User login
Real-world data favor dimethyl fumarate, fingolimod for MS
VANCOUVER – Dimethyl fumarate and fingolimod appear to have an edge over other disease-modifying therapies for multiple sclerosis (MS) in real-world practice, according to a comparative effectiveness study reported at the annual meeting of the American Academy of Neurology.
Dr. Jacqueline A. Nicholas, a neuroimmunologist and MS specialist with the OhioHealth Multiple Sclerosis Center, Riverside Methodist Hospital, Columbus, and her colleagues analyzed claims data from 5,004 commercially insured adults with MS in the United States who started treatment with any of five oral and injectable disease-modifying therapies.
Findings reported at the meeting showed that dimethyl fumarate netted the greatest reduction in annualized relapse rate, at one-third, followed by fingolimod, at about one-fourth. The adjusted risk of relapse in the year after drug initiation was significantly higher for interferon-beta, glatiramer acetate, and teriflunomide, compared with dimethyl fumarate.
“Right now, a lot of the data that we have to use in the clinic is based on clinical trials data. That’s often not what we see in the real world, the MS centers, and even the outpatient neurology setting,” Dr. Nicholas said in an interview. “This study is nice just because it points out that when you look at real-world data, it shows, yes, that these drugs work, and that some of the initial benefit for the oral disease-modifying therapies is what we thought. Obviously, we don’t have cross-trial comparisons to make from the clinical trials, so this is real data that we can actually use in our clinic setting.”
The findings are also helpful given changing health care models and ongoing issues with reimbursement and obtaining insurance approval to use various drugs, she added. “These are things that we can show to those payers as to why it’s important that we have these therapies and that we be able to decide as MS specialists what’s going to be best for the patient.
“Right now, the biggest challenge in the MS world is that obviously, as an MS specialist, you have a lot of experience and knowledge. And based on poor prognostic factors, when somebody comes in, you may not want to go with an escalation model [of treatment], where you are starting with something that a payer may think we should start with, an injectable,” Dr. Nicholas added. “Somebody may have more aggressive disease, and maybe you are going to want to start with an oral or an IV drug. But the payers are the ones right now who have the say. So it’s a lot of time and a lot of work [getting insurance approval], and while you are fighting to get what you know your patient needs, your patient’s suffering, accumulating disability, and possibly having more relapses.”
For the study, the investigators analyzed administrative data from the Truven MarketScan Commercial Claims Databases for 2012 through 2014.
Analyses were based on 2,564 patients treated with dimethyl fumarate (brand name Tecfidera), 735 with interferon-beta (Rebif, Avonex, Betaseron, and Extavia), 827 with glatiramer acetate (Copaxone), 417 with teriflunomide (Aubagio), and 461 with fingolimod (Gilenya).
Comparing the year before and the year after drug initiation, only dimethyl fumarate and fingolimod were associated with significant reductions in the annualized relapse rate, according to findings reported in a poster session. The reductions were 33% and 27%, respectively.
In the postinitiation year and with dimethyl fumarate as the comparator, the adjusted incidence rate ratio for relapse was similar for fingolimod but significantly higher for glatiramer acetate (1.28), interferon-beta (1.25), and teriflunomide (1.28).
“I don’t think that these findings are surprising,” Dr. Nicholas said. “I work in a large MS center and I would say this is generally what I see clinically in terms of the effectiveness. So it’s more reassuring to me than anything.”
She acknowledged that safety and tolerability will also come into play when selecting among disease-modifying therapies. “Those data are incredibly important, and we certainly balance that. With a health care claims database, that’s hard data to pull unless you are looking at one specific [adverse effect], but that’s something that needs to be very carefully weighed with the efficacy data for the patient,” she said.
In a companion study also reported in the poster session, the investigators compared the impact of starting the same five drugs on health care costs and utilization.
Results of that study showed that total health care costs rose in the postinitiation year for all five drugs, with the increase ranging from $38,801 for dimethyl fumarate to $52,352 for fingolimod.
However, total nonprescription medical costs decreased across the board, apparently driven by both less use of outpatient services and fewer inpatient hospital stays, with the greatest reduction seen for dimethyl fumarate.
Dr. Nicholas disclosed that she has received research funding from Genzyme, Novartis, Teva, Biogen, and Alexion, and has received consulting and speaking honoraria from Genzyme, Novartis, Teva, Biogen, and Medtronic. The study was supported by Biogen.
VANCOUVER – Dimethyl fumarate and fingolimod appear to have an edge over other disease-modifying therapies for multiple sclerosis (MS) in real-world practice, according to a comparative effectiveness study reported at the annual meeting of the American Academy of Neurology.
Dr. Jacqueline A. Nicholas, a neuroimmunologist and MS specialist with the OhioHealth Multiple Sclerosis Center, Riverside Methodist Hospital, Columbus, and her colleagues analyzed claims data from 5,004 commercially insured adults with MS in the United States who started treatment with any of five oral and injectable disease-modifying therapies.
Findings reported at the meeting showed that dimethyl fumarate netted the greatest reduction in annualized relapse rate, at one-third, followed by fingolimod, at about one-fourth. The adjusted risk of relapse in the year after drug initiation was significantly higher for interferon-beta, glatiramer acetate, and teriflunomide, compared with dimethyl fumarate.
“Right now, a lot of the data that we have to use in the clinic is based on clinical trials data. That’s often not what we see in the real world, the MS centers, and even the outpatient neurology setting,” Dr. Nicholas said in an interview. “This study is nice just because it points out that when you look at real-world data, it shows, yes, that these drugs work, and that some of the initial benefit for the oral disease-modifying therapies is what we thought. Obviously, we don’t have cross-trial comparisons to make from the clinical trials, so this is real data that we can actually use in our clinic setting.”
The findings are also helpful given changing health care models and ongoing issues with reimbursement and obtaining insurance approval to use various drugs, she added. “These are things that we can show to those payers as to why it’s important that we have these therapies and that we be able to decide as MS specialists what’s going to be best for the patient.
“Right now, the biggest challenge in the MS world is that obviously, as an MS specialist, you have a lot of experience and knowledge. And based on poor prognostic factors, when somebody comes in, you may not want to go with an escalation model [of treatment], where you are starting with something that a payer may think we should start with, an injectable,” Dr. Nicholas added. “Somebody may have more aggressive disease, and maybe you are going to want to start with an oral or an IV drug. But the payers are the ones right now who have the say. So it’s a lot of time and a lot of work [getting insurance approval], and while you are fighting to get what you know your patient needs, your patient’s suffering, accumulating disability, and possibly having more relapses.”
For the study, the investigators analyzed administrative data from the Truven MarketScan Commercial Claims Databases for 2012 through 2014.
Analyses were based on 2,564 patients treated with dimethyl fumarate (brand name Tecfidera), 735 with interferon-beta (Rebif, Avonex, Betaseron, and Extavia), 827 with glatiramer acetate (Copaxone), 417 with teriflunomide (Aubagio), and 461 with fingolimod (Gilenya).
Comparing the year before and the year after drug initiation, only dimethyl fumarate and fingolimod were associated with significant reductions in the annualized relapse rate, according to findings reported in a poster session. The reductions were 33% and 27%, respectively.
In the postinitiation year and with dimethyl fumarate as the comparator, the adjusted incidence rate ratio for relapse was similar for fingolimod but significantly higher for glatiramer acetate (1.28), interferon-beta (1.25), and teriflunomide (1.28).
“I don’t think that these findings are surprising,” Dr. Nicholas said. “I work in a large MS center and I would say this is generally what I see clinically in terms of the effectiveness. So it’s more reassuring to me than anything.”
She acknowledged that safety and tolerability will also come into play when selecting among disease-modifying therapies. “Those data are incredibly important, and we certainly balance that. With a health care claims database, that’s hard data to pull unless you are looking at one specific [adverse effect], but that’s something that needs to be very carefully weighed with the efficacy data for the patient,” she said.
In a companion study also reported in the poster session, the investigators compared the impact of starting the same five drugs on health care costs and utilization.
Results of that study showed that total health care costs rose in the postinitiation year for all five drugs, with the increase ranging from $38,801 for dimethyl fumarate to $52,352 for fingolimod.
However, total nonprescription medical costs decreased across the board, apparently driven by both less use of outpatient services and fewer inpatient hospital stays, with the greatest reduction seen for dimethyl fumarate.
Dr. Nicholas disclosed that she has received research funding from Genzyme, Novartis, Teva, Biogen, and Alexion, and has received consulting and speaking honoraria from Genzyme, Novartis, Teva, Biogen, and Medtronic. The study was supported by Biogen.
VANCOUVER – Dimethyl fumarate and fingolimod appear to have an edge over other disease-modifying therapies for multiple sclerosis (MS) in real-world practice, according to a comparative effectiveness study reported at the annual meeting of the American Academy of Neurology.
Dr. Jacqueline A. Nicholas, a neuroimmunologist and MS specialist with the OhioHealth Multiple Sclerosis Center, Riverside Methodist Hospital, Columbus, and her colleagues analyzed claims data from 5,004 commercially insured adults with MS in the United States who started treatment with any of five oral and injectable disease-modifying therapies.
Findings reported at the meeting showed that dimethyl fumarate netted the greatest reduction in annualized relapse rate, at one-third, followed by fingolimod, at about one-fourth. The adjusted risk of relapse in the year after drug initiation was significantly higher for interferon-beta, glatiramer acetate, and teriflunomide, compared with dimethyl fumarate.
“Right now, a lot of the data that we have to use in the clinic is based on clinical trials data. That’s often not what we see in the real world, the MS centers, and even the outpatient neurology setting,” Dr. Nicholas said in an interview. “This study is nice just because it points out that when you look at real-world data, it shows, yes, that these drugs work, and that some of the initial benefit for the oral disease-modifying therapies is what we thought. Obviously, we don’t have cross-trial comparisons to make from the clinical trials, so this is real data that we can actually use in our clinic setting.”
The findings are also helpful given changing health care models and ongoing issues with reimbursement and obtaining insurance approval to use various drugs, she added. “These are things that we can show to those payers as to why it’s important that we have these therapies and that we be able to decide as MS specialists what’s going to be best for the patient.
“Right now, the biggest challenge in the MS world is that obviously, as an MS specialist, you have a lot of experience and knowledge. And based on poor prognostic factors, when somebody comes in, you may not want to go with an escalation model [of treatment], where you are starting with something that a payer may think we should start with, an injectable,” Dr. Nicholas added. “Somebody may have more aggressive disease, and maybe you are going to want to start with an oral or an IV drug. But the payers are the ones right now who have the say. So it’s a lot of time and a lot of work [getting insurance approval], and while you are fighting to get what you know your patient needs, your patient’s suffering, accumulating disability, and possibly having more relapses.”
For the study, the investigators analyzed administrative data from the Truven MarketScan Commercial Claims Databases for 2012 through 2014.
Analyses were based on 2,564 patients treated with dimethyl fumarate (brand name Tecfidera), 735 with interferon-beta (Rebif, Avonex, Betaseron, and Extavia), 827 with glatiramer acetate (Copaxone), 417 with teriflunomide (Aubagio), and 461 with fingolimod (Gilenya).
Comparing the year before and the year after drug initiation, only dimethyl fumarate and fingolimod were associated with significant reductions in the annualized relapse rate, according to findings reported in a poster session. The reductions were 33% and 27%, respectively.
In the postinitiation year and with dimethyl fumarate as the comparator, the adjusted incidence rate ratio for relapse was similar for fingolimod but significantly higher for glatiramer acetate (1.28), interferon-beta (1.25), and teriflunomide (1.28).
“I don’t think that these findings are surprising,” Dr. Nicholas said. “I work in a large MS center and I would say this is generally what I see clinically in terms of the effectiveness. So it’s more reassuring to me than anything.”
She acknowledged that safety and tolerability will also come into play when selecting among disease-modifying therapies. “Those data are incredibly important, and we certainly balance that. With a health care claims database, that’s hard data to pull unless you are looking at one specific [adverse effect], but that’s something that needs to be very carefully weighed with the efficacy data for the patient,” she said.
In a companion study also reported in the poster session, the investigators compared the impact of starting the same five drugs on health care costs and utilization.
Results of that study showed that total health care costs rose in the postinitiation year for all five drugs, with the increase ranging from $38,801 for dimethyl fumarate to $52,352 for fingolimod.
However, total nonprescription medical costs decreased across the board, apparently driven by both less use of outpatient services and fewer inpatient hospital stays, with the greatest reduction seen for dimethyl fumarate.
Dr. Nicholas disclosed that she has received research funding from Genzyme, Novartis, Teva, Biogen, and Alexion, and has received consulting and speaking honoraria from Genzyme, Novartis, Teva, Biogen, and Medtronic. The study was supported by Biogen.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: In real-world care, dimethyl fumarate and fingolimod appear more effective than other disease-modifying therapies for MS.
Major finding: Dimethyl fumarate and fingolimod were associated 33% and 27% reductions, respectively, in the annualized relapse rate in the year after initiation of therapy.
Data source: A retrospective cohort study of 5,004 patients with MS treated with five oral or injectable disease-modifying therapies in routine clinical care.
Disclosures: Dr. Nicholas disclosed that she has received research funding from Genzyme; Novartis, the maker of fingolimod (Gilenya); Teva; Biogen, the maker of dimethyl fumarate (Tecfidera); and Alexion. She has received consulting and speaking honoraria from Genzyme, Novartis, Teva, Biogen, and Medtronic. The study was supported by Biogen.
TNF blocker safety may differ in RA and psoriasis patients
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: TNF-antagonists provoke different adverse events in patients with RA than in those with psoriasis, and safety data should be extrapolated with caution.
Major finding: The risk of serious adverse events associated with anti-TNF therapy was approximately twice as high in RA patients as in psoriasis patients (hazard ratio, 0.54).
Data source: A pair of prospective studies including 4,117 adults with RA or psoriasis who received anti-TNF agents.
Disclosures: The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
By Sharing Painkillers, Friends And Family Members Can Fuel Opioid Epidemic: Study
As lawmakers grapple with how best to combat the nation’s prescription painkiller abuse crisis, a recent survey is shedding light on how patients who get these medications — drugs such as OxyContin, methadone or Vicodin — sometimes share or mishandle them.
According to findings detailed in a research letter published Monday in JAMA Internal Medicine, about one in five people who were prescribed the highly addictive drugs reported having shared their meds with a friend, often to help the other person manage pain. Most people with a prescription either had or expected to have extra pills left after finishing treatment. And almost 50 percent didn’t know how to safely get rid of the drugs left over after their treatment was complete, or how to store them while going through treatment.
The study’s authors suggested that the results point to changes doctors could make in prescribing practices and counseling to help alleviate the problems.
“We’ve all been saying leftover medications are an issue,” said Wilson Compton, deputy director of the federal National Institute on Drug Abuse, who wasn’t involved with the study. “Now I have a number that is concerning.”
The survey was sent to a random sample of almost 5,000 people in 2015. Of the recipients, about 1,000 had used prescription painkillers in the past year. Almost all of the people in this group responded to the survey.
Public concerns about painkiller abuse are growing louder. About 2 million people were addicted to prescription opioids in 2014, the most recent year for which data is available, according to the Centers for Disease Control and Prevention. Overdoses kill 44 people per day, the U.S. Department of Health and Human Services estimates. Researchers say deaths in 2014 were almost four times as common as they were in 2000.
“There’s a growing awareness among medical advisers, policymakers and even members of the general public that these are medications that can do serious harm,” said Colleen Barry, one of the study’s authors. She is a professor of health policy at Johns Hopkins University and co-director of the university’s Center for Mental Health and Addiction Policy Research.
And it is not news that most people who use prescription painkillers for nonmedical reasons often get them through social channels rather than a physician. In 2013 — the most recent year for which this data is available — the National Survey on Drug Use and Health estimated that number to be more than 80 percent.
But this paper’s findings illustrate some of the forces behind drug-sharing, Barry said, and in turn indicate how to stop it. For instance, the authors recommend that doctors prescribe smaller amounts of drugs, to minimize leftovers that could be shared or stolen. That tracks with new opioid prescribing guidelines issued by the Centers for Disease Control and Prevention.
“We probably prescribe a little bit more than we need to, and it’s not like people throw these away afterward. The leftovers are something we’re not thinking about,” said Jonathan Chen, an instructor at Stanford University School of Medicine, who has researched opioid abuse. Chen, who was not involved in the study, is also a practicing physician.
Meanwhile, it’s still tough for people to get rid of the drugs when they finish with them, and few say they know about safe storage practices. That’s another avenue for prevention.
Most respondents, for instance, didn’t lock up the pills when storing them. That makes it easier for someone else to take them.
And the prevalence of sharing medications suggests consumers need to be better educated about how addictive prescription opioids are, Barry said.
Doctors, added NIDA’s Compton, also need to understand the risk that, when they prescribe pills, they could end up used by someone else.
