User login
Corticosteroid prophylaxis reduces GVHD in high-risk patients

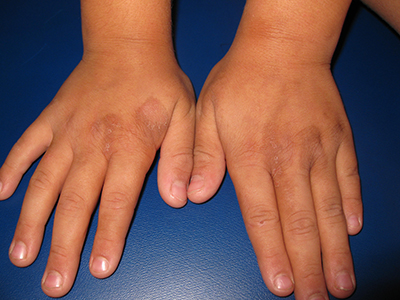
Image courtesy of PLOS ONE
A team of researchers has found that risk-stratified, low-dose corticosteroid prophylaxis can significantly reduce the incidence of acute graft-versus-host disease (GVHD), accelerate platelet recovery, and reduce adverse events without increasing infections in patients who undergo haploidentical transplantation.
They believe this is the first test of a novel risk stratification–directed prophylaxis strategy. The strategy effectively prevented acute GVHD among patients who were at high risk for GVHD, without unnecessarily exposing patients who were at low risk to excessive toxicity from additional immunosuppressive agents.
To evaluate the prophylaxis strategy, the team enrolled 228 patients who underwent haploidentical transplant onto this controlled, open-label, randomized trial.
Corresponding author Xiao-Jun Huang, MD, of Peking University in Beijing, China, and colleagues reported the results in JCO.
The team stratified patients as high risk or low risk for developing GVHD based on biomarkers.
They categorized patients as high risk if they had CD4:CD8 ratios of 1.15 or greater in bone marrow grafts or more than 1.9 x 106/kg CD56bright NK cells in allogeneic grafts.
Patients who did not qualify for the high-risk group were considered to be low risk.
The team randomly assigned the high-risk patients to either receive or not receive the investigational intervention of methylprednisolone (MP).
All patients received cyclosporine, mycophenolate mofetil, and methotrexate for GVHD prevention.
Patients in any arm who developed acute GVHD higher than grade II received MP. If they did not respond, they received basiliximab.
Study population
Of the 228 patients enrolled, 83 were in the low-risk group, 72 in the high-risk experimental prophylaxis group, and 73 in the high-risk control group.
Patient characteristics were similar across all cohorts, including the conditioning regimens, except for their biomarker status.
Patients were a median age of about 30 years, (range 14 – 58), about half were male, and most had a diagnosis of acute myeloid leukemia or acute lymphoblastic leukemia.
GVHD incidence
The cumulative, 100-day incidence of acute GVHD grades II to IV was 21% in the high-risk prophylaxis arm.
This was similar to the incidence of 26% in the low-risk arm, P=0.43.
Both cohorts were significantly lower than the incidence of 48% in the high-risk control group, P<0.001.
Multivariate analysis showed that risk-stratified prophylaxis with low-dose corticosteroid significantly reduced the incidence of acute GVHD compared with no low-dose corticosteroid, with a hazard ratio of 0.66, P=0.007.
However, investigators found no differences between cohorts in the incidence of grades III to IV GVHD.
Corticosteroid-refractory acute GVHD developed in 14% of high-risk patients in the experimental arm, 18% in the control arm, and 23% in the low-risk group. Basiliximab, an anti-CD25 antibody, was used to treat it.
Hematopoietic and immune recovery
The median times to myeloid and platelet recovery were significantly shorter in the high-risk prophylaxis group (P<0.05 for both) and the low-risk group (P<0.05) than in the high-risk control group.
And immune reconstitution was comparable among all 3 cohorts.
Safety
Cumulative incidences among the low-risk, high-risk prophylaxis, and high-risk control groups, respectively, of cytomegalovirus reactivation (80%, 82%, 81%), Epstein-Barr virus reactivation (7%, 14%, 14%), post-transplantation lymphoproliferative disorder (0%, 1%, 4%), hemorrhagic cystitis (56%, 45%, 47%), bacteremia (13%, 10%, 11%), and invasive fungal infection (11%, 8%, 8%) were similar across the cohorts.
Osteonecrosis of the femoral head (P=0.034) and secondary hypertension (P=0.015) were significantly lower in the high-risk prophylaxis group than in the high-risk control group.
Transplant outcomes
Transplant outcomes, both relapse and non-relapse mortality at 100 days and 1 year, were similar among the 3 cohorts.
After a median follow-up of 505 days, the cumulative incidence of chronic GVHD was 44% in the high-risk prophylaxis group, 64% in the low-risk group, and 59% in the high-risk control group.
However, moderate to severe chronic GVHD was lower in the high-risk prophylaxis group, at 21%, compared to 50% in the low-risk group and 36% in the high-risk control group.
Thirty-two patients died of non-relapse causes, and the 3-year probabilities of leukemia-free survival and overall survival were similar among the 3 groups. ![]()

Image courtesy of PLOS ONE
A team of researchers has found that risk-stratified, low-dose corticosteroid prophylaxis can significantly reduce the incidence of acute graft-versus-host disease (GVHD), accelerate platelet recovery, and reduce adverse events without increasing infections in patients who undergo haploidentical transplantation.
They believe this is the first test of a novel risk stratification–directed prophylaxis strategy. The strategy effectively prevented acute GVHD among patients who were at high risk for GVHD, without unnecessarily exposing patients who were at low risk to excessive toxicity from additional immunosuppressive agents.
To evaluate the prophylaxis strategy, the team enrolled 228 patients who underwent haploidentical transplant onto this controlled, open-label, randomized trial.
Corresponding author Xiao-Jun Huang, MD, of Peking University in Beijing, China, and colleagues reported the results in JCO.
The team stratified patients as high risk or low risk for developing GVHD based on biomarkers.
They categorized patients as high risk if they had CD4:CD8 ratios of 1.15 or greater in bone marrow grafts or more than 1.9 x 106/kg CD56bright NK cells in allogeneic grafts.
Patients who did not qualify for the high-risk group were considered to be low risk.
The team randomly assigned the high-risk patients to either receive or not receive the investigational intervention of methylprednisolone (MP).
All patients received cyclosporine, mycophenolate mofetil, and methotrexate for GVHD prevention.
Patients in any arm who developed acute GVHD higher than grade II received MP. If they did not respond, they received basiliximab.
Study population
Of the 228 patients enrolled, 83 were in the low-risk group, 72 in the high-risk experimental prophylaxis group, and 73 in the high-risk control group.
Patient characteristics were similar across all cohorts, including the conditioning regimens, except for their biomarker status.
Patients were a median age of about 30 years, (range 14 – 58), about half were male, and most had a diagnosis of acute myeloid leukemia or acute lymphoblastic leukemia.
GVHD incidence
The cumulative, 100-day incidence of acute GVHD grades II to IV was 21% in the high-risk prophylaxis arm.
This was similar to the incidence of 26% in the low-risk arm, P=0.43.
Both cohorts were significantly lower than the incidence of 48% in the high-risk control group, P<0.001.
Multivariate analysis showed that risk-stratified prophylaxis with low-dose corticosteroid significantly reduced the incidence of acute GVHD compared with no low-dose corticosteroid, with a hazard ratio of 0.66, P=0.007.
However, investigators found no differences between cohorts in the incidence of grades III to IV GVHD.
Corticosteroid-refractory acute GVHD developed in 14% of high-risk patients in the experimental arm, 18% in the control arm, and 23% in the low-risk group. Basiliximab, an anti-CD25 antibody, was used to treat it.
Hematopoietic and immune recovery
The median times to myeloid and platelet recovery were significantly shorter in the high-risk prophylaxis group (P<0.05 for both) and the low-risk group (P<0.05) than in the high-risk control group.
And immune reconstitution was comparable among all 3 cohorts.
Safety
Cumulative incidences among the low-risk, high-risk prophylaxis, and high-risk control groups, respectively, of cytomegalovirus reactivation (80%, 82%, 81%), Epstein-Barr virus reactivation (7%, 14%, 14%), post-transplantation lymphoproliferative disorder (0%, 1%, 4%), hemorrhagic cystitis (56%, 45%, 47%), bacteremia (13%, 10%, 11%), and invasive fungal infection (11%, 8%, 8%) were similar across the cohorts.
Osteonecrosis of the femoral head (P=0.034) and secondary hypertension (P=0.015) were significantly lower in the high-risk prophylaxis group than in the high-risk control group.
Transplant outcomes
Transplant outcomes, both relapse and non-relapse mortality at 100 days and 1 year, were similar among the 3 cohorts.
After a median follow-up of 505 days, the cumulative incidence of chronic GVHD was 44% in the high-risk prophylaxis group, 64% in the low-risk group, and 59% in the high-risk control group.
However, moderate to severe chronic GVHD was lower in the high-risk prophylaxis group, at 21%, compared to 50% in the low-risk group and 36% in the high-risk control group.
Thirty-two patients died of non-relapse causes, and the 3-year probabilities of leukemia-free survival and overall survival were similar among the 3 groups. ![]()

Image courtesy of PLOS ONE
A team of researchers has found that risk-stratified, low-dose corticosteroid prophylaxis can significantly reduce the incidence of acute graft-versus-host disease (GVHD), accelerate platelet recovery, and reduce adverse events without increasing infections in patients who undergo haploidentical transplantation.
They believe this is the first test of a novel risk stratification–directed prophylaxis strategy. The strategy effectively prevented acute GVHD among patients who were at high risk for GVHD, without unnecessarily exposing patients who were at low risk to excessive toxicity from additional immunosuppressive agents.
To evaluate the prophylaxis strategy, the team enrolled 228 patients who underwent haploidentical transplant onto this controlled, open-label, randomized trial.
Corresponding author Xiao-Jun Huang, MD, of Peking University in Beijing, China, and colleagues reported the results in JCO.
The team stratified patients as high risk or low risk for developing GVHD based on biomarkers.
They categorized patients as high risk if they had CD4:CD8 ratios of 1.15 or greater in bone marrow grafts or more than 1.9 x 106/kg CD56bright NK cells in allogeneic grafts.
Patients who did not qualify for the high-risk group were considered to be low risk.
The team randomly assigned the high-risk patients to either receive or not receive the investigational intervention of methylprednisolone (MP).
All patients received cyclosporine, mycophenolate mofetil, and methotrexate for GVHD prevention.
Patients in any arm who developed acute GVHD higher than grade II received MP. If they did not respond, they received basiliximab.
Study population
Of the 228 patients enrolled, 83 were in the low-risk group, 72 in the high-risk experimental prophylaxis group, and 73 in the high-risk control group.
Patient characteristics were similar across all cohorts, including the conditioning regimens, except for their biomarker status.
Patients were a median age of about 30 years, (range 14 – 58), about half were male, and most had a diagnosis of acute myeloid leukemia or acute lymphoblastic leukemia.
GVHD incidence
The cumulative, 100-day incidence of acute GVHD grades II to IV was 21% in the high-risk prophylaxis arm.
This was similar to the incidence of 26% in the low-risk arm, P=0.43.
Both cohorts were significantly lower than the incidence of 48% in the high-risk control group, P<0.001.
Multivariate analysis showed that risk-stratified prophylaxis with low-dose corticosteroid significantly reduced the incidence of acute GVHD compared with no low-dose corticosteroid, with a hazard ratio of 0.66, P=0.007.
However, investigators found no differences between cohorts in the incidence of grades III to IV GVHD.
Corticosteroid-refractory acute GVHD developed in 14% of high-risk patients in the experimental arm, 18% in the control arm, and 23% in the low-risk group. Basiliximab, an anti-CD25 antibody, was used to treat it.
Hematopoietic and immune recovery
The median times to myeloid and platelet recovery were significantly shorter in the high-risk prophylaxis group (P<0.05 for both) and the low-risk group (P<0.05) than in the high-risk control group.
And immune reconstitution was comparable among all 3 cohorts.
Safety
Cumulative incidences among the low-risk, high-risk prophylaxis, and high-risk control groups, respectively, of cytomegalovirus reactivation (80%, 82%, 81%), Epstein-Barr virus reactivation (7%, 14%, 14%), post-transplantation lymphoproliferative disorder (0%, 1%, 4%), hemorrhagic cystitis (56%, 45%, 47%), bacteremia (13%, 10%, 11%), and invasive fungal infection (11%, 8%, 8%) were similar across the cohorts.
Osteonecrosis of the femoral head (P=0.034) and secondary hypertension (P=0.015) were significantly lower in the high-risk prophylaxis group than in the high-risk control group.
Transplant outcomes
Transplant outcomes, both relapse and non-relapse mortality at 100 days and 1 year, were similar among the 3 cohorts.
After a median follow-up of 505 days, the cumulative incidence of chronic GVHD was 44% in the high-risk prophylaxis group, 64% in the low-risk group, and 59% in the high-risk control group.
However, moderate to severe chronic GVHD was lower in the high-risk prophylaxis group, at 21%, compared to 50% in the low-risk group and 36% in the high-risk control group.
Thirty-two patients died of non-relapse causes, and the 3-year probabilities of leukemia-free survival and overall survival were similar among the 3 groups. ![]()
Neoadjuvant chemo for advanced ovarian cancer opens ‘window of opportunity’ for immunotherapies
Presurgical neoadjuvant chemotherapy (NACT) altered the immune microenvironment in patients with stage IIIC/IV tubo-ovarian high-grade serous carcinoma (HGSC), perhaps enhancing the potential response to immunotherapy, investigators report.
“The results suggest that the effects of immunotherapy might be enhanced if given after chemotherapy, potentially improving disease control in patients with advanced HGSC and other cancer types,” wrote Dr. Steffen Bohm of Barts Cancer Institute in London and his associates (CCR. 2016. doi: 10.1158/1078-0432.CCR-15-2657).
Dr. Bohm and associates collected pre- and postchemotherapy biopsies and blood samples from 60 patients with stage IIIC or IV HGSC; 54 patients underwent platinum-based neoadjuvant chemotherapy and 6 received chemotherapy after debulking surgery. The investigators used immunohistochemistry and RNA sequencing to evaluate the changes before and after chemotherapy. The patients were grouped by their response to chemotherapy.
