User login
Most people who undergo gender reassignment surgery appreciate the results
ORLANDO – Gender reassignment surgery is the most extreme step for those transgender individuals who wish to complete the transformation to the opposite sex. While many transgender people do not opt to take this step, it may be an option for people who still have gender dysphoria after a thorough diagnostic work-up by a mental health professional, hormonal treatment, and having lived in the desired gender role as a “real-life test.”
Dr. Stan Monstrey, of Ghent University Hospital, Belgium, is an experienced gender reassignment surgeon and reported at the annual meeting of the American Academy of Clinical Endocrinology that between 1995 and 2005, he saw about 20-30 new patients a year. But now, he said, “We operate on a weekly basis between a minimum of three and sometimes six or seven transsexuals, so ... in our practice, probably between 90% and 95% are still going the whole way, still want what was called initially binary surgery.”
Transwomen: Male to female
The transformation procedure for male to female begins with feminizing aesthetic procedures, such as reducing the Adam’s apple (laryngeal prominence of the thyroid cartilage) and chin, frontal boss of the forehead, and possibly other facial work such as rhinoplasty. “Sometimes minor changes can have a huge effect on the face of the patient,” Dr. Monstrey said. “This is becoming, in our opinion, increasingly important for transwomen.”
Then, in about 75% of cases, Dr. Monstrey performs at least two surgeries under the same anesthesia – breast augmentation and perineal transformation. He said even after years of hormone therapy, most such patients have only a limited amount of breast tissue but want more prominent breasts. Implants can be placed behind or in front of the pectoralis muscle via inframammary, transaxillary, or occasionally periareolar approaches. Results are immediate, and complications are rare.
Another technique, which has become very popular over the past 5-10 years, is lipofilling to fill defects and depressions in the breasts. Stem cells contained in the fat may help soften scars. But when faced with a patient who had a BRCA1 mutation, the surgeons would not use lipofilling, fearing the potential for breast cancer, and would use prostheses instead (J Sex Med. 2014 Oct;11:2496-9). Still, questions remain about even using hormone treatments in such a patient.
Dr. Monstrey mentioned that in Belgium, breast augmentation for transwomen is considered reconstructive surgery and is always reimbursed whereas it is considered aesthetic surgery and never reimbursed for non-transwomen who want larger breasts. (For transmen, breast amputation is similarly reimbursed.)
The second operation is genital transformation. Basically, the interior of the penis is removed and the skin is invaginated to form a vagina of 8-18.5 cm and a scrotal flap, along with castration and removal of the penile bulb erectile tissue (corpus spongiosum) posteriorly. It is important to protect the rectal wall, which is not very strong. The foreskin becomes the new clitoral hood and inner side of the labia minora, and the clitoris is formed by reducing and transposing the penile glans. If the patient had a small penis and not enough tissue for the reconstruction, skin flaps from various other sites can be used.
Among more than 1,200 patients, 92% could achieve orgasm. Rectovaginal fistulas occurred in 4 patients, 19 needed repositioning of the urethra, 21 needed an operation to lengthen the vagina, and 95 needed aesthetic correction of the vulva. Dr. Monstrey said many patients have asked him when they should tell their new boyfriends about their transformation, meaning that the surgery was quite convincing even with penetrative sex.
If the first operation does not work, another technique is to use an isolated piece of colon or sigmoid bowel, which has been performed completely laparoscopically by a very skilled gastroenterologic surgeon at the hospital in Ghent.
Speaking to a roomful of endocrinologists, Dr. Monstrey told them, “I’ll be the first one to agree with you that indeed puberty blockers are a very good thing. However, we as surgeons are not so enthusiastic about them because … it is impossible to create a normal vagina” because of a lack of available tissue from the underdeveloped penis.
Transmen: Female to male
“Transmen react much better to hormonal therapy than do transwomen,” he said. “If they hide their breasts they really look like men. The disadvantage is that the surgical treatment is much more complex.” The most important operation for them is subcutaneous mastectomy and male contouring. A small, semiareolar incision leaves almost no scar. Most patients still require excision of redundant skin of the breasts.
Phalloplasty is a complex operation aimed at giving the patient an aesthetic phallus, a normal scrotum, the ability to void while standing, and to perform sexual intercourse, all while protecting erogenous sensation, with minimal morbidity and mortality. Dr. Monstrey reported that he has performed 600-700 phalloplasties.
The most common technique has been to use a free vascularized flap from another bodily site with the artery, vein, and nerves to reconnect at the phalloplasty site. Because the skin is very thin on the inner forearm, it is often used and allows forming an inner tube for the urethra and an outer tube for the penis. The surgery may have to be done in three or four stages for the best results. From pictures that Dr. Monstrey showed, it was obvious that the constructed penises were not absolutely natural in appearance, but he said most patients were “rather happy” with them, despite many of these patients being quite demanding. A scrotum is constructed from transposition of the labia minora.
Unfortunately, voiding while standing is often a problem, with 197 out of 562 patients (35%) having a fistula and urine leakage, but this issue frequently corrects itself. “More difficult to treat are the strictures with stenosis, which can be a problem voiding,” he said (occurring in 78/562). Other complications were 5 complete and 43 partial flap failures, 4 cases of compression syndrome, 58 cases of delayed wound healing, and 15 cases of transient ischemia. Flap failures occurred mainly in smokers, “so we don’t operate on smokers anymore,” he said.
One year after the constructive surgery, a penile prosthesis is implanted for those who want it, allowing sexual intercourse. Most individuals had orgasmic function, not because of reconnected nerves in the flap, but, Dr. Monstrey said he believes, because the clitoris, placed beneath the phallus, is denuded and stimulated during sexual activity. He said the problem is that the prostheses are usually intended for elderly men “who have sex a couple of times a month and who have a normal anatomy.” Young transmen may engage in more sexual activity, “so we have a lot of problems with exposure [of the prosthesis], infection, technical defects, and so on,” he said.
A technique gaining popularity is to use a skin flap from the groin area to make a urethra and one from the thigh to construct a penis. Although a penile transplant has recently been performed for a patient who had lost his penis to cancer, transplants are not being considered at this point, both for surgical technical reasons and because of a need for lifelong immunosuppressive drugs.
Proper referrals and counseling
The World Professional Association for Transgender Health in its Standard of Care guidelines 7 recommends one mental health professional referral for the breast surgery and two such referrals for genital surgery. The issue of possible parenthood should be discussed with patients, along with early counseling about fertility options. The age of majority and consent in different countries is important. Dr. Monstrey said genital surgery may be possible before the age of 18 years if all members of a multidisciplinary team of health professionals agree on a case by case basis that the adolescent can understand the risks, benefits, and alternatives to the surgery with the same degree of competence as someone 18 years of age or older.
Dr. Monstrey reported having no financial disclosures.
ORLANDO – Gender reassignment surgery is the most extreme step for those transgender individuals who wish to complete the transformation to the opposite sex. While many transgender people do not opt to take this step, it may be an option for people who still have gender dysphoria after a thorough diagnostic work-up by a mental health professional, hormonal treatment, and having lived in the desired gender role as a “real-life test.”
Dr. Stan Monstrey, of Ghent University Hospital, Belgium, is an experienced gender reassignment surgeon and reported at the annual meeting of the American Academy of Clinical Endocrinology that between 1995 and 2005, he saw about 20-30 new patients a year. But now, he said, “We operate on a weekly basis between a minimum of three and sometimes six or seven transsexuals, so ... in our practice, probably between 90% and 95% are still going the whole way, still want what was called initially binary surgery.”
Transwomen: Male to female
The transformation procedure for male to female begins with feminizing aesthetic procedures, such as reducing the Adam’s apple (laryngeal prominence of the thyroid cartilage) and chin, frontal boss of the forehead, and possibly other facial work such as rhinoplasty. “Sometimes minor changes can have a huge effect on the face of the patient,” Dr. Monstrey said. “This is becoming, in our opinion, increasingly important for transwomen.”
Then, in about 75% of cases, Dr. Monstrey performs at least two surgeries under the same anesthesia – breast augmentation and perineal transformation. He said even after years of hormone therapy, most such patients have only a limited amount of breast tissue but want more prominent breasts. Implants can be placed behind or in front of the pectoralis muscle via inframammary, transaxillary, or occasionally periareolar approaches. Results are immediate, and complications are rare.
Another technique, which has become very popular over the past 5-10 years, is lipofilling to fill defects and depressions in the breasts. Stem cells contained in the fat may help soften scars. But when faced with a patient who had a BRCA1 mutation, the surgeons would not use lipofilling, fearing the potential for breast cancer, and would use prostheses instead (J Sex Med. 2014 Oct;11:2496-9). Still, questions remain about even using hormone treatments in such a patient.
Dr. Monstrey mentioned that in Belgium, breast augmentation for transwomen is considered reconstructive surgery and is always reimbursed whereas it is considered aesthetic surgery and never reimbursed for non-transwomen who want larger breasts. (For transmen, breast amputation is similarly reimbursed.)
The second operation is genital transformation. Basically, the interior of the penis is removed and the skin is invaginated to form a vagina of 8-18.5 cm and a scrotal flap, along with castration and removal of the penile bulb erectile tissue (corpus spongiosum) posteriorly. It is important to protect the rectal wall, which is not very strong. The foreskin becomes the new clitoral hood and inner side of the labia minora, and the clitoris is formed by reducing and transposing the penile glans. If the patient had a small penis and not enough tissue for the reconstruction, skin flaps from various other sites can be used.
Among more than 1,200 patients, 92% could achieve orgasm. Rectovaginal fistulas occurred in 4 patients, 19 needed repositioning of the urethra, 21 needed an operation to lengthen the vagina, and 95 needed aesthetic correction of the vulva. Dr. Monstrey said many patients have asked him when they should tell their new boyfriends about their transformation, meaning that the surgery was quite convincing even with penetrative sex.
If the first operation does not work, another technique is to use an isolated piece of colon or sigmoid bowel, which has been performed completely laparoscopically by a very skilled gastroenterologic surgeon at the hospital in Ghent.
Speaking to a roomful of endocrinologists, Dr. Monstrey told them, “I’ll be the first one to agree with you that indeed puberty blockers are a very good thing. However, we as surgeons are not so enthusiastic about them because … it is impossible to create a normal vagina” because of a lack of available tissue from the underdeveloped penis.
Transmen: Female to male
“Transmen react much better to hormonal therapy than do transwomen,” he said. “If they hide their breasts they really look like men. The disadvantage is that the surgical treatment is much more complex.” The most important operation for them is subcutaneous mastectomy and male contouring. A small, semiareolar incision leaves almost no scar. Most patients still require excision of redundant skin of the breasts.
Phalloplasty is a complex operation aimed at giving the patient an aesthetic phallus, a normal scrotum, the ability to void while standing, and to perform sexual intercourse, all while protecting erogenous sensation, with minimal morbidity and mortality. Dr. Monstrey reported that he has performed 600-700 phalloplasties.
The most common technique has been to use a free vascularized flap from another bodily site with the artery, vein, and nerves to reconnect at the phalloplasty site. Because the skin is very thin on the inner forearm, it is often used and allows forming an inner tube for the urethra and an outer tube for the penis. The surgery may have to be done in three or four stages for the best results. From pictures that Dr. Monstrey showed, it was obvious that the constructed penises were not absolutely natural in appearance, but he said most patients were “rather happy” with them, despite many of these patients being quite demanding. A scrotum is constructed from transposition of the labia minora.
Unfortunately, voiding while standing is often a problem, with 197 out of 562 patients (35%) having a fistula and urine leakage, but this issue frequently corrects itself. “More difficult to treat are the strictures with stenosis, which can be a problem voiding,” he said (occurring in 78/562). Other complications were 5 complete and 43 partial flap failures, 4 cases of compression syndrome, 58 cases of delayed wound healing, and 15 cases of transient ischemia. Flap failures occurred mainly in smokers, “so we don’t operate on smokers anymore,” he said.
One year after the constructive surgery, a penile prosthesis is implanted for those who want it, allowing sexual intercourse. Most individuals had orgasmic function, not because of reconnected nerves in the flap, but, Dr. Monstrey said he believes, because the clitoris, placed beneath the phallus, is denuded and stimulated during sexual activity. He said the problem is that the prostheses are usually intended for elderly men “who have sex a couple of times a month and who have a normal anatomy.” Young transmen may engage in more sexual activity, “so we have a lot of problems with exposure [of the prosthesis], infection, technical defects, and so on,” he said.
A technique gaining popularity is to use a skin flap from the groin area to make a urethra and one from the thigh to construct a penis. Although a penile transplant has recently been performed for a patient who had lost his penis to cancer, transplants are not being considered at this point, both for surgical technical reasons and because of a need for lifelong immunosuppressive drugs.
Proper referrals and counseling
The World Professional Association for Transgender Health in its Standard of Care guidelines 7 recommends one mental health professional referral for the breast surgery and two such referrals for genital surgery. The issue of possible parenthood should be discussed with patients, along with early counseling about fertility options. The age of majority and consent in different countries is important. Dr. Monstrey said genital surgery may be possible before the age of 18 years if all members of a multidisciplinary team of health professionals agree on a case by case basis that the adolescent can understand the risks, benefits, and alternatives to the surgery with the same degree of competence as someone 18 years of age or older.
Dr. Monstrey reported having no financial disclosures.
ORLANDO – Gender reassignment surgery is the most extreme step for those transgender individuals who wish to complete the transformation to the opposite sex. While many transgender people do not opt to take this step, it may be an option for people who still have gender dysphoria after a thorough diagnostic work-up by a mental health professional, hormonal treatment, and having lived in the desired gender role as a “real-life test.”
Dr. Stan Monstrey, of Ghent University Hospital, Belgium, is an experienced gender reassignment surgeon and reported at the annual meeting of the American Academy of Clinical Endocrinology that between 1995 and 2005, he saw about 20-30 new patients a year. But now, he said, “We operate on a weekly basis between a minimum of three and sometimes six or seven transsexuals, so ... in our practice, probably between 90% and 95% are still going the whole way, still want what was called initially binary surgery.”
Transwomen: Male to female
The transformation procedure for male to female begins with feminizing aesthetic procedures, such as reducing the Adam’s apple (laryngeal prominence of the thyroid cartilage) and chin, frontal boss of the forehead, and possibly other facial work such as rhinoplasty. “Sometimes minor changes can have a huge effect on the face of the patient,” Dr. Monstrey said. “This is becoming, in our opinion, increasingly important for transwomen.”
Then, in about 75% of cases, Dr. Monstrey performs at least two surgeries under the same anesthesia – breast augmentation and perineal transformation. He said even after years of hormone therapy, most such patients have only a limited amount of breast tissue but want more prominent breasts. Implants can be placed behind or in front of the pectoralis muscle via inframammary, transaxillary, or occasionally periareolar approaches. Results are immediate, and complications are rare.
Another technique, which has become very popular over the past 5-10 years, is lipofilling to fill defects and depressions in the breasts. Stem cells contained in the fat may help soften scars. But when faced with a patient who had a BRCA1 mutation, the surgeons would not use lipofilling, fearing the potential for breast cancer, and would use prostheses instead (J Sex Med. 2014 Oct;11:2496-9). Still, questions remain about even using hormone treatments in such a patient.
Dr. Monstrey mentioned that in Belgium, breast augmentation for transwomen is considered reconstructive surgery and is always reimbursed whereas it is considered aesthetic surgery and never reimbursed for non-transwomen who want larger breasts. (For transmen, breast amputation is similarly reimbursed.)
The second operation is genital transformation. Basically, the interior of the penis is removed and the skin is invaginated to form a vagina of 8-18.5 cm and a scrotal flap, along with castration and removal of the penile bulb erectile tissue (corpus spongiosum) posteriorly. It is important to protect the rectal wall, which is not very strong. The foreskin becomes the new clitoral hood and inner side of the labia minora, and the clitoris is formed by reducing and transposing the penile glans. If the patient had a small penis and not enough tissue for the reconstruction, skin flaps from various other sites can be used.
Among more than 1,200 patients, 92% could achieve orgasm. Rectovaginal fistulas occurred in 4 patients, 19 needed repositioning of the urethra, 21 needed an operation to lengthen the vagina, and 95 needed aesthetic correction of the vulva. Dr. Monstrey said many patients have asked him when they should tell their new boyfriends about their transformation, meaning that the surgery was quite convincing even with penetrative sex.
If the first operation does not work, another technique is to use an isolated piece of colon or sigmoid bowel, which has been performed completely laparoscopically by a very skilled gastroenterologic surgeon at the hospital in Ghent.
Speaking to a roomful of endocrinologists, Dr. Monstrey told them, “I’ll be the first one to agree with you that indeed puberty blockers are a very good thing. However, we as surgeons are not so enthusiastic about them because … it is impossible to create a normal vagina” because of a lack of available tissue from the underdeveloped penis.
Transmen: Female to male
“Transmen react much better to hormonal therapy than do transwomen,” he said. “If they hide their breasts they really look like men. The disadvantage is that the surgical treatment is much more complex.” The most important operation for them is subcutaneous mastectomy and male contouring. A small, semiareolar incision leaves almost no scar. Most patients still require excision of redundant skin of the breasts.
Phalloplasty is a complex operation aimed at giving the patient an aesthetic phallus, a normal scrotum, the ability to void while standing, and to perform sexual intercourse, all while protecting erogenous sensation, with minimal morbidity and mortality. Dr. Monstrey reported that he has performed 600-700 phalloplasties.
The most common technique has been to use a free vascularized flap from another bodily site with the artery, vein, and nerves to reconnect at the phalloplasty site. Because the skin is very thin on the inner forearm, it is often used and allows forming an inner tube for the urethra and an outer tube for the penis. The surgery may have to be done in three or four stages for the best results. From pictures that Dr. Monstrey showed, it was obvious that the constructed penises were not absolutely natural in appearance, but he said most patients were “rather happy” with them, despite many of these patients being quite demanding. A scrotum is constructed from transposition of the labia minora.
Unfortunately, voiding while standing is often a problem, with 197 out of 562 patients (35%) having a fistula and urine leakage, but this issue frequently corrects itself. “More difficult to treat are the strictures with stenosis, which can be a problem voiding,” he said (occurring in 78/562). Other complications were 5 complete and 43 partial flap failures, 4 cases of compression syndrome, 58 cases of delayed wound healing, and 15 cases of transient ischemia. Flap failures occurred mainly in smokers, “so we don’t operate on smokers anymore,” he said.
One year after the constructive surgery, a penile prosthesis is implanted for those who want it, allowing sexual intercourse. Most individuals had orgasmic function, not because of reconnected nerves in the flap, but, Dr. Monstrey said he believes, because the clitoris, placed beneath the phallus, is denuded and stimulated during sexual activity. He said the problem is that the prostheses are usually intended for elderly men “who have sex a couple of times a month and who have a normal anatomy.” Young transmen may engage in more sexual activity, “so we have a lot of problems with exposure [of the prosthesis], infection, technical defects, and so on,” he said.
A technique gaining popularity is to use a skin flap from the groin area to make a urethra and one from the thigh to construct a penis. Although a penile transplant has recently been performed for a patient who had lost his penis to cancer, transplants are not being considered at this point, both for surgical technical reasons and because of a need for lifelong immunosuppressive drugs.
Proper referrals and counseling
The World Professional Association for Transgender Health in its Standard of Care guidelines 7 recommends one mental health professional referral for the breast surgery and two such referrals for genital surgery. The issue of possible parenthood should be discussed with patients, along with early counseling about fertility options. The age of majority and consent in different countries is important. Dr. Monstrey said genital surgery may be possible before the age of 18 years if all members of a multidisciplinary team of health professionals agree on a case by case basis that the adolescent can understand the risks, benefits, and alternatives to the surgery with the same degree of competence as someone 18 years of age or older.
Dr. Monstrey reported having no financial disclosures.
AACE 2016
Expert simplifies diagnosis of endocrine hypertension
ORLANDO – The diagnosis of hypertension with its origin in the endocrine system may appear complex, but it does not have to be. Primary aldosteronism may be underappreciated and underdiagnosed. On the other hand, catecholamine-secreting tumors are rare, but they often come to mind in making a diagnosis of endocrine hypertension. Dr. William Young Jr., professor of medicine at the Mayo Clinic, Rochester, Minn., presented cases in a lively session of audience participation at the annual meeting of the America Association of Clinical Endocrinologists. Later, Dr. Young summarized some of the key points in an interview, which has been edited for brevity.
Frontline Medical News: What is the endocrinologist’s role in working up the patient who has hypertension of suspected endocrine origin?
Dr. William Young Jr.: The first is knowing when to suspect endocrine hypertension. The most common form of endocrine hypertension is primary aldosteronism. So this is the adrenal-dependent autonomous production of aldosterone, which leads to high blood pressure, volume expansion, and sometimes hypokalemia. One of the concepts that many clinicians forget is that only about 30% of patients with primary aldosteronism present with hypokalemia. So 70% of patients with this disorder don’t have hypokalemia. They look like any other person with high blood pressure.
So when should we look for primary aldosteronism? Onset of high blood pressure at a young age, for example, less than age 30, drug resistant hypertension – so three drugs [with] poor control. Twenty percent of those patients will prove to have primary aldosteronism. Simply poorly controlled hypertension is another group; [or] family history of primary aldosteronism, so all first degree relatives should be tested. Or a patient who has hypertension and has had an incidental discovery of an adrenal mass should also be tested for primary aldosteronism.
