User login
Management Challenges in Sarcoidosis
From the New Cross Hospital, Wolverhampton, UK.
Abstract
- Objective: To discuss the management of sarcoidosis.
- Methods: Review of the literature.
- Results: Sarcoidosis is a challenging multisystem disorder of uncertain etiology characterized by granulomatous inflammation in the affected organs. Treatment is dependent on the severity of disease and organ involvement at the time of diagnosis. Glucocorticoids have traditionally been considered first-line pharmacologic treatment; however, a significant proportion of patients do not require drug treatment due to the propensity toward spontaneous disease remission. Treated patients who fail to respond to corticosteroids or develop significant adverse effects can be offered a second-line agent, eg, methotrexate. Anti-TNF therapy may be considered as a treatment option in carefully selected patients with refractory disease after discussion of potential adverse effects followed by close monitoring at a specialist center.
- Conclusion: Further research into therapeutic options is likely to unveil novel agents with different mechanisms of action and better safety profiles than those seen with currently available immunosuppressive regimens.
Sarcoidosis is a multisystem disorder of uncertain etiology characterized by granulomatous inflammation in the affected organs. The diagnosis of sarcoidosis is best supported by histological evidence of noncaseating granuloma formation. It is a disease with a generally good prognosis; less than 5% patients die from the disease, with cause of death usually secondary to respiratory failure or cardiac or neurologic involvement. This review aims to discuss the management of sarcoidosis with a special emphasis on the management challenges resulting from the myriad clinical manifestations and potential complications seen in this chronic multisystem disease.
Case Study
Initial Presentation
A 40-year-old African-American man presents to his primary care physician with symptoms of fatigue, dry cough, exertional breathlessness, dry and painful eyes, generalized arthralgia, and multiple skin lesions for 3 months. He has a history of essential hypertension and is a former smoker with 10 pack-year history. He is not on any regular medications. Examination reveals bilateral cervical lymphadenopathy and multiple skin lesions on trunk. The rest of the systemic examination (including respiratory and cardiovascular system) is normal.
Workup
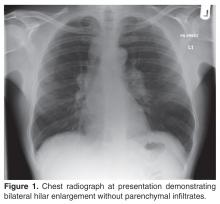
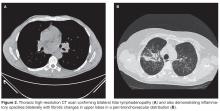
The patient’s initial bloodwork showed a mild degree of lymphopenia (1.1 × 109/L, normal range 1.5–4.5). Other bloodwork results, bone profile and immunology screen (including ANA, rheumatoid factor, immunoglobulins, and extractable nuclear antigen antibodies) were negative. Angiotensin-converting enzyme (ACE) was elevated at 149 U/L (normal range 5–58), while serum calcium and vitamin D levels (including vitamin D3) were normal. The findings on CT scan along with the biochemical profile suggest a plausible diagnosis of sarcoidosis.
What is the next step in the workup to establish the suspected diagnosis?
The radiological findings of hilar lymphadenopathy are not confirmatory. There are a number of entities in the differential diagnoses, including tuberculosis, malignancy, lymphoma, and other granulomatous disorders such as histoplasmosis, schistosomiasis, and blastomycosis. It is important to obtain histological evidence before a definitive diagnosis of sarcoidosis can be made. This is necessary as management differs for each of the diagnostic categories mentioned above. Furthermore, diagnostic confirmation would be helpful later in the disease course if the patient develops any associated complications such as pulmonary hypertension or respiratory failure and/or need for lung transplant assessment.
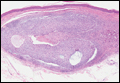
As this patient had palpable cervical lymphadenopathy, an ultrasound guided biopsy of the lymph node was obtained. The histological examination demonstrated evidence of noncaseating granulomas that were well formed and highly consistent with the suspected clinical and radiological diagnosis of sarcoidosis. In addition, the special stains for acid-fast bacilli and other infections including fungi were negative.
Our practice is to evaluate patients with suspected sarcoidosis with neck ultrasound and tru-cut biopsy of cervical lymph nodes (if appropriate) as a first-line investigation, as it is less invasive than bronchoscopy/endoscopic ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) or thoracoscopic lung biopsy. The diagnostic yield of EBUS-TBNA has been evaluated in a number of studies with variable results [1–5]. A large multicenter randomized clinical trial [5] of 304 patients investigated the diagnostic yield of endosonography (endobronchial and esophageal ultrasound) in comparison with bronchoscopy with transbronchial biopsy (TBB) and endobronchial biopsy. The study cohort was made up of patients with stage I/II sarcoidosis. The results showed that endosonography had a higher diagnostic yield to detect granulomas (80% vs 53%; P < 0.001) and there were no serious adverse events related to endoscopy. Hence, ultrasound-guided endoscopic procedures are becoming common first-line investigations for sarcoidosis in the absence of other readily identifiable biopsy sites such as peripheral lymph nodes in the cervical area.
What should be done about the cutaneous and ophthalmologic symptoms in this patient?
As sarcoidosis commonly involves eyes and skin (after pulmonary involvement, which is seen in 90% of cases), the patient was referred to ophthalmology and dermatology departments for further evaluation. These assessments confirmed him to have bilateral uveitis and skin involvement with granulomatous inflammation consistent with ocular and cutaneous sarcoidosis respectively. Hence, the diagnosis of multisystem sarcoidosis was made. At this stage, the patient also mentioned symptoms of intermittent palpitations for 3 months’ duration and feeling of missing a beat, so an urgent cardiological evaluation was undertaken that showed him to have ectopic beats on Holter monitoring. However, his trans-thoracic echocardiogram and a cardiac MRI scan were normal with good biventricular function, excluding cardiac sarcoidosis as a cause of his palpitations. Cardiac involvement with sarcoidosis is clinically apparent in only 5% of cases and presents as cardiomyopathy and or cardiac arrhythmias (both tachy and bradyarrythmias). As cardiac involvement with sarcoid granulomas is usually patchy, endomyocardial biopsy has a limited diagnostic yield of < 20% [6]. In this particular case, endomyocardial biopsy was not attempted in view of normal cardiac MR and echocardiography as well as no significant cardiac dysrythmia on holter monitoring.
Case Continued
The patient was started on corticosteroid eye drops and steroid ointment for his ophthalmologic and cutaneous sarcoidosis, respectively, and symptoms gradually improved over the next few months. As the patient was symptomatic with cough and breathlessness and there was evidence of reduction in FVC (along with reduced DLco), a trial of oral corticosteroids was considered to treat the pulmonary sarcoidosis.
What is first-line pharmacological treatment for sarcoidosis and when is it indicated?
Treatment of sarcoidosis is dependent upon the severity of disease and organ involvement at the time of the diagnosis. Glucocorticoids have traditionally been considered first-line pharmacological agents in selective cases, as a significant proportion of patients do not require drug treatment due to the propensity for spontaneous remission. Furthermore, sarcoidosis remains stable without anti-inflammatory/immunosuppressive therapies in a majority of patients. A number of clinical trials have evaluated the value of corticosteroids in the management of sarcoidosis, with variable outcomes [10–16]. The disease tends to be severe in patients of African descent compared with patients from other racial backgrounds. The European cohort of sarcoidosis patients generally have milder disease with less propensity for vital organ involvement such as cardiac or central nervous system (CNS) disease.
The decision to initiate corticosteroids for sarcoidosis is not a straightforward one as there is variability in symptom presentation, disease severity, and response to corticosteroids. We initiate first-line therapy with oral prednisolone in the following circumstances:
- Evidence of pulmonary impairment (forced vital capacity FVC < 80% predicted) with or without reduction in gas transfer (DLco) along with respiratory symptoms of cough, chest pain, and/or breathlessness (as seen in the case patient)
- Vital organ involvement such as cardiac, ophthalmic (such as panuveitis) or CNS sarcoidosis once confirmed by respective investigations
- Selective cases of sarcoid-associated pulmonary hypertension (SAPH) along with close liaison with pulmonary hypertension specialists
We recommend an initial starting dose of 20 to 40 mg of prednisolone for a period of 1 to 3 months, followed by maintenance dose of 10 mg or less for a further 6 to 9 months, aiming for a total duration of treatment of 12 months. However, the duration may vary depending on the response and any associated adverse effects with corticosteroids. It is usual practice to supplement with calcium and vitamin D when beginning patients on oral corticosteroids due to the potential risk of osteoporosis. However, this may result in significant hypercalcemia, which itself may be an endocrine manifestation of sarcoidosis. Hence, we recommend monitoring serum calcium during treatment and supplement vitamin D in patients who are vitamin D–deficient [17]. Furthermore, serum vitamin 1,25(OH)2 vitamin D3 has been demonstrated to the best available test to evaluate vitamin D status in sarcoidosis [18].
Case Continued
What are the preferred pharmacological agents for second-line treatment in sarcoidosis?
Alternative immunosuppression should be considered in the following circumstances in patients diagnosed with sarcoidosis:
- Failure or less than optimal response to oral corticosteroids
- Use as a steroid-sparing agent in patients requiring high doses of steroids for symptomatic control
- Failure to tolerate corticosteroids due to significant adverse effects such as excessive weight gain, steroid-induced psychosis, osteoporosis, and worsening diabetic control
A small trial of 11 patients examined azathioprine as a steroid-sparing agent and found it as an acceptable immunosuppressive agent for that purpose [19]. It was associated with good safety profile and adherence to treatment was 82% (9 out of 11 patients). However, the small sample size makes it difficult to draw firm conclusions from the findings of this study. In another trial evaluating methotrexate as a steroid-sparing agent in the first year after the diagnosis of sarcoidosis, Baughman and colleagues [20] reported that methotrexate is an attractive alternative to other immunosuppressive agents in term of a steroid sparer. In this double-blind randomized controlled trial (RCT), 15 patients were studied with at least 6 months of treatment with methotrexate vs placebo. There was a significantly reduced dosage of prednisolone observed in methotrexate group. However, the difference was not significant when data were analyzed for all patients, including the dropouts. More recently, a large international retrospective cohort study of 200 patients with sarcoidosis demonstrated that both methotrexate and azathioprine have similar efficacy and steroid-sparing capacity in sarcoidosis [21]. However, infection rates were significantly higher in the azathioprine group as compared to methotrexate (34.6% vs. 18.1%, P = 0.01). Hence methotrexate should be considered as a preferred second-line agent in sarcoidosis after detailed discussion about potential side effects.
Case Continued
The patient was started on methotrexate after discussion about the potential adverse effects of bone marrow suppression, hepatotoxicity, and pneumonitis. He was screened for latent tuberculosis and viral hepatitis prior to starting methotrexate. The dosage was 7.5 mg per week along with folic acid once a week. We gradually increase the dose in increments of 2.5 mg every 2 weeks with a view to reach 15 mg every week as maintenance therapy. In severely obese patients, a dose of up to 20 mg weekly is occasionally considered if 15 mg is suboptimal after careful clinical assessment.
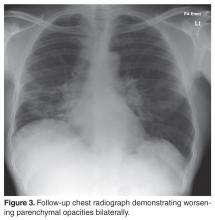
The patient failed to make significant progress after being on methotrexate for a period of 6 months and lung function tests continued to demonstrate a persistent decline with symptomatic worsening of dyspnea and cough.
What are treatment options in refractory sarcoidosis?
Options to consider in the setting of refractory sarcoidosis are leflunomide, hydroxychloroquine, or combination therapy of methotrexate and leflunomide. Leflunomide has been shown to be of similar efficacy to methotrexate as demonstrated by a retrospective analysis of 32 patients treated with the drug in a tertiary care center [22]. Complete or partial response was noted in 12 of 17 patients treated solely with leflunomide and 13 of 15 treated in conjunction with methotrexate. Hence, combination therapy has been suggested as a viable option for these patients who fail to respond to initial glucocorticoid agents and alternative immunosuppressive drugs, as combination therapy may enhance efficacy with reduced toxicity if considered in a rational manner after careful selection of patients [23].
How should the symptom of fatigue be addressed?
The patient had ongoing fatigue during the treatment period with corticosteroids and alternative immunosuppressants. Fatigue, noted in a majority of patients with sarcoidosis [24], is one of the commonest symptoms of sarcoidosis and one of the most difficult to treat. We recommend excluding alternative etiologies of fatigue when confronted with this symptom and evaluating for associated comorbidities such as thyroid dysfunction, vitamin D deficiency, and hypoadrenalism. Extra-pulmonary sarcoidosis seems to be associated with fatigue in comparison to sarcoidosis restricted to the pulmonary system [25]. Furthermore, there is a paucity of good quality data on the benefit of pharmacological intervention for treatment of fatigue. A small double-blind randomized study of 10 patients demonstrated a positive impact of treatment with dexmethylphenidate hydrochloride (d-MPH) for a period of 8 weeks [26]. However, the small sample and lack of long-term outcomes data make it difficult to draw firm conclusions based on the findings of this study and larger randomized trials are warranted to investigate this important aspect of sarcoidosis before recommending a pharmacological agent routinely for this disabling symptom.
What are the pharmacological agents for cutaneous sarcoidosis?
Corticosteroids (local and or systemic) are the mainstay of treatment in cutaneous sarcoidosis. However, patients who fail to respond to these agents or develop significant adverse effects should be offered second-line agents in the form of hydroxychloroquine/chloroquine, methotrexate, or leflunomide. It is important to acknowledge that the evidence of benefit for these agents is derived from uncontrolled studies [27–29]. Anti-malarial agents are usually well tolerated; patients do require a baseline ophthalmological assessment and subsequent periodic examinations to monitor for any ocular toxicity associated with their use. Leflunomide is also a second-line option in cutaneous disease and is associated with lesser toxicity than methotrexate [22]. More recently, topical tacrolimus has shown promising results in isolated case reports [30–33]. Hence, it may be considered a treatment option in refractory cutaneous sarcoidosis.
Is there a role for anti-TNF therapy in the management of sarcoidosis? Should it be considered for the case patient?
Tumour necrosis factor-α (TNF-α) is implicated in the pathogenesis of sarcoidosis and is believed to have a significant role in the inflammatory processes in sarcoidosis. It is released from alveolar macrophages of patients with active disease and is involved in formation and maintenance of granulomas in the lung tissue. There has been significant progress in therapeutic options alternative to traditional immunosuppressants such as corticosteroids and TNF-α inhibition has been on the horizon as a treatment strategy for few years. It may be considered as a steroid-sparing agent or a third-line treatment option in refractory cases.
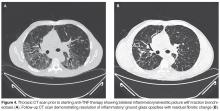
The initial evidence of benefit of anti-TNF therapy comes from case reports and small case series [34,35]. However, there have been 2 RCTs published so far invest-igating infliximab in sarcoidosis [36,37]. Baughman and colleagues [36] evaluated 138 patients with chronic pulmonary sarcoidosis in a double-blind study. There was a statistically significant improvement in FVC after 24 weeks of therapy with infliximab as compared to placebo (2.5% increase in mean FVC % predicted from baseline, P = 0.038). However, there was no improvement in any of the secondary outcome variables including SGRQ (St George’s Respiratory Questionnaire), 6-minute walk distance, and dyspnea scores. The benefit seemed to be more pronounced in the severe disease category on post-hoc analysis. The clinical significance of these findings are however unclear and it is difficult to draw firm conclusions based on the findings of this study alone.
The other RCT exploring the role of infliximab in sarcoidosis was conducted by Rossman et al [37]. This multi-center phase II study was conducted to evaluate the safety and tolerability of the drug in active pulmonary sarcoidosis (stage II to IV). The trial had a small number of participants with only 19 patients and failed to show a significant improvement in lung function after 6 weeks of treatment. Furthermore, 4 patients developed serious adverse events after treatment. Doty and colleagues [34] retrospectively analysed 10 patients treated with infliximab for refractory sarcoidosis and found subjective and objective evidence of improvement in all patients. This therapy resulted in reduction of corticosteroid dosage in 83% of cases (5 out of 6). However, adverse reactions were noted in 3 cases, including development of angioimmunoblastic lymphoma in one case. A retrospective study of 16 consecutive unselected cases of refractory sarcoidosis by Chapelon-Abric and colleagues [38] demonstrated a positive response in a majority of cases. However 38% of patients experienced a relapse. Furthermore, 44% of patients (7 out of 16) had infectious complications associated with anti-TNF therapy. Finally, infliximab may be used successfully in treating severe small fibre neuropathy [39] and should be considered in refractory cases where neuropathy is associated with autonomic dysfunction.
In summary, based on the findings of the aforementioned trials and case series, anti-TNF therapy may be considered as a treatment option in carefully selected patients after discussion of the potential adverse effects, followed by close monitoring at a specialist center for the management of sarcoidosis. Furthermore, anti-TNF therapy should not delay referral for lung transplant assessment, particularly when disease progression is relatively rapid. Duration of treatment and timing of thoracic imaging after anti-TNF therapy is subject to debate and should be individualized in close collaboration with a specialist center.
Case Continued
What is the role of newer biologics in the treatment of sarcoidosis? And what other therapies are on the horizon?
Although sarcoidosis is a T cell–mediated disease, humoral immunity has been implicated in sarcoidosis [40] and B cell depletion by rituximab (anti-CD 20+chimeric monoclonal antibody) has been successfully utilized in T cell–mediated diseases such as rheumatoid arthritis. Rituximab has been studied in phase I/II trial [41] in patients with refractory pulmonary sarcoidosis. The response to rituximab was inconsistent in these patients, with only a small group demonstrating > 5% improvement in FVC or walking distance. Hence, further studies are required to demonstrate a significant clinical benefit of this treatment in refractory sarcoidosis and potentially identify the characteristics of patients that may respond to B-cell depletion.
Adalimumab is another biologic agent that may be a potential option in refractory sarcoidosis as demonstrated in an open-label study of 11 patients [42]. This small trial of 52 weeks’ duration showed that adalimumab is well tolerated and may be considered in refractory pulmonary sarcoidosis when other treatment options have been exhausted.
Acthar had been used to treat pulmonary sarcoidosis in 1950s and there has been recent interest in evaluating the value of acthar gel therapy in the management of sarcoidosis. Baughman and colleagues [43] carried out a retrospective analysis of 47 patients with advanced sarcoidosis treated with acthar gel. The results showed that there was an objective improvement in approximately a third of patients receiving at least 3 months of treatment. Thirty-six percent of patients (n = 17) managed to reduce their oral corticosteroid dosage by more than 50% while on this therapy. However, a significant proportion of patients were unable to take ≥ 3 months of treatment, suggesting poor tolerance/adherence. The utility of acthar gel therapy would need to be examined in larger prospective randomized trial before it can be recommended for a treatment option in advanced disease.
Should pneumocystis pneumonia (PCP) prophylaxis be considered in sarcoidosis?
We do not routinely consider PCP prophylaxis in all sarcoidosis patients. However, it should be considered in the following clinical situations:
- Failure to reduce oral corticosteroid dose to less than 20 mg of prednisolone daily
- Concomitant use of anti-TNF agents with relatively high maintenance dose of oral corticosteroids (≥ 20 mg per day)
- Significant comorbid condition in association with sarcoidosis resulting in significant level of immunosuppression
As our patient did not fall in any of the above categories, we did not offer him PCP prophylaxis. However, clinicians treating patients with sarcoidosis with strong immunosuppressants should be aware of this potential complication and be vigilant about discussing prophylaxis with trimethoprim-sulphamethoxazole, which is the first-line agent for this purpose.
Is there a role of anti-tuberculous treatment in sarcoidosis?
Both mycobacterium tuberculosis (MTB) and non-mycobateria (NTM) have been implicated in sarcoidosis [44] and it is challenging to differentiate tuberculosis (TB) from sarcoidosis in certain clinical situations, such as when dealing with patients from countries with a high incidence of TB. The association of mycobacterium with sarcoidosis was explored in 2 recent trials of concomitant use of levofloxacin, ethambutol, azithromycin, and rifampin in cutaneous [45] and pulmonary sarcoidosis [46]. Drake and colleagues evaluated 15 chronic pulmonary sarcoidosis patients in an open-label trial to investigate if the combination of these drugs was associated with improvement in pulmonary sarcoidosis. The patients who completed 8 weeks of treatment had improvement in FVC at both 4 and 8 weeks of treatment. However, only 8 patients could complete 8 weeks of therapy, with significant adverse events. The small sample size limits our ability to draw meaningful conclusions and larger randomized trials are warranted to investigate this approach in sarcoidosis management.
Are there any valid serum biomarkers for sarcoidosis?
A biomarker is defined as a compound easily measurable in serum, urine, or other body fluids that can be used as indicator of presence and/or severity of particular disease state. Moreover, it helps in evaluation of effectiveness of drug therapy and useful to monitor the disease longitudinally. Unfortunately, there is no ideal serum biomarker in sarcoidosis. The most widely evaluated marker is serum angiotensin-converting enzyme (ACE). It has been found to be elevated in three quarters of patients with sarcoidosis [47]. However, it has poor diagnostic utility due to limited sensitivity and specificity. Furthermore, levels of serum ACE are reduced in patients taking ACE inhibitors and hence measurement of ACE levels in patients taking ACE inhibitors may lead to inaccurate interpretations [48].
A number of other serum biomarkers have been evaluated in sarcoidosis. Gungor and colleagues [49] evaluated 48 patients with sarcoidosis and 20 healthy controls. The biomarkers measured were ACE, adenosine deaminase (ADA), total IgE, serum amyloid-A (SAA), soluble interleukin-2 receptor (sIL2R), and C-reactive protein (CRP). This study showed that SAA was significantly elevated in sarcoidosis as compared to controls (P < 0.001). Furthermore, sIL2R levels were raised in extra-pulmonary sarcoidosis (P < 0.014). In another study, Grutters and co-workers [50] demonstrated elevated levels of sIL2R in 47 patients with active sarcoidosis. However, the levels did not correlate with radiolographic or physiological outcomes or response to treatment. Hence, the utility of these biomarkers in clinical practice is questionable and larger longitudinal studies are required to demonstrate the actual benefit of these biomarkers in everyday clinical practice.
There is a complex relationship between vitamin D and calcium metabolism and risk of osteoporosis in sarcoidosis. The value of active vitamin D metabolite 1, 25-dihydroxy vitamin D in relation to the degree of sarcoidosis disease chronicity was evaluated in a study of 59 patients with sarcoidosis [51]. It was noted that increased levels of 1,25 vitamin D were associated with increased risk of chronic phenotype and the need to require repeated treatments with immunosuppressive agents. Hence, serum levels of 1,25 vitamin D may have a prognostic value in sarcoidosis. It is important to note that both vitamin D deficiency as well as vitamin D excess can result in osteoporosis [52], and there is a risk of bone fragility and fractures in patients who may need long-term oral corticosteroid therapy. Hence, an optimal level of vitamin D is crucial; we recommend a value of serum 25(OH) vitamin D between 10 and 20 ng/mL as evidenced by a cross-sectional analysis of 142 consecutive patients with biopsy-proven sarcoidosis [53]. The above range of 25(OH) vitamin D was associated with higher bone mineral density and values above 20 ng/mL resulted in increased risk of fractures, demonstrating the need to keep vitamin D levels of these patients to be lower than those recommended for general population.
When should a patient with sarcoidosis be referred for lung transplantation?
Lung transplantation is a treatment option in advanced/end-stage disease after pharmacologic treatments have been exhausted and there is no evidence of progressive or severe extrapulmonary disease. The most critical decision regarding transplantation in pulmonary sarcoidosis is the timing of the referral and close liaison with the transplant center to ensure the maximal chance of success with lung transplantation. We recommend considering transplant referral when % predicted FVC approaches a value of 50% or less with or without significant pulmonary hypertension. Single lung transplant is appropriate for the majority of patients with sarcoidosis. However, bilateral transplant should be considered in bilateral mycetomas and bilateral bronchiectasis. Arcasoy and colleagues [54] analysed 43 patients listed for transplantation and found the following factors associated with increased risk of mortality:
- pulmonary hypertension
- hypoxia
- low cardiac output
- elevated right atrial pressure
It is important to note that pulmonary function parameters have not been found to be predictive of mortality in sarcoidosis [54,55]. Although granuloma recurrence in transplanted lung has been observed, it is a rare to have organ failure secondary to recurrence, and lung transplant should be considered in refractory cases of pulmonary sarcoidosis in the absence of contraindications and close liaison with the transplant center at the earliest opportunity is recommended.
What is the optimal duration of clinical follow-up in sarcoidosis?
There is no consensus on appropriate follow-up duration once a diagnosis of sarcoidosis is confirmed, and it is dependent on physician preference, vital organ involvement, disease progression, need for pharmacological treatment, and availability of resources. The American Thoracic Society statement on sarcoidosis suggested at least 3 years of follow-up after corticosteroid treatment is completed irrespective of radiographic stage [56]. Patients with serious extrapulmonary symptoms would require longer-term follow-up and a recent single-center Japanese study of corticosteroid-naive patients showed that the number of organs involved at the outset dictates the cumulative risk of subsequent progression of sarcoidosis [57]. This study of 150 patients with sarcoidosis with a median follow-up of 7.7 years demonstrated significantly increased risk of progression when there were > 3 organ systems involved as compared to ≤ 3 involved. These corticosteroid-naive patients may require longer follow-up (up to 10 years) than previously thought. However, the findings of this study would require confirmation in multi-center and multi-ethnic cohort of sarcoidosis patients.
Summary
Sarcoidosis presents as a fascinating and challenging disease with myriad clinical, radiological, and pathological manifestations. Despite extensive research in the last decade to increase our understanding of the mechanisms of disease evolution and progression, the underlying etiology remains unidentified. The treatment paradigm has changed over the last 10 years with the introduction of biological agents such as infliximab and adalimumab [42]; these anti-TNF drugs offer a treatment option in refractory cases of sarcoidosis with or without corticosteroids and other anti-metabolites. Methotrexate is the preferred first-line immunosuppressive agent after corticosteroids and has a safer adverse effect profile in comparison to azathioprine. Larger randomized studies to evaluate the efficacy of antimycobacterial agents and newer biologics are warranted before making strong recommendations for the use of these drugs, as there are significant potential toxic effects associated with their use.
Corresponding author: Dr. Ahmed Fahim, Dept. of Respiratory Medicine, McHale Centre New Cross Hospital, Wolverhampton, UK WV10 0QP, [email protected].
Financial disclosures: None.
1. Kitamura AY, Takiguchi K, Kurosu N, et al. Feasibility of cytological diagnosis of sarcoidosis with endobronchial US-guided transbronchial aspiration. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:82–9.
2. Chee A, Khalil M, Stather DR, et al. Cytologic assessment of endobronchial ultrasound-guided transbronchial needle aspirates in sarcoidosis. J Bronchology Interv Pulmonol 2012;19:24–8.
3. Oki M, Saka H, Kitagawa C, et al. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg 2012;143:1324–9.
4. Plit ML, Havryk AP, Hodgson A, et al. Rapid cytological analysis of endobronchial ultrasound-guided aspirates in sarcoidosis. Eur Respir J 2013;42:1302-8.
5. von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457–64.
6. Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302.
7. Ishimaru S, Tsujino I, Sakaue S, et al. Combination of 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in assessing cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:234–5.
8. Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241–8.
9. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36.
10. Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J 2013;41:1424–8.
11. James DG, Carstairs LS, Trowell J, Sharma OP. Treatment of sarcoidosis. Report of a controlled therapeutic trial. Lancet 1967;2:526–8.
12. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Study Group. Chest 1999;116:424–31.
13. Eule H, Roth I, Ehrke I, Weinecke W. Corticosteroid therapy of intrathoracic sarcoidosis stages I and II--results of a controlled clinical trial. Z Erkr Atmungsorgane 1977;149:142–7.
14. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis 1973;107:609–14.
15. Zaki MH, Lyons HA, Leilop L, Huang CT. Corticosteroid therapy in sarcoidosis. A five-year, controlled follow-up study. N Y State J Med 1987;87:496–9.
16. Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax 1996;51:238–47.
17. Kamphuis LS, Bonte-Mineur F, van Laar JA, et al. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res 2014;29:2498–503.
18. Baughman RP, Janovcik J, Ray M, et al. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:113–20.
19. Muller-Quernheim J, Kienast K, Held M, et al. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999;14:1117–22.
20. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:60–6.
21. Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate versus azathioprine in second line therapy of sarcoidosis. Chest 2013.
22. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:43–8.
23. Baughman RP, Ohmichi M, Lower EE. Combination therapy for sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001;18:133–7.
24. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J 2012;40:255–63.
25. Fleischer M, Hinz A, Brahler E, et al. Factors associated with fatigue in sarcoidosis. Respir Care 2014;59:1086–94.
26. Lower EE, Harman S, Baughman RP. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest 2008;133:1189–95.
27. Veien NK, Brodthagen H. Cutaneous sarcoidosis treated with methotrexate. Br J Dermatol 1977;97:213–6.
28. Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995;155:846–51.
29. Kaye O, Palazzo E, Grossin M, et al. Low-dose methotrexate: an effective corticosteroid-sparing agent in the musculoskeletal manifestations of sarcoidosis. Br J Rheumatol 1995;34:642–4.
30. Green CM. Topical tacrolimus for the treatment of cutaneous sarcoidosis. Clin Exp Dermatol 2007:32:457–8.
31. Vano-Galvan S, Fernandez-Guarino M, Carmona LP, et al. Lichenoid type of cutaneous sarcoidosis: great response to topical tacrolimus. Eur J Dermatol 2008;18:89–90.
32. Gutzmer R, Volker B, Kapp A, Werfel T. [Successful topical treatment of cutaneous sarcoidosis with tacrolimus]. Hautarzt 2003;54:1193–7.
33. Katoh N, Mihara H, Yasuno H. Cutaneous sarcoidosis successfully treated with topical tacrolimus. Br J Dermatol 2002;147:154–6.
34. Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127:1064–71.
35. Berrios I, Jun-O’Connell A, Ghiran S, Ionete C. A case of neurosarcoidosis secondary to treatment of etanercept and review of the literature. BMJ Case Rep 2015 Jul 6;2015.
36. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802.
37. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:201–8.
38. Chapelon-Abric D, Saadoun D, Biard L. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol 2015;33:509–15.
39. Hoitsma E, Faber CG, Santen-Hoeufft M, et al. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment with infliximab. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:73–7.
40. Lee NS, Barber L, Kanchwala A, et al. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol 2011;18:223–34.
41. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 2014;43:1525–8.
42. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy Results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:46–54.
43. Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med 2016;110:66–72.
44. Mangiapan G, Hance AJ. Mycobacteria and sarcoidosis: an overview and summary of recent molecular biological data. Sarcoidosis 1995;12:20-37.
45. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol 2013;149:1040–9.
46. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:201–11.
47. Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis--its value in present clinical practice. Ann Clin Biochem 1989;26 ( Pt 1):13–8.
48. Krasowski MD, Savage J, Ehlers A, et al. Ordering of the serum angiotensin-converting enzyme test in patients receiving angiotensin-converting enzyme inhibitor therapy: an avoidable but common error. Chest 2015;148:1447–53.
49. Gungor S, Ozseker F, Yalcinsoy M. Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol 2015;25:174–9.
50. Grutters JC, Fellrath JM, Mulder L, et al. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest 2003;124:186–95.
51. Kavathia D, Buckley JD, Rao D, et al. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med 2010;104:564–70.
52. Baughman RP, Lower EE. Goldilocks, vitamin D and sarcoidosis. Arthritis Res Ther 2014;16:111.
53. Saidenberg-Kermanac’h N, Semerano L, Nunes H, et al. Bone fragility in sarcoidosis and relationships with calcium metabolism disorders: a cross sectional study on 142 patients. Arthritis Res Ther 2014;16:R78.
54. Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 2001;120:873–80.
55. Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 2003;124:922–8.
56. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149–73.
57. Inoue Y, Inui N, Hashimoto D, et al. Cumulative incidence and predictors of progression in corticosteroid-naive patients with sarcoidosis. PLoS One 2015;10:e0143371.
From the New Cross Hospital, Wolverhampton, UK.
Abstract
- Objective: To discuss the management of sarcoidosis.
- Methods: Review of the literature.
- Results: Sarcoidosis is a challenging multisystem disorder of uncertain etiology characterized by granulomatous inflammation in the affected organs. Treatment is dependent on the severity of disease and organ involvement at the time of diagnosis. Glucocorticoids have traditionally been considered first-line pharmacologic treatment; however, a significant proportion of patients do not require drug treatment due to the propensity toward spontaneous disease remission. Treated patients who fail to respond to corticosteroids or develop significant adverse effects can be offered a second-line agent, eg, methotrexate. Anti-TNF therapy may be considered as a treatment option in carefully selected patients with refractory disease after discussion of potential adverse effects followed by close monitoring at a specialist center.
- Conclusion: Further research into therapeutic options is likely to unveil novel agents with different mechanisms of action and better safety profiles than those seen with currently available immunosuppressive regimens.
Sarcoidosis is a multisystem disorder of uncertain etiology characterized by granulomatous inflammation in the affected organs. The diagnosis of sarcoidosis is best supported by histological evidence of noncaseating granuloma formation. It is a disease with a generally good prognosis; less than 5% patients die from the disease, with cause of death usually secondary to respiratory failure or cardiac or neurologic involvement. This review aims to discuss the management of sarcoidosis with a special emphasis on the management challenges resulting from the myriad clinical manifestations and potential complications seen in this chronic multisystem disease.
Case Study
Initial Presentation
A 40-year-old African-American man presents to his primary care physician with symptoms of fatigue, dry cough, exertional breathlessness, dry and painful eyes, generalized arthralgia, and multiple skin lesions for 3 months. He has a history of essential hypertension and is a former smoker with 10 pack-year history. He is not on any regular medications. Examination reveals bilateral cervical lymphadenopathy and multiple skin lesions on trunk. The rest of the systemic examination (including respiratory and cardiovascular system) is normal.
Workup
The patient’s initial bloodwork showed a mild degree of lymphopenia (1.1 × 109/L, normal range 1.5–4.5). Other bloodwork results, bone profile and immunology screen (including ANA, rheumatoid factor, immunoglobulins, and extractable nuclear antigen antibodies) were negative. Angiotensin-converting enzyme (ACE) was elevated at 149 U/L (normal range 5–58), while serum calcium and vitamin D levels (including vitamin D3) were normal. The findings on CT scan along with the biochemical profile suggest a plausible diagnosis of sarcoidosis.
What is the next step in the workup to establish the suspected diagnosis?
The radiological findings of hilar lymphadenopathy are not confirmatory. There are a number of entities in the differential diagnoses, including tuberculosis, malignancy, lymphoma, and other granulomatous disorders such as histoplasmosis, schistosomiasis, and blastomycosis. It is important to obtain histological evidence before a definitive diagnosis of sarcoidosis can be made. This is necessary as management differs for each of the diagnostic categories mentioned above. Furthermore, diagnostic confirmation would be helpful later in the disease course if the patient develops any associated complications such as pulmonary hypertension or respiratory failure and/or need for lung transplant assessment.
As this patient had palpable cervical lymphadenopathy, an ultrasound guided biopsy of the lymph node was obtained. The histological examination demonstrated evidence of noncaseating granulomas that were well formed and highly consistent with the suspected clinical and radiological diagnosis of sarcoidosis. In addition, the special stains for acid-fast bacilli and other infections including fungi were negative.
Our practice is to evaluate patients with suspected sarcoidosis with neck ultrasound and tru-cut biopsy of cervical lymph nodes (if appropriate) as a first-line investigation, as it is less invasive than bronchoscopy/endoscopic ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) or thoracoscopic lung biopsy. The diagnostic yield of EBUS-TBNA has been evaluated in a number of studies with variable results [1–5]. A large multicenter randomized clinical trial [5] of 304 patients investigated the diagnostic yield of endosonography (endobronchial and esophageal ultrasound) in comparison with bronchoscopy with transbronchial biopsy (TBB) and endobronchial biopsy. The study cohort was made up of patients with stage I/II sarcoidosis. The results showed that endosonography had a higher diagnostic yield to detect granulomas (80% vs 53%; P < 0.001) and there were no serious adverse events related to endoscopy. Hence, ultrasound-guided endoscopic procedures are becoming common first-line investigations for sarcoidosis in the absence of other readily identifiable biopsy sites such as peripheral lymph nodes in the cervical area.
What should be done about the cutaneous and ophthalmologic symptoms in this patient?
As sarcoidosis commonly involves eyes and skin (after pulmonary involvement, which is seen in 90% of cases), the patient was referred to ophthalmology and dermatology departments for further evaluation. These assessments confirmed him to have bilateral uveitis and skin involvement with granulomatous inflammation consistent with ocular and cutaneous sarcoidosis respectively. Hence, the diagnosis of multisystem sarcoidosis was made. At this stage, the patient also mentioned symptoms of intermittent palpitations for 3 months’ duration and feeling of missing a beat, so an urgent cardiological evaluation was undertaken that showed him to have ectopic beats on Holter monitoring. However, his trans-thoracic echocardiogram and a cardiac MRI scan were normal with good biventricular function, excluding cardiac sarcoidosis as a cause of his palpitations. Cardiac involvement with sarcoidosis is clinically apparent in only 5% of cases and presents as cardiomyopathy and or cardiac arrhythmias (both tachy and bradyarrythmias). As cardiac involvement with sarcoid granulomas is usually patchy, endomyocardial biopsy has a limited diagnostic yield of < 20% [6]. In this particular case, endomyocardial biopsy was not attempted in view of normal cardiac MR and echocardiography as well as no significant cardiac dysrythmia on holter monitoring.
Case Continued
The patient was started on corticosteroid eye drops and steroid ointment for his ophthalmologic and cutaneous sarcoidosis, respectively, and symptoms gradually improved over the next few months. As the patient was symptomatic with cough and breathlessness and there was evidence of reduction in FVC (along with reduced DLco), a trial of oral corticosteroids was considered to treat the pulmonary sarcoidosis.
What is first-line pharmacological treatment for sarcoidosis and when is it indicated?
Treatment of sarcoidosis is dependent upon the severity of disease and organ involvement at the time of the diagnosis. Glucocorticoids have traditionally been considered first-line pharmacological agents in selective cases, as a significant proportion of patients do not require drug treatment due to the propensity for spontaneous remission. Furthermore, sarcoidosis remains stable without anti-inflammatory/immunosuppressive therapies in a majority of patients. A number of clinical trials have evaluated the value of corticosteroids in the management of sarcoidosis, with variable outcomes [10–16]. The disease tends to be severe in patients of African descent compared with patients from other racial backgrounds. The European cohort of sarcoidosis patients generally have milder disease with less propensity for vital organ involvement such as cardiac or central nervous system (CNS) disease.
The decision to initiate corticosteroids for sarcoidosis is not a straightforward one as there is variability in symptom presentation, disease severity, and response to corticosteroids. We initiate first-line therapy with oral prednisolone in the following circumstances:
- Evidence of pulmonary impairment (forced vital capacity FVC < 80% predicted) with or without reduction in gas transfer (DLco) along with respiratory symptoms of cough, chest pain, and/or breathlessness (as seen in the case patient)
- Vital organ involvement such as cardiac, ophthalmic (such as panuveitis) or CNS sarcoidosis once confirmed by respective investigations
- Selective cases of sarcoid-associated pulmonary hypertension (SAPH) along with close liaison with pulmonary hypertension specialists
We recommend an initial starting dose of 20 to 40 mg of prednisolone for a period of 1 to 3 months, followed by maintenance dose of 10 mg or less for a further 6 to 9 months, aiming for a total duration of treatment of 12 months. However, the duration may vary depending on the response and any associated adverse effects with corticosteroids. It is usual practice to supplement with calcium and vitamin D when beginning patients on oral corticosteroids due to the potential risk of osteoporosis. However, this may result in significant hypercalcemia, which itself may be an endocrine manifestation of sarcoidosis. Hence, we recommend monitoring serum calcium during treatment and supplement vitamin D in patients who are vitamin D–deficient [17]. Furthermore, serum vitamin 1,25(OH)2 vitamin D3 has been demonstrated to the best available test to evaluate vitamin D status in sarcoidosis [18].
Case Continued
What are the preferred pharmacological agents for second-line treatment in sarcoidosis?
Alternative immunosuppression should be considered in the following circumstances in patients diagnosed with sarcoidosis:
- Failure or less than optimal response to oral corticosteroids
- Use as a steroid-sparing agent in patients requiring high doses of steroids for symptomatic control
- Failure to tolerate corticosteroids due to significant adverse effects such as excessive weight gain, steroid-induced psychosis, osteoporosis, and worsening diabetic control
A small trial of 11 patients examined azathioprine as a steroid-sparing agent and found it as an acceptable immunosuppressive agent for that purpose [19]. It was associated with good safety profile and adherence to treatment was 82% (9 out of 11 patients). However, the small sample size makes it difficult to draw firm conclusions from the findings of this study. In another trial evaluating methotrexate as a steroid-sparing agent in the first year after the diagnosis of sarcoidosis, Baughman and colleagues [20] reported that methotrexate is an attractive alternative to other immunosuppressive agents in term of a steroid sparer. In this double-blind randomized controlled trial (RCT), 15 patients were studied with at least 6 months of treatment with methotrexate vs placebo. There was a significantly reduced dosage of prednisolone observed in methotrexate group. However, the difference was not significant when data were analyzed for all patients, including the dropouts. More recently, a large international retrospective cohort study of 200 patients with sarcoidosis demonstrated that both methotrexate and azathioprine have similar efficacy and steroid-sparing capacity in sarcoidosis [21]. However, infection rates were significantly higher in the azathioprine group as compared to methotrexate (34.6% vs. 18.1%, P = 0.01). Hence methotrexate should be considered as a preferred second-line agent in sarcoidosis after detailed discussion about potential side effects.
Case Continued
The patient was started on methotrexate after discussion about the potential adverse effects of bone marrow suppression, hepatotoxicity, and pneumonitis. He was screened for latent tuberculosis and viral hepatitis prior to starting methotrexate. The dosage was 7.5 mg per week along with folic acid once a week. We gradually increase the dose in increments of 2.5 mg every 2 weeks with a view to reach 15 mg every week as maintenance therapy. In severely obese patients, a dose of up to 20 mg weekly is occasionally considered if 15 mg is suboptimal after careful clinical assessment.
The patient failed to make significant progress after being on methotrexate for a period of 6 months and lung function tests continued to demonstrate a persistent decline with symptomatic worsening of dyspnea and cough.
What are treatment options in refractory sarcoidosis?
Options to consider in the setting of refractory sarcoidosis are leflunomide, hydroxychloroquine, or combination therapy of methotrexate and leflunomide. Leflunomide has been shown to be of similar efficacy to methotrexate as demonstrated by a retrospective analysis of 32 patients treated with the drug in a tertiary care center [22]. Complete or partial response was noted in 12 of 17 patients treated solely with leflunomide and 13 of 15 treated in conjunction with methotrexate. Hence, combination therapy has been suggested as a viable option for these patients who fail to respond to initial glucocorticoid agents and alternative immunosuppressive drugs, as combination therapy may enhance efficacy with reduced toxicity if considered in a rational manner after careful selection of patients [23].
How should the symptom of fatigue be addressed?
The patient had ongoing fatigue during the treatment period with corticosteroids and alternative immunosuppressants. Fatigue, noted in a majority of patients with sarcoidosis [24], is one of the commonest symptoms of sarcoidosis and one of the most difficult to treat. We recommend excluding alternative etiologies of fatigue when confronted with this symptom and evaluating for associated comorbidities such as thyroid dysfunction, vitamin D deficiency, and hypoadrenalism. Extra-pulmonary sarcoidosis seems to be associated with fatigue in comparison to sarcoidosis restricted to the pulmonary system [25]. Furthermore, there is a paucity of good quality data on the benefit of pharmacological intervention for treatment of fatigue. A small double-blind randomized study of 10 patients demonstrated a positive impact of treatment with dexmethylphenidate hydrochloride (d-MPH) for a period of 8 weeks [26]. However, the small sample and lack of long-term outcomes data make it difficult to draw firm conclusions based on the findings of this study and larger randomized trials are warranted to investigate this important aspect of sarcoidosis before recommending a pharmacological agent routinely for this disabling symptom.
What are the pharmacological agents for cutaneous sarcoidosis?
Corticosteroids (local and or systemic) are the mainstay of treatment in cutaneous sarcoidosis. However, patients who fail to respond to these agents or develop significant adverse effects should be offered second-line agents in the form of hydroxychloroquine/chloroquine, methotrexate, or leflunomide. It is important to acknowledge that the evidence of benefit for these agents is derived from uncontrolled studies [27–29]. Anti-malarial agents are usually well tolerated; patients do require a baseline ophthalmological assessment and subsequent periodic examinations to monitor for any ocular toxicity associated with their use. Leflunomide is also a second-line option in cutaneous disease and is associated with lesser toxicity than methotrexate [22]. More recently, topical tacrolimus has shown promising results in isolated case reports [30–33]. Hence, it may be considered a treatment option in refractory cutaneous sarcoidosis.