Results indicated that NACT induced T cell activation, with results most pronounced among those who had a good response to chemotherapy. Among a subset of 25 patients, omental metastases biopsies indicated CD8+ T cells and memory cells were present in the tumors after NACT. Proinflammatory cytokines decreased in all patients following NACT.
Immunohistochemical staining for PD-1 ligands showed that PD-L1 levels were significantly increased in post-NACT samples regardless of a patient’s response to chemotherapy.
“Our results suggest that NACT opens a window of opportunity for immunotherapies such as immune checkpoint blockade for patients with different levels of response to chemotherapy,” Dr. Bohm and associates said.
The study was funded by the Swiss Cancer League, the European Research Council, Cancer Research UK, and Barts and The London Charity. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
Presurgical neoadjuvant chemotherapy (NACT) altered the immune microenvironment in patients with stage IIIC/IV tubo-ovarian high-grade serous carcinoma (HGSC), perhaps enhancing the potential response to immunotherapy, investigators report.
“The results suggest that the effects of immunotherapy might be enhanced if given after chemotherapy, potentially improving disease control in patients with advanced HGSC and other cancer types,” wrote Dr. Steffen Bohm of Barts Cancer Institute in London and his associates (CCR. 2016. doi: 10.1158/1078-0432.CCR-15-2657).
Dr. Bohm and associates collected pre- and postchemotherapy biopsies and blood samples from 60 patients with stage IIIC or IV HGSC; 54 patients underwent platinum-based neoadjuvant chemotherapy and 6 received chemotherapy after debulking surgery. The investigators used immunohistochemistry and RNA sequencing to evaluate the changes before and after chemotherapy. The patients were grouped by their response to chemotherapy.
Results indicated that NACT induced T cell activation, with results most pronounced among those who had a good response to chemotherapy. Among a subset of 25 patients, omental metastases biopsies indicated CD8+ T cells and memory cells were present in the tumors after NACT. Proinflammatory cytokines decreased in all patients following NACT.
Immunohistochemical staining for PD-1 ligands showed that PD-L1 levels were significantly increased in post-NACT samples regardless of a patient’s response to chemotherapy.
“Our results suggest that NACT opens a window of opportunity for immunotherapies such as immune checkpoint blockade for patients with different levels of response to chemotherapy,” Dr. Bohm and associates said.
The study was funded by the Swiss Cancer League, the European Research Council, Cancer Research UK, and Barts and The London Charity. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
Presurgical neoadjuvant chemotherapy (NACT) altered the immune microenvironment in patients with stage IIIC/IV tubo-ovarian high-grade serous carcinoma (HGSC), perhaps enhancing the potential response to immunotherapy, investigators report.
“The results suggest that the effects of immunotherapy might be enhanced if given after chemotherapy, potentially improving disease control in patients with advanced HGSC and other cancer types,” wrote Dr. Steffen Bohm of Barts Cancer Institute in London and his associates (CCR. 2016. doi: 10.1158/1078-0432.CCR-15-2657).
Dr. Bohm and associates collected pre- and postchemotherapy biopsies and blood samples from 60 patients with stage IIIC or IV HGSC; 54 patients underwent platinum-based neoadjuvant chemotherapy and 6 received chemotherapy after debulking surgery. The investigators used immunohistochemistry and RNA sequencing to evaluate the changes before and after chemotherapy. The patients were grouped by their response to chemotherapy.
Results indicated that NACT induced T cell activation, with results most pronounced among those who had a good response to chemotherapy. Among a subset of 25 patients, omental metastases biopsies indicated CD8+ T cells and memory cells were present in the tumors after NACT. Proinflammatory cytokines decreased in all patients following NACT.
Immunohistochemical staining for PD-1 ligands showed that PD-L1 levels were significantly increased in post-NACT samples regardless of a patient’s response to chemotherapy.
“Our results suggest that NACT opens a window of opportunity for immunotherapies such as immune checkpoint blockade for patients with different levels of response to chemotherapy,” Dr. Bohm and associates said.
The study was funded by the Swiss Cancer League, the European Research Council, Cancer Research UK, and Barts and The London Charity. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
FROM CLINICAL CANCER RESEARCH
Key clinical point: Neoadjuvant chemotherapy alters the immune microenvironment in patients with advanced ovarian cancer.
Major finding: Neoadjuvant chemotherapy induced T cell activity in patients with stage IIIC/IV tubo-ovarian high-grade serous carcinoma. Proinflammatory cytokines decreased in all patients following neoadjuvant chemotherapy.
Data source: A longitudinal cohort study of 60 patients with stage IIIC/IV tubo-ovarian high-grade serous carcinoma.
Disclosures: The research was funded by the Swiss Cancer League, the European Research Council, Cancer Research UK, and Barts and The London Charity. The authors had no relevant disclosures to report.
Simplify cardiac risk assessment for rheumatologic conditions
LONDON – Cardiovascular disease (CVD) risk assessment for patients with rheumatic diseases can be simple and integrated into general practice or rheumatology clinics, experts said during an Outcomes Science Session at the European Congress of Rheumatology
Patients with rheumatoid arthritis have a 50% higher risk of heart disease than do their counterparts without the disease, but “just having RA on its own isn’t sufficient to render that individual at high risk,” Dr. Naveed Sattar, professor of metabolic medicine at the University of Glasgow’s Institute of Cardiovascular and Medical Sciences in Scotland, said in an interview.
It’s simple enough to use traditional CVD risk factors in an RA population by including a patient’s age, gender, smoking status, and family history of heart disease, in addition to measuring blood pressure and blood lipid levels. Most risk scores will compile those features into a 10-year risk of a fatal CVD event. To account for the contribution of RA, Dr. Sattar said, simply multiply that score by 1.5.
While “there’s a fixation in some parts of Europe for [measuring] fasting lipids,” it is not necessary, Dr. Sattar said. The two lipid parameters that go in risk scores tend to be cholesterol and HDL cholesterol, he said, which change only minimally in fasting versus nonfasting states.
“The evidence overwhelmingly shows that nonfasting lipids, which can be done easily on the same sample as other clinic tests, are just as predictive of CVD risk as fasting lipids,” he said. “That really matters because many of our patients with RA or other conditions come to the hospital when they’re not fasting, and we shouldn’t be sending them away to come back fasting to do risk scores for CVD. That just doesn’t make sense.”
Updated guidelines from the European Society of Cardiology and guidelines soon to be released from the European League Against Rheumatism suggest that risk scores can be calculated every 5 years for most patients, a change from previous recommendations to calculate risk annually. Risk scoring is not perfect, however, and there is some debate about whether additional blood tests or ultrasound scanning of the carotid artery could augment the ability to predict heart disease risk. “We’re not quite there yet,” Dr. Sattar said. “I think we should do the simple things first and do them well.”
CV risk raised in all inflammatory arthritic diseases
During the same session, Dr. Paola de Pablo of the University of Birmingham, England, focused on how immune-mediated diseases predispose to premature, accelerated atherosclerosis and subsequent increased cardiovascular morbidity and mortality.
Cardiovascular risk is not only elevated in those with RA, she observed, but also in those with systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, vasculitides, and inflammatory myopathies. The risk varies but as a rule is more than 50% higher than the rate seen in the general population.
The underlying mechanisms are not clear, but chronic inflammation is closely linked with atherosclerosis, which in turn ups the risk for myocardial infarction and cerebrovascular accident.
Despite treatment, the risk often remains, Dr. de Pablo said. She highlighted how treatment with methotrexate and anti–tumor necrosis factor (TNF)–alpha drugs in RA had been associated with a reduction in the risk for heart attack of 20% and 40%, respectively (Ann Rheum Dis. 2014;74:480–89) so targeting inflammation with these drugs may have positive effects, at least in RA.
Managing traditional cardiovascular risk factors remains important, Dr. de Pablo said. That was a sentiment echoed by Dr. Sattar and by rheumatologist Dr. Michael Nurmohamed of the VU Medical Center in Amsterdam. This includes controlling blood pressure with antihypertensive medications and blood lipids with statins, and advocating smoking cessation and perhaps other appropriate lifestyle changes such as increasing physical exercise and controlling weight.
Dr. Nurmohamed, who was involved in the 2015 update of the EULAR recommendations on cardiovascular risk management, noted that traditional risk factor management in patients with arthritis in current clinical practice is often poor and that strategies to address this were urgently needed.
Although treating to-target and preventing disease flares in the rheumatic diseases is important, it lowers but does not normalize cardiovascular risk. “This appears to be irrespective of the drug used,” Dr. Nurmohamed said. Rheumatologists need to be careful when tapering medication, particularly the biologics, as these are perhaps helping to temper cardiovascular inflammation, which could worsen when doses are reduced. “Antirheumatic treatment only is not good enough to decrease or normalize the cardiovascular risk of our patients”, he emphasized.
Norwegian project shows how to integrate CVD assessment into routine practice
In a separate presentation, Dr. Eirik Ikdahl, a PhD student at Diakonhjemmet Hospital in Oslo, discussed how some rheumatology clinics in Norway are successfully incorporating CVD risk screening.
Through the Norwegian Collaboration on Atherosclerotic disease in patients with Rheumatic joint diseases (NOCAR), which started in April 2014, annual cardiovascular disease risk evaluations of patients with inflammatory joint diseases are being implemented into the practices of 11 rheumatology outpatient clinics. While waiting for clinic appointments, patients are given electronic devices through which they can report CVD risk factors via an electronic patient journal program called GoTreatIt Rheuma. From there, the clinic can order nonfasting lipid measurements and nurses can record patients’ blood pressure.
Then, using the ESC Systematic Coronary Risk Evaluation (SCORE) algorithm, the program automatically calculates a patient’s 10-year risk of a fatal CVD event. If the SCORE estimate is 5% or greater, the rheumatologist forwards a note to the patient’s primary care physician or cardiologist saying there is an indication for initiation of CVD-preventive measures such as medication or lifestyle changes. Rheumatologists and rheumatology nurses also deliver brief advice regarding smoking cessation and healthy diet.
“The main aim of the project is to raise awareness of the cardiovascular burden that these patients experience, and to ensure that patients with inflammatory joint diseases receive guideline-recommended cardiovascular preventive treatment,” Dr. Ikdahl said.
Of 6,150 patients defined as eligible for the NOCAR project in three of the centers, 41% (n = 2,519) received a CVD risk assessment during the first year and a half of the program, officers found in a recent review. Of those, 1,569 had RA, 418 had ankylosing spondylitis, 350 had psoriatic arthritis, and 122 had other spondyloarthritides.
Through the program, “a large number of high-risk patients have received screening that they would not otherwise have been offered,” Dr. Ikdahl said.
The major obstacles to successful implementation were time scarcity, defining a date for annual CVD risk assessment among patients who visit the clinics multiple times per year, and making sure lipids were measured before seeing the rheumatologist, he said. “We acknowledge there is room for improvement. It is challenging to implement new work tasks in an already busy rheumatology outpatient clinic, and since the project does not offer financial incentives to the participating centers, we rely on a collective effort and voluntary work based on resources already available.”
Remember CVD, but don’t forget other comorbidities
Other research presented by Dr. Laure Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Pierre & Marie Curie University in Paris highlighted the importance of identifying all comorbidities and their risk factors in patients with rheumatic diseases, and not just cardiovascular disease.
Dr. Gossec presented the results of an initiative aiming to make the collection and management of comorbidities easier in routine rheumatologic practice. The aim was to develop a simple, more pragmatic form that could be used to help rheumatologists manage selected comorbidities, and know when to refer for other specialist assessment. The focus was on ischemic cardiovascular disease, malignancies, infections such as chronic bronchitis, gastrointestinal disease such as diverticulitis, osteoporosis, and depression.
A committee of 18 experts, both physicians and nurses, was convened to examine the results of a systematic literature review of recommendations on comorbidity management and come up with concise recommendations for rheumatologists. Each of their recommendations covered whether or not the comorbidity was present (yes/no/don’t know) and if screening had been undertaken, such as measurement of blood lipids, and when this had occurred if known. There was then guidance on how to interpret these findings, calculate risk, and what to do if findings were abnormal.
The project is ongoing, and so far the expert panel has developed a pragmatic document with forms to help collect, report, and manage each specific comorbidity and its known risk factors. But it is still perhaps too long to be feasibly used in everyday practice, Dr. Gossec conceded. So the aim is to create a short, 2-page form that could summarize the recommendations briefly, and also develop a questionnaire for the patient to fill out and understand how to self-manage some comorbidities.
“We feel that this is a way to disseminate and adapt to the national context for France the EULAR comorbidities initiative,” Dr. Gossec said. “It also defines exactly what rheumatologists should be doing and when they should refer, hopefully to the benefit of our patients.”
Dr. Sattar has participated in advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily and UCB. He has also consulted for Merck and is a member of Roche’s speakers’ bureau. Dr. de Paolo and Dr. Nurmohamed reported no relevant financial disclosures. Dr. Ikdahl has received speaker’s honoraria from Pfizer. Dr. Gossec and coauthors have received honoraria from Abbvie France.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Cardiovascular disease (CVD) risk assessment for patients with rheumatic diseases can be simple and integrated into general practice or rheumatology clinics, experts said during an Outcomes Science Session at the European Congress of Rheumatology
Patients with rheumatoid arthritis have a 50% higher risk of heart disease than do their counterparts without the disease, but “just having RA on its own isn’t sufficient to render that individual at high risk,” Dr. Naveed Sattar, professor of metabolic medicine at the University of Glasgow’s Institute of Cardiovascular and Medical Sciences in Scotland, said in an interview.
It’s simple enough to use traditional CVD risk factors in an RA population by including a patient’s age, gender, smoking status, and family history of heart disease, in addition to measuring blood pressure and blood lipid levels. Most risk scores will compile those features into a 10-year risk of a fatal CVD event. To account for the contribution of RA, Dr. Sattar said, simply multiply that score by 1.5.
