesion reductions between the provided time intervals was assumed. In this linear model, the TOA was calculated using the 2 extracted lesion count values between which the 25% lesion reduction was achieved as well as their corresponding given time points.9 Differences between the results in the active and vehicle study arms were calculated for a number of determinants.
How was pivotal trial data with clindamycin-BP 3.75% gel used to assess TOA?
A total of 498 patients with moderate to severe AV were randomized (1:1) to receive clindamycin-BP 3.75% gel or vehicle in a multicenter, double-blind, controlled, 12-week, 2-arm study.10 Before randomization, patients were stratified by acne severity based on a static Evaluator’s Global Severity Score (EGSS) ranging from 0 (clear) to 5 (very severe). Specifically, moderate AV (EGSS of 3) was described as predominantly noninflammatory lesions with evidence of multiple inflammatory lesions; several to many comedones, papules, and pustules; and no more than 1 small nodulocystic lesion. Severe AV (EGSS of 4) was characterized by inflammatory lesions; numerous comedones, papules, and pustules; and possibly a few nodulocystic lesions.10
Male and female patients aged 12 to 40 years with moderate to severe AV—defined as 20 to 40 inflammatory lesions (papules, pustules, nodules), 20 to 100 noninflammatory lesions (comedones), and no more than 2 nodules—were included in the study. Standard washout periods were required for patients using prior prescription and over-the-counter acne treatments.10
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10

This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).

Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13
There are limitations with this analysis. First, it is not possible to assess the contributions from each of the monads to the efficacy of clindamycin-BP 3.75% gel or TOA. Also, the data extraction method used assumes a linear progression model during the provided time points and was used to provide some comparison with calculations for other combination products.9 Although no strong deviations from the linear model are likely, calculations of TOA using other methodologies may give different results. The definition of a clinically meaningful benefit, defined here as a 25% reduction in the mean lesion count, has been used as a guide, but it has not been validated in clinical practice. It also is important to recognize that the initial visible perception of improvement of AV is likely to differ based on interpatient variability; that is, how different individuals perceive improvement. It also may be affected by differences in baseline severity of AV among different patients. Additionally, the TOA reflects an average duration of time, so it should not be described to patients as a suggestion of when they will definitely see visible improvement in their AV.
Conclusion
Unrealistic expectations of acne therapy or poor tolerability can lead to low adherence and poor clinical outcomes.1-4 The data on TOA reported here suggests that a clinically meaningful benefit with clindamycin-BP 3.75% gel may be seen in some patients within 2 to 3 weeks and maybe sooner in females or those with milder disease; however, longer durations may be required in some patients. This information can help clinicians and their staff in providing reasonable expectations and stress the importance of encouraging patients about the need to adhere to treatment.
Acknowledgments
The author thanks Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for publication support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis. The author did not receive funding or any form of compensation for authorship of this publication.
Acne vulgaris (AV) is a common skin disease that usually presents in adolescence and can persist into adulthood. Some cases may start in adulthood, especially in women. Acne vulgaris remains a challenge to treat successfully, both in teenagers and adults. Unrealistic expectations that therapy will rapidly clear and sustain clearance of AV completely can lead to incomplete adherence or complete cessation of treatment.1-4 Local tolerability reactions also may decrease adherence to topical medications. Suboptimal adherence to medications for AV is one of the major reasons for treatment failure.5 Acne vulgaris can strongly influence psychological well-being and self-esteem.6 In general, severe AV causes more psychological distress, but the adverse emotional impact of AV can be independent of its severity.7
An effective relationship between the patient and his/her physician and staff is believed to be important in setting realistic expectations, optimizing adherence, and achieving a positive therapeutic outcome. One component related to setting reasonable expectations is the discussion about when the patient may begin to visibly perceive that the treatment regimen is working. This article evaluates the time course of a clinically meaningful response using pivotal trial data with clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel for treatment of AV.
Are data available that evaluate the time course of a clinically relevant response to treatment of AV?
Unfortunately, data on what might be perceived as a clinically meaningful improvement in AV and how long it might take to achieve this treatment effect are limited. A meta-analysis of more than 4000 patients with moderate to severe AV suggested that a 10% to 20% difference in acne lesion counts from baseline as compared to a subsequent designated time point was clinically relevant.8 A review of 24 comparative studies of patients with mild to moderate AV used a primary outcome parameter of a 25% reduction in mean inflammatory lesion count to evaluate time to onset of action (TOA) to achieve a clinically meaningful benefit.9 This outcome was based on a previously identified threshold of clinical relevance and the authors’ clinical experience in a patient population with milder AV. In this same analysis, a difference of greater than 4 days between the active group and the vehicle group was considered to be relevant to the patient.9
A faster onset of visible improvement as perceived by the patient should be more desirable and is likely to improve treatment adherence, as long as it is not counterbalanced by an increase in adverse events.