Unfortunately, most primary care providers ... think that this is a complicated and dense endocrine disorder, and they frequently will not look for it, but it’s actually very simple. Some of the complexities are historical in nature in that when this disorder was first described, several rules were made for what medications a patient could be on, for example. And it’s difficult to comply with those rules. For example, if you have a patient who’s on five drugs and has poor control, you’re not going to switch him to the two drugs that are recommended because they are weak antihypertensives. It wouldn’t be ethical to do so. [The two drug classes are the calcium channel blocker verapamil and the alpha-1 antagonists doxazosin (Cardura) and terazosin (Hytrin).]
So the best thing to do regardless of what drugs the patient is on – it doesn’t matter if they’re on ACE inhibitors or angiotensin-receptor blockers or diuretics – just get a morning blood sample as your aldosterone and plasma renin activity. If aldosterone is high or generous, greater than 15 ng/dL, if the plasma renin activity is less than 1 ng/mL per hour, that’s a positive case detection test.
That doesn’t prove the patient has primary aldosteronism. The sensitivity/specificity of aldosterone and renin case detection testing is about 75%. So most patients need confirmatory testing, which would either be the saline infusion test or the 24-hour urine for aldosterone on a high-sodium diet. And once primary aldosteronism is confirmed, then we would do an adrenal-directed CT scan.
The problem with the findings in the adrenal glands on CT is that the prevalence of adrenal nodularity increases with age. So people in their 60s and 70s can have adrenal nodules that have nothing to do with aldosterone production. So whereas if the patient is less than age 35 and CT shows a unilateral macroadenoma, the contralateral adrenal is perfectly normal appearing, and the patient has a marked primary aldosteronism – so spontaneous hypokalemia, plasma aldosterone over 30 ng/dL – that subset of patients could go straight to surgery and skip adrenal vein sampling. However, everyone else over age 35 if they want to pursue the surgical option, adrenal vein sampling is a key test.
FMN: Is there anything that rules out primary aldosteronism?
Dr. Young: If the plasma aldosterone level is less than 10 ng/dL it makes primary aldosteronism very unlikely, and if the renin level is higher than 1 ng/mL per hour, that makes primary aldosteronism very unlikely.
FMN: What about working up pheochromocytoma?
Dr. Young: Clinicians, unlike with primary aldosteronism, where they don’t look for it enough, for pheochromocytoma they look for it a lot, and it’s really rare. Between 0.1 and 0.01% of the hypertensive population will prove to have pheochromocytoma.
The false positive rate with our case detection testing of plasma metanephrines about 15%. So based on how rare pheochromocytoma is and a 15% false positive rate with plasma metanephrines, 97% of patients with elevated plasma normetanephrines do not have pheochromocytoma.
So we have a real problem with case detection testing. The 24-hour urine metanephrines and catecholamines using appropriate reference ranges are probably a better way to do case detection testing for pheochromocytoma, but there’s still a false positive rate with urinary normetanephrine.
Never mistake a benign adrenal adenoma for a pheo. In terms of the imaging phenotype, pheos are dense and vascular. As they enlarge, they get cystic hemorrhagic areas within them.
FMN: What goes on with other paragangliomas?
Dr. Young: Pheochromocytoma is the term we use when you have a catecholamine-secreting tumor in the adrenal gland itself. It develops in the adrenal medulla. Paraganglioma is an identical tumor, but it’s outside of the adrenal gland. It’s somewhere in the pelvis, could be in the chest, could be in the skull base, or neck. Most commonly it’s in the abdomen. So the case detection testing is the same.
But patients we should consider testing for pheochromocytoma and paraganglioma are those with paroxysmal symptoms like episodes of pounding heartbeat, sweating, headache, tremor, and pallor. Young people with new onset hypertension, hypertension that’s poorly controlled, and vascular adrenal masses should also be tested for pheochromocytoma.
FMN: Are there things that can confound any of these tests we discussed or any drugs that should be noted that could get in the way?
Dr. Young: For pheochromocytoma, the good news is now that most reference labs use tandem mass spectrometry technology, the hypertension drugs that potentially interfered in the past like labetalol and sotalol no longer interfere. So these days the clinician doesn’t need to stop any blood pressure–related medications.
The medications that can cause false positive testing are primarily tricyclic antidepressants. Flexeril, which is cyclobenzaprine, is commonly used to treat fibromyalgia, and that is a tricyclic antidepressant, and that will cause false positive testing ... with norepinephrine and normetanephrine. Tricyclic antidepressants can increase those levels three, four, or fivefold. Levodopa, which is in Sinemet, can cause false positive testing. Antipsychotics can cause false positive testing, and MAO inhibitors ... So the clinician shouldn’t worry about blood pressure medications but should worry about the other medications the patient is taking.
FMN: When someone looks at laboratory values, should you be comparing these values to people with hypertension who do not have these conditions, and do labs have adjusted values?
Dr. Young: That’s a good question, and in the Mayo medical lab, our reference range that we use is based on patients who were tested for pheochromocytoma [and] proved not to have it. So our cutoffs are 50% to 100% higher than some other reference labs.
These other reference labs use normal laboratory volunteers who have normal blood pressure and who are taking no medications, and I’ve never tested such a patient for pheochromocytoma, so why would we use that group of people to determine our reference range? So we should use reference ranges based on patients tested for pheo but who prove to not have pheo. And that leads to higher accuracy of our case detection tests.
FMN: What are the treatments for these conditions and follow-up? I take it if there’s an adrenal mass, you get a surgeon, and I think you also noted that you need an experienced endocrine surgeon.
Dr. Young: For primary aldosteronism, if the patient has a unilateral aldosterone-producing adenoma, the outstanding treatment is laparoscopic adrenalectomy. Patients are in the hospital one night, [and] they’re back at work in 7-10 days, but that does require an expert laparoscopic adrenal surgeon. And in the United States we have a 1-year endocrine surgery program. It’s optimal that patients are referred to surgeons who have done that unique training.
For pheochromocytoma less than 8-9 cm, laparoscopic adrenalectomy with an experienced endocrine surgeon is an excellent treatment option. When the adrenal pheochromocytoma is larger than 8 or 9 cm, especially if it’s cystic, the surgeon may want to do it as open [surgery] because it’s critical that the capsule of the pheochromocytoma is not ruptured intraoperatively. If it is ruptured, a benign pheochromocytoma has just been transformed to malignant, incurable disease.
If it’s a paraganglioma, typically that requires an open operation whether it’s in the neck or the chest or the pelvis or lower abdomen.
FMN: What is the follow-up to any of these conditions?
Dr. Young: The follow-up once you’ve resected an adrenal pheochromocytoma depends on whether there is a germline mutation. If there is a germline mutation, for example, succinate dehydrogenase mutation [SDH], these patients are at higher risk for developing recurrent pheochromocytoma or paraganglioma, and they’re at risk for developing malignant pheochromocytoma or paraganglioma.
One of our challenges is when we resect a pheochromocytoma or paraganglioma, the pathologist doesn’t have the tools to tell us if it’s benign or malignant ... So all patients need lifelong biochemical follow-up, basically a 24-hour urine for metanephrines and catecholamines annually or plasma metanephrines for life.
If the patients have an underlying mutation like succinate dehydrogenase, they’re at risk for developing nonfunctioning paragangliomas. So these patients need periodic imaging in addition to the annual biochemical testing. For example, if a patient had an abdominal paraganglioma with an SDHB [succinate dehydrogenase complex iron sulfur subunit B], we would do abdominal MRI scans every 1-2 years. That would include the pelvis. We would screen for paragangliomas elsewhere with MRI of the skull base and neck and the chest every 3-5 years, and a total body scan every 5 years or so, either FDG-PET [18F-fluorodeoxyglucose positron emission tomography] scan or 123I-MIBG [metaiodobenzyl-guanidine] scan.
FMN: Is there anything that is particularly new in the past couple of years?
Dr. Young: Some of the innovations lately have been in the area of metastatic pheochromocytoma and paraganglioma. These are in patients who have limited metastatic disease that’s localized to bone or to liver, and we’ve been using ablative therapies. This includes cryoablation ... and radiofrequency ablation, which is killing the tumor with hot temperature, and that’s very effective for patients who have limited metastatic lesions in the bone or liver.
For patients with complex tumors in difficult areas of the body, for example, in the mediastinum or surrounding the heart, we’ve been using 3D printer technology to print [a replica of the structures and] the tumor preoperatively, and this assists in surgical planning.
FMN: And what do you see coming?
Dr. Young: I think we’re getting close to something near curative for patients with malignant pheochromocytoma and paraganglioma. We’re understanding the basic biology better [and] pathophysiology, and I think that’s going to lead to some novel treatments.
Also, what I see coming is that we’ll be able to use germline mutation information and somatic tumor mutation information to guide us on specific imaging modalities, to guide us on forms of preventative therapy so that we prevent the paraganglioma from ever developing and also provide us with additional treatment options.
ORLANDO – The diagnosis of hypertension with its origin in the endocrine system may appear complex, but it does not have to be. Primary aldosteronism may be underappreciated and underdiagnosed. On the other hand, catecholamine-secreting tumors are rare, but they often come to mind in making a diagnosis of endocrine hypertension. Dr. William Young Jr., professor of medicine at the Mayo Clinic, Rochester, Minn., presented cases in a lively session of audience participation at the annual meeting of the America Association of Clinical Endocrinologists. Later, Dr. Young summarized some of the key points in an interview, which has been edited for brevity.
Frontline Medical News: What is the endocrinologist’s role in working up the patient who has hypertension of suspected endocrine origin?
Dr. William Young Jr.: The first is knowing when to suspect endocrine hypertension. The most common form of endocrine hypertension is primary aldosteronism. So this is the adrenal-dependent autonomous production of aldosterone, which leads to high blood pressure, volume expansion, and sometimes hypokalemia. One of the concepts that many clinicians forget is that only about 30% of patients with primary aldosteronism present with hypokalemia. So 70% of patients with this disorder don’t have hypokalemia. They look like any other person with high blood pressure.
So when should we look for primary aldosteronism? Onset of high blood pressure at a young age, for example, less than age 30, drug resistant hypertension – so three drugs [with] poor control. Twenty percent of those patients will prove to have primary aldosteronism. Simply poorly controlled hypertension is another group; [or] family history of primary aldosteronism, so all first degree relatives should be tested. Or a patient who has hypertension and has had an incidental discovery of an adrenal mass should also be tested for primary aldosteronism.
Unfortunately, most primary care providers ... think that this is a complicated and dense endocrine disorder, and they frequently will not look for it, but it’s actually very simple. Some of the complexities are historical in nature in that when this disorder was first described, several rules were made for what medications a patient could be on, for example. And it’s difficult to comply with those rules. For example, if you have a patient who’s on five drugs and has poor control, you’re not going to switch him to the two drugs that are recommended because they are weak antihypertensives. It wouldn’t be ethical to do so. [The two drug classes are the calcium channel blocker verapamil and the alpha-1 antagonists doxazosin (Cardura) and terazosin (Hytrin).]
So the best thing to do regardless of what drugs the patient is on – it doesn’t matter if they’re on ACE inhibitors or angiotensin-receptor blockers or diuretics – just get a morning blood sample as your aldosterone and plasma renin activity. If aldosterone is high or generous, greater than 15 ng/dL, if the plasma renin activity is less than 1 ng/mL per hour, that’s a positive case detection test.
That doesn’t prove the patient has primary aldosteronism. The sensitivity/specificity of aldosterone and renin case detection testing is about 75%. So most patients need confirmatory testing, which would either be the saline infusion test or the 24-hour urine for aldosterone on a high-sodium diet. And once primary aldosteronism is confirmed, then we would do an adrenal-directed CT scan.
The problem with the findings in the adrenal glands on CT is that the prevalence of adrenal nodularity increases with age. So people in their 60s and 70s can have adrenal nodules that have nothing to do with aldosterone production. So whereas if the patient is less than age 35 and CT shows a unilateral macroadenoma, the contralateral adrenal is perfectly normal appearing, and the patient has a marked primary aldosteronism – so spontaneous hypokalemia, plasma aldosterone over 30 ng/dL – that subset of patients could go straight to surgery and skip adrenal vein sampling. However, everyone else over age 35 if they want to pursue the surgical option, adrenal vein sampling is a key test.
FMN: Is there anything that rules out primary aldosteronism?
Dr. Young: If the plasma aldosterone level is less than 10 ng/dL it makes primary aldosteronism very unlikely, and if the renin level is higher than 1 ng/mL per hour, that makes primary aldosteronism very unlikely.
FMN: What about working up pheochromocytoma?
Dr. Young: Clinicians, unlike with primary aldosteronism, where they don’t look for it enough, for pheochromocytoma they look for it a lot, and it’s really rare. Between 0.1 and 0.01% of the hypertensive population will prove to have pheochromocytoma.
The false positive rate with our case detection testing of plasma metanephrines about 15%. So based on how rare pheochromocytoma is and a 15% false positive rate with plasma metanephrines, 97% of patients with elevated plasma normetanephrines do not have pheochromocytoma.
So we have a real problem with case detection testing. The 24-hour urine metanephrines and catecholamines using appropriate reference ranges are probably a better way to do case detection testing for pheochromocytoma, but there’s still a false positive rate with urinary normetanephrine.
Never mistake a benign adrenal adenoma for a pheo. In terms of the imaging phenotype, pheos are dense and vascular. As they enlarge, they get cystic hemorrhagic areas within them.
FMN: What goes on with other paragangliomas?
Dr. Young: Pheochromocytoma is the term we use when you have a catecholamine-secreting tumor in the adrenal gland itself. It develops in the adrenal medulla. Paraganglioma is an identical tumor, but it’s outside of the adrenal gland. It’s somewhere in the pelvis, could be in the chest, could be in the skull base, or neck. Most commonly it’s in the abdomen. So the case detection testing is the same.
But patients we should consider testing for pheochromocytoma and paraganglioma are those with paroxysmal symptoms like episodes of pounding heartbeat, sweating, headache, tremor, and pallor. Young people with new onset hypertension, hypertension that’s poorly controlled, and vascular adrenal masses should also be tested for pheochromocytoma.
FMN: Are there things that can confound any of these tests we discussed or any drugs that should be noted that could get in the way?
Dr. Young: For pheochromocytoma, the good news is now that most reference labs use tandem mass spectrometry technology, the hypertension drugs that potentially interfered in the past like labetalol and sotalol no longer interfere. So these days the clinician doesn’t need to stop any blood pressure–related medications.
The medications that can cause false positive testing are primarily tricyclic antidepressants. Flexeril, which is cyclobenzaprine, is commonly used to treat fibromyalgia, and that is a tricyclic antidepressant, and that will cause false positive testing ... with norepinephrine and normetanephrine. Tricyclic antidepressants can increase those levels three, four, or fivefold. Levodopa, which is in Sinemet, can cause false positive testing. Antipsychotics can cause false positive testing, and MAO inhibitors ... So the clinician shouldn’t worry about blood pressure medications but should worry about the other medications the patient is taking.
FMN: When someone looks at laboratory values, should you be comparing these values to people with hypertension who do not have these conditions, and do labs have adjusted values?
Dr. Young: That’s a good question, and in the Mayo medical lab, our reference range that we use is based on patients who were tested for pheochromocytoma [and] proved not to have it. So our cutoffs are 50% to 100% higher than some other reference labs.
These other reference labs use normal laboratory volunteers who have normal blood pressure and who are taking no medications, and I’ve never tested such a patient for pheochromocytoma, so why would we use that group of people to determine our reference range? So we should use reference ranges based on patients tested for pheo but who prove to not have pheo. And that leads to higher accuracy of our case detection tests.
FMN: What are the treatments for these conditions and follow-up? I take it if there’s an adrenal mass, you get a surgeon, and I think you also noted that you need an experienced endocrine surgeon.
Dr. Young: For primary aldosteronism, if the patient has a unilateral aldosterone-producing adenoma, the outstanding treatment is laparoscopic adrenalectomy. Patients are in the hospital one night, [and] they’re back at work in 7-10 days, but that does require an expert laparoscopic adrenal surgeon. And in the United States we have a 1-year endocrine surgery program. It’s optimal that patients are referred to surgeons who have done that unique training.
For pheochromocytoma less than 8-9 cm, laparoscopic adrenalectomy with an experienced endocrine surgeon is an excellent treatment option. When the adrenal pheochromocytoma is larger than 8 or 9 cm, especially if it’s cystic, the surgeon may want to do it as open [surgery] because it’s critical that the capsule of the pheochromocytoma is not ruptured intraoperatively. If it is ruptured, a benign pheochromocytoma has just been transformed to malignant, incurable disease.
If it’s a paraganglioma, typically that requires an open operation whether it’s in the neck or the chest or the pelvis or lower abdomen.
FMN: What is the follow-up to any of these conditions?
Dr. Young: The follow-up once you’ve resected an adrenal pheochromocytoma depends on whether there is a germline mutation. If there is a germline mutation, for example, succinate dehydrogenase mutation [SDH], these patients are at higher risk for developing recurrent pheochromocytoma or paraganglioma, and they’re at risk for developing malignant pheochromocytoma or paraganglioma.
One of our challenges is when we resect a pheochromocytoma or paraganglioma, the pathologist doesn’t have the tools to tell us if it’s benign or malignant ... So all patients need lifelong biochemical follow-up, basically a 24-hour urine for metanephrines and catecholamines annually or plasma metanephrines for life.
If the patients have an underlying mutation like succinate dehydrogenase, they’re at risk for developing nonfunctioning paragangliomas. So these patients need periodic imaging in addition to the annual biochemical testing. For example, if a patient had an abdominal paraganglioma with an SDHB [succinate dehydrogenase complex iron sulfur subunit B], we would do abdominal MRI scans every 1-2 years. That would include the pelvis. We would screen for paragangliomas elsewhere with MRI of the skull base and neck and the chest every 3-5 years, and a total body scan every 5 years or so, either FDG-PET [18F-fluorodeoxyglucose positron emission tomography] scan or 123I-MIBG [metaiodobenzyl-guanidine] scan.
FMN: Is there anything that is particularly new in the past couple of years?
Dr. Young: Some of the innovations lately have been in the area of metastatic pheochromocytoma and paraganglioma. These are in patients who have limited metastatic disease that’s localized to bone or to liver, and we’ve been using ablative therapies. This includes cryoablation ... and radiofrequency ablation, which is killing the tumor with hot temperature, and that’s very effective for patients who have limited metastatic lesions in the bone or liver.
For patients with complex tumors in difficult areas of the body, for example, in the mediastinum or surrounding the heart, we’ve been using 3D printer technology to print [a replica of the structures and] the tumor preoperatively, and this assists in surgical planning.
FMN: And what do you see coming?
Dr. Young: I think we’re getting close to something near curative for patients with malignant pheochromocytoma and paraganglioma. We’re understanding the basic biology better [and] pathophysiology, and I think that’s going to lead to some novel treatments.
Also, what I see coming is that we’ll be able to use germline mutation information and somatic tumor mutation information to guide us on specific imaging modalities, to guide us on forms of preventative therapy so that we prevent the paraganglioma from ever developing and also provide us with additional treatment options.
ORLANDO – The diagnosis of hypertension with its origin in the endocrine system may appear complex, but it does not have to be. Primary aldosteronism may be underappreciated and underdiagnosed. On the other hand, catecholamine-secreting tumors are rare, but they often come to mind in making a diagnosis of endocrine hypertension. Dr. William Young Jr., professor of medicine at the Mayo Clinic, Rochester, Minn., presented cases in a lively session of audience participation at the annual meeting of the America Association of Clinical Endocrinologists. Later, Dr. Young summarized some of the key points in an interview, which has been edited for brevity.
Frontline Medical News: What is the endocrinologist’s role in working up the patient who has hypertension of suspected endocrine origin?
Dr. William Young Jr.: The first is knowing when to suspect endocrine hypertension. The most common form of endocrine hypertension is primary aldosteronism. So this is the adrenal-dependent autonomous production of aldosterone, which leads to high blood pressure, volume expansion, and sometimes hypokalemia. One of the concepts that many clinicians forget is that only about 30% of patients with primary aldosteronism present with hypokalemia. So 70% of patients with this disorder don’t have hypokalemia. They look like any other person with high blood pressure.
So when should we look for primary aldosteronism? Onset of high blood pressure at a young age, for example, less than age 30, drug resistant hypertension – so three drugs [with] poor control. Twenty percent of those patients will prove to have primary aldosteronism. Simply poorly controlled hypertension is another group; [or] family history of primary aldosteronism, so all first degree relatives should be tested. Or a patient who has hypertension and has had an incidental discovery of an adrenal mass should also be tested for primary aldosteronism.
Unfortunately, most primary care providers ... think that this is a complicated and dense endocrine disorder, and they frequently will not look for it, but it’s actually very simple. Some of the complexities are historical in nature in that when this disorder was first described, several rules were made for what medications a patient could be on, for example. And it’s difficult to comply with those rules. For example, if you have a patient who’s on five drugs and has poor control, you’re not going to switch him to the two drugs that are recommended because they are weak antihypertensives. It wouldn’t be ethical to do so. [The two drug classes are the calcium channel blocker verapamil and the alpha-1 antagonists doxazosin (Cardura) and terazosin (Hytrin).]
So the best thing to do regardless of what drugs the patient is on – it doesn’t matter if they’re on ACE inhibitors or angiotensin-receptor blockers or diuretics – just get a morning blood sample as your aldosterone and plasma renin activity. If aldosterone is high or generous, greater than 15 ng/dL, if the plasma renin activity is less than 1 ng/mL per hour, that’s a positive case detection test.