While “there’s a fixation in some parts of Europe for [measuring] fasting lipids,” it is not necessary, Dr. Sattar said. The two lipid parameters that go in risk scores tend to be cholesterol and HDL cholesterol, he said, which change only minimally in fasting versus nonfasting states.
“The evidence overwhelmingly shows that nonfasting lipids, which can be done easily on the same sample as other clinic tests, are just as predictive of CVD risk as fasting lipids,” he said. “That really matters because many of our patients with RA or other conditions come to the hospital when they’re not fasting, and we shouldn’t be sending them away to come back fasting to do risk scores for CVD. That just doesn’t make sense.”
Updated guidelines from the European Society of Cardiology and guidelines soon to be released from the European League Against Rheumatism suggest that risk scores can be calculated every 5 years for most patients, a change from previous recommendations to calculate risk annually. Risk scoring is not perfect, however, and there is some debate about whether additional blood tests or ultrasound scanning of the carotid artery could augment the ability to predict heart disease risk. “We’re not quite there yet,” Dr. Sattar said. “I think we should do the simple things first and do them well.”
CV risk raised in all inflammatory arthritic diseases
During the same session, Dr. Paola de Pablo of the University of Birmingham, England, focused on how immune-mediated diseases predispose to premature, accelerated atherosclerosis and subsequent increased cardiovascular morbidity and mortality.
Cardiovascular risk is not only elevated in those with RA, she observed, but also in those with systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, vasculitides, and inflammatory myopathies. The risk varies but as a rule is more than 50% higher than the rate seen in the general population.
The underlying mechanisms are not clear, but chronic inflammation is closely linked with atherosclerosis, which in turn ups the risk for myocardial infarction and cerebrovascular accident.
Despite treatment, the risk often remains, Dr. de Pablo said. She highlighted how treatment with methotrexate and anti–tumor necrosis factor (TNF)–alpha drugs in RA had been associated with a reduction in the risk for heart attack of 20% and 40%, respectively (Ann Rheum Dis. 2014;74:480–89) so targeting inflammation with these drugs may have positive effects, at least in RA.
Managing traditional cardiovascular risk factors remains important, Dr. de Pablo said. That was a sentiment echoed by Dr. Sattar and by rheumatologist Dr. Michael Nurmohamed of the VU Medical Center in Amsterdam. This includes controlling blood pressure with antihypertensive medications and blood lipids with statins, and advocating smoking cessation and perhaps other appropriate lifestyle changes such as increasing physical exercise and controlling weight.
Dr. Nurmohamed, who was involved in the 2015 update of the EULAR recommendations on cardiovascular risk management, noted that traditional risk factor management in patients with arthritis in current clinical practice is often poor and that strategies to address this were urgently needed.
Although treating to-target and preventing disease flares in the rheumatic diseases is important, it lowers but does not normalize cardiovascular risk. “This appears to be irrespective of the drug used,” Dr. Nurmohamed said. Rheumatologists need to be careful when tapering medication, particularly the biologics, as these are perhaps helping to temper cardiovascular inflammation, which could worsen when doses are reduced. “Antirheumatic treatment only is not good enough to decrease or normalize the cardiovascular risk of our patients”, he emphasized.
Norwegian project shows how to integrate CVD assessment into routine practice
In a separate presentation, Dr. Eirik Ikdahl, a PhD student at Diakonhjemmet Hospital in Oslo, discussed how some rheumatology clinics in Norway are successfully incorporating CVD risk screening.
Through the Norwegian Collaboration on Atherosclerotic disease in patients with Rheumatic joint diseases (NOCAR), which started in April 2014, annual cardiovascular disease risk evaluations of patients with inflammatory joint diseases are being implemented into the practices of 11 rheumatology outpatient clinics. While waiting for clinic appointments, patients are given electronic devices through which they can report CVD risk factors via an electronic patient journal program called GoTreatIt Rheuma. From there, the clinic can order nonfasting lipid measurements and nurses can record patients’ blood pressure.
Then, using the ESC Systematic Coronary Risk Evaluation (SCORE) algorithm, the program automatically calculates a patient’s 10-year risk of a fatal CVD event. If the SCORE estimate is 5% or greater, the rheumatologist forwards a note to the patient’s primary care physician or cardiologist saying there is an indication for initiation of CVD-preventive measures such as medication or lifestyle changes. Rheumatologists and rheumatology nurses also deliver brief advice regarding smoking cessation and healthy diet.
“The main aim of the project is to raise awareness of the cardiovascular burden that these patients experience, and to ensure that patients with inflammatory joint diseases receive guideline-recommended cardiovascular preventive treatment,” Dr. Ikdahl said.
Of 6,150 patients defined as eligible for the NOCAR project in three of the centers, 41% (n = 2,519) received a CVD risk assessment during the first year and a half of the program, officers found in a recent review. Of those, 1,569 had RA, 418 had ankylosing spondylitis, 350 had psoriatic arthritis, and 122 had other spondyloarthritides.
Through the program, “a large number of high-risk patients have received screening that they would not otherwise have been offered,” Dr. Ikdahl said.
The major obstacles to successful implementation were time scarcity, defining a date for annual CVD risk assessment among patients who visit the clinics multiple times per year, and making sure lipids were measured before seeing the rheumatologist, he said. “We acknowledge there is room for improvement. It is challenging to implement new work tasks in an already busy rheumatology outpatient clinic, and since the project does not offer financial incentives to the participating centers, we rely on a collective effort and voluntary work based on resources already available.”
Remember CVD, but don’t forget other comorbidities
Other research presented by Dr. Laure Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Pierre & Marie Curie University in Paris highlighted the importance of identifying all comorbidities and their risk factors in patients with rheumatic diseases, and not just cardiovascular disease.
Dr. Gossec presented the results of an initiative aiming to make the collection and management of comorbidities easier in routine rheumatologic practice. The aim was to develop a simple, more pragmatic form that could be used to help rheumatologists manage selected comorbidities, and know when to refer for other specialist assessment. The focus was on ischemic cardiovascular disease, malignancies, infections such as chronic bronchitis, gastrointestinal disease such as diverticulitis, osteoporosis, and depression.
A committee of 18 experts, both physicians and nurses, was convened to examine the results of a systematic literature review of recommendations on comorbidity management and come up with concise recommendations for rheumatologists. Each of their recommendations covered whether or not the comorbidity was present (yes/no/don’t know) and if screening had been undertaken, such as measurement of blood lipids, and when this had occurred if known. There was then guidance on how to interpret these findings, calculate risk, and what to do if findings were abnormal.
The project is ongoing, and so far the expert panel has developed a pragmatic document with forms to help collect, report, and manage each specific comorbidity and its known risk factors. But it is still perhaps too long to be feasibly used in everyday practice, Dr. Gossec conceded. So the aim is to create a short, 2-page form that could summarize the recommendations briefly, and also develop a questionnaire for the patient to fill out and understand how to self-manage some comorbidities.
“We feel that this is a way to disseminate and adapt to the national context for France the EULAR comorbidities initiative,” Dr. Gossec said. “It also defines exactly what rheumatologists should be doing and when they should refer, hopefully to the benefit of our patients.”
Dr. Sattar has participated in advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily and UCB. He has also consulted for Merck and is a member of Roche’s speakers’ bureau. Dr. de Paolo and Dr. Nurmohamed reported no relevant financial disclosures. Dr. Ikdahl has received speaker’s honoraria from Pfizer. Dr. Gossec and coauthors have received honoraria from Abbvie France.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Cardiovascular disease (CVD) risk assessment for patients with rheumatic diseases can be simple and integrated into general practice or rheumatology clinics, experts said during an Outcomes Science Session at the European Congress of Rheumatology
Patients with rheumatoid arthritis have a 50% higher risk of heart disease than do their counterparts without the disease, but “just having RA on its own isn’t sufficient to render that individual at high risk,” Dr. Naveed Sattar, professor of metabolic medicine at the University of Glasgow’s Institute of Cardiovascular and Medical Sciences in Scotland, said in an interview.
It’s simple enough to use traditional CVD risk factors in an RA population by including a patient’s age, gender, smoking status, and family history of heart disease, in addition to measuring blood pressure and blood lipid levels. Most risk scores will compile those features into a 10-year risk of a fatal CVD event. To account for the contribution of RA, Dr. Sattar said, simply multiply that score by 1.5.
While “there’s a fixation in some parts of Europe for [measuring] fasting lipids,” it is not necessary, Dr. Sattar said. The two lipid parameters that go in risk scores tend to be cholesterol and HDL cholesterol, he said, which change only minimally in fasting versus nonfasting states.
“The evidence overwhelmingly shows that nonfasting lipids, which can be done easily on the same sample as other clinic tests, are just as predictive of CVD risk as fasting lipids,” he said. “That really matters because many of our patients with RA or other conditions come to the hospital when they’re not fasting, and we shouldn’t be sending them away to come back fasting to do risk scores for CVD. That just doesn’t make sense.”
Updated guidelines from the European Society of Cardiology and guidelines soon to be released from the European League Against Rheumatism suggest that risk scores can be calculated every 5 years for most patients, a change from previous recommendations to calculate risk annually. Risk scoring is not perfect, however, and there is some debate about whether additional blood tests or ultrasound scanning of the carotid artery could augment the ability to predict heart disease risk. “We’re not quite there yet,” Dr. Sattar said. “I think we should do the simple things first and do them well.”
CV risk raised in all inflammatory arthritic diseases
During the same session, Dr. Paola de Pablo of the University of Birmingham, England, focused on how immune-mediated diseases predispose to premature, accelerated atherosclerosis and subsequent increased cardiovascular morbidity and mortality.
Cardiovascular risk is not only elevated in those with RA, she observed, but also in those with systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, vasculitides, and inflammatory myopathies. The risk varies but as a rule is more than 50% higher than the rate seen in the general population.
The underlying mechanisms are not clear, but chronic inflammation is closely linked with atherosclerosis, which in turn ups the risk for myocardial infarction and cerebrovascular accident.
Despite treatment, the risk often remains, Dr. de Pablo said. She highlighted how treatment with methotrexate and anti–tumor necrosis factor (TNF)–alpha drugs in RA had been associated with a reduction in the risk for heart attack of 20% and 40%, respectively (Ann Rheum Dis. 2014;74:480–89) so targeting inflammation with these drugs may have positive effects, at least in RA.
Managing traditional cardiovascular risk factors remains important, Dr. de Pablo said. That was a sentiment echoed by Dr. Sattar and by rheumatologist Dr. Michael Nurmohamed of the VU Medical Center in Amsterdam. This includes controlling blood pressure with antihypertensive medications and blood lipids with statins, and advocating smoking cessation and perhaps other appropriate lifestyle changes such as increasing physical exercise and controlling weight.
Dr. Nurmohamed, who was involved in the 2015 update of the EULAR recommendations on cardiovascular risk management, noted that traditional risk factor management in patients with arthritis in current clinical practice is often poor and that strategies to address this were urgently needed.
Although treating to-target and preventing disease flares in the rheumatic diseases is important, it lowers but does not normalize cardiovascular risk. “This appears to be irrespective of the drug used,” Dr. Nurmohamed said. Rheumatologists need to be careful when tapering medication, particularly the biologics, as these are perhaps helping to temper cardiovascular inflammation, which could worsen when doses are reduced. “Antirheumatic treatment only is not good enough to decrease or normalize the cardiovascular risk of our patients”, he emphasized.
Norwegian project shows how to integrate CVD assessment into routine practice
In a separate presentation, Dr. Eirik Ikdahl, a PhD student at Diakonhjemmet Hospital in Oslo, discussed how some rheumatology clinics in Norway are successfully incorporating CVD risk screening.
Through the Norwegian Collaboration on Atherosclerotic disease in patients with Rheumatic joint diseases (NOCAR), which started in April 2014, annual cardiovascular disease risk evaluations of patients with inflammatory joint diseases are being implemented into the practices of 11 rheumatology outpatient clinics. While waiting for clinic appointments, patients are given electronic devices through which they can report CVD risk factors via an electronic patient journal program called GoTreatIt Rheuma. From there, the clinic can order nonfasting lipid measurements and nurses can record patients’ blood pressure.
Then, using the ESC Systematic Coronary Risk Evaluation (SCORE) algorithm, the program automatically calculates a patient’s 10-year risk of a fatal CVD event. If the SCORE estimate is 5% or greater, the rheumatologist forwards a note to the patient’s primary care physician or cardiologist saying there is an indication for initiation of CVD-preventive measures such as medication or lifestyle changes. Rheumatologists and rheumatology nurses also deliver brief advice regarding smoking cessation and healthy diet.
“The main aim of the project is to raise awareness of the cardiovascular burden that these patients experience, and to ensure that patients with inflammatory joint diseases receive guideline-recommended cardiovascular preventive treatment,” Dr. Ikdahl said.
Of 6,150 patients defined as eligible for the NOCAR project in three of the centers, 41% (n = 2,519) received a CVD risk assessment during the first year and a half of the program, officers found in a recent review. Of those, 1,569 had RA, 418 had ankylosing spondylitis, 350 had psoriatic arthritis, and 122 had other spondyloarthritides.
Through the program, “a large number of high-risk patients have received screening that they would not otherwise have been offered,” Dr. Ikdahl said.
The major obstacles to successful implementation were time scarcity, defining a date for annual CVD risk assessment among patients who visit the clinics multiple times per year, and making sure lipids were measured before seeing the rheumatologist, he said. “We acknowledge there is room for improvement. It is challenging to implement new work tasks in an already busy rheumatology outpatient clinic, and since the project does not offer financial incentives to the participating centers, we rely on a collective effort and voluntary work based on resources already available.”
Remember CVD, but don’t forget other comorbidities
Other research presented by Dr. Laure Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Pierre & Marie Curie University in Paris highlighted the importance of identifying all comorbidities and their risk factors in patients with rheumatic diseases, and not just cardiovascular disease.
Dr. Gossec presented the results of an initiative aiming to make the collection and management of comorbidities easier in routine rheumatologic practice. The aim was to develop a simple, more pragmatic form that could be used to help rheumatologists manage selected comorbidities, and know when to refer for other specialist assessment. The focus was on ischemic cardiovascular disease, malignancies, infections such as chronic bronchitis, gastrointestinal disease such as diverticulitis, osteoporosis, and depression.
