User login
FDA panel narrowly endorses empagliflozin’s cardiovascular mortality benefit
ROCKVILLE, MD. – In a 12-11 vote, a Food and Drug Administration advisory panel just barely came down in favor of the agency adding a new labeling entry to the already-approved diabetes drug empagliflozin (Jardiance) that would say the drug reduces cardiovascular mortality.
While several members of the panel wished the FDA’s staff good luck in weighing both the evidence and the advisory committee’s closely split endorsement when deciding whether to grant this unprecedented labeling to a diabetes drug, the fact that a majority of panelists favored this course marked a watershed moment in the development of new agents for treating hyperglycemia.
“It’s the first time we have evidence that a diabetes drug can reduce cardiovascular risk. That’s never been seen before, and it’s huge,” said Marvin A. Konstam, MD, a temporary member of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) and chief physician executive of the cardiovascular center at Tufts Medical Center in Boston. “The question is whether or not this effect is real, and the vote was 50-50, but I think there is a good chance it’s real, and, if so, it’s a game changer,” Dr. Konstam said in an interview. He voted in favor of the new labeling, and, like many of his colleagues on the panel, he admitted to agonizing over the decision during the postvote comment period.
What he and the other committee members struggled with was a remarkably strong effect by empagliflozin on reducing cardiovascular mortality by a relative 38%, compared with placebo, in more than 7,000 patients with type 2 diabetes selected for their high cardiovascular disease risk. The major sticking point was that the study enrolled patients into a randomized, placebo-controlled trial that was primarily designed to test the drug’s cardiovascular safety and not its efficacy, and where cardiovascular death was not even a prespecified secondary endpoint.
“It’s very hard to go from safety to superiority in one study,” said Peter W.F. Wilson, MD, who voted against the added indication. Like many panel members who voted no, Dr. Wilson said that any claim to preventing cardiovascular mortality with empagliflozin should meet the standard FDA requirement to have consistent results from at least two studies. “This is the first drug in its class [the sodium-glucose cotransporter 2 inhibitors], and we should have a high bar for the quality of the evidence,” said Dr. Wilson, professor of medicine and public health at Emory University in Atlanta.
“There is substantial evidence [to support the mortality claim], but not yet to the extent to put it on the label,” said another voter on the no side, Judith Fradkin, MD, also a temporary committee member and director of the division of diabetes, endocrinology and metabolic diseases at the National Institutes of Health. The data collected so far in favor of the mortality claim “are very compelling, but what I couldn’t get past is my long-standing belief that a positive result to a study’s secondary outcome is hypothesis generating. We need a second study to put this on the label,” Dr. Fradkin said.
That dramatic and highly meaningful clinical effect of empagliflozin on cardiovascular mortality jumped out at the investigators who ran the EMPA-REG OUTCOME trial as well as to many others from the moment the results had their unveiling less than a year ago, at the annual meeting of the European Association for the Study of Diabetes in Stockholm, and in a concurrently published article (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The primary efficacy endpoint placed into the EMPA-REG OUTCOME safety trial as the study developed following the 2008 FDA mandate for cardiovascular safety trials for all new hypoglycemic drugs was a three-part, combined-outcome endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke. Although this primary, combined endpoint had a statistically significant but much more modest benefit with a 14% relative risk reduction, compared with placebo, “the benefit was all driven by the reduction in cardiovascular death that had an astonishing P value of less than .0001 with no suggestion of benefit or risk for MI or stroke,” said Stuart Pocock, PhD, professor of medical statistics at the London School of Hygiene and Tropical Medicine who appeared before the committee as a consultant brought in by the applicant, Boehringer Ingelheim.
The total 309 cardiovascular deaths seen during the study that led to this finding provided more data than most cardiovascular trials, and while in some respects, the cardiovascular benefit seemed “too good to be true,” it also turned out that “the data were so strong that they overwhelm skepticism,” Dr. Pocock told the panel while presenting some advanced statistical test results to prove this assertion. The trial results showed “overwhelming evidence of benefit, beyond a reasonable doubt,” and while cardiovascular death was just one part of the efficacy endpoint, “mortality merits special attention,” he said. The statistical analyses also showed an equally robust 32% relative risk reduction in all-cause mortality, and both the cardiovascular and all-cause death benefits seen in EMPA-REG OUTCOME were consistent across both dosages of empagliflozin tested in the study (10 mg and 25 mg daily) and across the sensitivity analyses applied by the investigators.
“These are convincing data, but I’m not comfortable enough that these robust data would be reproduced in a second trial,” said panel chair Robert J. Smith, MD, who voted against the indication.
An additional limitation acting against the proposed new labeling, according to several panel members, is that the mechanism by which empagliflozin might exert protection against cardiovascular death remains unknown, with no suggestion in the trial results that it acts by protecting patients against ischemic disease.
Current opinion also splits among clinicians on how empagliflozin, which has had FDA approval since 2014 as an option for treating type 2 diabetes, should be used in routine practice to treat diabetes patients with high cardiovascular risk who match those enrolled in the EMPA-REG OUTCOME trial. Dr. Smith urged a cautious approach.
“I think it’s important [for prescribers] to wait to hear from the FDA. If the cardiovascular mortality benefit was proven, then it would be an important option given the magnitude of cardiovascular disease and death as a consequence of type 2 diabetes. But people should be cautious in drawing their own interpretations of the data,” Dr. Smith, professor of medicine at Brown University in Providence, R.I., said in an interview. For the time being, metformin remains the top oral drug for most of these patients because of its proven effectiveness and low cost, he added.
But others have already been active in prescribing empagliflozin to at-risk patients with type 2 diabetes based on last year’s EMPA-REG OUTCOME report.
“I am using it in addition to metformin and aggressive lifestyle changes in patients with established cardiovascular disease and uncontrolled type 2 diabetes,” commented Alison L. Bailey, MD, a cardiologist at the Erlanger Health System and University of Tennessee in Chattanooga. “A patient’s health insurance status must be taken into account as empagliflozin can be a significant financial burden, but if all other things are equal and cost is not prohibitive, I am definitely using this in my patients with type 2 diabetes and cardiovascular disease. I think there are enough data to warrant its use first line in patients who can get the drug without a financial burden,” she said in an interview.
Dr. Konstam cautioned that “just because empagliflozin may have a cardiovascular effect does not make it a cardiovascular drug. As a cardiologist I am not comfortable prescribing this drug. When it comes to diabetes management ,you need to take many things into consideration, most notably blood sugar and hemoglobin A1c,” which are usually best managed by a diabetologist or experienced primary care physician, he said.
Dr. Konstam, Dr. Wilson, Dr. Fradkin, and Dr. Smith had no relevant financial disclosures. Dr. Pocock is a consultant to Boehringer Ingelheim. Dr. Bailey has received research grants from CSL Behring.
On Twitter@mitchelzoler
ROCKVILLE, MD. – In a 12-11 vote, a Food and Drug Administration advisory panel just barely came down in favor of the agency adding a new labeling entry to the already-approved diabetes drug empagliflozin (Jardiance) that would say the drug reduces cardiovascular mortality.
While several members of the panel wished the FDA’s staff good luck in weighing both the evidence and the advisory committee’s closely split endorsement when deciding whether to grant this unprecedented labeling to a diabetes drug, the fact that a majority of panelists favored this course marked a watershed moment in the development of new agents for treating hyperglycemia.
“It’s the first time we have evidence that a diabetes drug can reduce cardiovascular risk. That’s never been seen before, and it’s huge,” said Marvin A. Konstam, MD, a temporary member of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) and chief physician executive of the cardiovascular center at Tufts Medical Center in Boston. “The question is whether or not this effect is real, and the vote was 50-50, but I think there is a good chance it’s real, and, if so, it’s a game changer,” Dr. Konstam said in an interview. He voted in favor of the new labeling, and, like many of his colleagues on the panel, he admitted to agonizing over the decision during the postvote comment period.
What he and the other committee members struggled with was a remarkably strong effect by empagliflozin on reducing cardiovascular mortality by a relative 38%, compared with placebo, in more than 7,000 patients with type 2 diabetes selected for their high cardiovascular disease risk. The major sticking point was that the study enrolled patients into a randomized, placebo-controlled trial that was primarily designed to test the drug’s cardiovascular safety and not its efficacy, and where cardiovascular death was not even a prespecified secondary endpoint.
“It’s very hard to go from safety to superiority in one study,” said Peter W.F. Wilson, MD, who voted against the added indication. Like many panel members who voted no, Dr. Wilson said that any claim to preventing cardiovascular mortality with empagliflozin should meet the standard FDA requirement to have consistent results from at least two studies. “This is the first drug in its class [the sodium-glucose cotransporter 2 inhibitors], and we should have a high bar for the quality of the evidence,” said Dr. Wilson, professor of medicine and public health at Emory University in Atlanta.
“There is substantial evidence [to support the mortality claim], but not yet to the extent to put it on the label,” said another voter on the no side, Judith Fradkin, MD, also a temporary committee member and director of the division of diabetes, endocrinology and metabolic diseases at the National Institutes of Health. The data collected so far in favor of the mortality claim “are very compelling, but what I couldn’t get past is my long-standing belief that a positive result to a study’s secondary outcome is hypothesis generating. We need a second study to put this on the label,” Dr. Fradkin said.
That dramatic and highly meaningful clinical effect of empagliflozin on cardiovascular mortality jumped out at the investigators who ran the EMPA-REG OUTCOME trial as well as to many others from the moment the results had their unveiling less than a year ago, at the annual meeting of the European Association for the Study of Diabetes in Stockholm, and in a concurrently published article (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The primary efficacy endpoint placed into the EMPA-REG OUTCOME safety trial as the study developed following the 2008 FDA mandate for cardiovascular safety trials for all new hypoglycemic drugs was a three-part, combined-outcome endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke. Although this primary, combined endpoint had a statistically significant but much more modest benefit with a 14% relative risk reduction, compared with placebo, “the benefit was all driven by the reduction in cardiovascular death that had an astonishing P value of less than .0001 with no suggestion of benefit or risk for MI or stroke,” said Stuart Pocock, PhD, professor of medical statistics at the London School of Hygiene and Tropical Medicine who appeared before the committee as a consultant brought in by the applicant, Boehringer Ingelheim.
The total 309 cardiovascular deaths seen during the study that led to this finding provided more data than most cardiovascular trials, and while in some respects, the cardiovascular benefit seemed “too good to be true,” it also turned out that “the data were so strong that they overwhelm skepticism,” Dr. Pocock told the panel while presenting some advanced statistical test results to prove this assertion. The trial results showed “overwhelming evidence of benefit, beyond a reasonable doubt,” and while cardiovascular death was just one part of the efficacy endpoint, “mortality merits special attention,” he said. The statistical analyses also showed an equally robust 32% relative risk reduction in all-cause mortality, and both the cardiovascular and all-cause death benefits seen in EMPA-REG OUTCOME were consistent across both dosages of empagliflozin tested in the study (10 mg and 25 mg daily) and across the sensitivity analyses applied by the investigators.
“These are convincing data, but I’m not comfortable enough that these robust data would be reproduced in a second trial,” said panel chair Robert J. Smith, MD, who voted against the indication.
An additional limitation acting against the proposed new labeling, according to several panel members, is that the mechanism by which empagliflozin might exert protection against cardiovascular death remains unknown, with no suggestion in the trial results that it acts by protecting patients against ischemic disease.
Current opinion also splits among clinicians on how empagliflozin, which has had FDA approval since 2014 as an option for treating type 2 diabetes, should be used in routine practice to treat diabetes patients with high cardiovascular risk who match those enrolled in the EMPA-REG OUTCOME trial. Dr. Smith urged a cautious approach.
“I think it’s important [for prescribers] to wait to hear from the FDA. If the cardiovascular mortality benefit was proven, then it would be an important option given the magnitude of cardiovascular disease and death as a consequence of type 2 diabetes. But people should be cautious in drawing their own interpretations of the data,” Dr. Smith, professor of medicine at Brown University in Providence, R.I., said in an interview. For the time being, metformin remains the top oral drug for most of these patients because of its proven effectiveness and low cost, he added.
But others have already been active in prescribing empagliflozin to at-risk patients with type 2 diabetes based on last year’s EMPA-REG OUTCOME report.
“I am using it in addition to metformin and aggressive lifestyle changes in patients with established cardiovascular disease and uncontrolled type 2 diabetes,” commented Alison L. Bailey, MD, a cardiologist at the Erlanger Health System and University of Tennessee in Chattanooga. “A patient’s health insurance status must be taken into account as empagliflozin can be a significant financial burden, but if all other things are equal and cost is not prohibitive, I am definitely using this in my patients with type 2 diabetes and cardiovascular disease. I think there are enough data to warrant its use first line in patients who can get the drug without a financial burden,” she said in an interview.
Dr. Konstam cautioned that “just because empagliflozin may have a cardiovascular effect does not make it a cardiovascular drug. As a cardiologist I am not comfortable prescribing this drug. When it comes to diabetes management ,you need to take many things into consideration, most notably blood sugar and hemoglobin A1c,” which are usually best managed by a diabetologist or experienced primary care physician, he said.
Dr. Konstam, Dr. Wilson, Dr. Fradkin, and Dr. Smith had no relevant financial disclosures. Dr. Pocock is a consultant to Boehringer Ingelheim. Dr. Bailey has received research grants from CSL Behring.
On Twitter@mitchelzoler
ROCKVILLE, MD. – In a 12-11 vote, a Food and Drug Administration advisory panel just barely came down in favor of the agency adding a new labeling entry to the already-approved diabetes drug empagliflozin (Jardiance) that would say the drug reduces cardiovascular mortality.
While several members of the panel wished the FDA’s staff good luck in weighing both the evidence and the advisory committee’s closely split endorsement when deciding whether to grant this unprecedented labeling to a diabetes drug, the fact that a majority of panelists favored this course marked a watershed moment in the development of new agents for treating hyperglycemia.
“It’s the first time we have evidence that a diabetes drug can reduce cardiovascular risk. That’s never been seen before, and it’s huge,” said Marvin A. Konstam, MD, a temporary member of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) and chief physician executive of the cardiovascular center at Tufts Medical Center in Boston. “The question is whether or not this effect is real, and the vote was 50-50, but I think there is a good chance it’s real, and, if so, it’s a game changer,” Dr. Konstam said in an interview. He voted in favor of the new labeling, and, like many of his colleagues on the panel, he admitted to agonizing over the decision during the postvote comment period.
What he and the other committee members struggled with was a remarkably strong effect by empagliflozin on reducing cardiovascular mortality by a relative 38%, compared with placebo, in more than 7,000 patients with type 2 diabetes selected for their high cardiovascular disease risk. The major sticking point was that the study enrolled patients into a randomized, placebo-controlled trial that was primarily designed to test the drug’s cardiovascular safety and not its efficacy, and where cardiovascular death was not even a prespecified secondary endpoint.
“It’s very hard to go from safety to superiority in one study,” said Peter W.F. Wilson, MD, who voted against the added indication. Like many panel members who voted no, Dr. Wilson said that any claim to preventing cardiovascular mortality with empagliflozin should meet the standard FDA requirement to have consistent results from at least two studies. “This is the first drug in its class [the sodium-glucose cotransporter 2 inhibitors], and we should have a high bar for the quality of the evidence,” said Dr. Wilson, professor of medicine and public health at Emory University in Atlanta.
“There is substantial evidence [to support the mortality claim], but not yet to the extent to put it on the label,” said another voter on the no side, Judith Fradkin, MD, also a temporary committee member and director of the division of diabetes, endocrinology and metabolic diseases at the National Institutes of Health. The data collected so far in favor of the mortality claim “are very compelling, but what I couldn’t get past is my long-standing belief that a positive result to a study’s secondary outcome is hypothesis generating. We need a second study to put this on the label,” Dr. Fradkin said.
That dramatic and highly meaningful clinical effect of empagliflozin on cardiovascular mortality jumped out at the investigators who ran the EMPA-REG OUTCOME trial as well as to many others from the moment the results had their unveiling less than a year ago, at the annual meeting of the European Association for the Study of Diabetes in Stockholm, and in a concurrently published article (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The primary efficacy endpoint placed into the EMPA-REG OUTCOME safety trial as the study developed following the 2008 FDA mandate for cardiovascular safety trials for all new hypoglycemic drugs was a three-part, combined-outcome endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke. Although this primary, combined endpoint had a statistically significant but much more modest benefit with a 14% relative risk reduction, compared with placebo, “the benefit was all driven by the reduction in cardiovascular death that had an astonishing P value of less than .0001 with no suggestion of benefit or risk for MI or stroke,” said Stuart Pocock, PhD, professor of medical statistics at the London School of Hygiene and Tropical Medicine who appeared before the committee as a consultant brought in by the applicant, Boehringer Ingelheim.
The total 309 cardiovascular deaths seen during the study that led to this finding provided more data than most cardiovascular trials, and while in some respects, the cardiovascular benefit seemed “too good to be true,” it also turned out that “the data were so strong that they overwhelm skepticism,” Dr. Pocock told the panel while presenting some advanced statistical test results to prove this assertion. The trial results showed “overwhelming evidence of benefit, beyond a reasonable doubt,” and while cardiovascular death was just one part of the efficacy endpoint, “mortality merits special attention,” he said. The statistical analyses also showed an equally robust 32% relative risk reduction in all-cause mortality, and both the cardiovascular and all-cause death benefits seen in EMPA-REG OUTCOME were consistent across both dosages of empagliflozin tested in the study (10 mg and 25 mg daily) and across the sensitivity analyses applied by the investigators.
“These are convincing data, but I’m not comfortable enough that these robust data would be reproduced in a second trial,” said panel chair Robert J. Smith, MD, who voted against the indication.
An additional limitation acting against the proposed new labeling, according to several panel members, is that the mechanism by which empagliflozin might exert protection against cardiovascular death remains unknown, with no suggestion in the trial results that it acts by protecting patients against ischemic disease.