“One out of five people that I write a prescription to for opioids may share those with someone else. That’s a lot of people,” he said.
Physicians, meanwhile, haven’t historically been trained to counsel patients on safe drug disposal, meaning patients are often left unaware. Just under a quarter of respondents reported they remembered learning from the doctor or nurse about how to get rid of their meds safely. Chen said he couldn’t recall ever going over disposal practices with a patient. Even if he did, he said, it’s hard to know if patients would remember that information.
And when they are informed, it’s still difficult for consumers to easily get rid of pills they no longer need. The federal Drug Enforcement Administration sponsors “drug take-back days” twice a year. Some local law enforcement agencies hold similar events. But such events are often sporadic enough that it’s hard to make them a real habit, Barry noted.
Making those practices easier is essential, Barry said. And changing the culture around those drugs is key, so people understand the risk.
“Just the realization on the part of the public as well as physicians that these medications are not like Tylenol — these are highly addictive meds,” she said. “That message is starting to get out there.”
This story was produced by Kaiser Health News, which publishes California Healthline, a service of the California Health Care Foundation.
As lawmakers grapple with how best to combat the nation’s prescription painkiller abuse crisis, a recent survey is shedding light on how patients who get these medications — drugs such as OxyContin, methadone or Vicodin — sometimes share or mishandle them.
According to findings detailed in a research letter published Monday in JAMA Internal Medicine, about one in five people who were prescribed the highly addictive drugs reported having shared their meds with a friend, often to help the other person manage pain. Most people with a prescription either had or expected to have extra pills left after finishing treatment. And almost 50 percent didn’t know how to safely get rid of the drugs left over after their treatment was complete, or how to store them while going through treatment.
The study’s authors suggested that the results point to changes doctors could make in prescribing practices and counseling to help alleviate the problems.
“We’ve all been saying leftover medications are an issue,” said Wilson Compton, deputy director of the federal National Institute on Drug Abuse, who wasn’t involved with the study. “Now I have a number that is concerning.”
The survey was sent to a random sample of almost 5,000 people in 2015. Of the recipients, about 1,000 had used prescription painkillers in the past year. Almost all of the people in this group responded to the survey.
Public concerns about painkiller abuse are growing louder. About 2 million people were addicted to prescription opioids in 2014, the most recent year for which data is available, according to the Centers for Disease Control and Prevention. Overdoses kill 44 people per day, the U.S. Department of Health and Human Services estimates. Researchers say deaths in 2014 were almost four times as common as they were in 2000.
“There’s a growing awareness among medical advisers, policymakers and even members of the general public that these are medications that can do serious harm,” said Colleen Barry, one of the study’s authors. She is a professor of health policy at Johns Hopkins University and co-director of the university’s Center for Mental Health and Addiction Policy Research.
And it is not news that most people who use prescription painkillers for nonmedical reasons often get them through social channels rather than a physician. In 2013 — the most recent year for which this data is available — the National Survey on Drug Use and Health estimated that number to be more than 80 percent.
But this paper’s findings illustrate some of the forces behind drug-sharing, Barry said, and in turn indicate how to stop it. For instance, the authors recommend that doctors prescribe smaller amounts of drugs, to minimize leftovers that could be shared or stolen. That tracks with new opioid prescribing guidelines issued by the Centers for Disease Control and Prevention.
“We probably prescribe a little bit more than we need to, and it’s not like people throw these away afterward. The leftovers are something we’re not thinking about,” said Jonathan Chen, an instructor at Stanford University School of Medicine, who has researched opioid abuse. Chen, who was not involved in the study, is also a practicing physician.
Meanwhile, it’s still tough for people to get rid of the drugs when they finish with them, and few say they know about safe storage practices. That’s another avenue for prevention.
Most respondents, for instance, didn’t lock up the pills when storing them. That makes it easier for someone else to take them.
And the prevalence of sharing medications suggests consumers need to be better educated about how addictive prescription opioids are, Barry said.
Doctors, added NIDA’s Compton, also need to understand the risk that, when they prescribe pills, they could end up used by someone else.
“One out of five people that I write a prescription to for opioids may share those with someone else. That’s a lot of people,” he said.
Physicians, meanwhile, haven’t historically been trained to counsel patients on safe drug disposal, meaning patients are often left unaware. Just under a quarter of respondents reported they remembered learning from the doctor or nurse about how to get rid of their meds safely. Chen said he couldn’t recall ever going over disposal practices with a patient. Even if he did, he said, it’s hard to know if patients would remember that information.
And when they are informed, it’s still difficult for consumers to easily get rid of pills they no longer need. The federal Drug Enforcement Administration sponsors “drug take-back days” twice a year. Some local law enforcement agencies hold similar events. But such events are often sporadic enough that it’s hard to make them a real habit, Barry noted.
Making those practices easier is essential, Barry said. And changing the culture around those drugs is key, so people understand the risk.
“Just the realization on the part of the public as well as physicians that these medications are not like Tylenol — these are highly addictive meds,” she said. “That message is starting to get out there.”
This story was produced by Kaiser Health News, which publishes California Healthline, a service of the California Health Care Foundation.
As lawmakers grapple with how best to combat the nation’s prescription painkiller abuse crisis, a recent survey is shedding light on how patients who get these medications — drugs such as OxyContin, methadone or Vicodin — sometimes share or mishandle them.
According to findings detailed in a research letter published Monday in JAMA Internal Medicine, about one in five people who were prescribed the highly addictive drugs reported having shared their meds with a friend, often to help the other person manage pain. Most people with a prescription either had or expected to have extra pills left after finishing treatment. And almost 50 percent didn’t know how to safely get rid of the drugs left over after their treatment was complete, or how to store them while going through treatment.
The study’s authors suggested that the results point to changes doctors could make in prescribing practices and counseling to help alleviate the problems.
“We’ve all been saying leftover medications are an issue,” said Wilson Compton, deputy director of the federal National Institute on Drug Abuse, who wasn’t involved with the study. “Now I have a number that is concerning.”
The survey was sent to a random sample of almost 5,000 people in 2015. Of the recipients, about 1,000 had used prescription painkillers in the past year. Almost all of the people in this group responded to the survey.
Public concerns about painkiller abuse are growing louder. About 2 million people were addicted to prescription opioids in 2014, the most recent year for which data is available, according to the Centers for Disease Control and Prevention. Overdoses kill 44 people per day, the U.S. Department of Health and Human Services estimates. Researchers say deaths in 2014 were almost four times as common as they were in 2000.
“There’s a growing awareness among medical advisers, policymakers and even members of the general public that these are medications that can do serious harm,” said Colleen Barry, one of the study’s authors. She is a professor of health policy at Johns Hopkins University and co-director of the university’s Center for Mental Health and Addiction Policy Research.
And it is not news that most people who use prescription painkillers for nonmedical reasons often get them through social channels rather than a physician. In 2013 — the most recent year for which this data is available — the National Survey on Drug Use and Health estimated that number to be more than 80 percent.
But this paper’s findings illustrate some of the forces behind drug-sharing, Barry said, and in turn indicate how to stop it. For instance, the authors recommend that doctors prescribe smaller amounts of drugs, to minimize leftovers that could be shared or stolen. That tracks with new opioid prescribing guidelines issued by the Centers for Disease Control and Prevention.
“We probably prescribe a little bit more than we need to, and it’s not like people throw these away afterward. The leftovers are something we’re not thinking about,” said Jonathan Chen, an instructor at Stanford University School of Medicine, who has researched opioid abuse. Chen, who was not involved in the study, is also a practicing physician.
Meanwhile, it’s still tough for people to get rid of the drugs when they finish with them, and few say they know about safe storage practices. That’s another avenue for prevention.
Most respondents, for instance, didn’t lock up the pills when storing them. That makes it easier for someone else to take them.
And the prevalence of sharing medications suggests consumers need to be better educated about how addictive prescription opioids are, Barry said.
Doctors, added NIDA’s Compton, also need to understand the risk that, when they prescribe pills, they could end up used by someone else.
“One out of five people that I write a prescription to for opioids may share those with someone else. That’s a lot of people,” he said.
Physicians, meanwhile, haven’t historically been trained to counsel patients on safe drug disposal, meaning patients are often left unaware. Just under a quarter of respondents reported they remembered learning from the doctor or nurse about how to get rid of their meds safely. Chen said he couldn’t recall ever going over disposal practices with a patient. Even if he did, he said, it’s hard to know if patients would remember that information.
And when they are informed, it’s still difficult for consumers to easily get rid of pills they no longer need. The federal Drug Enforcement Administration sponsors “drug take-back days” twice a year. Some local law enforcement agencies hold similar events. But such events are often sporadic enough that it’s hard to make them a real habit, Barry noted.
Making those practices easier is essential, Barry said. And changing the culture around those drugs is key, so people understand the risk.
“Just the realization on the part of the public as well as physicians that these medications are not like Tylenol — these are highly addictive meds,” she said. “That message is starting to get out there.”
This story was produced by Kaiser Health News, which publishes California Healthline, a service of the California Health Care Foundation.
Olaparib benefit maintained long term
CHICAGO – Long-term maintenance monotherapy with olaparib following a response to platinum therapy in patients with recurrent high-grade serous ovarian cancer was associated with continued benefit vs. placebo in an updated analysis of the randomized phase II Study 19.
The new survival analysis supports prior Study 19 data showing a significant progression-free survival (PFS) advantage and a delay in time to first and second subsequent therapy in the 136 patients in the study with a BRCA 1/2 mutation who were treated with the approved PARP inhibitor, Dr. Jonathan A. Ledermann reported at the annual meeting of the American Society of Clinical Oncology.
The PFS in the overall study population of 265 patients in that prior analysis was 8.4 months in the olaparib group vs. 4.8 months in the placebo group (hazard ratio, 0.35). The PFS in the BRCA mutation subpopulation was 11.2 and 4.3 months in the groups, respectively (HR, 0.18), reported Dr. Ledermann of University College London.
“Time to first subsequent therapy or death was significantly improved with olaparib. This represents the time that women are free from the next line of chemotherapy,” he said, adding that time to second subsequent therapy or death was also significantly improved with olaparib.
“[These measures] can demonstrate the benefit beyond the next line of chemotherapy, and also help to address the confounding impact of crossover that occurs in many trials,” he explained.
Neither of two prior data analyses, the first with data maturity of 38% and the second with maturity of 58%, showed an improvement in overall survival in Study 19 participants.
In the current analysis, with a data cut-off of Sept. 31, 2015 (an additional 3 years of follow-up since the last analysis), and data maturity of 77%, overall survival was 29.8 and 27.8 months in 136 treatment group patients and 129 placebo group patients, respectively (HR, 0.73). In the BRCA mutation subgroup, the median overall survival was 34.9 months with treatment, vs. 30.2 months with placebo (HR, 0.62). For 118 patients with BRCA wild type, the hazard ratio was 0.83.
The differences did not meet the criteria for statistical significance, as the study was not powered to show a difference in overall survival, but a restricted means analysis to compare mean survival – a “useful additional way of looking at the data, rather than the point-estimated median PFS, particularly as the data mature” – showed a mean overall survival of 40.1 and 34.9 months in the olaparib and placebo patients, respectively (difference of 5.2 months), and 44.3 and 36.9 months in the BRCA mutation subgroup (difference of 7.4 months).
As for the median time to first subsequent therapy in BRCA mutation patients in the current analysis, the benefit of olaparib is maintained, with a 9.4-month difference between the treatment and placebo groups (15.6 vs. 6.2), and this was highly statistically significant (HR, 0.32; data maturity, 82%). For wild-type patients, the difference in the median was 6 months (12.9 vs. 6.9 months; HR, 0.45; maturity, 91%).
The same was true for the time to second subsequent treatment in BRCA mutation patients (22 vs. 15.3 months; HR, 0.41; data maturity, 79%), he said, noting that 23% of placebo patients crossed over to a PARP inhibitor at some point in their treatment.
A separation between the groups was also seen in BRCA wild-type patients (median of 17 vs. 14.7 months; HR, 0.63; data maturity, 90%), “perhaps maintained by those patients taking the drug for a long time,” Dr. Ledermann said.
At a median follow-up of 5.9 years, 15 patients were still receiving olaparib (11%), including 8 BRCA mutation patients and 7 BRCA wild-type patients. One patient was still receiving placebo.
“So 13% were on the drug for at least 5 years, 15% in the BRCA mutation subgroup were on it for at least 5 years, and 12% of patients with the BRCA wild type were on the drug for at least 5 years,” Dr. Ledermann said, noting that this represents “unprecedented long-term exposure to olaparib.”
Olaparib is a potent oral PARP inhibitor that traps PARP at sites of DNA damage, which blocks base-excision repair and leads to the collapse of DNA replication forks and the accumulation of DNA double-strand breaks, Dr. Ledermann explained, noting that the agent induces synthetic lethality in tumors with deficient hemologous recombination repair, which is most often seen with BRCA 1/2 mutations.
In Study 19, patients received 400 mg of olaparib twice daily or placebo after response to platinum-based therapy. BRCA mutation status was known for 254/265 patients (96%) from germline or tumor tests.
No new safety signals were observed with the longer follow-up, and the frequency of common adverse events, including nausea, fatigue, vomiting, and anemia was consistent with that seen in the overall population, with most adverse events initially reported during the first 2 years, he said.
The greatest benefits, in terms of overall survival and time to first and second subsequent treatment, were seen in patients with BRCA mutation, he concluded.
This study was sponsored by AstraZeneca. Dr. Ledermann reported that he has participated in advisory boards and lecture symposia and received institutional and personal fees from AstraZeneca, personal fees from Roche and Pfizer, and institutional fees from Clovis Oncology and Merck.
CHICAGO – Long-term maintenance monotherapy with olaparib following a response to platinum therapy in patients with recurrent high-grade serous ovarian cancer was associated with continued benefit vs. placebo in an updated analysis of the randomized phase II Study 19.
The new survival analysis supports prior Study 19 data showing a significant progression-free survival (PFS) advantage and a delay in time to first and second subsequent therapy in the 136 patients in the study with a BRCA 1/2 mutation who were treated with the approved PARP inhibitor, Dr. Jonathan A. Ledermann reported at the annual meeting of the American Society of Clinical Oncology.
The PFS in the overall study population of 265 patients in that prior analysis was 8.4 months in the olaparib group vs. 4.8 months in the placebo group (hazard ratio, 0.35). The PFS in the BRCA mutation subpopulation was 11.2 and 4.3 months in the groups, respectively (HR, 0.18), reported Dr. Ledermann of University College London.
“Time to first subsequent therapy or death was significantly improved with olaparib. This represents the time that women are free from the next line of chemotherapy,” he said, adding that time to second subsequent therapy or death was also significantly improved with olaparib.
“[These measures] can demonstrate the benefit beyond the next line of chemotherapy, and also help to address the confounding impact of crossover that occurs in many trials,” he explained.
Neither of two prior data analyses, the first with data maturity of 38% and the second with maturity of 58%, showed an improvement in overall survival in Study 19 participants.
In the current analysis, with a data cut-off of Sept. 31, 2015 (an additional 3 years of follow-up since the last analysis), and data maturity of 77%, overall survival was 29.8 and 27.8 months in 136 treatment group patients and 129 placebo group patients, respectively (HR, 0.73). In the BRCA mutation subgroup, the median overall survival was 34.9 months with treatment, vs. 30.2 months with placebo (HR, 0.62). For 118 patients with BRCA wild type, the hazard ratio was 0.83.
The differences did not meet the criteria for statistical significance, as the study was not powered to show a difference in overall survival, but a restricted means analysis to compare mean survival – a “useful additional way of looking at the data, rather than the point-estimated median PFS, particularly as the data mature” – showed a mean overall survival of 40.1 and 34.9 months in the olaparib and placebo patients, respectively (difference of 5.2 months), and 44.3 and 36.9 months in the BRCA mutation subgroup (difference of 7.4 months).
As for the median time to first subsequent therapy in BRCA mutation patients in the current analysis, the benefit of olaparib is maintained, with a 9.4-month difference between the treatment and placebo groups (15.6 vs. 6.2), and this was highly statistically significant (HR, 0.32; data maturity, 82%). For wild-type patients, the difference in the median was 6 months (12.9 vs. 6.9 months; HR, 0.45; maturity, 91%).
The same was true for the time to second subsequent treatment in BRCA mutation patients (22 vs. 15.3 months; HR, 0.41; data maturity, 79%), he said, noting that 23% of placebo patients crossed over to a PARP inhibitor at some point in their treatment.
A separation between the groups was also seen in BRCA wild-type patients (median of 17 vs. 14.7 months; HR, 0.63; data maturity, 90%), “perhaps maintained by those patients taking the drug for a long time,” Dr. Ledermann said.
At a median follow-up of 5.9 years, 15 patients were still receiving olaparib (11%), including 8 BRCA mutation patients and 7 BRCA wild-type patients. One patient was still receiving placebo.