A committee of 18 experts, both physicians and nurses, was convened to examine the results of a systematic literature review of recommendations on comorbidity management and come up with concise recommendations for rheumatologists. Each of their recommendations covered whether or not the comorbidity was present (yes/no/don’t know) and if screening had been undertaken, such as measurement of blood lipids, and when this had occurred if known. There was then guidance on how to interpret these findings, calculate risk, and what to do if findings were abnormal.
The project is ongoing, and so far the expert panel has developed a pragmatic document with forms to help collect, report, and manage each specific comorbidity and its known risk factors. But it is still perhaps too long to be feasibly used in everyday practice, Dr. Gossec conceded. So the aim is to create a short, 2-page form that could summarize the recommendations briefly, and also develop a questionnaire for the patient to fill out and understand how to self-manage some comorbidities.
“We feel that this is a way to disseminate and adapt to the national context for France the EULAR comorbidities initiative,” Dr. Gossec said. “It also defines exactly what rheumatologists should be doing and when they should refer, hopefully to the benefit of our patients.”
Dr. Sattar has participated in advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lily and UCB. He has also consulted for Merck and is a member of Roche’s speakers’ bureau. Dr. de Paolo and Dr. Nurmohamed reported no relevant financial disclosures. Dr. Ikdahl has received speaker’s honoraria from Pfizer. Dr. Gossec and coauthors have received honoraria from Abbvie France.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
Follicular lymphoma with histologic transformation may merit ASCT
Among patients with high tumor burden follicular lymphoma (FL) that responded to rituximab chemotherapy but then underwent histologic transformation, median overall survival was not reached when patients received autologous stem cell transplantation (ASCT), but was only 1.7 years otherwise, based on results of an ancillary study of a clinical trial.
In contrast, ASCT did not affect overall survival when patients progressed to untransformed FL, said Dr. Clémentine Sarkozy of Centre Hospitalier Lyon-Sud in Pierre Bénite, France, and her associates. Fully 58% of histologic transformations occurred in the first year of follow-up, highlighting “the necessity for biopsy at the first recurrence of FL,” they wrote online June 13 in the Journal of Clinical Oncology.
Histologic transformation in FL signifies progression to aggressive lymphoma. Studies of histologic transformation and subsequent overall survival in the rituximab era have been retrospective, with variable patient populations and initial management regimens, according to the investigators. Therefore, they followed 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial, which evaluated maintenance rituximab therapy among patients with symptomatic FL who had responded to induction chemotherapy plus rituximab (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.7163).
A total of 463 patients (45.5%) experienced disease recurrence or progression, and 194 (42%) were biopsied over a median follow-up time of 6 years. A total of 40 (20.6%) biopsies showed histologic transformation, while 154 (79.4%) had untransformed FL. Median time to recurrence was 9.6 months for patients with histologic transformation and 22.8 months for patients with untransformed FL (P = .02). Median overall survival with histologic transformation was worse than with untransformed FL (3.8 years vs. 6.4 years; hazard ratio, 3.9; 95% confidence interval, 2.2-6.9; P = .001). Furthermore, among patients who progressed within 12 months, median overall survival with histologic transformation was 2 years, compared with 6.4 years for patients with untransformed FL (P = .007).
After salvage therapy, 17 (42%) patients with histologic transformation underwent consolidation with high-dose chemotherapy and ASCT. Median overall survival for these patients was not reached, versus 1.7 years when they did not undergo ASCT. In contrast, ASCT did not improve overall survival among patients with untransformed FL. Results were similar after excluding patients with early progression and patients who were older than 65 years, the investigators reported.
Risk factors for histologic transformation in the univariate analysis included performance status, anemia, high lactate dehydrogenase level, “B” symptoms, histologic grade 3a, and high Follicular Lymphoma International Prognostic Index scores at diagnosis. However, only Eastern Cooperative Oncology Group performance status of 2 to 4 (HR, 5.6; 95% CI, 1.7-17.7), and anemia (HR, 3.7; 95% CI, 1.4-9.7) remained significant in the multivariate analysis. Neither the choice of induction regimen nor the quality of response seemed to affect the likelihood of histologic transformation, and rituximab maintenance therapy did not seem to alter response to salvage treatment or survival after histologic transformation. By necessity, the study excluded patients who did not respond to initial immunochemotherapy, which could have limited the generalizability of the findings, the investigators noted.
The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
In just 3 years, prospective observational studies and [this] clever ancillary analysis of a prospective clinical trial have better informed the lymphoma community about the expected incidence and timing of transformation in patients with follicular lymphoma after being treated with modern management strategies. But we are still limited by clumsy predictive tools for identifying patients at highest risk. Deeper understanding of biologic and genetic factors of FL subclonal populations as well as the tumor microenvironment will allow for more precise identification of patients truly at risk and potentially will provide actionable targets for abrogating that risk. Future [studies of] transformed lymphoma will hopefully replace variables such as anthracyclines, the Follicular Lymphoma International Prognostic Index, lactate dehydrogenase, and ASCT with promising new variables such as IRF-4, miR-31, bcl-2, pleuripotency, and nuclear factor kappa B pathway genes or new therapies that target these variables. Future analyses should not simply prognosticate who is at risk for transformation, but should predict a specific intervention to either prevent or treat such an event.
Dr. Brian K. Link is at the University of Iowa, Iowa City. He reported ties to AbbVie, Gilead Sciences, Genentech, Sandoz, Pharmacyclics, Millennium Pharmaceuticals, Genentech, Kite Pharma, Seattle Genetics, and Dynavax Technologies. These comments are from his editorial accompanying the report (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.67.4234).
In just 3 years, prospective observational studies and [this] clever ancillary analysis of a prospective clinical trial have better informed the lymphoma community about the expected incidence and timing of transformation in patients with follicular lymphoma after being treated with modern management strategies. But we are still limited by clumsy predictive tools for identifying patients at highest risk. Deeper understanding of biologic and genetic factors of FL subclonal populations as well as the tumor microenvironment will allow for more precise identification of patients truly at risk and potentially will provide actionable targets for abrogating that risk. Future [studies of] transformed lymphoma will hopefully replace variables such as anthracyclines, the Follicular Lymphoma International Prognostic Index, lactate dehydrogenase, and ASCT with promising new variables such as IRF-4, miR-31, bcl-2, pleuripotency, and nuclear factor kappa B pathway genes or new therapies that target these variables. Future analyses should not simply prognosticate who is at risk for transformation, but should predict a specific intervention to either prevent or treat such an event.
Dr. Brian K. Link is at the University of Iowa, Iowa City. He reported ties to AbbVie, Gilead Sciences, Genentech, Sandoz, Pharmacyclics, Millennium Pharmaceuticals, Genentech, Kite Pharma, Seattle Genetics, and Dynavax Technologies. These comments are from his editorial accompanying the report (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.67.4234).
In just 3 years, prospective observational studies and [this] clever ancillary analysis of a prospective clinical trial have better informed the lymphoma community about the expected incidence and timing of transformation in patients with follicular lymphoma after being treated with modern management strategies. But we are still limited by clumsy predictive tools for identifying patients at highest risk. Deeper understanding of biologic and genetic factors of FL subclonal populations as well as the tumor microenvironment will allow for more precise identification of patients truly at risk and potentially will provide actionable targets for abrogating that risk. Future [studies of] transformed lymphoma will hopefully replace variables such as anthracyclines, the Follicular Lymphoma International Prognostic Index, lactate dehydrogenase, and ASCT with promising new variables such as IRF-4, miR-31, bcl-2, pleuripotency, and nuclear factor kappa B pathway genes or new therapies that target these variables. Future analyses should not simply prognosticate who is at risk for transformation, but should predict a specific intervention to either prevent or treat such an event.
Dr. Brian K. Link is at the University of Iowa, Iowa City. He reported ties to AbbVie, Gilead Sciences, Genentech, Sandoz, Pharmacyclics, Millennium Pharmaceuticals, Genentech, Kite Pharma, Seattle Genetics, and Dynavax Technologies. These comments are from his editorial accompanying the report (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.67.4234).
Among patients with high tumor burden follicular lymphoma (FL) that responded to rituximab chemotherapy but then underwent histologic transformation, median overall survival was not reached when patients received autologous stem cell transplantation (ASCT), but was only 1.7 years otherwise, based on results of an ancillary study of a clinical trial.
In contrast, ASCT did not affect overall survival when patients progressed to untransformed FL, said Dr. Clémentine Sarkozy of Centre Hospitalier Lyon-Sud in Pierre Bénite, France, and her associates. Fully 58% of histologic transformations occurred in the first year of follow-up, highlighting “the necessity for biopsy at the first recurrence of FL,” they wrote online June 13 in the Journal of Clinical Oncology.
Histologic transformation in FL signifies progression to aggressive lymphoma. Studies of histologic transformation and subsequent overall survival in the rituximab era have been retrospective, with variable patient populations and initial management regimens, according to the investigators. Therefore, they followed 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial, which evaluated maintenance rituximab therapy among patients with symptomatic FL who had responded to induction chemotherapy plus rituximab (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.7163).
A total of 463 patients (45.5%) experienced disease recurrence or progression, and 194 (42%) were biopsied over a median follow-up time of 6 years. A total of 40 (20.6%) biopsies showed histologic transformation, while 154 (79.4%) had untransformed FL. Median time to recurrence was 9.6 months for patients with histologic transformation and 22.8 months for patients with untransformed FL (P = .02). Median overall survival with histologic transformation was worse than with untransformed FL (3.8 years vs. 6.4 years; hazard ratio, 3.9; 95% confidence interval, 2.2-6.9; P = .001). Furthermore, among patients who progressed within 12 months, median overall survival with histologic transformation was 2 years, compared with 6.4 years for patients with untransformed FL (P = .007).
After salvage therapy, 17 (42%) patients with histologic transformation underwent consolidation with high-dose chemotherapy and ASCT. Median overall survival for these patients was not reached, versus 1.7 years when they did not undergo ASCT. In contrast, ASCT did not improve overall survival among patients with untransformed FL. Results were similar after excluding patients with early progression and patients who were older than 65 years, the investigators reported.
Risk factors for histologic transformation in the univariate analysis included performance status, anemia, high lactate dehydrogenase level, “B” symptoms, histologic grade 3a, and high Follicular Lymphoma International Prognostic Index scores at diagnosis. However, only Eastern Cooperative Oncology Group performance status of 2 to 4 (HR, 5.6; 95% CI, 1.7-17.7), and anemia (HR, 3.7; 95% CI, 1.4-9.7) remained significant in the multivariate analysis. Neither the choice of induction regimen nor the quality of response seemed to affect the likelihood of histologic transformation, and rituximab maintenance therapy did not seem to alter response to salvage treatment or survival after histologic transformation. By necessity, the study excluded patients who did not respond to initial immunochemotherapy, which could have limited the generalizability of the findings, the investigators noted.
The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
Among patients with high tumor burden follicular lymphoma (FL) that responded to rituximab chemotherapy but then underwent histologic transformation, median overall survival was not reached when patients received autologous stem cell transplantation (ASCT), but was only 1.7 years otherwise, based on results of an ancillary study of a clinical trial.
In contrast, ASCT did not affect overall survival when patients progressed to untransformed FL, said Dr. Clémentine Sarkozy of Centre Hospitalier Lyon-Sud in Pierre Bénite, France, and her associates. Fully 58% of histologic transformations occurred in the first year of follow-up, highlighting “the necessity for biopsy at the first recurrence of FL,” they wrote online June 13 in the Journal of Clinical Oncology.
Histologic transformation in FL signifies progression to aggressive lymphoma. Studies of histologic transformation and subsequent overall survival in the rituximab era have been retrospective, with variable patient populations and initial management regimens, according to the investigators. Therefore, they followed 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial, which evaluated maintenance rituximab therapy among patients with symptomatic FL who had responded to induction chemotherapy plus rituximab (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.7163).
A total of 463 patients (45.5%) experienced disease recurrence or progression, and 194 (42%) were biopsied over a median follow-up time of 6 years. A total of 40 (20.6%) biopsies showed histologic transformation, while 154 (79.4%) had untransformed FL. Median time to recurrence was 9.6 months for patients with histologic transformation and 22.8 months for patients with untransformed FL (P = .02). Median overall survival with histologic transformation was worse than with untransformed FL (3.8 years vs. 6.4 years; hazard ratio, 3.9; 95% confidence interval, 2.2-6.9; P = .001). Furthermore, among patients who progressed within 12 months, median overall survival with histologic transformation was 2 years, compared with 6.4 years for patients with untransformed FL (P = .007).
After salvage therapy, 17 (42%) patients with histologic transformation underwent consolidation with high-dose chemotherapy and ASCT. Median overall survival for these patients was not reached, versus 1.7 years when they did not undergo ASCT. In contrast, ASCT did not improve overall survival among patients with untransformed FL. Results were similar after excluding patients with early progression and patients who were older than 65 years, the investigators reported.
Risk factors for histologic transformation in the univariate analysis included performance status, anemia, high lactate dehydrogenase level, “B” symptoms, histologic grade 3a, and high Follicular Lymphoma International Prognostic Index scores at diagnosis. However, only Eastern Cooperative Oncology Group performance status of 2 to 4 (HR, 5.6; 95% CI, 1.7-17.7), and anemia (HR, 3.7; 95% CI, 1.4-9.7) remained significant in the multivariate analysis. Neither the choice of induction regimen nor the quality of response seemed to affect the likelihood of histologic transformation, and rituximab maintenance therapy did not seem to alter response to salvage treatment or survival after histologic transformation. By necessity, the study excluded patients who did not respond to initial immunochemotherapy, which could have limited the generalizability of the findings, the investigators noted.
The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Histologic transformation of follicular lymphoma tends to occur early and may merit intensive salvage with autologous stem cell transplantation.