Current opinion also splits among clinicians on how empagliflozin, which has had FDA approval since 2014 as an option for treating type 2 diabetes, should be used in routine practice to treat diabetes patients with high cardiovascular risk who match those enrolled in the EMPA-REG OUTCOME trial. Dr. Smith urged a cautious approach.
“I think it’s important [for prescribers] to wait to hear from the FDA. If the cardiovascular mortality benefit was proven, then it would be an important option given the magnitude of cardiovascular disease and death as a consequence of type 2 diabetes. But people should be cautious in drawing their own interpretations of the data,” Dr. Smith, professor of medicine at Brown University in Providence, R.I., said in an interview. For the time being, metformin remains the top oral drug for most of these patients because of its proven effectiveness and low cost, he added.
But others have already been active in prescribing empagliflozin to at-risk patients with type 2 diabetes based on last year’s EMPA-REG OUTCOME report.
“I am using it in addition to metformin and aggressive lifestyle changes in patients with established cardiovascular disease and uncontrolled type 2 diabetes,” commented Alison L. Bailey, MD, a cardiologist at the Erlanger Health System and University of Tennessee in Chattanooga. “A patient’s health insurance status must be taken into account as empagliflozin can be a significant financial burden, but if all other things are equal and cost is not prohibitive, I am definitely using this in my patients with type 2 diabetes and cardiovascular disease. I think there are enough data to warrant its use first line in patients who can get the drug without a financial burden,” she said in an interview.
Dr. Konstam cautioned that “just because empagliflozin may have a cardiovascular effect does not make it a cardiovascular drug. As a cardiologist I am not comfortable prescribing this drug. When it comes to diabetes management ,you need to take many things into consideration, most notably blood sugar and hemoglobin A1c,” which are usually best managed by a diabetologist or experienced primary care physician, he said.
Dr. Konstam, Dr. Wilson, Dr. Fradkin, and Dr. Smith had no relevant financial disclosures. Dr. Pocock is a consultant to Boehringer Ingelheim. Dr. Bailey has received research grants from CSL Behring.
On Twitter@mitchelzoler
AT AN FDA EMDAC MEETING
Enlarged Facial Pores: An Update on Treatments
Enlarged facial pores are superficial skin structures that are visualized as small openings on the skin corresponding to the openings of the pilosebaceous apparatus. These openings may be impacted with horny follicular plugs consisting of sebaceous debris that appear as open comedones.1 Skin pores is a lay term that is poorly defined in the medical literature and often is categorized in terms of arbitrary circular diameters determined through cosmetic skin analyzers.2 The term refers to pilosebaceous follicular enlargements (with or without open comedonal horny impactions) that can be visualized by the naked eye, most commonly occurring on the face and scalp. These enlarged pores remain a pervasive cosmetic concern that impacts patient quality of life. Enlarged pores are difficult to treat, in part due to lack of knowledge of the pathophysiology; thus, we review the currently proposed causes of enlarged pilosebaceous openings and the treatments in the scope of this pathogenesis with a focus on therapeutic efficacy.
Pathogenesis of Enlarged Facial Pores
It is now thought that seborrhea, loss of skin elasticity and tension, and hair follicle size are most clinically relevant to the pathogenesis of enlarged pores.2 Other potential associated and causative factors include genetic predisposition, acne, comedogenic xenobiotics, chronic photodamage, chronic radiodermatitis, and vitamin A deficiency.1,3
The direct relationship between sebum output and pore size has been well established, particularly in men who generally have higher sebum output levels than women, which likely is testosterone driven.4,5 However, there are contradictory data on whether sex affects pore size, as females also exhibit contributory hormonal factors. Sebum output and pore size increase substantially during the ovulation phase of the female menstrual cycle, likely secondary to increased progesterone affecting sebaceous gland activity.2,4 The presence of acne also is associated with enlarged facial pores, though the extent of seborrhea as a confounding factor is unclear. Furthermore, acne severity does not correlate with increased pore size.5 However, the processes of acne and facial pores are interlinked, given the frequent occurrence of open comedones within the pores.
Skin elasticity and tensile strength when defined visually and mechanically has shown a negative correlation with facial pore size and density.5 It is well known that cutaneous aging and chronic photodamage cause perturbation in the collagen and elastin framework that allows for the skin to maintain its resilient properties.6 Aged and photodamaged skin also demonstrates decreased expression of microfibril-associated glycoprotein-1 (MAGP-1), a crucial component in elastic fiber assembly and skin elasticity in the dermis and perifollicular/pore areas.7
Pore density and size appears to range diversely across ethnicities, though Chinese women exhibit notably lower pore size and density across all ages as compared to other ethnicities.8 Black individuals have aberrant epidermal architecture, defined as the presence of stalagmitelike structures at the dermoepidermal junction, correlating with enlarged pore size compared to other ethnicities.2,8
Treating Enlarged Facial Pores
Treatments for enlarged facial pores primarily aim to decrease sebum production, rejuvenate skin, remove hair, and/or decrease follicular size. Evidence-based studies are limited, and many currently used therapies have not been studied with enlarged facial pores as a primary investigative outcome. Here, we include studies that report efficacy in decreasing pore size specifically. It is important to note the lack of a uniform and objective modality with which to report skin pore size. Studies use a wide range of techniques including patient self-reporting, physician observation, and software image analyzers.
Topical Therapies
Topical retinoids are vitamin A derivatives, and they are first-line therapies in reversing the aberrant collagen and elastin-associated epidermal and dermal changes that occur with chronological aging and photoaging. Tretinoin, isotretinoin, and tazarotene have shown efficacy in multiple parameters of skin rejuvenation, including facial pores, skin wrinkling, hyperpigmentation, skin laxity, and sebum production.9 However, it is important to note that retinoids treat keratinocyte atypia in acne, and efficacy in facial pores is confounded by improvement in follicular keratinization. Because studies have not distinctly uncoupled this association, it is erroneous to conclude that retinoids reduce facial pore size and density irrespective of concomitant acne vulgaris.
Tazarotene has been evaluated for use in reducing facial pore size. In one investigation, 568 patients with moderate wrinkling or hyperpigmentation were randomized to receive tazarotene cream 0.1% or placebo once daily for 24 weeks and were evaluated for enlarged facial pores as a secondary outcome using a double-blinded physician 5-point scale.10 At week 24, 42% of tazarotene-treated patients achieved improvement of at least 1 point compared to 20% of placebo-treated patients (P<.001). Adverse events were dermatitic, as can be expected of retinoids, leading to a 4% discontinuation rate in the tazarotene group compared to 1% in the placebo group.10
Tretinoin has long been used off label for antiaging treatments but has only recently shown efficacy for facial pores. In one study, 60 women who had previously sought antiaging procedures were treated with tretinoin cream 0.025% once daily and no other antiaging products or procedures for 90 days.11 Facial pore evaluations were determined by a modified dermatoscope with a polarized analyzer for clinical scoring using a photonumeric scale. Patients improved from a baseline average score of 3.2 in facial pores to a posttreatment average score of 2.0 (P<.05) at day 84. This improvement was sustained from day 28 of treatment and corresponded to patient self-perception. Adverse events included xerosis, desquamation, burning, and erythema, which led to 3 premature discontinuations.11
Various chemical peel formulations are used in skin rejuvenation and have shown application in enlarged facial pores. Chemical peels act at the epidermal or dermal level to induce temporary breakdown and regeneration of healthier cells and improved skin matrix.12 Twenty-two Japanese women applied glycolic acid (30% solution) every 2 weeks for a total of 5 treatments and exhibited reduced appearance of conspicuous, open, and dark pores, defined by surface area and shading as determined through dermatoscopic and software analysis, with mean improvement rates of 34.6%, 11%, and 34.3%, respectively. More than 70% of participants exhibited improvement in enlarged facial pores.13 A study involving a 40% glycolic acid and vitamin C formulation demonstrated significant improvement in facial pores (28.3%; P<.001).14
The newest topical therapies studied for use in minimizing facial pilosebaceous openings are natural plant-derived copper chlorophyllin complex sodium salt (CHLcu) and tetra-hydro-jasmonic acid (LR2412). Clinical trials of these botanicals are limited with small sample sizes but are included here as novel treatments requiring further investigation.
Chlorophyllin copper complex sodium salt is derived from chlorophyll, a green pigment found in plants, and has been investigated as a topical gel in liposomal dispersions for application in photodamaged and aged skin. Chlorophyllin copper complex sodium salt exerts in vitro hyaluronidase inhibitory activity to maintain hyaluronic acid in the extracellular matrix and counteract the structural breakdown of cutaneous aging.15 Two small single-center pilot trials enrolled 10 participants each in a 3-week study of CHLcu 0.1% twice daily and an 8-week study of CHLcu 0.066% twice daily.16,17 After 3 weeks, patients treated with CHLcu 0.1% exhibited a 22.2% improvement in facial pores by clinical assessment grading, though this improvement was not significant on software imaging analysis. Patients improved the most on parameters of facial seborrhea by clinical assessment.16 After 8 weeks, patients treated with CHLcu 0.066% exhibited 25.3% improvement in facial pores by clinical assessment grading.17 Treatments were reported to be well tolerated without noted adverse events in both studies.
Tetra-hydro-jasmonic acid is an analogue of jasmonic acid, a plant hormone derived from linoleic acid. Due to its favorable safety profile and bioavailability, penetration into epidermal and dermal layers, and potential effects in rejuvenating desquamation, LR2412 is currently being assessed for treatment of skin wrinkles, texture, and pores.18 Its effect is thought to relate to stimulation of laminin-5, collagen IV, and fibrillin deposition at the dermoepidermal junction.19 In an open-label trial of a topical preparation of LR2412, 15 participants were treated twice daily for 6 weeks and assessed through investigator clinical assessment scoring.20 Investigator scoring of pores improved by 25.2% from baseline (P<.05) after 6 weeks of treatment. Improvement in pores was seen as early as days 1 and 3. No serious adverse events were reported, though 2 participants developed acne on follow-up.20
Tetra-hydro-jasmonic acid also is formulated with retinol (retinol 0.2%/LR2412 2.0%) and demonstrated cosmetic efficacy in a noninferiority trial with tretinoin cream 0.025%.11 Sixty patients each were randomized to retinol/LR2412 or tretinoin at bedtime and treated for 90 days. At day 84, participants in the retinol/LR2412 group exhibited an improvement in investigator clinical assessment scoring from a baseline of 3.6 to 2.5 (P<.05). There were no significant differences in investigator-assessed efficacy between the treatment arms. Participants reported similar or better results and fewer side effects with retinol/LR2412 on self-questionnaires. Eight participants treated with retinol/LR2412 and 15 participants treated with tretinoin reported various incidences of skin irritation, burning, and desquamation.11
Oral Therapies
The most commonly used oral therapies for enlarged pores are antiandrogens, such as combined oral contraceptives, spironolactone, and cyproterone acetate, which modulate sebum production due to the presence of androgen receptors within sebaceous glands.21 Forty-four white women in an open-label, phase 4 study were treated with combined oral contraceptives containing chlormadinone acetate–ethinyl estradiol for 6 menstrual cycles, with standardized photography taken before and after the treatment period for software analysis. After 6 treatment cycles, 9.1% (4/44) of participants had visibly enlarged pores of the forehead and cheeks compared to 43.2% (19/44) of participants at baseline (P<.0001).22 The effects of other antiandrogens on facial pores have not been studied in this capacity.
Lasers, Radiofrequency, and Ultrasound Devices
The development of various devices that can deliver targeted thermal or ultrasound energy to the skin offers the newest and most robust modality in cosmetic therapy. The mechanism of their efficacy may be due to a combination of induced remodeling of collagen fibers near pilosebaceous openings to increase skin elasticity and decrease sebum production.2,23
Devices with established antiaging effects have been extensively reviewed and include the gold particle 800-nm diode laser, 1450-nm diode laser, microneedle apparatuses, fractional radiofrequency devices, 2790-nm erbium:YAG laser, nonablative 1410-nm fractionated erbium-doped fiber laser, and nonablative 1440-nm fractional laser.2
Literature on the use of these devices for minimizing facial pore size is limited. One treatment of intense focused ultrasound using a 3-mm transducer successfully improved overall pore appearance in 91% of sites at 6-week follow-up on a clinical grading scale.24 Three sessions of nonablative 1410-nm fractionated erbium-doped fiber laser treatments yielded facial skin pore minimization of greater than 51% in 14 of 15 participants.25
The nonablative 1440-nm diode fractional laser received 510(k) clearance by the US Food and Drug Administration in 2011 for aesthetic use in chronologically aged and photoaged skin. Twenty participants treated for 2 weeks and a total of 6 facial treatments with this laser system showed a 17% average improvement in facial pore score on software analysis (P≤.002). Adverse events were mild and included erythema and xerosis.26
Conclusion
The reliability of available literature on efficacy of various treatments in diminishing facial skin pores has been challenging given that most studies are low in power, lack control groups, use nonuniform methods of reporting outcomes, and do not report complete adverse events. Thus, all results should be interpreted with caution.
Overall, it is clear that the pathogenesis of enlarged facial pores is multifactorial and complex, necessitating a similar approach to therapeutics. Topical treatments offer a range of diverse therapies with proven benefit in facial pore reduction. The advent of lasers and devices offers constantly evolving therapeutic options with diffuse antiaging effects. Despite the numerous topical, oral, and device-oriented options, enlarged facial pores remain a challenging cosmetic concern. More robust efficacy studies on new treatments are necessary.
- Uhoda E, Pierard-Franchimont C, Petit L, et al. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3-7.
- Lee SJ, Seok J, Jeong SY, et al. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42:277-285.
- Pierard GE, Pierard-Franchimont C, Marks R, et al. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372-389.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890-894.
- Kim BY, Choi JW, Park KC, et al. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:E45-E53.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 suppl):S12-S16.
- Zheng Q, Chen S, Chen Y, et al. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317-323.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135-139.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327-348.
- Kang S, Krueger GG, Tanghetti EA, et al; Tazarotene Cream in Photodamage Study Group. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52:268-274.
- Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14:40-46.
- Fischer TC, Perosino E, Poli F, et al. Chemical peels in aesthetic dermatology: an update 2009 [published online September 8, 2009]. J Eur Acad Dermatol Venereol. 2010;24:281-292.
- Kakudo N, Kushida S, Tanaka N, et al. A novel method to measure conspicuous facial pores using computer analysis of digital-camera-captured images: the effect of glycolic acid chemical peeling. Skin Res Technol. 2011;17:427-433.
- Kim WS. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15:21-24.
- McCook JP, Dorogi PL, Vasily DB, et al. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin Cosmet Investig Dermatol. 2015;8:443-448.
- Stephens TJ, McCook JP, Herndon JH Jr. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J Drugs Dermatol. 2015;14:589-592.
- Sigler ML, Stephens TJ. Assessment of the safety and efficacy of topical copper chlorophyllin in women with photodamaged facial skin. J Drugs Dermatol. 2015;14:401-404.
- Alexiades M. Jasmonates and tetrahydrojasmonic acid: a novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-207.
- Tran C, Michelet JF, Simonetti L, et al. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J Eur Acad Dermatol Venereol. 2014;28:415-423.
- Alexiades M. Clinical assessment of a novel jasmonate cosmeceutical, LR2412-Cx, for the treatment of skin aging. J Drugs Dermatol. 2016;15:209-215.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Kerscher M, Reuther T, Bayrhammer J, et al. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28:703-711.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140:1373-1376.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73-77.
- Suh DH, Chang KY, Lee SJ, et al. Treatment of dilated pores with 1410-nm fractional erbium-doped fiber laser. Lasers Med Sci. 2015;30:1135-1139.
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118.
Enlarged facial pores are superficial skin structures that are visualized as small openings on the skin corresponding to the openings of the pilosebaceous apparatus. These openings may be impacted with horny follicular plugs consisting of sebaceous debris that appear as open comedones.1 Skin pores is a lay term that is poorly defined in the medical literature and often is categorized in terms of arbitrary circular diameters determined through cosmetic skin analyzers.2 The term refers to pilosebaceous follicular enlargements (with or without open comedonal horny impactions) that can be visualized by the naked eye, most commonly occurring on the face and scalp. These enlarged pores remain a pervasive cosmetic concern that impacts patient quality of life. Enlarged pores are difficult to treat, in part due to lack of knowledge of the pathophysiology; thus, we review the currently proposed causes of enlarged pilosebaceous openings and the treatments in the scope of this pathogenesis with a focus on therapeutic efficacy.
Pathogenesis of Enlarged Facial Pores
It is now thought that seborrhea, loss of skin elasticity and tension, and hair follicle size are most clinically relevant to the pathogenesis of enlarged pores.2 Other potential associated and causative factors include genetic predisposition, acne, comedogenic xenobiotics, chronic photodamage, chronic radiodermatitis, and vitamin A deficiency.1,3
The direct relationship between sebum output and pore size has been well established, particularly in men who generally have higher sebum output levels than women, which likely is testosterone driven.4,5 However, there are contradictory data on whether sex affects pore size, as females also exhibit contributory hormonal factors. Sebum output and pore size increase substantially during the ovulation phase of the female menstrual cycle, likely secondary to increased progesterone affecting sebaceous gland activity.2,4 The presence of acne also is associated with enlarged facial pores, though the extent of seborrhea as a confounding factor is unclear. Furthermore, acne severity does not correlate with increased pore size.5 However, the processes of acne and facial pores are interlinked, given the frequent occurrence of open comedones within the pores.
Skin elasticity and tensile strength when defined visually and mechanically has shown a negative correlation with facial pore size and density.5 It is well known that cutaneous aging and chronic photodamage cause perturbation in the collagen and elastin framework that allows for the skin to maintain its resilient properties.6 Aged and photodamaged skin also demonstrates decreased expression of microfibril-associated glycoprotein-1 (MAGP-1), a crucial component in elastic fiber assembly and skin elasticity in the dermis and perifollicular/pore areas.7
Pore density and size appears to range diversely across ethnicities, though Chinese women exhibit notably lower pore size and density across all ages as compared to other ethnicities.8 Black individuals have aberrant epidermal architecture, defined as the presence of stalagmitelike structures at the dermoepidermal junction, correlating with enlarged pore size compared to other ethnicities.2,8
Treating Enlarged Facial Pores
Treatments for enlarged facial pores primarily aim to decrease sebum production, rejuvenate skin, remove hair, and/or decrease follicular size. Evidence-based studies are limited, and many currently used therapies have not been studied with enlarged facial pores as a primary investigative outcome. Here, we include studies that report efficacy in decreasing pore size specifically. It is important to note the lack of a uniform and objective modality with which to report skin pore size. Studies use a wide range of techniques including patient self-reporting, physician observation, and software image analyzers.