“So 13% were on the drug for at least 5 years, 15% in the BRCA mutation subgroup were on it for at least 5 years, and 12% of patients with the BRCA wild type were on the drug for at least 5 years,” Dr. Ledermann said, noting that this represents “unprecedented long-term exposure to olaparib.”
Olaparib is a potent oral PARP inhibitor that traps PARP at sites of DNA damage, which blocks base-excision repair and leads to the collapse of DNA replication forks and the accumulation of DNA double-strand breaks, Dr. Ledermann explained, noting that the agent induces synthetic lethality in tumors with deficient hemologous recombination repair, which is most often seen with BRCA 1/2 mutations.
In Study 19, patients received 400 mg of olaparib twice daily or placebo after response to platinum-based therapy. BRCA mutation status was known for 254/265 patients (96%) from germline or tumor tests.
No new safety signals were observed with the longer follow-up, and the frequency of common adverse events, including nausea, fatigue, vomiting, and anemia was consistent with that seen in the overall population, with most adverse events initially reported during the first 2 years, he said.
The greatest benefits, in terms of overall survival and time to first and second subsequent treatment, were seen in patients with BRCA mutation, he concluded.
This study was sponsored by AstraZeneca. Dr. Ledermann reported that he has participated in advisory boards and lecture symposia and received institutional and personal fees from AstraZeneca, personal fees from Roche and Pfizer, and institutional fees from Clovis Oncology and Merck.
CHICAGO – Long-term maintenance monotherapy with olaparib following a response to platinum therapy in patients with recurrent high-grade serous ovarian cancer was associated with continued benefit vs. placebo in an updated analysis of the randomized phase II Study 19.
The new survival analysis supports prior Study 19 data showing a significant progression-free survival (PFS) advantage and a delay in time to first and second subsequent therapy in the 136 patients in the study with a BRCA 1/2 mutation who were treated with the approved PARP inhibitor, Dr. Jonathan A. Ledermann reported at the annual meeting of the American Society of Clinical Oncology.
The PFS in the overall study population of 265 patients in that prior analysis was 8.4 months in the olaparib group vs. 4.8 months in the placebo group (hazard ratio, 0.35). The PFS in the BRCA mutation subpopulation was 11.2 and 4.3 months in the groups, respectively (HR, 0.18), reported Dr. Ledermann of University College London.
“Time to first subsequent therapy or death was significantly improved with olaparib. This represents the time that women are free from the next line of chemotherapy,” he said, adding that time to second subsequent therapy or death was also significantly improved with olaparib.
“[These measures] can demonstrate the benefit beyond the next line of chemotherapy, and also help to address the confounding impact of crossover that occurs in many trials,” he explained.
Neither of two prior data analyses, the first with data maturity of 38% and the second with maturity of 58%, showed an improvement in overall survival in Study 19 participants.
In the current analysis, with a data cut-off of Sept. 31, 2015 (an additional 3 years of follow-up since the last analysis), and data maturity of 77%, overall survival was 29.8 and 27.8 months in 136 treatment group patients and 129 placebo group patients, respectively (HR, 0.73). In the BRCA mutation subgroup, the median overall survival was 34.9 months with treatment, vs. 30.2 months with placebo (HR, 0.62). For 118 patients with BRCA wild type, the hazard ratio was 0.83.
The differences did not meet the criteria for statistical significance, as the study was not powered to show a difference in overall survival, but a restricted means analysis to compare mean survival – a “useful additional way of looking at the data, rather than the point-estimated median PFS, particularly as the data mature” – showed a mean overall survival of 40.1 and 34.9 months in the olaparib and placebo patients, respectively (difference of 5.2 months), and 44.3 and 36.9 months in the BRCA mutation subgroup (difference of 7.4 months).
As for the median time to first subsequent therapy in BRCA mutation patients in the current analysis, the benefit of olaparib is maintained, with a 9.4-month difference between the treatment and placebo groups (15.6 vs. 6.2), and this was highly statistically significant (HR, 0.32; data maturity, 82%). For wild-type patients, the difference in the median was 6 months (12.9 vs. 6.9 months; HR, 0.45; maturity, 91%).
The same was true for the time to second subsequent treatment in BRCA mutation patients (22 vs. 15.3 months; HR, 0.41; data maturity, 79%), he said, noting that 23% of placebo patients crossed over to a PARP inhibitor at some point in their treatment.
A separation between the groups was also seen in BRCA wild-type patients (median of 17 vs. 14.7 months; HR, 0.63; data maturity, 90%), “perhaps maintained by those patients taking the drug for a long time,” Dr. Ledermann said.
At a median follow-up of 5.9 years, 15 patients were still receiving olaparib (11%), including 8 BRCA mutation patients and 7 BRCA wild-type patients. One patient was still receiving placebo.
“So 13% were on the drug for at least 5 years, 15% in the BRCA mutation subgroup were on it for at least 5 years, and 12% of patients with the BRCA wild type were on the drug for at least 5 years,” Dr. Ledermann said, noting that this represents “unprecedented long-term exposure to olaparib.”
Olaparib is a potent oral PARP inhibitor that traps PARP at sites of DNA damage, which blocks base-excision repair and leads to the collapse of DNA replication forks and the accumulation of DNA double-strand breaks, Dr. Ledermann explained, noting that the agent induces synthetic lethality in tumors with deficient hemologous recombination repair, which is most often seen with BRCA 1/2 mutations.
In Study 19, patients received 400 mg of olaparib twice daily or placebo after response to platinum-based therapy. BRCA mutation status was known for 254/265 patients (96%) from germline or tumor tests.
No new safety signals were observed with the longer follow-up, and the frequency of common adverse events, including nausea, fatigue, vomiting, and anemia was consistent with that seen in the overall population, with most adverse events initially reported during the first 2 years, he said.
The greatest benefits, in terms of overall survival and time to first and second subsequent treatment, were seen in patients with BRCA mutation, he concluded.
This study was sponsored by AstraZeneca. Dr. Ledermann reported that he has participated in advisory boards and lecture symposia and received institutional and personal fees from AstraZeneca, personal fees from Roche and Pfizer, and institutional fees from Clovis Oncology and Merck.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Long-term maintenance monotherapy with olaparib following a response to platinum therapy in patients with recurrent high-grade serous ovarian cancer was associated with a continued advantage vs. placebo in an updated analysis of Study 19 data.
Major finding: A restricted means analysis showed a mean survival of 44.3 and 36.9 months with olaparib vs. placebo in the BRCA mutation subgroup (difference of 7.4 months).
Data source: A randomized phase II study of 265 patients.
Disclosures: This study was sponsored by AstraZeneca. Dr. Ledermann reported that he has participated in advisory boards and lecture symposia and received institutional and personal fees from AstraZeneca, personal fees from Roche and Pfizer, and institutional fees from Clovis Oncology and Merck.
Nonbenzodiazepines reduce time to extubation, compared with benzodiazepines
The nonbenzodiazepines propofol and dexmedetomidine reduce the time to extubation, compared with benzodiazepines, suggest results of an observational study published in Chest.
“This study found that sedatives vary in their associations with [ventilator-associated events] and time to extubation but not in their associations with time to hospital discharge or mortality. Both propofol and dexmedetomidine were associated with less time to extubation, compared with benzodiazepines,” wrote Dr. Michael Klompas of the department of population medicine at Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston, and colleagues (Chest. 2016 Jun;149[6]:1373-9).
Current sedation guidelines for mechanical ventilation recommend using nonbenzodiazepines to lightly sedate patients, whenever possible.
Compared with the use of benzodiazepines, the uses of propofol and dexmedetomidine were associated with shorter times to extubation with hazard ratios of propofol vs. benzodiazepines and dexmedetomidine vs. benzodiazepines of 1.4 (P less than .0001) and 2.3 (P less than .0001), respectively. In the relatively few cases involving uses of dexmedetomidine that were available, this sedative was also associated with shorter time to extubation, compared with propofol (HR, 1.7; P less than .0001).
Uses of benzodiazepines and propofol were associated with increased risk for ventilator-associated events (VAEs), compared with regimens not involving them; for benzodiazepine use, the HR was 1.4 (P = .002) and for propofol, the HR was 1.3 (P = .003). Dexmedetomidine use, in contrast, was not associated with increased risk for VAEs (P = .92).
Regarding hazards for hospital discharges and hospital deaths, using each sedative or sedative class studied had similar outcomes.
The observational study involved 9,603 retrospectively identified mechanical ventilations. All consecutively occurring invasive mechanical ventilations lasting 3 days or longer in Boston’s Brigham and Women’s Hospital between July 1, 2006 and December 31, 2013 were studied. The researchers evaluated the impact that daily use of propofol, dexmedetomidine, and benzodiazepines have on VAEs, time to extubation, time to hospital discharge, and death in a large cohort of patients.
This study’s findings were similar to those of prior randomized controlled trials, especially concerning the time to extubation, the researchers said. “The large number of episodes of mechanical ventilation in our trial dataset, however, allowed us to extend conceivable but underpowered signals from randomized controlled trials.”
A limitation of this study is that it was a single-center retrospective analysis, which may have caused some of its findings to be attributable to “residual confounding and/or idiosyncratic local practice patterns.” Other limitations include the lack of measurements of patients’ total doses or adjusted doses per kilogram of body weight, a possible overtraining of the analysis model used to adjust for severity of illness, and a relatively low number of patients treated with dexmedetomidine, with most of such patients undergoing cardiac surgery.
Funding was provided by the Centers for Disease Control and Prevention. Dr. Klompas and the other researchers had no disclosures.
While sedatives are the most widely used pharmacologic compounds in the critical care of patients, data on the real-world patterns of sedative use are lacking,
Klompas et al. are to be commended for conducting an observational trial that addressed the real-world patterns of sedative use. “Their data speak to what many clinicians believe to be their clinical sedative administration experience.”
This is an important study that begins to address a basic pharmacologic issue. The researchers’ observations of the effects of the two nonbenzodiazepines (dexmedetomidine and propofol) and benzodiazepines on the patients studied will help to clarify whether such effects can be attributed to the specific drug used or the sedative effect that a drug had on a patient.
The study was limited by the relatively low number of patients who received dexmedetomidine. This limitation, which might have suggested a selection bias, made the conclusions less robust.
Dr. Yoanna Skrobik is from the faculty of medicine, department of medicine at the McGill University Health Centre, Montreal. She reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (Chest. 2016 Jun;149[6]:1355-6).
While sedatives are the most widely used pharmacologic compounds in the critical care of patients, data on the real-world patterns of sedative use are lacking,
Klompas et al. are to be commended for conducting an observational trial that addressed the real-world patterns of sedative use. “Their data speak to what many clinicians believe to be their clinical sedative administration experience.”
This is an important study that begins to address a basic pharmacologic issue. The researchers’ observations of the effects of the two nonbenzodiazepines (dexmedetomidine and propofol) and benzodiazepines on the patients studied will help to clarify whether such effects can be attributed to the specific drug used or the sedative effect that a drug had on a patient.
The study was limited by the relatively low number of patients who received dexmedetomidine. This limitation, which might have suggested a selection bias, made the conclusions less robust.
Dr. Yoanna Skrobik is from the faculty of medicine, department of medicine at the McGill University Health Centre, Montreal. She reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (Chest. 2016 Jun;149[6]:1355-6).
While sedatives are the most widely used pharmacologic compounds in the critical care of patients, data on the real-world patterns of sedative use are lacking,
Klompas et al. are to be commended for conducting an observational trial that addressed the real-world patterns of sedative use. “Their data speak to what many clinicians believe to be their clinical sedative administration experience.”
This is an important study that begins to address a basic pharmacologic issue. The researchers’ observations of the effects of the two nonbenzodiazepines (dexmedetomidine and propofol) and benzodiazepines on the patients studied will help to clarify whether such effects can be attributed to the specific drug used or the sedative effect that a drug had on a patient.
The study was limited by the relatively low number of patients who received dexmedetomidine. This limitation, which might have suggested a selection bias, made the conclusions less robust.
Dr. Yoanna Skrobik is from the faculty of medicine, department of medicine at the McGill University Health Centre, Montreal. She reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (Chest. 2016 Jun;149[6]:1355-6).
The nonbenzodiazepines propofol and dexmedetomidine reduce the time to extubation, compared with benzodiazepines, suggest results of an observational study published in Chest.
“This study found that sedatives vary in their associations with [ventilator-associated events] and time to extubation but not in their associations with time to hospital discharge or mortality. Both propofol and dexmedetomidine were associated with less time to extubation, compared with benzodiazepines,” wrote Dr. Michael Klompas of the department of population medicine at Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston, and colleagues (Chest. 2016 Jun;149[6]:1373-9).
Current sedation guidelines for mechanical ventilation recommend using nonbenzodiazepines to lightly sedate patients, whenever possible.
Compared with the use of benzodiazepines, the uses of propofol and dexmedetomidine were associated with shorter times to extubation with hazard ratios of propofol vs. benzodiazepines and dexmedetomidine vs. benzodiazepines of 1.4 (P less than .0001) and 2.3 (P less than .0001), respectively. In the relatively few cases involving uses of dexmedetomidine that were available, this sedative was also associated with shorter time to extubation, compared with propofol (HR, 1.7; P less than .0001).
Uses of benzodiazepines and propofol were associated with increased risk for ventilator-associated events (VAEs), compared with regimens not involving them; for benzodiazepine use, the HR was 1.4 (P = .002) and for propofol, the HR was 1.3 (P = .003). Dexmedetomidine use, in contrast, was not associated with increased risk for VAEs (P = .92).
Regarding hazards for hospital discharges and hospital deaths, using each sedative or sedative class studied had similar outcomes.
The observational study involved 9,603 retrospectively identified mechanical ventilations. All consecutively occurring invasive mechanical ventilations lasting 3 days or longer in Boston’s Brigham and Women’s Hospital between July 1, 2006 and December 31, 2013 were studied. The researchers evaluated the impact that daily use of propofol, dexmedetomidine, and benzodiazepines have on VAEs, time to extubation, time to hospital discharge, and death in a large cohort of patients.
This study’s findings were similar to those of prior randomized controlled trials, especially concerning the time to extubation, the researchers said. “The large number of episodes of mechanical ventilation in our trial dataset, however, allowed us to extend conceivable but underpowered signals from randomized controlled trials.”
A limitation of this study is that it was a single-center retrospective analysis, which may have caused some of its findings to be attributable to “residual confounding and/or idiosyncratic local practice patterns.” Other limitations include the lack of measurements of patients’ total doses or adjusted doses per kilogram of body weight, a possible overtraining of the analysis model used to adjust for severity of illness, and a relatively low number of patients treated with dexmedetomidine, with most of such patients undergoing cardiac surgery.
Funding was provided by the Centers for Disease Control and Prevention. Dr. Klompas and the other researchers had no disclosures.
The nonbenzodiazepines propofol and dexmedetomidine reduce the time to extubation, compared with benzodiazepines, suggest results of an observational study published in Chest.
“This study found that sedatives vary in their associations with [ventilator-associated events] and time to extubation but not in their associations with time to hospital discharge or mortality. Both propofol and dexmedetomidine were associated with less time to extubation, compared with benzodiazepines,” wrote Dr. Michael Klompas of the department of population medicine at Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston, and colleagues (Chest. 2016 Jun;149[6]:1373-9).
Current sedation guidelines for mechanical ventilation recommend using nonbenzodiazepines to lightly sedate patients, whenever possible.
Compared with the use of benzodiazepines, the uses of propofol and dexmedetomidine were associated with shorter times to extubation with hazard ratios of propofol vs. benzodiazepines and dexmedetomidine vs. benzodiazepines of 1.4 (P less than .0001) and 2.3 (P less than .0001), respectively. In the relatively few cases involving uses of dexmedetomidine that were available, this sedative was also associated with shorter time to extubation, compared with propofol (HR, 1.7; P less than .0001).
Uses of benzodiazepines and propofol were associated with increased risk for ventilator-associated events (VAEs), compared with regimens not involving them; for benzodiazepine use, the HR was 1.4 (P = .002) and for propofol, the HR was 1.3 (P = .003). Dexmedetomidine use, in contrast, was not associated with increased risk for VAEs (P = .92).
Regarding hazards for hospital discharges and hospital deaths, using each sedative or sedative class studied had similar outcomes.
The observational study involved 9,603 retrospectively identified mechanical ventilations. All consecutively occurring invasive mechanical ventilations lasting 3 days or longer in Boston’s Brigham and Women’s Hospital between July 1, 2006 and December 31, 2013 were studied. The researchers evaluated the impact that daily use of propofol, dexmedetomidine, and benzodiazepines have on VAEs, time to extubation, time to hospital discharge, and death in a large cohort of patients.