Is there a role for anti-TNF therapy in the management of sarcoidosis? Should it be considered for the case patient?
Tumour necrosis factor-α (TNF-α) is implicated in the pathogenesis of sarcoidosis and is believed to have a significant role in the inflammatory processes in sarcoidosis. It is released from alveolar macrophages of patients with active disease and is involved in formation and maintenance of granulomas in the lung tissue. There has been significant progress in therapeutic options alternative to traditional immunosuppressants such as corticosteroids and TNF-α inhibition has been on the horizon as a treatment strategy for few years. It may be considered as a steroid-sparing agent or a third-line treatment option in refractory cases.
The initial evidence of benefit of anti-TNF therapy comes from case reports and small case series [34,35]. However, there have been 2 RCTs published so far invest-igating infliximab in sarcoidosis [36,37]. Baughman and colleagues [36] evaluated 138 patients with chronic pulmonary sarcoidosis in a double-blind study. There was a statistically significant improvement in FVC after 24 weeks of therapy with infliximab as compared to placebo (2.5% increase in mean FVC % predicted from baseline, P = 0.038). However, there was no improvement in any of the secondary outcome variables including SGRQ (St George’s Respiratory Questionnaire), 6-minute walk distance, and dyspnea scores. The benefit seemed to be more pronounced in the severe disease category on post-hoc analysis. The clinical significance of these findings are however unclear and it is difficult to draw firm conclusions based on the findings of this study alone.
The other RCT exploring the role of infliximab in sarcoidosis was conducted by Rossman et al [37]. This multi-center phase II study was conducted to evaluate the safety and tolerability of the drug in active pulmonary sarcoidosis (stage II to IV). The trial had a small number of participants with only 19 patients and failed to show a significant improvement in lung function after 6 weeks of treatment. Furthermore, 4 patients developed serious adverse events after treatment. Doty and colleagues [34] retrospectively analysed 10 patients treated with infliximab for refractory sarcoidosis and found subjective and objective evidence of improvement in all patients. This therapy resulted in reduction of corticosteroid dosage in 83% of cases (5 out of 6). However, adverse reactions were noted in 3 cases, including development of angioimmunoblastic lymphoma in one case. A retrospective study of 16 consecutive unselected cases of refractory sarcoidosis by Chapelon-Abric and colleagues [38] demonstrated a positive response in a majority of cases. However 38% of patients experienced a relapse. Furthermore, 44% of patients (7 out of 16) had infectious complications associated with anti-TNF therapy. Finally, infliximab may be used successfully in treating severe small fibre neuropathy [39] and should be considered in refractory cases where neuropathy is associated with autonomic dysfunction.
In summary, based on the findings of the aforementioned trials and case series, anti-TNF therapy may be considered as a treatment option in carefully selected patients after discussion of the potential adverse effects, followed by close monitoring at a specialist center for the management of sarcoidosis. Furthermore, anti-TNF therapy should not delay referral for lung transplant assessment, particularly when disease progression is relatively rapid. Duration of treatment and timing of thoracic imaging after anti-TNF therapy is subject to debate and should be individualized in close collaboration with a specialist center.
Case Continued
What is the role of newer biologics in the treatment of sarcoidosis? And what other therapies are on the horizon?
Although sarcoidosis is a T cell–mediated disease, humoral immunity has been implicated in sarcoidosis [40] and B cell depletion by rituximab (anti-CD 20+chimeric monoclonal antibody) has been successfully utilized in T cell–mediated diseases such as rheumatoid arthritis. Rituximab has been studied in phase I/II trial [41] in patients with refractory pulmonary sarcoidosis. The response to rituximab was inconsistent in these patients, with only a small group demonstrating > 5% improvement in FVC or walking distance. Hence, further studies are required to demonstrate a significant clinical benefit of this treatment in refractory sarcoidosis and potentially identify the characteristics of patients that may respond to B-cell depletion.
Adalimumab is another biologic agent that may be a potential option in refractory sarcoidosis as demonstrated in an open-label study of 11 patients [42]. This small trial of 52 weeks’ duration showed that adalimumab is well tolerated and may be considered in refractory pulmonary sarcoidosis when other treatment options have been exhausted.
Acthar had been used to treat pulmonary sarcoidosis in 1950s and there has been recent interest in evaluating the value of acthar gel therapy in the management of sarcoidosis. Baughman and colleagues [43] carried out a retrospective analysis of 47 patients with advanced sarcoidosis treated with acthar gel. The results showed that there was an objective improvement in approximately a third of patients receiving at least 3 months of treatment. Thirty-six percent of patients (n = 17) managed to reduce their oral corticosteroid dosage by more than 50% while on this therapy. However, a significant proportion of patients were unable to take ≥ 3 months of treatment, suggesting poor tolerance/adherence. The utility of acthar gel therapy would need to be examined in larger prospective randomized trial before it can be recommended for a treatment option in advanced disease.
Should pneumocystis pneumonia (PCP) prophylaxis be considered in sarcoidosis?
We do not routinely consider PCP prophylaxis in all sarcoidosis patients. However, it should be considered in the following clinical situations:
- Failure to reduce oral corticosteroid dose to less than 20 mg of prednisolone daily
- Concomitant use of anti-TNF agents with relatively high maintenance dose of oral corticosteroids (≥ 20 mg per day)
- Significant comorbid condition in association with sarcoidosis resulting in significant level of immunosuppression
As our patient did not fall in any of the above categories, we did not offer him PCP prophylaxis. However, clinicians treating patients with sarcoidosis with strong immunosuppressants should be aware of this potential complication and be vigilant about discussing prophylaxis with trimethoprim-sulphamethoxazole, which is the first-line agent for this purpose.
Is there a role of anti-tuberculous treatment in sarcoidosis?
Both mycobacterium tuberculosis (MTB) and non-mycobateria (NTM) have been implicated in sarcoidosis [44] and it is challenging to differentiate tuberculosis (TB) from sarcoidosis in certain clinical situations, such as when dealing with patients from countries with a high incidence of TB. The association of mycobacterium with sarcoidosis was explored in 2 recent trials of concomitant use of levofloxacin, ethambutol, azithromycin, and rifampin in cutaneous [45] and pulmonary sarcoidosis [46]. Drake and colleagues evaluated 15 chronic pulmonary sarcoidosis patients in an open-label trial to investigate if the combination of these drugs was associated with improvement in pulmonary sarcoidosis. The patients who completed 8 weeks of treatment had improvement in FVC at both 4 and 8 weeks of treatment. However, only 8 patients could complete 8 weeks of therapy, with significant adverse events. The small sample size limits our ability to draw meaningful conclusions and larger randomized trials are warranted to investigate this approach in sarcoidosis management.
Are there any valid serum biomarkers for sarcoidosis?
A biomarker is defined as a compound easily measurable in serum, urine, or other body fluids that can be used as indicator of presence and/or severity of particular disease state. Moreover, it helps in evaluation of effectiveness of drug therapy and useful to monitor the disease longitudinally. Unfortunately, there is no ideal serum biomarker in sarcoidosis. The most widely evaluated marker is serum angiotensin-converting enzyme (ACE). It has been found to be elevated in three quarters of patients with sarcoidosis [47]. However, it has poor diagnostic utility due to limited sensitivity and specificity. Furthermore, levels of serum ACE are reduced in patients taking ACE inhibitors and hence measurement of ACE levels in patients taking ACE inhibitors may lead to inaccurate interpretations [48].
A number of other serum biomarkers have been evaluated in sarcoidosis. Gungor and colleagues [49] evaluated 48 patients with sarcoidosis and 20 healthy controls. The biomarkers measured were ACE, adenosine deaminase (ADA), total IgE, serum amyloid-A (SAA), soluble interleukin-2 receptor (sIL2R), and C-reactive protein (CRP). This study showed that SAA was significantly elevated in sarcoidosis as compared to controls (P < 0.001). Furthermore, sIL2R levels were raised in extra-pulmonary sarcoidosis (P < 0.014). In another study, Grutters and co-workers [50] demonstrated elevated levels of sIL2R in 47 patients with active sarcoidosis. However, the levels did not correlate with radiolographic or physiological outcomes or response to treatment. Hence, the utility of these biomarkers in clinical practice is questionable and larger longitudinal studies are required to demonstrate the actual benefit of these biomarkers in everyday clinical practice.
There is a complex relationship between vitamin D and calcium metabolism and risk of osteoporosis in sarcoidosis. The value of active vitamin D metabolite 1, 25-dihydroxy vitamin D in relation to the degree of sarcoidosis disease chronicity was evaluated in a study of 59 patients with sarcoidosis [51]. It was noted that increased levels of 1,25 vitamin D were associated with increased risk of chronic phenotype and the need to require repeated treatments with immunosuppressive agents. Hence, serum levels of 1,25 vitamin D may have a prognostic value in sarcoidosis. It is important to note that both vitamin D deficiency as well as vitamin D excess can result in osteoporosis [52], and there is a risk of bone fragility and fractures in patients who may need long-term oral corticosteroid therapy. Hence, an optimal level of vitamin D is crucial; we recommend a value of serum 25(OH) vitamin D between 10 and 20 ng/mL as evidenced by a cross-sectional analysis of 142 consecutive patients with biopsy-proven sarcoidosis [53]. The above range of 25(OH) vitamin D was associated with higher bone mineral density and values above 20 ng/mL resulted in increased risk of fractures, demonstrating the need to keep vitamin D levels of these patients to be lower than those recommended for general population.
When should a patient with sarcoidosis be referred for lung transplantation?
Lung transplantation is a treatment option in advanced/end-stage disease after pharmacologic treatments have been exhausted and there is no evidence of progressive or severe extrapulmonary disease. The most critical decision regarding transplantation in pulmonary sarcoidosis is the timing of the referral and close liaison with the transplant center to ensure the maximal chance of success with lung transplantation. We recommend considering transplant referral when % predicted FVC approaches a value of 50% or less with or without significant pulmonary hypertension. Single lung transplant is appropriate for the majority of patients with sarcoidosis. However, bilateral transplant should be considered in bilateral mycetomas and bilateral bronchiectasis. Arcasoy and colleagues [54] analysed 43 patients listed for transplantation and found the following factors associated with increased risk of mortality:
- pulmonary hypertension
- hypoxia
- low cardiac output
- elevated right atrial pressure
It is important to note that pulmonary function parameters have not been found to be predictive of mortality in sarcoidosis [54,55]. Although granuloma recurrence in transplanted lung has been observed, it is a rare to have organ failure secondary to recurrence, and lung transplant should be considered in refractory cases of pulmonary sarcoidosis in the absence of contraindications and close liaison with the transplant center at the earliest opportunity is recommended.
What is the optimal duration of clinical follow-up in sarcoidosis?
There is no consensus on appropriate follow-up duration once a diagnosis of sarcoidosis is confirmed, and it is dependent on physician preference, vital organ involvement, disease progression, need for pharmacological treatment, and availability of resources. The American Thoracic Society statement on sarcoidosis suggested at least 3 years of follow-up after corticosteroid treatment is completed irrespective of radiographic stage [56]. Patients with serious extrapulmonary symptoms would require longer-term follow-up and a recent single-center Japanese study of corticosteroid-naive patients showed that the number of organs involved at the outset dictates the cumulative risk of subsequent progression of sarcoidosis [57]. This study of 150 patients with sarcoidosis with a median follow-up of 7.7 years demonstrated significantly increased risk of progression when there were > 3 organ systems involved as compared to ≤ 3 involved. These corticosteroid-naive patients may require longer follow-up (up to 10 years) than previously thought. However, the findings of this study would require confirmation in multi-center and multi-ethnic cohort of sarcoidosis patients.
Summary
Sarcoidosis presents as a fascinating and challenging disease with myriad clinical, radiological, and pathological manifestations. Despite extensive research in the last decade to increase our understanding of the mechanisms of disease evolution and progression, the underlying etiology remains unidentified. The treatment paradigm has changed over the last 10 years with the introduction of biological agents such as infliximab and adalimumab [42]; these anti-TNF drugs offer a treatment option in refractory cases of sarcoidosis with or without corticosteroids and other anti-metabolites. Methotrexate is the preferred first-line immunosuppressive agent after corticosteroids and has a safer adverse effect profile in comparison to azathioprine. Larger randomized studies to evaluate the efficacy of antimycobacterial agents and newer biologics are warranted before making strong recommendations for the use of these drugs, as there are significant potential toxic effects associated with their use.
Corresponding author: Dr. Ahmed Fahim, Dept. of Respiratory Medicine, McHale Centre New Cross Hospital, Wolverhampton, UK WV10 0QP, [email protected].
Financial disclosures: None.
From the New Cross Hospital, Wolverhampton, UK.
Abstract
- Objective: To discuss the management of sarcoidosis.
- Methods: Review of the literature.
- Results: Sarcoidosis is a challenging multisystem disorder of uncertain etiology characterized by granulomatous inflammation in the affected organs. Treatment is dependent on the severity of disease and organ involvement at the time of diagnosis. Glucocorticoids have traditionally been considered first-line pharmacologic treatment; however, a significant proportion of patients do not require drug treatment due to the propensity toward spontaneous disease remission. Treated patients who fail to respond to corticosteroids or develop significant adverse effects can be offered a second-line agent, eg, methotrexate. Anti-TNF therapy may be considered as a treatment option in carefully selected patients with refractory disease after discussion of potential adverse effects followed by close monitoring at a specialist center.
- Conclusion: Further research into therapeutic options is likely to unveil novel agents with different mechanisms of action and better safety profiles than those seen with currently available immunosuppressive regimens.
Sarcoidosis is a multisystem disorder of uncertain etiology characterized by granulomatous inflammation in the affected organs. The diagnosis of sarcoidosis is best supported by histological evidence of noncaseating granuloma formation. It is a disease with a generally good prognosis; less than 5% patients die from the disease, with cause of death usually secondary to respiratory failure or cardiac or neurologic involvement. This review aims to discuss the management of sarcoidosis with a special emphasis on the management challenges resulting from the myriad clinical manifestations and potential complications seen in this chronic multisystem disease.
Case Study
Initial Presentation
A 40-year-old African-American man presents to his primary care physician with symptoms of fatigue, dry cough, exertional breathlessness, dry and painful eyes, generalized arthralgia, and multiple skin lesions for 3 months. He has a history of essential hypertension and is a former smoker with 10 pack-year history. He is not on any regular medications. Examination reveals bilateral cervical lymphadenopathy and multiple skin lesions on trunk. The rest of the systemic examination (including respiratory and cardiovascular system) is normal.
Workup
The patient’s initial bloodwork showed a mild degree of lymphopenia (1.1 × 109/L, normal range 1.5–4.5). Other bloodwork results, bone profile and immunology screen (including ANA, rheumatoid factor, immunoglobulins, and extractable nuclear antigen antibodies) were negative. Angiotensin-converting enzyme (ACE) was elevated at 149 U/L (normal range 5–58), while serum calcium and vitamin D levels (including vitamin D3) were normal. The findings on CT scan along with the biochemical profile suggest a plausible diagnosis of sarcoidosis.
What is the next step in the workup to establish the suspected diagnosis?
The radiological findings of hilar lymphadenopathy are not confirmatory. There are a number of entities in the differential diagnoses, including tuberculosis, malignancy, lymphoma, and other granulomatous disorders such as histoplasmosis, schistosomiasis, and blastomycosis. It is important to obtain histological evidence before a definitive diagnosis of sarcoidosis can be made. This is necessary as management differs for each of the diagnostic categories mentioned above. Furthermore, diagnostic confirmation would be helpful later in the disease course if the patient develops any associated complications such as pulmonary hypertension or respiratory failure and/or need for lung transplant assessment.
As this patient had palpable cervical lymphadenopathy, an ultrasound guided biopsy of the lymph node was obtained. The histological examination demonstrated evidence of noncaseating granulomas that were well formed and highly consistent with the suspected clinical and radiological diagnosis of sarcoidosis. In addition, the special stains for acid-fast bacilli and other infections including fungi were negative.
Our practice is to evaluate patients with suspected sarcoidosis with neck ultrasound and tru-cut biopsy of cervical lymph nodes (if appropriate) as a first-line investigation, as it is less invasive than bronchoscopy/endoscopic ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) or thoracoscopic lung biopsy. The diagnostic yield of EBUS-TBNA has been evaluated in a number of studies with variable results [1–5]. A large multicenter randomized clinical trial [5] of 304 patients investigated the diagnostic yield of endosonography (endobronchial and esophageal ultrasound) in comparison with bronchoscopy with transbronchial biopsy (TBB) and endobronchial biopsy. The study cohort was made up of patients with stage I/II sarcoidosis. The results showed that endosonography had a higher diagnostic yield to detect granulomas (80% vs 53%; P < 0.001) and there were no serious adverse events related to endoscopy. Hence, ultrasound-guided endoscopic procedures are becoming common first-line investigations for sarcoidosis in the absence of other readily identifiable biopsy sites such as peripheral lymph nodes in the cervical area.
What should be done about the cutaneous and ophthalmologic symptoms in this patient?
As sarcoidosis commonly involves eyes and skin (after pulmonary involvement, which is seen in 90% of cases), the patient was referred to ophthalmology and dermatology departments for further evaluation. These assessments confirmed him to have bilateral uveitis and skin involvement with granulomatous inflammation consistent with ocular and cutaneous sarcoidosis respectively. Hence, the diagnosis of multisystem sarcoidosis was made. At this stage, the patient also mentioned symptoms of intermittent palpitations for 3 months’ duration and feeling of missing a beat, so an urgent cardiological evaluation was undertaken that showed him to have ectopic beats on Holter monitoring. However, his trans-thoracic echocardiogram and a cardiac MRI scan were normal with good biventricular function, excluding cardiac sarcoidosis as a cause of his palpitations. Cardiac involvement with sarcoidosis is clinically apparent in only 5% of cases and presents as cardiomyopathy and or cardiac arrhythmias (both tachy and bradyarrythmias). As cardiac involvement with sarcoid granulomas is usually patchy, endomyocardial biopsy has a limited diagnostic yield of < 20% [6]. In this particular case, endomyocardial biopsy was not attempted in view of normal cardiac MR and echocardiography as well as no significant cardiac dysrythmia on holter monitoring.
Case Continued
The patient was started on corticosteroid eye drops and steroid ointment for his ophthalmologic and cutaneous sarcoidosis, respectively, and symptoms gradually improved over the next few months. As the patient was symptomatic with cough and breathlessness and there was evidence of reduction in FVC (along with reduced DLco), a trial of oral corticosteroids was considered to treat the pulmonary sarcoidosis.
What is first-line pharmacological treatment for sarcoidosis and when is it indicated?
Treatment of sarcoidosis is dependent upon the severity of disease and organ involvement at the time of the diagnosis. Glucocorticoids have traditionally been considered first-line pharmacological agents in selective cases, as a significant proportion of patients do not require drug treatment due to the propensity for spontaneous remission. Furthermore, sarcoidosis remains stable without anti-inflammatory/immunosuppressive therapies in a majority of patients. A number of clinical trials have evaluated the value of corticosteroids in the management of sarcoidosis, with variable outcomes [10–16]. The disease tends to be severe in patients of African descent compared with patients from other racial backgrounds. The European cohort of sarcoidosis patients generally have milder disease with less propensity for vital organ involvement such as cardiac or central nervous system (CNS) disease.
The decision to initiate corticosteroids for sarcoidosis is not a straightforward one as there is variability in symptom presentation, disease severity, and response to corticosteroids. We initiate first-line therapy with oral prednisolone in the following circumstances:
- Evidence of pulmonary impairment (forced vital capacity FVC < 80% predicted) with or without reduction in gas transfer (DLco) along with respiratory symptoms of cough, chest pain, and/or breathlessness (as seen in the case patient)
- Vital organ involvement such as cardiac, ophthalmic (such as panuveitis) or CNS sarcoidosis once confirmed by respective investigations
- Selective cases of sarcoid-associated pulmonary hypertension (SAPH) along with close liaison with pulmonary hypertension specialists
We recommend an initial starting dose of 20 to 40 mg of prednisolone for a period of 1 to 3 months, followed by maintenance dose of 10 mg or less for a further 6 to 9 months, aiming for a total duration of treatment of 12 months. However, the duration may vary depending on the response and any associated adverse effects with corticosteroids. It is usual practice to supplement with calcium and vitamin D when beginning patients on oral corticosteroids due to the potential risk of osteoporosis. However, this may result in significant hypercalcemia, which itself may be an endocrine manifestation of sarcoidosis. Hence, we recommend monitoring serum calcium during treatment and supplement vitamin D in patients who are vitamin D–deficient [17]. Furthermore, serum vitamin 1,25(OH)2 vitamin D3 has been demonstrated to the best available test to evaluate vitamin D status in sarcoidosis [18].
Case Continued
What are the preferred pharmacological agents for second-line treatment in sarcoidosis?
Alternative immunosuppression should be considered in the following circumstances in patients diagnosed with sarcoidosis:
- Failure or less than optimal response to oral corticosteroids
- Use as a steroid-sparing agent in patients requiring high doses of steroids for symptomatic control
- Failure to tolerate corticosteroids due to significant adverse effects such as excessive weight gain, steroid-induced psychosis, osteoporosis, and worsening diabetic control
A small trial of 11 patients examined azathioprine as a steroid-sparing agent and found it as an acceptable immunosuppressive agent for that purpose [19]. It was associated with good safety profile and adherence to treatment was 82% (9 out of 11 patients). However, the small sample size makes it difficult to draw firm conclusions from the findings of this study. In another trial evaluating methotrexate as a steroid-sparing agent in the first year after the diagnosis of sarcoidosis, Baughman and colleagues [20] reported that methotrexate is an attractive alternative to other immunosuppressive agents in term of a steroid sparer. In this double-blind randomized controlled trial (RCT), 15 patients were studied with at least 6 months of treatment with methotrexate vs placebo. There was a significantly reduced dosage of prednisolone observed in methotrexate group. However, the difference was not significant when data were analyzed for all patients, including the dropouts. More recently, a large international retrospective cohort study of 200 patients with sarcoidosis demonstrated that both methotrexate and azathioprine have similar efficacy and steroid-sparing capacity in sarcoidosis [21]. However, infection rates were significantly higher in the azathioprine group as compared to methotrexate (34.6% vs. 18.1%, P = 0.01). Hence methotrexate should be considered as a preferred second-line agent in sarcoidosis after detailed discussion about potential side effects.
Case Continued
The patient was started on methotrexate after discussion about the potential adverse effects of bone marrow suppression, hepatotoxicity, and pneumonitis. He was screened for latent tuberculosis and viral hepatitis prior to starting methotrexate. The dosage was 7.5 mg per week along with folic acid once a week. We gradually increase the dose in increments of 2.5 mg every 2 weeks with a view to reach 15 mg every week as maintenance therapy. In severely obese patients, a dose of up to 20 mg weekly is occasionally considered if 15 mg is suboptimal after careful clinical assessment.
The patient failed to make significant progress after being on methotrexate for a period of 6 months and lung function tests continued to demonstrate a persistent decline with symptomatic worsening of dyspnea and cough.
What are treatment options in refractory sarcoidosis?
Options to consider in the setting of refractory sarcoidosis are leflunomide, hydroxychloroquine, or combination therapy of methotrexate and leflunomide. Leflunomide has been shown to be of similar efficacy to methotrexate as demonstrated by a retrospective analysis of 32 patients treated with the drug in a tertiary care center [22]. Complete or partial response was noted in 12 of 17 patients treated solely with leflunomide and 13 of 15 treated in conjunction with methotrexate. Hence, combination therapy has been suggested as a viable option for these patients who fail to respond to initial glucocorticoid agents and alternative immunosuppressive drugs, as combination therapy may enhance efficacy with reduced toxicity if considered in a rational manner after careful selection of patients [23].
How should the symptom of fatigue be addressed?
The patient had ongoing fatigue during the treatment period with corticosteroids and alternative immunosuppressants. Fatigue, noted in a majority of patients with sarcoidosis [24], is one of the commonest symptoms of sarcoidosis and one of the most difficult to treat. We recommend excluding alternative etiologies of fatigue when confronted with this symptom and evaluating for associated comorbidities such as thyroid dysfunction, vitamin D deficiency, and hypoadrenalism. Extra-pulmonary sarcoidosis seems to be associated with fatigue in comparison to sarcoidosis restricted to the pulmonary system [25]. Furthermore, there is a paucity of good quality data on the benefit of pharmacological intervention for treatment of fatigue. A small double-blind randomized study of 10 patients demonstrated a positive impact of treatment with dexmethylphenidate hydrochloride (d-MPH) for a period of 8 weeks [26]. However, the small sample and lack of long-term outcomes data make it difficult to draw firm conclusions based on the findings of this study and larger randomized trials are warranted to investigate this important aspect of sarcoidosis before recommending a pharmacological agent routinely for this disabling symptom.
What are the pharmacological agents for cutaneous sarcoidosis?
Corticosteroids (local and or systemic) are the mainstay of treatment in cutaneous sarcoidosis. However, patients who fail to respond to these agents or develop significant adverse effects should be offered second-line agents in the form of hydroxychloroquine/chloroquine, methotrexate, or leflunomide. It is important to acknowledge that the evidence of benefit for these agents is derived from uncontrolled studies [27–29]. Anti-malarial agents are usually well tolerated; patients do require a baseline ophthalmological assessment and subsequent periodic examinations to monitor for any ocular toxicity associated with their use. Leflunomide is also a second-line option in cutaneous disease and is associated with lesser toxicity than methotrexate [22]. More recently, topical tacrolimus has shown promising results in isolated case reports [30–33]. Hence, it may be considered a treatment option in refractory cutaneous sarcoidosis.
Is there a role for anti-TNF therapy in the management of sarcoidosis? Should it be considered for the case patient?
Tumour necrosis factor-α (TNF-α) is implicated in the pathogenesis of sarcoidosis and is believed to have a significant role in the inflammatory processes in sarcoidosis. It is released from alveolar macrophages of patients with active disease and is involved in formation and maintenance of granulomas in the lung tissue. There has been significant progress in therapeutic options alternative to traditional immunosuppressants such as corticosteroids and TNF-α inhibition has been on the horizon as a treatment strategy for few years. It may be considered as a steroid-sparing agent or a third-line treatment option in refractory cases.
The initial evidence of benefit of anti-TNF therapy comes from case reports and small case series [34,35]. However, there have been 2 RCTs published so far invest-igating infliximab in sarcoidosis [36,37]. Baughman and colleagues [36] evaluated 138 patients with chronic pulmonary sarcoidosis in a double-blind study. There was a statistically significant improvement in FVC after 24 weeks of therapy with infliximab as compared to placebo (2.5% increase in mean FVC % predicted from baseline, P = 0.038). However, there was no improvement in any of the secondary outcome variables including SGRQ (St George’s Respiratory Questionnaire), 6-minute walk distance, and dyspnea scores. The benefit seemed to be more pronounced in the severe disease category on post-hoc analysis. The clinical significance of these findings are however unclear and it is difficult to draw firm conclusions based on the findings of this study alone.
The other RCT exploring the role of infliximab in sarcoidosis was conducted by Rossman et al [37]. This multi-center phase II study was conducted to evaluate the safety and tolerability of the drug in active pulmonary sarcoidosis (stage II to IV). The trial had a small number of participants with only 19 patients and failed to show a significant improvement in lung function after 6 weeks of treatment. Furthermore, 4 patients developed serious adverse events after treatment. Doty and colleagues [34] retrospectively analysed 10 patients treated with infliximab for refractory sarcoidosis and found subjective and objective evidence of improvement in all patients. This therapy resulted in reduction of corticosteroid dosage in 83% of cases (5 out of 6). However, adverse reactions were noted in 3 cases, including development of angioimmunoblastic lymphoma in one case. A retrospective study of 16 consecutive unselected cases of refractory sarcoidosis by Chapelon-Abric and colleagues [38] demonstrated a positive response in a majority of cases. However 38% of patients experienced a relapse. Furthermore, 44% of patients (7 out of 16) had infectious complications associated with anti-TNF therapy. Finally, infliximab may be used successfully in treating severe small fibre neuropathy [39] and should be considered in refractory cases where neuropathy is associated with autonomic dysfunction.
In summary, based on the findings of the aforementioned trials and case series, anti-TNF therapy may be considered as a treatment option in carefully selected patients after discussion of the potential adverse effects, followed by close monitoring at a specialist center for the management of sarcoidosis. Furthermore, anti-TNF therapy should not delay referral for lung transplant assessment, particularly when disease progression is relatively rapid. Duration of treatment and timing of thoracic imaging after anti-TNF therapy is subject to debate and should be individualized in close collaboration with a specialist center.
Case Continued
What is the role of newer biologics in the treatment of sarcoidosis? And what other therapies are on the horizon?
Although sarcoidosis is a T cell–mediated disease, humoral immunity has been implicated in sarcoidosis [40] and B cell depletion by rituximab (anti-CD 20+chimeric monoclonal antibody) has been successfully utilized in T cell–mediated diseases such as rheumatoid arthritis. Rituximab has been studied in phase I/II trial [41] in patients with refractory pulmonary sarcoidosis. The response to rituximab was inconsistent in these patients, with only a small group demonstrating > 5% improvement in FVC or walking distance. Hence, further studies are required to demonstrate a significant clinical benefit of this treatment in refractory sarcoidosis and potentially identify the characteristics of patients that may respond to B-cell depletion.
Adalimumab is another biologic agent that may be a potential option in refractory sarcoidosis as demonstrated in an open-label study of 11 patients [42]. This small trial of 52 weeks’ duration showed that adalimumab is well tolerated and may be considered in refractory pulmonary sarcoidosis when other treatment options have been exhausted.
Acthar had been used to treat pulmonary sarcoidosis in 1950s and there has been recent interest in evaluating the value of acthar gel therapy in the management of sarcoidosis. Baughman and colleagues [43] carried out a retrospective analysis of 47 patients with advanced sarcoidosis treated with acthar gel. The results showed that there was an objective improvement in approximately a third of patients receiving at least 3 months of treatment. Thirty-six percent of patients (n = 17) managed to reduce their oral corticosteroid dosage by more than 50% while on this therapy. However, a significant proportion of patients were unable to take ≥ 3 months of treatment, suggesting poor tolerance/adherence. The utility of acthar gel therapy would need to be examined in larger prospective randomized trial before it can be recommended for a treatment option in advanced disease.
Should pneumocystis pneumonia (PCP) prophylaxis be considered in sarcoidosis?
We do not routinely consider PCP prophylaxis in all sarcoidosis patients. However, it should be considered in the following clinical situations:
- Failure to reduce oral corticosteroid dose to less than 20 mg of prednisolone daily
- Concomitant use of anti-TNF agents with relatively high maintenance dose of oral corticosteroids (≥ 20 mg per day)
- Significant comorbid condition in association with sarcoidosis resulting in significant level of immunosuppression
As our patient did not fall in any of the above categories, we did not offer him PCP prophylaxis. However, clinicians treating patients with sarcoidosis with strong immunosuppressants should be aware of this potential complication and be vigilant about discussing prophylaxis with trimethoprim-sulphamethoxazole, which is the first-line agent for this purpose.
Is there a role of anti-tuberculous treatment in sarcoidosis?
Both mycobacterium tuberculosis (MTB) and non-mycobateria (NTM) have been implicated in sarcoidosis [44] and it is challenging to differentiate tuberculosis (TB) from sarcoidosis in certain clinical situations, such as when dealing with patients from countries with a high incidence of TB. The association of mycobacterium with sarcoidosis was explored in 2 recent trials of concomitant use of levofloxacin, ethambutol, azithromycin, and rifampin in cutaneous [45] and pulmonary sarcoidosis [46]. Drake and colleagues evaluated 15 chronic pulmonary sarcoidosis patients in an open-label trial to investigate if the combination of these drugs was associated with improvement in pulmonary sarcoidosis. The patients who completed 8 weeks of treatment had improvement in FVC at both 4 and 8 weeks of treatment. However, only 8 patients could complete 8 weeks of therapy, with significant adverse events. The small sample size limits our ability to draw meaningful conclusions and larger randomized trials are warranted to investigate this approach in sarcoidosis management.
Are there any valid serum biomarkers for sarcoidosis?
A biomarker is defined as a compound easily measurable in serum, urine, or other body fluids that can be used as indicator of presence and/or severity of particular disease state. Moreover, it helps in evaluation of effectiveness of drug therapy and useful to monitor the disease longitudinally. Unfortunately, there is no ideal serum biomarker in sarcoidosis. The most widely evaluated marker is serum angiotensin-converting enzyme (ACE). It has been found to be elevated in three quarters of patients with sarcoidosis [47]. However, it has poor diagnostic utility due to limited sensitivity and specificity. Furthermore, levels of serum ACE are reduced in patients taking ACE inhibitors and hence measurement of ACE levels in patients taking ACE inhibitors may lead to inaccurate interpretations [48].
A number of other serum biomarkers have been evaluated in sarcoidosis. Gungor and colleagues [49] evaluated 48 patients with sarcoidosis and 20 healthy controls. The biomarkers measured were ACE, adenosine deaminase (ADA), total IgE, serum amyloid-A (SAA), soluble interleukin-2 receptor (sIL2R), and C-reactive protein (CRP). This study showed that SAA was significantly elevated in sarcoidosis as compared to controls (P < 0.001). Furthermore, sIL2R levels were raised in extra-pulmonary sarcoidosis (P < 0.014). In another study, Grutters and co-workers [50] demonstrated elevated levels of sIL2R in 47 patients with active sarcoidosis. However, the levels did not correlate with radiolographic or physiological outcomes or response to treatment. Hence, the utility of these biomarkers in clinical practice is questionable and larger longitudinal studies are required to demonstrate the actual benefit of these biomarkers in everyday clinical practice.
There is a complex relationship between vitamin D and calcium metabolism and risk of osteoporosis in sarcoidosis. The value of active vitamin D metabolite 1, 25-dihydroxy vitamin D in relation to the degree of sarcoidosis disease chronicity was evaluated in a study of 59 patients with sarcoidosis [51]. It was noted that increased levels of 1,25 vitamin D were associated with increased risk of chronic phenotype and the need to require repeated treatments with immunosuppressive agents. Hence, serum levels of 1,25 vitamin D may have a prognostic value in sarcoidosis. It is important to note that both vitamin D deficiency as well as vitamin D excess can result in osteoporosis [52], and there is a risk of bone fragility and fractures in patients who may need long-term oral corticosteroid therapy. Hence, an optimal level of vitamin D is crucial; we recommend a value of serum 25(OH) vitamin D between 10 and 20 ng/mL as evidenced by a cross-sectional analysis of 142 consecutive patients with biopsy-proven sarcoidosis [53]. The above range of 25(OH) vitamin D was associated with higher bone mineral density and values above 20 ng/mL resulted in increased risk of fractures, demonstrating the need to keep vitamin D levels of these patients to be lower than those recommended for general population.
When should a patient with sarcoidosis be referred for lung transplantation?
Lung transplantation is a treatment option in advanced/end-stage disease after pharmacologic treatments have been exhausted and there is no evidence of progressive or severe extrapulmonary disease. The most critical decision regarding transplantation in pulmonary sarcoidosis is the timing of the referral and close liaison with the transplant center to ensure the maximal chance of success with lung transplantation. We recommend considering transplant referral when % predicted FVC approaches a value of 50% or less with or without significant pulmonary hypertension. Single lung transplant is appropriate for the majority of patients with sarcoidosis. However, bilateral transplant should be considered in bilateral mycetomas and bilateral bronchiectasis. Arcasoy and colleagues [54] analysed 43 patients listed for transplantation and found the following factors associated with increased risk of mortality:
- pulmonary hypertension
- hypoxia
- low cardiac output
- elevated right atrial pressure
It is important to note that pulmonary function parameters have not been found to be predictive of mortality in sarcoidosis [54,55]. Although granuloma recurrence in transplanted lung has been observed, it is a rare to have organ failure secondary to recurrence, and lung transplant should be considered in refractory cases of pulmonary sarcoidosis in the absence of contraindications and close liaison with the transplant center at the earliest opportunity is recommended.
What is the optimal duration of clinical follow-up in sarcoidosis?
There is no consensus on appropriate follow-up duration once a diagnosis of sarcoidosis is confirmed, and it is dependent on physician preference, vital organ involvement, disease progression, need for pharmacological treatment, and availability of resources. The American Thoracic Society statement on sarcoidosis suggested at least 3 years of follow-up after corticosteroid treatment is completed irrespective of radiographic stage [56]. Patients with serious extrapulmonary symptoms would require longer-term follow-up and a recent single-center Japanese study of corticosteroid-naive patients showed that the number of organs involved at the outset dictates the cumulative risk of subsequent progression of sarcoidosis [57]. This study of 150 patients with sarcoidosis with a median follow-up of 7.7 years demonstrated significantly increased risk of progression when there were > 3 organ systems involved as compared to ≤ 3 involved. These corticosteroid-naive patients may require longer follow-up (up to 10 years) than previously thought. However, the findings of this study would require confirmation in multi-center and multi-ethnic cohort of sarcoidosis patients.
Summary
Sarcoidosis presents as a fascinating and challenging disease with myriad clinical, radiological, and pathological manifestations. Despite extensive research in the last decade to increase our understanding of the mechanisms of disease evolution and progression, the underlying etiology remains unidentified. The treatment paradigm has changed over the last 10 years with the introduction of biological agents such as infliximab and adalimumab [42]; these anti-TNF drugs offer a treatment option in refractory cases of sarcoidosis with or without corticosteroids and other anti-metabolites. Methotrexate is the preferred first-line immunosuppressive agent after corticosteroids and has a safer adverse effect profile in comparison to azathioprine. Larger randomized studies to evaluate the efficacy of antimycobacterial agents and newer biologics are warranted before making strong recommendations for the use of these drugs, as there are significant potential toxic effects associated with their use.
Corresponding author: Dr. Ahmed Fahim, Dept. of Respiratory Medicine, McHale Centre New Cross Hospital, Wolverhampton, UK WV10 0QP, [email protected].
Financial disclosures: None.
1. Kitamura AY, Takiguchi K, Kurosu N, et al. Feasibility of cytological diagnosis of sarcoidosis with endobronchial US-guided transbronchial aspiration. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:82–9.
2. Chee A, Khalil M, Stather DR, et al. Cytologic assessment of endobronchial ultrasound-guided transbronchial needle aspirates in sarcoidosis. J Bronchology Interv Pulmonol 2012;19:24–8.
3. Oki M, Saka H, Kitagawa C, et al. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg 2012;143:1324–9.
4. Plit ML, Havryk AP, Hodgson A, et al. Rapid cytological analysis of endobronchial ultrasound-guided aspirates in sarcoidosis. Eur Respir J 2013;42:1302-8.
5. von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457–64.
6. Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302.
7. Ishimaru S, Tsujino I, Sakaue S, et al. Combination of 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in assessing cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:234–5.
8. Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241–8.
9. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36.
10. Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J 2013;41:1424–8.
11. James DG, Carstairs LS, Trowell J, Sharma OP. Treatment of sarcoidosis. Report of a controlled therapeutic trial. Lancet 1967;2:526–8.
12. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Study Group. Chest 1999;116:424–31.
13. Eule H, Roth I, Ehrke I, Weinecke W. Corticosteroid therapy of intrathoracic sarcoidosis stages I and II--results of a controlled clinical trial. Z Erkr Atmungsorgane 1977;149:142–7.
14. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis 1973;107:609–14.
15. Zaki MH, Lyons HA, Leilop L, Huang CT. Corticosteroid therapy in sarcoidosis. A five-year, controlled follow-up study. N Y State J Med 1987;87:496–9.
16. Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax 1996;51:238–47.
17. Kamphuis LS, Bonte-Mineur F, van Laar JA, et al. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res 2014;29:2498–503.
18. Baughman RP, Janovcik J, Ray M, et al. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:113–20.
19. Muller-Quernheim J, Kienast K, Held M, et al. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999;14:1117–22.
20. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:60–6.
21. Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate versus azathioprine in second line therapy of sarcoidosis. Chest 2013.
22. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:43–8.
23. Baughman RP, Ohmichi M, Lower EE. Combination therapy for sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001;18:133–7.
24. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J 2012;40:255–63.
25. Fleischer M, Hinz A, Brahler E, et al. Factors associated with fatigue in sarcoidosis. Respir Care 2014;59:1086–94.
26. Lower EE, Harman S, Baughman RP. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest 2008;133:1189–95.
27. Veien NK, Brodthagen H. Cutaneous sarcoidosis treated with methotrexate. Br J Dermatol 1977;97:213–6.
28. Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995;155:846–51.
29. Kaye O, Palazzo E, Grossin M, et al. Low-dose methotrexate: an effective corticosteroid-sparing agent in the musculoskeletal manifestations of sarcoidosis. Br J Rheumatol 1995;34:642–4.
30. Green CM. Topical tacrolimus for the treatment of cutaneous sarcoidosis. Clin Exp Dermatol 2007:32:457–8.
31. Vano-Galvan S, Fernandez-Guarino M, Carmona LP, et al. Lichenoid type of cutaneous sarcoidosis: great response to topical tacrolimus. Eur J Dermatol 2008;18:89–90.
32. Gutzmer R, Volker B, Kapp A, Werfel T. [Successful topical treatment of cutaneous sarcoidosis with tacrolimus]. Hautarzt 2003;54:1193–7.
33. Katoh N, Mihara H, Yasuno H. Cutaneous sarcoidosis successfully treated with topical tacrolimus. Br J Dermatol 2002;147:154–6.
34. Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127:1064–71.
35. Berrios I, Jun-O’Connell A, Ghiran S, Ionete C. A case of neurosarcoidosis secondary to treatment of etanercept and review of the literature. BMJ Case Rep 2015 Jul 6;2015.
36. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802.
37. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:201–8.
38. Chapelon-Abric D, Saadoun D, Biard L. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol 2015;33:509–15.
39. Hoitsma E, Faber CG, Santen-Hoeufft M, et al. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment with infliximab. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:73–7.
40. Lee NS, Barber L, Kanchwala A, et al. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol 2011;18:223–34.
41. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 2014;43:1525–8.
42. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy Results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:46–54.
43. Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med 2016;110:66–72.
44. Mangiapan G, Hance AJ. Mycobacteria and sarcoidosis: an overview and summary of recent molecular biological data. Sarcoidosis 1995;12:20-37.
45. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol 2013;149:1040–9.
46. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:201–11.
47. Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis--its value in present clinical practice. Ann Clin Biochem 1989;26 ( Pt 1):13–8.
48. Krasowski MD, Savage J, Ehlers A, et al. Ordering of the serum angiotensin-converting enzyme test in patients receiving angiotensin-converting enzyme inhibitor therapy: an avoidable but common error. Chest 2015;148:1447–53.
49. Gungor S, Ozseker F, Yalcinsoy M. Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol 2015;25:174–9.
50. Grutters JC, Fellrath JM, Mulder L, et al. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest 2003;124:186–95.
51. Kavathia D, Buckley JD, Rao D, et al. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med 2010;104:564–70.
52. Baughman RP, Lower EE. Goldilocks, vitamin D and sarcoidosis. Arthritis Res Ther 2014;16:111.
53. Saidenberg-Kermanac’h N, Semerano L, Nunes H, et al. Bone fragility in sarcoidosis and relationships with calcium metabolism disorders: a cross sectional study on 142 patients. Arthritis Res Ther 2014;16:R78.
54. Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 2001;120:873–80.
55. Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 2003;124:922–8.
56. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149–73.
57. Inoue Y, Inui N, Hashimoto D, et al. Cumulative incidence and predictors of progression in corticosteroid-naive patients with sarcoidosis. PLoS One 2015;10:e0143371.
1. Kitamura AY, Takiguchi K, Kurosu N, et al. Feasibility of cytological diagnosis of sarcoidosis with endobronchial US-guided transbronchial aspiration. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:82–9.
2. Chee A, Khalil M, Stather DR, et al. Cytologic assessment of endobronchial ultrasound-guided transbronchial needle aspirates in sarcoidosis. J Bronchology Interv Pulmonol 2012;19:24–8.
3. Oki M, Saka H, Kitagawa C, et al. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg 2012;143:1324–9.
4. Plit ML, Havryk AP, Hodgson A, et al. Rapid cytological analysis of endobronchial ultrasound-guided aspirates in sarcoidosis. Eur Respir J 2013;42:1302-8.
5. von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457–64.
6. Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302.
7. Ishimaru S, Tsujino I, Sakaue S, et al. Combination of 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in assessing cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:234–5.
8. Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241–8.
9. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36.
10. Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J 2013;41:1424–8.
11. James DG, Carstairs LS, Trowell J, Sharma OP. Treatment of sarcoidosis. Report of a controlled therapeutic trial. Lancet 1967;2:526–8.
12. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Study Group. Chest 1999;116:424–31.
13. Eule H, Roth I, Ehrke I, Weinecke W. Corticosteroid therapy of intrathoracic sarcoidosis stages I and II--results of a controlled clinical trial. Z Erkr Atmungsorgane 1977;149:142–7.
14. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis 1973;107:609–14.
15. Zaki MH, Lyons HA, Leilop L, Huang CT. Corticosteroid therapy in sarcoidosis. A five-year, controlled follow-up study. N Y State J Med 1987;87:496–9.
16. Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax 1996;51:238–47.
17. Kamphuis LS, Bonte-Mineur F, van Laar JA, et al. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res 2014;29:2498–503.
18. Baughman RP, Janovcik J, Ray M, et al. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:113–20.
19. Muller-Quernheim J, Kienast K, Held M, et al. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999;14:1117–22.
20. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:60–6.
21. Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate versus azathioprine in second line therapy of sarcoidosis. Chest 2013.
22. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:43–8.
23. Baughman RP, Ohmichi M, Lower EE. Combination therapy for sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001;18:133–7.
24. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J 2012;40:255–63.
25. Fleischer M, Hinz A, Brahler E, et al. Factors associated with fatigue in sarcoidosis. Respir Care 2014;59:1086–94.
26. Lower EE, Harman S, Baughman RP. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest 2008;133:1189–95.
27. Veien NK, Brodthagen H. Cutaneous sarcoidosis treated with methotrexate. Br J Dermatol 1977;97:213–6.
28. Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995;155:846–51.
29. Kaye O, Palazzo E, Grossin M, et al. Low-dose methotrexate: an effective corticosteroid-sparing agent in the musculoskeletal manifestations of sarcoidosis. Br J Rheumatol 1995;34:642–4.
30. Green CM. Topical tacrolimus for the treatment of cutaneous sarcoidosis. Clin Exp Dermatol 2007:32:457–8.
31. Vano-Galvan S, Fernandez-Guarino M, Carmona LP, et al. Lichenoid type of cutaneous sarcoidosis: great response to topical tacrolimus. Eur J Dermatol 2008;18:89–90.
32. Gutzmer R, Volker B, Kapp A, Werfel T. [Successful topical treatment of cutaneous sarcoidosis with tacrolimus]. Hautarzt 2003;54:1193–7.
33. Katoh N, Mihara H, Yasuno H. Cutaneous sarcoidosis successfully treated with topical tacrolimus. Br J Dermatol 2002;147:154–6.
34. Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127:1064–71.
35. Berrios I, Jun-O’Connell A, Ghiran S, Ionete C. A case of neurosarcoidosis secondary to treatment of etanercept and review of the literature. BMJ Case Rep 2015 Jul 6;2015.
36. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802.
37. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:201–8.
38. Chapelon-Abric D, Saadoun D, Biard L. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol 2015;33:509–15.
39. Hoitsma E, Faber CG, Santen-Hoeufft M, et al. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment with infliximab. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:73–7.
40. Lee NS, Barber L, Kanchwala A, et al. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol 2011;18:223–34.
41. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 2014;43:1525–8.
42. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy Results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:46–54.
43. Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med 2016;110:66–72.
44. Mangiapan G, Hance AJ. Mycobacteria and sarcoidosis: an overview and summary of recent molecular biological data. Sarcoidosis 1995;12:20-37.
45. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol 2013;149:1040–9.
46. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:201–11.
47. Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis--its value in present clinical practice. Ann Clin Biochem 1989;26 ( Pt 1):13–8.
48. Krasowski MD, Savage J, Ehlers A, et al. Ordering of the serum angiotensin-converting enzyme test in patients receiving angiotensin-converting enzyme inhibitor therapy: an avoidable but common error. Chest 2015;148:1447–53.
49. Gungor S, Ozseker F, Yalcinsoy M. Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol 2015;25:174–9.
50. Grutters JC, Fellrath JM, Mulder L, et al. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest 2003;124:186–95.
51. Kavathia D, Buckley JD, Rao D, et al. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med 2010;104:564–70.
52. Baughman RP, Lower EE. Goldilocks, vitamin D and sarcoidosis. Arthritis Res Ther 2014;16:111.
53. Saidenberg-Kermanac’h N, Semerano L, Nunes H, et al. Bone fragility in sarcoidosis and relationships with calcium metabolism disorders: a cross sectional study on 142 patients. Arthritis Res Ther 2014;16:R78.
54. Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 2001;120:873–80.
55. Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 2003;124:922–8.
56. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149–73.
57. Inoue Y, Inui N, Hashimoto D, et al. Cumulative incidence and predictors of progression in corticosteroid-naive patients with sarcoidosis. PLoS One 2015;10:e0143371.
Symptomatic Intracranial Atherosclerotic Disease
From the Emory University School of Medicine, Atlanta, GA.
Abstract
- Objective: To provide an overview of the diagnosis, clinical presentation, and management of symptomatic intracranial atherosclerotic disease (ICAD).
- Methods: Review of the current literature in the context of a clinical case.
- Results: ICAD is a common cause of ischemic strokes or transient ischemic attacks (TIAs), especially among Asian, black, and Hispanic patients. ICAD can be identified with noninvasive arterial imaging such as CT angiography, MR angiography, or transcranial Doppler ultrasound of the head when evaluating for the cause of an ischemic stroke or TIA. Aggressive medical management with dual antiplatelet therapy and lifestyle and risk factor modification has emerged as effective first-line therapy. In patients who have recurrent ischemic symptoms while on aggressive medical management, endovascular treatment can be considered.
- Conclusion: When symptomatic ICAD is identified early, aggressive medical management is effective in reducing the risk of recurrent ischemic events in this patient population.
Symptomatic intracranial atherosclerotic disease (ICAD) may represent the most common cause of ischemic stroke worldwide and the cause of approximately 8% to 10% of ischemic strokes in the United States [1–7]. It is a particularly important clinical entity due to the high recurrence rate of ischemic events in this population. The estimated recurrent stroke risk from symptomatic ICAD has been reported to be as high as 14.9% in the first year after an initial ischemic event [1].There are multiple risk factors for ICAD. Non-modifiable risk factors include race (particularly Asian, black, or Hispanic race), age, and family history of coronary artery disease or stroke; modifiable risk factors include diabetes, hypertension, and hyperlipidemia [2].
Case Study
Initial Presentation
A 78-year-old right-handed man presents to the outpatient clinic for follow-up evaluation after inpatient admission for acute ischemic stroke. The patient has an established medical history of hypertension, diabetes mellitus (diagnosed 10 years ago), dyslipidemia, and a 50 pack-year history of tobacco use.
Two weeks prior to the clinic visit, the patient presented to the emergency department (ED) via emergency medical services with right face and arm weakness and numbness and the inability to speak that had been ongoing for approximately 1 hour. The patient’s wife reported to ED providers that the patient had a similar episode 1 month prior that resolved completely in 30 to 45 minutes (and for which the patient never sought medical attention). Home medications at the time of admission included aspirin 81 mg and pravastatin 20 mg daily, both of which he takes intermittently.
The initial blood pressure was 185/76 mm Hg and blood glucose was 225 mg/dL. Initial exam was remarkable for the inability to answer orientation questions (but able to follow simple commands), forced gaze deviation to the left, right lower facial weakness, weakness in the right arm with no antigravity movement, moderately decreased sensation of the right face and arm, severe expressive aphasia, and severe dysarthria. A CT of the head without contrast showed no evidence of hemorrhage. Patient was suspected of having an acute ischemic stroke and was given intravenous tPA.
What are the possible mechanisms for this patient’s presentation with ischemic stroke?
This patient is presenting with right face and arm weakness with sensory loss, gaze deviation to the left, and expressive aphasia. Abnormalities of speech and gaze paresis that localize to the left frontal lobe with cortical involvement make a subcortical or brainstem lacunar ischemic event less likely. The syndrome is suggestive of a large artery occlusion of the left middle cerebral artery. This type of large artery occlusive disease in the anterior circulation is most often due to an embolus (artery-to-artery or cardiac origin) event and requires arterial imaging of the head and neck acutely as endovascular thrombectomy has been associated with reduced disability compared to intravenous tPA alone.
Further Evaluation
What imaging is recommended to identify patients with ICAD?
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial sought to evaluate the reliability of noninvasive imaging modalities to identify a 50%–99% stenosis of large proximal cerebral arteries as compared with the gold standard of conventional angiography [3]. Qualifying vessels included the M1 segment of the middle cerebral artery, the carotid siphon, and intracranial vertebral and basilar arteries. Imaging techniques included MRA head without contrast (time of flight) and TCD ultrasound. Both of these imaging modalities demonstrated a high negative predictive value (NPV) for the presence of a 50%–99% intracranial stenosis; however, the positive predictive value (PPV) for TCD and MRA was only 55% and 66%, respectively. Subsequent studies of contrast-enhanced MRA and CTA of the headhave shown high PPV (78%–94%) and NPV (95%–100%) when compared with cerebral angiography for the detection of 70%–99% ICAD [4,5]. Together these data suggest that TCD and MRA of the head without contrast can be used as screening tools to rule out ICAD when normal; however, subsequent contrast-enhanced MRA or CTA is required when abnormalities are identified.
When choosing the best noninvasive imaging modality for any given patient, concomitant medical issues including renal disease, age, presence of a pacemaker, implantable cardiac defibrillator or other metal, and claustrophobia are factors to consider.
What are the potential mechanisms of ischemic stroke in patients with symptomatic ICAD?
Stroke in the symptomatic ICAD group occurs as a result of either (1) thrombus formation at the site of a high-grade stenosis due to an unstable atherosclerotic plaque, (2) low flow through a narrow, stenotic artery (hypoperfusion) or (3) a combination of atheroembolism and hypoperfusion [6]. While a thrombus can occlude the parent vessel at the site of stenosis, more commonly the thrombus migrates distally as an artery-artery embolism to a smaller caliber artery.
What medical regimen is recommended for this patient with symptomatic ICAD?
Dual Antiplatelet Therapy
DAT with aspirin and clopidogrel is thought to reduce ischemic events related to regional thromboembolism. The best clinical evidence of the use of DAT in symptomatic ICAD is found in outcomes data for the SAMMPRIS trial where 30-day recurrent ischemic stroke rates in the aggressive medical management arm were significantly lower than patients treated with aspirin or warfarin monotherapy as part of the Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis (WASID) trial. A subgroup of patients enrolled in WASID with similar clinical characteristics to those in SAMMPRIS were found to have a 30-day rate of stroke or death of 10.7% and a collective 1-year rate of ischemic stroke, brain hemorrhage, or vascular death of 25% [2]. The corresponding rates in the SAMMPRIS medical management arm were 5.8% at 30 days and 17.5% at 1 year. Given that the recurrent stroke rates were significantly lower in as little as 30 days, these benefits have been hypothesized to be more likely secondary to the DAT regimen than other risk factor modification [7]. There is some evidence that longer term DAT with aspirin and clopidogrel up to 1 year may be associated with further reduction in stroke, MI, and vascular death with similar bleeding risk, but this needs to be evaluated in prospective studies [8].
Additional evidence of the benefit and potential mechanism from DAT in ICAD comes from CLAIR (Clopidogrel plus Aspirin versus Aspirin alone for Reducing Embolization in Patients with Acute Symptomatic Cerebral or Carotid Artery Stenosis Trial), a multicenter, randomized trial with blinded outcome assessment, with patients recruited at sites in Hong Kong, Singapore, China, Thailand, and Malaysia [9]. Patients were enrolled with an extracranial or intracranial stenosis (greater than or equal to 50%, as diagnosed by carotid duplex, transcranial Doppler, or magnetic resonance angiography) if they had a clinical diagnosis of acute ischemic stroke or transient ischemic attack (TIA) during the 7 days prior to enrollment and were found to have microembolic signals (suggesting microemboli of atherosclerotic origin) detected at baseline assessment with transcranial Doppler ultrasound. Patients were randomized to DAT with aspirin and clopidogrel versus aspirin monotherapy. Patients on DAT had significantly reduced microembolic signals compared with patients on aspirin monotherapy. Asymptomatic embolization with dual antiplatelet therapy may also proffer a reduction in clinical events in these patients with symptomatic ICAD.
Statin Therapy
Statin therapy is an integral part in the prevention of recurrent ischemic events in the symptomatic ICAD cohort. Post-hoc analyses from the WASID trial found that total cholesterol greater than 200 mg/dL was associated with an increased risk of ischemic stroke, myocardial infarction, or vascular death.
In 2013, the American Heart Association released new guidelines regarding the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults [10]. Clinical atherosclerotic cardiovascular disease (ASCVD) is the manifestation of systemic atherosclerotic disease and defined as acute coronary syndromes, a history of myocardial infarction, stable or unstable angina, a history of coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin. Recommendations for secondary prevention in patients with clinical ASCVD include the use of high-intensity statin therapy (atorvastatin 40 or 80 mg, rosuvastatin 20 or 40 mg) for patients less than 75 years of age or moderate-intensity statin therapy (atorvastatin 10 or 20 mg, rosuvastatin 5 or 10 mg, pravastatin 40 mg, or simvastatin 20 or 40 mg) for patients > 75 years of age. In the SAMMPRIS trial, an LDL cholesterol level less than 70 mg/dL was targeted [7].
Blood Pressure Management
Post-hoc analysis of data from WASID demonstrated a statistically significant increase in recurrent stroke risk with increasing mean systolic and diastolic blood pressure (BP) [11]. This was particularly true in patients with mean SBP > 160 mm Hg. This is contrary to the common perception that BP should be maintained higher in patients with intracranial stenosis. In multivariable analysis, systolic BP greater than 140 mm Hg was associated with an increased risk of ischemic stroke, myocardial infarction, or vascular death. In the SAMMPRIS trial, the recommended BP goals for patients with symptomatic ICAD were less than 140/90 mm Hg in non-diabetic patients and less than 130/80 mm Hg in diabetic patients [7]. The timing and pace of blood pressure normalization for a recently symptomatic patient with ICAD is still unclear and needs further study.
Lifestyle Modification and Secondary Risk Factors
The SAMMPRIS protocol incorporated a lifestyle coach for all patients enrolled in the study. Lifestyle modification to achieve smoking cessation, regular physical activity, weight reduction for overweight patients, and glucose control in diabetes (goal hemoglobin A1c < 7.0%) were complementary to the pharmacotherapy (aspirin, clopidogrel, statin, and antihypertensive regimen) prescribed [7].
Patients should be encouraged to participate in aerobic exercise for at least 30 minutes at least 3 times weekly. Dietary modifications modeled after the Mediterranean diet should be encouraged. These should be coupled with additional efforts to address excessive weight as needed.
Successful smoking cessation proves one of the most challenging lifestyle modifications for this group of patients and may require the employment of an extended support system with both family and medical providers. Nicotine supplementation is a common first-line aid for cessation, which may be provided in the form of gum or transdermal patches. Additional pharmacotherapy to address central mechanisms of addiction may be necessary. Many patients benefit from the addition of an antidepressant therapy such as bupropion or an adjunctive medication such as varenicline (a nicotine receptor partial agonist that helps with breaking nicotine addiction). It is important to establish a quit date and detailed, multistep plan for cessation [12].
Exceptions and Other Considerations
These evidence-based recommendations are directed toward a specific group of patients with high-grade stenosis (70%–99%), a single symptomatic vessel, and relatively short segments of stenosis (< 14 mm) based on the inclusion and exclusion criteria for the SAMMPRIS trial [7]. Additionally, these patients were not enrolled during the immediate period following an acute ischemic event. There is less evidence on the management of patients who present with < 70% symptomatic ICAD but we can infer that therapy with DAT, statins, and risk factor modification are still of benefit for patients with 50%–69% stenosis or those requiring treatment during the acute evaluation [13]. Additionally, some of these SAMMPRIS exclusion criteria were included to select patients who could be considered intracranial stenting candidates (eg, short segment stenosis, single vessel disease, etc) and are not considered to impact on the benefits of the aggressive medical management regimen.
Further Evaluation
The patient was initiated on antithrombotic therapy on hospital day 2 (24 hours after IV tPA administration) with aspirin 325 mg daily and clopidogrel 300 mg oral load followed by 75 mg daily, ranitidine 150 mg twice a day to reduce gastroesophageal reflux and atorvastatin 80 mg daily. On hospital day 3, his neurological exam remained stable and he was initiated on losartan 25 mg daily with a plan to titrate the dose up as an outpatient to achieve target blood pressure. The patient and his wife were advised to maintain a blood pressure log and provide it to his physicians at follow-up and was provided smoking cessation counseling with a plan to remain off tobacco after discharge utilizing nicotine patches. The patient was discharged from the hospital 4 days after admission with a diagnosis of acute ischemic stroke secondary to symptomatic ICAD from regional thromboembolism. Early outpatient follow-up was scheduled with his primary physician within 1 week after discharge to evaluate BP and a vascular neurologist within 1 month after discharge.
What factors predict recurrent stroke in patients with symptomatic ICAD?
As evidenced in the WASID post-hoc analysis, female sex, prior ischemic stroke (versus TIA), time from qualifying event to enrollment (≤ 17 days after ischemic event), severity of stenosis (≥ 70% stenosis) of the symptomatic vessel, and history of diabetes were identified as independent risk factors for recurrent stroke in this population [14].
How do we manage patients who continue to have symptoms despite optimal medical therapy?
When evaluating symptomatic ICAD patients with recur-rent neurologic symptoms, several important factors should be considered:
- Do the recurrent neurologic symptoms localize to the original ICAD location?
Patients with symptomatic ICAD often have multiple risk factors that put them at risk for other subtypes of ischemic stroke including lacunar stroke. Repeat neuro-imaging with brain MRI is recommended to confirm the localization of a recurrent stroke is inside or outside of the territory of the ICAD.
- Were the recurrent neurologic symptoms provoked?
Patients with ICAD can be uniquely sensitive to fluctuations in cerebral blood flow and oxygenation. Diabetic patients with ICAD can develop autonomic neuropathy that results in orthostatic hypotension. If patients have recurrent transient ischemic events associated with postural changes, adequate treatment of orthostasis can reduce recurrent ischemic events. Other provoked circumstances include patients who develop anemia and patients with untreated severe obstructive sleep apnea who may awake with recurrent ischemic events due to hypoxemia.
- Is the patient adhering to the medication regimen?
Patients with symptomatic ICAD frequently have multiple medications to take for treatment of their comorbidities. Discussion of the number of missed doses of medications over the prior month is important to ascertain adherence. Patients should also be counseled to avoid concomitant medications that may cause drug interactions; given the known drug interactions between nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin, specific counseling on the avoidance of NSAIDs should be encouraged.
- Are the patient’s risk factors optimally managed?
Risk factor control should be assessed on a routine (eg every 3-6 months) basis and again if an ICAD patient has recurrent ischemic symptoms. Regular review of blood pressure logs, interval assessment of lipids, optimization of glucose control with serial hemoglobin A1c, tobacco cessation, and attention to weight management are paramount.
- Does the patient have an appropriate metabolic response to antiplatelet therapy?
If risk factors are optimized and the patient reports adherence to their medication regimen, aspirin and clopidogrel response should be evaluated. Aspirin resistance can be assessed by measuring the urinary level of 11-dehydrothromboxane B2 [15]. A urine level >1500 pg/mg creatinine should be expected in healthy, aspirin-free individuals; however, if this level is identified in a patient who is prescribed aspirin, aspirin resistance can be diagnosed. Various causes of aspirin resistance have been reported including inadequate adherence to aspirin therapy, concomitant use of a NSAID, genetic mutations in the COX-1 gene, non-platelet sources of thromboxane A2, and high platelet turnover [15]. Aspirin dosage adjustments should be made in consultation with a hematologist.
Resistance to clopidogrel has been less widely evaluated, but one meta-analysis estimated a mean prevalence of clopidogrel non-responsiveness at 21% [16]. While there is limited data on the optimal assessment of clopidogrel responsiveness in stroke patients, on-treatment platelet reactivity has been measured in parallel by means of light transmittance aggregometry, Verifynow P2Y12 and Platelet works assays, and the IMPACT-R and PFA-100 system in one study of patients undergoing coronary stent implantation [17]; of the platelet function assays assessed, only light transmittance aggregometry, Verifynow, and Platelet works assays were significantly associated with clinical outcomes in patients undergoing coronary stent implantation. Various causes of clopidogrel resistance have been reported including inadequate adherence to clopidogrel therapy, concomitant use of medications that interfere with clopidogrel prodrug conversion in the liver to its active metabolite, and genetic mutations in the cytochrome p450 3A4 gene [18,19]. Clopidogrel dosage adjustments should be made in consultation with a hematologist.
While the majority of patients will have no recurrent ischemic symptoms on aggressive medical therapy, some patients may continue to experience recurrent ischemic stroke or TIA secondary to ICAD despite optimal medical management. Patients who have hypoperfusion resulting in borderzone infarctions may be at higher risk for recurrent ischemic symptoms despite optimal medical therapy [20]. The risks of intracranial stenting, including stroke and death, must be weighed against the potential benefits. In the angioplasty plus stenting arm of the SAMMPRIS trial, the risk of stroke or death at 30 days was 14.7% [7]. In the angioplasty plus stenting arm of the VISSIT clinical trial evaluating symptomatic ICAD patients, the 30-day risk of stroke or death was 24% [21]. While intracranial angioplasty without stenting has been proposed as an alternative, there have been no randomized clinical trials to evaluate its efficacy beyond medical therapy alone in symptomatic ICAD patients. If endovascular treatment is considered, neuro-interventionists with high volume experience appear to have lower peri-procedural complications than those with low volume experience [22].
Another strategy that may be considered in symptomatic ICAD patients who have recurrent ischemic cerebral events due to symptomatic intracranial stenosis despite optimal medical therapy is indirect revascularization via encephaloduroarteriosynangiosis (EDAS). A single center retrospective study of 36 patients with ICAD and recent (< 30 days) TIAs or nondisabling strokes in the territory of the stenotic vessel (degree of stenosis not stated) and evidence of hypoperfusion and poor collateral flow on MR perfusion and/or conventional angiogram underwent EDAS [23]. Over a 2-year follow-up period, 5.6% of patients had ischemic strokes (1 stroke was periprocedural), compared with the estimated 17.2% risk of stroke in the SAMMPRIS cohort.
While endovascular and surgical techniques for revascularization of symptomatic ICAD are options for cases with medically refractory ischemic events due to hypoperfusion, further studies are needed to determine the safety of and optimal timing for these treatments.
Post-Discharge Follow-up Evaluation
At 1 month after discharge, the patient denied any new or recurrent signs or symptoms of stroke. Home blood pressure logs showed that BP was at target. Orthostatic BPs were assessed and no evidence of hypotension on standing was identified. Repeat laboratory evaluation was remarkable for hemoglobin A1c of 6.8% and LDL cholesterol of 64 mg/dL. The patient and his wife have been walking briskly 3 times per week for 45 minutes and continue to work on dietary modifications modeled after the Mediterranean diet. He is scheduled to follow up with his vascular neurologist 3 months after the stroke to discuss transition from DAT to aspirin monotherapy.
Conclusion
Intracranial atherosclerotic disease is a common cause of ischemic stroke, particularly amongst Asian, black, and Hispanic patients. Identification of ICAD can be performed with noninvasive arterial imaging including CT angiography or contrast-enhanced MR angiography as part of an ischemic stroke workup. Optimal medical management with early DAT with aspirin and clopidogrel along with aggressive risk factor and lifestyle modification has emerged as an effective first-line therapy. In patients with recurrent ischemic symptoms while optimally medically managed, endovascular therapy with angioplasty with or without stenting may be considered.
Corresponding author: Fadi Nahab, MD, 1635 Clifton Rd., Clinic B, Suite 2200, Atlanta, GA 30322, [email protected].
Financial disclosures: None.
1. Derdeyn C, Chimowitz M, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial arterial stenosis (SAMMPRIS): the final results of a randomized trial. Lancet 2014;383:333–41.
2. Chimowitz, MI, Lynn MJ, Howlett-Smith H, et al. Comparision of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2004;351:1250–1.
3. Feldmann E, Wilterdink JL, Kosinski A, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology 2007;68:2099–106.
4. Willinek WA, von Falkenhausen M, Born M, et al. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: a prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke 2005;36:38–43.
5. Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol 2005;26:1012–21.
6. Qureshi A, Caplan L. Intracranial atherosclerosis. Lancet 2014;383:984–98.
7. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003.
8. Winningham M, Kasshout T, Bamford L, et al. Optimal duration of dual antiplatelet therapy for symptomatic intracranial stenosis. Stroke 2015;46:ATP100.
9. Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolization in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomized, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–97.
10. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
11. Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115:2969–75.
12. Agency for Healthcare Research and Quality. Clinical guidelines for prescribing pharmacotherapy for smoking cessation. Accessed 8 May 2016 at www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/prescrib.html.
13. Nahab F, Kingston C, Frankel MR, et al. Early aggressive medical management for patients with symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis 2013;22:87–91.
14. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555–63.
15. Quest Diagnostics. Aspirin resistance. Accessed 23 Mar 2016 at www.questdiagnostics.com/testcenter/testguide.action?dc=TS_Aspirin_Resistance.
16. Alberts MJ. Platelet function testing for aspirin resistance is reasonable to do. Stroke 2010;41:2400–401.
17. Feher G, Feher A, Pusch G, et al. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol 2010;26:171–86.
18. Taubert D, Kastrati A, Harlfinger S, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost 2004;92:311–6.
19. Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 2004;109:166–71.
20. Wabnitz AM, Derdeyn CP, Fiorella DJ, et al; for the SAMMPRIS Investigators. Infarct patterns in the anterior circulation as predictors of recurrent stroke in the medical arm of SAMMPRIS. Stroke 2016;47:A103.
21. Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240–8.
22. Nahab F, Lynn MJ, Kasner SE, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology 2009;72:2014–9.
23. Gonzalez N, Dusick J, Connolly M, et al. Encephaloduroarteriosynangiosis for adult intracranial arterial steno-occlusive disease: long-term single-center experience with 107 operations. J Neurosurg 2015;123:654–61.
From the Emory University School of Medicine, Atlanta, GA.
Abstract
- Objective: To provide an overview of the diagnosis, clinical presentation, and management of symptomatic intracranial atherosclerotic disease (ICAD).
- Methods: Review of the current literature in the context of a clinical case.
- Results: ICAD is a common cause of ischemic strokes or transient ischemic attacks (TIAs), especially among Asian, black, and Hispanic patients. ICAD can be identified with noninvasive arterial imaging such as CT angiography, MR angiography, or transcranial Doppler ultrasound of the head when evaluating for the cause of an ischemic stroke or TIA. Aggressive medical management with dual antiplatelet therapy and lifestyle and risk factor modification has emerged as effective first-line therapy. In patients who have recurrent ischemic symptoms while on aggressive medical management, endovascular treatment can be considered.
- Conclusion: When symptomatic ICAD is identified early, aggressive medical management is effective in reducing the risk of recurrent ischemic events in this patient population.
Symptomatic intracranial atherosclerotic disease (ICAD) may represent the most common cause of ischemic stroke worldwide and the cause of approximately 8% to 10% of ischemic strokes in the United States [1–7]. It is a particularly important clinical entity due to the high recurrence rate of ischemic events in this population. The estimated recurrent stroke risk from symptomatic ICAD has been reported to be as high as 14.9% in the first year after an initial ischemic event [1].There are multiple risk factors for ICAD. Non-modifiable risk factors include race (particularly Asian, black, or Hispanic race), age, and family history of coronary artery disease or stroke; modifiable risk factors include diabetes, hypertension, and hyperlipidemia [2].
Case Study
Initial Presentation
A 78-year-old right-handed man presents to the outpatient clinic for follow-up evaluation after inpatient admission for acute ischemic stroke. The patient has an established medical history of hypertension, diabetes mellitus (diagnosed 10 years ago), dyslipidemia, and a 50 pack-year history of tobacco use.
Two weeks prior to the clinic visit, the patient presented to the emergency department (ED) via emergency medical services with right face and arm weakness and numbness and the inability to speak that had been ongoing for approximately 1 hour. The patient’s wife reported to ED providers that the patient had a similar episode 1 month prior that resolved completely in 30 to 45 minutes (and for which the patient never sought medical attention). Home medications at the time of admission included aspirin 81 mg and pravastatin 20 mg daily, both of which he takes intermittently.
The initial blood pressure was 185/76 mm Hg and blood glucose was 225 mg/dL. Initial exam was remarkable for the inability to answer orientation questions (but able to follow simple commands), forced gaze deviation to the left, right lower facial weakness, weakness in the right arm with no antigravity movement, moderately decreased sensation of the right face and arm, severe expressive aphasia, and severe dysarthria. A CT of the head without contrast showed no evidence of hemorrhage. Patient was suspected of having an acute ischemic stroke and was given intravenous tPA.
What are the possible mechanisms for this patient’s presentation with ischemic stroke?
This patient is presenting with right face and arm weakness with sensory loss, gaze deviation to the left, and expressive aphasia. Abnormalities of speech and gaze paresis that localize to the left frontal lobe with cortical involvement make a subcortical or brainstem lacunar ischemic event less likely. The syndrome is suggestive of a large artery occlusion of the left middle cerebral artery. This type of large artery occlusive disease in the anterior circulation is most often due to an embolus (artery-to-artery or cardiac origin) event and requires arterial imaging of the head and neck acutely as endovascular thrombectomy has been associated with reduced disability compared to intravenous tPA alone.
Further Evaluation
What imaging is recommended to identify patients with ICAD?
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial sought to evaluate the reliability of noninvasive imaging modalities to identify a 50%–99% stenosis of large proximal cerebral arteries as compared with the gold standard of conventional angiography [3]. Qualifying vessels included the M1 segment of the middle cerebral artery, the carotid siphon, and intracranial vertebral and basilar arteries. Imaging techniques included MRA head without contrast (time of flight) and TCD ultrasound. Both of these imaging modalities demonstrated a high negative predictive value (NPV) for the presence of a 50%–99% intracranial stenosis; however, the positive predictive value (PPV) for TCD and MRA was only 55% and 66%, respectively. Subsequent studies of contrast-enhanced MRA and CTA of the headhave shown high PPV (78%–94%) and NPV (95%–100%) when compared with cerebral angiography for the detection of 70%–99% ICAD [4,5]. Together these data suggest that TCD and MRA of the head without contrast can be used as screening tools to rule out ICAD when normal; however, subsequent contrast-enhanced MRA or CTA is required when abnormalities are identified.
When choosing the best noninvasive imaging modality for any given patient, concomitant medical issues including renal disease, age, presence of a pacemaker, implantable cardiac defibrillator or other metal, and claustrophobia are factors to consider.
What are the potential mechanisms of ischemic stroke in patients with symptomatic ICAD?
Stroke in the symptomatic ICAD group occurs as a result of either (1) thrombus formation at the site of a high-grade stenosis due to an unstable atherosclerotic plaque, (2) low flow through a narrow, stenotic artery (hypoperfusion) or (3) a combination of atheroembolism and hypoperfusion [6]. While a thrombus can occlude the parent vessel at the site of stenosis, more commonly the thrombus migrates distally as an artery-artery embolism to a smaller caliber artery.
What medical regimen is recommended for this patient with symptomatic ICAD?
Dual Antiplatelet Therapy
DAT with aspirin and clopidogrel is thought to reduce ischemic events related to regional thromboembolism. The best clinical evidence of the use of DAT in symptomatic ICAD is found in outcomes data for the SAMMPRIS trial where 30-day recurrent ischemic stroke rates in the aggressive medical management arm were significantly lower than patients treated with aspirin or warfarin monotherapy as part of the Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis (WASID) trial. A subgroup of patients enrolled in WASID with similar clinical characteristics to those in SAMMPRIS were found to have a 30-day rate of stroke or death of 10.7% and a collective 1-year rate of ischemic stroke, brain hemorrhage, or vascular death of 25% [2]. The corresponding rates in the SAMMPRIS medical management arm were 5.8% at 30 days and 17.5% at 1 year. Given that the recurrent stroke rates were significantly lower in as little as 30 days, these benefits have been hypothesized to be more likely secondary to the DAT regimen than other risk factor modification [7]. There is some evidence that longer term DAT with aspirin and clopidogrel up to 1 year may be associated with further reduction in stroke, MI, and vascular death with similar bleeding risk, but this needs to be evaluated in prospective studies [8].
Additional evidence of the benefit and potential mechanism from DAT in ICAD comes from CLAIR (Clopidogrel plus Aspirin versus Aspirin alone for Reducing Embolization in Patients with Acute Symptomatic Cerebral or Carotid Artery Stenosis Trial), a multicenter, randomized trial with blinded outcome assessment, with patients recruited at sites in Hong Kong, Singapore, China, Thailand, and Malaysia [9]. Patients were enrolled with an extracranial or intracranial stenosis (greater than or equal to 50%, as diagnosed by carotid duplex, transcranial Doppler, or magnetic resonance angiography) if they had a clinical diagnosis of acute ischemic stroke or transient ischemic attack (TIA) during the 7 days prior to enrollment and were found to have microembolic signals (suggesting microemboli of atherosclerotic origin) detected at baseline assessment with transcranial Doppler ultrasound. Patients were randomized to DAT with aspirin and clopidogrel versus aspirin monotherapy. Patients on DAT had significantly reduced microembolic signals compared with patients on aspirin monotherapy. Asymptomatic embolization with dual antiplatelet therapy may also proffer a reduction in clinical events in these patients with symptomatic ICAD.
Statin Therapy
Statin therapy is an integral part in the prevention of recurrent ischemic events in the symptomatic ICAD cohort. Post-hoc analyses from the WASID trial found that total cholesterol greater than 200 mg/dL was associated with an increased risk of ischemic stroke, myocardial infarction, or vascular death.
In 2013, the American Heart Association released new guidelines regarding the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults [10]. Clinical atherosclerotic cardiovascular disease (ASCVD) is the manifestation of systemic atherosclerotic disease and defined as acute coronary syndromes, a history of myocardial infarction, stable or unstable angina, a history of coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin. Recommendations for secondary prevention in patients with clinical ASCVD include the use of high-intensity statin therapy (atorvastatin 40 or 80 mg, rosuvastatin 20 or 40 mg) for patients less than 75 years of age or moderate-intensity statin therapy (atorvastatin 10 or 20 mg, rosuvastatin 5 or 10 mg, pravastatin 40 mg, or simvastatin 20 or 40 mg) for patients > 75 years of age. In the SAMMPRIS trial, an LDL cholesterol level less than 70 mg/dL was targeted [7].
Blood Pressure Management
Post-hoc analysis of data from WASID demonstrated a statistically significant increase in recurrent stroke risk with increasing mean systolic and diastolic blood pressure (BP) [11]. This was particularly true in patients with mean SBP > 160 mm Hg. This is contrary to the common perception that BP should be maintained higher in patients with intracranial stenosis. In multivariable analysis, systolic BP greater than 140 mm Hg was associated with an increased risk of ischemic stroke, myocardial infarction, or vascular death. In the SAMMPRIS trial, the recommended BP goals for patients with symptomatic ICAD were less than 140/90 mm Hg in non-diabetic patients and less than 130/80 mm Hg in diabetic patients [7]. The timing and pace of blood pressure normalization for a recently symptomatic patient with ICAD is still unclear and needs further study.
Lifestyle Modification and Secondary Risk Factors
The SAMMPRIS protocol incorporated a lifestyle coach for all patients enrolled in the study. Lifestyle modification to achieve smoking cessation, regular physical activity, weight reduction for overweight patients, and glucose control in diabetes (goal hemoglobin A1c < 7.0%) were complementary to the pharmacotherapy (aspirin, clopidogrel, statin, and antihypertensive regimen) prescribed [7].
Patients should be encouraged to participate in aerobic exercise for at least 30 minutes at least 3 times weekly. Dietary modifications modeled after the Mediterranean diet should be encouraged. These should be coupled with additional efforts to address excessive weight as needed.
Successful smoking cessation proves one of the most challenging lifestyle modifications for this group of patients and may require the employment of an extended support system with both family and medical providers. Nicotine supplementation is a common first-line aid for cessation, which may be provided in the form of gum or transdermal patches. Additional pharmacotherapy to address central mechanisms of addiction may be necessary. Many patients benefit from the addition of an antidepressant therapy such as bupropion or an adjunctive medication such as varenicline (a nicotine receptor partial agonist that helps with breaking nicotine addiction). It is important to establish a quit date and detailed, multistep plan for cessation [12].
Exceptions and Other Considerations
These evidence-based recommendations are directed toward a specific group of patients with high-grade stenosis (70%–99%), a single symptomatic vessel, and relatively short segments of stenosis (< 14 mm) based on the inclusion and exclusion criteria for the SAMMPRIS trial [7]. Additionally, these patients were not enrolled during the immediate period following an acute ischemic event. There is less evidence on the management of patients who present with < 70% symptomatic ICAD but we can infer that therapy with DAT, statins, and risk factor modification are still of benefit for patients with 50%–69% stenosis or those requiring treatment during the acute evaluation [13]. Additionally, some of these SAMMPRIS exclusion criteria were included to select patients who could be considered intracranial stenting candidates (eg, short segment stenosis, single vessel disease, etc) and are not considered to impact on the benefits of the aggressive medical management regimen.
Further Evaluation
The patient was initiated on antithrombotic therapy on hospital day 2 (24 hours after IV tPA administration) with aspirin 325 mg daily and clopidogrel 300 mg oral load followed by 75 mg daily, ranitidine 150 mg twice a day to reduce gastroesophageal reflux and atorvastatin 80 mg daily. On hospital day 3, his neurological exam remained stable and he was initiated on losartan 25 mg daily with a plan to titrate the dose up as an outpatient to achieve target blood pressure. The patient and his wife were advised to maintain a blood pressure log and provide it to his physicians at follow-up and was provided smoking cessation counseling with a plan to remain off tobacco after discharge utilizing nicotine patches. The patient was discharged from the hospital 4 days after admission with a diagnosis of acute ischemic stroke secondary to symptomatic ICAD from regional thromboembolism. Early outpatient follow-up was scheduled with his primary physician within 1 week after discharge to evaluate BP and a vascular neurologist within 1 month after discharge.
What factors predict recurrent stroke in patients with symptomatic ICAD?
As evidenced in the WASID post-hoc analysis, female sex, prior ischemic stroke (versus TIA), time from qualifying event to enrollment (≤ 17 days after ischemic event), severity of stenosis (≥ 70% stenosis) of the symptomatic vessel, and history of diabetes were identified as independent risk factors for recurrent stroke in this population [14].
How do we manage patients who continue to have symptoms despite optimal medical therapy?
When evaluating symptomatic ICAD patients with recur-rent neurologic symptoms, several important factors should be considered:
- Do the recurrent neurologic symptoms localize to the original ICAD location?
Patients with symptomatic ICAD often have multiple risk factors that put them at risk for other subtypes of ischemic stroke including lacunar stroke. Repeat neuro-imaging with brain MRI is recommended to confirm the localization of a recurrent stroke is inside or outside of the territory of the ICAD.
- Were the recurrent neurologic symptoms provoked?
Patients with ICAD can be uniquely sensitive to fluctuations in cerebral blood flow and oxygenation. Diabetic patients with ICAD can develop autonomic neuropathy that results in orthostatic hypotension. If patients have recurrent transient ischemic events associated with postural changes, adequate treatment of orthostasis can reduce recurrent ischemic events. Other provoked circumstances include patients who develop anemia and patients with untreated severe obstructive sleep apnea who may awake with recurrent ischemic events due to hypoxemia.
- Is the patient adhering to the medication regimen?
Patients with symptomatic ICAD frequently have multiple medications to take for treatment of their comorbidities. Discussion of the number of missed doses of medications over the prior month is important to ascertain adherence. Patients should also be counseled to avoid concomitant medications that may cause drug interactions; given the known drug interactions between nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin, specific counseling on the avoidance of NSAIDs should be encouraged.
- Are the patient’s risk factors optimally managed?
Risk factor control should be assessed on a routine (eg every 3-6 months) basis and again if an ICAD patient has recurrent ischemic symptoms. Regular review of blood pressure logs, interval assessment of lipids, optimization of glucose control with serial hemoglobin A1c, tobacco cessation, and attention to weight management are paramount.
- Does the patient have an appropriate metabolic response to antiplatelet therapy?
If risk factors are optimized and the patient reports adherence to their medication regimen, aspirin and clopidogrel response should be evaluated. Aspirin resistance can be assessed by measuring the urinary level of 11-dehydrothromboxane B2 [15]. A urine level >1500 pg/mg creatinine should be expected in healthy, aspirin-free individuals; however, if this level is identified in a patient who is prescribed aspirin, aspirin resistance can be diagnosed. Various causes of aspirin resistance have been reported including inadequate adherence to aspirin therapy, concomitant use of a NSAID, genetic mutations in the COX-1 gene, non-platelet sources of thromboxane A2, and high platelet turnover [15]. Aspirin dosage adjustments should be made in consultation with a hematologist.
Resistance to clopidogrel has been less widely evaluated, but one meta-analysis estimated a mean prevalence of clopidogrel non-responsiveness at 21% [16]. While there is limited data on the optimal assessment of clopidogrel responsiveness in stroke patients, on-treatment platelet reactivity has been measured in parallel by means of light transmittance aggregometry, Verifynow P2Y12 and Platelet works assays, and the IMPACT-R and PFA-100 system in one study of patients undergoing coronary stent implantation [17]; of the platelet function assays assessed, only light transmittance aggregometry, Verifynow, and Platelet works assays were significantly associated with clinical outcomes in patients undergoing coronary stent implantation. Various causes of clopidogrel resistance have been reported including inadequate adherence to clopidogrel therapy, concomitant use of medications that interfere with clopidogrel prodrug conversion in the liver to its active metabolite, and genetic mutations in the cytochrome p450 3A4 gene [18,19]. Clopidogrel dosage adjustments should be made in consultation with a hematologist.
While the majority of patients will have no recurrent ischemic symptoms on aggressive medical therapy, some patients may continue to experience recurrent ischemic stroke or TIA secondary to ICAD despite optimal medical management. Patients who have hypoperfusion resulting in borderzone infarctions may be at higher risk for recurrent ischemic symptoms despite optimal medical therapy [20]. The risks of intracranial stenting, including stroke and death, must be weighed against the potential benefits. In the angioplasty plus stenting arm of the SAMMPRIS trial, the risk of stroke or death at 30 days was 14.7% [7]. In the angioplasty plus stenting arm of the VISSIT clinical trial evaluating symptomatic ICAD patients, the 30-day risk of stroke or death was 24% [21]. While intracranial angioplasty without stenting has been proposed as an alternative, there have been no randomized clinical trials to evaluate its efficacy beyond medical therapy alone in symptomatic ICAD patients. If endovascular treatment is considered, neuro-interventionists with high volume experience appear to have lower peri-procedural complications than those with low volume experience [22].
Another strategy that may be considered in symptomatic ICAD patients who have recurrent ischemic cerebral events due to symptomatic intracranial stenosis despite optimal medical therapy is indirect revascularization via encephaloduroarteriosynangiosis (EDAS). A single center retrospective study of 36 patients with ICAD and recent (< 30 days) TIAs or nondisabling strokes in the territory of the stenotic vessel (degree of stenosis not stated) and evidence of hypoperfusion and poor collateral flow on MR perfusion and/or conventional angiogram underwent EDAS [23]. Over a 2-year follow-up period, 5.6% of patients had ischemic strokes (1 stroke was periprocedural), compared with the estimated 17.2% risk of stroke in the SAMMPRIS cohort.
While endovascular and surgical techniques for revascularization of symptomatic ICAD are options for cases with medically refractory ischemic events due to hypoperfusion, further studies are needed to determine the safety of and optimal timing for these treatments.
Post-Discharge Follow-up Evaluation
At 1 month after discharge, the patient denied any new or recurrent signs or symptoms of stroke. Home blood pressure logs showed that BP was at target. Orthostatic BPs were assessed and no evidence of hypotension on standing was identified. Repeat laboratory evaluation was remarkable for hemoglobin A1c of 6.8% and LDL cholesterol of 64 mg/dL. The patient and his wife have been walking briskly 3 times per week for 45 minutes and continue to work on dietary modifications modeled after the Mediterranean diet. He is scheduled to follow up with his vascular neurologist 3 months after the stroke to discuss transition from DAT to aspirin monotherapy.