What is meant by TOA?
Time to onset of action refers to the duration required to achieve a 25% mean lesion count reduction from baseline, which is believed to correlate with the time point at which many patients would be able to perceive visible improvement when viewing their full face. Therefore, TOA represents an attempt to correlate data that is quantitative (based on lesion count reduction) with what is likely to be the average time that a patient may qualitatively observe an initial visible improvement in their AV. This concept may be useful as a tool when communicating with AV patients but should not be used in a way that will overpromise and underdeliver; rather, it is a guide for discussion with the patient and with a parent or guardian when applicable.
Consistent with the comparative AV study analysis that evaluated TOA, a linear course of lesion reductions between the provided time intervals was assumed. In this linear model, the TOA was calculated using the 2 extracted lesion count values between which the 25% lesion reduction was achieved as well as their corresponding given time points.9 Differences between the results in the active and vehicle study arms were calculated for a number of determinants.
How was pivotal trial data with clindamycin-BP 3.75% gel used to assess TOA?
A total of 498 patients with moderate to severe AV were randomized (1:1) to receive clindamycin-BP 3.75% gel or vehicle in a multicenter, double-blind, controlled, 12-week, 2-arm study.10 Before randomization, patients were stratified by acne severity based on a static Evaluator’s Global Severity Score (EGSS) ranging from 0 (clear) to 5 (very severe). Specifically, moderate AV (EGSS of 3) was described as predominantly noninflammatory lesions with evidence of multiple inflammatory lesions; several to many comedones, papules, and pustules; and no more than 1 small nodulocystic lesion. Severe AV (EGSS of 4) was characterized by inflammatory lesions; numerous comedones, papules, and pustules; and possibly a few nodulocystic lesions.10
Male and female patients aged 12 to 40 years with moderate to severe AV—defined as 20 to 40 inflammatory lesions (papules, pustules, nodules), 20 to 100 noninflammatory lesions (comedones), and no more than 2 nodules—were included in the study. Standard washout periods were required for patients using prior prescription and over-the-counter acne treatments.10
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10

This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).

Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13
There are limitations with this analysis. First, it is not possible to assess the contributions from each of the monads to the efficacy of clindamycin-BP 3.75% gel or TOA. Also, the data extraction method used assumes a linear progression model during the provided time points and was used to provide some comparison with calculations for other combination products.9 Although no strong deviations from the linear model are likely, calculations of TOA using other methodologies may give different results. The definition of a clinically meaningful benefit, defined here as a 25% reduction in the mean lesion count, has been used as a guide, but it has not been validated in clinical practice. It also is important to recognize that the initial visible perception of improvement of AV is likely to differ based on interpatient variability; that is, how different individuals perceive improvement. It also may be affected by differences in baseline severity of AV among different patients. Additionally, the TOA reflects an average duration of time, so it should not be described to patients as a suggestion of when they will definitely see visible improvement in their AV.
Conclusion
Unrealistic expectations of acne therapy or poor tolerability can lead to low adherence and poor clinical outcomes.1-4 The data on TOA reported here suggests that a clinically meaningful benefit with clindamycin-BP 3.75% gel may be seen in some patients within 2 to 3 weeks and maybe sooner in females or those with milder disease; however, longer durations may be required in some patients. This information can help clinicians and their staff in providing reasonable expectations and stress the importance of encouraging patients about the need to adhere to treatment.
Acknowledgments
The author thanks Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for publication support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis. The author did not receive funding or any form of compensation for authorship of this publication.
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(suppl 1):1-14.
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15:87-94.
- Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223:174-181.
- Zauli S, Caracciolo S, Borghi A, et al. Which factors influence quality of life in acne patients? J Eur Acad Dermatol Venereol. 2014;28:46-50.
- Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124-130.
- Gerlinger C, Stadtler G, Gotzelmann R, et al. A noninferiority margin for acne lesion counts. Drug Inf J. 2008;42:607-615.
- Jacobs A, Starke G, Rosumeck S, et al. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170:557-564.
- Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:611-617.
- Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
- Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160-166.
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(suppl 1):1-14.
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15:87-94.
- Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223:174-181.
- Zauli S, Caracciolo S, Borghi A, et al. Which factors influence quality of life in acne patients? J Eur Acad Dermatol Venereol. 2014;28:46-50.
- Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124-130.
- Gerlinger C, Stadtler G, Gotzelmann R, et al. A noninferiority margin for acne lesion counts. Drug Inf J. 2008;42:607-615.
- Jacobs A, Starke G, Rosumeck S, et al. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170:557-564.
- Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:611-617.
- Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
- Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160-166.
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
Practice Points
- Time to onset of action (TOA) refers to how long it takes after starting a therapy for a patient to perceive visible improvement.