Topical Therapies
Topical retinoids are vitamin A derivatives, and they are first-line therapies in reversing the aberrant collagen and elastin-associated epidermal and dermal changes that occur with chronological aging and photoaging. Tretinoin, isotretinoin, and tazarotene have shown efficacy in multiple parameters of skin rejuvenation, including facial pores, skin wrinkling, hyperpigmentation, skin laxity, and sebum production.9 However, it is important to note that retinoids treat keratinocyte atypia in acne, and efficacy in facial pores is confounded by improvement in follicular keratinization. Because studies have not distinctly uncoupled this association, it is erroneous to conclude that retinoids reduce facial pore size and density irrespective of concomitant acne vulgaris.
Tazarotene has been evaluated for use in reducing facial pore size. In one investigation, 568 patients with moderate wrinkling or hyperpigmentation were randomized to receive tazarotene cream 0.1% or placebo once daily for 24 weeks and were evaluated for enlarged facial pores as a secondary outcome using a double-blinded physician 5-point scale.10 At week 24, 42% of tazarotene-treated patients achieved improvement of at least 1 point compared to 20% of placebo-treated patients (P<.001). Adverse events were dermatitic, as can be expected of retinoids, leading to a 4% discontinuation rate in the tazarotene group compared to 1% in the placebo group.10
Tretinoin has long been used off label for antiaging treatments but has only recently shown efficacy for facial pores. In one study, 60 women who had previously sought antiaging procedures were treated with tretinoin cream 0.025% once daily and no other antiaging products or procedures for 90 days.11 Facial pore evaluations were determined by a modified dermatoscope with a polarized analyzer for clinical scoring using a photonumeric scale. Patients improved from a baseline average score of 3.2 in facial pores to a posttreatment average score of 2.0 (P<.05) at day 84. This improvement was sustained from day 28 of treatment and corresponded to patient self-perception. Adverse events included xerosis, desquamation, burning, and erythema, which led to 3 premature discontinuations.11
Various chemical peel formulations are used in skin rejuvenation and have shown application in enlarged facial pores. Chemical peels act at the epidermal or dermal level to induce temporary breakdown and regeneration of healthier cells and improved skin matrix.12 Twenty-two Japanese women applied glycolic acid (30% solution) every 2 weeks for a total of 5 treatments and exhibited reduced appearance of conspicuous, open, and dark pores, defined by surface area and shading as determined through dermatoscopic and software analysis, with mean improvement rates of 34.6%, 11%, and 34.3%, respectively. More than 70% of participants exhibited improvement in enlarged facial pores.13 A study involving a 40% glycolic acid and vitamin C formulation demonstrated significant improvement in facial pores (28.3%; P<.001).14
The newest topical therapies studied for use in minimizing facial pilosebaceous openings are natural plant-derived copper chlorophyllin complex sodium salt (CHLcu) and tetra-hydro-jasmonic acid (LR2412). Clinical trials of these botanicals are limited with small sample sizes but are included here as novel treatments requiring further investigation.
Chlorophyllin copper complex sodium salt is derived from chlorophyll, a green pigment found in plants, and has been investigated as a topical gel in liposomal dispersions for application in photodamaged and aged skin. Chlorophyllin copper complex sodium salt exerts in vitro hyaluronidase inhibitory activity to maintain hyaluronic acid in the extracellular matrix and counteract the structural breakdown of cutaneous aging.15 Two small single-center pilot trials enrolled 10 participants each in a 3-week study of CHLcu 0.1% twice daily and an 8-week study of CHLcu 0.066% twice daily.16,17 After 3 weeks, patients treated with CHLcu 0.1% exhibited a 22.2% improvement in facial pores by clinical assessment grading, though this improvement was not significant on software imaging analysis. Patients improved the most on parameters of facial seborrhea by clinical assessment.16 After 8 weeks, patients treated with CHLcu 0.066% exhibited 25.3% improvement in facial pores by clinical assessment grading.17 Treatments were reported to be well tolerated without noted adverse events in both studies.
Tetra-hydro-jasmonic acid is an analogue of jasmonic acid, a plant hormone derived from linoleic acid. Due to its favorable safety profile and bioavailability, penetration into epidermal and dermal layers, and potential effects in rejuvenating desquamation, LR2412 is currently being assessed for treatment of skin wrinkles, texture, and pores.18 Its effect is thought to relate to stimulation of laminin-5, collagen IV, and fibrillin deposition at the dermoepidermal junction.19 In an open-label trial of a topical preparation of LR2412, 15 participants were treated twice daily for 6 weeks and assessed through investigator clinical assessment scoring.20 Investigator scoring of pores improved by 25.2% from baseline (P<.05) after 6 weeks of treatment. Improvement in pores was seen as early as days 1 and 3. No serious adverse events were reported, though 2 participants developed acne on follow-up.20
Tetra-hydro-jasmonic acid also is formulated with retinol (retinol 0.2%/LR2412 2.0%) and demonstrated cosmetic efficacy in a noninferiority trial with tretinoin cream 0.025%.11 Sixty patients each were randomized to retinol/LR2412 or tretinoin at bedtime and treated for 90 days. At day 84, participants in the retinol/LR2412 group exhibited an improvement in investigator clinical assessment scoring from a baseline of 3.6 to 2.5 (P<.05). There were no significant differences in investigator-assessed efficacy between the treatment arms. Participants reported similar or better results and fewer side effects with retinol/LR2412 on self-questionnaires. Eight participants treated with retinol/LR2412 and 15 participants treated with tretinoin reported various incidences of skin irritation, burning, and desquamation.11
Oral Therapies
The most commonly used oral therapies for enlarged pores are antiandrogens, such as combined oral contraceptives, spironolactone, and cyproterone acetate, which modulate sebum production due to the presence of androgen receptors within sebaceous glands.21 Forty-four white women in an open-label, phase 4 study were treated with combined oral contraceptives containing chlormadinone acetate–ethinyl estradiol for 6 menstrual cycles, with standardized photography taken before and after the treatment period for software analysis. After 6 treatment cycles, 9.1% (4/44) of participants had visibly enlarged pores of the forehead and cheeks compared to 43.2% (19/44) of participants at baseline (P<.0001).22 The effects of other antiandrogens on facial pores have not been studied in this capacity.
Lasers, Radiofrequency, and Ultrasound Devices
The development of various devices that can deliver targeted thermal or ultrasound energy to the skin offers the newest and most robust modality in cosmetic therapy. The mechanism of their efficacy may be due to a combination of induced remodeling of collagen fibers near pilosebaceous openings to increase skin elasticity and decrease sebum production.2,23
Devices with established antiaging effects have been extensively reviewed and include the gold particle 800-nm diode laser, 1450-nm diode laser, microneedle apparatuses, fractional radiofrequency devices, 2790-nm erbium:YAG laser, nonablative 1410-nm fractionated erbium-doped fiber laser, and nonablative 1440-nm fractional laser.2
Literature on the use of these devices for minimizing facial pore size is limited. One treatment of intense focused ultrasound using a 3-mm transducer successfully improved overall pore appearance in 91% of sites at 6-week follow-up on a clinical grading scale.24 Three sessions of nonablative 1410-nm fractionated erbium-doped fiber laser treatments yielded facial skin pore minimization of greater than 51% in 14 of 15 participants.25
The nonablative 1440-nm diode fractional laser received 510(k) clearance by the US Food and Drug Administration in 2011 for aesthetic use in chronologically aged and photoaged skin. Twenty participants treated for 2 weeks and a total of 6 facial treatments with this laser system showed a 17% average improvement in facial pore score on software analysis (P≤.002). Adverse events were mild and included erythema and xerosis.26
Conclusion
The reliability of available literature on efficacy of various treatments in diminishing facial skin pores has been challenging given that most studies are low in power, lack control groups, use nonuniform methods of reporting outcomes, and do not report complete adverse events. Thus, all results should be interpreted with caution.
Overall, it is clear that the pathogenesis of enlarged facial pores is multifactorial and complex, necessitating a similar approach to therapeutics. Topical treatments offer a range of diverse therapies with proven benefit in facial pore reduction. The advent of lasers and devices offers constantly evolving therapeutic options with diffuse antiaging effects. Despite the numerous topical, oral, and device-oriented options, enlarged facial pores remain a challenging cosmetic concern. More robust efficacy studies on new treatments are necessary.
Enlarged facial pores are superficial skin structures that are visualized as small openings on the skin corresponding to the openings of the pilosebaceous apparatus. These openings may be impacted with horny follicular plugs consisting of sebaceous debris that appear as open comedones.1 Skin pores is a lay term that is poorly defined in the medical literature and often is categorized in terms of arbitrary circular diameters determined through cosmetic skin analyzers.2 The term refers to pilosebaceous follicular enlargements (with or without open comedonal horny impactions) that can be visualized by the naked eye, most commonly occurring on the face and scalp. These enlarged pores remain a pervasive cosmetic concern that impacts patient quality of life. Enlarged pores are difficult to treat, in part due to lack of knowledge of the pathophysiology; thus, we review the currently proposed causes of enlarged pilosebaceous openings and the treatments in the scope of this pathogenesis with a focus on therapeutic efficacy.
Pathogenesis of Enlarged Facial Pores
It is now thought that seborrhea, loss of skin elasticity and tension, and hair follicle size are most clinically relevant to the pathogenesis of enlarged pores.2 Other potential associated and causative factors include genetic predisposition, acne, comedogenic xenobiotics, chronic photodamage, chronic radiodermatitis, and vitamin A deficiency.1,3
The direct relationship between sebum output and pore size has been well established, particularly in men who generally have higher sebum output levels than women, which likely is testosterone driven.4,5 However, there are contradictory data on whether sex affects pore size, as females also exhibit contributory hormonal factors. Sebum output and pore size increase substantially during the ovulation phase of the female menstrual cycle, likely secondary to increased progesterone affecting sebaceous gland activity.2,4 The presence of acne also is associated with enlarged facial pores, though the extent of seborrhea as a confounding factor is unclear. Furthermore, acne severity does not correlate with increased pore size.5 However, the processes of acne and facial pores are interlinked, given the frequent occurrence of open comedones within the pores.
Skin elasticity and tensile strength when defined visually and mechanically has shown a negative correlation with facial pore size and density.5 It is well known that cutaneous aging and chronic photodamage cause perturbation in the collagen and elastin framework that allows for the skin to maintain its resilient properties.6 Aged and photodamaged skin also demonstrates decreased expression of microfibril-associated glycoprotein-1 (MAGP-1), a crucial component in elastic fiber assembly and skin elasticity in the dermis and perifollicular/pore areas.7
Pore density and size appears to range diversely across ethnicities, though Chinese women exhibit notably lower pore size and density across all ages as compared to other ethnicities.8 Black individuals have aberrant epidermal architecture, defined as the presence of stalagmitelike structures at the dermoepidermal junction, correlating with enlarged pore size compared to other ethnicities.2,8
Treating Enlarged Facial Pores
Treatments for enlarged facial pores primarily aim to decrease sebum production, rejuvenate skin, remove hair, and/or decrease follicular size. Evidence-based studies are limited, and many currently used therapies have not been studied with enlarged facial pores as a primary investigative outcome. Here, we include studies that report efficacy in decreasing pore size specifically. It is important to note the lack of a uniform and objective modality with which to report skin pore size. Studies use a wide range of techniques including patient self-reporting, physician observation, and software image analyzers.
Topical Therapies
Topical retinoids are vitamin A derivatives, and they are first-line therapies in reversing the aberrant collagen and elastin-associated epidermal and dermal changes that occur with chronological aging and photoaging. Tretinoin, isotretinoin, and tazarotene have shown efficacy in multiple parameters of skin rejuvenation, including facial pores, skin wrinkling, hyperpigmentation, skin laxity, and sebum production.9 However, it is important to note that retinoids treat keratinocyte atypia in acne, and efficacy in facial pores is confounded by improvement in follicular keratinization. Because studies have not distinctly uncoupled this association, it is erroneous to conclude that retinoids reduce facial pore size and density irrespective of concomitant acne vulgaris.
Tazarotene has been evaluated for use in reducing facial pore size. In one investigation, 568 patients with moderate wrinkling or hyperpigmentation were randomized to receive tazarotene cream 0.1% or placebo once daily for 24 weeks and were evaluated for enlarged facial pores as a secondary outcome using a double-blinded physician 5-point scale.10 At week 24, 42% of tazarotene-treated patients achieved improvement of at least 1 point compared to 20% of placebo-treated patients (P<.001). Adverse events were dermatitic, as can be expected of retinoids, leading to a 4% discontinuation rate in the tazarotene group compared to 1% in the placebo group.10
Tretinoin has long been used off label for antiaging treatments but has only recently shown efficacy for facial pores. In one study, 60 women who had previously sought antiaging procedures were treated with tretinoin cream 0.025% once daily and no other antiaging products or procedures for 90 days.11 Facial pore evaluations were determined by a modified dermatoscope with a polarized analyzer for clinical scoring using a photonumeric scale. Patients improved from a baseline average score of 3.2 in facial pores to a posttreatment average score of 2.0 (P<.05) at day 84. This improvement was sustained from day 28 of treatment and corresponded to patient self-perception. Adverse events included xerosis, desquamation, burning, and erythema, which led to 3 premature discontinuations.11
Various chemical peel formulations are used in skin rejuvenation and have shown application in enlarged facial pores. Chemical peels act at the epidermal or dermal level to induce temporary breakdown and regeneration of healthier cells and improved skin matrix.12 Twenty-two Japanese women applied glycolic acid (30% solution) every 2 weeks for a total of 5 treatments and exhibited reduced appearance of conspicuous, open, and dark pores, defined by surface area and shading as determined through dermatoscopic and software analysis, with mean improvement rates of 34.6%, 11%, and 34.3%, respectively. More than 70% of participants exhibited improvement in enlarged facial pores.13 A study involving a 40% glycolic acid and vitamin C formulation demonstrated significant improvement in facial pores (28.3%; P<.001).14
The newest topical therapies studied for use in minimizing facial pilosebaceous openings are natural plant-derived copper chlorophyllin complex sodium salt (CHLcu) and tetra-hydro-jasmonic acid (LR2412). Clinical trials of these botanicals are limited with small sample sizes but are included here as novel treatments requiring further investigation.
Chlorophyllin copper complex sodium salt is derived from chlorophyll, a green pigment found in plants, and has been investigated as a topical gel in liposomal dispersions for application in photodamaged and aged skin. Chlorophyllin copper complex sodium salt exerts in vitro hyaluronidase inhibitory activity to maintain hyaluronic acid in the extracellular matrix and counteract the structural breakdown of cutaneous aging.15 Two small single-center pilot trials enrolled 10 participants each in a 3-week study of CHLcu 0.1% twice daily and an 8-week study of CHLcu 0.066% twice daily.16,17 After 3 weeks, patients treated with CHLcu 0.1% exhibited a 22.2% improvement in facial pores by clinical assessment grading, though this improvement was not significant on software imaging analysis. Patients improved the most on parameters of facial seborrhea by clinical assessment.16 After 8 weeks, patients treated with CHLcu 0.066% exhibited 25.3% improvement in facial pores by clinical assessment grading.17 Treatments were reported to be well tolerated without noted adverse events in both studies.
Tetra-hydro-jasmonic acid is an analogue of jasmonic acid, a plant hormone derived from linoleic acid. Due to its favorable safety profile and bioavailability, penetration into epidermal and dermal layers, and potential effects in rejuvenating desquamation, LR2412 is currently being assessed for treatment of skin wrinkles, texture, and pores.18 Its effect is thought to relate to stimulation of laminin-5, collagen IV, and fibrillin deposition at the dermoepidermal junction.19 In an open-label trial of a topical preparation of LR2412, 15 participants were treated twice daily for 6 weeks and assessed through investigator clinical assessment scoring.20 Investigator scoring of pores improved by 25.2% from baseline (P<.05) after 6 weeks of treatment. Improvement in pores was seen as early as days 1 and 3. No serious adverse events were reported, though 2 participants developed acne on follow-up.20
Tetra-hydro-jasmonic acid also is formulated with retinol (retinol 0.2%/LR2412 2.0%) and demonstrated cosmetic efficacy in a noninferiority trial with tretinoin cream 0.025%.11 Sixty patients each were randomized to retinol/LR2412 or tretinoin at bedtime and treated for 90 days. At day 84, participants in the retinol/LR2412 group exhibited an improvement in investigator clinical assessment scoring from a baseline of 3.6 to 2.5 (P<.05). There were no significant differences in investigator-assessed efficacy between the treatment arms. Participants reported similar or better results and fewer side effects with retinol/LR2412 on self-questionnaires. Eight participants treated with retinol/LR2412 and 15 participants treated with tretinoin reported various incidences of skin irritation, burning, and desquamation.11
Oral Therapies
The most commonly used oral therapies for enlarged pores are antiandrogens, such as combined oral contraceptives, spironolactone, and cyproterone acetate, which modulate sebum production due to the presence of androgen receptors within sebaceous glands.21 Forty-four white women in an open-label, phase 4 study were treated with combined oral contraceptives containing chlormadinone acetate–ethinyl estradiol for 6 menstrual cycles, with standardized photography taken before and after the treatment period for software analysis. After 6 treatment cycles, 9.1% (4/44) of participants had visibly enlarged pores of the forehead and cheeks compared to 43.2% (19/44) of participants at baseline (P<.0001).22 The effects of other antiandrogens on facial pores have not been studied in this capacity.