Major finding: Median overall survival was not reached among patients who received ASCT and was 1.7 years in those who didn’t have ASCT.
Data source: A study of 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial.
Disclosures: The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
Dually active antiretrovirals protect HIV patients against HBV infection
Dually active antiretroviral therapy (DAART) for HIV-infected patients may be the best treatment option to also protect these individuals against hepatitis B virus (HBV) infection, according to results of a study in the Journal of Infectious Diseases.
“Vaccination against HBV remains the mainstay of preventing HBV acquisition both in HIV-infected and uninfected individuals,” states the study, led by Roger Kouyos, Ph.D., of University Hospital Zürich. “However, owing to HIV’s effect on the immune system, mounting and maintaining a protective immune response against HBV is sometimes unattainable, with a success rate between 18% and 71%.”
Dr. Kouyos and his colleagues collected CD4, CD8, and HIV-1 viral load data for 1,716 patients in the Swiss HIV Cohort Study – an ongoing, prospective cohort study that began in 1988 – and updated the data continuously during follow-up visits every 3 months, on average. Subjects were enrolled if they had negative baseline HBV serology and at least one additional HBV test after the initial one, and were either a man who had sex with men, a heterosexual, or an intravenous drug user – the three major groups examined by the study (J Infect Dis. 2016 May 18. doi:10.1093/infdis/jiw195).
DAART treatment consisted of tenofovir, lamivudine, and emtricitabine. Antiretroviral treatment history was also collected, and at follow-up examinations every 6 months, subjects were asked about occasional sex partners and frequency of condom use during the preceding 6 months. All were tested for at least one HBV marker: surface antigen (HBsAg), anti–hepatitis B core antibodies (AntiHBc), or hepatitis B virus DNA.
Of the 1,716 subjects enrolled, 177 had HBV. Of these, 86 (49%) were men who had sex with men. Most subjects underwent at least two HBV tests, with the median time between tests being 29 months (range of 12-58 months). In the transmission group, the overall incidence rate of HBV was 16 (95% confidence interval, 14-19), while it was 25 (21-31) for men who had sex with men, 9 (6-11) for heterosexuals, and 28 (21-38) for intravenous drug users.
DAART, however, was shown to significantly reduce HBV-infection risk. Univariate analysis yielded a hazard ratio of 0.4 (95% CI, 0.2-0.6), and multivariate analysis showed even more significant effects for intravenous drug users (3.8, 2.4-6.1), men who had sex with men (2.7, 1.7-4.2), and those who had unprotected sex (1.9, 1.4-2.6).
“Our study confirms the importance of viral suppression (and the implicit adherence) in reaching the protective effect of DAART,” the authors concluded, noting that “Our study adds to the growing body of evidence that early antiretroviral therapy initiation, regardless of CD4 counts, has a strong beneficial public health impact, including preexposure prophylaxis of HBV coinfections.”
The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
Dually active antiretroviral therapy (DAART) for HIV-infected patients may be the best treatment option to also protect these individuals against hepatitis B virus (HBV) infection, according to results of a study in the Journal of Infectious Diseases.
“Vaccination against HBV remains the mainstay of preventing HBV acquisition both in HIV-infected and uninfected individuals,” states the study, led by Roger Kouyos, Ph.D., of University Hospital Zürich. “However, owing to HIV’s effect on the immune system, mounting and maintaining a protective immune response against HBV is sometimes unattainable, with a success rate between 18% and 71%.”
Dr. Kouyos and his colleagues collected CD4, CD8, and HIV-1 viral load data for 1,716 patients in the Swiss HIV Cohort Study – an ongoing, prospective cohort study that began in 1988 – and updated the data continuously during follow-up visits every 3 months, on average. Subjects were enrolled if they had negative baseline HBV serology and at least one additional HBV test after the initial one, and were either a man who had sex with men, a heterosexual, or an intravenous drug user – the three major groups examined by the study (J Infect Dis. 2016 May 18. doi:10.1093/infdis/jiw195).
DAART treatment consisted of tenofovir, lamivudine, and emtricitabine. Antiretroviral treatment history was also collected, and at follow-up examinations every 6 months, subjects were asked about occasional sex partners and frequency of condom use during the preceding 6 months. All were tested for at least one HBV marker: surface antigen (HBsAg), anti–hepatitis B core antibodies (AntiHBc), or hepatitis B virus DNA.
Of the 1,716 subjects enrolled, 177 had HBV. Of these, 86 (49%) were men who had sex with men. Most subjects underwent at least two HBV tests, with the median time between tests being 29 months (range of 12-58 months). In the transmission group, the overall incidence rate of HBV was 16 (95% confidence interval, 14-19), while it was 25 (21-31) for men who had sex with men, 9 (6-11) for heterosexuals, and 28 (21-38) for intravenous drug users.
DAART, however, was shown to significantly reduce HBV-infection risk. Univariate analysis yielded a hazard ratio of 0.4 (95% CI, 0.2-0.6), and multivariate analysis showed even more significant effects for intravenous drug users (3.8, 2.4-6.1), men who had sex with men (2.7, 1.7-4.2), and those who had unprotected sex (1.9, 1.4-2.6).
“Our study confirms the importance of viral suppression (and the implicit adherence) in reaching the protective effect of DAART,” the authors concluded, noting that “Our study adds to the growing body of evidence that early antiretroviral therapy initiation, regardless of CD4 counts, has a strong beneficial public health impact, including preexposure prophylaxis of HBV coinfections.”
The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
Dually active antiretroviral therapy (DAART) for HIV-infected patients may be the best treatment option to also protect these individuals against hepatitis B virus (HBV) infection, according to results of a study in the Journal of Infectious Diseases.
“Vaccination against HBV remains the mainstay of preventing HBV acquisition both in HIV-infected and uninfected individuals,” states the study, led by Roger Kouyos, Ph.D., of University Hospital Zürich. “However, owing to HIV’s effect on the immune system, mounting and maintaining a protective immune response against HBV is sometimes unattainable, with a success rate between 18% and 71%.”
Dr. Kouyos and his colleagues collected CD4, CD8, and HIV-1 viral load data for 1,716 patients in the Swiss HIV Cohort Study – an ongoing, prospective cohort study that began in 1988 – and updated the data continuously during follow-up visits every 3 months, on average. Subjects were enrolled if they had negative baseline HBV serology and at least one additional HBV test after the initial one, and were either a man who had sex with men, a heterosexual, or an intravenous drug user – the three major groups examined by the study (J Infect Dis. 2016 May 18. doi:10.1093/infdis/jiw195).
DAART treatment consisted of tenofovir, lamivudine, and emtricitabine. Antiretroviral treatment history was also collected, and at follow-up examinations every 6 months, subjects were asked about occasional sex partners and frequency of condom use during the preceding 6 months. All were tested for at least one HBV marker: surface antigen (HBsAg), anti–hepatitis B core antibodies (AntiHBc), or hepatitis B virus DNA.
Of the 1,716 subjects enrolled, 177 had HBV. Of these, 86 (49%) were men who had sex with men. Most subjects underwent at least two HBV tests, with the median time between tests being 29 months (range of 12-58 months). In the transmission group, the overall incidence rate of HBV was 16 (95% confidence interval, 14-19), while it was 25 (21-31) for men who had sex with men, 9 (6-11) for heterosexuals, and 28 (21-38) for intravenous drug users.
DAART, however, was shown to significantly reduce HBV-infection risk. Univariate analysis yielded a hazard ratio of 0.4 (95% CI, 0.2-0.6), and multivariate analysis showed even more significant effects for intravenous drug users (3.8, 2.4-6.1), men who had sex with men (2.7, 1.7-4.2), and those who had unprotected sex (1.9, 1.4-2.6).
“Our study confirms the importance of viral suppression (and the implicit adherence) in reaching the protective effect of DAART,” the authors concluded, noting that “Our study adds to the growing body of evidence that early antiretroviral therapy initiation, regardless of CD4 counts, has a strong beneficial public health impact, including preexposure prophylaxis of HBV coinfections.”
The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: DAART for HIV and hepatitis B virus consisting of tenofovir, lamivudine, and emtricitabine can be an effective preexposure prophylaxis against HBV coinfection.
Major finding: HBV infection was negatively associated with DAART therapy (0.4, 95% CI, 0.2-0.6), with even stronger indications of protection after adjusting for condomless sex, drug use, and men having sex with men.
Data source: Prospective cohort study of 1,716 patients, with 177 incident HBV cases, from the Swiss HIV Cohort Study.
Disclosures: The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
Hormonal maintenance therapy prolonged PFS in LGSC
CHICAGO – Hormonal maintenance therapy after primary treatment was associated with significantly prolonged progression-free survival when compared with surveillance in a retrospective study of women with stage II-IV low-grade serous carcinoma of the ovary or peritoneum.
The findings are “potentially practice-changing,” Dr. David Marc Gershenson of the University of Texas MD Anderson Cancer Center, Houston, said at the annual meeting of the American Society of Clinical Oncology.
Of 204 women with newly diagnosed low-grade serous carcinoma (LGSC) who underwent primary cytoreductive surgery followed by platinum-based chemotherapy, 70 received hormonal maintenance therapy after completion of primary chemotherapy and 134 underwent surveillance. Median progression-free survival (PFS) was 64.9 vs. 27.3 months, respectively (33 months overall), Dr. Gershenson said.
“To date, 40% of the hormone maintenance therapy group has not relapsed, compared [with] only 12% in the surveillance group,” he said.
Median overall survival was not statistically different, but there was a trend toward an overall survival benefit in the hormonal maintenance therapy group (115.7 vs. 98.8 months). In a subset analysis in women who were clinically disease free at the completion of primary chemotherapy, the median PFS was 29.9 months in 121 women undergoing surveillance, vs. 81.1 months in 27 receiving hormone maintenance therapy. The mean overall survival was also statistically different in the subset analysis (106.8 vs. 191.3 months).
On multivariable analysis, four factors reached significance: hormone maintenance therapy vs. surveillance (hazard ratio, 0.23), peritoneum vs. ovary as primary site (HR, 0.45), no gross residual disease vs. gross residual disease (HR, 0.49),and persistent disease at completion of chemotherapy, vs. no evidence of disease (HR, 0.42).
Patients included in the study presented between 1981 and 2013. They had a median age of 47.6 years, about 3/4 had primary ovarian LGSC, and about 1/4 had primary peritoneal LGSC.
Patients in the hormonal therapy group were treated with letrozole (54%), tamoxifen (29%), anastrozole (3%), leuprolide acetate (7%), depot medroxyprogesterone acetate (1%), and leuprolide acetate + tamoxifen or letrozole (3% each). Median duration of hormonal maintenance therapy was 33.3 months (range of 1-223), and follow-up was at least 2 years in those who had not recurred.
LGSC is a rare subtype that accounts for about 10% of serous carcinomas of the ovary or peritoneum. These cancers may arise de novo or after a diagnosis of serous borderline tumor. LGSC, relative to high-grade serous carcinoma (HGSC), is characterized by younger age at diagnosis, chemoresistance, prolonged overall survival, and aberrations within the MAP kinase signaling pathway, Dr. Gershenson said.
The findings are of interest as primary postoperative platinum-based chemotherapy remains the standard for women with newly diagnosed metastatic ovarian or peritoneal LGSC despite multiple reports indicating that this subtype is relatively chemoresistant. For example, in the AGO database of 5,114 patients accrued to four separate phase III trials, the response rate to chemotherapy in women with suboptimally debulked LGSC was significantly lower than in women with suboptimally debulked HGSC (23% vs. 90%), he said.
However, no prospective clinical trials have been conducted in the front line setting in women with LGSC, he noted.
Recent reports of promising activity of hormonal therapy in the recurrent setting have led to an increase in interest in integrating the modality into the primary treatment setting. One study showed a high frequency of ER and PR expression in LGSC, and another showed that hormonal therapy for recurrent LGSC was associated with an objective response rate of 9%, stable disease rate of 66% and median progression-free survival of 7.4 months.
“Some patients derived several years of benefit,” he said. “In addition, low-grade serous carcinoma bears a striking resemblance to luminal breast cancer in that women who are 35 years of age or younger appear to have a significantly worse prognosis.”
Some have concluded, based on these findings, that platinum-based chemotherapy is of no value in the frontline treatment of LGSC and should be abandoned, Dr. Gershenson said.
The current findings, though limited by factors such as their retrospective nature, missing data, a long study period, and possible referral bias suggest that hormonal maintenance therapy deserves a closer look, Dr. Gershenson said.
“The findings of this hypotheses-generating study are potentially practice changing and warrant further investigation using a prospective trial design,” he concluded.
Dr. Gershenson reported stock ownership in AbbVie, Biogen Idec, Celgene, GlaxoSmithKline, Johnson & Johnson, Merck, and Pfizer; consulting or advisory roles with Clovis Oncology, and patents with Elsevier and UpToDate.
CHICAGO – Hormonal maintenance therapy after primary treatment was associated with significantly prolonged progression-free survival when compared with surveillance in a retrospective study of women with stage II-IV low-grade serous carcinoma of the ovary or peritoneum.
The findings are “potentially practice-changing,” Dr. David Marc Gershenson of the University of Texas MD Anderson Cancer Center, Houston, said at the annual meeting of the American Society of Clinical Oncology.
Of 204 women with newly diagnosed low-grade serous carcinoma (LGSC) who underwent primary cytoreductive surgery followed by platinum-based chemotherapy, 70 received hormonal maintenance therapy after completion of primary chemotherapy and 134 underwent surveillance. Median progression-free survival (PFS) was 64.9 vs. 27.3 months, respectively (33 months overall), Dr. Gershenson said.
“To date, 40% of the hormone maintenance therapy group has not relapsed, compared [with] only 12% in the surveillance group,” he said.
Median overall survival was not statistically different, but there was a trend toward an overall survival benefit in the hormonal maintenance therapy group (115.7 vs. 98.8 months). In a subset analysis in women who were clinically disease free at the completion of primary chemotherapy, the median PFS was 29.9 months in 121 women undergoing surveillance, vs. 81.1 months in 27 receiving hormone maintenance therapy. The mean overall survival was also statistically different in the subset analysis (106.8 vs. 191.3 months).