That doesn’t prove the patient has primary aldosteronism. The sensitivity/specificity of aldosterone and renin case detection testing is about 75%. So most patients need confirmatory testing, which would either be the saline infusion test or the 24-hour urine for aldosterone on a high-sodium diet. And once primary aldosteronism is confirmed, then we would do an adrenal-directed CT scan.
The problem with the findings in the adrenal glands on CT is that the prevalence of adrenal nodularity increases with age. So people in their 60s and 70s can have adrenal nodules that have nothing to do with aldosterone production. So whereas if the patient is less than age 35 and CT shows a unilateral macroadenoma, the contralateral adrenal is perfectly normal appearing, and the patient has a marked primary aldosteronism – so spontaneous hypokalemia, plasma aldosterone over 30 ng/dL – that subset of patients could go straight to surgery and skip adrenal vein sampling. However, everyone else over age 35 if they want to pursue the surgical option, adrenal vein sampling is a key test.
FMN: Is there anything that rules out primary aldosteronism?
Dr. Young: If the plasma aldosterone level is less than 10 ng/dL it makes primary aldosteronism very unlikely, and if the renin level is higher than 1 ng/mL per hour, that makes primary aldosteronism very unlikely.
FMN: What about working up pheochromocytoma?
Dr. Young: Clinicians, unlike with primary aldosteronism, where they don’t look for it enough, for pheochromocytoma they look for it a lot, and it’s really rare. Between 0.1 and 0.01% of the hypertensive population will prove to have pheochromocytoma.
The false positive rate with our case detection testing of plasma metanephrines about 15%. So based on how rare pheochromocytoma is and a 15% false positive rate with plasma metanephrines, 97% of patients with elevated plasma normetanephrines do not have pheochromocytoma.
So we have a real problem with case detection testing. The 24-hour urine metanephrines and catecholamines using appropriate reference ranges are probably a better way to do case detection testing for pheochromocytoma, but there’s still a false positive rate with urinary normetanephrine.
Never mistake a benign adrenal adenoma for a pheo. In terms of the imaging phenotype, pheos are dense and vascular. As they enlarge, they get cystic hemorrhagic areas within them.
FMN: What goes on with other paragangliomas?
Dr. Young: Pheochromocytoma is the term we use when you have a catecholamine-secreting tumor in the adrenal gland itself. It develops in the adrenal medulla. Paraganglioma is an identical tumor, but it’s outside of the adrenal gland. It’s somewhere in the pelvis, could be in the chest, could be in the skull base, or neck. Most commonly it’s in the abdomen. So the case detection testing is the same.
But patients we should consider testing for pheochromocytoma and paraganglioma are those with paroxysmal symptoms like episodes of pounding heartbeat, sweating, headache, tremor, and pallor. Young people with new onset hypertension, hypertension that’s poorly controlled, and vascular adrenal masses should also be tested for pheochromocytoma.
FMN: Are there things that can confound any of these tests we discussed or any drugs that should be noted that could get in the way?
Dr. Young: For pheochromocytoma, the good news is now that most reference labs use tandem mass spectrometry technology, the hypertension drugs that potentially interfered in the past like labetalol and sotalol no longer interfere. So these days the clinician doesn’t need to stop any blood pressure–related medications.
The medications that can cause false positive testing are primarily tricyclic antidepressants. Flexeril, which is cyclobenzaprine, is commonly used to treat fibromyalgia, and that is a tricyclic antidepressant, and that will cause false positive testing ... with norepinephrine and normetanephrine. Tricyclic antidepressants can increase those levels three, four, or fivefold. Levodopa, which is in Sinemet, can cause false positive testing. Antipsychotics can cause false positive testing, and MAO inhibitors ... So the clinician shouldn’t worry about blood pressure medications but should worry about the other medications the patient is taking.
FMN: When someone looks at laboratory values, should you be comparing these values to people with hypertension who do not have these conditions, and do labs have adjusted values?
Dr. Young: That’s a good question, and in the Mayo medical lab, our reference range that we use is based on patients who were tested for pheochromocytoma [and] proved not to have it. So our cutoffs are 50% to 100% higher than some other reference labs.
These other reference labs use normal laboratory volunteers who have normal blood pressure and who are taking no medications, and I’ve never tested such a patient for pheochromocytoma, so why would we use that group of people to determine our reference range? So we should use reference ranges based on patients tested for pheo but who prove to not have pheo. And that leads to higher accuracy of our case detection tests.
FMN: What are the treatments for these conditions and follow-up? I take it if there’s an adrenal mass, you get a surgeon, and I think you also noted that you need an experienced endocrine surgeon.
Dr. Young: For primary aldosteronism, if the patient has a unilateral aldosterone-producing adenoma, the outstanding treatment is laparoscopic adrenalectomy. Patients are in the hospital one night, [and] they’re back at work in 7-10 days, but that does require an expert laparoscopic adrenal surgeon. And in the United States we have a 1-year endocrine surgery program. It’s optimal that patients are referred to surgeons who have done that unique training.
For pheochromocytoma less than 8-9 cm, laparoscopic adrenalectomy with an experienced endocrine surgeon is an excellent treatment option. When the adrenal pheochromocytoma is larger than 8 or 9 cm, especially if it’s cystic, the surgeon may want to do it as open [surgery] because it’s critical that the capsule of the pheochromocytoma is not ruptured intraoperatively. If it is ruptured, a benign pheochromocytoma has just been transformed to malignant, incurable disease.
If it’s a paraganglioma, typically that requires an open operation whether it’s in the neck or the chest or the pelvis or lower abdomen.
FMN: What is the follow-up to any of these conditions?
Dr. Young: The follow-up once you’ve resected an adrenal pheochromocytoma depends on whether there is a germline mutation. If there is a germline mutation, for example, succinate dehydrogenase mutation [SDH], these patients are at higher risk for developing recurrent pheochromocytoma or paraganglioma, and they’re at risk for developing malignant pheochromocytoma or paraganglioma.
One of our challenges is when we resect a pheochromocytoma or paraganglioma, the pathologist doesn’t have the tools to tell us if it’s benign or malignant ... So all patients need lifelong biochemical follow-up, basically a 24-hour urine for metanephrines and catecholamines annually or plasma metanephrines for life.
If the patients have an underlying mutation like succinate dehydrogenase, they’re at risk for developing nonfunctioning paragangliomas. So these patients need periodic imaging in addition to the annual biochemical testing. For example, if a patient had an abdominal paraganglioma with an SDHB [succinate dehydrogenase complex iron sulfur subunit B], we would do abdominal MRI scans every 1-2 years. That would include the pelvis. We would screen for paragangliomas elsewhere with MRI of the skull base and neck and the chest every 3-5 years, and a total body scan every 5 years or so, either FDG-PET [18F-fluorodeoxyglucose positron emission tomography] scan or 123I-MIBG [metaiodobenzyl-guanidine] scan.
FMN: Is there anything that is particularly new in the past couple of years?
Dr. Young: Some of the innovations lately have been in the area of metastatic pheochromocytoma and paraganglioma. These are in patients who have limited metastatic disease that’s localized to bone or to liver, and we’ve been using ablative therapies. This includes cryoablation ... and radiofrequency ablation, which is killing the tumor with hot temperature, and that’s very effective for patients who have limited metastatic lesions in the bone or liver.
For patients with complex tumors in difficult areas of the body, for example, in the mediastinum or surrounding the heart, we’ve been using 3D printer technology to print [a replica of the structures and] the tumor preoperatively, and this assists in surgical planning.
FMN: And what do you see coming?
Dr. Young: I think we’re getting close to something near curative for patients with malignant pheochromocytoma and paraganglioma. We’re understanding the basic biology better [and] pathophysiology, and I think that’s going to lead to some novel treatments.
Also, what I see coming is that we’ll be able to use germline mutation information and somatic tumor mutation information to guide us on specific imaging modalities, to guide us on forms of preventative therapy so that we prevent the paraganglioma from ever developing and also provide us with additional treatment options.
EXPERT ANALYSIS AT AACE 2016
TAVR cerebral protection device appears safe, effective
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
PARIS – The TriGuard neuroprotection device for use during transcatheter aortic valve replacement effectively prevented strokes while raising no safety concerns in a pooled analysis of three controlled trials, according to Dr. Alexandra J. Lansky.
The TriGuard, which is investigational in the United States but approved in Europe, also significantly reduced the risk of central nervous system infarction, as assessed by diffusion-weighted MRI. Moreover, when imaging did show CNS infarcts in patients with the TriGuard in place during their TAVR (transcatheter aortic valve replacement), the total brain lesion volume was about 40% less than in controls without the neuroprotection device, according to Dr. Lansky, professor of medicine and director of the cardiovascular clinical research program at Yale University in New Haven, Conn.
“Essentially what’s happening is that we’re reducing with this device the frequency of CNS infarctions, and also reducing the size of the lesions when they are present,” she said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The TriGuard is designed to fill an unmet need for stroke protection in TAVR patients. The incidence of clinical stroke within 30 days after TAVR in recent randomized controlled trials is 1.5%-6%. But there is clear evidence of underreporting of stroke in these trials. When neurologists examine TAVR patients or the patients are evaluated by serial testing using the NIH Stroke Scale plus brain imaging, the 30-day stroke rates are 15%-28%, according to the cardiologist.
“We know that about 50% of these strokes happen in the periprocedural period, and stroke is one of the strongest predictors of mortality, conferring a three- to ninefold increased risk,” Dr. Lansky emphasized.
She presented a pooled analysis including 59 TriGuard recipients and 83 controls who underwent TAVR in the DEFLECT I and III trials and the NeuroTAVR registry. They were evaluated using the NIH Stroke Scale before TAVR and again at 4 and 30 days post procedure. In addition, they underwent brain imaging via diffusion-weighted MRI 4 days post TAVR.
Stroke as defined by the Valve Academic Research Consortium–2 (VARC2) criteria occurred in none of the TriGuard group but in 6% of controls. And stroke as defined by the American Stroke Association, which requires a worsening score on the serial NIH Stroke Scale measurements plus imaging evidence of CNS infarction, occurred in 0 TriGuard-protected patients and in 19% of controls.
The incidence of CNS infarction on MRI was 92% in controls and 72% in the TriGuard group. Thus, 28% of patients with the TriGuard in place developed no brain infarct lesions at all; that’s a first for any TAVR neuroprotection device, according to Dr. Lansky.
In patients with CNS lesions, the total lesion volume was 101 mm3 in the TriGuard group, compared with 174 mm3 in the controls. The average lesion volume was 25 mm3 in the TriGuard group versus 43 mm3 in the controls.
TriGuard is a relatively simple device consisting of a single-wire nitinol frame and mesh filter with a pore size of 130 mcm. It’s designed to deflect emboli during TAVR while allowing maximal cerebral blood flow. After being delivered by a 9 French sheath from the contralateral femoral artery, the device sits at the roof of the aortic arch. Importantly, it covers all three cerebral arteries, Dr. Lansky said. The device is held in position by a stabilizer in the innominate artery.
Although introducing an additional element into TAVR raises the theoretic possibility of safety concerns, no safety signal was seen in the pooled analysis. In-hospital major adverse event rates were similar in the two groups.
Asked why 72% of patients with the TriGuard in place nonetheless developed CNS infarcts, Dr. Lansky said she believes the device has gaps on the sides that allow smaller emboli to pass through. Future iterations of the TriGuard will address this.
The clinical significance of the CNS infarcts seen on MRI in TAVR patients is a controversial issue among interventional cardiologists. Some cardiologists consider these to be silent lesions of dubious clinical relevance. That’s not Dr. Lansky’s view.
“When you track these MRI lesions out to 30 days, many times they disappear. They don’t disappear because there’s no damage; they disappear because the cells die. When you talk to neurologists about the MRI lesions, they will tell you that they actually represent cell death and correlate with brain infarction,” she said.
Dr. Nicolo Piazza commented that he considered the pooled analysis findings hypothesis generating but not definitive because of baseline imbalances between the two study arms. The control group had numerically higher – albeit not statistically significantly so – rates of atrial fibrillation at hospital admission as well as higher Society of Thoracic Surgeons risk scores, both of which increase stroke risk, noted Dr. Piazza of McGill University in Montreal.
Dr. Lansky replied that the much larger ongoing pivotal randomized, phase III REFLECT trial should provide definitive answers.
She reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
AT EUROPCR 2016
Key clinical point: The TriGuard neuroprotection device for use in TAVR effectively prevented strokes.
Major finding: The 30-day incidence of stroke in TAVR patients with the TriGard embolic protection device in place was 0, compared with 6% or 19% in controls, depending upon the stroke definition used.
Data source: A post hoc analysis of pooled data on 59 TriGuard recipients and 83 controls in three trials.
Disclosures: The presenter reported receiving institutional research grant support from Keystone Heart, which produces the TriGuard device.
Skin Lesions in Patients Treated With Imatinib Mesylate: A 5-Year Prospective Study
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.
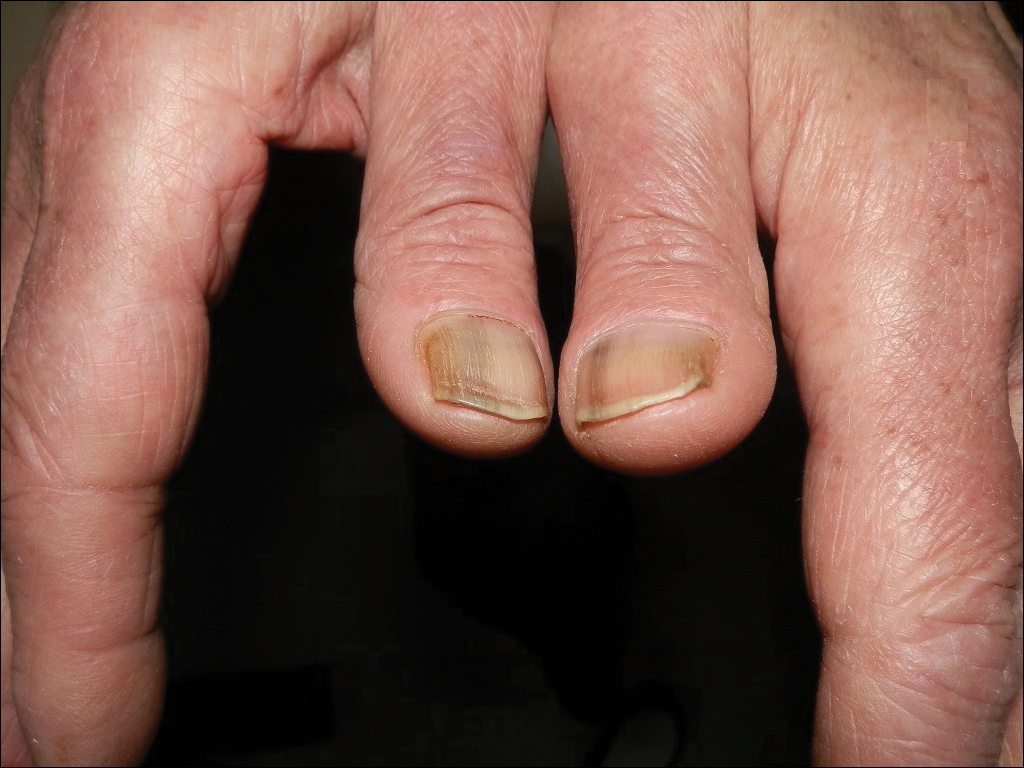
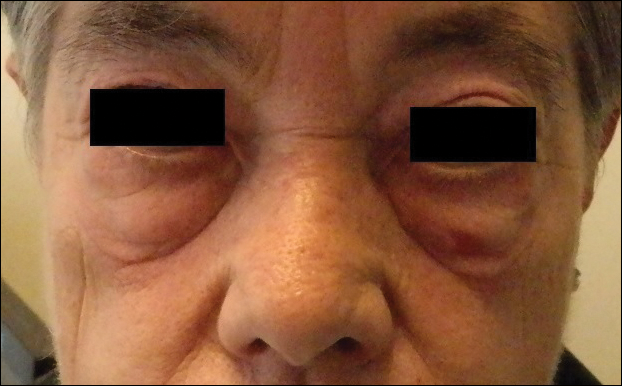
All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.
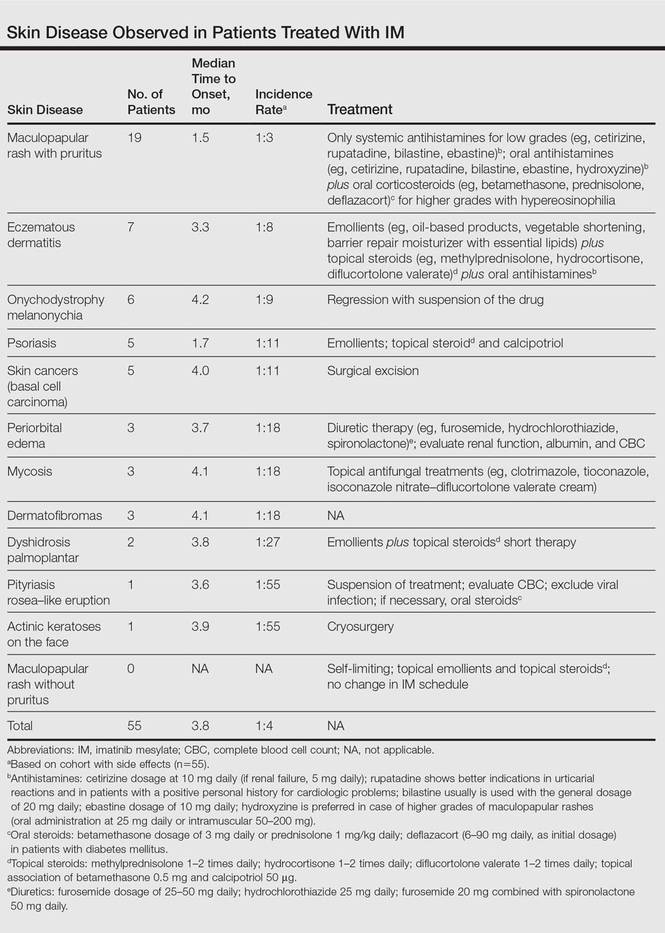
Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.




All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.

Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
Imatinib mesylate (IM) represents the first-line treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). Its pharmacological activity is related to a specific action on several tyrosine kinases in different tumors, including Bcr-Abl in CML, c-Kit (CD117) in GIST, and platelet-derived growth factor receptor in dermatofibrosarcoma protuberans.1,2
Imatinib mesylate has been shown to improve progression-free survival and overall survival2; however, it also has several side effects. Among the adverse effects (AEs), less than 10% are nonhematologic, such as nausea, vomiting, diarrhea, muscle cramps, and cutaneous reactions.3,4
We followed patients who were treated with IM for 5 years to identify AEs of therapy.
Methods
The aim of this prospective study was to identify and collect data regarding IM cutaneous side effects so that clinicians can detect AEs early and differentiate them from AEs caused by other medications. All patients were subjected to a median of 5 years’ follow-up. We included all the patients treated with IM and excluded patients who had a history of eczematous dermatitis, psoriasis, renal impairment, or dyshidrosis palmoplantar. Before starting IM, all patients presented for a dermatologic visit. They were subsequently evaluated every 3 months.
The incidence rate was defined as the ratio of patients with cutaneous side effects and the total patients treated with IM. Furthermore, we calculated the ratio between each class of patient with a specific cutaneous manifestation and the entire cohort of patients with cutaneous side effects related to IM.
When necessary, microbiological, serological, and histopathological analyses were performed.
Results
In 60 months, we followed 220 patients treated with IM. Among them, 55 (25%) developed cutaneous side effects (35 males; 20 females). The incidence rate of the patients with cutaneous side effects was 1:4. The median age of the entire cohort was 52.5 years. Fifty patients were being treated for CML and 5 for GISTs. All patients received IM at a dosage of 400 mg daily.
The following skin diseases were observed in patients treated with IM (Table): 19 patients with maculopapular rash with pruritus (no maculopapular rash without pruritus was detected), 7 patients with eczematous dermatitis such as stasis dermatitis and seborrheic dermatitis, 6 patients with onychodystrophy melanonychia (Figure 1), 5 patients with psoriasis, 5 patients with skin cancers including basal cell carcinoma (BCC)(Figure 2), 3 patients with periorbital edema (Figure 3), 3 patients with mycosis, 3 patients with dermatofibromas, 2 patients with dyshidrosis palmoplantar, 1 patient with pityriasis rosea–like eruption (Figure 4), and 1 patient with actinic keratoses on the face. No hypopigmentation or hyperpigmentation, excluding the individual case of melanonychia, was observed.




All cutaneous diseases reported in this study appeared after IM therapy (median, 3.8 months). The median time to onset for each cutaneous disorder is reported in the Table. During the first dermatologic visit before starting IM therapy, none of the patients showed any of these cutaneous diseases.
The adverse cutaneous reactions were treated with appropriate drugs. Generally, eczematous dermatitis was treated using topical steroids, emollients, and oral antihistamines. In patients with maculopapular rash with pruritus, oral corticosteroids (eg, betamethasone 3 mg daily or prednisolone 1 mg/kg) in association with antihistamine was necessary. Psoriasis was completely improved with topical betamethasone 0.5 mg and calcipotriol 50 µg. Skin cancers were treated with surgical excision with histologic examination. All treatments are outlined in the Table.