Lasers, Radiofrequency, and Ultrasound Devices
The development of various devices that can deliver targeted thermal or ultrasound energy to the skin offers the newest and most robust modality in cosmetic therapy. The mechanism of their efficacy may be due to a combination of induced remodeling of collagen fibers near pilosebaceous openings to increase skin elasticity and decrease sebum production.2,23
Devices with established antiaging effects have been extensively reviewed and include the gold particle 800-nm diode laser, 1450-nm diode laser, microneedle apparatuses, fractional radiofrequency devices, 2790-nm erbium:YAG laser, nonablative 1410-nm fractionated erbium-doped fiber laser, and nonablative 1440-nm fractional laser.2
Literature on the use of these devices for minimizing facial pore size is limited. One treatment of intense focused ultrasound using a 3-mm transducer successfully improved overall pore appearance in 91% of sites at 6-week follow-up on a clinical grading scale.24 Three sessions of nonablative 1410-nm fractionated erbium-doped fiber laser treatments yielded facial skin pore minimization of greater than 51% in 14 of 15 participants.25
The nonablative 1440-nm diode fractional laser received 510(k) clearance by the US Food and Drug Administration in 2011 for aesthetic use in chronologically aged and photoaged skin. Twenty participants treated for 2 weeks and a total of 6 facial treatments with this laser system showed a 17% average improvement in facial pore score on software analysis (P≤.002). Adverse events were mild and included erythema and xerosis.26
Conclusion
The reliability of available literature on efficacy of various treatments in diminishing facial skin pores has been challenging given that most studies are low in power, lack control groups, use nonuniform methods of reporting outcomes, and do not report complete adverse events. Thus, all results should be interpreted with caution.
Overall, it is clear that the pathogenesis of enlarged facial pores is multifactorial and complex, necessitating a similar approach to therapeutics. Topical treatments offer a range of diverse therapies with proven benefit in facial pore reduction. The advent of lasers and devices offers constantly evolving therapeutic options with diffuse antiaging effects. Despite the numerous topical, oral, and device-oriented options, enlarged facial pores remain a challenging cosmetic concern. More robust efficacy studies on new treatments are necessary.
- Uhoda E, Pierard-Franchimont C, Petit L, et al. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3-7.
- Lee SJ, Seok J, Jeong SY, et al. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42:277-285.
- Pierard GE, Pierard-Franchimont C, Marks R, et al. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372-389.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890-894.
- Kim BY, Choi JW, Park KC, et al. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:E45-E53.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 suppl):S12-S16.
- Zheng Q, Chen S, Chen Y, et al. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317-323.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135-139.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327-348.
- Kang S, Krueger GG, Tanghetti EA, et al; Tazarotene Cream in Photodamage Study Group. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52:268-274.
- Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14:40-46.
- Fischer TC, Perosino E, Poli F, et al. Chemical peels in aesthetic dermatology: an update 2009 [published online September 8, 2009]. J Eur Acad Dermatol Venereol. 2010;24:281-292.
- Kakudo N, Kushida S, Tanaka N, et al. A novel method to measure conspicuous facial pores using computer analysis of digital-camera-captured images: the effect of glycolic acid chemical peeling. Skin Res Technol. 2011;17:427-433.
- Kim WS. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15:21-24.
- McCook JP, Dorogi PL, Vasily DB, et al. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin Cosmet Investig Dermatol. 2015;8:443-448.
- Stephens TJ, McCook JP, Herndon JH Jr. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J Drugs Dermatol. 2015;14:589-592.
- Sigler ML, Stephens TJ. Assessment of the safety and efficacy of topical copper chlorophyllin in women with photodamaged facial skin. J Drugs Dermatol. 2015;14:401-404.
- Alexiades M. Jasmonates and tetrahydrojasmonic acid: a novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-207.
- Tran C, Michelet JF, Simonetti L, et al. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J Eur Acad Dermatol Venereol. 2014;28:415-423.
- Alexiades M. Clinical assessment of a novel jasmonate cosmeceutical, LR2412-Cx, for the treatment of skin aging. J Drugs Dermatol. 2016;15:209-215.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Kerscher M, Reuther T, Bayrhammer J, et al. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28:703-711.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140:1373-1376.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73-77.
- Suh DH, Chang KY, Lee SJ, et al. Treatment of dilated pores with 1410-nm fractional erbium-doped fiber laser. Lasers Med Sci. 2015;30:1135-1139.
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118.
- Uhoda E, Pierard-Franchimont C, Petit L, et al. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3-7.
- Lee SJ, Seok J, Jeong SY, et al. Facial pores: definition, causes, and treatment options. Dermatol Surg. 2016;42:277-285.
- Pierard GE, Pierard-Franchimont C, Marks R, et al. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372-389.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890-894.
- Kim BY, Choi JW, Park KC, et al. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:E45-E53.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 suppl):S12-S16.
- Zheng Q, Chen S, Chen Y, et al. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin Cosmet Investig Dermatol. 2013;6:317-323.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135-139.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327-348.
- Kang S, Krueger GG, Tanghetti EA, et al; Tazarotene Cream in Photodamage Study Group. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52:268-274.
- Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14:40-46.
- Fischer TC, Perosino E, Poli F, et al. Chemical peels in aesthetic dermatology: an update 2009 [published online September 8, 2009]. J Eur Acad Dermatol Venereol. 2010;24:281-292.
- Kakudo N, Kushida S, Tanaka N, et al. A novel method to measure conspicuous facial pores using computer analysis of digital-camera-captured images: the effect of glycolic acid chemical peeling. Skin Res Technol. 2011;17:427-433.
- Kim WS. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15:21-24.
- McCook JP, Dorogi PL, Vasily DB, et al. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin Cosmet Investig Dermatol. 2015;8:443-448.
- Stephens TJ, McCook JP, Herndon JH Jr. Pilot study of topical copper chlorophyllin complex in subjects with facial acne and large pores. J Drugs Dermatol. 2015;14:589-592.
- Sigler ML, Stephens TJ. Assessment of the safety and efficacy of topical copper chlorophyllin in women with photodamaged facial skin. J Drugs Dermatol. 2015;14:401-404.
- Alexiades M. Jasmonates and tetrahydrojasmonic acid: a novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-207.
- Tran C, Michelet JF, Simonetti L, et al. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J Eur Acad Dermatol Venereol. 2014;28:415-423.
- Alexiades M. Clinical assessment of a novel jasmonate cosmeceutical, LR2412-Cx, for the treatment of skin aging. J Drugs Dermatol. 2016;15:209-215.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Kerscher M, Reuther T, Bayrhammer J, et al. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28:703-711.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140:1373-1376.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73-77.
- Suh DH, Chang KY, Lee SJ, et al. Treatment of dilated pores with 1410-nm fractional erbium-doped fiber laser. Lasers Med Sci. 2015;30:1135-1139.
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118.
Practice Points
- The pathogenesis of enlarged facial pores is speculated to be associated with sebum production, skin aging and photodamage, and hair follicle size, among other factors.
- Current treatment modalities for enlarged facial pores target these factors and include topical retinoids, chemical peels, oral antiandrogens, lasers, radiofrequency, and ultrasound devices, with the latter devices offering the most novel and robust choices.
- New botanically derived topical treatments, specifically copper chlorophyllin complex sodium salt and tetra-hydro-jasmonic acid, are in development with initial positive results, though studies are still limited.
Attending religious services linked to lower suicide risk
New findings published June 29 suggest that women with frequent religious attendance had substantially lower suicide risk, compared with women who did not attend religious services as often.
“Our results do not imply that health care providers should prescribe attendance at religious services,” wrote Tyler J. VanderWeele, PhD, and his associates. “However, for patients who are already religious, service attendance might be encouraged as a form of meaningful social participation.”
The investigators examined 89,708 women from 1996-2010. Out of the 89,708 women studied, 17,028 attended services more than once per week, 36,488 attended once per week, 14,548 attended fewer than once per week, and 21,644 never attended. In the multivariable regression model, women who attended religious services one time per week or more in 1996 had an approximately fivefold lower rate of suicide (adjusted hazard ratio, 0.16).
Dr. VanderWeele and his associates also found that suicide risks differed between Catholic and Protestant participants. The religious attendance HR for women who attended Catholic services one time a week or more, compared with women who attended fewer than one time a week, was 0.05 (95% confidence interval, 0.006-0.48), which was approximately sevenfold smaller than the corresponding HR for Protestants (HR, 0.34; 95% CI, 0.10-1.10; P = .05 for heterogeneity).
Mediation analysis was used to evaluate whether depressive symptoms in 2000, alcohol consumption in 1998, and subsequent social integration score in 2000 played a role in religious service attendance and suicide. With depressive symptoms, alcohol consumption, and social integration score adjusted, the women who attended religious services fewer than once per week, compared with women who did not attend at all, changed from 0.85 to 0.94. However, the HR for women attending religious services more than once per week, compared with the women who did not attend at all, did not change (0.16).
Read the full study in Jama Psychiatry (doi: 10.1001/jamapsychiatry.2016.1243).
New findings published June 29 suggest that women with frequent religious attendance had substantially lower suicide risk, compared with women who did not attend religious services as often.
“Our results do not imply that health care providers should prescribe attendance at religious services,” wrote Tyler J. VanderWeele, PhD, and his associates. “However, for patients who are already religious, service attendance might be encouraged as a form of meaningful social participation.”
The investigators examined 89,708 women from 1996-2010. Out of the 89,708 women studied, 17,028 attended services more than once per week, 36,488 attended once per week, 14,548 attended fewer than once per week, and 21,644 never attended. In the multivariable regression model, women who attended religious services one time per week or more in 1996 had an approximately fivefold lower rate of suicide (adjusted hazard ratio, 0.16).
Dr. VanderWeele and his associates also found that suicide risks differed between Catholic and Protestant participants. The religious attendance HR for women who attended Catholic services one time a week or more, compared with women who attended fewer than one time a week, was 0.05 (95% confidence interval, 0.006-0.48), which was approximately sevenfold smaller than the corresponding HR for Protestants (HR, 0.34; 95% CI, 0.10-1.10; P = .05 for heterogeneity).
Mediation analysis was used to evaluate whether depressive symptoms in 2000, alcohol consumption in 1998, and subsequent social integration score in 2000 played a role in religious service attendance and suicide. With depressive symptoms, alcohol consumption, and social integration score adjusted, the women who attended religious services fewer than once per week, compared with women who did not attend at all, changed from 0.85 to 0.94. However, the HR for women attending religious services more than once per week, compared with the women who did not attend at all, did not change (0.16).
Read the full study in Jama Psychiatry (doi: 10.1001/jamapsychiatry.2016.1243).
New findings published June 29 suggest that women with frequent religious attendance had substantially lower suicide risk, compared with women who did not attend religious services as often.
“Our results do not imply that health care providers should prescribe attendance at religious services,” wrote Tyler J. VanderWeele, PhD, and his associates. “However, for patients who are already religious, service attendance might be encouraged as a form of meaningful social participation.”
The investigators examined 89,708 women from 1996-2010. Out of the 89,708 women studied, 17,028 attended services more than once per week, 36,488 attended once per week, 14,548 attended fewer than once per week, and 21,644 never attended. In the multivariable regression model, women who attended religious services one time per week or more in 1996 had an approximately fivefold lower rate of suicide (adjusted hazard ratio, 0.16).
Dr. VanderWeele and his associates also found that suicide risks differed between Catholic and Protestant participants. The religious attendance HR for women who attended Catholic services one time a week or more, compared with women who attended fewer than one time a week, was 0.05 (95% confidence interval, 0.006-0.48), which was approximately sevenfold smaller than the corresponding HR for Protestants (HR, 0.34; 95% CI, 0.10-1.10; P = .05 for heterogeneity).
Mediation analysis was used to evaluate whether depressive symptoms in 2000, alcohol consumption in 1998, and subsequent social integration score in 2000 played a role in religious service attendance and suicide. With depressive symptoms, alcohol consumption, and social integration score adjusted, the women who attended religious services fewer than once per week, compared with women who did not attend at all, changed from 0.85 to 0.94. However, the HR for women attending religious services more than once per week, compared with the women who did not attend at all, did not change (0.16).
Read the full study in Jama Psychiatry (doi: 10.1001/jamapsychiatry.2016.1243).
FROM JAMA PSYCHIATRY
Can a Diagnosis of Concussion Be Objective?
VANCOUVER—A diagnosis of concussion is based on clinical observation and testing, and it therefore is susceptible to error. Researchers are seeking biomarkers in CSF, in serum, and on imaging that could provide stronger grounds for a diagnosis of concussion, as well as a method of monitoring recovery, according to an overview provided at the 68th Annual Meeting of the American Academy of Neurology.
Concussion often occurs with no macroscopic evidence of injury. Symptoms of concussion can be subjective and nonspecific, and a patient report of symptoms is not sufficient grounds for a diagnosis of concussion. In addition, athletes may not report their symptoms to avoid being sidelined.
“What we need is an objective biomarker,” said David W. Dodick, MD, Professor of Neurology at Mayo Clinic in Phoenix, Arizona. “We need a biomarker for diagnosis, we need one for recovery, and we need one that could potentially prognosticate how these athletes are going to do over time and whether we should return that athlete ever to play.”
CSF Biomarkers
“The optimal biomarker, of course, would be in the CSF because it’s in direct contact with the extracellular matrix and interstitial fluid of the brain, and its composition reflects what’s going on biochemically in that organ,” said Dr. Dodick.
David W. Dodick, MD
In one study, investigators collected CSF from 30 Olympic boxers at one to six days after a bout and after 14 days of rest. The researchers found increased levels of tau, neurofilament light, and glial fibrillary acidic protein (GFAP) in more than 80% of the boxers after their bouts. Neurofilament light and GFAP remained elevated after the rest period and for more than three months after injury. This result “implies that there may be ongoing degeneration well after a bout,” said Dr. Dodick.
CSF biomarkers, however, are not pragmatic for acute concussion evaluation or management on a large scale, he added. “We’re not going to be pulling out a lumbar puncture tray on the sideline, or even in our office, for most individuals.” But studies like this one suggest which biomarkers might be relevant for the diagnosis of concussion.
Serum Biomarkers
Blood biomarkers are more pragmatic than CSF biomarkers for acute postconcussion evaluation, said Dr. Dodick. In 2014, investigators examined blood biomarkers after concussion in 288 professional hockey players. They found a statistically significant increase in total tau after concussion, compared with preseason measurements. In contrast, they found no significant difference in S100 beta or neuron-specific enolase. These biomarkers did increase after a friendly game without concussion, however.
“Total tau at one hour correlates with symptom duration, so the lower the concentration of total tau increase at one hour, the more likely that athlete was to become asymptomatic more quickly,” said Dr. Dodick. Total tau thus may be a biomarker for recovery as well as for brain injury. Total tau elevations at six days predicted the persistence of symptoms and the development of postconcussion syndrome, Dr. Dodick added.
In 2015, researchers examined total tau in military personnel with and without concussion during the previous six months. Compared with controls, personnel who self-reported concussion had a significant increase in total tau. Personnel with a diagnosis of concussion also had a statistically significant increase in total tau, compared with controls and with personnel with self-reported concussion. In addition, tau concentration was associated with postconcussion syndrome at months to years after concussion, independent of posttraumatic stress disorder and depressive symptoms.
A systematic review of research on S100 beta concluded that extracranial injury, physical activity, intoxication with alcohol, and medications affect the level of this biomarker. S100 beta has a short half-life, and a sample must be collected within 30 minutes of injury to be accurate. “All we can say now is that if you have high levels of S100 beta, that’s a cause for concern, and it may correlate with imaging changes,” said Dr. Dodick. “Maybe it could be used in conjunction with some of the other biomarkers like total tau if you can access the blood early enough.”
A 2013 study published in PLoS One included nine patients presenting to an emergency department with mild traumatic brain injury. Their levels of ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL1) were fivefold higher than those of normal controls, and their levels of GFAP were tenfold higher. Elevation in GFAP correlated with hemorrhage on susceptibility-weighted imaging. These biomarkers may identify patients for whom an MRI would be informative, said Dr. Dodick.
More recently, investigators took blood samples from 584 patients with trauma and found that GFAP and UCHL1 were detectable within one hour of injury. GFAP concentration peaked at 20 hours, declined slowly, and remained detectable at seven days. GFAP distinguished patients with traumatic brain injury from injured controls. The biomarker also correlated with CT lesions and the need for neurosurgical intervention. The researchers concluded that UCHL1 could be used as a point-of-care test at the scene of injury and that GFAP was useful in the subacute and in the acute phase of injury.
Blood biomarkers have limitations, however. Although they are highly expressed in CNS, they are detected in low concentrations in serum. In addition, blood biomarkers such as S100 beta have sources outside the brain, thus they are not specific to concussion. Finally, the precision of current immunoassays for these biomarkers needs improvement, said Dr. Dodick.
Imaging Biomarkers
Imaging biomarkers also could support a diagnosis of concussion. In one study, 142 patients with concussion underwent gadolinium-enhanced MRI scans within 48 hours of injury. Meningeal hemorrhage was seen on CT in about 13% of participants, but almost half had focal gadolinium enhancement on MRI. The results indicate a possible breakdown of the blood–brain barrier, said Dr. Dodick.
An investigation published in Annals of Neurology examined 135 patients with concussion and a normal CT of the head. Approximately 30% of participants had abnormal brain MRI scans. Findings included sulcal subarachnoid hemorrhage, hemosiderin deposition, and focal contusions.
In a study published in 2015 in Neurology, researchers found 60 microbleeds in 26 patients with concussion, compared with 15 microbleeds in 12 control subjects. Approximately 90% of the microbleeds in participants with concussion were cortical or subcortical, compared with 20% in controls. Patients with concussion and microbleeds have poor short-term memory and other neuropsychologic deficits on examination. Microbleeds resulting from concussion usually occur at the juncture of gray matter and white matter, said Dr. Dodick.
Lobar cerebral microbleeds appear to be a biomarker of severity and to indicate risk of adverse long-term outcomes. This biomarker can inform decision-making and is ready to be integrated into clinical practice, said Dr. Dodick.