This study’s findings were similar to those of prior randomized controlled trials, especially concerning the time to extubation, the researchers said. “The large number of episodes of mechanical ventilation in our trial dataset, however, allowed us to extend conceivable but underpowered signals from randomized controlled trials.”
A limitation of this study is that it was a single-center retrospective analysis, which may have caused some of its findings to be attributable to “residual confounding and/or idiosyncratic local practice patterns.” Other limitations include the lack of measurements of patients’ total doses or adjusted doses per kilogram of body weight, a possible overtraining of the analysis model used to adjust for severity of illness, and a relatively low number of patients treated with dexmedetomidine, with most of such patients undergoing cardiac surgery.
Funding was provided by the Centers for Disease Control and Prevention. Dr. Klompas and the other researchers had no disclosures.
FROM CHEST
Key clinical point: Dexmedetomidine and propofol reduce the time to extubation, compared with benzodiazepines.
Major finding: Compared with the use of benzodiazepines, the uses of propofol and dexmedetomidine were associated with shorter times to extubation with hazard ratios of propofol vs. benzodiazepines and dexmedetomidine vs. benzodiazepines of 1.4 and 2.3, respectively.
Data source: A observational study of 9,603 consecutive episodes of mechanical ventilation lasting 3 days or longer at a large medical center.
Disclosures: Funding for the study came from the Centers for Disease Control and Prevention. Dr. Klompas and his coauthors had no disclosures.
Lichen Planus Pemphigoides Associated With Pregnancy Mimicking Pemphigoid Gestationis
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
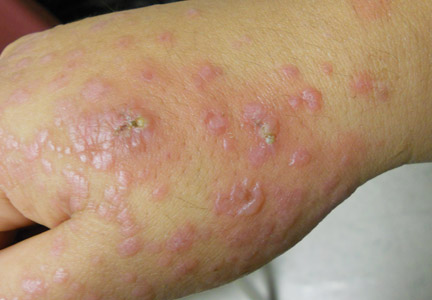
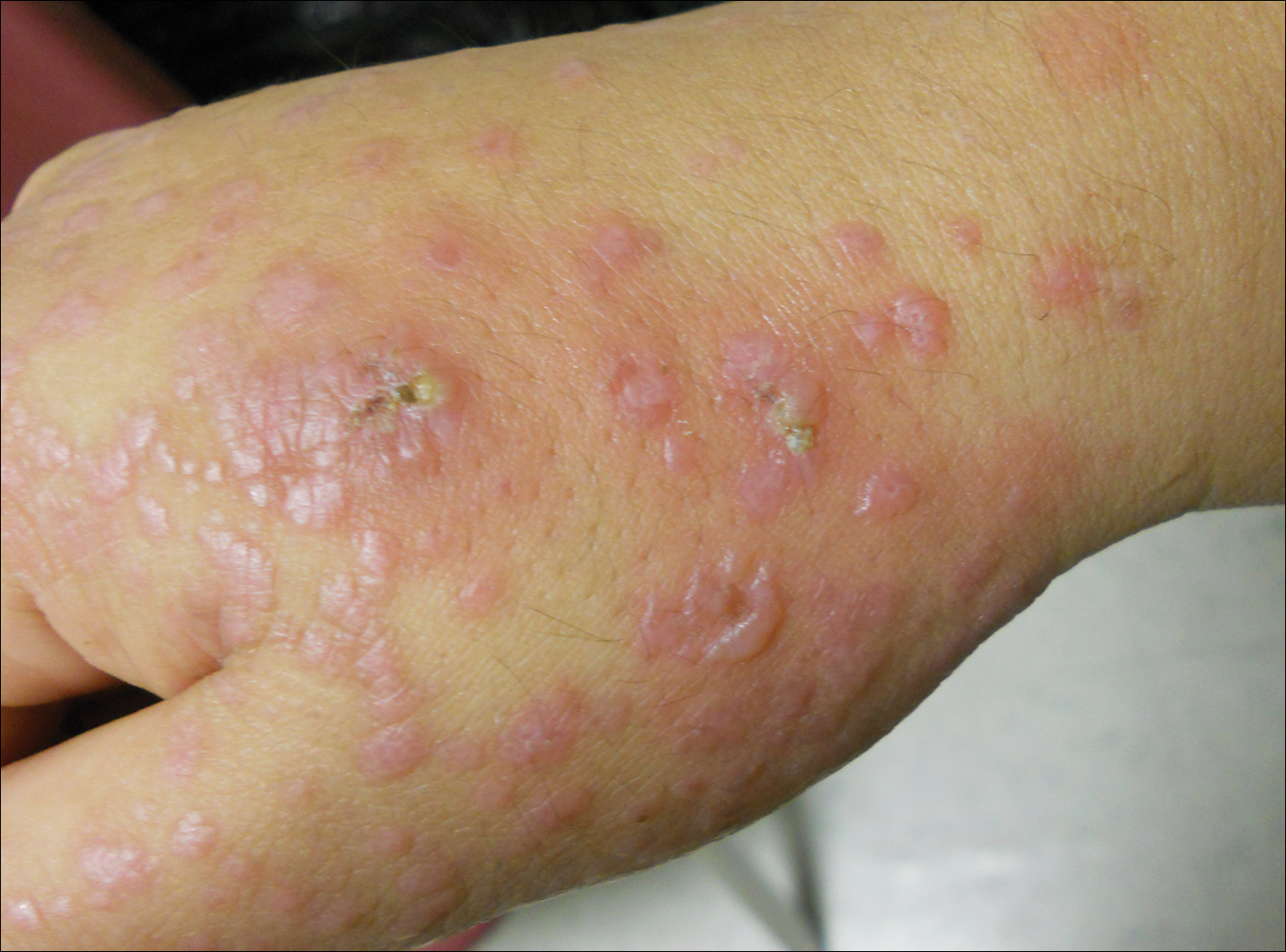
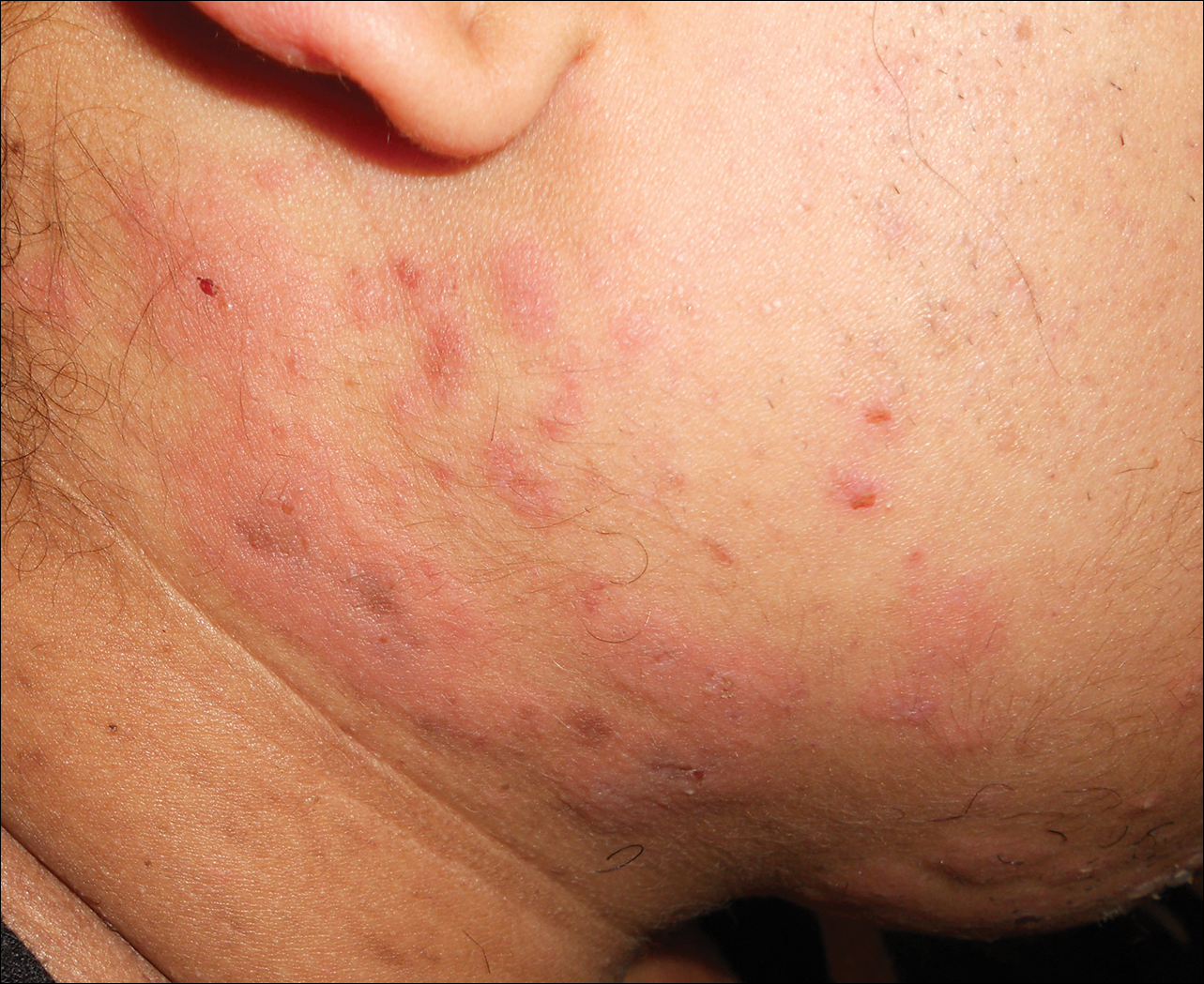
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.
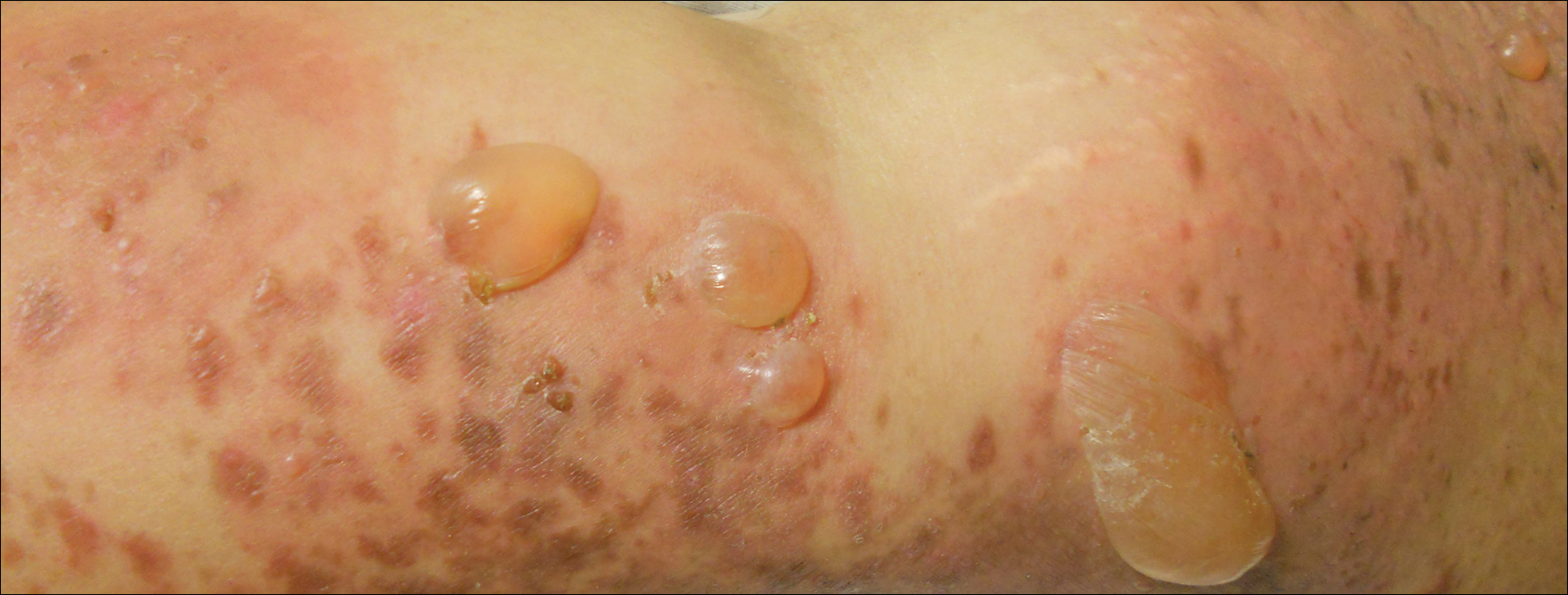
An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
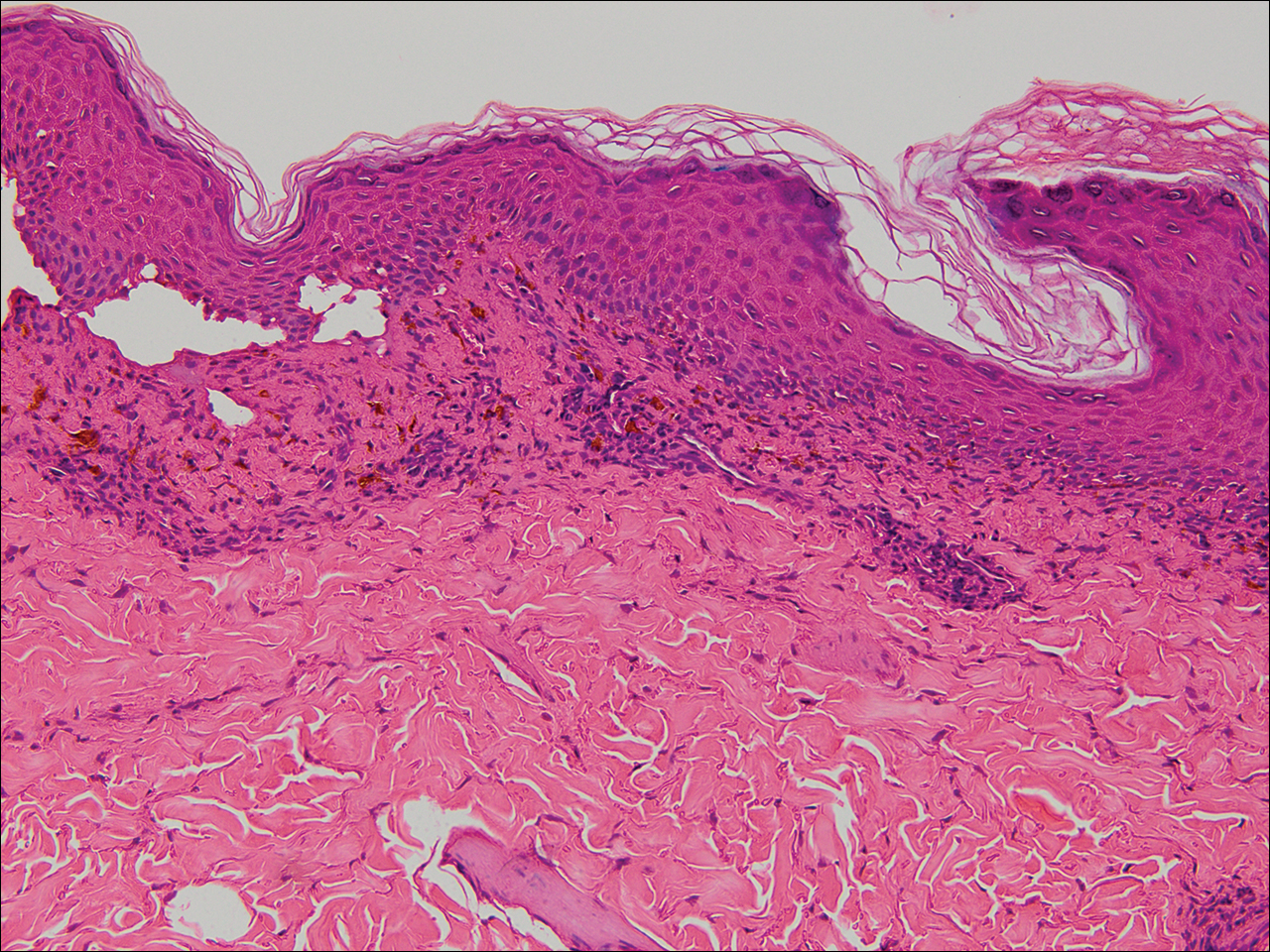
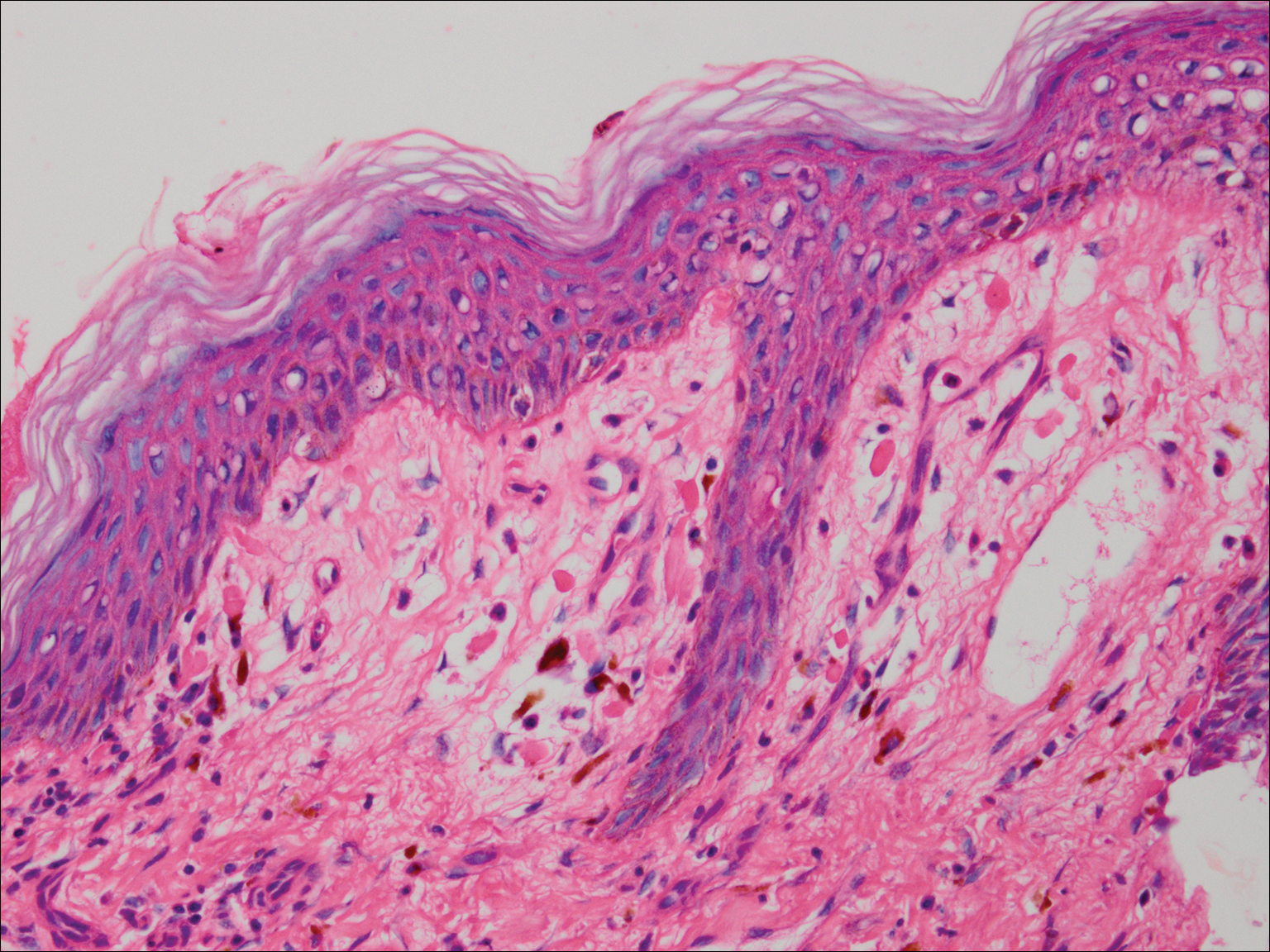
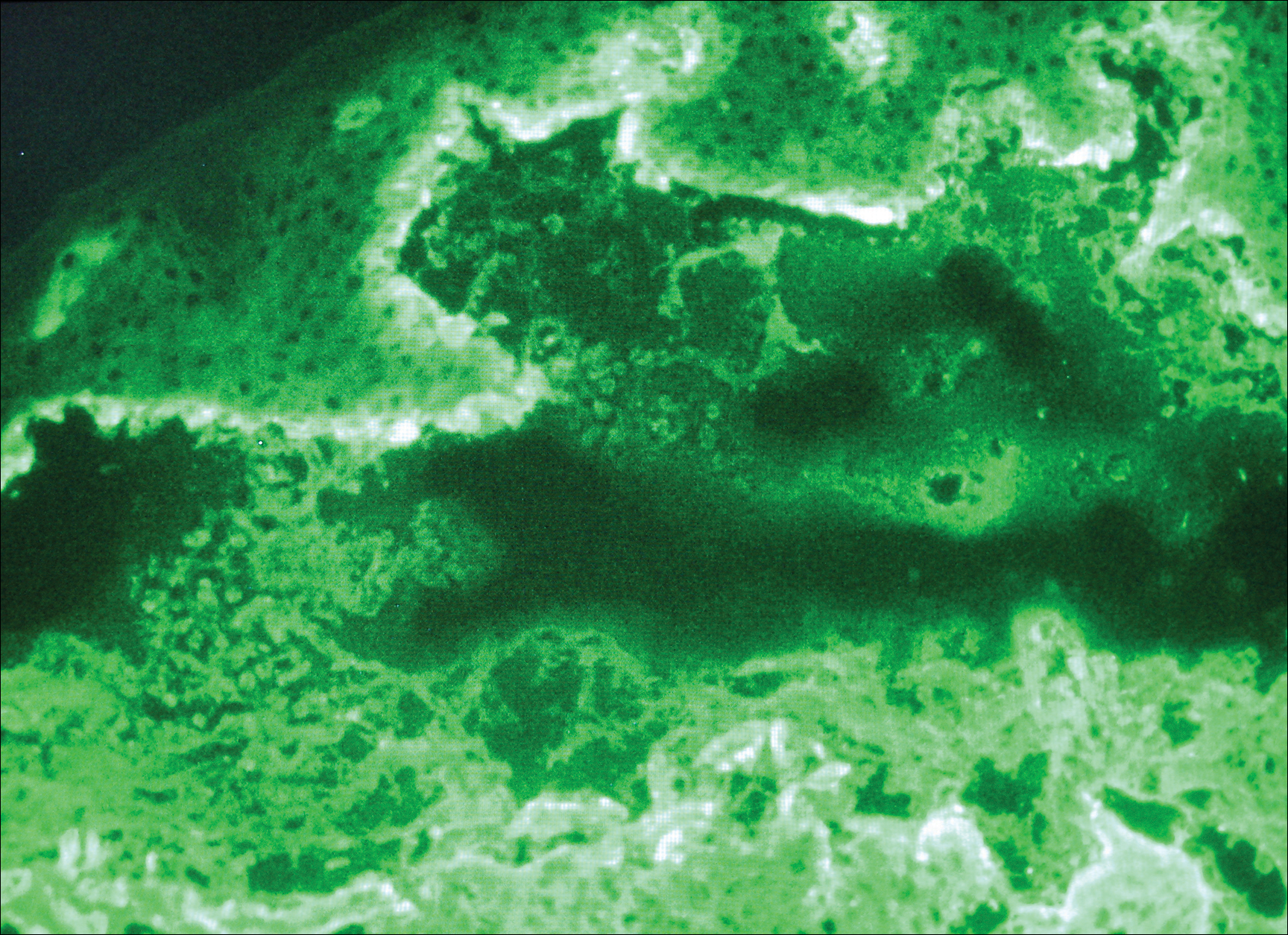
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.
Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.
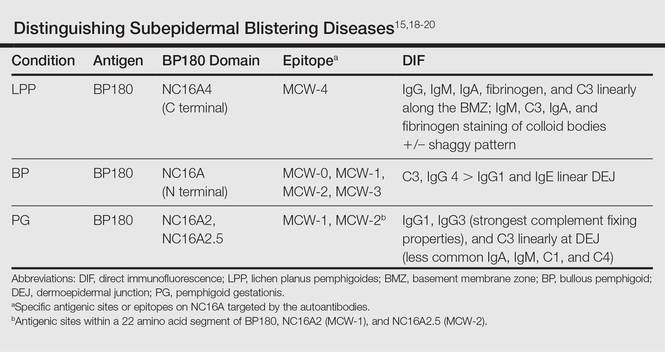
The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.



An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.



Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.

The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
Case Report
A 25-year-old woman with a 5-month history of severe lichen planus (LP) on the arms, legs, and trunk presented to the emergency department with generalized blisters and erythema over the entire body, including the face and soles, of 2 days’ duration. She was evaluated for the LP 1 week prior in a referral dermatology clinic, and in addition to topical corticosteroids, she received 1 injection of 40 mg intramuscular triamcinolone acetonide. Hours following the injection she developed nausea, vomiting, and fever. The patient reported that her last menstrual period was 3 weeks prior to the current presentation.
Physical examination revealed numerous lichenified, flat-topped, pink-violaceous, hyperpigmented, scaly papules and plaques (Figure 1), as well as tense, yellow, fluid-filled vesicles and bullae of various sizes on the neck, arms (Figure 2), legs, trunk, and dorsal aspect of the feet. The vesicles occurred on both normal skin and the lichenified plaques with a negative Nikolsky sign. There also were urticarial erythematous papules and plaques on the arms, trunk, neck, and face, some of which had vesicles or a violaceous dusky central hue (Figure 3). Vesicles were noted within both nostrils (nasal mucosa), and there were extremely tender erythematous patches and thick sheets of scales on the soles.



An elevated β human chorionic gonadotropin level and transvaginal ultrasonography confirmed an intrauterine pregnancy of 12 weeks’ gestation despite the patient’s report of the last menstrual period.
Histologic examination of a vesicle on the right arm revealed hyperkeratosis with hypergranulosis, vacuolar alteration of the basal layer with a paucicellular subepidermal vesicle, and melanophages in the superficial dermis consistent with vesicular LP (Figure 4). Histologic examination of an erythematous edematous plaque on the right upper leg revealed edema in the upper dermis with a perivascular and interstitial lymphocytic infiltrate with eosinophils. A third biopsy of a lichenoid flat-topped papule on the left arm revealed a mild bandlike infiltrate of lymphocytes and scattered eosinophils, eosinophilic colloid bodies and edema in the papillary dermis, and subepidermal vesicles and vacuolar alteration of the basal layer consistent with a vesicular lichenoid dermatitis (Figure 5). Direct immunofluorescence (DIF) of perilesional skin showed linear deposition of C3 and IgM along the basement membrane zone (BMZ) in addition to a shaggy pattern with cytoid bodies (Figure 6). There also was a faint linear deposit of IgA along the BMZ with cytoid bodies but negative for IgG. These results were interpreted as consistent with LP pemphigoides (LPP). Neither an enzyme-linked immunosorbent assay nor an immunoblot analysis was performed.



Because the patient was pregnant and had failed to respond to topical and intramuscular corticosteroids, she was started on intravenous methylprednisolone in the emergency department until new lesions stopped appearing. She was then discharged home on oral prednisone 50 mg (0.5 mg/kg/d), with close observation by her obstetrician. She also used clobetasol propionate ointment 0.05% for more severe lesions and triamcinolone acetonide cream 0.1% for less severe lesions until lesions resolved.
During treatment, the patient developed cellulitis on the leg that presented as pustules and erythema at a site of an eroded bulla, inframammary and axillary cutaneous candidiasis, and hyperglycemia at 19 weeks’ gestation. The cutaneous infections resolved with oral clindamycin 300 mg 3 times daily for 10 days. Topical mupirocin was used to treat the cellulitis and a mixture of zinc oxide, econazole cream, and desonide cream twice daily treated the candidiasis. Her obstetrician managed the hyperglycemia.