On multivariable analysis, four factors reached significance: hormone maintenance therapy vs. surveillance (hazard ratio, 0.23), peritoneum vs. ovary as primary site (HR, 0.45), no gross residual disease vs. gross residual disease (HR, 0.49),and persistent disease at completion of chemotherapy, vs. no evidence of disease (HR, 0.42).
Patients included in the study presented between 1981 and 2013. They had a median age of 47.6 years, about 3/4 had primary ovarian LGSC, and about 1/4 had primary peritoneal LGSC.
Patients in the hormonal therapy group were treated with letrozole (54%), tamoxifen (29%), anastrozole (3%), leuprolide acetate (7%), depot medroxyprogesterone acetate (1%), and leuprolide acetate + tamoxifen or letrozole (3% each). Median duration of hormonal maintenance therapy was 33.3 months (range of 1-223), and follow-up was at least 2 years in those who had not recurred.
LGSC is a rare subtype that accounts for about 10% of serous carcinomas of the ovary or peritoneum. These cancers may arise de novo or after a diagnosis of serous borderline tumor. LGSC, relative to high-grade serous carcinoma (HGSC), is characterized by younger age at diagnosis, chemoresistance, prolonged overall survival, and aberrations within the MAP kinase signaling pathway, Dr. Gershenson said.
The findings are of interest as primary postoperative platinum-based chemotherapy remains the standard for women with newly diagnosed metastatic ovarian or peritoneal LGSC despite multiple reports indicating that this subtype is relatively chemoresistant. For example, in the AGO database of 5,114 patients accrued to four separate phase III trials, the response rate to chemotherapy in women with suboptimally debulked LGSC was significantly lower than in women with suboptimally debulked HGSC (23% vs. 90%), he said.
However, no prospective clinical trials have been conducted in the front line setting in women with LGSC, he noted.
Recent reports of promising activity of hormonal therapy in the recurrent setting have led to an increase in interest in integrating the modality into the primary treatment setting. One study showed a high frequency of ER and PR expression in LGSC, and another showed that hormonal therapy for recurrent LGSC was associated with an objective response rate of 9%, stable disease rate of 66% and median progression-free survival of 7.4 months.
“Some patients derived several years of benefit,” he said. “In addition, low-grade serous carcinoma bears a striking resemblance to luminal breast cancer in that women who are 35 years of age or younger appear to have a significantly worse prognosis.”
Some have concluded, based on these findings, that platinum-based chemotherapy is of no value in the frontline treatment of LGSC and should be abandoned, Dr. Gershenson said.
The current findings, though limited by factors such as their retrospective nature, missing data, a long study period, and possible referral bias suggest that hormonal maintenance therapy deserves a closer look, Dr. Gershenson said.
“The findings of this hypotheses-generating study are potentially practice changing and warrant further investigation using a prospective trial design,” he concluded.
Dr. Gershenson reported stock ownership in AbbVie, Biogen Idec, Celgene, GlaxoSmithKline, Johnson & Johnson, Merck, and Pfizer; consulting or advisory roles with Clovis Oncology, and patents with Elsevier and UpToDate.
CHICAGO – Hormonal maintenance therapy after primary treatment was associated with significantly prolonged progression-free survival when compared with surveillance in a retrospective study of women with stage II-IV low-grade serous carcinoma of the ovary or peritoneum.
The findings are “potentially practice-changing,” Dr. David Marc Gershenson of the University of Texas MD Anderson Cancer Center, Houston, said at the annual meeting of the American Society of Clinical Oncology.
Of 204 women with newly diagnosed low-grade serous carcinoma (LGSC) who underwent primary cytoreductive surgery followed by platinum-based chemotherapy, 70 received hormonal maintenance therapy after completion of primary chemotherapy and 134 underwent surveillance. Median progression-free survival (PFS) was 64.9 vs. 27.3 months, respectively (33 months overall), Dr. Gershenson said.
“To date, 40% of the hormone maintenance therapy group has not relapsed, compared [with] only 12% in the surveillance group,” he said.
Median overall survival was not statistically different, but there was a trend toward an overall survival benefit in the hormonal maintenance therapy group (115.7 vs. 98.8 months). In a subset analysis in women who were clinically disease free at the completion of primary chemotherapy, the median PFS was 29.9 months in 121 women undergoing surveillance, vs. 81.1 months in 27 receiving hormone maintenance therapy. The mean overall survival was also statistically different in the subset analysis (106.8 vs. 191.3 months).
On multivariable analysis, four factors reached significance: hormone maintenance therapy vs. surveillance (hazard ratio, 0.23), peritoneum vs. ovary as primary site (HR, 0.45), no gross residual disease vs. gross residual disease (HR, 0.49),and persistent disease at completion of chemotherapy, vs. no evidence of disease (HR, 0.42).
Patients included in the study presented between 1981 and 2013. They had a median age of 47.6 years, about 3/4 had primary ovarian LGSC, and about 1/4 had primary peritoneal LGSC.
Patients in the hormonal therapy group were treated with letrozole (54%), tamoxifen (29%), anastrozole (3%), leuprolide acetate (7%), depot medroxyprogesterone acetate (1%), and leuprolide acetate + tamoxifen or letrozole (3% each). Median duration of hormonal maintenance therapy was 33.3 months (range of 1-223), and follow-up was at least 2 years in those who had not recurred.
LGSC is a rare subtype that accounts for about 10% of serous carcinomas of the ovary or peritoneum. These cancers may arise de novo or after a diagnosis of serous borderline tumor. LGSC, relative to high-grade serous carcinoma (HGSC), is characterized by younger age at diagnosis, chemoresistance, prolonged overall survival, and aberrations within the MAP kinase signaling pathway, Dr. Gershenson said.
The findings are of interest as primary postoperative platinum-based chemotherapy remains the standard for women with newly diagnosed metastatic ovarian or peritoneal LGSC despite multiple reports indicating that this subtype is relatively chemoresistant. For example, in the AGO database of 5,114 patients accrued to four separate phase III trials, the response rate to chemotherapy in women with suboptimally debulked LGSC was significantly lower than in women with suboptimally debulked HGSC (23% vs. 90%), he said.
However, no prospective clinical trials have been conducted in the front line setting in women with LGSC, he noted.
Recent reports of promising activity of hormonal therapy in the recurrent setting have led to an increase in interest in integrating the modality into the primary treatment setting. One study showed a high frequency of ER and PR expression in LGSC, and another showed that hormonal therapy for recurrent LGSC was associated with an objective response rate of 9%, stable disease rate of 66% and median progression-free survival of 7.4 months.
“Some patients derived several years of benefit,” he said. “In addition, low-grade serous carcinoma bears a striking resemblance to luminal breast cancer in that women who are 35 years of age or younger appear to have a significantly worse prognosis.”
Some have concluded, based on these findings, that platinum-based chemotherapy is of no value in the frontline treatment of LGSC and should be abandoned, Dr. Gershenson said.
The current findings, though limited by factors such as their retrospective nature, missing data, a long study period, and possible referral bias suggest that hormonal maintenance therapy deserves a closer look, Dr. Gershenson said.
“The findings of this hypotheses-generating study are potentially practice changing and warrant further investigation using a prospective trial design,” he concluded.
Dr. Gershenson reported stock ownership in AbbVie, Biogen Idec, Celgene, GlaxoSmithKline, Johnson & Johnson, Merck, and Pfizer; consulting or advisory roles with Clovis Oncology, and patents with Elsevier and UpToDate.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Hormonal maintenance therapy after primary treatment was associated with significantly prolonged PFS, when compared with surveillance, in a retrospective study of women with stage II-IV low-grade serous carcinoma of the ovary or peritoneum.
Major finding: Median PFS was 64.9 vs. 27.3 months with hormonal maintenance therapy vs. surveillance.
Data source: A retrospective cohort study of 204 women.
Disclosures: Dr. Gershenson reported stock ownership in AbbVie, Biogen Idec, Celgene, GlaxoSmithKline, Johnson & Johnson, Merck, and Pfizer; consulting or advisory roles with Clovis Oncology, and patents with Elsevier and UpToDate.
Increase infliximab dosing to heal Crohn’s fistulas
SAN DIEGO – It takes more than the usual 5 mg/kg of infliximab to heal perianal fistulas in some Crohn’s disease patients, according to a multicenter review of 117 cases.
The investigators aimed for trough levels of at least 10-20 mcg/mL, higher than the usual target of perhaps 7 mcg/mL or less. Doing so generally required 10 mg/kg every 8 weeks, but a few patients needed 10 mg/kg or even 15 mg/kg every 4 weeks. Fistulas healed in 63 (54%) patients and closed in 36 (31%).
Troughs of at least 10 mcg/mL and a history of dose escalation were both independent predictors of fistula healing, defined as the absence of drainage. Patients with fistula healing had significantly higher infliximab levels compared to those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001), and there was an incremental gain in healing with higher infliximab levels up to 50 mcg/mL. The association between infliximab levels and fistula healing had an area under the curve of 0.82 (P less than .0001) and the association with fistula closure was 0.69 (P = .014).
“Infliximab levels needed to achieve fistula healing were higher than what [has] been described for mucosal healing. Achieving higher infliximab levels in patients with Crohn’s disease and perianal fistulas may improve outcomes and should be considered in a treat-to-target strategy. At least 10-20 mcg/mL may be needed to achieve fistula healing in some patients,” concluded investigator Dr. Andres J. Yarur, a gastroenterologist at the Medical College of Wisconsin, Milwaukee.
“Obviously, in somebody with active perianal fistulas” at a trough of 30 mcg/mL, “it’s time to try something else, but I don’t give up on infliximab at a level of 7 mcg/mL, like a lot of people do. I’m kind of aggressive with these patients.” There wasn’t a formal assessment of adverse events, but “we have not really seen any side effects,” he said at the annual Digestive Disease Week meeting. One audience member remarked that the findings will likely change how he treats Crohn’s fistulas.
Dr. Yarur said he wasn’t surprised by the findings, “but I have more reassurance now” that aggressive dosing is the way to go for aggressive Crohn’s.
The patients were an average of 39 years old, with a mean disease duration of 12 years; about half were women. They were on infliximab for a median of 19 months. Infliximab antibodies dropped the likelihood of healing (odds ratio, 0.04; 95% confidence interval, 0.005-0.3; P less than .001) and closure (OR 0.038; 95% CI, 0.005-0.3; P less than .0001). About two-thirds of the patients were on concomitant immunomodulators, but they did not increase the odds of healing (OR 1.7; 95% CI, 0.8-3.7; P = .157).
“We usually start at a standard dose of 5 mg/kg, and then start checking troughs at week 14, before the first maintenance dose, and adjust” upward to hit the trough target. However, dosing also depends on “how much I think the patient needs. I don’t think we should standardize trough levels,” Dr. Yarur said. The team checks levels with the homogeneous mobility-shift assay from Prometheus Laboratories. “The type of assay you use is very important,” because interpretation of the results varies from one to the next.
De-escalation is on a case-by-case basis. “I would be very careful de-escalating somebody [who] has very aggressive disease. Many times patients don’t want to go down. They say ‘I feel good doc. I have no side effects. Leave me on my dose,’ ” he said.
There was a lack of cross-sectional imaging in the study; the investigators couldn’t distinguish simple fistulas from complex ones in their review.
Dr. Yarur had no disclosures. There was no industry funding for the work.
SAN DIEGO – It takes more than the usual 5 mg/kg of infliximab to heal perianal fistulas in some Crohn’s disease patients, according to a multicenter review of 117 cases.
The investigators aimed for trough levels of at least 10-20 mcg/mL, higher than the usual target of perhaps 7 mcg/mL or less. Doing so generally required 10 mg/kg every 8 weeks, but a few patients needed 10 mg/kg or even 15 mg/kg every 4 weeks. Fistulas healed in 63 (54%) patients and closed in 36 (31%).
Troughs of at least 10 mcg/mL and a history of dose escalation were both independent predictors of fistula healing, defined as the absence of drainage. Patients with fistula healing had significantly higher infliximab levels compared to those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001), and there was an incremental gain in healing with higher infliximab levels up to 50 mcg/mL. The association between infliximab levels and fistula healing had an area under the curve of 0.82 (P less than .0001) and the association with fistula closure was 0.69 (P = .014).
“Infliximab levels needed to achieve fistula healing were higher than what [has] been described for mucosal healing. Achieving higher infliximab levels in patients with Crohn’s disease and perianal fistulas may improve outcomes and should be considered in a treat-to-target strategy. At least 10-20 mcg/mL may be needed to achieve fistula healing in some patients,” concluded investigator Dr. Andres J. Yarur, a gastroenterologist at the Medical College of Wisconsin, Milwaukee.
“Obviously, in somebody with active perianal fistulas” at a trough of 30 mcg/mL, “it’s time to try something else, but I don’t give up on infliximab at a level of 7 mcg/mL, like a lot of people do. I’m kind of aggressive with these patients.” There wasn’t a formal assessment of adverse events, but “we have not really seen any side effects,” he said at the annual Digestive Disease Week meeting. One audience member remarked that the findings will likely change how he treats Crohn’s fistulas.
Dr. Yarur said he wasn’t surprised by the findings, “but I have more reassurance now” that aggressive dosing is the way to go for aggressive Crohn’s.
The patients were an average of 39 years old, with a mean disease duration of 12 years; about half were women. They were on infliximab for a median of 19 months. Infliximab antibodies dropped the likelihood of healing (odds ratio, 0.04; 95% confidence interval, 0.005-0.3; P less than .001) and closure (OR 0.038; 95% CI, 0.005-0.3; P less than .0001). About two-thirds of the patients were on concomitant immunomodulators, but they did not increase the odds of healing (OR 1.7; 95% CI, 0.8-3.7; P = .157).