In another study, 45 male and female university-level ice hockey players underwent susceptibility-weighted imaging before and after the playing season, as well as 72 hours, two weeks, and two months after a concussion. The investigators saw a significant increase in cerebral microbleeds in male athletes at two weeks after concussion, and a significant increase in microbleeds in males without concussion, compared with females, at the beginning and end of the season.
—Erik Greb
Suggested Reading
Helmer KG, Pasternak O, Fredman E, et al. Hockey Concussion Education Project, Part 1. Susceptibility-weighted imaging study in male and female ice hockey players over a single season. J Neurosurg. 2014;120(4):864-872.
Huang YL, Kuo YS, Tseng YC, et al. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology. 2015;84(6):580-585.
Kou Z, Gattu R, Kobeissy F, et al. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: results from a pilot study. PLoS One. 2013;8(11):e80296.
Neselius S, Brisby H, Theodorsson A, et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One. 2012;7(4):e33606.
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015;72(10):1109-1116.
Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.
Roth TL, Nayak D, Atanasijevic T, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223-228.
Schulte S, Podlog LW, Hamson-Utley JJ, et al. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71(6):684-692. Yuh EL, Mukherjee P, Lingsma HF, et al; TRACK-TBI Investigators. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73(2):224-235.
VANCOUVER—A diagnosis of concussion is based on clinical observation and testing, and it therefore is susceptible to error. Researchers are seeking biomarkers in CSF, in serum, and on imaging that could provide stronger grounds for a diagnosis of concussion, as well as a method of monitoring recovery, according to an overview provided at the 68th Annual Meeting of the American Academy of Neurology.
Concussion often occurs with no macroscopic evidence of injury. Symptoms of concussion can be subjective and nonspecific, and a patient report of symptoms is not sufficient grounds for a diagnosis of concussion. In addition, athletes may not report their symptoms to avoid being sidelined.
“What we need is an objective biomarker,” said David W. Dodick, MD, Professor of Neurology at Mayo Clinic in Phoenix, Arizona. “We need a biomarker for diagnosis, we need one for recovery, and we need one that could potentially prognosticate how these athletes are going to do over time and whether we should return that athlete ever to play.”
CSF Biomarkers
“The optimal biomarker, of course, would be in the CSF because it’s in direct contact with the extracellular matrix and interstitial fluid of the brain, and its composition reflects what’s going on biochemically in that organ,” said Dr. Dodick.
David W. Dodick, MD
In one study, investigators collected CSF from 30 Olympic boxers at one to six days after a bout and after 14 days of rest. The researchers found increased levels of tau, neurofilament light, and glial fibrillary acidic protein (GFAP) in more than 80% of the boxers after their bouts. Neurofilament light and GFAP remained elevated after the rest period and for more than three months after injury. This result “implies that there may be ongoing degeneration well after a bout,” said Dr. Dodick.
CSF biomarkers, however, are not pragmatic for acute concussion evaluation or management on a large scale, he added. “We’re not going to be pulling out a lumbar puncture tray on the sideline, or even in our office, for most individuals.” But studies like this one suggest which biomarkers might be relevant for the diagnosis of concussion.
Serum Biomarkers
Blood biomarkers are more pragmatic than CSF biomarkers for acute postconcussion evaluation, said Dr. Dodick. In 2014, investigators examined blood biomarkers after concussion in 288 professional hockey players. They found a statistically significant increase in total tau after concussion, compared with preseason measurements. In contrast, they found no significant difference in S100 beta or neuron-specific enolase. These biomarkers did increase after a friendly game without concussion, however.
“Total tau at one hour correlates with symptom duration, so the lower the concentration of total tau increase at one hour, the more likely that athlete was to become asymptomatic more quickly,” said Dr. Dodick. Total tau thus may be a biomarker for recovery as well as for brain injury. Total tau elevations at six days predicted the persistence of symptoms and the development of postconcussion syndrome, Dr. Dodick added.
In 2015, researchers examined total tau in military personnel with and without concussion during the previous six months. Compared with controls, personnel who self-reported concussion had a significant increase in total tau. Personnel with a diagnosis of concussion also had a statistically significant increase in total tau, compared with controls and with personnel with self-reported concussion. In addition, tau concentration was associated with postconcussion syndrome at months to years after concussion, independent of posttraumatic stress disorder and depressive symptoms.
A systematic review of research on S100 beta concluded that extracranial injury, physical activity, intoxication with alcohol, and medications affect the level of this biomarker. S100 beta has a short half-life, and a sample must be collected within 30 minutes of injury to be accurate. “All we can say now is that if you have high levels of S100 beta, that’s a cause for concern, and it may correlate with imaging changes,” said Dr. Dodick. “Maybe it could be used in conjunction with some of the other biomarkers like total tau if you can access the blood early enough.”
A 2013 study published in PLoS One included nine patients presenting to an emergency department with mild traumatic brain injury. Their levels of ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL1) were fivefold higher than those of normal controls, and their levels of GFAP were tenfold higher. Elevation in GFAP correlated with hemorrhage on susceptibility-weighted imaging. These biomarkers may identify patients for whom an MRI would be informative, said Dr. Dodick.
More recently, investigators took blood samples from 584 patients with trauma and found that GFAP and UCHL1 were detectable within one hour of injury. GFAP concentration peaked at 20 hours, declined slowly, and remained detectable at seven days. GFAP distinguished patients with traumatic brain injury from injured controls. The biomarker also correlated with CT lesions and the need for neurosurgical intervention. The researchers concluded that UCHL1 could be used as a point-of-care test at the scene of injury and that GFAP was useful in the subacute and in the acute phase of injury.
Blood biomarkers have limitations, however. Although they are highly expressed in CNS, they are detected in low concentrations in serum. In addition, blood biomarkers such as S100 beta have sources outside the brain, thus they are not specific to concussion. Finally, the precision of current immunoassays for these biomarkers needs improvement, said Dr. Dodick.
Imaging Biomarkers
Imaging biomarkers also could support a diagnosis of concussion. In one study, 142 patients with concussion underwent gadolinium-enhanced MRI scans within 48 hours of injury. Meningeal hemorrhage was seen on CT in about 13% of participants, but almost half had focal gadolinium enhancement on MRI. The results indicate a possible breakdown of the blood–brain barrier, said Dr. Dodick.
An investigation published in Annals of Neurology examined 135 patients with concussion and a normal CT of the head. Approximately 30% of participants had abnormal brain MRI scans. Findings included sulcal subarachnoid hemorrhage, hemosiderin deposition, and focal contusions.
In a study published in 2015 in Neurology, researchers found 60 microbleeds in 26 patients with concussion, compared with 15 microbleeds in 12 control subjects. Approximately 90% of the microbleeds in participants with concussion were cortical or subcortical, compared with 20% in controls. Patients with concussion and microbleeds have poor short-term memory and other neuropsychologic deficits on examination. Microbleeds resulting from concussion usually occur at the juncture of gray matter and white matter, said Dr. Dodick.
Lobar cerebral microbleeds appear to be a biomarker of severity and to indicate risk of adverse long-term outcomes. This biomarker can inform decision-making and is ready to be integrated into clinical practice, said Dr. Dodick.
In another study, 45 male and female university-level ice hockey players underwent susceptibility-weighted imaging before and after the playing season, as well as 72 hours, two weeks, and two months after a concussion. The investigators saw a significant increase in cerebral microbleeds in male athletes at two weeks after concussion, and a significant increase in microbleeds in males without concussion, compared with females, at the beginning and end of the season.
—Erik Greb
VANCOUVER—A diagnosis of concussion is based on clinical observation and testing, and it therefore is susceptible to error. Researchers are seeking biomarkers in CSF, in serum, and on imaging that could provide stronger grounds for a diagnosis of concussion, as well as a method of monitoring recovery, according to an overview provided at the 68th Annual Meeting of the American Academy of Neurology.
Concussion often occurs with no macroscopic evidence of injury. Symptoms of concussion can be subjective and nonspecific, and a patient report of symptoms is not sufficient grounds for a diagnosis of concussion. In addition, athletes may not report their symptoms to avoid being sidelined.
“What we need is an objective biomarker,” said David W. Dodick, MD, Professor of Neurology at Mayo Clinic in Phoenix, Arizona. “We need a biomarker for diagnosis, we need one for recovery, and we need one that could potentially prognosticate how these athletes are going to do over time and whether we should return that athlete ever to play.”
CSF Biomarkers
“The optimal biomarker, of course, would be in the CSF because it’s in direct contact with the extracellular matrix and interstitial fluid of the brain, and its composition reflects what’s going on biochemically in that organ,” said Dr. Dodick.
David W. Dodick, MD
In one study, investigators collected CSF from 30 Olympic boxers at one to six days after a bout and after 14 days of rest. The researchers found increased levels of tau, neurofilament light, and glial fibrillary acidic protein (GFAP) in more than 80% of the boxers after their bouts. Neurofilament light and GFAP remained elevated after the rest period and for more than three months after injury. This result “implies that there may be ongoing degeneration well after a bout,” said Dr. Dodick.
CSF biomarkers, however, are not pragmatic for acute concussion evaluation or management on a large scale, he added. “We’re not going to be pulling out a lumbar puncture tray on the sideline, or even in our office, for most individuals.” But studies like this one suggest which biomarkers might be relevant for the diagnosis of concussion.
Serum Biomarkers
Blood biomarkers are more pragmatic than CSF biomarkers for acute postconcussion evaluation, said Dr. Dodick. In 2014, investigators examined blood biomarkers after concussion in 288 professional hockey players. They found a statistically significant increase in total tau after concussion, compared with preseason measurements. In contrast, they found no significant difference in S100 beta or neuron-specific enolase. These biomarkers did increase after a friendly game without concussion, however.
“Total tau at one hour correlates with symptom duration, so the lower the concentration of total tau increase at one hour, the more likely that athlete was to become asymptomatic more quickly,” said Dr. Dodick. Total tau thus may be a biomarker for recovery as well as for brain injury. Total tau elevations at six days predicted the persistence of symptoms and the development of postconcussion syndrome, Dr. Dodick added.
In 2015, researchers examined total tau in military personnel with and without concussion during the previous six months. Compared with controls, personnel who self-reported concussion had a significant increase in total tau. Personnel with a diagnosis of concussion also had a statistically significant increase in total tau, compared with controls and with personnel with self-reported concussion. In addition, tau concentration was associated with postconcussion syndrome at months to years after concussion, independent of posttraumatic stress disorder and depressive symptoms.
A systematic review of research on S100 beta concluded that extracranial injury, physical activity, intoxication with alcohol, and medications affect the level of this biomarker. S100 beta has a short half-life, and a sample must be collected within 30 minutes of injury to be accurate. “All we can say now is that if you have high levels of S100 beta, that’s a cause for concern, and it may correlate with imaging changes,” said Dr. Dodick. “Maybe it could be used in conjunction with some of the other biomarkers like total tau if you can access the blood early enough.”
A 2013 study published in PLoS One included nine patients presenting to an emergency department with mild traumatic brain injury. Their levels of ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL1) were fivefold higher than those of normal controls, and their levels of GFAP were tenfold higher. Elevation in GFAP correlated with hemorrhage on susceptibility-weighted imaging. These biomarkers may identify patients for whom an MRI would be informative, said Dr. Dodick.
More recently, investigators took blood samples from 584 patients with trauma and found that GFAP and UCHL1 were detectable within one hour of injury. GFAP concentration peaked at 20 hours, declined slowly, and remained detectable at seven days. GFAP distinguished patients with traumatic brain injury from injured controls. The biomarker also correlated with CT lesions and the need for neurosurgical intervention. The researchers concluded that UCHL1 could be used as a point-of-care test at the scene of injury and that GFAP was useful in the subacute and in the acute phase of injury.
Blood biomarkers have limitations, however. Although they are highly expressed in CNS, they are detected in low concentrations in serum. In addition, blood biomarkers such as S100 beta have sources outside the brain, thus they are not specific to concussion. Finally, the precision of current immunoassays for these biomarkers needs improvement, said Dr. Dodick.
Imaging Biomarkers
Imaging biomarkers also could support a diagnosis of concussion. In one study, 142 patients with concussion underwent gadolinium-enhanced MRI scans within 48 hours of injury. Meningeal hemorrhage was seen on CT in about 13% of participants, but almost half had focal gadolinium enhancement on MRI. The results indicate a possible breakdown of the blood–brain barrier, said Dr. Dodick.
An investigation published in Annals of Neurology examined 135 patients with concussion and a normal CT of the head. Approximately 30% of participants had abnormal brain MRI scans. Findings included sulcal subarachnoid hemorrhage, hemosiderin deposition, and focal contusions.
In a study published in 2015 in Neurology, researchers found 60 microbleeds in 26 patients with concussion, compared with 15 microbleeds in 12 control subjects. Approximately 90% of the microbleeds in participants with concussion were cortical or subcortical, compared with 20% in controls. Patients with concussion and microbleeds have poor short-term memory and other neuropsychologic deficits on examination. Microbleeds resulting from concussion usually occur at the juncture of gray matter and white matter, said Dr. Dodick.
Lobar cerebral microbleeds appear to be a biomarker of severity and to indicate risk of adverse long-term outcomes. This biomarker can inform decision-making and is ready to be integrated into clinical practice, said Dr. Dodick.
In another study, 45 male and female university-level ice hockey players underwent susceptibility-weighted imaging before and after the playing season, as well as 72 hours, two weeks, and two months after a concussion. The investigators saw a significant increase in cerebral microbleeds in male athletes at two weeks after concussion, and a significant increase in microbleeds in males without concussion, compared with females, at the beginning and end of the season.
—Erik Greb
Suggested Reading
Helmer KG, Pasternak O, Fredman E, et al. Hockey Concussion Education Project, Part 1. Susceptibility-weighted imaging study in male and female ice hockey players over a single season. J Neurosurg. 2014;120(4):864-872.
Huang YL, Kuo YS, Tseng YC, et al. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology. 2015;84(6):580-585.
Kou Z, Gattu R, Kobeissy F, et al. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: results from a pilot study. PLoS One. 2013;8(11):e80296.
Neselius S, Brisby H, Theodorsson A, et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One. 2012;7(4):e33606.
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015;72(10):1109-1116.
Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.
Roth TL, Nayak D, Atanasijevic T, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223-228.
Schulte S, Podlog LW, Hamson-Utley JJ, et al. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71(6):684-692. Yuh EL, Mukherjee P, Lingsma HF, et al; TRACK-TBI Investigators. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73(2):224-235.
Suggested Reading
Helmer KG, Pasternak O, Fredman E, et al. Hockey Concussion Education Project, Part 1. Susceptibility-weighted imaging study in male and female ice hockey players over a single season. J Neurosurg. 2014;120(4):864-872.
Huang YL, Kuo YS, Tseng YC, et al. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology. 2015;84(6):580-585.
Kou Z, Gattu R, Kobeissy F, et al. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: results from a pilot study. PLoS One. 2013;8(11):e80296.
Neselius S, Brisby H, Theodorsson A, et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One. 2012;7(4):e33606.
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015;72(10):1109-1116.
Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.
Roth TL, Nayak D, Atanasijevic T, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223-228.
Schulte S, Podlog LW, Hamson-Utley JJ, et al. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71(6):684-692. Yuh EL, Mukherjee P, Lingsma HF, et al; TRACK-TBI Investigators. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73(2):224-235.
Restless Sleep in 11-year-olds Predicts Earlier Substance Use
DENVER – Poor sleep quality and shorter sleep duration in boys at age 11 years is associated with subsequent accelerated use and misuse of alcohol and cannabis during adolescence and emerging adulthood, Thomas B. Mike reported at the annual meeting of the Associated Professional Sleep Societies.
This association has been reported before in cross-sectional studies. But Mr. Mike presented new findings from the longitudinal prospective Pitt Mother and Child Project.
The study comprised 170 boys aged 11 years whose mothers filled out the Child Sleep Questionnaire, modified for pediatric use from the well known Pittsburgh Sleep Quality Index. At that time, the boys also were assessed for internalizing and externalizing behaviors, neighborhood dangerousness, and socioeconomic status – all of which are factors known to influence teen substance use.
When participants reached ages 20 and 22 years, they underwent detailed interviews including assessment via the validated Lifetime Drinking History and Lifetime Drug History questionnaires.
In a multivariate Cox regression analysis controlling for a history of child internalizing and externalizing problem behavior as well as socioeconomic factors, every hour of sleep duration less than 9.5 hours at age 11 years was associated with a 21% acceleration in the time to first use of alcohol and a 22% acceleration in first use of cannabis, reported Mr. Mike, a medical student at the University of Pittsburgh.
Moreover, every 1 point worse score for sleep quality on the Child Sleep Questionnaire, scored from 0 (best) to 21 (worst), was associated with a 9% acceleration in time to alcohol use and an 8% acceleration to cannabis use.
Subjects with poor sleep duration and quality as 11-year-olds by parental report also experienced intoxication at a younger age than did those who had been better sleepers at that age. And they reported more frequent use of alcohol and cannabis during adolescence.
In an interview, Mr. Mike, who is planning a career in pediatrics, said the study findings contain an important message for pediatricians and family physicians when they meet with the parents of young boys entering adolescence.
“Probably the biggest take-home message is, ‘sleep more, sleep better.’ We don’t have a lot of ways to intervene right now for kids who have poor sleep, but we know based on this and other studies that kids who don’t sleep enough are more likely to use drugs and more likely to engage in other risky behaviors. So any way the parents can intervene will help, perhaps by getting them to bed earlier or trying to get them on a more regular schedule. At a social policy level, having school start later would probably be the most effective measure, but that probably won’t happen for a very long time,” he said.
“The vast majority of people who end up addicted to drugs later in life started when they were kids, so any way we can reduce adolescent substance use should have a downstream effect, including a decreased total substance use burden,” Mr. Mike added.
Possible mechanisms for the observed longitudinal association between poor quality and duration of sleep at age 11 years and earlier and heavier use of alcohol and cannabis later in adolescence include attempted self-medication for sleep problems, reward regulation, and alterations in brain chemistry, making the boys more susceptible to using drugs later in life, perhaps because they obtain a bigger high than their peers do. The investigators plan to delve deeper into the role of self-regulation and mental health as possible mechanisms linking childhood sleep and later substance use, he said.