The bullous lesions and LP completely resolved after 2 months of treatment with oral prednisone 50 mg daily. The patient tolerated a corticosteroid taper (dose decreased by 5 mg every 2 weeks) until arriving at 10 mg, which was then decreased to 7.5 mg until delivery. A cesarean delivery was performed due to a large-for-gestational-age fetus, and an internist was consulted for the necessary precautions to increase the steroid dose during delivery due to the stress of the surgery and the risk for a hypothalamic crisis. There were no peripartum complications, and the baby was born without cutaneous lesions and remains healthy 1 year later. The patient remained disease free over 2 months postpartum, until new LP lesions developed without vesicles or bullae, which were then controlled with topical therapy. She was subsequently lost to follow-up.
Comment
Kaposi first described LPP in 1892 and used the term lichen ruber pemphigoides to describe a case of typical LP together with a widespread bullous eruption. Lichen planus pemphigoides is characterized by tense blisters that arise on lesions of LP as well as on skin unaffected by LP. In contrast, bullous LP blisters are confined to LP lesions only and occur from intense lichenoid inflammation and extensive liquefactive degeneration of basal keratinocytes. The vesicle formation in LPP is a result of autoantibodies to the bullous pemphigoid (BP) antigen BPAg2, which can be explained by the epitope spreading epiphenomenon whereby epidermotropic cytotoxic T cells damage the basal keratinocytes in LP by targeting unknown epidermal antigens, resulting in the exposure of BP180 and therefore instigating the autoimmune response.1 The process of epitope spreading takes months to develop; the mean duration of LP before LPP is 8 weeks in children and 12 weeks in adults,2 which is comparable to the current case.
Pathogenesis
Lichen planus pemphigoides usually is idiopathic; however, there have been cases reported in association with various medications including calcium channel blockers such as diltiazem, Chinese herbs,3 simvastatin,4 ramipril,5,6 captopril,7 psoralen plus UVA phototherapy,8 and cinnarizine.9 In addition, in a case-controlled study, the use of neuroleptics or diuretics was found to be a risk factor for LPP development.10
This case is unique because it shows an association of LPP with an intrauterine pregnancy. Despite the fact that we did not perform the required studies to determine the exact cause, there probably exists an association between LPP and the pregnancy, as the patient presented with a 5-month history of severe LP prior to vesicle formation. The patient only developed the vesicular lesions during pregnancy, which were later controlled with systemic steroids and then recurred postpartum only as LP lesions, suggesting that the patient’s pregnancy may have contributed in the pathogenesis as an inducing factor. We suspect that the LP was aggravated by the pregnancy and continued to worsen, so much as to cause epitope spreading and lead to the bullous eruption at the end of the first trimester.2
Differential Diagnosis
Initially, we suspected a diagnosis of pemphigoid gestationis (PG), previously known as herpes gestationis. The classic presentation of PG starts with an intense pruritus followed by the emergence of pruritic urticarial papules and plaques in the umbilical or periumbilical areas. The lesions may become targetlike or polycyclic and may spread to other areas of the trunk, arms, and legs, often including the palms and soles.11-15 Just as in our case, vesicles and bullous lesions appear at both the site of the urticarial plaques as well as on normal skin.16 The clinical features noted in our patient that were not typical of PG included the multiple lesions on the face and inside the nostrils. Only 20% of PG cases are associated with mucosal involvement,11,12,15 and there are no documented reports of PG occurring in a patient with LP, according to a PubMed search of articles indexed for MEDLINE using the search terms pemphigoid gestationis, herpes gestationis, and lichen planus.
Lichen planus pemphigoides can be easily differentiated from BP. Lichen planus pemphigoides occurs in younger patients, with a mean age of 35 years, unlike BP, which commonly affects elderly men.17 Lichen planus pemphigoides also is less severe and has a better response to treatment than BP. It also affects the palms and soles, which are rarely affected in BP. There are no reports in the literature of BP developing during pregnancy, according to a PubMed search using the terms bullous pemphigoid and pregnancy. However, LPP and BP share a common antibody, the BP180 antigen, and differences exist in the epitope where the antibody binds in each condition.18,19
Diagnosing LPP
In LPP, DIF typically shows linear deposits of IgG, IgM, IgA, fibrinogen, and C3 along the BMZ, of which IgG and C3 are most commonly seen.3 Our patient had linear deposition of C3, IgM, and IgA along the BMZ, which excluded bullous LP from the differential diagnosis. Bullous LP is not an autoimmune condition but rather is on the severe spectrum of LP where Max Joseph spaces become so large so as to lead to vesicle and bullae formation. In addition to the linear deposit at the BMZ, LPP typically reveals immunoglobulin (mainly IgM but also IgA), C3, and fibrinogen staining of colloid bodies in the papillary dermis on DIF; however, some cases of LPP only present with a linear deposition of C3 along the BMZ, which is why, similar to PG, these diagnoses by DIF are similar. Direct immunofluorescence of PG reveals linear IgG1 and IgG3 along the BMZ. IgG1 and IgG3 immunoglobulins are known to fix complement better than other immunoglobulins, thus linear C3 along the BMZ is the most consistently positive immunoreactant. Less common positive immunoreactivity with the same pattern has been seen with IgA, IgM, C1, and C4 (Table).14,15,18 The lack of linear IgG and the presence of IgM is more suggestive of LPP.