Conclusion
Intracranial atherosclerotic disease is a common cause of ischemic stroke, particularly amongst Asian, black, and Hispanic patients. Identification of ICAD can be performed with noninvasive arterial imaging including CT angiography or contrast-enhanced MR angiography as part of an ischemic stroke workup. Optimal medical management with early DAT with aspirin and clopidogrel along with aggressive risk factor and lifestyle modification has emerged as an effective first-line therapy. In patients with recurrent ischemic symptoms while optimally medically managed, endovascular therapy with angioplasty with or without stenting may be considered.
Corresponding author: Fadi Nahab, MD, 1635 Clifton Rd., Clinic B, Suite 2200, Atlanta, GA 30322, [email protected].
Financial disclosures: None.
From the Emory University School of Medicine, Atlanta, GA.
Abstract
- Objective: To provide an overview of the diagnosis, clinical presentation, and management of symptomatic intracranial atherosclerotic disease (ICAD).
- Methods: Review of the current literature in the context of a clinical case.
- Results: ICAD is a common cause of ischemic strokes or transient ischemic attacks (TIAs), especially among Asian, black, and Hispanic patients. ICAD can be identified with noninvasive arterial imaging such as CT angiography, MR angiography, or transcranial Doppler ultrasound of the head when evaluating for the cause of an ischemic stroke or TIA. Aggressive medical management with dual antiplatelet therapy and lifestyle and risk factor modification has emerged as effective first-line therapy. In patients who have recurrent ischemic symptoms while on aggressive medical management, endovascular treatment can be considered.
- Conclusion: When symptomatic ICAD is identified early, aggressive medical management is effective in reducing the risk of recurrent ischemic events in this patient population.
Symptomatic intracranial atherosclerotic disease (ICAD) may represent the most common cause of ischemic stroke worldwide and the cause of approximately 8% to 10% of ischemic strokes in the United States [1–7]. It is a particularly important clinical entity due to the high recurrence rate of ischemic events in this population. The estimated recurrent stroke risk from symptomatic ICAD has been reported to be as high as 14.9% in the first year after an initial ischemic event [1].There are multiple risk factors for ICAD. Non-modifiable risk factors include race (particularly Asian, black, or Hispanic race), age, and family history of coronary artery disease or stroke; modifiable risk factors include diabetes, hypertension, and hyperlipidemia [2].
Case Study
Initial Presentation
A 78-year-old right-handed man presents to the outpatient clinic for follow-up evaluation after inpatient admission for acute ischemic stroke. The patient has an established medical history of hypertension, diabetes mellitus (diagnosed 10 years ago), dyslipidemia, and a 50 pack-year history of tobacco use.
Two weeks prior to the clinic visit, the patient presented to the emergency department (ED) via emergency medical services with right face and arm weakness and numbness and the inability to speak that had been ongoing for approximately 1 hour. The patient’s wife reported to ED providers that the patient had a similar episode 1 month prior that resolved completely in 30 to 45 minutes (and for which the patient never sought medical attention). Home medications at the time of admission included aspirin 81 mg and pravastatin 20 mg daily, both of which he takes intermittently.
The initial blood pressure was 185/76 mm Hg and blood glucose was 225 mg/dL. Initial exam was remarkable for the inability to answer orientation questions (but able to follow simple commands), forced gaze deviation to the left, right lower facial weakness, weakness in the right arm with no antigravity movement, moderately decreased sensation of the right face and arm, severe expressive aphasia, and severe dysarthria. A CT of the head without contrast showed no evidence of hemorrhage. Patient was suspected of having an acute ischemic stroke and was given intravenous tPA.
What are the possible mechanisms for this patient’s presentation with ischemic stroke?
This patient is presenting with right face and arm weakness with sensory loss, gaze deviation to the left, and expressive aphasia. Abnormalities of speech and gaze paresis that localize to the left frontal lobe with cortical involvement make a subcortical or brainstem lacunar ischemic event less likely. The syndrome is suggestive of a large artery occlusion of the left middle cerebral artery. This type of large artery occlusive disease in the anterior circulation is most often due to an embolus (artery-to-artery or cardiac origin) event and requires arterial imaging of the head and neck acutely as endovascular thrombectomy has been associated with reduced disability compared to intravenous tPA alone.
Further Evaluation
What imaging is recommended to identify patients with ICAD?
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial sought to evaluate the reliability of noninvasive imaging modalities to identify a 50%–99% stenosis of large proximal cerebral arteries as compared with the gold standard of conventional angiography [3]. Qualifying vessels included the M1 segment of the middle cerebral artery, the carotid siphon, and intracranial vertebral and basilar arteries. Imaging techniques included MRA head without contrast (time of flight) and TCD ultrasound. Both of these imaging modalities demonstrated a high negative predictive value (NPV) for the presence of a 50%–99% intracranial stenosis; however, the positive predictive value (PPV) for TCD and MRA was only 55% and 66%, respectively. Subsequent studies of contrast-enhanced MRA and CTA of the headhave shown high PPV (78%–94%) and NPV (95%–100%) when compared with cerebral angiography for the detection of 70%–99% ICAD [4,5]. Together these data suggest that TCD and MRA of the head without contrast can be used as screening tools to rule out ICAD when normal; however, subsequent contrast-enhanced MRA or CTA is required when abnormalities are identified.
When choosing the best noninvasive imaging modality for any given patient, concomitant medical issues including renal disease, age, presence of a pacemaker, implantable cardiac defibrillator or other metal, and claustrophobia are factors to consider.
What are the potential mechanisms of ischemic stroke in patients with symptomatic ICAD?
Stroke in the symptomatic ICAD group occurs as a result of either (1) thrombus formation at the site of a high-grade stenosis due to an unstable atherosclerotic plaque, (2) low flow through a narrow, stenotic artery (hypoperfusion) or (3) a combination of atheroembolism and hypoperfusion [6]. While a thrombus can occlude the parent vessel at the site of stenosis, more commonly the thrombus migrates distally as an artery-artery embolism to a smaller caliber artery.
What medical regimen is recommended for this patient with symptomatic ICAD?
Dual Antiplatelet Therapy
DAT with aspirin and clopidogrel is thought to reduce ischemic events related to regional thromboembolism. The best clinical evidence of the use of DAT in symptomatic ICAD is found in outcomes data for the SAMMPRIS trial where 30-day recurrent ischemic stroke rates in the aggressive medical management arm were significantly lower than patients treated with aspirin or warfarin monotherapy as part of the Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis (WASID) trial. A subgroup of patients enrolled in WASID with similar clinical characteristics to those in SAMMPRIS were found to have a 30-day rate of stroke or death of 10.7% and a collective 1-year rate of ischemic stroke, brain hemorrhage, or vascular death of 25% [2]. The corresponding rates in the SAMMPRIS medical management arm were 5.8% at 30 days and 17.5% at 1 year. Given that the recurrent stroke rates were significantly lower in as little as 30 days, these benefits have been hypothesized to be more likely secondary to the DAT regimen than other risk factor modification [7]. There is some evidence that longer term DAT with aspirin and clopidogrel up to 1 year may be associated with further reduction in stroke, MI, and vascular death with similar bleeding risk, but this needs to be evaluated in prospective studies [8].
Additional evidence of the benefit and potential mechanism from DAT in ICAD comes from CLAIR (Clopidogrel plus Aspirin versus Aspirin alone for Reducing Embolization in Patients with Acute Symptomatic Cerebral or Carotid Artery Stenosis Trial), a multicenter, randomized trial with blinded outcome assessment, with patients recruited at sites in Hong Kong, Singapore, China, Thailand, and Malaysia [9]. Patients were enrolled with an extracranial or intracranial stenosis (greater than or equal to 50%, as diagnosed by carotid duplex, transcranial Doppler, or magnetic resonance angiography) if they had a clinical diagnosis of acute ischemic stroke or transient ischemic attack (TIA) during the 7 days prior to enrollment and were found to have microembolic signals (suggesting microemboli of atherosclerotic origin) detected at baseline assessment with transcranial Doppler ultrasound. Patients were randomized to DAT with aspirin and clopidogrel versus aspirin monotherapy. Patients on DAT had significantly reduced microembolic signals compared with patients on aspirin monotherapy. Asymptomatic embolization with dual antiplatelet therapy may also proffer a reduction in clinical events in these patients with symptomatic ICAD.
Statin Therapy
Statin therapy is an integral part in the prevention of recurrent ischemic events in the symptomatic ICAD cohort. Post-hoc analyses from the WASID trial found that total cholesterol greater than 200 mg/dL was associated with an increased risk of ischemic stroke, myocardial infarction, or vascular death.
In 2013, the American Heart Association released new guidelines regarding the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults [10]. Clinical atherosclerotic cardiovascular disease (ASCVD) is the manifestation of systemic atherosclerotic disease and defined as acute coronary syndromes, a history of myocardial infarction, stable or unstable angina, a history of coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin. Recommendations for secondary prevention in patients with clinical ASCVD include the use of high-intensity statin therapy (atorvastatin 40 or 80 mg, rosuvastatin 20 or 40 mg) for patients less than 75 years of age or moderate-intensity statin therapy (atorvastatin 10 or 20 mg, rosuvastatin 5 or 10 mg, pravastatin 40 mg, or simvastatin 20 or 40 mg) for patients > 75 years of age. In the SAMMPRIS trial, an LDL cholesterol level less than 70 mg/dL was targeted [7].
Blood Pressure Management
Post-hoc analysis of data from WASID demonstrated a statistically significant increase in recurrent stroke risk with increasing mean systolic and diastolic blood pressure (BP) [11]. This was particularly true in patients with mean SBP > 160 mm Hg. This is contrary to the common perception that BP should be maintained higher in patients with intracranial stenosis. In multivariable analysis, systolic BP greater than 140 mm Hg was associated with an increased risk of ischemic stroke, myocardial infarction, or vascular death. In the SAMMPRIS trial, the recommended BP goals for patients with symptomatic ICAD were less than 140/90 mm Hg in non-diabetic patients and less than 130/80 mm Hg in diabetic patients [7]. The timing and pace of blood pressure normalization for a recently symptomatic patient with ICAD is still unclear and needs further study.
Lifestyle Modification and Secondary Risk Factors
The SAMMPRIS protocol incorporated a lifestyle coach for all patients enrolled in the study. Lifestyle modification to achieve smoking cessation, regular physical activity, weight reduction for overweight patients, and glucose control in diabetes (goal hemoglobin A1c < 7.0%) were complementary to the pharmacotherapy (aspirin, clopidogrel, statin, and antihypertensive regimen) prescribed [7].
Patients should be encouraged to participate in aerobic exercise for at least 30 minutes at least 3 times weekly. Dietary modifications modeled after the Mediterranean diet should be encouraged. These should be coupled with additional efforts to address excessive weight as needed.
Successful smoking cessation proves one of the most challenging lifestyle modifications for this group of patients and may require the employment of an extended support system with both family and medical providers. Nicotine supplementation is a common first-line aid for cessation, which may be provided in the form of gum or transdermal patches. Additional pharmacotherapy to address central mechanisms of addiction may be necessary. Many patients benefit from the addition of an antidepressant therapy such as bupropion or an adjunctive medication such as varenicline (a nicotine receptor partial agonist that helps with breaking nicotine addiction). It is important to establish a quit date and detailed, multistep plan for cessation [12].
Exceptions and Other Considerations
These evidence-based recommendations are directed toward a specific group of patients with high-grade stenosis (70%–99%), a single symptomatic vessel, and relatively short segments of stenosis (< 14 mm) based on the inclusion and exclusion criteria for the SAMMPRIS trial [7]. Additionally, these patients were not enrolled during the immediate period following an acute ischemic event. There is less evidence on the management of patients who present with < 70% symptomatic ICAD but we can infer that therapy with DAT, statins, and risk factor modification are still of benefit for patients with 50%–69% stenosis or those requiring treatment during the acute evaluation [13]. Additionally, some of these SAMMPRIS exclusion criteria were included to select patients who could be considered intracranial stenting candidates (eg, short segment stenosis, single vessel disease, etc) and are not considered to impact on the benefits of the aggressive medical management regimen.
Further Evaluation
The patient was initiated on antithrombotic therapy on hospital day 2 (24 hours after IV tPA administration) with aspirin 325 mg daily and clopidogrel 300 mg oral load followed by 75 mg daily, ranitidine 150 mg twice a day to reduce gastroesophageal reflux and atorvastatin 80 mg daily. On hospital day 3, his neurological exam remained stable and he was initiated on losartan 25 mg daily with a plan to titrate the dose up as an outpatient to achieve target blood pressure. The patient and his wife were advised to maintain a blood pressure log and provide it to his physicians at follow-up and was provided smoking cessation counseling with a plan to remain off tobacco after discharge utilizing nicotine patches. The patient was discharged from the hospital 4 days after admission with a diagnosis of acute ischemic stroke secondary to symptomatic ICAD from regional thromboembolism. Early outpatient follow-up was scheduled with his primary physician within 1 week after discharge to evaluate BP and a vascular neurologist within 1 month after discharge.
What factors predict recurrent stroke in patients with symptomatic ICAD?
As evidenced in the WASID post-hoc analysis, female sex, prior ischemic stroke (versus TIA), time from qualifying event to enrollment (≤ 17 days after ischemic event), severity of stenosis (≥ 70% stenosis) of the symptomatic vessel, and history of diabetes were identified as independent risk factors for recurrent stroke in this population [14].
How do we manage patients who continue to have symptoms despite optimal medical therapy?
When evaluating symptomatic ICAD patients with recur-rent neurologic symptoms, several important factors should be considered:
- Do the recurrent neurologic symptoms localize to the original ICAD location?
Patients with symptomatic ICAD often have multiple risk factors that put them at risk for other subtypes of ischemic stroke including lacunar stroke. Repeat neuro-imaging with brain MRI is recommended to confirm the localization of a recurrent stroke is inside or outside of the territory of the ICAD.
- Were the recurrent neurologic symptoms provoked?
Patients with ICAD can be uniquely sensitive to fluctuations in cerebral blood flow and oxygenation. Diabetic patients with ICAD can develop autonomic neuropathy that results in orthostatic hypotension. If patients have recurrent transient ischemic events associated with postural changes, adequate treatment of orthostasis can reduce recurrent ischemic events. Other provoked circumstances include patients who develop anemia and patients with untreated severe obstructive sleep apnea who may awake with recurrent ischemic events due to hypoxemia.
- Is the patient adhering to the medication regimen?
Patients with symptomatic ICAD frequently have multiple medications to take for treatment of their comorbidities. Discussion of the number of missed doses of medications over the prior month is important to ascertain adherence. Patients should also be counseled to avoid concomitant medications that may cause drug interactions; given the known drug interactions between nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin, specific counseling on the avoidance of NSAIDs should be encouraged.
- Are the patient’s risk factors optimally managed?
Risk factor control should be assessed on a routine (eg every 3-6 months) basis and again if an ICAD patient has recurrent ischemic symptoms. Regular review of blood pressure logs, interval assessment of lipids, optimization of glucose control with serial hemoglobin A1c, tobacco cessation, and attention to weight management are paramount.
- Does the patient have an appropriate metabolic response to antiplatelet therapy?
If risk factors are optimized and the patient reports adherence to their medication regimen, aspirin and clopidogrel response should be evaluated. Aspirin resistance can be assessed by measuring the urinary level of 11-dehydrothromboxane B2 [15]. A urine level >1500 pg/mg creatinine should be expected in healthy, aspirin-free individuals; however, if this level is identified in a patient who is prescribed aspirin, aspirin resistance can be diagnosed. Various causes of aspirin resistance have been reported including inadequate adherence to aspirin therapy, concomitant use of a NSAID, genetic mutations in the COX-1 gene, non-platelet sources of thromboxane A2, and high platelet turnover [15]. Aspirin dosage adjustments should be made in consultation with a hematologist.
Resistance to clopidogrel has been less widely evaluated, but one meta-analysis estimated a mean prevalence of clopidogrel non-responsiveness at 21% [16]. While there is limited data on the optimal assessment of clopidogrel responsiveness in stroke patients, on-treatment platelet reactivity has been measured in parallel by means of light transmittance aggregometry, Verifynow P2Y12 and Platelet works assays, and the IMPACT-R and PFA-100 system in one study of patients undergoing coronary stent implantation [17]; of the platelet function assays assessed, only light transmittance aggregometry, Verifynow, and Platelet works assays were significantly associated with clinical outcomes in patients undergoing coronary stent implantation. Various causes of clopidogrel resistance have been reported including inadequate adherence to clopidogrel therapy, concomitant use of medications that interfere with clopidogrel prodrug conversion in the liver to its active metabolite, and genetic mutations in the cytochrome p450 3A4 gene [18,19]. Clopidogrel dosage adjustments should be made in consultation with a hematologist.
While the majority of patients will have no recurrent ischemic symptoms on aggressive medical therapy, some patients may continue to experience recurrent ischemic stroke or TIA secondary to ICAD despite optimal medical management. Patients who have hypoperfusion resulting in borderzone infarctions may be at higher risk for recurrent ischemic symptoms despite optimal medical therapy [20]. The risks of intracranial stenting, including stroke and death, must be weighed against the potential benefits. In the angioplasty plus stenting arm of the SAMMPRIS trial, the risk of stroke or death at 30 days was 14.7% [7]. In the angioplasty plus stenting arm of the VISSIT clinical trial evaluating symptomatic ICAD patients, the 30-day risk of stroke or death was 24% [21]. While intracranial angioplasty without stenting has been proposed as an alternative, there have been no randomized clinical trials to evaluate its efficacy beyond medical therapy alone in symptomatic ICAD patients. If endovascular treatment is considered, neuro-interventionists with high volume experience appear to have lower peri-procedural complications than those with low volume experience [22].
Another strategy that may be considered in symptomatic ICAD patients who have recurrent ischemic cerebral events due to symptomatic intracranial stenosis despite optimal medical therapy is indirect revascularization via encephaloduroarteriosynangiosis (EDAS). A single center retrospective study of 36 patients with ICAD and recent (< 30 days) TIAs or nondisabling strokes in the territory of the stenotic vessel (degree of stenosis not stated) and evidence of hypoperfusion and poor collateral flow on MR perfusion and/or conventional angiogram underwent EDAS [23]. Over a 2-year follow-up period, 5.6% of patients had ischemic strokes (1 stroke was periprocedural), compared with the estimated 17.2% risk of stroke in the SAMMPRIS cohort.
While endovascular and surgical techniques for revascularization of symptomatic ICAD are options for cases with medically refractory ischemic events due to hypoperfusion, further studies are needed to determine the safety of and optimal timing for these treatments.
Post-Discharge Follow-up Evaluation
At 1 month after discharge, the patient denied any new or recurrent signs or symptoms of stroke. Home blood pressure logs showed that BP was at target. Orthostatic BPs were assessed and no evidence of hypotension on standing was identified. Repeat laboratory evaluation was remarkable for hemoglobin A1c of 6.8% and LDL cholesterol of 64 mg/dL. The patient and his wife have been walking briskly 3 times per week for 45 minutes and continue to work on dietary modifications modeled after the Mediterranean diet. He is scheduled to follow up with his vascular neurologist 3 months after the stroke to discuss transition from DAT to aspirin monotherapy.
Conclusion
Intracranial atherosclerotic disease is a common cause of ischemic stroke, particularly amongst Asian, black, and Hispanic patients. Identification of ICAD can be performed with noninvasive arterial imaging including CT angiography or contrast-enhanced MR angiography as part of an ischemic stroke workup. Optimal medical management with early DAT with aspirin and clopidogrel along with aggressive risk factor and lifestyle modification has emerged as an effective first-line therapy. In patients with recurrent ischemic symptoms while optimally medically managed, endovascular therapy with angioplasty with or without stenting may be considered.
Corresponding author: Fadi Nahab, MD, 1635 Clifton Rd., Clinic B, Suite 2200, Atlanta, GA 30322, [email protected].
Financial disclosures: None.
1. Derdeyn C, Chimowitz M, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial arterial stenosis (SAMMPRIS): the final results of a randomized trial. Lancet 2014;383:333–41.
2. Chimowitz, MI, Lynn MJ, Howlett-Smith H, et al. Comparision of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2004;351:1250–1.
3. Feldmann E, Wilterdink JL, Kosinski A, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology 2007;68:2099–106.
4. Willinek WA, von Falkenhausen M, Born M, et al. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: a prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke 2005;36:38–43.
5. Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol 2005;26:1012–21.
6. Qureshi A, Caplan L. Intracranial atherosclerosis. Lancet 2014;383:984–98.
7. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003.
8. Winningham M, Kasshout T, Bamford L, et al. Optimal duration of dual antiplatelet therapy for symptomatic intracranial stenosis. Stroke 2015;46:ATP100.
9. Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolization in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomized, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–97.
10. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
11. Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115:2969–75.
12. Agency for Healthcare Research and Quality. Clinical guidelines for prescribing pharmacotherapy for smoking cessation. Accessed 8 May 2016 at www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/prescrib.html.
13. Nahab F, Kingston C, Frankel MR, et al. Early aggressive medical management for patients with symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis 2013;22:87–91.
14. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555–63.
15. Quest Diagnostics. Aspirin resistance. Accessed 23 Mar 2016 at www.questdiagnostics.com/testcenter/testguide.action?dc=TS_Aspirin_Resistance.
16. Alberts MJ. Platelet function testing for aspirin resistance is reasonable to do. Stroke 2010;41:2400–401.
17. Feher G, Feher A, Pusch G, et al. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol 2010;26:171–86.
18. Taubert D, Kastrati A, Harlfinger S, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost 2004;92:311–6.
19. Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 2004;109:166–71.
20. Wabnitz AM, Derdeyn CP, Fiorella DJ, et al; for the SAMMPRIS Investigators. Infarct patterns in the anterior circulation as predictors of recurrent stroke in the medical arm of SAMMPRIS. Stroke 2016;47:A103.
21. Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240–8.
22. Nahab F, Lynn MJ, Kasner SE, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology 2009;72:2014–9.
23. Gonzalez N, Dusick J, Connolly M, et al. Encephaloduroarteriosynangiosis for adult intracranial arterial steno-occlusive disease: long-term single-center experience with 107 operations. J Neurosurg 2015;123:654–61.
1. Derdeyn C, Chimowitz M, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial arterial stenosis (SAMMPRIS): the final results of a randomized trial. Lancet 2014;383:333–41.
2. Chimowitz, MI, Lynn MJ, Howlett-Smith H, et al. Comparision of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2004;351:1250–1.
3. Feldmann E, Wilterdink JL, Kosinski A, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology 2007;68:2099–106.
4. Willinek WA, von Falkenhausen M, Born M, et al. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: a prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke 2005;36:38–43.
5. Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol 2005;26:1012–21.
6. Qureshi A, Caplan L. Intracranial atherosclerosis. Lancet 2014;383:984–98.
7. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003.
8. Winningham M, Kasshout T, Bamford L, et al. Optimal duration of dual antiplatelet therapy for symptomatic intracranial stenosis. Stroke 2015;46:ATP100.
9. Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolization in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomized, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–97.
10. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45.
11. Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115:2969–75.
12. Agency for Healthcare Research and Quality. Clinical guidelines for prescribing pharmacotherapy for smoking cessation. Accessed 8 May 2016 at www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/prescrib.html.
13. Nahab F, Kingston C, Frankel MR, et al. Early aggressive medical management for patients with symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis 2013;22:87–91.
14. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555–63.
15. Quest Diagnostics. Aspirin resistance. Accessed 23 Mar 2016 at www.questdiagnostics.com/testcenter/testguide.action?dc=TS_Aspirin_Resistance.
16. Alberts MJ. Platelet function testing for aspirin resistance is reasonable to do. Stroke 2010;41:2400–401.
17. Feher G, Feher A, Pusch G, et al. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol 2010;26:171–86.
18. Taubert D, Kastrati A, Harlfinger S, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost 2004;92:311–6.
19. Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 2004;109:166–71.
20. Wabnitz AM, Derdeyn CP, Fiorella DJ, et al; for the SAMMPRIS Investigators. Infarct patterns in the anterior circulation as predictors of recurrent stroke in the medical arm of SAMMPRIS. Stroke 2016;47:A103.
21. Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240–8.
22. Nahab F, Lynn MJ, Kasner SE, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology 2009;72:2014–9.
23. Gonzalez N, Dusick J, Connolly M, et al. Encephaloduroarteriosynangiosis for adult intracranial arterial steno-occlusive disease: long-term single-center experience with 107 operations. J Neurosurg 2015;123:654–61.
Promoting Early Literacy in the Pediatrician’s Office: What Have We Learned?
From the Hasbro Children’s Hospital/Warren Alpert School of Medicine at Brown University, Providence, RI.
Abstract
- Objective: To describe current knowledge about the effects of promoting literacy and early language development in young children.
- Methods: Review of the literature.
- Results: Children who are exposed to literacy-promoting interventions in their pediatricians’ offices are more likely to be read to frequently by their caregivers and have improved language skills when compared to children who are not. Language disparities can have life-long consequences that are particularly important in children from disadvantaged socioeconomic backgrounds. The power of the intervention may lie in the fact that it begins in a parent's lap and helps build strong and nurturing parent-child relationships as well as language skills.
- Conclusion: Pediatric providers are in a unique position to positively influence a child’s life course by promoting literacy starting at birth.
Over the past few decades, pediatric providers and parents have been inundated with information about the importance of reading to children, starting at a young age. In fact, a national organization, Reach Out and Read (ROR), has been promoting this idea for the past 25 years. ROR began in 1989 at Boston City Hospital when it was noticed that the books brought in by staff for the pediatric waiting room area were disappearing. Pediatricians and staff members realized that this was likely the result of a lack of children’s books in homes of disadvantaged children, and they decided to provide quality children’s books and guidance about reading with young children as a component of their primary care [1,2]. Since then, ROR has proliferated, with now over 5000 sites throughout the nation. Millions of children between the ages of 6 months and 5 years are given books by their pediatricians at every well child visit. Their parents receive anticipatory guidance about the benefits and joys of reading aloud to their children.
Most pediatricians trained in the past 10 to 15 years cannot imagine a visit that will not include giving a book to a child and talking to his or her parents about the benefits of sharing books together. This practice was reinforced when in 2014 the American Academy of Pediatrics (AAP) released a policy statement making literacy promotion in pediatric practice the standard of care [3]. In this paper, we review the data supporting early literacy promoting interventions and the role that pediatricians have in improving children’s literacy environments. We also discuss the ROR model as well as the impact of electronic media on children’s language skills.
Early Brain Development and Literacy Interventions
About 90% of brain growth occurs before the age of 5. In the first year of life, the brain triples in volume and there is a dramatic increase in the number of synapses. As many as 700 new neural connections are formed every second, and the number grows exponentially from 50 trillion at birth to 1000 trillion by the time of the child’s first birthday. This period of rapid proliferation is followed by a phase of synaptic retraction or “pruning,” so that brain circuits become more efficient. The time course for synaptic “blooming and pruning” varies by brain region. Overproduction in the sensory pathways like those for basic vision and hearing peaks at about the 4th postnatal month and is followed by a gradual retraction that occurs until the middle-end of the preschool period. A similar pattern is observed in areas of the brain that govern development of early language skills but with a somewhat later time course observed, peaking at about 9 months, followed by decline and stabilization in the preschool years. The prefrontal cortex, involved in higher cognitive functions, is the last to develop, reaching a peak overproduction in synapses by age 1, and it is not until late adolescence to early adulthood that a more streamlined density of synapses is obtained [4,5].
Both genetic guidance and experiential exposure are important and play a crucial role in brain development. In fact, the purpose of synaptic overproduction is in part to capture and incorporate experience into the developing synaptic architecture of the brain. Exposure is particularly important during “critical” and “sensitive” periods of development. Critical periods are times during which a set of signals must be present for neural systems to differentiate normally. For example, exposure to patterned visual information in the first few years of life is crucial for stereoscopic vision to develop. Sensitive developmental periods are times when opportunity exists for experience to define patterns of synaptic connectivity, optimizing a child’s ability to adapt to specific environmental factors. Brain plasticity however decreases with age, and as the maturing brain becomes more specialized it is less capable of adapting to new or unexpected challenges. This makes early childhood an important sensitive period in a child’s life, during which experiences directly mold neuronal circuits, offering a critical window for learning [6–9].
Pediatric providers have the unique opportunity to intervene at a time in which the brain is absorbing information at an incredible pace. When children miss the chance to acquire foundational language skills at a very young age, they in turn are at risk for immediate struggles with literacy when they begin attending school. Therefore, for an intervention to have a significant impact on the development of early literacy skills, it has to start early. In the ROR model, pediatric providers start providing anticipatory guidance about the benefits of shared reading, talking, singing, and rhyming starting soon after birth.
Impact of the “Word Gap”
The term “word gap” was first coined by psychologists Betty Hart and Todd Risley in their 1995 book, Meaningful Differences in the Everyday Lives of Young American Children [10]. Their study included 42 healthy and intact young families: 13 high-income families (professional families), 23 families of middle/low socioeconomic status (working-class families), and 6 families who received welfare benefits. Monthly hour-long recordings of parent-child conversations and observations of each family were conducted from the time their index child was about 12 months old until they turned 3 years of age. Gender and race were balanced within the sample.
This study identified remarkable differences in the early vocabulary experiences of young children. The average child raised in a family receiving welfare was hearing half as many words per hour (616 words per hour) as was the average child in working-class family (1251 words per hour) and less than one-third as many than the average child raised in a professional family (2153 words per hour). By extrapolating these numbers in a linear fashion, their study found that the average child growing up in a family living in poverty would listen to about 13 million fewer words than the average child being raised by working class parents and 30 million fewer words than children living in higher income/professional families by the time they reached the age of 3.
To investigate if these findings had longer-term implications, 29 of the 42 families included in their initial study were recruited for follow-up when the children were in third grade. Researchers found that measures of accomplishment at age 3 were highly predictive of performance at the ages of 9 and 10 on several standardized vocabulary, language development, and reading comprehension measures. Thus, the foundation built at age 3 had a great bearing on their progress many years later [11]. This is important because it confirmed that vocabulary development during the toddler and preschool years is directly related to later reading skills and school success in general.
Outcomes of Poor Literacy
Poor early literacy skills are associated with lifelong academic, social, and income disparities. Studies have repeatedly shown that high school graduation rates are directly correlated to reading abilities by the end of 3rd grade. Poor early readers are at a much higher risk of dropping out of school later on. In turn, dropping out of high school is associated with higher risks of delinquency, substance abuse, and incarceration [12,13].
To break the cycle of poverty, we need to help our children—particularly children coming from low-income, disadvantaged homes—become better readers. One of the ways in which we can achieve this is by giving them the tools they need starting in infancy. By giving them books at every well child visit and by encouraging parents to read aloud with their children every day, we can strengthen their early literacy skills, providing a foundation for later success in school and ultimately impacting the quality of their lives.
As Nobel laureate economist James Heckman stated [14]:
Investment in early education for disadvantaged children from birth to age 5 helps reduce the achievement gap, reduce the need for special education, increase the likelihood of healthier lifestyles, lower the crime rate, and reduce overall social costs. In fact, every dollar invested in high-quality early childhood education produces a 7 to 10 percent per annum return on investment.
Why Books? What About Electronics and TV?
In an era of electronics par excellence, we have to look at what the data say about the effects of electronics on children’s brains and language development. To date, studies looking at the effects of electronic media on infant and toddler development have failed to show any benefits. In fact, heavy exposure to electronic devices has been linked to language delays [15]. The data is so strong that in 2011, the AAP released an update of the 1999 policy statement on media use in children. The revised policy stated once again that “pediatricians should urge parents to avoid television viewing in children less than 2 years of age.” The updated statement addresses (1) the lack of evidence supporting educational or developmental benefits for media use by children younger than 2 years, (2) the potential adverse health and developmental effects of media use by children younger than 2 years, and (3) adverse effects of parental media use (background media) on children younger than 2 years [16].
The existing literature suggests that media use does not promote language skills in infants and toddlers and that vocabulary growth is directly related to the amount of time parents spend speaking to and interacting with their children [17–19]. For example, a study comparing the quantity and quality of language interactions of 25 parent-infant dyads during a total of six 15-minute play sessions with electronic toys, traditional toys, and books showed that during play with electronic toys, there were fewer adult words, fewer conversational turns, fewer parental responses, and fewer productions of content-specific words than during play with traditional toys or books. Children vocalized less during play with electronic toys than during play with books. Parents produced fewer words during play with traditional toys than during play with books and use of content-specific words was lower during play with traditional toys than during play with books. This study included primarily college-educated white non-Hispanic parents [20].
Heavy television use in a household can interfere with a child’s language development likely because parents spend less time talking to their child. In turn, children who live in households with heavy media use spend less time being read to. In the short-term, children younger than 2 years who spend a significant amount of time watching television or videos have higher chances of having a language delay [21–23]. Children who are exposed to infant videos also develop fewer language skills than children who are read to [24,25]. What is clear from all of this work is that young children learn best by interacting with the caring people in their lives, not with screens.
Given these facts, the AAP continues to discourage media use among children younger than 2, encourages parents to spend time reading and playing with their children, and discourages parents from having the TV or other electronics on as “background noise” when their children are present, since it decreases the amount of talking and interacting between parents and their children [16].
Benefits of the Reach Out and Read Model
For the past 25 years, pediatricians have been promoting early literacy in their practices following the ROR model, which consists of the following components:
- Giving a new, colorful, age-appropriate book to babies, toddlers, and preschoolers at every well child visit starting at 6 months of age
- Providing anticipatory guidance to parents on the benefits of reading aloud to children starting at birth
- Having a literacy-rich waiting room area (which at times includes volunteers reading to the children)
The data supporting this very simple, inexpensive intervention is robust. Multiple studies have shown that children exposed to the ROR model have improved language skills when compared to children who are not. Parents also report a much higher frequency of reading with their children when exposed to ROR than parents who are not [26–28].
In a randomized controlled study of literacy promotion in Hispanic families, when parents were asked open-endedly “What are your 3 most favorite things to do with your child?,” parents who had received literacy-promoting anticipatory guidance and books reported “reading with my toddler” significantly more often than parents who had not (43% intervention vs. 13% controls). When asked about the frequency of reading to their toddlers, intervention parents were significantly more likely to report reading books with their children at least 3 days/week than controls (66% intervention vs. 24% controls). Applying a multiple logistic regression model controlling for child and parent age, parent reading habits, and English proficiency, we found that the odds of parents reading to their child at least 3 days/week were 10 times greater in intervention families (odds ratio [OR] 10.1, 95% confidence interval 4.0–25.6) than in controls [29].
In a parallel study with English-speaking low income families, when parents were asked open-endedly, “What are your child’s 3 most favorite activities?,” parents who had been exposed to the intervention, were significantly more likely to report “reading books” as one of their toddler’s 3 favorite activities than parents who were not exposed (27% intervention vs. 12% controls). Toddler expressive and receptive vocabulary scores were higher in intervention families and were associated with more frequent shared reading [30].
A multicenter study (19 clinical sites in 10 different states) that compared 730 children aged 6 to 72 months exposed to the ROR model with a comparison group of 917 matched children who did not participate in this literacy promoting model found significant associations between exposure to ROR and reading aloud as a favorite parent activity (adjusted OR 1.6, P < 0.001); reading aloud at bedtime (adjusted OR 1.5, P < 0.001); reading aloud 3 or more days per week (adjusted OR 1.8, P < 0.001); and ownership of 10 or more picture books (adjusted OR 1.6, P < 0.001) [31].
Across the world, others have been replicating and testing the ROR model. Interestingly, studies conducted in Taiwan and with immigrants from Latin America and Asia have all shown similar effects on parental literacy behaviors and on the development of children’s early oral language skills [32–35].
Parent-Child Bonding from Sharing Books
According to the 2014 AAP policy statement, literacy promotion is an essential component of pediatric primary care [3]. The statement emphasizes that parent-child shared reading is a “very personal and nurturing experience that promotes parent-child interaction, social-emotional development, and language and literacy skills during this critical period of early brain and child development.” It recognizes the importance of shared reading as a bonding experience that could start in early infancy. These early nurturing relationships are critical to promoting healthy child development [36].
Most studies of practice-based literacy promotion have asked parents what their favorite things are to do with their child. All of these studies have shown that parents who have received guidance around the importance of reading together and high-quality books to share with their infants, toddlers, and preschoolers include reading aloud as one of their 3 most favorite activities, compared to control families who did not receive this intervention [28–31]. When activities are favorites, they are enriched by this shared enjoyment and are far more likely to occur often and perhaps become treasured family routines. Children’s books and early play and discussions around the themes in these books stimulate increased interaction between caregivers and children [37]. These interactions build secure relationships that are key to children’s healthy cognitive, language, and social-emotional development [38–40].
The Effects on the Brain From Listening to Stories
In a recent study, 48 children aged 6 to 11 years were classified as early talkers (16), on-time talkers (16), or late talkers (16) by parental report [41]. Group assignments were based on whether the parent recalled their child making 2- to 3-word sentences early, on-time, or late. None of the “early talkers” had spoken their first sentences after 24 months, and none of the “late talkers” had spoken sentences before age 2. Utilizing functional MRI, researchers analyzed talker group differences in processing of speech and print and functional activation differences on auditory stimuli and when visualizing print. The groups were matched by age, gender, and performance IQ. This study showed strong group differences in the activation of several regions of the brain, including the left superior temporal gyrus, left putamen, globus pallidus, right putamen, left insula, and thalamus. In each of these areas, late talkers demonstrated significantly less activation that early talkers in both speech and print conditions (P < 0.001). Talker group status was strongly related to neural activation patterns during simple linguistic tasks. These cortical differences in activation are consistent with other studies that demonstrate the role of these regions in understanding speech [42] and processing print [43,44]. These findings highlight the importance of early language development on the formation of critical language and reading circuits and how these neural pathways are affected many years later [41].
In another study of nineteen 3- to 5-year-olds, researchers used functional MRI to examine the relationship between home reading environment and brain activity during a story listening task. The study showed that while listening to stories, children with greater home reading exposure exhibited higher activation of left-sided brain regions involved with processing of meaning. Higher reading exposure at home as measured by the StimQ-P Reading subscale score, was positively correlated with neural activation in the left-sided parietal-temporal-occipital association cortex, a region of the brain supporting semantic language processing, when controlling for household income (P < 0.05) [45].
Conclusion
Pediatric providers are in a unique position to impact a child’s life by promoting literacy starting at birth. The effects of shared reading and parent-child interactions on early language development, on the formation of brain circuitry, and on children’s ability to become better readers and arrive to school ready to learn is now known.
We have an obligation to not only make literacy promotion in pediatric encounters the standard of care, but to continue to expand these types of interventions to other settings to reach as many young children as possible. Children from disadvantaged socioeconomic backgrounds and those from immigrant families are at highest risk and should be the primary focus of our intervention efforts. However, data from the 2011–2012 National Survey of Children’s Health found that only 60% of US children raised in households with income > 400% of the federal poverty level were read to daily [46]. These data suggest that more affluent, professional families should also be counseled by their pediatricians about the benefits of shared reading and about the detrimental effects of “electronics” at this critical time in their child’s development.
More research is needed to fully understand the long-term impacts of literacy promotion interventions in primary care settings. Longitudinal studies directly measuring the potential effects of the ROR model on reading skills in 3rd grade, on high school graduation rates, and on other measures of social and academic success are lacking. However, the existing evidence suggests that this kind of program can fulfill the promise of child health supervision visits. While providing guidance and the tools aimed at improving the home environment, pediatric providers can shape the course of young children’s lives.
Corresponding author: Natalia Golova, MD, Hasbro Children’s Hospital, 593 Eddy St., Hasbro Lower Level, Providence, RI 02903, [email protected].
Financial disclosures: None.
1. Needlman R, Fried L, Morley D, et al. Clinic-based intervention to promote literacy. Am J Dis Child 1991;145:881–4.
2. Reach Out and Read: a national pediatric literacy program. Available at http://reachoutandread.org.
3. High PC, Klass P. Literacy promotion: an essential component of primary care pediatric practice. Council on Early Childhood. Pediatrics 2014;134:404–9.
4. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167–78.
5. Center on the Developing Child. The science of early childhood development (in brief); 2007. Accessed 6 May 2016 at www.developingchild.harvard.edu.
6. Connecting Science, Policy, and Practice: Zero to Three’s National Training Institute, 2015. Zero Three 2016;36(3).
7. Fox NA, Zeanah CH, Nelson CA. A matter of timing: enhancing positive change for the developing brain. Zero Three 2014;34(3):4–9.
8. Halfon N, Shulman E, Hochstein M. Brain development in early childhood. Technical report. UCLA Center for Healthier Children, Families and Communities. Aug 2001.
9. National Scientific Council on the Developing Child. The science of early childhood development: closing the gap between what we know and what we do. Center on the Developing Child. Harvard University; 2007.
10. Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995.
11. Hart B, Risley TR. The early catastrophe: the 30 million word gap by age 3. Am Educator 2003;27:4–9.
12. The Annie E. Casey Foundation. Double jeopardy: how third grade reading skills and poverty influence high school graduation. 2012. Accessed 21 Feb 2016 at www.aecf.org/resources/double-jeopardy.
13. The Annie E. Casey Foundation. Early warning confirmed: a research update on third grade reading. 2013 Nov. Accessed 23 Feb 2016 at www.aecf.org/m/resourcedoc/AECF-EarlyWarningConfirmed-2013.pdf
14. Heckman J. The economics of inequality: the value of early childhood education. Am Educator 2011;47:31–5.
15. Christakis DA. The effects of infant media usage: what do we know and what should we learn? Acta Paediatr 2009;98: 8–16.
16. American Academy of Pediatrics, Council on Communications and Media. Policy statement. Media use by children younger than 2 years. Pediatrics 2011;128:1040–5.
17. Linebarger DL, Walker D. Infants’ and toddlers’ television viewing and language outcomes. Am Behav Sci 2005;48:624–45.
18. Masako T, Okuma K, Kyoshima K. Television viewing and reduced parental utterance, and delayed speech development in infants and young children. Arch Pediatr Adolesc Med 2007;161:618–9.
19. Rideout VJ, Hamel E. The media family: electronic media in the lives of infants, toddlers, preschoolers, and their parents. Menlo Park, CA: Kaiser Family Foundation; 2006.
20. Sosa AV. Association of the type of toy used during play with the quantity and quality of parent-infant communication. JAMA Pediatr 2016;170:132–7.
21. Vandewater EA, Bickham DS, Lee JH et al. When the television is always on: heavy television exposure and young children’s development. Am Behav Sci 2005;48:562–77.
22. Zimmerman FJ, Christakis DA, Meltzoff AN. Associations between media viewing and language development in children under age two years. J Pediatr 2007;151:364–8.
23. Chonchaiya W, Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr 2008;97:977–82.
24. Robb MB, Richert RA, Wartella EA. Just a talking book? Word learning from watching baby videos. Br J Dev Psychol 2009;27(Pt 1):27–45.
25. DeLoache JS, Chiong C, Sherman K, et al. Do babies learn from baby media? Psychol Sci 2010;21:1570–4.
26. Mendelsohn A, Mogliner L, Dreyer B, et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics 2001;107:130–4.
27. Mendelsohn AL. Promoting language and literacy through reading aloud: the role of the pediatrician. Curr Probl Pediatr Adolesc Health Care 2002;32:183–210.
28. High P, Hopman M, LaGasse L, et al. Evaluation of a clinic-based program to promote book sharing and bedtime routines among low-income urban families with young children. Arch Pediatr Adolesc Med 1998;152:459–65.
29. Golova N, Alario A, Vivier P, et al. Literacy promotion for Hispanic families in a primary care setting: a randomized controlled trial. Pediatrics 1999;103:993–7.
30. High PC, LaGasse L, Becker S, et al. Literacy promotion in primary care pediatrics: can we make a difference? Pediatrics 2000;105:927–34.
31. Needlman R, Toker KH, Dreyer BP, et al. Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr 2005;5:209–15.
32. Wu SC, Lue HC, Tseng LL. A pediatric clinic-based approach to early literacy promotion--experience in a well-baby clinic in Taiwan. J Formos Med Assoc 2012;111:258–64.
33. Sanders LM, Gershon TD, Huffman LC, et al. Prescribing books for immigrant children: a pilot study to promote emergent literacy among the children of Hispanic immigrants. Arch Pediatr Adolesc Med 2000;154:771–7.