- Time to onset of action has been determined based on data to date to correlate overall with a 25% lesion reduction.
- The TOA for clindamycin phosphate 1.2%–benzoyl peroxide 3.75% gel applied once daily based on analysis of pivotal trial data is 3 weeks or less depending on the severity of acne vulgaris at baseline.
Andrew Hershey, MD, PhD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Imaging of the Breast 2016: Reducing the Confusion
Program Director
Steven R. Goldstein, MD, CCD, NCMP, FACOG
Professor of Obstetrics and Gynecology
New York University School of Medicine
Director of Gynecological Ultrasound
Co-Director of Bone Densitometry
Department of Obstetrics and Gynecology
New York University Medical Center
New York, New York
Authors/Editors
Reni S. Butler, MD
Assistant Professor, Diagnostic Radiology
Yale University
New Haven, Connecticut
Bonnie N. Joe, MD, PhD, FSBI
Professor of Radiology and Biomedical Imaging
Chief, Breast Imaging
University of California, San Francisco
San Francisco, California
Bruce A. Porter, MD, FACR
Clinical Associate Professor
Department of Radiology
University of Washington School of Medicine
Seattle, Washington
Michael J. Ulissey, MD, FACR
Adjunct Professor of Radiology
University of Texas
Health Sciences Center
San Antonio, Texas
Breast Diagnostic Centers
Auburn and Federal Way, Washington
Conflict of Interest Disclosure
Steven R. Goldstein, MD, CCD, NCMP, FACOG
Consultant: Cook OB/GYN, fees received
No Disclosures to Declare
Reni S. Butler, MD; Bonnie N. Joe, MD, PhD, FSBI;
Bruce A. Porter, MD, FACR; Michael J. Ulissey, MD, FACR;
Heidi M. Wilson, Course Director
OBG Manag. 2016 June;28 (6 suppl):S1-S8.
Program Director
Steven R. Goldstein, MD, CCD, NCMP, FACOG
Professor of Obstetrics and Gynecology
New York University School of Medicine
Director of Gynecological Ultrasound
Co-Director of Bone Densitometry
Department of Obstetrics and Gynecology
New York University Medical Center
New York, New York
Authors/Editors
Reni S. Butler, MD
Assistant Professor, Diagnostic Radiology
Yale University
New Haven, Connecticut
Bonnie N. Joe, MD, PhD, FSBI
Professor of Radiology and Biomedical Imaging
Chief, Breast Imaging
University of California, San Francisco
San Francisco, California
Bruce A. Porter, MD, FACR
Clinical Associate Professor
Department of Radiology
University of Washington School of Medicine
Seattle, Washington
Michael J. Ulissey, MD, FACR
Adjunct Professor of Radiology
University of Texas
Health Sciences Center
San Antonio, Texas
Breast Diagnostic Centers
Auburn and Federal Way, Washington
Conflict of Interest Disclosure
Steven R. Goldstein, MD, CCD, NCMP, FACOG
Consultant: Cook OB/GYN, fees received
No Disclosures to Declare
Reni S. Butler, MD; Bonnie N. Joe, MD, PhD, FSBI;
Bruce A. Porter, MD, FACR; Michael J. Ulissey, MD, FACR;
Heidi M. Wilson, Course Director
Program Director
Steven R. Goldstein, MD, CCD, NCMP, FACOG
Professor of Obstetrics and Gynecology
New York University School of Medicine
Director of Gynecological Ultrasound
Co-Director of Bone Densitometry
Department of Obstetrics and Gynecology
New York University Medical Center
New York, New York
Authors/Editors
Reni S. Butler, MD
Assistant Professor, Diagnostic Radiology
Yale University
New Haven, Connecticut
Bonnie N. Joe, MD, PhD, FSBI
Professor of Radiology and Biomedical Imaging
Chief, Breast Imaging
University of California, San Francisco
San Francisco, California
Bruce A. Porter, MD, FACR
Clinical Associate Professor
Department of Radiology
University of Washington School of Medicine
Seattle, Washington
Michael J. Ulissey, MD, FACR
Adjunct Professor of Radiology
University of Texas
Health Sciences Center
San Antonio, Texas
Breast Diagnostic Centers
Auburn and Federal Way, Washington
Conflict of Interest Disclosure
Steven R. Goldstein, MD, CCD, NCMP, FACOG
Consultant: Cook OB/GYN, fees received
No Disclosures to Declare
Reni S. Butler, MD; Bonnie N. Joe, MD, PhD, FSBI;
Bruce A. Porter, MD, FACR; Michael J. Ulissey, MD, FACR;
Heidi M. Wilson, Course Director
OBG Manag. 2016 June;28 (6 suppl):S1-S8.
OBG Manag. 2016 June;28 (6 suppl):S1-S8.