The differential diagnosis of the subepidermal autoimmune blistering diseases associated with antibodies against BP180, including BP, LPP, and PG, often is challenging.15 However, LPP can now be distinguished by immunological studies including immunoblot analysis of the immunodominant region of NC16A of the BP180 antigen and the immunoglobulin subclass that reacts to 180-, 200-,20 and 230-kDa antigens within the BMZ (Table).15,18-20 The Table summarizes the different autoantibodies, antigens, and epitopes to distinguish subepidermal autoimmune blistering diseases.
Despite not performing these studies in our patient, we concluded that the clinical, histological, and DIF findings of this case are more consistent with LPP than with the other subepidermal blistering diseases. However, we cannot exclude the possibility of the patient having a new entity with a unique antibody from epitope spreading.
Conclusion
We present a case of lichenoid papules and plaques consistent with LP, with the development of vesicles and bullae after the first trimester of pregnancy. The clinical, pathologic, and DIF findings were highly suggestive of LPP. Although the exact pathogenic mechanism is not fully known, we suspect that pregnancy may have contributed to the origin of the disease. Further evaluation of pregnant patients with lichenoid lesions who develop blisters are needed for the elucidation of the mechanism, which may be secondary to epitope spreading that led to new autoantibody formation.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
- Stingl G, Holubar K. Coexistence of lichen planus and bullous pemphigoid. an immunopathological study. Br J Dermatol. 1975;93:313-320.
- Paige DG, Bhogal BS, Black MM, et al. Lichen planus pemphigoides in a child—immunopathological findings. Clin Exp Dermatol. 1993;18:552-554.
- Xu HH, Xiao T, He CD, et al. Lichen planus pemphigoides associated with Chinese herbs. Clin Exp Dermatol. 2009;34:329-332.
- Stoebner PE, Michot C, Ligeron C, et al. Simvastatin induced lichen planus pemphigoides. Ann Dermatol Venereol. 2003;130:187-190.
- Zhu YI, Fitzpatrick JE, Kornfeld BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Ogg GS, Bhogal BS, Hashimoto T, et al. Ramipril-associated lichen planus pemphigoides. Br J Dermatol. 1997;136:412-414.
- Flageul B, Foldes C, Wallach D, et al. Captopril-induced lichen planus pemphigoides with pemphigus-like features. a case report. Dermatologica. 1986;173:248-255.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Miyagawa S, Ohi H, Muramatsu T, et al. Lichen planus pemphigoides-like lesions induced by Cinnarizine. Br J Dermatol. 1985;112:607-613.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265-275.
- DiZenzo G, Calabresi V, Grosso F, et al. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol. 2006;127:864-873.
- Jenkis RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigus gestationis. Clin Exp Dermatol. 1999;24:255-259.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45:55-70.
- Cobo MF, Santi CG, Maruta CW, et al. Pemphigoid gestationis: clinical and laboratory evaluation. Clinics. 2009;64:1042-1047.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180 kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Harjai B, Mendiratta V, Kakkar S, et al. Childhood lichen planus pemphigoides—a rare entity. J Eur Acad Dermatol Venereol. 2006;20:117-118.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. 2002;51:21-28.
- Davis AL, Bhogal BS, Whitehead P, et al. Lichen planus pemphigoides: its relationship to bullous pemphigoid. Br J Dermatol. 1991;125:263-271.
Practice Points
- Lichen planus pemphigoides (LPP) is characterized by tense blisters that arise not only on lichen planus lesions such as bullous lichen planus but also on skin unaffected by lichen planus.
- In LPP, the autoantibodies specifically target the MCW-4 epitope of the NC16A4 domain of the bullous pemphigoid antigen BPAg2, distinguishing it from other autoimmune blistering diseases against the NC16A domain.
Pemphigus Vulgaris Successfully Treated With Doxycycline Monotherapy
To the Editor:
Pemphigus vulgaris (PV) is an acquired autoimmune bullous disease with notable morbidity and mortality if not treated appropriately due to loss of epidermal barrier function and subsequent infection and loss of body fluids. Although the use of systemic corticosteroids and immunosuppressive agents has improved the prognosis, these drugs also may have severe adverse effects, especially in elderly patients. Hence, alternative and safer therapies with anti-inflammatory and immunomodulatory agents such as tetracyclines and nicotinamide have been used with variable results. We report a case of PV that was successfully treated with doxycycline.
An 81-year-old man presented with well-demarcated erosions with overlying yellow crust as well as vesicles and pustules on the scalp (Figure 1A), forehead, bilateral cheeks, and upper back (Figure 1B) of 6 months’ duration. He used topical fluorouracil in the month prior to presentation for suspected actinic keratosis but had stopped its use after 2 weeks. At the first visit, a diagnosis of a reaction to topical fluorouracil with secondary bacterial infection was made and he was prescribed doxycycline hyclate 100 mg twice daily. The patient returned 4 weeks later for follow-up and reported initial notable improvement with subsequent worsening of lesions after he ran out of doxycycline. On physical examination the lesions had considerably improved from the last visit, but he still had a few erosions on the scalp and a few in the oral mucosa. A 1-cm shallow erosion with minimal surrounding erythema on the forehead was present, along with fewer scattered, edematous, erythematous plaques on the back and chest. Pemphigus vulgaris was suspected and 2 shave biopsies from the lesions on the back and cheek were obtained for confirmation. Histopathologic examination revealed epidermal hyperplasia and suprabasal acantholysis as well as moderate perivascular and perifollicular lymphocytic infiltrate with several eosinophils and plasma cells, characteristic of PV (Figure 2). Direct immunofluorescence showed moderate intercellular deposition of IgG within the basal layer and to a lesser extent within suprabasal layers as well as moderate intercellular deposition of C3 within the basal layer, characteristic of PV. IgA and IgM were not present. Indirect immunofluorescence using monkey esophagus revealed no antibodies against the intercellular space of the basement membrane zone. Due to the dramatic response, he continued on doxycycline 100 mg twice daily and remained in complete remission. Ten months after initiating treatment he discontinued doxycycline for 2 days and developed a 1-cm lesion on the left cheek. He resumed treatment with clearing of lesions and was slowly tapered to 50 mg of doxycycline once daily, remaining in complete remission (Figure 3). Doxycycline was discontinued 16 months after initiation; he has remained clear at 13 weeks.
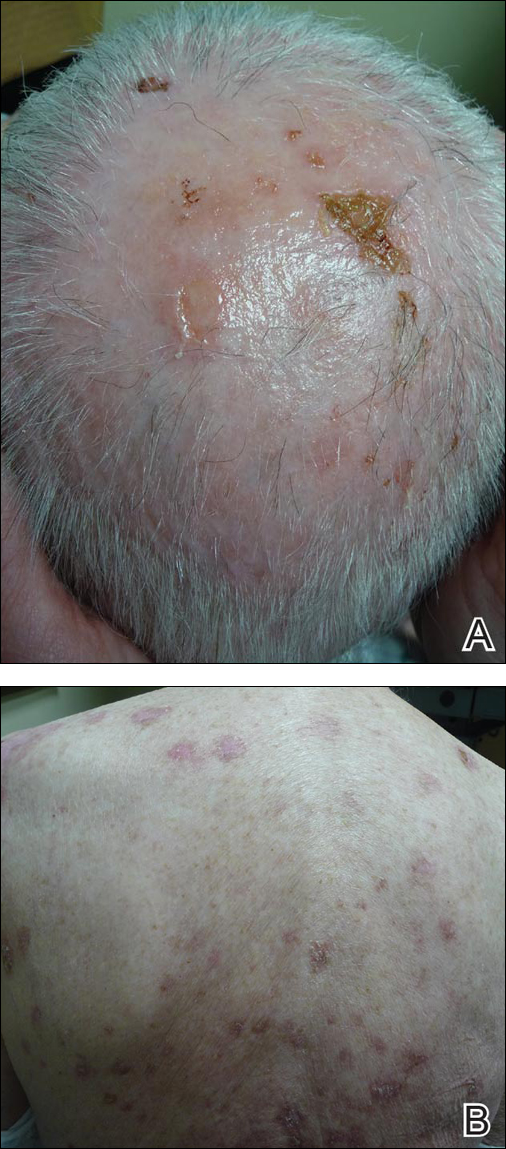
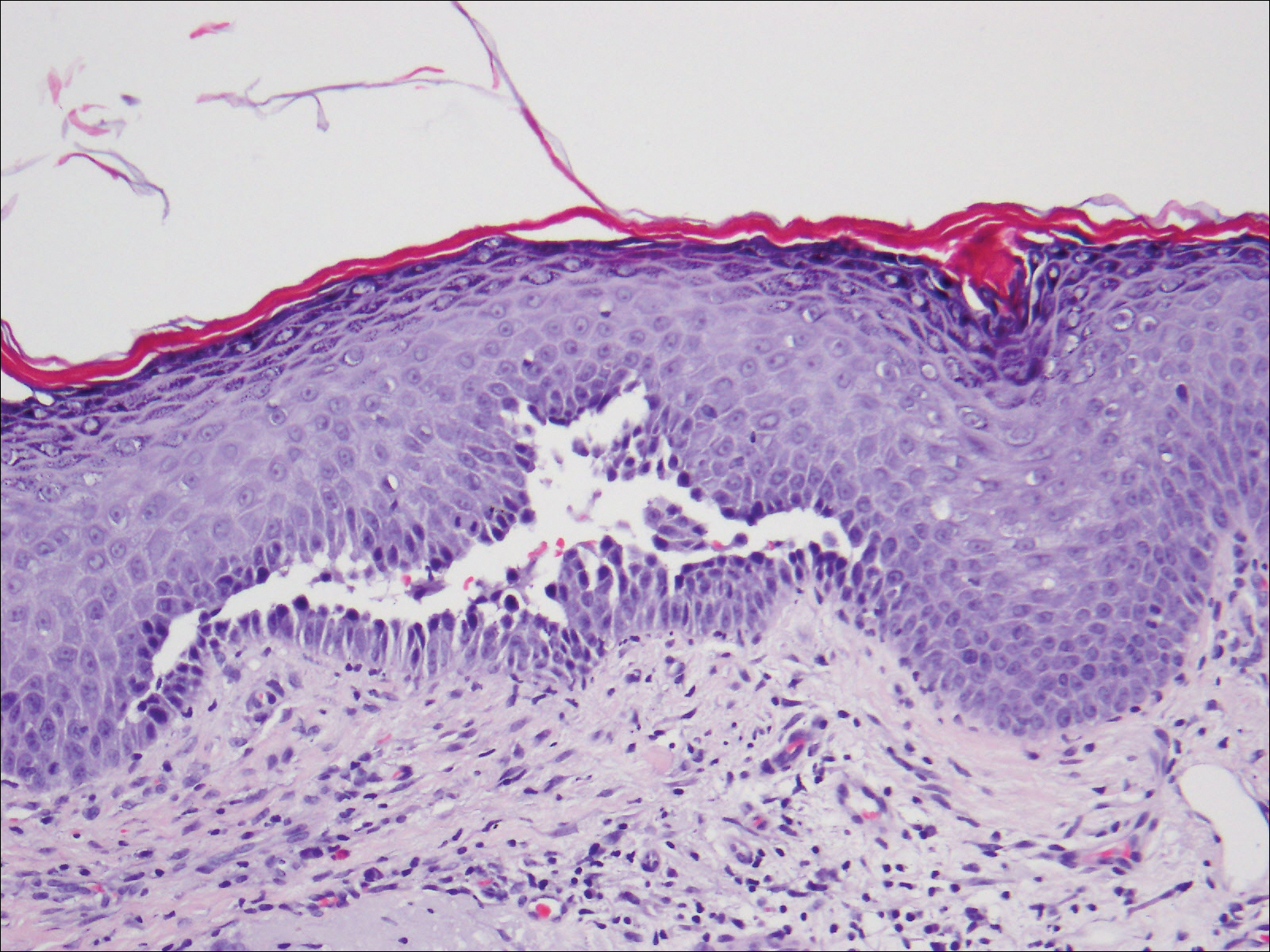
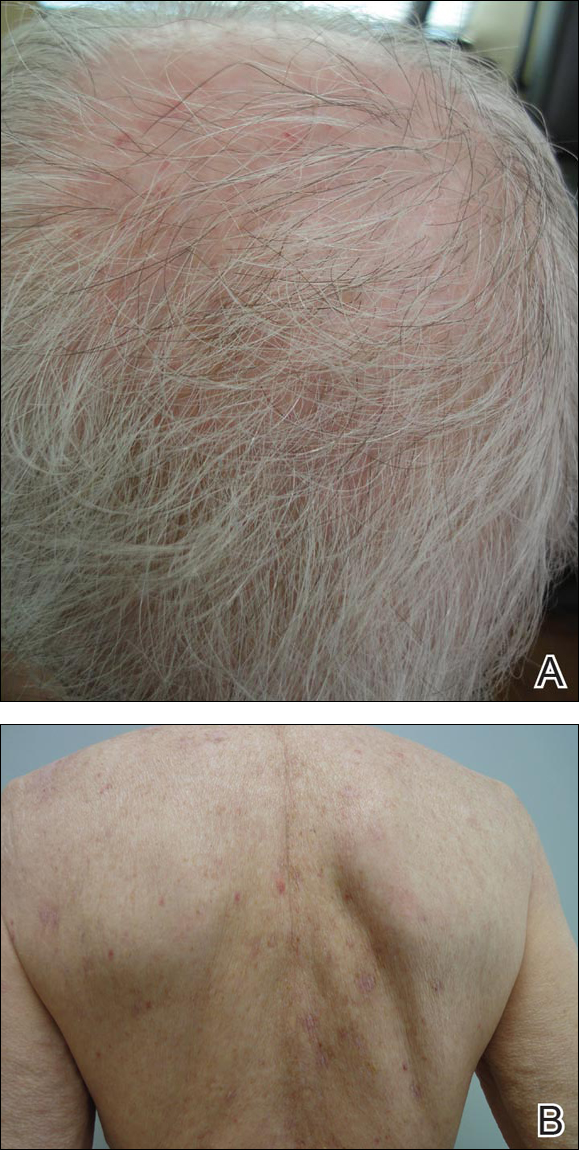
The treatment of PV is challenging given the multiple side effects of steroids, especially in elderly patients. Tetracyclines have an advantageous side-effect profile and they have been shown to be efficacious in treating PV when combined with nicotinamide or when used as adjuvant therapy to steroids.1-3 Our case shows a patient who was treated exclusively with doxycycline and achieved complete remission.
Tetracyclines have multiple biological activities in addition to their antimicrobial function that may provide a therapeutic benefit in PV. They possess immunomodulatory and anti-inflammatory effects by inhibiting leukocyte chemotaxis and activation4-8 and inhibiting cytokine release. They inhibit matrix metalloproteinases, which are the major enzymes responsible for breakdown of the extracellular matrix,9 and they indirectly inhibit neutrophil elastase by protecting α1-protease inhibitor from matrix metalloproteinase degradation.10 Additionally, tetracyclines increase the cohesion of the dermoepidermal junction11; whether they increase the adhesion between epidermal cells is unknown. It has been determined that CD4+ T cells play an essential role in the pathogenesis of PV by promoting anti-desmoglein 3 antibody production.12 Szeto et al13 reported that minocycline, a member of the tetracycline family, has suppressive effects on CD4+ T-cell activation by hindering the activation of nuclear factor of activated T cells (NFAT), a key regulatory factor in T-cell activation. We hypothesize that doxycycline exerted what appears to be immunomodulatory properties in our patient by suppressing CD4+ T-cell activity.
In conclusion, tetracyclines can be an effective and promising therapy for PV given their relatively few side effects and immunomodulating properties. However, further randomized controlled trials will be important to support our conclusion.
- Chaffins ML, Collison D, Fivenson DP. Treatment of pemphigus and linear IgA dermatosis with nicotinamide and tetracycline: a review of 13 cases. J Am Acad Dermatol. 1993;28:998-1000.
- Caelbotta A, Saenz AM, Gonzalez F, et al. Pemphigus vulgaris: benefits of tetracycline as adjuvant therapy in series of thirteen patients. Int J Dermatol. 1999;38:217-221.
- McCarty M, Fivenson D. Two decades of using the combination of tetracycline derivatives and niacinamide as steroid-sparing agents in the management of pemphigus defining a niche for these low toxicity agents. J Am Acad Dermatol. 2014;71:475-479.
- Majeski JA, McClellan MA, Alexander JW. Effect of antibiotics on the in vitro neutrophil chemotactic response. Am Surg. 1976;42:785-788.
- Esterly NB, Furley NL, Flanagan LE. The effect of antimicrobial agents on leukocyte chemotaxis. J Invest Dermatol. 1978;70:51-55.
- Gabler WL, Creamer HR. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26:52-58.
- Esterly NB, Koransky JS, Furey NL, et al. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol. 1984;120:1308-1313.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
- Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63:130-145.
- Golub LM, Evans RT, McNamara TF, et al. A nonantimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis–induced periodontitis in rats. Ann N Y Acad Sci. 1994;732:96-111.