Imatinib mesylate therapy was suspended in 2 patients with maculopapular rash with moderate to severe pruritus; however, despite the temporary suspension of the drug and the appropriate therapies (corticosteroids and antihistamines), cutaneous side effects reappeared 7 to 10 days after therapy resumed. Therefore, the treatment was permanently suspended in these 2 cases and IM was replaced with erlotinib, a second-generation Bcr-Abl tyrosine kinase inhibitor.
Comment
The introduction of IM for the treatment of GIST and CML has changed the history of these diseases. The drug typically is well tolerated and few patients have reported severe AEs. Mild skin reactions are relatively frequent, ranging from 7% to 21% of patients treated.3 In our case, the percentage was relatively higher (25%), likely because of close monitoring of patients, with an increase in the incidence rate.
Imatinib mesylate cutaneous reactions are dose dependent.4 Indeed, in all our cases, the cutaneous reactions arose with an IM dosage of 400 mg daily, which is compatible with the definition of dose-independent cutaneous AEs.
The most common cutaneous AEs reported in the literature were swelling/edema and maculopapular rash. Swelling is the most common AE described during therapy with IM with an incidence of 63% to 84%.5 Swelling often involves the periorbital area and occurs approximately 6 weeks after starting IM. Although its pathogenesis is uncertain, it has been shown that IM blocks the platelet-derived growth factor receptor expressed on blood vessels that regulates the transportation transcapillary. The inhibition of this receptor can lead to increased pore pressure, resulting in edema and erythema. Maculopapular eruptions (50% of cases) often affect the trunk and the limbs and are accompanied by pruritus. Commonly, these rashes arise after 9 weeks of IM therapy. These eruptions are self-limiting and only topical emollients and steroids are required, without any change in IM schedule. To treat maculopapular eruptions with pruritus, oral steroids and antihistamines may be helpful, without suspending IM treatment. When grade 2 or 3 pruriginous maculopapular eruptions arise, the suspension of IM combined with steroids and antihistamines may be necessary. When the readministration of IM is required, it is mandatory to start IM at a lower dose (50–100 mg/d), administering prednisolone 0.5 to 1.0 mg/kg daily. Then, the steroid gradually can be tapered.6 Critical cutaneous AEs that are resistant to supportive measures warrant suspension of IM therapy. However, the incidence of this event is small (<1% of all patients).7
Regarding severe cutaneous AEs from IM therapy, Hsiao et al8 reported the case of Stevens-Johnson syndrome. In this case, IM was immediately stopped and systemic steroids were started. Rarely, erythroderma (grade 4 toxicity) can develop for which a prompt and perpetual suspension of IM is necessary and supportive care therapy with oral and topical steroids is recommended.9
Hyperpigmentation induced by IM, mostly in patients with Fitzpatrick skin types V to VI and with a general prevalence of 16% to 40% in treated patients, often is related to a mutation of c-Kit or other kinases that are activated rather than inhibited by the drug, resulting in overstimulation of melanogenesis.10 The prevalence of Fitzpatrick skin types I to III determined the absence of pigmentation changes in our cohort, excluding melanonychia. Hyperpigmentation was observed in the skin as well as the appendages such as nails, resulting in melanonychia (Figure 1). However, Brazzelli et al11 reported hypopigmentation in 5 white patients treated with IM; furthermore, they found a direct correlation between hypopigmentation and development of skin cancers in these patients. The susceptibility to develop skin cancers may persist, even without a clear manifestation of hypopigmentation, as reported in the current analysis. We documented BCC in 5 patients, 1 patient developed actinic keratoses, and 3 patients developed dermatofibromas. However, these neoplasms probably were not provoked by IM. On the contrary, we did not note squamous cell carcinoma, which was reported by Baskaynak et al12 in 2 CML patients treated with IM.
The administration of IM can be associated with exacerbation of psoriasis. Paradoxically, in genetically predisposed individuals, tumor necrosis factor α (TNF-α) antagonists, such as IM, seem to induce psoriasis, producing IFN-α rather than TNF-α and increasing inflammation.13 In fact, some research shows induction of psoriasis by anti–TNF-α drugs.14-16 Two cases of IM associated with psoriasis have been reported, and both cases represented an exacerbation of previously diagnosed psoriasis.13,17 On the contrary, in our analysis we reported 5 cases of psoriasis vulgaris induced by IM administration. Our patients developed cutaneous psoriatic lesions approximately 1.7 months after the start of IM therapy.
The pityriasis rosea–like eruption (Figure 4) presented as nonpruritic, erythematous, scaly patches on the trunk and extremities, and arose 3.6 months after the start of treatment. This particular cutaneous AE is rare. In 3 case reports, the IM dosage also was 400 mg daily.18-20 The pathophysiology of this rare skin reaction stems from the pharmacological effect of IM rather than a hypersensitivity reaction.18
Deininger et al7 reported that patients with a high basophil count (>20%) rarely show urticarial eruptions after IM due to histamine release from basophils. Premedication with an antihistamine was helpful and the urticarial eruption resolved after normalization in basophil count.7
Given the importance of IM for patients who have limited therapeutic alternatives for their disease and the ability to safely treat the cutaneous AEs, as demonstrated in our analysis, the suspension of IM for dermatological complications is necessary only in rare cases, as shown by the low number of patients (n=2) who had to discontinue therapy. The cutaneous AEs should be diagnosed and treated early with less impact on chemotherapy treatments. The administration of IM should involve a coordinated effort among oncologists and dermatologists to prevent important complications.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Breccia M, Carmosimo I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121-123.
- Ugurel S, Hildebrand R, Dippel E, et al. Dose dependent severe cutaneous reactions to imatinib. Br J Cancer. 2003;88:1157-1159.
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukaemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201-206.
- Scott LC, White JD, Reid R, et al. Management of skin toxicity related to the use of imatinibnmesylate (STI571, GlivecTM) for advanced stage gastrointestinal stromal tumors. Sarcoma. 2005;9:157-160.
- Deininger MW, O’Brien SG, Ford JM, et al. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637-1647.
- Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620-622.
- Sehgal VN, Srivastava G, Sardana K. Erythroderma/exfoliative dermatitis: a synopsis. Int J Dermatol. 2004;43:39-47.
- Pietras K, Pahler J, Bergers G, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19.
- Brazzelli V, Prestinari F, Barbagallo T, et al. A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venerol. 2007;21:384-387.
- Baskaynak G, Kreuzer KA, Schwarz M, et al. Squamous cutaneous epithelial cell carcinoma in two CML patients with progressive disease under imatinib treatment. Eur J Haematol. 2003;70:231-234.
- Cheng H, Geist DE, Piperdi M, et al. Management of imatinib-related exacerbation of psoriasis in a patient with a gastrointestinal stromal tumor. Australas J Dermatol. 2009;50:41-43.
- Faillace C, Duarte GV, Cunha RS, et al. Severe infliximab-induced psoriasis treated with adalimumab switching. Int J Dermatol. 2013;52:234-238.
- Iborra M, Beltrán B, Bastida G, et al. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a aradoxical side effect. J Crohns Colitis. 2011;5:157-161.
- Fernandes IC, Torres T, Sanches M, et al. Psoriasis induced by infliximab. Acta Med Port. 2011;24:709-712.
- Woo SM, Huh CH, Park KC, et al. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724-726.
- Brazzelli V, Prestinari F, Roveda E, et al. Pytiriasis rosea-like eruption during treatment with imatinib mesylate. description of 3 cases. J Am Acad Dermatol. 2005;53:240-243.
- Konstantapoulos K, Papadogianni A, Dimopoulou M, et al. Pytriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002;205:172-173.
- Cho AY, Kim DH, Im M, et al. Pityriasis rosealike drug eruption induced by imatinib mesylate (Gleevec). Ann Dermatol. 2011;23(suppl 3):360-363.
Practice Points
- The most common cutaneous adverse reactions from imatinib mesylate (IM) are swelling and edema.
- Maculopapular rash with pruritus is one of the most common side effects from IM and can be effectively treated with oral or systemic antihistamines.
- The onset of periorbital edema requires a complete evaluation of renal function.
Low hematocrit in elderly portends increased bleeding post PCI
PARIS – A low hematocrit in an elderly patient who’s going to undergo percutaneous coronary intervention signals a markedly increased risk of major bleeding within 30 days of the procedure, according to Dr. David Marti.
“Analysis of hematocrit in elderly patients can guide important procedural characteristics, such as access site and antithrombotic regimen,” he said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
For example, studies have established that transradial artery access percutaneous coronary intervention (PCI) results in significantly less bleeding than the transfemoral route, said Dr. Marti, an interventional cardiologist at the University of Alcalá in Madrid.
He presented a prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital. Their mean age was 81.4 years, and slightly over half of them presented with an acute coronary syndrome.
All patients received dual-antiplatelet therapy in accord with current guidelines. Stent type and procedural anticoagulant regimen were left to the discretion of the cardiologist; 80% of the subjects received bivalirudin-based anticoagulation.
The primary study outcome was the 30-day incidence of major bleeding, as defined by a Bleeding Academic Research Consortium (BARC) type 3-5 event. The overall rate in this elderly PCI population was 5.5%. However, the rate varied markedly by baseline hematocrit tertile, in accord with the investigators’ study hypothesis.
Major bleeding occurred in 2.9% of patients with an Hct greater than 42% and 3.1% in those with an Hct of 38%-52%, and jumped to 10.6% in the one-third of subjects whose baseline Hct was below 38%, Dr. Marti reported.
Thus, a preprocedural Hct below 38% was associated with a 4.1-fold increased risk of major bleeding within 30 days following PCI. An Hct in this range was a stronger predictor of BARC type 3-5 bleeding risk than were other factors better known as being important, including advanced age, greater body weight, female sex, or an elevated serum creatinine indicative of chronic kidney disease. Indeed, an Hct below 38% was the only statistically significant predictor of major bleeding in this elderly population.
The likely explanation for the observed results is that a low Hct level in elderly patients usually reflects subclinical blood loss that can be worsened by antithrombotic therapies, the cardiologist explained.
The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
PARIS – A low hematocrit in an elderly patient who’s going to undergo percutaneous coronary intervention signals a markedly increased risk of major bleeding within 30 days of the procedure, according to Dr. David Marti.
“Analysis of hematocrit in elderly patients can guide important procedural characteristics, such as access site and antithrombotic regimen,” he said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
For example, studies have established that transradial artery access percutaneous coronary intervention (PCI) results in significantly less bleeding than the transfemoral route, said Dr. Marti, an interventional cardiologist at the University of Alcalá in Madrid.
He presented a prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital. Their mean age was 81.4 years, and slightly over half of them presented with an acute coronary syndrome.
All patients received dual-antiplatelet therapy in accord with current guidelines. Stent type and procedural anticoagulant regimen were left to the discretion of the cardiologist; 80% of the subjects received bivalirudin-based anticoagulation.
The primary study outcome was the 30-day incidence of major bleeding, as defined by a Bleeding Academic Research Consortium (BARC) type 3-5 event. The overall rate in this elderly PCI population was 5.5%. However, the rate varied markedly by baseline hematocrit tertile, in accord with the investigators’ study hypothesis.
Major bleeding occurred in 2.9% of patients with an Hct greater than 42% and 3.1% in those with an Hct of 38%-52%, and jumped to 10.6% in the one-third of subjects whose baseline Hct was below 38%, Dr. Marti reported.
Thus, a preprocedural Hct below 38% was associated with a 4.1-fold increased risk of major bleeding within 30 days following PCI. An Hct in this range was a stronger predictor of BARC type 3-5 bleeding risk than were other factors better known as being important, including advanced age, greater body weight, female sex, or an elevated serum creatinine indicative of chronic kidney disease. Indeed, an Hct below 38% was the only statistically significant predictor of major bleeding in this elderly population.
The likely explanation for the observed results is that a low Hct level in elderly patients usually reflects subclinical blood loss that can be worsened by antithrombotic therapies, the cardiologist explained.
The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
PARIS – A low hematocrit in an elderly patient who’s going to undergo percutaneous coronary intervention signals a markedly increased risk of major bleeding within 30 days of the procedure, according to Dr. David Marti.
“Analysis of hematocrit in elderly patients can guide important procedural characteristics, such as access site and antithrombotic regimen,” he said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
For example, studies have established that transradial artery access percutaneous coronary intervention (PCI) results in significantly less bleeding than the transfemoral route, said Dr. Marti, an interventional cardiologist at the University of Alcalá in Madrid.
He presented a prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital. Their mean age was 81.4 years, and slightly over half of them presented with an acute coronary syndrome.
All patients received dual-antiplatelet therapy in accord with current guidelines. Stent type and procedural anticoagulant regimen were left to the discretion of the cardiologist; 80% of the subjects received bivalirudin-based anticoagulation.
The primary study outcome was the 30-day incidence of major bleeding, as defined by a Bleeding Academic Research Consortium (BARC) type 3-5 event. The overall rate in this elderly PCI population was 5.5%. However, the rate varied markedly by baseline hematocrit tertile, in accord with the investigators’ study hypothesis.
Major bleeding occurred in 2.9% of patients with an Hct greater than 42% and 3.1% in those with an Hct of 38%-52%, and jumped to 10.6% in the one-third of subjects whose baseline Hct was below 38%, Dr. Marti reported.
Thus, a preprocedural Hct below 38% was associated with a 4.1-fold increased risk of major bleeding within 30 days following PCI. An Hct in this range was a stronger predictor of BARC type 3-5 bleeding risk than were other factors better known as being important, including advanced age, greater body weight, female sex, or an elevated serum creatinine indicative of chronic kidney disease. Indeed, an Hct below 38% was the only statistically significant predictor of major bleeding in this elderly population.
The likely explanation for the observed results is that a low Hct level in elderly patients usually reflects subclinical blood loss that can be worsened by antithrombotic therapies, the cardiologist explained.
The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
AT EUROPCR 2016
Key clinical point: Elderly patients scheduled for PCI have a fourfold greater risk of major bleeding within 30 days if their Hct is less than 38%.
Major finding: The 30-day incidence of BARC types 3-5 major bleeding was 10.9% in elderly patients with a pre-PCI Hct below 38%, compared with 2.9% in those in the top Hct tertile.
Data source: A prospective study of 212 consecutive patients aged 75 or older who underwent PCI at a single university hospital.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted without commercial support.
Adolescent obesity rose slightly, again
Nearly one in five young people in the United States are obese, and proportionally more adolescents have been obese during every time period measured since 1994, according to an analysis published online June 7 in JAMA.
But the most recent data suggest only a “small” rise in adolescent obesity since 2011, and stable rates among children during this time period, said Cynthia L. Ogden, Ph.D., of the National Center for Health Statistics at the Centers for Disease Control and Prevention.
Few studies of obesity in young people have teased out rates by age, according to Dr. Ogden and her associates. Using National Health and Nutrition Examination Survey data, they calculated rates of obesity and extreme obesity among 40,780 children and adolescents aged 2-19 years for the periods 1988-1994 through 2013-2014. They defined obesity as a body mass index (BMI) at or above the sex-specific 95th percentile on the CDC BMI-for-age growth charts, and they defined extreme obesity as a BMI at least 120% of the sex-specific 95th percentile on the charts (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6361).
Based on these definitions, 17% of children and adolescents were obese between 2011 and 2014, while 6% were extremely obese, the investigators reported. Furthermore, 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 11% during 1988-1994.
Rates for 6- to -11-year-olds have remained fairly stable in the high teens for more than a decade, while rates among 2- to 5-year-olds peaked in 2003-2004 at nearly 14% before dropping to about 9% during 2013-2014. The prevalence of obesity varied little by sex, but diverged substantially by race and ethnicity. For example, in 2011-2014, 23% of Hispanics and about 23% of black children were obese, and 9% and 12% were extremely obese, respectively the researchers reported. Rates for the same ages of non-Hispanic Asian children were 9% and 2%, respectively, and those for non-Hispanic whites were 20% and 7%, respectively.
“Body mass index is an imperfect measure of body fat and health risk,” the investigators cautioned. “There are racial and ethnic differences in body fat at the same BMI level. Among children and adolescents, the definition of obesity is statistical. Children and adolescents are compared with a group of U.S. children in the 1960s to early 1990s, so the prevalence of obesity is dependent on the characteristics of the age-specific population during that period. In addition, among young children, small changes in weight can lead to relatively large changes in BMI percentile”
The researchers reported no funding sources and had no disclosures.
Numerous foundations, industries, professional societies, and governmental agencies have provided hundreds of millions of dollars in funding to support basic science research in obesity, clinical trials, and observational studies, development of new drugs and devices, and hospital and community programs to help stem the tide of the obesity epidemic. In addition, communities, schools, places of worship, and professional societies have become active in attempting to counteract obesity – emphasizing exercise, better dietary choices, and nutritional labeling of foods.
Although it is impossible to know what the extent of the obesity epidemic would have been without these efforts, [these data] certainly do not suggest much success. Perhaps new incentives are needed to encourage the food industry to work with families and the medical community to prevent obesity. The stakes for the health of people in the United States are high, and creative solutions are needed.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are excerpted from their accompanying editorial (JAMA. 2016 Jun. doi: 10.1001/jama.2016.6190).
Numerous foundations, industries, professional societies, and governmental agencies have provided hundreds of millions of dollars in funding to support basic science research in obesity, clinical trials, and observational studies, development of new drugs and devices, and hospital and community programs to help stem the tide of the obesity epidemic. In addition, communities, schools, places of worship, and professional societies have become active in attempting to counteract obesity – emphasizing exercise, better dietary choices, and nutritional labeling of foods.
Although it is impossible to know what the extent of the obesity epidemic would have been without these efforts, [these data] certainly do not suggest much success. Perhaps new incentives are needed to encourage the food industry to work with families and the medical community to prevent obesity. The stakes for the health of people in the United States are high, and creative solutions are needed.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are excerpted from their accompanying editorial (JAMA. 2016 Jun. doi: 10.1001/jama.2016.6190).
Numerous foundations, industries, professional societies, and governmental agencies have provided hundreds of millions of dollars in funding to support basic science research in obesity, clinical trials, and observational studies, development of new drugs and devices, and hospital and community programs to help stem the tide of the obesity epidemic. In addition, communities, schools, places of worship, and professional societies have become active in attempting to counteract obesity – emphasizing exercise, better dietary choices, and nutritional labeling of foods.
Although it is impossible to know what the extent of the obesity epidemic would have been without these efforts, [these data] certainly do not suggest much success. Perhaps new incentives are needed to encourage the food industry to work with families and the medical community to prevent obesity. The stakes for the health of people in the United States are high, and creative solutions are needed.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are excerpted from their accompanying editorial (JAMA. 2016 Jun. doi: 10.1001/jama.2016.6190).
Nearly one in five young people in the United States are obese, and proportionally more adolescents have been obese during every time period measured since 1994, according to an analysis published online June 7 in JAMA.
But the most recent data suggest only a “small” rise in adolescent obesity since 2011, and stable rates among children during this time period, said Cynthia L. Ogden, Ph.D., of the National Center for Health Statistics at the Centers for Disease Control and Prevention.
Few studies of obesity in young people have teased out rates by age, according to Dr. Ogden and her associates. Using National Health and Nutrition Examination Survey data, they calculated rates of obesity and extreme obesity among 40,780 children and adolescents aged 2-19 years for the periods 1988-1994 through 2013-2014. They defined obesity as a body mass index (BMI) at or above the sex-specific 95th percentile on the CDC BMI-for-age growth charts, and they defined extreme obesity as a BMI at least 120% of the sex-specific 95th percentile on the charts (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6361).
Based on these definitions, 17% of children and adolescents were obese between 2011 and 2014, while 6% were extremely obese, the investigators reported. Furthermore, 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 11% during 1988-1994.
Rates for 6- to -11-year-olds have remained fairly stable in the high teens for more than a decade, while rates among 2- to 5-year-olds peaked in 2003-2004 at nearly 14% before dropping to about 9% during 2013-2014. The prevalence of obesity varied little by sex, but diverged substantially by race and ethnicity. For example, in 2011-2014, 23% of Hispanics and about 23% of black children were obese, and 9% and 12% were extremely obese, respectively the researchers reported. Rates for the same ages of non-Hispanic Asian children were 9% and 2%, respectively, and those for non-Hispanic whites were 20% and 7%, respectively.
“Body mass index is an imperfect measure of body fat and health risk,” the investigators cautioned. “There are racial and ethnic differences in body fat at the same BMI level. Among children and adolescents, the definition of obesity is statistical. Children and adolescents are compared with a group of U.S. children in the 1960s to early 1990s, so the prevalence of obesity is dependent on the characteristics of the age-specific population during that period. In addition, among young children, small changes in weight can lead to relatively large changes in BMI percentile”
The researchers reported no funding sources and had no disclosures.
Nearly one in five young people in the United States are obese, and proportionally more adolescents have been obese during every time period measured since 1994, according to an analysis published online June 7 in JAMA.
But the most recent data suggest only a “small” rise in adolescent obesity since 2011, and stable rates among children during this time period, said Cynthia L. Ogden, Ph.D., of the National Center for Health Statistics at the Centers for Disease Control and Prevention.