“We usually start at a standard dose of 5 mg/kg, and then start checking troughs at week 14, before the first maintenance dose, and adjust” upward to hit the trough target. However, dosing also depends on “how much I think the patient needs. I don’t think we should standardize trough levels,” Dr. Yarur said. The team checks levels with the homogeneous mobility-shift assay from Prometheus Laboratories. “The type of assay you use is very important,” because interpretation of the results varies from one to the next.
De-escalation is on a case-by-case basis. “I would be very careful de-escalating somebody [who] has very aggressive disease. Many times patients don’t want to go down. They say ‘I feel good doc. I have no side effects. Leave me on my dose,’ ” he said.
There was a lack of cross-sectional imaging in the study; the investigators couldn’t distinguish simple fistulas from complex ones in their review.
Dr. Yarur had no disclosures. There was no industry funding for the work.
SAN DIEGO – It takes more than the usual 5 mg/kg of infliximab to heal perianal fistulas in some Crohn’s disease patients, according to a multicenter review of 117 cases.
The investigators aimed for trough levels of at least 10-20 mcg/mL, higher than the usual target of perhaps 7 mcg/mL or less. Doing so generally required 10 mg/kg every 8 weeks, but a few patients needed 10 mg/kg or even 15 mg/kg every 4 weeks. Fistulas healed in 63 (54%) patients and closed in 36 (31%).
Troughs of at least 10 mcg/mL and a history of dose escalation were both independent predictors of fistula healing, defined as the absence of drainage. Patients with fistula healing had significantly higher infliximab levels compared to those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001), and there was an incremental gain in healing with higher infliximab levels up to 50 mcg/mL. The association between infliximab levels and fistula healing had an area under the curve of 0.82 (P less than .0001) and the association with fistula closure was 0.69 (P = .014).
“Infliximab levels needed to achieve fistula healing were higher than what [has] been described for mucosal healing. Achieving higher infliximab levels in patients with Crohn’s disease and perianal fistulas may improve outcomes and should be considered in a treat-to-target strategy. At least 10-20 mcg/mL may be needed to achieve fistula healing in some patients,” concluded investigator Dr. Andres J. Yarur, a gastroenterologist at the Medical College of Wisconsin, Milwaukee.
“Obviously, in somebody with active perianal fistulas” at a trough of 30 mcg/mL, “it’s time to try something else, but I don’t give up on infliximab at a level of 7 mcg/mL, like a lot of people do. I’m kind of aggressive with these patients.” There wasn’t a formal assessment of adverse events, but “we have not really seen any side effects,” he said at the annual Digestive Disease Week meeting. One audience member remarked that the findings will likely change how he treats Crohn’s fistulas.
Dr. Yarur said he wasn’t surprised by the findings, “but I have more reassurance now” that aggressive dosing is the way to go for aggressive Crohn’s.
The patients were an average of 39 years old, with a mean disease duration of 12 years; about half were women. They were on infliximab for a median of 19 months. Infliximab antibodies dropped the likelihood of healing (odds ratio, 0.04; 95% confidence interval, 0.005-0.3; P less than .001) and closure (OR 0.038; 95% CI, 0.005-0.3; P less than .0001). About two-thirds of the patients were on concomitant immunomodulators, but they did not increase the odds of healing (OR 1.7; 95% CI, 0.8-3.7; P = .157).
“We usually start at a standard dose of 5 mg/kg, and then start checking troughs at week 14, before the first maintenance dose, and adjust” upward to hit the trough target. However, dosing also depends on “how much I think the patient needs. I don’t think we should standardize trough levels,” Dr. Yarur said. The team checks levels with the homogeneous mobility-shift assay from Prometheus Laboratories. “The type of assay you use is very important,” because interpretation of the results varies from one to the next.
De-escalation is on a case-by-case basis. “I would be very careful de-escalating somebody [who] has very aggressive disease. Many times patients don’t want to go down. They say ‘I feel good doc. I have no side effects. Leave me on my dose,’ ” he said.
There was a lack of cross-sectional imaging in the study; the investigators couldn’t distinguish simple fistulas from complex ones in their review.
Dr. Yarur had no disclosures. There was no industry funding for the work.
AT DDW 2016
Key clinical point: Infliximab trough levels of at least 10-20 mcg/mL are needed to heal fistulas in some Crohn’s patients.
Major finding: Patients with fistula healing had significantly higher infliximab trough levels, compared with those with active fistulas (18.5 vs. 6.5 mcg/mL; P less than .0001).
Data source: Multicenter review of 117 patients
Disclosures: There was no industry funding for the work, and the presenter had no disclosures.
Pediatric Dermatology Consult - June 2016
Dr. Catalina Matiz and David Ginsberg discuss the diagnosis and treatment of phytophotodermatitis.
Phytophotodermatitis
The term phytophotodermatitis was first used in 1942 by Robert Klaber, but knowledge of this condition dates back as far as 1500 BC.1,2 It is a nonimmune reaction caused by exposure to chemicals called furocoumarins and psoralens, found in a variety of plants and fruits such as lemons, limes, celery, parsnips, figs, carrots, dill, mustard, and rindweed.1,2
When these chemicals get in contact with the skin and are then exposed to UV light from the sun, a phototoxic reaction occurs. This reaction leads to cell membrane damage and cell death, which after the acute insult has resolved results in postinflammatory hyperpigmentation that may last months and is not responsive to bleaching skin treatments.1,2
Studies have shown that long-wave length UV radiation is the best trigger to induce this irritating reaction.2 There have been rare reports of photosensitivity reactions due to ingestion of large quantities of plants containing furocoumarins and psoralens.3 Plants known to cause phytophotodermatitis are found in almost every country across the globe, and exposure does not have to just be to the fruit, but contact with the leaves or sap also can induce the reaction.2-4 Typically after contact with the plant, followed by sun exposure, erythema will begin within 1 to 2 days, followed by bullae and vesicles, which tend to coalesce and burst over the following days. The patient is left with hyperpigmentation.2-4
Differential diagnosis
This condition can be difficult to diagnose and is often confused with type IV hypersensitivity reaction, eczema, herpes simplex virus (HSV), and burns, potentially leading to suspicion of child abuse.4,5 A thorough and detailed history is essential to correctly making the diagnosis and ruling out other potential causes.
Because the cause in children is often due to exposure to certain plants, unsupervised time outdoors leading to a rash may lead parents to think of poison ivy or poison oak, which are type IV hypersensitivity reactions.4,5 Although the rash may appear in a similar time course, the evolution to hyperpigmented patches is a distinguishing feature that helps to make the diagnosis of phytophotodermatitis.4 The postinflammatory hyperpigmentation, along with the clinical course, also can help differentiate it from burns and HSV infections, which it sometimes is mistaken for due to the bullous and vesicular lesions.4,5
Treatment
Counseling for avoidance of the exposure in the future is the most important aspect of treatment in order to prevent recurrences. Depending on the extent of the inflammatory reaction prior to the hyperpigmentation, no treatment may be needed for mild cases, but for more extreme bullous reactions, systemic steroids may be used.4
Topical corticosteroids and sun avoidance are the mainstays for treating mild to moderate cases. UV avoidance can be difficult because the long wave-length UV radiation that causes this reaction is not blocked by windows, and therefore it is important to keep affected areas covered even while indoors during daylight hours.4
There is no effective treatment for the hyperpigmented lesions. Patients need to be informed that this may resolve in months.
References
- Br J Dermatol. 1942;54(7):193-211.
- Clin Dermatol. 1986 Apr-Jun;4(2):102-21.
- Arch Dermatol. 1990 Oct;126(10):1334-6.
- J Am Acad Dermatol. 2007 Nov;57(5 Suppl):S88-91.
- Arch Fam Med. 2000 Jan;9(1):88.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Dr. Catalina Matiz and David Ginsberg discuss the diagnosis and treatment of phytophotodermatitis.
Phytophotodermatitis
The term phytophotodermatitis was first used in 1942 by Robert Klaber, but knowledge of this condition dates back as far as 1500 BC.1,2 It is a nonimmune reaction caused by exposure to chemicals called furocoumarins and psoralens, found in a variety of plants and fruits such as lemons, limes, celery, parsnips, figs, carrots, dill, mustard, and rindweed.1,2
When these chemicals get in contact with the skin and are then exposed to UV light from the sun, a phototoxic reaction occurs. This reaction leads to cell membrane damage and cell death, which after the acute insult has resolved results in postinflammatory hyperpigmentation that may last months and is not responsive to bleaching skin treatments.1,2
Studies have shown that long-wave length UV radiation is the best trigger to induce this irritating reaction.2 There have been rare reports of photosensitivity reactions due to ingestion of large quantities of plants containing furocoumarins and psoralens.3 Plants known to cause phytophotodermatitis are found in almost every country across the globe, and exposure does not have to just be to the fruit, but contact with the leaves or sap also can induce the reaction.2-4 Typically after contact with the plant, followed by sun exposure, erythema will begin within 1 to 2 days, followed by bullae and vesicles, which tend to coalesce and burst over the following days. The patient is left with hyperpigmentation.2-4
Differential diagnosis
This condition can be difficult to diagnose and is often confused with type IV hypersensitivity reaction, eczema, herpes simplex virus (HSV), and burns, potentially leading to suspicion of child abuse.4,5 A thorough and detailed history is essential to correctly making the diagnosis and ruling out other potential causes.
Because the cause in children is often due to exposure to certain plants, unsupervised time outdoors leading to a rash may lead parents to think of poison ivy or poison oak, which are type IV hypersensitivity reactions.4,5 Although the rash may appear in a similar time course, the evolution to hyperpigmented patches is a distinguishing feature that helps to make the diagnosis of phytophotodermatitis.4 The postinflammatory hyperpigmentation, along with the clinical course, also can help differentiate it from burns and HSV infections, which it sometimes is mistaken for due to the bullous and vesicular lesions.4,5
Treatment
Counseling for avoidance of the exposure in the future is the most important aspect of treatment in order to prevent recurrences. Depending on the extent of the inflammatory reaction prior to the hyperpigmentation, no treatment may be needed for mild cases, but for more extreme bullous reactions, systemic steroids may be used.4
Topical corticosteroids and sun avoidance are the mainstays for treating mild to moderate cases. UV avoidance can be difficult because the long wave-length UV radiation that causes this reaction is not blocked by windows, and therefore it is important to keep affected areas covered even while indoors during daylight hours.4
There is no effective treatment for the hyperpigmented lesions. Patients need to be informed that this may resolve in months.
References
- Br J Dermatol. 1942;54(7):193-211.
- Clin Dermatol. 1986 Apr-Jun;4(2):102-21.
- Arch Dermatol. 1990 Oct;126(10):1334-6.
- J Am Acad Dermatol. 2007 Nov;57(5 Suppl):S88-91.
- Arch Fam Med. 2000 Jan;9(1):88.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Dr. Catalina Matiz and David Ginsberg discuss the diagnosis and treatment of phytophotodermatitis.
Phytophotodermatitis
The term phytophotodermatitis was first used in 1942 by Robert Klaber, but knowledge of this condition dates back as far as 1500 BC.1,2 It is a nonimmune reaction caused by exposure to chemicals called furocoumarins and psoralens, found in a variety of plants and fruits such as lemons, limes, celery, parsnips, figs, carrots, dill, mustard, and rindweed.1,2
When these chemicals get in contact with the skin and are then exposed to UV light from the sun, a phototoxic reaction occurs. This reaction leads to cell membrane damage and cell death, which after the acute insult has resolved results in postinflammatory hyperpigmentation that may last months and is not responsive to bleaching skin treatments.1,2
Studies have shown that long-wave length UV radiation is the best trigger to induce this irritating reaction.2 There have been rare reports of photosensitivity reactions due to ingestion of large quantities of plants containing furocoumarins and psoralens.3 Plants known to cause phytophotodermatitis are found in almost every country across the globe, and exposure does not have to just be to the fruit, but contact with the leaves or sap also can induce the reaction.2-4 Typically after contact with the plant, followed by sun exposure, erythema will begin within 1 to 2 days, followed by bullae and vesicles, which tend to coalesce and burst over the following days. The patient is left with hyperpigmentation.2-4
Differential diagnosis
This condition can be difficult to diagnose and is often confused with type IV hypersensitivity reaction, eczema, herpes simplex virus (HSV), and burns, potentially leading to suspicion of child abuse.4,5 A thorough and detailed history is essential to correctly making the diagnosis and ruling out other potential causes.
Because the cause in children is often due to exposure to certain plants, unsupervised time outdoors leading to a rash may lead parents to think of poison ivy or poison oak, which are type IV hypersensitivity reactions.4,5 Although the rash may appear in a similar time course, the evolution to hyperpigmented patches is a distinguishing feature that helps to make the diagnosis of phytophotodermatitis.4 The postinflammatory hyperpigmentation, along with the clinical course, also can help differentiate it from burns and HSV infections, which it sometimes is mistaken for due to the bullous and vesicular lesions.4,5
Treatment
Counseling for avoidance of the exposure in the future is the most important aspect of treatment in order to prevent recurrences. Depending on the extent of the inflammatory reaction prior to the hyperpigmentation, no treatment may be needed for mild cases, but for more extreme bullous reactions, systemic steroids may be used.4
Topical corticosteroids and sun avoidance are the mainstays for treating mild to moderate cases. UV avoidance can be difficult because the long wave-length UV radiation that causes this reaction is not blocked by windows, and therefore it is important to keep affected areas covered even while indoors during daylight hours.4
There is no effective treatment for the hyperpigmented lesions. Patients need to be informed that this may resolve in months.
References
- Br J Dermatol. 1942;54(7):193-211.
- Clin Dermatol. 1986 Apr-Jun;4(2):102-21.
- Arch Dermatol. 1990 Oct;126(10):1334-6.
- J Am Acad Dermatol. 2007 Nov;57(5 Suppl):S88-91.