34. Kitabayashi KM, Huang GY, Linskey KR, et al. Parent-child reading interactions among English and English as a second language speakers in an underserved pediatric clinic in Hawai’i. Hawaii Med J 2008;67:260–3.
35. Festa N, Loftus PD, Cullen MR, Mendoza FS. Disparities in early exposure to book sharing within immigrant families. Pediatrics. 2014;134:e162–8.
36. Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. National Research Council (US) and Institute of Medicine (US) Committee on Integrating the Science of Early Childhood Development. Washington, DC: National Academies Press; 2000.
37. Neuman SB. Guiding young children’s participation in early literacy development: a family literacy program for adolescent mothers. Early Child Dev Care 1997;127:119–29.
38. Tomopoulos S, Dreyer BP, Tamis-LeMonda C, et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr 2006;6:72–8.
39. Mendelsohn AL, Huberman HS, Berkule SB, et al. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med 2011;165:33–41.
40. Ginsburg K; American Academy of Pediatrics, Committee on Communications, Committee on Psychosocial Aspects of Child and Family Health. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007;119:182–91.
41. Preston JL, Frost SJ, Mencl WE, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain 2010;133:2185–95.
42. Hugdahl K, Gundersen H, Brekke C, et al. fMRI Brain activation in a Finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res 2004;47:162–72.
43. Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 2000;6:207–13.
44. Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001;34:479–92.
45. Hutton JS, Horowitz-Kraus T, Mendelsohn AL, et al. Home reading environment and brain activation in preschool children listening to stories. Pediatrics 2015;136:466–78.
46. Data Resource Center for Child and Adolescent Health. 2011/12 National Survey of Children’s Health. Accessed 28 Feb 2016 at www.nschdata.org.
From the Hasbro Children’s Hospital/Warren Alpert School of Medicine at Brown University, Providence, RI.
Abstract
- Objective: To describe current knowledge about the effects of promoting literacy and early language development in young children.
- Methods: Review of the literature.
- Results: Children who are exposed to literacy-promoting interventions in their pediatricians’ offices are more likely to be read to frequently by their caregivers and have improved language skills when compared to children who are not. Language disparities can have life-long consequences that are particularly important in children from disadvantaged socioeconomic backgrounds. The power of the intervention may lie in the fact that it begins in a parent's lap and helps build strong and nurturing parent-child relationships as well as language skills.
- Conclusion: Pediatric providers are in a unique position to positively influence a child’s life course by promoting literacy starting at birth.
Over the past few decades, pediatric providers and parents have been inundated with information about the importance of reading to children, starting at a young age. In fact, a national organization, Reach Out and Read (ROR), has been promoting this idea for the past 25 years. ROR began in 1989 at Boston City Hospital when it was noticed that the books brought in by staff for the pediatric waiting room area were disappearing. Pediatricians and staff members realized that this was likely the result of a lack of children’s books in homes of disadvantaged children, and they decided to provide quality children’s books and guidance about reading with young children as a component of their primary care [1,2]. Since then, ROR has proliferated, with now over 5000 sites throughout the nation. Millions of children between the ages of 6 months and 5 years are given books by their pediatricians at every well child visit. Their parents receive anticipatory guidance about the benefits and joys of reading aloud to their children.
Most pediatricians trained in the past 10 to 15 years cannot imagine a visit that will not include giving a book to a child and talking to his or her parents about the benefits of sharing books together. This practice was reinforced when in 2014 the American Academy of Pediatrics (AAP) released a policy statement making literacy promotion in pediatric practice the standard of care [3]. In this paper, we review the data supporting early literacy promoting interventions and the role that pediatricians have in improving children’s literacy environments. We also discuss the ROR model as well as the impact of electronic media on children’s language skills.
Early Brain Development and Literacy Interventions
About 90% of brain growth occurs before the age of 5. In the first year of life, the brain triples in volume and there is a dramatic increase in the number of synapses. As many as 700 new neural connections are formed every second, and the number grows exponentially from 50 trillion at birth to 1000 trillion by the time of the child’s first birthday. This period of rapid proliferation is followed by a phase of synaptic retraction or “pruning,” so that brain circuits become more efficient. The time course for synaptic “blooming and pruning” varies by brain region. Overproduction in the sensory pathways like those for basic vision and hearing peaks at about the 4th postnatal month and is followed by a gradual retraction that occurs until the middle-end of the preschool period. A similar pattern is observed in areas of the brain that govern development of early language skills but with a somewhat later time course observed, peaking at about 9 months, followed by decline and stabilization in the preschool years. The prefrontal cortex, involved in higher cognitive functions, is the last to develop, reaching a peak overproduction in synapses by age 1, and it is not until late adolescence to early adulthood that a more streamlined density of synapses is obtained [4,5].
Both genetic guidance and experiential exposure are important and play a crucial role in brain development. In fact, the purpose of synaptic overproduction is in part to capture and incorporate experience into the developing synaptic architecture of the brain. Exposure is particularly important during “critical” and “sensitive” periods of development. Critical periods are times during which a set of signals must be present for neural systems to differentiate normally. For example, exposure to patterned visual information in the first few years of life is crucial for stereoscopic vision to develop. Sensitive developmental periods are times when opportunity exists for experience to define patterns of synaptic connectivity, optimizing a child’s ability to adapt to specific environmental factors. Brain plasticity however decreases with age, and as the maturing brain becomes more specialized it is less capable of adapting to new or unexpected challenges. This makes early childhood an important sensitive period in a child’s life, during which experiences directly mold neuronal circuits, offering a critical window for learning [6–9].
Pediatric providers have the unique opportunity to intervene at a time in which the brain is absorbing information at an incredible pace. When children miss the chance to acquire foundational language skills at a very young age, they in turn are at risk for immediate struggles with literacy when they begin attending school. Therefore, for an intervention to have a significant impact on the development of early literacy skills, it has to start early. In the ROR model, pediatric providers start providing anticipatory guidance about the benefits of shared reading, talking, singing, and rhyming starting soon after birth.
Impact of the “Word Gap”
The term “word gap” was first coined by psychologists Betty Hart and Todd Risley in their 1995 book, Meaningful Differences in the Everyday Lives of Young American Children [10]. Their study included 42 healthy and intact young families: 13 high-income families (professional families), 23 families of middle/low socioeconomic status (working-class families), and 6 families who received welfare benefits. Monthly hour-long recordings of parent-child conversations and observations of each family were conducted from the time their index child was about 12 months old until they turned 3 years of age. Gender and race were balanced within the sample.
This study identified remarkable differences in the early vocabulary experiences of young children. The average child raised in a family receiving welfare was hearing half as many words per hour (616 words per hour) as was the average child in working-class family (1251 words per hour) and less than one-third as many than the average child raised in a professional family (2153 words per hour). By extrapolating these numbers in a linear fashion, their study found that the average child growing up in a family living in poverty would listen to about 13 million fewer words than the average child being raised by working class parents and 30 million fewer words than children living in higher income/professional families by the time they reached the age of 3.
To investigate if these findings had longer-term implications, 29 of the 42 families included in their initial study were recruited for follow-up when the children were in third grade. Researchers found that measures of accomplishment at age 3 were highly predictive of performance at the ages of 9 and 10 on several standardized vocabulary, language development, and reading comprehension measures. Thus, the foundation built at age 3 had a great bearing on their progress many years later [11]. This is important because it confirmed that vocabulary development during the toddler and preschool years is directly related to later reading skills and school success in general.
Outcomes of Poor Literacy
Poor early literacy skills are associated with lifelong academic, social, and income disparities. Studies have repeatedly shown that high school graduation rates are directly correlated to reading abilities by the end of 3rd grade. Poor early readers are at a much higher risk of dropping out of school later on. In turn, dropping out of high school is associated with higher risks of delinquency, substance abuse, and incarceration [12,13].
To break the cycle of poverty, we need to help our children—particularly children coming from low-income, disadvantaged homes—become better readers. One of the ways in which we can achieve this is by giving them the tools they need starting in infancy. By giving them books at every well child visit and by encouraging parents to read aloud with their children every day, we can strengthen their early literacy skills, providing a foundation for later success in school and ultimately impacting the quality of their lives.
As Nobel laureate economist James Heckman stated [14]:
Investment in early education for disadvantaged children from birth to age 5 helps reduce the achievement gap, reduce the need for special education, increase the likelihood of healthier lifestyles, lower the crime rate, and reduce overall social costs. In fact, every dollar invested in high-quality early childhood education produces a 7 to 10 percent per annum return on investment.
Why Books? What About Electronics and TV?
In an era of electronics par excellence, we have to look at what the data say about the effects of electronics on children’s brains and language development. To date, studies looking at the effects of electronic media on infant and toddler development have failed to show any benefits. In fact, heavy exposure to electronic devices has been linked to language delays [15]. The data is so strong that in 2011, the AAP released an update of the 1999 policy statement on media use in children. The revised policy stated once again that “pediatricians should urge parents to avoid television viewing in children less than 2 years of age.” The updated statement addresses (1) the lack of evidence supporting educational or developmental benefits for media use by children younger than 2 years, (2) the potential adverse health and developmental effects of media use by children younger than 2 years, and (3) adverse effects of parental media use (background media) on children younger than 2 years [16].
The existing literature suggests that media use does not promote language skills in infants and toddlers and that vocabulary growth is directly related to the amount of time parents spend speaking to and interacting with their children [17–19]. For example, a study comparing the quantity and quality of language interactions of 25 parent-infant dyads during a total of six 15-minute play sessions with electronic toys, traditional toys, and books showed that during play with electronic toys, there were fewer adult words, fewer conversational turns, fewer parental responses, and fewer productions of content-specific words than during play with traditional toys or books. Children vocalized less during play with electronic toys than during play with books. Parents produced fewer words during play with traditional toys than during play with books and use of content-specific words was lower during play with traditional toys than during play with books. This study included primarily college-educated white non-Hispanic parents [20].
Heavy television use in a household can interfere with a child’s language development likely because parents spend less time talking to their child. In turn, children who live in households with heavy media use spend less time being read to. In the short-term, children younger than 2 years who spend a significant amount of time watching television or videos have higher chances of having a language delay [21–23]. Children who are exposed to infant videos also develop fewer language skills than children who are read to [24,25]. What is clear from all of this work is that young children learn best by interacting with the caring people in their lives, not with screens.
Given these facts, the AAP continues to discourage media use among children younger than 2, encourages parents to spend time reading and playing with their children, and discourages parents from having the TV or other electronics on as “background noise” when their children are present, since it decreases the amount of talking and interacting between parents and their children [16].
Benefits of the Reach Out and Read Model
For the past 25 years, pediatricians have been promoting early literacy in their practices following the ROR model, which consists of the following components:
- Giving a new, colorful, age-appropriate book to babies, toddlers, and preschoolers at every well child visit starting at 6 months of age
- Providing anticipatory guidance to parents on the benefits of reading aloud to children starting at birth
- Having a literacy-rich waiting room area (which at times includes volunteers reading to the children)
The data supporting this very simple, inexpensive intervention is robust. Multiple studies have shown that children exposed to the ROR model have improved language skills when compared to children who are not. Parents also report a much higher frequency of reading with their children when exposed to ROR than parents who are not [26–28].
In a randomized controlled study of literacy promotion in Hispanic families, when parents were asked open-endedly “What are your 3 most favorite things to do with your child?,” parents who had received literacy-promoting anticipatory guidance and books reported “reading with my toddler” significantly more often than parents who had not (43% intervention vs. 13% controls). When asked about the frequency of reading to their toddlers, intervention parents were significantly more likely to report reading books with their children at least 3 days/week than controls (66% intervention vs. 24% controls). Applying a multiple logistic regression model controlling for child and parent age, parent reading habits, and English proficiency, we found that the odds of parents reading to their child at least 3 days/week were 10 times greater in intervention families (odds ratio [OR] 10.1, 95% confidence interval 4.0–25.6) than in controls [29].
In a parallel study with English-speaking low income families, when parents were asked open-endedly, “What are your child’s 3 most favorite activities?,” parents who had been exposed to the intervention, were significantly more likely to report “reading books” as one of their toddler’s 3 favorite activities than parents who were not exposed (27% intervention vs. 12% controls). Toddler expressive and receptive vocabulary scores were higher in intervention families and were associated with more frequent shared reading [30].
A multicenter study (19 clinical sites in 10 different states) that compared 730 children aged 6 to 72 months exposed to the ROR model with a comparison group of 917 matched children who did not participate in this literacy promoting model found significant associations between exposure to ROR and reading aloud as a favorite parent activity (adjusted OR 1.6, P < 0.001); reading aloud at bedtime (adjusted OR 1.5, P < 0.001); reading aloud 3 or more days per week (adjusted OR 1.8, P < 0.001); and ownership of 10 or more picture books (adjusted OR 1.6, P < 0.001) [31].
Across the world, others have been replicating and testing the ROR model. Interestingly, studies conducted in Taiwan and with immigrants from Latin America and Asia have all shown similar effects on parental literacy behaviors and on the development of children’s early oral language skills [32–35].
Parent-Child Bonding from Sharing Books
According to the 2014 AAP policy statement, literacy promotion is an essential component of pediatric primary care [3]. The statement emphasizes that parent-child shared reading is a “very personal and nurturing experience that promotes parent-child interaction, social-emotional development, and language and literacy skills during this critical period of early brain and child development.” It recognizes the importance of shared reading as a bonding experience that could start in early infancy. These early nurturing relationships are critical to promoting healthy child development [36].
Most studies of practice-based literacy promotion have asked parents what their favorite things are to do with their child. All of these studies have shown that parents who have received guidance around the importance of reading together and high-quality books to share with their infants, toddlers, and preschoolers include reading aloud as one of their 3 most favorite activities, compared to control families who did not receive this intervention [28–31]. When activities are favorites, they are enriched by this shared enjoyment and are far more likely to occur often and perhaps become treasured family routines. Children’s books and early play and discussions around the themes in these books stimulate increased interaction between caregivers and children [37]. These interactions build secure relationships that are key to children’s healthy cognitive, language, and social-emotional development [38–40].
The Effects on the Brain From Listening to Stories
In a recent study, 48 children aged 6 to 11 years were classified as early talkers (16), on-time talkers (16), or late talkers (16) by parental report [41]. Group assignments were based on whether the parent recalled their child making 2- to 3-word sentences early, on-time, or late. None of the “early talkers” had spoken their first sentences after 24 months, and none of the “late talkers” had spoken sentences before age 2. Utilizing functional MRI, researchers analyzed talker group differences in processing of speech and print and functional activation differences on auditory stimuli and when visualizing print. The groups were matched by age, gender, and performance IQ. This study showed strong group differences in the activation of several regions of the brain, including the left superior temporal gyrus, left putamen, globus pallidus, right putamen, left insula, and thalamus. In each of these areas, late talkers demonstrated significantly less activation that early talkers in both speech and print conditions (P < 0.001). Talker group status was strongly related to neural activation patterns during simple linguistic tasks. These cortical differences in activation are consistent with other studies that demonstrate the role of these regions in understanding speech [42] and processing print [43,44]. These findings highlight the importance of early language development on the formation of critical language and reading circuits and how these neural pathways are affected many years later [41].
In another study of nineteen 3- to 5-year-olds, researchers used functional MRI to examine the relationship between home reading environment and brain activity during a story listening task. The study showed that while listening to stories, children with greater home reading exposure exhibited higher activation of left-sided brain regions involved with processing of meaning. Higher reading exposure at home as measured by the StimQ-P Reading subscale score, was positively correlated with neural activation in the left-sided parietal-temporal-occipital association cortex, a region of the brain supporting semantic language processing, when controlling for household income (P < 0.05) [45].
Conclusion
Pediatric providers are in a unique position to impact a child’s life by promoting literacy starting at birth. The effects of shared reading and parent-child interactions on early language development, on the formation of brain circuitry, and on children’s ability to become better readers and arrive to school ready to learn is now known.
We have an obligation to not only make literacy promotion in pediatric encounters the standard of care, but to continue to expand these types of interventions to other settings to reach as many young children as possible. Children from disadvantaged socioeconomic backgrounds and those from immigrant families are at highest risk and should be the primary focus of our intervention efforts. However, data from the 2011–2012 National Survey of Children’s Health found that only 60% of US children raised in households with income > 400% of the federal poverty level were read to daily [46]. These data suggest that more affluent, professional families should also be counseled by their pediatricians about the benefits of shared reading and about the detrimental effects of “electronics” at this critical time in their child’s development.
More research is needed to fully understand the long-term impacts of literacy promotion interventions in primary care settings. Longitudinal studies directly measuring the potential effects of the ROR model on reading skills in 3rd grade, on high school graduation rates, and on other measures of social and academic success are lacking. However, the existing evidence suggests that this kind of program can fulfill the promise of child health supervision visits. While providing guidance and the tools aimed at improving the home environment, pediatric providers can shape the course of young children’s lives.
Corresponding author: Natalia Golova, MD, Hasbro Children’s Hospital, 593 Eddy St., Hasbro Lower Level, Providence, RI 02903, [email protected].
Financial disclosures: None.
From the Hasbro Children’s Hospital/Warren Alpert School of Medicine at Brown University, Providence, RI.
Abstract
- Objective: To describe current knowledge about the effects of promoting literacy and early language development in young children.
- Methods: Review of the literature.
- Results: Children who are exposed to literacy-promoting interventions in their pediatricians’ offices are more likely to be read to frequently by their caregivers and have improved language skills when compared to children who are not. Language disparities can have life-long consequences that are particularly important in children from disadvantaged socioeconomic backgrounds. The power of the intervention may lie in the fact that it begins in a parent's lap and helps build strong and nurturing parent-child relationships as well as language skills.
- Conclusion: Pediatric providers are in a unique position to positively influence a child’s life course by promoting literacy starting at birth.
Over the past few decades, pediatric providers and parents have been inundated with information about the importance of reading to children, starting at a young age. In fact, a national organization, Reach Out and Read (ROR), has been promoting this idea for the past 25 years. ROR began in 1989 at Boston City Hospital when it was noticed that the books brought in by staff for the pediatric waiting room area were disappearing. Pediatricians and staff members realized that this was likely the result of a lack of children’s books in homes of disadvantaged children, and they decided to provide quality children’s books and guidance about reading with young children as a component of their primary care [1,2]. Since then, ROR has proliferated, with now over 5000 sites throughout the nation. Millions of children between the ages of 6 months and 5 years are given books by their pediatricians at every well child visit. Their parents receive anticipatory guidance about the benefits and joys of reading aloud to their children.
Most pediatricians trained in the past 10 to 15 years cannot imagine a visit that will not include giving a book to a child and talking to his or her parents about the benefits of sharing books together. This practice was reinforced when in 2014 the American Academy of Pediatrics (AAP) released a policy statement making literacy promotion in pediatric practice the standard of care [3]. In this paper, we review the data supporting early literacy promoting interventions and the role that pediatricians have in improving children’s literacy environments. We also discuss the ROR model as well as the impact of electronic media on children’s language skills.
Early Brain Development and Literacy Interventions
About 90% of brain growth occurs before the age of 5. In the first year of life, the brain triples in volume and there is a dramatic increase in the number of synapses. As many as 700 new neural connections are formed every second, and the number grows exponentially from 50 trillion at birth to 1000 trillion by the time of the child’s first birthday. This period of rapid proliferation is followed by a phase of synaptic retraction or “pruning,” so that brain circuits become more efficient. The time course for synaptic “blooming and pruning” varies by brain region. Overproduction in the sensory pathways like those for basic vision and hearing peaks at about the 4th postnatal month and is followed by a gradual retraction that occurs until the middle-end of the preschool period. A similar pattern is observed in areas of the brain that govern development of early language skills but with a somewhat later time course observed, peaking at about 9 months, followed by decline and stabilization in the preschool years. The prefrontal cortex, involved in higher cognitive functions, is the last to develop, reaching a peak overproduction in synapses by age 1, and it is not until late adolescence to early adulthood that a more streamlined density of synapses is obtained [4,5].
Both genetic guidance and experiential exposure are important and play a crucial role in brain development. In fact, the purpose of synaptic overproduction is in part to capture and incorporate experience into the developing synaptic architecture of the brain. Exposure is particularly important during “critical” and “sensitive” periods of development. Critical periods are times during which a set of signals must be present for neural systems to differentiate normally. For example, exposure to patterned visual information in the first few years of life is crucial for stereoscopic vision to develop. Sensitive developmental periods are times when opportunity exists for experience to define patterns of synaptic connectivity, optimizing a child’s ability to adapt to specific environmental factors. Brain plasticity however decreases with age, and as the maturing brain becomes more specialized it is less capable of adapting to new or unexpected challenges. This makes early childhood an important sensitive period in a child’s life, during which experiences directly mold neuronal circuits, offering a critical window for learning [6–9].
Pediatric providers have the unique opportunity to intervene at a time in which the brain is absorbing information at an incredible pace. When children miss the chance to acquire foundational language skills at a very young age, they in turn are at risk for immediate struggles with literacy when they begin attending school. Therefore, for an intervention to have a significant impact on the development of early literacy skills, it has to start early. In the ROR model, pediatric providers start providing anticipatory guidance about the benefits of shared reading, talking, singing, and rhyming starting soon after birth.
Impact of the “Word Gap”
The term “word gap” was first coined by psychologists Betty Hart and Todd Risley in their 1995 book, Meaningful Differences in the Everyday Lives of Young American Children [10]. Their study included 42 healthy and intact young families: 13 high-income families (professional families), 23 families of middle/low socioeconomic status (working-class families), and 6 families who received welfare benefits. Monthly hour-long recordings of parent-child conversations and observations of each family were conducted from the time their index child was about 12 months old until they turned 3 years of age. Gender and race were balanced within the sample.
This study identified remarkable differences in the early vocabulary experiences of young children. The average child raised in a family receiving welfare was hearing half as many words per hour (616 words per hour) as was the average child in working-class family (1251 words per hour) and less than one-third as many than the average child raised in a professional family (2153 words per hour). By extrapolating these numbers in a linear fashion, their study found that the average child growing up in a family living in poverty would listen to about 13 million fewer words than the average child being raised by working class parents and 30 million fewer words than children living in higher income/professional families by the time they reached the age of 3.
To investigate if these findings had longer-term implications, 29 of the 42 families included in their initial study were recruited for follow-up when the children were in third grade. Researchers found that measures of accomplishment at age 3 were highly predictive of performance at the ages of 9 and 10 on several standardized vocabulary, language development, and reading comprehension measures. Thus, the foundation built at age 3 had a great bearing on their progress many years later [11]. This is important because it confirmed that vocabulary development during the toddler and preschool years is directly related to later reading skills and school success in general.
Outcomes of Poor Literacy
Poor early literacy skills are associated with lifelong academic, social, and income disparities. Studies have repeatedly shown that high school graduation rates are directly correlated to reading abilities by the end of 3rd grade. Poor early readers are at a much higher risk of dropping out of school later on. In turn, dropping out of high school is associated with higher risks of delinquency, substance abuse, and incarceration [12,13].
To break the cycle of poverty, we need to help our children—particularly children coming from low-income, disadvantaged homes—become better readers. One of the ways in which we can achieve this is by giving them the tools they need starting in infancy. By giving them books at every well child visit and by encouraging parents to read aloud with their children every day, we can strengthen their early literacy skills, providing a foundation for later success in school and ultimately impacting the quality of their lives.
As Nobel laureate economist James Heckman stated [14]:
Investment in early education for disadvantaged children from birth to age 5 helps reduce the achievement gap, reduce the need for special education, increase the likelihood of healthier lifestyles, lower the crime rate, and reduce overall social costs. In fact, every dollar invested in high-quality early childhood education produces a 7 to 10 percent per annum return on investment.
Why Books? What About Electronics and TV?
In an era of electronics par excellence, we have to look at what the data say about the effects of electronics on children’s brains and language development. To date, studies looking at the effects of electronic media on infant and toddler development have failed to show any benefits. In fact, heavy exposure to electronic devices has been linked to language delays [15]. The data is so strong that in 2011, the AAP released an update of the 1999 policy statement on media use in children. The revised policy stated once again that “pediatricians should urge parents to avoid television viewing in children less than 2 years of age.” The updated statement addresses (1) the lack of evidence supporting educational or developmental benefits for media use by children younger than 2 years, (2) the potential adverse health and developmental effects of media use by children younger than 2 years, and (3) adverse effects of parental media use (background media) on children younger than 2 years [16].
The existing literature suggests that media use does not promote language skills in infants and toddlers and that vocabulary growth is directly related to the amount of time parents spend speaking to and interacting with their children [17–19]. For example, a study comparing the quantity and quality of language interactions of 25 parent-infant dyads during a total of six 15-minute play sessions with electronic toys, traditional toys, and books showed that during play with electronic toys, there were fewer adult words, fewer conversational turns, fewer parental responses, and fewer productions of content-specific words than during play with traditional toys or books. Children vocalized less during play with electronic toys than during play with books. Parents produced fewer words during play with traditional toys than during play with books and use of content-specific words was lower during play with traditional toys than during play with books. This study included primarily college-educated white non-Hispanic parents [20].
Heavy television use in a household can interfere with a child’s language development likely because parents spend less time talking to their child. In turn, children who live in households with heavy media use spend less time being read to. In the short-term, children younger than 2 years who spend a significant amount of time watching television or videos have higher chances of having a language delay [21–23]. Children who are exposed to infant videos also develop fewer language skills than children who are read to [24,25]. What is clear from all of this work is that young children learn best by interacting with the caring people in their lives, not with screens.
Given these facts, the AAP continues to discourage media use among children younger than 2, encourages parents to spend time reading and playing with their children, and discourages parents from having the TV or other electronics on as “background noise” when their children are present, since it decreases the amount of talking and interacting between parents and their children [16].
Benefits of the Reach Out and Read Model
For the past 25 years, pediatricians have been promoting early literacy in their practices following the ROR model, which consists of the following components:
- Giving a new, colorful, age-appropriate book to babies, toddlers, and preschoolers at every well child visit starting at 6 months of age
- Providing anticipatory guidance to parents on the benefits of reading aloud to children starting at birth
- Having a literacy-rich waiting room area (which at times includes volunteers reading to the children)
The data supporting this very simple, inexpensive intervention is robust. Multiple studies have shown that children exposed to the ROR model have improved language skills when compared to children who are not. Parents also report a much higher frequency of reading with their children when exposed to ROR than parents who are not [26–28].
In a randomized controlled study of literacy promotion in Hispanic families, when parents were asked open-endedly “What are your 3 most favorite things to do with your child?,” parents who had received literacy-promoting anticipatory guidance and books reported “reading with my toddler” significantly more often than parents who had not (43% intervention vs. 13% controls). When asked about the frequency of reading to their toddlers, intervention parents were significantly more likely to report reading books with their children at least 3 days/week than controls (66% intervention vs. 24% controls). Applying a multiple logistic regression model controlling for child and parent age, parent reading habits, and English proficiency, we found that the odds of parents reading to their child at least 3 days/week were 10 times greater in intervention families (odds ratio [OR] 10.1, 95% confidence interval 4.0–25.6) than in controls [29].
In a parallel study with English-speaking low income families, when parents were asked open-endedly, “What are your child’s 3 most favorite activities?,” parents who had been exposed to the intervention, were significantly more likely to report “reading books” as one of their toddler’s 3 favorite activities than parents who were not exposed (27% intervention vs. 12% controls). Toddler expressive and receptive vocabulary scores were higher in intervention families and were associated with more frequent shared reading [30].
A multicenter study (19 clinical sites in 10 different states) that compared 730 children aged 6 to 72 months exposed to the ROR model with a comparison group of 917 matched children who did not participate in this literacy promoting model found significant associations between exposure to ROR and reading aloud as a favorite parent activity (adjusted OR 1.6, P < 0.001); reading aloud at bedtime (adjusted OR 1.5, P < 0.001); reading aloud 3 or more days per week (adjusted OR 1.8, P < 0.001); and ownership of 10 or more picture books (adjusted OR 1.6, P < 0.001) [31].
Across the world, others have been replicating and testing the ROR model. Interestingly, studies conducted in Taiwan and with immigrants from Latin America and Asia have all shown similar effects on parental literacy behaviors and on the development of children’s early oral language skills [32–35].
Parent-Child Bonding from Sharing Books
According to the 2014 AAP policy statement, literacy promotion is an essential component of pediatric primary care [3]. The statement emphasizes that parent-child shared reading is a “very personal and nurturing experience that promotes parent-child interaction, social-emotional development, and language and literacy skills during this critical period of early brain and child development.” It recognizes the importance of shared reading as a bonding experience that could start in early infancy. These early nurturing relationships are critical to promoting healthy child development [36].
Most studies of practice-based literacy promotion have asked parents what their favorite things are to do with their child. All of these studies have shown that parents who have received guidance around the importance of reading together and high-quality books to share with their infants, toddlers, and preschoolers include reading aloud as one of their 3 most favorite activities, compared to control families who did not receive this intervention [28–31]. When activities are favorites, they are enriched by this shared enjoyment and are far more likely to occur often and perhaps become treasured family routines. Children’s books and early play and discussions around the themes in these books stimulate increased interaction between caregivers and children [37]. These interactions build secure relationships that are key to children’s healthy cognitive, language, and social-emotional development [38–40].
The Effects on the Brain From Listening to Stories
In a recent study, 48 children aged 6 to 11 years were classified as early talkers (16), on-time talkers (16), or late talkers (16) by parental report [41]. Group assignments were based on whether the parent recalled their child making 2- to 3-word sentences early, on-time, or late. None of the “early talkers” had spoken their first sentences after 24 months, and none of the “late talkers” had spoken sentences before age 2. Utilizing functional MRI, researchers analyzed talker group differences in processing of speech and print and functional activation differences on auditory stimuli and when visualizing print. The groups were matched by age, gender, and performance IQ. This study showed strong group differences in the activation of several regions of the brain, including the left superior temporal gyrus, left putamen, globus pallidus, right putamen, left insula, and thalamus. In each of these areas, late talkers demonstrated significantly less activation that early talkers in both speech and print conditions (P < 0.001). Talker group status was strongly related to neural activation patterns during simple linguistic tasks. These cortical differences in activation are consistent with other studies that demonstrate the role of these regions in understanding speech [42] and processing print [43,44]. These findings highlight the importance of early language development on the formation of critical language and reading circuits and how these neural pathways are affected many years later [41].
In another study of nineteen 3- to 5-year-olds, researchers used functional MRI to examine the relationship between home reading environment and brain activity during a story listening task. The study showed that while listening to stories, children with greater home reading exposure exhibited higher activation of left-sided brain regions involved with processing of meaning. Higher reading exposure at home as measured by the StimQ-P Reading subscale score, was positively correlated with neural activation in the left-sided parietal-temporal-occipital association cortex, a region of the brain supporting semantic language processing, when controlling for household income (P < 0.05) [45].
Conclusion
Pediatric providers are in a unique position to impact a child’s life by promoting literacy starting at birth. The effects of shared reading and parent-child interactions on early language development, on the formation of brain circuitry, and on children’s ability to become better readers and arrive to school ready to learn is now known.
We have an obligation to not only make literacy promotion in pediatric encounters the standard of care, but to continue to expand these types of interventions to other settings to reach as many young children as possible. Children from disadvantaged socioeconomic backgrounds and those from immigrant families are at highest risk and should be the primary focus of our intervention efforts. However, data from the 2011–2012 National Survey of Children’s Health found that only 60% of US children raised in households with income > 400% of the federal poverty level were read to daily [46]. These data suggest that more affluent, professional families should also be counseled by their pediatricians about the benefits of shared reading and about the detrimental effects of “electronics” at this critical time in their child’s development.
More research is needed to fully understand the long-term impacts of literacy promotion interventions in primary care settings. Longitudinal studies directly measuring the potential effects of the ROR model on reading skills in 3rd grade, on high school graduation rates, and on other measures of social and academic success are lacking. However, the existing evidence suggests that this kind of program can fulfill the promise of child health supervision visits. While providing guidance and the tools aimed at improving the home environment, pediatric providers can shape the course of young children’s lives.
Corresponding author: Natalia Golova, MD, Hasbro Children’s Hospital, 593 Eddy St., Hasbro Lower Level, Providence, RI 02903, [email protected].
Financial disclosures: None.
1. Needlman R, Fried L, Morley D, et al. Clinic-based intervention to promote literacy. Am J Dis Child 1991;145:881–4.
2. Reach Out and Read: a national pediatric literacy program. Available at http://reachoutandread.org.
3. High PC, Klass P. Literacy promotion: an essential component of primary care pediatric practice. Council on Early Childhood. Pediatrics 2014;134:404–9.
4. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167–78.
5. Center on the Developing Child. The science of early childhood development (in brief); 2007. Accessed 6 May 2016 at www.developingchild.harvard.edu.
6. Connecting Science, Policy, and Practice: Zero to Three’s National Training Institute, 2015. Zero Three 2016;36(3).
7. Fox NA, Zeanah CH, Nelson CA. A matter of timing: enhancing positive change for the developing brain. Zero Three 2014;34(3):4–9.
8. Halfon N, Shulman E, Hochstein M. Brain development in early childhood. Technical report. UCLA Center for Healthier Children, Families and Communities. Aug 2001.
9. National Scientific Council on the Developing Child. The science of early childhood development: closing the gap between what we know and what we do. Center on the Developing Child. Harvard University; 2007.
10. Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995.
11. Hart B, Risley TR. The early catastrophe: the 30 million word gap by age 3. Am Educator 2003;27:4–9.
12. The Annie E. Casey Foundation. Double jeopardy: how third grade reading skills and poverty influence high school graduation. 2012. Accessed 21 Feb 2016 at www.aecf.org/resources/double-jeopardy.
13. The Annie E. Casey Foundation. Early warning confirmed: a research update on third grade reading. 2013 Nov. Accessed 23 Feb 2016 at www.aecf.org/m/resourcedoc/AECF-EarlyWarningConfirmed-2013.pdf
14. Heckman J. The economics of inequality: the value of early childhood education. Am Educator 2011;47:31–5.
15. Christakis DA. The effects of infant media usage: what do we know and what should we learn? Acta Paediatr 2009;98: 8–16.
16. American Academy of Pediatrics, Council on Communications and Media. Policy statement. Media use by children younger than 2 years. Pediatrics 2011;128:1040–5.
17. Linebarger DL, Walker D. Infants’ and toddlers’ television viewing and language outcomes. Am Behav Sci 2005;48:624–45.
18. Masako T, Okuma K, Kyoshima K. Television viewing and reduced parental utterance, and delayed speech development in infants and young children. Arch Pediatr Adolesc Med 2007;161:618–9.
19. Rideout VJ, Hamel E. The media family: electronic media in the lives of infants, toddlers, preschoolers, and their parents. Menlo Park, CA: Kaiser Family Foundation; 2006.
20. Sosa AV. Association of the type of toy used during play with the quantity and quality of parent-infant communication. JAMA Pediatr 2016;170:132–7.
21. Vandewater EA, Bickham DS, Lee JH et al. When the television is always on: heavy television exposure and young children’s development. Am Behav Sci 2005;48:562–77.
22. Zimmerman FJ, Christakis DA, Meltzoff AN. Associations between media viewing and language development in children under age two years. J Pediatr 2007;151:364–8.
23. Chonchaiya W, Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr 2008;97:977–82.
24. Robb MB, Richert RA, Wartella EA. Just a talking book? Word learning from watching baby videos. Br J Dev Psychol 2009;27(Pt 1):27–45.
25. DeLoache JS, Chiong C, Sherman K, et al. Do babies learn from baby media? Psychol Sci 2010;21:1570–4.
26. Mendelsohn A, Mogliner L, Dreyer B, et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics 2001;107:130–4.
27. Mendelsohn AL. Promoting language and literacy through reading aloud: the role of the pediatrician. Curr Probl Pediatr Adolesc Health Care 2002;32:183–210.
28. High P, Hopman M, LaGasse L, et al. Evaluation of a clinic-based program to promote book sharing and bedtime routines among low-income urban families with young children. Arch Pediatr Adolesc Med 1998;152:459–65.
29. Golova N, Alario A, Vivier P, et al. Literacy promotion for Hispanic families in a primary care setting: a randomized controlled trial. Pediatrics 1999;103:993–7.
30. High PC, LaGasse L, Becker S, et al. Literacy promotion in primary care pediatrics: can we make a difference? Pediatrics 2000;105:927–34.
31. Needlman R, Toker KH, Dreyer BP, et al. Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr 2005;5:209–15.
32. Wu SC, Lue HC, Tseng LL. A pediatric clinic-based approach to early literacy promotion--experience in a well-baby clinic in Taiwan. J Formos Med Assoc 2012;111:258–64.
33. Sanders LM, Gershon TD, Huffman LC, et al. Prescribing books for immigrant children: a pilot study to promote emergent literacy among the children of Hispanic immigrants. Arch Pediatr Adolesc Med 2000;154:771–7.
34. Kitabayashi KM, Huang GY, Linskey KR, et al. Parent-child reading interactions among English and English as a second language speakers in an underserved pediatric clinic in Hawai’i. Hawaii Med J 2008;67:260–3.
35. Festa N, Loftus PD, Cullen MR, Mendoza FS. Disparities in early exposure to book sharing within immigrant families. Pediatrics. 2014;134:e162–8.
36. Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. National Research Council (US) and Institute of Medicine (US) Committee on Integrating the Science of Early Childhood Development. Washington, DC: National Academies Press; 2000.
37. Neuman SB. Guiding young children’s participation in early literacy development: a family literacy program for adolescent mothers. Early Child Dev Care 1997;127:119–29.
38. Tomopoulos S, Dreyer BP, Tamis-LeMonda C, et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr 2006;6:72–8.
39. Mendelsohn AL, Huberman HS, Berkule SB, et al. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med 2011;165:33–41.
40. Ginsburg K; American Academy of Pediatrics, Committee on Communications, Committee on Psychosocial Aspects of Child and Family Health. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007;119:182–91.
41. Preston JL, Frost SJ, Mencl WE, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain 2010;133:2185–95.
42. Hugdahl K, Gundersen H, Brekke C, et al. fMRI Brain activation in a Finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res 2004;47:162–72.
43. Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 2000;6:207–13.
44. Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001;34:479–92.
45. Hutton JS, Horowitz-Kraus T, Mendelsohn AL, et al. Home reading environment and brain activation in preschool children listening to stories. Pediatrics 2015;136:466–78.
46. Data Resource Center for Child and Adolescent Health. 2011/12 National Survey of Children’s Health. Accessed 28 Feb 2016 at www.nschdata.org.
1. Needlman R, Fried L, Morley D, et al. Clinic-based intervention to promote literacy. Am J Dis Child 1991;145:881–4.
2. Reach Out and Read: a national pediatric literacy program. Available at http://reachoutandread.org.
3. High PC, Klass P. Literacy promotion: an essential component of primary care pediatric practice. Council on Early Childhood. Pediatrics 2014;134:404–9.
4. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167–78.
5. Center on the Developing Child. The science of early childhood development (in brief); 2007. Accessed 6 May 2016 at www.developingchild.harvard.edu.
6. Connecting Science, Policy, and Practice: Zero to Three’s National Training Institute, 2015. Zero Three 2016;36(3).
7. Fox NA, Zeanah CH, Nelson CA. A matter of timing: enhancing positive change for the developing brain. Zero Three 2014;34(3):4–9.
8. Halfon N, Shulman E, Hochstein M. Brain development in early childhood. Technical report. UCLA Center for Healthier Children, Families and Communities. Aug 2001.
9. National Scientific Council on the Developing Child. The science of early childhood development: closing the gap between what we know and what we do. Center on the Developing Child. Harvard University; 2007.
10. Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995.
11. Hart B, Risley TR. The early catastrophe: the 30 million word gap by age 3. Am Educator 2003;27:4–9.
12. The Annie E. Casey Foundation. Double jeopardy: how third grade reading skills and poverty influence high school graduation. 2012. Accessed 21 Feb 2016 at www.aecf.org/resources/double-jeopardy.
13. The Annie E. Casey Foundation. Early warning confirmed: a research update on third grade reading. 2013 Nov. Accessed 23 Feb 2016 at www.aecf.org/m/resourcedoc/AECF-EarlyWarningConfirmed-2013.pdf
14. Heckman J. The economics of inequality: the value of early childhood education. Am Educator 2011;47:31–5.
15. Christakis DA. The effects of infant media usage: what do we know and what should we learn? Acta Paediatr 2009;98: 8–16.
16. American Academy of Pediatrics, Council on Communications and Media. Policy statement. Media use by children younger than 2 years. Pediatrics 2011;128:1040–5.
17. Linebarger DL, Walker D. Infants’ and toddlers’ television viewing and language outcomes. Am Behav Sci 2005;48:624–45.
18. Masako T, Okuma K, Kyoshima K. Television viewing and reduced parental utterance, and delayed speech development in infants and young children. Arch Pediatr Adolesc Med 2007;161:618–9.
19. Rideout VJ, Hamel E. The media family: electronic media in the lives of infants, toddlers, preschoolers, and their parents. Menlo Park, CA: Kaiser Family Foundation; 2006.
20. Sosa AV. Association of the type of toy used during play with the quantity and quality of parent-infant communication. JAMA Pediatr 2016;170:132–7.
21. Vandewater EA, Bickham DS, Lee JH et al. When the television is always on: heavy television exposure and young children’s development. Am Behav Sci 2005;48:562–77.
22. Zimmerman FJ, Christakis DA, Meltzoff AN. Associations between media viewing and language development in children under age two years. J Pediatr 2007;151:364–8.
23. Chonchaiya W, Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr 2008;97:977–82.
24. Robb MB, Richert RA, Wartella EA. Just a talking book? Word learning from watching baby videos. Br J Dev Psychol 2009;27(Pt 1):27–45.
25. DeLoache JS, Chiong C, Sherman K, et al. Do babies learn from baby media? Psychol Sci 2010;21:1570–4.
26. Mendelsohn A, Mogliner L, Dreyer B, et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics 2001;107:130–4.
27. Mendelsohn AL. Promoting language and literacy through reading aloud: the role of the pediatrician. Curr Probl Pediatr Adolesc Health Care 2002;32:183–210.
28. High P, Hopman M, LaGasse L, et al. Evaluation of a clinic-based program to promote book sharing and bedtime routines among low-income urban families with young children. Arch Pediatr Adolesc Med 1998;152:459–65.
29. Golova N, Alario A, Vivier P, et al. Literacy promotion for Hispanic families in a primary care setting: a randomized controlled trial. Pediatrics 1999;103:993–7.
30. High PC, LaGasse L, Becker S, et al. Literacy promotion in primary care pediatrics: can we make a difference? Pediatrics 2000;105:927–34.
31. Needlman R, Toker KH, Dreyer BP, et al. Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr 2005;5:209–15.
32. Wu SC, Lue HC, Tseng LL. A pediatric clinic-based approach to early literacy promotion--experience in a well-baby clinic in Taiwan. J Formos Med Assoc 2012;111:258–64.
33. Sanders LM, Gershon TD, Huffman LC, et al. Prescribing books for immigrant children: a pilot study to promote emergent literacy among the children of Hispanic immigrants. Arch Pediatr Adolesc Med 2000;154:771–7.
34. Kitabayashi KM, Huang GY, Linskey KR, et al. Parent-child reading interactions among English and English as a second language speakers in an underserved pediatric clinic in Hawai’i. Hawaii Med J 2008;67:260–3.
35. Festa N, Loftus PD, Cullen MR, Mendoza FS. Disparities in early exposure to book sharing within immigrant families. Pediatrics. 2014;134:e162–8.
36. Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. National Research Council (US) and Institute of Medicine (US) Committee on Integrating the Science of Early Childhood Development. Washington, DC: National Academies Press; 2000.
37. Neuman SB. Guiding young children’s participation in early literacy development: a family literacy program for adolescent mothers. Early Child Dev Care 1997;127:119–29.
38. Tomopoulos S, Dreyer BP, Tamis-LeMonda C, et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr 2006;6:72–8.
39. Mendelsohn AL, Huberman HS, Berkule SB, et al. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med 2011;165:33–41.
40. Ginsburg K; American Academy of Pediatrics, Committee on Communications, Committee on Psychosocial Aspects of Child and Family Health. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007;119:182–91.
41. Preston JL, Frost SJ, Mencl WE, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain 2010;133:2185–95.
42. Hugdahl K, Gundersen H, Brekke C, et al. fMRI Brain activation in a Finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res 2004;47:162–72.
43. Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 2000;6:207–13.
44. Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001;34:479–92.
45. Hutton JS, Horowitz-Kraus T, Mendelsohn AL, et al. Home reading environment and brain activation in preschool children listening to stories. Pediatrics 2015;136:466–78.