Few studies of obesity in young people have teased out rates by age, according to Dr. Ogden and her associates. Using National Health and Nutrition Examination Survey data, they calculated rates of obesity and extreme obesity among 40,780 children and adolescents aged 2-19 years for the periods 1988-1994 through 2013-2014. They defined obesity as a body mass index (BMI) at or above the sex-specific 95th percentile on the CDC BMI-for-age growth charts, and they defined extreme obesity as a BMI at least 120% of the sex-specific 95th percentile on the charts (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6361).
Based on these definitions, 17% of children and adolescents were obese between 2011 and 2014, while 6% were extremely obese, the investigators reported. Furthermore, 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 11% during 1988-1994.
Rates for 6- to -11-year-olds have remained fairly stable in the high teens for more than a decade, while rates among 2- to 5-year-olds peaked in 2003-2004 at nearly 14% before dropping to about 9% during 2013-2014. The prevalence of obesity varied little by sex, but diverged substantially by race and ethnicity. For example, in 2011-2014, 23% of Hispanics and about 23% of black children were obese, and 9% and 12% were extremely obese, respectively the researchers reported. Rates for the same ages of non-Hispanic Asian children were 9% and 2%, respectively, and those for non-Hispanic whites were 20% and 7%, respectively.
“Body mass index is an imperfect measure of body fat and health risk,” the investigators cautioned. “There are racial and ethnic differences in body fat at the same BMI level. Among children and adolescents, the definition of obesity is statistical. Children and adolescents are compared with a group of U.S. children in the 1960s to early 1990s, so the prevalence of obesity is dependent on the characteristics of the age-specific population during that period. In addition, among young children, small changes in weight can lead to relatively large changes in BMI percentile”
The researchers reported no funding sources and had no disclosures.
FROM JAMA
Key clinical point:Nearly one in five children and adolescents are obese, and rates of adolescent obesity have risen during every time period measured since 1994.
Major finding: About 17% of children and adolescents in the United States were obese between 2011 and 2014 (95% confidence interval, 15.5%-18.6%). Nearly 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 10% during 1988-1994.
Data source: An analysis of the body mass indexes of 40,780 individuals aged 2-19 years from the National Health and Nutrition Examination Survey.
Disclosures: The researchers reported no funding sources and had no disclosures.
Immediate postpartum IUD insertion causes little pain, distress
WASHINGTON – Many women who underwent post-placental IUD insertion reported little or no pain, regardless of whether they had an epidural during childbirth, according to the findings of a small pilot study.
Dr. Shannon Carr and her colleagues at the University of New Mexico, Albuquerque, assessed 66 women using both a 100-mm visual analog pain scale (VAS) and a four-point Likert verbal rating scale (VRS). They also interviewed a subset of the participants to gather qualitative data about the experience. About half the group received an epidural (36 women) and half did not (30 women).
The VAS scores did not reveal normal distributions and the standard deviations were large, which was not statistically meaningful, according to Dr. Carr. The median scores were 40.5 mm and 2.8 mm in the no-epidural and epidural groups, respectively. But using the four-point pain scale – none, mild, moderate, and severe – the researchers found that 53% of women in the no-epidural group reported pain ranging from none to mild, while 89% of women in the epidural group reported pain in the none-to-mild range.
“What I saw basically reflected what we call the floor effect of the VAS scores in the epidural group. Most of the women hardly had any pain,” Dr. Carr said at the annual meeting of the American College of Obstetricians and Gynecologists. “Women who did not have an epidural reported scores that were all over the map.”
The results help to shed light on what is probably the most common question asked by women considering post-placental IUD insertion – Will it hurt?
“The more we learn about it, the more we can reassure women and counsel them appropriately about what to expect, not only clinically but on a personal level with their labor experience,” Dr. Carr said. “I think that’s really important, and it might promote uptake of the procedure and more women getting really effective contraception before leaving the hospital.”
Qualitative data, based on interviews with 9 women in the no-epidural group and 12 in the epidural group, showed that they had no regrets about the procedure, and most reported that it didn’t detract from their overall labor experience. Instead, most of the women in the study said that holding their newborn baby was a pleasant distraction from the placement of the IUD.
The primary driver for undergoing IUD insertion immediately post partum was convenience. “They wanted to have really good birth control on board before they left the hospital,” Dr. Carr said. “They recognize those logistical barriers to getting to that 6-week postpartum visit.”
The researchers did not report having any financial disclosures.
On Twitter @maryellenny
WASHINGTON – Many women who underwent post-placental IUD insertion reported little or no pain, regardless of whether they had an epidural during childbirth, according to the findings of a small pilot study.
Dr. Shannon Carr and her colleagues at the University of New Mexico, Albuquerque, assessed 66 women using both a 100-mm visual analog pain scale (VAS) and a four-point Likert verbal rating scale (VRS). They also interviewed a subset of the participants to gather qualitative data about the experience. About half the group received an epidural (36 women) and half did not (30 women).
The VAS scores did not reveal normal distributions and the standard deviations were large, which was not statistically meaningful, according to Dr. Carr. The median scores were 40.5 mm and 2.8 mm in the no-epidural and epidural groups, respectively. But using the four-point pain scale – none, mild, moderate, and severe – the researchers found that 53% of women in the no-epidural group reported pain ranging from none to mild, while 89% of women in the epidural group reported pain in the none-to-mild range.
“What I saw basically reflected what we call the floor effect of the VAS scores in the epidural group. Most of the women hardly had any pain,” Dr. Carr said at the annual meeting of the American College of Obstetricians and Gynecologists. “Women who did not have an epidural reported scores that were all over the map.”
The results help to shed light on what is probably the most common question asked by women considering post-placental IUD insertion – Will it hurt?
“The more we learn about it, the more we can reassure women and counsel them appropriately about what to expect, not only clinically but on a personal level with their labor experience,” Dr. Carr said. “I think that’s really important, and it might promote uptake of the procedure and more women getting really effective contraception before leaving the hospital.”
Qualitative data, based on interviews with 9 women in the no-epidural group and 12 in the epidural group, showed that they had no regrets about the procedure, and most reported that it didn’t detract from their overall labor experience. Instead, most of the women in the study said that holding their newborn baby was a pleasant distraction from the placement of the IUD.
The primary driver for undergoing IUD insertion immediately post partum was convenience. “They wanted to have really good birth control on board before they left the hospital,” Dr. Carr said. “They recognize those logistical barriers to getting to that 6-week postpartum visit.”
The researchers did not report having any financial disclosures.
On Twitter @maryellenny
WASHINGTON – Many women who underwent post-placental IUD insertion reported little or no pain, regardless of whether they had an epidural during childbirth, according to the findings of a small pilot study.
Dr. Shannon Carr and her colleagues at the University of New Mexico, Albuquerque, assessed 66 women using both a 100-mm visual analog pain scale (VAS) and a four-point Likert verbal rating scale (VRS). They also interviewed a subset of the participants to gather qualitative data about the experience. About half the group received an epidural (36 women) and half did not (30 women).
The VAS scores did not reveal normal distributions and the standard deviations were large, which was not statistically meaningful, according to Dr. Carr. The median scores were 40.5 mm and 2.8 mm in the no-epidural and epidural groups, respectively. But using the four-point pain scale – none, mild, moderate, and severe – the researchers found that 53% of women in the no-epidural group reported pain ranging from none to mild, while 89% of women in the epidural group reported pain in the none-to-mild range.
“What I saw basically reflected what we call the floor effect of the VAS scores in the epidural group. Most of the women hardly had any pain,” Dr. Carr said at the annual meeting of the American College of Obstetricians and Gynecologists. “Women who did not have an epidural reported scores that were all over the map.”
The results help to shed light on what is probably the most common question asked by women considering post-placental IUD insertion – Will it hurt?
“The more we learn about it, the more we can reassure women and counsel them appropriately about what to expect, not only clinically but on a personal level with their labor experience,” Dr. Carr said. “I think that’s really important, and it might promote uptake of the procedure and more women getting really effective contraception before leaving the hospital.”
Qualitative data, based on interviews with 9 women in the no-epidural group and 12 in the epidural group, showed that they had no regrets about the procedure, and most reported that it didn’t detract from their overall labor experience. Instead, most of the women in the study said that holding their newborn baby was a pleasant distraction from the placement of the IUD.
The primary driver for undergoing IUD insertion immediately post partum was convenience. “They wanted to have really good birth control on board before they left the hospital,” Dr. Carr said. “They recognize those logistical barriers to getting to that 6-week postpartum visit.”
The researchers did not report having any financial disclosures.
On Twitter @maryellenny
AT ACOG 2016
Key clinical point: Many women felt little to no pain during post-placental IUD insertion.
Major finding: More than half of women (53%) who did not have an epidural, and 89% of women who did, reported experiencing little to no pain during post-placental IUD insertion.
Data source: A mixed-methods pilot study of 66 women.
Disclosures: The researchers did not report having any financial disclosures.
High-tech pills help increase medication adherence
orlando – A digital device that patients swallow every time they take a medication may be an answer to improving compliance and disease control. An aim of this digital health system is to encourage patient engagement and provider interaction. Despite available and efficacious medicines, only slightly more than half of patients with type 2 diabetes mellitus have glycated hemoglobin (HbA1c), hypertension, or LDL cholesterol under good control. One study showed only 17% had all three under control (Endocr Pract. 2016;22:689-98).
“Important in this are the facts of medication nonadherence by the patient, poor patient engagement frequently in their care, as well as from a clinician perspective, therapeutic inertia,” said Dr. Juan Frias at the annual meeting of the American Association of Clinical Endocrinologists. Approximately half of patients with chronic diseases do not take their medications as prescribed (Circulation. 2013;128:29-41).
To improve treatment adherence, diagnose the reasons for a patient not reaching therapeutic goals, address those issues with patient education or counseling or changes in medication, and engage with the patient to reinforce adherence goals. A digital system called Proteus Discover has been designed to assist patients and clinicians with all these tasks.
Proteus uses ingestible sensor-detectable medications. Each medication and dose of medication has a unique marker. A compounding pharmacy co-encapsulates a tiny detectable chip about the size of a grain of sand with a medication as prescribed by a physician. The patient wears a Band-Aid sized skin patch sensor that transmits to a mobile device that uploads to a secure cloud server. The sensor can tell if a medication has been taken and also serves as a pedometer and activity gauge.
The information is available to the patient, who can gain insight into medication taking, activity, and rest, along with other health parameters that are entered into the system. Entering the system through a provider portal and with the patient’s permission, the clinician can see patient behavior patterns outside of the clinic, including medication adherence, which helps to determine the best treatment for that patient. The system provides a report to the clinician that can be a point of discussion when the patient visits the clinic.
Trial demonstrates better medication adherence and goal attainment
Dr. Frias, CEO and principal investigator of National Research Institute in Los Angeles, described a 12 week multicenter cluster-randomized pilot study involving 90 patients with uncontrolled hypertension and type 2 diabetes to investigate the effect of Proteus on blood pressure, HbA1c, and LDL cholesterol reduction. Other goals of the trial were to promote medication adherence and physical activity and alert providers to a need to make more medical decisions.
Cluster randomization meant that each of the 16 trial sites randomized patients to one of three treatment arms within that site. The arms were: Proteus Discover for 4 or 12 weeks or usual care. Patients had to have systolic blood pressure of 140 mm Hg or greater, have failed therapy with 2 or more antihypertensive agents, and have HbA1c at 7% or greater on metformin or a sulfonylurea. Two-thirds of the subjects also had dyslipidemia and were treated with statins. Subjects were excluded if they had a history of acute or chronic dermatitis, had a skin allergy or sensitivity to adhesive medical tape, or had secondary causes for uncontrolled hypertension or type 2 diabetes.
Usual care consisted of all standard interventions, including medication titrations, patient education, and lifestyle coaching. All subjects took medications for 12 weeks and were followed for 12 weeks after enrollment. Available medications were various doses of lisinopril, losartan, hydrochlorothiazide, amlodipine, atorvastatin, metformin, and glipizide.
All the arms were fairly well balanced as to age (58-62 years), ethnicity, employment, education, and income. About one quarter had incomes in the $20,000-$40,000 range, and about half had incomes of $20,000 or less. Body mass index was 32 kg/m2 in the Proteus and usual care arms. Total cholesterol was similar for the Proteus and usual care groups at 173-177 mg/dL, but LDL was slightly higher in the Proteus arms (103 vs. 95 mg/dL).
Better adherence and outcomes for patients using Proteus
Dr. Frias presented 4-week results for blood pressure and cholesterol reduction. The 4- and 12-week Proteus arms were combined for this analysis, since up to 4 weeks they received the same intervention. Blood pressure, LDL cholesterol, and total cholesterol were all significantly reduced in the Proteus group, compared with the usual care group. At week 4, 83% of the subjects in the Proteus group had reached a blood pressure goal of less than 140/90 mm Hg vs. 33% in the usual care arm (difference of 50%; 95% confidence interval, 24%, 76%). Across all 11 medications/doses, the adherence was between 80% and 89% for every one.
The system spurred clinicians to make more treatment decisions for their patients. Providers made more changes to treatment for the Proteus patients, compared with the usual care providers (50% vs. 36%), gave more adherence counseling (28% vs. 0%), and provided more patient education (42% vs. 9%). Patients using the Proteus Discover system expressed high levels of satisfaction and acceptance of the technology (83%-100%), including ease of use in one’s daily routine, learning to use it, motivation to manage one’s health, better discussions with providers, understanding their care plan, seeing how they take their medications, and applying and wearing the sensor patch.
Safety was excellent. Twenty-seven adverse effects occurred in the Proteus arms, none of them serious, and about half were attributed to the device, mostly self-limited rashes. Seven adverse effects were attributed to the medications, mostly gastrointestinal side effects. There were four adverse effects in the usual care group, two of them serious.
Overall, compared with about 50% typical medication adherence, Proteus users had 84% adherence, which was associated with better blood pressure and LDL cholesterol control, compared with usual care.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said the Proteus system may be very helpful for patients who are taking multiple medications to prompt them when to take them. “I could easily see somebody who has this system in place where they’re also able to measure their blood pressure and get that information to their provider and for their provider to make changes in their medicine dose,” he said.
This research study of 90 patients was quite manageable, but “what about when you have 300 people on this therapy and all those data are starting to come in? Who’s going to manage those data... and look at it all?” Dr. Lieb wondered. Patients may have to be taught to understand the data and make changes on their own to their medication behavior, exercise, and other factors within their control.
Half the patients in the study made $20,000 or less a year. “If you could help underserved patients with their compliance and all those other things... that would be fantastic. It’s a huge area of need,” he said. People will need internet access to upload their data to the cloud server.
Another question is how the data can interface with the various electronic health records in use and generate reports.
Proteus Discover is approved by the U.S. Food and Drug Administration and is available now.
orlando – A digital device that patients swallow every time they take a medication may be an answer to improving compliance and disease control. An aim of this digital health system is to encourage patient engagement and provider interaction. Despite available and efficacious medicines, only slightly more than half of patients with type 2 diabetes mellitus have glycated hemoglobin (HbA1c), hypertension, or LDL cholesterol under good control. One study showed only 17% had all three under control (Endocr Pract. 2016;22:689-98).
“Important in this are the facts of medication nonadherence by the patient, poor patient engagement frequently in their care, as well as from a clinician perspective, therapeutic inertia,” said Dr. Juan Frias at the annual meeting of the American Association of Clinical Endocrinologists. Approximately half of patients with chronic diseases do not take their medications as prescribed (Circulation. 2013;128:29-41).
To improve treatment adherence, diagnose the reasons for a patient not reaching therapeutic goals, address those issues with patient education or counseling or changes in medication, and engage with the patient to reinforce adherence goals. A digital system called Proteus Discover has been designed to assist patients and clinicians with all these tasks.
Proteus uses ingestible sensor-detectable medications. Each medication and dose of medication has a unique marker. A compounding pharmacy co-encapsulates a tiny detectable chip about the size of a grain of sand with a medication as prescribed by a physician. The patient wears a Band-Aid sized skin patch sensor that transmits to a mobile device that uploads to a secure cloud server. The sensor can tell if a medication has been taken and also serves as a pedometer and activity gauge.
The information is available to the patient, who can gain insight into medication taking, activity, and rest, along with other health parameters that are entered into the system. Entering the system through a provider portal and with the patient’s permission, the clinician can see patient behavior patterns outside of the clinic, including medication adherence, which helps to determine the best treatment for that patient. The system provides a report to the clinician that can be a point of discussion when the patient visits the clinic.
Trial demonstrates better medication adherence and goal attainment
Dr. Frias, CEO and principal investigator of National Research Institute in Los Angeles, described a 12 week multicenter cluster-randomized pilot study involving 90 patients with uncontrolled hypertension and type 2 diabetes to investigate the effect of Proteus on blood pressure, HbA1c, and LDL cholesterol reduction. Other goals of the trial were to promote medication adherence and physical activity and alert providers to a need to make more medical decisions.
Cluster randomization meant that each of the 16 trial sites randomized patients to one of three treatment arms within that site. The arms were: Proteus Discover for 4 or 12 weeks or usual care. Patients had to have systolic blood pressure of 140 mm Hg or greater, have failed therapy with 2 or more antihypertensive agents, and have HbA1c at 7% or greater on metformin or a sulfonylurea. Two-thirds of the subjects also had dyslipidemia and were treated with statins. Subjects were excluded if they had a history of acute or chronic dermatitis, had a skin allergy or sensitivity to adhesive medical tape, or had secondary causes for uncontrolled hypertension or type 2 diabetes.
Usual care consisted of all standard interventions, including medication titrations, patient education, and lifestyle coaching. All subjects took medications for 12 weeks and were followed for 12 weeks after enrollment. Available medications were various doses of lisinopril, losartan, hydrochlorothiazide, amlodipine, atorvastatin, metformin, and glipizide.
All the arms were fairly well balanced as to age (58-62 years), ethnicity, employment, education, and income. About one quarter had incomes in the $20,000-$40,000 range, and about half had incomes of $20,000 or less. Body mass index was 32 kg/m2 in the Proteus and usual care arms. Total cholesterol was similar for the Proteus and usual care groups at 173-177 mg/dL, but LDL was slightly higher in the Proteus arms (103 vs. 95 mg/dL).
Better adherence and outcomes for patients using Proteus
Dr. Frias presented 4-week results for blood pressure and cholesterol reduction. The 4- and 12-week Proteus arms were combined for this analysis, since up to 4 weeks they received the same intervention. Blood pressure, LDL cholesterol, and total cholesterol were all significantly reduced in the Proteus group, compared with the usual care group. At week 4, 83% of the subjects in the Proteus group had reached a blood pressure goal of less than 140/90 mm Hg vs. 33% in the usual care arm (difference of 50%; 95% confidence interval, 24%, 76%). Across all 11 medications/doses, the adherence was between 80% and 89% for every one.
The system spurred clinicians to make more treatment decisions for their patients. Providers made more changes to treatment for the Proteus patients, compared with the usual care providers (50% vs. 36%), gave more adherence counseling (28% vs. 0%), and provided more patient education (42% vs. 9%). Patients using the Proteus Discover system expressed high levels of satisfaction and acceptance of the technology (83%-100%), including ease of use in one’s daily routine, learning to use it, motivation to manage one’s health, better discussions with providers, understanding their care plan, seeing how they take their medications, and applying and wearing the sensor patch.
Safety was excellent. Twenty-seven adverse effects occurred in the Proteus arms, none of them serious, and about half were attributed to the device, mostly self-limited rashes. Seven adverse effects were attributed to the medications, mostly gastrointestinal side effects. There were four adverse effects in the usual care group, two of them serious.
Overall, compared with about 50% typical medication adherence, Proteus users had 84% adherence, which was associated with better blood pressure and LDL cholesterol control, compared with usual care.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said the Proteus system may be very helpful for patients who are taking multiple medications to prompt them when to take them. “I could easily see somebody who has this system in place where they’re also able to measure their blood pressure and get that information to their provider and for their provider to make changes in their medicine dose,” he said.
This research study of 90 patients was quite manageable, but “what about when you have 300 people on this therapy and all those data are starting to come in? Who’s going to manage those data... and look at it all?” Dr. Lieb wondered. Patients may have to be taught to understand the data and make changes on their own to their medication behavior, exercise, and other factors within their control.
Half the patients in the study made $20,000 or less a year. “If you could help underserved patients with their compliance and all those other things... that would be fantastic. It’s a huge area of need,” he said. People will need internet access to upload their data to the cloud server.
Another question is how the data can interface with the various electronic health records in use and generate reports.
Proteus Discover is approved by the U.S. Food and Drug Administration and is available now.
orlando – A digital device that patients swallow every time they take a medication may be an answer to improving compliance and disease control. An aim of this digital health system is to encourage patient engagement and provider interaction. Despite available and efficacious medicines, only slightly more than half of patients with type 2 diabetes mellitus have glycated hemoglobin (HbA1c), hypertension, or LDL cholesterol under good control. One study showed only 17% had all three under control (Endocr Pract. 2016;22:689-98).
“Important in this are the facts of medication nonadherence by the patient, poor patient engagement frequently in their care, as well as from a clinician perspective, therapeutic inertia,” said Dr. Juan Frias at the annual meeting of the American Association of Clinical Endocrinologists. Approximately half of patients with chronic diseases do not take their medications as prescribed (Circulation. 2013;128:29-41).
To improve treatment adherence, diagnose the reasons for a patient not reaching therapeutic goals, address those issues with patient education or counseling or changes in medication, and engage with the patient to reinforce adherence goals. A digital system called Proteus Discover has been designed to assist patients and clinicians with all these tasks.