The Pitt Mother and Child Project is supported by the National Institutes of Health. Mr. Mike reported having no financial conflicts.
DENVER – Poor sleep quality and shorter sleep duration in boys at age 11 years is associated with subsequent accelerated use and misuse of alcohol and cannabis during adolescence and emerging adulthood, Thomas B. Mike reported at the annual meeting of the Associated Professional Sleep Societies.
This association has been reported before in cross-sectional studies. But Mr. Mike presented new findings from the longitudinal prospective Pitt Mother and Child Project.
The study comprised 170 boys aged 11 years whose mothers filled out the Child Sleep Questionnaire, modified for pediatric use from the well known Pittsburgh Sleep Quality Index. At that time, the boys also were assessed for internalizing and externalizing behaviors, neighborhood dangerousness, and socioeconomic status – all of which are factors known to influence teen substance use.
When participants reached ages 20 and 22 years, they underwent detailed interviews including assessment via the validated Lifetime Drinking History and Lifetime Drug History questionnaires.
In a multivariate Cox regression analysis controlling for a history of child internalizing and externalizing problem behavior as well as socioeconomic factors, every hour of sleep duration less than 9.5 hours at age 11 years was associated with a 21% acceleration in the time to first use of alcohol and a 22% acceleration in first use of cannabis, reported Mr. Mike, a medical student at the University of Pittsburgh.
Moreover, every 1 point worse score for sleep quality on the Child Sleep Questionnaire, scored from 0 (best) to 21 (worst), was associated with a 9% acceleration in time to alcohol use and an 8% acceleration to cannabis use.
Subjects with poor sleep duration and quality as 11-year-olds by parental report also experienced intoxication at a younger age than did those who had been better sleepers at that age. And they reported more frequent use of alcohol and cannabis during adolescence.
In an interview, Mr. Mike, who is planning a career in pediatrics, said the study findings contain an important message for pediatricians and family physicians when they meet with the parents of young boys entering adolescence.
“Probably the biggest take-home message is, ‘sleep more, sleep better.’ We don’t have a lot of ways to intervene right now for kids who have poor sleep, but we know based on this and other studies that kids who don’t sleep enough are more likely to use drugs and more likely to engage in other risky behaviors. So any way the parents can intervene will help, perhaps by getting them to bed earlier or trying to get them on a more regular schedule. At a social policy level, having school start later would probably be the most effective measure, but that probably won’t happen for a very long time,” he said.
“The vast majority of people who end up addicted to drugs later in life started when they were kids, so any way we can reduce adolescent substance use should have a downstream effect, including a decreased total substance use burden,” Mr. Mike added.
Possible mechanisms for the observed longitudinal association between poor quality and duration of sleep at age 11 years and earlier and heavier use of alcohol and cannabis later in adolescence include attempted self-medication for sleep problems, reward regulation, and alterations in brain chemistry, making the boys more susceptible to using drugs later in life, perhaps because they obtain a bigger high than their peers do. The investigators plan to delve deeper into the role of self-regulation and mental health as possible mechanisms linking childhood sleep and later substance use, he said.
The Pitt Mother and Child Project is supported by the National Institutes of Health. Mr. Mike reported having no financial conflicts.
DENVER – Poor sleep quality and shorter sleep duration in boys at age 11 years is associated with subsequent accelerated use and misuse of alcohol and cannabis during adolescence and emerging adulthood, Thomas B. Mike reported at the annual meeting of the Associated Professional Sleep Societies.
This association has been reported before in cross-sectional studies. But Mr. Mike presented new findings from the longitudinal prospective Pitt Mother and Child Project.
The study comprised 170 boys aged 11 years whose mothers filled out the Child Sleep Questionnaire, modified for pediatric use from the well known Pittsburgh Sleep Quality Index. At that time, the boys also were assessed for internalizing and externalizing behaviors, neighborhood dangerousness, and socioeconomic status – all of which are factors known to influence teen substance use.
When participants reached ages 20 and 22 years, they underwent detailed interviews including assessment via the validated Lifetime Drinking History and Lifetime Drug History questionnaires.
In a multivariate Cox regression analysis controlling for a history of child internalizing and externalizing problem behavior as well as socioeconomic factors, every hour of sleep duration less than 9.5 hours at age 11 years was associated with a 21% acceleration in the time to first use of alcohol and a 22% acceleration in first use of cannabis, reported Mr. Mike, a medical student at the University of Pittsburgh.
Moreover, every 1 point worse score for sleep quality on the Child Sleep Questionnaire, scored from 0 (best) to 21 (worst), was associated with a 9% acceleration in time to alcohol use and an 8% acceleration to cannabis use.
Subjects with poor sleep duration and quality as 11-year-olds by parental report also experienced intoxication at a younger age than did those who had been better sleepers at that age. And they reported more frequent use of alcohol and cannabis during adolescence.
In an interview, Mr. Mike, who is planning a career in pediatrics, said the study findings contain an important message for pediatricians and family physicians when they meet with the parents of young boys entering adolescence.
“Probably the biggest take-home message is, ‘sleep more, sleep better.’ We don’t have a lot of ways to intervene right now for kids who have poor sleep, but we know based on this and other studies that kids who don’t sleep enough are more likely to use drugs and more likely to engage in other risky behaviors. So any way the parents can intervene will help, perhaps by getting them to bed earlier or trying to get them on a more regular schedule. At a social policy level, having school start later would probably be the most effective measure, but that probably won’t happen for a very long time,” he said.
“The vast majority of people who end up addicted to drugs later in life started when they were kids, so any way we can reduce adolescent substance use should have a downstream effect, including a decreased total substance use burden,” Mr. Mike added.
Possible mechanisms for the observed longitudinal association between poor quality and duration of sleep at age 11 years and earlier and heavier use of alcohol and cannabis later in adolescence include attempted self-medication for sleep problems, reward regulation, and alterations in brain chemistry, making the boys more susceptible to using drugs later in life, perhaps because they obtain a bigger high than their peers do. The investigators plan to delve deeper into the role of self-regulation and mental health as possible mechanisms linking childhood sleep and later substance use, he said.
The Pitt Mother and Child Project is supported by the National Institutes of Health. Mr. Mike reported having no financial conflicts.
AT SLEEP 2016
Restless sleep in 11-year-olds predicts earlier substance use
DENVER – Poor sleep quality and shorter sleep duration in boys at age 11 years is associated with subsequent accelerated use and misuse of alcohol and cannabis during adolescence and emerging adulthood, Thomas B. Mike reported at the annual meeting of the Associated Professional Sleep Societies.
This association has been reported before in cross-sectional studies. But Mr. Mike presented new findings from the longitudinal prospective Pitt Mother and Child Project.
The study comprised 170 boys aged 11 years whose mothers filled out the Child Sleep Questionnaire, modified for pediatric use from the well known Pittsburgh Sleep Quality Index. At that time, the boys also were assessed for internalizing and externalizing behaviors, neighborhood dangerousness, and socioeconomic status – all of which are factors known to influence teen substance use.
When participants reached ages 20 and 22 years, they underwent detailed interviews including assessment via the validated Lifetime Drinking History and Lifetime Drug History questionnaires.
In a multivariate Cox regression analysis controlling for a history of child internalizing and externalizing problem behavior as well as socioeconomic factors, every hour of sleep duration less than 9.5 hours at age 11 years was associated with a 21% acceleration in the time to first use of alcohol and a 22% acceleration in first use of cannabis, reported Mr. Mike, a medical student at the University of Pittsburgh.
Moreover, every 1 point worse score for sleep quality on the Child Sleep Questionnaire, scored from 0 (best) to 21 (worst), was associated with a 9% acceleration in time to alcohol use and an 8% acceleration to cannabis use.
Subjects with poor sleep duration and quality as 11-year-olds by parental report also experienced intoxication at a younger age than did those who had been better sleepers at that age. And they reported more frequent use of alcohol and cannabis during adolescence.
In an interview, Mr. Mike, who is planning a career in pediatrics, said the study findings contain an important message for pediatricians and family physicians when they meet with the parents of young boys entering adolescence.
“Probably the biggest take-home message is, ‘sleep more, sleep better.’ We don’t have a lot of ways to intervene right now for kids who have poor sleep, but we know based on this and other studies that kids who don’t sleep enough are more likely to use drugs and more likely to engage in other risky behaviors. So any way the parents can intervene will help, perhaps by getting them to bed earlier or trying to get them on a more regular schedule. At a social policy level, having school start later would probably be the most effective measure, but that probably won’t happen for a very long time,” he said.
“The vast majority of people who end up addicted to drugs later in life started when they were kids, so any way we can reduce adolescent substance use should have a downstream effect, including a decreased total substance use burden,” Mr. Mike added.
Possible mechanisms for the observed longitudinal association between poor quality and duration of sleep at age 11 years and earlier and heavier use of alcohol and cannabis later in adolescence include attempted self-medication for sleep problems, reward regulation, and alterations in brain chemistry, making the boys more susceptible to using drugs later in life, perhaps because they obtain a bigger high than their peers do. The investigators plan to delve deeper into the role of self-regulation and mental health as possible mechanisms linking childhood sleep and later substance use, he said.
The Pitt Mother and Child Project is supported by the National Institutes of Health. Mr. Mike reported having no financial conflicts.
DENVER – Poor sleep quality and shorter sleep duration in boys at age 11 years is associated with subsequent accelerated use and misuse of alcohol and cannabis during adolescence and emerging adulthood, Thomas B. Mike reported at the annual meeting of the Associated Professional Sleep Societies.
This association has been reported before in cross-sectional studies. But Mr. Mike presented new findings from the longitudinal prospective Pitt Mother and Child Project.
The study comprised 170 boys aged 11 years whose mothers filled out the Child Sleep Questionnaire, modified for pediatric use from the well known Pittsburgh Sleep Quality Index. At that time, the boys also were assessed for internalizing and externalizing behaviors, neighborhood dangerousness, and socioeconomic status – all of which are factors known to influence teen substance use.
When participants reached ages 20 and 22 years, they underwent detailed interviews including assessment via the validated Lifetime Drinking History and Lifetime Drug History questionnaires.
In a multivariate Cox regression analysis controlling for a history of child internalizing and externalizing problem behavior as well as socioeconomic factors, every hour of sleep duration less than 9.5 hours at age 11 years was associated with a 21% acceleration in the time to first use of alcohol and a 22% acceleration in first use of cannabis, reported Mr. Mike, a medical student at the University of Pittsburgh.
Moreover, every 1 point worse score for sleep quality on the Child Sleep Questionnaire, scored from 0 (best) to 21 (worst), was associated with a 9% acceleration in time to alcohol use and an 8% acceleration to cannabis use.
Subjects with poor sleep duration and quality as 11-year-olds by parental report also experienced intoxication at a younger age than did those who had been better sleepers at that age. And they reported more frequent use of alcohol and cannabis during adolescence.
In an interview, Mr. Mike, who is planning a career in pediatrics, said the study findings contain an important message for pediatricians and family physicians when they meet with the parents of young boys entering adolescence.
“Probably the biggest take-home message is, ‘sleep more, sleep better.’ We don’t have a lot of ways to intervene right now for kids who have poor sleep, but we know based on this and other studies that kids who don’t sleep enough are more likely to use drugs and more likely to engage in other risky behaviors. So any way the parents can intervene will help, perhaps by getting them to bed earlier or trying to get them on a more regular schedule. At a social policy level, having school start later would probably be the most effective measure, but that probably won’t happen for a very long time,” he said.
“The vast majority of people who end up addicted to drugs later in life started when they were kids, so any way we can reduce adolescent substance use should have a downstream effect, including a decreased total substance use burden,” Mr. Mike added.
Possible mechanisms for the observed longitudinal association between poor quality and duration of sleep at age 11 years and earlier and heavier use of alcohol and cannabis later in adolescence include attempted self-medication for sleep problems, reward regulation, and alterations in brain chemistry, making the boys more susceptible to using drugs later in life, perhaps because they obtain a bigger high than their peers do. The investigators plan to delve deeper into the role of self-regulation and mental health as possible mechanisms linking childhood sleep and later substance use, he said.
The Pitt Mother and Child Project is supported by the National Institutes of Health. Mr. Mike reported having no financial conflicts.
DENVER – Poor sleep quality and shorter sleep duration in boys at age 11 years is associated with subsequent accelerated use and misuse of alcohol and cannabis during adolescence and emerging adulthood, Thomas B. Mike reported at the annual meeting of the Associated Professional Sleep Societies.
This association has been reported before in cross-sectional studies. But Mr. Mike presented new findings from the longitudinal prospective Pitt Mother and Child Project.
The study comprised 170 boys aged 11 years whose mothers filled out the Child Sleep Questionnaire, modified for pediatric use from the well known Pittsburgh Sleep Quality Index. At that time, the boys also were assessed for internalizing and externalizing behaviors, neighborhood dangerousness, and socioeconomic status – all of which are factors known to influence teen substance use.
When participants reached ages 20 and 22 years, they underwent detailed interviews including assessment via the validated Lifetime Drinking History and Lifetime Drug History questionnaires.
In a multivariate Cox regression analysis controlling for a history of child internalizing and externalizing problem behavior as well as socioeconomic factors, every hour of sleep duration less than 9.5 hours at age 11 years was associated with a 21% acceleration in the time to first use of alcohol and a 22% acceleration in first use of cannabis, reported Mr. Mike, a medical student at the University of Pittsburgh.
Moreover, every 1 point worse score for sleep quality on the Child Sleep Questionnaire, scored from 0 (best) to 21 (worst), was associated with a 9% acceleration in time to alcohol use and an 8% acceleration to cannabis use.
Subjects with poor sleep duration and quality as 11-year-olds by parental report also experienced intoxication at a younger age than did those who had been better sleepers at that age. And they reported more frequent use of alcohol and cannabis during adolescence.
In an interview, Mr. Mike, who is planning a career in pediatrics, said the study findings contain an important message for pediatricians and family physicians when they meet with the parents of young boys entering adolescence.
“Probably the biggest take-home message is, ‘sleep more, sleep better.’ We don’t have a lot of ways to intervene right now for kids who have poor sleep, but we know based on this and other studies that kids who don’t sleep enough are more likely to use drugs and more likely to engage in other risky behaviors. So any way the parents can intervene will help, perhaps by getting them to bed earlier or trying to get them on a more regular schedule. At a social policy level, having school start later would probably be the most effective measure, but that probably won’t happen for a very long time,” he said.
“The vast majority of people who end up addicted to drugs later in life started when they were kids, so any way we can reduce adolescent substance use should have a downstream effect, including a decreased total substance use burden,” Mr. Mike added.
Possible mechanisms for the observed longitudinal association between poor quality and duration of sleep at age 11 years and earlier and heavier use of alcohol and cannabis later in adolescence include attempted self-medication for sleep problems, reward regulation, and alterations in brain chemistry, making the boys more susceptible to using drugs later in life, perhaps because they obtain a bigger high than their peers do. The investigators plan to delve deeper into the role of self-regulation and mental health as possible mechanisms linking childhood sleep and later substance use, he said.
The Pitt Mother and Child Project is supported by the National Institutes of Health. Mr. Mike reported having no financial conflicts.
AT SLEEP 2016
Key clinical point: Boys with poor sleep quality and quantity start using alcohol and cannabis earlier as teens.
Major finding: Every hour less of sleep duration at age 11 years was associated with a 21% acceleration in time to first use of alcohol and a 22% acceleration in first use of cannabis.
Data source: An analysis from the Pitt Mother and Child Project of 170 boys with sleep data recorded at age 11 and lifetime alcohol and cannabis use recalled at ages 20 and 22.
Disclosures: The Pitt Mother and Child Project is supported by the National Institutes of Health. The presenter reported having no financial conflicts.
Dopamine Antagonists Treat Migraine Effectively
STOWE, VT—Dopamine antagonists can be a beneficial adjunctive treatment for migraine, according to an overview provided at the 26th Annual Stowe Headache Symposium of the Headache Cooperative of New England. “We should consider dopamine antagonists as first-line IV treatments and second-line oral treatments for both acute treatment and prevention of headache,” said Lauren Natbony, MD. Among oral dopamine antagonists, olanzapine appears to be supported by the most compelling evidence, she added. Migraine treatment is an off-label use for most of these medications.
Lauren Natbony, MD
Dopamine modulates processes involved in migraine such as nociception, autonomic response, and vasoregulation. Dopamine also contributes to many of the symptoms of migraine, including nausea, vomiting, mood changes, and fatigue. Dopamine antagonists are appropriate for patients who have not responded to triptans and for patients who cannot use triptans, said Dr. Natbony, a headache fellow at the Icahn School of Medicine at Mount Sinai in New York. “For episodes of status migrainosus, these medications can be used continuously over a number of days. For prevention, we could consider these agents in patients who fail multiple medications.”
The three classes of dopamine antagonists used to treat headache are typical antipsychotics, atypical antipsychotics, and prokinetic agents. The typical antipsychotics tend to produce more sedation, have more anticholinergic effect, and entail a higher risk of extrapyramidal symptoms such as acute dystonia, compared with atypical antipsychotics. Because of concerns about side effects, the recommendation is to use typical antipsychotics for no more than 10 days per month. Atypical antipsychotics, on the other hand, are suitable for long-term treatment, said Dr. Natbony.
Typical Antipsychotics
Four studies have indicated that prochlorperazine is more effective for pain reduction than placebo, regardless of the route of administration. The drug also has proven more effective than ketorolac, magnesium, valproate, and sumatriptan. Data suggest that buccal prochlorperazine is more effective than oral ergotamine, but this formulation is not available in the United States. The best alternatives are oral prochlorperazine (5 mg to 10 mg) and rectal prochlorperazine (25 mg), said Dr. Natbony. “The dose can be used once for acute relief or can be repeated every six to eight hours for multiple days to abort status migrainosus.”