46. Data Resource Center for Child and Adolescent Health. 2011/12 National Survey of Children’s Health. Accessed 28 Feb 2016 at www.nschdata.org.
Assessment of Personal Medical History Knowledge in Adolescents with Sickle Cell Disease: A Pilot Study
From the Departments of Psychology (Ms. Zhao, Drs. Russell, Wesley, and Porter) and Hematology (Mss. Johnson and Pullen, Dr. Hankins), St. Jude Children’s Research Hospital, Memphis, TN.
Abstract
- Background: Children with sickle cell disease (SCD) are surviving into adulthood. Mastery of disease knowledge may facilitate treatment continuity in adult care.
- Objective: To assess the accuracy and extent of medical history knowledge among adolescents with SCD through the use of a personal health record (PHR) form.
- Methods: 68 adolescent patients with SCD (52.9% male; mean age, 16.8 years; 100% African American) completed a PHR listing significant prior medical events (eg, disease complications, diagnostic evaluations, treatments). Responses were compared against participants’ electronic medical record. An agreement percentage was calculated to determine accuracy of knowledge.
- Results: Most adolescents correctly reported their sickle cell genotype (100%), usage of penicillin (97.1%), prior hospitalizations (96.5%), history of prior blood transfusions (93.8%), usage of hydroxyurea (88.2%), and allergies (85.2%). Fewer adolescents accurately reported usage of opioids (52.9%), prior acute chest syndrome events (50.9%), baseline hemoglobin (41.8%), and hepatitis (43.3%), pneumovax (30.2%), and menactra (14.5%) vaccinations.
- Conclusion: Adolescents are aware of most but not all aspects of their medical history. The present findings can inform areas of knowledge deficits. Future targeted interventions for transition education and preparation may be tailored based on individual disease knowledge.
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal sickle hemoglobin resulting in chronic hemolytic anemia and vaso-occlusion [1]. More than 95% of children with SCD in the United States survive into adulthood; however, young adults (YAs) are at risk for mortality shortly after transfer to adult health care [2–5]. Specifically, YAs with SCD (ages 18 to 30) have increased hospital utilization, emergency department visits, and mortality compared to other age-groups [4–7]. During this critical period, transition preparation that includes improving disease literacy and ensuring medical history knowledge may be necessary for optimal outcomes.
In the extant YA literature, significant gaps in medical history knowledge during the transition period were observed in pediatric cancer and inflammatory bowel disease patients [8,9]. YAs often require multidisciplinary management of their chronic disease complications [10]. Therefore, possessing comprehensive knowledge of personal health history may facilitate communication with different adult care providers and promote continuity of care. In the SCD transition literature, transition readiness measures have been developed to assess several aspects of knowledge, including medical and disease knowledge; however, these measures are primarily self-reported perceptions of knowledge and do not evaluate the accuracy of knowledge [11,12]. The current pilot study addresses this gap with the aim of assessing medical history knowledge accuracy in adolescents with SCD.
Methods
Participants
From March 2011 to January 2014, adolescents (aged 15–18 years) with SCD (any genotype) were approached during their regular health maintenance visits by hematology social workers. They were invited to complete the Personal Health Record (PHR) as an implementation effort of transition preparation within our pediatric SCD program.
Personal Health Record
The PHR was developed through literature review and discussions with area adult hematologists. The form was modeled after first visit intake forms used in adult hematology clinics. It was reviewed by the hematology medical team and the institution’s patient education committee. Prior to implementation, the form was piloted to obtain patient feedback on format and content. The PHR consists of 33 questions with 168 possible items/data points covering 12 domains: personal information (eg, contact information, SCD genotype), health provider information, personal health history (ie, health diagnoses), blood transfusion history, sickle cell pain events, hospitalization history in the previous year, diagnostic testing history (eg, laboratory tests), current medications, immunizations, advance directives, resource information (eg, disability benefits), and activities of daily living. Some questions required patients to check “Yes” or “No” (eg, “Have you been hospitalized in the past year? Have you received flu vaccine?”) while some required a written response (eg, “What medicines do you currently take?”).
Adolescents were instructed to complete the PHR independently without the help of their caregivers. After completing the form, the social worker reviewed the answers and/or asked participants’ perspectives about communicating health information to providers. A copy of the completed PHR was provided to the adolescent to promote continued education regarding medical history knowledge. The retrospective review of the PHR answers and participants’ characteristics was approved by the institutional review board with a waiver of consent from participants.
Statistical Methods
PHR answers were compared with each individual’s electronic medical record (EMR) for accuracy of responses. PHR responses were considered accurate only if they matched the information in the EMR. PHR items absent in the EMR were not coded (inability to verify the accuracy of responses) to capture the most accurate depiction of adolescents’ medical history knowledge. Coding was checked by at least 2 coders for response accuracy. Due to lack of EMR information for certain items, we could not verify the accuracy of many PHR items. Therefore, only items with at least 75% of data verified (across all patients who completed the PHR) were included in subsequent analyses.
Using SPSS (version 18), an agreement percentage was calculated for each patient across verifiable items and used as the primary outcome measure of knowledge accuracy. We used t tests to investigate gender or genotype differences in medical history accuracy. To examine genotype differences, we stratified the sample by SCD genotype: HbSS/Sβ0 thalassemia and HbSC/Sβ+ thalassemia [13].
Results
Patient Characteristics
Knowledge Accuracy Among Adolescents with SCD
Seventeen items in 6 PHR domains had the highest number of data points (at least 75% verified), and therefore were the only items that could be analyzed. Analyzed items included information about sickle cell genotype, eye doctor care, comorbid health issues (eg, asthma), allergies, hospitalizations, surgeries, transfusions, acute chest syndrome (ACS) episodes, eye problems, baseline hemoglobin level, and vaccination history as well as adolescents’ knowledge of current medications, including hydroxyurea, penicillin, and opioid pain medications.
Gender was not significantly associated with overall accuracy (P = 0.36). A significant difference was found in sickle genotype such that individuals with HbSC/Sβ+ thalassemia genotype (mean number of items, 8.23; SD = 1.70) were more accurate reporters of their medical history than those with HbSS/Sβ0 thalassemia genotype (mean number of items, 7.14; SD = 1.75; t(65) = –2.59, P = 0.01). Specifically, those with HbSS/Sβ0 thalassemia genotype were significantly less accurate reporters of vaccination history (meningococcus t(60) = 3.55, P = 0.001; pneumococcus t(60) = 2.46, P = 0.02; hepatitis t(64) = 2.18, P = 0.03, eye problems t(62) = 3.62; P = 0.001, and surgical history t(62) = 2.14, P = 0.04).
Discussion
In the present study, we utilized the PHR to assess the accuracy of medical history knowledge of adolescents with SCD preparing to transition to adult care. Most adolescents were accurate reporters of important disease-relevant information (eg, genotype, transfusion history, hydroxyurea use), which may be a result of these topics being frequently discussed or recently encountered. For example, 97% of adolescents accurately reported penicillin use which may be related to our program’s emphasis on infection prevention education. However, disease knowledge of immunization history, prior ACS events, and opioid medication use might have been more difficult to recall due to the long interval from their occurrence until the completion of the PHR. Further, frequent changes in opioid medication use may have impacted the accuracy of adolescents’ answers with EMR data.
Individuals with HbSC/Sβ+ thalassemia genotype were more accurate reporters of their medical history, but the magnitude of difference was not large. These individuals tend to have fewer health issues and therefore less health information to recall, leading to higher accuracy. Furthermore, evidence demonstrates that individuals with HbSS/Sβ0 thalassemia genotype are at greater risk for cerebrovascular events and subsequent cognitive deficits [14], leading to more memory deficits and difficulty understanding and retaining health information [15]. The results suggest that patient health literacy, or an individual’s capacity to understand basic health information [16], may be a mediating factor in assessing for transition readiness. This is especially important given SCD risk for cognitive deficits [17].
Only 17 PHR items were analyzed due to conservative selection of items. Thus the present findings are not representative of the entire medical history. Additionally, the accuracy of medical history knowledge results may be limited by conservatism with abstracting information from the EMR (PHR information was considered accurate if it matched the information found in their EMR). Finally, we did not systematically assess the feasibility and utility of the PHR; ongoing participant feedback would aid in improving the PHR tool and implementation. It would be important to validate the PHR in a larger sample. However, our study is the first to our knowledge to systematically evaluate medical history knowledge among youth with SCD.
Conclusion and Practice Implications
The present study demonstrates that use of the PHR during regular health maintenance visits can help identify gaps in knowledge among adolescents with SCD who are approaching transfer to adult care. Sufficient knowledge of one’s medical history is an important aspect in transition preparation as it can facilitate the communication of medical information, thereby ensuring continuity of care [18,19]. The PHR could be used to teach medical history knowledge, assess a patient’s level of transition readiness at different time points, and identify areas for further targeted intervention. Knowledge tools, such as the PHR, can be investigated prospectively to assess the association of disease literacy and clinical outcomes, serving as a possible predictive instrument for transition health outcomes.
Corresponding author: Jerlym S. Porter, PhD, MPH, St. Jude Children’s Research Hospital, Dept. of Psychology, 262 Danny Thomas Pl., Mail Stop 740, Memphis, TN 38105, [email protected].
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02 (PI: Hankins).
Financial disclosures: None.
Author contributions: conception and design, MJ, AP, KMW, JSH, JSP; analysis and interpretation of data, MSZ, KMR, JSP; drafting of article, MSZ, JSP; critical revision of the article, MSZ, MJ, AP, KMW, JSH, JSP; provision of study materials or patients, MJ, AP; statistical expertise, KMR; obtaining of funding, JSH; collection and assembly of data, MSZ, MJ, AP, KMR, KMW.
1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013;60:1363–81.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:S512–21.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. de Montalembert M, Guitton C. Transition from paediatric to adult care for patients with sickle cell disease. Br J Haematol 2014;164:630–5.
5. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
6. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
7. Lanzkron S, Carroll CP, Haywood Jr C. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
8. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002:287:1832–9.
9. Hait EJ, Barendse RM, Arnold JH, et al. Transition of adolescents with inflammatory bowel disease from pediatric to adult care: a survey of adult gastroenterologists. J Pediatr Gastroenterol Nutr 2009;48:61–5.
10. Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr 2008;20:403–9.
11. Sobota A, Akinlonu A, Champigny M, et al. Self-reported transition readiness among young adults with sickle cell disease. J Pediatr Hematol Oncol 2014;36:389–94.
12. Treadwell M, Johnson S, Sisler I, et al. Development of a sickle cell disease readiness for transition assessment. Int J Adolesc Med Health 2016;28:193–201.
13. Dampier C, Ely B, Brodecki D, et al. Pain characteristics and age-related pain trajectories in infants and young children with sickle cell disease. Pediatr Blood Cancer 2014;61:291–6.
14. Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol 2014;120:1015–25.
15. Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol 2014;36:572–7.
16. Centers for Disease Control and Prevention. Health literacy. 2015. Accessed 26 Oct 2015 at www.cdc.gov/healthliteracy/index.html.
17. Armstrong FD, Thompson Jr RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97:864–70.
18. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev 2013;27:279–87.
19. Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011;86:116–2.
From the Departments of Psychology (Ms. Zhao, Drs. Russell, Wesley, and Porter) and Hematology (Mss. Johnson and Pullen, Dr. Hankins), St. Jude Children’s Research Hospital, Memphis, TN.
Abstract
- Background: Children with sickle cell disease (SCD) are surviving into adulthood. Mastery of disease knowledge may facilitate treatment continuity in adult care.
- Objective: To assess the accuracy and extent of medical history knowledge among adolescents with SCD through the use of a personal health record (PHR) form.
- Methods: 68 adolescent patients with SCD (52.9% male; mean age, 16.8 years; 100% African American) completed a PHR listing significant prior medical events (eg, disease complications, diagnostic evaluations, treatments). Responses were compared against participants’ electronic medical record. An agreement percentage was calculated to determine accuracy of knowledge.
- Results: Most adolescents correctly reported their sickle cell genotype (100%), usage of penicillin (97.1%), prior hospitalizations (96.5%), history of prior blood transfusions (93.8%), usage of hydroxyurea (88.2%), and allergies (85.2%). Fewer adolescents accurately reported usage of opioids (52.9%), prior acute chest syndrome events (50.9%), baseline hemoglobin (41.8%), and hepatitis (43.3%), pneumovax (30.2%), and menactra (14.5%) vaccinations.
- Conclusion: Adolescents are aware of most but not all aspects of their medical history. The present findings can inform areas of knowledge deficits. Future targeted interventions for transition education and preparation may be tailored based on individual disease knowledge.
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal sickle hemoglobin resulting in chronic hemolytic anemia and vaso-occlusion [1]. More than 95% of children with SCD in the United States survive into adulthood; however, young adults (YAs) are at risk for mortality shortly after transfer to adult health care [2–5]. Specifically, YAs with SCD (ages 18 to 30) have increased hospital utilization, emergency department visits, and mortality compared to other age-groups [4–7]. During this critical period, transition preparation that includes improving disease literacy and ensuring medical history knowledge may be necessary for optimal outcomes.
In the extant YA literature, significant gaps in medical history knowledge during the transition period were observed in pediatric cancer and inflammatory bowel disease patients [8,9]. YAs often require multidisciplinary management of their chronic disease complications [10]. Therefore, possessing comprehensive knowledge of personal health history may facilitate communication with different adult care providers and promote continuity of care. In the SCD transition literature, transition readiness measures have been developed to assess several aspects of knowledge, including medical and disease knowledge; however, these measures are primarily self-reported perceptions of knowledge and do not evaluate the accuracy of knowledge [11,12]. The current pilot study addresses this gap with the aim of assessing medical history knowledge accuracy in adolescents with SCD.
Methods
Participants
From March 2011 to January 2014, adolescents (aged 15–18 years) with SCD (any genotype) were approached during their regular health maintenance visits by hematology social workers. They were invited to complete the Personal Health Record (PHR) as an implementation effort of transition preparation within our pediatric SCD program.
Personal Health Record
The PHR was developed through literature review and discussions with area adult hematologists. The form was modeled after first visit intake forms used in adult hematology clinics. It was reviewed by the hematology medical team and the institution’s patient education committee. Prior to implementation, the form was piloted to obtain patient feedback on format and content. The PHR consists of 33 questions with 168 possible items/data points covering 12 domains: personal information (eg, contact information, SCD genotype), health provider information, personal health history (ie, health diagnoses), blood transfusion history, sickle cell pain events, hospitalization history in the previous year, diagnostic testing history (eg, laboratory tests), current medications, immunizations, advance directives, resource information (eg, disability benefits), and activities of daily living. Some questions required patients to check “Yes” or “No” (eg, “Have you been hospitalized in the past year? Have you received flu vaccine?”) while some required a written response (eg, “What medicines do you currently take?”).
Adolescents were instructed to complete the PHR independently without the help of their caregivers. After completing the form, the social worker reviewed the answers and/or asked participants’ perspectives about communicating health information to providers. A copy of the completed PHR was provided to the adolescent to promote continued education regarding medical history knowledge. The retrospective review of the PHR answers and participants’ characteristics was approved by the institutional review board with a waiver of consent from participants.
Statistical Methods
PHR answers were compared with each individual’s electronic medical record (EMR) for accuracy of responses. PHR responses were considered accurate only if they matched the information in the EMR. PHR items absent in the EMR were not coded (inability to verify the accuracy of responses) to capture the most accurate depiction of adolescents’ medical history knowledge. Coding was checked by at least 2 coders for response accuracy. Due to lack of EMR information for certain items, we could not verify the accuracy of many PHR items. Therefore, only items with at least 75% of data verified (across all patients who completed the PHR) were included in subsequent analyses.
Using SPSS (version 18), an agreement percentage was calculated for each patient across verifiable items and used as the primary outcome measure of knowledge accuracy. We used t tests to investigate gender or genotype differences in medical history accuracy. To examine genotype differences, we stratified the sample by SCD genotype: HbSS/Sβ0 thalassemia and HbSC/Sβ+ thalassemia [13].
Results
Patient Characteristics
Knowledge Accuracy Among Adolescents with SCD
Seventeen items in 6 PHR domains had the highest number of data points (at least 75% verified), and therefore were the only items that could be analyzed. Analyzed items included information about sickle cell genotype, eye doctor care, comorbid health issues (eg, asthma), allergies, hospitalizations, surgeries, transfusions, acute chest syndrome (ACS) episodes, eye problems, baseline hemoglobin level, and vaccination history as well as adolescents’ knowledge of current medications, including hydroxyurea, penicillin, and opioid pain medications.
Gender was not significantly associated with overall accuracy (P = 0.36). A significant difference was found in sickle genotype such that individuals with HbSC/Sβ+ thalassemia genotype (mean number of items, 8.23; SD = 1.70) were more accurate reporters of their medical history than those with HbSS/Sβ0 thalassemia genotype (mean number of items, 7.14; SD = 1.75; t(65) = –2.59, P = 0.01). Specifically, those with HbSS/Sβ0 thalassemia genotype were significantly less accurate reporters of vaccination history (meningococcus t(60) = 3.55, P = 0.001; pneumococcus t(60) = 2.46, P = 0.02; hepatitis t(64) = 2.18, P = 0.03, eye problems t(62) = 3.62; P = 0.001, and surgical history t(62) = 2.14, P = 0.04).
Discussion
In the present study, we utilized the PHR to assess the accuracy of medical history knowledge of adolescents with SCD preparing to transition to adult care. Most adolescents were accurate reporters of important disease-relevant information (eg, genotype, transfusion history, hydroxyurea use), which may be a result of these topics being frequently discussed or recently encountered. For example, 97% of adolescents accurately reported penicillin use which may be related to our program’s emphasis on infection prevention education. However, disease knowledge of immunization history, prior ACS events, and opioid medication use might have been more difficult to recall due to the long interval from their occurrence until the completion of the PHR. Further, frequent changes in opioid medication use may have impacted the accuracy of adolescents’ answers with EMR data.
Individuals with HbSC/Sβ+ thalassemia genotype were more accurate reporters of their medical history, but the magnitude of difference was not large. These individuals tend to have fewer health issues and therefore less health information to recall, leading to higher accuracy. Furthermore, evidence demonstrates that individuals with HbSS/Sβ0 thalassemia genotype are at greater risk for cerebrovascular events and subsequent cognitive deficits [14], leading to more memory deficits and difficulty understanding and retaining health information [15]. The results suggest that patient health literacy, or an individual’s capacity to understand basic health information [16], may be a mediating factor in assessing for transition readiness. This is especially important given SCD risk for cognitive deficits [17].
Only 17 PHR items were analyzed due to conservative selection of items. Thus the present findings are not representative of the entire medical history. Additionally, the accuracy of medical history knowledge results may be limited by conservatism with abstracting information from the EMR (PHR information was considered accurate if it matched the information found in their EMR). Finally, we did not systematically assess the feasibility and utility of the PHR; ongoing participant feedback would aid in improving the PHR tool and implementation. It would be important to validate the PHR in a larger sample. However, our study is the first to our knowledge to systematically evaluate medical history knowledge among youth with SCD.
Conclusion and Practice Implications
The present study demonstrates that use of the PHR during regular health maintenance visits can help identify gaps in knowledge among adolescents with SCD who are approaching transfer to adult care. Sufficient knowledge of one’s medical history is an important aspect in transition preparation as it can facilitate the communication of medical information, thereby ensuring continuity of care [18,19]. The PHR could be used to teach medical history knowledge, assess a patient’s level of transition readiness at different time points, and identify areas for further targeted intervention. Knowledge tools, such as the PHR, can be investigated prospectively to assess the association of disease literacy and clinical outcomes, serving as a possible predictive instrument for transition health outcomes.
Corresponding author: Jerlym S. Porter, PhD, MPH, St. Jude Children’s Research Hospital, Dept. of Psychology, 262 Danny Thomas Pl., Mail Stop 740, Memphis, TN 38105, [email protected].
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02 (PI: Hankins).
Financial disclosures: None.
Author contributions: conception and design, MJ, AP, KMW, JSH, JSP; analysis and interpretation of data, MSZ, KMR, JSP; drafting of article, MSZ, JSP; critical revision of the article, MSZ, MJ, AP, KMW, JSH, JSP; provision of study materials or patients, MJ, AP; statistical expertise, KMR; obtaining of funding, JSH; collection and assembly of data, MSZ, MJ, AP, KMR, KMW.
From the Departments of Psychology (Ms. Zhao, Drs. Russell, Wesley, and Porter) and Hematology (Mss. Johnson and Pullen, Dr. Hankins), St. Jude Children’s Research Hospital, Memphis, TN.
Abstract
- Background: Children with sickle cell disease (SCD) are surviving into adulthood. Mastery of disease knowledge may facilitate treatment continuity in adult care.
- Objective: To assess the accuracy and extent of medical history knowledge among adolescents with SCD through the use of a personal health record (PHR) form.
- Methods: 68 adolescent patients with SCD (52.9% male; mean age, 16.8 years; 100% African American) completed a PHR listing significant prior medical events (eg, disease complications, diagnostic evaluations, treatments). Responses were compared against participants’ electronic medical record. An agreement percentage was calculated to determine accuracy of knowledge.
- Results: Most adolescents correctly reported their sickle cell genotype (100%), usage of penicillin (97.1%), prior hospitalizations (96.5%), history of prior blood transfusions (93.8%), usage of hydroxyurea (88.2%), and allergies (85.2%). Fewer adolescents accurately reported usage of opioids (52.9%), prior acute chest syndrome events (50.9%), baseline hemoglobin (41.8%), and hepatitis (43.3%), pneumovax (30.2%), and menactra (14.5%) vaccinations.
- Conclusion: Adolescents are aware of most but not all aspects of their medical history. The present findings can inform areas of knowledge deficits. Future targeted interventions for transition education and preparation may be tailored based on individual disease knowledge.
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal sickle hemoglobin resulting in chronic hemolytic anemia and vaso-occlusion [1]. More than 95% of children with SCD in the United States survive into adulthood; however, young adults (YAs) are at risk for mortality shortly after transfer to adult health care [2–5]. Specifically, YAs with SCD (ages 18 to 30) have increased hospital utilization, emergency department visits, and mortality compared to other age-groups [4–7]. During this critical period, transition preparation that includes improving disease literacy and ensuring medical history knowledge may be necessary for optimal outcomes.
In the extant YA literature, significant gaps in medical history knowledge during the transition period were observed in pediatric cancer and inflammatory bowel disease patients [8,9]. YAs often require multidisciplinary management of their chronic disease complications [10]. Therefore, possessing comprehensive knowledge of personal health history may facilitate communication with different adult care providers and promote continuity of care. In the SCD transition literature, transition readiness measures have been developed to assess several aspects of knowledge, including medical and disease knowledge; however, these measures are primarily self-reported perceptions of knowledge and do not evaluate the accuracy of knowledge [11,12]. The current pilot study addresses this gap with the aim of assessing medical history knowledge accuracy in adolescents with SCD.
Methods
Participants
From March 2011 to January 2014, adolescents (aged 15–18 years) with SCD (any genotype) were approached during their regular health maintenance visits by hematology social workers. They were invited to complete the Personal Health Record (PHR) as an implementation effort of transition preparation within our pediatric SCD program.
Personal Health Record
The PHR was developed through literature review and discussions with area adult hematologists. The form was modeled after first visit intake forms used in adult hematology clinics. It was reviewed by the hematology medical team and the institution’s patient education committee. Prior to implementation, the form was piloted to obtain patient feedback on format and content. The PHR consists of 33 questions with 168 possible items/data points covering 12 domains: personal information (eg, contact information, SCD genotype), health provider information, personal health history (ie, health diagnoses), blood transfusion history, sickle cell pain events, hospitalization history in the previous year, diagnostic testing history (eg, laboratory tests), current medications, immunizations, advance directives, resource information (eg, disability benefits), and activities of daily living. Some questions required patients to check “Yes” or “No” (eg, “Have you been hospitalized in the past year? Have you received flu vaccine?”) while some required a written response (eg, “What medicines do you currently take?”).
Adolescents were instructed to complete the PHR independently without the help of their caregivers. After completing the form, the social worker reviewed the answers and/or asked participants’ perspectives about communicating health information to providers. A copy of the completed PHR was provided to the adolescent to promote continued education regarding medical history knowledge. The retrospective review of the PHR answers and participants’ characteristics was approved by the institutional review board with a waiver of consent from participants.
Statistical Methods
PHR answers were compared with each individual’s electronic medical record (EMR) for accuracy of responses. PHR responses were considered accurate only if they matched the information in the EMR. PHR items absent in the EMR were not coded (inability to verify the accuracy of responses) to capture the most accurate depiction of adolescents’ medical history knowledge. Coding was checked by at least 2 coders for response accuracy. Due to lack of EMR information for certain items, we could not verify the accuracy of many PHR items. Therefore, only items with at least 75% of data verified (across all patients who completed the PHR) were included in subsequent analyses.
Using SPSS (version 18), an agreement percentage was calculated for each patient across verifiable items and used as the primary outcome measure of knowledge accuracy. We used t tests to investigate gender or genotype differences in medical history accuracy. To examine genotype differences, we stratified the sample by SCD genotype: HbSS/Sβ0 thalassemia and HbSC/Sβ+ thalassemia [13].
Results
Patient Characteristics
Knowledge Accuracy Among Adolescents with SCD
Seventeen items in 6 PHR domains had the highest number of data points (at least 75% verified), and therefore were the only items that could be analyzed. Analyzed items included information about sickle cell genotype, eye doctor care, comorbid health issues (eg, asthma), allergies, hospitalizations, surgeries, transfusions, acute chest syndrome (ACS) episodes, eye problems, baseline hemoglobin level, and vaccination history as well as adolescents’ knowledge of current medications, including hydroxyurea, penicillin, and opioid pain medications.
Gender was not significantly associated with overall accuracy (P = 0.36). A significant difference was found in sickle genotype such that individuals with HbSC/Sβ+ thalassemia genotype (mean number of items, 8.23; SD = 1.70) were more accurate reporters of their medical history than those with HbSS/Sβ0 thalassemia genotype (mean number of items, 7.14; SD = 1.75; t(65) = –2.59, P = 0.01). Specifically, those with HbSS/Sβ0 thalassemia genotype were significantly less accurate reporters of vaccination history (meningococcus t(60) = 3.55, P = 0.001; pneumococcus t(60) = 2.46, P = 0.02; hepatitis t(64) = 2.18, P = 0.03, eye problems t(62) = 3.62; P = 0.001, and surgical history t(62) = 2.14, P = 0.04).
Discussion
In the present study, we utilized the PHR to assess the accuracy of medical history knowledge of adolescents with SCD preparing to transition to adult care. Most adolescents were accurate reporters of important disease-relevant information (eg, genotype, transfusion history, hydroxyurea use), which may be a result of these topics being frequently discussed or recently encountered. For example, 97% of adolescents accurately reported penicillin use which may be related to our program’s emphasis on infection prevention education. However, disease knowledge of immunization history, prior ACS events, and opioid medication use might have been more difficult to recall due to the long interval from their occurrence until the completion of the PHR. Further, frequent changes in opioid medication use may have impacted the accuracy of adolescents’ answers with EMR data.
Individuals with HbSC/Sβ+ thalassemia genotype were more accurate reporters of their medical history, but the magnitude of difference was not large. These individuals tend to have fewer health issues and therefore less health information to recall, leading to higher accuracy. Furthermore, evidence demonstrates that individuals with HbSS/Sβ0 thalassemia genotype are at greater risk for cerebrovascular events and subsequent cognitive deficits [14], leading to more memory deficits and difficulty understanding and retaining health information [15]. The results suggest that patient health literacy, or an individual’s capacity to understand basic health information [16], may be a mediating factor in assessing for transition readiness. This is especially important given SCD risk for cognitive deficits [17].
Only 17 PHR items were analyzed due to conservative selection of items. Thus the present findings are not representative of the entire medical history. Additionally, the accuracy of medical history knowledge results may be limited by conservatism with abstracting information from the EMR (PHR information was considered accurate if it matched the information found in their EMR). Finally, we did not systematically assess the feasibility and utility of the PHR; ongoing participant feedback would aid in improving the PHR tool and implementation. It would be important to validate the PHR in a larger sample. However, our study is the first to our knowledge to systematically evaluate medical history knowledge among youth with SCD.
Conclusion and Practice Implications
The present study demonstrates that use of the PHR during regular health maintenance visits can help identify gaps in knowledge among adolescents with SCD who are approaching transfer to adult care. Sufficient knowledge of one’s medical history is an important aspect in transition preparation as it can facilitate the communication of medical information, thereby ensuring continuity of care [18,19]. The PHR could be used to teach medical history knowledge, assess a patient’s level of transition readiness at different time points, and identify areas for further targeted intervention. Knowledge tools, such as the PHR, can be investigated prospectively to assess the association of disease literacy and clinical outcomes, serving as a possible predictive instrument for transition health outcomes.
Corresponding author: Jerlym S. Porter, PhD, MPH, St. Jude Children’s Research Hospital, Dept. of Psychology, 262 Danny Thomas Pl., Mail Stop 740, Memphis, TN 38105, [email protected].
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02 (PI: Hankins).
Financial disclosures: None.
Author contributions: conception and design, MJ, AP, KMW, JSH, JSP; analysis and interpretation of data, MSZ, KMR, JSP; drafting of article, MSZ, JSP; critical revision of the article, MSZ, MJ, AP, KMW, JSH, JSP; provision of study materials or patients, MJ, AP; statistical expertise, KMR; obtaining of funding, JSH; collection and assembly of data, MSZ, MJ, AP, KMR, KMW.
1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013;60:1363–81.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:S512–21.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. de Montalembert M, Guitton C. Transition from paediatric to adult care for patients with sickle cell disease. Br J Haematol 2014;164:630–5.
5. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
6. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
7. Lanzkron S, Carroll CP, Haywood Jr C. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
8. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002:287:1832–9.
9. Hait EJ, Barendse RM, Arnold JH, et al. Transition of adolescents with inflammatory bowel disease from pediatric to adult care: a survey of adult gastroenterologists. J Pediatr Gastroenterol Nutr 2009;48:61–5.
10. Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr 2008;20:403–9.
11. Sobota A, Akinlonu A, Champigny M, et al. Self-reported transition readiness among young adults with sickle cell disease. J Pediatr Hematol Oncol 2014;36:389–94.
12. Treadwell M, Johnson S, Sisler I, et al. Development of a sickle cell disease readiness for transition assessment. Int J Adolesc Med Health 2016;28:193–201.
13. Dampier C, Ely B, Brodecki D, et al. Pain characteristics and age-related pain trajectories in infants and young children with sickle cell disease. Pediatr Blood Cancer 2014;61:291–6.
14. Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol 2014;120:1015–25.
15. Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol 2014;36:572–7.
16. Centers for Disease Control and Prevention. Health literacy. 2015. Accessed 26 Oct 2015 at www.cdc.gov/healthliteracy/index.html.
17. Armstrong FD, Thompson Jr RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97:864–70.
18. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev 2013;27:279–87.
19. Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011;86:116–2.
1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013;60:1363–81.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:S512–21.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. de Montalembert M, Guitton C. Transition from paediatric to adult care for patients with sickle cell disease. Br J Haematol 2014;164:630–5.
5. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
6. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
7. Lanzkron S, Carroll CP, Haywood Jr C. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
8. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002:287:1832–9.
9. Hait EJ, Barendse RM, Arnold JH, et al. Transition of adolescents with inflammatory bowel disease from pediatric to adult care: a survey of adult gastroenterologists. J Pediatr Gastroenterol Nutr 2009;48:61–5.
10. Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr 2008;20:403–9.
11. Sobota A, Akinlonu A, Champigny M, et al. Self-reported transition readiness among young adults with sickle cell disease. J Pediatr Hematol Oncol 2014;36:389–94.
12. Treadwell M, Johnson S, Sisler I, et al. Development of a sickle cell disease readiness for transition assessment. Int J Adolesc Med Health 2016;28:193–201.
13. Dampier C, Ely B, Brodecki D, et al. Pain characteristics and age-related pain trajectories in infants and young children with sickle cell disease. Pediatr Blood Cancer 2014;61:291–6.
14. Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol 2014;120:1015–25.
15. Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol 2014;36:572–7.
16. Centers for Disease Control and Prevention. Health literacy. 2015. Accessed 26 Oct 2015 at www.cdc.gov/healthliteracy/index.html.
17. Armstrong FD, Thompson Jr RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97:864–70.
18. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev 2013;27:279–87.
19. Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011;86:116–2.
Weight Gain Prevention in Young Adults: A New Frontier for Primary Care?
Study Overview
Objective. To compare several behavioral strategies for weight gain prevention in young adults.
Study design. Randomized clinical trial.
Setting and participants. The study took place at 2 U.S. academic centers between 2010 and 2016. Participants were recruited using email and postal mailings if they were 18–35 years old, had a body mass index (BMI) between 21 and 30.9 (ie, they ranged from normal body weight to class I obesity), spoke English, had internet access, and did not have contraindications to participating in a behavioral weight management intervention (eg, eating disorders). Once recruited, participants were block randomized, stratified by site, sex, and ethnic group, in to 1 of 3 study arms. The control arm of the study consisted of a single in-person meeting where behavioral strategies to prevent weight gain were discussed, as well as quarterly newsletters and personalized reports on interim weight data during follow-up.
Intervention. There were 2 intervention arms in the study. Both intervention groups had 10 in-person group-based visits over the initial 4 months of the intervention, at which strategies to prevent weight gain were discussed. Additionally they received annual invitations to participate in online refresher courses and the same newsletter frequency and content as the control group. Advice to the 2 intervention groups differed, however. Those in the “small changes” group were advised to decrease caloric intake by about 100 kcal per day in order to prevent weight gain. Additionally they were given pedometers, with a goal of increasing their daily step counts by about 2000. In the “large changes” group, participants were given lower calorie targets and more aggressive physical activity goals, with a goal of producing weight loss over the first 4 months of follow-up (2.3 kg for those with normal baseline BMI, and 4.5 kg if overweight or obese at baseline). Participants in all groups were encouraged to engage in self-monitoring behaviors such as daily weighing, and to report these weights to study staff by email, text, or on the web. Aside from pre-specified study follow-up assessments, most follow-up beyond the initial 4 month “small” or “large” changes phase was done using email or web-based intervention.
Main outcome measures. All participants were scheduled for follow-up assessments at 4 months, 1 year, and 2 years, with some early participants having additional follow-ups at 3 and 4 years. The primary outcome of interest was change in weight from baseline through follow-up, with additional outcome measures including the proportion in each group who gained at least 0.45 kg, or developed obesity. Additionally, the investigators did a thorough evaluation of intervention implementation and delivery. Weight change was modeled using mixed effects linear models, adjusting for clinic site. They corrected for multiple measures using Bonferroni adjustment to minimize the risk of type I error and used multiple imputation to examine the impact of missing data on their results. Pre-specified subgroup comparisons between several groups of patients were conducted—those in the normal weight vs. overweight category at baseline, those younger vs. older than age 25 at baseline, and men vs. women.
Results. 599 participants were randomized to the control (n = 202), small changes (n = 200), or large changes groups (n = 197), with no significant differences between groups in terms of measured baseline characteristics. The majority of participants were women (78%) and non-Hispanic white (73%). Mean (SD) baseline age was 28.2 (4.4) years and BMI was 25.4 (2.6) kg/m2. The group as a whole was highly educated—between 77% and 82% had college degrees. The series of 10 intervention sessions in the first 4 months was very well-attended (87% attendance on average for large changes group, 86% for small changes group), and by 4 months of follow-up, a majority of participants in both intervention groups endorsed the behavior of daily self-weighing (75% in large changes, 72% in small changes).
Both intervention groups had statistically significant weight losses compared to control (average weight change in control +0.3 kg, in small change –0.6 kg, and in large change –2.4 kg, over an average of 3 years), with large change participants also having significantly greater average weight loss in follow-up than small change participants. Significantly fewer participants in the intervention groups went on to develop obesity than in the control group (16.9% incidence in control, vs. 7.9% incidence in small changes [P = 0.002] and 8.6% in large changes [P = 0.02]). Importantly, the trajectories of weight gain (or regain) after the initial 4-month intervention differed between the small and large change groups, with small change participants experiencing a more gradual rate of gain throughout follow-up, versus a steeper rate of gain in the large changes group, such that the groups were at very similar weights by the final time point. The investigators did not observe any differences in effect between subgroups according to participant baseline BMI, sex, age, or race.
Conclusion. The authors conclude that these scalable small- and large-change interventions reduced longer-term weight gain and even promoted weight loss in a group of young adults, with the large-change intervention having a greater impact on weight than the small-change intervention.
Commentary
Treatment of obesity is difficult, leading to frustration for many patients and clinicians. Although it is often possible to help patients lose weight with tools such as low-calorie diets and increased physical activity, the long-term maintenance of weight loss is quite challenging. There is a growing awareness that the difficulty in maintaining weight loss has strong physiologic underpinnings. The human body has complex energy regulatory systems that may oppose weight loss by lowering metabolic rate, increasing hunger cues, and limiting satiety cues, when faced with energy restriction or weight loss [1,2].
In order to decrease the number of patients who ultimately require treatment for obesity, an alternative approach may be to try to prevent weight gain in the first place. Young adults in the U.S. tend to gain weight steadily over time, yet this insidious pattern is unlikely to be addressed by physicians [3]. Given that gradual weight gain seems to be the norm for most young adults, it may be beneficial for primary care providers to advise all young adult patients to make small behavioral changes in order to prevent the onset of overweight or obesity. Preventing weight gain is an attractive approach for broad application because it may require lower intensity programs, and less behavioral commitment from patients, compared to what is required for weight loss [4].
In this randomized trial, Wing et al investigated several relatively low-intensity approaches for weight gain prevention. Strengths of the study include aspects of the design and analysis, including its randomized nature, the relatively long follow-up period, the use of multiple imputation to address missing data, and the use of statistical methods to account for the large number of comparisons made between groups over time (Bonferroni correction). More importantly, however, this study represents an important innovation in how physicians might think about obesity, with a shift toward prevention rather than treatment. Historically, many obesity prevention efforts have fallen in the domain of public health or population-level interventions, and it may be the case that physicians have felt they did not really have a role in prevention. On the other hand, doctors who have engaged in obesity treatment—trying to help patients lose weight—may have felt that they lacked the resources or training needed to implement successful programs to promote long-term weight loss. By testing several lower-intensity strategies for weight gain prevention, this study sheds light on what could possibly be a new role for primary care providers or health care systems who care for otherwise healthy young adults. As the authors point out, the methods they employed could also be easily scaled or disseminated using public health approaches and community organizations.
In addition to addressing an important topic, this study relied on intervention methods that would be relatively easy to replicate in clinical practice or in community settings. Aside from the initial 4-month intervention, which involved 10 face-to-face group sessions (which were very well attended by participants), the remainder of the ~3 year follow-up consisted mostly of contact that took place electronically using email and/or text messaging. These modes of communication align well with the move toward electronic health records (eg, e-visits) and are probably ideally suited for young adults, who as a group rely heavily on these methods of communication.
The study has several limitations, most of which are addressed by the authors in the discussion section of the paper. As with most studies of behavioral weight interventions, the majority of participants in this study were women, with relatively few racial and ethnic minorities. Furthermore this was a highly educated group of participants and it is unclear whether these results would generalize to a more diverse clinical population with fewer resources or lower health literacy. Given that the control arm of the study experienced less weight gain over time than would be expected based on population averages, it could be that the participants in this study were a select group of individuals who were more motivated around preventing long-term health problems than a general clinical population. One additional point of possible concern is that, while participants in the “large changes” group did, as per the design, lose weight at the beginning of the trial, they also went on to regain much of that weight and experienced a steeper trajectory of overall gain during follow-up compared to the “small changes” group, so that the 2 intervention groups were not statistically different from each other in terms of overall weight change from baseline by 2 years. Therefore, whether the “large changes” approach is truly more beneficial for long-term obesity prevention than the more modest “small changes” approach is not entirely clear from this study.
Applications for Clinical Practice
The identification of young adults who are gaining weight, but who are not yet obese, represents an opportunity for providers and health care systems. Efforts to promote modest dietary and physical activity changes in this population may prevent obesity, and may be achievable even in busy clinical practice settings. Whether weight-gain prevention programs should include an attempt to first foster a small amount of weight loss as a “buffer” against later gains is still not entirely clear.
—Kristina Lewis, MD, MPH
1. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–604.
2. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016 May 2.
3. Tang JW, Kushner RF, Thompson J, Baker DW. Physician counseling of young adults with rapid weight gain: a retrospective cohort study. BMC Fam Pract 2010;11:31.
4. Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med 2013;173:1770–7.
Study Overview
Objective. To compare several behavioral strategies for weight gain prevention in young adults.
Study design. Randomized clinical trial.
Setting and participants. The study took place at 2 U.S. academic centers between 2010 and 2016. Participants were recruited using email and postal mailings if they were 18–35 years old, had a body mass index (BMI) between 21 and 30.9 (ie, they ranged from normal body weight to class I obesity), spoke English, had internet access, and did not have contraindications to participating in a behavioral weight management intervention (eg, eating disorders). Once recruited, participants were block randomized, stratified by site, sex, and ethnic group, in to 1 of 3 study arms. The control arm of the study consisted of a single in-person meeting where behavioral strategies to prevent weight gain were discussed, as well as quarterly newsletters and personalized reports on interim weight data during follow-up.
Intervention. There were 2 intervention arms in the study. Both intervention groups had 10 in-person group-based visits over the initial 4 months of the intervention, at which strategies to prevent weight gain were discussed. Additionally they received annual invitations to participate in online refresher courses and the same newsletter frequency and content as the control group. Advice to the 2 intervention groups differed, however. Those in the “small changes” group were advised to decrease caloric intake by about 100 kcal per day in order to prevent weight gain. Additionally they were given pedometers, with a goal of increasing their daily step counts by about 2000. In the “large changes” group, participants were given lower calorie targets and more aggressive physical activity goals, with a goal of producing weight loss over the first 4 months of follow-up (2.3 kg for those with normal baseline BMI, and 4.5 kg if overweight or obese at baseline). Participants in all groups were encouraged to engage in self-monitoring behaviors such as daily weighing, and to report these weights to study staff by email, text, or on the web. Aside from pre-specified study follow-up assessments, most follow-up beyond the initial 4 month “small” or “large” changes phase was done using email or web-based intervention.
Main outcome measures. All participants were scheduled for follow-up assessments at 4 months, 1 year, and 2 years, with some early participants having additional follow-ups at 3 and 4 years. The primary outcome of interest was change in weight from baseline through follow-up, with additional outcome measures including the proportion in each group who gained at least 0.45 kg, or developed obesity. Additionally, the investigators did a thorough evaluation of intervention implementation and delivery. Weight change was modeled using mixed effects linear models, adjusting for clinic site. They corrected for multiple measures using Bonferroni adjustment to minimize the risk of type I error and used multiple imputation to examine the impact of missing data on their results. Pre-specified subgroup comparisons between several groups of patients were conducted—those in the normal weight vs. overweight category at baseline, those younger vs. older than age 25 at baseline, and men vs. women.
Results. 599 participants were randomized to the control (n = 202), small changes (n = 200), or large changes groups (n = 197), with no significant differences between groups in terms of measured baseline characteristics. The majority of participants were women (78%) and non-Hispanic white (73%). Mean (SD) baseline age was 28.2 (4.4) years and BMI was 25.4 (2.6) kg/m2. The group as a whole was highly educated—between 77% and 82% had college degrees. The series of 10 intervention sessions in the first 4 months was very well-attended (87% attendance on average for large changes group, 86% for small changes group), and by 4 months of follow-up, a majority of participants in both intervention groups endorsed the behavior of daily self-weighing (75% in large changes, 72% in small changes).
Both intervention groups had statistically significant weight losses compared to control (average weight change in control +0.3 kg, in small change –0.6 kg, and in large change –2.4 kg, over an average of 3 years), with large change participants also having significantly greater average weight loss in follow-up than small change participants. Significantly fewer participants in the intervention groups went on to develop obesity than in the control group (16.9% incidence in control, vs. 7.9% incidence in small changes [P = 0.002] and 8.6% in large changes [P = 0.02]). Importantly, the trajectories of weight gain (or regain) after the initial 4-month intervention differed between the small and large change groups, with small change participants experiencing a more gradual rate of gain throughout follow-up, versus a steeper rate of gain in the large changes group, such that the groups were at very similar weights by the final time point. The investigators did not observe any differences in effect between subgroups according to participant baseline BMI, sex, age, or race.