- Humbert P, Treffel P, Chapius JF, et al. The tetracyclines in dermatology. J Am Acad Dermatol. 1991;25:691-697.
- Nishifuji K, Amagai M, Kuwana M, et al. Detection of antigen-specific B cells in patients with pemphigus vulgaris by enzyme-linked immunospot assay: requirement of T cell collaboration for autoantibody production. J Invest Dermatol. 2000;114:88-94.
- Szeto GL, Pomerantz JL, Graham DRM, et al. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4 T Cells. J Biol Chem. 2011;286:11275-11282.
To the Editor:
Pemphigus vulgaris (PV) is an acquired autoimmune bullous disease with notable morbidity and mortality if not treated appropriately due to loss of epidermal barrier function and subsequent infection and loss of body fluids. Although the use of systemic corticosteroids and immunosuppressive agents has improved the prognosis, these drugs also may have severe adverse effects, especially in elderly patients. Hence, alternative and safer therapies with anti-inflammatory and immunomodulatory agents such as tetracyclines and nicotinamide have been used with variable results. We report a case of PV that was successfully treated with doxycycline.
An 81-year-old man presented with well-demarcated erosions with overlying yellow crust as well as vesicles and pustules on the scalp (Figure 1A), forehead, bilateral cheeks, and upper back (Figure 1B) of 6 months’ duration. He used topical fluorouracil in the month prior to presentation for suspected actinic keratosis but had stopped its use after 2 weeks. At the first visit, a diagnosis of a reaction to topical fluorouracil with secondary bacterial infection was made and he was prescribed doxycycline hyclate 100 mg twice daily. The patient returned 4 weeks later for follow-up and reported initial notable improvement with subsequent worsening of lesions after he ran out of doxycycline. On physical examination the lesions had considerably improved from the last visit, but he still had a few erosions on the scalp and a few in the oral mucosa. A 1-cm shallow erosion with minimal surrounding erythema on the forehead was present, along with fewer scattered, edematous, erythematous plaques on the back and chest. Pemphigus vulgaris was suspected and 2 shave biopsies from the lesions on the back and cheek were obtained for confirmation. Histopathologic examination revealed epidermal hyperplasia and suprabasal acantholysis as well as moderate perivascular and perifollicular lymphocytic infiltrate with several eosinophils and plasma cells, characteristic of PV (Figure 2). Direct immunofluorescence showed moderate intercellular deposition of IgG within the basal layer and to a lesser extent within suprabasal layers as well as moderate intercellular deposition of C3 within the basal layer, characteristic of PV. IgA and IgM were not present. Indirect immunofluorescence using monkey esophagus revealed no antibodies against the intercellular space of the basement membrane zone. Due to the dramatic response, he continued on doxycycline 100 mg twice daily and remained in complete remission. Ten months after initiating treatment he discontinued doxycycline for 2 days and developed a 1-cm lesion on the left cheek. He resumed treatment with clearing of lesions and was slowly tapered to 50 mg of doxycycline once daily, remaining in complete remission (Figure 3). Doxycycline was discontinued 16 months after initiation; he has remained clear at 13 weeks.



The treatment of PV is challenging given the multiple side effects of steroids, especially in elderly patients. Tetracyclines have an advantageous side-effect profile and they have been shown to be efficacious in treating PV when combined with nicotinamide or when used as adjuvant therapy to steroids.1-3 Our case shows a patient who was treated exclusively with doxycycline and achieved complete remission.
Tetracyclines have multiple biological activities in addition to their antimicrobial function that may provide a therapeutic benefit in PV. They possess immunomodulatory and anti-inflammatory effects by inhibiting leukocyte chemotaxis and activation4-8 and inhibiting cytokine release. They inhibit matrix metalloproteinases, which are the major enzymes responsible for breakdown of the extracellular matrix,9 and they indirectly inhibit neutrophil elastase by protecting α1-protease inhibitor from matrix metalloproteinase degradation.10 Additionally, tetracyclines increase the cohesion of the dermoepidermal junction11; whether they increase the adhesion between epidermal cells is unknown. It has been determined that CD4+ T cells play an essential role in the pathogenesis of PV by promoting anti-desmoglein 3 antibody production.12 Szeto et al13 reported that minocycline, a member of the tetracycline family, has suppressive effects on CD4+ T-cell activation by hindering the activation of nuclear factor of activated T cells (NFAT), a key regulatory factor in T-cell activation. We hypothesize that doxycycline exerted what appears to be immunomodulatory properties in our patient by suppressing CD4+ T-cell activity.
In conclusion, tetracyclines can be an effective and promising therapy for PV given their relatively few side effects and immunomodulating properties. However, further randomized controlled trials will be important to support our conclusion.
To the Editor:
Pemphigus vulgaris (PV) is an acquired autoimmune bullous disease with notable morbidity and mortality if not treated appropriately due to loss of epidermal barrier function and subsequent infection and loss of body fluids. Although the use of systemic corticosteroids and immunosuppressive agents has improved the prognosis, these drugs also may have severe adverse effects, especially in elderly patients. Hence, alternative and safer therapies with anti-inflammatory and immunomodulatory agents such as tetracyclines and nicotinamide have been used with variable results. We report a case of PV that was successfully treated with doxycycline.
An 81-year-old man presented with well-demarcated erosions with overlying yellow crust as well as vesicles and pustules on the scalp (Figure 1A), forehead, bilateral cheeks, and upper back (Figure 1B) of 6 months’ duration. He used topical fluorouracil in the month prior to presentation for suspected actinic keratosis but had stopped its use after 2 weeks. At the first visit, a diagnosis of a reaction to topical fluorouracil with secondary bacterial infection was made and he was prescribed doxycycline hyclate 100 mg twice daily. The patient returned 4 weeks later for follow-up and reported initial notable improvement with subsequent worsening of lesions after he ran out of doxycycline. On physical examination the lesions had considerably improved from the last visit, but he still had a few erosions on the scalp and a few in the oral mucosa. A 1-cm shallow erosion with minimal surrounding erythema on the forehead was present, along with fewer scattered, edematous, erythematous plaques on the back and chest. Pemphigus vulgaris was suspected and 2 shave biopsies from the lesions on the back and cheek were obtained for confirmation. Histopathologic examination revealed epidermal hyperplasia and suprabasal acantholysis as well as moderate perivascular and perifollicular lymphocytic infiltrate with several eosinophils and plasma cells, characteristic of PV (Figure 2). Direct immunofluorescence showed moderate intercellular deposition of IgG within the basal layer and to a lesser extent within suprabasal layers as well as moderate intercellular deposition of C3 within the basal layer, characteristic of PV. IgA and IgM were not present. Indirect immunofluorescence using monkey esophagus revealed no antibodies against the intercellular space of the basement membrane zone. Due to the dramatic response, he continued on doxycycline 100 mg twice daily and remained in complete remission. Ten months after initiating treatment he discontinued doxycycline for 2 days and developed a 1-cm lesion on the left cheek. He resumed treatment with clearing of lesions and was slowly tapered to 50 mg of doxycycline once daily, remaining in complete remission (Figure 3). Doxycycline was discontinued 16 months after initiation; he has remained clear at 13 weeks.



The treatment of PV is challenging given the multiple side effects of steroids, especially in elderly patients. Tetracyclines have an advantageous side-effect profile and they have been shown to be efficacious in treating PV when combined with nicotinamide or when used as adjuvant therapy to steroids.1-3 Our case shows a patient who was treated exclusively with doxycycline and achieved complete remission.
Tetracyclines have multiple biological activities in addition to their antimicrobial function that may provide a therapeutic benefit in PV. They possess immunomodulatory and anti-inflammatory effects by inhibiting leukocyte chemotaxis and activation4-8 and inhibiting cytokine release. They inhibit matrix metalloproteinases, which are the major enzymes responsible for breakdown of the extracellular matrix,9 and they indirectly inhibit neutrophil elastase by protecting α1-protease inhibitor from matrix metalloproteinase degradation.10 Additionally, tetracyclines increase the cohesion of the dermoepidermal junction11; whether they increase the adhesion between epidermal cells is unknown. It has been determined that CD4+ T cells play an essential role in the pathogenesis of PV by promoting anti-desmoglein 3 antibody production.12 Szeto et al13 reported that minocycline, a member of the tetracycline family, has suppressive effects on CD4+ T-cell activation by hindering the activation of nuclear factor of activated T cells (NFAT), a key regulatory factor in T-cell activation. We hypothesize that doxycycline exerted what appears to be immunomodulatory properties in our patient by suppressing CD4+ T-cell activity.
In conclusion, tetracyclines can be an effective and promising therapy for PV given their relatively few side effects and immunomodulating properties. However, further randomized controlled trials will be important to support our conclusion.
- Chaffins ML, Collison D, Fivenson DP. Treatment of pemphigus and linear IgA dermatosis with nicotinamide and tetracycline: a review of 13 cases. J Am Acad Dermatol. 1993;28:998-1000.
- Caelbotta A, Saenz AM, Gonzalez F, et al. Pemphigus vulgaris: benefits of tetracycline as adjuvant therapy in series of thirteen patients. Int J Dermatol. 1999;38:217-221.
- McCarty M, Fivenson D. Two decades of using the combination of tetracycline derivatives and niacinamide as steroid-sparing agents in the management of pemphigus defining a niche for these low toxicity agents. J Am Acad Dermatol. 2014;71:475-479.
- Majeski JA, McClellan MA, Alexander JW. Effect of antibiotics on the in vitro neutrophil chemotactic response. Am Surg. 1976;42:785-788.
- Esterly NB, Furley NL, Flanagan LE. The effect of antimicrobial agents on leukocyte chemotaxis. J Invest Dermatol. 1978;70:51-55.
- Gabler WL, Creamer HR. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26:52-58.
- Esterly NB, Koransky JS, Furey NL, et al. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol. 1984;120:1308-1313.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
- Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63:130-145.
- Golub LM, Evans RT, McNamara TF, et al. A nonantimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis–induced periodontitis in rats. Ann N Y Acad Sci. 1994;732:96-111.
- Humbert P, Treffel P, Chapius JF, et al. The tetracyclines in dermatology. J Am Acad Dermatol. 1991;25:691-697.
- Nishifuji K, Amagai M, Kuwana M, et al. Detection of antigen-specific B cells in patients with pemphigus vulgaris by enzyme-linked immunospot assay: requirement of T cell collaboration for autoantibody production. J Invest Dermatol. 2000;114:88-94.
- Szeto GL, Pomerantz JL, Graham DRM, et al. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4 T Cells. J Biol Chem. 2011;286:11275-11282.
- Chaffins ML, Collison D, Fivenson DP. Treatment of pemphigus and linear IgA dermatosis with nicotinamide and tetracycline: a review of 13 cases. J Am Acad Dermatol. 1993;28:998-1000.
- Caelbotta A, Saenz AM, Gonzalez F, et al. Pemphigus vulgaris: benefits of tetracycline as adjuvant therapy in series of thirteen patients. Int J Dermatol. 1999;38:217-221.
- McCarty M, Fivenson D. Two decades of using the combination of tetracycline derivatives and niacinamide as steroid-sparing agents in the management of pemphigus defining a niche for these low toxicity agents. J Am Acad Dermatol. 2014;71:475-479.
- Majeski JA, McClellan MA, Alexander JW. Effect of antibiotics on the in vitro neutrophil chemotactic response. Am Surg. 1976;42:785-788.
- Esterly NB, Furley NL, Flanagan LE. The effect of antimicrobial agents on leukocyte chemotaxis. J Invest Dermatol. 1978;70:51-55.
- Gabler WL, Creamer HR. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26:52-58.
- Esterly NB, Koransky JS, Furey NL, et al. Neutrophil chemotaxis in patients with acne receiving oral tetracycline therapy. Arch Dermatol. 1984;120:1308-1313.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
- Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63:130-145.
- Golub LM, Evans RT, McNamara TF, et al. A nonantimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis–induced periodontitis in rats. Ann N Y Acad Sci. 1994;732:96-111.
- Humbert P, Treffel P, Chapius JF, et al. The tetracyclines in dermatology. J Am Acad Dermatol. 1991;25:691-697.
- Nishifuji K, Amagai M, Kuwana M, et al. Detection of antigen-specific B cells in patients with pemphigus vulgaris by enzyme-linked immunospot assay: requirement of T cell collaboration for autoantibody production. J Invest Dermatol. 2000;114:88-94.
- Szeto GL, Pomerantz JL, Graham DRM, et al. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4 T Cells. J Biol Chem. 2011;286:11275-11282.
Practice Points
- The treatment of pemphigus vulgaris (PV) is challenging given the side-effect profile of commonly used systemic medications, including steroids, especially in elderly patients.
- Tetracyclines have an advantageous side-effect profile and may be efficacious in treating PV.
Preventing, Identifying, and Managing Cosmetic Procedure Complications, Part 1: Soft-Tissue Augmentation and Botulinum Toxin Injections
The primary cosmetic procedures that dermatology residents will perform or assist in performing during their training are soft-tissue augmentation, botulinum toxin injections, chemical peels, and laser therapy. Because complications can occur from these procedures, it is important for residents to learn how to prevent, identify, and manage them for optimal patient outcomes. In part 1 of this 2-part series, soft-tissue augmentation and botulinum toxin injections are discussed. Chemical peels and laser therapy will be addressed in part 2.
Soft-Tissue Augmentation
Soft-tissue fillers include those that are made from collagen (bovine or human), hyaluronic acid (HA), poly-L-lactic acid, calcium hydroxylapatite, silicone, and polymethylmethacrylate. In general, acute complications of soft-tissue filler injections include erythema, swelling, and bruising.1-3 Patients who take blood thinners or supplements (eg, vitamin E, ginseng, garlic, ginger) should be asked to discontinue use 1 week prior to the procedure. Patients who take blood thinners also should be counseled to expect some bruising. Prior to injection, the skin should be thoroughly cleansed to avoid introducing skin bacteria into the injection site and to reduce infection risk. Postinjection erythema may be related to mast cell activation, which is temporary and should resolve after a few days.1-3
If you find yourself injecting the filler too superficially, you may notice that the skin begins to take on a blue-gray hue1-3 that is known as the Tyndall effect and can be prevented by injecting the filler at the proper level. For example, collagen-based fillers should be placed at the mid dermis, thicker HA fillers should be placed in the deep dermis, and calcium hydroxylapatite should be placed at the junction of the dermis and subcutaneous tissue. Polymethylmethacrylate and poly-L-lactic acid should both be placed subdermally.1-3
The gravest immediate complications associated with soft-tissue filler injections are occlusion of the central retinal artery and/or skin necrosis.1-4 Residents should never inject filler to the glabella or to the nose.1-3 Injections at these sites are sometimes performed but should only be performed by experienced dermatologists. The perioral and tear trough regions also are high-risk injection areas that require a high degree of experience and should only be injected with proper supervision by an experienced dermatologist.1-3 Residents generally can avoid these complications, though not with a 100% guarantee, by avoiding injections in high-risk areas, aspirating to check for blood, and slowly injecting a small amount of filler into the treatment area.1-3 A consensus statement on management of injection-induced necrosis advises to apply a nitropaste ointment 2% to the treatment site or administer an oral aspirin if the patient develops severe pain; vision loss; or acute skin discoloration, especially blanching.4 For HA-based fillers, at least 200 U of hyaluronidase should be injected. It has been suggested that saline can be injected to flush out calcium hydroxylapatite fillers.3 Warm compresses should be placed on the involved area. Following these interventions, any patient with vision loss or orbital pain should immediately undergo ophthalmologic evaluation.3 The most important intervention occurs in the first 24 hours.3,4 After 24 hours, careful wound care, oral anticoagulants, and hyperbaric oxygen therapy have been suggested as management options.3
There are 2 major chronic complications of soft-tissue filler injection, including delayed-onset infection, which occurs 2 weeks or more postinjection, and granuloma formation.1-3 Chronic low-grade infection at the injection site may be indicative of biofilm formation. If an HA filler was used, it should be dissolved with hyaluronidase to help break up the biofilm nidus.3 A course of oral antibiotics also may be indicated.1-3 Intralesional steroids may be used but only after antibiotics have been administered. A biofilm that develops from more permanent fillers may be more difficult to manage. Atypical mycobacterial infections have been known to develop at injection sites, which should be considered in refractory cases.1-3,5
Calcium hydroxylapatite, polymethylmethacrylate, and silicone can stimulate chronic immune system activation, which makes them more prone to granuloma formation.1-3 Once infection is ruled out, granulomas may be treated with intralesional steroids, surgical excision (if the results would be cosmetically acceptable), laser therapy, or potentially local injection of an immunosuppressant (eg, methotrexate, 5-fluorouracil).3
Botulinum Toxin Injections
Patients who are pregnant, lactating, or have neuromuscular disease are not candidates for botulinum toxin injections. There also is a risk that patients taking calcium channel blockers or aminoglycoside antibiotics may experience potentiated effects of the botulinum toxin.6
Patients should be informed that a postinjection headache may occur and should be treated with over-the-counter medications.6 Complication-free botulinum toxin procedures depend heavily on the physician’s knowledge of facial anatomy.1,6 The diagrams provided by Hirsch and Stier1 offer an excellent guide on where to place the injections. Brow droop, eyelid ptosis, and “Spock brow” (eyebrows that are overarched) largely can be avoided by proper injection point placement. A Spock brow may be corrected by injecting the lateral upper forehead with a few units to correct the exaggerated arch.6,7 For eyelid ptosis, apraclonidine 0.05% drops (1–2 drops 3 times daily) should be used until the ptosis resolves.6 Phenylephrine hydrochloride drops may be used should a patient have a documented sensitivity to apraclonidine but should not be used in a patient with acute angle-closure glaucoma or aneurysms.6
Final Thoughts
Learning to perform soft-tissue augmentation and botulinum toxin injections can be a satisfying and fun part of dermatology residency. Preventing, identifying, and managing any complications that may occur is an integral part of performing these procedures.