Proteus uses ingestible sensor-detectable medications. Each medication and dose of medication has a unique marker. A compounding pharmacy co-encapsulates a tiny detectable chip about the size of a grain of sand with a medication as prescribed by a physician. The patient wears a Band-Aid sized skin patch sensor that transmits to a mobile device that uploads to a secure cloud server. The sensor can tell if a medication has been taken and also serves as a pedometer and activity gauge.
The information is available to the patient, who can gain insight into medication taking, activity, and rest, along with other health parameters that are entered into the system. Entering the system through a provider portal and with the patient’s permission, the clinician can see patient behavior patterns outside of the clinic, including medication adherence, which helps to determine the best treatment for that patient. The system provides a report to the clinician that can be a point of discussion when the patient visits the clinic.
Trial demonstrates better medication adherence and goal attainment
Dr. Frias, CEO and principal investigator of National Research Institute in Los Angeles, described a 12 week multicenter cluster-randomized pilot study involving 90 patients with uncontrolled hypertension and type 2 diabetes to investigate the effect of Proteus on blood pressure, HbA1c, and LDL cholesterol reduction. Other goals of the trial were to promote medication adherence and physical activity and alert providers to a need to make more medical decisions.
Cluster randomization meant that each of the 16 trial sites randomized patients to one of three treatment arms within that site. The arms were: Proteus Discover for 4 or 12 weeks or usual care. Patients had to have systolic blood pressure of 140 mm Hg or greater, have failed therapy with 2 or more antihypertensive agents, and have HbA1c at 7% or greater on metformin or a sulfonylurea. Two-thirds of the subjects also had dyslipidemia and were treated with statins. Subjects were excluded if they had a history of acute or chronic dermatitis, had a skin allergy or sensitivity to adhesive medical tape, or had secondary causes for uncontrolled hypertension or type 2 diabetes.
Usual care consisted of all standard interventions, including medication titrations, patient education, and lifestyle coaching. All subjects took medications for 12 weeks and were followed for 12 weeks after enrollment. Available medications were various doses of lisinopril, losartan, hydrochlorothiazide, amlodipine, atorvastatin, metformin, and glipizide.
All the arms were fairly well balanced as to age (58-62 years), ethnicity, employment, education, and income. About one quarter had incomes in the $20,000-$40,000 range, and about half had incomes of $20,000 or less. Body mass index was 32 kg/m2 in the Proteus and usual care arms. Total cholesterol was similar for the Proteus and usual care groups at 173-177 mg/dL, but LDL was slightly higher in the Proteus arms (103 vs. 95 mg/dL).
Better adherence and outcomes for patients using Proteus
Dr. Frias presented 4-week results for blood pressure and cholesterol reduction. The 4- and 12-week Proteus arms were combined for this analysis, since up to 4 weeks they received the same intervention. Blood pressure, LDL cholesterol, and total cholesterol were all significantly reduced in the Proteus group, compared with the usual care group. At week 4, 83% of the subjects in the Proteus group had reached a blood pressure goal of less than 140/90 mm Hg vs. 33% in the usual care arm (difference of 50%; 95% confidence interval, 24%, 76%). Across all 11 medications/doses, the adherence was between 80% and 89% for every one.
The system spurred clinicians to make more treatment decisions for their patients. Providers made more changes to treatment for the Proteus patients, compared with the usual care providers (50% vs. 36%), gave more adherence counseling (28% vs. 0%), and provided more patient education (42% vs. 9%). Patients using the Proteus Discover system expressed high levels of satisfaction and acceptance of the technology (83%-100%), including ease of use in one’s daily routine, learning to use it, motivation to manage one’s health, better discussions with providers, understanding their care plan, seeing how they take their medications, and applying and wearing the sensor patch.
Safety was excellent. Twenty-seven adverse effects occurred in the Proteus arms, none of them serious, and about half were attributed to the device, mostly self-limited rashes. Seven adverse effects were attributed to the medications, mostly gastrointestinal side effects. There were four adverse effects in the usual care group, two of them serious.
Overall, compared with about 50% typical medication adherence, Proteus users had 84% adherence, which was associated with better blood pressure and LDL cholesterol control, compared with usual care.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said the Proteus system may be very helpful for patients who are taking multiple medications to prompt them when to take them. “I could easily see somebody who has this system in place where they’re also able to measure their blood pressure and get that information to their provider and for their provider to make changes in their medicine dose,” he said.
This research study of 90 patients was quite manageable, but “what about when you have 300 people on this therapy and all those data are starting to come in? Who’s going to manage those data... and look at it all?” Dr. Lieb wondered. Patients may have to be taught to understand the data and make changes on their own to their medication behavior, exercise, and other factors within their control.
Half the patients in the study made $20,000 or less a year. “If you could help underserved patients with their compliance and all those other things... that would be fantastic. It’s a huge area of need,” he said. People will need internet access to upload their data to the cloud server.
Another question is how the data can interface with the various electronic health records in use and generate reports.
Proteus Discover is approved by the U.S. Food and Drug Administration and is available now.
AT AACE 2016
Key clinical point: Feedback from a system of digital-enabled pills enhanced medication adherence.
Major finding: Patients using Proteus had 84% adherence and better risk control.
Data source: Randomized unblinded study of 90 patients.
Disclosures: Dr. Frias has study grants from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk, Pfizer, and Sanofi and has consulting relationships with Proteus Digital Health, Johnson & Johnson, AstraZeneca, CeQur, and Sanofi.
Does medical marijuana work for PTSD, other psychiatric indications?
SCOTTSDALE, ARIZ. – There’s a rapidly growing list of states that have approved marijuana for medical use, but that does not mean that there’s also an expanding body of science to support marijuana’s use for psychiatric indications, according to Dr. Deepak Cyril D’Souza.
Though moderate evidence exists to support the use of medical marijuana for nausea and vomiting in chemotherapy, HIV/AIDS cachexia, neuropathic pain, and spasticity in multiple sclerosis, there’s scant evidence for some other uses. Little evidence exists to support the use of medical marijuana in Tourette syndrome, Crohn’s disease and ulcerative colitis, and epilepsy, and the data are even weaker for Parkinson’s disease, posttraumatic stress disorder, and agitation in Alzheimer’s disease, Dr. D’Souza said at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
Even so, he said, medical marijuana has been approved in several states for all of those conditions – and more.
Different states have required widely varying standards of evidence in making decisions about whether to approve medical marijuana use, and for which conditions. These range, in some cases, from relying on mere anecdotal evidence – as when individuals provide powerful but unscientific testimony about marijuana’s efficacy for a condition – to requiring the gold standard of randomized, double-blind, placebo-controlled trials.
Dr. D’Souza and his collaborators reviewed the data supporting the use of medical marijuana for several of the most commonly approved psychiatric indications, and found many of them hampered by poor design, poor execution, and an overreliance on the subjective effects of the studied compound.
For PTSD, only one randomized controlled study was found, and only one study had an active control, so the quality of evidence was rated as “very low” or “low” by Dr. D’Souza’s group. “Most of the studies were not blinded, and the sample sizes were generally small,” said Dr. D’Souza, professor of psychiatry at Yale University, New Haven, Conn. “It’s notable that many of these studies were with dronabinol, which is a synthetic THC analog. However, one take-home is that many of these studies reported improvement with sleep and a reduction in nightmares. This is something that should be followed up.”
For Tourette syndrome, there were just two studies by the same author, both with small sample sizes and of short duration. One study was rated “low” and the other “very low” for quality; in particular, the placebo effect could not be ruled out.
Four studies, said Dr. D’Souza, examined cannabinoids for dementia, and all had low quality of evidence. One published study had a sample size of two, he said. “The point here is that the only positive finding is that people who were diagnosed with dementia ate more and gained weight. And that wasn’t the objective of the study.”
“When we’re talking about medical marijuana, we’re talking about the whole plant,” he said. The marijuana plant has at least 450 known distinct constituent chemicals; these include about 70 cannabinoids as well as terpenoids and flavonoids. This means that the whole plant as dispensed represents a much more complex compound than medical tetrahydrocannabinol, for example, Dr. D’Souza said.
“We need to think about not just efficacy but side effects,” he said. These can include tolerance, abuse, and withdrawal syndrome. Marijuana’s cognitive effects may contribute to an increased risk of motor vehicle crashes. Though acute psychotic symptoms with quick resolution have long been noted, it’s also now thought that heavy marijuana exposure in adolescence more than doubles the risk for schizophrenia and might decrease adult IQ by about 10 points.
Though cannabis is “neither necessary nor sufficient for developing psychosis” but instead is a factor in a set of complex interactions, “what’s absolutely clear is that people with a psychotic disorder or at risk for developing one are very much more vulnerable to the effects of cannabinoids,” Dr. D’Souza said.
Despite all of those concerns, he said, “public demand and legislators have usurped the [Food and Drug Administration] approval process” when it comes to state-by-state approval of medical marijuana. The current state of affairs stands in contrast to the requirements for drug approvals for new indications, which require at least two adequately powered randomized clinical trials. When it comes to marijuana, Dr. D’Souza said, “For most of the indications the evidence fails to meet FDA standards.”
However, he said, the lack of high quality evidence may reflect the difficulty of conducting medical marijuana research in the United States. If this is so, he said, the “federal and state governments need to support and encourage research to generate high quality evidence to guide decisions.”
Dr. D’Souza reported a financial relationship with Insys Therapeutics, which develops pharmaceutical cannabinoid products.
On Twitter @karioakes
SCOTTSDALE, ARIZ. – There’s a rapidly growing list of states that have approved marijuana for medical use, but that does not mean that there’s also an expanding body of science to support marijuana’s use for psychiatric indications, according to Dr. Deepak Cyril D’Souza.
Though moderate evidence exists to support the use of medical marijuana for nausea and vomiting in chemotherapy, HIV/AIDS cachexia, neuropathic pain, and spasticity in multiple sclerosis, there’s scant evidence for some other uses. Little evidence exists to support the use of medical marijuana in Tourette syndrome, Crohn’s disease and ulcerative colitis, and epilepsy, and the data are even weaker for Parkinson’s disease, posttraumatic stress disorder, and agitation in Alzheimer’s disease, Dr. D’Souza said at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
Even so, he said, medical marijuana has been approved in several states for all of those conditions – and more.
Different states have required widely varying standards of evidence in making decisions about whether to approve medical marijuana use, and for which conditions. These range, in some cases, from relying on mere anecdotal evidence – as when individuals provide powerful but unscientific testimony about marijuana’s efficacy for a condition – to requiring the gold standard of randomized, double-blind, placebo-controlled trials.
Dr. D’Souza and his collaborators reviewed the data supporting the use of medical marijuana for several of the most commonly approved psychiatric indications, and found many of them hampered by poor design, poor execution, and an overreliance on the subjective effects of the studied compound.
For PTSD, only one randomized controlled study was found, and only one study had an active control, so the quality of evidence was rated as “very low” or “low” by Dr. D’Souza’s group. “Most of the studies were not blinded, and the sample sizes were generally small,” said Dr. D’Souza, professor of psychiatry at Yale University, New Haven, Conn. “It’s notable that many of these studies were with dronabinol, which is a synthetic THC analog. However, one take-home is that many of these studies reported improvement with sleep and a reduction in nightmares. This is something that should be followed up.”
For Tourette syndrome, there were just two studies by the same author, both with small sample sizes and of short duration. One study was rated “low” and the other “very low” for quality; in particular, the placebo effect could not be ruled out.
Four studies, said Dr. D’Souza, examined cannabinoids for dementia, and all had low quality of evidence. One published study had a sample size of two, he said. “The point here is that the only positive finding is that people who were diagnosed with dementia ate more and gained weight. And that wasn’t the objective of the study.”
“When we’re talking about medical marijuana, we’re talking about the whole plant,” he said. The marijuana plant has at least 450 known distinct constituent chemicals; these include about 70 cannabinoids as well as terpenoids and flavonoids. This means that the whole plant as dispensed represents a much more complex compound than medical tetrahydrocannabinol, for example, Dr. D’Souza said.
“We need to think about not just efficacy but side effects,” he said. These can include tolerance, abuse, and withdrawal syndrome. Marijuana’s cognitive effects may contribute to an increased risk of motor vehicle crashes. Though acute psychotic symptoms with quick resolution have long been noted, it’s also now thought that heavy marijuana exposure in adolescence more than doubles the risk for schizophrenia and might decrease adult IQ by about 10 points.
Though cannabis is “neither necessary nor sufficient for developing psychosis” but instead is a factor in a set of complex interactions, “what’s absolutely clear is that people with a psychotic disorder or at risk for developing one are very much more vulnerable to the effects of cannabinoids,” Dr. D’Souza said.
Despite all of those concerns, he said, “public demand and legislators have usurped the [Food and Drug Administration] approval process” when it comes to state-by-state approval of medical marijuana. The current state of affairs stands in contrast to the requirements for drug approvals for new indications, which require at least two adequately powered randomized clinical trials. When it comes to marijuana, Dr. D’Souza said, “For most of the indications the evidence fails to meet FDA standards.”
However, he said, the lack of high quality evidence may reflect the difficulty of conducting medical marijuana research in the United States. If this is so, he said, the “federal and state governments need to support and encourage research to generate high quality evidence to guide decisions.”
Dr. D’Souza reported a financial relationship with Insys Therapeutics, which develops pharmaceutical cannabinoid products.
On Twitter @karioakes
SCOTTSDALE, ARIZ. – There’s a rapidly growing list of states that have approved marijuana for medical use, but that does not mean that there’s also an expanding body of science to support marijuana’s use for psychiatric indications, according to Dr. Deepak Cyril D’Souza.
Though moderate evidence exists to support the use of medical marijuana for nausea and vomiting in chemotherapy, HIV/AIDS cachexia, neuropathic pain, and spasticity in multiple sclerosis, there’s scant evidence for some other uses. Little evidence exists to support the use of medical marijuana in Tourette syndrome, Crohn’s disease and ulcerative colitis, and epilepsy, and the data are even weaker for Parkinson’s disease, posttraumatic stress disorder, and agitation in Alzheimer’s disease, Dr. D’Souza said at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting.
Even so, he said, medical marijuana has been approved in several states for all of those conditions – and more.
Different states have required widely varying standards of evidence in making decisions about whether to approve medical marijuana use, and for which conditions. These range, in some cases, from relying on mere anecdotal evidence – as when individuals provide powerful but unscientific testimony about marijuana’s efficacy for a condition – to requiring the gold standard of randomized, double-blind, placebo-controlled trials.
Dr. D’Souza and his collaborators reviewed the data supporting the use of medical marijuana for several of the most commonly approved psychiatric indications, and found many of them hampered by poor design, poor execution, and an overreliance on the subjective effects of the studied compound.
For PTSD, only one randomized controlled study was found, and only one study had an active control, so the quality of evidence was rated as “very low” or “low” by Dr. D’Souza’s group. “Most of the studies were not blinded, and the sample sizes were generally small,” said Dr. D’Souza, professor of psychiatry at Yale University, New Haven, Conn. “It’s notable that many of these studies were with dronabinol, which is a synthetic THC analog. However, one take-home is that many of these studies reported improvement with sleep and a reduction in nightmares. This is something that should be followed up.”
For Tourette syndrome, there were just two studies by the same author, both with small sample sizes and of short duration. One study was rated “low” and the other “very low” for quality; in particular, the placebo effect could not be ruled out.
Four studies, said Dr. D’Souza, examined cannabinoids for dementia, and all had low quality of evidence. One published study had a sample size of two, he said. “The point here is that the only positive finding is that people who were diagnosed with dementia ate more and gained weight. And that wasn’t the objective of the study.”
“When we’re talking about medical marijuana, we’re talking about the whole plant,” he said. The marijuana plant has at least 450 known distinct constituent chemicals; these include about 70 cannabinoids as well as terpenoids and flavonoids. This means that the whole plant as dispensed represents a much more complex compound than medical tetrahydrocannabinol, for example, Dr. D’Souza said.
“We need to think about not just efficacy but side effects,” he said. These can include tolerance, abuse, and withdrawal syndrome. Marijuana’s cognitive effects may contribute to an increased risk of motor vehicle crashes. Though acute psychotic symptoms with quick resolution have long been noted, it’s also now thought that heavy marijuana exposure in adolescence more than doubles the risk for schizophrenia and might decrease adult IQ by about 10 points.
Though cannabis is “neither necessary nor sufficient for developing psychosis” but instead is a factor in a set of complex interactions, “what’s absolutely clear is that people with a psychotic disorder or at risk for developing one are very much more vulnerable to the effects of cannabinoids,” Dr. D’Souza said.
Despite all of those concerns, he said, “public demand and legislators have usurped the [Food and Drug Administration] approval process” when it comes to state-by-state approval of medical marijuana. The current state of affairs stands in contrast to the requirements for drug approvals for new indications, which require at least two adequately powered randomized clinical trials. When it comes to marijuana, Dr. D’Souza said, “For most of the indications the evidence fails to meet FDA standards.”
However, he said, the lack of high quality evidence may reflect the difficulty of conducting medical marijuana research in the United States. If this is so, he said, the “federal and state governments need to support and encourage research to generate high quality evidence to guide decisions.”
Dr. D’Souza reported a financial relationship with Insys Therapeutics, which develops pharmaceutical cannabinoid products.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE ASCP ANNUAL MEETING
Linea Aspera as Rotational Landmark for Tumor Endoprostheses: A Computed Tomography Study
The distal or proximal femur with tumor endoprosthesis is commonly replaced after segmental resections for bone tumors, complex trauma, or revision arthroplasty. In conventional joint replacements, correct rotational alignment of the component is referenced off anatomical landmarks in the proximal or distal femur. After tumor resection, however, these landmarks are often not available for rotational orientation. There are no reports of studies validating a particular method of establishing rotation in these cases.
To establish a guide for rotational alignment of tumor endoprostheses, we set out to define the natural location of the linea aspera (LA) based on axial computed tomography (CT) scans. The LA is often the most outstanding visible bony landmark on a cross-section of the femur during surgery, and it would be helpful to know its normal orientation in relation to the true anteroposterior (AP) axis of the femur and to the femoral version. We wanted to answer these 5 questions:
1. Is the prominence of the LA easily identifiable on cross-section at different levels of the femoral shaft?
2. Does an axis passing through the LA correspond to the AP axis of the femur?
3. If not, is this axis offset internally or externally and by how much?
4. Is this offset constant at all levels of the femoral shaft?
5. How does the LA axis relate to the femoral neck axis at these levels?
The answers determine if the LA can be reliably used for rotational alignment of tumor endoprostheses.
Materials and Methods
After this study received Institutional Review Board approval, we retrospectively reviewed whole-body fluorine-18-deoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) studies performed in our hospital between 2003 and 2006 to identify those with full-length bilateral femur CT scans. These scans were available on the hospital’s computerized picture archiving system (General Electric). Patients could be included in the study as long as they were at least 18 years old at time of scan and did not have any pathology that deformed the femur, broke a cortex, or otherwise caused any gross asymmetry of the femur. Of the 72 patients with full-length femur CT scans, 3 were excluded: 1 with a congenital hip dysplasia, 1 with an old, malunited femoral fracture, and 1 who was 15 years old at time of scan.
Axial Slice Selection
For each patient, scout AP films were used to measure femoral shaft length from the top of the greater trochanter to the end of the lateral femoral condyle. The levels of the proximal third, midshaft, and distal third were then calculated based on this length. The LA was studied on the axial slices nearest these levels. Next, we scrolled through the scans to identify an axial slice that best showed the femoral neck axis. The literature on CT measurement of femoral anteversion is varied. Some articles describe a technique that uses 2 superimposed axial slices, and others describe a single axial slice.1-3 We used 1 axial slice to draw the femoral neck axis because our computer software could not superimpose 2 images on 1 screen and because the CT scans were not made under specific protocols to measure anteversion but rather were part of a cancer staging work-up. Axial cuts were made at 5-mm intervals, and not all scans included a single slice capturing the head, neck, and greater trochanter. Therefore, we used a (previously described) method in which the femoral neck axis is drawn on a slice that most captured the femoral neck, usually toward its base.4 Last, in order to draw the posterior condyle (PC) axis, we selected an axial slice that showed the posterior-most aspects of the femoral condyles at the intercondylar notch.
Determining Anteroposterior and Posterior Condyle Axes of Femur
As we made all measurements for each femur off a single CT scan, we were able to use a straight horizontal line—drawn on-screen with a software tool—as a reference for measuring rotation. On a distal femur cut, the PC axis is drawn by connecting the posterior-most points of both condyles. The software calculates the angle formed—the PC angle (Figure 1). This angle, the degree to which the PC axis deviates from a straight horizontal line on-screen, can be used to account for gross rotation of the limb on comparison of images. The AP axis of the femur is the axis perpendicular to the PC axis. As such, the PC angle can also be used to determine degree of deviation of the AP axis from a straight vertical line on-screen. The AP axis was used when calculating the LA axis at the various levels of the femur (Figure 2).
Femoral Version
We used the software tool to draw the femoral neck axis. From the end of this line, a straight horizontal line is drawn on-screen (Figure 3). The software calculates the angle formed—the femoral neck axis angle. We assigned a positive value for a femoral head that pointed anteriorly on the image and a negative value for a head that pointed posteriorly. Adjusting for external rotation of the limb involved calculating the femoral version by subtracting the PC angle from the neck axis angle; adjusting for internal rotation involved adding these 2 angles.