Conclusion. The authors conclude that these scalable small- and large-change interventions reduced longer-term weight gain and even promoted weight loss in a group of young adults, with the large-change intervention having a greater impact on weight than the small-change intervention.
Commentary
Treatment of obesity is difficult, leading to frustration for many patients and clinicians. Although it is often possible to help patients lose weight with tools such as low-calorie diets and increased physical activity, the long-term maintenance of weight loss is quite challenging. There is a growing awareness that the difficulty in maintaining weight loss has strong physiologic underpinnings. The human body has complex energy regulatory systems that may oppose weight loss by lowering metabolic rate, increasing hunger cues, and limiting satiety cues, when faced with energy restriction or weight loss [1,2].
In order to decrease the number of patients who ultimately require treatment for obesity, an alternative approach may be to try to prevent weight gain in the first place. Young adults in the U.S. tend to gain weight steadily over time, yet this insidious pattern is unlikely to be addressed by physicians [3]. Given that gradual weight gain seems to be the norm for most young adults, it may be beneficial for primary care providers to advise all young adult patients to make small behavioral changes in order to prevent the onset of overweight or obesity. Preventing weight gain is an attractive approach for broad application because it may require lower intensity programs, and less behavioral commitment from patients, compared to what is required for weight loss [4].
In this randomized trial, Wing et al investigated several relatively low-intensity approaches for weight gain prevention. Strengths of the study include aspects of the design and analysis, including its randomized nature, the relatively long follow-up period, the use of multiple imputation to address missing data, and the use of statistical methods to account for the large number of comparisons made between groups over time (Bonferroni correction). More importantly, however, this study represents an important innovation in how physicians might think about obesity, with a shift toward prevention rather than treatment. Historically, many obesity prevention efforts have fallen in the domain of public health or population-level interventions, and it may be the case that physicians have felt they did not really have a role in prevention. On the other hand, doctors who have engaged in obesity treatment—trying to help patients lose weight—may have felt that they lacked the resources or training needed to implement successful programs to promote long-term weight loss. By testing several lower-intensity strategies for weight gain prevention, this study sheds light on what could possibly be a new role for primary care providers or health care systems who care for otherwise healthy young adults. As the authors point out, the methods they employed could also be easily scaled or disseminated using public health approaches and community organizations.
In addition to addressing an important topic, this study relied on intervention methods that would be relatively easy to replicate in clinical practice or in community settings. Aside from the initial 4-month intervention, which involved 10 face-to-face group sessions (which were very well attended by participants), the remainder of the ~3 year follow-up consisted mostly of contact that took place electronically using email and/or text messaging. These modes of communication align well with the move toward electronic health records (eg, e-visits) and are probably ideally suited for young adults, who as a group rely heavily on these methods of communication.
The study has several limitations, most of which are addressed by the authors in the discussion section of the paper. As with most studies of behavioral weight interventions, the majority of participants in this study were women, with relatively few racial and ethnic minorities. Furthermore this was a highly educated group of participants and it is unclear whether these results would generalize to a more diverse clinical population with fewer resources or lower health literacy. Given that the control arm of the study experienced less weight gain over time than would be expected based on population averages, it could be that the participants in this study were a select group of individuals who were more motivated around preventing long-term health problems than a general clinical population. One additional point of possible concern is that, while participants in the “large changes” group did, as per the design, lose weight at the beginning of the trial, they also went on to regain much of that weight and experienced a steeper trajectory of overall gain during follow-up compared to the “small changes” group, so that the 2 intervention groups were not statistically different from each other in terms of overall weight change from baseline by 2 years. Therefore, whether the “large changes” approach is truly more beneficial for long-term obesity prevention than the more modest “small changes” approach is not entirely clear from this study.
Applications for Clinical Practice
The identification of young adults who are gaining weight, but who are not yet obese, represents an opportunity for providers and health care systems. Efforts to promote modest dietary and physical activity changes in this population may prevent obesity, and may be achievable even in busy clinical practice settings. Whether weight-gain prevention programs should include an attempt to first foster a small amount of weight loss as a “buffer” against later gains is still not entirely clear.
—Kristina Lewis, MD, MPH
Study Overview
Objective. To compare several behavioral strategies for weight gain prevention in young adults.
Study design. Randomized clinical trial.
Setting and participants. The study took place at 2 U.S. academic centers between 2010 and 2016. Participants were recruited using email and postal mailings if they were 18–35 years old, had a body mass index (BMI) between 21 and 30.9 (ie, they ranged from normal body weight to class I obesity), spoke English, had internet access, and did not have contraindications to participating in a behavioral weight management intervention (eg, eating disorders). Once recruited, participants were block randomized, stratified by site, sex, and ethnic group, in to 1 of 3 study arms. The control arm of the study consisted of a single in-person meeting where behavioral strategies to prevent weight gain were discussed, as well as quarterly newsletters and personalized reports on interim weight data during follow-up.
Intervention. There were 2 intervention arms in the study. Both intervention groups had 10 in-person group-based visits over the initial 4 months of the intervention, at which strategies to prevent weight gain were discussed. Additionally they received annual invitations to participate in online refresher courses and the same newsletter frequency and content as the control group. Advice to the 2 intervention groups differed, however. Those in the “small changes” group were advised to decrease caloric intake by about 100 kcal per day in order to prevent weight gain. Additionally they were given pedometers, with a goal of increasing their daily step counts by about 2000. In the “large changes” group, participants were given lower calorie targets and more aggressive physical activity goals, with a goal of producing weight loss over the first 4 months of follow-up (2.3 kg for those with normal baseline BMI, and 4.5 kg if overweight or obese at baseline). Participants in all groups were encouraged to engage in self-monitoring behaviors such as daily weighing, and to report these weights to study staff by email, text, or on the web. Aside from pre-specified study follow-up assessments, most follow-up beyond the initial 4 month “small” or “large” changes phase was done using email or web-based intervention.
Main outcome measures. All participants were scheduled for follow-up assessments at 4 months, 1 year, and 2 years, with some early participants having additional follow-ups at 3 and 4 years. The primary outcome of interest was change in weight from baseline through follow-up, with additional outcome measures including the proportion in each group who gained at least 0.45 kg, or developed obesity. Additionally, the investigators did a thorough evaluation of intervention implementation and delivery. Weight change was modeled using mixed effects linear models, adjusting for clinic site. They corrected for multiple measures using Bonferroni adjustment to minimize the risk of type I error and used multiple imputation to examine the impact of missing data on their results. Pre-specified subgroup comparisons between several groups of patients were conducted—those in the normal weight vs. overweight category at baseline, those younger vs. older than age 25 at baseline, and men vs. women.
Results. 599 participants were randomized to the control (n = 202), small changes (n = 200), or large changes groups (n = 197), with no significant differences between groups in terms of measured baseline characteristics. The majority of participants were women (78%) and non-Hispanic white (73%). Mean (SD) baseline age was 28.2 (4.4) years and BMI was 25.4 (2.6) kg/m2. The group as a whole was highly educated—between 77% and 82% had college degrees. The series of 10 intervention sessions in the first 4 months was very well-attended (87% attendance on average for large changes group, 86% for small changes group), and by 4 months of follow-up, a majority of participants in both intervention groups endorsed the behavior of daily self-weighing (75% in large changes, 72% in small changes).
Both intervention groups had statistically significant weight losses compared to control (average weight change in control +0.3 kg, in small change –0.6 kg, and in large change –2.4 kg, over an average of 3 years), with large change participants also having significantly greater average weight loss in follow-up than small change participants. Significantly fewer participants in the intervention groups went on to develop obesity than in the control group (16.9% incidence in control, vs. 7.9% incidence in small changes [P = 0.002] and 8.6% in large changes [P = 0.02]). Importantly, the trajectories of weight gain (or regain) after the initial 4-month intervention differed between the small and large change groups, with small change participants experiencing a more gradual rate of gain throughout follow-up, versus a steeper rate of gain in the large changes group, such that the groups were at very similar weights by the final time point. The investigators did not observe any differences in effect between subgroups according to participant baseline BMI, sex, age, or race.
Conclusion. The authors conclude that these scalable small- and large-change interventions reduced longer-term weight gain and even promoted weight loss in a group of young adults, with the large-change intervention having a greater impact on weight than the small-change intervention.
Commentary
Treatment of obesity is difficult, leading to frustration for many patients and clinicians. Although it is often possible to help patients lose weight with tools such as low-calorie diets and increased physical activity, the long-term maintenance of weight loss is quite challenging. There is a growing awareness that the difficulty in maintaining weight loss has strong physiologic underpinnings. The human body has complex energy regulatory systems that may oppose weight loss by lowering metabolic rate, increasing hunger cues, and limiting satiety cues, when faced with energy restriction or weight loss [1,2].
In order to decrease the number of patients who ultimately require treatment for obesity, an alternative approach may be to try to prevent weight gain in the first place. Young adults in the U.S. tend to gain weight steadily over time, yet this insidious pattern is unlikely to be addressed by physicians [3]. Given that gradual weight gain seems to be the norm for most young adults, it may be beneficial for primary care providers to advise all young adult patients to make small behavioral changes in order to prevent the onset of overweight or obesity. Preventing weight gain is an attractive approach for broad application because it may require lower intensity programs, and less behavioral commitment from patients, compared to what is required for weight loss [4].
In this randomized trial, Wing et al investigated several relatively low-intensity approaches for weight gain prevention. Strengths of the study include aspects of the design and analysis, including its randomized nature, the relatively long follow-up period, the use of multiple imputation to address missing data, and the use of statistical methods to account for the large number of comparisons made between groups over time (Bonferroni correction). More importantly, however, this study represents an important innovation in how physicians might think about obesity, with a shift toward prevention rather than treatment. Historically, many obesity prevention efforts have fallen in the domain of public health or population-level interventions, and it may be the case that physicians have felt they did not really have a role in prevention. On the other hand, doctors who have engaged in obesity treatment—trying to help patients lose weight—may have felt that they lacked the resources or training needed to implement successful programs to promote long-term weight loss. By testing several lower-intensity strategies for weight gain prevention, this study sheds light on what could possibly be a new role for primary care providers or health care systems who care for otherwise healthy young adults. As the authors point out, the methods they employed could also be easily scaled or disseminated using public health approaches and community organizations.
In addition to addressing an important topic, this study relied on intervention methods that would be relatively easy to replicate in clinical practice or in community settings. Aside from the initial 4-month intervention, which involved 10 face-to-face group sessions (which were very well attended by participants), the remainder of the ~3 year follow-up consisted mostly of contact that took place electronically using email and/or text messaging. These modes of communication align well with the move toward electronic health records (eg, e-visits) and are probably ideally suited for young adults, who as a group rely heavily on these methods of communication.
The study has several limitations, most of which are addressed by the authors in the discussion section of the paper. As with most studies of behavioral weight interventions, the majority of participants in this study were women, with relatively few racial and ethnic minorities. Furthermore this was a highly educated group of participants and it is unclear whether these results would generalize to a more diverse clinical population with fewer resources or lower health literacy. Given that the control arm of the study experienced less weight gain over time than would be expected based on population averages, it could be that the participants in this study were a select group of individuals who were more motivated around preventing long-term health problems than a general clinical population. One additional point of possible concern is that, while participants in the “large changes” group did, as per the design, lose weight at the beginning of the trial, they also went on to regain much of that weight and experienced a steeper trajectory of overall gain during follow-up compared to the “small changes” group, so that the 2 intervention groups were not statistically different from each other in terms of overall weight change from baseline by 2 years. Therefore, whether the “large changes” approach is truly more beneficial for long-term obesity prevention than the more modest “small changes” approach is not entirely clear from this study.
Applications for Clinical Practice
The identification of young adults who are gaining weight, but who are not yet obese, represents an opportunity for providers and health care systems. Efforts to promote modest dietary and physical activity changes in this population may prevent obesity, and may be achievable even in busy clinical practice settings. Whether weight-gain prevention programs should include an attempt to first foster a small amount of weight loss as a “buffer” against later gains is still not entirely clear.
—Kristina Lewis, MD, MPH
1. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–604.
2. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016 May 2.
3. Tang JW, Kushner RF, Thompson J, Baker DW. Physician counseling of young adults with rapid weight gain: a retrospective cohort study. BMC Fam Pract 2010;11:31.
4. Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med 2013;173:1770–7.
1. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–604.
2. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016 May 2.
3. Tang JW, Kushner RF, Thompson J, Baker DW. Physician counseling of young adults with rapid weight gain: a retrospective cohort study. BMC Fam Pract 2010;11:31.
4. Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med 2013;173:1770–7.
PAWSS tool identifies alcohol withdrawal syndrome risk
ATLANTA – A new scale for predicting complicated alcohol withdrawal syndrome in hospitalized medically ill patients had high sensitivity and specificity in a prospective validation study.
The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) can help clinicians identify those at risk for complicated alcohol withdrawal syndrome (AWS), and either prevent or treat complicated AWS in a timely manner, Dr. José R. Maldonado of Stanford (Calif.) University said at the annual meeting of the American Psychiatric Association.
In 403 subjects hospitalized to general medicine and surgery units over a 12-month period, the PAWSS – along with the Clinical Institute Withdrawal Assessment–alcohol revised (CIWA-Ar) and clinical monitoring – was administered daily. Using a cutoff score of 4 on the 0-10 point PAWSS, the tool had sensitivity and positive predictive value of 93.1%, and specificity and negative predictive value of 99.5% for identifying complicated AWS, Dr. Maldonado said, noting that the tool also had excellent inter-rater reliability.
The findings are important because the prevalence of alcohol use disorders among hospitalized medically ill patients exceeds 40%, he said, noting that medically ill patients with AWS tend to have a significant number of complications, which makes them “not only very difficult to treat but also very high risk.”
Importantly, seizures – commonly known as “rum fits,” which occur in 5%-15% of cases – happen very early on in the course of AWS. This is a concern, because it has implications for prescribing, Dr. Maldonado said.
Another concern, and “probably the most dreadful of them all,” is delirium tremens (DTs), a severe symptom of alcohol withdrawal that occurs in about 10% of patients with AWS.
The overall mortality associated with DTs is 1% in non–medically ill patients, but in the medically ill, this figure increases to 20% because of the frequency of comorbidities, such as heart disease and diabetes in this population. Also, DTs tend to occur a few days into withdrawal, peaking on day 5, which could be problematic; a trauma patient who is intubated in the operating room, for example, still could go through withdrawal a few days later, as most medications being used in that patient are not going to prevent it, he explained.
Previously, no tool was available to predict complicated AWS to prevent seizures and DTs. Existing tools such as the CIWA and AWS scale assess AWS severity but do not predict who will withdraw, he noted, explaining that by the time the CIWA scale is positive, the patient already is in withdrawal.
One option is to treat everyone with benzodiazepines or other drugs that facilitate GABA (gamma-aminobutyric acid) transmission in patients at risk of AWS, but this unnecessarily puts 80% of patients at risk of numerous side effects, including excessive sedation, falls, respiratory depression, and medication-induced delirium.
A benzodiazepine-sparing protocol, which involved the use of alpha-2 agonists and anticonvulsants instead of benzodiazepines, was being studied at Stanford, comparing outcomes in patients treated with and without benzodiazepines.
“But before we started to use alpha-2 agonists and anticonvulsants ... we wanted to make sure that we were actually treating the population that really needs it. That was the main motivation for creating this tool,” he said. “The other thing is we wanted to make sure that we don’t scare people away from treating patients with potential alcohol withdrawal, because the consequences of withdrawal are dreadful, not only immediately but also into the future.”
Every time someone goes through withdrawal, it is more severe than before, and it lowers the threshold for DTs, he added.
An extensive literature review for anything associated with the various phases of alcohol withdrawal was performed to help develop the PAWSS, which includes 10 highly predictive questions for any patients who first indicate that they have had alcohol in the prior 30 days, or who is admitted with a positive blood alcohol level test.
A pilot study involving 70 patients yielded a sensitivity and specificity of 100% each, leading to the larger study of hospitalized patients, which was published last year in Alcohol and Alcoholism (2015 May 21. doi: 10.1093/alcalc/agv043).
A check of admission notes would have increased the ultimate sensitivity of the scale to 100%, as false answers provided on the scale were easily identified. Blood alcohol level testing also would help.
But PAWSS is meant to provide timely information, which is important in patients at risk, and another purpose for developing PAWSS was to provide an affordable tool that can be used anywhere, including rural community hospitals or clinics where other tests might not be available, Dr. Maldonado said.
Currently, he and his colleagues are evaluating whether all 10 items on the scale are needed to make a diagnosis, or whether a shorter version would be equally useful.
“The incidence of alcoholism is extremely high. It is the most common drug problem in the United States, and we know that many physicians do not feel comfortable dealing with patients who have alcohol withdrawal,” Dr. Maldonado said, adding that this tool will simplify management.
For the Stanford study, patients with a negative PAWSS (score below 4) receive no treatment specifically for AWS. If they test positive (score of 4 or more), it is assumed that they will withdraw, and the AWS scale is administered to discriminate patients who are withdrawing from those at high risk of withdrawal. Patients with a positive PAWSS and a negative AWS scale or CIWA are directed to a prophylactic treatment arm. Those with a positive PAWSS and a positive AWS scale or CIWA are directed into a treatment arm, which involves more aggressive management.
The validation study was supported by the Chase Research Fund. Dr. Maldonado reported having no disclosures.
ATLANTA – A new scale for predicting complicated alcohol withdrawal syndrome in hospitalized medically ill patients had high sensitivity and specificity in a prospective validation study.
The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) can help clinicians identify those at risk for complicated alcohol withdrawal syndrome (AWS), and either prevent or treat complicated AWS in a timely manner, Dr. José R. Maldonado of Stanford (Calif.) University said at the annual meeting of the American Psychiatric Association.
In 403 subjects hospitalized to general medicine and surgery units over a 12-month period, the PAWSS – along with the Clinical Institute Withdrawal Assessment–alcohol revised (CIWA-Ar) and clinical monitoring – was administered daily. Using a cutoff score of 4 on the 0-10 point PAWSS, the tool had sensitivity and positive predictive value of 93.1%, and specificity and negative predictive value of 99.5% for identifying complicated AWS, Dr. Maldonado said, noting that the tool also had excellent inter-rater reliability.
The findings are important because the prevalence of alcohol use disorders among hospitalized medically ill patients exceeds 40%, he said, noting that medically ill patients with AWS tend to have a significant number of complications, which makes them “not only very difficult to treat but also very high risk.”
Importantly, seizures – commonly known as “rum fits,” which occur in 5%-15% of cases – happen very early on in the course of AWS. This is a concern, because it has implications for prescribing, Dr. Maldonado said.
Another concern, and “probably the most dreadful of them all,” is delirium tremens (DTs), a severe symptom of alcohol withdrawal that occurs in about 10% of patients with AWS.
The overall mortality associated with DTs is 1% in non–medically ill patients, but in the medically ill, this figure increases to 20% because of the frequency of comorbidities, such as heart disease and diabetes in this population. Also, DTs tend to occur a few days into withdrawal, peaking on day 5, which could be problematic; a trauma patient who is intubated in the operating room, for example, still could go through withdrawal a few days later, as most medications being used in that patient are not going to prevent it, he explained.
Previously, no tool was available to predict complicated AWS to prevent seizures and DTs. Existing tools such as the CIWA and AWS scale assess AWS severity but do not predict who will withdraw, he noted, explaining that by the time the CIWA scale is positive, the patient already is in withdrawal.
One option is to treat everyone with benzodiazepines or other drugs that facilitate GABA (gamma-aminobutyric acid) transmission in patients at risk of AWS, but this unnecessarily puts 80% of patients at risk of numerous side effects, including excessive sedation, falls, respiratory depression, and medication-induced delirium.
A benzodiazepine-sparing protocol, which involved the use of alpha-2 agonists and anticonvulsants instead of benzodiazepines, was being studied at Stanford, comparing outcomes in patients treated with and without benzodiazepines.
“But before we started to use alpha-2 agonists and anticonvulsants ... we wanted to make sure that we were actually treating the population that really needs it. That was the main motivation for creating this tool,” he said. “The other thing is we wanted to make sure that we don’t scare people away from treating patients with potential alcohol withdrawal, because the consequences of withdrawal are dreadful, not only immediately but also into the future.”
Every time someone goes through withdrawal, it is more severe than before, and it lowers the threshold for DTs, he added.
An extensive literature review for anything associated with the various phases of alcohol withdrawal was performed to help develop the PAWSS, which includes 10 highly predictive questions for any patients who first indicate that they have had alcohol in the prior 30 days, or who is admitted with a positive blood alcohol level test.
A pilot study involving 70 patients yielded a sensitivity and specificity of 100% each, leading to the larger study of hospitalized patients, which was published last year in Alcohol and Alcoholism (2015 May 21. doi: 10.1093/alcalc/agv043).
A check of admission notes would have increased the ultimate sensitivity of the scale to 100%, as false answers provided on the scale were easily identified. Blood alcohol level testing also would help.
But PAWSS is meant to provide timely information, which is important in patients at risk, and another purpose for developing PAWSS was to provide an affordable tool that can be used anywhere, including rural community hospitals or clinics where other tests might not be available, Dr. Maldonado said.
Currently, he and his colleagues are evaluating whether all 10 items on the scale are needed to make a diagnosis, or whether a shorter version would be equally useful.
“The incidence of alcoholism is extremely high. It is the most common drug problem in the United States, and we know that many physicians do not feel comfortable dealing with patients who have alcohol withdrawal,” Dr. Maldonado said, adding that this tool will simplify management.
For the Stanford study, patients with a negative PAWSS (score below 4) receive no treatment specifically for AWS. If they test positive (score of 4 or more), it is assumed that they will withdraw, and the AWS scale is administered to discriminate patients who are withdrawing from those at high risk of withdrawal. Patients with a positive PAWSS and a negative AWS scale or CIWA are directed to a prophylactic treatment arm. Those with a positive PAWSS and a positive AWS scale or CIWA are directed into a treatment arm, which involves more aggressive management.
The validation study was supported by the Chase Research Fund. Dr. Maldonado reported having no disclosures.
ATLANTA – A new scale for predicting complicated alcohol withdrawal syndrome in hospitalized medically ill patients had high sensitivity and specificity in a prospective validation study.
The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) can help clinicians identify those at risk for complicated alcohol withdrawal syndrome (AWS), and either prevent or treat complicated AWS in a timely manner, Dr. José R. Maldonado of Stanford (Calif.) University said at the annual meeting of the American Psychiatric Association.
In 403 subjects hospitalized to general medicine and surgery units over a 12-month period, the PAWSS – along with the Clinical Institute Withdrawal Assessment–alcohol revised (CIWA-Ar) and clinical monitoring – was administered daily. Using a cutoff score of 4 on the 0-10 point PAWSS, the tool had sensitivity and positive predictive value of 93.1%, and specificity and negative predictive value of 99.5% for identifying complicated AWS, Dr. Maldonado said, noting that the tool also had excellent inter-rater reliability.
The findings are important because the prevalence of alcohol use disorders among hospitalized medically ill patients exceeds 40%, he said, noting that medically ill patients with AWS tend to have a significant number of complications, which makes them “not only very difficult to treat but also very high risk.”
Importantly, seizures – commonly known as “rum fits,” which occur in 5%-15% of cases – happen very early on in the course of AWS. This is a concern, because it has implications for prescribing, Dr. Maldonado said.
Another concern, and “probably the most dreadful of them all,” is delirium tremens (DTs), a severe symptom of alcohol withdrawal that occurs in about 10% of patients with AWS.
The overall mortality associated with DTs is 1% in non–medically ill patients, but in the medically ill, this figure increases to 20% because of the frequency of comorbidities, such as heart disease and diabetes in this population. Also, DTs tend to occur a few days into withdrawal, peaking on day 5, which could be problematic; a trauma patient who is intubated in the operating room, for example, still could go through withdrawal a few days later, as most medications being used in that patient are not going to prevent it, he explained.
Previously, no tool was available to predict complicated AWS to prevent seizures and DTs. Existing tools such as the CIWA and AWS scale assess AWS severity but do not predict who will withdraw, he noted, explaining that by the time the CIWA scale is positive, the patient already is in withdrawal.
One option is to treat everyone with benzodiazepines or other drugs that facilitate GABA (gamma-aminobutyric acid) transmission in patients at risk of AWS, but this unnecessarily puts 80% of patients at risk of numerous side effects, including excessive sedation, falls, respiratory depression, and medication-induced delirium.
A benzodiazepine-sparing protocol, which involved the use of alpha-2 agonists and anticonvulsants instead of benzodiazepines, was being studied at Stanford, comparing outcomes in patients treated with and without benzodiazepines.
“But before we started to use alpha-2 agonists and anticonvulsants ... we wanted to make sure that we were actually treating the population that really needs it. That was the main motivation for creating this tool,” he said. “The other thing is we wanted to make sure that we don’t scare people away from treating patients with potential alcohol withdrawal, because the consequences of withdrawal are dreadful, not only immediately but also into the future.”
Every time someone goes through withdrawal, it is more severe than before, and it lowers the threshold for DTs, he added.
An extensive literature review for anything associated with the various phases of alcohol withdrawal was performed to help develop the PAWSS, which includes 10 highly predictive questions for any patients who first indicate that they have had alcohol in the prior 30 days, or who is admitted with a positive blood alcohol level test.
A pilot study involving 70 patients yielded a sensitivity and specificity of 100% each, leading to the larger study of hospitalized patients, which was published last year in Alcohol and Alcoholism (2015 May 21. doi: 10.1093/alcalc/agv043).
A check of admission notes would have increased the ultimate sensitivity of the scale to 100%, as false answers provided on the scale were easily identified. Blood alcohol level testing also would help.
But PAWSS is meant to provide timely information, which is important in patients at risk, and another purpose for developing PAWSS was to provide an affordable tool that can be used anywhere, including rural community hospitals or clinics where other tests might not be available, Dr. Maldonado said.
Currently, he and his colleagues are evaluating whether all 10 items on the scale are needed to make a diagnosis, or whether a shorter version would be equally useful.
“The incidence of alcoholism is extremely high. It is the most common drug problem in the United States, and we know that many physicians do not feel comfortable dealing with patients who have alcohol withdrawal,” Dr. Maldonado said, adding that this tool will simplify management.
For the Stanford study, patients with a negative PAWSS (score below 4) receive no treatment specifically for AWS. If they test positive (score of 4 or more), it is assumed that they will withdraw, and the AWS scale is administered to discriminate patients who are withdrawing from those at high risk of withdrawal. Patients with a positive PAWSS and a negative AWS scale or CIWA are directed to a prophylactic treatment arm. Those with a positive PAWSS and a positive AWS scale or CIWA are directed into a treatment arm, which involves more aggressive management.
The validation study was supported by the Chase Research Fund. Dr. Maldonado reported having no disclosures.
AT THE APA ANNUAL MEETING
Key clinical point: A new scale for predicting complicated alcohol withdrawal syndrome in hospitalized medically ill patients had high sensitivity and specificity in a prospective validation study.
Major finding: The PAWSS had sensitivity and positive predictive value of 93.1%, and specificity and negative predictive value of 99.5% for identifying complicated AWS.
Data source: A prospective validation study in 403 hospitalized patients.
Disclosures: Dr. Maldonado reported having no disclosures.
Growing Papule on the Right Shoulder of an Elderly Man
Granular Cell Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human epithelial malignancy. There are several histologic variants, the rarest being granular cell BCC (GBCC).1 Granular cell BCC is reported most commonly in men with a mean age of 63 years. Sixty-four percent of cases develop on the face, with the remainder arising on the chest or trunk. Granular cell BCC has distinct histologic features but has no specific epidemiologic or clinical features that differentiate it from more common forms of BCC. Treatment of GBCC is identical to BCC and demonstrates similar outcomes. The presence of granular cells can make GBCC difficult to differentiate from other benign and malignant lesions that display similar granular histologic changes.1,2 Rarely, tumors that are histologically similar to human GBCC have been reported in animals.1
Histologically, GBCC commonly demonstrates the architecture of a nodular BCC or may extend from an existing nodular BCC (quiz images A and B). Granular cell BCC is comprised of large islands of basaloid cells extending from the epidermis with rare mitotic activity. Certain variants showing no epidermal attachments have been described,1,3 as in the current case. Classically, BCC and GBCC both demonstrate a peripheral palisade of blue basal cells; however, GBCC may lack this palisading feature in some cases. Therefore, GBCC may be comprised of granular cells only, which may be more easily confused with other tumors with granular cell differentiation. Even when GBCC retains the traditional peripheral palisade of blue basal cells, the central cells are filled with eosinophilic granules.1,2
Electron microscopy of GBCC usually reveals bundles of cytoplasmic tonofilaments and desmosomes in both granular cells and the peripherally palisaded cells. Electron microscopy imaging also demonstrates 0.1- to 0.5-µm membrane-bound lysosomelike structures. In certain reports, these structures show focal positivity for lysozymes.1,2 The etiology of the granules is unclear; however, they are thought to represent degenerative changes related to metabolic alteration and accumulation of lysosomelike structures. These lysosomelike structures have been highlighted with CD68 staining, which was negative in our case.1,2 The lesional cells in GBCC stain positively for cytokeratins, p63, and Ber-EP4, and negatively for S-100 protein, epithelial membrane antigen, and carcinoembryonic antigen. The granules in GBCC generally are positive on periodic acid–Schiff staining.1-4
The histologic differential diagnosis for GBCC includes granular cell tumor as well as other tumors that can present with granular cell changes such as ameloblastoma, leiomyoma, leiomyosarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, and granular cell trichoblastoma. Granular cell ameloblastomas have histologic features and staining patterns that are identical to GBCC; however, ameloblastomas are distinguished by their location within the oral cavity. Granular cell tumors and malignant peripheral nerve sheath tumors stain positive for S-100 protein, and angiosarcomas stain positive for D2-40 and CD31. Leiomyomas and leiomyosarcomas can be differentiated by staining with smooth muscle actin or desmin.1 Granular cell trichoblastomas can be differentiated by the follicular stem cell marker protein PHLDA1 positivity.5
Desmoplastic trichilemmoma is difficult to distinguish from BCC. These tumors are comprised of superficial lobules of basaloid cells with a perilobular hyaline mantel surrounding a central desmoplastic stroma (Figure 1). The basaloid cells in desmoplastic trichoepithelioma demonstrate clear cell change; however, granular features are not seen. The cells within the desmoplastic areas are arranged haphazardly in cords and nests and can mimic an invasive carcinoma; however, nuclear atypia and mitotic activity generally are absent in desmoplastic trichilemmoma.6
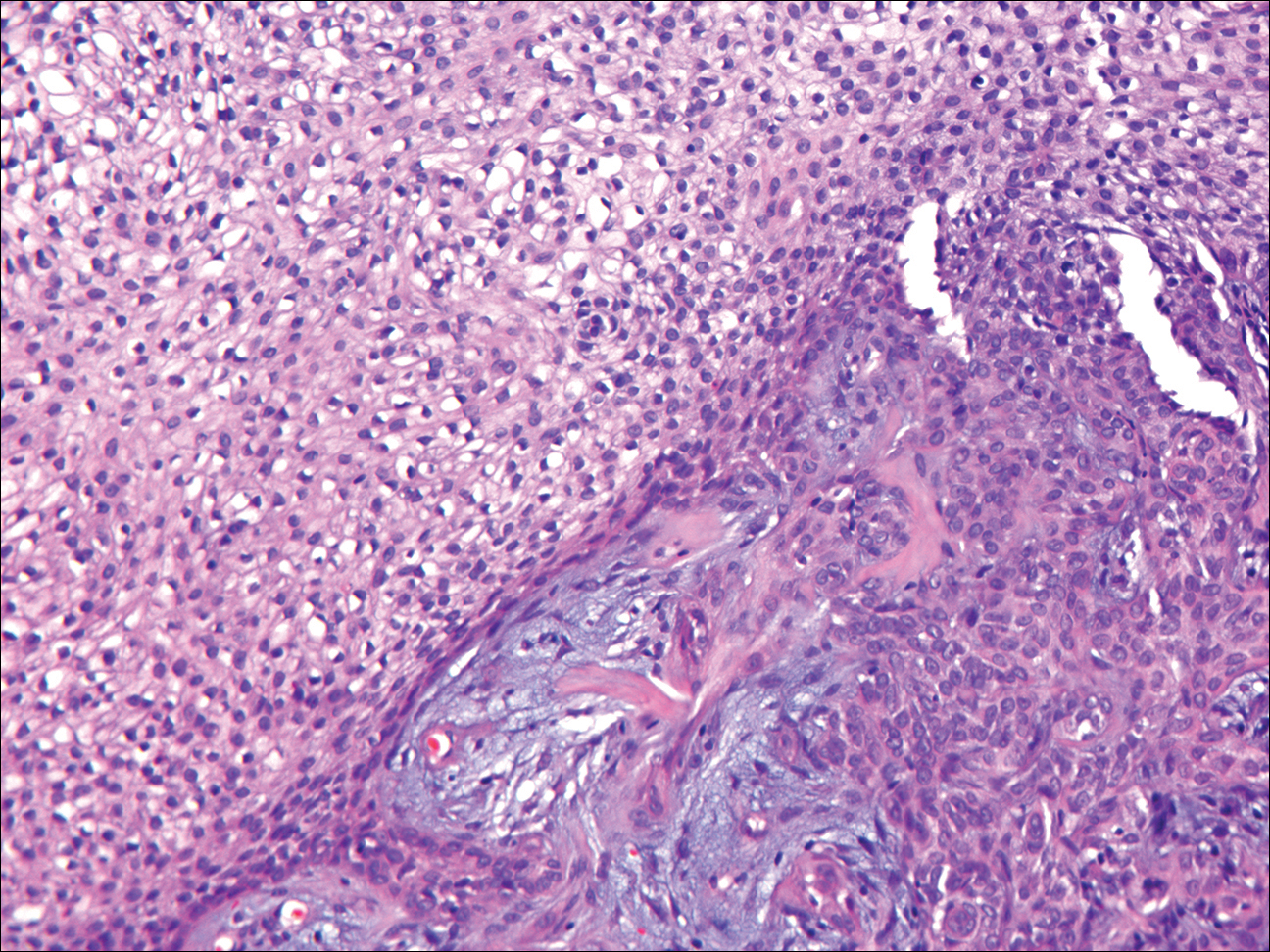
Granular cell tumors generally are poorly circumscribed dermal nodules comprised of large polygonal cells with an eosinophilic granular cytoplasm (Figure 2). The nuclei are generally small and round, and cytological atypia, necrosis, and mitotic activity are uncommon. The cells are positive for S-100 protein and neuron-specific enolase but negative for CD68. The granules are positive for periodic acid–Schiff stain and are diastase resistant. Rarely, these tumors can be malignant.7

Sebaceous adenoma is a well-circumscribed tumor comprised of lobules of characteristic mature sebocytes with bubbly or multivacuolated cytoplasm and crenated nuclei (Figure 3). There is an expansion and increased prominence of the peripherally located basaloid cells; however, in contrast to sebaceous epithelioma, less than 50% of the tumor usually is comprised of these basaloid cells.8
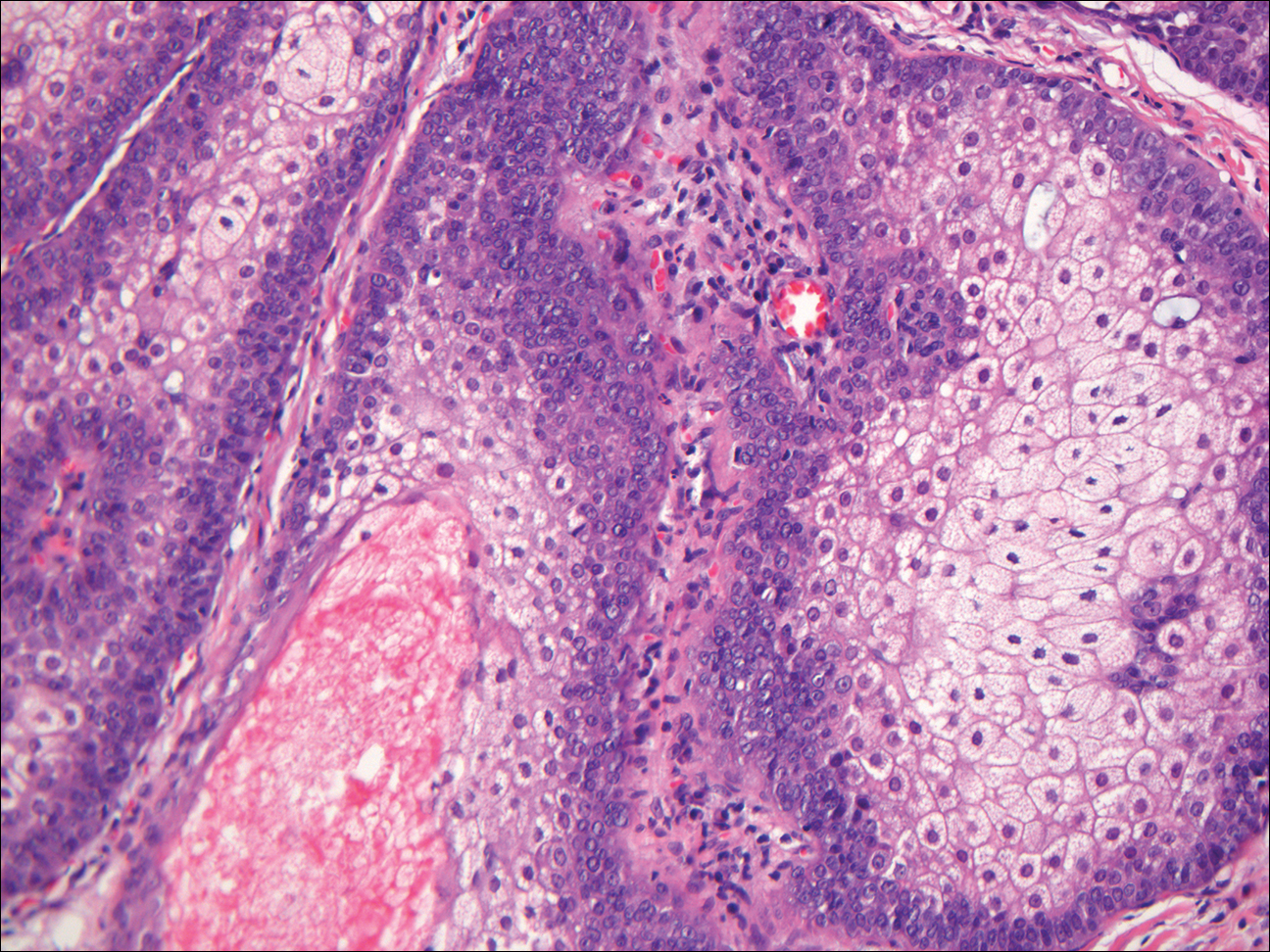
Xanthogranuloma demonstrates a dense collection of histiocytes in the dermis, commonly with Touton giant cell formation (Figure 4). The cells often have a foamy cytoplasm and cytoplasmic vacuoles are observed. The histiocytes are positive for factor XIIIa and CD68, and generally negative for S-100 protein and CD1a, which allows for differentiation from Langerhans cells.9
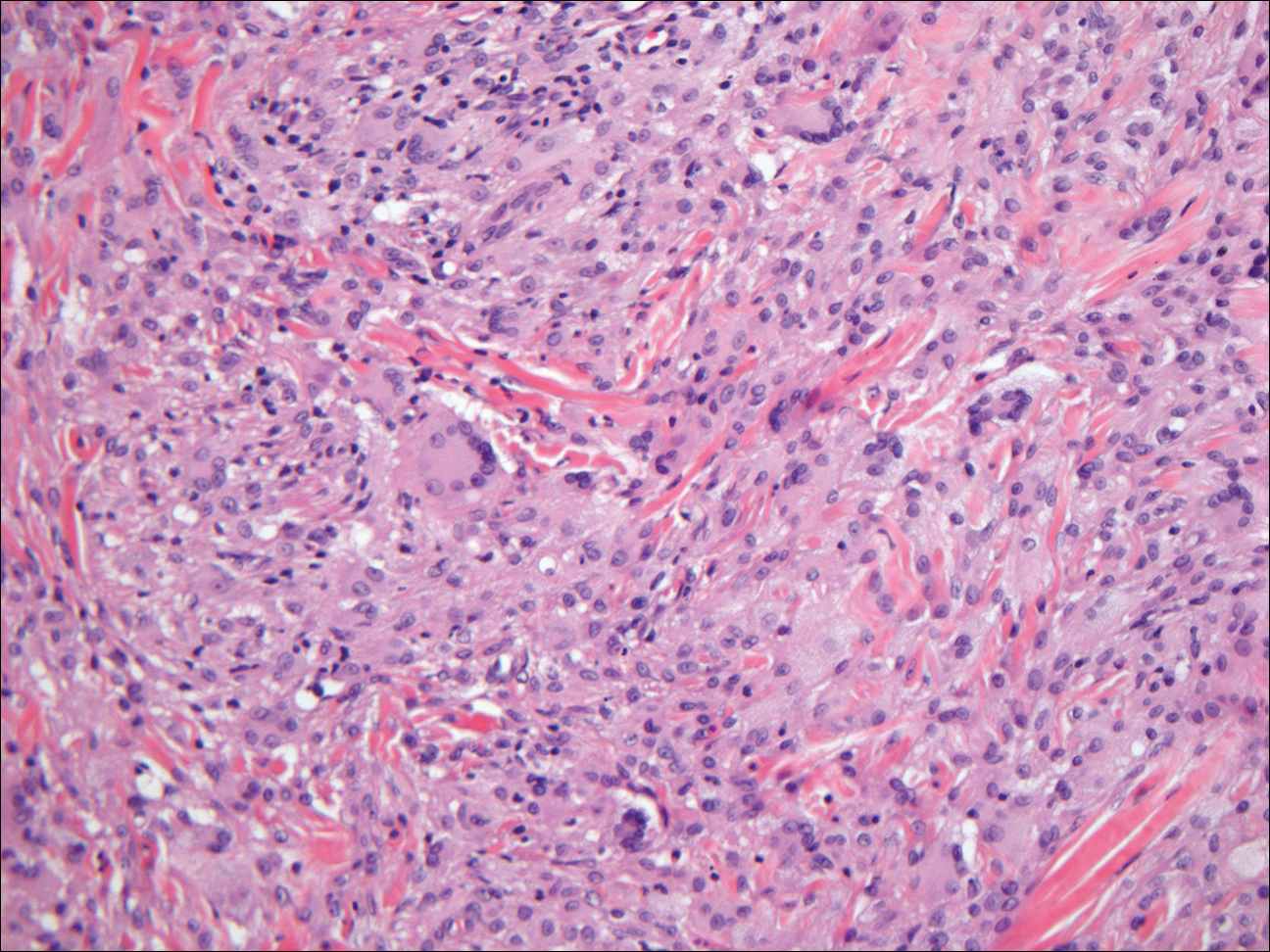
- Kanitakis J, Chouvet B. Granular-cell basal cell carcinoma of the skin. Eur J Dermatol. 2005;15:301-303.
- Dundr P, Stork J, Povysil C, et al. Granular cell basal cell carcinoma. Australas J Dermatol. 2004;45:70-72.
- Hayden AA, Shamma HN. Ber-EP4 and MNF-116 in a previously undescribed morphologic pattern of granular basal cell carcinoma. Am J Dermatopathol. 2001;23:530-532.
- Ansai S, Takayama R, Kimura T, et al. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39:688-692.
- Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36:643-650.
- Tellechea O, Reis JP, Baptista AP. Desmoplastic trichilemmoma. Am J Dermatopathol. 1992;14:107-114.
- Battistella M, Cribier B, Feugeas JP, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67:19-25.
- Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133-147.
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21-28.