- Hirsch R, Stier M. Complications and their management in cosmetic dermatology. Dermatol Clin. 2009;27:507-520.
- Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26:34-39.
- Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205-214.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler–induced impending necrosis with hyaluronidase: consensus recommendations [published online May 10, 2015]. Aesthet Surg J. 2015;35:844-849.
- Rodriguez JM, Xie YL, Winthrop KL, et al. Mycobacterium chelonae facial infections following injection of dermal filler. Aesthet Surg J. 2013;33:265-269.
- Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55:8-14.
- Carruthers A, Carruthers J. Update on the botulinum neurotoxins. Skin Therapy Lett. 2001;6:1-2.
The primary cosmetic procedures that dermatology residents will perform or assist in performing during their training are soft-tissue augmentation, botulinum toxin injections, chemical peels, and laser therapy. Because complications can occur from these procedures, it is important for residents to learn how to prevent, identify, and manage them for optimal patient outcomes. In part 1 of this 2-part series, soft-tissue augmentation and botulinum toxin injections are discussed. Chemical peels and laser therapy will be addressed in part 2.
Soft-Tissue Augmentation
Soft-tissue fillers include those that are made from collagen (bovine or human), hyaluronic acid (HA), poly-L-lactic acid, calcium hydroxylapatite, silicone, and polymethylmethacrylate. In general, acute complications of soft-tissue filler injections include erythema, swelling, and bruising.1-3 Patients who take blood thinners or supplements (eg, vitamin E, ginseng, garlic, ginger) should be asked to discontinue use 1 week prior to the procedure. Patients who take blood thinners also should be counseled to expect some bruising. Prior to injection, the skin should be thoroughly cleansed to avoid introducing skin bacteria into the injection site and to reduce infection risk. Postinjection erythema may be related to mast cell activation, which is temporary and should resolve after a few days.1-3
If you find yourself injecting the filler too superficially, you may notice that the skin begins to take on a blue-gray hue1-3 that is known as the Tyndall effect and can be prevented by injecting the filler at the proper level. For example, collagen-based fillers should be placed at the mid dermis, thicker HA fillers should be placed in the deep dermis, and calcium hydroxylapatite should be placed at the junction of the dermis and subcutaneous tissue. Polymethylmethacrylate and poly-L-lactic acid should both be placed subdermally.1-3
The gravest immediate complications associated with soft-tissue filler injections are occlusion of the central retinal artery and/or skin necrosis.1-4 Residents should never inject filler to the glabella or to the nose.1-3 Injections at these sites are sometimes performed but should only be performed by experienced dermatologists. The perioral and tear trough regions also are high-risk injection areas that require a high degree of experience and should only be injected with proper supervision by an experienced dermatologist.1-3 Residents generally can avoid these complications, though not with a 100% guarantee, by avoiding injections in high-risk areas, aspirating to check for blood, and slowly injecting a small amount of filler into the treatment area.1-3 A consensus statement on management of injection-induced necrosis advises to apply a nitropaste ointment 2% to the treatment site or administer an oral aspirin if the patient develops severe pain; vision loss; or acute skin discoloration, especially blanching.4 For HA-based fillers, at least 200 U of hyaluronidase should be injected. It has been suggested that saline can be injected to flush out calcium hydroxylapatite fillers.3 Warm compresses should be placed on the involved area. Following these interventions, any patient with vision loss or orbital pain should immediately undergo ophthalmologic evaluation.3 The most important intervention occurs in the first 24 hours.3,4 After 24 hours, careful wound care, oral anticoagulants, and hyperbaric oxygen therapy have been suggested as management options.3
There are 2 major chronic complications of soft-tissue filler injection, including delayed-onset infection, which occurs 2 weeks or more postinjection, and granuloma formation.1-3 Chronic low-grade infection at the injection site may be indicative of biofilm formation. If an HA filler was used, it should be dissolved with hyaluronidase to help break up the biofilm nidus.3 A course of oral antibiotics also may be indicated.1-3 Intralesional steroids may be used but only after antibiotics have been administered. A biofilm that develops from more permanent fillers may be more difficult to manage. Atypical mycobacterial infections have been known to develop at injection sites, which should be considered in refractory cases.1-3,5
Calcium hydroxylapatite, polymethylmethacrylate, and silicone can stimulate chronic immune system activation, which makes them more prone to granuloma formation.1-3 Once infection is ruled out, granulomas may be treated with intralesional steroids, surgical excision (if the results would be cosmetically acceptable), laser therapy, or potentially local injection of an immunosuppressant (eg, methotrexate, 5-fluorouracil).3
Botulinum Toxin Injections
Patients who are pregnant, lactating, or have neuromuscular disease are not candidates for botulinum toxin injections. There also is a risk that patients taking calcium channel blockers or aminoglycoside antibiotics may experience potentiated effects of the botulinum toxin.6
Patients should be informed that a postinjection headache may occur and should be treated with over-the-counter medications.6 Complication-free botulinum toxin procedures depend heavily on the physician’s knowledge of facial anatomy.1,6 The diagrams provided by Hirsch and Stier1 offer an excellent guide on where to place the injections. Brow droop, eyelid ptosis, and “Spock brow” (eyebrows that are overarched) largely can be avoided by proper injection point placement. A Spock brow may be corrected by injecting the lateral upper forehead with a few units to correct the exaggerated arch.6,7 For eyelid ptosis, apraclonidine 0.05% drops (1–2 drops 3 times daily) should be used until the ptosis resolves.6 Phenylephrine hydrochloride drops may be used should a patient have a documented sensitivity to apraclonidine but should not be used in a patient with acute angle-closure glaucoma or aneurysms.6
Final Thoughts
Learning to perform soft-tissue augmentation and botulinum toxin injections can be a satisfying and fun part of dermatology residency. Preventing, identifying, and managing any complications that may occur is an integral part of performing these procedures.
The primary cosmetic procedures that dermatology residents will perform or assist in performing during their training are soft-tissue augmentation, botulinum toxin injections, chemical peels, and laser therapy. Because complications can occur from these procedures, it is important for residents to learn how to prevent, identify, and manage them for optimal patient outcomes. In part 1 of this 2-part series, soft-tissue augmentation and botulinum toxin injections are discussed. Chemical peels and laser therapy will be addressed in part 2.
Soft-Tissue Augmentation
Soft-tissue fillers include those that are made from collagen (bovine or human), hyaluronic acid (HA), poly-L-lactic acid, calcium hydroxylapatite, silicone, and polymethylmethacrylate. In general, acute complications of soft-tissue filler injections include erythema, swelling, and bruising.1-3 Patients who take blood thinners or supplements (eg, vitamin E, ginseng, garlic, ginger) should be asked to discontinue use 1 week prior to the procedure. Patients who take blood thinners also should be counseled to expect some bruising. Prior to injection, the skin should be thoroughly cleansed to avoid introducing skin bacteria into the injection site and to reduce infection risk. Postinjection erythema may be related to mast cell activation, which is temporary and should resolve after a few days.1-3
If you find yourself injecting the filler too superficially, you may notice that the skin begins to take on a blue-gray hue1-3 that is known as the Tyndall effect and can be prevented by injecting the filler at the proper level. For example, collagen-based fillers should be placed at the mid dermis, thicker HA fillers should be placed in the deep dermis, and calcium hydroxylapatite should be placed at the junction of the dermis and subcutaneous tissue. Polymethylmethacrylate and poly-L-lactic acid should both be placed subdermally.1-3
The gravest immediate complications associated with soft-tissue filler injections are occlusion of the central retinal artery and/or skin necrosis.1-4 Residents should never inject filler to the glabella or to the nose.1-3 Injections at these sites are sometimes performed but should only be performed by experienced dermatologists. The perioral and tear trough regions also are high-risk injection areas that require a high degree of experience and should only be injected with proper supervision by an experienced dermatologist.1-3 Residents generally can avoid these complications, though not with a 100% guarantee, by avoiding injections in high-risk areas, aspirating to check for blood, and slowly injecting a small amount of filler into the treatment area.1-3 A consensus statement on management of injection-induced necrosis advises to apply a nitropaste ointment 2% to the treatment site or administer an oral aspirin if the patient develops severe pain; vision loss; or acute skin discoloration, especially blanching.4 For HA-based fillers, at least 200 U of hyaluronidase should be injected. It has been suggested that saline can be injected to flush out calcium hydroxylapatite fillers.3 Warm compresses should be placed on the involved area. Following these interventions, any patient with vision loss or orbital pain should immediately undergo ophthalmologic evaluation.3 The most important intervention occurs in the first 24 hours.3,4 After 24 hours, careful wound care, oral anticoagulants, and hyperbaric oxygen therapy have been suggested as management options.3
There are 2 major chronic complications of soft-tissue filler injection, including delayed-onset infection, which occurs 2 weeks or more postinjection, and granuloma formation.1-3 Chronic low-grade infection at the injection site may be indicative of biofilm formation. If an HA filler was used, it should be dissolved with hyaluronidase to help break up the biofilm nidus.3 A course of oral antibiotics also may be indicated.1-3 Intralesional steroids may be used but only after antibiotics have been administered. A biofilm that develops from more permanent fillers may be more difficult to manage. Atypical mycobacterial infections have been known to develop at injection sites, which should be considered in refractory cases.1-3,5
Calcium hydroxylapatite, polymethylmethacrylate, and silicone can stimulate chronic immune system activation, which makes them more prone to granuloma formation.1-3 Once infection is ruled out, granulomas may be treated with intralesional steroids, surgical excision (if the results would be cosmetically acceptable), laser therapy, or potentially local injection of an immunosuppressant (eg, methotrexate, 5-fluorouracil).3
Botulinum Toxin Injections
Patients who are pregnant, lactating, or have neuromuscular disease are not candidates for botulinum toxin injections. There also is a risk that patients taking calcium channel blockers or aminoglycoside antibiotics may experience potentiated effects of the botulinum toxin.6
Patients should be informed that a postinjection headache may occur and should be treated with over-the-counter medications.6 Complication-free botulinum toxin procedures depend heavily on the physician’s knowledge of facial anatomy.1,6 The diagrams provided by Hirsch and Stier1 offer an excellent guide on where to place the injections. Brow droop, eyelid ptosis, and “Spock brow” (eyebrows that are overarched) largely can be avoided by proper injection point placement. A Spock brow may be corrected by injecting the lateral upper forehead with a few units to correct the exaggerated arch.6,7 For eyelid ptosis, apraclonidine 0.05% drops (1–2 drops 3 times daily) should be used until the ptosis resolves.6 Phenylephrine hydrochloride drops may be used should a patient have a documented sensitivity to apraclonidine but should not be used in a patient with acute angle-closure glaucoma or aneurysms.6
Final Thoughts
Learning to perform soft-tissue augmentation and botulinum toxin injections can be a satisfying and fun part of dermatology residency. Preventing, identifying, and managing any complications that may occur is an integral part of performing these procedures.
- Hirsch R, Stier M. Complications and their management in cosmetic dermatology. Dermatol Clin. 2009;27:507-520.
- Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26:34-39.
- Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205-214.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler–induced impending necrosis with hyaluronidase: consensus recommendations [published online May 10, 2015]. Aesthet Surg J. 2015;35:844-849.
- Rodriguez JM, Xie YL, Winthrop KL, et al. Mycobacterium chelonae facial infections following injection of dermal filler. Aesthet Surg J. 2013;33:265-269.
- Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55:8-14.
- Carruthers A, Carruthers J. Update on the botulinum neurotoxins. Skin Therapy Lett. 2001;6:1-2.
- Hirsch R, Stier M. Complications and their management in cosmetic dermatology. Dermatol Clin. 2009;27:507-520.
- Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26:34-39.
- Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205-214.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler–induced impending necrosis with hyaluronidase: consensus recommendations [published online May 10, 2015]. Aesthet Surg J. 2015;35:844-849.
- Rodriguez JM, Xie YL, Winthrop KL, et al. Mycobacterium chelonae facial infections following injection of dermal filler. Aesthet Surg J. 2013;33:265-269.
- Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55:8-14.
- Carruthers A, Carruthers J. Update on the botulinum neurotoxins. Skin Therapy Lett. 2001;6:1-2.
A review of 2015 drug approvals: Safety in pregnancy and lactation
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Long-acting opioids increase cardiovascular deaths in noncancer patients
Long-acting opioids increased the risk of all-cause mortality in patients with chronic noncancer pain, based on 22,912 new episodes of long-acting opioid prescription and an equal number of control prescriptions during an average follow-up of 176 days and 128 days respectively.
In the follow-up period, 185 people given long-acting opioids and 87 in the control group died. The hazard ratio for total mortality in the opioid group was 1.64, according to Wayne Ray, Ph.D., of Vanderbilt University, Nashville, and his associates.
Out-of-hospital deaths accounted for 154 deaths in the opioid group and 60 in the control group, with a hazard ratio of 1.9. The hazard ratio in the opioid group for out-of-hospital deaths other than unintentional overdose was 1.72. Cardiovascular events were the most common cause of death, accounting for 79 deaths in the opioid group and for 53 deaths in the control group. The hazard ratio for cardiovascular death was 1.65.
“The study finding that prescription of long-acting opioids was associated with increased cardiovascular and other nonoverdose mortality adds to the already considerable known harms of the opioids and thus should be considered when assessing the benefits and harms of medications for chronic pain,” the investigators noted.
Find the full study in JAMA (doi: 10.1001/jama.2016.7789).
Long-acting opioids increased the risk of all-cause mortality in patients with chronic noncancer pain, based on 22,912 new episodes of long-acting opioid prescription and an equal number of control prescriptions during an average follow-up of 176 days and 128 days respectively.
In the follow-up period, 185 people given long-acting opioids and 87 in the control group died. The hazard ratio for total mortality in the opioid group was 1.64, according to Wayne Ray, Ph.D., of Vanderbilt University, Nashville, and his associates.
Out-of-hospital deaths accounted for 154 deaths in the opioid group and 60 in the control group, with a hazard ratio of 1.9. The hazard ratio in the opioid group for out-of-hospital deaths other than unintentional overdose was 1.72. Cardiovascular events were the most common cause of death, accounting for 79 deaths in the opioid group and for 53 deaths in the control group. The hazard ratio for cardiovascular death was 1.65.
“The study finding that prescription of long-acting opioids was associated with increased cardiovascular and other nonoverdose mortality adds to the already considerable known harms of the opioids and thus should be considered when assessing the benefits and harms of medications for chronic pain,” the investigators noted.
Find the full study in JAMA (doi: 10.1001/jama.2016.7789).
Long-acting opioids increased the risk of all-cause mortality in patients with chronic noncancer pain, based on 22,912 new episodes of long-acting opioid prescription and an equal number of control prescriptions during an average follow-up of 176 days and 128 days respectively.
In the follow-up period, 185 people given long-acting opioids and 87 in the control group died. The hazard ratio for total mortality in the opioid group was 1.64, according to Wayne Ray, Ph.D., of Vanderbilt University, Nashville, and his associates.
Out-of-hospital deaths accounted for 154 deaths in the opioid group and 60 in the control group, with a hazard ratio of 1.9. The hazard ratio in the opioid group for out-of-hospital deaths other than unintentional overdose was 1.72. Cardiovascular events were the most common cause of death, accounting for 79 deaths in the opioid group and for 53 deaths in the control group. The hazard ratio for cardiovascular death was 1.65.
“The study finding that prescription of long-acting opioids was associated with increased cardiovascular and other nonoverdose mortality adds to the already considerable known harms of the opioids and thus should be considered when assessing the benefits and harms of medications for chronic pain,” the investigators noted.
Find the full study in JAMA (doi: 10.1001/jama.2016.7789).
FROM JAMA