Data suggest that chlorpromazine effectively relieves migraine and associated symptoms, and prevents migraine recurrence more effectively than IV lidocaine or dihydroergotamine. “For acute migraine attacks, 25 mg orally can be used every six hours, for a total daily dose of 100 mg,” said Dr. Natbony. Neurologists can consider chlorpromazine for patients with cluster headache who are refractory to other treatments, but the study of the drug in this population had a small sample size and included a wide range of doses.
Researchers have studied promethazine as an adjunctive treatment, but not as a single agent. Intramuscular promethazine provides a similar level of pain relief whether given with meperidine or ketorolac. Oral promethazine plus sumatriptan provides a significant benefit, compared with sumatriptan alone. “Therefore, I would consider it an add-on medication to a triptan,” said Dr. Natbony. Promethazine is not administered IV or subcutaneously because of the risk of severe tissue injury such as gangrene.
Two studies indicate that droperidol is highly effective for intractable migraine. No oral formulation is available, but droperidol can be administered either IV or intramuscularly. For outpatient purposes, an intramuscular dose of 2.75 mg is effective and yields few side effects. Droperidol entails a risk of QT prolongation, however, and may be inappropriate for patients with cardiac disease.
In a study of 40 patients with migraine, 5 mg of IV haloperidol provided significantrelief, compared with placebo. Based on these data, Dr. Natbony and colleagues use 2 mg to 5 mg of oral haloperidol as an outpatient treatment to abort headache. No studies have examined oral haloperidol specifically, however.
Prokinetic Agents
Prokinetic agents can be used during a migraine prodrome to prevent an attack. Data suggest that IV metoclopramide is superior to placebo, but that intramuscular and rectal formulations are not. As a single agent, metoclopramide is inferior to prochlorperazine. Oral metoclopramide, when administered with an analgesic, is less effective than a triptan, “therefore it should only be considered in those who can’t use triptans,” said Dr. Natbony. A 10-mg tid oral dose is beneficial for prophylactic and abortive therapy. Metoclopramide is pregnancy category B and can be considered for pregnant and lactating women. The drug may stimulate gastrointestinal motility, and using it in combination with another agent (eg, a triptan) may improve absorption of that agent.
Domperidone, another prokinetic agent, effectively prevents migraine if taken in a 30-mg dose during the prodromal phase. Research also indicates that the oral formulation shortens the duration of a migraine attack without provoking adverse events. Domperidone is not available in the US, however, thus in this class of drugs, metoclopramide is the abortive treatment of choice, said Dr. Natbony.
Atypical Antipsychotics
Atypical antipsychotics are effective in migraine prophylaxis and generally are less sedating and less likely to produce movement disorders. Olanzapine has the best evidence in migraine among the atypical antipsychotics. The drug decreased the number of headache days and pain intensity in a study of chronic migraineurs who were refractory to more than four preventive agents. The study was retrospective and unblinded and had no placebo control, however. In practice, 5-mg to 10-mg doses are used at bedtime as a preventive treatment. A second study supported olanzapine’s efficacy as an abortive agent in daily doses of 2.5 mg to 5 mg. Neurologists may repeat dosing as needed to a daily maximum of 20 mg. “We have used 2.5 mg to 5 mg nightly for five to 10 nights to help break status migrainosus,” said Dr. Natbony.
Compared with olanzapine, quetiapine is less potent and entails a lower risk of movement disorders. In two studies of migraineurs who had failed standard preventive agents, quetiapine reduced headache frequency and severity, as well as the use of rescue medications. Doses administered in the study ranged from 25 mg to 75 mg daily.
The literature contains only case studies of aripiprazole in migraineurs. Three patients who received aripiprazole for psychiatric conditions reported spontaneous relief of migraine. One patient with medication overuse headache was treated successfully with aripiprazole. Doses range from 10 mg to 20 mg daily.
Like aripiprazole, ziprasidone has not been studied in randomized controlled trials. One case report described three patients with chronic daily headache who had improvements in headache frequency and severity with ziprasidone. For migraine prevention, neurologists can start with a dose of 20 mg bid and increase it to 80 mg bid, said Dr. Natbony. For acute treatment, doses range from 40 mg to 80 mg, with a maximum of 160 mg daily.
Comparative Efficacy
“The most beneficial oral medication appears to be olanzapine, followed by chlorpromazine” for acute treatment, said Dr. Natbony. IV prochlorperazine is also more effective than IV metoclopramide for acute treatment, “though metoclopramide should be considered as first-line treatment for prevention of a migraine attack and given during a prodrome,” she concluded.
—Erik Greb
Suggested Reading
Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902.
Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515-518.
Thomas MC, Musselman ME, Shewmaker J. Droperidol for the treatment of acute migraine headaches. Ann Pharmacother. 2015;49(2):233-240.
STOWE, VT—Dopamine antagonists can be a beneficial adjunctive treatment for migraine, according to an overview provided at the 26th Annual Stowe Headache Symposium of the Headache Cooperative of New England. “We should consider dopamine antagonists as first-line IV treatments and second-line oral treatments for both acute treatment and prevention of headache,” said Lauren Natbony, MD. Among oral dopamine antagonists, olanzapine appears to be supported by the most compelling evidence, she added. Migraine treatment is an off-label use for most of these medications.
Lauren Natbony, MD
Dopamine modulates processes involved in migraine such as nociception, autonomic response, and vasoregulation. Dopamine also contributes to many of the symptoms of migraine, including nausea, vomiting, mood changes, and fatigue. Dopamine antagonists are appropriate for patients who have not responded to triptans and for patients who cannot use triptans, said Dr. Natbony, a headache fellow at the Icahn School of Medicine at Mount Sinai in New York. “For episodes of status migrainosus, these medications can be used continuously over a number of days. For prevention, we could consider these agents in patients who fail multiple medications.”
The three classes of dopamine antagonists used to treat headache are typical antipsychotics, atypical antipsychotics, and prokinetic agents. The typical antipsychotics tend to produce more sedation, have more anticholinergic effect, and entail a higher risk of extrapyramidal symptoms such as acute dystonia, compared with atypical antipsychotics. Because of concerns about side effects, the recommendation is to use typical antipsychotics for no more than 10 days per month. Atypical antipsychotics, on the other hand, are suitable for long-term treatment, said Dr. Natbony.
Typical Antipsychotics
Four studies have indicated that prochlorperazine is more effective for pain reduction than placebo, regardless of the route of administration. The drug also has proven more effective than ketorolac, magnesium, valproate, and sumatriptan. Data suggest that buccal prochlorperazine is more effective than oral ergotamine, but this formulation is not available in the United States. The best alternatives are oral prochlorperazine (5 mg to 10 mg) and rectal prochlorperazine (25 mg), said Dr. Natbony. “The dose can be used once for acute relief or can be repeated every six to eight hours for multiple days to abort status migrainosus.”
Data suggest that chlorpromazine effectively relieves migraine and associated symptoms, and prevents migraine recurrence more effectively than IV lidocaine or dihydroergotamine. “For acute migraine attacks, 25 mg orally can be used every six hours, for a total daily dose of 100 mg,” said Dr. Natbony. Neurologists can consider chlorpromazine for patients with cluster headache who are refractory to other treatments, but the study of the drug in this population had a small sample size and included a wide range of doses.
Researchers have studied promethazine as an adjunctive treatment, but not as a single agent. Intramuscular promethazine provides a similar level of pain relief whether given with meperidine or ketorolac. Oral promethazine plus sumatriptan provides a significant benefit, compared with sumatriptan alone. “Therefore, I would consider it an add-on medication to a triptan,” said Dr. Natbony. Promethazine is not administered IV or subcutaneously because of the risk of severe tissue injury such as gangrene.
Two studies indicate that droperidol is highly effective for intractable migraine. No oral formulation is available, but droperidol can be administered either IV or intramuscularly. For outpatient purposes, an intramuscular dose of 2.75 mg is effective and yields few side effects. Droperidol entails a risk of QT prolongation, however, and may be inappropriate for patients with cardiac disease.
In a study of 40 patients with migraine, 5 mg of IV haloperidol provided significantrelief, compared with placebo. Based on these data, Dr. Natbony and colleagues use 2 mg to 5 mg of oral haloperidol as an outpatient treatment to abort headache. No studies have examined oral haloperidol specifically, however.
Prokinetic Agents
Prokinetic agents can be used during a migraine prodrome to prevent an attack. Data suggest that IV metoclopramide is superior to placebo, but that intramuscular and rectal formulations are not. As a single agent, metoclopramide is inferior to prochlorperazine. Oral metoclopramide, when administered with an analgesic, is less effective than a triptan, “therefore it should only be considered in those who can’t use triptans,” said Dr. Natbony. A 10-mg tid oral dose is beneficial for prophylactic and abortive therapy. Metoclopramide is pregnancy category B and can be considered for pregnant and lactating women. The drug may stimulate gastrointestinal motility, and using it in combination with another agent (eg, a triptan) may improve absorption of that agent.
Domperidone, another prokinetic agent, effectively prevents migraine if taken in a 30-mg dose during the prodromal phase. Research also indicates that the oral formulation shortens the duration of a migraine attack without provoking adverse events. Domperidone is not available in the US, however, thus in this class of drugs, metoclopramide is the abortive treatment of choice, said Dr. Natbony.
Atypical Antipsychotics
Atypical antipsychotics are effective in migraine prophylaxis and generally are less sedating and less likely to produce movement disorders. Olanzapine has the best evidence in migraine among the atypical antipsychotics. The drug decreased the number of headache days and pain intensity in a study of chronic migraineurs who were refractory to more than four preventive agents. The study was retrospective and unblinded and had no placebo control, however. In practice, 5-mg to 10-mg doses are used at bedtime as a preventive treatment. A second study supported olanzapine’s efficacy as an abortive agent in daily doses of 2.5 mg to 5 mg. Neurologists may repeat dosing as needed to a daily maximum of 20 mg. “We have used 2.5 mg to 5 mg nightly for five to 10 nights to help break status migrainosus,” said Dr. Natbony.
Compared with olanzapine, quetiapine is less potent and entails a lower risk of movement disorders. In two studies of migraineurs who had failed standard preventive agents, quetiapine reduced headache frequency and severity, as well as the use of rescue medications. Doses administered in the study ranged from 25 mg to 75 mg daily.
The literature contains only case studies of aripiprazole in migraineurs. Three patients who received aripiprazole for psychiatric conditions reported spontaneous relief of migraine. One patient with medication overuse headache was treated successfully with aripiprazole. Doses range from 10 mg to 20 mg daily.
Like aripiprazole, ziprasidone has not been studied in randomized controlled trials. One case report described three patients with chronic daily headache who had improvements in headache frequency and severity with ziprasidone. For migraine prevention, neurologists can start with a dose of 20 mg bid and increase it to 80 mg bid, said Dr. Natbony. For acute treatment, doses range from 40 mg to 80 mg, with a maximum of 160 mg daily.
Comparative Efficacy
“The most beneficial oral medication appears to be olanzapine, followed by chlorpromazine” for acute treatment, said Dr. Natbony. IV prochlorperazine is also more effective than IV metoclopramide for acute treatment, “though metoclopramide should be considered as first-line treatment for prevention of a migraine attack and given during a prodrome,” she concluded.
—Erik Greb
STOWE, VT—Dopamine antagonists can be a beneficial adjunctive treatment for migraine, according to an overview provided at the 26th Annual Stowe Headache Symposium of the Headache Cooperative of New England. “We should consider dopamine antagonists as first-line IV treatments and second-line oral treatments for both acute treatment and prevention of headache,” said Lauren Natbony, MD. Among oral dopamine antagonists, olanzapine appears to be supported by the most compelling evidence, she added. Migraine treatment is an off-label use for most of these medications.
Lauren Natbony, MD
Dopamine modulates processes involved in migraine such as nociception, autonomic response, and vasoregulation. Dopamine also contributes to many of the symptoms of migraine, including nausea, vomiting, mood changes, and fatigue. Dopamine antagonists are appropriate for patients who have not responded to triptans and for patients who cannot use triptans, said Dr. Natbony, a headache fellow at the Icahn School of Medicine at Mount Sinai in New York. “For episodes of status migrainosus, these medications can be used continuously over a number of days. For prevention, we could consider these agents in patients who fail multiple medications.”
The three classes of dopamine antagonists used to treat headache are typical antipsychotics, atypical antipsychotics, and prokinetic agents. The typical antipsychotics tend to produce more sedation, have more anticholinergic effect, and entail a higher risk of extrapyramidal symptoms such as acute dystonia, compared with atypical antipsychotics. Because of concerns about side effects, the recommendation is to use typical antipsychotics for no more than 10 days per month. Atypical antipsychotics, on the other hand, are suitable for long-term treatment, said Dr. Natbony.
Typical Antipsychotics
Four studies have indicated that prochlorperazine is more effective for pain reduction than placebo, regardless of the route of administration. The drug also has proven more effective than ketorolac, magnesium, valproate, and sumatriptan. Data suggest that buccal prochlorperazine is more effective than oral ergotamine, but this formulation is not available in the United States. The best alternatives are oral prochlorperazine (5 mg to 10 mg) and rectal prochlorperazine (25 mg), said Dr. Natbony. “The dose can be used once for acute relief or can be repeated every six to eight hours for multiple days to abort status migrainosus.”
Data suggest that chlorpromazine effectively relieves migraine and associated symptoms, and prevents migraine recurrence more effectively than IV lidocaine or dihydroergotamine. “For acute migraine attacks, 25 mg orally can be used every six hours, for a total daily dose of 100 mg,” said Dr. Natbony. Neurologists can consider chlorpromazine for patients with cluster headache who are refractory to other treatments, but the study of the drug in this population had a small sample size and included a wide range of doses.
Researchers have studied promethazine as an adjunctive treatment, but not as a single agent. Intramuscular promethazine provides a similar level of pain relief whether given with meperidine or ketorolac. Oral promethazine plus sumatriptan provides a significant benefit, compared with sumatriptan alone. “Therefore, I would consider it an add-on medication to a triptan,” said Dr. Natbony. Promethazine is not administered IV or subcutaneously because of the risk of severe tissue injury such as gangrene.
Two studies indicate that droperidol is highly effective for intractable migraine. No oral formulation is available, but droperidol can be administered either IV or intramuscularly. For outpatient purposes, an intramuscular dose of 2.75 mg is effective and yields few side effects. Droperidol entails a risk of QT prolongation, however, and may be inappropriate for patients with cardiac disease.
In a study of 40 patients with migraine, 5 mg of IV haloperidol provided significantrelief, compared with placebo. Based on these data, Dr. Natbony and colleagues use 2 mg to 5 mg of oral haloperidol as an outpatient treatment to abort headache. No studies have examined oral haloperidol specifically, however.
Prokinetic Agents
Prokinetic agents can be used during a migraine prodrome to prevent an attack. Data suggest that IV metoclopramide is superior to placebo, but that intramuscular and rectal formulations are not. As a single agent, metoclopramide is inferior to prochlorperazine. Oral metoclopramide, when administered with an analgesic, is less effective than a triptan, “therefore it should only be considered in those who can’t use triptans,” said Dr. Natbony. A 10-mg tid oral dose is beneficial for prophylactic and abortive therapy. Metoclopramide is pregnancy category B and can be considered for pregnant and lactating women. The drug may stimulate gastrointestinal motility, and using it in combination with another agent (eg, a triptan) may improve absorption of that agent.
Domperidone, another prokinetic agent, effectively prevents migraine if taken in a 30-mg dose during the prodromal phase. Research also indicates that the oral formulation shortens the duration of a migraine attack without provoking adverse events. Domperidone is not available in the US, however, thus in this class of drugs, metoclopramide is the abortive treatment of choice, said Dr. Natbony.
Atypical Antipsychotics
Atypical antipsychotics are effective in migraine prophylaxis and generally are less sedating and less likely to produce movement disorders. Olanzapine has the best evidence in migraine among the atypical antipsychotics. The drug decreased the number of headache days and pain intensity in a study of chronic migraineurs who were refractory to more than four preventive agents. The study was retrospective and unblinded and had no placebo control, however. In practice, 5-mg to 10-mg doses are used at bedtime as a preventive treatment. A second study supported olanzapine’s efficacy as an abortive agent in daily doses of 2.5 mg to 5 mg. Neurologists may repeat dosing as needed to a daily maximum of 20 mg. “We have used 2.5 mg to 5 mg nightly for five to 10 nights to help break status migrainosus,” said Dr. Natbony.
Compared with olanzapine, quetiapine is less potent and entails a lower risk of movement disorders. In two studies of migraineurs who had failed standard preventive agents, quetiapine reduced headache frequency and severity, as well as the use of rescue medications. Doses administered in the study ranged from 25 mg to 75 mg daily.
The literature contains only case studies of aripiprazole in migraineurs. Three patients who received aripiprazole for psychiatric conditions reported spontaneous relief of migraine. One patient with medication overuse headache was treated successfully with aripiprazole. Doses range from 10 mg to 20 mg daily.
Like aripiprazole, ziprasidone has not been studied in randomized controlled trials. One case report described three patients with chronic daily headache who had improvements in headache frequency and severity with ziprasidone. For migraine prevention, neurologists can start with a dose of 20 mg bid and increase it to 80 mg bid, said Dr. Natbony. For acute treatment, doses range from 40 mg to 80 mg, with a maximum of 160 mg daily.
Comparative Efficacy
“The most beneficial oral medication appears to be olanzapine, followed by chlorpromazine” for acute treatment, said Dr. Natbony. IV prochlorperazine is also more effective than IV metoclopramide for acute treatment, “though metoclopramide should be considered as first-line treatment for prevention of a migraine attack and given during a prodrome,” she concluded.
—Erik Greb
Suggested Reading
Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902.
Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515-518.
Thomas MC, Musselman ME, Shewmaker J. Droperidol for the treatment of acute migraine headaches. Ann Pharmacother. 2015;49(2):233-240.
Suggested Reading
Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902.
Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515-518.
Thomas MC, Musselman ME, Shewmaker J. Droperidol for the treatment of acute migraine headaches. Ann Pharmacother. 2015;49(2):233-240.