Linea Aspera Morphology
After viewing the first 20 CT scans, we identified 3 types of LA morphology. Type I presents as a thickening on the posterior cortex with a sharp apex; type II presents as a flat-faced but distinct ridge of bone between the medial and lateral lips; and in type III there is no distinct cortical thickening with blunted medial and lateral lips; the latter is always more prominent.
Linea Aspera Axis Offset
From the most posterior point of the LA, a line drawn forward bisecting the femoral canal defined the LA axis. In type I morphology, the posterior-most point was the apex; in type II, the middle of flat posterior surface was used as the starting point; in type III, the lateral lip was used, as it was sharper than the medial lip. This line is again referenced with a straight horizontal line across the image. The PC angle is then added to account for limb rotation, and the result is the LA angle. As the AP axis is perpendicular to the PC axis, the LA angle is subtracted from 90°; the difference represents the amount of offset of the LA axis from the AP axis. By convention, we assigned this a positive value for an LA lateral to the midpoint of the femur and a negative value for an LA medial to the midpoint (Figure 4).
Linea Aspera Axis and Femoral Neck Axis
The angle between the LA axis and the PC axis was measured. The femoral version angle was subtracted from that angle to obtain the arc between the LA axis and the femoral neck axis.
Statistical Analyses
All analyses were performed with SAS 9.1 (SAS Institute). All tests were 2-sided and conducted at the .05 significance level. No adjustments were made for multiple testing. Statistical analysis was performed with nonparametric tests and without making assumptions about the distribution of the study population. Univariate analyses were performed to test for significant side-to-side differences in femoral length, femoral version angle, and LA torsion angles at each level. A multivariate analysis was performed to test for interactions between sex, side, and level. In all analyses, P < .05 was used as the cutoff value for statistical significance.
Results
Femoral lengths varied by side and sex. The left side was longer than the right by a mean of 1.3 mm (P = .008). With multivariate analysis taking into account sex and age (cumulated per decade), there was still a significant effect of side on femoral length. Sex also had a significant effect on femoral length, with females’ femurs shorter by 21.7 mm (standard error, 5.0 mm). Mean (SD) anteversion of the femoral neck was 7.9° (12.7°) on the left and 13.3° (13.0°) on the right; the difference between sides was significant (P < .001). In a multivariate analysis performed to identify potential predictors of femoral version, side still had a significant (P < .001) independent effect; sex and age did not have an effect.
LA morphology varied according to femoral shaft level (Table 1). The morphology was type I in 75% of patients at the distal femur and 74% of patients at the midshaft femur, while only 53% of patients had a type I morphology at the proximal femur. The proportion of type III morphology was larger in the proximal femur (41%) than in the other locations.
The LA axis of the femur did not correspond exactly to the AP axis at all femoral levels. At the distal femur, mean (SD) lateral offset of the LA axis was 5.5° (7.5°) on the left and 8.3° (8.9°) on the right. At the midshaft, mean (SD) medial offset of the LA axis was 3.1° (8.4°) on the left and 1.2° (7.9°) on the right. At the proximal femur, mean (SD) lateral offset of the LA axis was 5.4° (9.2°) on the left and 6.2° (8.3°) on the right. The side-to-side differences were statistically significant for the distal femur and midshaft but not the proximal femur. Table 2 lists the 95% confidence intervals for the mean values. As the range of differences was small (0.7°-2.8°), and the differences may not be clinically detected on gross inspection during surgery, we pooled both sides’ values to arrive at a single mean for each level. The LA axis was offset a mean (SD) of 6.9° (8.3°) laterally at the distal femur, 2.2° (8.2°) medially at the midshaft, and 5.8° (8.6°) laterally at the proximal femur. Figure 5 shows the frequency of distribution of LA axis offset.Offset of the LA axis from the AP axis of the femur was significantly (P < .001) different for each femoral level, even when a multivariate analysis was performed to determine the effect of sex, age, or side. Age and sex had no significant effect on mean offset of LA axis from AP axis.
We compared the mean arc between femoral neck axis and LA axis after referencing both off the PC axis. At the distal femur, mean (SD) arc between these 2 axes was 76.6° (13.1°) on the left and 68.3° (13.6°) on the right (mean difference, 8.3°); at the midshaft, mean (SD) arc was 85.2° (13.5°) on the left and 77.9° (13.1°) on the right (mean difference, 7.4°); at the proximal femur, mean (SD) arc was 76.7° (11.9°) on the left and 70.5° (12.8°) on the right (mean difference, 6.2°). The side-to-side differences were statistically significant (P < .001) for all locations.
In multivariate analysis, sex and age did not have an effect on mean arc between the 2 axes. Side and femoral level, however, had a significant effect (P < .001).
Discussion
In total hip arthroplasty, the goal is to restore femoral anteversion, usually referenced to the remaining femoral neck segment.3 In total knee arthroplasty (TKA), proper rotation preserves normal patellofemoral tracking.5 Various landmarks are used, such as the PCs or the epicondyles. After tumor resections, these landmarks are often lost.6 However, there are no reports of studies validating a particular method of achieving proper rotational orientation of tumor endoprostheses, though several methods are being used. One method involves inserting 2 drill bits before osteotomy—one proximal to the intended level of resection on the anterior femur, and the other on the anterior tibial shaft. The straight line formed can establish a plane of rotation (and length), which the surgeon must aim to restore when the components are placed. This method is useful for distal femur resections but not proximal femur resections. Another method, based on the LA’s anatomical position on the posterior aspect of the femur,4 uses the prominence of the LA to align the prosthesis. With this method, the LA is assumed to be directly posterior (6 o’clock) on the femur. However, this assumption has not been confirmed by any study. A third method, described by Heck and Carnesale,5 involves marking the anterior aspect of the femur after resection and aligning the components to it. The authors cautioned against using the LA as a landmark, saying that its course is highly variable.
The LA is a narrow, elevated length of bone, with medial and lateral lips, that serves as an attachment site for muscles in the posterior thigh. Proximally, the LA presents with lateral, medial, and intermediate lips. In the midshaft, it is often elevated by an underlying bony ridge or pilaster complex. Distally, it diverges into 2 ridges that form the triangular popliteal surface.1,7 For the LA to be a reliable landmark, first it must be clearly identifiable on viewing a femoral cross-section. The LA that presents with type I or II morphology is distinctly identifiable, and an axis from its apex and bisecting the canal can easily be constructed. In our study, the LA presented with type I or II morphology in 82% of distal femoral sections and 99% of midshaft femoral sections. Therefore, the LA is a conspicuous landmark at these levels. In the proximal femur, 59% had type I or II morphology. Type III morphology could be identified on cross-sections by the persisting prominence of the lateral lip. However, it may be difficult to appreciate the LA with this morphology at surgery.
Once the LA is identified, its normal cross-sectional position must be defined. One way to do this is to establish the relationship of its axis (LA axis) to the true AP axis. Based on mean values, the LA axis is laterally offset 7º at the distal third of the femur, medially offset 2º at the midshaft, and laterally offset 6º at the proximal third. Therefore, for ideal placement with the LA used for orientation, the component must be internally rotated 7º relative to the LA for femoral resection at the distal third, externally rotated 2º for resection at the midshaft, and internally rotated 6º for resection at the proximal third. Studies have demonstrated that joint contact forces and mechanical alignment of the lower limb can be altered with as little as 5º of femoral malrotation.8,9 Although such a small degree of malrotation is often asymptomatic, it can have long-term effects on soft-tissue tension and patellar tracking.10,11 Rotating-platform mobile-bearing TKA designs can compensate for femoral malrotation, but they may have little to no effect on patellar tracking.12 Therefore, we think aligning the components as near as possible to their natural orientation can prove beneficial in long-term patient management.
Another way of defining the normal cross-sectional position of the LA is to relate it to the femoral neck axis. We measured the difference between these 2 axes. Mean differences were 72º (distal femur), 81.5º (midshaft), and 73.5º (proximal third). Mean arc differences at all levels were larger on the left side—a reflection of the femoral neck being less anteverted on that side in our measurements. Standard deviations were smaller for measurements of LA axis offset from AP axis (range, 7.5°-9.2°) than for measurements of arc between LA axis and femoral neck axis (range, 11.9°-13.6°). This finding indicates there is less variation in the former method, making it preferable for defining the cross-sectional position of the LA.
It has been said that the course of the LA is variable, and our data provide confirmation. The LA does not lie directly posterior (6 o’clock), and it does not trace a straight longitudinal course along the posterior femur, as demonstrated by the different LA axis offsets at 3 levels. However, we may still use it as a landmark if we remain aware how much the LA is offset from the AP axis at each femoral level. Figures 6A-6D, which show CT scans of a patient who underwent distal femoral resection and replacement with an endoprosthesis, illustrate how the LA axis was measured before surgery and how proper prosthesis placement was confirmed after surgery.
In hip arthroplasty, restoration of normal femoral version is the reference for endoprosthetic placement. The literature on “normal” femoral anteversion varies with the method used. In a review of studies on CT-measured adult femoral version, reported values ranged from 6.3° to 40°.2 Mean femoral version in our study ranged from 8° to 13°. Orthopedics textbooks generally put the value at 10° to 15º, and this seems to be the range that surgeons target.6 However, we found a statistically significant mean side-to-side difference of 5.4°. This finding is possibly explained by our large sample—it was larger than the samples used in other studies of CT-measured femoral version. Other studies have found mean side-to-side differences of up to 4.0º.5 Another explanation for our finding is that the studies may differ methodologically. The studies that established values for femoral anteversion were based on CT protocols—thinner slices (1-5 mm), use of foot holders to standardize limb rotation, use of 2 axial cuts in proximal femur to establish femoral neck axis2,13—designed specifically for this measurement. As the CT scans reviewed in our study are not designed for this purpose, errors in femoral version measurement may have been introduced, which may also explain why there is larger variation in measurements of the arc between the LA axis and the femoral neck axis.
Conclusion
The LA does not lie directly on the posterior surface of the femur. It deviates 6.9° laterally at the distal femur, 2.2° medially at the midshaft, and 6.9° laterally at the proximal third. As the LA is an easily identifiable structure on cross-sections of the femoral shaft at the midshaft and distal third of the femur, it may be useful as a rotational landmark for resections at these levels if these deviations are considered during tumor endoprosthetic replacements.
1. Desai SC, Willson S. Radiology of the linea aspera. Australas Radiol. 1985;29(3):273-274.
2. Kuo TY, Skedros JG, Bloebaum RD. Measurement of femoral anteversion by biplane radiography and computed tomography imaging: comparison with an anatomic reference. Invest Radiol. 2003;38(4):221-229.
3. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006;21(5):696-701.
4. Gray H. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1918.
5. Heck RK, Carnesale PG. General principles of tumors. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Vol 1. 10th ed. St. Louis, MO: Mosby; 2003:733-791.
6. Katz, MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16(3):301-305.
7. Pitt MJ. Radiology of the femoral linea aspera–pilaster complex: the track sign. Radiology. 1982;142(1):66.
8. Bretin P, O’Loughlin PF, Suero EM, et al. Influence of femoral malrotation on knee joint alignment and intra-articular contact pressures. Arch Orthop Trauma Surg. 2011;131(8):1115-1120.
9. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech. 2005;20(7):661-668.
10. Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA. The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res. 2010;28(9):1136-1141.
11. Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: an in vitro biomechanical study. J Bone Joint Surg Br. 2010;92(5):737-742.
12. Kessler O, Patil S, Colwell CW Jr, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41(16):3332-3339.
13. Strecker W, Keppler P, Gebhard F, Kinzl L. Length and torsion of the lower limb. J Bone Joint Surg Br. 1997;79(6):1019-1023.
The distal or proximal femur with tumor endoprosthesis is commonly replaced after segmental resections for bone tumors, complex trauma, or revision arthroplasty. In conventional joint replacements, correct rotational alignment of the component is referenced off anatomical landmarks in the proximal or distal femur. After tumor resection, however, these landmarks are often not available for rotational orientation. There are no reports of studies validating a particular method of establishing rotation in these cases.
To establish a guide for rotational alignment of tumor endoprostheses, we set out to define the natural location of the linea aspera (LA) based on axial computed tomography (CT) scans. The LA is often the most outstanding visible bony landmark on a cross-section of the femur during surgery, and it would be helpful to know its normal orientation in relation to the true anteroposterior (AP) axis of the femur and to the femoral version. We wanted to answer these 5 questions:
1. Is the prominence of the LA easily identifiable on cross-section at different levels of the femoral shaft?
2. Does an axis passing through the LA correspond to the AP axis of the femur?
3. If not, is this axis offset internally or externally and by how much?
4. Is this offset constant at all levels of the femoral shaft?
5. How does the LA axis relate to the femoral neck axis at these levels?
The answers determine if the LA can be reliably used for rotational alignment of tumor endoprostheses.
Materials and Methods
After this study received Institutional Review Board approval, we retrospectively reviewed whole-body fluorine-18-deoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) studies performed in our hospital between 2003 and 2006 to identify those with full-length bilateral femur CT scans. These scans were available on the hospital’s computerized picture archiving system (General Electric). Patients could be included in the study as long as they were at least 18 years old at time of scan and did not have any pathology that deformed the femur, broke a cortex, or otherwise caused any gross asymmetry of the femur. Of the 72 patients with full-length femur CT scans, 3 were excluded: 1 with a congenital hip dysplasia, 1 with an old, malunited femoral fracture, and 1 who was 15 years old at time of scan.
Axial Slice Selection
For each patient, scout AP films were used to measure femoral shaft length from the top of the greater trochanter to the end of the lateral femoral condyle. The levels of the proximal third, midshaft, and distal third were then calculated based on this length. The LA was studied on the axial slices nearest these levels. Next, we scrolled through the scans to identify an axial slice that best showed the femoral neck axis. The literature on CT measurement of femoral anteversion is varied. Some articles describe a technique that uses 2 superimposed axial slices, and others describe a single axial slice.1-3 We used 1 axial slice to draw the femoral neck axis because our computer software could not superimpose 2 images on 1 screen and because the CT scans were not made under specific protocols to measure anteversion but rather were part of a cancer staging work-up. Axial cuts were made at 5-mm intervals, and not all scans included a single slice capturing the head, neck, and greater trochanter. Therefore, we used a (previously described) method in which the femoral neck axis is drawn on a slice that most captured the femoral neck, usually toward its base.4 Last, in order to draw the posterior condyle (PC) axis, we selected an axial slice that showed the posterior-most aspects of the femoral condyles at the intercondylar notch.
Determining Anteroposterior and Posterior Condyle Axes of Femur
As we made all measurements for each femur off a single CT scan, we were able to use a straight horizontal line—drawn on-screen with a software tool—as a reference for measuring rotation. On a distal femur cut, the PC axis is drawn by connecting the posterior-most points of both condyles. The software calculates the angle formed—the PC angle (Figure 1). This angle, the degree to which the PC axis deviates from a straight horizontal line on-screen, can be used to account for gross rotation of the limb on comparison of images. The AP axis of the femur is the axis perpendicular to the PC axis. As such, the PC angle can also be used to determine degree of deviation of the AP axis from a straight vertical line on-screen. The AP axis was used when calculating the LA axis at the various levels of the femur (Figure 2).
Femoral Version
We used the software tool to draw the femoral neck axis. From the end of this line, a straight horizontal line is drawn on-screen (Figure 3). The software calculates the angle formed—the femoral neck axis angle. We assigned a positive value for a femoral head that pointed anteriorly on the image and a negative value for a head that pointed posteriorly. Adjusting for external rotation of the limb involved calculating the femoral version by subtracting the PC angle from the neck axis angle; adjusting for internal rotation involved adding these 2 angles.
Linea Aspera Morphology
After viewing the first 20 CT scans, we identified 3 types of LA morphology. Type I presents as a thickening on the posterior cortex with a sharp apex; type II presents as a flat-faced but distinct ridge of bone between the medial and lateral lips; and in type III there is no distinct cortical thickening with blunted medial and lateral lips; the latter is always more prominent.
Linea Aspera Axis Offset
From the most posterior point of the LA, a line drawn forward bisecting the femoral canal defined the LA axis. In type I morphology, the posterior-most point was the apex; in type II, the middle of flat posterior surface was used as the starting point; in type III, the lateral lip was used, as it was sharper than the medial lip. This line is again referenced with a straight horizontal line across the image. The PC angle is then added to account for limb rotation, and the result is the LA angle. As the AP axis is perpendicular to the PC axis, the LA angle is subtracted from 90°; the difference represents the amount of offset of the LA axis from the AP axis. By convention, we assigned this a positive value for an LA lateral to the midpoint of the femur and a negative value for an LA medial to the midpoint (Figure 4).
Linea Aspera Axis and Femoral Neck Axis
The angle between the LA axis and the PC axis was measured. The femoral version angle was subtracted from that angle to obtain the arc between the LA axis and the femoral neck axis.
Statistical Analyses
All analyses were performed with SAS 9.1 (SAS Institute). All tests were 2-sided and conducted at the .05 significance level. No adjustments were made for multiple testing. Statistical analysis was performed with nonparametric tests and without making assumptions about the distribution of the study population. Univariate analyses were performed to test for significant side-to-side differences in femoral length, femoral version angle, and LA torsion angles at each level. A multivariate analysis was performed to test for interactions between sex, side, and level. In all analyses, P < .05 was used as the cutoff value for statistical significance.
Results
Femoral lengths varied by side and sex. The left side was longer than the right by a mean of 1.3 mm (P = .008). With multivariate analysis taking into account sex and age (cumulated per decade), there was still a significant effect of side on femoral length. Sex also had a significant effect on femoral length, with females’ femurs shorter by 21.7 mm (standard error, 5.0 mm). Mean (SD) anteversion of the femoral neck was 7.9° (12.7°) on the left and 13.3° (13.0°) on the right; the difference between sides was significant (P < .001). In a multivariate analysis performed to identify potential predictors of femoral version, side still had a significant (P < .001) independent effect; sex and age did not have an effect.
LA morphology varied according to femoral shaft level (Table 1). The morphology was type I in 75% of patients at the distal femur and 74% of patients at the midshaft femur, while only 53% of patients had a type I morphology at the proximal femur. The proportion of type III morphology was larger in the proximal femur (41%) than in the other locations.
The LA axis of the femur did not correspond exactly to the AP axis at all femoral levels. At the distal femur, mean (SD) lateral offset of the LA axis was 5.5° (7.5°) on the left and 8.3° (8.9°) on the right. At the midshaft, mean (SD) medial offset of the LA axis was 3.1° (8.4°) on the left and 1.2° (7.9°) on the right. At the proximal femur, mean (SD) lateral offset of the LA axis was 5.4° (9.2°) on the left and 6.2° (8.3°) on the right. The side-to-side differences were statistically significant for the distal femur and midshaft but not the proximal femur. Table 2 lists the 95% confidence intervals for the mean values. As the range of differences was small (0.7°-2.8°), and the differences may not be clinically detected on gross inspection during surgery, we pooled both sides’ values to arrive at a single mean for each level. The LA axis was offset a mean (SD) of 6.9° (8.3°) laterally at the distal femur, 2.2° (8.2°) medially at the midshaft, and 5.8° (8.6°) laterally at the proximal femur. Figure 5 shows the frequency of distribution of LA axis offset.Offset of the LA axis from the AP axis of the femur was significantly (P < .001) different for each femoral level, even when a multivariate analysis was performed to determine the effect of sex, age, or side. Age and sex had no significant effect on mean offset of LA axis from AP axis.
We compared the mean arc between femoral neck axis and LA axis after referencing both off the PC axis. At the distal femur, mean (SD) arc between these 2 axes was 76.6° (13.1°) on the left and 68.3° (13.6°) on the right (mean difference, 8.3°); at the midshaft, mean (SD) arc was 85.2° (13.5°) on the left and 77.9° (13.1°) on the right (mean difference, 7.4°); at the proximal femur, mean (SD) arc was 76.7° (11.9°) on the left and 70.5° (12.8°) on the right (mean difference, 6.2°). The side-to-side differences were statistically significant (P < .001) for all locations.
In multivariate analysis, sex and age did not have an effect on mean arc between the 2 axes. Side and femoral level, however, had a significant effect (P < .001).
Discussion
In total hip arthroplasty, the goal is to restore femoral anteversion, usually referenced to the remaining femoral neck segment.3 In total knee arthroplasty (TKA), proper rotation preserves normal patellofemoral tracking.5 Various landmarks are used, such as the PCs or the epicondyles. After tumor resections, these landmarks are often lost.6 However, there are no reports of studies validating a particular method of achieving proper rotational orientation of tumor endoprostheses, though several methods are being used. One method involves inserting 2 drill bits before osteotomy—one proximal to the intended level of resection on the anterior femur, and the other on the anterior tibial shaft. The straight line formed can establish a plane of rotation (and length), which the surgeon must aim to restore when the components are placed. This method is useful for distal femur resections but not proximal femur resections. Another method, based on the LA’s anatomical position on the posterior aspect of the femur,4 uses the prominence of the LA to align the prosthesis. With this method, the LA is assumed to be directly posterior (6 o’clock) on the femur. However, this assumption has not been confirmed by any study. A third method, described by Heck and Carnesale,5 involves marking the anterior aspect of the femur after resection and aligning the components to it. The authors cautioned against using the LA as a landmark, saying that its course is highly variable.