- Arch Fam Med. 2000 Jan;9(1):88.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
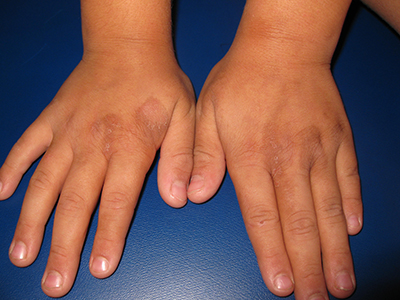
An 8-year-old healthy female presents with a 7-day history of a rash on the dorsum of her hands bilaterally. Her mother reports that it began as redness and swelling followed by blisters. She also reports that after the blisters popped and the redness went away, she noticed some darkened areas around where the blisters had been. The patient reports that there was some slight stinging and burning when the rash first appeared, which has been improving with time. She has had no fevers and has not been ill. The family has a dog, the patient has one older sibling who has atopic dermatitis, and the mother has asthma. The mother reports they live on a farm, and they have a vegetable and flower garden where the daughter plays every afternoon. She has no known allergies. The mother denies any recent travels or family history of bullous disease. Physical exam The patient is a well-appearing child who is attentive and in no apparent distress. On exam, there are several non-tender hyperpigmented patches between 1 cm and 3 cm on the dorsum of the hands, and intertriginous areas with overlying erosions. There are no other lesions on the skin. She is afebrile.
Comprehensive diabetic retinopathy screening challenging
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Some 71% of diabetes patients did not undergo screening for diabetic retinopathy.
Major finding: Only 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%.
Data source: An analysis of administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System in 2014.
Disclosures: The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
Edaravone slows progression of ALS when started early on
VANCOUVER – The antioxidant edaravone was associated with less deterioration in functional rating and quality of life scales when started early in the course of amyotrophic lateral sclerosis (ALS), based on results from a set of trials conducted in Japan and reported at the annual meeting of the American Academy of Neurology.
Edaravone is thought to confer neuroprotection in part through its free radical–scavenging activity and first garnered interest for the treatment of acute ischemic stroke, according to presenting author Dr. Joseph M. Palumbo, vice president and head of Clinical Research at Mitsubishi Tanabe Pharma Development America, the maker of edaravone. It is now approved in several countries for that indication.
In a pivotal randomized phase III trial, Dr. Palumbo and his colleagues studied 137 patients who had definite or probable ALS, were less than 2 years out from symptom onset, had normal respiratory function, and were able to perform most activities of daily living. All patients received standard of care, usually including riluzole (Rilutek), plus either edaravone (MCI-186) or placebo.
After 24 weeks of treatment, compared with placebo, edaravone was associated with a smaller decline in scores on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) (–5.01 vs. –7.50; difference between groups, 2.49; P = .0013), according to data reported in a poster session at the meeting.
Significant benefit was seen on the limb and bulbar subscales, and there was a trend favoring edaravone on the respiratory subscale. Additionally, patients given edaravone had comparatively less deterioration in quality of life as assessed with the 40-item Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) (P = .03). Grip strength did not differ between groups, and there were no deaths in either group.
“We are not aware of any other positive phase III study in maybe a generation, since riluzole, in ALS. So we are showing this data and we are hopeful that people are as excited as we are,” Dr. Palumbo said in an interview.
In the area of safety, edaravone and placebo did not differ significantly with respect to the rate of adverse events, with contusion, dysphagia, and constipation predominating. The most common serious adverse event was dysphagia, seen in 12% of each group.
The trial’s findings led to approval of edaravone for treatment of ALS in Japan, where it is marketed as Radicut. Additionally, the U.S. Food and Drug Administration (FDA) granted edaravone orphan drug designation for ALS but has not approved it for this indication.
An additional 24-week open-label extension study among 123 patients from the trial, in which all received the drug regardless of their initially assigned treatment, showed that the benefit of edaravone was durable. Patients who continued treatment with the drug had less of a decline from baseline in ALSFRS-R score than did peers switched to the drug from placebo (difference between groups, 4.17; P = .004), according to data reported in another poster. Also, the former had a lower risk of death (P = .019) and less decline in lung function. Meanwhile, the drug’s safety profile remained good.
The researchers are preparing their findings for journal submission and are revisiting the drug’s regulatory status in the United States, according to Dr. Palumbo. “We’re talking to the FDA now. We’ve got our fingers crossed.”
There is no compelling reason to think that the drug’s efficacy in the U.S. population would differ from that in the Japanese population, but that will ultimately be an issue for regulators to decide, he said.
It is difficult to compare edaravone with other ALS treatment options, as all patients in the trial concomitantly received standard of care, Dr. Palumbo explained. “We can only speak about standard of care, and we think that we’ve improved on standard of care here,” he maintained.
The initial phase III trial of edaravone in ALS, which enrolled a population having a wider range of disease severity, failed to meet its primary endpoint of improvement in ALSFRS-R score (Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610-7). An extension study in 181 patients, also reported in a poster at the meeting, supported post hoc findings hinting that the timing of drug initiation was important.
“We learned a tremendous amount in that study about who the patients were who would ultimately benefit,” Dr. Palumbo commented. “We had a number of hypotheses. One hypothesis was that if we found patients who in fact were still functional at baseline and who really had the diagnosis – they had either definite or probable ALS – and still had very good respiratory function, that we would likely find a signal there.”
Rounding out the set of trials was a small randomized placebo-controlled trial among 25 patients with more advanced ALS, done at the request of Japanese health authorities. Results showed that edaravone was safe in this population but had no clear benefit.
Dr. Palumbo disclosed that he is an employee of Mitsubishi Tanabe Pharma Development America, Inc. The trials were sponsored by Mitsubishi Tanabe Pharma Corporation.
VANCOUVER – The antioxidant edaravone was associated with less deterioration in functional rating and quality of life scales when started early in the course of amyotrophic lateral sclerosis (ALS), based on results from a set of trials conducted in Japan and reported at the annual meeting of the American Academy of Neurology.
Edaravone is thought to confer neuroprotection in part through its free radical–scavenging activity and first garnered interest for the treatment of acute ischemic stroke, according to presenting author Dr. Joseph M. Palumbo, vice president and head of Clinical Research at Mitsubishi Tanabe Pharma Development America, the maker of edaravone. It is now approved in several countries for that indication.
In a pivotal randomized phase III trial, Dr. Palumbo and his colleagues studied 137 patients who had definite or probable ALS, were less than 2 years out from symptom onset, had normal respiratory function, and were able to perform most activities of daily living. All patients received standard of care, usually including riluzole (Rilutek), plus either edaravone (MCI-186) or placebo.
After 24 weeks of treatment, compared with placebo, edaravone was associated with a smaller decline in scores on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) (–5.01 vs. –7.50; difference between groups, 2.49; P = .0013), according to data reported in a poster session at the meeting.
Significant benefit was seen on the limb and bulbar subscales, and there was a trend favoring edaravone on the respiratory subscale. Additionally, patients given edaravone had comparatively less deterioration in quality of life as assessed with the 40-item Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) (P = .03). Grip strength did not differ between groups, and there were no deaths in either group.
“We are not aware of any other positive phase III study in maybe a generation, since riluzole, in ALS. So we are showing this data and we are hopeful that people are as excited as we are,” Dr. Palumbo said in an interview.
In the area of safety, edaravone and placebo did not differ significantly with respect to the rate of adverse events, with contusion, dysphagia, and constipation predominating. The most common serious adverse event was dysphagia, seen in 12% of each group.
The trial’s findings led to approval of edaravone for treatment of ALS in Japan, where it is marketed as Radicut. Additionally, the U.S. Food and Drug Administration (FDA) granted edaravone orphan drug designation for ALS but has not approved it for this indication.
An additional 24-week open-label extension study among 123 patients from the trial, in which all received the drug regardless of their initially assigned treatment, showed that the benefit of edaravone was durable. Patients who continued treatment with the drug had less of a decline from baseline in ALSFRS-R score than did peers switched to the drug from placebo (difference between groups, 4.17; P = .004), according to data reported in another poster. Also, the former had a lower risk of death (P = .019) and less decline in lung function. Meanwhile, the drug’s safety profile remained good.
The researchers are preparing their findings for journal submission and are revisiting the drug’s regulatory status in the United States, according to Dr. Palumbo. “We’re talking to the FDA now. We’ve got our fingers crossed.”
There is no compelling reason to think that the drug’s efficacy in the U.S. population would differ from that in the Japanese population, but that will ultimately be an issue for regulators to decide, he said.
It is difficult to compare edaravone with other ALS treatment options, as all patients in the trial concomitantly received standard of care, Dr. Palumbo explained. “We can only speak about standard of care, and we think that we’ve improved on standard of care here,” he maintained.
The initial phase III trial of edaravone in ALS, which enrolled a population having a wider range of disease severity, failed to meet its primary endpoint of improvement in ALSFRS-R score (Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610-7). An extension study in 181 patients, also reported in a poster at the meeting, supported post hoc findings hinting that the timing of drug initiation was important.
“We learned a tremendous amount in that study about who the patients were who would ultimately benefit,” Dr. Palumbo commented. “We had a number of hypotheses. One hypothesis was that if we found patients who in fact were still functional at baseline and who really had the diagnosis – they had either definite or probable ALS – and still had very good respiratory function, that we would likely find a signal there.”
Rounding out the set of trials was a small randomized placebo-controlled trial among 25 patients with more advanced ALS, done at the request of Japanese health authorities. Results showed that edaravone was safe in this population but had no clear benefit.
Dr. Palumbo disclosed that he is an employee of Mitsubishi Tanabe Pharma Development America, Inc. The trials were sponsored by Mitsubishi Tanabe Pharma Corporation.
VANCOUVER – The antioxidant edaravone was associated with less deterioration in functional rating and quality of life scales when started early in the course of amyotrophic lateral sclerosis (ALS), based on results from a set of trials conducted in Japan and reported at the annual meeting of the American Academy of Neurology.
Edaravone is thought to confer neuroprotection in part through its free radical–scavenging activity and first garnered interest for the treatment of acute ischemic stroke, according to presenting author Dr. Joseph M. Palumbo, vice president and head of Clinical Research at Mitsubishi Tanabe Pharma Development America, the maker of edaravone. It is now approved in several countries for that indication.
In a pivotal randomized phase III trial, Dr. Palumbo and his colleagues studied 137 patients who had definite or probable ALS, were less than 2 years out from symptom onset, had normal respiratory function, and were able to perform most activities of daily living. All patients received standard of care, usually including riluzole (Rilutek), plus either edaravone (MCI-186) or placebo.
After 24 weeks of treatment, compared with placebo, edaravone was associated with a smaller decline in scores on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) (–5.01 vs. –7.50; difference between groups, 2.49; P = .0013), according to data reported in a poster session at the meeting.
Significant benefit was seen on the limb and bulbar subscales, and there was a trend favoring edaravone on the respiratory subscale. Additionally, patients given edaravone had comparatively less deterioration in quality of life as assessed with the 40-item Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) (P = .03). Grip strength did not differ between groups, and there were no deaths in either group.
“We are not aware of any other positive phase III study in maybe a generation, since riluzole, in ALS. So we are showing this data and we are hopeful that people are as excited as we are,” Dr. Palumbo said in an interview.
In the area of safety, edaravone and placebo did not differ significantly with respect to the rate of adverse events, with contusion, dysphagia, and constipation predominating. The most common serious adverse event was dysphagia, seen in 12% of each group.
The trial’s findings led to approval of edaravone for treatment of ALS in Japan, where it is marketed as Radicut. Additionally, the U.S. Food and Drug Administration (FDA) granted edaravone orphan drug designation for ALS but has not approved it for this indication.
An additional 24-week open-label extension study among 123 patients from the trial, in which all received the drug regardless of their initially assigned treatment, showed that the benefit of edaravone was durable. Patients who continued treatment with the drug had less of a decline from baseline in ALSFRS-R score than did peers switched to the drug from placebo (difference between groups, 4.17; P = .004), according to data reported in another poster. Also, the former had a lower risk of death (P = .019) and less decline in lung function. Meanwhile, the drug’s safety profile remained good.
The researchers are preparing their findings for journal submission and are revisiting the drug’s regulatory status in the United States, according to Dr. Palumbo. “We’re talking to the FDA now. We’ve got our fingers crossed.”
There is no compelling reason to think that the drug’s efficacy in the U.S. population would differ from that in the Japanese population, but that will ultimately be an issue for regulators to decide, he said.
It is difficult to compare edaravone with other ALS treatment options, as all patients in the trial concomitantly received standard of care, Dr. Palumbo explained. “We can only speak about standard of care, and we think that we’ve improved on standard of care here,” he maintained.
The initial phase III trial of edaravone in ALS, which enrolled a population having a wider range of disease severity, failed to meet its primary endpoint of improvement in ALSFRS-R score (Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610-7). An extension study in 181 patients, also reported in a poster at the meeting, supported post hoc findings hinting that the timing of drug initiation was important.
“We learned a tremendous amount in that study about who the patients were who would ultimately benefit,” Dr. Palumbo commented. “We had a number of hypotheses. One hypothesis was that if we found patients who in fact were still functional at baseline and who really had the diagnosis – they had either definite or probable ALS – and still had very good respiratory function, that we would likely find a signal there.”
Rounding out the set of trials was a small randomized placebo-controlled trial among 25 patients with more advanced ALS, done at the request of Japanese health authorities. Results showed that edaravone was safe in this population but had no clear benefit.
Dr. Palumbo disclosed that he is an employee of Mitsubishi Tanabe Pharma Development America, Inc. The trials were sponsored by Mitsubishi Tanabe Pharma Corporation.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: When added to standard of care, edaravone slows loss of function in patients with early-stage ALS.
Major finding: Compared with placebo, edaravone was associated with a smaller 24-week reduction in scores on the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (–5.01 vs. –7.50).
Data source: A randomized phase III trial among 137 patients with definite or probable ALS, normal respiratory function, and ability to perform most activities of daily living.
Disclosures: Dr. Palumbo disclosed that he is an employee of Mitsubishi Tanabe Pharma Development America the maker of edaravone. The studies were sponsored by Mitsubishi Tanabe Pharma Corporation.