Granular Cell Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human epithelial malignancy. There are several histologic variants, the rarest being granular cell BCC (GBCC).1 Granular cell BCC is reported most commonly in men with a mean age of 63 years. Sixty-four percent of cases develop on the face, with the remainder arising on the chest or trunk. Granular cell BCC has distinct histologic features but has no specific epidemiologic or clinical features that differentiate it from more common forms of BCC. Treatment of GBCC is identical to BCC and demonstrates similar outcomes. The presence of granular cells can make GBCC difficult to differentiate from other benign and malignant lesions that display similar granular histologic changes.1,2 Rarely, tumors that are histologically similar to human GBCC have been reported in animals.1
Histologically, GBCC commonly demonstrates the architecture of a nodular BCC or may extend from an existing nodular BCC (quiz images A and B). Granular cell BCC is comprised of large islands of basaloid cells extending from the epidermis with rare mitotic activity. Certain variants showing no epidermal attachments have been described,1,3 as in the current case. Classically, BCC and GBCC both demonstrate a peripheral palisade of blue basal cells; however, GBCC may lack this palisading feature in some cases. Therefore, GBCC may be comprised of granular cells only, which may be more easily confused with other tumors with granular cell differentiation. Even when GBCC retains the traditional peripheral palisade of blue basal cells, the central cells are filled with eosinophilic granules.1,2
Electron microscopy of GBCC usually reveals bundles of cytoplasmic tonofilaments and desmosomes in both granular cells and the peripherally palisaded cells. Electron microscopy imaging also demonstrates 0.1- to 0.5-µm membrane-bound lysosomelike structures. In certain reports, these structures show focal positivity for lysozymes.1,2 The etiology of the granules is unclear; however, they are thought to represent degenerative changes related to metabolic alteration and accumulation of lysosomelike structures. These lysosomelike structures have been highlighted with CD68 staining, which was negative in our case.1,2 The lesional cells in GBCC stain positively for cytokeratins, p63, and Ber-EP4, and negatively for S-100 protein, epithelial membrane antigen, and carcinoembryonic antigen. The granules in GBCC generally are positive on periodic acid–Schiff staining.1-4
The histologic differential diagnosis for GBCC includes granular cell tumor as well as other tumors that can present with granular cell changes such as ameloblastoma, leiomyoma, leiomyosarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, and granular cell trichoblastoma. Granular cell ameloblastomas have histologic features and staining patterns that are identical to GBCC; however, ameloblastomas are distinguished by their location within the oral cavity. Granular cell tumors and malignant peripheral nerve sheath tumors stain positive for S-100 protein, and angiosarcomas stain positive for D2-40 and CD31. Leiomyomas and leiomyosarcomas can be differentiated by staining with smooth muscle actin or desmin.1 Granular cell trichoblastomas can be differentiated by the follicular stem cell marker protein PHLDA1 positivity.5
Desmoplastic trichilemmoma is difficult to distinguish from BCC. These tumors are comprised of superficial lobules of basaloid cells with a perilobular hyaline mantel surrounding a central desmoplastic stroma (Figure 1). The basaloid cells in desmoplastic trichoepithelioma demonstrate clear cell change; however, granular features are not seen. The cells within the desmoplastic areas are arranged haphazardly in cords and nests and can mimic an invasive carcinoma; however, nuclear atypia and mitotic activity generally are absent in desmoplastic trichilemmoma.6

Granular cell tumors generally are poorly circumscribed dermal nodules comprised of large polygonal cells with an eosinophilic granular cytoplasm (Figure 2). The nuclei are generally small and round, and cytological atypia, necrosis, and mitotic activity are uncommon. The cells are positive for S-100 protein and neuron-specific enolase but negative for CD68. The granules are positive for periodic acid–Schiff stain and are diastase resistant. Rarely, these tumors can be malignant.7

Sebaceous adenoma is a well-circumscribed tumor comprised of lobules of characteristic mature sebocytes with bubbly or multivacuolated cytoplasm and crenated nuclei (Figure 3). There is an expansion and increased prominence of the peripherally located basaloid cells; however, in contrast to sebaceous epithelioma, less than 50% of the tumor usually is comprised of these basaloid cells.8

Xanthogranuloma demonstrates a dense collection of histiocytes in the dermis, commonly with Touton giant cell formation (Figure 4). The cells often have a foamy cytoplasm and cytoplasmic vacuoles are observed. The histiocytes are positive for factor XIIIa and CD68, and generally negative for S-100 protein and CD1a, which allows for differentiation from Langerhans cells.9

Granular Cell Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human epithelial malignancy. There are several histologic variants, the rarest being granular cell BCC (GBCC).1 Granular cell BCC is reported most commonly in men with a mean age of 63 years. Sixty-four percent of cases develop on the face, with the remainder arising on the chest or trunk. Granular cell BCC has distinct histologic features but has no specific epidemiologic or clinical features that differentiate it from more common forms of BCC. Treatment of GBCC is identical to BCC and demonstrates similar outcomes. The presence of granular cells can make GBCC difficult to differentiate from other benign and malignant lesions that display similar granular histologic changes.1,2 Rarely, tumors that are histologically similar to human GBCC have been reported in animals.1
Histologically, GBCC commonly demonstrates the architecture of a nodular BCC or may extend from an existing nodular BCC (quiz images A and B). Granular cell BCC is comprised of large islands of basaloid cells extending from the epidermis with rare mitotic activity. Certain variants showing no epidermal attachments have been described,1,3 as in the current case. Classically, BCC and GBCC both demonstrate a peripheral palisade of blue basal cells; however, GBCC may lack this palisading feature in some cases. Therefore, GBCC may be comprised of granular cells only, which may be more easily confused with other tumors with granular cell differentiation. Even when GBCC retains the traditional peripheral palisade of blue basal cells, the central cells are filled with eosinophilic granules.1,2
Electron microscopy of GBCC usually reveals bundles of cytoplasmic tonofilaments and desmosomes in both granular cells and the peripherally palisaded cells. Electron microscopy imaging also demonstrates 0.1- to 0.5-µm membrane-bound lysosomelike structures. In certain reports, these structures show focal positivity for lysozymes.1,2 The etiology of the granules is unclear; however, they are thought to represent degenerative changes related to metabolic alteration and accumulation of lysosomelike structures. These lysosomelike structures have been highlighted with CD68 staining, which was negative in our case.1,2 The lesional cells in GBCC stain positively for cytokeratins, p63, and Ber-EP4, and negatively for S-100 protein, epithelial membrane antigen, and carcinoembryonic antigen. The granules in GBCC generally are positive on periodic acid–Schiff staining.1-4
The histologic differential diagnosis for GBCC includes granular cell tumor as well as other tumors that can present with granular cell changes such as ameloblastoma, leiomyoma, leiomyosarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, and granular cell trichoblastoma. Granular cell ameloblastomas have histologic features and staining patterns that are identical to GBCC; however, ameloblastomas are distinguished by their location within the oral cavity. Granular cell tumors and malignant peripheral nerve sheath tumors stain positive for S-100 protein, and angiosarcomas stain positive for D2-40 and CD31. Leiomyomas and leiomyosarcomas can be differentiated by staining with smooth muscle actin or desmin.1 Granular cell trichoblastomas can be differentiated by the follicular stem cell marker protein PHLDA1 positivity.5
Desmoplastic trichilemmoma is difficult to distinguish from BCC. These tumors are comprised of superficial lobules of basaloid cells with a perilobular hyaline mantel surrounding a central desmoplastic stroma (Figure 1). The basaloid cells in desmoplastic trichoepithelioma demonstrate clear cell change; however, granular features are not seen. The cells within the desmoplastic areas are arranged haphazardly in cords and nests and can mimic an invasive carcinoma; however, nuclear atypia and mitotic activity generally are absent in desmoplastic trichilemmoma.6

Granular cell tumors generally are poorly circumscribed dermal nodules comprised of large polygonal cells with an eosinophilic granular cytoplasm (Figure 2). The nuclei are generally small and round, and cytological atypia, necrosis, and mitotic activity are uncommon. The cells are positive for S-100 protein and neuron-specific enolase but negative for CD68. The granules are positive for periodic acid–Schiff stain and are diastase resistant. Rarely, these tumors can be malignant.7

Sebaceous adenoma is a well-circumscribed tumor comprised of lobules of characteristic mature sebocytes with bubbly or multivacuolated cytoplasm and crenated nuclei (Figure 3). There is an expansion and increased prominence of the peripherally located basaloid cells; however, in contrast to sebaceous epithelioma, less than 50% of the tumor usually is comprised of these basaloid cells.8

Xanthogranuloma demonstrates a dense collection of histiocytes in the dermis, commonly with Touton giant cell formation (Figure 4). The cells often have a foamy cytoplasm and cytoplasmic vacuoles are observed. The histiocytes are positive for factor XIIIa and CD68, and generally negative for S-100 protein and CD1a, which allows for differentiation from Langerhans cells.9

- Kanitakis J, Chouvet B. Granular-cell basal cell carcinoma of the skin. Eur J Dermatol. 2005;15:301-303.
- Dundr P, Stork J, Povysil C, et al. Granular cell basal cell carcinoma. Australas J Dermatol. 2004;45:70-72.
- Hayden AA, Shamma HN. Ber-EP4 and MNF-116 in a previously undescribed morphologic pattern of granular basal cell carcinoma. Am J Dermatopathol. 2001;23:530-532.
- Ansai S, Takayama R, Kimura T, et al. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39:688-692.
- Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36:643-650.
- Tellechea O, Reis JP, Baptista AP. Desmoplastic trichilemmoma. Am J Dermatopathol. 1992;14:107-114.
- Battistella M, Cribier B, Feugeas JP, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67:19-25.
- Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133-147.
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21-28.
- Kanitakis J, Chouvet B. Granular-cell basal cell carcinoma of the skin. Eur J Dermatol. 2005;15:301-303.
- Dundr P, Stork J, Povysil C, et al. Granular cell basal cell carcinoma. Australas J Dermatol. 2004;45:70-72.
- Hayden AA, Shamma HN. Ber-EP4 and MNF-116 in a previously undescribed morphologic pattern of granular basal cell carcinoma. Am J Dermatopathol. 2001;23:530-532.
- Ansai S, Takayama R, Kimura T, et al. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39:688-692.
- Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36:643-650.
- Tellechea O, Reis JP, Baptista AP. Desmoplastic trichilemmoma. Am J Dermatopathol. 1992;14:107-114.
- Battistella M, Cribier B, Feugeas JP, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67:19-25.
- Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133-147.
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21-28.

The best diagnosis is:
a. desmoplastic trichilemmoma
b. granular cell basal cell carcinoma
c. granular cell tumor
d. sebaceous adenoma
e. xanthogranuloma
Continue to the next page for the diagnosis >>
VIDEO: Dr. William A. Gradishar and Dr. Hope S. Rugo discuss #ASCO16
CHICAGO – Do anthracyclines still have a role in treating breast cancer? What are the implications for resistance of extending adjuvant aromatase inhibitors to 10 years or beyond? How best to treat women with metastatic hormone receptor–positive breast cancer, in light of findings on CDK 4/6 and mTOR inhibitors? Does sequence matter? In the case of HER2-positive disease, can a trastuzumab biosimilar be as effective as trastuzumab? And does a regimen with TDM-1 do more than reduce toxicity?
Dr. William A. Gradishar and Dr. Hope S. Rugo reflect on these questions and more in a video roundtable at the annual meeting of the American Society of Clinical Oncology.
Dr. William A. Gradishar is the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago. He had no disclosures to report. Dr. Hope S. Rugo is professor of medicine at the University of California, San Francisco. She disclosed she is on the Speakers’ Bureau for Genomic Health and receives research funding (institutional) from Plexxikon, Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Lilly, GlaxoSmithKline, Genentech, Celsion, Nektar, and Merck.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @NikolaidesLaura
CHICAGO – Do anthracyclines still have a role in treating breast cancer? What are the implications for resistance of extending adjuvant aromatase inhibitors to 10 years or beyond? How best to treat women with metastatic hormone receptor–positive breast cancer, in light of findings on CDK 4/6 and mTOR inhibitors? Does sequence matter? In the case of HER2-positive disease, can a trastuzumab biosimilar be as effective as trastuzumab? And does a regimen with TDM-1 do more than reduce toxicity?
Dr. William A. Gradishar and Dr. Hope S. Rugo reflect on these questions and more in a video roundtable at the annual meeting of the American Society of Clinical Oncology.
Dr. William A. Gradishar is the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago. He had no disclosures to report. Dr. Hope S. Rugo is professor of medicine at the University of California, San Francisco. She disclosed she is on the Speakers’ Bureau for Genomic Health and receives research funding (institutional) from Plexxikon, Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Lilly, GlaxoSmithKline, Genentech, Celsion, Nektar, and Merck.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @NikolaidesLaura
CHICAGO – Do anthracyclines still have a role in treating breast cancer? What are the implications for resistance of extending adjuvant aromatase inhibitors to 10 years or beyond? How best to treat women with metastatic hormone receptor–positive breast cancer, in light of findings on CDK 4/6 and mTOR inhibitors? Does sequence matter? In the case of HER2-positive disease, can a trastuzumab biosimilar be as effective as trastuzumab? And does a regimen with TDM-1 do more than reduce toxicity?
Dr. William A. Gradishar and Dr. Hope S. Rugo reflect on these questions and more in a video roundtable at the annual meeting of the American Society of Clinical Oncology.
Dr. William A. Gradishar is the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago. He had no disclosures to report. Dr. Hope S. Rugo is professor of medicine at the University of California, San Francisco. She disclosed she is on the Speakers’ Bureau for Genomic Health and receives research funding (institutional) from Plexxikon, Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Lilly, GlaxoSmithKline, Genentech, Celsion, Nektar, and Merck.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @NikolaidesLaura
EXPERT ANALYSIS FROM THE 2016 ASCO ANNUAL MEETING
MyPathway: Targeted therapies show promise in nonindicated tumors
CHICAGO – Agents that target the HER2, BRAF, Hedgehog, or EGFR pathways show promise in nonindicated tumor types that harbor these molecular alterations, according to early findings from the MyPathway study.
Of 129 patients enrolled in the multicenter, open-label, phase IIa study, 29 had a major response, defined as tumor shrinkage of at least 30%, to such treatment. One of those patients had a complete response, and 28 had a partial response. An additional 40 patients had stable disease on treatment. Fourteen of the 29 patients progressed after a median of 6 months’ follow-up, and 15 responses were ongoing at up to 11 months, Dr. John D. Hainsworth reported at the annual meeting of the American Society of Clinical Oncology.
No new safety signals were observed, said Dr. Hainsworth of Sarah Cannon Research Institute in Nashville, Tenn.
Treatments evaluated in MyPathway included:
• Trastuzumab + pertuzumab, which targets the HER2 pathway and is currently indicated for breast cancer.
• Vemurafenib, which targets the BRAF pathway and is currently indicated for melanoma.
• Vismodegib, which targets the Hedgehog pathway and is currently indicated for basal cell carcinoma of the skin.
• Erlotinib, which targets the EGFR pathway and is indicated for non–small-cell lung cancer.
Responses have been seen with all four of the treatments, but the best responses were seen among patients with HER2 and BRAF abnormalities.
Among 61 cancers with HER2 amplification/overexpression, trastuzumab + pertuzumab provided a benefit for colorectal, bladder, biliary, non–small-cell lung, pancreas, and head/neck cancers.
Of 20 colorectal tumors, 7 (35%) showed complete or partial response, and 3 (15%) remained stable for at least 120 days (clinical benefit rate, 50%). Complete/partial responses and stable disease, respectively, were also seen in three and two of eight bladder tumors (clinical benefit rate, 63%), in three and three of six biliary tumors (clinical benefit rate, 100%), in two and zero of seven non–small-cell lung tumors (clinical benefit rate, 29%), one and zero of six pancreas tumors (clinical benefit rate, 17%), and one and zero of three head and neck tumors (34%). One of 11 other types of tumors showed disease stability at 120 days (clinical benefit rate, 9%). The overall clinical benefit rate in the study was 43%, Dr. Hainsworth said.
Among 33 cancers with the BRAF mutation, vemurafenib showed activity for non–small-cell lung, ovary, unknown primary, colorectal, pancreas, and head/neck tumors. Of 15 non–small-cell lung tumors, 3 (20%) showed complete or partial responses and 2 (13%) remained stable for at least 120 days (clinical benefit rate, 33%). Complete/partial responses and stable disease, respectively, were also seen in one and two of four ovary tumors (clinical benefit rate, 75%), and complete or partial responses were seen in one each of three unknown primary tumors, two colorectal tumors, two pancreas tumors, and one head/neck tumor (clinical benefit rates of 33%, 50%, 50%, and 100%, respectively). No benefit was seen with tumors at other sites (total clinical benefit rate, 36%), Dr. Hainsworth said.
“Of interest in this group [of patients with BRAF mutations], seven of the eight responses were in V600E mutations, and as you know, that’s the mutation that’s been specifically correlated with high response to BRAF inhibition in melanoma where this treatment is now approved,” he said, adding that the response rate in those patients was 38%.
Based on these early results, enrollment of patients with HER2 abnormalities and colorectal, bladder, or biliary cancer, and of patients with BRAF mutations and lung cancer, will be expanded, he said.
Subjects enrolled in MyPathway have advanced cancer showing abnormalities in any of the pathways of interest. The first 129 received a mean of three prior therapies, and in the 29 who responded, 12 different types of cancer responded to the targeted treatment.
“An increasing number of targeted agents for advanced cancer are in use now based on the presence of molecular abnormalities in the cancer. … We’ve known that the same mutations that are in those cancers are found in a wide variety of other cancers, although at a lower incidence, and it’s been difficult to test how effective these same treatments are for the other cancers due to the difficulty in identifying the patient population,” he said, explaining that an increase in comprehensive genomic profiling in recent years has allowed for identification of more and more of these mutations in other cancers.
“I think we’ve shown now that this trial design is feasible, where patients are selected on the basis of molecular abnormalities in their cancers rather than on their primary tumor type or primary site, and certainly offers opportunities for patients with these molecular abnormalities,” Dr. Hainsworth concluded.
Thus far, MyPathway has enrolled more than 200 patients, and is designed to accrue up to 500, with adjustment of treatment groups based on response rates. Emerging new regimens that target these pathways, such as the MEK inhibitor cobemetinib, will also be added, as will new agents targeting additional molecular abnormalities.
The study design, using this “tumor-agnostic approach,” mirrors that of the ASCO-led TAPUR trial, according to ASCO spokesperson Dr. Sumanta Kumar Pal.
The findings of these and other precision medicine trials may ultimately shift the longstanding cancer treatment paradigm, Dr. Pal said.
MyPathway received funding from Genentech. Dr. Hainsworth reported that his institution has received research funding from Astellas Pharma, AstraZeneca, Celgene, Genentech, Johnson & Johnson, Lilly, and Novartis.
CHICAGO – Agents that target the HER2, BRAF, Hedgehog, or EGFR pathways show promise in nonindicated tumor types that harbor these molecular alterations, according to early findings from the MyPathway study.
Of 129 patients enrolled in the multicenter, open-label, phase IIa study, 29 had a major response, defined as tumor shrinkage of at least 30%, to such treatment. One of those patients had a complete response, and 28 had a partial response. An additional 40 patients had stable disease on treatment. Fourteen of the 29 patients progressed after a median of 6 months’ follow-up, and 15 responses were ongoing at up to 11 months, Dr. John D. Hainsworth reported at the annual meeting of the American Society of Clinical Oncology.
No new safety signals were observed, said Dr. Hainsworth of Sarah Cannon Research Institute in Nashville, Tenn.
Treatments evaluated in MyPathway included:
• Trastuzumab + pertuzumab, which targets the HER2 pathway and is currently indicated for breast cancer.
• Vemurafenib, which targets the BRAF pathway and is currently indicated for melanoma.
• Vismodegib, which targets the Hedgehog pathway and is currently indicated for basal cell carcinoma of the skin.
• Erlotinib, which targets the EGFR pathway and is indicated for non–small-cell lung cancer.
Responses have been seen with all four of the treatments, but the best responses were seen among patients with HER2 and BRAF abnormalities.
Among 61 cancers with HER2 amplification/overexpression, trastuzumab + pertuzumab provided a benefit for colorectal, bladder, biliary, non–small-cell lung, pancreas, and head/neck cancers.
Of 20 colorectal tumors, 7 (35%) showed complete or partial response, and 3 (15%) remained stable for at least 120 days (clinical benefit rate, 50%). Complete/partial responses and stable disease, respectively, were also seen in three and two of eight bladder tumors (clinical benefit rate, 63%), in three and three of six biliary tumors (clinical benefit rate, 100%), in two and zero of seven non–small-cell lung tumors (clinical benefit rate, 29%), one and zero of six pancreas tumors (clinical benefit rate, 17%), and one and zero of three head and neck tumors (34%). One of 11 other types of tumors showed disease stability at 120 days (clinical benefit rate, 9%). The overall clinical benefit rate in the study was 43%, Dr. Hainsworth said.
Among 33 cancers with the BRAF mutation, vemurafenib showed activity for non–small-cell lung, ovary, unknown primary, colorectal, pancreas, and head/neck tumors. Of 15 non–small-cell lung tumors, 3 (20%) showed complete or partial responses and 2 (13%) remained stable for at least 120 days (clinical benefit rate, 33%). Complete/partial responses and stable disease, respectively, were also seen in one and two of four ovary tumors (clinical benefit rate, 75%), and complete or partial responses were seen in one each of three unknown primary tumors, two colorectal tumors, two pancreas tumors, and one head/neck tumor (clinical benefit rates of 33%, 50%, 50%, and 100%, respectively). No benefit was seen with tumors at other sites (total clinical benefit rate, 36%), Dr. Hainsworth said.
“Of interest in this group [of patients with BRAF mutations], seven of the eight responses were in V600E mutations, and as you know, that’s the mutation that’s been specifically correlated with high response to BRAF inhibition in melanoma where this treatment is now approved,” he said, adding that the response rate in those patients was 38%.
Based on these early results, enrollment of patients with HER2 abnormalities and colorectal, bladder, or biliary cancer, and of patients with BRAF mutations and lung cancer, will be expanded, he said.
Subjects enrolled in MyPathway have advanced cancer showing abnormalities in any of the pathways of interest. The first 129 received a mean of three prior therapies, and in the 29 who responded, 12 different types of cancer responded to the targeted treatment.
“An increasing number of targeted agents for advanced cancer are in use now based on the presence of molecular abnormalities in the cancer. … We’ve known that the same mutations that are in those cancers are found in a wide variety of other cancers, although at a lower incidence, and it’s been difficult to test how effective these same treatments are for the other cancers due to the difficulty in identifying the patient population,” he said, explaining that an increase in comprehensive genomic profiling in recent years has allowed for identification of more and more of these mutations in other cancers.
“I think we’ve shown now that this trial design is feasible, where patients are selected on the basis of molecular abnormalities in their cancers rather than on their primary tumor type or primary site, and certainly offers opportunities for patients with these molecular abnormalities,” Dr. Hainsworth concluded.
Thus far, MyPathway has enrolled more than 200 patients, and is designed to accrue up to 500, with adjustment of treatment groups based on response rates. Emerging new regimens that target these pathways, such as the MEK inhibitor cobemetinib, will also be added, as will new agents targeting additional molecular abnormalities.
The study design, using this “tumor-agnostic approach,” mirrors that of the ASCO-led TAPUR trial, according to ASCO spokesperson Dr. Sumanta Kumar Pal.
The findings of these and other precision medicine trials may ultimately shift the longstanding cancer treatment paradigm, Dr. Pal said.
MyPathway received funding from Genentech. Dr. Hainsworth reported that his institution has received research funding from Astellas Pharma, AstraZeneca, Celgene, Genentech, Johnson & Johnson, Lilly, and Novartis.
CHICAGO – Agents that target the HER2, BRAF, Hedgehog, or EGFR pathways show promise in nonindicated tumor types that harbor these molecular alterations, according to early findings from the MyPathway study.
Of 129 patients enrolled in the multicenter, open-label, phase IIa study, 29 had a major response, defined as tumor shrinkage of at least 30%, to such treatment. One of those patients had a complete response, and 28 had a partial response. An additional 40 patients had stable disease on treatment. Fourteen of the 29 patients progressed after a median of 6 months’ follow-up, and 15 responses were ongoing at up to 11 months, Dr. John D. Hainsworth reported at the annual meeting of the American Society of Clinical Oncology.
No new safety signals were observed, said Dr. Hainsworth of Sarah Cannon Research Institute in Nashville, Tenn.
Treatments evaluated in MyPathway included:
• Trastuzumab + pertuzumab, which targets the HER2 pathway and is currently indicated for breast cancer.
• Vemurafenib, which targets the BRAF pathway and is currently indicated for melanoma.
• Vismodegib, which targets the Hedgehog pathway and is currently indicated for basal cell carcinoma of the skin.
• Erlotinib, which targets the EGFR pathway and is indicated for non–small-cell lung cancer.
Responses have been seen with all four of the treatments, but the best responses were seen among patients with HER2 and BRAF abnormalities.
Among 61 cancers with HER2 amplification/overexpression, trastuzumab + pertuzumab provided a benefit for colorectal, bladder, biliary, non–small-cell lung, pancreas, and head/neck cancers.
Of 20 colorectal tumors, 7 (35%) showed complete or partial response, and 3 (15%) remained stable for at least 120 days (clinical benefit rate, 50%). Complete/partial responses and stable disease, respectively, were also seen in three and two of eight bladder tumors (clinical benefit rate, 63%), in three and three of six biliary tumors (clinical benefit rate, 100%), in two and zero of seven non–small-cell lung tumors (clinical benefit rate, 29%), one and zero of six pancreas tumors (clinical benefit rate, 17%), and one and zero of three head and neck tumors (34%). One of 11 other types of tumors showed disease stability at 120 days (clinical benefit rate, 9%). The overall clinical benefit rate in the study was 43%, Dr. Hainsworth said.
Among 33 cancers with the BRAF mutation, vemurafenib showed activity for non–small-cell lung, ovary, unknown primary, colorectal, pancreas, and head/neck tumors. Of 15 non–small-cell lung tumors, 3 (20%) showed complete or partial responses and 2 (13%) remained stable for at least 120 days (clinical benefit rate, 33%). Complete/partial responses and stable disease, respectively, were also seen in one and two of four ovary tumors (clinical benefit rate, 75%), and complete or partial responses were seen in one each of three unknown primary tumors, two colorectal tumors, two pancreas tumors, and one head/neck tumor (clinical benefit rates of 33%, 50%, 50%, and 100%, respectively). No benefit was seen with tumors at other sites (total clinical benefit rate, 36%), Dr. Hainsworth said.
“Of interest in this group [of patients with BRAF mutations], seven of the eight responses were in V600E mutations, and as you know, that’s the mutation that’s been specifically correlated with high response to BRAF inhibition in melanoma where this treatment is now approved,” he said, adding that the response rate in those patients was 38%.
Based on these early results, enrollment of patients with HER2 abnormalities and colorectal, bladder, or biliary cancer, and of patients with BRAF mutations and lung cancer, will be expanded, he said.
Subjects enrolled in MyPathway have advanced cancer showing abnormalities in any of the pathways of interest. The first 129 received a mean of three prior therapies, and in the 29 who responded, 12 different types of cancer responded to the targeted treatment.
“An increasing number of targeted agents for advanced cancer are in use now based on the presence of molecular abnormalities in the cancer. … We’ve known that the same mutations that are in those cancers are found in a wide variety of other cancers, although at a lower incidence, and it’s been difficult to test how effective these same treatments are for the other cancers due to the difficulty in identifying the patient population,” he said, explaining that an increase in comprehensive genomic profiling in recent years has allowed for identification of more and more of these mutations in other cancers.
“I think we’ve shown now that this trial design is feasible, where patients are selected on the basis of molecular abnormalities in their cancers rather than on their primary tumor type or primary site, and certainly offers opportunities for patients with these molecular abnormalities,” Dr. Hainsworth concluded.
Thus far, MyPathway has enrolled more than 200 patients, and is designed to accrue up to 500, with adjustment of treatment groups based on response rates. Emerging new regimens that target these pathways, such as the MEK inhibitor cobemetinib, will also be added, as will new agents targeting additional molecular abnormalities.
The study design, using this “tumor-agnostic approach,” mirrors that of the ASCO-led TAPUR trial, according to ASCO spokesperson Dr. Sumanta Kumar Pal.
The findings of these and other precision medicine trials may ultimately shift the longstanding cancer treatment paradigm, Dr. Pal said.
MyPathway received funding from Genentech. Dr. Hainsworth reported that his institution has received research funding from Astellas Pharma, AstraZeneca, Celgene, Genentech, Johnson & Johnson, Lilly, and Novartis.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Agents that target the HER2, BRAF, Hedgehog, or EGFR pathways show promise in nonindicated tumor types that harbor these molecular alterations, according to early findings from the MyPathway study.
Major finding: Twenty-nine patients had a major response, and an additional 40 remained stable on treatment.
Data source: The ongoing open-label, phase IIa MyPathway study, including results from the first 129 patients.
Disclosures: MyPathway received funding from Genentech. Dr. Hainsworth reported that his institution has received research funding from Astellas Pharma, AstraZeneca, Celgene, Genentech, Johnson & Johnson, Lilly, and Novartis.
Shoulder Arthroplasty: Disposition and Perioperative Outcomes in Patients With and Without Rheumatoid Arthritis
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
Shoulder arthroplasty (SA), including total SA (TSA) and reverse TSA, is an effective surgical treatment for fracture and primary or secondary degenerative disease of the shoulder.1 Over the past few decades, use of SA has increased dramatically, from about 5000 cases in 1990 to 7000 in 2000 and more than 26,000 in 2008.1,2
Complications associated with SA generally are classified as perioperative (occurring during the operative index) or long-term (postdischarge).3 Long-term complications include implant loosening, instability, revision, infection, rotator cuff tear, neural injury, and deltoid detachment.1,4,5 Perioperative complications, which are less commonly reported, include intraoperative fracture, infection, neural injury, venous thromboembolic events (VTEs, including pulmonary embolism [PE] and deep vein thrombosis [DVT]), transfusion, and death.3,6-10
SA is an attractive treatment option for patients with rheumatoid arthritis (RA), as the effects of pain on these patients are greater in the shoulder joint than in any other joint.11 Patients with RA pose unique orthopedic surgical challenges, including any combination of decreased bone mineralization, poor capsular tissue integrity, and osteonecrosis.3,12 In addition, RA patients may be taking immunosuppressive medications that have severe side effects, and they may require multiple surgeries.12,13 These factors predispose patients with RA to complications that include infection and wound dehiscence.3,5,12-14
The complex nature of RA has prompted investigators to examine outcome measures in this patient group. Hambright and colleagues3 used the Nationwide Inpatient Sample (NIS) to examine perioperative outcomes in RA patients who underwent TSA between 1988 and 2005.3 They found that TSA patients with RA had shorter and less costly hospital stays and were more likely to have a routine discharge.3 Using the same patient population drawn from the period 2006–2011, we conducted a study to determine if this unexpected trend persists as the number of TSAs and quality of postoperative care continue to increase. Given the potential for anemia of chronic disease and the systemic inflammatory nature of RA, we hypothesized that the perioperative complication profile of RA patients would be worse than that of non-RA patients.
Materials and Methods
NIS data were acquired for the period 2006–2011. The NIS is the largest publicly available all-payer inpatient database, with a random 20% sample of about 1000 US hospitals accounting for 7 to 8 million inpatient stays. The database supplies weights used to estimate national totals, at about 35 million inpatient visits per year. NIS inpatient data are limited to the operative index. Postdischarge information is not available. The NIS is managed by the Healthcare Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. The quality of NIS data is assessed and validated by an independent contractor. NIS data have been widely used to examine perioperative outcomes.15-17
NIS data cover patient and hospital demographics, hospital length of stay (LOS), discharge status, payer information, charges, and perioperative outcomes and procedure/diagnosis codes (ICD-9; International Classification of Diseases, Ninth Revision18).
As our Institutional Review Board (IRB) reviewed the database and determined the project was not human subject research, IRB involvement was not required. This study paralleled successful efforts with similar RA and non-RA patients who had shoulder and elbow surgery.3,19 SA patients were identified by ICD-9 procedure code 81.80, but this code does not specify whether the prosthesis was unconstrained, semiconstrained, or constrained. ICD-9 coding also does not specify whether the TSA was traditional or reverse. Patients with RA were identified by ICD-9 diagnosis codes 714.0, 714.1, and 714.2. Patients without one of these codes were placed in the non-RA cohort. Patients with codes associated with pathologic fractures secondary to metastatic cancer or bone malignant neoplasm as a secondary or primary diagnosis and patients who had revision surgery indicated by code 81.83 were excluded, as they have a disproportionately higher comorbidity burden.
After each cohort was defined, demographic data (age, sex, race, income quartile based on ZIP postal code) were compared, as were data on primary payer, hospital demographics, LOS (≤5 days, defined as perioperative index), discharge type, inflation-adjusted charges in 2014 dollars based on the Consumer Price Indexes (http://www.bls.gov/cpi/), and mortality. Perioperative complications—respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related (including embolism, fibrosis, hemorrhage, pain, stenosis, or thrombus caused by any device, implant, or graft), cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, postoperative infection complications, and intraoperative transfusions—were considered using ICD-9 codes (996.X-999.X and 99.X, respectively).20 Although commonly used to determine perioperative comorbidity burden using ICD-9 coding, the modified Charlson index was not considered because RA is a component of the index and would therefore bias the variable.3,21
Statistical analyses, including χ2 tests and 2-sample t tests, were performed for categorical and continuous variables, respectively. P < .05 was considered significant. Fisher exact test was used for cohorts with fewer than 5 occurrences. Multivariate logistic regression models were then calculated to determine the effect of RA on different outcomes and complications, with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. Statistical analyses were performed using the R statistical programming language.22
Results
Of the 34,970 patients who underwent SA between 2006 and 2011, 1674 (4.8%) had a diagnosis of RA and 33,296 (95.2%) did not. On average, patients with RA tended to be younger than patients without RA (66.4 vs 69.1 years; P < .001), and a larger percentage of RA patients were female (75.5% vs 54.4%; P < .001). Compared with non-RA patients, RA patients comprised a different ethnic group and had a different expected primary payer (P < .001). SA patients with and without RA did not differ in income quartile based on ZIP code, total number of hospital beds, hospital region, or hospital teaching status (P = .34, .78, .59, and .82, respectively) (Table 1).
LOS was significantly (P < .001) statistically longer for RA patients (2.196 days) than for non-RA patients (2.085 days). RA patients were significantly less likely to be discharged home (63.0% vs 67.6%; P < .001). (Routine discharge was defined as discharge home, whereas nonroutine discharge was defined as discharge to a short-term hospital, skilled nursing facility, intermediate care, another type of facility, home health care, against medical advice, or death.) In addition, inflation-adjusted charges associated with SA were significantly higher (P = .018) for RA patients ($54,284) than for non-RA patients ($52,663) (Table 1).
Regarding the rates of complications that occurred during the perioperative index, there were no significant differences between RA and non-RA cohorts. These complications included respiratory, gastrointestinal, genitourinary, accidental puncture/laceration, central nervous system, wound dehiscence, device-related, cardiac, hematoma/seroma, acute respiratory distress syndrome, postoperative shock, VTE, and postoperative infection (Table 2). In addition, there was no significant difference in mortality between the groups (P = .48).
In TSA, blood transfusions were more likely (P < .001) to be given to RA patients (9.00%) than to non-RA patients (6.16%). Multivariate regression analyses were performed with age, race, sex, hospital region, hospital type, number of hospital beds, primary payer, and hospital ownership as covariates. These analyses revealed that transfusion (P < .001), discharge type (P = .002), total inflation-adjusted charges (P < .001), and LOS (P = .047) remained significant (Table 3).
Discussion
Large national databases like NIS allow study of uncommon medical occurrences and help delineate risks and trends that otherwise might be indeterminable. Although it has been suggested that patients with RA may have poorer long-term outcomes after SA, the perioperative risk profile indicates that TSA is well tolerated in RA patients during the operative index.3,23-25
The data on this study’s 34,970 patients, drawn from the period 2006–2011, demonstrated no significant differences in safety profile with respect to the 14 perioperative complications and outcomes examined, except blood transfusion rate. Rates of postoperative infection (RA, 0.24%; non-RA, 0.14%; P = .303), VTE (RA, 0.30%; non-RA, 0.25%; P = .905), and transfusion (RA, 9.00%; non-RA, 6.16%; P < .001) are of particular interest because of the severity of these situations.
Postoperative infection is a potentially serious complication and often occurs secondary to diabetes, RA, lupus erythematosus, prior surgery, or a nosocomial or remote source.1 The often costly treatment options include antibiotic suppression, irrigation and debridement with implant retention, 1-stage exchange with antibiotic-impregnated cement fixation, staged reimplantation, resection arthroplasty, arthrodesis, and amputation.1 The overall 0.14% infection rate determined in this study is lower than the 0.7% reported for SA patients in the literature.1 Given the nature of the NIS database, this rate underestimates the true postoperative infection rate, as any infection that occurred after the perioperative period is not captured.26 The present study’s perioperative infection rates (RA, 0.24%; non-RA, 0.14%) for the period 2006–2011 are comparable to the rates (RA, 0.17%; non-RA, 0.24%) reported by Hambright and colleagues3 for the same patient population over the preceding, 18-year period (1988–2005) and similarly do not significantly differ between groups. Although infection is uncommon in the immediate perioperative period, the ICD-9 codes used refer specifically to infection resulting from surgery and do not represent concomitant infection.
VTEs, which include PEs and DVTs, are rare but potentially life-threatening surgical complications.27,28 Mechanical prophylaxis and chemical prophylaxis have been recommended for major orthopedic surgery, particularly lower extremity surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA).28,29 In the present study, VTE rates were low, 0.30% (RA) and 0.25% (non-RA), and not significantly different in bivariate or multivariate analyses. These rates are comparable to those found in other national-database SA studies.28 VTEs that occur outside the index hospital admission are not captured in this database. Therefore, the rates in the present study may be lower than the true incidence after SA. Mortality secondary to VTE usually occurs within 24 hours but may occur up to 90 days after surgery. DVT rates, on the other hand, are difficult to evaluate because of differences in screening practices.27,28,30,31
That RA patients were more likely than non-RA patients to receive perioperative blood transfusions supports prior findings that SA patients with RA were more likely than SA patients with osteoarthritis (OA) to receive perioperative blood transfusions.8 RA patients have been shown to have high rates of anemia of chronic disease, ranging from 22% to 77%.32 During joint replacement, these patients often require transfusions.32,33 However, these findings differ from prior findings of no differences between RA and non-RA patients in the same patient population during the period 1988–2005.3 This difference may be a product of the constantly changing transfusion guidelines and increased use; transfusion rates increased 140% between 1997 and 2007, making transfusions the fastest growing common procedure in the United States during that time.34 There was no difference between RA and non-RA patients in household income (as determined by ZIP code analysis), number of hospital beds, hospital region, or hospital teaching status. Compared with non-RA patients, RA patients were more likely to be younger, female, and of a difference race and to have a different expected primary payer (P < .001).These findings are consistent with previous findings in the literature.3 In the present SA study, however, RA patients were more likely than non-RA patients to have longer LOS, higher inflation-adjusted hospital charges, and nonroutine discharge. These findings deviate from those of the study covering the preceding 18 years (1988–2005).3 Despite the findings of a changing environment of care for RA patients, by Hambright and colleagues3 and Weiss and colleagues,35 the trend appears to have shifted. Both groups had shorter average LOS than either group from the preceding 18 years.3 Although statistically significant in bivariate analysis, the difference in LOS between the 2 groups differed by an average of 0.11 day (2 hours 24 minutes) and was not clinically relevant.
In addition, the higher charges for patients with RA represent a deviation from the preceding 18 years.3 Other studies have also shown that RA is associated with increased cost in TSA.36 Patients with RA often have rotator cuff pathology, indicating reverse SA may be used more frequently.37,38 The increased implant cost associated with reverse SA may account for the increased costs in RA patients.39 As mentioned, TSA type is not captured in the NIS database. In addition, that RA patients were less likely than non-RA patients to have routine discharge may indicate RA cases are more complex because of their complications.1,5,14,40 A recent study of complications in RA patients (1163 who underwent THA, 2692 who underwent TKA) found that THA patients with RA were significantly more likely than THA patients with OA to dislocate, and TKA patients with RA were significantly more likely than TKA patients with OA to develop an infection after surgery.41 Postoperative dislocation has been shown to increase hospital costs in other orthopedic procedures.42 Also, during TSA, patients with RA are more likely than patients with OA to receive intraoperative blood transfusions.8 These complications—combined with the fact that RA is a chronic, progressive, systemic inflammatory disease that can affect soft tissue and blood vessel wall healing and is associated with medications having potential side effects—could contribute to the apparent increased hospital charges and LOS.3,12,13,43 Factors that include surgeon preference, impact of primary payer, and hospital practice may also affect final charges. Total charges in the NIS database include administrative fees, hospital costs, device-related costs, operating room costs, and ancillary staff costs. Total charges do not include professional fees and differ from the total cost that represents the amount reimbursed by the payer. Charges tend to correlate with but overestimate the total costs.44
This study had several important limitations. As mentioned, only events that occur during the operative admission are captured in the NIS database, and thus postoperative complications or serious adverse events that lead to readmission cannot be identified. In addition, outpatient TSAs are not captured in the NIS database, and thus inclusion of only inpatient procedures yields higher average LOS and total charges.45 Given the limited granularity of ICD-9 coding, this study could not determine RA severity, estimated blood loss, length of surgery, complication severity, type of TSA procedure/prosthesis, or cause of death. Although commonly used to determine comorbidity burden, the modified Charlson index could not be used, and therefore could not be entered as a covariate in multivariate analysis. Furthermore, the NIS database does not include imaging or patient-reported outcomes information, such as improvements in pain or function, which are of crucial importance in considering surgery.
Conclusion
Our findings corroborated findings that the demographics and the perioperative safety profile for TSA were similar for patients with and without RA. The risk for complications or death in the perioperative period was low. Compared with non-RA patients, RA patients had significantly higher charges and longer LOS and were less likely to be discharged home after surgery. The 0.11-day difference in LOS, though statistically significant, was not clinically relevant. These findings differ from those for the preceding, 18-year period (1988–2005). Future research should focus on the causes of these changes.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.
1. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
4. van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35(6):426-434.
5. Wirth MA, Rockwood CA Jr. Complications of shoulder arthroplasty. Clin Orthop Relat Res. 1994;(307):47-69.
6. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):
1915-1923.
7. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206-216.
8. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
9. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689.
10. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
11. Tanaka E, Saito A, Kamitsuji S, et al. Impact of shoulder, elbow, and knee joint involvement on assessment of rheumatoid arthritis using the American College of Rheumatology core data set. Arthritis Rheum. 2005;53(6):864-871.
12. Nassar J, Cracchiolo A 3rd. Complications in surgery of the foot and ankle in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2001;(391):140-152.
13. den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689-695.
14. Sanchez-Sotelo J. (i) Shoulder arthroplasty for osteoarthritis and rheumatoid arthritis. Curr Orthop. 2007;21(6):405-414.
15. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). 2012. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 3, 2015.
16. Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85(9):1775-1783.
17. Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample database. Arch Intern Med. 2005;165(15):1756-1761.
18. World Health Organization. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Geneva, Switzerland: World Health Organization; 2008.
19. Cook C, Hawkins R, Aldridge JM 3rd, Tolan S, Krupp R, Bolognesi M. Comparison of perioperative complications in patients with and without rheumatoid arthritis who receive total elbow replacement. J Shoulder Elbow Surg. 2009;18(1):21-26.
20. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976.
21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
22. R: a language and environment for statistical computing [computer program]. Vienna, Austria: Foundation for Statistical Computing; 2012.
23. Cuomo F, Greller MJ, Zuckerman JD. The rheumatoid shoulder. Rheum Dis Clin North Am. 1998;24(1):67-82.
24. Kelly IG, Foster RS, Fisher WD. Neer total shoulder replacement in rheumatoid arthritis. J Bone Joint Surg Br. 1987;69(5):723-726.
25. Donigan JA, Frisella WA, Haase D, Dolan L, Wolf B. Pre-operative and intra-operative factors related to shoulder arthroplasty outcomes. Iowa Orthop J. 2009;29:60-66.
26. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
27. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
28. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):
764-770.
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2 suppl):e278S-e325S.
30. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888.
31. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599-603.
32. Wilson A, Yu H, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(7):50-57.
33. Mercuriali F, Gualtieri G, Sinigaglia L, et al. Use of recombinant human erythropoietin to assist autologous blood donation by anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Transfusion. 1994;34(6):501-506.
34. Shander A, Gross I, Hill S, et al. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193-202.
35. Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatology. 2008;47(4):491-494.
36. Davis DE, Paxton ES, Maltenfort M, Abboud J. Factors affecting hospital charges after total shoulder arthroplasty: an evaluation of the national inpatient sample database.
J Shoulder Elbow Surg. 2014;23(12):1860-1866.
37. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
38. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
39. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288.
40. Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134-144.
41. Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263.
42. Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(2):290-294.
43. Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50(4):1122-1131.
44. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14(9):2019-2027.
45. Goz V, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine J. 2015;15(5):959-965.