Universal decolonization reduces CLABSI rates in large health care system
Universal decolonization of adult intensive care unit patients in a large community health system significantly reduced the incidence of central line–associated bloodstream infections (CLABSIs), according to Dr. Edward Septimus and his associates.
A total of 136 ICUs at 95 hospitals were included in the study. Universal decolonization was completed within 6 months. A total of 672 CLABSIs occurred in the 24 months before intervention, and 181 CLABSIs occurred in the 8 months after intervention. The CLABSI rate was 1.1/1,000 central-line days before intervention and 0.87 after intervention, a reduction of 23.5%.
No evidence of a trend over time was found in either the pre- or postintervention period. The reduction in CLABSIs post intervention was not affected after adjustment for seasonality, number of beds in the ICU, or unit type. The gram-positive CLABSI rate was reduced by 28.7% after invention, and the rate of CLABSI due to Staphylococcus aureus was reduced by 31.9%.
“This rapid dissemination and implementation program demonstrated the utility of a protocol-specific toolkit, coupled with a multistep translation program to implement universal decolonization in a large, complex organization. Doing so demonstrated that use of CHG [chlorhexidine] bathing plus nasal mupirocin in routine practice reduced ICU CLABSIs at a level commensurate with that achieved within the framework of a clinical trial. This supports the use of universal decolonization in a wide variety of acute care facilities across the United States,” the investigators wrote.
Find the full study in Clinical Infectious Diseases (doi: 10.1093/cid/ciw282).
Universal decolonization of adult intensive care unit patients in a large community health system significantly reduced the incidence of central line–associated bloodstream infections (CLABSIs), according to Dr. Edward Septimus and his associates.
A total of 136 ICUs at 95 hospitals were included in the study. Universal decolonization was completed within 6 months. A total of 672 CLABSIs occurred in the 24 months before intervention, and 181 CLABSIs occurred in the 8 months after intervention. The CLABSI rate was 1.1/1,000 central-line days before intervention and 0.87 after intervention, a reduction of 23.5%.
No evidence of a trend over time was found in either the pre- or postintervention period. The reduction in CLABSIs post intervention was not affected after adjustment for seasonality, number of beds in the ICU, or unit type. The gram-positive CLABSI rate was reduced by 28.7% after invention, and the rate of CLABSI due to Staphylococcus aureus was reduced by 31.9%.
“This rapid dissemination and implementation program demonstrated the utility of a protocol-specific toolkit, coupled with a multistep translation program to implement universal decolonization in a large, complex organization. Doing so demonstrated that use of CHG [chlorhexidine] bathing plus nasal mupirocin in routine practice reduced ICU CLABSIs at a level commensurate with that achieved within the framework of a clinical trial. This supports the use of universal decolonization in a wide variety of acute care facilities across the United States,” the investigators wrote.
Find the full study in Clinical Infectious Diseases (doi: 10.1093/cid/ciw282).
Universal decolonization of adult intensive care unit patients in a large community health system significantly reduced the incidence of central line–associated bloodstream infections (CLABSIs), according to Dr. Edward Septimus and his associates.
A total of 136 ICUs at 95 hospitals were included in the study. Universal decolonization was completed within 6 months. A total of 672 CLABSIs occurred in the 24 months before intervention, and 181 CLABSIs occurred in the 8 months after intervention. The CLABSI rate was 1.1/1,000 central-line days before intervention and 0.87 after intervention, a reduction of 23.5%.
No evidence of a trend over time was found in either the pre- or postintervention period. The reduction in CLABSIs post intervention was not affected after adjustment for seasonality, number of beds in the ICU, or unit type. The gram-positive CLABSI rate was reduced by 28.7% after invention, and the rate of CLABSI due to Staphylococcus aureus was reduced by 31.9%.
“This rapid dissemination and implementation program demonstrated the utility of a protocol-specific toolkit, coupled with a multistep translation program to implement universal decolonization in a large, complex organization. Doing so demonstrated that use of CHG [chlorhexidine] bathing plus nasal mupirocin in routine practice reduced ICU CLABSIs at a level commensurate with that achieved within the framework of a clinical trial. This supports the use of universal decolonization in a wide variety of acute care facilities across the United States,” the investigators wrote.
Find the full study in Clinical Infectious Diseases (doi: 10.1093/cid/ciw282).
FROM CLINICAL INFECTIOUS DISEASES
The times I dig out my thank you note box
I get the occasional heartfelt thank you note from a patient. I also get hate mail, but fortunately the thank yous predominate.
I still have all of them, going back to residency, in an old Nike box. They sit in a closet at home, taken out here and there – on bad days.
You know what I mean. The days where you screwed up, or had an angry patient get on your nerves and/or in your face. Where the schedule was accidentally double booked and you were running behind from the start. When you question your abilities and wonder why you still do this to yourself.
At the end of those days, I go home, dig out the box, and quietly read a few of the notes. Their neatly folded pages of gratitude remind me why I’m here, why I chose this path, why I need to be clear and ready for the patients depending on me the next day. They help me realize that there’s more good than bad in this job; that an unhappy, albeit vocal, few don’t represent most patients; and that I really do know what I’m doing, regardless of what Mr. I’m-going-to-complain-about-you-on-Yelp says.
Of course, there are other reminders of what you have to be thankful for, like families and dogs. But sometimes you need a reminder directly from the people you make a difference for every day, to let you know that this isn’t just a job. It’s why you once volunteered at a hospital, fought through organic chemistry, wrote out 20 (or more) drafts of a personal statement and studied for the MCAT. Because, once upon a time, this job was just a dream.
I don’t spend a lot of time with the notes – maybe 10 minutes reading a randomly pulled handful – but it’s enough to get me out of a funk. Then the old shoe box is carefully returned to my closet. Until I need it again.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I get the occasional heartfelt thank you note from a patient. I also get hate mail, but fortunately the thank yous predominate.
I still have all of them, going back to residency, in an old Nike box. They sit in a closet at home, taken out here and there – on bad days.
You know what I mean. The days where you screwed up, or had an angry patient get on your nerves and/or in your face. Where the schedule was accidentally double booked and you were running behind from the start. When you question your abilities and wonder why you still do this to yourself.
At the end of those days, I go home, dig out the box, and quietly read a few of the notes. Their neatly folded pages of gratitude remind me why I’m here, why I chose this path, why I need to be clear and ready for the patients depending on me the next day. They help me realize that there’s more good than bad in this job; that an unhappy, albeit vocal, few don’t represent most patients; and that I really do know what I’m doing, regardless of what Mr. I’m-going-to-complain-about-you-on-Yelp says.
Of course, there are other reminders of what you have to be thankful for, like families and dogs. But sometimes you need a reminder directly from the people you make a difference for every day, to let you know that this isn’t just a job. It’s why you once volunteered at a hospital, fought through organic chemistry, wrote out 20 (or more) drafts of a personal statement and studied for the MCAT. Because, once upon a time, this job was just a dream.
I don’t spend a lot of time with the notes – maybe 10 minutes reading a randomly pulled handful – but it’s enough to get me out of a funk. Then the old shoe box is carefully returned to my closet. Until I need it again.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I get the occasional heartfelt thank you note from a patient. I also get hate mail, but fortunately the thank yous predominate.
I still have all of them, going back to residency, in an old Nike box. They sit in a closet at home, taken out here and there – on bad days.
You know what I mean. The days where you screwed up, or had an angry patient get on your nerves and/or in your face. Where the schedule was accidentally double booked and you were running behind from the start. When you question your abilities and wonder why you still do this to yourself.
At the end of those days, I go home, dig out the box, and quietly read a few of the notes. Their neatly folded pages of gratitude remind me why I’m here, why I chose this path, why I need to be clear and ready for the patients depending on me the next day. They help me realize that there’s more good than bad in this job; that an unhappy, albeit vocal, few don’t represent most patients; and that I really do know what I’m doing, regardless of what Mr. I’m-going-to-complain-about-you-on-Yelp says.
Of course, there are other reminders of what you have to be thankful for, like families and dogs. But sometimes you need a reminder directly from the people you make a difference for every day, to let you know that this isn’t just a job. It’s why you once volunteered at a hospital, fought through organic chemistry, wrote out 20 (or more) drafts of a personal statement and studied for the MCAT. Because, once upon a time, this job was just a dream.
I don’t spend a lot of time with the notes – maybe 10 minutes reading a randomly pulled handful – but it’s enough to get me out of a funk. Then the old shoe box is carefully returned to my closet. Until I need it again.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
High-Value, Cost-Conscious Evaluation for PCOS: Which Tests Should Be Routinely Ordered in Acne Patients?
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8
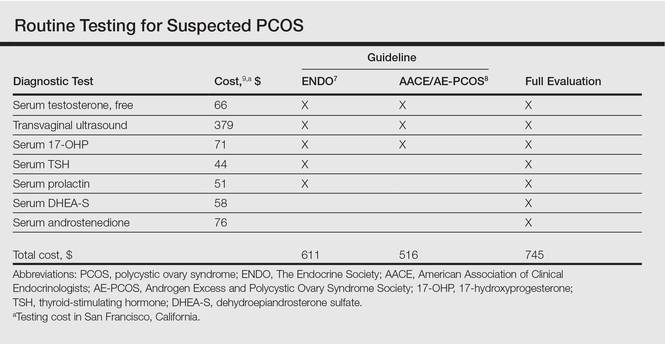
Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8

Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
The adult female patient presenting with severe acne vulgaris may raise special diagnostic concerns, including consideration of an underlying hormonal disorder. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with an estimated prevalence as high as 12%.1 Many women with undiagnosed PCOS may be referred to dermatologists for evaluation of its cutaneous manifestations of hyperandrogenism including acne, hirsutism, and androgenic alopecia.2 Given the prevalence of PCOS and its long-term health implications, dermatologists can play an important role in the initial evaluation of these patients. Acne and androgenic alopecia, however, are quite common, and in the absence of red flags such as menstrual irregularities, virilization, visual field deficits, or signs of Cushing syndrome,3 clinicians must decide when to pursue limited versus comprehensive evaluation.
Despite being common in patients with PCOS, a recent study suggests that acne is an unreliable marker of biochemical hyperandrogenism, and specific features of acne (ie, lesion counts, lesional types, distribution) cannot reliably discriminate women who meet PCOS diagnostic criteria from those who do not.4 Similarly, the study found that androgenic alopecia was not associated with biochemical hyperandrogenism and was no more common in women with PCOS than women of similar age in a high-risk population. Unlike acne and androgenic alopecia, however, the study identified hirsutism, especially truncal hirsutism, as a reliable indicator of hyperandrogenemia and PCOS. Hirsutism also is associated with metabolic sequelae of PCOS. These findings suggest that hirsutism, but not acne or androgenic alopecia, in a female of reproductive age warrants a workup for PCOS.4 This report is consistent with a recommendation from the Androgen Excess and Polycystic Ovary Syndrome Society (AE-PCOS) to pursue a diagnostic evaluation in any woman presenting with hirsutism.5 Acanthosis nigricans also was found to be a reliable indicator of hyperandrogenemia, PCOS, and associated metabolic derangement. Thus, although recent evidence indicates that acne as an isolated cutaneous finding does not warrant further diagnostic evaluation, acne in the setting of hirsutism, acanthosis nigricans, menstrual irregularities, or additional specific signs of endocrine dysregulation should prompt focused workup.4
Multiple clinical practice guidelines for the evaluation of hirsutism and PCOS based on literature review and expert opinion have been proposed5-8; however, these guidelines vary in recommendations for routine diagnostic steps to exclude mimickers of PCOS such as prolactinoma/pituitary adenoma and congenital adrenal hyperplasia (CAH)(Table). In 2009, an AE-PCOS task force suggested that routine testing of thyroid function and serum prolactin in the absence of additional clinical signs may not be necessary based on the low prevalence of thyroid disorders and hyperprolactinemia in patients presenting with hyperandrogenism.6 In 2013, the Endocrine Society’s (ENDO) clinical guideline, however, recommended routine measurement of serum thyroid-stimulating hormone (TSH) to exclude thyroid disease and serum prolactin to exclude hyperprolactinemia in all women before making a diagnosis of PCOS.7 In 2015, the AE-PCOS collaborated with the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology to publish an updated guideline for best practices, which was consistent with the prior AE-PCOS recommendation in 2009 for routine screening including to test 17-hydroxyprogesterone to exclude nonclassical CAH.8

Importantly, these recommendations for routine testing for mimickers of PCOS are based on the rare prevalence of these etiologies in multiple studies of women presenting for hyperandrogenism. One study included 873 women presenting to an academic reproductive endocrine clinic for evaluation of symptoms potentially related to androgen excess. In addition to cutaneous manifestations of hirsutism, acne, and alopecia, the study also included women presenting with oligomenorrhea/amenorrhea, ovulatory dysfunction, and even virilization.10 A second study included 950 women presenting to academic endocrine departments with hirsutism, acne, or androgenic alopecia.11 Both studies defined hirsutism as having a modified Ferriman-Gallwey score of 6 or greater. Both studies also only measured serum prolactin or TSH when clinically indicated (ie, patients with ovulatory dysfunction).10,11
The diagnostic yield of tests for mimickers of PCOS was exceedingly low in both studies. For example, of the patients evaluated, only 0.4% to 0.7% had thyroid dysfunction, 0% to 0.3% had hyperprolactinemia, 0.2% had androgen-secreting neoplasms, 2.1% to 4.3% had nonclassical CAH, 0.7% had CAH, and 3.8% had HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome.10,11 Because patients in both studies were only tested for hyperprolactinemia and thyroid dysfunction when clinically indicated, it is probable that routine screening without clinical indication would result in even lower yields.
Given the increasing importance of high-value, cost-conscious care,12 clinicians must consider the costs associated with testing in the face of low pretest probability. Although some studies have examined the cost-effectiveness of fertility treatments in PCOS,13,14 no studies have examined the cost-effectiveness of diagnostic strategies for PCOS. Cost-effectiveness studies are emerging to provide important guidance on high-value, cost-conscious diagnostic evaluation and monitoring15 and are much needed in dermatology.16,17
In the case of PCOS, the costs of some diagnostic tests are relatively low. For example, based on estimates from Healthcare Bluebook,9 serum TSH and prolactin tests in San Francisco, California, are $44 and $51, respectively. However, the cumulative costs for even the most stringent routine workup for PCOS recommended in the AACE/AE-PCOS guideline consisting of a free testosterone measurement, 17-hydroxyprogesterone, and transvaginal ultrasound would still cost a total of $516. Additional TSH and prolactin tests recommended by ENDO would increase the cost of PCOS testing by approximately 18%. Routine testing for additional serum androgens—dehydroepiandrosterone sulfate (DHEA-S) and androstenedione—would further increase this amount by an additional $134 to a total cost of $745. The ENDO guideline only recommends DHEA-S testing to assist in the diagnosis of an androgen-secreting tumor when signs of virilization are present, while the AACE/AE-PCOS guideline discourages routine testing for DHEA-S and androstenedione based on the low frequency of cases in which these androgens are elevated in isolation.7,8
Although the selection of tests influences total cost, the setting of tests (ie, hospitals, physician offices, independent test settings) also can contribute to wide variations in cost. For example, Healthcare Bluebook’s estimates for transvaginal ultrasound in Chicago, Illinois, range from $236 to more than $740.9 When the separate physician visit fees are included, the total cost of a routine diagnostic evaluation of a patient with acne or hirsutism concerning for PCOS is not trivial.
Large national clinical registries and formal cost-effectiveness analyses are necessary to shed light on this issue, but it is clear that clinicians should rely on their clinical judgment when ordering laboratory tests in the evaluation for PCOS given the apparent low yield of routine screening for PCOS mimickers in the absence of clinical indications. For example, a TSH would not be warranted in a patient without evidence of thyroid dysfunction (ie, weight gain, fatigue, constipation, menstrual irregularities). Similarly, clinicians should routinely consider the principle of high-value care: whether the results of a test will change management of the patient. For example, a woman with amenorrhea and severe acne who already meets diagnostic criteria for PCOS would benefit from a combined oral contraceptive for both acne and endometrial protection. An ovarian ultrasound may not be needed to confirm the diagnosis unless there is suspicion for an ovarian condition other than PCOS causing the symptoms.
Finally, clinicians should discuss testing options and involve patients in decisions around testing. Although PCOS treatments generally target individual symptoms rather than the syndrome as a whole, confirmation of a PCOS diagnosis importantly informs women of their risk for cardiovascular and metabolic disease. The ENDO recommends screening for impaired glucose tolerance, type 2 diabetes mellitus, obesity, family history of early cardiovascular disease, tobacco use, hypertension, dyslipidemia, and obstructive sleep apnea in all women with PCOS, including nonobese patients.7 Ongoing efforts to gain and understand evidence to support high-value, cost-conscious care should be prioritized and kept in balance with shared decision-making in individual patients suspected of having PCOS.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544-551.
- Sivayoganathan D, Maruthini D, Glanville JM, et al. Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Hum Fertil. 2011;14:261-265.
- Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2015;152:391-398.
- Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146-170.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Legro RS, Arslanian SA, Ehermann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline [published online October 22, 2013]. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
- Healthcare Bluebook. https://healthcarebluebook.com. Accessed June 13, 2016.
- Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453-462.
- Carmina E, Rosato F, Jannì A, et al. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism [published online November 1, 2005]. J Clin Endocrinol Metab. 2006;91:2-6.
- Owens DK, Qaseem A, Chou R, et al. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174-180.
- Nahuis MJ, Oude Lohuis E, Kose N, et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Hum Reprod Oxf Engl. 2012;27:3577-3582.
- Moolenaar LM, Nahuis MJ, Hompes PG, et al. Cost-effectiveness of treatment strategies in women with PCOS who do not conceive after six cycles of clomiphene citrate. Reprod Biomed Online. 2014;28:606-613.
- Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27:E35-E38.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Shinkai K, McMichael A, Linos E. Isotretinoin laboratory test monitoring—a call to decrease testing in an era of high-value, cost-conscious care. JAMA Dermatol. 2016;152:17-19.