The LA is a narrow, elevated length of bone, with medial and lateral lips, that serves as an attachment site for muscles in the posterior thigh. Proximally, the LA presents with lateral, medial, and intermediate lips. In the midshaft, it is often elevated by an underlying bony ridge or pilaster complex. Distally, it diverges into 2 ridges that form the triangular popliteal surface.1,7 For the LA to be a reliable landmark, first it must be clearly identifiable on viewing a femoral cross-section. The LA that presents with type I or II morphology is distinctly identifiable, and an axis from its apex and bisecting the canal can easily be constructed. In our study, the LA presented with type I or II morphology in 82% of distal femoral sections and 99% of midshaft femoral sections. Therefore, the LA is a conspicuous landmark at these levels. In the proximal femur, 59% had type I or II morphology. Type III morphology could be identified on cross-sections by the persisting prominence of the lateral lip. However, it may be difficult to appreciate the LA with this morphology at surgery.
Once the LA is identified, its normal cross-sectional position must be defined. One way to do this is to establish the relationship of its axis (LA axis) to the true AP axis. Based on mean values, the LA axis is laterally offset 7º at the distal third of the femur, medially offset 2º at the midshaft, and laterally offset 6º at the proximal third. Therefore, for ideal placement with the LA used for orientation, the component must be internally rotated 7º relative to the LA for femoral resection at the distal third, externally rotated 2º for resection at the midshaft, and internally rotated 6º for resection at the proximal third. Studies have demonstrated that joint contact forces and mechanical alignment of the lower limb can be altered with as little as 5º of femoral malrotation.8,9 Although such a small degree of malrotation is often asymptomatic, it can have long-term effects on soft-tissue tension and patellar tracking.10,11 Rotating-platform mobile-bearing TKA designs can compensate for femoral malrotation, but they may have little to no effect on patellar tracking.12 Therefore, we think aligning the components as near as possible to their natural orientation can prove beneficial in long-term patient management.
Another way of defining the normal cross-sectional position of the LA is to relate it to the femoral neck axis. We measured the difference between these 2 axes. Mean differences were 72º (distal femur), 81.5º (midshaft), and 73.5º (proximal third). Mean arc differences at all levels were larger on the left side—a reflection of the femoral neck being less anteverted on that side in our measurements. Standard deviations were smaller for measurements of LA axis offset from AP axis (range, 7.5°-9.2°) than for measurements of arc between LA axis and femoral neck axis (range, 11.9°-13.6°). This finding indicates there is less variation in the former method, making it preferable for defining the cross-sectional position of the LA.
It has been said that the course of the LA is variable, and our data provide confirmation. The LA does not lie directly posterior (6 o’clock), and it does not trace a straight longitudinal course along the posterior femur, as demonstrated by the different LA axis offsets at 3 levels. However, we may still use it as a landmark if we remain aware how much the LA is offset from the AP axis at each femoral level. Figures 6A-6D, which show CT scans of a patient who underwent distal femoral resection and replacement with an endoprosthesis, illustrate how the LA axis was measured before surgery and how proper prosthesis placement was confirmed after surgery.
In hip arthroplasty, restoration of normal femoral version is the reference for endoprosthetic placement. The literature on “normal” femoral anteversion varies with the method used. In a review of studies on CT-measured adult femoral version, reported values ranged from 6.3° to 40°.2 Mean femoral version in our study ranged from 8° to 13°. Orthopedics textbooks generally put the value at 10° to 15º, and this seems to be the range that surgeons target.6 However, we found a statistically significant mean side-to-side difference of 5.4°. This finding is possibly explained by our large sample—it was larger than the samples used in other studies of CT-measured femoral version. Other studies have found mean side-to-side differences of up to 4.0º.5 Another explanation for our finding is that the studies may differ methodologically. The studies that established values for femoral anteversion were based on CT protocols—thinner slices (1-5 mm), use of foot holders to standardize limb rotation, use of 2 axial cuts in proximal femur to establish femoral neck axis2,13—designed specifically for this measurement. As the CT scans reviewed in our study are not designed for this purpose, errors in femoral version measurement may have been introduced, which may also explain why there is larger variation in measurements of the arc between the LA axis and the femoral neck axis.
Conclusion
The LA does not lie directly on the posterior surface of the femur. It deviates 6.9° laterally at the distal femur, 2.2° medially at the midshaft, and 6.9° laterally at the proximal third. As the LA is an easily identifiable structure on cross-sections of the femoral shaft at the midshaft and distal third of the femur, it may be useful as a rotational landmark for resections at these levels if these deviations are considered during tumor endoprosthetic replacements.
The distal or proximal femur with tumor endoprosthesis is commonly replaced after segmental resections for bone tumors, complex trauma, or revision arthroplasty. In conventional joint replacements, correct rotational alignment of the component is referenced off anatomical landmarks in the proximal or distal femur. After tumor resection, however, these landmarks are often not available for rotational orientation. There are no reports of studies validating a particular method of establishing rotation in these cases.
To establish a guide for rotational alignment of tumor endoprostheses, we set out to define the natural location of the linea aspera (LA) based on axial computed tomography (CT) scans. The LA is often the most outstanding visible bony landmark on a cross-section of the femur during surgery, and it would be helpful to know its normal orientation in relation to the true anteroposterior (AP) axis of the femur and to the femoral version. We wanted to answer these 5 questions:
1. Is the prominence of the LA easily identifiable on cross-section at different levels of the femoral shaft?
2. Does an axis passing through the LA correspond to the AP axis of the femur?
3. If not, is this axis offset internally or externally and by how much?
4. Is this offset constant at all levels of the femoral shaft?
5. How does the LA axis relate to the femoral neck axis at these levels?
The answers determine if the LA can be reliably used for rotational alignment of tumor endoprostheses.
Materials and Methods
After this study received Institutional Review Board approval, we retrospectively reviewed whole-body fluorine-18-deoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) studies performed in our hospital between 2003 and 2006 to identify those with full-length bilateral femur CT scans. These scans were available on the hospital’s computerized picture archiving system (General Electric). Patients could be included in the study as long as they were at least 18 years old at time of scan and did not have any pathology that deformed the femur, broke a cortex, or otherwise caused any gross asymmetry of the femur. Of the 72 patients with full-length femur CT scans, 3 were excluded: 1 with a congenital hip dysplasia, 1 with an old, malunited femoral fracture, and 1 who was 15 years old at time of scan.
Axial Slice Selection
For each patient, scout AP films were used to measure femoral shaft length from the top of the greater trochanter to the end of the lateral femoral condyle. The levels of the proximal third, midshaft, and distal third were then calculated based on this length. The LA was studied on the axial slices nearest these levels. Next, we scrolled through the scans to identify an axial slice that best showed the femoral neck axis. The literature on CT measurement of femoral anteversion is varied. Some articles describe a technique that uses 2 superimposed axial slices, and others describe a single axial slice.1-3 We used 1 axial slice to draw the femoral neck axis because our computer software could not superimpose 2 images on 1 screen and because the CT scans were not made under specific protocols to measure anteversion but rather were part of a cancer staging work-up. Axial cuts were made at 5-mm intervals, and not all scans included a single slice capturing the head, neck, and greater trochanter. Therefore, we used a (previously described) method in which the femoral neck axis is drawn on a slice that most captured the femoral neck, usually toward its base.4 Last, in order to draw the posterior condyle (PC) axis, we selected an axial slice that showed the posterior-most aspects of the femoral condyles at the intercondylar notch.
Determining Anteroposterior and Posterior Condyle Axes of Femur
As we made all measurements for each femur off a single CT scan, we were able to use a straight horizontal line—drawn on-screen with a software tool—as a reference for measuring rotation. On a distal femur cut, the PC axis is drawn by connecting the posterior-most points of both condyles. The software calculates the angle formed—the PC angle (Figure 1). This angle, the degree to which the PC axis deviates from a straight horizontal line on-screen, can be used to account for gross rotation of the limb on comparison of images. The AP axis of the femur is the axis perpendicular to the PC axis. As such, the PC angle can also be used to determine degree of deviation of the AP axis from a straight vertical line on-screen. The AP axis was used when calculating the LA axis at the various levels of the femur (Figure 2).
Femoral Version
We used the software tool to draw the femoral neck axis. From the end of this line, a straight horizontal line is drawn on-screen (Figure 3). The software calculates the angle formed—the femoral neck axis angle. We assigned a positive value for a femoral head that pointed anteriorly on the image and a negative value for a head that pointed posteriorly. Adjusting for external rotation of the limb involved calculating the femoral version by subtracting the PC angle from the neck axis angle; adjusting for internal rotation involved adding these 2 angles.
Linea Aspera Morphology
After viewing the first 20 CT scans, we identified 3 types of LA morphology. Type I presents as a thickening on the posterior cortex with a sharp apex; type II presents as a flat-faced but distinct ridge of bone between the medial and lateral lips; and in type III there is no distinct cortical thickening with blunted medial and lateral lips; the latter is always more prominent.
Linea Aspera Axis Offset
From the most posterior point of the LA, a line drawn forward bisecting the femoral canal defined the LA axis. In type I morphology, the posterior-most point was the apex; in type II, the middle of flat posterior surface was used as the starting point; in type III, the lateral lip was used, as it was sharper than the medial lip. This line is again referenced with a straight horizontal line across the image. The PC angle is then added to account for limb rotation, and the result is the LA angle. As the AP axis is perpendicular to the PC axis, the LA angle is subtracted from 90°; the difference represents the amount of offset of the LA axis from the AP axis. By convention, we assigned this a positive value for an LA lateral to the midpoint of the femur and a negative value for an LA medial to the midpoint (Figure 4).
Linea Aspera Axis and Femoral Neck Axis
The angle between the LA axis and the PC axis was measured. The femoral version angle was subtracted from that angle to obtain the arc between the LA axis and the femoral neck axis.
Statistical Analyses
All analyses were performed with SAS 9.1 (SAS Institute). All tests were 2-sided and conducted at the .05 significance level. No adjustments were made for multiple testing. Statistical analysis was performed with nonparametric tests and without making assumptions about the distribution of the study population. Univariate analyses were performed to test for significant side-to-side differences in femoral length, femoral version angle, and LA torsion angles at each level. A multivariate analysis was performed to test for interactions between sex, side, and level. In all analyses, P < .05 was used as the cutoff value for statistical significance.
Results
Femoral lengths varied by side and sex. The left side was longer than the right by a mean of 1.3 mm (P = .008). With multivariate analysis taking into account sex and age (cumulated per decade), there was still a significant effect of side on femoral length. Sex also had a significant effect on femoral length, with females’ femurs shorter by 21.7 mm (standard error, 5.0 mm). Mean (SD) anteversion of the femoral neck was 7.9° (12.7°) on the left and 13.3° (13.0°) on the right; the difference between sides was significant (P < .001). In a multivariate analysis performed to identify potential predictors of femoral version, side still had a significant (P < .001) independent effect; sex and age did not have an effect.
LA morphology varied according to femoral shaft level (Table 1). The morphology was type I in 75% of patients at the distal femur and 74% of patients at the midshaft femur, while only 53% of patients had a type I morphology at the proximal femur. The proportion of type III morphology was larger in the proximal femur (41%) than in the other locations.
The LA axis of the femur did not correspond exactly to the AP axis at all femoral levels. At the distal femur, mean (SD) lateral offset of the LA axis was 5.5° (7.5°) on the left and 8.3° (8.9°) on the right. At the midshaft, mean (SD) medial offset of the LA axis was 3.1° (8.4°) on the left and 1.2° (7.9°) on the right. At the proximal femur, mean (SD) lateral offset of the LA axis was 5.4° (9.2°) on the left and 6.2° (8.3°) on the right. The side-to-side differences were statistically significant for the distal femur and midshaft but not the proximal femur. Table 2 lists the 95% confidence intervals for the mean values. As the range of differences was small (0.7°-2.8°), and the differences may not be clinically detected on gross inspection during surgery, we pooled both sides’ values to arrive at a single mean for each level. The LA axis was offset a mean (SD) of 6.9° (8.3°) laterally at the distal femur, 2.2° (8.2°) medially at the midshaft, and 5.8° (8.6°) laterally at the proximal femur. Figure 5 shows the frequency of distribution of LA axis offset.Offset of the LA axis from the AP axis of the femur was significantly (P < .001) different for each femoral level, even when a multivariate analysis was performed to determine the effect of sex, age, or side. Age and sex had no significant effect on mean offset of LA axis from AP axis.
We compared the mean arc between femoral neck axis and LA axis after referencing both off the PC axis. At the distal femur, mean (SD) arc between these 2 axes was 76.6° (13.1°) on the left and 68.3° (13.6°) on the right (mean difference, 8.3°); at the midshaft, mean (SD) arc was 85.2° (13.5°) on the left and 77.9° (13.1°) on the right (mean difference, 7.4°); at the proximal femur, mean (SD) arc was 76.7° (11.9°) on the left and 70.5° (12.8°) on the right (mean difference, 6.2°). The side-to-side differences were statistically significant (P < .001) for all locations.
In multivariate analysis, sex and age did not have an effect on mean arc between the 2 axes. Side and femoral level, however, had a significant effect (P < .001).
Discussion
In total hip arthroplasty, the goal is to restore femoral anteversion, usually referenced to the remaining femoral neck segment.3 In total knee arthroplasty (TKA), proper rotation preserves normal patellofemoral tracking.5 Various landmarks are used, such as the PCs or the epicondyles. After tumor resections, these landmarks are often lost.6 However, there are no reports of studies validating a particular method of achieving proper rotational orientation of tumor endoprostheses, though several methods are being used. One method involves inserting 2 drill bits before osteotomy—one proximal to the intended level of resection on the anterior femur, and the other on the anterior tibial shaft. The straight line formed can establish a plane of rotation (and length), which the surgeon must aim to restore when the components are placed. This method is useful for distal femur resections but not proximal femur resections. Another method, based on the LA’s anatomical position on the posterior aspect of the femur,4 uses the prominence of the LA to align the prosthesis. With this method, the LA is assumed to be directly posterior (6 o’clock) on the femur. However, this assumption has not been confirmed by any study. A third method, described by Heck and Carnesale,5 involves marking the anterior aspect of the femur after resection and aligning the components to it. The authors cautioned against using the LA as a landmark, saying that its course is highly variable.
The LA is a narrow, elevated length of bone, with medial and lateral lips, that serves as an attachment site for muscles in the posterior thigh. Proximally, the LA presents with lateral, medial, and intermediate lips. In the midshaft, it is often elevated by an underlying bony ridge or pilaster complex. Distally, it diverges into 2 ridges that form the triangular popliteal surface.1,7 For the LA to be a reliable landmark, first it must be clearly identifiable on viewing a femoral cross-section. The LA that presents with type I or II morphology is distinctly identifiable, and an axis from its apex and bisecting the canal can easily be constructed. In our study, the LA presented with type I or II morphology in 82% of distal femoral sections and 99% of midshaft femoral sections. Therefore, the LA is a conspicuous landmark at these levels. In the proximal femur, 59% had type I or II morphology. Type III morphology could be identified on cross-sections by the persisting prominence of the lateral lip. However, it may be difficult to appreciate the LA with this morphology at surgery.
Once the LA is identified, its normal cross-sectional position must be defined. One way to do this is to establish the relationship of its axis (LA axis) to the true AP axis. Based on mean values, the LA axis is laterally offset 7º at the distal third of the femur, medially offset 2º at the midshaft, and laterally offset 6º at the proximal third. Therefore, for ideal placement with the LA used for orientation, the component must be internally rotated 7º relative to the LA for femoral resection at the distal third, externally rotated 2º for resection at the midshaft, and internally rotated 6º for resection at the proximal third. Studies have demonstrated that joint contact forces and mechanical alignment of the lower limb can be altered with as little as 5º of femoral malrotation.8,9 Although such a small degree of malrotation is often asymptomatic, it can have long-term effects on soft-tissue tension and patellar tracking.10,11 Rotating-platform mobile-bearing TKA designs can compensate for femoral malrotation, but they may have little to no effect on patellar tracking.12 Therefore, we think aligning the components as near as possible to their natural orientation can prove beneficial in long-term patient management.
Another way of defining the normal cross-sectional position of the LA is to relate it to the femoral neck axis. We measured the difference between these 2 axes. Mean differences were 72º (distal femur), 81.5º (midshaft), and 73.5º (proximal third). Mean arc differences at all levels were larger on the left side—a reflection of the femoral neck being less anteverted on that side in our measurements. Standard deviations were smaller for measurements of LA axis offset from AP axis (range, 7.5°-9.2°) than for measurements of arc between LA axis and femoral neck axis (range, 11.9°-13.6°). This finding indicates there is less variation in the former method, making it preferable for defining the cross-sectional position of the LA.
It has been said that the course of the LA is variable, and our data provide confirmation. The LA does not lie directly posterior (6 o’clock), and it does not trace a straight longitudinal course along the posterior femur, as demonstrated by the different LA axis offsets at 3 levels. However, we may still use it as a landmark if we remain aware how much the LA is offset from the AP axis at each femoral level. Figures 6A-6D, which show CT scans of a patient who underwent distal femoral resection and replacement with an endoprosthesis, illustrate how the LA axis was measured before surgery and how proper prosthesis placement was confirmed after surgery.
In hip arthroplasty, restoration of normal femoral version is the reference for endoprosthetic placement. The literature on “normal” femoral anteversion varies with the method used. In a review of studies on CT-measured adult femoral version, reported values ranged from 6.3° to 40°.2 Mean femoral version in our study ranged from 8° to 13°. Orthopedics textbooks generally put the value at 10° to 15º, and this seems to be the range that surgeons target.6 However, we found a statistically significant mean side-to-side difference of 5.4°. This finding is possibly explained by our large sample—it was larger than the samples used in other studies of CT-measured femoral version. Other studies have found mean side-to-side differences of up to 4.0º.5 Another explanation for our finding is that the studies may differ methodologically. The studies that established values for femoral anteversion were based on CT protocols—thinner slices (1-5 mm), use of foot holders to standardize limb rotation, use of 2 axial cuts in proximal femur to establish femoral neck axis2,13—designed specifically for this measurement. As the CT scans reviewed in our study are not designed for this purpose, errors in femoral version measurement may have been introduced, which may also explain why there is larger variation in measurements of the arc between the LA axis and the femoral neck axis.
Conclusion
The LA does not lie directly on the posterior surface of the femur. It deviates 6.9° laterally at the distal femur, 2.2° medially at the midshaft, and 6.9° laterally at the proximal third. As the LA is an easily identifiable structure on cross-sections of the femoral shaft at the midshaft and distal third of the femur, it may be useful as a rotational landmark for resections at these levels if these deviations are considered during tumor endoprosthetic replacements.
1. Desai SC, Willson S. Radiology of the linea aspera. Australas Radiol. 1985;29(3):273-274.
2. Kuo TY, Skedros JG, Bloebaum RD. Measurement of femoral anteversion by biplane radiography and computed tomography imaging: comparison with an anatomic reference. Invest Radiol. 2003;38(4):221-229.
3. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006;21(5):696-701.
4. Gray H. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1918.
5. Heck RK, Carnesale PG. General principles of tumors. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Vol 1. 10th ed. St. Louis, MO: Mosby; 2003:733-791.
6. Katz, MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16(3):301-305.
7. Pitt MJ. Radiology of the femoral linea aspera–pilaster complex: the track sign. Radiology. 1982;142(1):66.
8. Bretin P, O’Loughlin PF, Suero EM, et al. Influence of femoral malrotation on knee joint alignment and intra-articular contact pressures. Arch Orthop Trauma Surg. 2011;131(8):1115-1120.
9. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech. 2005;20(7):661-668.
10. Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA. The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res. 2010;28(9):1136-1141.
11. Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: an in vitro biomechanical study. J Bone Joint Surg Br. 2010;92(5):737-742.
12. Kessler O, Patil S, Colwell CW Jr, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41(16):3332-3339.
13. Strecker W, Keppler P, Gebhard F, Kinzl L. Length and torsion of the lower limb. J Bone Joint Surg Br. 1997;79(6):1019-1023.
1. Desai SC, Willson S. Radiology of the linea aspera. Australas Radiol. 1985;29(3):273-274.
2. Kuo TY, Skedros JG, Bloebaum RD. Measurement of femoral anteversion by biplane radiography and computed tomography imaging: comparison with an anatomic reference. Invest Radiol. 2003;38(4):221-229.
3. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006;21(5):696-701.
4. Gray H. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1918.
5. Heck RK, Carnesale PG. General principles of tumors. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Vol 1. 10th ed. St. Louis, MO: Mosby; 2003:733-791.
6. Katz, MA, Beck TD, Silber JS, Seldes RM, Lotke PA. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16(3):301-305.
7. Pitt MJ. Radiology of the femoral linea aspera–pilaster complex: the track sign. Radiology. 1982;142(1):66.
8. Bretin P, O’Loughlin PF, Suero EM, et al. Influence of femoral malrotation on knee joint alignment and intra-articular contact pressures. Arch Orthop Trauma Surg. 2011;131(8):1115-1120.
9. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech. 2005;20(7):661-668.
10. Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA. The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res. 2010;28(9):1136-1141.
11. Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: an in vitro biomechanical study. J Bone Joint Surg Br. 2010;92(5):737-742.
12. Kessler O, Patil S, Colwell CW Jr, D’Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41(16):3332-3339.
13. Strecker W, Keppler P, Gebhard F, Kinzl L. Length and torsion of the lower limb. J Bone Joint Surg Br. 1997;79(6):1019-1023.